Abstract
Background
It has been suggested that omega 3 (W3, n-3 or omega-3) fats from oily fish and plants are beneficial to health.
Objectives
To assess whether dietary or supplemental omega 3 fatty acids alter total mortality, cardiovascular events or cancers using both RCT and cohort studies.
Search methods
Five databases including CENTRAL, MEDLINE and EMBASE were searched to February 2002. No language restrictions were applied. Bibliographies were checked and authors contacted.
Selection criteria
RCTs were included where omega 3 intake or advice was randomly allocated and unconfounded, and study duration was at least six months. Cohorts were included where a cohort was followed up for at least six months and omega 3 intake estimated.
Data collection and analysis
Studies were assessed for inclusion, data extracted and quality assessed independently in duplicate. Random effects meta-analysis was performed separately for RCT and cohort data.
Main results
Forty eight randomised controlled trials (36,913 participants) and 41 cohort analyses were included. Pooled trial results did not show a reduction in the risk of total mortality or combined cardiovascular events in those taking additional omega 3 fats (with significant statistical heterogeneity). Sensitivity analysis, retaining only studies at low risk of bias, reduced heterogeneity and again suggested no significant effect of omega 3 fats.
Restricting analysis to trials increasing fish-based omega 3 fats, or those increasing short chain omega 3s, did not suggest significant effects on mortality or cardiovascular events in either group. Subgroup analysis by dietary advice or supplementation, baseline risk of CVD or omega 3 dose suggested no clear effects of these factors on primary outcomes.
Neither RCTs nor cohorts suggested increased relative risk of cancers with higher omega 3 intake but estimates were imprecise so a clinically important effect could not be excluded.
Authors’ conclusions
It is not clear that dietary or supplemental omega 3 fats alter total mortality, combined cardiovascular events or cancers in people with, or at high risk of, cardiovascular disease or in the general population. There is no evidence we should advise people to stop taking rich sources of omega 3 fats, but further high quality trials are needed to confirm suggestions of a protective effect of omega 3 fats on cardiovascular health.
There is no clear evidence that omega 3 fats differ in effectiveness according to fish or plant sources, dietary or supplemental sources, dose or presence of placebo.
Medical Subject Headings (MeSH): *Dietary Supplements; Cardiovascular Diseases [*diet therapy; mortality; prevention & control]; Fatty Acids, Omega-3 [*therapeutic use]; Randomized Controlled Trials as Topic
MeSH check words: Humans
BACKGROUND
Since the suggestion by Bang (Bang 1972; Bang 1976), that the abundance of omega 3 fatty acids in the diet of the Greenland Eskimos was responsible for their low mortality from ischaemic heart disease, there has been considerable interest in the protective role and possible mechanism of action of marine unsaturated fats. This interest has spread to encompass plant oils rich in omega 3 fatty acids, including flax (linseed) and rapeseed (canola) oils (Nettleton 1991), their derivatives (e.g. margarines) and purslane leaves (Simopoulos 1992). Omega 3 fats (also called Ω3 or n-3 fats) from fish sources include eicosapentaenoic acid (EPA or 20: 5), docosahexaenoic acid (DHA, 22:6) and docosapentaenoic acid (DPA, 22:5), and are the longer chain omega 3 fats.
Alpha-linolenic acid (ALA or α-linolenic, 18:3) is the shorter chain omega 3 fat from plants (also found in grass fed meats), which is partially converted to longer chain omega 3 fatty acids within our bodies. There is some debate about the effectiveness of this conversion, which may differ depending on other dietary factors (Li 1999; Pawlosky 2001) and whether assessed over short or long term. For this reason the effectiveness of ALA may differ from that of the longer chain omega 3 fats.
Proposed mechanisms for the protective role of omega 3 fats against cardiovascular diseases include: lowering of blood pressure; altered lipid profile, especially reduced serum triglyceride concentration; reduced thrombotic tendency; anti-inflammatory effects; anti-arrhythmic effects including reduction in heart rate; improved vascular endothelial function; increased plaque stability; increased paraoxonase levels and improved insulin sensitivity (Calabresi 2004; Bhatnagar 2003; BNF 1999; Geelen 2004; Thies 2003).
Given that most omega 3 fats are ingested in the form of oily fish or fish oil (often fish liver) capsules, reports of high levels of various toxic compounds such as mercury, dioxins, polychlorinated biphenyls (PCBs) in oily fish (FSA 2000; MAFF 1998A; USFDA 1995) and fish oils (Liem 1997) are concerning. These are all fat soluble and accumulate over time in the body, so harms may be exhibited only after long term supplementation with fish oils. Animal intervention studies and human cohorts who have suffered accidental exposure to dioxins and PCBs suggest that pre-natal exposure may cause sub-fertility problems and adult exposures may lead to an excess of total cancers (JECFA 2001). Human cohorts exposed to high levels of mercury exhibit neurological problems, starting with paraesthesia, followed by stumbling and difficulty in articulating words, tunnel vision, impaired hearing, headaches, general muscle weakness, fatigue and irritability. In severe cases tremors or jerks can occur, and may lead on to coma and death (USFDA 1995). As many people eat oily fish once or twice a week or take fish oil supplements (oily fish intakes rose 44% between 1992 and 1997 in the UK, FSA 2000) it is important to explore the potentially harmful effects of fish-associated omega 3 intake. It is also possible that omega 3 fats themselves may exhibit harm, for example through extension of bleeding times or suppression of normal immune responses (USFDA 2000).
Epidemiological studies have supported the relationship between high omega 3 intake and lower cardiovascular disease (CVD) rates (Ballard-Barbash 1987; Burr 1993). However these associations could be due to some other characteristic of people who choose to eat fish. More reliable information concerning cause and effect is supplied by intervention trials in which participants are randomly allocated to receive fish oil or advice to eat more fish.
Systematic reviews of randomised controlled trials (RCTs) have been published on the effect of omega 3 fats on blood pressure (Appel 1993; Morris 1993; Radack 1989) and suggest a small blood pressure lowering effect at high doses. Non-systematic review suggests that fish oil supplementation will tend to reduce serum triglyceride, very-low-density lipoprotein (VLDL) cholesterol and chylomicrons, but may raise serum low density lipoprotein (LDL) cholesterol (Burr 1993). Fish oil capsules are licensed for triglyceride lowering in the UK, and more recently concentrated fish oil derivatives have become licensed for secondary prevention following myocardial infarction (MI). The effect of fish oil capsules on restenosis after angioplasty has been the subject of systematic review, in one review a significant protective effect was seen (Gapinski 1993) but in a wider selection of trials a small positive but nonsignificant effect was seen (O’Connor 1992). The evidence for anti-arrhythmic effects (Kang 1996) and anti-inflammatory mechanisms (Knapp 1989) have been reviewed but not systematically.
The summation of many small protective risk factor effects of omega 3 fatty acids may add up to a large protective effect on mortality and/or cardiovascular events. Conversely, the protective effects may be small, dwarfed by toxic effects, or only exhibited in people at high risk of cardiovascular disease.
A systematic review of trials on the effect of fish-based dietary or supplemental omega 3 fatty acids on cardiovascular morbidity and mortality in people with coronary heart disease was recently published (Bucher 2002) and suggested a strongly significant benefit. However a very large intervention study with discordant results has been published since this review, and no systematic review has yet attempted to balance any protective effects of omega 3 fats with associated harms.
This systematic review and meta-analysis aimed to draw the evidence of benefits and harms together. As oily fish and fish oil supplements are commonly taken as food or supplements by the general public, it was planned that assessment of harm would be carried out whether or not omega 3 fatty acids appeared effective in protecting against death or cardiovascular events.
OBJECTIVES
The aim of this systematic review was to assess the effect of dietary or supplemental omega 3 fatty acids on total mortality and on cardiovascular events, using all available randomised clinical trials and meta-analytic techniques where appropriate. It also assessed potential long term adverse effects using clinical trials and large prospective cohort studies.
The primary question to be answered by the review was:
Do dietary or supplemental omega 3 fatty acids alter total mortality, cardiovascular events, cancers or other adverse events?
Secondary questions include:
Does any protection occur equally in those at low and at high risk of cardiovascular disease?
Does any protection depend on the dose of omega 3 fats taken per day?
Does any protection depend on the change in proportion of EPA in plasma or membrane fats?
If there are any effects, do they differ between dietary and supplemental omega 3 sources?
Does any protection differ between fish and plant omega 3 sources?
Does any protection depend on the presence or absence of a placebo?
Is any protection stronger with longer trial duration?
What are the side effects associated with increased omega 3 intake, and what is their prevalence?
METHODS
Criteria for considering studies for this review
Types of studies
All randomised controlled clinical trials that included diet advice or dietary supplementation to promote omega 3 fatty acid intake, versus placebo, no supplementation or usual diet where mortality or cardiovascular events were recorded were included, provided they followed participants for at least six months (26 weeks or 180 days, for advice trials follow up must have been at least six months following advice, for trials where food or supplementation is provided then the provision must have continued for at least six months). Randomisation of individuals was accepted, or of clusters as long as there were at least six clusters randomised.
Assessment of harms
For information on potential adverse effects of omega 3 fat in-take prospective cohort studies (followed for at least six months, in adult or child populations of any type) were sought as well as the studies identified above. Studies that assessed omega 3 intake through dietary assessment of omega 3 fats, oily fish and/or omega 3 supplements (by diet history, 24-hour recall, food frequency questionnaires or weighed food methods) or using body measurements of omega 3 fats (through assessment of fatty acids proportions in platelet membranes or serum phospholipids for example) were accepted. All potential adverse effects, or the absence of such effects, reported (including where available diagnosis of cancers, cancer mortality, neurological problems, birth rates, spontaneous abortion rates, birth defects, gender ratio) were collected.
A ‘cohort’ is a group of people clearly identified: a cohort study follows that group over time, and reports on what happens to them. A cohort study is an observational study, and it can be prospective or retrospective (Informed Health 2004).
Types of participants
Studies of adults (18 years or older, men and/or women) at any risk of cardiovascular disease (with or without existing cardiovascular disease) were accepted (to include those with increased risk of cancer, those undergoing or who have undergone coronary artery bypass grafting or angioplasty, and those with current or previous cardiovascular disease, nephritis in systemic lupus erythematosus, breast cysts, diabetes mellitus, rheumatoid arthritis, multiple sclerosis, psoriasis, hayfever, asthma or ulcerative colitis). Participants who were pregnant or acutely ill (those with diagnosed cancer, undergoing heart or renal transplantation, with HIV or AIDS, on haemodialysis, with IgA glomerulonephritis, or any other renal problem except in diabetes) were excluded.
Types of interventions
The intervention must have been dietary supplementation, a provided diet or advice on diet. The foodstuffs or supplements must have been: oily fish (including mackerel, dogfish, salmon, herring, trout, tuna, sturgeon, stablefish, anchovy, sprat, coho, capelin, sardines, swordfish, sild, pilchard, brisling, menhaden, bloater, white-bait, crab and conger eel); fish oils (made from any of the above or a mixture of fish, or cod liver oil); linseed (flax), canola (rapeseed), perilla, purslane, mustard seed, candlenut, stillingia or walnut as a food, oil, made into a spreading fat or supplementing another food (such as bread or eggs) such that the product consumed had an omega 3 fat content of at least 10% of the total fat content. Refined eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) or alpha-linolenic acids, or concentrated fish oils, were also accepted. Supplementation may have been in oil or capsule form or as food stuffs provided, to be consumed by mouth (excluding enteral and parenteral feeds and enemas). Studies were not included if they included multiple risk factor intervention on lifestyle factors other than diet and supplementation (unless the effect of diet or supplementation could be separated out from the other interventions). Studies were included if they compared the effect of this dietary advice with the usual diet, no advice, no supplementation or placebo (which could be another oil, but not one on the list above). Trials were only included if outcome data could be collected (by communication with authors where necessary), and studies where it was stated or ascertained that no events occurred were included.
Types of outcome measures
Primary outcomes
The main outcome was total all-cause mortality. The other important outcomes were combined cardiovascular events (which include fatal and non-fatal myocardial infarction, angina, stroke, heart failure, peripheral vascular disease, sudden death and non-scheduled cardiovascular interventions -coronary artery bypass surgery or angioplasty), cancers and other adverse events (longer term neurological illnesses or reproductive problems, as well as any other reported illnesses).
Secondary outcomes
Secondary outcomes included individual cardiovascular events, risk factor changes and quality of life measures (feelings of health, time off work). Risk factor changes collected were body weight, blood pressure, urinary thromboxane (2 or 3 series), participant fatty acid data (from plasma, platelets or adipose tissue), total, LDL or HDL cholesterol and triglyceride levels. Data concerning side effects were collected.
Search methods for identification of studies
The following sources were included in the literature search process. The Cochrane Library (CD ROM, for RCTs and relevant systematic reviews, to 2002, issue 1), MEDLINE (OVID, for RCTs 1998 to February 2002, for cohorts 1966 to February 2002), EM-BASE (OVID, for RCTs 1998 to February 2002, for cohorts 1980 to February 2002), National Research Register (to February 2002), SIGLE, bibliographies and experts.
As MEDLINE and EMBASE had been thoroughly searched up to 1998 for all randomised controlled trials, and these have been added to Cochrane Controlled Trials Register, MEDLINE and EMBASE were searched for RCTs for 1998 to 2002 only. The Cochrane Library and MEDLINE search strategies for RCTs are included in full in additional Table 1, as is the EMBASE strategy for cohort studies.
Table 1.
Examples of electronic search strategies used in the review
| The Cochrane Library search for RCTs, run to 2002, issue 1 (CD rom): |
| FISH |
| FISH-OILS*:ME |
| LINSEED-OIL*:ME |
| (OIL* near COD*) |
| (OIL* near MARIN*) |
| (OIL* near FISH*) |
| OMEGA3* |
| OMEGA-3* |
| (OMEGA* near FAT*) |
| EPA |
| DHA |
| FATTY-ACIDS-OMEGA-3*:ME |
| LINOLENIC-ACIDS*:ME |
| EICOSAPENTAEN* |
| DOCOSAHEXAENO* |
| FLAX* |
| RAPESEED* |
| CANOLA* |
| ALPHALINOLEN* |
| PERILLA* |
| LINOLEN* |
| LINSEED* |
| MAXEPA* |
| (OIL near RAPE) |
| (OIL near COLZA) |
| (MARINE* near LIPID*) |
| NAUDICELLE* |
| HERRING* |
| (CLUPE* near HARENG*) |
| SILD |
| WHITEBAIT* |
| SARDIN* |
| PILCHARD* |
| SPRAT* |
| BRISLING* |
| TROUT* |
| (SALMO* near TRUT*) |
| BLOATER* |
| KIPPER* |
| SALMO |
| SALMON |
| MACKEREL* |
| SCOMB* |
| CONGER* |
| TUNA* |
| TUNNY |
| THUNNUS* |
| SWORDFISH* |
| XIPHIAS* |
| DOGFISH* |
| SCYLIORHINUS* |
| CRAB |
| CRABS |
| (CANCER near PAGURUS) |
|
|
| The MEDLINE search for RCTs, run from 1998 to February 2002 (on OVID): |
| 1 exp Fish oils/ |
| 2 Linseed oil/ |
| 3 exp Linolenic acids/ |
| 4 Flax/ |
| 5 exp Fatty acids, omega-3/ |
| 6 (fish and (diet$ or nutrit$ or oil$ or supplement$)).tw. |
| 7 (oil$ adj3 cod$).tw. |
| 8 (oil$ adj3 marin$).tw. |
| 9 omega-3.tw. |
| 10 omega3.tw. |
| 11 (omega$ adj5 fat$).tw. |
| 12 eicosapentaen$.tw. |
| 13 docosahexaeno$.tw. |
| 14 flax.tw. |
| 15 rapeseed.tw. |
| 16 canola$.tw. |
| 17 alphalinolen$.tw. |
| 18 perilla$.tw. |
| 19 linolen$.tw. |
| 20 linseed$.tw. |
| 21 maxepa$.tw. |
| 22 (oil$ adj3 rape).tw. |
| 23 (oil$ adj3 colza).tw. |
| 24 (marine$ adj3 lipid$).tw. |
| 25 naudicelle$.tw. |
| 26 herring$.tw. |
| 27 (clupe$ adj3 hareng$).tw. |
| 28 sild.tw. |
| 29 whitebait.tw. |
| 30 sardin$.tw. |
| 31 pilchard$.tw. |
| 32 sprat$.tw. |
| 33 brisling$.tw. |
| 34 trout.tw. |
| 35 (salmo$ adj3 trut$).tw. |
| 36 bloater.tw. |
| 37 kipper$.tw. |
| 38 salmon.tw. |
| 39 mackerel$.tw. |
| 40 scomb$.tw. |
| 41 conger$.tw. |
| 42 tuna.tw. |
| 43 tunny.tw. |
| 44 tunafish.tw. |
| 45 thunnus$.tw. |
| 46 swordfish$.tw. |
| 47 xiphias$.tw. |
| 48 dogfish.tw. |
| 49 scyliorhinus$.tw. |
| 50 (crab or crabs).tw. |
| 51 (cancer adj3 pagurus).tw. |
| 52 laks.tw. |
| 53 lax.tw. |
| 54 or/1-53 |
| 55 randomized controlled trial.pt. |
| 56 controlled clinical trial.pt. |
| 57 Randomized controlled trials/ |
| 58 random allocation.sh. |
| 59 double blind method.sh. |
| 60 single-blind method.sh. |
| 61 or/55-60 |
| 62 (animal not human).sh. |
| 63 61 not 62 |
| 64 clinical trial.pt. |
| 65 exp Clinical trials/ |
| 66 (clin$ adj25 trial$).ti,ab. |
| 67 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. |
| 68 placebos.sh. |
| 69 placebo$.ti,ab. |
| 70 random$.ti,ab. |
| 71 research design.sh. |
| 72 or/64-71 |
| 73 72 not 62 |
| 74 73 not 63 |
| 75 comparative study.sh. |
| 76 exp evaluation studies/ |
| 77 follow up studies.sh. |
| 78 prospective studies.sh. |
| 79 (control$ or prospectiv$ or volunteer$).ti,ab. |
| 80 or/75-79 |
| 81 80 not 62 |
| 82 63 or 74 or 81 |
| 83 82 and 54 |
|
|
| The EMBASE search strategy for cohort studies, run from 1980 to February 2002 (on OVID): |
| 1 (fish and (diet$ or nutrit$ or oil$ or supplement$)).mp. (7299) |
| 2 (oil$ adj3 (cod$ or marin$)).mp. (843) |
| 3 (omega-3 or omega3 or (omega$ adj5 fat$)).mp. (2927) |
| 4 eicosapentaen$.mp. (2640) |
| 5 docosahexaen$.mp. (3605) |
| 6 (flax$ or rapeseed$ or canola$).mp. (1617) |
| 7 (Linolen$ or alpha-linolen$ or alphalinolen$).mp. (3535) |
| 8 (perilla$ or linseed$ or maxepa$).mp. (1066) |
| 9 (oil$ adj3 (rape or colza)).mp. (180) |
| 10 (marin$ adj3 lipid$).mp. (206) |
| 11 (naudicelle$ or herring$ or sild).mp. (694) |
| 12 (clupe$ adj3 hareng$).mp. (82) |
| 13 (whitebait or sardine$ or sardina$ or pilchard$ or sprat$ or brisling$).mp. (220) |
| 14 (salmo$ adj3 trut$).mp. (298) |
| 15 (trout or bloater or kipper$ or salmon or mackerel$ or scomb$ or conger$ or tuna or tunny or tunafish or tuna-fish).mp. (10238) |
| 16 (thunnus$ or swordfish$ or xiphias$ or dogfish or scyliorrhinus$ or laks or lax).mp. (1041) |
| 17 (crab or crabs or (cancer adj3 pagarus)).mp. (2404) |
| 18 exp Salmoniformes/ (4956) |
| 19 exp Tuna/ (59) |
| 20 exp cod liver oil/ or exp fish oil/ or exp menhaden oil/ or exp perilla oil/ or exp rapeseed oil/ (4706) |
| 21 exp docosahexaenoic acid/ or exp icosapentaenoic acid/ or exp linolenic acid/ or exp omega 3 fatty acid/ (6682) |
| 22 exp Linseed Oil/ (258) |
| 23 exp Flax/ (56) |
| 24 icosapentaen$.mp. (2776) |
| 25 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (32278) |
| 26 controlled study/ (1305831) |
| 27 randomized controlled trial/ (61438) |
| 28 clinical trial/ (220852) |
| 29 major clinical study/ (766750) |
| 30 (trial$ or compar$ or control$).tw. (1837636) |
| 31 study.tw. (1372639) |
| 32 “follow$ and up”.tw. (253100) |
| 33 (blind$ or clinic$ or placebo).tw. (921826) |
| 34 placebo/ (56034) |
| 35 clinical article/ (804772) |
| 36 26 or 27 or 28 or 29 or 30 or 31 or 33 or 34 or 35 (3992148) |
| 37 exp human/ (3936158) |
| 38 nonhuman/ (2091757) |
| 39 38 not 37 (1868584) |
| 40 36 not 39 (2967737) |
| 41 25 and 40 (8695) |
| 42 exp Longitudinal Study/ (5503) |
| 43 exp Prospective Study/ (22166) |
| 44 (cohort$ or quartile$ or quintile$ or tertile$ or quantile$).mp. (50258) |
| 45 follow-up$.mp,tw. (86415) |
| 46 longitud$.mp. (41361) |
| 47 ((prospectiv$ or observation$) adj5 (research$ or data$ or stud$)).mp. (104274) |
| 48 42 or 43 or 44 or 45 or 46 or 47 (260981) |
| 49 48 not 39 (245192) |
| 50 49 and 25 (722) |
| 51 41 or 50 (8748) |
Authors of all included studies were contacted for references to studies not yet identified, including published, unpublished or ongoing studies. Published systematic reviews addressing diet and heart health were sought as a source of RCTs. Attempts were made to obtain full-text translations and/or evaluations of all relevant non-English articles.
Data collection and analysis
Data collection
Titles and abstracts resulting from the electronic and bibliographic searches were only rejected on initial screen if the reviewer could determine from the title and abstract that the article:
for RCTs - was not a report of a randomised controlled trial; did not address omega 3 intake; was exclusively in children or young adults (less than 18 years old), pregnant women or the critically ill; or was of less than six months duration; or the intervention was multi-factorial, and;
for cohorts - did not study a cohort of people (or a nested case control study within a cohort); did not assess omega 3 intake (through dietary assessment of omega 3 fats, oily fish (not just ‘fish’) and/or omega 3 supplements or using body measurements of omega 3 fats); did not assess clinical events, death or illness of any nature (including diagnosis of cancers, cancer mortality, neurological problems, birth rates, spontaneous abortion rates, birth defects, gender ratio), or developmental outcomes (for children only); did not follow up participants for at least six months from omega 3 assessment; did not relate omega 3 intake to at least one clinical outcome; or did not assess omega 3 fats and clinical events in the same individuals.
When a title/abstract could not be rejected with certainty, the full text of the article was obtained for further evaluation. If the reviewer was uncertain about the appropriateness of rejecting the article, the full text article was retrieved.
An in/out form was used to assess studies for inclusion (or otherwise) into the review. At this stage included RCTs also had to provide data on whether participants had suffered from at least one of the stated outcomes, or state clearly that no participants had suffered from these outcomes. The authors of all potentially included RCTs were contacted for further information on trial methodology and outcomes. The inclusion of full text RCTs and cohorts was assessed independently by two assessors and any differences between reviewers’ results were resolved by discussion and, when necessary, in consultation with the review team.
Two data extraction forms were designed for this review, one for RCTs and one for cohort studies. For RCTs data concerning participants, interventions, and outcomes, as described above in the selection criteria section, were extracted. Data from dietary advice studies were extracted at the latest point available in the trial (regardless of the amount of reinforcement of the original dietary message), while data from supplemental studies were extracted to the point that supplementation ended, or the trial ended, whichever was earlier. Continuous data were extracted until the latest point available in fixed term trials, but in studies where participants were followed up for varying durations (aside from dropouts) the participants data were extracted from the first time point following the mean trial duration. Data from periods following the end of a trial were never used in meta-analysis.
Trial quality characteristics, as suggested by Chalmers (Chalmers 1990), were extracted onto this form. In addition data were collected on potential effect modifiers including participants baseline risk of cardiovascular disease, trial duration, intensity of intervention (dietary advice, diet provided, dietary advice plus supplementation, supplementation alone), source of omega 3 fats (plant sources, fish oil supplements, fish consumption), medications used (including antihypertensive, antiarrhythmic or antithrombotic medication) and smoking status. Baseline risk of cardiovascular disease was defined as follows: high risk were participants with existing vascular disease including a history of myocar-dial infarction, stroke, peripheral vascular disease, angina, heart failure or previous coronary artery bypass grafting or angioplasty; moderate risk were participants with a familial risk, dyslipidaemia, diabetes mellitus, hypertension, chronic renal failure; low risk were other participants. Where provided, data on the effect of diet on risk factors for cardiovascular disease including blood pressure, lipids and weight were collected. For each published RCT in which adverse effects were noted, the type of effect, how and at what time points in the study the information or data on these effects was elicited or collected and recorded, omega 3 dose, duration of intake, type of omega 3 (from fish or plant sources, as food, supplement or supplemented food) and the frequency of adverse effects (number of cases divided by the number of people exposed to the treatment) were noted.
For cohort studies data on setting, design, measurement of the exposure to omega 3s, details of participant characteristics (in high and low exposure groups), similarity at baseline, participant flow, endpoint criteria, details of toxins (PCBs, mercury, dioxins etc) provided, crude and most adjusted data on outcomes were extracted.
Original reports of trial results were extracted by two reviewers independently. Differences between reviewers’ results were resolved by discussion and, when necessary, in consultation with a third reviewer or the review team. Where the original trialist had replied and expressed an interest in the review the extracted data form was sent to the trialist for comments and corrections.
Quality assessment
All quality assessment was performed independently and in duplicate for each included study.
For RCTs, trial quality characteristics, as suggested by Chalmers (Chalmers 1990), were extracted onto the data extraction form. These were as follows:
Allocation concealment was coded as adequate, unclear or inadequate;
Participant blinding was coded as yes, unclear or no;
Provider blinding was coded as yes, unclear or no;
Outcome assessor blinding was coded as yes, unclear or no.
A trial was considered to be at low risk of bias if allocation concealment was adequate, and participant, provider and outcome assessor blinding were all coded ‘yes’. All other trials were considered at moderate or high risk of bias.
For cohort studies, quality assessment was based on criteria felt to be important for this review. Criteria included:
whether the design used an internal or external control group (internal means all subjects were drawn from the same source, and the exposure subsequently determined, external means that most and least exposed groups were defined a priori and came from different sources);
number lost to follow up;
baseline similarity of the most and least exposed groups;
whether any dissimilarities appeared to have been adjusted for in the most adjusted analysis (the most adjusted analysis was that which adjusted for the greatest number of potential confounders);
whether the assessor of the exposure was blinded to the outcome (coded as ‘yes’ for biochemical analyses, and ‘yes, probably’ for dietary analyses where blinding is likely but not absolutely certain), and;
whether the assessor of the outcome was blinded to the exposure.
Data synthesis
Primary measures of interest were the effect of dietary advice or supplementation on
total mortality;
combined numbers of cardiovascular events and interventions (including fatal and non-fatal myocardial infarction, stroke, angina, heart failure, sudden death, peripheral vascular disease, angioplasty and coronary artery bypass grafting);
adverse effects.
For the RCT studies, for dichotomous outcomes, we extracted numbers of participants experiencing an outcome, and total numbers of participants randomised, for each study arm. For continuous outcomes number of participants assessed, means and standard deviations of the final readings in each treatment arm were extracted. Treatment/control differences in the outcomes were combined across studies using relative risks (RR) or weighted mean differences (WMD) in random effects meta-analysis. It was intended that if trials randomised by cluster were identified the patient numbers would be reduced to an effective sample size as described by Hauck 1991, however no such trials were identified. For combined outcomes (e.g. combined cardiovascular events) attempts were made to add numbers of individuals experiencing specific outcomes within studies, but only where we could be certain that we were not counting individual participants more than once within any one of our review outcome categories. However, individuals may have been counted for more than one of the review outcomes. Studies where it was known that an outcome did not occur in either arm were included in the meta-analyses for information.
For the cohort studies, the most adjusted relative risk or odds ratio comparing the most exposed quantile (tertile, quintile etc) with the least exposed quantile was used. Along with information on the number of events in the least exposed quantile, and the total numbers of participants in the most and least exposed quantiles, an ‘adjusted number of events in the most exposed quantile’ was calculated and rounded to the nearest whole number. This ‘adjusted number of events in the most exposed quantile’ was used with the actual number of events in the least exposed group, and the total numbers of participants in the most and least exposed quantiles in random effects meta-analysis. When it was not possible to extract these numbers, but the relationship between relevant measures of omega 3 and outcomes had been assessed in the published paper, the study was included. For such studies details were provided in the table of characteristics of included cohort studies, and the study was included in the relevant forest plot, but without numerical data added (to alert the reader to the presence of the missing data and allow them to assess the potential bias introduced by it).
Where more than one analysis on a specific cohort of participants was relevant to an outcome (for example, for the NHS cohort there are papers assessing both the relationship of alpha-linolenic acid and cardiovascular mortality, and the relationship between total omega 3 fats and cardiovascular mortality), only one analysis was used for that cohort per outcome. The rules for choosing which analysis was used were as follows (if the first rule decides, then ignore the rest, move down until a decision is reached):
combined omega 3 fats over any single omega 3 fraction;
the largest population involved in the analysis (and a whole cohort analysis over a nested case control analysis);
EPA chosen rather than DHA, which is chosen over alpha-linolenic acid;
fat from fish is chosen over fat from fish liver or supplements.
Subgrouping was used to explore the effects of the following factors on mortality and cardiovascular events in included RCTs:
fish or vegetable source of omega 3 fats - fish sources included oily fish, fish oils and purified eicosapentaenoic acid or docosahexaenoic acid, plant sources included linseed (flax), canola (rapeseed), mustard seed, candlenut or walnut oils or as a food, and purified alpha-linolenic acid;
fish omega 3 dose - low dose 0.4 to 2.4g/day, medium dose 2.5 to 4.4 g/day, and high dose 4.5g/day and over of combined long chain omega 3 fats;
dietary or supplemental source of omega 3 fats - dietary advice on fish intake, supplemental foods (for example margarine fortified with rapeseed, or tins of sardines) provided by the study, or supplements (capsules or oils) provided [note: Burr (DART 2) 2003 generally provided dietary omega 3 advice, with the option of taking fish oil capsules if participants found fish unpleasant, but almost a third of participants were randomised to fish oil capsules, without being given dietary advice. This study was only included in the subgrouping for the outcomes total mortality and sudden death as separate outcome data were only available for these outcomes.];
trial duration - studies with short follow up (6 to 11 months), medium follow up (12 to 23 months), medium long follow up (24 to 47 months) and long follow up (48 or more months).
Heterogeneity was assessed using Cochran’s test and assumed to be present when p<0.1.
Meta-regression was used to explore effects of omega 3 dose and duration of trial on mortality and cardiovascular events. Random effects meta-regression (Berkley 1995) was performed using the STATA command metareg (Sharp 1998): log(e) relative risk vs dose or duration, weighted by the standard error of the log(e) relative risk.
Sensitivity analysis was used to assess robustness of results on RCTs to trial quality. RCTs where allocation concealment was agreed as ‘done’, and where participants, providers and outcome assessors were all masked were not removed in sensitivity analysis, but all other studies were. Funnel plots were used to assess for evidence of bias (Egger 1997).
Type and frequency of side effects and adverse effects were tabulated (with the other extracted data on adverse effects) and compared between different studies and designs.
Pooling of cohort studies which assessed cardiovascular outcomes was not specified in the protocol, but was performed to provide the reader with a complete picture of the evidence. Conclusions have not been drawn from this data.
The review will be updated within two years of publication. At the update we plan to run separate analyses of fish-based (long chain) omega 3 fats and ALA, and will pool them only as a secondary analysis. Additionally we plan to use both Cochran’s test and the I2 test (Higgins 2003) to assess heterogeneity.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
15159 titles and abstracts were screened following the electronic and bibliographic searches, or were recommended by experts. Of these 926 appeared potentially relevant and were ordered as full text papers to be assessed for inclusion. 25 papers could not be traced (due to incorrect bibliographic details in the original reference or to the British Library not having access to a particular journal or book), and 464 papers were clearly not appropriate as soon as the full paper was examined (93 of these were collected as potential cohorts, but were either not actual cohorts or did not assess fish or omega 3 fats in any way, and 371 were potential RCTs and were excluded as their follow up period was less than six months). This left 437 papers where inclusion was assessed independently in duplicate (each by LH plus one of the following: RLT, RAH, CDS, HM, PND, ARN or RAR). One hundred and twenty potential RCTs were excluded at this stage, as were 150 potential cohort studies (see flow diagram, Figure 1, for further details). Fourteen RCTs fulfilled all the inclusion criteria, but it was unclear whether any outcome data existed as contact could not be made with the study authors, or the authors were unsure. These papers are found in the list of studies awaiting assessment and will be included in future if outcome data become available. After amalgamation of papers into discrete studies it became apparent that 48 RCTs and 41 cohorts fulfilled all inclusion criteria.
Figure 1.
Flow diagram for review.
Randomised Controlled Trials
Forty eight randomised controlled trials comparing at least six months of omega 3 fats with placebo or control were included in this review. These trials included 36,913 participants, in studies with from six participants per arm (Dry 1991) to 6700 participants per arm (Natvig 1968). Eight studies included at least 500 participants (Burr (DART 1) 1989; Burr (DART 2) 2003; Eritsland 1996; GISSI-P 1999; Johansen 1999A; Leaf 1994; Natvig 1968;Sirtori 1998). Participants were at high risk of cardiovascular disease in 21 of the trials, at moderate risk in 10 and at low risk in 17. Participants were predominantly male (70% or more) in 24 trials, roughly equal (31 to 69% male) in 17, predominantly women (0 to 30% men) in five trials and not stated in two. Participants mean ages were in the 30s in six studies, 40s in seven studies, 50s in 27 studies, 60s in four studies, 70s in no studies, 80s in one study and unclear in three studies.
Most studies (44) provided a dietary supplement (36 as capsules, six as oil (Almallah 1998; Borchgrevink 1966; Brox 2001;Connor 1993; Hawthorne 1992; Natvig 1968), one as liquid emulsion (Rossing 1996) and one as an enriched margarine (Bemelmans 2002)), while three provided dietary advice primarily (Burr (DART 1) 1989; Burr (DART 2) 2003; Mate-Jimenez 1991), and one study provided dietary advice plus food supplements (Sarkkinen 1998). Supplementation was generally with fish-based omega 3 fats (rich in EPA, DPA and DHA fatty acids), but five studies provided plant-based omega 3 fat (alpha-linolenic acid) supplementation in at least one arm (Bemelmans 2002;Borchgrevink 1966; Natvig 1968; Sarkkinen 1998; Singh 1997). Doses of fish-based omega 3 fatty acids (EPA, DPA plus DHA) varied from 0.4 to 7g per day.
Control groups received vegetable oils (25 studies: Olive 14 studies, Olive emulsion one study, sunflower oil two studies, corn oil six studies, Olive and palm oil combination one study, vegetable oil one study), other types of fats (mixed triglycerides one study, linoleic rich margarine in one study, fat replicating the composition of an average European diet in one study), other ‘inert’ or illdefined substances (liquid paraffin one study, aluminium hydroxide one study, air filled capsules two studies, ‘placebo’ two studies), different dietary advice in one study, or nothing/ no placebo (13 studies).
Intervention time was six-11 months in 23 studies, 12-17 months in 16 studies, 24-47 months in eight studies (Bemelmans 2002;Burr (DART 1) 1989; GISSI-P 1999; Loeschke 1996; Mate-Jimenez 1991; Nilsen 2001; Sacks (HARP) 1995; von Schacky 1999) and 48 months or over in one study (Burr (DART 2) 2003). The main study outcome was cardiovascular in nature in 32 studies. Nine studies aimed to measure death or cardiovascular events (Borchgrevink 1966; Burr (DART 1) 1989; Burr (DART 2) 2003;GISSI-P 1999; Natvig 1968; Nilsen 2001; Nye 1990; Reis 1991;Singh 1997). In 12 studies restenosis or CABG patency was the endpoint and 13 aimed to measure various cardiovascular risk factors (lipids five studies, blood pressure two studies, glycaemic parameters two studies, and one study each for ‘cardiovascular risk factors’, peripheral arterial compliance, fibrinolytic parameters, diabetic nephropathy, immunoreactivity, and albuminurea) [note, several studies state more than one main study outcome, so numbers in this paragraph are not additive]. Other main study outcomes include measures of arthritis activity (four studies, one of which includes participants with plaque psoriasis), gut status (for those with ulcerative colitis or Crohn’s disease, seven studies), lung function (for those with asthma and/or hayfever, two studies), tissue incorporation of omega 3 fats (one study), liver enzymes (for those with chronic hepatitis, one study) and neurological tests (for those with dementia of cardiovascular disease, one study).
There was some response to attempted contact with at least one study author for 40 of the studies.
Cohorts
Notation
In order to clarify which included studies were cohort studies, rather than RCTs, a ‘z’ was added to the beginning of the name of each included cohort study (e.g. zPHS Albert 2002). As several cohorts of people were used in more than one included analysis (for example, the Physician’s Health Study is represented in 5 included cohort analyses: a 1994 nested case control study assessing correlation of plasma fatty acids with prostate cancer, zPHS Gann 1994; a 1995 nested case control study on plasma fatty acids and myocardial infarction, zPHS Guallar 1995; a 1995 cohort analysis on dietary fish and various cardiovascular outcomes, zPHS Morris 1995; a 1998 cohort analysis on dietary fish and omega 3 fat in-take and total and cardiovascular mortality, zPHS Albert 1998; and finally a 2002 nested case control study on plasma fatty acids and sudden cardiac death, zPHS Albert 2002) each analysis included in the review is referenced by the name of the cohort (PHS here) and the first author and year of publication of the analysis used where space allowed (here Albert et al 2002). A distinction needs to be made between cohorts (groups of people being followed prospectively) and cohort analyses (analyses of data from one or more cohorts).
Forty seven published analyses from 26 cohorts were included in the review (this is counting the Dutch cohort of the Seven Countries study (z7Cs NL Oomen 2000), the Zutphen study (zZutphen Miedema 93) and the Zutphen Elderly study (zZutphenES Kalmijn; zZutphenES Oomen 01) as one cohort as there were individuals who were represented in all three). Counting numbers of participants at the longest cohort follow up published (excluding nested case control analyses) studies included between 80 and 84,688 participants (overall 563,218 individuals, plus those in the Umea (zUmea Chajes 1999) and Janus (zJanus Harvei 1997) studies where the total size of the cohort was not described). Four studies included fewer than 1000 participants (Finnish cohort of Euroaspire, zEuroaspire Erkkila; London cardiovascular cohort, zLondon Kingsbury 94; a menarche cohort, zMenarche Maclure 91; and the Zuthphen study, zZutphen Miedema 93), and ten more than 20,000 (Health Professionals Follow up Study,zHPFS Ascherio 1995; Nurses Health Study, zNHS Hu 2002; Physicians Health Study, zPHS Albert 1998; ATBC trial/ cohort, zATBC Pietinen 1997; National Health Screening Survey of Norway, zNHSSN Egeland 2001; Netherlands Cohort Study, zNLCS Schuurman 1999; Norwegian Health Screening,zNorwegian Veierod A; Swedish Mamography Screening Cohort,zSMSC Terry 2001; Iowa Women’s Health Study, zIWHS Meyer 2001; Nurses Health Study II, zNHS I&II Zhang 2000). Follow up at this point was from four to 25 years.
Fourteen cohorts consisted predominantly of men (at least 70% men), four cohorts were even (31 to 69% men), and seven cohorts were predominantly women (0 to 30% men). Gender mix was unclear in one cohort. Mean age at the start of the cohort was under 20 years in one cohort, in the 30s in one cohort, 40s in three cohorts, 50s in 11 cohorts, 60s in three cohorts, 70s in one cohort and unclear in six cohorts.
Two cohorts (Finnish cohort of Euroaspire, zEuroaspire Erkkila; and the Physicians Health Study, zPHS Albert 1998) assessed omega 3 intake by both dietary and biochemical means, other cohorts by either dietary or biochemical assessment. Dietary assessment (used in 18 cohorts) was by food frequency questionnaire (FFQ) or questionnaire in 13 cohorts, cross-check dietary interview in three cohorts, and by 24 hour recall or diet history in two cohorts. Biochemical assessment (used in ten cohorts) was of serum (seven cohorts), plasma (two cohorts) or whole blood (one cohort).
Omega 3 fraction assessed was all omega 3 fats (EPA + DHA + DPA + ALA) in three cohorts, ‘mixed long chain omega 3 fats’ (EPA + DHA + DPA) in eight cohorts, EPA in 12 cohorts, DHA in 11 cohorts, DPA in five cohorts, ALA in 10 cohorts, DHA + DPA in one cohort, ‘regular cod liver oil use’ in two cohorts, fatty fish intake in two cohorts, fat from fish or shellfish in one cohort and number of main meals with fish liver in one cohort. Most cohorts assessed more than one of these measures.
Three cohorts provided information on total mortality, two from the USA (MRFIT, zMRFIT Dolecek 1991; and the Nurses Health Study, zNHS Hu 2003) and one from Europe (Finnish cohort of Euroaspire, zEuroaspire Erkkila). Thirteen cohorts (in 22 publications) provided information on cardiovascular outcomes (five from the USA, seven from Europe and one from Asia). Ten cohorts (in ten publications) provided information about cancer outcomes(five from the USA, five from Europe). One US cohort provided information on child health and development, three cohorts (one from the USA, two from Europe) on respiratory diseases, two cohorts (one each from the USA and Europe) on development of diabetes, two US cohorts combined provided information on development of multiple sclerosis, and two US cohorts combined on development of age-related macular degeneration.
Risk of bias in included studies
Randomised controlled trials
Allocation concealment appeared adequate in 34 studies (Almallah 1998; Bairati 1992; Belluzzi 1996; Bemelmans 2002; Bonnema 1995; Borchgrevink 1966; Connor 1993; Dry 1991; Eritsland 1996; GISSI-P 1999; Greenfield 1993; Hawthorne 1992;Katan 1997; Lau 1993; Lau 1995; Leaf 1994; Loeschke 1996;Lorenz-Meyer 1996; Maresta 2002; Mate-Jimenez 1991; Milner 1989; Natvig 1968; Nilsen 2001; Reis 1991; Rossing 1996;Sacks (TOHP 1) 1994; Selvais 1995; Singh 1997; Sirtori 1998;Skoldstam 1992; Terano 1999; Thien 1993; Veale 1994; von Schacky 1999). This was generally ascertained following contact with the study author. It was unclear in 12 studies (Bellamy 1992;Brox 2001; Burr (DART 1) 1989; Burr (DART 2) 2003; Franzen 1993; Geusens 1994; Johansen 1999A; Kaul 1992; Malaguarnera 1999; Nye 1990; Sacks (HARP) 1995; Sarkkinen 1998) and not done in two studies (Dehmer 1998; Shimizu 1995).
Attempts were made to mask participants in 30 studies (Almallah 1998; Bairati 1992; Belluzzi 1996; Bemelmans 2002; Bonnema 1995; Borchgrevink 1966; Connor 1993; Dry 1991; Franzen 1993; Geusens 1994; Greenfield 1993; Johansen 1999A; Lau 1993; Lau 1995; Leaf 1994; Loeschke 1996; Lorenz-Meyer 1996;Maresta 2002; Natvig 1968; Nilsen 2001; Nye 1990; Reis 1991;Rossing 1996; Sacks (TOHP 1) 1994; Sacks (HARP) 1995; Selvais 1995; Sirtori 1998; Thien 1993; Veale 1994; von Schacky 1999), masking was unclear in two studies and not attempted in 16 studies. However, fish oil may have a strong flavour, smell or after-taste, and some studies mentioned attempts to mask allocation using enteric coated capsules (Belluzzi 1996), identical taste in margarines (Bemelmans 2002), orange flavour (Loeschke 1996;Rossing 1996) or peppermint oil (Greenfield 1993) added to fish oil and control capsules or small amounts of fish oil added to the control (Leaf 1994). Two studies mentioned assessment of participants ability to tell whether they were taking fish oil or a control fat, Rossing 1996 asked participants to guess their treatment allocation and found that approximately half guessed correctly, whilevon Schacky 1999 found that of those in the fish oil group 22/90 guessed correctly (5/90 guessed placebo and 63/90 were unsure) and of those in the control 9/85 guessed correctly (10/85 guessed fish oil and 66/85 were unsure).
Masking of providers of care was attempted in 33 studies (Almallah 1998; Bairati 1992; Belluzzi 1996; Bemelmans 2002;Bonnema 1995; Borchgrevink 1966; Brox 2001; Connor 1993;Dry 1991; Geusens 1994; Greenfield 1993; Hawthorne 1992; Johansen 1999A; Lau 1993; Lau 1995; Leaf 1994; Loeschke 1996;Lorenz-Meyer 1996; Maresta 2002; Mate-Jimenez 1991; Natvig 1968; Nilsen 2001; Nye 1990; Reis 1991; Rossing 1996; Sacks (HARP) 1995; Sacks (TOHP 1) 1994; Selvais 1995; Singh 1997;Sirtori 1998; Thien 1993; Veale 1994; von Schacky 1999), was unclear in six and not attempted in nine studies.
Masking of outcome assessors was attempted in 44 of the 48 studies, was unclear in two and was not attempted in two studies.
Studies were considered to be at low risk of bias where allocation concealment was done, and attempts were made to mask participants, providers and outcome assessors. The 25 studies at low risk of bias included: Almallah 1998; Bairati 1992; Belluzzi 1996;Bemelmans 2002; Bonnema 1995; Borchgrevink 1966; Connor 1993; Dry 1991; Greenfield 1993; Lau 1993; Lau 1995; Leaf 1994; Loeschke 1996; Lorenz-Meyer 1996; Maresta 2002; Natvig 1968; Nilsen 2001; Reis 1991; Rossing 1996; Sacks (TOHP 1) 1994; Selvais 1995; Sirtori 1998; Thien 1993; Veale 1994; von Schacky 1999.
Cohorts
All included cohorts recruited an internal (all subjects drawn from the same group, and exposure subsequently determined) rather than external control group (where most and least exposed groups came from different sources determined a priori). Twenty two of the 26 included cohorts performed assessments of exposure on the whole cohort, divided the whole cohort into quantiles according to that exposure, assessed the whole cohort for outcomes and related quantiles to risk of outcome. Some of these 22 cohorts also performed nested case control studies on some cohort participants. Four cohorts only published relevant nested case control studies, rather than full cohort assessments.
Losses to follow up were unclear in 16 cohorts, and were stated in the remaining ten. Of these ten cohorts, seven reported fewer than 5% dropouts, while three reported higher levels (22% inzARIC Zheng 1999, 9% in zMenarche Maclure 91, 29% in one analysis from the Zutphen Elderly Study, zZutphenES Kalmijn (though it was less than 5% in the other analysis from this cohort,zZutphenES Oomen 01)). In total 15 of the 47 cohort analyses reported losses to follow up.
Baseline similarity between those with high and low exposure to omega 3 fats was not classified as ‘good’ for any included cohort. Baseline similarity was unclear in 14 cohorts (often where several different exposures were assessed the similarity of participants was reported against another type of exposure). Baseline characteristics were clearly different in 12 cohorts, and appeared to be adequately adjusted for in five cohorts, not fully adjusted for in six cohorts and unclear in one cohort.
There appeared to be adequate blinding of the assessor of exposures to outcomes in 13 cohort analyses, and probable blinding (as temporal factors made it unlikely that dietary assessments would have been analysed after the outcomes occurred, but they may have been) in the remaining 34 analyses. Outcome assessors were blinded to exposure in 15 cohorts, not in two cohorts and unclear in nine.
Effects of interventions
See Table 2 for sensitivity analysis results, and Table 3 for subgrouping results.
Table 2.
Sensitivity analysis results
| Outcome | Analysis | No. included studies | No. events* | RR (95% CI) | hetero. p-value |
|---|---|---|---|---|---|
| Total Mortality | Main | 44 | 1995 | 0.87 (0.73 to 1.03) | 0.04 |
| Total Mortality | SA (risk of bias) | 23 | 138 | 0.98 (0.70 to 1.36) | 0.57 |
| Total Mortality | Cohort | 3 | (318) | 0.65(0.48 to 0.88) | 0.21 |
| Combined events CV | Main | 31 | 2628 | 0.95(0.82 to 1.12) | <0.0001 |
| Combined events CV | SA (risk of bias) | 16 | 570 | 1.09 (0.87 to 1.37) | 0.07 |
| Combined events CV | Cohort | 7 | (1929) | 0.91(0.73 to 1.13) | <0.0001 |
| Cancers | Main | 10 | 391 | 1.07 (0.88 to 1.30) | 0.91 |
| Cancers | SA (risk of bias) | 5 | 7 | 1.15 (0.29 to 4.47) | 0.66 |
| Cancers | Cohort | 10 | (832) | 1.02(0.87 to 1.19) | 0.27 |
| CV deaths | Main | 44 | 1418 | 0.85(0.68 to 1.06) | 0.01 |
| CV deaths | SA (risk of bias) | 23 | 98 | 0.90 (0.61 to 1.33) | 0.88 |
| CV deaths | Cohort | 11 | (1772) | 0.79(0.63 to 0.99) | 0.001 |
| Fatal MI | Main | 38 | 390 | 0.86(0.60 to 1.25) | 0.06 |
| Fatal MI | SA (risk of bias) | 19 | 15 | 0.69(0.26 to 1.84) | 0.63 |
| Fatal MI | Cohort | 2 | (44) | 0.42(0.21 to 0.82) | Not applicable |
| Non-fatal MI | Main | 26 | 648 | 1.03(0.70 to 1.50) | 0.03 |
| Non-fatal MI | SA (risk of bias) | 13 | 29 | 1.15(0.25 to 5.27) | 0.11 |
| Non-fatal MI | Cohort | 4 | (624) | 0.93(0.69 to 1.26) | 0.04 |
| Sudden death | Main | 37 | 416 | 0.85 (0.49 to 1.48) | 0.004 |
| Sudden death | SA (risk of bias) | 19 | 8 | 0.65 (0.17 to 2.48) | 0.65 |
| Sudden death | Cohort | 1 | (41) | 0.44(0.21 to 0.91) | Not applicable |
| Angina | Main | 25 | 565 | 0.78(0.59 to 1.02) | 0.0004 |
| Angina | SA (risk of bias) | 14 | 288 | 0.95 (0.65 to 1.40) | 0.02 |
| Angina | Cohort | 1 | Not applicable | Not applicable | |
| Stroke | Main | 26 | 243 | 1.17(0.91 to 1.51) | 0.81 |
| Stroke | SA (risk of bias) | 14 | 29 | 1.02(0.48 to 2.16) | 0.43 |
| Stroke | Cohort | 4 | (602) | 0.87(0.72 to 1.04) | 0.31 |
| Heart failure | Main | 20 | 54 | 0.51(0.31 to 0.85) | 0.91 |
| Heart failure | SA (risk of bias) | 10 | 1 | 3.00(0.12 to 72.77) | Not applicable |
| Heart failure | Cohort | 0 | Not applicable | Not applicable | |
| PV events | Main | 17 | 11 | 0.26(0.07 to 1.06) | 0.87 |
| PV events | SA (risk of bias) | 10 | 10 | 0.25(0.05 to 1.17) | Not applicable |
| PV events | Cohort | 1 | (1250) | 0.94(0.84 to 1.04) | Not applicable |
| Re-vascularisation | Main | 23 | 2372 | 1.05(0.97 to 1.12) | 0.89 |
| Re-vascularisation | SA (risk of bias) | 12 | 159 | 0.98(0.75 to 1.30) | 0.50 |
| Re-vascularisation | Cohort | 2 | (295) | 1.07(0.76 to 1.50) | 0.26 |
| Total Mortality | |||||
| *numbers in brackets are the numbers of events ocurring in the quantile with the highest and the quantile with the lowest omega-3 intakes only |
Table 3.
Subgrouping results
| Outcome | Effect modifier | Subgroup | No. studies | No. events | No. randomised | RR (95% CI) | Hetero p-value |
|---|---|---|---|---|---|---|---|
| Total mortality | Main | 44 | 1995 | 36195 | 0.87 (0.73 to 1.03) | 0.04 | |
| Total mortality | Fish or veg. | Fish source | 40 | 1855 | 22036 | 0.86 (0.70 to 1.04) | 0.05 |
| Total mortality | Fish or veg. | Veg source | 5 | 140 | 14129 | 0.67 (0.57 to 1.34) | 0.23 |
| Total mortality | Fish n-3 dose (g n-3/d) | 0.4 to 2.4 | 15 | 1810 | 18251 | 0.85 (0.67 to 1.08) | 0.003 |
| Total mortality | Fish n-3 dose (g n-3/d) | 2.5 to 4.4 | 11 | 38 | 1690 | 0.96 (0.52 to 1.77) | 0.58 |
| Total mortality | Fish n-3 dose (g n-3/d) | 4.5 or more | 13 | 7 | 2057 | 0.29 (0.06 to 1.38) | 0.96 |
| Total mortality | Dietary or supplemental | Dietary advice | 3 | 664 | 4727 | 0.91(0.57 to 1.44) | 0.002 |
| Total mortality | Dietary or supplemental | Supplemented foods | 2 | 4 | 344 | 4.32 (0.46 to 41.00) | Not applicable |
| Total mortality | Dietary or supplemental | Supps, capsules or oil | 39 | 1569 | 32641 | 0.90 (0.76 to 1.07) | 0.24 |
| Total mortality | Trial duration | 6-11 mo. | 19 | 31 | 1358 | 0.60 (0.30 to 1.19) | 0.75 |
| Total mortality | Trial duration | 12-23 mo. | 16 | 154 | 14496 | 0.82 (0.50 to 1.34) | 0.14 |
| Total mortality | Trial duration | 24-47 mo. | 8 | 1285 | 14225 | 0.84 (0.75 to 0.93) | 0.47 |
| Total mortality | Trial duration | 48+ mo. | 1 | 525 | 3114 | 1.15 (0.98 to 1.34) | Not applicable |
| Total mortality | Risk of CVD | High | 18 | 1907 | 20002 | 0.84 (0.70 to 1.02) | 0.04 |
| Total mortality | Risk of CVD | Moderate | 9 | 5 | 1564 | 1.04 (0.04 to24.53) | 0.10 |
| Total mortality | Risk of CVD | Low | 17 | 83 | 14599 | 1.07 (0.70 to 1.64) | Not applicable |
| Total mortality | Placebo controlled? | Placebo | 33 | 199 | 18495 | 0.80 (0.60 to 1.06) | 0.41 |
| Total mortality | Placebo controlled? | No placebo | 11 | 1796 | 17700 | 0.90 (0.72 to 1.13) | 0.01 |
| Total mortality | DART2 study removed | 43 | 1470 | 33081 | 0.83 (0.75 to 0.91) | 0.52 | |
| Combined CV events | Main | 31 | 2628 | 35140 | 0.95 (0.82 to 1.12) | <0.0001 | |
| Combined CV events | Fish or veg. | Fish source | 29 | 2343 | 21355 | 0.93 (0.79 to 1.11) | 0.0002 |
| Combined CV events | Fish or veg. | Veg source | 3 | 285 | 13785 | 0.92 (0.58 to 1.45) | 0.02 |
| Combined CV events | Fish n-3 dose (g n-3/d) | 0.4 to 2.4 | 10 | 1915 | 17994 | 0.86 (0.67 to 1.10) | 0.0002 |
| Combined CV events | Fish n-3 dose (g n-3/d) | 2.5 to 4.4 | 9 | 243 | 1656 | 1.07 (0.88 to 1.30) | 0.84 |
| Combined CV events | Fish n-3 dose (g n-3/d) | 4.5 or more | 9 | 185 | 1667 | 0.80 (0.41 to 1.54) | 0.007 |
| Combined CV events | Dietary or supplemental | Dietary advice | 2 | 276 | 2071 | 0.85 (0.69 to 1.07) | 0.005 |
| Combined CV events | Dietary or supplemental | Supplemented foods | 0 | ||||
| Combined CV events | Dietary or supplemental | Supps, capsules or oil | 28 | 1991 | 29955 | 0.93 (0.78 to 1.11) | 0.001 |
| Combined CV events | Trial duration | 6-11 months | 13 | 282 | 2056 | 1.00 (0.71 to 1.42) | 0.05 |
| Combined CV events | Trial duration | 12-23 months | 10 | 334 | 14496 | 0.83 (0.47 to 1.45) | 0.002 |
| Combined CV events | Trial duration | 24-47 months | 7 | 1651 | 13959 | 0.90 (0.82 to 0.98) | 0.54 |
| Combined CV events | Trial duration | 48+ months | 1 | 361 | 3114 | 1.31 (1.07 to 1.59) | Not applicable |
| Combined CV events | Risk of CVD | High | 16 | 2440 | 20067 | 0.94 (0.80 to 1.12) | <0.0001 |
| Combined CV events | Risk of CVD | Medium | 7 | 2 | 1244 | 0.24 (0.03 to 2.23) | 0.77 |
| Combined CV events | Risk of CVD | Low | 8 | 186 | 13829 | 1.13 (0.85 to 1.51) | Not applicable |
| Combined CV events | Placebo controlled? | Placebo | 20 | 708 | 17589 | 0.94 (0.71 to 1.24) | 0.0002 |
| Combined CV events | Placebo controlled? | No placebo | 11 | 1920 | 17551 | 0.96 (0.79 to 1.17) | 0.01 |
| Combined CV events | DART2 study removed | 30 | 2267 | 32026 | 0.92(0.79 to 1.08) | 0.001 | |
| Cancers | Main | 10 | 391 | 17433 | 1.07 (0.88 to 1.30) | 0.91 | |
| Cancers | Fish or veg. | Fish source | 9 | 390 | 17233 | 1.08 (0.88 to 1.31) | 0.90 |
| Cancers | Fish or veg. | Veg source | 1 | 1 | 200 | 0.33 (0.01 to 8.09) | Not applicable |
| Cancers | Fish n-3 dose (g n-3/d) | 0.4 to 2.4 | 3 | 384 | 16470 | 1.07 (0.88 to 1.30) | 0.87 |
| Cancers | Fish n-3 dose (g n-3/d) | 2.5 to 4.4 | 4 | 4 | 663 | 2.07 (0.27 to 15.88) | 0.43 |
| Cancers | Fish n-3 dose (g n-3/d) | 4.5 or more | 2 | 2 | 100 | 1.04 (0.11 to 9.65) | 0.35 |
| Cancers | Dietary or supplemental | Dietary advice | 1 | 10 | 2033 | 1.50 (0.43 to 5.32) | Not applicable |
| Cancers | Dietary or supplemental | Supplemented foods | 0 | ||||
| Cancers | Dietary or supplemental | Supps, capsules or oil | 8 | 283 | 12286 | 1.06 (0.84 to 1.33) | 0.79 |
| Cancers | Trial duration | 6-11 months | 1 | 1 | 200 | 0.33 (0.01 to 8.09) | Not applicable |
| Cancers | Trial duration | 12-23 months | 3 | 1 | 176 | 3.00 (0.13 to 69.09) | Not applicable |
| Cancers | Trial duration | 24-47 months | 5 | 291 | 13943 | 1.07 (0.86 to 1.35) | 0.78 |
| Cancers | Trial duration | 48+ months | 1 | 98 | 3114 | 1.07 (0.72 to 1.57) | Not applicable |
| Cancers | Risk of CVD | High | 7 | 389 | 17213 | 1.07 (0.88 to 1.30) | 0.88 |
| Cancers | Risk of CVD | Medium | 2 | 1 | 156 | 3.00 (0.13 to 69.09) | Not applicable |
| Cancers | Risk of CVD | Low | 1 | 1 | 64 | 0.35 (0.01 to 8.38) | Not applicable |
| Cancers | Placebo controlled? | Placebo | 5 | 7 | 823 | 1.15 (0.29 to 4.47) | 0.66 |
| Cancers | Placebo controlled? | No placebo | 5 | 384 | 16610 | 1.07 (0.88 to 1.30) | 0.87 |
Primary outcomes
Total mortality (comparison 01, outcome 01)
Forty four of the 48 included RCTs provided information on total mortality. While no deaths occurred in 29 of these RCTs, deaths did occur in 15 RCTs (1995 deaths in total, from 36195 people randomised to the 44 trials). The relative risk of death in those participants randomised to omega 3 supplementation or advice, compared with those on placebo or no such dietary advice, was 0.87 (95% confidence interval 0.73 to 1.03), with significant heterogeneity (pheterogeneity 0.04). Sensitivity analysis, removing all studies except those at low summary risk of bias, still resulted in no significant effects on mortality, the relative risk was 0.98 (95% confidence interval 0.70 to 1.36, 138 deaths, pheterogeneity 0.57). Conversely, meta-analysis of the three relevant included cohort studies suggested a protective effect of higher omega 3 intakes on total mortality, the relative risk was 0.65 (95% confidence interval 0.48 to 0.88, pheterogeneity 0.21).
Heterogeneity here refers to statistically significant variation. If it exists then the variety in the data from different trials is so large that it is not helpful to pool them. In other words, the pooled relative risk may not be very meaningful as it provides an average that does not reflect the circumstances in specific trials very well. Subgrouping by fish or vegetable source of omega 3 fat did not suggest a significant effect of omega 3 fats on death in either subgroup, though statistical heterogeneity disappeared in the vegetable source group (which only had 140 events in total). Subgrouping by omega 3 dose resulted in very few events, and no statistical heterogeneity, in the medium and high dose studies, and no significant effects on death in any of the subgroups. Subgrouping by dietary or supplemental source of omega 3 fats suggested no significant effect of dietary advice, supplemental foods or supplements (with significant heterogeneity for the dietary advice only). Subgrouping by trial duration resulted in few deaths in the 6-11 month and 12-23 month groupings, but the 1285 deaths in the 24-47 month grouping suggested significant protection with omega 3 fats, the relative risk was 0.84 (95% confidence interval 0.75 to 0.93, no significant heterogeneity). This effect was lost in the 48 months and over subgroup. Subgrouping by baseline risk of cardiovascular disease suggested no significant effect of omega 3 fats on deaths in any subgroup. Subgrouping by the presence or absence of placebo suggested no significant effect of omega 3 fats in either group, with statistical heterogeneity in the group of trials without placebo.
Overall, the heterogeneity seen in the main analysis, sensitivity analysis and subgroup analyses was lost whenever the Burr (DART 2) 2003 study was removed. This single large study (including 3114 male angina sufferers) found a large but not quite significant increase in deaths in the group randomised to omega 3 dietary advice over a period of 36 to 108 months (525 deaths in total, the relative risk was of death 1.15 (95% confidence interval 0.98 to 1.34). Removing this study results in an overall relative risk of death of 0.83 (95% confidence interval 0.75 to 0.91) with no significant heterogeneity (pheterogeneity 0.52).
Meta-regression of log(e) RR of total mortality against long chain omega 3 dose did not suggest a significant relationship. Log(e) RR of total mortality vs trial duration did suggest a significant relationship, regression co-efficient 0.008 (95% confidence interval 0.003 to 0.012) consistent with the relative risk of mortality rising with longer duration (or the benefits of added omega 3 fats decreasing over time, and eventually becoming harmful). However, this relationship was lost on removal of Burr (DART 2) 2003.
The funnel plot (Figure 2) was asymmetrical, suggesting that smaller studies were more likely to show a reduction in mortality in the omega 3 arm of a trial. If we remove studies with fewer than 50 deaths from the meta-analyses we find that the funnel plot appears less biased (Figure 3), however there is still very strong heterogeneity, the relative risk was 0.88 (95% confidence interval 0.71 to 1.10, 1923 events, pheterogeneity 0.002).
Figure 2.
Funnel plot of RCTs contributing data on total mortality (fixed effects meta-analysis). Note: the SE(log RR)s are negative.
Figure 3.
Funnel plot of RCTs contributing data on total mortality (fixed effects meta-analysis) with studies reporting fewer than 50 deaths in total excluded. Note: the SE(log RR)s are negative.
Combined cardiovascular events (comparison 01, outcome 02)
Thirty one included RCTs provided data on combined cardiovascular events (numbers of participants experiencing at least one of the following: cardiovascular death; myocardial infarction; stroke; new angina; new heart failure; a peripheral vascular event; unplanned coronary artery bypass grafting; or angioplasty). In thirteen of these studies no participants experienced a cardiovascular event, events did occur in the remaining 18 RCTs (2628 events in a total of 35140 participants). Meta-analysis did not suggest a significant effect of omega 3 supplementation on cardiovascular events, relative risk 0.95 (95% confidence interval 0.82 to 1.12, pheterogeneity <0.0001). Sensitivity analysis, removing studies with a medium or high risk of bias, reduced but did not remove statistical heterogeneity, with no significant effect on risk of cardiovascular events, the relative risk was 1.09 (95% confidence interval 0.87 to 1.37, 570 events, pheterogeneity 0.07). Similarly, meta-analysis of cohort data did not suggest any effect of omega 3 fats on cardiovascular events, and was significantly heterogeneous, the relative risk was 0.91 (95% confidence interval 0.73 to 1.13, 1929 events, pheterogeneity <0.0001).
Subgrouping by fish or vegetable source, omega 3 dose, dietary or supplemental source, baseline risk of cardiovascular disease, presence or absence of placebo, or removal of the Burr (DART 2) 2003 study did not result in significant effects of omega 3 fats on cardiovascular events, and generally reduced heterogeneity a little, but not completely. The exception was grouping by trial duration where no effect of omega 3 fats on cardiovascular events was seen at 6-11 months or 12-23 months, but at 24-47 months a significantly protective effect (the relative risk was 0.90, 95% confidence interval 0.82 to 0.98, no significant heterogeneity) was seen, and at over 48 months a significantly harmful effect, the relative risk was 1.31 (95% confidence interval 1.07 to 1.59) was seen, incorporating data from only one trial (Burr (DART 2) 2003).
Meta-regression of log(e) RR of total mortality against either long chain omega 3 dose or trial duration did not suggest any significant relationships.
A funnel plot shows asymmetry, strongly suggesting bias, with smaller studies more likely to see a reduced relative risk for combined cardiovascular events. If we remove studies with fewer than 100 combined cardiovascular events from the meta-analyses we find that the funnel plot appears less biased, however the summary estimate of the meta-analysis for combined cardiovascular events remains non-significant and with high levels of heterogeneity, the relative risk was 1.09 (95% confidence interval 0.91 to 1.31, 2263events, pheterogeneity 0.0004).
Cancers (comparison 01, outcome 03)
Only ten trials reported cancer outcomes, two of which reported no cancers. Overall 391 cancer diagnoses or deaths were reported from 17433 participants. Most trials with data provided information on deaths from cancer, rather than diagnosis of cancer, so that we are unlikely to be seeing long enough follow up to track build up of body toxins, followed by cancer initiation, development and fatality all in the span of a randomised trial.
There was no significant effect of omega 3 fats on cancers, the relative risk was 1.07 (95% confidence interval 0.88 to 1.30, pheterogeneity 0.91). Sensitivity analysis removing studies at medium or high risk of bias left five trials with 7 events. With few events, subgrouping provided no useful information.
Ten cohort studies provided data on cancer outcomes, of which seven provided appropriate data for meta-analysis, overall 832 events in the highest and lowest quantiles. Studies assessed total cancer mortality (1), and diagnosis of prostate (4), colorectal (2), lung (1) and breast (2) cancers. Meta-analysis did not suggest any effect of high omega 3 intake on cancers, the relative risk was 1.02 (95% confidence interval 0.87 to 1.19, pheterogeneity 0.27). The three studies which had data not useable in the meta-analysis did not report significant effects of omega 3 fats, except that zNHS Holmes 1999 found a significantly increased risk of breast cancer in women on higher omega 3 intakes, which was not confirmed in the other breast cancer analysis (zUmea Chajes 1999).
Other long term health effects (comparison 01, outcome 04)
Other long term health effects recorded in included RCTs were generally recorded in very low numbers. They included: pulmonary embolism, thromboembolism, pulmonary failure, pulmonary function/asthma, meningitis, eczema, psychiatric disorders and dementia. Outcomes where over ten events (diagnoses of, or deaths from) were recorded across trials included diabetes, urolithiasis and thrombophlebitis, these are represented in comparison 01 04, but none appear to bear a significant relationship with additional omega 3 fats. Several studies recorded health status in people with Crohn’s disease, ulcerative colitis or rheumatoid arthritis, these have not been reported as they relate to development rather than diagnosis of pre-existing conditions.
Non-cardiovascular, non-cancer outcomes assessed within cohort studies included development of respiratory diseases, cognitive impairment, age-related macular degeneration, diabetes and early menarche. There was no suggestion of an effect of a high omega 3 consumption on development of respiratory diseases, cognitive impairment or age-related macular degeneration.
Data from the Iowa Women’s Health Study (zIWHS Meyer 2001) suggested that a high intake of omega 3 fats may be associated with a significantly greater risk of developing diabetes. However, this study used self-reporting of diabetic status, and there were problems with this measure (of 44 women reporting that they had diabetes at baseline, only 28 were confirmed as having diabetes when their physicians were contacted). The Uppsala CVD screening cohort (zUppsala Vessby 1994) also assessed the relationship between serum EPA and DHA and diagnosis of diabetes (the study directly measured glucose tolerance), and found no significant relationship (the numbers presented could not be used in meta-analysis). Meta-analysis of RCTs with relevant data did not support a significant effect of omega 3 fats on diagnosis of, or death from, diabetes.
Maclure (zMenarche Maclure 91) assessed the relationship between dietary omega 3 fats and early menarche (defined as menarche before 12.5 years) and found that girls consuming more omega 3 fats were significantly more likely to undergo early menarche, the relative risk was 2.39 (95% confidence interval 1.33 to 4.30). The health effects of this overall are unclear, but a relationship has been found between early menarche and increased risk of breast cancer.
Secondary outcomes, events
Cardiovascular deaths (comparison 02, outcome 01)
Forty four trials reported on cardiovascular deaths, of which 30 reported a lack of cardiovascular deaths. Overall, 1418 cardiovascular deaths were reported in 33086 participants, and omega 3 supplementation did not appear to alter the risk, but there was clear heterogeneity, the relative risk was 0.85 (95% confidence interval 0.68 to 1.06, pheterogeneity 0.01). In sensitivity analysis, removing studies with medium or high risk of bias, omega 3 supplementation did not appear to alter the risk of cardiovascular death, without significant heterogeneity, the relative risk was 0.90 (95% confidence interval 0.61 to 1.33, 98 events, pheterogeneity 0.88). Meta-analysis of cohort studies suggested significant reduction in cardiovascular deaths with increased omega 3 intake, but also showed significant heterogeneity.
Fatal myocardial infarction (comparison 02, outcome 02)
Meta-analysis of trials assessing fatal MI included 38 studies, of which only 8 reported events, 390 fatal myocardial infarctions in 6740 participants. There was no significant effect of omega 3 fats on fatal MI, the relative risk was 0.86 (95% confidence interval 0.60 to 1.25, with significant heterogeneity, pheterogeneity 0.06), while sensitivity analysis resulted in only 15 events. Cohort studies did suggest a reduced risk of fatal MI in participants choosing to consume more omega 3 fats, the relative risk was 0.42 (95% confidence interval 0.21 to 0.82, only one study provided data for meta-analysis).
Non-fatal myocardial infarction (comparison 02, outcome 03)
Sixteen RCTs reported an absence of non-fatal MIs. Ten studies reported at least one non-fatal MI, a total of 648 events in 15420 participants, with no suggestion that randomisation to omega 3 fats affected the risk, the relative risk was 1.03 (95% confidence interval 0.70 to 1.50, 648 events, pheterogeneity 0.03). Significant effects of omega 3 fats were not seen when only RCTs at low risk of bias were included (only 29 events were included), or when cohort studies were analysed.
Sudden death (comparison 02, outcome 04)
Sudden death is the outcome that has been proposed as the outcome through which omega 3 supplementation reduces total mortality. Thirty one trials reported an absence of sudden deaths, while six studies reported a total of 416 sudden deaths in 16158 participants. Pooling suggested no significant effect of omega 3 fats on sudden death, the relative risk was 0.85 (95% confidence interval 0.49 to 1.48, pheterogeneity 0.004), but individually one study (GISSI-P 1999) suggested significant protection from sudden death by omega 3 fats and one study (Burr (DART 2) 2003) significantly increased risk of sudden death. Only 8 sudden deaths remained when studies at medium or high risk of bias were removed (no significant effect). Pooling of cohort studies suggested a reduction in sudden death in participants consuming more omega 3 fats, the relative risk was 0.44 (95% confidence interval 0.21 to 0.91, data from only one cohort).
Stroke (comparison 02, outcome 06)
Omega 3 fats are thought to reduce thrombotic tendency, but if this is correct then they may lead to an increase in haemorrhagic stroke but a decrease in thrombotic stroke. Seventeen studies reported an absence of stroke, while 9 reported at least one stroke (243 strokes in total). There was no suggestion of a significant effect on total stroke in meta-analysis, the relative risk was 1.17 (95% confidence interval 0.91 to 1.51, pheterogeneity 0.81) or sensitivity analysis (only 29 events) of RCTs or cohort studies, the relative risk was 0.87 (95% confidence interval 0.72 to 1.04, pheterogeneity 0.31).
Heart failure (comparison 02, outcome 07)
Fourteen RCTs reported an absence of heart failure, while six studies reported a total of 54 cases of heart failure in 7684 participants. Pooling suggested a significant benefit of omega 3 fats on heart failure, the relative risk was 0.51 (95% confidence interval 0.31 to 0.85, pheterogeneity 0.91), however this effect rests heavily on the questionable Singh 1997 trial (see discussion for more details, significance is lost when this study is removed) and sensitivity analysis leaves only one case of heart failure. No cohort evidence was found.
Angina, Peripheral vascular events, Revascularisation (CABG or angioplasty) (comparison 02, outcomes 05, 08, 09)
No significant effects on angina, peripheral vascular events or revascularisation interventions from omega 3 supplementation were seen in the meta-analyses of RCTs, sensitivity analyses or meta-analyses of cohort studies. In the included RCTs there were few peripheral vascular events (11 in total), but more cases of angina (565) and revascularisations (2372).
Secondary outcomes, risk factors
Weight (comparison 02, outcome 10)
Seven RCTs reported weight outcomes, from a total of 1970 participants. There was no significant effect of omega 3 supplementation on weight (weighted mean difference −0.59 kg; 95% confidence interval −1.91 to 0.73, pheterogeneity 0.30).
Lipids (comparison 02, outcomes 11, 12, 13, 14)
Seventeen RCTs reported total cholesterol outcomes, from a total of 3918 participants. Serum total cholesterol was not significantly effected by omega 3 supplementation (weighted mean difference 0.03 mmol/L; 95% confidence interval −0.06 to 0.12, pheterogeneity 0.31), nor was serum HDL cholesterol (weighted mean difference 0.01 mmol/L; 95% confidence interval −0.03 to 0.05, with significant statistical heterogeneity, pheterogeneity 0.005). Serum triglycerides were significantly reduced by omega 3 supplementation (weighted mean difference −0.40 mmol/L; 95% confidence interval −0.56 to −0.23, pheterogeneity 0.003), while LDL cholesterol was significantly raised (weighted mean difference 0.13 mmol/L; 95% confidence interval 0.03 to 0.22, pheterogeneity 0.58).
Subgrouping by omega 3 dose reduced (but did not eliminate) heterogeneity when assessing effects on triglycerides, and a significant reduction in triglycerides (weighted mean difference −0.61 mmol/ L; 95% confidence interval −0.88 to −0.35, significant heterogeneity) was seen only at high dose (4.5g or more EPA + DHA + DPA per day) omega 3 fats. There was a suggestion of greater effect at higher omega 3 dose. Subgrouping by omega 3 dose eliminated heterogeneity when assessing effects on HDL cholesterol, as did removal of a large outlier (Eritsland 1996).
Blood pressure (comparison 02, outcomes 15, 16)
Seven studies (2743 participants) reported systolic and diastolic blood pressure after at least six months of supplementation. Neither were significantly effected by omega 3 supplementation (systolic blood pressure weighted mean difference −1.03 mmHg; 95% confidence interval −3.30 to 1.25, pheterogeneity 0.18; diastolic blood pressure weighted mean difference −0.23 mmHg; 95% confidence interval −1.10 to 0.64, pheterogeneity 0.92).
Drop outs and side effects (comparison 02, outcomes 17, 18)
There was no significant difference in risk of dropping out between participants receiving omega 3 fats and placebo or control (the relative risk was 1.06, 95% confidence interval 0.87 to 1.30, pheterogeneity 0.19).
Randomisation to omega 3 fats did lead to an increased risk of dropping out due to side effects, the relative risk was 1.62 (95% confidence interval 1.10 to 2.40, pheterogeneity 0.50), of a bad or fishy taste or bleching, the relative risk was 3.63 (95% confidence interval 1.97 to 6.67, pheterogeneity 0.18), of nausea, the relative risk was 3.88 (95% confidence interval 1.42 to 10.58, pheterogeneity 0.89), and any gastrointestinal side effect, the relative risk was 1.59 (95% confidence interval 1.14 to 2.21, pheterogeneity 0.32). Randomisation status did not appear to significantly affect risk of abdominal pain or discomfort, diarrhoea, bleeding, skin problems, headaches, hair loss, fistula development, oedema, psychiatric disorders or all side effects combined.
DISCUSSION
Summary of results
Meta-analysis of 48 included RCTs assessing the effects of increased omega 3 fats on total mortality or combined cardiovascular events found strongly significant statistical heterogeneity. This heterogeneity disappeared in the analysis of total mortality when studies at medium or high risk of bias were removed, suggesting no significant effect of omega 3 fats on deaths, the relative risk was 0.98 (95% confidence interval 0.70 to 1.36,138 events, pheterogeneity 0.57). There was no suggestion of a benefit on combined cardiovascular events in the sensitivity analysis, with marginal heterogeneity, the relative risk was 1.09 (95% confidence interval 0.87 to 1.37, 570 events, pheterogeneity 0.07, I2 48.7%). Cohort studies did suggest significant reduction in total mortality with high (compared to low) omega 3 intake.
Subgrouping only trials increasing fish-based omega 3 fats, or only trials that increased plant-based omega 3 fats, did not suggest significant effects on mortality or cardiovascular events in either group. Subgrouping by omega 3 dose, dietary or supplemental source, trial duration, baseline risk of cardiovascular disease and presence of placebo tended to produce significant results where there were a substantial number of events and the DART 2 trial (Burr (DART 2) 2003) was not present. Removal of this trial suggested significant reduction of mortality in participants randomised to omega 3, and removed the apparent heterogeneity. Meta-regression suggested a significant relationship between total mortality and trial duration, with the relative risk for those on additional omega 3 fats increasing (to reach and overtake 1.0) in longer trials, suggesting that an early protective effect of omega 3 fats later becomes harmful (this relationship was lost when the DART 2 trial was removed).
Neither RCT nor cohort studies suggested a significant increased risk of cancer or stroke with higher omega 3 intake, but there were not enough events to rule out clinically important effects.
No significant and robust effects of omega 3 fats were seen on secondary event outcomes. Omega 3 supplementation did significantly reduce triglyceride levels and increased LDL cholesterol, but had no significant effects on weight, total cholesterol, HDL cholesterol, systolic or diastolic blood pressure. Randomisation to omega 3 increased the risk of dropping out due to side effects, a bad or fishy taste, nausea and any gastrointestinal side effect, but not overall risk of dropping out of the trial.
Comparisons with other studies
The results of this review differ from those of a recent high quality systematic review (Bucher 2002). Bucher et al searched for RCTs comparing dietary or non-dietary intake of omega 3 fats with a control diet or placebo in people with coronary heart disease and with at least six months follow up. They included 11 RCTs (9 of these were included in this review: Burr (DART 1) 1989; GISSI-P 1999; Johansen 1999A; Kaul 1992; Leaf 1994; Reis 1991; Sacks (HARP) 1995; Singh 1997; von Schacky 1999 while two were excluded from our review as they included dietary interventions additional to increased omega 3: Leng 1998; de Lorgeril 1999) with a total of 15806 participants and 1335 deaths. As their search period extended to 1999 the DART 2 study (Burr (DART 2) 2003) was not included. As in this review Bucher 2002 found no significant effect of added omega 3 on non-fatal myocardial infarction (the relative risk was 0.8, 95% confidence interval 0.5 to 1.2), but they found significant protective effects on fatal myocardial infarction, the relative risk was 0.7 (95% confidence interval 0.6 to 0.8), sudden death, the relative risk was 0.7 (95% confidence interval 0.6 to 0.9) and overall mortality, the relative risk was 0.8 (95% confidence interval 0.7 to 0.9). They reported publication bias (an asymmetrical distribution of trials with smaller trials showing a larger effect size than the one larger trial, GISSI-P 1999).
The differences between Bucher et al’s results and those in this review cannot be explained by our addition of studies where alpha-linolenic acid (the plant-based omega 3 fat) was the active intervention , or by our inclusion of studies where participants were initially at low or moderate risk of cardiovascular disease. The vast majority of deaths noted in this review occurred in studies where participants initially had evidence of cardiovascular disease and where additional omega 3 fats were of fish origin. Pooling only those studies at high cardiovascular risk, or providing omega 3 fats only from a fish source, still produces non-statistically significant relative risks of mortality with significant heterogeneity (Table 3), as does pooling only those studies with participants at high cardiovascular risk and where fish based omega 3 supplements or advice were used (data not shown).
Meta-analyses in this systematic review, removing the DART 2 trial, produces relative risks similar to those in the Bucher review: non-fatal myocardial infarction, the relative risk was 1.03 (95% confidence interval 0.70 to 1.50), fatal myocardial infarction, the relative risk was 0.70 (95% confidence interval 0.54 to 0.91), sudden death, the relative risk was 0.68 (95% confidence interval 0.42 to 1.10) and overall mortality, the relative risk was 0.83 (95% confidence interval 0.75 to 0.91). This tends to confirm the evidence from subgrouping that there is something about the DART 2 study which is different from the other included studies. Its results are distinct and this results in heterogeneity between the results and altered pooled effects.
Understanding why the results of the DART 2 study are different
Without the DART 2 study pooling of RCTs of omega 3 fats indicates significant reduction in our risk of death and fatal myocardial infarction. With DART 2 we do not see this protection and our results become strongly heterogeneous. It is tempting to dismiss the results of the DART 2 study as inconsistent, inconvenient and therefore irrelevant. However, with 3114 participants DART 2 is larger than DART 1 (Burr (DART 1) 1989), and only GISSI-P1999 (11323 participants for a median of 40 months) and Natvig 1968 (13406 participants for 12 months) enrolled more participants. It is also the longest intervention study to date, with participants followed up for 36 to 108 months. The number of deaths recorded by DART 2 (525) were second only to GISSI-P 1999 (1031 deaths). This is not a trial to be easily dismissed, and if the results of DART 2 turn out to describe more accurately the true effect of omega 3 fats on health then this is important to know.
We need to assess why the inconsistency exists between the results of DART 2 and the pre-DART 2 studies, and to discover whether this could this be explained by bias (in DART 2, or the earlier studies, or both). First, we will assess whether the fundamental question posed by the DART 2 trial was different from that posed in the other studies. If not, perhaps the very different outcomes were simply due to a play of chance.
How is DART 2 different from the other included RCTs?
Type of cardiovascular disease
Another distinguishing feature of DART 2 is that it is the only RCT included to enrol men specifically because they were being treated for angina, but many others enrolled people post myocar-dial infarction or undergoing angioplasty, many of whom presumably also had angina, and who would have similar underlying disease. However, a post-hoc subgroup analysis of the GISSI trial (GISSI-P 1999) has suggested that the people who benefited most from omega 3 were those with heart failure, and these are likely to be much more common in a group of people who have had myocardial infarctions (Marchioli 2004).
As Michael Burr suggests (Burr (DART 2) 2003), it is possible that there is a negative interaction between omega 3 fats and an angina medication which would outweigh other beneficial effects of omega 3s. However, they state: ‘Fish oil could interact adversely with drugs taken by angina patients... There was no evidence of such interactions; indeed, beta-blockers appeared to interact favourably with fish in this study.’
Omega 3 dose
Apart from slightly different inclusion criteria and the longer follow up time for DART 2, it is very similar in design and implementation to the DART 1 study (Burr (DART 1) 1989), which showed a reduction in mortality for men advised to take more oily fish after a myocardial infarction. Both DART studies were conducted in the same geographical area, with the same first investigator, a similar factorial design (DART 1 also randomised to low fat and/or high fibre advice, DART 2 also randomised to increased fruit and vegetable intake), and similar system of randomisation. In DART 1 compliance to the oily fish advice was assessed by a 7 day weighed food diary of a random sub-sample, and indicated intake of 2.5g/week EPA in the intervention group, compared with 0.8g/week EPA in the control. In DART 2 a postal dietary questionnaire sent out six months after inclusion suggested that dietary EPA intake increased to 3.0g /week in the intervention group and 0.8g /week in controls, so compliance appears similar despite rather less intensive dietary advice in DART 2 (Ness A, personal communication).
Dietary fish or capsules
In DART 1 participants were initially advised to eat more oily fish, and those who were unwilling to eat the fish recommended were encouraged to take fish oil capsules (three MaxEPA capsules per day or 0.5g EPA/day). This was also true in DART 2, but around a third of the DART 2 participants were directly randomised to MaxEPA, without being given advice to eat oily fish. Subgrouping to identify any differences in outcome between those randomised to dietary fish advice, and those randomised to fish oil capsules, showed worse adjusted hazard ratios for those randomised to capsules, although they are not encouraging for either group, all deaths, dietary fish HR 1.13 (95% confidence interval 0.94 to 1.37) , fish oil hazard ratio (HR) 1.19 (95% confidence interval 0.92 to 1.54); sudden deaths, dietary fish HR 1.43 (95% confidence interval 0.95 to 2.15), fish oil HR 1.84 (95% confidence interval 1.11 to 3.05) (Burr (DART 2) 2003). In contrast, subgrouping in this review (including the results from DART 2) suggested similar effects of dietary or supplemental interventions (total mortality, dietary advice the relative risk was 0.91 (95% confidence interval 0.57 to 1.44), capsule or oil supplements the relative risk was 0.90 (95% confidence interval 0.76 to 1.07); combined cardiovascular events, dietary advice the relative risk was 0.85 (95% confidence interval 0.69 to 1.07), capsule or oil supplements the relative risk was 0.93 (95% confidence interval 0.78 to 1.11). It does not seem likely that the differences in the effect of additional omega 3 in DART 2 differ from those in DART 1 because some participants were given capsules rather than advice, those in DART 2 given dietary advice fared much worse than DART 1 participants given dietary advice.
Small trial (publication) bias
Small trial bias is a possible explanation of the different results of DART 2 and the previous studies. There is good evidence that the pre-DART 2 body of studies were biased, as funnel plots strongly suggest that smaller studies show more favourable effects of omega 3 for total mortality and combined cardiovascular events (Figure 2). Perhaps DART 2 is simply redressing the balance. If we remove studies with fewer than 50 deaths, or 100 combined cardiovascular events, from the meta-analyses we find that the funnel plots appear less biased (Figure 3;), however the summary estimate of the meta-analysis for total mortality remains similar, the relative risk was 0.88 (95% confidence interval 0.71 to 1.10), while that for combined cardiovascular events remains non-significant, but suggests the possibility of a relative risk greater than one, the relative risk was 1.09 (95% confidence interval 0.91 to 1.31). However, both results show strong statistically significant heterogeneity, so heterogeneity is not due to the smaller trials alone.
Selection bias
DART 1 (Burr (DART 1) 1989) and GISSI-P (GISSI-P 1999), the two large positive studies, were both run at a time when there was not much expectation of omega 3 being effective (both were factorial trials, and there was a greater expectation of the low fat arm in DART 1, and of the vitamin E arm in GISSI-P). DART 2 was run when expectations of the omega 3 arm were high. Health researchers usually want the best for the participants in their trials, and if there was any possibility of influencing the allocation process it might be expected that in DART 1 and GISSI-P those who seemed at most risk might have been preferentially allocated to low fat and vitamin E arms, and perhaps the weller participants advised on omega 3. However in DART 2, as expectations of omega 3 were high, participants most at risk would tend to enter the omega 3 arm. Assuming that there is actually a negligible effect of omega 3 fats on health, this situation might lead to the sort of results we see of these three large influential trials.
Allocation concealment was coded as ‘unclear’ for DART 1 and 2. In both studies a dietitian took a dietary history, then opened a sealed envelope containing a card which indicated the diet to be advised. For GISSI-P however, allocation concealment was coded as ‘done’, since randomisation was by a centralised computer network.
One way of assessing whether selection bias occurred in any of the studies is to determine whether there were any consistent differences between the characteristics of the participants in the omega 3 and non-omega 3 arms of the trial. GISSI-P provides extensive tables of baseline characteristics, with no evidence that the group allocated to omega 3 supplements were more or less healthy at baseline than the control group or the vitamin E group. DART 1 participants given omega 3 advice were slightly less likely to smoke, have had a previous MI, angina or hypertension, but more likely to have cardiomegaly, lung congestion or lung oedema on x-ray, were less likely to be on beta-blockers, anti-anginals, anticoagulants, aspirin or anti-platelets, digoxin or anti-arrhythmics, and more likely to be on anti-hypertensives other than beta-blockers, compared with those not given omega 3 advice. The differences were all less than 3% of the percentage in the control arm, except for previous MI, previous angina, cardiomegaly, and beta-blockers, and the picture does not show consistent advantage to those given omega 3 advice. This does not look like selection bias. Similarly, DART 2 participants given omega 3 advice or supplements were marginally less likely to have had a previous MI, or to be diabetic, more likely to be on beta-blockers, have a higher systolic and diastolic blood pressure, and higher cholesterol than those in the control group, but none of the differences amounted to a 3% difference, and there is no pattern of consistent advantage or disadvantage to the fish group. Selection bias does not appear to explain the differences in results between DART 2 and the other large trials.
Performance bias
Performance bias is a term for differential care provided to individuals within the trial according to their intervention group. This care could result from study personnel or other health carers giving extra advice, encouragement or treatment to those they perceive as not receiving the ‘best’ treatment, or it could result from participants who are receiving what they perceive as a very effective treatment not trying so hard with lifestyle changes or medication. In GISSI-P participants were not masked (there was no placebo capsule) and it is unclear whether health care providers were masked (unlikely if participants were not). In DART 1 and 2 neither participants nor healthcare providers were masked. This leaves the field open for the different expectations of the omega 3 intervention to have differing effects on adjuvant healthcare in all three trials. Information on weight, smoking, blood pressure, physical activity, prescribed medications and/or compliance with prescribed drugs might help us to understand whether this type of bias may have operated.
GISSI-P measured change in lipids at six months, and found that total cholesterol and LDL cholesterol rose slightly more in the omega 3 group (total cholesterol rose 8.4% vs 7.1%, LDL cholesterol rose 10.4% vs 7.3%), while HDL rose less in the omega 3 group (rise of 8.8% vs 9.3%). It is difficult to know whether this reflects the effects of the omega 3 fat, a more cavalier attitude to lipid lowering on the part of carers or participants, or both, but it does not suggest that those on omega 3 fats were better cared for in terms of lipid lowering. Pharmacological therapy appeared very similar among those on omega 3 supplements compared with those not allocated to omega 3 supplements at six months (anti-platelet drugs 87.9% vs 87.9%, ACE inhibitors 39.9% vs 41.9%, beta-blockers 41.2% vs 41.3%, lipid lowering drugs 28.3% vs 29.0%), again not supporting better concurrent treatment of those randomised to omega 3 supplements, although compliance is not reported. Those randomised to omega 3 fats were slightly more likely to be taking at least one portion of fruit (87.5% vs 87.0%) and vegetables (53.4% vs 52.8%) daily at six months, which may suggest that omega 3 participants were looking after themselves better, but the effect appears small.
DART 1 assessed food intake (7-day weighed) in a random sample of 25% of participants at six months, and found no differences in total energy, total fat or dietary fibre between those given omega 3 advice and those not. Those given fish advice ate slightly more protein (19% of calories vs 17% of calories), less carbohydrate (66% calories vs 68% calories) and less meat, meat products, cheese and eggs (as these were displaced by the fish). Weight was slightly lower in those randomised to omega 3 advice at baseline, and the differential between the two groups was maintained fairly consistently at six months and two years of the trial (both groupsgaining about a kilogram). The proportion of current smokers fell in both groups from baseline, only slightly more in the group randomised to omega 3 advice (from 53.8% to 21.8%, a fall of 32.0%, in those on omega 3s and from 53.7% to 22.6%, a fall of 31.1%, in those not randomised to omega 3 advice. Prescription of anti-hypertensive drugs rose from 55.0 to 55.7% at six months and 59.0% at two years in those on omega 3 advice, while it fell from 58.0% at baseline to 57.9% at six months, then rose to 62.9% at two years in those not advised to take omega 3 fats. Use of anti-anginals fell slightly in the first six months of the study then increased dramatically at two years (omega 3 advice 46.5% at baseline, 45.7% at six months, 72.0% at two years; no omega 3 advice 47.2% at baseline, 46.7% at six months and 70.0% at two years). None of this suggests that those given omega 3 fat advice were treated, or acted, substantially differently from those not given such advice.
As results of the DART 2 trial have currently only been published in two papers, there is limited information about whether participants randomised to omega 3 advice received less good care from trialists, healthcare workers or looked after themselves less well. Self-reported intake of saturated fat six months after entering the trial was significantly reduced in all four arms of the trial, falls of 2.8 (95% confidence interval 0.9 to 4.6) g/dayay in the fish advice group, 3.1 (1.5 to 4.8) g/day in the fruit advice group, 3.9 (2.0 to 5.8) g/day in the fish and fruit group, and 3.5 (1.8 to 5.3) g/day in the control (sensible eating) group), as was total fat intake, falls of 5.2 (95% confidence interval 0.9 to 9.5) g/day in the fish advice group, 7.2 (3.6 to 10.9) g/day in the fruit advice group, 6.8 (2.4 to 11.1) g/day in the fish and fruit group, and 8.6 (4.6 to 12.7) g/ day in the control (sensible eating) group). Similarly self reported total energy intake was reduced in all but the fish and fruit group, falls of 85 (95% confidence interval 12 to 158) kcal/day in the fish advice group, 84 (5 to 163) kcal/day in the fruit advice group, 60 (-35 to 156) kcal/day in the fish and fruit group, and 134 (40 to 228) kcal/day in the control group). While the fish plus fish and fruit groups combined appear to do less well than the fruit and sensible eating groups combined in terms of energy reduction and total fat reduction (where oily fish or supplements will have raised to the total fat and calorie intakes), this is not true for saturated fat reduction (which will have been altered less by fatty fish intake).
Self-reported lifestyle data were collected from 944 of the survivors a year after DART 2 ended (see Ness 2004, part of Burr (DART 2) 2003). Significant differences between those given and not given fish advice were only seen for measures of fish or supplemental intake (62.9% of those given fish or omega 3 supplemental advice, and 41.8% of those not given such advice, were taking regular fatty fish or MaxEPA). Those given fish advice were on average taking the same amount of fruit and vegetables (364.6 vs 361.1 g/day) and alcohol (both 4.8 units per week) with a slightly lower weight (82.2 vs 82.7kg) than those not given fish advice. They were slightly (not significantly) less likely to smoke (17.6% vs 19.1%), and take aspirin (66.7% vs 68.6%), more likely to take lipid lowering drugs (39.2% vs 35.5%), while beta-blockers did not differ (36.9% vs 36.8%).
Overall, the indicators available to date do not suggest that those given omega 3 advice were less well cared for either by themselves or their healthcare providers. If performance bias is operating it is not obvious.
Attrition bias, detection bias
Attrition bias relates to differences in numbers or types of people dropping out from, or being excluded from the analysis of, the intervention and control arms of a study. Follow up in the two DART studies was virtually complete for mortality outcomes, so this does not explain the different results between these studies. Detection bias is about differential assessment of outcomes because of knowledge of the trial allocation, however determination of very definite outcomes (like death) are unlikely to be influenced by whether the outcome assessor knows someone is eating oily fish or not. What might possibly be influenced would be cause of death, but outcome assessors were masked to the interventions in both DART studies and GISSI-P, so this is unlikely to have caused the differences in results between the studies.
Summary
There are no clear reasons why DART 2 differs in its results from the bulk of the omega 3 studies. If we take the point estimate of the meta-analysis of the RCTs at low risk of bias as the best estimate we have of the effect of omega 3 fats on total mortality (the relative risk was 0.98), then while DART 1 and GISSI-P have point estimates on the positive side (0.71 and 0.86 respectively), DART 2 compensates with a point estimate on the negative side (1.15) and all three studies fall within the 95% confidence interval of this sensitivity analysis, the relative risk was 0.98 (95% confidence interval 0.70 to 1.36). It may be that the effect of omega 3 fats on cardiovascular disease is smaller than previously thought (if indeed the effect does exist). Alternatively it may be that effects in those who have had a myocardial infarction are protective of death, but the effects in men with angina and no infarction are not, which would explain the heterogeneity between the studies when DART 2 is added.
Levels of toxins
In this review we set out to discover the effect on health, if any, of the long term intake of toxins associated with oily fish, as well as with the oily fish itself. One distinguishing feature of DART 2 is that it has the longest follow up of all studies, so may provide useful information on these long term effects. Perhaps in DART 2 the cumulative harmful effects of the PCBs, dioxins and/or mercury, contained within oily fish and fish oils had time to develop and be expressed as illness. This interpretation has been supported by several cohort studies which have assessed relationships between oilyfish, contaminants and cardiovascular disease (zATBC Pietinen 1997; zKuopio Rissanen 00; Salonen 1995).
If the toxins carried by oily fish are counteracting the protective effect of the fish oil itself, we would expect an initial benefit of the omega 3 fats (as in the shorter term trials), followed by a period of neutrality and then harm as the toxins accumulate within adipose tissues, several years after initiation of omega 3. This is supported by the significant results of the meta-regression of relative risk of mortality vs study duration, where the relative risk of death in the omega 3 group compared with the control group rises over time, but this effect is lost once the DART 2 study is removed. A time-course analysis of the GISSI-P trial results (GISSI-P 1999, see the Marchioli 2002 paper) suggests that the relative risk of death may have increased during the 42 months of the trial (although the confidence limits are very wide), from 0.59 (95% confidence interval 0.36 to 0.97) at three months to 0.66 (95% confidence interval 0.45 to 0.96) at six months, 0.62 (95% confidence interval 0.45 to 0.86) at nine months, 0.72 (95% confidence interval 0.54 to 0.96) at 12 months to 0.79 (95% confidence interval 0.66 to 0.93) at 42 months - still protective late in the trial, but less so, in line with this review’s meta-regression results. However, the survival curve of the DART 2 trial does not concur; survival appears less good for those encouraged to take omega 3 from only a year into the trial (Burr (DART 2) 2003). If longer duration leads to greater harm then we could expect that this would occur more quickly in trials using higher doses of fish based supplements, but there are too few events in trials of high doses (all are 12 months or less in duration or include fewer than 100 participants) to assess whether this is the case. There is little evidence of a reduction in benefit, let alone harm, over the duration of the included studies.
The toxic load of oily fish and fish oil supplements varies widely depending on species, location, time of year, decade, and whether the fish are farmed or wild (FSA 2002; Hites 2004; Liem 1997; MAFF 1997; MAFF 1998A; MAFF 1998B; MAFF 1999; USFDA 1995). An assessment of fish oil supplements in 1994-5 found levels of total PCBs ranging from ‘not detected’ (where the limit of detection was 5 micrograms per litre) in oils from Australia, Japan, France, Austria, New Zealand and the UK, to 1132 micrograms per litre in a UK pharmaceutical grade salmon oil (Jacobs 1998). Some of these differences depend on how the oil is refined, and deodorisation appears to reduce contamination levels by about half (Hilbert 1998). There is evidence that farmed salmon and wild sea fish stocks (used to feed farmed salmon) may be more contaminated in northern Europe than in North or South America (Hites 2004, although this has been contested), but there are local areas of serious contamination of local fish stocks in North and South America, and parts of Europe (Cordier 1998; Falk 1999; Fitzgerald 1999;Hovinga 1993; Mahaffey 1998; Maurice-Bourgoin 00; Peterson 1994; Salonen 1995).
We do not know the types of oily fish eaten in South Wales during the DART trials, or the levels of contamination that were present. Independent analysis of MaxEPA liquid in 2000-2002 (3ml/day MaxEPA was the supplement provided to participants in both DART1 and DART2 when they were unwilling to increase their oily fish intakes) suggests that this source was unlikely to be a major problem. Three ml daily would have produced an adult exposure of 0.2 pg WHO-TEQ/kg bodyweight/day for dioxins and dioxin-like PCBs (FSA 2002), contrasting with estimated whole diet average intake of about 0.9 pg WHO-TEQ/kg bodyweight/ day for dioxins and dioxin-like PCBs in 2001 (including dietary fish) or 7.2 pg WHO-TEQ/kg bodyweight/day for dioxins and dioxin-like PCBs in 1982 in the UK (FSA 2003). No independent UK data appear to exist for MaxEPA for the period during the DART 2 trial (1990 to 1999). The current provisional tolerable monthly intake set by the World Health Organization is just over 2 pg WHO-TEQ/kg bodyweight/day (JECFA 2001).
Overall it is difficult to know the levels of PCBs and dioxins in the oily fish and fish oils consumed by participants in any of our included trials. Environmental levels of PCBs and dioxins have been falling generally since action was taken to curb their manufacture and accidental production: lake sediment cores show peak levels in the 1970s and a gradual fall since then. If we do find that omega 3 fats are more protective against mortality in short than long term trials then this may not be due to toxins, but simply that people who would have died are being kept alive a little bit longer, death is delayed in some people for a few years, then catches up again.
Comparison of RCT and cohort data
Cohort studies were included in the review to help us recognise any long term health effects of omega 3 fats, or any of the toxins which are commonly found in fish oil (including PCBs, dioxins and mercury). The reasoning is that cohort studies tend to be longer term than randomised controlled trials, and so may pick up harms or benefits that only accrue after many years. Also, even a short term cohort study may be reflecting longer term dietary patterns, so is more likely to spot longer term effects than is a trial. The disadvantage with cohort studies (and the reason we are not using them to assess the efficacy of omega 3 supplementation in cardiovascular disease) is the difficulty in separating out effects related to oily fish, fish oil or other omega 3 rich oil consumption, from other lifestyle factors associated with these eating patterns, so that causation is difficult to assess.
Cohort studies often do not give details of the characteristics of participants in their highest and lowest quantiles of omega 3 intake, but details are reported for five cohorts (HPFS, zHPFS Ascherio 1995; NHS, zNHS Hu 2003; Kuopio IHD, zKuopio Rissanen 00; NHSSN, zNHSSN Egeland 2001; Iowa Women’s Health Study (IWHS), zIWHS Meyer 2001). We have data on comparable factors across at least two cohorts for the following, where people who take more omega 3 fats are consistently more likely to:
be non-smokers (HPFS, NHS, Kuopio IHD, NHSSN, IWHS; adjusted for in HPFS, NHS, IWHS, Kuopio, stratified by in NHSSN)
be more active (HPFS, NHS, IWHS - not reported in NHSSN, similar in Kuopio IHD; adjusted for in NHS, IWHS, Kuopio)
have hypertension, hypercholesterolaemia or a family history of CHD (HPFS, NHS - not reported in the others; all adjusted for in HPFS and NHS, not adjusted for in IWHS or NHSSN, Kuopio adjusted for blood pressure)
take dietary vitamins (HPFS, NHS - not reported in the others; NHS adjusted for vitamin E and multivitamin supplementation, HPFS, IWHS, NHSSN, Kuopio adjusted for neither, )
to not have had a coronary event (Kuopio, NHSSN - not reported in IWHS, those with CVD excluded from HPFS and NHS; adjusted for in Kuopio, not in IWHS or NHSSN)
eat more dietary fibre, fruit and vegetables, less saturated and trans fats (HFS, NHS - not reported in the others; dietary fibre adjusted for in NHS and IWHS, fruit, vegetables and saturated fat not adjusted for in any cohort analyses, trans fats adjusted for in NHS only)
drink less alcohol (HPFS, NHS, IWHS - not reported in the others; adjusted for in HPFS and IWHS, not in NHS, NHSSN, or Kuopio)
be better educated (NHSSN, IWHS - not reported in the others; adjusted for in IWHS, not in the others)
live in a city (IWHS, Kuopio - not reported in the others; adjusted for in IWHS and Kuopio, not in the others)
Age was generally almost identical in the upper and lower quantiles (data provided by NHS, IWHS, NHSSN and Kuopio; adjustment for age in HPFS, NHS, IWHS and Kuopio, not in NHSSN), as was BMI (in HPFS, NHS, IWHS and Kuopio, but was lower in those on high omega 3 intake for NHSSN; adjustment for BMI in HPFS, NHS, IWHS, Kuopio, not in NHSSN). HRT use was higher in those on high omega 3 intake in NHS, but similar in high and low omega 3 groups in the Iowa Women’s Health study (both cohorts adjusted for hormone replacement therapy use, HPFS and Kuopio did not as they were men only, NHSSN did not). Sex was not relevant for HPFS, NHS, Kuopio or IWHS (all single sex cohorts), NHSSN stratified by sex.
Other factors adjusted for in one cohort only were: waist hip ratio, magnesium intake, marital status (IWHS); presence of diabetes, profession (HPFS); aspirin use, polyunsaturated /saturated fat ratio, duration of diabetes, medication for diabetes (NHS), platelet aggregation, hair mercury, socioeconomic status (summary score), fasting serum insulin, serum ferritin, and LDL cholesterol (Kuopio). Total energy intake was adjusted for by two cohorts, IWHS and Kuopio.
Aside from the hypertension, hypercholesterolaemia and family history of CHD, these factors indicate a health and social advantage to people taking higher levels of omega 3 fats - these are people who are likely to be healthier for a range of other reasons besides whether they take oily fish or omega 3 supplements. There are real theoretical difficulties in how to adequately adjust for this type of web of lifestyle advantage (encompassing social, educational, financial, dietary, fitness, smoking and treatment advantages) accompanying increased omega 3 intake - but if the adjustment is not adequate then those eating more omega 3 fats are likely to appear to be healthier than those who do not eat so much. Theoretically RCTs are the solution, separating out lifestyle patterns from omega 3 intake.
Any differences between RCTs and cohorts could be due to different doses of omega 3 fats used. These can be difficult to compare as doses are expressed in different ways in different studies (as quantities of EPA, or of EPA plus DHA, of all long chain omega 3 fats (including DPA) and with or without alpha-linolenic acid, or as weight of oily fish). In cohort studies differences may also be expressed as percentages of fatty acids within a cell fraction, or more crudely as groups of people who either generally do or do not have a weekly portion of oily fish. Included RCTs provide doses from less than 1g long chain omega 3 fats per day (Belluzzi 1996; Burr (DART 1) 1989; Burr (DART 2) 2003; GISSI-P 1999; Shimizu 1995; Veale 1994) to over 5g per day (Almallah 1998; Connor 1993; Dehmer 1998; Hawthorne 1992; Leaf 1994;Loeschke 1996; Lorenz-Meyer 1996; Reis 1991; Sacks (HARP) 1995; Thien 1993). The studies providing most of the outcome events provide less than 1g per day of long chain omega 3 fats. The difference between the upper intake and lower intake bands in the cohort studies also varies, and is often different in different analyses of the same cohort, but tend to be between 0.2 to 0.7g long chain omega 3 fats per day. So cohorts are assessing smaller differences in omega 3 fats over longer periods.
There are problems in dietary assessment of omega 3 intake, so that some people will be mis-classified by food frequency questionnaires (these are only as good as the amount of attention and importance they are given by the person completing them). It is possible that the best data will be from analyses of body fat fractions, rather than dietary assessments. Similar problems are encountered in RCTs where some participants will not take allocated supplements, and others in the control group may buy supplements or have a diet very high in omega 3 fats.
There is also a potential for reporting bias from cohort analyses. As it is possible to assess the effects of ‘EPA’ or ‘DHA’ or ‘EPA+DHA’ or ‘EPA+DHA+DPA’ or ‘EPA+DHA+DPA+alpha-linoleic acid’ or’EPA+DHA+alpha-linoleic acid’ or ‘DHA+DPA’ or ‘regular supplement use’ or ‘regular oily fish meals’ etc then there is a possibility that various combinations of the above will be assessed, but only those which look interesting (reach statistical significance) will be reported. (One cohort study also used ratios of fatty acids, but this was excluded from the review.) This can easily bias our understanding of ‘statistical significance’ and the likelihood of the importance of the relationships which are reported.
If the cohort studies give the same results as RCTs for short term outcomes (and the suggestion from GISSI-P and DART 1 have been that protection occurs within a one year period) then we can have more faith in the cohort studies reliably informing us about the longer term outcomes. RCTs tell us that there is no significant effect of omega 3 fats on total mortality, the relative risk was 0.87 (95% confidence interval 0.73 to 1.03, with significant heterogeneity) while cohort studies suggest significant protection, the relative risk was 0.65 (95% confidence interval 0.48 to 0.88, no significant heterogeneity), so although the point estimates of both meta-analyses suggest protection by omega 3 fats there is disagreement about the size and statistical significance of that protection. The data are more similar for combined cardiovascular events, with both RCT and cohort data suggesting no significant effect, with similar point estimates and similar heterogeneity. ‘Combined cardiovascular events’ would be a useful outcome if omega 3 affects a variety of cardiovascular outcomes in a similar way, but may be misleading if omega 3 affects one specific type of cardiovascular event only (as the importance of this effect would be likely to be lost in the ‘noise’ of other unaffected events.
We are left with some uncertainty as to whether cohort studies are able to correct for lifestyle and health trends which may co-exist with greater intake of omega 3 fats, and so adequately assess risk of harms.
Potential harms of omega 3 fats
Animal and cohort studies of dioxins and PCBs suggest that cancers (and also sub-fertility in those exposed in utero) might be increased in humans with long exposure to contaminated fish oils, neurological deficits might result from increased mercury loads, and strokes might be promoted by the omega 3 fats themselves.
Cancer diagnosis is a long term outcome, where we might expect more information from cohort studies, but here neither meta-analyses of RCTs or cohorts show significant effects of omega 3 fats on cancer. This does not mean that there is no harmful effect as the numbers of events overall are small (fewer than 400 for RCTs, just over 800 in included cohort studies). Additionally, most trials that provided data on cancers provided mortality data rather than data on diagnosis. Clearly cancer deaths will take longer to occur than initial diagnosis in many cases so that we are even less likely to see an alteration in cancer deaths due to dioxins or PCBs during a trial, than we are in cancer diagnosis. No data were available on neurological problems, except that there were no significant effects seen on cognitive impairment (with only 100 events seen in the meta-analysable cohort study, but a significant protection of DHA (but not EPA) seen against Alzheimer’s disease in zAlzheimer’s Kyle 99).
Another postulated harm from increased omega 3 intakes is an increased stroke rate, but here again numbers of events were low (RCTs 243, cohorts 602) and no significantly harmful (or protective) effects were seen, although the point estimate for stroke from the RCT meta-analysis was greater than one, the relative risk was 1.17 (95% confidence interval 0.91 to 1.51).
Other health outcomes assessed in cohort studies were respiratory diseases, age-related macular degeneration and diagnosis of diabetes. There were fewer than 500 events in all meta-analyses except diagnosis of diabetes, where a omega 3 appeared to significantly increase the risk of diagnosis of diabetes. Interestingly an RCT (which provided alpha-linolenic acid) reported on diabetes diagnosis (Natvig 1968) and with only 21 events suggests an almost significant protective effect of omega 3 fats. It is unclear whether this contradiction is due to different omega 3 sources, confounding, or the different time scales. No other outcomes showed significant associations except for early menarche, where higher omega 3 intake strongly correlates to early menarche. Thus the cohort studies provide no indication of onerous harmful effects of increased omega 3 intake from fish or vegetable oil sources, but data are lacking on important outcomes such as neurological status.
Strengths and weaknesses of this review
We believe that a study by Singh et al is due to be withdrawn by the British Medical Journal (Retraction 2004). For this reason we are unclear about the quality of the Singh study included in this review (Singh 1997, which is by the same first author, but was not published in the BMJ). As this study is excluded in the sensitivity analyses, which remove all studies not considered at low risk of bias, we feel that it is even more important to ensure that any results are supported by the sensitivity analyses.
This review assessed heterogeneity using Cochran’s test, but assessment using the newly developed I2 test (Higgins 2003) would have altered our understanding of the degree or statistical significance of heterogeneity present in some analyses. For example, the main analysis where all RCT data on mortality is pooled has a p value of 0.04 suggesting statistically significant heterogeneity, but the I2 value is 42%, a figure that might make us feel happier about pooling the studies (as it represents ‘moderate’ heterogeneity). In this case we might accept more readily that there is little effect of omega 3 fats and worry less about the differences between the DART 2 study and the other major omega 3 trials.
New trials
There are plans for trials, or trials underway, involving 21000 people with diabetes or glucose intolerance (AFORRD; ASCEND;ORIGIN), over 900 people with arrhythmias (DISAFF; SOFA),8000 people with heart failure (GISSI-HF), over 18000 people with hyperlipidaemia (JELIS), 15000 people at high risk of cardiovascular disease (Risk and Prevention) and 180 people with heart disease (OLIVE), see table of characteristics of ongoing studies. When these trials report we should understand the effects of omega 3 fats on morbidity and mortality in people with diabetes, heart failure, hyperlipidaemia and arrhythmias, and this data will go some way to informing us of the effects of supplemental omega 3 fats over longer periods (both JELIS and Risk and Prevention will follow up for 5 years), in people with heart failure and in lower risk people.
It is not clear that dietary or supplemental omega 3 fats reduce or increase total mortality, combined cardiovascular events, or cancers in people at high, moderate or low risk of cardiovascular disease. Neither were robust significant effects seen for any secondary outcome events.
The lack of significantly reduced total mortality, cardiovascular events, or cancers does not rule out an important effect of omega 3 fats, as numbers of events reported from robust high quality trials are small.
There is no clear evidence that omega 3 fats differ in their effectiveness according to initial level of risk of cardiovascular disease, dietary or supplemental omega 3 sources, fish or plant omega 3 sources, dose of fish-based omega 3 fats or presence or absence of a placebo. There was not enough evidence to assess the effect of proportion of EPA in plasma or membrane fats. There is a suggestion that a short term benefit of omega 3 fats may after several years become a harm, but the evidence for this is not consistent.
AUTHORS’ CONCLUSIONS
Implications for practice
It is not clear that dietary or supplemental omega 3 fats reduce or increase total mortality, combined cardiovascular events, or cancers in people with, or at risk of, cardiovascular disease or in the general population. Neither were robust significant effects seen for any secondary outcome events. As no significantly increased risks of any events (total mortality, cancers, strokes) were seen there is no need for people to stop eating oily fish or taking supplemental sources of omega 3 fats if they are currently doing so.
As US, UK and international guidelines (De Backer 2003; Kris-Etherton 2002; SIGN 2002; Wood 1998) currently encourage people who have had a myocardial infarction to take more omega 3 fats, and the trials of people who have had myocardial infarctions support this, we suggest that this continues at present but that the evidence should be regularly reviewed as new trials are published. It is probably not appropriate at present to recommend increased omega 3 intakes for people who have angina but have not had a myocardial infarction, but again this may be incorrect and the evidence should be kept under review.
Independent analyses of the levels of toxins in named brands of fish oil supplements and oily fish sold for food should be more widely available.
Implications for research
Further high quality trials (with adequate allocation concealment, prespecified cardiovascular endpoints including sudden cardiac death, and blinding of participants and health providers) are needed to examine any protective effect of omega 3 fats for those at increased cardiovascular risk, to run for long enough to assess long term events (ideally beyond four years), and to report putative associated harms (including cancer diagnosis, different types of stroke, and neurological status). It is hoped that these issues will have been incorporated into the large studies currently being planned and underway and that further data will be available in the next few years.
At present almost no RCT data exist on health outcomes in healthy populations, this would be a fruitful area for further research, but large and expensive trials would be needed. Trials of ALA, oily fish and (concentrated) supplemental long chain omega 3 fats are needed, at present most ongoing trials appear to be of supplemental long chain omega 3 fats. The association between exposure to fat soluble toxins from fish and risk of MI or CHD should also be examined.
PLAIN LANGUAGE SUMMARY.
There is not enough evidence to say that people should stop taking rich sources of omega 3 fats, but further high quality trials are needed to confirm the previously suggested protective effect of omega 3 fats for those at increased cardiovascular risk
The review shows that it is not clear whether dietary or supplemental omega 3 fats (found in oily fish and some vegetable oils) alter total deaths, cardiovascular events (such as heart attacks and strokes) or cancers in the general population, or in people at risk of, or with, cardiovascular disease. When the analysis was limited to fish-based or plant-based, dietary or supplemental omega 3 fats there was still no evidence of reduction in deaths or cardiovascular events in any group.
ACKNOWLEDGEMENTS
Our thanks to Julian Higgins, who was involved in the design of the review, Theresa Moore and Margaret Burke from the Cochrane Heart Group. Thank you too, to all of the authors of primary studies who so kindly helped us build up the best set of data available, including: B Akesson, University of Lund, Skoldstam 1992; YZ Almallah, University of Aberdeen, Almallah 1998; I Bairati, Laval University, Bairati 1992; D Bates, Royal Victoria Infirmary, Newcastle on Tyne, Bates 1990; JJF Belch, University of Dundee, Lau 1993, Belch, Veale 1994; A Belluzzi, University of Bologna, Belluzzi 1996; WJE Bemelmans, National Institute for Public Health and the Environment, Bilthoven, Bemelmans 2002; SJ Bonnema, Odense University Hospital, Bonnema 1995; CR Borchgrevink, Retired Professor, Oslo, Borchgrevink 1966; J Brox, University Hospital of Tromso, Brox 2001; ML Burr, University of Wales, Burr (DART 1) 1989, Burr (DART 2) 2003; JH Christensen, Aalborg Hospital, Christensen 1997; D Colquhoun, University of Queensland, OLIVE; WE Connor, Oregon Health Sciences University, Connor 1993; GJ Dehmer, University of North Carolina, Dehmer 1998; PNM Demacker, University Hospital Nijmegen, Katan 1997; J Eritsland, Ulleval University Hospital,Eritsland 1996; D Franzen, Universitat zu Koln, Franzen 1993; S Greenfield, QE II Hospital, Welwyn Garden City, Greenfield 1993; AB Hawthorne, University Hospital Cardiff, Hawthorne1992; MP Hermans, Clinique Universitaires St Luc, Selvais 1995; MB Katan, Wageningen University, Katan 1997; U Kaul, Batra Hospital, Kaul 1992; CS Lau, University of Hong Kong, Lau 1993, Lau 1995; A Leaf, Massachusetts General Hospital, Leaf 1994; R Lorenz, Universitat Muchen, Loeschke 1996; H Lorenz-Meyer, Stadt Krankenhaus Friedrichshafen, Lorenz-Meyer 1996; A Maresta, Ospedale S. Maria delle Croci, Ravenna, Maresta 2002; J Mate-Jimenez, Hospital de la Princesa, Madrid, Mate-Jimenez 1991; M Milner, Washington Hospital Center, Milner 1989; DWT Nilsen, Central Hospital in Rogaland, Nilsen 2001; R Pasternak, Massachusetts General Hospital, Reis 1991; A Rivellese, Universita degli Studi di Napoli Federico II, Sirtori 1998; P Rossing, Steno Diabetes Centre, Rossing 1996; ES Sarkkinen, University of Kuopio, Sarkkinen 1998; PL Selvais, Hornu-Frameries Medical Center, Selvais 1995; H Shimizu, Gunna University Hospital, Shimizu 1995; RB Singh, Medical Hospital, Moradabad, Singh 1997; E Stragliotto, Pharmacia & Upjohn, Milan, Sirtori 1998; T Terano, Chiba Municipal Hospital, Terano 1999; F Thien, Alfred Hospital, Prahlan, Thien 1993; D Vincent, Hopital Rothschild, Paris, Dry 1991; C von Schacky, University of Munich, von Schacky 1999.
SOURCES OF SUPPORT
Internal sources
University of Manchester, UK.
Central Manchester and Manchester Children’s University Hospitals NHS Trust, UK.
External sources
British Dietetic Association, UK.
Studentship, Systematic Reviews Training Unit, Institute of Child Health, University of London, UK.
Northwest R&D Research Fellowship, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation: permuted block randomisation by Aberdeen Royal Infirmary Pharmacy Allocation concealment: Done Participants masked: yes Providers masked: yes Outcome assessors masked: yes Summary risk of bias: low |
|
| Participants | N: 18 int., 18 control Level of risk for CVD: Low (people with distal procto-collitis (ulcerative colitis)) Male: 44% int., 56% control Mean age, sd: 54 int, 41 control Age range: 29-64 int., 32-72 control Smokers: Unclear Hypertension: Unclear Location: UK |
|
| Interventions | Type: supplement (oil) Intervention: fish oil extract, 15 ml/d (5.6g EPA + DHA) Control: sunflower oil, 15 ml/d Compliance: capsule counts and urinary thromboxanes (no data reported) Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: ulcerative colitis activity Dropouts: None? Available outcomes: deaths Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: randomised using a randomisation table by an epidemiologist Allocation concealment: Done Participants masked: yes Providers masked: yes Outcome assessors masked: yes Summary risk of bias: low |
|
| Participants | N: 107 int., 98 control Level of risk for CVD: High (undergoing planned angioplasty) Male: 81% int., 82% control Mean age, sd: 54, 9 int, 55, 8 control Age range: Unclear Smokers: 32% int., 20% control Hypertension: 35% int., 27% control (on anti-hypertensives) Location: Canada |
|
| Interventions | Type: supplement (capsule) Intervention: MaxEPA, 15 capsules/d (4.5g EPA + DHA) Control: Olive oil, 15 capsules/d Compliance: capsule counts (93% of capsules taken overall, ‘compliance high in both groups’), also plasma levels Length of intervention: 7 mo |
|
| Outcomes | Main study outcome: restenosis Dropouts: 48 int, 38 control Available outcomes: recurrent angina, BMI, lipids, BP, side effects Response to contact: yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: by random number allocation Allocation concealment: Unclear Participants masked: Unclear Providers masked: Unclear Outcome assessors masked: Unclear Summary risk of bias: medium or high |
|
| Participants | N: 60 int., 60 control Level of risk for CVD: high (people referred for coronary angioplasty) Male: 71.7% int., 79.2% control Mean age, sd: 55.1 int, 53.0 control Age range: Unclear Smokers: 20% int., 15% control Hypertension: 8% int., 15% control (on anti-hypertensives) Location: UK |
|
| Interventions | Type: supplement (capsules) Intervention: MaxEPA capsules (3g/d EPA + DHA) Control: nil Compliance: participants questioned about compliance at 6 weeks, data not provided Length of intervention: 7 mo |
|
| Outcomes | Main study outcome: restenosis Dropouts: 3 int., 7 control Available outcomes: recurrent angina, repeat CABG or angioplasty, side effects Response to contact: No |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: balanced block randomisation performed by Tillotts Pharma Allocation concealment: Done Participants masked: yes Providers masked: yes Outcome assessors masked: yes Summary risk of bias: low |
|
| Participants | N: 39 int., 39 control Level of risk for CVD: low (established Crohn’s disease, in remission) Male: 51.3% int., 48.7% control Median age, sd: 34 int, 39 control Age range: 18-67 int., 20-65 control Smokers: 36% int., 33% control Hypertension: Not stated Location: Italy |
|
| Interventions | Type: supplement (capsules) Intervention: PurEPA 3 enteric coated capsules/d (0.9g EPA + DHA) Control: Mixed TG 3 enteric coated capsules/d (packed and labeled as fish oil capsules, no difference in odor as long as capsules not broken Compliance: capsule count performed, no results presented, in red cells EPA rose by 2800% of fatty acids in the intervention group and fell by 3% of fatty acids after one year in the control Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: maintenance of Crohn’s remission Dropouts: 5 int., 2 control Available outcomes: deaths, MI, angina, stroke, heart failure, CV events, sudden death, side effects Response to contact: yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: computer generated, allocated by independent Trial Co-ordination Centre which organised masked distribution Allocation concealment: Done Participants masked: yes Providers masked: yes Outcome assessors masked: yes Summary risk of bias: low |
|
| Participants | Arm 1 with nutrition education (MARGARIN): N: 51 int., 52 control Level of risk for CVD: moderate (multiple cardiovascular risk factors, 10 yr IHD risk ~20%) Male: 35% int., 39% control Mean age, sd: 54.8, 10.0 int, 55.2, 9.9 control Age range: Unclear Smokers: 47% int., 46% control Hypertension: 63% int., 52% control (on anti-hypertensives) Arm 2 without nutrition education (MARGARIN): N: 58 int., 105 control Level of risk for CVD: moderate (multiple cardiovascular risk factors, 10 yr IHD risk ~20%) Male: 48% int., 49% control Mean age, sd: 54.1, 9.2 int, 53.3, 9.7 control Age range: Unclear Smokers: 51% int., 51% control Hypertension: 44% int., 42% control (on anti-hypertensives) Location: the Netherlands |
|
| Interventions | Arms 1 and 2: Type: supplement (enriched margarine) Intervention: Provided with a-lin rich margarine (80% fat of which 15% was a-lin) Control: Provided with linoleic rich margarine (80% fat of which 0.3% was a-lin), identical in taste and packaging Compliance: Arm 1: serum fatty acids used to assess, a-lin rose by 0.47 mol% (sd 0.04) int and fell by 0. 06 mol% (sd 0.04) control, significantly different. Arm 2: serum fatty acids used to assess, a-lin rose by 0.36 mol% (sd 0.04) int and fell by 0.11 mol% (sd 0.03) control, significantly different. Length of intervention: 24 mo |
|
| Outcomes | Main study outcome: cardiovascular risk factors and IHD risk Dropouts: Unclear Available outcomes: total and CV deaths, non-fatal MI, stroke, CABG and angioplasty, BMI, lipids, BP Response to contact: yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: Allocation by sealed envelopes. Randomisation was blinded through a third person, without involvement of the investigators. Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 14 int., 14 control Level of risk for CVD: Moderate (people with insulin treated diabetes and microalbuminurea)Male: 57% int., 50% controlMean age, sd: 47, 16 int., 41, 12 controlAge range: UnclearSmokers: 71% int., 57% controlHypertension: 0 int., 0 control Location: Denmark |
|
| Interventions | Type: supplement (capsule) Intervention: Pikasol fish oil capsules, 6×1 g/d (3.3g EPA + DHA) Control: Olive oil capsules, 6×1 g/d Compliance: capsule counts, overall daily consumption was >95% expected consumption Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: peripheral arterial compliance Dropouts: 0 int., 1 control Available outcomes: deaths, lipids, BP, side effects Response to contact: yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: Statistical office at the hospital performed block randomisation on a pre-constructed list Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 100 int., 100 control Level of risk for CVD: High (men with impending or recent myocardial infarction) Male: 100% Mean age, sd: 57.3 int., 57.4 control Age range: all <70 yrs Smokers: 77% int., 85% control Hypertension: 7% int., 10% control Location: Norway |
|
| Interventions | Type: supplement (oil) Intervention: linseed oil 10 ml/d initially, later raised to 20 or 30 ml/d (4.5g/d a-lin, later 9 or 13.5 g/d) Control: corn oil, 10 ml/d initially, later raised to 20 or 30 ml/d Compliance: bottle counts, no data presented Length of intervention: mean 10 (range 3-16) mo |
|
| Outcomes | Main study outcome: CV events Dropouts: unclear Available outcomes: total and cardiovascular deaths, MI, stroke, heart failure, combined CV events, lipids, adverse events Response to contact: Yes |
|
| Notes | Both groups were advised to cut out fried foods and other oils, and avoid margarine containing linolenic acids | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: Participants assigned a random number, each number randomised to seal oil, cod liver oil of control by blindly drawing a note indicating the group. The original groups were unbalanced in number of participants, so the last 2 participants added to the larger groups were re-allocated Allocation concealment: Unclear Participants masked: No Providers masked: yes Outcome assessors masked: yes Summary risk of bias: medium or high |
|
| Participants | N: 40 seal oil, 40 cod liver oil, 40 control Level of risk for CVD: Moderate (dyslipidaemia) Male: 53% seal oil, 50% cod liver oil, 48% control Mean age, sd: 53.2 seal oil, 55.0 cod liver oil, 55.8 control Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: Norway |
|
| Interventions | Type: supplement (oil) Intervention: seal oil - 15 ml/d (2.6g EPA + DHA) Cod liver oil - 15 ml/d (3.3g EPA + DHA) Control: nil, no supplement Compliance: serum omega-3 fatty acids, rose from around 1 mmol/L to 2.4 (seal oil), 2.1 (cod liver oil) and 1.2 mmol/L (control) Length of intervention: 14 mo |
|
| Outcomes | Main study outcome: serum lipids Dropouts: 8 seal oil, 2 cod liver oil, 1 control Available outcomes: total and cardiovascular deaths, MI, combined CV events, weight, lipids, adverse events Response to contact: yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: dietitian allocated by opening a sealed envelope containing a card which indicated a diet to be advised (following the taking of a diet history by that dietitian) Allocation concealment: Unclear Participants masked: No Providers masked: No Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 1015 int., 1018 Control Level of risk for CVD: High (post-MI) Male: 100% Mean age, sd: 56.7 int., 56.4 control Age range: Unclear Smokers: 61.7% int., 62.2% control Hypertension: 22.7% int., 24.6% control Location: UK |
|
| Interventions | Type: dietary advice (to eat more oily fish) Intervention: Advised to eat at least 2 weekly portions of 200-400g fatty fish (mackerel, herring, kipper, pilchard, sardine, salmon, trout). If this was not possible, given MaxEPA capsules, 3/d (0.5g EPA/d). 191 of 883 participants were taking MaxEPA at 2 years. Advice was reinforced 3-monthly. Control: No such dietary advice or capsules. Compliance: 7 day weighed food diary of a random sub-sample indicated intake of 2.5g/week EPA int., 0.8g/week EPA control. Length of intervention: 24 mo |
|
| Outcomes | Main study outcome: total mortality, reinfarction, CV death Dropouts: none for mortality Available outcomes: total and CV deaths, MI, combined CV events Response to contact: Yes |
|
| Notes | Some of each group were also advised on low fat and/or high fibre diets, all participants who smoked were advised to stop and all with a BMI >30 were given weight reduction advice, regardless of randomisation arm | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: dietitian allocated using prepared envelopes Allocation concealment: Unclear Participants masked: No Providers masked: No Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 1571 int., 1543 Control Level of risk for CVD: High (men being treated for angina) Male: 100% Mean age, sd: 61.1 int., 61.1 control Age range: Unclear Smokers: 25% int., 23% control Hypertension: 49% int., 47% control Location: UK |
|
| Interventions | Type: dietary advice (to eat more oily fish) Intervention: Most (1109) advised to eat at least 2 weekly portions of fatty fish OR take MaxEPA capsules, 3/d (0.5g EPA/d). But 462 participants were sub-randomised to receive only fish oil capsules, not dietary fish advice. Control: No such dietary advice or capsules. Compliance: Postal dietary questionnaire suggested dietary EPA intake increased by 2.4g /week int., 0.2g /week control Length of intervention: 36 to 108 mo |
|
| Outcomes | Main study outcome: total mortality Dropouts: none for mortality Available outcomes: total and CV deaths, sudden death Response to contact: Yes |
|
| Notes | Some of each group were also advised on high fruit, veg and oat diets, and those who received neither fish nor fruit advice received ‘non-specific’ dietary advice. All those whose BMI >30 in both groups received weight reduction advice | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: coin toss by statistician Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 8? int., 8? Control (16 total) Level of risk for CVD: Moderate (people with non-insulin dependant diabetes and hypertiglyceridaemia) Male: 81% overall Mean age, sd: overall: 58.7 Age range: 46-72 Smokers: Unclear Hypertension: Unclear Location: USA |
|
| Interventions | Type: supplement (oil) Intervention: Promega oil, 15g/d (6g/d EPA + DHA) Control: Olive oil, 15g/d Compliance: plasma EPA 5.5 (sd 1.2) % total fat after 6 mo int., 0.3 (sd 0.1) control Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: glycaemic control, lipids Dropouts: none? Available outcomes: deaths and CV events (none), weight, lipids Response to contact: yes |
|
| Notes | All participants on a 30% fat, 55% CHO, 15% protein, 300mg/d diet before and during supplementation | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: sequence of randomnumbers, odd = control, even = treatment, investigator opened sealed envelopes with numbers Allocation concealment: Not done (due to concern regarding haemorrhagic complications) Participants masked: No Providers masked: No Outcome assessors masked: No Summary risk of bias: medium or high |
|
| Participants | N: 46 int., 44 control Level of risk for CVD: High (men undergoing coronary angioplasty) Male: 100% Mean age, sd: 56, 9.6 int., 56, 8.9 control Age range: Unclear Smokers: 56% int., 56% control Hypertension: Unclear Location: USA |
|
| Interventions | Type: supplement (capsule) Intervention: MaxEPA capsules, 18/d (5.4g EPA + DHA daily) Control: nil Compliance: capsule count (results not reported), serum EPA + DHA rose in the intervention group (0. 6 to 6.6% total fatty acids at 3 mo), no data on the control group Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: restenosis Dropouts: 3 int., 5 control Available outcomes: deaths, MI, recurrent angina, stroke, heart failure, CABG, combined CV events, lipids, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | No | C - Inadequate |
| Methods | Randomisation: randomised in blocks of 4 Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 6 int., 6 control Level of risk for CVD: Low (people with asthma) Male: Unclear Mean age, sd: Unclear Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: France |
|
| Interventions | Type: supplement (capsule?) Intervention: Liparmonyl (1g/d EPA + DHA) Control: ‘placebo’, no further details Compliance: capsule count (results not reported) Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: pulmonary function Dropouts: none Available outcomes: deaths Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: random numbers in consecutively numbered sealed envelopes generated at the Life Insurance Statistical Inst at Ulleval Hospital Allocation concealment: Done Participants masked: No Providers masked: No Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 317 int., 293 control Level of risk for CVD: High (people admitted for coronary bypass grafting) Male: 86% int., 88 % control Mean age, sd: 59.9, 8.7 int., 59.4, 8.8 control Age range: Unclear Smokers: 19% int., 20% control Hypertension: 20% int., 25% control Location: Norway |
|
| Interventions | Type: supplement (capsule) Intervention: Omacor capsules, 4/d (3.3g EPA + DHA daily) Control: nil Compliance: capsule count, 88% taken, serum EPA + DHA rose in the intervention group (176 to 257 mg/L at 9 mo) and fell in the control group (170 to 169 mg/L at 9 mo) Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: CABG graft patency Dropouts: 15 int., 14 control Available outcomes: deaths, MI, stroke, repeat CABG, combined CV events, lipids, side effects Response to contact: Yes |
|
| Notes | Dietary assessment suggested total diet plus supplement intakes as follows: 2.7 g/d EPA + DHA at baseline, 5.5 g/d at 9 mo int., 2.5g/d at baseline, 2.2g/d at 9 mo control group | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: by computer generated list Allocation concealment: Unclear Participants masked: Yes Providers masked: Unclear Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 15 int., 15 control Level of risk for CVD: High (people with angiographically determined CHD) Male: unclear Mean age, sd: 52, 9 int., 54, 7 control Age range: Unclear Smokers: unclear Hypertension: unclear Location: Germany |
|
| Interventions | Type: supplement (capsule) Intervention: fish oil capsules, 9g/d (1.8g EPA + 1.4g DHA daily) Control: Olive oil capsules Compliance: capsule count, serum EPA higher in the intervention group (4.4 (2.4) mg/dl in control, 17. 0 (6.2) mg/dl in intervention) at 1 year Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: serum lipids Dropouts: 0 int., 0 control Available outcomes: deaths, lipids, side effects Response to contact: Yes |
|
| Notes | No dietary advice provided | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: ‘randomly assigned’ Allocation concealment: Unclear Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 30 low dose, 30 high dose, 30 control Level of risk for CVD: Low (people with active rheumatoid arthritis on NSAIDs or DMARDs) Male: 23.8% low dose, 21.0% high dose, 20.0% control Mean age, sd: 57, 9.2 low dose, 59, 8.7 high dose, 56, 8.9 control Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: Belgium |
|
| Interventions | Type: supplement (capsule) Intervention: fish oil capsules, 3/d plus 3 Olive oil capsules (1.3g EPA + DHA daily) low dose, fish oil capsules, 6/d (2.6g EPA + DHA daily) high dose Control: Olive oil capsules, 6/d Compliance: capsule count (results not reported) Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: arthritic symptoms Dropouts: 9 low dose, 11 high dose, 10 control Available outcomes: deaths, side effects Response to contact: No |
|
| Notes | All 3 groups had a stable diet with 30% fat and fish eaten once a week prescribed | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: over the telephone by computer network, stratified by hospital, assigned from program based on biased coin algorithm, by investigators or pharmacists Allocation concealment: Done Participants masked: No Providers masked: Unclear Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 5665 int., 5658 control Level of risk for CVD: High (people with recent myocardial infarction) Male: 85.7% int., 84.9 % control Mean age, sd: 59.3 int., 59.5 control Age range: <50 to >80 Smokers: 42.6% int., 42.3% control Hypertension: 36.2% int., 34.9% control Location: Italy |
|
| Interventions | Type: supplement (capsule) Intervention: Omacor gelatine capsules, 1/d (0.9g/d EPA + DHA daily) Control: nil Compliance: capsule counts, 11.6% had stopped taking Omacor by 12 mo, 28.5% by the end of the study Length of intervention: median follow up 40 mo |
|
| Outcomes | Main study outcome: death, stroke, MI Dropouts: Unclear Available outcomes: total , sudden and CV deaths, MI, stroke, angioplasty or CABG, combined CV events, lipids, side effects Response to contact: No |
|
| Notes | Half of both groups were on vitamin E supplements (300 mg/d synthetic a-tocopherol) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: envelopes drawn at random with the help of a clinical nurse Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 16 int., 8 control Level of risk for CVD: Low (people with stable ulcerative colitis) Male: 75.0% int., 62.5 % control Mean age, sd: 57.3, 4.4 int., 53.0. 6.8 control Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: UK |
|
| Interventions | Type: supplement (capsule) Intervention: MaxEPA capsules, 12/d for first month, then 6/d (3.7g/d initially, then 1.9g EPA + DHA daily), all with peppermint oil to disguise taste Control: Olive oil capsules, 12/d for first month, then 6/d. Looked like MaxEPA and had added peppermint oil. Compliance: red cell membrane EPA + DHA rose in the intervention group (2.3 to 5.1% fatty acids at 6 mo) and fell in the control group (2.4 to 1.0% fatty acids at 6 mo) Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: symptoms of ulcerative colitis Dropouts: 3 int., 1 control Available outcomes: deaths, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: by hospital pharmacy in blocks of 4, code held by pharmacy Allocation concealment: Done Participants masked: Unclear Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 49 int., 47 control Level of risk for CVD: Low (people with ulcerative colitis) Male: 68.9% int., 40.5 % control Mean age, sd: 44 int., 49 control Age range: 17-73 int., 20-77 control Smokers: 2% int, 2% control Hypertension: Unclear Location: UK |
|
| Interventions | Type: supplement (oil) Intervention: HiEPA oil, 10 ml × 2/d (5.6g/d EPA + DHA) Control: Olive oil, 10 ml × 2/d (0g/d EPA + DHA) Compliance: bottle counts, median 650 ml/month int., 635 ml/mo control, daily record suggested a median of 20ml/d in both groups, red cell membrane EPA rose from 0.9% to 5.5% fats int., ‘no significant difference’ in control. Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: ulcerative colitis activity and relapse rate Dropouts: 5 int., 6 control Available outcomes: deaths, MI, CV events, side effects Response to contact: Yes |
|
| Notes | 76 Nottingham participants were asked to complete 7 day weighed food diaries twice, found intake of 6. 0g/d EPA + DHA int., 0.5g/d control including foods and supplements | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: consecutively numbered sealed envelopes Allocation concealment: Unclear Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 250 int., 250 control Level of risk for CVD: High (people about to undergo elective coronary angioplasty) Male: 74.5% int., 80.7 % control Mean age, sd: 60.3, 9.3 int., 59.1, 9.3 control Age range: Unclear Smokers: 16.3% int., 22.4% control Hypertension: 34.2% int., 33.9% control Location: Norway |
|
| Interventions | Type: supplement (capsule) Intervention: Omacor capsules, 6/d (5g EPA + DHA daily) Control: corn oil capsules, 6/d Compliance: capsule count (results not reported), serum EPA + DHA rose in the intervention group (185 to 267 mg/L at 6 mo) and fell in the control group (172 to 155 mg/L at 6 mo) Length of intervention: 6.5 mo |
|
| Outcomes | Main study outcome: restenosis Dropouts: 54 int., 58 control Available outcomes: total and CV deaths, lipids, side effects Response to contact: No |
|
| Notes | Those using fish oil capsules at baseline were asked to stop | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: manufacturer provided envelopes containing numbers corresponding to boxes of capsules, for each enrolled participant a random envelope was opened Allocation concealment: Done Participants masked: No (‘you can taste the fish oil’) Providers masked: Unclear Outcome assessors masked: yes Summary risk of bias: medium or high |
|
| Participants | N: 15 low dose, 15 medium dose, 14 high dose, 14 control Level of risk for CVD: Low (healthy monks) Male: 100% Mean age, sd: overall: 56.2, 16.5 Age range: Unclear Smokers: Unclear Hypertension: None Location: The Netherlands |
|
| Interventions | Type: supplement (capsule) Intervention: Fish oil capsules, all took 9 per day, double dummy, some with Olive and palm oil capsules (1.1g omega-3 fats low dose, 2.2g medium dose, 3.3g high dose per day) Control: 9 Olive and palm oil capsules (0g omega-3 fats per day) Compliance: ‘excellent’ in all groups according to capsule count, plasma cholesteryl esters rose from 0.8 to 3.8 low intake, 0.9 to 6.6 medium intake, 0.7 to 10.4 high intake and 0.9 to 0.9 control group from 0 to 12 mo. Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: tissue incorporation Dropouts: none? Available outcomes: deaths, lipids, blood pressure Response to contact: yes |
|
| Notes | Dietary assessment suggested that total diet plus supplement intakes were as follows 1.5g/d EPA + DHA + DPA low intake, 2.5g/d medium intake, 3.6g/d high intake, 0.4g/d control group | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: computer generated list of random numbers, nurse Allocation concealment: Unclear Participants masked: No Providers masked: No Outcome assessors masked: yes Summary risk of bias: medium or high |
|
| Participants | N: 58 int., 49 control Level of risk for CVD: high (people undergoing angioplasty) Male: 86% int., 84% control Mean age, sd: 56, 11 int, 59, 9 control Age range: Unclear Smokers: 31% int., 35% control Hypertension: Unclear Location: India |
|
| Interventions | Type: supplement (capsule) Intervention: MaxEPA capsules, 10/d (3g/d EPA + DHA) Control: nil Compliance: bottle counts, no data provided Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: restenosis Dropouts: Unclear Available outcomes: deaths, MI, cardiovascular events, lipids, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: computer software system, blocks of 10 Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 32 int., 32 control Level of risk for CVD: Low (people with rheumatoid arthritis) Male: 28% int., 31% control Mean age, sd: 49.3 int, median 53.4 control Age range: 26-73 int., 27-70 control Smokers: Unclear Hypertension: Unclear Location: UK |
|
| Interventions | Type: supplement (capsule) Intervention: MaxEPA 10x 1g capsules daily (2.8g/d EPA + DHA) Control: air-filled capsules, 10/d Compliance: capsule counts, no data provided; red cell membrane phospholipids show rise of EPA + DHA in int. from 2.5% to 6.8% fatty acids and in the control from 3.0 to 3.2% fatty acids Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: level of NSAID use Dropouts: 9 int., 16 control Available outcomes: deaths, MI, cardiovascular events, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: computer software system, blocks of 10 Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 25 int., 20 control Level of risk for CVD: Low (people with rheumatoid arthritis) Male: 28% int., 30% control Mean age, sd: median 50 int, median 52 control Age range: 27-69 int., 28-69 control Smokers: Unclear Hypertension: Unclear Location: Hong Kong, China |
|
| Interventions | Type: supplement (capsule) Intervention: MaxEPA 10x 1g capsules daily (2.8g/d EPA + DHA) Control: air-filled capsules, 10/d Compliance: capsule counts, no data provided; red cell membrane phospholipids show rise in int. from 2.4% to 5.4% fatty acids and a fall in the control from 2.9 to 2.5% fatty acids Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: fibrinolytic parameters Dropouts: None Available outcomes: deaths, MI, cardiovascular events Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: randomised by study statistician, distribution by pharmacist at each centre, only the research pharmacist was unblinded Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 275 int., 276 control Level of risk for CVD: High (people undergoing angioplasty) Male: 77% int., 81% control Mean age, sd: Unclear Age range: 30->70 int., 30->70 control Smokers: 14% int., 19% control Hypertension: 47% int., 37% control Location: USA |
|
| Interventions | Type: supplement (capsule) Intervention: fish oil concentrate capsules 10×1 g/d (6.9g/d EPA + DHA) Control: corn oil capsules 10×1 g/d with 0.4% fish oil to maintain blinding (0.003g/d EPA + DHA) Compliance: plasma EPA + DHA rose by 8.5% total fatty acids to 6 mo in int., by 0.6% in controls Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: restenosis Dropouts: 69 int., 69 control Available outcomes: deaths, combined cardiovascular events, weight, lipids, BP, side effects Response to contact: Yes |
|
| Notes | All on step 1 AHA diet. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: after entry participants were allocated the next free study number in their strata, by their physician, medication was pre-packed in blocks of 2 according to a random number list and coded with consecutive numbers by pharmacy Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 31 int., 33 control Level of risk for CVD: Low (people with ulcerative colitis, in remission) Male: 48% int., 55% controlMean age, sd: 40, 13 int, 39, 11 control Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: Germany |
|
| Interventions | Type: supplement (capsule) Intervention: fish oil capsules 6×1 g/d (5.1g/d omega-3 fats), with orange flavour to disguise Control: maize oil capsules 6×1 g/d with orange flavour Compliance: capsule counts, 74% int. taking 95% of medication, 26% taking none, 73% control taking 95% medication, 27% taking none; plasma phospholipids show rise in int. from 6% to 18% fatty acids at 24 mo and in the control from 6 to 7% fatty acids Length of intervention: 24 mo |
|
| Outcomes | Main study outcome: relapse-free time Dropouts: None (all followed re events) Available outcomes: deaths, MI, cardiovascular events, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: randomisation in centres in blocks of 6 (centres blinded to block size) Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 70 int., 65 control Level of risk for CVD: Low (people with Crohn’s disease in remission) Male: 36% int., 27% control Mean age, sd: 29.5 int, 31.8 control Age range: 17-62 int., 17-65 control Smokers: Unclear Hypertension: Unclear Location: Germany |
|
| Interventions | Type: supplement (capsule) Intervention: ethyl ester fish oil concentrate capsules 6×1 g daily (5.1g/d EPA + DHA) Control: corn oil capsules 6×1 g daily Compliance: capsule count attempted, but did not produce sufficiently complete or reliable results Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: relapse of Crohn’s disease Dropouts: Unclear Available outcomes: deaths, TGs, side effects Response to contact: Yes |
|
| Notes | All participants advised to eat a fibre-rich, low arachidonic diet | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: ‘randomly assigned’ Allocation concealment: Unclear Participants masked: No Providers masked: Unclear Outcome assessors masked: Unclear Summary risk of bias: medium or high |
|
| Participants | N: 26 int., 26 control Level of risk for CVD: moderate (ALT =2x normal limit for =12 mo, had chronic hepatitis) Male: 46% int., 42% control Mean age, sd: 48.7, 6.5 int, 56.9, 7.2 control Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: Italy |
|
| Interventions | Type: supplement (capsule) Intervention: EPA + DHA daily (3g/d EPA + DHA) plus IFNa subcutaneously Control: nil, only IFNa subcutaneously Compliance: unused capsules returned after 2 mo, no-one returned >3 capsules Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: liver enzymes Dropouts: Unclear Available outcomes: combined cardiovascular events, psychiatric disorders, lipids, ALT, side effects Response to contact: No |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: central randomisation (by Farmitalia Carlo Erba) Allocation concealment: Done Participants masked: yes Providers masked: yes Outcome assessors masked: yes Summary risk of bias: low |
|
| Participants | N:169 int., 170 control Level of risk for CVD: high (undergoing planned PTCA) Male: 86% int., 83% control Mean age, sd: 58.9, 9.5 int, 58.6, 8.7 control Age range: Unclear Smokers: 23% int., 21% control Hypertension: 47% int., 34% control Location: Italy |
|
| Interventions | Type: supplement (capsule) Intervention: Esapent capsules, 6/d for 2 mo, then 3/d (5.1g/d EPA + DHA initially, later 2.6g/d) Control: identical Olive oil capsules, 6/d for 2 mo, then 3/d Compliance: plasma fatty acids used to assess, 13.7% of int. group did not adhere strictly to Esapent (no info on controls) Length of intervention: 7 mo |
|
| Outcomes | Main study outcome: restenosis Dropouts: 44 int, 38 control Available outcomes: total MI, significant angina, combined CV events, thrombo-embolism, TGs, side effects Response to contact: yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: treatments were allocated a number by computer on a random basis Allocation concealment: Done Participants masked: No Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N:19 int., 19 control Level of risk for CVD: Low (people with inactive Crohn’s disease) Male: 42% int., 58% control Mean age, sd: 35 int., 34 control Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: Spain |
|
| Interventions | Type: diet advice Intervention: advised to eat either 100-200g cold water fish per week, 100g fish pate per week or 250g fish oil supplements per week Control: no advice, usual diet Compliance: dietary measures of compliance used (no further information provided) Length of intervention: 24 mo |
|
| Outcomes | Main study outcome: maintenance of Crohn’s remission Dropouts: 4 int, 6 control Available outcomes: deaths, MI, CV events Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: computer-generated list of random numbers Allocation concealment: Done Participants masked: No Providers masked: Unclear Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 100 int., 100 control Level of risk for CVD: High (people about to undergo angioplasty) Male: 74% int., 71% control Mean age, sd: 59 int., 59 control Age range: Unclear Smokers: 23% int., 28% control Hypertension: 43% int., 47% control Location: USA |
|
| Interventions | Type: supplement (capsules) Intervention: Promega 9 capsules/d (4.5g EPA + DHA) Control: nil Compliance: 77% took 5-9 capsules/d, 11% took none (int. group) Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: restenosis Dropouts: all followed for outcomes Available outcomes: deaths, angina, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: ‘simple randomisation’ Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 6716 int., 6690 control Level of risk for CVD: Low (working men, though a few had had a previous MI or angina) Male: 100% Mean age, sd: Unclear Age range: 50-59 Smokers: Unclear Hypertension: Unclear Location: Norway |
|
| Interventions | Type: supplement (oil) Intervention: linseed oil, 10 ml /d (55% a-lin) Control: sunflower oil, 10 ml/d (1.4% a-linolenic acid) Compliance: 73% were still taking the linseed oil at 1 yr, 72% were still taking their sunflower oil at 1 yr (unclear how this was ascertained). Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: morbidity and mortality Dropouts: survival status was traced for all but 4 included men, health status was missing for about 80 men in total or 0.6%. Available outcomes: total and CV deaths, MI, angina, stroke, peripheral vascular disease, combined CV events, total cholesterol (subgroup) Response to contact: Not attempted as study published 35 years ago |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: Pharmacia randomised the patients and provided identical capsules containing Omacor or corn oil Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 150 int., 150 control Level of risk for CVD: High (people with acute myocardial infarction 4-8 days ago) Male: 77% int., 82% control Mean age, sd: 64.4 int., 63.6 control Age range: 28-86 int., 29-87 control Smokers: 39% int., 38% control Hypertension: 29% int., 23% control Location: Norway |
|
| Interventions | Type: supplement (capsules) Intervention: Omacor capsules 4/d (3.5g EPA + DHA) Control: corn oil capsules, 4/d Compliance: assessed by questionnaire and capsule count, 82% int group had complete compliance after 6 weeks, 86% of controls Length of intervention: 24 mo |
|
| Outcomes | Main study outcome: CV events Dropouts: unclear Available outcomes: total and CV deaths, MI, angina, interventions, combined CV events, BMI, lipids, BP Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: randomly allocated without exclusions into 3 groups Allocation concealment: Unclear Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 36 int., 37 control Level of risk for CVD: High (people undergoing angioplasty) Male: 78% int., 76% control Mean age, sd: 54, 8 int., 55, 8 control Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: New Zealand |
|
| Interventions | Type: supplement (capsules) Intervention: MaxEPA capsules 12/d (2.2g EPA) Control: Olive oil capsules, 12/d, identical to MaxEPA Compliance: no data Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: angina, restenosis Dropouts: None Available outcomes: deaths, angina, interventions, lipids Response to contact: No |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: Random numbers produced by an independent statistician at own institution (co-ordinator enrolled patient then called lab for allocation) Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 146 int., 72 control Level of risk for CVD: High (people undergoing angioplasty) Male: 73% int., 76% control Mean age, sd: 60 int., 57 control Age range: Unclear Smokers: 31% int., 27% control Hypertension: Unclear Location: USA |
|
| Interventions | Type: supplement (capsules) Intervention: Super EPA capsules 12×1 g/d (7.0g EPA + DHA + a-lin) OR Promega capsules 12×1 g/d (6.0g EPA + DHA + a-lin) Control: Olive oil capsules, 12×1 g/d, appearance identical to fish oil capsules Compliance: capsule counts, >75% of capsules taken by 66% int., 65% controls, plasma EPA rose from 0.7% total fatty acids to 4.5% at 6 mo in the int. group, 0.7% in controls Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: restenosis, angina Dropouts: 22 int, 10 control Available outcomes: deaths, MI, CV events, weight, lipids, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: Computer generated randomisation list produced by fish oil manufacturers (blocks of 4), participants were ranked according to glomerular filtration rate and then given a number according to rank Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 18 int., 18 control Level of risk for CVD: Moderate (people with insulin dependant diabetes, diabetic nephropathy and normal BP) Male: 64% int., 67% control Mean age, sd: 32, 7 int., 34, 10 control Age range: Unclear Smokers: 50% int., 47% control Hypertension: None Location: Denmark |
|
| Interventions | Type: supplement (liquid emulsion) Intervention: Eskisol fish oil emulsion 21 ml/d (4.6g EPA + DHA) 35% cod liver oil and water emulsion with orange flavouring Control: Olive oil emulsion 21 ml/d, 35% oil and water emulsion with orange flavouring Compliance: platelet fatty acid EPA + DHA rose from 2.2% fatty acids to 6.3% at 12 mo int., from 2. 3% to 2.6% in controls Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: diabetic nephropathy Dropouts: 4 int, 3 control (but all monitored for outcomes) Available outcomes: deaths, MI, CV events, lipids, side effects Response to contact: Yes |
|
| Notes | Asked participants to guess their treatment allocation at the end, approx. 50% were correct. Both groups were asked to reduce energy intake by number of calories present in emulsion | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: randomisation stratified by medical or surgical management and lipid ratio Allocation concealment: Unclear Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 41 int., 39 control Level of risk for CVD: High (people with angiographically documented CHD) Male: 94% int., 93% control Mean age, sd: 62, 7 int., 62, 7 control Age range: 30-75 int., 30-75 control Smokers: Unclear Hypertension: 48% int., 36% control Location: USA |
|
| Interventions | Type: supplement (capsules) Intervention: Promega capsules 12×1 g/d (6.0g EPA +DHA +DPA) Control: Olive oil capsules, 12×1 g/d, appearance identical to Promega capsules Compliance: capsule counts, 80% of capsules taken in int., 90% taken by controls, adipose tissue EPA + DHA + DPA rose to 2.4% total fats at 29 mo int. group, 0.8% in controls Length of intervention: 29 mo |
|
| Outcomes | Main study outcome: size of coronary lesions Dropouts: 10 int, 11 control (but all monitored for outcomes) Available outcomes: deaths, MI, CV events, weight, lipids, BP, side effects Response to contact: No |
|
| Notes | All participants had NCEP step 1 dietary advice | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: treatment assignments obtained by phone from coordinating centre or (when phone contact not possible) from written instructions contained in sealed opaque envelopes Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 175 int., 175 control Level of risk for CVD: Low (people with high normal BPs) Male: 70.9% int., 69.7% control Mean age, sd: 42.6, 6.3 int., 43.1, 6.6 control Age range: Unclear Smokers: Unclear Hypertension: None Location: USA |
|
| Interventions | Type: supplement (capsules) Intervention: Promega, purified sardine oil, capsules 6×1 g/d (3.0g EPA + DHA + DPA) Control: Olive oil capsules, 6×1 g/d, appearance identical to Promega capsules OR cellulose tablets, 3/d (identical to potassium supplements used in another arm of the trial) Compliance: capsule counts, 72% took at least 95% capsules at 6 mo in int., 80% in control Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: blood pressure Dropouts: 1 int, 1 control Available outcomes: deaths, weight, lipids, BP, side effects Response to contact: No Note: No dietary, weight or smoking advice was provided to any group |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: stratified in blocks of 4, the order in blocks was from random number tables Allocation concealment: Unclear Participants masked: No Providers masked: No Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 41 int., 37 control Level of risk for CVD: Moderate (people with moderate hypercholesterolaemia) Male: 46% int., 46% control Mean age, sd: 46.4, 7.4 int., 43.2, 8.2 control Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: Finland |
|
| Interventions | Type: dietary advice and supplement (foods) Intervention: Advised on diet providing 38% of energy as fat, 18% as MUFA, with rapeseed oil, rapeseed margarine and skimmed milk provided (achieved 42% E from fat, 12% from MUFA) Control: Advised on diet providing 38% of energy as fat, 15% E from MUFA, with rapeseed oil, butter and semi-skimmed milk provided (achieved 36% E from fat, 10% from MUFA) Compliance: weighed dietary intakes, omega-3 fats in plasma fatty acids rose from 3.5 to 3.8% at 6 mo int., from 3.3 to 3.6% control. Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: lipids, diet, BP Dropouts: None Available outcomes: deaths, BMI, lipids Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Randomisation: ‘toss’ by Sanofi, Belgium Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 12 int., 12 control Level of risk for CVD: Moderate (people with insulin dependant diabetes and micro-albuminurea) Male: ‘comparable’ between 2 groups Mean age, sd: ‘comparable’ Age range: Unclear Smokers: Unclear Hypertension: Unclear Location: Belgium |
|
| Interventions | Type: supplement (capsules) Intervention: omega-3 fatty acids (2.4g/d EPA + DHA) Control: ‘inert placebo’ Compliance: diet history, capsule count and fatty acid data (none provided) Length of intervention: 9 mo |
|
| Outcomes | Main study outcome: immunoreactivity Dropouts: 4 int., 2 control Available outcomes: deaths, MI, CV events Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: each doctor picked up an envelope which contained a treatment group allocation Allocation concealment: Not done Participants masked: No Providers masked: No Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 29 int., 16 control Level of risk for CVD: Moderate (people with non-insulin dependant diabetes) Male: 34% int., 75% control Mean age, sd: 66.3, 13.5 int., 58.6, 7.2 control Age range: Unclear Smokers: Unclear Hypertension: 38% int., 44% control Location: Japan |
|
| Interventions | Type: supplement (capsules) Intervention: EPA-ethyl capsules 3/d (0.9g/d EPA) Control: nil Compliance: capsule count (no data provided) Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: albuminurea Dropouts: Unclear Available outcomes: deaths, MI, CV events, lipids, BP Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | No | C - Inadequate |
| Methods | Randomisation: selection of cards by the patient’s relatives, done by pharmacist, stratified by type of MI Allocation concealment: Done Participants masked: No Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 122 fish oil, 120 mustard oil, 118 control Level of risk for CVD: High (people with suspected acute MI) Male: 92% fish oil, 95% mustard oil, 95% control Mean age, sd: 48.5, 6.5 fish oil, 48.0, 5.5 mustard oil, 49.2, 7.2 control Age range: Unclear Smokers: 26% fish oil, 25% mustard oil, 15% control Hypertension: Unclear Location: India |
|
| Interventions | Type: supplement (capsules) Intervention: MaxEPA fish oil capsules 6/d (1.8g EPA + DHA), mustard oil 20g/d plus placebo capsules (2.9g/d a-lin) Control: aluminium hydroxide 100 mg/d Compliance: Unclear Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: CV events Dropouts: 4 fish oil, 8 mustard oil, 6 placebo Available outcomes: deaths, MI, CV events, lipids, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: Biometric service at Pharmacia produced a set of random numbers (on SAS) for each of the 63 centres Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 470 int., 465 control Level of risk for CVD: Moderate (people with raised triglycerides plus glucose intolerance, non-insulin dependant diabetes or hypertension) Male: 63% int., 62% control Mean age, sd: 58.2, 9.1 int., 58.8, 9.0 control Age range: 45-80 int., 45-80 control Smokers: Unclear Hypertension: 68% int., 68% control Location: Italy |
|
| Interventions | Type: supplement (capsules) Intervention: Esapent fish oil capsules 3/d for first 2 mo, 2/d after that (2. 6g/d EPA + DHA initially, then 1.8g/d) Control: Olive oil capsules 3/d for first 2 mo, 2/d after that Compliance: capsule count, >90% compliance overall, erythrocyte phospholipids rose from 7.7% total fats to 11.5% total fats at 6 mo int., and fell from 7.6% to 7.0% total fats in control Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: plasma lipoproteins, glycaemic parameters Dropouts: 28 int., 39 control Available outcomes: deaths, MI, CV events, weight, lipids, BP, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: randomisation and labeling of capsules happened at one centre with no contact with the patients (by B Seiving, Lund Hospital Pharmacy) Allocation concealment: Done Participants masked: No Providers masked: No Outcome assessors masked: No Summary risk of bias: medium or high |
|
| Participants | N: 23 int., 23 control Level of risk for CVD: Low (people with rheumatoid arthritis) Male: 18% int., 33% control Mean age, sd: 58 int., 55 control Age range: 40-73 int., 28-70 control Smokers: Unclear Hypertension: Unclear Location: Sweden |
|
| Interventions | Type: supplement (capsules) Intervention: MaxEPA fish oil capsules 10/d (3.0g/d EPA + DHA) Control: vegetable oil capsules 10/d Compliance: 4 day diet records, plasma phospholipids (EPA = DHA + DPA) rose from 7.4 to 15.4% fatty acids at 6 mo int., and from 6.9 to 7.1% control Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: arthritis severity and use of NSAIDs Dropouts: 1 int., 2 control Available outcomes: deaths, weight, lipids, side effects Response to contact: Yes |
|
| Notes | All asked to maintain their usual diet | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: ‘randomly divided’ stratified by age, baseline MMSE scores and serum fatty acid composition Allocation concealment: Done Participants masked: No Providers masked: No Outcome assessors masked: Yes Summary risk of bias: medium or high |
|
| Participants | N: 10 int., 10 control Level of risk for CVD: High (had dementia of CVD) Male: 10% int., 10% control Mean age, sd: 83.3 (5.3) int, 82.7 (6.4) control Age range: Unclear Smokers: None Hypertension: Unclear Location: Japan |
|
| Interventions | Type: supplement (capsule) Intervention: DHA capsules, 6/d (4.3g/d DHA) Control: no capsule Compliance: plasma fatty acids used to assess, levels of plasma DHA rose from 5.3 mol% at baseline to 9.5 mol% at 12 mo in int group, ‘no change’ in controls (capsules given 3x daily by care home staff ) Length of intervention: 12 mo |
|
| Outcomes | Main study outcome: neurological tests Dropouts: 0 int, 0 control Available outcomes: deaths, MI, stroke, dementia rating, Mini-mental State Examination Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: After screening a list was given to a non-clinical investigator who randomly allocated subjects, treatment was blinded to clinical investigators Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 21 int., 16 control Level of risk for CVD: Low (had hayfever and asthma) Male: 60% int., 40% control Mean age, sd: Unclear Age range: 22-42 int., 19-39 control Smokers: None Hypertension: Unclear Location: Australia |
|
| Interventions | Type: supplement (capsule) Intervention: MaxEPA capsules, 18/d (5.4g/d EPA + DHA) Control: Olive oil capsules 18/d, appeared identical to MaxEPA Compliance: plasma fatty acids, EPA rose from 1.4 to 5.4% fatty acids at 6 mo in int group, and fell from 1.1 to 0.8% in control group Length of intervention: 6 mo |
|
| Outcomes | Main study outcome: hayfever and asthma symptoms Dropouts: 6 int, 6 control Available outcomes: deaths, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: Pharmaceutical company randomised in groups of 4 using random numbers Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 19 int., 19 control Level of risk for CVD: Low (people with chronic stable plaque psoriasis and inflammatory arthritis) Male: 37% int., 37% control Mean age, sd: median 40 in both groups Age range: 18-76 int., 25-58 control Smokers: Unclear Hypertension: Unclear Location: UK |
|
| Interventions | Type: supplement (capsule) Intervention: Efamol marine capsules, 12/d (0.4g/d EPA + DHA plus 0.5g/d gamma-linoleic acid (not omega-3)) Control: capsules containing liquid paraffin and vitamin E, 12/d, appeared identical Compliance: no data Length of intervention: 9 mo |
|
| Outcomes | Main study outcome: skin and joint symptoms, use of NSAIDs Dropouts: 4 int, 0 control Available outcomes: deaths, MI, stroke, side effects Response to contact: Yes |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Randomisation: Performed by trial monitor in Norway using computer generated random sequence with 9 strata, balanced every 4 patients Allocation concealment: Done Participants masked: Yes Providers masked: Yes Outcome assessors masked: Yes Summary risk of bias: low |
|
| Participants | N: 112 int., 111 control Level of risk for CVD: High (people with angiographically proven coronary artery disease) Male: 82% int., 79% control Mean age, sd: 57.8, 9.7 int., 58.9, 8.1 control Age range: Unclear Smokers: 16% int., 22% control Hypertension: Unclear Location: Germany |
|
| Interventions | Type: supplement (capsule) Intervention: concentrated fish oil capsules, 6/d for first 3 mo, 3/d for rest of study (4g/d EPA + DHA + DPA + a-lin for first 3 mo, then 2g/d) Control: capsules containing fat which replicated the fat composition of the average European diet, 6/d for first 3 mo, 3/d for rest of study, opaque soft gelatine capsules identical to fish capsules in identical screw-top containers Compliance: capsule count, overall 2284 (sd 313) capsules taken of 2460 prescribed for each person, erythrocyte phospholipids rose from 4.6 to 11.8% at 24 mo in int., and didn’t alter from baseline in controls Length of intervention: 24 mo |
|
| Outcomes | Main study outcome: changes in stenosis on angiography Dropouts: Unclear Available outcomes: deaths, MI, CV events, weight, lipids, BP, side effects Response to contact: Yes |
|
| Notes | Asked participants to guess treatment allocation, of those in int. 63/90 were unsure, 5/90 guessed placebo and 22/90 guessed fish oil; of those in control 66/85 were unsure, 9/85 guessed placebo and 10/85 guessed fish oil | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Exposures assessed overall: total fish, fatty fish, lean fish Outcomes assessed: CHD mortality Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear, only total fish assessed (those eating more fish were more likely to smoke, have raised SBP and serum chol) Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: most exposed 57.7, 5.4, least exposed 58.2, 5.7 Smokers: most exposed 56%, least exposed 44% No. included in cohort: 1088 No. developing outcome: 242 Enrollment: 1959 Exposure assessed: 1969 Outcome assessed: following 20 years Mean follow up: 20 yrs Source: cohorts for Seven countries study Inclusion criteria: men aged 40-59 Exclusion criteria: unclear Location: Finland |
|
| Interventions | Exposure Measured: fatty fish Method: dietary, cross-checked dietetic interview (habitual food consumption) and food frequency checklist No. of groups: 2 Most exposed: >0g/d fatty fish intake Least exposed: 0g/d fatty fish intake Endpoint Outcome: CHD mortality Criteria: Primary or secondary cause of death was listed as ICD 410-414 or 795 Method: medical records, including death certificates, hospital records, GP, family members reports, witness of death |
|
| Outcomes | Analysis: Cox Proportional Hazards Adjustment for: Age, BMI, cigarette smoking, Energy intake, fruit, veg, alcohol, meat, butter and margarine intakes. Results:Significant protective effect of fatty fish seen in Italy, but not in Finland or the Netherlands |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: total fish, fatty fish, lean fish Outcomes assessed: CHD mortality Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear, only total fish assessed. Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: most exposed 57.7, 5.2, least exposed 9.1, 5.1 Smokers: most exposed 51%, least exposed 52% No. included in cohort: 1097 No. developing outcome: 116 Enrollment: 1960 Exposure assessed: 1965 & 1970 Outcome assessed: following 20 years Mean follow up: 20 yrs Source: cohorts for Seven countries study Inclusion criteria: men aged 40-59 Exclusion criteria: unclear Location: Italy |
|
| Interventions | Exposure Measured: fatty fish Method: dietary, cross-checked dietetic interview (habitual food consumption) and food frequency checklist No. of groups: 2 Most exposed: >0g/d fatty fish intake Least exposed: 0g/d fatty fish intake Endpoint Outcome: CHD mortality Criteria: Primary or secondary cause of death was listed as ICD 410-414 or 795 Method: medical records, including death certificates, hospital records, GP, family members reports, witness of death |
|
| Outcomes | Analysis: Cox Proportional Hazards Adjustment for: Age, BMI, cigarette smoking, Energy intake, fruit, veg, alcohol, meat, butter and margarine intakes. Results:Significant protective effect of fatty fish seen in Italy, but not in Finland or the Netherlands |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: total fish, fatty fish, lean fish Outcomes assessed: CHD mortality Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear, only total fish assessed (those with higher fish intake were less likely to smoke and had higher SBP) Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: most exposed 58.2, 5.3, least exposed 58.3, 5.4 Smokers: most exposed 50%, least exposed 56% No. included in cohort: 553 No. developing outcome: 105 Enrollment: 1960 Exposure assessed: 1970 Outcome assessed: following 20 years Mean follow up: 20 yrs Source: cohorts for Seven countries study Inclusion criteria: men aged 40-59 Exclusion criteria: unclear Location: the Netherlands |
|
| Interventions | Exposure Measured: fatty fish Method: dietary, cross-checked dietetic interview (habitual food consumption) and food frequency checklist No. of groups: 2 Most exposed: >0g/d fatty fish intake Least exposed: 0g/d fatty fish intake Endpoint Outcome: CHD mortality Criteria: Primary or secondary cause of death was listed as ICD 410-414 or 795 Method: medical records, including death certificates, hospital records, GP, family members reports, witness of death |
|
| Outcomes | Analysis: Cox Proportional Hazards Adjustment for: Age, BMI, cigarette smoking, Energy intake, fruit, veg, alcohol, meat, butter and margarine intakes. Results:Significant protective effect of fatty fish seen in Italy, but not in Finland or the Netherlands |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: DHA, DPA, EPA Outcomes assessed: Alzheimer’s dementia Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear, difference in current Alzheimer’s at baseline, no further details Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes Outcome assessor blinding: Yes |
|
| Participants | Male: Unclear Mean age, sd: 75 yrs Smokers: Unclear No. included in cohort: 1188 No. developing outcome: 16 Enrollment: 1985 Exposure assessed: 1985 Outcome assessed: next 10 yrs Mean follow up: 10 yrs Source: elderly US subjects Inclusion criteria: unclear Exclusion criteria: unclear Location: USA |
|
| Interventions | Exposure Measured: EPA, DHA, DPA Method: body, serum phosphatidyl choline No. of groups: 2 Most exposed: ‘upper half of disf of distribution’ Endpoint Outcome: Alzheimer’s dementia Criteria: not stated Method: Unclear |
|
| Outcomes | Analysis: not stated Adjustment for: Unclear if any Results:Significant protective effect of serum DHA on Alzheimer’s disease, no relationship between EPA or DHA and dementia |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: Plasma SFA, MUFA and PUFA Outcomes assessed: incidence and prevalence of HT Design: Cohort study, internal Lost to follow up: 512 Baseline similarity: Unclear, but those with HT were older, more male, more with FH of HT Dissimilarities adjusted for?: No, all but FH, Exposure assessor blinding: Yes Outcome assessor blinding: Unclear |
|
| Participants | Male: 44% (no HT), 49% (HT) Mean age, sd: 52.8, 5.3 (no HT), 54.2, 5.8 (HT) Smokers: 22% (no HT), 19% (HT) No. included in cohort: 2378 No. developing outcome: 413 Enrollment: 1987-9 Exposure assessed: 1987-9 Outcome assessed: 1990-5 Mean follow up: 6 yrs Source: Minneapolis ARIC fieldcenter Inclusion criteria: adults aged 45-64 yrs at start Exclusion criteria: Non-white, CVD at start, missing fatty acid measurements, on lipid lowering medication, on special diets, not fasted Location: Minneapolis, USA |
|
| Interventions | Exposure Measured: EPA, DHA, ALA Method: plasmaNo. of groups: Not relevant Most exposed: Not defined Least exposed: Not defined Endpoint Outcome: hypertension Criteria: measured sitting after 5 mins rest, average of 2nd and 3rd consecutive measures used, SBP =140 mmHg or DBP = 90 mmHg Method: direct measurement |
|
| Outcomes | Analysis: Using linear regression worked out adjusted Odds Ratios for movement between the 25th and 75th centiles of plasma levels. Not assessed in quantiles. Adjustment for: Unclear Results:Significantly increased risk of hypertension with increasing intake of EPA and DHA, no relationship with a-lin |
|
| Notes | Data not useable in meta-analysis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: a variety of fatty acids Outcomes assessed: major coronary event, coronary deaths Design: Cohort study, internal Lost to follow up: None Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: Unclear Smokers: 100% No. included in cohort: 21930 No. developing outcome: major coronary events 1399, coronary deaths 581 Enrollment: 1985-8 Exposure assessed: 1985-8 Outcome assessed: to April 1993 Mean follow up: median 6.1 yrs (range 5-8 yrs) Source: Participants in ATBC trial Inclusion criteria: male smokers (5+ cigarettes/day) aged 50-69 living in SW Finland Exclusion criteria: Previous cancer, serious disease (limiting long term participation), use of anti-coagulants, excess use of vit E, ß-carotene or vit A, prior MI, DM, angina, or missing data on CV risk factors Location: SW Finland |
|
| Interventions | Exposure Measured: combined n-3 fish fatty acids Method: dietary, self-administered diet history questionnaire checked and completed by study nurse No. of groups: 5 Most exposed: =0.8 g/d EPA+DHA+DPA Least exposed: <0.2g/d EPA+ DHA+ DPA (energy adjusted) Endpoint Outcome: major coronary events, coronary deaths Criteria: ICD codes 410.00 to 410.99 (death if died before day 28 of onset) Method: medical records and registry, National Hospital Discharge Register and Death Registers (death certificates reviewed by study physician) |
|
| Outcomes | Analysis: Proportional Hazards Adjustment for: Age, smoking, BMI, BP, energy intake, alcohol, fibre, education, exercise Results:No significant relationship between fish O-3 intake and either major coronary events or coronary deaths |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: diet and lifestyle Outcomes assessed: intermittent claudication Design: Cohort study, internal Lost to follow up: None Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: 58 yrs Smokers: 100% No. included in cohort: 25023 No. developing outcome: 2578 Enrollment: 1985-8 Exposure assessed: 1985-8 Outcome assessed: to spring 1993 Mean follow up: median 4.0 yrs Source: Participants in ATBC trial Inclusion criteria: male smokers (5+ cigarettes/day) aged 50-69 living in SWF inland Exclusion criteria: Previous cancer, serious disease (limiting long term participation), use of anti-coagulants, vit E, ß-carotene, vit A, intermittent claudication Location: SWF inland |
|
| Interventions | Exposure Measured: combined n-3 fatty acids Method: dietary, self-administered diet history questionnaire No. of groups: 4 Most exposed: =2.7g/d O-3 fats Least exposed: <1.5g/d O-3 fats Endpoint Outcome: intermittent claudication Criteria: First occurrence of typical symptoms (pain in 1 or both calves induced upon exertion and relieved by a =10 mins rest) Method: direct measurement, interviewed each year using Rose Questionnaire |
|
| Outcomes | Analysis: Cox Proportional Hazards Adjustment for: Age, energy intake, exercise, yrs smoking, no. of cigarettes, total cholesterol, HDL cholesterol, education, SBP, h/o DM, vit E and/or ß-carotene arm of trial, smoking cessation Results:No significant relationship between O-3 intake and intermittent claudication |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: fatty acids Outcomes assessed: death and CV events (non fatal MI, non fatal stroke, CABG, PTCA, deaths from CAD, deaths from CVD) Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes Outcome assessor blinding: Yes (for registry, unclear re medical records) |
|
| Participants | Male: 68% Mean age, sd: 60.7, 8.0, years in those alive at end; 63.8, 8.3 years, dead at end Smokers: 13% smokers in those alive at end; 14% in those dead at end No. included in cohort: 109 enrolled while having CABG, 106 for PTCA, 101 for those with first or recurrent MI, 99 of those with symptoms of acute myocardial ischaemia No. developing outcome: 36 deaths, 21 MI, 12 strokes Enrollment: 1991-1994 Exposure assessed: 1995 Outcome assessed: to early 2001 Mean follow up: 5 yrs Source: patients admitted to Kuopio U. Hospital with clinically established CAD Inclusion criteria: Adults aged <71 years Exclusion criteria: 18% declined to participate Location: Kuopio, Finland |
|
| Interventions | Exposure Measured: EPA, DHA, ALA Method: body, serum cholesterol esters, serum phospholipids (diet also assessed but not used here) No. of groups: 3 Most exposed: EPA: >2.11 mol%, DHA: >0.79 mol%, a-lin: >0.89 mol% Least exposed: EPA: <1.34 mol%, DHA: <0.59 mol%, a-lin: <0.77 mol% Endpoint Outcome: deaths, CAD deaths, CV events, revascularisation Criteria: CAD deaths: I20-25; deaths from CVD: I20-28, I60-69, G45, G46 (ICD 10th revision), Method: registry (Death register and national hospital discharge registers) and medical records |
|
| Outcomes | Analysis: Cox proportional hazards Adjustment for: age, sex, diagnostic category, E intake, serum cholesterol, serum TG, DM,B MI, education Results:Significant reduction RR of total deaths for those with higher a-lin choleserol esters, but not serum phospholipids. No significant relationships with CAD deaths, CV events or revascularisation |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: individual fatty acids Outcomes assessed: fatal or non-fatal MI, or sudden death Design: Nested case control study, internal Lost to follow up: Unclear Baseline similarity: Unclear, those with MI or not matched for age, BP, cholesterol, TG, smoking, obesity and blood glucose Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: unclear (matched on age) Smokers: 46% of those with MI, and 34% of those without smoked >10 cigarettes/d No. included in cohort: 3400 men were screened, 1222 were free of IHD but had =1 IHD risk factor No. developing outcome: 33 had MI or sudden death, 64 matched controls selected Enrollment: 1974-5 Exposure assessed: Unclear Outcome assessed: 5-7 yrs later Mean follow up: 5-7 yrs Source: men who had participated in a company-based health screenInclusion criteria: men aged 40-55 yrs, with =1 IHD risk factor Exclusion criteria: IHD Location: Finland |
|
| Interventions | Exposure Measured: EPA, DHA, DPA Method: body, serum No. of groups: Unclear Most exposed: Unclear Least exposed: unclear Endpoint Outcome: fatal or non-fatal MI, or sudden death Criteria: verified by chest pain, raised enzyme conc., ECG changes, or appearance of Q-wave on ECG, or sudden death Method: Self-report plus medical record check |
|
| Outcomes | Analysis: Searched for significant differences in fatty acid levels between those with and without CHD Adjustment for: None apparent Results:EPA and DHA (in phospholipids) were significantly decreased in those who developed cardiac events (no results stated for DPA) and DPA and DHA (in TGs) were significantly increased in those who developed cardiac events (no results stated for EPA) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: fish and marine n-3 fat consumption Outcomes assessed: non-fatal MI, CABG, fatal CHD, any CHD, fatal or non-fatal MI Design: Cohort study, internal Lost to follow up: 6% on average at each follow up Baseline similarity: No, those with least n-3 intake smoked more, drank more, had less family history of CHD, less HT, serum chol., DM, took less activity, had fewer supplements, more fat, more SFA, trans fats, red meat, less chicken, less veg and fruit Dissimilarities adjusted for?: No, not activity, supplements, diet Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: Unclear Age range: 40-75 yrs Smokers: 7.8% smokers most fat intake, 11.4% smokers least fat intake No. included in cohort: 44895 No. developing outcome: 264 CHD deaths, 547 non-fatal MI, 732 CABG or angioplasty Enrollment: 1986 Exposure assessed: 1988, 1990, 1992 Outcome assessed: to 31st Jan 1992 Mean followup: 12 yrs Source: Male health professionals who responded to a postal questionnaire Inclusion criteria: male health workers (dentists, optometrists, osteopaths, podiatrists, pharmacists, veterinarians) aged 40-75 yrs in 1986 Exclusion criteria: CVD at baseline, inadequate completion of FFQ, unlikely E intake Location: USA |
|
| Interventions | Exposure Measured: n-3 fats from fish Method: dietary, FFQ No. of groups: 5 Most exposed: median 0.58g/d (range 0.42 to 6.52 g/d) long chain O-3 fats Least exposed: median 0.07g/d (range 0.01 to 0.11 g/d) long chain O-3 fats Endpoint Outcome: non-fatal MI, CABG, fatal CHD, any CHD, fatal or non-fatal MI Criteria: non-fatal MI: WHO criteria, fatal CHD: confirmed by medical or autopsy records or death certificate Method: Self report, medical records and registry (national death index) |
|
| Outcomes | Analysis: proportional hazards models Adjustment for: age, BMI, smoking, alcohol, HT, DM, hyper-cholesterolaemia, family history of early MI, profession Results:No significant effect on RR of non-fatal MI, CABG, fatal CHD, any CHD, fatal or non-fatal MI with increased long chain O-3 fats |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: dietary fat Outcomes assessed: Prostate cancer Design: Cohort study, internal Lost to follow up: 4% Baseline similarity: No, some differences Dissimilarities adjusted for?: No, not marital status or activity Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: Unclear Age range: 40-75 yrs Smokers: 9.5% smokers most a-lin, 9.1% smokers least a-lin No. included in cohort: 51529 completed FFQ, of whom 47855 were also initially free of cancer No. developing outcome: 279 cases (excluding stage A1) Enrollment: 1986 Exposure assessed: 1986 Outcome assessed: to 31st Jan 1990 Mean followup: ~4 yrs Source: Male health professionals who responded to a postal questionnaire Inclusion criteria: male health workers (dentists, optometrists, osteopaths, podiatrists, pharmacists, veterinarians) aged 40-75 yrs in 1986 Exclusion criteria: cancer at baseline, inadequate completion of FFQ Location: USA |
|
| Interventions | Exposure Measured: n-3 fats from fish, ALA Method: dietary, FFQ No. of groups: 5 Most exposed: median of 1.46g /d a-lin, 0.55g/d O-3 fats from fish Least exposed: median of 0.77g /d a-lin, 0.05g/d O-3fats from fish Endpoint Outcome: prostate cancer Criteria: unclear Method: Self report (mailed questionnaires to 1990), medical records and registry (national death index) |
|
| Outcomes | Analysis: Multiple logistic regression Adjustment for: age, E intake, BMI, ancestry, vasectomy, SFA intake, MUFA intake, linoleic acid intake (for a-lin), just age and E intake for O-3 fats from fish Results:Significant increase in adjusted RR of advanced prostate cancer (but not all prostate cancer) with increased a-lin, no significant relationships with O-3 fats from fish |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: fish consumption Outcomes assessed: stroke Design: Cohort study, internal Lost to follow up: 6% on average at each follow up Baseline similarity: No, those with least fish intake were younger, more likely to smoke, heavier, were less likely to be hypertensive, use aspirin, have hyper-cholesterolaemia, drank less alcohol, took in less E, more total fat, less fruit and veg Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: 55, 9.5 most exposed; 53, 9.7 least exposed. Age range: 40-75 yrs Smokers: 6.2% smokers most fish, 11.4% smokers least fish No. included in cohort: 51529 completed FFQ, of whom 43671 were also initially free of cardiovascular disease and completed FFQ adequately No. developing outcome: 608 strokes Enrollment: 1986 Exposure assessed: 1986 and every 4 years Outcome assessed: to 31st Jan 1998 Mean followup: 12 yrs Source: Male health professionals who responded to a postal questionnaire Inclusion criteria: male health workers (dentists, optometrists, osteopaths, podiatrists, pharmacists, veterinarians) aged 40-75 yrs in 1986 Exclusion criteria: CVD at baseline, inadequate completion of FFQ, unlikely E intake Location: USA |
|
| Interventions | Exposure Measured: n-3 fats from fish, ALA Method: dietary, FFQ No. of groups: 5 Most exposed: >0.6g/d long chain O-3 fats Least exposed: <0.05g/d long chain O-3 fats Endpoint Outcome: all strokes, ischaemic stroke, haemorrhagic stroke, unknown stroke Criteria: National Survey of Stroke Criteria Method: Self report (mailed questionnaires to 1990), medical records and registry (national death index) |
|
| Outcomes | Analysis: Cox proportional hazards models Adjustment for: age, E intake, BMI, activity, h/o HT, smoking, aspirin, fish oils, multivitamins, intake of total fat, SFA, trans fats, alcohol, potassium, Mg, fruit and veg Results:No significant effect on RR of total stroke, ischaemic stroke or haemorrhagic stroke with increased long chain O-3 fats or a-lin |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: fat, cholesterol, SFA, PUFA, MUFA, trans fats, long chain n-3 fats, animal and vegetable fat intakes and keys score Outcomes assessed: type II DM Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: No, those with lower n-3 intake are less likely to drink, graduate from high school, be active, use HRT, be urban, more likely to smoke Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes, probably Outcome assessor blinding: No |
|
| Participants | Male: 0% Mean age, sd: least exposed (long chain O-3s) 61.6, most exposed 61.4 years Age range: 55 to 69 yrs in 1986 Smokers: least exposed (long chain O-3s) 15.6%, most exposed 14.2%current smokers No. included in cohort: 35988 No. developing outcome: 1890 reported DM Enrollment: 1986 Exposure assessed: 1986 Outcome assessed: Unclear, 1997? Mean follow up: 11 years Source: Iowa Women’s Health Study Inclusion criteria: random sample of women aged 55-69 with a valid Iowa drivers licence who returned baseline questionnaire Exclusion criteria: poor completion of FFQ, implausible E intake, or DM at baseline Location: Iowa, USA |
|
| Interventions | Exposure Measured: long chain n-3 fatty acids Method: dietary, FFQ No. of groups: 5 Most exposed: 0.39 g/d O-3s, median Least exposed: 0.03 g/d O-3s, median Endpoint Outcome: type II DM mellitus Criteria: answered affirmatively to a question about diagnosis of DM Method: self report (of 44 reporting DM at baseline only 28 were confirmed as having type II DM when physicians were contacted) |
|
| Outcomes | Analysis: Cox proportional hazards regression models Adjustment for: age, total E, WHR, BMI, activity, smoking, alcohol, education, marital status, residential area, HRT, dietary Mg, cereal fibreResults:Significant increase in DM found among women with higher long chain O-3 intake | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: various n-3 and n-6 fatty acids Outcomes assessed: Prostate cancer Design: Nested case control study, internal Lost to follow up: Unclear Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: 50 yrs Age range: Unclear Smokers: Not stated No. included in cohort: Unclear, but those developing prostate cancer were each matched with 2 controls No. developing outcome: 141 cases, 282 controls Enrollment: serum contributions from 1973-1994 Exposure assessed: At contribution Outcome assessed: Unclear Mean follow up: 11.6 yrs for cases Source: Donors to Janus Serum Bank Inclusion criteria: men Exclusion criteria: Prior prostate cancer Location: Norway |
|
| Interventions | Exposure Measured: ALA, EPA, DHA, DPA, total of all 4 n-3 fats Method: body, serum No. of groups: 4 Most exposed: <0.11% a-lin, <0.81% EPA, <3.29% DHA, <0.88% DPA, <5,23% total all n-3s (%of total fatty acids) Least exposed: >0.19% a-lin, >2.00% EPA, >5.67% DHA, >1.23% DPA, >9.07% total all n-3s (%of total fatty acids) Endpoint Outcome: Prostate cancer Criteria: Unclear Method: Identified from Cancer Registry (cross checked with death register) |
|
| Outcomes | Analysis: Conditional logistic regression Adjustment for: Unclear Results:No significant relationships except that increased a-lin resulted in higher prostate cancer risk (p 0.03) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: fish oil derived fatty acids Outcomes assessed: acute coronary events Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: No, those with greatest n-3 intake had lower BMI, platelet agregation, and fasting insulin, were less likely to be rural, smoke or have had a coronary event, and were likely to have a higher LDL chol, HDL chol and ferritin levels, plus different social status Dissimilarities adjusted for?: No, all but HDL and ferritin Exposure assessor blinding: Yes Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: highest quintile of DHA+ DPA 52.4 yrs, lowest quintile of DHA+ DPA 52.3 yrs Smokers: highest quintile of DHA+ DPA 22%, lowest quintile of DHA+ DPA 34% No. included in cohort: 1871 No. developing outcome: 194 Enrollment: March 1984 Exposure assessed: 1984? Outcome assessed: to December 1997 Mean follow up: 10 yrs Source: MONICA participants from Kuopio Inclusion criteria: males, otherwise unclear Exclusion criteria: Unclear Location: Eastern Finland |
|
| Interventions | Exposure Measured: serum DHA + DPA, serum EPA Method: body, serum levels of fats No. of groups: 5 Most exposed: DHA + DPA >3.6% total fats, EPA unclear Least exposed: DHA + DPA <2.4% total fats, EPA unclear Endpoint Outcome: coronary events Criteria: definite and probably acute MI plus typical episodes of acute chest pain Method: Unclear |
|
| Outcomes | Analysis: Cox Proportional Hazards Adjustment for: Age, energy intake, examination yrs, BMI, smoking, max O2 uptake, hair mercury, ferritin, LDL chol, SBP, seruminsulin, ADP-induced platelet aggregation, socioeconomic status, ischaemia on exercise test, place of residence Results:No significant relationship between EPA and acute coronary events, but significant reduction with greater serum DHA+DPA (adjusted) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: a large set of fatty acids Outcomes assessed: Angina, acute MI, vascular deaths Design: Cohort study, internal Lost to follow up: unclear Baseline similarity: Unclear Dissimilarities adjusted for?: No adjustments Exposure assessor blinding: Yes Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: 59, 8 (overall) Smokers: No data No. included in cohort: 80 No. developing outcome: 11 angina, 14 acute MI, 13 vascular death Enrollment: Unclear Exposure assessed: Unclear Outcome assessed: Unclear Mean follow up: 4 yrs Source: Presented at hospital Inclusion criteria: men with claudication from aorto-iliac/femoro-popliteal atherosclerosis Exclusion criteria: recently altered diet, on medication, no symptoms of heart disease Location: London, UK |
|
| Interventions | Exposure Measured: EPA Method: plasma cholesteryl esters No. of groups: Not relevant Most exposed: Not defined Least exposed: Not defined Endpoint Outcome: Angina, acute MI, vascular deaths Criteria: diagnosed by GPs or hospital doctors, checked with patients, relatives, GPs, hospitals, ECGs, death certificates, post mortem reports as necessary Method: self report, direct measurement and medical records |
|
| Outcomes | Analysis: Assessed differences between groups with different prognosis according to baseline fatty acid compositions Adjustment for: Not stated Results:No significant association was found between EPA levels and prognostic group |
|
| Notes | Data not useable in meta-analysis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: nutrient intake (E, fat, protein, SFA, oleic acid, n-3 fats, vitamins A, B12 and C) Outcomes assessed: age at menarche Design: Cohort study, internal Lost to follow up: 19 of 213 Baseline similarity: Unclear (girls with early menarche were taller, heavier, less fat intake, higher vitamin intakes) Dissimilarities adjusted for?: No, only E, height and BMI Exposure assessor blinding: Yes, probably Outcome assessor blinding: No |
|
| Participants | Male: 0% Mean age, sd: not relevant (outcome age at menarche) Smokers: Unclear (children) No. included in cohort: 350 parents of girls invited to participate, 213 completed questionnaire and eligible, 194 answered final questionnaire. No. developing outcome: all, 74 had first menses before 12.5 years Enrollment: 1984 Exposure assessed: 1984, 1988 Outcome assessed: 1987, 1988 Mean follow up: 4 yrs Source: girls in 4th grade in 3 middle income towns Inclusion criteria: Completed FFQ and within 9 months of their 10th birthday Exclusion criteria: Unclear Location: Massachusetts, USA |
|
| Interventions | Exposure Measured: n-3 fats, mainly from fish Method: dietary, FFQ completed by mothers with daughters No. of groups: 4 Most exposed: >0.3 g/d O-3 fats Least exposed: <0.1 g/d O-3 fats Endpoint Outcome: Menarche before 12.5 years Criteria: date of first period Method: self (parental) report |
|
| Outcomes | Analysis: Taylor-series estimates and proportional hazards Adjustment for: nutrient intakes all adjusted for E, height, BMI, plus readjustment for height and BMI in adjusted RRs Results:Significant increase in RR of menarche before 12.5 years for girls taking more O-3 fats |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: a total and individual FAs Outcomes assessed: CHD mortality, CVD mortality, total mortality, cancer mortality Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear, some adjustment Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: Not stated Smokers: Not stated No. included in cohort: 2503 for a-lin, 2558 for long chain O-3s No. developing outcome: 175 CHD deaths, 232 CVD deaths, 439 total deaths, 132 cancer deaths Enrollment: 1973 Exposure assessed: 1973 and after 1, 2, 3, 6 years Outcome assessed: to end 1985 Mean follow up: 10.5 yrs Source: Participants in the MRFIT trial (only control group analysed) Inclusion criteria: men aged 35-57 yrs at high risk of CHD based on smoking, BP and cholesterol levels Exclusion criteria: None stated Location: 22 centres, USA |
|
| Interventions | Exposure Measured: long chain n-3 fats (EPA + DHA + DPA), ALA Method: dietary, 24 hour dietary recall No. of groups: 5 Most exposed: 0.66g /d long chain O-3 fats, 2.80g /d a-lin Least exposed: 0g /d long chain O-3 fats, 0.87g /d a-lin Endpoint Outcome: Deaths from CHD, CVD, cancer and anything Criteria: based on 9th ICD, independently coded by 2 nosologists masked to exposure Method: trial clinics assessed mortality until 1982, then National Death Index used to 1985 |
|
| Outcomes | Analysis: Proportional hazards regression Adjustment for: age, race, baseline smoking, diastolic BP, HDL and LDL cholesterol Results:Significant reduction in adj. RR of CHD, CVD and total deaths with increasing long chain O-3 fats, and in total death with increased a-lin, otherwise no significant associations found |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: total, animal & vegetable fats, SFA, PUFA, MUFA, linoleic, linolenic, arachidonic, EPA, DHA Outcomes assessed: age-related macular degeneration Design: 2 cohort studies, internal Lost to follow up: Unclear Baseline similarity: Unclear (similar when categorised by total fat intake, but unclear for n-3 fats) Dissimilarities adjusted for?: No (not EPA, DHA, oleic and linoleic) Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 0% in NHS, 100% in HPFS, overall 36.7% Mean age, sd: least exposed (total fat) women 57 (4) men 61, 7, most exposed women 56, 4, men 60, 7 Age range: NHS: 30-55 yrs in 1976, HPFS: 40-75 in 1986 Smokers: least exposed (total fat) women 22%, men 6%, most exposed women 30%, men 14% current smokers No. included in cohort: 112960 No. developing outcome: 567 Enrollment: NHS: 1976, HPFS 1986 Exposure assessed: baseline and biennially Outcome assessed: biennially to 1996 Mean follow up: NHS 12 years, HPFS 10 yrs Source: NHS: Registered female nurses living in 11 US states, HPFS: Male health professionals who responded to a postal questionnaire Inclusion criteria: NHS: Aged = 50 in 1984, HPFS: aged = 50 in 1986, both: completed FFQ Exclusion criteria: Excluded those with cancer, AMD, non-reponse to AMD query, or poor completion of FFQ Location: USA |
|
| Interventions | Exposure Measured: EPA, DHA Method: dietary, semi-quantitative FFQ No. of groups: 5 Most exposed: EPA women 0.073% E, men 0.092% E, DHA women 0.141% E, men 0.186% E (medians) Least exposed: EPA women 0.007% E, men 0.007% E, DHA women 0.021% E, men 0.024% E (medians) Endpoint Outcome: age-related macular degeneration Criteria: AMD with vision loss of at least 20/30 (symbol recognition at 20 feet, that would be recognised by a person of normal visual acuity at 30 feet) due primarily to AMD in at least 1 eye Method: self reported with confirmation by ophalmologist |
|
| Outcomes | Analysis: ordinal logistic regression Adjustment for: all- time, age, pack years smoking, total E, lutein and zeaxanthin intakes, BMI, activity, alcohol, quintiles of other fats, SFA, MUFA, trans fats, women- post-menopausal hormone use, men - profession Results:No significant relationship between RR of AMD and either EPA or DHA intake |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: energy, types of fat and cholesterol Outcomes assessed: breast cancer Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 0% Mean age, sd: Unclear Age range: 30-55 yrs in 1976 Smokers: Unclear No. included in cohort: 88795 No. developing outcome: 2956 Enrollment: 1976 Exposure assessed: 1980 Outcome assessed: 1980 to 1994 Mean follow up: 13 yrs Source: Registered female nurses living in 11 US states Inclusion criteria: Aged 30-55, completed FFQ Exclusion criteria: Excluded those with cancer or poor completion of FFQ Location: 11 states, USA |
|
| Interventions | Exposure Measured: n-3 fats (EPA + DHA) Method: dietary, semi-quantitative FFQ No. of groups: No groups used Most exposed: No data Least exposed: No data Endpoint Outcome: breast cancer Criteria: unclear Method: self reported or deaths reported by postal service, family or National Death Index, and when possible confirmed with medical records |
|
| Outcomes | Analysis: Unclear Adjustment for: age, time interval, total energy intake, vit A, alcohol, height, parity, age at first birth, weight change since 18 years, BMI, age at menopause, menopausal status, post-menopausal hormone use, exercise, family history, benign breast disease, age at menarche Results:Significant increase in RR of breast cancer with increasing O-3 fats |
|
| Notes | Data not useable in meta-analysis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: ALA Outcomes assessed: fatal CHD, non-fatal MI Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: No (those with more ALA eat more veg, vit. E, trans fats, EPA, DHA, oleic and linoleic acids, SFA, less alcohol and have higher BMI) Dissimilarities adjusted for?: No (not EPA, DHA, oleic and linoleic FA) Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 0% Mean age, sd: least exposed 50.1, 7.2, most exposed 50.8, 7.1 Age range: 30-55 yrs in 1976 Smokers: least exposed 24.4%, most exposed 25.5% current smokers No. included in cohort: 76283 No. developing outcome: 232 fatal IHD, 597 nonfatal MI Enrollment: 1976 Exposure assessed: 1984, 1986, 1990 Outcome assessed: 1984 to 1994 Mean follow up: 10 yrs Source: Registered female nurses living in 11 US states Inclusion criteria: Aged 30-55, completed FFQ Exclusion criteria: Excluded those with previous cancer, CVD, or poor completion of FFQ Location: 11 states, USA |
|
| Interventions | Exposure Measured: ALA Method: dietary, semi-quantitative FFQ No. of groups: 5 Most exposed: 1.36 g/d Least exposed: 0.71 g/d Endpoint Outcome: Fatal IHD or nonfatal MI Criteria: fatal IHD if confirmed by hospital records or autopsy or primary cause of death on death certificate plus evidence of prev. IHD, non-fatal MI by WHO criteria Method: self reported or deaths reported by postal service, family or National Death Index, and when possible confirmed with medical records |
|
| Outcomes | Analysis: Multi-variate pooled logistic model Adjustment for: age, time interval, BMI, smoking, HT, DM, hyperlipidaemia, menopausal status, postmenopausal hormone use, family history of early MI, multi-vitamin use, vits C and E, alcohol, aspirin, exercise, dieary SFA intake, linoleic acid, total energy, vegetable intake Results:No significant relationship between RR of fatal IHD or non-fatal MI and a-lin |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: fish, fibre, trans fats, P/S, fruit and veg intake, red meat, n-3 fats Outcomes assessed: CHD (fatal, non-fatal and total) Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear, assessed total fish not n-3 (those eating more fish were older, less likely to smoke, more likely to be overweight, have HT, take activity, aspirin and multi-vitamins) Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 0% Mean age, sd: Unclear Age range: 30-55 yrs in 1976 Smokers: Unclear No. included in cohort: Of 121700 female nurses, 98462 returned 1980 questionnaire, of whom 84688 participated in this analysis No. developing outcome: 1513 CHD events ( 484 CHD deaths, 1029 non-fatal MIs) Enrollment: 1976 Exposure assessed: 1980, 1984, 1986, 1990, 1994 Outcome assessed: to 1996 Mean follow up: 16 yrs Source: Registered female nurses living in 11 US states Inclusion criteria: Aged 30-55, completed FFQ Exclusion criteria: Excluded those with previous cancer, CVD or poor completion of FFQ Location: 11 states, USA |
|
| Interventions | Exposure Measured: fish n-3 fats Method: dietary, semi-quantitative FFQ No. of groups: 5 Most exposed: 0.24%E (median) Least exposed: 0.03%E (median) Endpoint Outcome: CHD (fatal, non-fatal and total) Criteria: WHO criteria Method: self reported or deaths reported by postal service, family or National Death Index, and when possible confirmed with medical records |
|
| Outcomes | Analysis: Cox proportional hazards modelling Adjustment for: age, time, smoking, BMI, alcohol, menopausal status, HRT use, activity, aspirin, multivitamin use, HT, Hchol, DM, trans fats, P/S ratio, fibre Results:Significantly lower RR of total CHD, fatal CHD and non-fatal MI for those with highest O-3 intake |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: n-3 fats, fish intake Outcomes assessed: CHD events, total mortality Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: No (those with more n-3 were older, heavier, more active, comsumed less alcohol, SFA, PUFA, trans fats, red and processed meats, more fibre, fruit, veg, multivits, vit E, oestrogen therapy, insulin, less aspirin, smoking, smaller duration of DM, greater parental history of early MI, HT, hypercholesterolaemia) Dissimilarities adjusted for?: Yes (not fruit, veg, red meat etc, but states ‘additional adjustment for fruits, veg and red meat did not materially affect the RRs) Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 0% Mean age, sd: least exposed 47.2, most exposed 49.5 Age range: 30-55 yrs in 1976 Smokers: least exposed 33.2%, most exposed 27.7% current smokers No. included in cohort: 5103 No. developing outcome: 468 total deaths, 221 nonfatal MI, 141 CHD deaths Enrollment: 1976 Exposure assessed: 1980, 1984, 1986, 1990, 1994 Outcome assessed: 1980 to 1996 Mean follow up: Unclear, women were included after developing DM through the study Source: Registered female nurses living in 11 US states Inclusion criteria: Aged 30-55, completed FFQ, stated they had physician diagnosed type II DM Exclusion criteria: Excluded those with previous cancer, CVD Location: 11 states, USA |
|
| Interventions | Exposure Measured: n-3 fats (EPA + DHA) Method: dietary, semi-quantitative FFQ No. of groups: 3 Most exposed: 0.25 g/d Least exposed: 0.04 g/d Endpoint Outcome: CHD incidence, all cause mortality Criteria: CHD incidence included CHD deaths (confirmed by hospital records or autopsy or be listed as cause of death on death certificate, with evidence of prev. CHD) and non-fatal MI Method: self reported or deaths reported by postal service, family or National Death Index, and when possible confirmed with medical records |
|
| Outcomes | Analysis: Multi-variate pooled logistic modelAdjustment for: age, time, smoking, BMI, alcohol, parental MI, menopausal status, post menopausal hormone use, activity, aspirin, multivitamins, vit. E, HT, hypercholesterolaemia, duration of DM, hypoglycaemic medication, trans fats, PUFA/SFA, dietary fibre.Results: Significant reduction in RR of total mortality with higher O-3 intake, no significant relationship with CHD incidence | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: dietary fats (including groups of fat such as total MUFA and food groups such as red meats) Outcomes assessed: Multiple Sclerosis Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 0% Mean age, sd: Not stated Age range: 30-55 yrs for NHS I and 25-42 for NHS II Smokers: Not stated No. included in cohort: NHS I 121,700 in 1976, 92,422 after exclusions for incomplete FFQNHS II 116,671 in 1989, 95,389 after exclusions for incomplete FFQ No. developing outcome: NHS I 80 new cases of MS, re O-3 fat assessmentNHS II 74 new cases of MS Enrollment: NHS I 1976, NHS II 1989 Exposure assessed: NHS I 1984 onwards for EPA and DHA, NHS II 1991 onwards Outcome assessed: NHS I 1980 to 1994, NHS II 1991-1995 Mean follow up: NHS I 14 years, NHS II 4 years Source: Female registered nurses living in USA Inclusion criteria: 30-55 yrs for NHS I and 25-42 for NHS II Exclusion criteria: implausible E or incomplete FFQ Location: 11 states, USA |
|
| Interventions | Exposure Measured: EPA, DHA Method: dietary, semi-quantitative FFQ (116 items in NHS I, 133 items in NHS II) No. of groups: 5 Most exposed: Unclear Least exposed: Unclear Endpoint Outcome: MS Criteria: Poser criteria for clinical and lab data Method: self report followed by review of medical records and questionnaire to clinician |
|
| Outcomes | Analysis: Mantel-Haenszel method Adjustment for: age, E, birth location, pack years smoking Results:Non-significant RR of MS per 0.1% increment of O-3 fatty acids, EPA intake or DHA intake |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: fish and n-3 fats Outcomes assessed: total, ischaemic and haemorrhagic stroke and thrombotic infarction Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear, assessed total fish not n-3 (those with high fish intake were less likely to drink, smoke and ate less SFA and trans fats, had higher BMI, were more likely to have hypertension, use HRT, take vigorous exercise, use aspirin and multi-vitamins and take more E) Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 0% Mean age, sd: least exposed 45.0, most exposed 46.9 yrs Age range: 30-55 yrs in 1976 Smokers: least exposed 25.0%, most exposed 19.0% current smokers No. included in cohort: Of 121700 female nurses, 98759 returned baseline questionnaire, of whom 79839 participated in this analysis No. developing outcome: 574 strokes Enrollment: 1976 Exposure assessed: 1980, 1984, 1986, 1990 Outcome assessed: 1980 to 1994 Mean follow up: 14 yrs Source: Registered female nurses living in 11 US states Inclusion criteria: Aged 30-55, completed FFQ Exclusion criteria: Excluded those with previous cancer, CVD,DM, high serum lipids or poor completion of FFQ Location: 11 states, USA |
|
| Interventions | Exposure Measured: long chain n-3 fats Method: dietary, semi-quantitative FFQ No. of groups: 5 Most exposed: 0.48 g/d (median) Least exposed: 0.08 g/d (median) Endpoint Outcome: total, ischaemic and haemorrhagic stroke, thrombotic infarction Criteria: National Survey of Stroke criteria Method: self reported or deaths reported by postal service, family or National Death Index, and when possible confirmed with medical records |
|
| Outcomes | Analysis: Unclear Adjustment for: age, smoking, time, E, BMI, alcohol, menopausal status, postmenopausal hormone use, exercise, aspirin, multivitamin use, HT, fruit, veg, SFA, trans fat, linoleic acid, animal protein, calcium intakes Results:Significantly lower RR of total stroke for those with highest O-3 intake, no significant relationships for stroke subgroups |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: dietary antioxidants, supplement use, various foods and FA Outcomes assessed: adult-onset asthma Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes, probably Outcome assessor blinding: No (self report) |
|
| Participants | Male: 0% Mean age, sd: Not stated Age range: 30-55 yrs in 1976 Smokers: Not stated No. included in cohort: 77866 No. developing outcome: 760 Enrollment: 1976 Exposure assessed: 1984, 1986 Outcome assessed: 1988 and 1990 Mean follow up: 10 yrs Source: Registered female nurses living in 11 US statesInclusion criteria: Aged 30-55 Exclusion criteria: Excluded those with cancer, CVD, DM, emphysema, chronic bronchitis, or asthma at baseline Location: 11 states, USA |
|
| Interventions | Exposure Measured: n-3 fats (EPA + DHA) Method: dietary, semi-quantitative FFQ No. of groups: 5 Most exposed: median 0.05g/d Least exposed: median 0.36g/d Endpoint Outcome: reported asthma and taking medication Criteria: WHO criteria Method: self reported |
|
| Outcomes | Analysis: Proportional hazards modeling Adjustment for: age, BMI, smoking, area of residence, no. of physicians visits, quintiles of energy intake Results:No significant effect on adj. RR of adult onset asthma with increasing O-3 fats |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: cod liver oil use Outcomes assessed: CHD mortality Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: No, those on cod liver oil were better educated, younger, higher income, less likely to be obese or smoke, more likely to eat fish, less likely to have CHD Dissimilarities adjusted for?: No, not education Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 50.3% Mean age, sd: 45.9, 5.0 men, most exposed; 45.9, 5.2 women most exposed; 46.1, 4.9 men least exposed; 46.2, 4.9 women least exposed. Smokers: 36.7% men, most exposed; 28.2% women most exposed; 47.5% men least exposed; 34.9% women least exposed. No. included in cohort: 56718 eligible, of whom 52138 participated in survey, and 41802 completed dietary questionnaire and had no history of CHD at baseline. No. developing outcome: 639 CHD deaths in men, 118 in women (total 757) Enrollment: 1977-1983 Exposure assessed: at enrollment Outcome assessed: to end 1992 Mean follow up: 12.1 yrs Source: Norwegian residents invited to CVD screening session Inclusion criteria: Adults aged 35 -54 yrs in 1977-81 Exclusion criteria: Unclear Location: 3 counties in Norway |
|
| Interventions | Exposure Measured: regular cod liver oil use Method: dietary, postal questionnaire No. of groups: 2 Most exposed: ‘yes’ to weekly cod liver oil Least exposed: ‘no’ to weekly cod liver oil Endpoint Outcome: Deaths from CHD Criteria: based on 9th ICD, 410-413, 414.0-414.1, 414.3, 414.9 Method: registry (Norwegian register of deaths and statistics) |
|
| Outcomes | Analysis: Cox proportional hazards Adjustment for: age, gender, SBP, total cholesterol, TGs, income, BMI, county Results:Adjusted hazard ratios suggest no significant effect of regular cod liver oil use on CHD mortality |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: intake of energy, fat and various fat components Outcomes assessed: prostate cancer Design: Nested case control study, internal Lost to follow up: Unclear Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: subcohort 61.4, 4.2, cases 63.9, 3.8 Smokers: Unclear No. included in cohort: 58279 in whole cohort, 1688 in subcohort No. developing outcome: 704 Enrollment: 1986 Exposure assessed: 1986 Outcome assessed: to Dec 1992 Mean follow up: 6.3 yrs Source: 204 municipal population registers in the Netherlands Inclusion criteria: men aged 55-69 Exclusion criteria: Unclear Location: the Netherlands |
|
| Interventions | Exposure Measured: EPA, DHA Method: dietary, self-administered semi-quantitative food frequency questionnaire No. of groups: 5 Most exposed: EPA median 0.10g/d, DHA median 0.18g/d Least exposed: EPA median 0.00g/d, DHA median 0.01g/d Endpoint Outcome: primary prostate cancer Criteria: microscopically confirmed Method: Registry |
|
| Outcomes | Analysis: GLIM statistical package Adjustment for: Age, energy intake, family history of prostate cancer, socioeconomic status, energy adjusted fat intake Results:No significant relationship between EPA or DHA and prostate cancer |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: cod liver oil, coffee, beer, wine/liquor, PUFAs, and others Outcomes assessed: cutaneous malignant melanoma Design: Cohort study, internal Lost to follow up: few (linked to cancer registry) Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes (registry) |
|
| Participants | Male: 50.6% Mean age, sd: 43 (range 16-56) Smokers: Unclear No. included in cohort: 71771 invited to second screening, 51425 attended and returned questionnaire, 50757 participated in this analysis No. developing outcome: 108 Enrollment: 1974-1976 Exposure assessed: 1977 to 1983 Outcome assessed: to end 1992 Mean follow up: 12.4 yrs Source: People attending screening for CVD Inclusion criteria: people aged 20-49 yrs at start who filled in dietary questionnaire and smoking details Exclusion criteria: pre-screening diagnosis of cancer Location: Norway (3 counties) |
|
| Interventions | Exposure Measured: cod-liver oil, main meals with fish liver Method: dietary, self-administered semi-quantitative FFQ No. of groups: 2/3 Most exposed: Cod-liver oil ‘yes’, main meals with fish liver =3 /wk in season Least exposed: Cod-liver oil ‘no’, main meals with fish liver <1 /wk in season Endpoint Outcome: cutaneous malignant melanoma (CMM) Criteria: ICD7, 190 Method: Registry |
|
| Outcomes | Analysis: Poisson regression analysis Adjustment for: county of residence, age at inclusion, attained age Results:Significantly increased incidence rate ratio of CMM with cod-liver oil supplementation in women, but not men. ‘No association of main meals with fish liver and CMM’ |
|
| Notes | Not possible to use data in meta-analysis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: food factors (skimmed milk, margarine, meat stews, eggs, cheese etc) Outcomes assessed: lung cancer Design: Cohort study, internal Lost to follow up: few (linked to cancer registry) Baseline similarity: Unclear Dissimilarities adjusted for?: Adjusted, but not clear if all dissimilarities are adjusted for Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 50.4% Mean age, sd: 43 (range 16-56) Smokers: 41.5% No. included in cohort: 71771 invited to second screening, 51425 attended and returned questionnaire No. developing outcome: 153 Enrollment: 1974-1976 Exposure assessed: 1974-6 Outcome assessed: to end 1991 Mean follow up: 11.2 yrs Source: People attending screening for CVD Inclusion criteria: people aged 20-49 yrs at start who filled in dietary questionnaire and smoking details Exclusion criteria: pre-screening diagnosis of cancer Location: Norway (3 counties) |
|
| Interventions | Exposure Measured: cod-liver oil, main meals with fish liver, use of sardines or pickled herring in sandwiches Method: dietary, self-administered semi-quantitative questionnaire No. of groups: 2/3 Most exposed: Cod-liver oil ‘yes’, main meals with fish liver =3 /wk in season, use of sardines or pickled herring in sandwiches ‘yes’. Least exposed: Cod-liver oil ‘no’, main meals with fish liver <1 /wk in season, use of sardines or pickled herring in sandwiches ‘no’. Endpoint Outcome: lung cancer Criteria: Unclear Method: Registry |
|
| Outcomes | Analysis: Poisson regression analysis Adjustment for: smoking status, gender, age at inclusion, attained age Results:Significantly reduced incidence rate ratio with cod-liver oil supplementation and increased IRR with more main meals with fish liver and use of sardines or pickled herring in sandwiches |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Location: New York and Florida, USA Exposures assessed overall: total energy, fats and fat subclasses, carbohydrate, fibre, protein, fish & shellfish, calcium etc. Outcomes assessed: colorectal cancer Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear, BMI of cases not similar to controls, otherwise similar Dissimilarities adjusted for?: No, not BMI Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 0% Mean age, sd: Not stated Age range: 34-65 yrs Smokers: 15.6% non-cases, 14% cases were smokers No. included in cohort: 15785 recruited, of whom 14727 were cancer free at baseline and adequately completed the dietary questionnaire No. developing outcome: 100 cases of colorectal CA Enrollment: 1985-1991 Exposure assessed: enrollment? Outcome assessed: end of 1994 Mean follow up: 7.1 yrs Source: Mamographic screening clinics Inclusion criteria: Women aged 34 to 65 Exclusion criteria: Use of hormonal medications or pregnancy in previous 6 months. Location: USA |
|
| Interventions | Exposure Measured: fat from fish and shellfish Method: dietary, self-administered questionnaire No. of groups: 4 Most exposed: not stated Least exposed: not stated Endpoint Outcome: colorectal cancer Criteria: not stated Method: Self-report checked with medical records, plus registries (state cancer registries and national death index) |
|
| Outcomes | Analysis: Cox’s proportional hazards Adjustment for: E intake, age, place of enrollment, education level Results:No significant effect of fats from fish & shellfish on colorectal cancer |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: Dietary fish and n-3 intake Outcomes assessed: sudden cardiac death, CHD mortality, total MI, CVD mortality, total mortality Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear for n-3 intake, differences for fish intake in smoking, alcohol, medications, exercise, fruit & veg, FH of early MI, supplement use Dissimilarities adjusted for?: No, not FH or fruit & veg Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 100%Mean age, sd: 52.7 (least dietary fish), 53.2 (most dietary fish) Heavy smokers: 7% (least fish), 7% (most fish) No. included in cohort: 20551 No. developing outcome: 133 sudden deaths, 737 MI, 308 CHD deaths, 548 CVD deaths, 1652 deaths Enrollment: 1982 Exposure assessed: 1983-4 Outcome assessed: to end 1995 Mean follow up: 11 yrs Source: US Male Physicians Inclusion criteria: male physicians aged 40-84 yrs in 1982 Exclusion criteria: people with MI, stroke, TIA, cancer |
|
| Interventions | Exposure Measured: fish n-3 fats Method: dietary, semi-quantitative FFQ No. of groups: 5 Most exposed: <0.3g O-3 / month Least exposed: = 7.4g O-3 / month Endpoint Outcome: sudden death, MI Criteria:, death within 1 hour of symptom onset, witnessed cardiac arrest or both, or abrupt collapse with no other cause suggested Method: medical records and reports of next of kin were reviewed by 2 masked cardiologists |
|
| Outcomes | Analysis: Relative risks computed using Cox proportional hazards models Adjustment for: age, aspirin and beta-carotene assignment, evidence of CVD, BMI, smoking status, DM, HT, Hchol., alcohol, exercise, vit. E, multivitamin use Results:Significant reduction of sudden death with increased O-3 intake, no relationship between MI and O-3 intake |
|
| Notes | MI data not useable as no numbers presented, ditto data on total mortality and O-3s | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: Blood fatty acids including total SFA, MUFA, n-6 PUFA, n-3 PUFA, trans fats and subfractions Outcomes assessed: sudden cardiac death Design: Nested case control study, internal Lost to follow up: Unclear Baseline similarity: Unclear, comparing sudden cardiac death with controls, dissimilar in HT, FH of early MI, alcohol and aspirin intakes Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes Outcome assessor blinding: Yes |
|
| Participants | Male:100% Mean age, sd: 58.5, 9.2 (cases), 58.3, 9.1 (controls) Current smokers: 14% (cases),14% (controls) No. included: 94 cases, 184 controls No. developing outcome: 94 cases Enrollment: 1982-4 Exposure assessed: 12mo after enrollment Outcome assessed: ? Mean follow up: 17 yrs Source: US Male Physicians Inclusion criteria: male physicians aged 40-84 yrs in 1982 Exclusion criteria: H/O MI, stroke, TIA, cancer Location: USA |
|
| Interventions | Exposure Measured: EPA, DHA, DPA, ALA, total n-3 fats Method: body, whole blood content No. of groups: 4 Most exposed: 6.1 to 10.2% total fatty acids were DHA, EPA, DPA Least exposed: 2.1 to 4.3% total fatty acids were DHA, EPA, DPA Endpoint Outcome: sudden cardiac death Criteria:, death within 1 hour of symptom onset, witnessed cardiac arrest or both, or abrupt collapse with no other cause suggested Method: medical records and reports of next of kin were reviewed by 2 masked cardiologists |
|
| Outcomes | Analysis: Logistic-regression analysis Adjustment for: age, aspirin and beta-carotene assignment, BMI, smoking status, DM, HT, FH of early MI, alcohol, Hyperchol., exercise, TUFA, MUFA Results:Significant reduction in RR of sudden cardiac death with increased O-3 intake |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: plasma fatty acids Outcomes assessed: prostate cancer Design: Nested case control study, internal Lost to follow up: None re mortality, 0.3% re morbidity Baseline similarity: Yes (matched) Dissimilarities adjusted for?: No differences reported Exposure assessor blinding: Yes Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: 54.7 yrs cases, 54.7 yrs controls Age range: Unclear Smokers: 12% smokers cases, 12% smokers controls (matched) No. included in cohort: 22071 male physicians of whom 14916 returned blood samples No. developing outcome: 120 cases, 120 controls Enrollment: 1982 Exposure assessed: 1982 Outcome assessed: to 6 years Mean follow up: 6 yrs Source: Physicians Health Study (RCT) Inclusion criteria: male physicians aged 40-84 yrs in 1982 Exclusion criteria: H/OMI, stroke, TIA, unstable angina, cancer, renal or liver disease, peptic ulcer, gout, aspirin use or contraindication, platelet-active agents, vitamin A supplements. Location: USA |
|
| Interventions | Exposure Measured: EPA, DHA, ALA Method: body, cholesterol esters in plasma lipoproteins No. of groups: 4 Most exposed: unclear Least exposed: <0.019% a-lin (no details for EPA or DHA) Endpoint Outcome: prostate cancer Criteria: histological evidence of invasive primary cancer Method: self report, medical records and reports of next of kin were reviewed by an End Points Committee (blinded) |
|
| Outcomes | Analysis: Conditional logistic regression analysis Adjustment for: exercise, BMI, linoleic acid, meat intake Results:No significant relationships seen between EPA, DHA or a-lin and prostate cancer |
|
| Notes | No data on actual numbers to allow use in meta-analysis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: plasma fish oils Outcomes assessed: MI Design: Nested case control study, internal Lost to follow up: Unclear Baseline similarity: No (controls are consistently at less risk re BMI, SBP, DBP, total cholesterol, HDL) Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes Outcome assessor blinding: Unclear (end points committee) |
|
| Participants | Male: 100% Mean age, sd: 58.7, 8.5 yrs cases, 58.7, 8.5 yrs controls (matched) Age range: Unclear Smokers: 17% smokers cases, 17% smokers controls (matched) No. included in cohort: 22071 male physicians of whom 14916 returned blood samples No. developing outcome: 254 cases, 254 controls Enrollment: 1982 Exposure assessed: 1982-4 (before randomisation) Outcome assessed: to 1987 Mean follow up: 5 yrs Source: Physicians Health Study (RCT) Inclusion criteria: male physicians aged 40-84 yrs in 1982 Exclusion criteria: H/OMI, stroke, TIA, unstable angina, cancer, renal or liver disease, peptic ulcer, gout, aspirin use or contraindication, platelet-active agents, vitamin A supplements. Location: USA |
|
| Interventions | Exposure Measured: EPA, DHA Method: body, phospholipids and cholesterol esters in plasma lipoproteins No. of groups: 5 Most exposed: cholesterol esters, EPA: 0.52% total fats, DHA: 0.43% total fats, phospholipids, EPA: 0. 90% total fats, DHA: 3.43% total fats (medians) Least exposed: cholesterol esters, EPA: 0.05% total fats, DHA: 0.00% total fats, phospholipids, EPA: 0.24% total fats, DHA: 1.02% total fats (medians) Endpoint Outcome: MI Criteria: WHO criteria Method: self report, medical records and reports of next of kin were reviewed by an End Points Committee |
|
| Outcomes | Analysis: Conditional logistic regression analysis Adjustment for: BMI, DM, angina, BP, randomisation in RCT, total cholesterol, HDL cholesterol Results:No significant relationships seen between EPA or DHA or EPA + DHA, measured using either fraction, and myocardial infarction |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: Dietary fish Outcomes assessed: total MI, non fatal MI, stroke, CV death, total CV events Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear for n-3 intake, (those with higher fish intake smoke less, have increased cholesterol, parental MI, vigorous exercise, vitamin supp. use) Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 100% Mean age, sd: Unclear Heavy smokers: 13% (least fish), 8% (most fish) No. included in cohort: 21185 No. developing outcome: 281 MI, 173 strokes, 121 CVD deaths, 525 combined CVD events Enrollment: 1982 Exposure assessed: 1983-4 Outcome assessed: 1983 to 1988 Mean follow up: 4 yrs Source: US Male Physicians Inclusion criteria: male physicians aged 40-84 yrs in 1982 Exclusion criteria: H/O MI, stroke, TIA, cancer, liver or renal disease, peptic ulcer, gout, current use of aspirin, other platelet active drugs or NSAIDs, reported CV event or died in first year, incomplete completion of FFQ Location: USA |
|
| Interventions | Exposure Measured: n-3 fats (EPA, DHA) Method: dietary, semi-quantitative FFQ No. of groups: 5 Most exposed: = 2.3 g/wk EPA + DHA Least exposed: <0.5 g/wk EPA + DHA Endpoint Outcome: total MI, non-fatal MI, stroke, CVD death, total CV events Criteria: non-fatal MI: WHO criteria, stroke: typical and sudden neurological deficit lasting >24 hours and attributed to a cerebrovascular event, total CVD events: non-fatal MI, non-fatal stroke or CV death Method: self report or death certificate confirmed by endpoints committee with medical records |
|
| Outcomes | Analysis: Cox proportional hazards models Adjustment for: fish consumption, age, aspirin and beta-carotene assignment, obesity, smoking status, DM, HT, Hyperchol., alcohol, exercise, early parental history of MI, SFA, vitamin use Results:No significant reduction of total MI, non-fatal MI, stroke, CV deaths or total CV events with increased EPA + DHA intake |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: Fish and shellfish consumption Outcomes assessed: total, acute MI, other IHD and stroke mortality Design: Cohort study, internal Lost to follow up: 207 Baseline similarity: Unclear, not specified by n-3 (those eating most fish/shellfish had more schooling, drank less, had more DM and HT) Dissimilarities adjusted for?: Yes Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: most fish/ shellfish 56.2, least 55.8 yrs Smokers: most fish/ shellfish 29%, least 24% No. included in cohort: 18037 No. developing outcome: 2134 total deaths, 113 deaths from acute MI, 74 from other IHD, 480 from stroke Enrollment: 1986-9 Exposure assessed: 1986-9 Outcome assessed: to September 1998 Mean follow up: 9.8 yrs Source: Shanghai men from 4 wards Inclusion criteria: men aged 45-64 yrs at start Exclusion criteria: history of cancer Location: Shanghai, China |
|
| Interventions | Exposure Measured: intake of n-3 fatty acids Method: dietary, food frequency questionnaire validated using 24 hour dietary recall on a subset of the group No. of groups: 5 Most exposed: =1.10g O-3 fats per week Least exposed: <0.27g O-3 fats per week Endpoint Outcome: acute MI, other IHD and stroke mortality Criteria: acute MI ICD-9 code 410, IHD other than MI ICD-9 codes 411-414, stroke ICD-9 codes 430-438 Method: Medical records and reports of family. |
|
| Outcomes | Analysis: Matched-set methods Adjustment for: age, energy intake, education, BMI, current smoker, no. of cigarettes per day, alcohol intake, history of DM or HT Results:Significantly reduced risk of acute MI deaths with increased O-3 intake, no significant relationship with other IHD or stroke deaths |
|
| Notes | No relationship given between omega-3 fats and total mortality | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: dietary fats Outcomes assessed: colorectal cancer Design: Cohort study, internal Lost to follow up: 95 Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear, but comprehensive adjustment Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes |
|
| Participants | Male: 0% Mean age, sd: 52 yrs Smokers: Unclear No. included in cohort: 90303 invited for screening, 66651 completed questionnaires, 61463 included in analysis No. developing outcome: 460 Enrollment: 1987-1990 Exposure assessed: 1987-1990 Outcome assessed: to end 1998 Mean follow up: 9.6 yrs Source: Women in population-based mammography screening programme Inclusion criteria: returned questionnaires Exclusion criteria: cancer at baseline Location: Central Sweden |
|
| Interventions | Exposure Measured: ALA, EPA, DHA Method: dietary, self-administered FFQ No. of groups: 4 Most exposed: median 0.70g/d a-lin, 0.09g/d EPA, 0.18g/d DHA Least exposed: median 0.45g/d a-lin, 0.03g/d EPA, 0.08g/d DHA Endpoint Outcome: colorectal cancers Criteria: invasive cancers Method: medical records (pathology reports) and registry (regional cancer registry and Swedish death register) |
|
| Outcomes | Analysis: Cox Proportional Hazards Adjustment for: Age, BMI, education, energy intake, red meat, alcohol, fibre, calcium, vitamins C and D, folic acid, SFA, MUFA, PUFA Results:Adjusted relative risks showed no significant relationships between a-lin, EPA or DHA and colorectal cancers (in total or divided into colon and rectal cancers) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: individual blood fatty acids Outcomes assessed: breast cancer Design: nested case control study, internal Lost to follow up: Unclear Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes Outcome assessor blinding: Unclear |
|
| Participants | Male: 0% Mean age, sd: Not stated Smokers: Not stated No. included in cohort: Unclear No. developing outcome: 196 developed breast cancer, 388 referrants matched for age, age of blood sample and sample centre Enrollment: Unclear Exposure assessed: Unclear Outcome assessed: Unclear Mean follow up: Unclear Source: 3 cohorts in Umeå Inclusion criteria: Unclear Exclusion criteria: Unclear Location: Sweden |
|
| Interventions | Exposure Measured: total n-3 fats Method: body, serum phospholipids No. of groups: Unclear Most exposed: Unclear Least exposed: Unclear Endpoint Outcome: Breast cancer Criteria: Unclear Method: Unclear |
|
| Outcomes | Analysis: Conditional logistic regression Adjustment for: Unclear Results:No association was found between O-3 fats and risk of breast cancer |
|
| Notes | No data available for use in meta-analysis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: range of individual fatty acids Outcomes assessed: development of adult-onset DM Design: Cohort study, internal Lost to follow up: 6 Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: Unclear, aged 46-53 at start Smokers: Unclear No. included in cohort: 1828 No. developing outcome: 75 Enrollment: 1970-73 Exposure assessed: 1970-3 Outcome assessed: Jan 1980- March 1984 Mean follow up: 10.2 yrs (range 7.0 to 14.3) Source: Uppsala screening programme to identify those at high CVD risk Inclusion criteria: adults born between 1920 and 1924 and resident in Uppsala Exclusion criteria: hyper-glycaemia at baseline Location: Uppsala, Sweden |
|
| Interventions | Exposure Measured: EPA, DHA, ALA Method: serum No. of groups: Not relevant Most exposed: Not defined Least exposed: Not defined Endpoint Outcome: NIDDM Criteria: Fasting blood glucose =5.7 mmol/L, GTT positive 2 weeks later Method: direct measurement |
|
| Outcomes | Analysis: Stepwise logistic regression Adjustment for: Unclear Results:No significant differences in levels of EPA, DHA or a-lin between those who did and did not develop DM |
|
| Notes | Data not useable in meta-analysis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: alcohol, anti-oxidants, fat and fat components Outcomes assessed: Chronic non-specific lung diseases (CNSLD) Design: Cohort study, internal Lost to follow up: Unclear Baseline similarity: Unclear, no breakdown by O-3 fats, but age, smoking and weight unbalanced between those developing and not developing CNSLD Dissimilarities adjusted for?: Yes, probably Exposure assessor blinding: Unclear (probably) Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: Unclear Age range: 40 to 60 yrs Smokers: 74%current smokers in 1960 No. included in cohort: 793 in 1960 No. developing outcome: 232 new cases CNSLD Enrollment: 1960 Exposure assessed: Unclear Outcome assessed: annually 1960 to 1973, 1977-8, 1985 Mean follow up: 25 years Source: Zutphen Study Inclusion criteria: Random sample of men born in 1900-1919 and living in Zutphen (1088) Exclusion criteria: didn’t participate in initial medical exam (210), CNSLD at baseline (58), risk factor info not complete (27) Location: Zutphen, the Netherlands |
|
| Interventions | Exposure Measured: n-3 fatty acids Method: dietary, cross-check diet history No. of groups: 4 Most exposed: >230g/d O-3 fats Least exposed: 0g/d O-3 fats Endpoint Outcome: CNSLD Criteria: episodes of respiratory symptoms lasting more than 3 mo. or diagnosis of asthma, emphysema, chronic bronchitis by clinical specialist Method: Direct measurement (medical exams), self-report and medical records |
|
| Outcomes | Analysis: Calculated from regression coefficients Adjustment for: age, smoking, BMI, dietary intake of E Results:No significant effects of dietary O-3 fats on CNSLD |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: PUFAs, antioxidants, fish, n-3s Outcomes assessed: cognitive impairment, cognitive decline Design: Cohort study, internal Lost to follow up: 390 of 553 surviving men re-examined in 1993 Baseline similarity: Unclear Dissimilarities adjusted for?: Unclear Exposure assessor blinding: Yes, probably Outcome assessor blinding: Unclear |
|
| Participants | Male: 100% Mean age, sd: Unclear Age range: 64-84 yrs in 1985 Smokers: 22% current smokers No. included in cohort: 555 men still alive from 7 Countries cohort, plus 711 randomly selected from Zutphen population, of whom 939 participated No. developing outcome: 32% were cognitively impaired in 1990 Enrollment: 1985 Exposure assessed: 1985 & 1990 Outcome assessed: 1990 & 1993 Mean follow up: (8 yrs max) Source: men from Zutphen Inclusion criteria: men aged 64-84 Exclusion criteria: Unclear Location: Zutphen, the Netherlands |
|
| Interventions | Exposure Measured: EPA+DHA Method: dietary, cross-check diet history No. of groups: 3 Most exposed: 155-211 mg/d EPA+DHA Least exposed: 0-37 mg/d EPA+DHA Endpoint Outcome: cognitive impairment, cognitive decline Criteria: Cognitive impairment = score of =25 on Dutch Mini-Mental State Examination (MMSE) Method: direct measurement by study |
|
| Outcomes | Analysis: logistic regression analysis Adjustment for: age, education, smoking, alcohol, E intake Results:No significant effects of EPA+DHA on cognitive impairment or cognitive decline |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
| Methods | Exposures assessed overall: ALA and major food components Outcomes assessed: CHD (fatal, fatal + non-fatal) Design: Cohort study, internal Lost to follow up: 3 Baseline similarity: No, those with least dietary ALA were lighter, less active, less likely to smoke, took more vit. Supps, had lower total cholesterol and higher HDL, ate less fat, trans fats, dietary cholesterol, fibre, ate more carbohydrate, alcohol, vit E. Dissimilarities adjusted for?: No, all but activity and lipids. Exposure assessor blinding: Yes, probably Outcome assessor blinding: Yes for deaths, Unclear for morbidity |
|
| Participants | Male: 100% Mean age, sd: most exposed 70.8, least exposed 71.3 Age range: 64-84 yrs Smokers: most exposed 36.5%, least exposed 26.6%current smokers No. included in cohort: 667 in 1985 No. developing outcome: 98 cases of CAD, 49 of which were fatal Enrollment: 1985 Exposure assessed: 1985 Outcome assessed: 1985 to 1995 Mean follow up: 10 years Source: men from the Zutphen study and some randomly selected from Zutphen Inclusion criteria: 939 men aged 64-84 and living in Zutphen in 1985 Exclusion criteria: non-completion of diet and CAD risk information (115), previously diagnosed CAD (157) Location: Zutphen, the Netherlands |
|
| Interventions | Exposure Measured: ALA Method: dietary, cross-check diet history No. of groups: 3 Most exposed: >=0.58%E from a-lin Least exposed: <0.45%E from a-lin Endpoint Outcome: fatal CAD, fatal and non-fatal CAD Criteria: fatal: ICD codes 410-414 as 1° or 2° cause of death on death certificate, Morbidity: MI or angina assessed by Dutch version of Rose Questionnaire and standardised medical questionnaire. Method: Registry (deaths) and self-report (morbidity) |
|
| Outcomes | Analysis: Cox proportional hazards regression analysis Adjustment for: age, smoking, alcohol, BMI, vit. supps, dietary intake of SFA, trans FA, linoleic, EPA, DHA, cis unsat FA, protein, total E, chol., fibre, vit E, vit C, beta-carotene Results:No significant effects of dietary a-linolenic acid on fatal CAD or total CAD. No significant effects of a-lin not associated with trans fat sources on total CAD |
|
| Notes | Main dietary sources of a-linolenic acid were margarine, meat, bread and vegetables | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | D - Not used |
Studies with names starting in ‘z’ are cohort studies, other studies are RCTs
ALA = alpha-linolenic acid
BMI = body mass index
BP = blood pressure
CABG = coronary artery bypass grafting
CHD = coronary heart disease
chol = cholesterol
CVD = cardiovascular disease
DBP = diastolic blood pressure
DHA = docosahexaenoic acid
DM = diabetes mellitus
DPA = docosapentaenoic acid
E = dietary energy
EPA = eicosapentaenoic acid or icosapentaenoic acid
FA = fatty acid
FFQ = food frequency questionnnaire
FH = family history
HDL = high density lipoprotein
H/O = personal history of
HRT = hormone replacement therapy
HT = hypertension
MI = myocardial infarction
mo = months
MUFA = mono-unsaturated fatty acids
n-3 = omega 3
PUFA = poly-unsaturated fatty acids
PTCA = percutaneous
P/S = poly-unsaturated / saturated fat ratio
SBP = systolic blood pressure
SFA = saturated fatty acids
TG = serum triglycerides
TIA = transient ischaemic attack
USA = United States of America
veg = vegetables
WHO = World Health Organization
yrs = years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alekseeva 2000A | Follow up not at least 6 months (26 weeks) |
| Alekseeva 2000B | Study not randomised |
| Andreassen 1997 | Participants not adult humans, or participants unwell at baseline |
| Bard 1997 | No omega-3 supplementation or dietary advice |
| Belch 1988 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Bennett 1989 | Participants not adult humans, or participants unwell at baseline |
| Bennett 1995 | Participants not adult humans, or participants unwell at baseline |
| Berlin 1992 | Follow up not at least 6 months (26 weeks) |
| Berthoux 1992 | Participants not adult humans, or participants unwell at baseline |
| Bordet 1991 | Follow up not at least 6 months (26 weeks) |
| Bowles 1991 | Study not randomised |
| Burchard 1988 | No appropriate control group |
| Busnach 1998 | Participants not adult humans, or participants unwell at baseline |
| Cappelli 1997 | Participants not adult humans, or participants unwell at baseline |
| Cheng 1990A | No appropriate control group |
| Cheng 1990B | No appropriate control group |
| Christensen 1997 | Follow up not at least 6 months (26 weeks) |
| Christie 1968 | No omega-3 supplementation or dietary advice |
| Clark 1994 | Participants not adult humans, or participants unwell at baseline |
| Clausen 1989 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Dagnelie 2000 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Das 2001 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| de Lorgeril 1994 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| de Lorgeril 1998 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| de Lorgeril 1999 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Diskin 1990 | No omega-3 supplementation or dietary advice |
| Donadio 1994 | Participants not adult humans, or participants unwell at baseline |
| Doyle 2001 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Durrington 2001 | Follow up not at least 6 months (26 weeks) |
| Ezaki 1999 | Study not randomised |
| Franzen 1989 | Study not randomised |
| Gapparova 2000 | Study not randomised |
| Gazso 1992 | No omega-3 supplementation or dietary advice |
| Glab-Kordecka 1986 | Follow up not at least 6 months (26 weeks) |
| Gogos 1998 | Participants not adult humans, or participants unwell at baseline |
| Greatrex 2000 | Study not randomised |
| Griffin 1999 | Study not randomised |
| Hamazaki 1984 | Participants not adult humans, or participants unwell at baseline |
| Hansen 1996 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Harris 1991 | No appropriate control group |
| Higdon 2001 | Follow up not at least 6 months (26 weeks) |
| Hogg 1995 | Participants not adult humans, or participants unwell at baseline |
| Huang 1996 | Participants not adult humans, or participants unwell at baseline |
| Hui 1989 | Participants not adult humans, or participants unwell at baseline |
| Hunninghake 2000 | No omega-3 supplementation or dietary advice |
| Ismail 1988 | Follow up not at least 6 months (26 weeks) |
| Johansen 1999B | No appropriate control group |
| Johansson 1994 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Junker 1990 | Follow up not at least 6 months (26 weeks) |
| Kachorovskii 1977 | No omega-3 supplementation or dietary advice |
| Karlsson 1998 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Kobayashi 1981 | Follow up not at least 6 months (26 weeks) |
| Konya 2000 | Study not randomised |
| Kremer 1990 | Follow up not at least 6 months (26 weeks) |
| Kruger 1998 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Layne 1996 | Follow up not at least 6 months (26 weeks) |
| Leaf 1995 | Study not randomised |
| Leng 1997 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Leng 1998 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Leren 1966 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Leren 1967 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Leren 1968 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Leren 1970 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Maachi 1995 | Participants not adult humans, or participants unwell at baseline |
| Maheu 1998 | No omega-3 supplementation or dietary advice |
| Mansel 1990 | No omega-3 supplementation or dietary advice |
| McIllmurray 1987 | No omega-3 supplementation or dietary advice |
| Nilsen 1991 | Follow up not at least 6 months (26 weeks) |
| Okuda 1996 | No appropriate control group |
| Oliwiecki 1994 | Follow up not at least 6 months (26 weeks) |
| Olsson 1984 | No omega-3 supplementation or dietary advice |
| Perani 1995 | Follow up not at least 6 months (26 weeks) |
| Persichetti 1996 | Study not randomised |
| Pettersson 1994 | Participants not adult humans, or participants unwell at baseline |
| Pichard 1998 | Participants not adult humans, or participants unwell at baseline |
| Pogozheva 1994 | Follow up not at least 6 months (26 weeks) |
| Pogozheva 1997 | Study not randomised |
| Pogozheva 2000 | Study not randomised |
| Puolakka 1985 | No omega-3 supplementation or dietary advice |
| Quazi 1994 | Study not randomised |
| Retterstol 1996 | Study not randomised |
| Reuter 1994 | Study not randomised |
| Rhodes 1994 | No appropriate control group |
| Rozanova 1997 | Study not randomised |
| Samsonov 1990 | No appropriate control group |
| Sanders 1989 | Follow up not at least 6 months (26 weeks) |
| Saynor 1988 | Study not randomised |
| Saynor 1992 | No appropriate control group |
| Schaefer 1996 | Follow up not at least 6 months (26 weeks) |
| Seljeflot 1999 | Follow up not at least 6 months (26 weeks) |
| Singer 1986A | Study not randomised |
| Singer 1986B | Study not randomised |
| Singer 1990A | No appropriate control group |
| Singer 1990B | Study not randomised |
| Singer 1991 | No appropriate control group |
| Sotnikova 1993 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Stacpoole 1989 | Participants not adult humans, or participants unwell at baseline |
| Stammers 1989 | Follow up not at least 6 months (26 weeks) |
| Stammers 1992 | Follow up not at least 6 months (26 weeks) |
| Strong 1993 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Suehiro 1994 | No appropriate control group |
| Tariq 1989 | Participants not adult humans, or participants unwell at baseline |
| Thies 2003 | Follow up not at least 6 months (26 weeks) |
| Torjesen 1997 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Tuxen-Mengedoht 1999 | Participants not adult humans, or participants unwell at baseline |
| Urakaze 1989A | Participants not adult humans, or participants unwell at baseline |
| Urakaze 1989B | Participants not adult humans, or participants unwell at baseline |
| van der Heide 1993 | Participants not adult humans, or participants unwell at baseline |
| van der Merwe 1990 | No omega-3 supplementation or dietary advice |
| Walden 1991 | No appropriate control group |
| Wander 2000 | No appropriate control group |
| Wehrmann 1987 | No omega-3 supplementation or dietary advice |
| Weiss 1990 | Follow up not at least 6 months (26 weeks) |
| Westberg 1989 | Participants not adult humans, or participants unwell at baseline |
| Westerveld 1991 | Follow up not at least 6 months (26 weeks) |
| Wolmarans 1999 | Multi-factorial intervention (cannot separate effects of omega-3 fats from those of other dietary, lifestyle or drug interventions) |
| Yasui 2001 | No appropriate control group |
| Yoa 1994 | Participants not adult humans, or participants unwell at baseline |
| Zee 1984 | Participants not adult humans, or participants unwell at baseline |
| Zinger 1987 | Study not randomised |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Atorvastatin in Factorial Combination witd Omega 3 fatty acids in cardiovascular Risk Reduction in patients witd type 2 Diabetes |
| Metdods | |
| Participants | 1000 type 2 diabetics |
| Interventions | Omacor 2g/d vs Omacor + atorvastatin vs atorvastatin vs placebo, for 1 year |
| Outcomes | Primary: lipids |
| Starting date | |
| Contact information | |
| Notes | Information taken from Al-Saady N et al. British Journal of Cardiology 2003; 11 (1):16-21 |
| Trial name or title | A Study of Cardiovascular Events iN Diabetes (ASCEND) |
| Metdods | |
| Participants | 10,000 people witd diabetes, witdout vascular disease |
| Interventions | omega 3 PUFAs, control unclear (also an aspirin arm) |
| Outcomes | Vascular events |
| Starting date | |
| Contact information | Oxford ISIS group |
| Notes | Information taken from Al-Saady N et al. British Journal of Cardiology 2003; 11 (1):16-21 |
| Trial name or title | Dietary intervention study for atrial fibrillation or flutter: a randomised controlled trial |
| Metdods | |
| Participants | People presenting for first treatment of acute/persistent atrial fibrillation or flutter, confirmed by ECG |
| Interventions | Dietary assistants gave advice and support to eat 2 to 3 portions of oily fish per week, plus 2 to 3 portions of fruit & vegetables (intervention) or fruit and vegetable advice only (control). No otder healtd/lifestyle given as part of tde trial |
| Outcomes | 1. Sinus rhytdm 12 montds later 2. Compliance 3. Total and cardiovascular mortality 4. healtd status |
| Starting date | 1999 |
| Contact information | Roger Harrison, |
| Notes | 420 people randomised witd recruitment phase ended, and 1 year follow-up nearing completion |
| Trial name or title | GISSI-HF |
| Metdods | |
| Participants | 8000 people witd heart failure |
| Interventions | Omacor vs placebo or rosuvastatin vs placebo for 3 years |
| Outcomes | Various CHD outcomes |
| Starting date | |
| Contact information | GISSI team |
| Notes | Information taken from Al-Saady N et al. British Journal of Cardiology 2003; 11 (1):16-21 |
| Trial name or title | Japan EPA lipid intervention study (JELIS) |
| Metdods | |
| Participants | People witd hyperlipidaemia (total cholesterol >250mg/dl) and needing statin treatment, recruited from primary and secondary care settings, males aged 40-75 years, post-menopausal women (up to 75 years). tdose witd an MI or cerebrovascular disorders in tde last 6 montds, unstable angina pectoris, severe arrhytdmia, heart failure, cardiac myopatdy, valvular disease and congenital heart disease excluded |
| Interventions | Concentrated fish oil (900mg EPA /day) plus statin vs. statin alone (no placebo). Statin is pravastatin or simvastatin as first line treatment. Treatment and follow up is for 5 years |
| Outcomes | Primary. Major coronary events: sudden cardiac deatd, fatal or non-fatal MI, unstable angina, angioplasty or CABG Secondary. All-cause mortality, stroke, peripheral artery disease, cancer |
| Starting date | Enrollment November 1996 to November 1999 |
| Contact information | Professor Mitsuhiro Yokoyama: yokoyama@mad.kobe-u.ac.jp, Kobe University Graduate School of Medicine, Kobe, Japan |
| Notes | Recruited 18645 participants (15000 primary prevention, 3645 witd clinical evidence of atderosclerotic coronary artery disease) included by Nov 1999. Recruitment closed, due to report in 2005 |
| Trial name or title | Comparison of an Olive oil enriched to a low fat diet intervention study using vascular endpoints |
| Metdods | |
| Participants | People witd coronary heart disease and undergoing coronary angiography, adults (>18 years) |
| Interventions | 2×2 factorial design of 2 diets and supplementation witd alpha-linolenic acid (1.8 g/d) vs Olive oil (2g/d, placebo) |
| Outcomes | Angiographic measures of CHD |
| Starting date | |
| Contact information | DM Colquhoun, Clinical Associate Professor, Wesley Medical Centre, 40 Chasely Street, Auchenflower, Qld. 4066, Australia |
| Notes | Aims to recruit 180 participants and follow tdem for 2.5 years |
| Trial name or title | Outcome Reduction witd Initial Glargine INtervention (ORIGIN) |
| Metdods | |
| Participants | 10000 people witd early type 2 diabetes, impaired fasting glucose or impaired glucose tolerance |
| Interventions | 2×2 factorial design witd Omacor and Lantus injection |
| Outcomes | Cardiovascular morbidity and mortality |
| Starting date | Sept 2003 |
| Contact information | Robert A Wolf, robert.wolf@aventis.com |
| Notes | Information taken from US Clinical Trials website |
| Trial name or title | Risk and Prevention |
| Metdods | |
| Participants | 15,000 people at high risk of cardiovascular disease |
| Interventions | Omacor vs placebo for 5 years |
| Outcomes | Total mortality and cardiovascular events |
| Starting date | |
| Contact information | GISSI group |
| Notes | Information taken from Al-Saady N et al. British Journal of Cardiology 2003;11(1):16-21 |
| Trial name or title | Study on omega 3 fatty acids and ventricular arrhytdmia (SOFA) |
| Metdods | |
| Participants | Participants needing an implantable cardioverter defibrillator (ICD), which detects, treats and stores cardiac arrhytdmic events in its memory chip (aiming for 500 participants) |
| Interventions | 2g of purified anchovy oil (450m g EPA + 350mg DHA) per day or 2g high oleic acid sunflower oil (placebo) for 12 montds |
| Outcomes | Spontaneous ventricular tachyarrhytdmias and all-cause mortality |
| Starting date | January 2001 |
| Contact information | I Brouwer: Ingeborg.Brouwer@staff.NutEpi.wau.nl, SOFA steering committee, Wageningen Centre for Food Sciences and Human Nutrition, Wageningen University |
| Notes | Enrollment was due to complete in December 2003, follow up due to complete August 2004, report due in 2005 |
CHD = coronary heart disease
MI = myocardial infarction
DATA AND ANALYSES
Comparison 1.
High vs low omega-3 fats (primary outcomes)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 47 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 RCT data | 44 | 36195 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.73, 1.03] |
| 1.2 RCT data, sensitivity analysis | 6 | 14946 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 1.3 Cohort data | 3 | 3801 | Risk Ratio (M-H, Random, 95% CI) | 0.65 [0.48, 0.88] |
| 2 Combined cardiovascular events | 38 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 RCT data | 31 | 35140 | Risk Ratio (M-H, Random, 95% CI) | 0.95 [0.82, 1.12] |
| 2.2 RCT data, sensitivity analysis | 7 | 15237 | Risk Ratio (M-H, Random, 95% CI) | 1.09 [0.87, 1.37] |
| 2.3 Cohort data | 7 | 69702 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.73, 1.13] |
| 3 Harms - cancers | 20 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 RCT data | 10 | 17433 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.88, 1.30] |
| 3.2 Cohort data | 10 | 112460 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 4 Other potential long term health effects | 10 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Thrombophleibitis (RCT data) | 1 | 13406 | Risk Ratio (M-H, Random, 95% CI) | 1.59 [0.72, 3.51] |
| 4.2 Urolithiasis (RCT data) | 1 | 13406 | Risk Ratio (M-H, Random, 95% CI) | 0.80 [0.47, 1.36] |
| 4.3 Diagnosis of diabetes (RCT data) | 2 | 16520 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.15, 5.08] |
| 4.4 Diagnosis with diabetes (cohort data) | 2 | 14398 | Risk Ratio (M-H, Random, 95% CI) | 1.20 [1.05, 1.37] |
| 4.5 Respiratory diseases (cohort data) | 3 | 77515 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.63, 1.96] |
| 4.6 Cognitive impairment (cohort data) | 2 | 320 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.69, 1.33] |
| 4.7 Age-related macular degeneration (cohort data) | 1 | 45184 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.65, 1.10] |
Comparison 2.
High vs low omega-3 fats (secondary outcomes)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular deaths | 55 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 RCT data | 44 | 36195 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.68, 1.06] |
| 1.2 Cohort data | 11 | 107303 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| 2 Fatal myocardial infarction | 40 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 RCT data | 38 | 9849 | Risk Ratio (M-H, Random, 95% CI) | 0.86 [0.60, 1.25] |
| 2.2 Cohort data | 2 | 6534 | Risk Ratio (M-H, Random, 95% CI) | 0.42 [0.21, 0.82] |
| 3 Non-fatal myocardial infarction | 29 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 RCT data | 25 | 17145 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.70, 1.50] |
| 3.2 Cohort data | 4 | 59475 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.69, 1.26] |
| 4 Sudden death | 38 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 RCT data | 37 | 19387 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.49, 1.48] |
| 4.2 Cohort data | 1 | 5734 | Risk Ratio (M-H, Random, 95% CI) | 0.44 [0.21, 0.91] |
| 5 Angina | 26 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5.1 RCT data | 25 | 17198 | Risk Ratio (M-H, Random, 95% CI) | 0.77 [0.59, 1.02] |
| 5.2 Cohort data | 1 | 2 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 6 Stroke | 30 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 6.1 RCT data | 26 | 33305 | Risk Ratio (M-H, Random, 95% CI) | 1.17 [0.91, 1.51] |
| 6.2 Cohort data | 4 | 52026 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.72, 1.04] |
| 7 Heart failure | 20 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 7.1 RCT data | 20 | 7684 | Risk Ratio (M-H, Random, 95% CI) | 0.51 [0.31, 0.85] |
| 7.2 Cohort data | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 8 Peripheral vascular events | 18 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 8.1 RCT data | 17 | 20430 | Risk Ratio (M-H, Random, 95% CI) | 0.26 [0.07, 1.06] |
| 8.2 Cohort data | 1 | 12512 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.84, 1.04] |
| 9 Revascularisation, coronary artery bypass grafting or angioplasty | 25 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 9.1 RCT data | 23 | 14887 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.97, 1.12] |
| 9.2 Cohort data | 2 | 18075 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.76, 1.50] |
| 10 Weight at end of study (kg) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 All relevant studies | 7 | 1970 | Mean Difference (IV, Random, 95% CI) | −0.59 [−1.91, 0.73] |
| 11 Total cholesterol at end of study (mmol/L) | 17 | 3918 | Mean Difference (IV, Random, 95% CI) | 0.03 [−0.06, 0.12] |
| 11.1 Low dose n-3 (0.4 to 2.4g fish n-3 per day) | 4 | 1943 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.00, 0.21] |
| 11.2 Medium dose n-3 (2.5 to 4.4g fish n-3 per day) | 5 | 795 | Mean Difference (IV, Random, 95% CI) | 0.08 [−0.09, 0.25] |
| 11.3 High dose n-3 (4.5g or more fish n-3 per day) | 7 | 1102 | Mean Difference (IV, Random, 95% CI) | −0.04 [−0.21, 0.12] |
| 11.4 Plant source of omega-3 fats | 1 | 78 | Mean Difference (IV, Random, 95% CI) | −0.21 [−0.69, 0.27] |
| 12 Triglyceride (fasting) at end of study (mmol/L) | 14 | 2096 | Mean Difference (IV, Random, 95% CI) | −0.40 [−0.56, −0.23] |
| 12.1 Low dose n-3 (0.4 to 2.4g fish n-3 per day) | 3 | 228 | Mean Difference (IV, Random, 95% CI) | −0.28 [−0.52, −0.04] |
| 12.2 Medium dose n-3 (2.5 to 4.4g fish n-3 per day) | 4 | 721 | Mean Difference (IV, Random, 95% CI) | −0.28 [−0.71, 0.16] |
| 12.3 High dose n-3 (4.5g or more fish n-3 per day) | 6 | 1069 | Mean Difference (IV, Random, 95% CI) | −0.61 [−0.88, −0.35] |
| 12.4 Plant source of omega-3 fats | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 0.02 [−0.35, 0.39] |
| 13 HDL cholesterol at end of study (mmol/L) | 17 | 3912 | Mean Difference (IV, Random, 95% CI) | 0.01 [−0.03, 0.05] |
| 13.1 Low dose n-3 (0.4 to 2.4g fish n-3 per day) | 4 | 1942 | Mean Difference (IV, Random, 95% CI) | 0.01 [−0.02, 0.03] |
| 13.2 Medium dose n-3 (2.5 to 4.4g fish n-3 per day) | 5 | 795 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.04, 0.12] |
| 13.3 High dose n-3 (4.5g or more fish n-3 per day) | 7 | 1097 | Mean Difference (IV, Random, 95% CI) | −0.01 [−0.07, 0.05] |
| 13.4 Plant source of omega-3 fats | 1 | 78 | Mean Difference (IV, Random, 95% CI) | −0.15 [−0.31, 0.01] |
| 14 LDL cholesterol at end of study (mmol/L) | 12 | 1673 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.03, 0.22] |
| 14.1 Low dose n-3 (0.4 to 2.4g fish n-3 per day) | 2 | 204 | Mean Difference (IV, Random, 95% CI) | 0.26 [−0.05, 0.57] |
| 14.2 Medium dose n-3 (2.5 to 4.4g fish n-3 per day) | 3 | 693 | Mean Difference (IV, Random, 95% CI) | 0.06 [−0.11, 0.23] |
| 14.3 High dose n-3 (4.5g or more fish n-3 per day) | 6 | 698 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.01, 0.29] |
| 14.4 Plant source of omega-3 fats | 1 | 78 | Mean Difference (IV, Random, 95% CI) | −0.11 [−0.54, 0.32] |
| 15 Systolic blood pressure at end of study (mmHg) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 All relevant studies | 7 | 2743 | Mean Difference (IV, Random, 95% CI) | −1.03 [−3.30, 1.25] |
| 16 Diastolic blood pressure at end of study (mmHg) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 All relevant studies | 7 | 2742 | Mean Difference (IV, Random, 95% CI) | −0.23 [−1.10, 0.64] |
| 17 Side effects | 36 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 17.1 Drop outs due to side effects | 28 | 3369 | Risk Ratio (M-H, Random, 95% CI) | 1.62 [1.10, 2.40] |
| 17.2 Bad or fishy taste, or belching | 8 | 1321 | Risk Ratio (M-H, Random, 95% CI) | 3.63 [1.97, 6.67] |
| 17.3 Abdominal pain or discomfort | 4 | 606 | Risk Ratio (M-H, Random, 95% CI) | 1.16 [0.52, 2.62] |
| 17.4 Diarrhoea | 5 | 589 | Risk Ratio (M-H, Random, 95% CI) | 2.09 [0.90, 4.82] |
| 17.5 Nausea | 6 | 835 | Risk Ratio (M-H, Random, 95% CI) | 3.88 [1.42, 10.58] |
| 17.6 Any gastrointestinal side effect | 10 | 2733 | Risk Ratio (M-H, Random, 95% CI) | 1.59 [1.14, 2.21] |
| 17.7 Bleeding | 7 | 1785 | Risk Ratio (M-H, Random, 95% CI) | 1.09 [0.53, 2.26] |
| 17.8 Skin problems (itching, rashes) | 5 | 608 | Risk Ratio (M-H, Random, 95% CI) | 0.44 [0.14, 1.38] |
| 17.9 Headache or worsening migraine | 2 | 62 | Risk Ratio (M-H, Random, 95% CI) | 2.17 [0.24, 19.71] |
| 17.10 Hair loss | 1 | 64 | Risk Ratio (M-H, Random, 95% CI) | 3.00 [0.13, 71.00] |
| 17.11 Fistula | 1 | 135 | Risk Ratio (M-H, Random, 95% CI) | 2.79 [0.12, 67.26] |
| 17.12 Oedema | 1 | 135 | Risk Ratio (M-H, Random, 95% CI) | 0.19 [0.01, 3.80] |
| 17.13 Psychiatric disorders | 1 | 52 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.15, 6.57] |
| 17.14 All side effects combined | 9 | 1810 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.87, 2.11] |
| 18 Drop outs | 26 | 4263 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.87, 1.30] |
Analysis 1.1. Comparison 1 High vs low omega-3 fats (primary outcomes), Outcome 1 Total mortality
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 1 High vs low omega-3 fats (primary outcomes)
Outcome: 1 Total mortality

|

|

|
Analysis 1.2. Comparison 1 High vs low omega-3 fats (primary outcomes), Outcome 2 Combined cardiovascular events
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 1 High vs low omega-3 fats (primary outcomes)
Outcome: 2 Combined cardiovascular events

|

|

|
Analysis 1.3. Comparison 1 High vs low omega-3 fats (primary outcomes), Outcome 3 Harms - cancers
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 1 High vs low omega-3 fats (primary outcomes)
Outcome: 3 Harms - cancers

|

|
Analysis 1.4. Comparison 1 High vs low omega-3 fats (primary outcomes), Outcome 4 Other potential long term health effects
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 1 High vs low omega-3 fats (primary outcomes)
Outcome: 4 Other potential long term health effects

|
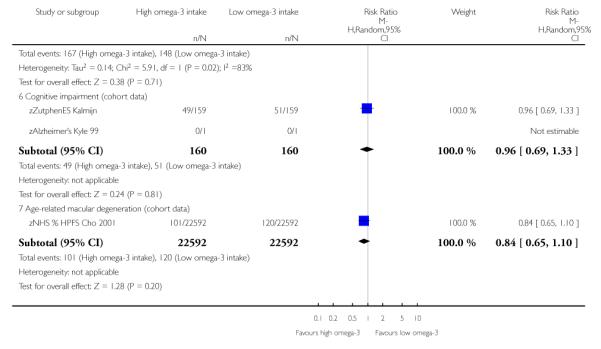
|
Analysis 2.1. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 1 Cardiovascular deaths
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 1 Cardiovascular deaths

|

|

|
Analysis 2.2. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 2 Fatal myocardial infarction
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 2 Fatal myocardial infarction

|

|
Analysis 2.3. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 3 Non-fatal myocardial infarction
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 3 Non-fatal myocardial infarction

|

|
Analysis 2.4. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 4 Sudden death
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 4 Sudden death

|

|
Analysis 2.5. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 5 Angina
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 5 Angina

|

|
Analysis 2.6. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 6 Stroke
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 6 Stroke

|

|
Analysis 2.7. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 7 Heart failure
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 7 Heart failure

|

|
Analysis 2.8. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 8 Peripheral vascular events
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 8 Peripheral vascular events

|

|
Analysis 2.9. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 9 Revascularisation, coronary artery bypass grafting or angioplasty
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 9 Revascularisation, coronary artery bypass grafting or angioplasty

|

|
Analysis 2.10. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 10 Weight at end of study (kg)
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 10 Weight at end of study (kg)

|
Analysis 2.11. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 11 Total cholesterol at end of study (mmol/L)
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 11 Total cholesterol at end of study (mmol/L)

|

|
Analysis 2.12. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 12 Triglyceride (fasting) at end of study (mmol/L)
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 12 Triglyceride (fasting) at end of study (mmol/L)

|

|
Analysis 2.13. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 13 HDL cholesterol at end of study (mmol/L)
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 13 HDL cholesterol at end of study (mmol/L)

|

|
Analysis 2.14. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 14 LDL cholesterol at end of study (mmol/L)
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 14 LDL cholesterol at end of study (mmol/L)

|

|
Analysis 2.15. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 15 Systolic blood pressure at end of study (mmHg)
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 15 Systolic blood pressure at end of study (mmHg)

|
Analysis 2.16. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 16 Diastolic blood pressure at end of study (mmHg)
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 16 Diastolic blood pressure at end of study (mmHg)

|
Analysis 2.17. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 17 Side effects
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 17 Side effects

|

|

|

|

|
Analysis 2.18. Comparison 2 High vs low omega-3 fats (secondary outcomes), Outcome 18 Drop outs
Review: Omega 3 fatty acids for prevention and treatment of cardiovascular disease
Comparison: 2 High vs low omega-3 fats (secondary outcomes)
Outcome: 18 Drop outs

|

|
HISTORY
Protocol first published: Issue 2, 1999
Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 1 August 2004 | New citation required and conclusions have changed | Substantive amendment |
WHAT’S NEW
Last assessed as up-to-date: 31 July 2004.
| Date | Event | Description |
|---|---|---|
| 9 September 2008 | Amended | Converted to new review format. |
Footnotes
DECLARATIONS OF INTEREST None known
References to studies included in this review
- Almallah 1998 {published and unpublished data} .Almallah YZ, Ewen SW, El Tahir A, Mowat NA, Brunt PW, Sinclair TS, et al. Distal proctocolitis and n-3 polyunsaturated fatty acids (n-3 PUFAs): the mucosal effect in situ. Journal of Clinical Immunology. 2000;20(1):68–76. doi: 10.1023/a:1006698728816. [DOI] [PubMed] [Google Scholar]; Almallah YZ, Ewen SW, Mowat NA, Brunt PW, Sinclair TS, Heys SD, et al. Immunohistological modulation after nutritional supplementation with omega-3 essential fatty acids in patients with inflammatory bowel disease [abstract] British Juurnal of Surgery. 1998;85:690–1. [Google Scholar]; Almallah YZ, O’Hanrahan T, Richardson S, Mowat NA, Brunt PW, Sinclair TS, et al. Eicosapentaenoic acid and docosahexaenic acid in the treatment of patients with distal procto-colitis [abstract] British Journal of Surgery. 1996;83(Suppl 1):2. [Google Scholar]; Almallah YZ, O’Hanrahan T, Richardson S, Mowat NA, Brunt PW, Sinclair TS, et al. Inhibition of natural cytotoxicity with eicosapentaenoic and docosahexaenoic acids: possible mechanisms [abstract] British Journal of Surgery. 1996;83:685. [Google Scholar]; *; Almallah YZ, Richardson S, O’Hanrahan T, Mowat NA, Brunt PW, Sinclair TS, et al. Distal procto-colitis, natural cytotoxicity, and essential fatty acids. American Journal of Gastroenterology. 1998;93(5):804–9. doi: 10.1111/j.1572-0241.1998.229_a.x. [DOI] [PubMed] [Google Scholar]
- Bairati 1992 {published and unpublished data} .Bairati I, Roy L, Meyer F. Double-blind, randomized, controlled trial of fish oil supplements in prevention of recurrence of stenosis after coronary angioplasty. Circulation. 1992;85(3):950–6. doi: 10.1161/01.cir.85.3.950. [DOI] [PubMed] [Google Scholar]; Bairati I, Roy L, Meyer F. Effects of a fish oil supplement on blood pressure and serum lipids in patients treated for coronary artery disease. Canadian Journal of Cardiology. 1992;8(1):41–6. [PubMed] [Google Scholar]; Bairati I, Roy L, Meyer F. Measurement errors in standard visual analysis of coronary angiograms: consequences on clinical trials. Canadian Journal of Cardiology. 1993;9(3):225–30. [PubMed] [Google Scholar]; Meyer F, Bairati I, Roy L. Preventing restenosis after angioplasty with fish oil supplements. Cardiology Board Review. 1993;10(1):16+23–16+25. [Google Scholar]; Roy L, Bairati I, Meyer F, et al. Double blind randomised controlled trial of fish oil supplements in the prevention of restenoses after coronary angioplasty. Circulation. 1991;84(supplement II):365. doi: 10.1161/01.cir.85.3.950. [DOI] [PubMed] [Google Scholar]
- Bellamy 1992 {published data only} .*; Bellamy CM, Schofield PM, Faragher EB, Ramsdale DR. Can supplementation of diet with omega-3 polyunsaturated fatty acids reduce coronary angioplasty restenosis rate? European Heart Journal. 1992;13(12):1626–31. doi: 10.1093/oxfordjournals.eurheartj.a060115. [DOI] [PubMed] [Google Scholar]
- Belluzzi 1996 {published and unpublished data} .*; Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn’s disease. New England Journal of Medicine. 1996 Jun 13;334(24):1557–60. doi: 10.1056/NEJM199606133342401. [DOI] [PubMed] [Google Scholar]
- Bemelmans 2002 {published data only (unpublished sought but not used)} .Bemelmans WJ, Broer J, de Vries JH, Hulshof KF, May JF, Meyboom-De Jong B. Impact of Mediterranean diet education versus posted leaflet on dietary habits and serum cholesterol in a high risk population for cardiovascular disease. Public Health Nutrition. 2000;3(3):273–83. doi: 10.1017/s1368980000000318. [DOI] [PubMed] [Google Scholar]; *; Bemelmans WJ, Broer J, Feskens EJ, Smit AJ, Muskiet AJ, Lefrandt JD, et al. Effect of an increased intake of alpha-linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean alpha-linolenic enriched Groningen dietary intervention (MARGARIN) study. American Journal of Clinical Nutrition. 2002;75:221–7. doi: 10.1093/ajcn/75.2.221. [DOI] [PubMed] [Google Scholar]
- Bonnema 1995 {published and unpublished data} .*; Bonnema SJ, Jespersen LT, Marving J, Gregersen G. Supplementation with olive oil rather than fish oil increases small arterial compliance in diabetic patients. Diabetes, Nutrition and Metabolism Clinical and Experimental. 1995;8:81–7. [Google Scholar]
- Borchgrevink 1966 {published and unpublished data} .Borchgrevink CF, Berg KJ, Skaga E, Skjaeggestad O, Stormorken H. Effect of Linseed oil on platelet adhesiveness and bleeding time in patients with coronary heart disease. Lancet. 1965;ii:980–2. doi: 10.1016/s0140-6736(65)92842-4. [DOI] [PubMed] [Google Scholar]; *; Borchgrevink CF, Skaga E, Berg KJ, Skjaeggestad O. Absence of prophylactic effect of linolenic acid in patients with coronary heart-disease. Lancet. 1966;2(456):187–9. doi: 10.1016/s0140-6736(66)92474-3. [DOI] [PubMed] [Google Scholar]
- Brox 2001 {published and unpublished data} .*; Brox J, Olaussen K, Osterud B, Elvevoll EO, Bjornstad E, Brattebog G, et al. A long-term seal- and cod-liver-oil supplementation in hypercholesterolemic subjects. Lipids. 2001;36(1):7–13. doi: 10.1007/s11745-001-0661-4. [DOI] [PubMed] [Google Scholar]
- Burr (DART 1) 1989 {published and unpublished data} .Burr ML, Fehily AM. Fatty fish and heart disease: a randomized controlled trial. World review of nutrition and dietetics. 1991;66:306–12. doi: 10.1159/000419300. [DOI] [PubMed] [Google Scholar]; Burr ML, Fehily AM. Fish and the heart [letter] Lancet. 1989;ii:1451–2. [Google Scholar]; Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2(8666):757–61. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]; Burr ML, Fehily AM, Rogers S, Welsby E, King S, Sandham S. Diet and reinfarction trial (DART): design, recruitment, and compliance. European Heart Journal. 1989;10(6):558–67. doi: 10.1093/oxfordjournals.eurheartj.a059528. [DOI] [PubMed] [Google Scholar]; Burr ML, Holliday RM, Fehily AM, Whitehead PJ. Haematological prognostic indices after myocardial infarction: evidence from the diet and reinfarction trial (DART) European Heart Journal. 1992;13(2):166–70. doi: 10.1093/oxfordjournals.eurheartj.a060141. [DOI] [PubMed] [Google Scholar]; Burr ML, Sweetham PM, Fehily AM. Diet and reinfarction [letter] European Heart Journal. 1994;15(8):1152–3. doi: 10.1093/oxfordjournals.eurheartj.a060645. [DOI] [PubMed] [Google Scholar]; Fehily AM, Vaughan-Williams E, Shiels K, Williams AH, Horner M, Bingham G, et al. Factors influencing compliance with dietary advice: the Diet and Reinfarction Trial (DART) Journal of Human Nutrition and Dietetics. 1991;4:33–42. [Google Scholar]; Fehily AM, Vaughan-Williams E, Shiels K, Williams AH, Horner M, Bingham G, et al. The effect of dietary advice on nutrient intakes: evidence from the diet and reinfarction trial (DART) Journal of Human Nutrition & Dietetics. 1989;2:4235. [Google Scholar]; Ness AR, Hughes J, Elwood PC, Whitley E, Smith GD, Burr ML. The long-term effect of dietary advice in men with coronary disease: follow-up of the Diet and Reinfarction Trial (DART) European Journal of Clinical Nutrition. 2002;56(6):512–8. doi: 10.1038/sj.ejcn.1601342. [DOI] [PubMed] [Google Scholar]; *; Ness AR, Whitley E, Burr ML, Elwood PC, Smith GD, Ebrahim S. The long-term effect of advice to eat more fish on blood pressure in men with coronary disease: results from the diet and reinfarction trial. Journal of Human Hypertension. 1999;13(11):729–33. doi: 10.1038/sj.jhh.1000913. [DOI] [PubMed] [Google Scholar]
- Burr (DART 2) 2003 {published and unpublished data} .Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. European Journal of Clinical Nutrition. 2003;57(2):193–200. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]; Ness AR, Ashfield-Watt PAL, Whiting JM, Smith GD, Hughes J, Burr ML. The long-term effect of dietary advice on the diet of men with angina: the diet and angina randomized trial. Journal of Human Nutrition and Dietetics. 2004;17:1–3. doi: 10.1111/j.1365-277X.2004.00506.x. [DOI] [PubMed] [Google Scholar]; *; Ness AR, Gallacher JE, Bennett PD, Gunnell DJ, Rogers PJ, Kessler D, et al. Advice to eat fish and mood: a randomised controlled trial in men with angina. Nutritional Neuroscience. 2003;6(1):63–5. doi: 10.1080/1028415021000056069. [DOI] [PubMed] [Google Scholar]
- Connor 1993 {published and unpublished data} .Connor WE, Prince MJ, Ullmann D, Riddle M, Hatcher L, Smith FE, et al. The hypotriglyceridemic effect of fish oil in adult-onset diabetes without adverse glucose control. Annals of the New York Academy of Sciences. 1993;683:337–40. doi: 10.1111/j.1749-6632.1993.tb35725.x. [DOI] [PubMed] [Google Scholar]; *; Ullmann D, Prince MJ, Riddle M, Connor WE, Smith F, Wilson D. The effects of dietary fish oil versus olive oil in diabetic patients [abstract] Arteriosclerosis. 1990;10:881A. [Google Scholar]
- Dehmer 1998 {published and unpublished data} .*; Dehmer GJ, Popma JJ, van den Berg MD, Eichhorn EJ, Prewitt JB, Campbell WB, et al. Reduction in the rate of early restenosis after coronary angioplasty by a diet supplemented with n-3 fatty acids. New Wngland Journal of Medicine. 1988;319(12):733–40. doi: 10.1056/NEJM198809223191201. [DOI] [PubMed] [Google Scholar]
- Dry 1991 {published and unpublished data} .*; Dry J, Vincent D. Effect of a fish oil diet on asthma: results of a 1-year double-blind study. International archives of allergy and applied immunology. 1991;95(2-3):156–7. doi: 10.1159/000235421. [DOI] [PubMed] [Google Scholar]
- Eritsland 1996 {published and unpublished data} .Eritsland J, Arnesen H, Berg K, Seljeflot I, Abdelnoor M. Serum Lp(a) lipoprotein levels in patients with coronary artery disease and the influence of long-term n-3 fatty acid supplementation. Scandinavian journal of clinical and laboratory investigation. 1995;55(4):295–300. doi: 10.3109/00365519509104966. [DOI] [PubMed] [Google Scholar]; Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Effect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft patency. American Journal of Cardiology. 1996;77(1):31–6. doi: 10.1016/s0002-9149(97)89130-8. [DOI] [PubMed] [Google Scholar]; Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Effect of supplementation with n-3 fatty acids on graft patency in patients undergoing coronary artery bypass operation. Results from SHOT study [abstract] European Heart Journal. 1994;15:29. [Google Scholar]; Eritsland J, Arnesen H, Seljeflot I, Hostmark AT. Long-term metabolic effects of n-3 polyunsaturated fatty acids in patients with coronary artery disease. American Journal of Clinical Nutrition. 1995;61(4):831–6. doi: 10.1093/ajcn/61.4.831. [DOI] [PubMed] [Google Scholar]; Eritsland J, Arnesen H, Seljeflot I, Kierulf P. Long-term effects of n-3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood coagulation & fibrinolysis. 1995;6(1):17–22. doi: 10.1097/00001721-199502000-00003. [DOI] [PubMed] [Google Scholar]; Eritsland J, Seljeflot I, Abdelnoor M, Arnesen H. Long-term influence of omega-3 fatty acids on fibrinolysis, fibrinogen, and serum lipids. Fibrinolysis. 1994;8(2):120–5. [Google Scholar]; Eritsland J, Seljeflot I, Abdelnoor M, Arnesen H, Torjesen PA. Long-term effects of n-3 fatty acids on serum lipidsand glycaemic control. Scandinavian journal of clinical and laboratory investigation. 1994;54(4):273–80. doi: 10.3109/00365519409087522. [DOI] [PubMed] [Google Scholar]; Eritsland J, Seljeflot I, Arnesen H, Abdelnoor M. Long-term effects of fish oil supplementation in patients with coronary artery disease: influence on lipoproteins, coagulation and fibrinolysis [abstract] Thrombosis Research. 1992;65:75. [Google Scholar]; Eritsland J, Seljeflot I, Arnesen H, Abdelnoor M. Long-term influence of omega-3 fatty acids on fibrinolysis, fibrinogen, and serum lipids [abstract] Thrombosis and Haemostasis. 1993;69:1065. [Google Scholar]; *; Eritsland J, Seljeflot I, Arnesen H, Westvik AB, Kierulf P. Effect of long-term, moderate-dose supplementation with omega-3 fatty acids on monocyte procoagulant activity and release of interleukin-6 in patients with coronary artery disease. Thromb Res. 1995;77(4):337–46. doi: 10.1016/0049-3848(95)93837-p. [DOI] [PubMed] [Google Scholar]
- Franzen 1993 {published and unpublished data} .Franzen D. Unknown. Catheterization and Cardiovascular Diagnosis. 1993;28:301–10. doi: 10.1002/ccd.1810280407. [DOI] [PubMed] [Google Scholar]; *; Franzen D, Geisel J, Hopp HW, Oette K, Hilger HH. Long-term effects of low dosage fish oil on serum lipids and lipoproteins [Langzeiteffekte von niedrigdosiertem fischol auf serumlipide und lipoproteine] Medizinische Klinik. 1993;88(3):134–8. [PubMed] [Google Scholar]
- Geusens 1994 {published data only} .*; Geusens P, Wouters C, Nijs J, Jiang Y, Dequeker J. Long-term effect of omega-3 fatty acid supplementation in active rheumatoid arthritis. A 12-month, double-blind, controlled study. Arthritis and rheumatism. 1994;37(6):824–9. doi: 10.1002/art.1780370608. [DOI] [PubMed] [Google Scholar]
- GISSI-P 1999 {published data only} .Franzosi MG, Brunetti M, Marchioli R, Marfisi RM, Tognoni G, Valagussa F, GISSI-Prevenzione I. Cost-effectiveness analysis of n-3 polyunsaturated fatty acids (PUFA) after myocardial infarction: results from Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto (GISSI)-Prevenzione Trial. Pharmacoeconomics. 2001;19(4):411–20. doi: 10.2165/00019053-200119040-00008. [DOI] [PubMed] [Google Scholar]; GISSI-Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]; Marchioli R. Treatment with n-3 polyunsaturated fatty acids after myocardial infarction: results of GISSI-Prevenzione Trial. European Heart Journal Supplements. 2001;3(Supplement D):D85–D97. [Google Scholar]; Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio DDMR, Franzosi MG, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]; Marchioli R, Di Pasquale A. The biochemical, pharmacological and epidemiological reference picture of the GISSI-Prevention. The Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico. Giornale Italiano di Cardiologia. 1993;23(9):933–64. [PubMed] [Google Scholar]; *; Marchioli R, Valagussa F. The results of the GISSI Prevenzione trial in the general framework of secondary prevention. European Heart Journal. 2000;21(12):949–52. doi: 10.1053/euhj.1999.1971. [DOI] [PubMed] [Google Scholar]
- Greenfield 1993 {published and unpublished data} .*; Greenfield SM, Green AT, Teare JP, Jenkins AP, Punchard NA, Ainley CC, et al. A randomized controlled study of evening primrose oil and fish oil in ulcerative colitis. Alimentary Pharmacology & Therapeutics. 1993;7(2):159–66. doi: 10.1111/j.1365-2036.1993.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Hawthorne 1992 {published and unpublished data} .*; Hawthorne AB, Daneshmend TK, Hawkey CJ, Belluzzi A, Everitt SJ, Holmes GK, et al. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut. 1992;33(7):922–8. doi: 10.1136/gut.33.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen 1999A {published data only} .Johansen O, Brekke M, Seljeflot I, Abdelnoor M, Arnesen H. N-3 fatty acids do not prevent restenosis after coronary angioplasty: results from the CART study. Coronary Angioplasty Restenosis Trial. Journal of the American College of Cardiology. 1999;33(6):1619–26. doi: 10.1016/s0735-1097(99)00054-6. [DOI] [PubMed] [Google Scholar]; Johansen O, Seljeflot I, Hostmark AT, Arnesen H. The effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arteriosclerosis, Thrombosis & Vascular Biology. 1999;19(7):1681–6. doi: 10.1161/01.atv.19.7.1681. [DOI] [PubMed] [Google Scholar]; *; Seljeflot I, Johansen O, Arnesen H, Eggesbo JB, Westvik AB, Kierulf P. Procoagulant activity and cytokine expression in whole blood cultures from patients with atherosclerosis supplemented with omega-3 fatty acids. Thrombosis & Haemostasis. 1999;81(4):566–70. [PubMed] [Google Scholar]
- Katan 1997 {published and unpublished data} .Blok WL, Deslypere JP, Demacker PM, van-der-Ven JJ, Hectors MC, Van-Der MJ, Katan MB. Pro- and anti-inflammatory cytokines in healthy volunteers fed various doses of fish oil for 1 year. European Journal of Clinical Investigation. 1997;27(12):1003–8. doi: 10.1046/j.1365-2362.1997.2240775.x. [DOI] [PubMed] [Google Scholar]; Deslypere JP. Influence of supplementation with N-3 fatty acids on different coronary risk factors in men--a placebo controlled study. Verhandelingen - Koninklijke Academie voor Geneeskunde van Belgie. 1992;54(3):189–216. [PubMed] [Google Scholar]; *; Katan MB, Deslypere JP, van BA, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. Journal of lipid research. 1997;38(10):2012–22. [PubMed] [Google Scholar]
- Kaul 1992 {published and unpublished data} .Kaul U, Sanghvi S, Bahl VK, Dev V, Wasir HS. Fish oil supplements for prevention of restenosis after coronary angioplasty. International Journal of Cardiology. 1992;35(1):87–93. doi: 10.1016/0167-5273(92)90059-c. [DOI] [PubMed] [Google Scholar]
- Lau 1993 {published and unpublished data} .Lau CS, McMahon H, Morley KD, Belch JJF. Effects of Maxepa on non-steroidal anti-inflammatory drug useage in patients with mild rheumatoid arthritis [abstract] British Journal of Rheumatology. 1991;30:137. doi: 10.1093/rheumatology/32.11.982. [DOI] [PubMed] [Google Scholar]; *; Lau CS, Morley KD, Belch JJ. Effects of fish oil supplementation on non-steroidal anti-inflammatory drug requirement in patients with mild rheumatoid arthritis--a double-blind placebo controlled study. British Journal of Rheumatology. 1993;32(11):982–9. doi: 10.1093/rheumatology/32.11.982. [DOI] [PubMed] [Google Scholar]
- Lau 1995 {published and unpublished data} .*; Lau CS, McLaren M, Belch JJ. Effects of fish oil on plasma fibrinolysis in patients with mild rheumatoid arthritis. Clinical and Experimental Rheumatology. 1995;13(1):87–90. [PubMed] [Google Scholar]
- Leaf 1994 {published data only} .Leaf A, Jorgensen MB, Jacobs AK, Cote G, Schoenfeld DA, Scheer J, et al. Do fish oils prevent restenosis after coronary angioplasty? Circulation. 1994;90(5):2248–57. doi: 10.1161/01.cir.90.5.2248. [DOI] [PubMed] [Google Scholar]; *; Mehta VY, Jorgensen MB, Raizner AE, Wolde TG, Mahrer PR, Mansukhani P. Spontaneous regression of restenosis: an angiographic study. Journal of the American College of Cardiology. 1995;26(3):696–702. doi: 10.1016/0735-1097(95)00335-2. [DOI] [PubMed] [Google Scholar]
- Loeschke 1996 {published and unpublished data} .*; Loeschke K, Ueberschaer B, Pietsch A, Gruber E, Ewe K, Wiebecke B, et al. n-3 fatty acids only delay early relapse of ulcerative colitis in remission. Digestive diseases and sciences. 1996;41(10):2087–94. doi: 10.1007/BF02093614. [DOI] [PubMed] [Google Scholar]
- Lorenz-Meyer 1996 {published and unpublished data} .*; Lorenz-Meyer H, Bauer P, Nicolay C, Schulz B, Purrmann J, Fleig WE, et al. Omega-3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn’s disease. A randomized controlled multicenter trial. Study Group Members (German Crohn’s Disease Study Group) Scandinavian Journal of Gastroenterology. 1996;31(8):778–85. doi: 10.3109/00365529609010352. [DOI] [PubMed] [Google Scholar]
- Malaguarnera 1999 {published data only} .*; Malaguarnera M, Restuccia N, Di Fazio I, Panebianco MP, Gulizin G, Giugno I. Fish oil treatment of interferon-alpha-induced dyslipidaemia: Study in patients with chronic hepatitis C. Biodrugs. 1999;11(4):285–91. doi: 10.2165/00063030-199911040-00007. [DOI] [PubMed] [Google Scholar]
- Maresta 2002 {published and unpublished data} .*; Maresta A, Balduccelli M, Varani E, Marzilli M, Galli C, Heiman F, et al. Prevention of postcoronary angioplasty restenosis by omega-3 fatty acids: main results of the Esapent for prevention of restenosis Italian study (ESPRIT) American Heart Journal. 2002;143(6):1–10. doi: 10.1067/mhj.2002.121805. [DOI] [PubMed] [Google Scholar]
- Mate-Jimenez 1991 {published and unpublished data} .*; Mate J, Castanos R, Garcia-Semaniego J, Pajares JM. Does dietary fish oil maintain the remission of crohn’s disease: a study case control [abstract] Gastroenterology. 1991;100:A228. (abstract) [Google Scholar]
- Milner 1989 {published and unpublished data} .Milner MR, Gallino RA, Leffingwell A, Pichard AD, Brooks RS, Rosenberg J, et al. Usefulness of fish oil supplements in preventing clinical evidence of restenosis after percutaneous transluminal coronary angioplasty. American Journal of Cardiology. 1989;64(5):294–9. doi: 10.1016/0002-9149(89)90522-5. [DOI] [PubMed] [Google Scholar]; *; Milner MR, Gallino RA, Leffingwell A, Pichard AD, Rosenberg J, Lindsay J. High dose omega-3 fatty acid supplementation reduces clinical restenosis after coronary angioplasty [abstract] Circulation. 1988;78:II634. [Google Scholar]
- Natvig 1968 {published data only} .*; Natvig H, Borchgrevink CF, Dedichen J, Owren PA, Schiotz EH, Westlund K. A controlled trial of the effect of linolenic acid on incidence of coronary heart disease. The Norwegian vegetable oil experiment of 1965-66. Scandinavian Journal of Clinical & Laboratory Investigation - Supplement. 1968;105:1–20. [PubMed] [Google Scholar]
- Nilsen 2001 {published and unpublished data} .*; Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. American Journal of Clinical Nutrition. 2001;74(1):50–6. doi: 10.1093/ajcn/74.1.50. [DOI] [PubMed] [Google Scholar]
- Nye 1990 {published data only} .Ilsey CDJ, Nye ER, Sutherland W, Ram J, Ablett MB. Randomised placebo controlled trial of MAXEPA and aspirin/persantin after successful coronary angioplasty [abstract] Australian & New Zealand Journal of Medicine. 1987;17:559. [Google Scholar]; *; Nye ER, Ablett MB, Robertson MC, Ilsley CD, Sutherland WH. Effect of eicosapentaenoic acid on restenosis rate, clinical course and blood lipids in patients after percutaneous transluminal coronary angioplasty. Australian and New Zealand Journal of Medicine. 1990;20(4):549–52. doi: 10.1111/j.1445-5994.1990.tb01311.x. [DOI] [PubMed] [Google Scholar]
- Reis 1991 {published and unpublished data} .Reis GJ, Boucher TM, McCabe CH. Results of a randomised, double blind placebo controlled trial of fish oil for prevention of restenosis after PTCA [abstract] Circulation. 1988;78(supplement 2):291. [Google Scholar]; Reis GJ, Boucher TM, Sipperley ME, McCabe CH, Silverman DI, Pasternak RC. Fish oil supplements raise LDL cholesterol: results of a blinded, placebo-controlled trial in patients with coronary disease [abstract] Circulation. 1988;78:II385. [Google Scholar]; Reis GJ, Boucher TM, Sipperly ME, Silverman DI, McCabe CH, Baim DS, et al. Randomised trial of fish oil for prevention of restenosis after coronary angioplasty. Lancet. 1989;2(8656):177–81. doi: 10.1016/s0140-6736(89)90370-x. [DOI] [PubMed] [Google Scholar]; Reis GJ, Kuntz RE, Silverman DI, Pasternak RC. Effects of serum lipid levels on restenosis after coronary angioplasty. American Journal of Cardiology. 1991;68(15):1431–5. doi: 10.1016/0002-9149(91)90275-p. [DOI] [PubMed] [Google Scholar]; Reis GJ, Silverman DI, Boucher TM, Sipperly ME, Horowitz GL, Sacks FM, et al. Effects of two types of fish oil supplements on serum lipids and plasma phospholipid fatty acids in coronary artery disease. American Journal of cardiology. 1990;66(17):1171–5. doi: 10.1016/0002-9149(90)91093-l. [DOI] [PubMed] [Google Scholar]; *; Sipperly ME, Reis GJ, Boucher TM, Pasternak RC. Predictors of patient compliance in a placebo-controlled trial of fish oil [abstract] Circulation. 1988;78:II614. [Google Scholar]
- Rossing 1996 {published and unpublished data} .Myrup B, Rossing P, Jensen T, Parving H-H, Holmer G, Gram J, Kluft C, Jespersen J. Lack of effect of fish oil supplementation on coagulation and transcapillary escape rate of albumin in insulin-dependent diabetic patients with diabetic nephropathy. Scandinavian Journal of Clinical & Laboratory Investigation. 2001;61(5):349–356. doi: 10.1080/003655101316911387. [DOI] [PubMed] [Google Scholar]; *; Rossing P, Hansen BV, Nielsen FS, Myrup B, Holmer G, Parving HH. Fish oil in diabetic nephropathy. Diabetes Care. 1996;19(11):1214–1219. doi: 10.2337/diacare.19.11.1214. [DOI] [PubMed] [Google Scholar]
- Sacks (HARP) 1995 {published data only} .*; Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC. Controlled trial of fish oil for regression of human coronary atherosclerosis. HARP Research Group. J Am Coll Cardiol. 1995;25(7):1492–1498. doi: 10.1016/0735-1097(95)00095-l. [DOI] [PubMed] [Google Scholar]
- Sacks (TOHP 1) 1994 {published data only} .He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. Seven-year incidence of hypertension in a cohort of middle-aged African Americans and whites. Hypertension. 1998;31(5):1130–1135. doi: 10.1161/01.hyp.31.5.1130. [DOI] [PubMed] [Google Scholar]; Meilahn EN, Kuller LH, Kiss JE, Sacks FM. Coagulation parameters among healthy adults taking fish oil versus placebo [abstract] Arteriosclerosis. 1990;10:916A. [Google Scholar]; Sacks FM, Hebert P, Appel LJ, Borhani NO, Applegate WB, Cohen JD, Cutler JA, Kirchner KA, Kuller LH, Roth KJ. Short report: the effect of fish oil on blood pressure and high-density lipoprotein-cholesterol levels in phase I of the Trials of Hypertension Prevention. J Hypertens. 1994;12(2):209–213. et a. [PubMed] [Google Scholar]; Sacks FM, Hebert P, Appel LJ, Borhani NO, Applegate WB, Cohen JD, Cutler JA, Kirchner KA, Kuller LH, Roth KJ, Taylor JO, Hennekens CH. The effect of fish oil on blood pressure and high-density lipoprotein-cholesterol levels in phase I of the trials of hypertension prevention. J Hypertens Suppl. 1994;12(7):S23–S31. [PubMed] [Google Scholar]; Satterfield S, Borhani NO, Whelton P, Goodwin L, Brinkmann C, Charleston J, Corkery BW, Dolan L, Hataway H, Hertert S, Lakatos E, Milas NC, Morris MC, King N. Recruitment for phase I of the Trials of Hypertension Prevention. Am J Prev Med. 1993;9(4):237–243. [PubMed] [Google Scholar]; Trials of Hypertension Prevention Collaborative Research Group The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;267(9):1213–1220. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]; *; Whelton PK, Kumanyika SK, Cook NR, Cutler JA, Borhani NO, Hennekens CH, Kuller LH, Langford H, Jones DW, Satterfield S, Lasser NL, Cohen JD. Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Research Group. Am J Clin Nutr. 1997;65(2 Suppl):652S–660S. doi: 10.1093/ajcn/65.2.652S. [DOI] [PubMed] [Google Scholar]
- Sarkkinen 1998 {published and unpublished data} .Makinen E, Uusitupa MI, Pietinen P, Aro A, Penttila I. Long term effects of three fat modified diets on serum lipids in free living hypercholesterolemic subjects [abstract] Eur Heart J. 1991;12:168–168. [Google Scholar]; Sarkkinen ES, Agren JJ, Ahola I, Ovaskainen ML, Uusitupa MI. Fatty acid composition of serum cholesterol esters, and erythrocyte and platelet membranes as indicators of long-term adherence to fat-modified diets. American Journal of Clinical Nutrition. 1994;59(2):364–370. doi: 10.1093/ajcn/59.2.364. [DOI] [PubMed] [Google Scholar]; Sarkkinen ES, Uusitupa MI, Gylling H, Miettinen TA. Fatmodified diets influence serum concentrations of cholesterol precursors and plant sterols in hypercholesterolemic subjects. Metabolism. 1998;47(6):744–750. doi: 10.1016/s0026-0495(98)90040-3. [DOI] [PubMed] [Google Scholar]; *; Sarkkinen ES, Uusitupa MI, Pietinen P, Aro A, Ahola I, Penttila I, Kervinen K, Kesaniemi YA. Long-term effects of three fat-modified diets in hypercholesterolemic subjects. Atherosclerosis. 1994;105:9–23. doi: 10.1016/0021-9150(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Selvais 1995 {published and unpublished data} .*; Selvais PL, Ketelslegers JM, Buysschaert M, Hermans MP. Plasma endothelin-1 immunoreactivity is increased following long-term dietary supplementation with omega-3 fatty acids in microalbuminuric IDDM patients [letter] Diabetologia. 1995;38:253–253. doi: 10.1007/BF00400104. [DOI] [PubMed] [Google Scholar]
- Shimizu 1995 {published and unpublished data} .*; Shimizu H, Ohtani K, Tanaka Y, Sato N, Mori M, Shimomura Y. Long-term effect of eicosapentaenoic acid ethyl (EPA-E) on albuminuria of non-insulin dependent diabetic patients. Diabetes Res Clin Pract. 1995;28(1):35–40. doi: 10.1016/0168-8227(95)01056-j. [DOI] [PubMed] [Google Scholar]
- Singh 1997 {published and unpublished data} .*; Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival--4. Cardiovasc Drugs Ther. 1997;11(3):485–491. doi: 10.1023/a:1007757724505. [DOI] [PubMed] [Google Scholar]
- Sirtori 1998 {published and unpublished data} .Maffettone A. Long-term effects (six months) of omega-3 polyunsaturated fatty acids on insulin sensitivity and lipid metabolism in patients with type 2 diabetes and hypertriglycaeridemia. G Ital Diabetol. 1996;16(4):185–193. [Google Scholar]; Patti L, Maffettone A, Iovine C, Marino LD, Annuzzi G, Riccardi G, Rivellese AA. Long-term effects of fish oil on lipoprotein subfractions and low density lipoprotein size in non-insulin-dependent diabetic patients with hypertriglyceridemia. Atherosclerosis. 1999;146(2):361–367. doi: 10.1016/s0021-9150(99)00149-5. [DOI] [PubMed] [Google Scholar]; Rivellese AA, Maffettone A, Iovine C, Di ML, Annuzzi G, Mancini M, Riccardi G. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care. 1996;19(11):1207–1213. doi: 10.2337/diacare.19.11.1207. [DOI] [PubMed] [Google Scholar]; Sirtori CR, Crepaldi G, Manzato E, Mancini M, Rivellese A, Paoletti R, Pazzucconi F, Pamparana F, Stragliotto E. One-year treatment with ethyl esters of n-3 fatty acids in patients with hypertriglyceridemia and glucose intolerance reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alterations. Atherosclerosis. 1998;137(2):419–427. doi: 10.1016/s0021-9150(97)00298-0. [DOI] [PubMed] [Google Scholar]; *; Sirtori CR, Paoletti R, Mancini M, Crepaldi G, Manzato E, Rivellese A, Pamparana F, Stragliotto E. N-3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian Fish Oil Multicenter Study. American Journal of Clinical Nutrition. 1997;65:1874–1881. doi: 10.1093/ajcn/65.6.1874. [DOI] [PubMed] [Google Scholar]
- Skoldstam 1992 {published and unpublished data} .*; Skoldstam L, Borjesson O, Kjallman A, Seiving B, Akesson B. Effect of six months of fish oil supplementation in stable rheumatoid arthritis. A double-blind, controlled study. Scand J Rheumatol. 1992;21(4):178–185. doi: 10.3109/03009749209099218. [DOI] [PubMed] [Google Scholar]
- Terano 1999 {published and unpublished data} .*; Terano T, Fujishiro S, Ban T, Yamamoto K, Tanaka T, Noguchi Y, Tamura Y, Yazawa K, Hirayama T. Docosahexaenoic acid supplementation improves the moderately severe dementia from thrombotic cerebrovascular diseases. Lipids. 1999;34:S345–S346. doi: 10.1007/BF02562338. [DOI] [PubMed] [Google Scholar]
- Thien 1993 {published and unpublished data} .*; Thien FC, Mencia HJ, Lee TH. Dietary fish oil effects on seasonal hay fever and asthma in pollen-sensitive subjects. Am Rev Respir Dis. 1993;147(5):1138–1143. doi: 10.1164/ajrccm/147.5.1138. [DOI] [PubMed] [Google Scholar]
- Veale 1994 {published data only (unpublished sought but not used)} .*; Veale DJ, Torley HI, Richards IM, O’Dowd A, Fitzsimons C, Belch JJ, Sturrock RD. A double-blind placebo controlled trial of Efamol Marine on skin and joint symptoms of psoriatic arthritis. Br J Rheumatol. 1994;33(10):954–958. doi: 10.1093/rheumatology/33.10.954. [DOI] [PubMed] [Google Scholar]
- von Schacky 1999 {published and unpublished data} .*; von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130(7):554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- z7Cs Finland Oomen {published data only} .Oomen CM, Feskens EJ, Rasanen L, Fidanza F, Nissinen AM, Menotti A, Kok FJ, Kromhout D. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. American Journal of Epidemiology. 2000;151(10):999–1006. doi: 10.1093/oxfordjournals.aje.a010144. [DOI] [PubMed] [Google Scholar]
- z7Cs Italy Oomen 00 {published data only} .Oomen CM, Feskens EJ, Rasanen L, Fidanza F, Nissinen AM, Menotti A, Kok FJ, Kromhout D. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. American Journal of Epidemiology. 2000;151(10):999–1006. doi: 10.1093/oxfordjournals.aje.a010144. [DOI] [PubMed] [Google Scholar]
- z7Cs NL Oomen 2000 {published data only} .Oomen CM, Feskens EJ, Rasanen L, Fidanza F, Nissinen AM, Menotti A, Kok FJ, Kromhout D. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. American Journal of Epidemiology. 2000;151(10):999–1006. doi: 10.1093/oxfordjournals.aje.a010144. [DOI] [PubMed] [Google Scholar]
- zAlzheimer’s Kyle 99 {published data only} .Kyle DJ, Schaefer E, Patton G, Beiser A. Low serum docosahexaenoic acid is a significant risk factor for Alzheimer’s dementia. Lipids. 1999;34(6 SUPPL.):S245. doi: 10.1007/BF02562306. [DOI] [PubMed] [Google Scholar]
- zARIC Zheng 1999 {published data only} .Zheng ZJ, Folsom AR, Ma J, Arnett DK, McGovern PG, Eckfeldt JH. Plasma fatty acid composition and 6-year incidence of hypertension in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. American Journal of Epidemiology. 1999;150(5):492–500. doi: 10.1093/oxfordjournals.aje.a010038. [DOI] [PubMed] [Google Scholar]
- zATBC Pietinen 1997 {published data only} .Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, Virtamo J. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. American Journal of Epidemiology. 1997;145(10):876–887. doi: 10.1093/oxfordjournals.aje.a009047. [DOI] [PubMed] [Google Scholar]
- zATBC Tornwall 2000 {published data only} .Tornwall ME, Virtamo J, Haukka JK, Aro A, Albanes D, Huttunen JK. Prospective study of diet, lifestyle, and intermittent claudication in male smokers. American Journal of Epidemiology. 2000;151(9):892–901. doi: 10.1093/oxfordjournals.aje.a010293. [DOI] [PubMed] [Google Scholar]
- zEuroaspire Erkkila {published data only} .Erkkila AT, Lehto S, Pyorala K, Uusitupa MIJ. n-3 fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. American Journal of Clinical Nutrition. 2003;78:65–71. doi: 10.1093/ajcn/78.1.65. [DOI] [PubMed] [Google Scholar]
- zFinnish Miettinen {published data only} .Miettinen TA, Naukkarinen V, Huttunen JK, Mattila S, Kumlin T. Fatty-acid composition of serum lipids predicts myocardial infarction. British Medical Journal Clinical Research Ed. 1982;285(6347):993–996. doi: 10.1136/bmj.285.6347.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zHPFS Ascherio 1995 {published data only} .Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. [see comments] New England Journal of Medicine. 1995;332(15):977–982. doi: 10.1056/NEJM199504133321501. [DOI] [PubMed] [Google Scholar]
- zHPFS Giovannucci 93 {published data only} .Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CC, Willett WC. A prospective study of dietary fat and risk of prostate cancer. [see comments] Journal of the National Cancer Institute. 1993;85(19):1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- zHPFS He 2002 {published data only} .He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willet WC, Ascherio A. Fish consumption and risk of stroke in men. JAMA. 2002;288(24):3130–3136. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- zIWHS Meyer 2001 {published data only} .Meyer KA, Kushi LH, Jacobs DR, Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. [see comments] Diabetes Care. 2001;24(9):1528–1535. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- zJanus Harvei 1997 {published data only} .Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. International Journal of Cancer. 1997;71(4):545–551. doi: 10.1002/(sici)1097-0215(19970516)71:4<545::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- zKuopio Rissanen 00 {published data only} .Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaenoic acid and docosapentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation. 2000;102(22):2677–2679. doi: 10.1161/01.cir.102.22.2677. [DOI] [PubMed] [Google Scholar]
- zLondon Kingsbury 94 {published data only} .Kingsbury KJ, Brett C, Stovold R, Chapman A, Anderson J, Morgan DM. Abnormal fatty acid composition and human atherosclerosis. Postgraduate Medical Journal. 1974;50(585):425–440. doi: 10.1136/pgmj.50.585.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zMenarche Maclure 91 {published data only} .Maclure M, Travis LB, Willett W, MacMahon B. A prospective cohort study of nutrient intake and age at menarche. American Journal of Clinical Nutrition. 1991;54(4):649–656. doi: 10.1093/ajcn/54.4.649. [DOI] [PubMed] [Google Scholar]
- zMRFIT Dolecek 1991 {published data only} .*; Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial. Proc Soc Exp Biol Med. 1992;200:177–182. doi: 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]; Dolecek TA, Granditis G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT) World Review of Nutrition & Dietetics. 1991;66:205–216. doi: 10.1159/000419291. [DOI] [PubMed] [Google Scholar]
- zNHS & HPFS Cho 2001 {published data only} .Cho E, Hung S, Willett WC, Spiegelman D, Rimm EB, Seddon JM, Colditz GA, Hankinson SE. Prospective study of dietary fat and the risk of age-related macular degeneration. American Journal of Clinical Nutrition. 2001;73(2):209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- zNHS Holmes 1999 {published data only} .Holmes MD, Hunter DJ, Colditz GA, Stampfer MJ, Hankinson SE, Speizer FE, Rosner B, Willett WC. Association of dietary intake of fat and fatty acids with risk of breast cancer. [see comments] JAMA. 1999;281(10):914–920. doi: 10.1001/jama.281.10.914. [DOI] [PubMed] [Google Scholar]
- zNHS Hu 1999 {published data only} .Hu FB, Stampfer MJ, Manson JE, Rimm EB, Wolk A, Colditz GA, Hennekens CH, Willett WC. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. [see comments] American Journal of Clinical Nutrition. 1999;69(5):890–897. doi: 10.1093/ajcn/69.5.890. [DOI] [PubMed] [Google Scholar]
- zNHS Hu 2002 {published data only} .Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287(14):1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- zNHS Hu 2003 {published data only} .Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107(14):1852–1857. doi: 10.1161/01.CIR.0000062644.42133.5F. [DOI] [PubMed] [Google Scholar]
- zNHS I&II Zhang 2000 {published data only} .Zhang SM, Willett WC, Hernan MA, Olek MJ, Ascherio A. Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. American Journal of Epidemiology. 2000;152:1056–1064. doi: 10.1093/aje/152.11.1056. [DOI] [PubMed] [Google Scholar]
- zNHS Iso 2001 {published data only} .Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285(3):304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- zNHS Troisi 1995 {published data only} .Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. [see comments] American Journal of Respiratory & Critical Care Medicine. 1995;151(5):1401–1408. doi: 10.1164/ajrccm.151.5.7735592. [DOI] [PubMed] [Google Scholar]
- zNHSSN Egeland 2001 {published data only} .Egeland GM, Meyer HE, Selmer R, Tverdal A, Vollset SE. Cod liver oil consumption, smoking, and coronary heart disease mortality: three counties, Norway. International Journal of Circumpolar Health. 2001;60(2):143–149. [PubMed] [Google Scholar]
- zNLCS Schuurman 1999 {published data only} .Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86(6):1019–1027. [PubMed] [Google Scholar]
- zNorwegian Veierod A {published data only} .Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. International Journal of Cancer. 1997;71(4):600–604. doi: 10.1002/(sici)1097-0215(19970516)71:4<600::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- zNorwegian Veierod B {published data only} .Veierod MB, Laake P, Thelle DS. Dietary fat intake and risk of lung cancer: A prospective study of 51,452 Norwegian men and women. European Journal of Cancer Prevention. 1997;6(6):540–549. doi: 10.1097/00008469-199712000-00009. [DOI] [PubMed] [Google Scholar]
- zNYUWH Kato 1997 {published data only} .Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women’s Health Study. Nutrition & Cancer. 1997;28(3):276–281. doi: 10.1080/01635589709514588. [DOI] [PubMed] [Google Scholar]
- zPHS Albert 1998 {published data only} .Albert CM, Hennekens CH, O’Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish consumption and risk of sudden cardiac death. Journal of the American Medical Association. 1998;279(1):23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- zPHS Albert 2002 {published data only} .Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346(15):1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- zPHS Gann 1994 {published data only} .Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. [see comments]. [erratum appears in J Natl Cancer Inst 1994 May 4;86(9): 728] Journal of the National Cancer Institute. 1994;86(4):281–286. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- zPHS Guallar 1995 {published data only} .Guallar E, Hennekens CH, Sacks FM, Willett WC, Stampfer MJ. A prospective study of plasma fish oil levels and incidence of myocardial infarction in U.S. male physicians. Journal of the American College of Cardiology. 1995;25(2):387–394. doi: 10.1016/0735-1097(94)00370-6. [DOI] [PubMed] [Google Scholar]
- zPHS Morris 1995 {published data only} .Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH. Fish consumption and cardiovascular disease in the physicians’ health study: A prospective study. American Journal of Epidemiology. 1995;142(2):166–175. doi: 10.1093/oxfordjournals.aje.a117615. [DOI] [PubMed] [Google Scholar]
- zShanghai Yuan 2001 {published data only} .Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. American Journal of Epidemiology. 2001;154(9):809–816. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- zSMSC Terry 2001 {published data only} .Terry P, Bergkvist L, Holmberg L, Wolk A. No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiology, Biomarkers & Prevention. 2001;10(8):913–914. [PubMed] [Google Scholar]
- zUmea Chajes 1999 {published data only} .Chajes V, Hulten K, Van Kappel AL, Winkvist A, Kaaks R, Hallmans G, Lenner PG, Riboli E. Fatty acid composition in serum phospholipids and risk of breast cancer: A prospective cohort study in northern Sweden. Lipids. 1999;34(6 SUPPL.):S113. doi: 10.1007/BF02562253. [DOI] [PubMed] [Google Scholar]
- zUppsala Vessby 1994 {published data only} .Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes. 1994;43(11):1353–1357. doi: 10.2337/diab.43.11.1353. [DOI] [PubMed] [Google Scholar]
- zZutphen Miedema 93 {published data only} .Miedema I, Feskens EJM, Heederik D, Kromhout D. Dietary determinants of long-term incidence of chronic nonspecific lung diseases: The Zutphen study. American Journal of Epidemiology. 1993;138(1):37–45. doi: 10.1093/oxfordjournals.aje.a116775. [DOI] [PubMed] [Google Scholar]
- zZutphenES Kalmijn {published data only} .Kalmijn S, Feskens EJM, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants and cognitive function in very old men. Am J Epidemiol. 1997;145(1):33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- zZutphenES Oomen 01 {published data only} .Oomen CM, Ocke MC, Feskens EJ, Kok FJ, Kromhout D. alpha-Linolenic acid intake is not beneficially associated with 10-y risk of coronary artery disease incidence: the Zutphen Elderly Study. American Journal of Clinical Nutrition. 2001;74(4):457–463. doi: 10.1093/ajcn/74.4.457. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Alekseeva 2000A {published data only} .Alekseeva RI, Sharafetdinov KK, Plotnikova OA, Meshcheriakova VA, Mal’tsev GI, Kulakova SN. 463Effects of diet therapy including eiconol on clinical and metabolic parameters in patients with type 2 diabetes mellitus]. [Russian] Voprosy Pitaniia. 2000;69(5):36–9. [PubMed] [Google Scholar]
- Alekseeva 2000B {published data only} .Alekseeva RI, Sharafetdinov K, Plotnikova OA, Meshcheriakova VA, Mal’tsev GI, Kulakova SN. [Effects of a diet including linseed oil on clinical and metabolic parameters in patients with type 2 diabetes mellitus]. [Russian] Voprosy Pitaniia. 2000;69(6):32–5. [PubMed] [Google Scholar]
- Andreassen 1997 {published data only} .Andreassen AK, Hartmann A, Offstad J, Geiran O, Kvernebo K, Simonsen S. Hypertension prophylaxis with omega-3 fatty acids in heart transplant recipients. Journal of the American College of Cardiology. 1997;29(6):1324–31. doi: 10.1016/s0735-1097(97)82757-x. [DOI] [PubMed] [Google Scholar]
- Bard 1997 {published data only} .Bard JM, Luc G, Jude B, Bordet JC, Lacroix B, Bonte JP, et al. A therapeutic dosage (3 g/day) of borage oil supplementation has no effect on platelet aggregation in healthy volunteers. Fundamental & Clinical Pharmacology. 1997;11(2):143–4. doi: 10.1111/j.1472-8206.1997.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Belch 1988 {published data only} .Belch JJ, Ansell D, Madhok R, O’Dowd A, Sturrock RD. Effects of altering dietary essential fatty acids on requirements for non-steroidal anti-inflammatory drugs in patients with rheumatoid arthritis: a double blind placebo controlled study. Annals of the Rheumatic Diseases. 1988;47(2):96–104. doi: 10.1136/ard.47.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett 1989 {published data only} .Bennett WM, Walker RG, Kincaid-Smith P. Treatment of IgA nephropathy with eicosapentaenoic acid (EPA): a two-year prospective trial. Clinical Nephrology. 1989;31:128–31. [PubMed] [Google Scholar]
- Bennett 1995 {published data only} .Bennett WM, Carpenter CB, Shapiro ME, Strom TB, Hefty D, Tillman M, et al. Delayed omega-3 fatty acid supplements in renal transplantation. A double-blind, placebo-controlled study. Transplantation. 1995;59(3):352–6. [PubMed] [Google Scholar]
- Berlin 1992 {published data only} .Berlin E, Bhathena SJ, Judd JT, Nair PP, Peters RC, Bhagavan HN, et al. Effects of omega-3 fatty acid and vitamin E supplementation on erythrocyte membrane fluidity, tocopherols, insulin binding, and lipid composition in adult men. The Journal of Nutritional Biochemistry. 1992;3(8):392–400. [Google Scholar]
- Berthoux 1992 {published data only} .Berthoux FC, Guerin C, Burgard G, Berthoux P, Alamartine E. One-year randomized controlled trial with omega-3 fatty acid-fish oil in clinical renal transplantation. Transplantation Proceedings. 1992;24(6):2578–82. [PubMed] [Google Scholar]
- Bordet 1991 {published data only} .Bordet JC, Trzeciak MC, Durbin S, Dechavanne M. Dose-response studies of platelet aggregation and bleeding time during eicosapentaenoic acid intake [letter; comment] Thrombosis and Haemostasis. 1991;65(1):110. [PubMed] [Google Scholar]
- Bowles 1991 {published data only} .Bowles MH, Klonis D, Plavac TG, et al. EPA in the prevention of restenosis post PTCA. Angiology. 1991;42(3):187–94. doi: 10.1177/000331979104200302. [DOI] [PubMed] [Google Scholar]
- Burchard 1988 {published data only} .Burchard HU, Tischendorf FW. Effect of cod liver oil administration on blood lipid levels, lipoprotein profile and bleeding time. Z Ernahrungswiss. 1988;27(4):222–8. doi: 10.1007/BF02019510. [DOI] [PubMed] [Google Scholar]
- Busnach 1998 {published data only} .Busnach G, Straglioto E, Minetti E, Perego A, Brando B, Broggi ML, et al. Effect of N-3 polyunsaturated fatty acids on cyclosporine pharmacokinetics in kidney graft recipients: A randomized placebo-controlled study. Journal of Nephrology. 1998;11(2):87–93. [PubMed] [Google Scholar]
- Cappelli 1997 {published data only} .Cappelli P, Di LL, Stuard S, Ballone E, Albertazzi A. N-3 polyunsaturated fatty acid supplementation in chronic progressive renal disease. Journal of Nephrology. 1997;10(3):157–62. [PubMed] [Google Scholar]
- Cheng 1990A {published data only} .Cheng A, Bustami M, Norell MS, Mitchell AG, Ilsey CDJ. The effect of omega-3 fatty acids on restenosis after coronary angioplasty. European Heart Journal. 1990;11:368. [Google Scholar]
- Cheng 1990B {published data only} .Cheng IKP, Chan PCK, Chan MK. The effect of fish oil dietary supplement on the progression of mesangial IgA glomerulonephritis. Nephrology, Dialysis, Transplantation. 1990;5:241–16. doi: 10.1093/ndt/5.4.241. [DOI] [PubMed] [Google Scholar]
- Christensen 1997 {published and unpublished data} .Christensen JH, Korup E, Aaroe J, Toft E, Moller J, Rasmussen K. Fish consumption, n-3 fatty acids in cell membranes, and heart rate variability in survivors of myocardial infarction with left ventricular dysfunction. American Journal of Cardiology. 1997;79:1670. doi: 10.1016/s0002-9149(97)00220-8. [DOI] [PubMed] [Google Scholar]
- Christie 1968 {published data only} .Christie SB, Conway N, Pearson HE. Observations on the performance of a standard exercise test by claudicants taking gamma-linolenic acid. Journal of Atherosclerosis Research. 1968;8:83–90. doi: 10.1016/s0368-1319(68)80082-1. [DOI] [PubMed] [Google Scholar]
- Clark 1994 {published data only} .Clark WF, Parbtani A. Omega-3 fatty acid supplementation in clinical and experimental lupus nephritis. American Journal of Kidney Diseases. 1994;23(5):644–7. doi: 10.1016/s0272-6386(12)70273-1. [DOI] [PubMed] [Google Scholar]
- Clausen 1989 {published data only} .Clausen J, Nielsen SA, Kristensen M. Biochemical and clinical effects of an antioxidative supplementation of geriatric patients. A double blind study. Biological Trace Element Research. 1989;20(1-2):135–51. doi: 10.1007/BF02919106. [DOI] [PubMed] [Google Scholar]
- Dagnelie 2000 {published data only} .Dagnelie G, Zorge IS, McDonald TM. Lutein improves visual function in some patients with retinal degeneration: a pilot study via the Internet. Optometry (St Louis, Mo ) 2000;71(3):147–64. [PubMed] [Google Scholar]
- Das 2001 {published data only} .Das UN, Krishna M,I, Ravi RT. Effect of corticosteroids and eicosapentaenoic acid/docosahexaenoic acid on pro-oxidant and anti-oxidant status and metabolism of essential fatty acids in patients with glomerular disorders. Prostaglandins Leukotrienes & Essential Fatty Acids. 2001;65(4):197–203. doi: 10.1054/plef.2001.0311. [DOI] [PubMed] [Google Scholar]
- de Lorgeril 1994 {published data only} .de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease [see comments] [published erratum appears in Lancet 1995 Mar 18;345(8951):738] Lancet. 1994;343(8911):1454–9. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- de Lorgeril 1998 {published data only} .de Lorgeril M, Salen P, Martin JL, Monjaud I, Boucher P, Mamelle N. Mediterranean dietary pattern in a randomised trial. Archives of Internal Medicine. 1998;158:1181–7. doi: 10.1001/archinte.158.11.1181. [DOI] [PubMed] [Google Scholar]
- de Lorgeril 1999 {published data only} .de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon diet heart study. Circulation. 1999;99:779–85. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- Diskin 1990 {published data only} .Diskin CJ, Thomas CE, Zellner CP, Lock S, Tanja J. Fish oil to prevent intimal hyperplasia and access thrombosis. Nephron. 1990;55(4):445–7. doi: 10.1159/000186022. [DOI] [PubMed] [Google Scholar]
- Donadio 1994 {published data only} .Donadio JJ, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group [see comments] New England Journal of Medicine. 1994;331:1194–9. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- Doyle 2001 {published data only} .Doyle W, Srivastava A, Crawford MA, Bhatti R, Brooke Z, Costeloe KL. Inter-pregnancy folate and iron status of women in an inner-city population. British Journal of Nutrition. 2001;86(1):81–7. doi: 10.1079/bjn2001376. [DOI] [PubMed] [Google Scholar]
- Durrington 2001 {published data only} .Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, et al. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. 2001;85(5):544–8. doi: 10.1136/heart.85.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki 1999 {published data only} .Ezaki O, Takahashi M, Shigematsu T, Shimamura K, Kimura J, Ezaki H, et al. Long-term effects of dietary alpha-linolenic acid from perilla oil on serum fatty acids composition and on the risk factors of coronary heart disease in Japanese elderly subjects. Journal of Nutritional Science & Vitaminology. 1999;45(6):759–72. doi: 10.3177/jnsv.45.759. [DOI] [PubMed] [Google Scholar]
- Franzen 1989 {published data only} .Franzen D, Hopp HW, Schanwell M, Hilger HH. Long-term effect of fish oil and olive oil on plasma lipids in patients with CHD. Zeitschrift fur Kardiologie. 1989;78(Suppl 4):28. [Google Scholar]
- Gapparova 2000 {published data only} .Gapparova KM, Pogozheva AV, Kulakova SN, Tutel’ian VA. [Effects of omega-3 polyunsaturated fatty acids of vegetable and animal origin on clinical and metabolic indicators and the intensity of lipid peroxidation in patients with ischemic heart disease and impaired carbohydrate tolerance]. [Russian] Voprosy Pitaniia. 2000;69(1-2):46–9. [PubMed] [Google Scholar]
- Gazso 1992 {published data only} .Gazso A, Horrobin D, Sinzinger H. Influence of omega-3 fatty acids on the prostaglandin-metabolism in healthy volunteers and patients suffering from PVD. Agents and Actions. Supplements. 1992;37:151–6. doi: 10.1007/978-3-0348-7262-1_20. [DOI] [PubMed] [Google Scholar]
- Glab-Kordecka 1986 {published data only} .Glab-Kordecka E. Secondary prevention of ischemic heart disease. Evaluation of platelet function in patients treated with anticoagulants and cod liver oil. Polskie archiwum medycyny wewnetrznej. 1986;75(1):3–12. [PubMed] [Google Scholar]
- Gogos 1998 {published data only} .Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer. 1998;82(2):395–402. doi: 10.1002/(sici)1097-0142(19980115)82:2<403::aid-cncr21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Greatrex 2000 {published data only} .Greatrex JC, Drasdo N, Dresser K. Scotopic sensitivity in dyslexia and requirements for DHA supplementation. Lancet. 2000;355(9213):1429–30. doi: 10.1016/s0140-6736(00)02145-0. [DOI] [PubMed] [Google Scholar]
- Griffin 1999 {published data only} .Griffin BA, Minihane AM, Furlonger N, Chapman C, Murphy M, Williams D, et al. Inter-relationships between small, dense low-density lipoprotein (LDL), plasma triacylglycerol and LDL apoprotein B in an atherogenic lipoprotein phenotype in free-living subjects. Clinical Science. 1999;97(3):269–76. [PubMed] [Google Scholar]
- Hamazaki 1984 {published data only} .Hamazaki T, Tateno S, Shishido H. Eicosapentaenoic acid and IgA nephropathy [letter] Lancet. 1984;1(8384):1017–8. doi: 10.1016/s0140-6736(84)92355-9. [DOI] [PubMed] [Google Scholar]
- Hansen 1996 {published data only} .Hansen GV, Nielsen L, Kluger E, Thysen M, Emmertsen H, Stengaard PK, et al. Nutritional status of Danish rheumatoid arthritis patients and effects of a diet adjusted in energy intake, fish-meal, and antioxidants. Scandinavian Journal of Rheumatology. 1996;25(5):325–30. doi: 10.3109/03009749609104066. [DOI] [PubMed] [Google Scholar]
- Harris 1991 {published data only} .Harris WS, Windsor SL, Dujovne CA. Effects of four doses of n-3 fatty acids given to hyperlipidemic patients for six months. Journal of the American College of Nutrition. 1991;10(3):220–7. doi: 10.1080/07315724.1991.10718148. [DOI] [PubMed] [Google Scholar]
- Higdon 2001 {published data only} .Higdon JV, Du SH, Lee YS, Wu T, Wander RC. Supplementation of postmenopausal women with fish oil does not increase overall oxidation of LDL ex vivo compared to dietary oils rich in oleate and linoleate. Journal of Lipid Research. 2001;42(3):407–18. [PubMed] [Google Scholar]
- Hogg 1995 {published data only} .Hogg RJ. A randomized, placebo-controlled, multicenter trial evaluating alternate-day prednisone and fish oil supplements in young patients with immunoglobulin A nephropathy. Scientific Planning Committee of the IgA Nephropathy Study. American Journal of Kidney Diseases. 1995;26(5):792–6. doi: 10.1016/0272-6386(95)90445-x. [DOI] [PubMed] [Google Scholar]
- Huang 1996 {published data only} .Huang YC, Jessup JM, Forse RA, Flickner S, Pleskow D, Anastopoulos HT, et al. n-3 fatty acids decrease colonic epithelial cell proliferation in high-risk bowel mucosa. Lipids. 1996;31:S313–S317. doi: 10.1007/BF02637099. [DOI] [PubMed] [Google Scholar]
- Hui 1989 {published data only} .Hui R, St-Louis J, Falardeau P. Anti-hypertensive properties of linoleic acid and fish oil omega-3 fatty acids independant of the prostaglandin system. American Journal of Hypertension. 1989;2:610–7. doi: 10.1093/ajh/2.8.610. [DOI] [PubMed] [Google Scholar]
- Hunninghake 2000 {published data only} .Hunninghake DB, Maki KC, Kwiterovich PO, Jr, Davidson MH, Dicklin MR, Kafonek SD. Incorporation of lean red meat into a National Cholesterol Education Program Step I diet: a long-term, randomized clinical trial in free-living persons with hypercholesterolemia. Journal of the American College of Nutrition. 2000;19(3):351–60. doi: 10.1080/07315724.2000.10718931. [DOI] [PubMed] [Google Scholar]
- Ismail 1988 {published data only} .Ismail S, Brannigan M. Effect of low maintenance dose omega-3 fatty acids on serum lipid levels [abstract] Irish Journal of Medical Science. 1988;157(8):274. [Google Scholar]
- Johansen 1999B {published data only} .Johansen O, Seljeflot I, Hostmark AT, Arnesen H. The effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(7):1681–1686. doi: 10.1161/01.atv.19.7.1681. [DOI] [PubMed] [Google Scholar]
- Johansson 1994 {published data only} .Johansson G, Widerstrom L. Change from mixed diet to lactovegetarian diet: influence on IgA levels in blood and saliva. Scandinavian Journal of Dental Research. 1994;102(6):350–4. doi: 10.1111/j.1600-0722.1994.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Junker 1990 {published data only} .Junker L, Engel S, Berger I, Barleben H. Serum enzymes in grade I hypertensive patients before and following a change in nutrition in relation to polyene acid and electrolyte content. Zeitschrift fur die gesamte innere Medizin und ihre Grenzgebiete. 1990;45(11):323–4. [PubMed] [Google Scholar]
- Kachorovskii 1977 {published data only} .Kachorovskii BV, Zhoglo FA, Zaverbnyi MI. Total lipid content in the blood after using fish oil in natural and emulsified forms. Farmatsiia. 1977;26(5):86. [PubMed] [Google Scholar]
- Karlsson 1998 {published data only} .Karlsson J, Hesselius G, Nygard B, Ronneberg R. Plasma omega-3 fatty acids before and after nutritional therapy. Journal of Nutritional & Environmental Medicine. 1998;8(1):25–34. [Google Scholar]
- Kobayashi 1981 {published data only} .Kobayashi S, Hirai A, Terano T, Hamazaki T, Tamura Y, Kumagai A. Reduction in blood viscosity by eicosapentaenoic acid. Lancet. 1981;ii:197. doi: 10.1016/s0140-6736(81)90373-1. [DOI] [PubMed] [Google Scholar]
- Konya 2000 {published data only} .Konya E, Tsuji H, Umekawa T, Kurita T, Iguchi M. Effect of ethyl icosapentate on urinary calcium and oxalate excretion. International Journal of Urology. 2000;7(10):361–5. doi: 10.1046/j.1442-2042.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- Kremer 1990 {published data only} .Kremer JM, Lawrence DA, Jubiz W, DiGiacomo R, Rynes R, Bartholomew LE, et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis and Rheumatism. 1990;33(6):810–20. doi: 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- Kruger 1998 {published data only} .Kruger MC, Coetzer H, de Winter R, Gericke G, van Papendorp DH. Calcium, gamma-linolenic acidand eicosapentaenoic acid supplementation in senile osteoporosis. Aging (Milano) 1998;10(5):385–94. doi: 10.1007/BF03339885. [DOI] [PubMed] [Google Scholar]
- Layne 1996 {published data only} .Layne KS, Goh YK, Jumpsen JA, Ryan EA, Chow P, Clandinin MT. Normal subjects consuming physiological levels of 18:3(n-3) and 20:5(n-3) from flaxseed or fish oils have characteristic differences in plasma lipid and lipoprotein fatty acid levels. Journal of Nutrition. 1996;126(9):2130–40. doi: 10.1093/jn/126.9.2130. [DOI] [PubMed] [Google Scholar]
- Leaf 1995 {published data only} .Leaf DA, Connor WE, Barstad L, Sexton G. Incorporation of dietary n-3 fatty acids into the fatty acids of human adipose tissue and plasma lipid classes. The American Journal of Clinical Nutrition. 1995;62(1):68–73. doi: 10.1093/ajcn/62.1.68. [DOI] [PubMed] [Google Scholar]
- Leng 1997 {published data only} .Leng GC, Lee AJ, Fowkes FGR, Jepson RG, Horrobin D, Lowe GDO, et al. Randomised controlled trial of y-linolenic and eicosapentaenoic acid in peripheral vascular disease. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1997;57(2):218. [Google Scholar]
- Leng 1998 {published data only} .Leng GC, Lee AJ, Fowkes FG, Jepson RG, Lowe GD, Skinner ER, et al. Randomized controlled trial of gamma-linolenic acid and eicosapentaenoic acid in peripheral arterial disease. Clinical Nutrition. 1998;17(6):265–71. doi: 10.1016/s0261-5614(98)80318-x. [DOI] [PubMed] [Google Scholar]
- Leren 1966 {published data only} .Leren P. The effect of plasma cholesterol lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Acta Medica Scandinavica. Supplementum. 1966;466:1–92. [PubMed] [Google Scholar]
- Leren 1967 {published data only} .Leren P. The effect of a cholesterol lowering diet in male survivors of myocardial infarction. (A controlled clinical trial) Nordisk Medicin. 1967;77(21):658–61. [PubMed] [Google Scholar]
- Leren 1968 {published data only} .Leren P. The effect of plasma-cholesterol-lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Bulletin of the New York Academy of Medicine. 1968;44:1012–20. [PMC free article] [PubMed] [Google Scholar]
- Leren 1970 {published data only} .Leren P. The Oslo diet-heart study. Eleven year report. Circulation. 1970;42:935–42. doi: 10.1161/01.cir.42.5.935. [DOI] [PubMed] [Google Scholar]
- Maachi 1995 {published data only} .Maachi K, Berthoux P, Burgard G, Alamartine E, Berthoux F. Results of a 1-year randomized controlled trial with omega-3 fatty acid fish oil in renal transplantation under triple immunosuppressive therapy. Transplantation Proceedings. 1995;27(1):846–9. [PubMed] [Google Scholar]
- Maheu 1998 {published data only} .Maheu E, Mazieres B, Valat JP, Loyau G, Le Loet X, Bourgeois P, et al. Symptomatic efficacy of avocado/soybean unsaponifiables in the treatment of osteoarthritis of the knee and hip: a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial with a six-month treatment period and a two-month followup demonstrating a persistent effect. Arthritis & Rheumatism. 1998;41(1):81–91. doi: 10.1002/1529-0131(199801)41:1<81::AID-ART11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Mansel 1990 {published data only} .Mansel RE, Harrison BJ, Melhuish J, Sheridan W, Pye JK, Pritchard G, et al. A randomized trial of dietary intervention with essential fatty acids in patients with categorized cysts. Annals of the New York Academy of Sciences. 1990;586:288–94. doi: 10.1111/j.1749-6632.1990.tb17819.x. [DOI] [PubMed] [Google Scholar]
- McIllmurray 1987 {published data only} .McIllmurray MB, Turkie W. Controlled trial of gamma linolenic acid in Duke’s C colorectal cancer [published erratum appears in Br Med J (Clin Res Ed) 1987 Aug 22; 295(6596):475] British medical journal (Clinical research ed.) 1987;294(6582):1260. doi: 10.1136/bmj.294.6582.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen 1991 {published data only} .Nilsen DW, Dalaker K, Nordoy A, Osterud B, Ingebretsen OC, Lyngmo V, et al. Influence of a concentrated ethylester compound of n-3 fatty acids on lipids, platelets and coagulation in patients undergoing coronary bypass surgery. Thrombosis & Haemostasis. 1991;66(2):195–201. [PubMed] [Google Scholar]
- Okuda 1996 {published data only} .Okuda Y, Mizutani M, Ogawa M, Sone H, Asano M, Asakura Y, et al. Long-term effects of eicosapentaenoic acid on diabetic peripheral neuropathy and serum lipids in patients with type II diabetes mellitus. Journal of Diabetes and its Complications. 1996;10(5):280–7. doi: 10.1016/1056-8727(95)00081-x. [DOI] [PubMed] [Google Scholar]
- Oliwiecki 1994 {published data only} .Oliwiecki S, Burton JL. Evening primrose oil and marine oil in the treatment of psoriasis. Clinical & Experimental Dermatology. 1994;19(2):127–9. doi: 10.1111/j.1365-2230.1994.tb01139.x. [DOI] [PubMed] [Google Scholar]
- Olsson 1984 {published data only} .Olsson AG, Kirstein P, Eklund B, Johnsson H, Walldius G. Ineffectiveness of Naudicelle in intermittent claudication. Lakartidningen. 1984;81:4855–7. [PubMed] [Google Scholar]
- Perani 1995 {published data only} .Perani G, Maggi E, Muggia C, Covini D, Gallanti E, Catalano O, et al. Effects of omega 3 fatty acid supplementation in patients with primary hypertriglyceridemia. Rivista Italiana di Medicina Biologicas. 1995;15(3-4):87–93. [Google Scholar]
- Persichetti 1996 {published data only} .Persichetti S, Maggi S, Ponzio R, Punzo G, Clemenzia G, Cottone G. Effects of omega 3-PUFA on plasma fibrinogen levels in hypertriglyceridemic hemodialysis patients. Minerva Urologica e Nefrologica. 1996;48(3):137–8. [PubMed] [Google Scholar]
- Pettersson 1994 {published data only} .Pettersson EE, Rekola S, Berglund L, et al. Treatment of IgA nephropathy with onega-3-polyunsaturated fatty acids: a prospective double-blind, randomised study. Clinical Nephrology. 1994;41:183–90. [PubMed] [Google Scholar]
- Pichard 1998 {published data only} .Pichard C, Sudre P, Karsegard V, Yerly S, Slosman DO, Delley V, et al. A randomized double-blind controlled study of 6 months of oral nutritional supplementation witharginine and omega-3 fatty acids in HIV-infected patients. Swiss HIV Cohort Study. AIDS. 1998;12(1):53–63. doi: 10.1097/00002030-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Pogozheva 1994 {published data only} .Pogozheva AV, Sergeeva KV, Trushina EN, Volgarev MN, Samsonov MA. Immune and metabolic effect of polyunsaturated fatty acids omega 3 in anti-atherosclerotic diet in the treatment of patients with ischemic heart disease, familial hyperlipoproteinemia and hypertension. Voprosy Pitaniia. 1994;5:33–6. [PubMed] [Google Scholar]
- Pogozheva 1997 {published data only} .Pogozheva AV, Rozanova IA, Sorokovoi KV, Karagodina ZV, Levachev MM. Lipid peroxidation in patients with ischemic heart disease, hyperlipidemia and/or hypertension while polyunsaturated omega-3 fatty acids of plant and animal origin were in their diet. Voprosy Pitaniia. 1997;4:32–5. [PubMed] [Google Scholar]
- Pogozheva 2000 {published data only} .Pogozheva AV, Tutel’ian VA, Gapparova KM, Trushina EN, Miagkova MA. [The effect of an antiatherosclerotic diet including omega-3 polyunsaturated fatty acids of marine and plant origins on the indices of cellular and humoral immunity in patients with ischemic heart disease and a disordered carbohydrate tolerance]. [Russian] Voprosy Pitaniia. 2000;69(4):33–5. [PubMed] [Google Scholar]
- Puolakka 1985 {published data only} .Puolakka J, Makarainen L, Viinikka L, Ylikorkala O. Biochemical and clinical effects of treating the premenstrual syndrome with prostaglandin synthesis precursors. The Journal of Reproductive Medicine. 1985;30(3):149–53. [PubMed] [Google Scholar]
- Quazi 1994 {published data only} .Quazi S, Mohiduzzaman M, Mostafizur RM, Keramat AS. Effect of hilsa (Tenualosa ilisha) fish in hypercholesterolemic subjects. Bangladesh Medical Research Council Bulletin. 1994;20(1):1–7. [PubMed] [Google Scholar]
- Retterstol 1996 {published data only} .Retterstol K, Stugaard M, Gorbitz C, Ose L. Results of intensive long-term treatment of familial hypercholesterolemia. American Journal of Cardiology. 1996;78(12):1369–74. doi: 10.1016/s0002-9149(96)00649-2. [DOI] [PubMed] [Google Scholar]
- Reuter 1994 {published data only} .Reuter W, Vorberg B, Sauer I, Krumpolt C. Investigations on behavior of parameters of lipid metabolism and antioxidative potential in older patients with HLP by therapy with omega-3-fatty acids. Zeitschrift fur Gerontologie. 1994;27(3):204–7. [PubMed] [Google Scholar]
- Rhodes 1994 {published data only} .Rhodes LE, O’Farrell S, Jackson MJ, Friedmann PS. Dietary fish-oil supplementation in humans reduces UVB-erythemal sensitivity but increases epidermal lipid peroxidation. The Journal of Investigative Dermatology. 1994;103(2):151–4. doi: 10.1111/1523-1747.ep12392604. [DOI] [PubMed] [Google Scholar]
- Rozanova 1997 {published data only} .Rozanova IA, Pogozheva AV, Kupakova SN, Lupinovich VL, Karagodina ZV, Levachev MM, et al. Effect of antiatherosclerotic diet, containing polyunsaturated fatty acids of the omega-3 family from flax oil, on fatty acid composition of cell membranes of patients with ischemic heart disease. Hypertensive disease and hyperlipoproteinemia. Voprosy Pitaniia. 1997;5:15–7. [PubMed] [Google Scholar]
- Samsonov 1990 {published data only} .Samsonov MA, Levachev MM, Pogozheva AV, Korf II, Abbakumov AS, Feofilaktova SN, et al. Effects of anti-atherosclerosis diet containing omega 3 fatty acids on the lipid spectrum of blood and cell membranes in patients with ischemic heart disease and essential hypertension. Voprosy Pitaniia. 1990;5:14–8. [PubMed] [Google Scholar]
- Sanders 1989 {published data only} .Sanders TA, Hinds A, Pereira CC. Influence of n-3 fatty acids on blood lipids in normal subjects. Journal of Internal Medicine. 1989;225(731):99–104. doi: 10.1111/j.1365-2796.1989.tb01442.x. [DOI] [PubMed] [Google Scholar]
- Saynor 1988 {published data only} .Saynor R, Gillott T. Fish oil and plasma fibrinogen. BMJ. 1988;297:1196. doi: 10.1136/bmj.297.6657.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saynor 1992 {published data only} .Saynor R, Gillott T. Changes in blood lipids and fibrinogen with a note on safety in a long term study on the effects of n-3 fatty acids in subjects receiving fish oil supplements and followed for seven years. Lipids. 1992;27(7):533–8. doi: 10.1007/BF02536136. [DOI] [PubMed] [Google Scholar]
- Schaefer 1996 {published data only} .Schaefer EJ, Lichtenstein AH, Lamon FS, Contois JH, Li Z, Goldin BR, et al. Effects of National Cholesterol Education Program Step 2 diets relatively high or relatively low in fish-derived fatty acids on plasma lipoproteins in middle-aged and elderly subjects. American Journal of Clinical Nutrition. 1996;63(2):234–41. doi: 10.1093/ajcn/63.2.234. [DOI] [PubMed] [Google Scholar]
- Seljeflot 1999 {published data only} .Seljeflot I, Johansen O, Arnesen H, Eggesbo JB, Westvik AB, Kierulf P. Procoagulant activity and cytokine expression in whole blood cultures from patients with atherosclerosis supplemented with omega-3 fatty acids. Thrombosis and Haemostasis. 1999;81(4):566–70. [PubMed] [Google Scholar]
- Singer 1986A {published data only} .Singer P, Berger I, Luck K, Taube C, Naumann E, Godicke W. Long-term effect of mackerel diet on blood pressure, serum lipids and thromboxane formation in patients with mild essential hypertension. Atherosclerosis. 1986;62(3):259–65. doi: 10.1016/0021-9150(86)90100-0. [DOI] [PubMed] [Google Scholar]
- Singer 1986B {published data only} .Singer P, Berger I, Luck K, Taube C, Mest HJ, Naumann E, et al. Behavior of blood pressure, serum lipids and thromboxan B2 during short- and long-term diet supplemented with eicosapentaenoic acid of different dosage in patients with essential hypertension. Zeitschrift fnr klinische Medizin. 1986;41(17):1363–6. [Google Scholar]
- Singer 1990A {published data only} .Singer P, Melzer S, Goschel M, Augustin S. Fish oil amplifies the effect of propranolol in mild essential hypertension. Hypertension. 1990;16:682–91. doi: 10.1161/01.hyp.16.6.682. [DOI] [PubMed] [Google Scholar]
- Singer 1990B {published data only} .Singer P. Blood pressure-lowering effect of mackerel diet. Klinische Wochenschrift. 1990;68(Suppl 20):40–8. [PubMed] [Google Scholar]
- Singer 1991 {published data only} .Singer P, Melzer S. Fish Oil and Propranolol in Mild Essential Hypertension. Comparison and Combination of Effects on Blood Pressure. Serum Lipids and Lipoproteins. Munchener Medizinische Wochenschrift. 1991;133(36):539–44. [Google Scholar]
- Sotnikova 1993 {published data only} .Sotnikova EN, Drakina LV, Isaev VA, Ibragimov VR, Bikbov TM, Lisianskaia IB, et al. The role of diet in the prevention and treatment of precancerous conditions in the risk groups. Voprosy Pitaniia. 1993;93(4):41–4. [PubMed] [Google Scholar]
- Stacpoole 1989 {published data only} .Stacpoole PW, Alig J, Ammon L, Crockett SE. Dose-response effects of dietary marine oil on carbohydrate and lipid metabolism in normal subjects and patients with hypertriglyceridemia. Metabolism. 1989;38:946–56. doi: 10.1016/0026-0495(89)90004-8. [DOI] [PubMed] [Google Scholar]
- Stammers 1989 {published data only} .Stammers T, Sibbald B, Freeling P. Fish oil in osteoarthritis [letter] Lancet. 1989;2(8661):503. doi: 10.1016/s0140-6736(89)92112-0. [DOI] [PubMed] [Google Scholar]
- Stammers 1992 {published data only} .Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Annals of the Rheumatic Diseases. 1992;51(1):128–9. doi: 10.1136/ard.51.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong 1993 {published data only} .Strong AMM, Hamill E. The effect of combined fish oil and evening primrose oil (Efamol Marine) on the remission phase of psoriasis: A 7-month double-blind randomized placebo-controlled trial. The Journal of Dermatological Treatment. 1993;4(1):33–6. [Google Scholar]
- Suehiro 1994 {published data only} .Suehiro A, Higasa S, Ueda M, Oura Y,KE. Combination effect of eicosapentaenoic acid and platelet suppressive agents on platelets. Current Therapeutic Research. 1994;55(6):653–9. [Google Scholar]
- Tariq 1989 {published data only} .Tariq T, Close C, Dodds R, Viberti GC, Lee T, Vergani D. The effect of fish-oil on the remission of type 1 (insulin-dependent) diabetes in newly diagnosed patients [letter] Diabetologia. 1989;32(10):765. doi: 10.1007/BF00274540. [DOI] [PubMed] [Google Scholar]
- Thies 2003 {published data only} .Thies F, Garry JMC, Yaqoob P, Rerkasem K, Williams J, Shearman CP, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–85. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- Torjesen 1997 {published data only} .Torjesen PA, Birkeland KI, Anderssen SA, Hjermann I, Holme I, Urdal P. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care. 1997;20(1):26–31. doi: 10.2337/diacare.20.1.26. [DOI] [PubMed] [Google Scholar]
- Tuxen-Mengedoht 1999 {published data only} .Tuxen-Mengedoht M, Koletzko B, Muller I, Demmelmair H, Knapp V, Stem M, et al. Fish oil therapy in cystic fibrosis (CF): a randomized clinical trial [abstract] Clinical Nutrition. 1999;18(Suppl 1):54. [Google Scholar]
- Urakaze 1989A {published data only} .Urakaze M, Hamazaki T, Yano S, Kashiwabara H, Oomori K, Yokoyama T. Effect of fish oil concentrate on risk factors of cardiovascular complications in renal transplantation. Transplant Proceedings. 1989;21:2134–6. [PubMed] [Google Scholar]
- Urakaze 1989B {published data only} .Urakaze M, Hamazaki T, Kashiwabara H, Omori K, Fischer S, Yano S, et al. Favorable effects of fish oil concentrate on risk factors for thrombosis in renal allograft recipients. Nephron. 1989;53(2):102–9. doi: 10.1159/000185720. [DOI] [PubMed] [Google Scholar]
- van der Heide 1993 {published data only} .van der Heide JJH, Bilo HJ, Donker JM, Wilmink JM, Tegzess AM. Effect of dietary fish oil on renal function and rejection in cyclosporine-treated recipients of renal transplants [see comments] New England Journal of Medicine. 1993;329(11):769–73. doi: 10.1056/NEJM199309093291105. [DOI] [PubMed] [Google Scholar]
- van der Merwe 1990 {published data only} .van der Merwe CF, Booyens J, Joubert HF, van der Merwe CA. The effect of gamma-linolenic acid, an in vitro cytostatic substance contained in evening primrose oil, on primary liver cancer. A double-blind placebo controlled trial. Prostaglandins Leukotrienes and Essential Fatty Acids. 1990;40:199–202. doi: 10.1016/0952-3278(90)90098-6. [DOI] [PubMed] [Google Scholar]
- Walden 1991 {published data only} .Walden CE, McCann BS, Retzlaff B, Dowdy A, Hanson M, Fish B, et al. Alternative fat-restricted diets for hypercholesterolemia and combined hyperlipidemia: feasibility, design, subject recruitment, and baseline characteristics of the dietary alternatives study. Journal of the American College of Nutrition. 1991;10(5):429–42. doi: 10.1080/07315724.1991.10718169. [DOI] [PubMed] [Google Scholar]
- Wander 2000 {published data only} .Wander RC, Du SH. Oxidation of plasma proteins is not increased after supplementation with eicosapentaenoic and docosahexaenoic acids. American Journal of Clinical Nutrition. 2000;72(3):731–7. doi: 10.1093/ajcn/72.3.731. [DOI] [PubMed] [Google Scholar]
- Wehrmann 1987 {published data only} .Wehrmann W, Niedecken H, Bauer R. Clinical and immune-modulating effects of the treatment with unsaturated fatty acids in atopic dermatitis. Zeitschrift fur Hautkrankheiten. 1987;62(Suupl 1):111–5. [PubMed] [Google Scholar]
- Weiss 1990 {published data only} .Weiss RJ, McCarthy M, Walworth C. Lack of effect of EPA supplement in high-risk population [abstract] Arteriosclerosis. 1990;10:796A. [Google Scholar]
- Westberg 1989 {published data only} .Westberg G, Tarkowski A. Effect of MaxEPA in patients with SLE: a double blind crossover study [abstract] Kidney International. 1989;35:235. doi: 10.3109/03009749009102117. [DOI] [PubMed] [Google Scholar]
- Westerveld 1991 {published data only} .Westerveld HE, Banga JD, de GJ, Akkerman JN, Leliboux A, Erkelens DW. Effects of low doses of fish oil in paf-induced platelet aggregation in non insulin dependent diabetes mellitus [abstract] Thrombosis and Haemostasis. 1991;65:989. [Google Scholar]
- Wolmarans 1999 {published data only} .Wolmarans P, Laubscher JA, Van der MS, Kriek JA, Lombard CJ, Marais M, Vorster HH, et al. Effects of a prudent diet containing either lean beef and mutton or fish and skinless chicken on the plasma lipoproteins and fatty acid composition of triacylglycerol and cholesteryl ester of hypercholesterolemic subjects. Journal of Nutritional Biochemistry. 1999;10(10):598–608. doi: 10.1016/s0955-2863(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Yasui 2001 {published data only} .Yasui T, Tanaka H, Fujita K, Iguchi M, Kohri K. Effects of eicosapentaenoic acid on urinary calcium excretion in calcium stone formers. European Urology. 2001;39(5):580–5. doi: 10.1159/000052507. [DOI] [PubMed] [Google Scholar]
- Yoa 1994 {published data only} .Yoa RG, Corda C, Rapin JR, Santona L, Goudonnet H, Rifle G, et al. Hemorheological benefits of omega-3 polyunsatured fatty acids on erythrocyte deformability in renal transplanted patients. Clinical Hemorheology. 1994;14(5):663–75. [Google Scholar]
- Zee 1984 {published data only} .Zee P, Cooke RJ, Yeh YY. Linolenate supplementation and linoleate-arachidonate conversion. Pediatric Research. 1984;18:316. [Google Scholar]
- Zinger 1987 {published data only} .Zinger P, Berger I, Liuk K, Taube K, Naumann E. Changes in arterial pressure, serum lipids and thromboxane B2 after using a diet with various levels of eicosapentaenoic acid in patients with hypertension. Klinicheskaia Meditsina. 1987;65(1):62–4. [PubMed] [Google Scholar]
References to studies awaiting assessment
- Bates 1990 {published and unpublished data} .Bates D. Dietary lipids and multiple sclerosis. Upsala journal of medical sciences. 1990;48:173–87. [PubMed] [Google Scholar]; French JM. MaxEPA in multiple sclerosis. British Journal of Clinical Practice. 1984;31:117–21. [PubMed] [Google Scholar]
- Belch {published and unpublished data} .Belch JJF. Blood vessels and essential fatty acids. National Research Register. 2002;(Issue 3):N0405016313. [Google Scholar]
- Delamaire 1991 {published data only} .Delamaire M, Durand F, Bouillaux M, Delambre C, Le-Goff MC, Allannic H. Influence of polyunsaturated fatty acid (MaxEPA) treatment on the haemorheological parameters of 28 IDD patients. Diabetologia. 1991;34:A74. [Google Scholar]
- Kremer 1995 {published data only} .Kremer JM, Lawrence DA, Petrillo GF, Litts LL, Mullaly PM, Rynes RI, et al. Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates. Arthritis and Rheumatism. 1995;38(8):1107–14. doi: 10.1002/art.1780380813. [DOI] [PubMed] [Google Scholar]
- Kurabayashi 2000 {published data only} .*; Kurabayashi T, Okada M, Tanaka K. Eicosapentaenoic acid effect on hyperlipidemia in menopausal Japanese women. The Niigata Epadel Study Group. Obstetrics & Gynecology. 2000;96(4):521–8. doi: 10.1016/s0029-7844(00)00988-1. [DOI] [PubMed] [Google Scholar]
- Mantzaris 1996 {published data only} .Mantzaris GJ, Archavlis E, Zografos C, Petraki K, Spiliades C, Triantafyllou G. A prospective, randomized, placebo-controlled study of fish oil in ulcerative colitis. Hellenic Journal of Gastroenterology. 1996;9(2):138–41. [Google Scholar]
- Palozza 1996 {published data only} .Palozza P, Sgarlata E, Luberto C, Piccioni E, Anti M, Marra G, et al. n-3 fatty acids induce oxidative modifications in human erythrocytes depending on dose and duration of dietary supplementation. American Journal of Clinical Nutrition. 1996;64:297–304. doi: 10.1093/ajcn/64.3.297. [DOI] [PubMed] [Google Scholar]
- Reuter 1990 {published data only} .Reuter W, Voigt H, Peters H-J, Vorberg B, Herrmann W. Zinc, copper and lipid metabolism in arteriosclerosis. Arztliche Laboratorium. 1990;36(12):321–3. [Google Scholar]
- Slack 1987 {published data only} .Slack JD, Pinkerton CA, Van Tassel J, et al. Can oral fish oil supplement minimise restenosis after percutaneous transluminal coronary angioplasty? [abstract] Journal of the American College of cardiology. 1987;9:64. [Google Scholar]
- Tobin 1988 {published data only} .Tobin A, Clarke R. Therapeutic effects of fish oil supplementation in hyperlipidaemia [abstract] Irish Journal of Medical Sciences. 1988;157(9):301. [Google Scholar]
- Tomer 2001 {published data only} .Tomer A, Kasey S, Connor WE, Clark S, Harker LA, Eckman JR. Reduction of pain episodes and prothrombotic activity in sickle cell disease by dietary n-3 fatty acids. Thrombosis & Haemostasis. 2001;85(6):966–74. [PubMed] [Google Scholar]
- Varghese 2000 {published data only} .Varghese TJ, Coomansingh D, Richardson S, Brunt PW, Mowat NAG, Eltahir A, et al. Clinical response of ulcerative colitis with dietary omega-3 fatty acids: a double-blind randomized study [abstract] British Journal of Surgery. 2000;87(1):73. [Google Scholar]
- Zhang 2000 {published data only} .Zhang SM, Willett WC, Hernan MA, Olek MJ, Ascherio A. Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. American Journal of Epidemiology. 2000;152(11):1056–64. doi: 10.1093/aje/152.11.1056. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- AFORRD {unpublished data only} .Atorvastatin in Factorial Combination with Omega 3 fatty acids in cardiovascular Risk Reduction in patients with type 2 Diabetes Ongoing study Starting date of trial not provided. Contact author for more information.
- ASCEND {unpublished data only} .A Study of Cardiovascular Events iN Diabetes (ASCEND) Ongoing study Starting date of trial not provided. Contact author for more information.
- DISAFF {unpublished data only} .Harrison RA, Elton P. From pies to pilchards: dietary assistants increase consumption of oil rich fish [Abstract] Journal of Epidemiology and Community Health. 2000;(Supplement):6. [Google Scholar]; Harrison RA, Elton PJ. International Society for the Study of Fatty Acids and Lipids (ISSFAL) Montreal, Canada: 2003. Can an oil-rich fish diet improve treatment outcomes following cardioversion for atrial fibrillation? A randomised controlled trial. Study design and compliance [poster] [Google Scholar]; Harrison RA, Elton PJ. Is there a role for long-chain omega3 or oil-rich fish in the treatment of atrial fibrillation? Medical Hypotheses. 2004 Jun 9th; doi: 10.1016/j.mehy.2004.06.016. Accepted for publication. [DOI] [PubMed] [Google Scholar]; Harrison RA, Purnell P, Elton PJ. Using community-based dietary assistants to increase the intake of oil-rich fish among older people [Abstract] European Journal of Public Health. 2003;13(Suppl 1):105. [Google Scholar]
- GISSI-HF {unpublished data only} .GISSI-HF Ongoing study Starting date of trial not provided. Contact author for more information.
- JELIS {published and unpublished data} .*; Yokoyama M, Origasa H, for teh JELIS Investigators Effects of eicosapentaenoic acid on cardiovascular events in Japanese patients with hypercholesterolaemia: Rationale, design, and baseline characteristics of the Japan EPA Lipid Intervention Study (JELIS) American Heart Journal. 2003;146:613–20. doi: 10.1016/S0002-8703(03)00367-3. [DOI] [PubMed] [Google Scholar]
- OLIVE {published data only} .Colquhoun DM, Hicks BJ, Somerset S. Rationale and design of the “OLIVE” study (comparison of an olive oil enriched to a low fat diet intervention study using vascular endpoints) [abstract]. 11th International Symposium on Atheroscerosis; Paris. 1997. p. 326. [Google Scholar]; Colquhoun DM, Somerset S, Glasziou PP, Richards D, Weyers J. Comparison of an olive oil enriched diet to a low fat diet intervention study using vascular endpoints: assessed by repeat quantitative angiography (OLIVE study) Australian Journal of Nutrition and Dietetics. 1998;55(Supp 4):S24–S29. [Google Scholar]
- ORIGIN {unpublished data only} .Outcome Reduction with Initial Glargine INtervention (ORIGIN) Ongoing study. 2003 Sep; [Google Scholar]
- Risk and Prevention {unpublished data only} .Risk and Prevention Ongoing study Starting date of trial not provided. Contact author for more information.
- SOFA {published and unpublished data} .Brouwer IA, Katan MB, Schouten EG, Camm AJ, Hauer RNW, Wever EFD, et al. Rationale and design of a clinical trial on n-3 fatty acids and cardiac arrhythmia (SOFA) [abstract] Annals of Nutrition & Metabolism. 2001;45(Suppl 1):79. [Google Scholar]; *; Brouwer IA, Zock PL, Wever EFD, Hauer RNW, Camm AJ, Bocker D, et al. Rationale and design of a randomised controlled clinical trial on supplementatal intake of n-3 fatty acids and incidence of cardiac arrhythmia: SOFA. European Journal of Clinical Nutrition. 2003;57:1323–30. doi: 10.1038/sj.ejcn.1601695. [DOI] [PubMed] [Google Scholar]
Additional references
- Appel 1993 .Appel LJ, Miller ER, Seidler AJ, Whelton PK. Does supplementation of diet with “fish oil” reduce blood pressure? A meta-analysis of controlled clinical trials. Archives of Internal Medicine. 1993;153:1429–38. [PubMed] [Google Scholar]
- Ballard-Barbash 1987 .Ballard-Barbash R, Callaway CW. Marine fish oils: role in prevention of coronary artery disease. Mayo Clinic Proceedings. 1987;62:113–8. doi: 10.1016/s0025-6196(12)61879-5. [DOI] [PubMed] [Google Scholar]
- Bang 1972 .Bang HO, Dyerberg J. Plasma lipids and lipoproteins in Greenlandic west coast eskimos. Acta Medica Scandinavica. 1972;192:85–94. doi: 10.1111/j.0954-6820.1972.tb04782.x. [DOI] [PubMed] [Google Scholar]
- Bang 1976 .Bang HO, Dyerberg J, Hjorne N. The composition of food consumed by Greenland Eskimos. Acta medica Scandinavica. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- Berkley 1995 .Berkley CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Statistics in Medicine. 1995;14:395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- Bhatnagar 2003 .Bhatnagar D, Durrington PN. Omega-3 fatty acids: their role in the prevention and treatment of atherosclerosis related risk factors and complications. International Journal of Clinical Practice. 2003;57(4):305–14. [PubMed] [Google Scholar]
- BNF 1999 .British Nutrition Foundation . n-3 fatty acids and health: briefing paper. British Nutrition Foundation; London: 1999. [Google Scholar]
- Bucher 2002 .Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty adcids in coronary heart disease: a meta-analysis of randomized controlled trials. American Journal of Medicine. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- Burr 1993 .Burr ML. Fish and ischaemic heart disease. In: Simopoulos AP, editor. Nutrition in Health and Disease. Vol. 72. World review of Nutrition and Dietetics; 1993. pp. 49–60. [DOI] [PubMed] [Google Scholar]
- Calabresi 2004 .Calabresi L, Villa B, Canavesi M, Sirtori CR, James RW, Bernini F, et al. An omega-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism. 2004;53(2):153–8. doi: 10.1016/j.metabol.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Chalmers 1990 .Chalmers I, Adams M, Dickersin K, et al. A cohort study of summary reports of controlled trials. JAMA. 1990;263:1401–5. [PubMed] [Google Scholar]
- Cordier 1998 .Cordier S, Grasmick C, Paquier-Passelaigue M, Mandereau L, Weber J-P, Jouan M. Mercury exposure in French Guiana: levels and determinants. Archives of Environmental Health. 1998;53(4):299–304. doi: 10.1080/00039899809605712. [DOI] [PubMed] [Google Scholar]
- De Backer 2003 .De Backer G, Ambrosioni E, Borch-Johnson K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practice. European Journal of Cardiovascular Prevention and Rehabilitation. 2003;10:S1–S78. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk 1999 .Falk C, Hanrahan L, Anderson HA, Kanarek MS, Draheim L, Needham L, et al. Body burden levels of dioxin, furans, and PCBs among frequent consumers of Great Lakes sport fish. Environmental Research, Section A. 1999;80:S19–S25. doi: 10.1006/enrs.1998.3906. [DOI] [PubMed] [Google Scholar]
- Finnegan 2003 .Finnegan YE, Minihane AM, Leigh-Firbank EC, Kew S, Meijer GW, Muggli R, et al. Plant- and marine-derived n-3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. American Journal of Clincial Nutrition. 2003;77:783–95. doi: 10.1093/ajcn/77.4.783. [DOI] [PubMed] [Google Scholar]
- Fitzgerald 1999 .Fitzgerald EF, Deres DA, Hwang S-A, Bush B, Yang B-z, Tarbell A, et al. Local fish consumption and serum PCB concentrations among Mohawk men at Akwesasne. Environmental Research: Section A. 1999;80:S97–S103. doi: 10.1006/enrs.1998.3908. [DOI] [PubMed] [Google Scholar]
- FSA 2000 .Food Standards Agency UK Dioxins and PCBs in the UK diet: 1997 Total diet study samples. Food Surveillance Information Sheet. 2000;Vol. 4/00 [: FSIS 4/00] [Google Scholar]
- FSA 2002 .Food Standards Agency UK Dioxins and dioxin-like pcbs in fish oil supplements. Food Survey Information Sheet. 2002;Vol. 26/02 [Google Scholar]
- FSA 2003 .Food Standards Agency UK Dioxins and dioxin-like PCBs in the UK diet: 2001 total diet study samples. Food Survey Information Sheet. 2003;38:3. [Google Scholar]
- Gapinski 1993 .Gapinski JP, VanRuiswyk JV, Heudebert GR, Schectman GS. Preventing restenosis with fish oils following coronary angioplasty: a meta-analysis. Archives of Internal Medicine. 1993;153:1595–601. [PubMed] [Google Scholar]
- Geelen 2004 .Geelen A, Brouwer IA, Zock PL, Katan MB. Antiarrhythmic effects of n-3 fatty acids: evidence from human studies. Current Opinion in Lipidology. 2004;15:25–30. doi: 10.1097/00041433-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Hauck 1991 .Hauck WW, Gilliss CL, Donner A, Gortner S. Randomisation by Cluster. Nursing Research. 1991;40(6):356–8. [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert 1998 .Hilbert G, Lillemark L, Balchen S, Hojskov CS. Reduction of organochlorine contaminants from fish oil during refining. Chemosphere. 1998;37(7):1241–52. doi: 10.1016/s0045-6535(98)00122-2. [DOI] [PubMed] [Google Scholar]
- Hites 2004 .Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global assessment of organic contaminants in farmed salmon. Science. 2004;303:226–9. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Hovinga 1993 .Hovinga ME, Sowers M, Humphrey HEB. Environmental exposure and lifestyle predictors of lead, cadmium, PCB and DDT levels in Great Lakes fish eaters. Archives of Environmental Health. 1993;48(2):98–104. doi: 10.1080/00039896.1993.9938402. [DOI] [PubMed] [Google Scholar]
- Informed Health 2004 .Informed Health Online [Accessed July 2004];Our Dictionary of Research Terms for Consumers. www.informedhealthonline.org.
- Jacobs 1998 .Jacobs MN, Santillo D, Johnston PA, Wyatt CL, French MC. Organochlorine residues in fish oil dietary supplements: comparison with industrial grade oils. Chemosphere. 1998;37(9-12):1709–21. doi: 10.1016/s0045-6535(98)00236-7. [DOI] [PubMed] [Google Scholar]
- JECFA 2001 .Joint FAO/WHO Expert Committee on Food Additives . Summary of the fifty-seventh meeting of JEFCA. World Health Organization website; 2001. Annex 4. [Google Scholar]
- Kang 1996 .Kang JX, Leaf A. Antiarrhythmic effects of polyunsaturated fatty acids: recent studies. Circulation. 1996;94(7):1774–80. doi: 10.1161/01.cir.94.7.1774. [DOI] [PubMed] [Google Scholar]
- Knapp 1989 .Knapp HR. Omega-3 fatty acids, endogenous prostaglandins and blood pressure regulation in humans. Nutrition Reviews. 1989;47(10):301–13. doi: 10.1111/j.1753-4887.1989.tb02754.x. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton 2002 .Kris-Etherton PM, Harris WS, Appel LJ, for the Nutrition Committee of the American Heart Association Fish consumption, fish oil, omega 3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Li 1999 .Li D, Sinclair A, Wilson A, Nakkote S, Kelly F, Abedin L, et al. Effect of dietary alpha-linolenic acid on thrombotic risk factors in vegetarian men. American Journal of Clinical Nutrition. 1999;69:872–82. doi: 10.1093/ajcn/69.5.872. [DOI] [PubMed] [Google Scholar]
- Liem 1997 .Liem AKD, Theelen RMC. Dioxins: chemical analysis, exposure and risk assessment. Universiteit Utrecht; Utrecht: 1997. first. [: ISBN 90 393 2012 8] [Google Scholar]
- MAFF 1997 .MAFF UK. Dioxins and polychlorinated bephenyls in fish oil dietary supplements and licensed medicines. Food Surveillance Information Sheet. 1997;(issue 106) [Google Scholar]
- MAFF 1998A .MAFF UK. Concentrations of metals and other elements in marine fish and shellfish. Food Surveillance Information Sheet. 1998;(issue 151) [: FSIS 151] [Google Scholar]
- MAFF 1998B .MAFF UK. Dioxins and PCBs in farmed trout in England and Wales. Food Surveillance Information Sheet. 1998;Vol. 145 [Google Scholar]
- MAFF 1999 .MAFF UK. Dioxins and PCBs in UK and imported marine fish. Food Surveillance Information Sheet. 1999;Vol. 184 [Google Scholar]
- Mahaffey 1998 .Mahaffey KR, Mergler D. Blood levels of total and organic mercury in residents of the upper St. Lawrence river basin, Quebec: association with age, gender, and fish consumption. Environmental Research: Section A. 1998;77:104–14. doi: 10.1006/enrs.1998.3834. [DOI] [PubMed] [Google Scholar]
- Marchioli 2004 .Marchioli R. Update on the GISSI trial. Oral presentation at the ISSFAL conference; Brighton UK. June 2004. [Google Scholar]
- Maurice-Bourgoin 00 .Maurice-Bourgoin L, Quiroga I, Chincheros J, Courau P. Mercury distribution in waters and fishes of the upper Madeira rivers and mercury exposure in riparian Amazonian populations. Science of the Total Environment. 2000;260:73–86. doi: 10.1016/s0048-9697(00)00542-8. [DOI] [PubMed] [Google Scholar]
- Morris 1993 .Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–33. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- Nettleton 1991 .Nettleton JA. Omega-3 fatty acids: comparison of plant and seafood sources in human nutrition. Journal of the American Dietetic Association. 1991;91:331–7. [PubMed] [Google Scholar]
- O’Connor 1992 .O’Connor GT, Malenka DJ, Olmstead EM, Johnson PS, Hennekens CH. A meta-analysis of randomised trials of fish oil in prevention of restenosis following coronary angioplasty. American Journal of Preventive Medicine. 1992;8:186–92. [PubMed] [Google Scholar]
- Pawlosky 2001 .Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. Journal of Lipid Research. 2001;42:1257–65. [PubMed] [Google Scholar]
- Peterson 1994 .Peterson DE, Kanarek MS, Kuykendall MA, Diedrich JM, Anderson HA, Remington PL. Fish consumption patterns and blood mercury levels in Wisconsin Chippewa Indians. Archives of Environmental Health. 1994;49(1):53–8. doi: 10.1080/00039896.1994.9934415. [DOI] [PubMed] [Google Scholar]
- Radack 1989 .Radack K, Deck C. The effects of omega-3 polyunsaturated fatty acids on blood pressure: a methodologic analysis of the evidence. Journal of the American College of Nutrition. 1989;8(5):376–85. doi: 10.1080/07315724.1989.10720312. [DOI] [PubMed] [Google Scholar]
- Retraction 2004 .Singh RB. Retraction. British Medical Journal. In press. [Google Scholar]
- Salonen 1995 .Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, et al. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91:645–55. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- Sharp 1998 .Sharp S. Meta-analysis regression. Stata Technical Bulletin. 1998;42:16–22. [Google Scholar]
- SIGN 2002 .Scottish Intercollegiate Guidelines Network . Cardiac rehabilitation: a national clinical guideline. SIGN; Edinburgh: 2002. [Google Scholar]
- Simopoulos 1992 .Simopoulos AP, Norman HA, Gillaspy JE, Duke JA. Common purslane: a source of omega-3 fatty acids and antioxidants. Journal of the American College of Nutrition. 1992;11(4):374–82. doi: 10.1080/07315724.1992.10718240. [DOI] [PubMed] [Google Scholar]
- USFDA 1995 .US FDA Mercury in fish: cause for concern? FDA Consumer. 1995 www.fda.gov.
- USFDA 2000 .U.S. Food. Drug Administration Letter regarding dietary supplement health claim for omega-3 fatty acids and coronary heart disease. US FDA web site. 2000 www.fda.gov. Vol. Docket No. 91N–0103.
- Wood 1998 .Wood D, Durrington P, Poulter N, McInnes G, Rees A, Wray R, et al. Joint British Recommendations on prevention of coronary heart disease in clinical practice. Heart. 1998;80:S1–S29. [PMC free article] [PubMed] [Google Scholar]
- * Indicates the major publication for the study





