SUMMARY
Background
Excess weight is an established risk factor for several cancers but there are sparse data from Asian populations in whom overweight and obesity is increasing rapidly and adiposity can be substantially greater for the same body mass index (BMI) compared to Caucasians.
Methods
We examined associations of adult BMI with cancer mortality (overall and 20 sites) in geographic populations from Asia and Australia/New Zealand (ANZ) within the Asia Pacific Cohort Studies Collaboration using Cox regression. Pooled data from 39 cohorts (recruitment 1961-99, median follow-up 4 years) were analyzed for 424 519 participants (77% Asian; 41% female; mean recruitment age 48 years) with individual data on BMI.
Findings
After excluding follow-up < 3 years, 4872 cancer deaths occurred in 401 215 participants. Hazard ratios (95% CI) for cancer sites with increased mortality risk in the obese (≥30 kg/m2) relative to the normal weight (18.5-24.9 kg/m2) were: 1.21 (1.09-1.36) for all-cause cancer (excluding lung and upper-aero digestive tract), 1.50 (1.13-1.99) for colon, 1.68 (1.06-2.67) for rectum, 1.63 (1.13-2.35) for breast in women aged ≥ 60 years, 2.62 (1.57-4.37) for ovary, 4.21 (1.89-9.39) for cervix, 1.45 (0.97-2.19) for prostate, and 1.66 (1.03-2.68) for leukaemia with the increased risk associated with a 5-unit increment in BMI ≥ 18.5 kg/m2 ranging from 1.13 (0.91-1.40) for rectum to 1.45 (1.00-2.11) for cervix. There was little evidence of regional differences in relative risk except for oropharynx and larynx where the association was inverse in ANZ but absent in Asia.
Interpretation
Overweight and obese individuals in populations across the Asia-Pacific region are at significantly increased risk of mortality from cancer. Strategies to prevent overweight and obesity across Asia are required to reduce the burden of cancer expected to occur if the obesity epidemic continues.
Funding
The APCSC has been funded by the National Health and Medical Research Council of Australia, the Health Research Council of New Zealand and Pfizer Inc., through an unrestricted medical grant.
Keywords: body mass index, obesity, overweight, cancer, mortality, Asia-Pacific
INTRODUCTION
Overweight and obesity affect more than 1 billion people worldwide and is a major risk factor for chronic diseases, including some cancers.1 Due to both its widespread prevalence and impact on a large number of health conditions, excess weight is ranked as the seventh most important contributor to mortality worldwide.2 As in the West, many Asian countries such as China, South Korea, Thailand and Singapore are experiencing a steep rise in the prevalence of overweight and obesity in their populations3 although, compared with the West, the prevalence remains low.4,5
Overweight and obesity are related to increased risk of several site-specific cancers where a 5 kg/m2 higher body mass index (BMI) is typically associated with risk ratios in the range 1.10 – 1.60.6 The evidence linking overweight and obesity to cancer has come primarily from Caucasian populations, with few data from Asians7-9 in whom adiposity can be substantially greater for the same BMI.10-11 Furthermore, the background prevalence of other dietary and lifestyle risk factors for cancer may also differ between Western and Asian populations12 which potentially may impact on the relationship between excess weight and subsequent cancer risk. Recent meta-analyses of the few Asian data, predominantly from Japan or Hawaii, indicate that higher BMI associated relative risks of cancer of the female breast5 and colo-rectum13 in populations from Asia or the Asia-Pacific compared to North America, Europe and Australia.
Using data from the Asia Pacific Cohort Studies Collaboration (APCSC), the purpose of the present study is two-fold: first, to examine the associations between BMI and site-specific cancer mortality in populations of the Asia Pacific region; and second, to determine whether the magnitude and direction of the associations are consistent between geographic populations in Asia and outside Asia (Australia and New Zealand) which, to the best of our knowledge, has not previously been systematically examined using individual participant data.
SUBJECTS AND METHODS
The APCSC is a large collaborative data pooling project involving data from over 600,000 participants in the Asia-Pacific region. Details of the collaboration have been described elsewhere.14-15 Briefly, studies were eligible for inclusion if they met the following criteria: 1) a study population from the Asia-Pacific region; 2) a prospective cohort study design; 3) at least 5000 person-years of follow-up; 4) date of birth (or age), sex, and blood pressure recorded at baseline; 5) date of death or age at death recorded during follow-up. Studies were excluded if entry was dependent on a particular condition or risk factor. Studies were classified as Asian if their participants were recruited from mainland China, Hong Kong, Taiwan, Japan, South Korea, Singapore, or Thailand, and as ANZ if from Australia or New Zealand. We did not have individual-level data on ethnicity, but given that immigration from Asia to ANZ is a relatively recent occurrence and unlikely to have predated the cohorts that were established from the mid-1960s to mid-1980’s, it is unlikely that the ANZ cohorts would have comprised a significant proportion of Asians. Since the APCSC is based on existing data, no ethics approval was needed for the present study.
Study sample
BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Baseline data on height and weight (or BMI) and at least one cancer event were available from 39 cohorts in the APCSC database. Of the 575 458 participants aged ≥ 20 years in these 39 cohorts, a total of 26% were excluded due to missing follow-up for cancer mortality (n = 1062), missing BMI values (n = 149 861), or a reported BMI of < 12 kg/m2 (n = 9) or > 60 kg/m2 (n = 7). Thus, a total of 424 519 participants with baseline data on age, sex, BMI, and cancer mortality comprised the present analytical sample.
Cancer outcomes
Cancer mortality was classified according to the ninth or tenth revision of the International Classification of Diseases (ICD-9/ICD-10). Older ICD-7 codes reported by some studies were recoded into version 9 or 10 by the project secretariat. Five small studies did not use ICD codes (Capitol Iron, Beijing Aging, EGAT, Tanno Sobetsu and Aito Town), contributing < 5% (n = 343) of all cancer deaths (n = 7211), that were grouped into an “other” category in the majority of cases (> 80%) for other specified, or unspecified sites, which was included in the analysis of “all” cancer, or into specified categories by the project secretariat using all available information. Summary reports were referred back to principal investigators of each collaborating study for review and confirmation. Histological subtypes are not available in the APCSC.
Statistical analyses
Eligible participants contributed person years from study recruitment until death from cancer, death from another cause, the last known date that they were alive, or end of follow-up – whichever came first. Associations between BMI and cancer mortality were estimated from Cox proportional hazard regression models that were stratified by study and, where appropriate, also by sex and study with adjustment for age at baseline and using elapsed time since participant’s measurement at study baseline as time scale. To investigate the potential effect of confounding by pre-existing disease and weight loss at baseline on the observed associations (i.e. reverse causality), the age and smoking adjusted HRs were estimated both before and after three years left-censoring of the data. Unless otherwise stated, the results will only describe the left-censored data. We estimated hazard ratios (HRs) for four categories of BMI using the World Health Organization (WHO) classification: BMI < 18.5 kg/m2 (underweight), 18.5 - 24.9 kg/m2 (normal range), 25.0 - 29.9 kg/m2 (overweight) and ≥ 30 (obese) kg/m2, taking the normal range as the reference category. As the majority of participants fell within the normal range, this category was further subdivided using the intermediate WHO cut points16 of 18.5 - 22.9 kg/m2 and 23 - 24.9 kg/m2 to investigate changes in the shape of the risk pattern for five versus four categories of BMI. The 95% confidence intervals (CI) for categories of BMI were obtained by the method of floating absolute risks.17
In separate analyses, further adjustments for smoking status (not current versus current smoking) and drinking status (not current versus current alcohol consumption) were made. Participants with missing values were assigned to a separate category for those variables. The main Cox model was adjusted for age at baseline and smoking status with missing smoking as a separate category. Attained age < 60 or ≥ 60 years was taken as a proxy for menopausal status (no direct data were available) when estimating the risk of fatal breast cancer (adjustment for attained age and smoking status). This age cut-point was chosen as it is similar to other studies of breast cancer mortality18 to account for the relatively high survival19,20 and the fact that few events occurred before age 50 years. After excluding the underweight category, we tested for a log-linear trend in HR across BMI categories by fitting the main Cox model with BMI as a continuous variable, using three levels on an ordinal scale. To ease comparability with other studies we calculated the overall effect of a 5 kg/m2 increase in BMI > 18.5 kg/m2 for all cancer sites. We tested for regional differences in risk by adding interaction terms between region (Asia versus ANZ) and BMI to the Cox models for categorical (18.5 to 24.9 vs. ≥ 25.0 kg/m2) and continuous (> 18.5 kg/m2) effects after left censoring. Further interaction testing was limited to testing for sex differences to reduce the potential for Type I errors. We also examined the effect of restricting analyses to participants with known values for all covariates; 94% of participants (n = 400 277) had data on smoking status and 91% (n = 388 109) had data on both smoking and drinking status. As the effect was negligible (Web Table 4), the decision was made to conduct the analyses on the entire sample. The main Cox model was adjusted for age at baseline and smoking status with missing smoking as a separate category, without additional adjustment for drinking status. All statistical analyses were performed using SAS version 9.1.3 for Windows. Plots were created with R software. All tests were two-sided with statistical significance set at p < 0.05.
Role of the funding source
The APCSC data pooling project has been funded by the National Health and Medical Research Council of Australia, the Health Research Council of New Zealand and Pfizer Inc., through an unrestricted medical grant. The sponsors had no role in the conduct of the study or the writing of this report. CLP and FB had full access to the raw data as analysts. The corresponding author (CLP) had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Study sample characteristics at baseline
Individual participant data from 39 cohorts (Web Table 1) comprised 424 519 people of which 77% (n = 326 387) were from Asian cohorts and 41% (n = 175 364) were female; mean age was 48 years; and median year of recruitment was 1992 in cohorts from both Asia and ANZ. Comparing baseline characteristics with the Asian cohorts, individuals from ANZ had higher BMI: the mean (SD) BMI was 26.6 (3.7) kg/m2 in men and 26.0 (4.9) kg/m2 in women versus 23.0 (2.7) kg/m2 in men and 22.6 (3.1) kg/m2 in women from Asia; were generally older, and had a lower proportion of current smokers in men (20% ANZ versus 60% Asia), but higher in women (14% ANZ versus 6% Asia). Selected baseline characteristics and cancer follow-up of study participants (before left censoring) by sex and categories of BMI are presented separately for Asia (Table 1) and ANZ (Table 2).
Table 1. Baseline characteristics and cancer follow-up by sex and categories of body mass index (BMI) in study participants (n = 326 387) compared to excluded subjects with missing BMI (n = 149 304) from 31 Asian cohorts in the Asia Pacific Cohort Studies Collaboration.
| Body mass index (kg/m2) |
|||||
|---|---|---|---|---|---|
| Asian cohorts | Missing | 12.0-18.4 | 18.5-24.9 | 25.0-29.9 | 30.0-60.0 |
| Males | |||||
| No. of participants | 118246 | 7209 | 143292 | 42947 | 1996 |
| No. of cancer deaths | 1008 | 287 | 2150 | 461 | 42 |
| Person yrs, follow-up | 871441 | 47860 | 801239 | 216162 | 10884 |
| Age (yrs) at baseline, mean (SD) | 41.9 (6.9) | 52.3 (13.7) | 46.5 (9.2) | 46.7 (8.7) | 49.4 (11.3) |
| BMI (kg/m2), mean (SD) | - | 17.5 (0.9) | 22.1 (1.7) | 26.5 (1.2) | 31.8 (2.6) |
| Weight (kg), mean (SD) | 60.0 (9.0) | 48.3 (4.7) | 62.3 (6.6) | 75.1 (6.1) | 88.8 (9.6) |
| Height (m), mean (SD) | 1.69 (0.05) | 1.66 (0.07) | 1.67 (0.06) | 1.68 (0.06) | 1.67 (0.07) |
| Smoking status (%) | |||||
| Not current smoker | 40.7 | 31.3 | 36.7 | 44.1 | 47.0 |
| Current smoker | 58.8 | 66.1 | 58.5 | 50.5 | 49.3 |
| Unknown | 0.5 | 2.6 | 4.7 | 5.4 | 3.7 |
| Drinking status (%) | |||||
| Not current drinker | 75.1 | 44.5 | 32.5 | 30.3 | 41.3 |
| Current drinker | 24.2 | 49.6 | 61.6 | 64.1 | 54.2 |
| Unknown | 0.7 | 5.8 | 6.0 | 5.7 | 4.5 |
| Females | |||||
| No. of participants | 31058 | 8355 | 96814 | 22861 | 2913 |
| No. of cancer deaths | 149 | 156 | 780 | 224 | 30 |
| Person yrs, follow-up | 239421 | 54565 | 550748 | 131004 | 17027 |
| Age (yrs) at baseline, mean (SD) | 41.1 (7.3) | 48.3 (14.6) | 45.1 (10.1) | 49.2 (10.5) | 52.1 (11.6) |
| BMI (kg/m2), mean (SD) | - | 17.4 (0.9) | 21.8 (1.7) | 26.6 (1.3) | 32.3 (2.8) |
| Weight (kg), mean (SD) | 54.0 (8.5) | 42.1 (4.3) | 53.2 (5.6) | 64.3 (5.6) | 76.5 (8.8) |
| Height (m), mean (SD) | 1.59 (0.05) | 1.55 (0.06) | 1.56 (0.06) | 1.55 (0.06) | 1.54 (0.07) |
| Smoking status (%) | |||||
| Not current smoker | 97.4 | 80.6 | 83.6 | 83.4 | 84.3 |
| Current smoker | 1.5 | 11.9 | 4.4 | 5.8 | 10.6 |
| Unknown | 1.1 | 7.5 | 11.9 | 10.8 | 5.0 |
| Drinking status (%) | |||||
| Not current drinker | 95.0 | 82.6 | 80.1 | 81.1 | 86.0 |
| Current drinker | 3.8 | 7.6 | 9.2 | 8.6 | 7.3 |
| Unknown | 1.2 | 9.8 | 10.7 | 10.3 | 6.7 |
Sub-sample of participants with missing BMI, but available data on either weight or height
Table 2. Baseline characteristics and cancer follow-up by sex and categories of body mass index (BMI) in study participants (n = 98 132) compared to excluded subjects with missing BMI (n = 557) from 8 Australia/New Zealand (ANZ) cohorts in the Asia Pacific Cohort Studies Collaboration.
| Body mass index (kg/m2) |
|||||
|---|---|---|---|---|---|
| ANZ cohorts | Missing | 12.0-18.4 | 18.5-24.9 | 25.0-29.9 | 30.0-60.0 |
| Males | |||||
| No. of participants | 284 | 248 | 17888 | 26692 | 8883 |
| No. of cancer deaths | 38 | 15 | 671 | 965 | 300 |
| Person yrs, follow-up | 3939 | 2019 | 167895 | 214069 | 63895 |
| Age (yrs) at baseline, mean (SD) | 53.0 (20.6) | 58.0 (19.0) | 52.3 (17.0) | 56.0 (14.4) | 56.8 (13.5) |
| BMI (kg/m2), mean (SD) | - | 17.4 (1.0) | 23.0 (1.5) | 27.2 (1.4) | 32.6 (2.7) |
| Weight (kg), mean (SD) | 76.3 (10.4) | 52.0 (5.9) | 69.7 (7.2) | 81.4 (7.6) | 96.5 (11.5) |
| Height (m), mean (SD) | 1.75 (0.08) | 1.72 (0.08) | 1.74 (0.07) | 1.73 (0.07) | 1.72 (0.07) |
| Smoking status (%) | |||||
| Not current smoker | 73.9 | 62.1 | 74.9 | 82.0 | 82.0 |
| Current smoker | 23.9 | 37.9 | 25.0 | 17.9 | 17.9 |
| Unknown | 2.1 | 0.0 | 0.1 | 0.1 | 0.1 |
| Drinking status (%) | |||||
| Not current drinker | 37.0 | 42.3 | 37.4 | 44.2 | 47.0 |
| Current drinker | 62.3 | 54.0 | 56.0 | 51.2 | 50.7 |
| Unknown | 0.7 | 3.6 | 6.5 | 4.6 | 2.4 |
| Females | |||||
| No. of participants | 273 | 661 | 20967 | 14698 | 8095 |
| No. of cancer deaths | 17 | 18 | 496 | 381 | 235 |
| Person yrs, follow-up | 4173 | 7859 | 230986 | 146164 | 75643 |
| Age (yrs) at baseline, mean (SD) | 51.8 (21.2) | 45.7 (16.3) | 48.1 (13.5) | 53.8 (11.6) | 54.4 (10.5) |
| BMI (kg/m2), mean (SD) | - | 17.6 (0.8) | 22.4 (1.6) | 27.1 (1.4) | 33.9 (3.7) |
| Weight (kg), mean (SD) | 63.1 (11.9) | 46.6 (4.5) | 58.8 (6.0) | 69.4 (6.4) | 85.1 (11.4) |
| Height (m), mean (SD) | 1.62 (0.06) | 1.62 (0.07) | 1.62 (0.06) | 1.60 (0.06) | 1.58 (0.07) |
| Smoking status (%) | |||||
| Not current smoker | 74.0 | 72.2 | 83.2 | 87.8 | 89.7 |
| Current smoker | 23.8 | 27.5 | 16.6 | 12.1 | 10.2 |
| Unknown | 2.2 | 0.3 | 0.2 | 0.1 | 0.1 |
| Drinking status (%) | |||||
| Not current drinker | 52.7 | 51.3 | 60.5 | 73.5 | 80.7 |
| Current drinker | 45.8 | 41.3 | 34.3 | 23.4 | 17.9 |
| Unknown | 1.5 | 7.4 | 5.2 | 3.2 | 1.4 |
Sub-sample of participants with missing BMI, but available data on either weight or height
Distribution of cancer deaths by site
During the 2 738 020 person-years of follow-up (median 4 years), there were 7211 cancer deaths of which 57% (n = 4130) occurred in Asian cohorts. The most common cancer site overall was lung (20%, n = 1478), followed by stomach (12%, n = 855), liver (11%, n = 774), and large intestine (9%, n = 668), but the distribution varied by sex and region (Table 3). After excluding the first three years of follow-up, there were 401 215 study participants (95% of study sample) and 4872 cancer deaths, of which 77% of participants (n = 309 263) and 54% of deaths (n = 2627) were from Asian cohorts. Of the excluded cancer deaths (n = 2339), 25% (n = 588) were from lung cancer.
Table 3. Site-specific number of cancer deaths and % distribution by sex within region in the Asia Pacific Cohort Studies Collaboration participants, n = 424 519.
| Asia |
Australia/New Zealand |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All |
Male |
Female |
Male |
Female |
||||||
| Cancer site (ICD-9/ICD-10) | No. | % | No. | % | No. | % | No. | % | No. | % |
| All cancers* | 7211 | 100% | 2940 | 100% | 1190 | 100% | 1951 | 100% | 1130 | 100% |
| Upper aero-digestive tract (UADT)* | 388 | 5% | 207 | 7% | 59 | 5% | 99 | 6% | 23 | 2% |
| Oesophagus (150/C15) | 229 | 3% | 117 | 4% | 42 | 4% | 52 | 3% | 18 | 2% |
| Oropharynx (140-149/C00-C14) & larynx (161/C32) | 159 | 2% | 90 | 3% | 17 | 1% | 47 | 3% | 5 | 0.4% |
| Stomach (151/C16) | 855 | 12% | 503 | 17% | 222 | 19% | 100 | 12% | 30 | 3% |
| Large intestine (153-154/C18-21) * | 668 | 9% | 128 | 4% | 85 | 7% | 263 | 8% | 192 | 17% |
| Colon (153/C18) | 429 | 6% | 52 | 2% | 44 | 4% | 183 | 5% | 150 | 13% |
| Rectum (154/C18-20) | 233 | 3% | 73 | 2% | 39 | 3% | 79 | 3% | 42 | 4% |
| Liver (155/C22) | 774 | 11% | 610 | 21% | 108 | 9% | 38 | 13% | 18 | 2% |
| Pancreas (157/C25) | 301 | 4% | 110 | 4% | 48 | 4% | 84 | 4% | 59 | 5% |
| Lung (162/C33-34) | 1478 | 20% | 706 | 24% | 196 | 16% | 442 | 23% | 134 | 12% |
| Skin, malignant melanoma (172/C43) | 88 | 1% | 5 | 0.2% | 0 | 0% | 58 | 1% | 25 | 2% |
| Breast, female (174/C50) | 324 | 4% | - | - | 98 | 8% | - | - | 226 | 20% |
| Female genital organs (179-183/C53-56) * | 198 | 3% | - | - | 98 | 8% | - | - | 100 | 9% |
| Uterus (179,182/C55) | 37 | 1% | - | - | 17 | 1% | - | - | 20 | 2% |
| Cervix (180/C53) | 60 | 1% | - | - | 45 | 4% | - | - | 15 | 1% |
| Ovaries (183/C56) | 99 | 1% | - | - | 34 | 3% | - | - | 65 | 6% |
| Prostate (185/C61) | 278 | 4% | 37 | 1% | - | - | 241 | 12% | - | - |
| Bladder (188/C67) | 120 | 2% | 43 | 1% | 6 | 1% | 55 | 3% | 16 | 1% |
| Kidney (189/C64-65) | 93 | 1% | 26 | 1% | 6 | 1% | 43 | 2% | 18 | 2% |
| Brain & nervous system (191-192/C70-72) | 191 | 3% | 56 | 2% | 22 | 2% | 77 | 4% | 36 | 3% |
| Haematological cancers* | 454 | 6% | 113 | 4% | 46 | 4% | 186 | 10% | 109 | 10% |
| Lymphoma (200-202/C81-85) | 201 | 3% | 50 | 2% | 24 | 2% | 81 | 4% | 46 | 4% |
| Myeloma (203/C90) | 69 | 1% | 11 | 0.4% | 6 | 1% | 33 | 2% | 19 | 2% |
| Leukaemia (204-208/C91-95) | 184 | 3% | 52 | 2% | 16 | 1% | 72 | 4% | 44 | 4% |
| Other and unspecified sites | 1001 | 14% | 396 | 13% | 196 | 16% | 265 | 14% | 144 | 13% |
All cancers include all specified sites and “Other and unspecified sites”. UADT includes the sub-categories “oesophagus” and “oropharynx and larynx”; large intestine includes the sub-categories “colon”, “rectum”, 4 cases of anal cancer, and 2 cases with missing ICD code; female genital organ cancers include the sub-categories “uterus”, “cervix”, “ovaries”, 1 case of placenta cancer and one case with missing ICD code; haematological cancers include the sub-categories “lymphoma”, “myeloma”, and “leukaemia”.
Association between BMI and all cancer mortality
The assumptions for the Cox proportional hazard models were met when examining the relationship between categories of BMI and cancer mortality in those >18.5 kg/m2 for all cancers and for all site-specific cancers. In age and smoking-adjusted analyses, there was a U-shaped relation between BMI and mortality from all cancers combined; in both the underweight (< 18.5 kg/m2) and obese (≥ 30 kg/m2) categories there was an increased risk of mortality (HR of 1.13 and 1.11, respectively) compared with the normal weight (18.5 to 24.9 kg/m2) reference group (Table 4). However, the overall trend between BMI and all cancers in individuals > 18.5 kg/m2 was weak and non-significant (ptrend = 0.32). This was due to the opposing effects of BMI with cancers of the lung and upper-aero digestive tract (UADT) which after excluding (n = 1866 cases), resulted in a positive and significant association between BMI and all cancers in individuals > 18.5 kg/m2: the HR per 5 kg/m2 increment in BMI was 1.09 (95% CI 1.04 - 1.14; ptrend = 0.003). In this analysis, overweight and obese individuals had a greater risk of mortality from cancer compared with those of normal weight: HR 1.06 (95% CI 1.00 - 1.12) and HR 1.21 (95% CI 1.09 - 1.36), respectively; Table 4. We conducted a sensitivity analysis to examine the impact of the large Korean study (KMIC) on all cancers and the results were not materially altered by the exclusion of this study: the HR (95%CI) per 5-unit increment in BMI for all cancers was 1.03 (0.99 - 1.08) versus 1.05 (1.00 - 1.10) after exclusion of KMIC.
Table 4. Age and smoking adjusted hazard ratios (HR) with 95% confidence intervals (CI) for cancer mortality according to body mass index before (n = 424 519) and after (n = 401 215) left censoring at 3 years* in the Asia Pacific Cohort Studies Collaboration.
Models stratified by study and sex.
| Body mass index (kg/m2) |
Trend ≥ 18.5 (kg/m2) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left cens. years | 12.0-18.4 | 18.5-24.9 | 25.0-29.9 | 30.0-60.0 | Per 5 units | |||||||
|
|
|
|||||||||||
| Cancer site | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | Ptrend | |
| All Cancer | 0 | 1.25 | (1.13, 1.38) | 1.00 | (0.97, 1.03) | 0.94 | (0.90, 0.98) | 1.03 | (0.95, 1.12) | 1.00 | (0.97, 1.04) | 0.62 |
| 3 | 1.13 | (1.00, 1.28) | 1.00 | (0.96, 1.04) | 0.97 | (0.91, 1.02) | 1.11 | (1.00, 1.23) | 1.03 | (0.99, 1.08) | 0.32 | |
| All Cancer** | 0 | 1.18 | (1.04, 1.33) | 1.00 | (0.96, 1.04) | 1.01 | (0.96, 1.06) | 1.12 | (1.02, 1.23) | 1.05 | (1.01, 1.10) | 0.09 |
| 3 | 1.12 | (0.96, 1.31) | 1.00 | (0.96, 1.04) | 1.06 | (1.00, 1.12) | 1.21 | (1.09, 1.36) | 1.09 | (1.04, 1.14) | 0.003 | |
| Upper aero-digestive | 0 | 1.52 | (1.08, 2.15) | 1.00 | (0.87, 1.14) | 0.83 | (0.68, 1.03) | 0.67 | (0.41, 1.10) | 0.82 | (0.68, 0.99) | 0.09 |
| 3 | 1.12 | (0.70, 1.81) | 1.00 | (0.85, 1.17) | 0.72 | (0.55, 0.96) | 0.69 | (0.38, 1.24) | 0.78 | (0.62, 0.98) | 0.07 | |
| Oesophagus | 0 | 1.39 | (0.89, 2.16) | 1.00 | (0.84, 1.20) | 1.05 | (0.80, 1.37) | 0.93 | (0.51, 1.69) | 1.00 | (0.80, 1.25) | 0.99 |
| 3 | 1.26 | (0.72, 2.19) | 1.00 | (0.81, 1.23) | 0.79 | (0.55, 1.14) | 0.80 | (0.37, 1.74) | 0.87 | (0.65, 1.16) | 0.36 | |
| Oropharynx, larynx | 0 | 1.79 | (1.03, 3.12) | 1.00 | (0.82, 1.23) | 0.61 | (0.43, 0.86) | 0.39 | (0.16, 0.97) | 0.60 | (0.44, 0.82) | 0.009 |
| 3 | 0.86 | (0.34, 2.19) | 1.00 | (0.78, 1.28) | 0.65 | (0.43, 0.98) | 0.56 | (0.23, 1.41) | 0.66 | (0.46, 0.95) | 0.09 | |
| Stomach | 0 | 1.31 | (1.02, 1.67) | 1.00 | (0.91, 1.09) | 0.89 | (0.77, 1.03) | 0.99 | (0.69, 1.44) | 0.98 | (0.86, 1.11) | 0.41 |
| 3 | 1.19 | (0.87, 1.62) | 1.00 | (0.89, 1.12) | 1.05 | (0.88, 1.25) | 1.04 | (0.67, 1.63) | 1.10 | (0.95, 1.28) | 0.66 | |
| Large intestine | 0 | 0.78 | (0.48, 1.27) | 1.00 | (0.90, 1.12) | 1.20 | (1.05, 1.37) | 1.48 | (1.20, 1.83) | 1.13 | (1.02, 1.25) | 0.001 |
| 3 | 0.73 | (0.39, 1.35) | 1.00 | (0.88, 1.13) | 1.25 | (1.07, 1.45) | 1.57 | (1.23, 2.00) | 1.15 | (1.02, 1.29) | 0.001 | |
| Colon | 0 | 0.67 | (0.33, 1.39) | 1.00 | (0.87, 1.15) | 1.13 | (0.96, 1.33) | 1.45 | (1.13, 1.86) | 1.14 | (1.01, 1.29) | 0.02 |
| 3 | 0.63 | (0.26, 1.56) | 1.00 | (0.86, 1.17) | 1.13 | (0.94, 1.36) | 1.50 | (1.13, 1.99) | 1.14 | (0.99, 1.31) | 0.02 | |
| Rectum | 0 | 0.93 | (0.48, 1.79) | 1.00 | (0.83, 1.20) | 1.28 | (1.03, 1.60) | 1.44 | (0.96, 2.16) | 1.07 | (0.89, 1.29) | 0.07 |
| 3 | 0.86 | (0.37, 2.02) | 1.00 | (0.80, 1.25) | 1.44 | (1.11, 1.86) | 1.68 | (1.06, 2.67) | 1.13 | (0.91, 1.40) | 0.03 | |
| Liver | 0 | 1.23 | (0.93, 1.64) | 1.00 | (0.91, 1.10) | 1.00 | (0.86, 1.17) | 1.07 | (0.69, 1.66) | 1.07 | (0.94, 1.22) | 0.79 |
| 3 | 1.13 | (0.78, 1.64) | 1.00 | (0.89, 1.13) | 1.06 | (0.87, 1.30) | 1.10 | (0.63, 1.91) | 1.11 | (0.94, 1.31) | 0.58 | |
| Pancreas | 0 | 0.71 | (0.38, 1.31) | 1.00 | (0.86, 1.16) | 0.93 | (0.75, 1.15) | 0.75 | (0.48, 1.18) | 0.93 | (0.78, 1.11) | 0.24 |
| 3 | 0.82 | (0.41, 1.63) | 1.00 | (0.84, 1.19) | 1.06 | (0.83, 1.36) | 0.90 | (0.54, 1.49) | 1.02 | (0.83, 1.25) | 0.88 | |
| Lung | 0 | 1.30 | (1.08, 1.56) | 1.00 | (0.94, 1.07) | 0.72 | (0.64, 0.80) | 0.81 | (0.66, 1.00) | 0.87 | (0.79, 0.94) | 0.0005 |
| 3 | 1.11 | (0.86, 1.44) | 1.00 | (0.92, 1.09) | 0.68 | (0.59, 0.79) | 0.83 | (0.64, 1.08) | 0.86 | (0.77, 0.96) | 0.003 | |
| Skin, melanoma | 0 | 1.91 | (0.45, 8.05) | 1.00 | (0.75, 1.33) | 0.58 | (0.40, 0.85) | 0.75 | (0.44, 1.30) | 0.95 | (0.71, 1.27) | 0.15 |
| 3 | 3.21 | (0.76, 13.5) | 1.00 | (0.70, 1.43) | 0.68 | (0.44, 1.05) | 1.03 | (0.57, 1.86) | 1.13 | (0.82, 1.54) | 0.77 | |
| Breast, female < 60 yrs | 0 | 0.19 | (0.03, 1.34) | 1.00 | (0.83, 1.20) | 1.26 | (0.89, 1.79) | 1.11 | (0.59, 2.09) | 1.18 | (1.03, 1.35) | 0.33 |
| 3 | 0.27 | (0.04, 1.95) | 1.00 | (0.80, 1.25) | 1.13 | (0.72, 1.76) | 0.93 | (0.42, 2.09) | 1.13 | (0.97, 1.33) | 0.84 | |
| Breast, female ≥ 60 yrs | 0 | 1.29 | (0.63, 2.64) | 1.00 | (0.83, 1.20) | 1.21 | (0.95, 1.53) | 1.53 | (1.10, 2.13) | 1.21 | (1.06, 1.36) | 0.02 |
| 3 | 0.71 | (0.22, 2.24) | 1.00 | (0.81, 1.23) | 1.13 | (0.85, 1.50) | 1.63 | (1.13, 2.35) | 1.19 | (1.03, 1.38) | 0.03 | |
| Female genital organs | 0 | 1.63 | (0.96, 2.75) | 1.00 | (0.82, 1.23) | 1.21 | (0.92, 1.59) | 2.32 | (1.61, 3.34) | 1.32 | (1.12, 1.56) | 0.0008 |
| 3 | 1.25 | (0.62, 2.49) | 1.00 | (0.79, 1.26) | 1.41 | (1.05, 1.90) | 2.65 | (1.77, 3.98) | 1.37 | (1.15, 1.64) | 0.0002 | |
| Ovaries | 0 | 0.86 | (0.27, 2.75) | 1.00 | (0.75, 1.33) | 1.24 | (0.86, 1.78) | 2.14 | (1.33, 3.47) | 1.28 | (1.03, 1.59) | 0.01 |
| 3 | - | - | 1.00 | (0.72, 1.39) | 1.46 | (0.98, 2.16) | 2.62 | (1.57, 4.37) | 1.38 | (1.09, 1.73) | 0.003 | |
| Uterus | 0 | 1.21 | (0.29, 5.15) | 1.00 | (0.63, 1.57) | 1.07 | (0.58, 1.99) | 1.77 | (0.74, 4.23) | 1.38 | (0.95, 1.99) | 0.33 |
| 3 | 1.76 | (0.40, 7.65) | 1.00 | (0.58, 1.72) | 1.45 | (0.75, 2.80) | 1.41 | (0.42, 4.67) | 1.31 | (0.83, 2.06) | 0.44 | |
| Cervix | 0 | 2.55 | (1.33, 4.89) | 1.00 | (0.68, 1.47) | 1.30 | (0.74, 2.27) | 3.64 | (1.75, 7.56) | 1.42 | (1.01, 2.00) | 0.02 |
| 3 | 2.11 | (0.93, 4.77) | 1.00 | (0.65, 1.55) | 1.29 | (0.68, 2.46) | 4.21 | (1.89, 9.39) | 1.45 | (1.00, 2.11) | 0.02 | |
| Prostate | 0 | 1.39 | (0.65, 2.97) | 1.00 | (0.83, 1.20) | 1.51 | (1.26, 1.82) | 1.49 | (1.05, 2.11) | 1.22 | (1.03, 1.45) | 0.01 |
| 3 | 1.13 | (0.44, 2.90) | 1.00 | (0.82, 1.23) | 1.41 | (1.14, 1.74) | 1.45 | (0.97, 2.19) | 1.18 | (0.96, 1.44) | 0.046 | |
| Bladder | 0 | 1.48 | (0.75, 2.92) | 1.00 | (0.79, 1.27) | 1.06 | (0.77, 1.46) | 0.53 | (0.23, 1.20) | 1.00 | (0.76, 1.33) | 0.46 |
| 3 | 1.39 | (0.51, 3.76) | 1.00 | (0.75, 1.34) | 1.11 | (0.75, 1.65) | 0.72 | (0.29, 1.79) | 1.09 | (0.78, 1.54) | 0.87 | |
| Kidney | 0 | 0.77 | (0.18, 3.22) | 1.00 | (0.74, 1.34) | 1.21 | (0.86, 1.72) | 1.67 | (0.94, 2.96) | 1.23 | (0.93, 1.63) | 0.14 |
| 3 | 1.17 | (0.28, 4.97) | 1.00 | (0.70, 1.43) | 1.42 | (0.96, 2.12) | 1.59 | (0.78, 3.24) | 1.20 | (0.86, 1.66) | 0.19 | |
| Brain & nervous system | 0 | 1.19 | (0.54, 2.59) | 1.00 | (0.82, 1.21) | 0.96 | (0.75, 1.24) | 0.89 | (0.55, 1.45) | 0.93 | (0.75, 1.15) | 0.67 |
| 3 | 1.32 | (0.56, 3.07) | 1.00 | (0.80, 1.25) | 0.95 | (0.70, 1.28) | 0.99 | (0.57, 1.73) | 0.97 | (0.75, 1.24) | 0.89 | |
| Hematologic cancers | 0 | 1.42 | (0.88, 2.31) | 1.00 | (0.88, 1.14) | 1.13 | (0.96, 1.32) | 1.19 | (0.90, 1.57) | 1.11 | (0.98, 1.27) | 0.16 |
| 3 | 1.21 | (0.64, 2.27) | 1.00 | (0.85, 1.17) | 1.18 | (0.97, 1.43) | 1.28 | (0.93, 1.78) | 1.15 | (0.99, 1.34) | 0.12 | |
| Lymphoma | 0 | 1.85 | (0.99, 3.46) | 1.00 | (0.82, 1.22) | 1.17 | (0.92, 1.47) | 0.84 | (0.52, 1.36) | 0.99 | (0.81, 1.21) | 0.97 |
| 3 | 1.29 | (0.51, 3.30) | 1.00 | (0.78, 1.29) | 1.35 | (1.00, 1.81) | 0.97 | (0.54, 1.73) | 1.07 | (0.84, 1.36) | 0.57 | |
| Myeloma | 0 | 1.64 | (0.48, 5.56) | 1.00 | (0.72, 1.39) | 0.98 | (0.64, 1.49) | 1.14 | (0.58, 2.21) | 1.06 | (0.76, 1.47) | 0.76 |
| 3 | 1.94 | (0.57, 6.68) | 1.00 | (0.70, 1.43) | 0.87 | (0.54, 1.41) | 1.20 | (0.59, 2.43) | 1.05 | (0.73, 1.50) | 0.78 | |
| Leukaemia | 0 | 0.82 | (0.29, 2.26) | 1.00 | (0.81, 1.23) | 1.14 | (0.89, 1.47) | 1.65 | (1.10, 2.47) | 1.27 | (1.05, 1.54) | 0.047 |
| 3 | 0.82 | (0.25, 2.67) | 1.00 | (0.78, 1.28) | 1.18 | (0.87, 1.60) | 1.66 | (1.03, 2.68) | 1.29 | (1.03, 1.61) | 0.09 | |
Number of events in each BMI category is presented in Web Table 2. Hyphened cells indicate no events.
All cancers after excluding lung and upper aero-digestive tract cancers.
Positive associations between BMI and site-specific cancers
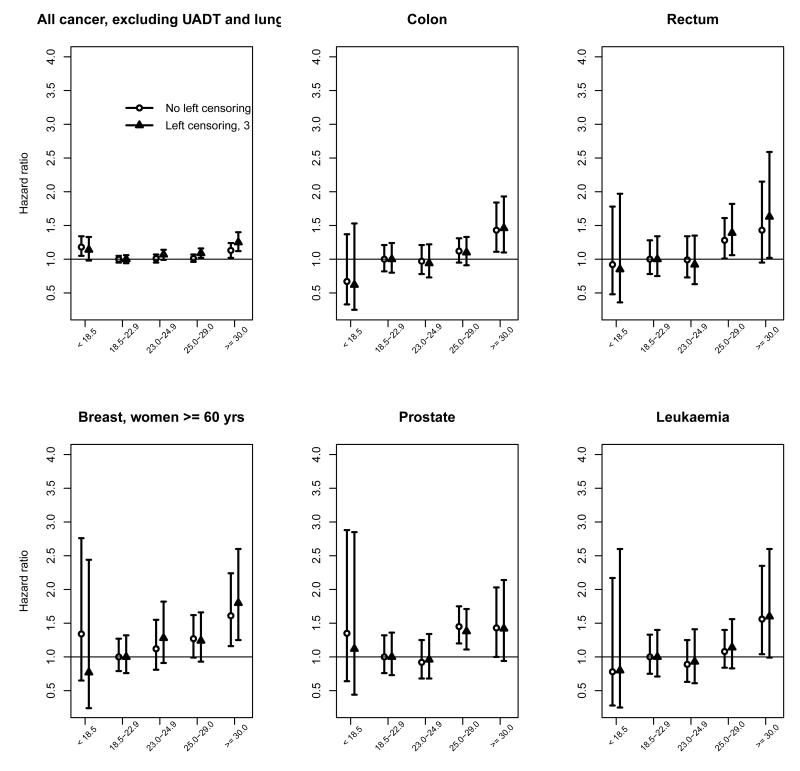
In six of the twenty cancer sites examined, namely cancers of the colon, rectum, breast in women aged ≥ 60 years, ovary, cervix and prostate, there was evidence of a significant log-linear trend in HR with BMI after controlling for age and smoking status (Table 4; all p-values for trend < 0.05). There was also a borderline significant trend for leukaemia (Table 4; ptrend = 0.09). As the total number of leukaemia cases (n = 129, left censored) was too small to conduct separate analyses, myeloid (which comprised 71 cases, 55%) and lymphoid (27 cases, 21%) leukaemias were combined with other specified or unspecified cell types in this analysis. An increased risk was also evident in the overweight category (≥ 25 to 29.9 kg/m2) for rectum and prostate (Table 4), but not in the upper (23.0 to 24.9 kg/m2) relative to lower (18.5 to 22.9 kg/m2) end of the normal BMI range (Figure 1). The HR for mortality associated with a 5 kg/m2 increase in BMI > 18.5 kg/m2 ranged from 1.13 (for rectal cancer) to 1.45 (for cervical cancer; Table 4).
Figure 1a.
Figure 1b.
Inverse associations between BMI and site-specific cancers
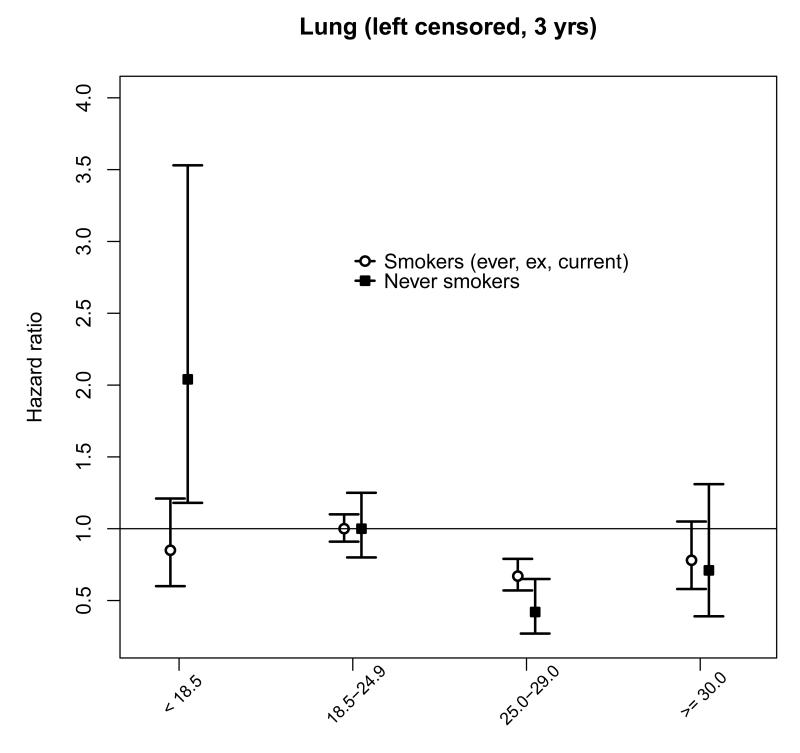
For cancer sites where there was an inverse association with BMI (Table 4), the reduced risk was significant for cancers of the upper aero-digestive tract (UADT), including oropharynx and larynx (analyzed together), and lung cancer in the overweight relative to the normal weight category. As these cancers are largely due to smoking, and thereby to avoid the possibility of confounding (smokers tend to have lower BMI compared with never smokers), we also examined the association in never-smokers (n = 181 920, 68% women) compared with ever, ex, or current smokers (n = 178 943, 11% women). Among people who had never smoked cigarettes, the inverse association in the overweight category persisted for lung cancer (Figure 2) with a HR (95% CI) of 0.42 (0.27 - 0.65; ptrend = 0.01) compared with 0.67 (0.57 - 0.79) in smokers (ptrend < 0.01), but not for UADT overall: the HR (95% CI) was 1.17 (0.75 - 1.83) in never smokers (ptrend = 0.91) compared with 0.54 (0.37 - 0.79) in smokers (ptrend = 0.01), or for oropharynx and larynx: the HR (95% CI) was 1.62 (0.91 to 2.89) in never smokers (ptrend = 0.76) compared with 0.36 (0.20 to 0.65) in smokers (ptrend < 0.01). All p-values for trend are for BMI > 18.5 kg/m2 (results by smoking status presented in Web Table 4).
Figure 2.
No clear relationships were found between BMI and other cancer sites, i.e. oesophagus, stomach, liver, pancreas, melanoma (skin), breast in all women or women aged < 60 years, bladder, kidney, uterus, brain and nervous system, lymphoma, myeloma, or all haematological cancers combined.
BMI and site-specific cancers by region and by sex
There was little evidence of a regional difference in the associations for BMI > 18.5 (kg/m2), except for oropharynx and larynx, where the association was inverse in ANZ, but absent in Asia (Table 5): the HR (95% CI) for a 5 kg/m2 increase in BMI was 0.46 (0.27 to 0.77) for ANZ compared with 0.99 (0.60 to 1.62) in Asia (pregion = 0.04). The association with lung cancer was similar: 0.86 (0.75 to 0.99) in ANZ compared with 0.85 (0.71 to 1.02) in Asia (pregion = 0.95) per 5 kg/m2.
Table 5. Region-specific age and smoking adjusted hazard ratios (HR) with 95% confidence intervals (CI) for cancer mortality according to body mass index after left censoring at 3 years (n = 401 215) in the Asia Pacific Cohort Studies Collaboration.
Models stratified by study and sex.
| Body mass index (kg/m2) |
Trend > 18.5 (kg/m2) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18.5-24.9 |
25.0-29.9 |
30.0-60.0 |
< 25 vs. ≥ 25 |
Per 5 units |
|
|||||||
| Cancer site | Region | HR | 95% CI | HR | 95% CI | HR | 95% CI | Pregion † | HR | 95% CI | Pregion † | Ptrend |
| All Cancer | ANZ | 1.00 | (0.93, 1.07) | 0.96 | (0.90, 1.03) | 1.12 | (1.01, 1.24) | 0.80 | 1.05 | (1.00, 1.11) | 0.29 | 0.20 |
| Asia | 1.00 | (0.95, 1.06) | 0.98 | (0.89, 1.07) | 1.07 | (0.81, 1.43) | 1.00 | (0.93, 1.08) | 0.93 | |||
| All Cancer* | ANZ | 1.00 | (0.92, 1.08) | 1.08 | (1.01, 1.16) | 1.23 | (1.10, 1.37) | 0.43 | 1.10 | (1.04, 1.17) | 0.39 | 0.004 |
| Asia | 1.00 | (0.94, 1.07) | 1.04 | (0.94, 1.15) | 1.20 | (0.87, 1.65) | 1.06 | (0.97, 1.15) | 0.29 | |||
| Upper aero-digestive | ANZ | 1.00 | (0.72, 1.38) | 0.58 | (0.40, 0.82) | 0.61 | (0.33, 1.14) | 0.16 | 0.69 | (0.50, 0.95) | 0.28 | 0.046 |
| Asia | 1.00 | (0.80, 1.26) | 0.93 | (0.63, 1.39) | 0.76 | (0.19, 3.06) ** | 0.88 | (0.64, 1.21) | 0.66 | |||
| Oesophagus | ANZ | 1.00 | (0.64, 1.57) | 0.72 | (0.44, 1.17) | 0.84 | (0.37, 1.89) | 0.75 | 0.92 | (0.61, 1.39) | 0.69 | 0.49 |
| Asia | 1.00 | (0.74, 1.35) | 0.89 | (0.52, 1.51) | 0.56 | (0.08, 4.03) ** | 0.82 | (0.54, 1.24) | 0.53 | |||
| Oropharynx, larynx | ANZ | 1.00 | (0.63, 1.58) | 0.45 | (0.27, 0.76) | 0.42 | (0.16, 1.13) ** | 0.08 | 0.46 | (0.27, 0.77) | 0.04 | 0.03 |
| Asia | 1.00 | (0.71, 1.42) | 1.01 | (0.55, 1.85) | 1.15 | (0.16, 8.35) ** | 0.99 | (0.60, 1.62) | 0.93 | |||
| Stomach | ANZ | 1.00 | (0.71, 1.40) | 0.89 | (0.66, 1.20) | 0.99 | (0.60, 1.63) | 0.45 | 1.12 | (0.87, 1.45) | 0.87 | 0.85 |
| Asia | 1.00 | (0.88, 1.14) | 1.11 | (0.90, 1.39) | 1.01 | (0.45, 2.26) | 1.09 | (0.91, 1.31) | 0.49 | |||
| Large intestine | ANZ | 1.00 | (0.83, 1.21) | 1.27 | (1.09, 1.48) | 1.55 | (1.23, 1.96) | 0.82 | 1.16 | (1.02, 1.32) | 0.62 | 0.004 |
| Asia | 1.00 | (0.80, 1.26) | 1.18 | (0.83, 1.67) | 2.21 | (0.97, 5.03) | 1.07 | (0.80, 1.44) | 0.11 | |||
| Liver | ANZ | 1.00 | (0.58, 1.73) | 1.18 | (0.76, 1.85) | 0.97 | (0.43, 2.19) | 0.87 | 1.04 | (0.70, 1.53) | 0.70 | 0.93 |
| Asia | 1.00 | (0.87, 1.15) | 1.04 | (0.84, 1.30) | 1.27 | (0.63, 2.56) | 1.13 | (0.93, 1.36) | 0.56 | |||
| Pancreas | ANZ | 1.00 | (0.72, 1.38) | 1.15 | (0.88, 1.51) | 0.88 | (0.53, 1.47) | 0.70 | 1.06 | (0.84, 1.34) | 0.55 | 0.89 |
| Asia | 1.00 | (0.77, 1.30) | 0.91 | (0.57, 1.48) | 1.35 | (0.33, 5.51) ** | 0.93 | (0.63, 1.35) | 0.95 | |||
| Lung | ANZ | 1.00 | (0.86, 1.17) | 0.65 | (0.54, 0.77) | 0.84 | (0.64, 1.09) | 0.57 | 0.86 | (0.75, 0.99) | 0.95 | 0.02 |
| Asia | 1.00 | (0.88, 1.14) | 0.76 | (0.60, 0.97) | 0.78 | (0.37, 1.65) | 0.85 | (0.71, 1.02) | 0.06 | |||
| Skin, melanoma | ANZ | 1.00 | (0.67, 1.49) | 0.58 | (0.38, 0.90) | 0.97 | (0.55, 1.73) | - | 1.08 | (0.78, 1.49) | 0.10 | 0.57 |
| Asia | - | - | - | - | - | - | - | - | - | |||
|
| ||||||||||||
| Breast, female | ANZ | 1.00 | (0.79, 1.27) | 0.99 | (0.77, 1.28) | 1.26 | (0.90, 1.74) | 0.67 | 1.10 | (0.94, 1.29) | 0.78 | 0.35 |
| Asia | 1.00 | (0.71, 1.40) | 0.88 | (0.49, 1.57) | 1.28 | (0.31, 5.29) ** | 1.03 | (0.66, 1.60) | 0.93 | |||
| Female genital organs | ANZ | 1.00 | (0.67, 1.48) | 1.56 | (1.11, 2.21) | 2.83 | (1.92, 4.16) | 0.27 | 1.43 | (1.17, 1.74) | 0.38 | 0.0003 |
| Asia | 1.00 | (0.70, 1.43) | 1.17 | (0.67, 2.02) | 2.09 | (0.65, 6.70) ** | 1.16 | (0.76, 1.77) | 0.30 | |||
| Prostate | ANZ | 1.00 | (0.76, 1.32) | 1.42 | (1.18, 1.71) | 1.42 | (0.96, 2.11) | 0.99 | 1.14 | (0.92, 1.41) | 0.31 | 0.07 |
| Asia | 1.00 | (0.60, 1.66) | 1.21 | (0.45, 3.26) ** | 3.89 | (0.53, 28.3) ** | 1.58 | (0.87, 2.87) | 0.34 | |||
| Bladder | ANZ | 1.00 | (0.64, 1.56) | 0.95 | (0.63, 1.41) | 0.69 | (0.29, 1.69) ** | 0.16 | 1.08 | (0.74, 1.57) | 0.86 | 0.53 |
| Asia | 1.00 | (0.54, 1.85) | 2.18 | (1.01, 4.71) | - | - | 1.17 | (0.52, 2.60) | 0.24 | |||
| Kidney | ANZ | 1.00 | (0.58, 1.71) | 1.42 | (0.95, 2.12) | 1.26 | (0.59, 2.69) | 0.80 | 1.12 | (0.77, 1.62) | 0.38 | 0.47 |
| Asia | 1.00 | (0.54, 1.85) | 1.20 | (0.46, 3.13) ** | 6.38 | (1.52, 26.9) ** | 1.56 | (0.81, 3.01) | 0.11 | |||
| Brain & nervous system | ANZ | 1.00 | (0.70, 1.43) | 0.86 | (0.62, 1.21) | 1.04 | (0.61, 1.78) | 0.71 | 1.02 | (0.77, 1.36) | 0.43 | 0.94 |
| Asia | 1.00 | (0.70, 1.44) | 1.15 | (0.66, 2.01) | - | - | 0.80 | (0.47, 1.37) | 0.88 | |||
| Hematologic cancers | ANZ | 1.00 | (0.78, 1.28) | 1.21 | (0.99, 1.47) | 1.27 | (0.93, 1.74) | 0.94 | 1.11 | (0.94, 1.30) | 0.20 | 0.19 |
| Asia | 1.00 | (0.74, 1.35) | 1.12 | (0.71, 1.78) | 2.02 | (0.63, 6.52) ** | 1.41 | (1.01, 1.99) | 0.35 | |||
| Lymphoma | ANZ | 1.00 | (0.68, 1.48) | 1.32 | (0.98, 1.78) | 0.94 | (0.53, 1.68) | 0.63 | 1.00 | (0.76, 1.31) | 0.26 | 0.85 |
| Asia | 1.00 | (0.60, 1.65) | 1.53 | (0.82, 2.84) | 1.52 | (0.20, 11.4) ** | 1.39 | (0.83, 2.31) | 0.32 | |||
| Myeloma | ANZ | 1.00 | (0.61, 1.63) | 0.73 | (0.45, 1.19) | 1.00 | (0.50, 2.03) | 0.15 | 0.92 | (0.61, 1.37) | 0.07 | 0.80 |
| Asia | 1.00 | (0.46, 2.19) | 1.88 | (0.64, 5.54) ** | 5.55 | (0.67, 46.0) ** | 2.13 | (0.93, 4.90) | 0.12 | |||
| Leukaemia | ANZ | 1.00 | (0.66, 1.51) | 1.43 | (1.06, 1.94) | 1.85 | (1.18, 2.91) | 0.14 | 1.30 | (1.02, 1.66) | 0.81 | 0.04 |
| Asia | 1.00 | (0.67, 1.50) | 0.65 | (0.27, 1.54) ** | 1.64 | (0.23, 11.9) ** | 1.21 | (0.68, 2.14) | 0.62 | |||
ANZ = Australia and New Zealand; Asia = mainland China, Hong Kong, Taiwan, Japan, South Korea, Singapore, and Thailand.
All cancers after excluding lung and upper aero-digestive tract cancers.
HR based on ≤ 5 events. Hyphened cells denote too few or no events. Number of events in each BMI category is presented in Web Table 3.
P-value of interaction term.
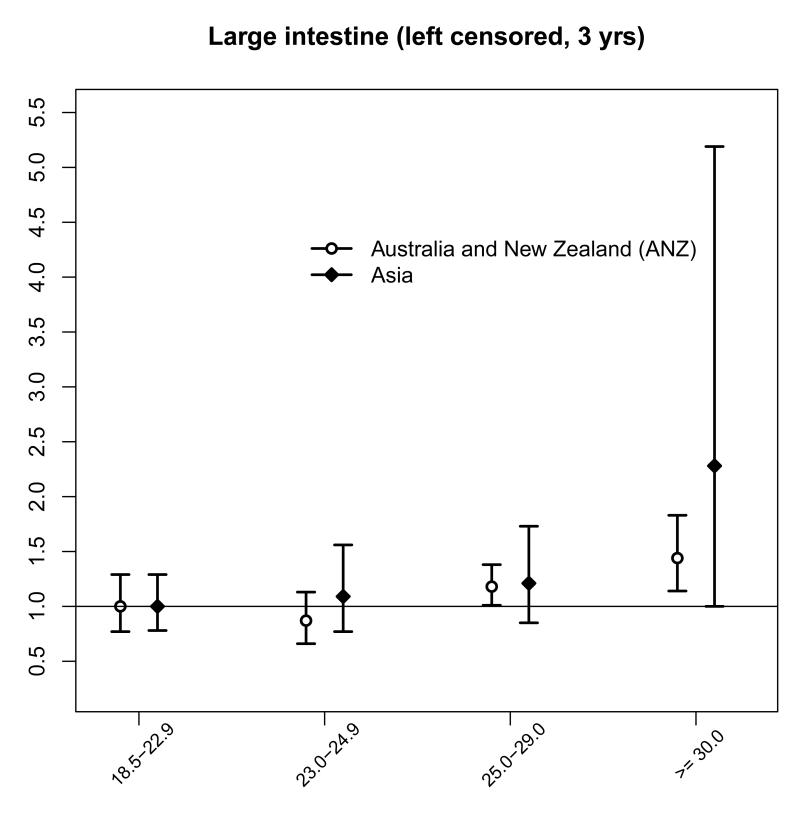
For all cancers and sub-sites positively associated with BMI in the main analysis, the region-specific HRs (Table 5) for ANZ, categorical and continuous effects, were similar (large intestine, prostate), or slightly strengthened (female genital organs, leukaemia), compared with the overall, left censored, HRs (Table 4). The HRs for Asia were generally lower and non-significant with wider CIs compared with ANZ. After sub-dividing the normal range, there was little evidence of increased risk in Asia compared with ANZ in the upper range (23.0 to 24.9 kg/m2), but there were relatively few sub-sites with a sufficient number of events in the reference category (18.5 to 22.9 kg/m2) for both regions (Web Table 3). Results are only shown for large intestine (Figure 3). Breast cancer by age-group was not further stratified by region due to small numbers in Asia.
Figure 3.
In the left-censored data there was little evidence of a sex difference in the observed BMI-cancer associations (Web Table 5). For large intestine a sex difference was indicated, but only in the categorical analysis (psex = 0.06): the HR (95% CI) for mortality among the obese relative to those of normal weight was 1.99 (1.45 to 2.73) in men compared to 1.28 (0.91 to 1.78) in women.
DISCUSSION
In the present study of 7211 cancer deaths in > 400 000 individuals from 39 prospective cohort studies within the Asia-Pacific region, we observed that among individuals with a BMI in excess of 18.5 kg/m2 there was a positive and continuous association between BMI and all cancer mortality such that a 5 kg/m2 increment in BMI was associated with a relative risk (HR) of about 1.10 (after excluding cases of lung and UADT cancer which had a negative association with BMI). Compared with individuals of normal weight, the relative risk of dying from cancer was about 1.05 for those who were overweight and 1.20 for those who were obese. The positive association between BMI and all cancer mortality was primarily driven by cancers of the large intestine, breast (in women over 60 years of age), ovary, cervix, prostate, and with leukaemia. For each of these cancers, in individuals above 18.5 kg/m2, a 5 kg/m2 higher BMI was associated with an increased relative risk of about 1.15 for both rectum and colon, 1.20 for breast and for prostate, 1.30 for leukaemia, 1.40 for ovaries, and 1.45 for cervix. Of particular note and contrary to previous findings6,13 there was little evidence of regional differences in relative risks between cohorts from Asia and ANZ. This regional consistency in the magnitude of association between excess weight and cancer is consistent with previous findings from the APCSC which have shown that the relationships between numerous cardiovascular risk factors with cardiovascular disease are broadly similar 20.
The effect sizes (per 5 kg/m2 or the obese category) for colon, rectal, breast (in older women), and prostate are largely in line with previous reports from other large studies of cancer mortality in predominantly Caucasian populations, such as the Million Women Study from the UK21 and the Prospective Studies Collaboration (PSC)18.
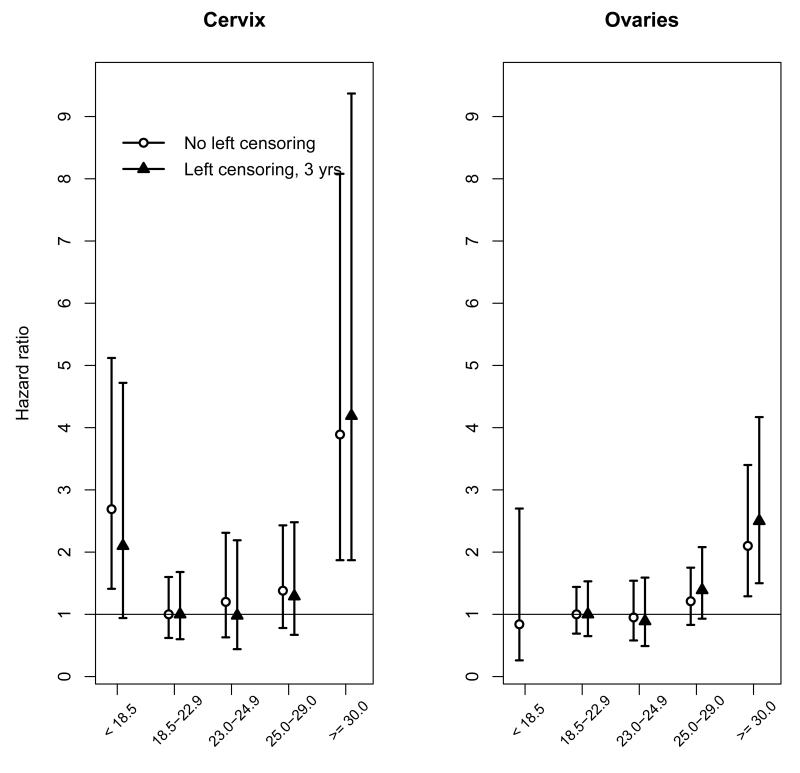
The current study confirms that obesity increases the risk of prostate cancer mortality in contrast to studies of incidence, which have often reported conflicting findings on the association between BMI and prostate cancer22. Recent evidence indicates that obesity may increase risks of aggressive disease, recurrence, and subsequent death, which may, in part, be explained by late detection and surgical difficulties during radical prostatectomy23. Our study also contributes to the growing evidence that excess weight is associated with increased risk of several haematological cancers.18,22,25,26. For example, in contrast to the PSC study18, but consistent with studies of UK women21 and US men,24 we observed an association between BMI and mortality from leukaemia. Moreover, we did not observe a statistically significant association between BMI and mortality from uterine cancer, which is considered to be strongly related with obesity, possibly because of the small number of events in our study (n = 37). Interestingly however, we did observe positive and significant associations between BMI and cancers of the ovaries and cervix, relationships that previously have been reported inconsistently in the literature 6,8,18,22,25, 27,28 .
The inverse associations between BMI and cancers of the UADT (mainly driven by oropharynx and larynx) and lung that we observe in the current study are a commonly reported finding6,8,9,22 . Some reassurance that these associations are unlikely to be mainly a result of reverse causality (as a consequence of only excluding the first three years of follow-up) is proffered from a recent study from the Prospective Studies Collaboration which reported similar inverse relationships after exclusion of the first 10 years of follow-up18. In contrast to most studies of BMI and lung cancer in Asian 8,9 and Western populations18,22,25 the association remained negative in non-smokers. One possibility is that causes of the high background mortality from lung cancer in countries such as China, e.g. indoor fuel use patterns,29 may be socio-economically correlated to BMI, but this remains speculative.
Our study was not able to confirm some commonly reported associations between BMI and specific cancer sites, including oesophagus, pancreas, and kidney. Some possible explanations include lack of histological data to distinguish between oesophageal adenocarcinoma and squamous cell carcinoma, for which BMI may have opposite effects6,22; a potentially stronger effect of central obesity than of BMI on pancreatic cancer30; and few renal cancer events.
Our study provided relatively many cases of stomach and liver cancer in contrast to studies from predominantly Caucasian populations.18,31 We could not confirm previous reports of positive associations with excess body weight 8,18,31, possibly indicating that the majority of stomach cancers are of the non-cardia type, for which H pylori infection and intake of salt or certain salted foods19 are more important risk factors than obesity, which may be more strongly related to adenocarcinoma of the gastric cardia (gastro-oesophageal junction).32 Similarly, infection with the hepatitis B or C viruses causing an estimated > 75% of liver cancer cases worldwide19, could be the predominant risk factor in Asia.
Regional differences
The observed BMI-cancer associations were generally consistent across Asia and ANZ, except for oropharynx and larynx. The association was inverse in ANZ but absent in Asia, which could be a chance finding, or reflect the comparatively early stage of the “smoking epidemic” in large parts of Asia29,33,34, as no association was found in never smokers. However, a regional difference due to smoking was not observed for lung cancer, possibly because the inverse association with BMI persisted in never smokers. Although smoking is a common risk factor for cancers of the oropharynx, larynx, and lung, their aetiologies also differ, e.g. for laryngeal cancer there is a multiplicative effect between smoking and alcohol consumption19, and cancers of the oropharynx may be related to human papilloma virus infection.35 Thus, other risk factors than smoking, if associated with BMI, may explain the discrepant regional difference for oropharynx and larynx, and lung cancer.
In the current study, including 31 cohorts from Asia, we did not observe a higher relative risk of mortality from colorectal cancer in Asians within the normal BMI range (23.0 to 24.9 kg/m2), as reported by Ning et al. in a recent meta-analysis of both prospective (incidence and mortality) and retrospective studies from ten Asian populations.13 A systematic review and meta-analysis of BMI and cancer incidence by Renehan et al.6 showed similar estimates for colon cancer risk in the Asia-Pacific and North America, but possibly higher compared to Europe and Australia.6 However, the association between BMI with breast cancers in younger and older women within the Asia–Pacific region (five studies) was stronger compared to North America, Europe and Australia. 6 We did not have enough events to analyse breast cancer by age-group and region as comparatively few breast cancers (about 25%) occurred in Asian populations, but there was no evidence of such a difference in the current analysis of fatal breast cancer overall.
There is still a paucity of data on BMI and cancer risk from Asian populations, and several challenges when comparing estimates across regions. Current studies of BMI and cancer risk are predominantly from Japan6 where national screening programs for colorectal and female breast cancer recommended from age 40 years36 may inflate incidence rates in younger age groups with lower BMI. In studies of cancer mortality, a higher risk in Asian compared to Western populations for the same level of BMI could also reflect poorer survival, at least for cancers where early diagnosis and/or therapy can markedly influence outcomes, such as large intestine and female breast.19
Study strengths and weaknesses
The principal strengths of the present study are its prospective design, and the relatively large sample size with more than three quarters of the participants from Asian populations for which data on BMI-cancer associations remain very limited. It lends credibility to the APCSC data that the observed regional distribution of cancer sites, such as a lower proportion of cancers associated with Western lifestyles, including large intestine and prostate, but a higher proportion of cancers of the stomach and liver in Asia compared to ANZ, are in accordance with global cancer statistics.19 However, the large regional difference in cancer occurrence and BMI level made regional comparisons difficult, with few events and very wide confidence intervals in Asia for “Western” cancers, in particular among the obese.
We were also unable to examine whether the associations of cancer mortality with other measures of obesity, such as waist circumference or waist:hip ratio, have a different relationship across ethnic groups. There has been considerable speculation in the literature regarding potential differences in the magnitude of the relationships between obesity with cardiovascular disease with some, but not all studies37 suggesting that the relationships are steeper in Asians compared with Caucasians possibly as a consequence of Asians having a higher percent body fat per given level of BMI and a greater propensity towards central obesity (which is metabolically more unfavourable than global obesity as measured by BMI). 38 In the current study, there was little evidence of any regional difference in the magnitude of the associations between BMI and cancer mortality as indicated by the test for regional interaction being non-significant for all but one (oropharynx and larynx) of the twenty sub-sites of cancer examined. However, given that the test for interaction has relatively low power to detect a difference, there is a high probability of type II error.
Other weaknesses of the APCSC relate to non-standardization of data collection. For smoking and drinking status, the “not current” category was included in several cohorts and therefore used in the analysis to preserve events, although these variables may only provide a crude adjustment. However, we addressed the issue of residual confounding by smoking by repeating the analyses of cancer sites inversely associated with BMI in never smokers. Further, the APCSC database does not have data on histological sub-types of cancer and there is limited and non-systematic information on socioeconomic status, a parameter that incorporates differences in a wide array of potential confounders including dietary factors and levels of physical activity. Moreover, we had no information on hormone replacement therapy or menopausal status in women. Thus, results should be interpreted with some caution. Moreover, as one quarter of the cohort were excluded due to missing values for BMI there is the potential for selection bias to distort the observed associations. However, given that our principal findings are largely in agreement with estimates from other meta-analyses and large prospective studies, we consider that it is unlikely to be a significant source of error. Finally, many of the cohorts included are occupational or from non-random samples of the population. This is unlikely however, to have had any appreciable effect on the results and conclusions of the current study given that the primary purpose of the paper was not to report on the prevalence but rather the magnitude of the relationships between BMI and cancer mortality which are unlikely to differ between populations from occupational cohorts or nationally representative studies.
Due to the short follow-up only the baseline data on BMI were used. Previous calculations based on APCSC studies with repeated measurements, indicate that BMI has a regression dilution coefficient close to one, so the current results were not adjusted.15 Similarly, due to the relatively short follow-up we were only able to left-censor the data by three years which may have been an insufficient length of time to exclude all underlying cancers (particularly those with a long latency period) and hence the possibility of reverse causality remains. However, some reassurance is gained by our observing comparable results with studies that were able to censor the data by 10 years.18
Conclusion
Many countries of the Asia-Pacific region are experiencing a steep increase in the prevalence of overweight and obesity in their populations largely as a consequence of the shift in traditional to more Western lifestyles characterized in large part by excess energy consumption, reduced levels of physical activity, and more affluent lifestyles.4 Effective strategies to prevent the increasing prevalence of overweight and obesity across Asia need to be developed and evaluated in order to reduce the burden of cancer that would be expected to occur if the obesity epidemic continues. However, the current study does not indicate a greater relative risk for cancer mortality in Asian populations for the same level of BMI, as has been suggested for diabetes and cardiovascular disease.16
Supplementary Material
Acknowledgement
CLP was supported by the Norwegian Foundation for Health and Rehabilitation via the Norwegian Cancer Society, GDB was supported by the UK Wellcome Trust, SHJ was supported by Seoul City Research and Business Development Program (10526), and RH was supported by a New South Wales and Heart Foundation Career Development Award. All personal funding sources are unrelated to funding of the APCSC study.
Dedication: Dedicated to the memory of our colleague, Professor Konrad Jamrozik, who passed away March 23, 2010.
Footnotes
Conflict of interest statement: The authors declared no conflicts of interest.
REFERENCES
- 1.World Cancer Research Fund. American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington, DC: 2007. [Google Scholar]
- 2.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 3.Asia Pacific Cohort Studies Collaboration. Lee C, Martiniuk A, Woodward M, Feigin V, Gu D, Jamrozik K, Lam T, Ni Mhurchu C, Pan W, Suh I, Ueshema H, Woo J, Huxley R. The burden of overweight and obesity in the Asia-Pacific region. Obes Rev. 2007;8:191–196. [Google Scholar]
- 4.Wu Y. Overweight and obesity in China. BMJ. 2006;333:362–363. doi: 10.1136/bmj.333.7564.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asia Pacific Cohort Studies Collaboration The burden of overweight and obesity in the Asia-Pacific region. Obes Rev. 2007;8:191–196. doi: 10.1111/j.1467-789X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 7.Kuriyama S, Tsubono Y, Hozawa A, Shimazu T, Suzuki Y, Koizumi Y, Suzuki Y, Ohmori K, Nishino Y, Tsuji I. Obesity and risk of cancer in Japan. Int J Cancer. 2005;113:148–157. doi: 10.1002/ijc.20529. [DOI] [PubMed] [Google Scholar]
- 8.Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, Ohrr H, Samet JM. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123:1892–1896. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Yang G, Zhou M, Smith M, Ge H, Boreham J, Hu Y, Peto R, Wang J, Chen Z. Body mass index and mortality from lung cancer in smokers and nonsmokers: A nationally representative prospective study of 220,000 men in China. Int J Cancer. 2009 doi: 10.1002/ijc.24527. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 11.Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr. 2009;102:632–641. doi: 10.1017/S0007114508207221. [DOI] [PubMed] [Google Scholar]
- 12.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116:963–971. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 13.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2009;11:19–30. doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 14.Asia Pacific Cohort Studies Collaboration Determinants of Cardiovascular Disease in the Asian Pacific Region: Protocol for a Collaborative Overview of Cohort Studies. CVD Prevention. 1999;2:281–289. [Google Scholar]
- 15.Woodward M, Barzi F, Martiniuk A, Fang X, Gu DF, Imai Y, Lam TH, Pan WH, Rodgers A, Suh I, Jee SH, Ueshima H, Huxley R. Cohort profile: the Asia Pacific Cohort Studies Collaboration. Int J Epidemiol. 2006;35:1412–1416. doi: 10.1093/ije/dyl222. [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 17.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 18.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 20.Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen T, Micheli A, Sant M, Weir HK, Elwood JM, Tsukuma H, Koifman S, E Silva GA, Francisci S, Santaquilani M, Verdecchia A, Storm HH, Young JL. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 21.Ni Mhurchu C, Rodgers A, Pan WH, Gu DF, Woodward M. Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310 000 participants. Int J Epidemiol. 2004;33:751–758. doi: 10.1093/ije/dyh163. [DOI] [PubMed] [Google Scholar]
- 22.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 24.Freedland SJ, Banez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: an analysis of the duke prostate center database. Prostate Cancer Prostatic Dis. 2009 doi: 10.1038/pcan.2009.11. [DOI] [PubMed] [Google Scholar]
- 25.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 26.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122:1418–1421. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 27.Schouten LJ, Rivera C, Hunter DJ, Spiegelman D, Adami HO, Arslan A, Beeson WL, van den Brandt PA, Buring JE, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, Hankinson SE, Lacey JV, Jr., Leitzmann M, Lukanova A, Marshall JR, Miller AB, Patel AV, Rodriguez C, Rohan TE, Ross JA, Wolk A, Zhang SM, Smith-Warner SA. Height, body mass index, and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2008;17:902–912. doi: 10.1158/1055-9965.EPI-07-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez C, Calle EE, Fakhrabadi-Shokoohi D, Jacobs EJ, Thun MJ. Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:822–828. [PubMed] [Google Scholar]
- 29.Liu BQ, Peto R, Chen ZM, Boreham J, Wu YP, Li JY, Campbell TC, Chen JS. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–1422. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansary-Moghaddam A, Huxley R, Barzi F, Lawes C, Ohkubo T, Fang X, Jee SH, Woodward M. The effect of modifiable risk factors on pancreatic cancer mortality in populations of the Asia-Pacific region. Cancer Epidemiol Biomarkers Prev. 2006;15:2435–2440. doi: 10.1158/1055-9965.EPI-06-0368. [DOI] [PubMed] [Google Scholar]
- 31.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–878. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 33.Huxley R, Jamrozik K, Lam TH, Barzi F, Ansary-Moghaddam A, Jiang CQ, Suh I, Woodward M. Impact of smoking and smoking cessation on lung cancer mortality in the Asia-Pacific region. Am J Epidemiol. 2007;165:1280–1286. doi: 10.1093/aje/kwm002. [DOI] [PubMed] [Google Scholar]
- 34.Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9:655–664. doi: 10.1038/nrc2703. [DOI] [PubMed] [Google Scholar]
- 35.Heck JE, Berthiller J, Vaccarella S, Winn DM, Smith EM, Shan’gina O, Schwartz SM, Purdue MP, Pilarska A, Eluf-Neto J, Menezes A, McClean MD, Matos E, Koifman S, Kelsey KT, Herrero R, Hayes RB, Franceschi S, Wunsch-Filho V, Fernandez L, Daudt AW, Curado MP, Chen C, Castellsague X, Ferro G, Brennan P, Boffetta P, Hashibe M. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39:166–181. doi: 10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore MA, Sobue T. Cancer research and control activities in Japan: contributions to international efforts. Asian Pac J Cancer Prev. 2009;10:183–200. [PubMed] [Google Scholar]
- 37.Huxley R, James WP, Barzi F, Patel JV, Lear SA, Suriyawongpaisal P, Janus E, Caterson I, Zimmet P, Prabhakaran D, Reddy S, Woodward M, Obesity in Asia Collaboration Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9(Suppl 1):53–61. doi: 10.1111/j.1467-789X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Chen X, Jang Y, et al. Obesity, coronary heart disease risk factors and diabetes in Chinese: an approach to the criteria of obesity in the Chinese population. Obes Rev. 2002;3:167–72. doi: 10.1046/j.1467-789x.2002.00067.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.