Abstract
This paper describes the current cancer burden and time trends, discusses dominant risk factors and prevention and control strategies, and makes future projections for the top eight cancers (stomach, lung, liver, colon/rectum, esophagus, breast, cervix, and leukemia) in the Asian Pacific Rim region. The future cancer trends through to the year 2050 are projected based on population dynamics, including population growth and ageing. In 2000, the Asian Pacific Rim had over 3 million new cancer cases, over 2 million cancer deaths, and 5.4 million people living with cancer. In 2050, 7.8 million new cancer cases and 5.7 million deaths from cancer are projected. The current cancer burden and the future projection provide facts that cancer is and will be a very serious public health problem in the Asian Pacific Rim region and will assist public health officers and cancer researchers in the design and establishment of public health policies, prioritization of future research, and application of current knowledge in the prevention and control of cancer.
Keywords: Cancer burden, risk factors, time trends, prevention, cancer projections, Asian Pacific Rim
Introduction
With the control of infectious diseases in the countries of the Asian Pacific Rim, the importance of noncommunicable diseases such as cancer is sure to increase. This paper will describe the current burden of cancer such as incidence, mortality, and prevalence, discuss the dominant risk factors, time trends, and prevention routes for the top cancers, and project future cancer trends in the Asian Pacific Rim region. We will be focusing on Asia and the Pacific Islands of the Pacific Rim. The data will be divided into five regions: Eastern Asia (Japan), Eastern Asia (South and North Korea), Eastern Asia (China), South-Eastern Asia, and the Pacific Islands. Japan is separated as its own region because it is generally categorized as a ‘developed’ country, whereas the rest of Eastern Asia, as well as the rest of the Asian Pacific Rim, is considered ‘developing’ (World Bank, 1993). We further chose to separate China and Korea to prevent China's results from overshadowing those of South and North Korea due to the much larger population size of China. The Pacific Islands includes the islands of Melanesia, Micronesia, and Polynesia.
Indices of “Burden”
There are many indices for measuring burden, or impact, of a disease. The three indices we will use in this analysis to assess the burden of cancer are incidence, mortality, and prevalence. Other indices that will not be further discussed but are used as well are years of life lost (YLLs), disability-adjusted life years (DALYs), and quality-adjusted life years (QALYs).
Incidence measures the frequency of new cases in a population. Often, it is expressed as an absolute number of newly diagnosed cases in the defined population or as a rate of new cases per 100,000 persons per year. The incidence rate gives an estimate of the average risk of developing the cancer in question. Also, it allows for comparisons of risk of developing the cancer among different populations. For evaluating the effectiveness of primary prevention strategies, incidence would be the most appropriate indicator.
Mortality measures the frequency of deaths in a population. Like incidence, mortality is also commonly expressed as an absolute number of deaths in a defined population or as a rate per 100,000 persons per year. Mortality is a reflection of both the incidence and survival (which will be further explained below) of a given cancer, such that the higher the incidence and the shorter the survival of a cancer the higher the mortality will be. Thus, the mortality rate gives an estimate of the average population risk of dying from the cancer in question. Mortality is often chosen as an indicator because the data for it is generally more readily available.
Prevalence specifically measures the number of people alive in a population who have ever been diagnosed with a given cancer in the past. Prevalence by this definition, however, is not particularly practical for healthcare planning purposes since the regimen for treating newly diagnosed patients is quite different from that for long-term survivors, whom often may be considered ‘cured’ if the survival time is very long. Prevalence, therefore, is commonly given as a partial prevalence, or the number of cases diagnosed within a fixed time period in the past. It is often expressed as the prevalence within 5 years since patients of most cancers who live beyond 5 years after diagnosis are considered to be ‘cured’ of the cancer.
Survival is the time between cancer diagnosis and death. It is commonly expressed as a 5-year survival, which is the percentage of patients alive after 5 years of follow-up from the diagnosis date.
As previously mentioned, incidence, mortality, and prevalence data will be used in this paper to assess the burden of cancer. Incidence and mortality data will be presented by the absolute number of people developing and dying, respectively, from cancer and as a rate per 100,000 persons per year. Incidence and mortality rates will be age-standardized to the world standard population (Doll et al., 1967) to allow for comparisons between different populations. Age-standardization accounts for differences in the age structures between populations. Prevalence will be presented within a 5-year period. For the site-specific cancers that are further discussed later in the paper, the following ICD-10 codes were used unless specified otherwise: stomach (C16), lung (C33 and C34), liver (C22), colon/rectum (C18-C20), esophagus (C15), female breast (C50), cervix uteri (C53), and leukemia (C91-95) (World Health Organization, 1992).
Data Sources
Data for this review were retrieved primarily from six sources. Current incidence, mortality, prevalence, and future trend estimates were obtained from the GLOBOCAN 2000 database (Ferlay et al., 2001). Survival information was obtained from ‘Cancer Survival in Developing Countries’ (Sankaranarayanan et al., 1998), the Osaka Tumor Registry (Department of Cancer Control and Statistics, 2003), and ‘Cancer Facts & Figures’ (American Cancer Society, 2002). Age-standardized incidence rates for time trends were obtained from ‘Cancer Incidence in Five Continents’ (Waterhouse et al., 1982; Muir et al., 1987; Parkin et al., 1992; Parkin et al., 1997; Parkin et al., 2002). Population projections were taken from ‘World Population Prospects: The 2000 Revision’ (United Nations, 2001).
Current Cancer Burden
In 2000, the Asian Pacific Rim contained 24% of the world's total population. The Asian Pacific Rim had over 3 million new cases (Table 1), over 2 million deaths (Table 2), and 5.4 million people living with cancer (Table 3), which accounts for 30% of the world's new cases and 34% of the world's cancer deaths. The most commonly occurring new cancers are stomach (16.4%), lung (15.8%), and liver (13.6%). The top three killers include lung (19.9%), liver (18.9%), and stomach (16.3%). Cancers of the stomach (16.4%), breast (13.0%), and colon/rectum (12.3%) are the most prevalent cancers. Non-melanoma skin cancer is excluded from the total because of the lack of data and difficulty of measurement for this tumor.
Table 1.
Estimated New Cancer Cases, Asian Pacific Rim 2000
| Cancer | Male | Female | Both sexes | % |
|---|---|---|---|---|
| Oral cavity | 20212 | 13914 | 34126 | 1.1 |
| Nasopharynx | 33133 | 12395 | 45528 | 1.5 |
| Other Pharynx | 8629 | 2472 | 11101 | 0.4 |
| Esophagus | 167595 | 74264 | 241859 | 7.9 |
| Stomach | 332171 | 169196 | 501367 | 16.4 |
| Colon/Rectum | 157138 | 123284 | 280422 | 9.2 |
| Liver | 304665 | 113210 | 417875 | 13.6 |
| Pancreas | 39092 | 29732 | 68824 | 2.2 |
| Larynx | 23479 | 3151 | 26630 | 0.9 |
| Lung | 342358 | 141628 | 483986 | 15.8 |
| Melanoma of skin | 2807 | 2625 | 5432 | 0.2 |
| Breast | 0 | 199815 | 199815 | 6.5 |
| Cervix uteri | 0 | 92168 | 92168 | 3.0 |
| Corpus uteri | 0 | 27875 | 27875 | 0.9 |
| Ovary etc. | 0 | 44614 | 44614 | 1.5 |
| Prostate | 36689 | 0 | 36689 | 1.2 |
| Testis | 5593 | 0 | 5593 | 0.2 |
| Bladder | 44163 | 11930 | 56093 | 1.8 |
| Kidney etc. | 23492 | 12645 | 36137 | 1.2 |
| Brain, nervous system | 29396 | 22214 | 51610 | 1.7 |
| Thyroid | 8974 | 27533 | 36507 | 1.2 |
| Non-Hodgkin lymphoma | 38639 | 23368 | 62007 | 2.0 |
| Hodgkin's disease | 3513 | 1804 | 5317 | 0.2 |
| Multiple myeloma | 7394 | 5519 | 12913 | 0.4 |
| Leukemia | 43443 | 32504 | 75947 | 2.5 |
| All sites but skin | 1784314 | 1279658 | 3063972 | 100.0 |
Table 2.
Estimated Cancer Deaths, Asian Pacific Rim 2000
| Cancer | Male | Female | Both sexes | % |
|---|---|---|---|---|
| Oral cavity | 9582 | 6544 | 16126 | 0.8 |
| Nasopharynx | 18955 | 7343 | 26298 | 1.3 |
| Other Pharynx | 5320 | 1623 | 6943 | 0.3 |
| Esophagus | 125107 | 56873 | 181980 | 8.7 |
| Stomach | 223281 | 118274 | 341555 | 16.3 |
| Colon/Rectum | 81241 | 66137 | 147378 | 7.0 |
| Liver | 287971 | 108043 | 396014 | 18.9 |
| Pancreas | 34671 | 28068 | 62739 | 3.0 |
| Larynx | 12185 | 2013 | 14198 | 0.7 |
| Lung | 294513 | 121601 | 416114 | 19.9 |
| Melanoma of skin | 1388 | 1316 | 2704 | 0.1 |
| Breast | 0 | 64237 | 64237 | 3.1 |
| Cervix uteri | 0 | 46612 | 46612 | 2.2 |
| Corpus uteri | 0 | 7060 | 7060 | 0.3 |
| Ovary etc. | 0 | 23687 | 23687 | 1.1 |
| Prostate | 20302 | 0 | 20302 | 1.0 |
| Testis | 1476 | 0 | 1476 | 0.1 |
| Bladder | 18895 | 6301 | 25196 | 1.2 |
| Kidney etc. | 11023 | 6071 | 17094 | 0.8 |
| Brain, nervous system | 18021 | 14273 | 32294 | 1.5 |
| Thyroid | 2351 | 4973 | 7324 | 0.3 |
| Non-Hodgkin lymphoma | 22810 | 14253 | 37063 | 1.8 |
| Hodgkin's disease | 1455 | 756 | 2211 | 0.1 |
| Multiple myeloma | 5687 | 4117 | 9804 | 0.5 |
| Leukemia | 31244 | 23077 | 54321 | 2.6 |
| All sites but skin | 1296999 | 796878 | 2093877 | 100.0 |
Table 3.
Estimated 5-year Prevalence of Cancer, Asian Pacific Rim 2000
| Cancer | Male | Female | Both sexes | % |
|---|---|---|---|---|
| Oral cavity | 53184 | 31442 | 84626 | 1.6 |
| Nasopharynx | 86009 | 32920 | 118928 | 2.2 |
| Other Pharynx | 17060 | 4563 | 21623 | 0.4 |
| Esophagus | 164127 | 68917 | 233044 | 4.3 |
| Stomach | 600141 | 291625 | 891766 | 16.4 |
| Colon/Rectum | 372772 | 296342 | 669113 | 12.3 |
| Liver | 142752 | 51954 | 194707 | 3.6 |
| Pancreas | 18613 | 13441 | 32054 | 0.6 |
| Larynx | 59164 | 7848 | 67012 | 1.2 |
| Lung | 406294 | 153351 | 559645 | 10.3 |
| Melanoma of skin | 7857 | 7767 | 15624 | 0.3 |
| Breast | 0 | 707195 | 707195 | 13.0 |
| Cervix uteri | 0 | 266100 | 266100 | 4.9 |
| Corpus uteri | 0 | 106662 | 106662 | 2.0 |
| Ovary etc. | 0 | 121531 | 121531 | 2.2 |
| Prostate | 85677 | 0 | 85677 | 1.6 |
| Testis | 18161 | 0 | 18161 | 0.3 |
| Bladder | 109384 | 30506 | 139890 | 2.6 |
| Kidney etc. | 54535 | 28648 | 83183 | 1.5 |
| Brain, nervous system | 52479 | 37182 | 89661 | 1.7 |
| Thyroid | 36621 | 114057 | 150678 | 2.8 |
| Non-Hodgkin lymphoma | 76662 | 48554 | 125216 | 2.3 |
| Hodgkin's disease | 9199 | 4663 | 13862 | 0.3 |
| Multiple myeloma | 11138 | 9105 | 20243 | 0.4 |
| Leukemia | 49782 | 38979 | 88761 | 1.6 |
| All sites but skin | 2687032 | 2738589 | 5425621 | 100.0 |
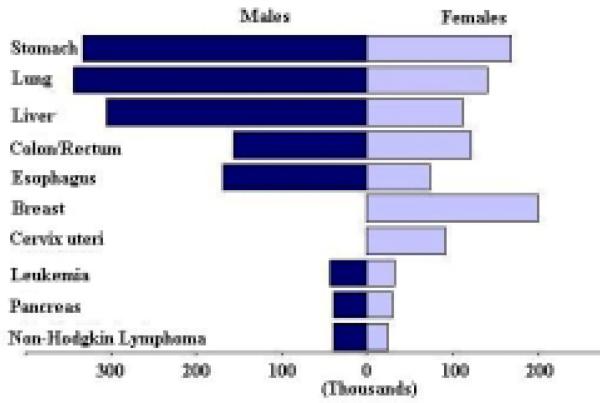
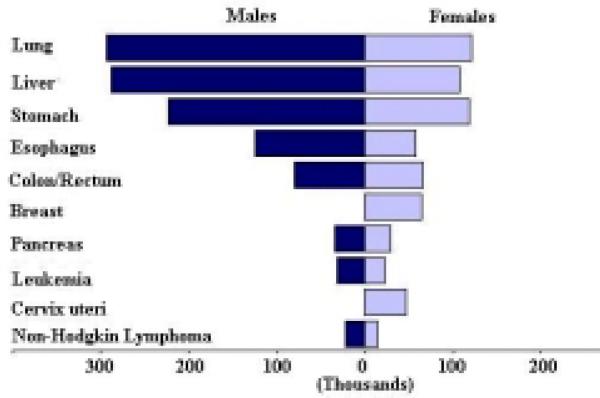
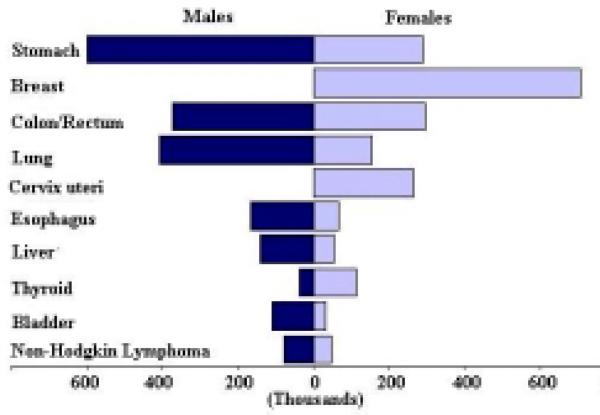
The rankings of these cancers in males are different from the rankings in females. The ten most common cancers by sex based on the number of new cases is shown in Figure 1, the ten top cancer killers based on the number of deaths is shown in Figure 2, and the ten most prevalent cancers based on the five-year prevalence is shown in Figure 3. The mortality to incidence ratio can be used as an indicator of case fatality. When comparing Figures 1 and 2, the high case fatality and magnitude of liver and lung cancer underlines their importance in the Asian Pacific Rim, with mortality to incidence ratios of 0.95 and 0.86, respectively. The ratio between prevalence and incidence can be used as an indicator of prognosis. Comparing Figures 1 and 3, we see that although breast cancer falls below liver and lung cancer in terms of new cases, its prevalence far exceeds that of those two cancers, indicating a much better prognosis for breast cancer. The five-year survival for the major cancers is shown in Table 4, and we see that breast cancer does in fact have a much better survival relative to the other cancers, and liver and lung cancer have low survival. Stomach cancer, however, has the highest number of new cases, the third highest number of deaths, and the highest number of prevalent cases.
Figure 1.
Number of New Cases (in thousands) for the 10 most Common Cancers, 2000
Figure 2.
Number of Deaths (in thousands) for the 10 most Common Cancers, 2000
Figure 3.
Five-year Prevalence (in thousands) for the 10 most Prevalent Cancers, 2000
Table 4.
Five-year Survival Rates for the Major Cancers Among Populations in the US, Japan, China and Thailand
| Cancer site | US White |
Japan Osaka (1994) | China |
Thailand |
|||
|---|---|---|---|---|---|---|---|
| (1986-91) | (1992-98) | Qidong (1982-91) | Shanghai (1988-91) | Chiang Mai (1983-92) | Khon Kaen (1985-92) | ||
| Stomach | 20 | 21 | 48 | 17 | 28 | 9 | 17 |
| Lung | 16 | 15 | 13 | 4 | 14 | 3 | 9 |
| Liver | 10 | 7 | 14 | 2 | 5 | 1 | 10 |
| Colon/rectum | 62 | 62 | 58* | 30 | 46 | 36 | 29 |
| Esophagus | 13 | 15 | 18 | 5 | 15 | 3 | 27 |
| Female breast | 84 | 88 | 80 | 56 | 73 | 63 | 47 |
| Cervix | 70 | 72 | 68** | 42 | 62 | 65 | 55 |
| Leukemia | 48 | 47 | 29 | 5 | 17 | 10 | 17 |
Includes ICD-10 C18-C21
Includes ICD-10 C53-C55
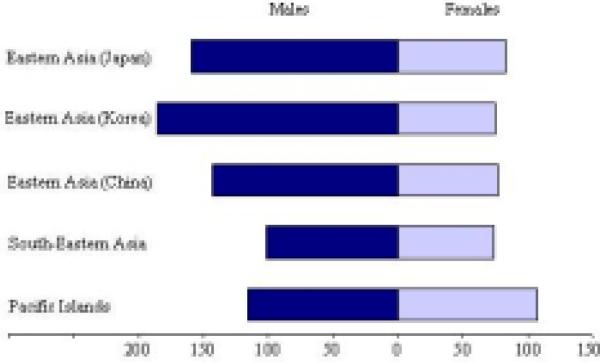
Figure 4 shows the sex-specific age-standardized incidence rates per 100,000 for all cancer sites by Asian Pacific Rim region, and Figure 5 shows the sex-specific age-standardized mortality rates (per 100,000) by region. Among males, Japan and Korea have the highest incidence rates in the region, followed by China, the Pacific Islands, and South-Eastern Asia, respectively. Korea also has the highest mortality rate among men of the region, followed by Japan, China, the Pacific Islands, and South-Eastern Asia, respectively. Among females, the Pacific Islands and Japan have the highest incidence and mortality rates in the region. Korea, China, and South-Eastern Asia have lower rates comparable to each other among females.
Figure 4.
Age-standardized Incidence Rates (per 100,000) for All Cancer Sites by Pacific Rim Region, 2000
Figure 5.
Age-standardized Mortality Rates (per 100,000) for All Cancer Sites by Pacific Rim Region, 2000
The Major Cancers: Burden, Risk Factors, and Prevention
In this section, we will expand upon the eight most common new cancers in the Asian Pacific Rim today. We will further discuss their current burden, geographical distribution, time trends, most important risk factors in these areas, and a brief summary of the most current and promising prevention strategies to address the control of the cancers.
Stomach Cancer
Burden 2000
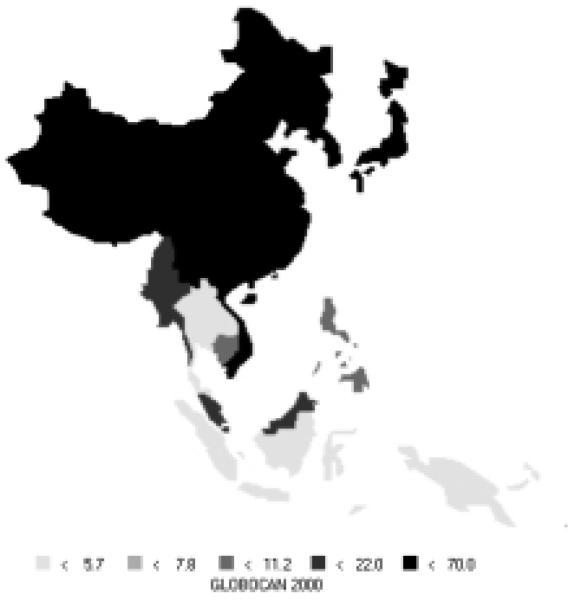
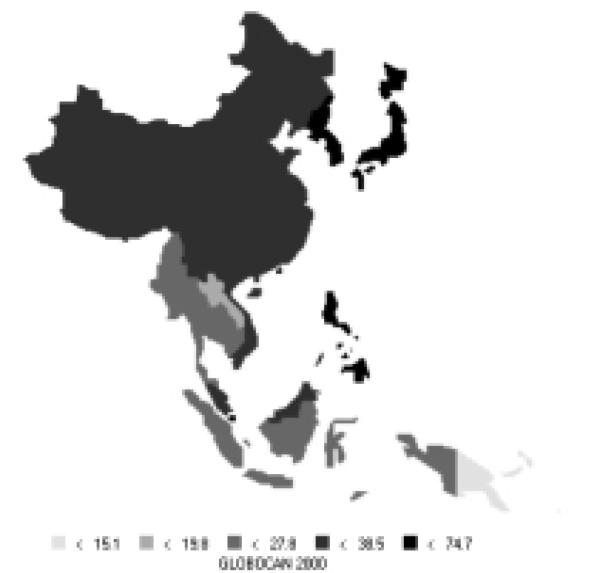
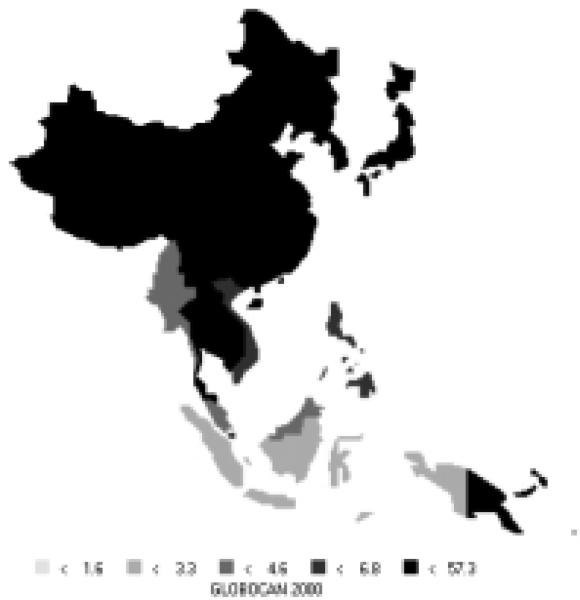
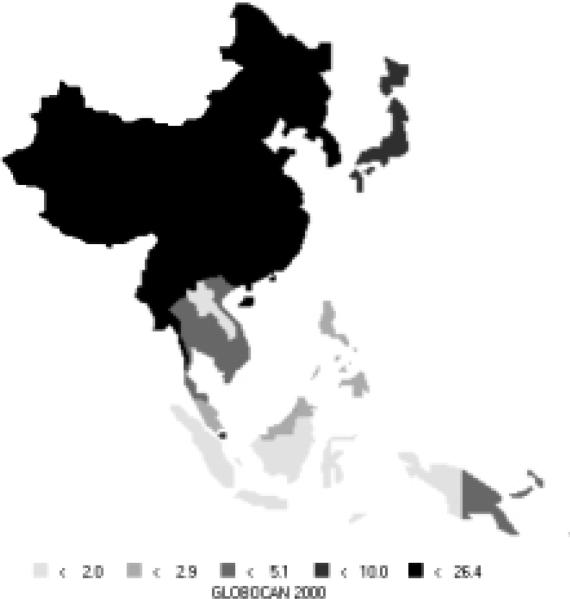
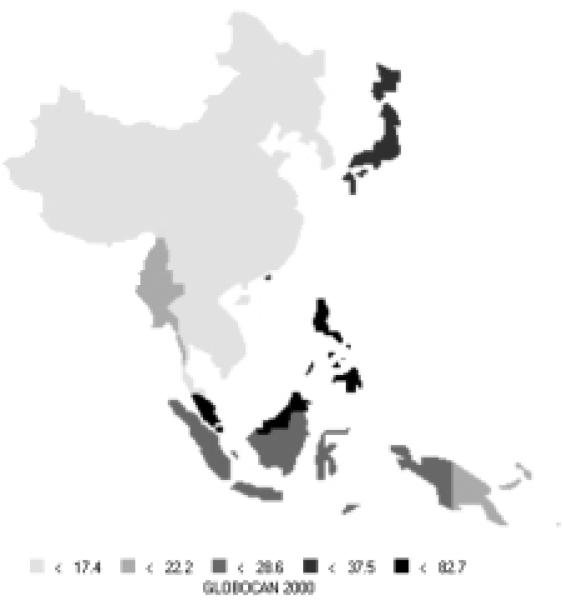
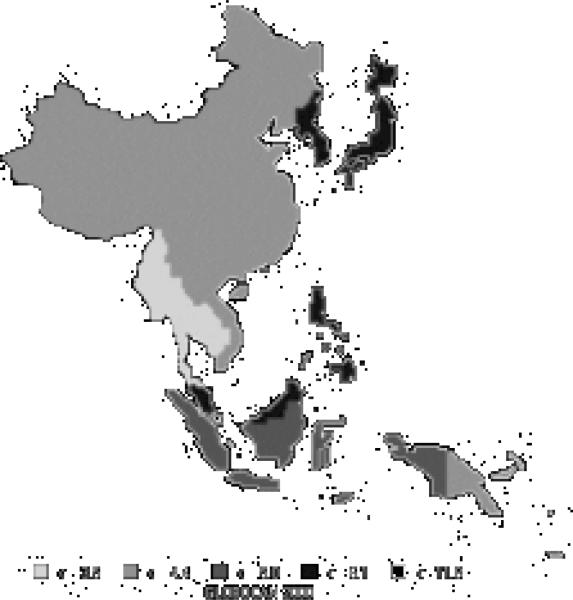
Stomach cancer is currently the most common cancer in the Asian Pacific Rim, with over 501,000 new cases, comprising over 16% of all new cases in the area. It is the third most common cause of death from cancer (340,000 deaths annually). Stomach cancer occurs more commonly in men, with 66% of new cases being male. Incidence rates for men (Figure 6) and women (Figure 7) are especially high in China, Korea, and Japan. The rates of men are generally twice those of females (Table 5). Relative to the rest of the world, the Asian Pacific Rim is among the highest-risk areas.
Figure 6.
Incidence of Stomach Cancer: Age-standardized Rates (per 100,000) - Males (all ages)
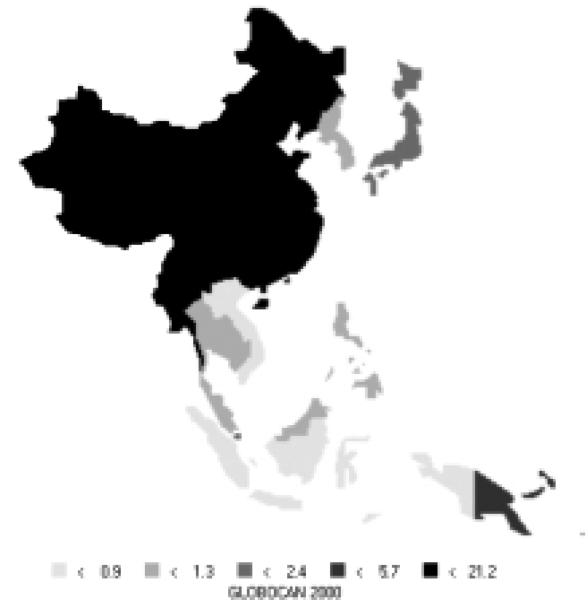
Figure 7.
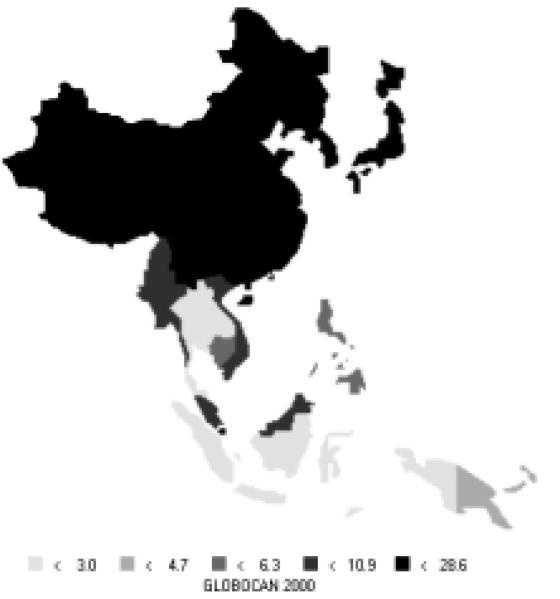
Incidence of Stomach Cancer: Age-standardized Rates (per 100,000) - Females (all ages)
Table 5.
Stomach Cancer Age-standardized Rates (per 100,000) by Sex, 2000
| Region | Incidence | Mortality | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Eastern Asia (Japan) | 69.2 | 28.6 | 31.2 | 13.8 |
| Eastern Asia (Korea) | 70.0 | 25.7 | 43.3 | 17.9 |
| Eastern Asia (China) | 36.0 | 17.4 | 26.9 | 13.0 |
| South-Eastern Asia | 8.7 | 4.8 | 7.4 | 4.1 |
| Pacific Islands | 6.6 | 4.4 | 5.7 | 3.7 |
| Pacific Rim | 36.1 | 16.6 | 24.5 | 11.5 |
Risk Factors
Helicobacter pylori infection is an important risk factor for stomach cancer because of its high prevalence in developing countries (80-90%) (IARC, 1994b). It is linked to low socio-economic settings because crowded living conditions promote acquisition. Infection usually occurs at a young age and may persist throughout life. Over 40% of new stomach cancer cases in the world are attributed to H. pylori infection because its infection is so prevalent (Parkin et al., 1999b). Only a handful of studies in Asian populations have found a positive association between H. pylori infection and risk of stomach cancer because of the high prevalence of H. pylori infection and lack of variation in the population.
In addition to H. pylori infection, dietary habits also contribute to risk for stomach cancer. Diets high in fruits and vegetables are protective, whereas diets high in salt increase risk. Additionally, green tea drinking and intake of garlic and allium vegetables may decrease risk, and intake of fish sources may increase risk (Cai et al., 2001; Setiawan et al., 2001).
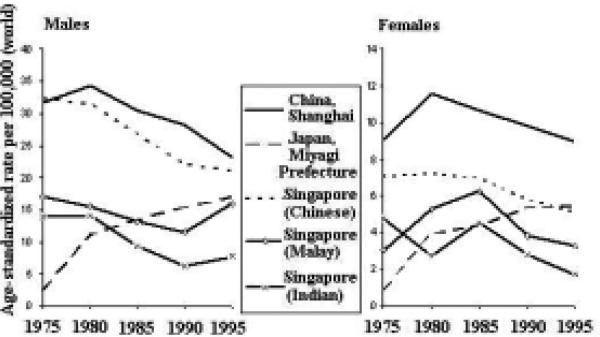
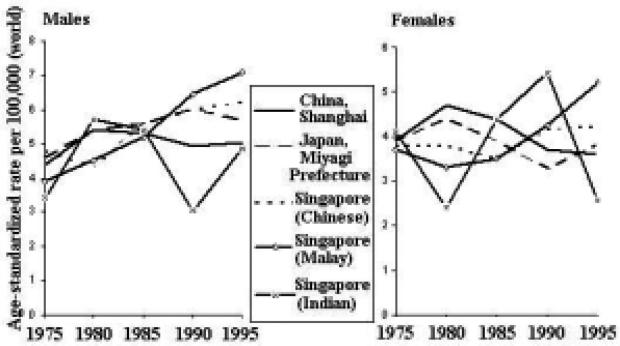
Time Trends
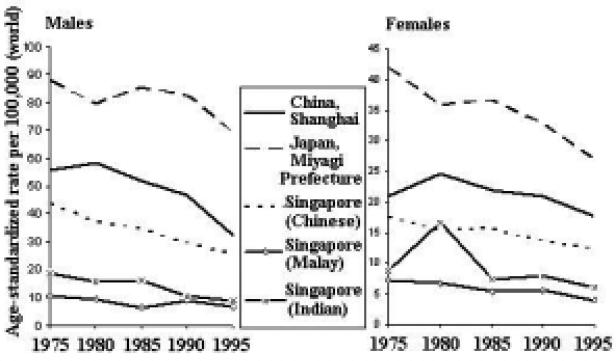
The incidence of stomach cancer is generally decreasing throughout the Asian Pacific region (Figure 8). Furthermore, the decline is more dramatic among males than females, across countries.
Figure 8.
Stomach Cancer Incidence Trends by Sex
Prospects for Prevention
With many environmental risk factors, prevention of stomach cancer seems a possibility. Migrant studies have given evidence that environmental changes can reduce stomach cancer risk. Studies of Japanese migrants to Hawaii and the United States show a decline in rates between the first and subsequent generations (Kolonel et al., 1981). Given the dietary risk/protective factors, increasing the consumption of fresh fruits and vegetables and decreasing consumption of salty foods should be considered as effective preventive measures. In addition, the use of refrigerator for food storage at home may keep food fresh and reduced consumption of salty foods may also be an effective method for prevention. Eradication of H. pylori is another possible route of prevention that has been considered. The effectiveness of eradication, however, might be questioned since a large proportion of the population is infected yet only a relatively small proportion will develop stomach cancer. Vaccinations against H. pylori infection may be a more reasonable route, but the development of an effective vaccine will take some time. In addition, investigators in the People's Republic of China have been conducting randomized intervention trials involving amoxicillin/ omeprazole, vitamins C and E and selenium, and garlic oil/ extract since 1995 (Gail et al., 1998). Japan also has X-ray screening followed by gastroscopy to detect early cancers which, although resource intensive, has shown promise in decreasing mortality rates (Parkin et al., 1999a).
Lung Cancer
Burden 2000
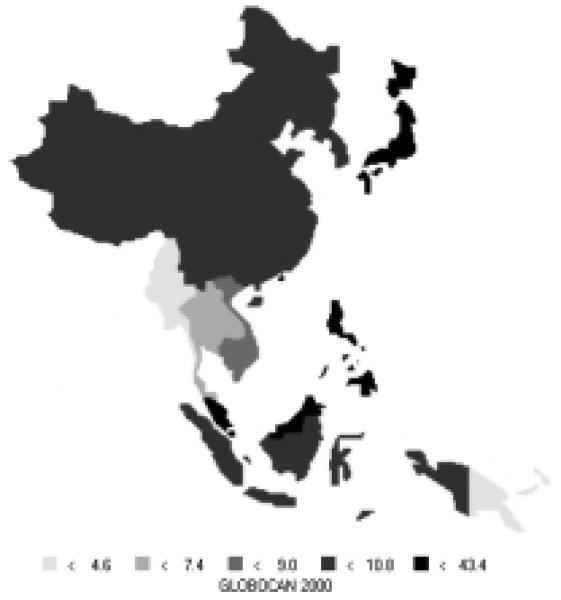
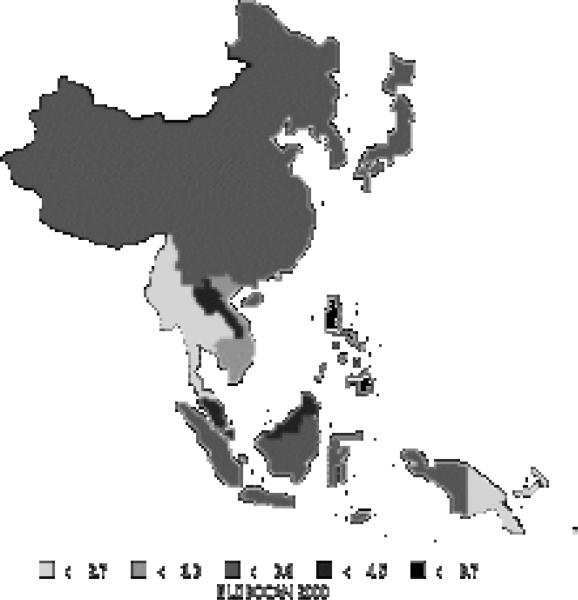
Lung cancer is currently the second most common new cancer in the Asian Pacific Rim, with 484,000 new cases, comprising about 16% of all new cases in the area. It causes 416,000 deaths annually, making it the most common cause of death from cancer in the region. Most lung cancer cases are men (71% of new cases). High-risk areas for men include Japan, Korea, and Philippines (Figure 9). High rates for women are found in all of Eastern Asia, as well as in parts of South-Eastern Asia (Figure 10). The rates of men are over 2.5 times those of females (Table 6). Relative to other parts of the world, the Asian Pacific Rim currently has moderate-level risk. Considering the high prevalence of tobacco smoking in Asian men, the relatively low incidence and mortality rates are probably due to the immaturity of the cigarette smoking and lung cancer relationship because the Asian population started smoking cigarettes approximately 20-30 years later than populations in North America and Western European countries. It was projected that the incidence and mortality of lung cancer in Asian populations in the next 20-25 years will reach today's high levels of incidence and mortality of lung cancer in the United States and United Kingdom.
Figure 9.
Incidence of Lung Cancer: Age-standardized Rates (per 100,000) - Males (all ages)
Figure 10.
Incidence of Lung Cancer: Age-standardized Rates (per 100,000) - Females (all ages)
Table 6.
Lung Cancer Age-standardized Rates (per 100,000) by Sex, 2000
| Region | Incidence | Mortality | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Eastern Asia (Japan) | 40.3 | 12.1 | 33.1 | 9.6 |
| Eastern Asia (Korea) | 48.4 | 12.1 | 40.2 | 9.6 |
| Eastern Asia (China) | 38.7 | 15.8 | 33.4 | 13.5 |
| South-Eastern Asia | 27.8 | 9.1 | 25.7 | 8.4 |
| Pacific Islands | 11.2 | 5.0 | 10.4 | 3.5 |
| Pacific Rim | 37.4 | 13.9 | 32.3 | 11.9 |
Risk Factors
The most important risk factor for lung cancer is clearly tobacco smoking. It is believed that 80-90% of lung cancer in men and 40-50% of lung cancer in women may be attributed to tobacco smoking in the world (Parkin et al., 1994; Parkin et al., 2001). Lifetime smokers have 20-30 times the risk of developing lung cancer compared to non-smokers, and there is a clear dose-response relationship between number of cigarettes smoked per day, degree of inhalation, and age at initiation and risk for lung cancer. Alarmingly, two-thirds of men in China smoke (Zhang et al., 2003). Importation of tobacco from the United States into the Asian Pacific Rim region, beginning in 1986 with Japan, drove smoking prevalences to increase as a result of the aggressive advertising strategies of the American tobacco companies (Chen et al., 1990; Connolly, 1992; Honjo et al., 2000). Additionally, passive exposure to tobacco smoke (ETS) is also believed to increase risk by about 25% (Zhong et al., 2000).
Although smoking is fairly uncommon among Chinese women, their rates are higher than in other ethnicities. This increase in risk is partly attributed to exposure to environmental risk factors, such as passive smoking (Zhong et al., 1999a), cooking fumes (Zhong et al., 1999b; Ko et al., 2000) and indoor smoky coal emissions (Xu et al., 1989).
Another important risk factor in the Asian Pacific region is occupational exposure to harmful materials, such as asbestos and rubber exposure. Occupational exposure to asbestos, including among factory workers and miners, has been shown to have an excess of death from lung cancer. Additionally, the effects of asbestos and rubber exposure work synergistically with the effects of cigarette smoking in increasing risk for lung cancer (Zhang et al., 1989; Morinaga et al., 2001).
Diet also contributes to risk for lung cancer. Diets high in vegetables, especially green vegetables and carrots, and fruits can decrease risk (IARC, 2003). Clinical trials, however, found that β-carotene, in fact, did not prevent lung cancer but actually increased risk in high-risk individuals, including heavy smokers (IARC, 1998).
Time Trends
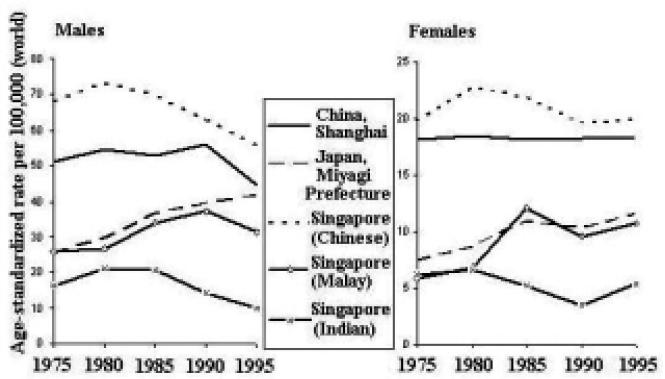
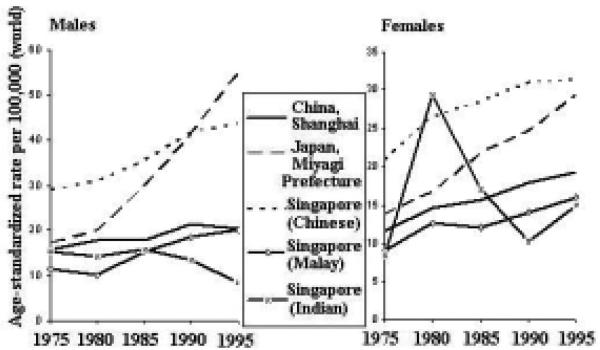
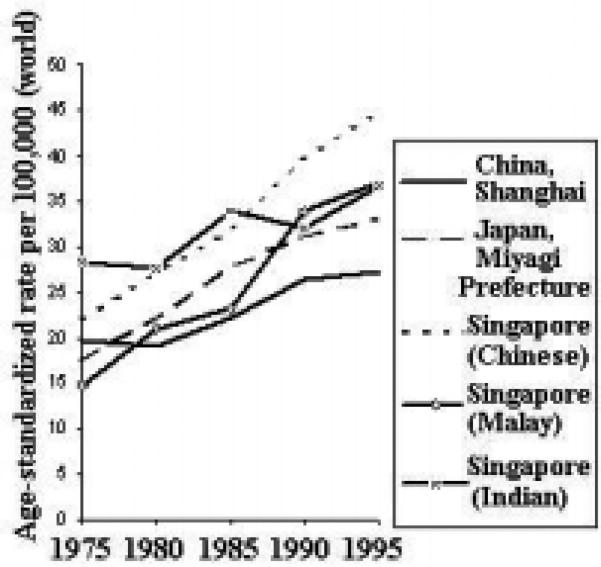
The incidence rates of lung cancer are increasing in some areas of the Asian Pacific Rim and decreasing in others (Figure 11). Among males, rates in Japan and Singapore (Malay) have been generally increasing, whereas rates in China and Singapore (Chinese and Indian) have been decreasing. The rates among females are generally increasing throughout the region, with the possible exception of Singapore (Indian).
Figure 11.
Lung Cancer Incidence Trends by Sex
Prospects for Prevention
The promotion of smoking cessation is the most cost-effective campaign against lung and other smoking-related cancers and diseases. The risk of lung cancer will progressively decline with duration of cessation relative to a continuation in smoking; yet it still remains unclear whether the increased risk of former smokers will ever drop back to that of ‘never-smokers’ (Doll et al., 1976). Therefore, another prospect for prevention involves programs to persuade adolescents not to start smoking. Social pressure to make smoking socially unappealing and legislation to make smoking financially less accessible are important measures for prevention. These preventive measures have been shown to be effective and successful in the United States (Fishman et al., 1999; Weir et al., 2003).
Control of other risk factors, such as workplace exposure associated with the increase of lung cancer, environmental tobacco smoking, and radon exposure in residences may also lead to a reduction in lung cancer. Also, sputum cytology and chest radiographs are not recommended for lung cancer screening because no favorable impact of the screening on lung cancer mortality could be demonstrated (Zhang, 2002). Spiral computerized topography, however, another screening method that may be able to detect small, asymptomatic lung cancers in heavy smokers aged over 60 years has received increasing interest (Henschke et al., 1999). Additionally, recent developments have pointed out that molecular genetic alterations associated with progression toward lung cancer, such as p53 mutations in sputum samples, may be employed to identify high-risk individuals for early detection and chemoprevention (Samet, 1995).
Liver Cancer
Burden 2000
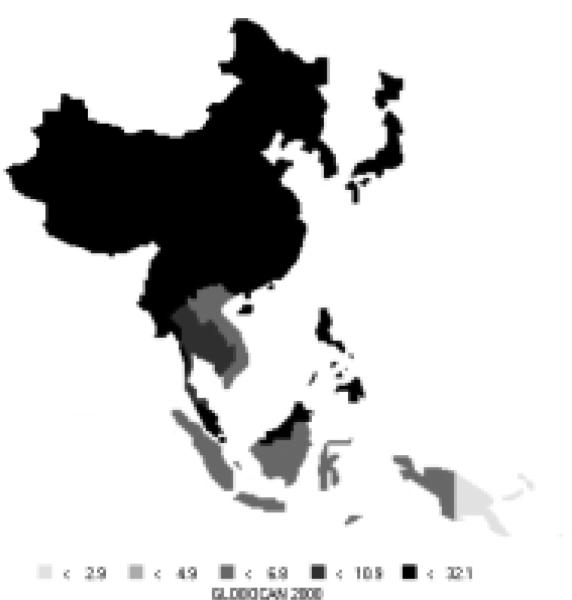
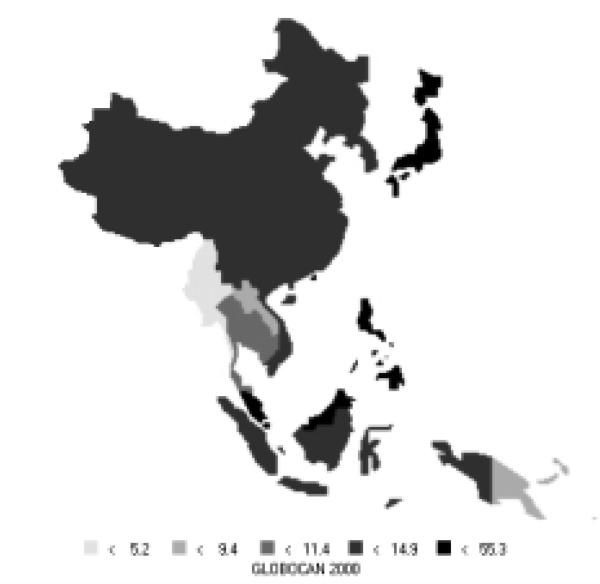
Liver cancer comprises over 13% of all new cases in the Asian Pacific region (418,000 new cases), now making it the third most common cancer in the area. It is the second most common cause of death from cancer in the Asian Pacific Rim, responsible for 396,000 deaths annually. About 73% of new cases are men. High-risk areas among men include all of Eastern Asia and parts of the Pacific Islands; parts of South-Eastern Asia are moderate to high-risk areas (Figure12). Areas with high rates for women include all of Eastern Asia, parts of South-Eastern Asia, and parts of the Pacific Islands (Figure 13). Interestingly, men have rates that are nearly 3 times those of females (Table 7). The Asian Pacific Rim has very high risk relative to the rest of the world.
Figure 12.
Incidence of Liver Cancer: Age-standardized Rates (per 100,000) - Males (all ages)
Figure 13.
Incidence of Liver Cancer: Age-standardized Rates (per 100,000) - Females (all ages)
Table 7.
Liver Cancer Age-standardized Rates (per 100,000) by Sex, 2000
| Region | Incidence | Mortality | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Eastern Asia (Japan) | 29.2 | 8.1 | 22.5 | 6.7 |
| Eastern Asia (Korea) | 48.8 | 11.6 | 39.8 | 9.7 |
| Eastern Asia (China) | 35.2 | 13.3 | 34.4 | 13.0 |
| South-Eastern Asia | 18.3 | 5.7 | 17.4 | 5.4 |
| Pacific Islands | 18.3 | 9.4 | 17.2 | 8.8 |
| Pacific Rim | 32.1 | 11.2 | 30.3 | 10.7 |
Risk Factors
The major environmental risk factors for liver cancer are Hepatitis B and C virus and aflatoxin B1 exposure. Hepatitis B virus (HBV) chronic carriers have a 20-fold increase in risk compared with non-carriers. The prevalence of HBV chronic infection is 10-15% in China and South-Eastern Asia (Parkin et al., 2001). About two-thirds of liver cancers in these areas are attributed to HBV infection (Parkin et al., 1999b). The dominant route of transmission among children is vertical transmission (mother to child), whereas the dominant routes among adults are through sexual transmission or blood transfusion (IARC, 1994a). Although the mechanism by which HBV infection may increase risk for liver cancer is still unclear, two possible pathways are through viral DNA integration, which promotes genetic instability in the host (Brechot, 1998), and inflammation (Butel et al., 1995; Ghebranious et al., 1998).
Hepatitis C virus (HCV) infection increases risk for liver cancer 25-fold compared with those never infected. The prevalence of HCV in Japan is among the highest in the world. Unlike HBV, it is transmitted primarily by blood and blood products through transfusions or injection with contaminated equipment. HCV appears to cause liver cancer through chronic hepatitis and cirrhosis, both known as precursors of liver cancer, through the intense hepatocyte regeneration occurring in these conditions (Rocken et al., 2001).
Aflatoxin B1, a toxin found in mildewed grains and nuts, has shown to increase risk for liver cancer in several studies in China (Yeh et al., 1989; Qian et al., 1994). It is believed to cause point mutations in the tumor-suppressor gene TP53 (Aguilar et al., 1993). Microcystins (MC), a hepatotoxic peptide produced by water bloom algae, contaminate the drinking water in the endemic areas of primary liver cancer in China. It was believed that intake of MC in drinking water may be related to primary liver cancer in China (Ueno et al., 1996). Other environmental risk factors include alcohol consumption (Chen et al., 1991; Goodman et al., 1995) and cigarette smoking (Doll, 1996).
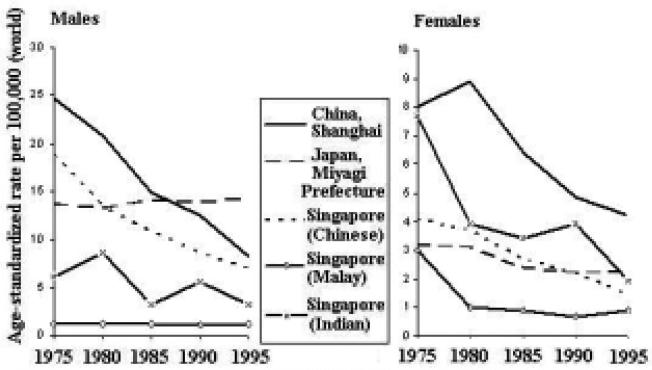
Time trends
There have been large increases in liver cancer incidence and mortality in Japan (Figure 14); this has been ascribed to increasing alcohol consumption (in men) (Makimoto et al., 1999) and to an increasing prevalence of HCV infection (Tanaka et al., 1991). Transmission of the virus, by non-sterile transfusions and injections, was at a maximum in the years after the second World War, and the risk of liver cancer in Osaka (which has one of the highest rates in the world) has decreased in successive birth cohorts, born since around 1931-35, along with prevalence of infection with HCV (Tsukuma et al., 1999). Incidence rates in China have also been decreasing, more dramatically among males than females. Rates in Singapore have also generally been decreasing.
Figure 14.
Liver Cancer Incidence Trends by Sex
Prospects for Prevention
Given the large proportion of liver cancers attributed to HBV, the vaccine against HBV renders great promise for the prevention of liver cancer. Studies have shown that 90-95% of transmission in neonates can be prevented if Hepatitis B immune globin (HBIG) is given in conjunction with the vaccine (Beasley et al., 1983; Wong et al., 1984). To establish the effectiveness of the vaccine in preventing liver cancers later in life, a study in Qidong County, China is currently underway, but it will be many years before results are available (Sun et al., 1991). In Taiwan, mass vaccination against Hepatitis B was introduced in the 1980s, first to neonates born of HBsAg positive mothers, then, in 1984, for all new-borns. By 1994, it was possible to compare liver cancer incidence in children aged 6-9 born before vaccination was introduced and after. There was a four-fold difference in incidence (Chang et al., 1997). Vaccination against HBV, therefore, provides great hope in liver cancer prevention. In addition, improvements in conditions for food storage and avoidance of drinking contaminated water may also be effective in reducing incidence of primary liver cancer.
Colorectal Cancer
Burden 2000
Cancer of the colon/rectum is currently the fourth most common new cancer in the Asian Pacific Rim, with over 280,000 new cases, making up about 9% of all new cases in the area. It is the cause of over 147,000 deaths annually, making it the fifth most common cause of death from cancer. Colorectal cancer cases are split almost evenly between men and women (56% of new cases are men). High-risk areas for both men and women include Japan and parts of South-Eastern Asia (Figures 15 and 16). Moderate-level risk areas include the remainder of Eastern Asia and other parts of South-Eastern Asia. The age-standardized mortality rates are nearly half the incidence rates (Table 8), indicating a relatively good prognosis. Relative to the rest of the world, the Pacific Rim has moderate to low-level risk, with the exception of Japan which is considered to be a high-risk area.
Figure 15.
Incidence of Colorectal Cancer: Age-standardized Rates (per 100,000) - Males (all ages)
Figure 16.
Incidence of Colorectal Cancer: Age-standardized Rates (per 100,000) - Females (all ages)
Table 8.
Colorectal Cancer Age-standardized Rates (per 100,000) by Sex, 2000
| Region | Incidence | Mortality | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Eastern Asia (Japan) | 43.2 | 25.3 | 17.6 | 11.0 |
| Eastern Asia (Korea) | 14.9 | 10.3 | 8.8 | 5.7 |
| Eastern Asia (China) | 13.2 | 9.9 | 7.2 | 5.4 |
| South-Eastern Asia | 12.6 | 10.0 | 8.1 | 6.4 |
| Pacific Islands | 10.4 | 5.8 | 6.7 | 3.8 |
| Pacific Rim | 16.9 | 12.1 | 8.8 | 6.4 |
Risk Factors
Migrant studies of Japanese immigrants to the United States have shown that risk for colorectal cancer increases greatly in the migrants, lending support to the large role of environmental factors in the risk for colorectal cancer (Shimizu et al., 1987). These environmental factors include diet and exercise. Diets rich in vegetables and unrefined plant foods, like cereals and legumes, protect against colorectal cancer, whereas, diets rich in red meat increase risk (World Cancer Research Fund Panel, 1997). The mechanism by which these foods confer risk, however, remains unclear. Alcohol also increases risk for colorectal cancer. Regular physical exercise has been shown to decrease risk, and obesity may increase risk (Le Marchand et al., 1997; Giacosa et al., 1999).
Time Trends
In the Asian Pacific Rim region, incidence rates for colorectal cancer in both men and women have been rising, with the possible exception of Singapore (Indian) (Figure 17). Rates in Japan and China have had a more dramatic increase.
Figure 17.
Colorectal Cancer Incidence Trends by Sex
Prospects for Prevention
Given the dietary and behavioral risk factors for colorectal cancer, improvements in diet and exercise frequency are a prospect for prevention. Diets rich in vegetables and unrefined plant foods and moderate in red and processed meat, as well as regular exercise and weight control, should be promoted. Diets rich in fiber have been hypothesized to be protective against colorectal cancer. Recent trials, however, have found little evidence to support this hypothesis (Alberts et al., 2000; Schatzkin et al., 2000). Another potential prevention route is screening. Colorectal cancer can be screened by tests for occult blood in stool, sigmoidoscopy, or colonoscopy. Any of these methods can be used for early detection of colorectal adenomatous polyps, which are precursors of colorectal cancer, and localized cancers.
Esophageal Cancer
Burden 2000
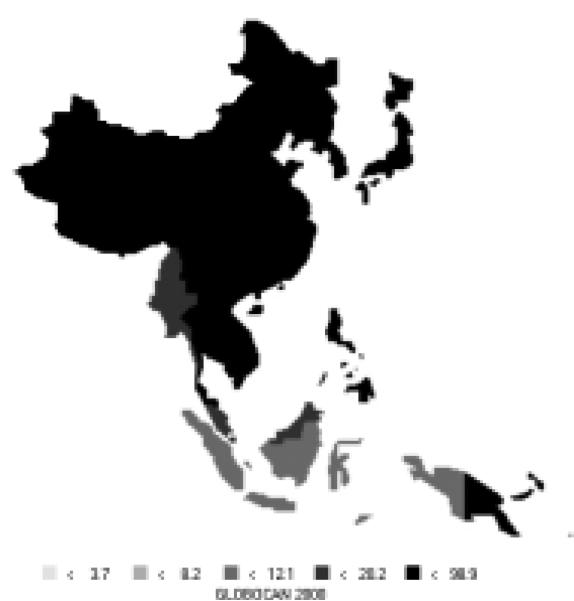
Esophageal cancer is currently ranked fifth in terms of new cancers (242,000 new cases) and fourth in cancer deaths (182,000 deaths annually). It makes up about 8% of the new cancers in the Pacific Rim, and most cases are men (69% of new cases). Recently, there has been an increase in incidence of adenocarcinoma of the esophagus in the United States and European countries, which accounts for approximately 50% of esophageal cancers (Powell et al., 2002; Vizcaino et al., 2002). However, squamous-cell carcinoma is still the major histological type of esophageal cancer in the Asian Pacific Rim. In China, over 90% of cases with esophageal cancer are squamous-cell carcinoma (Chang et al., 2002). Among men, China and Korea are high-risk areas, whereas Japan has moderate-level risk (Figure 18). Incidence rates are also high in China among women (Figure 19). The risk in China remains high even when compared to other parts of the world. The rates of men are 2.5 times those of females (Table 9).
Figure 18.
Incidence of Esophageal Cancer: Age-standardized Rates (Pacific Rim, per 100,000) - Males (all ages)
Figure 19.
Incidence of Esophageal Cancer: Age-standardized Rates (Pacific Rim, per 100,000) - Females (all ages)
Table 9.
Esophageal Cancer Age-standardized Rates (per 100,000) by Sex, 2000
| Region | Incidence | Mortality | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Eastern Asia (Japan) | 10.0 | 1.6 | 7.4 | 1.1 |
| Eastern Asia (Korea) | 10.1 | 1.0 | 7.5 | 0.7 |
| Eastern Asia (China) | 24.4 | 10.9 | 18.4 | 8.3 |
| South-Eastern Asia | 3.1 | 1.3 | 2.7 | 1.1 |
| Pacific Islands | 3.2 | 2.2 | 2.7 | 1.9 |
| Pacific Rim | 18.3 | 7.4 | 13.8 | 5.6 |
Risk Factors
Diet and lifestyle are very important when considering risk for esophageal cancer in the Asian Pacific Rim. Pickled vegetables increase risk, whereas fresh fruits and vegetables, specifically citrus fruits and green leafy vegetables, decrease risk. Tobacco and alcohol consumption also increase risk for esophageal cancer. Additionally, drinking and eating foods at very high temperatures increase risk (Hu et al., 1994), whereas drinking green tea at a moderate temperature may be protective (Yang et al., 1993; Gao et al., 1994).
Time Trends
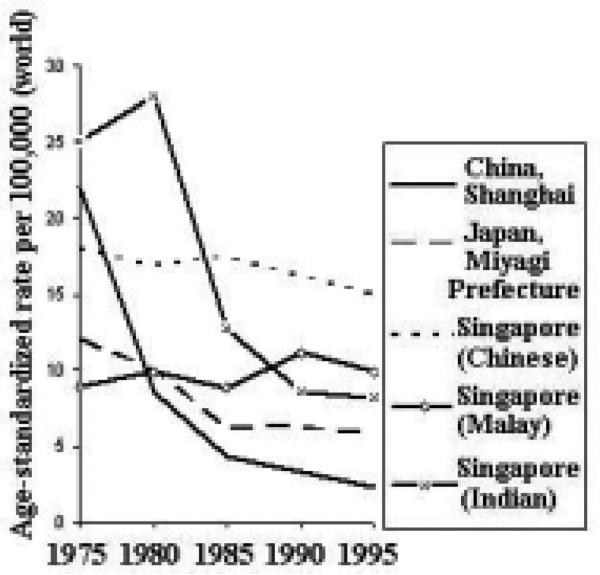
Incidence trends for esophageal cancer have been generally decreasing throughout the Asian Pacific Rim (Figure 20). The decline has been most dramatic among Chinese males. Among females, the decline has been most dramatic in Singapore (Indian) and China. Furthermore, the decline has been more dramatic among males than females.
Figure 20.
Esophageal Cancer Incidence Trends by Sex
Prospects for Prevention
Studies of Chinese immigrants in Singapore have shown that risk for esophageal cancer is greatly decreased among second generations relative to their China-born counterparts (Lee et al., 1992), giving evidence that esophageal cancer can be prevented. Based on the risk factors, the most promising preventive interventions include, primarily, improvements in diet to be high in nutrients and, secondarily, smoking cessation and control of alcohol consumption. Even though nutrient supplementation seems to be a good prevention route, micronutrient supplementation trials have not been very promising. Although they have shown a slight decrease in risk, the decreases have not been very significant (Munoz et al., 1985; Li et al., 1993; Blot et al., 1993a; Blot et al., 1993b).
Breast Cancer
Burden 2000
Breast cancer is currently ranked sixth in the Pacific Rim according to number of new cancers, but it is the leading cause of cancer among women (200,000 new cases). It comprises only about 6% of all new cases in the area but makes up 13% of all prevalent cases, indicating a relatively good prognosis. It is the cause of over 64,000 deaths annually, making it the sixth most common cause of death from cancer in the Asian Pacific Rim but the fifth most common among women in this area. Within the Asian Pacific Rim, relatively high incidence rates are found in parts of South-Eastern Asia; Japan has moderate-level risk (Figure 21 and Table 10). Compared with the rest of the world, however, the Pacific Rim has fairly low risk.
Figure 21.
Incidence of Breast Cancer: Age-standardized Rates (Pacific Rim, per 100,000) - Females (all ages)
Table 10.
Female Breast Cancer Age-standardized Rates (per 100,000), 2000
| Region | Incidence | Mortality |
|---|---|---|
| Females | Females | |
| Eastern Asia (Japan) | 31.4 | 7.7 |
| Eastern Asia (Korea) | 12.5 | 4.0 |
| Eastern Asia (China) | 16.5 | 4.5 |
| South-Eastern Asia | 25.6 | 11.5 |
| Pacific Islands | 25.7 | 11.6 |
| Pacific Rim | 19.8 | 6.4 |
Risk Factors
Migrant studies have provided evidence that environmental and lifestyle factors play a large role in conferring risk for breast cancer. Studies of Japanese, Chinese, and Korean migrants to the United States show a progressive increase in risk in successive generations, demonstrating that the former culture may possess behavioral factors that protect against breast cancer (Ziegler et al., 1993). Those factors that impact reproductive and hormonal patterns have been found to influence risk most. For example, factors associated with increased levels of endogenous estrogens, such as early menarche, late age at first birth, low parity, and late menopause, increase risk for breast cancer. Additionally, obesity and alcohol consumption increase risk (Heck et al., 1997).
There also appears to be a genetic/familial component in assessing risk for breast cancer. BRCA1 mutations are related to early occurrence of breast cancer (Ho et al., 2000; Patmasiriwat et al., 2002). Additionally, women with a family history of breast cancer have an increase in risk. For example, in Korea, women with a mother and/or sister previously diagnosed with breast cancer have a 2- to 3-fold increase in risk relative to those with no family history of breast cancer (Yoo et al., 2002).
Time Trends
Incidence rates for breast cancer are rising in the Asian Pacific Rim (Figure 22). The increasing trend is very dramatic and rather consistent in different parts of the region. There are many possible explanations: decreasing age at menarche, increasing age at menopause, decreasing fertility and increasing age at first birth, and increases in height and weight, as well as changes in diet.
Figure 22.
Female Breast Cancer Incidence Trends
Prospects for Prevention
At present, the most practical approach to improving the burden of breast cancer is by decreasing the mortality rate through early detection by screening. Regular mammographic screening may reduce mortality by about 25% in women above 50 years of age (de Koning, 2003). The benefit in younger women, however, is not as clear-cut. Given the resource-intensity of screening by mammography, it may not be a feasible option for many countries in the Pacific Rim. Clinical breast examination (CBE) and breast self-examination (BSE) may be other options for low-resource settings. BSE requires relatively few resources, at least for the screening process, making it very appealing, but a randomized controlled trial of BSE in China found no reduction in mortality (Thomas et al., 2002). CBE has been introduced as a single screening modality in Japan. There is some suggestion that, where coverage by such screening is high, breast cancer mortality rates have declined more than in other areas (Kuroishi et al., 2000), although a case-control study was inconclusive (Kanemura et al., 1999).
Cervical Cancer
Burden 2000
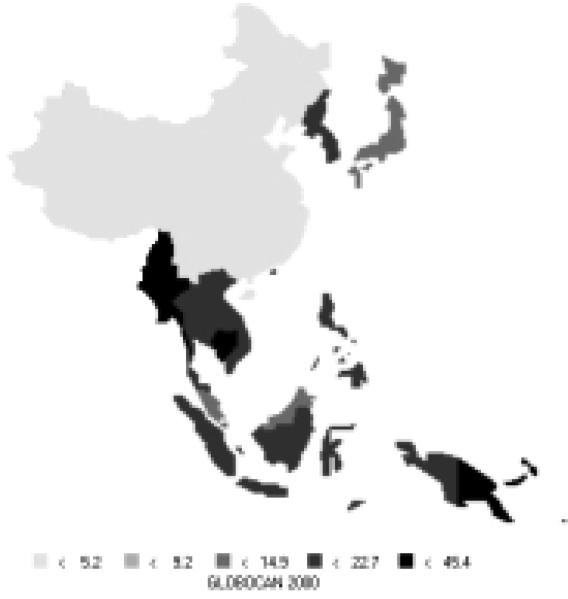
Cervical cancer is currently the seventh most common new cancer in the Asian Pacific Rim and sixth most common cancer among females in the area, with 92,000 new cases in 2000. It is responsible for 47,000 deaths annually, making it the seventh most common cause of death from cancer among females in the Pacific Rim and the ninth most common among both sexes combined. South-Eastern Asia and the Pacific Islands are moderate to high-risk areas where cervical cancer plays a much larger role relative to other cancers (Figure 23). In South-Eastern Asia, cervical cancer is actually ranked second, behind breast cancer, in number of new cancers and deaths among females. In the Pacific Islands, cervical cancer is the most common cancer in terms of both number of new cancers and number of deaths among women. The incidence and mortality rates in the Pacific Islands far exceed those of any other area in the Pacific Rim (Table 11). Relative to the other parts of the world, the Pacific Rim is generally a low-risk region with pockets of high-risk areas, like the Pacific Islands.
Figure 23.
Incidence of Cervical Cancer: Age-standardized Rates (Pacific Rim, per 100,000) - Females (all ages)
Table 11.
Cervical Cancer Age-standardized Rates (per 100,000), 2000
| Region | Incidence | Mortality |
|---|---|---|
| Females | Females | |
| Eastern Asia (Japan) | 11.1 | 3.0 |
| Eastern Asia (Korea) | 15.3 | 5.6 |
| Eastern Asia (China) | 5.3 | 3.1 |
| South-Eastern Asia | 18.3 | 9.6 |
| Pacific Islands | 40.3 | 21.8 |
| Pacific Rim | 9.1 | 4.6 |
Risk Factors
The major risk factor for cervical cancer is infection with human papillomavirus (HPV), specifically, the high-risk types (especially 16 and 18). It is transmitted sexually and has been associated with increases in risk of over 100-fold. For the most part, it is considered to be a necessary but not sufficient cause of cervical cancer. The co-factors that work with HPV infection to produce cervical cancer include high parity and tobacco smoking (Castellsague et al., 2003).
Time Trends
Incidence rates for cervical cancer are generally decreasing in the Asian Pacific Rim region (Figure 24). There have been dramatic declines in China in particular, especially in urban populations, although the trend has reversed recently in younger women (Yang et al., 2003). The declines have been attributed to Pap smear screening, treatment programs and improved genital hygiene, while the increased rates among younger women may reflect changing economic circumstances and sexual mores, with a greater prevalence of infection with HPV and other agents (Li et al., 2000).
Figure 24.
Cervical Cancer Incidence Trends
Prospects for Prevention
There are two major possible approaches to prevention. The first, screening, is already commonly practiced and is oriented toward detection of pre-invasive disease or precursor lesions. Screening methods include cytological tests (the Pap Smear) and ‘aided visual inspections’ with dilute acetic acid (VIA) or Lugol's iodine (VILI). Both have equivalently high sensitivities, but the specificity of the cytological test is slightly higher than the latter (Cullins et al., 1999; Sankaranarayanan et al., 2003). There are several advantages to VIA and VILI as screening tests in low-resource settings, as in some Pacific Rim countries. They are simple and inexpensive tests and do not require a sophisticated laboratory infrastructure. The immediate availability of the test result permits diagnostic procedures (colposcopy with or without biopsy) and treatment (either cryotherapy or electrosurgical excision (LEEP)) to be performed at the time of the screening visit. This avoids the inevitable loss to follow-up that occurs when women must be recalled following positive cytology (Herdman et al., 2000). It has been estimated that cervical cancer mortality in Asia and the Pacific Islands could be reduced by at least 30% with early detection and appropriate treatment (Pisani et al., 1999). Tests that detect HPV DNA may be used to detect high-risk groups on which screening programs could be focused. The second route of prevention, although still in its very early stages, involves vaccination against HPV.
Leukemia
Burden 2000
Leukemia is currently the eighth most common new cancer in the Asian Pacific Rim, with almost 76,000 new cases, making up about 2.5% of all new cases in the area. Leukemia is also the eighth most common cause of death from cancer, with over 54,000 deaths annually. Rates among men are slightly more elevated than among women (Table 12). Hong Kong, Singapore, and parts of the Pacific Islands, including Samoa, are relatively high-risk areas while Korea, Japan, and other parts of South-Eastern Asia are moderate-risk areas for leukemia among males (Figure 25). Among females, Hong Kong and parts of South-Eastern Asia (Philippines) and the Pacific Islands (Samoa) are relatively high-risk areas (Figure 26). Chinese women also have relatively moderate-level risk for leukemia, but the risk among Chinese men is relatively low compared to other areas in the Asian Pacific Rim. Relative to the rest of the world, males of the Pacific Rim have moderate to low risk. Females from Hong Kong and the Philippines, on the other hand, have fairly high risk, while females from other areas of the Asian Pacific Rim have moderate to low risk, relative to the rest of the world.
Table 12.
Leukemia Age-standardized Rates (per 100,000), 2000
| Region | Incidence | Mortality | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Eastern Asia (Japan) | 5.8 | 3.8 | 4.1 | 2.6 |
| Eastern Asia (Korea) | 5.2 | 3.5 | 3.5 | 2.6 |
| Eastern Asia (China) | 4.3 | 3.3 | 2.8 | 2.0 |
| South-Eastern Asia | 4.3 | 3.4 | 3.6 | 2.8 |
| Pacific Islands | 4.3 | 3.0 | 3.2 | 1.9 |
| Pacific Rim | 4.5 | 3.4 | 3.2 | 2.3 |
Figure 25.
Incidence of Leukemia: Age-standardized Rates (Pacific Rim, per 100,000) - Males (all ages)
Figure 26.
Incidence of Leukemia: Age-standardized Rates (Pacific Rim, per 100,000) - Females (all ages)
Risk Factors
Environmental risk factors for leukemia include benzene, tobacco smoking, radiation, radiotherapy, chemotherapy, and human T-cell lymphotropic virus type I (HTLV-I). Benzene exposure has been associated with a significant 2.6 fold increase in risk for leukemia among a cohort of Chinese workers. Occupations with benzene exposure include painting, printing, and the manufacture of footwear, paint, and other chemicals (Yin et al., 1996). Tobacco smoking has been associated with slight increases in leukemia, especially myeloid leukemia (Siegel, 1993; Friedman, 1993; Doll, 1996). High-dose radiation exposure, especially from the atomic bomb in Hiroshima and Nagasaki, also increases risk for leukemia (Preston et al., 1994). Radiotherapy and chemotherapy, especially involving alkylating agents (Tucker et al., 1987), for treating other cancers, including cervical and uterine cancer (Boice, Jr. et al., 1987), breast cancer (Curtis et al., 1992), and Hodgkin's disease (Tucker et al., 1988; Kaldor et al., 1990) are associated with an increase in risk for leukemia as a second cancer.
Adult T-cell leukemia is etiologically associated to HTLV-I, which is endemic in certain areas of the Asian Pacific Rim, including Southwestern and Northern Japan. The lifetime risk for ATL is about 5% for HTLV-I carriers, with a latency period of 30 years or more (Kondo et al., 1989). A dominant transmission route for HTLV-I is vertical transmission, believed to be primarily due to breast-feeding (Sugiyama et al., 1986; Kusuhara et al., 1987). Additionally, vertical transmission is significantly higher among children breast-fed for over three months than among children breast-fed under three months (Hirata et al., 1992; Oki et al., 1992).
In addition to environmental risk factors, there are also familial and genetic factors that increase risk of leukemia. Family studies have shown an increase in risk of leukemia among close relatives of a leukemia patient (Linet, 1985; Pottern et al., 1991). Chromosomal abnormalities, including Down's syndrome (trisomy of chromosome 21) (Robison, 1992), ataxia telangiectasia (chromosomal breakage syndrome) (Linet et al., 1996), and translocations, also increase risk for leukemia, especially among children. Some chromosomal abnormalities may result from exposure to environmental risk factors. For example, benzene is believed to cause t(8;21) translocations (Smith, 1996).
Time Trends
Leukemia incidence rates have been increasing in some parts of the Asian Pacific Rim and decreasing in other parts (Figure 27). Among males, the rates have been generally increasing except in Singapore (Indian). Among females, the trend is increasing in Singapore (Malay and Chinese) but decreasing in China and Japan. The trend in Singapore among Indians is very erratic and difficult to generalize.
Figure 27.
Leukemia Incidence Trends by Sex
Prospects for Prevention
Given the environmental risk factors for leukemia, there are strategies that may lead to prevention. Prevention of vertical transmission of human T-cell lymphotropic virus type I is the best strategy for prevention of adult T-cell leukemia. Prevention of vertical transmission of HTLV-I involves screening pregnant women for HTLV-I antibodies in areas where HTLV-I is endemic, including Southwestern and Northern Japan, and advising seropositive women to avoid breast-feeding their newborn (Bittencourt, 1998). This prevention strategy has had great success in preventing vertical transmission in Nagasaki, Japan (Hino et al., 1996; Hino et al., 1997). Additionally, control of other risk factors, including tobacco smoking and occupational exposures associated with the increased risk for leukemia, may also lead to reduced risk for leukemia.
Future Trends
When making projections, changes in the annual number of cases (or deaths) are due to three components: changes in the size of the population at risk, ageing of the population, and changes in the age-specific rates. Trends in the past may not, however, be a good guide to what will happen in the future due to improvements in prevention, early detection, and treatment. Projections may therefore be highly inaccurate, especially over 50 years, if past patterns are incorporated. In addition, there are only limited data on past trends in cancer incidence from the Asian Pacific Rim region. The projections in this section incorporate only the demographic changes resulting in population growth and ageing. Age is a strong determinant of risk for cancer, with risk of epithelial cancers generally increasing approximately as a fifth power of age (Armitage et al., 1954). We will look at projections for all cancers combined and for the eight most common cancers in 2000 discussed in detail in the previous section. We will, however, briefly examine the effects of past trends in projections for all cancer sites combined for the Asian Pacific Rim region. For the major cancers, we will also give a note on how the burden projections might be modified based on past trends.
Population Projections
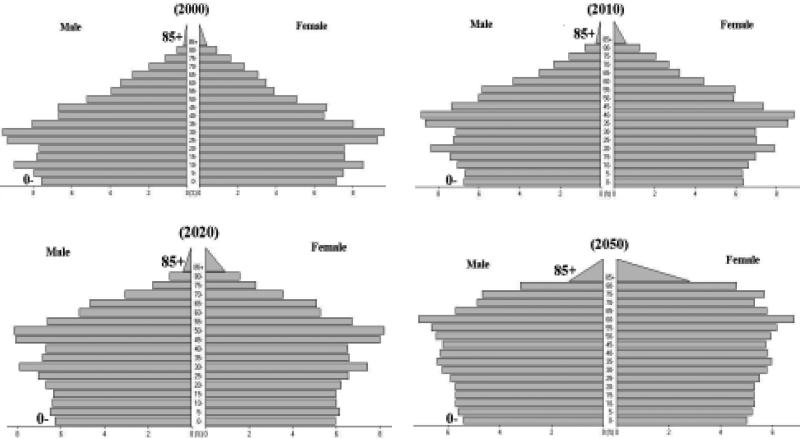
Over the next fifty years, the population is projected to change due to a gradual decline in fertility and extension of life expectancy. According to these projections, the population is expected to become “top-heavy,” with a growing elderly population (people 65 years of age or older) and a relatively shrinking child population, as shown in Figure 28. The population of the Pacific Rim is projected to grow by 23% between 2000 and 2050 (Table 13). In Japan, however, the growth rate will actually be negative over the fifty years. The elderly population (65 and above) of the Pacific Rim is expected to increase from 7% to 21% over this time period. In Japan, where the elderly proportion is already relatively large (17%), nearly one out of three people are expected to be elderly in 2050.
Figure 28.
Projected Population Pyramids of the Pacific Rim in 2000, 2010, 2020 and 2050
Table 13.
Population Growth and Ageing: 2000-2050
| Region | Total number of people (in millions) (% elderly people) | |||
|---|---|---|---|---|
| 2000 | 2010 | 2020 | 2050 | |
| Eastern Asia (Japan) | 126.7 (17.1%) | 127.3 (21.5%) | 123.9 (26.2%) | 104.9 (31.8%) |
| Eastern Asia (Korea) | 70.9 (6.2%) | 76.4 (8.5%) | 80.3 (11.3%) | 82.0 (23.3%) |
| Eastern Asia (China) | 1284.5 (6.9%) | 1380.5 (8.1%) | 1462.2 (11.5%) | 1484.4 (22.6%) |
| South-Eastern Asia | 518.5 (4.7%) | 588.2 (5.6%) | 653.4 (7.1%) | 785.6 (16.7%) |
| Pacific Islands | 7.6 (3.4%) | 9.4 (3.9%) | 11.1 (4.9%) | 15.2 (11.3%) |
| Pacific Rim | 2008.3 (6.9%) | 2181.7 (8.2%) | 2330.8 (11.0%) | 2472.1 (21.1%) |
Cancer Burden Projections
The population growth and ageing will not only impact the dynamics of support and care for the elderly but will also affect the burden of cancer. Age is a very strong predictor of risk for cancer; generally, cancer risk rises with increasing age. Future trends in cancer burden were estimated by applying the currently estimated rates of the year 2000 to the projected populations for 2010, 2020, and 2050, taking into account the age and sex distributions of each year.
The number of new cases (Table 14) and deaths (Table 15) from all cancers is expected to more than double from 2000 to 2050. In 2050, there are expected to be 7.8 million new cancer cases and 5.7 million deaths from cancer. Although Japan is expected to have a high elderly population, we do not see a great increase in the number of new cases and deaths from cancers because of a 30% decrease in the non-elderly population (and number of new cases and deaths) and a 50% increase in the elderly population over the 50 years. Overall, the burden of cancer will greatly increase in the decades to come with the ‘aging of the population’, putting a great deal of pressure on the need for future expansion of resources.
Table 14.
Number of New Cases of All Cancers (in thousands)
| Region | 2000 | 2010 | 2020 | 2050 |
|---|---|---|---|---|
| Eastern Asia (Japan) | 518.7 | 610.4 | 670.8 | 644.0 |
| Eastern Asia (Korea) | 127.7 | 175.6 | 236.9 | 346.4 |
| Eastern Asia (China) | 1914.0 | 2477.7 | 3292.5 | 4721.8 |
| South-Eastern Asia | 495.3 | 656.8 | 875.3 | 1611.5 |
| Pacific Islands | 8.2 | 11.1 | 15.2 | 34.5 |
| Pacific Rim | 3064.0 | 3945.0 | 5169.7 | 7830.2 |
Table 15.
Number of Deaths from All Cancers (in thousands)
| Region | 2000 | 2010 | 2020 | 2050 |
|---|---|---|---|---|
| Eastern Asia (Japan) | 300.4 | 361.8 | 404.4 | 395.8 |
| Eastern Asia (Korea) | 81.3 | 113.6 | 155.4 | 240.2 |
| Eastern Asia (China) | 1369.3 | 1788.1 | 2426.2 | 3683.7 |
| South-Eastern Asia | 337.7 | 449.9 | 606.5 | 1154.4 |
| Pacific Islands | 5.3 | 7.2 | 9.9 | 23.1 |
| Pacific Rim | 2093.9 | 2716.0 | 3613.3 | 5720.9 |
When including the effects of past trends, the projected number of new cases in the Asian Pacific Rim region in the year 2020 for all cancer sites is 4.77 million (a decrease of about 7.7% compared with the projection using constant rates); the number of new cases decreases among men by 204,000 (a total of 2.8 million cases) and decreases among women by 194,000 (a total of 1.9 million cases). The Asian Pacific Rim region is projected to have 3.36 million deaths from all cancers in 2020 when including effects of past trends (a decrease of about 7.1% compared with the projection using constant rates); the number of deaths decreases by 139,000 among males (a total of 2.1 million deaths) and decreases by over 118,000 among females (a total of over 1.2 million deaths). The decrease in new cancer cases and deaths when including past trends is due to an overall decrease in rates from stomach, esophageal, cervical cancer, and leukemia. The cancers that are projected to have an increase because of increasing past trends are lung and breast cancer. For liver cancer, rural populations have increasing past trends, whereas urban populations have experienced a decreasing trend. On the other hand, rural populations have decreasing trends for colorectal cancer, whereas urban populations have increasing trends. The annual percent changes used in the projections were estimated from urban-and rural-specific cancer mortality time trends in China (Yang et al., 2003) and urbanization projections (United Nations Population Division, 2002). Our projections with past trends, therefore, are limited to the appropriateness of using mortality trends, which reflects changes in both incidence and treatment, in the projection of incidence and how representative China is of the whole Asian Pacific Rim region. Future discussion on projections will be based upon constant rates.
Projections based upon constant rates do not, of course, change the relative importance of the different cancer types much. Stomach cancer remains the most common new cancer (Table 16), and lung cancer is the biggest killer from cancer over the fifty years (Table 17). The number of new cases from stomach cancer is expected to increase from 501,000 in 2000 to 878,000 in 2020 and 1.4 million in 2050. The number of deaths is expected to increase almost threefold over the fifty years. These projections for new stomach cancers and deaths, however, may be an overestimation because the introduction of a vaccine for H. pylori is likely to reduce rates.
Table 16.
Projected Demographic Effects on Cancer Burden: Number of New Cases (in thousands)
| 2000 | 2010 | 2020 | 2050 | |
|---|---|---|---|---|
| Stomach cancer | ||||
| Eastern Asia (Japan) | 115.3 | 136.6 | 150.5 | 145.1 |
| Eastern Asia (Korea) | 30.0 | 41.8 | 57.5 | 87.0 |
| Eastern Asia (China) | 330.9 | 435.5 | 590.3 | 885.7 |
| South-Eastern Asia | 25.0 | 33.6 | 46.2 | 95.3 |
| Pacific Islands | 0.2 | 0.3 | 0.5 | 1.2 |
| Pacific Rim | 501.4 | 655.9 | 877.7 | 1398.8 |
| Lung cancer | ||||
| Eastern Asia (Japan) | 65.2 | 79.7 | 90.1 | 89.2 |
| Eastern Asia (Korea) | 17.9 | 25.7 | 36.2 | 58.9 |
| Eastern Asia (China) | 334.7 | 441.4 | 604.7 | 936.1 |
| South-Eastern Asia | 65.8 | 89.3 | 124.5 | 258.5 |
| Pacific Islands | 0.3 | 0.5 | 0.7 | 1.7 |
| Pacific Rim | 484.0 | 634.7 | 855.5 | 1392.7 |
| Liver cancer | ||||
| Eastern Asia (Japan) | 44.5 | 53.2 | 57.5 | 55.3 |
| Eastern Asia (Korea) | 19.1 | 26.9 | 36.5 | 49.4 |
| Eastern Asia (China) | 308.4 | 397.7 | 519.4 | 703.5 |
| South-Eastern Asia | 45.1 | 60.7 | 82.7 | 157.2 |
| Pacific Islands | 0.7 | 0.9 | 1.2 | 2.8 |
| Pacific Rim | 417.9 | 539.2 | 701.1 | 1015.8 |
| Colorectal cancer | ||||
| Eastern Asia (Japan) | 83.2 | 98.4 | 108.0 | 103.7 |
| Eastern Asia (Korea) | 8.1 | 11.3 | 15.5 | 22.8 |
| Eastern Asia (China) | 146.1 | 188.8 | 252.3 | 373.2 |
| South-Eastern Asia | 42.7 | 57.5 | 78.6 | 156.4 |
| Pacific Islands | 0.4 | 0.5 | 0.7 | 1.7 |
| Pacific Rim | 280.4 | 364.0 | 483.5 | 765.1 |
| Esophageal cancer | ||||
| Eastern Asia (Japan) | 13.5 | 16.2 | 17.4 | 16.7 |
| Eastern Asia (Korea) | 3.3 | 4.7 | 6.7 | 10.1 |
| Eastern Asia (China) | 217.3 | 288.0 | 392.9 | 593.7 |
| South-Eastern Asia | 7.7 | 10.4 | 14.8 | 31.6 |
| Pacific Islands | 0.1 | 0.2 | 0.2 | 0.6 |
| Pacific Rim | 241.9 | 318.8 | 427.8 | 674.3 |
| Female breast cancer | ||||
| Eastern Asia (Japan) | 31.1 | 32.1 | 33.4 | 28.5 |
| Eastern Asia (Korea) | 4.7 | 5.8 | 6.8 | 7.0 |
| Eastern Asia (China) | 107.4 | 134.5 | 165.9 | 181.4 |
| South-Eastern Asia | 55.9 | 74.5 | 95.4 | 145.5 |
| Pacific Islands | 0.7 | 0.9 | 1.2 | 2.7 |
| Pacific Rim | 199.8 | 250.0 | 307.8 | 368.2 |
| Cervical cancer | ||||
| Eastern Asia (Japan) | 11.7 | 12.7 | 13.4 | 12.1 |
| Eastern Asia (Korea) | 5.8 | 7.3 | 9.0 | 11.2 |
| Eastern Asia (China) | 34.0 | 44.0 | 57.2 | 71.3 |
| South-Eastern Asia | 39.6 | 52.9 | 68.2 | 104.0 |
| Pacific Islands | 1.1 | 1.4 | 1.9 | 4.0 |
| Pacific Rim | 92.2 | 116.7 | 146.2 | 187.8 |
| Leukemia | ||||
| Eastern Asia (Japan) | 8.2 | 9.1 | 9.6 | 8.9 |
| Eastern Asia (Korea) | 3.0 | 3.4 | 4.0 | 4.8 |
| Eastern Asia (China) | 46.9 | 53.1 | 62.1 | 72.3 |
| South-Eastern Asia | 17.7 | 20.8 | 24.8 | 38.0 |
| Pacific Islands | 0.2 | 0.3 | 0.4 | 0.7 |
| Pacific Rim | 75.9 | 87.0 | 101.9 | 128.5 |
Table 17.
Projected Demographic Effects on Cancer Burden: Number of Deaths (in thousands)
| 2000 | 2010 | 2020 | 2050 | |
|---|---|---|---|---|
| Stomach cancer | ||||
| Eastern Asia (Japan) | 55.8 | 67.3 | 75.5 | 74.1 |
| Eastern Asia (Korea) | 19.0 | 26.7 | 36.8 | 59.1 |
| Eastern Asia (China) | 245.3 | 323.4 | 446.2 | 705.9 |
| South-Eastern Asia | 21.3 | 28.7 | 39.5 | 82.3 |
| Pacific Islands | 0.2 | 0.3 | 0.4 | 1.0 |
| Pacific Rim | 341.6 | 447.5 | 605.5 | 1002.6 |
| Lung cancer | ||||
| Eastern Asia (Japan) | 53.9 | 66.3 | 75.5 | 75.2 |
| Eastern Asia (Korea) | 14.6 | 21.0 | 29.7 | 49.4 |
| Eastern Asia (China) | 286.7 | 378.3 | 523.0 | 831.9 |
| South-Eastern Asia | 60.6 | 82.2 | 114.9 | 238.8 |
| Pacific Islands | 0.3 | 0.4 | 0.6 | 1.5 |
| Pacific Rim | 416.1 | 546.1 | 740.1 | 1228.2 |
| Liver cancer | ||||
| Eastern Asia (Japan) | 35.6 | 42.8 | 46.8 | 45.3 |
| Eastern Asia (Korea) | 15.8 | 22.0 | 29.6 | 40.8 |
| Eastern Asia (China) | 301.5 | 389.0 | 508.1 | 688.3 |
| South-Eastern Asia | 42.5 | 57.2 | 78.2 | 148.9 |
| Pacific Islands | 0.6 | 0.8 | 1.2 | 2.7 |
| Pacific Rim | 396.0 | 510.6 | 663.6 | 960.7 |
| Colorectal cancer | ||||
| Eastern Asia (Japan) | 36.4 | 43.8 | 48.9 | 47.8 |
| Eastern Asia (Korea) | 4.6 | 6.5 | 8.9 | 14.3 |
| Eastern Asia (China) | 78.7 | 102.5 | 141.1 | 225.5 |
| South-Eastern Asia | 27.4 | 36.9 | 50.4 | 101.8 |
| Pacific Islands | 0.2 | 0.3 | 0.5 | 1.1 |
| Pacific Rim | 147.4 | 191.6 | 258.1 | 428.1 |
| Esophageal cancer | ||||
| Eastern Asia (Japan) | 10.1 | 12.2 | 13.4 | 13.0 |
| Eastern Asia (Korea) | 2.4 | 3.4 | 4.9 | 7.8 |
| Eastern Asia (China) | 162.7 | 215.5 | 299.6 | 481.2 |
| South-Eastern Asia | 6.7 | 9.1 | 12.9 | 27.7 |
| Pacific Islands | 0.1 | 0.1 | 0.2 | 0.5 |
| Pacific Rim | 182.0 | 240.0 | 326.5 | 542.8 |
| Female breast cancer | ||||
| Eastern Asia (Japan) | 8.3 | 8.9 | 9.3 | 8.2 |
| Eastern Asia (Korea) | 1.5 | 1.9 | 2.3 | 2.7 |
| Eastern Asia (China) | 29.2 | 37.7 | 49.0 | 63.3 |
| South-Eastern Asia | 25.0 | 33.3 | 43.0 | 65.9 |
| Pacific Islands | 0.3 | 0.4 | 0.5 | 1.2 |
| Pacific Rim | 64.2 | 81.7 | 103.0 | 133.7 |
| Cervical cancer | ||||
| Eastern Asia (Japan) | 3.7 | 4.2 | 4.6 | 4.4 |
| Eastern Asia (Korea) | 2.1 | 2.8 | 3.6 | 5.0 |
| Eastern Asia (China) | 19.8 | 25.9 | 34.6 | 48.0 |
| South-Eastern Asia | 20.5 | 27.6 | 36.1 | 58.1 |
| Pacific Islands | 0.6 | 0.8 | 1.0 | 2.3 |
| Pacific Rim | 46.6 | 59.9 | 76.9 | 105.6 |
| Leukemia | ||||
| Eastern Asia (Japan) | 6.8 | 7.7 | 8.4 | 7.9 |
| Eastern Asia (Korea) | 2.1 | 2.5 | 2.9 | 3.6 |
| Eastern Asia (China) | 30.4 | 36.2 | 43.8 | 54.6 |
| South-Eastern Asia | 14.9 | 17.5 | 21.0 | 33.0 |
| Pacific Islands | 0.2 | 0.2 | 0.3 | 0.4 |
| Pacific Rim | 54.3 | 64.2 | 77.2 | 102.9 |
The number of new cases from lung cancer is expected to increase over 2.5-fold, and the number of deaths is expected to increase by nearly 3 times over the fifty years. In 2050, lung cancer is projected to have almost 1.4 million new cases and be responsible for 1.2 million deaths. Given the high prevalence of smoking, especially in China, however, these projections may be underestimations.
Liver cancer is projected to have 1 million new cases and be the cause of 960,000 deaths in the year 2050. This is over a two-fold increase from the year 2000. Given the availability of a vaccination for HBV, these projections may be an overestimation.
Cancer of the colon/rectum is projected to increase 2.5-fold over the fifty years. The number of new cancers from colorectal cancer is expected to increase from 280,000 in 2000 to 765,000 in 2050. These estimates, however, may be underestimated when taking into account that the rates for colorectal cancer in the area have been increasing.
In 2050, esophageal cancer is projected to have 674,000 new cancers, a 2.5-fold increase from 2000 (242,000 new cases). This projection, however, may be an overestimation since the rates for esophageal cancer in the area have been dropping, with the exception of Japan (based on Miyagi Prefecture).
The number of new cancers from breast cancer is projected to increase from 200,000 in 2000 to 308,000 in 2020 and 368,000 in 2050. The number of deaths attributed to breast cancer will increase 2-fold by 2050, with 134,000 deaths, compared with 2000 (64,000 deaths). Furthermore, this may be underestimated since incidence and mortality rates for breast cancer have been increasing in the Pacific Rim.
The number of new cancers and deaths from cervical cancer is projected to increase 2-fold over the next fifty years. The number of new cancers is expected to increase from 92,000 to 188,000 between 2000 and 2050. These estimates, however, may drop with advances in screening and treatment, especially for low-resource settings, and with a readily available and effective vaccine against HPV.
Leukemia is projected to have almost 129,000 new cases and be the cause of 103,000 deaths in the year 2050. This is over a 1.5-fold increase from the year 2000. Given the increasing trend of leukemia, though, this projection may be an underestimation.
Conclusions
The relative importance of non-communicable diseases, such as cancer, is increasing with the control of infectious diseases. Cancer prevention strategies covered in this paper involve control of exposures to risk factors and/or screening for pre-invasive disease or precursor lesions. A topic that is gaining increasing interest that was not touched upon greatly in this paper is the molecular epidemiology of cancer. Molecular epidemiology of cancer involves finding the genetic components of risk, or susceptibility genes, for cancers. There are numerous genetic markers that are currently under study. We chose, however, to focus on the risk factors that had the most potential to be altered, environmental and lifestyle factors, and would lead to the most promising and feasible preventive interventions for cancers in the Asian Pacific Rim region.
We have summarized the burden and trends of cancer in the Asian Pacific Rim. Additionally, for the eight top cancers, we summarized the major risk factors and presented future burden trends through to the year 2050 based on population dynamics. The facts and data contained within this paper will hopefully assist in the prioritization of future cancer prevention and control strategies, as well as cancer research, and in the application of current knowledge in order to prevent and treat cancer.
Acknowledgements
This project is supported by Grant CA09412 and CA 90388 from the National Cancer Institute, ES11667 from National Institute of Environment Health Sciences, NIH, Department of Health and Human Services, the Helene G. Brown Award and the Alper Program of UCLA Comprehensive Cancer Center.
References
- Aguilar F, Hussain SP, Cerutti P. Aflatoxin B1 induces the transversion of G-->T in codon 249 of the p53 tumor suppressor gene in human hepatocytes. Proc Natl Acad Sci U S A. 1993;90:8586–90. doi: 10.1073/pnas.90.18.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts DS, Martinez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med. 2000;342:1156–62. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Cancer Facts & Figures 2002. American Cancer Society, Inc.; Atlanta, GA.: 2002. [Google Scholar]
- Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 1954;8:1–12. doi: 10.1038/bjc.1954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099–102. doi: 10.1016/s0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- Bittencourt AL. Vertical transmission of HTLV-I/II: a review. Rev Inst Med Trop Sao Paulo. 1998;40:245–51. doi: 10.1590/s0036-46651998000400008. [DOI] [PubMed] [Google Scholar]
- Blot WJ, Devesa SS, Fraumeni JF., Jr Continuing climb in rates of esophageal adenocarcinoma: an update. JAMA. 1993a;270:1320. [PubMed] [Google Scholar]
- Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993b;85:1483–92. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Blettner M, Kleinerman RA, et al. Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst. 1987;79:1295–311. [PubMed] [Google Scholar]
- Brechot C. Molecular mechanisms of hepatitis B and C viruses related to liver carcinogenesis. Hepatogastroenterology. 1998;45(Suppl 3):1189–96. [PubMed] [Google Scholar]
- Butel JS, Lee TH, Slagle BL. Viral co-factors in liver cancer: lessons from hepatitis B virus. Princess Takamatsu Symp. 1995;25:185–98. [PubMed] [Google Scholar]
- Cai L, Yu SZ, Zhan ZF. Cytochrome P450 2E1 genetic polymorphism and gastric cancer in Changle. Fujian Province. World J Gastroenterol. 2001;7:792–5. doi: 10.3748/wjg.v7.i6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis--role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31:20–8. [PubMed] [Google Scholar]
- Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- Chang SS, Lu CL, Chao JY, et al. Unchanging trend of adenocarcinoma of the esophagus and gastric cardia in Taiwan: a 15-year experience in a single center. Dig Dis Sci. 2002;47:735–40. doi: 10.1023/a:1014771429546. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Liang KY, Chang AS, et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13:398–406. [PubMed] [Google Scholar]
- Chen TT, Winder AE. The opium wars revisited as US forces tobacco exports in Asia. Am J Public Health. 1990;80:659–62. doi: 10.2105/ajph.80.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly GN. Worldwide expansion of transnational tobacco industry. J Natl Cancer Inst Monogr. 1992;12:29–35. [PubMed] [Google Scholar]
- Cullins VE, Wright TC, Beattie DJ, Pollack AE. Cervical cancer prevention using visual screening methods. Reproductive Health Matters. 1999;7:134–43. [Google Scholar]
- Curtis RE, Boice JD, Jr, Stovall M, et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745–51. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- de Koning HJ. Mammographic screening: evidence from randomised controlled trials. Ann Oncol. 2003;14:1185–9. doi: 10.1093/annonc/mdg319. [DOI] [PubMed] [Google Scholar]
- Department of Cancer Control and Statistics OMCfCaCD Osaka Cancer Registry. 2003 http://www_mc_pref_osaka_jp/ocr_e/ocr/#survival.
- Doll R. Cancers weakly related to smoking. Br Med Bull. 1996;52:35–49. doi: 10.1093/oxfordjournals.bmb.a011531. [DOI] [PubMed] [Google Scholar]
- Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer. 1967;2:269–79. doi: 10.1002/ijc.2910020310. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R. Mortality in relation to smoking: 20 years' observations on male British doctors. Br Med J. 1976;2:1525–36. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. IARC Press; Lyon, France: 2001. IARC CancerBase No. 5. [Google Scholar]
- Fishman JA, Allison H, Knowles SB, et al. State laws on tobacco control--United States, 1998. MMWR CDC Surveill Summ. 1999;48:21–40. [PubMed] [Google Scholar]
- Friedman GD. Cigarette smoking, leukemia, and multiple myeloma. Ann Epidemiol. 1993;3:425–8. doi: 10.1016/1047-2797(93)90071-b. [DOI] [PubMed] [Google Scholar]
- Gail MH, You WC, Chang YS, et al. Factorial trial of three interventions to reduce the progression of precancerous gastric lesions in Shandong, China: design issues and initial data. Control Clin Trials. 1998;19:352–69. doi: 10.1016/s0197-2456(98)00016-6. [DOI] [PubMed] [Google Scholar]
- Gao YT, McLaughlin JK, Blot WJ, et al. Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–8. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Sell S. Hepatitis B injury, male gender, aflatoxin, and p53 expression each contribute to hepatocarcinogenesis in transgenic mice. Hepatology. 1998;27:383–91. doi: 10.1002/hep.510270211. [DOI] [PubMed] [Google Scholar]
- Giacosa A, Franceschi S, La Vecchia C, et al. Energy intake, overweight, physical exercise and colorectal cancer risk. Eur J Cancer Prev. 1999;8(Suppl 1):S53–S60. [PubMed] [Google Scholar]
- Goodman MT, Moriwaki H, Vaeth M, et al. Prospective cohort study of risk factors for primary liver cancer in Hiroshima and Nagasaki, Japan. Epidemiology. 1995;6:36–41. doi: 10.1097/00001648-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Heck KE, Pamuk ER. Explaining the relation between education and postmenopausal breast cancer. Am J Epidemiol. 1997;145:366–72. doi: 10.1093/oxfordjournals.aje.a009114. [DOI] [PubMed] [Google Scholar]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- Herdman C, Sherris J. Program for Appropriate Technology in Health. Seattle, WA: 2000. Planning appropriate cervical prevention programs. [Google Scholar]
- Hino S, Katamine S, Miyata H, et al. Primary prevention of HTLV-I in Japan. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl 1):S199–S203. doi: 10.1097/00042560-199600001-00030. [DOI] [PubMed] [Google Scholar]
- Hino S, Katamine S, Miyata H, et al. Primary prevention of HTLV-1 in Japan. Leukemia. 1997;11(Suppl 3):57–9. [PubMed] [Google Scholar]
- Hirata M, Hayashi J, Noguchi A, et al. The effects of breastfeeding and presence of antibody to p40tax protein of human T cell lymphotropic virus type-I on mother to child transmission. Int J Epidemiol. 1992;21:989–94. doi: 10.1093/ije/21.5.989. [DOI] [PubMed] [Google Scholar]
- Ho GH, Phang BH, Ng IS, et al. Novel germline BRCA1 mutations detected in women in singapore who developed breast carcinoma before the age of 36 years. Cancer. 2000;89:811–6. doi: 10.1002/1097-0142(20000815)89:4<811::aid-cncr13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Honjo K, Kawachi I. Effects of market liberalisation on smoking in Japan. Tob Control. 2000;9:193–200. doi: 10.1136/tc.9.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Nyren O, Wolk A, et al. Risk factors for oesophageal cancer in northeast China. Int J Cancer. 1994;57:38–46. doi: 10.1002/ijc.2910570108. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer . Evaluation of Carcinogenic Risks to Humans. Vol. 59. IARC; Lyon, France: 1994a. Hepatitis Viruses. [Google Scholar]
- International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 61. IARC; Lyon, France: 1994b. Schistosomes, liver flukes and helicobacter pylori. [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer . IARC Handbooks of Cancer Prevention. Vol. 2. IARC; Lyon, France: 1998. Carotenoids. [Google Scholar]
- International Agency for Research on Cancer . IARC Handbooks of Cancer Prevention. Vol. 8. IARC; Lyon, France: 2003. Fruit and Vegetables. [Google Scholar]
- Kaldor JM, Day NE, Clarke EA, et al. Leukemia following Hodgkin's disease. N Engl J Med. 1990;322:7–13. doi: 10.1056/NEJM199001043220102. [DOI] [PubMed] [Google Scholar]
- Kanemura S, Tsuji I, Ohuchi N, et al. A case control study on the effectiveness of breast cancer screening by clinical breast examination in Japan. Jpn J Cancer Res. 1999;90:607–13. doi: 10.1111/j.1349-7006.1999.tb00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YC, Cheng LS, Lee CH, et al. Chinese food cooking and lung cancer in women nonsmokers. Am J Epidemiol. 2000;151:140–7. doi: 10.1093/oxfordjournals.aje.a010181. [DOI] [PubMed] [Google Scholar]
- Kolonel LN, Nomura AM, Hirohata T, et al. Association of diet and place of birth with stomach cancer incidence in Hawaii Japanese and Caucasians. Am J Clin Nutr. 1981;34:2478–85. doi: 10.1093/ajcn/34.11.2478. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kono H, Miyamoto N, et al. Age- and sex-specific cumulative rate and risk of ATLL for HTLV-I carriers. Int J Cancer. 1989;43:1061–4. doi: 10.1002/ijc.2910430618. [DOI] [PubMed] [Google Scholar]
- Kuroishi T, Hirose K, Suzuki T, Tominaga S. Effectiveness of mass screening for breast cancer in Japan. Breast Cancer. 2000;7:1–8. doi: 10.1007/BF02967181. [DOI] [PubMed] [Google Scholar]
- Kusuhara K, Sonoda S, Takahashi K, et al. Mother-to-child transmission of human T-cell leukemia virus type I (HTLV-I): a fifteen-year follow-up study in Okinawa, Japan. Int J Cancer. 1987;40:755–7. doi: 10.1002/ijc.2910400607. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Wilkens LR, Kolonel LN, et al. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57:4787–94. [PubMed] [Google Scholar]
- Lee HP, Chia KS, Shanmugaratnam K. Cancer Incidence in Singapore 1983-87. Singapore Cancer Registry; Singapore: 1992. [Google Scholar]
- Li B, Taylor PR, Li JY, et al. Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann Epidemiol. 1993;3:577–85. doi: 10.1016/1047-2797(93)90078-i. [DOI] [PubMed] [Google Scholar]
- Li H, Jin S, Xu H, Thomas DB. The decline in the mortality rates of cervical cancer and a plausible explanation in Shandong, China. Int J Epidemiol. 2000;29:398–404. [PubMed] [Google Scholar]
- Linet MS. The Leukemias: Epidemiologic Aspects. Oxford University Press; New York: 1985. [Google Scholar]
- Linet MS, Cartwright RA. The Leukemias. In: Schottenfeld D, Fraumeni JF Jr., editors. Cancer Epidemiology and Prevention. Oxford University Press; New York: 1996. pp. 841–92. [Google Scholar]
- Makimoto K, Higuchi S. Alcohol consumption as a major risk factor for the rise in liver cancer mortality rates in Japanese men. Int J Epidemiol. 1999;28:30–4. doi: 10.1093/ije/28.1.30. [DOI] [PubMed] [Google Scholar]
- Morinaga K, Kishimoto T, Sakatani M, et al. Asbestos-related lung cancer and mesothelioma in Japan. Ind Health. 2001;39:65–74. doi: 10.2486/indhealth.39.65. [DOI] [PubMed] [Google Scholar]
- Muir C, Waterhouse J, Mack T, et al. Cancer Incidence in Five Continents. V. IARC; Lyon, France: 1987. IARC Scientific Publications No. 88. [Google Scholar]
- Munoz N, Wahrendorf J, Bang LJ, et al. No effect of riboflavine, retinol, and zinc on prevalence of precancerous lesions of oesophagus. Randomised double-blind intervention study in high-risk population of China. Lancet. 1985;2:111–4. doi: 10.1016/s0140-6736(85)90223-5. [DOI] [PubMed] [Google Scholar]
- Oki T, Yoshinaga M, Otsuka H, et al. A sero-epidemiological study on mother-to-child transmission of HTLV-I in southern Kyushu, Japan. Asia Oceania J Obstet Gynaecol. 1992;18:371–7. doi: 10.1111/j.1447-0756.1992.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Muir CS, Whelan SL, et al. Cancer Incidence in Five Continents. VI. IARC; Lyon, France: 1992. IARC Scientific Publications No. 120. [Google Scholar]
- Parkin DM, Pisani P. Gastric cancer. In: Kramer BR, Gohagan JK, Prorok P, editors. Cancer Screening. Theory and Practice. Dekker; New York: 1999a. pp. 515–29. [Google Scholar]
- Parkin DM, Pisani P, Lopez AD, Masuyer E. At least one in seven cases of cancer is caused by smoking. Global estimates for 1985. Int J Cancer. 1994;59:494–504. doi: 10.1002/ijc.2910590411. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Pisani P, Munoz N, Ferlay J. The global health burden of infection associated cancers. Cancer Surv. 1999b;33:5–33. [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, et al. Cancer Incidence in Five Continents. VII. IARC; Lyon, France: 1997. IARC Scientific Publications No. 143. [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, et al. Cancer Incidence in Five Continents. VIII. IARC; Lyon, France: 2002. IARC Scientific Publications No. 155. [Google Scholar]
- Patmasiriwat P, Bhothisuwan K, Sinilnikova OM, et al. Analysis of breast cancer susceptibility genes BRCA1 and BRCA2 in Thai familial and isolated early-onset breast and ovarian cancer. Hum Mutat. 2002;20:230. doi: 10.1002/humu.9049. [DOI] [PubMed] [Google Scholar]
- Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Pottern LM, Linet M, Blair A, et al. Familial cancers associated with subtypes of leukemia and non-Hodgkin's lymphoma. Leuk Res. 1991;15:305–14. doi: 10.1016/0145-2126(91)90005-e. [DOI] [PubMed] [Google Scholar]
- Powell J, McConkey CC, Gillison EW, Spychal RT. Continuing rising trend in oesophageal adenocarcinoma. Int J Cancer. 2002;102:422–7. doi: 10.1002/ijc.10721. [DOI] [PubMed] [Google Scholar]
- Preston DL, Kusumi S, Tomonaga M, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950-1987. Radiat Res. 1994;137:S68–S97. [PubMed] [Google Scholar]
- Qian GS, Ross RK, Yu MC, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3:3–10. [PubMed] [Google Scholar]
- Robison LL. Down syndrome and leukemia. Leukemia. 1992;6(Suppl 1):5–7. [PubMed] [Google Scholar]
- Rocken C, Carl-McGrath S. Pathology and pathogenesis of hepatocellular carcinoma. Dig Dis. 2001;19:269–78. doi: 10.1159/000050693. [DOI] [PubMed] [Google Scholar]
- Samet JM. Lung Cancer. In: Greenwald P, Kramer BS, Weed DL, editors. Cancer Prevention and Control. Marcel Dekker Inc.; New York: 1995. pp. 561–84. [Google Scholar]
- Sankaranarayanan R, Basu P, Wesley RS, et al. Visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. IARC; Lyon, France: 2003. (in press) [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Black RJ, Parkin DM, editors. Cancer Survival in Developing Countries. IARC; Lyon, France: 1998. IARC Scientific Publications No. 145. [PubMed] [Google Scholar]
- Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342:1149–55. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- Setiawan VW, Zhang ZF, Yu GP, et al. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. Int J Cancer. 2001;92:600–4. doi: 10.1002/ijc.1231. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Mack TM, Ross RK, Henderson BE. Cancer of the gastrointestinal tract among Japanese and white immigrants in Los Angeles County. J Natl Cancer Inst. 1987;78:223–8. [PubMed] [Google Scholar]
- Siegel M. Smoking and leukemia: evaluation of a causal hypothesis. Am J Epidemiol. 1993;138:1–9. doi: 10.1093/oxfordjournals.aje.a116772. [DOI] [PubMed] [Google Scholar]
- Smith MT. The mechanism of benzene-induced leukemia: a hypothesis and speculations on the causes of leukemia. Environ Health Perspect. 1996;104(Suppl 6):1219–25. doi: 10.1289/ehp.961041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H, Doi H, Yamaguchi K, et al. Significance of postnatal mother-to-child transmission of human T-lymphotropic virus type-I on the development of adult T-cell leukemia/lymphoma. J Med Virol. 1986;20:253–60. doi: 10.1002/jmv.1890200307. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhu Y, Stjernsward J, et al. Design and compliance of HBV vaccination trial on newborns to prevent hepatocellular carcinoma and 5-year results of its pilot study. Cancer Detect Prev. 1991;15:313–8. [PubMed] [Google Scholar]
- Tanaka K, Hirohata T, Koga S, et al. Hepatitis C and hepatitis B in the etiology of hepatocellular carcinoma in the Japanese population. Cancer Res. 1991;51:2842–7. [PubMed] [Google Scholar]
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst. 2002;94:1445–57. doi: 10.1093/jnci/94.19.1445. [DOI] [PubMed] [Google Scholar]
- Tsukuma H, Ajiki W, Oshima A. Cancer epidemiology and control in the Asia-Pacific region International Center for Medical Research. Kobe University School of Medicine; Japan: 1999. Cancer incidence and survival in Osaka. [Google Scholar]
- Tucker MA, Coleman CN, Cox RS, et al. Risk of second cancers after treatment for Hodgkin's disease. N Engl J Med. 1988;318:76–81. doi: 10.1056/NEJM198801143180203. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Meadows AT, Boice JD, Jr., et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78:459–64. [PubMed] [Google Scholar]
- Ueno Y, Nagata S, Tsutsumi T, et al. Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis. 1996;17:1317–21. doi: 10.1093/carcin/17.6.1317. [DOI] [PubMed] [Google Scholar]
- United Nations . The 2000 Revision. United Nations; New York: 2001. World Population Prospects. Ref Type: Data File. [Google Scholar]
- United Nations Population Division . World Urbanization Prospects: The 2001 Revision. United Nations; New York: 2002. [Google Scholar]
- Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer. 2002;99:860–8. doi: 10.1002/ijc.10427. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Muir C, Shanmugaratnam K, Powell J. Cancer Incidence in Five Continents. IV. IARC; Lyon, France: 1982. IARC Scientific Publications No. 42. [Google Scholar]
- Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–99. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet. 1984;1:921–6. doi: 10.1016/s0140-6736(84)92388-2. [DOI] [PubMed] [Google Scholar]
- World Bank . World development report 1993. Investing in Health. Oxford University Press; New York: 1993. [Google Scholar]
- World Cancer Research Fund (WCRF) Panel . Diet, Nutrition, and the Prevention of Cancer: A Global Perspective. World Cancer Research Fund; Washington, USA: 1997. [Google Scholar]
- World Health Organization . International Classification on Diseases and Related Health Problems, 1989 Revision. WHO; Geneva: 1992. [Google Scholar]
- Xu ZY, Blot WJ, Xiao HP, et al. Smoking, air pollution, and the high rates of lung cancer in Shenyang, China. J Natl Cancer Inst. 1989;81:1800–6. doi: 10.1093/jnci/81.23.1800. [DOI] [PubMed] [Google Scholar]
- Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–49. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- Yang L, Parkin DM, Li L, Chen Y. Time trends in cancer mortality in China: 1987-1999. Int J Cancer. 2003;106:771–83. doi: 10.1002/ijc.11300. [DOI] [PubMed] [Google Scholar]
- Yeh FS, Yu MC, Mo CC, et al. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989;49:2506–9. [PubMed] [Google Scholar]
- Yin SN, Hayes RB, Linet MS, et al. An expanded cohort study of cancer among benzene-exposed workers in China. Benzene Study Group. Environ Health Perspect. 1996;104(Suppl 6):1339–41. doi: 10.1289/ehp.961041339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo KY, Kang D, Park SK, et al. Epidemiology of breast cancer in Korea: occurrence, high-risk groups, and prevention. J Korean Med Sci. 2002;17:1–6. doi: 10.3346/jkms.2002.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cai B. The impact of tobacco on lung health in China. Respirology. 2003;8:17–21. doi: 10.1046/j.1440-1843.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- Zhang ZF. Lung Cancer. In: Breslow L, editor. Encyclopedia of Public Health. Macmillan Reference USA; New York: 2002. pp. 702–4. [Google Scholar]
- Zhang ZF, Yu SZ, Li WX, Choi BC. Smoking, occupational exposure to rubber, and lung cancer. Br J Ind Med. 1989;46:12–5. doi: 10.1136/oem.46.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Goldberg MS, Gao YT, Jin F. A case-control study of lung cancer and environmental tobacco smoke among nonsmoking women living in Shanghai, China. Cancer Causes Control. 1999a;10:607–16. doi: 10.1023/a:1008962025001. [DOI] [PubMed] [Google Scholar]
- Zhong L, Goldberg MS, Gao YT, Jin F. Lung cancer and indoor air pollution arising from Chinese-style cooking among nonsmoking women living in Shanghai, China. Epidemiology. 1999b;10:488–94. [PubMed] [Google Scholar]
- Zhong L, Goldberg MS, Parent ME, Hanley JA. Exposure to environmental tobacco smoke and the risk of lung cancer: a meta-analysis. Lung Cancer. 2000;27:3–18. doi: 10.1016/s0169-5002(99)00093-8. [DOI] [PubMed] [Google Scholar]
- Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–27. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]