Abstract
Aims/hypothesis
While there are plausible biological mechanisms linking oral health with cardiovascular disease (CVD) and mortality, to our knowledge, no study has examined this association in a representative population of people with type 2 diabetes.
Methods
We used the Action in Diabetes and Vascular disease: Preterax and Diamicron Modified-Release Controlled Evaluation study, a large, detailed, randomised controlled trial amongst a general population of individuals with type 2 diabetes. A total of 10,958 men and women, aged 55-88 years, with type 2 diabetes participated in a baseline medical examination when they counted their number of natural teeth and reported the number of days that their gums had bled over the preceding year. Study members were followed up for mortality and morbidity experience over 5 years. For the purposes of the present analyses, data from the trial are utilised using a prospective cohort study design.
Results
After control for a range of potential confounding factors, relative to the group with the most teeth (>=22 teeth), the group with no teeth had a marked increased risk of death due to all-causes (hazard ratio; 95% confidence interval: 1.48; 1.24, 1.78), CVD (1.35; 1.05, 1.74) and non-CVD (1.64; 1.26, 2.13). Frequency of bleeding gums was not associated with any of the outcomes of interest. There was no suggestion that either treatment group or gender modified these relationships.
Conclusions/interpretations
In people with type 2 diabetes, oral disease, as indexed by fewer teeth, was related to an increased risk of total mortality, and death due to both CVD and non-CVD.
Keywords: cardiovascular disease, coronary heart disease, epidemiology, oral disease, stroke
Introduction
Bacterial infection was first implicated in the aetiology of cardiovascular disease over a century ago.[1] Although oral disease is the most common type of infectious challenge in humans,[2] it is only in the last twenty years that investigators have explored its relationship with cardiovascular disease (CVD) and mortality in a modest series of studies.[3-9] This association has some plausibility. One possibility is that a local oral bacterial infection may produce systemic effects, leading to an elevation in inflammatory activity which has itself been implicated in atherothrombogenesis.[10;11] An alternative, non-causal explanation is that poor oral health is simply a marker of significant co-morbidity and/or poverty, and it is these confounding variables that are generating the relationship with CVD.
Oral disease is substantially more common in people with type 2 diabetes than the general population.[12] Thus, any long term consequences of oral disease in this group will represent a significant public health burden. Oral disease has recently been linked with an elevated risk of future CVD in individuals with type 2 diabetes.[13] However, that study sampled only Pima Indians[13] so it is unclear if the results are applicable to a general population of people with type 2 diabetes.
Accordingly, we utilise cohort analyses of The Action in Diabetes and Vascular disease: Preterax and Diamicron Modified-Release Controlled Evaluation (ADVANCE) study,[14] a large, detailed, randomised controlled trial amongst a general population of individuals with type 2 diabetes, to examine the relationship of oral health at study induction with subsequent mortality and morbidity experience.
Methods
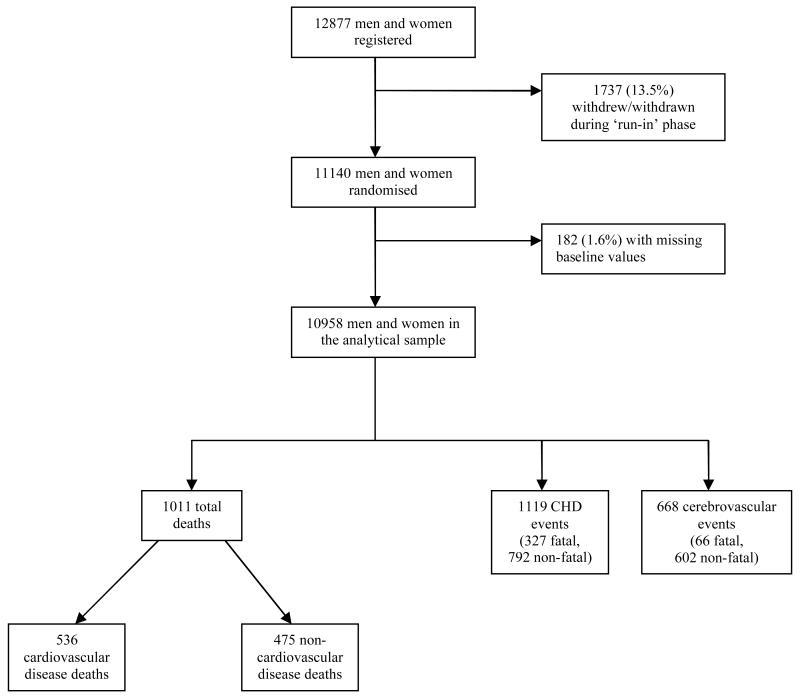
The ADVANCE trial – described in detail elsewhere[14] – was established to investigate the separate effects of routine blood pressure lowering and intensive glucose control on vascular outcomes in people with type 2 diabetes. In brief, between 2001 and 2003, 11,140 men and women aged 55-88 years with type 2 diabetes and a history of major macro- or microvascular disease, or at least one other cardiovascular risk factor, were recruited from 215 centres (20 countries). Using a factorial design, patients were randomised to perindopril-indapamide or placebo, and to intensive glucose control based on Gliclazide MR or to standard glucose control. The flow of subjects through the trial is depicted in figure 1. For the purposes of the present analyses, data from the trial are utilised using a prospective cohort study design; an approach we have taken elsewhere.[15] Approval to conduct the trial was obtained from the ethics committee of each study centre; all participants provided written informed consent.
Figure 1. Flow of study participants through the ADVANCE trial.
At study induction, participants responded to questionnaire enquiries and took part in a medical examination. Individuals with a baseline Mini Mental State Examination[16] score of less than 24, or where dementia was suspected, were referred to a medically-qualified specialist for diagnosis of dementia.[17] Given concerns regarding the accuracy of self-reported information in people who were cognitively challenged, individuals with either a contemporaneous or prior diagnosis of dementia did not enter the study. Glycated haemoglobin (HbA1c), blood cholesterol (and fractions), blood pressure, resting heart rate, and serum creatinine were measured using standard protocols.10 Height and weight were used to derive body mass index (weight[kg]/height[m]2). Research staff administered a series of questions regarding ethnicity, educational attainment, physical activity, alcohol intake, cigarette smoking habit, illicit drug use, major chronic disease, assistance with activities of daily living, and quality of life (‘EuroQol5d’).[18]
Study members responded to two questions concerning the presence of oral disease. During the medical examination, they were asked to count the number of natural teeth in their mouth. Artificial teeth were not included but any tooth, or part of a tooth, that was visible in the mouth and connected to the gum or jawbone was counted as one tooth. Study members also reported the number of days their teeth had bled in the preceding year. This included spontaneous bleeding, bleeding on cleaning the teeth, and bleeding on eating food but not bleeding associated with dental treatment, tooth loss or facial trauma. Lower numbers of natural teeth and higher numbers of days of gum bleeding indicated poorer oral health.
Ascertainment of cardiovascular disease during follow-up
A range of fatal and non-fatal cardiovascular disease outcomes were ascertained using a variety of sources. Information on cause of death (certification, autopsy report, clinical notes) were scrutinised by an independent Endpoint Adjudication Committee and a coding made according to the 10th revision of the International Classification of Diseases.[19] For non-fatal outcomes, where applicable, clinical notes, computed tomography and magnetic resonance imaging reports (for suspected cerebrovascular disease), laboratory biomarkers (e.g., creatine kinase, troponins) and ECG reports (for suspected myocardial infarction) were utilised. A CHD event was denoted by death due to this condition (including sudden death), non-fatal myocardial infarction, silent myocardial infarction, coronary revascularisation, or hospital admission for unstable angina.[20] A cerebrovascular event was denoted by death due to this condition or non-fatal stroke, transient ischaemic attack, or subarachnoid haemorrhage.[20]
Statistical analyses
With 182 study members having at least one item of missing data, the analytical sample comprised 10,958 (figure 1). Data for both markers of oral health were skewed. We therefore created three groups for both number of natural teeth (0; 1-21; ≥22) and days of bleeding gums (0; <12 days; ≥12 days) by taking values above zero and separating at the median. Differences in baseline characteristics across these oral health groups were tested. For categorical variables (e.g., sex), we used the Chi-square test; for continuous variables with normal distribution (e.g., systolic blood pressure) we used an ANOVA; and for continuous variables with a skewed distribution (e.g., Mini Mental State Examination), we used Kruskal-Wallis test.
Having first ascertained that the proportional hazards assumption had not been violated, hazard ratios with accompanying 95% confidence intervals were used to summarise the association between the two markers of oral disease and the various study endpoints.[21] In these analyses, the group with the best oral health (>=22 teeth; 0 days with bleeding gums in the last year) represented the reference categories.
The relation between oral disease and the various health outcomes was first computed separately in the treatment and placebo groups, and in men and women. With no indication that either treatment allocation (p-value for interaction >0.1) or sex (p-value for interaction>0.1) modified the association of either marker of oral disease with any of the outcomes, the data were pooled and all analyses were adjusted for treatment, sex and age. The relation of oral disease with each endpoint was further adjusted for an array of possible confounding factors which, after controlling for basic covariates (age, sex and randomized treatment allocation), were organised by the themes of existing illness (use of Metformin/beta-blockers, history of macrovascular or microvascular disease, or those requiring assistance with daily activities, plus diabetes duration); behavioural CVD risk factors (cigarette smoking, alcohol intake, vigorous physical activity in previous week); physiological CVD risk factors (haemoglobin A1c, Creatinine, BMI, total cholesterol, HDL cholesterol, resting heart rate, SBP, DBP); psychological CVD risk factors (quality of life [EQ-5d score], and Mini Mental State Exam Score); and socio-economic CVD risk factors (age at completion of highest level of education, height). Multiple adjustment was for all these covariates. All analyses performed using SAS version 9.1 (SAS Institute, Inc, Cary, NC).
Results
In table 1 we present baseline characteristics according to the two markers of oral disease. At study entry, around one fifth (21%) of study members reported complete absence of teeth, while 6.5% indicated that their gums had bled for 12 days or more in the preceding year. People with fewer natural teeth generally had less favourable biological, social, behavioural and psychological characteristics at study induction. That is, relative to study members with more teeth, those in the groups with fewer teeth were more likely to be older, Caucasian, less well educated, less physically active, heavier, have elevated systolic blood pressure and serum creatinine, reduced HDL cholesterol, and have marginally poorer cognitive function and quality of life. They were also more likely to smoke cigarettes, report vascular disease, and require assistance with activities of daily living. There was no apparent relationship between number of teeth and either glycosylated haemoglobin or diabetes duration. The association of days of bleeding gums with study characteristics was less clear. On the one hand, people who reported more bleeding days relative to fewer were somewhat younger, better educated, taller, had lower creatinine values, and smoked less; however, they were also marginally heavier, and had a higher diastolic systolic blood pressure and alcohol intake. In general, the magnitude of associations between the two markers of oral disease and the various covariates was modest with statistical significance often reached owing to the high study power.
Table 1. Oral health and baseline characteristics in ADVANCE (N=10,958 men and women).
| Number of natural teeth | P-value | Days of bleeding gums | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| >=22 teetha (n=4476) | 1-21 teeth (n=4174) | 0 teeth (n=2308) | 0 days* (n=9553) | <12 days (n=686) | >=12 days (n=719) | |||
| Age at baseline examination (yr)b | 63.91 (5.9) | 66.25 (6.2) | 68.62 (6.4) | 0.001 | 66.08 (6.4) | 64.16 (6.0) | 63.59 ( 5.93) | 0.001 |
| Age at completion of education (yr) | 19.34 (7.59) | 18.26 (7.2) | 17.02 (6.5) | 0.001 | 18.30 (7.2) | 19.42 (7.93) | 19.45 (7.61) | 0.001 |
| Haemoglobin A1c (%) | 7.53 (1.6) | 7.53 (1.6) | 7.47 (1.5) | 0.18 | 7.50 (1.55) | 7.57 (1.54) | 7.64 (1.62) | 0.012 |
| Height (cm) | 165.6 (9) | 166.0 (9.4) | 165.6 (10.0) | 0.58 | 165.7 (9.43) | 166.0 (9.14) | 166.5 (9.12) | 0.01 |
| Body mass index (kg/m2) | 27.35 (4.8) | 28.88 (5.3) | 29.24 (5.4) | 0.001 | 28.24 (5.17) | 28.42 (5.08) | 29.47 (5.37) | 0.001 |
| Total cholesterol (mmol/L) | 5.20 (1.2) | 5.22 (1.2) | 5.12 (1.1) | 0.02 | 5.19 (1.19) | 5.11 (1.19) | 5.27 (1.25) | 0.41 |
| High Density Lipoprotein (mmol/L) | 1.27 (0.4) | 1.25 (0.3) | 1.24 (0.3) | 0.001 | 1.26 (0.35) | 1.24 (0.36) | 1.25 (0.35) | 0.29 |
| Systolic blood pressure (mmHg) | 142.0 (20.6) | 146.3 (21.6) | 148.4 (22.5) | 0.001 | 145.2 (21.56) | 142.8 (20.63) | 144.4 (21.91) | 0.05 |
| Diastolic blood pressure (mmHg) | 80.42 (10.6) | 81.01 (11.2) | 80.22 (11.1) | 0.95 | 80.48 (10.90) | 80.84 (10.55) | 82.01 (11.48) | 0.001 |
| Resting heart rate (BPM) | 74.58 (11.8) | 74.11 (12.1) | 73.16 (12.5) | 0.001 | 74.12 (12.09) | 74.59 (12.21) | 73.40 (11.69) | 0.32 |
| Serum creatinine (umol/L) | 84.42 (25.5) | 87.52 (26.1) | 89.05 (23.7) | 0.001 | 86.87 (25.98) | 85.08 (21.28) | 84.10 (20.57) | 0.001 |
| Cognitive function (MMSE) | 28.79 (1.8) | 28.37 (1.9) | 28.20 (2.1) | 0.001 | 28.50 (1.92) | 28.56 (1.77) | 28.61 (1.73) | 0.08 |
| Quality of life (EQ-5d) | 0.84 (0.2) | 0.81 (0.2) | 0.80 (0.2) | 0.001 | 0.82 (0.19) | 0.81 (0.20) | 0.79 (0.21) | 0.001 |
| Diabetes duration (years) | 7.89 (6.1) | 8.01 ( 6.5) | 7.89 (6.5) | 0.82 | 7.97 (6.40) | 7.98 (5.93) | 7.43 (6.22) | 0.05 |
| No. occasions exercise ≤15 mins/week | 3.78 (6.4) | 3.18 (5.6) | 3.00 (5.0) | 0.001 | 3.39 ( 5.68) | 3.23 ( 5.98) | 3.54 (7.33) | 0.72 |
| Current number of drinks/week | 2.92 (8.16) | 3.07 (7.82) | 3.00 (7.4) | 0.57 | 2.92 (7.8) | 3.61 (8.2) | 3.35 (8.9) | 0.035 |
| Femalec | 1795 (40.1) | 1751 (42.0) | 1099 (47.6) | 0.001 | 4063 (42.5) | 271 (39.5) | 311 (43.3) | 0.27 |
| Caucasian/European ethnicity | 1712 (38.2) | 2917 (69.9) | 1920 (83.2) | 0.001 | 5677 (59.4) | 399 (58.2) | 473 (65.8) | 0.001 |
| Current cigarettes smoker | 554 (12.4) | 613 (14.7) | 351 (15.2) | 0.004 | 1362 (14.3) | 95 (13.8) | 61 (8.5) | 0.001 |
| Use of Metformin or Beta-blocker | 3120 (69.7) | 2973 (71.2) | 1645 (71.3) | 0.22 | 6766 (70.8) | 482 (70.3) | 490 (68.2) | 0.24 |
| Require assistance with daily activities | 94 (2.1) | 141 (3.4) | 133 (5.8) | 0.001 | 324 (3.4) | 16 (2.3) | 28 (3.9) | 0.24 |
| History of major macrovascular disease | 1339 (29.9) | 1385 (33.2) | 800 (34.7) | 0.001 | 3064 (32.1) | 213 (31.0) | 247 (34.4) | 0.36 |
| History of major microvascular disease | 426 (9.5) | 448 (10.7) | 271 (11.7) | 0.013 | 998 (10.4) | 80 (11.7) | 67 (9.3) | 0.36 |
| History of major diabetic disease | 309 (6.9) | 298 (7.1) | 180 (7.8) | 0.40 | 688 (7.2) | 51 (7.4) | 48 (6.7) | 0.84 |
better oral health
results presented here and below are mean (SD)
results presented here and below are N (%)
In table 2, hazards ratios for the two indicators of oral health (number of teeth and bleeding gums) in relation to total mortality and various CVD outcomes during follow-up are depicted. In the simplest model (age-, sex-, and treatment-adjusted), the group with no teeth experienced almost twice the risk of total mortality (hazard ratio; 95% CI: 1.78; 1.50, 2.11) relative to those with 22 teeth or more. This effect was incremental across the teeth groups (p for trend: <0.0001) such that people with an intermediate number of teeth had intermediate risk (1.41; 1.20, 1.65). Controlling separately for a series of covariates had very little impact on these effects estimates, however, adding all potentially confounding factors simultaneously to the multivariable model did lead to some attenuation, although statistical significance at conventional levels (p<0.05) was retained. When fatal and non-fatal CHD (combined) events were the outcome of interest, the strength of the association with number of teeth in age-, sex-, and treatment-adjusted analyses, while again inverse, was of lower magnitude than that evident for the analyses featuring total mortality. Controlling for individual risk factors again had little impact on this gradient, but in the multiple adjusted analyses the association was eliminated. There was no apparent link between number of teeth and cerebrovascular disease (largely comprising stroke) in any of our analyses.
Table 2. Hazard ratio (95% confidence intervals) for the relation of baseline oral health with health outcomes in ADVANCE (N=10,958 men and women).
| Adjustments | Number of natural teeth | P-trend | Days of bleeding gums/year | P-trend | ||||
|---|---|---|---|---|---|---|---|---|
| >=22* (n=4476) | 1-21 (n=4174) | 0 (n=2308) | 0 days* (n=9553) | <12 days (n= 686) | >=12 days (n= 719) | |||
| Total mortality (1011 deaths) | ||||||||
| Age, sex + treatment (‘base’ model) | 1 (ref) | 1.41 (1.20, 1.65) | 1.78 (1.50, 2.11) | 0.001 | 1 (ref) | 1.03 (0.79, 1.35) | 0.92 (0.69, 1.22) | 0.67 |
| Base + ethnicity | 1 | 1.44 (1.23, 1.69) | 1.84 (1.54, 2.20) | 0.001 | 1 | 1.03 (0.79, 1.35) | 0.92 (0.69, 1.22) | 0.66 |
| Base + quality of life | 1 | 1.36 (1.16, 1.59) | 1.70 (1.44, 2.02) | 0.001 | 1 | 1.02 (0.78, 1.34) | 0.87 (0.66, 1.16) | 0.45 |
| Base + existing illness | 1 | 1.36 (1.16, 1.59) | 1.70 (1.44, 2.02) | 0.001 | 1 | 1.00 (0.76, 1.31) | 0.94 (0.70, 1.25) | 0.69 |
| Base + behavioural CVD risk factors | 1 | 1.38 (1.18, 1.62) | 1.73 (1.46, 2.05) | 0.001 | 1 | 1.05 (0.80, 1.37) | 0.95 (0.71, 1.26) | 0.86 |
| Base + physiological CVD risk factors | 1 | 1.36 (1.16, 1.59) | 1.71 (1.44, 2.03) | 0.001 | 1 | 1.05 (0.80, 1.38) | 0.93 (0.70, 1.24) | 0.77 |
| Base + psychological CVD risk factors | 1 | 1.33 (1.14, 1.56) | 1.66 (1.40, 1.97) | 0.001 | 1 | 1.03 (0.79, 1.35) | 0.88 (0.66, 1.17) | 0.47 |
| Base + socio-economic CVD risk factors | 1 | 1.40 (1.20, 1.63) | 1.76 (1.48, 2.08) | 0.001 | 1 | 1.06 (0.81, 1.38) | 0.95 (0.71, 1.26) | 0.86 |
| Multiple adjusted | 1 | 1.24 (1.05, 1.46) | 1.48 (1.24, 1.78) | 0.001 | 1 | 1.08 (0.82, 1.41) | 0.96 (0.72, 1.28) | 1.00 |
| All CHD events (1119 events) | ||||||||
| Age, sex + treatment (‘base’ model) | 1 (ref) | 1.27 (1.11, 1.46) | 1.38 (1.17, 1.62) | 0.001 | 1 (ref) | 1.21 (0.96, 1.52) | 1.07 (0.84, 1.36) | 0.26 |
| Base + ethnicity | 1 | 1.17 (1.01, 1.35) | 1.22 (1.03, 1.45) | 0.02 | 1 | 1.21 (0.96, 1.52) | 1.04 (0.81, 1.32) | 0.38 |
| Base + quality of life | 1 | 1.24 (1.08, 1.42) | 1.32 (1.13, 1.55) | 0.001 | 1 | 1.19 (0.95, 1.50) | 1.03 (0.81, 1.31) | 0.44 |
| Base + existing illness | 1 | 1.21 (1.05, 1.39) | 1.26 (1.07, 1.49) | 0.003 | 1 | 1.22 (0.97, 1.54) | 1.08 (0.85, 1.38) | 0.22 |
| Base + behavioural CVD risk factors | 1 | 1.28 (1.11, 1.47) | 1.39 (1.18, 1.64) | 0.001 | 1 | 1.21 (0.96, 1.52) | 1.06 (0.83, 1.35) | 0.29 |
| Base + physiological CVD risk factors | 1 | 1.20 (1.05, 1.38) | 1.25 (1.06, 1.47) | 0.004 | 1 | 1.23 (0.98, 1.54) | 1.06 (0.83, 1.35) | 0.27 |
| Base + psychological CVD risk factors | 1 | 1.23 (1.07, 1.41) | 1.31 (1.12, 1.54) | 0.001 | 1 | 1.19 (0.95, 1.50) | 1.03 (0.81, 1.31) | 0.44 |
| Base + socio-economic CVD risk factors | 1 | 1.27 (1.11, 1.46) | 1.37 (1.16, 1.61) | 0.001 | 1 | 1.22 (0.97, 1.54) | 1.09 (0.85, 1.39) | 0.20 |
| Multiple adjusted | 1 | 1.24 (0.98, 1.56) | 1.04 (0.81, 1.32) | 0.34 | 1 | 1.24 (0.98, 1.56) | 1.04 (0.81, 1.32) | 0.34 |
| All cerebrovascular disease events (668 events) | ||||||||
| Age, sex + treatment (‘base’ model) | 1 (ref) | 1.10 (0.92, 1.31) | 0.93 (0.75, 1.15) | 0.68 | 1 (ref) | 1.18 (0.88, 1.60) | 1.08 (0.79, 1.47) | 0.39 |
| Base + ethnicity | 1 | 1.34 (1.12, 1.60) | 1.26 (1.00, 1.58) | 0.02 | 1 | 1.19 (0.88, 1.61) | 1.15 (0.84, 1.58) | 0.21 |
| Base + quality of life | 1 | 1.07 (0.90, 1.28) | 0.90 (0.73, 1.12) | 0.47 | 1 | 1.17 (0.87, 1.59) | 1.05 (0.77, 1.43) | 0.51 |
| Base + existing illness | 1 | 1.06 (0.89, 1.27) | 0.88 (0.71, 1.09) | 0.35 | 1 | 1.19 (0.88, 1.60) | 1.08 (0.79, 1.48) | 0.38 |
| Base + behavioural CVD risk factors | 1 | 1.09 (0.92, 1.30) | 0.93 (0.75, 1.15) | 0.66 | 1 | 1.19 (0.88, 1.61) | 1.08 (0.79, 1.48) | 0.38 |
| Base + physiological CVD risk factors | 1 | 1.12 (0.94, 1.33) | 0.95 (0.77, 1.19) | 0.87 | 1 | 1.22 (0.90, 1.64) | 1.13 (0.82, 1.54) | 0.25 |
| Base + psychological CVD risk factors | 1 | 1.06 (0.89, 1.26) | 0.89 (0.72, 1.10) | 0.39 | 1 | 1.18 (0.87, 1.59) | 1.05 (0.77, 1.43) | 0.51 |
| Base + socio-economic CVD risk factors | 1 | 1.10 (0.92, 1.31) | 0.93 (0.75, 1.15) | 0.68 | 1 | 1.20 (0.89, 1.62) | 1.11 (0.81, 1.52) | 0.20 |
| Multiple adjusted | 1 | 1.24 (1.03, 1.49) | 1.10 (0.87, 1.38) | 0.29 | 1 | 1.24 (0.91, 1.67) | 1.16 (0.84, 1.58) | 0.18 |
| CVD mortality (536 deaths) | ||||||||
| Age, sex + treatment (‘base’ model) | 1 (ref) | 1.53 (1.24, 1.89) | 1.67 (1.32, 2.12) | 0.001 | 1 (ref) | 1.21 (0.86, 1.70) | 1.01 (0.69, 1.48) | 0.62 |
| Base + ethnicity | 1 | 1.58 (1.27, 1.97) | 1.76 (1.37, 2.27) | 0.001 | 1 | 1.21 (0.86, 1.71) | 1.01 (0.69, 1.47) | 0.63 |
| Base + quality of life | 1 | 1.46 (1.18, 1.80) | 1.58 (1.25, 2.00) | 0.001 | 1 | 1.20 (0.85, 1.69) | 0.95 (0.65, 1.39) | 0.87 |
| Base + existing illness | 1 | 1.44 (1.16, 1.78) | 1.55 (1.22, 1.96) | 0.001 | 1 | 1.16 (0.83, 1.64) | 1.04 (0.71, 1.52) | 0.58 |
| Base + behavioural CVD risk factors | 1 | 1.51 (1.22, 1.87) | 1.66 (1.30, 2.10) | 0.001 | 1 | 1.22 (0.87, 1.73) | 1.02 (0.70, 1.49) | 0.57 |
| Base + physiological CVD risk factors | 1 | 1.47 (1.19, 1.82) | 1.60 (1.26, 2.04) | 0.001 | 1 | 1.24 (0.88, 1.76) | 1.02 (0.70, 1.49) | 0.55 |
| Base + psychological CVD risk factors | 1 | 1.43 (1.16, 1.77) | 1.54 (1.21, 1.95) | 0.001 | 1 | 1.21 (0.86, 1.71) | 0.95 (0.65, 1.39) | 0.85 |
| Base + socio-economic CVD risk factors | 1 | 1.52 (1.23, 1.89) | 1.66 (1.31, 2.11) | 0.001 | 1 | 1.24 (0.88, 1.76) | 1.05 (0.72, 1.53) | 0.46 |
| Multiple adjusted | 1 | 1.32 (1.06, 1.65) | 1.35 (1.05, 1.74) | 0.02 | 1 | 1.28 (0.91, 1.81) | 1.04 (0.71, 1.52) | 0.47 |
| Non-CVD mortality (475 deaths) | ||||||||
| Age, sex + treatment (‘base’ model) | 1 (ref) | 1.28 (1.02, 1.61) | 1.90 (1.49, 2.42) | 0.001 | 1 (ref) | 0.83 (0.54, 1.28) | 0.81 (0.52, 1.27) | 0.25 |
| Base + ethnicity | 1 | 1.29 (1.01, 1.63) | 1.93 (1.49, 2.49) | 0.001 | 1 | 0.84 (0.55, 1.29) | 0.81 (0.52, 1.26) | 0.24 |
| Base + quality of life | 1 | 1.24 (0.99, 1.57) | 1.84 (1.45, 2.35) | 0.001 | 1 | 0.83 (0.54, 1.27) | 0.79 (0.51, 1.22) | 0.19 |
| Base + existing illness | 1 | 1.27 (1.01, 1.60) | 1.88 (1.48, 2.40) | 0.001 | 1 | 0.81 (0.53, 1.25) | 0.82 (0.53, 1.27) | 0.24 |
| Base + behavioural CVD risk factors | 1 | 1.24 (0.99, 1.57) | 1.82 (1.42, 2.32) | 0.001 | 1 | 0.85 (0.55, 1.31) | 0.86 (0.55, 1.34) | 0.37 |
| Base + physiological CVD risk factors | 1 | 1.24 (0.98, 1.56) | 1.84 (1.44, 2.35) | 0.001 | 1 | 0.85 (0.55, 1.30) | 0.83 (0.53, 1.28) | 0.29 |
| Base + psychological CVD risk factors | 1 | 1.23 (0.97, 1.55) | 1.81 (1.42, 2.30) | 0.001 | 1 | 0.83 (0.54, 1.28) | 0.79 (0.51, 1.22) | 0.19 |
| Base + socio-economic CVD risk factors | 1 | 1.26 (1.00, 1.59) | 1.86 (1.46, 2.37) | 0.001 | 1 | 0.85 (0.55, 1.31) | 0.83 (0.53, 1.29) | 0.29 |
| Multiple adjusted | 1 | 1.15 (0.91, 1.47) | 1.64 (1.26, 2.13) | 0.001 | 1 | 0.86 (0.56, 1.33) | 0.86 (0.56, 1.35) | 0.40 |
Of the 1119 CHD events, 327 were fatal and 792 non-fatal; of the 668 cerebrovascular events, 66 were fatal and 602 non-fatal. All analyses are adjusted for age, sex and randomized treatment allocation. Existing illness: comprises one or more of the following: use of Metformin/beta-blockers, history of macrovascular or microvascular disease, or those requiring assistance with daily activities, plus diabetes duration; Behavioural CVD risk factors: Cigarette smoking, alcohol intake, vigorous physical activity in previous week; Physiological CVD risk factors: Haemoglobin A1c, Creatinine, BMI, total cholesterol, HDL cholesterol, resting heart rate, SBP, DBP; Psychological CVD risk factors: Quality of life (EQ-5d score), and Mini Mental State Exam Score; Socio-economic CVD risk factors: Age at completion of highest level of education, height; Multiple adjusted: All above covariates.
better oral health
Men and women with fewer teeth experienced an elevated risk of death from both CVD and non-CVD in age-, sex-, and treatment-adjusted analyses. Although these gradients were weakened after control for potential confounding factors, particularly for CVD deaths, they remained robust to full adjustment and there was again evidence of a dose-response effect. In none of our analyses did days of bleeding gums show any relationship with the five study outcomes.
In analyses using age rather than calendar time as the time scale, our Cox models revealed the same results as those described above. While there were too few events to stratify by each of the 215 study Centres, we were able to do so by the 5 regions (Australasia and south east Asia, Canada, China, Europe – continental, Europe – Northern) in which each Centre was located. There was no suggestion that this modified the impact of oral disease on any of the outcomes.
Discussion
The main finding of this study was that, following adjustment for a range of confounding variables, oral disease, as indexed by a lower number of teeth, was associated with total mortality and mortality ascribed to both CVD and non-CVD, such that the highest risk was apparent in men and women who reported the fewest teeth. The association between a lower number of teeth and CHD was evident in most analyses but was lost on multiple adjustment. Our other marker of oral disease – days of bleeding gums – was unrelated to any of these outcomes. This may be because few people reported any gum bleeding, so limiting statistical power, or that, in comparison to tooth loss, gum bleeding does not capture oral disease severe enough to yield an effect on the study endpoints. It is also plausible that enquiring about bleeding gums over the preceding 12 months is asking too much of even the most attentive study member. A relationship between tooth loss and an increased risk of non-CVD mortality was also apparent in our analyses. Given that this outcome partially comprises malignancies, some which have been linked with inflammatory markers,[22] this association does not rule out systemic inflammation as the causal process linking oral disease with CVD.
Alternative (non-causal) explanations
The two most likely alternative explanations for the observation that having fewer teeth is related to an excess disease risk are reverse causality and confounding. Although the prospective design of this cohort study largely rules out reverse causality, it is plausible that some participants entered the study with oral disease caused by existing CVD (and associated risk factors) – either diagnosed or hidden – and this generated a positive oral disease-CVD gradient. We examine this issue in two ways: first, we excluded study members with diagnosed CVD at study induction and repeated our analyses; second, we dropped individuals who registered events in the first 2 years of follow-up and again repeated our analyses. The latter approach is based on the assumption that people entering the study with CVD or other important but occult co-morbidity would have been most likely to die from their condition in the early stages of follow-up. In both cases our results were essentially unchanged (results available upon request).
The apparent detrimental effect of poor oral health on these outcomes was generally robust to the adjustment of a wide range of covariates (cardiovascular disease risk factors, psychological wellbeing, socioeconomic adversity) which have been implicated in the aetiology of our disease endpoints, although some attenuation of risk was evident. Since marked attenuation following adjustment was apparent, and given the association with coronary heart disease became non-significant with full statistical control, it is possible, as in all observational studies, that the gradients we found would be explained by unmeasured covariates even in this well characterised study, or perhaps more precise measurements of existing ones. In a related point, an alternative approach to examine the link between oral disease and mortality in type 2 diabetes would be extended follow-up for CVD events in large scale randomised controlled trials of treatments for oral disease where confounding is not a concern.
That our results may not be completely ascribed to the above alternative explanations, at least signals the possibility that tooth loss may be mechanistically linked to both CVD and non-CVD deaths. Reduced masticatory capacity impairs nutritional intake and this may in turn be a risk factor for CVD.[23] We did not collect data on dietary intake with which to explore this possibility of mediation, however, in, to our knowledge, the only study to capture information on food intake, adjusting for this behaviour did not eliminate the association between oral health and coronary artery disease.[24] As described, inflammation resulting from poor oral health has also been implicated in the aetiology of CVD,[10] although, again, we did not have data on markers of systemic inflammation to test such a hypothesis.
Study strengths and limitations
While this study has several strengths – large sample size, high number of events, and the sampling of a general population of type 2 diabetics – it is not without its shortcomings. That the measures of oral health were both self-reported raises concerns regarding validity. While the enquiry regarding tooth loss is widely used in the field of dental epidemiology,[9] the measure of gums bleeding is less common. However, having decided a priori to investigate the association of the latter with CVD and other health outcomes, we did not want to omit it from our manuscript simply because the results were null. This would lead to publication bias, a major problem in modern epidemiology.[25]
In conclusion, in the present study of people with type 2 diabetes, oral disease, as indexed by fewer teeth, was related to an increased risk of total mortality, and death due to CVD and non-CVD.
Acknowledgments and Funding
The ADVANCE trial was funded by grants from Servier and the National Health and Medical Research Council of Australia. These sponsors had no role in the design of the study, data collection, data analysis, data interpretation, and the writing of the manuscript. Study data were not made available to the sponsors. The Management Committee, whose membership did not include any sponsor representatives, had final responsibility for the decision to submit this manuscript for publication. The authors had full access to the study data and take responsibility for the accuracy of the analysis. The Medical Research Council (MRC) Social and Public Health Sciences Unit receives funding from the UK MRC and the Chief Scientist Office at the Scottish Government Health Directorates. David Batty is a Wellcome Trust Career Development Fellow (WBS U.1300.00.006.00012.01). Sébastien Czernichow holds a Fellowship awarded by the Institut Servier-France and Assistance Publique – Hôpitaux de Paris, France.
Duality of interest: John Chalmers holds research grants from Servier; John Chalmers, Bruce Neal, Anushka Patel, Sophia Zoungas and Mark Woodward have received lecturing fees from Servier.
Abbreviations
- CVD
cardiovascular disease
- CHD
coronary heart disease
References
- 1.Osler W. Diseases of the arteries. In: Osler W, editor. Modern medicine: its theory and practice in original contributions by americans and foreign authors. 4 ed. Lead and Fabiger; Philadelphia, Pa: 1908. [Google Scholar]
- 2.Marcus SE, Drury TF, Brown LJ, Zion GR. Tooth retention and tooth loss in the permanent dentition of adults: United States, 1988-1991. J Dent.Res. 1996;75(Spec No):684–95. doi: 10.1177/002203459607502S08. [DOI] [PubMed] [Google Scholar]
- 3.Genco R, Offenbacher S, Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am.Dent.Assoc. 2002;133(Suppl):14S–22S. doi: 10.14219/jada.archive.2002.0375. [DOI] [PubMed] [Google Scholar]
- 4.Janket SJ, Baird AE, Chuang SK, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg.Oral Med.Oral Pathol.Oral Radiol.Endod. 2003;95:559–69. doi: 10.1067/moe.2003.107. [DOI] [PubMed] [Google Scholar]
- 5.Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev.Oral Biol.Med. 2004;15:403–13. doi: 10.1177/154411130401500606. [DOI] [PubMed] [Google Scholar]
- 6.Mattila KJ, Pussinen PJ, Paju S. Dental infections and cardiovascular diseases: a review. J Periodontol. 2005;76:2085–8. doi: 10.1902/jop.2005.76.11-S.2085. [DOI] [PubMed] [Google Scholar]
- 7.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 8.Geismar K, Stoltze K, Sigurd B, Gyntelberg F, Holmstrup P. Periodontal disease and coronary heart disease. J Periodontol. 2006;77:1547–54. doi: 10.1902/jop.2006.050405. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen.Intern.Med. 2008;23:2079–86. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahekar AA, Singh S, Saha S, Molnar J, Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am.Heart J. 2007;154:830–7. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Dave S, Van DT. The link between periodontal disease and cardiovascular disease is probably inflammation. Oral Dis. 2008;14:95–101. doi: 10.1111/j.1601-0825.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 12.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull.World Health Organ. 2005;83:661–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27–32. doi: 10.2337/diacare.28.1.27. [DOI] [PubMed] [Google Scholar]
- 14.The ADVANCE Collaborative group Study rationale and design of ADVANCE: action in diabetes and vascular disease--preterax and diamicron MR controlled evaluation. Diabetologia. 2001;44:1118–20. doi: 10.1007/s001250100612. [DOI] [PubMed] [Google Scholar]
- 15.Kengne AP, Czernichow S, Huxley R, et al. Blood Pressure Variables and Cardiovascular Risk. New Findings From ADVANCE. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.109.133041. [DOI] [PubMed] [Google Scholar]
- 16.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–91. [PubMed] [Google Scholar]
- 17.American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychological Association; Washington DC: 1994. [Google Scholar]
- 18.The EuroQol Group EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 19.Anon . International Statistical Classification of Diseases and Related Health Problems (10th revision) WHO; Geneva: 1992. [Google Scholar]
- 20.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N.Engl.J.Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life-tables. J R Stat Soc [Ser B] 1972;34:187–220. [Google Scholar]
- 22.Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin.Pharmacol.Ther. 2010;87:401–6. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 23.Mann JI. Diet and risk of coronary heart disease and type 2 diabetes. Lancet. 2002;360:783–9. doi: 10.1016/s0140-6736(02)09901-4. [DOI] [PubMed] [Google Scholar]
- 24.Geerts S, Legrand V, Charpentier J, et al. Further evidence of the association between periodontal conditions and coronary artery disease. J Periodontol. 2004;75:1274–80. doi: 10.1902/jop.2004.75.9.1274. [DOI] [PubMed] [Google Scholar]
- 25.Davey Smith G. Reflections on the limitations to epidemiology. Journal of Clinical Epidemiology. 2001;54:325–31. doi: 10.1016/s0895-4356(00)00334-6. [DOI] [PubMed] [Google Scholar]