Abstract
Altered neurogenesis in adult hippocampus is implicated in cognition impairment and depression. Inflammation is a potent inhibitor of neurogenesis. The cyclin-dependent kinase inhibitor p21Cip1 (p21) restrains cell cycle progression and arrests the cell in the G1 phase. We recently showed that p21 is expressed in neuronal progenitors and regulates proliferation of these cells in the subgranular zone of the dentate gyrus of hippocampus where adult neurogenesis occurs. The current study suggests that p21 is induced in vivo in the hippocampus of WT mice in response to acute systemic inflammation caused by LPS injections, restrains neuronal progenitor proliferation and protects these cells from inflammation-induced apoptosis. In intact p21-/- hippocampus, neuronal progenitors proliferate more actively as assessed by BrdU incorporation, and give rise to increased number of DCX positive neuroblasts. However, when mice were treated with LPS, the number of neuroblasts decreased due to induced subgranular zone apoptosis.
In vitro, differentiating Tuj-1 positive neuroblasts isolated from p21-/- hippocampus exhibited increased proliferation rate, measured by Ki-67 staining, as compared to WT cells (p<0.05). In WT neuronal progenitors treated with IL-6, the number of p21-positive cells was increased (p<0.05), and this led to Tuj-1+ cell proliferation restraint, whereas the number of proliferating GFAP+ astrocytes was increased ∼ 2-fold. Thus, when p21 is intact, inflammation might divert neuronal progenitors towards astrogliogenesis by inducing p21. At the same time, when p21 is lacking, no effects of IL-6 on proliferation of Tuj-1+ cells or GFAP+ cells are detected in differentiating p21-/- neuronal progenitors. These results underscore the important role of p21 controlling hippocampal neuronal differentiation during inflammation.
Keywords: inflammation, IL-6, Cdk inhibitor p21, hippocampal neuronal progenitors, differentiation
Introduction
Functional granule neurons are generated in the subgranular zone (SGZ) of the dentate gyrus of the hippocampus throughout adult life. Within these region progenitor cells differentiate into neurons, astroglia or oligodendrocytes (van Praag et al., 2002). Neuronal stem cells are radial glia cells that express glial fibrillary acidic protein (GFAP) (Doetsch, 2003). SOX2 and nestin are markers that are expressed in neural stem cells and early neuronal progenitors. In a process of differentiation, the cells that are committed to a neuronal lineage stop expressing GFAP, nestin and SOX2 and start expressing neuronal markers such as doublecortin (DCX) and βIII-tubulin, both microtubule-associated proteins. Therefore, in the hippocampus, neuronal progenitor cells (NPC) constitute slow dividing quiescent radial “stem” cells (GFAP+/SOX2+ or GFAP+/nestin+ type 1 cells), transit amplifying progenitors (GFAP-/SOX2+ and GFAP-/nestin+ type 2a cells, or GFAP-/nestin+/DCX+ type 2b cells), and neuroblasts (DCX+/βIII-tubulin+), but not mature neurons (NeuN+; type 3 cells) (Encinas et al., 2006; Kempermann et al., 2003). Cells that are destined to become astrocytes express GFAP (Encinas et al., 2011; Ming and Song, 2011). Studies have suggested significant contribution of hippocampal neurogenesis to learning process and hippocampus-dependent memory (Denny et al., 2011; Drew and Hen, 2007; Fabel and Kempermann, 2008; Lagace et al., 2010; Sahay et al., 2011; Saxe et al., 2006; Saxe et al., 2007; Scharfman and Hen, 2007; van Praag, 2008; Winocur et al., 2006). In humans, low proliferation and differentiation capacity of adult neuronal progenitors correlates with memory dysfunction (Coras et al., 2010). Reduced neurogenesis have been reported in depressed patients and in animal models of stress-related depression (David et al., 2010; Duman, 2004; Eisch and Petrik, 2012; Kempermann et al., 2008; Malberg and Duman, 2003; Sapolsky, 2004; Snyder et al., 2011). At the same time, antidepressants have been shown to induce hippocampal neurogenesis (Encinas et al., 2006; Malberg et al., 2000; Sahay and Hen, 2007; Sahay et al., 2011).
Bacterial endotoxin lipopolysaccharide (LPS) mimics the infection of Gram–negative bacteria, and LPS injection results in marked up-regulation of circulating and brain proinflammatory cytokines including IL-6 and IL-1β (Bluthe et al., 1994; Laye et al., 1994). LPS treatment causes sharp decline in hippocampal neurogenesis (Ekdahl et al., 2009; Monje et al., 2003). Cytokines play a pivotal role in suppressing neurogenesis (Ben-Hur et al., 2003; Iosif et al., 2006; Koo and Duman, 2009b). At the same time, LPS–activated microglia reduced NPC survival and prevented neuronal differentiation in vitro (Cacci et al., 2008), and transgenic mice with chronic astroglial expression of IL-6 demonstrate a substantial decrease in the production of new neurons (Vallieres et al., 2002). IL-1β has also been shown to decrease adult hippocampal neurogenesis (Goshen et al., 2008; Koo and Duman, 2009a; Kuzumaki et al., 2010; Zunszain et al., 2012). Association between increased cytokine expression and depression have been reported in depressed patients and animal models of depression (Anisman and Merali, 1999; Dantzer et al., 2008; Howren et al., 2009; Koo and Duman, 2009b; Koo et al., 2010; Raison et al., 2010). Therefore, the disruptive effects of pro-inflammatory cytokines on neurogenesis might be a key link between inflammation and changes in behavior. However, molecular mechanisms linking inflammation and reduced neurogenesis are not fully understood.
In mammalian cells, the control of cellular proliferation is achieved primarily in the G1-phase of the cell cycle. The cyclin-dependent kinase (Cdk) inhibitor p21Cip1 (p21) restrains cell cycle progression and arrests the cell in the G1 phase (Sherr and Roberts, 1999). Previously, we reported that p21 is expressed exclusively in the subgranular zone (SGZ) of the dentate gyrus of hippocampus, restrains hippocampal neurogenesis in the adult mouse, and that antidepressant-induced stimulation of neurogenesis is associated with p21 suppression (Pechnick et al., 2011; Pechnick et al., 2008). We extended our study and demonstrate here that in vivo, acute systemic inflammation markedly increased p21 expression in hippocampus, restraining neuronal progenitor cell proliferation and protecting them from apoptosis. In vitro, IL-6-induced p21 arrests proliferation of neuroblasts, whereas cells destined to become astrocytes continue to proliferate. These results imply that p21 plays a critical role in hippocampal neurogenesis during inflammation.
Methods
Experimental animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center. p21-/- (Cdk1atm1Tyj) mice were obtained from The Jackson Laboratory. These mice were backcrossed to the C57Bl/6 genetic background 6 times prior to testing. p21+/- females and males were used for breeding, and both wild-type (WT) and p21-/- animals were obtained from the same litters. Two-month old male mice were used for the experiments. All mice were kept under standard laboratory conditions and had free access to water and standard mouse diet. For Western blot analyses and for obtaining NPC, mice were sacrificed by cervical dislocation, the brains removed and rapidly cooled in ice-cold saline and the hippocampi were dissected out (Paxinos G, 2001). For the immunohistochemistry studies mice were anesthetized with isoflurane and perfused with paraformaldehyde (4% in 0.1M phosphate buffer, pH 7.4).
LPS injections
LPS (Sigma-Aldrich, St. Louis, MO) (1 mg/kg, in 100 μl of normal saline, i.p.) was injected once a day for 5 days. Control mice receive normal saline (NS). Note that 100% of the mice survive this procedure in our laboratory.
Protein isolation and Western blot analysis
Hippocampus was dissected out (Paxinos G, 2001) and protein was isolated using Immunoprecipitation Kit (Roche Diagnostics, Indianapolis, IN) according to manufacturer's instruction. For Western blot analysis, 25 μg protein lysate was resolved by SDS-PAGE, electroblotted onto PVDF Millipore membrane (Millipore, Temecula, CA). The membrane was blocked by 5% nonfat dry milk in TBST (50mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.05% Tween 20) and incubated overnight with primary antibodies at 4°C, followed by incubation with corresponding secondary antibodies (Sigma-Aldrich, St. Louis, MO) for 2 hours at room temperature. Immunoreactive bands were detected using BioRad Molecular Imager® ChemiDoc™ XRS And Image Lab™ Software (BioRad Laboratories, Hercules, CA). The following antibodies were used: p21 (1:300, Cell Signaling Technology, Danvers, MA), p27 (1:300), β-actin (1:10,000), DCX (1:1,000), all from Santa Cruz Biotechnology (Santa Cruz, CA), GFAP (1:1,000, Millipore, Billerica, MA), and cleaved caspase 3 (1:1,000, Abcam, Cambridge, MA).
Quantitative Real Time PCR
Total RNA was isolated from hippocampi with Trizol reagent (Invitrogen, Carlsbad, CA). After DNAse I treatment (TURBO DNA free, Ambion, Austin, TX), cDNA was synthesized from 3 μg of purified RNA by the SuperScript II First-Strand cDNA synthesis system (Invitrogen, Grand Island, NY) according to manufacturer's instructions. Quantitative PCR was performed in 20 μl reaction using IQ SYBR Green Master Mix in BioRad IQ5 instrument (BioRad Laboratories, Hercules, CA). Specific validated primers for murine IL-1β, IL-6, TNF-α, and p21 were purchased from SuperArray (Qiagen, Germantown, MD). Triplicate PCR reactions yielded threshold cycle (Ct) average, with coefficient of variance of <0.05%, and used to determine ΔCt values [ΔCt=Ct of the target gene minus Ct of the housekeeping actin gene]. A comparative threshold cycle (CT) method was used for relative gene expression quantification. All experiments included template-free (water) and reverse transcriptase-minus controls to ensure no contamination. Relative quantities of mRNAs in experimental samples were determined, normalized to actin and GAPDH, and expressed in arbitrary units as fold difference from control (control was taken as one).
Bromodeoxyuridine (BrdU) incorporation
BrdU incorporation was assessed as described previously (Pechnick et al., 2008). The mice were injected every two hours with BrdU (Sigma-Aldrich, St. Louis, MO, 100 mg/kg/i.p) for a total of three injections, and then sacrificed 24 hr after the first BrdU injection. Twenty four hours have been reported to be sufficient for newborn cells to complete one cycle (Takahashi et al., 1992). The entire left half of the brain was cut into sagittal sections (5 μm) and processed using a BrdU Labeling and Detection Kit (Roche Applied Science, Indianapolis, IN). Sections were coded for blind data analysis. Cutting of sections from 0.36 to 0.6 mm lateral to the midline was carried out (Paxinos G, 2001). Every third section (total 30 sections) was counted under a × 100 objective and the sum was multiplied by 3 to estimate the total number of BrdU-positive cells in the region. Cells were counted if they were in or touching the SGZ and were excluded if they were more than two cell diameters away from the GCL (Malberg et al., 2000). Some sections were double-labeled to detect DCX, and BrdU-positive cells were examined by confocal microscopy to determine co-localization with DCX.
Immunohisto- and cytochemistry studies
Slide mounted brain paraffin sections (5 μM) or coverslips were double-labeled with primary antibodies conjugated with Alexa 488 and Alexa 568 fluorescent dyes (1:400, Invitrogen, Grand Island, NY) (Chesnokova et al., 2005). Antigen retrieval was performed in 10 mM sodium citrate, and control reactions were devoid of primary antibodies or stained with blocking antibodies. The following primary antibodies were used: p21 (1:50, BD Biosciences, San Jose, CA), SOX2 (1:100 or 1:30) and GFAP (1:50) (both Millipore, Burlington, MA), BrdU (1:50), DCX (1:20), both Santa Cruz Biotechnology (Santa Cruz, CA), Ki-67 (1:1,000) (Abcam, Cambridge, MA) and Tuj-1 (1:30, Stemcell Technologies, Vancouver, BC, Canada), cleaved caspase 3 (1:30, Abcam, Cambridge, MA). DNA (nuclei) was stained with DAPI (Prolong Gold, Invitrogen, Grand Island, NY). Three independent experiments were performed for each antibody. In vitro, immunoreactive cells were determined by immunocytochemistry in 3-10 random fields (total number of cells between 500 and 5,000 depending on experiment). Samples were imaged with a Leica TCS/SP spectral confocal scanner (Leica Microsystems, Mannheim, Germany) in dual emission mode to distinguish autofluorescence from specific staining.
Adult NPC cultures
Cultures were prepared and maintained according to published protocols (Doetsch, 2003; Ray et al., 1993; Zhao et al., 2003). Two-month old WT and p21-/- mice were sacrificed, the hippocampi dissected and dissociated using Papain Dissociation System (Worthington Biochemical, Lakewood, NJ). The NPC cells were isolated and cultured using Neural Stem Cell Expansion Kit Neurosphere System in serum-free neurobasal A-medium (R&D Systems, Minneapolis, MN). The single-cell suspension was resuspended in DMEM/F-12 medium supplemented with N-2 Plus Media Supplement, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 ng/ml FGF-2 and 20 ng/ml EGF. Under these conditions only stem/neuronal progenitors survive and form spheres. Cells were dispersed and passaged weekly and passages 2 -4 were used for the experiments. Differentiation was induced by growing cells in Complete NeuroCult NSC Differentiation Medium (Stemcell Technologies, Vancouver, Canada) in the absence of FGF-2 and EGF. 5 × 104 cells/ml were plated on ECL cell attachment matrix coated coverslips (5-10 μg/cm2, Millipore, Burlington, MA) in 24 well plates and cultured for 8 days in the presence of 50 ng/ml IL-6 (R&D Systems, Minneapolis, MN) with half the medium changed and fresh cytokine added every other day. Cells were then fixed in 4% paraformaldehyde and immunocytochemistry performed to detect neuronal markers.
Statistical analysis
The data were analyzed using unpaired t tests. The levels of significance for all analysis was set at p<0.05.
Results
Acute systemic inflammation triggers SGZ p21 expression in hippocampus
We induced inflammation by injecting WT male mice with LPS (1 mg/kg BW, i.p.) or normal saline once a day for 5 days in order to mimic acute and intensive inflammation produced by bacterial infection (i.e. sepsis). Three hours after the last injection animals were sacrificed, RNA and protein were isolated from hippocampi, and cytokine expression was measured by real time PCR. Proinflammatory cytokines IL-1β, TNF-α and IL-6 were upregulated in the hippocampus of LPS-treated mice, reflecting systemic inflammation (Fig. 1A). We demonstrated previously that p21 was expressed in the SGZ of hippocampus. p21 was co-localized with SOX2 and nestin, both markers of neuronal stem cells, and with DCX, a marker of committed neuroblast (Pechnick et al., 2011; Pechnick et al., 2008). Here we show that p21 mRNA and protein levels were strongly up-regulated in hippocampus of LPS treated mice (Fig. 1B,C), and this induction was accompanied by the increase in GFAP, a marker of astroglia and also activated microglia (Fig. 1C). At the same time, no change in hippocampal DCX expression was observed, and the levels of Cdk inhibitor p27, which belongs to the same Cip1/Kip1 family of inhibitors, were not altered by LPS injection (Fig. 1C), indicating specific effect of inflammation on p21. These results were confirmed by immunohistochemistry, showing increased number of SGZ cells expressing p21 after LPS treatment (Fig. 1D).
Fig. 1.
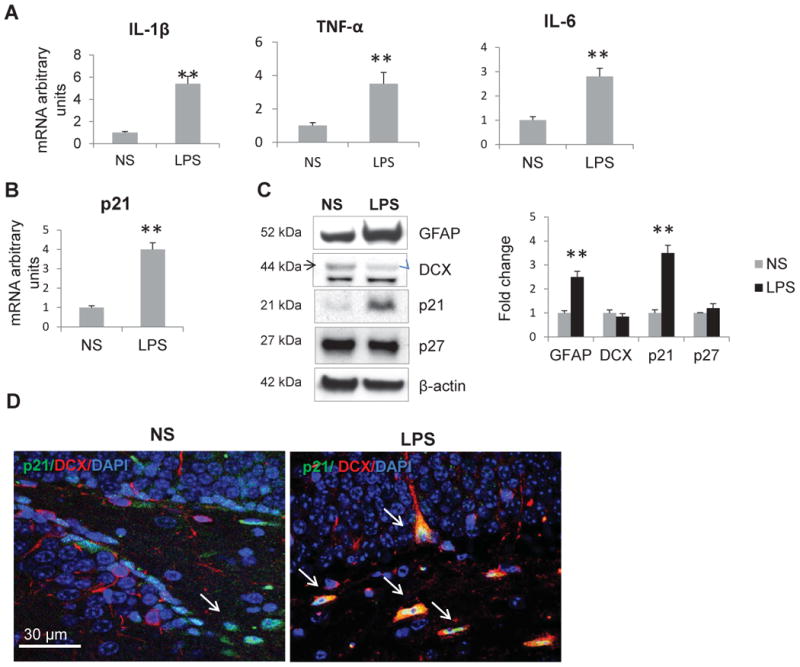
Acute systemic inflammation triggers proinflammatory cytokines and p21 expression in the hippocampus of WT mice. mRNA expression levels of A) cytokines; B) of p21. Results are expressed in fold change vs. control as average of 3 independent experiments. Data are shown as mean ± SEM; C) Left panel: Western blot analysis of neuronal markers and Cdk inhibitors; representative blots are shown. Right panel: intensities of protein bands were quantified from 3 independent experiments, normalized to β-actin and presented as fold change relative to control animals. Values represent mean ± SE; **, p<0.01. NS, normal saline. D) The confocal image shows that p21 (green, nuclear) expression is markedly increased in the SGZ of the dentate gyrus of LPS treated mice. Cells expressing p21 are marked with arrows. Note multiple cells co-expressing DCX (cytoplasmic, red) and p21 in LPS-treated group. Here and in other confocal images nuclei are stained with the DNA specific dye DAPI (blue).
Acute systemic inflammation decreases hippocampal DCX in p21-/- mice
To examine the role of p21 in SGZ during inflammation the p21-/- mice were treated with LPS as described above. Similar to WT animals, proinflammatory cytokines were up-regulated in hippocampus of LPS-treated animals (Fig. 2A). As in WT mice, Western blot analysis detected increase in GFAP expression. We also observed down-regulated DCX protein levels (Fig. 2B).
Fig. 2.
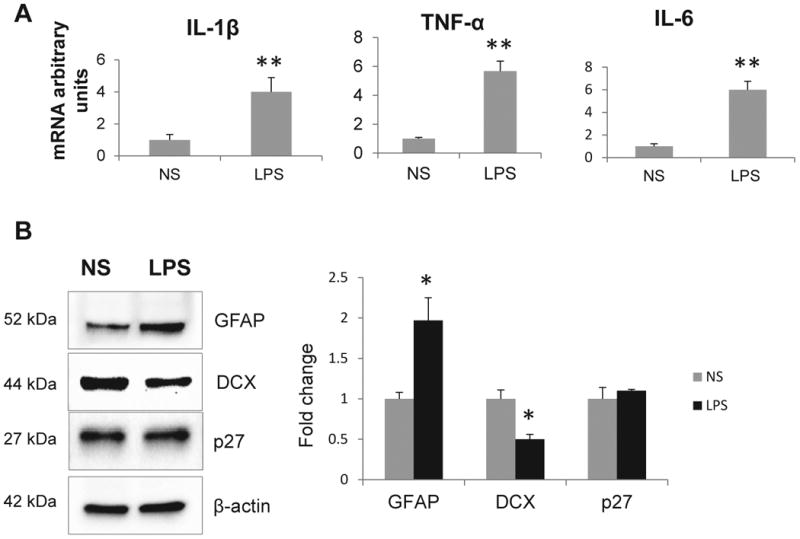
Acute systemic inflammation induces proinflammatory cytokine and decreases DCX expression in the hippocampus of p21-/- mice. A) mRNA expression levels of cytokines. Results are expressed in fold change vs. control as average of 3 independent experiments. Data are shown as mean ±SEM. B) Left panel: Western blot analysis of neuronal markers and Cdk inhibitor p27; representative blots are shown. Right panel: intensities of protein bands were quantified from 3 independent experiments, normalized to β-actin and presented as fold change relative to control animals. Values represent mean ± SE; *,p<0.05; **, p<0.01.
Acute systemic inflammation decreases proliferation of neuronal progenitors in both WT and p21-/- mice
Confocal microscopic analysis showed that the number of DCX+ cells is higher in untreated p21-deficient mice (Fig. 3A), confirming our earlier observations (Pechnick et al., 2008). We also tested how inflammation affected neuronal progenitor proliferation. WT and p21-/- mice were injected with LPS as described above. Immediately after the last LPS (or saline) treatment all mice received 3 BrdU injections, 2 hours apart, and were sacrificed 24 hours after the first BrdU injection. The number of BrdU+ (proliferating) cells and the percentage of DCX+ cells that co-express BrdU were determined by immunofluorescent double staining and confocal microscopy. The number of DCX+ cells was markedly higher in saline treated p21-/- as compared to WT mice, whereas LPS treatment resulted in decreased number of DCX+ cells in hippocampus of these animals. In contrast, LPS did not affect the number of DCX+ cells in WT hippocampus (Fig. 3B).
Fig. 3.
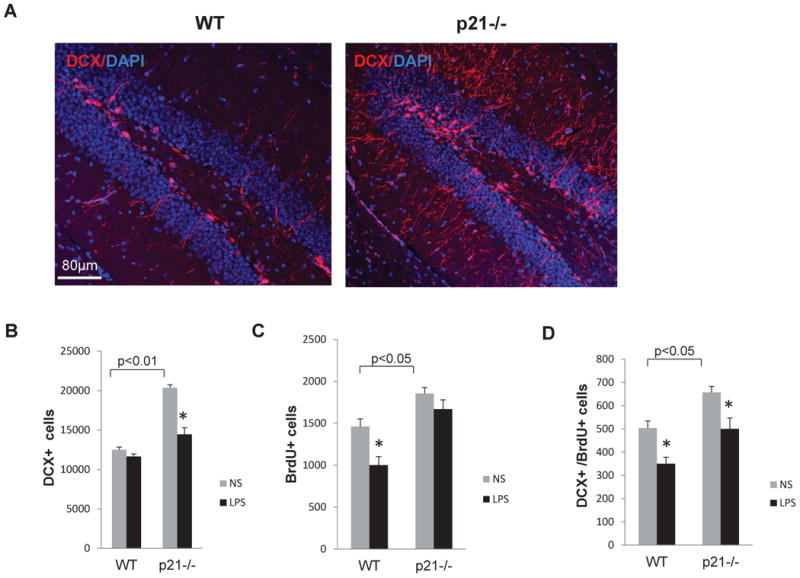
Acute systemic inflammation decreases proliferation of DCX+ cells in the SGZ of WT and p21-/- mice. A) The confocal image shows that DCX+ cells (cytoplasmic, red) are more abundant in the SGZ of p21-/- hippocampus; B) Number of DCX+ cells; C) Number of BrdU+ cells; D) Number of DCX+ BrdU+ cells in WT and p21-/- SGZ after LPS treatment. For each sample hippocampi from 5 mice/group were analyzed. Data are presented as a mean ± SEM, *, p<0.05; NS, normal saline.
The total number of proliferating BrdU+ cells was higher in saline-treated p21-/- as compared to WT hippocampus. However, LPS did not significantly change the number of proliferating cells in these animals, whereas in WT hippocampus, the number of proliferating cells was decreased (Fig. 3C). We also assessed the DCX+BrdU+ double positive cells and found that this number is higher in p21-/- control mice than in WT control mice; and in both genotypes LPS decreased the number of proliferating neuronal progenitors (Fig. 3D).
Acute systemic inflammation triggers apoptosis in the SGZ of p21-/- mice
As p21 exerts proliferation restrain, it is likely that p21 induction underlies a decrease in proliferating neuronal progenitors (DCX+BrdU+ cells) in WT hippocampus. We therefore tested mechanisms underlying decrease in DCX+ neuroblast proliferation in mice lacking p21. In apoptotic cells, caspase 3 is cleaved to a shorter isoform to trigger the apoptotic process (Dou and An, 1998). Immunofluorescent staining of hippocampal specimens derived from LPS-treated mice showed that cleaved caspase 3 expression in the SGZ of p21-/- hippocampus was strongly increased (Fig. 4A). This observation was also supported by Western blot analysis showing that after LPS treatment, cleaved caspase 3 levels were markedly induced in hippocampus of p21-/- mice, whereas this protein was undetectable in WT animals. These results indicate that LPS treatment induced apoptosis in SGZ of p21-/-, but not in WT mice. LPS treatment also resulted in downregulation of SOX2 protein levels in p21-/- hippocampus (Fig. 4B).
Fig. 4.
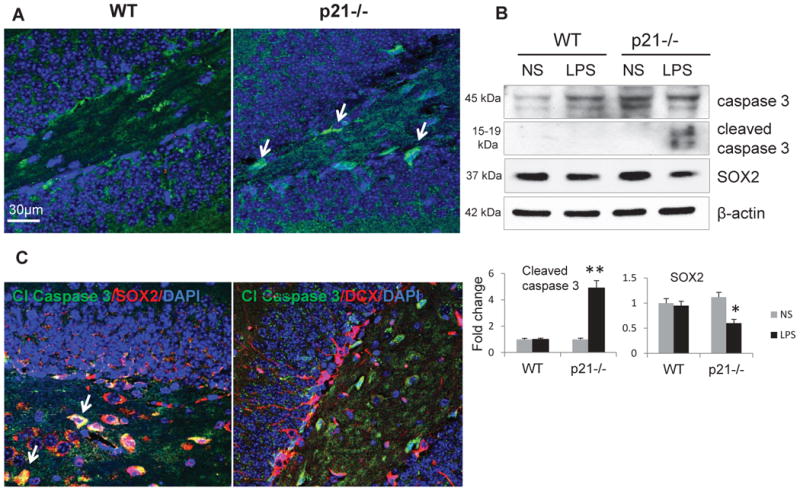
Acute systemic inflammation triggers apoptosis in the SGZ of p21-/- mice. A) The confocal image shows cleaved (Cl) caspase 3 (green, both nuclei and cytoplasmic) induction in the SGZ of p21-/- mice after LPS treatment; cells expressing cleaved caspase 3 are marked with arrows. B) Upper panel: Western blot analysis of cleaved caspase 3 and SOX2 after LPS treatment; representative blots are shown. Lower panel: intensities of protein bands were quantified from 3 independent experiments, normalized to β-actin and presented as fold change relative to control animals. Values represent mean ± SE; *, p<0.05; **, p<0.01; C) The confocal images showing co-localization of cleaved caspase 3 (green) with SOX2 (nuclear, red), but not with DCX (cytoplasmic, red) in the SGZ of p21-/- mice treated with LPS. Cells co-expressing both cleaved caspase 3 and SOX2 appear yellow and marked with arrows.
Next, we examined what types of cells undergo apoptosis in the LPS-treated p21-/- hippocampus. Double immunofluorescent staining for caspase 3-, and SOX2 or DCX revealed that cleaved caspase 3 was expressed in SOX2+ neuronal progenitors, but not in DCX+ neuroblasts (Fig. 4C). As SOX2 protein levels were decreased in these animals (Fig. 4B), the results suggest that systemic inflammation might lead to apoptosis of p21-deficient SOX2-positive early neuronal progenitors resulting in subsequent decrease in the number of more mature DCX+ and in double DCX+BrdU+ positive cells in p21-/- SGZ.
By inducing p21, IL 6 decreases neurogenesis and increases astrogliogenesis in NPC
Inflammation suppresses new neuron development (Ekdahl et al., 2003; Monje et al., 2003). As IL-6 is a part of LPS-induced inflammatory response (Carpentier and Palmer, 2009; Uematsu and Akira, 2006) and a key factor released by activated microglia (Kohman and Rhodes, 2013; Monje et al., 2003; Vallieres et al., 2002), we tested its direct effects on differentiation of hippocampal neuronal progenitors. NPC were isolated from WT hippocampus using the neurosphere assay as described (Pechnick et al., 2011). Sphere-forming floating neural progenitors were cultured in serum-free neurobasal A-medium under proliferating conditions for 7 days, then the spheres were collected and dispersed to individual cells, plated on polyornithine-covered culture dishes, and after 3 passages medium was changed to Differentiation Medium. Cells were allowed to differentiate for 8 days with no treatment (control) or in a presence of IL-6 (50 ng/ml). Under these differentiating conditions NPC lose nestin and SOX2, and primarily generate neuroblasts and astroglia (Gage, 2000; Morshead et al., 1994; Reynolds and Weiss, 1992). Cells were double-stained for βIII-tubulin, a marker of committed neuroblasts that is recognized by antibodies to Tuj-1, and for p21 or Ki-67. Another portion of cells was stained for GFAP, a marker for differentiated astroglia in this condition, and for p21 or Ki-67. The numbers of cells co-expressing Tuj-1, and p21 or Ki-67, or GFAP, and p21 and Ki-67 were counted in three separate experiments and a total 3,000 cells were counted for each antibody. p21 was expressed in 17 ± 2.1 % of Tuj-1+ control cells. However, after IL-6 treatment, the number of cells expressing p21 was increased to 27.3±3.3% (p<0.05), whereas the number of Ki-67+ proliferating cells was decreased by 50% (Fig. 5A). Confirming the in vivo observations (Pechnick et al., 2011), p21 was not detected in GFAP+ cells (astroglia) (not shown); and in these cells IL-6 treatment induced proliferation (the number of GFAP+Ki-67+ cells) ∼2-fold (p<0.05) (Fig. 5B), likely due to p21 absence. These results were supported by immunocytochemistry studies showing increased expression of p21 and concordant decreased expression of Ki-67 in Tuj-1+ cells after IL-6 treatment (Fig. 5C, D). In contrast, the number of proliferating GFAP+Ki-67+cells was increased by IL-6 (Fig. 5E). Thus, IL-6 triggers p21 expression in progenitors committed to a neuronal lineage, and p21 induction negatively correlates with proliferation rate in these cells. At the same time, IL-6 stimulates proliferation of GFAP+ cells that don't express p21.
Fig. 5.
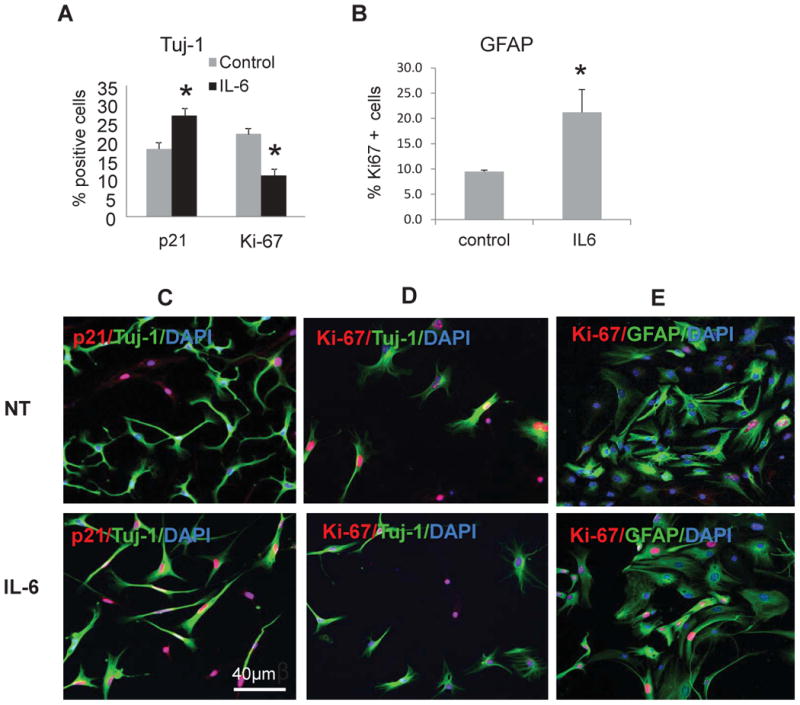
IL-6 decreases neurogenesis and increases astrogliogenesis in vitro. One thousand WT neuronal progenitor cells were plated and kept in differentiating conditions for 8 days. Cells were cultured in 24-well plates in triplicate. A) Cells were co-stained for Tuj-1, and p21 or Ki-67. The graph depicts the percentage of p21+ or Ki-67+ cells among Tuj-1+ neuroblasts; B) Cells were co-stained with GFAP and Ki-67. The graph depicts the percentage of Ki-67+ among GFAP+ cells. Counting was performed in triplicates (>500 cells per sample). Data are presented as a mean ± SEM, *, p<0.05. Three independent experiments were conducted, and representative results are shown; C-E) Representative confocal images of neuronal progenitors non-treated (NT) or treated with IL-6. C) The cells were stained for Tuj-1 (cytoplasmic, green) and p21 (nuclear, red). Note the increased number of p21 positive nuclei after IL-6 treatment; D) The cells were stained for Tuj-1 (cytoplasmic, green) and Ki-67 (nuclear, red). Note the decreased number of Ki-67 positive nuclei after IL-6 treatment; E) The cells were stained for GFAP (cytoplasmic, green) and Ki-67 (nuclear, red). Note the increased number of Ki-67 positive nuclei after IL-6 treatment.
To further confirm the role of p21 in neuroblast proliferation, we isolated neuronal progenitor cells from the hippocampus of p21-/- mice as described above, and compared them to WT cells. Equal amounts of cells from both genotypes were allowed to differentiate. As described above, we observed higher number of proliferating Tuj-1+ neuroblasts among control p21-/- as compared to WT cells (34.5±3.5 vs. 16.5 ±2.2%, p<0.01), and treatment with IL-6 led to marked decrease in a number of proliferating (Ki67+) cells among WT Tuj-1+ differentiating neuroblasts (from 16.5 ± 2.0% to 9 ± 2.2, p<0.01), but not in p21-/- Tuj-1+ cells (34.5 ± 3.5 to 32.5 ± 4.6 %) (Fig. 6A). Similar to Tuj-1, the number of GFAP+ Ki67+ cells was higher in control p21-/- cells (17.5 ±3 vs. 9.2 ±2.3 in WT, p<0.05). IL-6 treatment led to increased number of proliferating GFAP+Ki-67+WT cells (from 9.2 ±0.3 to 17 ±2.1, p<0.05) whereas no significant changes were observed in the number of GFAP+Ki-67+ p21-/- cells (17.5 ± 2.6 to 14 ± 2.6) (Fig. 6B). Thus, p21 deficiency results in an increase in both Tuj-1+ Ki-67+and GFAP+ Ki-67+ proliferating cells. While IL-6 restrains proliferation of WT neuroblasts, this cytokine fails to restrain proliferation of p21-/- neuroblasts. These results support our hypothesis that p21 induced in the course of inflammation, facilitates proliferation arrest of the progenitors destined to become neurons.
Fig. 6.
IL-6 did not affect proliferation in differentiating p21-/- neuronal progenitors. One thousand WT and p21-/- neuronal progenitor cells were plated in 24-well plates in triplicates, and kept in differentiating conditions for 8 days. A) Cells were co-stained for Tuj-1 and Ki-67. The graph depicts the percentage of Ki-67+ cells among Tuj-1+ neuroblasts. B) Cells were co-stained with GFAP and Ki-67. The graph depicts the percentage of Ki-67+ among GFAP+ cells. Counting was performed in triplicates (>500 cells per sample). Data are presented as a mean ± SEM, *, p<0.05. Three independent experiments were conducted, and representative results are shown.
Discussion
Adult neurogenesis is influenced by pathological conditions affecting the brain. Engagement of immune system-to-brain communication leads to the activation of resident microglia (Ekdahl et al., 2009). Microglia express a wide repertoire of Toll-like receptors that recognize bacterial products such as LPS (Okun et al., 2009), and activated microglia are a major source of pro-inflammatory cytokines (Ekdahl et al., 2009). Activated microglia can both diminish the production and survival of new neurons, or protect developing neurons from apoptosis depending on the duration of inflammation, levels of activation, and on the secreted cytokine profile (Butovsky et al., 2006; Chen et al., 2012; Ekdahl et al., 2003; Lazarini et al., 2012). Different groups demonstrated that systemic administration of LPS induced the expression levels of pro-inflammatory cytokine mRNA and protein in the brain (Dantzer et al., 2008; Laye et al., 1994; Quan et al., 1999; van Dam et al., 1992). Pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α, contribute to neurodegeneration after brain injury (Allan and Rothwell, 2001) or inflammatory stress (Venters et al., 2000). Our results support these observations and show that in both WT and p21-/- animals hippocampal cytokines including IL-1β, IL-6 and TNF-α, are all induced after LPS treatments. As p21 expression is stimulated by proinflammatory cytokines (Gartel and Radhakrishnan, 2005; Osawa et al., 2000; Scatizzi et al., 2009), it is likely that these cytokines activate p21 mRNA and protein expression observed in the hippocampus of LPS-treated WT animals. In addition, transcription factor Notch 1 is expressed in neuronal stem cells (Breunig et al., 2007); it is also known to suppress cell proliferation by stimulating p21 expression (Rangarajan et al., 2001). In response to cytokine signaling, Notch 1 can induce p21 in neuronal progenitors, suppressing neurogenesis and stimulating generation of astrocytes as it was demonstrated (Nagao et al., 2007). The specificity of p21 induction is evident from our data showing no effects of LPS on the expression of p27, which belongs to the same Cip1/Kip1 family of Cdk inhibitors.
We showed that p21 is expressed in early neuronal progenitors and neuroblasts (but not in mature neurons or astroglia) (Pechnick et al., 2011). In these cells, induced p21 might have a dual effect, keeping cells in quiescent state via G1 proliferation arrest (Sherr and Roberts, 1999), and protecting them from apoptosis (Gartel and Tyner, 2002). Indeed, confocal microscopy analysis revealed that at baseline or after treatment with normal saline, the numbers of DCX+ cells, and the number of DCX+ BrdU+ cells are greater in the SGZ of p21-/- mice than of WT animals, confirming our earlier observations of the increased number of DCX-positive neuroblasts in these mice (Pechnick et al., 2011; Pechnick et al., 2008).
In the WT SGZ, acute systemic inflammation resulted in decreased total number of BrdU+ cells, and of DCX+BrdU+ proliferating neuroblasts, likely due to the induced p21 expression. In contrast, in the SGZ of p21-/- mice, this treatment did not affect the number of proliferating BrdU+ cells which continued to be higher than in WT mice. However, LPS treatment resulted in a decreased number of DCX+ and double DCX+BrdU+ neuroblasts in p21-/- hippocampus. These results are in agreement with Western blot data showing decreased DCX protein levels in the hippocampus of p21-/-, but not of WT mice in response to LPS treatment. This reduction may be a consequence of decreased number of SOX2+ early neuronal progenitors, as SOX2 protein expression is markedly diminished in p21-/- hippocampus after LPS treatment. It is plausible that these cells (transient amplifying type 1 and type 2a progenitors) are undergoing apoptosis, evident by the appearance of cleaved caspase 3 in LPS-treated p21-/- hippocampus. In hippocampus, apoptosis occurs mostly in proliferating early neuronal progenitors (Biebl et al., 2000; Sierra et al., 2010). Indeed, confocal analysis confirmed that cleaved caspase 3 is expressed in SOX2-positive early neuronal progenitors, but not in DCX+ neuroblasts. We showed recently that p21 is expressed in SOX2 positive cells (Pechnick et al., 2011). Antiapoptotic properties of p21 were described in several cell types (Gartel and Tyner, 2002), and we observed the antiapoptotic effects of p21 in hippocampal neuronal progenitors in vivo (Pechnick et al., 2011). It is reasonable to suggest that, in the absence of p21, early progenitors become vulnerable to LPS-induced apoptosis and this results in decreased numbers of DCX+ and DCX+BrdU+ cells in p21-/- hippocampus after LPS treatment. p21 might also directly interact with and inactivate caspase 3, and its downstream apoptotic pathway (Suzuki et al., 1999), and when p21 is absent, caspase 3 is activated and apoptosis is induced. In p21-/- hippocampus, activation of cleaved caspase 3 in SOX2+ cells suggests that LPS triggers apoptosis of SOX2 positive early progenitors that are destined to become neuroblasts, resulting in the subsequent decrease in DCX+ cells. In WT hippocampus, up-regulated p21 not only arrest this cell proliferation, but also protects them from LPS-induced cell death. Although proliferation of DCX+ cells was decreased in WT SGZ after 5 days of LPS treatment, it was probably not enough time to change the total number of existing DCX+ cells detected in our experiments. In p21-/- mice, cleaved caspase 3 is expressed in Sox2 positive cells, but is not expressed in DCX positive cells. Cleaved caspase 3 eliminate Sox2+ cells, but a fraction of DCX+ cells (not expressing cleaved caspase 3) continue to proliferate because p21 is not induced in these cells. As an outcome, the total number of BrdU+, or BrdU+DCX+ cells in p21-/- mice represents a balance between increased number of proliferating DCX+ cells and decreased number of early Sox2+ cells that would not be able to progress to DCX+ stage.
Thus, systemic inflammation, and cytokines released by activated resident microglia can shift neurogenesis towards astrogliogenesis. These results are in agreement with the data showing decreased neurogenesis in human hippocampal progenitor cells in response to IL-1β treatment (Zunszain et al., 2012). However, when p21 is absent, these cells proliferate more, and likely undergo more apoptosis in response to inflammatory stimuli.
We showed previously that p21 plays a key role in early neuronal progenitor proliferation (Pechnick et al., 2011; Pechnick et al., 2008). Here we took advantage of in vitro model of neurogenesis allowing analysis of the role of p21 in inflammation-affected neuronal progenitor differentiation. As mentioned above, p21 is expressed in transient amplifying progenitors, where it is co-localized with Sox2 and nestin. Upon differentiation, GFAP+ cells do not express p21, whereas DCX+ and Tuj-1+ neuroblasts do (Pechnick et al., 2011). IL-6 is induced in hippocampus in response to LPS treatment (Dantzer et al., 2008; Ekdahl et al., 2003; Ekdahl et al., 2009; Monje et al., 2003). In WT cells, IL-6 treatment increases the number of Tuj-1+ neuroblasts expressing p21, and high levels of p21 limit these cells' proliferation evidenced by lower expression of a proliferation marker Ki-67. In contrast, the number of proliferating GFAP+ cells that don't express p21, was increased after IL-6 treatment. These results are in agreement with studies showing direct effects of IL-6 on adult NPC differentiation, activating astrocyte production and decreasing neuron production (Monje et al., 2003; Nakanishi et al., 2007). It is likely that IL-6-induced p21 increase in neuroblasts underlies these effects. Further support for our hypothesis comes from experiments where we compared WT and p21-/- cells NPC. In control p21-/- cells, proliferation rate of Tuj-1+ neuroblasts is higher than in WT Tuj-1+ cells; moreover, treatment with IL-6 does not affect Tuj-1+ p21-/- cell proliferation. These results underscore the important role of p21 in regulating proliferation of already committed neuroblasts.
In summary, during acute systemic inflammation, cytokines up-regulated in hippocampus, trigger p21 induction in cells of neuronal linage, where induced p21 limits proliferation of neuroblasts, protecting these cells from apoptosis. At the same time, proliferation of cells destined to become astroglia increases. Thus, LPS-induced GFAP expression in hippocampus might signal not only activated microglia, but also the increased number of astrocytes, as these cells don't express p21. These data strongly support our hypothesis that p21 is a key regulator of neuronal lineage during acute inflammation. Induced p21 and decreased neurogenesis may, at least in part, account for the mechanisms underlying clinical depression prevalent in patients with chronic inflammatory diseases (Dantzer et al., 2008; Williams and Steptoe, 2007).
Acknowledgments
The authors would like to thank Alex Ljubimov for his critical comments and help with editing the manuscript.
Funding: This work was supported by NIH Grant MH79988 and NARSAD Independent Investigator Award (to V.C).
Footnotes
Conflict of interest. The authors declare no conflict of interest.
References
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2(10):734–44. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24(3):623–31. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291(1):17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317(6):499–503. [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(51):20558–63. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31(1):149–60. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56(4):412–25. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64(1):79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012;32(34):11706–15. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Kovacs K, Castro AV, Zonis S, Melmed S. Pituitary hypoplasia in Pttg-/- mice is protective for Rb+/- pituitary tumorigenesis. Mol Endocrinol. 2005;19(9):2371–9. doi: 10.1210/me.2005-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, Villmann C, Hahnen E, Neuhuber W, Weigel D, et al. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010;133(11):3359–72. doi: 10.1093/brain/awq215. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Wang J, Samuels BA, Rainer Q, David I, Gardier AM, Hen R. Implications of the functional integration of adult-born hippocampal neurons in anxiety-depression disorders. Neuroscientist. 2010;16(5):578–91. doi: 10.1177/1073858409360281. [DOI] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2011 doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–34. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Dou QP, An B. RB and apoptotic cell death. Front Biosci. 1998;3:d419–30. doi: 10.2741/a288. [DOI] [PubMed] [Google Scholar]
- Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets. 2007;6(3):205–18. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56(3):140–5. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338(6103):72–5. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100(23):13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158(3):1021–9. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8(5):566–79. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103(21):8233–8. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008;10(2):59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65(10):3980–5. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1(8):639–49. [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13(7):717–28. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26(38):9703–12. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21(3):290–5. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain Behav Immun. 2013;27(1):22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr Opin Investig Drugs. 2009a;10(7):664–71. [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett. 2009b;456(1):39–43. doi: 10.1016/j.neulet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107(6):2669–74. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, et al. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64(9):721–8. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107(9):4436–41. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27(1):157–62. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Gabellec MM, Torquet N, Lledo PM. Early activation of microglia triggers long-lasting impairment of adult neurogenesis in the olfactory bulb. J Neurosci. 2012;32(11):3652–64. doi: 10.1523/JNEUROSCI.6394-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9):1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13(5):1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Nagao M, Sugimori M, Nakafuku M. Cross talk between notch and growth factor/cytokine signaling pathways in neural stem cells. Mol Cell Biol. 2007;27(11):3982–94. doi: 10.1128/MCB.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25(3):649–58. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59(2):278–92. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa Y, Hachiya M, Araki S, Kusama T, Matsushima K, Aoki Y, Akashi M. IL-1 induces expression of p21(WAF1) independently of p53 in high-passage human embryonic fibroblasts WI38. J Biochem. 2000;127(5):883–93. doi: 10.1093/oxfordjournals.jbchem.a022683. [DOI] [PubMed] [Google Scholar]
- Paxinos GFK. The Mouse Brain in Stereotaxic Coordinates. Academic Press; 2001. [Google Scholar]
- Pechnick RN, Zonis S, Wawrowsky K, Cosgayon R, Farrokhi C, Lacayo L, Chesnokova V. Antidepressants stimulate hippocampal neurogenesis by inhibiting p21 expression in the subgranular zone of the hipppocampus. PLoS ONE. 2011;6(11):e27290. doi: 10.1371/journal.pone.0027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V. p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci U S A. 2008;105(4):1358–63. doi: 10.1073/pnas.0711030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Stern EL, Whiteside MB, Herkenham M. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93(1-2):72–80. doi: 10.1016/s0165-5728(98)00193-3. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20(13):3427–36. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M, Gage FH. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci U S A. 1993;90(8):3602–6. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–5. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011 doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56(3):137–9. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104(11):4642–6. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM, Ruderman EM, Samways DS, Corbett JA, Egan TM, et al. The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol. 2009;39(3):820–5. doi: 10.1002/eji.200838683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315(5810):336–8. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–95. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–61. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Tsutomi Y, Miura M, Akahane K. Caspase 3 inactivation to suppress Fas-mediated apoptosis: identification of binding domain with p21 and ILP and inactivation machinery by p21. Oncogene. 1999;18(5):1239–44. doi: 10.1038/sj.onc.1202409. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr BUdR as an S-phase marker for quantitative studies of cytokinetic behaviour in the murine cerebral ventricular zone. J Neurocytol. 1992;21(3):185–97. doi: 10.1007/BF01194977. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med (Berl) 2006;84(9):712–25. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22(2):486–92. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588(2):291–6. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and Exercise: Past and Future Directions. Neuromolecular Med. 2008 doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venters HD, Dantzer R, Kelley KW. Tumor necrosis factor-alpha induces neuronal death by silencing survival signals generated by the type I insulin-like growth factor receptor. Ann N Y Acad Sci. 2000;917:210–20. doi: 10.1111/j.1749-6632.2000.tb05385.x. [DOI] [PubMed] [Google Scholar]
- Williams ED, Steptoe A. The role of depression in the etiology of acute coronary syndrome. Curr Psychiatry Rep. 2007;9(6):486–92. doi: 10.1007/s11920-007-0066-y. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16(3):296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2003;100(13):7925–30. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–49. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]


