Abstract
Social rejection impairs self-regulation, yet the neural mechanisms underlying this relationship remain unknown. The right ventrolateral prefrontal cortex (rVLPFC) facilitates self-regulation and plays a robust role in regulating the distress of social rejection. However, recruiting this region’s inhibitory function during social rejection may come at a self-regulatory cost. As supported by prominent theories of self-regulation, we hypothesized that greater rVLPFC recruitment during rejection would predict a subsequent self-regulatory imbalance that favored reflexive impulses (i.e., cravings), which would then impair self-regulation. Supporting our hypotheses, rVLPFC activation during social rejection was associated with greater subsequent nucleus accumbens (NAcc) activation and lesser functional connectivity between the NAcc and rVLPFC to appetitive cues. Over seven days, the effect of daily felt rejection on daily self-regulatory impairment was exacerbated among participants who showed a stronger rVLPFC response to social rejection. This interactive effect was mirrored in the effect of daily felt rejection on heightened daily alcohol cravings. Our findings suggest that social rejection likely impairs self-regulation by recruiting the rVLPFC, which then tips the regulatory balance towards reward-based impulses.
Keywords: social rejection, self-regulation, strength model of self-regulation, balance theory, rVLPFC
Social rejection is not merely an inconvenience, it has been a long-standing and profound threat to health and reproduction throughout human history and into modernity (Baumeister & Leary, 1995; Cacioppo & Patrick, 2008; Williams, 2007). Social rejection threatens human needs to belong, maintain a favorable self-view, exert control over the environment, and feel that one’s existence is meaningful (Williams, 1997, 2009). In addition to these threats, social rejection reduces individuals’ efforts towards self-regulation and subsequently leads to self-regulation failures (Baumeister, DeWall, Ciarocco, & Twenge, 2005; DeWall, Baumeister, & Vohs, 2008; Oaten, Williams, Jones, & Zadro, 2008). For instance, compared to their non-rejected counterparts, rejected participants persisted less when faced with failure and ate more unhealthy food (Baumeister et al., 2005). Rejection’s deleterious effect on self-regulation is particularly important to understand because the ability to successfully engage in self-regulation is a uniquely powerful predictor of life outcomes such as criminality, academic performance, and interpersonal relationship health (Gottfredson & Hirschi, 1990; Tangney, Baumeister, & Boone, 2004). Indeed, many societal problems (e.g., substance abuse, violence) can be readily construed as stemming directly from self-regulatory failure (Baumeister & Vohs, 2003).
To date, the neuroscientific literature is relatively silent in explaining the link between social rejection and impaired self-regulation. We propose to fill this gap by combining fMRI and longitudinal methodologies to assess the potential role that recruitment of the lateral prefrontal cortex during social rejection may play in the effect of rejection on self-regulatory failure.
Theories of Self-Regulation Failure: Strength, Motivation, and Balance
Completing a task that that requires greater self-regulatory effort often leads to subsequent self-regulatory impairment (e.g., Baumeister, Bratslavsky, Muraven, & Tice, 1998). One of the leading explanations for this phenomenon is the strength model of self-regulation, which posits that self-regulation relies upon a reservoir of regulatory ability that can be fatigued much like a muscle (Baumeister & Heatherton, 1996). According to the strength model, self-regulatory impairment occurs when this top-down, inhibitory, regulatory resource is fatigued by other demanding tasks. This model has received substantial empirical support (Hagger, Wood, Stiff, & Chatzisarantis, 2010; Hofmann, Vohs, & Baumeister, 2012).
Neuroscientific research has identified the neuroanatomical seat of this regulatory resource in the lateral prefrontal cortex (lateral PFC; Cohen, Berkman, & Lieberman, 2012; Cohen & Lieberman, 2010; Heatherton & Wagner, 2011; Lieberman, 2011). Just as the strength model would predict, the more individuals tend to use the lateral PFC to regulate their impulses (e.g., racial bias), the less regulatory effort they then exert on subsequent tasks (Richeson et al., 2003). As would be predicted by the strength model, the lateral PFC likely becomes ‘fatigued’ due to greater initial use, predicting greater subsequent self-regulatory impairment. Crucially, this is not to say the lateral PFC is unable to exert self-regulatory influence it is just less likely to do so, much like a muscle that can function after intense exercise, yet would require more motivation for to do so (e.g., an oncoming car). Indeed, the seminal research on the link between social rejection and self-regulatory impairment found that the link could be broken when participants were given adequate incentives for their performance (Baumeister et al., 2005).
An alternative account of self-regulatory failure has arisen which de-emphasizes the notion that self-regulation is a resource that can become fatigued and instead posits that self-regulatory exertion shifts motivation, attention, and emotion away from superordinate goals (e.g., weight loss) and towards impulses (e.g., food cravings; Inzlicht & Schmeichel, 2012; Inzlicht, Schmeichel, & Macrae, 2014). Much like the strength model, this motivational model of self-regulation would predict that greater lateral PFC use during a self-regulatory task, an index of self-regulatory effort, would lead to lesser subsequent activation of this region as motivation and attention shifted to more impulsive, subcortical neural substrates (e.g., the nucleus accumbens).
Findings from cognitive neuroscience have been used to incorporate and expand upon models of self-regulation, taking the form of balance theory (Heatherton & Wagner, 2011). The balance perspective integrates literature on the role of the lateral PFC in facilitating self-regulation by inhibiting subcortical activity that often undermines self-regulation, stemming from regions such as the amygdala and nucleus accumbens. According to balance theory, self-regulation involves a tenuous balance between the activity of bottom-up, subcortical neural regions and top-down, prefrontal neural regions. Self-regulatory failure occurs when the balance is tipped in favor of the subcortical regions. Supporting this notion, individuals who experience self-regulatory fatigue show greater bottom-up reward activation to appetitive targets and reduced connectivity between the nucleus accumbens and lateral prefrontal regions (Wagner, Altman, Boswell, Kelley, & Heatherton, 2013). Integrating these theories and findings, social rejection is thus likely to impair self-regulation by recruiting the neural seat of self-regulation, the lateral PFC, which may subsequently tip the brain’s self-regulatory balance towards the activity of subcortical regions and the impulses they elicit. These impulses may then overpower top-down, inhibitory processes and relate to later self-regulatory impairment. Thus, enhanced activation in the lateral PFC to social rejection may place people at risk for self-regulation impairments, specifically those that stem from bottom-up cravings such as alcohol consumption.
The rVLPFC: Involvement in the Regulation of Social Rejection
Seminal neuroscientific research on social rejection has shown that the right ventrolateral PFC (rVLPFC) occupies the inferior frontal gyrus and plays a robust regulatory role during instances of exclusion (Eisenberger, Lieberman, & Williams, 2003). Across several studies, rVLPFC activation during rejection predicted less self-reported distress and activation in neural regions that subserve painful distress, suggesting a regulatory function (Eisenberger et al., 2003; Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007; Onoda et al., 2009). Confirming this regulatory role, electrical stimulation of the rVLPFC during social rejection attenuated participants’ reports of distress and aggressive responses (Riva, Romero Lauro, DeWall, & Bushman, 2012; Riva, Romero Lauro, DeWall, Chester, & Bushman, in press). These findings fit well with other neuroimaging research that identify the rVLPFC as a neural region that generally subserves inhibition and top-down control of the amygdala and nucleus accumbens in the service of effective self-regulation (Berkman & Lieberman, 2009; Berkman, Kahn, & Merchant, 2014; Cohen et al., 2012; Lieberman, 2011; Ochsner & Gross, 2005; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008; Wagner et al., 2013).
These findings support the prediction that social rejection may impair self-regulation by recruiting the rVLPFC to manage the aversive experience of social rejection. This recruitment would then, if partially, reduce the amount of self-regulatory exertion on a subsequent self-regulatory task, as shown in previous research on the lateral PFC (e.g., Richeson et al., 2003). As predicted by balance theory, this self-regulatory impairment would tip the neural balance in favor of subcortical neural regions that generate affective and reward-based impulses (Heatherton & Wagner, 2011). Neuroimaging research has implicated the nucleus accumbens (NAcc) as a crucial substrate of cravings and reward-based impulses in response to appetitive cues and possesses strong regulatory ties to the VLPFC (e.g., food; Wagner et al., 2013).
Reflecting an impaired regulatory tendency, we predicted that greater rVLPFC activation during social rejection would be associated with greater subsequent activation of the nucleus accumbens to appetitive cues. Providing evidence of a regulatory imbalance, we further predicted that rejection-specific rVLPFC activation would predict reduced functional connectivity between the rVLPFC and NAcc. Functional connectivity estimates the degree to which neural regions’ activity synchronizes or de-synchronizes over the time and across situations with greater coupling suggesting an interaction between two regions and lesser coupling suggesting the two regions function more orthogonally (Rogers, Morgan, Newton, & Gore, 2007).
Reflecting a growing trend in using neural signatures to predict outcomes in everyday life (i.e., the brain-as-predictor approach; Berkman & Falk, 2013; Berkman, Falk, & Lieberman, 2011; Falk, Berkman, & Lieberman, 2012), we sought to test these predictions combining functional magnetic resonance imaging (fMRI) with a daily diary approach. We hypothesized that daily reports of perceived social rejection would be associated with self-regulatory impairment among individuals who expressed a relatively higher level of rVLPFC activation during social rejection. Based on our balance theory perspective, we also hypothesized that daily reports of perceived social rejection would be associated with greater cravings for appetitive items (i.e., alcohol) among individuals who expressed a relatively higher level of rVLPFC activation during social rejection. Alcohol use was selected because it is a particularly acute self-regulation issue for undergraduates and has substantial consequences for life outcomes (Crawford & Novak, 2006).
To do so, participants completed 7 days of daily diaries and then entered our fMRI scanner where they were socially accepted and then rejected and then passively viewed appetitive, drug, and neutral stimuli while undergoing fMRI. The fMRI scan was performed last because we did not want the experimental induction of social rejection to contaminate subsequent daily reports of rejection. We conceptualized the fMRI scan as a measure akin to a personality questionnaire in which rank-order differences in neural activation obtained from this scan were assumed to be durable across time. This assumption is based on a considerable amount of evidence showing that neural responses obtained with fMRI correspond to such durable characteristics as Big Five personality trait clusters (DeYoung, 2010) and long-term behavioral outcomes such as smoking cessation (Berkman et al., 2011).
Materials and Methods
Participants
Forty undergraduates who reported being neurologically and psychologically healthy participated in the study for course credit and money. Due to the confined and magnetic nature of the MRI environment, we excluded obese, claustrophobic, color blind, and pregnant individuals from participating as well as individuals who reported metal inside of their bodies, the use of psychoactive medication, or a history of seizures.
One participant distorted their fMRI data during the Cyberball task by repeatedly itching their face with the response glove. Two more participants failed to pass quality assurance items on their daily diaries in which they were asked to select a given number to ensure their attention to the instructions and content of each item. Therefore, only the 37 remaining participants had their data submitted for analysis (19 females; Age: M = 18.92, SD = 1.32).
Procedure
Questionnaires
Participants completed a computerized battery of personality questionnaires that included scales relevant to self-regulation and social rejection: the Conscientiousness subscale of the Big Five Inventory (John, Donahue, & Kentle, 1991; John, Naumann, & Soto, 2008), the Brief Self Control Scale (Tangney et al., 2004), the Rejection Sensitivity Questionnaire (Downey & Feldman, 1996), and the Timeline Follow-Back Calendar (Sobell & Sobell, 1992), which measured participants’ alcohol drinking behavior over the past year.
Daily reports
For the seven days following the questionnaire session, participants received an internet questionnaire in the evening. Each daily survey contained the following components.
Daily felt rejection
To measure daily perceptions of rejection, participants responded to the item “How rejected did you feel today?” Responses were made on a scale from 1 = not at all to 7 = extremely and were averaged across the 7 days
Daily self-regulation
To measure daily self-regulation, participants completed five of the highest loading items from the Brief Self-Control Scale (Tangney et al., 2004). They were “I had a hard time resisting temptation today (reverse-scored),” “Today, I was able to meet most of my goals,” “My emotions got the best of me today (reverse-scored),” “I didn’t have much self-discipline today (reverse-scored),” and “I had a lot of mental focus and concentration today.” Responses were made on a scale from 1 = not at all to 7 = extremely. The internal reliability of the averaged items was adequate across all 7 days (α = .91) and therefore responses were averaged to create composite daily self-regulation scores across the 7 days.
Daily alcohol craving
To measure daily alcohol craving, participants responded to the question, “Today how strong was your urge to use alcohol?” Responses were made on a scale from 1 = not at all to 7 = very. Participants also used the same scale to report how much they craved marijuana and polydrugs (e.g., cocaine).
Daily control of alcohol craving
To measure how well participants controlled their alcohol craving, they answered the question, “Today, how much were you able to control your urge to use alcohol?” Responses were made on a scale from 1 = not at all to 7 = very. Participants also used the same scale to report how much they were able to control their cravings to use marijuana and polydrugs.
Social rejection task
Participants were socially accepted then rejected via the Cyberball task (as in Chester et al., 2014; Williams, Cheung, & Choi, 2000). Cyberball was implemented as a three block-design (60 seconds per block). Prior to each block, participants were instructed to rest for 10 seconds and then saw a 2 second screen which instructed them to “get ready” for the next block. Participants received an equal amount of ball tosses (i.e., ~33%) throughout the first 2.5 blocks (i.e., 150 seconds; acceptance condition). Then, participants stopped receiving the ball for the last 30 seconds of the last task (i.e., rejection condition). Although 30 seconds is a relatively short block duration, assessing this initial reaction to social rejection allowed us to capture the distress of rejection before other psychological processes begin to activate in response to the distress. For an outline of these responses to rejection, see the temporal need threat model of ostracism (Williams, 2009).
Cue reactivity task
Participants then passively viewed a series of alcohol, marijuana, polydrugs, appetitive, and neutral images while undergoing fMRI. This cue reactivity task contained 21 blocks: 3 alcohol, 3 marijuana, 3 polydrugs, 9 appetitive, and 3 neutral. Each 30 second block sequentially presented 5 images within the given condition (4 seconds per image) which were then followed by a 10 second fixation cross which modeled baseline neural activation. The order of the blocks was randomized yet held constant across participants.
Alcohol, marijuana, and polydrug stimuli were acquired from previous research on the appetitive nature of drugs and alcohol (Mun, von Eye, Bates, & Vaschillo, 2008; Buckman, White, & Bates, 2010; Ray, Hanson, Hanson, & Bates, 2010). Appetitive and neutral images were acquired from the International Affective Picture Set (IAPS; Lang, Bradley, & Cuthbert, 2008). All images were pre-rated in the IAPS technical report along a 1 – 9 Likert scale on the dimensions of pleasantness, with higher values indicating higher pleasantness (i.e., appetitiveness; Lang et al., 2008). Appetitive images included a diverse array of stimuli, such as pictures of appetizing food, smiling faces, and beautiful landscapes. The appetitive images selected for the cue reactivity task were selected due to the fact that they were rated as highly pleasant (M = 7.53, SD = 0.43). Neutral images depicted household items, bland landscapes, and mundane social scenes and were rated close to the midpoint of the pleasantness scale (i.e., 5; M = 4.91, SD = 0.26).
Post scan
After a series of anatomical scans, participants were removed from the scanner and completed the 20-item Need Threat Scale, which measured participants’ level of social distress due to Cyberball (Williams, 2009). However, this was done approximately 45 to 60 minutes after the social rejection manipulation. Finally, participants were administered a three-item suspicion probe to assess whether they believed the Cyberball manipulation.
MRI Data Acquisition, Preprocessing, and Analysis
All MRI data were obtained using a 3.0-tesla Siemens Magnetom Trio scanner. Echo planar BOLD images were acquired with a T2*-weighted gradient across the entire brain with a 3D shim (matrix size = 64 × 64, field of view = 224mm, echo time = 28ms, repetition time = 2.5s, slice thickness = 3.5mm, 40 interleaved axial slices, flip angle = 90°). To allow for registration to native space, a coplanar T1-weighted MP-RAGE was also acquired from each participant (1mm3 isotropic voxel size, echo time = 2.56ms, repetition time = 1.69s, flip angle = 12°).
The Oxford Center for Functional MRI of the Brain (FMRIB)’s Software Library (FSL version 5.0) was used to conduct all preprocessing and fMRI analyses (Smith et al., 2004; Woolrich et al, 2009). Reconstructed functional volumes underwent head motion correction to the middle functional volume using FSL’s MCFLIRT tool (Jenkinson, Bannister, Brady, & Smith, 2002). FSL’s Brain Extraction Tool was then used to remove non-brain tissue from all functional and structural volumes (Smith, 2002). After a series of data quality checks, functional volumes underwent slice-timing correction, pre-whitening, were smoothed with a 5-mm FWHM Gaussian kernel, and were high-pass filtered (120s cutoff).
Preprocessed fMRI data from the Cyberball task were then analyzed using a two-level general linear model approach. First, each participant’s BOLD signal was modeled with a fixed-effects analysis which separately modeled acceptance and rejection blocks as regressor using a canonical double-gamma hemodynamic response function with a temporal derivative. Instructions screens and all six motion parameters were also included as regressors-of-no-interest into the analysis. Rest blocks were not modeled in this analysis. A linear contrast then compared these two conditions (rejection > acceptance). Resulting contrast images from this analysis were first linearly registered to native space structural volumes and then spatially normalized to an MNI stereotaxic space template image using FSL’s FLIRT tool (Jenkinson & Smith, 2001; Jenkinson et al., 2002). Second, each participant’s contrast volumes were fed into a group-level, mixed-effects analysis which created group average maps. Cluster-based thresholding (Heller, Stanley, Yekutieli, Rubin, & Benjamini, 2006; Worsley, 2001) was applied to each image (cluster Z statistic threshold: 2.3). Family-wise error correction was then applied to all voxels within the rVLPFC region-of-interest (ROI) mask (cluster significance threshold: p < .005).The rVLPFC mask was constructed from the Automated Anatomical Labeling (AAL) atlas, utilizing the opercular, orbital, and triangular portions of the right inferior frontal gyrus (Tzourio-Mazoyer et al., 2009). To assess the specificity of the rVLPFC we also, separately, constrained our analyses to ROIs from the AAL atlas in the left VLPFC (i.e., inferior gyrus), left and right dorsolateral PFC (i.e., middle frontal gyrus), and left and right dorsomedial PFC (i.e., superior frontal gyrus).
Analyses were largely identical for the cue reactivity task. Alcohol, marijuana, polydrug, appetitive, and neutral blocks were modeled as regressors and fixation trials were left unmodeled. Four linear contrasts compared the alcohol, marijuana, polydrug, and appetitive blocks, separately, to the neutral block. Five additional linear contrasts compared each of the five conditions to the fixation baseline condition for later use in functional connectivity analyses. Group level analyses and thresholding were identical to those described in the above paragraph.
Results
Daily Diary Results
Replicating previous research (Baumeister et al., 2005; Oaten et al., 2008), the more daily felt rejection participants reported experiencing, the more they also reported self-regulation impairments across the 7-days, r(35) = −.36, p = .028. Felt rejection was also associated with greater alcohol craving, r(35) = .33, p = .049. Although in the expected direction, felt rejection was not significantly associated with control over alcohol cravings, r(35) = −.22, p = .184. Descriptive statistics for each of the types of daily reports are provided in the table below (Tables 1 and 2). The overwhelming majority of participants reported no cravings of marijuana (79%) or polydrugs (95%) across all 7 days. Thus, these cravings measures and their association control measures were not analyzed.
Table 1.
Descriptive information for daily self-reports of felt rejection and self-regulation. Scores can range from 1 to 7.
| Felt Rejection (1 item) |
Self-Regulation (5 items) |
||||||
|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | α | |
| Day 1 | 2.00 | 1.37 | 1 – 6 | 5.13 | 1.01 | 2.0 – 6.8 | .61 |
| Day 2 | 2.36 | 1.46 | 1 – 5 | 5.22 | 1.17 | 2.0 – 7.0 | .75 |
| Day 3 | 2.09 | 1.56 | 1 – 7 | 5.04 | 1.05 | 2.6 – 6.6 | .61 |
| Day 4 | 1.81 | 1.24 | 1 – 5 | 5.48 | 1.19 | 1.2 – 6.8 | .78 |
| Day 5 | 1.97 | 1.34 | 1 – 5 | 5.23 | 1.09 | 2.2 – 6.6 | .75 |
| Day 6 | 1.89 | 1.30 | 1 – 7 | 5.23 | 0.93 | 3.0 – 7.0 | .65 |
| Day 7 | 1.92 | 1.50 | 1 – 7 | 5.29 | 1.03 | 3.0 – 7.0 | .70 |
| Mean | 1.95 | 0.86 | 1.0 – 4.3 | 5.23 | 0.74 | 3.4 – 6.7 | .91 |
Table 2.
Descriptive information for daily alcohol self-reports. Scores can range from 1 to 7.
| Alcohol Craving (1 item) |
Alcohol Control (1 item) |
||||||
|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | ||
| Day 1 | 1.69 | 1.53 | 1 – 7 | 6.36 | 1.74 | 1 – 7 | |
| Day 2 | 1.39 | 0.93 | 1 – 3 | 6.47 | 1.50 | 1 – 7 | |
| Day 3 | 1.54 | 1.38 | 1 – 7 | 6.49 | 1.50 | 4 – 7 | |
| Day 4 | 1.19 | 0.82 | 1 – 3 | 6.56 | 1.52 | 1 – 7 | |
| Day 5 | 1.19 | 0.74 | 1 – 4 | 6.76 | 1.09 | 6 – 7 | |
| Day 6 | 1.11 | 0.67 | 1 – 2 | 6.69 | 1.28 | 6 – 7 | |
| Day 7 | 1.22 | 0.80 | 1 – 3 | 6.56 | 1.44 | 1 – 7 | |
| Mean | 1.32 | 0.56 | 1.0 – 3.1 | 6.57 | 1.10 | 4.7 – 7.0 | |
Neuroimaging Results
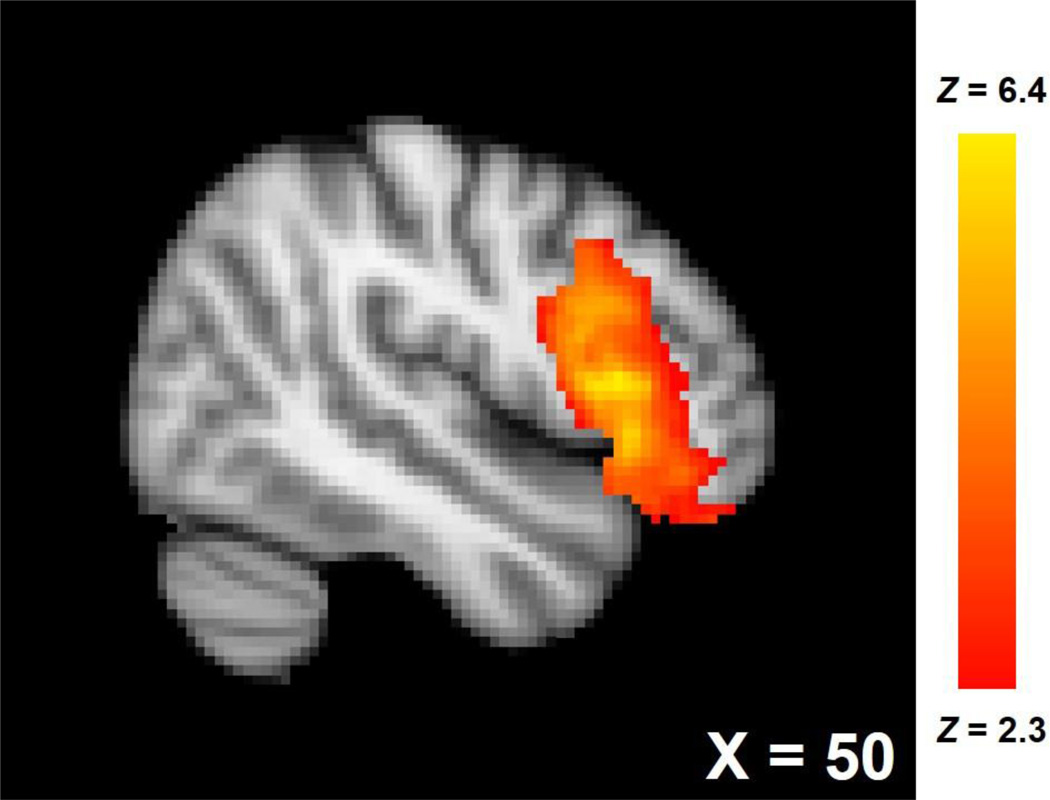
Validating the social rejection manipulation, participants reported average Need Threat Scale scores (Cronbach α = 0.91), an indicator of social distress, above the midpoint of the scale (i.e., 4), M = 4.46, SD = 0.89, t(36) = 3.16, p = .003, d = 0.73. Actual social distress during the Cyberball task was likely higher than the need threat scores suggest, as self-reports of social distress tend to diminish over the time-course of the anatomical scans that followed the rejection induction (Zadro, Boland, & Richardson, 2006). Also, no participants reported any suspicion of the task during the suspicion probe. Social rejection, compared to social acceptance, was associated with increased activity in the rVLPFC (Figure 1; 3,369 voxels, peak Z = 6.43, peak MNI coordinates: x = 50, y = 16, z = 8; rejection > acceptance contrast).
Figure 1.
rVLPFC activation associated with rejection > acceptance.
Functional data from this activated main effect cluster of the rVLPFC were converted to units of percent signal change, averaged and extracted from each participant (Mumford, J., http://mumford.bol.ucla.edu/perchange_guide.pdf). To assess the specificity of the rVLPFC, percent signal change units from this contrast were extracted from all voxels of the following prefrontal ROIs that have been implicated in successful self-regulation using AAL masks: left VLPFC (lVLPFC; inferior frontal gyrus); left and right dorsolateral PFC (DLPFC; middle frontal gyrus); left and right dorsomedial PFC (DMPFC; medial aspect of the superior frontal gyrus).
Percent signal change units from this region during social rejection were positively correlated with scores on the Brief Self-Control Scale (Cronbach α = 0.79), r(35) = .334, p = .043, and unassociated with Rejection Sensitivity Questionnaire scores (Cronbach α = 0.75), r(35) = −.155, p = .360, or scores from the Conscientiousness subscale of the Big Five Inventory (Cronbach α = 0.80), r(35) = .190, p = .259.
Correlations with Cue Reactivity
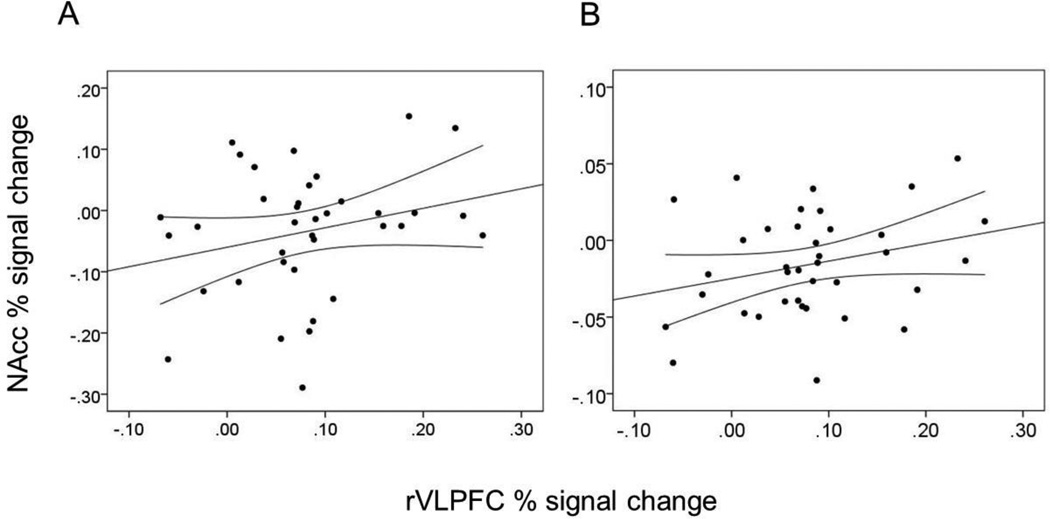
Functional data from the alcohol > neutral, marijuana > neutral, polydrug > neutral, and appetitive > neutral contrasts were converted to percent signal change units and extracted from and averaged across the left and right nucleus accumbens (NAcc). The NAcc ROI masks were acquired from the Wake Forest University Pickatlas (Maldjian, Laurienti, Kraft, & Burdette, 2003). NAcc activation from each of the four contrasts were separately regressed onto rVLPFC percent signal change units and trait measures of self-control and conscientiousness. After controlling for trait self-control and conscientiousness (to ensure rVLPFC activation was not indexing domain-general inhibitory tendencies), rejection-specific rVLPFC activation was associated with greater bilateral NAcc activation to appetitive images, β = .35, t(33) = 2.07, p = .047. After further controlling for alcohol consumption over the past year (to ensure NAcc reactivity was not a mere function of familiarity), rVLPFC activation was marginally associated with greater bilateral NAcc activation to alcohol images, β = .34, t(32) = 1.74, p = .092 (Figure 2). Correlations with the marijuana and polydrug conditions were non-significant, βs < .17, ps > .35.
Figure 2.
Correlations between percent signal change units averaged across the activated rVLPFC cluster from the rejection > acceptance contrast and (A) percent signal change units in the bilateral NAcc from the alcohol > neutral contrast, (B) percent signal change units in the bilateral NAcc from the appetitive > neutral contrast. Straight lines represent regression lines whereas curved lines represent 95% confidence intervals of that regression line.
In attempting to understand the marginal and null associations with alcohol, marijuana, and polydrug cues, an independent sample (n = 12) from the same population as the study participants rated each cue on its pleasantness along a 1 (not at all) to 9 (very much so) scale. Alcohol cues were rated as rather unpleasant (M = 3.78, SD = 2.05), and more so for the marijuana (M = 1.98, SD = 1.43), and polydrug cues (M = 1.68, SD = 1.13). Thus the alcohol, marijuana, and polydrug cues did not appear to be appetitive, with these ratings being below the IAPS technical report ratings of the neutral cues (M = 4.91, SD = 0.26) and far below the appetitive cues (M = 7.53, SD = 0.43). In the Discussion, we explain possible reasons why our undergraduate participants rated the alcohol, marijuana, and polydrug cues as relatively unpleasant.
Correlations with Functional Connectivity During Cue Reactivity
To assess the possible influence that rVLPFC activation during social rejection might have on the regulatory balance between the rVLPFC and NAcc, we extracted functional connectivity estimates from each condition of the cue reactivity task. To do so, we extracted the time-series of the cue reactivity task for each participant from the bilateral NAcc and rVLPFC, using the ROI masks described previously. After segregating the time-series by condition (i.e., alcohol, marijuana, polydrug, appetitive, neutral), we correlated the rVLPFC and NAcc time-series yielding a Pearson’s r coefficient for each participant and for each condition (as in Denson, Dobson-Stone, Ronay, von Hippel, & Schira, in press). We then used multiple linear regression to correlate these r values with rVLPFC signal change units from the rejection > acceptance contrast, separately for each cue reactivity condition.
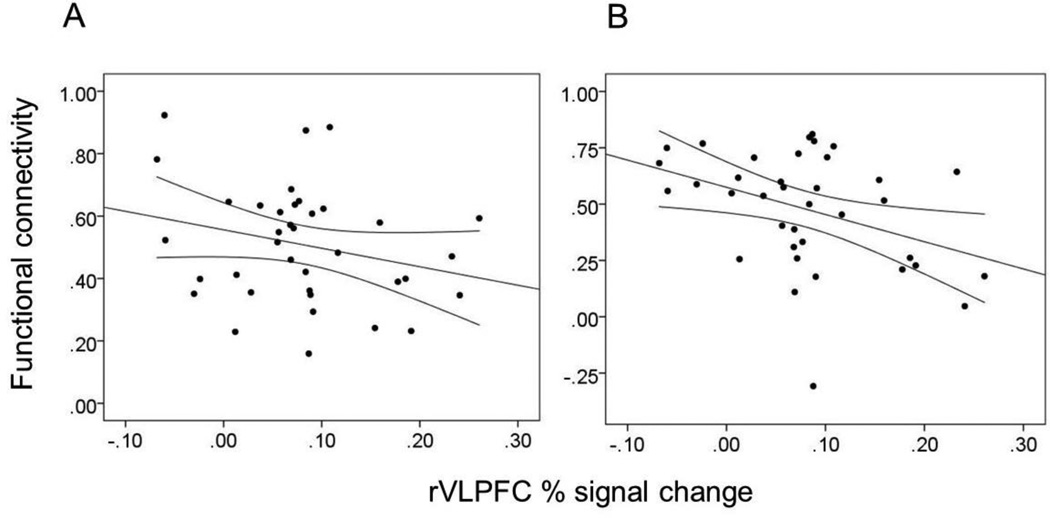
After controlling for trait conscientiousness and self-control, rVLPFC activation acquired from the rejection > acceptance contrast was negatively associated with functional connectivity between the rVLPFC and bilateral NAcc while participants viewed appetitive cues, β = −.34, t(33) = 2.08, p = .045, and polydrug cues, β = −.39, t(33) = 2.34, p = .025 (Figure 3). Associations with connectivity estimates from the alcohol, marijuana, polydrug, and neutral conditions did not reach significance, βs < .07, ps > .70.
Figure 3.
Correlation between percent signal change units averaged across the activated rVLPFC cluster from the rejection > acceptance contrast and functional connectivity estimates between the rVLPFC and the bilateral NAcc from the appetitive condition and (B) polydrug conditions. Straight lines represents the regression line whereas curved lines represent 95% confidence intervals of that regression line.
Moderation Analysis: Self-Regulation Failure
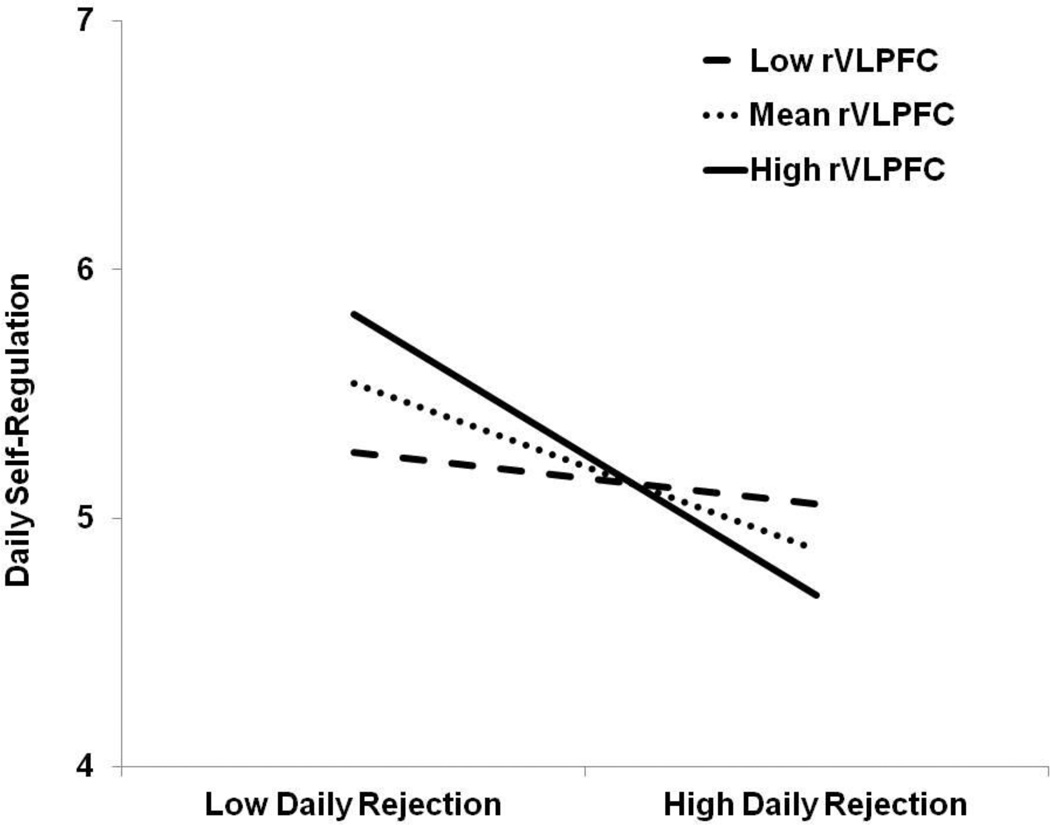
A multiple linear regression model was used to regress the composite score of self-regulation onto the main effect terms of composite felt rejection score, rejection-specific rVLPFC activity, and their interaction term simultaneously. Daily felt rejection was significantly association with lesser daily self-regulation, β = −.46, t(33) = −2.94, p = .006. No main effect was observed for rVLPFC activation on daily self-regulation, β = .10, t(33) = 0.61, p = .548. These main effects were qualified by an interaction between daily felt rejection and rVLPFC activation associated with social rejection, β = −.36, t(33) = −2.17, p = .037 (Figure 4). At low levels (-1 SD) of rVLPFC activation, felt rejection was unassociated with self-regulation, β = −.12, t(33) = −0.76, p = .454. However, at mean levels, β = −.33, t(33) = −2.86, p = .007, and high levels (+1 SD) of rVLPFC activation, β = −.57, t(33) = −3.19, p = .003, felt rejection was negatively associated with self-regulation. Thus, the greater rVLPFC activation participants showed when perceiving social rejection, the more they reported experiencing self-regulation impairments.
Figure 4.
The interactive effect of felt rejection (averaged across 7 days) and rejection-specific rVLPFC activation on successful self-regulation (averaged across 7 days). ‘Low’ labels refer to 1 standard deviation below the mean and ‘High’ labels refer to 1 standard deviation above the mean.
Next, we conducted analyses to demonstrate the specificity of the rVLPFC above and beyond a domain-general indicator of dispositional ability to effectively self-regulate. We performed a multiple linear regression analysis on self-regulation reports in which conscientiousness and trait self-control were entered simultaneously as covariates alongside the main effect and interaction terms of felt rejection and rVLPFC activation. The felt rejection by rVLPFC interaction remained significant, β = −.33, t(33) = −2.13, p = .041, even after controlling for both conscientiousness and trait self-control. Further, this interaction with the rVLPFC (not controlling for conscientiousness and trait self-control) was not observed for other regions of the prefrontal cortex that have been implicated in successful self-regulation: lVLPFC, β = −.30, t(33) = −1.37, p = .180; rDLPFC, β = −.28, t(33) = −1.62, p = .114; lDLPFC, β = −.17, t(33) = −0.94, p = .355; rDMPFC, β = −.17, t(33) = −0.91, p = .372; lDMPFC, β = −.07, t(33) = −0.43, p = .671.
Moderation Analysis: Balance Between Cravings and Control
Our next set of analyses tested predictions derived from balance theory (Heatherton & Wagner, 2011), in which self-regulatory exertion inhibits activation in subcortical areas that govern behaviors that bring immediate pleasure, such as alcohol consumption. We predicted that the more rVLPFC activation participants showed while experiencing social rejection (vs. social acceptance), the more they would report daily alcohol cravings.
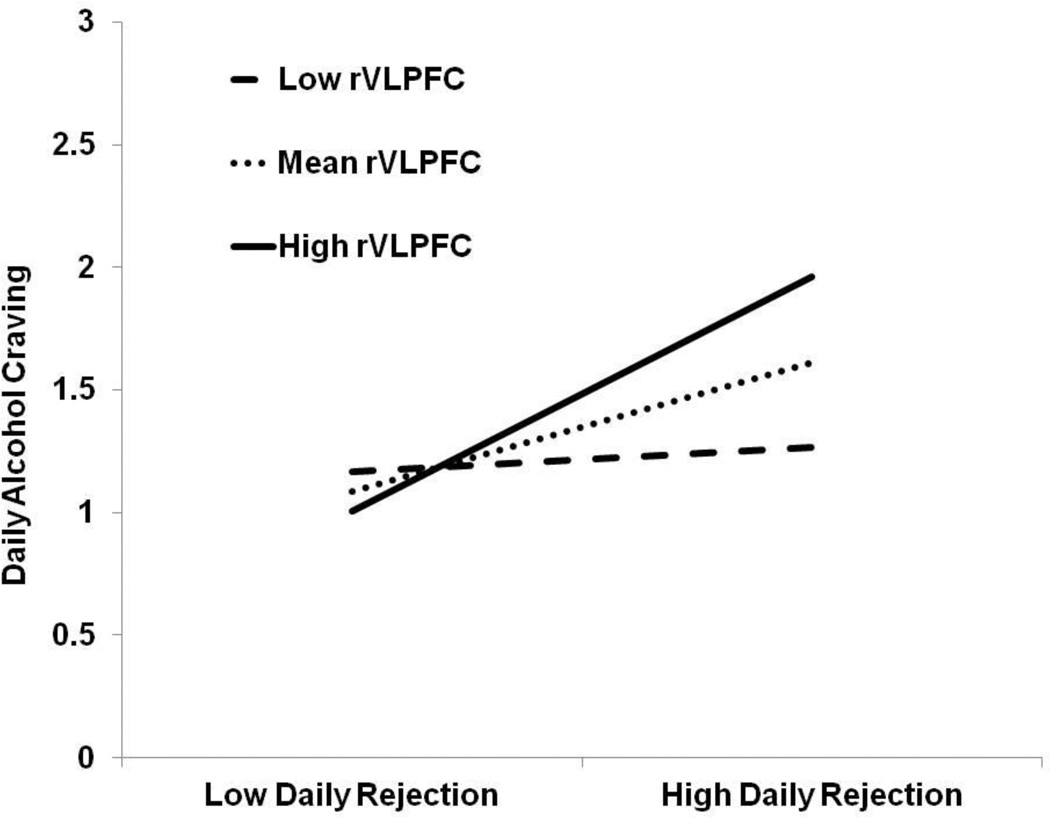
A multiple linear regression model was used to regress the composite score of alcohol craving onto the main effect terms of composite felt rejection score, rejection-specific rVLPFC activity, and their interaction term simultaneously. Daily felt rejection was significantly association with greater daily alcohol craving, β = .47, t(33) = 3.31, p = .002. No main effect was observed for rVLPFC activation on daily alcohol craving, β = .12, t(33) = 1.51, p = .140. These main effects were qualified by an interaction between daily felt rejection and rVLPFC activation associated with social rejection, β = .44, t(33) = 2.93, p = .006 (Figure 5). At low levels (-1 SD) of rVLPFC activation, felt rejection was unassociated with alcohol craving, β = −.04, t(33) = 0.54, p = .600. However, at mean levels, β = .20, t(33) = 3.30, p = .002, and high levels of rVLPFC activation (+1 SD), β = .36, t(33) = 3.94, p < .001, felt rejection was positively associated with alcohol craving. The interaction term remained significant, β = .43, t(33) = 2.83, p = .008, even after controlling for both conscientiousness and trait self-control. Further, this interaction with the rVLPFC (not controlling for conscientiousness and trait self-control) was not observed for other regions of the prefrontal cortex that have been implicated in successful self-regulation: lVLPFC, β = .37, t(33) = 1.63, p = .113; rDLPFC, β = .04, t(33) = 0.23, p = .823; lDLPFC, β = −.02, t(33) = −0.11, p = .914; rDMPFC, β = . 14, t(33) = 0.71, p = .482; lDMPFC, β = −.06, t(33) = −0.32, p = .753. This interaction between felt rejection and rVLPFC activation to rejection was not observed for control over alcohol craving scores, β = .07, t(33) = 0.41, p = .685.
Figure 5.
The interactive effect of felt rejection (averaged across 7 days) and rejection-specific rVLPFC activation on alcohol craving (averaged across 7 days). ‘Low’ labels refer to 1 standard deviation below the mean and ‘High’ labels refer to 1 standard deviation above the mean.
Discussion
Social rejection leads to a host of problematic consequences for human behavior. Impaired self-regulation due to social rejection may be one of its most impactful yet poorly understood effects. Shedding light on the neural contributors to the link between social rejection and self-regulation failure may help alleviate this gap in the literature. Towards that end, we demonstrated that rVLPFC activation during social rejection predicted greater reactivity of the NAcc to appetitive cues such as alcohol and appetitive images. Further, the more individuals recruited the rVLPFC during social rejection, the greater this same region was functionally decoupled from the NAcc while processing the same appetitive cues. This pattern of decoupling also held for polydrug cues despite their low perceived pleasantness. This polydrug-specific effect may be due to the novelty of such substances to our participants who, as undergraduates, are more likely to be exposed to alcohol and marijuana and not polydrugs. However, this remains speculative. It appears that after social rejection, those that exerted greater self-regulatory effort via the rVLPFC were then vulnerable to appetitive stimuli as the rewarding nature of these stimuli were then increased and dysregulated.
In our longitudinal daily diaries, we replicated the effect whereby perceived social rejection was associated with less successful self-regulation over 7 days. We showed that the negative association between felt rejection and self-regulation was maintained at mean levels of rVLPFC activation during rejection, exacerbated at high levels, and eliminated at low levels. Daily felt rejection also predicted greater alcohol cravings, though this was only observed a mean and high levels of rejection-specific rVLPFC activation. Balance and strength models of self-regulation would have predicted similar decrements in control over such alcohol cravings, yet these were not observed. This may be due to the skewed nature of our control over craving reports, though future research should explore this possibility. Each of these effects were obtained after accounting for dispositional levels of self-regulation. Thus, the rVLPFC effects we observed were not an artifact of a personality or domain-general tendencies to inhibit and self-regulate. Taken together, these findings implicate the rVLPFC as a crucial neural mechanism underlying the effect of rejection on self-regulatory impairment.
The ability of rVLPFC activity in response to social rejection to exacerbate the effect of social rejection on regulatory outcomes was obtained for bottom-up contributors to self-regulatory failure (i.e., NAcc reactivity to appetitive images, self-reported alcohol cravings). These findings suggest that the general self-regulatory failures we observed in relation to social rejection may be due to a self-regulatory imbalance that favors greater bottom-up cravings and impulses. Contemporary self-regulation research has and will benefit greatly from dissecting self-regulatory failures with such a dual process approach (e.g., Fujita, 2011; Hofmann, Baumeister, Förster, & Vohs, 2012).
The interaction between daily felt rejection and rVLPFC activation on daily self-regulation exhibited a positive relationship between rVLPFC activation during social rejection and successful daily self-regulation when daily felt rejection was low (see Figure 4). These findings suggest that rVLPFC recruitment during social rejection may be beneficial, but it becomes maladaptive when rejection is felt as a relatively more frequent experience. Much as sprinting is an adaptive strategy when the distance is small and leads to excess fatigue when the distance is long, prefrontal inhibition must be tailored to the self-regulatory situation. Recent neuroscience research has shown that rVLPFC activation during inhibitory tasks can readily subserve successful self-regulation (e.g., Berkman et al., 2014). These findings also fit within the body of literature on self-regulation which shows that people are aware of these self-regulatory economics and conserve and expend self-regulatory resources to the extent of the perceived demand (Muraven, Shmueli, & Burkley, 2006).
Our findings support the strength model of self-regulation (Baumeister & Heatherton, 1996) in that the greater an individual’s use of a regulatory resource (e.g., rVLPFC), the more that rejection was associated self-regulation impairment. Using activation of the PFC as a measure of self-regulatory resource fatigue is an under-used methodological approach to self-regulation research that represents a potential contribution that neuroimaging can make to psychological research questions. These findings might also be explained within the framework of the mechanistic model of self-regulation (Inzlicht & Schmeichel, 2012; Inzlicht et al., 2014), in which greater recruitment of the rVLPFC during social rejection shifts individuals’ motivational states to act on bottom-up impulses. Indeed, motivation appears to be a central element of the rejection-regulation link as this effect can be removed when extrinsic performance rewards are present (Baumeister et al., 2005).
These results also support a central tenet of balance theory which is that self-regulatory fatigue biases the brain in terms of bottom-up impulses (Heatherton & Wager, 2011). Indeed, we found that self-regulatory effort during social rejection was associated with shifts in reward reactivity to appetitive cues, dysregulation of this reactivity, and self-reported increases in alcohol cravings, all of which are bottom-up sources of self-regulatory failure. Previous neuroimaging research supporting the balance theory of self-regulation had participants engage in a fatiguing self-regulatory task outside the scanner and then imaged participants (Wagner et al., 2013). Our study was novel in that it imaged participants while they engaged in a self-regulatory task and used the degree of effort (as indexed by the rVLPFC) to predict subsequent outcomes. Future research would benefit from adopting this approach where neuroimaging is acquired during and after a self-regulatory task.
Our findings have several practical implications. Perhaps counter-intuitively, our results potentially imply that a buffer against the effect of social rejection on self-regulatory impairment is to reduce inhibitory, suppressive effort during the rejection incident, though this somewhat conjectural given the issues with reverse inference in fMRI. It may be that interventions designed at increasing emotional suppression might backfire in cases of socially rejected individuals, exacerbating their self-regulatory deficits. Instead, interventions designed at reducing the top-down inhibition of affect and increasing the acceptance of the distress, such as mindfulness-based therapies may be effective (e.g., Creswell, Pacilio, Lindsay, & Brown, 2014). Further, these results suggest that substance abuse that results from social rejection is driven by bottom-up urges. Thus, people with substance abuse problems who have poor social connections may benefit most from interventions targeted at managing cravings.
Limitations and Future Directions
A key assumption of these findings is that the degree of rVLPFC activation assessed at the fMRI session is a meaningful indicator of individual differences in the response to social rejection the ‘real-world’. Previous research has indeed shown substantial intra-individual variability in neural activation (Bennett & Miller, 2010). However, decades of personality research have shown that intra-individual variability in a given measure is not necessarily evidence that the given measurement does not relate to stable, individual differences that generalize over time (Costa & McCrae, 1994; Epstein, 1979; Fishbein & Ajzen, 1974; Funder, 2006). Although individuals may differ from time to time in the degree to which they exhibit rVLPFC activation during social rejection, the rank order individual differences that are captured by fMRI are assumed to be stable over time. This assumption has been substantiated by a growing movement in neuroscience, coined as the brain-as-predictor approach (Berkman & Falk, 2013). In this burgeoning methodology, neural activations from the scanner are assessed as predictors of longitudinally-assessed behaviors in the real world that skirt the bias inherent in self-report measures. Striking results have been obtained from this approach, with neural signatures from the scanner predicting outcomes such as the efficacy of smoking cessation advertisements and social media use (Berkman et al., 2011; Falk, Morelli, Welborn, Dambacher, & Lieberman, 2013). The ability of neural activations measured in the scanner to predict such outcomes can be taken as an indicator that these individual differences hold fidelity into the real world. Hence, our assumption about rVLPFC activation’s temporal stability and predictive validity is likely well-founded.
The Cyberball task possessed a potential confound in that the acceptance block involved a motor response and the rejection block did not. Thus, it is possible that a portion of our rVLPFC activation represented the inhibition of a motor response. However, the task does not possess the usual features of a motor inhibition task in that the button press was not prepotent, inhibition of the motor response was not difficult because the task did not advance quickly, and it is clear that no motor response was required when the ball was not passed to the participant. Despite this, future research should adopt methodological designs that de-confound this aspect of the Cyberball task. Additionally, the effects we observed in regards to NAcc reactivity and connectivity were marginal or null for alcohol, marijuana, and polydrug cues. These null relations are not evidence against our hypotheses as these cues were perceived as grossly un-appetitive and thus were unlikely to have elicited the cravings we expected the cue reactivity task to elicit. These results are likely due to the nature of our sample, which consisted of healthy, young adults who were not pre-selected on the basis of their alcohol and substance use history. Future research must correct these methodological flaws and use cues that are equivalent in their ability to elicit cravings and reward-based impulses.
Yet this speaks to another limitation of our research in that we rely on reverse inference to assume that the rVLPFC cluster we observed represented self-regulatory effort. The positive association between this cluster and trait self-control lends some support for this notion. Because trait self-control is multifaceted it is difficult to tease apart which aspect of self-control this region represented. Future research should design rejection tasks that disentangle the various elements of self-regulation (e.g., inhibition of prepotent responses, reappraisal).
Daily reports of perceived social rejection, alcohol cravings, and control over those cravings were skewed towards low or high ends of their potential distributions. This skew and restriction of range limit our findings. However, this issue with daily reports would likely serve only to make it more difficult to obtain our results as the effects must be relatively strong to emerge among a distribution with relatively little variance. Thus our data provided a conservative test of our hypotheses. Additionally, we measured felt rejection and rVLPFC activation instead of experimentally manipulating them, which reduces our ability to make causal inferences. With the advent of brain stimulation techniques, future research should assess whether our findings are causal in nature. The use of measuring ‘felt’ and perceived social rejection has inherent issues as two individuals may experience the same objective level of exclusion yet perceive it differently. Thus, we are unable to determine whether individuals who reported feeling more rejected actually were. Future research should use more objective measure of social rejection. Notwithstanding these limitations, our research extends theory, suggests intervention strategies, and lends novel insight into the neural substrates of rejection’s ability to impair self-regulation.
Highlights.
rVLPFC response to rejection predicted greater NAcc response to reward cues
rVLPFC response to rejection predicted less rVLPFC-NAcc connectivity to reward cues
rVLPFC response to rejection magnified effect of daily rejection on daily selfcontrol failure
rVLPFC response to rejection magnified effect of daily rejection on daily alcohol craving
Acknowledgments
We are deeply grateful to Naomi Eisenberger and her laboratory for their help in designing, programming, and sharing the Cyberball task we used in this study. We also thank David Powell for his technical help in the running of this study, Richard Pond Jr. and Stephanie Richman for help with data collection, and Ian Boggero for his thoughtful insight and help with earlier versions of this manuscript.
This experiment was funded by grants to the last author from the University of Kentucky’s Center for Drug Abuse Research Translation (Sponsor: National Institute on Drug Abuse, Grant number: DA005312) and from the National Science Foundation (Grant number: BCS1104118).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74(5):1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. Journal of Personality and Social Psychology. 2005;88(4):589–604. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: An overview. Psychological Inquiry. 1996;7(1):1–15. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD. Self-regulation and the executive function of the self. In: Leary MR, Tangney JP, editors. Handbook of self and identity. New York: Guilford Press; 2003. pp. 197–217. [Google Scholar]
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Annals of the New York Academy of Sciences. 2010;1191(1):133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Falk EB. Beyond brain mapping using neural measures to predict real-world outcomes. Current Directions in Psychological Science. 2013;22(1):45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: Neural correlates of breaking the link between craving and smoking. Psychological Science. 2011;22(4):498–506. doi: 10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Kahn LE, Merchant JS. Training-induced changes in inhibitory control network activity. The Journal of Neuroscience. 2014;34(1):149–157. doi: 10.1523/JNEUROSCI.3564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Using neuroscience to broaden emotion regulation: Theoretical and methodological considerations. Social and Personality Psychology Compass. 2009;3(4):475–493. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JF, White HR, Bates ME. Psychophysiological reactivity to emotional picture cues two years after college students were mandated for alcohol interventions. Addictive Behaviors. 2010;35:786–790. doi: 10.1016/j.addbeh.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Patrick W. Loneliness: human nature and the need for social connection. New York: W. W. Norton & Company; 2008. [Google Scholar]
- Chester DS, Eisenberger NI, Pond RS, Richman SB, Bushman BJ, DeWall CN. The interactive effect of social pain and executive functioning on aggression: An fMRI experiment. Social Cognitive and Affective Neuroscience. 2014;9(5):699–704. doi: 10.1093/scan/nst038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Berkman ET, Lieberman MD. Ventrolateral PFC as a self-control muscle and how to use it without trying. Principles of Frontal Lobe Functions. 2012 [Google Scholar]
- Cohen JR, Lieberman MD. The common neural basis of exerting self-control in multiple domains. Self control in society, mind, and brain. 2010:141–162. [Google Scholar]
- Costa PT, Jr, McCrae RR. Set like plaster? Evidence for the stability of adult personality. In: Heatherton TF, Weinberger JL, editors. Can personality change? Washington, DC: American Psychological Association; 1994. pp. 21–40. [Google Scholar]
- Crawford L, Novak K. Alcohol abuse as a rite of passage: The effect of beliefs about alcohol and the college experience on undergraduates’ drinking behaviors. Journal of Drug Education. 2006;36(3):193–212. doi: 10.2190/F0X7-H765-6221-G742. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1–12. doi: 10.1016/j.psyneuen.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Denson TF, Dobson-Stone C, Ronay R, von Hippel W, Schira MM. A functional polymorphism of the MAOA gene is associated with neural responses to induced anger control. Journal of Cognitive Neuroscience. doi: 10.1162/jocn_a_00592. in press. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Baumeister RF, Vohs KD. Satiated with belongingness? Effects of acceptance, rejection, and task framing on self-regulatory performance. Journal of Personality and Social Psychology. 2008;95(6):1367–1382. doi: 10.1037/a0012632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG. Personality neuroscience and the biology of traits. Social and Personality Psychology Compass. 2010;4(12):1165–1180. [Google Scholar]
- Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology. 1996;70(6):1327–1343. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S. The stability of behavior: On predicting most of the people much of the time. Journal of Personality and Social Psychology. 1979;37:1097–1126. [Google Scholar]
- Falk EB, Berkman ET, Lieberman MD. From neural responses to population behavior: Neural focus group predicts population-level media effects. Psychological Science. 2012;23(5):439–445. doi: 10.1177/0956797611434964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Morelli SA, Welborn BL, Dambacher K, Lieberman MD. Creating buzz: The neural correlates of effective message propagation. Psychological Science. 2013;24(7):1234–1242. doi: 10.1177/0956797612474670. [DOI] [PubMed] [Google Scholar]
- Fishbein M, Ajzen I. Attitudes towards objects as predictors of single and multiple behavioral criteria. Psychological Review. 1974;81(1):59–74. [Google Scholar]
- Fujita K. On conceptualizing self-control as more than the effortful inhibition of impulses. Personality and Social Psychology Review. 2011;15(4):352–366. doi: 10.1177/1088868311411165. [DOI] [PubMed] [Google Scholar]
- Funder DC. Towards a resolution of the personality triad: Persons, situations, and behaviors. Journal of Research in Personality. 2006;40:21–34. [Google Scholar]
- Gottfredson MR, Hirschi T. A general theory of crime. Stanford: Stanford University Press; 1990. [Google Scholar]
- Hagger MS, Wood C, Stiff C, D L. Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin. 2010;136(4):495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hassin R, Ochsner K, Trope Y. Self control in society, mind, and brain. New York: Oxford University Press; 2010. [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15(3):132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y. Cluster-based analysis of fMRI data. NeuroImage. 2006;33(2):599–608. doi: 10.1016/j.neuroimage.2006.04.233. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Baumeister RF, Förster G, Vohs KD. Everyday temptations: An experience sampling study of desire, conflict, and self-control. Journal of Personality and Social Psychology. 2012;102(6):1318–1335. doi: 10.1037/a0026545. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Vohs KD, Baumeister RF. What people desire, feel conflicted about, and try to resist in everyday life. Psychological Science. 2012;23(6):582–588. doi: 10.1177/0956797612437426. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Schmeichel BJ. What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspectives on Psychological Science. 2012;7(5):450–463. doi: 10.1177/1745691612454134. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Schmeichel BJ, Macrae CN. Why self-control seems (but may not be) limited. Trends in Cognitive Sciences. 2014;18(3):127–133. doi: 10.1016/j.tics.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- John OP, Donahue EM, Kentle RL. The Big Five Inventory--Versions 4a and 54. Berkeley, CA: University of California, Berkeley, Institute of Personality and Social Research; 1991. [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big Five trait taxonomy: History, measurement, and conceptual issues. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. New York: Guilford Press; 2008. pp. 114–158. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida; 2008. Technical Report A-8. [Google Scholar]
- Lieberman MD. Why symbolic processing of affect can disrupt negative affect: Social cognitive and affective neuroscience investigations. In: Todorov A, Fiske ST, Prentice DA, editors. Social neuroscience: Toward understanding the underpinnings of the social mind. New York: Oxford University Press; 2011. pp. 188–209. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mun EY, von Eye A, Bates ME, Vaschillo EG. Finding groups using model-based cluster analysis: Heterogeneous emotional self-regulatory processes and heavy alcohol use risk. Developmental Psychology. 2008;44(2):481–495. doi: 10.1037/0012-1649.44.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Shmueli D, Burkley E. Conserving self-control strength. Journal of Personality and Social Psychology. 2006;91(3):524–537. doi: 10.1037/0022-3514.91.3.524. [DOI] [PubMed] [Google Scholar]
- Oaten M, Williams KD, Jones A, Zadro L. The effects of ostracism on self-regulation in the socially anxious. Journal of Social and Clinical Psychology. 2008;27(5):471–504. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima KI, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Ray S, Hanson S, Hanson C, Bates ME. fMRI bold response in high risk college students: During exposure to alcohol, marijuana, polydrug and emotional picture cues. Alcohol and Alcoholism. 2010;45:437–443. doi: 10.1093/alcalc/agq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, Shelton JN. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;6(12):1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Riva P, Lauro LJR, DeWall CN, Bushman BJ. Buffer the pain away stimulating the right ventrolateral prefrontal cortex reduces pain following social exclusion. Psychological Science. 2012;23(12):1473–1475. doi: 10.1177/0956797612450894. [DOI] [PubMed] [Google Scholar]
- Riva P, Romero-Lauro LJ, DeWall CN, Chester DS, Bushman BJ. Reducing aggressive responses to social exclusion using transcranial direct current stimulation. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsu053. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magnetic Resonance Imaging. 2007;25(10):1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Humana Press; 1992. pp. 41–72. [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Altman M, Boswell RG, Kelley WM, Heatherton TF. Self-regulatory depletion enhances neural responses to rewards and impairs top-down control. Psychological Science. 2013;24(11):2262–2271. doi: 10.1177/0956797613492985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD. Aversive interpersonal behaviors. New York: Plenum Press; 1997. Social ostracism; pp. 133–170. [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58(1):425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism: A temporal need-threat model. In: Zanna Mark P, editor. Advances in Experimental Social Psychology. Vol. 41. Academic Press; 2009. pp. 275–314. [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: Effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79(5):748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Smith SM. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. Functional MRI: an introduction to methods. 2001;14:251–270. [Google Scholar]
- Zadro L, Boland C, Richardson R. How long does it last? The persistence of the effects of ostracism in the socially anxious. Journal of Experimental Social Psychology. 2006;42(5):692–697. [Google Scholar]