Abstract
Patients with pancreatic adenocarcinoma have the lowest 5 year survival rate and yearly rates of incidence are nearly equal to the mortality rates. Long term cure rates by standard therapies are disappointing owing to disseminated disease at diagnosis and chemotherapeutic resistance. New therapeutic targets are necessary to decrease the progression of pancreatic cancer and the ability to identify targets specific to metastasis would improve patient care. We evaluated the levels of micro-RNA of metastatic and non-metastatic cell lines. The expression levels of microRNAs and mRNAs were determined using microarray analysis to examine and compare five pancreatic cancer cell lines, two that can metastasize in vivo (S2VP10 and S2CP9) and three that do not metastasize (MiaPaCa2, Panc-1 and ASPC-1). MicroRNA analysis indicated an increase in miR-100 and a decrease in miR-138 expression in metastatic cancer cells. Microarray analysis of different expressions of mRNAs in metastatic and non-metastatic pancreatic cell lines also indicated significantly increased insulin growth factor-1 receptor (IGF1-R) expression in metastatic pancreatic cancer cell lines compared to non-metastatic pancreatic cancer cell lines. To confirm microarray analysis results, western blot and immunocytochemistry were performed. Western blot revealed that IGF1-R expression exhibited in metastatic cancer cell lines a seven-fold increase compared to non-metastatic cell lines. In addition, downstream expressions of the proteins, GRB2 and phosphorylated PI3K, also were increased in aggressive cancer cell lines. Immunocytochemistry confirmed the linkage of IGF1-R to miR-100, because cells transfected with miR-100 inhibitor showed a decrease in IGF1-R. Cells transfected with a miR-138 mimic, however, did not affect IGF1-R expression.
Keywords: IGF1-receptor, metastasis, microRNA-100, microRNA-138, pancreatic cancer
Pancreatic ductal adenocarcinoma, also known as pancreatic cancer, is one of the most devastating diseases in the world. In 2008, 99.1% of all patients diagnosed with pancreatic cancer in the United States died from the disease (Jemal et al. 2008). The five year survival rate of patients diagnosed with pancreatic cancer is 5% and median survival is approximately 6 months (Couch et al. 2007). Current treatment for pancreatic cancer includes chemotherapy, radiation therapy, and surgery; however, all therapies for pancreatic cancer are disappointing. Recently, scientists have focused on a new approach to target specific genes that decrease the progression and metastasis of pancreatic cancer.
MicroRNAs (miRNAs) are short non-coding RNAs that control post-transcriptional gene expression (Bentwich et al. 2005, McNally et al. 2013). MiRNAs are important regulators of cellular processes including proliferation, apoptosis, differentiation, motility and morphogenesis. Several miRNAs are classified as proto-oncogenes or tumor suppressors (Esquela-Kerscher et al. 2006, Calin et al. 2006). Specifically, miRNA-100 has been shown to have an anti-angiogenic function (Grundmann et al. 2011) and to act as a tumor suppressor in human bladder carcinoma (Oliveira et al. 2011). MiRNA-138 has been linked to repair of DNA damage and is involved in several types of cancer (Liu et al. 2011, Landgraf et al. 2007). The therapeutic effects of miRNA-100 and -138 on pancreatic adenocarcinoma remain unclear.
Insulin growth factor-1 receptor (IGF1-R) has been shown to control cell proliferation and down-regulation of IGF1-R can lead to cell death (Baserga et al. 2003a). Earlier studies have shown that high circulating levels of IGF1-R are associated with breast (Hankinson et al. 1998), prostate (Chan et al. 1998), lung (Yu et al. 1999), pancreatic (Li et al. 2003) and colorectal cancer (Ma et al. 1999). When IGF1-R is up-regulated, proliferation of cancer cells is accelerated and this may play a role in cancer cell metastasis (Lopez et al. 2002).
We examined the expression levels of miRNA-100 and miRNA-138 and their involvement with IGF1-R in pancreatic cancer cell lines that can metastasize in vivo. We found that miRNA-100 is over-expressed and miRNA-138 is under-expressed in potentially metastatic pancreatic cancer cell lines. It is interesting that transfection of miRNA-100 inhibitor into S2VP10 pancreatic cancer cells caused decreased IGF1-R expression. Transfection of S2VP10 cells with miRNA-138 mimic, however, did not affect IGF1-R expression. These findings indicate that miRNA-100, and not miRNA-138, is associated with regulating IGF1-R in pancreatic cell lines.
Materials and methods
Cell lines
Potentially metastatic pancreatic adenocarcinoma cell lines, S2VP10 and S2CP9, and non-metastatic pancreatic adenocarcinoma cell lines, Panc-1, ASPC-1, and Miapaca-2, were cultured with RPMI 1640 and DMEM (Invitrogen, Grand Island, NY), respectively, the media were supplemented with 10% FBS (Atlanta Biological, Atlanta GA) and 1% L-glutamine, and the cells were grown at 37° C in a humidified incubator with 5% CO2.
MiRNA and mRNA array
RNA was extracted from S2VP10, S2CP9, Miap-PaCa-2, Panc-1, and Aspc-1 cells using an Ambion RNAqueous isolation kit according to the manufacturer’s instructions (LifeTechnologies, Grand Island, NY). MiRNA expression profiles were evaluated from RNA samples. Affymetrix miRNA array (Flashtag HSR, #HSR10FTA Santa Clara, CA) was used to measure miRNA and mRNA expression was measured using the Affymetrix GeneChip miRNA 2.0 Array for each cell line. Diana micro-T4.0 is an algorithm based on several parameters calculated individually for each miRNA; it combines conserved and non-conserved miRNA recognition elements into a final prediction score of miRNA + target gene interactions. Downstream target proteins of selected miRNAs were identified using Diana micro-T4.0 and were correlated to the mRNA data. Likelihood scores compared metastatic cell lines to non-metastatic cell lines.
Western blot
Cells were lysed in a buffer solution containing 1% NP-40, 1% phosphatase inhibitor and 1% protease inhibitor in nuclease free-water. Lysates were centrifuged at 13.3 × g for 10 min at 4° C. The total protein in the lysates was quantified using the Bradford assay (Amm et al. 2011). Approximately 50 µg of protein from each sample was dissolved in loading buffer. Proteins were separated by NuPage 4–12% Bis-Tris gel, then transferred onto nitrocellulose membranes using iBlot (LifeTechnologies). The membranes were blocked with Li-Cor blocking buffer (Li-Cor, Lincoln, NE). Proteins were incubated with IGF1-R (ABcam, Cambridge, UK), PI3K p110 (Epitomics, Burlingame, CA), and GRB2 (ABcam) at a dilution of 1:1000. The membranes were incubated overnight at 4° C, then washed three times using TBS and incubated with secondary antibodies, anti-mouse or anti-rabbit IgG (Li-Cor) diluted 1:2500 at room temperature. Membranes were scanned and analyzed using Li-Cor Odyssey.
Transfection of miRNA 138 and 100
S2VP10 was transfected with PreMir-138, a miR-138 mimic, and MirZip-100, a miR-100 inhibitor, using Superfect Transfection Reagent (Qiagen, Valencia, CA) according to manufacturer’s instructions. Plasmids of PreMir-138 and MirZip-100 were obtained from SBI System Bioscience (Mountain View, CA). PreMir-138 and Mirzip-100 plasmids were tagged with green fluorescent protein (GFP) to indicate positive transfection of cells.
Immunocytochemistry
S2VP10 transfected with PreMir-138 and MirZip-100 were cultured in multi-well gaskets on microscope slides for 24 h. Cells were fixed with 10% neutral buffered formalin for 12 h, then incubated with 2% Bovine Serum Albumin in Tris Buffered Saline. IGF1-R antibody (ABcam) diluted 1:50 was added and incubated at 4° C overnight. Cells then were washed with TBS and secondary goat anti-rabbit IgG (H + L), DyLight 633 antibodies diluted 1:1000 (Pierce Biotechnology, Rockford, IL) were added and incubated at room temperature for 1 h. Cells then were washed with TBS and hard mounting media with DAPI was added (Vector Laboratories, Burlingame, CA).
Statistical analysis
Data were analyzed using the statistical software package, SAS9.1 (SAS Institute, Cary, NC). Differentially expressed miRNAs identified between the cells that can metastasize in vivo and the cells that do not were analyzed using a Wilcoxon test with correction using Benjamini and Hochberg False Discovery Rate. This was done to correct for the occurrence of false positives, i.e., that are found to be statistically different between conditions secondary to multiple comparisons. A two-tailed value of P < 0.05 was considered statistically significant. MiRNAs were sorted in ascending order by P-value. Pearson’s correlation coefficients were calculated for mRNA expression and miR expression. Nonparametric rank tests to give an estimate for R were performed.
Results
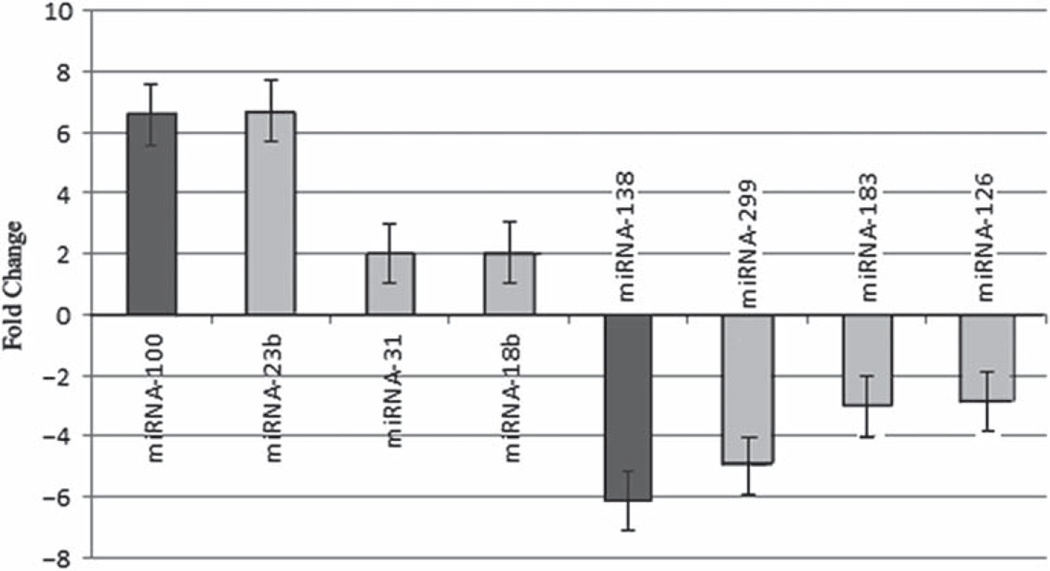
MiRNA and mRNA array analysis indicated differential expression of specific miRNAs between metastatic and non-metastatic pancreatic cancer cell lines as shown in Fig. 1. MiRNA-100, -23b, -31, and -18b showed greater expression in metastatic pancreatic cancer cell lines than in non-metastatic cell lines (p = 0.0001, 0.001, 0.001, 0.003, respectively). MiR-NA-138, -299, -183, and -126 showed less expression in metastatic pancreatic cancer cell lines than in non-metastatic cancer cell lines (p = 0.04, 0.07, 0.002, 0.0007, respectively). Diana micro-T4.0 identified miRNA-100 and miRNA-138 as potential modulators of IGF1-R. MiRNA-100 levels were 6.6 greater in metastatic cell lines (p = 0.0001), while miRNA-138 levels were 6.1 times lower (p = 0.04) in metastatic pancreatic cancer cell lines compared to non-metastatic pancreatic cancer cell lines.
Fig. 1.
MiRNA array analysis of pancreatic cancer cell lines that can metastasize in vivo. Graph shows miRNA differences between potentially metastatic and non-metastatic pancreatic cancer cell lines. MiRNA-100, -23b, -31, and -18b show greater expression in metastatic pancreatic cancer cell lines than in non-metastatic cancer cell lines (p = 0.0001, 0.001, 0.001, 0.003, respectively). MiRNA-138, -299, -183, and -126 have lower expressions in metastatic pancreatic cancer cells compared to non-metastatic cancer cells (p = 0.04, 0.07, 0.002, 0.0007, respectively).
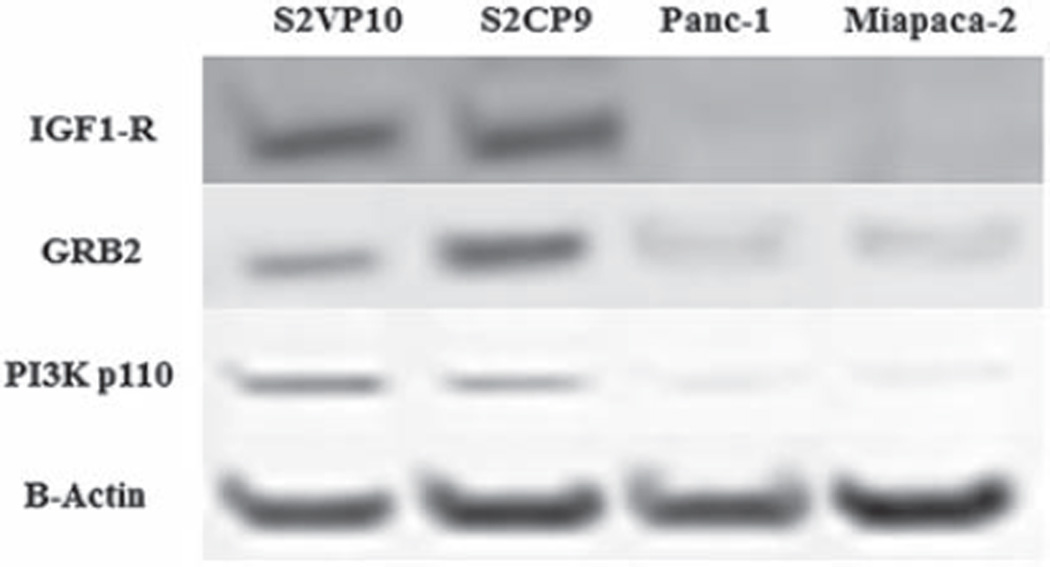
To confirm that IGF1-R was greater in metastatic pancreatic cancer cell lines, western blots were performed on both the metastatic and non-metastatic pancreatic cancer cell lines. In western blots, IGF1-R expression data was increased seven times in metastatic pancreatic cancer cell lines as shown in Fig. 2. The levels of protein expression downstream from IGF1-R, including GRB2 and phosphorylated PI3K, also were increased in metastatic pancreatic cancer cell lines.
Fig. 2.
Western blot analysis of S2VP10, S2CP9, Panc-1, and Miapaca-2. Metastatic pancreatic cancer cell lines (S2VP10, S2CP9) in vitro demonstrated up-regulation of IGF1-R compared to non-metastatic pancreatic cancer cell lines (Panc-1, Miapaca-2). The downstream signals of IGF1-R, including GRB2 and PI3K, showed increased protein expression in metastatic pancreatic cancer cell lines.
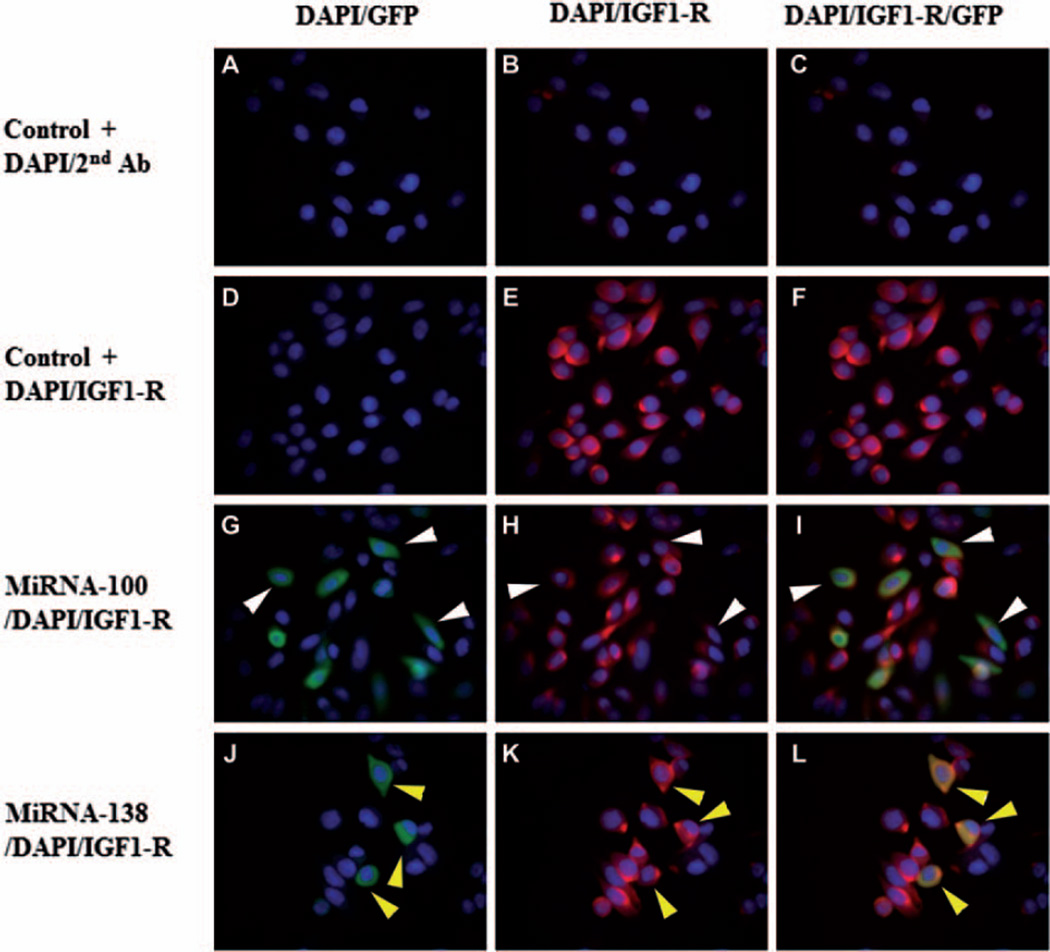
S2VP10 cells were transfected with miRNA-100 inhibitor or miRNA-138 mimic to test whether the expression of IGF1-R was affected. Immunocytochemistry was used to test for IGF1-R expression in transfected S2VP10 cells. As a control, the cells were stained with DAPI and secondary antibody (Fig. 3A–C). Also as a control, S2VP10 cells were stained with IGF1-R and DAPI (Fig. 3D–F). As shown in Fig. 3G–I, cells transfected with miRNA-100 (GFP) had less IGF1-R expression than the controls. As shown in Fig. 3J–L, however, there was no change in IGF1-R expression after transfection of S2VP10 cells with miRNA-138 mimic.
Fig. 3.
Immunocytochemistry of S2VP10 cancer cells. Blue indicates DAPI, red indicates IGF1-R, and green indicates miRNA transfected cells. A–C show controls, i.e., S2VP10 cells stained with DAPI and secondary antibody. D–F show additional controls, i.e., S2VP10 cells stained with IGF1-R. E, F) S2VP10 cells show red fluorescence, which indicates that IGF1-R is present in these cells. G–I) S2VP10 cells transfected with miRNA-100. Transfected cells (GFP positive) show less intense red fluorescence, which indicates that IGF1-R is down-regulated in S2VP10 cells transfected with miR-100. J–L) S2VP10 cells transfected with miR-138. Red fluorescence in transfected S2VP10 cells (GFP positive) is similar to S2VP10 control cells, which indicates that IGF1-R expression was not down-regulated in S2VP10 cells transfected with miR-138.
Discussion
Metastasis is the main cause of death from cancer. For metastatic pancreatic cancer, chemotherapy provides only palliative benefits. Novel therapies for treating metastatic pancreatic cancer must be developed (Chambers et al. 2002, Li et al. 2004). Currently, a variety of strategic targets are under development for clinical use. These targets include PSCA (Wente et al. 2005), MEK (Chung et al. 2009), Src (Rajeshkumar et al. 2009), and the hedgehog pathway (Olive et al. 2009). Identifying new biomarkers and understanding the tumor microenvironment also are crucial for developing new treatments. We have found that IGF1-R is over-expressed in metastatic pancreatic cancer cell lines that metastasize in vivo and one of these cell lines, S2VP10, is regulated by miR-100.
Previous studies have shown that IGF1-R plays an important role in cell longevity, adhesion and proliferation (Mauro et al. 2002, Baserga et al. 2003b). An increased level of IGF1-R expression in cells is associated with increased risk of breast, prostate, lung, pancreatic and colorectal cancers (Taromaru et al. 2012, Ouban et al. 2003, Sekharam et al. 2003). Previous studies have shown that high levels of IGF1-R correspond to the ability of pancreatic cancer cells to metastasize in vivo (Jaquish et al. 2011). The cells of metastatic islet tumors also have been reported to demonstrate greater IGF1-R expression, which suggests that IGF1-R signaling is involved in cancer cell invasion and metastasis (Lopez et al. 2002). In our study, western blot analysis showed a seven-fold increase in IGF1-R expression in metastatic pancreatic cancer cell lines compared to pancreatic cancer cell lines that do not metastasize in vivo. Protein signals downstream of IGF1-R, including GRB2 and phosphorylated PI3K, showed greater expression in the metastatic pancreatic cancer cell lines. These findings suggest that IGF1-R expression may be an important biomarker for the severity of pancreatic cancer.
Functional analyses of miRNAs have demonstrated their important roles in initiation, invasion, and progression of cancer (Esquela-Kerscher et al. 2006). Our results indicated altered expressions of eight miRNAs between metastatic and non-metastatic pancreatic cancer cell lines. Because each miRNA may have hundreds of target mRNAs, we focused on identifying functional linkages between these miRNAs and IGF1-R.
The specific involvement of miR-100 in the development of neoplasia appears to be organ-specific, because high levels of miRNA-100 are linked to the development of prostate cancer (Leite et al. 2011), but low levels are associated with ovarian (Peng et al. 2012) and bladder carcinomas (Oliveira et al. 2011). By contrast, inhibition of miRNA-138 has been reported to cause apoptosis and cell cycle arrest in cell lines of head and neck squamous cell carcinomas (Liu et al. 2009). Other studies have shown that down-regulation of miRNA-138 increases the proliferation of squamous cell carcinoma cells of the tongue (Jiang et al. 2011). Our studies show that miRNA-100 is over-expressed and miRNA-138 is under-expressed in metastatic pancreatic cancer cell lines compared to non-metastatic pancreatic cancer cell lines. To determine the role of miRNA-100 and -138 in regulating IGF1-R, we transfected a miRNA-100 inhibitor or -138 mimic into the S2VP10 cell line. Immunocytochemistry showed that IGF1-R was decreased in S2VP10 cells transfected with miR-100. Down-regulation of IGF1-R was not detected in S2VP10 cells transfected with miR-138.
Our investigation indicates that IGF1-R is increased in metastatic pancreatic cancer cell lines. Inhibition of miRNA-100 S2VP10 cells decreased IGF1-R expression. IGF1-R may be an important molecular feature and a useful biomarker for diagnosis and treatment of metastatic pancreatic cancer.
Acknowledgments
This work was supported by NCI grant CA139050. The authors thank Ying Song and Chuanlin Ding for technical assistance.
Footnotes
Declaration of interest: The authors declare no conflicts of interest.
References
- Amm HM, Zhou T, Steg AD, Kuo H, Li Y, Buchsbaum DJ. Mechanisms of drug sensitization to TRA-8, an agonistic death receptor 5 antibody, involve modulation of the intrinsic apoptotic pathway in human breast cancer cells. Mol. Cancer Res. 2011;9:403–417. doi: 10.1158/1541-7786.MCR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int. J. Cancer. 2003a;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- Baserga R, Prisco M, Yuan T. IGF-1 Receptor Signaling in Health and Disease. Georgetown, TX: Landes Bioscience; 2003b. pp. 120–140. [Google Scholar]
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Metastasis: dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- Chung EJ, Brown AP, Asano H, Mandler M, Burgan WE, Carter D, Camphausen K, Citrin D. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase ½ kinase. Clin. Cancer Res. 2009;15:3050–3057. doi: 10.1158/1078-0432.CCR-08-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch FJ, Johnson MR, Rabe KG, Brune K, Andrade M, Goggins M, Rothenmund H, Gallinger S, Klein A, Petersen GM, Hruban RH. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs–microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Grundmann S, Hans FP, Kinniry S, Heinke J, Helbing T, Bluhm F, Sluijter JP, Hoefer I, Pasterkamp G, Bode C, Moser M. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011;123:999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- Jaquish DV, Yu PT, Shields DJ, French RP, Maruyama KP, Niessen S, Hoover HA, Cheresh D, Cravatt B, Lowy AM. IGF1-R signals through the RON receptor to mediate pancreatic cancer cell migration. Carcinogenesis. 2011;32:1151–1156. doi: 10.1093/carcin/bgr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jiang L, Dai Y, Liu X, Wang C, Wang A, Chen Z, Heidbreder CE, Kolokythas A, Zhou X. Identification and experimental validation of G protein alpha inhibiting activity polypeptide 2 (GNAI2) as a microRNA-138 target in tongue squamous cell carcinoma. Hum. Genet. 2011;129:189–197. doi: 10.1007/s00439-010-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, Vita GD, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Lauro RD, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite KRM, Tomiyama A, Reis ST, Sousa-Canavez JM, Sanudo A, Dall’Oglio MF, Camara-Lopes LH. Micro-RNA-100 expression is independently related to biochemical recurrence of prostate cancer. J. Urol. 2011;183:1118–1122. doi: 10.1016/j.juro.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- Li J, Kleeff J, Guo J, Fischer L, Giese N, Büchler MW, Friess H. Effects of STI571 (gleevec) on pancreatic cancer cell growth. Molec. Cancer. 2003;2:32. doi: 10.1186/1476-4598-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jiang L, Wang A, Yu J, Shi F, Zhou X. Micro-RNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009;286:217–222. doi: 10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang C, Chen Z, Jin Y, Wang Y, Kolokythas A, Dai Y, Zhou X. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem. J. 2011;440:23–31. doi: 10.1042/BJ20111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J. Natl. Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- Mauro L, Salerno ME, Morelli C, Roterberg T, Bracke ME, Surmacz E. Role of the IGF-1 receptor in the regulation of cell-cell adhesion: implications in cancer development and progression. J. Cell. Physiol. 2002;94:108–116. doi: 10.1002/jcp.10207. [DOI] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, Mclntyre DH, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, DeNicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JC, Brassesco MS, Morales AG, Pezuk JA, Fedatto PF, Silva GN, Scrideli CA, Tone LG. MicroRNA-100 acts as a tumor suppressor in human bladder carcinoma 5637 cells. Asian Pacific J. Cancer Prev. 2011;12:3001–3004. [PubMed] [Google Scholar]
- Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum. Pathol. 2003;34:803–808. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- Peng DX, Luo M, Qiu LW, He YL, Wang XF. Prognostic implications of microRNA-100 and its functional roles in human epithelial ovarian cancer. Oncol. Rep. 2012;27:1238–1244. doi: 10.3892/or.2012.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar NV, Tan AC, De Oliveira E, Womack C, Wombwell H, Morgan S, Warren MV, Walker J, Green TP, Jimeno A, Messersmith WA, Hidalgo M. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin. Cancer Res. 2009;15:4138–4146. doi: 10.1158/1078-0432.CCR-08-3021. [DOI] [PubMed] [Google Scholar]
- Sekharam M, Zhao H, Sun M, Fang Q, Zhang Q, Yuan Z, Dan HC, Boulware D, Cheng JQ, Coppola D. Insulin-like growth factor 1 receptor enhances invasion and induces resistance to apoptosis of colon cancer cells through the Akt/Bcl-x(L) pathway. Cancer Res. 2003;63:7708–7716. [PubMed] [Google Scholar]
- Taromaru GC, DE Oliveira VM, Silva MA, Montor WR, Bagnoli F, Rinaldi JF, Aoki T. Interaction between cyclooxygenase-2 and insulin-like growth factor in breast cancer: a new field for prevention and treatment. Oncol. Lett. 2012;3:682–688. doi: 10.3892/ol.2011.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente MN, Jain A, Kono E, Berberat PO, Giese T, Reber HA, Friess H, Buchler MW, Reiter RE, Hines OJ. Prostate stem cell antigen is a putative target for immunotherapy in pancreatic cancer. Pancreas. 2005;31:119–125. doi: 10.1097/01.mpa.0000173459.81193.4d. [DOI] [PubMed] [Google Scholar]
- Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J. Natl. Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]