Abstract
Background
Higher prevalence of nonalcoholic fatty liver disease (NAFLD) in men and postmenopausal women than in premenopausal women has suggested a potential role of sex hormones in the pathogenesis of the disease. We sought to evaluate the association between oral contraceptive pills (OCP) and NAFLD and to determine whether adiposity mediates any effect.
Methods
We included 4338 women aged 20–60 years who were enrolled in the Third National Health and Nutrition Examination Survey from 1988 to 1994 in a population-based cross-sectional study. We defined NA-FLD as moderate–severe steatosis on ultrasonography in women without excessive alcohol use or other identifiable causes. OCP use was based on self-report and was categorized as never, former or current use.
Results
The overall weighted prevalence of NAFLD was 11.6 % but lower in current (6.7 %) than in former (12.0 %) or never users (15.6 %, P = 0.016). In the multivariable model, current OCP users experienced a 50 % lower odds of NAFLD than never users (adjusted odds ratio 0.50; 95 % confidence interval 0.26, 0.98) after adjusting for age, race/ethnicity, smoking status, history of diabetes or hypertension and education. Further adjustment for body mass index or waist circumference significantly attenuated the OCP–NAFLD relationship.
Conclusions
In this large US-representative population, OCP use was associated with reduced odds of NAFLD. However, this association could be mediated or confounded by adiposity. Prospective studies are needed to further clarify the causal role of sex hormone.
Keywords: Adiposity, Nonalcoholic fatty liver disease, Obesity, Oral contraceptive pill, Sex hormone
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become the most common liver disease in developed countries over the past two decades. Due to an increased prevalence of obesity, type 2 diabetes and hyperlipidemia, NAFLD and its liver-related consequences are expected to become public health problems, and thus identification of factors that can prevent the development of NAFLD is key [1, 2].
While higher prevalence of NAFLD is commonly found in Hispanics than in non-Hispanic whites [2] or in African Americans [3–5], NAFLD has largely been associated with older age, inflammatory biomarkers involved in hepatocellular injury, impaired glucose and lipoprotein metabolism and anthropometric measures that reflect central obesity [1, 2]. In addition, NAFLD is most frequently found in men and postmenopausal women without hormone replacement therapy (HRT) [2], suggesting a role of sex hormones, particularly estrogen, in the pathogenesis of NAFLD [6, 7].
Animal studies and clinical observations indicate that high levels of estrogen, as normally seen in premenopausal women, preferentially increase peripheral distribution of fat, thereby reducing the risk of central obesity; whereas the fall in the levels of estrogen observed during menopause, is associated with loss of subcutaneous fat and a gain in visceral fat [8–10]. Similarly, observational studies have described more pronounced deposition of fat mass in the waist relative to the hip and high waist-to-hip ratio in postmenopausal compared to premenopausal women [11, 12]. Previous studies have reported that menopause increased the risk of NAFLD and that postmenopausal women on HRT were less likely to have NAFLD than those not treated with hormones [9, 13–15]. However, there is limited evidence on whether the use of oral contraceptive pills (OCP), another source of exogenous hormones, can also protect younger women from the risk of NAFLD. Therefore, we sought to determine the association between OCP use and prevalent NAFLD in women aged 20–60 who were enrolled in the Third National Health and Nutrition Examination Survey (NHANES III) from 1988 to 1994. We hypothesized that women taking OCPs would have lower odds of NAFLD than those who never used OCP. We also examined whether the association could be explained by the postulated effects of hormones on adiposity.
Methods
Study design and population
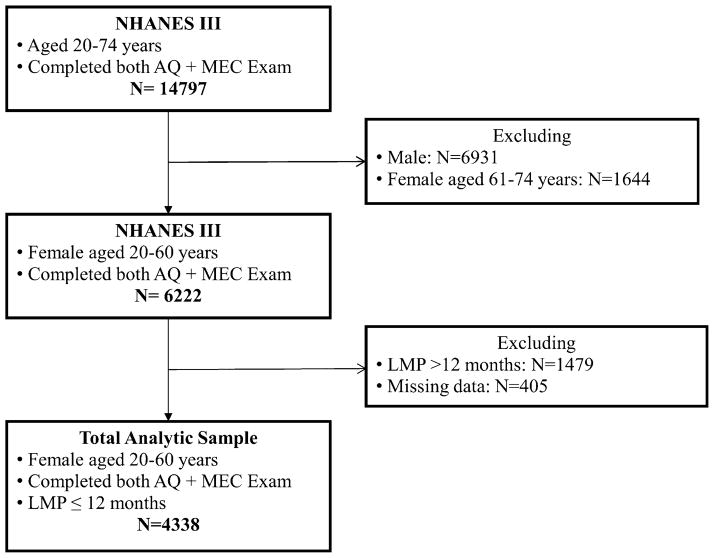
NHANES III was conducted between 1988 and 1994 as part of a program consisting of cross-sectional population-based studies to assess the health and nutritional status of non-institutionalized adults and children in the US. Extensive documentation regarding the data collection procedure and contents of questionnaire for adults in NHANES III is available elsewhere [16]. Briefly, in addition to demographic, socioeconomic and dietary information, detailed lifestyle and behavioral questionnaire for adults aged 20 or older were documented through household interview as well as comprehensive physical examinations and blood samples collection performed in mobile examination centers (MEC). Overall, 33,944 participants were interviewed in NHANES III, of which 14,797 adults aged 20–74 completed household interview, physical examinations and a gallbladder ultrasonography. For the current study, we included 4338 females aged 20–60 whose anthropometric measures were available and who provided their reproductive health information regarding the last menstrual period (LMP) and OCP use. Women who had LMP within the prior 12 months were considered regularly menstruating (Fig. 1). Considering the distinct profiles of endogenous hormones in women of twenties through sixties, we further grouped women into a younger, reproductive group (age 20–34) and a mid-adult, perimenopausal group (age 35–60). Detailed menstruation history was unavailable for staging women’s perimenopausal status according to the 2011 Stages of Reproductive Aging Workshop (STRAW) + 10 criteria [17].
Fig. 1.
Flowchart of participant selection. Among 33,944 participants who were interviewed in NHANES III in 1988–1994, 14,797 adults aged 20–74 completed adult questionnaire (AQ) and received physical examinations at the mobile examination center (MEC) where blood samples collection and a gallbladder ultrasonography were also performed. In the current analysis, we included 4338 females aged 20–60 who reported their history of oral contraceptive pills use and had the last menstrual period (LMP) within the prior 12 months. Women without any of the following anthropometric measures were excluded: body mass index, waist circumference or waist-to-hip ratio
Data collection
In 2009–2010, hepatic steatosis was systematically determined from archived gallbladder ultrasounds using modified criteria by Hamaguchi et al. [18] and included the assessment of: liver parenchyma brightness, liver-to-kidney contrast, deep beam attenuation, bright vessel walls and thickness of gallbladder wall. Comprehensive descriptions about the review protocol are available elsewhere [19]. NAFLD was defined as moderate or severe hepatic steatosis in women who did not use zidovudine or didanosine and who reported daily consumption of one alcohol drink (14 g alcohol) or less in the prior 12 months [20]. The intra- and inter-rater reliability of the ultrasound-defined hepatic steatosis was 0.77 [95 % confidence interval (CI) 0.73–0.82] and 0.70 (95 % CI 0.64–0.76), respectively [19].
Women aged 20–60 were asked: “Have you ever taken birth-control/estrogen pills?”, “How old when you first took birth-control/estrogen pills?”, “How many months ago did you stop taking birth control pills or are you still taking them?” and “Not counting any time when you stopped taking them, for how many months have you taken/did you take birth-control/estrogen pills?” Based on responses to these answers, women were categorized as: never, former or current users of OCP. In addition, we examined the age of initiation, the duration of OCP use (excluding intervals not taking the pills among former and current users), and time since stopping OCP (in months) in former users only.
Statistical analysis
Descriptive analyses were performed to compare socio-demographic characteristics and laboratory profiles among participants who never, formerly or currently used OCP at the time of the household interview. To assess unadjusted associations, design-based chi-square statistics were used for categorical variables, and survey-based linear regression analyses were conducted for continuous covariates. Covariates that were considered in multivariable models included age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American and others), past medical history of diabetes mellitus (DM) or hypertension, smoking behavior (never, former or current smokers), socio-economic indicators such as highest educational level obtained (high school or higher vs. less than high school), insurance coverage status (yes vs. no) and income level (at or above poverty ratio, 1 vs. below poverty ratio, 1); liver enzymes including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) as well as plasma levels of glucose and insulin. Among women who fasted for 8 h or longer, we also compared serum levels of triglyceride (TG) by OCP use and estimated the degree of insulin resistance by calculating the homeostasis model assessment-estimated insulin resistance index (HOMA-IR) [21]. Stratified analysis and formal statistical tests for interaction were used to decide whether OCP–NAFLD association differed by age, race, DM or hypertension history. When no such effect modification was identified among the above mentioned variables, multivariable logistic regression was then built to determine the independent association between OCP use and NAFLD while adjusting for all potential confounders.
In the mediation analysis, anthropometric variables that measure different aspects of adiposity, such as body mass index (BMI), waist circumference (WC), waist-to-hip ratio and total body percent fat [22], were first examined by inclusion of each variable into the base multivariable model to assess whether the measure of association between OCP use and NAFLD was attenuated by at least 10–15 % on the log scale [23]. Assumptions for valid mediation analysis, such as whether OCP users were distributed equally across different levels of each of the potential mediators, were examined empirically [23, 24] before calculating the absolute proportion of exposure effect explained (aPEE) for each potential mediator. Odds ratios (OR) and 95 % CI were reported at a two-tailed significance level of 0.05. All analyses were performed in Stata 11 [25], employing appropriate sampling weights to account for the stratified, probability sampling and non-responses in the survey.
Results
Characteristics of subjects
Characteristics of the study population and by OCP use history are summarized in Table 1. Compared to never users, current OCP users were younger (mean 27.6 vs. 34.1 years), less likely to be Mexican-American (5.3 vs. 9.5 %) or to have diabetes (2.0 vs. 5.6 %) or hypertension (11.6 vs. 17.9 %). Current users were also more likely to have a higher educational attainment (88.3 vs. 75.9 %) and income (86.6 vs. 79.6 % were at or above poverty line); access to care as indicated by insurance coverage (88.5 vs. 80.9 %); as well as being current or former smokers (40.7 vs. 34.0 %). In addition, more women taking OCPs had a BMI <25 kg/m2 (67.9 vs. 52.9 %) or a normal level of serum ALT (≤31 U/L) than those who never used OCPs (97.8 vs. 93.4 %). However, current OCP users were less likely to have an elevated serum concentration of insulin or insulin resistance (HOMA-IR ≥2.60) than never users (14.4 vs. 21.6 %) whereas former users had a comparable level of HOMA-IR to never users. Women had similar levels of serum TG and plasma glucose regardless of OCP use status. As shown in Table 2, current OCP users began to take OCP at a similar age to those who reported former use (20.0 vs. 20.2 years). However, 19.9 % of current users reported having been on OCP for more than a decade while 21.7 % of former users had stopped taking oral pills within the first year of use (Table 2).
Table 1.
Characteristics of premenopausal women aged 20–60 in NHANES III (N = 4338) by OCP Use History, 1988–1994
| All (N = 4338) | Never use (N = 960) | Former use (N = 2604) | Current use (N = 774) | |
|---|---|---|---|---|
| NAFLD | 11.6 % | 15.6 % | 12.0 % | 6.7 % |
| Demographic | ||||
| Age (years): mean (SEa) | 33.7 (0.3) | 34.1 (0.6) | 35.5 (0.2) | 27.6 (0.4) |
| 20–34 | 55.0 % | 54.2 % | 45.0 % | 87.7 % |
| 35–60 | 45.1 % | 45.9 % | 55.0 % | 12.3 % |
| Race/ethnicity | ||||
| Non-Hispanic white | 72.9 % | 59.3 % | 75.7 % | 76.8 % |
| Non-Hispanic black | 12.3 % | 11.7 % | 12.8 % | 11.6 % |
| Mexican-American | 5.7 % | 9.5 % | 4.8 % | 5.3 % |
| Others | 9.0 % | 19.5 % | 6.7 % | 6.3 % |
| Socio-economic status | ||||
| Education | ||||
| High school or higher | 83.4 % | 75.9 % | 84.2 % | 88.3 % |
| Income level | n = 3996 | n = 849 | n = 2431 | n = 716 |
| At or above poverty line | 85.2 % | 79.6 % | 86.4 % | 86.6 % |
| Insurance coverage | n = 4148 | n = 913 | n = 2491 | n = 744 |
| Yes | 86.5 % | 80.9 % | 87.5 % | 88.5 % |
| Anthropometrics | ||||
| BMI (kg/m2) | ||||
| Mean (SEa) | 25.8 (0.2) | 26.6 (0.7) | 26.0 (0.2) | 24.1 (0.3) |
| <25 | 55.8 % | 52.9 % | 52.9 % | 67.9 % |
| 25–29.9 | 22.5 % | 21.0 % | 24.7 % | 17.2 % |
| ≥30 | 21.7 % | 26.1 % | 22.4 % | 14.9 % |
| Waist circumference (cm) | ||||
| >88 | 35.2 % | 40.8 % | 38.1 % | 20.6 % |
| Waist–hip ratio | ||||
| ≥0.8 | 69.8 % | 72.4 % | 74.2 % | 53.4 % |
| Total percent body fat (%) | n = 3808 | n = 831 | n = 2255 | n = 722 |
| ≥40 | 22.3 | 24.6 | 24.3 | 14.1 |
| Risk factors (%) | ||||
| Diabetes | 4.4 | 5.6 | 4.7 | 2.0 |
| Hypertension | 18.6 | 17.9 | 21.0 | 11.6 |
| Smoking (%) | ||||
| Never | 55.8 | 66.1 | 51.6 | 59.3 |
| Former smoker | 16.1 | 9.7 | 18.3 | 15.4 |
| Current smoker | 28.1 | 24.3 | 30.1 | 25.3 |
| Cholesterol (mg/dL) | n = 4133 | n = 909 | n = 2487 | n = 737 |
| ≥240 mg/dL | 9.9 % | 10.1 % | 9.9 % | 9.6 % |
| Triglycerideb (mg/dL) | n = 2674 | n = 594 | n = 1593 | n = 487 |
| ≥150 mg/dL | 16.0 % | 18.3 % | 15.9 % | 14.0 % |
| AST (U/L) | n = 4102 | n = 900 | n = 2472 | n = 730 |
| >31 | 3.4 % | 3.9 % | 3.7 % | 2.0 % |
| ALT (U/L) | n = 4102 | n = 900 | n = 2472 | n = 730 |
| >31 | 4.4 % | 6.6 % | 4.5 % | 2.2 % |
| Plasma glucosec (mg/dL) | n = 4167 | n = 915 | n = 2512 | n = 740 |
| ≥200 | 0.6 % | 0.7 % | 0.7 % | 0.1 % |
| Serum insulin (μU/mL) | n = 4136 | n = 912 | n = 2491 | n = 733 |
| Mean (SEa) | 9.6 (0.2) | 11.0 (0.7) | 9.4 (0.2) | 9.1 (0.3) |
| <10 | 69.7 % | 68.0 % | 70.1 % | 70.2 % |
| 10–20 | 24.4 % | 22.9 % | 24.3 % | 26.3 % |
| ≥20 | 5.9 % | 9.2 % | 5.7 % | 3.5 % |
| HOMA-IRd | n = 2528 | n = 540 | n = 1463 | n = 451 |
| HOMA-IR ≥2.6 | 20.6 % | 21.6 % | 22.3 % | 14.4 % |
ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, HOMA-IR the homeostasis model assessment-estimated insulin resistance index, OCP oral contraceptive pill, SE standard error
Survey-based mean estimation with linearized standard error
Only included participants who fasted ≥8 h
3.9 % of participants missing plasma level of glucose; included participants who fasted ≥8 h (65.1 %),<8 h (34.8 %) and 4 participants with unknown fasting status
Calculated only among participants who fasted ≥8 h and had plasma levels of glucose and insulin available (58.3 %)
Table 2.
Former and current OCP users among premenopausal women, NHANES III, 1988–1994
| OCP use | Former use (N = 2604) | Current user (N = 774) |
|---|---|---|
| Age (years): mean (SEa) | 35.5 (0.2) | 27.6 (0.4) |
| Age at first use of OCP (years): mean (SEa) | 20.2 (0.1) | 20.0 (0.3) |
| Years of taking OCP: mean (SEa) | 4.3 (0.2) | 6.7 (0.6) |
| <1 | 21.7 % | 8.0 % |
| 1–5 | 41.0 % | 37.7 % |
| 5–10 | 24.7 % | 34.4 % |
| ≥10 | 12.6 % | 19.9 % |
| Years since stopping OCP: mean (SEa) | 9.8 (0.2) | |
| <1 | 8.2 % | |
| 1–5 | 21.3 % | |
| 5–10 | 20.8 % | |
| ≥10 | 49.7 % |
OCP oral contraceptive pill, SD standard deviation
Survey-based mean estimation with linearized standard error
Associations between OCP and NAFLD
Overall, a protective effect of current OCP use remained significant after adjusting for age, race/ethnicity, smoking history, co-morbidities and the highest educational level (Model 1 in Table 3), with a 50 % lower odds of NAFLD in current versus never users [adjusted odds ratio (AOR) 0.50; 95 % CI 0.26, 0.98]. In Model 2, obesity as defined by BMI ≥30 kg/m2 was found to be strongly associated with NAFLD compared to their normal-BMI counterparts, overweight females showed a 1.3-fold odds of NAFLD (AOR 1.31; 95 % CI 0.91, 1.89) and obese individuals had a 3.3-fold higher odds of NAFLD (AOR 4.27; 95 % CI 2.88, 6.34). BMI significantly attenuated the relationship between current OCP use and NAFLD (AOR 0.55; 95 % CI 0.27, 1.13), by about 14 % [absolute PEE (aPEE) was 14.0 %]. In other words, slightly more than one-tenth of the association between OCP use and NAFLD could have been explained by BMI alone. Similarly, women with a WC >88 cm (34.6 in.) also exhibited a twofold higher odds of NAFLD than those with smaller waist (AOR 3.21; 95 % CI 2.25, 4.58). WC also substantially attenuated the OCP–NAFLD association with an estimated aPEE 21.3 % (Model 3 in Table 3). On the other hand, the waist-to-hip ratio and total percent body fat appeared to exert only minimal influence (aPEE <15 %) (Models 4 and 5, Table 3).
Table 3.
Odds ratios of NAFLD by oral contraceptive pill use, premenopausal women in NHANES III (N = 4338), 1988–1994
| Model 1 Adjusted for age, race, smoking, DM, hypertension and educationb AOR (95 % CI) |
Model 2 Adjusted for age, race, smoking, DM, hypertension, educationb and BMI AOR (95 % CI) |
Model 3 Adjusted for age, race, smoking, DM, hypertension, educationb and WC AOR (95 % CI) |
Model 4 Adjusted for age, race, smoking, DM, hypertension, educationb and WHR AOR (95 % CI) |
Model 5a Adjusted for age, race, smoking, DM, hypertension, educationb and PBF AOR (95 % CI) |
|
|---|---|---|---|---|---|
| OCP use | |||||
| Never | 1 | 1 | 1 | 1 | 1 |
| Former | 0.81 (0.54, 1.20) | 0.84 (0.54, 1.28) | 0.83 (0.55, 1.26) | 0.80 (0.53, 1.19) | 0.86 (0.57, 1.30) |
| Current | 0.50 (0.26, 0.98) | 0.55 (0.27, 1.13) | 0.58 (0.29, 1.17) | 0.52 (0.26, 1.03) | 0.53 (0.24, 1.16) |
| Ptrend | 0.031 | 0.087 | 0.104 | 0.042 | 0.095 |
| Anthropometrics | |||||
| BMI (kg/m2) | |||||
| <25 | |||||
| 25–29.9 | 1.31 (0.91, 1.89) | ||||
| ≥30 | 4.27 (2.88, 6.34) | ||||
| WC >88 cm | 3.21 (2.25, 4.58) | ||||
| WHR ≥0.8 | 1.40 (1.03, 1.91) | ||||
| PBF ≥40 % | 2.67 (1.88, 3.78) | ||||
| aPEEc (%) | – | 14.0 % | 21.3 % | 5.4 % | 12.4 % |
AOR adjusted odds ratio, aPEE absolute proportion of exposure effect, BMI body mass index, CI confidence interval, DM self-reported diagnosis of type 2 diabetes, PBF total percent body fat, WC waist circumference, WHR waist-to-hip ratio
Women without bio-impedance measure were not included (n = 530)
Age was entered as a continuous variable; race grouped as non-Hispanic white, non-Hispanic black, Mexican-American and others; smoking as a categorical variable that indicated never, former and current smoking status; education as a binary indicator for a high-school or higher educational level
Absolute proportion of exposure effect (aPEE) was estimated by calculating percent change in regression coefficients of current versus never users between the extended and the base models, using the same estimation sample
The low prevalence of NAFLD associated with current OCP use seemed particularly prominent in women aged 20–34 (AOR 0.41, 95 % CI 0.20, 0.86) but not in women aged 35 or older (AOR 1.58, 95 % CI 0.51, 4.91). However, there was no statistical evidence for the differential effect of age on the OCP–NAFLD association (P values for interaction >0.05). Nor did the OCP–NAFLD association significantly differ among women with or without diabetes or hypertension (P values for interaction >0.05, data not shown). Moreover, we found that the association between OCP use and NAFLD was differentially affected by race, with a protective effect of current OCP use observed only among non-Hispanic White (AOR 0.41, 95 % CI 0.18, 0.97) after adjusting for age, smoking history, co-morbidities and the highest educational level (Table 4). Notably, African American women who never used OCP had a 54 % lower odds of NAFLD than their non-Hispanic White counterparts (AOR 0.46, 95 % CI 0.25, 0.84).
Table 4.
OCP–NAFLD association by race, premenopausal women aged 20–60 years in NHANES III (N = 4338), 1988–1994
| Non-Hispanic white
|
Non-Hispanic black
|
Mexican-American
|
Others
|
|||||
|---|---|---|---|---|---|---|---|---|
| AORa | 95 % CI | AORa | 95 % CI | AORa | 95 % CI | AORa | 95 % CI | |
| OCP use | ||||||||
| Never | 1 | – | 0.46 | 0.25, 0.84b | 1.60 | 0.96, 2.69 | 1.30 | 0.60, 2.81 |
| Former* | 0.74 | 0.41, 1.33 | 0.77 | 0.43, 1.40 | 1.30 | 0.75, 2.24 | 0.87 | 0.31, 2.47 |
| Current* | 0.41 | 0.18, 0.97c | 0.68 | 0.26, 1.82 | 1.01 | 0.55, 1.85 | 0.61 | 0.15, 2.44 |
AOR adjusted odds ratio, CI confidence interval, OCP oral contraceptive pill
P values for interaction: 0.024 for former versus never users; 0.011 for current versus never users
Adjusted for age, smoking status, history of diabetes or hypertension and highest educational level
P value for interaction 0.04
P value for interaction 0.017
Discussion
In this population-based study, we found an independent association between current OCP use and NAFLD among women of reproductive age. This study finding extends the accumulating literature that exogenous sex hormones may provide a protective effect against NAFLD in women. Current literature on the relationship of exogenous sex hormones and adiposity remains inconclusive [2, 6, 12, 15]. Particularly, in a recent systematic review, most included trials were of short duration (<1 year) [26] whereas more than 90 % of current and 75 % of former OCP users in the current study reported that they had taken OCP for more than 1 year at the time of the interview or before quitting. As such, we hypothesized that adiposity situated on the pathway between OCP use and NAFLD as a mediator, rather than as a confounder. Nevertheless, the directionality of the OCP-obesity relationship can only be clarified in a prospective manner.
While female sex hormones are known to modulate immune responses underlying some viral infections and chronic inflammatory disorders [27, 28], several mechanisms have been proposed by which estrogen may exert a hepatoprotective effect [7, 27]. Both in vitro and clinical studies have shown that estradiol (E2) at the pregnancy level can block the secretion of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 [27, 29, 30]. E2 is also known to be potent anti-oxidant acting through estrogen receptors on hepatic cells [31]. Conceivably, low levels of E2 may be associated with impaired beta-oxidation of fatty acids that are deposited in hepatocytes, worsening the steatosis in patients with NAFLD [7, 32]. In contrast, research on the potential involvement of progesterone in adipocyte development and differentiation has been relatively little and the results are often inconsistent [33, 34]. Future investigations to further delineate the relative contributions of estrogen and progesterone in the pathogenesis of NAFLD are warranted.
Given the low prevalence of current OCP users among participants aged 35 or older (weighted prevalence 5.3 % vs. 30.8 % in those aged 20–34 years), our finding that the protective effect of current OCP use against NAFLD was most evident in younger (age <35 years) versus older women was not unexpected. This also likely explains our lack of power to detect a significant modifying effect of age on the OCP–NAFLD association. As early work has revealed that women taking OCPs generally have a low concentration of plasma estrogen [35, 36], we believe that endogenous estrogen may not be involved in this differential relationship by age in current OCP users.
Consistent with other work showing that African Americans tended to have a reduced risk of nonalcoholic steatohepatitis (NASH) [3, 37], we found that African American women who never used OCP had a reduced odds of NAFLD as compared to non-Hispanic White. Possible explanations for this risk reduction by race abound yet none is more favorable than others. Among a cohort of obese women requiring bariatric surgery, Solga et al. [38] found that African American women were less likely to be affected by moderate–severe hepatic steatosis or liver fibrosis than their Caucasian counterparts, despite of similarly high prevalence of risk factors for cardio-metabolic disorders. Solga et al. [38] suggested a gene–environmental interaction that modified women’s immune responses to an altered adiposity status. Besides, several observational studies have demonstrated that African American women tend to have less visceral fat than Caucasian women of similar BMI values, suggesting race-specific metabolic pathways in utilization and storage of adipose tissue [39, 40]. As studies have shown that E2 levels were generally comparable among African American women, non-Hispanic White and Hispanic women [41–43], it is possible that estrogen differentially modulates the immune system under an oxidative stress in the liver of African American women as compared to non-Hispanic Whites. However, whether this OCP–NAFLD association truly differs by race needs further research. Longitudinal studies that monitor changes in immune cells and cytokines along with hormone levels in a multi-racial population may help elucidate the interplay among the immune, endocrine and metabolic aspects of this particular liver disease.
Several limitations need to be considered in light of our findings. First, the cross-sectional nature makes it impossible to ascertain causality. Nevertheless, our findings contribute to the existing literature that exogenous sex hormone may independently protect women against NA-FLD by providing evidence from a large, representative female population in the US. Second, while performing mediation analysis, theoretical work in the causal inference has shown that it is imperative to make sure the mediator-outcome relationship is well-adjusted by including most, if not all, measured confounders in the statistical model [24]. Although NHANES III has extremely comprehensive data collection, the risk for omitted variables still exists and cannot be determined by empirical analysis. Lastly, women’s exposure to OCP relied on self-report in the current analysis. Regiments or doses of OCP were not available to verify the relative contribution of estrogen versus progesterone or to evaluate the dose– or duration–response relationship with NAFLD.
In this US-representative sample of women aged 20–60 years, we found that current use of OCP was independently associated with decreased odds of NAFLD. A significant proportion of such effect could be attributed to adiposity as measured by waist measure or BMI. Additional investigations using prospective cohorts, with very well characterized measures of adiposity are needed to determine whether a causal relationship between OCP use and NAFLD exists.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Su-Hsun Liu, Department of Epidemiology, Johns Hopkins Bloomberg, School of Public Health, 2024 E. Monument St., Suite 2-600, Baltimore, MD 21287, USA.
Mariana Lazo, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, 2024 E. Monument St., Suite 2-600, Baltimore, MD 21287, USA.
Ayman Koteish, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, USA.
W. H. Linda Kao, Department of Epidemiology, Johns Hopkins Bloomberg, School of Public Health, 2024 E. Monument St., Suite 2-600, Baltimore, MD 21287, USA
Ming-Hsiung Shih, Department of Family Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan.
Susanne Bonekamp, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University, School of Medicine, Baltimore, MD, USA.
Ruben Hernaez, Department of Epidemiology, Johns Hopkins Bloomberg, School of Public Health, 2024 E. Monument St., Suite 2-600, Baltimore, MD 21287, USA. Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, USA. Department of Medicine, Washington Hospital Center/Georgetown University Hospital, Washington, DC, USA.
Jeanne M. Clark, Email: jmclark@jhmi.edu, Department of Epidemiology, Johns Hopkins Bloomberg, School of Public Health, 2024 E. Monument St., Suite 2-600, Baltimore, MD 21287, USA. Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, USA
References
- 1.Angulo P. Nonalcoholic fatty liver disease. NEJM. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–50. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 3.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 4.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 5.Mohanty SR, Troy TN, Huo D, O’Brien BL, Jensen DM, Hart J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50:797–804. doi: 10.1016/j.jhep.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez-Grobe Y, Ponciano-Rodriguez G, Ramos MH, Uribe M, Mendez-Sanchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, postmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402–9. [PubMed] [Google Scholar]
- 7.Shimizu I, Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatol Res. 2007;37:239–47. doi: 10.1111/j.1872-034X.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 8.Elbers JM, de Roo GW, Popp-Snijders C, Nicolaas-Merkus A, Westerveen E, Joenje BW, et al. Effects of administration of 17beta-oestradiol on serum leptin levels in healthy postmenopausal women. Clin Endocrinol (Oxf) 1999;51:449–54. doi: 10.1046/j.1365-2265.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- 9.Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism. 1991;40:1323–6. doi: 10.1016/0026-0495(91)90037-w. [DOI] [PubMed] [Google Scholar]
- 10.Witteman J, Grobbee D, Kok F, Hofman A, Valkenburg H. Increased risk of atherosclerosis in women after the menopause. Br Med J. 1989;298:642–4. doi: 10.1136/bmj.298.6674.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjoerkelund C, Lissner L, Andersson S, Lapidus L, Bengtsson C. Reproductive history in relation to relative weight and fat distribution. Int J Obes. 1996;20:213–9. [PubMed] [Google Scholar]
- 12.Saruç M, Yüceyar H, Ayhan S, Türkel N, Tuzcuoglu I, Can M. The association of dehydroepiandrosterone, obesity, waist–hip ratio and insulin resistance with fatty liver in postmenopausal women—a hyperinsulinemic euglycemic insulin clamp study. Hepatogastroenterology. 2003;50:771–4. [PubMed] [Google Scholar]
- 13.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–57. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2006;65:40–4. doi: 10.1111/j.1365-2265.2006.02543.x. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138–43. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. [Accessed 16 Nov 2012];NHANES III data files, documentation and codebooks. 2011 http://www.cdc.gov/nchs/nhanes/nh3data.htm.
- 17.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–68. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–15. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Alcohol and Public Health. [Accessed 16 Nov 2012];Frequently asked questions: what is a standard drink in the United States? 2012 http://www.cdc.gov/alcohol/faqs.htm#standDrink.
- 20.National Center for Health Statistics. [Accessed on 16 Nov 2012];Third National Health and Nutrition Survey: hepatic steatosis assessment procedure manual. 2010 http://www.cdc.gov/nchs/data/nhanes/nhanes3/Hepatic_Steatosis_Ultrasound_Procedures_Manual.pdf.
- 21.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 22.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 23.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11:167–78. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]
- 24.VanderWeele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172:1339–48. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp. Stata Statistical Software: release 11. College Station; StataCorp LP: 2009. [Google Scholar]
- 26.Gallo MF, Lopez LM, Grimes DA, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2011;(9) doi: 10.1002/14651858.CD003987.pub4. [DOI] [PubMed] [Google Scholar]
- 27.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 28.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–49. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 30.Rogers AER. The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29:30–4. doi: 10.1016/s8756-3282(01)00468-9. [DOI] [PubMed] [Google Scholar]
- 31.Lacort M, Leal AM, Liza M, Martín C, Martínez R, Ruiz-Larrea MB. Protective effect of estrogens and catecholestro-gens against peroxidative membrane damage in vitro. Lipids. 1995;30:141–6. doi: 10.1007/BF02538267. [DOI] [PubMed] [Google Scholar]
- 32.Omoya T, Shimizu I, Zhou Y, Okamura Y, Inoue H, Lu G, et al. Effects of idoxifene and estradiol on NF-kappaB activation in cultured rat hepatocytes undergoing oxidative stress. Liver. 2001;21:183–91. doi: 10.1034/j.1600-0676.2001.021003183.x. [DOI] [PubMed] [Google Scholar]
- 33.Caprio M, Zennaro MC, Feve B, Mammi C, Fabbri A, Rosano G. Potential role of progestogens in the control of adipose tissue and salt sensitivity via interaction with the mineralocorticoid receptor. Climacteric. 2008;11:258–64. doi: 10.1080/13697130802162608. [DOI] [PubMed] [Google Scholar]
- 34.Lacasa D, Le Liepvre X, Ferre P, Dugail I. Progesterone stimulates adipocyte determination and differentiation 1/sterol regulatory element-binding protein 1c gene expression. Potential mechanism for the lipogenic effect of progesterone in adipose tissue. J Biol Chem. 2001;276:11512–6. doi: 10.1074/jbc.M008556200. [DOI] [PubMed] [Google Scholar]
- 35.Kjeld JM, Puah CM, Joplin GF. Changed levels of endogenous sex steroids in women on oral contraceptives. Br Med J. 1976;2:1354–6. doi: 10.1136/bmj.2.6048.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey-Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983;27:577–90. doi: 10.1016/0010-7824(83)90023-9. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? Am J Gastroenterol. 2002;97:1496–500. doi: 10.1111/j.1572-0241.2002.05795.x. [DOI] [PubMed] [Google Scholar]
- 38.Solga SF, Clark JM, Alkhuraishi AR, Torbenson M, Tabesh A, Schweitzer M, et al. Race and comorbid factors predict nonal-coholic fatty liver disease histopathology in severely obese patients. Surg Obes Relat Dis. 2005;1:6–11. doi: 10.1016/j.soard.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61:765–71. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 40.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–24. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 41.Randolph JF, Jr, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–22. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 42.Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–61. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 43.Ukkola O, Gagnon J, Rankinen T, Thompson PA, Hong Y, Leon AS, et al. Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol. 2001;145:1–9. doi: 10.1530/eje.0.1450001. [DOI] [PubMed] [Google Scholar]