Abstract
Cellular studies suggest sphingolipids may cause or accelerate amyloid-beta (Aβ) and tau pathology but in vivo human studies are lacking. We determined cerebrospinal fluid (CSF) levels of sphingolipids (ceramides, sphingomyelins), amyloid-beta (Aβ1–42, AβX-38, AβX-40, AβX-42) and tau (T-tau, p-tau181) in 91 cognitively normal individuals, aged 36–69 years, with a parental history of Alzheimer's disease (AD). The 18-carbon acyl chain length ceramide species was associated with AβX-38 (r = 0.312, p = 0.003), AβX-40 (r = 0.327, p = 0.002), and T-tau (r = 0.313, p = 0.003) but not with AβX-42 (r = 0.171, p = 0.106) or p-tau (r = 0.086, p = 0.418). All sphingomyelin species correlated (most p < 0.001) with all Aβ species and T-tau; many also correlated with p-tau. Results remained in regression models after controlling for age and APOE genotype. These results suggest in vivo relationships between CSF ceramides and sphingomyelins and Aβ and tau levels in cognitively normal individuals at increased risk for AD, indicating these sphingolipids may be associated with early pathogenesis.
Keywords: Sphingolipids, Ceramide, Sphingomyelin, Cerebrospinal fluid, Beta-amyloid, Tau, Alzheimer's disease
1. Introduction
As evidenced by the many failed treatment trials for Alzheimer's disease (AD), there appears to be minimal treatment benefit in the fully symptomatic stage of the disease. The pathogenesis of amyloid-beta (Aβ) plaques and neurofibrillary tangles begins decades before the emergence of clinical symptoms (Jack, et al., 2010; Reiman, et al., 2012) and impacts multiple cellular pathways. While much research has focused on Aβ as a biomarker and therapeutic target for AD, there is limited understanding of the cellular pathways that contribute to, or are caused by, Aβ aggregation and deposition or tau phosphorylation. Understanding the early mechanisms associated with AD pathology may be especially important for identifying disease-modifying therapeutic targets.
Lipidomic and targeted approaches have identified pathways and products of sphingolipid metabolism that are altered early in the course of AD and contribute to AD neuropathology (Haughey, et al., 2010; Mielke and Haughey, 2012). Sphingolipid metabolism is a dynamic process that modulates the formation of a number of bioactive metabolites. Sphingomyelins are among the most abundant lipids in many mammalian cells and tissues. They are central to the creation of lipid rafts and ordered membrane domains (Quinn and Wolf, 2009; Simons and Ikonen, 1997; Simons and Vaz, 2004), the functional regulation of membrane-spanning proteins (Contreras, et al., 2012), act as important regulators of plasma membrane and cell cholesterol homeostasis (Gatt and Bierman, 1980; Slotte and Bierman, 1988), and are precursors for other sphingolipids such as ceramides. Ceramides, the central molecular species of the sphingolipids pathway, have important structural roles in cell membranes and function as second messengers for critical intra- and inter-cellular signaling affecting cellular growth, differentiation, proliferation, and apoptosis. While ceramides are important for cell survival, injury-induced cytokine production, and activate stress-signaling protein phosphatases and kinases (Hannun, 1996), at high levels ceramides inhibit cell division and induce cellular dysfunction and apoptosis (Goodman and Mattson, 1996).
Several lines of evidence suggest both direct and indirect associations between ceramides and Aβ levels at the cellular level (Mattson, et al., 2005; Tamboli, et al., 2011). Recent studies have also suggested that ceramides, particularly the 18-carbon acyl chain length species, modulate tau phosphorylation (Chalfant, et al., 1999; Dobrowsky, et al., 1993; Goedert, et al., 1995; Gong, et al., 1994; Mukhopadhyay, et al., 2009). While one post-mortem study reported correlations between sphingomyelinase activity, Aβ and phosphorylated tau,(He, et al., 2010) CSF studies of sphingolipids in AD have not examined the in vivo relationship between sphingolipids and AD pathology (Kosicek, et al., 2012; Satoi, et al., 2005). Therefore, we examined the association between CSF ceramides and sphingomyelins, Aβ, and tau in cognitively normal individuals aged 36 to 69 years at increased risk for AD due to a parental family history.
2. Methods
2.1. Participants and recruitment
Participants included 91 cognitively normal middle-aged adults with parental history of AD enrolled in the ESPRIT (Evaluating Simvastatin's Potential Role in Therapy) trial who had available CSF Aβ, tau, and sphingolipid measures at baseline, prior to randomization. The ESPRIT study is a prospective, randomized, placebo-controlled clinical trial that aimed to determine the effects of nine months of simvastatin therapy on CSF biomarkers for AD including Aβ and phospho-tau. Participants were recruited from the Wisconsin Registry for Alzheimer's Prevention (WRAP) (Sager, et al., 2005) and also from the community. Prior to enrollment into the ESPRIT trial, the diagnosis of AD in one or both parents was confirmed for each participant by using the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria through clinical evaluation and/or chart review by Wisconsin Alzheimer's Disease Research Center physicians and neuropsychologists. Exclusion criteria included current use of cholesterol-lowering medications or medications known to interact with simvastatin, contraindication to lumbar puncture, active liver disease, diabetes mellitus, past adverse reaction to a statin, or elevated creatine kinase. The study was approved by the Institutional Review Board of the University of Wisconsin. Subjects provided written informed consent prior to participating. This clinical trial was registered with ClinicalTrials.gov (NCT00486044).
2.2. Study procedures
CSF collection was completed in the morning at approximately the same time at each visit. A Sprotte 22- or 25-gauge spinal needle was inserted into the L3-4 or L4-5 interspace and between 8 to 22 mL of CSF was removed. CSF samples were centrifuged (to remove red blood cells [RBCs]) at the University of Wisconsin Clinical Research Unit within 30 minutes of collection, aliquoted into 0.5 mL polypropylene storage tubes, and stored in a −80°C freezer until simultaneous analysis was conducted at the conclusion of the study. As ceramide and sphingomyelin levels can be elevated in RBCs, we also quantified RBCs in the CSF and found the concentrations to be low (mean 15.55, SD 82.5, median 1.00). This suggests that remaining RBCs would have little impact on our results.
2.3. Cognitive testing
All participants underwent cognitive testing by a trained technician according to protocols established for each test. The cognitive test battery targeted four domains: memory and learning (Hopkins Verbal Learning Test [HVLT] (Brandt, 1991); New York University [NYU] Paragraph Recall (Kluger, et al., 1999); Modified Taylor Complex Figure delayed recall (Hubley, 1996); Visual Spatial Learning Test [VSLT]) (Malec, et al., 1997); language (Controlled Oral Word Association Test) (Benton and Hamsher, 1989); visual-motor skills (Modified Taylor Complex Figure – Copy (Hubley, 1996); Grooved Pegboard) (Kløve, 1963); and executive functioning (Ruff 2 and 7 Selective Attention Test (Ruff, et al., 1986); Stroop Color-Word Test (Spreen and Strauss, 1998); Color Trails Test (D'Elia, et al., 1996); Wechsler Memory Scale – Third Edition [WMS-III] Mental Control, Letter-Number Sequencing, and Spatial Span subtests (Wechsler, 1997); Wechsler Adult Intelligence Scale [WAIS]-III Symbol Search and Digit Symbol Substitution Subtests) (Wechsler, 1991). Mini-Mental State Exam was administered to assess global cognitive function (Folstein, et al., 1975). To reduce multiple comparisons among cognitive measures, six cognitive variables were prospectively identified targeting memory, learning, and executive function: 1) HVLT total learning summary score (sum of trials 1–3); 2) HVLT delayed recall score; 3) WAIS-III Processing Speed Index; 4) WMS-III Mental Control total score; 5) WMS-III Working Memory score; and 6) Stroop Color-Word score.
2.4. Laboratory evaluation
Blood tests were collected following a 12-hour fast. Lipoproteins were analyzed by NMR spectroscopy (LipoScience, Inc., Raleigh, NC) (Otvos, 2002). CK, AST, and ALT levels were measured using enzymatic precipitation techniques. APOE genotype was measured using standard PCR and DNA sequencing techniques.
2.5. Sphingolipid assays
A crude lipid extraction of CSF was conducted using a modified Bligh and Dyer procedure as previously described (Haughey, et al., 2004). To control for slight differences in extraction efficiencies, and day to day variations in the efficiency of the mass spectrometer, purified standards of sphingomyelin C12:0 and ceramide C12:0 (10 nM each; Avanti Polar Lipids) were added directly to samples prior to extraction. Extractions were performed by the addition of 900 μl methanol containing ammonium formate (53 mM) to 300 μl of CSF. The mixture was vortexed, and 1200 μl of chloroform was added. The mixture was centrifuged at 1,000 g for 10 minutes. The chloroform (bottom) layer was carefully removed and dried in a nitrogen evaporator (OASYS model 11848. Organomation Associates, Inc.). Dried extracts were sealed and stored at −80°C. The dried extracts were re-suspended in 100% methanol prior to analysis.
Analyses of sphingolipids were performed on a high-performance liquid chromatography coupled electrospray ionization tandem mass spectrometer (LC/ESI/MS/MS) (API3000s, Sciex Inc., Thornhill, Ontario, Canada) using methods similar to those described in previous studies (Haughey, et al., 2004). Quantitation of sphingomyelins (d18:1/C16:0-C26:1) and simple and complex ceramides (d18:1/C16:0-C26:1) were conducted by multiple reaction monitoring (MRM). Samples were injected using a CTC PAL autosampler (LEAP technologies, Inc.) into a PerkElmer HPLC equipped with a 2.6 μm C18 100 Å LC Column 50 × 2.1 mm (Kinetex) and a guard column with identical packing material (Phenomenex). For a typical run, the LC column was first pre-equilibrated for 0.5 min with the first mobile phase consisting of 85% methanol, 15% H20, and 5 mM ammonium formate. The column was then eluted with the second mobile phase consisting of 99% methanol, 1% formic acid, and 5 mM ammonium formate at the flow rate of 400.0 μl/min. The eluted sample was injected into the ion source where the detection of each analyte was conducted by ESI/MS/MS in MRM mode monitoring the precursor, and products by ion scan.
Area under the curve was used to quantitate each sphingomyelin and simple and complex ceramide species using MultiQuant (AB Sciex). The resulting data were normalized to the corresponding internal standard for sphingomyelin (C18:1/C12:0) or ceramides (C18:1/C12:0). Each molecular species was analyzed separately to determine whether there were chain-specific associations within each sphingolipid species. A summary variable of all species within each class (sphingomyelins, ceramides) was also examined. Quantitation was reported as counts per second (cps). As concentrations were skewed to the right, similar to previous studies (Mielke, et al., 2010a; Mielke, et al., 2012), they were log-transformed prior to analyses.
2.6. CSF amyloid-beta and tau assays
Levels of β-amyloid1-42 (Aβ1-42), total-tau (T-tau) and phosphorylated tau 181 (ptau) in CSF were analyzed using the xMAP platform with the INNO-BIA AlzBio3 assay, as described previously in detail (Olsson, et al., 2005). Levels of CSF AβX-38, AβX-40 and AβX-42 were measured using Meso Scale Discovery (MSD®) electrochemiluminescence detection technology and the MSD Human/Rodent (4G8) Abeta Triplex Assay on a SECTOR™ Imager 2400 instrument as described by the manufacturer (Meso Scale Discovery, Gaithersburg, MD, USA). This assay employs Cterminally specific antibodies to capture AβX-38, AβX-40 and AβX-42, respectively, and a SULFO-TAG-labeled 4G8 antibody (directed against the Aβ mid-domain) to quantify them. All analyses were performed batch-wise by board-certified laboratory technicians who were blinded to all clinical data.
2.7. Statistical analysis
The relationship between the CSF sphingolipids and demographics, health characteristics, cognitive performance, and CSF Aβ and tau levels were examined using Spearman rank correlation for continuous variables and t-tests for dichotomous variables. Linear regression models were used to examine the relationship between the CSF sphingolipids and CSF Aβ and tau, controlling for age and APOE E4 genotype. Linear regression models were also used to examine the relationship between the CSF sphingolipids and performance on each cognitive test, controlling for age, education and APOE genotype. The a priori p-value was set at p < 0.05. All analyses were conducted using STATA Version 12.1 (StataCorp, College Station, TX).
3. Results
Characteristics of the 91 individuals included in this study are described in Table 1. On average, participants were 53.4 (SD = 7.9) years of age, had 16 years of education, were overweight (mean BMI 27.8, SD = 5.5) and had normal levels of cholesterol, triglycerides, and blood glucose. There were 63 women (69.2%); 33 participants (36.3%) had at least one APOE E4 allele. All individuals were considered cognitively normal and had levels of Aβ and tau that are thought to be in the `normal' range. There was a significant, positive correlation between Aβ1-42 using the xMAP assay and AβX-42 using the MSD assay (r = 0.766, p < 0.0001).
Table 1.
Characteristics of sample
| Characteristics | N | Mean (SD)/n (%) | Range |
|---|---|---|---|
| Age, years | 91 | 53.4 (7.9) | 36–69 |
| Female | 91 | 63 (69.2%) | |
| Education, years | 91 | 16.3 (2.9) | 8–23 |
| Any E4 allele | 91 | 33 (36.3%) | |
| Body mass index, kg/m2 | 91 | 27.8 (5.5) | 19.2–47.8 |
| Vitamin C supplements | 91 | 22 (24.2%) | |
| Vitamin E supplements | 91 | 20 (22.0%) | |
| Blood pressure medications | 91 | 11 (12.1%) | |
| Systolic blood pressure, mm Hg | 91 | 121.6 (13.4) | 94–161 |
| Total cholesterol, mg/dL | 91 | 190.7 (32.4) | 114–299 |
| HDL cholesterol, mg/dL | 91 | 56.0 (16.5) | 31–122 |
| LDL cholesterol, mg/dL | 91 | 118.8 (29.6) | 41–215 |
| Trigylcerides, mg/dL | 91 | 99.7 (43.8) | 46–246 |
| Glucose, mg/dL | 90 | 83.4 (7.2) | 72–109 |
| MMSE, points of 30 | 90 | 29.5 (0.7) | 27–30 |
| HVLT-total learning score | 91 | 29.6 (3.4) | 20–35 |
| HVLT-delayed recall score | 91 | 10.5 (1.8) | 3–12 |
| Processing speed index | 91 | 90.8 (12.6) | 57–119 |
| Mental control total score | 91 | 30.3 (4.9) | 19–40 |
| Working memory composite score | 91 | 26.4 (4.2) | 14–37 |
| Stroop color-word score | 91 | 43.1 (7.7) | 26–69 |
| CSF ABx-38 by MSD, ng/L | 91 | 1575.7 (661.7) | 405–4042 |
| CSF ABx-40 by MSD, ng/L | 91 | 6662.9 (2015.8) | 2710–13,235 |
| CSF ABx-42 by MSD, ng/L | 91 | 524.6 (202.9) | 155–1315 |
| CSF AB1-42 by xMAP, ng/L | 91 | 344.1 (69.0) | 150–510 |
| CSF tau by xMAP, ng/L | 91 | 63.4 (25.0) | 27–132 |
| CSF p-tau by xMAP, ng/L | 91 | 36.4 (18.6) | 13–127 |
| CSF nucleated cells, cells/uL | 91 | 2.05 (2.42) |
Key: MMSE, Mini-Mental State Examination. HVLT, Hopkins Verbal Learning Test. CSF, Cerebrospinal Fluid. AB, amyloid-beta. MSD, Meso Scale Discovery electrochemiluminescence. xMAP. INNO-BIA AlzBio3.
Levels of CSF very long chain ceramides with chain lengths of C20–C26 were higher in APOE E4 carriers compared to non-carriers (p-values range from 0.016–0.045). However, levels of other ceramide species (C16:0 and C18:0) or sphingomyelins (C16:0-C26:1) did not vary by APOE genotype (all p > 0.10, data not shown). There were positive correlations between all sphingomyelins (total and each species) and age (e.g., total CSF sphingomyelin: r = 0.246, p = 0.019), but not between ceramides and age. Neither sphingomyelin nor ceramide levels differed by sex. There were also no associations between any sphingomyelins nor ceramides and use of vitamins or blood pressure medications, body mass index, and fasting serum cholesterol, triglycerides, or glucose (data not shown).
3.1. Relationship between CSF sphingolipids and CSF Aβand tau
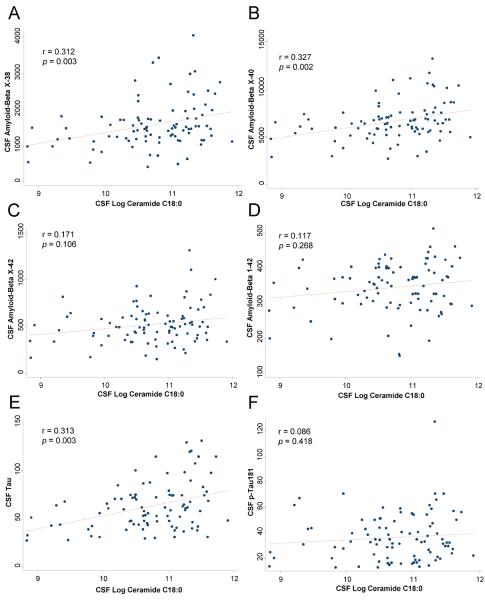
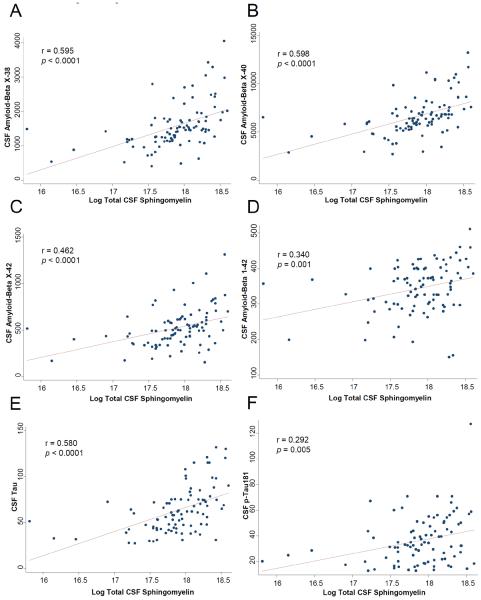
Correlations between total ceramides, sphingomyelins and each individual species within these lipid classes and CSF Aβ and tau are shown in Table 2. Only ceramide C18:0 consistently correlated with Aβ and tau (Table 2 and Fig. 1). Notably, there were positive, significant, correlations with AβX-38 (r = 0.312, p = 0.003) and AβX-40 (r = 0.327, p = 0.002), but not with AβX-42 (r = 0.171, p = 0.106) or with Aβ1-42 (r = 0.117, p = 0.268). In contrast to the specific carbon-chain length findings for ceramides, virtually all CSF sphingomyelins were positively correlated with all CSF Aβ measures, and also with tau, p-tau, and the tau/Aβ1-42 ratio (Table 2 and Fig. 2).
Table 2.
Correlation between CSF sphingolipids and CSF amyloid-beta and tau measures
| ABX38a | ABX40a | ABX42a | AB1-42b | taub | p-tau181b | tau/AB1-42b | p-tau181/AB1-42b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log CSF sphinqolipid | r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | r | p value |
| Ceramides | ||||||||||||||||
| d18:1–C16:0 | ||||||||||||||||
| d18:1–C18:0 | 0.312 | 0.003 | 0.327 | 0.002 | 0.313 | 0.003 | 0.306 | 0.003 | ||||||||
| d18:1–C20:0 | ||||||||||||||||
| d18:1–C22:0 | ||||||||||||||||
| d18:1–C24:0 | ||||||||||||||||
| d18:1–C24:1 | 0.221 | 0.035 | ||||||||||||||
| d18:1–C26:0 | ||||||||||||||||
| Total ceramidesc | ||||||||||||||||
| Sphingomyelins | ||||||||||||||||
| d18:1–C16:0 | 0.404 | 0.0001 | 0.412 | <0.0001 | 0.301 | 0.004 | 0.259 | 0.013 | 0.410 | <0.0001 | 0.311 | 0.003 | ||||
| d18:1–C16:1 | 0.484 | <0.0001 | 0.500 | <0.0001 | 0.369 | <0.001 | 0.253 | 0.016 | 0.441 | <0.0001 | 0.277 | 0.008 | 0.331 | 0.001 | ||
| d18:1–C18:0 | 0.558 | <0.0001 | 0.554 | <0.0001 | 0.428 | <0.0001 | 0.333 | 0.001 | 0.565 | <0.0001 | 0.245 | 0.019 | 0.446 | <0.0001 | ||
| d18:1–C18:1 | 0.552 | <0.0001 | 0.559 | <0.0001 | 0.419 | <0.0001 | 0.281 | 0.007 | 0.535 | <0.0001 | 0.306 | 0.003 | 0.426 | <0.0001 | 0.214 | 0.042 |
| d18:1–C20:0 | 0.652 | <0.0001 | 0.642 | <0.0001 | 0.505 | <0.0001 | 0.358 | 0.001 | 0.627 | <0.0001 | 0.332 | 0.001 | 0.483 | <0.0001 | 0.208 | 0.048 |
| d18:1–C20:1 | 0.421 | <0.0001 | 0.456 | <0.0001 | 0.334 | 0.001 | 0.266 | 0.011 | 0.359 | <0.001 | 0.238 | 0.023 | ||||
| d18:1–C22:0 | 0.613 | <0.0001 | 0.611 | <0.0001 | 0.488 | <0.0001 | 0.354 | <0.001 | 0.605 | <0.0001 | 0.317 | 0.002 | 0.463 | <0.0001 | ||
| d18:1–C22:1 | 0.386 | <0.001 | 0.427 | <0.0001 | 0.319 | 0.002 | 0.255 | 0.015 | 0.353 | <0.001 | 0.239 | 0.023 | ||||
| d18:1–C24:0 | 0.297 | 0.004 | 0.305 | 0.003 | 0.239 | 0.023 | 0.249 | 0.017 | 0.294 | 0.005 | 0.273 | 0.009 | 0.282 | 0.007 | ||
| d18:1–C24:1 | 0.387 | <0.001 | 0.421 | <0.001 | 0.326 | 0.002 | 0.257 | 0.014 | 0.384 | <0.001 | ||||||
| Total sphinogmyelinsc | 0.595 | <0.0001 | 0.598 | <0.0001 | 0.462 | <0.0001 | 0.34 | 0.001 | 0.580 | <0.0001 | 0.29 | 0.005 | 0.443 | <0.0001 | 0.271 | 0.010 |
Assays conducted using Meso Scale Discovery (MSD) electrochemiluminescence.
Assays conducted using INNO-BIO AlzBio3 (xMAP).
Total refers to the sum of all carbon-chain lengths within the specific lipid class.
Fig. 1.
Correlations between CSF ceramide C18:0 and CSF levels of: A) AβX-38; B) AβX-40; C) AβX-42; D) Aβ1-42; E) tau; and F) p-tau181. In F (correlation between ceramide C18:0 and p-tau181), excluding the one outlier with p-tau181 level >121 had little effect on the results (excluding outlier: r = 0.068, p = 0.524).
Fig. 2.
Correlations between CSF total sphingomyelin and CSF levels of: A) AβX-38; B) AβX-40; C) AβX-42; D) Aβ1-42; E) tau; and F) p-tau181. In F (correlation between total sphingomyelins and p-tau181), excluding the one outlier with p-tau181 level >121 slightly reduced the significance of the results (excluding outlier: r = 0.270, p = 0.010).
3.2. Linear regression models and the effects of age and APOE genotype
Based on the above-described correlations, we focused on ceramide C18 in multivariate analyses because this compound was consistently associated with both CSF Aβ and tau. In contrast, since all sphingomyelins species were significantly associated with CSF Aβ and tau, linear regression models only examined total sphingomyelin levels. The associations between CSF ceramide C18:0 or sphingomyelins and CSF Aβ and tau remained in linear regression models controlling for age and APOE genotype (Table 3). Notably, the association between ceramide C18 and AβX-42 (b = 67.24, p = 0.031) and Aβ1-42 (b = 19.12, p = 0.071) were also stronger in the multivariate models.
Table 3.
Cross-sectional associations between CSF ceramides and sphingomyelins and CSF amyloid-beta and tau using multivariate linear regression
| All individuals (n = 91)a | Age <54 (n = 44) | Age ≥54 (n = 47) | No APOE E4 (n = 58) | APOE E4 (n = 33) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Log sphingolipid | b (SE) | p value | b (SE) | p value | b (SE) | p value | b (SE) | p value | b (SE) | p value | |
| Ceramide C 18:0 | ABX-38b | 302.75 (96.57) | 0.002 | 163.11 (158.45) | 0.309 | 418.91 (120.88) | 0.001 | 286.68 (103.30) | 0.008 | 358.88 (204.89) | 0.090 |
| ABX-40b | 983.20 (291.75) | 0.001 | 549.37 (474.95) | 0.254 | 1349.24 (365.49) | 0.001 | 868.16 (341.89) | 0.014 | 1298.58 (549.95) | 0.025 | |
| ABX-42b | 67.24 (30.58) | 0.031 | 21.21 (49.84) | 0.522 | 90.07 (38.76) | 0.025 | 66.01 (36.62) | 0.077 | 69.02 (57.31) | 0.238 | |
| AB1-42c | 19.12 (10.44) | 0.071 | 14.46 (14.37) | 0.320 | 21.19 (15.10) | 0.168 | 19.49 (11.88) | 0.107 | 18.47 (21.07) | 0.388 | |
| tauc | 13.44 (3.49) | <0.001 | 7.61 (5.41) | 0.167 | 18.18 (4.63) | <0.001 | 13.70 (4.03) | 0.001 | 12.77 (6.91) | 0.075 | |
| p-tau 181c | 2.80 (2.84) | 0.327 | 0.81 (4.77) | 0.867 | 4.38 (3.55) | 0.224 | 0.94 (2.92) | 0.750 | 6.37 (6.24) | 0.315 | |
| tau/AB1-42c | 0.03 (0.01) | 0.039 | 0.01 (0.01) | 0.244 | 0.04 (0.02) | 0.062 | 0.03 (0.01) | 0.025 | 0.03 (0.03) | 0.391 | |
| Total sphingomyelin | ABX-38b | 653.29 (128.14) | <0.001 | 3.89 (202.17) | 0.021 | 816.50 (158.79) | <0.001 | 450.22 (149.83) | 0.004 | 1152.83 (216.49) | <0.001 |
| ABX-40b | 2037.57 (387.32) | <0.001 | 1502.38 (605.20) | 0.017 | 2553.43 (481.13) | <0.001 | 1455.48 (492.0) | 0.005 | 3490.53 (548.05) | <0.001 | |
| ABX-42b | 173.49 (41.01) | <0.001 | 158.65 (62.64) | 0.015 | 184.46 (53.72) | 0.001 | 129.12 (52.42) | 0.017 | 266.29 (66.48) | <0.001 | |
| AB1-42c | 47.00 (14.40) | 0.002 | 45.39 (18.24) | 0.017 | 45.43 (21.68) | 0.042 | 35.79 (17.17) | 0.042 | 71.80 (26.93) | 0.012 | |
| tauc | 24.56 (4.73) | <0.001 | 18.69 (6.86) | 0.009 | 30.68 (6.44) | <0.001 | 18.22 (6.01) | 0.004 | 37.74 (7.58) | <0.001 | |
| p-tau181c | 10.85 (3.92) | 0.007 | 13.48 (6.06) | 0.032 | 9.79 (5.11) | 0.062 | 4.53 (4.24) | 0.290 | 22.48 (7.91) | 0.008 | |
| tau/AB1-42c | 0.05 (0.02) | 0.007 | 0.03 (0.01) | 0.053 | 0.08 (0.03) | 0.020 | 0.03 (0.02) | 0.068 | 0.09 (0.04) | 0.037 | |
Key: CSF, cerebrospinal fluid. SE, standard error.
Models control for age and APOE E4 allele; Models stratified by age control for APOE E4 genotype.
Assays conducted using Meso Scale Discovery (MSD) electrochemiluminescence.
Assays conducted using INNO-BIO AlzBio3 (xMAP).
In additional analyses, we separately stratified the above models by median age (≥54 vs <54 years) and the presence of an APOE E4 allele to further determine how these factors affected the above associations. Almost all relationships between CSF ceramide C18 or sphingomyelin and CSF Aβ and tau were stronger among individuals aged ≥54 compared to those less than 54 years (Table 3). While there were only 33 individuals with an APOE E4 allele, the associations between ceramide C18:0 and AβX38 and AβX40, and between total sphingomyelins and all measures were also stronger within this group compared to those without an E4 allele.
3.3. Relationship between CSF sphingolipids and cognitive performance
While all participants were cognitively normal, we did see some associations between CSF ceramides and cognitive performance, specifically in domains of memory performance. Using linear regression models and controlling for age, education, and APOE E4 genotype, higher ceramide C20:0 (b = −0.67, p = 0.034) and C22:0 (b = −0.69, p = 0.026) were associated with worse performance on the HVLT-delayed recall. Higher ceramide C26:0 (b = −1.32, p = 0.049) was also associated with worse performance on the Working Memory Composite Score. Examining Aβ and tau measures and controlling for the same variables, CSF tau was associated with better performance on Working Memory (b = 0.04, p = 0.042) while p-tau was associated with worse performance on the HVLT-immediate recall (b = −0.04, p = 0.047).
4. Discussion
In the present study, we found cross-sectional, positive associations between CSF ceramide C18:0 and total sphingomyelins and CSF Aβ and tau levels in a cohort of cognitively normal individuals aged 36–69 with a confirmed parental history of AD. These associations were stronger among individuals that were older than the median sample age (54 years) and among those with at least one APOE E4 allele. These results suggest in vivo associations between CSF sphingolipids and CSF Aβ and tau levels and, while cross-sectional, may suggest new pathways for the treatment or prevention of AD.
Cellular and animal studies have shown direct and indirect associations between sphingolipids and Aβ metabolism (Cutler, et al., 2004; Grimm, et al., 2005; Kalvodova, et al., 2005; Lee, et al., 2004; Mattson, et al., 2005; Puglielli, et al., 2003). Ceramides regulate Aβ(1-42) production by modulating the physical location of APP with beta-secretase, and stabilizing the activity of gamma-secretase (Kalvodova, et al., 2005; Puglielli, et al., 2003). Exposure of cultured neurons to Aβ(1-42) increases ceramide levels by activating sphingomyelinase (Grimm, et al., 2005; Lee, et al., 2004); blocking this ceramide increase protects neurons from Aβ-induced cell death (Cutler, et al., 2004). Lastly, Aβ(1-42) indirectly increases ceramides through an oxidative stress-mediated mechanism (Cutler, et al., 2004; Mattson, et al., 2005). Studies have also linked long-chain ceramides, specifically the 18-carbon acyl chain length species, to tau phosphorylation through modulation of PP2A activity (Chalfant, et al., 1999; Dobrowsky, et al., 1993; Goedert, et al., 1995; Gong, et al., 1994; Mukhopadhyay, et al., 2009). In this study, we extend these findings to humans to show in vivo correlations between CSF sphingolipids and CSF Aβ and tau measures. Notably, only ceramide C18:0 was associated with Aβ and tau levels, which is a direct in vivo translation of animal and cellular studies. However, further investigation is needed to determine why ceramide C18:0, the most abundant CSF ceramide species, was only associated with total tau and not phospho-tau.
An intriguing finding is that ceramide C18:0 was most strongly associated with Aβx-38 and Aβx-40. While the associations with Aβx-42 or Aβ1-42 were stronger among older individuals (>54 years) and those with an APOE E4 allele, most associations still did not reach statistical significance. These results suggest that the relationship between ceramides and Aβ may vary by Aβ isomer length. Alternatively, as these individuals are all cognitively normal and Aβ42 is the more toxic species of amyloid, the relationship between ceramide C18:0 and Aβ42 may strengthen with increasing pathology. Additional research is needed to replicate these findings and to longitudinally examine this association in persons with MCI.
Interestingly, while much basic science research, as described above, has focused on the associations between ceramides and Aβ and tau, we observed the strongest correlations between CSF sphingomyelins and CSF Aβ and tau. A previous study reported that sphingomyelin levels were elevated in prodromal AD and then decreased in mild to moderate AD (Kosicek, et al., 2012). However, the reason for the mechanism behind this sphingomyelin increase is not currently clear. As the authors of this study speculate (Kosicek, et al., 2012), it is possible that the increased sphingomyelin could be the result of a cellular response to initially increased ceramide. As ceramide can be catabolized to sphingomyelin, levels of this lipid would increase. An alternative explanation is that sphingomyelin is elevated as a result of brain atrophy and cellular degeneration since sphingomyelin is primarily a structural lipid located in membranes.
Previous clinical and epidemiological studies have reported associations of both CSF and plasma sphingolipids with AD severity. Two studies reported that CSF ceramide (Satoi, et al., 2005) and sphingomyelin levels varied by AD severity (Kosicek, et al., 2012), but they did not examine associations between these lipids and markers of AD pathology, including CSF Aβ or tau. High levels of plasma ceramides have also been associated with an increased risk of cognitive impairment and AD among cognitively normal individuals (Mielke, et al., 2010a; Mielke, et al., 2012), memory decline and hippocampal volume loss among patients with mild cognitive impairment (Mielke, et al., 2010b), and faster rates of cognitive decline among AD patients (Mielke, et al., 2011). A shotgun lipidomic approach also reported differences between plasma sphingolipids in AD patients and controls (Han, et al., 2011). This is one of the first studies to extend this research to examine associations between these sphingolipids and CSF biomarkers of AD. Examining the relationship between CSF sphingolipids and cognition, ceramides C20:0 and C22:0 were associated with worse performance on the HVLT-delayed recall and C26:0 with worse performance on the Working Memory Composite Score. There were also very few associations between Aβ and tau levels and cognitive performance. These limited associations might be expected in this group of relatively young (age 36–69) cognitively normal individuals. Notably, the associations between CSF sphingolipids and CSF Aβ and tau were stronger in individuals aged 54 years or older, at the time when AD pathology is thought to begin (Jack, et al., 2010), and also in APOE E4 carriers. This may suggest, in line with cellular studies, that sphingolipids are involved early in the etiopathogenesis of AD and may be viable therapeutic targets.
Serial measures of CSF and brain imaging are needed to better understand the temporality of these associations in humans. Similarly, back translational research in transgenic animals to understand the mechanism and timing by which CSF sphingomyelins and ceramide increase in relation amyloid is also critical. This research is particularly important to understand alterations in sphingolipids in the context of the current biomarker ordering of AD, with the hypothesis that amyloid is deposited first, followed by tau accumulation, neurodegeneration, and then cognitive symptoms (Jack, et al., 2013a). Although, recent research suggests that, among some individuals, neurodegeneration may appear prior to significant amyloid deposition (Jack, et al., 2013b).
Limitations of the study warrant consideration. First, the sample size was relatively small. However, despite this, we still detected significant associations between CSF sphingolipids and CSF Aβ and tau levels. Second, the sample consisted of cognitively normal individuals with `normal' levels of CSF Aβ and tau. While this is an advantage from the standpoint of understanding the earliest pathological changes, it is not known whether these correlations will be as robust in patients with MCI and AD. Such knowledge will help to determine whether CSF sphingolipids levels could be utilized as diagnostic or prognostic markers of disease severity. Third, the study was cross-sectional and cannot generate causal inferences. Longitudinal studies to assess the relationships between changes in CSF sphingolipids and changes in AD pathology are also needed to validate the pathological implications of these cross-sectional findings. Lastly, given the associations between plasma ceramides and cognitive decline across severities of AD (Mielke, et al., 2010a; Mielke, et al., 2012; Mielke, et al., 2010b), further research should be directed to elucidate the mechanistic relationship between plasma sphingolipids and CSF Aβ and tau.
Acknowledgements
This study was supported in part by the National Institutes of Health (grants AG026752, AG027161, 1UL1RR025011, AG033514, U01 AG037526, AA0017408, MH077542, MH075673, AG034849) and pilot funding from the University of Wisconsin, Department of Medicine.
Disclosure statement Dr. Mielke receives research support from the NIH/NIA and has served as a consultant for Eli Lilly. Dr. Haughey receives grant funding from NIH. Dr. Bandaru, Dr. Zetterberg, Dr. Andreasson, Dr. Gleason and Ms. Blazel have no disclosures to report. Dr. Johnson receives grant funding from NIH and the Department of Veterans Affairs. Dr. Puglielli receives grant funding from NIH and the Department of Veterans Affairs. Dr. Sager receives grant funding from NIH and the Helen Bader Foundation, Milwaukee, WI. Dr. Asthana receives grant funding from NIH and serves as a site PI for Alzheimer's disease treatment trials sponsored by Elan, Merck and Co., Inc., Pfizer, and Wyeth. Dr. Carlsson receives research support from the NIH/NIA, NIH/NINR, HRSA, and the University of Wisconsin, Department of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benton A, Hamsher K. Multilingual Aphasia Examination. AJA Associates; Iowa City, IA: 1989. [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5(2):125–142. [Google Scholar]
- Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J. Biol. Chem. 1999;274(29):20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- Contreras FX, Ernst AM, Haberkant P, Bjorkholm P, Lindahl E, Gonen B, Tischer C, Elofsson A, von Heijne G, Thiele C, Pepperkok R, Wieland F, Brugger B. Molecular recognition of a single sphingolipid species by a protein's transmembrane domain. Nature. 2012;481(7382):525–529. doi: 10.1038/nature10742. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2004;101(7):2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test: Professional Manual. Psychological Assessment Resources; Odessa, FL: 1996. [Google Scholar]
- Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem. 1993;268(21):15523–15530. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gatt S, Bierman EL. Sphingomyelin suppresses the binding and utilization of low density lipoproteins by skin fibroblasts. J. Biol. Chem. 1980;255(8):3371–3376. [PubMed] [Google Scholar]
- Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J. Neurochem. 1995;65(6):2804–2807. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- Gong CX, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer's disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience. 1994;61(4):765–772. doi: 10.1016/0306-4522(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Mattson MP. Ceramide protects hippocampal neurons against excitotoxic and oxidative insults, and amyloid beta-peptide toxicity. J. Neurochem. 1996;66(2):869–872. doi: 10.1046/j.1471-4159.1996.66020869.x. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat. Cell Biol. 2005;7(11):1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Han X, Rozen S, Boyle SH, Hellegers C, Cheng H, Burke JR, Welsh-Bohmer KA, Doraiswamy PM, Kaddurah-Daouk R. Metabolomics in early Alzheimer's disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE. 2011;6(7):e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim. Biophys. Acta. 2010;1801(8):878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann. Neurol. 2004;55(2):257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol. Aging. 2010;31(3):398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubley AM. Edgeworth Series in Quantitative Behavioural Science. University of Northern British Columbia, Prince George, B.C.; Canada: 1996. Modification of the Taylor Complex Figure: A comparable figure to the Rey-Osterrieth Figure? Paper No. ESQBS-96-7. [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013a;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurolology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, Mielke MM, Jones DT, Senjem ML, Gunter JL, Gregg BE, Pankratz VS, Petersen RC. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013b;81(20):1732–1740. doi: 10.1212/01.wnl.0000435556.21319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, Simons K. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J. Biol. Chem. 2005;280(44):36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- Kløve H, editor. Clinical Neuropsychology. Saunders; New York: 1963. [Google Scholar]
- Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J. Geriatr. Psychiatry Neurol. 1999;12(4):168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- Kosicek M, Zetterberg H, Andreasen N, Peter-Katalinic J, Hecimovic S. Elevated cerebrospinal fluid sphingomyelin levels in prodromal Alzheimer's disease. Neurosci. Lett. 2012;516(2):302–305. doi: 10.1016/j.neulet.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J. Cell Biol. 2004;164(1):123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec JF, Ivnik RJ, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Visual Spatial Learning Test: Normative data and further validation. Psychol Assess. 1997;4(4):433–441. [Google Scholar]
- Mattson MP, Cutler RG, Jo DG. Alzheimer peptides perturb lipid-regulating enzymes. Nat. Cell Biol. 2005;7(11):1045–1047. doi: 10.1038/ncb1105-1045. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol. Aging. 2010a;31(1):17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VVR, Xia J, Haughey NJ, Fried LP, Yasar S, Albert M, Varma V, Harris G, Schneider EG, Rabins PV, Bandeen-Roche K, Lyketsos CG, Carlson MC. Serum ceramides increase the risk of Alzheimer disease: the Women's Health and Aging Study II. Neurology. 2012;79:633–641. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ. Could plasma sphingolipids be diagnostic or prognostic biomarkers for Alzheimer's disease? Clin. Lipidol. 2012;7(5):525–536. doi: 10.2217/clp.12.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ, Bandaru VV, Weinberg DD, Darby E, Zaidi N, Pavlik V, Doody RS, Lyketsos CG. Plasma sphingomyelins are associated with cognitive progression in Alzheimer's disease. J. Alzheimers Dis. 2011;27(2):259–269. doi: 10.3233/JAD-2011-110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ, Ratnam Bandaru VV, Schech S, Carrick R, Carlson MC, Mori S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimer's Dement. 2010b;6(5):378–385. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnold HK, Sears RC, Hannun YA, Ogretmen B. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23(3):751–763. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, Rosengren L, Vanmechelen E, Blennow K. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin. Chem. 2005;51(2):336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin. Lab. 2002;48(3–4):171–180. [PubMed] [Google Scholar]
- Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J. Biol. Chem. 2003;278(22):19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- Quinn PJ, Wolf C. The liquid-ordered phase in membranes. Biochim. Biophys. Acta. 2009;1788(1):33–46. doi: 10.1016/j.bbamem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Sperling RA, Dickerson B, Stern CE, Tirado V, Munoz C, Reiman RA, Huentelman MJ, Alexander GE, Langbaum JB, Kosik KS, Tariot PN, Lopera F. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11(12):1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM, Evans RW, Light RH. Automatic detection vs controlled search: a paper-and-pencil approach. Percept. Mot. Skills. 1986;62(2):407–416. doi: 10.2466/pms.1986.62.2.407. [DOI] [PubMed] [Google Scholar]
- Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J. Geriatr. Psychiatry Neurol. 2005;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, Oka N, Akiguchi I, Furuya S, Hirabayashi Y, Okazaki T. Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130(3):657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- Slotte JP, Bierman EL. Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem. J. 1988;250(3):653–658. doi: 10.1042/bj2500653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. Second edition Oxford University Press; New York: 1998. [Google Scholar]
- Tamboli IY, Hampel H, Tien NT, Tolksdorf K, Breiden B, Mathews PM, Saftig P, Sandhoff K, Walter J. Sphingolipid storage affects autophagic metabolism of the amyloid precursor protein and promotes Abeta generation. J. Neurosci. 2011;31(5):1837–1849. doi: 10.1523/JNEUROSCI.2954-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Wechsler Adult Intelligence Scale-III. Third edition Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-III. Third Edition Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]