Abstract
Background
Magnetic resonance (MR) technology offers non-invasive methods for in vivo assessment of neuroabnormalities.
Methods
A comprehensive neuropsychological/psychiatric battery, coupled with MR imaging, (MRI), MR spectroscopy (MRS), and functional MRI (fMRI) assessments, were administered to children with fetal alcohol spectrum disorders (FASD) to determine if global and/or focal abnormalities could be identified, and distinguish diagnostic subclassifications across the spectrum. The four study groups included: 1. FAS/Partial FAS; 2. Static Encephalopathy/Alcohol Exposed (SE/AE); 3. Neurobehavioral Disorder/Alcohol Exposed (ND/AE) as diagnosed with the FASD 4-Digit Code; and 4. healthy peers with no prenatal alcohol exposure. Presented here are the MRI assessments used to compare the sizes of brain regions between the four groups. The neuropsychological/behavioral, MRS, and fMRI outcomes are reported separately.
Results
Progressing across the four study groups from Controls to ND/AE to SE/AE to FAS/PFAS, the mean absolute size of the total brain, frontal lobe, caudate, putamen, hippocampus, cerebellar vermis, and corpus callosum length decreased incrementally and significantly. The FAS/PFAS group (the only group with the 4-Digit FAS facial phenotype) had disproportionately smaller frontal lobes relative to all other groups. The FAS/PFAS and SE/AE groups (the two groups with the most severe CNS dysfunction) had disproportionately smaller caudate regions relative to the ND/AE and Control groups. The prevalence of subjects in the FAS/PFAS, SE/AE, and ND/AE groups that had one or more brain regions, two or more standard deviations below the mean size observed in the Control group was78%, 58%, and 43%, respectively . Significant correlations were observed between size of brain regions and level of prenatal alcohol exposure, magnitude of FAS facial phenotype, and level of CNS dysfunction.
Conclusions
MRI provided further validation that ND/AE, SE/AE, and FAS/PFAS, as defined by the FASD 4-Digit Code, are three clinically distinct and increasingly more affected diagnostic subclassifications under the umbrella of FASD. Neurostructural abnormalities are present across the spectrum. MRI could importantly augment diagnosis of conditions under the umbrella of FASD, once population-based norms for structural development of the human brain are established.
Keywords: Fetal alcohol spectrum disorder (FASD), Magnetic resonance imaging (MRI), FASD 4-Digit Diagnostic Code
Fetal alcohol syndrome (FAS) is a permanent birth defect syndrome caused by maternal alcohol consumption during pregnancy. FAS is defined by growth deficiency, a unique cluster of minor facial anomalies, and central nervous system (CNS) dysfunction and/or structural brain abnormalities (Smith, 1979). The cognitive/behavioral problems in this condition stem from prenatal brain damage. Not all individuals with prenatal alcohol exposure present with measurable CNS dysfunction or structural brain abnormalities, and not all who present with measurable CNS dysfunction or structural brain abnormalities have FAS. Recently, the term Fetal Alcohol Spectrum Disorders (FASD) was coined to depict the spectrum of outcomes observed among individuals with prenatal alcohol exposure. The degree of brain damage among individuals with prenatal alcohol exposure may vary from microcellular and neurochemical aberrations to gross structural anomalies. Similarly, cognitive/behavioral dysfunction varies along the full continuum from mild developmental delay or learning disabilities to global developmental disability (Astley et al., 2009b). The specificity of the FAS facial phenotype to prenatal alcohol exposure lends credence to the clinical judgment that the cognitive and behavioral dysfunction observed in individuals with FAS is due, at least in part, to brain damage caused by a teratogen (Aase et al., 1995; Astley et al., 2002; Astley and Clarren, 2001). Unfortunately, without the unique facial phenotype of FAS or at least a severe or clinically obvious expression of brain damage, the neurodevelopmental disabilities of an individual affected by prenatal alcohol exposure often go unrecognized and inappropriately served (Streissguth et al., 1993).
Many individuals with prenatal alcohol exposure exhibit cognitive difficulties and significant maladaptation that prevent them from leading productive, independent lives (Stratton et al., 1996; Streissguth et al., 2004). Across the population, the profile of cognitive dysfunction among individuals with prenatal alcohol exposure is highly variable, though there are some commonalities in functional compromise among subgroups, and conceptual models of overarching deficits have been proposed (Kodituwakku, 2007). However, no single behavioral phenotype specific to alcohol teratogenicity has been described. Without a behavioral phenotype specific to the teratogen alcohol, attributing an alcohol-exposed child’s dysfunction to brain damage is often questionable at a clinical level (Aase et al., 1995). If indisputable evidence of brain damage (e.g., alterations in neurostructure, neurometabolites and/or neuroactivation) could be found in these individuals, and linked to behavioral deficit, diagnostic efforts could be improved. The “disability” of these alcohol-exposed children would be clearly established, and help facilitate eligibility for needed services. Further, if specific alterations in neurostructure, neurometabolites, and/or neuroactivation could be linked to clinically meaningful, discrete neuropsychological deficits, development of appropriate intervention programs could be accelerated.
The overall goal of this research study was to determine if magnetic resonance imaging, (MRI), magnetic resonance spectroscopy (MRS), and/or functional MRI (fMRI) could serve as non-invasive methods for definitively identifying global and/or focal brain abnormality across the full continuum of FASD, and distinguish diagnostic subclassifications within the spectrum. The results of this comprehensive study are presented in four separate reports: MRI (presented here), and the neuropsychological/behavioral (Astley et al., 2009b), MRS (Astley et al., 2009), and fMRI (Astley et al., 2009a) outcomes reported separately. The focus on FASD diagnostic methodology in this report directly responds to the following Research Recommendations for Diagnostic Criteria published in the Institute of Medicine report on FASD (Stratton et al., 1996): 1) Research to evaluate the utility, reliability, and validity of schemes for classification and diagnosis. 2) Research to identify potential structural or functional brain abnormalities and other neurobiological indices that may be associated with, or distinguish FAS, Alcohol-related Neurodevelopmental Disorder (ARND), or Alcohol-related Birth Defect (ARBD), and to relate these abnormalities and indices to cognitive and behavioral correlates.
MRI allows for very sensitive assessment of size, shape, spatial orientation, and even tissue composition of selected brain regions. Numerous FASD MRI studies have been conducted to date (Archibald et al., 2001; Bookstein et al., 2002b; Mattson et al., 2001; Miller et al., 1999; Riley et al., 1995; Sowell et al., 2001b; Sowell et al., 2002a; Sowell et al., 2002b). Documented abnormalities include reduction in overall brain size, reduction in absolute size of selected brain regions (basal ganglia, caudate, cerebellum, and anterior/posterior regions of the corpus callosum), disproportionate reduction of the caudate, alterations in shape and spatial orientation of the corpus callosum, and white matter hypoplasia in the parietal and temporal lobes.
The majority of FASD MRI studies published to date have enrolled study groups diagnosed or classified as FAS, Fetal Alcohol Effects (FAE), ARND, or Prenatal Exposure to Alcohol (PEA) prior to the establishment of comprehensive, case-defined FASD diagnostic guidelines that are quickly becoming best practice (Astley, 2004; Bertrand et al., 2004; Chudley et al., 2005). The specific diagnostic criteria used to establish the FASD study groups (e.g., level of growth deficiency; type, number and severity of facial anomalies; breadth and magnitude of neuropsychological impairment; type of neurostructural anomaly present), providing confirmation that FASD diagnostic subgroups are clinically and statistically distinct from each other, are typically not reported. Absence of rigorous diagnostic methods can lead to diagnostic misclassification. Astley and Clarren (Astley and Clarren, 2000) and Hoyme et al. (Hoyme et al., 2005) have both demonstrated that individuals diagnosed with FAS by a gestalt approach often lose that diagnostic classification when more rigorous diagnostic guidelines are applied. Misclassification impacts study validity and reduces the power of a study to detect clinically meaningful differences between FASD subgroups. If specific diagnostic features that define the FASD study groups are not clearly reported, this limits the ability to compare outcomes across studies.
In general, most FASD MRI studies have found significant differences between FAS and control groups, regardless of diagnostic system used; but have not always found differences between clinical subgroups on the fetal alcohol spectrum. In the current study, the sizes of brain regions were compared between three FASD diagnostic subgroups and a healthy control group with no prenatal alcohol exposure. An important goal of this MRI study was to determine if meaningful neurostructural differences do exist between FASD subgroups when the subgroups, including FAS, are rigorously case-defined (Astley, 2004) and confirmed to be clinically and statistically distinct from one another (Astley et al., 2009b).
MATERIALS AND METHODS
Subjects and Study Groups
The protocol was approved by the University of Washington Human Subjects Review Board. The goal of the study was to create three clinically distinct and increasingly less affected FASD study groups and one healthy unexposed control group that fell along the following ordinal scale: 1) significant brain damage/dysfunction with the FAS facial phenotype; 2) significant brain damage/dysfunction without the FAS facial phenotype; 3) mild to moderate brain dysfunction without the FAS facial phenotype and 4) healthy with no prenatal alcohol exposure. The three FASD groups were selected from among 1,200 patients previously diagnosed by an interdisciplinary team in the WA State FAS Diagnostic & Prevention Network (FAS DPN) of clinics using a practical, comprehensive diagnostic system called the FASD 4-Digit Code (Astley, 2004; Astley and Clarren, 2000). Briefly, the 4 digits of the FASD 4-Digit Code reflect the magnitude of expression of the 4 key diagnostic features of FASD, in the following order: (1) growth deficiency, (2) characteristic FAS facial phenotype, (3) CNS structural/functional abnormalities, and (4) prenatal alcohol exposure (Figure 1). The magnitude of expression of each feature is ranked independently on a 4-point Likert scale, with 1 reflecting complete absence of the FASD feature and 4 reflecting a strong “classic” presence of the FASD feature. Each Likert rank is specifically case defined. There are 256 possible 4-digit diagnostic codes, ranging from 1111 to 4444. Each 4-digit diagnostic code falls into 1 of 22 unique clinical diagnostic categories (labeled A through V). Seven of the 22 diagnostic categories (4-Digit Categories A–C and E–H) fall under the umbrella of FASD (A. FAS/Alcohol Exposed, B. FAS/Alcohol Exposure Unknown, C. Partial FAS/Alcohol Exposed, E-F. Static Encephalopathy/Alcohol Exposed, and G-H. Neurobehavioral Disorder/Alcohol Exposed). The three FASD study groups in the current study represent these FASD diagnostic categories. This diagnostic system is currently being used by a wide variety of diagnostic teams in the USA and other countries. The control population for this study was selected primarily from a large cohort of children enrolled at birth in a University of Washington study of typical development conducted through the Department of Speech and Hearing Sciences. With the enrollment of each child in the FAS/PFAS group, a child matched on age (within 6 months), gender, and race was randomly identified and invited to enroll from the eligible SE/AE, ND/AE and Control populations. The enrollment goal was 80 subjects (20 per group).
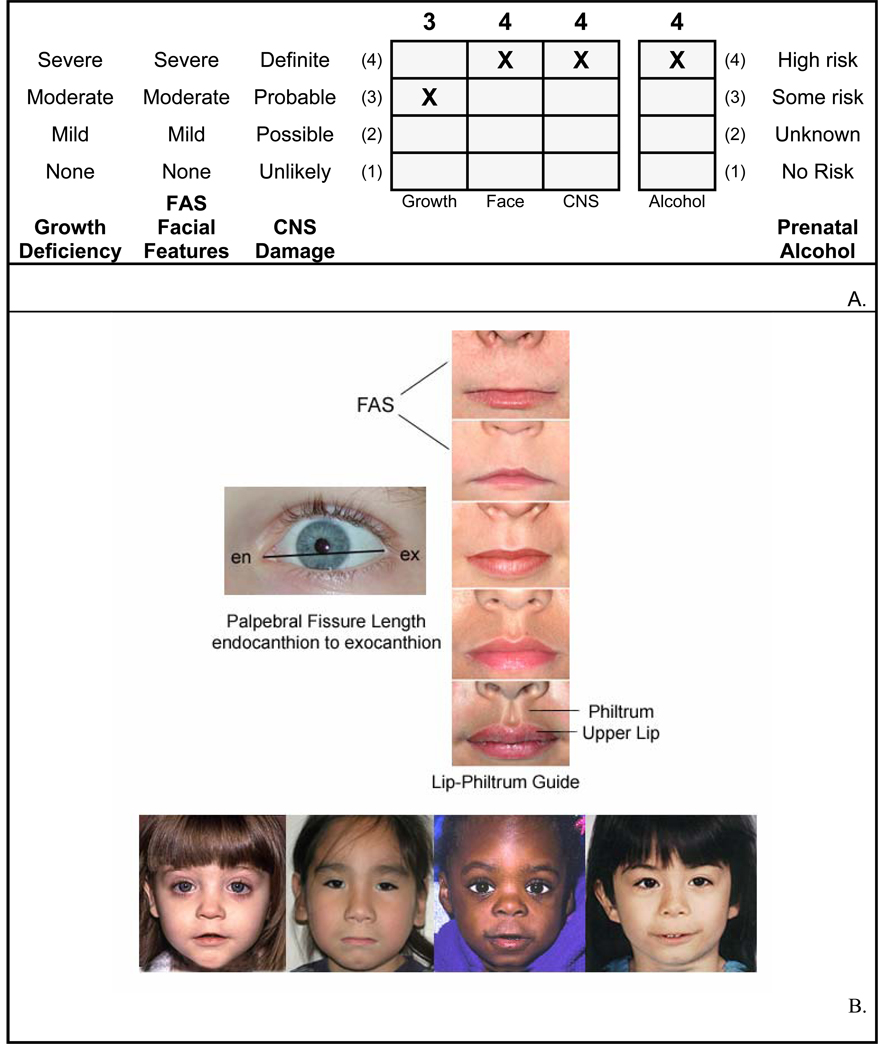
Figure 1.
A. FASD 4-Digit Diagnostic Code grid. FASD is defined by growth deficiency, specific FAS facial features, evidence of CNS damage and prenatal alcohol exposure. The 4-Digit Code ranks each of these areas on 4-point, case-defined, Likert scales. The 4-Digit Code (3444) inserted in the grid is 1 of 12 codes that meet the diagnostic criteria for FAS (Astley, 2004). B. FASD 4-Digit Code FAS facial phenotype. The Rank 4 FAS facial phenotype determined with the 4-Digit Diagnostic Code requires the presence of all 3 of the following anomalies: (1) palpebral fissure length 2 or more standard deviations below the norm; (2) smooth philtrum (Rank 4 or 5 on the Lip-Philtrum Guide), an (3) thin upper lip (Rank 4 or 5 on the Lip-Philtrum Guide). Examples of the Rank 4 FAS facial phenotype for Caucasian, Native American, African American, and Asian American children are shown.
The study enrollment procedure produced a sample of 81 children of diverse ethnicity. The age range (8 to 15.9 years) included the broadest age range of children that could be administered a comparable psychometric assessment battery and be reasonably capable of participating in the MR scanning. Each of the four study groups had 16–24 subjects successfully balanced on age, gender, and race. The 61 children with FASD were highly representative of the entire clinic sample of 1,200 from which they were drawn. The clinic population of 1,200 was 43% female, 51% Caucasian, with 40% between 8 and 15.9 years of age. The 4-Digit Codes of all 81 children are presented in the neuropsychological/behavioral report for this study (Astley et al., 2009b). The diagnostic features specific to each group were as follows:
Children in Group 1 had a 4-Digit diagnosis of FAS or Partial FAS (FAS/PFAS) (e.g., 4-Digit Diagnostic Categories A,B,C: with Growth Ranks 1–4, Face Ranks 3–4, CNS Ranks 3 and/or 4, and Alcohol Ranks 2–4) (Figure 1). Alcohol Rank 2 (unknown exposure) could only be present if the child had a diagnosis of full FAS because the Rank 4 FAS facial features are so specific to prenatal alcohol exposure (Astley et al., 2002; Astley and Clarren, 1996). Since the only clinical difference between FAS and PFAS in this study was the presence of growth deficiency in the former, FAS and PFAS were combined. In summary, children in Group 1 had severe cognitive/behavioral dysfunction and the FAS facial phenotype.
Children in Group 2 had a 4-Digit diagnosis of Static Encephalopathy/Alcohol Exposed (SE/AE) (e.g., 4-Digit Diagnostic Categories E and F: with Growth Ranks 1–4, Face Ranks 1–2, CNS Ranks 3 and/or 4, and Alcohol Ranks 3–4). In summary, children in Group 2 had severe cognitive/behavioral dysfunction, comparable to Group 1, but did not have the FAS facial phenotype.
Children in Group 3 had a 4-Digit diagnosis of Neurobehavioral Disorder / Alcohol Exposed (ND/AE) (e.g. 4-Digit Diagnostic Categories G and H: with Growth Ranks 1–4, Face Ranks 1–2, CNS Rank 2, and Alcohol Ranks 3–4). In summary, children in Group 3 had prenatal alcohol exposure comparable to Groups 1 and 2, but had only mild to moderate cognitive/behavioral dysfunction, and did not have the FAS facial phenotype.
Children in Group 4 (Healthy Controls/No Alcohol Exposure) were selected based on parental report that the child was healthy, had no academic concerns, and no prenatal alcohol exposure (e.g., 4-Digit Diagnostic Category V: with Growth Ranks 1–2, FAS Face Ranks (no restrictions), CNS Rank 1, Alcohol Rank **1). In summary, these were non-exposed, healthy, average to high-functioning controls.
Using the FASD terminology introduced by the Stratton et al. (Stratton et al., 1996), the SE/AE and ND/AE groups most closely reflect the severe and mild expressions of ARND, respectively. A comprehensive analysis of the between-group differences of these diagnostic features is presented in the neuropsychological/psychiatric report for this study (Astley et al., 2009b).
Within our FASD participants, one subject with PFAS had agenesis of the corpus callosum (ACC) and one subject with FAS had hypogenesis of the corpus callosum (HCC). That these subjects had callosal abnormalities was known prior to study enrollment. Indeed these two subjects with ACC/HCC are the only documented cases of ACC/HCC in the 2,040 patients with prenatal alcohol exposure diagnosed to date at the WA State FAS DPN clinics. MRIs are typically only available when clinically indicated (e.g., evidence of neurological abnormalities). As such, only 204 (10%) of the 2,040 patients evaluated at the FAS DPN had a previous MRI evaluation summarized in their medical record and 76% of the 204 MRI evaluations were interpreted as normal by the patient’s neuroradiologist. Although ACC/HCC has been observed in individuals with FASD (Riley et al., 1995), ACC/HCC is not specific to prenatal alcohol exposure. The prevalence of ACC among developmentally disabled populations is estimated to be 2–3 per 100 (Jeret et al., 1986). Thus, a causal link between ACC/HCC and prenatal alcohol exposure in these two individuals should not be assumed; nor can it be ruled-out. Since all current FASD diagnostic guidelines (Astley, 2004; Bertrand et al., 2004; Chudley et al., 2005; Hoyme et al., 2005) list ACC/HCC as one of the many types of structural abnormalities that meet the CNS criteria for a FAS/PFAS diagnosis, it would be clinically invalid to exclude ACC/HCC from the FAS/PFAS study group. While they may represent a very small fraction of all alcohol-exposed patients evaluated in the FAS DPN clinic, they represent two of all 41 patients with FAS/PFAS from the FAS DPN clinic who met the 8–15 year-old eligibility criteria for enrollment into this study.
Study Participation
Participation in the study involved five visits over a 4 to 6 week study period. The psychological and sociodemographic data were collected during visits 1 and 2. The MR data were collected during visits 3 and 4. Outcomes of the psychological assessments were shared with the caregivers on visit 5, and submitted to the child’s medical record with caregiver consent.
Sociodemographic and Clinical Assessments
A comprehensive sociodemographic and health/medication history of each child was obtained by parent interview and record review. Information included birth data, growth, and all prenatal and lifetime exposures and adverse events. For subjects with FASD, most information was obtained at the time of their FASD diagnostic evaluation. All controls had a reported absence of prenatal alcohol exposure. All children had a standardized digital facial photograph taken at the time of enrollment. The facial photographs were analyzed using the FAS Facial Analysis Software (Astley, 2003) to document the magnitude of expression of the FAS facial phenotype (Astley and Clarren, 1996). A more detailed methodology and analysis of the sociodemographic and FASD diagnostic outcomes, including prenatal alcohol exposure histories, are presented in the neuropsychological/behavioral report from this study (Astley et al., 2009b).
Neuropsychological and Psychiatric Assessments
A detailed description of the assessment battery and a comprehensive analysis of the between-group differences in neuropsychological outcome are presented in the neuropsychological/behavioral report for this study (Astley et al., 2009b). Briefly, a comprehensive, standardized assessment battery was administered to each child and their primary caregiver by a psychologist masked to group assignment. The assessment battery was designed to capture the domains of potential neuropsychological impairment seen as the result of the typically diffuse brain damage arising from alcohol teratogenesis (Bertrand et al., 2004; Chudley et al., 2005; Kodituwakku, 2007; Mattson and Riley, 1998; Olson et al., 1998; Roebuck et al., 1999). The neuropsychological/behavioral outcomes served to profile the study groups and confirm the groups were clinically and statistically distinct from one another; fundamental to the interpretation of the MR outcomes. The neuropsychological data will also be used to assess correlations between brain structure and function. These correlations will be reported separately.
It is important to note that the mean full scale IQ of the healthy Control group (123 + 7 SD) was higher than the population-based mean of 100 ± 15 SD. This was anticipated since children with potential threats to brain development were screened out and recruitment was through a University community. Other population-based MRI and FASD-MRI studies enrolling healthy controls have reported mean FSIQs ranging from 110 to 127 (Bookstein et al., 2002b; Haier et al., 2004; Sowell et al., 2008; Waber et al., 2007). Most FASD-MRI studies do not report the IQ or neuropsychological profile of their healthy control population. In spite of the Control group’s relatively high IQ, however, many of the memory, executive function, language and adaptive behavior scores assessed for this Control group were, on average, solidly within normal limits compared to age peers (Table 1) (Astley et al., 2009b). The ND/AE group had a mean FSIQ (99.2 + 11.3 SD) equivalent to the population-based mean, despite their multiple prenatal/postnatal risk factors and significant adaptive/behavioral deficits.
Table 1.
Sociodemographic and FASD 4-Digit Diagnostic Code profiles of the four study groups.
| Characteristic | Groups | Statistics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. FAS/PFAS A |
2. SE/AE |
3. ND/AE |
4. Control |
ANOVA | Chi2 | ||||||||
| Overall | Post Hoc |
A Priori LT |
|||||||||||
| N = 20 | N = 24 | N = 21 | N = 16 | F (p) B | Duncan | F (p) C | Chi2 (p) | ||||||
| Gender n (%) | |||||||||||||
| female | 10 | (50.0) | 8 | (33.3) | 10 | (47.6) | 8 | (50.0) | 1.7 (.63) | ||||
| Age at enrollment in years: mean (SD) | 12.7 | (2.4) | 12.2 | (2.0) | 12.4 | (2.3) | 12.4 | (2.7) | 0.1 (.96) | 0.1 (.79) | |||
| Race: n (%) | |||||||||||||
| Caucasian | 12 | (60.0) | 11 | (45.8) | 12 | (57.1) | 13 | (81.3) | D 5.0 (.17) | ||||
| African American | 6 | (30.0) | 4 | (16.7) | 6 | (28.6) | 2 | (12.6) | |||||
| Native American | 2 | (10.0) | 7 | (29.2) | 2 | (9.5) | 0 | (0.0) | |||||
| Other | 0 | (0.0) | 2 | (8.3) | 1 | (4.8) | 1 | (6.3) | |||||
| Growth Rank from 4-Digit Code: n (%) | |||||||||||||
| 1 none | 10 | (50.0) | 15 | (62.5) | 13 | (61.8) | 15 | (93.7) | E 10.3 (.02) | ||||
| 2 mild | 2 | (10.0) | 2 | (8.3) | 6 | (28.6) | 1 | (6.3) | |||||
| 3 moderate | 5 | (25.0) | 3 | (12.5) | 1 | (4.8) | 0 | (0.0) | |||||
| 4 severe | 3 | (15.0) | 4 | (16.7) | 1 | (4.8) | 0 | (0.0) | |||||
| Current height percentile: mean (SD) | 33.5 | (31.6) | 40.7 | (30.4) | 34.7 | (30.9) | 60.6 | (34.2) | 2.7 (.05) | 132,24 | 5.2 (.03) | ||
| Current weight percentile: mean (SD) | 51.7 | (33.4) | 50.4 | (34.2) | 46.6 | (31.2) | 67.6 | (24.5) | 1.5 (.22) | 1.7 (.19) | |||
| Face Rank from 4-Digit Code: n (%) c | |||||||||||||
| 1 none | 0 | (0.0) | 4 | (16.7) | 7 | (33.3) | 10 | (62.5) | |||||
| 2 mild | 0 | (0.0) | 20 | (83.3) | 14 | (66.7) | 6 | (37.5) | |||||
| 3 moderate | 4 | (20.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |||||
| 4 severe | 16 | (80.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |||||
| Facial D-Score | 1.2 | (0.9) | −0.4 | (0.9) | −0.8 | (0.7) | −1.5 | (0.9) | 31.6 (.000) | 1,23,4 | 84.4 (.000) | ||
| CNS Ranks 1–3 from 4-Digit Code | |||||||||||||
| Level of functional impairment: n (%) | |||||||||||||
| 1. none | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 16 | (100) | |||||
| 2. moderate | 0 | (0.0) | F 3 | (12.5) | 21 | (100) | 0 | (0.0) | |||||
| 3. severe | 20 | (100) | 21 | (87.5) | 0 | (0.0) | 0 | (0.0) | |||||
| CNS Rank 4 from 4-Digit Code | |||||||||||||
| Structural / Neurologic Abnormality: n (%) | |||||||||||||
| Present | 13 | (65.0) | 6 | (25.0 | 0 | (0.0) | 0 | (0.0) | G 31 (.000) | ||||
| Microcephaly (OFC ≤ −2 SD): n, % | 10 | (50.0) | 2 | (8.3) | 0 | (0.0) | 0 | (0.0) | H26(.000) | ||||
| Alcohol Rank from 4-Digit Code: n (%) | |||||||||||||
| 1. No exposure | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 16 | (100) | |||||
| 2. Unknown exposure | 1 | (5.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |||||
| 3. Confirmed exposure. Level moderate or Unk. | 7 | (35.0) | 12 | (50.0) | 11 | (52.4) | 0 | (0.0) | |||||
| 4. Confirmed exposure: Level high | 12 | (60.0) | 12 | (50.0) | 10 | (47.6) | 0 | (0.0) | |||||
| Alcohol use prior to pregnancy | |||||||||||||
| Days/week: mean (SD), range | 5.4 (1.7) | 2–7 | 4.0 (2.2) | 1–7 | 5.3 (2.1) | 1–7 | 0.9 (1.0) | 0–3 | 19.8 (.000) | 123,4 | 31.0 (.000) | ||
| Most drinks/occasion: mean (SD), range | 23.1 (24.8) | 8–78 | 19.8 (26.3) | 2–96 | 12.7 (7.7) | 4–24 | 1.7 (1.5) | 0–5 | 3.8 (.018) | 123,4 | 8.7 (.005) | ||
| Alcohol use during pregnancy | |||||||||||||
| Days/week: mean (SD), range | 5.5 (1.7) | 3–7 | 3.9 (2.1) | 1–7 | 5.3 (2.1) | 1–7 | 0 (0) | 0–0 | 35.9 (.000) | 123,4 | 35.8 (.000) | ||
| Most drinks/occasion: mean (SD), range | 11.6 (7.1) | 5–24 | 14.1 (8.9) | 3–26 | 11.7 (7.3) | 4–24 | 0 (0) | 0–0 | 14.2 (.000) | 123,4 | 17.8 (.000) | ||
| Drank all 3 trimesters: n (valid %) | 13 | (77) | 13 | (59) | 7 | (50) | 0 | (0) | 21 (.000) | ||||
| Neuropsychological. Selected Tests | Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |||||
| WISC-III: FSIQ (ss) | 77.5 | (14.4) | 79.3 | (10.5) | 99.2 | (11.3) | 123.9 | (6.5) | 67.6 (.000) | 12,3,4 | 183.4 (.000) | ||
| VABS: Adaptive Behavior Composite (ss) | 59.0 | (17.5) | 55.0 | (14.2) | 65.4 | (21.1) | 95.3 | (12.3) | 21.1 (.000) | 123,4 | 46.3 (.000) | ||
| D-KEFS: Tower, Total Achievement (ss) | 7.6 | (2.3) | 8.3 | (2.5) | 9.6 | (2.1) | 10.8 | (2.1) | 7.0 (.000) | 12,23,34 | 20.5 (.000) | ||
| VMI: Total (ss) | 76.2 | (12.7) | 81.4 | (9.2) | 90.9 | (11.8) | 102.7 | (12.9) | 17.8 (.000) | 12,3,4 | 51.6 (.000) | ||
Abbreviations: Chi2 chi-square test across four study groups, unless otherwise specified. D-KEFS: Delis-Kaplan Executive Function System, Tower, Total Achievement (Delis et al., 2000). Duncan: Multiple comparison range test. Reported if the overall ANOVA is statistically significant. Commas separate groups with homogeneous means at p < 0.05. F: F statistic. LT: ANOVA unweighted linear trend. OFC: occipital frontal circumference. Overall: Overall assessment of between-group means using ANOVA. p: p-value. SD: standard deviation. Unk: unknown. VABS: Vineland Adaptive Behavior Scales, Adaptive Behavior Composite (Sparrow et al., 1984). VMI: Beery Buktenica Developmental Test of Visual-Motor Integration (Beery, 1997). WISC-III Full Scale IQ (Wechsler, 1996).
Notations:
Six of the 20 subjects in Group 1 had full FAS using the 4-Digit Code. Ten of the 14 PFAS had Rank 4 Faces, but received a diagnosis of PFAS because they had no growth deficiency (Growth Rank 1).
Between groups degrees of freedom = 3; within groups df = total sample size minus 4.
Between groups linear term degrees of freedom = 1; within groups df = total sample size minus 4.
Caucasian versus not Caucasian.
No growth deficiency versus mild-severe growth deficiency.
All 3 children with moderate functional impairment had structural evidence of brain abnormality (microcephaly).
FAS/PFAS versus SE/AE (Chi-square = 7.1, p = .008).
FAS/PFAS versus SE/AE (Chi-square = 9.6, p = .002).
MR Scanner
All scans (MRI, MRS and fMRI) were acquired under the direction of Dr. Richards using a General Electric 1.5 Tesla scanner in the Diagnostic Imaging Sciences Center (DISC) at the University of Washington.
MRS and fMRI
The MRS (Astley et al., 2009) and fMRI (Astley et al., 2009a) components of this study are reported separately. Briefly, MRS was used to measure the concentrations of three neurometabolites: 1) choline, a marker of cell membrane stability and myelination, 2) N-acetyl aspartate, a neuronal or axonal marker, and 3) creatine, a marker of metabolic activity, in selected brain regions. fMRI was used to assess neuroactivation in selected brain regions during performance of N-back working memory tasks.
Structural MRI Acquisition
An initial sagittal series was obtained first for orientation of subsequent series (TE=8, TR=400, flip angle = 25°, FOV =24 × 24, matrix = 256 × 160, thickness = 4.0mm, gap = 1.0mm). A high resolution 3-D T1-weighted fast gradient echo image of the whole brain was then performed in the axial plane (TE= 3, TR= 18, FOV =24 × 18, matrix = 256 × 256, NEX = 2, thickness = 1.5mm, no gap). This acquisition allows images to be reformatted into any plane, which, in turn, allows measurement of each structure in the plane that is optimal for its visualization. This also allows brain scans to be realigned to ensure that all scans are being measured in a standardized format. An exact midsagittal slice was reconstructed, allowing for area measures of corpus callosum and cerebellar vermis. Total scanning time for the structural series was approximately 15 minutes. Each scan was clinically reviewed by the neuroradiologist (masked to group assignment) to determine if there were any gross structural abnormalities.
Structural MRI Image Processing
MRI measures were performed, under the direction of Dr. Aylward, on a PC workstation, using the MEASURE program (Barta et al., 1997). Each scan was rotated in three-dimensional space so that the axial images were parallel to the line connecting the anterior and posterior commissures and perpendicular to the interhemispheric fissure (AC-PC plane). Coronal slices were reconstructed with a thickness of 0.9375mm and positioned perpendicular to the AC-PC plane. Based on prior FASD MRI literature (Archibald et al., 2001; Mattson et al., 2001; Miller et al., 1999; Sowell et al., 2001b), volume measurements focused on the hippocampus, caudate, putamen, frontal lobe, gray matter and white matter of the frontal lobe, and total brain volume from the 1.5mm SPGR. Area measures included cerebellar vermis and its subregions, corpus callosum and its subregions, and total brain area in the midsagittal slice. For the hippocampus, caudate, putamen, and frontal lobe volume measures, area of each structure was outlined manually, using a mouse-controlled cursor, in each slice. Areas within each slice were calculated, summed across slices, and multiplied by slice thickness, resulting in approximate structure volumes. Semi-automated methods were used to measure total brain volume and a stereology point-counting method was used to measure gray and white matter within the frontal lobe. Specific methods of measurement for each structure were as follows:
Hippocampus
The rules for defining boundaries of the hippocampus were developed by Aylward et al. (Aylward et al., 1999). Briefly, measurement was made on the reconstructed coronal slices, beginning in the most posterior coronal slice in which the hippocampus is viewed. Boundaries of the hippocampus were traced manually, with the choroid fissure as the superior boundary, the inferior temporal horn of the lateral ventricle as the lateral boundary, and the white matter of the parahippocampal gyrus as the inferior boundary. The hippocampus forms a natural boundary around the edge of the medial temporal lobe. Both the alveus and the subiculum were included in hippocampal measurements. Anteriorly, when a clear demarcation between the hippocampus and amygdala was not seen coronally, the sagittal view was consulted to determine the border between the hippocampus and amygdala. Inter-rater reliability for the hippocampus yielded an intraclass correlation of 0.92.
Basal Ganglia
Volumes of putamen and caudate were obtained on the axial images, using rules previously described (Aylward et al., 1997a). Briefly, measurement of putamen and caudate began in the most inferior slice in which these structures were clearly separated by the internal capsule. Measurement continued in a superior direction until the body of the caudate was no longer observed. The borders of the caudate were defined laterally by the anterior limb of the internal capsule and medially by the frontal horn or body of the lateral ventricle. The borders of the putamen were defined laterally by the external capsule. At more inferior levels, the medial borders of the putamen were defined by the globus pallidus; at more superior levels, the medial borders were defined by the internal capsule. Intra-rater reliability yielded intraclass correlations of .99 for both caudate and putamen. Inter-rater reliability yielded intraclass correlations of 0.97 for caudate and 0.94 for putamen.
Frontal Lobe Volume
The procedure used to measure the volume of the frontal lobes (Aylward et al., 1997b) and of the gray and white matter within the frontal lobes was based on the identification of sulcal-gyral landmarks on the surface of a 3-D reconstruction of the 1.5mm coronal slices. For frontal lobe measures, this 3-D reconstruction, which could be viewed in any orientation, was used to identify the precentral gyrus and sylvian fissure. Using the MEASURE software, the surfaces of these regions were “painted” on the 3-D reconstruction. The brain imaging data was then resliced in the axial plane, starting at the most superior level, and the paint (which remains on the surface of the recreated slices) was used to guide, as the rater cut away portions of the brain posterior to the paint. Inter-rater reliability yielded an intraclass correlation of .99 for frontal lobe volume. After the frontal lobe was thus identified, a stereological point-counting method was used to measure gray and white volumes within the frontal lobe, as described by Aylward et al. (Aylward et al., 1995). Inter-rater reliability yielded an intraclass correlation of 0.92 for the frontal cortex.
Total Brain Volumes
Regional volumes were divided by total brain volume to allow correction for overall brain size. Total brain volume was measured using semi-automated thresholding procedures for segmenting brain tissue from cerebral spinal fluid (CSF) on the 1.5 mm axial images. Briefly, this procedure allowed the user to set the contrast such that all pixels above a certain value were highlighted, thus eliminating CSF (which in these images was black). A mask of this region was saved, and a command of “erode” was executed that “shrunk” the highlighted areas by a specified number of pixels. This performed automatic cutting of “bridges” between highlighted brain and non-brain (e.g., dura, muscle) tissue regions. The cursor was then placed inside the brain region, and an “isolate blob” command then identified only those pixels that were connected (in any plane) with the pixel at the cursor location. This allowed the brain to be isolated from non-brain tissue. Finally a “dilate” command was executed, which restored pixels that were eroded previously, but no further than the original mask. Raters modified the procedure to ensure that only non-brain tissue was removed. Intra-rater reliability for obtaining brain volumes with this procedure yielded an intraclass correlation of 0.99. At each step, modifications were made manually to ensure inclusion of all brain tissue and exclusion of nonbrain regions.
Midsagittal Area Measures
The 3-D brain was rotated so that the interhemispheric fissure was perfectly positioned in the vertical plane. As described above, the slices were reformatted from the 1.5mm axial series, yielding sagittal slices that were 0.9375mm thick. The sagittal slice yielding the clearest visualization of the cerebral aqueduct was selected for the midsagittal measures. Midsagittal area measures were performed for cerebellar vermis and its three subsections (Lobules I–V, Lobules VI–VII, and Lobules VIII–X), corpus callosum and five subregions, and total brain (Aylward and Reiss, 1991; Aylward et al., 1994; Reiss et al., 1991).Inter-rater reliability for obtaining midsagittal areas yielded intraclass correlations ranging from 0.87 to 0.94. Midsagittal area of the total brain was also measured to allow correction of the corpus callosum and cerebellar vermis measures for overall brain size. The CC length (cm) was the distance from the most anterior and posterior borders of the CC that intersected with a line perpendicular to the ac-pc plane. B. The CC was transected into five equiangular regions (1 genu, 2 anterior body, 3 posterior body, 4 isthmus, 5 splenium, using the mammilary body as a reference point (Riley et al., 1995).
MRI Hypotheses
In this report the analyses focus on the MRI comparisons between the four study groups. The following primary hypotheses were derived from the current MRI FASD literature (Archibald et al., 2001; Astley and Clarren, 2001; Bookstein et al., 2002b; Mattson et al., 2001; Miller et al., 1999; Sowell et al., 2001b). Note again that the neuropsychological/psychiatric report for this study (Astley et al., 2009b) confirmed the four study groups were clinically distinct and increasingly more affected progressing across the four groups from the Controls to the FAS/PFAS.
The mean absolute and/or relative size of the brain as a whole and its regions will become increasingly smaller progressing across the four study groups from Controls to ND/AE to SE/AE to FAS/PFAS.
The mean absolute and/or relative size of the brain as a whole and its regions will be significantly smaller in the FAS/PFAS group (with the FAS facial phenotype) compared to the SE/AE group (without the FAS facial phenotype). This hypothesis specifically addresses a common clinical question: Do individuals with FASD and the FAS facial phenotype have more severe brain abnormality than individuals with FASD and no FAS facial phenotype?
The prevalence of subjects with one or more brain regions two or more standard deviations below the mean size of the Control group will increase significantly progressing from the ND/AE group, to the SE/AE group, to the FAS/PFAS group.
Statistical Analysis
The statistical analyses used to confirm the four study groups were effectively balanced on age, gender, and race are described and presented in the neuropsychological / psychiatric report (Astley et al., 2009b). Primary analyses: ANOVA was used to determine if differences in mean size of brain regions existed among the four study groups. If significant differences existed, the Duncan post hoc range test was used to identify which group means differed. The Duncan test makes pairwise comparisons using a stepwise procedure. Means are ordered from highest to lowest, and extreme differences are tested first. The Duncan test sets a protection level for the error rate for the collection of tests and identifies homogeneous subsets of means that are not different from one another at the p = 0.05 level. To specifically test primary hypothesis 1, an a priori test for linear trend was included in the ANOVA to determine if the mean size of selected brain regions became increasingly smaller progressing across the four study groups from Control, to ND/AE, to SE/AE, to FAS/PFAS. To test primary hypothesis 2, an a priori contrast (t-test) between the FAS/PFAS and SE/AE groups was specified in the ANOVA. To test primary hypothesis 3, a chi-square test for trend was used to compare the prevalence of structural anomalies across the three FASD groups relative to the Control group. Two-tailed p-values of 0.05 were used throughout the analyses. Secondary analyses: Secondary analyses using Pearson Correlations Coefficients and ANOVA tests for linear trends were conducted to determine if the size of brain regions decreased with increasing quantity, frequency, and/or duration of reported prenatal alcohol exposure among the three FASD groups.
Two adjustments are used throughout the analysis of the MRI data: 1) adjustment for total brain volume (or midsagittal area) and 2) inclusion or exclusion of the two subjects in the FAS/PFAS group with ACC/HCC (Figures 2A, B). Adjustment 1): To determine if some brain regions were disproportionately reduced in size, given that overall brain size is often reduced among individuals with prenatal alcohol exposure, relative measures of brain regions were computed by dividing the volume of the region by total brain volume, or the midsagittal area of the brain region by the midsagittal area of the total brain. The terms ‘relative’ and ‘absolute’ are used to distinguish the two types of measures. Adjustment 2): The two subjects with ACC/HCC in FAS/PFAS group had CCs that were substantially smaller than the CCs of the other members of that group. The midsagittal area of the CC was 0.3 cm2 in the subject with agenesis and 1.95 cm2 in the subject with hypogenesis (Figures 2A, B). These are 6% and 42% respectively of the mean midsagittal area of the CC (4.64 cm2) for the remaining 18 members of the FAS/PFAS group. Inclusion of these two subjects in the FAS/PFAS group could influence some between-group differences (particularly differences in the mean midsagittal area of the CC). As such, it was important to confirm statistically significant group differences were not driven primarily by these two lowest measures (which represent outliers in the FASD sample). Although the size of the CC for the subject with ACC was a statistical outlier (more than 2 SDs below the mean of the FAS/PFAS group), it would not be clinically valid to exclude this case from the study. All published FASD diagnostic guidelines (Astley, 2004; Bertrand et al., 2004; Chudley et al., 2005; Hoyme et al., 2005) list ACC as an example of a CNS structural abnormality that meets the CNS diagnostic criteria for FASD. Thus, all analyses were conducted with inclusion of these two subjects. If their exclusion resulted in a statistical outcome that was discordant with the statistical outcome when the two subjects were included, both analyses are presented.
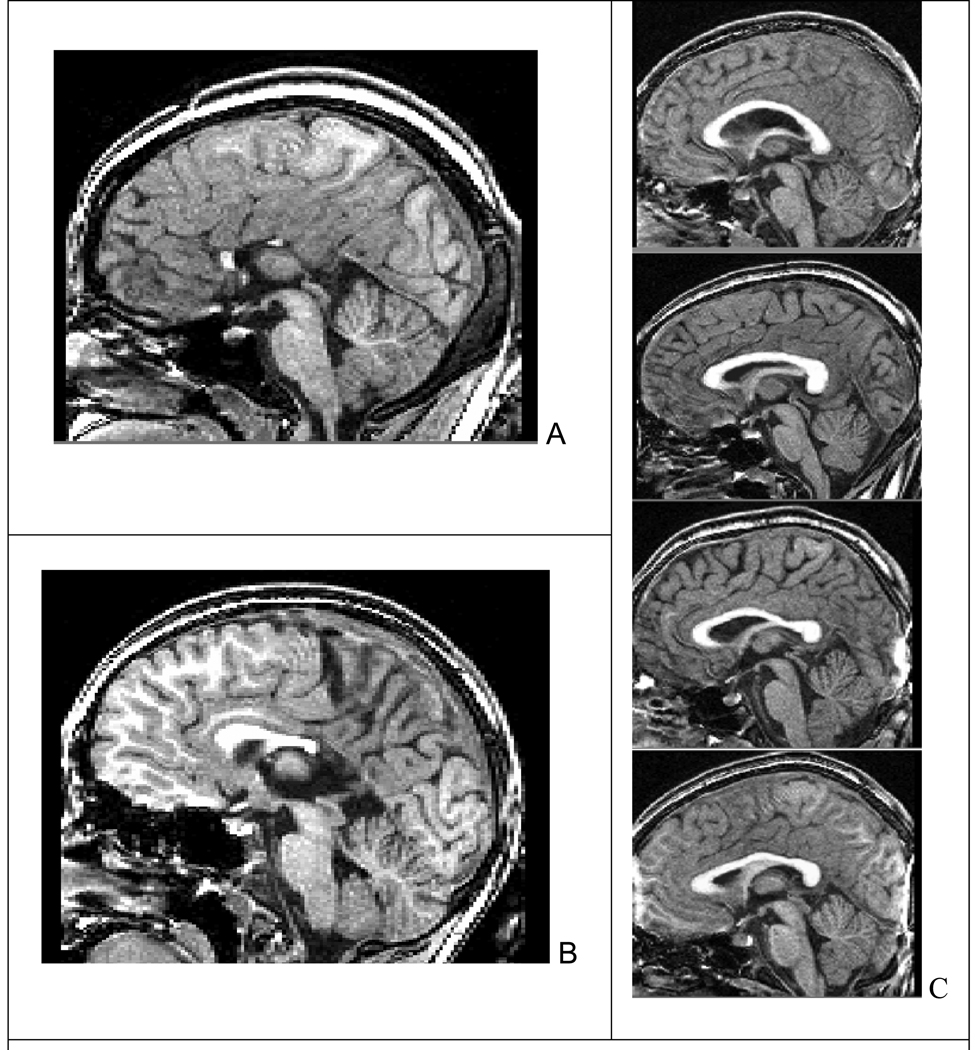
Figure 2.
Two subjects with (A) agenesis and (B) hypogenesis of the corpus callosum in the FAS/PFAS group. C. Variability in corpus callosum shape among Controls with no prenatal alcohol exposure.
Power/Sample Size
This study had 80% power or greater to detect the following effect sizes at a two-tailed alpha level of 0.05: 1) a difference in means equal to or greater than the standard deviation of the mean difference; 2) a correlation coefficient of 0.30 or greater; and 3) a 35-point or greater difference in proportions between two groups.
RESULTS
Size of Brain Regions across the Four Groups (Primary Hypotheses 1–2)
Total Brain
The mean total brain volume decreased significantly progressing across the four study groups from Control to ND/AE to SE/AE to FAS/PFAS (Table 2). The linear trend remained significant with exclusion of the Control group, confirming total brain size decreased incrementally across the three FASD groups. Indeed, all significant linear trends by brain region, reported in Table 2, remained significant with exclusion of the Control group. The mean total brain volume of the FAS/PFAS group was significantly smaller (11% smaller) than the mean of the Control group. The prevalence of microcephaly (OFC ≤ −2 SD) was significantly higher in the FAS/PFAS group with the FAS facial phenotype than in the SE/AE group without the FAS facial phenotype (83.1% versus 16.7%: Chi-square = 7.6, two-tailed p = 0.006) (Astley et al., 2009b). This group difference is not influenced by the 4-Digit Code diagnostic criteria for CNS abnormality. The CNS criteria for FAS, PFAS, and SE/AE are identical (e.g., presence of structural and/or significant functional abnormality). Is brain size reduced simply because individuals with FASD are typically growth deficient? Although FAS is the only FASD subclassification in the 4-Digit Code that requires the presence of some level of growth deficiency (height and/or weight below the 10th percentile), all three FASD groups were significantly shorter in stature (but not lower in weight) than the Controls (Table 1). Despite the growth deficiency among the FASD, there was no significant correlation between height or weight percentile and total brain size across the 81 subjects.
Table 2.
Mean size of brain regions across the four study groups.
| Brain Region | Group | ANOVA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. FAS/PFAS |
2. SE/AE |
3. ND/AE |
4. Control |
Overall | Post Hoc |
A Priori Contrasts | ||||||||||
| LT | Groups 1 vs 2C |
|||||||||||||||
| N | Mean | (SD) | N | Mean | (SD) | N | Mean | (SD) | N | Mean | (SD) | F (p) B | Duncan C | F (p) D | T (p) D | |
| Total Brain volume (cm3) | 20 | 1217.8 | (183.4 | 24 | 1284.7 | (180.0) | 21 | 1305.0 | (95.1) | 16 | 1370.5 | (99.3) | 3.2 (.03) | 123,234 | 9.3 (.003) | 1.5 (.14) |
| Total Brain midsaggital area (cm2) | 20 | 6.7 | (.8) | 24 | 7.1 | (.8) | 21 | 7.2 | (.4) | 14 | 7.6 | (.7) | 5.0 (.003) | 12,234 | 14.1 (.000) | 2.1 (.04) |
| Frontal Lobe volume (cm3) | 19 | 346.1 | (61.7) | 23 | 385.7 | (59.7) | 20 | 391.3 | (41.1) | 16 | 419.8 | (45.6) | 5.7 (.001) | 1,234 | 16.0 (.000) | 2.4 (.02) |
| A Frontal Lobe/Total Brain | 17 | .283 | (.03) | 23 | .301 | (.03) | 20 | .300 | (.02) | 16 | .306 | (.02) | 3.0 (.03) | 1,234 | 5.4 (.02) | 2.0 (.05) |
| Frontal Lobe/Total Brain | 19 | .286 | (.03) | 23 | .301 | (.03) | 20 | .300 | (.02) | 16 | .306 | (.02) | 1.9 (.14) | 4.5 (.04) | ||
| Frontal Lobe gray matter volume (cm3) | 19 | 190.6 | (38.1) | 22 | 216.2 | (33.7) | 20 | 220.8 | (28.6) | 15 | 235.4 | (28.3) | 5.7 (.001) | 1,234 | 15.5 (.000) | 2.5 (.01) |
| Frontal Lobe % gray matter | 19 | .550 | (.04) | 22 | .564 | (.03) | 20 | .563 | (.04) | 15 | .559 | (.03) | 0.7 (.53) | 0.5 (.50) | ||
| Frontal Lobe white matter volume (cm3) | 19 | 155.4 | (28.6) | 22 | 167.5 | (31.8) | 20 | 171.0 | (22.9) | 15 | 186.0 | (24.7) | 3.5 (.002) | 123,234 | 10.3 (.002) | 1.4 (.17) |
| Frontal Lobe %white matter | 19 | .450 | (.04) | 22 | .436 | (.03) | 20 | .437 | (.04) | 1 | .441 | (.03) | 0.7 (.53) | 0.5 (.50) | ||
| R. Caudate volume (cm3) | 19 | 3.8 | (.5) | 24 | 3.9 | (.9) | 21 | 4.5 | (.4) | 16 | 4.8 | (.5) | 12.2 (.000) | 12,34 | 33.7 (.000) | 0.7 (.46) |
| L. Caudate volume (cm3) | 19 | 3.7 | (.5) | 24 | 3.9 | (.9) | 21 | 4.4 | (.6) | 16 | 4.8 | (.6) | 10.2 (.000) | 12,3,4 | 29.4 (.000) | 0.9 (.36) |
| Total Caudate volume (cm3) | 19 | 7.4 | (1.0) | 24 | 7.8 | (1.7) | 21 | 8.8 | (.9) | 16 | 9.6 | (1.1) | 11.6 (.000) | 12,34 | 32.8 (.000) | 0.9 (.39) |
| Total Caudate/Total Brain (10−2) | 19 | .618 | (.09) | 24 | .608 | (.12) | 21 | .679 | (.78) | 16 | .705 | (.86) | 4.6 (.005) | 12,23,34 | 10.5 (.002) | 0.4 (.73) |
| Caudate + Putamen volume (cm3) | 19 | 14.0 | (2.3) | 24 | 15.2 | (2.6) | 21 | 16.4 | (1.8) | 16 | 17.2 | (2.0) | 7.2 (.000) | 12,23,34 | 8.8 (.04) | 1.8 (.08) |
| R. Putamen volume (cm3) | 19 | 3.3 | (.8) | 24 | 3.7 | (.9) | 21 | 3.8 | (.6) | 16 | 3.8 | (.7) | 2.3 (.08) | 5.2 (.03) | ||
| L. Putamen volume (cm3) | 19 | 3.3 | (.8) | 24 | 3.8 | (.7) | 21 | 3.8 | (.7) | 16 | 3.7 | (.7) | 1.8 (.15) | 2.7 (.11) | ||
| Total Putamen volume (cm3) | 19 | 6.6 | (1.5) | 24 | 7.4 | (1.6) | 21 | 7.6 | (1.2) | 16 | 7.6 | (1.3) | 2.9 (.04) | 12,234 | 4.4 (.04) | 2.0 (.04) |
| A Total Putamen volume (cm3) | 17 | 6.7 | (1.5) | 24 | 7.4 | (1.6) | 21 | 7.6 | (1.2) | 16 | 7.6 | (1.3) | 1.7 (.18) | 3.4 (.07) | ||
| Total Putamen /Total Brain (10−2) | 19 | .546 | (.12) | 24 | .582 | (.12) | 21 | .584 | (.09) | 16 | .550 | (.08) | 0.8 (.53) | 0.1 (.91) | ||
| R. Hippocampus volume (cm3) | 20 | 2.9 | (.5) | 24 | 3.0 | (.4) | 21 | 3.1 | (.5) | 16 | 3.4 | (.5) | 5.1 (.003) | 123,4 | 14.7 (.000) | 1.0 (.29) |
| L. Hippocampus volume (cm3) | 20 | 2.9 | (.5) | 24 | 2.9 | (.4) | 21 | 3.1 | (.4) | 16 | 3.4 | (.6) | 4.5 (.006) | 123,4 | 12.6 (.001) | 0.5 (.63) |
| Total Hippocampus volume (cm3) | 20 | 5.7 | (1.0) | 24 | 5.9 | (.8) | 21 | 6.2 | (.8) | 16 | 6.8 | (1.0) | 5.1 (.003) | 123,4 | 14.6 (.000) | 0.8 (.43) |
| Total Hippocampus / Total Brain (10−2) | 20 | .473 | (.07) | 24 | .470 | (.09) | 21 | .478 | (.08) | 16 | .501 | (.07) | 0.6 (.64) | 1.3 (.26) | ||
| CC: midsagittal area (cm2) | 20 | 4.12 | (1.3) | 24 | 4.45 | (.8) | 21 | 4.35 | (.7) | 16 | 4.64 | (.7) | 1.0 (.38) | 2.3 (.13) | ||
| CC: Region 1 (genu) (cm2) | 20 | 1.15 | (.4) | 24 | 1.21 | (.3) | 21 | 1.19 | (.2) | 16 | 1.34 | (.2) | 1.5 (.22) | 123,234 | 3.6 (.06) | |
| CC: Region 2 (cm2)D | 20 | .74 | (.2) | 24 | .76 | (.2) | 21 | .72 | (.1) | 16 | .76 | (.2) | 0.2 (.90) | 0.1 (.92) | ||
| CC: Region 3 (cm2) | 20 | .63 | (.2) | 24 | .64 | (.1) | 21 | .63 | (.1) | 16 | .70 | (.1) | 0.6 (.58) | 1.4 (.25) | ||
| CC: Region 4 (cm2) | 20 | .55 | (.2) | 24 | .62 | (.1) | 21 | .61 | (.2) | 16 | .63 | (.2) | 0.8 (.48) | 1.6 (.21) | ||
| CC: Region 5 (splenium) (cm2) | 20 | 1.04 | (.4) | 24 | 1.21 | (.3) | 21 | 1.18 | (.3) | 16 | 1.22 | (.2) | 1.5 (.23) | 2.4 (.13) | ||
| A CC: Length (cm) | 18 | 6.75 | (.7) | 24 | 6.75 | (.8) | 21 | 6.99 | (.4) | 16 | 7.41 | (.5) | 3.9 (.01) | 123,4 | 9.9 (.002) | 0.1 (.99) |
| CC: Length (cm) | 20 | 6.26 | (1.8) | 24 | 6.75 | (.8) | 21 | 6.99 | (.4) | 16 | 7.41 | (.5) | 3.9 (.01) | 123,234 | 11.5 (.001) | 1.6 (.13) |
| CC / Brain midsaggital Area | 20 | .614 | (.18) | 24 | .631 | (.12) | 21 | .602 | (.09) | 14 | .611 | (.10) | 0.3 (.83) | 0.1 (.80) | ||
| CC / Brain midsaggital area: Region 1 genu | 20 | .171 | (.05) | 24 | .171 | (.04) | 21 | .165 | (.03) | 16 | .175 | (.02) | 0.3 (.85) | 0.1 (.84) | ||
| A CC / Brain midsaggital area: Region 2 | 18 | .117 | (.02) | 24 | .108 | (.04) | 21 | .100 | (.02) | 16 | .099 | (.02) | 1.5 (.22) | 5.4 (.02) | ||
| CC / Brain midsaggital area: Region 2 | 20 | .111 | (.04) | 24 | .108 | (.04) | 21 | .100 | (.02) | 16 | .099 | (.02) | 0.7 (.57) | 2.0 (.16) | ||
| CC / Brain midsaggital area: Region 3 | 20 | .092 | (.03) | 24 | .092 | (.02) | 21 | .088 | (.02) | 16 | .091 | (.02) | 0.2 (.93) | 0.1 (.71) | ||
| CC / Brain midsaggital area: Region 4 | 20 | .082 | (.03) | 24 | .089 | (.02) | 21 | .085 | (.02) | 16 | .084 | (.02) | 0.4 (.44) | 0.0 (.96) | ||
| CC /Brain midsaggital area: Region 5 | 20 | .154 | (.06) | 24 | .172 | (.04) | 21 | .163 | (.03) | 14 | .161 | (.04) | 0.8 (.49) | 0.1 (.79) | ||
| Total CV: midsagittal area (cm2) | 20 | 7.4 | (.8) | 24 | 8.4 | (1.6) | 21 | 7.9 | (1.3) | 16 | 8.4 | (1.6) | 3.4 (.02) | 13,234 | 5.2 (.03) | 2.7 (.009) |
| CV: Lobules I–V midsagittal area (cm2) | 20 | 2.9 | (.4) | 24 | 3.3 | (.5) | 21 | 3.1 | (.4) | 16 | 3.4 | (.5) | 5.4 (.002) | 13,23,24 | 8.2 (.005) | 3.3 (.002) |
| CV: Lobules VI–VII midsagittal area (cm2) | 20 | 2.1 | (.4) | 24 | 2.4 | (.6) | 21 | 2.4 | (1.0) | 16 | 2.4 | (.5) | 1.1 (.35) | 1.9 (.18) | ||
| CV: Lobules VIII–X midsagittal area (cm2) | 20 | 2.5 | (.3) | 24 | 2.7 | (.7) | 21 | 2.4 | (.3) | 16 | 2.7 | (.4) | 2.5 (.07) | 1.6 (.21) | ||
| Total CV / Brain midsaggital area. | 20 | 1.1 | (.2) | 24 | 1.2 | (.2) | 21 | 1.1 | (.2) | 14 | 1.1 | (.1) | 0.9 (.41) | 0.1 (.79) | ||
Abbreviations: CC: corpus callosum. CV: cerebellar vermis. Duncan: multiple comparison range test; commas separate groups with homogeneous means at p < 0.05. F: f statistic. L: Left. LT: ANOVA unweighted linear trend across the four study groups. Note, all significant linear trends remain significant with exclusion of the Control group. p: p-value. SD: standard deviation. R: right. T: t statistic from ANOVA a priori contrast. FAS, fetal alcohol syndrome; PFAS, partial FAS; SE, static encephalopathy; AE, alcohol exposed; ND, neurobehavioral disorder.
Notations:
Two subjects with agenesis and hypogenesis of the corpus callosum removed from FAS/PFAS group.
Numerator degrees of freedom = 3; denominator df = total sample size minus 4.
Only reported when overall ANOVA is statistically significant.
Numerator degrees of freedom = 1; denominator df = total sample size minus 4.
Frontal Lobe
Absolute Volume: The mean absolute volume of the frontal lobe decreased incrementally and significantly, progressing across the four study groups from Controls to FAS/PFAS (Table 2). The mean absolute volume of the frontal lobe for the FAS/PFAS group was significantly smaller (11%–18% smaller) than each of the other groups. Relative Volume: The mean relative volume of the frontal lobe decreased significantly progressing across the four study groups from Control to FAS/PFAS (with and without inclusion of the two subjects with ACC/HCC). The mean volume of the frontal lobe for the FAS/PFAS group with the two subjects with ACC/HCC removed was 352.3 (62.1 SD). When the subjects with ACC/HCC were included in the FAS/PFAS group, no significant pair-wise group differences were observed. When the two subjects with ACC/HCC were excluded from the FAS/PFAS group, the mean relative volume of the frontal lobe for the FAS/PFAS group was significantly smaller (6–9% smaller) than each of the three other groups. Gray/White Matter: The mean absolute volume of white and gray matter in the frontal lobe decreased significantly progressing across the four study groups from Control to ND/AE to SE/AE to FAS/PFAS. The mean absolute volume of the frontal gray matter for the FAS/PFAS group was also significantly smaller (19% smaller) than the mean of the SE/AE group. The mean percent white matter and percent gray matter in the frontal lobes were comparable across the four study groups.
Caudate
Absolute Volume: The mean absolute volume of the caudate decreased significantly progressing across the four study groups from Control to FAS/PFAS. The mean absolute volume of the caudate in both the FAS/PFAS and SE/AE groups were comparable to one another, but significantly smaller (on average 23% and 19% smaller, respectively) than the mean of the Control group (Table 2). Relative Volume: The mean relative volume of the caudate in both the FAS/PFAS and SE/AE groups were comparable to one another and significantly smaller (12% and 14%, respectively) than the mean of the Control group. Right versus Left: No differences in size were observed between the right and left caudate.
Putamen
Absolute Volume: The mean absolute volume of the putamen decreased significantly progressing across the four study groups from Control to FAS/PFAS (Table 2). This trend was no longer statistically significant (F = 3.4; df 3,74; p = 0.07) after removal of the two subjects with ACC and HCC. The mean volume of the putamen for the FAS/PFAS group was significantly smaller (13% smaller) than the mean of the Control group. When the two subjects with ACC/HCC were removed from the FAS/PFAS group, the mean volume of the putamen was 12% smaller than the mean of the Control group. This difference was no longer statistically significant (t = 1.8, p = 0.08). Similar outcomes were observed in the left putamen. Relative Volume: The mean relative volume of the putamen was comparable across all four study groups. Right versus Left: No differences in size were observed between the right and left putamen.
Hippocampus
Absolute Volume: The mean absolute volume of the hippocampus decreased significantly progressing across the four study groups from Control to FAS/PFAS (Table 2). The mean absolute volumes of the hippocampus for each of the three FASD groups were significantly smaller (9–16% smaller) than the mean of the Control group. Relative Volume: The mean relative volume of the hippocampus was comparable across the four study groups. Right versus Left: No differences in size were observed between the right and left hippocampus.
Corpus Callosum
Absolute Area: The midsagittal area of the CC and each of the five subregions were 6% to 15% smaller in size (although not significantly) in each of the FASD groups relative to the Control group (Table 2). Exclusion of the two subjects with ACC/HCC did not alter these outcomes. When the three alcohol-exposed groups were combined, the mean midsagittal area of the genu (region 1) was significantly smaller (9% smaller) (mean 1.22 cm2, 0.2 SD) relative to the Control group (mean 1.34 cm2, 0.2 SD) (t = 2.0; p = 0.03). This difference remained significant with exclusion of the two subjects with ACC/HCC. The mean absolute length of the corpus callosum decreased significantly progressing across the four study groups from Control to FAS/PFAS (with and without the ACC/HCC). The mean length of the corpus callosum for the FAS/PFAS group was significantly shorter (16%) than the mean of the Control group. The mean length of the corpus callosum for the FAS/PFAS group remained significantly shorter (9%) than the mean of the Control group, after removal of the two subjects with ACC/HCC. Visual inspection of the CCs revealed substantial variability in the shape of the CC, even within the Control group (Figure 2C). Relative Area: The mean relative midsaggital area of the CC and its five subregions (after adjustment for total brain midsaggital area) were comparable across all four groups. When the two subjects with ACC/HCC were removed from the FAS/PFAS group, a significant increase in the relative size of Region 2 was observed, progressing across the four study groups from the Control group to the FAS/PFAS group.
Cerebellar Vermis
Absolute Area: The mean absolute midsagittal areas of the CV and lobules I–V were significantly smaller in the FAS/PFAS group (12% and 15% respectively) than the means of each area in the Control group (Table 2). Removal of the two subjects with ACC/HCC had no impact on these outcomes. Relative Area: The mean relative midsagittal area of the CV and its three regions were comparable across all four groups.
Radiologist Review of MRI
Visual inspection of the study MRIs by the neuroradiologist on the investigative team identified the following prevalence of structural abnormalities: Control (n = 0, 0.0%), ND/AE (n = 0, 0.0%); SE/AE (n = 3, 12.5%), FAS/PFAS (n = 3, 15.0%). Three subjects in the FAS/PFAS presented with the following anomalies:1 ACC, 1 HCC, and 1 with a 13 mm lesion in the left cerebellar hemisphere and slight deformity of the 4th ventricle. Three subjects in the SE/AE group presented with Chiari 1 malformations. Only the ACC and HCC were known to be present prior to the subjects’ enrollment in the study. The neuroradiologist was masked to the subject’s FASD diagnosis and study group assignment.
Size of Brain Regions between FASD Subjects With and Without the FAS Facial Phenotype (Primary Hypothesis 2)
The mean absolute size of the frontal lobe, putamen, hippocampus, and CV were significantly smaller in the FAS/PFAS group than the SE/AE group (Table 2). The children with FAS/PFAS also had disproportionately smaller frontal lobes than the children with SE/AE (Table 2, Figure 3). It is important to note that the only diagnostic feature that distinguishes the FAS/PFAS group from the SE/AE group in this study is the presence of the FAS facial phenotype in the former. Both groups have comparably severe CNS dysfunction (Astley et al., 2009b). The FAS Facial D-Score is a continuous measure of the magnitude of expression of the FAS facial features: the higher the D-Score, the more severe the expression of the FAS facial features (Astley and Clarren, 2001). The size of the following brain regions decreased significantly (Pearson Correlation Coefficients with two-tailed p-values < 0.05) as the FAS Facial D-Score increased: frontal lobe volume (absolute size −.261, relative size −.215); midsagittal area of cerebellar vermis (absolute size−.299); and caudate volume (absolute size−.362, relative size−.262).
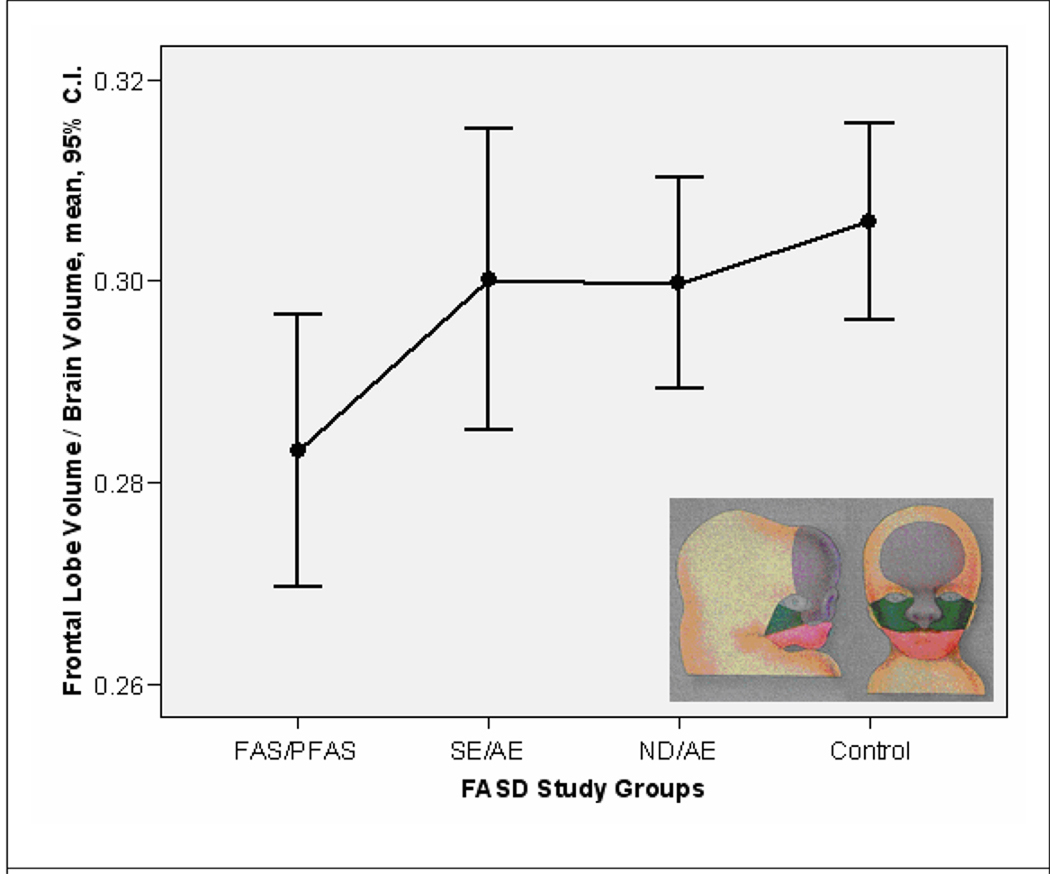
Figure 3.
The relative volume (cc) of the frontal lobe was significantly smaller in the FAS/PFAS group (after exclusion of the two subjects with ACC/HCC) compared to each of the other study groups. The FAS/PFAS group is the only group with the full FAS facial phenotype. Morphogenesis of the middle and upper face is heavily influenced by signals emanating from the forebrain to the frontonasal prominence (Marcucio et al., 2005). The frontonasal prominence is the purple region in the insert depicting a 5-week (left) and 10-week (right) fetus (Moore et al., 1994). C.I.: confidence interval.
Structural Abnormality Correlations with FASD Group and CNS Function (Primary Hypothesis 3)
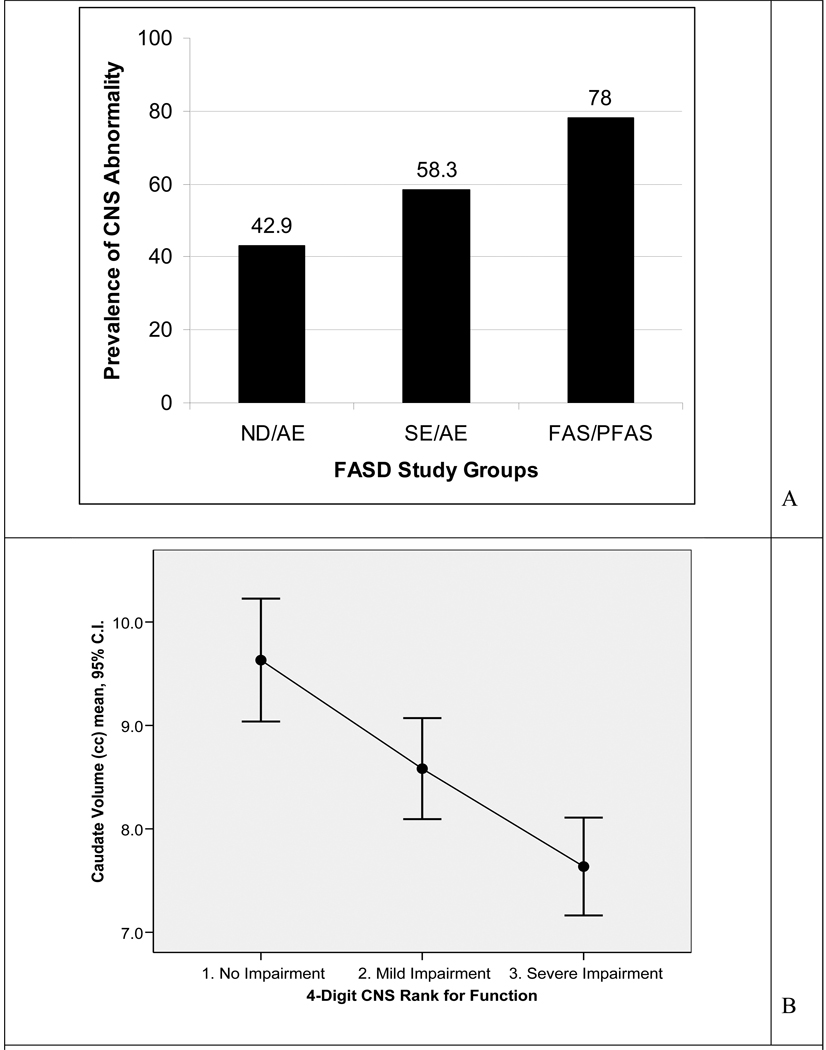
The number of subjects in each FASD study group, with one or more brain regions that were 2 or more SDs below the mean of the Control group, increased significantly as one advanced from the ND/AE to the SE/AE to the FAS/PFAS group (chi-square test for trend 4.8, p = 0.03) (Figure 4A). Importantly, this demonstrated that even when the FAS facial phenotype is missing and the level of brain dysfunction is mild to moderate (4-Digit CNS Rank 2 in ND/AE group), there is a significant increased risk of underlying structural brain abnormality relative to the Control group (Fisher exact test, p = 0.005). Further evidence of structural brain abnormality along the full continuum of mild to severe brain dysfunction was observed by dividing the 81 subjects into three groups based on their 4-Digit CNS Rank: Rank 1 (no dysfunction), Rank 2 (mild-moderate dysfunction), and Rank 3 (severe dysfunction). The absolute size of all brain regions (with the exception of the CC, putamen, and CV) decreased significantly and incrementally progressing from CNS Rank 1 to 2 to 3. The only measures of the CC that decreased significantly with increasing CNS Rank were CC length and CC region 1 midsagittal area. Figure 4B serves to illustrate one of these findings: the significant decrease in the mean absolute volume of the caudate progressing from CNS Rank 1 to 2 to 3 (ANOVA overall F = 13.5; df 2,77; p <.001: unweighted linear trend F = 25.8; df 1,74; p < .001). The Duncan range test confirmed all three mean caudate volumes were significantly distinct from one another.
Figure 4.
A. Prevalence of subjects in each FASD study group that had one or more brain regions, two or more standard deviations below the mean size observed in the Control group. B. Mean (95% confidence interval) absolute caudate volume decreased as a global measure of brain function (4-Digit Code CNS Rank) increased in impairment. C.I.: confidence interval.
Size of Brain Regions and Alcohol Exposure
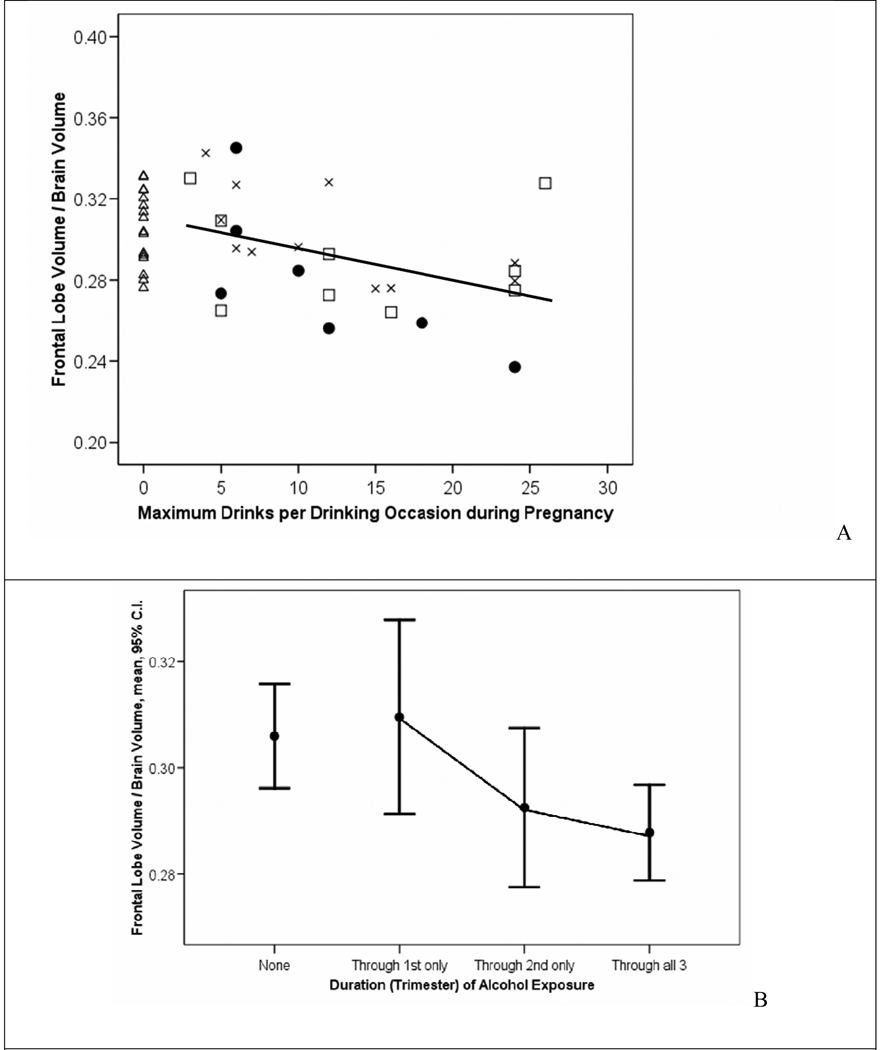
Reported prenatal alcohol exposure patterns (frequency, quantity, and duration) across the three FASD study groups are presented in detail in the neuropsychological/psychiatric report for this study (Astley et al., 2009b) and briefly in Table 1. Of the 65 subjects with FASD, 64 had confirmed prenatal alcohol exposure and one with full FAS had an unknown exposure. More detailed information on quantity, frequency, and/or trimester of alcohol use was available on 53 of the 65 alcohol-exposed subjects. The size of various brain regions decreased significantly and incrementally among the subjects with FASD with increasing frequency, quantity, and/or duration of reported alcohol exposure. Days per week: Significant inverse correlations (Pearson Correlation Coefficients with p-values < 0.05) were observed between the average number of days per week of drinking during pregnancy and the size of the following brain regions: midsagittal area of the brain (−.306); absolute (−.435) and relative (−.306) volumes of the hippocampus, and CC length (−.327). Maximum drinks per drinking occasion: Significant inverse correlations were also observed between the maximum number of alcoholic drinks consumed per drinking occasion during pregnancy and the size of the following brain regions: relative volume of the frontal lobe (−.430) (Figure 5A), relative volume of the caudate (−.373), and the absolute (−.463) and relative (−.538) volumes of the hippocampus. Trimester of Exposure: A significant linear decrease in the mean relative volume of the frontal lobe was also observed when the FASD study sample was divided into groups based on their duration of exposure (exposure through 1st trimester only, (n = 10, mean .31, SD .03); through the 2nd trimester only (n = 9, mean .29, SD .02); and through all 3 trimesters (n = 32, mean, ..28, SD .02) (ANOVA overall F = 3.1; df 2,50; p = 0.05; unweighted linear trend, F = 6.1; df 1,50; p = 0.017) (Figure 5B). Post hoc comparison tests confirmed the mean relative volume of the frontal lobe was significantly smaller among those with 3 trimesters of exposure relative to those with exposure through just the 1st trimester. Interestingly, the mean relative volume of the frontal lobe of the unexposed Control group (mean .31, SD .02, n = 16) was comparable to the two FASD groups with exposure through the 1st and 2nd trimesters, but was significantly larger than the group with exposure through all 3 trimesters.
Figure 5.
A. The relative volume of the frontal lobe decreased significantly with increasing maximum number of alcohol drinks per drinking occasion during pregnancy among subjects with FASD (Pearson Correlation Coefficient−.430, p .02). B. A significant linear decrease in the mean relative volume of the frontal lobe was also observed when the FASD study sample was divided into groups based on their duration of exposure (exposure through 1st trimester only, (n = 10, mean .31, SD .03); through the 2nd trimester only (n = 9, mean .29, SD .02); and through all 3 trimesters (n = 32, mean, .28, SD .02) (ANOVA overall F = 3.1; df 2,50; p = 0.05; unweighted linear trend, F = 6.1; df 1,50; p = 0.017). The unexposed Control group is plotted for visual comparison. Circle = FAS/PFAS, square = SE/AE, X = ND/AE, triangle = control.
DISCUSSION
Overall, all three primary hypotheses were supported in this study. Hypothesis 1: Progressing across the four study groups from Controls to ND/AE to SE/AE to FAS/PFAS, the mean absolute size of most brain regions decreased significantly in size. The frontal lobe and caudate were disproportionately reduced in size. Hypothesis 2: Even though the FAS/PFAS and SE/AE groups had comparably severe levels of brain dysfunction (CNS Rank 3), those with the FAS facial phenotype had significantly smaller frontal lobes, frontal lobe gray matter volume, and CV. Hypothesis 3: The risk of underlying structural abnormalities increased linearly as one advanced from ND/AE to SE/AE to FAS/PFAS, relative to Controls. Most notably, 43% of subjects in the ND/AE group had one or more brain regions that were two or more standard deviations below the mean of the Control group, despite only mild to moderate brain dysfunction, no FAS facial phenotype, and the absence of microcephaly. MRI provided further validation that ND/AE, SE/AE, and FAS/PFAS, as defined by the FASD 4-Digit Code, are three clinically distinct and increasingly more affected diagnostic subclassifications under the umbrella of FASD. The significant inverse correlations between quantity, frequency, and timing of alcohol exposure and size of selected brain regions provide further evidence of a potential causal association between alcohol exposure and structural brain abnormality observed in this study population. It is important to note that the findings reported in this study are reflective of the 4-Digit Code definitions used to generate the FASD study groups. While all FASD diagnostic systems (Astley, 2004; Bertrand et al., 2004; Chudley et al., 2005; Hoyme et al., 2005) share some common clinical terminology (e.g., FAS and PFAS), the criteria used to define these diagnostic classifications are not the same.
FASD Diagnostic Classification Systems
When comparing outcomes across published FASD MRI studies, it is important to note that not all investigations use the same FASD diagnostic criteria to establish their FASD study groups. In many fields, readers of research data can draw their own conclusions about the comparability of diagnostic subgroups because the diagnostic criteria are widely established or, in newer fields of study, the diagnostic methods would be presented in sufficient detail. At present the FASD MRI literature lacks the diagnostic detail necessary when a field has not yet settled on common diagnostic standards. Most FASD MRI studies to date have classified their subjects into one or more of the following groups: 1) an alcohol-exposed group with FAS facial features (FAS), 2) an alcohol-exposed group without FAS facial features (e.g., ARND or FAE); or 3) a group in which all FASD subgroups are combined into one group with prenatal exposure to alcohol (PEA). Criteria used to define FAS groups are typically described qualitatively (e.g., known prenatal exposure to alcohol, craniofacial anomalies consistent with FAS, prenatal or postnatal growth deficiency or both, and CNS dysfunction). Criteria used to define ARND groups are also described qualitatively (e.g. exposed to high levels of alcohol, but display few or none of the facial features characteristic of FAS, and do not show growth retardation). But these qualitative descriptions do not adequately convey what goes into the classifications of FAS, ARND, or FAE. Missing are parameters such as, what level of growth deficiency must be present, what level of CNS dysfunction must be present, which FAS facial features must be evident, and how severe the facial anomalies had to be. Without this information, it is not clear if clinically distinct study groups have been established, or how they are clinically distinct. Nor can it be determined if the FAS/D group in one study is comparable to the FAS/D group in another study. As the field has matured, there is now clear consensus that more rigorous diagnostic methods should be used and several more rigorous methods have been published (Astley, 2004; Bertrand et al., 2004; Chudley et al., 2005; Stratton et al., 1996). Two groups of investigators (Astley and Clarren, 2000; Hoyme et al., 2005) have demonstrated in two large clinical populations that the majority (50% to 87%) of FAS diagnoses rendered using gestalt diagnostic methods did not meet FAS criteria when more rigorous diagnostic criteria were applied.
To help bridge the gap between the different diagnostic terms used in the FASD literature, our ND/AE and SE/AE groups would be most comparable to mild and severe ARND, respectively. Our FAS/PFAS group will differ from other groups labeled FAS or PFAS if the diagnostic criteria used to establish the groups differ. Our FAS/PFAS, SE/AE, and NE/AE groups span the entire continuum of FASD. The effort here was to describe groups across the full fetal alcohol spectrum and to retain sufficient statistical power to detect clinically meaningful differences between diagnostic subgroups. Combining possible subtypes into one larger alcohol-exposed group may obscure actual differences between these subtypes. In the current study, most neuroimaging differences would not have been revealed if the three FASD study groups had been combined in any way and compared to the Controls. We suggest the following questions as standards for review and interpretation of current and future literature on neuroimaging and behavioral outcomes in FASD: 1) What diagnostic criteria were used to establish the study groups? 2) Has the magnitude of growth deficiency, FAS facial features, CNS structural/functional abnormality, and alcohol exposure been reported to confirm that study groups are clinically distinct? 3) When significant differences between groups are not observed, did the study have clinically distinct groups and sufficient sample size/power to support null findings? 4) When outcomes between FAS groups differ between studies, is this because outcomes differ or the definition of FAS differs?
MRI FASD Literature
In general, the FASD MRI literature documents significant reductions in the size of many, but not all brain regions studied, when comparing full FAS to a healthy control group. The current study supports and extends these findings. The FASD MRI literature also documents marginal effects in PEA, FAE, or ARND groups relative to FAS or healthy controls, but these differences are rarely statistically significant. The current study provides definitive evidence that when these nondysmorphic FASD clinical subgroups are more rigorously defined and subdivided (e.g., SE/AE and ND/AE) (Astley, 2004), significant differences are observed, not only when each of these FASD groups is compared to a healthy control group, but also when each is compared to the other. These findings provide compelling evidence that clinically meaningful and distinct subgroups do exist under the umbrella of FASD, and at least two distinct subgroups exist under the broad classification ARND (e.g., SE/AE and ND/AE). Key outcomes in the current study are discussed below and compared to the FASD MRI literature, with emphasis on findings specific to FASD clinical subgroups and how subgroups were clinically defined.
Total Brain
In the current study, total brain size became incrementally smaller progressing across the four study groups from Control to FAS/PFAS. This was influenced, in part, by the criteria used to construct the four groups. Microcephaly is a sufficient, but not necessary CNS criterion for diagnosis of FAS, PFAS, and Static Encephalopathy/Alcohol exposed. Of note, however, is the finding that the prevalence of microcephaly was four-fold greater in the FAS/PFAS group relative to the SE/AE group. Both groups were required to meet the same enrollment criteria for CNS damage/dysfunction (CNS Rank 3 and/or 4). The only difference between the enrollment criteria for the two groups was the FAS/PFAS group had to have FAS facial features (Face Ranks 3 or 4) and SE/AE group had to have normal facial features (Face Ranks 1 and 2). Thus, the fact that microcephaly was far more prevalent in the group with the FAS facial features is further evidence that 4-Digit Code definition of the FAS face is a marker for underlying structural abnormality. The impact of alcohol on reduction of overall brain size is well documented in the animal and human FASD literature (Archibald et al., 2001; Hoyme et al., 2005; Mattson and Riley, 1995; Sowell et al., 2001b; Stratton et al., 1996). The current study provided definitive evidence of the continuum of effects across the full spectrum, not just among those with FAS.
Frontal Lobe
The most notable and novel finding in the current study was a significant reduction in absolute and relative volume of the frontal lobe in the FAS/PFAS relative to all other groups including the SE/AE group. This latter finding indicates individuals with FASD and the FAS facial features have smaller frontal lobes than those with FASD, but no facial features, despite the fact that both groups had comparable cognitive/behavioral impairment (4-Digit CNS Rank 3: three or more domains of function, 2 or more standard deviations below the mean) (Astley et al., 2009b). Archibald et al. (Archibald et al., 2001) (using a study sample of 14 FAS and 12 PEA by gestalt diagnosis, and 41 controls) and Sowell et al. (Sowell et al., 2001b) (using a subset of the Archibald study sample, 14 FAS and 7 PEA by gestalt diagnosis, and 21 controls) were unable to detect significant differences in absolute or relative measures of frontal lobe volumes between FAS, PEA, and controls. Further analysis of the Sowell et al (2001b) study population (Sowell et al., 2002a) using surface based image analysis revealed a significant reduction in the absolute volume and a significant increase in the relative volume of the frontal lobe, when the 14 FAS and 7 PEA were combined and compared to 21 controls. Wass et al. (Wass et al., 2001) reported significant reductions in the absolute size of the frontal cortex associated with prenatal alcohol exposure in an ultrasonographic study of 167 pregnant women. The percent of fetuses with a frontal cortex below the 10th percentile increased from 4% for non-exposed fetuses to 23% for heavily exposed fetuses. Frontal Lobe Gray-White Matter: Although we observed significantly smaller absolute volumes of white matter and gray matter in the frontal lobes among subjects with FAS/PFAS relative to Controls, the proportion of the frontal lobe that was white matter did not vary significantly across the four study groups. These findings are consistent with the literature. Archibald et al (Archibald et al., 2001) identified a significant proportional reduction of white matter across the entire cerebrum among subjects with a gestalt diagnosis of FAS relative to Controls. When their analysis was repeated for each lobe (frontal, parietal, temporal and occipital), a significant proportional reduction of white matter was only observed in the parietal lobe. When subsets of these children were further assessed by Sowell et al (Sowell et al., 2001b; Sowell et al., 2002a) proportional reductions in white matter were observed in the left hemisphere perisylvian cortices of the temporal and parietal lobes (not in the frontal lobes) among alcohol-exposed subjects relative to Controls.
Caudate
The caudate, lenticular nuclei, some subthalamic nuclei, and the substantia nigra are subcortical gray-matter structures that form the basal ganglia (Cote and Crutcher, 1990). The caudate nucleus has extensive neural connections to the frontal lobes and is thought to mediate higher cognitive and executive function. Like the frontal lobe, the absolute and relative volume of the caudate was significantly reduced in the current study, but unlike the frontal lobe, this reduction in size was observed in both the FAS/PFAS and SE/AE groups relative to the Control group. Archibald et al. (Archibald et al., 2001) report the absolute and relative size of the caudate was significantly reduced in 14 children with a gestalt diagnosis of FAS relative to 41 controls. But no significant caudate reductions were observed among their group of 12 children with PEA. Cortese et al. (Cortese et al., 2006) assessed caudate size in an fMRI study with a very small sample of children (7 with FAS and 4 with FAE by gestalt diagnosis, and 4 controls). They report significantly smaller absolute, but not relative, caudate volumes in the FAS group relative to controls, but the unconventional use of 1-tailed p-values increased the probability of achieving statistical significance. No caudate differences were detected between their FAE and control groups. It is noteworthy that no significant differences in intracranial volume were detected between their FAS, FAE, and control groups.
Putamen
The putamen (part of the lenticular nuclei) together with the caudate form the striatum. The putamen is innervated by neurons from the primary motor, premotor, supplementary motor, and somotosensory cortices, whereas the caudate receives input from frontal eye fields and association areas of the frontal and parietal lobes (Cote and Crutcher, 1990; Giedd et al., 1994). In the present study, the absolute, but not relative volume of the putamen was significantly smaller in the FAS/PFAS group relative to the SE/AE, ND/AE and Control groups. Mattson et al. (Mattson et al., 1996), in a study of 6 children with FAS (by gestalt diagnosis), report the absolute, but not relative, volume of the lenticular nuclei was significantly smaller relative to seven controls. Archibald et al. (Archibald et al., 2001) report no significant absolute or relative reduction in the size of the lenticular nucleus among 14 FAS or 12 PEA (by gestalt diagnosis) when compared to 41 controls.
Hippocampus
The hippocampus is a structure of the limbic system involved in learning, memory storage and retrieval (Eichenbaum et al., 1992). In the present study, the mean absolute volume of the hippocampus decreased significantly, progressing across the four study groups from Control to FAS/PFAS. In contrast, Archibald et al. (Archibald et al., 2001) report a disproportionate sparing of the hippocampus in an otherwise hypoplastic brain among 14 FAS participants (by gestalt diagnosis) relative to 41 controls.
Corpus callosum
The CC is a large bundle of nerve fibers connecting the two hemispheres of the brain. CC deficits have been linked to deficits including intellectual functioning, learning, memory, executive function and attention. In this study, the absolute midsagittal area of the genu (Region 1) and the length of the CC were comparably small across the FASD groups and significantly smaller relative to the Control group. No significant differences were observed after adjustment for midsagittal brain area. Riley et al.(Riley et al., 1995) reported significant decreases in the absolute midsagittal area of the CC and regions 1, 3, 4, and 5 among 11 alcohol-exposed subjects (9 with FAS by gestalt diagnosis) relative to 12 controls. After adjustment for midsaggital brain area, regions 1 (genu), 4, and 5 (splenium) remained significantly smaller, but overall midsagittal area did not. The more severe outcomes reported by Riley et al are not due to differences in diagnostic methodology, since our outcomes reflect our entire alcohol-exposed group. It does appear the alcohol-exposed group in the Riley et al study was more severely impaired than our alcohol-exposed group. They have a substantially lower FSIQ and were drawn from a sample with a higher estimated prevalence of ACC (3/44 or 6.8%). In a more recent study, Sowell et al. (Sowell et al., 2001a) looked at size, shape, and location of the CC among 20 alcohol-exposed subjects (13 with FAS by gestalt diagnosis) and 21 controls. Again, in contrast to our findings, they reported significant reductions in the absolute midsagittal area of the CC and all five subregions in their alcohol-exposed group relative to their control group. Their more severe findings could be explained by the much higher prevalence of microcephaly in their alcohol-exposed population (they report “most” were microcephalic). Only 18% of our alcohol-exposed group was microcephalic. After adjustment for brain volume, only the splenium in their alcohol-exposed group remained significantly smaller. Their key finding was a significant anterior-inferior displacement of the splenium in the alcohol-exposed relative to controls. Based on their published Figures 2 and 3 illustrating this effect, this finding would appear commensurate to our finding of a shorter CC. Autti-Ramo et al. (Autti-Ramo, 2000) reported the CC was significantly shorter (7.4%), the midsagittal area significantly smaller (13.8%), and the width of the splenium significantly smaller (12%) among 17 alcohol-exposed subjects (5 with FAS by gestalt diagnosis) relative to 17 controls. These differences were no longer significant after adjustment for midsagittal area of the brain. Forty-one percent of their alcohol-exposed population was microcephalic. Bookstein et al. (Bookstein et al., 2002a; Bookstein et al., 2002b) report a variety of shape, but not size differences in the CC of subjects with gestalt diagnoses of FASD versus controls. It is important to remember that brain regions are inherently highly variable in shape and size, even in typically developing individuals (Steen et al., 2007). This is clearly evident in our Control population. They present with extraordinary variability in CC shape (Figure 2C), despite their high level of cognitive function and absence of alcohol exposure. One final comparison with the literature is warranted. Miller (Miller et al., 1999) reported that the absolute size of the CC was significantly larger in ethanol-exposed nonhuman primates relative to unexposed controls. The number of axons (not the axon size or myelin thickness) was significantly greater, especially in the rostral half of the CC (anterior to where the fornix joins the CC). It is important to note that total brain volume was comparable between the exposed and unexposed groups, no animal had microcephaly, and the alcohol-exposed animals had mild to moderate cognitive dysfunction with possibly only one animal having full FAS. Several human studies, including ours, report sparing of some anterior callosal regions in their alcohol-exposed group. We observed a significant relative sparing of CC Region 2. Riley et al (Riley et al., 1995) observed significant absolute and relative sparing of CC Region 2. And Sowell (Sowell et al., 2001a) reported relative, but not absolute sparing of anterior callosal regions .
What appears to be one recurring theme across several FASD studies is that the CC is reduced in length and the regions most impacted are the genu and splenium. These findings are consistent with the developmental spectrum of ACC. CC abnormalities are present in a multitude of syndromes and disease entities (Paul et al., 2007). ACC is not specific to prenatal alcohol exposure. ACC is present in 2–3 / 100 individuals with general developmental disability (Jeret et al., 1986). The CC is the major commissure forming a junction between the cerebral hemispheres. The formation of the CC starts with the development of the genu in the 11th week; the body, isthmus and splenium develop at a later stage through the twentieth week of gestation (Paul et al., 2007). If the normal developmental process is disturbed, the CC may be absent completely or partially (‘hypogenetic’). Since the developmental process starts from the anterior part and progresses front to rear, when the CC is hypogenetic, it is usually the posterior portion that is affected the most (the posterior body and the splenium) (Figure 2B).
Cerebellar vermis
The cerebellum is located at the base of the brain and is involved in motor, cognitive and affective functions (Vidal et al., 2006). The absolute, but not the relative, midsagittal area of the CV and lobules I–V were significantly smaller in the FAS/PFAS group relative to the Controls and SE/AE in the current study. Sowell et al. (Sowell et al., 1996) reported a significant reduction in the absolute midsagittal area of lobules I–V in a group of alcohol-exposed subjects (6 FAS and 3 PEA by gestalt diagnosis) relative to 24 controls. They did not report if these findings remained significant when adjusted for overall brain size. More recently, O’Hare et al. (O’Hare et al., 2005) reported significant reductions in the midsagittal area of lobules I–V and the VIII–X in a group of alcohol-exposed subjects (14 FAS and 7 PEA by gestalt diagnosis) relative to 21 controls. After correction for brain size, lobule I–V remained significantly reduced in size. Goodlett et al. (Goodlett et al., 1990), using an animal model, demonstrated that the number of Purkinje cells was significantly reduced in earlier maturing regions of the CV (lobules I–V and VIII–X) after a single critical day of neonatal alcohol exposure. Notably, the Purkinje cells in the later maturing neocerebellar vermis (lobules VI and VII) were apparently spared.
Prevalence of CNS Structural Abnormalities among FASD Clinical Subgroups
From a FASD diagnostic perspective, it is interesting to note how many subjects within all three FASD clinical subgroups had one or more brain regions, two or more SDs below the mean of the Control group. Although we report the prevalence of anomalies in our FASD study groups using our research control group as the reference, this would not be an appropriate practice in a clinical setting. Norms for the size of brain regions, by gender and age, must be established using large, representative, population-based samples, rather than small, convenient research control samples. The National Institutes of Health (NIH) MRI Study of Normal Brain Development (Waber et al., 2007) is a landmark study that is documenting structural brain development and behavior longitudinally from birth to young adulthood in a large population-based sample of healthy children targeted to the United States 2000 census distribution .
Validation of the FASD 4-Digit Diagnostic Code
This study further validated (Astley et al., 2002; Astley and Clarren, 1996; Astley and Clarren, 2000; Astley and Clarren, 2001) the measurement scales and procedures for diagnostic classification used in the FASD 4-Digit Diagnostic Code. Statistically significant differences in neuropsychological outcomes (Astley et al., 2009b) and measures of brain structure were observed between the FAS/PFAS, SE/AE and ND/AE groups, confirming these groups reflect three clinically distinct and increasingly more affected diagnostic subclassifications under the umbrella of FASD. The prevalence/severity of structural brain abnormality increased significantly as dysfunction increased from CNS Rank 1 (no dysfunction) to Rank 2 (mild-moderate dysfunction) to Rank 3 (severe dysfunction). Indeed, when these CNS Ranks were first defined ten years ago (Astley and Clarren, 2000), the underlying principle was that as the magnitude and breadth of functional impairment increased, the probability of underlying structural abnormality would increase. It is for this reason that the 4-Digit CNS Ranks 1, 2, and 3 were labeled “unlikely”, “possible”, and “probable” underlying CNS abnormality, respectively (Figure 1). The finding that the subjects in the FAS/PFAS group with the 4-Digit FAS facial phenotype had significantly smaller frontal lobes than the SE/AE group with no FAS facial phenotype, provides the most compelling data yet that this facial phenotype truly is a marker for underlying brain abnormality. Midline FAS facial features and the frontal lobe share the same embryological origin (the frontonasal prominence) (Figure 3) (Moore et al., 1994). Several animal and clinical studies have documented correlations between midline facial anomalies and underlying brain abnormality caused by prenatal alcohol exposure (Astley and Clarren, 1996; Astley and Clarren, 2001; Johnston, 1975; Sulik, 2005; Sulik and Johnston, 1982; Sulik et al., 1981; Sulik et al., 1984).
The primary limitation of this study is the presence of other prenatal and/or postnatal risk factors, in addition to alcohol, that are capable of adversely impacting brain development. Clinical records for this FASD study sample document that up to 83% of the mothers reportedly smoked during pregnancy, up to 67% reportedly used illicit drugs during pregnancy, and over 75% of the children were in foster/adoptive care (Astley et al., 2009b). While the significant dose-response associations observed between alcohol exposure and neurostructural alterations in this study provide compelling evidence of alcohol’s potential causal role, one cannot rule-out the impact of the other risk factors.
In conclusion, the results of this study confirm that significant differences in the sizes of many brain regions exist between children with FASD and their healthy peers. More importantly, when the FASD 4-Digit Code is used to create clinically distinct diagnostic subgroups under the umbrella of FASD (FAS/PFAS, SE/AE, and ND/AE), statistically significant differences in neurostructure are identified between the FASD diagnostic subgroups. Structural abnormalities were prevalent across the full spectrum of FASD. MRI could importantly augment the diagnosis of FASD, once population-based norms for structural development of the human brain are established.
Acknowledgments
This research was supported by NIAAA grant R01- AA12915-01A1 to SJA. Support was also received from the Center on Human Development and Disability, University of Washington (National Institute of Child Health and Human Development grant P30 HD02274). We would like to acknowledge Katherine Field, Olivia Liang, Winslow Johnson, and Katherine Keller for their assistance in the measurement of MRI scans. Special thanks are extended to the children and their families who so kindly contributed time and effort to this study.
References
- Aase JM, Jones KL, Clarren SK. Do we need the term “FAE”? Pediatrics. 1995;95:428–430. [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Ganst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Astley S, Stachowiak J, Clarren S, Clausen C. Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. J Pediatr. 2002;141(5):712–717. doi: 10.1067/mpd.2002.129030. [DOI] [PubMed] [Google Scholar]
- Astley SJ. In: Fetal Alcohol Syndrome Facial Photograph Analysis Software. 1.0 ed. Astley SJ, editor. Seattle: University of Washington; 2003. [Google Scholar]
- Astley SJ. Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code. 3rd ed. Seattle WA: University of Washington Publication Services; 2004. [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Functional magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Journal Neurodevelopmental Disorders. 2009a;1:61–80. doi: 10.1007/s11689-009-9004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. A case definition and photographic screening tool for the facial phenotype of fetal alcohol syndrome. J Pediatr. 1996;129:33–41. doi: 10.1016/s0022-3476(96)70187-7. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol exposed individuals: Introducing the 4-Digit Diagnostic Code. Alcohol Alcohol. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Measuring the facial phenotype of individuals with prenatal alcohol exposure: correlations with brain dysfunction. Alcohol Alcohol. 2001;36:147–159. doi: 10.1093/alcalc/36.2.147. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Olson HC, Kerns K, Brooks A, Aylward EH, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Neuropsychological and behavioral outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Canadian Journal of Pharmacology. 2009b;16(1):e178–e201. [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Richards T, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K. Magnetic resonance spectroscopy outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Magnetic Resonance Imaging. 2009;27:760–778. doi: 10.1016/j.mri.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autti-Ramo I. Twelve-year follow-up of children exposed to alcohol in utero. Dev Med Child Neurol. 2000;42:406–411. doi: 10.1017/s0012162200000748. [DOI] [PubMed] [Google Scholar]
- Aylward E, Brettschneider PD, McArthur JC, Harris GJ, Schlaepfer TE, Henderer JD, Barta PE, Tien AY, Pearlson GD. Magnetic resonance imaging measurement of gray matter volume reductions in HIV-1 dementia. Am J Psychiatry. 1995;152:987–994. doi: 10.1176/ajp.152.7.987. [DOI] [PubMed] [Google Scholar]
- Aylward E, Li Q, Habbak R, Warren A, Pulsifer M, Barta P, Jerram M, Pearlson G. Basal ganglia volume in adults with Down syndrome. Psychiatry Res. 1997a;74:73–82. doi: 10.1016/s0925-4927(97)00011-5. [DOI] [PubMed] [Google Scholar]
- Aylward E, Reiss A. Area and volume measurement of posterior fossa structures in MRI. J Psychiatr Res. 1991;25:159–168. doi: 10.1016/0022-3956(91)90020-b. [DOI] [PubMed] [Google Scholar]
- Aylward E, Reiss A, Barta P, Tien A, Han W, Lee J, Pearlson G. Magnetic resonance imaging measurement of posterior fossa structures in schizophrenia. Am J Psychiatry. 1994;151:1448–1452. doi: 10.1176/ajp.151.10.1448. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Augustine A, Li Q, Barta PE, Pearlson GD. Measurement of frontal lobe volume on magnetic resonance imaging scans. Psychiatry Res. 1997b;75:23–30. doi: 10.1016/s0925-4927(97)00026-7. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Li Q, Honeycutt NA, Warren AC, Pulsifer MB, Barta PE, Chang MD, Smith PD, Jerram M, Pearlson GD. MRI volumes of hippocampus and amygdala in adults with Down’s syndrome with and without dementia. Am J Psychiatry. 1999;156:564–568. doi: 10.1176/ajp.156.4.564. [DOI] [PubMed] [Google Scholar]
- Barta PE, Powers RE, Aylward EH, Chase GA, Harris GJ, Rabins PV, Tune LE, Pearlson GD. Quantitative MRI volume changes in late onset schizophrenia and Alzheimer's disease compared to normal controls. Psychiatry Res. 1997;68(2–3):65–75. doi: 10.1016/s0925-4927(96)02751-5. [DOI] [PubMed] [Google Scholar]
- Beery KE. The Beery-Buktenica developmental test of visual-motor integration. 4th ed. Parsippany, NJ: Modern Curriculum Press; 1997. [Google Scholar]
- Bertrand J, Floyd RL, Weber MK, O’Connor M, Riley EP, Johnson KA, Cohen DE. National Task Force on FAS/FAE Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. Atlanta GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec. 2002a;269:162–174. doi: 10.1002/ar.10110. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002b;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Conroy J, Cook JL, Loock C, Rosales T, LeBlanc N. Public Health Agency of Canada’s National Advisory Committee on Fetal Alcohol Spectrum Disorder Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. Can Med Assoc J. 2005;172:S1–S21. doi: 10.1503/cmaj.1040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, Moore GJ, Bailey BA, Jacobson SW, Delaney-Black V, Hannigan JH. Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: Preliminary findings in the caudate nucleus. Neurotoxicol Teratol. 2006;28:597–606. doi: 10.1016/j.ntt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Cote L, Crutcher MD. The basal ganaglia. In: Kandel ER, Schwartz JH, Jessell M, editors. Subcortical Dementia. New York: Oxford University Press; 1990. pp. 647–659. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH, Ober BA. Delis-Kaplan Executive Function Scale. San Antonio TX: The Psychological Corporation; 2000. [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampus—what does it do? Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Casey BJ, Kozuch P, King AC, Hamburger SD, Rapoport JL. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:665–669. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol Clin Exp Res. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Haier R, Jung R, Yeo R, Head K, Alkire M. Structural brain variation and general intelligence. Neuroimage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeret JS, Serur D, Wisniewski K, Fisch C. Frequency of agenesis of the corpus callosum in the developmentally disabled population as determined by computerized tomography. Pediatr Neurosci. 1986;12:101–103. doi: 10.1159/000120229. [DOI] [PubMed] [Google Scholar]
- Johnston MC. The neural crest in abnormalities of the face and brain. In: Bergsma D, editor. Morphogenesis and Malformation of Face and Brain. Vol. 11. 1975. pp. 1–18. [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev. 2007;31(2):192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Develop Bio. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Prenatal exposure to alcohol. What images reveal. Alcohol Health Res World. 1995;19:273–278. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of neurobehavioral deficits in children with FAS or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with FAS. Alcohol Clin Exp Res. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Research & Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Astley SJ, Clarren SK. Number of axons in the corpus callosum of the mature Macaca nemestrina: Increases caused by prenatal exposure to ethanol. J Comp Neurol. 1999;412:123–131. [PubMed] [Google Scholar]
- Moore KL, Persaud TVN, Shiota K. Color Atlas of Clinical Embryology. Philidelphia PA: W B Saunders Co; 1994. [Google Scholar]
- O’Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16:1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Olson HC, Feldman J, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits among adolescents with fetal alcohol syndrome: Clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic developmental and functional aspects of connectivity. Nature Reviews. Neuroscience. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Reiss A, Aylward E, Freund L, Bryan PJN. Neuroanatomy of Fragile X syndrome I: The posterior fossa. Ann Neurol. 1991;29:26–32. doi: 10.1002/ana.410290107. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 1999;23:1070–1076. [PubMed] [Google Scholar]
- Smith DW. The fetal alcohol syndrome. Hosp Pract. 1979;14(10):121–128. doi: 10.1080/21548331.1979.11707631. [DOI] [PubMed] [Google Scholar]
- Sowell E, Johnson A, Kan E, Lu L, Horn JV, Toga A, O'Connor M, Bookheimer S. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. Journal of Neuroscience. 2008;28(6):1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: Size reduction in lobules I through V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: Effects of heavy prenatal alcohol exposure. Neurology. 2001a;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001b;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002a;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002b;17:1807–1903. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview Edition Survey Form Manual. Circle Pines MN: American Guidance Service; 1984. [Google Scholar]
- Steen RG, Hamer RM, Lieberman JA. Measuring Brain Volume by MR Imaging: Impact of Measurement Precision and Natural Variation on Sample Size Requirements. American Journal of Neuroradiology. 2007;28:1119–1125. doi: 10.3174/ajnr.A0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis Epidemiology Prevention and Treatment.Institute of Medicine. Washington DC: National Academy Press; 1996. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O'Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25(4):228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PC, Barr HM. The enduring effects of prenatal alcohol exposure on child development: Birth through seven years a partial least squares solution. Ann Arbor MI: University of Michigan Press; 1993. [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med. 2005;230(6):366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC. Embryonic origin of holoprosencephaly: interrelationship of the developing brain and face. Scan Electron Microsc. 1982;1:309–322. [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Lauder JM, Dehart DB. Brain malformations in prenatal mice following acute maternal ethanol administration. Int J Dev Neurosci. 1984;2:203–214. doi: 10.1016/0736-5748(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, Williamson PC, Rajakumar N, Sui Y, Dutton RA, Toga AW, Thompson PM. Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biol Psychiatry. 2006;60:218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Waber DP, Moor CD, Forbes PW, Almli CR, Botteron KN, Leonard G, Milovan D, Puas T, Rumsey J Group TBDC. The NIH MRI Study of Normal Brain Development: Performance of a Population Based Sample of Healthy Children Aged 6 to 18 Years on a Neuropsychological Battery. J Int Neuropsychol Soc. 2007;13:1–18. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wass TS, Persutte WH, Hobbins JC. The impact of prenatal alcohol exposure on frontal cortex development in utero. Am J Obstet Gynecol. 2001;185:737–742. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-III Manual. San Antonio TX: Psychological Corporation; 1996. [Google Scholar]