Abstract
Background
The Food and Drug Administration has approved devices for endovascular management of thoracic endovascular aortic aneurysm repair (TEVAR); however, limited data exist describing the outcomes of TEVAR for aneurysms attributable to chronic type B aortic dissection (cTBAD). This study was undertaken to determine the results of endovascular treatment of cTBAD with aneurysmal degeneration.
Methods
A retrospective analysis of all patients treated for cTBAD with aneurysmal degeneration at the University of Florida from 2004 to 2011 was performed. Computed tomograms with centerline reconstruction were analyzed to determine change in aortic diameter, relative proportions of aortic treatment lengths, and false lumen perfusion status. Reintervention and mortality were estimated using life-tables. Cox regression analysis was completed to predict mortality.
Results
Eighty patients underwent TEVAR for aneurysm due to cTBAD (mean age [± standard deviation], 60 ± 13 years [male, 87.5%; n = 70]; median follow-up, 26 [range, 1–74] months). Median time from diagnosis of TBAD to TEVAR was 16 (range, 1–72) months. Prior aortic root/arch replacement had been performed in 29% (n = 23) at a median interval of 28.5 (range, 0.5–312) months. Mean preoperative aneurysm diameter was 62.0 ± 9.9 mm. In 75% (n = 60) of cases, coverage was proximal to zone 3, and 24% (n = 19) underwent carotid-subclavian bypass or other arch debranching procedure. Spinal drains were used in 78% (pre-op 71%, n = 57; post-op 6%, n = 5). Length of stay was 6.5 ± 4.7 days with a composite morbidity of 26% and in-hospital mortality of 2.5% (n = 2). Overall neurologic event rate was 17% (spinal cord ischemia 10% [n = 8], with a permanent deficit observed in 6.2% [n = 5]; stroke 7.5%). Aneurysm diameter reduced or stabilized in 65%. The false lumen thrombosed completely within the thoracic aorta in 52%, and reintervention within the treated aortic segment was required in 16% (n = 13). One- and 3-year freedom from reintervention (with 95% confidence interval [CI]) was 80% (range, 68%–88%) and 70% (range, 57%–80%), respectively. Survival at 1 and 5 years was 89% (range, 80%–94%) and 70% (range, 55%–81%) and was not significantly different among patients requiring reintervention or experiencing favorable aortic remodeling. Multivariable analysis identified coronary artery disease (hazard ratio [HR], 6.4; 95% CI, 2.3–17.7; P < .005), prior infrarenal aortic surgery (HR, 8.6; 95% CI, 2.3–31.7; P = .001), and congestive heart failure (HR, 11.9; 95% CI, 1.9–73.8; P = .008) as independent risk factors for mortality. Hyperlipidemia was found to be protective (HR, 0.2; 95% CI, 0.05–0.6; P = .004). No significant difference in predictors of mortality were found between patients who underwent reintervention vs those who did not (P = .2).
Conclusions
TEVAR for cTBAD with aneurysmal degeneration can be performed safely but spinal cord ischemia rates may be higher than previously reported. Liberal use of procedural adjuncts to reduce this complication, such as spinal drainage, is recommended. Reintervention is common, but long-term survival does not appear to be impacted by remediation.
Thoracic endovascular aortic aneurysm repair (TEVAR) is increasingly performed to treat a variety of acute and chronic aortic pathologies1,2; however, its role in the management of dissection-related diseases remains controversial. Although there are large clinical trials and registries examining outcomes of patients with acute aortic dissection,3,4 results of chronic dissection treated with TEVAR are primarily retrospective, single center experiences comprised of heterogeneous cohorts of acute, subacute, and chronic presentations with varying operative indications (eg, pain, malperfusion, false lumen [FL] aneurysm). Patients who develop chronic thoracic or thoracoabdominal FL aneurysmal dilatation have traditionally been treated with open surgery, but substantial morbidity and mortality have been described with these repairs.5,6 Given the success of TEVAR in the management of degenerative aneurysms, this therapy offers an attractive alternative to open surgery for chronic dissection with aneurysmal dilatation.7,8 However, the presence of a mature, rigid dissection flap and multiple re-entry tears are thought to potentiate failure of endovascular therapy, and the long-term durability of endovascular management remains in question.9
The primary objective of managing aneurysms resulting from chronic type B aortic dissection (cTBAD) is to prevent patient death from rupture. This clinical end point is frequently associated with the ability of TEVAR to trigger favorable aortic remodeling and, consequently, it is thought that an important predictor of outcome is the status of FL patency.10 Because of the need for reintervention in 18%–50% of patients, a consensus definition of `clinical success' remains elusive, particularly in patients with dissection extending into the visceral segment.7,11 Although short-term outcomes of TEVAR for cTBAD have been reported to have high technical success rates and acceptable morbidity, concerns over precipitation of retrograde dissection or visceral ischemia remain.12,13 Because of these concerns over efficacy, safety, and durability, Food and Drug Administration approval of chronic dissection as an indication for TEVAR has not yet occurred.
The purpose of this analysis is to provide midterm results of patients treated electively for cTBAD complicated by aneurysmal dilatation. Specifically, results of perioperative outcomes, secondary intervention, mortality, and impact of aortic remodeling will be described.
METHODS
Database and study cohort
A prospectively maintained endovascular aortic registry at the University of Florida was queried for all patients undergoing TEVAR from January 2004 to December 2011. Patients treated for degenerative and trauma-related pathology (including aneurysm, penetrating ulcer, atheromatous disease, pseudoaneurysm, and traumatic transection) were excluded. Patients with dissection-related indications were further reviewed, and only elective procedures performed for aneurysmal degeneration of descending aortic dissection were included. Patients with thoracoabdominal FL aneurysm managed with chimney, fenestration, or visceral debranching procedures were omitted. However, patients who received a distal aortic stent (graft) and/or renal/visceral stent graft to treat a large secondary entry tear in an attempt to further reduce FL perfusion of the thoracic aneurysm were included. Patients with a primary entry tear located distal to the left subclavian artery and concomitant descending thoracic FL aneurysm comprised the study cohort (Supplementary Fig, online only). While acute proximal dissections (Stanford A14 or Debakey I/II15) were not included, patients treated for type A dissection with residual cTBAD with aneurysm formation were analyzed. Additionally, patients requiring bypass or stenting for revascularization of great vessels to augment the proximal landing zone for descending dissection cases were included. Indication for TEVAR was maximal thoracic aneurysm diameter ≥6.0 cm or documented growth rate ≥1.0 cm on serial centerline computed tomography (CTA) measurements over 12 months.
The electronic medical record was reviewed to collect demographics, comorbidities, procedure specific details, reinterventions, and complications. Comorbidities were defined based on previously published definitions from the Society of Vascular Surgery.16,17 Reintervention was defined as any aortic-related endovascular or open surgical procedure that occurred after the index TEVAR either at the intended treatment zone or at remote aortic sites. Patient mortality was confirmed utilizing the Social Security Death Masterfile.
Clinical practice
All operations were performed electively in a hybrid operating room using regional or general anesthesia. Need for preoperative or intraoperative adjuncts (eg, carotid-subclavian bypass, open/endovascular access vessel conduit) were determined selectively by each surgeon. Preoperative spinal drainage was used selectively according to previously published guidelines from our group.18,19 Intravascular ultrasound was used in all cases to confirm traversal of the wire through the true lumen, evaluate septal mobility pre- and post-stent deployment, assist endograft positioning, and evaluate expansion of the endograft after deployment. All patients undergoing repair were administered systemic heparin (100 U/Kg) to achieve an activated clotting time of ≥300 seconds. Devices were oversized 10%–15% relative to the diameter of the normal aorta proximal to the dissection, with little consideration given to sizing of the distal graft given the often very small true lumen diameter. Compliant balloon angioplasty of the proximal or distal stent was not routinely performed but selectively employed in the event of type I endoleak. Technical success was defined as deployment of the endograft at the intended aortic segment with absence of antegrade flow into the FL/aneurysm at case completion.
Coverage length was chosen with the goal of excluding the entire dissected aorta and all fenestrations in the cases of Debakey IIIa dissection.15 For Debakey IIIb dissections,15 there was some variability in treatment length based on surgeon's discretion. Early in our experience, coverage length was often determined intraoperatively based on the goal of covering the primary fenestration, as well as any obvious large fenestrations noted on intravascular ultrasound, and the ability to incite sluggish or no evident FL flow on aortography after endograft deployment. Because of the frequent need for reintervention, our current practice has evolved to more aggressive aortic coverage, and we now generally treat down to the celiac artery in all patients.
Postoperative care occurred in a cardiovascular intensive care unit, and management of the spinal drain was based on a previously published protocol.18,19 Spinal cord ischemia (SCI) was defined as any new impairment in lower extremity motor and/or sensory deficit not attributable to intracranial pathology, peripheral neuropathy, or neuropraxia. The diagnosis was determined by the treating physician and confirmatory imaging (eg, magnetic resonance imaging [MRI]), and clinical consultation with a neurologist were employed in selected cases when the diagnosis was not clear.
Reintervention was performed for multiple indications including persistent FL flow into the treated segment with further aneurysmal enlargement, type I or III endoleak, erosion of the graft through the dissection septum with pain or recurrent FL flow, device failure (eg, infolding), and aneurysmal degeneration of the untreated proximal or distal aorta.
Imaging protocol, aortic measurements, and definitions
Preoperative imaging for all patients included CTA with 1–2 mm cuts, including arterial and venous phase images, which was further analyzed on an Aquarius workstation (TeraRecon, Inc, San Mateo, Calif). Postoperative surveillance was based on a similar protocol with imaging at 1 month, 6 months, and annually thereafter. Postoperative centerline of flow reconstructions were completed from the sinotubular junction (STJ) to the aortic bifurcation. Measurements lengths included STJ→aortic bifurcation (total aortic length: [TAL]), left carotid artery (LCCA)→celiac artery (thoracic aortic length [ThAL]), and total coverage length of the thoracic endograft(s) (TCL). Percentage of total aortic coverage (TAC) and thoracic aortic coverage (ThAC) were calculated as follows: %TAC = (TCL/TAL) × 100; %ThAC = (TCL/ThAL) × 100.
FL patency was defined by contrast within the FL on arterial or venous phase of CTA. FL thrombosis was characterized by the following zone designations: complete (no contrast blush in any segment of the aorta), proximal thoracic (site of the TEVAR treatment zone or proximal to T6), distal thoracic (below T6, but above the celiac artery), visceral segment (between celiac axis and lowest renal artery), infrarenal aortic (lowest renal artery to aortic bifurcation), and iliac (aortic bifurcation to iliac bifurcation). Using this classification, further analysis of the status of residual FL patency was completed by categorizing patients into the following subgroups: entire FL thrombosed except (1) isolated segment of infrarenal aorta FL, (2) visceral segment FL patent but complete thrombosis of thoracic segment, or (3) distal thoracic aortic FL patent but thrombosis of the proximal, treated segment of the thoracic aorta. Finally, trans-aortic diameters were measured on preoperative and most recent postoperative CTA at multiple sites within the thoracic aorta. Favorable aortic remodeling was defined by a ≥5 mm thoracic aneurysm diameter reduction and no need for aortic-related reintervention.
Statistical analysis
Categorical variables were summarized using frequencies and percentages. Continuous variables were analyzed with means and standard deviations if normally distributed; otherwise medians with range were applied. Comparisons of patient- or procedure-related characteristics in subgroup analysis were performed using Fischer exact test, two-sample t-test, Wilcoxon rank sum text, and ANOVA, when appropriate. Patient survival and reintervention rates were estimated using Kaplan-Meier methodology and compared between groups using the log-rank test. Cox regression analysis was utilized to develop a model to predict mortality. Variables found on univariate analysis to have P < .2 were included in the multivariable model, which was refined using stepwise backward Cox regression and log-likelihood ratio testing. All statistical analysis was performed using STATA 11 software (Stata-Corp, College Station, Tex).
RESULTS
From 2004 to 2011, 635 patients were treated with TEVAR for a variety of aortic pathologies, of whom 80 underwent repair for asymptomatic FL aneurysms attributable to cTBAD. Specifically, the indication for intervention was thoracic aneurysm ≥6.0 cm (n = 51; 63.8%) or aneurysm expansion ≥1.0 cm during a 12-month interval (n = 29; 36.2%). Median time from diagnosis of acute dissection to TEVAR for chronic aneurysm was 16 (range, 1–74) months. Most patients were male (n = 70; 88%; mean age 60 ± 13 years), and median follow-up was 24 (range, 1–72) months. Demographics and preoperative characteristics are further highlighted in Table I.
Table I.
Patient demographics, comorbidities, dissection morphology, and previous aortic surgery history
| Feature | % (n = 80) |
|---|---|
| Age (mean ± SD), years | 60 ± 13 |
| Male | 88 |
| Hypertension | 95 |
| Dyslipidemia | 53 |
| Tobacco | 51 |
| CRI(eGFR <50) | 30 |
| Coronary artery disease | 19 |
| COPD | 16 |
| Arrhythmia | 11 |
| Marfan's | 9 |
| Peripheral artery disease | 9 |
| Cerebrovascular disease | 8 |
| Dissection extenta | |
| Debakey IIIa | 24 |
| Debakey IIIb | 76 |
| Previous aortic operation | |
| Infrarenal AAA repair | 10 |
| Type A repairb | 9 |
| Asc. and arch repair | 9 |
| Elephant trunk | 5 |
Procedural details and perioperative outcomes
Technical success was achieved in 79 cases (99%), with one intraoperative type Ia endoleak. Two patients (2.5%) had type 1a endoleak on their 1-month postoperative CTA. In 75% (n = 60) of patients, coverage occurred proximal to zone 3, and 30% (n = 18) of these patients underwent subclavian revascularization. Additional data regarding technical conduct of the TEVAR procedure are detailed in Table II. All patients were treated with covered stent grafts, and no self or balloon expandable bare metal stents were employed to augment radial force in an effort to improve true lumen expansion. Mean proportion of total aortic and thoracic aortic coverage length were 44.8 ± 11.9% and 78.2 ± 19.3%, respectively.
Table II.
Operative details
| Feature | % (n = 80) |
|---|---|
| Preoperative aneurysm diameter (mean ± SD), mm | 62 ± 10 |
| Stent graft type | |
| Gore TAG | 50 |
| Cook TX2 | 50 |
| Procedure details | |
| Spinal anesthesia | 51 |
| ASA score (III/IV) | 99 |
| Cerebrospinal fluid drain | |
| Preoperative | 71 |
| Postoperative | 6 |
| Left subclavian coverage | 75 |
| Carotid subclavian bypass | 20 |
| Intraoperative adjunct (any) | 39 |
| Visceral/renal stent graft | 20 |
| Simultaneous aortic stent graft | 16 |
| Iliac artery stent graft (to treat distal fenestration) | 15 |
| Left subclavian embolization | 6 |
| Access vessel conduit | 4 |
| Arch vessel stent graft | 3 |
| Palmaz stent | 3 |
ASA, American Society of Anesthesiologists; SD, standard deviation.
Spinal drains were used in 78% (n = 62) of the procedures with 57/62 (92%) placed preoperatively, and 8% (n = 5) implanted postoperatively. Length of stay was 6.5 ± 4.7 days, and 21(26%) patients experienced some complication. Further details regarding outcomes and complications are demonstrated in Table III. Notably, a total of 14(17%) patients experienced some form of neurologic morbidity. A permanent deficit (either from SCI or stroke) was documented in 11.2% of patients. More specifically, SCI developed in 10% (n = 8), with a permanent deficit observed in 6.2% (n = 5). The remaining neurologic events (n = 6; 7.5%) were MRI-evident cerebrovascular infarctions, and four (5%) cases resulted in permanent disability. For the entire cohort, there were two inhospital deaths (2.5%). One of the patients experienced a devastating posterior stroke and subsequently died on postoperative day 15. The second patient suffered multiple complications including SCI and respiratory failure, and support was withdrawn on postoperative day 11.
Table III.
Clinical outcomes and complications
| Feature | % (n = 80) |
|---|---|
| LOS (mean ± SD), days | 6.5 ± 4.7 |
| 30-day mortality | 2.5 |
| Complication | 26 |
| Spinal cord ischemia | |
| Any SCI | 10 |
| Permanent | 6 |
| Stroke | 8 |
| Pulmonary | 4 |
| Cardiac | 3 |
| Bleeding (access vessel) | 3 |
| Renal (25 ↓ eGFR) | 1 |
| Gastrointestinal | 1 |
eGFR, Estimated glomerular filtration rate; LOS, length of stay; SCI, spinal cord ischemia; SD, standard deviation.
A decreased incidence of SCI was noted in patients who underwent preoperative spinal drain placement (5.3%; n = 57) compared with those who did not (21.7%; n = 23; P = .04). No significant association was found between SCI and planned left carotid-subclavian bypass (P = .3), although there was a trend toward a lower incidence with carotid-subclavian bypass (0% in 16 patients compared with 12.5% in 64). Finally, there was no difference in ThAC between patients who developed SCI and those who did not (83% vs 78%; P =.6).
Aortic remodeling and secondary intervention
Clinical and radiographic follow-up was available in 74 (93%) patients with median clinical and radiographic follow-up times of 25.8 (range, 7–38) and 18.1 (range, 1–34) months, respectively. Postoperative CTA beyond 12 months was available in 63 (79%) patients (noncontrast CT, n = 13; no postoperative CT available = 4). Aneurysm diameter decreased or stabilized in 65% (n = 52) whereas the FL thrombosed completely within the thoracic aorta in 51% (n = 41). For the entire cohort, mean decrease in diameter of the treated thoracic aortic segment was 2.6 ± 10.1 mm. The impact of FL thrombosis on changes in aortic diameter is depicted in Fig 1. A significant difference (P = .02) in the mean diameter decrease is noted between patients experiencing complete vs partial FL thrombosis.
Fig 1.
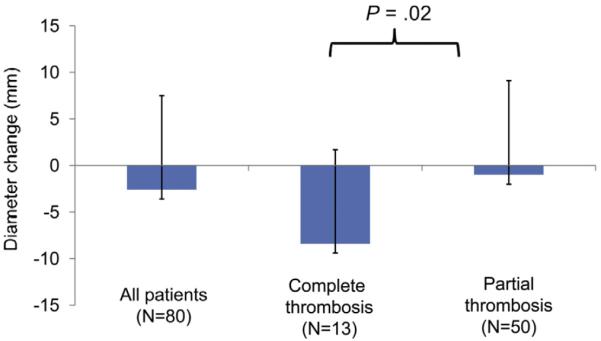
This figure demonstrates the relative change in maximal descending thoracic aortic diameter after thoracic endovascular aortic aneurysm repair (TEVAR) for chronic type B aortic dissection (cTBAD) withaneurysm. The differential impact of partial vs complete thrombosis of the false lumen (FL) led to significant differences (P = .02) in average diameter reduction after endograft repair.
Further analysis of the association of FL thrombosis to aortic remodeling is highlighted in Fig 2. The majority of patients did not have complete FL thrombosis (50/63; 79%) because of involvement of the visceral aorta. Those without complete FL thrombosis had varying segments of residual FL patency. When comparing mean thoracic aortic diameter change after TEVAR, a significant difference (P < .0005) was noted between groups with persistent FL perfusion isolated to the distal thoracic aorta (increase 5.5 ± 1.4 mm), involving the visceral aorta (decrease 2.2 ± 1.7 mm), or localized to the infrarenal aorta (decrease 6.3 ± 3.4 mm).
Fig 2.
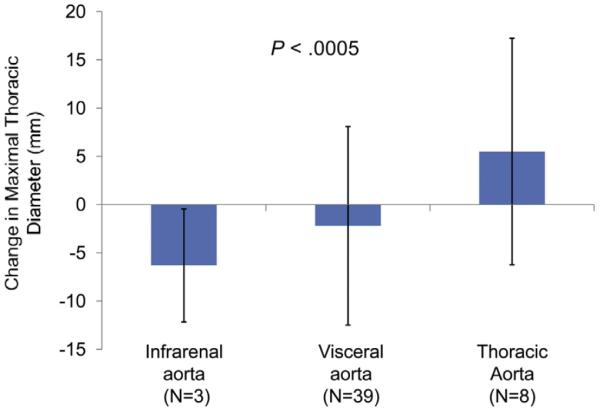
This demonstrates a comparison of the average maximal thoracic aortic diameter change after thoracic endovascular aortic aneurysm repair (TEVAR) for chronic type B aortic dissection (cTBAD) with aneurysm formation as a function of residual false lumen (FL) patency. Patients with isolated infrarenal FL patency vs visceral aortic segmental patency vs distal descending thoracic aortic FL patency are compared. A significant difference (P < .0005) in diameter outcome is noted and primarily impacted by persistent perfusion of the FL in the distal descending thoracic aorta resulting in progression of aneurysm size.
Twenty-three (29%) patients required some form of reintervention, with reintervention within the original treated aortic segment occurring in 16% (n = 13). Median time to reintervention was 17 (range, 4–33) months with 1- and 3-year freedom from reintervention (95% confidence interval [CI]) of 80% (range, 68%–88%) and 70% (range, 57%–80%), respectively (Fig 3). There were six open conversions requiring TEVAR explantation (one suspected aortoesophageal fistula and five patients with persistent FL expansion from proximal or distal endoleak), and a variety of remedial procedures were employed to treat residual degeneration of the FL at remote aortic sites (Table IV). Six patients underwent more than one secondary intervention (two reinterventions, n = 3; three reinterventions, n = 3). Of note, no delayed FL ruptures or distal endograft collapse occurred in any patient, and none of the reinterventions resulted in perioperative mortality. No significant association was found between time from diagnosis of dissection to index TEVAR and subsequent reintervention (P = .7) or favorable aortic remodeling (P = .4). Furthermore, incidence of favorable aortic remodeling was similar across all quartiles of time from dissection diagnosis to TEVAR (P = .4).
Fig 3.
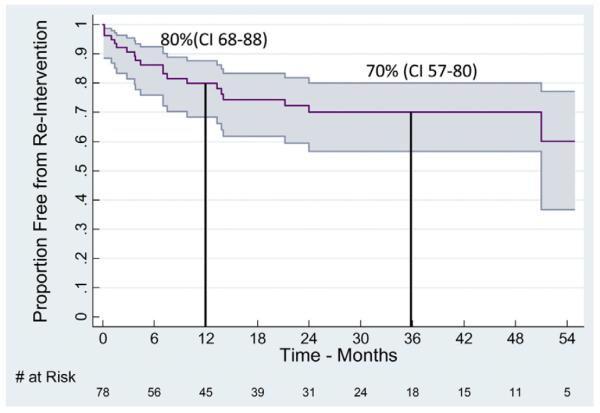
Kaplan-Meier curve with 95% confidence intervals (CIs) of secondary intervention after thoracic endovascular aortic aneurysm repair (TEVAR) for chronic type B aortic dissection (cTBAD) with aneurysm. All displayed intervals have ≤10% standard error of the mean.
Table IV.
Secondary interventions after TEVAR for cTBAD with aneurysm
| Feature | % (n = 80) |
|---|---|
| Time to reintervention (median), months | 17 |
| Patients | 29 |
| Open surgery | |
| Descending thoracic aorta | 8 |
| Ascending/arch | 4 |
| Carotid subclavian bypass | 1 |
| Endovascular | |
| Extension (proximal/distal) | 6 |
| Embolization | 6 |
| Visceral, renal, and/or iliac stent graft | 4 |
cTBAD, Chronic type B aortic dissection; TEVAR, thoracic endovascular aortic aneurysm repair.
Mortality
Estimated actuarial 1- and 5-year survival was 89% (range, 80%–94%) and 70% (range, 55%–81%), respectively (Fig 4). To study the impact of reintervention and aortic remodeling on mortality, patients were further analyzed based on need of secondary aortic-related procedures, as well as presence or absence of positive aortic remodeling. Reintervention and favorable aortic remodeling did not have a significant influence on survival (Figs 5 and 6). However, survival analysis suggested a trend toward improved survival in patients with reintervention.
Fig 4.
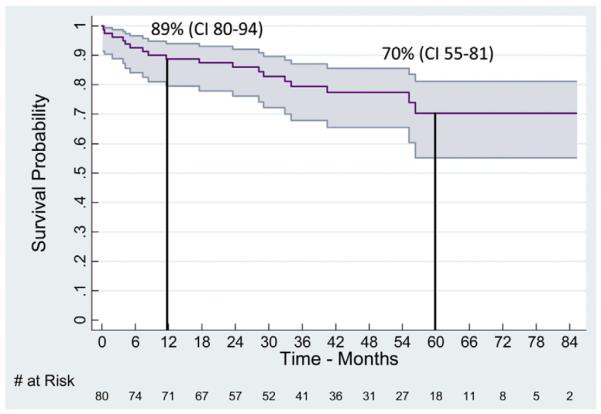
Life table estimate of long-term survival with 95% confidence intervals (CIs) after thoracic endovascular aortic aneurysm repair (TEVAR) for chronic type B aortic dissection (cTBAD) for aneurysm. All displayed intervals have ≤10% standard error of the mean.
Fig 5.
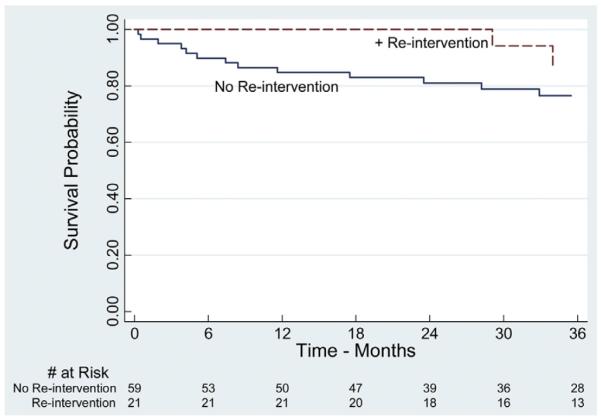
This figure compares the proportion of patients surviving between cohorts requiring reintervention and those who did not. No statistically significant difference was noted between the two subgroups (log-rank, P = .48). All displayed intervals have ≤10% standard error of the mean.
Fig 6.
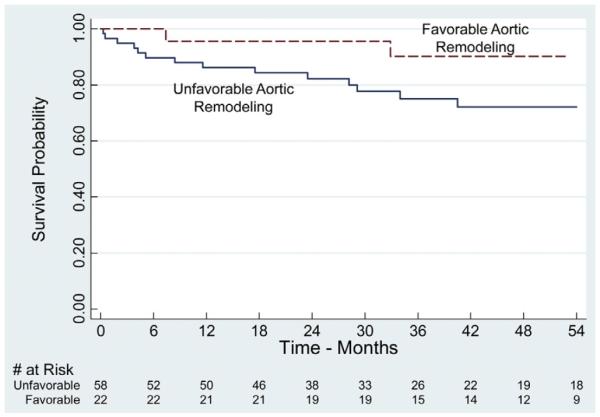
Kaplan-Meier estimates of survival dichotomized by presence or absence of favorable aortic remodeling. Favorable aortic remodeling was defined as a ≥5 mm decrease in maximal descending thoracic aortic diameter and no need for aortic reintervention either at the intended treatment zone or remote aortic sites. No difference in estimated long-term survival is present between the two groups (log-rank, P = .18). All displayed intervals have ≤10% standard error of the mean.
In an attempt to understand differences in the patient populations of those requiring reintervention and those who did not, a multivariable prediction model for long-term mortality was created. This model identified coronary artery disease (hazard ratio [HR], 6.4; 95% CI, 2.3–17.7; P < .005), prior infrarenal aortic surgery (HR, 8.6; 95% CI, 2.3–31.7; P = .001), and congestive heart failure (HR, 11.9; 95% CI, 1.9–73.8; P = .008) as independent risk factors associated with increased mortality. Hyperlipidemia was found to be protective (HR, 0.2; 95% CI, 0.05–0.6; P = .004). No significant difference in predictors of survival were found between patients undergoing reintervention vs those who did not (P = .2), although a trend was noted toward increased risk factors in patients not requiring reintervention.
DISCUSSION
This report is one of the largest series to date that highlights the perioperative outcomes, midterm reintervention, and long-term survival of a cohort of patients treated with TEVAR for FL aneurysm formation secondary to cTBAD. Perioperative morbidity and mortality are similar to other TEVAR series for degenerative8,20 and dissection-related pathology; however, rates of spinal cord injury were higher than previously reported.7,21 A majority of patients had thrombosis of the intended treatment site within the thoracic aortic segment with aneurysm stabilization or reduction. Secondary intervention was common, but remediation or lack of favorable aortic remodeling did not appear to significantly impact long-term survival.
Most centers advocate selective intervention for acute TBAD, with medical management being the cornerstone of therapy in uncomplicated cases.22,23 Medical therapy for uncomplicated chronic descending thoracic aortic dissection has previously been shown to be equivalent to endovascular treatment; however, the 2-year event-free survival rate for the medical arm was only 74%.24 This underscores the need for long-term radiographic surveillance attributable to FL expansion in 25%–40%, with 10%–20% of patients experiencing late rupture as a result of aneurysm formation.9 Results of open surgical repair for TBAD have variable outcomes depending on acuity, patient comorbidity profile, and hospital volume, with a reported in-hospital mortality rate ranging from 5% to 20%.25,26 In this study, there was no open cohort for comparison, and given the risk of complications and reintervention, open therapy may still be an attractive option for selected patients. Interestingly, Svensson and colleagues12 performed a review of contemporary open surgical series for elective descending thoracic aortic aneurysm repair, including those attributable to chronic dissection, and reported an average permanent lower extremity paralysis rate of 3.4%, stroke rate of 2.7%, and mortality of 4.8% leading to a recommendation of open surgical repair in low-risk patients.
The role of TEVAR for cTBAD remains undefined because of the lack of consensus regarding definitions of clinical success and concerns about treatment failure.12,13 A variety of methods have been described to define aortic remodeling after TEVAR for cTBAD, including false:true lumen ratios, volumetry, and diameter changes over time.4,27,28 Unlike degenerative aneurysms, complete aneurysm exclusion in dissection patients is often difficult to achieve because of distal septal tears. Despite this limitation, a similar number of patients undergoing TEVAR for cTBAD (15%–30%) require adjunctive procedures or need future reintervention as patients with degenerative aneurysm (12%–22%),7,9,27,29 and the majority of patients achieve FL thrombosis parallel to the stent graft, similar to our series.7,11,27 Given the low risk of reintervention and the apparent lack of adverse impact on mortality, diligent follow-up and judicious reintervention have become important aspects of our management algorithm.
Additional concerns regarding TEVAR management of cTBAD aneurysms are the potential for endograft collapse because of a noncompliant dissection flap, visceral vessel ischemia (if branch vessels receive dual or sole perfusion from the FL), and intimomedial erosion.30 Despite the frequent presence of a small distal true lumen diameter and rigid dissection septum, these complications were rare in our experience. One patient in this series experienced late distal endograft collapse that resulted in lower extremity claudication and postprandial symptoms suggestive of chronic mesenteric ischemia. This patient was successfully managed with TEVAR relining and Palmaz stent placement.
Perhaps the most dreaded complication from these interventions is precipitation of retrograde dissection, which has been reported to occur in up to 4% of cases.27,30 In our series, a single retrograde dissection was noted (1.2%) and occurred 3 months after the index TEVAR. This patient presented with acute chest pain and was managed by arch replacement with the Dacron graft sewn to the endograft in the proximal descending thoracic aorta. These events underscore the current limitations of stent graft design for chronic dissection because, in the majority of cases, treatment extends to the left common carotid artery, and the endograft frequently must adapt to significant differences in lumen diameter between the proximal and compressed distal true lumen. The next generation of stent graft design will likely have pathology-specific device modifications to address these challenges (eg, Provisional ExTension To Induce COmplete ATtachment [PETTICOAT31] technique now being evaluated for acute dissection).
Composite perioperative morbidity was 26%, similar to results from other series; however, the rate of stroke and SCI was higher than the 0%–4% incidence reported in the literature.7, 9,12,27,29 This may be a reflection of the aggressive posture that is taken in managing FL aneurysms at our institution whereby the majority of the thoracic aorta (78.2 ± 19.3%) was covered. Of note, the incidence of SCI was lower in patients who had a preoperative spinal drain placed. Regarding known risk factors for SCI with TEVAR, four of the SCI patients had previous infrarenal aortic surgery, all had patent internal iliac vessels, and seven of the eight individuals required zone 2 coverage but did not undergo carotid-subclavian bypass. All of these cases occurred before 2009 and since that time, 34 TEVAR procedures for cTBAD have been completed without SCI. Over this interval, we implemented a protocol with routine cerebrospinal fluid drainage and subclavian revascularization for all cTBAD with aneurysm cases.
Notwithstanding the significant reintervention and perioperative morbidity, long-term survival in this series was excellent. The 1- and 5-year survival rates of 80% ± 4% and 70% ± 6% are similar to other series.7,27 These outcomes occurred despite a relatively high-risk patient population, with 83% (n = 66) having an American Society of Anesthesiologists (ASA) score of 4 preoperatively. Interestingly, patients who required reintervention were noted to have less overall comorbidity than those who did not and may partially explain the trend toward a survival advantage (Fig 5), however, this was not statistically significant. Finally, no significant difference in predictors of mortality were found between patients who underwent reintervention vs those who did not (P = .2).
This report has several important limitations including that it is a single center, retrospective analysis without an open surgical cohort for comparison. Although no significant mortality difference was noted when considering reintervention or favorable aortic remodeling, the modest number of events and duration of the follow-up interval underscores the potential of type II error. Cause of late death is not known, and it is conceivable that some of these events were dissection-related. Although we performed a multivariable prediction model for survival in an attempt to understand the impact of reintervention on outcome, comorbidity severity and medication history (eg, statin utilization) were not accounted for in the regression analysis. There was no standardized treatment algorithm in place over the entire study interval regarding patient selection and procedural conduct, although since 2009 our practice has become more uniform.
Because of the lack of consensus on definition of clinical success or favorable aortic remodeling, the results highlighted in this series may not be directly comparable to other reports of TEVAR for cTBAD. Additionally, postoperative CTA was not available for all patients and only two timepoints were analyzed to discern FL morphology and aortic diameter. Moreover, no volumetric data were abstracted, which may offer a more comprehensive and precise assessment of aortic remodeling for dissection-related pathology.27 FL patency status was detected from the arterial and venous phase of postoperative CTA, and it is conceivable that this is not the most sensitive or specific method for analyzing this end point given reports on efficacy of various dynamic MRI techniques.32,33
In conclusion, thoracic endovascular repair of FL aneurysmal degeneration resulting from cTBAD can be performed with a high degree of technical success with acceptable perioperative morbidity and mortality. Spinal cord ischemia may occur at a higher incidence than previously reported, and liberal use of adjuncts such as spinal drainage and subclavian revascularization is recommended to reduce the risk of this complication. Lack of uniform aneurysm diameter reduction, frequent need for reintervention, and variable FL thrombosis underscore the need for longer-term follow-up. Despite the need for reintervention and potential procedural morbidity, good long-term survival can be anticipated.
Supplementary Material
Acknowledgments
Author conflict of interest: Dr Feezor has received educational grant support from Cook Medical and Medtronic, Inc. Dr Beck has received grant support from Cook Medical, Medtronic, Inc, and W. L. Gore. Dr Beck is a consultant for Cook Medical and Medtronic Inc.
Footnotes
Presented at the Thirty-ninth Annual Meeting of the New England Society of Vascular Surgery, Boston, Mass, September 22, 2012.
AUTHOR CONTRIBUTIONS Conception and design: SS, RF
Analysis and interpretation: SS, RF, CC, DS, AB
Data collection: SS
Writing the article: SS, RF, CC
Critical revision of the article: SS, RF, CC, DS, PH, TM, TH, AB
Final approval of the article: SS, RF, CC, DS, PH, TM, TH, AB
Statistical analysis: CC
Obtained funding: Not applicable
Overall responsibility: SS
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Brown KE, Eskandari MK, Matsumura JS, Rodriguez H, Morasch MD. Short and midterm results with minimally invasive endovascular repair of acute and chronic thoracic aortic pathology. J Vasc Surg. 2008;47:714–22. doi: 10.1016/j.jvs.2007.12.003. discussion: 722. [DOI] [PubMed] [Google Scholar]
- 2.Chaikof EL, Mutrie C, Kasirajan K, Milner R, Chen EP, Veeraswamy RK, et al. Endovascular repair for diverse pathologies of the thoracic aorta: an initial decade of experience. J Am Coll Surg. 2009;208:802–16. doi: 10.1016/j.jamcollsurg.2008.12.021. discussion: 816. [DOI] [PubMed] [Google Scholar]
- 3.Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Bossone E, et al. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2006;114(1 Suppl):I357–64. doi: 10.1161/CIRCULATIONAHA.105.000620. [DOI] [PubMed] [Google Scholar]
- 4.Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519–28. doi: 10.1161/CIRCULATIONAHA.109.886408. [DOI] [PubMed] [Google Scholar]
- 5.Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann Thorac Surg. 2008;85:965–70. doi: 10.1016/j.athoracsur.2007.11.013. discussion: 970. [DOI] [PubMed] [Google Scholar]
- 6.Elefteriades JA, Hartleroad J, Gusberg RJ, Salazar AM, Black HR, Kopf GS, et al. Long-term experience with descending aortic dissection: the complication-specific approach. Ann Thorac Surg. 1992;53:11–20. doi: 10.1016/0003-4975(92)90752-p. discussion: 20. [DOI] [PubMed] [Google Scholar]
- 7.Parsa CJ, Schroder JN, Daneshmand MA, McCann RL, Hughes GC. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg. 2010;89:97–102. doi: 10.1016/j.athoracsur.2009.09.029. discussion: 102–4. [DOI] [PubMed] [Google Scholar]
- 8.Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP. Gore TAG Investigators. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008;47:912–8. doi: 10.1016/j.jvs.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Atkins MD, Jr, Black JH, III, Cambria RP. Aortic dissection: perspectives in the era of stent-graft repair. J Vasc Surg. 2006;43(Suppl A):30A–43A. doi: 10.1016/j.jvs.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez JA, Olsen DM, Lucas L, Wheatley G, Ramaiah V, Diethrich EB. Aortic remodeling after endografting of thoracoabdominal aortic dissection. J Vasc Surg. 2008;47:1188–94. doi: 10.1016/j.jvs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Manning BJ, Dias N, Ohrlander T, Malina M, Sonesson B, Resch T, et al. Endovascular treatment for chronic type B dissection: limitations of short stent grafts revealed at midterm follow-up. J Endovasc Ther. 2009;16:590–7. doi: 10.1583/09-2717.1. [DOI] [PubMed] [Google Scholar]
- 12.Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85:S1–41. doi: 10.1016/j.athoracsur.2007.10.099. [DOI] [PubMed] [Google Scholar]
- 13.Thrumurthy SG, Karthikesalingam A, Patterson BO, Holt PJ, Hinchliffe RJ, Loftus IM, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;42:632–47. doi: 10.1016/j.ejvs.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Coady MA, Ikonomidis JS, Cheung AT, Matsumoto AH, Dake MD, Chaikof EL, et al. Surgical management of descending thoracic aortic disease: open and endovascular approaches: a scientific statement from the American Heart Association. Circulation. 2010;121:2780–4. doi: 10.1161/CIR.0b013e3181e4d033. [DOI] [PubMed] [Google Scholar]
- 15.Debakey ME, Henly WS, Cooley DA, Morris GC, Jr, Crawford ES, Beall AC., Jr Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–49. [PubMed] [Google Scholar]
- 16.Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–6. doi: 10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 17.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards. Reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg. 2010;52:1022–33. doi: 10.1016/j.jvs.2010.07.008. 1033.e1015. [DOI] [PubMed] [Google Scholar]
- 18.Feezor RJ, Lee WA. Strategies for detection and prevention of spinal cord ischemia during TEVAR. Semin Vasc Surg. 2009;22:187–92. doi: 10.1053/j.semvascsurg.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Martin DJ, Martin TD, Hess PJ, Daniels MJ, Feezor RJ, Lee WA. Spinal cord ischemia after TEVAR in patients with abdominal aortic aneurysms. J Vasc Surg. 2009;49:302–6. doi: 10.1016/j.jvs.2008.08.119. discussion: 306–7. [DOI] [PubMed] [Google Scholar]
- 20.Fairman RM, Criado F, Farber M, Kwolek C, Mehta M, White R, et al. Pivotal results of the medtronic vascular talent thoracic stent graft system: the VALOR trial. J Vasc Surg. 2008;48:546–54. doi: 10.1016/j.jvs.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Akin I, Kische S, Ince H, Nienaber CA. Indication, timing and results of endovascular treatment of type B dissection. Eur J Vasc Endovasc Surg. 2009;37:289–96. doi: 10.1016/j.ejvs.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Schor JS, Yerlioglu ME, Galla JD, Lansman SL, Ergin MA, Griepp RB. Selective management of acute type B aortic dissection: long-term follow-up. Ann Thorac Surg. 1996;61:1339–41. doi: 10.1016/0003-4975(96)00105-1. [DOI] [PubMed] [Google Scholar]
- 23.Hata M, Shiono M, Inoue T, Sezai A, Niino T, Negishi N, et al. Optimal treatment of type B acute aortic dissection: long-term medical follow-up results. Ann Thorac Surg. 2003;75:1781–4. doi: 10.1016/s0003-4975(03)00113-9. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg RK, Lu Q, Roselli EE, Svensson LG, Moon MC, Hernandez AV, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118:808–17. doi: 10.1161/CIRCULATIONAHA.108.769695. [DOI] [PubMed] [Google Scholar]
- 25.Brunt ME, Egorova NN, Moskowitz AJ. Propensity score-matched analysis of open surgical and endovascular repair for type B aortic dissection. Int J Vasc Med. 2011;2011:364046. doi: 10.1155/2011/364046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachs T, Pomposelli F, Hagberg R, Hamdan A, Wyers M, Giles K, et al. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample. J Vasc Surg. 2010;52:860–6. doi: 10.1016/j.jvs.2010.05.008. discussion: 866. [DOI] [PubMed] [Google Scholar]
- 27.Andacheh ID, Donayre C, Othman F, Walot I, Kopchok G, White R. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J Vasc Surg. 2012;56:644–50. doi: 10.1016/j.jvs.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 28.Stanley GA, Murphy EH, Knowles M, Ilves M, Jessen ME, Dimaio JM, et al. Volumetric analysis of type B aortic dissections treated with thoracic endovascular aortic repair. J Vasc Surg. 2011;54:985–92. doi: 10.1016/j.jvs.2011.03.263. discussion: 992. [DOI] [PubMed] [Google Scholar]
- 29.Eggebrecht H, Nienaber CA, Neuhauser M, Baumgart D, Kische S, Schmermund A, et al. Endovascular stent graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27:489–98. doi: 10.1093/eurheartj/ehi493. [DOI] [PubMed] [Google Scholar]
- 30.Yang CP, Hsu CP, Chen WY, Chen IM, Weng CF, Chen CK, et al. Aortic remodeling after endovascular repair with stainless steel-based stent graft in acute and chronic type B aortic dissection. J Vasc Surg. 2012;55:1600–10. doi: 10.1016/j.jvs.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Nienaber CA, Kische S, Zeller T, Rehders TC, Schneider H, Lorenzen B, et al. Provisional extension to induce complete attachment after stent graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther. 2006;13:738–46. doi: 10.1583/06-1923.1. [DOI] [PubMed] [Google Scholar]
- 32.Tomiguchi S, Morishita S, Nakashima R, Hara M, Oyama Y, Kojima A, et al. Usefulness of turbo-FLASH dynamic MR imaging of dissecting aneurysms of the thoracic aorta. Cardiovasc Intervent Radiol. 1994;17:17–21. doi: 10.1007/BF00197909. [DOI] [PubMed] [Google Scholar]
- 33.Karmonik C, Duran C, Shah DJ, Anaya-Ayala JE, Davies MG, Lumsden AB, et al. Preliminary findings in quantification of changes in septal motion during follow-up of type B aortic dissections. J Vasc Surg. 2012;55:1419–26. doi: 10.1016/j.jvs.2011.10.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


