Abstract
The aim of this study was to determine whether the cumulative effects of head impacts from a season of high school football produce magnetic resonance imaging (MRI) measureable changes in the brain in the absence of clinically diagnosed concussion. Players from a local high school football team were instrumented with the Head Impact Telemetry System (HITS™) during all practices and games. All players received pre- and postseason MRI, including diffusion tensor imaging (DTI). Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) was also conducted. Total impacts and risk-weighted cumulative exposure (RWE), including linear (RWELinear), rotational (RWERotational), and combined components (RWECP), were computed from the sensor data. Fractional, linear, planar, and spherical anisotropies (FA, CL, CP, and CS, respectively), as well as mean diffusivity (MD), were used to determine total number of abnormal white matter voxels defined as 2 standard deviations above or below the group mean. Delta (post-preseason) ImPACT scores for each individual were computed and compared to the DTI measures using Spearman's rank correlation coefficient. None of the players analyzed experienced clinical concussion (N=24). Regression analysis revealed a statistically significant linear relationship between RWECP and FA. Secondary analyses demonstrated additional statistically significant linear associations between RWE (RWECP and RWELinear) and all DTI measures. There was also a strong correlation between DTI measures and change in Verbal Memory subscore of the ImPACT. We demonstrate that a single season of football can produce brain MRI changes in the absence of clinical concussion. Similar brain MRI changes have been previously associated with mild traumatic brain injury.
Key words: : diffusion tensor imaging, football, Head Impact Telemetry System, mild traumatic brain injury, risk-weighted cumulative exposure
Introduction
There are over 5 million athletes playing organized football in the United States, with the vast majority in the youth and high school leagues.1–3 The effects of head impacts on these vulnerable younger players have not been well studied. Studies of head impacts are typically related to concussion. Football has the highest concussion rate, compared to other contact sports.4 Concussion in football players occurs at a wide range of impact magnitudes,5 and clinical measures of symptom severity are independent of impact magnitude and location.5,6 Concussions at the low end of magnitude present with as many clinical deficits as the higher end of magnitude. Whereas concussion can represent a serious and immediate outward manifestation of any head impact, the more indolent effects of repeated subconcussive impacts are largely unknown.
Conventional neuroimaging methods are typically unremarkable in the setting of mild traumatic brain injury (mTBI)7, whereas diffusion tensor imaging (DTI) has been successful in revealing subtle underlying changes in white matter (WM) integrity.8 The reproducibility of DTI metrics (e.g., fractional anisotropy [FA] and mean diffusivity [MD]) is sufficient to track relatively small longitudinal changes in single subjects9 within limits of motion and noise. Most studies of TBI and mTBI have reported decreases in FA and increases in MD as a result of demyelination or disruption of tissue structure.10–17 There is, however, a growing body of evidence, especially in the realm of sports-related mTBI, that FA values increase in both acute and chronic phases,18–22 with abnormalities typically identified in the corpus callosum (CC). Voxel-wise and region of interest–based analyses of DTI data have typically been used to compare FA values across time and subjects. A central assumption to these methods is that the location of injury is similar across subjects.23,24 The sensitivity to subtle DTI changes, especially for subconcussive impacts, can be improved by using methods that are not dependent on spatial co-localization of the injury.25,26
In order to relate changes in imaging data or cognitive function to head impacts, an effective method of measuring the underlying biomechanical response is required. This information can be provided by the Head Impact Telemetry System (HITS™). The HITS was developed by SIMBEX with the assistance of Virginia Tech from 2000 to 2003 and has been implemented in several high schools and colleges to collect real-time data in practices and games.5,27–30 It consists of a base unit, charging station, and MX Encoders for each helmet, which measure translational and rotational accelerations of the skull, not of the helmet. The HITS is placed beside the game field and collects real-time data on number of impacts, duration of impact, time between impacts, as well as linear and rotational acceleration, which can be analyzed in terms of the peak gs, impact location, and other biomechanical indicators.
Research on the biomechanics of impact has focused primarily on collegiate football players.5,27,28,30,31 Studies of head impact exposure for high school and youth football populations are only now beginning to emerge.3,32–34 These demonstrate accelerations, even for the youngest players, approaching those of collegiate athletes. A recent study of asymptomatic (clinically nonconcussed) high school football players (∼50 players over two seasons) related HITS findings to cognition and functional magnetic resonance imaging (fMRI) n-back memory testing. Significant associations were found between pre- and postseason fMRI changes, number of head blows, and functional impairment (change in ImPACT testing in at least one domain).35,36 Remarkably, 50% of nonconcussed players were found to have functional impairments. In a study relating HITS findings to DTI, 10 football and ice hockey players were imaged at baseline and within 10 days postconcussion.19 Using subject-specific finite element modeling (FEM) employing HITS data, change in FA and MD values in the CC were significantly related to strain and strain rate estimated by the FEM.
The primary aim of this study is to determine if the cumulative effects of head impacts from a season of high school varsity football produce changes in the brain in the absence of clinically diagnosed concussion.
Methods
Protocol overview
All participants were instrumented with the HITS system for acquisition of real-time biomechanical exposure data during all practices and games. All participants also received baseline pre- and postseason MRI and Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT). The data for this study are part of an ongoing study of high school and youth football players involving biomechanics, MRI, magnetoencephalography, and cognitive testing.31,32
Subjects
Players from a local Winston-Salem varsity high school football team were enrolled to participate in the study for the 2012 season. This study was approved by the Wake Forest School of Medicine (Winston-Salem, NC) Institutional Review Board Committee. Exclusion criteria included any history of previous neurological illness, psychiatric illness, brain tumor, concussion within the past 6 months, and/or contraindication to MRI. A certified athletic trainer (ATC) was present during all games and practices and evaluated all players for clinical signs of concussion using the Sports Concussion Assessment Tool version 2. Players identified by the ATC with suspected concussion were then evaluated by a sports-medicine physician experienced in the clinical diagnosis and treatment of concussion. During the season, 2 players had clinically diagnosed concussions and were excluded from further analysis. Before the season, 1 player had a clinically diagnosed concussion and was excluded from further analysis. This provided 24 complete pre- and postseason imaging data sets from players without clinical evidence of concussion. These subjects were all male, with a mean age (in years) of 16.9 (standard deviation [SD], 0.6), a mean body mass index (BMI) of 26.3 (SD, 3.7), and mean time between pre- and postseason scans of 4.9 months (SD, 0.6).
Magnetic resonance imaging acquisition and processing
MRI data were acquired in accordance with the National Institute of Neurological Disorders and Stroke Common Data Elements advanced protocol recommendations on a 3T Siemens Skyra MRI scanner using a high-resolution 20-channel head/neck coil (Siemens Healthcare, Erlangen, Germany). T1-weighted anatomical images were obtained using a three-dimensional volumetric magnetization prepared rapid gradient echo sequence with isotropic resolution of 0.9 mm (repetition time [TR]=1900 msec; echo time [TE]=2.93 msec; inversion time=900 msec; flip angle=9 degrees; and 176 slices). DTI images were acquired using a two-dimensional single-shot echo planar imaging sequence (TR=10,500 msec; TE=99 msec; flip angle=90 degrees; spatial resolution=2.2×2.2 mm; slice thickness=3 mm; 54 slices; 10 b=0 volumes; and 15 diffusion directions with b=1000/2000 each). Structural T1 images were normalized to Montreal Neurologic Imaging (MNI) space using the Dartel high-dimensional warping and the SPM837 new segment procedure, as implemented in the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html). Diffusion tensor preprocessing was performed using the Functional MRI of the Brain Software Library (FSL).38 Eddy current correction of the diffusion tensor images was performed using FSL dti_eddy by normalizing each image to the baseline (B0) image using the mutual information registration algorithm. DTI scalar metrics, including FA and MD, as well as shape anisotropy coefficients, including linear anisotropy (CL), planar anisotropy (CP), and spherical anisotropy (CS), were computed using DTI-TK.39 Distortion correction was performed by normalizing the B0 image to the T1 image and applying this transformation to the computed maps. The resulting scalar maps were then normalized to MNI space based on the parameters computed from the structural normalization. All normalizations and scalar maps were visually inspected to ensure the quality of normalization procedures.
Head Impact Telemetry System data collection
All players were fitted with Riddell Revolution or Riddell Revolution Speed football helmets containing MxEncoders that fit into the spaces between padding in the helmet. The MX Encoder weighs 4 ounces and includes six nonorthogonally mounted single-axis accelerometers, a digital encoder, and instrumentation to transmit signal to the base unit. Riddell helmets incorporating the HITS have been certified by the National Operating Committee on Standards for Athletic Equipment. Trained research assistants monitored the HITS at all hitting practices and games. Acceleration threshold was set at 10g. Once this threshold was achieved, information from all six accelerometers was collected at 1 kHz for a period of 40 ms (8 ms before the trigger and 32 ms after the trigger). All games and practices were also videotaped to remove any false impacts, such as a dropped helmet. A complete description of the processing algorithm and validation of the HITS has been previously described.40
Head Impact Telemetry System data analysis
Quantitative HITS data include number of impacts, peak linear acceleration in gs, peak impact rotational acceleration in radians per second,2 as well as azimuth and elevation for measures of impact location (e.g., angle >60=top; 45 from sagittal anterior/posterior=front/back; and 45 from frontal plane=side). The time history of acceleration is also obtained for the resultant translational acceleration. The biomechanical metric computed from the HITS data for this study was the risk-weighted cumulative exposure (RWE).31 This metric is defined as the collected risk of concussion over the course of the season. The risk of concussion for each impact for each player was calculated using three different risk functions previously described in the literature. The three risk functions include the logistic regression equations and regression coefficients for 1) linear acceleration,41 2) rotational acceleration,42 and 3) the combined probability from linear and rotational acceleration43 (Table 1). The risk for each respective head acceleration specific to the given risk function for a single player was summed to generate the RWE for the season. RWE is favorable to previously-described exposure metrics in football head impacts because it is based on the player specific distribution of impacts and the associated risk of concussion for each impact during the season for each player. The RWE calculated from each respective risk function is simply referred to as RWELinear, RWERotational, and RWECP. Because RWECP is comprised of both linear and rotational forces that are experienced with every head impact, our primary hypothesis relates to changes in the brain associated with RWECP. The equations for RWE are provided in Table 2.
Table 1.
Logistic Regression Equations and Regression Coefficients of the Three Injury Risk Functions Utilized in the Risk Calculation for Each Impacta
| Equation | Logistic regression equation | Risk function | Regression coefficients |
|---|---|---|---|
| (1) |  |
Linear | α=−9.805, β=0.0510 |
| Rotational | α=−12.531, β=0.0020 |
||
| (2) |  |
Combined probability (CP) | β0=−10.2, β1=0.0433, β2=0.000873, β3=−9.2E-07 |
α and β are the regression coefficients and x is the measured acceleration for the linear and rotational risk functions. β0, β1, β2, and β3 are the regression coefficients, a is the measured linear acceleration, and α is the measured rotational acceleration for the combined probability risk function.
Table 2.
Risk-Weighted Cumulative Exposure (RWE) Equationsa
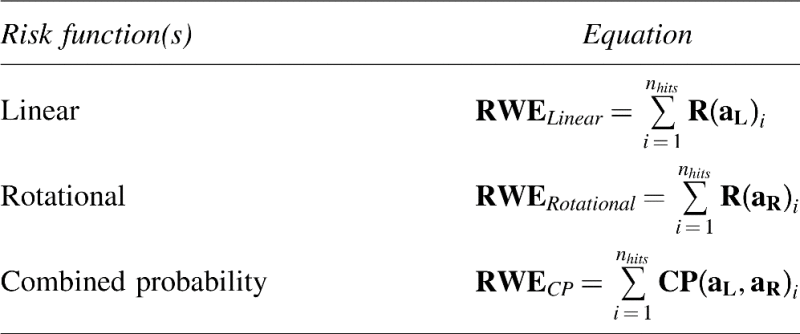 |
aL is the measured peak linear acceleration, aR is the measured peak rotational acceleration, and nhits is the number of head impacts in a season for a given player.
Diffusion tensor imaging scalar z-scores
Delta post-preseason maps were computed for each DTI scalar metric (FA, MD, CL, CP, and CS). The group mean and standard deviation (SD) of the delta maps were used to create a normative reference for each scalar metric. Voxel-wise z-scores were computed using the normative reference. The z-maps were thresholded at 2 SDs above the mean and 2 SDs below the mean to identify any abnormally high or low scalar values. A cluster threshold requiring a minimum 1-mL contiguous volume was applied to reduce false positives. The total number of abnormal voxels for each subject and scalar metric was computed for regression analyses. A sensitivity analysis was conducted to examine for threshold effects, with analyses repeated at a range of SD cutoffs (0.5–3.0).
Comparison of Head Impact Telemetry System and imaging data
Linear regression analyses were performed to examine the relationships between RWE metrics and brain imaging using JMP software (SAS Institute Inc., Cary, NC). Our primary hypothesis was that there would be an association between RWECP and FA. Secondary analyses were performed to better characterize any associations between the biomechanical metrics (RWELinear, RWERotational, and RWECP) and other DTI measures, including MD and subcomponents of the FA (CL, CP, and CS). For the primary linear regression analysis, a model was constructed for RWECP and FA. Number of abnormal FA voxels was used as the dependent variable. Log transformation was applied to satisfy assumptions of normality. Age at preseason, BMI, and time between scans were used as covariates. The proportion of variance (r2) in FA associated with RWECP, without and with covariate adjustment, was used to portray the strength of relationships. Secondary analyses were performed in a similar fashion using FA, MD, CL, CP, CS, and all biomechanical metrics (RWELinear, RWERotational, and RWECP). For each linear regression performed, the Cook's distance of each point was calculated and analyzed for potential outliers.
Immediate Post-Concussion Assessment and Cognitive Testing
Neuropsychological testing using version 2.1 of the ImPACT computer-administered test battery was successfully completed pre- and postseason for 14 of the 24 subjects. The test consists of multiple modules that assess different measures of cognitive function reported as composite scores, including verbal memory, visual memory, visual motor (processing speed), and reaction time. The test takes approximately 25 min to complete and was administered in the school's computer laboratory under the supervision of the team's athletic trainer. These 14 subjects had a mean age (in years) of 17.03 (SD, 0.6), mean BMI of 23.2 (SD, 4.1), and mean time between pre- and postseason scans of 4.8 months (SD, 0.6).
Immediate Post-Concussion Assessment and Cognitive Testing delta scores
Delta post-preseason scores for each individual were computed using the four ImPACT composite measures (verbal memory, visual memory, visual motor, and reaction time). These four delta scores were then compared to the number of abnormal DTI voxels using Spearman's rank-correlation coefficient.
Results
As seen in Table 3, the combined metric, RWECP, demonstrated a significant association with changes in all of the DTI scalars. No data points were found to be outliers in our analysis. Our primary hypothesis focused on RWECP-associated changes in FA. RWECP and number of abnormal delta FA voxels explained 49.5% of the total variance (p=0.0001). Covariate adjustment for age, BMI, and time between scans increased the strength of the relationship (R2 adjusted=0.4985; p<0.0001). The most significant relationship, however, was between RWECP and CL (Fig. 1), explaining 50.4% of the variance (p=0.0001). RWEcp explained 33.7% of the variance in abnormal delta MD voxels (p=0.0030), 42.9% of the variance with CP (p=0.0005), and 46.9% of the variance with Cs (p=0.0002). In each case, covariate adjustment for age, BMI, and time between scans increased the strength of the relationship.
Table 3.
Associations between HITS Metrics and Changes in DTI Measures, without and with Adjustment for Age, BMI, and Time between MRI Scans
| Without covariate adjustment | Covariate adjustment for age, BMI, and time between scans | |||
|---|---|---|---|---|
| R2 | p value | Adjusted R2 | p value | |
| FA vs. RWEcp | 0.4949 | 0.0001* | 0.4985 | <0.0001* |
| MD vs. RWEcp | 0.3366 | 0.0030* | 0.3816 | 0.0006* |
| CL vs. RWEcp | 0.5043 | 0.0001* | 0.5626 | <0.0001* |
| CP vs. RWEcp | 0.4286 | 0.0005* | 0.5956 | <0.0001* |
| CS vs. RWEcp | 0.4694 | 0.0002* | 0.5275 | <0.0001* |
| FA vs. RWELinear | 0.2479 | 0.0133* | 0.1656 | 0.0127* |
| MD vs. RWELinear | 0.1705 | 0.0449* | 0.1081 | 0.0252* |
| CL vs. RWELinear | 0.2789 | 0.0080* | 0.2250 | 0.0071* |
| CP vs. RWELinear | 0.2269 | 0.0186* | 0.3294 | 0.0108* |
| CS vs. RWELinear | 0.2477 | 0.0133* | 0.2331 | 0.0065* |
| FA vs. RWERotational | 0.0139 | 0.5834 | −0.1282 | 0.4294 |
| MD vs. RWERotational | 0.0770 | 0.1892 | −0.1064 | 0.3134 |
| CL vs. RWERotational | 0.0287 | 0.4287 | −0.0966 | 0.3661 |
| CP vs. RWERotational | 0.0176 | 0.5363 | 0.0480 | 0.9736 |
| CS vs. RWERotational | 0.0057 | 0.7261 | −0.1062 | 0.4351 |
| FA vs. total impacts | 0.0082 | 0.6732 | −0.1624 | 0.7893 |
| MD vs. total impacts | 0.0087 | 0.6652 | −0.1680 | 0.9042 |
| CL vs. total impacts | 0.0337 | 0.3905 | −0.1262 | 0.5688 |
| CP vs. total impacts | 0.0189 | 0.5222 | 0.0676 | 0.5341 |
| CS vs. total impacts | 0.0072 | 0.6935 | −0.1343 | 0.7043 |
p<0.05.
HITS, Head Impact Telemetry System; DTI, diffusion tensor imaging; BMI, body mass index; MRI, magnetic resonance imaging; FA, fractional anisotropy; MD, mean diffusivity; CL, linear anisotropy; CP, planar anisotropy; CS, spherical anisotropy.
FIG. 1.
RWECP versus linear anisotropy. A strong linear relationship is demonstrated between combined risk-weighted exposure and number of abnormal voxels based on linear anisotropy. RWE-CP, risk-weighted cumulative exposure and combined components.
RWELinear also had strong associations with changes in DTI scalars, explaining 24.8% of the variance in abnormal delta FA voxels (p=0.0133), 17.0% of the variance in abnormal delta MD voxels (p=0.0449), 27.9% of the variance in CL (p=0.008), 22.7% of the variance in CP (p=0.0186), and 24.8% of the variance in Cs (p=0.0133). Covariate adjustment for age, BMI, and time between scans also increased the strength of these relationships.
RWERotational and the number of head impacts did not demonstrate a statistically significant association with changes in any of the DTI scalars, without or with covariate adjustment.
Pre- and postseason ImPACT composite scores are presented in Table 4. Spearman's rank correlation revealed a statistically significant association between the magnitude of verbal memory score decrease (post-pre) and the number of abnormal voxels for each DTI scalar. Specifically, there was a strong statistically significant inverse relationship between delta verbal memory composite score and number of abnormal MD voxels (rs(12)=−0.65; p=0.0116) and CP voxels (rs(12)=−0.75; p=0.0022). There was also a trend toward statistical significance for an inverse relationship between delta verbal memory composite score and number of abnormal FA voxels (rs(12)=−0.49; p=0.0749) and Cs voxels (rs(12)=−0.48; p=0.0843).
Table 4.
ImPACT Composite Scores Pre- and Postseason
| Preseason | Postseason | |||||
|---|---|---|---|---|---|---|
| Range | Mean | SD | Range | Mean | SD | |
| Verbal memory composite score | 65–99 | 89.64 | 10.71 | 61–99 | 85.29 | 10.96 |
| Visual memory composite score | 61–97 | 81.93 | 11.65 | 42–97 | 73.79 | 15.92 |
| Visual motor composite score | 27.64–50.25 | 40.00 | 6.78 | 20.85–47.08 | 37.20 | 9.67 |
| Reaction time composite score | 0.44–0.62 | 0.53 | 0.06 | 0.49–0.99 | 0.63 | 0.17 |
Range is for lowest to highest score.
ImPACT, Immediate Post-Concussion Assessment and Cognitive Testing; SD, standard deviation.
No other associations between delta ImPACT composite scores (visual memory, visual motor, and reaction time) and number of abnormal DTI voxels achieved statistical significance. Sensitivity analyses demonstrated findings to be similar across a wide range of SD cutoffs (0.5–3.0).
Discussion
In this study, we directly compared head impact exposure in the form of risk-weighted cumulative acceleration experienced in one season of high school football with pre- and postseason imaging and cognitive data. The number of abnormal DTI voxels for each subject was measured using a method independent of spatial relationships. The number of abnormal voxels in each DTI scalar was shown to have a statistically significant relationship to RWECP and RWELinear. This is the first report of a quantitative relationship between head impact metrics and DTI scalars in nonconcussed subjects.
RWE reflects both frequency and severity of impacts; therefore, it captures wide variances in exposure within subjects. RWELinear represents the risk associated with the peak linear acceleration, whereas RWERotational represents the risk associated with the peak rotational acceleration. RWECP combines both linear and rotational acceleration. The relationship between the RWE metrics and DTI scalars can be thought of as a correlation between increased head impact exposure and changes in WM integrity. It has been shown that as frequency and severity of impacts increase, the amount of brain injury increases.43–45 It is also well documented that mTBI events in children, as well as adults, have led to changes in DTI scalars, FA in particular.22,25,26,46–48 Our study shows changes in DTI scalars that are significantly associated with RWE metrics in the absence of a clinical diagnosis of concussion or clinically apparent mTBI.
RWECP explained more variance in the DTI scalars than RWELinear or RWERotational, suggesting that both linear and rotational acceleration contribute to the prediction of brain injury. The DTI scalars did not demonstrate a significant relationship with the total number of impacts, indicating that weighting impacts according to associated risk may be important. Simply counting impacts does not account for impact severity and directional components that may be critical in producing brain injury. RWECP includes the magnitude of both linear and rotational acceleration as well as the total number of impacts in order to create an accurate assessment of each player's risk of concussion for the season. TBI is caused by neither a purely linear nor a purely rotational force.29,43,49,50 Linear and rotational accelerations are associated with different injury mechanisms. Linear accelerations are associated with transient intracranial pressure gradients, whereas rotational accelerations are associated more strongly with a strain response or brain deformation.51,52 The primary contributor to brain injuries and concussion is still a matter of debate, with some studies suggesting linear acceleration49 and some suggesting rotational acceleration.29 RWELinear in our study was the second most significant RWE metric, whereas RWERotational did not show a significant relationship with any of the DTI scalars. This suggests that pure linear acceleration explained variance in the subconcussive changes in brain DTI metrics better than pure rotational acceleration, but it is well known that most real-world impacts involve both linear and rotational components. Conclusions on the involvement of rotational versus linear acceleration in concussion, however, cannot be determined from our study.
In our study, the delta DTI metrics of each subject were compared against the entire group to compute z-scores. This generates a measure of the change in DTI metric over the season while accounting for any baseline differences in DTI values and effects of maturation. Both increases and decreases in DTI metrics were studied and no significant relationship was found when compared to RWE or ImPACT scores. There is variability in the TBI literature regarding the time course and direction of change in DTI metrics after injury. Studies of TBI and mTBI have typically demonstrated decreases in FA and increases in MD. Recent evidence on sports-related concussions and nonconcussive impacts; however, supports increased FA and decreased MD in the acute (days to weeks) and chronic (up to 6 months) phases.18–20 These changes in DTI metrics are likely sensitive to the time interval between injury and postinjury imaging.11,47,48 Our study used both ends of the distribution in order to account for the uncertainty in the precise temporal evolution and direction of change in DTI measures.
FA can be separated into linear (CL), planar (Cp), and spherical (Cs) anisotropy components to further explain the shape of the diffusion ellipsoid.53,54 These diffusivity metrics allow a closer look at the microstructural causes of abnormal FA. The linear measure represents diffusion along two orthogonal directions, also referred to as “cigar” shaped. Major WM tracts, such as the CC, have high linear diffusivity. Planar diffusion is restricted to a plane (or saucer shaped). Planar diffusivity is often associated with crossing, or “kissing,” fibers. Spherical diffusion is purely isotropic diffusion. The highest spherical measures are observed in cerebrospinal fluid, where diffusion is unbounded.55 Axial (λ1) and radial ((λ2+λ3)/2) diffusivity are other DTI scalars used in the literature55 computed from the principle directions of the diffusion tensor. Axial diffusivity is the most similar to linear diffusivity in association with WM tracts.57 Radial diffusivity has been associated with demyelination and axonal swelling.14,57 Previous studies have shown changes in geometrical tensors to be especially sensitive to changes in TBI.14,46,58 Changes in linear, planar, or spherical diffusivity likely represent different responses to mTBI and WM integrity. However, the pathological implication of the changes in these DTI parameters is still not well understood. In our study, RWECP and RWELinear explained more variance and were more significant in their association with the number of abnormal CL voxels than any other DTI metric. It is known that mTBI causes a focal disruption to multiple axons, which can then lead to swelling and tearing of those axons. A change in linear diffusivity (CL) suggests a focal change in the WM tracts related to disconnection. When this process becomes widespread, it is referred to as diffuse axonal injury. In our study, the stronger correlation with linear diffusivity fits the characteristics of axotomy, or axonal tearing. The secondary relationships with spherical and planar diffusivities suggest axonal swelling and, possibly, demyelination in addition to the primary damage of disconnection. It is important to note that the time course and reversibility of these changes were not studied.
We found a statistically significant association between the magnitude of delta (post-pre) ImPACT verbal memory composite score and number of abnormal voxels for two of the five DTI scalars (MD and CP), with a trend toward statistical significance for two others (FA and Cs). In a study of concussed high school and college athletes, verbal memory measured with the ImPACT has been shown to decrease after a single season of football.59 In our study, the correlation between diminished verbal memory score and number of abnormal DTI voxels was found in athletes without clinically diagnosed concussion. The cognitive data further support our imaging findings that cumulative head impacts from a season of high school varsity football can produce changes in the brain, even in the absence of clinically diagnosed concussion.
Our study has several limitations that must be considered. Its sample size was relatively small; however, it is the largest study to date of high school football that includes biomechanical metrics, MRI DTI measures, and cognitive testing. Our delta DTI metric used the entire group mean and SD to compute z-scores. An alternative method would be to use a control group of noncontact sport athletes to provide an effective comparison group. Both methods have validity, although our approach accounts for differences in baseline DTI values. Longitudinal evaluations of the subjects would be helpful to determine reversibility of the findings. The selected 2-SD cutoff is somewhat arbitrary, but was chosen because it has been previously used in the mTBI literature and facilitates comparisons with other data sets. In order to evaluate for threshold effects, we conducted a sensitivity analysis, repeating the analyses at a range of SD Z-map cutoffs (0.5–3.0). This demonstrated the findings to be robust across these threshold ranges. In addition, the HITS used for the collection of biomechanical data is associated with some error in measurement for linear and rotational acceleration. In Beckwith and colleagues, the HITS overestimated linear acceleration by an average of 1% and underestimated rotational acceleration by an average of 6%, when compared to acceleration data measured from a biomechanical headform instrumented with a nine accelerometer package (Hybrid III).60 However, the correlation between the HITS and the Hybrid III headform was significant for linear and rotational acceleration.
Conclusion
We demonstrate a significant relationship between changes in DTI measures and cumulative impacts using biomechanical metrics in the absence of clinical concussion. In this study, we show that a single season of football can produce MRI measureable brain changes that have been previously associated with mTBI. Finally, we demonstrate that these impact-related changes in the brain have a strong association with a postseason change in cognitive function. Taken together, these data add to the growing body of literature providing evidence that a season of play in a contact sport can show brain changes in the absence of concussion or clinical findings. Studies relating the biomechanics of head impacts with brain imaging and cognitive function may allow equipment designers, researchers, and clinicians to prevent, mitigate, identify, and treat injuries to help make football a safer activity for millions of children.
Acknowledgments
The authors thank the Reagan High School, especially Ashley Lake, ATC (Reagan High School), Corbin Ratcliffe, Lauren Smith, and the football program. The authors also thank Ben Wagner, Elizabeth Lillie, and all those who contributed to the study development. The authors extend a special thanks to the Childress Institute for Pediatric Trauma at Wake Forest Baptist Medical Center for providing support for this study. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship (under grant no. DGE-0907738). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation. Support for this research was also provided by the National Institutes of Health (grant no.: NS082453; to J.A.M. and J.D.S.).
Author Disclosure Statement
Dr. Gioia is a coauthor of Pediatric ImPACT and, in the future, may receive royalties, but has no financial interest in the adolescent/mid-range version presented here. The remaining authors declare that they have no conflict of interest.
References
- 1.Guskiewicz K.M., Weaver N.L., Padua D.A., and Garrett W.E., Jr. (2000). Epidemiology of concussion in collegiate and high school football players. Am. J. Sports Med. 28, 643–650 [DOI] [PubMed] [Google Scholar]
- 2.Powell J.W., and Barber-Foss K.D. (1999). Traumatic brain injury in high school athletes. JAMA 282, 958–963 [DOI] [PubMed] [Google Scholar]
- 3.Daniel R.W., Rowson S., and Duma S.M. (2012). Head impact exposure in youth football. Ann. Biomed. Eng. 40, 976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessel L.M., Fields S.K., Collins C.L., Dick R.W., and Comstock R.D. (2007). Concussions among United States high school and collegiate athletes. J. Athl. Train 42, 495–503 [PMC free article] [PubMed] [Google Scholar]
- 5.Guskiewicz K.M., Mihalik J.P., Shankar V., Marshall S.W., Crowell D.H., Oliaro S.M., Ciocca M.F., and Hooker D.N. (2007). Measurement of head impacts in collegiate football players: relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurgery 61, 1244–1252; discussion, 1252–1253. [DOI] [PubMed] [Google Scholar]
- 6.Guskiewicz K.M., and Mihalik J.P. (2011). Biomechanics of sport concussion: quest for the elusive injury threshold. Exerc. Sport Sci. Rev. 39, 4–11 [DOI] [PubMed] [Google Scholar]
- 7.Belanger H.G., Vanderploeg R.D., Curtiss G., and Warden D.L. (2007). Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 19, 5–20 [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee P., Chung S.W., Berman J.I., Hess C.P., and Henry R.G. (2008). Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR Am. J. Neuroradiol. 29, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell J.A., Landman B.A., Jones C.K., Smith S.A., Prince J.L., van Zijl P.C., and Mori S. (2007). Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. J. Magn. Reson. Imaging 26, 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arfanakis K., Haughton V.M., Carew J.D., Rogers B.P., Dempsey R.J., and Meyerand M.E. (2002). Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am. J. Neuroradiol. 23, 794–802 [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar R., Gupta R.K., Husain M., Chaudhry C., Srivastava A., Saksena S., and Rathore R.K. (2009). Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 23, 675–685 [DOI] [PubMed] [Google Scholar]
- 12.Miles L., Grossman R.I., Johnson G., Babb J.S., Diller L., and Inglese M. (2008). Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 22, 115–122 [DOI] [PubMed] [Google Scholar]
- 13.Newcombe V.F., Williams G.B., Nortje J., Bradley P.G., Chatfield D.A., Outtrim J.G., Harding S.G., Coles J.P., Maiya B., Gillard J.H., Hutchinson P.J., Pickard J.D., Carpenter T.A., and Menon D.K. (2008). Concordant biology underlies discordant imaging findings: diffusivity behaves differently in grey and white matter post acute neurotrauma. Acta Neurochirurg. Suppl. 102, 247–251 [DOI] [PubMed] [Google Scholar]
- 14.Newcombe V.F., Williams G.B., Nortje J., Bradley P.G., Harding S.G., Smielewski P., Coles J.P., Maiya B., Gillard J.H., Hutchinson P.J., Pickard J.D., Carpenter T.A., and Menon D.K. (2007). Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br. J. Neurosurg. 21, 340–348 [DOI] [PubMed] [Google Scholar]
- 15.Wilde E.A., Ramos M.A., Yallampalli R., Bigler E.D., McCauley S.R., Chu Z., Wu T.C., Hanten G., Scheibel R.S., Li X., Vasquez A.C., Hunter J.V., and Levin H.S. (2010). Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev. Neuropsychol. 35, 333–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wozniak J.R., and Lim K.O. (2006). Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci. Biobehav. Rev. 30, 762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wozniak J.R., Krach L., Ward E., Mueller B.A., Muetzel R., Schnoebelen S., Kiragu A., and Lim K.O. (2007). Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 22, 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry L.C., Tremblay J., Tremblay S., Lee A., Brun C., Lepore N., Theoret H., Ellemberg D., and Lassonde M. (2011). Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma 28, 2049–2059 [DOI] [PubMed] [Google Scholar]
- 19.McAllister T.W., Ford J.C., Ji S., Beckwith J.G., Flashman L.A., Paulsen K., and Greenwald R.M. (2012). Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann. Biomed. Eng. 40, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazarian J.J., Zhu T., Blyth B., Borrino A., and Zhong J. (2012). Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn. Reson. Imaging 30, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bazarian J.J., Zhong J., Blyth B., Zhu T., Kavcic V., and Peterson D. (2007). Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma 24, 1447–1459 [DOI] [PubMed] [Google Scholar]
- 22.Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., and Levin H.S. (2008). Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70, 948–955 [DOI] [PubMed] [Google Scholar]
- 23.Levin H.S., Wilde E.A., Chu Z., Yallampalli R., Hanten G.R., Li X., Chia J., Vasquez C., and Hunter J.V. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 23, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ptak T., Sheridan R.L., Rhea J.T., Gervasini A.A., Yun J.H., Curran M.A., Borszuk P., Petrovick L., and Novelline R.A. (2003). Cerebral fractional anisotropy score in trauma patients: a new indicator of white matter injury after trauma. AJR Am. J. Roentgenol. 181, 1401–1407 [DOI] [PubMed] [Google Scholar]
- 25.Davenport N.D., Lim K.O., Armstrong M.T., and Sponheim S.R. (2012). Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage 59, 2017–2024 [DOI] [PubMed] [Google Scholar]
- 26.Ling J.M., Peña A., Yeo R.A., Merideth F.L., Klimaj S., Gasparovic C., and Mayer A.R. (2012). Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 135, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brolinson P.G., Manoogian S., McNeely D., Goforth M., Greenwald R., and Duma S. (2006). Analysis of linear head accelerations from collegiate football impacts. Curr. Sports Med. Rep. 5, 23–28 [DOI] [PubMed] [Google Scholar]
- 28.Duma S.M., Manoogian S.J., Bussone W.R., Brolinson P.G., Goforth M.W., Donnenwerth J.J., Greenwald R.M., Chu J.J., and Crisco J.J. (2005). Analysis of real-time head accelerations in collegiate football players. Clin. J. Sport Med. 15, 3–8 [DOI] [PubMed] [Google Scholar]
- 29.Broglio S.P., Sosnoff J.J., Shin S., He X., Alcaraz C., and Zimmerman J. (2009). Head impacts during high school football: a biomechanical assessment. J. Athl. Train. 44, 342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnebel B., Gwin J.T., Anderson S., and Gatlin R. (2007). In vivo study of head impacts in football: a comparison of National Collegiate Athletic Association Division I versus high school impacts. Neurosurgery 60, 490–495; discussion 495–496. [DOI] [PubMed] [Google Scholar]
- 31.Urban J.E., Davenport E.M., Golman A.J., Maldjian J.A., Whitlow C.T., Powers A.K., and Stitzel J.D. (2013). Head impact exposure in youth football: high school ages 14 to 18 years and cumulative impact analysis. Ann. Biomed. Eng. 41, 2474–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobb B.R., Urban J.E., Davenport E.M., Rowson S., Duma S.M., Maldjian J.A., Whitlow C.T., Powers A.K., and Stitzel J.D. (2013). Head impact exposure in youth football: elementary school ages 9–12 years and the effect of practice structure. Ann. Biomed. Eng. 41, 2463–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniel R.W., Rowson S., and Duma S.M. (2014). Head impact exposure in youth football: middle school ages 12 to 14 years. J. Biomech. Eng. June1. doi: 10.1115/1.4027872. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Young T.J., Daniel R.W., Rowson S., and Duma S.M. (2013). Head impact exposure in youth football: elementary school ages 7 to 8 years and the effect of returning players. Clin. J. Sports Med. December9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Breedlove E.L., Robinson M., Talavage T.M., Morigaki K.E., Yoruk U., O'Keefe K., King J., Leverenz L.J., Gilger J.W., and Nauman E.A. (2012). Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J. Biomech. 45, 1265–1272 [DOI] [PubMed] [Google Scholar]
- 36.Talavage T.M., Nauman E., Breedlove E.L., Yoruk U., Dye A.E., Morigaki K., Feuer H., and Leverenz L.J. (2014). Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma 31, 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashburner J., and Friston K.J. (2000). Voxel-based morphometry—the methods. NeuroImage 11, 805–821 [DOI] [PubMed] [Google Scholar]
- 38.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., and Matthews P.M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, Suppl. 1, S208–S219 [DOI] [PubMed] [Google Scholar]
- 39.Zhang H., Yushkevich P.A., Alexander D.C., Gee J.C. (2006). Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med. Image Anal. 10, 764–785 [DOI] [PubMed] [Google Scholar]
- 40.Crisco J.J., Chu J.J., and Greenwald R.M. (2004). An algorithm for estimating acceleration magnitude and impact location using multiple nonorthogonal single-axis accelerometers. J. Biomech. Eng. 126, 849–854 [DOI] [PubMed] [Google Scholar]
- 41.Rowson S., and Duma S.M. (2011). Development of the STAR evaluation system for football helmets: integrating player head impact exposure and risk of concussion. Ann. Biomed. Eng. 39, 2130–2140 [DOI] [PubMed] [Google Scholar]
- 42.Rowson S., Duma S.M., Beckwith J.G., Chu J.J., Greenwald R.M., Crisco J.J., Brolinson P.G., Duhaime A.C., McAllister T.W., and Maerlender A.C. (2012). Rotational head kinematics in football impacts: an injury risk function for concussion. Ann. Biomed. Eng. 40, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowson S., and Duma S.M. (2013). Brain injury prediction: assessing the combined probability of concussion using linear and rotational head acceleration. Ann. Biomed. Eng. 41, 873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ommaya A.K., and Hirsch A.E. (1971). Tolerances for cerebral concussion from head impact and whiplash in primates. J. Biomech. 4, 13–21 [DOI] [PubMed] [Google Scholar]
- 45.Ono K., Kikuchi A., Nakamura M., Kobayashi H., and Nakamura N. (1980). Human head tolerance to sagittal impact. Reliable estimation deduced from experimental head injury using subhuman primates and human cadaver skulls. Proceedings of the 24th Stapp car Crash Conference, Troy, Michigan, October15–17, SAE paper no. 801303. [Google Scholar]
- 46.Mayer A.R., Ling J.M., Yang Z., Pena A., Yeo R.A., and Klimaj S. (2012). Diffusion abnormalities in pediatric mild traumatic brain injury. J. Neurosci. 32, 17961–17969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu Z., Wilde E.A., Hunter J.V., McCauley S.R., Bigler E.D., Troyanskaya M., Yallampalli R., Chia J.M., and Levin H.S. (2010). Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am. J. Neuroradiol. 31, 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer A.R., Ling J., Mannell M.V., Gasparovic C., Phillips J.P., Doezema D., Reichard R., and Yeo R.A. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74, 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellman E.J., Viano D.C., Tucker A.M., Casson I.R., and Waeckerle J.F. (2003). Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery 53, 799–812; discussion, 812–794. [DOI] [PubMed] [Google Scholar]
- 50.Wang H.-C., Duan Z.-X., Wu F.-F., Xie L., Zhang H., and Ma Y.-B. (2010). A new rat model for diffuse axonal injury using a combination of linear acceleration and angular acceleration. J. Neurotrauma 27, 707–719 [DOI] [PubMed] [Google Scholar]
- 51.King A.I., Yang K.H., Zhang L., Hardy W., and Viano D.C. (2003). Is head injury caused by linear or angular acceleration? Proceedings of the IRCOBI International Research Council on the Biomechanics of Impact Conference, Lisbon, Portugal, September2003, Introduction. [Google Scholar]
- 52.Unterharnscheidt F.J. (1972). Translational versus rotational acceleration: animal experiments with measured input. Scand. J. Rehabil. Med. 4, 24–26 [PubMed] [Google Scholar]
- 53.Farhadi H.F., and Lonser R.R. (2013). Diffusion tensor imaging in the spotlight on concussion. World Neurosurg. 80, 794–795 [DOI] [PubMed] [Google Scholar]
- 54.Westin C.F., Peled S., Gudbjartsson H., Kikinis R., and Jolesz F.A. (1997). Geometrical diffusion measures for {MRI} from tensor basis analysis, in: ISMRM ’97, Vancouver, British Columbia, Canada, April1997 [Google Scholar]
- 55.Westin C.F., Maier S.E., Mamata H., Nabavi A., Jolesz F.A. and Kikinis R. (2002). Processing and visualization for diffusion tensor MRI. Med. Image Anal. 6, 93–108 [DOI] [PubMed] [Google Scholar]
- 56.Basser P.J. (1995). Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 8, 333–344 [DOI] [PubMed] [Google Scholar]
- 57.Song S.-K., Sun S.-W., Ramsbottom M.J., Chang C., Russell J., and Cross A.H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17, 1429–1436 [DOI] [PubMed] [Google Scholar]
- 58.Farbota K.D., Bendlin B.B., Alexander A.L., Rowley H.A., Dempsey R.J., and Johnson S.C. (2012). Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front. Hum. Neurosci. 6, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iverson G.L., Lovell M.R., and Collins M.W. (2003). Interpreting change on ImPACT following sport concussion. Clin. Neuropsychol. 17, 460–467 [DOI] [PubMed] [Google Scholar]
- 60.Beckwith J.G., Greenwald R.M., and Chu J.J. (2012). Measuring head kinematics in football: correlation between the Head Impact Telemetry System and Hybrid III Headform. Ann. Biomed. Eng. 40, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]



