Abstract
Background
Suppression of anger is linked to subsequent pain intensity among chronic low back patients, but it is not clear whether anger regulation style (trait anger-out, anger-in) moderates these effects or if aroused anger accounts for links between anger regulation style and pain.
Method
Chronic low back pain patients (N=58) were assigned to Suppression or No Suppression conditions for a task with harassing confederate and then underwent structured pain behavior procedures. Spielberger Anger Expression Inventory tapped trait anger-out (AOS) and anger-in (AIS).
Results
Regressions tested Emotion Regulation condition × AOS and AIS effects on outcomes. AOS was related to grimacing and sighing for Suppression condition patients. AIS was related negatively to guarding and bracing for Suppression condition patients. Anger report partly mediated effects for AOS and AIS.
Conclusions
Anger regulation style moderated effects of state anger suppression on subsequent pain behaviors, effects that were partly explained by aroused anger.
Keywords: Anger suppression, Anger regulation style, Chronic pain, Pain behaviors
Introduction
Anger is related to both acute [1–4] and chronic pain intensity [1, 5–7]. Findings suggest that the manner in which anger is regulated—for example, suppression (anger-in) or expression (anger-out)—is consistently correlated with chronic pain severity [1, 5, 6]. An enduring psychosomatic hypothesis holds that the suppression or inhibition of strong emotions has detrimental effects on physical health. Thus, a number of studies of anger regulation and pain have focused on suppressed anger. Relying on correlations between trait measures of anger-in and pain, these studies have not provided well-defined accounts of how the actual process of suppressing anger during upsetting events may affect pain. To redress this shortcoming, we have proposed and tested an ironic process model of anger suppression and pain based on Wegner's [8] ironic process theory of mental control [9].
Wegner and colleagues [10–13] showed that attempts to suppress unwanted thoughts have the ironic effect of rendering these thoughts highly accessible to consciousness. They proposed that attempts to suppress thoughts involve two processes [8]. First, an effortful “operating process” works to avoid unwanted thoughts through conscious use of distracters. A second process, however, occurs that is less conscious and deliberate. This unconscious “monitoring process” searches for mental content that signals a failure to suppress the unwanted material. Ironically, by searching for failures to achieve mental control, the cognitive accessibility of the undesired thoughts and feelings actually increase. Much research across a wide diversity of populations supports the ironic process model (e.g., [14–17]).
Our ironic process model of anger suppression and pain holds that suppression of anger may render anger-related content highly accessible to conscious awareness because the monitoring process works to find more and more instances of failure to avoid or be rid of this content. Although suppression may subdue angry feelings and behavior initially, it may in the long run paradoxically increase the cognitive accessibility of anger, in turn leading to “contamination” [18] of appraisals of subsequent pain with heightened feelings of irritation and annoyance. Results of our recent studies support this model [9, 19, 20]. Findings generally show that participants instructed to suppress anger-related thoughts and feelings during anger induction reveal significantly greater pain intensity during subsequent pain induction than participants who underwent anger induction but were not instructed to suppress, both in healthy adults and chronic low back pain patients. Results generally indicate that when self-reported anger following anger or pain induction is statistically controlled, differences in pain intensity are attenuated between groups instructed to suppress compared to those given a standardized “think anything” control instruction [9, 20]. These findings support the notion that initially suppressed anger can contaminate appraisals of a subsequent painful stimulus in a measureable and potentially clinically meaningful way.
Despite our emphasis on examining effects of actual and deliberate attempts to suppress anger, it remains important for several reasons to consider the role of trait anger regulation style. This trait describes the way in which anger is typically managed by an individual and has been assessed almost exclusively with the anger-in and anger-out subscales of the Spielberger Anger Expression Inventory [21]. However, for a host of reasons, a person may not use their preferred strategy for regulating anger in any given circumstance, and so he or she may be compelled to use a different, possibly less-preferred tactic. Brosschot and Thayer [22] have argued that the actual inhibition of anger may play a large role in producing or exacerbating physical disorder because of social norms that discourage the full expression of anger, a factor that may affect dispositional anger expressers quite dramatically. Indeed, Engebretson et al. [23] proposed a “matching” model in which adaptive responses to anger provocation would occur among people most often when they could use their preferred mode of anger regulation. A “mismatch” would arise when, for example, habitual anger expressers are forced by circumstances—lab situations or real-life social constraints—to suppress their anger. Evidence generally supports mismatch models with regard to physiological stress reactivity and recovery [23–26], as well as measures of pain intensity [27]. Most relevant for our purposes here, Burns et al. [27] found that healthy normal participants characterized by high trait anger-out, who underwent anger induction while trying to suppress—a mismatch situation—reported greater sustained pain intensity following later pain induction than high anger-out participants who did not try to suppress during anger induction and all low anger-out participants as well. Moreover, these individuals showed delayed systolic blood pressure (SBP) recovery following the pain stimulus. Thus, high anger-out people forced to suppress anger-related thoughts and feelings during anger arousal may show a particularly acute vulnerability to later noxious stimulation. However, whether such trait-situation mismatches affect clinical pain has, to our knowledge, not been examined.
In the present study, we conducted additional analyses of data reported in Burns et al. [20]. In that study, chronic low back pain patients underwent anger induction under conditions in which they were instructed to suppress thoughts and feelings about the episode and to not reveal any signs of how they felt, or they were not given these instructions. All patients then underwent pain induction using a structured pain behavior task, adapted from Keefe, Williams, and Smith [28], which has been shown to elicit significant increases in low back pain among chronic low back pain patients [28, 29]. We found that patients in the Suppression condition reported greater pain intensity and revealed more pain behaviors during the structured pain behavior task than those in the No Suppression condition and that the differences in pain behaviors were largely accounted for by the greater anger reports of the Suppression condition patients.
Here, we examined whether trait anger-out further moderated these effects such that patients in mismatched situations would report greater pain and exhibit more pain behaviors than patients not in mismatched conditions. If the mismatch model extends to chronic pain patients and their clinical pain, then attempts to suppress during anger induction should lead high anger-out patients to report greater pain and show more pain behaviors during the subsequent structured pain behavior task than high anger-out patients not instructed to suppress. Further, rather than focus analyses only on the total frequency of pain behaviors summed across categories, as in the original report, we examined Emotion Regulation condition × anger-out effects on component pain behaviors (e.g., grimacing, bracing). Investigators [30, 31] have shown that pain behaviors cluster into empirically distinct categories. Recently, it has been argued that such behavior may be grouped into two broad classes [32, 33]—communicative (e.g., grimacing, sighing) and protective (e.g., guarding, bracing, rubbing)—with evidence suggesting that these kinds of behaviors are exhibited to different degrees depending on situational demands [34] and may have different implications for the development of pain-related disability [35]. We speculate that communicative pain behaviors like grimacing and sighing may be more akin to facial expressions of anger than engaging in protective behaviors such as guarding and bracing or rubbing a body part. If so, then we expected trait anger-out for patients in the Suppression condition to predict grimacing and sighing during the later structured pain behavior task more strongly than protective behaviors. We also evaluated the notion that feelings of anger, in particular, may mediate the link between trait anger-out and later pain intensity by testing whether self-reported anger, anxiety, and sadness following anger-induction partly accounted for any mismatch effects shown by high anger-out patients. That is, we examined whether trait anger-out affected later pain behaviors through the degree to which anger was aroused during the maze task. Finally, we examined whether trait anger-in also moderated effects of suppression versus no suppression on later pain intensity.
Method
Participants
Participants were 60 chronic low back pain patients recruited through advertisements and postings at pain clinics. They were paid $40. Exclusion criteria were: (a) any current cardiovascular disorder; (b) current use of medications that affect cardiovascular function (i.e., beta blockers); (c) chronic pain stemming from malignant conditions (i.e., cancer); (d) current alcohol or substance abuse problems; (e) a history of psychotic or bipolar disorders; (f) daily use of narcotic analgesic medication; (g) inability to understand and speak English well enough to take instructions from a confederate (see below). Inclusion criteria were: (a) musculoskeletal pain of the lower back stemming from degenerative processes, muscular or ligamentous strain, or disk herniation as determined by a physician; (b) pain duration of at least 6 months. The final sample was 58 chronic low back pain patients due to equipment malfunction for two participants. Those who reported occasional use of opioid-based medication were asked not to take these substances on the morning of their appointments. Women comprised 51.7% (n=30) of the sample. Descriptive information appears in Table 1.
Table 1. Descriptive data (N=58).
| Variables | Statistics | |||
|---|---|---|---|---|
|
| ||||
| M | SD | % | n | |
| Age (years) | 39.2 | 9.7 | ||
| At least 12-years of education | 90.0% | 52 | ||
| Ethnicity | ||||
| Caucasian | 67.2% | 39 | ||
| Hispanic | 10.3% | 6 | ||
| African American | 15.5% | 9 | ||
| Asian | 1.7% | 1 | ||
| Native American | 5.2 | 3 | ||
| Pain duration (months) | 48.4 | 45.2 | ||
| Opioid-based | 20.7% | 12 | ||
| Nonsteroidal anti-inflammatory | 32.8% | 53 | ||
| Muscle relaxants | 34.5% | 20 | ||
| Antidepressants | 8.6% | 5 | ||
| Pain behavior: guard | 1.2 | 1.9 | ||
| Pain behavior: brace | 1.5 | 2.1 | ||
| Pain behavior: rub | .5 | 1.3 | ||
| Pain behavior: grimace | 1.2 | 1.8 | ||
| Pain behavior: sigh | .8 | 1.5 | ||
Design Overview
A mixed between- × within-subjects design was used. The between-subjects factor was two Emotion Regulation conditions (Suppression; No suppression). Participants were assigned randomly to these conditions. The within-subjects factor consisted of participants undergoing an anger-induction procedure while attempting to suppress or not (see below), followed by the structured pain behavior task (see below) in a fixed order.
Measures
Anger Regulation Style
The tendencies to express and inhibit anger were assessed with the Anger Expression Inventory [24]. This inventory provides scales to measure anger expressive style (Anger-out Scale) and anger inhibition style (Anger-in Scale) for which Spielberger et al. [24] report adequate internal consistency coefficients. Further, Faber and Burns [25] found that Anger-out Scale scores predicted the degree to which anger was expressed verbally during provocation.
Negative Effect and Pain Intensity Checklists
Eleven-point numeric rating scales [36] tapped self-reported pain intensity (0=None and 10=Most Severe Possible), and the degree to which participants felt tense, nervous, sad, irritated, and angry (0=Not at all; 10=Extremely). The angry and irritated items following anger induction (see below) were correlated (r=.85), and the tense and nervous items were correlated (r=.70). The angry and irritated items were summed to give anger composite scores, and values for the tense and nervous items were summed to give anxiety composite scores.
Anger-Induction Task
Anger was induced by having a participant take instructions from a confederate during a demanding task. The task was described to participants as a collaborative task for two people (i.e., participant and confederate, who was a trained assistant), the object of which was for one person to move a computer cursor from the entry to the exit of a computer-generated maze. This person, however, was not able to see the maze but instead moved a computer mouse across a white pad—representing the boundary of the maze—according to instructions issued by the other person who was able to see the computer screen. Thus, one person told the other to move the mouse in certain directions and distances in an effort to exit the maze. Unbeknown to the participant, he or she was always assigned to move the mouse and the confederate always instructed. The participant was also told that they and the other person “need to act as a team to do well,” that he/she cannot speak to the other person (confederate), and that after the participant has completed the second part of the experiment, he or she would have a turn as the instructor and the other person (confederate) would move the mouse.
However, the confederate assumed an unfriendly attitude from the outset. In addition to the instructions described above, the participant was told that “errors” resulted from bumping the cursor into maze walls or having to reverse direction. During the task, the confederate followed a semi-standard script that included instructions to move the cursor in certain directions, reversing directions, exclamations about errors, derogatory comments about the participant's ability, and comments indicating that the confederate blamed the participant for all mistakes. This task and the harassment manipulation were adopted from Engebretson, Matthews, and Scheier [23]. Male and female experimenters served as confederates. To avoid confounds involving participant-confederate gender matches, equal numbers of same sex, male participant-female confederate, and female participant-male confederate matches were used.
Emotion Regulation Manipulations
Suppression
In this condition, participants were told to suppress thoughts about their feelings during the maze task and not to show any behavioral signs that betrayed how they felt. Full details of the procedure are provided in Burns et al. [20]. In brief, patients were asked to give their “best effort on the maze task,” were told that working with a partner “can bring up a lot of thoughts and feelings,” and that they were supposed to suppress what they were thinking and feeling about the task and to hide how they felt at all times. Participants were told not to speak to the other person.
No Suppression
In this condition, participants were told to think anything they wanted and to reveal their feelings if they wanted without speaking to the confederate or standing. In brief, they were asked to give their best effort on the task, were told that working with a partner “can bring up a lot of thoughts and feelings,” and that they could “deal with your thoughts and feelings in any way” they chose and that they should “feel free to think about and/or to show your feelings at any time,” but without speaking to the other person.
Structured Pain Behavior Task
A structured pain behavior task [28, 29] was used as a naturalistic pain-induction manipulation that allows assessment of both self-reported pain intensity and observable pain behaviors (e.g., grimacing). This task involves sitting, standing, walking, reclining, and bending to lift a light-weight object (see below); everyday activities that typically produce mild pain in chronic low back pain patients.
The procedure, variables, and data reduction for the structured pain behavior task are described in detail in Burns et al. [20] and followed the procedures described in Keefe and Block [29]. In brief, participants engaged in 1-and 2-min sitting and standing periods, and two 1-min reclining and walking periods. We added a separate bending and lifting sequence, in which participants picked up a pencil (placed at their feet) from the floor, stood erect, and then replaced it on the floor. The order of positions and activities was varied randomly across participants. The tester spoke to participants only to request activity changes. The 11-min session was videotaped.
Behaviors coded were guarding, bracing, rubbing, grimacing, and sighing. Keefe and Block [29] reported excellent inter-rater agreement in coding these behaviors, ranging from 93% to 99%. Test–retest reliability of intervals up to 6 months was also adequate [28]. For our data, three graduate students in clinical psychology-coded behaviors. Videotapes were prepared for interval recording following the procedure of Keefe et al. [28]. In Burns et al. [20], we used the total frequency of combined behaviors in analyses. For the present study, we analyzed the component behaviors separately. Two raters coded all 58 videotapes separately. Inter-rater reliabilities ranged from r=.88 for “bracing” to r=.96 for “grimacing.”
Procedure
Participants were screened for exclusion criteria and asked not to consume caffeine, alcohol, or nicotine during the 6 h prior to their appointments. When they arrived at the laboratory, procedures and risks were explained, including information about the “other participant” (i.e., confederate) “who will arrive shortly.” All participants reported compliance with the opioid-based medication, caffeine, alcohol, and nicotine restrictions. Informed consent was obtained. Participants sat upright throughout the maze task. A 5-min resting adaptation period was followed by the entry of the confederate, who sat 2 m from the participant on the opposite side of the computer table. They were told not to speak. Another 5-min resting period proceeded. The participant completed pain and affect numeric rating scales to rate current pain and emotional state, while the confederate did likewise as part of the ruse. Instructions for the maze task and emotion regulation instructions, depending on condition, were given. After the 5-min maze task, the confederate left the room and the participant again completed pain and affect numeric rating scales. When finished, the participant was brought to an adjoining room, instructions for the structured pain behavior task were given, and this task began approximately 2.5 min after the maze task ended. After the 11-min task, the participant completed pain and affect numeric rating scales. They were debriefed, especially with regard to the deception of the confederate. All participants were asked whether they believed the confederate was indeed another patient and was also, like them, a participant in the study. All indicated that they believed the confederate was as portrayed.
Data Analysis
In Burns et al. [20], we reported that anger, anxiety, and sadness increased significantly during the maze task, but that Emotion Regulation condition moderated the effect for anger such that patients in the Suppression condition reported more anger following the maze task than No Suppression patients. We showed also that while pain intensity increased significantly from baseline to the structured pain behavior task for both Suppression and No Suppression patients, the former reported greater increases in pain than the latter. Finally, we showed that the Suppression condition group exhibited more total pain behaviors than the No Suppression group.
In the present study, we generated correlation coefficients between the five component pain behaviors to determine whether there was some degree of independence among these pain behaviors indexes. We conducted ANOVAs to determine whether the Suppression and No Suppression conditions differed on the five component pain behaviors. We then performed hierarchical regressions to test whether AOS and/or AIS scores moderated associations between Emotion Regulation condition and pain indexes. Emotion Regulation condition was dummy-coded (Suppression=“1” and No Suppression=“0”). Interaction terms were computed by multiplying the Emotion Regulation condition term by AOS and AIS scores. Analyses were performed by entering the main effect terms (Emotion Regulation condition, AOS or AIS scores) simultaneously in the first step, followed by the interaction term in the second step. Significant interactions were revealed by a significant R2 increment in the second step with the interaction term entered. These interactions were dissected by regressing dependent variables on AOS or AIS scores separately for each Emotion Regulation condition, following recommendations of Keppel and Zedeck [37]. For self-reported anger, anxiety, sadness, and pain intensity, residualized change scores were computed by regressing post-maze or structure pain behavior task values on baseline values. These variables were used as dependent variables in the regressions, as well as the five pain behaviors.
In Burns et al. [20], we found that anger report following anger induction significantly mediated the effect of Emotion Regulation condition on total pain behaviors such that greater anger in the Suppression condition partly accounted for the greater frequency of pain behaviors revealed by this group. Anxiety and sadness did not. In the present study, affect changes were again evaluated as potential mediators, but this time focusing on the relationships between AOS and/or AIS scores and pain indexes. The guidelines for testing mediation provided by Baron and Kenny [38] were followed in general. In cases where the Emotion Regulation condition × AOS or AIS interactions were significant for pain indexes, and simple effects tests showed that a pain-dependent variable was significantly predicted by AOS or AIS scores only among participants in one condition: mediation effects were evaluated just for that group. For example, in a case where AOS scores are related to a negative effect change score and are also related to a component pain behavior but among participants in only one of the two Emotion Regulation conditions, mediation tests proceeded only for that group. Sobel tests were then performed to determine whether a significant degree of mediation had occurred.
Results
Zero-Order Correlations
Correlation coefficients were generated among the five component behaviors and pain intensity change scores and are shown in Table 2. For pain behaviors, the smallest correlations tended to be between the so-called communicative behaviors (e.g., grimacing) and protective behaviors (e.g., rubbing), whereas the largest correlations tended to be between indexes within the same category (e.g., grimacing and sighing). We squared the r coefficients to get r2 coefficients and then computed mean r2 within and between each behavior category. The relationship within the two communicative behaviors was described by an r2 of .37, the relationships within the three protective behaviors was described by a mean r2 of .13, and the relationships between the communicative and protective behaviors was described by a mean r2 of .07. Thus, results suggest some degree of coherence within each group and some degree of independence between communicative and protective pain behaviors, supporting claims that these behaviors are somewhat distinct and may serve different purposes [32–35]. At the very least, our findings support the potential usefulness of examining effects of Emotion Regulation condition and anger regulation style on the five pain behavior indexes separately.
Table 2. Zero-order correlations among pain behavior component values and pain intensity.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Guard | – | |||||
| Brace | .42 | – | ||||
| Rub | .38 | .28 | – | |||
| Grimace | .42 | .31 | .11 | – | ||
| Sigh | .31 | .32 | .12 | .61 | – | |
| Pain intensity changes | .27 | .26 | .27 | .31 | .20 | – |
Correlation coefficients equal to or greater than r=.26 are significant at p<.05
The correlations between the five pain behaviors and changes in self-reported pain intensity were no more than moderate. Although these factors are related, findings also suggest that self-reports of pain intensity and observable pain behaviors are somewhat distinct constructs.
A correlation coefficient was generated between AOS and AIS scores, which was r=.08 (ns), indicating that anger-out and anger-in styles were largely independent in this sample.
Condition Effects on Component Pain Behaviors
One-way ANOVAs were conducted for the five component pain behaviors with Emotion Regulation condition (Suppression, No Suppression) as the between-subject factor. A significant main effect was found [F(1,57)=6.00; p<.02; η2=.10] only for grimacing such that participants in the Suppression condition (M=1.8; SD=2.1) displayed more grimaces than their counterparts in the No Suppression condition (M=.7; SD=1.3). However, effects for guarding [F(1,57)=3.40; p<.07; η2=.06] and sighing [F(1,57)=2.87; p<.09; η2=.05] were in the same direction but did not reach conventional levels of significance. Thus, the largest effect for the anger suppression manipulation was for facial displays of grimacing, an index that appears akin to behaviorally expressed anger.
Tests of Emotion Regulation Condition × AOS Score Interactions
Pain Behaviors
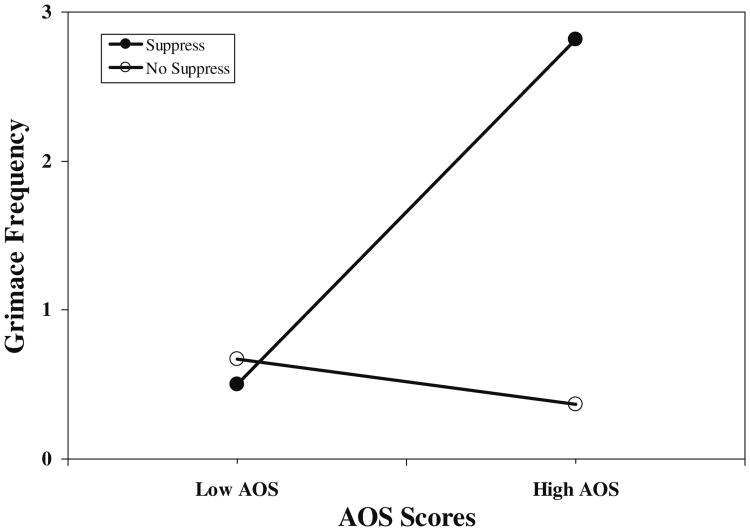
Results of hierarchical regressions revealed significant Emotion Regulation condition × AOS score interactions for grimacing (R2 increment=.08; p<.02) and sighing (R2 increment=.06; p<.05). Interactions for guarding, bracing, and rubbing were nonsignificant. The significant interaction for grimacing was dissected by regressing grimacing values on AOS scores separately for the Suppression and No Suppression groups while using the error term from the overall interaction analysis in determining significance, as recommended by Keppel and Zedeck [37]. For the Suppression condition, the beta weight describing the relationship between AOS scores and frequency of grimaces was significant (beta=.56; p<.002), whereas for the No Suppression condition, the beta weight was nonsignificant (beta=−.02; p>.10). Similarly for sighing, the beta weight for the Suppression condition was significant (beta=.46; p<.01), whereas the beta weight was nonsignificant for the No Suppression groups (beta=−.05; p>.10). The interaction for grimacing values is depicted in Fig. 1 to provide a sample illustration of these effects. Values shown are results of solving the regression equation for hypothetical AOS scores (+1 SD and/or −1 SD from the sample mean). Results show strong positive relationships between AOS scores and the frequency of grimaces and sighs during pain induction but only among patients who were instructed to suppress awareness of angry thoughts and feelings during the maze task. That is, high anger-out patients in a mismatch situation showed the most grimaces and uttered the most sighs during a later painful event.
Fig. 1.
Emotion regulation condition × AOS scores. Low AOS anger-out subscale values −1 SD from mean; High AOS anger-out subscale values +1 SD from mean; Suppress suppression condition; No Suppress no suppression condition
Pain Intensity
The Emotion Regulation condition × AOS score interaction for pain intensity changes from baseline to the structured pain behavior task was nonsignificant (R2 increment=.01; p>.10), and the main effect for AOS scores also did not reach significance (beta=.21; p>.10).
Negative Affect
The Emotion Regulation condition × AOS scores interactions for anger, anxiety, and sadness changes were nonsignificant (R increments<.02; p>.10). However, the main effects for AOS scores on anger (beta=.35; p<.007) and sadness changes (beta=.29; p<.03) were significant, whereas the main effect for anxiety changes was not (beta=.11; p>.10). Results indicate that AOS scores were related positively to negative effect changes following the maze task irrespective of whether patients suppressed or not.
Tests of Mediation for AOS Scores
Because AOS scores were related significantly to grimacing and sighing values only among Suppression condition participants, our goal here was to test whether negative effect, anger in particular, accounted for these associations. Following analyses were conducted only for those in the Suppression condition. Results reported above indicate that AOS scores—the “independent” variable in these mediation models—significantly predicted only anger and sadness changes, and so these affect factors were considered the potential mediators. Although we reported in Burns et al. [20] that anger, anxiety, and sadness change scores were correlated significantly with total pain behaviors, we conducted additional analyses here to determine whether anger and sadness change scores were correlated significantly with grimacing and sighing values. Anger changes were correlated significantly with both grimacing (r=.34; p<.008) and sighing (r=.42; p<.001), whereas sadness changes were not (r=.17, p>.10 and r=.24, p<.08; respectively). Thus, only anger change scores were considered further as a potential mediator among participants in the Suppression condition.
A regression was performed to determine whether anger change scores accounted for a significant portion of unique variance in grimacing and sighing with AOS scores held constant. Anger change scores were related significantly to grimacing (beta=.31; p<.05) and sighing (beta=.48; p<.003) with AOS scores controlled. Further tests for mediation indicated that when anger changes were controlled, the effect of AOS scores on grimacing remained significant (beta=.38; p<.02), whereas the effect of AOS scores on sighing was reduced to nonsignificance (beta=.21; p>.10). The Sobel coefficient for grimacing did not reach conventional levels of significance (1.74; p<.08), whereas the Sobel coefficient for sighing did (2.07; p<.03). Results indicate that anger changes significantly mediated the link between AOS scores and frequency of sighing during the structured pain behavior task, but only among patients who attempted to suppress. Thus, the greater frequency of sighs during pain induction shown by participants scoring high on trait anger-out compared to those scoring low was partly explained by their greater experience of anger following the anger-inducing maze task.
Tests of Emotion Regulation Condition × AIS Score Interactions
Pain Behaviors
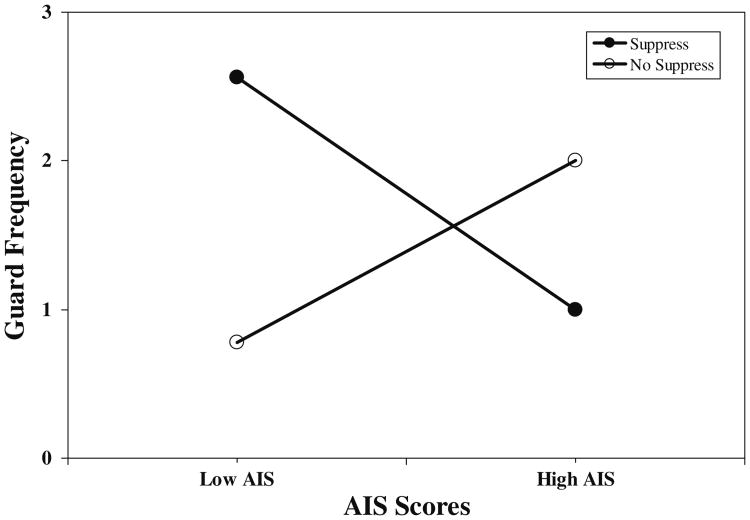
Results of hierarchical regressions revealed significant Emotion Regulation condition × AIS score interactions for guarding (R2 increment=.11; p<.009) and bracing (R2 increment=.12; p<.009). Interactions for rubbing, grimacing, and sighing were nonsignificant. The significant interaction for guarding was dissected by regressing guarding values on AIS scores separately for the Suppression and No Suppression groups while using the error term from the overall interaction analysis in determining significance, as recommended by Keppel and Zedeck [37]. For the Suppression condition, the beta weight describing the relationship between AIS scores and frequency of guarding behaviors was significant (beta=−.37; p<.05), whereas for the No Suppression condition the beta weight did not reach conventional levels of significance (beta=.33; p<.08). For bracing, the beta weight for the Suppression condition was again significant (beta=−.37; p<.05), whereas the beta weight for the No Suppression group again missed conventional levels of significance (beta=.32; p<.08). The interaction for guarding values is depicted in Fig. 2 to provide a sample illustration of these effects. Values shown are results of solving the regression equation for hypothetical AIS scores (+1 SD and/or −1 SD from the sample mean). Results show an interesting pattern. For patients instructed to suppress, AIS scores were related negatively to guarding and bracing, suggesting that this condition may have constituted a match for high anger-in people. Although the beta weights for the No Suppression patients were not significant at the p<.05 level, the positive direction of effect suggests that this condition was possibly perceived as a mismatch for high anger-in patients.
Fig. 2.
Emotion regulation condition × AIS scores. Low AIS anger-in subscale values −1 SD from mean; High AIS anger-in subscale values +1 SD from mean; Suppress suppression condition; No Suppress no suppression condition
Pain Intensity
The Emotion Regulation condition × AIS score interaction for pain intensity changes from baseline to the structured pain behavior task was nonsignificant (R2 increment=.01; p>.10), and the main effect for AIS scores also did not reach significance (beta=.14; p>.10).
Negative Effect
The Emotion Regulation condition × AIS score interaction for anger change was significant (R2 increment=.12; p<.003). However, these interactions were not significant for anxiety and sadness changes (R2 increments<.02; p>.10), nor were the main effects for AIS scores significant. The significant interaction for anger was dissected by regressing anger change scores on AIS scores separately for the Suppression and No Suppression groups. For the Suppression condition, the beta weight describing the relationship between AIS scores and anger did not reach conventional levels of significance (beta=−.31; p<.10), whereas for the No Suppression condition, the beta weight was significant (beta=.45; p<.01). Results parallel those for guarding and bracing and suggest that attempts to suppress among high anger-in people may represent a match situation whereas the converse is true for not suppressing.
Tests of Mediation for AIS Scores
Although AIS scores were related significantly and negatively to guarding and bracing only among Suppression condition participants, the pattern of findings involving the crossing interactions—with AIS scores related to these pain indexes in a positive direction—are unique and intriguing. Thus, our goals here were to test whether anger changes, in particular, accounted for both these divergent associations. Results reported above indicate that AIS scores—the “independent” variable in these mediation models—predicted significantly (or marginally) only anger changes, and so only this factor was evaluated as a potential mediator. Although we reported in Burns et al. [20] that anger change scores were correlated significantly with total pain behaviors, we conducted additional analyses here to determine whether these scores correlated significantly with guarding and bracing values. Anger changes were indeed correlated significantly with both guarding (r=.45; p<.001) and bracing (r=.47; p<.001), and so met conditions to be considered further as a potential mediator.
Mediation tests were performed for each condition separately. For participants in the Suppression condition, regressions were performed to determine whether anger change scores accounted for significant portions of unique variance in guarding and bracing with AIS scores held constant. Anger change scores accounted for significant portions of unique variance in guarding (beta=.44; p<.01) and bracing (beta=.40; p<.02) with AIS scores controlled. Further tests for mediation indicated that when anger changes were controlled, the effects of AIS scores on guarding (beta=−.21; p>.10) and bracing were reduced to nonsignificance (beta=−.21; p>.10). The Sobel coefficients for guarding (−2.02; p<.04) and bracing (−1.95; p<.05) were significant.
Among participants in the No Suppression condition, anger change scores did not account for significant portions of unique variance in guarding and bracing with AIS scores held constant. Thus, further tests of mediation were not pursued.
Results indicate that anger changes significantly mediated the link between AIS scores and frequency of guarding and bracing during the structured pain behavior task, but only among patients who attempted to suppress. More specifically, results suggest that high anger-in patients who attempted to suppress experienced low levels of anger during the maze task, which in turn contributed to a relatively low frequency of guarding and bracing pain behaviors.
Discussion
To address shortcomings in current approaches to understanding whether and how suppression of anger may affect pain, we developed an ironic process model of anger suppression and pain [9, 19] that adapted Wegner's original theory [8]. We showed in previous studies that suppression of anger-related thoughts and feelings during anger provocation appears to contaminate appraisals of a later painful event such that pain intensity and the frequency of observable pain behaviors may be increased among chronic low back pain patients [20]. We have shown as well that people who prefer to regulate anger through overt verbal or physical expression may be particularly vulnerable to ironic effects of suppression when obliged to suppress anger [26, 27]. In this study, we examined whether this mismatch conceptualization would extend to chronic low back pain patients and induced clinical pain. We expected that high anger-out patients who attempted to suppress during provocation—contrary to their preferred style—would report greater pain intensity and show more pain behaviors during subsequent pain induction than high anger-out people not told to suppress. Effects for high anger-in patients were also examined. In addition, we expected mismatch effects for anger regulation style to be partly accounted for by the degree of anger reported immediately following anger-induction. Results generally supported hypotheses.
Burns et al. [27] found that healthy people characterized by high trait anger-out instructed to suppress thoughts and feelings during provocation reported slower pain recovery following subsequent pain induction (i.e., a cold pressor) than high anger-out participants who did not suppress. Here, we replicated and extended those results by showing that high trait anger-out chronic low back pain patients who attempted to suppress anger during provocation exhibited more pain behaviors during a naturalistic pain task—one that mimicked routine daily movements—than patients who did not suppress. It is noteworthy that in these two studies, a similar pattern of mismatch effects emerged across different methods of anger induction, different methods of pain induction, and distinct sample populations. Such consistent findings using multi-method operationalizations of anger induction and multi-method assessment of pain intensity increase confidence in the validity of the mismatch model for trait anger-out, ironic effects of anger suppression, and pain. Put otherwise, high anger-out patients who, through force of circumstance, attempt to suppress anger may be more prone to incur the ironic effects of suppression (on later pain sensitivity) than low anger-out patients who also try to suppress.
As we reported in Burns et al. [20], anger report was higher among patients in the suppression condition, suggesting that attempts to suppress anger-related thoughts and feelings during harassment resulted in greater anger than was felt by patients who were also harassed but did not try to suppress. Moreover, because trait anger-out was related significantly and positively to state anger report, albeit irrespective of emotion regulation condition, we were able to evaluate whether amplified feelings of anger represented a suppression-induced pathway by which people high on trait anger-out came to reveal greater pain behaviors. Indeed, statistically controlling for post-maze anger significantly reduced the effect of trait anger-out on grimacing and sighing among patients in the Suppression condition, suggesting that the increased anger experienced by high anger-out patients following attempts to suppress colored their responses to the next noxious event. That is, the greater subsequent grimacing and sighing during movements typical of daily life, shown by high anger-out patients who attempted to suppress, may have been due in part to delayed effects exerted by ironically enhanced accessibility of thoughts and feelings of anger. Patients characterized by a pronounced tendency to express anger who, again by force of circumstances, end up suppressing anger may be more vulnerable to delayed effects of suppression (on pain perception) than their low anger-out counterparts because of ironically augmented feelings of anger immediately following the angering event. Anxiety and sadness, also inspired by the maze task and the antagonistic partner, did not account for the effects of trait anger-out on later pain behaviors, lending support to the notion that anger aroused while suppressing during harassment was the specific negative emotion linking the tendency to express anger with subsequent responses.
We also observed some moderation effects for trait anger-in. In our previous studies examining determinants of pain intensity among healthy people and muscle tension reactivity among chronic low back pain patients, we did not find evidence of match or mismatch situation effects for people scoring high on trait anger-in. Typically, trait anger-in was found to be related to pain indexes regardless of experimental condition (e.g., 3). Here, we found what could be construed as a potential match effect, with high anger-in patients who were allowed and encouraged to suppress anger, revealing fewer subsequent pain behaviors than low anger-in patients. Further, this effect was significantly mediated by post-maze reports of anger. Specifically, low levels of anger reported by high anger-in patients in the suppression condition appeared to contribute to their low frequency of subsequent pain behaviors. Thus, people who have a pronounced predisposition to suppress or inhibit anger may feel most comfortable in a situation which actively calls for this kind of anger regulation. Delayed effects on later responses to noxious stimulation may be minimal because the accessibility of anger is not ironically enhanced, despite their efforts to suppress.
Before too much store is placed in these anger-in findings, some important issues should be considered. The only other reported significant match-mismatch effect for trait anger-in, to our knowledge, was that by Engebretson et al. [23], who found that high trait anger-in participants showed faster SBP recovery from harassment when told to write positive—as opposed to negative—descriptions of their antagonist. As authors speculated, this apparent match situation led to adaptive blood pressure responses for high anger-in subjects. The majority of reported condition/situation × trait anger-in findings have been nonsignificant across diverse manipulations, populations, and dependent variables [3, 24, 26, 27, 39]. We have discussed some of the limitations of the most common trait anger-in measure—from the Spielberger Anger Expression Inventory—including a high degree of statistical overlap with measures of broad negative affect; a source of variance that is typically not evaluated by partitioning it into common and unique components [40]. Thus, some important psychometric problems with the trait anger-in scale could have contributed to the many null findings. In pain studies, we have pointed to the additional difficulty that many studies have relied on self-report methods to assess both trait anger-in and pain indexes. This problem of shared method variance may dilute or even conceal actual effects of anger suppression on pain. The nonsignificant condition × trait anger-in effects we have reported for pain [3, 26, 27, 39] may be rooted in these methodological shortcomings. Promising, however, was our use in the present study of observer-rated pain behaviors during a standardized stimulus. This break from strictly self-report assessment of constructs—purported causes and effects alike—may have allowed formerly obscured effects to emerge. Consider that the correlations between the five discrete pain behavior types and self-reported pain intensity were no more than moderate, suggesting some degree of distinctiveness between these modes of assessment. Thus, true effects of a predominant anger-in style on pain—particularly under certain conditions—may be best detected when the “pain” is revealed through observable behaviors. Future research, which is certainly called for to replicate and extend these match-mismatch findings for trait anger-in, may benefit immensely from multi-method assessment.
Results underscore the importance of considering person × situation factor interactions in general when evaluating whether a given emotion regulation or coping strategy is adaptive or maladaptive. From the diverse literatures ranging from coping (e.g., [41]), to cognitive vulnerability for depression (e.g., [42]), to anger/hostility and reactivity to stress (e.g., [43]), and now here with respect to anger regulation and later pain intensity, accumulating evidence supports the proposition that certain individual difference traits moderate effects of state responses to situations. Failure to take into account joint effects of both person traits and the coping or regulation strategy used in any given situation may run the risk of missing crucial processes that govern responses and of losing important information regarding the health effects—positive and negative—of these tactics. To take an example from our findings, the main effect for emotion regulation condition we reported in Burns et al. [20], in which those in the Suppression condition exhibited more pain behaviors subsequently, masked the strong interaction effect involving trait anger-out. Considering Fig. 1, it appears that the bulk of the effect on subsequent pain behaviors for the suppression manipulation was confined to high anger-out patients. Low anger-out patients in the Suppression condition appeared to grimace and sigh at rates comparable to low and high anger-out patients in the No Suppression condition. Thus, the ill effects of any given emotion regulation strategy may be magnified among those poorly suited to use it. Infrequent person × situation mismatches may simply make for temporary discomfort. To the extent that environments or social situations place persistent and enduring demands to regulate emotions and behaviors in certain ways, however, mismatches may exert larger cumulative effects. As Broschott and Thayer argue [22], social constraints typically place boundaries and limits on the magnitude and frequency of anger expressive behaviors. High anger-out people who are chronically constrained from expressing in their family, community, and work environments may be prone to increased health risks.
These results, and those of Burns et al. [27] and Wegner and Zanakos [44], also raise the possibility that suppression of thoughts and feelings does not work the same way for all people. In Study 6 described by Wegner and Zanakos [44], the delayed effects on skin conductance reactivity from attempts to suppress thoughts of a still desired “old flame” were largely confined to subjects with high levels of trait thought suppression. One way to interpret results for low trait anger-out people here and in Burns et al. [27] and for low trait thought suppression people in Wegner and Zanakos [44] is that suppression—with the alleged activation of the unconscious monitoring process—may not produce detrimental ironic effects in the same way for all people. The monitoring process may work to great disadvantage for people who typically want to express anger, find that they cannot or should not in certain circumstances, and then try to drive these very strong and almost reflexive urges from mind. Alternatively, the monitoring process may be largely innocuous for someone who does not want to express anger at all (a high trait anger-in) is, therefore, not invested in rooting out unwanted urges to express and who then finds that certain situational constraints or norms encourage or require inhibition.
This last point leads us back to some of the problematic issues with trait anger-in. Although suppression and inhibition of strong emotions are regarded as poor tactics overall, high anger-in patients who were instructed to suppress—a match situation—appeared to benefit in that they exhibited relatively fewer subsequent pain behaviors than high anger-in patients not instructed to suppress. These findings raise the intriguing possibility that suppression of anger per se may not necessarily be linked to negative outcomes for people with high trait anger-in. If trait anger-in is indeed linked to detrimental outcomes, but the actual process of anger suppression—a supposedly defining characteristic of those who keep anger in—is not a negative process for them, then what features or processes of trait anger-in account for links between it and poor physical health? As we have suggested, trait anger-in measures may be confounded by the broader construct of negative effect so that observed associations between trait anger-in, and for instance, pain are actually proxies for underlying links between negative effect and pain [40]. Alternatively, we may want to take a closer look at the specific situation created by our experimental manipulation. In the Suppression condition, we allowed, indeed, encouraged subjects to suppress. Borrowing on the related literatures on low assertiveness and social anxiety [45–47], high trait anger-in people may be beset by the complex interplay of a desire to somehow let people know they are angry, awareness of the broad social prescription to do so (i.e., constructively communicate feelings and wishes), and fear of the potential consequences of expressing (i.e., rejection, retaliation). Unlike high trait anger-out people, high trait anger-in people may want to avoid the many thoughts and feelings regarding how they probably should express themselves, and the thoughts and feelings of what may happen if anger is actually expressed and revealed. Put otherwise, high trait anger-in people during anger provocation may be suppressing a wide range of thoughts and feelings, of which actually suppressing anger is but one small part. A situation in which suppression or inhibition is actually sanctioned may reduce the pressure for high trait anger-in people to not think about how they should express but are afraid to do so, and so relieve them of one burden. Disentangling what may be complex intertwined layers of suppression and avoidance of conflicting desires for those characterized by high trait anger-in awaits future research.
Some final considerations may be fruitful regarding the effects on the separate pain behavior components. As part of a larger debate on the communicative functions of pain behavior, investigators have argued that [32–35] there are distinct kinds of pain behaviors. Our findings support this argument by showing evidence of relative statistical independence between so-called communicative and protective behaviors and by showing differential effects of trait anger-out and trait anger-in on separate indexes. Results hint that high trait anger-out patients who attempted to suppress anger manifested later pain intensity mostly through frequent facial and nonverbal displays. Although speculative at this point, these behaviors seem more like anger expressive behaviors than protective behaviors and may be part of the pain communicative repertoire of people who typically physically or verbally express anger. Consistent with our ironic process model, as well, these potentially anger expressive (pain) behaviors may have been specifically amplified among high trait anger-out patients by delayed contamination effects of suppressing anger. Although only marginally significant, high trait anger-in patients who did not try to suppress anger revealed pain intensity during the structured pain behavior task through frequent protective behaviors. Again, this is speculative at this point, and we did not have a priori hypotheses for trait anger-in effects on specific pain behaviors. Still, high trait anger-in people may typically inhibit obvious facial or verbal expressions, and may instead be prone to body posture changes or fidgeting; that is, they may tend to reveal indirect signs of emotion. Thus, their pain behavior repertoire may also be confined to the kinds of body movements described as protective pain behaviors. These speculations about correspondences between anger and pain expressions will need to be pursued through additional research.
Some limitations of the present study should be delineated. First, we did not use a condition to arouse a negative emotion other than anger. In Quartana and Burns [9], we used distinct anxiety- and anger-induction conditions crossed with suppression manipulations. Thus, we were able to compare effects of anxiety suppression and anger suppression on pain intensity. Results showed that participants in the anger suppression conditions reported greater subsequent pain intensity than those in the anxiety suppression conditions, and so we included only anger induction in the present design. Despite prior results, we still cannot definitively conclude that effects on pain behaviors during the naturalistic structured pain behavior task were specific to the suppression of anger. Second, we inferred the presence of suppression-induced accessibility of anger based solely on self-report ratings of angry feelings immediately after the maze task. Thus, we did not directly measure whether high trait anger-out patients across the emotion regulation conditions differed in their levels of the cognitive accessibility of anger-related content prior to or during pain induction. More direct measures of cognitive accessibility could be based on interference effects during an emotional Stroop task, or on attention bias indexes derived from dot-probe procedures. Third, this study was not originally designed to examine moderation effects of individual difference factors, and so the sample size is small for the kinds of analyses we performed here. Not only should caution be taken when interpreting statistically significant findings with a small, perhaps unrepresentative sample, but we may also have missed a number of important findings because of low power.
Here, we extend our prior findings of trait anger regulation × state anger regulation match–mismatch effects on later pain intensity among healthy nonpatients undergoing a cold pressor [27] to include chronic low back pain patients. Present findings suggest that attempts to suppress anger during emotionally arousing events (i.e., state anger suppression) may disproportionately affect certain patients in quite distinctive ways. It is important to note that our pain-induction task was not a typical laboratory procedure involving heat, pressure, or forearm ischemia, but instead involved patients performing everyday activities, like sitting and standing, which could aggravate low back pain. For pain patients who prefer to express anger, attempts to suppress anger under certain circumstances may lead to amplification of low back pain during subsequent engagement in ordinary movements. These findings may have important implications for understanding the processes by which low back pain is aggravated among chronic low back pain patients even during the most innocuous activities. As Broschott and Thayer [22] have argued, the act of suppressing or inhibiting anger may play a large role in producing or exacerbating physical disorder because of social norms that discourage full anger expression. Because of these social norms, chronic low back pain patients who are dispositional anger expressers may be at increased risk for frequent bouts of intensified pain. A fuller understanding of how, under what conditions and among whom in particular anger suppression detrimentally affects both emotional and physical hurt appears essential for the development of interventions to relieve suffering among those afflicted with chronic, painful conditions.
Acknowledgments
This research was supported in part by Grants NS37164 from the National Institute of Neurological Disorders and Stroke (John W. Burns, Ph.D.), MH071260 from the National Institute of Mental Health (John W. Burns, Ph.D. and Stephen Bruehl, Ph.D.), NS050578 from the National Institute of Neurological Disorders and Stroke (Stephen Bruehl, Ph.D.).
The authors gratefully acknowledge the encouragement and generosity of Kenneth Lofland, Ph.D., and the hard work of dedicated graduate students Wesley Gilliam, Erika Gray, Justin Matsuura, Carla Nappi, and Brandy Wolfe, without which this study would not have been possible.
Footnotes
Conflict of interest statement The authors have no conflicts of interest to disclose.
Contributor Information
John W. Burns, Email: john_burns@Rush.edu, Department of Behavioral Science, Rush University Medical Center, 1645 W. Jackson Blvd. Suite 400, Chicago, IL 60612, USA.
Phillip Quartana, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Stephen Bruehl, Vanderbilt University School of Medicine, Nashville, TN, USA
References
- 1.Bruehl S, Burns J, Chung OY, Ward P, Johnson P. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: The role of endogenous opioids. Pain. 2002;99:223–233. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 2.Burns JW, Bruehl S, Caceres C. Anger management style, blood pressure reactivity and acute pain sensitivity: Evidence for a “trait × situation” model. Ann Behav Med. 2004;27:195–204. doi: 10.1207/s15324796abm2703_7. [DOI] [PubMed] [Google Scholar]
- 3.Burns JW, Kubilus A, Bruehl S. Emotion-induction moderates effects of anger management style on acute pain sensitivity. Pain. 2003;106:109–118. doi: 10.1016/s0304-3959(03)00298-7. [DOI] [PubMed] [Google Scholar]
- 4.Janssen SJ, Spinhoven P, Brosschot JF. Experimentally induced anger, cardiovascular reactivity, and pain sensitivity. J Psychosom Res. 2001;51:479–485. doi: 10.1016/s0022-3999(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 5.Burns JW, Johnson BJ, Mahoney N, Devine J, Pawl R. Anger management style, hostility and spouse responses: Gender differences in predictors of adjustment among chronic pain patients. Pain. 1996;64:445–453. doi: 10.1016/0304-3959(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 6.Kerns RD, Rosenberg R, Jacob MC. Anger expression and chronic pain. J Behav Med. 1994;7:57–67. doi: 10.1007/BF01856882. [DOI] [PubMed] [Google Scholar]
- 7.Wade JB, Price DD, Hamer RM, Schwartz SM, Hart RP. An emotional component analysis of chronic pain. Pain. 1990;40:303–310. doi: 10.1016/0304-3959(90)91127-5. [DOI] [PubMed] [Google Scholar]
- 8.Wegner DM. Ironic processes of mental control. Psychol Rev. 1994;101:34–52. doi: 10.1037/0033-295x.101.1.34. [DOI] [PubMed] [Google Scholar]
- 9.Quartana PJ, Burns JW. The painful consequences of anger suppression. Emotion. doi: 10.1037/1528-3542.7.2.400. in press. [DOI] [PubMed] [Google Scholar]
- 10.Wegner DM, Erber R. The hyperaccessibility of suppressed thoughts. J Pers Soc Psychol. 1992;63:903–912. [Google Scholar]
- 11.Wegner DM, Erber R, Zanakos S. Ironic processes in mental control of mood and mood related thought. J Pers Soc Psychol. 1993;65:1093–1104. doi: 10.1037//0022-3514.65.6.1093. [DOI] [PubMed] [Google Scholar]
- 12.Wegner DM, Shortt JW, Blake AW, Page MS. The suppression of exciting thoughts. J Pers Soc Psychol. 1990;58:409–418. doi: 10.1037//0022-3514.58.3.409. [DOI] [PubMed] [Google Scholar]
- 13.Wegner DM, Gold DB. Fanning old flames: Emotional and cognitive effects of suppressing thoughts of a past relationship. J Pers Soc Psychol. 1995;68:782–792. doi: 10.1037//0022-3514.68.5.782. [DOI] [PubMed] [Google Scholar]
- 14.Wenzlaff RM, Rude SS, Taylor CJ, Stultz CH, Sweatt RA. Beneath the veil of thought suppression: Attentional bias and depression risk. Cogn Emot. 2001;15:435–452. [Google Scholar]
- 15.Newman LS, Duff KJ, Baumeister RF. A new look at defensive projection: Thought suppression, accessibility, and biased person perception. J Pers Soc Psychol. 1997;72:980–1001. doi: 10.1037//0022-3514.72.5.980. [DOI] [PubMed] [Google Scholar]
- 16.Petrie KJ, Booth RJ, Pennebaker JW. The immunological effects of thought suppression. J Pers Soc Psychol. 1998;75:1264–1272. doi: 10.1037//0022-3514.75.5.1264. [DOI] [PubMed] [Google Scholar]
- 17.Tolin DF, Abramowitz JS, Przeworski A, Foa EB. Thought suppression in obsessive-compulsive disorder. Behav Res Ther. 2002;40:1255–1274. doi: 10.1016/s0005-7967(01)00095-x. [DOI] [PubMed] [Google Scholar]
- 18.Cioffi D, Holloway J. Delayed costs of suppressed pain. J Pers Soc Psychol. 1993;64:274–282. doi: 10.1037//0022-3514.64.2.274. [DOI] [PubMed] [Google Scholar]
- 19.Quartana PJ, Yoon KL, Burns JW. Anger suppression, ironic processes and pain. J Behav Med. 2007;30:455–470. doi: 10.1007/s10865-007-9127-2. [DOI] [PubMed] [Google Scholar]
- 20.Burns JW, Quartana PJ, Gilliam W, Gray E, Matsuura J, Nappi C, Wolfe B, Lofland K. Effects of anger suppression on pain severity and pain behaviors among chronic pain patients: Evaluation of an ironic process model. Health Psychol. 2008;27:645–652. doi: 10.1037/a0013044. [DOI] [PubMed] [Google Scholar]
- 21.Spielberger CD, Johnson EH, Russell SF, Crane RJ, Jacobs GA, Worden TJ. The experience and expression of anger: construction and validation of an anger expression scale. In: Chesney MA, Rosenman RH, editors. Anger and Hostility in Cardiovascular and Behavioral Disorders. Washington, DC: Hemisphere Publishing Corp; 1985. pp. 5–30. [Google Scholar]
- 22.Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: A model of the link between hostility and cardiovascular disease. Annals Behav Med. 1998;20:326–332. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- 23.Engebretson TO, Matthews KA, Scheider MF. Relations between anger expression and cardiovascular reactivity: Reconciling inconsistent findings through a matching hypothesis. J Pers Soc Psychol. 1989;57:513–521. doi: 10.1037//0022-3514.57.3.513. [DOI] [PubMed] [Google Scholar]
- 24.Lai JY, Linden W. Gender, anger expression style, and opportunity for anger release determine cardiovascular reaction to and recovery from anger provocation. Mod Trends Psychosom Med. 1992;54:297–310. doi: 10.1097/00006842-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Faber SD, Burns JW. Anger management style, degree of expressed anger, and gender influence cardiovascular recovery from interpersonal harassment. J Behav Med. 1996;19:31–53. doi: 10.1007/BF01858173. [DOI] [PubMed] [Google Scholar]
- 26.Burns JW, Holly A, Quartana PJ, Wolff B, Bruehl S. Trait anger management style moderates effects of actual (“state”) anger regulation on symptom-specific reactivity and recovery among chronic low back pain patients. Mod Trends Psychosom Med. doi: 10.1097/PSY.0b013e3181835cb7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns JW, Quartana PJ, Bruehl S. Anger management style moderates effects of emotion suppression during stress on pain and cardiovascular responses during pain-induction. Annals Behav Med. 2007;34:154–165. doi: 10.1007/BF02872670. [DOI] [PubMed] [Google Scholar]
- 28.Keefe FJ, Williams DA, Smith SJ. Assessment of pain behaviors. In: Turk DC, Melzack R, editors. Handbook of pain assessment. 2nd. New York, NY: Guilford Press; 2001. pp. 170–190. [Google Scholar]
- 29.Keefe FJ, Block AR. Development of an observation method for assessing pain behavior in chronic low back pain patients. Behav Ther. 1982;13:363–375. [Google Scholar]
- 30.Turk DC, Wack JT, Kerns RD. An empirical examination of the “pain behavior” construct. J Behav Med. 1985;8:119–130. doi: 10.1007/BF00845516. [DOI] [PubMed] [Google Scholar]
- 31.Kerns RD, Haythornthwaite J, Rosenberg R, Southwick S, Giller EL, Jacob MC. The Pain Behavior Checklist (PBCL): Factor structure and psychometric properties. J Behav Med. 1991;14:155–167. doi: 10.1007/BF00846177. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan MJL. Toward a biopsychomotor conceptualization of pain: Implications for research and intervention. Clin J Pain. 2008;24:281–290. doi: 10.1097/AJP.0b013e318164bb15. [DOI] [PubMed] [Google Scholar]
- 33.Hadjistavropoulus T, Craig K. A theoretical framework for understanding self-report and observational measures of pain: A communication model. Behav Res Ther. 2002;40:551–570. doi: 10.1016/s0005-7967(01)00072-9. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan MJL, Thibault P, Savard A, Catchlove R, Kozey J, Stanish WD. The influence of communication goals and physical demands on different dimensions of pain behavior. Pain. 2006;125:270–277. doi: 10.1016/j.pain.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Prkachin KM, Schultz IZ, Hughes E. Pain behavior and the development of disability: The importance of guarding. Clin J Pain. 2007;23:270–277. doi: 10.1097/AJP.0b013e3180308d28. [DOI] [PubMed] [Google Scholar]
- 36.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 1992. pp. 135–157. [Google Scholar]
- 37.Keppel G, Zedeck S. Data analysis for research designs: Analysis of variance and multiple regression/correlation approaches. New York, NY: W.H. Freeman and Co; 1989. [Google Scholar]
- 38.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 39.Burns JW, Bruehl S, Caceres C. Anger management style, blood pressure reactivity and acute pain sensitivity: Evidence for a “trait × situation” model. Annals Behav Med. 2004;27:195–204. doi: 10.1207/s15324796abm2703_7. [DOI] [PubMed] [Google Scholar]
- 40.Burns JW, Quartana P, Bruehl S. Anger inhibition and pain: conceptualizations, evidence and new directions. J Behav Med. 2008;31:259–279. doi: 10.1007/s10865-008-9154-7. [DOI] [PubMed] [Google Scholar]
- 41.Dunkley DM, Zuroff DC, Blankstein KR. Self-critical perfectionism and daily affect: Dispositional and situational influences on stress and coping. J Pers Soc Psychol. 2003;84:234–252. [PubMed] [Google Scholar]
- 42.Iacoviello BM, Grant DA, Alloy LB, Abramson LY. Cognitive personality characteristics impact the course of depression: A prospective test of sociotropy, autonomy and domain-specific life events. Cogn Ther Res. 2009;33:187–198. doi: 10.1007/s10608-008-9197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Zimmerman EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: The role of interpersonal challenge. Psychosomatic Research. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Wegner DM, Zanakos S. Chronic thought suppression. J Person. 1994;62:615–640. doi: 10.1111/j.1467-6494.1994.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 45.Weber H, Wiedig M, Freyer J, Gralher J. Social anxiety and anger regulation. Eur J Pers. 2004;18:573–590. [Google Scholar]
- 46.Voncken MJ, Alden LE, Bögels SM, Roelofs J. Social rejection in social anxiety disorder: The role of performance deficits, evoked negative emotions and dissimilarity. Br J Clin Psychol. 2008;47:439–450. doi: 10.1348/014466508X334745. [DOI] [PubMed] [Google Scholar]
- 47.Bögels SM, Voncken M. Social skills training versus cognitive therapy for social anxiety disorder characterized by fear of blushing, trembling, or sweating. Int J Colorectal Dis. 2008;1:138–150. [Google Scholar]