Abstract
Background
The observation that taller people experience an increased risk of selected cancers is largely restricted to Caucasian cohorts. These associations may plausibly differ in Asian populations. For the first time, we make direct comparison of the associations between height and a series of malignancies in Australasian (Caucasian) and Asian populations.
Methods
Analyses were based on the Asia Pacific Cohort Studies Collaboration of 506, 648 male and female study participants (408,381 Asia, 98267 Australasia) drawn from 38 population-based cohort studies. Cox proportional hazards regression was used to estimate the relationship between height and cancer rates.
Results
A total of 3,272,600 person years of follow-up gave rise to 7497 cancer deaths (5232 in men, 2265 in women). After multiple adjustments and left censoring, taller individuals experienced increased rates of carcinoma of the intestine (men and women); all cancers, liver, lung, breast, ‘other’ malignancies (all women); and prostate and bladder (men). No consistent regional (Asia vs. Australasia) or sex-differences were observed.
Conclusions
In the present study, taller men and women had an elevated risk of selected malignancies. These associations did not differ appreciably between Asian and Caucasian populations.
Keywords: Asia, body height, stature, cancer, malignancy
Introduction
Over the last decade there has been a revitalisation of interest in the early life origins of chronic disease in later life.(1) With a scarcity of studies offering extended follow-up of children with which to explore these associations, investigators have instead examined the influence of adult proxies of early life exposures. One of the most commonly used is height which, although typically measured in middle-age and inevitably under a large degree of genetic control, may nonetheless capture a combination of pre-adult illness, nutrition, socio-economic status, and psychosocial stress.(2)
In a series of population-based studies, taller people has been shown to experience a lower risk of coronary heart disease (CHD).(3-8) As the cohorts on which these observations are based have matured, investigators have recently been able to examine the association between height and a malignancy sub-types. Typically, the opposite gradient to that apparent for CHD is reported such that taller people have an elevated risk of carcinoma in general, and breast (in women), prostate, colorectal cancer, and melanoma in particular.(9) Various mechanisms have been advanced to explain the apparent relationship,(10) including: increased stature is an indicator of childhood overfeeding;(9) stature is a proxy for cell numbers, so raising the possibility of dividing stem cells undergoing transformation to malignancy in taller persons;(11) and positive associations between height and insulin-like growth factors (IGF), which are themselves determinants of selected cancers.(12)
Conclusions about the association between stature and malignancies other than those described above are not currently possible either because results are inconsistent across studies (e.g., renal, pancreas) or, with few exceptions,(13) there is a paucity of data for specific endpoints (e.g., bladder, haematopoietic).(9) Further, methodological shortcomings hamper interpretation of data in many height-cancer studies. These include an absence of multivariable analyses to ascertain the impact of confounding variables;(14) a low number of cancer events, so reducing statistical power to detect what are typically modest height-cancer effects; and a focus on one type of malignancy which limits conclusions about specificity of association(15) and therefore insights into causality. A preponderance of case-control studies also raises the possibility of biased estimates. That is, in these studies, height is assessed after the occurrence of cancer which may have led to osteoporotic vertical collapse (i.e., ‘shrinkage’). This is likely to result in an artifactual elevation of cancer risk in shorter study members and therefore an underestimation of the true magnitude of any height-cancer relationship.
Finally, and most importantly, most analyses of the association of height with cancer risk are restricted to Western cohorts, with very few studies of Asian people.(16;17) There are prima facie reasons to anticipate that height may have different relationships with cancer risk in Asia. First, in Western cohorts, relative to shorter study members, taller individuals have a lower prevalence of cigarette smoking and heavy alcohol consumption,(18) both risk factors for selected cancers. In Asian populations, however, these associations are less consistent, particularly in men.(16) Second, western populations are characterised by different body frames, environmental exposures, genetic background, and socio-economic conditions in comparison to Asians. To our knowledge, no previous study has had the capacity to examine this issue by making direct comparison of height-cancer gradients in Asian versus Western populations.
The Asia Pacific Cohort Studies Collaboration (APCSC) is a large scale collaborative project consisting of over 600,000 participants from over 40 prospective cohort studies drawn from Asia and Australia or New Zealand (‘Western’-style populations). In addition to allowing us to contrast the height-malignancy association in these ethnically diverse populations, we are also able to address the afore-described methodological shortcomings and modest evidence base.
Methods
Details of the 44 studies that comprise APCSC have been described elsewhere.(19;20) In brief, a study was eligible for inclusion if it met the following criteria: 1) the population was drawn from the Asia Pacific region; 2) it was of prospective cohort study design; 3) it had accumulated at least 5000 person-years of follow-up; 4) date of birth (or age), sex, and blood pressure recorded at baseline; 5) date of death or age at death recorded during follow-up.
At baseline, height and weight were ascertained from direct measurement; body mass index was computed using the standard formulae (weight [kg]/height2 [m]). Study members also responded to enquiries regarding cigarette smoking habits (current smoker/non-smoker) and educational attainment (none/not completed primary; completed primary [age 10 years]; completed secondary [age 17/18 years]; or completed tertiary). Cohorts were classified as Asian if the participants were recruited from mainland China, Hong Kong, Japan, Korea, Singapore, Taiwan or Thailand; and as Australasian if the participants were drawn from Australia or New Zealand. This classification largely represented a dichotomy by ethnicity into Asians and non-Asians.
Cancer outcomes
Cancer mortality was classified according to the ninth(21) or tenth(22) revision of the International Classification of Diseases: stomach (ICD-9; ICD-10: 151; C16); large intestine or colorectum (153-154; C18-21); liver (155, 197.7; C22, C78.7); pancreas (157; C25); lung (162; C33-34); melanoma (172; C43); female breast (174; C50); ovary and uterus (179-183; C53-56); prostate (185; C61); bladder (188; C67); kidney (189; C64-65); brain and central nervous system (191-192; C70-72); and leukaemia (204-208; C91-95). Malignancies of the upper aero-digestive tract (UADT) were analysed by combining cancer of the oropharynx, oesophagus, and larynx.
Statistical analyses
Only those participants aged ≥20 years at the time of the baseline survey with complete data on age, ethnicity, year of birth, sex, height, and death due to specific malignancies were included in analyses. This resulted in an analytical sample of 506, 648 (328, 405 men, 178, 243 women) drawn from 38 of the 44 eligible studies.(19) Their characteristics are presented in table 1.
Table 1. Summary of study characteristics in the Asia Pacific Cohort Studies Collaboration.
| Study | N | Baseline year(s) | Median follow-up | % female | Age (yr) | Height (cm) | No. total cancer deaths | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||||||
| mean | SD | mean | SD | mean | SD | |||||||
| ALSA | 1566 | 92 - 93 | 4.7 | 48 | 78 | 6 | 169 | 7 | 156 | 6 | 44 | 23 |
| ANHF | 9271 | 89 - 90 | 8.3 | 51 | 43 | 13 | 175 | 7 | 162 | 6 | 102 | 52 |
| Busselton | 7463 | 66 - 81 | 26.5 | 52 | 45 | 17 | 173 | 7 | 161 | 6 | 395 | 294 |
| Fletcher Challenge | 10344 | 92 - 94 | 5.8 | 28 | 44 | 15 | 176 | 7 | 163 | 7 | 91 | 43 |
| Melbourne | 41267 | 90 - 94 | 8.5 | 59 | 55 | 9 | 172 | 7 | 160 | 7 | 595 | 517 |
| Newcastle | 5927 | 83 - 94 | 8.9 | 50 | 52 | 10 | 173 | 7 | 161 | 6 | 135 | 80 |
| Perth | 10226 | 78 - 94 | 14.4 | 48 | 45 | 13 | 175 | 7 | 162 | 6 | 189 | 122 |
| WA AAA Screenees | 12203 | 96 - 99 | 3.2 | 0 | 72 | 4 | 171 | 7 | - | - | 400 | - |
| Australasia | 98267 | 66-99 | 8.3 | 45 | 53 | 14 | 173 | 7 | 161 | 7 | 1951 | 1131 |
| Aito Town | 1711 | 80 - 83 | 15.2 | 57 | 51 | 9 | 163 | 7 | 151 | 6 | 38 | 24 |
| Akabane | 1834 | 85 - 86 | 11.0 | 56 | 54 | 8 | 161 | 6 | 149 | 5 | 32 | 25 |
| Anzhen | 8377 | 91 | 4.3 | 55 | 54 | 13 | 169 | 6 | 157 | 6 | 36 | 30 |
| Beijing Aging | 2085 | 92 | 4.8 | 51 | 70 | 9 | 165 | 7 | 153 | 6 | 21 | 27 |
| Capital Iron & Steel Company | 5132 | 74 - 80 | 12.5 | 0 | 45 | 8 | 169 | 6 | - | - | 113 | - |
| CISCH | 2166 | 92 - 93 | 3.3 | 51 | 44 | 7 | 170 | 6 | 159 | 6 | 3 | - |
| Civil Service Workers | 9316 | 90 - 92 | 6.7 | 33 | 47 | 5 | 166 | 6 | 154 | 5 | 53 | 8 |
| CVDFACTS | 5716 | 88 - 96 | 6.0 | 55 | 47 | 15 | 166 | 6 | 155 | 6 | 39 | 26 |
| East Beijing | 1087 | 77 - 94 | 17.1 | 52 | 43 | 15 | 171 | 7 | 160 | 6 | 10 | 5 |
| EGAT | 3492 | 85 | 11.4 | 23 | 43 | 5 | 165 | 6 | 155 | 5 | 38 | 5 |
| Fangshan | 2608 | 91 - 92 | 3.6 | 67 | 47 | 10 | 168 | 7 | 156 | 6 | 4 | 4 |
| Guangzhou Occupational | 106180 | 85 - 97 | 7.1 | 11 | 41 | 6 | 169 | 5 | 158 | 5 | 522 | 24 |
| Hisayama | 1569 | 61 | 24.6 | 56 | 56 | 11 | 157 | 6 | 146 | 6 | 110 | 89 |
| Hong Kong | 2881 | 85 - 91 | 2.5 | 57 | 78 | 7 | 162 | 7 | 148 | 7 | 71 | 54 |
| Huashan | 1859 | 90 - 92 | 2.8 | 52 | 53 | 12 | 168 | 7 | 156 | 6 | 2 | 2 |
| Kinmen | 1268 | 93-96 | 2.9 | 47 | 63 | 9 | 166 | 7 | 154 | 6 | 25 | 7 |
| KMIC | 183391 | 92 | 4.0 | 37 | 44 | 7 | 169 | 5 | 157 | 4 | 1021 | 211 |
| Konan | 1194 | 87-95 | 6.4 | 55 | 52 | 16 | 165 | 8 | 152 | 7 | 12 | 13 |
| Miyama | 1034 | 88 - 90 | 6.6 | 55 | 60 | 9 | 160 | 6 | 149 | 6 | 26 | 9 |
| Ohasama | 2196 | 92 - 93 | 4.1 | 63 | 59 | 11 | 161 | 7 | 149 | 6 | 20 | 10 |
| Saitama | 3602 | 86 - 90 | 11.0 | 62 | 54 | 12 | 161 | 7 | 150 | 6 | 94 | 51 |
| Seven Cities Cohorts | 10791 | 87 | 2.7 | 55 | 54 | 12 | 167 | 6 | 156 | 6 | 98 | 77 |
| Shibata | 2335 | 77 | 20.0 | 58 | 57 | 11 | 159 | 6 | 147 | 7 | 122 | 85 |
| Shigaraki Town | 3738 | 91-97 | 4.4 | 59 | 57 | 14 | 164 | 7 | 151 | 7 | 36 | 19 |
| Singapore Heart | 2312 | 82-97 | 14.6 | 49 | 41 | 13 | 166 | 6 | 154 | 6 | 25 | 10 |
| Singapore NHS92 | 3304 | 92 | 6.2 | 52 | 39 | 12 | 168 | 6 | 155 | 6 | 10 | 12 |
| Six Cohorts | 19367 | 82 - 86 | 9.0 | 47 | 45 | 7 | 165 | 6 | 154 | 6 | 240 | 141 |
| Tanno/Soubetsu | 1983 | 77 | 16.4 | 53 | 51 | 7 | 162 | 6 | 151 | 5 | 53 | 37 |
| Tianjin | 9273 | 84 | 6.1 | 51 | 55 | 12 | 168 | 7 | 156 | 7 | 169 | 128 |
| Yunnan | 6580 | 92 | 4.5 | 3 | 56 | 9 | 162 | 6 | 154 | 5 | 238 | 1 |
| Asia | 408381 | 61-97 | 4.0 | 33 | 45 | 10 | 168 | 6 | 156 | 6 | 3281 | 1134 |
| Overall | 506648 | 61-99 | 5.7 | 35 | 48 | 11 | 169 | 6 | 157 | 6 | 5232 | 2265 |
ALSA = Australian Longitudinal Study of Aging; ANHF = Australian National Heart Foundation; WA AAA Screenees = Western Australian AAA Screenees; CISCH = Capital Iron and Steel Company Hospital; EGAT = Electricity Generating Authority of Thailand; KMIC = Korean Medical Insurance Corporation; NHS92 = National Health Study 1992; CVDFACTS = Cardiovascular Disease Risk Factors Tow-Township Study
Using the floating absolute risks technique(23) based on Cox proportional hazards regression, we computed hazard ratios with accompanying for 95% confidence intervals for cancer type in relation to height expressed as both a one standard deviation increase (6 cm in both men and women) and sex-specific tertiles. Initially, hazard ratios were adjusted for age, year of birth and study. We then added other confounding variables to the multivariable models: smoking, educational attainment, and body mass index which, because of its low correlation with height (r = 0.11), did not raise concerns regarding colinearity. All analyses were stratified by gender and region (Asia and Australasia [Australia and New Zealand]) and effect modification tested by adding an interaction term to the multivariable model. To explore the possibility that existing illness at study entry may result in vertical shrinkage and therefore attenuate the height-cancer relation, we excluded cancer deaths occurring in the first three years of follow-up (“left censoring”) and repeated the above-described analyses. In doing so, we reasoned that most deaths due to sub-clinical malignancy at study induction would have occurred during this time frame. All statistical analyses were performed using SAS 9.1 for Windows (SAS Institute Inc., USA).
Results
The mean age of the combined cohorts was 48 years; on average, men (166 cm) were 12 cm taller than women (154 cm). Compared with individuals from Asian cohorts, those from Australasia were generally older and taller (table 1). During mortality surveillance, a total of 3,272,600 person years of follow-up (2,064,115 in men; 1,208,485 in women) gave rise to 21,604 deaths, 7497 of which were ascribed to cancer (5232 in men, 2265 in women). All hazard ratios (95% confidence intervals) presented below are adjusted for age, birth year and study. Additional control for educational attainment, smoking and BMI had essentially no impact on the magnitude of the height-cancer association (results not shown but available upon request).
We present analyses of height in relation to cancer in tables 2 and 3. Taller men had a lower risk of total mortality but an elevated rate of all neoplasms combined. For site-specific carcinomas, taller men and women experienced an increased risk of the intestine, liver (women only), lung, bladder cancer and melanoma (men). However, some confidence intervals arising from these analyses included unity. When we examined if the strength of the height-cancer association differed by region separately in men and women, there was a suggestion that, in women only, stature was positively related to carcinoma of the liver (p-value for interaction: 0.074) and breast (p-value for interaction: 0.044) in women from Australasia, whereas in Asia it was essentially null. The opposite was evident for kidney malignancy, whereby taller Asian women experienced an elevated risk that was not seen in Australasian females.
Table 2. Hazard ratio (95% confidence intervals) for a 1 standard deviation (6 cm) increase in height in relation to selected outcomes – stratified by region and gender.
| Sex | No. of deaths* | Overall (N=506, 648) | Australasia (N=98267) | Asia (N=408,381) | P-value for interaction by region | |
|---|---|---|---|---|---|---|
| All deaths | M F |
14614 6990 |
0.97 (0.95, 0.98) 0.98 (0.96, 1.01) |
0.97 (0.95, 0.99) 0.99 (0.95, 1.02) |
0.96 (0.94, 0.98) 0.98 (0.95, 1.01) |
0.64 0.76 |
| All cancers | M F |
5232 2265 |
1.05 (1.02, 1.08) 1.09 (1.05, 1.14) |
1.06 (1.02, 1.10) 1.11 (1.05, 1.17) |
1.04 (1.00, 1.08) 1.08 (1.01, 1.14) |
0.41 0.46 |
| Intestine cancer | M F |
401 276 |
1.09 (1.00, 1.20) 1.11 (0.99, 1.25) |
1.11 (1.00, 1.24) 1.16 (1.01, 1.33) |
1.05 (0.88, 1.24) 0.99 (0.80, 1.23) |
0.56 0.24 |
| Liver cancer | M F |
800 126 |
0.97 (0.90, 1.04) 1.26 (1.05, 1.52) |
0.84 (0.64, 1.11) 1.78 (1.17, 2.71) |
0.98 (0.90, 1.06) 1.17 (0.96, 1.43) |
0.31 0.074 |
| Lung cancer | M F |
1226 332 |
1.06 (1.00, 1.12) 1.08 (0.97, 1.21) |
1.02 (0.94, 1.11) 1.03 (0.87, 1.21) |
1.09 (1.01, 1.17) 1.13 (0.98, 1.30) |
0.21 0.38 |
| Breast cancer | M F |
** 318 |
1.11 (0.99, 1.24) |
1.18 (1.04, 1.33) |
0.91 (0.73, 1.13) |
0.044 |
| Stomach cancer | M F |
608 233 |
1.04 (0.96, 1.13) 1.11 (0.96, 1.27) |
0.99 (0.83, 1.18) 0.96 (0.69, 1.35) |
1.05 (0.96, 1.15) 1.13 (0.98, 1.32) |
0.56 0.38 |
| Prostate cancer | M F |
274*** | 1.06 (0.95, 1.18) |
1.06 (0.94, 1.18) |
1.12 (0.80, 1.56) |
0.74 |
All hazard ratios are age, study, and year of birth adjusted
number of events overall
too few events to run analyses
not applicable
Table 3. Hazard ratio (95% confidence intervals) for a 1 standard deviation (6 cm) increase in height in relation to selected cancer outcomes – stratified by region and gender.
| Sex | No. of deaths* | Overall (N=506, 648) | Australasia (N=98267) | Asia (N=408,381) | P-value interaction by region | |
|---|---|---|---|---|---|---|
| Pancreas | M F |
194 100 |
1.08 (0.94, 1.24) 0.99 (0.82, 1.21) |
1.18 (0.98, 1.43) 0.97 (0.76, 1.24) |
0.97 (0.80, 1.18) 1.04 (0.76, 1.43) |
0.16 0.72 |
| Leukaemia | M F |
130 59 |
1.03 (0.87, 1.21) 1.01 (0.79, 1.31) |
0.97 (0.79, 1.18) 0.99 (0.75, 1.32) |
1.16 (0.87, 1.54) 1.09 (0.64, 1.86) |
0.30 0.75 |
| Bladder | M F |
97 22 |
1.31 (1.09, 1.58) 1.43 (0.97, 2.13) |
1.35 (1.07, 1.71) 1.59 (1.02, 2.50) |
1.25 (0.93, 1.68) 1.06 (0.50, 2.26) |
0.68 0.30 |
| Brain | M F |
141 57 |
1.06 (0.90, 1.25) 0.96 (0.74, 1.25) |
1.03 (0.85, 1.26) 0.94 (0.69, 1.28) |
1.12 (0.86, 1.46) 1.02 (0.64, 1.65) |
0.62 0.75 |
| Kidney | M F |
67 23 |
1.04 (0.83, 1.31) 1.21 (0.81, 1.83) |
1.07 (0.82, 1.39) 1.02 (0.65, 1.59) |
0.99 (0.65, 1.50) 2.53 (1.11, 5.75) |
0.75 0.053 |
| Ovary/uterus | M F |
*** 195 |
1.08 (0.94, 1.25) |
1.10 (0.91, 1.33) |
1.06 (0.85, 1.31) |
0.78 |
| Melonoma | M F |
63 25 |
1.44 (1.15, 1.79) 1.04 (0.71, 1.52) |
1.46 (1.16, 1.83) 1.04 (0.71, 1.52) |
1.10 (0.42, 2.87)** | 0.57 |
| Upper Aero digestive Tract | M F |
329 79 |
0.93 (0.84, 1.04) 1.16 (0.92, 1.45) |
1.01 (0.85, 1.20) 1.04 (0.70, 1.55) |
0.89 (0.78, 1.02) 1.21 (0.93, 1.57) |
0.26 0.53 |
| Other | M F |
546 228 |
1.02 (0.94, 1.11) 1.11 (0.96, 1.27) |
1.12 (0.97, 1.30) 1.25 (1.00, 1.57) |
0.98 (0.88, 1.08) 1.03 (0.87, 1.23) |
0.13 0.18 |
All hazard ratios are age, study, and year of birth adjusted
number of events overall
too few events to run analyses
not applicable
The results after three years left-censoring are depicted in tables 4 and 5 where we also include the hazards ratios from the total population from the prior table for the purposes of comparison. For several malignancies, there was a suggestion of an increase in the magnitude of the height-malignancy association. This was particularly evident for carcinoma of the intestine (men and women); all cancers, liver, lung, breast, ‘other’ malignancies (all women only); and prostate and bladder (both men). There were too few deaths to explore effects by region in these analyses.
Table 4. Hazard ratio (95% confidence intervals) for a 1 standard deviation (6 cm) increase in height in relation to selected cancer outcomes – overall and with 3 yr left censoring.
| Sex | Overall (N=506,648) | With 3 yr. left censoring (N=482,438) |
|||
|---|---|---|---|---|---|
| No. of deaths | HR (95% CI) | No. of deaths | HR (95% CI) | ||
| All malignancies | M F |
5232 2265 |
1.05 (1.02, 1.08) 1.09 (1.05, 1.14) |
3479 1664 |
1.08 (1.03, 1.14) 1.21 (1.12, 1.30) |
| Intestine | M F |
401 276 |
1.09 (1.00, 1.20) 1.11 (0.99, 1.25) |
293 220 |
1.16 (1.01, 1.33) 1.27 (1.06, 1.52) |
| Liver | M F |
800 126 |
0.97 (0.90, 1.04) 1.26 (1.05, 1.52) |
511 80 |
0.96 (0.83, 1.12) 2.02 (1.32, 3.10) |
| Lung | M F |
1226 332 |
1.06 (1.00, 1.12) 1.08 (0.97, 1.21) |
738 229 |
1.10 (1.00, 1.21) 1.24 (1.04, 1.49) |
| Breast | M F |
* 318 |
1.11 (0.99, 1.24) |
* 228 |
1.23 (1.04, 1.45) |
| Stomach | M F |
608 233 |
1.04 (0.96, 1.13) 1.11 (0.96, 1.27) |
391 |
1.06 (0.87, 1.29) 1.02 (0.72, 1.43) |
| Prostate | M F |
274** | 1.06 (0.95, 1.18) |
207** | 1.16 (0.98, 1.37) |
All hazard ratios are age, study, and year of birth adjusted
too few events to run analyses
not applicab
Table 5. Hazard ratio (95% confidence intervals) for a 1 standard deviation (6 cm) increase in height in relation to selected cancer outcomes – overall and with 3 yr left censoring.
| Sex | Overall (N=506,648) | With 3 yr. left censoring (N=482,438) | |||
|---|---|---|---|---|---|
| No. of deaths | HR (95% CI) | No. of deaths | HR (95% CI) | ||
| Pancreas | M F |
194 100 |
1.08 (0.94, 1.24) 0.99 (0.82, 1.21) |
133 82 |
1.19 (0.95, 1.50) 1.01 (0.73, 1.40) |
| Leukaemia | M F |
130 59 |
1.03 (0.87, 1.21) 1.01 (0.79, 1.31) |
85 48 |
0.94 (0.72, 1.22) 1.11 (0.75, 1.64) |
| Bladder | M F |
97 22 |
1.31 (1.09, 1.58) 1.43 (0.97, 2.13) |
63 * |
1.55 (1.09, 2.19) |
| Brain | M F |
141 57 |
1.06 (0.90, 1.25) 0.96 (0.74, 1.25) |
103 45 |
0.93 (0.72, 1.20) 0.72 (0.46, 1.12) |
| Kidney | M F |
67 23 |
1.04 (0.83, 1.31) 1.21 (0.81, 1.83) |
44 20 |
1.04 (0.83, 1.31) 1.45 (0.80, 2.62) |
| Ovary/uterus | M F |
** 195 |
1.08 (0.94, 1.25) |
* 59 |
1.29 (1.00, 1.65) |
| Melonoma | M F |
63 25 |
1.44 (1.15, 1.79) 1.04 (0.71, 1.52) |
43* | 1.23 (0.89, 1.68) |
| Upper Aero digestive Tract | M F |
329 79 |
0.93 (0.84, 1.04) 1.16 (0.92, 1.45) |
212 57 |
0.95 (0.78, 1.15) 1.26 (0.74, 2.15) |
| Other | M F |
546 228 |
1.02 (0.94, 1.11) 1.11 (0.96, 1.27) |
424 168 |
1.15 (0.96, 1.37) 1.44 (1.13, 1.85) |
All hazard ratios are age, study, and year of birth adjusted
too few events to run analyses
not applicable
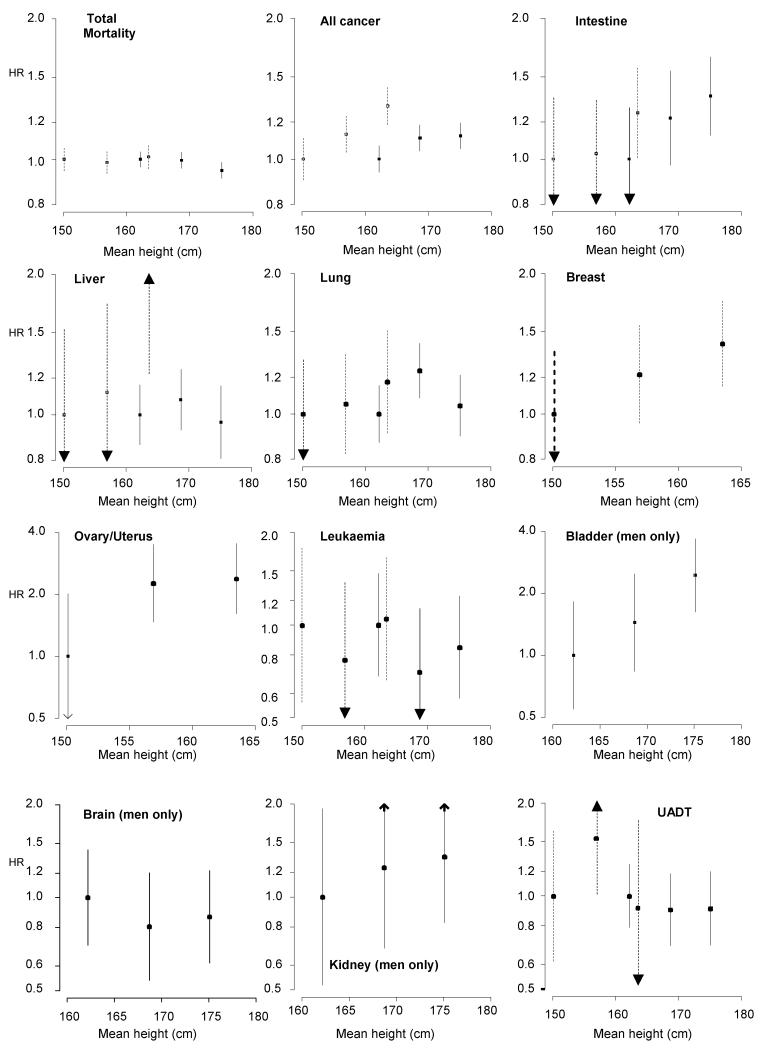
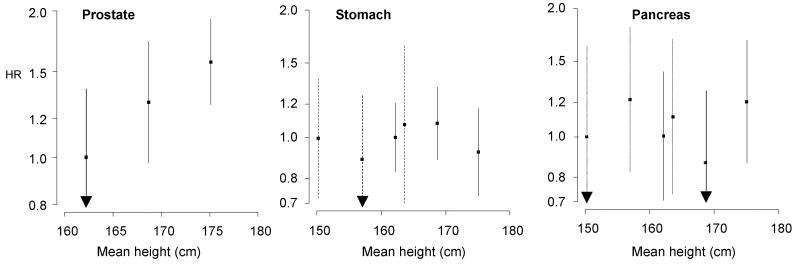
Finally, we examined the ‘shape’ of relation stature-cancer association by categorising height into tertiles. These results are depicted in figure 1 and are also based on the 3 year left-censored analyses. There was evidence of a positive stepwise effect for height in relation to carcinoma of the intestine (men only), lung (women), breast, bladder (men), kidney (men) and prostate.
Figure 1. Hazard ratios (95% confidence intervals) for the relation of height tertiles with cancer outcomes.
Dotted lines denote women, solid lines men. Results are missing from these graphs either because there were too few events (breast in men; bladder, brain and kidney in women) or the endpoints were not applicable to men (ovary/uterus) or women (prostate). All hazard ratios are age, study, and year of birth adjusted.
Discussion
The main findings of this large scale prospective cohort study – the first to explore differences in height-cancer effects according to Asian/non-Asian countries – was that taller individuals experienced increased rates of cancer of carcinoma of the intestine (men and women); all cancers, liver, lung, breast, ‘other’ malignancies (all women); and prostate and bladder (men). Contrary to our expectations, there was no consistent suggestion that these effects varied by region, or indeed sex. Several height-cancer associations increased in magnitude after left censoring. In general, the magnitude of the height-cancer relationships, though statistically significant at conventional levels, was modest and therefore concordant with existing literature. Thus, on comparing the highest height tertile with the lowest, there was, on average around a 50% increased risk of dying from cancer.
Alternative explanations
Alternative, non-causal explanations for the height-cancer gradient include chance, confounding and reverse causality. Given that this large cohort study allowed us to explore the link between stature and fourteen specific cancer endpoints, we necessarily conducted a large number of statistical tests, particularly when, based on our a priori analytical strategy, we stratified by both region and sex. It is therefore possible that some of the significant associations we report could have surfaced by chance alone. We controlled for a series of covariates which may have confounded the observed gradients – smoking, obesity and socioeconomic disadvantage (indexed by education) – and there was no suggestion of either positive or negative confounding. This is perhaps unsurprising because, although these covariates have been shown to be related to cancer risk in the APCSC,(24-26) they were very weakly related to stature (results not shown but available upon request), so effectively breaking any confounding structure. In the context of a positive height-cancer relationship, any effect is unlikely to be explained by reverse causality which has much more relevance to explaining the observation that taller people have a reduced rate of cardiovascular disease.(19) Many of the associations between taller people and increased cancer risk only emerged convincingly in analyses in which we excluded deaths occurring in the early stages of follow-up. This suggests that, for a proportion of these excluded individuals, height was assessed after the occurrence of sub-clinical cancer which may have led to osteoporotic vertical collapse (i.e., shrinkage). Prior to these exclusions, this seems to have resulted in an artifactual elevation of cancer risk in shorter cohort members and therefore an underestimation of the magnitude of the overall height-cancer gradient. This may have occurred in other cohorts but, to date, has largely been unexplored.
Plausible mechanisms
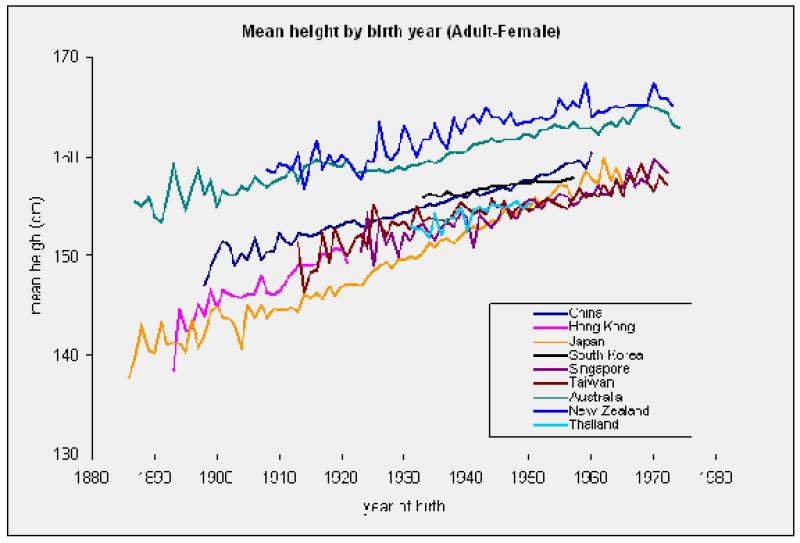
That the reported relations between stature and cancer are unlikely to be artifactual raises a number of mechanistic possibilities. Height itself cannot of course be a risk factor for malignancy but, although under a degree of genetic control, it is likely to capture pre-adult environmental exposures that may be. Evidence for the importance of environmental factors in general, whatever they may be, is found in the universal observation of secular increases in height across a multitude of populations in the twentieth century for which we found support in the present study. Thus, in women in APCSC there was a marked, persistent year-on-year increase in adult stature with advancing birth year (figure 2; P[trend] = 0.001). The same results were apparent in men (available on request). These secular increases in height are very unlikely to be explained by changes in the gene pool as the time frame is simply too narrow.
Figure 2. Mean height according to birth year in women in the APCSC.
Given that cancer is not a single disease entity, there is unlikely to be a single unifying environmental factor that would link height to every cancer sub-type; instead a range of possibilities exists. First, stature is an indicator of organ size: the larger the person the larger the organ. As such, the chances of dividing stem cells undergoing transformation to malignancy is raised in taller people.(11) However, if this is the case, a strong positive association would have been anticipated between height and skin cancer but this was not clear in the present analyses. Second, in a related point, childhood energy intake, associated with physical growth, may be an important dietary determinant of cancer. It has long been recognized that animals fed a calorie-constrained, but otherwise micronutrient-balanced diet, subsequently experience lower cancer incidence and longer life expectancy than their overfed counterparts,(27;28) again, perhaps because of reduced cell proliferation. Third, it has been proposed that underlying the height-malignancy relation may be reduced levels of insulin-like growth factors (IGF) which correlate directly with caloric intake (in animals),(29) height in children,(30) and risk of colorectal and prostate cancer in adult humans.(31) In support of the IGF-malignancy link, there is a positive relation of stature with carcinoma of the prostate and colorectum in the present study and elsewhere.(9) Finally, some genetic factors linked with height may also be tied to tumour risk(32) but we did not have the capacity to explore this possibility herein.
Study strengths and limitations
While the present study has its strengths, including its prospective design, its size, its capacity to explore effect modification by region and sex, and the range of confounding variables, it is not of course without its limitations. First, we did not have information on diet or potentially emerging risk factors for cancer, such as markers of inflammation,(33) which maybe additional candidate confounders. Second, despite the size of the study, on occasion, particularly when stratifying by region and gender, some analyses involved small numbers of events for certain cancer sub-types which may have reduced the power to detect an association if one exists. Third, we did not have re-survey data on height with which to test our shrinkage explanation more directly. Finally, a recent advance in this field has been to explore the relation of height components – trunk and leg length – with cancer outcomes,(34-36) however, we did not have data on these characteristics in the present cohort.
In conclusion, in the present study, taller men and women experienced an increased risk of death from selected malignancies with little evidence of differences in effect by sex or region. Given that this is the first study to examine regional differentials, and because they may plausibly occur, further examination is required.
Acknowledgments
Funding: This work is supported by the National Health and Medical Research Council of Australia [402903 to C.M.Y.L., 358395 to APCSC]. Rachel Huxley is supported by a Career Development Award from the National Heart Foundation of Australia. The Medical Research Council (MRC) Social and Public Health Sciences Unit receives funding from the MRC and the Chief Scientist Office at the Scottish Government Health Directorates. David Batty is a UK Wellcome Trust Research Career Development Fellow; this manuscript was written while he was a visiting fellow at The George Institute for International Health, Sydney.
Footnotes
Conflicts of interest: None
References
- 1.Kuh D, Ben Shlomo Y. A lifecourse approach to chronic disease epidemiology. Oxford Medical Publications; Oxford: 2004. [Google Scholar]
- 2.Gunnell D. Can adult anthropometry be used as a ‘biomarker’ for prenatal and childhood exposures? Int J Epidemiol. 2002;31:390–4. [PubMed] [Google Scholar]
- 3.Langenberg C, Shipley MJ, Batty GD, Marmot MG. Adult socioeconomic position and the association between height and coronary heart disease mortality: findings from 33 years of follow-up in the whitehall study. Am J Public Health. 2005;95:628–32. doi: 10.2105/2004.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannam JP, Levy D, Larson M, Wilson PW. Short stature and risk for mortality and cardiovascular disease events. The Framingham Heart Study. Circulation. 1994;90:2241–7. doi: 10.1161/01.cir.90.5.2241. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor DA, Taylor M, Davey SG, Gunnell D, Ebrahim S. Associations of components of adult height with coronary heart disease in postmenopausal women: the British women’s heart and health study. Heart. 2004;90:745–9. doi: 10.1136/hrt.2003.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JN, Kagan A, Pattison DC, Gardner MJ. Incidence and prediction of ischaemic heart-disease in London busmen. Lancet. 1966;2:553–9. doi: 10.1016/s0140-6736(66)93034-0. [DOI] [PubMed] [Google Scholar]
- 7.Wannamethee SG, Shaper AG, Whincup PH, Walker M. Adult height, stroke and coronary heart disease. American Journal of Epidemiology. 1998;148:1069–76. doi: 10.1093/oxfordjournals.aje.a009584. [DOI] [PubMed] [Google Scholar]
- 8.Yarnell JW, Limb ES, Layzell JM, Baker IA. Height: a risk marker for ischaemic heart disease: prospective results from the Caerphilly and Speedwell Heart Disease Studies. Eur.Heart J. 1992;13:1602–5. doi: 10.1093/oxfordjournals.eurheartj.a060111. [DOI] [PubMed] [Google Scholar]
- 9.Gunnell D, Okasha M, Davey Smith G, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23:313–42. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 10.Batty GD, Shipley M, Gunnell D, Kivimaki M, Woodward M, Man Ying, Lee C, et al. Height, Wealth, and Health: An Overview With New Data From Three Longitudinal Studies. Econ.Hum.Biol. 2009 doi: 10.1016/j.ehb.2009.06.004. in press. [DOI] [PubMed] [Google Scholar]
- 11.Albanes D, Winick M. Are cell number and cell proliferation risk factors for cancer? J.Natl.Cancer Inst. 1988;80:772–4. doi: 10.1093/jnci/80.10.772. [DOI] [PubMed] [Google Scholar]
- 12.Holly JM, Gunnell DJ, Davey SG. Growth hormone, IGF-I and cancer. Less intervention to avoid cancer? More intervention to prevent cancer? J Endocrinol. 1999;162:321–30. doi: 10.1677/joe.0.1620321. [DOI] [PubMed] [Google Scholar]
- 13.Batty GD, Shipley MJ, Langenberg C, Marmot MG, Davey Smith G. Adult height in relation to mortality from 14 cancer sites in men in London (UK): evidence from the original Whitehall study. Ann.Oncol. 2006;17:157–66. doi: 10.1093/annonc/mdj018. [DOI] [PubMed] [Google Scholar]
- 14.Batty GD. Confounding effect of socioeconomic position in the study of height in relation to prostate cancer risk (letter) Br.J Cancer. 2004;90:1875. doi: 10.1038/sj.bjc.6601799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss NS. Can the “specificity” of an association be rehabilitated as a basis for supporting a causal hypothesis? Epidemiology. 2002;13:6–8. doi: 10.1097/00001648-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Song YM, Davey Smith G, Sung J. Adult height and cause-specific mortality: a large prospective study of South Korean men. Am J Epidemiol. 2003;158:479–85. doi: 10.1093/aje/kwg173. [DOI] [PubMed] [Google Scholar]
- 17.Song YM, Sung J. Adult height and the risk of mortality in South Korean women. Am.J.Epidemiol. 2008;168:497–505. doi: 10.1093/aje/kwn187. [DOI] [PubMed] [Google Scholar]
- 18.Davey Smith G, Hart C, Upton M. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. Journal of Epidemiology and Community Health. 2000;54:97–103. doi: 10.1136/jech.54.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CM, Barzi F, Woodward M, Batty GD, Giles GG, Wong JW, et al. Adult height and the risks of cardiovascular disease and major causes of death in the Asia-Pacific region: 21 000 deaths in 510 000 men and women. Int.J Epidemiol. 2009 doi: 10.1093/ije/dyp150. in press. [DOI] [PubMed] [Google Scholar]
- 20.Woodward M, Barzi F, Martiniuk A, Fang X, Gu DF, Imai Y, et al. Cohort profile: the Asia Pacific Cohort Studies Collaboration. Int J Epidemiol. 2006;35:1412–6. doi: 10.1093/ije/dyl222. [DOI] [PubMed] [Google Scholar]
- 21.Anon . Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death (ninth revision) WHO; Geneva: 1977. [Google Scholar]
- 22.Anon . Classification of diseases. 10th revision. Danish National Board of Health; Copenhagen: 1993. [Google Scholar]
- 23.Woodward M. Epidemiology: Study Design and Data Analysis. Chapman & Hall/CRC; Boca Raton, Florida: 2005. [Google Scholar]
- 24.Huxley R, Jamrozik K, Lam TH, Barzi F, Ansary-Moghaddam A, Jiang CQ, et al. Impact of smoking and smoking cessation on lung cancer mortality in the Asia-Pacific region. Am J Epidemiol. 2007;165:1280–6. doi: 10.1093/aje/kwm002. [DOI] [PubMed] [Google Scholar]
- 25.Huxley R. The impact of modifiable risk factors on mortality from prostate cancer in populations of the Asia-Pacific region. Asian Pac.J Cancer Prev. 2007;8:199–205. [PubMed] [Google Scholar]
- 26.Ansary-Moghaddam A, Huxley R, Barzi F, Lawes C, Ohkubo T, Fang X, et al. The effect of modifiable risk factors on pancreatic cancer mortality in populations of the Asia-Pacific region. Cancer Epidemiol.Biomarkers Prev. 2006;15:2435–40. doi: 10.1158/1055-9965.EPI-06-0368. [DOI] [PubMed] [Google Scholar]
- 27.Hart RW, Turturro A. Dietary restrictions and cancer. Environ.Health Perspect. 1997;105(Suppl 4):989–92. doi: 10.1289/ehp.97105s4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimokawa I, Higami Y. Modulation of aging processes by dietary restriction. CRC Press; London: 1994. Effect of dietary restriction on pathological processes; pp. 247–66. [Google Scholar]
- 29.Ruggeri BA, Klurfeld DM, Kritchevsky D, Furlanetto RW. Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 1989;49:4130–4. [PubMed] [Google Scholar]
- 30.Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin.Endocrinol.Metab. 1994;78:744–52. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- 31.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 32.Hebert PR, Ajani U, Cook NR, Lee IM, Chan KS, Hennekens CH. Adult height and incidence of cancer in male physicians (United States) Cancer Causes Control. 1997;8:591–7. doi: 10.1023/a:1018442329319. [DOI] [PubMed] [Google Scholar]
- 33.Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J.Epidemiol.Community Health. 2007;61:824–33. doi: 10.1136/jech.2006.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunnell D, May M, Ben Shlomo Y, Yarnell J, Davey Smith G. Height, leg length, and cancer: the Caerphilly Study. Nutr.Cancer. 2003;47:34–9. doi: 10.1207/s15327914nc4701_4. [DOI] [PubMed] [Google Scholar]
- 35.Gunnell DJ, Davey Smith G, Holly JMP, Frankel S. Leg length and risk of cancer in the Boyd Orr cohort. British Medical Journal. 1998;317:1350–1. doi: 10.1136/bmj.317.7169.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunnell DJ, Davey Smith G, Frankel S, Nanchahal K, Braddon FE, Pemberton J, et al. Childhood leg length and adult mortality: follow up of the Carnegie (Boyd Orr) Survey of Diet and Health in Pre-war Britain. J Epidemiol Community Health. 1998;52:142–52. doi: 10.1136/jech.52.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]