Abstract
Amyotrophic lateral sclerosis (ALS) is a chronic neurodegenerative disease characterized by progressive degeneration of the motor neurons in the cortex, brainstem, and spinal cord. The etiology and mechanisms of selective motor neuron loss in ALS remain unknown. Wnt signaling is involved in neurodegenerative processes but little is known about the kinetic changes in Wnt signaling during ALS progression. In this study we used transcriptional microarray analysis to examine the expression of Wnt signaling components in the spinal cords of ALS transgenic SOD1G93A mice at different stages. We found that ALS onset led to the upregulation of Wnt signaling components and target genes involved in growth regulation and proliferation. We also determined the expression of Wnt inhibitory factor-1 (Wif1) and Wnt4 in the spinal cord of ALS transgenic mice at different stages by Western blot and immunofluorescence analysis. The protein levels of Wif1 and Wnt4 in the spinal cords of ALS transgenic mice were upregulated compared to those in wild-type mice. Moreover, the expression of Wif1 and Wnt4 in mature GFAP+ astrocytes was increased at the end stage of ALS. Our findings demonstrate that Wnt signaling is altered by spinal cord neuronal dysfunction in adult ALS transgenic mice, which provides new insight into ALS pathogenesis.
Keywords: Amyotrophic lateral sclerosis, Wnt, PCR array, Spinal cord, Transgenic mice
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive fatal neurodegenerative disease that affects motor neurons in the brainstem, spinal cord and motor cortex [1]. Although the causes of most cases of ALS are not well defined, ALS is characterized by multiple perturbations of cellular function in motor neurons, incriminating excessive excitatory tone, protein misfolding, impaired energy production, abnormal calcium metabolism, altered axonal transport, and activation of proteases and nucleases. The mechanisms underlying neurodegeneration in ALS are multifactorial and involve multiple molecular and genetic pathways [2, 3]. Roughly 20–25 % of familial ALS cases arise due to the mutations in the copper/zinc superoxide dismutase 1 (SOD1) gene [4, 5]. Transgenic mice expressing human mutant SOD1 (mSOD1) develop age-dependent clinical and pathological features closely mirroring those in human ALS and thus provide an excellent model to investigate the pathogenic mechanisms of ALS [6]. Using this mouse model we previously observed significant stem cell proliferation in the spinal cord in response to neurodegeneration in ALS, but the underlying mechanism remains elusive [7].
Wnt signaling is known to play important role in the regulation of cell survival, proliferation, and differentiation [8]. Wnt signaling is crucially implicated in neurogenesis and neurodegenerative processes such as Alzheimer’s disease and Huntington’s disease [9–11]. Recently, the activation of Wnt signaling was shown to stimulate the proliferation and neurogenesis of spinal neural precursors and promote neurite outgrowth. Wnt signaling pathways can be divided into canonical pathway and noncanonical pathway. The canonical pathway is initiated by binding of Wnt to Fzd (Frizzeld)/LRP5/6 (low-density lipoprotein receptor-related proteins 5 and 6) and is mediated by the transcriptional activity of β-catenin. Accumulated cytosolic β-catenin translocates to the nucleus, where it interacts with TCF (T cell factor)/LEF (lymphocyte-enhancing factor) and activates transcription of target genes including Cyclin D1, c-Myc, which then regulate cell fate and morphogenesis. The distinct pathways that are activated by Fzd/Wnt but do not involve β-catenin are referred to as noncanonical pathways, which include Wnt/calcium signaling and planar cell polarity (PCP) pathway [12, 13]. Interestingly, our recent results showed that the expression of canonical Wnt signaling components Wnt3a, β-catenin and target gene Cyclin D1 in mature GFAP+ astrocytes was increased in the spinal cord of ALS transgenic mice [14]. Therefore, we hypothesized that Wnt/β-catenin pathway regulates cell proliferation in response to neurodegeneration in ALS. To gain a wide view of Wnt signaling in the pathogenesis of ALS, in this study we examined the expression of Wnt signaling components in the spinal cord of ALS transgenic mice at different stages by transcriptional microarray analysis.
Materials and Methods
Animal and Tissue Preparation
Transgenic hSOD1-G93A mice (The Jackson Laboratory, Bar Harbor, ME, USA) were crossbred. Offspring were genotyped using a polymerase chain reaction (PCR) assay on tail DNA as suggested by the Jackson Laboratory genotyping protocol. All animals were assessed with a set of behavioral tests as described previously. All experiments were carried out in accordance with Institutional Animal Care and Use Committee guidelines. According to our previous study [7], the mice were initially evaluated at the early symptomatic stage of the disease (95 days). ALS and wild-type mice were anesthetized deeply at the early stage (95 days), middle stage (108 days), or end stage (122 days), and spinal cords were removed. For immunofluorescence analysis, lumbar spinal cords were fixed in 4 % paraformaldehyde overnight at 4 °C and then transferred to 30 % sucrose for cryopreservation. Spinal cords were cut into 5 mm coronal segments and embedded with OCT mounting medium. They were further sectioned coronally on a cryostat (CM1900; Leica, Germany) into 10 μm slices. For PCR and Western blotting analysis, lumbar spinal cords were immediately frozen in liquid nitrogen and stored until use.
Gene Expression Profiling PCR Array
Total RNA was isolated from the homogenized frozen spinal cord tissue of the mice using TRIzol reagent (Invitrogen) and then purified using RNeasy® MinElute™ Cleanup Kit (Qiagen). The integrity of total RNA was examined by 1 % agarose gel electrophoresis. In addition, the spectrophotometric absorbance of RNA samples at 230, 260 and 280 nm and ratios of D260:D280 and D260:D230 were measured spectrophotometrically (NanoDrop Spectrophotometer ND-1000, USA), to determine the purity and concentration of total RNA. RNA was reverse-transcribed using SuperScriptIII Reverse Transcriptase (Invitrogen), and then quantitative real-time PCR analysis of 84 genes related to Wnt signaling was performed using Mouse Wnt Signaling Pathway RT2 Profiler™ PCR Array (SA Biosciences, Frederick, MD, USA) following the manufacturer’s protocol. Relative gene expression was calculated using the 2−ΔΔCt method, in which Ct indicated the fractional cycle number at which the fluorescent signal reached detection threshold. The normalized ΔCt value of each sample was calculated using a total of five endogenous control genes. Fold change values were presented as average fold change = 2(average ΔΔCt) for genes in ALS transgenic mice relative to those in wild-type mice. The statistical significance of differences in gene expression between ALS and wild-type groups was calculated using a two-tailed Student’s t test (p < 0.05).
Western Blotting Analysis
Spinal cords were removed and homogenized at 4 °C in cell lysis buffer (50 mM Tris–HCl, pH 7.4, 50 mM NaCl, 1 % Triton-100, 1 mM EDTA, pH 8.0, 100 μg/ml PMSF, 1:1,000 cocktail). Lysates were centrifuged at 12,000g for 15 min to collect supernatant. Protein concentration in the lysates was measured by the Bradford dye-binding procedure. Proteins were run on 12 % SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). The membranes were blocked with 5 % milk–Tris-buffered saline Tween-20 at 20 °C for 1 h, then incubated with rabbit anti-Wif1 IgG (1:400, Santa Cruz), goat anti-Wnt4 IgG (2 μg/ml, R&D systems), and mouse anti-γ-tubulin antibody (1:20,000, Sigma), followed by incubation with peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch). The immunoreactive bands were detected with enhanced chemiluminescence reagents (Amersham Pharmacia Biotech) and analyzed using lab-works software (Ultra-Violet Products, Cambridge, UK).
Immunofluorescence
Cryostat sections were blocked by soaking in 5 % normal goat serum for 1 h. Primary antibodies were simultaneously applied overnight at 4 °C. The following primary antibodies were used at the suggested dilutions: rabbit anti-Wif1 IgG (Santa Cruz), rabbit anti-Wnt4 IgG (ProteinTech Group), chicken anti-Tuj1 IgY (Abcam), and chicken anti-GFAP IgY (Abcam). Immunolabelling was visualized using cy3 goat anti-rabbit IgG (Jackson Immunoresearch) and goat anti-chicken IgY-H&L (DyLight® 488) (Abcam), and sections were examined by confocal microscopy (Olympus FV500, Japan).
Statistical Analysis
Statistical analyses were performed using SPSS 13.0 for Windows. All data were expressed as means with standard deviations and analyzed using one-way analysis of variance. Differences with p < 0.05 were considered statistically significant. Hierarchical clustering was performed using MEV software (v4.6, TIGR).
Results
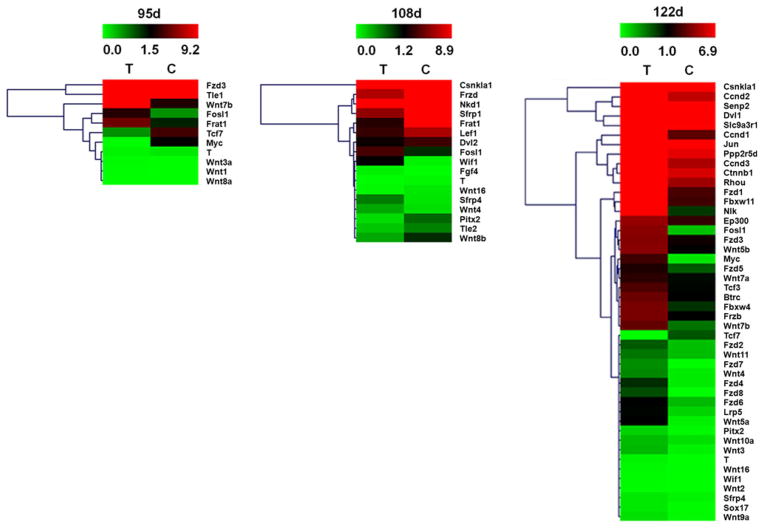
We sought to identify differentially regulated Wnt signaling molecules by comparing the transcription profiles of spinal cord from ALS and wild-type mice at different stages. We only selected transcripts that showed an increase/decrease of at least twofold with statistical significance in ALS mice compared to wild-type mice (Table 1). At 95 days, total 11 transcripts were identified with statistically differential expression: 8 transcripts were up-regulated and 3 transcripts were down-regulated. At 108 days, total 17 transcripts were statistically differentially expressed: 5 transcripts were up-regulated and 12 transcripts were down-regulated. At 122 days, 46 genes were differentially expressed: 45 transcripts were up-regulated and 1 transcript was down-regulated (Fig. 1). We found that canonical Wnt signaling molecules including Wnt1, Wnt3a, Wnt7b, and Wnt8a were up-regulated at 95 days; Wnt10a, Wnt16, Wnt2, Wnt3, Wnt7a, Wnt7b, and Wnt9a were all up-regulated at 122 days; while Wnt16 and Wnt8b were down-regulated at middle stage. Meanwhile, non-canonical Wnt signaling molecules such as Wnt4 were up-regulated at 108 days, and Wnt5a, Wnt5b, and Wnt11 were up-regulated at 122 days. Wnt inhibitors and antagonists including Wif1 and sfrp4 were up-regulated from 108 days. Frizzled receptors including Fzd1 to Fzd8 and coreceptor Lrp5 were all up-regulated at 122 days. At the end stage of the disease, Wnt target genes including Ccnd1, Ccnd2, Ccnd3, Ep300, Fosl1, NIk, and Pitx2 were all up-regulated.
Table 1.
Dysregulated components of Wnt signaling in the spinal cords of ALS mice compared to WT mice
| Symbol | Description | Fold difference
|
||
|---|---|---|---|---|
| 95 days | 108 days | 122 days | ||
| Wnt ligands | ||||
| Wnt1 | Wingless-related MMTV integration site 1 | 2.10 | −1.69 | 1.23 |
| Wnt10a | Wingless related MMTV integration site 10a | 1.79 | −1.52 | 2.08 |
| Wnt11 | Wingless-related MMTV integration site 11 | −1.06 | −1.76 | 2.07 |
| Wnt16 | Wingless-related MMTV integration site 16 | 1.80 | −3.42 | 2.39 |
| Wnt2 | Wingless-related MMTV integration site 2 | 1.37 | 1.74 | 8.38 |
| Wnt3 | Wingless-related MMTV integration site 3 | −1.64 | −1.78 | 5.40 |
| Wnt3a | Wingless-related MMTV integration site 3A | 7.47 | 1.40 | 1.01 |
| Wnt4 | Wingless-related MMTV integration site 4 | 1.64 | 2.59 | 5.76 |
| Wnt5a | Wingless-related MMTV integration site 5A | 1.41 | −1.10 | 15.65 |
| Wnt5b | Wingless-related MMTV integration site 5B | 1.12 | −1.47 | 3.65 |
| Wnt7a | Wingless-related MMTV integration site 7A | 1.99 | 1.79 | 2.11 |
| Wnt7b | Wingless-related MMTV integration site 7B | 3.91 | 1.44 | 6.10 |
| Wnt8a | Wingless-related MMTV integration site 8A | 2.78 | −1.55 | 1.23 |
| Wnt8b | Wingless related MMTV integration site 8b | −1.11 | −2.38 | −1.12 |
| Wnt9a | Wingless-type MMTV integration site 9A | 1.91 | −1.02 | 5.32 |
| Receptors | ||||
| Fzd1 | Frizzled homolog 1 (Drosophila) | 1.67 | 1.05 | 3.42 |
| Fzd2 | Frizzled homolog 2 (Drosophila) | −1.21 | 1.10 | 2.59 |
| Fzd3 | Frizzled homolog 3 (Drosophila) | 2.23 | −1.05 | 3.03 |
| Fzd4 | Frizzled homolog 4 (Drosophila) | 1.00 | −1.05 | 10.11 |
| Fzd5 | Frizzled homolog 5 (Drosophila) | −1.13 | −1.48 | 2.56 |
| Fzd6 | Frizzled homolog 6 (Drosophila) | 1.95 | 1.80 | 3.70 |
| Fzd7 | Frizzled homolog 7 (Drosophila) | 1.04 | −1.94 | 29.03 |
| Fzd8 | Frizzled homolog 8 (Drosophila) | 1.73 | 1.18 | 70.87 |
| Lrp5 | Low density lipoprotein receptor-related protein 5 | 1.34 | 1.80 | 5.76 |
| Downstream molecules | ||||
| Btrc | Beta-transducin repeat containing protein | −1.24 | −1.86 | 3.36 |
| Ccnd1 | Cyclin D1 | −1.17 | 1.37 | 7.71 |
| Ccnd2 | Cyclin D2 | −1.34 | −1.03 | 7.24 |
| Ccnd3 | Cyclin D3 | −1.28 | −1.34 | 2.41 |
| Csnk1a1 | Casein kinase 1, alpha 1 | 1.30 | −2.96 | 2.46 |
| Ctnnb1 | Catenin (cadherin associated protein), beta 1 | 1.30 | −1.25 | 2.18 |
| Dvl1 | Dishevelled, dsh homolog 1 (Drosophila) | 1.04 | −1.55 | 4.70 |
| Dvl2 | Dishevelled 2, dsh homolog (Drosophila) | −1.13 | −2.01 | 1.04 |
| Ep300 | E1A binding protein p300 | −1.32 | −1.45 | 2.04 |
| Fbxw11 | F-box and WD-40 domain protein 11 | −1.03 | −1.46 | 2.74 |
| Fbxw4 | F-box and WD-40 domain protein 4 | −1.24 | 1.03 | 4.69 |
| Fgf4 | Fibroblast growth factor 4 | −1.19 | 12.13 | 1.23 |
| Fosl1 | Fos-like antigen 1 | 4.06 | 3.34 | 17.00 |
| Frat1 | Frequently rearranged in advanced T-cell lymphomas | 3.64 | −3.72 | 1.13 |
| Frzb | Frizzled-related protein | 1.22 | −2.90 | 3.70 |
| Jun | Jun oncogene | 1.07 | −1.03 | 2.44 |
| Lef1 | Lymphoid enhancer binding factor 1 | 1.27 | −2.19 | −1.34 |
| Myc | Myelocytomatosis oncogene | −829.19 | 1.63 | 21.92 |
| Nkd1 | Naked cuticle 1 homolog (Drosophila) | 1.25 | −2.08 | 1.07 |
| Nlk | Nemo like kinase | −1.17 | −1.56 | 10.28 |
| Pitx2 | Paired-like homeodomain transcription factor 2 | −1.53 | −4.24 | 7.35 |
| Ppp2r5d | Protein phosphatase 2, regulatory subunit B (B56), delta isoform | 1.30 | −1.06 | 2.91 |
| Rhou | Ras homolog gene family, member U | 1.30 | −1.63 | 3.17 |
| Senp2 | SUMO/sentrin specific peptidase 2 | 1.07 | −1.04 | 4.68 |
| Sfrp1 | Secreted frizzled-related protein 1 | −1.03 | −3.97 | −1.27 |
| Sfrp4 | Secreted frizzled-related protein 4 | −1.66 | 5.13 | 2.03 |
| Slc9a3r1 | Solute carrier family 9 (sodium/hydrogen exchanger), member 3 regulator 1 | −1.09 | 1.09 | 2.48 |
| Sox17 | SRY-box containing gene 17 | 1.78 | −1.23 | 6.74 |
| T | Brachyury | −2.25 | −3.66 | 25.27 |
| Tcf3 | Transcription factor 3 | 1.15 | −1.26 | 2.91 |
| Tcf7 | Transcription factor 7, T-cell specific | −5.36 | −1.35 | −298.38 |
| Tle1 | Transducin-like enhancer of split 1, homolog of Drosophila E(spl) | 2.27 | 1.01 | 1.63 |
| Tle2 | Transducin-like enhancer of split 2, homolog of Drosophila E(spl) | −1.48 | −2.17 | −1.34 |
| Wif1 | Wnt inhibitory factor 1 | −1.11 | 24.80 | 2.13 |
Positive fold difference indicated up-regulated while negative fold difference indicated down-regulated
Bold values indicate transcripts that showed an increase/decrease of at least twofold with statistical significance in ALS mice compared to WT mice
Fig. 1.
Hierarchical clustering of the signal value of differentially expressed genes of Wnt signaling in the spinal cords of ALS mice and wild-type (WT) mice at different stages. The dendrogram was produced by hierarchical clustering of the signal value of differentially expressed genes (rows) in three ALS mice and three WT mice (columns). Green indicated down-regulated genes, black indicated intermediate change, and red indicated up-regulated genes. The hierarchical clustering successfully discriminated the signal value of genes between ALS mice (samples T) and WT mice (samples C) (Color figure online)
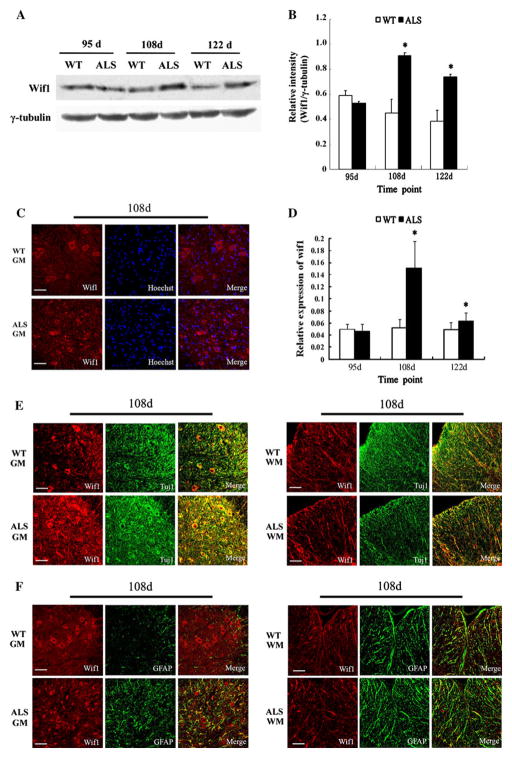
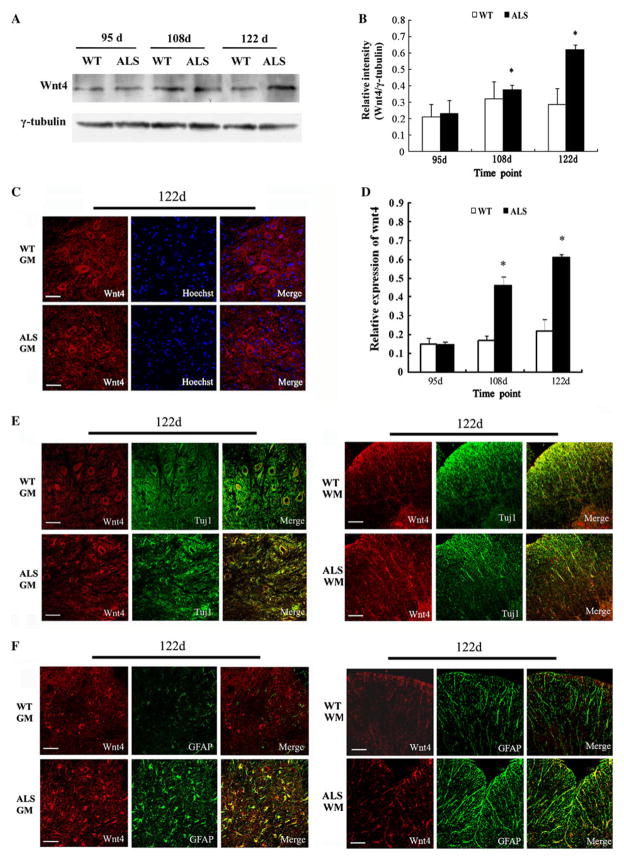
To confirm that Wnt signaling was activated in the later stage of ALS, we selected Wif1 and Wnt4 to detect their protein levels. Western blot analysis showed highly increased protein level of Wif1 at 108 days (p < 0.05), which was further increased at 122 days (p < 0.05) in the spinal cords of ALS mice compared to the wild-type group, but there was no statistical difference at 95 days (Fig. 2a, b). Compared to wild-type mice, the spinal cords of ALS mice demonstrated increased levels of Wnt4 protein at 108 days (p < 0.05), which was further increased at 122 days (p < 0.05) but not at 95 days (Fig. 3a, b).
Fig. 2.
Upregulation of Wif1 in the spinal cords of ALS mice compared with wild-type (WT) mice. a Immunoblotting of Wif1 level in the spinal cord samples at different stages. γ-tubulin was used as loading control. b The relative level of Wif1 protein detected by Western blot analysis (n = 5); *p < 0.05 versus WT littermates. c Staining of spinal cord sections showed the distribution of Wif1-positive cells in ALS mice and WT mice at 108 days, bar 50 μm. d Quantitative analysis of the number of Wif1-positive cells in ALS versus WT mice at different disease stages (n = 5), *p < 0.05 versus. WT littermates. e Wif1 (red) and Tuj1 (green) double-positive cells in gray matter (GM) and white matter (WM) at 108 days; bar 50 μm. f Wif1 (red) and GFAP (green) double-positive cells in gray matter (GM) and white matter (WM) at 108 days; bar 50 μm (Color figure online)
Fig. 3.
Upregulation of Wnt4 in the spinal cords of ALS mice compared with wild-type (WT) mice. a Immunoblotting of Wnt4 level in the spinal cord samples at different stages. γ-tubulin was used as loading control. b The relative level of Wnt4 protein detected by Western blot analysis (n = 5); *p < 0.05 vs. WT littermates. c Staining of spinal cord sections showed the distribution of Wnt4-positive cells in ALS mice and WT mice at 122 days, bar 50 μm. d Quantitative analysis of the number of Wnt4-positive cells in ALS versus wild-type mice at different disease stages (n = 5), *p < 0.05 vs. WT littermates. e Wnt4 (red) and Tuj1 (green) double-positive cells in gray matter (GM) and white matter (WM) at 122 days; bar 50 μm. f Wnt4 (red) and GFAP (green) double-positive cells in gray matter (GM) and white matter (WM) at 122 days; bar 50 μm (Color figure online)
Next we investigated the spatial differential expression of Wnt singlaing molecules in the spinal cord of ALS mice compared to wild-type mice. Immunofluorescence staining showed that Wif1-positive cells were detected in gray matter, white matter, and the central canal in wild-type mice. Wif1 expression in ALS mice was identified in a large number of cells, especially within the gray matter; the vast majority of Wif1-positive cells were in the ventral horn, the locus of neurodegeneration; and a few positive cells were detected in the dorsal horns. Wif1 expression was also detected in ependymal cells around the central canal and the nerve fibers within the white matter. At 108 and 122 days, more Wif1-positive cells were found in ALS mice than in their wild-type counterparts (Fig. 2c, d). Furthermore, Wif1/Tuj1 and Wif1/GFAP double immunofluorescence staining were performed to demonstrate the localization of Wif1 in differentiated cells (Fig. 2e, f). The results showed that in ALS mice most of the Wif1-positive cells were immunopositive for Tuj1, and a few of the Wif1-positive cells were immunopositive for GFAP. However, Wif1/GFAP double-positive cells in ALS mice were increased at middle stage (108 days) and end stage (122 days), compared to wild-type mice.
Wnt4-positive cells were sparsely distributed in grey matter, white matter, and the central zones of the spinal cord in wild-type mice, but not among the ependymal cells around the central canal. At 108 and 122 days, more Wnt4-positive cells were found in ALS mice than in wild-type mice (Fig. 3c, d). Within the gray matter, a lot of Wnt4-positive cells were detected in the ventral horn, the locus of neurodegeneration. Wnt4 was mainly expressed in Tuj1+ neurons, with a small amount in mature GFAP+ astrocytes (Fig. 3e, f). However, Wnt4/GFAP double-positive cells were markedly increased in ALS mice compared to wild-type mice, especially at the end stage (122 days).
Discussion
Substantial evidence suggests that Wnt signaling plays a critical role in determining the balance between neuronal survival and death in a variety of degenerative states [15–17]. Previous studies have identified differentially expressed and alternatively spliced genes involved in ALS, which function in cell adhesion, immune-inflammatory response and lipid metabolism [17, 18]. These data suggest a multi-factor mechanism in ALS development. Using PCR array, we found that 58 genes associated with Wnt signaling exhibited significant changes of expression in ALS mice compared to wild-type mice. Among them, 15 belong to Wnt ligands, 9 are Wnt receptor or co-receptor, 34 are downstream signaling molecules. In addition, regulators of Wnt signaling are included as well as downstream target genes. These data suggest that different kinds of Wnt signaling components show altered expression in ALS.
Wnt signaling is involved in the development of the brain and spinal cord and the extension of numerous subpopulations of sensory and motor neurons. Moreover, some CNS-related diseases in adulthood have been associated with disregulated Wnt signaling [10]. According to our results, the canonical Wnt signaling was upregulated in response to ALS pathological processes, especially Wnt3a at the early stage of this disease. The activation of the Wnt pathway is under tight control through β-catenin degradation and aberrant Wnt signaling may result in abnormal cell proliferation and tumorigenesis. The frizzled secreted ligands (such as Wnt1 and Wnt3a) triggers signaling that leads to the inhibition of β-catenin proteolytic degradation [19]. It has been shown that the upregulation of Wnt3a induced neurogenesis in the neocortex [20]. We have observed previously that Wnt3a mRNA was upregulated from the beginning stage in the adult spinal cord of ALS transgenic mice. In addition, β-catenin is translocated from the cell membrane to the nucleus and subsequently activates the transcription of target gene Cyclin D1 [14]. Our data of PCR array demonstrate that almost all Fzd family and Lrp5 had elevated expression in the spinal cord of ALS mice compared to wild-type mice at the end stage, indicating that Fzds and Lrp5 interpret canonical Wnt signals during the process of ALS disease. Only transcription factor Tcf7 was downregulated during ALS pathology. TCF/LEF members undergo a transcriptional switch from repression to activation. Indeed, the cellular processes are under the control of feedback mechanisms and crosstalk with various signaling pathways such as nuclear receptors and p53 family members to modulate Wnt signaling [21]. In this study, we found that Ccnd1 (Cyclin D1), Myc, Fosl1 and Pitx2, which are representative target genes of canonical Wnt signaling cascade, were upregulated in the spinal cord of ALS mice at the end stage. Myc and Cyclin D1 promote cellular proliferation via cell cycle progression and cell cycle proteins have been shown to be activated after traumatic brain injury and regulate neuron death and the proliferation of astrocytes [22, 23]. Fos-like antigen-1 (Fosl1) is a target gene of the NMDAR-mediated β-catenin signaling system regulating neuronal function [24]. Nemo-like kinase (NLK) positively regulates the transcriptional activity of LEF1, which regulates Wnt/β-catenin signaling to control cell proliferation and fate decision [25]. The bicoid-related transcription factor (Pitx2) is rapidly induced by Wnt/β-catenin pathway and is required for effective cell-type-specific proliferation by directly activating specific growth-regulating genes [26]. Taken together, these data indicate the possibility that Wnt/β-catenin signaling promotes the proliferation of astrocyte during ALS, and the increase in astrocytes in response to neurodegeneration is involved in the regulation of neurogenesis.
Wnt also activates non-canonical pathways to regulate planar cell polarity, cell adhesion, and motility [27]. In this study, we found that non-canonical Wnt signaling was also upregulated in the spinal cord of ALS mice at the end stage. Meanwhile, we confirmed that the protein level of Wnt4 was upregulated from the middle stage. Wnt4 can elicit non-canonical pathways, transmitting through the planar cell polarity receptor Fzd6 in murine hematopoietic progenitor cells [28, 29]. In ALS mice, especially at 122 days, the expression of Wnt4 in mature GFAP+ astrocytes increased and the expression of Wnt4 in Tuj1+ neurons decreased, perhaps due to the loss of neurons at the end stage of neurodegenerative disease. These results suggest that both canonical and non-canonical Wnt pathways are involved in astrocyte proliferation during ALS neurodegeneration. In addition, studies based on SOD1 mice model indicate that non-neuronal cells play important role in neurodegeneration through non-cell autonomous mechanism. The impaired astrocytic functions such as the clearance of extracellular glutamate and the release of neurotrophic factors are implicated in ALS [30]. Recent findings indicate that Wnts released by astrocytes act via canonical Wnt/β-catenin pathway to dictate the neurogenic behavior in the adult CNS both in normal brain or in mouse models of neuodegenration disease [16, 31]. Based on these results, we propose that Wnt signaling may act as a regulatory pathway in spinal cord astrocyte proliferation to modulate neuronal fate commitment or the proliferation of neuronally committed precursor cells.
Wnt/β-catenin signaling is regulated at many levels. Numerous soluble antagonists of Wnt signaling, such as secreted Fz related proteins (sfrps), Wnt inhibitory factor (Wif) and Dickkopf homolog-1(DKK-1) have been identified that antagonize Wnt signaling either by blocking the binding of Wnt ligands to the cell surface receptors or by direct interaction with the receptor complex. In addition, Wnt signaling is regulated by a complex network of intracellular feedback loops, such as the expression of canonical Wnt target gene naked cuticle (nkd), which blocks the activity of Dvl/Dsh, or by dual function of the kinases CK1 and GSK3, which act as antagonists at the level of the β-catenin destruction complex, but also act as agonists at the plasma membrane by sequentially phosphorylating LRP6 [32, 33]. We found that Sfrp4 and wif1 were upregulated in the spinal cord of ALS mice, especially wif1 at the middle stage. Moreover, Wif1 expression was markedly increased in ALS mice during the symptomatic stage (108 days). Wif1 is known to be involved in a feedback mechanism to regulate Wnt signaling [34, 35]. Interestingly, we found that the vast majority of Wif1-positive cells were in the locus of neurodegeneration, and Wif1 was expressed predominantly in neurons. It is known that during ALS disease onset and progression, neural progenitor cells in the ependymal zone surrounding the central canal migrate initially toward the dorsal horn, and then to the ventral horn regions, where motor neurons have degenerated [36]. Therefore, these findings suggest that Wif1 may inhibit the neurogenesis process by inhibiting Wnt signaling in response to ALS.
In summary, in this study we demonstrate that Wnt signaling is altered or involved in the pathophysiology of ALS, which provides new insight into the pathogenesis of ALS. However, further studies are needed to characterize the individual role of Wnt signaling components in ALS in order to identify novel therapeutic targets for ALS.
Acknowledgments
We thank Dr. Li at Tongji Medical College of Huazhong University of Science and Technology. This work was supported by grants from the National Natural Science Foundation of China (Y.G., 30871314, 81271413), the Shandong Provincial Science and Technology Department of China (2012GSF11827), and the National Institutes of Health/National Institute of Neurological Disorders and Stroke (X.W.).
Contributor Information
Li Yu, Department of Histology and Embryology, Weifang Medical University, Weifang 261042, Shandong, China.
Yingjun Guan, Email: yjunguan@163.com, Guanyj@wfmc.edu.cn, Department of Histology and Embryology, Weifang Medical University, Weifang 261042, Shandong, China.
Xin Wu, Department of Histology and Embryology, Weifang Medical University, Weifang 261042, Shandong, China.
Yanchun Chen, Department of Histology and Embryology, Weifang Medical University, Weifang 261042, Shandong, China.
Zhijun Liu, Department of Medical Microbiology, Weifang Medical University, Weifang, Shandong, China.
Hongmei Du, Department of Histology and Embryology, Weifang Medical University, Weifang 261042, Shandong, China.
Xin Wang, Email: xwang@rics.bwh.havard.edu, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, 221 Longwood Ave, Boston, MA 02115, USA.
References
- 1.Tripathi VB, Al-Chalabi A. Molecular insights and therapeutic targets in amyotrophic lateral sclerosis. CNS Neurol Disord: Drug Targets. 2008;7:11–19. doi: 10.2174/187152708783885110. [DOI] [PubMed] [Google Scholar]
- 2.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 3.Usuki S, Kamitani T, Matsuo Y, et al. Pathobiochemical effect of acylated steryl-beta-glucoside on aggregation and cytotoxicity of alpha-synuclein. Neurochem Res. 2012;37:1261–1266. doi: 10.1007/s11064-011-0662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 5.Kuzma-Kozakiewicz M, Kwiecinski H. New therapeutic targets for amyotrophic lateral sclerosis. Expert Opin Ther Targets. 2011;15:127–143. doi: 10.1517/14728222.2011.542152. [DOI] [PubMed] [Google Scholar]
- 6.Gama Sosa MA, De Gasperi R, Elder GA. Modeling human neurodegenerative diseases in transgenic systems. Hum Genet. 2011;131:535–563. doi: 10.1007/s00439-011-1119-1. [DOI] [PubMed] [Google Scholar]
- 7.Guan YJ, Wang X, Wang HY, et al. Increased stem cell proliferation in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. J Neurochem. 2007;102:1125–1138. doi: 10.1111/j.1471-4159.2007.04610.x. [DOI] [PubMed] [Google Scholar]
- 8.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 9.Boonen RA, van Tijn P, Zivkovic D. Wnt signaling in Alzheimer’s disease: up or down, that is the question. Ageing Res Rev. 2009;8:71–82. doi: 10.1016/j.arr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 11.Godin JD, Poizat G, Hickey MA, et al. Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington’s disease. EMBO J. 2010;29:2433–2445. doi: 10.1038/emboj.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–2109. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- 13.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Guan Y, Liu H, et al. Activation of the Wnt/β-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun. 2012;420:397–403. doi: 10.1016/j.bbrc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Clevers H, Nusse R. Wnt/beta-Catenin Signaling and Disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 16.L’Episcopo F, Serapide MF, Tirolo C, et al. A Wnt1 regulated Frizzled-1/beta-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol Neurodegener. 2011;6:49. doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Guo Y, Hu M, et al. Differential expression and alternative splicing of genes in lumbar spinal cord of an amyotrophic lateral sclerosis mouse model. Brain Res. 2010;1340:52–69. doi: 10.1016/j.brainres.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 18.Kudo LC, Parfenova L, Vi N, et al. Integrative gene-tissue microarray-based approach for identification of human disease biomarkers: application to amyotrophic lateral sclerosis. Hum Mol Genet. 2010;19:3233–3253. doi: 10.1093/hmg/ddq232. [DOI] [PubMed] [Google Scholar]
- 19.Kaur P, Mani S, Cros MP, et al. Epigenetic silencing of sFRP1 activates the canonical Wnt pathway and contributes to increased cell growth and proliferation in hepatocellular carcinoma. Tumour Biol. 2012;33:325–336. doi: 10.1007/s13277-012-0331-5. [DOI] [PubMed] [Google Scholar]
- 20.Munji RN, Choe Y, Li G, et al. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J Neurosci. 2011;31:1676–1687. doi: 10.1523/JNEUROSCI.5404-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao CD, Byers SW. Cell-context dependent TCF/LEF expression and function: alternative tales of repression, derepression and activation potentials. Crit Rev Eukaryot Gene Expr. 2011;21:207–236. doi: 10.1615/critreveukargeneexpr.v21.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh M. Network of WNT and other regulatory signaling cascades in pluripotent stem cells and cancer stem cells. Curr Pharm Biotechnol. 2011;12:160–170. doi: 10.2174/138920111794295710. [DOI] [PubMed] [Google Scholar]
- 23.Di Giovanni S, Movsesyan V, Ahmed F, et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci USA. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Ota S, Ishitani S, Shimizu N, et al. NLK positively regulates Wnt/beta-catenin signalling by phosphorylating LEF1 in neural progenitor cells. EMBO J. 2012;31:1904–1915. doi: 10.1038/emboj.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enjin A, Rabe N, Nakanishi ST, et al. Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as markers for fast motor neurons and partition cells. J Comp Neurol. 2010;518:2284–2304. doi: 10.1002/cne.22332. [DOI] [PubMed] [Google Scholar]
- 27.Katoh M. WNT signaling in stem cell biology and regenerative medicine. Curr Drug Targets. 2008;9:565–570. doi: 10.2174/138945008784911750. [DOI] [PubMed] [Google Scholar]
- 28.Kleber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 29.KMH Wnt4 enhances murine hematopoietic progenitor cell expansion through a planar cell polarity-like pathway. PLoS ONE. 2011;6:e19279. doi: 10.1371/journal.pone.0019279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lasiene J, Yamanaka K. Glial cells in amyotrophic lateral sclerosis. Neurol Res Int. 2011;2011:718987. doi: 10.1155/2011/718987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 32.Bovolenta P, Esteve P, Ruiz JM, et al. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 33.Buechling T, Boutros M. Wnt signaling at and above the receptor level. Curr Top Dev Biol. 2011;97:21–53. doi: 10.1016/B978-0-12-385975-4.00008-5. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh JC, Kodjabachian L, Rebbert ML, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 35.Hu YA, Gu X, Liu J, et al. Expression pattern of Wnt inhibitor factor 1(Wif1) during the development in mouse CNS. Gene Expr Patterns. 2008;8:515–522. doi: 10.1016/j.gep.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Chi L, Ke Y, Luo C, et al. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells. 2006;24:34–43. doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]