Abstract
Background
The long-acting bronchodilator tiotropium and single inhaler combination therapy of inhaled corticosteroids and long-acting beta2-agonists are both commonly used for maintenance treatment of chronic obstructive pulmonary disease. Combining these treatments, which have different mechanisms of action, may be more effective than the individual components. However, the benefits and risks of using tiotropium and combination therapy together for the treatment of chronic obstructive pulmonary disease are unclear.
Objectives
To assess the relative effects of inhaled corticosteroid and long-acting beta2-agonist combination therapy in addition to tiotropium compared to tiotropium or combination therapy alone in patients with chronic obstructive pulmonary disease.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials (July 2010) and reference lists of articles.
Selection criteria
We included parallel, randomised controlled trials of three months or longer, comparing inhaled corticosteroid and long-acting beta2-agonists combination therapy in addition to inhaled tiotropium against tiotropium alone or combination therapy alone.
Data collection and analysis
We independently assessed trials for inclusion and then extracted data on trial quality and outcome results. We contacted study authors for additional information. We collected information on adverse effects from the trials.
Main results
Three trials (1021 patients) were included comparing tiotropium in addition to inhaled corticosteroid and long-acting beta2-agonist combination therapy to tiotropium alone. The duration, type of combination treatment and definition of outcomes varied. The limited data led to wide confidence intervals and there was no significant statistical difference in mortality, participants with one or more hospitalisations, episodes of pneumonia or adverse events. The results on exacerbations were heterogeneous and were not combined. The mean health-related quality of life and lung function were significantly different when combination therapy was added to tiotropium, although the size of the average benefits of additional combination therapy were small, St George’s Respiratory Questionnaire (MD −2.49; 95% CI −4.04 to −0.94) and forced expiratory volume in one second (MD 0.06 L; 95% CI 0.04 to 0.08).
One trial (60 patients) compared tiotropium plus combination therapy to combination therapy alone. The results from the trial were insufficient to draw firm conclusions for this comparison.
Authors’ conclusions
To date there is uncertainty regarding the long-term benefits and risks of treatment with tiotropium in addition to inhaled corticosteroid and long-acting beta2-agonist combination therapy on mortality, hospitalisation, exacerbations of COPD and pneumonia. The addition of combination treatment to tiotropium has shown improvements in average health-related quality of life and lung function.
Medical Subject Headings (MeSH): Administration, Inhalation; Adrenergic beta-2 Receptor Agonists [*administration & dosage]; Bronchodilator Agents [*administration & dosage]; Drug Therapy, Combination [methods]; Glucocorticoids [*administration & dosage]; Pulmonary Disease, Chronic Obstructive [*drug therapy; mortality]; Randomized Controlled Trials as Topic; Scopolamine Derivatives [administration & dosage]
MeSH check words: Humans
BACKGROUND
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a general term referring to chronic bronchitis and emphysema, or both. COPD occurs when airflow to the lungs is restricted because of narrowing of the airways. Symptoms include cough, breathlessness and reduced exercise capacity. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines describe COPD as a preventable and treatable condition that is not fully reversible. Worldwide, the main cause of COPD is tobacco smoking, however air pollution is also a risk factor. The prevalence, morbidity and mortality of the disease vary across populations and cause a substantial economic and social burden.
There are a number of commonly used pharmacological treatments in COPD management that are used to relieve symptoms, improve exercise tolerance and quality of life, reduce mortality, and prevent and treat exacerbations. COPD exacerbations impair patients’ quality of life and a large part of the economic burden of COPD is attributed to the cost of managing exacerbations, particularly those resulting in use of acute care services or hospitalisations (Hutchinson 2010). Appropriate pharmacological management of the disease is therefore important to reduce and prevent exacerbations. COPD management tends to begin with one treatment and additional therapies are introduced as necessary to control symptoms (GOLD). Self-management education and rehabilitation can accompany these pharmacological interventions (Effing 2007; Lacasse 2006).
Description of the intervention
The first pharmacological step in treating COPD is the use of short-acting bronchodilators for symptom control when needed. These include short-acting beta2-agonists (SABA) and the shortacting anticholinergic ipratropium. For managing persistent COPD symptoms, long-acting bronchodilators can be introduced (GOLD). Regular treatment with long-acting bronchodilators is both more efficient and convenient than treatment with regular short-acting bronchodilators (Beeh 2010). Long-acting bronchodilators include long-acting beta2-agonists (LABA) and the long-acting anticholinergic agent tiotropium. Tiotropium bromide has gained widespread acceptance as a once daily maintenance therapy in COPD (Barr 2005; GOLD). Tiotropium reduces COPD exacerbations and related hospitalisations compared to ipratropium (Barr 2005). Most long-acting beta2-agonists are taken twice daily. They improve lung function compared to ipratropium, but there is little difference in improving COPD symptoms and exercise tolerance (Appleton 2006). For symptomatic patients with severe or very severe COPD (FEV1 < 50% predicted) and with repeated exacerbations, GOLD recommends the addition of inhaled corticosteroids (ICS) to bronchodilator treatment. Inhaled corticosteroids are licensed as combination inhalers with long-acting beta2-agonists. The most common combinations of inhaled corticosteroid and long-acting beta2-agonist in combination inhalers are fluticasone and salmeterol and budes-onide and formoterol. Combination therapy reduces exacerbation rates and all-cause mortality compared to inhaled corticosteroid alone (Nannini 2007). Also compared to long-acting beta2-agonists alone, combination therapy is more effective in reducing exacerbation rates, but there is no significant difference in mortality (Nannini 2007b). The benefits of combination inhalers should be viewed against the possible increased risk of pneumonia (Nannini 2007b). The potential risks or benefits of treatment with combination inhaler compared to tiotropium are uncertain (Welsh 2010), as are the risks or benefits of treatment with combination inhaler in addition to tiotropium, which will be explored in this review.
How the intervention might work
Tiotropium
Tiotropium is a long-acting anticholinergic agent that targets bronchospasm in COPD by relaxing airway smooth muscle. Tiotropium is structurally related to ipratropium, a short-acting anticholinergic agent that binds to M1, M2 and M3 muscarinic receptors which in turn open the bronchi (Barr 2005). Although tiotropium binds to the same receptors as ipratropium, it has a different kinetic selectivity. Tiotropium dissociates slowly from M1 and M3 receptors giving a bronchodilator effect lasting over 24 hours, but rapidly from M2 receptors. It appears that M2 receptors are feedback inhibitory receptors, and blocking them (as is the case for ipratropium) releases acetylcholine rather than reducing it as desired (Barr 2005). Benefits of tiotropium, in comparison with placebo, include reduced COPD exacerbations and exacerbation-related hospitalisations, and improved health-related quality of life and symptom scores among patients with moderate and severe disease (Barr 2005). Anticholinergic side effects can occur with tiotropium and include dry mouth, constipation and tachycardia.
Combination inhalers
Inhaled beta2-agonists activate beta2-receptors in the smooth muscle of the airway, releasing adenylate cyclase and increasing intracellular cAMP (cyclic adenosine monophosphate) which leads to a cascade of reactions resulting in bronchodilation. Beta2-agonists may act through other mechanisms such as respiratory muscle function or mucociliary clearance, because patients have shown improvement in symptoms whilst showing no improvement in lung function tests. Beta2-agonists are particularly useful because they reverse bronchoconstriction regardless of the initial cause of the bronchoconstriction. Side effects include muscle tremors, nervousness and occasional insomnia but, as with all inhaled medications, systemic side effects are minimised by giving a comparatively low dose directly to the lungs. Inhaled corticosteroids are anti-inflammatory drugs. They reduce the rate of exacerbations and the rate of decline in quality of life compared to placebo, without effect on overall mortality or the long-term decline in FEV1 (GOLD; Yang 2007). Combination inhalers including inhaled corticosteroids and long-acting beta2-agonists reduce exacerbation rates and all-cause mortality, and improve lung function and quality of life compared to placebo (Nannini 2007a). These effects are thought to be greater for combination inhalers than from the component preparations (GOLD). Inhaled corticosteroids, alone or in combination with beta2-agonists, potentially increase the risk of pneumonia (GOLD; Yang 2007).
Why it is important to do this review
Although both tiotropium and combination therapy inhalers are recommended for treatment of COPD, the relative effects of combination therapy compared to tiotropium on patients with COPD are unclear (Welsh 2010). However, it has been hypothesised that combining bronchodilators with different mechanisms and duration of action may be more effective than the individual components in improving bronchodilation without increasing side effects. For example a combination of salbutamol and ipratropium has been shown to improve FEV1 without an associated increase in tachyphylaxis (GOLD). Recent trials have been published on adding tiotropium to combined inhalers, and this review is necessary to show whether there is a benefit from this treatment regime compared to tiotropium or combination therapy alone.
OBJECTIVES
To assess the relative effects of the following treatments on markers of exacerbations, symptoms, quality of life, and lung function in patients with COPD:
Tiotropium plus LABA/ICS versus tiotropium
Tiotropium plus LABA/ICS versus LABA/ICS
METHODS
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials with a parallel group design of at least three months’ duration. Studies were not excluded on the basis of blinding.
Types of participants
Populations with a diagnosis of COPD. We included only studies that used an external set of criteria to screen participants for this condition (e.g. GOLD; ATS; BTS; TSANZ).
Types of interventions
Inhaled combination corticosteroid and long-acting beta2-agonist (such as fluticasone/salmeterol, budesonide/formoterol, beclomethasone/formoterol) and tiotropium bromide versus
Inhaled tiotropium bromide alone
Inhaled combination corticosteroid and long-acting beta2-agonist
Types of outcome measures
Primary outcomes
Mortality (all cause)
Hospital admissions; all cause and due to exacerbations
Exacerbations; all cause, requiring short burst oral corticosteroids or antibiotics as defined by agreed criteria
Pneumonia
Health-related quality of life (measured with a validated scale for COPD, e.g. St George’s Respiratory Questionnaire, Chronic Respiratory Disease Questionnaire)
Secondary outcomes
Symptoms
Forced expiratory volume in one second (FEV1)
Non-fatal serious adverse events
Adverse events
Side effects
Withdrawals
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Specialised Register coded as ‘COPD’ were searched using the following terms:
(tiotropium or spiriva) AND (((budesonide or fluticasone or beclomethasone or mometasone or steroid* or corticosteroid*) and (*formoterol or salmeterol or indacterol or (beta* and agonist*))) or (symbicort or viani or seretide or advair or foster or fostair or inuvair or fostex or kantos or combination*))
The search was conducted in July 2010.
Searching other resources
We reviewed reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and we asked them to identify other published or unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (CK and CJC) screened the titles and abstracts of citations retrieved through literature searches and obtained those deemed to be potentially relevant. Each reference was assigned to a study identifier and assessed against the inclusion criteria of this protocol.
Data extraction and management
We extracted information from each study for the following characteristics:
Design (design, total duration study and run in, number of study centres and location, withdrawals, date of study).
Participants (N, mean age, age range, gender, COPD severity, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria).
Interventions (run-in, intervention treatment and inhaler type, control treatment and inhaler type).
Outcomes (primary and secondary outcomes specified and collected, time points reported).
Two authors (CK and CJC) extracted data from the studies into data collection forms. Any discrepancies in the data were discussed and resolved between the authors. The data were then transferred from data collection forms into Review Manager 5.
Assessment of risk of bias in included studies
We assessed all included studies for the risk of bias according to recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) for the following items:
Allocation sequence generation
Concealment of allocation
Blinding of participants and investigators
Incomplete outcome data
Selective outcome reporting
Other sources of bias have been noted. We graded each potential source of bias as yes, no or unclear, relating to whether the potential for bias was low, high or unknown respectively.
Measures of treatment effect
We analysed dichotomous variables (such as mortality, participants with at least one hospital admission etc.) using the Mantel-Haenszel fixed odds ratio with 95% confidence intervals, unless events were rare, in which case we employed the Peto odds ratio (since this does not require a continuity correction for zero cells).
We analysed continuous outcome data (such as quality of life and FEV1) as fixed-effect mean differences with 95% confidence intervals as the same scale was used. Where treatment effects were reported as a mean difference with 95% confidence intervals, we entered the mean difference and standard errors calculated from 95% confidence intervals and analysed the data using the generic inverse variance (GIV) tool in Review Manager 5. For data which were not reported as the number of participants experiencing an event, we entered the natural log of reported rate ratios along with the standard error calculated from 95% confidence intervals into Review Manager 5 using the GIV function.
Unit of analysis issues
We analysed dichotomous data using participants as the unit of analysis (rather than events) to avoid counting the same participant more than once.
Dealing with missing data
We contacted investigators and study sponsors in order to verify key study characteristics and obtain missing numerical outcome data.
Assessment of heterogeneity
We assessed the amount of statistical variation between the study results with the I2 measurement.
Assessment of reporting biases
We minimised reporting bias from non-publication of studies or selective outcome reporting by using a broad search strategy, contacting study authors directly and checking references of included studies. We planned to assess reporting bias by visual inspection of funnel plots.
Data synthesis
We combined dichotomous data using Mantel-Haenszel odds ratios with a fixed-effect model and compared this to the randomeffects model. We combined rate ratios and hazard ratios using generic inverse variance using a fixed-effect model and compared to the random-effects model. We planned to calculate the numbers needed to treat for an additional beneficial outcome from the pooled odds ratio and its confidence interval, and apply to appropriate levels of baseline risk. We have presented the findings of our primary outcomes in Summary of findings for the main comparison generated using GradePro software.
Subgroup analysis and investigation of heterogeneity
We planned to subgroup studies according to:
Type of combination therapy
Severity of disease at baseline
Sensitivity analysis
We intended to assess the sensitivity of our primary outcomes to degree of bias by comparing the overall results with those exclusively from trials assessed as being at low risk of bias.
RESULTS
Description of studies
Results of the search
The initial search identified 101 references. Of these we identified 24 as potentially relevant, which we obtained in full text for further assessment. Fourteen of these were eligible for inclusion and belonged to three studies (Aaron 2007; Cazzola 2007; Welte 2009) (see Characteristics of included studies). Peer review identified one further potentially eligible study and is noted in Studies awaiting classification (Fang 2008).
Included studies
Study design
Two of the studies had a treatment duration of three months (Cazzola 2007; Welte 2009) and the third was a one year study (Aaron 2007). Both Aaron 2007 and Welte 2009 were multi-centre studies. The centres for the Aaron 2007 study were all located in Canada, whereas the Welte 2009 study centres were spread across nine different countries.
Sample size
There were 1051 participants randomised to the relevant treatments in the included studies; tiotropium + LABA/ICS (504), tiotropium (517), and LABA/ICS (30). These included two larger studies; Welte 2009 (n = 660) and Aaron 2007 (n = 301), and one smaller; Cazzola 2007 (n = 90).
Participants
The mean age of participants varied from 62 to 68 years. The gender distribution varied from 56% males in Aaron 2007 to 89% in Cazzola 2007. The severity of COPD varied from moderate to very severe according to GOLD guideline definitions of COPD. However, the baseline lung function for the participants was similar in the included studies with the mean FEV1 predicted averaging 38% to 39%.
Interventions
All included studies used 18 μg of tiotropium (Handihaler), one inhalation daily. In Welte 2009 the LABA/ICS combination used was budesonide/formoterol 320/9 μg (Turbuhaler), one inhalation twice daily. Both Aaron 2007 and Cazzola 2007 used fluticasone/salmeterol (Diskus). In Aaron 2007 the dose used was 250/25 μg, two inhalations twice daily, and in Cazzola 2007 the concentration was 500/50 μg, one inhalation twice daily.
Permitted co-treatment
In Aaron 2007 participants were instructed to use inhaled albuterol when necessary to relieve symptoms. Respiratory medications such as oxygen, antileukotrienes, and methylxanthines, were continued in all patient groups. In Welte 2009 terbutaline was used as needed for symptom relief in both treatment groups. Cazzola 2007 permitted supplemental salbutamol for relief of symptoms, which was monitored throughout the study. Stable regimens of theophylline were also allowed.
Outcomes
The primary outcome for Aaron 2007 was the proportion of patients suffering one or more COPD exacerbations. Both Welte 2009 and Cazzola 2007 studied the change in FEV1 from randomisation to the end of the study (three months) as their primary outcome.
Funding
The Welte 2009 study was sponsored by AstraZeneca, the producer of the LABA/ICS combination budesonide/formoterol (Symbicort Turbuhaler) used in the trial. The Aaron 2007 study was funded by the Canadian Institutes of Health Research and the Ontario Thoracic Society. We were not able to obtain information about funding for the Cazzola 2007 study.
Excluded studies
A total of eight studies failed to meet the eligibility criteria for the review (see Characteristics of excluded studies). Four of these compared tiotropium alone with combination therapy (LABA/ICS) (Ando 2008; Bateman 2008; Golabi 2006; Hara 2007) and one study compared tiotropium with LABA alone (Petroianni 2008). The remaining three studies were all shorter than three months (Biscione 2009; Perng 2006), and one of these was also of cross-over design (Singh 2008).
Risk of bias in included studies
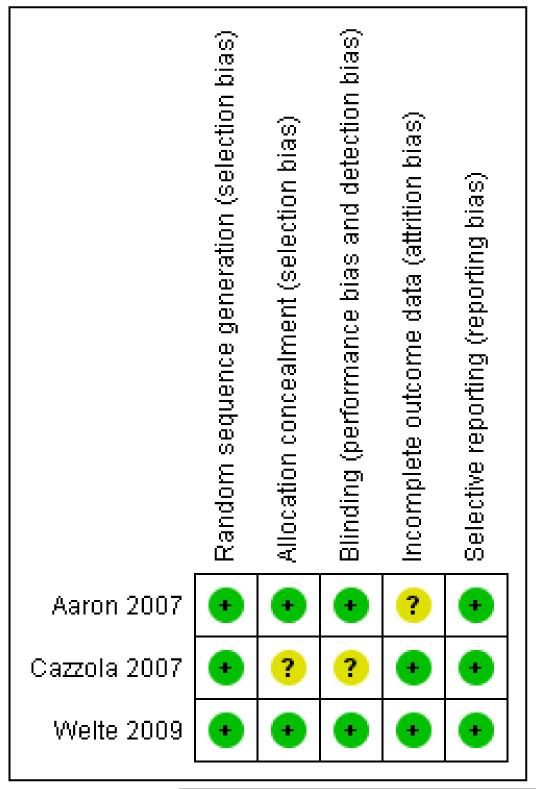
An assessment of the risk of bias is presented in the Characteristics of included studies table, and an overview of the findings is shown in Figure 1.
Figure 1. Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
Aaron 2007 and Welte 2009 reported adequate sequence generation and allocation concealment. Details for Welte 2009 were supplied on request. For both studies randomisation was computergenerated through central allocation and both research staff and patients were blinded to the treatment assignment until the end of study. Cazzola 2007 did not report full details regarding sequence generation and allocation concealment in the study report.
Blinding
The blinding in Aaron 2007 and Welte 2009 was adequate. Cazzola 2007 did not report full details of blinding procedures. In Aaron 2007, the different inhalers containing placebo, salmeterol, and fluticasone/salmeterol were identical in taste and appearance, and they were enclosed in identical tamper-proof blinding devices. The medication canisters within the blinding devices were stripped of any identifying labelling. Clinical data for suspected exacerbations were reviewed by a blinded committee judging whether the data met the study definition of COPD exacerbation. Blinding of patients was not broken for patients who prematurely discontinued treatment with study medications, and the statistician who performed the analysis was initially blinded to patient group assignments. In Welte 2009, treatment assignment was concealed as active and placebo inhalers were of identical appearance and both clinicians and patients were blinded to treatment until completion of the study.
Incomplete outcome data
Cazzola 2007 and Welte 2009 did not suffer from incomplete outcome data. Aaron 2007 suffered from high and uneven withdrawal rates in the different study groups (74 patients (47%) withdrew from the tiotropium + placebo group and 37 patients (26%) on tiotropium + LABA/ICS). High withdrawal rates are common in COPD trials over six months in length.
For most patients, data were recorded throughout the one-year trial period regardless of whether patients discontinued treatment with study medications. The rate of patients who stopped therapy and did not complete the trial, however, was still relatively large and unevenly distributed between the intervention groups (30 patients (19%) tiotropium + placebo and 15 patients (10%) tiotropium + LABA/ICS). Mortality data were obtained for all participants with the exception of 2/145 (1.4%) on tiotropium + LABA/ICS and 4/156 (2.6%) on tiotropium + placebo who withdrew and declined further study.
Selective reporting
All three trials adequately reported outcome data for the primary and secondary outcomes that they had pre-specified in the study record.
Effects of interventions
Because of the low number of eligible studies for the two comparisons (tiotropium + LABA/ICS versus tiotropium alone or versus LABA/ICS alone), no subgroup analysis of disease severity or type of combination therapy were possible.
Tiotropium plus LABA/ICS versus tiotropium plus placebo
Two authors (CK and CJC) independently extracted and analysed results for all data. Dichotomous data including mortality, exacerbations, pneumonia, adverse events and withdrawals were analysed as end of study measurements as this was the only time point for which these data were available. Continuous data were analysed at three months for Cazzola 2007 and Welte 2009 (end of study) and at both five months (20 weeks) and one year for Aaron 2007.
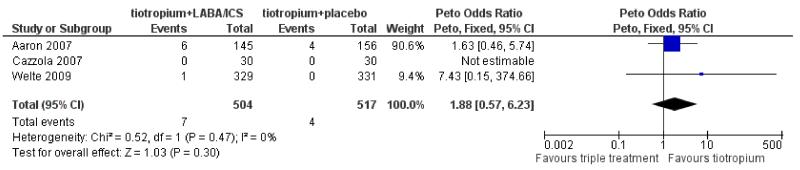
Primary outcome: mortality (all causes)
Mortality data were available from two trials involving 1021 participants, which reported mortality as a secondary outcome (Aaron 2007; Welte 2009). The third study, Cazzola 2007 (60 participants), reported zero serious adverse events and therefore we assumed there were no mortalities during the study. Taken together, there was a greater number of deaths in the tiotropium + LABA/ICS group (7/504) than in the tiotropium + placebo group (4/517), however, there was no statistically significant difference in mortality between the groups (Peto odds ratio (OR) 1.88; 95% confidence interval (CI) 0.57 to 6.23) as shown in Figure 2. The number of withdrawals from each of the arms in Aaron 2007, which adds most weight to the comparison, was six times larger than the number of deaths for participants on tiotropium + LABA/ICS and 19 times larger for participants on tiotropium + placebo. This uncertainty about the results is not reflected in the confidence interval for the odds ratio.
Figure 2. Forest plot of comparison: 1 tiotropium + LABA/ICS combination vs tiotropium + placebo, outcome: 1.1 Mortality (all cause).
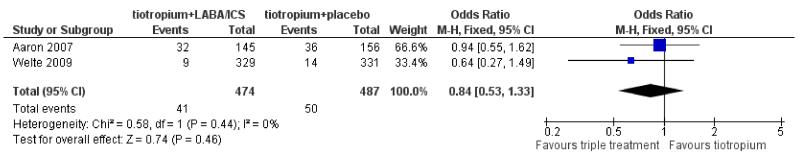
Primary outcome: hospital admissions (all cause)
Data regarding all cause hospital admissions were available from two trials involving 961 participants. The data were kindly supplied by Aaron 2007 and by AstraZeneca (for Welte 2009) on request. The number of patients admitted to hospital from Welte 2009 did not include any due to exacerbation, as the sponsors had recorded hospitalisations for COPD separately from other causes and were not able to provide data on the overlap between these two groups. Overall there were fewer patients admitted to hospital in the tiotropium + LABA/ICS group (41/474) than in the tiotropium + placebo group (50/487), however, there was no statistically significant difference between the groups when analysed as dichotomous data (OR 0.84; 95% CI 0.53 to 1.33), as shown in Figure 3.
Figure 3. Forest plot of comparison: 1 tiotropium + LABA/ICS combination vs tiotropium + placebo, outcome: 1.2 Hospital admission (all causes).
However, as presented in the paper by Aaron 2007, when analysed as a rate ratio the number of hospitalisations for any cause was significantly lower among patients on tiotropium + LABA/ICS (41 hospitalisations/137.1 patient years) compared to patients treated with tiotropium alone (62 hospitalisations/138.0 patient years), (RR 0.67; 95% CI 0.45 to 0.99). Welte 2009 did not report rate ratios of hospital admissions.
Primary outcome: hospital admissions (exacerbations)
Data regarding hospital admissions due to exacerbations were available from two trials involving 961 participants. The data were kindly supplied by Aaron 2007 and by AstraZeneca (for Welte 2009) on request. The number of patients admitted to hospital due to exacerbations were higher in the tiotropium + placebo group (38/487) than in the tiotropium + LABA/ICS group (25/474) (OR 0.66; 95% CI 0.39 to 1.13; Figure 4), although the difference was not statistically significant for the pooled result or the result from individual studies.
Figure 4. Forest plot of comparison: 1 tiotropium + LABA/ICS combination vs tiotropium + placebo, outcome: 1.3 Hospital admission (exacerbation).
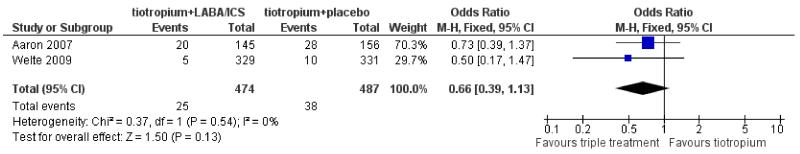
Similarly to all-cause hospital admissions, Aaron 2007 showed that when analysed as rate ratio there was a statistically significant reduction in hospitalisation rates due to exacerbation among patients on tiotropium + LABA/ICS (26 hospitalisations/137.1 patient years) compared to patients treated with tiotropium alone (49 hospitalisations/138.0 patient years), (rate ratio 0.53; 95% CI 0.33 to 0.86). Welte 2009 also reported significantly lower rates of hospitalisations/emergency room visits for exacerbations among patients treated with tiotropium + LABA/ICS (0.028 hospitalisations/patient/3 months) compared to patients on tiotropium alone (0.080 hospitalisations/patient/3 months), (rate ratio 0.35; 95% CI 0.16 to 0.78).
Primary outcome: exacerbations
Aaron 2007 and Welte 2009 reported the number of patients that experienced exacerbations (961 patients). Cazzola 2007 withdrew patients experiencing exacerbations during the study period without reporting the number of withdrawals due to exacerbation. In Aaron 2007 exacerbation was defined as a sustained worsening of the patient’s respiratory condition necessitating short-term use of either oral or intravenous steroids, oral or intravenous antibiotics, or both therapies. In Welte 2009 exacerbation was defined as worsening of COPD leading to treatment with systemic corticosteroids (oral or parenteral) and/or hospitalisation/emergency room visits. In both studies fewer patients on tiotropium + LABA/ICS had one or more exacerbations compared to the group treated with tiotropium + placebo. In the Welte 2009 study the difference was statistically significant (OR 0.36; 95% CI 0.22 to 0.60) with a baseline risk of 0.18. The result from Aaron 2007 did not reach statistical significance (OR 0.89; 95% CI 0.56 to 1.41) (baseline risk 0.63). See Figure 5.
Figure 5. Forest plot of comparison: 1 tiotropium + LABA/ICS combination vs tiotropium + placebo, outcome: 1.4 Exacerbation.
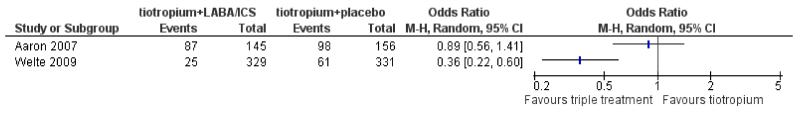
These results were consistent with the reported analysis of the individual study data as rate ratios. Welte 2009 showed significantly fewer exacerbations in the group treated with tiotropium + LABA/ICS (0.124 exacerbations/patient/3 months) compared to the group treated with tiotropium + placebo (0.326 exacerbations/patient/3 months), (OR 0.38; 95% CI 0.25 to 0.57). Aaron 2007 showed no significant difference in the number of exacerbations between the two groups; tiotropium + LABA/ICS (188 exacerbations/137.1 patient years), tiotropium + placebo (222 exacerbations/138.0 patient years), (OR 0.85; 95% CI 0.65 to 1.11). We did not pool the results because of considerable statistical heterogeneity across the studies (I2 = 85%).
Primary outcome: pneumonia
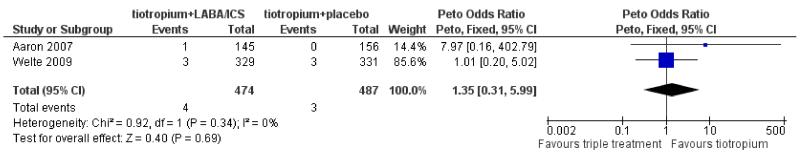
Aaron 2007 and Welte 2009 (961 patients) reported the number of patients suffering from pneumonia during the trials although Aaron 2007 reported only the cases of pneumonia leading to mechanical ventilation or death. The number of events was low and there was no statistically significant difference between the tiotropium + LABA/ICS group and the tiotropium + placebo group (Peto OR 1.35; 95% CI 0.31 to 5.99) as shown in Figure 6.
Figure 6. Forest plot of comparison: 1 tiotropium + LABA/ICS combination vs tiotropium + placebo, outcome: 1.5 Pneumonia.
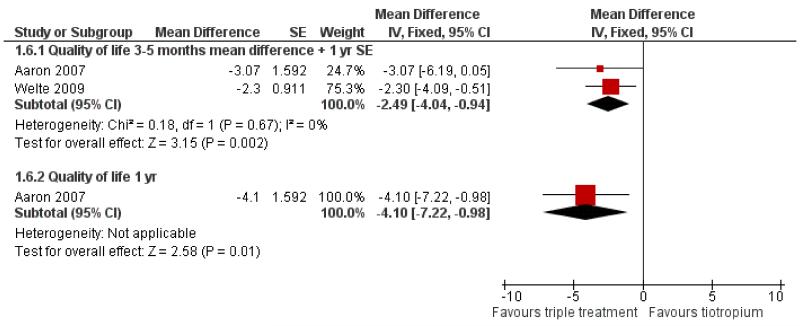
Primary outcome: quality of life
Both Aaron 2007 and Welte 2009 studied changes in health-related quality of life (961 patients) using the St George’s Respiratory Questionnaire (SGRQ). We extracted and analysed the mean change in health-related quality of life at three months (end of study) for Welte 2009 and five months (20 weeks) (reported time point closest to three months, data kindly supplied on request) and one year for Aaron 2007. Standard errors and standard deviations were not available for the mean difference at five months for Aaron 2007. We assumed that these values were relatively constant between five months and one year, and imputed the standard error for the mean difference at one year. At three to five months the combination of tiotropium + LABA/ICS had a significantly larger positive effect on the quality of life compared to tiotropium + placebo (MD −2.49; 95% CI −4.04 to −0.94), as shown in Figure 7. This was below the threshold of four units for clinically significant difference (SGRQ-C manual 2008). However, Welte 2009 reported the percentage of patients with improvements in SGRQ score of more than four units, which was significantly higher in the tiotropium + LABA/ICS group (49.5%) than in the tiotropium + placebo group (40.0%) (P = 0.016). The percentage of patients with a deterioration in SGRQ score of more than four units were similar in the two groups (tiotropium + LABA/ICS 27.6%, tiotropium + placebo group 29.7%) (Welte 2009).
Figure 7. Forest plot of comparison: 1 tiotropium plus LABA/ICS combination versus tiotropium plus placebo, outcome: 1.6 Quality of life SGRQ scale.
Secondary outcome: symptoms
Welte 2009 was the only included study reporting changes in COPD symptom scores for breathlessness (MD −0.142; 95% CI −0.214 to −0.069), night awakening (MD −0.157; 95% CI −0.222 to −0.092), chest tightness (MD −0.142; 95% CI −0.212 to −0.072) and cough (MD −0.161; 95% CI −0.238 to −0.084); 660 patients. The scores for all symptoms were in favour of the tiotropium + LABA/ICS group compared to the tiotropium + placebo group (P < 0.001).
Secondary outcome: forced expiratory volume in one second (FEV1)
Changes in FEV1 over the treatment period were measured and reported in all three included studies (1021 patients). Tiotropium in combination with LABA/ICS improved FEV1 significantly compared to tiotropium + placebo (MD 0.06; 95% CI 0.04 to 0.08) at three (Cazzola 2007, Welte 2009) and five months (Aaron 2007). This was below the threshold of 100 to 140 mL which is considered to be a clinically important increase (Cazzola 2008). FEV1 data at five months were kindly supplied on request by Aaron 2007. The standard error of the change from baseline in FEV1 for Welte 2009 was calculated from the reported P value < 0.001.
Secondary outcome: serious adverse events (non-fatal)
In the three included studies (1021 patients) there were fewer patients suffering non-fatal serious adverse events in the tiotropium + LABA/ICS group (12/504) than in patients on tiotropium + placebo (20/517) (OR 0.60; 95% CI 0.29 to 1.25), although the difference was not statistically significant.
Secondary outcome: adverse events
In the three included studies (1021 patients) there was a slightly larger number of patients suffering adverse events on tiotropium + LABA/ICS (140/504) than on patients on tiotropium + placebo (132/517) (OR 1.12; 95% CI 0.85 to 1.49), again the difference was not statistically significant.
Secondary outcome: side effects
Side effects were not reported in any of the included studies.
Secondary outcome: withdrawal
All three studies (1021 patients) reported the withdrawals from the study. There were many withdrawals for any reason from the longer of the three studies (Aaron 2007) and in this study the withdrawal rate was significantly higher in the tiotropium + placebo group (47%) than in the tiotropium + LABA/ICS group (26%) (OR 0.38; 95% CI 0.23 to 0.62). The two shorter studies had fewer withdrawals and they were more evenly distributed between the tiotropium + LABA/ICS groups (Cazzola 2007, 3/13% and Welte 2009 8/9% in the tiotropium + LABA/ICS/tiotropium + placebo group respectively). Both Aaron 2007 and Welte 2009 reported the breakdown of the reasons for withdrawals, which showed that the difference between the number withdrawing due to adverse events (OR 0.92; 95% CI 0.46 to 1.83) was not statistically significant, whereas the difference between the number withdrawing due to lack of efficacy was significantly higher in the tiotropium + placebo group than in the tiotropium + LABA/ICS group (OR 0.36; 95% CI 0.22 to 0.59).
Tiotropium plus LABA/ICS versus LABA/ICS plus placebo
Cazzola 2007 was the only eligible study identified comparing tiotropium + LABA/ICS versus LABA/ICS + placebo (60 patients). The study reported results for the following outcomes of interest for this review:
Primary outcome: mortality (all causes)
Cazzola 2007 reported zero serious adverse events and therefore we assumed there were no deaths during the study.
Secondary outcome: forced expiratory volume in one second (FEV1)
Tiotropium in combination with LABA/ICS improves FEV1 significantly compared to LABA/ICS + placebo (MD 0.05; 95% CI 0.00 to 0.09), but the mean difference and confidence interval were below the minimal clinically important difference of 100 to140 mL.
Secondary outcome: serious adverse events (non-fatal)
There were no serious adverse events in either intervention group.
Secondary outcome: adverse events
There were more adverse events in the tiotropium + LABA/ICS group (15/30) than in the tiotropium + placebo group (8/30), but the confidence interval was wide, due to small numbers of events (OR 2.75; 95% CI 0.93 to 8.10).
Secondary outcome: withdrawal
There were fewer withdrawals in the tiotropium + LABA/ICS group (1/30) than the tiotropium + placebo group (4/30), but the number of events was small and not statistically significant (OR 0.22; 95% CI 0.02 to 2.14).
DISCUSSION
Summary of main results
This systematic review set out to investigate the long-term (≥ three months) effects of tiotropium in combination with LABA/ICS compared to either LABA/ICS alone or tiotropium alone, for the treatment of COPD. Three randomised, double-blind, placebocontrolled trials with 1021 participants were identified. All three studied the effects of tiotropium in combination with LABA/ICS compared to tiotropium alone, whereas only one of these studies (60 participants) also looked at tiotropium in combination with LABA/ICS compared to LABA/ICS (Cazzola 2007).
Tiotropium plus LABA/ICS versus tiotropium plus placebo
The results from this review did not show any significant difference between tiotropium + LABA/ICS and tiotropium + placebo in mortality, the number of patients suffering from pneumonia, or having one or more hospital admissions. However, the individual study authors’ analyses of rate ratios showed a significant benefit of tiotropium + LABA/ICS treatment compared to tiotropium alone for the total number of hospital admissions (Aaron 2007), and for exacerbations leading to hospitalisations or emergency room visits (Aaron 2007; Welte 2009). We did not pool data for number of patients suffering one or more exacerbations due to heterogeneity. This review did find that tiotropium in combination with LABA/ICS significantly improved FEV1 for COPD patients compared to treatment with tiotropium alone. However, the mean increase was below what may be considered a clinically significant difference (100 to 140 mL). Similarly mean change in quality of life scores was lower than a four unit change (which is considered to be clinically significant), although the change, favouring tiotropium + LABA/ICS treatment, was statistically significant. One included study reported significantly more patients with a clinically significant improvement in their quality of life score in the tiotropium + LABA/ICS group than in the group on tiotropium alone (Welte 2009).
The effect of tiotropium + LABA/ICS combination treatment on mortality and pneumonia remains uncertain due to the low numbers of events, which were small in comparison to the high numbers of withdrawals and participants lost to follow-up. Also, Aaron 2007 reported only pneumonia leading to mechanical ventilation or death as the study took place at a time when the authors were unaware of any association of pneumonia with the use of inhaled corticosteroids.
Even though there was no significant difference in the number of patients admitted to hospital due to exacerbations or all causes, Aaron 2007 reported a significant difference in all cause hospitalisation (rate ratio 0.67; 95% CI 0.45 to 0.99) and hospital admissions due to exacerbation (rate ratio 0.53; 95% CI 0.33 to 0.86) when data were analysed as rate ratios. Similarly Welte 2009 reported significantly lower numbers of hospitalisations/emergency room visits for exacerbations in the tiotropium + LABA/ICS group compared to the tiotropium + placebo group (rate ratio 0.35; 95% CI 0.16 to 0.78). There are many ways to analyse exacerbation/hospitalisation rates and all have different advantages and disadvantages. Looking at the number of patients suffering one or more exacerbation will show the direction of the intervention effect but it does not give any information about potential difference in exacerbation frequency in the same patient and it does not take into account variable lengths of study time (Keene 2008). Using the rate ratio of exacerbations is more informative about exacerbation rates in the trial populations, but the various different statistical methods used to calculate this means that one has to be careful when combining/pooling the results from different trials. There are many possible reasons for the discrepancies in statistical significance between the results when they are analysed in different ways. There may be a difference in power between the methods, and chance could lead to a significant difference using one method and a non-significant difference using another.
Welte 2009 showed significantly fewer exacerbations in the group treated with tiotropium + LABA/ICS compared to the group treated with tiotropium + placebo (OR 0.38; 95% CI 0.25 to 0.57), whereas Aaron 2007 showed no significant difference between the two groups (OR 0.85; 95% CI 0.65 to 1.11). The difference between the study results was large enough to introduce considerable heterogeneity, and the two study results were therefore not combined. The reason for the heterogeneity is unknown but there are considerable differences between the two studies which could have an influence including; type of combination treatment, length of study, baseline risk, and notably definition of exacerbation. Aaron 2007 defined exacerbation as a worsening of COPD leading to treatment with systemic steroids and/or antibiotics. Welte 2009 defined exacerbation as a worsening of COPD leading to treatment with systemic steroids and/or hospitalisation/emergency room visits.
Treatment with tiotropium + LABA/ICS led to a greater improvement in health-related quality of life than treatment with tiotropium alone. However, the mean difference in quality of life score was below the limit of clinical significance (less than four units) although the difference between the intervention groups was statistically significant (MD −2.49; 95% CI −4.04 to −0.94). However, Welte 2009 showed a statistically significant difference in the number of patients who had a clinically significant improvement in quality of life score (tiotropium + LABA/ICS 49.5%, tiotropium + placebo 40.0%, P = 0.016), whereas there was no significant difference in the number whose quality of life score deteriorated (tiotropium + LABA/ICS 27.6%, tiotropium + placebo 29.7%). This illustrates that a small mean difference does not rule out the possibility of additional combination treatment being of benefit in some patients. This possibility does not only cover health-related quality of life but also changes in FEV1.
The difference in serious adverse events between the intervention groups was not statistically significant. The numbers were low in total and compared to the number of withdrawals and participants lost to follow-up. The withdrawals from the studies did not seem to be linked to adverse events but to the efficacy of the treatment.
LABA/ICS plus tiotropium versus LABA/ICS plus placebo
The one pilot study looking at the effect of LABA/ICS + tiotropium versus LABA/ICS + placebo showed a significantly larger improvement in FEV1 with tiotropium + LABA/ICS treatment compared to LABA/ICS treatment (Cazzola 2007), however the mean difference in FEV1 was not clinically significant. All other outcomes of interest were either not studied, had no events or did not achieve a statistically significant difference.
Overall completeness and applicability of evidence
For the comparison of the benefits and risks of treatment with tiotropium + LABA/ICS versus LABA/ICS one smaller study was eligible (Cazzola 2007), which did not look at or report any of the primary outcomes specified in this review except for mortality. Therefore there was little applicable evidence for this comparison from this review.
For the comparison of the benefits and risks of treatment with tiotropium + LABA/ICS versus tiotropium, the total number of patients in the included studies was insufficient and the differences between the studies too many (type of combination treatment, study length, definition of outcomes) to show any relevant statistically significant difference for several of the outcomes.
One limitation of the included studies was their duration. Two out of three studies (Cazzola 2007; Welte 2009) were only three months and the third was one year (Aaron 2007). A minimum of six months’ treatment would be advisable to be able to judge longterm benefits and risks of the studied interventions. However such a criteria also limits the number of eligible studies and leads to larger numbers of withdrawals in the included studies, which in turn will lead to an increased risk of bias.
There were too few eligible studies to break down the result in subgroups of disease severity or type of combination therapy.
Quality of the evidence
Aaron 2007 and Welte 2009 were of good methodological quality. However, long COPD trials (longer than six months) like Aaron 2007, which are essential to study long-term efficacy and risks with COPD interventions, will inevitably suffer from large numbers of withdrawals (Welsh 2010). Aaron 2007 did address this issue by sensitivity analyses for their primary outcome; COPD exacerbation. The authors assumed that all patients who were lost to follow-up in both intervention groups either did not have an exacerbation, had an exacerbation or had exacerbations at the same rate as those who continued in the study. However, they did not investigate the effect of the greatest possible difference between the intervention groups; assuming that all patients lost to followup in one intervention groups had an exacerbation and that all patients lost to follow-up in the other intervention group did not have an exacerbation. Even though the issue of withdrawals was addressed, it could introduce bias.
Cazzola 2007 could not provide additional information regarding allocation concealment, blinding, funding and withdrawals and therefore introduced an unknown risk of bias. However, a sensitivity analysis removing the Cazzola 2007 data from the FEV1 analysis did not change the outcome substantially.
Potential biases in the review process
The issue of large and/or uneven numbers of withdrawals, as mentioned above (Quality of the evidence), will, even if addressed, possibly introduce bias as there is no consensus on how to handle participants for whom no data are available.
We analysed available data as specified in the protocol. However, we did expand the review question from the protocol to include the comparison of tiotropium + LABA/ICS versus LABA/ICS + placebo. We also highlighted rate ratios for hospital admissions and percentage of patients with a clinically significant change in health-related quality of life reported by the authors although this was not specified in Measures of treatment effect.
Agreements and disagreements with other studies or reviews
No reviews looking at the long-term efficacy and adverse effects of tiotropium + LABA/ICS treatment compared to tiotropium or LABA/ICS have been identified. However, a systematic review looking at LABA/ICS combination treatment compared to placebo has shown that combination treatment significantly reduces mortality and exacerbation rates, and improves lung function (Nannini 2007). LABA/ICS also increases the risk of pneumonia compared with placebo. Tiotropium on its own has been shown to reduce COPD exacerbations and related hospitalisations compared to placebo (Barr 2005). Tiotropium also improves health-related quality of life among patients with moderate and severe disease. Although no conclusion has been drawn regarding the effect of tiotropium on mortality rates and change in FEV1 (Barr 2005), these reviews may give an indication of the treatment efficacy that can be anticipated from treatment with tiotropium + LABA/ICS compared to tiotropium alone.
AUTHORS’ CONCLUSIONS
Implications for practice
The total patient number from the included studies was insufficient and the differences between the studies too great to draw any general conclusions from the results regarding the long-term effects and risks of tiotropium + LABA/ICS treatment. The relative efficacy and safety of tiotropium + LABA/ICS treatment therefore remains uncertain.
Implications for research
Additional large, long-term randomised controlled trials are required to reduce the uncertainty about the efficacy and risks of tiotropium in combination with LABA/ICS for COPD patients.
PLAIN LANGUAGE SUMMARY.
Are tiotropium plus combination inhalers better than tiotropium or combination inhalers alone for the treatment of COPD?
Chronic obstructive pulmonary disease (COPD) is a lung disease which includes the conditions chronic bronchitis and/or emphysema. COPD is characterised by blockage or narrowing of the airways. The symptoms include breathlessness and chronic cough. COPD is an irreversible disease that is usually brought on by airway irritants, such as smoking or inhaled dust.
Inhalers with bronchodilators and/or anti-inflammatory agents are commonly used to ease symptoms and minimise the long-term decline in health caused by COPD. Examples of these treatments are tiotropium which is a bronchodilator and combination inhalers which contain another type of bronchodilator (long-acting beta-agonists) together with anti-inflammatory agents (steroids). These treatments work in different ways and therefore might be more beneficial if used together.
This review found three studies, involving 1021 patients, comparing the long-term efficacy and side effects of combining tiotropium with combination inhalers for treating COPD. In these studies there were not enough patients and the studies were too different from each other for us to be able to draw any firm conclusions as to whether combining tiotropium with combination inhalers is better or worse than using either drug alone for mortality, hospitalisation and pneumonia. The addition of combination inhalers to tiotropium did show small benefits in quality of life and lung function tests.
In order to better understand the effect of treatment with tiotropium and combination inhaler more long-term studies need to be done.
ACKNOWLEDGEMENTS
We are grateful to Susan Hansen for help designing the search strategy and to Emma J Welsh who drafted the protocol.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
SOURCES OF SUPPORT
Internal sources
St George’s University of London, UK.
External sources
NIHR, UK.
National Institute for Health Research supported this work through funding for both authors.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Design: A randomised, double-blind, placebo-controlled, parallel group trial from October 2003 to January 2006. The trial included 27 Canadian medical centres; 20 centres were academic hospital-based pulmonary clinics, 5 were community-based pulmonary clinics, and 2 were community-based primary care clinics | |
| Participants |
Population: 449 adults with a clinical history of moderate or severe COPD as defined by ATS and GOLD guidelines Baseline Characteristics: Mean age 68 years. COPD severity moderate to severe with mean FEV1 predicted of 39%. 44% women. Inclusion Criteria: At least 1 exacerbation of COPD that required treatment with systemic steroids or antibiotics within the 12 months before randomisation; age older than 35 years; a history of 10 pack-years or more of cigarette smoking; documented chronic airflow obstruction, with an FEV1/FVC ratio less than 0.70 and a post-bronchodilator FEV1 less than 65% of the predicted value. Exclusion Criteria: History of physician-diagnosed asthma before 40 years of age; history of physician-diagnosed chronic congestive heart failure with known persistent severe left ventricular dysfunction; those receiving oral prednisone; those with a known hypersensitivity or intolerance to tiotropium, salmeterol, or fluticasone-salmeterol; history of severe glaucoma or severe urinary tract obstruction, previous lung transplantation or lung volume reduction surgery, or diffuse bilateral bronchiectasis; and those who were pregnant or were breastfeeding |
|
| Interventions |
|
|
| Outcomes |
Primary: Proportion of patients with one or more exacerbation of COPD Secondary: Mean number of COPD exacerbations per patient-year; the total number of exacerbations that resulted in urgent visits to a healthcare provider or emergency department; the number of hospitalisations for COPD; the total number of hospitalisations for all causes; changes in health-related quality of life, dyspnoea, lung function |
|
| Notes | Co-medication: All study patients were provided with inhaled albuterol and were instructed to use it when necessary to relieve symptoms. Any treatment with inhaled corticosteroids, long-acting 2-agonists, and anticholinergics that the patient may have been using before entry was discontinued on entry into the study. Therapy with other respiratory medications, such as oxygen, antileukotrienes, and methylxanthines, was continued in all patient groups | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done through central allocation of a randomisation schedule that was prepared from a computer-generated random listing of the 3 treatment allocations, blocked in variable blocks of 9 or 12 and stratified by site |
| Allocation concealment (selection bias) | Low risk | Neither research staff nor patients were aware of the treatment assignment before or after randomisation |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | The metered-dose inhalers containing placebo, salmeterol, and fluticasone-salmeterol were identical in taste and appearance, and they were enclosed in identical tamper-proof blinding devices. The medication canisters within the blinding devices were stripped of any identifying labelling |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | The number of people who stopped drug therapy was high, with large variations between the groups (74 (47%) tiotropium + placebo and 37 (26%) tiotropium + LABA/ICS comb.). However, the number of people who did not complete the trial was lower, although there was still large variations between the groups (30 (19%) tiotropium + placebo and 15 (10%) tiotropium + LABA/ICS comb.). The issue of incomplete data was however addressed by sensitivity analyses of the data comprising alternative assumptions for patients who prematurely withdrew from treatment |
| Selective reporting (reporting bias) | Low risk | Results for all listed primary and secondary outcomes were reported |
| Methods | Design: A randomised, double-blind, double-dummy, parallel group trial over 12 weeks | |
| Participants |
Population: 90 patients with well-controlled COPD. Baseline Characteristics: Mean age 66 years. COPD severity severe to very severe with mean FEV1 predicted of 38%. 11% women. Inclusion Criteria: Baseline FEV1 of less than 50% of predicted, and a post-bronchodilator FEV1/FVC < 70% following salbutamol 400 mg according to the GOLD criteria of severity Exclusion Criteria: Current evidence of asthma as primary diagnosis; unstable respiratory disease requiring oral/parenteral corticosteroids within 4 weeks prior to beginning the study; upper or lower respiratory tract infection within 4 weeks ofthe screening visit; unstable angina or unstable arrhythmias; concurrent use of medications that affected COPD; and evidence of alcohol abuse |
|
| Interventions |
|
|
| Outcomes | Mean change from baseline in predose FEV1 after 3-month treatment, change from baseline in VAS score assessing dyspnoea and in supplemental salbutamol | |
| Notes | Run-in: Patients entered a 2-week run-in period during which their regular treatment for COPD (all were under regular treatment with a long-acting beta2-agonist and an inhaled corticosteroid, many (81 out of 90) with also theophylline) was stopped with the exception of stable regimens of theophylline (no change in dose for 1 month prior to screening) and they received salbutamol for relief of breakthrough symptoms. Use of all other inhaled or oral bronchodilators, systemic corticosteroids, ipratropium bromide, oxitropium bromide, or leukotriene modifiers was prohibited | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to receive FSC, tiotropium or their combination by a computer-generated list. Randomisation was performed in blocks of 9 |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Dropout rate 10% |
| Selective reporting (reporting bias) | Low risk | Results for all listed outcomes were reported |
| Methods | Design: A randomised, double-blind, parallel-group, multicenter trial from May 2007 to June 2008. The trial included 102 centres in 9 countries: Australia (10 centres), Canada (16), France (12), Germany (12), Hungary (13), Poland (10), Slovakia (13), Spain (6) and Sweden (10) | |
| Participants |
Population: 660 patients with COPD eligible for LABA/ICS combination therapy, with a pre-bronchodilator FEV1 not exceeding 50% of the predicted normal value and a history of exacerbations requiring systemic steroids and/or antibiotics, were studied Baseline Characteristics: Mean age 62 years. COPD severity, moderate, severe to very severe with mean FEV1 predicted of 38%. 25% women. Inclusion Criteria: Patients with COPD eligible for inhaled corticosteroid/long-acting beta2-agonist (LABA/ICS) combination therapy aged ≥ 40 years, with a clinical diagnosis of COPD and symptoms for at least 2 years, at least one COPD exacerbation in the previous 12 months requiring systemic steroids and/or antibiotics, current or previous smokers with a smoking history of ≥ 10 pack-years, forced expiratory volume in 1 second (FEV1) ≤ 50% of predicted normal value and FEV1 / vital capacity < 70% pre-dose. Exclusion Criteria: Worsening of COPD during run-in or within 4 weeks prior to visit 2 requiring hospitalisation, a course of oral and/or inhaled steroids and/or antibiotics, use of ICS within 2 weeks prior to visit 2, use of oral/parenteral glucocorticosteroids within 4 weeks prior to visit 2, a history of asthma or any significant disease/disorder which, in the opinion of the investigator, may put the patient at risk or influence results |
|
| Interventions |
|
|
| Outcomes |
Primary: Change in predose FEV1 from randomisation (Week 0) to the full treatment period (mean FEV1 at 1, 6, and 12 wk of treatment) Secondary: Pre- and post-dose spirometry measurements (predose FVC and inspiratory capacity and post-treatment FEV1 (5 and 60 min), forced vital capacity (5 and 60 min) , and inspiratory capacity (60 min)) and the St. George’s Respiratory Questionnaire for COPD (SGRQ-C) |
|
| Notes |
Run-in: Before entering the study, patients stopped their LABA and ICS medication (4 weeks and 2 weeks prior to run-in, respectively). During the 2-week run-in period, all patients used tiotropium (Spiriva HandiHaler, Boehringer Ingelheim, Germany) 18 μg once daily. Terbutaline 0.5 mg/inhalation (Bricanyl® Turbuhaler®, AstraZeneca, Lund, Sweden) was used as needed for symptom relief during the run-in period Co-medication: Terbutaline 0.5 mg/inhalation (Bricanyl® Turbuhaler®, AstraZeneca, Lund, Sweden) was used as needed for symptom relief during the treatment period in both treatment arms |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were sequentially assigned to patients from a computer-generated list at AstraZeneca R&D, Lund, Sweden, as they became eligible |
| Allocation concealment (selection bias) | Low risk | The investigators were provided with a blinded randomisation code for each patient. Both clinicians and patients were blinded to treatment until completion of the study. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Treatment assignment was concealed as active and placebo Turbuhalers were of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | The dropout rates were 9% in the tiotropium + placebo and 8% in the tiotropium + LABA/ICS comb group respectively |
| Selective reporting (reporting bias) | Low risk | All collected data reported. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ando 2008 | Effects of tiotropium alone versus salmeterol/fluticasone combination |
| Bateman 2008 | Effects of tiotropium alone versus salmeterol/fluticasone combination |
| Biscione 2009 | 4 weeks of treatment |
| Golabi 2006 | Effects of tiotropium alone versus salmeterol/fluticasone combination |
| Hara 2007 | Effects of tiotropium alone versus salmeterol/fluticasone combination |
| Perng 2006 | 1 month of treatment and crossover design |
| Petroianni 2008 | Effects of tiotropium treatment alone versus formoterol treatment alone |
| Singh 2008 | 14 days of treatment and of crossover design |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: randomised, parallel-group, 12 months’ treatment |
| Participants | 126 patients (M/F: 92/34) with COPD |
| Interventions | salmeterol/fluticasone (50/250 μg) twice daily and tiotropium 18 μg once daily (n = 33, M/F: 23/10) salmeterol/fluticasone (50/250 μg) twice daily (n = 32, M/F: 24/8) tiotropium 18 μg once daily (n = 32, M/F: 23/9) blank control group (n = 29, M/F: 22/7), patients in this group did not receive any inhaled anticholinergic drugs, long-acting beta2 agonists or glucocorticoid therapy |
| Outcomes | Symptoms, health status, use of rescue medication, frequency of exacerbations, and FEV1 |
| Notes |
DATA AND ANALYSES
Comparison 1. tiotropium plus LABA/ICS combination versus tiotropium plus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all cause) | 3 | 1021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.88 [0.57, 6.23] |
| 2 Hospital admission (all causes) | 2 | 961 | Odds Ratio (M-H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 3 Hospital admission (exacerbation) | 2 | 961 | Odds Ratio (M-H, Fixed, 95% CI) | 0.66 [0.39, 1.13] |
| 4 Exacerbation | 2 | Odds Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 5 Pneumonia | 2 | 961 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.31, 5.99] |
| 6 Quality of life SGRQ scale | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 6.1 Quality of life 3-5 months mean difference + 1 yr SE | 2 | Mean Difference (Fixed, 95% CI) | −2.49 [−4.04, −0.94] | |
| 6.2 Quality of life 1 yr | 1 | Mean Difference (Fixed, 95% CI) | −4.1 [−7.22, −0.98] | |
| 7FEV1 | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 FEV1 3-5 months mean difference + 1yr SE | 3 | Mean Difference (Fixed, 95% CI) | 0.06 [0.04, 0.08] | |
| 7.2 FEV1 1 yr | 1 | Mean Difference (Fixed, 95% CI) | 0.06 [2.01, 0.12] | |
| 8 Serious adverse event (non-fatal) | 3 | 1021 | Odds Ratio (M-H, Fixed, 95% CI) | 0.60 [0.29, 1.25] |
| 9 Adverse event | 3 | 1021 | Odds Ratio (M-H, Fixed, 95% CI) | 1.12 [0.85, 1.49] |
| 10 Withdrawal | 3 | Odds Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Total number of subjects withdrawn | 3 | 1021 | Odds Ratio (M-H, Fixed, 95% CI) | 0.54 [0.38, 0.77] |
| 10.2 Due to adverse events | 2 | 961 | Odds Ratio (M-H, Fixed, 95% CI) | 0.92 [0.46, 1.83] |
| 10.3 Due to lack of efficacy | 2 | 961 | Odds Ratio (M-H, Fixed, 95% CI) | 0.36 [0.22, 0.59] |
| 11 Hospital admission (all causes) rate ratio | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 12 Hospital admission (exacerbation) rate ratio | 2 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 13 Clinically relevant change in quality of life | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Totals not selected |
Comparison 2. LABA/ICS combination plus tiotropium versus LABA/ICS combination plus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 GIV | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Adverse event | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 3 Withdrawal | 1 | Odds Ratio (M-H, Fixed, 95% CI) | Totals not selected | |
| 4 FEV1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
WHAT’S NEW
Last assessed as up-to-date: 2 August 2010.
| Date | Event | Description |
|---|---|---|
| 11 April 2013 | Amended | NIHR acknowledgment added |
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
Tiotropium plus LABA/ICS combination compared to Tiotropium plus placebo for chronic obstructive pulmonary disease
Patient or population: patients with chronic obstructive pulmonary disease
Settings:
Intervention: Tiotropium plus LABA/ICS combination
Comparison: Tiotropium plus placebo
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Tiotropium plus placebo | Tiotropium plus LABA/ICS combination | |||||
|
Mortality (all cause) Follow-up: 3 to 12 months |
8 per 1000 |
15 per 1000 (5 to 48) |
OR 1.88 (0.57 to 6.23) |
1021 (2 studies) |
⊕⊕○○ low1,2 |
|
|
Hospital admission (all causes) Follow-up: 3 to 12 months |
103 per 1000 |
88 per 1000 (57 to 132) |
OR 0.84 (0.53 to 1.33) |
961 (2 studies) |
⊕⊕○○ low1,2 |
|
|
Hospital admission (exacerbation) Follow-up: 3 to 12 months |
78 per 1000 |
53 per 1000 (32 to 87) |
OR 0.66 (0.39 to 1.13) |
961 (2 studies) |
⊕⊕○○ low1,2 |
|
| Exacerbation | See comment | See comment | Not estimable | 961 (2 studies) |
See comment | Results not combined as there was too much heterogeneity between the results of the two included studies |
|
Pneumonia Follow-up: 3 to 12 months |
6 per 1000 |
8 per 1000 (2 to 35) |
OR 1.35 (0.31 to 5.99) |
961 (2 studies) |
⊕⊕○○ low1,2 |
One trial only reported pneumonia leading to mechanical ventilation or death |
| Quality of life SGRQ scale - Quality of life 3-5 months mean difference + 1 yr SE | The mean Quality of life SGRQ scale - Quality of life 3-5 months mean difference + 1 yr SE in the intervention groups was 2.49 lower (4.04 to 0.94 lower) |
961 (2 studies) |
⊕⊕○○ low1,2 |
|||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Unequal loss to follow-up
Wide confidence intervals
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We included the comparison of treatment with tiotropium plus LABA/ICS versus LABA/ICS. In response to a peer review comment we changed the health-related quality of life outcome from secondary to primary.
Footnotes
DECLARATIONS OF INTEREST
None known.
References to studies included in this review
- Aaron 2007 {published data only} .Aaron SD, Vandemheen K, Ferguson D, FitzGerald M, Maltais F, Boureau J, et al. The Canadian optimal therapy of COPD trial: Design, organization and patient recruitment. Canadian Respiratory Journal. 2004;11(8):581–5. doi: 10.1155/2004/394710. [DOI] [PubMed] [Google Scholar]
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine. 2007;146(8):545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [see comment][summary for patients in Ann Intern Med. 2007 Apr 17;146(8):I12; PMID: 17310044] [DOI] [PubMed] [Google Scholar]; *
- Kaplan A. Effects of tiotropium combined with either salmeterol or salmeterol/fluticasone in moderate to severe COPD. Primary Care Respiratory Journal. 2007;16(4):258–60. doi: 10.3132/pcrj.2007.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafzadeh M, Marra CA, Sadatsafavi M, Aaron SD, Sullivan SD, Vandemheen KL, et al. Cost effectiveness of therapy with combinations of long acting bronchodilators and inhaled steroids for treatment of COPD. Thorax. 2008;63(11):962–7. doi: 10.1136/thx.2007.089557. [DOI] [PubMed] [Google Scholar]
- Roisman G. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. A randomized trial. Revue de Pneumologie Clinique. 2007;63(6):390–1. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- Cazzola 2007 {published data only} .Cazzola M, Ando F, Santus P, Ruggeri P, Di Marco F, Sanduzzi A, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulmonary Pharmacology and Therapeutics. 2007;20(5):556–61. doi: 10.1016/j.pupt.2006.06.001. [DOI] [PubMed] [Google Scholar]; *
- D’Amato M, Ando F, Santus P, Ruggeri P, Di Marco F, Cazzola M. Clinical effects of adding fluticasone propionate/salmeterol (FSC) and tiotropium (TIO) in severe-to-very severe COPD [Abstract] European Respiratory Journal. 2005;26(Suppl 49) Abstract No. 218. [Google Scholar]
- Welte 2009 {published data only} .Welte T, Hartman L, Polanowski T, Hernandez P. Budesonide/formoterol added to tiotropium is well tolerated and reduces risk of severe exacerbations in COPD patients [Abstract]; American Thoracic Society International Conference; San Diego. May 15-20, 2009. 2009:A6188 [Poster #215] [Google Scholar]
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Addition of budesonide/formoterol to tiotropium reduces the number of exacerbation days compared with tiotropium alone. Chest. 2009;136(4):26S–f. [Google Scholar]
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Budesonide/formoterol added to tiotropium provides rapid improvements in lung function and ability to undertake morning activities. Chest. 2009;136(4):24S–g. [Google Scholar]
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2009;180(8):741–50. doi: 10.1164/rccm.200904-0492OC. [DOI] [PubMed] [Google Scholar]
- Welte T, Miravitlles M, Hernandez P, Hartman L, Polanowski T, Kessler R. Budesonide/formoterol added to tiotropium improves lung function, health status, symptoms & morning activities in COPD patients [Abstract]; European Respiratory Society Annual Congress; Vienna, Austria. 2009.Sep 12-16, p. P2005. [Google Scholar]
- Welte T, Miravitlles M, Hernandez P, Peterson S, Polanowski T, Kessler R. Budesonide/formoterol added to tiotropium improves exacerbations and exacerbation-related antibiotic use in patients with COPD [Abstract]; European Respiratory Society Annual Congress; Vienna, Austria. 2009.Sep 12-16, p. P2012. [Google Scholar]
- Welte T, Miravitlles M, Peterson S, Polanowski T, Kessler R. Budesonide/formoterol added to tiotropium improves the management of COPD patients [Abstract]; American Thoracic Society International Conference; San Diego. 2009; May 15-20, 2009:A6192 [Poster #216] [Google Scholar]
References to studies excluded from this review
- Ando 2008 {published data only} .Ando F, Ruggeri P, Girbino G, Cazzola M. Tiotropium and salmeterol/fluticasone combination do not cause oxygen desaturation in COPD. Respiratory Medicine. 2008;102(6):815–8. doi: 10.1016/j.rmed.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Bateman E, Van-Dyk M, Chang A. A pilot study comparing tiotropium to salmeterol plus fluticasone in moderate COPD [Abstract] European Respiratory Journal. 2005;26(Suppl 49) Abstract No. 851. [Google Scholar]
- Bateman ED, van Dyk M, Sagriotis A. Comparable spirometric efficacy of tiotropium compared with salmeterol plus fluticasone in patients with COPD: A pilot study. Pulmonary Pharmacology and Therapeutics. 2008;21(1):20–5. doi: 10.1016/j.pupt.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Biscione 2009 {published data only} .Biscione G, Crigna G, Auciello L, Pasqua F, Cazzola M. Addition of tiotropium (T) to a regular treatment with long-acting beta-agonist + inhaled corticosteroid (LABA + ICS) in patients with severe to very-severe COPD under inpatient pulmonary rehabilitation program (PRP) [Abstract]; European Respiratory Society Annual Congress; Vienna, Austria. 2009.Sep 12-16, p. P526. [Google Scholar]
- Golabi 2006 {published data only} .Golabi P, Topaloglu N, Karakurt S, Celikel T. Effects of tiotropium and salmeterol/fluticasone combination on lung hyperinflation dyspnea and exercise tolerance in COPD [Abstract] European Respiratory Journal. 2006;28(Suppl 50):33s. E304. [Google Scholar]
- Hara 2007 {published data only} .Hara K, Kurashima K, Tokunaga D, Ueno M, Aoyagi K, Isobe Z, et al. Single blind comparison of tiotropium and salmeterol plus fluticasone propionate of treatment in patients with chronic obstructive pulmonary disease (COPD) [Abstract]; American Thoracic Society International Conference; San Francisco, California, USA. 2007; May 18-23, 2007:Poster #A1. [Google Scholar]
- Perng 2006 {published data only} .Perng DW, Wu CC, Su KC, Lee YC, Perng RP, Tao CW. Additive benefits of tiotropium in COPD patients treated with long-acting beta2 agonists and corticosteroids. Respirology. 2006;11(5):598–602. doi: 10.1111/j.1440-1843.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- Petroianni 2008 {published data only} .Petroianni A, Ceccarelli D, Conti V, Graziani E, Terzano C. Evening administration of tiotropium during combination therapy reduces night symptoms in COPD patients [Abstract]; European Respiratory Society Annual Congress; Berlin, Germany. 2008; Oct 4-8, E4282. [Google Scholar]
- Singh 2008 {published data only} .Singh D, Brooks J, Hagan G, Cahn A, O’Connor BJ. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63(7):592–8. doi: 10.1136/thx.2007.087213. [DOI] [PubMed] [Google Scholar]
- Singh D, Hagan G, Cahn A, Leonard TB, Riley JH, O’Connor BJ. Individual and combined responses to salmeterol/fluticasone propionate combination (SFC) and tiotropium (Tio) shown in a COPD clinical trial [Abstract]; American Thoracic Society International Conference; Toronto. 2008; May 16-21, 2008:A648[#F10] [Google Scholar]
References to studies awaiting assessment
- Fang 2008 {published data only} .Fang L, Liang X, Zhang F, Liu L, Fu W, Zhao Z, et al. Combination of inhaled salmeterol/fluticasone and tiotropium in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Zhonghua Jiehe He Huxi Zazhi [Chinese Journal of Tuberculosis and Respiratory Diseases] 2008;31(11):811–4. [PubMed] [Google Scholar]
Additional references
- Appleton 2006 .Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, et al. Ipratropium bromide versus long-acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD006101. DOI: 10.1002/14651858.CD006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr 2005 .Barr RG, Bourbeau J, Camargo CA. Tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD002876.pub2. DOI: 10.1002/14651858.CD002876.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeh 2010 .Beeh KM, Beier J. The short, the long and the “ultra-long”: why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy. 2010;27(3):150–9. doi: 10.1007/s12325-010-0017-6. [DOI] [PubMed] [Google Scholar]
- Cazzola 2008 .Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal. 2008;31(2):416–69. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- Effing 2007 .Effing T, Monninkhof EEM, van der Valk PP, Zielhuis GGA, Walters EH, van der Palen JJ, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD002990.pub2. DOI: 10.1002/14651858.CD002990.pub2. [DOI] [PubMed] [Google Scholar]
- GOLD . [Accessed 4th February 2011];Global Initiative for Chronic Obstructive Lung Disease. http://www.goldcopd.com
- Higgins 2009 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration; 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Hutchinson 2010 .Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medical Journal. 2010;40(5):364–71. doi: 10.1111/j.1445-5994.2010.02195.x. 1445-5994: (Electronic) [DOI] [PubMed] [Google Scholar]
- Keene 2008 .Keene ON, Calverley PM, Jones PW, Vestbo J, Anderson JA. Statistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisited. European Respiratory Journal. 2008;32(1):17–24. doi: 10.1183/09031936.00161507. 1399-3003: (Electronic) [DOI] [PubMed] [Google Scholar]
- Lacasse 2006 .Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2006;(4) doi: 10.1002/14651858.CD003793.pub2. DOI: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- Nannini 2007 .Nannini LJ, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD006826. DOI: 10.1002/14651858.CD006826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannini 2007a .Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD003794.pub3. DOI: 10.1002/14651858.CD003794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannini 2007b .Nannini LJ, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta-agonist in one inhaler versus long-acting beta-agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD006829. DOI: 10.1002/14651858.CD003794.pub2. [DOI] [PubMed] [Google Scholar]
- Review Manager 5 .Copenhagen The. Review Manager (RevMan). 5.0. The Cochrane Collaboration; Copenhagen, The Nordic Cochrane Centre: 2008. Nordic Cochrane Centre: The Cochrane Collaboration. [Google Scholar]
- SGRQ-C manual 2008 .Paul Jones. St George’s Respiratory Questionnaire for COPD Patients (SGRQ-C) St George’s, University of London; 2008. [Google Scholar]
- Welsh 2010 .Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long-acting beta2-agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2010;(5) doi: 10.1002/14651858.CD007891.pub2. DOI: 10.1002/14651858.CD007891.pub2. [DOI] [PubMed] [Google Scholar]
- Yang 2007 .Yang IA, Fong K, Sim EHA, Black PN, Lasserson TJ. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD002991.pub2. DOI: 10.1002/14651858.CD002991.pub2. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study