Abstract
Background
Peripartum cardiomyopathy (PPCM or PCMO) is a rare disease of unknown etiology, characterised by an acute onset of heart failure in women in the late stage of pregnancy or in the early months postpartum.
Objectives
To assess the effectiveness and safety of any intervention for the care of women and/or their babies with a diagnosis of peripartum cardiomyopathy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (27 July 2010) and the reference lists of identified studies.
Selection criteria
Randomised and quasi-randomised controlled trials of any intervention for treating peripartum cardiomyopathy. Such interventions include: drugs; cardiac monitoring and treatment; haemodynamic monitoring and treatments; supportive therapies and heart transplant.
Data collection and analysis
Two authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. Data entry was checked.
Main results
We identified and included one pilot study, involving 20 women, undertaken in South Africa. Women were diagnosed postnatally and included in the study within 24 hours of diagnosis.
Authors’ conclusions
There are insufficient data to draw any firm conclusions. Treatment with bromocriptine appears promising, although women would be unable to breastfeed due to suppression of lactation.
Medical Subject Headings (MeSH): Acute Disease; Bromocriptine [adverse effects; *therapeutic use]; Cardiotonic Agents [adverse effects; *therapeutic use]; Heart Failure [* drug therapy]; Pilot Projects; Postoperative Period; Pregnancy Complications, Cardiovascular [* drug therapy]
MeSH check words: Female, Humans, Infant, Pregnancy
BACKGROUND
Peripartum cardiomyopathy (sometimes abbreviated to PCMO or PPCM) is a rare disorder in which an enlarged and weakened heart, which cannot pump blood efficiently, is diagnosed within the last month of pregnancy or within five months after birth, without other identifiable causes for dysfunction of the heart. Decreased heart function affects the lungs, liver, and other body systems. Symptoms usually include: orthopnoea (difficulty in breathing while lying flat); dyspnoea (shortness of breath on exertion); pitting oedema (swelling); palpitations (increased heart rate); chest pain; cough; frequent night-time urination; and excessive weight gain during the last month of pregnancy. Women have considerable morbidity and increased risk of mortality, and generally need to be looked after in specialist heart wards or in intensive care units. Babies can also be affected by the condition, either directly due to the side effects of the medications used to treat the heart problem or as a result of a medically induced preterm birth that may be necessary to improve the mother’s condition.
Description of the condition
Peripartum cardiomyopathy was first described by Gouley in 1937 (Gouley 1937) but the association between cardiac failure and the puerperium was first noted early in the 19th century (Ritchie 1850). The accepted definition is cardiomyopathy that develops in the last month of pregnancy or in the first five months postpartum, in the absence of pre-existing heart disease or any identifiable cause (Demakis 1971a). Pregnancy associated cardiomyopathy is an identical condition that has been described as presenting between 17 and 36 weeks’ gestation (Elkayam 2005) and other cases have been reported up to six months postpartum; therefore, it is likely that all three conditions are part of the same clinical spectrum.
The classical criteria for the diagnosis of peripartum cardiomyopathy were first described by Demakis (Demakis 1971b); however, more recently, Hibbard has proposed that additional criteria should include objective echocardiographic measurements of left ventricular dysfunction (ejection fraction less than 45%, fractional shortening of 30% or both and end-diastolic dimension of greater than 2.7 cm/m2 body surface area) (Hibbard 1999). Women usually present acutely with classical signs and symptoms of congestive cardiac failure; therefore, all possible aetiologies and other causes of cardiomyopathy must be excluded before peripartum cardiomyopathy is accepted as the definitive diagnosis.
The true incidence of peripartum cardiomyopathy is difficult to ascertain due to variations between individual studies, populations and the criteria used for diagnosis, but an accepted incidence is approximately one per 3000 to 4000 pregnant women or 1000 to 1300 cases each year in the USA (Pearson 2000). It is relatively rare in Europe (Ferriere 1990), more common in West Africa (Cenac 1998) and the Republic of South Africa (Desai 1995) (one in 1000), and higher still in Haiti at one in 299 (Fett 2002; Fett 2004a; Fett 2004b). The highest reported incidence is in the Hausa tribe of Nigeria where up to one in 100 women are affected, possibly due a combination of postpartum hypertension, the cultural practice of eating sodium rich kanwa leading to volume overload, and the cardiovascular demands of heat, both climatic and that traditionally self-imposed (Davidson 1978).
Risk factors may include age, multiparity, black race, pre-eclampsia or hypertension, multiple pregnancy and prolonged use of tocolysis (Demakis 1971b; Elkayam 2005; Fett 2005; Pearson 2000; Sliwa 2000). Although extremes of age, particularly older women of higher parity are associated with increased risk, up to one-third of cases may occur in young primigravidae (Demakis 1971b; Demakis 1971a; Desai 1995; Elkayam 2005; Fett 2005; Sliwa 2000; Sliwa 2006a).
The aetiology of peripartum cardiomyopathy is unknown, although many hypotheses have been presented including viral myocarditis, immune-mediated injury and the haemodynamic stresses of pregnancy. The possible relationship between viral myocarditis and peripartum cardiomyopathy was first reported by Farber and Glasgow in 1968 demonstrating a susceptibility to viral infections during pregnancy, with the greatest viral multiplication seen within the heart (Farber 1970). The physiological changes of pregnancy may also predispose to viral infection (Farber 1970; Lyden 1984) and result in more severe forms of myocarditis when infected (O’Connell 1986). Although myocarditis has been demonstrated in endomyocardial biopsies in several small studies, there is significant variation between studies with positive results ranging from 8.8% to 76% (Felker 2000; Melvin 1982; Midei 1990; O’Connell 1986; Rizeq 1994; Sanderson 1986), perhaps due to timing of biopsies in relation to the onset of symptoms or failure to adhere to Dallas criteria for histological diagnosis. One study has clearly linked the presence of viral genomic material in biopsies from patients with idiopathic cardiomyopathy and, more importantly, demonstrated improvements in left ventricular function with viral clearing (Bultmann 2005). If further research provides stronger evidence for a viral aetiology, biopsy may become increasingly important in the management of women with peripartum cardiomyopathy. A possible genetic predisposition has now also been suggested (van Spaendonck-Zwarts 2010).
Immune mediated mechanisms have also been associated with the development of peripartum cardiomyopathy. Increased concentrations of inflammatory mediators such as tumour necrosis factor α, C-reactive protein, interleukin six and soluble Fas receptors (an apoptosis signalling receptor) have been reported and correlate with echocardiographic measures of impaired left ventricular function (Sliwa 2000; Sliwa 2006a).
The most recent developments have focused on the key role of unbalanced oxidative stresses and the generation of a cardiotoxic prolactin sub fragment in experiments on mice, thus opening up novel, disease-specific treatments using prolactin blockade (Hilfiker-Kleiner 2007). This has led to a pilot study of the dopamine antagonist, bromocriptine, in pregnant women who developed cardiomyopathy within a month of giving birth (Sliwa 2010).
In summary, it is unlikely that one single mechanism is responsible for the development of peripartum cardiomyopathy, but rather the interaction of multiple processes within susceptible individuals.
Additional considerations for care
Multidisciplinary teams compromising maternal fetal medicine specialists, cardiologists, intensivists and obstetric anaesthetists are essential to optimise care of these women. Investigations such as echocardiogram (ECHO), which is essential for diagnosis, and magnetic resonance imaging (MRI) scans can provide clinically useful information.
The use of uterotonics in any of the three stages of labour needs careful consideration, due to their effects on central and peripheral haemodynamics (Sultatos 1997). Syntocinon can cause profound peripheral vasodilatation and in women with, or at risk of, cardiac disease should not be administered with large volumes of fluid or given as a rapid bolus (Hendricks 1970; Pinder 2002; Tamhane 2006). Ergot derivatives also have the potential to significantly increase maternal blood pressure and should generally be avoided in women with cardiac problems (Liabsuetrakul 2007). Newer agents, such as prostaglandin F2-alpha and misoprostol, are also vasoactive and their use should be balanced against the potential risks involved (Chong 1997; Gülmezoglu 2007).
Intrapartum analgesics can be used, but care should be taken to avoid large doses of opiates which may depress respiratory and myocardial function (Bricker 2002). Regional anaesthesia can cause significant sympathetic blockade and, therefore, needs to be managed at a senior level and only after consultation with specialist obstetric anaesthetists and the attending cardiologist (Pinder 2002; Tamhane 2006). Any additional pregnancy complications will add to the complexity of the woman’s management and these must be considered when planning for the birth and postpartum care.
Description of the intervention
Interventions are complex because they include:
drugs (primary treatment);
place of care;
monitoring techniques;
supportive therapies such as ventricular assist devices and, ultimately, transplantation.
Drug treatments
Most treatment protocols for peripartum cardiomyopathy are similar to those used in congestive cardiac failure (Sliwa 2006b), with the emphasis on reducing afterload/preload and improving coronary blood flow (Pearson 2000).
Angiotensin converting enzyme (ACE) inhibitors and the newer receptor blockers are the mainstay of treatment postpartum, but fetal toxicity limits their use in pregnancy; therefore, nitrates (such as hydralazine) are preferred prior to birth.
Beta-blockers (β-blockers) reduce heart rate, thus improving left ventricular diastolic function and also protect against arrhythmias. Carvedilol has certainly demonstrated improvements in the outcomes of pregnant women with dilated cardiomyopathy, but whether or not the same applies to peripartum cardiomyopathy is unclear and the lack of safety data for this β-blocker will probably limit its use. Safer alternatives such as metoprolol are more commonly used during pregnancy. The inotrope digoxin can also be used to maximise contractility and rate control, although caution is advised when used in females as higher serum concentrations can worsen prognosis (Adams 2005a; Rathore 2002). Diuretics are relatively safe and act to decrease volume overload and pulmonary congestion, thereby giving symptomatic relief.
A high incidence of thromboembolic events (Bennani 2003; Box 2004; Damorou 2000; Kaufman 2003; Nishi 2002; Quinn 2004; Sanchez-Rubio Lezcano 2004) and bedrest as an adjunct to medical and supportive therapy has also encouraged the widespread use of thromboprophylaxis in these patients, although this practice is based on expert opinion rather than any data from experimental studies (Abboud 2007).
Due to the theories surrounding immune-based mechanisms in the aetiology of peripartum cardiomyopathy, attention has also focused on immune modulation therapies in an attempt to modify maternal responses. Intravenous immunoglobulin has been explored as an alternative treatment option, based on the reported improvements noted in left ventricular function in patients with dilated cardiomyopathy and myocarditis. In theory, this type of intervention may ameliorate the degree of myocardial damage, thus accelerating recovery and restoration of normal ventricular function, but data thus far are equivocal. These types of therapy may seem logical, although thus far they have failed to produce significant improvements in outcome in the management of acute cardiomyopathy (McNamara 2001) or myocarditis (Mason 1995). A single small pilot trial using intravenous immunoglobulin for peripartum cardiomyopathy showed significant improvements in cardiac function when compared with a group of historical controls (Bozkurt 1999); therefore, this approach may still warrant further assessment.
Inhibitors of inflammatory mediators may also have a role in the management of peripartum cardiomyopathy as tumour necrosis factor-α (TNF-α) levels are elevated in these patients, and the xanthine-derived inhibitor of TNF-α (pentoxifylline) has been investigated in a prospective controlled trial, demonstrating measurable improvements in outcome (Sliwa 2002). Although a recent systematic review of several small trials suggested that pentoxifylline may be of benefit in other forms of cardiomyopathy, larger studies are recommended to confirm both efficacy and safety in peripartum cardiomyopathy (Batchelder 2005).
Dopamine-receptor antagonists may have a role. People with acute peripartum cardiomyopathy have increased serum levels of oxLDL, activated cathepsin D and 16kDa prolactin, which suggest the presence of a systemically activated cascade triggered by oxidative stress (Hilfiker-Kleiner 2007). Activation of this cascade may, therefore, be an important factor in the pathophysiology of peripartum cardiomyopathy, raising the possibility of prolactin blockade as a potentially new therapy. A single study has demonstrated the potential value of bromocryptine (a dopamine-receptor antagonist) when used prophylactically in pregnant women with a past history of peripartum cardiomyopathy. Six women who received bromocryptine in addition to standard cardiac failure therapy had uneventful follow ups, while all six women in the non-bromocryptine group suffered with recurrence and three died (Hilfiker-Kleiner 2007). As there have been reported cases of myocardial infarction due to thromboembolic events in postpartum women taking bromocryptine (Hopp 1996), thromboprophylaxis is recommended as a co-therapy. Larger controlled studies are awaited to confirm this promising new treatment.
Cardiac monitoring and treatments
All but the mildest cases will require some form of cardiovascular monitoring. This will vary according to the severity of the woman’s condition but may include continuous ECG (electrocardiogram) in a coronary care unit or full invasive haemodynamic monitoring, such as pulmonary catheters, pulse contour cardiac output (PiCCO) or lithium dilution cardiovascular output monitoring (LiDCO) in an intensive care setting.
The concept of ‘goal targeted therapy’ has been around for some time since Shoemaker’s original study in the 1980s (Shoemaker 1983). In simple terms, individual haemodynamic parameters are optimised in order to improve overall prognosis, or more commonly, to speed recovery. As far as we are aware, this strategy has yet to be considered or assessed in clinical trials in the treatment of peripartum cardiomyopathy.
Haemodynamic monitoring and treatments
Management in the coronary care unit (CCU) or intensive care unit is usually required due to the severity of the condition. This opens up possibilities for even more interventions, not just pharmacological but also haemodynamic monitoring and cardiovascular supportive therapy, such as ventricular assist devices and a variety of ventilatory strategies.
Supportive therapies
During the most critical phase of the disease process, additional supportive therapies are often required to allow time for targeted medical treatments to improve ventricular function. These may include intravenous inotropic support such as catecholamines (adrenaline/noradrenaline) or dobutamine to increase cardiac output and critical tissue perfusion, or more invasive methods such as balloon pumps also known as ‘ventricular assist devices’. Intraaortic balloon pump therapy helps restore the balance between the supply of oxygen-rich blood the heart receives from the coronary arteries, and the amount of oxygen the heart needs to pump. The intra-aortic balloon pump assists the heart during both its rest phase and its work phase. In the rest phase, the balloon inflates, increasing the supply of oxygen-rich blood to the coronary arteries. In the work phase, the balloon deflates, decreasing the workload on the heart. The decrease in workload results in a decrease in the amount of oxygen the heart needs to pump. These devices are often used in the short term or to enable transfer to units with cardiac bypass facilities when the situation becomes critical or as a bridge to transplantation (Sayer 2009). Currently, we think it unlikely that such methods are used routinely in the management of peripartum cardiomyopathy.
Cardiac transplant
In the most severe and refractory cases, cardiac transplantation is used as a last resort. Several authors have published outcomes following transplantation for peripartum cardiomyopathy reporting higher than expected incidences of rejection and infection (Aravot 1987; Keogh 1994).
How the intervention might work
Standard medical interventions for the treatment of acute heart failure utilise their basic pharmacological actions. In simple terms, most of these drugs act either by reducing pre- and afterload, or by relieving congestion This combination of effects act to optimise function and increase contractility. In contrast to chronic heart failure, therapeutic advances in the treatment of acute heart failure syndromes (AHFS) have been limited. This situation is slowly being resolved with the some recent clinical trials and the development of large acute heart failure registries in the non-pregnant population (Adams 2005b; Cleland 2003; Fonarow 2007). These have combined to help provide a better understanding of the population and identify certain features that may drive individualised therapy.
Regardless of the clinical situation in the non-pregnant groups, the initial management should always focus on:
identification and treatment of the precipitant of AHFS, e.g. ischaemia, atrial fibrillation, infarction;
symptom improvement;
haemodynamic optimisation;
achieving euvolaemia;
adequate oxygenation;
ventilatory support.
The approach to the management of suspected peripartum cardiomyopathy is as above, with some alterations dependent on whether the woman is still pregnant. Standard therapy includes diuretics, nitrates, β-blockers, digoxin and ACE inhibitors. Whilst some of these medications have evidence of benefit outside pregnancy, the rationale for their use in peripartum cardiomyopathy is limited to expert opinion due to the lack of adequately controlled clinical trials. In more severe cases, potent inotropes such as dopamine and dobutamine are needed during the supportive phase.
Management in high-dependency areas becomes necessary when multi-system support is required. This may be a combination of ventilation, invasive monitoring guided inotropic therapy and more intensive treatments, such as balloon pumps. These types of interventions are primarily concerned with gaining time during the most severe phase of the disease, in order to allow the medical therapies time to take effect. Once volume overload has been corrected - and this may take several days - therapy moves onto the chronic phase, with several months often needed before full cardiac function is restored. In some cases, residual functional deficits can persist, and it is these cases that should be advised to avoid any future pregnancy due to the significant risk of maternal death.
Why it is important to do this review
Despite optimal medical management, morbidity and mortality remain significant, although more recent data describe improved outcomes compared to older studies (Amos 2006; Brar 2007; Mielniczuk 2006). As previously discussed, the management of peripartum cardiomyopathy is largely extrapolated from established treatments of acute heart failure in non-pregnant adults. It is possible that the unique cardiovascular physiology of pregnancy may alter the effectiveness of these interventions or that the aetiology of peripartum cardiomyopathy, largely unknown, means that modified or completely new treatment strategies are needed in order to achieve the best prognosis.
Interventions beyond medical therapies are also used in the treatment of peripartum cardiomyopathy, again on the basis that pump failure is the endpoint common to acute heart failure and the pregnancy related disease. These may include coronary or intensive care regimes, incorporating invasive monitoring, with or without inotropic support and more complex interventions such as cardiopulmonary bypass and ventricular assist devices. A variety of ventilatory strategies are also used to manage women with peripartum cardiomyopathy, but these are not necessarily supported by a solid evidence base.
The clinical significance of peripartum cardiomyopathy, its relationship with maternal health and the uncertainties which surround both aetiology and treatment generate a very strong argument for the need to review the current intervention and management strategies.
OBJECTIVES
To assess the effectiveness and safety of any intervention for the care of women and/or their babies with a diagnosis of peripartum cardiomyopathy.
METHODS
Criteria for considering studies for this review
Types of studies
We included randomised and quasi-randomised trials, as long as they reported clinically meaningful outcomes and presented results on an intention-to-treat (ITT) basis. In future studies, where authors do not report clinically meaningful outcomes or present results on an ITT basis, we shall write to request further data and information. We excluded cluster and crossover trials. In future, we will include studies published only as conference abstracts, and we shall write to authors seeking further data and information.
Types of participants
Women with a confirmed diagnosis of peripartum cardiomyopathy. If data are identified in future, we shall analyse separately women who have had a previous cardiomyopathy.
Types of interventions
Any clinical or other intervention specifically designed to improve the care of women or their babies following a diagnosis of peripartum cardiomyopathy. Such interventions might include: early birth of the baby, different medical treatments and monitoring systems - invasive or otherwise - high dependency/intensive care, and a variety of ventilatory modalities.
Comparisons include:
drug treatments (e.g. ACE inhibitors, B-blockers, thromboprophylaxis, immune modulation therapies) versus standard care;
cardiovascular monitoring and treatments versus standard care;
haemodynamic monitoring and treatments versus standard care;
supportive therapies such as ventricular assist devices versus standard care;
heart transplantation versus standard care.
Where standard care is that described in the hospital where the study was undertaken.
Types of outcome measures
Indices of maternal outcome (e.g. death, chronic heart failure, renal failure, length of stay in coronary care or intensive care, venous thromboembolism, impact on future fertility and pregnancies, emotional status).
Fetal outcome (e.g. death, short- and long-term disability, length of stay in neonatal intensive care unit), and obstetric care (e.g. caesarean section, admission to intensive care unit).
Primary outcomes
Maternal death.
Chronic heart failure.
Time to recovery of left ventricular function to baseline or ejection fraction greater than 40%.
Secondary outcomes
Maternal
Caesarean section.
Operative vaginal birth.
Organ failure other than cardiac.
Time spent in high dependency area - HDU/CCU/ICU.
Time to discharge.
Fetal and infant
Perinatal mortality.
Fetal death.
Neonatal death.
Birth before 37 weeks’ gestation.
Birth before 32 weeks’ gestation.
Birth before 28 weeks’ gestation.
Admission to neonatal intensive care.
Neonatal length of hospital stay.
Major neurodevelopmental delay at childhood follow up.
Additional outcome
Cost.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (27 July 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved papers.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors (A Carlin (AC), G Gyte (GG)) independently assessed for inclusion the one study we identified as a result of the search strategy (Sliwa 2010). There was no disagreement about its inclusion and no need, therefore, to consult the third author.
Data extraction and management
We designed a form to extract data. Two review authors (AC, GG) extracted the data using the agreed form. We resolved discrepancies through discussion or, had it been required, we would have consulted the third author. We entered the data into Review Manager software (RevMan 2008) and checked them for accuracy.
Since some of the information regarding the above was unclear, or data were missing on many of our specified outcomes, we are writing to the authors requesting further information.
Assessment of risk of bias in included studies
Two review authors (AC, GG) independently assessed risk of bias for the included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We resolved any disagreements by discussion and had it been required, we would have consulted the third author.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should have produce comparable groups.
We assessed the methods as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non-opaque envelopes; alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We describe for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We also provide information on whether the intended blinding was effective. Where blinding was not possible, we have assessed whether the lack of blinding was likely to have introduced bias. Blinding was assessed separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We describe for each included study and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. In future, where sufficient information was reported or supplied by the trial authors, we will re-include missing data in the analyses which we undertake.
In future, we shall discuss whether missing data greater than 20% might impact on outcomes acknowledging that, with long-term follow up, complete data are difficult to attain.
(5) Selective reporting bias
We describe for each included study how the possibility of selective outcome reporting bias was examined by us and what we found. We assessed the methods as:
adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre-specified outcomes have been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We describe for each included study any important concerns we have about other possible sources of bias, e.g. whether the study was stopped early and reporting the reason, baseline imbalances and differential diagnoses.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2009). With reference to (1) to (6) above, we have assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We would have explored the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis) had there been sufficient studies and data.
Measures of treatment effect
Dichotomous data
When further studies are included then, for dichotomous data, we shall present results as summary risk ratio with 95% confidence intervals.
Continuous data
When further studies are included then, for continuous data, we shall use the mean difference if outcomes are measured in the same way between trials. We shall use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
We have excluded cluster trials and crossover trials as we feel they are not appropriate designs for this topic.
Dealing with missing data
For the included study, we noted the level of attrition. In future, when further studies are included, we shall explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we have carried out analyses, as far as possible, on an intention-to-treat basis, i.e. we have included all participants randomised in the analyses, and we have analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. In future updates, the denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing (i.e. ‘available case’ analysis).
Assessment of heterogeneity
In future updates, when further studies have been included, we will assess statistical heterogeneity in each meta-analysis using the T2, I2 and Chi2 statistics. We will regard heterogeneity as substantial if T2 is greater than zero and either I2 is greater than 30% or there is a low P-value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in a meta-analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes, we will use the test proposed by Egger 1997 and, for dichotomous outcomes, we will use the tests proposed by Harbord 2006. If asymmetry is detected by any of these tests or is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out the statistical analysis using the Review Manager software (RevMan 2008). In future, when further studies are included, we will use fixed-effect meta-analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random-effects analysis to produce an overall summary if this is considered clinically meaningful. If an average treatment effect across trials is not clinically meaningful, we will not combine heterogeneous trials. If we use random-effects analyses, the results will be presented as the average treatment effect and its 95% confidence interval, the 95% prediction interval for the underlying treatment effect, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
In future updates when further studies are included, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful and, if it is, use random-effects analysis to produce it.
In future updates when more data are available, we plan to carry out the following subgroup analyses:
gestation less than or equal to 37 weeks versus gestation greater than 37 weeks;
maternal age less than or equal to 40 years at presentation versus greater than 40 years at presentation;
presentation antenatally versus presentation postpartum;
women with no pre-existing medical conditions versus women with pre-existing medical condition, e.g. diabetes, hypertension.
Only primary outcomes will be used in subgroup analysis.
For fixed-effect inverse variance meta-analysis, we will assess differences between the subgroups by interaction tests (Deeks 2001). For random-effects analyses and fixed-effect analyses using methods other than inverse variance, we will assess differences between subgroups by inspection of the subgroups and confidence intervals; non-overlapping confidence intervals indicate a difference in treatment effect between the subgroups.
Sensitivity analysis
In future updates, we will carry out sensitivity analysis to explore the effect of trial quality for important outcomes in the review. Where there is high risk of bias associated with a particular aspect of study quality (e.g. quasi-randomised controlled trials or inadequate sequence generation and allocation concealment), we will explore this by sensitivity analysis. We will only use primary outcomes in the sensitivity analyses.
RESULTS
Description of studies
See: Characteristics of included studies.
We identified and included one pilot study, involving 20 women, undertaken in South Africa (Sliwa 2010). All women in this trial were recruited in the postnatal period (see Characteristics of included studies).
Risk of bias in included studies
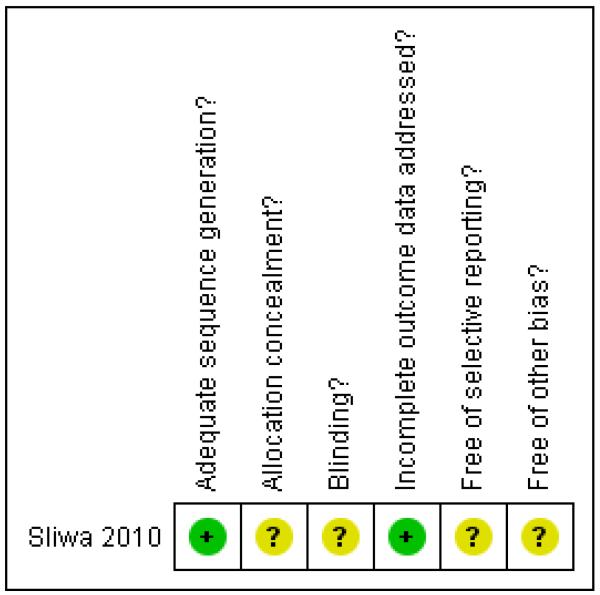
The one included study was assessed as having low risk of bias for sequence generation, although it was unclear whether the allocation was concealed. There was complete reporting of outcome data, although blinding, selective reporting bias and other possible sources of bias were unclear (Figure 1).
Figure 1.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Effects of interventions
1. Drug treatments plus standard care versus standard care alone (one study, 20 women)
a) Bromocriptine for eight weeks
The one pilot study identified assessed an eight weeks of treatment with bromocriptine (a dopamine-receptor antagonist) against standard care for heart failure (see Characteristics of included studies). This study included only women diagnosed postnatally. These women were recruited to the study within 24 hours of diagnosis.
Primary outcomes
There was no statistically significant difference identified in maternal mortality, risk ratio (RR) 0.25, 95% confidence interval (CI) 0.03 to 1.86, one study, 20 women, Analysis 1.1). The other primary outcomes were not assessed.
Secondary outcomes
None of the secondary outcomes were assessed.
Outcomes not pre-specified
The study authors reported a number of laboratory measurements and these have not been included because we were specifically interested in clinical outcomes. ‘Poor outcome’, assessed as a composite outcome, was measured; it included death, New York Heart Association (NYHA) functional class III/IV and left ventricular ejection fractions less than 35% at six months. We are writing for further information, and once we have confirmed that the composite outcome only counted each woman once, we will include these data.
2. Cardiovascular monitoring plus treatment versus standard care alone (no studies)
We identified no studies for this comparison.
3. Haemodynamic monitoring plus treatment versus standard care alone (no studies)
We identified no studies for this comparison.
4. Supportive therapies plus standard care versus standard care alone (no studies)
We identified no studies for this comparison.
5. Heart transplant versus standard care (no studies)
We identified no studies for this comparison.
DISCUSSION
There is only one small pilot study involving 20 women with peripartum cardiomyopathy, who were treated with bromocriptine for eight weeks compared with standard care. The study is limited in that it only included women diagnosed postnatally, and maternal mortality was the single outcome measure on which data were available. It is important to consider that women given bromocriptine postpartum will be unable to breastfeed because bromocriptine suppresses lactation (Oladapo 2009), and far more safety data are required to assess the potential adverse effects of this treatment (Ruch 1989). Overall, there was insufficient evidence in this small study to make any firm recommendations, but the authors report that larger-scale multicentred, blinded studies are in progress to test the strategy more robustly.
AUTHORS’ CONCLUSIONS
Implications for practice
The clinical management of peripartum cardiomyopathy has to rely on information other than that obtained from randomised controlled trials.
Implications for research
There is an urgent need for further study into the management of peripartum cardiomyopathy. Large International multicentre randomised controlled clinical intervention trials may provide important guidance for the management of this serious maternal disease and we suggest that these should include outcomes from this review as a minimum dataset. It will be important to collect outcome data on the babies as well as the mothers.
PLAIN LANGUAGE SUMMARY.
Interventions for treating pregnant women or new mothers with heart failure of unknown cause (peripartum cardiomyopathy)
Very rarely, some women suffer from heart failure (without any known cause) in late pregnancy or as a new mother. The heart muscle becomes large and weakened, and is unable to pump blood properly round the body. This affects the lungs, liver, and other body systems. Symptoms include: difficulty in breathing, shortness of breath, the heart racing or skipping beats. There can also be chest pain, swelling, and excessive weight gain during the last month of pregnancy. Women need to be cared for in intensive care wards. Labour is often medically induced earlier than normal if the problem arises late in pregnancy. These babies then suffer the problems of being born too early (prematurely). This review looked at interventions which might reduce harm for women with this condition The interventions included drugs, heart or blood monitoring, supportive therapies and heart transplants. We found only one pilot study, involving 20 women with heart failure after giving birth, that looked at bromocriptine given over a period of eight weeks. There were not enough data to provide a clear answer on the number of mothers dying, but the drug looked promising. Biochemical measurements were also made. Women need to be informed that the drug stops the production of breastmilk, making breastfeeding impossible. We found no trials on other possible interventions. Large trials are needed to decide the best treatment for these women and their babies.
ACKNOWLEDGEMENTS
The review authors would like to acknowledge the support of Mrs Sonja Henderson, Managing Editor, and Ms Lynn Hampson, Trials Search Co-ordinator, in the preparation of this review.
As part of the pre-publication editorial process, this review has been commented on by two peers (an editor and referee who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
The University of Liverpool, UK.
External sources
National Institute for Health Research, UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial - 2 arms. | |
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intervention: bromocriptine + standard heart failure therapy.
Comparison: standard heart failure therapy.
|
|
| Outcomes | Maternal
Infant
|
|
| Notes | Setting: Chris Hani Baragwanath Hospital, Cape Town, South Africa. Women were referred there from local clinics and secondary hospitals Subgroups
Additional information for documentation
We are writing to the authors for further information on their study |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | “…computer generated randomization list…”. |
| Allocation concealment? | Unclear | No information provided. |
| Blinding? All outcomes |
Unclear | “All open-label efficacy assessments were made by independent blinded investigators.” Also described as ‘open label’ with “…blinded clinical, hemodynamic, and echocardiographical assessments…” Blinding seems to be not possible because the women on bromocriptine cannot breastfeed because the drug dries up breastmilk production. However, it is reported that investigators were blinded for data analysis |
| Incomplete outcome data addressed? All outcomes |
Yes | No women were excluded after randomisation. Data were complete for mortality, but not all women received all the tests |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Unclear |
PPCM-BR: Enalapril 5 mg/d (range 5-10); Carvedilol 6.25 mg twice daily (range 6.25-25); Furosamide at 6 months was 80 mg/d (range 80-120) PPCM-Std: Enalapril 10 mg/d (range 5-10); Carvedilol 12.25 mg twice daily (range 6.25-25); Furosamide at 6 months was 80 mg/d (range 80-120) |
ACE: angiotensin converting enzyme
CPR: cardiac pulmonary resistance
FBC: full blood count
hsCPR: high-sensitivity
LFT: liver function tests
LV: left ventricular
LVEF: left ventricular ejection fraction
MRI: magnetic resonance imaging
NYHA: New York Heart Association
PPCM: peripartum cardiomyopathy
DATA AND ANALYSES
Comparison 1.
Drug treatments versus standard care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death (primary) | 1 | 20 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
| 1.1 Bromocriptine | 1 | 20 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
| 2 Chronic heart failure (primary) | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Time to recovery of left ventricular function (primary) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.1 Bromocriptine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 4 Caesarean section | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Operative vaginal birth | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Organ failure other than cardiac | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Time in high-dependency area | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 7.1 Bromocriptine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 8 Time to discharge | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 8.1 Bromocriptine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 9 Perinatal mortality | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Fetal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Neonatal death | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Birth before 37 weeks’ gestation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13 Birth before 34 weeks’ gestation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Birth before 28 weeks’ gestation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15 Admission to neonatal intensive care unit | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16 Length of neonatal hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16.1 Bromocriptine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17 Major neurodevelopmental delay in childhood | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17.1 Bromocriptine | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18 Cost | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18.1 Bromocriptine | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Analysis 1.1. Comparison 1 Drug treatments versus standard care, Outcome 1 Maternal death (primary)
Review: Interventions for treating peripartum cardiomyopathy to improve outcomes for women and babies
Comparison: 1 Drug treatments versus standard care
Outcome: 1 Maternal death (primary)
 |
HISTORY
Protocol first published: Issue 7, 2010
Review first published: Issue 9, 2010
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The title has been changed from ‘Interventions for treating peripartum cardiomyopathy’ to ‘Interventions for treating peripartum cardiomyopathy to improve outcomes for women and babies’.
Footnotes
DECLARATIONS OF INTEREST No known conflicts of interest.
References to studies included in this review
- Sliwa 2010 {published data only} .Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121(13):1465–73. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
Additional references
- Abboud 2007 .Abboud J, Murad Y, Chen-Scarabelli C, Saravolatz L, Scarabelli TM. Peripartum cardiomyopathy: a comprehensive review. International Journal of Cardiology. 2007;118(3):295–303. doi: 10.1016/j.ijcard.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Adams 2005a .Adams KF, Jr, Patterson JH, Gattis WA, O’Connor CM, Lee CR, Schwartz TA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. Journal of the American College of Cardiology. 2005;46(3):497–504. doi: 10.1016/j.jacc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- Adams 2005b .Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American Heart Journal. 2005;149(2):209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Amos 2006 .Amos AM, Jaber WA, Russel SD. Improved outcomes in peripartum cardiomyopathy with contemporary. American Heart Journal. 2006;152(3):509–13. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Aravot 1987 .Aravot DJ, Banner NR, Dhalla N, Fitzgerald M, Khaghani A, Radley-Smith R, et al. Heart transplantation for peripartum cardiomyopathy. Lancet. 1987;2(8566):1024. doi: 10.1016/s0140-6736(87)92585-2. [DOI] [PubMed] [Google Scholar]
- Batchelder 2005 .Batchelder K, Mayosi BM. Pentoxifylline for heart failure: a systematic review. South African Medical Journal. 2005;95(3):171–5. [PubMed] [Google Scholar]
- Bennani 2003 .Bennani SL, Loubaris M, Lahlou I, Haddour N, Badidi M, Bouhouch R, et al. Postpartum cardiomyopathy revealed by acute lower limb ischemia. Annales de Cardiologie et Angeiologie. 2003;52(6):382–5. doi: 10.1016/s0003-3928(03)00059-3. [DOI] [PubMed] [Google Scholar]
- Box 2004 .Box LC, Hanak V, Arciniegas JG. Dual coronary emboli in peripartum cardiomyopathy. Texas Heart Institute Journal. 2004;31(4):442–4. [PMC free article] [PubMed] [Google Scholar]
- Bozkurt 1999 .Bozkurt B, Villaneuva FS, Holubkov R, Tokarczyk T, Alvarez RJ, Jr, MacGowan GA, et al. Intravenous immune globulin in the therapy of peripartum cardiomyopathy. Journal of the American College of Cardiology. 1999;34(1):177–80. doi: 10.1016/s0735-1097(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Brar 2007 .Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, Hsu JW, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. American Journal of Cardiology. 2007;100(2):302–4. doi: 10.1016/j.amjcard.2007.02.092. [DOI] [PubMed] [Google Scholar]
- Bricker 2002 .Bricker L, Lavender T. Parenteral opioids for labor pain relief: a systematic review. American Journal of Obstetrics and Gynecology. 2002;186(5):s94–s109. doi: 10.1067/mob.2002.121549. [DOI] [PubMed] [Google Scholar]
- Bultmann 2005 .Bultmann BD, Klingel K, Näbauer M, Wallwiener D, Kandolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. American Journal of Obstetrics and Gynecology. 2005;193(2):363–5. doi: 10.1016/j.ajog.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Cenac 1998 .Cenac A, Djibo A. Postpartum cardiac failure in Sudanese-Sahelian Africa: clinical prevalence in western Niger. American Journal of Tropical Medicine and Hygiene. 1998;58(3):319–23. doi: 10.4269/ajtmh.1998.58.319. [DOI] [PubMed] [Google Scholar]
- Chong 1997 .Chong YS, Chua S, Arulkumaran S. Severe hyperthermia following oral misoprostol in the immediate postpartum period. Obstetrics and Gynecology. 1997;90(4):703–4. doi: 10.1016/s0029-7844(97)00275-5. [DOI] [PubMed] [Google Scholar]
- Cleland 2003 .Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme-- a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. European Heart Journal. 2003;24(5):442–63. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- Damorou 2000 .Damorou FJ, Kane A, Damorou FJ, Kane A, Napporn G, Thiam O, et al. Biventricular thrombus complicating peripartum cardiomyopathy. A case report. Dakar Medical. 2000;45(2):199–201. [PubMed] [Google Scholar]
- Davidson 1978 .Davidson NM, Parry EH. Peri-partum cardiac failure. Quarterly Journal of Medicine. 1978;47(188):431–61. [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. BMJ Books; London: 2001. [Google Scholar]
- Demakis 1971a .Demakis JG, Rahimtoola SH, Sutton GC, Meadows WR, Szanto PB, Tobin JR, et al. Natural course of peripartum cardiomyopathy. Circulation. 1971;44(6):1053–61. doi: 10.1161/01.cir.44.6.1053. [DOI] [PubMed] [Google Scholar]
- Demakis 1971b .Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44(5):964–8. doi: 10.1161/01.cir.44.5.964. [DOI] [PubMed] [Google Scholar]
- Desai 1995 .Desai D, Moodley J, Naidoo D. Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Tropical Doctor. 1995;25(3):118–23. doi: 10.1177/004947559502500310. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkayam 2005 .Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111(16):2050–5. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- Farber 1970 .Farber PA, Glasgow LA. Viral myocarditis during pregnancy: encephalomyocarditis virus infection in mice. American Heart Journal. 1970;80(1):96–102. doi: 10.1016/0002-8703(70)90042-6. [DOI] [PubMed] [Google Scholar]
- Felker 2000 .Felker GM, Jaeger CJ, Klodas E, Thiemann DR, Hare JM, Hruban RH, et al. Myocarditis and long-term survival in peripartum cardiomyopathy. American Heart Journal. 2000;140(5):785–91. doi: 10.1067/mhj.2000.110091. [DOI] [PubMed] [Google Scholar]
- Ferriere 1990 .Ferriere M, Sacrez A, Bouhour JB, Cassagnes J, Geslin P, Dubourg O, et al. Cardiomyopathy in the peripartum period: current aspects. A multicenter study. 11 cases. Archives des Maladies du Coeur et de Vaisseaux. 1990;83(10):1563–9. [PubMed] [Google Scholar]
- Fett 2002 .Fett JD, Carraway RD, Dowell DL, King ME, Pierre R. Peripartum cardiomyopathy in the Hospital Albert Schweitzer District of Haiti. American Journal Obstetrics and Gynecology. 2002;186(5):1005–10. doi: 10.1067/mob.2002.122423. [DOI] [PubMed] [Google Scholar]
- Fett 2004a .Fett JD, Dowell DL, Carraway RD, Sundstrom JB, Ansari AA. One hundred cases of peripartum cardiomyopathy… and counting: what is going on? International Journal of Cardiology. 2004;97(3):571–3. doi: 10.1016/j.ijcard.2003.10.068. [DOI] [PubMed] [Google Scholar]
- Fett 2004b .Fett J, Christie LG, Carraway R, Ansari A, Sundstrom J, Murphy J. Learning from a population-based peripartum cardiomyopathy registry. Circulation. 2004;110(111):727. [Google Scholar]
- Fett 2005 .Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clinic Proceedings. 2005;80(12):1602–6. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- Fonarow 2007 .Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. Journal of the American College of Cardiology. 2007;50(8):768–77. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- Gouley 1937 .Gouley BA, McMillan TM, Bellet S. Idiopathic myocardial degeneration associated with pregnancy and especially the peripartum. American Journal of Medical Sciences. 1937;19:185–99. [Google Scholar]
- Gülmezoglu 2007 .Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews. 2007;(Issue 3) doi: 10.1002/14651858.CD000494.pub3. [DOI: 10.1002/14651858.CD000494.pub3] [DOI] [PubMed] [Google Scholar]
- Harbord 2006 .Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Hendricks 1970 .Hendricks CH, Brenner WE. Cardiovascular effects of oxytocic drugs used post partum. American Journal of Obstetrics and Gynecology. 1970;108:751–60. doi: 10.1016/0002-9378(70)90542-9. [DOI] [PubMed] [Google Scholar]
- Hibbard 1999 .Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstetrics and Gynecology. 1999;94(2):311–6. doi: 10.1016/s0029-7844(99)00293-8. [DOI] [PubMed] [Google Scholar]
- Higgins 2009 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration; 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Hilfiker-Kleiner 2007 .Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Hopp 1996 .Hopp L, Haider B, Iffy L. Myocardial infarction postpartum in patients taking bromocriptine for the prevention of breast engorgement. International Journal of Cardiology. 1996;57(3):227–32. doi: 10.1016/s0167-5273(96)02789-1. [DOI] [PubMed] [Google Scholar]
- Kaufman 2003 .Kaufman I, Bondy R, Benjamin A. Peripartum cardiomyopathy and thromboembolism; anesthetic management and clinical course of an obese, diabetic patient. Canadian Journal of Anaesthesia. 2003;50(2):161–5. doi: 10.1007/BF03017850. [DOI] [PubMed] [Google Scholar]
- Keogh 1994 .Keogh A, Macdonald P, Spratt P, Marshman D, Larbalestier R, Kaan A. Outcome in peripartum cardiomyopathy after heart transplantation. Journal of Heart and Lung Transplantation. 1994;13(2):202–7. [PubMed] [Google Scholar]
- Liabsuetrakul 2007 .Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews. 2007;(Issue 2) doi: 10.1002/14651858.CD005456.pub2. [DOI: 10.1002/14651858.CD005456.pub2] [DOI] [PubMed] [Google Scholar]
- Lyden 1984 .Lyden DC, Huber SA. Aggravation of coxsackievirus, group B, type 3-induced myocarditis and increase in cellular immunity to myocyte antigens in pregnant Balb/c mice and animals treated with progesterone. Cell Immunology. 1984;87(2):462–72. doi: 10.1016/0008-8749(84)90015-7. [DOI] [PubMed] [Google Scholar]
- Mason 1995 .Mason JW, O’Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. New England Journal of Medicine. 1995;333(5):269–75. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- McNamara 2001 .McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre-Amione G, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103(18):2254–9. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- Melvin 1982 .Melvin KR, Richardson PJ, Olsen EG, Daly K, Jackson G. Peripartum cardiomyopathy due to myocarditis. New England Journal of Medicine. 1982;307(12):731–4. doi: 10.1056/NEJM198209163071207. [DOI] [PubMed] [Google Scholar]
- Midei 1990 .Midei MG, DeMent SH, Feldman AM, Hutchins GM, Baughman KL. Peripartum myocarditis and cardiomyopathy. Circulation. 1990;81(3):922–8. doi: 10.1161/01.cir.81.3.922. [DOI] [PubMed] [Google Scholar]
- Mielniczuk 2006 .Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, Green MS, et al. Frequency of peripartum cardiomyopathy. American Journal of Cardiology. 2006;97(12):1765–8. doi: 10.1016/j.amjcard.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Nishi 2002 .NishiI, Ishimitsu T, Ishizu T, Ueno Y, Suzuki A, Seo Y. Peripartum cardiomyopathy and biventricular thrombi. Circulation Journal. 2002;66(9):863–5. doi: 10.1253/circj.66.863. [DOI] [PubMed] [Google Scholar]
- O’Connell 1986 .O’Connell JB, Costanzo-Nordin MR, Subramanian R, Robinson JA, Wallis DE, Scanlon PJ, et al. Peripartum cardiomyopathy: clinical, hemodynamic, histologic and prognostic characteristics. Journal of the American College of Cardiology. 1986;8(1):52–6. doi: 10.1016/s0735-1097(86)80091-2. [DOI] [PubMed] [Google Scholar]
- Oladapo 2009 .Oladapo OT, Fawole B. Treatments for suppression of lactation. Cochrane Database of Systematic Reviews. 2009;(Issue 1) doi: 10.1002/14651858.CD005937.pub2. [DOI: 10.1002/14651858.CD005937.pub2] [DOI] [PubMed] [Google Scholar]
- Pearson 2000 .Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283(9):1183–8. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- Pinder 2002 .Pinder AJ, Dresner M, Calow C, ORiordan J, Johnson R. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. International Journal of Obstetric Anaesthesia. 2002;11:156–9. doi: 10.1054/ijoa.2002.0970. [DOI] [PubMed] [Google Scholar]
- Quinn 2004 .Quinn B, Doyle B, McInerney J. Postnatal pre-cordial pain. Pulmonary embolism or peripartum cardiomyopathy. Emergency Medicine Journal. 2004;21(6):746–7. doi: 10.1136/emj.2003.005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore 2002 .Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. New England Journal of Medicine. 2002;347(18):1403–11. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- RevMan 2008 .The Nordic Cochrane Centre, The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Ritchie 1850 .Ritchie C. Clinical contribution to the patho-diagnosis and treatment of certain chronic diseases of the heart. Edinburgh Medical Journal. 1850;2:2. [PMC free article] [PubMed] [Google Scholar]
- Rizeq 1994 .Rizeq MN, Rickenbacher PR, Fowler MB, Billingham ME. Incidence of myocarditis in peripartum cardiomyopathy. American Journal of Cardiology. 1994;74(5):474–7. doi: 10.1016/0002-9149(94)90906-7. [DOI] [PubMed] [Google Scholar]
- Ruch 1989 .Ruch A, Duhring JL. Postpartum myocardial infarction in a patient receiving bromocriptine. Obstetrics and Gynecology. 1989;74(3, part 2):448–51. [PubMed] [Google Scholar]
- Sanchez-Rubio Lezcano 2004 .Sánchez-Rubio Lezcano J, Galache Osuna JG, Marquina Barcos A, Calvo Cebollero I, Diarte de Miguel JA, Placer Peralta LJ. Peripartum cardiomyopathy with biventricular thrombi. Anales de Medicina Interna. 2004;21(10):498–500. doi: 10.4321/s0212-71992004001000008. [DOI] [PubMed] [Google Scholar]
- Sanderson 1986 .Sanderson JE, Olsen EG, Gatei D. Peripartum heart disease: an endomyocardial biopsy study. British Heart Journal. 1986;56(3):285–91. doi: 10.1136/hrt.56.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer 2009 .Sayer G, Naka Y, Jorde UP. Ventricular assist device therapy. Cardiovascular Therapy. 2009;27(2):140–50. doi: 10.1111/j.1755-5922.2009.00081.x. [DOI] [PubMed] [Google Scholar]
- Shoemaker 1983 .Shoemaker WC, Appel P, Bland R. Use of physiologic monitoring to predict outcome and to assist in clinical decisions in critically ill postoperative patients. American Journal of Surgery. 1983;146(1):43–50. doi: 10.1016/0002-9610(83)90257-x. [DOI] [PubMed] [Google Scholar]
- Sliwa 2000 .Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, Sareli P. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. Journal of the American College of Cardiology. 2000;35(3):701–5. doi: 10.1016/s0735-1097(99)00624-5. [DOI] [PubMed] [Google Scholar]
- Sliwa 2002 .Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. European Journal of Heart Failure. 2002;4(3):305–9. doi: 10.1016/s1388-9842(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Sliwa 2006a .Sliwa K, Förster O, Libhaber E, Fett JD, Sundstrom JB, Hilfiker-Kleiner D, et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. European Heart Journal. 2006;27(4):441–6. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- Sliwa 2006b .Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368(9536):687–93. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- Sultatos 1997 .Sultatos LG. Mechanism of drugs that affect uterine motility. Journal of Nurse-Midwifery. 1997;42(4):367–70. doi: 10.1016/s0091-2182(97)60134-2. [DOI] [PubMed] [Google Scholar]
- Tamhane 2006 .Tamhane P, OSullivan G, Reynolds F. Oxytocin in parturients with cardiac disease. International Journal of Obstetric Anaesthesia. 2006;15:332–3. doi: 10.1016/j.ijoa.2006.04.006. [DOI] [PubMed] [Google Scholar]
- van Spaendonck-Zwarts 2010 .van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JDH, Paulus WJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010;121:2169–75. doi: 10.1161/CIRCULATIONAHA.109.929646. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study