Abstract
Background
Cluster headache is an uncommon, but severely painful and disabling condition, with rapid onset. Validated treatment options are limited, and first-line therapy includes inhaled oxygen. Alternative therapies such as intranasal lignocaine and ergotamine are not as commonly used and are less well studied. Triptans are successfully used to treat migraine attacks and, because of this, they may also be useful for cluster headache.
Objectives
To determine the efficacy and tolerability of triptans for the acute treatment of cluster headaches.
Search methods
We searched Cochrane CENTRAL, MEDLINE and EMBASE for studies through 22 January 2010.
Selection criteria
Randomised, double-blind, placebo-controlled studies of triptans for acute treatment of cluster headache episodes.
Data collection and analysis
Two review authors independently assessed study quality and extracted data. Numbers of participants with different levels of pain relief, requiring rescue medication and experiencing adverse events and headache-associated symptoms in treatment and control groups were used to calculate relative risk and numbers needed to treat (NNT) and harm (NNH).
Main results
All six included studies used a single dose of triptan to treat an attack of moderate to severe pain intensity. In total 231 participants received zolmitriptan 5 mg, 223 received zolmitriptan 10 mg, 131 received sumatriptan 6 mg, 88 received sumatriptan 12 mg, and 326 received placebo. Zolmitriptan was administered either orally or intranasally, and sumatriptan either subcutaneously or intranasally.
Overall, the triptans studied were better than placebo for headache relief and pain-free responses, with an NNT of 2.4 for 15 minute pain relief with subcutaneous sumatriptan 6 mg (75% with sumatriptan and 32% with placebo), and 2.8 for 30 minute pain relief with intranasal zolmitriptan 10 mg (62% with zolmitriptan and 26% with placebo). Fewer participants need rescue medication with triptan than with placebo, but more experienced adverse events.
Authors’ conclusions
Zolmitriptan and sumatriptan are effective in the acute treatment of cluster headaches and may provide a useful treatment option, potentially offering convenience over oxygen therapy and a better safety and tolerability profile than ergotamine. Non-oral routes of administration are likely to provide better and more rapid responses.
Medical Subject Headings (MeSH): Cluster Headache [*drug therapy], Oxazolidinones [therapeutic use], Randomized Controlled Trials as Topic, Serotonin Receptor Agonists [*therapeutic use], Sumatriptan [therapeutic use], Tryptamines [*therapeutic use]
MeSH check words: Humans
BACKGROUND
Description of the condition
Cluster headache is a most painful form of primary headache. Diagnosis is clinical with emphasis on pain, periodicity and autonomic features. The diagnostic criteria of the International Headache Society (IHS), which are used in clinical trials, describe cluster headache as ‘attacks of severe, strictly unilateral pain which is orbital, supraorbital, temporal or in any combination of these sites, lasting 15 to 180 minutes and occurring from once every other day to eight times per day’ and associated with one or more of various ipsilateral symptoms (conjunctival injection, lacrimation, nasal congestion, rhinorrhoea, forehead and facial sweating, miosis, ptosis or eyelid oedema) (IHS 2004; Appendix 1).
It is important to note a few key definitions used in the descriptions of cluster headache. An ‘attack’ is an individual episode of headache, and a ‘cluster period’ is a series of such attacks. The major division of cluster headache is between episodic and chronic types. Episodic cluster headache is defined as ‘cluster headache attacks occurring in periods lasting 7 days to 1 year separated by pain-free periods [remissions] lasting 1 month or longer’, while chronic cluster headache is defined as ‘attacks occurring for more than 1 year without remission or with remissions lasting less than 1 month’ (IHS 2004).
The prevalence of cluster headache is estimated to be 0.02% to 0.4%, with a male preponderance (D’Alessandro 1986; Torelli 2005). Onset is commonest between 20 to 40 years of age (Ekbom 1978). Cluster headache pain has a rapid onset and lasts from 15 minutes to 3 hours.
Treatment for cluster headache attacks can be either preventative or acute. Preventative treatment aims to decrease the incidence of attacks; acute therapy is directed at alleviating the symptoms of an individual attack when it occurs. This review will look at acute therapies only. Given the severity, rapid onset and short time to peak intensity, treatment has to be swift and effective. Validated treatment options are limited, and first-line therapy includes inhaled oxygen. Alternative therapies such as intranasal lignocaine and ergotamine are not as commonly used and are less well studied.
Description of the intervention
Triptans are selective agonists at the ligand gated, G-protein linked serotonergic, or 5-hydroxytriptamine (5-HT, or serotonin) receptors. Several drugs within the ‘triptan’ class are licensed for use in primary headache. These drugs have been extensively studied for the acute treatment of migraine. Because cluster headache shares some clinical features with migraine, triptans have also been studied for the acute treatment of cluster headache using the oral and the more rapid intranasal or subcutaneous routes of administration (Plosker 1994; Rapoport 2007).
How the intervention might work
There is no current unifying theory to explain cluster headaches. Positron emission tomography (PET) has been used to look at regional cerebral changes in blood flow during acute attacks. From these imaging studies, it appears that areas in the brain stem such as the hypothalamus may act as a neurological origin for cluster headache (May 1998). Alcohol, nitrates and carbon dioxide are known to precipitate cluster headache. Similar inflammatory metabolic intermediaries such as calcitonin gene related peptide (CGRP), substance P, somatostatin and histamine, all with vasodilatory properties, are also known to provoke attacks (Cluster Headache Study Group 1991). Despite this, such evidence does not fully answer why cluster headaches have circadian rhythmicity, are unilateral and have a male preponderance.
Cluster headaches are therefore thought to be a neurovascular phenomenon with contributions from central and peripheral mechanisms. The hypothalamus, or closely related structure, is involved as a candidate site of origin. This could cause severe unilateral pain by activation of the trigeminal nerve complex with autonomic symptoms as a result of perivascular activation and increased cranial parasympathetic outflow.
Serotoninergic receptors are distributed ubiquitously around the brain stem and trigeminal complex. Agonism of these receptors by the triptan class of drugs is thought to be responsible for their therapeutic effect. This effect could be mediated by inhibition of trigeminal nerve endings in large cerebral vessels, direct vasoconstriction of these associated vessels and neuronal inhibition of more central hypothalamic lesions.
Route of administration may of particular importance in cluster headache because of the need for rapid relief. The subcutaneous route might be expected to provide the best results since there is rapid absorption, followed by intranasal or sublingual routes (where absorption may be partly enteral and partly parenteral), with oral tablets likely to be least rapid due to slower absorption. In practice this may limit the choice of triptan for cluster headache, since only sumatriptan is available as a subcutaneous formulation, only zolmitriptan as a nasal spray, and only rizatriptan as a melt wafer. All are available as oral tablets.
Why it is important to do this review
Although cluster headache affects a relatively small proportion of people, it is an extremely painful and disabling condition. There is currently no systematic review of the evidence for the efficacy and tolerability of the triptan class of drugs for acute cluster headache. The present review fills this gap using methods that allow comparison between individual drugs and routes of administration in standardised trials.
OBJECTIVES
The objective of this review is to assess the efficacy and tolerability of the triptan class of drugs compared to placebo and other active interventions in the acute treatment of episodic and chronic cluster headache in adult patients.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised, double-blind, placebo-controlled studies using a triptan to treat a discrete cluster headache episode were included. Studies had to have a minimum of 10 participants per treatment arm and report dichotomous data for at least one of the primary outcomes specified below. Studies reporting treatment of consecutive headache episodes were accepted if outcomes for the first, or each, episode were reported separately. Cross-over studies were accepted if there was adequate washout between treatments.
Types of participants
Studies had to include adults (at least 18 years of age) with cluster headache. The diagnosis of cluster headache specified by the International Headache Society was used (IHS 1988 or IHS 2004), although other definitions were considered if they conformed in general to IHS diagnostic criteria. There were no restrictions on cluster headache frequency, duration or type (episodic or chronic). Participants taking stable prophylactic therapy to reduce headache frequency were accepted; details on the prophylactic therapy prescribed or allowed are provided in the Characteristics of included studies table.
Types of interventions
Included studies had to use either a single dose of any triptan to treat a cluster headache episode when pain was of moderate to severe intensity, or investigate different dosing strategies and/or timing of the first dose in relation to headache intensity. There was no restriction on dose or route of administration, provided the medication could be self-administered.
A placebo comparator is essential to demonstrate that triptans are effective in this condition, and the main comparison considered was triptan versus placebo. Where studies had both a placebo and active comparator, data were sought for direct comparison of triptan versus the active comparator. Active-controlled trials without a placebo, such as equivalence trials, were considered as secondary evidence if the trial was judged to have validity by accepted criteria (McAlister 2001). Studies to demonstrate prophylactic efficacy in reducing the number or frequency of cluster headaches were not included.
Types of outcome measures
Measures of pain intensity or pain relief had to be made by the patient (not the investigator or carer) using a standard 5-point categorical scale (pain intensity: none, mild, moderate, severe, very severe/excruciating; pain relief: none, a little, some, a lot, complete) or 100 mm visual analogue scale (VAS) (IHS Trial Guidelines 1995; Moore 2003).
Primary outcomes
The primary outcomes considered (see Differences between protocol and review) are:
Headache relief (assessed on the 5-point pain relief scale described above or defined as a reduction in pain intensity from moderate/severe/very severe to none/mild) at 15, 30, or 60 minutes, without use of rescue medication;
Pain-free at 15, 30, or 60 minutes, without use of rescue medication.
Secondary outcomes
Secondary outcomes to be considered were:
Relief of headache-associated symptoms;
Time to headache relief;
Time to pain-free;
Use of rescue medication;
Adverse events: any, serious, withdrawal, reported within 24 hours post-dose;
Recurrence of headache (defined as the return of headache to a moderate or severe pain intensity within 24 hours of initial medication).
Search methods for identification of studies
Electronic searches
The following databases were searched:
Cochrane CENTRAL (22 January 2010)
MEDLINE (via OVID) (22 January 2010)
EMBASE (via OVID) (22 January 2010)
See Appendix 2 for the search strategy for MEDLINE (via OVID), Appendix 3 for the search strategy for EMBASE and Appendix 4 for the search strategy for CENTRAL. There were no language restrictions.
Searching other resources
Reference lists of retrieved studies and review articles were searched for additional studies. Grey literature and abstracts were not searched.
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. Titles and abstracts of all studies identified were viewed on screen, and any that clearly did not satisfy inclusion criteria were excluded. Full copies of the remaining studies were read to identify those suitable for inclusion. Disagreements were settled by discussion with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. Disagreements were settled by discussion with a third review author. Data were entered into RevMan 5.0 by one author.
Assessment of risk of bias in included studies
Methodological quality was assessed using the Oxford Quality Scale (Jadad 1996).
The scale is used is as follows:
Is the study randomised? If yes, give one point.
Is the randomisation procedure reported and is it appropriate? If yes, add one point; if no, deduct one point.
Is the study double blind? If yes, add one point.
Is the double blind method reported and is it appropriate? If yes, add one point; if no, deduct one point.
Are the reasons for patient withdrawals and dropouts described? If yes, add one point.
The scores for each study are reported in the Characteristics of included studies table.
A risk of bias table was also completed for each study, using assessments of randomisation, allocation concealment and blinding.
Measures of treatment effect
Dichotomous data
Relative risk (or ‘risk ratio’, RR) was used to establish statistical difference. Numbers needed to treat (NNT) and pooled percentages were used as absolute measures of benefit or harm.
The following terms were used to describe adverse outcomes in terms of harm or prevention of harm:
When significantly fewer adverse outcomes occurred with triptans than with control (placebo or active) we used the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occurred with triptans than with control (placebo or active) we used the term the number needed to harm or cause one event (NNH).
Time-to-event data
Time-to-event data were weighted by study size and presented as the weighted mean of the median or mean.
Unit of analysis issues
We accepted randomisation to individual patient only.
Dealing with missing data
The most likely source of missing data is in cross-over studies. Where this was an issue, only first-period data were used.
Assessment of heterogeneity
Heterogeneity of studies was assessed visually (L’Abbe 1987).
Assessment of reporting biases
No formal tests were planned.
Data synthesis
We planned to analyse studies using a single dose of a triptan in established pain of at least moderate intensity separately from studies in which medication was taken before pain was well established or in which a second dose of medication was permitted. In fact, however, no studies were identified that examined early treatment of attacks before pain was of at least moderate intensity; similarly, none of the included studies employed multiple dosing strategies for a single attack.
Effect sizes were calculated and data combined for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). Relative risk of benefit or harm was calculated with 95% confidence intervals (CIs) using a fixed-effect model (Morris 1995). NNT, NNTp and NNH with 95% CIs were calculated using the pooled number of events by the method of Cook and Sackett (Cook 1995). Using this method, a statistically significant difference from control is assumed when the 95% CI of the relative benefit does not include the number one.
Data from comparisons and outcomes with only one study or fewer than 200 participants are described in the summary tables for information and comparison, but were not analysed quantitatively.
Subgroup analysis and investigation of heterogeneity
Issues for subgroup analysis were drug, dose and route of administration.
Sensitivity analysis
Sensitivity analysis was anticipated for study quality (Oxford Quality score of 2 versus 3 or more), and for headache type (episodic versus chronic cluster headache). A minimum of two studies and 200 participants had to be available for any sensitivity analysis.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Searching identified eight potentially relevant studies.
Included studies
Six studies satisfied inclusion criteria (Bahra 2000; Cittadini 2006; Ekbom 1991; Ekbom 1993; Rapoport 2007; Van Vliet 2003) and all were cross-over in design. One of these studies (Van Vliet 2003) treated two headache episodes with each study medication and did not provide data for each episode separately. We decided to include the study, subject to a sensitivity analysis, but since it was the only study using sumatriptan 20 mg, data from it were not pooled; results are presented where appropriate. In the remaining five studies, one headache episode was treated with each study medication. These five studies all included adequate washout periods between cross-over periods/treatments (18 to 24 hours), and all reported negative tests for period effect, so we combined data from all periods/headaches treated with each medication in our analyses.
All of the included studies used a single dose of a triptan when the headache was of at least moderate intensity; all used a similar 5-point scale to assess headache pain intensity. No studies were identified examining early treatment of attacks before pain was of at least moderate intensity. Similarly, no studies employed multiple dosing strategies for a single attack.
All studies were multicentred and diagnosed cluster headache according to IHS criteria. Most required that participants had previously tolerated treatment with a triptan, and/or had no contraindications, but Ekbom 1991 and Ekbom 1993 recruited only sumatriptan-naïve participants. In four studies participants self-treated their headaches at home (Bahra 2000; Cittadini 2006; Rapoport 2007; Van Vliet 2003), while in the other two medication was administered by a physician or nurse in hospital (Ekbom 1991; Ekbom 1993). We included these hospital-based studies because although the intervention was not self-administered in these cases, subcutaneous sumatriptan can be, and is commonly, self-administered.
The mean age of participants ranged from 40 to 44, and between 60% to 87% were men. The studies included participants with diagnoses of both episodic and chronic types of cluster headache, with between 56% and 75% having episodic-type headaches. Two studies reported some results for episodic and chronic headaches separately (Bahra 2000; Cittadini 2006). No participants were using prophylactic medication, and, as noted above, all studies had an appropriate washout period between study doses, co-analgesics or rescue medication.
All studies compared a triptan with placebo. No study directly compared one drug with another. Oral zolmitriptan 5 mg and 10 mg were tested in one study (Bahra 2000), and intranasal zolmitriptan 5 mg and 10 mg in two studies (Cittadini 2006; Rapoport 2007). Subcutaneous sumatriptan 6 mg was tested in two studies (Ekbom 1991; Ekbom 1993), subcutaneous sumatriptan 12 mg in one (Ekbom 1993), and intranasal sumatriptan 20 mg in one study (Van Vliet 2003). In total, 231 participants received zolmitriptan 5 mg, 223 received zolmitriptan 10 mg, 131 received sumatriptan 6 mg, 88 received sumatriptan 12 mg, 77 received sumatriptan 20 mg and 430 received placebo.
The outcomes reported by individual studies are listed in the Characteristics of included studies table. Most studies evaluated headache relief and pain-free response at 30 minutes as the primary outcome measure. Two of the six studies evaluated headache relief and pain-free response as the primary outcome measure at an earlier time of 15 minutes (Ekbom 1991; Ekbom 1993). One study (Rapoport 2007) reported these outcomes at various time points up to 60 minutes. Dichotomous data on the use of rescue medication were reported for all trials. Two studies did not report numerical data on adverse events (Cittadini 2006; Van Vliet 2003). All studies except Rapoport 2007 reported data on relief of associated symptoms, but not in a consistent way. No data were presented on headache recurrence. Only one study reported data on time to a response: initial headache relief (Van Vliet 2003).
Details are provided in the Characteristics of included studies table.
Excluded studies
Two publications describing three trials were excluded (Goadsby 1994; Hardebo 1998). Details are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Methodological quality, assessed using the Oxford Quality Scale, was good in all studies. Three studies scored 5/5 (Bahra 2000; Ekbom 1991; Rapoport 2007), and three scored 4/5 (Cittadini 2006; Ekbom 1993; Van Vliet 2003). Points were lost due to failure to adequately report the method of randomisation or blinding. All studies reported withdrawals and dropouts. Full details are provided in the Characteristics of included studies table.
Sequence generation, allocation concealment and blinding were assessed using the ‘Risk of bias’ tool. No studies were at high risk of bias (Figure 1).
Figure 1. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
Details of select efficacy outcomes (headache relief and pain-free at 15 and 30 minutes, headache recurrence) for individual studies are provided in Appendix 5.
Primary outcomes (Summary of results A)
For the primary outcomes of interest, we focused on results from 30 minutes and (to a lesser degree) 15 minutes post-treatment. The limited data available from 60 minutes are described in the text for completeness’ sake, but are not included in the summary tables or analyses (see Differences between protocol and review).
Headache relief at 30 minutes
Zolmitriptan
Three studies provided data. One (Bahra 2000) used an oral, and two (Cittadini 2006, Rapoport 2007) used an intranasal formulation. All tested both 5 mg and 10 mg doses; 231 participants were treated with zolmitriptan 5 mg, 223 with zolmitriptan 10 mg, and 226 with placebo.
Zolmitriptan (oral and nasal spray) 5 mg versus placebo
The proportion of patients experiencing headache relief at 30 minutes with zolmitriptan 5 mg was 50% (115/231; range 42% to 54%);
The proportion of participants experiencing headache relief at 30 minutes with placebo was 35% (79/226; range 23% to 43%);
The relative benefit of treatment compared with placebo was 1.4 (1.2 to 1.8), giving an NNT for headache relief at 30 minutes of 6.7 (4.2 to 17) (Figure 2).
Figure 2. Forest plot of comparison: 1 Zolmitriptan 5 mg versus placebo, outcome: 1.1 Participants with headache relief at 30 minutes.
For the two studies using the intranasal formulation only, the relative benefit compared with placebo was 1.7 (1.2 to 2.5), giving an NNT for headache relief at 30 minutes of 5.2 (3.2 to 14).
Zolmitriptan (oral and nasal spray) 10 mg versus placebo
The proportion of patients experiencing headache relief at 30 minutes with zolmitriptan 10 mg was 57% (128/223; range 53% to 63%);
The proportion of participants experiencing headache relief at 30 minutes with placebo was 35% (79/226; range 23% to 43%);
The relative benefit of treatment compared with placebo was 1.7 (1.3 to 2.0), giving an NNT for headache relief at 30 minutes of 4.5 (3.2 to 7.4) (Figure 3).
Figure 3. Forest plot of comparison: 2 Zolmitriptan 10 mg versus placebo, outcome: 2.1 Participants with headache relief at 30 minutes.
For the two studies using the intranasal formulation only, the relative benefit compared with placebo was 2.4 (1.7 to 3.3), giving an NNT for headache relief at 30 minutes of 2.8 (2.1 to 4.3).
Zolmitriptan (nasal spray) 10 mg versus 5 mg
The two studies using intranasal zolmitriptan 5 mg and 10 mg provided sufficient data for direct comparison of the two doses.
The proportion of participants experiencing headache relief at 30 minutes with zolmitriptan 10 mg was 62% (69/112; range 60% to 63%);
The proportion of patients experiencing headache relief at 30 minutes with zolmitriptan 5 mg was 45% (53/117; range 42% to 50%);
The relative benefit of 10 mg compared with 5 mg was 1.4 (1.1 to 1.7), giving an NNT for headache relief at 30 minutes of 6.1 (3.4 to 28) (Analysis 3.1).
When the study using the oral formulation was included in the analysis, the difference between the 10 mg and 5 mg doses failed to reach the 5% level of significance (relative benefit 1.2 (0.97 to 1.4)).
Sumatriptan
Only one study, using intranasal sumatriptan 20 mg, reported this outcome (Van Vliet 2003); 44/77 participants treated with sumatriptan and 20/77 treated with placebo achieved headache relief at 30 minutes (Appendix 5). There were insufficient data for analysis.
Headache relief at 15 minutes
Zolmitriptan
Two studies using the intranasal formulation provided data (Cittadini 2006; Rapoport 2007). Both tested 5 mg and 10 mg doses; 117 participants were treated with zolmitriptan 5 mg, 112 with zolmitriptan 10 mg, and 111 with placebo.
Zolmitriptan (nasal spray) 5 mg versus placebo
The proportion of patients experiencing headache relief at 15 minutes with zolmitriptan 5 mg was 15% (18/117; range 9% to 23%);
The proportion of participants experiencing headache relief at 15 minutes with placebo was 7% (8/111, range 2% to 14%);
The relative benefit of treatment compared with placebo was 2.2 (0.99 to 4.7; Analysis 1.3). The NNT was not calculated.
Zolmitriptan (nasal spray) 10 mg versus placebo
The proportion of patients experiencing headache relief at 15 minutes with zolmitriptan 10 mg was 28% (31/112; range 19% to 39%);
The proportion of participants experiencing headache relief at 15 minutes with placebo was 7% (8/111; range 2% to 14%);
The relative benefit of treatment compared with placebo was 3.9 (1.9 to 8.0; Analysis 2.3), giving an NNT for headache relief at 15 minutes of 4.9 (3.3 to 9.2).
Sumatriptan
Sumatriptan (subcutaneous) 6 mg versus placebo
Two studies using subcutaneous sumatriptan provided data (Ekbom 1991; Ekbom 1993); 131 participants were treated with sumatriptan and 127 with placebo.
The proportion of patients experiencing headache relief at 15 minutes with sumatriptan 6 mg was 75% (98/131; range 74% to 75%);
The proportion of participants experiencing headache relief at 15 minutes with placebo was 32% (41/127; range 26% to 35%);
The relative benefit of treatment compared with placebo was 2.3 (1.8 to 3.0; Figure 4), giving an NNT for headache relief at 15 minutes of 2.3 (1.9 to 3.2).
Figure 4. Forest plot of comparison: 4 Sumatriptan 6 mg versus placebo, outcome: 4.1 Participants with headache relief at 15 minutes.
Sumatriptan (subcutaneous) 12 mg versus placebo
One study compared subcutaneous sumatriptan 12 mg with placebo (Ekbom 1993); 70/88 participants treated with sumatriptan and 31/88 treated with placebo achieved this outcome. There were insufficient data for analysis.
Comparing results for intranasal zolmitriptan 10 mg with subcutaneous sumatriptan 6 mg gave z = 2.96, P = 0.003, indicating superiority of subcutaneous sumatriptan.
Headache relief at 60 minutes
Zolmitriptan
One study (Rapoport 2007) reported on headache relief at 60 minutes: 80% of participants with intranasal zolmitriptan 10 mg, and 58% with intranasal zolmitriptan 5 mg had headache relief compared with 56% with placebo. There were insufficient data for analysis.
Sumatriptan
There were no studies of sumatriptan reporting on participants who had headache relief at times greater than 30 minutes.
Pain-free at 30 minutes
Zolmitriptan
Two studies using the intranasal formulation provided data (Cittadini 2006; Rapoport 2007). Both tested 5 mg and 10 mg doses; 117 participants were treated with zolmitriptan 5 mg, 112 with zolmitriptan 10 mg, and 111 with placebo.
Zolmitriptan (nasal spray) 5 mg versus placebo
The proportion of participants pain-free at 30 minutes with zolmitriptan 5 mg was 32% (38/117; range 28% to 38%);
The proportion of participants pain-free at 30 minutes with placebo was 18% (20/111, range 16% to 20%);
The relative benefit of treatment compared with placebo was 1.8 (1.1 to 2.9; Figure 5), giving an NNT for pain-free response at 30 minutes of 6.9 (3.9 to 30).
Figure 5. Forest plot of comparison: 1 Zolmitriptan 5 mg versus placebo, outcome: 1.2 Participants pain-free at 30 minutes.
Zolmitriptan (nasal spray) 10 mg versus placebo
The proportion of participants pain-free at 30 minutes with zolmitriptan 10 mg was 48% (54/112; range 47% to 49%);
The proportion of participants pain-free at 30 minutes with placebo was 18% (20/111; range 16% to 20%);
The relative benefit of treatment compared with placebo was 2.7 (1.7 to 4.2; Analysis 2.2), giving an NNT for pain-free response at 30 minutes of 3.3 (2.4 to 5.4).
Zolmitriptan (nasal spray) 10 mg versus 5 mg
The two studies using intranasal zolmitriptan 5 mg and 10 mg provided sufficient data for direct comparison of the two doses for this outcome.
The proportion of participants experiencing pain-free response at 30 minutes with zolmitriptan 10 mg was 48% (54/112; range 47% to 49%);
The proportion of patients experiencing pain-free response at 30 minutes with zolmitriptan 5 mg was 32% (38/117; range 28% to 38%);
The relative benefit of 10 mg compared with 5 mg was 1.5 (1.1 to 2.1; Analysis 3.2), giving an NNT for pain-free response at 30 minutes of 6.4 (3.5 to 31.5).
Sumatriptan
One study, using intranasal sumatriptan 20 mg, reported this outcome (Van Vliet 2003); 36/77 participants treated with sumatriptan, and 14/77 treated with placebo achieved this outcome (Appendix 5). There were insufficient data for analysis.
Pain-free at 15 minutes
Zolmitriptan
Two studies using the intranasal formulation provided data (Cittadini 2006; Rapoport 2007). Both tested 5 mg and 10 mg doses; 117 participants were treated with zolmitriptan 5 mg, 112 with zolmitriptan 10 mg, and 111 with placebo.
Zolmitriptan (nasal spray) 5 mg versus placebo
The proportion of participants pain-free at 15 minutes with zolmitriptan 5 mg was 8% (9/117; range 2% to 15%);
The proportion of participants pain-free at 15 minutes with placebo was 3% (3/111, range 0% to 6%);
The relative benefit of treatment compared with placebo was 2.6 (0.80 to 8.5; Analysis 1.4). The NNT was not calculated.
Zolmitriptan (nasal spray) 10 mg versus placebo
The proportion of participants pain-free at 15 minutes with zolmitriptan 10 mg was 12% (13/112; range 3% to 22%);
The proportion of participants pain-free at 15 minutes with placebo was 3% (3/111; range 0% to 6%);
The relative benefit of treatment compared with placebo was 3.9 (1.3 to 12; Analysis 2.4), giving an NNT for pain-free response at 30 minutes of 11 (6.4 to 49).
Sumatriptan
Sumatriptan (subcutaneous) 6 mg versus placebo
Two studies using subcutaneous sumatriptan 6 mg provided data (Ekbom 1991; Ekbom 1993); 131 participants were treated with sumatriptan and 127 with placebo.
The proportion of patients pain-free at 15 minutes with sumatriptan 6 mg was 48% (63/131; range 46% to 49%);
The proportion of participants pain-free at 15 minutes with placebo was 17% (22/127; range 10% to 20%);
The relative benefit of treatment compared with placebo was 2.8 (1.8 to 4.2; Figure 6), giving an NNT for pain-free at 15 minutes of 3.3 (2.4 to 5.0).
Figure 6. Forest plot of comparison: 4 Sumatriptan 6 mg versus placebo, outcome: 4.2 Participants pain-free at 15 minutes.
Sumatriptan (subcutaneous) 12 mg versus placebo
One study compared subcutaneous sumatriptan 12 mg with placebo (Ekbom 1993); 53/88 participants treated with sumatriptan and 18/88 treated with placebo achieved this outcome (Appendix 5). There were insufficient data for analysis.
Comparing results for intranasal zolmitriptan 10 mg with subcutaneous sumatriptan 6 mg gave z = 3.38, P < 0.0008, indicating superiority of subcutaneous sumatriptan.
Pain-free at 60 minutes
Zolmitriptan
One study (Rapoport 2007) reported on participants who were pain-free at 60 minutes: 59% of participants with intranasal zolmitriptan 10 mg, and 49% (estimated from graph) with intranasal zolmitriptan 5 mg were pain-free compared with 36% with placebo. There were insufficient data for analysis.
Sumatriptan
There were no studies of sumatriptan reporting on participants who were pain-free at times greater than 30 minutes.
| Summary of results A: headache relief and pain-free | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Dose (mg) | Route | Studies | Participants | Triptan % | Placebo % | NNT (95% CI) |
| Headache relief at 30 minutes | |||||||
| Zolmitriptan | 5 | Oral, intranasal | 3 | 457 | 50 | 35 | 6.7 (4.2 to 17) |
| Zolmitriptan | 5 | Intranasal | 2 | 228 | 45 | 26 | 5.2 (3.2 to 14) |
| Zolmitriptan | 10 | Oral, intranasal | 3 | 449 | 57 | 35 | 4.5 (3.2 to 7.4) |
| Zolmitriptan | 10 | Intranasal | 2 | 223 | 62 | 26 | 2.8 (2.1 to 4.3) |
| *Sumatriptan | 20 | Intranasal | 1 | 154 | 57 | 26 | Not calculated |
| Headache relief at 15 minutes | |||||||
| Zolmitriptan | 5 | Intranasal | 2 | 228 | 15 | 7 | 12 (6.1 to 1700) |
| Zolmitriptan | 10 | Intranasal | 2 | 223 | 28 | 7 | 4.9 (3.3 to 9.2) |
| Sumatriptan | 6 | Subcutaneous | 2 | 258 | 75 | 32 | 2.4 (1.9 to 3.2) |
| Sumatriptan | 12 | Subcutaneous | 1 | 176 | 80 | 35 | Not calculated |
| Pain-free at 30 minutes | |||||||
| Zolmitriptan | 5 | Intranasal | 2 | 228 | 32 | 18 | 6.9 (3.9 to 30) |
| Zolmitriptan | 10 | Intranasal | 2 | 223 | 48 | 18 | 3.3 (2.4 to 5.4) |
| Sumatriptan* | 20 | Intranasal | 1 | 154 | 47 | 18 | Not calculated |
| Pain-free at 15 minutes | |||||||
| Zolmitriptan | 5 | Intranasal | 2 | 228 | 8.1 | 3.2 | Not calculated |
| Zolmitriptan | 10 | Intranasal | 2 | 223 | 12 | 3.2 | 11 (6.4 to 49) |
| Sumatriptan | 6 | Subcutaneous | 2 | 258 | 48 | 17 | 3.3 (2.4 to 5.0) |
| Sumatriptan | 12 | Subcutaneous | 1 | 176 | 60 | 20 | Not calculated |
This study reported the mean of outcomes for two headache episodes.
Sensitivity analysis of the primary outcomes
All studies had scores for methodological quality of ≥3/5, so no sensitivity analysis was carried out for this criterion. There were insufficient data to compare different routes of administration. Two studies reported data for episodic and chronic headaches separately for the outcome of headache relief at 30 minutes. One (Bahra 2000) used oral zolmitriptan and the other (Cittadini 2006) used intranasal zolmitriptan, both at 5 mg and 10 mg doses. There were insufficient data for analysis, but results suggest there may be a better response in participants with episodic-type headache than with chronic-type headache in these studies.
Secondary outcomes
Relief of headache associated symptoms (zolmitriptan and sumatriptan)
Details of associated symptom outcomes for individual studies are provided in Appendix 6.
Three studies (Bahra 2000; Cittadini 2006; Van Vliet 2003) reported on the percentage of participants experiencing ‘improvement’ in various symptoms commonly associated with cluster headache at 30 minutes after treatment. The percentage was of those with symptoms at baseline, but this baseline number was not reported. Bahra 2000 gave data for participants with episodic-type headaches only. The symptoms were conjunctival injection/lacrimation, nasal congestion, ptosis/eyelid oedema, miosis and photophobia. Numerically, there was generally more improvement with triptan than placebo, and with zolmitriptan 10 mg than 5 mg, but it was not possible to assess the clinical significance of the differences observed.
Ekbom 1991 reported the presence of conjunctival injection at 15 and 30 minutes, with substantial differences between treatment and placebo groups at both time points. Ekbom 1993 reported the presence of conjunctival injection and ‘normal function’ at both baseline and 15 minutes. There were substantial improvements, of clinical relevance, for both outcomes in the treatment group.
Time to headache relief or pain-free (zolmitriptan and sumatriptan)
Two studies (Cittadini 2006; Rapoport 2007) using intranasal zolmitriptan 5 mg and 10 mg reported numbers of participants with headache relief and pain-free response at 5, 10, 15, 20 and 30 minutes post-dose. Rapoport also reported results at 60 minutes. Based on these limited data, the 10 mg dose is better than placebo from about 10 minutes, and the 5 mg dose from about 20 minutes. By 60 minutes only the 10 mg dose remained more effective than placebo, presumably because more headaches had resolved spontaneously by that time.
Two studies (Ekbom 1991; Ekbom 1993) using subcutaneous sumatriptan 6 mg and 12 mg reported numbers of participants with headache relief and pain-free response at 5, 10 and 15 minutes post-dose. At all time points sumatriptan was better than placebo, with no significant difference between the two doses.
No studies reported data for median or mean time to predefined pain relief or pain-free outcomes. Van Vliet 2003 reported the mean time to ‘initial relief’ with sumatriptan as 12.4 ± 6 minutes, and with placebo as 17.6 ± 12 minutes.
Use of rescue medication (Summary of results B)
Details of use of rescue medication in individual studies are presented in Appendix 5.
Zolmitriptan
Three studies provided data for zolmitriptan. One (Bahra 2000) used an oral, and two (Cittadini 2006; Rapoport 2007) used an intranasal formulation. All tested both 5 mg and 10 mg doses; 231 participants were treated with zolmitriptan 5 mg, 223 with zolmitriptan 10 mg, and 226 with placebo. Bahra 2000 and Cittadini 2006 permitted use of rescue medication (oxygen or analgesic) after 30 minutes, and Rapoport 2007 after 60 minutes. There was no obvious difference in response rates between these two times, so we combined the results for analysis.
Zolmitriptan (oral and nasal spray) 5 mg versus placebo
The proportion of participants using rescue medication with zolmitriptan 5 mg was 30% (69/231; range 26% to 35%);
The proportion of participants using rescue medication with placebo was 42% (95/226; range 38% to 49%);
The relative risk of treatment compared with placebo was 0.71 (0.55 to 0.91; Analysis 1.5), giving an NNTp of 8.2 (4.8 to 29).
Zolmitriptan (oral and nasal spray) 10 mg versus placebo
The proportion of participants using rescue medication with zolmitriptan 10 mg was 27% (61/223; range 27% to 29%);
The proportion of participants using rescue medication with placebo was 42% (95/226; range 38% to 49%);
The relative risk of treatment compared with placebo was 0.65 (0.50 to 0.85; Analysis 2.5), giving an NNTp of 6.8 (4.3 to 16).
Analysis of the two studies using the intranasal formulation alone, or the two studies reporting at 30 minutes did not substantially change the result.
Sumatriptan
Sumatriptan (subcutaneous) 6 mg and 12 mg versus placebo
Two studies using subcutaneous sumatriptan provided data. Rescue medication (100% oxygen) was permitted after 15 minutes. Ekbom 1991 used 6 mg, and Ekbom 1993 used 6 mg and 12 mg. These studies were combined for analysis.
The proportion of participants using rescue medication with sumatriptan 6 mg and 12 mg was 12% (27/219; range 10% to 14%);
The proportion of participants using rescue medication with placebo was 41% (52/127; range 38% to 49%);
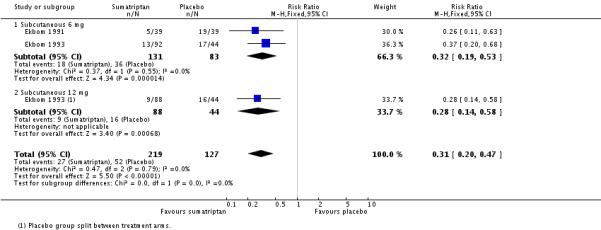
The relative risk of treatment compared with placebo was 0.31 (0.20 to 0.47; Analysis 4.3), giving an NNTp of 3.5 (2.6 to 5.3).
Analysis of the 6 mg dose alone did not substantially change the result.
Sumatriptan (nasal spray) 20 mg versus placebo
Van Vliet 2003, using intranasal sumatriptan 20 mg, permitted use of rescue medication (oxygen or analgesic) after 30 minutes, with 28/77 (36%) participants with sumatriptan, and 40/77 (52%) participants with placebo, requiring rescue medication (Appendix 5). There were insufficient data for analysis.
| Summary of results B: use of rescue medication | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Dose (mg) | Time (min) | Studies | Participants | Triptan % | Placebo % | NNTp (95% CI) |
| Zolmitriptan | 5 | 30 | 2 | 355 | 30 | 43 | 7.4 (4.3 to 27) |
| Zolmitriptan | 5 | 60 | 1 | 102 | 31 | 38 | Not calculated |
| Zolmitriptan | 5 | 30, 60 | 3 | 457 | 30 | 42 | 8.2 (4.8 to 29) |
| Zolmitriptan | 10 | 30 | 2 | 350 | 27 | 43 | 6.2 (3.8 to 16) |
| Zolmitriptan | 10 | 60 | 1 | 99 | 27 | 42 | Not calculated |
| Zolmitriptan | 10 | 30, 60 | 3 | 449 | 27 | 42 | 6.8 (4.3 to 17) |
| Sumatriptan | 6, 12 | 15 | 3 | 346 | 12 | 41 | 3.5 (2.6 to 5.3) |
| Sumatriptan | 6 | 15 | 2 | 214 | 14 | 43 | 3.4 (2.4 to 5.7) |
| Sumatriptan | 20 | 30 | 1 | 154 | 36 | 52 | Not calculated |
Any adverse event (Summary of results C)
Details of adverse events in individual studies are provided in Appendix 7.
Zolmitriptan
Two studies reported the numbers of participants experiencing any adverse event with zolmitriptan. One (Bahra 2000) specified that the duration over which these data were collected was the 24 hours following dosing, while the other did not specify the time period. Bahra 2000 used an oral formulation, and Rapoport 2007 used the intranasal formulation. Both tested 5 mg and 10 mg doses.
Zolmitriptan (oral and nasal spray) 5 mg versus placebo
The proportion of participants experiencing an adverse event with zolmitriptan 5 mg was 27% (45/166; range 25% to 28%);
The proportion of participants using rescue medication with placebo was 15% (25/165; range 15% to 16%);
The relative risk of treatment compared with placebo was 1.8 (1.2 to 2.8; Analysis 1.6), giving an NNH of 8.4 (4.8 to 31).
Zolmitriptan (oral and nasal spray) 10 mg versus placebo
The proportion of participants using rescue medication with zolmitriptan 10 mg was 37% (59/160; range 33% to 39%)
The proportion of participants using rescue medication with placebo was 15% (25/165; range 15% to 16%)
The relative risk of treatment compared with placebo was 2.4 (1.6 to 3.7; Analysis 2.6), giving an NNH of 4.6 (3.2 to 8.0).
The NNH was lower (worse) with the higher dose, but the difference was not statistically significant, with wide and overlapping confidence intervals.
Zolmitriptan (oral and nasal spray) 10 mg versus 5 mg
Direct comparison of the 10 mg and 5 mg doses just failed to show a statistically significant difference: 59/160 participants had an adverse event with zolmitriptan 10 mg, and 45/166 with zolmitriptan 5 mg, giving a relative risk of 1.4 (0.99 to 1.9; Analysis 3.3)
Sumatriptan
Two studies reported the numbers of participants experiencing any adverse event with sumatriptan, but did not specify the time over which the data were collected. Ekbom 1991 used 6 mg subcutaneously, and Ekbom 1993 used 6 mg and 12 mg subcutaneously.
Sumatriptan (subcutaneous) 6 mg versus placebo
The proportion of participants experiencing adverse events with sumatriptan 6 mg was 34% (51/150; range 34% to 35%);
The proportion of participants experiencing adverse events with placebo was 19% (27/143; range 16% to 26%);
The relative risk of treatment compared with placebo was 1.8 (1.2 to 2.7; Analysis 4.4), giving an NNH of 6.6 (4.0 to 19).
Sumatriptan (subcutaneous) 12 mg versus placebo
With sumatriptan 12 mg, 42/94 participants (45%) experienced adverse events compared to 15/96 (16%) with placebo. There were insufficient data for analysis.
Sumatriptan (subcutaneous) 12 mg versus 6 mg
There were insufficient data to compare 12 mg and 6 mg directly, although the results are compatible with more adverse events with the higher dose.
| Summary of results C: any adverse event | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Dose (mg) | Route | Studies | Participants | Triptan % | Placebo % | NNH (95% CI) |
| Zolmitriptan | 5 | Oral, nasal spray | 2 | 331 | 27 | 15 | 8.4 (4.8 to 31) |
| Zolmitriptan | 10 | Oral, nasal spray | 2 | 325 | 37 | 15 | 4.6 (3.2 to 8.0) |
| Sumatriptan | 6 | Subcutaneous | 2 | 293 | 34 | 19 | 6.6 (4.0 to 19) |
| Sumatriptan | 12 | Subcutaneous | 1 | 190 | 45 | 16 | Not calculated |
Severity of adverse events
Most adverse events were mild or moderate in intensity. Where specifically reported, chest symptoms were of mild or moderate intensity, of short duration and resolved spontaneously. Fourteen severe adverse events occurred with subcutaneous sumatriptan 6 mg and 12 mg (Ekbom 1993). These were mostly localised reactions around the injection site, and none led to withdrawal.
Specific adverse events
Details of specific adverse events were not consistently reported in these single dose studies (Appendix 8). There were too few data for analysis.
Serious adverse events
One study using oral zolmitriptan 10 mg reported a serious adverse event. This was exacerbation of cluster headache, which occurred 58 days after medication and was considered unrelated to the study medication by the investigators (Bahra 2000).
Withdrawals
Details of withdrawals and exclusions in individual studies are provided in Appendix 7.
Participants who took rescue medication were classified as withdrawals due to lack of efficacy, and details are reported under ‘Use of rescue medication’, above.
Three withdrawals due to adverse events were reported. One was the result of the serious adverse event reported above (Bahra 2000), one participant withdrew because of shortness of breath and rheumatic pain following 5 mg intranasal zolmitriptan (Cittadini 2006), and one participant withdrew after experiencing moderate pressure on the neck following administration of placebo (Ekbom 1993).
Participants who were excluded from analyses after randomisation were mostly due to major protocol violations or end of cluster period (no medication taken, or cross-over not completed), and were generally well reported. Numbers of participants lost to follow-up’, or withdrawing due for unspecified reasons were small and unlikely to influence results.
Recurrence
None of the included studies reported data on headache recurrence (Appendix 5).
DISCUSSION
Summary of main results
This review included six studies comparing either zolmitriptan or sumatriptan with placebo for the acute treatment of cluster headache. Studies used different doses of drug and routes of administration. Zolmitriptan, either orally or as a nasal spray, was given to 454 participants at doses of 5 mg and 10 mg in comparisons with 302 participants given placebo. Sumatriptan, either subcutaneous or intranasal, was given to 296 participants at doses of 6 mg, 12 mg and 20 mg in comparisons with 204 participants given placebo.
Overall, triptans were better than placebo for headache relief and pain-free responses. There were insufficient data to establish a clear dose response with the doses used. NNTs did, however, tend to be lower (better) for the higher dose and intranasal route for zolmitriptan, with 10 mg intranasal zolmitriptan being significantly better than 5 mg for headache relief at 30 minutes. For intranasal zolmitriptan 10 mg the NNT for headache relief at 30 minutes was 2.8, and for pain-free at 30 minutes was 3.3. For subcutaneous sumatriptan 6 mg the NNT for headache relief at 15 minutes was 2.4, and for pain-free at 15 minutes was 3.3. For every three participants treated with either triptan in these studies, at least one would experience headache relief within 30 minutes who would not have done so with placebo, and for every four treated at least one would be pain-free within 30 minutes. Comparing results for intranasal zolmitriptan 10 mg with subcutaneous sumatriptan 6 mg for headache relief and pain-free responses at 15 minutes demonstrated superiority of subcutaneous sumatriptan (p = 0.003 and < 0.0008 respectively). The available data support the oral route as being the least significant route of administration and favour the parenteral route, with subcutaneously administered triptans being the most effective for acute cluster headache attack. Rescue medication was used significantly less often with triptan than with placebo. For every seven participants treated with zolmitriptan 10 mg, one would not need rescue medication who would have done with placebo, and for every seven treated with subcutaneous sumatriptan 6 mg or 12 mg, two would not need rescue medication who would have done with placebo.
Adverse events were more common with triptan than placebo, and for zolmitriptan tended to affect more participants at higher doses. For every five individuals treated with zolmitriptan 10 mg and every seven treated with sumatriptan 6 mg, at least one would experience adverse event(s) who would not have done with placebo. Adverse events were generally of mild or moderate severity and rarely led to withdrawal. Participants reported transient local injection site reactions following subcutaneous sumatriptan, some of which were considered severe and could compromise acceptability of that route of administration. Chest symptoms were of mild or moderate intensity, of short duration, and resolved spontaneously. Details of specific adverse events and relief of associated symptoms were inconsistently reported, preventing any analysis. Single dose studies are underpowered for investigating specific adverse events, and studies of longer duration will be needed to determine the adverse event profiles of triptans in cluster headache. The safety and tolerability of triptans in migraine is, however, well established and likely to be relevant to this condition also (Dodick 2004; Tepper 2003; Welch 2000). The same contraindications in patients with ischaemic heart disease, history of myocardial infarction, uncontrolled hypertension, and the elderly will apply.
Overall completeness and applicability of evidence
Interpretation of these results is limited by the small number of studies. Only two drugs were tested, both at two doses, and via two routes of administration. There were inadequate numbers of participants and events to draw firm conclusions about any possible differences between individual drugs, different doses or routes of administration of the same drug, or between subgroups within the treated population. Intranasal and, particularly, subcutaneous administration should theoretically be better than oral administration of the same dose because they avoid problems of hepatic first-pass metabolism. Both the number of responders and the speed of response may be improved. In both studies that analysed data separately for episodic and chronic type headaches, a better response rate was observed for episodic type. This is compatible with the findings of a 1-year study in which fewer patients with chronic cluster headache responded with sumatriptan, and the time to response was longer (Gobel 1998). There were too few participants with chronic type headaches in the included studies to draw firm conclusions.
Recruitment for the studies was through headache clinics, which may select participants with cluster headaches that are more severe, frequent or resistant to treatment than in the population as a whole. If anything, this is likely to underestimate treatment effect. On the other hand, these participants may be more motivated than the general population.
The triptans in these trials were used in commonly prescribed doses which may mean that attacks can be treated safely and without toxicity whilst still experiencing clinically useful levels of efficacy. A limitation on effective treatment may be the currently recommended ceiling doses; a fact that may be addressed clinically by combining optimum dosing with adjunctive treatment such as inhaled oxygen.
Quality of the evidence
Methodological quality was good, with all studies scoring above the minimum required to minimise bias. IHS criteria were used for diagnosis of cluster headache in all studies, and well-defined, clinically useful outcomes were reported for efficacy. Detailed reporting of data on associated symptoms and adverse events was inconsistent, but this type of data is not well-captured in single dose studies.
Agreements and disagreements with other studies or reviews
A recent review of medical treatments for cluster headache (Tyagi 2009) identified the same studies involving triptans as this review, but did not carry out any meta-analyses, while a meta-analysis of the two included studies of intranasal zolmitriptan (Hedlund 2009) is in agreement with findings in this review. A recent Cochrane review of oxygen therapy for migraine and cluster headache (Bennett 2008), found weak evidence for efficacy of normobaric oxygen therapy in treating acute cluster headache based on 19 participants in a double blind cross-over study comparing oxygen therapy and air, and 50 participants in an open cross-over study comparing oxygen therapy with ergotamine tartrate. In the comparison with air, 9/16 and 1/14 participants experienced at least significant reduction in headache intensity with oxygen and air respectively. While these numbers are too small for indirect comparison, results are compatible with those for triptans in this review.
AUTHORS’ CONCLUSIONS
Implications for practice
This review suggests that triptans may be a useful treatment option in acute cluster headache, offering convenience over oxygen therapy, and a better safety and tolerability profile compared with ergotamine.
Implications for research
Further studies of adequate size and methodological quality are needed to establish differences between individual triptans and between different doses and routes of administration. Within-study comparisons, with placebo controls for internal validity, would provide the most useful data; long-term studies are needed to determine safety and tolerability. Further studies comparing triptans with alternative therapy, such as oxygen, are needed to determine relative benefits and harms.
PLAIN LANGUAGE SUMMARY.
Triptans for acute cluster headache
The triptans are a class of drugs used to treat cluster headaches. They can be administered orally, nasally or by subcutaneous injection. This review found that sumatriptan administered subcutaneously and zolmitriptan taken intranasally, in particular, were significantly more effective than placebo at relieving acute cluster headache in adults. Within 15 to 30 minutes of using an intranasal or subcutaneous triptan about two-thirds had no worse than mild pain, and about half were pain-free. The evidence was strongest for 6 mg subcutaneous sumatriptan and 10 mg intranasal zolmitriptan. The triptans compared favourably to other therapies such as inhaled oxygen. Adverse events were more common with triptans than with placebo but they were generally of mild to moderate severity and rarely led to withdrawal.
Acknowledgments
SOURCES OF SUPPORT
Internal sources
Pain Research Funds, UK.
External sources
NHS Cochrane Collaboration Program Grant scheme, UK.
NIHR Biomedical Research Centre Program, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Participants recruited from neurology departments; 13 international centres; out-patient based R, DB, three-period cross-over; 20 h washout One headache episode treated for each treatment |
|
| Participants | IHS diagnosis of cluster headache for at least three months; able to recognise an attack; excluded if taking any other medications to treat or prevent headache N = 153 randomised, 124 took ≥1 medication, 123 in efficacy analysis Mean age 44 ± 11 years 86% men Headache type: 73% episodic |
|
| Interventions | Zolmitriptan 5 mg, oral, n = 114 Zolmitriptan 10 mg, oral, n = 111 Placebo, n = 115 Patients instructed to take single dose of trial medication within 10 min of onset if headache was of at least moderate intensity Escape medication permitted after 30 min |
|
| Outcomes | Primary: Pain relief to mild or none at 30 min on a five-point scale Secondary: Pain-free at 30 min; relief of associated symptoms; adverse events/withdrawals; use of rescue medication |
|
| Notes | Results for primary outcome reported for episodic and chronic subgroups Oxford Quality Score: 5 (R2, DB2, W1) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated randomisation sequence |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | Colour-matched placebo tablets. Four tablets taken in appropriate combination of zolmitriptan 2.5 mg and placebo |
| Methods | Participants recruited from five international study centres; out-patient based R, DB, three-period cross-over; 24 h washout One headache episode treated for each treatment |
|
| Participants | Established IHS diagnosis of cluster headache N = 92 randomised, 69 took ≥ 1 medication Mean age 40 ±10 years 87% men Headache type: 64% episodic |
|
| Interventions | Zolmitriptan 5 mg, nasal spray, n = 65 Zolmitriptan 10 mg, nasal spray, n = 63 Placebo, n = 61 Patients instructed to take medication when headache reached moderate intensity Escape medication permitted after 30 min |
|
| Outcomes | Primary: Pain relief to mild or none at 30 min on a five-point scale Secondary: Pain-free at 30 min; relief of associated symptoms; adverse events/withdrawals; use of rescue medication |
|
| Notes | Results for primary outcome reported for episodic and chronic subgroups Oxford Quality Score: 4 (R1, DB2, W1) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Yes | Randomization schedule provided by sponsor and kept by pharmacies until study completed |
| Blinding? All outcomes |
Yes | Matching placebo |
| Methods | Participants recruited from 12 neurology departments; in-patient based study R, DB, two-period cross-over; 24 h washout One headache episode treated with each treatment |
|
| Participants | Known IHS diagnosis of cluster headache N = 49 randomised, 39 in efficacy analysis Mean age 42 ± 10 years 79% men Headache type: 56% episodic |
|
| Interventions | Sumatriptan 6 mg, subcutaneous, n = 39 Placebo, n = 39 Patients received 0.5 mL injection within 10 min of headache episode of at least moderate intensity Rescue medication permitted after 15 min |
|
| Outcomes | Primary: Pain relief to mild or none at 15 min on a five-point scale Secondary: Pain relief at 5 and 10 min; pain-free at 10 and 15 min; associated symptoms; overall functional disability; adverse events/withdrawals; use of rescue medication |
|
| Notes | Oxford Quality Score: 5 (R2, DB2, W1) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Sequence generated with PACT software programme |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | Matching ampules |
| Methods | Participants recruited from 33 international neurology centres; in-patient based study R, DB, three-period cross-over study; 18 h washout One headache episode treated with each of two treatments |
|
| Participants | IHS diagnosis of cluster headache N = 157 randomised, 134 in efficacy analysis Mean age 41 ± 9 years 87% men Headache type: 72% episodic |
|
| Interventions | Sumatriptan 6 mg, subcutaneous, n = 92 Sumatriptan 12 mg, subcutaneous, n = 88 Placebo, n = 88 Patients received 0.5 mL injection within 10 min of headache episode of at least moderate intensity Rescue medication permitted after 15 min |
|
| Outcomes | Primary: Pain relief to mild or none at 10 min on a five-point scale Secondary: Headache relief at 5 and 15 min; pain-free at 15 min; associated symptoms; overall functional disability; adverse events/withdrawals; use of rescue medication |
|
| Notes | Oxford Quality Score: 4 (R2, DB1, W1) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Generated by computer |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Unclear | Not described |
| Methods | Participants recruited from four headache centres in USA; out-patient based R, DB, three-period cross-over study; 24 h washout One headache episode treated with each of three treatments |
|
| Participants | Established IHS diagnosis of cluster headache N = 83 randomised, 52 in efficacy analysis Mean age 45 ±11 years 60% men Headache type: 71% episodic |
|
| Interventions | Zolmitriptan 5 mg, nasal spray, n = 52 attacks Zolmitriptan 10 mg, nasal spray, n = 49 attacks Placebo, n = 50 attacks Patients instructed to take medication when headache reached at least moderate intensity Escape medication permitted after 60 min |
|
| Outcomes | Primary: Pain relief to mild or none at 30 min on a five-point scale Secondary: Complete pain relief (pain-free) at 30 min; headache relief and pain-free at 5, 10, 15, 20 and 60 min; use of rescue medication; adverse events/withdrawals |
|
| Notes | Oxford Quality Score: 5 (R2, DB2, W1) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number generation programme |
| Allocation concealment? | Yes | Individual who generated sequence was blinded to other procedures. Schedules kept by study centre pharmacies until study completion |
| Blinding? All outcomes |
Yes | Matching placebo |
| Methods | Participants recruited from 5 international headache centres; out-patient based R, DB, two-period cross-over study; 24 h washout Two headache episodes treated with each of two treatments |
|
| Participants | Established IHS diagnosis of cluster headache N = 118 randomised, 85 in efficacy analysis Mean age 43 ± 11 years 82% men Headache type: 75% episodic |
|
| Interventions | Sumatriptan 20 mg, nasal spray, n = 77 Placebo, n = 77 Patients instructed to take medication when headache reached at least moderate intensity Escape medication permitted after 30 min |
|
| Outcomes | Primary: Pain relief to mild or none at 30 min on a five-point scale Secondary: Pain-free at 30 min; relief of associated symptoms; time to initial relief; use of rescue medication; adverse events; withdrawals |
|
| Notes | Oxford Quality Score: 4 (R1, DB2, W1) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | Matching placebo |
Abbreviations: DB - double blind; N - number of participants in study; n - number of participants in treatment group; R - randomised; W - withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Goadsby 1994 | Not original research. Uses in its analysis the data from Ekbom 1991 and Ekbom 1993 |
| Hardebo 1998 | Open study, not double blinded |
DATA AND ANALYSES
Comparison 1. Zolmitriptan 5 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with headache relief at 30 minutes | 3 | 457 | Risk Ratio (M-H, Fixed, 95% CI) | 1.43 [1.15, 1.78] |
| 1.1 Oral | 1 | 229 | Risk Ratio (M-H, Fixed, 95% CI) | 1.25 [0.96, 1.63] |
| 1.2 Nasal spray | 2 | 228 | Risk Ratio (M-H, Fixed, 95% CI) | 1.74 [1.20, 2.51] |
| 2 Participants pain-free at 30 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nasal spray | 2 | 228 | Risk Ratio (M-H, Fixed, 95% CI) | 1.81 [1.12, 2.90] |
| 3 Participants with headache relief at 15 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nasal spray | 2 | 228 | Risk Ratio (M-H, Fixed, 95% CI) | 2.15 [0.99, 4.67] |
| 4 Participants pain-free at 15 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nasal spray | 2 | 228 | Risk Ratio (M-H, Fixed, 95% CI) | 2.60 [0.80, 8.46] |
| 5 Participants using rescue medication | 3 | 457 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.55, 0.91] |
| 5.1 after 30 minutes | 2 | 355 | Risk Ratio (M-H, Fixed, 95% CI) | 0.68 [0.52, 0.90] |
| 5.2 after 60 minutes | 1 | 102 | Risk Ratio (M-H, Fixed, 95% CI) | 0.81 [0.47, 1.39] |
| 6 Participants with any adverse event | 2 | 331 | Risk Ratio (M-H, Fixed, 95% CI) | 1.79 [1.15, 2.77] |
Comparison 2. Zolmitriptan 10 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with headache relief at 30 minutes | 3 | 449 | Risk Ratio (M-H, Fixed, 95% CI) | 1.65 [1.33, 2.04] |
| 1.1 Oral | 1 | 226 | Risk Ratio (M-H, Fixed, 95% CI) | 1.22 [0.93, 1.60] |
| 1.2 Nasal spray | 2 | 223 | Risk Ratio (M-H, Fixed, 95% CI) | 2.36 [1.67, 3.34] |
| 2 Participants pain-free at 30 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nasal spray | 2 | 223 | Risk Ratio (M-H, Fixed, 95% CI) | 2.68 [1.72, 4.17] |
| 3 Participants with headache relief at 15 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nasal spray | 2 | 223 | Risk Ratio (M-H, Fixed, 95% CI) | 3.90 [1.90, 8.01] |
| 4 Participants pain-free at 15 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nasal spray | 2 | 223 | Risk Ratio (M-H, Fixed, 95% CI) | 3.90 [1.26, 12.05] |
| 5 Participants using rescue medication | 3 | 449 | Risk Ratio (M-H, Fixed, 95% CI) | 0.65 [0.50, 0.85] |
| 5.1 after 30 minutes | 2 | 350 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 [0.46, 0.84] |
| 5.2 after 60 minutes | 1 | 99 | Risk Ratio (M-H, Fixed, 95% CI) | 0.75 [0.43, 1.33] |
| 6 Participants with any adverse event | 2 | 325 | Risk Ratio (M-H, Fixed, 95% CI) | 2.43 [1.61, 3.68] |
Comparison 3. Zolmitriptan 10 mg versus 5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with headache relief at 30 minutes | 3 | 454 | Risk Ratio (M-H, Fixed, 95% CI) | 1.15 [0.97, 1.37] |
| 1.1 Oral | 1 | 225 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.77, 1.25] |
| 1.2 Nasal spray | 2 | 229 | Risk Ratio (M-H, Fixed, 95% CI) | 1.36 [1.06, 1.74] |
| 2 Participants pain-free at 30 minutes | 2 | 229 | Risk Ratio (M-H, Fixed, 95% CI) | 1.49 [1.07, 2.06] |
| 3 Participants with any adverse event | 2 | 326 | Risk Ratio (M-H, Fixed, 95% CI) | 1.36 [0.99, 1.87] |
Comparison 4. Sumatriptan versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with headache relief at 15 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Subcutaneous 6 mg | 2 | 258 | Risk Ratio (M-H, Fixed, 95% CI) | 2.31 [1.77, 3.03] |
| 2 Participants pain-free at 15 minutes | 2 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Subcutaneous 6 mg | 2 | 258 | Risk Ratio (M-H, Fixed, 95% CI) | 2.77 [1.82, 4.21] |
| 3 Participants using rescue medication | 2 | 346 | Risk Ratio (M-H, Fixed, 95% CI) | 0.31 [0.20, 0.47] |
| 3.1 Subcutaneous 6 mg | 2 | 214 | Risk Ratio (M-H, Fixed, 95% CI) | 0.32 [0.19, 0.53] |
| 3.2 Subcutaneous 12 mg | 1 | 132 | Risk Ratio (M-H, Fixed, 95% CI) | 0.28 [0.14, 0.58] |
| 4 Participants with any adverse event | 2 | 293 | Risk Ratio (M-H, Fixed, 95% CI) | 1.80 [1.20, 2.70] |
| 4.1 Subcutaneous 6 mg | 2 | 293 | Risk Ratio (M-H, Fixed, 95% CI) | 1.80 [1.20, 2.70] |
Analysis 1.1. Comparison 1 Zolmitriptan 5 mg versus placebo, Outcome 1 Participants with headache relief at 30 minutes.
Review: Triptans for acute cluster headche
Comparison: 1 Zolmitriptan 5 mg versus placebo
Outcome: 1 Participants with headche relief at 30 minutes
 |
Analysis 1.2. Comparison 1 Zolmitriptan 5 mg versus placebo, Outcome 2 Participants pain-free at 30 minutes.
Review: Triptans for acute cluster headche
Comparison: 1 Zolmitriptan 5 mg versus placebo
Outcome: 2 Participants pain-free at 30 minutes
 |
Analysis 1.3. Comparison 1 Zolmitriptan 5 mg versus placebo, Outcome 3 Participants with headache relief at 15 minutes.
Review: Triptans for acute cluster headche
Comparison: 1 Zolmitriptan 5 mg versus placebo
Outcome: 3 Participants with headche relief at 15 minutes
 |
Analysis 1.4. Comparison 1 Zolmitriptan 5 mg versus placebo, Outcome 4 Participants pain-free at 15 minutes.
Review: Triptans for acute cluster headche
Comparison: 1 Zolmitriptan 5 mg versus placebo
Outcome: 4 Participants pain-free at 15 minutes
 |
Analysis 1.5. Comparison 1 Zolmitriptan 5 mg versus placebo, Outcome 5 Participants using rescue medication.
Review: Triptans for acute cluster headche
Comparison: 1 Zolmitriptan 5 mg versus placebo
Outcome: 5 Participants using rescue medication
 |
Analysis 1.6. Comparison 1 Zolmitriptan 5 mg versus placebo, Outcome 6 Participants with any adverse event.
Review: Triptans for acute cluster headche
Comparison: 1 Zolmitriptan 5 mg versus placebo
Outcome: 6 Participants with any adverse event
 |
Analysis 2.1. Comparison 2 Zolmitriptan 10 mg versus placebo, Outcome 1 Participants with headache relief at 30 minutes.
Review: Triptans for acute cluster headche
Comparison: 2 Zolmitriptan 10 mg versus placebo
Outcome: 1 Participants with headche relief at 30 minutes
 |
Analysis 2.2. Comparison 2 Zolmitriptan 10 mg versus placebo, Outcome 2 Participants pain-free at 30 minutes.
Review: Triptans for acute cluster headche
Comparison: 2 Zolmitriptan 10 mg versus placebo
Outcome: 2 Participants pain-free at 30 minutes
 |
Analysis 2.3. Comparison 2 Zolmitriptan 10 mg versus placebo, Outcome 3 Participants with headache relief at 15 minutes.
Review: Triptans for acute cluster headche
Comparison: 2 Zolmitriptan 10 mg versus placebo
Outcome: 3 Participants with headche relief at 15 minutes
 |
Analysis 2.4. Comparison 2 Zolmitriptan 10 mg versus placebo, Outcome 4 Participants pain-free at 15 minutes.
Review: Triptans for acute cluster headche
Comparison: 2 Zolmitriptan 10 mg versus placebo
Outcome: 4 Participants pain-free at 15 minutes
 |
Analysis 2.5. Comparison 2 Zolmitriptan 10 mg versus placebo, Outcome 5 Participants using rescue medication.
Review: Triptans for acute cluster headche
Comparison: 2 Zolmitriptan 10 mg versus placebo
Outcome: 5 Participants using rescue medication
 |
Analysis 2.6. Comparison 2 Zolmitriptan 10 mg versus placebo, Outcome 6 Participants with any adverse event.
Review: Triptans for acute cluster headche
Comparison: 2 Zolmitriptan 10 mg versus placebo
Outcome: 6 Participants with any adverse event
 |
Analysis 3.1. Comparison 3 Zolmitriptan 10 mg versus 5 mg, Outcome 1 Participants with headache relief at 30 minutes.
Review: Triptans for acute cluster headche
Comparison: 3 Zolmitriptan 10 mg versus 5 mg
Outcome: 1 Participants with headche relief at 30 minutes
 |
Analysis 3.2. Comparison 3 Zolmitriptan 10 mg versus 5 mg, Outcome 2 Participants pain-free at 30 minutes.
Review: Triptans for acute cluster headche
Comparison: 3 Zolmitriptan 10 mg versus 5 mg
Outcome: 2 Participants pain-free at 30 minutes
 |
Analysis 3.3. Comparison 3 Zolmitriptan 10 mg versus 5 mg, Outcome 3 Participants with any adverse event.
Review: Triptans for acute cluster headche
Comparison: 3 Zolmitriptan 10 mg versus 5 mg
Outcome: 3 Participants with any adverse event
 |
Analysis 4.1. Comparison 4 Sumatriptan versus placebo, Outcome 1 Participants with headache relief at 15 minutes.
Review: Triptans for acute cluster headche
Comparison: 4 Sumatriptan versus placebo
Outcome: 1 Participants with headche relief at 15 minutes
 |
Analysis 4.2. Comparison 4 Sumatriptan versus placebo, Outcome 2 Participants pain-free at 15 minutes.
Review: Triptans for acute cluster headche
Comparison: 4 Sumatriptan versus placebo
Outcome: 2 Participants pain-free at 15 minutes
 |
Analysis 4.3. Comparison 4 Sumatriptan versus placebo, Outcome 3 Participants using rescue medication.
Review: Triptans for acute cluster headche
Comparison: 4 Sumatriptan versus placebo
Outcome: 3 Participants using rescue medication
 |
Analysis 4.4. Comparison 4 Sumatriptan versus placebo, Outcome 4 Participants with any adverse event.
Review: Triptans for acute cluster headche
Comparison: 4 Sumatriptan versus placebo
Outcome: 4 Participants with any adverse event
 |
Appendix 1. International Headache Society criteria for diagnosis of cluster headache
At least 5 attacks fulfilling criteria B-D.
Severe or very severe unilateral orbital, supraorbital and/or temporal pain lasting 15-180 minutes if untreated.
- Headache is accompanied by at least one of the following:
- ipsilateral conjunctival injection and/or lacrimation
- ipsilateral nasal congestion and/or rhinorrhoea
- ipsilateral eyelid oedema
- ipsilateral forehead and facial sweating
- ipsilateral miosis and/or ptosis
- a sense of restlessness or agitation
Attacks have a frequency from one every other day to 8 per day.
Not attributed to another disorder.
Appendix 2. Search strategy for MEDLINE (via OVID)
exp Cluster Headache/
((cluster adj4 headache*) or (ciliary adj4 neuralgi*) or (neuralgi* adj4 migraine*) or (histamin* adj4 cephalgi*) or (horton* adj2 syndrome*)).mp.
1 or 2
exp Tryptamines/
(triptan* or tryptamin*).mp.
(almotriptan or eletriptan or frovatriptan or naratriptan or rizatriptan or sumatriptan or zolmitriptan).mp.
or/4-6
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/8-15
Animals.sh. not (humans.sh. and animals.sh.)
16 not 17
3 and 7 and 18
Appendix 3. Search strategy for EMBASE (via OVID)
exp Cluster Headache/
((cluster adj4 headache*) or (ciliary adj4 neuralgi*) or (neuralgi* adj4 migraine*) or (histamin* adj4 cephalgi*) or (horton* adj2 syndrome*)).mp.
1 or 2
(exp Tryptamines/) or (exp triptans derivative/)
(triptan* or tryptamin*).mp.
(almotriptan or eletriptan or frovatriptan or naratriptan or rizatriptan or sumatriptan or zolmitriptan).mp.
or/4-6
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double-blind procedure.sh.
(clin* adj25 trial*)
((doubl* or trebl* or tripl*) adj25 (blind* or mask*))
placebo*
random*
or/8-15
Appendix 4. Search strategy for CENTRAL
exp MeSH descriptor Cluster Headache
((cluster adj4 headache*) or (ciliary adj4 neuralgi*) or (neuralgi* adj4 migraine*) or (histamin* adj4 cephalgi*) or (horton* adj2 syndrome*)):ti,ab,kw
1 or 2
exp MeSH descriptor Tryptamines
(triptan* or tryptamin*):ti,ab,kw
(almotriptan or eletriptan or frovatriptan or naratriptan or rizatriptan or sumatriptan or zolmitriptan):ti,ab,kw
or/4-6
Randomized controlled trial:pt
MeSH descriptor Double-blind Method
random*:ti,ab,kw.
or 8-10
3 and 7 and 11
Limit 12 to Clinical Trials (CENTRAL)
Appendix 5. Summary of outcomes: efficacy and use of rescue medication
| Study ID | Treatment | HR 30 | PF 30 | HR 15 | PF 15 | Headache recurrence | Rescue medication |
|---|---|---|---|---|---|---|---|
| Bahra 2000 |
|
Episodic; chronic
|
No data | No data | No data | No data | After 30 min:
Episodic; chronic
Up to 180 min |
| Cittadini 2006 |
|
Episodic; chronic
|
|
|
|
No data | After 30 min:
|
| Ekbom 1991 |
Not self-administered (phys/hurse) |
No data | No data |
|
|
No data | After 15 min:
|
| Ekbom 1993 |
Not self-administered (phys/nurse) |
No data | No data |
|
|
No data | After 15 min:
|
| Rapoport 2007 |
|
|
|
|
|
No data | After 60 min:
|
| Van Vliet 2003 |
|
|
|
No data | No data | No data | After 30 min:
|
HR - headache relief; IN - intranasal; n - number of participants; PF - pain-free; SC - subcutaneous
Appendix 6. Summary of outcomes: associated symptoms
| Study ID | Treatment | Definition | Conjunctival injection/lacrimation | Nasal congestion | Ptosis/eyelid oedema | Forehead sweating | Miosis | Photophobia | Normal function (FD score ≥1) |
|---|---|---|---|---|---|---|---|---|---|
| Bahra 2000 |
|
Episodic pts only Improvement at 30 min (from graph) % of those with symptom at baseline (denominator not reported) |
|
|
|
|
Blurred vision
|
No data | No data |
| Cittadini 2006 |
|
Improvement at 30 min (from graph) % of those with symptom at baseline (denominator not reported) |
|
|
|
|
|
No data | No data |
| Ekbom 1991 |
Not self-administered (phys/nurse) |
Symptom present at 15 and 30 min post-dose | 15 min:
30 min:
|
No usable data | |||||
| Ekbom 1993 |
Not self-administered (phys/nurse) |
Symptom present at 15 min post-dose Baseline:
|
|
From graph Baseline:
At 15 min:
|
|||||
| Rapoport 2007 |
|
No data | |||||||
| Van Vliet 2003 |
|
Improvement at 30 min (from graph) % of those with symptom at baseline (denominator not reported) |
|
|
|
|
|
|
No data |
IN - intranasal; n - number of participants; pts - participants; SC - subcutaneous
Appendix 7. Summary of outcomes: adverse events and withdrawals
| Study ID | Treatment | Any AE | Serious AE | AE withdrawal | Other withdrawal |
|---|---|---|---|---|---|
| Bahra 2000 |
|
Within 24 h:
AE details reported for ITT population, not safety population |
1 in (2), but 58 days after treatment, and not considered related | None in ITT population within 24 h of treatment One in safety population - no details |
20 pts did not have three episodes |
| Cittadini 2006 |
|
No data | None |
|
6 pts lost to follow up, 2 pts without clear reason after treating one episode |
| Ekbom 1991 |
|
No time specified:
|
None reported | None | 8 pts excluded due to protocol violations, 2 pts had only one episode |
| Ekbom 1993 |
|
No time specified:
(over 90% mild/mod) |
None reported | (3) 1/101 (pressure on neck) | 21 pts had only one episode |
| Rapoport 2007 |
|
No time specified:
(mild, non-specific, typical of triptans) |
None | None | 12 pts lost to follow up, 5 withdrew consent, 9 did not have an episode |
| Van Vliet 2003 |
|
No data | None | No data | 7 pts lost to follow up, 10 treated attacks of mild intensity |
AE - adverse event; IN - intranasal; ITT - intention-to-treat; n - number of participants; pts - participants; SC - subcutaneous
Appendix 8. Summary of outcomes: specific adverse events
| Study ID | Treatment | General | Nausea ± vomiting | Local reaction | Pain/stiffness/tightness | Temperature sensation | Feeling of heaviness | Dizziness | Somnolence |
|---|---|---|---|---|---|---|---|---|---|
| Bahra 2000 |
|
Detailed list (ITT pop not safety pop) for pts with specific events occurring in > 2%, and all chest symptoms |
|
Paresthesia (NB oral route):
Most common AE |
Pain:
Tightness:
|
Sweating:
|
|
|
|
| Cittadini 2006 |
|
No details of specific AEs | |||||||
| Ekbom 1991 |
Not self-administered (phys/nurse) |
Primarily two types:
|
|||||||
| Ekbom 1993 |
Not self-administered (phys/nurse) |
14 events of severe intensity (injection site reaction, flushing, sweating, warm sensation, malaise/fatigue, prickling sensation) Chest symptoms mild or moderate, resolve ≥ 30 min (pressure 2 suma 6 mg, tightness 1 suma 6 mg and 1 suma 12 mg) AEs in ≥ 5% of participants |
|
Injection site reaction:
|
|
|
|
||
| Rapoport 2007 |
|
Mild, nonspecific, typical of triptan sensations |
|
Bad taste:
Discomfort of nasal cavity:
|
Throat:
Chest:
Neck:
|
|
|
||
| Van Vliet 2003 |
|
Very limited data | Bitter taste:
(most common event) |
Chest pressure:
|
IN - intranasal; n - number of participants; pts - participants; SC - subcutaneous
HISTORY
Protocol first published: Issue 4, 2009
Review first published: Issue 4, 2010
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
In the published protocol for this review, we stated that we would consider pain outcomes assessed at 30 minutes post-treatment or later. For the full review, we decided to include 15-minute data as well. Our reasoning was as follows: Four of the six included studies reported data for 15 minutes. Since rapid relief is important in this disabling condition, and there were relatively few data for analysis from 30 minutes or later, we thought the 15-minute data would improve the relevance and usefulness of the review and should be included. Some data were available for other early time points (5, 10, 20 minutes) for some studies, but these were insufficient for analysis, and we felt they did not add significantly to our understanding of efficacy.
Relatively few pain outcomes were reported at 60 minutes post-treatment. Because of this, and because many cluster headache episodes may resolve spontaneously by 60 minutes, we have de-emphasised results for this time point in our descriptions of results for pain outcomes.
Footnotes
DECLARATIONS OF INTEREST
RAM has consulted for various pharmaceutical companies. RAM has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. RAM and SD have received research support from charities, government and industry sources at various times. Support for this review was from Pain Research Funds, the NHS Cochrane Collaboration Programme Grant Scheme, and the NIHR Biomedical Research Centre Programme. None had any input into the review at any stage.
References to studies included in this review
- Bahra 2000 {published data only} .Bahra A, Gawel MJ, Hardebo JE, Millson D, Breen SA, Goadsby PJ. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology. 2000;54(9):1832–9. doi: 10.1212/wnl.54.9.1832. [DOI] [PubMed] [Google Scholar]
- Cittadini 2006 {published data only} .Cittadini E, May A, Straube A, Evers S, Bussone G, Goadsby PJ. Effectiveness of intranasal zolmitriptan in acute cluster headache: a randomized, placebo-controlled, double-blind crossover study. Archives of Neurology. 2006;63(11):1537–42. doi: 10.1001/archneur.63.11.nct60002. DOI: 10.1001/archneur.63.11.nct60002. [DOI] [PubMed] [Google Scholar]
- Ekbom 1991 {published data only} .The Sumatriptan Cluster Headache Study Group Treatment of acute cluster headache with sumatriptan. New England Journal of Medicine. 1991;325(5):322–6. doi: 10.1056/NEJM199108013250505. [DOI] [PubMed] [Google Scholar]
- Ekbom 1993 {published data only} .Ekbom K, Monstad I, Prusinski A, Cole JA, Pilgrim AJ, Noronha D, The Sumatriptan Cluster Headache Study Group Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. Acta Neurologica Scandinavica. 1993;88(1):63–9. doi: 10.1111/j.1600-0404.1993.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Rapoport 2007 {published data only} .Rapoport AM, Mathew NT, Silberstein SD, Dodick D, Tepper SJ, Sheftell FD, et al. Zolmitriptan nasal spray in the acute treatment of cluster headache: a double-blind study. Neurology. 2007;69(9):821–6. doi: 10.1212/01.wnl.0000267886.85210.37. DOI: 10.1212/01.wnl.0000267886.85210.37. [DOI] [PubMed] [Google Scholar]
- Van Vliet 2003 {published data only} .Van Vliet JA, Bahra A, Martin V, Ramadan N, Aurora SK, Mathew NT, et al. Intranasal sumatriptan in cluster headache: randomized placebo-controlled double-blind study. Neurology. 2003;60(4):630–3. doi: 10.1212/01.wnl.0000046589.45855.30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Goadsby 1994 {published data only} .Goadsby PJ. The clinical profile of sumatriptan: cluster headache. European Neurolology. 1994;34(Suppl 2):35–9. doi: 10.1159/000119530. [DOI] [PubMed] [Google Scholar]
- Hardebo 1998 {published data only} .Hardebo JE, Dahlöf C. Sumatriptan nasal spray (20 mg/dose) in the acute treatment of cluster headache. Cephalalgia. 1998;18(7):487–9. doi: 10.1046/j.1468-2982.1998.1807487.x. [DOI] [PubMed] [Google Scholar]
Additional references
- Bennett 2008 .Bennett MH, French C, Schnabel A, Wasiak J, Kranke P. Normobaric and hyperbaric oxygen therapy for migraine and cluster headache. Cochrane Database of Systematic Reviews. 2008;(3) doi: 10.1002/14651858.CD005219.pub2. DOI: 10.1002/14651858.CD005219.pub2. [DOI] [PubMed] [Google Scholar]
- Cluster Headache Study Group 1991 .The Sumatriptan Cluster Headache Study Group Treatment of acute cluster headache with sumatriptan. New England Journal of Medicine. 1991;325(5):322–6. doi: 10.1056/NEJM199108013250505. [DOI] [PubMed] [Google Scholar]
- Cook 1995 .Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452–4. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro 1986 .D’Alessandro R, Gamberini G, Benassi G, Morganti G, Cortelli Lugaresi E. Cluster headache in the Republic of San Marino. Cephalalgia. 1986;6(3):159–62. doi: 10.1046/j.1468-2982.1986.0603159.x. [DOI] [PubMed] [Google Scholar]
- Dodick 2004 .Dodick DW, Martin VT, Smith T, Silberstein S. Cardiovascular tolerability and safety of triptans: a review of clinical data. Headache. 2004;44(Suppl 1):S20–30. doi: 10.1111/j.1526-4610.2004.04105.x. [DOI] [PubMed] [Google Scholar]
- Ekbom 1978 .Ekbom K, Ahlborg B, Schéle R. Prevalence of migraine and cluster headache in Swedish men of 18. Headache. 1978;18(1):9–19. doi: 10.1111/j.1526-4610.1978.hed1801009.x. [DOI] [PubMed] [Google Scholar]
- Gobel 1998 .Göbel H, Lindner V, Heinze A, Ribbat M, Deuschl G. Acute therapy for cluster headache with sumatriptan: findings of a one-year long-term study. Neurology. 1998;51(3):908–11. doi: 10.1212/wnl.51.3.908. [DOI] [PubMed] [Google Scholar]
- Hedlund 2009 .Hedlund C, Rapoport AM, Dodick DW, Goadsby PJ. Zolmitriptan nasal spray in the acute treatment of cluster headache: a meta-analysis of two studies. Headache. 2009;49(9):1315–23. doi: 10.1111/j.1526-4610.2009.01518.x. DOI: 10.1111/j.1526-4610.2009.01518.x. [DOI] [PubMed] [Google Scholar]
- IHS 1988 .Headache Classification Committee of the International Headache Society Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(Suppl 7):1–96. [PubMed] [Google Scholar]
- IHS 2004 .Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders. 2nd edition. Cephalalgia. 2004;24(Suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- IHS Trial Guidelines 1995 .Lipton RB, Micieli G, Russell D, Solomon S, Tfelt-Hansen P, Waldenlind E, for the International Headache Society Committee on Clinical Trials in Cluster Headache Guidelines for controlled trials of drugs in cluster headache. Cephalalgia. 1995;15(6):452–62. [PubMed] [Google Scholar]
- Jadad 1996 .Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- L’Abbe 1987 .L’Abbé KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine. 1987;107(2):224–33. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- May 1998 .May A, Bahra A, Büchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352(9124):275–8. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- McAlister 2001 .McAlister FA, Sackett DL. Active-control equivalence trials and antihypertensive agents. American Journal of Medicine. 2001;111(7):553–8. doi: 10.1016/s0002-9343(01)00900-7. [DOI] [PubMed] [Google Scholar]
- Moore 1998 .Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78(3):209–16. doi: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Moore 2003 .Moore A, Edwards J, Barden J, McQuay H. Bandolier’s little book of pain. Oxford University Press; Oxford: 2003. [Google Scholar]
- Morris 1995 .Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratio and standardized ratios and rates. In: Gardner MJ, Altman DG, editors. Statistics with confidence - confidence intervals and statistical guidelines. British Medical Journal; London: 1995. pp. 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosker 1994 .Plosker GL, McTavish D. Sumatriptan. A reappraisal of its pharmacology and therapeutic efficacy in the acute treatment of migraine and cluster headache. Drugs. 1994;47(4):622–51. doi: 10.2165/00003495-199447040-00006. [DOI] [PubMed] [Google Scholar]
- Rapoport 2007 .Rapoport AM, Mathew NT, Silberstein SD, Dodick D, Tepper SJ, Sheftell FD, et al. Zolmitriptan nasal spray in the acute treatment of cluster headache: a double-blind study. Neurology. 2007;69(21):821–6. doi: 10.1212/01.wnl.0000267886.85210.37. [DOI] [PubMed] [Google Scholar]
- Tepper 2003 .Tepper SJ, Millson D. Safety profile of the triptans. Expert Opinion on Drug Safety. 2003;2(2):123–32. doi: 10.1517/14740338.2.2.123. [DOI] [PubMed] [Google Scholar]
- Torelli 2005 .Torelli P, Beghi E, Manzoni GC. Cluster headache prevalence in the Italian general population. Neurology. 2005;64(3):469–74. doi: 10.1212/01.WNL.0000150901.47293.BC. [DOI] [PubMed] [Google Scholar]
- Tyagi 2009 .Tyagi A, Matharu M. Evidence base for the medical treatments used in cluster headache. Current Pain and Headache Reports. 2009;13(2):168–78. doi: 10.1007/s11916-009-0029-6. [DOI] [PubMed] [Google Scholar]
- Welch 2000 .Welch KM, Mathew NT, Stone P, Rosamond W, Saiers J, Gutterman D. Tolerability of sumatriptan: clinical trials and post-marketing experience. Cephalalgia. 2000;20(8):687–95. doi: 10.1111/j.1468-2982.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study








