Abstract
Previous research provides little information about variables that determine which elements of contextual cues gain associative control over behavior in the conditioned place preference (CPP) procedure. These studies examined the effect of external visual-spatial cues on CPP when tactile cues served as the conditioned stimuli. DBA/2J mice were trained in the dark (Experiment 1) or light (Experiment 2) using unbiased procedures in which the spatial location of an ethanol-paired tactile cue during training was relevant (two-compartment procedure) or irrelevant (one-compartment procedure). All groups developed CPP, but it was weakest after one-compartment training in the light. In Experiment 3, tactile cues were tested either in the same locations used during training or reversed after two-compartment training in either the dark or light. CPP was unaffected by cue location reversal in the dark, but it was reduced when cue locations changed in the light. Mice in Experiment 4 also received two-compartment training in either the light or dark, but the spatial locations of the drug- and vehicle-paired cues alternated over trials, making external visual-spatial cues irrelevant. In this case, lighting had no effect on CPP. These studies show that cue location does not affect CPP when tactile cue training occurs in the dark. Moreover, they suggest that external visual-spatial cues might enhance CPP when those cues are relevant, but not when an alternating two-compartment procedure is used. The cue reversal effect suggests that relevant external visual-spatial cues acquire associative strength when combined with tactile cues in a two-compartment procedure in the light. Overall, these studies improve our understanding of how external visual-spatial cues interact with tactile cues during drug-induced conditioning, which could have important implications for studies that use CPP to study the neurobiological bases of drug seeking and drug reward.
Keywords: ethanol, place conditioning, stimulus modality, tactile, visual, spatial, reward, light, locomotor activity, inbred mice (DBA/2J)
1. Introduction
The conditioned place preference (CPP) procedure typically involves pairing a drug with distinctive contextual cues (Cunningham et al., 2006a, 2011; Tzschentke, 1998, 2007). After exposure to this Pavlovian conditioning procedure, animals are given a spatial choice between those cues and a different set of cues previously paired with a control treatment (e.g., vehicle injection). The animal’s approach toward or avoidance of the drug-paired cues is widely used to draw inferences about the drug’s rewarding or aversive effects (Tzschentke, 1998, 2007) as well as the learning and memory processes that are thought to underlie this form of conditioned behavior (e.g., Bernardi et al., 2006; Young et al., 2014).
Despite the procedure’s popularity, little is known about the stimuli that control CPP. Based on the practices of most investigators, the consensus appears to be that using multiple stimuli from several different modalities (e.g., visual, tactile, olfactory) is better than using a single cue from one modality, a sentiment echoed in early reviews of the literature. Indeed, Carr et al. (1989) suggested that using contextual cues that varied, “on more than one stimulus dimension may provide a more sensitive measure” (p. 268). However, this suggestion was based primarily on anecdotal reports of difficulty in establishing CPP with visual cues alone (Barr et al., 1985; Mucha et al., 1982) rather than direct experimental comparisons between single and multi-modality contextual cues. Several studies showed that reliable CPP could be obtained with several different drugs using just visual cues (Asin & Wirtshafter, 1985; Cunningham et al., 2006b; Glimcher et al., 1984; White et al., 2005) or just tactile cues conditioned either in the light (Cunningham et al., 2006b) or in the dark (Cunningham et al., 1991, 1992; Roma & Riley, 2005; Vezina & Stewart, 1987a, 1987b), but the ability of visual cues to control CPP was later found to depend critically on use of a two-compartment training procedure. CPP was not seen when visual cues were used in a one-compartment training procedure, an outcome attributed to the lack of unique spatial locations for those cues during training (Cunningham et al., 2006b).
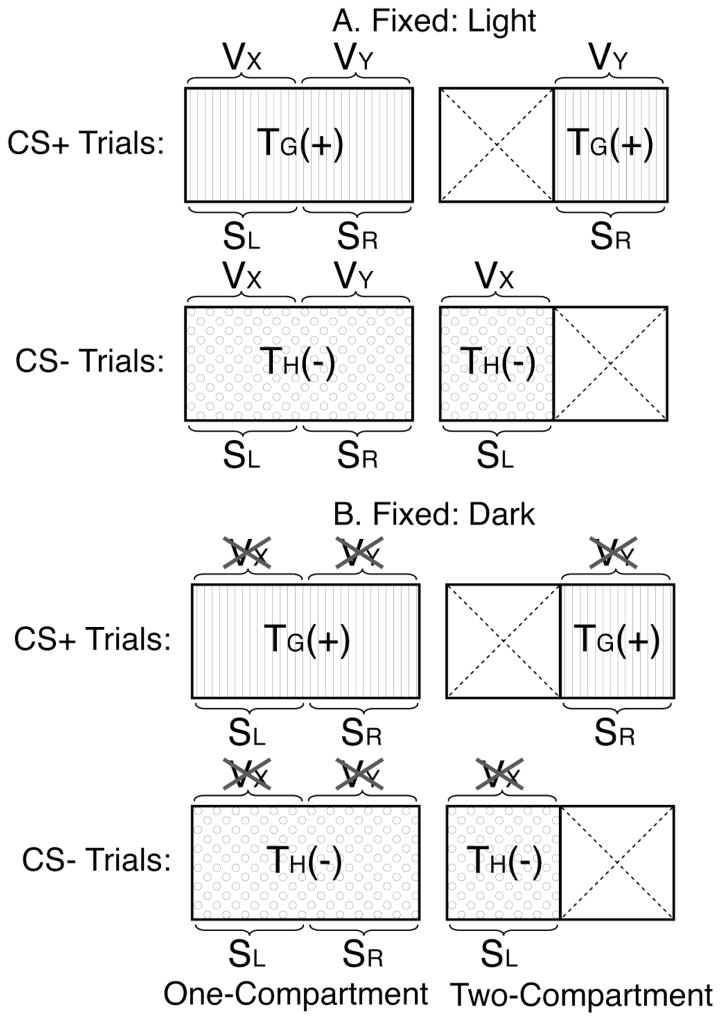
The similarities and differences between one- and two-compartment training procedures are illustrated in Figure 1, which depicts CPP training in each procedure using distinctive tactile cues (labeled TG or TH) as the primary conditioned stimuli (CSs) in an illuminated (Fig. 1A) or darkened (Fig. 1B) apparatus. As can be seen, one tactile CS is consistently paired with drug [TG (+)] while the other is consistently paired with vehicle [TH (−)] in both procedures under both lighting conditions (i.e., a Pavlovian discrimination training procedure). Although both procedures are expected to produce conditioned preference for the drug-paired tactile cue (Cunningham et al., 2006b), further analysis suggests that other cues present within each procedure might acquire associative strength and thereby affect the ability of the tactile cues, per se, to influence behavior. For example, drug and vehicle are experienced in consistent spatial locations (depicted simply as the left (SL) or right (SR) side) only in the two-compartment procedure, which confines the animal to one side of the apparatus on conditioning trials. In contrast, drug and vehicle are potentially experienced in either location in the one-compartment procedure because the animal has access to the entire apparatus on all trials. Thus, spatial location cues are more consistently predictive of drug exposure in the two- than in the one-compartment procedure. Though not explicitly manipulated by the experimenter, visual cues are also present in an illuminated CPP apparatus (Fig. 1A), but not in a darkened apparatus (indicated by X’s over the visual cues in Fig. 1B). These visual cues could include those provided by the tactile CSs themselves as well as any other cues that might be viewed at a distance through the walls or ceiling of the apparatus. For simplicity, these visual cues are depicted here as compound stimuli comprised of cues located primarily on the left (VX) or right (VY) sides of the apparatus. When present in an illuminated apparatus, these visual cues are more consistently predictive of drug exposure in the two- than in the one-compartment procedure.
Figure 1.
This figure shows the conditioned stimuli assumed to be available in a one- (left column) or two-compartment (right column) CPP procedure. Rows 1 (CS+ trials) and 2 (CS− trials) show cues available when training occurs in the light (Panel A); rows 3 and 4 show cues available when training occurs in the dark (Panel B). TG and TH represent that tactile-grid and tactile-hole floors, respectively. VX and VY represent external visual cues that are unique to each side of the apparatus while SL and SR represent spatial location cues on the left and right sides, respectively. The large dashed X’s in the two-compartment diagrams depict the compartment that the mouse is unable to enter on conditioning trials. The X’s shown over VX and VY in Panel B indicate that visual cues are not available in the dark. The (+) and (−) indicate pairing with ethanol or saline injection, respectively.
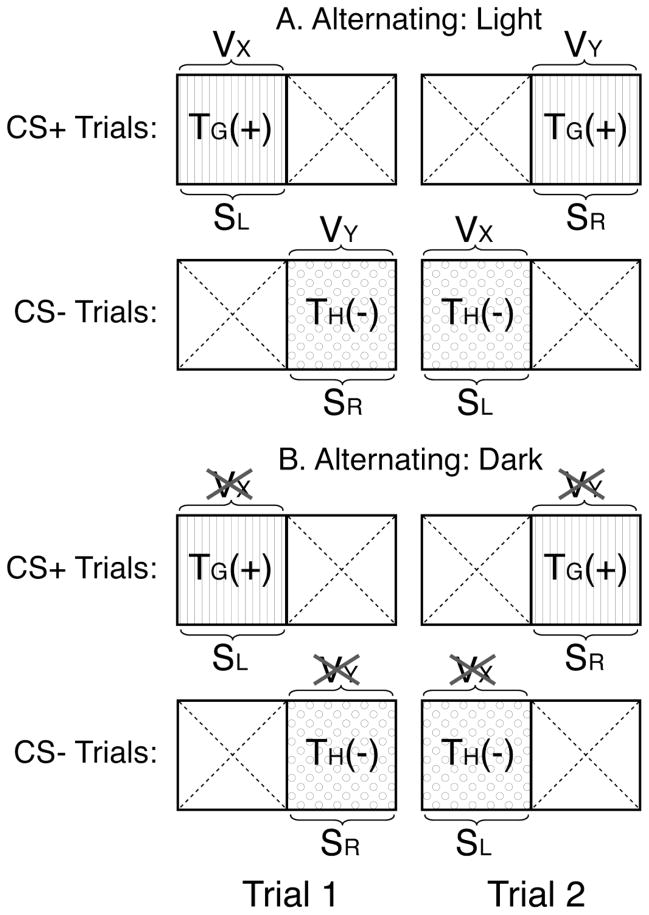
The roles played by such spatial and visual cues are difficult to determine in a conventional two-compartment apparatus. However, by using an apparatus that allowed direct comparison of the one- and two-compartment procedures, the present studies tested effects of adding visual and spatial cues during the conditioning of tactile cues in the procedures depicted in Figure 1. Our main interest was in understanding how the addition of spatial or visual-spatial cues affected stimulus control of CPP in each configuration. Exps. 1 and 2 directly compared one- vs. two-compartment conditioning of tactile cues in the absence of visual cues (Fig. 1B) or in the presence of visual cues (Fig. 1A), respectively. Exp. 3 examined the effect of switching the spatial locations of the tactile cues at the time of testing in groups trained either in the light or in the dark using a two-compartment procedure. Finally, Exp. 4 tested the effect of alternating the spatial locations of the tactile cues across conditioning trials in a two-compartment procedure conducted either in the dark or in the light (Figure 2).
Figure 2.
This figure shows the conditioned stimuli assumed to be available in a two-compartment procedure in which the spatial locations of the tactile cues are alternated over trials (Exp. 4). The labels are identical to those defined in the caption for Fig. 1.
Stimulus-element theories like the Rescorla-Wagner model (Rescorla & Wagner, 1972) offer a convenient conceptual framework for making predictions about interactions among the elements of multi-modality contextual stimuli. In general, the model states that the change in associative strength of each potential conditioned stimulus (CS) on each trial depends, in part, on the current combined associative strengths of all stimuli present on the trial. In other words, concurrent “background” cues like the visual and spatial stimuli shown in Fig. 1A can influence the development of associative strength to tactile CSs by acquiring and competing for associative strength. This competition is not usually apparent in a two-compartment procedure because the various stimuli unique to each compartment and drug condition are always presented together. However, this competition might interfere with control of CPP by tactile cues in a one-compartment procedure because visual and spatial cues on both sides of the apparatus are potentially paired with drug on half of the conditioning trials.
Since we assume that external visual-spatial cues have relatively low salience in the dark, the model predicts that there should be minimal competition with tactile cues for associative strength and therefore little or no difference in tactile-cue CPP between mice conditioned in one- or two-compartment procedures (Exp. 1). In contrast, the model predicts that two-compartment training in the light might yield stronger tactile-cue CPP than one-compartment training in the light (Exp. 2) because visual-spatial cues would acquire associative strengths that enhance overall associative strength of drug-paired cues in the two-compartment procedure or that impair associative control of behavior by drug-paired tactile cues in the one-compartment procedure.
For Exp. 3, the model predicts that reversing the spatial locations of the tactile cues should have a greater detrimental effect on CPP expression in the group trained in the light because visual-spatial cues in the originally rewarded location would now compete with the repositioned tactile cue for associative control of approach behavior. Finally, for Exp. 4, the model predicts that the addition of extraneous visual cues in an illuminated apparatus should make the two-compartment procedure more like a one-compartment procedure when spatial cues are made irrelevant by alternating the locations of the tactile cues across trials (see Figure 2). Thus, as in the one-compartment case, training in the light might interfere with tactile cue CPP in the two-compartment alternating-side procedure early in training because partially reinforced cues on the opposite side of the apparatus could elicit excitatory conditioned responses that compete with those evoked by the tactile CS+. In general, the model predicts that CPP should be altered when tactile cues are combined with visual cues or with both visual and spatial cues, but not when they are combined only with spatial cues.
2. Methods
2.1 Subjects
Male DBA/2J mice were shipped from the Jackson Laboratory (Sacramento, CA) at 6–7 weeks of age about 2 weeks before training began (Exp. 1 and 2: n = 48/experiment; Exp. 3: n = 96; Exp 4: n = 46). This strain was selected on the basis of previous research showing them to be sensitive to ethanol reward in the CPP procedure (Cunningham, 2014; Cunningham et al., 1992). A previous multi-strain study also showed this strain to perform well (in the “normal” range) in visual detection and pattern discrimination learning tasks (Wong & Brown, 2006), confirming their sensitivity to visual stimuli. Mice were housed in polycarbonate cages (3–4 mice/cage) in a ventilated Thoren rack in a room maintained at an ambient temperature of 21±1 °C on a 12-h light-dark cycle (lights on at 0700 h); experiments were carried out during the light portion of the cycle. Food and water were available in the home cage. The Oregon Health & Science University IACUC approved the protocol, which followed the National Institutes of Health (NIH) “Principles of Laboratory Animal Care.”
2.2 Apparatus
Rectangular conditioning boxes (30 × 15 × 15 cm) were constructed from aluminum end panels attached to clear acrylic walls and lid (Cunningham et al., 2006a, 2006b). The animal’s general activity and position (left vs. right side) were detected by six infrared emitter/detector pairs mounted 2.2 cm above the floor at 5 cm intervals. Time spent on each side of the box and activity (photobeam breaks) were recorded automatically by microcomputer. Each conditioning box was placed in a separate ventilated, light- and sound-attenuating enclosure (Coulbourn Model E10-20). In the “lights-on” condition, 4-in (10.2-cm) Mini-Moon lights (Model 73060; AmerTac, Saddle River, NJ; 3 VDC) illuminated these enclosures. These lights were placed facing upward on the floor of the enclosure between the conditioning box and the back wall of the enclosure. White paper (75 g/m2, 92 brightness) was taped to the outside of the walls of the conditioning box to diffuse the light. However, this paper covered only the upper portion of the long walls of the box (above the photodetectors), allowing mice to also see internal visual features of the sound enclosure through the lid and lower acrylic walls when the enclosure was illuminated (e.g., enclosure door, fan baffle, cables). With the light on, luminance measured in the center of the apparatus (with the light-meter sensor head pointed up) was about 4.9 lux (Model MT-4007 digital light-meter, Prokit’s Industries Co., Taiwan).
The tactile cues were those used in our previous studies of ethanol-induced CPP in DBA/2J mice (Cunningham et al., 2006a, 2006b). The grid floor was made from 2.3 mm stainless-steel rods mounted 6.4 mm apart in acrylic rails. The hole floor was made from perforated stainless steel (16 GA) with 6.4-mm round holes on 9.5-mm staggered centers. This particular combination of tactile cues was selected on the basis of many previous studies showing that groups of saline-injected DBA/2J mice spend about half their time on each cue during choice tests (e.g., Cunningham et al., 2003). The apparatus and floors were wiped with a damp sponge after each animal.
In the one-compartment configuration (Exps. 1–2), the same floor cue was placed on both sides of the conditioning box (i.e., grid/grid, hole/hole) during conditioning trials and the mouse was allowed to move back and forth across both sides of the box. For the two-compartment configuration (Exps. 1–4), a clear acrylic barrier (Cunningham et al., 2006b) confined the mouse to one side of the apparatus with the assigned floor cue (counterbalanced within each group); the other floor cue was placed on the opposite side of the apparatus. During preference tests, a different cue was presented on each side of the box (i.e., grid/hole) and there was no barrier.
2.3 Procedure
All experiments included three phases: habituation (1 session), conditioning (8 sessions), and testing (1 to 5 sessions). Sessions were conducted 4–5 days per week.
2.3.1 Habituation
The purpose of this session was to familiarize mice with general aspects of the daily procedure. Each mouse was transported to the conditioning room, weighed, injected with saline (12.5 ml/kg IP) and placed into the conditioning box on a white paper floor for 5 min before it was returned to the home cage. The tactile cues and barrier were not present during this session.
2.3.2 Conditioning
Mice in all experiments received an unbiased place conditioning procedure (Cunningham et al., 2003). Within each of the main treatment groups (see Design section below), mice were randomly assigned to one of two conditioning subgroups (n = 11–12/subgroup). For mice in the GRID+ subgroups, exposures to the grid floor were preceded by injection of ethanol (2 g/kg, 20% v/v in saline, 12.5 ml/kg) whereas exposures to the hole floor were preceded by injection of saline (12.5 ml/kg). These drug-floor pairings were reversed for mice in the GRID− subgroups, i.e., the grid floor was paired with saline injection while the hole floor was paired with ethanol. Thus, GRID+ mice were expected to spend more time on the grid floor during testing than GRID− mice (conversely, GRID− mice were expected to spend more time on the hole floor than GRID+ mice). The difference between these counterbalanced conditioning subgroups is used to index CPP strength in this design (Cunningham et al., 2003). All mice received four 5-min conditioning trials of each type (Cunningham & Prather, 1992). Ethanol and saline trials were alternated across training days and the order of exposure was counterbalanced within each conditioning subgroup.
2.3.3 Preference Tests
Each experiment concluded with one or more preference tests (30–60 min). Mice were weighed and injected with saline before placement in the box for testing. The first test was given 24–72 h after the last conditioning trial and subsequent tests occurred at 24-h intervals. Previous studies in our lab suggest that delays within this range generally have little impact on the expression of ethanol-induced CPP in similarly trained groups. Importantly, the delay was identical for all groups within each experiment.
2.4 Design
2.4.1 Experiments 1 and 2: One vs. two-compartment conditioning in the dark vs. light
The purpose of these experiments was to determine whether combining tactile cues with spatial cues (Exp. 1) or with both visual and spatial cues (Exp. 2) would enhance CPP. These experiments differed only in the illumination of the sound enclosure, with lights off in Exp. 1 and lights on in Exp. 2. The dark and light conditions were studied in separate experiments in order to test mice under identical conditions within each experiment and to avoid comparisons between groups that differed in test session activity due to illumination differences (Gremel & Cunningham, 2007). For mice in the one-compartment groups, the left-right positions of the floor cues were randomly assigned for testing (counterbalanced within conditioning subgroups). For mice in the two-compartment groups, the left-right positions of each cue were randomly assigned at the beginning of the conditioning phase (counterbalanced within subgroups) and those positions remained the same throughout conditioning and testing. All groups in both studies received five 60-min tests at 24-h intervals. Long test durations were used in these studies to allow response weakening (i.e., extinction produced by cue exposures in the absence of ethanol), which might increase the likelihood of observing group differences in case there are ceiling effects on performance (Groblewski et al., 2008).
2.4.2. Experiment 3: Reversing the spatial locations of tactile cues during testing
Exp. 3 was designed to provide an alternative test of the role played by external visual-spatial cues by reversing the test locations of the tactile cues after two-compartment conditioning in the dark or light. All mice received their tactile cues in unique spatial locations during conditioning. During testing, the Same groups received tactile cues in the same spatial locations used during training. However, the test positions of the tactile cues were opposite to those assigned during conditioning for the Different groups. The preference test in Exp. 3 was only 30 min long because our primary interest was in the immediate impact of changing the spatial location cue on CPP expression. If the external visual-spatial cues unique to each compartment had acquired associative value during conditioning in the light, one would expect that switching the spatial location of the tactile cues during testing would have an immediate and greater detrimental effect on CPP expression in the groups conditioned and tested in the light than in the groups conditioned and tested in the dark.
2.4.3. Experiment 4: Alternating the spatial locations of tactile cues during conditioning
Experiment 4 was designed to test the effect of adding external visual cues (i.e., lights on) to tactile cues using an alternative strategy for making external visual-spatial cues irrelevant. Specifically, all mice were trained in a two-compartment procedure and the spatial location of each mouse was varied across trials (LLRRRRLL or RRLLLLRR) while maintaining a consistent relationship between the specific tactile cues that were paired with ethanol or saline. For example, mice in GRID+ subgroups received a total of four ethanol-grid pairings: two trials occurred while the mouse was confined on the left side and two trials while it was confined on the right side. These mice also received four saline-hole pairings, with two trials on the left side and two trials on the right side. The alternating-side procedure is similar to a one-compartment procedure in providing a stronger contingency between ethanol and the assigned tactile cue than between ethanol and any specific visual-spatial cue.
One group was trained and tested in the dark while the other group was trained and tested in the light. The left-right positions of the floor cues were randomly assigned for the first test (counterbalanced within conditioning subgroups) and a total of three 30-min preference tests were conducted with the floors in those positions. Based on the idea that the alternating two-compartment procedure is functionally similar to a one-compartment procedure, we predicted that CPP conditioned in the light would be weaker than CPP conditioned in the dark.
2.5 Data Analysis
The primary dependent variable was the time spent on the grid floor during preference testing. The number of s spent on the grid floor was divided by the test session duration in min (i.e., 30) to create a dependent variable indexed in s/min. The primary advantage of this simple transformation is that results are easily compared with the full range of possible outcomes (e.g., 0 s/min = complete aversion to grid; 60 s/min = complete preference for grid). It also facilitates comparison across experiments with different total test durations. In our unbiased counterbalanced design, the difference between conditioning subgroups (i.e., GRID+ vs. GRID−) is indicative of place conditioning (Cunningham et al., 2003). Data were analyzed by factorial analysis of variance (ANOVA) using Conditioning Subgroup (GRID+ vs. GRID−) as a between-group factor. Number of Compartments (one vs. two), Lighting Condition (dark vs. light) and Test Floor Position (same vs. different) were also treated as between-group factors when they were included in the experimental design. Where appropriate, repeated measures ANOVA included Test session as a within-group factor. The alpha level for all analyses was set at .05. P values for follow-up comparisons between conditioning subgroups were Bonferroni-corrected to limit overall alpha for each experiment.
3. Results
Due to poor health or procedural problems, data were not available for three mice in Exp. 1 and one mouse in Exp. 2.
3.1. Experiments 1 and 2: One- vs. two-compartment conditioning in the dark vs. light
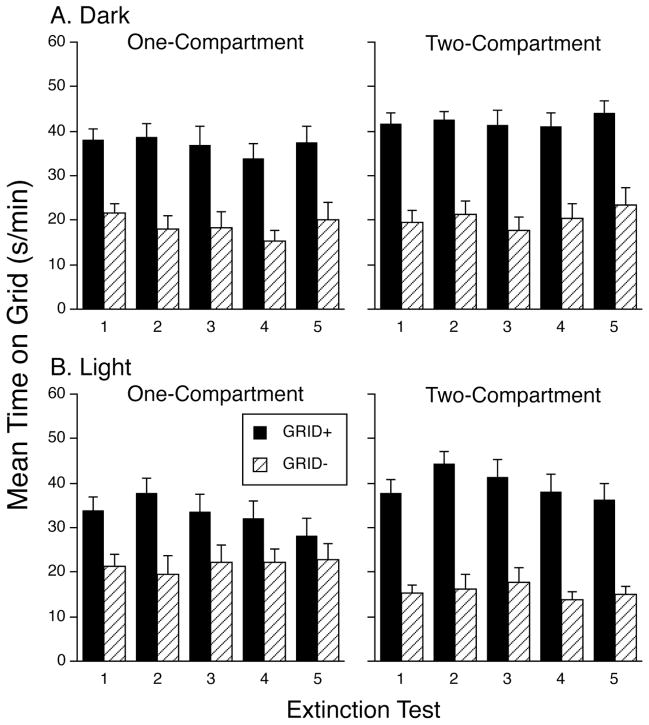
Test data from Exps. 1 and 2 are depicted in panels A and B of Figure 3, respectively. Each panel plots mean time (sec/min ± SEM) spent on the grid floor during the five consecutive 60-min choice tests. As can be seen, the GRID+ conditioning subgroups spent more time on the grid floor than the GRID− subgroups, indicating development of CPP (Cunningham et al., 2003). When training and testing occurred in the dark (Fig. 3A), the one- and two-compartment procedures yielded similarly strong levels of place preference, with little evidence of response weakening (extinction) over the five tests. However, when training and testing occurred in the light, place preference was generally weaker in the one-compartment condition than in the two-compartment condition (Fig. 3B).
Figure 3.
Mean time (±SEM) spent on the grid floor during five daily 60-min test sessions. The top panels depict Exp. 1 in which mice were trained and tested in the dark; the bottom panels depict Exp. 2 in which mice were trained and tested in the light. In one-compartment procedures, mice had access to the entire apparatus during conditioning trials (no barrier between sides) and the same floor cue was on both sides. In the two-compartment procedures, mice were confined to the left or right side of the apparatus on the assigned floor. Only the main effect of Conditioning Subgroup was significant (p < .0001) in the dark condition. That main effect (p < .0001) and the Conditioning Subgroup x Number of Compartments interaction (p < .05) were significant in the light condition. Each conditioning subgroup contained 11–12 mice.
The foregoing observations were supported by three-way (Number of Compartments x Conditioning Subgroup x Test) ANOVAs that were conducted separately on the grid time data from each experiment. The analysis of Exp. 1 yielded only a significant main effect of Conditioning Subgroup [F(1,41) = 62.3, p < .0001], confirming development of a significant place preference in both compartment conditions, with little change across tests. For Exp. 2, however, both the main effect of Conditioning Subgroup [F(1,43) = 37.3, p < .0001] and the interaction [Number of Compartments x Conditioning Subgroup: F(1,43) = 4.6, p < .05] were significant, reflecting the development of weaker place preference in the one-compartment group. Post-hoc comparisons (collapsed across tests) showed that the GRID+ and GRID− subgroups differed significantly in both the one- and two-compartment conditions (Bonferroni-correct p’s < .02), indicating that the addition of visual-spatial cues did not prevent development of CPP in the one-compartment condition. The three-way ANOVA for Exp. 2 also produced a significant Conditioning Subgroup x Test interaction [F(4,172) = 3.3, p < .02], which reflected a significant Test effect for the GRID+ subgroups [F(4,92) = 5.7, p < .001], but not for the GRID− subgroups [F < 1]. However, the three-way interaction was not significant (F(4,172) < 1), indicating that the effect of compartment number on CPP did not vary across tests. A two-way (Number of Compartments x Conditioning Subgroup) ANOVA on data from the first 30 min of the first test session was also conducted to more directly address whether compartment number affected the initial expression of CPP in the light condition. That analysis showed a significant main effect of Conditioning Subgroup [F(1,43) = 35.0, p < .001] but only a marginal interaction [F(1,43) = 3.6, p = .06], indicating a non-significant trend toward a compartment effect early in testing.
Test session activity was generally higher when mice were trained and tested in the dark (Exp. 1: 30.3 ± 0.7 counts/min) than when mice were trained and tested in the light (Exp. 2: 24.4 ± 0.7 counts/min). Also, activity decreased across tests under both lighting conditions (Exp. 1: first test = 34.8 ± 0.9 counts/min, last test = 27.7 ± 1.0 counts/min; Exp.2: first test = 27.8 ± 0.7 counts/min, last test = 22.5 ± 0.7 counts/min). Two-way (Number of Compartments x Test) ANOVAs applied separately to test activity data from each experiment yielded only significant main effects of Test [Exp. 1: F(4,172) = 18.0, p < .0001; Exp. 2: F(4,180) = 17.0, p < .0001]. Thus, there was no effect of the number of training compartments on subsequent test activity.
3.2. Experiment 3: Reversing the spatial locations of tactile cues during testing
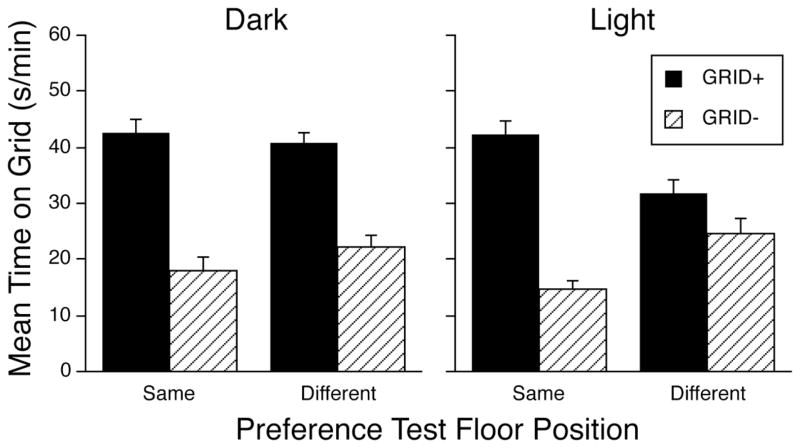
As can be seen in the left panel of Figure 4, when two-compartment training and testing occurred in the dark, reversing the positions of the tactile cues at the time of testing had little effect on conditioned preference. However, reversing the floor positions reduced the expression of preference when training and testing occurred in the light (Fig. 4, right panel). These conclusions were supported by a three-way ANOVA (Lighting Condition x Test Floor Position x Conditioning Subgroup) that revealed a significant three-way interaction [F(1,87) = 5.1, p < .03] as well as a significant main effect of conditioning Subgroup [F(1,87) = 149.8, p < .0001] and a significant Test Floor Position x Conditioning Subgroup interaction [F(1,87) = 16.8, p < .0001]. To interpret the 3-way interaction, we conducted two-way ANOVAs (Test Floor Position x Conditioning Subgroup) separately for each lighting condition. These analyses showed that the two-way interaction was significant for the light condition [F(1,44) = 19.2, p < .0001], but not for the dark condition [p = .19]. Pair-wise comparisons between the GRID+ and GRID− conditioning subgroups in each treatment condition indicated a significant conditioned place preference in all cases [Bonferroni-corrected p’s < .001] except for the light-different condition [Bonferroni-corrected p = .10]. As seen earlier, test session activity was higher when mice were trained and tested in the dark (39.2 ± 1.3 counts/min) than when they were trained and tested in the light (28.3 ± 0.7 counts/min; F(1,91) = 51.4, p < .0001). Test floor position had no effect on test session activity.
Figure 4.
Mean time (±SEM) spent on the grid floor during a 30-min test session. Two-compartment training and testing were conducted in the dark (left panel) or light (right panel). The Same groups received the assigned tactile cues in the same spatial locations used during the conditioning, whereas the cue locations were switched for mice in the Different groups. Overall ANOVA yielded a significant 3-way interaction (p < .03); follow-up 2-way ANOVAs indicated a significant main effect of Conditioning Subgroup (p < .0001) in the dark condition and a significant Conditioning Subgroup x Test Floor Position interaction (p < .0001) in the light condition. Post-hoc comparisons showed a significant effect of Conditioning Subgroup in the Light-Same group (p < .0001), but not in the Light-Different group. Each conditioning subgroup contained 11–12 mice.
3.3. Experiment 4: Alternating the spatial locations of tactile cues during conditioning
Figure 5 shows that a strong and persistent place preference was established when the spatial positions of the tactile cues were alternated across trials in a two-compartment procedure, regardless of the lighting condition. A three-way ANOVA (Lighting Condition x Conditioning Subgroup x Test) supported this conclusion, yielding a significant main effect of Conditioning Subgroup [F(1,42) = 85.0, p < .0001], but no other effects or interactions. Mice were generally more active during test sessions in the dark (33.0±1.2 counts/min) than in the light (23.5±1.0 counts/min), and they were slightly more active during the first test (30.5±1.1 counts/min) than during the third test (27.1±1.0 counts/min). A two-way ANOVA (Lighting Condition x Test) of test activity showed significant main effects of Lighting Condition [F(1,44) = 37.6, p < .0001] and Test [F(2,88) = 14.6, p < .0001] as well as the interaction [F(2,88) = 4.0, p < .03].
Figure 5.
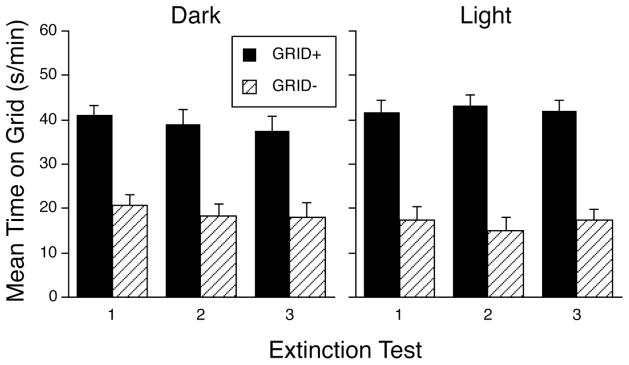
Mean time (±SEM) spent on the grid floor during three consecutive 30-min test sessions. All conditioning subgroups were trained in a two-compartment procedure in which the spatial locations of the tactile cues alternated over trials. Training and testing were conducted in the dark (left panel) or light (right panel). Overall ANOVA showed only a significant main effect of Conditioning Subgroup (p < .0001). Each conditioning subgroup contained 11–12 mice.
4. Discussion
These studies illustrate the important role that apparatus illumination can play in determining stimulus control of CPP when tactile cues are used as CSs. When lights were off, there was no difference between CPP across five consecutive tests in the one- vs. two-compartment procedures (Exp. 1). However, when lights were on, CPP was generally weaker in the one-compartment procedure than in the two-compartment procedure (Exp. 2). Using a two-compartment procedure, Exp. 3 showed that apparatus illumination also determines whether visual-spatial cues gain control over behavior. When training occurred in the dark, reversing the spatial locations of the tactile cues had no effect on CPP expression. In contrast, when training occurred in the light, CPP was markedly reduced by reversal of the tactile cue locations. However, apparatus illumination did not always affect CPP as shown by the absence of an effect when the locations of the tactile cues were alternated over conditioning trials (Exp. 4).
These findings extend our previous work on tactile cue CPP in several ways. First, Exp. 1 offers the first direct comparison between one- and two-compartment procedures in the dark, showing that CPP was not altered across five consecutive tests when spatial location was made relevant by using a two-compartment procedure. Second, Exp. 2 showed that CPP trained in a one-compartment procedure was significantly reduced compared to a two-compartment procedure when training occurred in the light. A previous study had failed to show this difference (see Fig. 6, Cunningham et al., 2006b), but testing was limited to three 30-min tests. By extending the number and duration of CPP tests (to five 60-min tests), Exp. 2 provided a better opportunity to detect differences. Our results suggest that CPP is generally stronger when tactile cues are combined with visual-spatial cues that are uniquely predictive of ethanol exposure (two-compartment procedure) than when visual-spatial cues are only partially predictive (one-compartment procedure). Observation of this effect only during a more extended period of testing raises the possibility that the added visual-spatial cues altered CPP extinction rather than acquisition. However, statistical analysis did not support the conclusion that this effect emerged across time during extinction. Given the possibility of a ceiling effect on CPP performance (Groblewski et al., 2008), an alternative interpretation is that response weakening during extinction simply unmasked an underlying difference in associative strength produced during acquisition.
The finding that reversal of tactile cue location had no effect in dark-tested mice (Exp. 3) replicates a previous observation (see Fig. 7, Cunningham et al., 2006b). However, the finding that cue reversal completely interfered with tactile-cue CPP expression in light-trained mice is new, highlighting the important role that apparatus illumination can play in determining stimulus control of CPP. If external visual-spatial cues played no role during light training, reversing tactile cue locations should have had no effect on CPP, i.e., mice would have continued to show a strong preference for the ethanol-paired tactile cue in its new location (as seen in dark trained mice). On the other hand, if tactile cues played no role during training in the light (i.e., if only the external visual-spatial cues had acquired associative strength), then mice should have remained on the side that was originally paired with ethanol (i.e., showing what would appear to be an aversion for the tactile cue now located on the opposite side). The fact that CPP was eliminated by tactile cue reversal is perhaps best explained by concluding that both tactile and visual-spatial cues had acquired associative strength during two-compartment training in the light. Finally, while the finding of a strong CPP when tactile cue location was alternated across training trials in the dark replicated a previous result (see Fig. 7, Cunningham et al., 2006b), the finding of a similarly strong CPP when such training occurred in the light (Exp. 4) was both novel and unexpected given the outcome of Exp. 2.
Most of these findings can be understood within the framework of conditioning theories like the Rescorla-Wagner model. This model asserts that elements of a compound stimulus compete for associative strength in proportion to their relative salience (Rescorla & Wagner, 1972). For example, the finding that spatial location has little impact on CPP conditioned to tactile cues in the dark can be explained by assuming that spatial location cues in a darkened apparatus have very low salience and are completely overshadowed by tactile cues. Thus, combining location and tactile cues during training (Exp. 1) or reversing tactile cue locations during testing (Exp. 3) should not affect CPP. However, when training and testing occurred in the light, visual-spatial cues competed with tactile cues, and all of these cues acquired associative value. In the two-compartment procedure, the unique visual-spatial cues on each side (VX/SL vs. VY/SR) were differentially associated with drug and vehicle exposure, just like the tactile cues. CPP expression should be strong as long as all cues remain in their original locations. However, CPP should be reduced if tactile cue locations are changed (Exp. 3) because conditioned visual-spatial cues in the original (drug-paired) location will elicit an approach response that competes with the response evoked by the tactile CS+ in its new location. In contrast, because a one-compartment procedure exposes mice to visual-spatial cues on both sides of the apparatus on all trials, those cues are not uniquely predictive of drug exposure. In other words, visual-spatial cues are partially reinforced during training (i.e., paired with drug on CS+ trials, but paired with vehicle on CS− trials). Consequently, the difference between the net associative values of the combined cues on each side of the apparatus is potentially smaller in the one-compartment procedure than in the two-compartment procedure, which might explain the compartment effect in Exp. 2.
The model’s prediction of enhanced overall associative strength of drug-paired cues in the two-compartment procedure applies primarily to early training trials before conditioning is asymptotic. The prediction of impaired associative conditioning to tactile cues in the one-compartment procedure also applies primarily to early training trials when partially reinforced cues on the opposite side of the apparatus might elicit excitatory conditioned responses that compete with those evoked by the tactile CS+. In both cases, however, the model predicts that differences between the two procedures should diminish after a large number of trials because the differences between the overall associative values of the cues on each side of the apparatus will eventually become the same in each procedure. The prediction could be tested in future studies by extending the length of the training phase before testing.
The similar CPP in the alternating cue groups trained in the dark or light in Exp. 4 is not consistent with predictions based on the Rescorla-Wagner model. Since the alternating cue procedure partially reinforced visual-spatial cues on both sides of the apparatus, the two-compartment procedure was functionally similar to the one-compartment procedure and would therefore be expected to show weaker CPP in the light, which did not occur. One possible explanation is that, in contrast to a fixed cue procedure, the alternating cue procedure reduced the relative salience of external visual-spatial cues, thereby reducing the associative strength acquired by those cues. In that case, the difference between the net associative values of the combined cues on each side of the apparatus would be more similar in the dark and light groups, yielding a comparable CPP. Regardless of the explanation, the results of Exp. 4 are important because they suggest that the alternating cue procedure is an effective way to eliminate differences produced by apparatus lighting and reduce the impact of external visual-spatial cues.
In addition to effects of apparatus lighting on the learning and retrieval of cue-drug associations, consideration must also be given to the possibility that effects on CPP were secondary to illumination-related differences in locomotor activity or anxiety. Previous studies have shown that CPP expression is inversely related to test session activity level (Gremel & Cunningham, 2007; Vezina & Stewart, 1987b), suggesting that mice tested in the light (less active) might be expected to express stronger CPP than mice tested in the dark (more active). Contrary to this prediction, however, the group showing the weakest CPP in Experiments 1–2 was the two-compartment group trained in the light. Similarly, the group showing the weakest CPP in Experiment 3 was tested in the light. Moreover, CPP differences were observed between groups that did not differ in test activity in both Exps. 2 and 3. Overall, this pattern of findings does not support an alternative interpretation based on lighting-related differences in test activity.
Consideration must also be given to the possibility that lighting-related differences in anxiety level might have affected CPP, independent of changes in activity level. For example, based on the assumption that rodent anxiety is greater in the light than in the dark (Crawley & Goodwin, 1980), CPP conditioned in an illuminated apparatus might be stronger due to greater negative reinforcement (i.e., alleviation of anxiety) by ethanol or to arousal-induced enhancement of memory consolidation. Alternatively, CPP conditioned in an illuminated apparatus might be weaker due to anxiety-induced disruption of learning, memory retrieval or performance. Although it is possible that light-induced anxiety generally increased the salience of spatial cues (explaining why spatial cues were generally more effective in the light than in the dark), none of the anxiety hypotheses can readily explain differences between groups tested under the same illumination condition.
The impact of apparatus lighting on the ability of visual-spatial cues to participate in stimulus-drug associations has potentially important implications for studies that use the CPP procedure to examine the neurobiological mechanisms that underlie drug-seeking behavior elicited by drug-paired stimuli. This possibility is well illustrated by a previous study showing that different brain areas were involved in morphine-induced CPP depending on whether rats had access to distal visual-spatial cues (White et al., 2005). One system (including lateral nucleus of the amygdala and nucleus accumbens) was thought to mediate conditioned approach (sign-tracking) responses, whereas the other system (fimbria fornix) was thought to mediate conditioned reinforcement. Since many (if not most) CPP apparatuses use distinctive tactile cues in a fixed two-compartment procedure and lighting conditions are not always well specified, the results of Exp. 3 are especially relevant in this context because they show that apparatus lighting determines whether tactile cues share stimulus control of CPP with external visual-spatial cues. When our findings are considered together with those of White et al. (2005), one might speculate that visual-spatial cues presented in the light are more likely to engage the conditioned approach-sign tracking system while tactile cues presented in the dark are more likely to engage the conditioned reinforcement system. Future studies should address this hypothesis.
Regardless of the underlying mechanisms, the present findings highlight the importance of understanding the conditions in which commonly used cues are effective in the CPP procedure. Using several different combinations of apparatus configuration, lighting conditions, and training procedures, these studies showed that apparatus lighting affects stimulus control of drug-seeking behavior when external visual-spatial cues are available during CPP training with tactile cues. Although interpretation of some CPP manipulations might not depend on knowing which of several stimulus elements control CPP, the nature of the controlling stimuli (i.e., tactile vs. visual-spatial) might determine the underlying neurobiological system (White et al., 2005). Overall, these studies improve our understanding of how cues from different modalities interact during the learning and expression of conditioned rewarding effects induced by drugs. Moreover, they encourage the use of CPP procedures in which the controlling stimuli have been well characterized.
Research Highlights.
One- or two-compartment training produced similar CPP in the dark
Two-compartment training produced stronger CPP in the light
Cue location reversal reduced CPP after training in the light, but not in the dark
External visual-spatial cues affect CPP conditioned to tactile cues
An alternating two-compartment procedure eliminates that interference
Acknowledgments
Research described here was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R01AA007702. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Charlene Voorhees for her assistance with Experiment 4. We also thank Tara Fidler, Peter Groblewski, Lauren Dobbs, Emily Young and Melanie Pina for helpful comments and suggestions on earlier drafts of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asin KE, Wirtshafter D. Clonidine produces a conditioned place preference in rats. Psychopharmacology. 1985;85:383–385. doi: 10.1007/BF00428206. [DOI] [PubMed] [Google Scholar]
- Barr GA, Paredes W, Bridger WH. Place conditioning with morphine and phencyclidine: Dose dependent effects. Life Sciences. 1985;36:363–368. doi: 10.1016/0024-3205(85)90122-5. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. Neuropharmacological basis of reward. New York: Oxford; 1989. pp. 264–319. [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behav Neurosci. 2014 doi: 10.1037/a0036459. Advance online publication, http://dx.doi.org/10.1037/a0036459. [DOI] [PMC free article] [PubMed]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature Protocols. 2006a;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Groblewski PA, Voorhees CM. Place conditioning. In: Olmstead MC, editor. Animal models of drug addiction. Totowa, NJ: Humana Press; 2011. pp. 167–189. [Google Scholar]
- Cunningham CL, Hallett CL, Niehus DR, Hunter JS, Nouth L, Risinger FO. Assessment of ethanol’s hedonic effects in mice selectively bred for sensitivity to ethanol-induced hypothermia. Psychopharmacology (Berl) 1991;105:84–92. doi: 10.1007/BF02316868. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Patel P, Milner L. Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav Neurosci. 2006b;120:1115–1132. doi: 10.1037/0735-7044.120.5.1115. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Prather LK. Conditioning trial duration affects ethanol-induced conditioned place preference in mice. Animal Learning & Behavior. 1992;20:187–194. [Google Scholar]
- Glimcher PW, Margolin DH, Giovino AA, Hoebel BG. Neurotensin: A new “reward peptide”. Brain Research. 1984;291:119–124. doi: 10.1016/0006-8993(84)90657-7. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191:195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology (Berl) 2008;201:97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Research. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Roma PG, Riley AL. Apparatus bias and the use of light and texture in place conditioning. Pharmacol Biochem Behav. 2005;82:163–169. doi: 10.1016/j.pbb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioned locomotion and place preference elicited by tactile cues paired exclusively with morphine in an open field. Psychopharmacology. 1987a;91:375–380. doi: 10.1007/BF00518195. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Morphine conditioned place preference and locomotion: The effect of confinement during training. Psychopharmacology. 1987b;93:257–260. doi: 10.1007/BF00179944. [DOI] [PubMed] [Google Scholar]
- White NM, Chai SC, Hamdani S. Learning the morphine conditioned cue preference: cue configuration determines effects of lesions. Pharmacol Biochem Behav. 2005;81:786–796. doi: 10.1016/j.pbb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Dreumont SE, Cunningham CL. Role of nucleus accumbens dopamine receptor subtypes in the learning and expression of alcohol-seeking behavior. Neurobiol Learn Mem. 2014;108:28–37. doi: 10.1016/j.nlm.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]