Abstract
A model of the CA3 region of the hippocampus was used to simulate the P50 auditory-evoked potential response to repeated stimuli in order to study the neuronal circuits involved in a sensory-processing deficit associated with schizophrenia. Normal subjects have a reduced P50 auditory-evoked potential amplitude in response to the second of two paired auditory click stimuli spaced 0.5 s apart. However, schizophrenic patients do not gate or reduce their response to the second click. They have equal auditory-evoked response amplitudes to both clicks. When schizophrenic patients were medicated with traditional neuroleptics, the evoked potential amplitude to both clicks increased, but gating of the second response was not restored or improved. Animal studies suggest a role for septohippocampal cholinergic activity in sensory gating. We used a computational model of this system in order to study the relative contributions of local processing and afferent activity in sensory gating. We first compared the effect of information representation as average firing rate to information representation as cell assemblies in order to evaluate the best method to represent the response of hippocampal neurons to the auditory click. We then studied the effects of nicotinic cholinergic input on the response of the network and the effect of GABAB receptor activation on the ability of the local network to suppress the test response. The results of our model showed that nicotinic cholinergic input from the septum to the hippocampus can control the flow of sensory information from the cortex into the hippocampus. In addition, postsynaptic GABAB receptor activation was not sufficient to suppress the test response when the interstimulus interval was 500 ms. However, presynaptic GABAB receptor activity may be responsible for the suppression of the test response at this interstimulus interval.
1 Introduction
It has been hypothesized that inhibitory control or gating of neuronal responses to afferent inputs has is necessary for maintaining attention in a changing environment or for sharpening of criteria for appropriate response to stimuli (Hubel and Wiesel 1959). One method used to study auditory sensory gating in humans is to measure the amplitude of the P50 auditory-evoked potential during a conditioning-testing paradigm (Adler et al. 1982; Freedman et al. 1987; Braff and Geyer 1990). The P50 auditory-evoked potential is a positive waveform in the surface EEG recorded approximately 50 ms after an auditory stimulus. When a normal subject is presented with two auditory click stimuli spaced 0.5 s apart, the amplitude of the P50 evoked potential in response to the second, or test, click is typically reduced by over 60% when compared to the first, or conditioning, click. The ratio of the amplitude of the test response to the amplitude of the conditioning response is a measure of sensory gating.
1.1 Loss of sensory gating in patients with schizophrenia
In contrast to normal patients, schizophrenic patients (and approximately half of their first-degree relatives) are not able to suppress the test response (Adler et al. 1982; Siegel et al. 1984; Waldo et al. 1988). Reduced gating generally implies that the test amplitude (i.e., amplitude in response to the second stimulus) increased, although a reduction in the conditioning (or first) amplitude has also been found. Due to increased incidence of smoking in the schizophrenic population and the effect of smoking on patients, it was hypothesized that nicotine may modulate sensory gating. This hypothesis that increasing nicotinic receptor (Nic-R) activation would improve gating was tested in schizophrenic patients and their nongating relatives (Adler et al. 1992, 1993). Both groups showed a transient improvement in gating after smoking cigarettes or administration of nicotine-containing gum. Anatomical studies on postmortem tissue (Freedman et al. 1993) showed more nicotinic cholinergic receptors on the inhibitory interneurons than on the pyramidal cells, suggesting a role for nicotine modulation of inhibitory processes in the hippocampus.
1.2 Animal studies
Results from animal studies were essential in furthering our understanding of the mechanisms of sensory gating. We identified a waveform in the rat, the rat N40, analogous to the human P50 (Adler et al. 1986) (Fig. 1). The N40 is a negative waveform that peaks approximately 40 ms after the click stimulus. Like the human P50, a rat’s N40 evoked response to the second of two paired auditory stimuli was smaller than the response to the first (Fig. 1a). Results from these animal studies indicate that the inability to gate sensory input may be due to a defect in the cholinergic input at the Nic-R subtype. One possible source of this N40 waveform has been localized to the CA3 region of the hippocampus (Bickford-Wimer et al. 1990). The largest source of cholinergic input to the hippocampus is from the septum via the fimbria/fornix (Alkondon and Albuquerque 1991; Baisden et al. 1984). Lesioning this cholinergic input in the rat (Bickford and Wear 1994, 1996), or blocking Nic-Rs in the hippocampus with α-bungarotoxin (an α – 7 nicotinic antagonist) impaired gating (Luntz-Leybman et al. 1992) (Fig. 1b). Nicotinic agonists could restore gating in the lesioned rat (Bickford and Wear 1996). In contrast, blockade of muscarinic receptors did not modify the P50 response to the conditioning or testing stimulus (Luntz-Leybman et al. 1992). The impairment in gating that resulted from blocking the Nic-R was due to two factors: (1) a decrease in the amplitudes of the conditioning response (CR) and (2) a concurrent increase in the amplitude of the test response (TR). Additional studies to examine the role of local inhibitory activity on sensory gating suggested that there may be a local GABAB-R-receptor-mediated suppression of the test response (Nagamoto et al. 1991; Hershman et al. 1995; Miller and Freedman 1995).
Fig. 1.
A,B. Four averaged auditory-evoked potentials recorded from the hippocampus of an awake, freely moving rat in response to two sets of paired auditory click stimuli spaced 0.5 s apart. Gating was quantitatively expressed as the amplitude of the evoked potential recorded in response to the second (test) click divided by the amplitude of the response to the first (conditioning) click, or T/C ratio. A Two N40 auditory-evoked potentials recorded in response to the paired-click stimuli. The first waveform, or conditioning response, has a greater amplitude than the second waveform or test response. These data are from a session during which the animal markedly suppressed or gated the hippocampal response to the second click. In this case, a T/C ratio of 0.28 indicated that the response to the test click was 72% smaller than the response to the conditioning click. B Data recorded from a session during which the animals did not gate or suppress their response to the test stimuli. In this case, a T/C ratio of 0.72 indicated that the amplitude of the response to the test click was only 28% smaller than the amplitude of the conditioning response. For both A and B these data represented responses to 80 pairs of clicks that were averaged. Presentation of pairs occurred every 15 s. Computer-generated marks below each tracing indicate the peak of the N40 wave. The auditory stimulus occurs at the beginning of each trace. The horizontal calibration is 20 ms; vertical is 1.0 mV
Studies in awake, freely moving rats showed two main characteristics of the response properties of CA3 pyramidal cells in gating and nongating rats. The first characteristic was that less than 15% of the cells responded to the conditioning click stimulus. The second important phenomenon was that, when individual trials were examined, cells that were found to have responded to the test click were also found to have responded to the conditioning, or first, click (Moxon et al. 1999). These data suggested that certain cells are set to respond to the click stimulus while others are not and that during the test click the response of many of these cells is inhibited.
1.3 Earlier modeling studies
In addition to these animal studies, we also developed a computer model of the CA3 region of the hippocampus to test our hypotheses about the role of Nic-R in sensory gating (Flach et al. 1996). Our initial results from these modeling efforts were consistent with animal and human experiments. In agreement with anatomical studies in humans (see above), the model required more nicotinic cholinergic receptors on the inhibitory interneurons than on the pyramidal cells to achieve normal gating. The modeling studies of auditory sensory gating also suggested a role for local GABAB-receptor (GABAB-R)-mediated suppression of the test response. This result has been supported by animal studies suggesting that there may be a local GABAB-R-receptor-mediated suppression of the test response (see Animal studies, above). A second result of the previous model, consistent with animal studies, was that more pyramidal cells fired in response to the conditioning click than to the test click. Finally, in this computer model, blocking nicotinic cholinergic input to the CA3 network impaired gating. However, simulating a nicotinic agonist by maintaining the same level of Nic-R activation throughout the simulation did not restore gating as well as it did in the animal studies. This negative result of the model suggested that the model did not include a necessary mechanism involved in sensory gating. In the study presented here, we expand the previous model to test the hypothesis that, in addition to the role of Nic-R activation on the suppression of the test response, local GABAB-R activation created by increased activity of local CA3 interneurons suppresses the test response.
1.4 Model hypothesis
The negative result of our earlier model suggested that the model did not include a necessary sensory-gating mechanism. In the study presented here, we expand the previous model to test the hypothesis that, in addition to the role of Nic-R activation on the suppression of the test response, local GABAB-R activation created by increased activity of local CA3 interneurons suppresses the test response.
To examine the effects of local GABAB-R-mediated suppression of the test response, we needed to ensure that our simulated response to the auditory stimuli was accurate. In particular, we needed to address the question of whether the response of the local CA3 network was due to an increase in firing rate across the population or to the activation of a specific subpopulation of cells. The type of response the network produces will have consequences for the effectiveness of GABAB-R activity. Two main characteristics of the response properties of CA3 pyramidal cells were suggested by animal studies. The first characteristic was that less than 15% of the cells responded to the conditioning click stimulus. The second important phenomenon was that, when individual trials were examined, cells that that were found to have responded to the test click were also found to have responded to the conditioning, or first, click (Moxon et al. 1999). These data suggested that certain cells are set to respond to the click stimulus while others are not and that during the test click the response of many of these cells is inhibited. In the model presented here, we tested two methods of how the response of neurons in the hippocampus represent the response to the auditory click stimulus to determine which method more closely simulated the auditory click response of the CA3 hippocampal network. For the first method, average firing rate, information is represented as an increase in average firing rate across a population of cells. In this method, the response of any particular cell is not important (Salinas and Abbott 1995). The response of the network to a stimulus is measured by an increase in average firing rate across the population of cells.
The second method we tested for modeling how neurons in the hippocampus may respond to the auditory click stimuli is a modified Hebb cell assembly, or sequential configuration, first simulated in a computer model by MacGregor (1991). This theoretical model of information representation in local neural circuits considers sensory information to be maintained in coordinated, spatiotemporal firing patterns (Hebb 1949; Gerstein et al. 1986; Abeles 1991; MacGregor and Gerstein 1991; MacGregor 1993). In general, the way in which these assemblies come about and their size and spatial characteristics have not yet been fully elucidated. However, it is likely that both the spatial and temporal aspects of these patterns are important (MacGregor and Gerstein 1991; Deadwyler and Hampson 1995). In addition, it is likely that cells participate in more than one pattern. The temporal firing of a cell in relation to other cell firings in the network would then be a reflection of the response of the network to a particular stimulus.
Because our previous modeling effort, which relied solely on Nic-R activation in the CA3 region of the hippocampus to produce sensory gating, was not completely successful, in this paper we explore the addition of putative mechanisms of local GABAB-R-mediated suppression of the test response. In particular, we compare pre- vs. postsynaptic GABAB-R-mediated activity on the response of the local CA3 network to the auditory click stimulus. To accomplish this, we first explored two different representations of the CA3 network’s response to the click stimuli to determine which was more consistent with data from experimental records. The mechanisms include a comparison of representing the CA3 network’s response as average firing rate or as a modified Hebb assembly.
2 Methods
2.1 Anatomical and physiological studies of the CA3 hippocampus
The local recurrent network of the hippocampus is an ideal structure to model because of the enormous amount of anatomical and physiological data available on this structure and the fact that extensive models of this system have been previously developed (Traub 1982; Pinsky and Rinzel 1994; Flach et al. 1996). There are two main cell types in the hippocampus: excitatory pyramidal cells and inhibitory interneurons (Schwartzkroin and Mathers 1978; Schwerdtfeger and Buhl 1986). Pyramidal cells synapse onto each other (i.e., recurrent excitation) (Blackstad 1956; Andersen et al. 1966; Chronister and DeFrance 1979; Amaral et al. 1984) and onto the interneurons (Frotscher and Zimmer 1983; Frotscher et al. 1984; Witter 1989; Amaral and Witter 1989).
In addition to this local network, there are two major input systems to this region. The first input, from cortical sources, includes input from the entorhinal cortex (EC) and the dentate gyrus (DG) (Deadwyler et al. 1975). This input represents highly processed cognitive information from the association areas of the cerebral cortex that enters the CA3 region via the EC (Beckstead 1978; Witter and Amaral 1991) and the DG (Van Hoesen and Pandya 1972; Deadwyler et al. 1975). The second input, from the septum (Benardo and Prince 1982a; Buhl et al. 1989; Freund and Antal 1988) includes two distinct types of septal fibers: nonmyelinated, or thinly myelinated, cholinergic fibers (Frotscher and Leranth 1985; Frotscher et al. 1989; Nyakas et al. 1986) and myelinated GABAergic fibers (Baisden et al. 1984; Freund and Antal 1988). The septal input enters the CA3 region via the fornix (Kiss et al. 1990; Wyss et al. 1979) and may regulate arousal levels. GABAergic cells in the septum did not respond to the auditory click stimulus and are therefore not considered to modulate the response to the click stimuli.
The septal cholinergic fibers activated nicotinic and muscarinic receptors. Each of these cholinergic-fiber populations contacted both CA3 pyramidal cells and CA3 interneurons (Miettinen and Freund 1992). The Nic-R, the glutamate receptor, and the GABAA receptor (GABAA-R) are ligand-gated ion channels (Bernardo and Prince 1982a,b; Aracava et al. 1987; Dodd et al. 1981) and therefore have relatively fast time courses. Nicotine and glutamate regulate channels mostly permeable to sodium and calcium, but other ions may also enter. The opening of the channel increases the conductance to these ions. GABAA-R, on the other hand, modulate channels permeable to chloride ions. Increased permeability to sodium and calcium pushes the membrane potential toward a reversal potential of about 0 mV (Alkondon and Albuquerque 1991; Kandel et al. 1960), while chloride ions have reversal potential around −65 mV. Both the DG and the EC afferents activate glutamate receptors on both CA3 pyramidal cells and interneurons. GABAA-R chloride channels that hyperpolarize the cell also have a relatively fast time course. Activation of GABAA-R initiates current flow to chloride ions that peaks within 5 ms and lasts for less than 100 ms (Pearce 1993; Thompson and Gahwiler 1992). GABAB-R and muscarinic receptors activate second messenger systems within the postsynaptic cell and therefore have relatively slow time courses. Muscarinic receptors have more complex effects; they can either hyperpolarize or depolarize the cell (Bernardo and Prince 1982a,b; Halliwell 1990; Gahwiler and Dreifuss 1982). However, since our animal studies have clearly shown that muscarinic antagonists do not modulate P50 sensory gating, we used a single mechanism for the effect of muscarinic receptor activation (see Methods).
Animal studies indicated that most cholinergic neurons in the septum increase their firing rate more in response to the first click than to the second (Miller and Freedman 1993). In contrast, septal GABAergic cells do not seem to be directly involved in N40 auditory sensory gating because they do not fire in response to the auditory stimuli (Miller and Freedman 1993). Cells in the EC also do not seem to become habituated to sensory stimuli (Vinogradova 1975) and therefore probably respond equally to both clicks.
2.2 Structure of the CA3 hippocampal model network
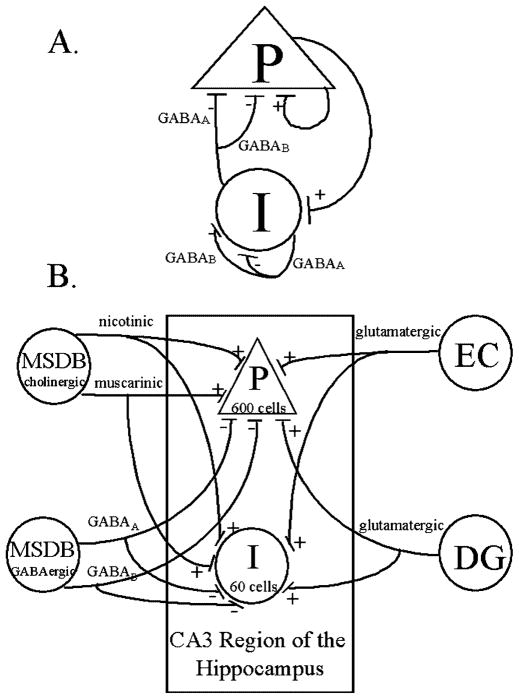
Our model of the CA3 region of the hippocampus can be broken down into the local recurrent network (Fig. 2a) and the main afferents (Fig. 2b). We scaled the model to include 600 individually modeled CA3 pyramidal cells and 60 CA3 interneurons (Kawaguchi and Hama 1987; Danos et al. 1991). The model pyramidal cells synapsed onto each other (i.e., recurrent excitation) and onto the interneurons at simulated glutamatergic postsynaptic potentials (PSPs) when a model CA3 pyramidal cell fired an action potential (Torp et al. 1992). The model interneurons contacted each other and the model pyramidal cells at two types of model inhibitory synapses, GABAA receptors (GABAA-R), and GABAB-R (Pearce 1993).
Fig. 2.
A,B. Schematic representation of the CA3 region of the hippocampus modeled in this study. The model included 600 individually modeled pyramidal cells (P) and 60 interneuron cells (I). A Recurrent activity between these two cell populations included excitatory activity between pyramidal cells and onto the interneurons and inhibitory activity between interneurons and pyramidal cells. Connections were made based on patterns embedded in the network (see text for details). Each interneuron had both GABAA and GABAB terminals. B Afferent inputs modeled in this study. Inputs from the entorhinal cortex (EC) and dentate gyrus (DG) were excitatory onto both the pyramidal cells and the interneuron cells. Inputs from the medial septum-diagonal band complex (MSDB) were of two types. MSDB cholinergic input was onto both pyramidal cells and interneuron cells at nicotinic and muscarinic receptors. MSDB GABAergic input was onto both pyramidal cells and interneurons, but this input was constant throughout the simulations (see text for details). Each of these inputs to the CA3 network was modeled using biologically relevant postsynaptic conductance changes
Two main afferent systems of the hippocampus were included in the model: cortical and septal. We modeled entorhinal and dentate gyrus cortical inputs by simulating the postsynaptic potential in response to glutamatergic receptor activation onto both the pyramidal cells and the interneurons. The septal input consisted of cholinergic fibers that activated model nicotinic and muscarinic receptors. Each of these cholinergic-fiber populations contacted both CA3 pyramidal cells and CA3 interneurons (Miettinen and Freund 1992). The septal GABAergic fibers activated both GABAA-R and GABAB-R. Both the CA3 interneurons and the CA3 pyramidal cells had septal GABAA-R and septal GABAB-R. The septal GABAergic receptor input was tonic throughout the simulation (see Subsect. Simulation of evoked potential, in Methods).
2.3 Equivalent circuit model of individual cells
In addition to modeling the overall structure of the CA3 network, we simulated individual neurons using a model that produces relatively realistic spiking model neurons similar to experimental records (MacGregor 1987). Pyramidal cells were modeled using a two-compartment equivalent circuit representation (Fig. 3), and the interneurons were modeled using a single compartment. Although detailed models of CA3 pyramidal cells using up to 28 compartments have been extensively simulated, Traub (1982) and Pinsky and Rinzel (1994) have shown that two compartments are sufficient to model the input-output dynamics of CA3 pyramidal cells.
Fig. 3.
Model of CA3 pyramidal cell. The model of the CA3 pyramidal cell had two compartments. There were nine synaptic inputs: recurrent excitation, local GABAA and local GABAB, excitatory input from the dentate gyrus (DG) and the entorhinal cortex (EC), and muscarinic and nicotinic cholinergic input from the septum. Inputs to the cell were distributed as shown. In addition, there was a voltage-dependent potassium conductance in the soma (GK) and a calcium-dependent potassium current in the dendrite (GKD). Finally, there was a voltage-dependent calcium current in the dendrite (GCA)
In the model, the rate of change of the potential of the soma, ES, was proportional to the sum of the currents across the membrane (Eq. 1). These included the synaptic currents, Gi, which are further described in the next section, Methods, a potassium current, GKS, that was responsible for the cell’s after-hyperpolarization, and the conductance from the dendritic compartment, GDS. If the potential of the cell exceeded threshold, the cell generated an action potential and projected synaptic activation to downstream cells.
| (1) |
The relative volume of the soma and dendritic compartments determined the value of GDS. Based on previous modeling studies (Traub et al. 1982; Pinsky and Rinzel 1994) we chose a value of 5 for this parameter. TS was the time constant of the soma membrane set to 0.5 ms. In addition, the equilibrium potentials of the different ionic currents were specified. EK, the potassium equilibrium potential, was −10 mV relative to resting. The EQi represented the equilibrium potentials for the different receptor types (i.e., glutamatergic, cholinergic, or GABAergic). The potassium current in the soma, GKS, was voltage dependent and partially responsible for the cell’s after-hyperpolarization. When the potential of the soma reached threshold, GKS was increased by an amount B and decayed with time constant τGK(Eq. 2).
| (2) |
ED represented the potential of the dendritic compartment. The rate of change of the potential of the dendritic compartment was also proportional to the sum of the conductances across the membrane. These included synaptic conductances, Gi, a voltage-dependent calcium current, GCA, a calcium-dependent potassium current, GKD, and the conductance from the soma compartment, GSD (Eq. 3).
| (3) |
ECA represented the calcium equilibrium potential, taken to be about 50 mV above resting, and GDS represented the conductance from the dendrites to the soma. This factor was related to the relative size of the dendrites to the soma. We used a value of 5.0 for GDS and 1.0 for GSD because the volume of the dendrites is approximately five times the size of the soma.
The conductance to calcium, GCA, was voltage dependent (Eq. 4). When the potential of the dendritic compartment exceeded the dendritic threshold, THD, the calcium conductance was increased by an amount D and decayed with time constant TGC.
| (4) |
The potassium current, GKD, was voltage dependent. The calcium concentration, CA, was proportional to the conductance to calcium (Eq. 5). A represented the amplitude of calcium-concentration rise in proportion to calcium conductance, and TCA was the time constant of calcium-concentration rise. CA0 was the threshold level of calcium concentration required to trigger potassium conductance in the dendrites.
| (5) |
When calcium conductance in the dendrites increased the concentration of calcium above CA0, there was an increase in dendritic potassium conductance, GKD (Eq. 6). BD, the amplitude of potassium-conductance increase, corresponded to the given level of CA. In the model, GKD increased incrementally (in proportion to BD) as long as CA was above CA0. As GKD rose, it allowed current to leak out of the model neuron, driving the potential of the model neuron toward the potassium equilibrium potential of −10 mV. When the membrane potential of the dendritic compartment dropped below its threshold, THD, the calcium conductance was no longer driven upward and tended to decay with time constant TGC toward zero. As GCA approached zero, the calcium concentration also decreased. When it fell below CA0, then GKD was no longer being augmented and it in turn began to decay exponentially back to zero. The net result of this mechanism was a relatively slow calcium spike of the order of 3 to 5 ms and a relatively long-lasting after-hyperpolarization, both of which are shown in Fig. 4.
Fig. 4.
A–C. The response of a model pyramidal cell to synaptic input. Each trace shows the time course of somatic voltage, ES. A The response of the model pyramidal cell to excitatory and inhibitory synaptic input. The inputs were onto both the somatic and dendritic compartment and resulted in low-frequency bursting. B Strong current injection into the soma resulted in high-frequency somatic spiking. C Strong current injection into the dendrites produced a combination of somatic spikes and weak dendritic bursts
| (6) |
2.4 Validation of single cell properties
The membrane equations were numerically integrated (MacGregor 1987), and the result was a reasonable simulation of neural activity from different types of synaptic input (Fig. 4). Moderate excitatory and inhibitory input to both compartments of the cell produced a series of action potentials riding on a relatively slow calcium spike followed by a relatively long-lasting after-hyperpolarization (Fig. 4a). Strong current injection into the soma generated somatic spiking with no calcium spike (Fig. 4b). A similar current injection into the dendrites (Fig. 4c) generated a combination of somatic spikes and weak calcium-mediated dendritic bursts. These data are consistent with previous models of hippocampal neurons (Pinsky and Rinzel 1994).
2.5 Synaptic effects between cells in the model
The postsynaptic potentials (PSPs) generated by activation of a receptor are modeled using a dual alpha-function equation (Eq. 7). The parameters that distinguish the different PSPs (i.e., resulting from the activation of a glutamate receptor as opposed to a GABA receptor) are the amplitude of the PSP, gmax, and the time course, described by τ1 and τ2 (Table 1). However, the resulting PSPs, when measured at the soma of the pyramidal cells, were different for DG as compared to EC inputs (Scharfman and Schwartzkroin 1990). The EC input contacts the distal dendrites, while the input from the DG contacts the proximal dendrites. To model this efficiently, we place the EC glutamatergic receptors on the dendritic compartment and the DG glutamatergic input on the soma compartment.
Table 1.
Model parameters for each synaptic type described in the model
| Synapse Type | τ1 | τ2(ms) | Equilibrium Potential (mV) |
|---|---|---|---|
| 1. Entorhinal Cortex | 5.00 | 1.00 | 70.0 |
| 2. Dentate Gyrus | 5.00 | 1.00 | 70.0 |
| 3. Recurrent Excitation | 5.00 | 1.00 | 70.0 |
| 4. Recurrent GABAa | 5.00 | 2.00 | −10.0 |
| 5. Recurrent GABAb | 60.00 | 50.00 | −10.0 |
| 6. Septal Nicotinic | 5.00 | 4.00 | 70.0 |
| 7. Septal Muscarinic | 40.00 | 30.00 | 70.0 |
| 8. Septal GABAa | 5.00 | 3.00 | −10.0 |
| 9. Septal GABAb | 60.00 | 50.00 | −10.0 |
| (7) |
In the model, GABAB-R activation (like GABAA) hyperpolarized the cell (Dingledine and Langmoen 1985). The PSP resulting from the activation of a GABAB-R took longer to peak (approximately 180 ms) (Peet and McLennan 1986) than the activation of a GABAA-R. The duration of the resulting chloride-ion current was more than 300 ms (see Dutar and Nicoll 1999 for a complete description). Similarly, the time course of the muscarinic input in the model was slower than that of the nicotinic input (Misgeld et al. 1980; Dodd et al. 1981). Since our animal studies have clearly shown that muscarinic antagonists do not modulate P50 sensory gating, we used a single mechanism for the effect of muscarinic receptor activation. The effect of muscarinic receptor activation in the model was to generate a postsynaptic response that hyperpolarized the cell. Based on the above information, we have chosen parameters for the equilibrium potentials and the synaptic time constants (Table 1).
2.6 Simulation of evoked potentials
The evoked potentials were modeled by simulating experimental data collected from rats during the presentation of paired auditory clicks that measured the firing rates of septal, hippocampal, and EC cells (Moxon et al. 1999). Using these data, we simulated the click stimuli by increasing the septal cholinergic input and the cortical input in the following manner. The simulation began 100 ms before the first click. Each fiber population maintained a small amount of background activity throughout the simulation. To simulate the first click stimulus, we increased the activity of the fiber populations known to reach the hippocampus after the click stimulus (Miller and Freedman 1995). The septal cholinergic fibers were activated first. Their firing rate remained increased for 5 ms. Two milliseconds after the start of the cholinergic input, the EC input was activated for 2 ms. The DG was activated 1 ms after the start of the EC input and lasted for 2 ms. The test click was simulated similarly to the conditioning click. However, the amount of septal cholinergic activity was half of the activity during the conditioning click.
The evoked response of the model simulated the activity recorded from the pyramidal cell layer during animal studies that appear to correlate with surface EEG electrode recordings in human studies. In the model, we simulated the evoked response of the network, VT, by summing the membrane potential, E, of every cell in the network. If a cell fired an action potential, a maximum spike potential, EQspike, represented the changes in the voltage drop across the membrane due to the action potential. EQspike was equal to 70 mV in all of these simulations. After summing the membrane potential of all the cells at each instance of time and including the firing factor, we divided this total by the number of cells in the population, N. The representation of the evoked response included a strength factor, AVR, to compensate for the reduced number of cells simulated (Flach et al. 1996) and a time constant for decay through the extracellular resistance, TVR (Eq. 8). The value of AVR was chosen so that the amplitude of the normal conditioning response was consistent with experimental data (approximately 2 μV amplitude for conditioning response in rats). It remained constant throughout all of the simulations (refer to Fig. 11 for an illustration of the computer-simulated P50 auditory-evoked response to conditioning and test stimuli).
Fig. 11.
A–C. Effect of modulating nicotinic cholinergic activity in the model. A Normal P50 auditory sensory gating, described in Fig. 10 is reproduced here for convenience. B Blocking nicotinic receptors in the model reduced sensory gating by producing a large decrease in the conditioning amplitude as compared to the normal case (A). Therefore, when nicotine was blocked, the T/C ratio was increased to 92.5%. C Simulating a nicotinic agonist restored gating. This decreased the T/C ratio to 12.0%, which was similar to the normal gating T/C ratio of 16.9%. Calibration bars are the same for each evoked potential: vertical calibration bar is 0.5 mV, horizontal calibration bar is 50 ms
| (8) |
where
VT(t) = total evoked potential at time t
TVR = decay constant for the evoked potentials
AVR = strength factor representing network structure and external resistance
E(i, k) = generator potential of the kth cell inthe ith population
N(i) = number of cells in the ith population
2.7 Representation of information in the model
Two theories regarding information representation in networks were modeled: (1) representation of information as cell assemblies and (2) representation of information as average firing rate. The results simulating these models were compared to the response characteristices of CA3 cells recorded during the P50 auditory gating paradigm to determine which model best simulated the data. These data from animal studies (Moxon et al. 1999) revealed two characteristics of the response properties of CA3 hippocampal cells to the auditory click stimuli. First, less than 15% of the cells responded to the first or conditioning click. Second, when individual trials were examined, only cells that responded to the first click stimulus were likely to respond to the second stimulus.
2.7.1 Representation of information as cell assemblies
To represent information as cell assemblies, we first had to define the cell assembly or pattern. All patterns or cell assemblies used in these simulations consisted of 15 cells firing (10 excitatory CA3 pyramidal cells and 5 inhibitory CA3 interneurons) at each time step for 5 ms (each time step is 1 ms). The patterns had a temporal as well as spatial dimension and consisted of ordered sequences of sets of cells. At each time step, a set of cells, called a link, fire an action potential and project coordinated input to cells in the next set of links. For example, each pattern in the model presented here consisted of five links. Each link consisted of ten excitatory CA3 pyramidal cells and five inhibitory CA3 interneurons. The identity of the cells in each link that constituted a pattern was generated randomly except for one test pattern that was created to be easily visualized (Fig. 5a). For this easily observed pattern, excitatory cells 1–10 fired at time t = 1, cells 11–20 fired at t = 2, etc., while inhibitory cells 1–5 fired at t = 1, inhibitory cells 6–10 fired at t = 2, etc. Therefore, each pattern has both a spatial and a temporal dimension. The temporal dimension defines a set of sender links and a set of receiver links at each time step.
Fig. 5.
A,B. Diagram of the cell assemblies in the model. A Representation of a single pattern embedded in the network. Cells 1–10 constitute one link, while cells 11–20 are the second link, cells 21–30 the third link, 31–40 the fourth link, and cells 41–50 the final link. The cells in the first link (cells 1–10) are synaptically connected to each of the other links. The value of the synaptic strengths are modified according to Eq. 10. For example, the magnitude of the synaptic-strength modification for cells between the first and second link is 1.0. The magnitude of the synaptic-strength modification between the first and third link is 0.605, and that between the first and fourth link is 0.368. Cells in the second link are synaptically connected to the third and fourth links (cells 21–40), while cells in the third link are synaptically connected to the fourth link. The pattern can be recalled by stimulating the first link of the pattern. For example, if the network is stimulated with the first link (i.e., all cells in the first link fire simultaneously), each of the cells in the first link will project synaptic activation to cells 11–40 according to Eq. 10. This synaptic activation is sufficient to cause the cells in the second link to fire at the next time step. These cells then provide synaptic input to the third link, which fires at the next time step. If only one pattern is embedded in the network, the pattern will respond with no noise and the cells will fire as defined by the pattern. B Effect of sharing synapses among patterns embedded in the network. When three more patterns were embedded in the network, several synapses were shared among the patterns, and therefore cross-talk activity caused the recall of the pattern to be noisy. Cells may fire earlier or later then their appointed time or they may not fire at all. In addition, cells not in the pattern (e.g., cells 56, 72, 73, etc.) received enough excitation to fire action potentials
In order for each link to fire in consecutive order, the receiving link must receive sufficient activation. When the patterns were embedded in the model, the synaptic strengths were modified by an amount DELTA (Eq. 9). Each receiving link receives synaptic activation from more than one of the previous sending links. LNKD represents the number of sender links that project to a given receiver link (Eq. 9). ℓ represents the time between the sending link and the receiving link. The value of LINKD and, therefore, number of sender links projecting synaptic activation to a receiving link was determined by measuring the number of PSPs necessary to raise the membrane potential of the cells in the receiving link above threshold (MacGregor 1991). In all of the simulations for this study, the value of LNKD for all of these simulations was 2. The number of cells that fired in each link was equal to 10 for the pyramidal cells and 5 for the interneurons.
| (9) |
As a result of these synaptic modifications, the magnitude of the synaptic change between the sender link and the receiver link increased as the time between the sending link and the receiving link decreased. The rate of change of the magnitude of the synaptic strength was exponential with decay factor DC.DC was equal to 2.0 for all of these simulations, so that the change is approximately equally distributed across the four links (Eq. 10).
To embed the patterns in the model, the synaptic strengths were modified by DELTA from an initial starting point of minimum excitation (i.e., 0.0) and maximum inhibition (i.e., 1.0). The excitatory synaptic strengths were initially set to 0.0, while the inhibitory synaptic strengths were set to 1.0. As the patterns were embedded in the network, excitatory synapses between adjacent links were modified from 0.0 by the amount determined by DELTA, while the inhibitory strengths were decreased from 1.0 by the amount DELTA. Since LNKD=4 in these simulations, there were four different values that the synaptic modification DELTA could take depending on how far away the cells were in time (Eq. 10).
| (10) |
The deltaex represent the excitatory synaptic modifications, and the deltain represent the inhibitory synaptic modifications that were set when only one trace was embedded in the network. For all other synapses, deltaex = 0.0 and deltain = 1.0. Therefore, any cell that did not participate in the pattern was maximally inhibited and received no excitation. The result of these synaptic modifications was to produce a ramp of increasing excitation and decreasing inhibition in the LNKD time steps before the link was scheduled to fire.
The computer program used here had two distinct parts, an embedding phase and a recalling phase. During the embedding phase the synaptic strengths were set using Eq. 9, depending on the patterns the user predefined. Specific mechanisms for how the network actually learned these patterns were not included. Each cell in the first link of the excitatory population experienced an increase in the strength of its synapses to all of the cells in the next four (LNKD) links in both the excitatory and inhibitory populations. Each cell in the first link of the inhibitory population saw the strength of its synapses to all of the cells in the next four (LNKD) links in both the excitatory and inhibitory populations decrease from a starting value of 1.0. This continued for each link in the pattern. The recalling phase consisted of stimulating the first two links to fire in consecutive order. If only one pattern was embedded in the model, cells in the pattern received maximum inhibition and no excitation until two time steps (LNKD) before the cell was due to fire. Gradually the inhibition was released and the excitation increased until one time unit before the cell was scheduled to fire. At this point, excitatory input was at a maximum and inhibition was at a minimum. The cells in the receiving link fired simultaneously and projected activation to the next LNKD sets, thereby propagating the pattern (Fig. 5a).
As more patterns were embedded and a synapse was used in more than one pattern, the maximum value for the excitatory synapse and the minimum value for the inhibitory synapse were used. In both cases, this maintained the greatest change in synaptic strength from the initial starting value. These shared synapses caused disruption of the pattern when one of the patterns was recalled (Fig. 5b). Cells did not fire due to increased inhibition from the shared synapses, or cells fired earlier than expected due to increased excitation from the shared synapses. In addition to changes in the firing times of cells that were part of the pattern being recalled, embedding new patterns created new synapses. The result, when the pattern was recalled, was that some cells not in the current pattern received enough excitation to fire an action potential, even though they were not part of the pattern being recalled (e.g., cells 56, 72, 73, etc. in Fig. 5b). This noise was due to cross-talk activation of extraneous synapses from postsynaptic potentials incident on cells not in the current pattern due to new synapses formed from additional patterns embedded in the network (MacGregor 1991).
2.7.2 Information representation as average firing rate
A second method for the representation of the CA3 network’s response to the click stimulus was average firing rate. In this case, the average firing rate of all the cells in the network represented the response to the stimulus, not the response of any particular cell. The synaptic strengths between cells in the model were set at random. Each CA3 pyramidal cell in the model contacted 25 of its neighboring pyramidal cells and 10 of its neighboring interneurons. Connectivity between cells in the network fell off exponentially the further its neighbor was away spatially (Traub 1982). Inputs from EC and DG contacted a random set of pyramidal cells and interneurons. The simulated click stimulus activated a subset of EC and DG input fibers. The number of EC and DG fibers activated was determined so that 15% of the CA3 cells responded to the simulated click stimulus.
2.8 Mechanism for presynaptic GABAB-receptor activation
The activation of presynaptic GABAB-R by nicotinic cholinergic input may be partially responsible for the reduction of the test response in normal gating (Nagamoto et al. 1991; Hershman et al. 1995; Flach et al. 1996). Several mechanisms have been proposed to account for this. It has been shown that presynaptic GABAB-R activation can reduce the amount of excitatory neurotransmitter released by reducing calcium (Ca++) current in the synaptic terminal (Hirata et al. 1992). Alternatively, it is also possible that GABAB affects transmitter release indirectly by first increasing a K+ conductance that then results in a change in Ca++ conductance (Gahwiler and Brown 1985). The result of both of these mechanisms is a smaller excitatory postsynaptic potential (EPSP) in the postsynaptic cell. We simulated a presynaptic mechanism for GABAB-R activation on CA3 pyramidal cells. This is consistent with recent data (Mizukami et al. 2000) showing a relatively larger loss of GABAB-R immunoreactivtity on pyramidal cells when compared to interneurons within the hippocampus of subjects with schizophrenia.
To minimize computational constraints, presynaptic GABAB-R activation in the model has a direct effect on EPSPs. We hypothesized that increased nicotinic input from the septum in response to the conditioning stimuli increased the activity of interneurons between the paired stimuli. The effect of activating these presynaptic GABAB-R was a reduction in EPSP amplitude from both recurrent activity and excitatory afferents from the EC (Fig. 6). Therefore, in the model, the Nic-R activity during the conditioning response stimulated presynaptic GABAB-R that subsequently reduced the amplitude of EPSPs during the test response. The effect of presynaptic GABAB-R activation in the model was to reduce the amplitude of EPSPs from recurrent activity and cortical input during the test response, but only when there was Nic-R activation during the conditioning response.
Fig. 6.
Schematic representation of presynaptic mechanisms of GABAB. Presynaptic GABAB acted at cortical (EC) and recurrent excitatory synapses (P) in the model by reducing the synaptic strength of the synapse. The GABAB was released from the interneurons (I) when the cells were stimulated by septal nicotinic input
3 Results – testing of model hypotheses
3.1 Test of average firing rate vs. cell assemblies
To simulate the auditory-evoked response and test contribution of pre- vs. post- synaptic GABAB on sensory gating, it was necessary to first ensure an accurate response to the simulated input. To test the model, we compared the responses of individual cells when average firing rate was modeled to the responses when cell assemblies were to determine the best model for representing the response of the network to the auditory stimuli.
When information representation as average firing rate was simulated, the simulated click input to the network was equally likely to activate any of the CA3 hippocampal cells. An increase in the average firing rate of the network represented the information. Eighty separate simulations were performed. For each simulation, only the random number seeds, which determined the input fibers that were activated, were changed; everything else remained the same. Enough fibers were activated during each simulation to cause about 15% of the CA3 pyramidal cells to fire in response to the click stimulus. Therefore, because the particular set of input fibers that are activated was selected randomly on each trial, a different, random set of CA3 cells responded on each trial (Fig. 7a). Two results from these simulations were most notable. First, each cell was equally likely to respond to the stimulus. This is not consistent with data recorded from awake, freely moving rats (Moxon et al. 1999). Second, on any given trial, a cell is equally likely to fire to the test stimulus regardless of whether or not it fired in response to the conditioning response. This result is also inconsistent with experimental data from awake, freely moving rats (Moxon et al. 1999). Therefore, information representation as average firing rate is not a good model of the response of the CA3 network to the click stimulus.
Fig. 7.
A,B. A. Poststimulus time histograms for pyramidal cells in the model when information was represented as average firing rate A and when information was represented as cell assemblies B. The stimulus is a single, simulated auditory click stimulus. A Response of four cells representing the population. These responses were representative of all cells in the network. All cells showed a small response to the stimulus. B Poststimulus time histograms for four pyramidal cells in the model when information was represented as cell assemblies. The top two responses were cells that were part of the pattern being recalled. There was a large response approximately 20 ms after the simulated input. The bottom two responses were from two cells not part of the pattern. These cells did not response to the stimulus. Bin sizes were 1 ms. The arrow points to the simulated stimulus input. The results were the sums for 80 trials
When information representation as cell assemblies was simulated, four patterns and one test pattern were embedded in the network. The results of these simulations are shown in Fig. 7b. Again, we ran 80 simulations. However, in this case the input from the EC was specific to activate the test pattern. The connectivity between cells did not change during each simulation, similar to the average firing rate case described above. These results (Fig. 7b) were more consistent with data collected from CA3 pyramidal cells (Moxon et al. 1999; Miller and Freedman 1995). Only 12% of the cells are consistently responsive to the click, which is similar to data recorded from awake, freely moving rats (Moxon et al. 1999). In addition, on any given trial the cells in the pattern were 85% more likely to respond to the test stimulus if they responded to the conditioning stimulus, and cells not in the pattern were unlikely to fire in response to either stimuli.
The results of these simulations suggest that information represented as cell assemblies generates cell responses that are more similar to data recorded from freely moving animals than model cell responses when information is represented as average firing rate. Therefore, for subsequent simulation in this study, information is represented as cell assemblies.
3.2 Test of GABAB-R activation on suppression of the test response
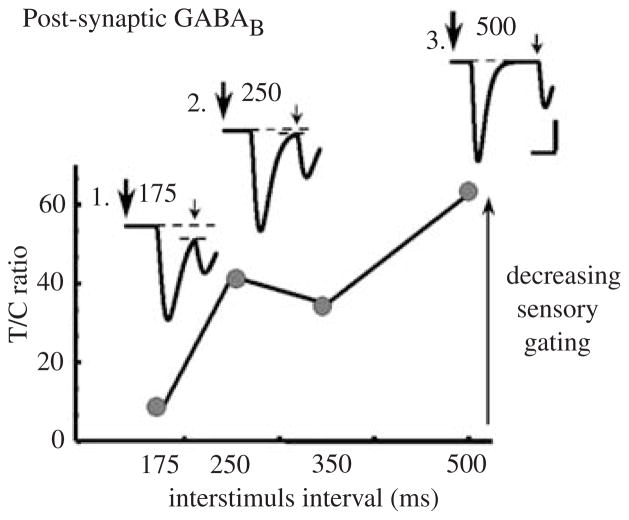
Using the model for auditory-evoked potential responses developed above, we now wanted to compare the effects of pre- and post-GABAB-R activity on sensory gating. To examine the effects of postsynaptic GABAB-R activation, we varied the time between the clicks from 175 ms to 500 ms and measured the suppression of the test response (Fig. 8). When only a postsynaptic mechanism for GABAB-R activation was used (Fig. 8a), at 175 ms, the test response was adequately suppressed. The postsynaptic GABAB-R activation initiated an inhibitory current that was still active in the postsynaptic pyramidal cells when the second stimulus was delivered (Fig. 8). The time constant of the inhibitory current produced by the activation of postsynaptic GABAB-R was 250 ms. Therefore, at 175 ms, when the test stimulus was simulated, the response was suppressed by the inhibitory current generated by the conditioning stimulus (Inset 1, Fig. 8). But as the interstimulus interval increased, the inhibitory current activated during the conditioning response continued to decay (Insets 2 and 3, Fig. 8). It was evident that postsynaptic GABAB-R activation of the inhibitory current was not sufficient to adequately (greater than 60%) suppress the test response when the test response was delivered more than 250 ms after the conditioning response. When the interstimulus interval was increased to 250 ms, the suppression was reduced to 60%. At the 500-ms interval there was less than 40% suppression of the test response. These results suggested that in addition to the afferent input that activates postsynaptic GABAB-R during the conditioning response and initiates an inhibitory current that is partially responsible for the suppression of the test response, there may be some additional local processing within the CA3 region of the hippocampus that contributed to the suppression of the test response. This local processing possibly involves presynaptic GABABR mechanisms (Hershman et al. 1995; Flach et al. 1996).
Fig. 8.
Ratio of the test response to the conditioning response (T/C ratio) as a function of interstimulus interval. When the interstimulus interval is 175 ms, local postsynaptic GABAb activity is sufficient to suppress the response to the test click. However, as the interstimulus interval is increased, the response to the test response also increases. Insets show the total GABAb current generated. The large arrow marks time of the first click. Inset 1: GABAB current generated when interstimulus interval is 175 ms. Inset 2: GABAB current generated when interstimulus interval is 250 ms. Inset 3: GABAB current generated when interstimulus interval is 500 ms. At 175 ms and 250 ms, the postsynaptic GABAB-receptor-mediated inhibitory current did not completely decay away before the second or test stimulus was activated (refer to dotted lines below second, smaller arrow). Therefore, postsynaptic GABAB currents contributed to the suppression of the test response when the interstimulus interval was 250 ms or less. When the interstimulus interval was 500 ms, the GABAB postsynaptic current was completely decayed away before the onset of the second stimulus. pS is picosiemens. Vertical calibration bar: 100 pS. Horizontal calibration bar: 200 ms
When the presynaptic mechanism for GABAB-R activation was added to the model, the amount of GABAB-R activation was proportional to the amount of Nic-R activation during the conditioning response. Nic-R activation during normal gating produces the greatest amount of GABAB-R activation. In this case, the suppression of the test response remained greater than 60% for up to a 500-ms interstimulus interval (Fig. 9). We now want to use this improved model (which includes Hebb assembly representation of the CA3 network response and a presynaptic mechanism for GABAB-R activation) to simulate normal P50 sensory gating, P50 sensory gating when Nic-R is blocked, restoration of gating from a nicotine agonist, and gating when GABAB-R is blocked.
Fig. 9.

Ratio of the test response to the conditioning response (T/C ratio) as a function of interstimulus interval when a presynaptic mechanism is included in the model. The test response is sufficiently suppressed for all interstimulus intervals tested, including 0.5 s
4 Results – validation of final model
The final version of the model included information representation as cell assemblies (refer to Sect. 2.7.1) and presynaptic mechanism for GABAB (refer to Sect. 2.8). To test the evoked response of the model, we measured the amplitude of the conditioning and test response of the simulated evoked potential as the largest negative deflection from baseline after the start of the simulated click. As in previous reports (Adler et al. 1982, 1986), the ratio of the amplitude of the test to the conditioning response (T/C ratio) was used to quantitatively measure the amount of auditory gating in the model. To simulate the conditioning response, nicotinic cholinergic input from the septum excites the cells in the CA3 network. Two milliseconds later, a pattern of information representing the click stimulus enters the hippocampus from the association areas of the cortex through the EC and stimulates the test pattern previously embedded in the network. The embedded pattern was recalled and generated a simulated P50 auditory-evoked potential.
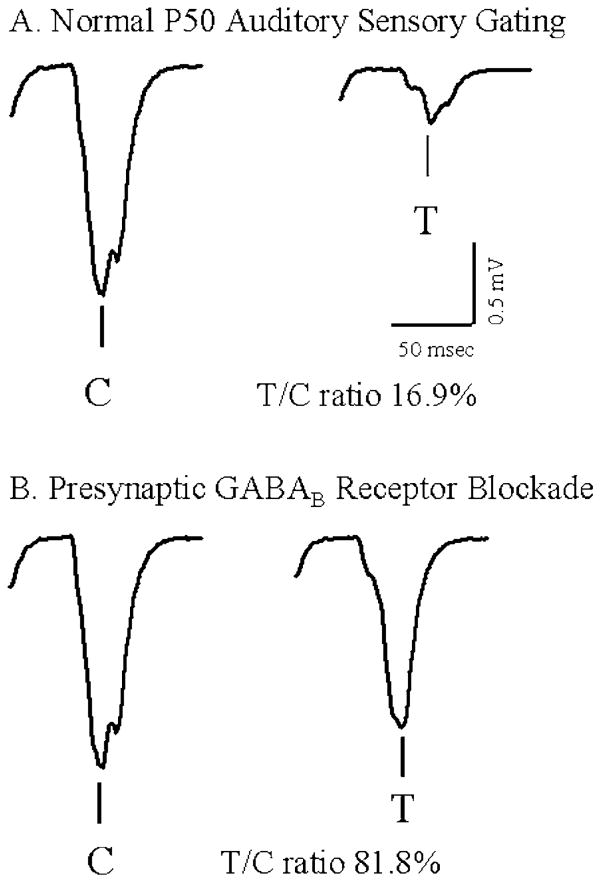
During the test response, the amount of cortical stimulation remained the same. The cholinergic input was reduced by 50% and the DG input by 25%. In addition, the presynaptic GABAB-R mechanism was initiated in response to nicotinic cholinergic input during the conditioning response that reduced the EPSPs by 50%. The result was a reduced simulated P50 evoked potential (Fig. 10a). The ratio of the test response to the conditioning response was 16.9%. These results are similar to human and animal studies.
Fig. 10.
A,B. Computer simulations of the P50 auditory-evoked potential to two paired-click stimuli. A Normal gating was simulated by increasing cholinergic activity from the septum and stimulating one of the four patterns embedded in the network via the EC input. The amplitude of the conditioning respones (C) was greater than the amplitude of the test response (T) by 83.1% (T/C ratio was 16.9%). T was modeled by reducing the cholinergic input to half of the input during the C and decreasing the EPSP by half to simulate presynaptic GABAB activity. B When presynaptic GABAB receptors were blocked, the amplitude of C was unchanged. However, the amplitude of T increased, reducing sensory gating. The T/C ratio was 81.8%. Calibration bars are the same for each evoked potential: vertical calibration bar is 0.5 mV, horizontal calibration bar is 50 ms
5 Simulation of P50 gating
5.1 Blocking GABAB
When the presynaptic effects of GABAB-R activation were incorporated into the model (Fig. 10), normal gating was simulated. Connections between cells in the CA3 network are made based on three random patterns and one test pattern, which represented the network’s response to the tone. To simulate a GABAB antagonist, the GABAB-R mechanism outlined in Sect. 2.8 was disabled in the model and the simulation repeated. In this case, the amplitude of the test response was greatly increased, and gating was reduced to 81.8% (Fig. 10b).
5.2 Effect of blocking nicotine and simulating a nicotinic agonist
To simulate blocking nicotinic input, we deactivated the Nic-Rs in the model (Fig. 11). While Nic-Rs were blocked, there was a slightly smaller simulated response of the network to the conditioning click and a much larger simulated response to the test click (Fig. 11b) when compared to the normal case (Fig. 11a). When Nic-Rs are inactivated in the model, the presynaptic GABAB-R mechanism during the test response was not initiated because of a lack of nicotinic input during the conditioning response, and therefore there was a larger simulated test response than in the normal case. The result was a loss of simulated gating due to a decrease in the conditioning amplitude and an increase in the test amplitude. The ratio of the test response to the conditioning response was 92.5% (Fig. 11b) compared to 16.9% during normal gating (Fig. 11a). This result was consistent with both animal models (Luntz-Leybman et al. 1992; Bickford and Wear 1994, 1996) and human studies of the effects of nicotine on sensory gating (Adler et al. 1992, 1993) that demonstrated that lesioning or blocking nicotinic input to the CA3 region of the hippocampus interferes with gating by a significant increase in the amplitude of the test response and concomitant decrease in the conditioning response.
Next, we simulated a nicotinic agonist by activating the Nic-Rs throughout the simulation and restoring the presynaptic GABAB-R mechanism during the test response (Fig. 11c). The Nic-R activation was increased at the start of the simulation and remained at the same level throughout as opposed to the activity during normal gating. The conditioning amplitude was increased approxiametely 10% as compared to the conditioning amplitude when Nic-Rs were blocked. To simulate the test response during a nicotinic activation, the nicotinic cholinergic input was the same level as the conditioning input and lasted throughout the simulation. The GABAB-R mechanism was restored due to the restoration of nicotinic input during the conditioning response. The result was a 50% decrease in the test amplitude when compared to the test amplitude when the Nic-Rs were blocked. Sensory gating was restored, and the ratio of the test response to the conditioning response was 12%, which is similar to the T/C ratio of 16.9% during normal gating.
5.3 Effect of simulating GABAB agonist
To study the effects of presynaptic GABAB-R activation without the nicotinic cholinergic input, we simulated a GABAB agonist when the Nic-Rs were blocked (Fig. 12). With Nic-Rs blocked, the addition of a GABAB-R agonist resulted in a reduced amplitude of the conditioning response when compared to the normal conditioning response (Fig. 12a, first trace) and a reduced amplitude of the conditioning response when compared to the response when Nic-Rs alone were blocked (Fig. 12b, first trace). The amplitude of the test response was smaller than the normal test response (Fig. 12a, second trace) and smaller than the test response when Nic-Rs were blocked (Fig. 12b, second trace). The amplitude of the test response is a function of the number of activated presynaptic GABAB receptors. Simulating a GABAB agonist activates more receptors and therefore reduces the size of the evoked potential to both the conditioning and test stimuli (Fig. 12c).
Fig. 12.
A–C. Effect of modulating GABAB presynaptic receptors and nicotinic cholinergic activity in the model. A Normal P50 auditory sensory gating, described in Fig. 10, is reproduced here for convenience. B The effect of blocking nicotinic receptors, described in Fig. 11, is reproduced here for comparison. C Simulating a presynaptic GABAB agonist improved gating by decreasing the amplitude of the test response (T) but had no effect on the conditioning response (C). This decreased the T/C ratio to 36.0%. Calibration bars are the same for each evoked potential: vertical calibration bar is 0.5 mV, horizontal calibration bar is 50 ms
6 Discussion
Gating of sensory information is thought to be one of the essential underlying mechanisms of information processing (Hubel and Wiesel 1959). P50 auditory sensory gating, modeled here, is characterized by a decreased P50 auditory-evoked response to a repeated stimulus, and a lack of P50 gating has been shown to be a trait deficit for schizophrenia (Waldo et al. 1988). In fact, schizophrenics and approximately half of their first-degree relatives do not gate (Adler et al. 1982). Furthermore, traditional neuroleptics (typically dopamine receptor antagonists) do not restore gating in the majority of schizophrenic subjects, whereas nicotinic cholinergic agonists show a transient improvement in P50 gating (Adler et al. 1992). Further human studies (Adler et al. 1982), which measure the P50 auditory-evoked response in the hippocampus, and rodent studies (Bickford-Wimer et al. 1990), which measure the analogous N40 auditory-evoked response, have provided several kinds of data that suggest putative mechanisms for sensory gating. First, it is clear that Nic-R activation is essential for normal gating (i.e., a greater than 60% reduction in the second (test) response as compared to the first (conditioning) response (Adler et al. 1982). Blocking nicotinic neuronal transmission, either pharmacologically (Bickford-Wimer et al. 1990) or with lesions (Luntz-Leybman et al. 1992), results in a significant increase in the amplitude of the evoked test response and a decrease in the amplitude of the conditioning response.
The computational model presented here confirms and extends our understanding of the underlying mechanisms of sensory gating. While our previous computer models also indicated that Nic-R activation is essential for normal gating (Flach et al. 1996), these computational models also suggested that Nic-R activation alone is not sufficient to account for all of the data. These results, combined with pharmocological response studies (Nagamoto et al. 1991; Hershman et al. 1995; Miller and Freedman 1995), suggested that local inhibition within the CA3 region of the hippocampus was also essential for normal gating. To test this possibility, we expanded our previous model of sensory gating to examine the effects of local inhibition within the CA3 network of the hippocampus and explored the effect of two different GABAB-R mechanisms – one presynaptic and the other postsynaptic. When we used the present computer model to simulate direct activation of postsynaptic GABAB-Rs by local interneurons, there was not enough inhibitory current, at the 500-ms interstimulus interval, to suppress the evoked test response (refer to Fig. 8a) and simulate normal gating. Therefore, the model suggests that neither a postsynaptic GABABR mechanism nor a Nic-R mechanism (Flach et al. 1996) is, by itself, sufficient to produce normal gating.
On the other hand, when we used the present computer model to simulate presynaptic GABAB-R activation that resulted from indirect activation of presynaptic GABAergic transmission by the septal nicotinic cholinergic input, we could simulate normal gating. As a result, we propose a mechanism whereby the septal nicotinic fibers indirectly activate the presynaptic GABAB-Rs and produce the local recurrent inhibitory activity that is necessary for normal gating. This hypothesis has two primary advantages over our previous model. First, this model can explain the somewhat paradoxical phenomenon that lesioning or blocking the nicotinic pathway can interrupt normal gating, both in vivo and in the computer model, but that simulating nicotinic agonist in the earlier computer model (Flach et al. 1996) did not simulate the restoration of normal gating that is evident in in vivo studies (Bickford-Wimer et al. 1990). Second, the model presented here may be useful in the future development of therapeutic agents for the treatment of schizophrenia. While human studies indicate that nicotine itself may not be a clinically useful therapeutic agent because of its transient effects in vivo (Adler et al. 1992, 1993), our model can help explain the unique effects of atypical neuroleptics such as clozapine. Clozapine, hypothesized to increase the blockade of serotonin receptors more than dopamine receptors (low D2/5HT2 ratio) (Meltzer 1989; Hoyer et al. 1989; Costall and Naylor 1992; Palreyman et al. 1992), can also restore normal gating in schizophrenic patients (Nagamoto et al. 1993). Blockade of the serotonin receptor may increase cholinergic neuronal transmission (Grassi et al. 1993; Barnes et al. 1989) and enhance the activity of GABAB inhibitory interneurons (Freund et al. 1990; Segal 1990). Previous models that do not include indirect activation of presynaptic GABAB-Rs by septal nicotinic cholinergic fibers cannot address the effectiveness of the atypical neuroleptics or the differing clinical profiles of the typical neuroleptics that have diverse pharmacological mechanisms. This model also predicts that direct pharmacological manipulation of the presynaptic GABAB-R alone may not be an appropriate method of restoring normal gating. However, since recent data (Mizukami et al. 2000) have shown a reduction of GABAB-Rs on hippocampal pyramidal cells, patients with schizophrenia not responding to traditional medication may benefit from both Ach-R and GABAB-R activation. Such a suggestion is consistent with recent hypotheses that have proposed a role for altered GABAergic transmission in the etiology of several mental illnesses, including schizophrenia, and cannot be explained by previous models.
While gating was restored using this presynaptic GABAB-R mechanism, we have no direct evidence that presynaptic GABAB affects EC input to the CA3 hippocampus, although it likely affects input from the DG and recurrent activation from local CA3 cells. In addition, it is possible that the presynaptic GABAB-Rs that we hypothesized to be located in the pyramidal cell layer are autoreceptors and regulate the amount of GABA released and not the amplitude of EPSPs. In this case, rather than a reduction in the amplitude of EPSPs, there would be an increase in the amplitude of inhibitory PSPs. These issues require further experimentation.
The use of a computational model to explore the mechanisms of sensory-information processing also have other advantages that cannot be replicated in an in vivo model. For example, there is much controversy over the way in which the networks themselves are fundamentally organized and the way in which information is represented in neuronal networks. Two of the primary alternatives to describe information representation are the Hebbian cell assembly and the average firing rate. While other groups have used an average firing rate model (Salinas and Abbott 1995) to describe information representation in neuronal systems, the results from our present computational model indicate that the Hebbian-type assembly results in a more accurate simulation of hippocampal response to auditory stimuli. In the Hebbian cell assembly model used in these simulations, cells are defined to be part of a particular pattern by their spatiotemporal firing pattern. In contrast, the average firing rate model requires only that a fraction of the cells fire in response to each stimulus presentation, and no cell is more or less likely to fire in response to the stimulus. In fact, multiunit in vivo recordings demonstrated that only 15% of hippocampal pyramidal cells respond to the conditioning stimulus (Moxon et al. 1993) and cells that respond to the conditioning stimulus also respond to the test response. This is consistent with the Hebb cell assembly model in that only a fraction of the cells in the hippocampus are set to fire to the stimulus; the others (85%) are not involved in the auditory response. If the average firing rate model were the correct model, one would expect all cells in the model to have a low but equal probability of firing in response to the conditioning and test stimulus.
In summary, the model shows that the Hebbian cell assembly is a better model than average firing rate to describe the response of hippocampal pyramidal cells to auditory stimuli. The model further suggests that indirect activation of GABAB presynaptic receptors are required for normal P50 auditory sensory gating. These results provide an understanding for the effectiveness of atypical neuroleptics in improving sensory gating in patients with schizophrenia. This model can be further used to model other mental illnesses that result from deficits in information processing in the hippocampus due to problems with inhibitory control.
Acknowledgments
Supported by USPHS, MH01245, MH58414, MH-50787, MH-01121, and research grants from the Department of Veterans Affairs and the National Alliance for Research on Schizophrenia and Depression.
References
- Abeles M. Corticonics: neural circuits of the cerebral cortex. Cambridge University Press; Boston, MA: 1991. [Google Scholar]
- Adler LE, Pachtman E, Fanks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Adler LE, Rose G, Freedman R. Neurophysiological studies of sensory gating in rats: effects of amphetamine, phencyclidine and haloperidol. Biol Psychiatry. 1986;21:767–798. doi: 10.1016/0006-3223(86)90244-1. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Griffith J, Waldo M, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenia. Biol Psychiatry. 1992;748:178–188. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;12:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Aertsen A, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res. 1985;340:341–354. doi: 10.1016/0006-8993(85)90931-x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Initial characterization of the nicotinic acetylcholine receptors in rat hippocampal neurons. J Pharm Exp Ther. 1991;11(6):1001–1021. doi: 10.3109/10799899109064693. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The commissural connections of the monkey hippocampal formation. J Comp Neurol. 1984;224:307–336. doi: 10.1002/cne.902240302. [DOI] [PubMed] [Google Scholar]
- Amaral D, Insausti R, Cowan WM. Entorhinal cortex of the monkey: I cytoarchitectonic organization. J Comp Neurol. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Ishixuka N, Clairborne B. Neurons, numbers and the hippocampal network. In: Storm-Mathisen J, Zimmer J, Ottersen OP, editors. Understanding the brain through the hippocampus. Elsevier Press; New York: 1990. pp. 1–12. [Google Scholar]
- Amaral DG, Witter MP. The three dimensional organization of the hippocampal formation: areview of anatomical data. Neuroscience. 1989;34:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andersen P, Blackstad TW, Lomo T. Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp Brain Res. 1966;1:236–248. doi: 10.1007/BF00234344. [DOI] [PubMed] [Google Scholar]
- Aracava Y, Deshpande SS, Swanson KL, Rapoport H, Wonnacott GL, Albuquerque EX. Nicotinic acetylcholine receptors in cultured neurons from the hippocampus and brain stem of the rat characterized by single channel recording. FEBS. 1987;222(1):63–70. doi: 10.1016/0014-5793(87)80192-8. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Ault JG, Lin JV. Baclofen selectively inhibits transmission at synapses made by axons of CA3 pyramidal cell in the hippocampal slice. J Pharmacol Exp Ther. 1982;223(2):291–297. [PubMed] [Google Scholar]
- Baisden RH, Woodruff ML, Hoover DB. Cholinergic and non-cholinergic septo-hippocampal projections: a double-label horseradish peroxidase-acetylcholinesterase study in the rabbit. Brain Res. 1984;290:146–151. doi: 10.1016/0006-8993(84)90745-5. [DOI] [PubMed] [Google Scholar]
- Baker N, Adler LE, Franks RD, Walso M, Berry S, Nagamoto H, Muckle A, Freedom R. Neurophysiological assessment of sensory gating in psychiatric inpatients: comparison between schizophrenia and other diagnoses. Biol Psychiatry. 1987;22:603–617. doi: 10.1016/0006-3223(87)90188-0. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Naylor RJ, Tyers MB. 5-HT3 receptors mediate inhibition of acetylcholine release in cortical tissues. Nature. 1989;338:762–763. doi: 10.1038/338762a0. [DOI] [PubMed] [Google Scholar]
- Beckstead R. Afferent connections of the entorhinal area in the rat as demonstarted by retrograde cell-labelling with horseradish peroxidase. Brain Res. 1978;152:249–264. doi: 10.1016/0006-8993(78)90254-8. [DOI] [PubMed] [Google Scholar]
- Benardo LS, Prince DA. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982a;249:315– 331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Benardo LS, Prince DA. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982b;249:315–331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Wear K. Fimbria-fornix lesions disrupt auditory sensory gating in the rat hippocampus. Neurosci Abstr. 1994;20:343. [Google Scholar]
- Bickford PC, Wear KD. Restoration of habituation of auditory evoked responses by nicotine in fimbria-fornix lesioned rats. Brain Res. 1996;705:235–240. doi: 10.1016/0006-8993(95)01157-9. [DOI] [PubMed] [Google Scholar]
- Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, Freedman R. Auditory sensory gating in hippocampal neurons: a model system in the rat. Biol Psychiatry. 1990;27:183–1192. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- Blackstad TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- Braff DL, Greyer MA. Sensorimotor gating and schizophrenia: Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Schwerdtfeger WK, Germroth P. New anatomical approaches to reveal afferent and efferent hippocampal circuitry. In: Chan-Palay V, Kohler C, editors. The hippocampus: new vistas. Alan R Liss; New York: 1989. pp. 71–83. [Google Scholar]
- Buzaki G. Feed-forward inhibition in the hippocampal formation. Prog Neurobiol. 1984;22:131–154. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Chronister RB, DeFrance JF. Organization of projection neurons of the hippocampus. Exp Neurol. 1979;66:509–523. doi: 10.1016/0014-4886(79)90198-5. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. 5-HT receptors and antipsychotic drugs. In: Marsden CA, Heal DJ, editors. Central serotonin receptors and psychotropic drugs. Blackwell Scientific Publishers; Oxford: 1992. pp. 214–224. [Google Scholar]
- Danos P, Frotscher M, Freund TF. Non-pyramidal cells in the CA3 region of the rat hippocamus: relationships of fine structure, synaptic input and chemical characteristics. Brain Res. 1991;546:195–202. doi: 10.1016/0006-8993(91)91481-f. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Ensemble actvity and behavior: what’s the code? Science. 1995;270:1316–1318. doi: 10.1126/science.270.5240.1316. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, West JR, Cotmanm CW, Lynch G. Physiological studies of the reciprocal connections between the hippocapus and entorhinal cortex. Exp Neurol. 1975;49:35–37. doi: 10.1016/0014-4886(75)90194-6. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Langmoen IA. Conductance changes and inhibitory actions of hippocampal recurrent IPSPs. Brain Res. 1985;185:277–287. doi: 10.1016/0006-8993(80)91068-9. [DOI] [PubMed] [Google Scholar]
- Dodd J, Dingledine R, Kelly JS. The excitatory action of acetylcholine on hippocampal neurones of the guinea pig and rat maintained in vitro. Brain Res. 1981;207:109–127. doi: 10.1016/0006-8993(81)90682-x. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. Pre- and postsynaptic GABAb receptors in the hippocampus have different pharmacological properties. Neuron. 1985;1:585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Flach KA, Adler LE, Gerhardt G, Miller C, Bickford P, MacGregor R. Sensory gating in a computer model of the CA3 neural network of the hippocampus. Biol Psychiatry. 1996;40:1230– 1245. doi: 10.1016/0006-3223(95)00624-9. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, Drebig C, Nagamoto H, Bickford-Wimer P, Franks R. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987;13(4):669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- Freedman R, Wetmore C, Stroberg I, Leonard S, Olson L. Alpha-bugarotoxin binding to hippocampal interneurons: immunocytochemical characteriztion and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas AL, Acsady L, Gorcs T, Toth K. Serotoninergic control of the hippocampus via local inhibitory neurons. Proc Natl Acad Sci USA. 1990;87:8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;293:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C, Lubbers K, Oertel WH. Commissural afferents innervate glutamate decarboxylase immunoreactive non-pyramidal neurons in the guinea pig hippcampus. Neurosci Lett. 1984;46:137–143. doi: 10.1016/0304-3940(84)90431-2. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Nitsch R, Leranth C. Cholinergic innervation of identified neurons in the hippocampus: electron microscopic double labeling. In: Chan-Palay V, Kohler C, editors. The hippocampus: new vistas. Alan R Liss; New York: 1989. pp. 85–96. [Google Scholar]
- Frotscher M, Zimmer J. Commissural fibers terminate on non-pyramidal neurons in the guinea pig hippocampus-a combined Golgi/EM degeneration study. Brain Res. 1983;265:289– 293. doi: 10.1016/0006-8993(83)90344-x. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Brown DA. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cell in hippocampal culture. Proc Natl Acad Sci USA. 1985;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahwiler BH, Dreifuss JJ. Multiple actions of acetycholine on hippocampal pyramidal cells in organotypic explant cultures. Neuroscience. 1982;7(5):1243–1255. doi: 10.1016/0306-4522(82)91131-9. [DOI] [PubMed] [Google Scholar]
- Germroth P, Schwerdtfeger WK, Buhl EH. Morphology of identified entorhinal neurons projecting to the hippocampus: a light microscopic study combining retrograde tracing and intracelluluar injection. Neuroscience. 1989;30:683–691. doi: 10.1016/0306-4522(89)90161-9. [DOI] [PubMed] [Google Scholar]
- Gerstein GL, Perkel DH, Dayhoff JE. Cooperative firing activity in simultaneously recorded populations of neurons: detection and measurement. J Neurosci. 1986;5(4):881–889. doi: 10.1523/JNEUROSCI.05-04-00881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochin PM, Colombo M, Dorfman GA, Gerstein GL, Gross CG. Neural ensemble coding in inferior temporal cortex. J Neurophysiol. 1994;6:2325–2337. doi: 10.1152/jn.1994.71.6.2325. [DOI] [PubMed] [Google Scholar]
- Grassi F, Polenzani L, Mileo AM, Caratsch CG, Eusebi F, Miledi R. Blockage of nicotinic acetylcholine receptors by 5-hydroxytryptamine. J Neurosci Res. 1993;34:562–570. doi: 10.1002/jnr.490340508. [DOI] [PubMed] [Google Scholar]
- Halliwell JV. Physiological mechanisms of cholinergic action in the hippocampus. Prog Brain Res. 1990;84:255–272. doi: 10.1016/s0079-6123(08)60910-3. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synaptses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15(7):5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Hebb DO. Organization of behavior. McGraw-Hill; New York: 1949. [Google Scholar]
- Hershman KM, Freedman R, Bickford PC. GABAb antagonists diminish the inhibitory gating of auditory response in the rat hippocampus. Neursci Lett. 1995;190:133–136. doi: 10.1016/0304-3940(95)11523-y. [DOI] [PubMed] [Google Scholar]
- Hirata K, Sawada S, Yamamoto C. Quantal analysis of suppressing action of baclofen on mossy fiber synapses in guinea pig hippocampus. Brain Res. 1992;578(1–2):33–40. doi: 10.1016/0006-8993(92)90226-y. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Golzlan H, Bolanos F, Schechter LE, Hamon M. Interaction of psychotrophic drugs with central 5-HT3 recognition sites: fact or artifact? Eur J Pharmacol. 1989;171:137–139. doi: 10.1016/0014-2999(89)90438-x. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J Physiol Lond. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Spencer WA, Brinley FJ. Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1960;24:225–241. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Hama K. Fast-spiking non-pyramidal cells in the hippocampal CA3 region, dentate gyrus, and subiculum of rats. Brain Res. 1987;425:351–355. doi: 10.1016/0006-8993(87)90518-x. [DOI] [PubMed] [Google Scholar]
- Kiss J, Patel AJ, Baimbridge KG, Freund TF. Topographical localization of neurons containing parvalbumin and choline acetyltransferase in the medial septum-diagonal band region of rat. Neuroscience. 1990;36:61–72. doi: 10.1016/0306-4522(90)90351-4. [DOI] [PubMed] [Google Scholar]
- Kohler C. Projection from the deep layers of the entorhinal area to the hippocampal formation in the rat brain. Neurosci Lett. 1985;56:13–16. doi: 10.1016/0304-3940(85)90433-1. [DOI] [PubMed] [Google Scholar]
- Kohler C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol. 1986;246:149–169. doi: 10.1002/cne.902460202. [DOI] [PubMed] [Google Scholar]
- Kohler C. Intrinsic connections of the retrohippocampal region in the rat brain. III. The lateral entorhinal area. J Comp Neurol. 1988;271:208–228. doi: 10.1002/cne.902710204. [DOI] [PubMed] [Google Scholar]
- Lorento de No R. Studies on the structure of the cerebral cortex. I. The area entorhinalis. J Psychol Neurol. 1933;45:381– 438. [Google Scholar]
- Luntz-Leybman V, Bickford P, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- MacGregor RJ. Neural and brain modeling. Academic Press; San Diego: 1987. [Google Scholar]
- MacGregor RJ. Sequential configuration model for firing patterns in local neural networks. Biol Cybern. 1991;65:339–349. doi: 10.1007/BF00216967. [DOI] [PubMed] [Google Scholar]
- MacGregor R. Theoretical mechanics of biological neural networks. Academic Press; Boston: 1993. [Google Scholar]
- MacGregor RJ, Gerstein GL. Cross-talk theory of memory capacity in neural networks. Biol Cybern. 1991;65:351–355. doi: 10.1007/BF00216968. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–838. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology. 1989;99:S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- Miettinen R, Freund TF. Convergence and segregation of septal and median raphe inputs onto different subsets of hippocampal inhibitory interneurons. Brain Res. 1992;594:263–272. doi: 10.1016/0006-8993(92)91133-y. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. Medial septal neuron acitivty in relation to an auditory sensory gating pardigm. Neuroscience. 1993;55(2):373–380. doi: 10.1016/0306-4522(93)90506-b. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69(2):371–381. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Frotscher M. Postsynaptic-GABAergic inhibition of non-pyramidal neurons in the guinea-pig hippocampus. Neuroscience. 1986;19(1):193–206. doi: 10.1016/0306-4522(86)90015-1. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Weiler MH, Bak IJ. Intrinsic cholinergic excitation in the rat neostriatum: nicotinic and muscarinic receptors. Exp Brain Res. 1980;39:401–409. doi: 10.1007/BF00239304. [DOI] [PubMed] [Google Scholar]
- Mizukami K, Sasaki M, Ishikawa M, Iwakiri M, Hidaka S, Shiraishi H, Iritani S. Immunohistochemical lcalization of gamma-aminobutyric acidB receptor in the hippocampus of subjects with schizophrenia. Neurosci Lett. 2000;283:101–104. doi: 10.1016/s0304-3940(00)00939-3. [DOI] [PubMed] [Google Scholar]
- Moxon KA, Gerhardt GA, Bickford PC, Austin KA, Rose GM, Woodward DJ, Adler LE. Multiple single units and population responses during inhibitory gating of hippocampal auditory response in freely-moving rats. Brain Res. 1999;825:75–85. doi: 10.1016/s0006-8993(99)01187-7. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Griggith J, Freedman R. Gating of auditory response in schizophrenics and normal controls: effects of recording site and stimulation interval on the P50 wave. Schizophr Res. 1991;4:31–40. doi: 10.1016/0920-9964(91)90007-e. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Heal RA, Hoffer LD, Freedman R. Gating of auditory P50 in schizophrenia: trait and state related phenomena. Society of Biological Psychiatry Annual Meeting. Biol Psychiatry. 1993;33:120A. [Google Scholar]
- Newberry NR, Nicoll RA. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984;384:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MAL, Lin RCS, Woodward DJ, Chapin JK. Dynamic and distributed properties of many-neuron ensembles in the ventral posterior medial thalamus of wake rats. Proc Natl Acad Sci USA. 1993;90:2212–2216. doi: 10.1073/pnas.90.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MAL, Baccala LA, Lin RCS, Chapin JK. Sensorimotor encoding by synchrounous neural ensemble activity at multiple levels of the somatosensory system. Science. 1995;268:1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- Nyakas C, Luiten GM, Spencer DG, Traber J. Detailed projection patterns of septal and diagonal band efferents to the hippocampus in the rat with emphasis on innervation of CA1 and the dentate gyrus. Brain Res Bull. 1986;18:533–545. doi: 10.1016/0361-9230(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Palreyman MG, Sorensen SM, Baron BM, Humphreys TM, Moser P, Dudley MW. Antipsychotic potential of 5-HT3 antagonists: preclinical data. In: Meltzer HY, editor. Novel antipsychotic drugs. Raven Press; New York: 1992. pp. 211–223. [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAa responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Peet MJ, McLennan H. Pre- and postsynaptic actions of baclofen: blockade of the late synaptically-evoked hyperpolarization of CA1 hippocampal neurones. Exp Brain Res. 1986;61:657–574. doi: 10.1007/BF00237582. [DOI] [PubMed] [Google Scholar]
- Pinsky PR, Rinzel J. Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. J Comput Neurosci. 1994;1:39–60. doi: 10.1007/BF00962717. [DOI] [PubMed] [Google Scholar]
- Salinas E, Abbott LF. Transfer of coded information from sensory to motor networks. J Neurosci. 1995;15(10):6461–6474. doi: 10.1523/JNEUROSCI.15-10-06461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Consequences of pro-longed afferent stimulation of the rat fascia dentata: epileptiform activity in area CA3 of hippocampus. Neuroscience. 1990;35(3):505–517. doi: 10.1016/0306-4522(90)90325-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Mathers LH. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978;157:1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger WK, Buhl E. Various types of non-pyramidal hippocampal neurons project to the septum and contralateral hippocampus. Brain Res. 1986;386:146–154. doi: 10.1016/0006-8993(86)90151-4. [DOI] [PubMed] [Google Scholar]
- Segal M. Serotonin attenuates a slow inhibitory postsynaptic potential in rat hippocampal neurons. Neuroscience. 1990;36:631– 641. doi: 10.1016/0306-4522(90)90006-p. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Tohyama M, Shoitani Y. Hippocampofugal gamma-aminobutyric acid (GABA)-containing neuron system in the rat: a study using a double-labeling method that combines retrograde tracing and immunocytochemistry. Brain Res. 1987;409:181–186. doi: 10.1016/0006-8993(87)90757-8. [DOI] [PubMed] [Google Scholar]
- Siegel C, Waldo M, Mizner G, Adler LE, Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Arch Gen Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- Spencer WA, Kandel ER. Electrophysiology of hippocampal neurons. III. Firing level and time contant. J Neurophysiol. 1961;24:260–261. doi: 10.1152/jn.1961.24.3.260. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Wear KD. Normalizing effects of nicotine and a novel nicotinic agonist on hippocampal auditory gating in two animal models. Pharmacol Biochem Behav. 1997;57:869–874. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comput Neurol. 1976;167:215–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kohler C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neuroscience. 1986;6:3010–3023. doi: 10.1523/JNEUROSCI.06-10-03010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp R, Haug FM, Tonder N, Zimmer J, Ottersen OP. Neuroactive amino acids in organotypic slice cultures of the rat hippocampus: an immunocytochemical study of the distribution of GABA, glutamate, glutamine, and taurine. Neuroscience. 1992;46(4):807–823. doi: 10.1016/0306-4522(92)90187-7. [DOI] [PubMed] [Google Scholar]
- Traub RD. Simulation of intrinsic bursting in CA3 hippocampal neurons. Neuroscience. 1982;7:1233–1242. doi: 10.1016/0306-4522(82)91130-7. [DOI] [PubMed] [Google Scholar]
- Traub RD, Miles R. Neuronal networks of the hippocampus. Cambridge University Press; Cambridge, UK: 1991. [Google Scholar]
- Traub RD, Dudek FE, Snow RW, Knowles WD. Computer simulations indicate that electrical field effects contribute to the shape of the epileptiform field potential. Neuroscience. 1985;15:947– 958. doi: 10.1016/0306-4522(85)90245-3. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Dingledine R. Presynaptic inhibitory effect of acetycholine in the hippocampus. J Neurosci. 1981;1(7):784–792. doi: 10.1523/JNEUROSCI.01-07-00784.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Cortical afferents to the entorhinal cortex of the Rhesus monkey. Science. 1972;175:1471–1473. doi: 10.1126/science.175.4029.1471. [DOI] [PubMed] [Google Scholar]
- Venables P. Input dysfunction in schizophrenia. In: Maher BA, editor. Progress in experimental personality research. Academic Press; Orlando: 1964. pp. 1–47. [PubMed] [Google Scholar]
- Vinogradova OS. Functional organization of the limbic system in the process of registration of information: facts and hypotheses. In: Isaacson RL, Pribram KH, editors. The hippocampus. Vol. 2. Plenum Press; New York: 1975. pp. 3–69. [Google Scholar]
- Waldo MC, Adler LE, Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophr Res. 1988;1:19–24. doi: 10.1016/0920-9964(88)90035-7. [DOI] [PubMed] [Google Scholar]
- Witter MP. Connectivity of the rat hippocampus. In: Chan-Palay V, Kohler C, editors. The hippocampus: new vistas. Alan R Liss; New York: 1989. pp. 53–69. [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey, V: Projections to the dentate gyrus, hippocampus, and the subicular complex. J Comp Neurol. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Wyss JM. An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol. 1981;199:495–512. doi: 10.1002/cne.901990405. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Swanson LW, Cowan WM. A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience. 1979;4:463–465. doi: 10.1016/0306-4522(79)90124-6. [DOI] [PubMed] [Google Scholar]