Abstract
Background
Gabapentin is an antiepileptic drug, also used in the treatment of neuropathic pain, which is the subject of a Cochrane review, currently under revision. Its efficacy in treating established acute postoperative pain has not been demonstrated.
Objectives
To assess the efficacy and safety of single dose oral gabapentin compared with placebo in established acute postoperative pain using methods that permit comparison with other analgesics.
Search methods
We searched Cochrane CENTRAL, MEDLINE, EMBASE, and the Oxford Pain Relief Database. Additional studies were sought from reference lists of retrieved articles and reviews. Clinical trials databases were searched for unpublished studies; clinical trial reports of several unpublished studies have been made public following litigation in the US.
Selection criteria
Single oral dose, randomised, double‐blind, placebo‐controlled trials of gabapentin for relief of established moderate to severe postoperative pain in adults.
Data collection and analysis
Studies were assessed for methodological quality and data extracted by two review authors independently. Numbers of participants with at least 50% of maximum possible total pain relief (TOTPAR) or summed pain intensity difference (SPID) with gabapentin or placebo were calculated and used to derive relative benefit (RB) or risk (RR), and number‐needed‐to‐treat‐to‐benefit (NNT). Numbers of participants using rescue medication, and time to its use, were sought as additional measures of efficacy. Information on adverse events and withdrawals was collected.
Main results
Four unpublished studies met inclusion criteria; in three, participants had pain following dental surgery, and one followed major orthopaedic surgery; 177 participants were treated with a single dose of gabapentin 250 mg, 21 with gabapentin 500 mg, and 172 with placebo. At least 50% pain relief over 6 hours was achieved by 15% with gabapentin 250 mg and 5% with placebo; giving a RB of 2.5 (95% CI 1.2 to 5.0) and an NNT of 11 (6.4 to 35). Significantly fewer participants needed rescue medication within 6 hours with gabapentin 250 mg than with placebo; NNT to prevent use 5.8. About one third of participants reported adverse events with both gabapentin 250 mg and placebo. No serious adverse events occurred with gabapentin.
Authors' conclusions
Gabapentin 250 mg is statistically superior to placebo in the treatment of established acute postoperative pain, but the NNT of 11 for at least 50% pain relief over 6 hours with gabapentin 250 mg is of limited clinical value and inferior to commonly used analgesics. Gabapentin 250 mg is not clinically useful as a stand‐alone analgesic in established acute postoperative pain, though this is probably the first demonstration of analgesic effect of an antiepileptic in established acute pain.
Plain language summary
Gabapentin for acute postoperative pain in adults
Gabapentin is a medicine used primarily to treat epilepsy and also pain caused by damage to nerves (neuropathic pain). Gabapentin is not normally used to treat pain due to injury or pain after an operation; it is debatable whether gabapentin is an effective pain medicine under such circumstances. We aimed to investigate whether gabapentin is effective in the treatment of acute postoperative pain in adults. We identified four unpublished clinical trials with 370 participants who received either gabapentin or placebo (sugar pill). Gabapentin 250 mg does provide some relief in acute postoperative pain but it is not as good as some other medicines commonly used in this setting, particularly ibuprofen, diclofenac, and naproxen, and probably paracetamol (acetaminophen) alone or in combination with a weak opioid.
However, from a scientific point of view, it is interesting that a medicine originally developed to treat epilepsy has any effect at all in postoperative pain. Research questions that need addressing now include finding the optimal dose, and whether combining gabapentin with conventional pain medicines might be better for postoperative pain than these conventional pain medicines on their own.
Background
Acute pain occurs as a result of tissue damage either accidentally due to an injury or as a result of surgery. Acute postoperative pain is a manifestation of inflammation due to tissue injury. The management of postoperative pain and inflammation is a critical component of patient care.
The aim of this series of reviews is to present evidence for relative analgesic efficacy through indirect comparisons with placebo, in very similar trials performed in a standard manner, with very similar outcomes, and over the same duration. Such relative analgesic efficacy does not in itself determine choice of drug for any situation or patient, but guides policy‐making at the local level.
Recently published reviews from the series include well established analgesics such as paracetamol (Toms 2008), naproxen (Derry 2009b), diclofenac (Derry 2009), and ibuprofen (Derry 2009a), and newer cyclo‐oxygenase‐2 selective analgesics, such as celecoxib (Derry 2008), etoricoxib (Clarke 2009), and parecoxib (Lloyd 2009).
Acute pain trials
Single dose trials in acute pain are commonly short in duration, rarely lasting longer than 12 hours. The numbers of participants are small, allowing no reliable conclusions to be drawn about safety. To show that the analgesic is working, it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, commonly called rescue analgesia, if the pain has not diminished after about an hour. This is reasonable, because not all participants given an analgesic will have significant pain relief. Approximately 18% of participants given placebo will have significant pain relief (Moore 2006), and up to 50% may have inadequate analgesia with active medicines. The use of additional or rescue analgesia is hence important for all participants in the trials.
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years. Trials have to be randomised and double blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity scales immediately before the intervention, and then using pain intensity and pain relief scales over the following 4 to 6 hours for shorter acting drugs, and up to 12 or 24 hours for longer acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome. For patients given rescue medication it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over 4 to 6 hours (Moore 2005). Patients usually remain in the hospital or clinic for at least the first 6 hours following the intervention, with measurements supervised, although they may then be allowed home to make their own measurements in trials of longer duration.
Knowing the relative efficacy of different analgesic drugs at various doses can be helpful. An example is the relative efficacy in the third molar extraction pain model (Barden 2004).
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are prescribed on a routine basis for a range of mild to moderate pain and are the most commonly prescribed analgesic medications worldwide. Their efficacy for treating acute pain has been well demonstrated (Moore 2003). Antiepileptic drugs (also known as anticonvulsants) have been used in pain management since the 1960s, very soon after they were first used for their original indication in medicine. They have most often been used in chronic pain conditions, particularly neuropathic pain (like painful diabetic neuropathy). Their efficacy in acute nociceptive pain is not established.
Gabapentin
This review looks at gabapentin. Gabapentin (original trade name Neurontin, but also now available as generic products in some parts of the world) is an antiepileptic drug which is now licensed for the treatment of peripheral and central neuropathic pain in adults in the UK, and has marketing approval in the US for postherpetic neuralgia, other painful neuropathies, and nerve related pain. An earlier review (Wiffen 2005) looked at gabapentin for acute and chronic pain. This updated review will evaluate its efficacy in acute pain only; efficacy in chronic neuropathic pain will be evaluated in a separate review (Wiffen 2009).
Gabapentin is thought to act by binding to calcium channels and modulating calcium influx. This mode of action confers antiepileptic, analgesic and anxiolytic effects, and might provide useful analgesia in acute postoperative pain. In chronic neuropathic pain the maximum recommended (approved) dose is 3600 mg/day. Some patients are started or treated at doses as low as 100 mg/day, often with titration from lower to higher doses over days, or possibly weeks. However there is no convincing evidence that any analgesic effects of gabapentin are dose‐related, whereas toxicity is probably dose‐dependent.
The use of gabapentin in acute pain is uncommon, and its efficacy questionable. Unanswered questions include whether gabapentin has analgesic efficacy in standard tests in participants with established pain of moderate or severe intensity. This is an established, classical, method of detecting analgesic activity in acute pain, different from neuropathic pain efficacy. Another question is whether use of gabapentin immediately before or immediately after surgery (perioperatively, so before pain is established) reduces the requirement for analgesia in the postoperative period (Tiippana 2007). This is much more difficult, and the methods used to establish any effects have never been validated, and have been heavily criticised (Kissin 2009; McQuay 2008). Other questions include whether less common but more serious adverse events are likely, and how gabapentin compares as a perioperative analgesic with conventional analgesics used in everyday surgery and anaesthesia.
This review concentrates on whether gabapentin has analgesic activity in established postoperative pain.
Objectives
To assess the efficacy and adverse effects of single dose oral gabapentin for established acute postoperative pain using methods that permit comparison with other analgesics evaluated in standardised trials using almost identical methods and outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Studies were included if they were double blind trials of single dose oral gabapentin compared with placebo for the treatment of established moderate to severe postoperative pain in adults, with at least 10 participants randomly allocated to each treatment group. Multiple dose studies were included if appropriate data from the first dose were available. Cross‐over studies would be included provided that data from the first arm were presented separately. No language restriction was applied to the search for studies.
The following were excluded:
review articles, case reports, and clinical observations;
studies of experimental pain;
studies where pain relief is assessed only by clinicians, nurses or carers (i.e., not patient‐reported);
studies of less than 4 hours duration or studies that fail to present data over 4 to 6 hours post‐dose.
For postpartum pain, studies would be included if the pain investigated was due to episiotomy or Caesarean section irrespective of the presence of uterine cramps; studies investigating pain due to uterine cramps alone would be excluded.
Types of participants
Studies of adult participants (> 15 yrs) with established postoperative pain of moderate to severe intensity following day surgery or in‐patient surgery were included. For studies using a visual analogue scale (VAS), pain of at least moderate intensity was equated to greater than 30 mm on a 100 mm scale (Collins 1997).
Types of interventions
Gabapentin or matched placebo administered as a single oral dose for postoperative pain.
Types of outcome measures
Data collected included the following:
participant characteristics;
patient reported pain at baseline (physician, nurse or carer reported pain will not be included in the analysis);
patient reported pain relief expressed at least hourly over 4 to 6 hours using validated pain scales (pain intensity and pain relief in the form of VAS or categorical scales, or both);
patient global assessment of efficacy (PGE), using a standard categorical scale;
time to use of rescue medication;
number of participants using rescue medication;
number of participants with one or more adverse events;
number of participants with serious adverse events;
number of withdrawals (all cause, adverse events).
Search methods for identification of studies
To identify studies for inclusion in this review, the following electronic databases were searched:
Cochrane CENTRAL (issue 4, 2010);
MEDLINE via Ovid (February 2010);
EMBASE via Ovid (February 2010);
Oxford Pain Relief Database (Jadad 1996a).
A search strategy was developed in co‐operation with the Cochrane Pain, Palliative Care and Supportive Care Cochrane Review Group. The subject search used a combination of controlled vocabulary and free text terms. See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy and Appendix 3 for the CENTRAL search strategy.
Additional studies were sought from the reference lists of retrieved articles and reviews.
Language
No language restriction was applied.
Unpublished studies
Clinical trials databases were searched for unpublished studies. Clinical trial reports of a number of unpublished studies have already been made public following litigation in the US. No request for unpublished trials was made directly to any pharmaceutical company or author.
Data collection and analysis
Selection of studies
Two review authors independently assessed and agreed the search results for studies to be included in the review.
Quality assessment
Two review authors independently assessed the included studies for quality using the Oxford Quality Scale, a five‐point scale (Jadad 1996b) that considers randomisation, blinding, and study withdrawals and dropouts.
The scale used is as follows.
Is the study randomised? If yes, add one point.
Is the randomisation procedure reported and is it appropriate? If yes, add one point, if no, deduct one point.
Is the study double blind? If yes, add one point.
Is the double blind method reported and is it appropriate? If yes, add one point, if no, deduct one point.
Are the reasons for patient withdrawals and dropouts described? If yes, add one point.
The results are described in the Results section below, and 'Characteristics of included studies' table.
Data management
Data were extracted by two review authors and recorded on a standard data extraction form. Data suitable for pooling were entered into RevMan 5.
Data analysis
For each study, the mean TOTPAR, SPID, VAS TOTPAR or VAS SPID (Appendix 4) values for active and placebo were converted to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). The proportion of participants in each treatment group who achieved at least 50%maxTOTPAR or 50%maxSPID was calculated using verified equations (Moore 1996; Moore 1997a; Moore 1997b). These proportions were then converted into the number of participants achieving at least 50%maxTOTPAR or 50%maxSPID by multiplying by the total number of participants in the treatment group. Information on the number of participants with at least 50%maxTOTPAR or 50%maxSPID for active and placebo was then used to calculate relative benefit (RB) or relative risk (RR), and number‐needed‐to‐treat‐to‐benefit (NNT).
Pain measures accepted for the calculation of TOTPAR or SPID were:
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
VAS for pain relief;
VAS for pain intensity.
If none of these measures were available, the number of participants reporting "very good or excellent" on a five‐point categorical global scale with the wording "poor, fair, good, very good, excellent" would be used for the number of participants achieving at least 50% pain relief (Collins 2001).
The number of participants reporting treatment‐emergent adverse effects was extracted for each treatment group. RB or RR estimates were calculated with 95% confidence intervals (CI) using a fixed‐effect model (Morris 1995). NNT and number‐needed‐to‐treat‐to‐harm (NNH) and 95% CI were calculated using the pooled number of events using the method devised by Cook and Sackett (Cook 1995). A statistically significant difference from control was assumed when the 95% CI of the RR or RB did not include the number one. Homogeneity was examined visually using L'Abbé plots (L'Abbe 1987).
It was planned to analyse different doses separately. Sensitivity analyses were planned to determine the effect of presenting condition (pain model), and high versus low (two versus three or more) quality trials. A minimum of two studies and 200 participants had to be available in any sensitivity analysis (Moore 1998).
Further details of the scales and derived outcomes are in the glossary (Appendix 4).
Results
Description of studies
Searches revealed no published studies, but three completed trials were listed in a clinical trials database (www.clinicaltrials.gov). We had been provided with clinical trial reports of these three unpublished studies, and one other, which were all carried out in the period 1999 to 2000. They were Phase 2 clinical studies designed primarily to investigate the analgesic efficacy of gabapentin in combination with either an NSAID (naproxen) or opioid (hydrocodone) analgesic. All met our inclusion criteria. Three were conducted in the third molar extraction pain model (720‐04378; 720‐04455; 720‐04483) and one investigated pain relief after major orthopaedic surgery (720‐04471). Across studies, 177 participants were treated with a single dose of gabapentin 250 mg, 21 with a single dose of gabapentin 500 mg, and 172 participants received placebo. Studies lasted eight (720‐04455; 720‐04471; 720‐04483) or 12 (720‐04378) hours. Typical exclusion criteria for the dental pain model were (among others): history or clinical evidence of renal disease; occurrence of an oral surgery complication; breastfeeding; history of serious adverse reaction to any analgesic agent or any medication to be used in the operative procedure or postoperative period; history of any bleeding disorder; patient gabapentin use within the past 6 months; and patient use of any analgesic, centrally acting, or anti‐inflammatory medication within 24 hours of surgery.
Details are in the 'Characteristics of included studies' table.
Risk of bias in included studies
All included studies achieved the maximum score of five on the Oxford Quality Scale. A risk of Bias table was completed for randomisation, allocation concealment and blinding, and indicated low risk of bias in all studies (Figure 1).
1.
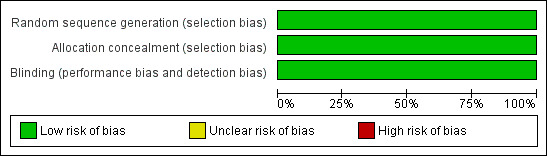
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
Three studies treated 177 participants with gabapentin 250 mg and provided data suitable for pooling. Only one study used gabapentin 500 mg, in 21 participants. There were insufficient data for analysis of this dose. Efficacy outcomes and adverse events and withdrawals for individual studies are presented in Appendix 5 and Appendix 6.
Number of participants achieving at least 50% pain relief
All three studies using gabapentin 250 mg (228 participants in dental studies, and 99 in an orthopaedic study) provided data for this analysis.
The proportion of participants experiencing at least 50% pain relief over 6 hours with gabapentin 250 mg was 15% (26/177; range 12% to 17%).
The proportion of participants experiencing at least 50% pain relief over 6 hours with placebo was 5% (8/150; range 0% to 12%).
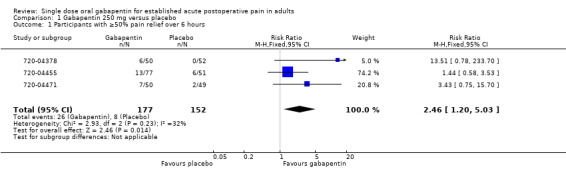
The relative benefit of treatment compared with placebo was 2.5 (95% CI 1.2 to 5.0), giving an NNT for at least 50% pain relief over 6 hours of 11 (95% CI 6.4 to 35) (Figure 2).
2.
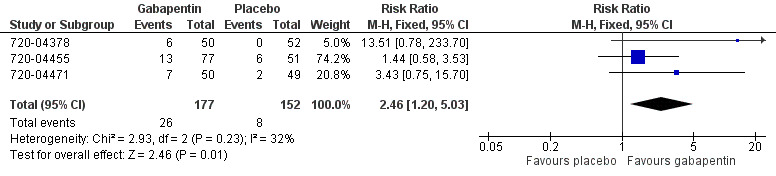
Forest plot of comparison: 1 Gabapentin 250 mg versus placebo, outcome: 1.1 Participants with ≥50% pain relief over 6 hours.
In the study using gabapentin 500 mg, 1/21 participants experienced at least 50% pain relief with gabapentin, and 1/20 with placebo.
Sensitivity analysis of primary outcome
Methodological quality
All studies achieved the maximum quality scores of 5. Therefore no sensitivity analysis could be carried out for this criterion.
Pain model: dental versus other surgery
Two studies (720‐04378; 720‐04455) with 230 participants receiving gabapentin 250 mg or placebo were in dental surgery. One study (720‐04471) with 99 participants was in orthopaedic surgery. Therefore there were insufficient data for a comparison between pain models.
Use of rescue medication
Proportion of participants using rescue medication within 6 hours
All three studies using gabapentin 250 mg reported this outcome.
The proportion of participants using rescue medication with gabapentin 250 mg was 69% (122/177; range 64% to 78%).
The proportion of participants using rescue medication with placebo was 86% (131/152; range 75% to 98%).
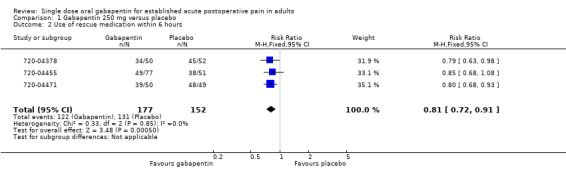
The relative benefit of treatment compared with placebo was 0.81 (95% CI 0.72 to 0.91), giving a number needed to treat to prevent re medication (NNTp) of 5.8 (95% CI 3.8 to 12) (Figure 3).
3.
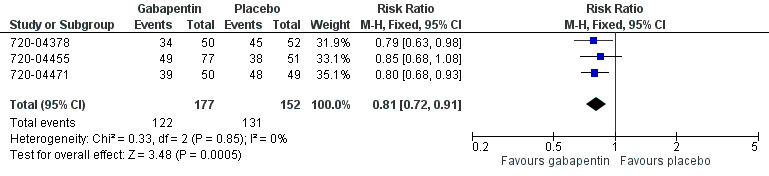
Forest plot of comparison: 1 Gabapentin 250 mg versus placebo, outcome: 1.2 Use of rescue medication within 6 hours.
In the study using gabapentin 500 mg, 16/21 participants needed rescue medication within 6 hours with gabapentin, and 16/20 with placebo.
Time to use of rescue medication
All three studies using gabapentin 250 mg reported the median time to use of rescue medication. The weighted mean of the median times to re medication was 2.4 hours in the gabapentin 250 mg arms and 2.1 hours in the placebo arms of the trials.
In the study using gabapentin 500 mg, the median time to use of rescue medication was less than 2 hours in both treatment arms.
Adverse events
Any adverse event
All three studies using gabapentin 250 mg provided data for this outcome over 8 or 24 hours.
The number of participants experiencing at least one adverse event rate with gabapentin 250 mg was 28% (49/177, range 18% to 50%).
The number of participants experiencing at least one adverse event rate with placebo was 32% (49/152, range 25% to 44%).
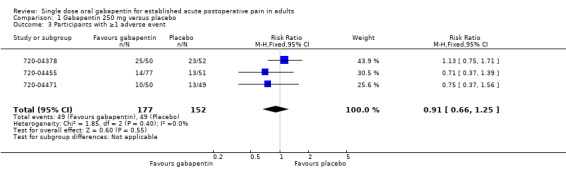
The relative risk of treatment compared to placebo was 0.91 (95% CI 0.66 to 1.3) (Figure 4). The NNH was not calculated.
4.
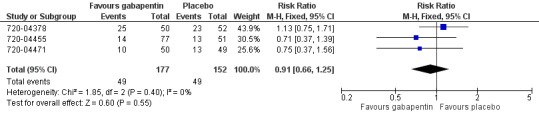
Forest plot of comparison: 1 Gabapentin 250 mg versus placebo, outcome: 1.3 Participants with ≥ adverse event.
In the single study using gabapentin 500 mg, 9/21 participants experienced at least one adverse event with gabapentin, and 2/20 with placebo.
Serious adverse events
Three studies reported that there were no serious adverse events (720‐04378; 720‐04455; 720‐04483). The fourth study (720‐04471) reported that there were two serious adverse events in the placebo group and none in the gabapentin 250 mg group. One participant died due to "heart arrest" occurring one day after study completion. The other had an accidental injury (fractured femur) during physical therapy. The accident occurred one day after study completion, and the patient recovered after surgery.
Withdrawals
Four participants treated with gabapentin 250 mg withdrew before completion of the study. Three withdrew during double‐blind treatment due to an adverse event. In each case the adverse event was fever, and in no case was the adverse event considered to be associated with treatment. The fourth patient was reported to not have withdrawn due to an adverse event but to have left clinic before 12 hours post dose (lack of compliance). One participant treated with gabapentin 500 mg left the clinic before 8 hours post dose (lack of compliance). No participants withdrew in placebo arms in any study.
| Summary of results: gabapentin 250 mg versus placebo | |||||
| Outcome | Studies | Participants | Gabapentin (%) | Placebo (%) | Summary statistic |
| Number of participants with ≥ 50% pain relief over 6 hours | 3 | 329 | 15 | 5 | NNT: 11 (6.4 to 35) |
| Number of participants using rescue medication over 6 hours | 3 | 329 | 68 | 86 | NNTp: 5.8 (3.8 to 12) |
| Number of participants with ≥1 adverse event | 3 | 329 | 28 | 32 | NNH: not calculated |
Discussion
Summary of main results
We identified four completed unpublished studies (720‐04378; 720‐04455; 720‐04471; 720‐04483) comparing gabapentin with placebo in established acute postoperative pain. Three used gabapentin 250 mg and provided data suitable for pooling. The other used gabapentin 500 mg in only 21 participants, with insufficient data for analysis. Gabapentin 250 mg provided some pain relief, with an NNT of 11 for at least 50% pain relief over 6 hours, and reduced the need for rescue analgesia compared with placebo. Indirect comparison with other analgesics reviewed using identical methods show that gabapentin 250 mg is inferior to commonly used analgesics. For comparison the NNT for at least 50% pain relief over 4 to 6 hours is 1.9 (1.7 to 2.1) with etoricoxib 120 mg (Clarke 2009), 2.5 (2.4 to 2.6) with ibuprofen 400 mg (Derry 2009a), 2.7 (2.3 to 3.2) with naproxen 500 mg (Derry 2009b), and 3.6 (3.2 to 4.1) with paracetamol 1000 mg (Toms 2008).
The weighted mean of the median times to re medication was 2.4 hours in the gabapentin 250 mg arms of the trials, compared to 2.1 hours with placebo, and the NNT to prevent remedication over 6 hours was of 5.8. Again the alternatives are superior: etoricoxib 120 mg (Clarke 2009; median time to rescue medication > 20 hours, NNTp at 24 hours 2.4); naproxen 500 mg (Derry 2009b; 8.9 hours, NNTp at 12 hours 6.9); ibuprofen 400 mg (Derry 2009a; 5.6 hours, NNTp at 6 hours 2.7) and paracetamol 1000 mg (Toms 2008; 3.8 hours, NNTp at 4 to 6 hours 5.2).
The single small study using gabapentin 500 mg in dental pain did not demonstrate any analgesic efficacy over placebo, but the number of participants was too small to draw any conclusions.
All studies reported on adverse events, and while the usefulness of single dose studies for assessing adverse events is questionable, it is still reassuring that no increase was demonstrated with gabapentin 250 mg or 500 mg compared to placebo. Longer term use of higher doses of gabapentin for indications other than acute pain is associated with adverse events such as somnolence and dizziness (Striano 2008) but it is unclear to what extent these observations apply to the question that this review addresses.
This is to the best of our knowledge the first demonstration of an analgesic effect of an antiepileptic or antidepressant (used to treat neuropathic pain) in the acute pain setting and is therefore theoretically interesting ,but the effect is clinically unremarkable. Our finding blurs the distinction between established acute pain, commonly thought of as largely nociceptive, and (chronic) neuropathic pain. It suggests that these two types of pain, usually considered distinct entities, might share part of their aetiopathological mechanism. The practical implication of this finding is that it provides a rationale for exploring the effect of antiepileptics and antidepressants in acute nociceptive pain scenarios and for investigating combinations of these drugs with conventional analgesics.
In addition to a postoperative analgesic effect of gabapentin, this drug has been suggested to have a role in the pre‐ and perioperative setting as a multimodal agent. Beneficial effects with regard to postoperative analgesia, preoperative anxiolysis, attenuation of the haemodynamic response to laryngoscopy and intubation, as well as the prevention of chronic postsurgical pain, postoperative nausea and vomiting, and delirium have been suggested (Kong 2007). The usefulness of perioperative gabapentin is controversial (McQuay 2008) and is beyond the scope of this review.
Overall completeness and applicability of evidence
We believe that all the trials of gabapentin in established acute postoperative pain have been identified. The amount of data available for analysis was limited in numbers of trials and participants, and doses of gabapentin used.
The drug was not developed for use in acute pain and did not, in the doses investigated, show promise in this setting, where there are already a number of well‐established and considerably more effective drugs available. It is likely that the relatively poor results in these Phase 2 trials stopped further development in this area.
Quality of the evidence
All trials were of high methodological quality, with a great deal of detail available in the clinical trial reports that is not commonly available in published reports. There were few withdrawals, and outcomes were both well defined and clinically relevant.
Potential biases in the review process
Exhaustive searches were carried out to identify relevant studies, and use of clinical trial reports minimises risk of reporting bias. Data extraction and analysis followed well established methods. We do not think there are any biases in the review process. The statistical benefit of gabapentin 250 mg over placebo in this setting appears reasonably strong ‐ three similarly sized trials with a null effect would be required to overturn statistical significance.
Agreements and disagreements with other studies or reviews
We are not aware of any other reviews or published studies of gabapentin for established acute postoperative pain. The earlier review of gabapentin in acute and chronic pain (Wiffen 2005) did not identify any studies in this setting. Anecdotally, gabapentin is perceived as ineffective in established acute pain, and certainly inferior to other established analgesics.
Authors' conclusions
Implications for practice.
Somewhat unexpectedly, gabapentin demonstrated an analgesic effect in a limited number of individuals with acute nociceptive pain. As a single agent, in the doses investigated, it is inferior to a number of other drugs that can be used in this setting, and would not be recommended as a first line therapy.
Implications for research.
The questions that now need addressing are whether a dose response can be demonstrated, and whether the combination of conventional analgesics and gabapentin for acute pain gives better results than conventional analgesics alone.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 11 May 2017 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 5, 2010
| Date | Event | Description |
|---|---|---|
| 25 April 2012 | Review declared as stable | The authors believe that there is unlikely to be any new studies over at least the next five years and will check for any update on this situation at that time. |
Notes
A restricted search in May 2017 did not identify any potentially relevant studies. The review question is now of historical interest only. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We are grateful to Dr Thomas L Perry for bringing our attention to the fact that these trials were in the public domain in the USA as a result of ongoing litigation.
Appendices
Appendix 1. MEDLINE search strategy (via OVID)
Gabapentin/
(gabapentin OR Neurontin OR neurotonin*).mp
OR/1‐2
Pain, postoperative/
((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ adj4 pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")).mp
((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)).mp
(("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")).mp
(("post surg$" or post‐surg$) AND (pain$ or discomfort)).mp
((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")).mp
((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")).mp
OR/4‐10
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
OR/12‐19
humans.sh.
20 AND 21
3 AND 11 AND 22
Appendix 2. EMBASE search strategy
Gabapentin/
(gabapentin OR Neurontin OR neurotonin*).mp
OR/1‐2
Postoperative pain/
((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ adj4 pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")).mp
((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)).mp
(("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")).mp
(("post surg$" or post‐surg$) AND (pain$ or discomfort)).mp
((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")).mp
((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")).mp
OR/4‐10
clinical trials.sh
controlled clinical trials.sh
randomized controlled trial.sh
double‐blind procedure.sh
(clin$ adj25 trial$)
((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$))
placebo$
random$
OR/12‐19
3 AND 11 AND 20
Appendix 3. CENTRAL search strategy
MESH descriptor Gabapentin
(gabapentin OR Neurontin OR neurotonin*):ti,ab,kw.
OR/1‐2
MESH descriptor Pain, postoperative
((postoperative near/4 pain*) or (post‐operative near/4 pain*) or post‐operative‐pain* or (post* near/4 pain*) or (postoperative near/4 analgesi*) or (post‐operative near/4 analgesi*) or ("post‐operative analgesi*")):ti,ab,kw.
((post‐surgical near/4 pain*) or ("post surgical" near/4 pain*) or (post‐surgery near/4 pain*)):ti,ab,kw.
(("pain‐relief after surg*") or ("pain following surg*") or ("pain control after")):ti,ab,kw.
(("post surg*" or post‐surg*) AND (pain* or discomfort)):ti,ab,kw.
((pain* near/4 "after surg*") or (pain* near/4 "after operat*") or (pain* near/4 "follow* operat*") or (pain* near/4 "follow* surg*")):ti,ab,kw.
((analgesi* near/4 "after surg*") or (analgesi* near/4 "after operat*") or (analgesi* near/4 "follow* operat*") or (analgesi* near/4 "follow* surg*")):ti,ab,kw.
OR/4‐10
3 AND 11
Limit 12 to Clinical Trials (CENTRAL)
Appendix 4. Glossary
Categorical rating scale:
The commonest is the five category scale (none, slight, moderate, good or lots, and complete). For analysis numbers are given to the verbal categories (for pain intensity, none = 0, mild = 1, moderate = 2 and severe = 3, and for relief none = 0, slight = 1, moderate = 2, good or lots = 3 and complete = 4). Data from different subjects is then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. Good correlation was found, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
VAS:
Visual analogue scale: For pain intensity, lines with left end labelled "no pain" and right end labelled "worst pain imaginable", and for pain relief lines with left end labelled "no relief of pain" and right end labelled "complete relief of pain", seem to overcome the limitation of forcing patient descriptors into particular categories. Patients mark the line at the point which corresponds to their pain or pain relief. The scores are obtained by measuring the distance between the no relief end and the patient's mark, usually in millimetres. The main advantages of VAS are that they are simple and quick to score, avoid imprecise descriptive terms and provide many points from which to choose. More concentration and coordination are needed, which can be difficult post‐operatively or with neurological disorders.
TOTPAR:
Total pain relief (TOTPAR) is calculated as the sum of pain relief scores over a period of time. If a patient had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the composite trapezoidal rule. This is a simple method that approximately calculates the definite integral of the area under the pain relief curve by calculating the sum of the areas of several trapezoids that together closely approximate to the area under the curve.
SPID:
Summed pain intensity difference (SPID) is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the trapezoidal rule. VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID. See “Measuring pain” in Bandolier’s Little Book of Pain, Oxford University Press, Oxford. 2003; pp 7‐13 (Moore 2003).
Appendix 5. Summary of outcomes: analgesia and rescue medication
| Analgesia | Rescue medication | |||||
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE "Responder" * | Median time to use (h) | % using at 6 h |
| 720‐04378 | (1) Gabapentin 250 mg, n = 50 (2) Placebo, n = 52 |
SPID 6: (1) 1.63 (2) 0.04 |
(1) 6/50 (2) 0/52 |
(1) 12/50 (2) 9/52 |
(1) 2.0 (2) 1.7 |
(1) 34/50 (2) 45/52 |
| 720‐04455 | (1) Gabapentin 250 mg, n = 77 (2) Placebo, n = 51 |
TOTPAR 6: (1) 5.06 (2) 4.34 |
(1) 13/77 (2) 6/51 |
(1) 24/77 (2) 13/51 |
(1) 2.1 (2) 2.0 |
(1) 49/77 (2) 38/51 |
| 720‐04471 | (1) Gabapentin 250 mg, n = 50 (2) Placebo, n = 49 |
TOTPAR 6: (1) 4.65 (2) 2.81 |
(1) 7/50 (2) 2/49 |
(1) 18/50 (2) 9/49 |
(1) 3.3 (2) 2.6 |
(1) 39/50 (2) 48/49 |
| 720‐04483 | (1) Gabapentin 500 mg, n = 21 (2) Placebo, n = 20 |
TOTPAR 6: (1) 3.25 (2) 2.53 |
(1) 1/21 (2) 1/20 |
(1) 5/21 (2) 2/20 |
(1) <2.0 (2) <2.0 |
(1) 16/21 (2) 16/20 |
Appendix 6. Summary of outcomes:adverse events and withdrawals
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other |
| 720‐04378 | (1) Gabapentin 250 mg, n = 50 (2) Placebo, n = 52 |
(1) 25/50 (2) 23/52 |
(1) 0/50 (2) 0/52 |
(1) 0/50 (2) 0/52 |
(1) 1/50 (lack of compliance) (2) 0/52 |
| 720‐04455 | (1) Gabapentin 250 mg, n = 77 (2) Placebo, n = 51 |
(1) 14/77 (2) 13/51 |
(1) 0/77 (2) 0/51 |
(1) 0/77 (2) 0/51 |
(1) 0/77 (2) 0/51 |
| 720‐04471 | (1) Gabapentin 250 mg, n = 50 (2) Placebo, n = 49 |
(1) 10/50 (2) 13/49 |
(1) 0/50 (2) 2/49 (one heart arrest, one accidental injury (fractured femur)) |
(1) 3/50 (all due to fever) (2) 0/49 |
(1) 0/50 (2) 0/49 |
| 720‐04483 | (1) Gabapentin 500 mg, n = 21 (2) Placebo, n = 20 |
(1) 9/21 (2) 2/20 |
(1) 0/21 (2) 0/20 |
(1) 0/21 (2) 0/20 |
(1) 1/21 (lack of compliance) (2) 0/20 |
Data and analyses
Comparison 1. Gabapentin 250 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with ≥50% pain relief over 6 hours | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.20, 5.03] |
| 2 Use of rescue medication within 6 hours | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.91] |
| 3 Participants with ≥1 adverse event | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.25] |
1.1. Analysis.
Comparison 1 Gabapentin 250 mg versus placebo, Outcome 1 Participants with ≥50% pain relief over 6 hours.
1.2. Analysis.
Comparison 1 Gabapentin 250 mg versus placebo, Outcome 2 Use of rescue medication within 6 hours.
1.3. Analysis.
Comparison 1 Gabapentin 250 mg versus placebo, Outcome 3 Participants with ≥1 adverse event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
720‐04378.
| Methods | Randomised. Double blind. Parallel group. Single dose. For inclusion: pain moderate or severe on a 4‐point categorical scale and ≥ 45 mm on a 100 mm visual analogue scale Pain assessments at 0, 0.5, 1 h and hourly up to 12 h |
|
| Participants | Surgical removal of 1 or 2 third molars N = 483 M = 197, F = 286 Mean age 23 years |
|
| Interventions | Gabapentin 250 mg, n = 50 Placebo, n = 52 also naproxen at various doses and gabapentin/naproxen combinations |
|
| Outcomes | PI: standard 4 point scale PR: standard 5 point scale (but no TOTPAR calculated) Overall assessment of study medication Time use of rescue medication Adverse events: any, serious Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Parke‐Davis Clinical Pharmacy Operations generated the randomisation code |
| Allocation concealment (selection bias) | Low risk | Patients assigned to prenumbered study medication by Parke‐Davis (remote allocation) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | matching placebo capsule |
720‐04455.
| Methods | Randomised. Double blind. Parallel group. Single dose. For inclusion: pain moderate or severe on a 4‐point categorical scale and ≥ 45 mm on a 100 mm visual analogue scale Pain assessments at 0, 0.33, 0.66, 1 h and hourly up to 8 h |
|
| Participants | Surgical removal of 1 or 2 third molars N = 325 M = 164, F = 161 Mean age 23 years |
|
| Interventions | Gabapentin 250 mg, n = 77 Placebo, n = 51 also hydrocodone, gabapentin/hydrocodone, and paracetamol/hydrocodone groups |
|
| Outcomes | PI: standard 4 point scale PR: standard 5 point scale Overall assessment of study medication Time use of rescue medication Adverse events: any, serious Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Parke‐Davis Clinical Pharmacy Operations generated the randomisation code |
| Allocation concealment (selection bias) | Low risk | Patients assigned to prenumbered study medication by Parke‐Davis (remote allocation) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | matching placebo capsule |
720‐04471.
| Methods | Randomised. Double blind. Parallel group. Single dose. For inclusion: pain moderate or severe on a 4‐point categorical scale Pain assessments at 0, 0.33, 0.66, 1 h and hourly up to 8 h |
|
| Participants | Major inpatient orthopaedic surgery N = 200 M = 98, F = 102 Mean age 63 years |
|
| Interventions | Gabapentin 250 mg, n = 50 Placebo, n = 49 also hydrocodone and gabapentin/hydrocodone groups |
|
| Outcomes | PI: standard 4 point scale PR: standard 5 point scale Overall assessment of study medication Time use of rescue medication Adverse events: any, serious Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The Pfizer Global Research & Development Biometrics Department generated the randomisation code |
| Allocation concealment (selection bias) | Low risk | Patients assigned to prenumbered study medication by Parke‐Davis remote allocation (remote allocation) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | matching placebo capsule |
720‐04483.
| Methods | Randomised. Double blind. Parallel group. Single dose. For inclusion: pain moderate or severe on a 4‐point categorical scale and ≥ 45 mm on a 100 mm visual analogue scale Pain assessments at 0, 0.33, 0.66, 1 h and hourly up to 8 h |
|
| Participants | Surgical removal of 1 or 2 third molars N = 101 M = 55, F = 46 Mean age 24 years |
|
| Interventions | Gabapentin 500 mg, n = 21 Placebo, n = 20 also gabapentin/hydrocodone groups |
|
| Outcomes | PI: standard 4 point scale PR: standard 5 point scale Overall assessment of study medication Time use of rescue medication Adverse events: any, serious Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Parke‐Davis Clinical Pharmacy Operations generated the randomization code |
| Allocation concealment (selection bias) | Low risk | Patients assigned to prenumbered study medication by Parke‐Davis (remote allocation); while this protocol does not give specific details of allocation concealment, it indicates that it follows same procedure as protocol 720‐0445. It is an amendment of this protocol. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | identical capsules |
N ‐ number of participants in study; n = number of participants in treatment arm
Contributions of authors
RAM and SD carried out searching. SS and SD carried out data extraction and analysis, including assessment of study quality. RAM acted as arbitrator. RAM, SD, PW and HJM were involved with writing the protocol. All review authors contributed to the writing of the final review.
Sources of support
Internal sources
Oxford Pain Research Funds, Other.
External sources
NHS Cochrane Collaboration Grant, UK.
-
NIHR Biomedical Research Centre Programme, UK.
[RAM]
-
UK National Institute of Health Research, UK.
[PW]
Declarations of interest
SD, SS, RAM & HJM have received research support from charities, government, academic, and industry sources at various times. This work was supported by NHS Cochrane Collaboration Grant and NIHR Biomedical Research Centre Programme. RAM and HJM have consulted for various pharmaceutical companies. RAM and HJM have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. PW is a full time employee of the UK Cochrane Centre.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
720‐04378 {unpublished data only}
720‐04455 {unpublished data only}
720‐04471 {unpublished data only}
720‐04483 {unpublished data only}
Additional references
Barden 2004
- Barden J, Edwards JE, McQuay HJ, Wiffen PJ. Relative efficacy of oral analgesics after third molar extraction. British Dental Journal 2004;197(7):407‐11. [DOI] [PubMed] [Google Scholar]
Clarke 2009
- Clarke R, Derry S, Moore RA, McQuay HJ. Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004309.pub2] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins SL, Edwards J, Moore RA, Smith LA, McQuay HJ. Seeking a simple measure of analgesia for mega‐trials: is a single global assessment good enough?. Pain 2001;91(1‐2):189‐94. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. The design of analgesic clinical trials. Advances in Pain Research Therapy 1991;18:117‐24. [Google Scholar]
Derry 2009
- Derry P, Derry S, Moore RA, McQuay HJ. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2008
- Derry S, Moore RA, McQuay HJ. Single dose oral celecoxib for acute postoperative pain. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004233] [DOI] [PubMed] [Google Scholar]
Derry 2009a
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2009b
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral naproxen and naproxen sodium for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore RA, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kissin 2009
- Kissin I. Patient‐controlled‐analgesia analgesimetry and its problems. Anesthesia and Analgesia 2009;108(6):1945‐9. [DOI: 10.1213/ane.0b013e3181a1a481] [DOI] [PubMed] [Google Scholar]
Kong 2007
- Kong VK, Irwin MG. Gabapentin: a multimodal perioperative drug?. British Journal of Anaesthesia 2007;99(6):775‐86. [DOI: 10.1093/bja/aem316] [DOI] [PubMed] [Google Scholar]
L'Abbe 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
Lloyd 2009
- Lloyd R, Derry S, Moore RA, McQuay HJ. Intravenous parecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004771.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2005
- McQuay HJ, Moore RA. Placebo. Postgraduate Medical Journal 2005;81:155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2008
- McQuay HJ, Poon KH, Derry S, Moore RA. Acute pain: combination treatments and how we measure their efficacy. British Journal of Anaesthesia 2008;101(1):69‐76. [DOI: 10.1093/bja/aen108] [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. [DOI: 10.1016/0304-3959(96)03032-1] [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69(1‐2):127‐30. [DOI: 10.1016/S0304-3959(96)03251-4] [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. [DOI: 10.1016/S0304-3959(96)03306-4] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2003
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. [DOI: 10.1016/j.pain.2005.05.001] [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratio and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with confidence ‐ confidence intervals and statistical guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Striano 2008
- Striano P, Striano S. Gabapentin: a Ca2+ channel alpha 2‐delta ligand far beyond epilepsy therapy. Drugs Today (Barcelona) 2008;44(5):353‐68. [DOI: 10.1358/dot.2008.44.5.1186403] [DOI] [PubMed] [Google Scholar]
Tiippana 2007
- Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesthesia and Analgesia 2007;104(6):1545‐56. [DOI: 10.1213/01.ane.0000261517.27532.80] [DOI] [PubMed] [Google Scholar]
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2009
- Wiffen PJ, Moore RA, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007938] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wiffen 2005
- Wiffen, PJ, McQuay HJ, Rees J, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005452] [DOI] [PubMed] [Google Scholar]


