Abstract
Health care–associated viral respiratory infections, common among hospitalized children, also occur among adults and institutionalized persons and result in increased patient morbidity, mortality, and health care costs. Approximately 20% of patients with health care–associated pneumonia have viral respiratory infections, with 70% of these infections caused by adenovirus, influenza virus, parainfluenza virus, and respiratory syncytial virus (RSV).1 These infections typically reflect the level of viral activity within the community.1,2 This article focuses on the epidemiology, transmission, and control of health care–associated RSV and influenza virus.
RSV
Epidemiology
RSV is the most common cause of pneumonia and bronchiolitis in infants3 and is a common pathogen in older and high-risk adults.4 Outbreaks of RSV have occurred in a variety of pediatric and adult health care settings.5–12 Secondary attack rates of 19% to 45% have been reported among patients when limited or no infection control measures are implemented.6,7,13 Similarly, 34% to 56% of personnel on infant wards may become infected.6,7,13 Most infected personnel are symptomatic, and many may be absent from work.14 However, many symptomatic personnel continue to work, and asymptomatic shedding of RSV occurs in 15% to 20% of infected personnel.14 Therefore, these infected personnel may play a role in transmission to hospitalized patients.7,15
Transmission
Transmission of RSV occurs via inoculation of the eye and nose16 and through close contact via direct inoculation of large droplets or self-inoculation after touching contaminated fomites.17 RSV has been recovered on countertops for up to 6 hours, rubber gloves for up to 2 hours, and on cloth gowns and hands for 15 to 60 minutes after contamination with infected nasal secretions.18 The duration of viral shedding among hospitalized infants averages 6.7 days but can be as long as 21 days.19 Infants with a lower respiratory tract disease and a compromised immune status have more prolonged shedding and shed greater quantities of the virus.19 Finally, because neonates may have atypical illness, the disease may be overlooked, thus facilitating transmission.7
Prevention and Control
Numerous studies have evaluated the effectiveness of various measures to prevent RSV transmission among patients and personnel. Studies evaluating the use of gowns and masks to prevent RSV transmission have shown mixed results. In a before-after design, the rate of health care–associated RSV infection among infants during the period when gowns and masks were routinely worn by staff was not statistically different from the rate during the period when gowns and masks were not used (32% vs 41%).20 A second prospective randomized study failed to show that the use of gowns and masks prevented respiratory illness among personnel.21 One possibility for the apparent ineffectiveness of gowns and masks to prevent health care–associated RSV transmission in earlier studies is the lack of adherence to the use of personal protective equipment (PPE) among staff. In another study, as compliance with the use of gowns and gloves increased from 39% to 95%, the incidence of health care–associated RSV decreased from 6.4 to 3.1 per 1000 patient-days.22 However, others have expressed concerns that gowns and gloves may facilitate transmission by serving as fomites, particularly given the prolonged survival of RSV on rubber gloves compared to skin.18 One study of 7 Canadian pediatric hospitals actually noted an increased risk of transmission with the use of gowns, thought to be because of the decreased adherence to other infection control measures related to the overuse of gowns.23
Another possibility for the lack of benefit from gowns and masks in RSV transmission may be the failure to protect against the eye as a portal of entry. Two studies suggested that wearing eye protection is beneficial.24,25 In a before-after study, staff wore disposable eye-nose goggles during routine care of patients with RSV and the proportion of susceptible infants and staff developing the infection was 6% and 5%, respectively.24 When the goggles were no longer used, the proportions increased to 43% and 34%. Similarly, only 5% of health care workers who wore goggles and masks when caring for RSV-infected children developed the infection compared with 61% of health care workers who did not wear PPE.25
Other studies have evaluated the effectiveness of a variety of measures in combination to prevent health care–associated RSV infection. A combination of both cohort nursing and routine use of gowns and gloves significantly reduced RSV transmission compared with either intervention alone.26 An intervention consisting of education, hand washing, consistent use of gowns and gloves, isolating or cohorting patients, restriction of visitors, and cohort nursing was associated with a 39% reduction in health care–associated RSV.27 Transmission of RSV in a special care nursery ended after instituting cohort nursing; active surveillance; patient cohorting; a strict policy limiting visitation in the winter; construction of segregate areas; and the use of gown, gloves, and masks during all patient contact.28 A similar intervention that included isolation or cohorting infected infants, hand washing, use of gowns, cohort nursing, isolation of asymptomatic high-risk infants, and limitation of visitors seemed to be effective in reducing transmission among infants from 45% in the previous year to 19% following the intervention.13
Recommendations for RSV infection control
In addition to the standard precautions, the Centers for Disease Control and Prevention (CDC) recommend contact precautions to prevent health care–associated RSV (Tables 1 and 2).1,29 Contact precautions should continue for the duration of illness but may be extended for immunocompromised patients because of prolonged viral shedding. Additional interventions to prevent health care–associated RSV include cohort nursing and the exclusion from the hospital of ill health care workers and visitors.
Table 1.
Precautions for Preventing Transmission of Respiratory Infections[29]
| Precautions | Component | Recommendation |
|---|---|---|
| Standard | Hand hygiene | Wash hands with soap and water or use an alcohol-based hand rub:
|
| Respiratory hygiene |
Instruct staff and visitors with signs and symptoms of a respiratory infection to:
|
|
| Gloves | Wear when contact with respiratory secretions could occur. | |
| Gowns | Wear during procedures and activities when contact of clothing or exposed skin with respiratory secretions is anticipated. |
|
| Masks and eye protection |
Wear during procedures and activities likely to generate splashes or sprays of respiratory secretions. |
|
| Contact* | Patient placement |
Place patient in a single-patient room, if possible, or cohort with other patients infected with the same organism. Limit patient movement to medically-necessary purposes. |
| Gloves and gowns |
Wear upon room entry whenever contact is likely with the patient, patient’s respiratory secretions, or potentially contaminated items in the patient’s vicinity, including equipment and environmental surfaces. |
|
| Masks and eye protection |
As per Standard Precautions. | |
| Droplet* | Patient placement |
Place patient in a single-patient room, if possible, or cohort with other patients infected with the same organism. Limit patient movement to medically-necessary purposes, and patients should wear a mask and follow respiratory hygiene during transport. |
| Gloves, gowns, and eye protection |
As per Standard Precautions. | |
| Masks | Wear a surgical mask upon room entry if close contact (e.g., < 3 feet) with the patient is anticipated. |
|
| Airborne* | Patient placement |
Place infected patients in a single-patient airborne infection isolation room.** Limit patient movement to medically-necessary purposes, and patients should wear a mask and follow respiratory hygiene during transport. |
| Gloves, gowns, and eye protection |
As per Standard Precautions. | |
| Masks | Wear a fit-tested N95 respirator prior to room entry. |
Contact, Droplet, and Airborne Precautions include hand hygiene and respiratory hygiene as per Standard Precautions.
Airborne infection isolation room consists of negative pressure relative to the surrounding area, 6 – 12 air changes per hour, and air is exhausted directly to the outside or recirculated through high-efficiency particulate air (HEPA) filtration before return.
Table 2.
Infection Control Recommendations for Viral Respiratory Pathogens
| Common measures for reducing transmission in the healthcare setting |
|---|
| Hand hygiene |
| Respiratory hygiene/cough etiquette |
| Standard precautions |
| Restrict ill visitors1 |
| Restrict ill personnel for caring for patients at high risk for complications from infection |
| Cohort nursing |
| Prompt diagnosis of respiratory infections among patients by rapid diagnostic tests2 |
| Restrict elective admissions of patients during outbreaks in the community and/or facility |
| Surveillance for an increase in activity of viral infections within the community |
|
|
| Measures for reducing transmission of specific pathogens in the healthcare setting |
| Influenza |
||||||
|---|---|---|---|---|---|---|
| Intervention | RSV | Adenovirus | Parainfluenza Virus |
Seasonal | 2009 H1N1 |
H5N1 |
| Precautions | ||||||
| Contact | • | • | • | • | • | |
| Droplet | • | • | ||||
| Airborne | • 3 | • | ||||
| Eye protection | • | |||||
| Vaccination of personnel | • | • | ||||
| Chemoprophylaxis | ○ 4 | ○ 5 | ○ 5 | • | ||
RSV, respiratory syncytial virus. Closed circles (•) denote recommended measures. Open circles (○) denote measures recommended in certain circumstances.
Institutions may restrict only young children and/or screen all visitors for illness by using a trained healthcare worker to assess for signs and symptoms or by using an educational patient information list to advise ill visitors.
To control outbreaks, institutions may perform pre-admission screening of patients for infection.
The Centers for Disease Control and Prevention recommends a N95 respirator for healthcare workers entering the room of patients infected with 2009 H1N1, but patients do not require placement in an airborne infection isolation room, except when undergoing aerosol-generating procedures.
In addition to other infection control measures, palivizumab prophylaxis of high-risk infants has been used to control outbreaks in the neonatal intensive care unit.
During a facility outbreak of influenza, administer antiviral chemoprophylaxis to all patients in the involved unit, regardless of vaccination status, and to unvaccinated personnel working in the involved unit. If feasible, administer facility-wide chemoprophylaxis for all residents in long-term care facilities. Chemoprophylaxis may also be administered to personnel when the outbreak strain is not well-matched by the vaccine.
Rapid RSV antigen screening has also been proposed to prevent health care–associated RSV. Rapid screening of symptomatic children on admission resulted in a greater than 50% decrease in the proportion of health care–associated RSV infections.30 Screening of all pediatric admissions, regardless of the presence of symptoms, with cohorting of infected patients reduced the incidence of health care–associated RSV from 7.2 to less than 1 per 1000 patient-days.31 However, rapid antigen detection is an insensitive method for diagnosing RSV infection in adults.32 Several reports described the administration of palivizumab to susceptible infants to control outbreaks in neonatal intensive care units (ICU).9,33 Palivizumab is a humanized mouse IgG monoclonal antibody that is effective in preventing hospitalizations caused by RSV infections.34,35 Palivizumab is licensed for the prevention of RSV infection in infants born at 35 weeks’ gestation or earlier, in infants with chronic lung disease of prematurity, or in infants with congenital heart disease. However, at present there are no guidelines for the use of this drug in controlling outbreaks of health care–associated RSV infections. Furthermore, use of palivizumab for controlling hospital outbreaks is limited to case reports, often with implementation of other infection control measures. There are no trials that have evaluated the individual effect of palivizumab in controlling nosocomial RSV transmission.
SEASONAL INFLUENZA
Epidemiology
Influenza infects approximately 5% to 20% of the US population annually, resulting in 226,000 hospitalizations and 36000 deaths.36,37 Transmission of influenza has been reported in a variety of pediatric and adult health care settings, and health care workers may be often implicated in the outbreaks.37 Health care workers are at an increased risk of acquiring influenza because of exposure to infection in both the health care and community settings,38 and they often fail to recognize that they are infected. In one study, 23% of health care workers demonstrated serologic evidence of influenza infection during a single influenza season; however, 59% of those infected could not recall influenzalike illness (ILI) and 28% were asymptomatic.39
Asymptomatic and mildly symptomatic health care workers may also shed influenza virus, potentially transmitting the infection to patients or other personnel.40 Finally, ill health care workers often continue to work despite the presence of symptoms.41,42 Secondary attack rates as high as 50% have been reported among both health care workers and patients.38 Such high attack rates can result in health care worker absenteeism and the subsequent disruption of patient care, particularly during periods of increased health care use. A 2003-2004 survey of 221 hospital epidemiologists from all regions in the United States indicated that a substantial number of hospitals experienced staffing shortages (34%), bed shortages (28%), ICU bed shortages (43%), and diversion of patients (9%) during the peak influenza activity.43
Transmission
The typical incubation period for influenza in healthy volunteers is 1 to 3 days.40 Viral shedding begins before the appearance of symptoms and within the first 24 hours after inoculation, peaks on the second day after inoculation, and usually declines rapidly thereafter.44,45 The virus is typically no longer detectable after 6 to 10 days after inoculation. However, prolonged viral shedding has been documented by culture for up to 21 days in children46 and up to 44 days in immunocompromised adults.47 Furthermore, the quantity of virus shed correlates with the severity of illness.44,45 The potential for hospitalized children and immunocompromised or severely ill adults to shed greater amounts of virus and for prolonged durations has important implications on influenza transmission within the health care setting.
Transmission via contact with fomites has been also suggested by the recovery of influenza on porous surfaces (eg, cloth, paper, tissues) for 8 to 12 hours and on nonporous surfaces (eg, steel, plastic) for 24 to 48 hours after inoculation.48 Transfer of virus from environmental surfaces to hands was also demonstrated. Likewise, influenza virus has been recovered from 23% and 53% of inanimate objects present in day care centers during fall and spring months, respectively.49 Although no study has clearly documented infection resulting from contact with fomites, one nursing home outbreak suggested a link between the hands of health care workers and influenza transmission.50 Nurses routinely had ungloved contact with patients’ oral secretions while administering medications and tube feedings. Thirty-eight percent of patients who were tube fed or frequently suctioned contracted influenza compared to 13% of other patients (P 5 .08), and no illness was detected among personnel.
Several studies have suggested that influenza may be transmitted by droplet nuclei (<5 mm) or small particle aerosols between infected and noninfected animals. Transmission of influenza occurred among ferrets in wire mesh cages separated by at least 1.5 m despite the susceptible ferrets being placed at a higher elevation.51 To control for air currents in the laboratory, additional experiments were conducted using cages connected by an S- or a U-shaped duct. Infection occurred at low air speeds using both types of ducts, which prevented transmission of coarse droplets. Murine experiments provided similar results on the effect of ventilation and physical separation. Twenty-four hours after their exposure to an aerosol spray of influenza, infected mice were placed in the same cage as noninfected mice in a closed chamber for 24 hours.52 Transmission was not affected by physical separation with a wire screen. However, transmission was inversely correlated with airflow. Because transmission was affected by air currents rather than by separation, the investigators concluded that droplet nuclei are the principal means of influenza transmission. Recovery of infectious particles less than 10 mm from the air surrounding the infected mice provided additional support for droplet nuclei transmission.53
Studies have also examined the ability to infect humans with influenza via experimental aerosol. Twenty-three healthy men were exposed to various doses of influenza via aerosolized particles of 1 to 3 mm.54 Half of the men with absent titers before inoculation became infected with a dose that was 40- to 500-fold lower than that required to cause disease when administered by nasal drops.40 Albeit to a lesser extent, another study similarly demonstrated that smaller doses were required to cause illness among normal volunteers when administered as an aerosol rather than as nasal drops.55 Although the lower respiratory tract seems to be a more efficient route of infection than the upper respiratory tract, the proportion of natural influenza infections acquired by aerosols remains unknown.
Several studies demonstrated that the size of particles produced during various activities is within the range in which deposition in the lower respiratory tract can occur. Loudon and Roberts56 demonstrated that 8% and 50% of particles produced during talking and coughing, respectively, were less than 5 mm in diameter. Papineni and Rosenthal57 similarly demonstrated that 36% of exhaled particles were less than or equal to 1 mm in diameter but 80% to 90% of the total particle concentration consisted of particles less than or equal to 1 mm in diameter. Furthermore, a recent sampling of aerosol particles in an emergency room demonstrated that 53% of influenza virus particles detected by polymerase chain reaction were less than or equal to 4 mm in diameter.58
Two commonly cited epidemiologic studies have suggested that influenza may be spread by droplet nuclei. During the 1957-1958 influenza outbreak, 150 patients with tuberculosis were housed in a building at a Veterans Administration Hospital with upper air ultraviolet radiation.59 An additional 250 patients with tuberculosis were housed in a separate building with nonradiated air. Serologic evidence of influenza infection was found in only 2% of the patients in the radiated building compared with 19% of the patients in the nonradiated building and 18% of personnel. Patients in both radiated and nonradiated building were equally exposed to the infected personnel, and all patients were assumed susceptible because the influenza strain was new and antigenically distinct from the previous strains. Because upper air radiation would not disinfect large respiratory droplets, transmission of influenza via droplet nuclei was proposed. However, the possibility that influenza was not introduced into the radiated building cannot be excluded. The second outbreak occurred in 1977 when 54 persons aboard a commercial airliner were grounded for 3 hours during which time the ventilation system was inoperative.60 A single index person became ill within 15 minutes after boarding. Within 72 hours, 72% of the remaining passengers developed ILI. The high illness rates and epidemic curve suggest airborne transmission from a single point source. However, passengers were allowed to move about the plane freely and the index patient sat immediately adjacent to the lavatory and galley areas, so droplet and contact transmission cannot be excluded. Conversely, other observations have suggested that large respiratory droplets (>10 mm) play a more significant role in transmission than droplet nuclei. During the 1958-1959 pandemic, a single patient with influenza was hospitalized in a general medical ward before the appearance of influenza in the community61 During the next 3 days 16 patients and staff became ill. The epidemic curve suggested an initial source with subsequent person-to-person spread. Furthermore, no patient in a single occupancy room was infected as would have been expected of airborne transmission. Similarly, most health care–associated influenza cases at the University of Rochester Medical Center were reported in patients housed in the same room adjacent to the patients with the same infection.62 Patients across the hall from infected patients were less likely to acquire influenza despite open doors and nonutilization of airborne infection isolation rooms (AIIR). Salgado and colleagues38 reported that health care–associated influenza was rare, although most patients were placed in private, positive pressure rooms. Finally, the lack of reports of outbreak of health care–associated influenza during annual influenza seasons supports the lack of widespread airborne transmission in health care settings. Although transmission by droplet nuclei may play a role in certain conditions, large respiratory droplets are likely the primary mode of transmission within a health care setting, an environment in which frequent air changes and exhaust ventilation occur.
Prevention and Control
Guidelines for seasonal influenza infection control
In addition to the standard precautions, the CDC recommends implementation of droplet precautions to prevent health care–associated influenza (see Tables 1 and 2).1,29 Droplet precautions should continue for at least 5 days but may be extended for immunocompromised patients because of prolonged viral shedding. In addition to vaccination and antiviral chemoprophylaxis, interventions to prevent health care–associated influenza include early identification of suspected patients with source control (ie wearing a mask), cohort nursing, exclusion of ill health care workers and visitors, and rapid diagnostic testing of symptomatic children. Similar to RSV infection, rapid antigen detection is an insensitive method for diagnosing influenza infection in most adults.63
Influenza vaccination of health care workers
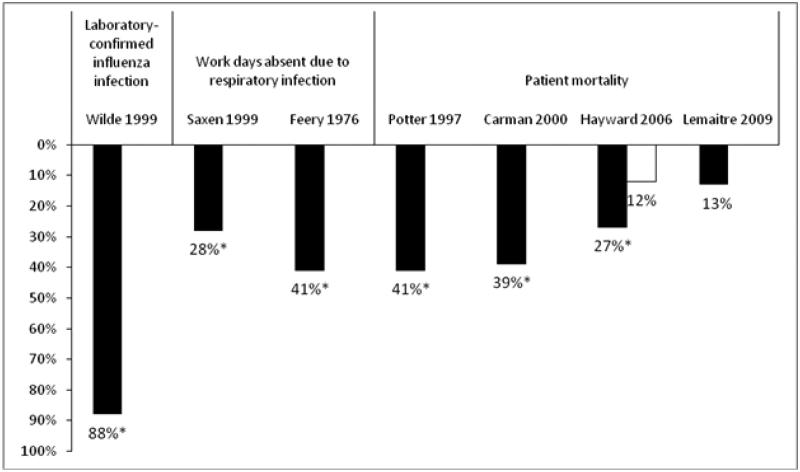
Vaccination is the most effective strategy for preventing influenza and is recommended for health care workers for several reasons.64 First, vaccination has been shown to be 88% effective in preventing laboratory-confirmed influenza in health care workers (Fig. 1).65 Second, health care worker absenteeism can stress the health system during influenza epidemics, and influenza vaccination has resulted in a statistically significant 28% to 41% decrease in work days lost because of respiratory illness (see Fig. 1).66,67 Third, health care workers have frequent contact with patients at high risk for complications from influenza and may transmit influenza to susceptible patients, resulting in increased patient morbidity and mortality. Improved health care worker vaccination rates have been linked with decreased health care–associated influenza among patients and personnel.68 Three cluster randomized trials demonstrated that health care worker vaccination was associated with a statistically significant decrease in mortality among nursing home patients (see Fig. 1).69–71 Similarly, health care worker vaccination led to a nonsignificant 13%decrease in patient mortality during a mild influenza season in a fourth cluster randomized trial.72 However, the study was likely underpowered and the vaccination rate was higher than expected among patients in both arms (>80%) and among staff in the control arm (31%). Nevertheless, a multivariate analysis showed that staff vaccination was a significant independent predictor of patient mortality (odds ratio 0.80; 95% confidence interval, 0.67–0.97). Despite recommendations for vaccination and evidence supporting its use, only 45% of health care workers were vaccinated during the 2007–2008 season.73 Barriers to vaccination include fear of needles and vaccine side effects, inconvenience, failure of the employer to pay for the vaccine, doubt about the risk of influenza, perceived lack of vaccine effectiveness, and failure to recognize the role of health care workers in transmission of influenza to patients.37,74 Strategies for improving acceptance of vaccine by health care workers have been published.37,74 Many health care organizations have adopted a policy requiring annual influenza vaccination of all health care workers as a condition of employment because of the patient safety ramifications of an unvaccinated workforce.75 The state of New York and the US Department of Defense have adopted similar requirements.75,76 However, mandatory vaccination programs should only be put into practice after careful planning and full assessment of the logistic barriers to successful implementation.
Figure 1. Percent Reduction in Noted Outcomes in Healthcare Workers Receiving Influenza Vaccination.
Adapted from Talbot et al.[37] Values marked by an asterisk (*) were statistically significant (p < 0.05) compared to an unvaccinated control group. Patient mortality data from Hayward et al. is from 2 different seasons.[71] A multivariate analysis of the Lemaitre study showed that staff vaccination was a significant independent predictor of patient mortality.[72]
Chemoprophylaxis
Data from observational studies and controlled trials support recommendations to provide antiviral chemoprophylaxis to residents in long-term care facilities, regardless of their vaccination status, during an institutional influenza outbreak.77 Moreover, because outbreaks may continue when prophylaxis is limited to residents of affected wards, facility-wide chemoprophylaxis is ideal.78 Chemoprophylaxis may also be considered for unvaccinated staff or when the vaccine is likely to be ineffective because of strain mismatch; however, no studies have evaluated the effect of employee chemoprophylaxis on patient outcomes.78 In the setting of an institutional outbreak, chemoprophylaxis should be continued for 14 days or for 7 to 10 days after the onset of symptoms in the last person infected, whichever is longer.77,78 After an unprotected exposure to someone with influenza, postexposure chemoprophylaxis can be considered for unvaccinated health care workers or for patients at high risk for complications from influenza.79 Chemoprophylaxis is not recommended if more than 48 hours have elapsed since the last contact with the infectious person or if the exposure occurred outside that person’s infectious period, defined as 1 day before symptom onset until 24 hours after fever resolution. Postexposure chemoprophylaxis should be continued for 10 days after the last known exposure. At present, the neuraminidase inhibitors, oseltamivir and zanamivir, are the recommended drugs for influenza chemoprophylaxis, but these recommendations may change depending on the antiviral resistance of circulating influenza strains.79
PANDEMIC INFLUENZA
Pandemic influenza results when a novel viral strain to which the population has little or no immunity achieves the ability to spread easily between humans, resulting in rapid spread across several continents. A novel virus emerges as a result of reassortment of human influenza genes with those of avian or swine strains. During the past century, there have been 4 major pandemics: 1918–1919 Spanish influenza (H1N1), 1957–1958 Asian influenza (H2N2), 1968–1969 Hong Kong influenza (H3N2), and 2009 H1N1 influenza A. Although there may be some differences between the seasonal and pandemic influenza strains, many of the basic infection control recommendations for the prevention of health care–associated seasonal influenza also apply for pandemic strains.
The 2009 Influenza A (H1N1) Pandemic
On April 21, 2009, the CDC reported that 2 children in California were infected with a novel influenza A (H1N1) virus of swine origin80 and the virus subsequently spread rapidly across the globe. Transmission among health care workers has been reported.81 According to the CDC’s interim recommendations to prevent the transmission of 2009 H1N1 influenza in health care settings, contact precautions and eye protection should be used for all patient care activities in addition to the standard precautions (see Tables 1 and 2).82 At the outset of the pandemic, the CDC also recommended that all health care workers entering the room should wear respiratory protection at least as protective as a fit-tested disposable N95 respirator. Patients should be placed in single-patient rooms. However, for aerosol-generating procedures (eg, bronchoscopy, intubation, open airway suctioning), patients should be placed in an AIIR with negative pressure air handling and 6 to 12 air changes per hour. These enhanced precautions (eg, use of N95 respirators during routine care) were the result of the lack of an effective vaccine for 2009 H1N1 influenza at the pandemic’s outset, the increased susceptibility of patients to 2009 H1N1 influenza, and the limited knowledge of the severity and transmissibility of the novel virus. Transmission-based precautions should be continued for 7 days after onset of illness or until resolution of symptoms.82 Similar to seasonal influenza, postexposure chemoprophylaxis for 10 days should be considered for patients at high risk for complications from influenza and health care workers exposed to a patient infected with 2009 H1N1 influenza.79
Following the CDC recommendations, the Society for Healthcare Epidemiology of America, the Healthcare Infection Control Practices Advisory Committee, and the World Health Organization released recommendations for infection control precautions for 2009 H1N1 influenza infections, endorsing the same practices as recommended for seasonal influenza, which did not include the use of N95 respirators by health care workers during routine patient care.83–85 Use of an N95 respirator and an AIIR, however, were still recommended for aerosol-generating procedures because of an increased risk of transmission to health care workers during these procedures. To help resolve doubts about the optimal use of PPE for 2009 H1N1 influenza infections, an Institute of Medicine panel was convened in August 2009. The panel recommended that health care workers wear a fit-tested N95 respirator when caring for patients infected with 2009 H1N1 influenza because of experimental studies demonstrating droplet nuclei as a possible route of transmission and studies demonstrating the efficacy of respirators and masks.86 However, the panel did not consider availability of respirators and other implementation issues when issuing the recommendation. Furthermore, a subsequent randomized controlled trial demonstrated that masks were noninferior to respirators in protecting health care workers from influenza.87 Although a second randomized controlled trial initially reported a difference between respirators and surgical masks, adjustment for the clustered randomization and multiple outcomes yielded no statistical significance between the 2 types of respiratory protection.88 In 2010 the CDC updated the interim guidance on infection control measures for 2009 H1N1 influenza. Similar to seasonal influenza, the CDC now recommends that health care workers should adhere to droplet precautions for routine patient care. However, airborne precautions are still recommended for aerosol-generating procedures.89
Avian Influenza
A highly pathogenic avian influenza A (H5N1) virus was first reported to cause human infections in 1997 in China.90 However, no other human infections were reported until the virus reemerged in Hong Kong in 2003. Since then, this virus has caused several hundred infections worldwide with a case-fatality rate of 60%.91 Several family clusters of disease suggest probable human-to-human transmission.92,93 A study of the 1997 cluster indicated that 3.7% of exposed health care workers had serologic evidence of infection94; however, more recent serologic surveys have not identified any cases among exposed personnel despite the lack of appropriate infection control precautions.95,96 The inefficient spread among humans may be explained by recent evidence showing a preferential binding of the H5N1 virus in the lower respiratory tract of humans.97 Avian influenza should be suspected in patients presenting with a severe respiratory illness within 10 days of travel to a country with avian influenza activity.98 Contact and airborne precautions should be used for patients with suspected avian influenza (see Tables 1 and 2).99 In addition, all health care workers should wear eye protection when entering the patient’s room. Transmission-based precautions for avian influenza should be continued for 14 days after the onset of illness. Antiviral chemoprophylaxis for 7 to 10 days should also be considered for health care workers with unprotected exposures.100
OTHER VIRUSES
Adenovirus
Health care–associated outbreaks of respiratory tract infections caused by adenovirus have been reported from pediatric and adult health care settings.101–104 Attack rates among patients have ranged from 15% to 56%.101,102 Health care workers were often infected, and many continued to provide patient care while ill.103 Similar to influenza, adenovirus is transmitted through large respiratory droplets. Transmission also occurs via self-inoculation after contact with contaminated fomites because adenovirus can survive on nonporous environmental surfaces for up to 49 days.105 In one outbreak, adenovirus was recovered from 19% of environmental surfaces before terminal cleaning.104 In addition to the standard precautions, the CDC recommends contact and droplet precautions to prevent health care–associated adenovirus infection (see Tables 1 and 2).1,29
Parainfluenza
Transmission of parainfluenza has been documented in pediatric wards,5 neonatal nurseries,8 and adult transplant units.106 Transmission of parainfluenza is similar to that of RSV and primarily occurs by direct person-to-person contact. Parainfluenza can survive for up to 4 hours on porous surfaces and up to 10 hours on nonporous surfaces.107 However, viral recovery from hand decreases rapidly, with only 5% detected after 10 minutes.108 The CDC recommends contact precautions, in addition to standard precautions, for the prevention of health care–associated parainfluenza infection (see Tables 1 and 2).1,29
SUMMARY
Transmission of viral respiratory infections occurs in a variety of pediatric and adult health care settings, resulting in increased patient morbidity and health care costs. Transmission may occur via aerosol, large respiratory droplets, or self-inoculation after touching contaminated fomites. Different viruses have different modes of transmission, and prevention of transmission requires early recognition of symptomatic patients and prompt institution of appropriate transmission-based precautions in addition to adherence to basic infection control practices such as hand hygiene. In addition to administrative and environmental controls, influenza vaccination of health care workers is an effective means of prevention of health care–associated influenza infection.
Acknowledgments
Funding support: H.K.T. received funding support from the National Institute of Allergy and Infectious Diseases grant K23 AI074863–03 and a T. Franklin Williams Scholarship Award, which is funded by Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the Infectious Diseases Society of America.
H.K.T. has received funding from Protein Sciences Corporation, Wyeth, Sanofi Pasteur, VaxInnate, the National Institutes of Health, and the Centers for Disease Control and Prevention (CDC). T.R.T. has received influenza vaccine donated by Sanofi Pasteur for a study funded by the CDC and serves as a consultant for Joint Commission Resources.
Footnotes
Disclosures: W.P.G. has no disclosures.
REFERENCES
- 1.Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-careassociated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 2.Hall CB. Nosocomial viral respiratory infections: perennial weeds on pediatric wards. Am J Med. 1981;70(3):670–6. doi: 10.1016/0002-9343(81)90594-5. [DOI] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282(15):1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 5.Gardner PS, Court SD, Brocklebank JT, et al. Virus cross-infection in paediatric wards. Br Med J. 1973;2(5866):571–5. doi: 10.1136/bmj.2.5866.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall CB, Douglas RG, Jr, Geiman JM, et al. Nosocomial respiratory syncytial virus infections. N Engl J Med. 1975;293(26):1343–6. doi: 10.1056/NEJM197512252932604. [DOI] [PubMed] [Google Scholar]
- 7.Hall CB, Kopelman AE, Douglas RG, Jr, et al. Neonatal respiratory syncytial virus infection. N Engl J Med. 1979;300(8):393–6. doi: 10.1056/NEJM197902223000803. [DOI] [PubMed] [Google Scholar]
- 8.Meissner HC, Murray SA, Kiernan MA, et al. A simultaneous outbreak of respiratory syncytial virus and parainfluenza virus type 3 in a newborn nursery. J Pediatr. 1984;104(5):680–4. doi: 10.1016/s0022-3476(84)80943-9. [DOI] [PubMed] [Google Scholar]
- 9.Halasa NB, Williams JV, Wilson GJ, et al. Medical and economic impact of a respiratory syncytial virus outbreak in a neonatal intensive care unit. Pediatr Infect Dis J. 2005;24(12):1040–4. doi: 10.1097/01.inf.0000190027.59795.ac. [DOI] [PubMed] [Google Scholar]
- 10.Guidry GG, Black-Payne CA, Payne DK, et al. Respiratory syncytial virus infection among intubated adults in a university medical intensive care unit. Chest. 1991;100(5):1377–84. doi: 10.1378/chest.100.5.1377. [DOI] [PubMed] [Google Scholar]
- 11.Englund JA, Anderson LJ, Rhame FS. Nosocomial transmission of respiratory syncytial virus in immunocompromised adults. J Clin Microbiol. 1991;29(1):115–9. doi: 10.1128/jcm.29.1.115-119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathur U, Bentley DW, Hall CB. Concurrent respiratory syncytial virus and influenza A infections in the institutionalized elderly and chronically ill. Ann Intern Med. 1980;93(1):49–52. doi: 10.7326/0003-4819-93-1-49. [DOI] [PubMed] [Google Scholar]
- 13.Hall CB, Geiman JM, Douglas RG, Jr, et al. Control of nosocomial respiratory syncytial viral infections. Pediatrics. 1978;62(5):728–32. [PubMed] [Google Scholar]
- 14.Hall CB. Nosocomial respiratory syncytial virus infections: the “Cold War” has not ended. Clin Infect Dis. 2000;31(2):590–6. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- 15.Hall CB. Respiratory syncytial virus: its transmission in the hospital environment. Yale J Biol Med. 1982;55(3/4):219–23. [PMC free article] [PubMed] [Google Scholar]
- 16.Hall CB, Douglas RG, Jr, Schnabel KC, et al. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33(3):779–83. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall CB, Douglas RG., Jr Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99(1):100–3. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 18.Hall CB, Douglas RG, Jr, Geiman JM. Possible transmission by fomites of respiratory syncytial virus. J Infect Dis. 1980;141(1):98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 19.Hall CB, Douglas RG, Jr, Geiman JM. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. J Pediatr. 1976;89(1):11–5. doi: 10.1016/s0022-3476(76)80918-3. [DOI] [PubMed] [Google Scholar]
- 20.Hall CB, Douglas RG., Jr Nosocomial respiratory syncytial viral infections. Should gowns and masks be used? Am J Dis Child. 1981;135(6):512–5. doi: 10.1001/archpedi.1981.02130300012006. [DOI] [PubMed] [Google Scholar]
- 21.Murphy D, Todd JK, Chao RK, et al. The use of gowns and masks to control respiratory illness in pediatric hospital personnel. J Pediatr. 1981;99(5):746–50. doi: 10.1016/S0022-3476(81)80401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leclair JM, Freeman J, Sullivan BF, et al. Prevention of nosocomial respiratory syncytial virus infections through compliance with glove and gown isolation precautions. N Engl J Med. 1987;317(6):329–34. doi: 10.1056/NEJM198708063170601. [DOI] [PubMed] [Google Scholar]
- 23.Langley JM, LeBlanc JC, Wang EE, et al. Nosocomial respiratory syncytial virus infection in Canadian pediatric hospitals: a Pediatric Investigators Collaborative Network on Infections in Canada Study. Pediatrics. 1997;100(6):943–6. doi: 10.1542/peds.100.6.943. [DOI] [PubMed] [Google Scholar]
- 24.Gala CL, Hall CB, Schnabel KC, et al. The use of eye-nose goggles to control nosocomial respiratory syncytial virus infection. JAMA. 1986;256(19):2706–8. [PubMed] [Google Scholar]
- 25.Agah R, Cherry JD, Garakian AJ, et al. Respiratory syncytial virus (RSV) infection rate in personnel caring for children with RSV infections. Routine isolation procedure vs routine procedure supplemented by use of masks and goggles. Am J Dis Child. 1987;141(6):695–7. doi: 10.1001/archpedi.1987.04460060111049. [DOI] [PubMed] [Google Scholar]
- 26.Madge P, Paton JY, McColl JH, et al. Prospective controlled study of four infection-control procedures to prevent nosocomial infection with respiratory syncytial virus. Lancet. 1992;340(8827):1079–83. doi: 10.1016/0140-6736(92)93088-5. [DOI] [PubMed] [Google Scholar]
- 27.Macartney KK, Gorelick MH, Manning ML, et al. Nosocomial respiratory syncytial virus infections: the cost-effectiveness and cost-benefit of infection control. Pediatrics. 2000;106(3):520–6. doi: 10.1542/peds.106.3.520. [DOI] [PubMed] [Google Scholar]
- 28.Snydman DR, Greer C, Meissner HC, et al. Prevention of nosocomial transmission of respiratory syncytial virus in a newborn nursery. Infect Control Hosp Epidemiol. 1988;9(3):105–8. doi: 10.1086/645804. [DOI] [PubMed] [Google Scholar]
- 29.Siegel JD, Rhinehart E, Jackson M, et al. 2007 guideline for isolation precautions:preventing transmission of infectious agents in health care settings. doi: 10.1016/j.ajic.2007.10.007. Available at: http://www.cdc.gov/hicpac/2007IP/2007isolationPrecautions.html. Accessed December 3, 2010. [DOI] [PMC free article] [PubMed]
- 30.Karanfil LV, Conlon M, Lykens K, et al. Reducing the rate of nosocomially transmitted respiratory syncytial virus. Am J Infect Control. 1999;27(2):91–6. doi: 10.1016/s0196-6553(99)70087-8. [DOI] [PubMed] [Google Scholar]
- 31.Krasinski K, LaCouture R, Holzman RS, et al. Screening for respiratory syncytial virus and assignment to a cohort at admission to reduce nosocomial transmission. J Pediatr. 1990;116(6):894–8. doi: 10.1016/s0022-3476(05)80646-8. [DOI] [PubMed] [Google Scholar]
- 32.Falsey AR, McCann RM, Hall WJ, et al. Evaluation of four methods for the diagnosis of respiratory syncytial virus infection in older adults. J Am Geriatr Soc. 1996;44(1):71–3. doi: 10.1111/j.1532-5415.1996.tb05641.x. [DOI] [PubMed] [Google Scholar]
- 33.Kurz H, Herbich K, Janata O, et al. Experience with the use of palivizumab together with infection control measures to prevent respiratory syncytial virus outbreaks in neonatal intensive care units. J Hosp Infect. 2008;70(3):246–52. doi: 10.1016/j.jhin.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 34.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3):531–7. [PubMed] [Google Scholar]
- 35.Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–40. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan KM, Monto AS, Longini IM., Jr Estimates of the US health impact of influenza. Am J Public Health. 1993;83(12):1712–6. doi: 10.2105/ajph.83.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talbot TR, Bradley SE, Cosgrove SE, et al. Influenza vaccination of healthcare workers and vaccine allocation for healthcare workers during vaccine shortages. Infect Control Hosp Epidemiol. 2005;26(11):882–90. doi: 10.1086/502512. [DOI] [PubMed] [Google Scholar]
- 38.Salgado CD, Farr BM, Hall KK, et al. Influenza in the acute hospital setting. Lancet Infect Dis. 2002;2(3):145–55. doi: 10.1016/s1473-3099(02)00221-9. [DOI] [PubMed] [Google Scholar]
- 39.Elder AG, O’Donnell B, McCruden EA, et al. Incidence and recall of influenza in a cohort of Glasgow healthcare workers during the 1993-4 epidemic: results of serum testing and questionnaire. BMJ. 1996;313(7067):1241–2. doi: 10.1136/bmj.313.7067.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douglas RG., Jr . Influenza in man. In: Kilbourne ED, editor. The influenza viruses and influenza. Academic Press; New York: 1975. pp. 395–447. [Google Scholar]
- 41.Lester RT, McGeer A, Tomlinson G, et al. Use of, effectiveness of, and attitudes regarding influenza vaccine among house staff. Infect Control Hosp Epidemiol. 2003;24(11):839–44. doi: 10.1086/502146. [DOI] [PubMed] [Google Scholar]
- 42.Weingarten S, Riedinger M, Bolton LB, et al. Barriers to influenza vaccine acceptance. A survey of physicians and nurses. Am J Infect Control. 1989;17(4):202–7. doi: 10.1016/0196-6553(89)90129-6. [DOI] [PubMed] [Google Scholar]
- 43.Poland GA, Tosh P, Jacobson RM. Requiring influenza vaccination for health care workers: seven truths we must accept. Vaccine. 2005;23(17/18):2251–5. doi: 10.1016/j.vaccine.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 44.Murphy BR, Chalhub EG, Nusinoff SR, et al. Temperature-sensitive mutants of influenza virus. III. Further characterization of the ts-1[E] influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973;128(4):479–87. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- 45.Hayden FG, Fritz R, Lobo MC, et al. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101(3):643–9. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall CB, Douglas RG., Jr Nosocomial influenza infection as a cause of intercurrent fevers in infants. Pediatrics. 1975;55(5):673–7. [PubMed] [Google Scholar]
- 47.Englund JA, Champlin RE, Wyde PR, et al. Common emergence of amantadine and rimantadine-resistant influenza A viruses in symptomatic immunocompromised adults. Clin Infect Dis. 1998;26(6):1418–24. doi: 10.1086/516358. [DOI] [PubMed] [Google Scholar]
- 48.Bean B, Moore BM, Sterner B, et al. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146(1):47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 49.Boone SA, Gerba CP. The occurrence of influenza A virus on household and day care center fomites. J Infect. 2005;51(2):103–9. doi: 10.1016/j.jinf.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Morens DM, Rash VM. Lessons from a nursing home outbreak of influenza A. Infect Control Hosp Epidemiol. 1995;16(5):275–80. doi: 10.1086/647107. [DOI] [PubMed] [Google Scholar]
- 51.Andrewes CH, Glover RE. Spread of infection from the respiratory tract of the ferret. I. Transmission of influenza A virus. Br J Exp Pathol. 1941;22(2):91–7. [Google Scholar]
- 52.Schulman JL, Kilbourne ED. Airborne transmission of influenza virus infection in mice. Nature. 1962;195:1129–30. doi: 10.1038/1951129a0. [DOI] [PubMed] [Google Scholar]
- 53.Schulman JL. Experimental transmission of influenza virus infection in mice. IV. Relationship of transmissibility of different strains of virus and recovery of airborne virus in the environment of infector mice. J Exp Med. 1967;125(3):479–88. doi: 10.1084/jem.125.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alford RH, Kasel JA, Gerone PJ, et al. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966;122(3):800–4. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 55.Knight V. Viruses as agents of airborne contagion. Ann N Y Acad Sci. 1980;353:147–56. doi: 10.1111/j.1749-6632.1980.tb18917.x. [DOI] [PubMed] [Google Scholar]
- 56.Loudon RG, Roberts RM. Droplet expulsion from the respiratory tract. Am Rev Respir Dis. 1967;95(3):435–42. doi: 10.1164/arrd.1967.95.3.435. [DOI] [PubMed] [Google Scholar]
- 57.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10(2):105–16. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 58.Blachere FM, Lindsley WG, Pearce TA, et al. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2009;48(4):438–40. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 59.McLean RL. The effect of ultraviolet radiation upon the transmission of epidemic influenza in long-term hospital patients. Am Rev Respir Dis. 1961;83(2):36–8. [Google Scholar]
- 60.Moser MR, Bender TR, Margolis HS, et al. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110(1):1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 61.Blumenfeld HL, Kilbourne ED, Louria DB, et al. Studies on influenza in the pandemic of 1957–1958. I. An epidemiologic, clinical and serologic investigation of an intrahospital epidemic, with a note on vaccination efficacy. J Clin Invest. 1959;38(1):199–212. doi: 10.1172/JCI103789. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37(8):1094–101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 63.Steininger C, Kundi M, Aberle SW, et al. Effectiveness of reverse transcription-PCR, virus isolation, and enzyme-linked immunosorbent assay for diagnosis of influenza A virus infection in different age groups. J Clin Microbiol. 2002;40(6):2051–6. doi: 10.1128/JCM.40.6.2051-2056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR–8):1–52. [PubMed] [Google Scholar]
- 65.Wilde JA, McMillan JA, Serwint J, et al. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA. 1999;281(10):908–13. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- 66.Saxen H, Virtanen M. Randomized, placebo-controlled double blind study on the efficacy of influenza immunization on absenteeism of health care workers. Pediatr Infect Dis J. 1999;18(9):779–83. doi: 10.1097/00006454-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Feery BJ, Evered MG, Morrison EI. Different protection rates in various groups of volunteers given subunit influenza virus vaccine in 1976. J Infect Dis. 1979;139(2):237–41. doi: 10.1093/infdis/139.2.237. [DOI] [PubMed] [Google Scholar]
- 68.Salgado CD, Giannetta ET, Hayden FG, et al. Preventing nosocomial influenza by improving the vaccine acceptance rate of clinicians. Infect Control Hosp Epidemiol. 2004;25(11):923–8. doi: 10.1086/502321. [DOI] [PubMed] [Google Scholar]
- 69.Potter J, Stott DJ, Roberts MA, et al. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis. 1997;175(1):1–6. doi: 10.1093/infdis/175.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomized controlled trial. Lancet. 2000;355(9198):93–7. doi: 10.1016/S0140-6736(99)05190-9. [DOI] [PubMed] [Google Scholar]
- 71.Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ. 2006;333(7581):1241. doi: 10.1136/bmj.39010.581354.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lemaitre M, Meret T, Rothan-Tondeur M, et al. Effect of influenza vaccination of nursing home staff on mortality of residents: a cluster-randomized trial. J Am Geriatr Soc. 2009;57(9):1580–6. doi: 10.1111/j.1532-5415.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- 73.Schiller JS, Euler GL, Centers for disease control and prevention Vaccination coverage estimates from the National Health Interview Survey: United States. 2008 Available at: http://www.cdc.gov/nchs/data/hestat/vaccine_coverage.htm. Accessed October 8, 2009.
- 74.The Joint Commission Providing a safer environment for health care personnel and patients through influenza vaccination: strategies from research and practice. 2009 Available at: http://www.jointcommission.org/assets/1/18/Flu_Monograph.pdf. Accessed December 6, 2010.
- 75.Immunization Action Coalition Honor roll for patient safety. Mandatory influenza vaccination policies for healthcare workers. Available at: http://www.immunize.org/laws/influenzahcw.asp. Accessed November 19, 2009.
- 76.New York State Department of Health Health care personnel influenza vaccination requirements - emergency regulation. Available at: http://www.health.state.ny.us/regulations/emergency/2009-08-13_health_care_personnel_influenza_vaccination_requirements.htm. Accessed November 19, 2009. [Google Scholar]
- 77.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR–7):1–60. [PubMed] [Google Scholar]
- 78.Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children–diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(8):1003–32. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Centers for Disease Control and Prevention Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. Available at: http://www.cdc.gov/h1n1flu/recommendations.htm. Accessed October 6, 2009.
- 80.Centers for Disease Control and Prevention Swine influenza A (H1N1) infection in two children–Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58(15):400–2. [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention Novel influenza A (H1N1) virus infections among health-care personnel - United States, April–May 2009. MMWR Morb Mortal Wkly Rep. 2009;58(23):641–5. [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention Interim guidance for infection control for care of patients with confirmed or suspected novel influenza A (H1N1) virus infection in a healthcare setting. Available at: http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed July 27, 2009.
- 83.The Society for Healthcare Epidemiology of America SHEA position statement: interim guidance on infection control precautions for novel swine-origin influenza A H1N1 in healthcare facilities. Available at: http://www.shea-online.org/Assets/ files/policy/061209_H1N1_Statement.pdf. Accessed July 27, 2009.
- 84.Centers for Disease Control and Prevention Summary of Healthcare Infection Control Practices Advisory Committee (HICPAC) recommendations for care of patients with confirmed or suspected 2009 H1N1 influenza infection in healthcare settings. 2009 Jul 23; Available at: http://www.cdc.gov/ncidod/dhqp/hicpac_h1n1.html. Accessed September 17, 2009.
- 85.World Health Organization Infection prevention and control in health care for confirmed or suspected cases of pandemic (H1N1) 2009 and influenza-like illnesses, interim guidance. Available at: http://www.who.int/csr/resources/publications/20090429_infection_control_en.pdf. Accessed August 8, 2009.
- 86.IOM (Institute of Medicine) Respiratory protection for healthcare workers in the workplace against novel H1N1 influenza A: a letter report. The National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 87.Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302(17):1865–71. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 88.Macintyre CR, Wang Q, Cauchemez S, et al. The first randomised, controlled clinical trial of surgical masks compared to fit-tested and non-fit tested N95 masks in the prevention of respiratory virus infection in hospital health care workers in Beijing, China [abstract 1247]. Final program of the 47th Annual Meeting of the Infectious Diseases Society of America; Philadelphia. Oct 31, 2009. p. 40. [Google Scholar]
- 89.Centers for Disease Control and Prevention Prevention strategies for seasonal influenza in healthcare settings. Available at: http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. Accessed December 6, 2010.
- 90.World Health Organization H5N1 avian influenza: timeline of major events. 2009 Mar 23; Available at: http://www.who.int/csr/disease/avian_influenza/Timeline_09_03_23.pdf. Accessed July 27, 2009.
- 91.World Health Organization Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. 2009 Sep 24; Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_09_24/en/index.html. Accessed October 8, 2009.
- 92.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352(4):333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Feng Z, Shu Y, et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008;371(9622):1427–34. doi: 10.1016/S0140-6736(08)60493-6. [DOI] [PubMed] [Google Scholar]
- 94.Buxton Bridges C, Katz JM, Seto WH, et al. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181(1):344–8. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 95.Liem NT, Lim W. Lack of H5N1 avian influenza transmission to hospital employees, Hanoi, 2004. Emerg Infect Dis. 2005;11(2):210–5. doi: 10.3201/eid1102.041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schultsz C, Dong VC, Chau NV, et al. Avian influenza H5N1 and healthcare workers. Emerg Infect Dis. 2005;11(7):1158–9. doi: 10.3201/eid1107.050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shinya K, Ebina M, Yamada S, et al. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440(7083):435–6. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 98.Centers for Disease Control and Prevention Interim recommendations for infection vontrol in health-care facilities caring for patients with known or suspected avian influenza. Available at: http://www.cdc.gov/flu/avian/professional/infectcontrol.htm. Accessed July 27, 2009.
- 99.World Health Organization Infection control recommendations for avian influenza in health-care facilities. Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/EPR_AM1_E5.pdf. Accessed July 27, 2009.
- 100.Beigel JH, Farrar J, Han AM, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353(13):1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 101.Brummitt CF, Cherrington JM, Katzenstein DA, et al. Nosocomial adenovirus infections: molecular epidemiology of an outbreak due to adenovirus 3a. J Infect Dis. 1988;158(2):423–32. doi: 10.1093/infdis/158.2.423. [DOI] [PubMed] [Google Scholar]
- 102.James L, Vernon MO, Jones RC, et al. Outbreak of human adenovirus type 3 infection in a pediatric long-term care facility–Illinois, 2005. Clin Infect Dis. 2007;45(4):416–20. doi: 10.1086/519938. [DOI] [PubMed] [Google Scholar]
- 103.Gerber SI, Erdman DD, Pur SL, et al. Outbreak of adenovirus genome type 7d2 infection in a pediatric chronic-care facility and tertiary-care hospital. Clin Infect Dis. 2001;32(5):694–700. doi: 10.1086/319210. [DOI] [PubMed] [Google Scholar]
- 104.Lessa FC, Gould PL, Pascoe N, et al. Health care transmission of a newly emergent adenovirus serotype in health care personnel at a military hospital in Texas, 2007. J Infect Dis. 2009;200(11):1759–65. doi: 10.1086/647987. [DOI] [PubMed] [Google Scholar]
- 105.Gordon YJ, Gordon RY, Romanowski E, et al. Prolonged recovery of desiccated adenoviral serotypes 5, 8, and 19 from plastic and metal surfaces in vitro. Ophthalmology. 1993;100(12):1835–9. doi: 10.1016/s0161-6420(93)31389-8. [discussion: 39–40] [DOI] [PubMed] [Google Scholar]
- 106.Zambon M, Bull T, Sadler CJ, et al. Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. J Clin Microbiol. 1998;36(8):2289–93. doi: 10.1128/jcm.36.8.2289-2293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brady MT, Evans J, Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control. 1990;18(1):18–23. doi: 10.1016/0196-6553(90)90206-8. [DOI] [PubMed] [Google Scholar]
- 108.Ansari SA, Springthorpe VS, Sattar SA, et al. Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol. 1991;29(10):2115–9. doi: 10.1128/jcm.29.10.2115-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]