Abstract
Background
Owing to the increasing prevalence of obesity and diabetes in Asia, and the paucity of studies, we examined the influence of raised blood glucose and diabetes on cancer mortality risk.
Methods
Thirty-six cohort Asian and Australasian studies provided 367,361 participants (74% from Asia); 6% had diabetes at baseline. Associations between diabetes and site-specific cancer mortality were estimated using time-dependent Cox models, stratified by study and sex, and adjusted for age.
Results
During a median follow-up of 4.0 years, there were 5,992 deaths due to cancer (74% Asian; 41% female). Participants with diabetes had 23% greater risk of mortality from all-cause cancer compared with those without: hazard ratio (HR) 1.23 (95% CI 1.12, 1.35). Diabetes was associated with mortality due to cancer of the liver (HR 1.51, 95% CI 1.19, 1.91), pancreas (HR 1.78, 95% CI 1.20, 2.65), and, less strongly, colorectum (HR 1.32, 95% CI 0.98, 1.78). There was no evidence of sex- or region-specific differences in these associations. The population attributable fractions for cancer mortality due to diabetes were generally higher for Asia compared with non-Asian populations.
Conclusion
Diabetes is associated with increased mortality from selected cancers in Asian and non-Asian populations.
Keywords: Diabetes Mellitus, Cancer Mortality, Epidemiology, Asia-Pacific
INTRODUCTION
A substantial proportion of cancer deaths are attributed to unhealthy lifestyles and behaviours including poor diet [1], obesity [2], smoking [3], and alcohol [4]. Mortality rates for specific cancers are known to vary significantly by geographical region and country. Mortality from cancers of the colon and rectum, which are considered to be due in large part to poor diet and lifestyle, is higher for industrialised nations, such as Australia, North America, and Western Europe, as compared with many Asian countries. By comparison, mortality from liver cancer is far more common across Asia, and particularly China, than in the West, due mainly to the high prevalence of chronic hepatitis B and C infections that account for a large majority of all liver cancers worldwide [5].
Previous studies have reported that diabetes status is associated with a 20-30% increased risk of total cancer mortality [6-10]. A positive association between abnormal glucose tolerance and the risk of cancer mortality has also been demonstrated for Western countries [11-13]. With regard to site-specific cancer mortality, many studies have shown positive associations between diabetes and the risk of mortality from cancers of the pancreas, liver, colorectum, and prostate [12-19]. . Most of these studies were conducted in Western populations, with scarce results from Asia. Among the few Asian studies, positive associations between diabetes and cancers of the pancreas, liver, and colorectum, as well as non-Hodgkin lymphoma, have been reported [6, 8, 20-24]. Given increasing life expectancy and urbanization [25], the increasing prevalence of obesity [26], and the growing prevalence of diabetes in Asian populations [27, 28], more studies are needed to clarify the evidence of the possible effect of diabetes on cancer mortality in Asia.
The Asia Pacific Cohort Studies Collaboration (APCSC) is a large-scale collaborative project with previous reports on the relationship between diabetes and major causes of death [20-22, 29]. A systematic analysis of mortality from specific cancers in relation to diabetes and blood glucose in the APCSC has not yet been reported. The aims of the present study are two-fold. First, to examine associations between fasting blood glucose levels, diabetes, and site-specific cancer mortality for which sufficient numbers of fatal events were available for analysis; and second, to estimate the population attributable fractions (PAFs) of cancer mortality due to diabetes within countries in the region. These give crude measures of the percentages of deaths that are expected to be due to diabetes, should there be a causal relationship [30].
MATERIAL AND METHODS
Participating studies
The APCSC is a pooled-analysis of individual data from cohort studies conducted in the Asia-Pacific region; details have been published elsewhere [31]. Studies were eligible for inclusion if the following criteria were met: study sample was drawn from the Asia-Pacific region; study was of a prospective cohort design; study had at least 5,000 person-years of follow-up. Studies were not eligible if entry was dependent on having a particular medical condition or risk factor. At a minimum, studies must have had data on date of birth or age, sex, blood pressure, and date or age of death. Outcome data included mortality from specific cancers. Cohorts were classified as Asian if study members were recruited from mainland China, Hong Kong, Japan, Korea, Singapore, South Korea, Taiwan, or Thailand; and as Australasian if from Australia or New Zealand. This classification largely represents a dichotomy by ethnicity into Asians and non-Asians.
Baseline assessment
Study participants’ diabetes status were determined on the basis of self-reported history of diabetes, or by applying the World Health Organization (WHO) diagnostic criteria to blood glucose levels at baseline [32]. Diabetes status according to glucose levels was positive if fasting whole blood glucose was ≥ 6.1 mmol/L (110 mg/dL) or plasma glucose ≥ 7 mmol/L (126 mg/dL); or if non-fasting whole blood glucose was ≥ 10 mmol/L (180 mg/dL) or plasma glucose ≥ 11.1 mmol/L (200 mg/dL) (information on glucose-lowering medication not available). All data on cigarette smoking were self-reported as either current smoker or non-smoker at the time of study entry. Height and weight were ascertained from direct measurements; body mass index (BMI) was calculated as weight (kg) divided by height (m)squared. Systolic blood pressure was measured using a sphygmomanometer. Participants also reported alcohol use habits (current alcohol user/non-alcohol user), exercise habits (‘none or almost none’ as sedentary lifestyle/‘any exercise’ as active exercise), and educational attainment (none/at least primary school).
Outcomes
Cancer deaths were classified according to the ninth [33] or tenth [34] revision of the International Classification of Diseases (ICD): bladder (ICD-9; ICD-10: 188; C67); brain and central nervous system (191-192; C70-72); breast (174; C50); colon and rectum (153-154; C18-21); leukaemia (204-208; C91-95); liver (155, 197.7; C22, C78.7); lung (162; C33-34); Non-Hodgkin’s lymphoma (200, 202; C82, C85); melanoma (172; C43); multiple myeloma (203; C90); ovary and uterus (179-183; C53-56); pancreas (157; C25); prostate (185; C61); kidney (189; C64); and stomach (151; C16). Malignancies of the upper aero-digestive tract (UADT) were analysed by combining cancers of the oropharynx, oesophagus, and larynx (ICD-9; ICD-10: 140-150, 161, C00-C15, C32).
Statistical analyses
Analyses were restricted to participants aged ≥ 20 years at the time of the baseline survey with complete data on diabetes status and site-specific cancer mortality. Cox proportional hazards regression models, stratified by study cohort and sex, and adjusted for age, were used to compute hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for those with and without diabetes, as well as for those with various fasting serum glucose levels. Further adjustments were made in multivariable models which included a priori potential confounding variables: BMI, height [35], education, smoking status, and alcohol use at baseline. Statistical significance of effect modification across groups defined by geographical area (Asia and Australasia) and sex were tested using the likelihood ratio test [30]. Differences between region and sex were tested for statistical significance using likelihood ratio tests. The PAFs for mortality from site-specific cancer mortality due to diabetes were calculated for each of the countries in the APCSC using previously published prevalence estimates of diabetes that were adjusted to a world standard population [36] by the formula [30]:
To explore the possibility of participants with a pre-existing malignancy (e.g. pancreatic cancer) at time of study entry contributing to the analyses, and potentially attenuating the diabetes-cancer relation (i.e. reverse causality), deaths from cancer in the first two years of follow-up were excluded (“left censored”) in a sensitivity analysis. In doing so, we reasoned that most deaths due to sub-clinical malignancy at study entry would have occurred during the first two years.
Trends were explored through analyses of fasting serum glucose levels according to tertiles (< 4.8 mmol/L; 4.8-5.4 mmol/L; > 5.4 mmol/L). Trends were tested for statistical significance using likelihood ratio tests. All statistical analyses were performed using STATA version 10.1 for Windows (StataCorp, College Station, Texas).
RESULTS
A total of 44 studies involving 600,443 participants were recruited to the APCSC by the end of 2006 [31]. Figure 1 shows the selection process of the analytical sample for this study. Information on diabetes at baseline and site-specific cancer mortality was available from 36 of the 44 eligible studies involving 367,361 participants, of whom 41% were female, 74% were Asian, and 6.4% had type 2 diabetes mellitus at study entry (Table 1). A summary of the characteristics of the included studies are shown in Table 1. The median follow-up time was 4.0 years and the mean age of participants was 48 years. Participants from the Australasian cohorts were older than those from Asian cohorts. During mortality follow up, a total of 2,223,958 person-years of follow-up gave rise to 17,413 deaths of which 5,992 were ascribed to cancer (31% female, 51% Asian).
Figure 1. Selection of Analytical Sample.
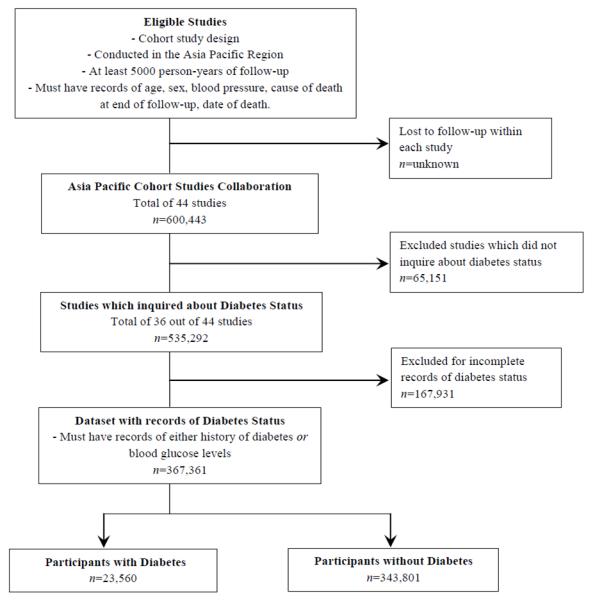
Table 1. Summary Characteristics of Participating Studies* from the Asia Pacific Cohort Studies Collaboration (APCSC).
| Cohort Name | Country | n | Median follow-up (years) | Range of follow-up (years) | Female (%) | Age (years) mean SD | Diabetes Mellitus (%) | No. total cancer deaths | |
|---|---|---|---|---|---|---|---|---|---|
| Australasia | |||||||||
| ALSA | Australia | 1,557 | 4.9 | 0.04-9.04 | 47.7 | 78 | 6 | 8.2 | 68 |
| ANHF | Australia | 9,272 | 8.3 | 0.15-8.63 | 51.0 | 43 | 13 | 1.9 | 153 |
| Busselton | Australia | 5,976 | 23.5 | 0.51-35.50 | 52.2 | 46 | 17 | 3.5 | 602 |
| Canberra | Australia | 712 | 9.2 | 0.04-13.27 | 45.3 | 77 | 5 | 6.6 | 100 |
| Fletcher Challenge | New Zealand | 10,366 | 5.8 | 0.02-7.33 | 28.0 | 44 | 15 | 2.6 | 135 |
| Melbourne | Australia | 41,286 | 8.5 | 0.02-11.39 | 58.9 | 55 | 9 | 5.4 | 1112 |
| Newcastle | Australia | 3,462 | 4.7 | 0.10-10.25 | 50.0 | 53 | 11 | 3.5 | 83 |
| Perth | Australia | 10,222 | 14.4 | 0.12-19.56 | 48.3 | 45 | 13 | 2.1 | 310 |
| WA AAA Screenees | Australia | 12,203 | 3.2 | 0.03-4.71 | 0.0 | 72 | 4 | 11.6 | 400 |
| Subtotal | 95,056 | 8.2 | 0.02-35.50 | 42.4 | 57 | 14 | 5.0 | 2963 | |
| Asia | |||||||||
| Aito Town | Japan | 1,717 | 15.2 | 0.73-16.96 | 56.7 | 51 | 9 | 2.7 | 62 |
| Akabane | Japan | 1,828 | 11.0 | 0.45-12.92 | 55.7 | 54 | 8 | 2.5 | 57 |
| Anzhen | China | 4,122 | 3.0 | 0.72-3.00 | 51.0 | 47 | 8 | 11.1 | 0 |
| Beijing Aging | China | 2,092 | 4.8 | 0.01-4.99 | 50.6 | 70 | 9 | 24.7 | 48 |
| CISCH | China | 2,162 | 3.3 | 0.24-3.90 | 51.0 | 44 | 7 | 2.4 | 3 |
| Civil Service | |||||||||
| Workers | Japan | 9,319 | 6.7 | 0.13-7.78 | 32.7 | 47 | 5 | 1.7 | 61 |
| CVDFACTS | Taiwan | 5,730 | 6.0 | 0.08-9.44 | 55.3 | 47 | 15 | 2.7 | 65 |
| East Beijing | China | 1,128 | 17.1 | 1.02-20.49 | 51.4 | 44 | 15 | 5.6 | 20 |
| EGAT | Thailand | 3,131 | 11.4 | 0.09-12.39 | 23.3 | 43 | 5 | 2.4 | 43 |
| Fangshan | China | 821 | 2.7 | 1.75-3.58 | 67.6 | 47 | 9 | 7.1 | 4 |
| Guangzhou | China | 5,796 | 7.9 | 1.17-13.55 | 34.2 | 44 | 7 | 10.5 | 35 |
| Occupational | |||||||||
| Hong Kong | Hong Kong | 2,953 | 2.5 | 0.04-5.04 | 57.1 | 79 | 7 | 8.6 | 127 |
| Huashan | China | 1,649 | 2.9 | 0.34-3.71 | 54.4 | 53 | 11 | 13.0 | 4 |
| Kinmen | Taiwan | 2,453 | 2.9 | 0.09-5.28 | 48.7 | 63 | 10 | 8.8 | 41 |
| KMIC | South Korea | 183,581 | 4.0 | 0.01-5.00 | 37.0 | 44 | 7 | 7.7 | 1236 |
| Konan | Japan | 1,226 | 6.4 | 0.02-10.42 | 55.4 | 52 | 16 | 12.6 | 26 |
| Miyama | Japan | 1,072 | 6.6 | 0.15-8.06 | 55.8 | 61 | 10 | 5.1 | 36 |
| Ohasama | Japan | 2,240 | 4.1 | 0.08-5.29 | 63.8 | 60 | 11 | 10.9 | 30 |
| Saitama | Japan | 3,624 | 11.0 | 0.06-12.00 | 62.2 | 55 | 12 | 1.7 | 147 |
| Seven Cities Cohorts | China | 10,731 | 2.7 | 0.04-11.50 | 54.5 | 54 | 12 | 1.2 | 174 |
| Shibata | Japan | 2,349 | 20.0 | 0.07-20.00 | 57.7 | 57 | 11 | 1.1 | 208 |
| Shigaraki Town | Japan | 3,757 | 4.4 | 0.07-6.44 | 59.5 | 57 | 14 | 7.2 | 55 |
| Shirakawa | Japan | 4,640 | 17.5 | 0.13-20.51 | 54.3 | 48 | 12 | 0.9 | 165 |
| Singapore Heart | Singapore | 2,325 | 14.6 | 0.14-16.31 | 49.0 | 41 | 13 | 11.4 | 35 |
| Singapore NHS92 | Singapore | 3,305 | 6.2 | 0.09-6.32 | 51.8 | 39 | 12 | 9.7 | 22 |
| Tanno/Soubetsu | Japan | 1,973 | 16.4 | 0.42-18.92 | 53.2 | 51 | 7 | 7.2 | 86 |
| Yunnan | China | 6,581 | 4.5 | 0.02-5.18 | 3.1 | 56 | 9 | 0.5 | 239 |
| Subtotal | 272,305 | 4.0 | 0.01-20.51 | 49.9 | 47 | 10 | 6.9 | 3029 | |
| Total | 367,361 | 4.0 | 0.01-35.50 | 41.3 | 48 | 12 | 6.4 | 5992 | |
ALSA = Australian Longitudinal Study of Aging; ANHF = Australian National Heart Foundation; WA AAA Screenees = Western Australian AAA Screenees; CISCH = Capital Iron and Steel Company Hospital; EGAT = Electricity Generating Authority of Thailand; KMIC = Korean Medical Insurance Corporation; NHS92 = National Health Study 1992; CVDFACTS = Cardiovascular Disease Risk Factors Two-Township Study
Restricted to studies and participants with information on history of diabetes or blood glucose levels at baseline and site-specific cancer mortality.
Study baseline characteristics
Of the 367,361 participants, 23,560 (24% female, 80% Asian) were classified as having diabetes at baseline. Both Asian and Australasian participants with diabetes were older, had higher levels of BMI, systolic blood pressure, total cholesterol, and triglycerides in both Asia and Australasia. Those from either region with diabetes were also more likely to be male, physically inactive, and have lower levels of education.
Outcomes
The age-adjusted, sex and study stratified, HR for death from all cancers was 1.23 (95% CI: 1.12, 1.35) for individuals with diabetes compared with individuals without diabetes (Table 2). This remained largely unchanged after two year left-censoring (HR 1.19, 95% CI 1.06, 1.32). Additional adjustment for BMI, height, education, smoking, and alcohol use had no material effect on the magnitude of the diabetes-cancer association. Analysis of the diabetes-cancer association by sex and region showed no evidence of any difference (p-value for interaction > 0.1). For mortality from specific cancers, diabetes was associated with an increased risk of cancers of the liver (HR 1.51, 95% CI: 1.19, 1.91) and pancreas (HR 1.78, 95% CI: 1.20, 2.65), compared with those without diabetes (Table 2). These also persisted after two year left-censoring: HR 1.52 (95% CI: 1.15, 2.01) and 1.66 (95% CI: 1.04, 2.63), respectively. An increase in the risk of mortality from colorectal cancer was also observed both before and after left-censoring: HR 1.32 (95% CI: 0.98, 1.78) and HR 1.34 (95% CI: 0.96, 1.87), respectively.
Table 2. Hazard ratio for diabetes in relation to causes of mortality.
| Site-Specific Cancer Mortality | APCSC (n=367,361) |
|
|---|---|---|
| No .of deaths | §Hazard Ratio (95% CI) | |
| Bladder | 105 | 1.42 (0.70, 2.86) |
| Brain | 168 | 0.96 (0.51, 1.79) |
| Breast | 299 | 0.75 (0.39, 1.47) |
| Colorectum | 596 | 1.32 (0.98, 1.78) ** |
| Kidney | 75 | 0.64 (0.23, 1.80) |
| Leukaemia | 167 | 1.18 (0.67, 2.06) |
| Liver | 561 | 1.51 (1.19, 1.91) * |
| Lung | 1227 | 0.88 (0.69, 1.13) |
| Melanoma | 82 | 1.60 (0.76, 3.37) |
| Multiple Myeloma | 65 | 1.89 (0.80, 4.47) |
| Non-Hodgkin’s Lymphoma | 161 | 1.00 (0.55, 1.82) |
| Ovarian & Uterine | 148 | 0.63 (0.23, 1.71) |
| Pancreas | 254 | 1.78 (1.20, 2.65) * |
| Prostate | 284 | 1.27 (0.84, 1.93) |
| Stomach | 662 | 1.17 (0.89, 1.54) |
| Upper Aero-Digestive Tract | 266 | 1.04 (0.67, 1.63) |
|
| ||
| All Cancers | 5992 | 1.23 (1.12, 1.35)* |
Hazard ratios are age-adjusted, sex and study stratified.
p-value < 0.01
p-value = 0.07
Additional analyses were performed for diabetes at baseline among individuals with cancer mortality with at least 8 years of follow-up. Information on diabetes at baseline and site-specific cancer mortality was reduced to 15 of the 44 eligible studies involving 95,979 participants, of whom 53% were female, 30% were Asian, and 4.4% had type 2 diabetes mellitus at study entry (Table 1a). The new median follow-up time was 9.1 years and the mean age of participants was 50 years. During this longer mortality follow up, 3,135 deaths were ascribed to cancer.
Table 1a. Summary Characteristics of Asia Pacific Cohort Studies Collaboration (APCSC) Participating Studies* with follow-up of 8 years or greater.
| Cohort Name | Country | n | Median follow-up (years) | Range of follow-up (years) | Female (%) | Age (years) mean SD | Diabetes Mellitus (%) | No. total cancer deaths | |
|---|---|---|---|---|---|---|---|---|---|
| Australasia | |||||||||
| ANHF | Australia | 9,272 | 8.3 | 0.15-8.63 | 51.0 | 43 | 13 | 1.9 | 153 |
| Busselton | Australia | 5,976 | 23.5 | 0.51-35.50 | 52.2 | 46 | 17 | 3.5 | 602 |
| Canberra | Australia | 712 | 9.2 | 0.04-13.27 | 45.3 | 77 | 5 | 6.6 | 100 |
| Melbourne | Australia | 41,286 | 8.5 | 0.02-11.39 | 58.9 | 55 | 9 | 5.4 | 1112 |
| Perth | Australia | 10,222 | 14.4 | 0.12-19.56 | 48.3 | 45 | 13 | 2.1 | 310 |
| Subtotal | 67,468 | 8.5 | 0.02-35.50 | 55.5 | 51 | 12 | 4.2 | 2277 | |
| Asia | |||||||||
| Aito Town | Japan | 1,717 | 15.2 | 0.73-16.96 | 56.7 | 51 | 9 | 2.7 | 62 |
| Akabane | Japan | 1,828 | 11.0 | 0.45-12.92 | 55.7 | 54 | 8 | 2.5 | 57 |
| East Beijing | China | 1,128 | 17.1 | 1.02-20.49 | 51.4 | 44 | 15 | 5.6 | 20 |
| EGAT | Thailand | 3,131 | 11.4 | 0.09-12.39 | 23.3 | 43 | 5 | 2.4 | 43 |
| Guangzhou | China | 5,796 | 7.9 | 1.17-13.55 | 34.2 | 44 | 7 | 10.5 | 35 |
| Occupational | |||||||||
| Saitama | Japan | 3,624 | 11.0 | 0.06-12.00 | 62.2 | 55 | 12 | 1.7 | 147 |
| Shibata | Japan | 2,349 | 20.0 | 0.07-20.00 | 57.7 | 57 | 11 | 1.1 | 208 |
| Shirakawa | Japan | 4,640 | 17.5 | 0.13-20.51 | 54.3 | 48 | 12 | 0.9 | 165 |
| Singapore Heart | Singapore | 2,325 | 14.6 | 0.14-16.31 | 49.0 | 41 | 13 | 11.4 | 35 |
| Tanno/Soubetsu | Japan | 1,973 | 16.4 | 0.42-18.92 | 53.2 | 51 | 7 | 7.2 | 86 |
| Subtotal | 28,511 | 11.4 | 0.06-20.51 | 47.7 | 48 | 11 | 4.8 | 858 | |
| Total | 95,979 | 9.1 | 0.02-35.50 | 53.2 | 50 | 12 | 4.4 | 3135 | |
ANHF = Australian National Heart Foundation; EGAT = Electricity Generating Authority of Thailand
Restricted to studies with median follow-up of ≥ 8.0 years and participants with information on history of diabetes or blood glucose levels at baseline and site-specific cancer mortality.
The age-adjusted, sex and study stratified HR for death from all cancers was 1.31 (95% CI: 1.13, 1.51) for individuals with diabetes compared with individuals without diabetes (Table 2a). Additional adjustment for BMI, height, education, smoking, and alcohol use had no substantial effect on the magnitude of the diabetes-cancer association. For mortality from specific cancers, diabetes was associated with an increased risk of cancers of the colorectum (HR 1.50, 95% CI: 1.03, 2.18), liver (HR 2.14, 95% CI: 1.08, 4.25), and pancreas (HR 1.85, 95% CI: 1.03, 3.31) compared with those without diabetes (Table 2a). In addition, diabetes was associated with an increased risk of cancers of the stomach among those with longer follow-up period (HR 1.90, 95% CI: 1.17, 3.08).
Table 2a. Hazard ratio for diabetes in relation to causes of mortality for studies with follow-up of 8 years or greater.
| Site-Specific Cancer Mortality |
#APCSC (n=95,979) |
|
|---|---|---|
| No .of deaths | §Hazard Ratio (95% CI) | |
| Bladder | 63 | 1.39 (0.55, 3.51) |
| Brain | 103 | 0.98 (0.39, 2.43) |
| Breast | 226 | 0.69 (0.30, 1.56) |
| Colorectum | 404 | 1.50 (1.03, 2.18)* |
| Kidney | 47 | 0.78 (0.19, 3.25) |
| Leukaemia | 98 | 0.70 (0.25, 1.92) |
| Liver | 93 | 2.14 (1.08, 4.25)* |
| Lung | 493 | 1.15 (0.80, 1.67) |
| Melanoma | 65 | 2.11 (0.94, 4.71) |
| Multiple Myeloma | 47 | 2.21 (0.85, 5.76) |
| Non-Hodgkin’s Lymphoma | 86 | 0.97 (0.39, 2.44) |
| Ovarian & Uterine | 106 | 0.75 (0.24, 2.39) |
| Pancreas | 161 | 1.85 (1.03, 3.31)* |
| Prostate | 205 | 1.32 (0.79, 2.22) |
| Stomach | 291 | 1.90 (1.17, 3.08)** |
| Upper Aero-Digestive Tract | 122 | 1.13 (0.52, 2.46) |
|
| ||
| All Cancers | 3135 | 1.31 (1.13, 1.51)*** |
Restricted to studies with median follow-up of ≥ 8.0 years and participants with information on history of diabetes or blood glucose levels at baseline and site-specific cancer mortality.
Hazard ratios are age-adjusted, sex and study stratified.
p-value < 0.05
p-value = 0.01
p-value< 0.0001
Association between fasting serum glucose levels and mortality from cancer
Fasting serum glucose levels were available from 202,681 participants with a total of 1490 cancer deaths of whom 8% (16,439/202,682) were classified as having diabetes. Based on this smaller sub-sample, there were no significant linear associations between glucose levels and all-cause cancer mortality (p for trend = 0.39; Table 3) or with any of the site-specific cancers. There was however, some evidence of a weak positive association with liver cancer (p-value for trend = 0.06) (Table 3). Analysis of trend in those who died from melanoma and multiple myeloma were not possible due to insufficient numbers.
Table 3. Age-adjusted, sex and study stratified hazard ratios of cancer mortality with respect to fasting serum glucose levels in Asia Pacific Cohort Studies Collaboration (APCSC).
| Site-Specific Cancer Mortality | Adjusted§ Hazard Ratio (95% CI) |
*P-value for trend | |||||
|---|---|---|---|---|---|---|---|
| Fasting Serum Blood Glucose Levels | |||||||
| n | 1st Tertile (< 4.8 mmol/L) | n | 2nd Tertile (4.8-5.4 mmol/L) | n | 3rd Tertile (> 5.4 mmol/L) | ||
| Bladder | 4 | 1.0 (ref) | 4 | 0.73 (0.18, 2.97) | 2 | 0.33 (0.06, 1.86) | 0.20 |
| Brain | 13 | 1 | 17 | 1.25 (0.60,2.60) | 10 | 0.84 (0.36, 1.96) | 0.72 |
| Breast | 22 | 1 | 20 | 1.02 (0.55, 1.90) | 7 | 0.67 (0.27, 1.65) | 0.47 |
| Colorectum | 26 | 1 | 29 | 0.89 (0.52, 1.54) | 19 | 0.64 (0.35, 1.20) | 0.17 |
| Kidney | 5 | 1 | 3 | 0.46 (0.11, 1.95) | 7 | 1.02 (0.31, 3.30) | 0.86 |
| Leukaemia | 14 | 1 | 11 | 0.74 (0.33, 1.63) | 10 | 0.78 (0.34, 1.79) | 0.53 |
| Liver | 116 | 1 | 120 | 0.98 (0.76, 1.26) | 152 | 1.26 (0.98, 1.61) | 0.06 |
| Lung | 86 | 1 | 63 | 0.63 (0.45, 0.87) | 74 | 0.75 (0.54, 1.03) | 0.08 |
| Non-Hodgkin’s Lymphoma | 10 | 1 | 9 | 0.82 (0.33, 2.04) | 7 | 0.72 (0.26, 1.98) | 0.52 |
| Ovarian & Uterine | 11 | 1 | 15 | 1.58 (0.71, 3.50) | 7 | 1.39 (0.51, 3.77) | 0.42 |
| Pancreas | 21 | 1 | 18 | 0.72 (0.38, 1.36) | 21 | 0.87 (0.47, 1.63) | 0.68 |
| Prostate | 6 | 1 | 6 | 0.45 (0.14, 1.43) | 10 | 0.62 (0.22, 1.73) | 0.52 |
| Stomach | 90 | 1 | 94 | 1.02 (0.76, 1.36) | 91 | 1.06 (0.79, 1.44) | 0.68 |
| Upper Aero-Digestive Tract | 25 | 1 | 25 | 0.87 (0.49, 1.51) | 33 | 1.05 (0.62, 1.80) | 0.81 |
|
| |||||||
| All Cancers | 487 | 1 | 473 | 0.90 (0.94, 1.28) | 530 | 1.06 (0.93, 1.20) | 0.39 |
p-values from Likelihood Ratio Test
Hazard ratios are age-adjusted, sex and study stratified.
Hazard ratios & 95% CI cannot be estimated due to an insufficient number of events
Population attributable fractions of cancer mortality due to diabetes
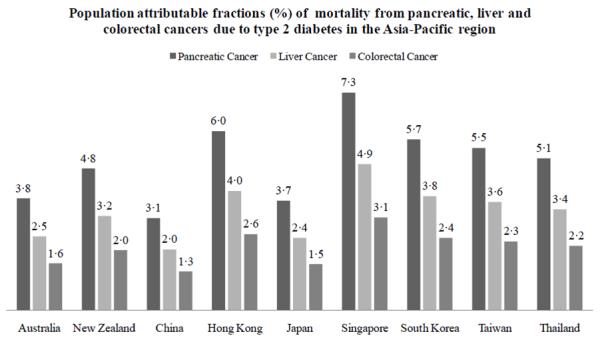
Figure 2 shows the PAF of mortality from specific cancers that seem to have notable associations with diabetes in the APCSC: pancreas, liver, and colorectum. These PAFs differed substantially across the Asia-Pacific region, and were generally higher for Asia than Australasia (Figure 2). Overall, the PAF ranged from 3.1% to 7.3% for pancreatic cancer, 2.0% to 4.9% for liver cancer, and 1.3% to 3.1% for colorectal cancer.
Figure 2. Estimated population attributable fractions for mortality from pancreatic, liver, and, colorectal cancers due to diabetes in the Asia-Pacific region.
DISCUSSION
To our knowledge, the present study is the first to systematically examine the associations between diabetes and mortality from specific cancers for the diverse populations of the Asia-Pacific region. The results from our large collaborative study indicate that individuals with diabetes have an approximately 20% greater risk of mortality from all-cause cancer compared with those without the condition. Specifically, diabetes is independently associated with mortality from pancreatic, liver and, possibly, colorectal cancer. These associations did not vary by region or by sex, and were adjusted for BMI, height, education, smoking, and alcohol use. The majority of cases of diabetes in this report are likely to be type 2 diabetes but an undetermined proportion may have diabetes secondary to pancreatic disease and some will have type 1 diabetes especially in the non-Asian cohorts.
We found a nearly 78% increased risk of death from pancreatic cancer for those with diabetes, which is comparable with other reports [6, 13, 14, 16, 20, 37], and a previous meta-analysis [38]. Other studies have also shown abnormal glucose metabolism to be associated with pancreatic cancer mortality [12, 37]. Though our findings suggest diabetes to be a risk factor for pancreatic cancer, the diabetic state is also a potential consequence of pancreatic malignancy [24, 38-41].
The earlier meta-analysis reported a 50% lower excess risk ratio of pancreatic cancer for individuals with greater than five year history of diabetes, compared with those with a shorter duration of diabetes [38]. Recent studies have also shown that onset of diabetes mellitus of less than two years of duration was more prevalent for patients with pancreatic cancer and, therefore, more likely to be induced by malignancy [24, 39]. Our finding of a 50% increased risk of liver cancer mortality for those with diabetes, compared with those without, is consistent with previous reports [6, 13-15, 42]. Our results also showed a marginally non-significant trend in risk of liver cancer mortality from fasting serum glucose levels (p-value for trend = 0.06). Previous studies [6, 14, 43-45] as well as a meta-analysis [46] have shown increased risk of colorectal cancer mortality for those with diabetes as suggested in the present study as a possible 30% increased risk of colorectal cancer mortality upon participants with diabetes.
Plausible mechanisms
It has been suggested that the increased risk of cancer mortality for those with type 2 diabetes might reflect metabolic and hormonal changes of compensatory hyperinsulinemia, and elevated levels of insulin-like growth factors (IGFs) in response to reduced insulin sensitivity [8, 37, 47-49]. In this regard, IGF-1 has been shown to stimulate cellular proliferation in the pancreas, liver, and colon [48-50]. In addition, increased levels of circulating insulin may activate IGF-1 receptor and thereby promote cellular growth and cell cycle progression [8, 37, 47]. Glucose-lowering therapy, such as exogenous insulin, has been shown to increase cancer risk in a large retrospective cohort study [51]. In these diabetic patients, glucose lowering therapies with sulphonylurea drugs or insulin were associated with increased cancer risk, as compared to treatment with metformin. In the subjects on insulin, the hazard ratio for solid tumour incidence was 1.42 and for pancreatic cancers 4.63, these conditions occurring in < 2.0% and < 0.2% of cases respectively. These were a multiplicity of confounders and no causal association can be assumed [51]. The present study, however, did not have information regarding glucose-lowering medications and our reported results may misrepresent the strength of the overall diabetes-cancer association. Similar to the diabetes-pancreatic cancer association, plausible biological mechanisms for the diabetes-liver cancer association may also involve glucose lowering therapy. Donadon et al have reported an approximately three-fold increased risk of hepatocellular carcinoma for individuals with diabetes on insulin or sulphonylurea treatment [52]. Another putative pathway between diabetes and liver cancer is the occurrence of fatty liver disease. Non-alcoholic fatty liver disease can progress to non-alcoholic steatohepatitis, which may develop subsequently into irreversible cirrhosis, and ultimately hepatocellular carcinoma [53].
Strengths and limitations
The key strengths of the APCSC include its prospective design, its capacity to adjust for several possible confounders, and its large sample size, which allows reliable estimates of associations with deaths from rare cancers to be estimated.
One limitation is that information regarding duration of diabetes was not available. Instead, to explore the possibility of reverse causality, the data were two-year left-censored, with a negligible effect on the original estimates. Given the long latency period between diabetes onset and death from cancer, it is possible that left-censoring the data by two years was insufficient to fully eliminate the effects of reverse causality. In this study, the median follow-up time available for analysis was only four years duration so we were unable to explore this issue further. This relatively short median duration of follow-up might not capture mortality from specific cancers that have longer average survival periods. Information regarding cancer incidence, rather than mortality, would be more useful for assessing cancers with low fatality rates (such as prostate cancer) as a smaller proportion of incidence of such cancers would be included in an analysis of cancer mortality alone. There is certainly a distinction between whether diabetes may cause cancer and whether diabetes may increase individual risk of mortality once a particular cancer is acquired – the present study is limited to exploring the latter question as only cancer mortality data were collected. We are, therefore, unable to determine to what extent the increased risk of cancer mortality represents an etiologic role for diabetes or an early manifestation or consequence of cancer.
Another limitation of this study is the diagnostic criteria used for diabetes. While some participants had provided self-reported history of diabetes, approximately two-thirds (65%) of the analytical sample had only laboratory measurements of blood glucose levels to identify their diabetes status. Sole reliance on records of blood glucose levels, without information on self-reported history of diabetes or medication history, might underestimate the true effect of diabetes on cancer mortality as the dataset does not preclude blood glucose levels within normal ranges to be the outcomes of glucose-lowering treatment. The effects of misclassification as such would be conservative and alter the observed effect towards null. However, misclassification of self-reported diabetes may also alter the observed effect away from null as certain participants who reported themselves as ‘non-diabetic’ may have elevated glucose levels if blood samples were not taken from them during the study. Moreover, the effects of glucose-lowering treatment on normalizing blood glucose levels, leading to a misclassification of someone with diabetes as non-diabetic, may be more pronounced among those with diabetes of longer duration, which may have a different relation with cancer mortality than diabetes of shorter duration; thereby making prediction of the direction of misclassification bias difficult. Lastly, some significant associations reported in the present study may be due to chance alone, given the large number of statistical tests performed for 17 specific cancer endpoints.
Our comparisons of glucose categories also have potential misclassification issues. Individuals in the normoglycaemic group could have had diabetes since, for those analyses, we were not able to identify individuals who were treated with glucose-lowering therapy. Such misclassifications will attenuate the dose response relationship between fasting serum glucose levels and liver cancer mortality reported in this study. Finally the possibility of residual confounding remains. We adjusted for BMI but not measures of central obesity which might be more strongly related to some cancers. In particular, heavy alcohol consumption is associated both with diabetes and with cancers of the liver and colorectum, but our measure of alcohol intake was crude and unable to differentiate between amount, type and duration of alcohol consumed.
CONCLUSIONS
The present study adds to the growing body of literature concerning the long-term co-morbidities of diabetes and provides insight into future patterns of diabetes-related cancer mortality. Our findings suggest diabetes is positively associated with cancer mortality for both Asian and Australasian populations. The relative effect of diabetes on the mortality risk from specific cancers for Asian populations is comparable to those for the largely Caucasian populations of Australasia. Given the increasing diabetes epidemic in both regions [54], and if the associations were causal, mortality from pancreatic, liver, and, possibly, colorectal cancers may be expected to rise, given that these cancers had the greatest percentage of deaths explained by diabetes. The large number of people living in Asia, particularly China, suggests this will be a public health problem of importance. Concerted interventions that target the control and reduction of type 2 diabetes in populations of the Asia-Pacific region may have considerable benefits on reducing mortality from cancer, in addition to that from other chronic illnesses.
ACKNOWLEDGMENTS
APCSC Executive Committee: M. Woodward (Chair), R. Huxley, X. Fang, D.F. Gu, Y. Imai, T.H. Lam, W.H. Pan, A. Rodgers, I. Suh, H.C. Kim, H. Ueshima.
Participating Studies and Principal Collaborators in APCSC: Aito Town: A. Okayama, H. Ueshima; H. Maegawa; Akabane: M. Nakamura, N. Aoki; Anzhen02: Z.S. Wu; Anzhen: C.H. Yao, Z.S. Wu; Australian Longitudinal Study of Aging: Mary Luszcz; Australian National Heart Foundation: T.A. Welborn and S.S. Dhaliwal; Beijing Aging: Z. Tang; Beijing Steelworkers: L.S. Liu, J.X. Xie; Blood Donors’ Health: R. Norton, S. Ameratunga, S. MacMahon, G. Whitlock; Busselton: M.W. Knuiman; Canberra-Queanbeyan: H. Christensen; Capital Iron and Steel Company: X.G. Wu; CISCH: J. Zhou, X.H. Yu; Civil Service Workers: A. Tamakoshi; CVDFACTS: W.H. Pan; East Beijing: Z.L. Wu, L.Q. Chen, G.L. Shan; Electricity Generating Authority of Thailand: P. Sritara; Fangshan: D.F. Gu, X.F. Duan; Fletcher Challenge: S. MacMahon, R. Norton, G. Whitlock, R. Jackson; Guangzhou: Y.H. Li; Guangzhou Occupational: T.H. Lam, C.Q. Jiang; Hisayama: Y. Kiyohara, H. Arima, M. Iida; Hong Kong: J. Woo, S.C. Ho; Huashan: Z. Hong, M.S. Huang, B. Zhou; Kinmen: J.L. Fuh; Konan: H. Ueshima, Y. Kita, S.R. Choudhury; KMIC: I. Suh, S.H. Jee, I.S. Kim; Melbourne: G.G. Giles; Miyama: T. Hashimoto, K. Sakata; Newcastle: A. Dobson; Ohasama: Y. Imai, T. Ohkubo, A. Hozawa; Perth: K. Jamrozik, M. Hobbs, R. Broadhurst; Saitama: K. Nakachi; Seven Cities: X.H. Fang, S.C. Li, Q.D. Yang; Shanghai Factory Workers: Z.M. Chen; Shibata: H. Tanaka; Shigaraki Town: Y. Kita, A. Nozaki, H. Ueshima; Shirakawa: H. Horibe, Y. Matsutani, M. Kagaya; Singapore Heart: K. Hughes, J. Lee; Singapore NHS92: D. Heng, S.K. Chew; Six Cohorts: B.F. Zhou, H.Y. Zhang; Tanno/Soubetsu: K. Shimamoto, S. Saitoh; Tianjin: Z.Z. Li, H.Y. Zhang; Western Australia AAA Screenees: P. Norman, K. Jamrozik; Xi’an: Y. He, T.H. Lam; Yunnan: S.X. Yao.
Funding: R.R. Huxley is supported by a Heart Foundation of Australia Career Development Award. A.L.C. Martiniuk is supported by a Canadian Institutes of Health Research Fellowship. E Lam is supported by the Queen’s University Community Medicine and Family Medicine post-graduate medical residency program.
Abbreviations
- APCSC
Asia Pacific Cohort Studies Collaboration
- DM
Diabetes Mellitus
- ICD
International Classification of Diseases
- IFG
Impaired Fasting Glucose
- PAF
Population Attributable Fractions
Footnotes
Conflict of interest: y Roche, for preparation of a paper by Servier, and for speaking at meetings by Servier, AstraZeneca and Pfizer.
REFERENCES
- 1.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 2.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 4.Boffetta P, Hashibe M, La Vecchia C, et al. The burden of cancer attributable to alcohol drinking. Int J Cancer. 2006;119:884–887. doi: 10.1002/ijc.21903. [DOI] [PubMed] [Google Scholar]
- 5.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean men and women.[see comment] JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 7.Grote VA, Becker S, Kaaks R. Diabetes mellitus type 2 - an independent risk factor for cancer? Exp Clin Endocrinol Diabetes. 2010;118:4–8. doi: 10.1055/s-0029-1243193. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, Iwasaki M, Otani T, et al. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Archives of Internal Medicine. 2006;166:1871–1877. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 9.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010;47:87–95. doi: 10.1007/s00592-010-0187-3. [DOI] [PubMed] [Google Scholar]
- 10.Schiel R, Beltschikow W, Steiner T, Stein G. Diabetes, insulin, and risk of cancer. Methods Find Exp Clin Pharmacol. 2006;28:169–175. doi: 10.1358/mf.2006.28.3.985230. [DOI] [PubMed] [Google Scholar]
- 11.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. American Journal of Epidemiology. 2003;157:1092–1100. doi: 10.1093/aje/kwg100. [DOI] [PubMed] [Google Scholar]
- 12.Smith GD, Egger M, Shipley MJ, Marmot MG. Post-challenge glucose concentration, impaired glucose tolerance, diabetes, and cancer mortality in men. American Journal of Epidemiology. 1992;136:1110–1114. doi: 10.1093/oxfordjournals.aje.a116576. [DOI] [PubMed] [Google Scholar]
- 13.Batty GD, Shipley MJ, Marmot M, Smith GD. Diabetes status and post-load plasma glucose concentration in relation to site-specific cancer mortality: findings from the original Whitehall study. Cancer Causes and Control. 2004;15:873–881. doi: 10.1007/s10552-004-1050-z. [DOI] [PubMed] [Google Scholar]
- 14.Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. American Journal of Epidemiology. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 15.Davila JA, Morgan RO, Shaib Y, et al. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calle EE, Murphy TK, Rodriguez C, et al. Diabetes mellitus and pancreatic cancer mortality in a prospective cohort of United States adults.[see comment] Cancer Causes and Control. 1998;9:403–410. doi: 10.1023/a:1008819701485. [DOI] [PubMed] [Google Scholar]
- 17.Adami HO, McLaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes and Control. 1991;2:307–314. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 18.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. Journal of the National Cancer Institute. 1997;89:1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 19.Chow WH, Gridley G, Nyren O, et al. Risk of pancreatic cancer following diabetes mellitus: a nationwide cohort study in Sweden. Journal of the National Cancer Institute. 1995;87:930–931. doi: 10.1093/jnci/87.12.930. [DOI] [PubMed] [Google Scholar]
- 20.Ansary-Moghaddam A, Huxley R, Barzi F, et al. The effect of modifiable risk factors on pancreatic cancer mortality in populations of the Asia-Pacific region. Cancer Epidemiology, Biomarkers and Prevention. 2006;15:2435–2440. doi: 10.1158/1055-9965.EPI-06-0368. [DOI] [PubMed] [Google Scholar]
- 21.Ansary-Moghaddam A, Huxley R, Barzi F, et al. The impact of modifiable risk factors on mortality from prostate cancer in populations of the Asia-Pacific Region. Asian Pacific Journal of Cancer Prevention. 2007;8:199–205. [PubMed] [Google Scholar]
- 22.Ansary-Moghaddam A, Huxley R, Lam TH, et al. The role of lifestyle risk factors on mortality from colorectal cancer in populations of the Asia Pacific Region. Asian Pacific Journal of Cancer Prevention. 2007;8:191–198. [PubMed] [Google Scholar]
- 23.Khan M, Mori M, Fujino Y, et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pacific Journal of Cancer Prevention. 2006;7:253–259. [PubMed] [Google Scholar]
- 24.Kuang TT, Jin da Y, Wang DS, et al. Clinical epidemiological analysis of the relationship between pancreatic cancer and diabetes mellitus: data from a single institution in China. Journal of Digestive Diseases. 2009;10:26–29. doi: 10.1111/j.1751-2980.2008.00359.x. [DOI] [PubMed] [Google Scholar]
- 25.Lopez AD, Begg S, Bos E. Demographic and Epidemiological Characteristics of Major Regions, 1990-2001. In: Lopez AD, Mathers CD, Ezzati M, editors. Global Burden of Disease and Risk Factors, Edition. The World Bank and Oxford University Press; New York: 2006. [Google Scholar]
- 26.Seidell JC. Obesity, insulin resistance and diabetes--a worldwide epidemic. British Journal of Nutrition. 2000;83(Suppl 1):S5–8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran A, Wan Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–418. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 28.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 29.Woodward M, Zhang X, Barzi F, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003;26:360–366. doi: 10.2337/diacare.26.2.360. [DOI] [PubMed] [Google Scholar]
- 30.Woodward M. Epidemiology; Study Design and Data Analysis. Chapman & Hall/CRC; Boca Raton, Florida: 2005. [Google Scholar]
- 31.Woodward M, Barzi F, Martiniuk A, et al. Cohort profile: the Asia Pacific Cohort Studies Collaboration. International Journal of Epidemiology. 2006;35:1412–1416. doi: 10.1093/ije/dyl222. [DOI] [PubMed] [Google Scholar]
- 32.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation.[see comment] Diabetic Medicine. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization . International classification of diseases. Manual of the international statistical classification of diseases, injuries, and causes of death. Ninth Revision. World Health Organization; Geneva, Switzerland: 1977. [Google Scholar]
- 34.Anon. Classification of diseases. 10th revision. Copenhagen; Danish National Board of Health; 1993. [Google Scholar]
- 35.Batty GD, Barzi F, Woodward M, et al. Adult height and cancer mortality in Asia: the Asia Pacific Cohort Studies Collaboration. Ann Oncol. 2009 doi: 10.1093/annonc/mdp363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.International Diabetes Federation . Diabetes Atlas - Prevalence estimates of diabetes 20-79 years, 2007 (adjusted to world population) 2007. [Google Scholar]
- 37.Gapstur SM, Gann PH, Lowe W, et al. Abnormal glucose metabolism and pancreatic cancer mortality.[see comment] JAMA. 2000;283:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 38.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British Journal of Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Permert J, Adrian TE, Jacobsson P, et al. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? American Journal of Surgery. 1993;165:61–66. doi: 10.1016/s0002-9610(05)80405-2. Discussion 66-67. [DOI] [PubMed] [Google Scholar]
- 41.Basso D, Valerio A, Seraglia R, et al. Putative pancreatic cancer-associated diabetogenic factor: 2030 MW peptide. Pancreas. 2002;24:8–14. doi: 10.1097/00006676-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 42.La Vecchia C, Negri E, Decarli A, Franceschi S. Diabetes mellitus and the risk of primary liver cancer. International Journal of Cancer. 1997;73:204–207. doi: 10.1002/(sici)1097-0215(19971009)73:2<204::aid-ijc7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46:595–607. doi: 10.1007/s00125-003-1109-5. [DOI] [PubMed] [Google Scholar]
- 44.Hu FB, Manson JE, Liu S, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women.[see comment] Journal of the National Cancer Institute. 1999;91:542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 45.Will JC, Galuska DA, Vinicor F, Calle EE. Colorectal cancer: another complication of diabetes mellitus? American Journal of Epidemiology. 1998;147:816–825. doi: 10.1093/oxfordjournals.aje.a009534. [DOI] [PubMed] [Google Scholar]
- 46.Huxley RR, Ansary-Moghaddam A, Clifton P, et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 47.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocrine Reviews. 2000;21:215–244. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 48.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. Journal of Surgical Research. 1996;63:310–313. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 49.Macaulay VM. Insulin-like growth factors and cancer. British Journal of Cancer. 1992;65:311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding XZ, Fehsenfeld DM, Murphy LO, et al. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas. 2000;21:310–320. doi: 10.1097/00006676-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 52.Donadon V, Balbi M, Ghersetti M, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World Journal of Gastroenterology. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smedile A, Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9:291–293. [PubMed] [Google Scholar]
- 54.Lee CM, Huxley RR, Lam TH, et al. Prevalence of diabetes mellitus and population attributable fractions for coronary heart disease and stroke mortality in the WHO South-East Asia and Western Pacific regions. Asia Pacific Journal of Clinical Nutrition. 2007;16:187–192. [PubMed] [Google Scholar]


