Abstract
Background
Postprandial triglyceridemia predicts cardiovascular events. Niacin might lower postprandial triglycerides (TG) by restricting free fatty acid (FFA). Immediate-release niacin reduced postprandial TGs, but extended-release niacin failed to do so when dosed the night before a fat challenge.
Aims
1) Determine whether extended-release niacin dosed before a fat challenge suppresses postprandial TG. 2) Determine whether postprandial TG is related to FFA restriction.
Methods
Double-blinded, placebo-controlled, random-order crossover experiment, where healthy volunteers took 2 g extended-release niacin or placebo 1 hour before heavy cream. We sampled blood over 12 hours, and report TG and FFA as means±SD for incremental area under the curve (iAUC) and nadir.
Results
Combining 43 fat challenges from 22 subjects, postprandial TG iAUC was +312±200 on placebo vs +199±200 mg/dL*h on extended-release niacin (33% drop, p= 0.02). The incremental nadir for FFA was −0.07±0.15 on placebo vs −0.27±0.13 mmol/L on extended-release niacin (p<0.0001), and FFA iAUC fell from +2.9±1.5 to +1.5±1.5 mmol/L*h on extended-release niacin (20% drop, p=0.0015). The TG iAUC was strongly related to the post-dose drop in FFA (r=+0.58, p=0.0007).
Conclusions
Given right before a fat meal, even a single dose of extended-release niacin suppresses postprandial triglyceridemia. This establishes that postprandial TG suppression is an acute pharmacodynamic effect of extended-release niacin, probably the result of marked FFA restriction. Further study is warranted to determine whether mealtime dosing would augment the clinical efficacy of extended-release niacin therapy.
Keywords: adult, African Americans, clinical trial, dietary fats, drug, evaluation, free fatty acids, humans, hydroxybutyrate, ketones, lipoprotein, lipids, niacin, niacin/pharmacology, niacin/therapeutic use, postprandial, randomized controlled trial, triglycerides
Hypertriglyceridemia is associated with premature coronary heart disease.(1;2) Although triglycerides (TGs) peak after meals, they are measured fasting for convenience. Non-fasting TGs predict coronary heart disease events better than fasting TGs, consistent with the atherogenic potential of alimentary TG-rich lipoproteins (TRLs) and cholesterol-rich remnants.(3–6) Moreover, postprandial triglyceridemia predicts endothelial dysfunction and early atherosclerosis.(7;8) Statins and fibrates suppress postprandial TG (ppTG), perhaps enhancing cardiovascular benefits.(9;10)
Immediate-release niacin suppresses ppTGs.(11–14) Though it takes several days to lower cholesterol,(15) immediate-release niacin suppresses ppTGs within hours of the first dose, indicating this is an acute pharmacodynamic response.(16) Surprisingly, extended-release niacin had no such benefit.(17) This disparity is relevant because extended-release niacin dominates clinical use, even though only immediate-release niacin prevented hard cardiovascular outcomes.(18;19) If suppression of postprandial TRLs retards atherosclerosis, extended-release niacin may prove less atheroprotective than immediate-release niacin. Adding impact, recent cardiovascular outcome studies of niacin+statin exclusively utilized extended-release niacin.(20;21) Clinical dosing differs strikingly by formulation: immediate-release niacin thrice daily with meals vs extended-release niacin once daily at bedtime--ie, before the major/only daily fast. Since TGs peak post-meal, one would expect dosing pre-fast to undermine efficacy if an acute dosing effect suppresses ppTG.(16). We suspect extended-release niacin misses an opportunity for efficacy because its short-lived TG-suppressive effects occur after bedtime, dissipating before breakfast. Conversely, we hypothesized that dosing extended-release niacin before a meal would suppress ppTG.
Methods
Objectives
Determine whether extended-release niacin before a fat challenge suppresses postprandial triglyceridemia.
Determine whether restricted supply of FFA or its metabolite hydroxybutyrate (HBA) predicts postprandial TG suppression.
Design
Double-blinded, random-order, crossover experiment of a single 2 g dose of extended-release niacin or placebo in niacin-naïve subjects lacking all elements of Metabolic Syndrome (cf. Supplemental Figure 1).
Protocol
Subjects presented after a 12-hour overnight fast and took 2 g extended-release niacin (Kos Pharmaceuticals, Miami, FL) or matching placebo (hour 0). After 1 hour, they drank heavy cream 50 g/m2 surface area within 20min, per the oral fat tolerance test (OFTT) of Cabezas et al.. (22). We sampled blood from an antecubital intravenous catheter hourly for 12 h. Subjects crossed to alternative treatment after ≥1 week. Some also provided 12 hours of fasting samples as a physiologic reference.
Laboratory Analysis
Within 30 min of collection into chilled EDTA tubes, we separated plasma from whole blood in a 4°C centrifuge, storing at −70°C until assaying runs by subject for TGs, FFA, and hydroxybutyrate (HBA)enzymatically on a Hitachi912 autoanalyzer (Roche Diagnostic Systems Inc, Indianapolis, IN) using Sigma reagents (Sigma-Aldrich, St. Louis, MO). The respective intra-assay and interassay coefficients of variation for TGs were 1.5% and 1.8%, for FFA 0.75% and 0.75%, and for HBA hydroxybutyrate 10% and 5%.
Statistics
We calculated area under the curve (AUC) over 12h using the trapezoidal rule, baseline by averaging −20, −10, and 0 minute samples, and incremental AUC (iAUC) as AUC-(baselineX12h). Though the recommended sample size for ppTG studies is at least 10 subjects,(23) our power calculations suggested a need of 22 subjects based on related literature(22)(24–26) Assuming baseline TG AUC of 2482 mg/dL*h, we needed 21 subjects for 80% power to detect a 615 mg/dL*h (25%) drop with a standard deviation (SD) of 691 mg/dL*h for the study’s significance threshold: a two-tailed alpha at <0.05. We performed all analyses in Stata® v10.0 (StataCorp), comparing iAUC by mixed-effects regression, adjusting for sex, African-American (Black) status, and body mass index (BMI). Typically, Blacks have lower fasting(27) and ppTGs,(28) so we tested for race interaction. We report mean, SD, and 95% confidence intervals (CI).
Results
Participants
Since 22 subjects received both OFTTs, we analyzed 44 OFTT studies (Supplemental Figure 1/Table 1).
Extended-release niacin suppresses postprandial triglycerides
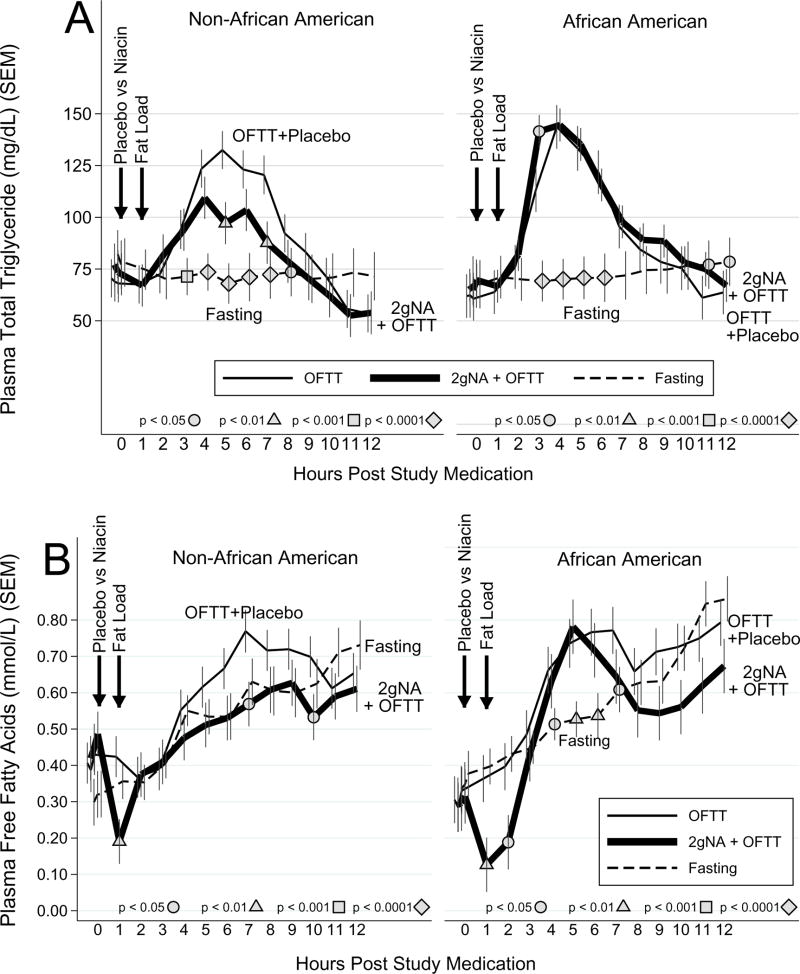
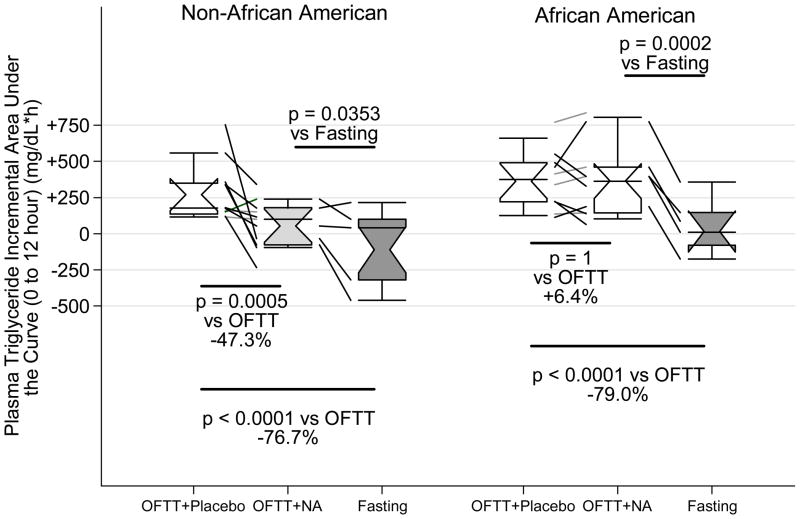
On placebo, ppTG increased +82±35mg/dL, peaking at 150±49 mg/dL after 5.6±2.5h, and normalizing by 9 h (Figure 1A). This bell-shaped curve is typical of ppTG, whose ascending phase indicates TRL accumulation, and descending phase TRL clearance.(23) On extended-release niacin, ppTG increased +72±41 mg/dL, peaking at 143±49 mg/dL after 4.8±2.4h, and normalizing by 9h. During the accumulation phase the rise in TGs was super-imposable. In contrast, extended-release niacin decreased ppTG levels during the post-peak clearance phase, significantly at 5h and 7h (both p<0.01 vs placebo). On placebo, TG iAUC was +312±200 vs +199±200 mg/dL*h on extended-release niacin (p=0.02), a median drop of 82 mg/dL (−33%) from the OFTT alone (Figure 2, Table 1).
Figure 1. Effect niacin on postprandial triglycerides.
Panel A: Time course of postprandial triglycerides.
Panel B: Time course of postprandial free fatty acids.
Filled shapes denote a significant comparison to OFTT+Placebo at same hour. SEM=standard error of the mean, OFTT=oral fat tolerance test, NA=niacin
Figure 2. Effect of niacin on triglyceride incremental area under the curve.
Whiskers delimit 10th and 90th percentiles, enclosed region the interquartile range (IQR), horizontal line the median, notches the 95% confidence interval and mean. Diagonal lines depict change in each individual. Percent change is the median of individuals’ change.
Table 1.
Postprandial lipid changes
| Effect of Niacin on Selected Outcomes | ||||
|---|---|---|---|---|
| Oral Fat Tolerance Test | 2g ER Niacin + OFTT | Fasting Alone | Fasting vs Niacin+OFTT | |
| Plasma Triglyceride Total Area Under the Curve (0 to 12 hour) (mg/dL*h) in Non-African Americans | ||||
| Mean (95% CI) | 1102.0 (921.0,1283.0) | 917.6 (732.6,1102.5) | 914.7 (688.1,1141.4) | p = 0.6638 |
| p vs OFTT | Ref | p = 0.0099 | p = 0.02 | |
| Median Delta (IQR) | −94.5 mg/dL*h (−469.5,+11.0) | −286.9 mg/dL*h (−369.4,−187.3) | ||
| Median %Change (IQR) | −11.6% (−30.3,+1.8%) | −28.4% (−31.5, −16.5%) | ||
|
| ||||
| Plasma Triglyceride Total Area Under the Curve (0 to 12 hour) (mg/dL*h) in African Americans (Interaction p = 0.0488) | ||||
| Mean (95% CI) | 1125.1 (935.0,1315.3) | 1145.4 (955.2,1335.5) | 856.8 (640.5,1073.0) | p = 0.045 |
| p vs OFTT | Ref | p = .8 | p = 0.031 | |
| Median Delta (IQR) | +50.3 mg/dL*h (−153.5,+117.5) | −149.9 mg/dL*h (−254.9, −66.0) | ||
| Median %Change (IQR) | +4.0% (−13.7,+14.8%) | −11.0% (−19.9, −9.7%) | ||
|
| ||||
| Plasma Triglyceride Incremental Area Under the Curve (0 to 12 hour) (mg/dL*h) in Non-African Americans | ||||
| Mean (95% CI) | +270.0 (+159.3,+380.7) | +53.7 (−61.1,+168.5) | −111.1 (−269.8,+47.5) | p = 0.0353 |
| p vs OFTT | Ref | p = 0.0005 | p < 0.0001 | |
| Median Delta (IQR) | −160.0 mg/dL*h (−414.5, −16.0) | −133.4 mg/dL*h (−798.9, −123.2) | ||
| Median %Change (IQR) | −47.3% (−123.0, −13.9%) | −76.7% (−142.3, −36.5%) | ||
|
| ||||
| Plasma Triglyceride Incremental Area Under the Curve (0 to 12 hour) (mg/dL*h) in African Americans (Interaction p = 0.0174) | ||||
| Mean (95% CI) | +362.8 (+243.2,+482.5) | +363.5 (+243.8,+483.1) | +14.2 (−127.6,+156.0) | p = 0.0002 |
| p vs OFTT | Ref | p = 1 | p < 0.0001 | |
| Median Delta (IQR) | +26.0 mg/dL*h (−152.5,+62.5) | −326.9 mg/dL*h (−329.4, −286.0) | ||
| Median %Change (IQR) | +6.4% (−33.1,+16.9%) | −79.0% (−97.4, −73.2%) | ||
|
| ||||
| Plasma Free Fatty Acids Area Under the Curve (0 to 12 hour) (mmol/L*h) Pooled Across Race (Interaction p = 0.9037) | ||||
| Mean (95% CI) | 7.326 (6.749,7.904) | 6.014 (5.428,6.600) | 6.638 (6.038,7.238) | p = 0.0296 |
| p vs OFTT | Ref | p < 0.0001 | p = 0.059 | |
| Median Delta (IQR) | −1.615 mmol/L*h (−2.075, −0.515) | −0.815 mmol/L*h (−1.466, −0.005) | ||
| Median %Change (IQR) | −23.7% (−30.3, −5.6%) | −14.0% (−22.7, −0.1%) | ||
|
| ||||
| Plasma Free Fatty Acids Incremental Area Under the Curve (0 to 12 hour) (mmol/L*h) Pooled Across Race (Interaction p = 0.5578) | ||||
| Mean (95% CI) | +2.930 (+2.311,+3.550) | +1.492 (+0.857,+2.126) | +2.474 (+1.807,+3.141) | p = 0.1762 |
| p vs OFTT | Ref | p = 0.0015 | p = 0.09 | |
| Median Delta (IQR) | −0.585 mmol/L*h (−3.235,+0.040) | −0.340 mmol/L*h (−2.355,+1.079) | ||
| Median %Change (IQR) | −20.0% (−80.3,+3.1%) | −9.3% (−40.8,+32.5%) | ||
|
| ||||
| Plasma Hydroxybutyrate Area Under the Curve (0 to 12 hour) (umol/L*h) Pooled Across Race (Interaction p = 0.4994) | ||||
| Mean (95% CI) | 3930.7 (2898.0,4963.5) | 3328.9 (2291.0,4366.8) | 2643.3 (1606.1,3680.4) | p = 0.2305 |
| p vs OFTT | Ref | p = 0.032 | p = 0.0027 | |
| Median Delta (IQR) | −547.5 umol/L*h (−982.0,+92.5) | −1438.5 umol/L*h (−1938.5, −1069.8) | ||
| Median %Change (IQR) | −19.9% (−36.1,+5.0%) | −62.8% (−72.1, −29.6%) | ||
|
| ||||
| Plasma Hydroxybutyrate Incremental Area Under the Curve (0 to 12 hour) (umol/L*h) Pooled Across Race (Interaction p = 0.5785) | ||||
| Mean (95% CI) | +3094.4 (+1999.3,+4189.4) | +2530.9 (+1492.0,+3569.9) | +1829.7 (+875.5,+2784.0) | p = 0.0921 |
| p vs OFTT | Ref | p = 0.07 | p < 0.0001 | |
| Median Delta (IQR) | −790.0 umol/L*h (−1370.0, −105.5) | −1154.6 umol/L*h (−1573.4, −817.8) | ||
| Median %Change (IQR) | −37.6% (−65.0, −11.2%) | −67.5% (−79.5, −24.4%) | ||
OFTT=oral fat tolerance test
Note that a negative incremental area under the curve is the same as –(incremental area over the curve) and represents a decrease from baseline.
On extended-release niacin we found significant interactions by race. During TRL accumulation Blacks reach peak TG faster. Post-peak, during the TRL clearance phase, extended-release niacin failed to suppress TG among Blacks (interaction p= 0.01, Figure 1). Strikingly, the median percent change in TG iAUC was −47% on extended-release niacin (interquartile range [IQR] −123 to −14%) among non-Blacks vs +4% (IQR − 14 to +15%) among Blacks. Thus, the 33% drop in TG iAUC pooling races obscures a marked disparity, falsely implying a benefit in Blacks and underestimating benefit in others. Accordingly, we recommend that outcomes be considered separately since interaction is present. The TG iAUC among non-Blacks was +270±196 on placebo vs +54±194 mg/dL*h on niacin (p=0.0005, median drop 160 mg/dL*h), driven by a drop during the TRL clearance phase from 5–7h. In contrast, TG iAUC among Blacks was +363±193 on placebo and 364±193 mg/dL*h on niacin (+26mg/dL*h, p=1). Moreover niacin failed to reduce ppTG from placebo at any time, and even exceeded placebo at 3h (p<0.05).
Effect of niacin on postprandial free fatty acids and hydroxybutyrate
On placebo, postprandial FFA increased 0.561±0.480 mmol/L peaking at 7.3±2.7h. The FFA did not normalize since even fasting raises FFA (Figure 1B). In non-Blacks postprandial FFA did not differ from the fasting reference. In Blacks, postprandial FFA exceeded fasting levels at hours 4 through 7 (all p<0.05). On niacin, the classic anti-lipolytic effect was seen, as FFA dropped 0.274±0.134 to an absolute nadir of 0.108±0.109 mmol/L at 3.9±4.0h post-niacin. At hour 1 the nadir dropped below baseline irrespective of race (p<0.01), and at hour 2 remained lower in Blacks (p<0.05, Figure 1B). Among non-Blacks post-nadir FFA tracked with postprandial and fasting FFA except for a few drops after hour 7. Among Blacks, postprandial FFA exceeded fasting FFA at 4–6h (all p<0.03) as well postprandial FFA for non-Blacks (all p<0.05). This suggests FFA rebound in Blacks prevented niacin from suppressing ppTG. Irrespective of race, on placebo FFA-iAUC was +2.93±1.48 vs +1.49±1.48 mmol/L*h on -release niacin over 12h (p=0.0015), a drop of 0.59 mmol/L*h (-20%, Table 1).
Since only hepatocytes convert FFA to HBA, plasma HBA reflects hepatic FFA exposure and corroborates FFA substrate availability for hepatic TG assembly. Postprandial HBA resembled FFA, with a nadir of 63.4.±50.1 on placebo and abruptly dropping to 32.5±50.1 μmol/L on niacin (p=0.006). Like FFA, among Blacks HBA rebounded between 4–8h, but gradually peaked at 12h in others (Supplemental Figure 2). Irrespective of race, on placebo HBA-iAUC was +3931±2472 vs +3329±2427 μmol/L*h on niacin (p=0.032), a drop of 548 μmol/L*h (−20%, Table 1).
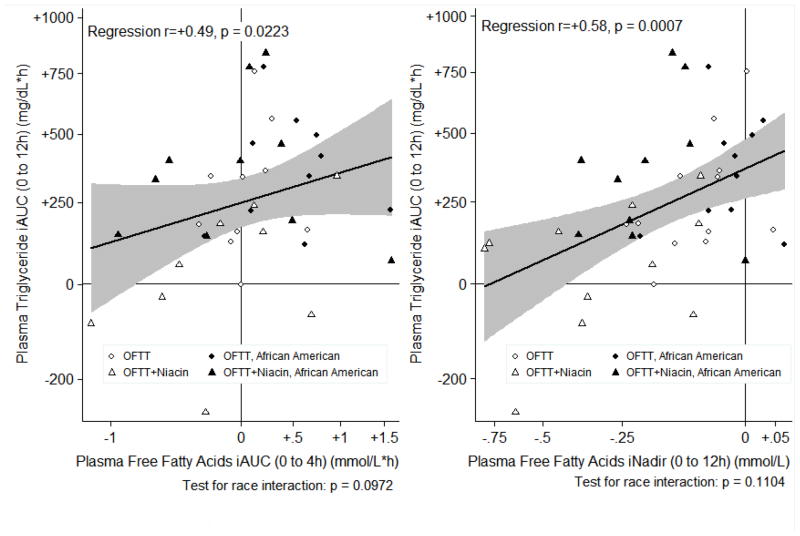
Restricted fatty acids predict suppressed postprandial triglyceride
Since the liver depends on adipose for FFA to make TG, niacin-induced FFA restriction could limit hepatic TG assembly by substrate limitation. Accordingly, changes in FFA or HBA predicted subsequent changes in TG-iAUC (Figure 3, Supplemental Table 2). As expected, regression often revealed a stronger relationship than Spearman’s correlation coefficient, since the former adjusted for race (Supplemental Table). TG-iAUC was strongly predicted by the incremental nadirs of FFA (r=+0.58, p=0.0007) and HBA (r=+0.52, p=0.0011) suggesting abruptly restricting FFA or HBA drives subsequent TG suppression. Time to HBA nadir inversely correlated with TG-iAUC (r= −0.55, p=0.05), implying prolonged restriction of hepatic FFA supply predicts greater ppTG suppression. The FFA-iAUC strongly correlated with TG-iAUC (r=+0.49). In summary, restricted FFA supply predicts suppressed ppTG.
Figure 3.
Relationship between postprandial fatty acids and postprandial triglycerides
iAUC= incremental area under curve, iNadir = incremental nadir. We transformed TG iAUC to reduce heteroskedasticity and skew.
Discussion
We are the first to demonstrate that extended-release niacin suppresses postprandial triglyceridemia. Frequent sampling revealed acutely-dosed extended-release niacin suppresses TGs during the post-peak TRL clearance phase only, like immediate-release niacin.(11;12) Our pooled 33% reduction in TG-iAUC by 2g extended-release niacin resembles reductions on chronic immediate-release niacin (Supplemental Table 3).(12–14) Others found immediate-release niacin thrice daily with meals suppressed diurnal/ppTGs (9am–9pm), as well as nocturnal/post-absorptive TGs (10pm–9am), and 24h AUC.(12) We show that extended-release niacin also suppresses diurnal/ppTGs for 12h, provided niacin is given pre-meal; however, the full 24h effect remains unknown. Irrespective of formulation, niacin reduces TG-AUC 21–41%.(12–14) Statins suppress ppTG 2–33%(29) and fibrates 33–55%,(30) suggesting 2–3g niacin has intermediate potency. Additive effects are reported with statin+gemfibrozil (31) and statin+immediate-release niacin.(14). Since postprandial TRLs are considered atherogenic, we believe further study of combination therapy is warranted.
Our results vary from Plaisance et al., whose bedtime dosing of <1500mg extended-release niacin for 6 weeks failed to suppress ppTGs the next day, adversely distinguishing extended-release niacin from immediate-release niacin.(17) Our results may differ for several reasons. Perhaps their Metabolic Syndrome subjects took medications interfering with ppTG suppression not used by our healthier cohort. They employed chronic niacin therapy; conceivably, the first-exposure ppTG response is greater than long-term response (ie, tachyphylaxis). If so, our suppression might not translate to chronic therapy. Contrariwise, immediate-release niacin chronically suppresses ppTG. Moreover, TG suppression grows more potent at the end of the first week vs first exposure to immediate-release niacin.(15) Perhaps they enrolled more Blacks or other non-responders. Differences in OFTT might explain disparate results; however their carbohydrate-enriched OFTT should amplify/prolong FFA and HBA restriction by insulin’s anti-lipolytic effect, hence deepening TG-suppression and not accounting for the discrepancy.
The simplest explanation may be that niacin’s ability to suppress ppTG depends on acute post-dose pharmacodynamics. Accordingly, we speculate bedtime dosing squanders an opportunity, and suggest comparing bedtime to pre-prandial extended-release niacin to test this. Further study is justified because counter-physiologic dosing could diminish extended-release niacin’s efficacy as an atheroprotective therapy, as nocturnally-dosed extended-release niacin fails to suppress atherogenic postprandial remnant lipoproteins. A recent study found that cardiovascular benefits of ≤3g mealtime immediate-release niacin did not materialize when ≤2g bedtime extended-release niacin was compared to placebo, with aggressive statin titration and/or addition of ezetimibe therapy in both groups.(20) Disappointing cardiovascular effects of bedtime extended-release niacin may involve failure to suppress atherogenic TRLs/remnants during the postprandial phase that dominates the 24h period. By completely dissociating acute from chronic effects, we challenge the notion that niacin suppresses postprandial triglyceridemia by merely lowering baseline/fasting TG.(17)
Froberg proposed 3 candidate mechanisms for niacin-induced ppTG suppression: 1) accelerating chylomicron (CM) or very low density lipoprotein (VLDL) catabolism (eg, by enhancing lipoprotein lipase [LPL] activity); 2) retarding intestinal CM production; or 3) retarding VLDL production (eg, FFA restriction).(12) The third is best supported, and we offer an expanded mechanistic hypothesis (cf. Supplemental Figure 3). A dose of niacin rapidly suppresses hormone sensitive lipase (HSL),(32) by stimulating adipocyte GPR109A.(33–35) Thus, niacin restricts restricts lipolysis of stored TG to FFA, prompting a precipitous drop in adipose-derived plasma FFA within minutes,(36)restricting FFA delivery to the liver, largely via the portal vein.(16;32;33) Since the liver depends on adipose-derived FFA for TG synthesis, restricted FFA supply suppresses TG synthesis, halting VLDL production as early as 1 hour post-dose.(37) With a half-life of 1–2 hours,(37) arrested VLDL production takes several hours to shrink the VLDL- and total-TG pools, consistent with observed TG suppression 4 hours post-dose.(16) Niacin-induced -FFA restriction is thought to initiate VLDL and plasma TG suppression.(38;39). Sustained suppression during plasma FFA rebound suggests additional mechanisms perpetuate the initial halt in VLDL production,(37;40–42) or that restored production simply lags plasma FFA rebound.
Regardless of how niacin suppresses VLDL-TG production, the resulting post-dose reduction in the VLDL-TG pool could be exploited clinically to reduce postprandial CM TG by simply taking extended-release niacin at mealtime. Catabolism of CM/VLDL-TG is rate-limited by LPL, facilitating dissolution of TG to FFA. Since CM and VLDL compete for LPL, post-dose restriction of VLDL-TG leaves more LPL available for CM-TG catabolism, facilitating ppTG clearance. Moreover, FFA inhibits LPL activity;(43) hence, restricted FFA disinhibits LPL activity, another way mealtime niacin might accelerate TRL catabolism. Accordingly, in our study restricted FFA preceded ppTG suppression during the clearance phase of the curve 5 to 7 hours post-niacin, with robust correlations between rapid FFA restriction (r=+0.58) and/or HBA (r=+0.52) and subsequent ppTG suppression, which to our knowledge are novel. Though FFA restriction has long been invoked to explain TG suppression by virtue of biological plausibility, strong correlations in our study advance the case for a causal relationship.
Plasma FFA may not reflect the totality of hepatic exposure to FFA by under-representing portal FFA delivery to the liver.(44) Since hepatocytes convert FFA to HBA, plasma HBA may thereby provide a more specific marker for hepatic (ie, portal) FFA flux than peripheral vein FFA.(44) Thus, HBA provides an important and novel corroboration of the concept that restricted FFA mediates ppTG suppression.
The major clinical implication is that the recommended dosing strategy for extended-release niacin undermines its efficacy, especially since our results accord with studies of peri-prandial immediate-release niacin whereas the study of pre-fast extended-release niacin had no effect on ppTG. We propose that dosing extended-release niacin at bedtime undermines efficacy by 1) ensuring the rapid drop in FFA is long gone by the time of the next day’s meal, an opportunity cost, and 2) risking timing breakfast during the FFA rebound, an active interference with benefit. Indeed, bedtime dosing raises fasting FFA well into the next morning, reflecting nocturnal FFA rebound which may promote VLDL production, thereby undermining TG suppression.(45–47) Alternatively, pre-meal dosing of extended-release niacin could fully exploit a postprandial benefit, and even forestall nocturnal FFA rebound, deepening fasting TG suppression. A theoretical benefit has been used to justify bedtime dosing of extended-release niacin (48) since Type IV triglyceridemics exposed to a high-carbohydrate meal and niacin infusion had diminished nocturnal FFA rebound and TGs.(49)A more practical reason to initiate extended-release niacin at bedtime is to time the disagreeable dermal response with sleep.(50) We propose that after developing tolerance to the latter, bedtime dosing is neither obligatory nor advantageous. Indeed, results of Plaisance and our group imply nocturnal dosing undermines a potentially atheroprotective benefit of the extended-release formulation. If switching the timing conferred additional 24h efficacy, perhaps extended-release niacin would achieve similar fasting and postprandial efficacy as immediate-release niacin, a proposition worthy of additional study.
The Black population has lower fasting TGs and ppTGs.(28) This may reflect increased LPL activity and superior TRL clearance. Unexpectedly, fasting and ppTGs did not vary by race on OFTT+placebo in our study. Perhaps the expected racial differences depend on variations in metabolic defects, so selecting fit subjects abrogated differences. Strikingly, on extended-release niacin in Blacks, FFA rebound quickly followed FFA restriction, suggesting FFA rebound prevented niacin from accelerating TRL clearance. (47) To our knowledge this is the first study demonstrating significant inefficacy of extended-release niacin in Blacks, but does not speak to inefficacy of long-term therapy or for fasting lipoproteins.
Our study is subject to several limitations. We limited niacin to a single exposure in drug-naive subjects to separate acute pharmacodynamic from chronic therapy effects, thus better representing pharmacodynamic effects at the expense of generalizability to chronic therapy. We limited intra-individual variability by enrolling healthy individuals at the expense of generalizability to dyslipidemia. We selected an OFTT with minimal insulin effects.(51) Though this minimizes confounding by a second anti-lipolytic, it limits generalizability to mixed meals. Strengths include randomized, double-blinded, placebo-controlled design, larger sample size than prior studies,(11–14;17) high-resolution sampling, and robust participation of Blacks, which allowed us to detect interaction by race.
Conclusions
We found a single exposure to extended-release niacin suppressed postprandial triglyceridemia in drug-naïve subjects, in contrast to a report where bedtime extended-release niacin failed to suppress postprandial triglyceridemia at breakfast. This indicates niacin suppresses postprandial triglyceridemia by an acute pharmacodynamic effect, probably by restricted FFA supply limiting VLDL and accelerating chylomicron catabolism. Clinically, this challenges the conventional wisdom of dosing extended-release niacin before a prolonged fast, which may undermine lipid if not atherosclerosis benefits.
Supplementary Material
Acknowledgments
Support provided by NIH grants K23HL091130 and 5-K12-RR-017625-05 (R.L. Dunbar), SCCOR P50-HL-083799 (D.J. Rader), and RFA-HL-05-002 (H.U. Usman) from the NHLBI; AHA grants 09SDG2180013 and 0725480U (R.L. Dunbar), and UL1RR024134 from the NCRR, including a Junior Investigator Pilot Grant (R.L. Dunbar) by the UPenn Institute for Diabetes, Obesity, and Metabolism and the UPenn Institute for Translational Medicine and Therapeutics. The content is solely the responsibility of the authors and not sponsoring institutions.
We gratefully acknowledge Lorraine Norfleet RN, Rhoda McCollick RN, the nurses and nutritionists of the UPenn CTRCs, Andrew Cucchiara Ph.D. for statistical assistance, and Joseph Jalkiewicz of Good Word Communications, LLC for editorial assistance, and especially the study participants.
Funding Source: No extramural project support
Role of Authors:
All authors had access to the data and a role in writing the manuscript, and have seen and approved the submitted version. Each author was involved in the conception or design of the study or the analysis of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 2.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–219. [PubMed] [Google Scholar]
- 3.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 4.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 5.Sharrett AR, Chambless LE, Heiss G, Paton CC, Patsch W. Association of postprandial triglyceride and retinyl palmitate responses with asymptomatic carotid artery atherosclerosis in middle-aged men and women. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 1995;15(12):2122–2129. doi: 10.1161/01.atv.15.12.2122. [DOI] [PubMed] [Google Scholar]
- 6.Ryu JE, Howard G, Craven TE, Bond MG, Hagaman AP, Crouse JR., III Postprandial triglyceridemia and carotid atherosclerosis in middle-aged subjects. Stroke. 1992;23(6):823–828. doi: 10.1161/01.str.23.6.823. [DOI] [PubMed] [Google Scholar]
- 7.Boquist S, Ruotolo G, Tang R, Bjorkegren J, Bond MG, de FU, et al. Alimentary lipemia, postprandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation. 1999;100(7):723–728. doi: 10.1161/01.cir.100.7.723. [DOI] [PubMed] [Google Scholar]
- 8.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79(3):350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 9.Kolovou GD, Kostakou PM, Anagnostopoulou KK, Cokkinos DV. Therapeutic effects of fibrates in postprandial lipemia. Am J Cardiovasc Drugs. 2008;8(4):243–255. doi: 10.2165/00129784-200808040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kolovou GD, Anagnostopoulou KK, Salpea KD, Daskalopoulou SS, Mikhailidis DP. The effect of statins on postprandial lipemia. Curr Drug Targets. 2007;8(4):551–560. doi: 10.2174/138945007780362809. [DOI] [PubMed] [Google Scholar]
- 11.Nikkila EA. Effect of nicotinic acid on adipose lipoprotein lipase and removal rate of plasma triglycerides. In: Gey KF, Carlson LA, editors. Metabolic Effects of Nicotinic Acid and its Derivatives. Bern: Hanss Huber Publishers; 1971. pp. 487–496. [Google Scholar]
- 12.Froberg S, Boberg J, Carlson LA, Eriksson M. Effect of nicotinic acid on the diurnal variation of plasma levels of glucose, free fatty acids, triglycerides and cholesterol and of urinary excretion of catecholamines. In: Gey KF, Carlson LA, editors. Metabolic Effects of Nicotinic Acid and its Derivatives. Bern: Hanss Huber Publishers; 1971. pp. 167–181. [Google Scholar]
- 13.King JM, Crouse JR, Terry JG, Morgan TM, Spray BJ, Miller NE. Evaluation of effects of unmodified niacin on fasting and postprandial plasma lipids in normolipidemic men with hypoalphalipoproteinemia. The American Journal of Medicine. 1994;97(4):323–331. doi: 10.1016/0002-9343(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 14.O’Keefe JH, Jr, Harris WS, Nelson J, Windsor SL. Effects of pravastatin with niacin or magnesium on lipid levels and postprandial lipemia. Am J Cardiol. 1995;76(7):480–484. doi: 10.1016/s0002-9149(99)80134-9. [DOI] [PubMed] [Google Scholar]
- 15.Carlson LA, Oro L, Ostman J. Effect of nicotinic acid on plasma lipids in patients with hyperlipoproteinemia during the first week of treatment. Journal of Atherosclerosis Research. 1968;8(4):667–677. doi: 10.1016/s0368-1319(68)80025-0. [DOI] [PubMed] [Google Scholar]
- 16.Carlson LA, Oro L, Ostman J. Effect of a single dose of nicotinic acid on plasma lipids in patients with hyperlipoproteinemia. Acta Med Scand. 1968;183(5):457–465. doi: 10.1111/j.0954-6820.1968.tb10508.x. [DOI] [PubMed] [Google Scholar]
- 17.Plaisance EP, Mestek ML, Mahurin AJ, Taylor JK, Moncada-Jimenez J, Grandjean PW. Postprandial triglyceride responses to aerobic exercise and extended-release niacin. Am J Clin Nutr. 2008;88(1):30–37. doi: 10.1093/ajcn/88.1.30. [DOI] [PubMed] [Google Scholar]
- 18.The Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA. 1975;231(4):360–381. [PubMed] [Google Scholar]
- 19.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8(6):1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 20.The AIM-HIGH Investigators. New England Journal of Medicine. 2011. Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. [DOI] [PubMed] [Google Scholar]
- 21.HPS2-Thrive: A randomized trial of the long-term clinical effects of raising HDL cholesterol with extended-release niacin/laropiprant. http://wwwthrivestudyorg [ 2012 Available from: URL: http://www.thrivestudy.org.
- 22.Cabezas MC, de Bruin TW, Jansen H, Kock LA, Kortlandt W, Erkelens DW. Impaired chylomicron remnant clearance in familial combined hyperlipidemia. Arterioscler Thromb. 1993;13(6):804–814. doi: 10.1161/01.atv.13.6.804. [DOI] [PubMed] [Google Scholar]
- 23.Lairon D, Lopez-Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr. 2007;61(10):1145–1161. doi: 10.1038/sj.ejcn.1602749. [DOI] [PubMed] [Google Scholar]
- 24.Birjmohun RS, Hutten BA, Kastelein JJP, Stroes ESG. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: A meta-analysis of randomized controlled trials. Journal of the American College of Cardiology. 2005;45(2):185–197. doi: 10.1016/j.jacc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Insull W, Jr, McGovern ME, Schrott H, Thompson P, Crouse JR, Zieve F, et al. Efficacy of extended-release niacin with lovastatin for hypercholesterolemia: assessing all reasonable doses with innovative surface graph analysis. Arch Intern Med. 2004;164(10):1121–1127. doi: 10.1001/archinte.164.10.1121. [DOI] [PubMed] [Google Scholar]
- 26.Vega GL, Cater NB, Meguro S, Grundy SM. Influence of Extended-Release Nicotinic Acid on Nonesterified Fatty Acid Flux in the Metabolic Syndrome With Atherogenic Dyslipidemia. The American Journal of Cardiology. 2005;95(11):1309–1313. doi: 10.1016/j.amjcard.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 27.Florez H, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Castillo-Florez S, Lee D, et al. Increased apolipoprotein C-III levels associated with insulin resistance contribute to dyslipidemia in normoglycemic and diabetic subjects from a triethnic population. Atherosclerosis. 2006;188(1):134–141. doi: 10.1016/j.atherosclerosis.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Friday KE, Srinivasan SR, Elkasabany A, Dong C, Wattigney WA, Dalferes E, Jr, et al. Black-white differences in postprandial triglyceride response and postheparin lipoprotein lipase and hepatic triglyceride lipase among young men. Metabolism. 1999;48(6):749–754. doi: 10.1016/s0026-0495(99)90175-0. [DOI] [PubMed] [Google Scholar]
- 29.van Oostrom AJ, van WJ, Cabezas MC. Lipaemia, inflammation and atherosclerosis: novel opportunities in the understanding and treatment of atherosclerosis. Drugs. 2004;64 (Suppl 2):19–41. doi: 10.2165/00003495-200464002-00004. [DOI] [PubMed] [Google Scholar]
- 30.Fredrik K. Postprandial lipemia-effect of lipid-lowering drugs. Atherosclerosis Supplements. 2002;3(1):41–46. doi: 10.1016/s1567-5688(01)00004-6. [DOI] [PubMed] [Google Scholar]
- 31.Cabezas CM, Erkelens DW, Kock LA, de Bruin TW. Postprandial apolipoprotein B100 and B48 metabolism in familial combined hyperlipidaemia before and after reduction of fasting plasma triglycerides. Eur J Clin Invest. 1994;24(10):669–678. doi: 10.1111/j.1365-2362.1994.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 32.Carlson LA. Studies on the effect of nicotinic acid on catecholamine stimulated lipolysis in adipose tissue in vitro. Acta Med Scand. 1963;173:719–722. doi: 10.1111/j.0954-6820.1963.tb17457.x. [DOI] [PubMed] [Google Scholar]
- 33.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9(3):352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 34.Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278(11):9869–9874. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 35.Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, et al. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun. 2003;303(1):364–369. doi: 10.1016/s0006-291x(03)00342-5. [DOI] [PubMed] [Google Scholar]
- 36.Carlson LA, Oro L. The effect of nicotinic acid on the plasma free fatty acid; demonstration of a metabolic type of sympathicolysis. Acta Med Scand. 1962;172:641–645. doi: 10.1111/j.0954-6820.1962.tb07203.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Basinger A, Neese RA, Shane B, Myong SA, Christiansen M, et al. Effect of nicotinic acid administration on hepatic very low density lipoprotein-triglyceride production. Am J Physiol Endocrinol Metab. 2001;280(3):E540–E547. doi: 10.1152/ajpendo.2001.280.3.E540. [DOI] [PubMed] [Google Scholar]
- 38.Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review J Intern Med. 2005;258(2):94–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 39.Lukasova M, Hanson J, Tunaru S, Offermanns S. Nicotinic acid (niacin): new lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol Sci. 2011;32(12):700–707. doi: 10.1016/j.tips.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Grundy SM, Mok HY, Zech L, Berman M. Influence of nicotinic acid on metabolism of cholesterol and triglycerides in man. J Lipid Res. 1981;22(1):24–36. [PubMed] [Google Scholar]
- 41.Ganji SH, Tavintharan S, Zhu D, Xing Y, Kamanna VS, Kashyap ML. Niacin non-competitively inhibits diacylglycerol acyltransferase-2 (DGAT2) but not DGAT1 activity in HepG2 cells. J Lipid Res. 2004 doi: 10.1194/jlr.M300403-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez C, Molusky M, Li Y, Li S, Lin JD. Regulation of hepatic ApoC3 expression by PGC-1beta mediates hypolipidemic effect of nicotinic acid. Cell Metab. 2010;12(4):411–419. doi: 10.1016/j.cmet.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bengtsson G, Olivecrona T. Lipoprotein lipase. Mechanism of product inhibition. Eur J Biochem. 1980;106(2):557–562. doi: 10.1111/j.1432-1033.1980.tb04603.x. [DOI] [PubMed] [Google Scholar]
- 44.Miles JM, Haymond MW, Nissen SL, Gerich JE. Effects of free fatty acid availability, glucagon excess, and insulin deficiency on ketone body production in postabsorptive man. J Clin Invest. 1983;71(6):1554–1561. doi: 10.1172/JCI110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauring B, Taggart AK, Tata JR, Dunbar RL, Caro L, Cheng K, et al. Niacin Lipid Efficacy is Independent of both the Niacin Receptor GPR109A and Free Fatty Acid Suppression. Science Translational Medicine. 2012 doi: 10.1126/scitranslmed.3003877. (In Press) [DOI] [PubMed] [Google Scholar]
- 46.Klein S, Young VR, Blackburn GL, Bistrian BR, Wolfe RR. Palmitate and glycerol kinetics during brief starvation in normal weight young adult and elderly subjects. J Clin Invest. 1986;78(4):928–933. doi: 10.1172/JCI112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sniderman AD, Cianflone K. Substrate delivery as a determinant of hepatic apoB secretion. Arterioscler Thromb. 1993;13(5):629–636. doi: 10.1161/01.atv.13.5.629. [DOI] [PubMed] [Google Scholar]
- 48.Knopp RH, Alagona P, Davidson M, Goldberg AC, Kafonek SD, Kashyap M, et al. Equivalent efficacy of a time-release form of niacin (Niaspan) given once-a-night versus plain niacin in the management of hyperlipidemia. Metabolism. 1998;47(9):1097–1104. doi: 10.1016/s0026-0495(98)90284-0. [DOI] [PubMed] [Google Scholar]
- 49.Schlierf G, Dorow E. Diurnal patterns of triglycerides, free fatty acids, blood sugar, and insulin during carbohydrate-induction in man and their modification by nocturnal suppression of lipolysis. J Clin Invest. 1973;52(3):732–740. doi: 10.1172/JCI107235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunbar RL, Gelfand JM. Seeing red: flushing out instigators of niacin-associated skin toxicity. J Clin Invest. 2010;120(8):2651–2655. doi: 10.1172/JCI44098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Oostrom AJ, van Dijk H, Verseyden C, Sniderman AD, Cianflone K, Rabelink TJ, et al. Addition of glucose to an oral fat load reduces postprandial free fatty acids and prevents the postprandial increase in complement component 3. Am J Clin Nutr. 2004;79(3):510–515. doi: 10.1093/ajcn/79.3.510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.