Abstract
Objective:
To evaluate the role of diffusion-weighted MRI (DW-MRI) as an imaging biomarker for upper urinary tract cancer (UUTC) that has already metastasized or will metastasize soon.
Methods:
61 patients clinically diagnosed with UUTC were prospectively enrolled in this study. All the patients underwent MRI, including DW-MRI, prior to any interventions. Correlations between apparent diffusion coefficient (ADC) and other clinicopathological variables, including metastasis-free survival, were analysed.
Results:
Median follow-up period was 938 days. Of the 61 patients, 12 had any metastases at the initial diagnosis. 11 patients developed metastases during the follow-up period. These 23 patients were categorized as “Metastatic”. Of the remaining 38 patients, 35 with a follow-up period longer than 400 days were categorized as “Localized”. ADC was significantly lower in the Metastatic category than in the Localized (p = 0.0002) category. Multivariate analysis of pre-operative variables identified ADC (cut-off value, 1.08 × 10−3 mm2 s−1) and clinical T stage based on T2 weighted MRI as an independent predictive factor of metastatic UUTC. 46 patients without any metastases during the initial diagnosis were stratified into a high-risk group (16 patients with low ADC and clinical T3–4) and a low-risk group (30 patients with high ADC or clinical Ta-2). The 3-year metastasis-free survivals were 45% and 93%, respectively.
Conclusion:
In the current study, UUTC with lower ADC value is more likely to have metastatic potential. Incorporating ADC with clinical T stage helps to differentiate metastatic UUTC at the initial diagnosis.
Advances in knowledge:
DW-MRI is a potential imaging biomarker reflecting metastatic propensity of UUTC.
Upper urinary tract cancer (UUTC) is a potentially lethal disease. The prognosis remains poor even when standard care, radical nephroureterectomy (RNU) is performed, and almost one-third of the patients die within 5 years.1–3 In the management of localized UUTC, adjuvant chemotherapy has no impact on survival, particularly owing to the impaired post-surgical renal function or comorbidity.4 However, neoadjuvant chemotherapy, which showed a survival benefit in bladder cancer,5 may have a similar benefit in UUTC.
Neoadjuvant chemotherapy can be considered an option for locally advanced disease at diagnosis. Two nomograms are available for predicting locally advanced UUTC in the pre-operative setting: one includes tumour histological grade, architecture and location and the other includes histological grade and radiological clinical stage.6,7 “Localized disease” at the initial diagnosis that will develop metastasis soon after RNU can also be a candidate for neoadjuvant chemotherapy. However, identifying these occult or developing metastases pre-operatively remains a challenge.
Diffusion-weighted MRI (DW-MRI) is a functional imaging technique that reveals physiological information by quantifying the diffusion of water molecules in tissues.8 The extent of water diffusion is quantified as the apparent diffusion coefficient (ADC). In 2009, a consensus meeting was held on the use of DW-MRI as a cancer imaging biomarker.9 An extraordinary opportunity for DW-MRI to evolve into a clinically valuable imaging tool was indicated. This imaging technique has been incorporated into general oncological imaging practices, including tissue characterization, monitoring the treatment response and predicting treatment outcome, in various cancers.8,10–14
Previous studies demonstrated the role of the ADC as a marker for the biological aggressiveness of UUTC by showing a correlation of the ADC with the histological grade and the Ki-67 labelling index.14,15 Furthermore, the ADC was significantly associated with the cancer-specific survival after RNU.15 Therefore, we hypothesized that the ADC can be used as a marker to reflect the metastatic potential of UUTC, as has been reported in bladder cancer.16 The aim of this study is to show that the ADC can predict UUTC that has already metastasized or will metastasize soon. We first evaluated ADC values of the biologically metastatic UUTC and non-metastatic UUTC. Secondarily, we analysed the potential of the ADC to predict the development of metastasis.
METHODS AND MATERIALS
Patients
At our single academic centre in Tokyo between January 2007 and February 2012, 123 consecutive Japanese patients suspected of having UUTC on the basis of clinical information, including urinary cytology, ultrasound and/or CT images, were enrolled in an institutional prospective study to evaluate the clinical role of DW-MRI for management of UUTC. Multisequence MRI, including DW-MRI, was performed prior to any interventions that can affect the findings of DW-MRI, such as ureteroscopy and RNU. Of the 123 patients, 68 were diagnosed with UUTC. Of the 68 patients who were diagnosed clinically with UUTC, only those with lesions detectable on DW-MRI were eligible for the current analysis, and we excluded 4 patients diagnosed with dominant CIS. Three patients who were lost to follow-up and did not undergo CT or MRI soon after the diagnosis were also excluded from this analysis. The remaining 61 patients were eligible for this analysis.
After the surgery, patients were subsequently followed up every 3 months over the first 2 years, every 6 months over the next 3 years and annually thereafter. Routine assessments included blood counts, blood chemistry, urinalysis, urine cytology, cystoscopy and CT of the chest, abdomen and pelvis. An institutional review board of Tokyo Medical and Dental University, Tokyo, Japan approved this study, and we obtained written informed consent from all patients.
MRI technique
Multisequence MRI was carried out by using a 1.5-T MR imager (Intera Achieva; Philips, Best, Netherlands) under free-breathing conditions with a four-channel sensitivity encoding body coil. The maximum gradient strength was 33 mT m−1, and the slew rate was 160 T m−1 s−1. The imaging parameters for DW-MRI with a single shot spin-echo planar imaging sequence were set as follows: repetition time, 5000 ms; echo time, 65 ms; matrix, 128 × 126/256 × 256 zip; field of view, 35 cm; slice thickness, 5 mm; interslice gap, 0.5 mm; slice number, 24; number of excitations, 8; bandwidth, 1880 Hz per pixel; 3 different diffusion gradient b-values (b = 0, 400 or 800 s mm−2); number of motion probing direction gradients, 3; number of averages, 9; fat suppression, spectral-attenuated inversion recovery; and total acquisition time, approximately 3 min 40 s. The MRI protocols in this study consisted of routine T1 weighted and T2 weighted (T2W) MRI and DW-MRI.
Image analysis
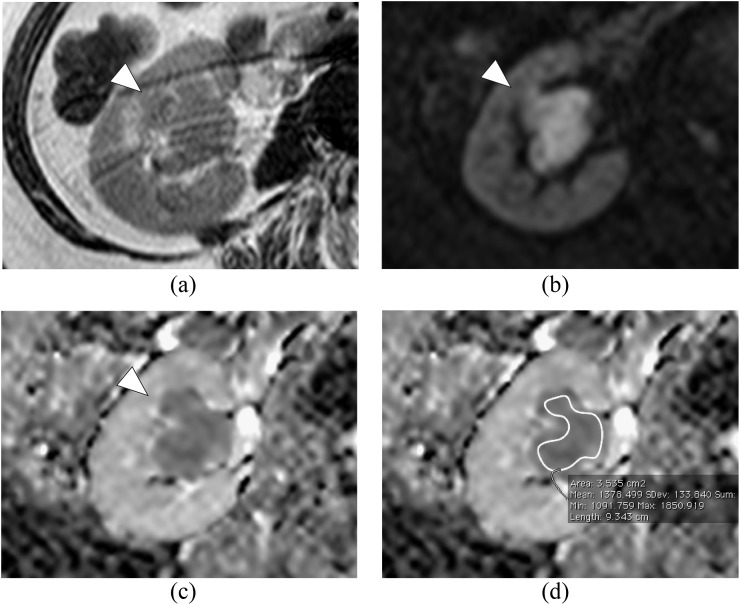
In the current study, two radiologists (each with 7 years' experience in reading abdominal DW-MRI data), blinded to clinical symptoms and the results of urinary cytology and other forms of imaging data, independently interpreted imaging data on a workstation (Doctor PACS v. 3, Doctor Net). In cases of discrepancy in diagnosis, a consensus was made. Then, one urologist with 13 years' experience in treating UUTC and 7 years' experience in reading DW-MRI data of UUTC, who was given the radiological diagnoses made by the two radiologists, positioned a region of interest (ROI) and calculated the ADC in all the cases. In patients with multiple tumours, the ADC value of an index lesion with the highest clinical stage was set as the representative value. The ADC value was calculated by using the following formula: ADC = −ln (S/S0)/(b−b0), where S0 and S are the signal intensities obtained with two different diffusion gradient values (b0 and b: 400 and 800 s mm−2, respectively). ADC maps were reconstructed at a workstation (View Forum v. R4.1; Philips Healthcare), and the ADC of the lesion corresponding to the tumour was calculated to quantitatively analyse the degree of diffusion. A ROI was positioned so that it did not extend over the tumour on the transverse ADC map at the slice that showed maximal tumour diameter (Figure 1). The ADC of each pixel in the ROI was quantified. In this study, the ADC of a tumour represented the mean ADC within the ROI.
Figure 1.
Right renal pelvic cancer in a 76-year-old male who was categorized into “Localized disease”. (a) Axial T2 weighted image shows renal pelvic cancer suspected of renal parenchyma invasion (arrowhead). (b) Axial diffusion-weighted image with b-value of 800 s mm−2 shows an area of high intensity. (c) Apparent diffusion coefficient (ADC) map shows restricted diffusion of the tumour. (d) Region of interest (ROI) was positioned as shown. The mean ADC value within the ROI is 1.39 × 10−3 mm2 s−1. Max, maximum, Min, minimum; SDev, standard deviation.
T staging and histological grading
The T stage was determined according to the 2002 TNM system. For the clinical T stage, UUTCs were classified into two groups based on the findings of T2W-MRI: organ confined (clinical Ta-2) and locally advanced (clinical T3–4). Tumours that were radiologically confined within the muscle layers of the UUTC were classified as clinical Ta-2, and those invading beyond the muscle layers into the peripelvic fat, periureteric fat, renal parenchyma, perinephric fat and adjacent organs were classified as clinical T3–4. Tumour histological grading was evaluated according to the 1973 World Health Organization classification.17
Reference standard
The final diagnoses were established based on pathological reports when patients underwent nephroureterectomy (NU). Otherwise, the final diagnoses were made on the basis of serial imaging tests and urinary cytology findings under at least 1-year follow-up.
Statistical analysis
Differences between groups were assessed by the Wilcoxon test for continuous variables and the Fisher's exact test for nominal variables. The optimal threshold ADC for detecting metastatic UUTC was determined by a partition analysis using JMP v. 10.0 (SAS Institute Inc., Cary, NC). Metastasis-free survival was analysed with the Kaplan–Meier method and the log-rank test. Associations between the variables and the metastatic potential were evaluated by logistic regression analysis. Age was divided into two groups at the median age of the patients. The period of metastasis-free survival was defined as the time from the diagnosis of localized UUTC to that of metastasis. All p-values were two sided and were considered statistically significant when <0.05.
RESULTS
Patient and tumour characteristics
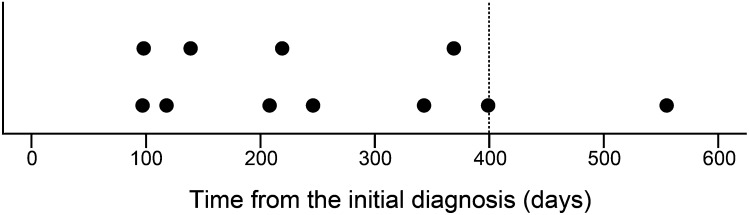
At the initial diagnosis, 12 patients had metastases to the lymph nodes or other organs, and these patients were categorized as “Macroscopic metastasis”. 49 patients had localized cancer without any visible metastases at the initial diagnosis, and all of these patients underwent NU. Of these 49 patients, we categorized 11 patients whose metastases appeared during the follow-up period as “Microscopic metastasis”. The remaining 38 patients did not develop metastasis during the follow-up period. As 91% (10/11) of Microscopic metastasis developed within 400 days (Figure 2), we hypothesized that the primary tumours in 35 of the remaining 38 patients, whose follow-up period was longer than 400 days, did not have metastatic potential, whereas the primary tumours in Macroscopic/Microscopic metastasis had metastatic potential. Therefore, we categorized these 35 patients as Localized disease. Three patients, whose follow-up period was shorter than 400 days, were omitted from the present analysis. The characteristics of 58 patients are described in Table 1. Their median follow-up period was 938 days (84–2150 days). Of these 58 patients, 23 (40%) and 35 (60%) patients were diagnosed with clinical Ta-2 and clinical T3–4 disease, respectively. Of the 50 patients who underwent surgical treatment, 21 (42%) and 29 (58%) patients were diagnosed with pathological Ta-2 and pathological T3–4 disease, respectively. Of the 29 patients with pathological T3–4 diseases, 7 patients were understaged as having not more than clinical T2 disease. On the other hand, 6 of the 21 patients with pathological Ta-2 were overstaged as having clinical T3 disease or greater. The concordance between clinical and pathological T staging was fair (κ = 0.43). Histopathological diagnoses were UC in all the patients with the exception of one patient with a large cell neuroendocrine carcinoma. The remaining eight patients who did not undergo surgical treatment were diagnosed with UUTC based on the imaging and urinary cytology findings. The locations of the metastases in Macroscopic metastasis and Microscopic metastasis cases are described in Table 2.
Figure 2.
Plot of time from the initial diagnosis to recurrence revealed in 11 patients of “Microscopic metastasis”.
Table 1.
Patient and tumour characteristics of 58 upper urinary tract cancer patients
| Variable | Number (%) |
|---|---|
| Age (years)a | 70 (47–84) |
| Sex | |
| Female | 18 (31) |
| Male | 40 (69) |
| Clinical T stage | |
| Ta-2 | 23 (40) |
| T3–4 | 35 (60) |
| Tumour status | |
| Localized disease | 35 (60) |
| Macroscopic metastasis | 12 (21) |
| Microscopic metastasis | 11 (19) |
| Pathological T stage | |
| Ta-2 | 21 (42) |
| T3–4 | 29 (58) |
| Histological grade | |
| Grade 1–2 | 26 (52) |
| Grade 3 | 24 (48) |
| Apparent diffusion coefficient value (×10−3 mm2 s−1)a | 0.94 (0.65–1.58) |
Continuous variables are expressed as median (range). Pathological T stage and histological grade are revealed only in patients who underwent curative surgeries, and the total number of patients described at the variables is 50.
Table 2.
Locations of metastases
| Location | Macroscopic metastasis | Microscopic metastasis |
|---|---|---|
| Regional lymph node | 11 | 3 |
| Juxta-regional lymph node | 2 | 0 |
| Lung | 2 | 5 |
| Liver | 2 | 1 |
| Bone | 1 | 1 |
| Others | 0 | 2a |
Multiple metastases in one identical patient are counted independently.
Subcutaneous metastasis and intrapelvic metastasis, respectively.
Characteristics of the apparent diffusion coefficient in upper urinary tract cancer
The ADC of UUTC ranged from 0.65 to 1.58 × 10−3 mm2 s−1 (median, 0.94 × 10−3 mm2 s−1). We evaluated the association between the ADC and the characteristics of UUTC. The clinical T stage, pathological T stage and the histological grade were related to the ADC, whereas the ADC was not significantly different according to age and sex (Table 3). The ADC was lower in clinical T3–4 and pathological T3–4 cancers than in clinical Ta-2 and pathological Ta-2 cancers, respectively (p = 0.030 and 0.040, respectively). Similarly, grade 3 cancers had a significantly lower ADC compared with grade 1 or 2 cancers (p = 0.009). The ADC of Macroscopic/Microscopic metastasis was significantly lower than that of Localized disease (median, 0.85 × 10−3 mm2 s−1 vs 1.14 × 10−3 mm2 s−1; p = 0.0002), whereas no significant difference was found between Macroscopic metastasis and Microscopic metastasis (median, 0.79 × 10−3 mm2 s−1 vs 0.86×10−3 mm2 s−1).
Table 3.
The apparent diffusion coefficient (ADC) according to clinicopathological characteristics in 58 upper urinary tract cancer patients
| Variable | Number | ADC value (range) | p-value |
|---|---|---|---|
| Age (years) | |||
| <70 | 28 | 0.99 (0.72–1.58) | 0.0950 |
| ≥70 | 30 | 0.86 (0.65–1.55) | |
| Sex | |||
| Female | 18 | 1.00 (0.69–1.55) | 0.1200 |
| Male | 40 | 0.87 (0.65–1.58) | |
| Clinical T stage | |||
| Ta-2 | 23 | 1.08 (0.68–1.58) | 0.0300 |
| T3–4 | 35 | 0.87 (0.65–1.36) | |
| Pathological T stage | |||
| Ta-2 | 21 | 1.11 (0.68–1.58) | 0.0400 |
| T3–4 | 29 | 0.87 (0.65–1.41) | |
| Histological grade | |||
| Grade 1–2 | 26 | 1.10 (0.71–1.58) | 0.0090 |
| Grade 3 | 24 | 0.86 (0.65–1.41) | |
| Tumour status 1 | |||
| Localized disease | 35 | 1.14 (0.68–1.58) | 0.0002 |
| Metastatic disease | 23 | 0.85 (0.65–1.30) | |
| Tumour status 2 | |||
| Macroscopic metastasis | 12 | 0.79 (0.66–1.30) | 0.6900 |
| Microscopic metastasis | 11 | 0.86 (0.65–1.05) | |
ADC value is expressed as median. Pathological T stage and histological grade are revealed only in patients who underwent curative surgeries, and the total number of patients described at the variables is 50.
Apparent diffusion coefficient as an indicator of the metastatic potential
Next, we evaluated the ability of the ADC to evaluate metastatic potential of UUTC. Partition analysis determined that the best cut-off value of the ADC for differentiating these 35 patients from Macroscopic/Microscopic metastasis was 1.08 × 10−3 mm2 s−1. Using this cut-off value, 20 (34%) and 38 (66%) patients were categorized into the high ADC group and the low ADC group, respectively. The patient and tumour characteristics of each group are shown in Table 4. Of the 20 patients in the high ADC group, 1 (5%) was categorized as Macroscopic metastasis and the remaining 19 (95%) were categorized as Localized disease. In the low ADC group, 11 (29%)/11 (29%)/16 (42%) were Macroscopic metastasis/Microscopic metastasis/Localized disease, respectively.
Table 4.
Patient and tumour characteristics categorized by apparent diffusion coefficient (ADC) of 58 upper urinary tract cancer patients
| Variable | High ADC group | Low ADC group | p-value |
|---|---|---|---|
| Age (years) | 66 (47–76) | 70 (58–84) | 0.031 |
| Sex | |||
| Female | 8 (14%) | 10 (17%) | 0.372 |
| Male | 12 (21%) | 28 (48%) | |
| Clinical T stage | |||
| Ta-2 | 12 (21%) | 11 (19%) | 0.027 |
| T3–4 | 8 (14%) | 27 (47%) | |
| Pathological T stage | |||
| Ta-2 | 11 (22%) | 10 (20%) | 0.087 |
| T3–4 | 8 (16%) | 21 (42%) | |
| Histological grade | |||
| Grade 1–2 | 13 (26%) | 13 (26%) | 0.087 |
| Grade 3 | 6 (12%) | 18 (36%) | |
Age is expressed as median (range) and categorical variables are expressed as number (%). Pathological T stage and histological grade are revealed only in patients who underwent curative surgeries, and the total number of patients described at the variables is 50.
Among the pre-operative variables, including age, sex, clinical T stage and ADC, the clinical T stage and the ADC were identified as significant predictive factors of the metastatic potential (p < 0.001 and p = 0.003, respectively; Table 5). Multivariate analysis revealed that a low ADC (<1.08 × 10−3 mm2 s−1) and a clinical T3–4 grade were independent and significant predictive factors of the metastatic potential with odds ratios of 23.2 (p = 0.005) and 14.0 (p = 0.003), respectively (Table 5).
Table 5.
Univariate and multivariate analysis of variables predicting upper urinary tract cancer that has already metastasized or will metastasize soon
| Variable | Univariate | Multivariate |
|
|---|---|---|---|
| p-value | Odds ratio | p-value | |
| Age (years) | |||
| ≥70 vs <70 | 0.260 | – | – |
| Sex | |||
| Male vs female | 0.076 | – | – |
| Clinical T stage | |||
| T3–4 vs Ta-2 | <0.001 | 14.0 | 0.003 |
| Apparent diffusion coefficient value | |||
| Low vs high | 0.003 | 23.2 | 0.005 |
Apparent diffusion coefficent as a predictor for metastasis development
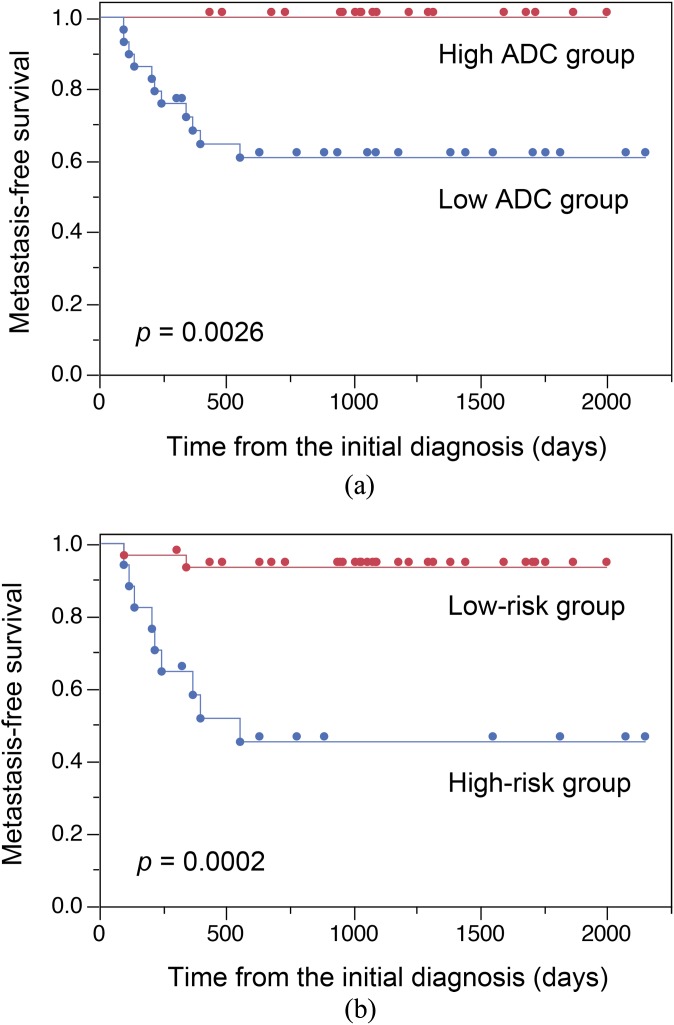
In 46 patients of Microscopic metastasis/Localized disease, 27 have low ADC and 19 have high ADC. Low ADC discriminated Microscopic metastasis from Localized disease with a sensitivity of 40%, a specificity of 100% and an accuracy of 65%. As shown in Figure 3a, the metastasis-free survival was significantly shorter in patients of low ADC than those of high ADC (p = 0.0026; the 3-year metastasis-free survival rate, 61% vs 100%). Then, we investigated whether incorporating the clinical T stage with the ADC improved the predictability of the metastasis-free survival. We defined 16 patients staged as clinical T3–4 disease in the low ADC group as the high-risk group and the remaining 30 patients as the low-risk group. Categorization of the high-risk group discriminated Microscopic metastasis from Localized disease with a sensitivity of 60%, a specificity of 86% and an accuracy of 80%. As shown in Figure 3b, patients in the high-risk group had a significantly shorter metastasis-free survival than those in the low-risk group (p = 0.0002; the 3-year metastasis-free survival of 45% vs 93%).
Figure 3.
(a) Kaplan–Meier analysis shows comparison of metastasis-free survival between high apparent diffusion coefficient (ADC) group (upper line) and low ADC group (lower line). (b) Kaplan–Meier analysis shows comparison of metastasis-free survival between low-risk group (upper line) and high-risk group (lower line).
DISCUSSION
In the current study, we performed two analyses. First, we tried to discriminate the biologically metastatic UUTC from the non-metastatic UUTC. As we considered UUTC that have metastasis at the initial diagnosis or develop metastasis during follow-up have biologically metastatic potential, we evaluated the difference of ADC values between Macroscopic/Microscopic metastasis and Localized disease. As a result, there was significant difference between Localized disease and Macroscopic/Microscopic metastasis. However, interestingly, we could not find any significant difference between Macroscopic metastasis and Microscopic metastasis. Therefore, we concluded that the ADC value of the UUTC with biologically metastatic potential is lower than that of the UUTC without the potential. This result encouraged us to conduct a second analysis to evaluate the potential of the ADC in predicting development of metastasis from the localized UUTC at the initial diagnosis.
The excellent diagnostic performance of DW-MRI for urothelial cancer has been reported since 2007,18 and recent evidence has emerged showing its potential as an imaging biomarker.9 However, the underlying mechanism, especially for metastatic potential, is not fully understood. Classically, the restricted diffusion in malignant lesions has been attributed to their high cellularity, tissue disorganization and decreased extracellular space.8 In view of the feasibility of the ADC as a biomarker for malignant cancer cells, the DW-MRI signal potentially has a biological implication with regard to the characteristics of cancer cells.
The clinical benefit of neoadjuvant chemotherapy for localized UUTC has not been confirmed.4 However, given the higher metastatic potential and the unfavourable prognosis of UUTC exhibiting lower ADC, the application of neoadjuvant chemotherapy may be a treatment option for localized UUTC with lower ADC value.15 The association between a higher Ki-67 level and favourable chemosensitivity in breast cancer19,20 and chemoradiosensitivity in bladder cancer13 and the inverse correlation between the Ki-67 level and the ADC13,21,22 suggests that UUTC with lower ADC value would show a favourable response to chemotherapy; however, we have no evidence to confirm this postulation.
Incorporating the morphological information assessed by T2W-MRI into the DW-MRI information may further improve the ability to stratify potentially metastatic disease. DW-MRI can be readily obtained with a routine MRI examination for most current clinical MRI scanners without any additional equipment, contrast enhancement or interventions. Therefore, the addition of DW-MRI to a routine MRI study may allow us to make qualitative and morphological evaluations in a non-invasive manner for the pre-operative risk assessment of developing metastatic disease.
The limitations of the current study include the small sample size and the short follow-up period of the cohort. However, the subjects of this analysis were prospectively enrolled to evaluate the role of DW-MRI in the diagnosis and management of UUTC. Although we set the best cut-off ADC value to identify the disease having metastatic potential in patients with a longer follow-up period, we cannot rule out the possibility that a case developing a late metastasis is included in the Localized disease. In addition, we set the best cut-off ADC value optimized for our cohort. Analysis of external validation data is needed to confirm our results. Another limitation of this study is the lack of standardization in the ADC management. An intrinsic issue of the ADC measurement is that the ADC depends on imaging conditions, which include MRI systems and protocols that differ between institutions. Thus, standardization of the imaging conditions is required in order to eliminate interinstitutional disparity. Moreover, inter- and intraobserver agreement of the ADC also needs to be evaluated. To confirm, reproducibility of the ADC is required, as indicated in the previous report.9
In conclusion, the present study demonstrated that UUTC exhibiting lower ADC value has a high risk of developing metastasis. Thus, the DW-MRI may be used as a useful adjunct to assess the metastatic potential of UUTC, although confirmatory studies are mandatory.
REFERENCES
- 1.Abouassaly R, Alibhai SM, Shah N, Timilshina N, Fleshner N, Finelli A. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology 2010; 76: 895–901. doi: 10.1016/j.urology.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 2.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology 1998; 52: 594–601. [DOI] [PubMed] [Google Scholar]
- 3.Olgac S, Mazumdar M, Dalbagni G, Reuter VE. Urothelial carcinoma of the renal pelvis: a clinicopathologic study of 130 cases. Am J Surg Pathol 2004; 28: 1545–52. [DOI] [PubMed] [Google Scholar]
- 4.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester R, Burger M, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 2013; 63: 1059–71. [DOI] [PubMed] [Google Scholar]
- 5.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349: 859–66. doi: 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 6.Favaretto RL, Shariat SF, Savage C, Godoy G, Chade DC, Kaag M, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int 2012; 109: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margulis V, Youssef RF, Karakiewicz PI, Lotan Y, Wood CG, Zigeuner R, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol 2010; 184: 453–8. [DOI] [PubMed] [Google Scholar]
- 8.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007; 188: 1622–35. doi: 10.2214/AJR.06.1403 [DOI] [PubMed] [Google Scholar]
- 9.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009; 11: 102–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akita H, Jinzaki M, Kikuchi E, Sugiura H, Akita A, Mikami S, et al. Preoperative T categorization and prediction of histopathologic grading of urothelial carcinoma in renal pelvis using diffusion-weighted MRI. AJR Am J Roentgenol 2011; 197: 1130–6. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Koga F, Yoshida S, Masuda H, Ishii C, Tanaka H, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol 2011; 21: 2178–86. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S, Koga F, Kawakami S, Ishii C, Tanaka H, Numao N, et al. Initial experience of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology 2010; 75: 387–91. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida S, Koga F, Kobayashi S, Ishii C, Tanaka H, Tanaka H, et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys 2012; 83: e21–7. doi: 10.1016/j.ijrobp.2011.11.065 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S, Masuda H, Ishii C, Tanaka H, Fujii Y, Kawakami S, et al. Usefulness of diffusion-weighted MRI in diagnosis of upper urinary tract cancer. AJR Am J Roentgenol 2011; 196: 110–16. doi: 10.2214/AJR.10.4632 [DOI] [PubMed] [Google Scholar]
- 15.Yoshida S, Kobayashi S, Koga F, Ishioka J, Ishii C, Tanaka H, et al. Apparent diffusion coefficient as a prognostic biomarker of upper urinary tract cancer: a preliminary report. Eur Radiol 2013; 23: 2206–14. doi: 10.1007/s00330-013-2805-2 [DOI] [PubMed] [Google Scholar]
- 16.Rosenkrantz AB, Mussi TC, Spieler B, Melamed J, Taneja SS, Huang WC. High-grade bladder cancer: association of the apparent diffusion coefficient with metastatic disease: preliminary results. J Magn Reson Imaging 2012; 35: 1478–83. [DOI] [PubMed] [Google Scholar]
- 17.Mostofi FK, Sobin LH, Torloni H. Histological typing of urinary bladder tumours. Geneva Switzerland: World Health Organisation; 1973. [Google Scholar]
- 18.Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol 2007; 17: 201–4. doi: 10.1007/s00330-006-0281-7 [DOI] [PubMed] [Google Scholar]
- 19.Liedtke C, Packeisen J, Hess KR, Vogt U, Kiesel L, Kersting C, et al. Systematic analysis of in vitro chemosensitivity and mib-1 expression in molecular breast cancer subtypes. Eur J Cancer 2012; 48: 2066–74. doi: 10.1016/j.ejca.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 20.Nishimura R, Osako T, Okumura Y, Hayashi M, Arima N. Clinical significance of Ki-67 in neoadjuvant chemotherapy for primary breast cancer as a predictor for chemosensitivity and for prognosis. Breast cancer 2010; 17: 269–75. doi: 10.1007/s12282-009-0161-5 [DOI] [PubMed] [Google Scholar]
- 21.Bae H, Yoshida S, Matsuoka Y, Nakajima H, Ito E, Tanaka H, et al. Apparent diffusion coefficient value as a biomarker reflecting morphological and biological features of prostate cancer. Int Urol Nephrol 2014; 46: 555–61. doi: 10.1007/s11255-013-0557-1 [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi S, Koga F, Kajino K, Yoshida S, Ishii C, Tanaka H, et al. Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging 2014; 39: 172–8. doi: 10.1002/jmri.24148 [DOI] [PubMed] [Google Scholar]