Abstract
Trans-sodium crocetinate (TSC) is a novel carotenoid compound capable of enhancing the diffusion of small molecules in aqueous solutions. TSC improves the diffusion of oxygen and glucose, and increases oxygenation in ischemic brain tissue. TSC also dampens the intensity of an ischemic challenge during an ongoing ischemic event. The current study examined the impact of TSC in rat models of ischemic and hemorrhagic stroke. Rat three vessel occlusion (3VO), and combined 3VO and one vessel occlusion (3VO/1VO) models of ischemic stroke were evaluated for structural and behavioral outcomes. The effects of TSC were also tested in a rat model of intracerebral hemorrhage (ICH). Delayed treatment with TSC reduced infarct volume in a rodent model of transient focal ischemia involving either 2 or 6 hours of ischemia. Neurological outcomes, based on a multi-scale assessment and automated gait analysis, also were improved by TSC treatment. Additionally, TSC reduced edema and hemorrhagic volume in a rat model of ICH. An optimal therapeutic candidate for early intervention in ischemic stroke should be effective when administered on a delayed basis and should not aggravate outcomes associated with hemorrhagic stroke. The current findings demonstrate that delayed TSC treatment improves outcomes in experimental models of both ischemic and hemorrhagic stroke. Together, these findings suggest that TSC may be a safe and beneficial therapeutic modality for early stroke intervention, irrespective of the type of stroke involved.
Keywords: stroke, rat, therapy, ischemia, hemorrhage, trans sodium crocetinate
1. Introduction
Emergency care for stroke must discriminate between ischemic and hemorrhagic strokes in order to safely manage the patient. Tissue plasminogen activator (t-PA) is currently the only FDA-approved therapeutic for the treatment of acute ischemic stroke (NINDs rt-PA STROKE STUDY GROUP, 1995). However, critical limitations to the utility of this therapy exist. First, t-PA can aggravate outcomes in hemorrhagic strokes and increase hemorrhagic transformation in some ischemic strokes (NINDs t-PA STROKE STUDY GROUP 1997; (Larrue et al. 2001; Hacke et al. 2004). Second, many patients are ineligible for t-PA treatment because they arrive in emergency departments after the FDA-approved therapeutic window of 3 hours, although there remains hope for extending this window (Edlow et al. 2013). Finally, the delay to recanalization after t-PA treatment is highly variable and can be rapid, delayed, complete, partial, or absent (Ribo et al. 2006). Considerable efforts are therefore being directed toward extending the window for t-PA treatment, identifying more effective thrombolytic agents, and limiting the progression of neural injury.
A complementary approach to limiting ischemic injury is to reinstate metabolic supply prior to clot dissolution. During ischemic stroke, areas of partial perfusion can maintain tissue integrity for a few hours and it may be possible to extend this period by increasing the levels of metabolic substrates in the residual flow of blood. Hyperoxic ventilation and/or the administration of compounds that increase the oxygen carrying capacity of blood continue to be tested in this regard (Singhal 2007). An alternative approach is to enhance the access of metabolic substrates to cells by increasing the diffusion of small molecules into the ischemic tissue. Trans-sodium crocetinate (TSC) is a derivative of the carotenoid crocetin, and can improve the diffusion of oxygen and glucose in aqueous solutions (Laidig et al. 1998; Stennett et al. 2006). Both crocetin and TSC have been shown to improve tissue oxygenation (Seyde et al. 1986; Okonkwo et al. 2003) and enhanced tissue oxygenation can occur without affecting blood flow rates (Holloway and Gainer 1988). Recent work from our laboratory demonstrated that TSC increases tissue oxygenation in the ischemic penumbra of a rodent model of focal ischemia (Manabe et al. 2010). Moreover, TSC treatment improves structural and behavioral outcomes in animal models of focal cerebral ischemia (Lapchak 2010a; Manabe et al. 2010). To be considered as a potential therapeutic for stroke, a treatment must be effective when initiated on a delayed basis after the onset of ischemia. Another desirable feature would be a benign or beneficial effect on hemorrhagic stroke outcomes, obviating the need for, and attendant delay associated with, the diagnosis of ischemic versus hemorrhagic strokes. Consequently, the current studies examined the impact of delayed TSC treatment in rat models of both ischemic and hemorrhagic stroke.
2. RESULTS
2.1 Focal Cerebral Ischemia
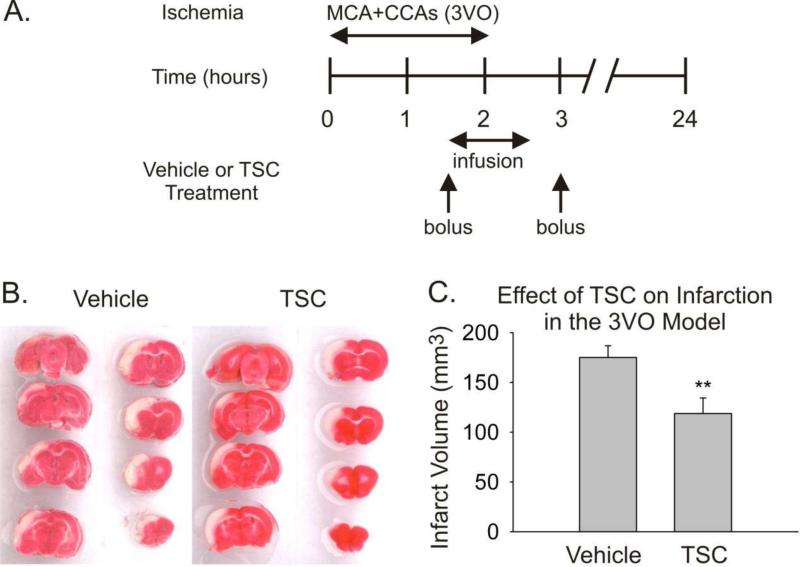
The effect of TSC was first tested in a model of ischemia-reperfusion involving 2 hours of ischemia (3VO) followed by 22 hours of reperfusion (Fig 1). Treatment with TSC (n=7) or saline (n=6) was initiated 1½ hours after the onset of ischemia. The volume of cerebral infarction was significantly reduced by 32% in the TSC-treated group (Fig 1). No differences in blood gas levels, blood pressure, or rectal temperature were observed between groups during the surgical procedure.
Fig 1.
Treatment with TSC is protective in a rat protocol of temporary (2 hours) focal cerebral ischemia when administered 1½ hours after the onset of ischemia. A. The timeline depicts the period of ischemia (3VO), and the treatment protocol for administering Vehicle or TSC. B. Serial sections stained with 2,3,5 triphenyltetrazolium chloride are shown from the brains of representative animals from the Vehicle and TSC groups. The pinkish-red staining represents healthy tissue, while the white areas are regions of infarction. C. The bar graph presents infarction volumes for the two groups measured at 24 hours post-ischemic onset. Values are means and SEMs. The difference in infarct volume between the Vehicle Group (n=6) and the TSC Group (n=7) was statistically significant (** p<0.01, Student's t-test)
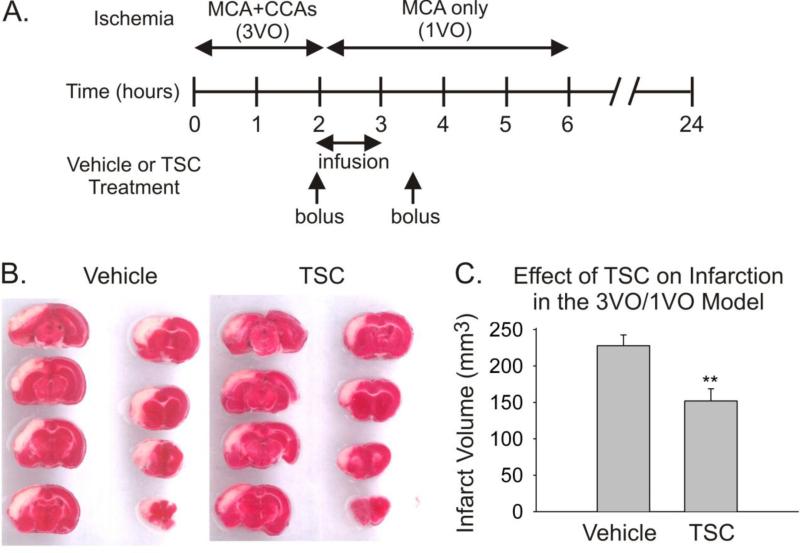
The effect of TSC was next tested using the 3VO/1VO model (Fig 2). Treatment with TSC (n=6) or saline (n=6) was initiated 2 hours after the onset of ischemia, i.e. at the cessation of 3VO, but four hours prior to the cessation of 1VO. The volume of cerebral infarction at 24 hours was significantly reduced by 33% in the TSC-treated group (Fig 2). No differences in blood gas levels, blood pressure, or rectal temperature were observed between groups during the surgical procedure.
Fig 2.
Treatment with TSC is protective in a rat protocol of temporary cerebral ischemia, involving partial reperfusion followed by delayed-complete reperfusion (3VO/1VO). A. The timeline depicts the periods of ischemia for 3VO and 1VO, and shows the treatment protocol for administering Vehicle or TSC. B. Serial sections stained with 2,3,5 triphenyltetrazolium chloride are shown from the brains of representative animals from the Vehicle and TSC groups. The pinkish-red staining represents healthy tissue, while the white areas are regions of infarction. C. The bar graph presents infarction volumes for the two groups measured at 24 hours post-ischemic onset. Values are means and SEMs. The difference in infarct volume between the Vehicle Group (n=6) and the TSC Group (n=6) was statistically significant (** p<0.01, Student's t-test)
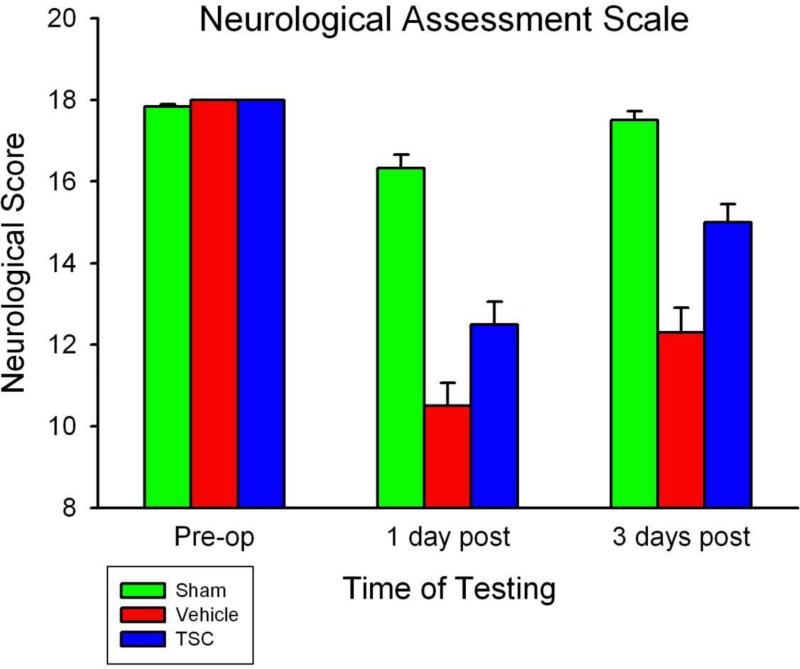
Another experiment utilizing the 3VO/1VO protocol was undertaken, in which animals were allowed to survive for three days, in order to assess neurological function. Vehicle-treated and TSC-treated animals were tested using both an 18-point neurological scale and an automated gait analysis system prior to surgery and on the first and third days post-surgery. As shown in Fig 3, TSC-treated animals demonstrated better outcomes on the neurological scale than vehicle-treated animals. Similarly, performance on the CatWalk gait analysis task was significantly better in TSC-treated animals (Fig 4). No differences in blood gas levels, blood pressure, or rectal temperature were observed between groups during the surgical procedure. Body temperature tested at 6, 12, and 24 hours after surgery also did not differ between vehicle-treated and TSC-treated groups.
Fig 3.
TSC improves post-ischemic performance on an 18-point neurological assessment scale. No significant differences were observed between groups prior to surgery. Significant impairments were observed on both post-operative days in the Vehicle and TSC Groups, as compared with: (a) the pre-operative baseline within a group, and (b) the Sham Group at the matched post-surgical time point. TSC treatment improved performance significantly at both post-operative time points, as compared with the Vehicle Group. P-values of less than 0.01 were obtained for all of these comparisons. Values shown are means and SEMs; n=6 animals per group
Fig 4.
TSC improves gait function after 3VO/1VO ischemia, as assessed by the CatWalk system. Multiple gait function parameters were impaired by the 3VO/1VO challenge, including Step Sequence Regularity Index, Speed, Cadence, Swing Speed, Stand and Stride Length in all four paws, Base of Support, Support, Phase Dispersions, and Max Intensity. Notably, post-ischemic performance by the 3VO/1VO+TSC Group was significantly better than that of the 3VO/1VO+Saline Group on several measures. Outcomes of statistical comparisons are shown below. Abbreviations: L=Left, R=Right, F=Front, H=Hind
1. Spatial parameters related to individual paws: Max Contact (%) RH p=0.042, LF p=0.017 ; Max Contact Area RF P<0.001; Max Contact Mean Intensity RF p<0.001, LF p<0.001; Print Length RH p<0.018; Print Area RF p<0.001, LF p<0.001; Max Intensity At (%) RF p=0.002 LF p<0.001; Min Intensity RF p=0.01, LH p=0.007 .
2. Relative spatial relationship between different paws: Base of Support FP p=0.003; HP p<0.001; Stride Length RF, RH, LF, LH: all p<0.001 ; Swing speed RF p<0.001 ; Duty Cycle(%) RF p<0.001, RH p=0.007, LF p<0.001, LH p=0.001.
3. Interlimb coordination: Phase Dispersions LH-RH Cstat p=0.007, LF-RF Cstat p=0.005, RF-RH C stat p<0.001 ; Support Lateral p<0.001.
4. Temporal parameters: Speed p<0.001; Cadence p<0.001; Stand(s) RF p<0.001 ; Stand Index RF, RH, LF, LH: all p<0.001
2.2 Intracerebral Hemorrhage (ICH)
A collagenase-injection model of ICH was used to assess the effect of TSC on hematoma volume, hemispheric swelling, and hemorrhage volume. A large hematoma was observed in the collagenase-injected hemispheres of both vehicle-treated and TSC-treated animals (Fig 5). Hematoma volume did not differ significantly between the vehicle-treated group and TSC-treated group, although there was a trend toward smaller hematoma size in the TSC-treated group (Fig 5). Hemispheric swelling was observed in the injected hemispheres of both groups, but the amount of swelling was significantly smaller in the TSC-treated group (Fig 5). Hemorrhage volume, as estimated by tissue hemoglobin content in the injected hemisphere, was also reduced significantly in the TSC-treated group (Fig 5).
Fig 5.
TSC improved outcomes after ICH. Photographs show examples of serial sections from a vehicle-treated and a TSC-treated animal. Hematomas appear dark-red in these unstained, thick sections. Hematoma volume was reduced slightly in the TSC Group; however, this difference did not achieve statistical significance (n=6 for each group). Treatment with TSC reduced significantly hemispheric swelling by a 46% and hemorrhage volume by 20% (* p<0.05, Student's t-test; n=6 for each group). Values are means and SEMs
3. Discussion
The supply of metabolic substrates to the brain is precisely regulated in order to serve the high metabolic demand of neurons. Key factors regulating this supply include the rate of blood flow, composition of the blood/plasma, and the ability to deliver metabolic substrates from the blood to the brain cells. The last of these factors is often overlooked because it is generally treated as a fixed component of the supply chain. However, it is important to recognize that the transfer of small molecules from blood to tissue involves a series of resistances that are mutable. A key limiting resistance in this series is diffusion through the plasma boundary layer (Huxley and Kutchai 1981; Huxley and Kutchai 1983; Yamaguchi et al. 1985). Consistent with Fick's laws of diffusion, oxygen delivery is directly proportional to both the concentration gradient between blood and tissue, and diffusivity, which is a measure of the ease with which a molecule diffuses through a medium. It is therefore possible to enhance substrate delivery to tissue not only by increasing the concentration of a metabolic substrate in the blood (e.g. blood hyperoxia), but also by enhancing the diffusion of substrates by modifying diffusivity.
An enhancement of metabolic substrate diffusion is postulated to be the primary mechanism by which TSC mitigates ischemic brain injury (Seyde et al. 1986; Manabe et al. 2010). In areas of partial tissue perfusion, such as an ischemic penumbra, neurons that are located more distant from their nearest microvessel experience more severe consequences than neurons located closer to a microvessel (Mabuchi et al. 2005). The difference in distances between the severely-affected neurons and the healthier neurons is on the order of 5 to 10 micrometers, which raises the possibility that modifying diffusion could increase the availability of metabolic substrates to at-risk cells. TSC increases the diffusion rates of oxygen and glucose through aqueous media (Laidig et al. 1998; Stennett et al. 2006). Although baseline diffusivities for oxygen and glucose in aqueous media differ, TSC increases both by a similar percentage (30-35%) (Stennett et al. 2006). Thus, TSC does not selectively affect oxygen or glucose. Rather, TSC increases hydrogen bonding of neighboring water molecules, resulting in a condition known as “structure-building”, which reduces the resistance to the diffusion of small molecules. Recent studies have demonstrated that TSC can increase oxygen levels in normal brain and in brain tumors (Okonkwo et al. 2003; Sheehan et al. 2009). Moreover, TSC was shown to reduce the depth of hypoxia in the ischemic penumbra of a 3VO model by approximately 55% (Manabe et al. 2010). This effective blunting of an ischemic challenge has been termed “metabolic reflow” to underscore the concept that TSC improves metabolic supply to tissue under conditions of ongoing partial perfusion (Manabe et al. 2011). Thus, metabolic reflow does not target vascular reperfusion or specific cellular injury cascades, but rather reduces the overall metabolic impact of an ischemic challenge. Inasmuch as the extent of ischemic injury is related to the depth of an ischemic event, improved tissue oxygenation would reasonably be expected to slow or limit the progression of most, if not all, ischemia-induced injury cascades.
The current findings demonstrate that structural and functional injury produced by focal ischemia is reduced when TSC is administered on a delayed basis after the onset of ischemia. In addition, the findings provide the first evidence of improved outcomes in a model of hemorrhagic stroke after TSC treatment. Together, these findings suggest that TSC is an attractive candidate therapy for treating stroke, irrespective of whether the stroke is ischemic or hemorrhagic in nature. However, when considering the potential translatability of any candidate therapy for stroke, it is essential to review critically all studies that have tested the therapeutic. To date, three studies have evaluated the impact of TSC in animal models of stroke: Lapchak (Lapchak 2010a), Manabe and associates (Manabe et al. 2010), and Wang and associates (current study). The study by Lapchak (Lapchak 2010a) tested TSC in a rabbit small clot embolic (RSCE) stroke model. The RSCE model entails the injection of small blood clots into the internal carotid artery and the assessment of behavioral outcomes using a clinical rating score (Lapchak 2010b; Lapchack 2012). In a dose-ranging experiment, a single bolus injection of TSC was delivered 5 minutes post-embolization via the ear vein at dosages ranging from 0.03 to 0.50 mg/kg. Behavioral outcomes improved significantly at dosages of 0.20 and 0.25 mg/kg, with testing performed at 1 day post-embolization. An optimally-effective dosage of 0.25 mg/kg was identified and used for subsequent experiments. Delayed administration of TSC, either alone or in combination with t-PA, also improved behavioral outcomes in this model. As a monotherapy, TSC was effective when administered at 1 hour, but not at 3 hours post-embolization. When administered in combination with t-PA, TSC was protective at delays of 1 and 3 hours post-embolization. Importantly, TSC was effective when administered contemporaneously with t-PA at 3 hours post-embolization. These findings suggest that TSC can be effective both prior to t-PA-induced recanalization and during the period of t-PA-induced recanalization. A key strength of this study is the use of neurological outcomes in a well-documented and clinically-relevant model for ischemic stroke.
The study by Manabe and associates (Manabe et al. 2010) tested TSC in a rat 3VO model, using both permanent and temporary occlusion protocols. A dose-ranging experiment was performed with the permanent 3VO model using 8 dosages of TSC, ranging from 0.023 to 4.58 mg/kg. TSC was administered via the femoral vein beginning 10 minutes after the onset of ischemia using a bolus-infusion-bolus protocol. The primary outcome used for this study was infarct volume measured 24 hours after the onset of ischemia. TSC significantly reduced infarct volume at dosages ranging from 0.023 to 0.229 mg/kg. Although dosages at or above 0.458 mg/kg did not significantly reduce infarct volume, the drug was well tolerated by the animals up to the highest dosage tested (4.58 mg/kg). Based on the dose-response data, an optimally-effective dosage of 0.092 mg/kg was identified, and this dosage reduced infarct volume by approximately 60%. The effect of 0.092 mg/kg TSC was also tested using a temporary 3VO model. When TSC was administered 10 minutes after the onset of a 2-hour period of ischemia, infarct volume was significantly reduced by 45%. A key weakness of the Manabe et al. study with respect to its applicability for stroke is that TSC was administered 10 minutes after the onset of ischemia in both the permanent and temporary 3VO protocols. Clearly, this is too brief of a delay to be relevant for the clinical treatment of stroke. Nonetheless, this treatment protocol is quite relevant for other applications in which brain ischemia can be anticipated, such as certain neurosurgical procedures, or for conditions in which ischemia is a chronic challenge. An important strength of this study is that the postulated mechanism of TSC action was directly examined, and TSC was shown to increase tissue oxygenation during ongoing ischemia in the penumbra of the temporary 3VO model. This demonstrated that the postulated mechanism of TSC action responsible for protection , i.e. modified supply of a metabolic substrate, is indeed affected.
The current study tested TSC using rat models of temporary focal ischemia and intracerebral hemorrhage. The first temporary focal ischemia experiment utilized a 2-hour period of 3VO with TSC administration initiated 1.5 hours after the onset of ischemia using a bolus-infusion-bolus protocol. TSC reduced significantly infarct volume by 32%. The second temporary focal ischemia experiment employed a 2-hour period of 3VO, followed by an additional 4 hour period of one vessel (middle cerebral artery) occlusion. In this 3VO/1VO model, TSC treatment was initiated 2 hours after the onset of ischemia using a bolus-infusion-bolus protocol. TSC significantly reduced infarct volume by 34% and significantly improved behavioral outcomes as assessed by both a neurological scale and an automated gait analysis system at 1 and 3 days post-ischemia. It is therefore notable that TSC treatment was effective in this experiment even when complete reflow was not established until 6 hours after the onset of ischemia. The 3VO/1VO protocol warrants specific comment in this context because it is not a common protocol for preclinical studies of transient focal cerebral ischemia. Although partial reperfusion is common after human ischemic stroke and can persist for many hours, even after the administration of t-PA (Ribo et al. 2006), this type of ischemic challenge is not typically evaluated in preclinical stroke studies. The purpose of the 3VO/1VO model was to mimic partial reperfusion and then to test the effects of TSC against this type of prolonged ischemic challenge. The 6-hour period of ischemia in the 3VO/1VO protocol is a relatively long challenge, as compared with most standard animal models of ischemia-reperfusion. In fact, a comparison of the infarct sizes produced in the Vehicle groups of the 3VO-only and 3VO/1VO protocols (Figures 1 and 2) demonstrates that the added period of partial ischemia in the 3VO/1VO protocol produces more extensive damage to the supply field of the middle cerebral artery than does the 3VO-only protocol. Importantly, despite the added ischemic challenge of the 3VO/1VO protocol, TSC was capable of reducing both structural and functional injury.
The final experiment in the current study examined the effects of TSC in a model of ICH in which the drug was administered at a dosage of 0.092 mg/kg beginning 3 hours after collagenase injection using a bolus-infusion-bolus protocol. Hemispheric swelling and hemorrhage volume were reduced significantly by TSC treatment; hematoma volume was reduced slightly, but not significantly. It is unclear whether the dosage of TSC utilized in this experiment produces a maximal protective effect. Selection of the TSC dosage used for this experiment was based on optimal outcomes obtained in a previous dose-response study of focal ischemia (Manabe et al. 2010). The rationale for this approach was to determine whether an effective dosage for mitigating ischemic injury might also impact post-hemorrhagic outcomes. If post-ICH outcomes were aggravated under these conditions, then the use of TSC for the emergent treatment of a stroke, i.e. prior to diagnosis of ischemic versus hemorrhagic stroke, would be contraindicated. However, the TSC dosage utilized actually improved post-ICH outcomes, suggesting that TSC could be beneficial for early treatment of stroke, regardless of the type of stroke. Nonetheless, it will be important for future studies to define the dose-response relation for TSC and hemorrhagic injury. Moreover, it will be valuable to test multiple models of ICH, as each experimental model possesses its own strengths and weaknesses for mimicking ICH. Another important goal will be to test the effects of TSC on long-term neurological outcomes after ICH. Extending the survival of animals to weeks to months would provide additional insights into the permanence of the protective effects of TSC for ICH. Despite the need for additional future studies, it is important to note the current findings provide the first evidence that TSC does not aggravate injury in a model of hemorrhagic stroke, but actually improves certain outcomes.
Taken together, the findings from these three studies portray TSC as a promising therapeutic candidate for treating acute ischemic stroke. TSC improved behavioral and structural outcomes using multiple, challenging animal models of stroke. The drug was evaluated in two different species and in two different laboratories. In one of the studies, TSC was shown to be effective when administered in combination with t-PA, the standard therapeutic for human ischemic stroke. The postulated mechanism of action underpinning ischemic protection, i.e. enhanced metabolic supply, was demonstrated. The dose-efficacy findings were similar for the RSCE model and the 3VO model. While these are promising results, a few key issues remain to be addressed. Perhaps the most important of these issues concerns the persistence of neurological protection provided by TSC for the treatment for acute ischemic stroke. The two studies examining behavioral outcomes evaluated very brief follow-up periods. It will be important for future studies to extend these observations to a period of weeks to months. The therapeutic window of opportunity also remains to be defined. Successful protection has been shown after delays to treatment of 2 to 3 hours. It is conceivable that this window is wider, and this could be evaluated by testing longer delays to treatment. Additionally, the validity of TSC as a therapeutic candidate would be greatly substantiated by successful testing in a primate model of acute ischemic stroke.
Novel therapies can also raise the specter of novel undesirable side-effects. In the case of TSC, a key concern was the possibility of a magnified oxidative burst and free radical damage during the reflow phase following ischemia. It is possible that the enhancement of tissue oxygenation by TSC during ischemia (Manabe et al. 2010) could amplify the deleterious effects of reperfusion injury. However, this is not the case. Using direct measurements of tissue oxygenation in the ischemic penumbra of the rat 3VO model, hyperoxia that occurs upon reperfusion was actually reduced by TSC (Manabe et al. 2010). The mechanistic basis for the attenuation of reperfusion hyperoxia is unknown, but may simply reflect the fact that the tissue is healthier in the TSC-treated animals.
Another issue associated with the development of any drug-based therapy is the possibility that multiple protective mechanisms contribute to the improved outcomes. This is a possibility that is difficult to rule out entirely. Even though the postulated mechanism of action (i.e. increased tissue oxygenation) has been directly demonstrated (Manabe et al. 2010), carotenoid molecules possess anti-oxidant activity, which could conceivably contribute to the protective actions of TSC. However, although TSC can serve as an anti-oxidant, this effect occurs at dosages much higher than those needed to produce an effect on diffusivity (Stennett et al. 2007). In addition, the dose-response curve for the protective effect of TSC on ischemic damage is U-shaped (Manabe et al. 2010). Thus, the capability of TSC to limit cerebral infarction actually diminishes as the effective dosage range is achieved for exerting anti-oxidative effects in the tissue. This does not eliminate other possible mechanisms of action, and it will be important for future studies to consider whether influences in addition to increased tissue oxygenation contribute to the protective effects of TSC. It should also be noted that, although there is a U-shaped dose-response curve for the protective actions of TSC, higher dosages do not appear to have an attendant increase in risk or side effects (Manabe et al. 2010), as is the case for many therapeutics, including t-PA.
Based on these encouraging results from preclinical models, it is reasonable to consider the feasibility of developing metabolic reflow into a clinical therapy for stroke. For instance, if clot-lysis therapy is available, then why would metabolic reflow therapy be of additional value? Several lines of reasoning support the potential clinical importance of metabolic reflow. First, clot-lysis therapy can take time to be effective. In fact, the rate of clot-lysis is a key factor dictating the therapeutic window for t-PA treatment. In this scenario, metabolic reflow could bridge the gap between the onset of t-PA treatment and clot dissolution. Second, clot-lysis therapy often results in only partial or minimal recanalization. As shown here, TSC treatment can limit tissue damage and improve behavioral outcomes even when vascular perfusion is not fully re-established in a timely manner. In this setting, TSC might be able to extend the therapeutic window for clot-lysis therapy. Third, it is plausible that metabolic reflow therapy can be safely implemented prior to diagnosis of the type of stroke that is under way. Unlike clot-lysis therapy, which increases the risk of re-bleeding and hemorrhagic transformation, metabolic reflow therapy does not appear to impart additional risk. In fact, our current findings indicate that it might actually be beneficial for both ischemic and hemorrhagic strokes. Fourth, blood flow may not be fully re-established in an ischemic penumbra, even after complete reflow of a large occluded vessel is established. In this scenario, a prolonged partial ischemia may persist in the downstream penumbra even after the dissolution of a clot upstream. Such a condition could also benefit from metabolic reflow therapy.
A final consideration is whether TSC can be safely used in the human population. TSC has already been evaluated in two Phase I/II clinical trials. The first trial evaluated safety and exercise performance in patients with peripheral artery disease (PAD) (Mohler et al. 2011). Patients were randomized to placebo or TSC groups, and 8 dosing levels ranging from 0.25 to 2.0 mg/kg were administered intravenously each day for a period of 5 days. No dose-response effect for TSC was observed for serious adverse events for any organ system. The interpretation of the overall safety outcomes in the trial was that TSC appears to be a safe treatment and the data support the further development of extended dosing schemes (Mohler et al. 2011). The development of extended dosing will ultimately be important for conditions of chronic ischemia, such as PAD. While the use of a prolonged treatment regimen may be less relevant to acute ischemic stroke therapy, it might prove of value for other applications such as repetitive transient ischemic attacks. Notably, TSC showed promise in improving walking performance in the PAD patients, supporting the value of undertaking a more comprehensive Phase II trial. The second clinical trial involving TSC (CLINICAL TRIAL IDENTIFIER: NCT01465347) is ongoing and is evaluating the safety and efficacy of TSC as an adjunct for radiotherapy (RT) in treating glioblastoma multiforme (GBM). The rationale for this trial is that glioma cells in hypoxic zones of the tumors are quite radioresistant, rendering the GBM refractory to RT. Elevating intra-tumor oxygen could reestablish radiosensitivity in glioma cells, thus making the tumors less resistant to RT. Sheehan and associates have provided evidence that TSC can increase intratumor tissue oxygenation and enhance the effects of RT in a rat syngeneic model of GBM (Sheehan et al. 2008; Sheehan et al. 2009; Sheehan et al. 2010). The Phase I/II clinical trial testing this strategy is currently in progress with an initial enrollment of 60 patients at 18 sites. This trial also involves multiple intravenous injections of TSC, timed to correspond with a fractionated RT protocol. No primary outcomes are as yet available from the trial. However, the drug administration stage of the trial has been completed, and the initial safety outcomes indicated that no severe adverse effects observed in the patients were attributable to an effect of TSC. Together, the findings of these two clinical trials portray TSC as a safe drug for use in humans, which further strengthens the potential translatability of the drug for use in stroke.
In summary, the development of optimal thrombolytics, neuroprotective compounds, and devices remains a key goal for translational stroke research. Metabolic reflow using TSC could be highly complementary to these approaches. This strategy might extend the therapeutic windows for thrombolysis and neuroprotection by improving the general health of ischemic tissue during an ongoing ischemic event. In addition, TSC may be suitable for early intervention irrespective of whether a stroke is ischemic or hemorrhagic in nature. This novel therapy might thus serve as a bridge to keep ischemic tissue healthy long enough for other primary therapies to be effective.
4. Experimental Procedure
4.1 Focal Cerebral Ischemia
All animal procedures were approved by the University of Virginia's Animal Care and Use Committee. In all studies, animals were randomized to groups and the investigators performing outcome analyses were blinded to the group identity of the animals. Focal cerebral ischemia is produced by three-vessel occlusion (3VO) surgery blocking one middle cerebral artery (MCA) and both common carotid arteries (CCAs) in adult male Sprague-Dawley rats. This technique has been described previously in detail (Hiramatsu et al. 1993). Two variants of this technique were used. The first protocol examined the effects of TSC administered beginning 1½ hours after the onset of a 2-hour period of 3VO. This protocol was used to mimic treatment given prior to complete reflow during an ischemic stroke. Animals were treated with vehicle (saline) (n=6) or TSC (n=7). The second 3VO protocol entailed 3VO for 2 hours followed by continued clipping of the MCA for an additional 4 hours. This protocol, termed 3VO/1VO, was used to mimic partial reflow followed by delayed complete reflow. Animals were treated with vehicle (saline) (n=6) or TSC (n=6). Both of these reflow scenarios mimic events observed in the human stroke population (Ribo et al. 2006).
TSC (Diffusion Pharmaceuticals, Charlottesville, VA) or vehicle (saline) was administered via the femoral vein beginning at 1½ hours after the onset of ischemia in the 3VO experiment and at 2 hours after the onset of ischemia in the 3VO/1VO experiment. Vehicle or TSC was administered using a “bolus-infusion-bolus” protocol (Okonkwo et al. 2003; Manabe et al. 2010). The total dosage of TSC administered was 0.092 mg/kg, which was selected based on a dose-response assessment of the protective actions of TSC (Manabe et al. 2010). Blood gas levels, blood pressure, and rectal temperature were monitored during surgery.
Animals were euthanized 24 hours after the onset of ischemia and coronal sections of the brain were stained with 2,3,5 triphenyltetrazolium chloride (TTC) to visualize areas of cerebral infarction. Infarct volume, corrected for swelling, was calculated for each animal. Statistical comparisons between vehicle-treated and TSC-treated groups utilized Student's t-test, and a p-value of less than 0.05 was accepted as statistically significant.
An additional experiment utilizing the 3VO/1VO protocol assessed neurological outcomes in vehicle-treated (n=6), TSC-treated (n=6), and Sham-operated (n=6) groups. Animals were tested before surgery and on days 1 and 3 after surgery. An 18-point neurological assessment scale, which is a standard metric for assessing deficits after MCAO, was used (Garcia et al. 1995). In addition, an automated gait analysis system (CatWalk, Noldus), which measures numerous parameters of gait, was utilized. Previous studies using CatWalk (Wang et al. 2008; Encarnacion et al. 2011) have identified multiple MCAO-sensitive gait parameters.
4.2 Intracerebral Hemorrhage
A rat model of ICH involving the injection of collagenase into the brain (Rosenberg et al. 1990; Elger et al. 1994) was used to examine the effect of TSC on hemorrhagic injury. Adult male Sprague-Dawley rats were anesthetized with Isoflurane and ventilated with Isoflurane using an FiO2 of 50%. Blood pressure was monitored and blood gases were sampled via the tail artery. Five microliters of collagenase (0.05 U bacteria collagenase; type IV, Sigma Chemical Co.) were injected stereotactically into the caudate putamen over 5 minutes. Three hours after collagenase injection, TSC (0.092 mg/kg) or saline was administered via the femoral vein. Blood pressure was controlled by adjusting Isoflurane levels to maintain the mean arterial pressure at 100-110 mm Hg. Body temperature was monitored with a rectal thermometer and maintained at 37°C using a heating pad.
Three outcomes were assessed for the ICH model: hematoma volume, brain edema, and brain hemoglobin levels. Hematoma volume and brain edema were measured in thick (2mm) brain sections of TSC-treated (n=6) and vehicle-treated animals (n=6) that were euthanized 48 hours after collagenase infusion. Brain hemoglobin levels were measured in a separate group of animals utilizing a spectrophotometric assay in order to estimate the amount of intracerebral bleeding (n=6 for each group) (Choudhri et al. 1997). Animals were euthanized 48 hours after collagenase infusion and whole brain was assayed.
Trans-sodium crocetinate (TSC) improves the supply of oxygen to ischemic brain tissue.
TSC treatment improves outcomes in animal models of ischemic and hemorrhagic stroke.
This strategy, termed Metabolic Reflow, prolongs tissue viability during ongoing ischemia.
Metabolic Reflow could extend the therapeutic windows for thrombolysis and neuroprotection.
Acknowledgements
Matthew Anzivino contributed importantly to the editing of this work. The drug trans-sodium crocetinate was provided by Diffusion Pharmaceuticals.
Sources of Funding: This work was supported by R01NS057168 and T32GM08238.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements:
Dr. Yi Wang does not have a conflict of interest with the National Institutes of Health, which funded the study or with Diffusion Pharmaceuticals, which provided the drug used for the study.
Dr. Ryo Yoshimura does not have a conflict of interest with the National Institutes of Health, which funded the study or with Diffusion Pharmaceuticals, which provided the drug used for the study.
Dr. Hiroaki Manabe does not have a conflict of interest with the National Institutes of Health, which funded the study or with Diffusion Pharmaceuticals, which provided the drug used for the study.
Ms. Catherine Schretter does not have a conflict of interest with the National Institutes of Health, which funded the study or with Diffusion Pharmaceuticals, which provided the drug used for the study.
Mr. Ryon Clarke does not have a conflict of interest with the National Institutes of Health, which funded the study or with Diffusion Pharmaceuticals, which provided the drug used for the study.
Dr. Yu Cai does not have a conflict of interest with the National Institutes of Health, which funded the study or with Diffusion Pharmaceuticals, which provided the drug used for the study.
Dr. Mark Fitzgerald does not have a conflict of interest with the National Institutes of Health, which funded the study or with Diffusion Pharmaceuticals, which provided the drug used for the study.
Dr. Kevin S. Lee does not have a conflict of interest with the National Institutes of Health, which funded the study or with Diffusion Pharmaceuticals, which provided the drug used for the study. Dr. Lee has served as a consultant for Diffusion Pharmaceuticals, but he was unpaid for that service and holds no financial stake in the company. Dr. Lee is a member of the Safety Monitoring Committee for Clinical Trial NCT01465347, entitled, “Safety and efficacy study of trans-sodium crocetinate (TSC) with concomitant radiation therapy and Temozolomide in newly diagnosed glioblastoma (GBM).” This service by Dr. Lee is unpaid and he has received no financial interest in the company sponsoring the trial (Diffusion Pharmaceuticals) as a result of this service.
Authors’ Contributions to this project:
Dr. Yi Wang contributed to the design of the ICH experiments. He performed the collagenase injection surgeries and the outcomes assessments for the ICH experiments. He contributed to the writing of the manuscript.
Dr. Ryo Yoshimura performed surgeries for the focal ischemia experiments.
Dr. Hiroaki Manabe participated in the design of the focal ischemia studies and performed surgeries in the first series of ischemia experiments.
Ms. Catherine Schretter assisted in the performance of outcome measures in the focal ischemia experiments.
Mr. Ryon Clarke assisted in assessing outcome measures in the ICH experiments and contributed to manuscript preparation..
Dr. Yu Cai assisted in the performance of the focal ischemia surgeries..
Dr. Mark Fitzgerald participated in the design and outcome assessment of the focal ischemia experiments..
Dr. Kevin S. Lee designed and oversaw all of the studies. He participated in the writing of the manuscript.
Animal Care and Usage: Rats were used for the experiments for the studies described in this manuscript. All institutional and national guidelines for the care and use of laboratory animals were followed. The studies were approved by the University of Virginia Animal Care and Use Committee.
Human Subjects: No human subjects were utilized as part of this study.
REFERENCES
- Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr., Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- Edlow JA, Smith EE, Stead LG, Gronseth G, Meese SR, et al. Clinical Policy: Use of intravenous tPA for the management of acute ischemic stroke in the emergency department. Ann Emerg Med. 2013;61:225–243. doi: 10.1016/j.annemergmed.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Elger B, Seega J, Brendel R. Magnetic resonance imaging study on the effect of levemopamil on the size of intracerebral hemorrhage in rats. Stroke. 1994;25:1836–1841. doi: 10.1161/01.str.25.9.1836. [DOI] [PubMed] [Google Scholar]
- Encarnacion A, Horie N, Keren-Gill H, Bliss TM, Steinberg GK, et al. Long-term behavioral assessment of function in an experimental model for ischemic stroke. J Neurosci Methods. 2011;196:247–257. doi: 10.1016/j.jneumeth.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K, Kassell NF, Goto Y, Soleau S, Lee KS. A reproducible model of reversible, focal, neocortical ischemia in Sprague-Dawley rat. Acta Neurochir (Wien) 1993;120:66–71. doi: 10.1007/BF02001472. [DOI] [PubMed] [Google Scholar]
- Holloway GM, Gainer JL. The carotenoid crocetin enhances pulmonary oxygenation. J Appl Physiol (1985) 1988;65:683–686. doi: 10.1152/jappl.1988.65.2.683. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Kutchai H. The effect of the red cell membrane and a diffusion boundary layer on the rate of oxygen uptake by human erythrocytes. J Physiol. 1981;316:75–83. doi: 10.1113/jphysiol.1981.sp013773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley VH, Kutchai H. Effect of diffusion boundary layers on the initial uptake of O2 by red cells. Theory versus experiment. Microvasc Res. 1983;26:89–107. doi: 10.1016/0026-2862(83)90058-4. [DOI] [PubMed] [Google Scholar]
- Laidig KE, Gainer JL, Daggett V. Altering diffusivity in biological solutions through modification of solution structure and dynamics. Journal of the American Chemical Society. 1998;120:9394–9395. [Google Scholar]
- Lapchack PA. A clinically relevant rabbit embolic stroke model for acute ischemic stroke therapy development: mechanisms and targets. In: Lapchack PA, Zhang JH, editors. Translational Stroke Research: From Target Selection to Clinical Trials. Springer; 2012. pp. 541–584. [Google Scholar]
- Lapchak PA. Efficacy and safety profile of the carotenoid trans sodium crocetinate administered to rabbits following multiple infarct ischemic strokes: a combination therapy study with tissue plasminogen activator. Brain Res. 2010a;1309:136–145. doi: 10.1016/j.brainres.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Translational stroke research using a rabbit embolic stroke model: a correlative analysis hypothesis for novel therapy development. Transl Stroke Res. 2010b;1:96–107. doi: 10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Lucero J, Feng A, Koziol JA, del Zoppo GJ. Focal cerebral ischemia preferentially affects neurons distant from their neighboring microvessels. J Cereb Blood Flow Metab. 2005;25:257–266. doi: 10.1038/sj.jcbfm.9600027. [DOI] [PubMed] [Google Scholar]
- Manabe H, Okonkwo DO, Gainer JL, Clarke RH, Lee KS. Protection against focal ischemic injury to the brain by trans-sodium crocetinate. Laboratory investigation. J Neurosurg. 2010;113:802–809. doi: 10.3171/2009.10.JNS09562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe H, Wang Y, Yoshimura R, Cai Y, Fitzgerald M, et al. Metabolic reflow as a therapy for ischemic brain injury. Acta Neurochir Suppl. 2011;110:87–91. doi: 10.1007/978-3-7091-0356-2_16. [DOI] [PubMed] [Google Scholar]
- Mohler ER, 3rd, Gainer JL, Whitten K, Eraso LH, Thanaporn PK, et al. Evaluation of trans sodium crocetinate on safety and exercise performance in patients with peripheral artery disease and intermittent claudication. Vasc Med. 2011;16:346–353. doi: 10.1177/1358863X11422742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo DO, Wagner J, Melon DE, Alden T, Stone JR, et al. Trans-sodium crocetinate increases oxygen delivery to brain parenchyma in rats on oxygen supplementation. Neurosci Lett. 2003;352:97–100. doi: 10.1016/j.neulet.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Ribo M, Alvarez-Sabin J, Montaner J, Romero F, Delgado P, et al. Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue reperfusion techniques. Stroke. 2006;37:1000–1004. doi: 10.1161/01.STR.0000206443.96112.d9. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Seyde WC, McKernan DJ, Laudeman T, Gainer JL, Longnecker DE. Carotenoid compound crocetin improves cerebral oxygenation in hemorrhaged rats. J Cereb Blood Flow Metab. 1986;6:703–707. doi: 10.1038/jcbfm.1986.126. [DOI] [PubMed] [Google Scholar]
- Sheehan J, Cifarelli CP, Dassoulas K, Olson C, Rainey J, et al. Trans-sodium crocetinate enhancing survival and glioma response on magnetic resonance imaging to radiation and temozolomide. J Neurosurg. 2010;113:234–239. doi: 10.3171/2009.11.JNS091314. [DOI] [PubMed] [Google Scholar]
- Sheehan J, Ionescu A, Pouratian N, Hamilton DK, Schlesinger D, et al. Use of trans sodium crocetinate for sensitizing glioblastoma multiforme to radiation: laboratory investigation. J Neurosurg. 2008;108:972–978. doi: 10.3171/JNS/2008/108/5/0972. [DOI] [PubMed] [Google Scholar]
- Sheehan J, Sherman J, Cifarelli C, Jagannathan J, Dassoulas K, et al. Effect of trans sodium crocetinate on brain tumor oxygenation. Laboratory investigation. J Neurosurg. 2009;111:226–229. doi: 10.3171/2009.3.JNS081339. [DOI] [PubMed] [Google Scholar]
- Singhal AB. A review of oxygen therapy in ischemic stroke. Neurol Res. 2007;29:173–183. doi: 10.1179/016164107X181815. [DOI] [PubMed] [Google Scholar]
- Stennett AK, Dempsey GL, Gainer JL. Trans-Sodium crocetinate and diffusion enhancement. Journal of Physical Chemistry B. 2006;110:18078–18080. doi: 10.1021/jp064308+. [DOI] [PubMed] [Google Scholar]
- Stennett AK, Murray RJ, Roy JW, Gainer JL. Trans-sodium crocetinate and hemorrhagic shock. Shock. 2007;28:339–344. doi: 10.1097/shk.0b013e3180487b2d. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bontempi B, Hong SM, Mehta K, Weinstein PR, et al. A comprehensive analysis of gait impairment after experimental stroke and the therapeutic effect of environmental enrichment in rats. J Cereb Blood Flow Metab. 2008;28:1936–1950. doi: 10.1038/jcbfm.2008.82. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nguyen-Phu D, Scheid P, Piiper J. Kinetics of O2 uptake and release by human erythrocytes studied by a stopped-flow technique. J Appl Physiol (1985) 1985;58:1215–1224. doi: 10.1152/jappl.1985.58.4.1215. [DOI] [PubMed] [Google Scholar]