Abstract
Background
Consensus statements recommend the addition of long‐acting inhaled ß2‐agonists (LABA) only in asthmatic patients who are inadequately controlled on inhaled corticosteroids (ICS). It is not uncommon for some patients to be commenced on ICS and LABA together as initial therapy.
Objectives
To compare the efficacy of combining inhaled corticosteroids with long‐acting ß2‐agonists (ICS+LABA) with inhaled corticosteroids alone (ICS alone) in steroid‐naive children and adults with persistent asthma. We assessed two protocols: (1) LABA + ICS versus a similar dose of ICS (comparison 1) and (2) LABA + ICS versus a higher dose of ICS (comparison 2).
Search methods
We identified randomised controlled trials through electronic database searches (May 2008).
Selection criteria
Randomised trials comparing ICS + LABA with ICS alone in children and adults with asthma who had no inhaled corticosteroids in the preceding 28 days prior to enrolment.
Data collection and analysis
Each author assessed studies independently for risk of bias and extracted data. We obtained confirmation from the trialists when possible. The primary endpoint was rate of patients with one or more asthma exacerbations requiring rescue systemic corticosteroids. Results are expressed as relative risks (RR) for dichotomous data and as mean differences (MD) or standardised mean differences (SMD) for continuous data.
Main results
Twenty‐eight study comparisons drawn from 27 trials (22 adult; five paediatric) met the review entry criteria (8050 participants). Baseline data from the studies indicated that trial populations had moderate or mild airway obstruction (FEV1≥65% predicted), and that they were symptomatic prior to randomisation. In comparison 1, the combination of ICS and LABA was not associated with a significantly lower risk of patients with exacerbations requiring oral corticosteroids (RR 1.04; 95% confidence interval (CI) 0.73 to 1.47) or requiring hospital admissions (RR 0.38; 95% CI 0.09 to 1.65) compared to a similar dose of ICS alone. The combination of LABA and ICS led to a significantly greater improvement from baseline in FEV1 (0.12 L/sec; 95% CI 0.07 to 0.17), in symptoms (SMD ‐0.26; 95% CI ‐0.37 to ‐0.14) and in rescue ß2‐agonist use (‐0.41 puffs/day; 95% CI ‐0.73 to ‐0.09) compared with a similar dose of ICS alone. There was no significant group difference in the risk of serious adverse events (RR 1.15; 95% CI 0.64 to 2.09), any adverse events (RR 1.02; 95% CI 0.96 to 1.09), study withdrawals (RR 0.95; 95% CI 0.82 to 1.11), or withdrawals due to poor asthma control (RR 0.94; 95% CI 0.63 to 1.41).
In comparison 2, the combination of LABA and ICS was associated with a higher risk of patients requiring oral corticosteroids (RR 1.24; 95% CI 1 to 1.53) and study withdrawal (RR 1.31; 95% CI 1.07 to 1.59) than a higher dose of ICS alone. For every 100 patients treated over 43 weeks, nine patients using a higher dose ICS compared to 11 (95% CI 9 to 14) on LABA and ICS suffered one or more exacerbations requiring rescue oral corticosteroids. There was a high level of statistical heterogeneity for FEV1 and morning peak flow. There was no statistically significant group difference in the risk of serious adverse events. Due to insufficient data we could not aggregate results for hospital admission, symptoms and other outcomes.
Authors' conclusions
In steroid‐naive patients with mild to moderate airway obstruction, the combination of ICS and LABA does not significantly reduce the risk of patients with exacerbations requiring rescue oral corticosteroids over that achieved with a similar dose of ICS alone. However, it significantly improves lung function, reduces symptoms and marginally decreases rescue ß2‐agonist use. Initiation of a higher dose of ICS is more effective at reducing the risk of exacerbations requiring rescue systemic corticosteroids, and of withdrawals, than combination therapy. Although children appeared to respond similarly to adults, no firm conclusions can be drawn regarding combination therapy in steroid‐naive children, given the small number of children contributing data.
Keywords: Adult; Child; Humans; Administration, Inhalation; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Adrenergic beta‐Agonists; Adrenergic beta‐Agonists/administration & dosage; Airway Obstruction; Airway Obstruction/drug therapy; Anti‐Asthmatic Agents; Anti‐Asthmatic Agents/administration & dosage; Asthma; Asthma/drug therapy; Drug Therapy, Combination; Randomized Controlled Trials as Topic
Plain language summary
The effect of adding a long‐acting beta‐agonist to inhaled steroids in people not previously treated with inhaled steroids
In patients with asthma who require daily anti‐inflammatory therapy, there is insufficient evidence to support initiating therapy with a combination of inhaled corticosteroids (ICS) and long‐acting ß2‐agonist (LABA) rather than with inhaled corticosteroids alone. Most consensus statements recommend the addition of LABA as second line therapy, only in asthmatic individuals who remain insufficiently controlled on maintenance inhaled corticosteroids. Yet, many physicians initiate combination therapy in patients with asthma, without a prior trial of inhaled corticosteroids alone. The purpose of this review was to compare the benefit and safety profile of initiating treatment with the combination of ICS and LABA as compared to a (1) similar and (2) higher dose of ICS alone in asthmatic patients who had not received ICS previously. This review identified 28 randomised controlled trials. The combination of ICS and LABA did not reduce the risk of patients with exacerbations requiring rescue oral corticosteroids but improved lung function, symptoms and minimally reduced the use of rescue ß2‐agonists as compared to a similar dose of ICS alone. Initiating ICS at a higher dose than that used with LABA in the control group significantly reduced the risk of exacerbations and study withdrawals over that observed with the combination of LABA and a lower dose of ICS; there is insufficient evidence to comment on the impact on lung function, symptoms and use of rescue ß2‐agonists. The current evidence does not support use of combination therapy with LABA and ICS as first line treatment in adults and children with asthma, without a prior trial of inhaled corticosteroids.
Background
The cornerstone of asthma management is the use of inhaled corticosteroids (ICS) to alleviate the inflammatory reaction that characterises asthma (Adams 2007; Adams 2008a; Adams 2008b). Short‐acting ß2‐agonists are the primary agents in the management of acute asthma symptoms. This class of medication provides rapid onset bronchodilation by interaction with specific ß2‐adrenergic receptors (Abramson 2003). Long‐acting ß2‐agonists (LABA), such as formoterol and salmeterol, were initially used in persistent asthmatics with severe nocturnal symptoms. Because of their lipophilicity, these agents achieve sustained bronchodilation for up to 12 hours (D'Alonzo 1997). Since bronchodilation with these agents is long‐lasting, they are of potential use in managing the symptoms of asthma.
The use of salmeterol in combination with inhaled corticosteroids has been found to be superior to an increased dose of inhaled corticosteroids for improving symptoms and reducing exacerbations in patients with moderate to severe persistent asthma (Shrewsbury 2000). However, a subsequent larger systematic review found an absence of a statistically significant group difference in the rate of exacerbations requiring systemic steroids, while significantly greater improvements in lung function, symptoms and use of rescue ß2‐agonists were documented with combination therapy than with higher ICS dose. Interestingly, a subgroup analysis suggested the superiority of a higher dose of ICS over combination therapy in patients with prolonged (> six months) therapy (Greenstone 2005). However, monotherapy with long‐acting ß2‐agonists alone had been associated with significant adverse events (Cates 2008a; Cates 2008b; Walters 2007).
Current national and international guidelines for asthma recommend long‐acting ß2‐agonists as an adjunctive therapy to inhaled corticosteroids in patients who are not controlled by inhaled corticosteroids alone (BTS 2008; GINA 2007; Lemiere 2004; NAEPP 2007). More specifically, guidelines recommend the initiation of therapy with low or moderate doses of inhaled corticosteroids alone in patients with mild or moderate persistent asthma, respectively. The addition of long‐acting ß2‐agonists to inhaled corticosteroids is generally recommended once a trial of inhaled corticosteroids alone has been insufficient to achieve adequate asthma control (GINA 2007). In fact, some national consensus statements have formally advised against the use of long‐acting ß2‐agonists without a prior trial of inhaled corticosteroids (Lemiere 2004).
Despite current guideline recommendations, data from observational studies indicate that the introduction of a long‐acting ß2‐agonist in mild asthma is still common in adults and children (Sazonov‐Kocevar 2006; Stockl 2008). Perhaps because of the perception of greater efficacy of combination therapy, there is an increasing tendency for practitioners to initiate a combination of inhaled corticosteroids and long‐acting ß2‐agonists in patients with mild or moderate airway obstruction, without a prior trial of inhaled corticosteroids alone. The recent development of single inhalers delivering both an inhaled corticosteroid and long‐acting ß2‐agonist may have further facilitated this practice.
Objectives
The objective of this review was to examine the safety and efficacy of initiating a combination of long‐acting ß2‐agonists and inhaled corticosteroids compared to a similar dose or a higher dose of inhaled corticosteroids alone, in steroid‐naive children and adults with persistent asthma.
More specifically, we wished to compare the impact of both treatment options on asthma control measured as exacerbations requiring systemic corticosteroids (main outcome), asthma symptoms, lung function, quality of life, withdrawals from the study, inflammatory mediator levels and adverse health events. We aimed to examine whether any observed benefit may be influenced by factors such as severity of baseline airway obstruction, age, dose of inhaled corticosteroids, use of one or two devices to deliver combination therapy, the long‐acting ß2‐agonist preparation used and trial duration.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials in which the combination of inhaled corticosteroids and long‐acting ß2‐agonists (ICS+LABA) was compared to a similar dose of inhaled corticosteroid (same ICS dose alone: Comparison 01), and to a higher dose of ICS (higher ICS dose alone: Comparison 02). Controlled studies with or without placebo were considered. Because of the requirement of participants to be steroid‐naive, we excluded cross‐over trials.
Types of participants
Adults and/or children aged two years and above with persistent asthma of any severity who were steroid‐naive; that is, who had not received inhaled corticosteroids in the month preceding enrolment.
Types of interventions
Long‐acting ß2‐agonist (e.g. salmeterol or formoterol) plus inhaled steroids versus a similar dose of inhaled corticosteroids alone (+/‐ placebo) administered for four weeks or more (Comparison 1). We included trials that compared different inhaled corticosteroids at the same equivalent dose. Inhaled short‐acting ß2‐agonists and short courses of systemic corticosteroids were considered as rescue medications.
Long‐acting ß2‐agonist (e.g. salmeterol or formoterol) plus inhaled steroids versus a higher dose of inhaled corticosteroids alone (+/‐ placebo) administered for four weeks or more (Comparison 2). We included trials that compared different inhaled corticosteroids in each arm, at doses higher than the dose used in combination with LABA. Inhaled short‐acting ß2‐agonists and short courses of systemic corticosteroids were considered as rescue medications.
We only considered fixed‐dose treatment arms, since maintenance and reliever therapy with budesonide and formoterol is subject to review elsewhere (Cates 2009).
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of participants who experienced exacerbations of asthma requiring a short course of systemic corticosteroids (5 to 10 days).
Secondary outcomes
Hospital admission
Pulmonary function tests
Symptoms
Quality of life assessed with a validated questionnaire
Use of rescue short‐acting ß2‐agonists.
Measures of inflammation such as expired nitric oxide, serum eosinophils, serum eosinophil cationic protein, and sputum eosinophils
Rates of clinical and biochemical adverse effects
Withdrawals
Search methods for identification of studies
Electronic searches
We carried out a search in the Cochrane Airways Group Specialised Register of trials which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearched respiratory journals and meeting abstracts (please see the Airways Group Module for further details). This register contains a variety of studies published in foreign languages. We did not exclude trials on the basis of language.
All records in the Specialised Register coded as 'asthma' were searched using the following terms:
(((beta* and agonist*) and long‐acting or "long acting") or ((beta* and adrenergic*) and long‐acting or "long acting") or (bronchodilat* and long‐acting or "long acting") or (salmeterol or formoterol or advair or symbicort)) and (((steroid* or glucocorticoid* or corticosteroid*) and inhal*) or (budesonide or beclomethasone or fluticasone or triamcinolone or flunisolide)).
The most recent search was conducted in May 2008.
Searching other resources
We reviewed reference lists of all included studies and of reviews to identify potentially relevant citations.
We also made enquiries regarding other published or unpublished studies known to the authors of the included studies or to pharmaceutical companies who produce the agents, namely GlaxoSmithKline (GSK) and AstraZeneca.
We handsearched the clinical trials websites of pharmaceutical firms which manufacture formoterol (AstraZeneca) and salmeterol (GSK). We undertook an additional search of Clinical Study Results. We conducted these additional handsearches in May 2008.
Data collection and analysis
Selection of studies
From the title, abstract, or descriptors, one of the authors (MNC, IG or TL) independently reviewed the literature searches. We excluded all studies that were clearly not randomised controlled trials or that clearly did not fit the inclusion criteria. Two authors ((MNC or TL ) and FMD) reviewed all other citations independently in full text, assessing for inclusion based on study design, population, intervention and outcome.
Data extraction and management
Two authors (TL, MNC or FMD) independently extracted data for the trials and entered data into The Cochrane Collaboration software program Review Manager 5.0 (RevMan 2008). For the update in 2008, TL performed data extraction and corresponded with trialists and study sponsors to obtain missing data. FMD provided checks for accuracy of the data analysed in the primary outcome.
Assessment of risk of bias in included studies
We assessed the risk of bias for the allocation, blinding and the handling of missing data in the studies. This is in line with the recommendations made in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2008).
Dealing with missing data
We contacted study investigators or study sponsors to verify data extraction for our primary outcome of exacerbations requiring systemic corticosteroids where this was reported in study publications. For study publications where no information was given on exacerbations, we attempted to establish the number of participants in each treatment group who had experienced one ore more oral steroid‐treated exacerbation.
For partially reported continuous data endpoints (such as lung function outcomes where no or incomplete summary data were available), we sought necessary numerical values from study investigators or sponsors.
Where necessary, we performed expansions of graphic reproductions and estimations from other data presented in the papers.
Assessment of heterogeneity
We tested homogeneity of effect size between the studies being pooled the DerSimonian and Laird method with I² ≥ 25% (Higgins 2003) being used as the threshold to prompt exploration of possible sources of variation. If heterogeneity was suggested, we applied the DerSimonian and Laird random‐effects model to the summary estimates. Unless otherwise specified we reported the fixed‐effect model.
Data synthesis
For dichotomous variables, we calculated individual and pooled statistics as relative risks with 95% confidence intervals. For continuous outcomes we calculated individual and pooled statistics as weighted mean differences or standardised mean differences, as indicated, with 95% confidence intervals.
We set limits of treatment equivalence a priori at +/‐ 0.10 on either side of the no‐difference line for our primary outcome, the risk of exacerbations requiring oral corticosteroids. The null hypothesis tested whether the confidence interval for the difference between the two treatments included one of these limits.
Subgroup analysis and investigation of heterogeneity
For each outcome, we stratified trials according to the severity of baseline airway obstruction as determined by the mean percent predicted forced expiratory volume in one second (FEV1) where an FEV1 equal to, or greater than, 80% of predicted was indicative of mild obstruction; an FEV1 61% to 79% of predicted, indicative of moderate obstruction; and an FEV1 equal to or less than 60% considered as severe obstruction (GINA 2007).
We recorded as a 'User defined order' the mean daily dose of inhaled corticosteroid in both groups reported in chlorofluorocarbon (CFC) propelled 'beclomethasone‐equivalent', where 1 µg of beclomethasone dipropionate was equivalent to 1 µg of budesonide or 0.5 µg fluticasone propionate, irrespective of delivery device used (NAEPP 2007). All doses of inhaled corticosteroids were reported based on ex‐valve rather than ex‐inhaler values.
The following a priori defined subgroups were examined to explore influence on the magnitude of effect (effect modification), irrespective of the presence or absence of heterogeneity.
Severity of airway obstruction at baseline (FEV1: 80% of predicted and above; 61% to 79% of predicted ; 60% of predicted or less) (GINA 2007).
Children versus adults.
-
Dose of inhaled corticosteroids, reported in CFC‐propelled beclomethasone or equivalent (µg/day) and portrayed as the user‐defined number, was examined as the:
Mean dose (ex‐valve) used in both groups in studies where both groups used a similar dose of ICS, reported in CFC‐propelled beclomethasone or equivalent (µg/day), portrayed as the user‐defined number.
Dose difference between groups in studies where a different ICS dose was used in the LABA + ICS versus ICS alone groups.
Use of one or two devices to deliver the combination of ICS plus LABA.
Long‐acting ß2‐agonist used (salmeterol versus formoterol).
Trial duration.
Sensitivity analysis
We performed sensitivity analyses to investigate the potential effect of:
risk of bias (blinding and completeness of outcome reporting);
publication status (data available from full text source versus non‐full‐text journal source (e.g. web‐based company trial report, data made available on request or conference abstract);
funding source (producers of tested interventions versus independent source);
use of the same ICS versus similar dose‐equivalent ICS on the study results.
We used funnel plots to test for the presence of possible publication bias (Egger 1997). The fail‐safe N test was used to assess the robustness of the results (Gleser 1996).
Results
Description of studies
Results of the search
We considered 35 new studies for eligibility in this update of the review. Eighteen new studies met the review entry criteria, combining with the previous nine trials to yield a total of 27 included studies (reported in 100 citations). One study contributed two between‐group comparisons: Pearlman 1999b; Pearlman 1999a, hereafter counted as two different studies for a total of 28 study comparisons. For full details of search history see Table 1, and for a literature flow diagram see Figure 1. The included trials randomised 8050 participants.
1. Search history.
| Search year | Detail |
| All years to April 2004 | Citations identified: 594
Excluded: 576 due to:
(1) duplicate references (N = 209);
(2) not a randomised controlled trial (N = 68), or an ongoing trial (N = 14);
(3) participants were not asthmatics (N = 4);
(4) no consistent intervention with inhaled corticosteroids in all participants (N = 41);
(5) intervention was not maintenance, inhaled long‐acting ß2‐agonists (N = 19);
(6) control intervention was not maintenance, inhaled corticosteroids alone (N = 64);
(7) duration of intervention was less than 30 days (N = 45);
(8) outcome measures did not reflect asthma control (N = 8);
(9) treatment and intervention groups compared the same medications either in combination or with different delivery devices (N = 30);
(10) co‐intervention with non‐permitted agent (N = 1);
(11) patients were not steroid‐naive, or did not examine the same dose of inhaled corticosteroids in each group (N = 73). Unique studies identified meeting entry criteria: 9 References pertaining to these studies: 16 |
| April 2004 to May 2007 | Citations identified: 293
Excluded: 231 due to:
(1) duplicate references (N = 50);
(2) not a randomised controlled trial (N = 14), or an ongoing trial (N = 0);
(3) crossover study (N = 17)
(4) participants were not asthmatics (N = 4);
(5) study conducted in children (N = 8)
(6) no consistent intervention with inhaled corticosteroids in all participants (N = 4);
(7) intervention was not maintenance, inhaled long‐acting ß2‐agonists (N = 16);
(8) control intervention was not maintenance, inhaled corticosteroids alone (N = 76);
(9) duration of intervention was less than 30 days (N = 2);
(10) outcome measures did not reflect asthma control (N = 8);
(11) treatment and intervention groups compared the same medications either in combination or with different delivery devices (N = 7);
(12) co‐intervention with non‐permitted agent (N = 0);
(13) patients were not steroid‐naive, or did not examine the same dose of inhaled corticosteroids in each group (N = 25). References identified of relevance to the review: 62 New unique studies identified meeting entry criteria: 10 |
1.

Flow diagram of literature added to update of the review (April 2004 to May 2008)
Included studies
There were two main comparisons: (1) the combination of LABA and ICS compared to a similar dose of ICS (N = 24 studies): Boonsawat 2008; Chuchalin 2002; Creticos 1999; Di Franco 1999; GOAL; Grutters 1999; Karaman 2007; Kerwin 2008; Miraglia del Giudice 2007; Murray 2004; Nelson 2003; O'Byrne 2001; Overbeek 2005; Pearlman 1999a; Pearlman 1999b; Prieto 2005; Rojas 2007; SAS30015; SAS30021; SAS40068; SLGF75; Stelmach 2008; Strand 2004; Weersink 1997) and (2) LABA + ICS versus higher dose ICS (N = four studies: Chuchalin 2008; SAM40034; SAM40036; Sorkness 2007). Assessment of the risk of bias and meta‐analysis results are provided for each comparison.
Participants
Age
Five studies recruited children with mean ages of between 8 and 12 years. The youngest participants eligible for these studies was six years, and the oldest was 18 years (Karaman 2007; Miraglia del Giudice 2007; SAS30021; Sorkness 2007; Stelmach 2008). Twenty‐three studies recruited adults with a mean age varying between 26 (Grutters 1999) and 45 years (Chuchalin 2002). Fifteen adult studies permitted the enrolment of an unspecified number of adolescents aged 12 years and above (Chuchalin 2008; Di Franco 1999; GOAL; Nelson 2003; O'Byrne 2001; Overbeek 2005; Pearlman 1999a; Pearlman 1999b; SAS30015; Murray 2004; Kerwin 2008; Boonsawat 2008; Rojas 2007; SAM40036; SAS40068). The gender distribution varied from 25% males in Chuchalin 2002 to 61% in Di Franco 1999.
Prior maintenance treatment
Participants were all naive to both long‐acting ß2‐agonists and inhaled corticosteroids; that is, they had never received inhaled corticosteroids (Creticos 1999; GOAL (stratum 1); Karaman 2007; Nelson 2003; Prieto 2005), had not received any inhaled corticosteroids for a minimum of one to six months (Boonsawat 2008; Di Franco 1999; Grutters 1999; Kerwin 2008; Miraglia del Giudice 2007; Murray 2004; O'Byrne 2001; Overbeek 2005; Pearlman 1999a; Pearlman 1999b; Rojas 2007; SAS30021; SAS40068; SLGF75; Sorkness 2007; Stelmach 2008; Strand 2004; Weersink 1997), or had abstained from corticosteroids for an unspecified period (Chuchalin 2002; Chuchalin 2008). In an additional study, the participants were described as uncontrolled at step 1 of the British Thoracic Society (BTS) guidelines (SAS30015), and therefore we considered them to be steroid naive, since these guidelines do not recommend the introduction of inhaled corticosteroids until step 2.
Asthma control
All participants had inadequate asthma control prior to enrolment, with ongoing symptoms and use of rescue short‐acting ß2‐agonists.
Ten studies (Chuchalin 2002; GOAL; Kerwin 2008; Miraglia del Giudice 2007; Murray 2004; Nelson 2003; Overbeek 2005; Pearlman 1999a; Pearlman 1999b; Rojas 2007) recruited patients with moderate airway obstruction (mean baseline FEV1 of 66% to 79% of predicted), whilst 12 trials recruited patients with minimal airway obstruction, for example, a mean baseline FEV1 of predicted 80% to 105% of predicted (Boonsawat 2008; Chuchalin 2008; Creticos 1999; Di Franco 1999; Grutters 1999; O'Byrne 2001; Prieto 2005; SAM40034; SAM40036; Sorkness 2007; Stelmach 2008; Weersink 1997). For six studies (including four accessed from the GSK trials register), we were unable to determine baseline FEV1 predicted (Karaman 2007; SAS30015; SAS30021; SAS40068; SLGF75; Strand 2004). Strand 2004 reported baseline peak expiratory flow (PEF) predicted of 79%.
The presence of atopy was discussed in seven studies with three studies enrolling only atopic patients (Grutters 1999; Prieto 2005; Weersink 1997) and four reporting a 58%, 69%, 75% and 85% prevalence of atopy respectively (Di Franco 1999; GOAL; Overbeek 2005; Sorkness 2007).
Intervention
Type of LABA and ICS dosing
The long‐acting ß2‐agonist preparation was salmeterol xinafoate (50 µg twice daily) in 22 studies and formoterol (12 µg twice daily) in the remaining six trials (Chuchalin 2002; Karaman 2007; Miraglia del Giudice 2007; O'Byrne 2001; Overbeek 2005; Stelmach 2008). The dose and type of inhaled corticosteroid varied among the studies.
Fourteen studies tested the combination of LABA and low doses of inhaled corticosteroids (i.e. 200 to 400 µg/day of beclomethasone, or equivalent: Boonsawat 2008; Chuchalin 2002; Chuchalin 2008; Creticos 1999; Murray 2004; Nelson 2003; O'Byrne 2001; Overbeek 2005; Pearlman 1999a; Prieto 2005; SAS30015; SAS40068; SLGF75; Strand 2004). One study assessed LABA added to 500 mcg of beclomethasone equivalent (Kerwin 2008) and nine studies used high doses (i.e. 800 to 1000 mcg/day of BDP, or equivalent: Di Franco 1999; Grutters 1999; GOAL; Karaman 2007; Pearlman 1999b; Rojas 2007; SAM40034; Sorkness 2007; Weersink 1997). In the studies assessing adjunctive LABA therapy against a higher ICS dose, the control group received at least double the dose of ICS in the LABA group, with a BDP equivalent differential dose of 200 mcg (Chuchalin 2008 control group dose: 400 mcg BDP equivalent; Sorkness 2007 control group dose: 400 mcg BDP equivalent); or 300 mcg (SAM40034 control group dose: 1000 mcg BDP equivalent; SAM40036 control group dose: 400 mcg BDP equivalent).
Studies assessed the addition of LABA to beclomethasone (three studies: Di Franco 1999; Grutters 1999; SAS30015), budesonide (seven studies: Chuchalin 2002; Karaman 2007; Miraglia del Giudice 2007; O'Byrne 2001; Overbeek 2005; SAM40036; Stelmach 2008), triamcinolone (one study: Creticos 1999) or fluticasone (17 studies: Boonsawat 2008; Chuchalin 2008; GOAL; Kerwin 2008; Murray 2004; Nelson 2003; Pearlman 1999a; Pearlman 1999b; Prieto 2005; Rojas 2007; SAM40034; SAS30021; SAS40068; SLGF75; Sorkness 2007; Strand 2004; Weersink 1997).
Inhaler devices
Fifteen studies tested the combination of long‐acting ß2‐agonist and corticosteroid administered in a single inhaler (Boonsawat 2008; Chuchalin 2008; GOAL; Grutters 1999; Kerwin 2008; Murray 2004; Nelson 2003; Prieto 2005; Rojas 2007; SAM40034; SAM40036; SAS30015; SAS30021; SAS40068; Strand 2004). Thirteen studies used two separate inhalers (Chuchalin 2002; Creticos 1999; Di Franco 1999; Karaman 2007; Miraglia del Giudice 2007; O'Byrne 2001; Overbeek 2005; Pearlman 1999a; Pearlman 1999b; SLGF75; Sorkness 2007; Stelmach 2008; Weersink 1997). Compliance was monitored during the intervention period in only five studies (Di Franco 1999; Grutters 1999; Pearlman 1999a; Pearlman 1999b; Sorkness 2007).
Co‐treatment and duration
Co‐intervention with other prophylactic medications such as xanthines and sodium cromoglycate was clearly not permitted in four of the studies (Chuchalin 2002; Di Franco 1999; Nelson 2003; O'Byrne 2001) and unreported in the remaining studies. Rescue medication such as inhaled short‐acting ß2‐agonist was permitted in all trials.
Study duration varied: four to eight weeks (Grutters 1999; Karaman 2007; Miraglia del Giudice 2007; Overbeek 2005; Pearlman 1999a; Pearlman 1999b; Prieto 2005; Stelmach 2008; Weersink 1997), 12 weeks (Boonsawat 2008; Chuchalin 2002; GOAL; Kerwin 2008; Murray 2004; Nelson 2003; Rojas 2007; SAM40034; SAM40036; SAS30015; SAS30021), 24 weeks (Creticos 1999; SAS40068; Strand 2004), 48 weeks (Sorkness 2007) and 52 weeks (Chuchalin 2008; Di Franco 1999; O'Byrne 2001). One study of uncertain duration was included since it was reported to be longer than 12 weeks in a recent meta‐analysis from GlaxoSmithKline (GSK) (SLGF75).
Outcomes
Eleven studies contributed data to our main outcome (number of patients with exacerbations requiring systemic corticosteroids) for Comparison 01 (Boonsawat 2008; Di Franco 1999; Kerwin 2008; Murray 2004; Nelson 2003; O'Byrne 2001; Rojas 2007; SAS30015; SAS30021; SAS40068; Strand 2004), and three studies to the same outcome under Comparison 02 (Chuchalin 2008; SAM40036; Sorkness 2007). We were able to obtain data relating to exacerbations requiring systemic corticosteroids for nine GSK‐funded studies following correspondence with the study sponsors.
Most trials reported changes in lung function, albeit using various parameters, use of rescue ß2‐agonists, cause‐specific and all‐cause withdrawals and overall adverse health events. Improvement in symptoms was reported in different ways (symptom score, percent symptom‐free days, percent days with symptoms, percent night awakenings) using many parameters (average value, final value at endpoint, percent change, change in percent values) so aggregation could only be done on a few variables. Only one trial (Grutters 1999) reported the impact of treatment on inflammatory markers, serum eosinophils, eosinophilic cationic protein, platelet‐activating factor and total IgE. Unfortunately, it failed to report change from baseline and could not be aggregated as no other trials reported these outcomes.
Funding status
Eighteen studies were funded by producers of long‐acting ß2‐agonists, namely GlaxoSmithKline (Boonsawat 2008; Chuchalin 2008; GOAL; Grutters 1999; Kerwin 2008; Murray 2004; Nelson 2003; Pearlman 1999a; Pearlman 1999b; Rojas 2007; SAM40034; SAM40036; SAS30015; SAS30021; SAS40068; SLGF75; Strand 2004; Weersink 1997) and AstraZeneca (O'Byrne 2001; Overbeek 2005). Two studies received funding from a charitable source (Sorkness 2007; Stelmach 2008). Source of funding was unspecified in the remaining six studies.
Excluded studies
We have listed the reason for the exclusion of 293 studies (411 citations) that did not meet the eligibility of the review in 'Characteristics of excluded studies'.
Risk of bias in included studies
See Figure 2 for a summary of our assessment of the risk of bias for each study.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Comparison 01: LABA and ICS versus a similar dose of ICS alone
Based on correspondence with GSK who sponsored the salmeterol studies, we were able to verify that appropriate methods of randomisation had been undertaken for a total of 17 (71%) of 24 studies (see Appendix 1).
Nineteen (79%) studies were reported as double blind with an appropriate means of blinding (Boonsawat 2008; Chuchalin 2002; Creticos 1999; GOAL; Grutters 1999; Kerwin 2008; Murray 2004; Nelson 2003; O'Byrne 2001; Overbeek 2005; Pearlman 1999a; Pearlman 1999b; Prieto 2005; SAS30015; Rojas 2007; SAS40068; SLGF75; Strand 2004; Weersink 1997); one study was not blinded (Di Franco 1999).
The data was analysed by intention‐to‐treat in 15 (62.5%) studies, although detailed descriptions of how this was done when data was missing were infrequently available. One small study described its intention‐to‐treat analysis as one based on the last observation carried forward (SLGF75).
Only one study reported the proportion of the screened patients that were enrolled in the run‐in period (GOAL: 67%). Only two trials reported the proportion of patients who were successfully randomised after the run‐in period: Chuchalin 2002: 99%; Nelson 2003: 54%. The reasons for non‐randomisation were not provided.
Comparison 02: LABA and ICS versus a higher dose of ICS alone
Based on correspondence with GSK who sponsored the salmeterol studies, we were able to verify that appropriate methods of randomisation had been undertaken for three of four studies (see Appendix 1).
Blinding of treatment was sufficient to categorise all four studies as being at a low risk of detection bias.
As with the studies under Comparison 01 the description of intention‐to‐treat analysis populations was not clear enough for us to determine how missing data were handled.
Only Sorkness 2007 provided information on the percentage of participants randomised from the screening population (44).
Effects of interventions
Comparison 01: LABA plus ICS versus a similar dose of ICS alone (24 studies)
Primary outcome: Patients with exacerbations requiring oral corticosteroids
In 12 (11 adult and one paediatric) trials contributing data to this outcome, there was no statistically significant group difference in the risk of patients requiring rescue oral steroids (RR 1.04; 95% CI 0.73 to 1.47; Figure 3).
3.
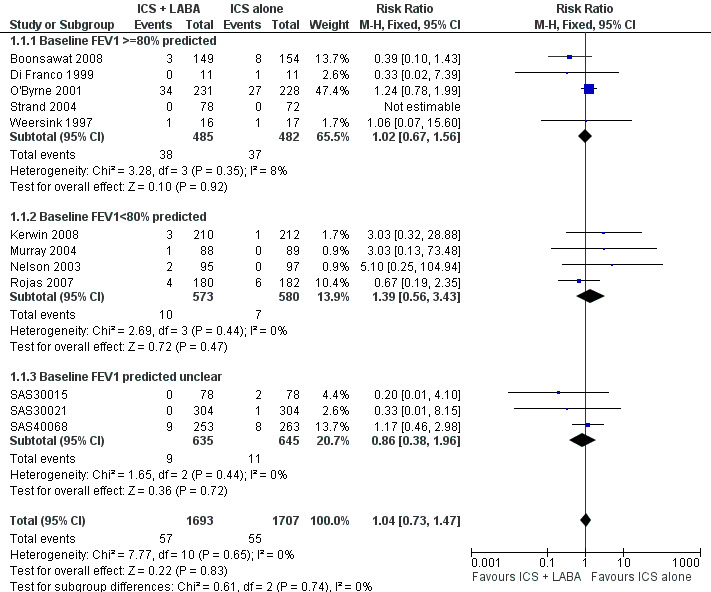
Forest plot of comparison: 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, outcome: 1.1 # patients with exacerbations requiring systemic steroids.
Although there was some variation in the characteristics of the studies (enrolment of patients with mild and moderate airway obstruction, doses of inhaled corticosteroids varying between 200 µg/day to 1000 µg/day of beclomethasone or equivalent), we did not observe any statistical variation between the study results (I² = 0%). The subgroup analyses did not identify patient, intervention or study characteristics that might explain modify the magnitude of response. The Egger test did not support significant bias (‐0.20; 95% CI ‐0.31 to 0.31).
Restricting the analyses to studies with a low or unclear risk of bias for blinding and those with acceptable proportion of follow up made little difference to our effect estimates (Analysis 4.1; Analysis 4.2). Removing from the analysis studies without a full‐text publication also did not affect the direction of the effect (Analysis 4.3).
4.1. Analysis.

Comparison 4 Sensitivity analysis (comparison 01), Outcome 1 # patients with exacerbations requiring systemic steroids (low or unclear risk of detection bias).
4.2. Analysis.

Comparison 4 Sensitivity analysis (comparison 01), Outcome 2 # patients with exacerbations requiring systemic steroids (low or unclear risk of bias in completeness of follow up).
4.3. Analysis.

Comparison 4 Sensitivity analysis (comparison 01), Outcome 3 # patients with exacerbations requiring systemic steroids.
Secondary outcomes
Exacerbations requiring hospitalisation
Three studies contributed data to the outcome measuring patients with exacerbations requiring hospitalisation which showed no significant difference between treatment regimens (RR 0.38; 95% CI 0.09 to 1.65; Analysis 1.2).
1.2. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 2 # patients with exacerbations requiring hospitalisation.
Lung function & diary recorded peak flow
There was a significant group difference in favour of LABA with regards to the improvement from baseline in FEV1 (11 studies: 0.12 litres; 95% CI 0.07 to 0.17; random‐effects modelling; Analysis 1.3), in morning peak expiratory flow (PEF) (11 studies: WMD 19.50 L/min; 95% CI 16.19 to 22.82; random‐effects model; Analysis 1.6) and in evening PEF (eight studies: 10.45 L/min; 95% CI 7.08 to 13.82; Analysis 1.7). There was no statistically significant group difference in the morning PEF measured at endpoint (19.34 L/min; 95% CI ‐10.75 to 49.42; Analysis 1.8) or in the change in PEF variability (four studies: SMD ‐0.04; 95% CI ‐0.50 to 0.41; random‐effects model; Analysis 1.12). There was an insufficient number of trials to allow aggregation of data pertaining to FEV1 measured at endpoint and in airway hyperreactivity (measured as PC20).
1.3. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 3 Change in FEV1 at endpoint.
1.6. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 6 Change in morning PEF (L/min) at endpoint.
1.7. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 7 Change in evening PEF (L/min) at endpoint.
1.8. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 8 Morning PEF at endpoint.
1.12. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 12 Change in PEF variability at endpoint.
We performed subgroup analyses on the change from baseline in FEV1. When restricting the analysis to the eight trials in which the average baseline FEV1 was reported, there was no significant group difference in the magnitude of effect between patients with a baseline FEV1 61% to 79% of predicted compared to those with FEV1 of >= 80% predicted (0.14 versus 0.12 L; P = 0.77 (Analysis 1.3). Similarly, the ICS dose to which LABA was added did not explain the statistical heterogeneity between the studies (<= 500: 0.11 L versus > 800 mcg: 0.18 L; P = 0.315 ) (Analysis 3.5). When studies were stratified by trial duration, there was a statistically significant group difference showing a weaker effect on FEV1 at 24 weeks compared with 12 weeks (mean difference: 0.08 L; P = 0.0101). With the small number of trials, it was impossible to perform a meta‐regression to disentangle the independent effect of baseline severity, ICS dose, and study duration on the magnitude of effect on FEV1. Finally, there were insufficient studies to examine the effects of the type of LABA, age and number of devices to administer the combination therapy in the magnitude of improvement in FEV1.
3.5. Analysis.

Comparison 3 Subgroup analyses (comparison 01), Outcome 5 Change in FEV1 at endpoint by ICS dose.
Symptoms and rescue medication use
Patients treated with LABA experienced significantly greater improvements from baseline in symptom score (seven studies: SMD ‐0.26; 95% CI ‐0.37 to ‐0.14; random‐effects modelling; Analysis 1.17) and in night‐time symptom score (SMD ‐0.16; 95% CI ‐0.32 to 0.00; Analysis 1.19). There was no significant difference in change from baseline in night‐time awakening (Analysis 1.21).
1.17. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 17 Change in symptom score at endpoint.
1.19. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 19 Change in night‐time symptoms at endpoint.
1.21. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 21 Change in % nights with no awakenings at 12 weeks.
There was also a significant group difference in favour of combination therapy in reducing the use of rescue short‐acting ß2‐agonists (eight studies: WMD ‐0.41 puffs/day; 95% CI ‐0.73 to ‐0.09; random‐effects model; Analysis 1.29) and in the increase in rescue‐free days (9.29%; 95% CI 4.52 to 14.05; Analysis 1.24). There were insufficient data to report aggregated estimates for night‐time awakenings (Analysis 1.22), percentage of symptom‐free days (Analysis 1.25; Analysis 1.26) rescue‐free days at endpoint (Analysis 1.23) or quality of life (Analysis 1.32; Analysis 1.33).
1.29. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 29 Change in use of rescue fast‐acting b2‐agonists (puffs/24 hrs) at endpoint.
1.24. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 24 Change in mean % rescue‐free days at 12 weeks.
1.22. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 22 % nights with symptoms at endpoint.
1.25. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 25 % 24 hrs with symptoms at endpoint.
1.26. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 26 % symptom‐free days.
1.23. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 23 Mean % rescue‐free days at endpoint.
1.32. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 32 Change in quality of life (AQLQ score) at 12 weeks.
1.33. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 33 Paediatric AQLQ scores.
Inflammation
With only one trial (Grutters 1999) reporting inflammatory markers, the impact of either treatment option on airway inflammation could not be examined.
Withdrawals & tolerability
There was no statistically significant difference, nor equivalence, in the risk of serious adverse events between treatment options (10 studies; RR 1.15; 95% CI 0.64 to 2.09; Analysis 1.34).
1.34. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 34 Serious adverse events.
The overall risk of withdrawals (18 trials; RR 0.95; 95% CI 0.82 to 1.11; Analysis 1.35) and withdrawals due to poor asthma control (13 studies; RR 0.94; 95% CI 0.63 to 1.41; Analysis 1.36) were not statistically different between groups. With regards to side effects, there were no statistically significant differences between treatments in the risk of any adverse effects (13 studies: RR 1.02; 95% CI 0.96 to 1.09; Analysis 1.38), reaching our a priori definition of equivalence. There was no significant group difference in withdrawals due to adverse effects (11 studies: RR 1.07; 95% CI 0.67 to 1.71; Analysis 1.37), oral candidiasis (six studies: RR 0.91; 0.39 to 2.12; Analysis 1.40), headache (11 studies: RR 1.03; 95% CI 0.86 to 1.23; Analysis 1.39) or hoarseness (three studies: RR 1.97; 95% CI 0.49 to 7.88; Analysis 1.41). There was a significant increase in the risk of tremor associated with the use of LABA (four studies: RR 4.71; 95% CI 1.38 to 16.08; Analysis 1.42). Other potential adverse effects such as tachycardia (Analysis 1.43) and adverse cardiovascular events (Analysis 1.44) could not be examined reliably due to insufficient trials reporting these outcomes. There were no reported deaths.
1.35. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 35 Total withdrawals.
1.36. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 36 # patients withdrawing due to poor asthma control or exacerbation.
1.38. Analysis.
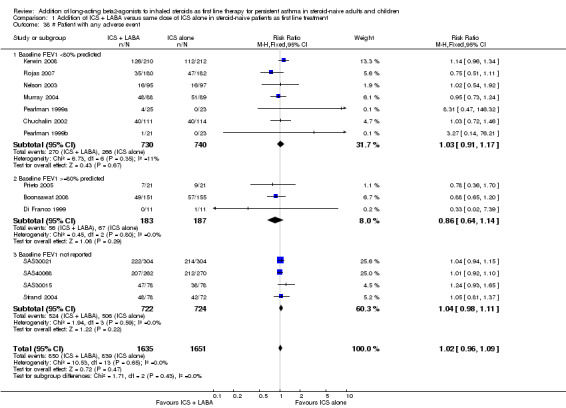
Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 38 # Patient with any adverse event.
1.37. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 37 # patient withdrawals due to adverse effects.
1.40. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 40 # patients with oral thrush.
1.39. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 39 # patients with headache.
1.41. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 41 # patients with hoarseness.
1.42. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 42 # patients with tremor.
1.43. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 43 # patients with tachycardia or palpitations.
1.44. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 44 # patients with adverse cardiovascular events.
Comparison 02: LABA plus ICS versus higher dose ICS alone (four studies)
Primary outcome: Patients with exacerbations requiring oral corticosteroids
In two adult and one paediatric trial, the combination of LABA and ICS in steroid‐naive participants led to a higher risk of patients with exacerbations requiring oral corticosteroids compared with those treated with a higher ICS dose alone (RR 1.24; 95% CI 1.00 to 1.53; Figure 4; Analysis 2.1), a group difference at the limit of statistical significance. For every 100 patients treated over 43 weeks, nine patients using a higher dose ICS compared to 11 (95% CI 9 to 14) on LABA and ICS required rescue oral corticosteroids for an exacerbation (Figure 5.) Three studies (two adult and one pediatric) contributed data to this outcome. Of note, the data were available only from trials in which patients had a mean baseline FEV1 of 80% or more of predicted. Three trials showing no group difference would reverse this conclusion (Gleser 1996).
4.

Forest plot of comparison: 5 Addition of ICS + LABA versus higher dose of ICS alone in steroid‐naive patients as first line treatment, outcome: 5.1 # patients with exacerbations requiring systemic steroids.
2.1. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 1 # patients with exacerbations requiring systemic steroids.
5.

In the higher dose ICS group 9 people out of 100 had exacerbations requiring oral corticosteroids over 43 weeks, compared to 11 (95% CI 9 to 14) out of 100 for the LABA + ICS group.
Given the low number of studies contributing data to this outcome, we did not undertake subgroup analyses.
Secondary outcomes
Exacerbations requiring hospitalisation
There was no group difference in the risk of patients with exacerbations requiring hospital admission (RR 1.00; 95% CI 0.31 to 3.25; Analysis 2.2).
2.2. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 2 # patients with exacerbations requiring hospitalisation.
Lung function & diary recorded peak flow
There was a high level of statistical heterogeneity between two studies contributing estimates of change in FEV1 (I² 72%; pooled random‐effects model: 0.07 L; 95% CI ‐0.02 to 0.15; Analysis 2.3). Similarly, the findings for change in morning PEF indicated a high level of statistical heterogeneity (I² 97%) (Analysis 2.6). This may be related to the design of Chuchalin 2008 in which fluticasone twice daily was compared to combination therapy administered once daily in the morning, before which PEF was measured (24 hours after previous dose).
2.3. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 3 Change in FEV1 at endpoint.
2.6. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 6 Change in morning PEF at endpoint.
Change in evening PEF significantly favoured LABA compared with a higher ICS dose (15.57 L/min; 95% CI 3.8 to 27.35; Analysis 2.8).
2.8. Analysis.
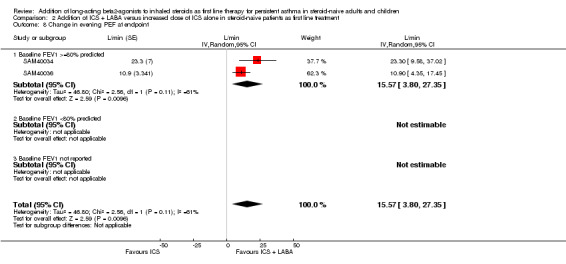
Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 8 Change in evening PEF at endpoint.
Symptoms and rescue medication use
Data for these outcomes could not be aggregated as they were only available for single studies (Analysis 2.10; Analysis 2.11).
2.10. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 10 % symptom‐free days at endpoint.
2.11. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 11 Absolute (or %) change in # rescue inhalations (per 24 hrs) at endpoint.
Airway hyperreactivity
No aggregation of data was possible for these outcomes. A paediatric trial (Sorkness 2007) reported significantly fewer doubling doses of methacholine to induce a 20% fall in FEV1 following higher dose ICS (Analysis 2.18).
2.18. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 18 Change in PC20.
Withdrawals & tolerability
There was no statistically significant difference in the risk of serious adverse events between treatments (four studies: RR 1.03; 95% CI 0.63 to 1.69; Analysis 2.12), with insufficient power to reach equivalence.
2.12. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 12 Serious adverse events.
All‐cause withdrawals were more likely with the combination of LABA and ICS than with a higher ICS dose (RR 1.31; 95% CI 1.07 to 1.59; Analysis 2.13). Withdrawals due to adverse events were not significantly different between treatments (RR 1.00; 95% CI 0.54 to 1.84; Analysis 2.14). The risk of headache was not significantly different between treatments (RR 0.97; 95% CI 0.80 to 1.17; Analysis 2.16). Due to insufficient trials reporting hoarseness (Analysis 2.17) or other adverse health events, we were unable to perform meta‐analysis for additional safety endpoints.
2.13. Analysis.
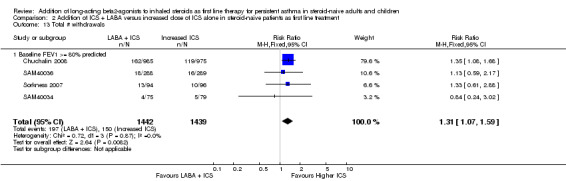
Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 13 Total # withdrawals.
2.14. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 14 # withdrawals due to adverse events.
2.16. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 16 # patients with headache.
2.17. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 17 # patients with hoarseness.
Discussion
In symptomatic steroid‐naive asthmatic patients, the combination of long acting ß2‐agonist (LABA) and inhaled corticosteroids does not significantly reduce the risk of patients with exacerbations requiring rescue systemic corticosteroids as compared to using a similar dose of ICS alone; however combination therapy improves lung function, symptoms and, marginally, the use of rescue ß2‐agonists. Of the few specific adverse events recorded in the trials, significant group differences were only documented for tremor, which was more than four‐fold more frequent with combination therapy than with ICS alone. Initiation of ICS at a slightly higher dose (by 200 to 300 mcg/day) was more effective than the combination of LABA and ICS at a lower dose reducing by 25% the risk of patients experiencing exacerbations requiring systemic corticosteroids and study withdrawals. Combination therapy achieved a higher evening PEF than a higher dose ICS, with no significant difference in improvement from baseline in FEV1 or morning PEF. Given the small number of children contributing data, no firm conclusions can be drawn regarding combination therapy in steroid‐naive children although no age group differences are apparent.
Comparison 1: LABA + ICS versus same dose ICS
When comparing ICS alone with the combination of LABA with a similar dose of ICS, the available data did not show a statistically significant group difference between these strategies for the main outcome, namely patients with one or more exacerbations requiring rescue oral corticosteroids; however, the confidence interval exceeds our predefined limits of equivalence and thus we could not exclude clinically meaningful superiority of either strategy. Subgroup analyses did not detect differences in the magnitude of effect associated with the severity of baseline airway obstruction, the choice of LABA, the dose of ICS, or the duration of treatment. Our findings contrast with that of the steroid‐naive stratum of GOAL (1098 patients). The GOAL data could not be included in this review because of the inability to obtain data pertaining only to rescue oral steroids. Using a composite definition of exacerbations including hospital admission, emergency visits and oral steroid, the GOAL study identified a small, but significant reduction in the overall rate of exacerbations over the 12 months, favouring combination therapy (P < 0.009). In contrast to included trials, the GOAL study design included step‐up therapy with inhaled corticosteroid until asthma control was achieved, whereas we examined the effects of introducing LABA to a stable dose of ICS. Thus, in addition to the different study design, the definition of exacerbations also differed to our endpoint, which may explain the apparent discrepancy.
In this review, there was no group difference in the risk of hospital admission, but the rarity of the event with only four contributing trials prevents firm conclusion. In contrast, the combination of LABA and ICS was associated with a significantly greater improvement from baseline in lung function, by a magnitude of 0.12 L in FEV1 and 19.5 L/min in morning PEF as compared to those treated with a similar dose of ICS alone. Following the addition of five new studies contributing data to the change in FEV1, the magnitude of improvement in FEV1 due to combination therapy decreased from 210 mL in the original review, to 120 mL in this updated review. While this downward trend probably results from a more representative sample of the population and treatment protocols in which combination therapy can be used, the magnitude of improvement in FEV1 appeared to be significantly affected by trial duration, with data from 24‐week or longer studies showing a smaller effect than those reporting outcome data at 12 weeks or less, suggesting that the benefit of combination therapy on lung function appears to wane with time. Because of the lack of power, the effect of the choice of LABA (i.e. formoterol versus salmeterol), and the number of devices to deliver combination therapy, on the improvement in FEV1 could not be examined.
Use of LABA and ICS also translated into significant improvements in the percentage of days without symptoms and in symptom scores over those observed with a similar ICS dose. It was also associated with a modest reduction of rescue fast‐acting ß2‐agonists (by less than a half‐puff per day) compared to inhaled corticosteroids alone. With only one trial reporting data, the impact on airway inflammation could not be examined.
The risk of overall adverse events showed no group difference, meeting our a priori definition of equivalence. With the exception of tremor, which was almost five times more frequent in the combination therapy, there was also no group difference in specific adverse effects. Use of ICS and LABA therapy was not associated with a reduced risk of withdrawals due to either adverse effects or all reasons combined. However, due to the small number of trials, the absence of group difference did not meet our a priori definition of equivalence.
Comparison 2: LABA + ICS versus higher dose ICS
When comparing the combination of LABA and ICS to a higher (two‐fold) dose of ICS, there is a statistically significant difference in favour of higher doses of ICS in reducing the risk of children and adults with exacerbations requiring oral corticosteroids. The findings were not particularly robust since only three additional trials with no group difference could change the conclusion. This finding is based on three (two adult and one paediatric) trials which all tested salmeterol in patients with a mean baseline FEV1 of 80% of predicted or higher. Whether the findings would be more or less positive in patients with more severe airway obstruction at baseline, receiving a higher ICS dose, or with other characteristics remains to be determined. The findings were supported by the superiority of a higher ICS dose for preventing study withdrawals.
With only four trials contributing few events, no firm conclusion could be made regarding the superiority of either treatment option for reducing the risk of patients with exacerbations requiring hospital admission. There was no significant group difference in most lung function tests, which displayed significant heterogeneity between studies in FEV1 and morning PEF; only the change from baseline in evening PEF favoured combination therapy in two trials with patients with mild airway obstruction. No data could be aggregated for other secondary outcomes.
Again, the risk of adverse events was not significantly different between groups, with insufficient power to prove equivalence and to rule out rare serious adverse health events. Due to the small number of trials leading to large confidence intervals, no equivalence in the safety profile can be assumed. Furthermore, the careful evaluation of the impact of a higher dose of corticosteroid requires the documentation of relevant outcomes such as bone mineral density, adrenal function and, in children, growth in studies of long duration (≥ 26 to 52 weeks).
The non‐superiority of LABA and ICS patients over ICS alone in steroid‐naive patients is interesting in view of three previous large Cochrane Reviews. Indeed, as compared to ICS alone, the combination of LABA and ICS was clearly associated with a greater reduction in exacerbations in patients already on daily inhaled corticosteroids and who remained poorly controlled, in other words, when combination therapy was added at step 3 of the Global Initiative for Asthma guidelines (Ni Chroinin 2005). Moreover, the superiority of a higher ICS dose over the combination of LABA and ICS in steroid‐naive patients is intriguing as no group difference was found when these strategies were compared in a large Cochrane Review focusing on patients at step 3 (Greenstone 2005). Bateman 2008, which summarised the evidence derived only from GlaxoSmithKline studies involving both steroid‐naive and non steroid‐naive patients, reported a significant reduction in the odds of exacerbations requiring systemic steroids in favour of adding LABA. Our linked Cochrane Reviews would indicate that the impact of LABA needs to be assessed separately in these different populations. Indeed, the divergence in response to LABA between steroid‐naive patients and patients who remain poorly controlled while on ICS, exemplifies the heterogeneity in asthma sub‐populations and reinforces the need for careful evaluation of all treatment strategies at each GINA step as well as within different subgroups.
The divergence in response for patients at step 2 versus step 3 suggests that, in steroid‐naive patients, asthma control is achieved in the majority of patients with ICS alone. This assumption is supported by the negligible reduction in the need for rescue ß2‐agonists with LABA (< 1/2 puff/day) despite baseline ß2‐agonist use varying between 1 puff/day (O'Byrne 2001) and 2.5 to 4 puffs/day (Chuchalin 2002; Nelson 2003; Pearlman 1999a; Pearlman 1999b), thus leaving room for improvement. These observations confirm that the single most important intervention in steroid‐naive patients, irrespective of severity of asthma, is to initiate inhaled corticosteroids at low or moderate doses.
Within each protocol, we were unable to demonstrate the impact of varying dose of ICS, LABA and duration of treatment on the magnitude of effects for our primary outcome. This may be explained by the small number of trials which under‐powered the subgroup analyses, as well as by the relatively flat dose response of ICS. Indeed, the flat dose‐response with inhaled corticosteroids indicates that the major part of the beneficial effect of inhaled corticosteroids is conferred at a low ICS dose, with minimal additional gain at higher doses (Powell 2003). Additional large trials with varying start‐up doses of ICS are needed to clarify the relative efficacy of both treatment options and to characterise responders on age, severity of airway obstruction, smoking status etc.
Many proponents of initial treatment with combination therapy may argue that the rapid improvement in lung function, symptom control and reduction in ß2‐agonists will lead to better compliance with treatment because of the patient's perception of immediate benefit with LABA. The validity of this argument could not be examined because compliance with treatment was infrequently reported and not apparently analysed in the trials. If compliance was indeed superior with the combination of ICS and LABA, it did not translate into a significant reductions in the risk of asthma exacerbation or a meaningful reduction use of rescue ß2‐agonists, whether compared to the same or a higher dose of ICS.
The results of this review must be interpreted in light of the following strengths and limitations. Our review included studies examining the relative efficacy of three different strategies in asthma management for patients with no prior controller medication; that is at step 2 of the Global Initiative for Asthma guidelines. Unfortunately, a notable number of trials did not contribute to our primary outcome, because data on exacerbations treated with systemic steroids was not made available to us. However, we obtained a considerable amount of unpublished information directly from trialists and study sponsors that would not have been otherwise available. With regards to generalisability of study results, only one study reported the proportion of eligible patients amongst those approached and, in the two trials reporting the proportion of randomised patients among those enrolled in the run‐in, this varied from 54% (Nelson 2003) to 99% (Chuchalin 2002). In view of this poor reporting, it is impossible to comment on how far the observed results may be replicated in clinical practice. However one must take note that patients included in the included trials were symptomatic and demonstrated significant (>= 12%) reversibility in FEV1 with a short‐acting ß2 agonist. The reversibility to bronchodilator would tend to favour combination therapy with LABA over inhaled corticosteroids alone and may seriously limit generalisability since reversibility to bronchodilator is a criterion met in less than 10% of patients at a given point in time (Storms 2003), leading to regression towards the mean. We recognise that over‐representation of short trials (<= 12 weeks) in the first comparison and the small number of trials in the second comparison may have limited the ability to identify group differences in specific adverse health events. Moreover, the review was not sufficiently powered to examine rare serious adverse health events. The long duration (>= 48 weeks) of two of the three trials contributing data to the main outcome probably explain the precision achieved for the second comparison. Paediatric trials represented 22% of identified studies, yet only one study in each comparison contributed data to the main outcome, thus preventing any subgroup analyses on age. While children seem to respond similarly to adults, no firm conclusion could be made with respect to the relative effectiveness of both treatment options in youth. The conclusion should not be generalised to preschool‐aged children, who were not included in any identified trial.
Authors' conclusions
Implications for practice.
In steroid‐naive asthmatic patients who are symptomatic and exhibit mild or moderate airway obstruction, the risk in exacerbations requiring oral corticosteroids is similar between adding long‐acting ß2‐agonists (LABA) to inhaled steroids (ICS) and ICS alone. It does not provide sufficient justification for initiating a combination of ICS and LABA without a prior trial of ICS as a means of reducing exacerbations requiring systemic steroids. However, greater improvement in lung function and symptoms, and minimal reduction in use of rescue ß2‐agonist would be expected with combination therapy. Interestingly, the benefits observed in lung function appear to wane by 24 weeks. Moreover, the use of higher dose ICS is superior to initiating combination therapy for preventing exacerbations and study withdrawals. The analyses are insufficiently powered to identify characteristics of patients (such as age group) or treatment modalities that may or may not modify the magnitude of effect on the risk of rescue corticosteroids. Insufficient reporting of relevant outcomes and insufficient power preclude firm conclusions as to the relative safety profile of both treatment strategies, including rare serious adverse health events.
Implications for research.
Long‐term studies >= 24 to 52 weeks are needed to examine the relative safety profile of both treatment options and the characteristics of patients responders (age, gender, smoking status, airway obstruction) or treatment modalities (dose of ICS, number of inhalers, duration of treatment) associated with each treatment strategy (ICS versus the combination of LABA and ICS) as step 2 therapy in steroid naive patients. The safety profile, including serious adverse health events, adrenal function, bone mineralization and, in children, growth, are crucial. Trials in children are of a high priority, with 12‐month duration to assess growth. These studies need to be adequately powered and preferably randomised by subgroups with subgroup analyses to identify factors which may modify outcomes (effect modifiers).
Given the flat dose‐response curve of inhaled corticosteroids, future trials should focus on the comparison of long‐acting ß2‐agonists as:
Add‐on to a low‐dose of inhaled corticosteroids in patients with mild obstruction (or stratified on the severity of baseline obstruction);
Add‐on to moderate doses of inhaled corticosteroids in patients with moderate or severe airway obstruction (or stratified on the severity of baseline obstruction).
Future trials should aim for the following design characteristics.
Enrol patients with asthma in whom the current reversibility with ß2‐agonists is not a pre‐requisite (in other words, asthma documented by provocation tests, prior documented reversibility with ß2‐agonists or inhaled/oral corticosteroids).
Report separately the number of patients with exacerbations requiring systemic corticosteroids and patients requiring hospital admission, as these outcomes are less influenced by the LABA effect on smooth muscle than lung function, use of rescue ß2‐agonists and symptoms.
Double blinding, adequate randomisation and complete reporting of withdrawals and drop‐outs, with intention‐to‐treat analyses.
Parallel‐groups
A minimal intervention period of 24 weeks or preferably more to properly assess the impact of treatment on exacerbations requiring systemic corticosteroids, and the possibility of an effect modification associated with treatment duration.
Clear reporting of the percentage of non‐eligibility (with reasons) of approached patients and of those enrolled in the run‐in period to assess the generalisability of findings.
Careful monitoring and reporting of compliance to treatment.
Complete reporting of continuous (denominators, mean change and mean standard deviation of change) and dichotomous (denominators and rate) data.
Systematic documentation of reasons for withdrawals and adverse effects, including those associated with inhaled corticosteroids, such as oral candidiasis, osteopenia, adrenal suppression and growth suppression.
Reporting of cost effectiveness of the use of combination inhalers as compared to inhaled corticosteroids alone.
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2013 | Amended | NIHR acknowledgement added |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 14 January 2010 | Amended | New title; minor spelling mistakes corrected |
| 12 June 2008 | New citation required and conclusions have changed | We added 19 studies to this review in the June 2008 update; four trials added data to our primary outcome. Confidence intervals tightened around pooled effect. We added an additional comparison comparing long‐acting inhaled ß2‐agonists (LABA) and inhaled corticosteroids (ICS) to increased dose of ICS, indicating that higher dose ICS is more effective than combining ICS with a LABA in reducing exacerbations. |
| 2 May 2008 | New search has been performed | New literature search performed (2004 to 2008). |
| 30 April 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the Cochrane Airways Group namely Liz Arnold, Karen Blackhall, Veronica Stewart and Bettina Reuben for the literature search and ongoing support, and Christopher Cates for editorial review and constructive commentary. We are indebted to Professor P.L. Paggiaro and Christine Sorkness for providing information and data about their studies; Richard Follows, Sheilesh Patel, Rob Pearson and Karen Richardson from GlaxoSmithKline; and Robin von Maltzan, Klas Svensson and Nils Grundstrom from AstraZeneca who assisted with our requests for information.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Details of GSK randomisation processes
The procedures for randomising GSK sponsored studies has been detailed in correspondence between Richard Follows and TL, the details of which are given below:
The randomisation software is a computer‐generated, centralised programme (RandAll). After verification that the randomisation sequence is suitable for the study design (cross‐over, block or stratification), Clinical Supplies then package the treatments according the randomisation list generated. Concealment of allocation is maintained by a third party, since the sites phone in and are allocated treatments on that basis. Alternatively a third party may dispense the drug at the sites. Unblinding of data for interim analyses can only be done through RandAll, and are restricted so that only those reviewing the data are unblinded to treatment group allocation.
Data and analyses
Comparison 1. Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 # patients with exacerbations requiring systemic steroids | 12 | 3400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.47] |
| 1.1 Baseline FEV1 >=80% predicted | 5 | 967 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.56] |
| 1.2 Baseline FEV1<80% predicted | 4 | 1153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.56, 3.43] |
| 1.3 Baseline FEV1 predicted unclear | 3 | 1280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.38, 1.96] |
| 2 # patients with exacerbations requiring hospitalisation | 10 | 2806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.65] |
| 2.1 Baseline FEV1 >=80% predicted | 2 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.27] |
| 2.2 Baseline FEV1 <80% predicted | 3 | 1009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.31] |
| 2.3 Baseline FEV1 not reported | 5 | 1472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.08, 4.38] |
| 3 Change in FEV1 at endpoint | 11 | 3014 | L (Random, 95% CI) | 0.12 [0.07, 0.17] |
| 3.1 Baseline FEV1 <80% predicted | 7 | 2172 | L (Random, 95% CI) | 0.14 [0.08, 0.20] |
| 3.2 Baseline FEV1 >=80% predicted | 3 | 370 | L (Random, 95% CI) | 0.12 [0.00, 0.25] |
| 3.3 Baseline FEV1 not reported | 1 | 472 | L (Random, 95% CI) | 0.06 [0.01, 0.11] |
| 4 Change in FEV1 predicted at endpoint | 2 | 489 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [0.20, 3.29] |
| 4.1 Baseline FEV1 <80% predicted | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Baseline FEV1 >/= 80% predicted | 2 | 489 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [0.20, 3.29] |
| 4.3 Baseline FEV1 not reported | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 FEV1 predicted at endpoint | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 4.39 [‐1.27, 10.05] |
| 5.1 Baseline FEV1 <80% predicted | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 4.39 [‐1.27, 10.05] |
| 5.2 Baseline FEV1 >/= 80% predicted | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Baseline FEV1 not reported | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Change in morning PEF (L/min) at endpoint | 11 | 2894 | Mean Difference (IV, Fixed, 95% CI) | 19.50 [16.19, 22.82] |
| 6.1 Baseline FEV1 < 80% predicted | 7 | 1465 | Mean Difference (IV, Fixed, 95% CI) | 23.03 [17.95, 28.10] |
| 6.2 Baseline FEV1 >=80% predicted | 2 | 765 | Mean Difference (IV, Fixed, 95% CI) | 15.32 [9.63, 21.00] |
| 6.3 Baseline FEV1 not reported | 2 | 664 | Mean Difference (IV, Fixed, 95% CI) | 19.15 [12.27, 26.03] |
| 7 Change in evening PEF (L/min) at endpoint | 8 | 2725 | Mean Difference (IV, Fixed, 95% CI) | 14.16 [11.48, 16.84] |
| 7.1 Baseline FEV1 <80% predicted | 4 | 1149 | Mean Difference (IV, Fixed, 95% CI) | 20.88 [15.68, 26.09] |
| 7.2 Baseline FEV1 >=80% predicted | 1 | 306 | Mean Difference (IV, Fixed, 95% CI) | 19.8 [11.34, 28.26] |
| 7.3 Baseline FEV1 not reported | 3 | 1270 | Mean Difference (IV, Fixed, 95% CI) | 10.45 [7.08, 13.82] |
| 8 Morning PEF at endpoint | 3 | L/min (Fixed, 95% CI) | 19.34 [‐10.75, 49.42] | |
| 8.1 Baseline FEV1 <80% predicted | 2 | L/min (Fixed, 95% CI) | 20.72 [‐21.47, 62.91] | |
| 8.2 Baseline FEV1 not reported | 1 | L/min (Fixed, 95% CI) | 17.9 [‐23.00, 60.80] | |
| 9 Evening PEF (L/min) at endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Baseline FEV1 <80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Baseline FEV1 not reported | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change in am PEF predicted (%) | 3 | 1207 | Mean Difference (IV, Fixed, 95% CI) | 3.41 [2.24, 4.58] |
| 10.1 Baseline FEV1 <80% predicted | 2 | 599 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [3.37, 6.43] |
| 10.2 Baseline FEV1 >=80% predicted | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Baseline FEV1 not reported | 1 | 608 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.52, 3.12] |
| 11 Change in pm PEF predicted (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Baseline FEV1 <80% predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Baseline FEV1 not reported | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Change in PEF variability at endpoint | 4 | 292 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.50, 0.41] |
| 12.1 Baseline FEV1 <80% predicted | 3 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.42, 0.06] |
| 12.2 Baseline FEV1 >=80% predicted | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 1.02 [0.12, 1.92] |
| 13 Diurnal PEF variability at endpoint | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Baseline FEV1 <80% predicted | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Baseline FEV1 >=80% predicted | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Baseline FEV1 not reported | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 % days with symptoms at endpoint | 2 | 593 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐4.47, 4.10] |
| 14.1 Baseline FEV1 <80% predicted | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Baseline FEV1 >=80% predicted | 1 | 459 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐6.20, 3.00] |
| 14.3 Baseline FEV1 not reported | 1 | 134 | Mean Difference (IV, Fixed, 95% CI) | 9.20 [‐2.65, 21.05] |
| 15 Change in % symptom‐free days at endpoint | 4 | 795 | Mean Difference (IV, Fixed, 95% CI) | 8.72 [3.75, 13.68] |
| 15.1 Baseline FEV1<80% predicted | 3 | 284 | Mean Difference (IV, Fixed, 95% CI) | 10.74 [1.86, 19.62] |
| 15.2 Baseline FEV1 >=80% predicted | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Baseline FEV1 not reported | 1 | 511 | Mean Difference (IV, Fixed, 95% CI) | 7.80 [1.82, 13.78] |
| 16 Day symptom score at endpoint | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Baseline FEV1 <80% predicted | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Baseline FEV1 >=80% predicted | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Baseline FEV1 not reported | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Change in symptom score at endpoint | 7 | 1464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.37, ‐0.14] |
| 17.1 Baseline FEV1 <80% predicted | 7 | 1464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.37, ‐0.14] |
| 17.2 Baseline FEV1 >=80% predicted | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Baseline FEV1 not reported | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 % nights with awakenings at endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Baseline FEV1 <80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Baseline FEV1 >=80% predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Change in night‐time symptoms at endpoint | 2 | 584 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.32, 0.00] |
| 19.1 Baseline FEV1 <80% predicted | 1 | 359 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.31, 0.11] |
| 19.2 Baseline FEV1 >=80% predicted | 1 | 225 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.51, 0.01] |
| 19.3 Baseline FEV1 not reported | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Night symptom score at endpoint | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Baseline FEV1 <80% predicted | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Baseline FEV1 >=80% predicted | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Baseline FEV1 not reported | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Change in % nights with no awakenings at 12 weeks | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 3.53 [‐2.98, 10.05] |
| 21.1 Baseline FEV1 <80% predicted | 2 | 369 | Mean Difference (IV, Fixed, 95% CI) | 3.53 [‐2.98, 10.05] |
| 21.2 Baseline FEV1 >=80% predicted | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 % nights with symptoms at endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 22.1 Baseline FEV1 <80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Baseline FEV1 not reported | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Mean % rescue‐free days at endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 23.1 Baseline FEV1 <80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.3 Baseline FEV1 not reported | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 Change in mean % rescue‐free days at 12 weeks | 2 | 703 | Mean Difference (IV, Fixed, 95% CI) | 9.29 [4.52, 14.05] |
| 24.1 Baseline FEV1 <80% predicted | 1 | 192 | Mean Difference (IV, Fixed, 95% CI) | 13.5 [2.06, 24.94] |
| 24.2 Baseline FEV1 >=80% predicted | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Baseline FEV1 not reported | 1 | 511 | Mean Difference (IV, Fixed, 95% CI) | 8.40 [3.15, 13.65] |
| 25 % 24 hrs with symptoms at endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 25.1 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Baseline FEV1 <80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.3 Baseline FEV1 not reported | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 % symptom‐free days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 26.1 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Baseline FEV1 <80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Baseline FEV1 not reported | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 Use of rescue fast‐acting b2‐agonists (puffs/24 hrs) at endpoint | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 27.1 Baseline FEV1 <80% predicted | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.3 Baseline FEV1 not reported | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28 Change in awakenings requiring SABA/nt | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 28.1 Baseline FEV1 <80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.3 Baseline FEV1 not reported | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 29 Change in use of rescue fast‐acting b2‐agonists (puffs/24 hrs) at endpoint | 8 | 2172 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.73, ‐0.09] |
| 29.1 Baseline FEV1 <80% predicted | 6 | 1105 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐0.94, ‐0.41] |
| 29.2 Baseline FEV1 >=80% predicted | 1 | 459 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 29.3 Baseline FEV1 not reported | 1 | 608 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 30 Change in daytime rescue medication (puffs) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 30.1 Baseline FEV1 <80% predicted | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 30.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 30.3 Baseline FEV1 not reported | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 31 Change in night‐time rescue medication (puffs) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 31.1 Baseline FEV1 <80% predicted | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 31.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 31.3 Baseline FEV1 not reported | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 32 Change in quality of life (AQLQ score) at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 32.1 Baseline FEV1 <80% predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 32.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 Paediatric AQLQ scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 33.1 FEV1 not reported | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 34 Serious adverse events | 15 | 3751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.64, 2.09] |
| 34.1 Baseline FEV1 >=80% predicted | 3 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.53, 4.45] |
| 34.2 Baseline FEV1 <80% predicted | 7 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.47, 3.90] |
| 34.3 Baseline FEV1 not reported | 5 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.28, 2.10] |
| 35 Total withdrawals | 18 | 3658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.11] |
| 35.1 Baseline FEV1 >=80% predicted | 5 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.60, 1.17] |
| 35.2 Baseline FEV1 <80% predicted | 7 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.80, 1.56] |
| 35.3 Baseline FEV1 not reported | 6 | 1530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.76, 1.16] |
| 36 # patients withdrawing due to poor asthma control or exacerbation | 13 | 3350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.63, 1.41] |
| 36.1 Baseline FEV1 <80% predicted | 6 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.40, 2.10] |
| 36.2 Baseline FEV1 >=80% predicted | 4 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.45, 4.42] |
| 36.3 Baseline FEV1 not reported | 3 | 1289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.52, 1.44] |
| 37 # patient withdrawals due to adverse effects | 13 | 3470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.71] |
| 37.1 Baseline FEV1<80% predicted | 6 | 1244 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.70, 6.55] |
| 37.2 Baseline FEV1 >=80% predicted | 3 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.55, 3.51] |
| 37.3 Baseline FEV1 not reported | 4 | 1439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.39] |
| 38 # Patient with any adverse event | 14 | 3286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.09] |
| 38.1 Baseline FEV1 <80% predicted | 7 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 38.2 Baseline FEV1 >=80% predicted | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.14] |
| 38.3 Baseline FEV1 not reported | 4 | 1446 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.11] |
| 39 # patients with headache | 11 | 2863 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.86, 1.23] |
| 39.1 Baseline FEV1 <80% predicted | 6 | 1245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.41] |
| 39.2 Baseline FEV1 >=80% predicted | 2 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.35] |
| 39.3 Baseline FEV1 not reported | 3 | 1290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.89, 1.35] |
| 40 # patients with oral thrush | 6 | 1325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.39, 2.12] |
| 40.1 Baseline FEV1<80% predicted | 4 | 1153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.34, 2.36] |
| 40.2 Baseline FEV1 >=80% predicted | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.39] |
| 40.3 Baseline FEV1 not reported | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.17, 19.93] |
| 41 # patients with hoarseness | 3 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.49, 7.88] |
| 41.1 Baseline FEV1 <80% | 2 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.28, 7.12] |
| 41.2 Baseline FEV1 not reported | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.23, 94.64] |
| 42 # patients with tremor | 5 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.71 [1.38, 16.08] |
| 42.1 Baseline FEV1 <80% predicted | 4 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.71 [1.38, 16.08] |
| 42.2 Baseline FEV1 >=80% predicted | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 43 # patients with tachycardia or palpitations | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.12, 64.76] |
| 43.1 Baseline FEV1 <80% predicted | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.12, 64.76] |
| 43.2 Baseline FEV1 >=80% predicted | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 44 # patients with adverse cardiovascular events | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.12, 64.76] |
| 44.1 Baseline FEV1 <80% | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.12, 64.76] |
| 45 Deaths | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 45.1 Baseline FEV1 <80% predicted | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 45.2 Baseline FEV1 >=80% predicted | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 46 Change in PC20 (methacholine) at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 46.1 Baseline FEV1 <80% predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 46.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 47 PC20 (methacholine) at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 47.1 Baseline FEV1 <80% predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 47.2 Baseline FEV1 >=80% predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 1 # patients with exacerbations requiring systemic steroids.
1.4. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 4 Change in FEV1 predicted at endpoint.
1.5. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 5 FEV1 predicted at endpoint.
1.9. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 9 Evening PEF (L/min) at endpoint.
1.10. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 10 Change in am PEF predicted (%).
1.11. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 11 Change in pm PEF predicted (%).
1.13. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 13 Diurnal PEF variability at endpoint.
1.14. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 14 % days with symptoms at endpoint.
1.15. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 15 Change in % symptom‐free days at endpoint.
1.16. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 16 Day symptom score at endpoint.
1.18. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 18 % nights with awakenings at endpoint.
1.20. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 20 Night symptom score at endpoint.
1.27. Analysis.
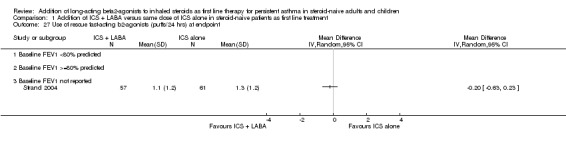
Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 27 Use of rescue fast‐acting b2‐agonists (puffs/24 hrs) at endpoint.
1.28. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 28 Change in awakenings requiring SABA/nt.
1.30. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 30 Change in daytime rescue medication (puffs).
1.31. Analysis.
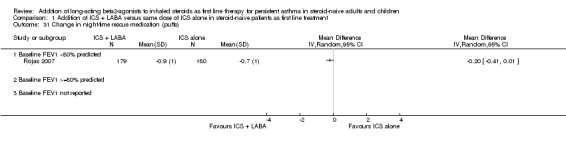
Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 31 Change in night‐time rescue medication (puffs).
1.45. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 45 Deaths.
1.46. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 46 Change in PC20 (methacholine) at 8 weeks.
1.47. Analysis.

Comparison 1 Addition of ICS + LABA versus same dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 47 PC20 (methacholine) at 8 weeks.
Comparison 2. Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 # patients with exacerbations requiring systemic steroids | 3 | 2709 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.00, 1.53] |
| 1.1 Baseline FEV1 >=80% predicted | 3 | 2709 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.00, 1.53] |
| 1.2 Baseline FEV1<80% predicted | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Baseline FEV1 predicted unclear | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 # patients with exacerbations requiring hospitalisation | 4 | 2864 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.31, 3.25] |
| 2.1 Baseline FEV1 >=80% predicted | 4 | 2864 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.31, 3.25] |
| 2.2 Baseline FEV1 <80% predicted | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Baseline FEV1 not reported | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in FEV1 at endpoint | 2 | L (Random, 95% CI) | 0.07 [‐0.02, 0.15] | |
| 3.1 Baseline FEV1 >=80% predicted | 2 | L (Random, 95% CI) | 0.07 [‐0.02, 0.15] | |
| 3.2 Baseline FEV1 <80% predicted | 0 | L (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Baseline FEV1 not reported | 0 | L (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in FEV1 predicted at endpoint | 1 | L (Random, 95% CI) | Totals not selected | |
| 4.1 Baseline FEV1 >=80% predicted | 1 | L (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Baseline FEV1 <80% predicted | 0 | L (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Baseline FEV1 not reported | 0 | L (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Morning PEF at endpoint | 1 | L/min (Random, 95% CI) | Totals not selected | |
| 5.1 Baseline FEV1 >=80% predicted | 1 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Baseline FEV1 <80% predicted | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Baseline FEV1 not reported | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in morning PEF at endpoint | 3 | 2642 | L/min (Random, 95% CI) | 13.27 [‐8.60, 35.15] |
| 6.1 Baseline FEV1 >=80% predicted | 3 | 2642 | L/min (Random, 95% CI) | 13.27 [‐8.60, 35.15] |
| 6.2 Baseline FEV1 <80% predicted | 0 | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Baseline FEV1 not reported | 0 | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Change in morning PEF predicted at endpoint | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 7.1 Baseline FEV1 >=80% predicted | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Baseline FEV1 <80% predicted | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Baseline FEV1 not reported | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in evening PEF at endpoint | 2 | L/min (Random, 95% CI) | 15.57 [3.80, 27.35] | |
| 8.1 Baseline FEV1 >=80% predicted | 2 | L/min (Random, 95% CI) | 15.57 [3.80, 27.35] | |
| 8.2 Baseline FEV1 <80% predicted | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Baseline FEV1 not reported | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Change in evening PEF predicted at endpoint | 1 | % (Random, 95% CI) | Totals not selected | |
| 9.1 Baseline FEV1 >=80% predicted | 1 | % (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Baseline FEV1 <80% predicted | 0 | % (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Baseline FEV1 not reported | 0 | % (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 % symptom‐free days at endpoint | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Baseline FEV1 >= 80 % predicted | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Baseline FEV1 61%‐79% predicted | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Baseline FEV1 % predicted not reported | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Absolute (or %) change in # rescue inhalations (per 24 hrs) at endpoint | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Baseline FEV1 >= 80% predicted | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Baseline FEV1 61%‐79% predicted | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Baseline FEV1 <= 60% predicted | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Baseline FEV1 not reported | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Serious adverse events | 4 | 2864 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.63, 1.69] |
| 12.1 Baseline FEV1 >= 80% predicted | 4 | 2864 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.63, 1.69] |
| 13 Total # withdrawals | 4 | 2881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.07, 1.59] |
| 13.1 Baseline FEV1 >= 80% predicted | 4 | 2881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.07, 1.59] |
| 14 # withdrawals due to adverse events | 3 | 2691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.84] |
| 14.1 Baseline FEV1 >= 80% predicted | 3 | 2691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.84] |
| 15 # withdrawals due to poor asthma control or exacerbation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 Baseline FEV1 >= 80% predicted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 # patients with headache | 3 | 2674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.17] |
| 16.1 Baseline FEV1 >= 80% predicted | 3 | 2674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.17] |
| 17 # patients with hoarseness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1 Baseline FEV1 >/=80% predicted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Change in PC20 | 1 | Doubl'g doses (Fixed, 95% CI) | Totals not selected | |
| 18.1 Baseline FEV1 >= 80% predicted | 1 | Doubl'g doses (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Baseline FEV1 61%‐79 % predicted | 0 | Doubl'g doses (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Baseline FEV1 <= 60% predicted | 0 | Doubl'g doses (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Baseline FEV1 not reported | 0 | Doubl'g doses (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Growth (paediatric data) | 1 | cm (Random, 95% CI) | Totals not selected |
2.4. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 4 Change in FEV1 predicted at endpoint.
2.5. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 5 Morning PEF at endpoint.
2.7. Analysis.
Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 7 Change in morning PEF predicted at endpoint.
2.9. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 9 Change in evening PEF predicted at endpoint.
2.15. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 15 # withdrawals due to poor asthma control or exacerbation.
2.19. Analysis.

Comparison 2 Addition of ICS + LABA versus increased dose of ICS alone in steroid‐naive patients as first line treatment, Outcome 19 Growth (paediatric data).
Comparison 3. Subgroup analyses (comparison 01).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 # patients with exacerbations requiring systemic steroids, stratified on LABA | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Formoterol 12 mcg bid | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.78, 1.99] |
| 1.2 Salmeterol 50 mcg bid | 11 | 2941 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.51, 1.44] |
| 2 # patients with exacerbations requiring systemic steroids, stratified on ICS dose | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low ICS dose (<=400 mcg/day of BDP or equivalent) | 8 | 2561 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.73, 1.55] |
| 2.2 Moderate dose of ICS (>400 to <800 mcg) | 1 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.32, 28.88] |
| 2.3 High ICS dose (>=800 mcg/day of BDP or equivalent) | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.23, 1.88] |
| 3 # patients with exacerbations requiring systemic steroids, stratified on duration of intervention | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 <24 weeks | 8 | 2253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.41, 1.48] |
| 3.2 ≥24 weeks | 4 | 1147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.80] |
| 4 # patients with exacerbations requiring systemic steroids, stratified on number of inhaler devices | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 One inhaler device | 9 | 2886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 4.2 Two inhaler devices | 3 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.75, 1.88] |
| 5 Change in FEV1 at endpoint by ICS dose | 11 | L (Random, 95% CI) | Subtotals only | |
| 5.1 200‐500 mcg/day of CFC‐BDP or equivalent | 8 | L (Random, 95% CI) | 0.11 [0.06, 0.17] | |
| 5.2 800‐1000 mcg/day of CFC‐BDP or equivalent | 3 | L (Random, 95% CI) | 0.18 [0.05, 0.30] | |
| 6 Change in FEV1 (L) at endpoint by LABA | 11 | L (Random, 95% CI) | Subtotals only | |
| 6.1 Formoterol | 1 | L (Random, 95% CI) | 0.16 [0.00, 0.32] | |
| 6.2 Salmeterol | 10 | L (Random, 95% CI) | 0.12 [0.07, 0.17] | |
| 7 Change in FEV1 (L) at endpoint by trial duration | 11 | L (Random, 95% CI) | Subtotals only | |
| 7.1 4 +/‐ 2 weeks | 3 | L (Random, 95% CI) | 0.22 [‐0.04, 0.49] | |
| 7.2 12 +/‐ 4 weeks | 6 | L (Random, 95% CI) | 0.14 [0.10, 0.17] | |
| 7.3 24 +/‐ 4 weeks | 2 | L (Random, 95% CI) | 0.06 [0.01, 0.11] |
3.1. Analysis.
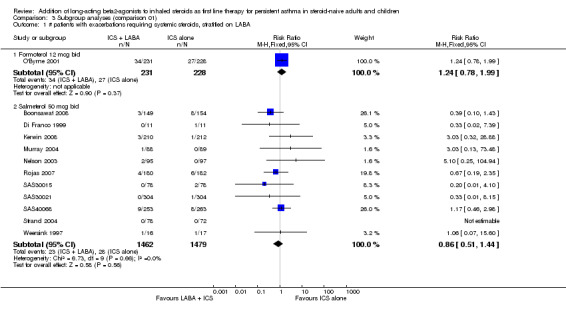
Comparison 3 Subgroup analyses (comparison 01), Outcome 1 # patients with exacerbations requiring systemic steroids, stratified on LABA.
3.2. Analysis.

Comparison 3 Subgroup analyses (comparison 01), Outcome 2 # patients with exacerbations requiring systemic steroids, stratified on ICS dose.
3.3. Analysis.

Comparison 3 Subgroup analyses (comparison 01), Outcome 3 # patients with exacerbations requiring systemic steroids, stratified on duration of intervention.
3.4. Analysis.

Comparison 3 Subgroup analyses (comparison 01), Outcome 4 # patients with exacerbations requiring systemic steroids, stratified on number of inhaler devices.
3.6. Analysis.
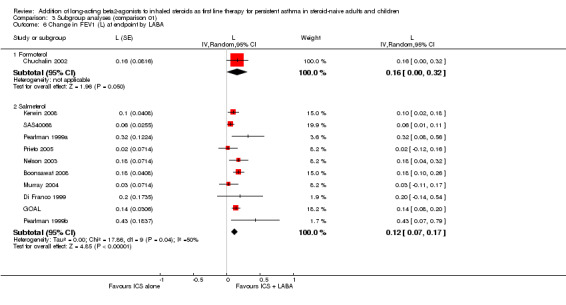
Comparison 3 Subgroup analyses (comparison 01), Outcome 6 Change in FEV1 (L) at endpoint by LABA.
3.7. Analysis.

Comparison 3 Subgroup analyses (comparison 01), Outcome 7 Change in FEV1 (L) at endpoint by trial duration.
Comparison 4. Sensitivity analysis (comparison 01).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 # patients with exacerbations requiring systemic steroids (low or unclear risk of detection bias) | 11 | 3378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.50] |
| 2 # patients with exacerbations requiring systemic steroids (low or unclear risk of bias in completeness of follow up) | 11 | 3223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.72, 1.45] |
| 3 # patients with exacerbations requiring systemic steroids | 9 | 2120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.74, 1.60] |
Comparison 5. WMD archive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morning PEF (L/min) at endpoint | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Baseline FEV1 <80% predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Baseline FEV1 not reported | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in FEV1 (L) at endpoint | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Baseline FEV1 <80% predicted | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Baseline FEV1 >=80% predicted | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Baseline FEV1 not reported | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.

Comparison 5 WMD archive, Outcome 1 Morning PEF (L/min) at endpoint.
5.2. Analysis.

Comparison 5 WMD archive, Outcome 2 Change in FEV1 (L) at endpoint.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boonsawat 2008.
| Methods | Parallel group, 69 centres in Australia, Thailand, Philippines and Europe. 3 treatment groups: FP/SAL; FP and placebo | |
| Participants | Asthmatic adults on short‐acting beta‐agonists alone % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: 67 RANDOMISED: 306 (FP/SAL: 151; FP: 155) WITHDRAWALS: FP/SAL: 5; FP: 9 AGE: mean (SD): 34 (13.6) GENDER: (% male): 44 SEVERITY: Mild to moderate BASELINE % PRED. FEV1 (mean): 95 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: 12 to 80 years of age; documented history of asthma for at least 6 months; treatment with short‐acting beta‐agonists only; symptomatic during run‐in EXCLUSION CRITERIA: ICS treatment within 12 weeks of run‐in; treatment with LABA, sodium cromoglycate, nedocromil, anticholinergic; upper/lower RTI; recent acute exacerbation; smoking history > 10 pack years |
|
| Interventions | PROTOCOL: Combination FP/SAL versus SAME DOSE FP OUTCOMES: 12 weeks RUN‐IN: 2 weeks DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 12 weeks TEST GROUP: Combination fluticasone and salmeterol 200/100 OD CONTROL GROUP: Fluticasone 200 OD DEVICE: HFA‐MDI NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF*; pm PEF; FEV1 SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Symptom‐free days; rescue medication use; well controlled asthma; exacerbations requiring oral corticosteroids INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported by treatment group WITHDRAWALS: Reported by treatment group *Primary outcome |
|
| Notes | Unpublished full data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Not obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatments given via identical inhaler devices |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although the intention‐to‐treat population was described in the trial report, it was not clear how this was composed. Withdrawal in the study was low, however (<5%) |
| Selective reporting (reporting bias) | Low risk | Data on primary outcome were available from full‐text publication |
Chuchalin 2002.
| Methods | Parallel group, multicentre study. 3 treatment groups of which 2 considered for this review | |
| Participants | Asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: 99% RANDOMISED: 338 randomised but 333 entered treatment period (F9/BUD: 111; BDP: 114; investigators choice = 108) WITHDRAWALS: Not described MEAN AGE years (RANGE): 46 (19 to 66) GENDER: (% male): 25 SEVERITY: Mild to moderate BASELINE FEV1 L (range): 1.96 (0.93 to 3.99) ASTHMA DURATION (range in years): Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: Adult patients; diagnosis of asthma minimum 6 months; FEV1 50% to 85% predicted normal; 15% reversibility post‐bronchodilator EXCLUSION CRITERIA: Current or recent users of inhaled, oral or parenteral corticosteroids; oral leukotriene antagonists; nedocromil sodium; sodium cromoglycate; betablockers including eye drops; smokers with a history of smoking > or = 10 pack years; all female patients were required to be post‐menopausal, sterile or using contraception CRITERIA FOR RANDOMISATION DURING RUN‐IN: No additional criteria |
|
| Interventions | LABA +ICS vs SAME dose of ICS OUTCOMES: reported monthly RUN‐IN PERIOD: 2 weeks DOSE OF ICS DURING RUN‐IN: No ICS during run‐in DOSE OPTIMISATION PERIOD: None INTERVENTION PERIOD: 12 weeks TEST GROUP: Formoterol 9 mcg bid and budesonide 200 mcg bid CONTROL GROUP: Budesonide 200 mcg bid DEVICE: Turbuhaler NUMBER OF DEVICES: 2 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1 predicted; am PEF; pm PEF SYMPTOM SCORES: Score of 0 to 3 recorded in patient diary card FUNCTIONAL STATUS: Rescue medication use; contact with healthcare provider; inability to work or conduct normal activities; quality of life score INFLAMMATORY MARKERS: None ADVERSE EFFECTS: Reported WITHDRAWALS: Not described Primary outcome measure: Not reported |
|
| Notes | Full‐text publication Source of funding: Not stated Confirmation of methodology and data: Not obtained User‐defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis stated, but explicit description of its composition not available and information on withdrawals was not reported |
| Selective reporting (reporting bias) | Unclear risk | No information on primary outcome available; exacerbation data were collected in the study but it is not clear if steroid‐treated exacerbations were recorded |
Chuchalin 2008.
| Methods | Parallel group, multicentre study. 175 centres in Australasia, South‐East Asia, Middle East, Eastern Europe. 3 treatment groups of which 2 considered in the review. JADAD quality score = 4 |
|
| Participants | Mild asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 1964 (316 randomised to placebo not considered in this review) FP/SAL: 985; FP: 979 WITHDRAWALS: FP/SAL: 162; FP: 119 AGE: mean: 34 GENDER (% male): 58 SEVERITY: Mild BASELINE % PRED. FEV1(mean): 96 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: 12 to 79 years of age; clinical history of asthma > 6 months; treatment with SABA prn only; symptomatic during run‐in (symptom score > 1 on 3 to 6 days of last 7 days of run‐in); > 15% reversibility in PEF post‐SABA OR mean PEF < 85% predicted post‐SABA EXCLUSION CRITERIA: Not reported |
|
| Interventions | LABA + ICS versus HIGHER dose ICS OUTCOMES: TIMING 52 weeks RUN‐IN: 2 weeks DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 52 weeks TEST GROUP: Combination fluticasone and salmeterol 100/50 OD CONTROL GROUP: Fluticasone 100 mcg bid DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF*; FEV1 SYMPTOM SCORES: % symptom‐free days FUNCTIONAL STATUS: Exacerbation rates; rescue medication use INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported WITHDRAWALS: Reported *Primary outcome |
|
| Notes | Full text article. Unpublished data available from GSK trial registry Source of funding: GSK Confirmation of methodology and data: Obtained for methods, not obtained for data User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaled devices |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Analysis populations for this study included the ITT Population (all subjects randomised to study treatment who had taken at least one dose of study medication), and the PP Population (subjects in the ITT Population who had no major protocol violations." |
| Selective reporting (reporting bias) | Low risk | OCS‐treated exacerbations available on request from study sponsor |
Creticos 1999.
| Methods | Parallel group study | |
| Participants | Symptomatic asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 46 WITHDRAWALS: Not described AGE: mean: 35 GENDER (% male): 43.5 SEVERITY: Mild‐moderate BASELINE FEV1 L (mean): 2.8 ASTHMA DURATION: Not described ATOPY (%): Not described ELIGIBILITY CRITERIA: FEV1 >= 65%; >= 12% reversibility; bronchodilator use >= 4 days/week EXCLUSION CRITERIA: None reported |
|
| Interventions | LABA + ICS vs. SAME dose of ICS OUTCOMES: Not described RUN‐IN: 2 weeks DOSE OF ICS DURING RUN‐IN: Zero INTERVENTION PERIOD: 6 months TEST GROUP: (TAA400 mcg bid + salm 50 mcg bid) Triamcinalone 400 mcg bid salmeterol 50 mcg bid CONTROL GROUP: (TAA400) Triamcinalone 400 mcg bid DEVICE: Not reported NUMBER OF DEVICES: 2 COMPLIANCE: Not reported CO‐TREATMENT: Not described |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1*; PEF SYMPTOM SCORES: Score of 0 to 4 FUNCTIONAL STATUS: Not described INFLAMMATORY MARKERS: Not described ADVERSE EFFECTS: Not described WITHDRAWALS: Not reported *Primary outcome |
|
| Notes | Full text publication Source of funding: Not reported Confirmation of methodology and data not obtained User defined number: 400 (TAA 400 bid X 0.5) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | No information on primary outcome available; not clear if exacerbation data were collected in the study |
Di Franco 1999.
| Methods | Parallel group, single centre. 3 groups of which 2 are considered in this review | |
| Participants | Symptomatic asthmatics teenagers and adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 22 (BDP/Sal: 11; BDP: 11) WITHDRAWALS: BDP/Sal: 1; BDP: 6 AGE mean (range): 37 (14 to 68) GENDER (% male): 59 SEVERITY: Mild to moderate BASELINE % PRED. FEV1: 96 BASELINE DOSE OF ICS: No ICS in the last 4 weeks before the study ASTHMA DURATION mean years (range): 10 (1 to 30) ATOPY (%): 68 ELIGIBILITY CRITERIA: A documented historical bronchial reversibility of at least 15% in FEV1 to 200 mg of salbutamol EXCLUSION CRITERIA: Well controlled asthma; previous treatment with corticosteroids; respiratory tract infections in the previous 4 weeks before ELIGILITY CRITERIA FOR RANDOMISATION DURING RUN‐IN: Daily symptom score >/= 2 or within‐day variation of at least 20% on at least 2 out of 7 days during the second baseline week |
|
| Interventions | LABA + ICS vs SAME dose of ICS OUTCOMES: Reported at 3, 6 and 12 months RUN‐IN PERIOD: 2 weeks DOSE OF ICS DURING RUN‐IN: Zero TREATMENT DURATION: 12 months DOSE OPTIMISATION PERIOD: None INTERVENTION PERIOD: 12 months TEST GROUP: (BDP 500 mg+ SALM 50 mg) beclomethasone dipropionate 500 mcg bid + salmeterol 50 mcg bid CONTROL GROUP: (BDP 500 mcg bid) Beclomethasone dipropionate 500 mcg bid DEVICE: Metered‐dose aerosol inhaler NUMBER OF DEVICES: 2 COMPLIANCE: MDI inhalers weighed to assess compliance CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: Diurnal variation in PEF (%); FEV1 % predicted; PC 20 SYMPTOM SCORES: Measured but not reported FUNCTIONAL STATUS: Rescue medication use (measured but not reported); exacerbations requiring oral corticosteroids INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported WITHDRAWALS: Described *Primary outcome: not reported |
|
| Notes | Full‐text publication Source of funding: Not reported Confirmation of methodology and data obtained from Dr. Di Franco User defined number: 1000 (mean ICS dose in LABA group in mcg/day of BDP‐equivalent: 1000 BDP) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers schedule |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Available case for spirometry outcomes |
| Selective reporting (reporting bias) | Low risk | Primary outcome data available |
GOAL.
| Methods | Parallel group, 326 centres in Europe, North America, Latin America and Asia Pacific | |
| Participants | Uncontrolled asthmatic adults % ELIGIBLE OF SCREENED POPULATION: 67 % RUN‐IN PARTICIPANTS RANDOMISED: Not clear RANDOMISED: 3416 (of which 1098 were in stratum one with no ICS in previous 6 months) FP/SAL: 1707; FP: 1709 WITHDRAWALS: FP/SAL: 162; FP: 215 AGE mean (SD): 40 (16) GENDER (% male): 42 SEVERITY: Moderate BASELINE % PRED. FEV1 (mean): 77 BASELINE DOSE OF ICS: Divided into 3 strata: 0; 500 mcg/d or less; between 500 and 1000 mcg/d ASTHMA DURATION: 0 to 1 year: FP/SAL: 56; FP: 97; 1 to 10 years: FP/SAL: 649; FP: 647; > 10 years: FP/SAL: 1004; FP: 992 ATOPY (%): 58 ELIGIBILITY CRITERIA: 12 to 80 years of age ;6‐month history of asthma; FEV1 reversibility of 15%; smoking history of less than 10 pack years; no use of LABA or oral beta‐agonists in previous 2 weeks EXCLUSION CRITERIA: Not reported |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES: End of phase 1 (12 weeks) RUN‐IN: 4 weeks DOSE OF ICS DURING RUN‐IN: Usual maintenance dose of ICS (including 0 for participants not treated with ICS) INTERVENTION PERIOD: Two different phases: I = Dose step‐up until total asthma control achieved, or until maximum dose of study drug given for 12 weeks; II = Constant dose of final dose of study drug until 52 weeks since randomisation had elapsed. TEST GROUP: Combination fluticasone and salmeterol 50/100; 50/250 or 50/500 mcg bid CONTROL GROUP: Fluticasone 100, 250 or 500 mcg bid DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1 SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: N achieving total asthma control*; exacerbations INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported by treatment group WITHDRAWALS: Reported by treatment group *Primary outcome |
|
| Notes | Full text publication Source of funding: GSK Confirmation of methodology and data: Not obtained User defined number: 1000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule. Participants allocated to stratum according to pre‐trial treatment (0 ICS, low dose & high dose). See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | Central system maintained by telephone. See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study population described as intention‐to‐treat; it is not clear whether last observation carried forward was applied: "...a minimum of 4 weeks of evaluable data were required to make an assessment of control... All unassessable patients were classified as uncontrolled." |
| Selective reporting (reporting bias) | Low risk | Primary outcome reported as a mean rate; dichotomous data requested and obtained from study sponsors |
Grutters 1999.
| Methods | Parallel group, 2‐centre study. 3 treatment arms of which 2 are considered for this review | |
| Participants | Stable asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICPANTS RANDOMISED: Not reported RANDOMISED: 40 (SALM 50 mcg + 400 BDP bid: 12; BDP 400 bid = 15) WITHDRAWALS: Not reported AGE: mean: 27 GENDER (% males): 52 SEVERITY: Moderate BASELINE % PRED. FEV1 mean: 84 BASELINE DOSE OF ICS (before start of run‐in): 0 ASTHMA DURATION: Not reported ATOPY (%): 100 ELIGIBILITY CRITERIA: Adults; history of wheezing, impaired lung function, verified in GP or hospital records; regular rescue medication; no oral corticosteroids during 12 months prior to study; no systemic disease or respiratory illness; FEV1 at baseline >= 60% of its predicted value; PC 20 < 4.0 mg/ml; 15% reversibility following bronchodilator; blood eosinophilia > 5%; raised Total IgE and specific antibodies to certain allergens and positive skin tests EXCLUSION CRITERIA: History of hospitalisation for asthma; change in medication for acute exacerbation in 2 months prior to study |
|
| Interventions | LABA + ICS vs SAME dose of ICS OUTCOMES: At days 12, 14, 15, 43, 69, 71 and 72 RUN‐IN PERIOD: 2 weeks DOSE OF ICS DURING RUN‐IN: 0 DOSE OPTIMISATION PERIOD: None INTERVENTION PERIOD: 8 weeks TEST GROUP (LABA + SAME DOSE ICS): BDP 400 mcg bid + salmeterol 50 mcg bid CONTROL GROUP: BDP 400 mcg bid DEVICE: Diskhaler NUMBER OF DEVICES: 1 COMPLIANCE: Study medication counted CO‐TREATMENT: Not stated |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1 SYMPTOM SCORES: Not given FUNCTIONAL STATUS: Not assessed INFLAMMATORY MARKERS: Serum ECP; respiratory burst defined as rate of oxygen uptake of eosinophils; release of PAF before and after allergen inhalation challenge ADVERSE EFFECTS: Not reported WITHDRAWALS: Not reported Primary outcome: Not reported |
|
| Notes | Full‐text publication Supported by GlaxoWellcome Research and Development Confirmation of methodology and data extraction not obtained User defined number: 800 (mean ICS dose in LABA group in mcg/day of BDP‐equivalent: 800) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | No information on primary outcome available; not clear if exacerbation data were collected in the study |
Karaman 2007.
| Methods | Parallel group trial, single centre in Turkey | |
| Participants | Asthmatic children without prior treatment N RANDOMISED: 90 (60 for this review) N COMPLETED: 67 (46 for this review) GENDER (% MALE): 52 MEAN AGE: 10 years BASELINE FEV1 not reported ATOPY (%): 54 INCLUSION CRITERIA: 7 to 17 years; GINA diagnosed asthma; no prior treatment with anti‐asthma medication; diagnosed within 3 months EXCLUSION: Mild or severe persistent asthma; hospitalisation in preceding 4 weeks; previous intubation |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES: At 8 weeks RUN‐IN PERIOD: 0 INTERVENTION PERIOD: 8 weeks TEST GROUP: Budesonide 400 mcg bid, plus 9 mcg formoterol bid CONTROL: Budesonide 400 mcg bid NUMBER OF INHALER DEVICES: 2 CO‐TREATMENT: Not reported |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1; FVC; PEF SYMPTOMS: Not reported FUNCTIONAL STATUS: Change in paediatric AQLQ INFLAMMATORY MARKERS: Eosinophil counts Primary outcome: Not stated |
|
| Notes | Full‐text article Additional data sought from trialists, but not forthcoming Funding source: not disclosed User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study completers analysed for outcomes |
| Selective reporting (reporting bias) | Unclear risk | No information on primary outcome available; not clear if exacerbation data were collected in the study |
Kerwin 2008.
| Methods | Parallel group, multicentre study (121 centres in USA and Canada) | |
| Participants | Mildly asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 844 (FP/SAL bid: 210 (not considered for this review); FP/SAL qd: 212; FP qd: 212; placebo: 212 (not considered for this review)) WITHDRAWALS: FP/SAL qd: 36; FP: 30 AGE mean (SD): 33 (13) GENDER: (% male): 46 SEVERITY: Mild BASELINE % PRED. FEV1 (mean): 74 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: > 12 years of age; 50% to 80% predicted; >/= 15% reversibility post‐SABA; pm PEF 50% to 90% normal; symptom score of more than 2 on 4 or more days in week prior to randomisation; treatment with SABA alone; use of SABA on 4 or more days in week prior to randomisation EXCLUSION CRITERIA: Not reported |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES TIMING: 12 weeks RUN‐IN: Not reported DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 12 weeks TEST GROUP: Combination fluticasone and salmeterol 250/50mcg qd CONTROL GROUP: Fluticasone 250mcg qd DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF predicted; pm PEF predicted* SYMPTOM SCORES: Combined symptoms FUNCTIONAL STATUS: Rescue medication use INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported WITHDRAWALS: Reported *Primary outcome |
|
| Notes | Unpublished full data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: obtained User defined number: 500 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis stated, but explicit description of its composition not available |
| Selective reporting (reporting bias) | Low risk | Data on OCS‐treated exacerbations available on request from study sponsor |
Miraglia del Giudice 2007.
| Methods | Parallel group, single centre in Italy | |
| Participants | % ELIGIBLE OF SCREENED POPULATION: 94 % RUN‐IN PARTICIPANTS RANDOMISED: Not stated RANDOMISED: 48 (N relevant to comparisons in this review: 24) WITHDRAWALS: All completed AGE mean (range) or mean (SD): 7 to 11 years SEVERITY: Moderate BASELINE % PRED. FEV1: 76% BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not stated ATOPY (%): 100 ELIGIBILITY CRITERIA: 7 to 11 years; HDM‐sensitive; > 12% increase in FEV1 post‐SABA EXCLUSION CRITERIA: Use of ICS, OCS or LTRAs in previous 4 weeks ELIGIBILITY CRITERIA DURING RUN‐IN: Not applicable |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES: 4 weeks RUN‐IN PERIOD: 0 DOSE OPTIMISATION PERIOD: Not applicable INTERVENTION PERIOD: 4 weeks TEST GROUP: Budesonide 200 mcg bid + formoterol 9 mcg bid CONTROL GROUP: Budesonide 200 mcg bid NUMBER OF DEVICES: 2 COMPLIANCE: Not assessed CO‐TREATMENT: SABA prn |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1 predicted SYMPTOM SCORES: NA FUNCTIONAL STATUS: NA INFLAMMATORY MARKERS: FEno ADVERSE EFFECTS: Not reported WITHDRAWALS: Reported |
|
| Notes | Full text article Funding: Non‐commercial source Confirmation of methodology and data: Not obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not reported |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Non‐identical placebo (confectionary with similar shape to anti‐leukotriene agent) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study completers analysed for outcomes |
| Selective reporting (reporting bias) | Unclear risk | No information on primary outcome available; not clear if exacerbation data were collected in the study |
Murray 2004.
| Methods | Parallel group, multicentre study (33 centres in USA). 3 treatment groups: FP/SAL; FP; SAL (not considered by this review) | |
| Participants | Asthmatic adults not treated with inhaled corticosteroids % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 177 (FP/SAL: 88; FP: 89) WITHDRAWALS: FP/SAL: 12; FP: 11 AGE mean (SD): 33 (13.5) GENDER (% male): 49 SEVERITY: Moderate BASELINE % PRED. FEV1 (mean): 66 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: ATS defined asthma for at least 6 months prior to screening; treatment with as‐needed short‐acting beta‐agonists only for at least 1 month; FEV1 40% to 85% predicted EXCLUSION CRITERIA: Use of ICS or OCS within 1 month of study entry; use of LABA within 72 hours of study entry |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES: 12 weeks RUN‐IN: Not reported DOSE OF ICS DURING RUN‐IN: Not reported INTERVENTION PERIOD: 12 weeks TEST GROUP: Combination fluticasone and salmeterol 100/50 mcg bid CONTROL GROUP: Fluticasone 100 mcg bid DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF; pm PEF; FEV1* SYMPTOM SCORES: Combined scores FUNCTIONAL STATUS: Rescue medication usage; night‐time awakenings; withdrawal due to worsening asthma INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported by treatment group WITHDRAWALS: Reported by treatment group *Primary outcome |
|
| Notes | Full data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Not obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Described as intention‐to‐treat analysis, last observation carried forward used: ITT population "...defined as all randomised patients who had taken at least one dose of the study drug. To minimize the potential bias due to different withdrawal rates among the treatment groups, end point analyses were used when appropriate." |
| Selective reporting (reporting bias) | Low risk | Data on exacerbations available on request from GSK |
Nelson 2003.
| Methods | Parallel‐group, multicentre study (33 centres). 3 treatment groups of which 2 are considered here | |
| Participants | Symptomatic asthmatic patients over 12 years % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: 54% (Of 525 patients screened 242 were not randomised reasons not stated) RANDOMISED: 192 total randomised to groups of interest (SAL + ICS: 95; placebo + ICS: 97) WITHDRAWALS: SAL + ICS: 9; placebo + ICS: 8 AGE: mean(range): 31.4 (12 to 76) GENDER (% male): 53 SEVERITY: Mild to moderate BASELINE FEV1 % PRED. MEAN : 66 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY(%): Not reported ELIGIBILITY CRITERIA: Asthma (ATS criteria) of at least 6 months duration; required pharmacotherapy for at least 6 months before study; FEV1 between 40% to 85%; 15% improvement in FEV1 post‐bronchodilator; female patients negative pregnancy test, surgically sterile, post‐menopausal or using birth control EXCLUSION CRITERIA: History of life threatening asthma; hypersensitivity reaction to sympathomimetic drugs or corticosteroids; smoking in year before study or smoking history of > 10 pack years; received a course of systemic corticosteroids in 6 months before study of use of any other prescription or OTC medication that could affect asthma or interact with other medications; abnormal CXR or EKG; history of diabetes glaucoma, hypertension CRITERIA FOR RANDOMISATION DURING RUN‐IN: Unstable asthma during run‐in periods, i.e. more than 3 nights with awakenings, during 7 days before randomisation; more than 12 puffs of rescue medication/day for more than 3 days; FEV1 not within 15% of value obtained at beginning of screening |
|
| Interventions | LABA + ICS vs SAME dose of ICS OUTCOMES: Reported weekly weeks 1 to 4 and thereafter 2‐weekly RUN‐IN PERIOD: 2 weeks DOSE OF ICS DURING RUN‐IN: 0 DOSE OPTIMISATION PERIOD: None INTERVENTION PERIOD: 12 weeks TEST GROUP: (SAL 50 + ICS) Salmeterol 50 mg bid + ICS FP 100 mcg bid CONTROL GROUP: (placebo+ICS) placebo + ICS FP 100 mcg bid DEVICE: MDI NUMBER OF DEVICES: 2 COMPLIANCE: Evaluated using diary cards (96% to 97%) CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF; pm PEF; FEV1 SYMTPOM SCORES: Mean change in patient‐rated daily diary card asthma symptom scores (score 0 to 5) FUNCTIONAL STATUS: Symptom‐free days; night awakenings; rescue medication use INFLAMMATORY MARKERS: Not described ADVERSE EFFECTS: Described WITHDRAWALS: Described |
|
| Notes | Full‐text publication Funded by GSK Confirmation of methodology obtained from Shailesh Patel User‐defined number: 400 (400 mcg/day BDP equivalent in control group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebos used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis: unclear how population composed |
| Selective reporting (reporting bias) | Low risk | Data on exacerbations available on request from GSK |
O'Byrne 2001.
| Methods | Parallel group, multicentre trial. 7 groups of which 2 considered here | |
| Participants | Symptomatic asthmatic teenagers and adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 459 (F/BUD: 231; BUD: 228) WITHDRAWALS: Not reported by subgroup AGE mean: 31 GENDER (% male): 38 SEVERITY: Mild BASELINE % PRED. FEV1: 90 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY(%): Not reported ELIGIBILITY CRITERIA: >= 12 years of age with mild asthma; taking no inhaled corticosteroids for >/= 3 months; FEV1 >= 80% predicted normal after terbutaline EXCLUSION CRITERIA: Experienced 3 severe exacerbations during the initial 6 months or 5 exacerbations in total; 2 poorly controlled asthma days, defined as days with morning PEF values >= 2 above baseline, or with asthma awakening CRITERIA FOR RANDOMISATION DURING RUN‐IN: Randomised patients demonstrated a need for 2 or more inhalations per week of rescue medication during the last 2 weeks of run‐in; a >= 15% variability in peak expiratory flows; or a >= 12% increase in FEV1 after terbutaline |
|
| Interventions | LABA + ICS vs SAME dose ICS OUTCOMES: Reported at 52 weeks RUN‐IN PERIOD: 4 weeks DOSE OF ICS DURING RUN‐ IN: 0 DOSE OPTIMISATION PERIOD: None INTERVENTION PERIOD: 52 weeks TEST GROUP: Formoterol 4.5 mcg and budesonide 100 mcg bid CONTROL GROUP: Budesonide 100 mcg bid DEVICE: Turbuhaler NUMBER OF DEVICES: 2 COMPLIANCE: Not reported CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF; FEV1 SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Symptom‐free days; nocturnal awakenings; rescue medication use; severe exacerbations (rate) INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported WITHDRAWAL: Reported *Primary outcome: time to the first severe asthma exacerbation defined as need for treatment with oral corticosteroids or hospital admission or emergency treatment for worsening asthma or a decrease in morning PEF > 25% from baseline |
|
| Notes | Full‐text publication Funded by AstraZeneca Confirmation of methodology and data obtained User‐defined order: 200 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Opaque consecutive numbered envelopes containing assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Use of identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis stated, but explicit description of its composition not available |
| Selective reporting (reporting bias) | Low risk | Primary outcome data available from study publication |
Overbeek 2005.
| Methods | Parallel‐group, single centre study | |
| Participants | Asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 40 WITHDRAWALS: 0 MEAN AGE years: 28.8 GENDER: (% male) 53 SEVERITY: Mild to moderate BASELINE FEV1 PREDICTED: 78% BASELINE DOSE OF ICS: 0 ASTHMA DURATION (range in years): Not reported ATOPY (%): 85 ELIGIBILITY CRITERIA: Non‐smokers; receiving maximum of 800 mcg ICS/d prior to steroid‐free run‐in; 18 to 55 years of age; FEV1 between 50% and 90% of predicted; provocative concentration of methacholine causing 20% fall in FEV1 of 8 mg/mL EXCLUSION CRITERIA: Not reported CRITERIA FOR RANDOMISATION DURING RUN‐IN: Not reported |
|
| Interventions | LABA + ICS vs SAME dose ICS OUTCOMES: Reported at 8 and 16 weeks RUN‐IN PERIOD: 4 weeks DOSE OF ICS DURING RUN‐IN: No ICS during run‐in DOSE OPTIMISATION PERIOD: 0 INTERVENTION PERIOD: 8 weeks (ICS doubled between weeks 8 and 16) TEST GROUP: Formoterol 12 mcg bid and budesonide 100 mcg bid CONTROL GROUP: Budesonide 100 mcg bid DEVICE: Turbuhaler NUMBER OF DEVICES: 2 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1 predicted SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MARKERS: Bronchoprovocation test*; exhaled nitric oxide ADVERSE EFFECTS: None WITHDRAWALS: None *Primary outcome measure |
|
| Notes | Full‐text publication Funded by AstraZeneca Data and methodology confirmation: Not obtained User‐defined number: 200 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No information on primary outcome available; not clear if exacerbation data were collected in the study |
Pearlman 1999a.
| Methods | Parallel group, multicentre study (11 centres). 6 treatment groups of which 4 are considered for this review and 2 are described here | |
| Participants | Asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 48 (FP/SAL 25; FP: 23) WITHDRAWALS: FP/SAL: 2; FP: 1 AGE: mean (range): 30 (13 to 60) GENDER (% male): 57 SEVERITY: Moderate BASELINE % PRED. FEV1: 68 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: >= 6 months & < 1 year = 0 >= 1 year & < 5 years = 11 >= 5 years & < 10 years = 7 >= 10 years & < 15 years = 7 >= 15 years = 23 ATOPY (%): Not recorded ELIGIBILITY CRITERIA: >= 12 years; FEV1 between 50% and 80% of predicted value for age, sex, height and race; medical history of asthma of at least 6 months requiring pharmacotherapy; >= 15% increase in FEV1 15 minutes after 2 puffs of inhaled albuterol; being treated with daily or as‐needed short‐acting beta‐sympathomimetic bronchodilators; females had negative pregnancy tests, surgically sterile, post‐menopausal for at least 1 year; or using acceptable birth control for at least 1 month prior to participation EXCLUSION CRITERIA: History of life threatening asthma; hypersensitivity reaction to sympathomimetic drugs or corticosteroids; smoking within the previous year or a history of > 10 pack‐years; use of oral, inhaled, injectable, or intranasal corticosteroid therapy within the previous month; use of daily oral corticosteroid treatment within the previous 6 months; use of any other prescription or over‐the‐counter medication that may affect the course of asthma or interact with sympathomimetic amines; abnormal chest X‐rays; clinically significant abnormal 12‐lead electrocardiograms; history of significant concurrent disease CRITERIA FOR RANDOMISATION DURING RUN‐IN: Completion of daily dairy cards and report medication compliance; patients were not eligible for inclusion if they used 12 or more puffs of albuterol daily for more than 2 days or if they had more than 2 night‐time awakenings due to asthma requiring treatment with albuterol during the 7 days immediately preceding the randomisation period; FEV 1 had to be between 50% and 80% of the predicted value and within 15% of the FEV1 obtained at the beginning of the screening period |
|
| Interventions | LABA + ICS vs SAME dose ICS OUTCOMES: reported at 2 and 4 weeks RUN‐IN PERIOD: 2 weeks DOSE OF ICS DURING RUN‐IN: Same as usual DOSE OPTIMISATION PERIOD: None INTERVENTION PERIOD: 4 weeks TEST GROUP: (SL50 + FP100) salmeterol 50 mg bid + fluticasone propionate 100 mg bid CONTROL GROUP: (FP 100) Fluticasone propionate 100 mg bid DEVICE: Metered‐dose inhaler NUMBER OF DEVICES: 2 COMPLIANCE: Evaluated CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1; am PEF SYMPTOM SCORE: Score of 0 to 4 mean change from baseline FUNCTIONAL STATUS: Rescue medication use; night awakenings; symptom‐free days INFLAMMATORY MARKERS: Not measured ADVERSE EFFECTS: Reported WITHDRAWALS: Reported *Primary outcome measure ( not reported) |
|
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data confirmed User defined number: 400 (F100 x 2 bid) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers "Lowest available treatment number in accordance with their chronological presentation to the investigator" |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Use of identical placebo (double dummy design) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat population defined as "all randomized subjects exposed to the study drug". Handling of withdrawals not explicit |
| Selective reporting (reporting bias) | Low risk | Exacerbations not assessed |
Pearlman 1999b.
| Methods | See Pearlman 1999a | |
| Participants | As for Pearlman 1999a, except for: RANDOMISED: 44 (FP/SAL: 21; FP: 23) WITHDRAWALS: FP/SAL: 0; FP: 1 AGE: mean (range) 29 (13 to 61) GENDER: (% male) 62 SEVERITY: Moderate BASELINE % PRED. FEV1: 67 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: >= 6 months & < 1 year = 1 >= 1 year & < 5 years = 5 >= 5 years & < 10 years = 6 >= 10 years & < 15 years = 8 >= 15 years = 24 |
|
| Interventions | As for Pearlman 1999a, except for TEST GROUP: (SL50 + FP250) salmeterol 50 mg bid and fluticasone propionate 250 mg bid CONTROL GROUP: (FP 250) Fluticasone propionate 250 mg bid DEVICE: Metered‐dose inhaler NUMBER OF DEVICES: 2 |
|
| Outcomes | See Pearlman 1999a | |
| Notes | As for Pearlman 1999a, except for: User defined number: 1000 (F250 x 2 bid) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers "Lowest available treatment number in accordance with their chronological presentation to the investigator" |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Use of identical placebo (double dummy design) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat population defined as "all randomized subjects exposed to the study drug". Handling of withdrawals not explicit |
| Selective reporting (reporting bias) | Low risk | Exacerbations not assessed |
Prieto 2005.
| Methods | Parallel group, single centre in Spain. | |
| Participants | Mild asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 42 (FP/SAL: 21; FP: 21) WITHDRAWALS: 0 AGE: mean: 41 GENDER (% male): 45 SEVERITY: Mild BASELINE % PRED. FEV1 (mean): 105 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): 100 ELIGIBILITY CRITERIA: 18 to 72 years; sensitised to pollen; lifelong non‐smoker EXCLUSION CRITERIA: Requirement for asthma therapy; symptoms outside pollen season |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES: 6 weeks RUN‐IN: None DOSE OF ICS DURING RUN‐IN: NA INTERVENTION PERIOD: 6 weeks TEST GROUP: Combination fluticasone and salmeterol 100/50 mcg bid CONTROL GROUP: Fluticasone 100 mcg bid DEVICE: Accuhaler NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: Not reported |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1 SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MARKERS: PC20* ADVERSE EFFECTS: Reported WITHDRAWALS: Reported *Primary outcome |
|
| Notes | Full text publication and unpublished data available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Not obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No information on primary outcome available; not clear if exacerbation data were collected in the study |
Rojas 2007.
| Methods | Parallel group, 48 centres in Argentina & Europe | |
| Participants | Moderately severe asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 362 (FP/SAL: 182; FP: 180) WITHDRAWALS: FP/SAL: 7; FP: 5 AGE mean (SD): 40 (15) GENDER (% male): 42 SEVERITY: Moderate BASELINE % PRED FEV1 (mean): 72 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: 12 to 80 years of age; history of asthma of more than 6 months; FEV1 60% to 80% predicted; >/= 15% reversibility FEV1 post‐SABA OR mean PEF <85% predicted post‐SABA over last 7 days of run‐in EXCLUSION CRITERIA: Use of corticosteroids within 12 weeks; anti‐leukotrienes within 4 weeks; LABAs/nedocromil sodium/ketotifen/methylxanthines/anticholinergics within 2 weeks; acute exacerbation of asthma within 6 weeks |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES: 12 weeks RUN‐IN: 2 weeks DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 12 weeks TEST GROUP: Combination fluticasone and salmeterol 250/50 mcg bid CONTROL GROUP: Fluticasone 250 mcg bid DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF*; pm PEF; FEV1 SYMPTOM SCORES: Daytime symptoms; night‐time symptoms FUNCTIONAL STATUS: Rescue medication use INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported by treatment group WITHDRAWALS: Reported by treatment group *Primary outcome |
|
| Notes | Unpublished full data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Obtained User defined number: 1000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis stated, but explicit description of its composition not available |
| Selective reporting (reporting bias) | Low risk | Data on OCS‐treated exacerbations available on request from study sponsor |
SAM40034.
| Methods | Parallel group, 27 centres in Scandinavia JADAD quality score = 4 |
|
| Participants | Symptomatic mildly asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 154 (FP/SAL: 75; FP: 79) WITHDRAWALS: FP/SAL: 4; FP: 5 AGE: mean (range) or mean: 37 GENDER (% male): 39 SEVERITY: Mild BASELINE % PRED. FEV1 (mean): 91 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: 18 to 60 years; symptoms of asthma for at least 3 months; treatment with SABA only EXCLUSION CRITERIA: ICS treatment |
|
| Interventions | LABA+ICS versus HIGHER dose of ICS OUTCOMES TIMING: 4, 8 & 12 weeks RUN‐IN: Not reported DOSE OF ICS DURING RUN‐IN: NA INTERVENTION PERIOD: 12 weeks TEST GROUP: Combination fluticasone and salmeterol 100/50 mcg bid CONTROL GROUP: Fluticasone 250 mcg bid DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not reported CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF*; pm PEF; FEV1 SYMPTOM SCORES: NA FUNCTIONAL STATUS: NA INFLAMMATORY MARKERS: NA ADVERSE EFFECTS: Reported WITHDRAWALS: Reported *Primary outcome |
|
| Notes | Full unpublished data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Obtained for methods, not obtained for data User defined number: 1000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler device |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "The primary population of patients analysed for safety and efficacy was the Intent‐to‐Treat (ITT) population that consisted of all subjects randomised to study treatment who received at least one dose of study treatment." |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on OCS‐treated exacerbations were collected. Request for data from study sponsors has not been successful. |
SAM40036.
| Methods | Parallel group, multicentre study (74 centres in Europe) JADAD quality score = 4 |
|
| Participants | Mildly asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 577 (FP/SAL: 288; BUDL: 289) WITHDRAWALS: FP/SAL: 18; BUD: 16 AGE mean: 37 GENDER (% male): 43 SEVERITY: Mild BASELINE % PRED. FEV1: 95.4 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: 12 to 80 years; diagnosis of asthma; treatment with inhaled short‐acting beta‐agonists alone EXCLUSION CRITERIA: Not reported |
|
| Interventions | LABA+ICS versus HIGHER dose ICS OUTCOMES TIMING: 12 weeks RUN‐IN: 2 weeks DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 12 weeks TEST GROUP: Combination fluticasone and salmeterol (100/50 mcg) once daily CONTROL GROUP: Budesonide 400 mcg once daily DEVICE: FP/SAL: Diskus; BUD: Turbuhaler NUMBER OF DEVICES: 1 (double‐dummy design) COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF*; FEV1 SYMPTOM SCORES: Daytime symptoms; night‐time symptoms FUNCTIONAL STATUS: Rescue medication use INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported WITHDRAWALS: Reported *Primary outcome |
|
| Notes | Unpublished full data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Obtained for methods; not obtained for data User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double dummy design with identical inhaler devices |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "The Intent‐to‐Treat (ITT) population representing all subjects randomised to treatment who had taken at least one dose of study medication was also used for safety analyses." |
| Selective reporting (reporting bias) | Low risk | OCS‐treated exacerbations available on request from study sponsor. |
SAS30015.
| Methods | Parallel group, multicentre study (37 centres in UK) | |
| Participants | Poorly controlled asthmatic adults at step 1 of BTS guidelines % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 156 (FP/SAL: 78; BDP: 78) WITHDRAWALS: FP/SAL: 9; BDP: 17 AGE mean (SD): 35 (15) GENDER (% male): 54 SEVERITY: Mild to moderate BASELINE % PRED. FEV1 (mean): Not reported BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: Step 1 of BTS asthma guidelines; am PEF 50% to 85% predicted over last 7 days of run‐in; rescue medication use >/= 2 occasions on 3 or more days of last 7 of run‐in period/symptom score >/= 1 on 3 of last 7 days of run‐in EXCLUSION CRITERIA: Not reported |
|
| Interventions | LABA + ICS versus EQUIVALENT dose of BDP OUTCOMES: 12 weeks RUN‐IN: 2 weeks DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 12 weeks TEST GROUP: Combination fluticasone and salmeterol 100/50 mcg bid CONTROL GROUP: Beclomethasone 200 mcg bid DEVICE: FP/SAL: HFA‐MDI; BDP: CFC‐MDI NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF*; pm PEF SYMPTOM SCORES: Combined symptoms FUNCTIONAL STATUS: Symptom‐free days; % SABA‐free nights; exacerbations; loss of control INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported by treatment group WITHDRAWALS: Reported by treatment group *Primary outcome |
|
| Notes | Full unpublished data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis stated, but explicit description of its composition not available |
| Selective reporting (reporting bias) | Low risk | Data on OCS‐treated exacerbations available on request from study sponsor |
SAS30021.
| Methods | Parallel group, multicentre study | |
| Participants | Steroid‐naive asthmatic children % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 608 (FP/SAL 304; FP: 304) WITHDRAWAL: FP/SAL: 56; FP: 63 AGE mean: 7.8 years GENDER (male %): 61% ASTHMA SEVERITY: Mild‐moderate BASELINE % PRED. FEV1: Not reported ASTHMA DURATION: Not reported ATOPY(%): Not reported ELIGIBILITY CRITERIA: Non‐ICS controller medication for 6 months prior to entry; 50% to 85% predicted am PEF; 50% to 90% predicted PEF at screening visit >/= 15% response to beta‐agonist EXCLUSION CRITERIA: Not reported CRITERIA FOR RANDOMISATION DURING RUN‐IN: Symptomatic in week before study entry (score >/= 2 or used SABA on >/= 4 days of preceding week) |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES: reported at 3 months RUN‐IN PERIOD: Unclear DOSE OF ICS DURING RUN‐IN: 0 DOSE OPTIMISATION PERIOD: None reported INTERVENTION PERIOD: 3 months TEST GROUP: Combination salmeterol 50/fluticasone 100 mcg once daily CONTROL GROUP: Fluticasone 100 mcg once daily DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not reported CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF predicted; pm PEF predicted* SYMPTOM SCORES: % Symptom‐free days FUNCTIONAL STATUS: Use of reliever medication; exacerbations (undefined) INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported WITHDRAWAL: Reported *Primary outcome |
|
| Notes | Data downloaded from GSK trials web site (http://www.ctr.gsk.co.uk) Source of funding: GSK Confirmation of methodology and data: Obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "An Intent‐to‐Treat Population was defined as all subjects who were randomized to treatment and received at least one dose of study drug. This population was used for all analyses of data from this trial (demographic, efficacy, and safety)." |
| Selective reporting (reporting bias) | Low risk | Data on OCS‐treated exacerbations available on request from study sponsor |
SAS40068.
| Methods | Parallel group, multicentre study (58 centres in Canada) | |
| Participants | Asthmatic adults not adequately controlled on SABA alone % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 532 (FP/SAL: 262; FP: 270) WITHDRAWALS: FP/SAL: 53; FP: 46 AGE mean: 34.6 GENDER (% male): 36 SEVERITY: Mild to moderate BASELINE % PRED. FEV1(mean): Not reported BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: > 12 years; FEV1 > 80% over last 7 days of run‐in; symptom score >/= 2 on 3 days of run‐in; SABA use on more than 4 days of last 7 days of run‐in EXCLUSION CRITERIA: ICS, anti‐leukotriene agent, LABA in 1 month prior to study entry; smoking history of > 10 pack years; emergency room treatment within 6 weeks of study entry & hospitalization within 12 weeks |
|
| Interventions | ICS and LABA versus SAME DOSE ICS OUTCOMES: 24 weeks RUN‐IN: Reported but duration not described DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 24 weeks TEST GROUP: Combined fluticasone and salmeterol 100/50 mcg bid CONTROL GROUP: Fluticasone 100 mcg bid DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not reported CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF*; pm PEF; FEV1 SYMPTOM SCORES: % symptom‐free days; % rescue‐free days FUNCTIONAL STATUS: Exacerbation rate INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported by treatment group WITHDRAWALS: Reported by treatment group *Primary outcome |
|
| Notes | Unpublished data set available from http://www.ctr.gsk.co.uk Source of funding GSK Confirmation of methodology and data: Obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis stated, but explicit description of its composition not available |
| Selective reporting (reporting bias) | Low risk | Data on OCS‐treated exacerbations available on request from study sponsor |
SLGF75.
| Methods | Parallel group, 7 centres in Italy | |
| Participants | % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not clear RANDOMISED: 31 (FP+SAL: 14; FP: 17) WITHDRAWALS: 4 (FP+SAL: 2; fp: 2) AGE mean (range) or mean (SD): 42 SEVERITY: Mild to moderate BASELINE % PRED. FEV1: Not reported BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not reported ATOPY (%): Not reported ELIGIBILITY CRITERIA: 16 to 65 years; asthma > 6 months duration; FEV1 > 60% predicted EXCLUSION CRITERIA: ICS within 3 months; upper RTI within 1 month ELIGIBILITY CRITERIA DURING RUN‐IN: Not reported |
|
| Interventions | LABA + ICS versus SAME DOSE ICS OUTCOMES: 12 weeks RUN‐IN PERIOD: 2 to 4 weeks DOSE OPTIMISATION PERIOD: NA TEST GROUP: Salmeterol 50 mcg bid plus fluticasone 100 mcg bid CONTROL GROUP: Fluticasone 100 mcg bid NUMBER OF DEVICES: 2 (DPI) CO‐TREATMENT: SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: NA SYMPTOM SCORES: NA FUNCTIONAL STATUS: Admission to hospital INFLAMMATORY MARKERS: Eosinophil count ADVERSE EFFECTS: Stated WITHDRAWALS: Stated |
|
| Notes | Unpublished data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Not obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Last observation carried forward: "Safety population: subjects randomised and with at least one dose of administered study drug. ITT population: subjects randomised and with at least one dose of administered study drug with eosinophils >5% were used for primary efficacy analysis. PP population: all subjects of ITT without any major protocol violation were used for secondary efficacy analysis." |
| Selective reporting (reporting bias) | Unclear risk | No information on primary outcome available |
Sorkness 2007.
| Methods | Parallel group, multicentre study. 3 treatment groups (FP/SAL; FP and montelukast) JADAD quality score = 4 |
|
| Participants | Mildly asthmatic children % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED 44 RANDOMISED: 190 (FP/SAL: 94; FP: 96. Montelukast not considered by this review: 95) WITHDRAWALS: FP/SAL: 13; FP: 10 AGE mean: 10 GENDER (% male): 62 SEVERITY: Mild BASELINE % PRED. FEV1: 97 BASELINE DOSE OF ICS: Not consistent (55% on ICS at baseline) ASTHMA DURATION: Not reported ATOPY (%): 75 ELIGIBILITY CRITERIA: Physician‐diagnosed asthma; 6 to 14 years; ability to perform reproducible spirometry; post‐dose FEV1 >/= 80% predicted normal at screening & >/= 70% predicted normal at randomisation; PC20 </= 12.5 mg/mL; mild‐moderate persistent asthma (defined by symptoms or SABA use) or peak flows < 80% calculated from mean of morning and evening peak flows obtained during last week of run‐in, on average >/= 3 times per week EXCLUSION CRITERIA: Other lung diseases; respiratory tract infection/asthma; exacerbation/systemic corticosteroid use within 4 weeks; 2 or more asthma hospitalisations in past year; history of life‐threatening asthma exacerbation; >/= 4 courses of systemic corticosteroids in past year; cigarette smoking within the past year‐pregnancy/lactation; adverse reactions to study medication; use of controller medications for at least 2 weeks before randomisation; inability to use study drug delivery systems/or adherence </= 75% of doses during the run‐in |
|
| Interventions | LABA+ICS versus versus higher dose ICS OUTCOMES TIMING: 48 weeks RUN‐IN: 4 weeks DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 48 weeks TEST GROUP: Combination fluticasone/salmeterol 100/50 mcg qd + salmeterol qd CONTROL GROUP: Fluticasone 100 mcg bid DEVICE: Diskus NUMBER OF DEVICES: 2 COMPLIANCE: 95% adherence (diary card entry) CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF; pm PEF; FEV1 SYMPTOM SCORES: Not stated FUNCTIONAL STATUS: Asthma control days (defined as: day without SABA rescue use; use of oral corticosteroids for asthma; use of non‐study asthma medications; daytime symptoms; night‐time awakenings; unscheduled health care visits, emergency department visits, or hospitalisations for asthma; and school absenteeism for asthma)*; episode‐free days INFLAMMATORY MARKERS: Exhaled nitric oxide ADVERSE EFFECTS: Growth WITHDRAWALS: Stated *Primary outcome |
|
| Notes | Full text publication Source of funding: NHLBI Confirmation of methodology and data: Obtained for data User defined number: 800 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified on lung function (method of randomisation/sequence generation not described) |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "All analyses were performed under the intent‐to‐treat paradigm." |
| Selective reporting (reporting bias) | Low risk | Study presented adjusted estimates; n/N data for OCS‐treated exacerbations available on request from investigators |
Stelmach 2008.
| Methods | DESIGN: Parallel group; single centre study (Poland) | |
| Participants | % ELIGIBLE OF SCREENED POPULATION: 67 % RUN‐IN PARTICIPANTS RANDOMISED: NA RANDOMISED: 40 WITHDRAWALS: BUD/F: 2; BUD/MON: 3 AGE mean (range) or mean (SD): 12 years (6 to 18) SEVERITY: Not stated BASELINE % PRED. FEV1: 91 BASELINE DOSE OF ICS: 0 ASTHMA DURATION: Not stated ATOPY (%): Not stated ELIGIBILITY CRITERIA: Age 6 to 18 years; diagnosis of bronchial asthma for at least 6 months; resting FEV1 of greater than 70%; a documented decrease in FEV1 of at least 20% post‐exercise challenge test. EXCLUSION CRITERIA: Active upper respiratory tract infection 3 weeks before study entry; sinus disease requiring antibiotic treatment within 1 month; intubation, or asthma hospitalisation in last 3 months; other clinically significant diseases; participants taking beta‐blockers and oral corticosteroids within 1 month before study; participants receiving immunotherapy ELIGIBILITY CRITERIA DURING RUN‐IN: Not applicable |
|
| Interventions | LABA + ICS versus SAME dose ICS OUTCOMES: 8 weeks RUN‐IN PERIOD: 4 weeks (LABAs, LTRAs and ICS stopped during run‐in) DOSE OPTIMISATION PERIOD: NA INTERVENTION PERIOD: 8 weeks TEST GROUP: Budesonide 100 mcg bid/formoterol 9 mcg bid CONTROL GROUP: Budesonide 100 mcg bid NUMBER OF INHALER DEVICES: 2 CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: AUC; fall in FEV1 post‐exercise SYMPTOM SCORES: Not assessed FUNCTIONAL STATUS: Not assessed INFLAMMATORY MARKERS: Not assessed ADVERSE EFFECTS: Not reported WITHDRAWALS: Stated |
|
| Notes | Full text article Funding: Non‐commercial source Confirmation of methodology and data: Not obtained User defined number: 200 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Low risk | Undertaken by third party (hospital pharmacy) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completers analysed for outcomes |
| Selective reporting (reporting bias) | Low risk | Participants who experienced OCS‐treated exacerbations were described in the study report, but distribution among treatment groups was not given. Correspondence has not been successful in retrieving these data. |
Strand 2004.
| Methods | Parallel group multicentre study (45 centres in Denmark) | |
| Participants | Asthmatic adults poorly controlled on SABA alone % ELIGIBLE OF SCREENED POPULATION: 68 RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 150 (FP/SAL: 78; FP: 72) WITHDRAWALS: FP/SAL: 11; FP: 13 AGE mean (SD): 39 (15) GENDER (% male): 43 SEVERITY: Mild to moderate BASELINE % PRED. FEV1 (mean): Not reported BASELINE DOSE OF ICS: 0 ASTHMA DURATION: 12 years ATOPY (%): Not reported ELIGIBILITY CRITERIA: > 18 years; medical history of ATS defined asthma for at least 3 months; use of SABA only once per week for 2 months prior to visit 1; diary data during run‐in for 11 days & 11 nights; PEF diurnal variation >/= 20% on > 2 days OR FEV1 reversibility > 15% within 3 years, PC20 </= 4 mg/mL, diurnal variation in PEF >/= 20%; SABA relief medication >/=once per week. Day or night symptom score >/= 1 once/week during run‐in EXCLUSION CRITERIA: Use of ICS within 2 months prior to visit 1; use of OCS within1 month of visit 1; upper/lower RTI or middle ear infection within 1 month of visit; inadequate inhaler technique; lung diseases other than asthma; serious comorbid disease |
|
| Interventions | LABA + ICS versus SAME DOSE ICS OUTCOMES: 24 weeks RUN‐IN: 2 weeks DOSE OF ICS DURING RUN‐IN: 0 INTERVENTION PERIOD: 24 weeks TEST GROUP: Combined fluticasone and salmeterol 100/50 mcg bid CONTROL GROUP: Fluticasone 100 mcg bid DEVICE: Diskus NUMBER OF DEVICES: 1 COMPLIANCE: Not assessed CO‐TREATMENT: prn SABA |
|
| Outcomes | PULMONARY FUNCTION TEST: am PEF; pm PEF; diurnal variation in PEF SYMPTOM SCORES: Day symptoms; night symptoms FUNCTIONAL STATUS: Rescue medication use; % 24‐hour days without symptoms*; episode‐free 24 hours INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Reported by treatment group WITHDRAWALS: Reported by treatment group *Primary outcome |
|
| Notes | Full text article, with unpublished data set available from http://www.ctr.gsk.co.uk Source of funding: GSK Confirmation of methodology and data: Not obtained User defined number: 400 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Identical inhaler devices used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat population defined as: "All efficacy parameters were analysed on an intent‐to‐treat basis and data from all patients with at least one dose of study drug were included in the analysis." |
| Selective reporting (reporting bias) | Low risk | Primary outcome data available in study publication |
Weersink 1997.
| Methods | Parallel group, single centre study. 3 treatment groups of which 2 are considered for this review | |
| Participants | Stable asthmatic adults % ELIGIBLE OF SCREENED POPULATION: Not reported % RUN‐IN PARTICIPANTS RANDOMISED: Not reported RANDOMISED: 33 (SAL + FP: 16; FP: 17) WITHDRAWALS: SAL + FP: 2; FP: 1 AGE years mean: 27 GENDER (% males): 47 SEVERITY: Mild BASELINE % PRED. FEV1: 86 BASELINE DOSE OF ICS : All ICS discontinued if taken at least 1 month before study commenced ASTHMA DURATION: Not reported ATOPY (%): 100 ELIGIBILITY CRITERIA: Non‐smoking, atopic asthmatic subjects 18 to 45 years; circadian variation in PEF >/= 15%; history of episodic dyspnea or wheezing consistent with clinical diagnosis of asthma and no concomitant diseases; BHR to methacholine bromide (PC20 < 9.6 mg/ml); elevated specific immunoglobulin E (IgE) against house dust mite (RAST > 2) or positive intracutaneous tests against house dust mite or 2 other common aeroallergens; no use of oral corticosteroids; no respiratory tract infection or acute asthma during the 2 months prior to the study; inhaled corticosteroids if used were stopped 4 weeks before onset of the study whereas nedocromil sodium and long‐acting beta2‐agonists were discontinued 2 weeks before. Short‐acting beta 2 agonists were allowed for symptom relief during 4‐week period before study. EXCLUSION CRITERIA: History of hospitalisation for asthma; change in medication for acute exacerbation in 2 months prior to study |
|
| Interventions | LABA + ICS vs SAME dose of ICS OUTCOMES: At days 1, 2 and at 6 weeks RUN‐IN PERIOD: None DOSE OF ICS DURING RUN‐IN: NA DOSE OPTIMISATION PERIOD: None INTERVENTION PERIOD: 6 weeks TEST GROUP: Fluticasone 250 mcg bid and salmeterol 50 mcg bid CONTROL GROUP: Fluticasone propionate 250 mcg bid DEVICE: Diskhaler NUMBER OF DEVICES: 2 COMPLIANCE: Not reported CO‐TREATMENT: Not stated |
|
| Outcomes | PULMONARY FUNCTION TEST: FEV1 % predicted; circadian variation in PEF; PC20 methacholine SYMPTOM SCORES: Not given FUNCTIONAL STATUS: Not assessed INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS: Not reported WITHDRAWALS: Not reported Primary outcome: Not reported |
|
| Notes | Full‐text publication Supported by Glaxo BV Netherlands Confirmation of methodology and data extraction: Not obtained User defined number: 1000 (mean ICS dose in LABA group in mcg/day of BDP‐equivalent: 1000) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Appendix 1 |
| Allocation concealment (selection bias) | Low risk | See Appendix 1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo device used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Handling of missing data not described |
| Selective reporting (reporting bias) | Low risk | Primary outcome data available |
AQLQ = Asthma quality of life questionnaire
ATS = American Thoracic Society
AUC = Area under the curve
BDP = Beclomethasone dipropionate
BHR = Bronchial hyperresonsiveness
bid = Twice a day
BTS = British Thoracic Society
BUD = Budesonide
CFC‐MDI = Chlorofluorocarbon metered dose inhaler
CXR = Chest X‐ray
ECP = Eosinophil cationic protein
EKG (or ECG): Electrocardiogram
FeNO: Fixed exhalation nitric oxide
FEV1 = Forced expiratory volume in one second
FP = Fluticasone
FVC = Forced vital capacity
GINA = Global Initiative for Asthma
GSK = GlaxoSmithKline
HDM = House dust mite
HFA‐MDI = Hydrofluoroalkane metered dose inhaler
ICS = Inhaled corticosteroid
IgE = Immunoglobulin E
LABA = long‐acting inhaled ß2‐agonist
LTRA = Leukotriene receptor antagonist (anti‐leukotriene)
MDI = Metered dose inhaler
NA = not applicable
OCS = Oral corticosteroids
OD = Once daily
OTC = Over the counter
PAF = Platelet‐activating factor
PC20 = Provocative concentration of adenosine 5'‐monophosphate producing a 20% decline in FEV1
PEF = Peak expiratory flow
prn = As needed
qd = Once daily
RAST = Radioallergosorbent test
RTI = Respiratory tract infection
SABA = Short‐acting ß2‐agonist
SAL = Salmeterol
vs = Versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aalbers 2004 | No group with inhaled corticosteroids alone |
| Adinoff 1998 | No consistent use of inhaled corticosteroids in either the intervention or control groups ‐ co‐intervention with other non‐steroidal anti‐asthmatic drugs not stable during the intervention period |
| Akpinarli 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Ankerst 2003 | Cross‐over study of inadequate duration |
| Anonymous 1999 | Not relevant comparison |
| Anonymous 2003a | Control intervention not ICS alone |
| Anonymous 2003b | Duplicate citation |
| Anonymous 2003c | Duplicate publication of SMART study of salmeterol in asthma |
| Arvidsson 1991 | Control intervention not inhaled corticosteroids alone |
| ASSURE | Fixed versus adjustable maintenance dosing of combination LABA/ICS |
| Aubier 1999 | Patients were not steroid‐naive |
| Aziz 1998 | Intervention duration < 30 days |
| Aziz 1999a | Intervention duration < 30 days |
| Aziz 1999b | Outcome measure did not reflect asthma control |
| Aziz 2000 | Duration of intervention < 30 days |
| Bacci 2002 | No consistent co‐intervention with ICS |
| Baki 1998 | No consistent intervention with ICS |
| Balachandran 2001 | Review article |
| Balzano 2002 | Review article |
| Baraniuk 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Bateman 1998 | Combination versus concomitant delivery of LABA and ICS |
| Bateman 2000 | Comparison between different delivery devices |
| Bateman 2001 | Prior ICS exposure |
| Bateman 2003a | Patients on inhaled corticosteroids prior to study commencement |
| Bateman 2003b | Comparison between combination therapy and montelukast in addition to ICS |
| Baumgarten 2002 | Not randomised |
| Beeh 2002 | Not randomised |
| Behling 1999 | Inadequate study duration |
| Bennett 2002 | Review article |
| Bensch 2002 | No concurrent ICS therapy |
| Berggren 2001 | Intervention not regular but prn inhaled long‐acting beta2‐agonists |
| Bergmann 2004 | Control group received a higher dose of ICS than was given in the intervention group |
| Berlinski 2001 | Comparison of different spacers |
| Bernstein 2002 | Correspondence |
| Bessmertny 2002 | Intervention not LABAs |
| Bijl‐Hofland 2001 | No consistent co‐treatment with ICS |
| Bjermer 2000 | Control intervention not inhaled corticosteroids alone but montelukast |
| Bjermer 2003 | LABA not compared to ICS alone |
| Bloom 2003 | Comparison of LABA/ICS with higher dose of ICS |
| Boonsawat 2003 | Outcome measures not asthma control |
| Booth 1993 | No consistent co‐intervention with ICS |
| Boskovska 2001 | Not a RCT |
| Bouchard 2000 | Not steroid‐naive at baseline |
| Boulet 1998 | No concurrent ICS therapy |
| Boulet 2003 | Patients on inhaled corticosteroids prior to study commencement |
| Bouros 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Boyd 1995 | Not steroid‐naive at baseline |
| Brambilla 1994 | Control intervention not ICS but rather slow‐release oral beta2‐agonist |
| Brambilla 2003 | No regular LABA |
| Braunstein 2002 | Review article |
| Brenner 1998 | Intervention not regular inhaled long‐acting beta2‐agonists |
| Britton 1992 | Control intervention not inhaled corticosteroids alone |
| Britton 1998 | ICS/LABA combination compared with separate administration of the same drugs |
| Brogden 1991 | Review article |
| Buchvald 2002 | Control intervention was not maintenance inhaled corticosteroids alone (it was a leukotriene receptor antagonist) |
| Buchvald 2003 | Control intervention was not maintenance inhaled corticosteroids alone (it was a leukotriene receptor antagonist) |
| Buhl 2003a | Patients on inhaled corticosteroids prior to study commencement |
| Buhl 2003b | Not a RCT |
| Busse 1999 | Control intervention not inhaled corticosteroids alone |
| Busse 2003 | Patients on inhaled corticosteroids prior to study commencement |
| Byrnes 2000 | Control intervention not inhaled corticosteroids alone |
| Calhoun 2001 | Control intervention is not ICS (but rather anti‐leukotrienes) |
| Calverley 2002 | Study in COPD |
| Cazzola 2000 | Study in COPD |
| Chalmers 1999 | Study of methacholine induced asthma |
| Chan 2001 | Intervention not regular inhaled long‐acting beta2‐agonist |
| Chapman 1999 | Tx and intervention compared LABA and ICS but in combined vs concurrent devices |
| Cheer 2003 | Review article |
| Cloosterman 2001 | No consistent co‐intervention with ICS Control intervention is not ICS alone (but rather regular short‐acting beta2‐agonist) |
| Condemi 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Condemi 2001 | Control intervention not ICS alone (but rather another LABA) |
| Crompton 1999 | Control intervention not ICS alone but oral bambuterol |
| Currie 2003a | Duration of intervention < 30 days |
| Currie 2003b | Co‐intervention with non‐permitted treatment |
| Currie 2003c | Duration < 1 month |
| D'Alonzo 1994 | No consistent co‐intervention with ICS ‐ approximately 1/4 of participants were taking regular inhaled corticosteroids at baseline. Control intervention was a short‐acting beta2‐agonist. |
| D'Urzo 2001 | Patients on inhaled corticosteroids prior to study commencement |
| Dahl 1989 | Intervention not inhaled LABA |
| Dahl 1991 | No consistent co‐treatment with ICS |
| Dal Negro 2001a | Comparison of LABA with ICS/LABA |
| Dal Negro 2001b | Comparison of combination LABA and ICS with LABA and ICS administered via 2 separate inhalers |
| Davis 2001 | Not a RCT |
| Dekhuijzen 2002 | Review article |
| Del Rio‐Navarro 2001 | Outcome measures do not reflect asthma control (but rather serum potassium, CPK‐MB, and ECG) |
| Del‐Rio‐Navarro 2001 | Outcome measures do not reflect asthma control (but rather saliva flow and IgA) |
| Dempsey 2000 | Assessment of antileukotriene agent in asthma |
| Dente 2001 | Not a RCT |
| Dicpinigaitis 2002 | Intervention not regular inhaled long‐acting beta2‐agonist |
| Didier 1997 | Control intervention is not ICS: this is a randomised, open, parallel‐group, multicentre study comparing salmeterol with an oral bronchodilator, terbutaline |
| Djordjevic 1999 | Not randomised |
| Dorinsky 2001 | Wrong comparison |
| Dorinsky 2002 | Not steroid‐naive at baseline |
| Durham 1999 | Review article |
| Ek 2000 | Study in healthy volunteers |
| Eliraz 2001 | Both the treatment and control group compared ICS with LABA with different inhaler devices |
| Everden 2002 | Different LABAs compared (formoterol versus salmeterol) |
| Faurschou 1994 | Duration < 30 days |
| Faurschou 1996 | Control intervention not ICS alone (but regular SABA) |
| Fish 2001 | Control intervention is not ICS (but rather anti‐leukotrienes) |
| Fitzgerald 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Fitzpatrick 1990 | Duration of intervention < 30 days: the treatment period was only 2 weeks. No consistent intervention with ICS in all patients: 19/20 participants were taking regular ICS and 6 were taking oral steroids at baseline. Both treatment groups received different doses of long‐acting beta2‐agonists. |
| Fowler 2002 | Patients on inhaled corticosteroids prior to study commencement |
| Fuglsang 1995 | Duration < 30 days |
| Garcia‐Marcos 2002 | Review article |
| Gardiner 1994 | Patients on inhaled corticosteroids prior to study commencement |
| Gessner 2003 | Not randomised |
| Giannini 1998a | Duration < 30 days |
| Giannini 1998b | Duration < 30 days |
| Giannini 1999 | Duration < 30 days |
| Giannini 2000 | Duration < 30 days |
| Giannini 2001 | Duration < 30 days |
| Giannini 2002 | Duration < 30 days |
| Gizycki 2000 | Duration < 30 days |
| Gold 2001 | Control intervention not inhaled corticosteroids alone |
| Green 2003 | Not steroid‐naive at baseline |
| Greening 1994 | Patients on inhaled corticosteroids prior to study commencement |
| Grootendorst 2001 | Wrong comparison |
| Gustafsson 1994 | Tx and intervention compared ICS + LABA combination therapy using 2 different devices |
| Hacki 2001 | Review article |
| Hasani 2003 | No consistent intervention with inhaled corticosteroids in all subjects |
| Heuck 2000 | Patients on inhaled corticosteroids prior to study commencement |
| Heyneman 2002 | Systematic review |
| Hultquist 2000 | Study of LABA & ICS versus increased dose ICS |
| Ind 2002 | Formoterol versus SABA as relief medication |
| Ind 2003 | Patients on inhaled corticosteroids prior to study commencement |
| Isabelle 2001 | Comparison of 2 different devices to deliver ICS & LABA |
| Jarvis 1999 | Review article |
| Jeffery 2002 | Control intervention not inhaled corticosteroids alone |
| Jenkins 1995 | Control intervention is not ICS (but LABA delivered with new propellant HFA134a) |
| Jenkins 2000 | Patients on inhaled corticosteroids prior to study commencement |
| Jenkins 2002 | Comparison of combination ICS & LABA versus separate administration |
| Johansson 2001 | Patients on inhaled corticosteroids prior to study commencement |
| Johnson 1998 | Not steroid‐naive at baseline |
| Jones 1994 | No consistent intervention with ICS (< 1/3 of participants were taking regular ICS at entry) |
| Juniper 1995 | No consistent co‐intervention with ICS (80% were taking regular ICS at entry). No subgroup analyses available. |
| Juniper 1999 | Duplicate of Pauwels's study (NEJM 1997;337:1405‐11) |
| Kalberg 1998 | Patients on inhaled corticosteroids prior to study commencement |
| Kalra 1996 | Duration < 30 days |
| Kardos 2001 | Tx and intervention compared ICS + LABA in a fixed vs flexible schedule |
| Kavuru 2000 | ICS permitted |
| Keith 2001 | Not a RCT |
| Kelsen 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Kemp 1984 | Wrong comparison |
| Kemp 1998 | Not steroid‐naive at baseline |
| Ketchell 2002 | Duration of intervention < 30 days |
| Kidney 1995 | No consistent intervention with inhaled corticosteroids in all subjects |
| Kips 2000 | Patients on inhaled corticosteroids prior to study commencement |
| Kirby 2000 | Subjects not asthmatics |
| Knobil 1998 | Not steroid‐naive at baseline |
| Knobil 2000 | Assessment of LABA versus anti‐leukotriene |
| Knorr 2001 | Intervention is not LABA (but rather an anti‐leukotriene agent: montelukast) |
| Kraft 2003 | Not a RCT |
| LaForce 1994 | Not a RCT |
| Lai 1995 | Control intervention was not ICS alone but regular short‐acting beta2‐agonist instead of placebo Duration of intervention < 30 days: the treatment period was only 2 weeks long Co‐intervention with non‐permitted drugs: oral steroids |
| Lalloo 2003 | Patients on inhaled corticosteroids prior to study commencement |
| Lange 2001 | Inadequate duration |
| Langton‐Hewer 1995 | Patients on inhaled corticosteroids prior to study commencement |
| Lazarus 2001 | No consistent co‐intervention with ICS ‐ intervention is monotherapy with LABA |
| Leblanc 1996 | Patients on inhaled corticosteroids prior to study commencement |
| Lemanske 2001 | Complicated protocol. No data provided for comparison groups of interest. |
| Lenney 1995 | Not a RCT |
| LHSRG 2000 | Subjects not asthmatics (but rather have COPD) |
| Li 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Lindqvist 2001 | No consistent co‐treatment with ICS |
| Lipworth 1998 | Duration < 30 days |
| Lipworth 1999 | Duration < 30 days |
| Lipworth 2000a | Duration < 30 days |
| Lipworth 2000b | Duration < 30 days |
| Lockey 1999 | No consistent co‐intervention with inhaled corticosteroids |
| Lowhagen 2002 | Intervention not regular inhaled long‐acting beta2‐agonists |
| Lundbäck 2006 | Mixture of ICS and non‐ICS users |
| Lötvall 2002 | Comparison of different ICS/LABA combinations |
| Magadle 2001 | Duration < 30 days |
| Malmqvist‐Granlund 2000 | Not a RCT |
| Malolepszy 2002 | Control intervention not ICS (but oral theophylline) |
| Matz 2001 | Duplicate publication of 2 RCTS, namely that of Condemi JJ (Ann Allergy Asthma Immunol 1999;82:383‐9) and of Kalberg CJ (J Allergy Clin Immunol 1998;101 (Suppl):S6 |
| McCarthy 2000 | Control intervention not inhaled corticosteroids alone |
| McCarthy 2001 | Not randomised |
| Mcivor 1998 | No consistent co‐treatment with a stable dose of ICS (tapering) |
| Meier 1997 | Case control study |
| Meijer 1995 | Patients on inhaled corticosteroids prior to study commencement |
| Michel 2000 | Duration < 30 days |
| Midgren 1992 | Control intervention not ICS alone |
| Mitchell 2000 | Study of LABA+ICS versus double‐dose ICS |
| Molimard 2001 | Study recruited patients who were taking ICS |
| Murray 1998 | Inadequate duration |
| Murray 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Nagel 2002 | Duplicate |
| Nathan 1995 | No consistent co‐intervention with ICS in all patients: only 1/4 of participants were taking regular ICS at entry The usual dose of inhaled corticosteroids taken by participants was not stated in the manuscript The control intervention was not ICS but a short‐acting beta2‐agonist |
| Nathan 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Nathan 2006 | Participants recruited who were prior users of ICS |
| Nelson 1999 | Duration < 30 days |
| Nelson 2000 | Control intervention is not ICS alone (but rather ICS with an anti‐leukotriene agent (montelukast)) |
| Nelson 2001 | Control intervention not ICS alone (but LTRA‐ zafirlukast) |
| Newnham 1995 | No consistent co‐treatment with ICS |
| Nielsen 1999 | Participants pre‐treated with steroids |
| Nightingale 2002 | Comparison of different LABAs (formoterol versus salmeterol) |
| Norhaya 1999 | Participants pre‐treated with ICS |
| Nsouli 2001 | Control intervention not inhaled corticosteroids alone |
| O'Brian 2001 | Duration of intervention < 30 days |
| O'Byrne 2005 | Comparison between combination ICS/LABA and higher dose ICS |
| O'Connor 2002 | Retrospective design |
| Odeback 1998 | Participants were pre‐treated with ICS |
| Ortega‐Cisneros 1998 | Patients on inhaled corticosteroids prior to study commencement |
| Palmer 1992 | Control intervention is not ICS alone: both treatment groups received long‐acting beta2‐agonists but in different doses |
| Palmqvist 2001 | Both the treatment and control groups compared ICS and LABA with different drugs and inhaler devices |
| Paterson 1999 | Comparison of anti‐leukotriene agent with LABA |
| Pauwels 1997 | Patients on inhaled corticosteroids prior to study commencement |
| Pauwels 1998a | DUPLICATE REPORT ‐ this study is a review of the FACET study which is already included in this analysis (Pauwels 1997) |
| Pauwels 1998b | Intervention not LABA but another ICS |
| Pearlman 1992 | No consistent co‐intervention with ICS (< 1/2 the participants were taking regular inhaled corticosteroids at entry) Control intervention was not ICS but short‐acting beta2‐agonist |
| Pearlman 1994 | No consistent co‐treatment with ICS 26% |
| Pearlman 2002 | Control intervention is not ICS alone (but anti‐leukotriene ‐ montelukast ‐ as maintenance) |
| Pearlman 2004 | Mixed population of ICS and non‐ICS users |
| Perez 2000 | Wrong comparison |
| Peters 2000 | CONTROL intervention is not ICS alone (but oral steroids, SABA and anticholinergics ‐ in hospital setting) |
| Pieters 1998 | Participants pre‐treated with ICS |
| Pinnas 1998 | No consistent intervention with inhaled corticosteroids in all subjects |
| Pizzichini 1996 | Duration < 4 weeks |
| Pljaskic‐Kamenov 2000 | Pre‐treatment with steroids |
| Price 2002 | Patients on inhaled corticosteroids prior to study commencement |
| Pujet 1995 | Intervention is not LABA (but theophylline) |
| Rance 2002 | Comparison of combined and concomitant inhaled ICS and LABA |
| Rickard 2001 | Control intervention not inhaled corticosteroids alone |
| Rijssenbeek‐Nouwens 2002 | Intervention is not LABA (but anti‐allergic casing) |
| Ringbaek 1996 | Control intervention not ICS alone but oral SABA as maintenance |
| Ringdal 2002 | Treatment and intervention groups compared the same medications either in combination or with different delivery devices |
| Ringdal 2003 | Control intervention no inhaled corticosteroids alone |
| Rocca‐Serra 2002 | Intervention not regular long‐acting beta2‐agonist |
| Rosenhall 2002 | Treatment and intervention groups compared the same medications either in combination or with different delivery devices |
| Rosenhall 2003 | Treatment and intervention groups compared the same medications either in combination or with different delivery devices |
| Rosenthal 1999 | No consistent co‐intervention with ICS |
| Russell 1995 | Participants pre‐treated with ICS |
| Saari 2002 | Inadequate duration |
| SAM40004 | ICS treatment permitted prior to study entry |
| SAM40104 | Prior treatment with ICS |
| SAS10006 | Cross‐over study |
| SAS30013 | Prior treatment with ICS |
| Schreurs 1996 | No consistent co‐intervention with ICS ‐ 90% used regular ICS at entry |
| Scicchitano 2004 | Combination given as maintenance as well as relief inhaler |
| Sears 2003 | Fixed versus adjustable dosing regimen |
| Serrier 2003 | Treatment and intervention groups compared the same medications either in combination or with different delivery devices |
| Shapiro 2000 | Participants pre‐treated with ICS |
| Shapiro 2001 | Intervention is not LABA |
| Sheth 2002 | Control intervention not inhaled corticosteroids alone |
| Sienra‐Monge 2001 | Comparison of LABA & ICS delivered as combination or concomitant therapy |
| Simons 1997a | Patients on inhaled corticosteroids prior to study commencement |
| Simons 1997b | No consistent co‐intervention with inhaled corticosteroids. Treatment groups compared ICS to long‐acting beta2‐agonist alone. |
| SNS | Comparison of salmeterol with salbutamol |
| Sovani 2008 | Assessment of combination therapy with usual care |
| Staehr 1995 | Control intervention not ICS (but SABA maintenance) |
| Stanford 2002 | Assessment of combination therapy with an anti‐leukotriene agent |
| Stelmach 2001 | The treatment and intervention groups compared the same medications either in combination or with different delivery devices |
| Stelmach 2002a | No co‐intervention with ICS |
| Stelmach 2002b | No co‐intervention with ICS |
| Stelmach 2007 | Prior ICS exposure |
| Stojkovic‐Andjelkovi 2001 | No comparison with ICS alone |
| Tal 2003 | Participants pre‐treated with ICS |
| Tan 1997 | Outcomes measures did not reflect asthma control |
| Tattersfield 2001 | Intervention is not daily LABA (but rather on‐demand LABA) |
| Trautmann 2001 | Study did not assess equivalent ICS dose in control arm; participants pre‐treated with ICS |
| Turner 1998 | No consistent co‐intervention with ICS |
| Ullman 1990 | Duration < 30 days |
| Van den Berg 2000 | No consistent co‐intervention with LABA ‐ both groups received LABA but compared delivery devices |
| van der Molen 1997 | Patients on inhaled corticosteroids prior to study commencement |
| van der Woude 2001 | Inadequate duration |
| van Noord 1999 | Patients on inhaled corticosteroids prior to study commencement |
| van Noord 2001 | Different propellants used to deliver FP & FP/SAL in the treatment groups |
| van Schayck 2002 | No concurrent ICS treatment |
| Verberne 1997 | No consistent co‐intervention with ICS ‐ approximately 20% were taking regular ICS at entry |
| Verberne 1998 | Patients on inhaled corticosteroids prior to study commencement |
| Vermetten 1999 | Patients on inhaled corticosteroids prior to study commencement |
| Vestbo 2000 | Patients are not asthmatics (but rather have COPD) |
| Vickers 2000 | The intervention is not LABA but placebo No consistent co‐intervention with ICS Ongoing study ‐ protocol only published |
| Vilsvik 2001 | Outcome measures did not reflect asthma control |
| Von Berg 1989 | Duration < 30 days |
| Wallaert 1999 | Control intervention not ICS alone (but another LABA) |
| Wallin 1990 | Control intervention not ICS alone (but regular SABA) |
| Wallin 1998 | No consistent co‐treatment with ICS |
| Wallin 2003 | Patients on inhaled corticosteroids prior to study commencement |
| Weinstein 1998 | No consistent co‐intervention with ICS ‐ only 57% were on ICS |
| Wempe 1992 | No consistent co‐treatment with ICS |
| Wilcke 1998 | Duration < 30 days |
| Wilding 1997 | Cross‐over study design |
| Wilson 2001 | Control intervention is not ICS alone (but rather ICS with an anti‐leukotriene agent ‐ montelukast) |
| Wong 1992 | Duration < 30 days |
| Woolcock 1996 | Patients on inhaled corticosteroids prior to study commencement |
| Yates 1995 | Duration < 30 days. No co‐treatment with ICS |
| Yates 1996 | Duration < 30 days |
| Youngchaiyud 1995 | Intervention not LABA (but theophylline) |
| Yurdakul 2002 | Control intervention not regular inhaled long‐acting beta2‐agonists alone |
| Zarkovic 1998 | No consistent co‐intervention with ICS Control intervention is placebo |
| Zetterstrom 2003 | Participants pre‐treated with ICS |
| Zimmerman 2004 | Patients were not steroid‐naive |
COPD = chronic obstructive pulmonary disease ECG = electrocardiogram ICS = inhaled corticosteroid LABA = long‐acting inhaled ß2‐agonist RCT = randomised controlled trial SABA = short‐acting ß2‐agonist Tx = treatment vs = versus
Differences between protocol and review
This review has now been amended to include to two treatment comparisons:
the addition of LABA to ICS versus the same dose of ICS;
the addition of LABA to ICS versus a higher dose of ICS.
We have continued to require that the participants be steroid‐naive prior to study entry.
We have incorporated a new method to assess the risk of bias, and based sensitivity analyses on sources of bias relating to blinding and completeness of follow up. Jadad scores have still been calculated for each study, but these findings are not the primary source of assessing the credibility of the results for each study.
Contributions of authors
Muireann Ni Chroinin, under the supervision of Francine Ducharme, identified and reviewed the full‐text publication of all citations of potential or potentially eligible RCTs identified in the 2004 literature search, extracted the methodology and data, analysed and interpreted results of the meta‐analysis and wrote the review. Ilana Greenstone participated in the selection of trials from the literature search. Toby Lasserson assessed studies for eligibility from the 2008 literature and company website search update, extracted and entered data, and wrote up the results.
Francine Ducharme supervised Muireann Ni Chroinin, Ilana Greenstone and Toby Lasserson. She conceived the protocol, supervised the literature search, participated in the selection of trials, methodology assessment and data extraction, corresponded with authors and/or the pharmaceutical companies to identify other possibly relevant trials, verify methodology and data extraction and request additional information, analysed and interpreted results of the meta‐analysis and supervised the writing up of the review.
Sources of support
Internal sources
Canadian Cochrane Network ‐ McGill University, Canada.
External sources
Francine Ducharme was supported by a senior clinical scientist award from the Fonds de la Santé du Québec, Canada.
Declarations of interest
Francine M. Ducharme has received travel support for meeting attendance, research funds, fees for speaking and/or consulting fees from AstraZeneca (producer of formoterol and budesonide), GlaxoSmithKline (producer of fluticasone, beclomethasone, salmeterol) and Novartis (producer of formoterol). Muireann Ni Chroinin has received some research funds and fees for speaking from AstraZeneca and has attended CME conferences with support from GlaxoSmithKline. Toby Lasserson and Ilana Greenstone report no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Boonsawat 2008 {published and unpublished data}
- Boonsawat W, Goryachkina L, Jacques L, Frith L. Combined salmeterol/fluticasone propionate versus fluticasone propionate alone in mild asthma. Clinical Drug Investigation 2008;28(2):101‐11. [DOI] [PubMed] [Google Scholar]
- Boonsawat W, Goryachkina L, Milns H, Balsara S. The efficacy and safety of Seretide/Advair once daily (50/100mcg) compared fluticasone propionate (100mcg) once daily and placebo as initial maintenance therapy in mild asthma. American Journal of Respiratory & Critical Care Medicine 2004;169(7):A86. [Google Scholar]
- Goryachkina L, Boonsawat W, Milns H, Balsara S. Seretide/Advair once daily (50/100mcg) is effective in patients with mild asthma. American Journal of Respiratory & Critical Care Medicine 2004;169(7):A86. [Google Scholar]
- SAS30023. A 12‐week multicentre, randomised, double‐blind, placebo‐controlled parallel group study to compare the efficacy and tolerability of fluticasone propionate/salmeterol combination (SERETIDE/VIANI/ADVAIR) 88/42mcg once daily in the morning with fluticasone propionate 88mcg once daily in the morning and placebo (short‐acting ß2‐agonist as required only) once daily in the morning, all via the HFA MDI as initial maintenance therapy in mild asthmatic subjects. http:www.ctr.gsk.co.uk 2004.
Chuchalin 2002 {published data only}
- Chuchalin AG, Ovcharenko SI, Goriachkina LA, Sidorenko IV, Tsoi AN. Formoterol (Oxis®) Turbuhaler® plus budesonide turbuhaler® is more effective than current non‐steroid therapy and budesonide alone in mild to moderate asthma in Russia. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
- Chuchalin AG, Ovcharenko SI, Goriachkina LA, Sidorenko IV, Tsoi AN. The safety and efficacy of formoterol (Oxis) turbuhaler plus budesonide (Pulmicort) turbuhaler in mild to moderate asthma: a comparison with budesonide turbuhaler alone and current non‐corticosteroid therapy in Russia. International Journal of Clinical Practice 2002;56(1):15‐20. [PubMed] [Google Scholar]
- Chuchalin AG, Stahl E, Svensson K, Ovcharenko SI, Goriachkina LA, Sidorenko IV, et al. Formoterol (oxis®) turbuhaler® plus budesonide turbuhaler® and budesonide alone improve health‐related quality of life vs non‐steroid therapy in mild to moderate asthma in Russia. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
- Chuchalin AG, Svensson K, Stahl E, Ovcharenko SI, Goriachkina LA, Sidorenko IV, et al. A health‐related quality‐of‐life comparison of formoterol (Oxis(R)) Turbuhaler(R) plus budesonide (Pulmicort(R)) Turbuhaler(R) with budesonide Turbuhaler(R) alone and noncorticosteroid treatment in asthma: a randomized clinical study in Russia. Respiration 2002;69(5):427‐33. [DOI] [PubMed] [Google Scholar]
Chuchalin 2008 {published and unpublished data}
- Chuchalin A, Jacques L, Frith L. Salmeterol/fluticasone propionate via Diskus® once daily versus fluticasone propionate twice daily in patients with mild asthma not previously receiving maintenance corticosteroids EMBASE 2008079067. Clinical Drug Investigation 2008;28(3):169‐81. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline (SAS30024). A 52‐week multicentre, randomised, double‐blind, double dummy, placebo‐controlled parallel group study to compare the efficacy and tolerability of salmeterol/fluticasone propionate combination (SERETIDE/VIANI/ADVAIR) 50/100mcg once daily in the morning with fluticasone propionate (FLIXOTIDE/FLOVENT) 100mcg twice daily and placebo twice daily, all via the DISKUS/ACCUHALER as initial maintenance therapy in mild asthmatic subject. http://ctr.gsk.co.uk 2005.
Creticos 1999 {published data only}
- Creticos PS, Freidhoff LR, Bernstein DI, Chu T, Khattignavong AP, Pasatiempo AM. Comparison of an inhaled corticosteroid (triamcinolone acetonide) to a long‐acting bronchodilator (salmeterol), the combination, and placebo in mild‐moderate adult asthmatic patients. International Archives of Allergy and Immunology 1999;118:345‐6. [DOI] [PubMed] [Google Scholar]
Di Franco 1999 {published and unpublished data}
- Franco A, Giannini D, Bacci E, Dente FL, Vagaggini B, Paggiaro PL. Comparison of different long‐term asthma treatments in subjects with mild‐to‐moderate asthma. Monaldi Archives of Chest Disease 1999;54(5):390‐3. [PubMed] [Google Scholar]
GOAL {published and unpublished data}
- Anonymous. GSK asthma trial suggests total control is possible. Pharmaceutical Journal 2004;273(7322):594. [Google Scholar]
- Arthurs R. Gaining optimal asthma control. Practice Nurse 2004;Supplement:3‐8. [Google Scholar]
- Bateman E, Boushey H, Bousquet J, Busse W, Clark T, Pauwels R, et al. Achievement for guideline based asthma control with salmeterol/fluticasone propionate compared with fluticasone propionate alone; results of GOAL study. Triennial World Asthma Meeting, Thailand (16‐19 February). 2004.
- Bateman E, Boushey H, Bousquet J, Busse W, Clark T, Pauwels R, et al. Achieving and maintaining guideline defined asthma control with salmeterol/fluticasone propionate versus fluticasone propionate alone: the results of the GOAL study. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A87. [DOI] [PubMed] [Google Scholar]
- Bateman E, Pauwels R, Boushey H, Bousquet J, Busse W, Clark T, et al. Aiming for total control of asthma significantly improves asthma‐related quality of life: salmeterol/fluticasone propionate versus fluticasone propionate alone. Amercian Journal of Respiratory and Critical Care Medicine 2004;169(7):A87. [Google Scholar]
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline‐defined asthma control be achieved? The Gaining Optimal Asthma ControL study. American Journal of Respiratory & Critical Care Medicine 2004;170(8):836‐44. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline‐defined asthma control be achieved? The Gaining Optimal Asthma ControL study. American Respiratory and Critical Care Medicine 2004:Online data supplement. [DOI] [PubMed]
- Bateman ED, Bousquet J, Keech ML, Busse WW, Clark TJ, Pedersen SE. The correlation between asthma control and health status: the GOAL study. European Respiratory Journal 2007;29(1):56‐62. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Edin HM, Sondhi S, Gul N. Asthma‐related quality of life in the GOAL study: baseline results. European Respiratory Journal 2002;20 Suppl 38:46s. [Google Scholar]
- Bons J, Cordier JF, Godard P, Prud’Homme A, Celli I, Bousquet J. Aiming for total control of asthma: the GOAL study design. Revue Française d’Allergologie et d’Immunologie Clinique 2004;44(367):TT13. [Google Scholar]
- Boushey H, Bateman E, Bousquet J, Busse W, Clark T, Pauwels R, et al. Achieving total control of asthma with salmeterol/fluticasone propionate versus fluticasone propionate alone: GOAL study results. Triennial World Asthma Meeting, Thailand (16‐19 February). 2004.
- Boushey H, Bateman E, Bousquet J, Busse W, Clark T, Pauwels R, et al. Improvements in asthma outcomes following 1 year of treatment with salmeterol/fluticasone or fluticasone alone when stepped up to achieve guideline‐defined total control. Journal of Allergy and Clinical Immunology 2004;113(2 suppl 1):114s‐5s. [Google Scholar]
- Boushey HA, Pedersen S, Bateman E, Clark T, Busse W, Bousquet J, et al. Improved exacerbation rates and asthma control in current and former smokers treated with salmeterol/fluticasone propionate: results of the GOAL study. Journal of Allergy & Clinical Immunology 2005;115(Suppl 2):S59. [Google Scholar]
- Bousquet J. Is asthma control achievable?. European Respiratory Review 2004;13(92):102‐4. [Google Scholar]
- Bousquet J, Bateman E, Boushey H, Busse W, Clark T, Pauwels R, et al. The effect of oral corticosteroids and high‐dose combination therapy on achieving control of refractory asthma. Journal Allergy and Clinical Immunology 2004;113(2 suppl 1):113s. [Google Scholar]
- Bousquet J, Bons J, Godard P, Cordier JF, Desfougeres JL, Prud’Homme A. Aiming for total control of asthma: the GOAL study results. Revue Française d’Allergologie et d’Immunologie Clinique 2004;44(367):TT16. [Google Scholar]
- Busse W, Bateman E, Boushey H, Bousquet J, Clark T, Pauwels R, et al. Achieving GINA/NIH guideline‐based asthma control with salmeterol/fluticasone compared with fluticasone alone: the results of the GOAL study. Journal of Allergy and Clinical Immunology 2004;113(2 suppl 1):114s. [Google Scholar]
- Busse W, Bateman E, Boushey H, Bousquet J, Clark T, Pauwels R, et al. Aiming to achieve total control of asthma with salmeterol/fluticasone propionate and fluticasone propionate alone is well tolerated: GOAL 1 year safety data. Triennial World Asthma Meeting, Thailand (16‐19 February). 2004.
- Clark T, Bateman E, Boushey H, Bousquet J, Busse W, Pauwels R, et al. Salmeterol/fluticasone and fluticasone alone are well tolerated over 1 year of treatment stepped‐up to achieve total control: safety results of the GOAL study. Journal of Allergy and Clinical Immunology 2004;113(2 suppl 1):115s. [Google Scholar]
- Clark T, Bateman E, Boushey H, Bousquet J, Busse W, Pauwels R, et al. Time course of achievement of individual clinical goals of asthma treatment: the results of the GOAL study. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A318. [Google Scholar]
- Clark TJ, Bousquet J, Bateman ED, James MH. GOAL (gaining optimal asthma control): a study to assess asthma control. European Respiratory Journal 2001;18 Suppl 33:175‐6s. [Google Scholar]
- Clark TJH, Bateman ED on behalf of the GOAL Steering Committee. Aiming for total control of asthma in ICS‐free patients improves traditional outcomes: results of the Gaining Optimal Asthma controL (GOAL) study [abstract]. 23rd European Academy of Allergology and Clinical Immunology Meeting, June 12‐16. Amsterdam, The Netherlands. 2004; Vol. 204:669.
- Clark TJH, Bateman ED, James MH, on behalf of the GOAL Steering Committee. Assessing asthma control using a composite measure based on GINA/NIH guidelines: an analysis of GOAL baseline data. European Respiratory Journal 2002;20 Suppl 38:47s. [Google Scholar]
- Cordier JF, Bousquet J, Boucot I, Prud’Homme A, Godard P. Which dose for achieving total control of asthma? The results of the GOAL study. Revue Française d’Allergologie et d’Immunologie Clinique 2004;44(367):TT14. [Google Scholar]
- Godard P, Prud’Homme A, Cordier JF, Sohier B, Bousquet J. Time course of achievement of asthma control: The results of the GOAL study. Revue Française d’Allergologie et d’Immunologie Clinique 2004;44(367):TT15. [Google Scholar]
- Juniper EF, Bateman ED, Sondhi S, Gul N. Asthma Control Questionnaire (ACQ) differentiates between levels of clinical control in a large scale trial. Amercian Journal of Respiratory and Critical Care Medicine 2003;167(7):A37. [Google Scholar]
- Pauwels R, Bateman E, Boushey H, Bousquet J, Busse W, Clark T, et al. Addition of oral corticosteroids to combination therapy has little impact on achieving total control of asthma. 4th Triennial World Asthma Meeting Abstract Book, Bangkok, Thailand, February 16‐19. 2004:135.
- Pauwels R, Bateman E, Boushey H, Bousquet J, Busse W, Clark T, et al. Aiming for total control of asthma reduces the risk of exacerbations: a comparison of salmeterol/fluticasone propionate versus fluticasone propionate alone. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A87. [Google Scholar]
- Pauwels R, Bateman E, Boushey H, Bousquet J, Busse W, Clark T, et al. Can total control of asthma be achieved?: the results of the GOAL study. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl 1):114s. [Google Scholar]
- Pedersen SE. Is guideline‐defined asthma control achievable? The Gaining Optimal Asthma ControL (GOAL) Study [Er guideline‐defineret astmakontrol opnaelig?]. Ugeskrift for Laeger 2005;167(38):3595‐7. [PubMed] [Google Scholar]
- Pedersen SE, Bateman ED on behalf of the GOAL Steering Committee. Aiming for Total Control of asthma in patients taking inhaled corticosteroids improves traditional outcomes: Results of the Gaining Optimal Asthma controL (GOAL) study. [abstract]. 3rd European Academy of Allergology and Clinical Immunology Meeting, June 12‐16. Amsterdam, The Netherlands. 2004; Vol. 204:670.
- Pederson S, Bateman E, Boushey H, Bousquet J, Busse W, Clark T, et al. Aiming for guideline defined total control of asthma improves one‐year asthma outcomes: results of GOAL study. Triennial World Asthma Meeting, Thailand (16‐19 February). 2004.
- SAM40027. Gaining Optimal Asthma ControL (GOAL): a multi‐centre, stratified, randomised, double‐blind, parallel‐group, step‐up comparison of the level of asthma control achieved with salmeterol/fluticasone propionate combination DISKUS (ACCUHALER) dry powder inhaler compared with fluticasone propionate DISKUS (ACCUHALER) alone in adults and adolescents. http://www.ctr.gsk.co.uk 2004.
Grutters 1999 {published data only}
- Grutters J, Brinkman L, Koenderman L, Bosch J, Lammers JW. The effect of treatment of allergic asthmatics with salmeterol (SLM), beclomethasone (BDP) or the combination on lung function and bronchial hyperresponsiveness (BHR) after allergen challenge. European Respiratory Journal 1997;10(Suppl 25):474s. [Google Scholar]
- Grutters JC, Brinkman L, Aslander MM, Bosch JMM, Koenderman L, Lammers JWJ. Asthma modulates priming ‐associated blood eosinophil responsiveness in allergic asthmatics. European Respiratory Journal 1999;14:915‐22. [DOI] [PubMed] [Google Scholar]
Karaman 2007 {published data only (unpublished sought but not used)}
- Karaman O, Arli O, Uzuner N, Islekel H, Babayigit A, Olmez D, et al. The effectiveness of asthma therapy alternatives and evaluating the effects of asthma therapy by interleukin‐13 and interferon gamma levels in children. Allergy & Asthma Proceedings 2007;28(2):204‐9. [DOI] [PubMed] [Google Scholar]
Kerwin 2008 {published and unpublished data}
- Dorinsky P, Kerwin E, Schoaf L, Ellsworth A, House K. Effectiveness and safety of fluticasone propionate/salmeterol 250/50mcg administered once daily to patients with persistent asthma [Abstract]. European Respiratory Journal 2004;24 Suppl 48:309s. [Google Scholar]
- Dorinsky P, Schoaf L, House K, Ellsworth A. The efficacy and safety of FP/salmeterol 250/50mcg once daily compared with FP/salmeterol 100/50mcg twice daily. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A149. [Google Scholar]
- Kerwin EM, Nathan RA, Meltzer EO, Ortega HG, Yancey SW, Schoaf L, et al. Efficacy and safety of fluticasone propionate/salmeterol 250/50 mcg Diskus administered once daily. Respiratory Medicine 2008;102(4):495‐504. [DOI] [PubMed] [Google Scholar]
- SAS30022. A randomized, double‐blind, placebo‐controlled, parallel‐group, 12‐week trial evaluating the efficacy and safety of the fluticasone propionate/salmeterol DISKUS combination product 250/50mcg once daily versus fluticasone propionate/salmeterol DISKUS combination product 100/50mcg twice daily versus fluticasone propionate DISKUS 250mcg once daily versus placebo in symptomatic adolescent and adult subjects with asthma that is not controlled on short acting beta2‐agonists alone. http://ctr.gsk.co.uk 2005.
Miraglia del Giudice 2007 {published data only}
- Miraglia del Giudice M, Piacentini GL, Capasso M, Capristo C, Maiello N, et al. Formoterol, montelukast, and budesonide in asthmatic children: effect on lung function and exhaled nitric oxide. Respiratory Medicine 2007;101(8):1809‐13. [DOI] [PubMed] [Google Scholar]
Murray 2004 {published and unpublished data}
- Edin HM, Lang ML, Vandermeer AK, House KW, Shah TP. Fluticasone propionate/salmeterol diskus combination product improves asthma‐related quality of life compared with individual components in asthma patients symptomatic on ß2‐agonists alone. Journal of Allergy and Clinical Immunology 2002;109(1):241s. [Google Scholar]
- Lange ML, House KW, Scott A, Shah TP, Akveld MLM. The salmeterol/fluticasone propionate combination 50/100µg bid is effective as initial maintenance therapy in mild and moderate asthmatics. European Respiratory Journal 2001;18 Suppl 33:263s. [Google Scholar]
- Murray J, Rosenthal R, Somerville L, Blake K, House K, Baitinger L, et al. Fluticasone propionate and salmeterol administered via diskus compared with salmeterol or fluticasone propionate alone in patients suboptimally controlled with short‐acting beta2‐agonists. Annals of Allergy, Asthma, & Immunology 2004;93(4):351‐9. [DOI] [PubMed] [Google Scholar]
- Rosenthal RR, Blake K, Strek M, Lange M, House KW, Vandermeer AK. Fluticasone propionate/salmeterol diskus combination product provides superior asthma control compared with fluticasone propionate and salmeterol alone in patients previously receiving PRN short‐acting beta‐agonists alone. Journal of Allergy and Clinical Immunology 2002;109(1):245s. [Google Scholar]
- SAS30017. A randomised, double‐blind, active‐controlled, parallel‐group, 12‐week trial evaluating the safety and efficacy of the salmeterol 50mcg/fluticasone propionate 100mcg diskus combination product bid compared with salmeterol 50mcg via diskus bid and fluticasone propionate 100mcg via diskus bid in symptomatic adult and adolescent subjects with asthma on short‐acting beta2‐agonist therapy. http://www.ctr.gsk.co.uk 2004.
- Schoaf L, Emmett A, House K, Matthews T, Dorinsky P. Treatment response to fluticasone/salmeterol combination in three ethnic groups. American Journal of Respiratory and Critical Care Medicine 2002;165(8):A568. [Google Scholar]
Nelson 2003 {published and unpublished data}
- Nelson HS, Chervinsky P, Greos L, Pleskow W, Baitinger L, Scott C, et al. The salmeterol/fluticasone propionate combination product improves asthma control compared with the individual products in asthmatics treated with PRN short‐acting beta2‐agonists alone. American Journal of Respiratory and Critical Care Medicine 2000;161(3 part 2 suppl 1):A196. [Google Scholar]
- Nelson HS, Wolfe JD, Gross D, Geos LS, Baitinger L, Scott C, et al. Efficacy and safety of fluticasone propionate 44mcg/salmeterol 21mcg administered in a hydrofluoroalkane metered‐dose inhaler as an initial asthma maintenance treatment. Annals of Allergy and Clinical Immunology 2003;91(3):263‐9. [DOI] [PubMed] [Google Scholar]
- Pyke SD, Frith L, Pritchard J, Johnson M, Theophilus A, Shah T. Synergy with salmeterol and fluticasone propionate after administration from a single inhaler (Seretide™). European Respiratory Journal 2001;18 Suppl 33:176s. [Google Scholar]
- SAS30001. A randomised, double‐blind, active‐controlled, parallel‐group, 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in HFA 134a MDI, 42/88mcg BID, and salmeterol in propellant 11/12 MDI, 42mcg BID, and fluticasone propionate in propellant 11/12 MDI, 88mcg BID, in adolescent and adult subjects with asthma. http://www.ctr.gsk.co.uk 2004.
O'Byrne 2001 {published data only}
- Barnes PJ, O'Byrne PM, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Oxis and Pulmicort turbuhaler in the management of asthma OPTIMA international study group. Treatment of mild persistent asthma with low doses of inhaled Budesonide alone or in combination with Formoterol. Thorax 2000;55(Suppl 3):A4. [Google Scholar]
- Barnes PJ, O'Byrne PM, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Treatment of mild persistent asthma with low doses of inhaled budesonide alone or in combination with formoterol. For the Oxis and Pulmicort Turbuhaler in the Management of Asthma (OPTIMA) international study group. Thorax 2000;55(Suppl 3):s5. [Google Scholar]
- Grosser D, Smith B. Low‐dose budesonide improved asthma control in mild asthma; adding formoterol improved control in corticosteroid‐treated patients. ACP Journal Club 2002;137(1):19. [PubMed] [Google Scholar]
- Jonsson B, Berggren F, Svensson K, O'Byrne PM. An economic evaluation of combination treatment with budesonide and formoterol in patients with mild‐to‐moderate persistent asthma. Respiratory Medicine 2004;98(11):1146‐54. [DOI] [PubMed] [Google Scholar]
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Budesonide and formoterol in mild persistent asthma compared with doubling the dose of budesonide ‐ a cost‐effectiveness analysis. European Respiratory Journal. 2001;18 Suppl 33:517s. [Google Scholar]
- O'Byrne PM, Barnes PJ, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: The OPTIMA randomized trial. American Journal of Repiratory and Critical Care Medicine 2001;164(8):1392‐97. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Barnes PJ, Rodriguez‐Roisin R, Sandtröm T, Tattersfield AE, Runnerström EM, et al. Addition of formoterol Turbuhaler® to budesonide Tubuhaler® is safe and well tolerated in the long‐term treatment of mild asthma: results from the OPTIMA trial.. European Respiratory Journal 2001;18 Suppl 33:330s. [Google Scholar]
Overbeek 2005 {published data only}
- Overbeek SE, Mulder PG, Baelemans SM, Hoogsteden HC, Prins JB. Formoterol added to low‐dose budesonide has no additional antiinflammatory effect in asthmatic patients. Chest 2005;128(3):1121‐7. [DOI] [PubMed] [Google Scholar]
- Overbeek SE, Mulder PGM, Baelemans SMI, Hoogsteden HC, Prines JB. Comparison of budesonide plus formoterol with budesonide alone on airway inflammation in mild asthmatics. American Thoracic Society 99th International Conference. 2003:D034 [Poster C36].
Pearlman 1999a {published and unpublished data}
- Pearlman DS, Stricker W, Weistein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: a study comparing monotherapy and combination therapy in asthma. Annals of Allergy, Asthma & Immunology 1999;82:257‐65. [DOI] [PubMed] [Google Scholar]
Pearlman 1999b {published and unpublished data}
- Pearlman DS, Stricker W, Weistein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: a study comparing monotherapy and combination therapy in asthma. Annals of Allergy, Asthma & Immunology 1999;82:257‐65. [DOI] [PubMed] [Google Scholar]
Prieto 2005 {published and unpublished data}
- Prieto L, Gutierrez V, Perez‐Frances C, Badiola C, Lanuza A, Bruno L, et al. Effect of fluticasone propionate‐salmeterol therapy on seasonal changes in airway responsiveness and exhaled nitric oxide levels in patients with pollen‐induced asthma. Annals of Allergy, Asthma, & Immunology 2005;95(5):452‐61. [DOI] [PubMed] [Google Scholar]
- SAM40092. Effect of salmeterol/fluticasone propionate combination product on seasonal changes in airway responsiveness and exhaled nitric oxide in subjects with pollen‐induced asthma. http://www.ctr.gsk.co.uk 2005. [DOI] [PubMed]
Rojas 2007 {published and unpublished data}
- Barnes N, Rojas R, Palga I, Goldfrad C, Duggan M. Efficacy and safety of fluticasone propionate/salmeterol (250/50ug bd) in a single diskus device compared with fluticasone propionate diskus alone (250ug) as initial maintenance therapy in moderate asthma. American Thoracic Society International Conference; May 20‐25; San Diego, California. 2005:Poster G13.
- Rojas RA, Paluga I, Goldfrad CH, Duggan MT. Fluticasone propionate/salmeterol 250/50ug BD is significantly superior to fluticasone propionate 250ug BD as initial maintenance therapy in moderate asthma. American Thoracic Society International Conference; May 20‐25; San Diego, California. 2005:Poster G14.
- Rojas RA, Paluga I, Goldfrad CH, Duggan MT, Barnes N. Initiation of maintenance therapy with salmeterol/fluticasone propionate combination therapy in moderate asthma: a comparison with fluticasone propionate. Journal of Asthma 2007;44(6):437‐41. [DOI] [PubMed] [Google Scholar]
- SAS30039. A 12‐week, multi‐centre, randomised, double‐blind, parallel‐group study to compare the efficacy and tolerability of salmeterol/fluticasone propionate combination (SERETIDE™/VIANI™/ADVAIR™) 50/250µg twice‐daily with fluticasone propionate 250µg twice‐daily, all via the DISKUS®/ACCUHALER® as initial maintenance therapy in moderate persistent asthma. http://www.ctr.gsk.co.uk 2005.
SAM40034 {published data only}
- GlaxoSmithKline (SAM40034). A double‐blind, randomised, parallel group, 12‐week comparison of fluticasone propionate/salmeterol combination Diskus 100/50mcg BID with fluticasone propionate (FP) 250mcg BID as initial maintenance treatment in persistent asthma (Seretide Nordic Jump‐Up Study). http://www.ctr.gsk.co.uk 2004.
- Kotaniemi J, Tiling B, Oien T. A double‐blind randomised, parallel group study over 12 weeks comparing seretide (50/100mcg bd Diskus) versus flixotide ( 250 mcg bd Diskus) as first line regular treatment for steroid‐naive adult patients. American Journal of Respiratory and Critical Care Medicine. 2003; Vol. 167, issue 7:A893.
- Oien T, Tilling B, Kontaniemi J. 12 weeks comparing salmeterol/fluticasone propionate (SFC, 50/100mcg bd diskus) versus fluticasone propionate (FP, 250mcg bd diskus) as first‐line regular asthma treatment. European Respiratory Journal 2003;22(Suppl 22):236s. [Google Scholar]
SAM40036 {published data only}
- GlaxoSmithKline (SAM40036). A 12‐week multicentre, randomised, double‐blind, double‐dummy, parallel group study to compare the efficacy and tolerability of once daily (QD) salmeterol/fluticasone propionate combination (salm/FP) 50/100mcg at night via the DISKUS/ACCUHALER with QD budesonide (BUD) 400mcg at night via a breath‐actuated dry powder inhaler (BADPI) as initial maintenance therapy in mild‐to‐moderate asthmatic subjects. http://www.ctr.gsk.co.uk 2004.
- Pauwels R, Smiltena I, Bagdonas A, Eliraz E, Firth R. Seretide once daily is more effective than budesonide 400mcg once daily in mild asthma. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A86. [Google Scholar]
SAS30015 {unpublished data only}
- McCarthy TP, Edin HM, House K, Vandermeer AK. Quality of life and asthma control assessment in patients previously on inhaled corticosteroids (ICS) treated with salmeterol/fluticasone combination (SFC) metered dose inhaler (MDI). Thorax 2001;56(Suppl 3):iii 63. [Google Scholar]
- McCarthy TP, Greening AP, Holgate SK, Whitehead C, Rice L. Salmeterol/fluticasone propionate combination (SFC) is more effective that beclometasone dipropionate (BDP) in patients not well controlled on bronchodilators alone. American Journal of Respiratory and Critical Care Medicine 2002;16(Suppl 8):A566. [Google Scholar]
- McCarthy TP, Greening AP, Holgate SK, Whitehead C, Rice L. The efficacy of salmeterol/fluticasone propionate combination (SFC) metered dose inhaler compared with beclomethasone dipropionate (BDP) in patients not well controlled at step 1 of the British guidelines on asthma management (BGAM). Thorax 2001;56:iii 62. [Google Scholar]
- SAS30015. A phase IIIB, multi‐centre, double‐blind, parallel group, randomised study to compare the efficacy of the salmeterol/fluticasone propionate combination (25/50 mcg strength), 2 inhalations bd via HFA‐MDI with beclomethasone dipropionate (BDP) 200mcg bd via metered dose inhaler (MDI) in adolescents and adults with asthma. http://www.ctr.gsk.co.uk 2004.
- Tolley K, Martin A, Rice L, McCarthy TP. Salmeterol/fluticasone propionate combination (SFC) demonstrates improved health outcomes and good cost effectiveness compared with beclometasone dipropionate. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A112. [Google Scholar]
SAS30021 {unpublished data only}
- GlaxoSmithKline (SAS30021). A stratified, randomized, double‐blind, placebo‐controlled, parallel‐group, 12‐week trial evaluating the safety and efficacy of the fluticasone propionate/salmeterol DISKUS combination product 100/50mcg once daily versus fluticasone propionate DISKUS 100mcg once daily and placebo in symptomatic pediatric subjects (4‐11 years) with asthma. http://www.ctr.gsk.co.uk 2004 (accessed 1 May 2008).
SAS40068 {unpublished data only}
- Renzi PM, Franssen E, Stat P, Watson EG. Salmeterol/fluticasone propionate diskus® (advair®) 50/100 mcg bid improves asthma outcomes compared with fluticasone propionate (Flovent®) Diskus® 100 mcg bid when used as initial maintenance treatment in adult and adolescent subjects with symptomatic persistent asthma. American Thoracic Society. 2005:A628.
- SAS40068. A 24 week, multicentre, randomized, double‐blind, parallel group trial to compare the efficacy and tolerability of salmeterol/fluticasone propionate (Advair) diskus inhalation device 50/100 mcg bid with fluticasone propionate diskus inhalation device 100 mcg bid as initial maintenance treatment in adult and adolescent subjects with symptomatic, persistent asthma not controlled on short‐acting bronchodilators alone (program of advair control and effectiveness ‐ initial maintenance treatment, PACE ‐ IMT study). http://www.ctr.glaxowellcome.co.uk 2005.
SLGF75 {unpublished data only}
- SLGF75. Salmeterol plus low‐dose fluticasone propionate (FP) versus high‐dose fluticasone propionate (FP) in naive patients with mild to moderate asthma: effects on pulmonary function, and inflammatory markers of induced sputum. www.ctr.gsk.co.uk 2005 (accessed 4 June 2008).
Sorkness 2007 {published data only}
- Sorkness CA, Lemanske Jr RF, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long‐term comparison of 3 controller regimens for mild‐moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. Journal of Allergy & Clinical Immunology 2007;119(1):64‐72. [DOI] [PubMed] [Google Scholar]
Stelmach 2008 {published data only (unpublished sought but not used)}
- Stelmach I, Grzelewski T, Jerzynska J, Kuna P. A randomized, double‐blind trial on the effect of treatment with montelukast, budesonide, montelukast with budesonide, formoterol with budesonide on lung function and clinical symptoms in children with asthma [Abstract]. Journal of Allergy & Clinical Immunology 2005;115(Suppl 2):S151. [Google Scholar]
- Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise‐induced bronchoconstriction in children with asthma. Journal of Allergy and Clinical Immunology 2007; Epub ahead of print (accessed 17 January 2008). [DOI] [PubMed]
Strand 2004 {published and unpublished data}
- SAM40049. A Danish, multi‐centre, comparative, parallel‐group study to determine whether initiation of combination treatment with Seretide™ (salmeterol + fluticasone propionate) 50/100 mg bd offers better asthma control than monotherapy with Flixotide™ (fluticasone propionate) 100 mg bd to adult asthmatic subjects uncontrolled on short‐acting bronchodilator alone. http://www.ctr.gsk.co/uk 2005.
- Strand AM. Initiation of treatment with the salmeterol/fluticasone propionate combination product is better than inhaled steroid alone (FP) in asthmatic patients symptomatic on short‐acting bronchodilator alone. European Respiratory Journal 2003;22(45):410s. [Google Scholar]
- Strand AM, Luckow A, on behalf of the DINA group (Danish INitiative for Asthma treatment). Initiation of maintenance treatment of persistent asthma: salmeterol/fluticasone propionate combination treatment is more effective than inhaled steroid alone. Respiratory Medicine 2004;98(10):1008‐15. [DOI] [PubMed] [Google Scholar]
Weersink 1997 {published data only}
- Weersink EJ, Zomeren EH, Koeter GH, Postma DS. Treatment of nocturnal airway obstruction improves daytime cognitive performance in asthmatics. American Journal of Respiratory & Critical Care Medicine. 1997;156(4 pt 1):1144‐50. [DOI] [PubMed] [Google Scholar]
- Weersink EJM, Doouma RR, Postma DS, Koeter GH. Fluticasone propionate, salmeterol xinafoate and their combination in the treatment of nocturnal asthma. American Journal of Respiratory and Critical Care Medicine 1997;155(4):1241‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aalbers 2004 {published data only}
- Aalbers R, Backer V, Kava TTK, Omenaas ER, Sandstrom T, Jorup C, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed‐dose salmeterol/fluticasone in moderate to severe asthma. Current Medical Research & Opinion 2004;20(2):225‐40. [DOI] [PubMed] [Google Scholar]
- Aalbers R, Backer V, Kava TTK, Welte T, Omenaas ER, Bergqvist PBF, et al. Adjustable dosing with budesonide/formoterol reduces the rate of asthma exacerbations compared with fixed dosing salmeterol/fluticasone. European Respiratory Society. 2003:p2‐20.
- Aalbers R, Backer V, Kava TTK, Welte T, Omenaas ER, Bergqvist PBF, et al. Improvements in FEV1 are greater with budesonide/formoterol than with salmeterol/fluticasone. European Respiratory Society. 2003:P2‐19.
- Aalbers R, Backer V, Kava TTK, Welte T, Omenaas ER, Bergqvist PBF, et al. Is well‐controlled asthma weeks a useful measure? Fewer exacerbations in patients treated with budesonide/formoterol than salmeterol/fluticasone. European Respiratory Society. 2003:P2‐18.
- Welte T, Aalbers R, Naya I. Budesonide/formoterol adjustable maintenance dosing (B/F AMD) reduces the burden of asthma more effectively than fixed‐dosing (FD) with B/F or salmeterol/fluticasone (S/FL). European Respiratory Journal 2004;24 Suppl 48:508s. [Google Scholar]
Adinoff 1998 {published data only}
- Adinoff AD, Schwartz HJ, Rickard KA, Yancey SW, Swearingen BE. Salmeterol compared with current therapies in chronic asthma. Journal of Family Practice 1998;47(4):278‐84. [PubMed] [Google Scholar]
Akpinarli 1999 {published data only}
- Akpinarli A, Tuncer A, Saraclar Y, Sekerel BE, Kalayci O. Effect of formoterol on clinical parameters and lung functions in patients with bronchial asthma: a randomised controlled trial. Archives of Disease in Childhood 1999;81:45‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ankerst 2003 {published data only}
- Ankerst J, Persson G, Weibull E. Cardiovascular effects of a high dose of the budesonide/formoterol single inhaler in asthmatic patients. European Respiratory Journal 2001;18 Suppl 33:53s. [Google Scholar]
- Ankerst J, Persson G, Weibull E. Tolerability of a high dose of budesonide/formoterol in a single inhaler in patients with asthma. Pulmonary Pharmacology & Therapeutics 2003;16:147‐51. [DOI] [PubMed] [Google Scholar]
Anonymous 1999 {published data only}
- Anonymous. Levalbuterol for asthma. Drugs & Therapeutics 1999;41(1054):51‐3. [PubMed] [Google Scholar]
Anonymous 2003a {published data only}
- Anonymous. Flexible dosing of combination inhaler cuts asthma exacerbations. Pharmaceutical Journal 2003;271(7271):535. [Google Scholar]
Anonymous 2003b {published data only}
- Anonymous. Asthma control with combination therapy. Fortschritte Der Medizin 2003;145(46):46‐7. [PubMed] [Google Scholar]
Anonymous 2003c {published data only}
- Anonymous. Excess mortality with salmeterol as single‐agent therapy. Prescriber International 2003;12(66):142. [PubMed] [Google Scholar]
Arvidsson 1991 {published data only}
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Svedmyr N, Wahlander L. Inhaled formoterol during one year in asthma: a comparison with salbutamol. European Respiratory Journal 1991;4(10):1168‐73. [PubMed] [Google Scholar]
ASSURE {published data only}
- Haughney J, Cotton P, Rosen JP, Rosen JP, Morrison K, Price D. The use of a modification of the Patient Enablement Instrument in asthma. CN‐00587268. Primary Care Respiratory Journal: Journal of the General Practice Airways Group 2007;16(2):89‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ind PW, Haughney J, Price D, Rosen JP, Kennelly J. Adjustable and fixed dosing with budesonide/formoterol via a single inhaler in asthma patients: the ASSURE study. Respiratory Medicine 2004;98(5):464‐75. [DOI] [PubMed] [Google Scholar]
Aubier 1999 {published data only}
- Aubier M, Pieters WR, Schlosser NJJ, Steinmetz KO. Salmeterol/fluticasone propionate (50/500 mug) in combination in a Diskus(TM) inhaler (Seretide(TM)) is effective and safe in the treatment of steroid‐dependent asthma. Respiratory Medicine 1999;93(12):876‐84. [DOI] [PubMed] [Google Scholar]
- Pieters WR, Lundback B, Sondhi S, Price MJ. Cost effectiveness analysis of salmeterol/fluticasone propionate 50/500mcg vs fluticasone propionate 500mcg in patients with corticosteroid‐dependent asthma. Pharmacoeconomics 1999;16(Suppl 2):29‐34. [Google Scholar]
- Pieters WR, Sondhi S, Price MJ, Thwaites RM, Nyth A. The cost effectiveness of salmeterol/fluticasone propionate 50/500 microgram combination inhaler versus fluticasone propionate 500 microgram in patients with chronic asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:P2458.
- Pieters WR, Wilson KK, Smith HCE, Tamminga JJ. Cost‐effectiveness of fluticasone propionate/salmeterol combination product and fluticasone propionate/montelukast in asthma. Annual Thoracic Society 97th International Conference; San Francisco CA , May 18‐23. 2001.
- SFCB30019. A multicentre randomized, double‐blind, double‐dummy, parallel‐group comparison of the salmeterol/fluticasone propionate combination product (50/500mcg strength) BD via one DISKUS/Accuhaler inhaler with salmeterol 50mcg BD via one DISKUS/Accuhaler and fluticasone propionate 500mcg BD via another DISKUS/Accuhaler and with fluticasone propionate 500mcg BD via one DISKUS/Accuhaler in adolescents and adults with reversible airways obstruction. http://www.ctr.gsk.co.uk 2004.
- Schlosser NJ, Steinmetz KO, Aubier M, Gomez E, Wixon C. Evaluation of long‐term safety of salmeterol/fluticasone propionate (50/500µg) combination inhaler in patients with reversible airways obstruction. European Respiratory Journal 1998;12 Suppl 28:35s. [Google Scholar]
Aziz 1998 {published data only}
- Aziz I, Hall IP, McFarlane LC, Lipworth BJ. Beta2‐adrenoceptor regulation and bronchodilator sensitivity after regular treatment with formoterol in subjects with stable asthma. Journal of Allergy & Clinical Immunology 1998;101(3):337‐41. [DOI] [PubMed] [Google Scholar]
Aziz 1999a {published data only}
- Aziz I, Lipworth BJ. A bolus of inhaled budesonide rapidly reverses airway sub‐sensitivity and beta2‐adrenoceptor down‐regulation after regular inhaled formoterol. Chest 1999;115(3):623‐8. [DOI] [PubMed] [Google Scholar]
Aziz 1999b {published data only}
- Aziz I, Lipworth BJ. In vivo effect of albuterol on methacholine‐contracted bronchi in conjunction with salmeterol and formoterol. Journal of Allergy & Clinical Immunology 1999;103(5 pt 1):816‐22. [DOI] [PubMed] [Google Scholar]
Aziz 2000 {published data only}
- Aziz I, Wilson AM, Lipworth BJ. Effects of formoterol (FM) and budesonide (BUD) alone or in combination (FM+BUD) on inflammatory markers in asthmatic patients. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:2854.
- Aziz I, Wilson AM, Lipworth BJ. Effects of once‐daily formoterol and budesonide given alone or in combination on surrogate inflammatory markers in asthmatic adults. Chest 2000;118(4):1049‐58. [DOI] [PubMed] [Google Scholar]
Bacci 2002 {published data only}
- Bacci E, Franco A, Bartoli ML, Carnevali S, Cianchetti S, Dente FL, et al. Comparison of anti‐inflammatory and clinical effects of beclomethasone dipropionate and salmeterol in moderate asthma. European Respiratory Journal 2002;20(1):66‐72. [DOI] [PubMed] [Google Scholar]
Baki 1998 {published data only}
- Baki A, Karaguzel G. Short‐term effects of budesonide, nedocromil sodium and salmeterol on bronchial hyperresponsiveness in childhood asthma. Acta Paediatrica Japonica 1998;40(3):247‐51. [DOI] [PubMed] [Google Scholar]
Balachandran 2001 {published data only}
- Balachandran A, Shivbalan S, Subramanyam L. Drug therapy of childhood asthma. Indian Journal of Pediatrics 2001;68(Suppl 4):S12‐16. [PubMed] [Google Scholar]
Balzano 2002 {published data only}
- Balzano G, Fuschillo S, Gaudiosi C. Leukotriene receptor antagonists in the treatment of asthma: an update. Allergy 2002;57(Suppl 72):16‐9. [DOI] [PubMed] [Google Scholar]
Baraniuk 1999 {published data only}
- Braniuk J, Murray JJ, Nathan RA, Berger WE, Johnson M, Edwards LD, et al. Fluticasone alone or in combination with salmeterol vs triamcinolone in asthma. Chest 1999;116(3):625‐32. [DOI] [PubMed] [Google Scholar]
Bateman 1998 {published data only}
- Bateman ED, Britton M, Carrillo J, Almeida J, Wixon C. Salmeterol/fluticasone combination inhaler. A new, effective and well tolerated treatment for asthma. Clinical Drug Investigation 1998;16(3):193‐201. [DOI] [PubMed] [Google Scholar]
Bateman 2000 {published data only}
- Bateman ED, Beasley R, Silins V, Bogolubov M. Comparison of salmeterol/fluticasone propionate combination 50/100 bid delivered via metered dose inhaler or diskus in patients with reversible airways obstruction. American Thoracic Society 2000 International Conference; May 5‐10; Toronto, Canada 2000;161(Suppl 3):A197. [Google Scholar]
Bateman 2001 {published data only}
- Bateman ED, Bantje TA, Gomes M, Toumbis M, Huber R, Eliraz A, et al. Symbicort (budesonide and formoterol in a single inhaler) is a more effective treatment than fluticasone in asthma patients. American Thoracic Society Abstracts. 2001.
- Bateman ED, Silins V, Bogolubov M. Clinical equivalence of salmeterol/fluticasone propionate in combination (50/100 mcg twice daily) when administered via a chlorofluorocarbon‐free metered dose inhaler or dry powder inhaler to patients with mild‐to‐moderate asthma. Respiratory Medicine 2001;95(2):136‐46. [DOI] [PubMed] [Google Scholar]
- SFCB3022. A multicentre, randomised, double‐blind, double‐dummy, parallel‐group, three‐month comparison of the salmeterol/fluticasone propionate combination product (2x25/50mcg strength) bd via the pressurised metered dose inhaler with salmeterol/fluticasone propionate combination product (1x50/100mcg strength) bd via the Diskus/Accuhaler™ inhaler and with fluticasone propionate (2x 50mcg strength) alone bd via the pressurised metered dose inhaler in adolescents and adults with reversible airways obstruction. http://www.ctr.gsk.co.uk 2004.
Bateman 2003a {published data only}
- Bateman ED, Bantje TA Joao Gomes M, Toumbis M, Huber R, Eliraz A, et al. Symbicort (budesonide/eformoterol) turbohaler controls asthma more effectively than fluticasone diskus. Thorax 2001;56(Suppl 3):iii 63. [Google Scholar]
- Bateman ED, Bantje TA, Gomes MJ, Toumbis MG, Huber RM, Naya I, et al. Combination therapy with a single inhaler budesonide/formoterol compared with high dose fluticasone propionate alone in patients with moderate persistent asthma. American Journal of Respiratory Medicine 2003;2(3):275‐81. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Bantje TA, Joao Gomes M, Toumbis M, Huber R, Eliraz A. Early and sustained benefits of budesonide and formoterol in a single inhaler vs fluticasone in moderate asthma. European Respiratory Journal. 2001;18 Suppl 33:157s. [Google Scholar]
- Ericsson K, Bantje TA, Huber H, Borg S. Symbicort® turbuhaler® is more cost effective than fluticasone diskus™ in the treatment of asthma. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
- Ericsson K, Bantje TA, Huber R, Borg S, Anderson F. Cost‐effectiveness of budesonide and formoterol in a single inhaler compared to fluticasone in the treatment of asthma. European Respiratory Journal 2001;18 Suppl 33:157s. [Google Scholar]
- Ericsson K, Bantje TA, Huber R, Borg S, Andersson F. Symbicort turbohaler is more effective than fluticasone diskus in the treatment of asthma. Thorax 2001;56(Suppl 3):iii63. [Google Scholar]
Bateman 2003b {published data only}
- Bateman ED, Akveld M, Ho M. Greater responder rate to fluticasone propionate/salmeterol combination over montelukast plus fluticasone in asthma. American Thoracic Society 99th International Conference; B036 Poster H90. 2003.
Baumgarten 2002 {published data only}
- Baumgarten C, Geldszus R, Behre U, Peslis N, Trautmann M, Viani‐Initial Study Group. Initial treatment of symptomatic mild to moderate bronchial asthma with the salmeterol/fluticasone propionate (50/250 microg) combination product. European Journal of Medical Research 2002;7(1):1‐7. [PubMed] [Google Scholar]
Beeh 2002 {published data only}
- Beeh KM, Beier J, Kornmann O, Wiewrodt R, Buhl R. Efficacy and safety of salmeterol (50 mcg) and fluticasone (250 mcg) in a single inhaler device (Diskus(R)) in patients with mild to moderate asthma. Pneumologie 2002;56(2):91‐7. [DOI] [PubMed] [Google Scholar]
- SAM40011 (GlaxoSmithKline). Efficacy and safety of salmeterol/fluticasone propionate (VIANI®) 50/250μg twice daily via Diskus in patients with mild to moderate asthma. http://www.ctr.gsk.co.uk 2005 (accessed 2 May 2008).
Behling 1999 {published data only}
- Behling B, Matthys H. Comparison of efficacy and safety of formoterol with terbutaline in children with mild to moderate asthma. European Respiratory Society; 1999 Oct 9‐13; Madrid, Spain. 1999:369.
Bennett 2002 {published data only}
- Bennett WD. Effect of beta‐adrenergic agonists on mucociliary clearance. Journal of Allergy & Clinical Immunology 2002;110(Suppl 6, part 2):s291‐7. [DOI] [PubMed] [Google Scholar]
Bensch 2002 {published data only}
- Bensch G, Berger WE, Blokhnn M, Socolovshy AL, Thomson MH, Till MD, et al. One year efficacy and safety of inhaled formoterol dry powder in children with persistent asthma. Annals of Allergy Asthma and Immunology 2002;89:180‐90. [DOI] [PubMed] [Google Scholar]
- Berger W, Bensch G, Blokhin BM, Socolovsky AL, Thompson MH, Till D. Addition of formoterol (Foradil®) improves lung function and symptoms in children with persistent asthma not controlled by inhaled corticosteroids. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
Berggren 2001 {published data only}
- Berggren F, Ekstrom T. A cost‐effectiveness study comparing the as‐needed use of formoterol (Oxis) and terbutaline (Bricanyl) in patients with moderate to severe asthma. Respiratory Medicine 2001;95(9):753‐8. [DOI] [PubMed] [Google Scholar]
Bergmann 2004 {published data only}
- Bergmann KC, Lindemann L, Braun R, Steinkamp G. Salmeterol/fluticasone propionate (50/250 mug) combination is superior to double dose fluticasone (500 mug) for the treatment of symptomatic moderate asthma: a prospective, double‐blind trial. Swiss Medical Weekly 2004;134(3‐4):50‐8. [DOI] [PubMed] [Google Scholar]
Berlinski 2001 {published data only}
- Berlinski A, Waldrep JC. Metering performance of several metered‐dose inhalers with different spacers/holding chambers. Journal of Aerosol Medicine 2001;14(4):427‐32. [DOI] [PubMed] [Google Scholar]
Bernstein 2002 {published data only}
- Bernstein IL. Beta2‐agonists: Deja vu all over again: the second‐generation controversy. Chest 2002;122(3):763‐5. [DOI] [PubMed] [Google Scholar]
Bessmertny 2002 {published data only}
- Bessmertny O, DiGregorio RV, Cohen H, Becker E, Looney D, Golden J, et al. A randomized clinical trial of nebulized magnesium sulfate in addition to albuterol in the treatment of acute mild‐to‐moderate asthma exacerbations in adults. Annals of Emergency Medicine 2002;39(6):585‐91. [DOI] [PubMed] [Google Scholar]
Bijl‐Hofland 2001 {published data only}
- Bijl‐Hofland ID, Cloosterman SG, Folgering HT, Elshout FJ, Weel C, Schayck CP. Inhaled corticosteroids, combined with long‐acting beta(2)‐agonists, improve the perception of bronchoconstriction in asthma. American Journal of Respiratory & Critical Care Medicine 2001;164(5):764‐9. [DOI] [PubMed] [Google Scholar]
Bjermer 2000 {published data only}
- Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening A, Haahtela T, et al. Montelukast or salmeterol combined with an inhaled steroid in adult asthma: design and rationale of a randomized, double‐blind comparative study (the IMPACT Investigation of Montelukast as a Partner Agent for Complementary Therapy trial). Respiratory Medicine 2000;94(6):612‐21. [DOI] [PubMed] [Google Scholar]
- Bjermer L, Greening A, Haahtela T, Bousquet J, Holgate ST, Picado C, et al. Addition of montelukast or salmeterol to fluticasone in patients with uncontrolled asthma: results of the IMPACT trial. Chest Conference, San Diego, CA. 2002:434.
Bjermer 2003 {published data only}
- Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening AP, Haahtela T, et al. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: one year, double blind, randomised, comparative trial. BMJ 2003;327(7420):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 2003 {published data only}
- Bloom J, Calhoun W, Koenig S, Yancey S, Reilly D, Edwards L, et al. Fluticasone propionate/salmeterol 100/50mcg is inhaled steroid sparing in patients who require fluticasone propionate 250mcg for asthma stability. American Thoracic Society 99th International Conference. 2003:D034 Poster C33.
Boonsawat 2003 {published data only}
- Boonsawat W, Charoenratanakul S, Pothirat C, Sawanyawisuth K, Seearamroongruang T, Bengtsson T, et al. Formoterol (OXIS) Turbuhaler as a rescue therapy compared with salbutamol pMDI plus spacer in patients with acute severe asthma. Respiratory Medicine 2003;97(9):1067‐74. [DOI] [PubMed] [Google Scholar]
Booth 1993 {published data only}
- Booth H, Fishwick K, Harkawat R, Devereux G, Hendrick DJ, Walters EH. Changes in methacholine induced bronchoconstriction with the long acting beta 2 agonist salmeterol in mild to moderate asthmatic patients. Thorax 1993;48(11):1121‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boskovska 2001 {published data only}
- Boskovska MI, Dokic D, Busletic‐Bozinovska K, Arbutina S, Goseva Z. Concomitant use of low‐dose inhaled corticosteroids and a long‐acting bronchodilator vis a vis doubling the dose of inhaled corticosteroid in asthma patients. European Respiratory Journal 2001;16(Suppl 33):98s. [Google Scholar]
Bouchard 2000 {published data only}
- Bouchard J, Arkinstall W, Tesarowski D. Efficacy of salmeterol and fluticasone propionate (FP) combination therapy versus FP alone in mild/moderate asthma. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A197. [Google Scholar]
Boulet 1998 {published data only}
- Boulet LP, Turcotte H, Cartier A, Milot J, Cote J, Malo JL, et al. Influence of beclomethasone and salmeterol on the perception of methacholine‐induced bronchoconstriction. Chest 1998;114(2):373‐9. [DOI] [PubMed] [Google Scholar]
Boulet 2003 {unpublished data only}
- Boulet LP, Chapman K, Roberts J, Watson EG. Efficacy of salmeterol/fluticasone propionate HFA MDI versus high dose fluticasone propionate HFA MDI in adolescent and adult asthma. European Respiratory Society Meeting 2003.
Bouros 1999 {published data only}
- Bouros D, Bachlitzanakis N, Kottakis J, Pfister P, Polychronopoulos V, Papadakis E, et al. Foromoterol and beclomethasone versus higher dose beclomethasone as maintenance therapy in adult asthma. European Respiratory Journal 1999;14:627‐32. [DOI] [PubMed] [Google Scholar]
Boyd 1995 {published data only}
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy. European Respiratory Journal 1995;8:1494‐8. [PubMed] [Google Scholar]
Brambilla 1994 {published data only}
- Brambilla C, Chastang C, Georges D, Bertin L. Salmeterol compared with slow‐release terbutaline in nocturnal asthma. Allergy 1994;49(6):421‐6. [DOI] [PubMed] [Google Scholar]
Brambilla 2003 {published data only}
- Brambilla C, Gros V, Bourdeix I. Formoterol 12 mug BID administered via single‐dose dry powder inhaler in adults with asthma suboptimally controlled with salmeterol or on‐demand salbutamol: a multicenter, randomized, open‐label, parallel‐group study. Clinical Therapeutics 2003;25(7):2022‐36. [DOI] [PubMed] [Google Scholar]
Braunstein 2002 {published data only}
- Braunstein G. Optimization of asthma control with fluticasone/salmeterol (seretide) combination: new clinical data. Revue De Pneumologie Clinique 2002;58(3 part 2):s12‐5. [PubMed] [Google Scholar]
Brenner 1998 {published data only}
- Brenner M, Berkowitz R, Marshall N, Strunk RC. Need for theophylline in severe steroid‐requiring asthmatics. Clinical Allergy 1988;18(2):143‐50. [DOI] [PubMed] [Google Scholar]
Britton 1992 {published data only}
- Britton MG, Earnshaw JS, Palmer JBD. A twelve month comparison of salmeterol with salbutamol in asthmatic patients. European Respiratory Journal 1992;5(9):1062‐7. [PubMed] [Google Scholar]
Britton 1998 {published data only}
- Britton MG, Carrillo T Almeida J Wixon C. Combined serevent™ and fluticasone propionate (50/100µg strength) bd via one diskus™ (accuhaler™) inhaler compared with salmeterol 50µg and fluticasone propionate 100µg bd via two separate diskus inhalers. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A415. [Google Scholar]
Brogden 1991 {published data only}
- Brogden‐RN, Faulds‐D. Salmeterol xinafoate. A review of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs 1991;42(5):895‐912. [DOI] [PubMed] [Google Scholar]
Buchvald 2002 {published data only}
- Buchvald FF, Bisgaard H. Comparison of add‐on of leukotriene receptor antagonist vs. long‐acting beta2‐agonist on FeNO in asthmatic children on regular inhaled budesonide. European Respiratory Society Annual Congress. 2002:p2736.
Buchvald 2003 {published data only}
- Buchvald F, Bisgaard H. Comparisons of the complementary effect on exhaled nitric oxide of salmeterol vs montelukast in asthmatic children taking regular inhaled budesonide. Annals of Allergy, Asthma, & Immunology 2003;91(3):309‐13. [DOI] [PubMed] [Google Scholar]
Buhl 2003a {published data only}
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Improved and maintained asthma control with once‐daily budesonide/formoterol single inhaler in mild‐to‐moderate persistent asthma. European Respiratory Journal. 2001;18(Suppl 33):21s. [Google Scholar]
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Once daily symbicort (budesonide/eformoterol in a single inhaler) is effective in moderate‐persistent asthma. Thorax 2001;56(Suppl 3):iii 62. [Google Scholar]
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Once‐daily budesonide/formoterol via a single inhaler is effective in mild‐to‐moderate persistent asthma. European Respiratory Journal 2001;18(Suppl 33):21s. [Google Scholar]
- Buhl R, Creemers JPHM, Vondra V, Martelli NA. Symbicort® (budesonide and formoterol in a single inhaler) administered once daily is effective in mild to moderate asthma. Annual Thoracic Society 97th International Conference; San Francisco CA , May 18‐23. 2001.
- Buhl R, Creemers JPHM, Vondra V, Martelli NA, Naya IP, Eksstrom T. Once daily budesonide /formoterol in a single inhaler in adults with moderate persistent asthma. Respiratory Medicine 2003;97:323‐30. [DOI] [PubMed] [Google Scholar]
- Buhl R, Zetterstrom O, Mellem H, Perpina M, Hedman J, O'Neill S, et al. Improved asthma control with budesonide/formoterol via a single inhaler compared with budesonide alone, in moderate persistent asthma. European Respiratory Journal 2001;18 Suppl 33:48s. [DOI] [PubMed] [Google Scholar]
Buhl 2003b {published data only}
- Buhl R. Budesonide/formoterol for the treatment of asthma. Expert Opinion in Pharmacotherapy 2003;4(8):1393‐406. [DOI] [PubMed] [Google Scholar]
Busse 1999 {published data only}
- Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. Journal of Allergy & Clinical Immunology 1999;103(6):1075‐80. [DOI] [PubMed] [Google Scholar]
Busse 2003 {published data only}
- Busse W, Koenig SM, Oppenheimer J, Sahn SA, Yancey SW, Reilly D, et al. Steroid‐sparing effects of fluticasone propionate 100mcg and salmeterol 50mcg administered twice daily in a single product in patients previously controlled with fluticasone propionate 250mcg administered twice daily. Journal of Allergy & Clinical Immunology 2003;111(2):57‐65. [DOI] [PubMed] [Google Scholar]
- Sahn S, Yancey S, Reilly D, Edwards L, Rickard K, Dorinsky P. The fluticasone propionate/salmeterol (FSC) combination product 100/50 mcg BID is steroid sparing in patients who require FP250 mcg BID for asthma stability. Chest Conference, San Diego, CA. 2002.
Byrnes 2000 {published data only}
- Byrnes C, Shrewsbury S, Barnes PJ, Bush A. Salmeterol in paediatric asthma. Thorax 2000;55(9):780‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calhoun 2001 {published data only}
- Calhoun WJ, Nelson HS, Nathan RA, Pepsin PJ, Kalberg C, Emmett A, et al. Comparison of fluticasone propionate‐salmeterol combination therapy and montelukast in patients who are symptomatic on short‐acting 2‐agonists alone. American Journal of Respiratory and Critical Care Medicine 2001;164(5):759‐63. [DOI] [PubMed] [Google Scholar]
Calverley 2002 {published data only}
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components in COPD. American Journal of Respiratory and Critical Care Medicine 2002; Vol. 165, issue Suppl 8:A226.
Cazzola 2000 {published data only}
- Cazzola M, Lorenzo G, Perna F, Calderaro F, Testi R, Centanni S. Additive effects of salmeterol and fluticasone or theophylline in COPD. Chest 2000;118(6):1576‐81. [DOI] [PubMed] [Google Scholar]
Chalmers 1999 {published data only}
- Chalmers GW, Macleod KJ, Thomson LJ, Little SA, Patel KR, McSharry C, et al. Sputum cellular and cytokine responses to inhaled endothelin‐1 in asthma. Clinical & Experimental Allergy 1999;29(11):1526‐31. [DOI] [PubMed] [Google Scholar]
Chan 2001 {published data only}
- Chan JS, Cowie RL, Lazarenko GC, Little C, Scott S, Ford GT. Comparison of intramuscular betamethasone and oral prednisone in the prevention of relapse of acute asthma. Canadian Respiratory Journal 2001;8(3):147‐52. [DOI] [PubMed] [Google Scholar]
Chapman 1999 {published data only}
- Chapman KR, Ringdal N, Backer V, Palmqvist M, Saarelainen S, Briggs M. Salmeterol and fluticasone propionate (50/250 mug) administered via combination Diskus inhaler: as effective as when given via separate Diskus inhalers. Canadian Respiratory Journal 1999;6(1):45‐51. [DOI] [PubMed] [Google Scholar]
Cheer 2003 {published data only}
- Cheer SM, Warner GT, Easthope SE. Formoterol delivered by Turbuhaler: in paediatric asthma. Paediatric Drugs 2003;5(1):63‐8. [DOI] [PubMed] [Google Scholar]
Cloosterman 2001 {published data only}
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, Akkermans RP, Elshout FJ, Folgering HT, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta(2)‐agonists in allergic asthmatic patients. Chest 2001;119(5):1306‐15. [DOI] [PubMed] [Google Scholar]
Condemi 1999 {published data only}
- Baker J, Yancey S, Kalberg C, Petrocella V, Emmett A, Bowers B, et al. Added salmeterol versus increased‐dose fluticasone in patients symptomatic on low‐dose fluticasone. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A406. [Google Scholar]
- Condemi JJ, Goldstein S, Kalberg C, Yancey S, Emmett A, Rickard K. The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Annals of Allergy and Asthma Immunology 1999;82:383‐9. [DOI] [PubMed] [Google Scholar]
Condemi 2001 {published data only}
- Condemi JJ. Comparison of the efficacy of formoterol and salmeterol in patients with reversible obstructive airway disease: a multicenter, randomized, open‐label trial. Clinical Therapeutics 2001;23(9):1529‐41. [DOI] [PubMed] [Google Scholar]
Crompton 1999 {published data only}
- Crompton GK, Ayres JG, Basran G, Schiraldi G, Brusasco V, Eivindson A, et al. Comparison of oral bambuterol and inhaled salmeterol. American Journal of Respiratory & Critical Care Medicine 1999;159(3):824‐8. [DOI] [PubMed] [Google Scholar]
Currie 2003a {published data only}
- Currie GP, Lee DKC, Haggart K, Bates CE, Lipworth BJ. Effects of montelukast on surrogate inflammatory markers in corticosteroid‐treated patients with asthma. American Journal of Respiratory and Critical Care Medicine 2003;167(9):1232‐8. [DOI] [PubMed] [Google Scholar]
Currie 2003b {published data only}
- Currie GP, Bates CE, Lee DKC, Jackson CM, Lipworth BJ. Effects of fluticasone plus salmeterol versus twice the dose of fluticasone in asthmatic patients. European Journal of Clinical Pharmacology 2003;59(1):11‐15. [DOI] [PubMed] [Google Scholar]
Currie 2003c {published data only}
- Currie GP, Lee DK, Fowler SJ, Cowan LM, Lipworth BJ. A proof of concept study to evaluate putative benefits of montelukast in moderate persistent asthmatics. British Journal of Pharmacology 2003;55(6):609‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Alonzo 1994 {published data only}
- D'Alonzo GE, Nathan RA, Henochowicz, Morris RJ, Ratner P, Rennard SI. Salmetrol xinafoate as maintenance therapy compared with albuterol in patients with asthma. JAMA 1994;271:1412‐16. [PubMed] [Google Scholar]
D'Urzo 2001 {published data only}
- D'Urzo AD, Chapman KR, Cartier A, Hargreave FE, Fitzgeerald M, Tesarowski D. Effectiveness and safety of salmeterol in non‐specialist practice settings. Chest 2001;119:714‐9. [DOI] [PubMed] [Google Scholar]
Dahl 1989 {published data only}
- Dahl R, Pedersen B, Hagglof B. Nocturnal asthma: effect of treatment with oral sustained‐release terbutaline, inhaled budesonide, and the two in combination. Journal of Allergy & Clinical Immunology 1989;83(4):811‐5. [DOI] [PubMed] [Google Scholar]
Dahl 1991 {published data only}
- Dahl R, Earnshaw JS, Palmer JBD. Salmeterol: a four week study of a long‐acting beta‐adrenoceptor agonist for the treatment of reversible airways disease. European Respiratory Journal 1991;4(10):1178‐84. [PubMed] [Google Scholar]
Dal Negro 2001a {published data only}
- Dal Negro R, Micheletto C, Tognella S, Trevisan F, Pomari C. Short‐term bronchodilation following salmeterol 50mcg and combined salmeterol + fluticasone (50/250mcg) via diskus: a randomized, double blind cross‐over study in reversible airway obstruction. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
Dal Negro 2001b {published data only}
- Dal Negro RW, Micheletto C, Pomari C, Trevisan F, Tognella S. The combination salmeterol (S) + fluticasone propionate (F) in mild‐to‐moderate asthma. European Respiratory Journal 2001;18(Suppl 33):426s. [Google Scholar]
Davis 2001 {published data only}
- Davis ES, Bowers B, Pepsin P, Kalberg C, Dorinsky P. The impact of fluticasone propionate/salmeterol combination product compared to oral montelukast on asthma related quality of life. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Dekhuijzen 2002 {published data only}
- Dekhuijzen PNR, Diamant Z. The role of montelukast, a leukotriene receptor antagonist, in the treatment of asthma. Pharmaceutisch Weekblad 2002;137(1):38‐9. [Google Scholar]
Del Rio‐Navarro 2001 {published data only}
- Rio Navarro BE, Sienra‐Monge JJL, Alvarez‐Amador M, Reyes‐Ruiz N, Arevalo‐Salas A, Berber A. Serum potassium levels, CPK‐MB and ECG in children suffering asthma treated with beclomethasone or beclomethasone‐salmeterol. Allergologia et Immunopathologia 2001;29(1):16‐21. [DOI] [PubMed] [Google Scholar]
Del‐Rio‐Navarro 2001 {published data only}
- Rio Navarro BE, Coron‐Hernandez L, Fragoso‐Rios R, Berber A, Torres‐Alcantara S, Cuairan‐Ruidiaz V, et al. Effect of salmeterol and salmeterol plus beclomethasone on saliva flow and IgA in patients with moderate‐persistent chronic asthma. Annals of Allergy and Asthma Immunology 2001;87(5):420‐3. [DOI] [PubMed] [Google Scholar]
- Rio Navarro BE, Corona L, Fregosa R, Berber A, Magana J, Sienra‐Monge JL. Effect of salmeterol and salmeterol plus beclomethasone on the saliva flow and saliva IgA levels on patients with chronic moderate persistent asthma (CMPA). Journal of Allergy and Clinical Immunology 2001;107(2):s11. [DOI] [PubMed] [Google Scholar]
Dempsey 2000 {published data only}
- Dempsey OJ, Wilson AM, Sims EJ, Lipworth BJ. Additive anti‐inflammatory effects of montelukast but not salmeterol in asthmatics suboptimally controlled on inhaled steroids. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A198. [Google Scholar]
Dente 2001 {published data only}
- Dente FL, Scuotri L, Bacci E, DeSanctis M, Franco A, Giannini D, et al. Combined treatment with fluticasone plus salmeterol protects against allergen‐induced asthmatic responses better than each drug alone. European Respiratory Journal 2001;18 Suppl 33:349s. [Google Scholar]
- Dente FL, Scuotri L, Bacci E, Franco A, Giannini D, Taccola M, et al. Effects of combined treatment ‐ fluticasone plus salmeterol ‐ on allergen‐induced asthmatic responses. American Journal of Respiratory and Critical Care Medicine 2001;463(Suppl 5):A419. [Google Scholar]
Dicpinigaitis 2002 {published data only}
- Dicpinigaitis PV, Dobkin JB, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough‐variant asthma. Journal of Asthma 2002;39(4):291‐7. [DOI] [PubMed] [Google Scholar]
Didier 1997 {published data only}
- Didier A, Campos Oriola R. A two‐month comparison of salmeterol/beclomethasone and slow‐release terbutaline/budesonide in moderate asthma management. Clinical Drug Investigation 1997;14(1):1‐11. [Google Scholar]
Djordjevic 1999 {published data only}
- Djordjevic D, Zickovic D, Stankovic I, Pejcic T, Ducic J, Rancic M, et al. Comparative study of three months treatment in combination of salmeterol and beclomethasone dipropionate (BDP) with doubling the dose of BDP in mild asthma. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999:p849.
Dorinsky 2001 {published data only}
- Dorinsky P, Kalberg C, Pepsin P, Emmett A, Bowers B, Rickard K. The fluticasone/salmeterol combination product is superior to montelukast as first‐line asthma control. European Respiratory Journal 2001;18 Suppl 33:263s. [Google Scholar]
- Dorinsky PM, Kalberg C, Pepsin P, Emmett A, Rickard K. Greater onset of improvement in clinical efficacy measures with first line use of the fluticasone/salmeterol combination product compared to montelukast. Annual Thoracic Society 97th International Conference; San Francisco CA May 18‐23. 2001.
Dorinsky 2002 {published data only}
- Dorinsky P, Jones S, Kalberg C, Emmett A, Rickard K. Sustained protection against activity‐induced bronchospasm (AIB) during chronic treatment with the fluticasone propionate/salmeterol combination (FSC). American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A568. [Google Scholar]
Durham 1999 {published data only}
- Durham S. Long acting inhaled beta2‐agonists: Anti‐inflammatory effects not evident during treatment of day to day asthma. European Respiratory Journal 1999;14(2):249‐50. [DOI] [PubMed] [Google Scholar]
Ek 2000 {published data only}
- Ek A, Palmberg L, Larsson K. Influence of fluticasone and salmeterol on airway effects of inhaled organic dust; an in vivo and ex vivo study. Clinical & Experimental Immunology 2000;121(1):11‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eliraz 2001 {published data only}
- Eliraz A, Ramirez‐Rivera A, Ferranti P, Holzer R, Garcia JM, Turcotte C, et al. Similar efficacy following four weeks treatment of asthmatics with formoterol 12 mcg BD delivered by two different dry powder inhalers; differences in inhaler handling. International Journal of Clinical Practice 2001;55(3):164‐70. [PubMed] [Google Scholar]
Everden 2002 {published data only}
- Everden P, Lloyd A, Hutchinson J, Plumb J. Cost‐effectiveness of eformoterol Turbohaler(R) versus salmeterol Accuhaler(R) in children with symptomatic asthma. Respiratory Medicine 2002;96(4):250‐8. [DOI] [PubMed] [Google Scholar]
Faurschou 1994 {published data only}
- Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy 1994;49(40):827‐32. [DOI] [PubMed] [Google Scholar]
Faurschou 1996 {published data only}
- Faurschou P. Use of salmeterol in moderate to severe asthmatic patients already receiving high dose inhaled steroids. European Respiratory Journal 1993;6 Suppl 17:419s. [DOI] [PubMed] [Google Scholar]
- Faurschou P, Steffensen I, Jacques L. Effect of addition of inhaled salmeterol to the treatment of moderate‐to‐severe asthmatics uncontrolled on high‐dose inhaled steroids. European Respiratory Journal 1996;9(9):1885‐90. [DOI] [PubMed] [Google Scholar]
Fish 2001 {published data only}
- Fish J, Boone R, Emmett A, Yancey S, Knobil K, Rickard K. Salmeterol added to inhaled corticosteroids (ICS) provides greater asthma control compared to montelukast. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A203. [Google Scholar]
- Fish JE, Israel E, Murray JJ, Emmett A, Boone R, Yancey SW, et al. Salmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapy. Chest 2001;120(2):423‐30. [DOI] [PubMed] [Google Scholar]
Fitzgerald 1999 {published data only}
- Fitzgerald JM, Chapman KR, Cioppa GD, Stubbing D, Fairbarn MS, Till DT, et al. Sustained bronchoprotection, bronchodilatation and symptom control during regular formoterol use in asthma of moderate or greater severity. Journal of Allergy and Clinical Immunology 1999;103(3 pt 1):427‐35. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 1990 {published data only}
- Fitzpatrick MF, Mackay T, Driver H, Douglas NJ. Salmeterol in nocturnal asthma: a double‐blind, placebo controlled trial of long‐acting inhaled beta2 agonist. BMJ 1990;301:1365‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fowler 2002 {published data only}
- Fowler SJ, Currie PC, Lipworth BJ. Step down therapy with low dose fluticasone‐salmeterol combination or medium dose hydrofluoroalkane 134a‐beclomethasone alone. Journal of Allergy and Clinical Immunology 2002;109(6):929‐35. [DOI] [PubMed] [Google Scholar]
Fuglsang 1995 {published data only}
- Fuglsang G, Agertoft L, Vikre‐Jorgensen J, Pedersen S. Influence of budesonide on the response to inhaled terbutaline in children with mild asthma. Pediatric Allergy and Immunology 1995;6(2):103‐8. [DOI] [PubMed] [Google Scholar]
Garcia‐Marcos 2002 {published data only}
- Garcia‐Marcos L, Schuster A, Cobos Barroso N. Inhaled corticosteroids plus long‐acting beta(2)‐agonists as a combined therapy in asthma. Expert Opinion in Pharmacotherapy 2002;4(1):23‐39. [DOI] [PubMed] [Google Scholar]
Gardiner 1994 {published data only}
- Gardiner PV, Ward C, Booth H, Allison A, Hendrick DJ, Walters EH. Bronchoalveolar lavage inflammatory indices in asthmatics. American Journal of Respiratory & Critical Care Medicine 1994;150:1006‐11. [DOI] [PubMed] [Google Scholar]
Gessner 2003 {published data only}
- Gessner C, Stenglein S, Brautigam M, Muller A, Schauer J. Miflonide/Foradil via Aerolizer compared with other anti‐inflammatory and anti‐obstructive therapeutic regimens. Pneumologie 2003;57(3):137‐43. [DOI] [PubMed] [Google Scholar]
Giannini 1998a {published data only}
- Giannini D, Carletti A, Dente FL, Testi R, Bacci D, Bancalari L, et al. Effect of inhaled beclomethasone dipropionate (BDP) on tolerance to salmeterol (S) in allergen induced bronchoconstriction. European Respiratory Journal 1996;9(23):272s. [Google Scholar]
- Giannini D, Franco A, Bacci E, Conti I, Dente FL, Kotopulos C, et al. Long‐term treatment with salmeterol and inhaled corticosteroids does not induce tolerance to the protective effect of salmeterol on allergen challenge. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A414. [Google Scholar]
Giannini 1998b {published data only}
- Giannini D, Franco A, Bacci E, Conti I, Dente FL, Kotopulos C, et al. One‐week regular treatment with salmeterol induces tolerance to the protective effect of salmeterol on allergen challenge only in subjects not regularly treated with salmeterol and inhaled corticosteroid. European Respiratory Journal 1998;12 Suppl 28:156s. [Google Scholar]
Giannini 1999 {published data only}
- Giannini D, Bacci E, Dente FL, Franco A, Vagaggini B, Testi R, et al. Inhaled beclomethasone dipropionate reverts tolerance to the protective effect of salmeterol on allergen challenge. Chest 1999;115(3):629‐34. [DOI] [PubMed] [Google Scholar]
Giannini 2000 {published data only}
- Giannini D, Franco A, Bacci E, Dente FL, Taccola M, Vagaggini B, et al. The protective effect of salbutamol inhaled using different devices on methacholine bronchoconstriction. Chest 2000;117(5):1319‐23. [DOI] [PubMed] [Google Scholar]
Giannini 2001 {published data only}
- Giannini D, Tonelli M, Franco A, Bacci E, Conti I, Dente FL, et al. Tolerance to the protective effect of salmeterol on allergen challenge in mild untreated asthmatics and in moderate asthmatics on inhaled corticosteroid treatment. European Respiratory Journal 2001;18 Suppl 33:103s. [Google Scholar]
Giannini 2002 {published data only}
- Giannini D, Tonelli M, Franco A, Bacci E, Conti I, Dente FL, et al. Tolerance to the protective effect of salmeterol + fluticasone combination (50/250 µg) on allergen challenge in mild untreated asthmatics. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A566. [Google Scholar]
Gizycki 2000 {published data only}
- Gizycki MJ, Venge P, Dahl R, Jeffery PK. Comparison of the effects of six weeks treatment with fluticasone or salmeterol on the late phase response (LPR) in mild asthma ‐ a bronchial biopsy study. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A203. [Google Scholar]
Gold 2001 {published data only}
- Gold M, Jõgi R, Mulder PGH, Akveld MLM. Salmeterol/fluticasone propionate combination 50/100µg bid is more effective than fluticasone propionate 100µg bid plus montelukast 10 mg once daily in reducing exacerbations. European Respiratory Journal 2001;18 Suppl 33:262s. [Google Scholar]
Green 2003 {published data only}
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Pavord ID. Placebo controlled comparison of formoterol, montelukast or higher dose of inhaled budesonide in subjects with symptomatic asthma despite treatment with low dose inhaled budesonide. American Thoracic Society 99th International Conference. 2003:B036 [Poster H82].
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Wardlaw AJ, et al. A placebo controlled comparison of formoterol, montelukast or higher dose of inhaled corticosteroids in subjects with symptomatic asthma despite treatment with low dose inhaled corticosteroids. Thorax 2002;57(Supp III):iii 11. [Google Scholar]
Greening 1994 {published data only}
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher‐dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994;344:219‐24. [DOI] [PubMed] [Google Scholar]
- Hyland ME, Crocker GR. Validation of an asthma quality of life diary in a clinical trial. Thorax 1995;50(7):724‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grootendorst 2001 {published data only}
- Grootendorst DC, Dahlen SE, Bos JW, Duiverman EJ, Veselic‐Charvat M, Vrijlandt EJ, et al. Benefits of high altitude allergen avoidance in atopic adolescents with moderate to severe asthma, over and above treatment with high dose inhaled steroids. Clinical and Experimental Allergy 2001;31(3):400‐8. [DOI] [PubMed] [Google Scholar]
Gustafsson 1994 {published data only}
- Gustafsson PM, Von BA, Jenkins MM. Salmeterol 50 mug twice daily in the treatment of mild‐to‐moderate asthma in childhood ‐ a comparison of two inhalation devices. European Journal of Clinical Research 1994;5:63‐73. [Google Scholar]
Hacki 2001 {published data only}
- Hacki M, Knoblauch A, Leuppi J. Asthma treatment. Pharma‐Kritik 2001;23(16):61‐4. [Google Scholar]
Hasani 2003 {published data only}
- Hasani A, Toms N, O'Connor J, Dilworth JP, Agnew JE. Effect of salmeterol xinafoate on lung mucociliary clearance in patients with asthma. Respiratory Medicine 2003;97(6):667‐71. [DOI] [PubMed] [Google Scholar]
Heuck 2000 {published data only}
- Heuck C, Heickendorff L, Wolthers OD. A randomized controlled trial of short term growth and collagen turnover in asthmatics with inhaled formoterol and budesonide. Archives of Disease in Childhood 2000;83:334‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuck C, Heickendorff L, Wolthers OD, Sygehus S. Short term growth and collagen turnover in asthmatics treated inhaled formoterol and budesonide. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999:364.
Heyneman 2002 {published data only}
- Heyneman CA, Crafts R, Holland J, Arnold AD. Fluticasone versus salmeterol/low‐dose fluticasone for long‐term asthma control. Annals of Pharmacotherapy 2002;36(12):1944‐9. [DOI] [PubMed] [Google Scholar]
Hultquist 2000 {unpublished data only}
- AstraZeneca. Personal communication 2000.
Ind 2002 {published data only}
- Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. European Respiratory Journal 2002;20(4):859‐66. [DOI] [PubMed] [Google Scholar]
Ind 2003 {published data only}
- Ind P, Haughney J, Price D, Rosen JP, Kennelly J. Four months adjustable or fixed BD dosing with budesonide/formoterol in a single inhaler reduces symptom severity. Thorax 2002;57(Suppl 3):iii 88. [Google Scholar]
- Ind PW, Dal Negro R, Colman N, Fletcher CP, Browning DC, James MH. Inhaled fluticasone propionate and salmeterol in moderate adult asthma I: lung function and symptoms. American Journal of Respiratory and Critical Care Medicine. 1998;157(Suppl 3):A416. [Google Scholar]
- Ind PW, Dal Negro R, Colman N, Fletcher CP, Browning DC, James MH. Inhaled fluticasone propionate and salmeterol in moderate adult asthma II: exacerbations. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A415. [Google Scholar]
- Ind PW, Dal Negro R, Colman NC, Fletcher CP, Browning D, James MH. Addition of salmeterol to fluticasone propionate treatment in moderate to severe asthma. Respiratory Medicine 2003;97:555‐62. [DOI] [PubMed] [Google Scholar]
Isabelle 2001 {published data only}
- Isabelle P, Bjamer D, Neuparth N, Desfougeres JL. Efficacy and safety of salmeterol/fluticasone combination 50/100mug bd via two different powder devices in children. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
Jarvis 1999 {published data only}
- Jarvis B, Faulds D. Inhaled fluticasone propionate. A review of its therapeutic efficacy at dosages <= 500 mug/day in adults and adolescents with mild to moderate asthma. Drugs 1999;57(5):769‐803. [DOI] [PubMed] [Google Scholar]
Jeffery 2002 {published data only}
- Jeffery PK, Venge P, Gizycki MJ, Egerod I, Dahl R, Faurschou P. Effects of salmeterol on mucosal inflammation in asthma: a placebo‐controlled study. European Respiratory Journal 2002;20(6):1378‐85. [DOI] [PubMed] [Google Scholar]
Jenkins 1995 {published data only}
- Jenkins M. Clinical evaluation of CFC‐free metered dose inhalers. Journal of Aerosol Medicine 1995;8(Suppl 1):s41‐7. [DOI] [PubMed] [Google Scholar]
Jenkins 2000 {published data only}
- Becker I, Kielborn A, Price MJ, Volmer T, Lloyd AC. Cost‐effectiveness of salmeterol/fluticasone combination product and budesonide in asthma patients in Germany. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999:854.
- Jenkins C, Woolcock A, James M. Superior overall control of moderate to severe asthma with salmeterol/fluticasone propionate (FP) combination (50/250 mcg bd) compared with three‐fold‐higher dose of budesonide (800mcg bd). European Respiratory Journal 2000;16 Suppl 31:456s. [Google Scholar]
- Jenkins C, Woolcock AJ, Saarelainen P, Lundback B, James MH. Salmeterol /fluticasone propionate combination therapy 50/250mcgs twice daily is more effective than budesonide 800 twice daily in treating moderate to severe asthma. Repiratory Medicine 2000;94:715‐23. [DOI] [PubMed] [Google Scholar]
- Lundback B, Jenkins C, Price MJ, Thwaites RM. Cost‐effectiveness of salmeterol/fluticasone propionate combination product 50/250 microg twice daily and budesonide 800 microg twice daily in the treatment of adults and adolescents with asthma. Respiratory Medicine 2000;94(7):724‐32. [DOI] [PubMed] [Google Scholar]
- Lundback B, Ronmark E, Jonsson AC. Treatment effectiveness and exacerbations during one year with Seretide compared to fluticasone propionate and salmeterol in mild to moderate asthma. European Respiratory Journal 2001;18 Suppl 33:176s. [Google Scholar]
- Parnaby A, Lloyd A, Browning D, McCarthy TP. A comparison of the cost‐effectiveness of salmeterol/fluticasone combination inhaler and budesonide in the management of asthma. Thorax 2000;55(Suppl 3):A64. [Google Scholar]
- SAS40006. A randomised, double‐blind, double‐dummy, parallel‐group comparison of seretide diskus/accuhaler (50/250µg Strength) b.i.d. with budesonide 800µg b.i.d. in adolescents and adults with reversible airways obstruction. http://www.ctr.gsk.co.uk 2004.
Jenkins 2002 {published data only}
- Jenkins C. Combination therapy with fluticasone and salmeterol for symptomatic asthma produces similar benefits when given by accuhaler in a single or two separate devices over 24 weeks. Respirology 2002;7(Suppl):A20 [P22]. [Google Scholar]
- Jenkins C, Wilson J, Rutherford C, Perry AS, Whitehead PJ. Asthma management costs are lower with combination fluticasone/salmeterol (25/50 mcg BD) in a single inhaler than with budesonide (800 mcg BD) plus eformoterol (12 mcg BD) via separate inhalers. Respirology 2002; Vol. 7, issue Suppl:A20 [P23].
Johansson 2001 {published data only}
- Johansson G, McIvor RA, D'Ambrosio FP, Gratziou C, James MH. Comparison of salmeterol/fluticasone propionate combination with budesonide in patients with mild‐to‐moderate asthma. Clinical Drug Investigation 2001;21(9):633‐42. [Google Scholar]
Johnson 1998 {published data only}
- Cook D, Srebro SH, Rogenes PR, Rickard K, Edwards L, Johnson MC. A comparison of the safety and efficacy of fluticasone, triamcinolone, and fluticasone plus salmeterol in patients with mild to moderate asthma. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A416. [Google Scholar]
- Johnson MC, Srebro SH, Rogenes PR, Rickard K, Edwards L. A comparison of physician‐rated and patient‐rated outcomes in a study with fluticasone, triamcinolone, and fluticasone plus salmeterol. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A414. [Google Scholar]
Jones 1994 {published data only}
- Jones KP. Salmeterol xinafoate in the treatment of mild to moderate asthma in primary care. Thorax 1994;49:971‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juniper 1995 {published data only}
- Juniper EF, Johnston PR, Borkhoff CM, Guyatt GH, Boulet LP, Haukioja A. Quality of life in asthma clinical trials: comparison of salmeterol and salbutamol. Amercian Journal of Respiratory and Critical Care Medicine 1995;151(1):66‐70. [DOI] [PubMed] [Google Scholar]
Juniper 1999 {published data only}
- Juniper EF, Svenson K, O'Byrne PM, Barnes PJ, Bauer CA, Lofdahl CG, et al. Asthma quality of life during 1 year of treatment with budesonide with or without formoterol. European Respiratory Journal 1999;14:1038‐43. [DOI] [PubMed] [Google Scholar]
Kalberg 1998 {published data only}
- Kalberg CJ, Nelson H, Yancey S, Petrocella V, Emmett AH, Rickard KA, et al. A comparison of added salmeterol versus increased‐dose fluticasone in patients symptomatic on low‐dose fluticasone [abstract]. Journal of Allergy and Clinical Immunology 1998;101(Suppl):S6. [Google Scholar]
Kalra 1996 {published data only}
- Kalra S, Swystun VA, Bhagat R, Cockcroft DW. Inhaled corticosteroids do not prevent the development of tolerance to the bronchoprotective effect of salmeterol. Chest 1996;109(4):953‐6. [DOI] [PubMed] [Google Scholar]
Kardos 2001 {published data only}
- Kardos P, Bruggenjurgen B, Martin A, Meyer‐Sabellek W, Richter K, et al. Treatment of bronchial asthma using a new adjustable combination treatment plan: Asthma Control Plan (ATACO). Pneumologie. 2001;55(5):253‐7. [DOI] [PubMed] [Google Scholar]
Kavuru 2000 {published data only}
- Edin HM, Payne E, Herrle MR, Schoaf L, Mather DB, Scott CA, et al. Salmeterol/fluticasone propionate combination via HFA MDI improves quality of life in asthma patients. Journal of Allergy and Clinical Immunology 2001;107(2):s246. [Google Scholar]
- Edin HM, Prillaman B, Baitinger LA, House K, Shah TP. Improved ability to perform strenuous activities after treatment with fluticasone propionate‐salmeterol combination. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A112. [Google Scholar]
- Edwards T, Gross G, Mitchell D, Chervinsky P, Woodring A, Baitinger L, et al. The salmeterol xinafoate/fluticasone propionate dry powder combination product via diskus® inhaler improves asthma control compared to salmeterol xinafoate or fluticasone propionate dry powder alone. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A414. [Google Scholar]
- Gross G, Woodring A, Prillaman B, House K, Shah T. Efficacy and safety of the salmeterol/fluticasone propionate (50/100 µg) dry powder combination inhaler in patients with asthma. European Respiratory Journal 1998;12 Suppl 28:156s. [Google Scholar]
- Johansson G, Price MJ, Sondhi S. Cost‐effectiveness analysis of salmeterol/fluticasone propionate 50/100mug vs fluticasone propionate 100mug in adults and adolescents with asthma III. Pharmacoeconomics 1999;16(Suppl 2):15‐21. [Google Scholar]
- Kavuru M, Melamed J, Gross G, Laforce C, House K, Prilaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomised, double blind,placebo controlled trial. Journal of Allergy & Clinical Immunology 2000;105(6):1108‐16. [DOI] [PubMed] [Google Scholar]
- Nathan RA, Dorinsky P, Carranza JR, Rosenzweig C, Shah T, et al. Improved ability to perform strenuous activities after treatment with fluticasone propionate/salmeterol combination in patients with persistent asthma. Journal of Asthma 2003;40(7):815‐22. [DOI] [PubMed] [Google Scholar]
Keith 2001 {published data only}
- Keith P, D Urzo A, Stepner N. Fluticasone/salmeterol combination (FSC) is safe and provides effective long‐term (52 week) control in the management of patients with persistent asthma (PA). European Respiratory Journal 2001;18(Suppl 33):176s. [Google Scholar]
Kelsen 1999 {published data only}
- Kelsen SG, Church NL, Gillman SA, Lanier BQ, Emmett AH, Rickard KA, et al. Salmeterol added to inhaled corticosteroids therapy is superior to doubling the dose of inhaled corticosteroids: a randomized clinical trial. Journal of Asthma 1999;36(8):703‐15. [DOI] [PubMed] [Google Scholar]
Kemp 1984 {published data only}
- Kemp JP, Chervinsky P, Orgel HA, Meltzer EO, Noyes JH, et al. Concomitant bitolterol mesylate aerosol and theophylline for asthma therapy, with 24 hr electrocardiographic monitoring. Journal of Allergy & Clinical Immunology 1984;73(1 pt 1):32‐43. [DOI] [PubMed] [Google Scholar]
Kemp 1998 {published data only}
- Kemp JP, Cook DA, Incaudo GA, Corren J, Kalberg C, Emmett A, et al. Salmeterol improves quality of life in patients with asthma requiring inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1998;101:188‐95. [DOI] [PubMed] [Google Scholar]
Ketchell 2002 {published data only}
- Ketchell RI, Jensen MW, Spina D, O'Connor BJ. Dose‐related effects of formoterol on airway responsiveness to adenosine 5'‐monophosphate and histamine. European Respiratory Journal 2002;19(4):611‐6. [DOI] [PubMed] [Google Scholar]
Kidney 1995 {published data only}
- Kidney J, Pizzichini MMM, Wong B, Morris MM, Efthimadis A, Dolovich J, et al. Salmeterol compared with beclomethasone and placebo on allergen induced asthmatic and inflammatory responses. European Respiratory Journal. 1995;19(Suppl 8):336s. [DOI] [PubMed] [Google Scholar]
Kips 2000 {published data only}
- Kips JC, O'Connor BJ, Inman MD, Svenson K, Pauwels RA, O'Byrne PM. A long‐term study of the antiinflammatory effect of low‐dose budesonide plus formoterol versus high‐dose budesonide in asthma. American Journal of Respiratory and Critical Care Medicine 2000;161:996‐1001. [DOI] [PubMed] [Google Scholar]
Kirby 2000 {published data only}
- Kirby S, Falcoz C, Daniel MJ, Milleri S, Squassante L, Ziviani L, et al. Salmeterol and fluticasone propionate given as a combination: lack of systemic pharmacodynamic and pharmacokinetic interactions. European Journal of Clinical Pharmacology 2000;56(11):781‐91. [DOI] [PubMed] [Google Scholar]
Knobil 1998 {published data only}
- Knobil K, Kalberg C, Emmett A, Rickard K. Adding salmeterol is more effective than increasing the dose of fluticasone for patients with asthma who are symptomatic on low dose fluticasone. European Respiratory Journal 1998;12 Suppl 29:19s [P160]. [Google Scholar]
Knobil 2000 {published data only}
- Knobil K, Dorinsky P, Yancey S, Emmett A, Rickard K. Salmeterol is superior to montelukast as add‐on therapy to inhaled corticosteroids. European Respiratory Journal 2000;16 Suppl 31:457s. [Google Scholar]
Knorr 2001 {published data only}
- Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001;108(3):E48. [DOI] [PubMed] [Google Scholar]
Kraft 2003 {published data only}
- Kraft M, Martin RJ, Lazarus SC, Fahy JV, Boushey HA, Lemanske Jr RF, et al. Airway tissue mast cells in persistent asthma: predictor of treatment failure when patients discontinue inhaled corticosteroids. Chest 2003;124(1):42‐50. [DOI] [PubMed] [Google Scholar]
LaForce 1994 {published data only}
- LaForce C, Liddle RF, Yancey SW. Salmeterol response in asthmatic patients using inhaled corticosteroids and in those not using inhaled corticosteroids. Annals of Allergy 1994;72:100. [Google Scholar]
Lai 1995 {published data only}
- Lai CKW, Chan CHS, Ho SS, Hui ACF, Lai KN. Inhaled salmeterol and albuterol in asthmatic patients receiving high‐dose inhaled corticosteroids. Chest 1995;108:36‐40. [DOI] [PubMed] [Google Scholar]
Lalloo 2003 {published data only}
- Lalloo UG, Bantje TA, Kozma D, Krofta K, Ankerst J, Johansen B, et al. Low‐dose Symbicort (budesonide / formoterol) is more effective than double‐dose inhaled corticosteroid in mild asthma. Allergy Clinical Immunology International. 122 2000; Vol. Suppl 2.
- Lalloo UG, Malolepsky J, Kozma D, Krofta K, Ankerst J, Johansen B, et al. Budesonide and formoterol in a single inhaler controls exacerbations more effectively than a higher dose of inhaled corticosteroids alone, in mild‐moderate persistent asthma. European Respiratory Journal 2001;18 Suppl 33:43s. [Google Scholar]
- Lalloo UG, Malolepszy D, Kozma K, Krofta J, Ankerst B, Johanasen NC, et al. Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild to moderate asthma. Chest 2003;123:1480‐7. [DOI] [PubMed] [Google Scholar]
- Lalloo UG, Malolepszy J, Kozma D, Krofta K, Ankerst J, Johansen B, et al. Budesonide and formoterol in a single inhaler is more effective than a higher dose of inhaled corticosteroid in mild‐moderate persistent asthma. European Respiratory Journal 2001;18 Suppl 33:159s. [Google Scholar]
- Lalloo UG, Malolepszy J, Kozma D, Krofta K, Ankerst J, Johansen B, et al. Symbicort® (budesonide and formoterol in a single inhaler) is more effective than increasing the dose of inhaled corticosteroids in mild asthma. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
Lange 2001 {published data only}
- Lange ML, House KW, Scott CA, Shah TP, Akveld MLM. The salmeterol/fluticasone propionate combination 50/100µg bid is effective as initial maintenance therapy in mild and moderate asthmatics. European Respiratory Journal 2001;18 Suppl 33:176s. [Google Scholar]
Langton‐Hewer 1995 {published data only}
- Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrims progress: the effect of salmeterol in older children with chronic severe asthma. Respiratory Medicine 1995;89:435‐40. [DOI] [PubMed] [Google Scholar]
Lazarus 2001 {published data only}
- Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, et al. Long‐acting beta2 agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285(20):2583‐93. [DOI] [PubMed] [Google Scholar]
Leblanc 1996 {published data only}
- Leblanc P, Knight A, Kreisman H, Borkhoff CM, Johnston PR. A placebo‐controlled, crossover comparison of salmeterol and salbutamol in patients with asthma. American Journal of Respiratory and Critical Care Medicine 1996;154:324‐8. [DOI] [PubMed] [Google Scholar]
Lemanske 2001 {published data only}
- Lemanske RF, Sorkness C, Mauger E, Lazarus S, Boushey H, Fahy J, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001;285(20):2594‐603. [DOI] [PubMed] [Google Scholar]
Lenney 1995 {published data only}
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins MM, on behalf of an International Study Group. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154:983‐90. [DOI] [PubMed] [Google Scholar]
LHSRG 2000 {published data only}
- The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1902‐9. [DOI] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li X, Ward C, Thien F, Bish R, Bamford T, Bao X, et al. An antiinflammatory effect of salmeterol, a long‐acting B2‐agonist, assessed in airway biopsies and bronchoalveolar lavage in asthma. American Journal of Respiratory and Crtitical Care Medicine 1999;160:1493‐9. [DOI] [PubMed] [Google Scholar]
- Orsida BE, Ward C, Li X, Bish R, Wilson JW, Thien F, et al. Effect of a long‐acting beta(2)‐agonist over three months on airway wall vascular remodeling in asthma. American Journal of Respiratory and Critical Care Medicine 2001;164(1):117‐21. [DOI] [PubMed] [Google Scholar]
- Reid DW, Ward C, Wang N, Zheng L, Bish R, Orsida B, et al. Possible anti‐inflammatory effect of salmeterol against interleukin‐8 and neutrophil activation in asthma in vivo. European Respiratory Journal 2003;21(6):994‐9. [DOI] [PubMed] [Google Scholar]
Lindqvist 2001 {published data only}
- Lindqvist AE, Karjalainen EM, Laitinen LA, Kava T, Altraja A, Pulkkinen M, et al. Salmeterol (sim), fluticasone propionate (fp) or disodium cromoglycate (dscg) in the treatment of newly diagnosed asthma. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
Lipworth 1998 {published data only}
- Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. American Journal of Medicine 1998;104(5):431‐8. [DOI] [PubMed] [Google Scholar]
Lipworth 1999 {published data only}
- Lipworth BJ. Does genetic polymorphism of b2‐adrenoceptors determine airway sensitivity to regular long‐acting b2‐agonist therapy?. Clinical Pharmacology, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, Scotland UK (Tayside Research Consortium).
- Lipworth BJ, Hall IP, Aziz I, Tan KS, Wheatley A. Beta2‐adrenoceptor polymorphism and bronchoprotective sensitivity with regular short‐ and long‐acting beta2‐agonist therapy. Clinical Science 96;3:253‐9. [PubMed] [Google Scholar]
Lipworth 2000a {published data only}
- Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous glycine‐16 beta(2)‐adrenoceptor polymorphism. Chest 2000;118(2):321‐8. [DOI] [PubMed] [Google Scholar]
Lipworth 2000b {published data only}
- Lipworth BJ, Dempsey OJ, Aziz I, Wilson AM. Effects of adding a leukotriene antagonist or a long‐acting beta(2)‐agonist in asthmatic patients with the glycine‐16 beta(2)‐adrenoceptor genotype. American Journal of Medicine 2000;109(2):114‐21. [DOI] [PubMed] [Google Scholar]
Lockey 1999 {published data only}
- Lockey RF, DuBuske LM, Friedman B, Petrocella V, Cox F, Rickard K. Nocturnal asthma: effect of salmeterol on quality of life and clinical outcomes. Chest 1999;115(3):666‐73. [DOI] [PubMed] [Google Scholar]
Lötvall 2002 {published data only}
- Lötvall J, Woude HJ, Palmqvist M, Arvidsson P, Beckman O, Boorsma M, et al. More rapid onset of action of budesonide/formoterol (Symbicort®) than salmeterol/fluticasone (Seretide™). American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A567. [Google Scholar]
Lowhagen 2002 {published data only}
- Lowhagen O, Wever AMJ, Lusuardi M, Moscato G, Backer WA, Gandola L, et al. The inflammatory marker serum eosinophil cationic protein (ECP) compared with PEF as a tool to decide inhaled corticosteroid dose in asthmatic patients. Respiratory Medicine 2002;96(2):95‐101. [DOI] [PubMed] [Google Scholar]
Lundbäck 2006 {published and unpublished data}
- FAS40008. An interventional three year study for asthma control ‐ In what way and in what kind of population is it possible to get asthmatic patients free from symptoms, keep the patients in work, restore a normal lung function, diminish hyperreactivity and normalise quality of life?. http:www.ctr.gsk.co.uk 2005.
- Lundback B, Ronmark E, Jonsson AC, Larsson LG, Lindberg A, Petavy F, et al. Fluticasone propionate/salmeterol combination improves airway hyperresponsiveness and clinical outcomes compared with fluticasone or salmeterol alone in mild to moderate asthma. American Thoracic Society 99th International Conference. 2003:D034 [Poster C28].
- Lundbäck B, Rönmark E, Lindberg A, Jonsson A‐C, Larsson L‐G, Pétavy F, et al. Control of mild to moderate asthma over 1‐year with the combination of salmeterol and fluticasone propionate. Respiratory Medicine 2006;100(1):2‐10. [DOI] [PubMed] [Google Scholar]
Magadle 2001 {published data only}
- Magadle R, Berar‐Yanay N, Weiner P. Long‐acting bronchodilators in premenstrual exacerbation of asthma. Respiratory Medicine 2001;95(9):740‐3. [DOI] [PubMed] [Google Scholar]
Malmqvist‐Granlund 2000 {published data only}
- Malmqvist‐Granlund K, Asking L, Lindbald T, Rollwage U, Steckel H. An in vitro comparison of budesonide/formoterol and fluticasone/salmeterol in dry powder inhalers. European Respiratory Journal 2000;16 Suppl 31:455s. [Google Scholar]
Malolepszy 2002 {published data only}
- Malolepszy J. Efficacy and tolerability of oral theophylline slow‐release versus inhaled formoterol in moderate asthma poorly controlled on low‐dose steroids. Atemwege und Lungenkrankheiten 2002;28(2):78‐87. [Google Scholar]
Matz 2001 {published data only}
- Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low‐dose fluticasone versus higher‐dose fluticasone: an analysis of asthma exacerbations. Journal of Allergy and Clinical Immunology 2001;107(5):783‐9. [DOI] [PubMed] [Google Scholar]
McCarthy 2000 {published data only}
- McCarthy TP, Boone R, Yancey S, Rickard K. Salmeterol compared to montelukast as adjunctive therapy to inhaled corticosteroids. Thorax 2000;55(Suppl 3):A63. [Google Scholar]
McCarthy 2001 {published data only}
- McCarthy TP, Edin HM, House K, Vandermeer AK. Quality of life and asthma control assessment in patients previously on inhaled corticosteroids (ICS) treated with salmeterol/fluticasone combination (SFC) metered dose inhaler (MDI). Thorax 2001;56(Suppl 3):iii63. [Google Scholar]
- McCarthy TP, Edin HM, House K, Vandermeer AK. Salmeterol/fluticasone 50/100 (SFC) dry powder (DPI) provides improved control and quality of life in patients symptomatic on inhaled corticosteroids (ICS). European Respiratory Journal 2002;20 Suppl 38:47s. [Google Scholar]
- McCarthy TP, Edin HM, House K, Vandermeer AK, Scott C. Low dose salmeterol/fluticasone propionate combination (SFC) via metered dose inhaler (MDI) improves asthma control and quality of life in patients not well controlled on inhaled steroids (ICS). European Respiratory Journal 2002;20 Suppl 38:47s. [Google Scholar]
- McCarthy TP, Edin HM, House K, Yan SK, Vandermeer AK. The effects of salmeterol/fluticasone combination (SFC) dry powder inhaler (DPI) on asthma control and quality of life in patients previously treated with inhaled corticosteroids (ICS). Thorax 2001;56(Suppl 3):iii63. [Google Scholar]
Mcivor 1998 {published data only}
- Mcivor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 1998;158(3):924‐30. [DOI] [PubMed] [Google Scholar]
Meier 1997 {published data only}
- Meier CR, Jick H. Drug use and pulmonary death rates in increasingly symptomatic asthma patients in the UK. Thorax 1997;52(7):612‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meijer 1995 {published data only}
- Meijer FG, Postma DS, Mulder PGH, Aalderen WMC. Long‐term circadian effects of salmeterol in asthmatic children treated with inhaled corticosteroids. American Journal of Respiratory and Critical Care Medicine 1995;152:1887‐92. [DOI] [PubMed] [Google Scholar]
Michel 2000 {published data only}
- Michel O, Olbrecht J, Moulard D, Sergysels R. Effect of anti‐asthmatic drugs on the response to inhaled endotoxin. Annals of Allergy, Asthma, & Immunology 2000;85(4):305‐10. [DOI] [PubMed] [Google Scholar]
Midgren 1992 {published data only}
- Midgren B, Melander B Persson G. Formoterol, a new long‐acting beta 2 agonist, inhaled twice daily, in stable asthmatic subjects. Chest 1992;101(4):1019‐22. [DOI] [PubMed] [Google Scholar]
Mitchell 2000 {published data only}
- Mitchell C, Jenkins C, Scicchitano R, Rubinfeld A. Adding formoterol is more effective and safer than doubling the dose of inhaled steroids in moderately severe asthma. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A197. [Google Scholar]
Molimard 2001 {published data only}
- Molimard M, Bourcereau J, Gros V, Bourdeix I, Leynadier F, Duroux P. Comparison between formoterol 12 ug bid and on‐demand salbutamol in moderate persistent asthma. Respiratory Medicine 2001;95(1):64‐70. [DOI] [PubMed] [Google Scholar]
Murray 1998 {published data only}
- Murray JJ, Hagaman DD, Dworski R, Keane B, Sheller JR. Inhibition by salmeterol and beclomethasone of late phase response to segmental antigen challenge in asthmatics. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A872. [Google Scholar]
Murray 1999 {published data only}
- Murray JJ, Church NL, Anderson WH, Bernstein DI, Wenzel SE, Emmett A, et al. Concurrent use of Salmeterol with inhaled corticosteroids is more effective than inhaled corticosteroid dose increases. Allergic Asthma Proceedings 1999;20(3):173‐80. [DOI] [PubMed] [Google Scholar]
Nagel 2002 {published data only}
- Nagel MW, Wiersema KJ, Bates SL, Mitchell JP. Performance of large‐ and small‐volume valved holding chambers with a new combination long‐term bronchodilator/anti‐inflammatory formulation delivered by pressurized metered dose inhaler. Journal of Aerosol Medicine 2002;15(4):427‐33. [DOI] [PubMed] [Google Scholar]
Nathan 1995 {published data only}
- Nathan RA, Seltzer JM, Kemp JP, Chervinsky P, Alexander WJ, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma and Immunology 1995;75:243‐8. [PubMed] [Google Scholar]
Nathan 1999 {published data only}
- Nathan R, Woodring A, Baitinger L, Prillaman B, Faris M, House K, et al. The salmeterol/fluticasone propionate diskus combination decreases the incidence of exacerbations compared to treatment with salmeterol or fluticasone propionate alone. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999:848.
- Nathan RA, Pinnas JL, Schwartz HJ, Grossman J, Yancey SW, Emmett AH, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy, Asthma, & Immunology 1999;82:521‐9. [DOI] [PubMed] [Google Scholar]
Nathan 2006 {published and unpublished data}
- Edin HM, Payne E, Herrle MR, Schoaf L, Mather DB, Scott CA, et al. Salmeterol/fluticasone propionate combination via HFA MDI improves quality of life. Journal of Allergy & Clinical Immunology 2001;107(2):S246. [Google Scholar]
- Nathan RA, Mitchell D, Condemi J, Heller A, Schoaf L, Herrle M, et al. Cardiovascular and hypothalamic‐pituitary‐adrenal axis safety of fluticasone propionate/salmeterol HFA MDI in adolescent and adult patients with asthma. American Journal for Respiratory and Critical Care Medicine 2001;163(5):A863. [Google Scholar]
- Nathan RA, Rooklin A, Schoaf L, Scott C, Ellsworth A, House K, et al. Efficacy and tolerability of fluticasone propionate/salmeterol administered twice daily via hydrofluoroalkane 134a metered‐dose inhaler in adolescent and adult patients with persistent asthma: a randomized, double‐blind, placebo‐controlled, 12‐week study. Clinical Therapeutics 2006;28(1):73‐85. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Kent E, Lanz MJ, Peden D, Baitinger L, Herrle M, et al. Fluticasone propionate/salmeterol HFA MDI has a rapid onset of effect in asthmatics treated with short or long‐acting beta‐agonists (BA) or inhaled corticosteroids (ICS). American Journal of Respiratory and Critical Care Medicine 2001;163(5):A865. [Google Scholar]
- Rooklin A, Elkayam D, Weiler J, Windom H, Schoaf L, Scott C, et al. The fluticasone propionate/salmeterol HFA MDI is significantly more efficacious in treating asthma than placebo HFA MDI, fluticasone propionate CFC MDI or salmeterol CFC MDI. Journal of Allergy and Clinical Immunology 2001;107(2):100s. [Google Scholar]
- SAS30004. A randomized, double‐blind, placebo‐controlled, parallel‐group 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in GR106642X MDI, 50/250mcg BID, and salmeterol in propellant 11/12 MDI, 50mcg BID, fluticasone propionate in propellant 11/12 MDI, 250mcg BID, and placebo propellant GR106642X MDI in adult and adolescent subjects with asthma. http://ctr.gsk.co.uk 2005.
Nelson 1999 {published data only}
- Nelson HS, Berkowitz RB, Tinkelman DA, Emmett AH, Rickard KA, Yancey SW. Lack of sub‐sensitivity to albuterol after treatment with salmeterol in patients with asthma. American Journal of Respiratory and Critical Care Medicine 1999;159(5 pt 1):1556‐61. [DOI] [PubMed] [Google Scholar]
Nelson 2000 {published data only}
- Nelson H, Chervinsky P, Greos L, Pelskow W, Baitinger L, Scott C, et al. The salmeterol/fluticasone propionate combination product improves asthma control compared with the individual products in asthmatics treated with prn short‐acting beta2‐agonists alone. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A196. [Google Scholar]
- Nelson HS, Baitinger L, Scott C, House K, Payne E, Shah T. Salmeterol/fluticasone propionate (50/100µg dose) non‐CFC metered dose inhaler is safe and effective in patients with asthma using short‐acting ß²‐agonists alone. European Respiratory Journal 2000;16(31):53s. [Google Scholar]
- Nelson HS, Busse WW, Kerwin E, Church N, Emmett A, Rickard K, et al. Fluticasone propionate/salmeterol combination provides more effective asthma control than low‐dose inhaled corticosteroid plus montelukast. Journal Allergy and Clinical Immunology 2000;106(6):1088‐95. [DOI] [PubMed] [Google Scholar]
Nelson 2001 {published data only}
- Nelson HS, Nathan RA, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in asthmatic patients using concomitant inhaled corticosteroids. Medscape General Medicine 2001;3(4):3. [PubMed] [Google Scholar]
Newnham 1995 {published data only}
- Newnham‐DM, Grove A, McDevitt DG, Lipworth‐BJ. Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax 1995;50(5):497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 1999 {published data only}
- Nielsen LP, Pedersen B, Faurschou P, Madsen F, Wilcke JTR, Dahl R. Salmeterol reduces the need for inhaled corticosteroids in steroid‐dependent asthmatics. Respiratory Medicine 1999;93:863‐8. [DOI] [PubMed] [Google Scholar]
Nightingale 2002 {published data only}
- Nightingale JA, Rogers DF, Barnes PJ. Comparison of the effects of salmeterol and formoterol in patients with severe asthma. Chest 2002;121(5):1401‐6. [DOI] [PubMed] [Google Scholar]
Norhaya 1999 {published data only}
- Norhaya MR, Yap TM, Zainudin BMZ. Addition of inhaled salmeterol to inhaled corticosteroids in patients with poorly controlled nocturnal asthma. Repirology 1999;4:77‐81. [DOI] [PubMed] [Google Scholar]
Nsouli 2001 {published data only}
- Nsouli SM, McNutt WJ. The additive effects of montelukast and salmeterol in moderate asthmatics who are uncontrolled on a low dose on inhaled corticosteroids. Annals of Allergy, Asthma & Immunology 2001;86:81. [Google Scholar]
O'Brian 2001 {published data only}
- O'Brian J, Carlos‐Palma A, Bogolubov M, Davies P, Payne E. Benefits of fluticasone propionate/salmeterol [fp/s] HFA MDI are apparent on the first day of dosing. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
O'Byrne 2005 {published data only}
- Bruce SA, Scherer YK. Maintenance and symptom relief with budesonide plus formoterol reduced severe asthma exacerbations. Evidence‐Based Nursing 2005;8(3):78. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Bisgaard H, Godard PP. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. American Journal of Respiratory and Critical Care Medicine 2005;171:129‐36. [DOI] [PubMed] [Google Scholar]
O'Connor 2002 {published data only}
- O'Connor RD, O'Donnell JC, Pinto LA, Wiener DJ, Legorreta AP. Two‐year retrospective economic evaluation of three dual‐controller therapies used in the treatment of asthma. Chest 2002;121(4):1028‐35. [DOI] [PubMed] [Google Scholar]
Odeback 1998 {published data only}
- Odeback P. Is the addition of salmeterol more effective than doubling the dose of budesonide in mild asthma?. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A417. [Google Scholar]
Ortega‐Cisneros 1998 {published data only}
- Ortega‐Cisnero M, Maldonado‐Alaniz ML, Rosas Vargas MA, Sierra‐Monge JJL. Salmeterol and inhaled beclomethasone versus high dose inhaled beclomethasone in the control of pediatric patients with moderate asthma. Annals of Allergy and Asthma Immunology 1998;80:131. [Google Scholar]
Palmer 1992 {published data only}
- Palmer JBD, Stuart AM, Shepherd GL, Viskum K. Inhaled salmeterol in the treatment of patients with moderate to severe reversible obstructive airways disease ‐ a 3 month comparison of the efficacy and safety of twice‐daily salmeterol (100 mcg) with salmeterol (50 mcg). Respiratory Medicine 1992;86:409‐17. [DOI] [PubMed] [Google Scholar]
Palmqvist 2001 {published data only}
- Palmqvist M, Arvidsson P, Beckman O, Peterson S, Lotvall J. Onset of bronchodilation of budesonide/formoterol versus salmeterol/fluticasone in single inhalers. Pulmonary Pharmacology & Therapeutics 2001;14(1):29‐34. [DOI] [PubMed] [Google Scholar]
Paterson 1999 {published data only}
- Paterson MC, Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. The effect of combination therapy with salmeterol and montelukast in asthmatic patients receiving inhaled corticosteroids. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999:3490.
Pauwels 1997 {published data only}
- Pauwels RA, Lofdahl CG, Postma DA, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine 1997;337(20):1405‐11. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. American Journal of Respiratory & Critical Care Medicine 1999;160(2):594‐9. [DOI] [PubMed] [Google Scholar]
Pauwels 1998a {published data only}
- Pauwels R. Additive effects of inhaled formoterol and budesonide in reducing asthma exacerbations. Allergy 1998;53:20‐3. [DOI] [PubMed] [Google Scholar]
Pauwels 1998b {published data only}
- Pauwels RA, Yernault JC, Demedts MG, Geusens P. Safety and efficacy of fluticasone and beclomethasone in moderate to severe asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3 pt 1):827‐32. [DOI] [PubMed] [Google Scholar]
Pearlman 1992 {published data only}
- Pearlman DA, Chervinsky P, LaForce C, Seltzer JM, Southern DL, Kemp JP, et al. A comparison of salmeterol with albuterol in the treatment of mild to moderate asthma. New England Journal of Medicine 1992;327:1420‐5. [DOI] [PubMed] [Google Scholar]
Pearlman 1994 {published data only}
- Pearlman DS, Liddle R. Controlling asthma symptoms: Salmeterol compared with salbutamol in large‐scale multicentre studies. European Respiratory Review 1994;4(21):301‐5. [Google Scholar]
Pearlman 2002 {published data only}
- Pearlman DS, White MV, Lieberman AK, Pepsin PJ, Kalberg C, Emmett A, et al. Fluticasone propionate/salmeterol combination compared with montelukast for the treatment of persistent asthma. Annals of Allergy and Asthma Immunology 2002;88(2):227‐35. [DOI] [PubMed] [Google Scholar]
Pearlman 2004 {published and unpublished data}
- McCarthy TP, Edin HM, House K, Yan SK, Vandermeer AK. The effects of salmeterol/fluticasone combination (SFC) dry powder inhaler (DPI) on asthma control and quality of life in patients previously treated with inhaled corticosteroids (ICS). Thorax 2001;56(Suppl 3):iii 63. [Google Scholar]
- Pearlman DS, Peden D, Condemi JJ, Weinstein S, White M, Baitinger L, et al. Efficacy and safety of fluticasone propionate/salmeterol HFA 134A MDI in patients with mild‐to‐moderate persistent asthma. Journal of Asthma 2004;41(8):797‐806. [DOI] [PubMed] [Google Scholar]
- SAS30003. A stratified, randomized, double‐blind, placebo‐controlled, parallel‐group, 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in HFA 134a MDI, 42/88mcg BID, and salmeterol in propellant 11/12 MDI, 42mcg BID, fluticasone propionate in propellant 11/12 MDI, 88mcg BID, and placebo propellant HFA 134a MDI in adult and adolescent subjects with asthma. http://ctr.gsk.co.uk 2005.
- Weinstein SF, Pearlman DS, Condemi JJ, Herrle MR, Scott CA, Payne JE, et al. Superior efficacy of the fluticasone propionate/salmeterol 88/42mcg HFA‐MDI combination product versus the individual components in asthmatics previously treated with either short‐ or long‐acting beta2‐agonists or inhaled corticosteroids. Journal of Allergy & Clinical Immunology 2001;107(2):S102. [Google Scholar]
- White M, Scott C, Herrle MR, Pearlman D, Payne E, House K, et al. Salmeterol/fluticasone propionate (42/88mcg) HFA‐mdi improves asthma control in asthmatics previously treated with short‐ or long‐acting beta2‐agonists or inhaled corticosteroids. Annals of Allergy, Asthma & Immunology 2001;86(1):81. [Google Scholar]
Perez 2000 {published data only}
- Perez O. Montelukast treatment in children with asthma. Revista Alergia Mexico 2000;47(1):30‐2. [PubMed] [Google Scholar]
Peters 2000 {published data only}
- Peters JI, Shelledy DC, Jones AP Jr, Lawson RW, Davis CP, LeGrand TS. A randomized, placebo‐controlled study to evaluate the role of salmeterol in the in‐hospital management of asthma. Chest 2000;118(2):313‐20. [DOI] [PubMed] [Google Scholar]
Pieters 1998 {published data only}
- Pieters WR, Sondhi S, Price MJ, Thwaites RM, Nyth A. The cost effectiveness of salmeterol/fluticasone propionate 50/500 microgram combination inhaler versus fluticasone propionate 500 microgram in patients with chronic asthma. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999:2458.
- Pieters WR, Steinmetz KO, Aubier M, Johnson L, Gomez E, Bogolubov M. Effectiveness of a new salmeterol/fluticasone propionate (50/500µg) combination inhaler in patients with reversible airways obstruction. European Respiratory Journal 1998;28:35s. [Google Scholar]
Pinnas 1998 {published data only}
- Pinnas JL, Schwartz H, Yancey SW, Rickard K. Six month comparison of beclomethasone versus salmeterol or placebo in adults with asthma. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A417. [Google Scholar]
Pizzichini 1996 {published data only}
- Pizzichini MM, Kidney JC, Wong BJ, Morris MM, Efthimiadis A, Dolovich J, et al. Effect of salmeterol compared with beclomethasone on allergen‐induced asthmatic and inflammatory responses. European Respiratory Journal 1996;9(3):449‐55. [DOI] [PubMed] [Google Scholar]
Pljaskic‐Kamenov 2000 {published data only}
- Pljaskic‐Kamenov SS, Filipovic MD, Kamenov BA. Comparison of addition of salmeterol xinafoate to budesonide with budesonide alone on symptoms and quality of life in asthmatic children. European Respiratory Journal 2000;16(Suppl 31):518s. [Google Scholar]
Price 2002 {published data only}
- Price D, Dutchman D, Mawson A, Bodalia B, Duggan S, Todd P. FLOW (Eformoterol in the management of mild asthma‐formoterol Turbohaler with budesonide turbohaler) Research group. Thorax 2002;57:791‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MJ, Briggs AH. Development of an economic model to assess the cost effectiveness of asthma management strategies. Pharmacoeconomics 2002; Vol. 20, issue 3:183‐94. [DOI] [PubMed]
- Price MJ, Sondhi S, Yan S, Nyth A, House K. Salmeterol/fluticasone propionate combination inhaler is more cost effective than fluticasone propionate in patients with asthma. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999.
Pujet 1995 {published data only}
- Pujet JC, Evano CI. A randomized double‐blind study comparing inhaled beclomethasone with long‐acting theophylline for the first‐line treatment of moderate asthma. Semaine Des Hopitaux 1995;71(27‐28):865‐72. [Google Scholar]
Rance 2002 {published data only}
- Rance L, Musin L. Asthma management costs in Canada are lower with combination fluticasone propionate/salmeterol (250/50mcg BID) in a single inhaler than with budesonide 800mcg BID plus eformoterol 12 mcg BID via separate inhalers. Chest Conference; San Diego, CA. 2002:s6.
Rickard 2001 {published data only}
- Rickard K, Dorinsky PM, Knobil K, Pepsin P, Akveld MLM. The salmeterol/fluticasone propionate combination 50/100µg bid is more effective than oral montelukast 10mg of as a first line therapy in mild and moderate asthmatics. European Respiratory Journal 2001;18 Suppl 33:262s. [Google Scholar]
Rijssenbeek‐Nouwens 2002 {published data only}
- Rijssenbeek‐Nouwens LHM, Oosting AJ, Monchy JGR, Bregman I, Postma DS, Bruin‐Weller MS. The effect of anti‐allergic mattress encasings on house dust mite‐induced early‐ and late‐airway reactions in asthmatic patients. A double‐blind, placebo‐controlled study. Clinical & Experimental Allergy 2002;32(1):117‐25. [DOI] [PubMed] [Google Scholar]
Ringbaek 1996 {published data only}
- Ringbaek TJ, Soes‐Petersen U, Christensen M, Iversen ET, Rasmussen FV. Salmeterol improves the control of disease in patients with moderate asthma. A comparative study of inhaled salmeterol 50 mg and salbutamol depot tablets 8 mg, both administered twice daily. Ugeskrift for Laeger 1996;158(27):3940‐3. [PubMed] [Google Scholar]
Ringdal 2002 {published data only}
- Alonso JF, Badiola C Kielhorn A. Economic evaluation of salmeterol/fluticasone combination vs budesonide plus formoterol in Spain. European Respiratory Journal 2001;18(Suppl 33):49s. [Google Scholar]
- Chuchalin AG, Chovan L, Ringdal N, Whitehead PJ. Advair™ seretide™ (250/50 mu‐g bid) shows nocturnal benefit over budesonide 800mu‐g + formoterol 12mu‐g bid in moderate to severe asthma. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
- Ringdal N, Chuchalin A, Chovan L, Tudoric N, Maggi E, Whitehead PJ. EDICT Investigators. Evaluation of different inhaled combinations therapies (EDICT); a randomised, double blind comparison of Seretide (ro/250) microg bd Diskus vs formoterol (12 microg bd) and budesonide (800 microg bd) given concurrently (both with turbuhaler) in patients with moderate to severe asthma. Respiratory Medicine 2002;96(11):851‐61. [DOI] [PubMed] [Google Scholar]
- Ringdal N, Chuchalin AG, Chovan L, Whitehead PJ. A comparison of Advair™/Seretide™ (salmeterol 50 mcg/fluticasone propionate 250 mcg bid) with formoterol 12 mcg + budesonide 800 mcg bid in moderate‐severe asthma. American Journal of Respiratory and Critical Care Medicine 2000;161(3 part 2 Suppl 1):A196. [Google Scholar]
- Ringdal NR, Chovan L, Chuchalin AG, Whitehead PJ. Advair™/Seretide™ (250/50mu‐g bid) shows exacerbation benefit over budesonide plus formoterol bid in moderate‐severe asthma. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
Ringdal 2003 {published data only}
- Ringdal N, Eliraz A, Pruzinec R, Weber HH, Mulder PG, Akveld M, et al. The salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthma. Respiratory Medicine 97;3:234‐41. [DOI] [PubMed] [Google Scholar]
Rocca‐Serra 2002 {published data only}
- Rocca‐Serra JP, Vicaut E, Lefrancois G, Umile A, Lefrancois G, Chiesi SA, et al. Efficacy and tolerability of a new non‐extrafine formulation of beclomethasone HFA‐134a in patients with asthma: comparison with beclomethasone CFC. Clinical Drug Investigation 2002;22(10):653‐65. [Google Scholar]
Rosenhall 2002 {published data only}
- Rosenhall L, Heinig JH, Lindqvist A, Leegaard J, Stahl E, Bergqvist PB. Budesonide/formoterol (Symbicort) is well tolerated and effective in patients with moderate persistent asthma. International Journal of Clinical Practice 2002;56(6):427‐33. [PubMed] [Google Scholar]
- Rosenhall L, Heinig JH, Lindqvist A, Leegard J, Bergqvist PBF. Symbicort (budesonide/eformoterol in a single inhaler) is safe and effective in the treatment of asthma. Thorax 2001;56(Suppl 3):iii 63. [Google Scholar]
Rosenhall 2003 {published data only}
- Rosenhall L, Elvstrand A, Tilling B, Vinge I, Jemsby P, Stahl E, et al. One‐year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort Turbuhaler ) for the treatment of asthma. Respiratory Medicine 2003;97(6):702‐8. [DOI] [PubMed] [Google Scholar]
- Rosenhall L, Heinig JH, Lindqvist A, Leegard J, Bergqvist PBF. Budesonide and formoterol in a single inhaler is safe and effective in the treatment of asthma. European Respiratory Journal 2001;18(33):159s. [Google Scholar]
- Rosenhall L, Stahl E, Heinig JH, Lindquist A, Leegard J, Bergqvist PBF. Health‐related quality of life and asthma control in patients using symbicort® (budesonide and formoterol in a single inhaler). Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
- Rosenhall L, Stahl E, Heinig JH, Lindqvist A, Leegard J, Bergqvist PBF. Health‐related quality of life and asthma control in patients treated with budesonide and formoterol in a single inhaler. European Respiratory Journal 2001;18 Suppl 33:46s. [Google Scholar]
Rosenthal 1999 {published data only}
- Rosenthal RR, Busse WW, Kemp JP, Baker JW, Kalberg C, Emmett A, et al. Effect of long‐term salmeterol therapy compared with as‐needed albuterol use on airway hyperresponsiveness. Chest 1999;116(3):595‐602. [DOI] [PubMed] [Google Scholar]
Russell 1995 {published data only}
- Russell G, Williams DAJ, Weller P, Price JF. Salmeterol xinafoate on children on high dose inhaled steroids. Annals of Allergy, Asthma and Immunology 1995;75:423‐8. [PubMed] [Google Scholar]
Saari 2002 {published data only}
- Saari SM, Vidgren MT, Herrala J, Turjanmaa VMH, Koskinen MO, Nieminen MM. Possibilities of formoterol to enhanced the peripheral lung deposition of the inhaled liposome corticosteroids. Respiratory Medicine 2002;96(12):999‐1005. [DOI] [PubMed] [Google Scholar]
SAM40004 {published data only}
- Beckett P, Hewitt L, Woodcock A, Smith J, Seghal N, Rice L, et al. Improvement in airway hyper‐responsiveness (AHR) and lung function with salmeterol/fluticasone propionate combination (SFC) in persistent asthma [abstract]. American Thoracic Society 99th International Conference. 2003:D034 Poster C27.
- SAM40004 (Glaxo Smith Kline). A multi‐centre, randomised, double‐blind, placebo‐controlled parallel group study to compare the effect on airway inflammation and remodelling of treatment with salmeterol/fluticasone propionate combination product (50/100μg strength) bd via the Accuhaler inhaler, or fluticasone propionate 100μg bd via the Accuhaler inhaler or placebo via the Accuhaler inhaler for 16 weeks, followed by double‐blind treatment for 52 weeks with the salmeterol/fluticasone propionate combination product (50/100μg strength) bd via the Accuhaler inhaler or fluticasone propionate 100μg bd via the Accuhaler inhaler, in adults with reversible airways obstruction (SIRIAS ‐ Seretide in Inflammation and Remodelling In Asthma Study). http://www.ctr.gsk.co.uk 2004 (accessed 2 May 2008).
SAM40104 {unpublished data only}
- Houghton CM, Wixon C, Yoxall S, Langley SJ, Singh D, Woodcock AA. Specific airways resistance (sRaw) provides a sensitive measure of bronchodilation in mild asthmatic adult patients with near normal lung function. American Thoracic Society Annual Meeting. 2005, issue A378.
- SAM40104. Single centre, randomised, double‐blind, comparator study to demonstrate superiority of salmeterol/fluticasone propionate combination product 50/100mcg bd over fluticasone propionate 100mcg bd with respect to improvements in airway physiology in adults with persistent asthma treated for 4 weeks. http://www.ctr.gsk.co.uk 2006.
SAS10006 {published data only}
- Fueki N, Fueki M, Makino S, Takemoto Y, Yasuda K, Nishioka Y, et al. The pharmacokinetics and pharmacodynamics of salmeterol/fluticasone propionate via diskus in Japanese and Caucasian asthma patients. 4th Triennual World Asthma Meeting, Bangkok, Thailand. 2004.
- SAS10006. A 12‐week, randomized, double‐blind, placebo‐controlled, 3‐way crossover study in adult subjects with asthma aged 18‐55 years to examine the pharmacodynamics and pharmacokinetics of fluticasone propionate (FP) administered twice daily via the DISKUS™ (FP 100mcg) and the FP/Salmeterol (SALM) combination product (FP 100mcg/SALM 50mcg) administered twice daily via the DISKUS. http://www.ctr.gsk.co.uk 2005.
SAS30013 {unpublished data only}
- SAS30013. A study to compare the long term effects on airway inflammation of Seretide versus Flixotide in adult subjects with asthma. http://www.ctr.gsk.co.uk 2004.
Schreurs 1996 {published data only}
- Schreurs AJM, Sinninghe Damste HEJ, Graaff CS, Greefhorst APM. A dose‐response study with formoterol Turbuhaler as maintenance therapy in asthmatic patients. European Respiratory Journal 1996;9:1678‐83. [DOI] [PubMed] [Google Scholar]
Scicchitano 2004 {published and unpublished data}
- SD‐039‐0668. Efficacy and safety of Symbicort (budesonide/formoterol) Turbuhaler as single therapy in subjects with moderate‐severe asthma. Comparison with conventional asthma therapy, Pulmicort (budesonide) Turbuhaler, as regular treatment complemented with Bricanyl (Terbutaline) Turbuhaler. www.astrazeneca.com 2005.
- Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Current Medical Research and Opinion 2004;20(9):1403‐18. [DOI] [PubMed] [Google Scholar]
Sears 2003 {published data only}
- Sears MR, McIvor A, Becker A, Fitzgearld JM, Boulet LP, Ernsy P, et al. Budesonide/formoterol adjustable maintenance dosing effectively improves asthma symptom severity: a multicentre Canadian study. European Respiratory Journal 2003;22 Suppl 45:258s. [Google Scholar]
Serrier 2003 {published data only}
- Serrier P, Roche N, Pello JY, Larguier JS, Mezzi K. Asthma control achieved with inhaled corticosteroids and long‐acting beta2‐agonists in a free or fixed combination: Results of the ALISE survey. Presse Medicale 2003;32(11):493‐7. [PubMed] [Google Scholar]
Shapiro 2000 {published data only}
- Shapiro G, Lumry W, Wolfe J, Given J, White M, Woodring A, et al. Combined salmeterol 50mcg and fluticasone propionate 250mcg in the diskus device for the treatment of asthma. American Journal of Respiratory and Critical Care Medicine 2000;161:527‐34. [DOI] [PubMed] [Google Scholar]
Shapiro 2001 {published data only}
- Shapiro GG, Mendelson LM, Pearlman DS. Once‐daily budesonide inhalation powder (Pulmicort Turbuhaler) maintains pulmonary function and symptoms of asthmatic children previously receiving inhaled corticosteroids. Annals of Allergy, Asthma, & Immunology 2001;86(6):633‐40. [DOI] [PubMed] [Google Scholar]
Sheth 2002 {published data only}
- Sheth K, Borker R, Emmett A, Rickard K, Dorinsky P. Cost‐effectiveness comparison of salmeterol/fluticasone propionate versus montelukast in the treatment of adults with persistent asthma. Pharmacoeconomics 2002;20(13):909‐18. [DOI] [PubMed] [Google Scholar]
Sienra‐Monge 2001 {published data only}
- Sienra‐Monge JJL, Rio BE, Alvarez ME, Magana AJ. Comparison of quality of life and pulmonary function on moderate asthmatic children treated with Beclomethasone and Beclomethasone plus Salmeterol. Journal of Allergy and Clinical Immunology 2001; Vol. 107, issue 2:s263.
Simons 1997a {published data only}
- Simons F, Estelle R, Gerstner TV, Cheang MS. Tolerance to the bronchoprotective effect of salmeterol in adolescents with exercise‐induced asthma using concurrent inhaled glucocorticoid treatment. Pediatrics 1997;99(5):655‐9. [DOI] [PubMed] [Google Scholar]
Simons 1997b {published data only}
- Simons FER. A comparison of beclomethasone, salmeterol and placebo in children with asthma. New England Journal of Medicine 1997;337:1659‐65. [DOI] [PubMed] [Google Scholar]
SNS {published data only}
- Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ 113;306:1034‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sovani 2008 {published data only}
- MP Sovani. The effect of providing a single combination inhaler on steroid use and asthma control. Nottingham City Hospital NHS Trust.
- Sovani MP, Whale CI, Oborne J, Cooper S, Mortimer K, Ekström T, et al. Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?. British Journal of General Practice 2008;58(546):37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staehr 1995 {published data only}
- Staehr P, Vestbo I. Salmeterol improves control in asthmatic patients treated in general practice. A comparative study of salmeterol (Serevent) and salbutamol. Ugeskrift for Laeger 1995;157(1):36‐40. [PubMed] [Google Scholar]
Stanford 2002 {published data only}
- Stanford RH, Borker R, Dorinsky P, Pepsin P, Kalberg C, Emmett A, et al. The costs and efficacy of fluticasone propionate/salmeterol combination versus montelukast in the treatment of adults with persistent asthma. Chest Conference; San Diego, CA. 2002:422.
Stelmach 2001 {published data only}
- Stelmach I, Grzelewski T, Majak P, Majak J, Bobrowska M, Jerzynska J, et al. The effect of triamcinolone, montelukast and formoterol on serum levels of il‐4, IgE and clinical parameters in children with asthma. Polski Merkuriusz Lekarski 2001;11(63):247‐51. [PubMed] [Google Scholar]
Stelmach 2002a {published data only}
- Stelmach I, Jerzynska J, Majak P, Grzelewski T, Gorski P, Stelmach W, et al. Effect of triamcinolone acetonide, montelukast, nedocromil sodium, formoterol on serum levels of sICAM‐1, sIL‐2R and clinical parameters of asthma in children. Polski Merkuriusz Lekarsk 2002;12(68):99‐103. [PubMed] [Google Scholar]
Stelmach 2002b {published data only}
- Stelmach I, Jerzynska J, Kuna P. A randomized, double‐blind trial of the effect of glucocorticoid, antileukotriene and [beta]‐agonist treatment on IL‐10 serum levels in children with asthma. Clinical & Experimental Allergy 2002;32(2):264‐9. [DOI] [PubMed] [Google Scholar]
Stelmach 2007 {published data only (unpublished sought but not used)}
- Stelmach I, Grzelewskia T, Bobrowska‐Korzeniowska M, Stelmach P, Kuna P. A randomized, double‐blind trial of the effect of anti‐asthma treatment on lung function in children with asthma. Pulmonology Pharmacology & Therapeutics 2007;20:691‐700. [DOI] [PubMed] [Google Scholar]
Stojkovic‐Andjelkovi 2001 {published data only}
- Stojkovic‐Andjelkovic AK, Pajovic DM, Protrka OJ, Ugrinovic BS, Obradovic SM, Pavicevic MD. Effect of combination fluticasone propionate and salmeterol diskhaler in treatment of moderate to severe asthma: comparison of initial high dose, constant medium dose and placebo. European Respiratory Journal 2001; Vol. 18 Suppl 33:123s.
Tal 2003 {published data only}
- Tal A, Simon G, Vermeulen JH. Symbicort® (budesonide and formoterol in a single inhaler) is effective and well tolerated in children with asthma. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard MI, et al. Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatric Pulmonology 2002;34:342‐50. [DOI] [PubMed] [Google Scholar]
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Rapid and sustained improvements in lung function and symptom control with budesonide/ formoterol in adolescent asthma. European Respiratory Journal 2001;18 Suppl 33:494s. [Google Scholar]
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Symbicort (budesonide and formoterol in a single inhaler) improves lung function in children with asthma. International Paediatric Respiratory and Allergy Congress, April 1‐4; Prague. 2001:85.
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Symbicort (budesonide and formoterol in a single inhaler) is more effective that budesonide alone in children with asthma. International Paediatric Respiratory and Allergy Congress, April 1‐4; Prague. 2001:84‐5.
- Vermeulen JH, Simon G, Tal A. Symbicort® (budesonide and formoterol in a single inhaler) improves lung function in asthmatic children aged 4‐17 years. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Tan 1997 {published data only}
- Tan S, Hall IP, Dewar J, Dow E, Lipworth B. Association between beta 2‐adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet 1997;350(9083):995‐9. [DOI] [PubMed] [Google Scholar]
Tattersfield 2001 {published data only}
- Tattersfield AE, Lofdahl CG, Postma DS, Eivindson A, Schreurs AG, Rasidakis A, et al. Comparisons of formoterol and terbutaline for as‐needed treatment of asthma: a randomised trial. Lancet 2001;357(9252):257‐61. [DOI] [PubMed] [Google Scholar]
Trautmann 2001 {published data only}
- Trautmann M. Treatment with salmeterol/fluticasone propionate (50/250g) inhaler improves lung function, asthma symptoms and quality of life in a large group of patients with mild to moderate asthma. American Journal of Respiratory and Critical Care Medicine 2001;163(5):A864. [Google Scholar]
Turner 1998 {published data only}
- Turner MO, Johnston PR, Pizzichini E, Pizzichini MMM, Hussack PA, Hargreave FE. Anti‐inflammatory effects of salmeterol compared with beclomethasone in eosinophilic mild exacerbations of asthma: a randomized, placebo controlled trial. Canadian Respiratory Journal 1998;5(4):261‐8. [DOI] [PubMed] [Google Scholar]
Ullman 1990 {published data only}
- Ullman A, Hedner J, Svedmyr N. Inhaled salmeterol and salbutamol in asthmatic patients. An evaluation of asthma symptoms and the possible development of tachyphylaxis. American Review of Respiratory Disease 1990;142(3):571‐5. [DOI] [PubMed] [Google Scholar]
Van den Berg 2000 {published data only}
- Berg NJ, Ossip MS, Hederos CA, Anttila H, Ribeiro BL, Davies PI. Salmeterol/fluticasone propionate (50/100 ug) in combination in Diskus inhaler (Seretide) is effective and safe in children with asthma. Pediatric Pulmonology 2000;30:97‐105. [DOI] [PubMed] [Google Scholar]
van der Molen 1997 {published data only}
- Molen T, Postma DS, Kraan J, Chapman K, Grossman R, Turner MO, et al. No influence of six months treatment with formoterol on airway hyperresponsiveness in asthma subjects using inhaled corticosteroids. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 157, issue Suppl 3:A400.
- Molen T, Postma DS, Schreurs AJ, Bosveld HE, Sears MR, Meyboom de Jong‐B. Discriminative aspects of two generic and two asthma‐specific instruments: relation with symptoms, bronchodilator use and lung function in patients with mild asthma. Quality of Life Research 1997;6(4):353‐61. [DOI] [PubMed] [Google Scholar]
- Molen T, Postma DS, Turner MO, Meyboom‐de Jong B, Malo JL, Chapman K, et al. Effects of the long acting beta agonist formoterol on asthma control in asthmatic patients using inhaled corticosteroids. Thorax 1996;52:535‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molen T, Sears MR, Graaff CS, Postma DS, Meyboom‐de Jong B. Quality of life during formoterol treatment: comparison between asthma‐specific and generic questionnaires. European Respiratory Journal 1998;12(1):30‐4. [DOI] [PubMed] [Google Scholar]
van der Woude 2001 {published data only}
- Woude HJ, Winter TH, Boorsma M, Bergqvist PBF, Aalbers A. Symbicort® (budesonide and formoterol in a singe inhaler) provides rapid relief on methacholine‐induced bronchoconstriction. Annual Thoracic Society 97th International Conference; San Francisco CA, May 18‐23. 2001.
- Woude HJ, Winter TH, Boorsma M, Bergqvist PBF, Aalbers R. More rapid relief of methacholine‐induced bronchoconstriction with budesonide/formoterol than with salmeterol/fluticasone. European Respiratory Journal 2001;18 Suppl 33:53s. [Google Scholar]
van Noord 1999 {published data only}
- Schreurs AJ, Noord JA, Mulder PG. Fluticasone propionate (FP) and salmeterol xinafoate (SLM) in patients with mild to moderate asthma. European Respiratory Journal 1998;12 Suppl 29:19 s [F159]. [Google Scholar]
- Noord JA, Schreurs AJM, Mol SJM, Mulder PGH. Addition of salmeterol versus doubling the dose of fluticasone propionate in patients with mild to moderate asthma. Thorax 1999;54:207‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Noord 2001 {published data only}
- SFCB3023. A multicentre, randomised, double‐blind, double‐dummy, parallel‐group, three‐month comparison of the salmeterol/fluticasone propionate combination product (2x25/250mcg strength) bd via the pressurised metered dose inhaler with salmeterol/fluticasone propionate combination product (1x50/500mcg strength) bd via the Diskus/Accuhaler™ inhaler and with fluticasone propionate (2x 250mcg strength) alone bd via the pressurised metered dose inhaler in adolescents and adults with reversible airways obstruction. http://www.ctr.gsk.co.uk 2004.
- Noord JA, Lill H, Carrillo Diaz T, Greefhorst AP, Davies P. Clinical equivalence of a salmeterol/fluticasone propionate combination product delivered via a chlorofluorocarbon‐free metered‐dose inhaler with the Diskus in patients with moderate to severe asthma. Clinical Drug Investigation 2001;21(4):243‐55. [Google Scholar]
- Noord JA, Lill H, Carrillo T, Davies P. Clinical equivalence of salmeterol/fluticasone propionate combination 50/500 bid delivered via metered dose inhaler (MDI) or Diskus™ in patients with reversible airways obstruction. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A197. [Google Scholar]
van Schayck 2002 {published data only}
- Schayck CP, Cloosterman SG, Bijl‐Hofland ID, Hoogen H, Folgering HT, Weel C. Is the increase in bronchial responsiveness or FEV1 shortly after cessation of beta2‐agonists reflecting a real deterioration of the disease in allergic asthmatic patients? A comparison between short‐acting and long‐acting beta2‐agonists. Respiratory Medicine 2002;96(3):155‐62. [DOI] [PubMed] [Google Scholar]
Verberne 1997 {published data only}
- Verberne AAPH, Frost C, Roorda RJ, Laag H, Kerrebijn KF. One year treatment with salmeterol compared with beclomethasone in children with asthma. American Journal of Respiratory and Critical Care Medicine 1997;156:688‐95. [DOI] [PubMed] [Google Scholar]
Verberne 1998 {published data only}
- Verberne AAPH, Frost C, Duiverman EJ, Grol MH, Kerribijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. American Journal of Respiratory and Critical Care Medicine 1998;158:213‐19. [DOI] [PubMed] [Google Scholar]
Vermetten 1999 {published data only}
- Vermetten AM, Boermans JM, Luiten WDVF, Mulder PGH, Vermue NA. Comparison of salmeterol with beclomethasone in adult patients with mild persistent asthma who are already on low‐dose inhaled steroids. Journal of Asthma 1999;36(1):97‐106. [DOI] [PubMed] [Google Scholar]
Vestbo 2000 {published data only}
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in patients with mild to moderate chronic obstructive lung disease. Ugeskrift for Laeger 2000;162(4):493‐7. [PubMed] [Google Scholar]
Vickers 2000 {unpublished data only}
- Vickers M. Assessment of long‐term efficacy of early introduction of inhaled steroids in asthma. National Health Technology Assessment (NCCHTA) 2000.
Vilsvik 2001 {published data only}
- Vilsvik J, Ankerst J, Palmqvist M, Persson G, Schaanning J, Schwabe G, et al. Protection against cold air and exercise‐induced bronchoconstriction while on regular treatment with Oxis. Respiratory Medicine 2001;95(6):484‐90. [DOI] [PubMed] [Google Scholar]
Von Berg 1989 {published data only}
- Berg A, Berdel D. Formoterol and salbutamol metered aerosols: comparison of a new and an established beta‐2‐agonist for their bronchodilating efficacy in the treatment of childhood bronchial asthma. Pediatric Pulmonology 1989;7(2):89‐93. [DOI] [PubMed] [Google Scholar]
Wallaert 1999 {published data only}
- Wallaert B, Brun P, Ostinelli J, Murciano D, Champel F, Blaive B, et al. A comparison of two long‐acting beta‐agonists, oral bambuterol and inhaled salmeterol, in the treatment of moderate to severe asthmatic patients with nocturnal symptoms. The French Bambuterol Study Group. Respiratory Medicine 1999;93(1):33‐8. [DOI] [PubMed] [Google Scholar]
Wallin 1990 {published data only}
- Wallin A, Melander B, Rosenhall L, Sandstrom T, Wahlander L. Formoterol, a new long acting beta 2 agonist for inhalation twice daily, compared with salbutamol in the treatment of asthma. Thorax 1990;45(4):259‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallin 1998 {published data only}
- Wallin A, Sandstrom T, Soderberg M, Howarth P, Djukanovic R, Wilson S, et al. Effects of formoterol, budesonide and placebo treatment on asthmatic airway inflammation. Annals of Allergy, Asthma and Immunology 1998;80:88. [Google Scholar]
- Wallin A, Sandstrom T, Soderberg M, Howarth P, Lundback B, Della‐Cioppa G, et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. American Journal of Respiratory & Critical Care Medicine 1998;158(1):79‐86. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Wallin A, Della‐Cioppa G, Sandstrom T, Holgate ST. Effects of budesonide and formoterol on NF‐kappaB, adhesion molecules, and cytokines in asthma. American Journal of Respiratory & Critical Care Medicine 2001;164(6):1047‐52. [DOI] [PubMed] [Google Scholar]
Wallin 2003 {published data only}
- Sue‐Chu M, Wallin A, Wilson S, Ward J, Sandstrom T, Djukanovic R, et al. Bronchial biopsy study in asthmatics treated with low and high dose fluticasone propionate (FP) compared to low dose FP combined with salmeterol. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999.
- Wallin A, Sandstrom T, Cioppa GD, Holgate S, Wilson S. The effects of regular inhaled formoterol and budesonide on preformed Th‐2 cytokines in mild asthmatics. Respiratory Medicine 2002;96(12):1021‐5. [DOI] [PubMed] [Google Scholar]
- Wallin A, Sue Chu M, Bjermer L, Ward J, Sanstrom T, Lindberg A, et al. Effect of inhaled fluticasone with and without salmeterol on airway inflammation in asthma. Journal of Allergy and Clinical Immunology 2003;112(1):72‐8. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Ward JA, Djukanovic R, Wallin A, Sue‐Chu M, Sandstrom, et al. Effects of high and low dose inhaled fluticasone propionate (FP) compared to low dose FP combined with salmeterol (SAL) on airway inflammation in asthma. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A196. [Google Scholar]
Weinstein 1998 {published data only}
- Weinstein SF, Pearlman DS, Bronsky EA, Byrne A, Arledge T, Liddle R, et al. Efficacy of salmeterol xinafoate powder in children with chronic persistent asthma. Annals of Allergy and Asthma Immunology 1998;81:51‐8. [DOI] [PubMed] [Google Scholar]
Wempe 1992 {published data only}
- Wempe JB, Tammeling EP, Koeter GH, Hakansson L, Venge P, Postma DS. Blood eosinophil numbers and activity during 24 hours: effects of treatment with budesonide and bambuterol. Journal of Allergy and Clinical Immunology 1992;90(5):757‐65. [DOI] [PubMed] [Google Scholar]
Wilcke 1998 {published data only}
- Wilcke JT, Iversen ET, Kok Jensen A. Effect of salmeterol is independent of previously inhaled salbutamol: a clinical controlled study. Lung 1998;176(2):133‐9. [DOI] [PubMed] [Google Scholar]
Wilding 1997 {published data only}
- Wilding P, Clark M, Thompson‐Coon J, Lewis S, Rushton L, Bennett J, et al. Effect of long term treatment with salmeterol on asthma control: a double‐blind, randomised crossover study. BMJ 1997;314:1441‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2001 {published data only}
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Evaluation of salmeterol or montelukast as second‐line therapy for asthma not controlled with inhaled corticosteroids. Chest 2001;119(4):1021‐6. [DOI] [PubMed] [Google Scholar]
Wong 1992 {published data only}
- Wong BJ, Dolovich J, Ramsdale EH, O'Byrne P, Gontovnick L, Denburg JA, et al. Formoterol compared with beclomethasone and placebo on allergen‐induced asthmatic responses. American Review of Respiratory Disease 1992;146(5 pt 1):1156‐60. [DOI] [PubMed] [Google Scholar]
Woolcock 1996 {published data only}
- Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling the dose of inhaled steroids. American Journal of Respiratory and Critical Care Medicine 1996;153:1481‐8. [DOI] [PubMed] [Google Scholar]
Yates 1995 {published data only}
- Yates DH, Sussman HS, Shaw MJ, Barnes PJ, Chung KF. Regular formoterol treatment in mild asthma: effect on bronchial responsiveness during and after treatment. American Journal of Respiratory and Critical Care Medicine 1995;152(41):1170‐4. [DOI] [PubMed] [Google Scholar]
Yates 1996 {published data only}
- Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the broncho‐protective effect of a long‐acting inhaled beta 2‐agonist. American Journal of Respiratory & Critical Care Medicine 1996;154(6 pt 1):1603‐7. [DOI] [PubMed] [Google Scholar]
Youngchaiyud 1995 {published data only}
- Youngchaiyud P, Permpikul C, Suthamsmai T, Wong E. A double‐blind comparison of inhaled budesonide, long‐acting theophylline, and their combination in treatment of nocturnal asthma. Allergy 1995;50(1):28‐33. [DOI] [PubMed] [Google Scholar]
Yurdakul 2002 {published data only}
- Yurdakul AS, Calisir HC, Tunctan B, Ogretensoy M. Comparison of second controller medications in addition to inhaled corticosteroid in patients with moderate asthma. Respiratory Medicine 2002;96(5):322‐9. [DOI] [PubMed] [Google Scholar]
Zarkovic 1998 {published data only}
- Zarkovic J, Gotz MH, Holgate ST, Taak NK. Effect of long‐term regular salmeterol treatment in children with moderate asthma. Clinical Drug Investigation 1998;15(3):169‐75. [Google Scholar]
Zetterstrom 2003 {published data only}
- Zetterstrom O, Buhl R, Mellem H. Efficacy and safety of Symbicort® (budesonide and formoterol in a single inhaler) in adults with asthma. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
- Zetterstrom O, Buhl R, Mellem H, Perpina M, Hedman J, O'Neill S, et al. The new single inhaler product containing both budesonide/formoterol improves asthma control in adults.. European Respiratory Journal 2000; Vol. 16 Suppl 31:455s. [DOI] [PubMed]
- Zetterstrom O, Buhl R, Perpina M, Hedman J, Neill SO, Ekstrom T. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. European Respiratory Journal 2001;18:262‐8. [DOI] [PubMed] [Google Scholar]
- Zetterström O, Buhl R, Mellem H, Perpiñá M, Hedman J, O'Neill S, Ekström T. Efficacy and safety of a new single inhaler product containing both budesonide and formoterol in adult asthma. European Respiratory Journal 2000; Vol. 16, issue 31:455s. [DOI] [PubMed]
Zimmerman 2004 {published data only}
- Zimmerman B. Efficacy and tolerability of formoterol turbuhaler® compared with placebo in children (6‐11 years) with asthma poorly controlled with inhaled corticosteroids. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):AA476. [Google Scholar]
- Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol turbuhaler(R) when added to inhaled corticosteroid treatment in children with asthma. Pediatric Pulmonology 2004;37(2):122‐7. [DOI] [PubMed] [Google Scholar]
- Zimmerman B, D'Urzo A, Berube D. Efficacy and tolerability of formoterol Turbuhaler in 6‐11 year old children with asthma, not adequately controlled with inhaled corticosteroids. European Respiratory Society Annual Congress 2002. 2002:P2734.
Additional references
Abramson 2003
- Abramson MJ, Walters J, Walters EH. Adverse effects of beta agonists: are they clinically relevant?. American Journal of Respiratory Medicine 2003;2(4):287‐97. [DOI] [PubMed] [Google Scholar]
Adams 2007
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002310.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2008a
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] [DOI] [Google Scholar]
Adams 2008b
- Adams NP, Bestall JC, Jones P, Lasserson TJ, Griffiths B, Cates CJ. Fluticasone at different doses for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003534.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 2008
- Bateman E, Nelson H, Bousquet J, Kral K, Sutton L, Ortega H, et al. Meta‐analysis: effects of adding salmeterol to inhaled corticosteroids on serious asthma‐related events. www.annals.org 2008;149(1):Epub (accessed 5 June 2008). [DOI] [PubMed] [Google Scholar]
BTS 2008
- British Thoracic Society. British Guidelines on Asthma Management. Thorax 2008; Vol. 63, issue Suppl 1.
Cates 2008a
- Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006363.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2008b
- Cates CJ, Lasserson T, Cates MJ. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006923.pub2] [DOI] [PubMed] [Google Scholar]
Cates 2009
- Cates CJ, Lasserson TJ. Combination formoterol and budesonide as maintenance and reliever therapy versus inhaled steroid maintenance for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007313] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Alonzo 1997
- D'Alonzo GE, Tolep KA. Salmeterol in the treatment of chronic asthma. American Family Physician 1997;56(2):558‐62. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2007
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Available at: http://www.ginasthma.com 2007.
Gleser 1996
- Gleser LJ, Olkin I. Models for estimating the number of unpublished studies. Statistics in Medicine 1996;15:2493‐507. [DOI] [PubMed] [Google Scholar]
Greenstone 2005
- Greenstone IR, Ni Chroinin MN, Masse V, Danish A, Magdalinos H, Zhang X, et al. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005533] [DOI] [PubMed] [Google Scholar]
Handbook 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemiere 2004
- Lemiere C, Bai T, Balter M, Bayliff C, Becker A, Boulet LP, et al. Adult Asthma Guidelines Update 2003. Canadian Respiratory Journal 2004;11(Suppl A):9A‐18A. [DOI] [PubMed] [Google Scholar]
NAEPP 2007
- National Asthma Education and Prevention Program. Expert Panel Report Guidelines for the Diagnosis and Management of Asthma. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/index.htm. Bethesda, MD: National Heart, Lung and Blood Institute, 2007. [Google Scholar]
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] [DOI] [PubMed] [Google Scholar]
Powell 2003
- Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: an evidence‐based approach. Medical Journal of Australia 2003;178:223‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Sazonov‐Kocevar 2006
- Sazonov‐Kocevar V, Laforest L, Travier N, Yin DD, Ganse E. Asthma and allergy medication use and costs among pediatric primary care patients on asthma controller therapy. Pediatric Allergy and Immunology 2006;17:620‐8. [DOI] [PubMed] [Google Scholar]
Shrewsbury 2000
- Shrewsbury S, Pyke S, Britton M. Meta‐analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA). BMJ 2000;320:1368‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockl 2008
- Stockl KM, Le L, Harada AS, Zhang S. Use of controller medications in patients initiated on a long‐acting beta2‐adrenergic agonist before and after safety alerts. American Journal of Health‐System Pharmacy 2008;65(16):1533‐8. [DOI] [PubMed] [Google Scholar]
Storms 2003
- Storms W. Clinical trials: are these your patients?. Journal of Allergy and Clinical Immunology 2003;112(5 Suppl 1):107s‐11s. [DOI] [PubMed] [Google Scholar]
Walters 2007
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD003901] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ni Chroinin 2004
- Ni Chroinin M, Greenstone IIG, Ducharme F. Addition of inhaled long‐acting beta2‐agonists to inhaled steroids as first line therapy for persistent asthma in steroid‐naive adults. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD005307] [DOI] [PubMed] [Google Scholar]


