Abstract
Purpose
An update on pharmacotherapy for achieving and maintaining abstinence and mitigating hepatic damage in patients with alcoholic liver disease (ALD) is presented.
Summary
Currently there are limited pharmacotherapy options for managing ALD, which encompasses a broad spectrum of disorders ranging from steatosis and alcoholic hepatitis to fibrosis, cirrhosis, and hepatocellular cancer. Individual variation in the severity, presentation, and complex pathologenesis of ALD defines barriers to effective treatment. Scoring of disease severity using validated assessment instruments should guide treatment approaches; abstinence and proper nutrition continue to be the cornerstones of management. A literature search (through December 31, 2013) identified no reports of randomized controlled trials using Food and Drug Administration (FDA)-approved medications for the treatment of alcohol dependence in ALD-spectrum disorders. Disulfiram, acamprosate, and naltrexone (oral and intramuscular), while approved by FDA for treatment of alcohol dependence, are not currently approved for use in patients with ALD. Baclofen (also not FDA-approved for use in ALD) is the only medication available in the United States with demonstrated safety and efficacy in reducing alcoholic behavior that has been formally tested in clinical trials in patients with ALD. Pharmacotherapy of alcoholic hepatitis using glucocorticoids or pentoxifylline has shown promise, but these options are reserved for severe ALD only.
Conclusion
Although various treatments have been investigated for ALD in patients with alcoholism, complete abstinence from alcohol is currently the only recommended form of hepatoprotection for the entire spectrum of ALD diagnoses.
In 2000, the Centers for Disease Control and Prevention ranked alcohol consumption as the third leading cause of death in the United States.1 Alcohol use disorders account for about one U.S. death every 5 minutes; this toll includes 32% of all traffic fatalities, with an average of one fatality related to alcohol every 39 minutes.2,3 The respective prevalence estimates for alcohol abuse and alcohol dependence (as defined by Diagnostic and Statistical Manual of Mental Disorders criteria) in 2001–02 were 4.65% and 3.81%.4-7 When combined with psychosocial treatments, medications can improve outcomes for some individuals with alcohol abuse and dependence disorders, but these treatments are unsuccessful for many others.8
Alcohol is one of the most widely used and socially acceptable hepatotoxins worldwide. The excessive use of alcohol leads to many complications, resulting in health care costs for alcohol-related disorders reaching over $26 billion annually.9,10 The World Health Organization has identified alcohol as one of the leading risk factors for increased mortality in both the United States and Europe.10,11 Alcohol is associated with over 230 medical conditions and is considered the fourth most common cause of disease burden (as measured by disability-adjusted life-years) in the world.12 The most common health problem associated with chronic alcoholism is alcoholic liver disease (ALD). In developed countries, the histopathologic consequences of alcoholic consumption are the leading cause of alcoholic liver cirrhosis, which results in over 14,000 deaths each year in the United States.10 One study noted that the consumption of alcohol dramatically increased mortality, potentially contributing to 5-10% of years of life lost in both men and women.12
The spectrum of liver changes resulting in ALD ranges from steatosis, acute alcoholic hepatitis, and alcoholic steatohepatitis (ASH) to more permanent changes such as fibrosis, cirrhosis, and hepatocellular carcinoma.13 Oftentimes, once ALD progresses to hepatitis, few pharmacotherapy options exist; for advanced fibrosis and cirrhosis, the only treatment may be a liver transplant.
The quantity of alcohol ingested and the frequency of consumption, as well as the harmful episodic pattern of excessive consumption known as binge drinking, are the most important factors that affect the severity, prognosis, and treatment of ALD.14 While the daily dose of alcohol affects the probability of developing ALD, the progression of ALD is unique in each person, as it also varies with additional risk factors such as gender, ethnicity, comorbidity, nutrition, and genetics.14
Women absorb and metabolize alcohol differently from men. Women have less body water than men and, compared by weight with men, achieve higher concentrations of alcohol in the blood after consuming the same amounts of alcohol.15 Moreover, women also appear to eliminate alcohol from the blood faster than men, potentially due to women’s higher liver volume per unit of lean body mass. As a result, women are more sensitive to the effects of alcohol and its metabolites and are also more likely to develop severe ALD at lower doses.
African-American individuals are more likely to develop alcoholic cirrhosis, and Hispanic individuals have a higher mortality rate from this disease than the rest of the population.14 Individuals of Chinese ethnicity are more likely to develop ALD at lower daily doses.10 Mortality is significantly higher in malnourished patients than in those with normal nutrition, while obesity also increases the risk of developing ALD.10,14 Concomitant infection with the hepatitis C virus results in severe liver disease at an earlier age, with a decreased survival rate, and a higher likelihood of developing cirrhosis and hepatic carcinoma.14 Genetic factors also play a role, with mutations affecting alcohol metabolism having a large impact on how the body responds to alcohol and contributing to possible ethnic differences in ALD risk.10,14
Although the prevalence of ALD is not precisely known, the National Institute on Alcohol Abuse and Alcoholism estimates that more than 2 million individuals in the United States suffer from ALD.16 In patients with steatosis secondary to alcohol consumption, studies indicate that 20% are likely to develop cirrhosis within 10 years.10 According to some estimates, 20% of heavy drinkers will develop acute alcoholic hepatitis, with mortality in the first 28 days of around 15%.17 Therefore, initiating treatment as soon as possible is essential to reduce mortality. The high mortality rate is attributed to the development of hepatorenal syndrome and subsequent multisystem organ failure.17 The European Association for the Study of the Liver defines alcoholic hepatitis as an exacerbation of ALD, usually with progressive jaundice and marked elevation of values in liver function tests (LFTs).18
The two main priorities for successful reversal of liver damage are abstinence from alcohol and nutritional support.19 In addition, pharmacologic treatment varies based on the severity of alcohol intake and should take into account whether or not there is evidence of ALD. Determining the severity of ALD includes using a scoring scale (discussed later in this article) that may help to delineate the kind of pharmacotherapy that might be most appropriate to help the patient stop drinking or promote abstinence; other essential considerations include the patient’s motivation to remain abstinent at the start of treatment, contraindications to medication use, any history of allergies to potential treatments, and any medical history features indicating success or failure with the use of a particular drug.
Medications that have a Food and Drug Administration (FDA)–approved indication for treatment of alcoholism are naltrexone, acamprosate, and disulfiram.20 The goal of this review is to describe clinically researched pharmacologic treatment options for alcohol dependence in patients with various stages of ALD, as well as options that might be useful in treating patients with alcoholic hepatitis. Moreover, while reviewing the pathophysiology of ALD, we also provide basic information intended to enhance clinicians’ understanding of pharmacologic management of patients with evidence of ALD attributed to alcohol use disorders.
Notably, while this review highlights pharmacotherapy options only, it is well-known that behavioral therapies play a major role when treating alcohol-dependent individuals and that pharmacotherapy alone is not sufficient to produce the most positive outcomes.8 Susceptibility to ALD differs considerably among individuals, so that even among people drinking similar amounts of alcohol, only some progress to the later stages of ALD. Understanding the mechanisms of these differences may help clinicians identify and treat patients at increased risk for advanced liver damage. Currently, the treatment of alcoholism in patients with ALD remains medically challenging secondary to the complexity of the pathogenesis.
Methods
We searched the MEDLINE and Google Scholar databases for articles on randomized controlled trials using pharmacotherapy for alcohol dependence in patients with ALD and articles on the pharmacologic treatment of alcoholic hepatitis, ASH, alcoholic fibrosis, and alcoholic cirrhosis. We also examined the reference lists of the reports of these trials. The search was conducted using combinations of the terms alcoholism, alcoholic liver disease, alcoholic steatosis, alcoholic hepatitis, alcoholic steatohepatitis, pharmacotherapy, medication, drug, and treatment. The search covered the period January 1, 1990, to December 31, 2013.
No published trials of FDA-approved medications for the treatment of alcohol dependence in ALD were located. There are drugs for alcoholism available in the United States and Europe (acamprosate, baclofen, gabapentin, ondansetron, and topiramate) or only in Europe (metadoxine) that appear to be safe to use “off label” in patients with ALD. However, except for baclofen in the United States and Europe and metadoxine in Europe, no medications for alcoholism have even been formally tested in this population via controlled trials. In theory, because of its first-pass metabolism, naltrexone intramuscular injection may also provide some benefit for ALD; however, as with naltrexone tablets and disulfiram, liver function still must be carefully monitored.
In addition, there are no FDA-approved medications for alcoholic hepatitis, which is at the distal end of the pharmacotherapy continuum for ALD. While there is strong evidence for efficacy, currently prednisolone (as a first-line therapy) and pentoxifylline (as a second-line therapy) are used off label for the treatment of alcoholic hepatitis. In general, however, these drugs only provide modest survival gains, as abstinence and nutritional support remain the foundation of ALD management. Ultimately, for many patients (e.g., those with severe alcoholic hepatitis who are nonresponsive to treatment), either liver transplantation (if available) or death is often the outcome. Liver transplantation for endstage liver disease is lifesaving but expensive and dangerous. Obviously, more research using drugs approved by FDA for alcoholism in patients with ALD, as well as new treatment approaches that address the entire continuum of ALD, is needed.
Pathophysiology of ALD
ALD ranges from mild steatosis (fatty liver), a relatively minor condition with few or no symptoms, to hepatitis, cirrhosis, hepatic carcinoma, and liver failure requiring transplantation. Steatosis is the buildup of fatty acids in hepatocytes as the result of the toxic effects of alcohol; it must be differentiated from steatosis that can result from nonalcoholic fatty liver disease. The majority of patients abusing alcohol develop steatosis, which generally resolves on its own with abstinence.10,14
Continued long periods of harmful alcohol consumption may result in progression to alcoholic hepatitis or cirrhosis.21 Alcoholic hepatitis is an acute condition characterized by hepatocyte injury and mild-to-severe symptoms such as jaundice and liver failure. Less severe forms of acute alcoholic hepatitis frequently respond to abstinence from alcohol, whereas severe acute alcoholic hepatitis is characterized by a poor prognosis: up to 40-60% of these patients may die within six months.21 Cirrhosis is defined as the development of scar tissue in the liver and is a chronic condition present in half of patients with acute alcoholic hepatitis. Fibrosis, resulting from chronic inflammation and tissue necrosis, is the precursor to cirrhosis.10,14
Alcohol is metabolized in the liver via alcohol dehydrogenase (ADH), cytochrome P-450 isozyme 2E1 (CYP2E1), and mitochondrial cata-lase. In early alcohol consumption, metabolism is primarily conducted by ADH. ADH is also found outside of the liver within the gastric mucosa.10 During the chronic consumption of alcohol as well as instances in which alcohol is ingested in large quantities, metabolism is primarily carried out by CYP2E1, resulting in the formation of reactive oxygen species, causing increased hydroxyl free radicals and ultimately perpetuating hepatocellular damage.20 Finally, alcohol is metabolized into the highly toxic compound acetaldehyde, whose presence in the liver leads to steatosis.20 In addition to the reactive oxygen species derived from alcohol metabolism via CYP2E1, microsomal and peroxisomal pathways produce free radicals that can perpetuate hepatocyte damage.10 Additionally, metabolized alcohol depletes glutathione levels and causes a further increase in the development of free radicals, ultimately leading to ALD.21
The development of ALD is also influenced by proinflammatory cytokines activating stellate and Kupffer cells that cause hepatocellular injury.22 The accumulation of triglycerides within hepatocytes along with the proliferation of proinflammatory cytokines is an example of the “two-hit” model hypothesis for the development of steatosis. The “first hit” results in steatosis, which is only complicated by inflammation if a “second hit” occurs. The components of the first hit include the release of free fatty acids from central adipose tissue, which, along with adipokines, drain into the portal vein and cause insulin resistance. Together, these processes result in reduced hepatic fatty acid oxidation and increased fatty acid synthesis. The second hit is caused by an additional stressor such as increased oxidative stress, lipid peroxidation, decreased levels of antioxidants, adipocytokines, transforming growth factor, and peroxisomes.23
Evidence for genetic susceptibility to ALD has also been demonstrated and was derived from a study of twins demonstrating that the concordance rate for alcoholic cirrhosis was three times higher in monozygotic versus dizygotic twin pairs.24,25 Moreover, indirect evidence of a genetic component to disease risk comes from the observation that the death rate from ALD is subject to wide ethnic variations not entirely explained by variations in the prevalence of alcohol abuse.26 Individuals of Hispanic origin appear to be at particularly high risk. For example, one group of investigators reported the coexistence of nonalcoholic steatohepatitis (NASH) and cryptogenic cirrhosis in seven of the eight groups of Hispanic relatives studied,27 while other researchers reported that 18% of 90 patients with NASH had an affected first-degree relative.28 Two other studies examining ethnic differences in the prevalence of nonalcoholic fatty liver disease and related cryptogenic cirrhosis found that the prevalence of cryptogenic cirrhosis was three times higher in Hispanics and four times lower in African-American patients compared with European-American patients.29,30
Diagnosis and assessment of ALD
In the early stages of ALD, including ASH, patients are either asymptomatic or have an enlarged liver, discomfort in the right upper quadrant of the abdomen, nausea, vomiting, and signs of jaundice. Patients may also have more advanced symptoms such as spider nevi, abdominal distention, portal hypertension, splenomegaly, ascites, encephalopathy, and asterixis.10,14 Even when liver disease is suspected, it is difficult to distinguish ALD from other types of liver disease such as NASH without additional information.31 It is important to obtain an accurate patient history to determine risk factors for ALD and to assess alcohol use history. Tools useful in detecting alcohol abuse or dependence such as the CAGE32 and Alcohol Use Disorders Identification Test33 questionnaires should be used to identify at-risk patients.
LFTs are used to assess for liver damage; in particular, concentrations of aspartate transaminase (AST) and alanine transaminase (ALT) should be measured. The γ-glutamyltransferase (GGT) level can be useful simply to verify if someone is drinking and over time to monitor changes in drinking behavior, yet the GGT level alone is not useful in gauging how much someone is actually drinking. Alcoholic fatty liver is characterized by elevations in AST, ALT, and GGT, as well as elevated triglycerides and occasional elevations in bilirubin. Patients with alcoholic hepatitis usually have AST or ALT values two to seven times the upper limit of normal, and AST levels typically exceed ALT levels. An AST or ALT value over 400 IU/L is uncommon in ALD and indicates an additional or alternative hepatic problem.10,14,31 The AST:ALT ratio, however, is sometimes useful in differentiating between causes of liver damage.34 The AST:ALT ratio is typically 1 or more, with a ratio exceeding 3 being highly indicative of ALD. A ratio of 2 or more may be associated with alcoholic hepatitis or hepatocellular carcinoma, and a ratio of greater than 1 but less than 2 may be associated with cirrhosis (by contrast, in viral hepatitis the ratio may be less than 1, but the levels of both AST and ALT are high). Generally, the AST:ALT ratio is less useful when liver enzymes are not elevated or concurrent conditions exist. Poly-morphonuclear cell counts exceeding 5500/μL are predictive of severe alcoholic hepatitis.31
In general, the diagnosis of ALD can be made when a patient exhibits severe liver dysfunction and a consistent history of alcohol abuse and alternative causes of liver disease are excluded.35 Imaging is important in staging the severity of disease, though is not as useful as laboratory values in identifying alcohol as the cause of liver disease. Biopsy is a useful tool for resolving diagnostic uncertainty in liver disease, for disease management, and for staging the severity of disease.36 While biopsy is the only way to confirm the extent of liver disease, the use of biopsy in liver disease is hampered by the invasive quality of the procedure.
It is vital to assess for disease in order to stratify the severity of ALD and approach treatment appropriately. The Maddrey Discriminant Function (MDF) uses prothrombin time and bilirubin to stratify the severity of illness.36 An MDF score of 32 or higher signals the highest risk of mortality (30-50% over one month); patients with concomitant hepatic encephalopathy are at even higher risk for mortality. Other prognostic measures, such as the Model for End-Stage Liver Disease (MELD) score,37-39 the Glasgow Alcoholic Hepatitis Score (GAHS),37 and the ABIC score (which incorporates age, serum bilirubin, International Normalized Ratio, and serum creatinine), can be used to stratify the risk of death in patients with alcoholic hepatitis at 90 days and one year.40
Fatty liver carries a good prognosis if the patient becomes abstinent and the condition clears up after four to six weeks.38-40 In cases of alcoholic hepatitis, liver function typically returns to normal in approximately two thirds of patients if they abstain from drinking, while continued drinking accelerates mortality.40 Medical calculators to facilitate the use of the MELD, GAHS, and ABIC scoring systems can be found on the Internet.41,42
Nutritional support
Individuals abusing or dependent on alcohol are likely to suffer from protein-calorie malnutrition and be deficient in important vitamins and minerals.14 Health care providers should encourage patients with known cirrhosis to eat small meals frequently, including night-time snacks, as that may prevent muscle wasting and improve lean muscle mass, ultimately improving overall health.10
Nutritional therapy should begin with the supplementation of micronutrients.22 Alcoholic patients are given folic acid, thiamine, and pyridoxine in both acute care and outpatient settings.10,14,40 In addition, any electrolyte deficiencies should be corrected. Hypoalbuminemia and malnutrition are common issues in the acute management of alcohol abuse and have important implications for long-term survival. Enteral feedings in the acute care setting have been shown to have a positive impact on mortality in cirrhotic patients relative to a standard oral diet (12% versus 47% mortality).40 Further evidence suggests that enteral feedings are comparable in effectiveness to corticosteroids in the acute care setting and may improve one-year mortality.40 Patients should be given a high calorie intake (40 kcal/kg daily), with supplementation with protein that includes branched-chain amino acids (1.5 g/kg daily) in cases of hepatic encephalopathy.14 Data suggest that in the setting of acute alcoholic hepatitis, patients who receive enteral nutrition have a decreased nitrogen balance and increased survival rates.13 During acute alcoholic hepatitis, patients treated with enteral nutritional support showed improvement in biochemical markers of liver injury. However, there was not a statistically significant improvement in mortality.18 Earlier deaths secondary to acute alcoholic hepatitis were noted when patients only received enteral nutrition instead of treatment combining nutritional support with corticosteroids.17
The presence and extent of protein-calorie malnutrition play an important role in determining the outcome of patients with ALD. Mortality increases in proportion to the extent of malnutrition, approaching 80% in patients with severe malnutrition (i.e., a nutrition level less than 50% of normal).14 Micronutrient abnormalities, such as hepatic vitamin A depletion and depressed vitamin E levels, may also potentially aggravate liver disease. Diets rich in polyunsaturated fats promote alcohol-induced liver disease in animals, whereas diets high in saturated fats may be protective. Obesity and excess body weight have been associated with an increased risk of ALD.14
Evidence-based pharmacotherapies for alcoholic hepatitis
There is limited evidence of a treatment that improves the hepatic destruction found in ALD.42,43 All patients at risk for developing ALD should be advised to abstain from alcohol, as there is no treatment as effective as abstinence, and the only recommended form of hepatoprotection is complete abstinence.10,14,22,41,43 Even patients with histological evidence of irreversible hepatic cellular changes should be advised to abstain from alcohol in an effort to slow the further progression of ALD. In patients with decompensated cirrhosis, complete abstinence from alcohol is associated with 60% five-year survival, compared with 30% five-year survival in patients who continue to drink alcohol.10
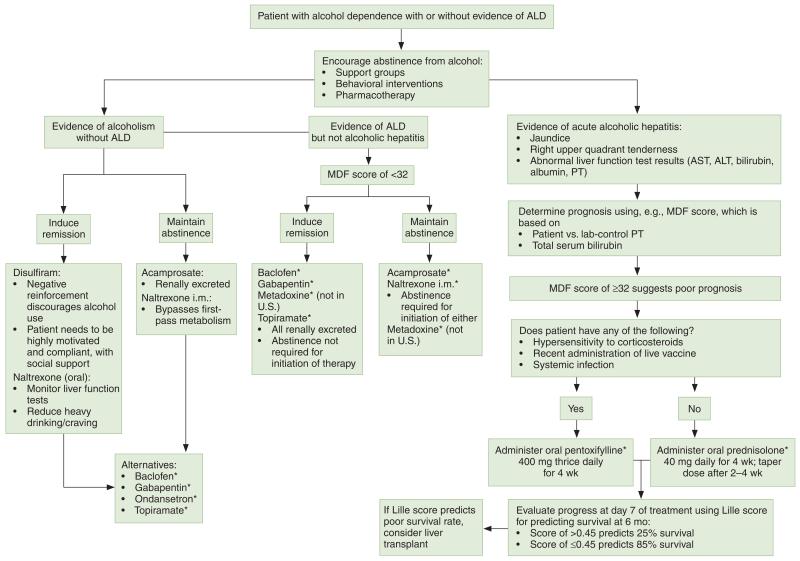
Although not supported by FDA-approved indications, two pharmacologic options for decreasing mortality in severe acute hepatitis have been frequently studied. In order to determine which patients would benefit from initiation and maintenance of either corticosteroids (primarily prednisolone) or pentoxifylline, the MDF and Lille scores are used (Figure 1). The Lille score is used for early identification of patients with severe (MDF score of ≥32) alcoholic hepatitis not responding to corticosteroids and incorporates values for serum bilirubin, serum albumin, prothrombin time, serum creatinine, and age.44 The score is used to determine the advisability of continuing corticosteroid treatment beyond seven days with the intent of improving survival. Patients with a Lille score of greater than 0.45 have a six-month survival probability of about 25%, compared with 85% in patients with lower scores.
Figure 1.
Suggested treatment algorithm for alcoholic liver disease (ALD) and alcoholic hepatitis. AST = aspartate transaminase, ALT = alanine transaminase, PT = prothrombin time, MDF = Maddrey Discriminant Function. Asterisk denotes lack of a Food and Drug Administration–approved indication
Corticosteroids
O’Shea and colleagues14 outlined the 40-year history of clinical trials using corticosteroids for the treatment of acute alcoholic hepatitis in patients for whom corticosteroids were not contraindicated. The consensus was that using corticosteroids for patients with an MDF score of 32 or higher decreased the short-term mortality rate; thus, these agents are considered the first-line treatment for acute alcoholic hepatitis. The use of 40 mg of prednisolone orally for 28 days was determined to be the most effective course of treatment for patients with acute alcoholic hepatitis.17 The data suggested a significant decrease in short-term (30-day) mortality in patients randomized to prednisolone, but only in those with more severe liver dysfunction, as manifested by hepatic encephalopathy or a markedly abnormal MDF score. The adverse effects of corticosteroid use must be also taken into consideration prior to administration, since adverse effects may include hyperglycemia, Cushing’s syndrome, and a heightened risk of infection.10
It is important to recognize that because of the exclusion criteria for clinical trials for alcoholic hepatitis, the efficacy of corticosteroids has not been fully evaluated in patients with severe alcoholic hepatitis and simultaneous pancreatitis, gastrointestinal bleeding, renal failure, or active infection. Despite the improvements in short-term survival, there is a lack of statistically significant data to support the benefit of long-term corticosteroid use for ALD.23 Therefore, health care providers are urged to use the Lille score for determining the length of treatment with corticosteroids.44 Ultimately, however, it is quite possible that treatment with prednisolone may not be effective at later stages of the disease.14
Pentoxifylline
Pentoxifylline, a phosphodiesterase inhibitor that also inhibits the production of tumor necrosis factor α (TNF-α), has an FDA-approved indication for the treatment of intermittent claudication. Based on the ability of pentoxifylline to inhibit the production of TNF-α, researchers investigated the potential benefit of using pentoxifylline 400 mg orally three times a day for four weeks in patients with an MDF score of 32 or higher for whom corticosteroids were contraindicated.10,14 As a result, pentoxifylline is considered the second-line pharmacotherapy for acute alcoholic hepatitis. Researchers reported that pentoxifylline decreased mortality from acute alcoholic hepatitis by 40% and reduced the likelihood of patients developing hepatorenal syndrome.45 (In patients with acute alcoholic hepatitis, the development of hepatorenal syndrome has been reported to account for 50% of deaths.16) Pentoxifylline was found to have renoprotective effects and ultimately proved to be a superior pharmacotherapy for acute alcoholic hepatitis.10 Despite the beneficial evidence of pentoxifylline, it is still recommended that corticosteroids be used as the primary treatment option in acute alcoholic hepatitis unless corticosteroid use is contraindicated.14
A recent study sought to determine if the addition of pentoxifylline to prednisolone was more effective than prednisolone alone in patients with alcoholic hepatitis.46 Patients (n = 270) in this multicenter double-blind study were randomly assigned to receive either a combination of 40 mg of prednisolone daily and 400 mg of pentoxifylline three times a day for 28 days or 40 mg of prednisolone daily and a matching placebo for 28 days. In this trial, treatment with pentoxifylline and prednisolone, compared with prednisolone alone, did not result in improved six-month survival. However, a lower frequency of hepatorenal syndrome in the pentoxifylline group may have limited the results of this study.
Therapies for alcoholic hepatitis lacking sufficient evidence for efficacy
Pioglitazone
NASH and ALD have the same histological features as progressive liver disease; however, the etiology of NASH differs since it is thought to be related to insulin resistance.47 As an FDA-approved hypoglycemic (based on its ability to improve insulin sensitivity), pioglitazone was studied for the potential to improve biochemical and histological features in NASH. A study was performed in 18 nondiabetic patients with biopsy-proven NASH who received pioglitazone (30 mg orally daily) for 48 weeks.48 Tests of insulin sensitivity and body composition, as well as liver biopsies, were performed before and after treatment. By 48 weeks, ALT values fell to normal in 72% of patients, and two thirds of patients had histological improvements.
In addition to the therapeutic benefits of pioglitazone for liver disease, there is also evidence of a direct effect on the brain. A preclinical study showed that the oral administration of pioglitazone resulted in activation of peroxisome proliferator-activated receptor γ and ultimately reduced consumption of alcohol, relapse, and alcohol withdrawal symptoms.49 Therefore, pioglitazone appears to have multiple mechanisms of action against ALD that warrant further research. However, at this time there is insufficient evidence to suggest a role for pioglitazone in the treatment of ALD.
Anti-TNF-α antibodies
Based on the role that TNF-α plays in the pathophysiology of ALD, researchers examined the efficacy of anti-TNF-α antibodies in ALD. The safety and efficacy of infliximab or etanercept alone were compared with the use of either agent in combination with prednisolone in patients with acute alcoholic hepatitis, but three separate studies yielded disappointing results. In one study, the use of a single 5-mg/kg dose of infliximab with or without 40 mg of prednisolone showed no statistically significant differences in mortality50; another study involving the use of 10 mg of infliximab with 40 mg of prednisolone ended prematurely due to seven patient deaths,51,52 and a study examining the efficacy of using etanercept failed to produce any difference in mortality.54 There was an overall increased risk of developing a life-threatening infection when patients received higher doses of etanercept.53 Despite the support for the theoretical use of anti-TNF-α antibodies in patients with ALD, the increased risks of infection and mortality outweigh the benefits for most of these drugs.
Antioxidants
Based on the pathophysiologic effects of oxidative stress on hepatocytes, researchers investigated the safety and efficacy of using antioxidants in ALD. Oxidative stress, one of the main stressors associated with the second hit, is one of the most popular potential mechanisms of hepatocellular insult.54
There is some inconsistent evidence of the benefits of antioxidants. Alcoholic fatty liver has been shown to be associated with inhibition of sirtuin 1 and adenosine monophosphate (AMP)-activated kinase. Resveratrol, a major component of red wine, has been identified as a potent activator of both sirtuin 1 and AMP-activated kinase and was shown to alleviate fatty liver in mice.55 Researchers found that resveratrol prevented free radicals and proinflammatory cytokines from causing oxidative damage; however, there was a lack of clinically significant efficacy in using resveratrol to treat or improve ALD.10
Liver injury has been noted after depletion of S-adenosylhomocysteine and subsequent depletion of glutathione.22 Abdelmalek and colleagues56 noted that betaine had the potential for improving liver injury since it was an alternative source for methyl group donation by substituting itself for S-adenosylhomocysteine. Hepatic protection via betaine was thought to occur by elevation of hepatic glutathione, downregulation of TNF-α, upregulation of interleukin 10, and inhibition of hepatocyte apoptosis. The results of the randomized placebo-controlled study demonstrated that there were no histological, pathological, or laboratory value improvements in liver injury associated with betaine use compared with placebo use. Other antioxidant therapies investigated in the treatment of alcoholic hepatitis but not found to provide any benefit include vitamin E,57 S-adenosyl-l-methionine,58 and silymarin,59 the active ingredient in milk thistle.
Miscellaneous
Several other therapies have been investigated in the treatment of alcoholic hepatitis specifically but have not been demonstrated to have any efficacy. These treatments include propylthiouracil,60 insulin,61 glucagon,62 and calcium channel blockers.63 Acetylcysteine in combination with prednisolone 40 mg a day was found to significantly improve the one-month survival of patients with severe alcoholic hepatitis; however, the six-month survival rate was not improved.64 As a result, until there are more data, there is currently not enough support for the use of any of these pharmacotherapies.
Evidence-based pharmacotherapies to reduce drinking or promote abstinence: Drugs approved in the United States
A search of the literature found that no U.S.-approved medications for use in treating alcohol dependence have been formally tested in patients with ALD. Given their safety, several other medications such as topiramate, ondansetron, baclofen, and gabapentin have the potential to be useful in treating alcoholism in this population65 (Figure 1). Of the medications available in the United States, only baclofen has been tested in double-blind, placebo-controlled trials in patients with ALD.65 Therefore, the medications currently approved for the treatment of alcohol dependence remain the primary treatment options to facilitate abstinence in patients with ALD. The mechanisms by which these medications reduce drinking are largely unknown, though it is compelling to consider that they work by reducing alcohol craving. Greater craving has been associated with the increased probability of alcoholism relapse. Reversal of craving, which is understood to be a symptom of protracted abstinence, offers the possibility of preventing relapses and treating alcoholism. While various medications have been researched for effectiveness in reducing alcohol craving, the results obtained from clinical studies have been conflicting.66
Acamprosate
Clinical trials suggest that acamprosate calcium 666 mg three times a day orally is an efficacious treatment for alcoholism since it has no abuse potential or significant drug interactions with naltrexone, antidepressants, anxiolytics, and hypnotics, which are commonly used in patients with alcoholism.16 Of the FDA-approved medications for alcohol dependence, acamprosate was found to be the only medication, because of its renal excretion, not associated with liver toxicity,67 and a Cochrane review reported diarrhea as the only significant adverse effect of acamprosate.68 By acting as a coagonist of N-methyl-d-aspartate receptors, acamprosate creates a balance between inhibitory and excitatory neurotransmitters, which ultimately aids in abstinence from alcohol.16 Acamprosate was found to be far better for maintaining abstinence than for inducing remission of alcoholism.14 In a study of patients with comorbid bipolar disorder in whom there was known baseline glutamate dysregulation, acamprosate therapy was found to be safe and well tolerated.67 A meta-analysis of data on its use for the treatment of alcohol use disorder suggested that acamprosate may be most effective in inducing and promoting abstinence among patients who have already undergone alcohol detoxification.69
Opioid antagonists
The opioid antagonist naltrexone, in both oral and injectable formulations, is approved for treating alcohol dependence. Initially developed as a β-endorphin antagonist primarily acting on the μ-opioid receptor that governs opiate dependence, oral naltrexone hydrochloride 12.5-100 mg per day gained an FDA-approved indication for alcohol dependence in the mid-1990s.14 Naltrexone blocks the uptake of endorphins when alcohol is consumed, resulting in an attenuation of dopamine release at the nucleus accumbens thought to be crucially important to positive reinforcement and reward in those who may be predisposed. Naltrexone 380 mg once monthly intramuscularly was demonstrated to be more effective than placebo use in reducing alcohol consumption, particularly in men and in patients who were already abstinent at randomization, and is recommended at the initiation of treatment.70 As 6-β-naltrexol is the primary metabolite of naltrexone, significantly less 6-β-naltrexol is produced after intramuscular versus oral naltrexone administration due to a reduction in first-pass metabolism after injection, yet hepatotoxicity remains a concern and should be monitored.20 Oral naltrexone has been noted to decrease cravings and reduce heavy drinking.71 Naltrexone is contraindicated in patients with acute hepatitis or liver failure because of the entero-hepatic recycling of its metabolites.13 Additionally, naltrexone should be avoided in patients with renal failure due to active renal tubular secretion during glomerular filtration.22
Disulfiram
The first pharmacotherapy with an approved indication for alcoholism, disulfiram (250-500 mg per day orally) acts as a negative reinforcement to discourage alcohol use.14 Disulfiram is an irreversible acetaldehyde dehydrogenase inhibitor that blocks the second stage of alcohol metabolism, creating a buildup of the toxic intermediate acetaldehyde.71 The inhibition reinforces an individual’s desire to stop drinking by providing a psychological and physical disincentive associated with the increase in acetaldehyde, which produces hypotension, flushing, nausea, and vomiting when alcohol is consumed. Notably, successful use of disulfiram is highly dependent on patient compliance and motivation; however, disulfiram use should be avoided in patients with advanced ALD due to the potential for hepatotoxicity.
Evidence-based pharmacotherapies to reduce drinking or promote abstinence: Drugs not approved in the United States
Nalmefene
The efficacy of as-needed targeting of the opioid system with the aim of reducing alcohol consumption in alcohol-dependent patients has been established with nalmefene, an opioid antagonist with a longer half-life, greater oral bioavailability, and no observed dose-dependent liver toxicity relative to naltrexone.72 Nalmefene was recently approved by the European Medicines Agency for as-needed use in the treatment of alcoholism.
Gamma-hydroxybutyric acid
Gamma-hydroxybutyric acid (GHB) is approved in a few European countries for the treatment of alcoholism. There is some evidence that GHB may be helpful in reducing alcohol consumption and promoting abstinence73,74; however, due to the scarcity of relevant well-run clinical trials, there is insufficient evidence indicating that GHB could be an effective treatment.75 Also, given its potential for addiction and requirement for significant medical oversight, GHB in its present dosage form would most likely have difficulty gaining U.S. approval for this indication.
Pharmacotherapies to reduce drinking or promote abstinence in patients with ALD
Baclofen
As a selective agonist of the γ-aminobutyric acid type B (GABAB) receptor, baclofen (titrated up to a standard dosage of 10 mg orally thrice daily) is approved by FDA (maximum dosage, 80 mg/day) for controlling symptoms of reversible spasticity. Baclofen is the only drug with simultaneous evidence for efficacy as an off-label treatment for alcoholism and as a useful agent for treating patients in various stages of ALD, including cirrhosis. GABAB-receptor activation by baclofen was investigated for its potential role in alcohol withdrawal symptoms. Investigators found rapid suppression of withdrawal symptoms when bac lofen was administered at a dose of 25 mg (given every eight hours), with no return of symptoms seen when the dose was tapered down to 10 mg.76 The use of baclofen in alcohol withdrawal was proven beneficial in comparison to the gold-standard therapy, diazepam78; there were no significant differences between the two pharmacotherapies in any evaluated outcome, and baclofen conferred an additional benefit in that patients did not develop any euphoric effect from the medication.
Baclofen was investigated for its potential role in managing alcoholism in patients with ALD. Preclinical research suggested that baclofen reduced alcohol consumption, as well as the motivational and reinforcing properties of alcohol abuse, by suppressing the release of dopamine.77 Clinical trials in humans discovered decreased cravings for alcohol when baclofen was administered at a dosage of 10 mg every eight hours for the first 3 days, with the dosage increased to 30 mg every eight hours for a subsequent 27 days.77 These data were not replicated by Garbutt and colleages,78 and differences in the severity of dependence have been highlighted as possible reasons for the conflicting results.76 When compared with placebo use, baclofen therapy was more effective in achieving and maintaining abstinence from alcohol when the oral dosage was raised from 10 mg twice daily to 20 mg twice daily.79 In cirrhotic alcoholic patients, baclofen was safe, promoted total abstinence and an increase in abstinent days, and improved LFT results.79 These findings were confirmed in a sub-group of alcoholic patients who also had hepatitis C virus infection.80 Additionally, there were no negative hepatic effects noted with the use of baclofen in any studies of patients with existing ALD.66
Metadoxine
While not available in the United States, metadoxine is approved for use in several European countries, India, the Russian Federation, and Brazil in treating alcoholism and alcohol toxicity and has been tested for ALD. The effect of metadoxine after alcohol ingestion was demonstrated to be an overall increase in metabolism via increased activity of acetaldehyde dehydrogenase.81,82 Metadoxine is of particular interest in treating acute alcohol intoxication since it is rapidly absorbed orally and has a half-life of 40-60 minutes. In acute alcohol intoxication, the median time to onset of recovery was reported to be 0.95 hour with metadoxine compared with 2.34 hours with placebo use (p = 0.013).83 The effects of treatment on blood alcohol levels were paralleled by a significant decrease in the rating of toxic clinical symptoms, and patients were noted to have significant improvement in the symptoms of acute alcohol intoxication. The increased metabolism of alcohol with metadoxine use was investigated to determine if it was beneficial in ALD.81 In mice that were not exposed to alcohol, metadoxine increased the hepatic concentrations of adenosine triphosphate. Mice that were given metadoxine prior to alcohol exposure demonstrated hepatoprotection against depleted glutathione, decreased ADH, and oxidative damage. Metadoxine further decreased cytokine damage to hepatocytes by inhibiting the increase in proinflammatory levels of TNF-α.81 Additionally, metadoxine aided in improving ALD by inhibiting fatty-acid esters in the liver of mice exposed to alcohol, which ultimately corrected the balance between saturated and unsaturated fats. The administration of metadoxine prior to ingestion of alcohol inhibits the accumulation of free fatty acids in various organs.66
Caballeria and colleagues82 demonstrated in a clinical trial that metadoxine was beneficial relative to placebo use in treating ASH. Within one month of being treated with oral metadoxine 500 mg twice daily, patients had improvement of LFT results. Within three months of the initiation of metadoxine treatment, LFT results were normalized. LFT results were not the only indicator of outcomes, as ultrasound revealed resolution of steatosis in 70% of patients taking metadoxine compared with 20% of placebo recipients.
Metadoxine was also found to have an additional benefit in mitigating ALD. Since cessation of alcohol is currently the only recommended form of hepatoprotection, a drug that would help maintain abstinence would be of significant importance for patients with ALD. Metadoxine was found to maintain abstinence and reduce the intake of and cravings for alcohol in alcohol-dependent patients.81 A retrospective review of the medical records of patients with ALD found that metadoxine was not only safe to use but improved the signs of liver damage.83
Conclusion
Although various treatments have been investigated for ALD in patients with alcoholism, complete abstinence from alcohol is currently the only recommended form of hepatoprotection for the entire spectrum of ALD diagnoses.
Acknowledgments
Drs. Kenna and Swift are currently receiving or have received support from the National Institute on Alcohol Abuse and Alcoholism from the following grants: R01-AA016079 (drugs under study, ondansetron and sertraline), R03AA020169 (baclofen), R21AA019709 (ghrelin), R01-AA015753 (aripiprazole and topiramate), R21AA019994 (doxazosin), and R21AA021128 (metadoxine). Drs. Kenna and Swift have received consultant fees from Laboratorio Farmaceutico CT Srl (drug under study, GET73). Dr. Swift has received fees from D & A Pharma.
Footnotes
The other authors have declared no potential conflicts of interest.
Contributor Information
Cynthia L. Vuittonet, Department of Internal Medicine, Warren Alpert Medical School, Brown University, Providence, RI.
Michael Halse, South County Hospital, Wakefield, RI.
Lorenzo Leggio, Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology, Laboratory of Clinical and Translational Studies, National Institute on Alcohol Abuse and Alcoholism, and Section Chief, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, and Center for Alcohol and Addiction Studies, Brown University.
Samuel B. Fricchione, Center for Alcohol and Addiction Studies, Brown University.
Michael Brickley, Center for Alcohol and Addiction Studies, Brown University.
Carolina L. Haass-Koffler, Center for Alcohol and Addiction Studies, Brown University.
Tonya Tavares, Center for Alcohol and Addiction Studies, Brown University.
Robert M. Swift, Center for Alcohol and Addiction Studies, Brown University, Deputy Director of Research, Providence Veterans Affairs Medical Center, Providence, RI, and Department of Psychiatry and Human Behavior, Warren Alpert Medical School, Brown University.
George A. Kenna, Center for Alcohol and Addiction Studies, and Department of Psychiatry and Human Behavior, Warren Alpert Medical School, Brown University.
References
- 1.Mokdad A, Marks J, Stroup D, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.National Highway Traffic Safety Administration [accessed 2014 Apr 28];Traffic safety facts: 2006 data. Alcohol-impaired driving. www.nrd.nhtsa.dot.gov/Pubs/810801.PDF.
- 3.Chou SP, Dawson DA, Stinson FS, et al. The prevalence of drinking and driving in the United States, 2001-2002: results from the national epidemiological survey on alcohol and related conditions. Drug Alcohol Depend. 2006;83:137–46. doi: 10.1016/j.drugalcdep.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Harford TC, Dawson DA. Prevalence of DSM-IV alcohol abuse and dependence: United States, 1992. Alcohol Health Res World. 2000;18:243–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Harford TC, Grant BF. Prevalence and population validity of DSM-III-R alcohol abuse and dependence: the 1989 National Longitudinal Survey on Youth. J Subst Abuse. 1994;6:37–44. doi: 10.1016/s0899-3289(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 6.Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–73. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- 7.Grant BF, Dawson DA, Stinson FS, et al. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211–20. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- 9.Harwood HJ, of The Lewin Group, for the National Institute on Alcohol Abuse and Alcoholism [accessed 2014 Apr 28];Updating estimates of the economic costs of alcohol abuse in the United States: estimates, update methods, and data. 2000 Dec; http://pubs.niaaa.nih.gov/publications/economic-2000/alcoholcost.PDF.
- 10.Bruha R, Dvorak K, Petrtyl J. Alcoholic liver disease. World J Hepatol. 2012;4:81–90. doi: 10.4254/wjh.v4.i3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–46. doi: 10.1016/j.cld.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Shield KD, Taylor B, Kehoe T, et al. Mortality and potential years of life lost attributable to alcohol consumption in Canada in 2005. BMC Public Health. 2012;12:91. doi: 10.1186/1471-2458-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addolorato G, Russell M, Albano E, et al. Understanding and treating patients with alcoholic cirrhosis: an update. Alcohol Clin Exp Res. 2009;33:1136–44. doi: 10.1111/j.1530-0277.2009.00956.x. [DOI] [PubMed] [Google Scholar]
- 14.O’Shea RS, Dasarathy S, McCullough AJ. for the Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 15.Li TK, Beard JD, Orr WE, et al. Gender and ethnic differences in alcohol metabolism. Alcohol Clin Exp Res. 1998;22:771–2. [Google Scholar]
- 16.Witkiewitz KA, Donovan DM, Hartzler B. Drink refusal training as part of a combined behavioral intervention: effectiveness and mechanisms of change. J Consult Clin Psychol. 2012;80:440–9. doi: 10.1037/a0026996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39–45. doi: 10.1016/S0168-8278(12)60005-1. [DOI] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol. 2012;2012:853175. doi: 10.1155/2012/853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards SM, Kenna GA, Swift RM, et al. Current and promising pharmacotherapies, and novel research target areas in the treatment of alcohol dependence: a review. Cur Pharm Design. 2011;17:1323–32. doi: 10.2174/138161211796150765. [DOI] [PubMed] [Google Scholar]
- 21.Testino G. Alcoholic hepatitis. J Med Life. 2013;6:161–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Kershenobich D, Corona DL, Kersheno vich R, et al. Management of alcoholic liver disease: an update. Alcohol Clin Exp Res. 2011;35:804–5. doi: 10.1111/j.1530-0277.2010.01402.x. [DOI] [PubMed] [Google Scholar]
- 23.Harris AH, Oliva E, Bowe T, et al. Pharmacotherapy of alcohol use disorders by the Veterans Health Administration: patterns of receipt and persistence. Psychiatr Serv. 2012;63:679–85. doi: 10.1176/appi.ps.201000553. [DOI] [PubMed] [Google Scholar]
- 24.Anstee QM, Daly AK, Day CP. Genetics of alcoholic and nonalcoholic fatty liver disease. Semin Liver Dis. 2011;31:128–46. doi: 10.1055/s-0031-1276643. [DOI] [PubMed] [Google Scholar]
- 25.Hrubec Z, Omenn GS. Evidence for genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcohol Clin Exp Res. 1981;5:207–15. doi: 10.1111/j.1530-0277.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- 26.Stinson FS, Grant BF, Dufour MC. The critical dimension of ethnicity in liver cirrhosis mortality statistics. Alcohol Clin Exp Res. 2001;25:1181–7. [PubMed] [Google Scholar]
- 27.Caetano R, Clark CL. Trends among alcohol related problems among whites, blacks, and Hispanics: 1984-1995. Alcohol Clin Exp Res. 1998;22:534–8. [PubMed] [Google Scholar]
- 28.Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9–13. doi: 10.1016/s0002-9343(99)00315-0. [DOI] [PubMed] [Google Scholar]
- 29.Willner IR, Waters B, Patil SR, et al. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–61. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- 30.Browning JD, Kumar KS, Saboorian MH, et al. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99:292–8. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 31.Zakhari S. Bermuda Triangle for the liver: alcohol, obesity, and viral hepatitis. J Gastroenterol Hepatol. 2013;28(suppl 1):18–25. doi: 10.1111/jgh.12207. [DOI] [PubMed] [Google Scholar]
- 32.Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252:1905–7. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 33.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. second edition Department of Mental Health and Substance Dependence, World Health Organization; Geneva: 2001. The Alcohol Use Disorders Identification Test: guidelines for use in primary care. [Google Scholar]
- 34.De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis: the transaminase serum activities. Clin Chim Acta. 2006;369:148–52. doi: 10.1016/j.cca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 35.McCullough AJ, O’Shea RS, Dasarathy S. Diagnosis and management of alcoholic liver disease. J Dig Dis. 2011;12:257–62. doi: 10.1111/j.1751-2980.2010.00470.x. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. for the Practice Guidelines Committee of American Association for Study of Liver Diseases and Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764–76. doi: 10.1053/j.gastro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Singal AK, Chaha KS, Rasheed K, et al. Liver transplantation in alcoholic liver disease: current status and controversies. World J Gastroenterol. 2013;19:5953–63. doi: 10.3748/wjg.v19.i36.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addolorato G, Mirijello A, Leggio L, et al. for the OLT Group. Liver transplantation in alcoholic patients: impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res. 2013;37:1601–8. doi: 10.1111/acer.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilg H, Day CP. Management strategies in alcoholic liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:24–34. doi: 10.1038/ncpgasthep0683. [DOI] [PubMed] [Google Scholar]
- 41.MDCalc. [accessed 2013 Dec 26]; www.mdcalc.com.
- 42. [accessed 2013 Dec 26];Alcoholic hepatitis score calculator. http://potts-uk.com/livercalculator.html.
- 43.Stickel F, Seitz HK. Update on the management of alcoholic steatohepatitis. J Gastrointestin Liver Dis. 2013;22:189–97. [PubMed] [Google Scholar]
- 44.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–54. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 45.Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–48. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 46.Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033–41. doi: 10.1001/jama.2013.276300. [DOI] [PubMed] [Google Scholar]
- 47.Lutchman G, Promrat K, Kleiner DE, et al. Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: relationship to histological improvement. Clin Gastroenterol Hepatol. 2006;4:1048–52. doi: 10.1016/j.cgh.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–96. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 49.Stopponi S, Somaini L, Cippitelli A, et al. Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry. 2011;69:642–9. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Spahr L, Rubbia-Brandt L, Frossard JL, et al. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37:448–55. doi: 10.1016/s0168-8278(02)00230-1. [DOI] [PubMed] [Google Scholar]
- 51.Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–7. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 52.Mookerjee RP, Tilg H, Williams R, et al. Infliximab and alcoholic hepatitis. Hepatology. 2004;40:499–500. doi: 10.1002/hep.20344. [DOI] [PubMed] [Google Scholar]
- 53.Menon KV, Stadheim L, Kamath P, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol. 2004;99:255–60. doi: 10.1111/j.1572-0241.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- 54.Basaranoglu M, Basaranoglu G, Sentürk H. From fatty liver to fibrosis: a tale of “second hit”. World J Gastroenterol. 2013;19:1158–65. doi: 10.3748/wjg.v19.i8.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ajmo JM, Liang X, Rogers CQ, et al. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–42. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdelmalek MF, Sanderson SO, Angulo P, et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50:1818–26. doi: 10.1002/hep.23239. [DOI] [PubMed] [Google Scholar]
- 57.Mezey E, Potter JJ, Rennie-Tankersley L, et al. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40:40–6. doi: 10.1016/s0168-8278(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 58.Rambaldi A, Gluud C. S-adenosyl-L-methionine for alcoholic liver diseases. Cochrane Database Syst Rev. 2006:2CD002235. doi: 10.1002/14651858.CD002235.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Rambaldi A, Jacobs BP, Iaquinto G, et al. Milk thistle for alcoholic and/or hepatitis B or C liver diseases—a systematic cochrane hepato-biliary group review with meta-analyses of randomized clinical trials. Am J Gastroenterol. 2005;100:2583–91. doi: 10.1111/j.1572-0241.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 60.Fede G, Germani G, Gluud C, et al. Propylthiouracil for alcoholic liver disease. Cochrane Database Syst Rev. 2011;6:CD002800. doi: 10.1002/14651858.CD002800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bird G, Lau JY, Koskinas J, et al. Insulin and glucagon infusion in acute alcoholic hepatitis: a prospective randomized controlled trial. Hepatology. 1991;14:1097–101. [PubMed] [Google Scholar]
- 62.Trinchet JC, Balkau B, Poupon RE, et al. Treatment of severe alcoholic hepatitis by infusion of insulin and glucagon: a multicenter sequential trial. Hepatology. 1992;15:76–81. doi: 10.1002/hep.1840150115. [DOI] [PubMed] [Google Scholar]
- 63.Bird GL, Prach AT, McMahon AD, et al. Randomised controlled double-blind trial of the calcium channel antagonist amlodipine in the treatment of acute alcoholic hepatitis. J Hepatol. 1998;28:194–8. doi: 10.1016/0168-8278(88)80005-9. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–9. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 65.Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27:287–99. doi: 10.1007/s40263-013-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haass-Koffler C, Leggio L, Kenna GA. Pharmacological approaches to reducing craving for alcohol in alcohol-dependent patients. CNS Drugs. doi: 10.1007/s40263-014-0149-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tolliver BK, Desantis SM, Brown DG, et al. A randomized, double-blind, placebo-controlled clinical trial of acamprosate in alcohol-dependent individuals with bipolar disorder: a preliminary report. Bipolar Disord. 2012;14:54–63. doi: 10.1111/j.1399-5618.2011.00973.x. [DOI] [PubMed] [Google Scholar]
- 68.Rösner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010;9:CD004332. doi: 10.1002/14651858.CD004332.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–93. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 71.Kenna GA, McGeary JE, Swift RM. Pharmacotherapy, pharmacogenomics, and the future of alcohol dependence treatment, part 1. Am J Health-Syst Pharm. 2004;61:2272–9. doi: 10.1093/ajhp/61.21.2272. [DOI] [PubMed] [Google Scholar]
- 72.Mann K, Bladstrom A, Torup L, et al. Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol Psychiatry. 2013;73:706–13. doi: 10.1016/j.biopsych.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 73.Addolorato G, Castelli E, Stefanini GF, et al. An open multicentric study evaluating 4 hydroxybutyric acid sodium salt (GHB) in the medium term treatment of 179 alcohol dependent patients. Alcohol Alcohol. 1996;31:341–5. doi: 10.1093/oxfordjournals.alcalc.a008160. [DOI] [PubMed] [Google Scholar]
- 74.Addolorato G, Cibin M, Capristo E, et al. Maintaining abstinence from alcohol with gamma-hydroxybutyric acid. Lancet. 1998;351:38. doi: 10.1016/s0140-6736(05)78088-0. [DOI] [PubMed] [Google Scholar]
- 75.Leone MA, Vigna-Taglianti F, Avanzi G, et al. Gammahydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses. Cochrane Database Syst Rev. 2010;2:CD006266. doi: 10.1002/14651858.CD006266.pub2. [DOI] [PubMed] [Google Scholar]
- 76.Leggio L, Garbutt JC, Addolorato G. Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets. 2010;9:33–44. doi: 10.2174/187152710790966614. [DOI] [PubMed] [Google Scholar]
- 77.Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46:312–7. doi: 10.1093/alcalc/agr017. [DOI] [PubMed] [Google Scholar]
- 78.Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2010;34:1849–57. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–22. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- 80.Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37:561–4. doi: 10.1016/j.addbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gutiérrez-Ruiz MC, Bucio L, Correa A, et al. Metadoxine prevents damage produced by ethanol and acetaldehyde in hepatocyte and hepatic stellate cells in culture. Pharmacol Res. 2001;44:431–6. doi: 10.1006/phrs.2001.0883. [DOI] [PubMed] [Google Scholar]
- 82.Caballería J, Parés A, Brú C, et al. for the Spanish Group for the Study of Alcoholic Fatty Liver Metadoxine accelerates fatty liver recovery in alcoholic patients: results of a randomized double-blind, placebo-control trial. J Hepatol. 1998;28(1):54–60. doi: 10.1016/s0168-8278(98)80202-x. [DOI] [PubMed] [Google Scholar]
- 83.Leggio L, Kenna GA, Ferrulli A, et al. Preliminary findings on the use of metadoxine for the treatment of alcohol dependence and alcoholic liver disease. Human Psychopharm. 2011;26:554–9. doi: 10.1002/hup.1244. [DOI] [PubMed] [Google Scholar]