Abstract
Aims
The aim of this study was to compare the strength of associations and discrimination capability of body mass index (BMI), waist circumference (WC) and waist-to-hip ratio (WHR) with cardiovascular disease risk in individuals with type-2-diabetes.
Methods and results
11,140 men and women were followed for a mean of 4.8 years. Cox proportional hazard models were used to compute the hazard ratios (HR) and 95% confidence intervals (95% CI) for one standard deviation (SD) increase in baseline BMI (SD: 5 kg/m2), WC (SD: 13 cm) and WHR (SD: 0.08) with cardiovascular disease risk. After adjustment, HR (95% CI) for WC were 1.10 (1.03-1.18) for cardiovascular events, 1.13 (1.03-1.24) for coronary events, and 1.08 (0.98-1.19) for cardiovascular deaths. Estimates for WHR were 1.12 (1.05-1.19), 1.17 (1.08-1.28) and 1.19 (1.09-1.31). BMI was not related to any of these outcomes. While the receiver operating characteristic curve could not differentiate between anthropometric variables (p-values ≥ 0.24), the relative integrated discrimination improvement statistic showed an enhancement in the discrimination capabilities of models using WHR for cardiovascular outcomes, except for cerebrovascular events.
Conclusion
Strengths of associations and discrimination statistics suggested that WHR was the best predictor of cardiovascular events and mortality in patients with type-2-diabetes and BMI the worst.
Keywords: body mass index, waist circumference, waist-to-hip ratio, type 2 diabetes, cardiovascular disease
Introduction
In the last two decades there have been marked secular increases in the prevalence of obesity in the majority of countries worldwide [1]. More than 1.1 billion individuals meet current definitions for overweight or obesity [2] which puts them at increased risk for number of chronic diseases including cardiovascular diseases and type 2 diabetes.
In large scale observational studies, the degree of adiposity is typically assessed using the following indicators: waist-to-hip ratio (WHR), waist circumference (WC), or, most commonly, body mass index (BMI). In non-diabetic populations, the magnitude of the association between obesity and cardiovascular disease is suggested to be stronger for WHR than with either WC or BMI [3-6]. However, prospective cohort studies comparing the associations in individuals with type 2 diabetes are sparse [7] and reveal inconsistent findings [8-19].
To our knowledge, no study has prospectively assessed the relative discriminative capability of a range of different anthropometric markers on the risk of cardiovascular disease in a cohort of individuals with type-2-diabetes. Identifying the best clinical anthropometric marker to predict this risk is critical in individuals with type-2-diabetes since it has been suggested that modifications in body composition, especially in visceral adipose tissue, may modify this association [20].
The primary objective of the present analyses was to assess the magnitude of association of each anthropometric marker (BMI, WC and WHR) for cardiovascular disease risk among participants in the ADVANCE trial (Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation) [21,22]. A secondary objective was to compare the discrimination capability of these markers on the same risk.
METHODS
The study protocol for ADVANCE has been reported in detail elsewhere.25-28 In brief, ADVANCE was a 2×2 factorial randomised controlled trial of blood pressure and glucose lowering on the incidence of microvascular and macrovascular events among individuals with type-2-diabetes. A total of 11,140 patients were randomly allocated to a fixed combination of perindopril and indapamide or matching placebo, and an intensive glicazide modified release (MR)-based glucose control regimen or standard blood glucose control. Mean duration of follow-up was 4.8 years.
Baseline assessment
Data were collected on medical history, current medical treatment, and major risk factors using standard protocols. The baseline anthropometric markers presented here are those obtained at the initial registration visit. Height and weight were measured without shoes, and without outdoor or heavy clothing. BMI was defined as weight (kg)/height (m2). WC was measured midway between the inferior margin of the last rib and the crest of the ileum and hip circumference (HC) around the pelvis at the point of maximum protrusion of the buttocks, both in a horizontal plane, without compressing the soft tissues. WC and HC were recorded to the nearest cm and WHR was defined as a ratio of WC to HC.
Ascertainment of cardiovascular disease outcomes
Outcomes were restricted to the first event recorded during follow-up. Major cardiovascular disease was a composite of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke. Major coronary events included death from coronary heart disease, sudden death and non-fatal myocardial infarction. Major cerebrovascular events included death from cerebrovascular events and non-fatal stroke. Outcomes were coded according to the 10th revision of the International Classification of Diseases (ICD-10), and major events (suspected myocardial infarction, suspected stroke and all deaths) were centrally validated by an independent endpoint committee.
Statistical methods
Cox proportional hazard regression models were used to estimate the hazard ratio (HR) and 95% confidence interval (95% CI) for a one standard deviation (SD) increase in each anthropometric risk factor in relation to cardiovascular disease outcomes. HRs (95% CI) for the participants in the fifths of anthropometric variables distribution were compared and a linear trend was computed. The corresponding confidence intervals were calculated by the floating absolute risk method [23]. Models adjusted for age-, sex-, ethnicity-, current smoking and treatment allocation as well as further adjusted for systolic blood pressure, total cholesterol, HDL cholesterol, HbA1c and statin use, aspirin use, other antiplatelets and blood pressure lowering medications classes (beta blockers, calcium channel blocker, ACE inhibitors, ARA II, diuretics, others) (Supplementary Table 1) are presented. Interactions between ethnicity, sex or randomised treatment group and each anthropometric variable were tested for each outcome. Potential quadratic interaction terms for each anthropometric variable with the same outcomes were also investigated.
The ability of anthropometric variables to discriminate between participants who developed an event during follow-up and those who did not was assessed using area under the receiver operating characteristic curves and the relative integrated discrimination improvement (RIDI) which measures the percentage increased discrimination when an extra variable is added to a prediction model [24-27]. Area under the receiver operating characteristic curve (AUC) comparisons were examined with nonparametric methods [28]. Bootstrap methods were used to derive the 95% CI for the RIDI estimates, which were based on 1000 replications. The likelihood ratio χ2 statistics for each event category were calculated by comparing multivariate regression models with and without a single anthropometric variable to assess improvement in model fit. Secondary analyses were conducted testing for the combination of anthropometric variables (BMI+WC or BMI+WHR) in Cox models. All analyses used SAS software v.9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics of the study population are given in Table 1. Mean BMI was 28 kg/m2 (SD = 5), WC was 98 (13) cm and WHR was 0.93 (0.08). The Pearson correlation coefficients for the anthropometric variables were: 0.83 (BMI vs. WC), 0.62 (WC vs. WHR), 0.42 (BMI vs. WHR) in men, and 0.81 (BMI vs. WC), 0.44 (WC vs. WHR) and 0.11 (BMI vs. WHR) in women respectively. During follow-up (n, cumulative incidence %), 1147 major cardiovascular events (10.3%), 647 major coronary events (5.8%), 584 major cerebrovascular events (4.3%) and 542 cardiovascular deaths (4.9%) were recorded.
Table 1.
Baseline characteristics of the population (n=11,140)
| Variables | |
|---|---|
| Age (years), mean (SD) | 65.8 (6.4) |
| Women, (%) | 42.5% |
| Body mass index (kg/m2), mean (SD) | 28 (5) |
| Waist circumference (cm), mean (SD) | 98 (13) |
| Waist to hip ratio, mean (SD) | 0.93 (0.08) |
| Blood pressure (mmHg), mean (SD) | |
| Systolic blood pressure | 145 (22) |
| Diastolic blood pressure | 81 (11) |
| Known duration of diabetes (years)* | 7 (3-11) |
| Total cholesterol (mmol/L), mean (SD) | 5.2 (1.2) |
| Current smoker, (%) | 14% |
| Use of statins, (%) | 28% |
| Caucasians, (%) | 63% |
| History of major macrovascular disease, (%) | 32% |
Median (25-75th percentiles)
BMI was not significantly related to any of the cardiovascular outcomes (all p-values > 0.16, Figure 1), although there was some suggestion of an inverse association with cerebrovascular events (p for linear trend = 0.04). A positive continuous association was observed for WC with cardiovascular and coronary events (both p-values < 0.05). These relationships were linear (both p values for linear trend < 0.05). A similar continuous positive association was observed for WHR with cardiovascular, coronary events and cardiovascular death (all p-values ≤ 0.001), but not with cerebrovascular events (p = 0.13), with all associations being linear (p values for linear trend < 0.05).
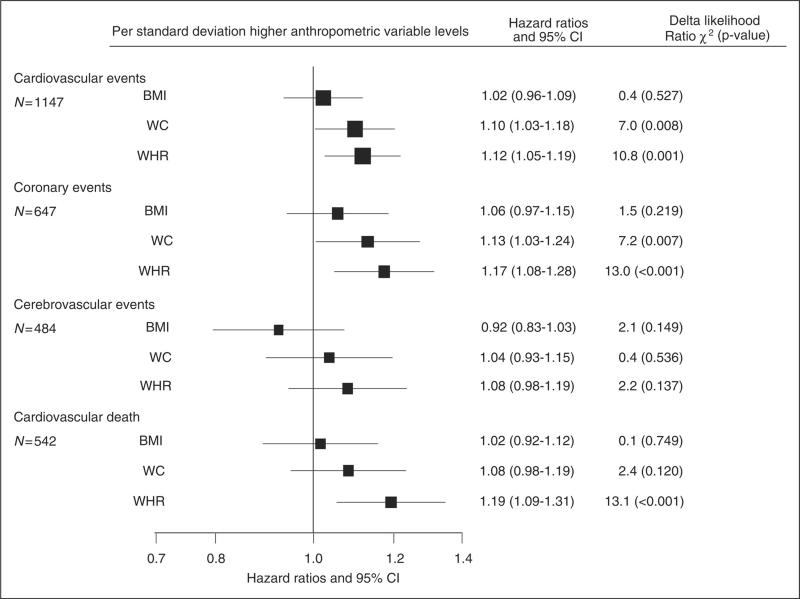
Figure 1.
Adjusted hazard ratios and 95% confidence intervals (95% CI) for major cardiovascular outcomes per standard deviation increment in anthropometric variables
All analyses were adjusted by age, gender, smoking status, treatment allocation and ethnicity. Boxes are for the point estimates (hazard ratios) and the horizontal bars represent the 95% confidence intervals. The size of the box is proportional to the inverse variance of the natural logarithm of the hazard ratio.
Delta likelihood Ratio χ2 = difference in the likelihood ratio χ2 statistics for each event category calculated by comparing multivariate regression models without and with a single anthropometric variable to assess improvement in model fit.
BMI = body mass index, WC = waist circumference, WHR = waist-to-hip ratio. Standard deviations are 5 kg/m2 for BMI, 13 cm for WC and 0.08 for WHR.
The adjusted HRs (95% CI) for a one SD increment in BMI in the risk of cerebrovascular events was 0.92 (0.83 - 1.03) (Figure 1). Multivariate HR (95% CI) associated with a one higher SD for cardiovascular, coronary events and cardiovascular death were 1.10 (1.03-1.18), 1.13 (1.03-1.24) and 1.08 (0.98-1.19) for WC, respectively and 1.12 (1.05-1.19), 1.17 (1.08-1.28) and 1.19 (1.09-1.31) for WHR.
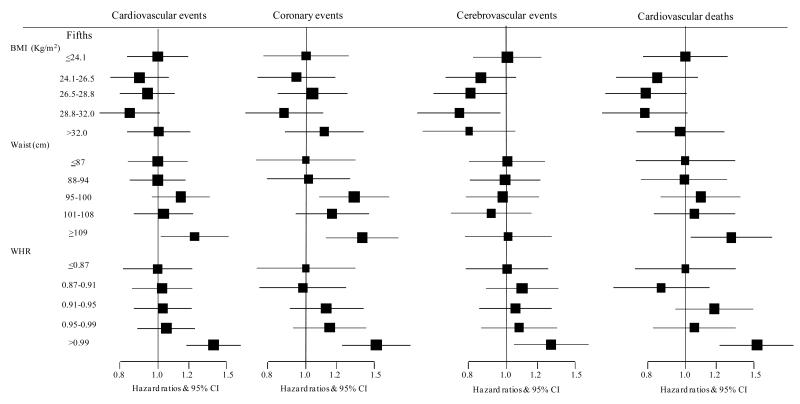
The difference in likelihood-ratio χ2 tests indicated that for every outcome, the association was always stronger with WHR compared to WC or BMI. All the above associations were similar when the fifths of the distribution of each anthropometric variable were compared (Figure 2). Sensitivity analyses excluding patients with macrovascular disease at baseline and with further adjustment did not materially alter these results (Supplementary Table 1).
Figure 2.
Adjusted hazard ratios and 95% confidence interval (95% CI) for major cardiovascular outcomes comparing the fifths for each anthropometric variable.
All analyses were adjusted by age, gender, smoking status, treatment allocation and ethnicity. Boxes are for the point estimates (hazard ratios) and the horizontal bars represent the 95% confidence intervals. The size of the box is proportional to the inverse variance of the natural logarithm of the hazard ratio.
BMI = body mass index, WC = waist circumference, WHR = waist-to-hip ratio.
There was no consistent interaction either between the three anthropometric variables and sex, except for BMI and major cardiovascular events, due to opposite associations in men and women, as follows: multivariate HR (95% CI) associated with a one higher SD were 1.08 (1.00-1.17) and 0.93 (0.82-1.05). Similarly, there was a significant interaction between WHR and ethnicity for the risk of coronary events, resulting from a significant positive association in non-Caucasians [HR (95%) for one SD higher WHR: 1.33 (1.16-1.51)] and a borderline association in Caucasians, HR (95% CI): 1.11 (0.99-1.23). Further, there was no significant interaction with randomised treatments (all p ≥ 0.45) and no consistent quadratic interaction (BMI2, WC2 or WHR2) for major cardiovascular events (all p ≥ 0.17).
For prediction of any of the outcomes, there was no significant difference in the area under the receiver operating characteristic curves between the three anthropometric variables (all overall p-value for differences ≥ 0.24; Table 2). On the other hand, using the RIDI statistics (Table 3), a significant increase of 2.8% (major cardiovascular), 3.2% (major coronary) and 1.3% (cardiovascular death) of the RIDI was observed when baseline waist circumference was used instead of baseline BMI. These results indicate an enhancement in the discriminative capability of the models using waist circumference instead of body mass index. A similar and stronger pattern was observed when using waist-to-hip ratio instead of body mass index. Results for major cerebrovascular events indicated either a worsening in the predictive value (-2.5% with WC vs. BMI) or a null effect (WHR vs. BMI). The advantage of using WHR instead of WC was apparent for all outcomes (2.0 to 5.6%).
Table 2.
Area under the receiver operating characteristic curves and 95% confidence intervals (95% CI) for the prediction of major cardiovascular events, major coronary events, major cerebrovascular events and cardiovascular death based on baseline anthropometric variables
| Variables | Major cardiovascular (n = 1147) | Major coronary (n = 647) | Major cerebrovascular (n = 584) | Cardiovascular death (n = 542) |
|---|---|---|---|---|
| BMI (A) | 0.62 (0.60 to 0.64) | 0.65 (0.63 to 0.67) | 0.62 (0.60 to 0.65) | 0.66 (0.64 to 0.69) |
| WC (B) | 0.62 (0.61 to 0.64) | 0.65 (0.63 to 0.67) | 0.62 (0.60 to 0.65) | 0.66 (0.64 to 0.69) |
| WHR (C) | 0.62 (0.61 to 0.64) | 0.65 (0.63 to 0.68) | 0.62 (0.60 to 0.65) | 0.67 (0.65 to 0.69) |
| BMI + WC (D) | 0.62 (0.61 to 0.64) | 0.65 (0.63 to 0.67) | 0.63 (0.60 to 0.65) | 0.67 (0.64 to 0.69) |
| BMI + WHR (E) | 0.62 (0.61 to 0.64) | 0.65 (0.63 to 0.68) | 0.62 (0.60 to 0.65) | 0.67 (0.65 to 0.69) |
| P value differences | ||||
| A-B | 0.28 | 0.44 | 0.53 | 0.52 |
| A-C | 0.15 | 0.14 | 0.69 | 0.10 |
| A-D | 0.20 | 0.50 | 0.13 | 0.48 |
| A-E | 0.15 | 0.14 | 0.20 | 0.09 |
| B-C | 0.46 | 0.25 | 0.21 | 0.10 |
| B-D | 0.38 | 0.77 | 0.11 | 0.65 |
| B-E | 0.46 | 0.23 | 0.23 | 0.11 |
| C-D | 0.92 | 0.29 | 0.26 | 0.14 |
| C-E | 0.65 | 0.78 | 0.41 | 0.60 |
| D-E | 0.61 | 0.29 | 0.48 | 0.12 |
| Overall p | 0.57 | 0.50 | 0.39 | 0.34 |
BMI, body mass index; WC, waist circumference; WHR, waist to hip ratio; Overall p, p-value for the difference in the AUC for the four models
Models are adjusted for age, sex, current smoking, ethnicity and treatment allocation
Table 3.
Relative integrated discrimination improvement statistics (RIDI, %) comparing models with a given anthropometric variable with models for which the variable has been replaced by another anthropometric variable or their combination*
| Models | RIDI (95% confidence interval) ** | ||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Major cardiovascular (n = 1147) | Major coronary (n = 647) | Major Cerebrovascular (n = 584) | Cardiovascular death (n= 542) |
| BMI | WC | 2.8 (2.7 to 3.0) | 3.2 (3.0 to 3.3) | −2.5 (−2.8 to −2.2) | 1.3 (1.2 to 1.4) |
| WHR | 4.9 (4.6 to 5.1) | 6.2 (6.0 to 6.5) | −0.3 (−0.7 to 0.1)† | 7.0 (6.8 to 7.3) | |
| BMI + WC | 5.8 (5.6 to 6.1) | 4.6 (4.4 to 4.8) | 9.0 (8.6 to 9.4) | 2.9 (2.7 to 3.0) | |
| BMI + WHR | 5.2 (5.2 to 5.7) | 7.0 (6.8 to 7.3) | 4.7 (4.4 to 5.1) | 7.8 (7.6 to 8.1) | |
| WC | WHR | 2.0 (1.8 to 2.2) | 3.0 (2.7 to 3.2) | 2.3 (2.1 to 2.6) | 5.6 (5.4 to 5.8) |
| BMI + WC | 2.9 (2.7 to 3.0) | 1.3 (1.2 to 1.4) | 12.1 (11.6 to 12.6) | 1.6 (1.4 to 1.7) | |
| BMI + WHR | 2.6 (2.4 to 2.7) | 3.7 (3.5 to 4.0) | 7.7 (7.2 to 8.2) | 6.4 (6.2 to 6.7) | |
| WHR | BMI + WC | 1.0 (0.8 to 1.2) | −1.5 (−1.7 to −1.3) | 9.6 (9.1 to 10.1) | −3.8 (−3.9 to −3.6) |
| BMI + WHR | 0.6 (0.5 to 0.6) | 0.8 (0.7 to 0.8) | 5.3 (4.9 to 5.6) | 0.8 (0.7 to 0.8) | |
| BMI + WC | BMI + WHR | −0.3 (−0.5 to −0.1) | −5.0 (−5.2 to −4.8) | −3.8 (−4.0 to −3.5) | 4.8 (4.6 to 5.0) |
BMI, body mass index; WC, waist circumference; WHR, waist to hip ratio; RIDI, relative integrated discrimination improvement.
Models are adjusted for age, sex, current smoking, ethnicity and treatment allocation
For each line the models compared the replacement of the second with the first anthropometric variable in the first column;
All comparisons significant at p<0.001
Non-significant (p=0.07)
Models combining two anthropometric variables such as BMI and WC or BMI and WHR provided some improvements in prediction of CVD. However, these combined models were not superior to WHR alone, except for the prediction of cerebrovascular event where BMI + WC did marginally better based on Akaike’s Information Criterion comparison and RIDI analyses (Table 3).
DISCUSSION
We have presented a comparison of the ability of different anthropometric markers to predict the risk of major cardiovascular diseases in individuals with type-2-diabetes. Positive, linear and continuous associations were observed between WC and WHR and cardiovascular outcomes. The relative magnitudes of the associations were systematically higher when WHR was considered, in particular for cardiovascular death. By comparison, BMI performed the least well out of all three measures at predicting vascular risk in this population. Using the RIDI statistics, WHR exhibited enhanced predictive capability compared to both body mass index and waist circumference.
Many studies in populations without diabetes have indicated a positive association between markers of abdominal obesity, either WHR or WC, and cardiovascular disease events [4,5]. With the exception of one study [7], we are not aware of any other prospective cohort studies of participants with diabetes in which the discrimination capabilities of different markers of abdominal obesity to predict the risk of cardiovascular disease have been compared.
Data on the relationship between BMI and coronary disease outcomes in populations without diabetes are numerous and largely in agreement [29,30], whereas reports of the association between BMI and stroke risk are conflicting [30-33]. In the two largest European surveys, BMI was variously unrelated to cerebrovascular disease mortality [5], inversely related in the lower range or positively related in the upper range of BMI [34]. The true nature of the association between BMI and the risk of stroke remains unclear.
Few prospective cohort studies have investigated these associations in populations with type-2-diabetes and these have yielded inconsistent findings. In some, BMI was associated with increased risk of cardiovascular disease or total mortality in a variety of sub-groups [8-13], whereas others have not found such an association [14-17]. The association of body mass index with the risk of stroke remains unclear in cohorts with type-2-diabetes [18,19].
The role of anthropometric markers in predicting CVD risk in individuals with or without diabetes may not have the same strength of association. Premature stiffening of arteries, release of pro-inflammatory markers or even modification in body composition could modify these associations [20,35,36]. Other studies have also shown that visceral fat is more closely related to WHR or even WC than to BMI [37], and as a consequence may have a stronger influence on cardiovascular disease risk [20,38,39]. Furthermore, relationships between BMI and CVD risk may be attributable to the relationship between BMI and diabetes. In individuals with established diabetes, the predictive value of BMI may add little additional information, whereas measures of central obesity that are more closely related to other metabolic abnormalities, such as blood lipids may remain predictive. In a cross-sectional study of subjects with type 2 diabetes, higher cholesterol VLDL and LDL particle number, larger VLDL particles and smaller LDL and HDL particles were associated with higher visceral adipose tissue [40]. This study emphasizes the heterogeneity of the phenotype of type 2 diabetes patients, in terms of metabolic profile, as shown before for the difference in the adipose tissue repartition in diabetic individuals versus healthy controls [36].
In conclusion, this was the first cohort study to assess the relative importance of different adiposity markers in predicting cardiovascular disease risk in a large population of individuals with type 2 diabetes. Using the RIDI statistics, but not the AUC/ROC approach, there was a suggestion that markers of abdominal obesity, particularly WHR, were the best predictor of future CVD events.
Supplementary Material
Supplementary table 1. Fully-adjusted hazard ratios and 95% confidence intervals (95% CI) for major cardiovascular outcomes per standard deviation increment in anthropometric variables in participants without prior history of cardiovascular disease at baseline
Acknowledgements/sources of Funding
The ADVANCE study was funded by grants from Servier and the National Health and Medical Research Council of Australia. Sébastien Czernichow holds a Fellowship awarded by the Institut Servier-France and Assistance Publique – Hôpitaux de Paris, France. Rachel Huxley is a recipient of a Heart Foundation of Australia Career Development Award. The Medical Research Council (MRC) Social and Public Health Sciences Unit receives funding from the UK MRC and the Chief Scientist Office at the Scottish Government Health Directorates. David Batty is a Welcome Trust Career Development Fellow (WBS U.1300.00.006.00012.01).
Footnotes
Conflicts of Interest/Disclosures Statement John Chalmers holds research grants from Servier, administered through the University of Sydney as Co-Principal Investigator for ADVANCE. John Chalmers, Bruce Neal, Mark Woodward and Sophia Zoungas have received lecturing fees from Servier.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 4.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur. Heart J. 2007;28:850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 5.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 6.van D, I, Kromhout D, Geleijnse JM, Boer JM, Verschuren WM. Body mass index and waist circumference predict both 10-year nonfatal and fatal cardiovascular disease risk: study conducted in 20,000 Dutch men and women aged 20-65 years. Eur. J. Cardiovasc. Prev. Rehabil. 2009;16:729–734. doi: 10.1097/HJR.0b013e328331dfc0. [DOI] [PubMed] [Google Scholar]
- 7.Sone H, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Ishibashi S, et al. Waist circumference as a cardiovascular and metabolic risk in Japanese patients with type 2 diabetes. Obesity. (Silver. Spring) 2009;17:585–592. doi: 10.1038/oby.2008.481. [DOI] [PubMed] [Google Scholar]
- 8.Cho E, Manson JE, Stampfer MJ, Solomon CG, Colditz GA, Speizer FE, et al. A prospective study of obesity and risk of coronary heart disease among diabetic women. Diabetes Care. 2002;25:1142–1148. doi: 10.2337/diacare.25.7.1142. [DOI] [PubMed] [Google Scholar]
- 9.Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Batty GD, Kivimaki M, Smith GD, Marmot MG, Shipley MJ. Obesity and overweight in relation to mortality in men with and without type 2 diabetes/impaired glucose tolerance: the original Whitehall Study. Diabetes Care. 2007;30:2388–2391. doi: 10.2337/dc07-0294. [DOI] [PubMed] [Google Scholar]
- 11.Pettitt DJ, Lisse JR, Knowler WC, Bennett PH. Mortality as a function of obesity and diabetes mellitus. Am. J. Epidemiol. 1982;115:359–366. doi: 10.1093/oxfordjournals.aje.a113313. [DOI] [PubMed] [Google Scholar]
- 12.Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005;28:799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- 13.Zoppini G, Verlato G, Leuzinger C, Zamboni C, Brun E, Bonora E, et al. Body mass index and the risk of mortality in type II diabetic patients from Verona. Int. J. Obes. Relat Metab Disord. 2003;27:281–285. doi: 10.1038/sj.ijo.802199. [DOI] [PubMed] [Google Scholar]
- 14.Rosengren A, Welin L, Tsipogianni A, Wilhelmsen L. Impact of cardiovascular risk factors on coronary heart disease and mortality among middle aged diabetic men: a general population study. BMJ. 1989;299:1127–1131. doi: 10.1136/bmj.299.6708.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford ES, DeStefano F. Risk factors for mortality from all causes and from coronary heart disease among persons with diabetes. Findings from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Am. J. Epidemiol. 1991;133:1220–1230. doi: 10.1093/oxfordjournals.aje.a115834. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi N, Fuller JH. Mortality risk by body weight and weight change in people with NIDDM. The WHO Multinational Study of Vascular Disease in Diabetes. Diabetes Care. 1995;18:766–774. doi: 10.2337/diacare.18.6.766. [DOI] [PubMed] [Google Scholar]
- 17.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA, et al. Risk of stroke in people with type 2 diabetes in the UK: a study using the General Practice Research Database. Diabetologia. 2006;49:2859–2865. doi: 10.1007/s00125-006-0493-z. [DOI] [PubMed] [Google Scholar]
- 19.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Nunez L, Gudbjornsdottir S, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52:65–73. doi: 10.1007/s00125-008-1190-x. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu P, Poirier P, Pibarot P, Lemieux I, Despres JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 21.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 22.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 23.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat. Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 24.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann. Intern. Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 27.Kengne AP, Czernichow S, Huxley R, Grobbee D, Woodward M, Neal B, et al. Blood Pressure Variables and Cardiovascular Risk. New Findings From ADVANCE. Hypertension. 2009;54:399–404. doi: 10.1161/HYPERTENSIONAHA.109.133041. [DOI] [PubMed] [Google Scholar]
- 28.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 29.Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch. Intern. Med. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 30.Ni MC, Rodgers A, Pan WH, Gu DF, Woodward M. Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310 000 participants. Int. J. Epidemiol. 2004;33:751–758. doi: 10.1093/ije/dyh163. [DOI] [PubMed] [Google Scholar]
- 31.Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Rich-Edwards JW, et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277:1539–1545. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 32.Kurth T, Gaziano JM, Rexrode KM, Kase CS, Cook NR, Manson JE, et al. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005;111:1992–1998. doi: 10.1161/01.CIR.0000161822.83163.B6. [DOI] [PubMed] [Google Scholar]
- 33.Park JW, Lee SY, Kim SY, Choe H, Jee SH. BMI and stroke risk in Korean women. Obesity. (Silver. Spring) 2008;16:396–401. doi: 10.1038/oby.2007.67. [DOI] [PubMed] [Google Scholar]
- 34.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, et al. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am. J. Clin. Nutr. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am. J. Clin. Nutr. 2002;75:683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 38.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br. Med. J. (Clin. Res. Ed) 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, et al. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 40.Sam S, Haffner S, Davidson MH, D’Agostino RB, Sr., Feinstein S, Kondos G, et al. Relationship of abdominal visceral and subcutaneous adipose tissue with lipoprotein particle number and size in type 2 diabetes. Diabetes. 2008;57:2022–2027. doi: 10.2337/db08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. Fully-adjusted hazard ratios and 95% confidence intervals (95% CI) for major cardiovascular outcomes per standard deviation increment in anthropometric variables in participants without prior history of cardiovascular disease at baseline