Abstract
A human laboratory model of intravenous methamphetamine self-administration may facilitate study of putative treatments for methamphetamine addiction. We conducted a double-blind, placebo-controlled, between groups investigation of the acetylcholinesterase (AChE) inhibitor rivastigmine in non-treatment-seeking volunteers who met criteria for methamphetamine abuse or dependence. Safety and subjective effects data derived from days 1–10 of this protocol are described in a separate publication. In this report, we describe self-administration outcomes in participants randomized to treatment with rivastigmine (0 mg, N=7; 1.5 mg, N=6; 3 mg, N=9); data that were collected on days 11–15 of the inpatient protocol. On day 11, participants sampled two infusions of methamphetamine (0 and 30 mg, IV). On days 12–15, participants made ten choices each day to receive an infusion of either methamphetamine (3 mg, IV) or saline or a monetary alternative ($0.05 – $16). The study design allowed for evaluation of differences in behavior on days in which infusions were performed by the physician (experimenter-administered) versus by the participant using a PCA pump (self-administered), and when monetary alternatives were presented in either ascending or descending sequence. The data show that rivastigmine (1.5 and 3 mg), as compared to placebo, did not significantly alter total choices for methamphetamine (p=0.150). Importantly, the number of infusion choices was greater when methamphetamine was available then when saline was available (p<0.0001), and the number of money choices was greater when saline was available then when methamphetamine was available (p<0.0001). The total number of choices for methamphetamine was not altered as a function of a participant’s preferred route of methamphetamine use (p=0.57), and did not differ significantly whether they were experimenter-administered or self-administered (p=0.30). In addition, total choices for methamphetamine were similar made when money was available in an ascending versus descending sequence (p=0.49). The participants’ years of methamphetamine use, recent use of methamphetamine (in the past 30 days), or baseline craving (indexed here as “Desire”) on the day of the self-administration task was not predictive of number of choices for methamphetamine. In a subset of participants (N=8) for which data was available, individual does of methamphetamine (3×3 mg, IV) produced significant increases in positive subjective effects, and a preliminary analysis revealed that 3mg rivastigmine was associated with reductions in these responses, as compared to placebo. In summary, the current report indicates that there were no effects of rivastigmine on total choices for methamphetamine, that there were low levels of methamphetamine self-administration but these were 8 times greater than saline, and that choice behavior was insensitive to alternative reinforcers. In addition, we showed that rivastigmine may reduce the positive subjective effects produced by methamphetamine during self-administration.
Introduction
Methamphetamine abuse and addiction are significant public health problems, both in the U.S. and worldwide. At present, no medication has been shown to be effective as treatment for methamphetamine addiction, highlighting a critical need to identify and test potentially efficacious compounds. Though the gold standard for evaluating treatment efficacy is the randomized clinical trial, suitably powered trials are extremely expensive, signaling the need for more efficient approaches (Perkins et al. 2006). One alternative approach is provided by phase I/II trials, during which effects of medications are evaluated in participants with the target condition and markers thought to relate to efficacy are assessed. In addiction research, the most common markers are self-reported subjective effects. A complementary approach may be to evaluate the effects of a putative medication treatment on the reinforcing effects of the targeted drug of abuse using self-administration procedures (Fischman and Foltin 1992).
Several models of cocaine self-administration have been developed for use in animals (Ahmed and Koob 1999; Deroche et al. 1999) and in humans (Donny et al. 2003; 2004; Fischman and Foltin 1992; Fischman et al. 1990; Fischman and Schuster 1982; Haney et al. 1999; Haney et al. 1998; Haney et al. 2001; Higgins et al. 1994; Walsh et al. 2001; Ward et al. 1997a; b). Most recently, patient-controlled analgesia (PCA) pumps have been utilized in cocaine self-administration paradigms (Lynch et al. 2006; Sughondhabirom et al. 2005). Preclinical models of amphetamine and methamphetamine self-administration have also been developed (Clemens et al. 2006; Hiranita et al. 2006; Kitamura et al. 2006; Moffett and Goeders 2005; Shepard et al. 2004; Shepard et al. 2006; Winger et al. 1994; Woolverton et al. 1984).
In humans, amphetamine (Comer et al. 1996; de Wit et al. 1987; Mitchell et al. 1996) and methamphetamine (Hart et al. 2001) have been shown to self-administered by the oral route. De Wit and colleagues compared overweight and depressed participants to healthy controls and reported that most participants chose amphetamine more frequently then placebo, and that this was independent of diagnostic group. Comer and colleagues (Comer et al. 2001) showed that when given choices between placebo and 10mg amphetamine, participants chose amphetamine over placebo more than three-quarters of the time. Hart and colleagues (Hart et al. 2001) presented participants with eight blinded choices between $1 and oral doses of placebo, 5mg, and 10mg doses methamphetamine. On average, participants chose 4 doses of methamphetamine and 1 dose of placebo. Although these participants were experienced stimulant users, it appears that none met criteria for methamphetamine dependence, so the implications of these studies for addicted participants are unclear. Taken together, the above studies provided the basis for the development of a model of intravenous methamphetamine self-administration. Because methamphetamine use by the intravenous route is common, we reasoned that the development of such a model would facilitate study of methamphetamine addiction.
The context for the present report was a laboratory study of the effects of rivastigmine, an acetylcholinesterase (AChE) inhibitor, on the safety and subjective effects produced by methamphetamine. Acetylcholine (ACh) is involved in brain reward and learning functions, and perturbations to this neurotransmitter system may contribute to substance abuse disorders. For example, increasing ACh in the nucleus accumbens, by ablation of cholinergic neurons, attenuated the reinforcing effects of cocaine in mice (Hikida et al. 2003), and administration of nicotine and the AChE inhibitor donepezil attenuated reinstatement induced by exposure to methamphetamine cues and by administration of priming doses of methamphetamine in rats (Hiranita et al. 2006). The outcomes obtained in the laboratory study of rivastigmine on the safety and subjective effects produced by methamphetamine have been submitted elsewhere for publication. In brief, these data indicate that rivastigmine 3 mg significantly attenuated methamphetamine-induced increases in diastolic blood pressure, and self-reports of “anxious” and “desire”. The primary aim of this report, which involved the same participants, was to establish a model of intravenous methamphetamine self-administration in humans, and secondarily to evaluate the effects of rivastigmine on methamphetamine self-administration and subjective effects.
Materials and Methods
Participants
Participants were recruited through advertisements and paid for their participation. During screening, participants completed the Mini International Neuropsychiatric Interview (MINI), which is a short, structured diagnostic interview developed in 1990 by psychiatrists and clinicians in the United States and Europe for DSM-IV and ICD-10 psychiatric disorders (Sheehan et al. 1998). The MINI is the structured psychiatric interview of choice for psychiatric evaluation and outcome tracking in clinical psychopharmacology trials and epidemiological studies, and is the most widely used psychiatric structured diagnostic interview instrument in the world. This instrument was used to determine whether the subject met DSM-IV criteria for drug dependence and to rule out any major psychiatric disorders (e.g., affective disorders, schizophrenia). All participants met DSM-IV-TR criteria for methamphetamine abuse (N=4) or dependence (N=18), and were not seeking treatment at the time of study entry.
Following admission to the Clinical Research Center (CRC), participants completed a standard battery of assessments including measures of addiction severity and mood. Other inclusion criteria included age between 18 and 55, participants must have used methamphetamine via the smoked and/or intravenous routes, and they must have screened positive for methamphetamine prior to intake. In addition, participants must have had normal vital signs and laboratory findings. Exclusion criteria included a history of seizure disorder, head trauma, prior adverse reaction to methamphetamine, or the presence of any other Axis I psychiatric disorder or significant medical illness. Concomitant use of psychotropic medications, other than zolpidem for sleep or acetaminophen for pain, was not allowed. Exclusion criteria also included dependence on other drugs aside from nicotine. Lack of dependence on other substances was initially ascertained using a drug use questionnaire and these answers were judged against DSM-IV TR criteria.
This study was approved by the University of California at Los Angeles (UCLA) medical institutional review board and all participants gave informed consent after being fully informed about potential risks of participation.
Study Design
From days 1–10, infusions of methamphetamine (15 mg and 30 mg, IV) and placebo were given to ensure that participants tolerated the drug in a hospital setting. Randomization to rivastigmine (0, 1.5, or 3 mg, p.o.) occurred on day 7 and daily treatment continued through day 15. The preliminary safety and subjective effects data (comprising days 1–10) have been submitted elsewhere for publication.
The current report focuses on outcomes from self-administration experiments, which were conducted on days 11–15. An overview of the experimental procedure is shown in Table 1. Given that this was the first attempt to develop a human model of intravenous methamphetamine self-administration, we employed a variety of experimental approaches that have been previously applied with success in humans involved in cocaine self-administration experiments. Not knowing which approach would produce the most useful outcomes for methamphetamine, we simply sought to replicate the best features from several approaches, and these are described below.
Table 1.
Experimental Design
| Study Day | Session Type | Infusion Type | *Money Sequence |
|---|---|---|---|
| 11 | Sample | Experimenter | none |
| 12 | Choice | Experimenter | Ascending |
| 13 | Choice | Experimenter | Descending |
| 14 | Choice | Self | Ascending |
| 15 | Choice | Self | Descending |
Days 12/13 and 14/15 were counterbalanced among participants with respect to ascending vs. descending money sequence.
During the sample session, on day 11, participants received methamphetamine 0 mg (saline) and 30 mg under double-blind conditions at either 11:30 a.m. or 2:30 p.m. A 120 min time period between infusions was selected on the basis of our previous data (Newton et al. 2005b), which indicates that peak subjective and cardiovascular responses occur by this time. Concerns about potential “carryover effects” from one infusion period to another were also mitigated by counterbalancing conditions among participants, and by using a subtraction from baseline to determine actual effects (see data analysis).
Sample infusions were coded as either “red” or “green”. Participants were instructed to write down any positive or negative subjective effects produced by the infusion, and encouraged to reference these notes prior to and during subsequent choice sessions. Participants and investigators were blinded as to the coding of methamphetamine or saline as the red or green infusion. This coding was counterbalanced among participants, but kept constant throughout the duration of the study for each individual.
Choice sessions were held twice per day on days 12, 13, 14, and 15. On each day, one session was held at 11:30 a.m. and involved choices for methamphetamine or saline and the other was held at 2:30 p.m. and involved choices for the alternate condition (methamphetamine or saline). Participants made a series of choices between money options of increasing value or 1/10 of the red or green infusion from the day before (i.e. 3 mg methamphetamine or saline). At 2:30 p.m. participants completed identical procedures except that the counter-condition (red or green infusion) was available. The 10 monetary options included $.05, $.05, $.05, $.05, $1, $4, $7, $10, $14, and $16. All choices were separated by 15 min and a research assistant verbally prompted choices. If the participant chose money, the cash selected was immediately placed into an envelope within the participants view. If the participant chose an infusion, the study doctor performed this as a slow push over 2-min. On day 13 at 11:30, the participant participated in the same procedure as on day 12 except the participant was offered decreasing monetary options (starting at $16 and decreasing to the choices for $.05). On day 14 at 11:30, the participant performed the same procedure as on day 12 except that a PCA pump was used. In addition, the research assistant did not approach the participant every 15 min, rather the participant was told (at the beginning of the experiment) that they would have to make a choice on the PC tablet computer every 15 min. If the participant chose money, the cash selected was immediately placed into an envelope within the participants view. If the participant chose an infusion, he/she actuated the PCA pump him/herself. The PCA pump had a 13-min lock-out following the 2-min infusion, thus preventing repeated dosing prior to the completion of the 13 min lockout. On day 15 at 11:30 a.m., the procedure was similar to day 13 (with decreasing monetary options) and also incorporated the PCA pump procedure similar to day 14.
Subjective Measures
Subjective effects data were not collected for all participants (due to a lack of foresight to include it in the original human subjects application), but once appropriate approvals were obtained these data were acquired from the remaining participants (N=8) enrolled in the study. During the sample session, visual analog scale (VAS) forms were administered at baseline (T=−15) and at 5-min time points after each choice opportunity. Using the VAS participants reported the degree to which they felt “any drug effect”, “high”, “good effects”, “desire for methamphetamine”, “stimulated”, and “likely to use” on a continuous scale digitized between 0 to 100. Monetary value (in dollars) was also evaluated by asking “how much would you pay for the infusion you just received”.
Drugs
Rivastigmine
Rivastigmine is a carbamate derivative that inhibits both AChE and butyrylcholinesterase with equal potency and has selectivity for central activity (Williams et al. 2003). Rivastigmine has no affinity for muscarinic, adrenergic, dopaminergic, or opioid receptors (Bentue-Ferrer et al. 2003), but blocks voltage-activated potassium currents in dissociated rat hippocampal neurons (Pan et al. 2003). Rivastigmine is primarily degraded by cholinesterase-mediated hydrolysis with minimal cytochrome P450 metabolism. The decarbamylated metabolite resulting from hydrolysis undergoes N-demethylation and conjugation (Polinsky 1998). Following oral dosing, the plasma half-lives of rivastigmine and its primary metabolite are roughly 1 hour and 2 hours, respectively; however, cholinesterase inhibition lasts much longer (~10 hours) than the plasma half-life indicates.
Commercially available rivastigmine tablets (1.5 mg) were encapsulated in gelatin capsules by the UCLA research pharmacy, and placebo was prepared in a similar manner. Rivastigmine or matched placebo pills were administered orally at 7:00 a.m. and 7:00 p.m. (0+0 mg, 1.5+0 mg, or 1.5+1.5 mg) beginning on day 7 and continuing through day 15 of the protocol.
Methamphetamine
A NIDA contractor provided sterile methamphetamine solution for human use and a saline solution of equal volume and appearance was used as the control. An IND was obtained from the FDA for the use of rivastigmine and methamphetamine in this study. The half-life of methamphetamine is ~11–12 hours.
On day 11, methamphetamine (o or 30 mg, IV) was administered over 2 min using an infusion pump activated by a study physician. A single bolus of 30mg methamphetamine was used in the sample session since it has been associated with significant increases in positive subjective effects as well as increases in blood pressure and heart rate (Newton et al. 2005a; Newton et al. 2005b; Newton 2005; Newton et al. 2005c; 2006), though these changes are not accompanied by adverse events greater than observed after saline administration. The participants were made aware that the 30 mg dose reflected the maximum effects the participants could anticipate to achieve if they selected all 10 infusions during choice session.
On days 12, 13, 14 and 15, methamphetamine infusions (0 or 3 mg, IV) were administered over 2 min using a PCA pump activated by the participant. The 3 mg dosage for methamphetamine was selected as a safe increment that could be self-administered repeatedly and since previous data from our lab showed that 15 mg produced significant increases in positive subjective effects (Newton et al. 2005b). In addition, given that this was the first time intravenous self-administration procedures were being tested in our lab, we were encouraged by the UCLA IRB to not exceed the 30 mg dosage given previously as a bolus.
Given this information, the dosages and timing of drug administrations were deemed appropriate for this study.
Data analysis
Analysis of variance (ANOVA) was used to assess differences among the three rivastigmine treatment groups along demographic and drug use variables. A two-factor ANOVA was used to assess differences in total infusions taken as a function of rivastigmine dose (0, 1.5 and 3 mg) and methamphetamine dose (0 and 30 mg). Self-administration data were also analyzed according to the total amount of money chosen. This analytic approach was based upon that described previously by Walsh and Bigelow (Walsh et al. 2001).
Simple linear regression was used to evaluate three a priori selected factors hypothesized to account for methamphetamine choice behavior in the laboratory. These included years of use of methamphetamine, recent use of methamphetamine, and desire for methamphetamine 15 min prior to the start of the self-administration session.
Subjective effects (VAS) data were not collected for all participants and therefore unavailable for analysis. An exploratory analysis was conducted on data from a subset of participants (N=8) using a repeated measures ANOVA as a function of infusion choice (methamphetamine 3 mg vs. no infusion)× time (5, 20 and 35 min). In addition, we performed an initial analysis of the effects of rivastigmine dose on subjective effects using a one-way ANOVA at each time point. Significance was set at p<0.05. If significant findings were observed, post-hoc tests were conducted to identify specific differences across groups.
Results
Participant Characteristics
Detailed demographic information and drug use data are provided in Table 2. Thirty-one participants began the inpatient portion of the rivastigmine protocol, but only 22 completed the self-administration experiments. Seven were non-completers who were discharged prior to study drug randomization. The specific reasons included 1) cocaine-positive urine, 2) untreated asthma, 3) preexisting condition of AIDS, 4) irregular electrocardiogram, 5) chest pain, 6) methamphetamine-positive urine, and 7) high blood pressure occurring after 5-days of treatment with rivastigmine 3 mg. One additional participant completed the inpatient protocol, but chose not to participate in the self-administration sessions, and one participant completed only 2 of 4 self-administration days and his data were therefore excluded from analysis. As a result, the final sample size for this part of the rivastigmine study was 22.
Table 2.
Participant Characteristics
| Values represent mean ±S.E.M., *p<0.05 | Rivastigmine Groups | ||
|---|---|---|---|
| 0 mg (n=7) | 1.5 mg (n=6) | 3 mg (n=9) | |
| Gender | |||
| Male (%) | 100 | 100 | 78 |
| Female (%) | 0 | 0 | 22 |
| Ethnicity | |||
| White (Not Hispanic) (%) | 29 | 62 | 78 |
| Hispanic or Latino (%) | 43 | 25 | 22 |
| African American (%) | 14 | 12 | 0 |
| Asian American (%) | 14 | 0 | 0 |
| Age | 33.6±4.2 | 36.5±3.0 | 34.1±3.0 |
| Education | 12.3±0.4 | 13.8±0.7 | 13.3±0.8 |
| Substance Use | |||
| Methamphetamine | |||
| Years of Use | 6.7±1.3 | 7.2±2.2 | 8.7±2.6 |
| # Days Use Last 30 | 23.4±1.6* | 13.8±3.3 | 13.0±2.5 |
| Preferred Route of Use | |||
| Smoke (%) | 71 | 63 | 33 |
| Nasal (%) | 14 | 25 | 22 |
| Intravenous (%) | 14 | 13 | 22 |
| Oral (%) | 0 | 0 | 22 |
| Current Nicotine use (%) | 100 | 63 | 67 |
| Current Alcohol use (%) | 71 | 75 | 56 |
| Current Marijuana use (%) | 29 | 63 | 67 |
Current use percentages are a reflection of the number of participants in each treatment group who reported any use of that substance during the last 30 days.
ANOVA revealed that participants in the three dosage groups were statistically similar for all demographic and drug use variables, with the exception of recent methamphetamine use (F2,18=5.5, p=0.0139). Post-hoc analysis revealed that participants in the rivastigmine 0 mg group used significantly more methamphetamine in the last 30 days as compared to those in the 1.5 mg (p=0.0233) and 3 mg groups (p=0.0059). Despite this, a linear regression indicated that recent use of methamphetamine did not predict choices for methamphetamine on days 12–15 (r2=0.018, p=0.23).
Rivastigmine Does Not Alter Choices for Methamphetamine
As specified above, on each day (days 12, 13, 14 and 15) one session (at either 11:30 AM or 2:30 PM) involved 10 choices for methamphetamine and one session (the opposite time, at either 11:30 AM or 2:30 PM) involved 10 choices for saline. Considering mean number of infusions chosen each day (of a maximum of 10 for either methamphetamine or saline), there were no significant effects of rivastigmine dose (F2,165=0.86, p=0.4253), though one was present for methamphetamine dose (F1,165=48.3, p<0.0001), and no significant interaction of rivastigmine dose * methamphetamine dose (F2,165=1.9, p=0.150)(Figure 1). In addition, choices for an infusion were not altered by rivastigmine as a function of the monetary alternative (low [$0.05], medium [$1–7] or high [$10–16] dollar choices; data not shown). Given these outcomes, all other data were analyzed without respect to rivastigmine dose.
Figure 1.

Average total methamphetamine (filled bars) or saline (open bars) infusions selected. Data represent the mean (±s.e.m.) of choices from a maximum possible of 10 made by participants on each of all self-administration days (days 12, 13, 14 and 15). Choices are plotted with regard to rivastigmine dosage group (0 mg, N=7; 1.5 mg, N=6; 3 mg, N=9).
Characteristics of Methamphetamine Self-Administration
The majority of participants chose to self-administer methamphetamine in the laboratory. Taking into consideration all choice sessions and conditions, 18/22 participants (82%) chose at least one (but usually several) methamphetamine dose, though 4/22 participants (18%) did not choose any methamphetamine doses. Importantly, the data reveal that the average number of infusion choices (of 10 maximum) was greater when methamphetamine was available then when saline was available (3.32±0.36 vs. 0.41±0.16)(F1,169=55.0, p<0.0001), and that the average number of money choices (of 10 maximum) was greater when saline was available then when methamphetamine was available (9.59±0.16 vs. 6.67±0.36)(F1,169=55.4, p<0.0001). The mean number of choices for methamphetamine was not altered as a function of a participant’s preferred route of methamphetamine use (F3,81=0.68, p=0.57). Specifically, intravenous users chose 3.94±0.97 methamphetamine doses, oral users chose 4.57±0.84 methamphetamine doses, users who smoked chose 2.98±0.47 doses, and nasal users chose 3.1±0.93 doses. Total choices for methamphetamine did not differ significantly whether they were experimenter-administered (2.96±0.50) or self-administered (3.71±0.53)(F1,83=1.08, p=0.30). In addition, total choices for methamphetamine were similar when money was available in an ascending (3.57±0.54) versus a descending sequence (3.01±0.48)(F1, 83=0.48, p=0.49).
Considering monetary alternatives and methamphetamine dose more generally, the data reveal that when methamphetamine was available, participants selected an infusion over money if the monetary alternative was low (e.g., $0.05), but money was selected if the monetary alternative was high (e.g., $16). When saline was available, participants selected money in more than 95% of choices.
Predictors of Methamphetamine Self-Administration
Using linear regression, the data indicate that recent use of methamphetamine (r2=0.018, p=0.23), years of use of methamphetamine (r2=0.005, p=0.53), and self-reported “desire” for methamphetamine 15 min prior to the choice session on the day of the test (r2=0.057, p=0.11) were not associated with increased choices for methamphetamine in the self-administration procedure.
Reinforcing Effects of Individual Doses of Methamphetamine
Over the four-day choice session period, no participant chose all methamphetamine infusions that were made available. Across groups, the mean choices for methamphetamine was 3.2±0.4 out of 10 available per session. Given this relatively modest number of methamphetamine choices, an important question is whether 3 mg methamphetamine produced sufficient subjective effects that were distinguishable from saline or no infusion. Figure 2 shows the group means for subjective responses and monetary value from a subset of participants (N=8) recorded after three consecutive infusions of 3 mg doses of methamphetamine, as compared to three consecutive non-infusion choices. The optimal statistical comparison would have been change in subjective effects after methamphetamine versus saline infusions, yet in this subset of individuals, only 2 participants made choices for a saline infusion. Clearly, saline was not perceived as reinforcing by the majority of participants, and even these two individuals only chose saline infusions during the lowest monetary alternative ($0.05). At 5 and 20 min post-infusion of saline, both participants rated Any Drug Effect, High, Desire, Stimulated, and Monetary Value at zero. At 25 min post-infusion 1 of the participants ranked each of the VAS adjectives at 10 (out of 100) and the monetary value at $5. However limited, these data clearly indicate that saline was neither associated with positive subjective effects nor assigned a monetary value comparable to that produced by methamphetamine.
Figure 2.
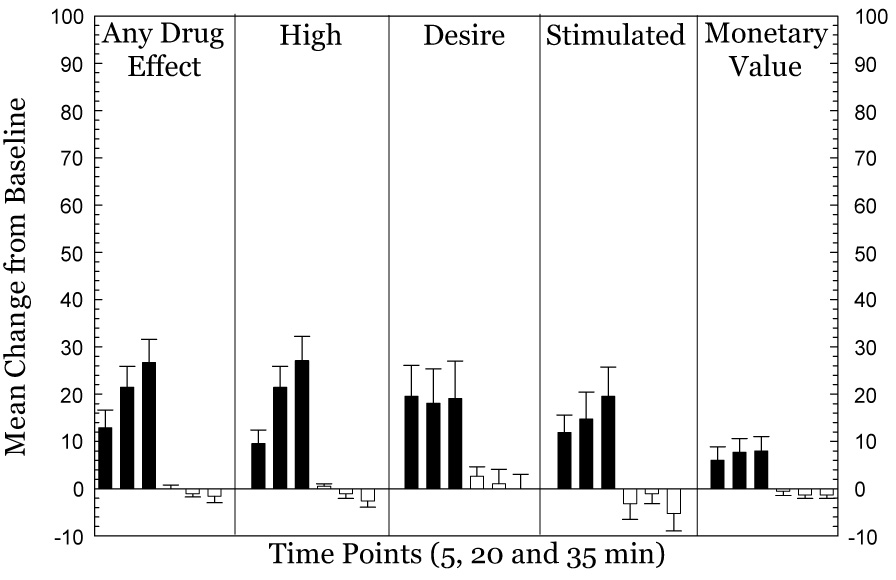
Increases in subjective effects and monetary value produced by 3 mg doses of methamphetamine (N=8). Data represent change from baseline after the first, second, and third dose of methamphetamine (filled bars) as compared to a non-infusion choice (open bars) during the self-administration procedures. Data are mean ± s.e.m.
In the subset of participants (N=8), subjective responses were documented 5 min after each infusion, and the data presented reflect mean responses obtained after each individual infusion or no infusion choice. The data reveal increases in Any Drug Effect, High, Desire, Stimulated, and Monetary Value after methamphetamine exposure. For Any Drug Effect, repeated measures ANOVA indicated a significant effect of infusion choice (3 mg methamphetamine vs. saline) (F1,38=23.5, p<0.0001), a significant effect for time course (F2,76=7.1, p=0.0015), and a significant interaction of infusion choice * time course (F2,76=11.3, p<0.0001). For High, repeated measures ANOVA indicated a significant effect of infusion choice (F1,38=25.1, p<0.0001), a significant effect for time course (F2,76=8.6, p=0.0004), and a significant interaction of infusion choice * time course (F2,76=17.2, p<0.0001). For Desire, repeated measures ANOVA indicated a significant effect of infusion choice (F1,38=5.3, p=0.027), no effect for time course (F2,76=0.32, p=0.73), and no interaction of infusion choice * time course (F2,76=0.15, p=0.86). For Stimulated, repeated measures ANOVA indicated a significant effect of infusion choice (F1,38=10.2, p=0.0028), no effect for time course (F2,76=0.96, p=0.39), and no interaction of infusion choice * time course (F2,76=3.1, p=0.0532). For Monetary Value, repeated measures ANOVA indicated a significant effect of infusion choice (F1,38=6.8, p=0.0129), no effect for time course (F2,76=0.75, p=0.4749), and a significant interaction of infusion choice * time course (F2,76=4.7, p=0.0124).
Because of the limited sample size (4, 1, and 3 participants randomized to rivastigmine doses 0, 1.5 and 3 mg, respectively), and outcomes from the main analysis indicating that rivastigmine did not alter overall choice behavior, rivastigmine dose was not originally included as a factor in VAS analyses. However, in a separate report, we showed that rivastigmine 3 mg attenuated some positive subjective effects produced by methamphetamine (O'Laco et al. 2006) and upon recommendation from one referee we performed a preliminary analysis of VAS outcomes with respect to rivastigmine dose. The preliminary data generally indicate that the positive subjective effects associated with methamphetamine infusions were reduced by rivastigmine 3 mg (Figure 3), though these did not reach statistical significance. Specifically, for Any Drug Effect, ANOVA did not indicate a significant effect at 5 min (F2,18=3.1, p=0.072), at 20 min (F2,18=0.68, p=0.52), or at 35 min (F2,18=0.86, p=0.44). For High, ANOVA did not indicate a significant effect at 5 min (F2,18=3.0, p=0.076), at 20 min (F2,18=0.50, p=0.62), or at 35 min (F2,18=0.45, p=0.65). For Desire, ANOVA did not indicate a significant effect at 5 min (F2,18=2.2, p=0.14), at 20 min (F2,18=3.3, p=0.06), or at 35 min (F2,18=3.0, p=0.076). For Stimulated, ANOVA did not indicate a significant effect at 5 min (F2,18=1.7, p=0.206), at 20 min (F2,18=2.7, p=0.096), or at 35 min (F2,18=1.38, p=0.28). Finally, for Monetary Value, ANOVA indicated a significant effect at 5 min (F2,18=3.8, p=0.042), though was not detected at 20 min (F2,18=3.49, p=0.053), or at 35 min (F2,18=2.2, p=0.142).
Figure 3.

Increases in subjective effects and monetary value produced by 3 mg doses of methamphetamine as a function of rivastigmine dose (0 mg-open bars, N=4; 1.5 mg-hatched bars, N=1; 3 mg filled bars, N=3). Data represent change from baseline after the first, second, and third dose of methamphetamine only. Data are mean ± s.e.m.
Discussion
The primary aim of this report was to establish a model of intravenous methamphetamine self-administration in humans and the outcomes indicate this was accomplished. The majority of participants chose to self-administer methamphetamine in the laboratory, and the data show that the number of infusion choices was ~8 times greater when methamphetamine was available as opposed to when saline was available. This outcome is similar to that obtained by Hart and colleagues (Hart et al. 2001), in which participants chose 4.4 doses of oral methamphetamine (10 mg) and 1.3 doses of placebo. In addition, the current finding coincides with reports in rodents and non-human primates showing that methamphetamine serves as a positive reinforcer (Anggadiredja et al. 2004; Clemens et al. 2006; Hiranita et al. 2006; Kitamura et al. 2006; Moffett and Goeders 2005; Shepard et al. 2006; Woolverton et al. 1984).
Self-report data (especially VAS “any drug effect”, but also verbal conversations with participants) indicate that these participants were able to discriminate the presence or absence of 3 mg methamphetamine subsequent to an intravenous infusion. This is not surprising, but given the small dose of methamphetamine utilized, it is worth emphasizing. Some have questioned the validity and usefulness of laboratory-based human experiments given that administration of methamphetamine in a laboratory environment may not duplicate the subjective effects experienced by users in a naturalistic environment. However, the current results demonstrate that methamphetamine-dependent participants report increases in positive subjective effects after exposure to low doses of methamphetamine, and these were similar in magnitude to that obtained by Hart and colleagues (Hart et al. 2001).
The data indicate that that the number of infusions selected was independent of whether the drug was self-administered or experimenter-administered. The intent was to examine the effect of perceived self-control over self-administration behavior.
When methamphetamine was available in the presence of low monetary alternatives, participants tended to choose an infusion over money. As the value of the monetary options increased, participants were more likely to select money rather than an infusion. When saline was available, participants selected the monetary alternative almost every time (>95%).
Of interest, participants exhibited similar patterns of responding when the monetary alternatives were presented in an ascending ($0.05→16.00) or descending ($16.00→0.05) sequence. These results differ from that observed in studies of cocaine users. Namely, Walsh and colleagues (Walsh et al. 2000) have shown that the sequence of money presentation influenced outcomes for self-administration of cocaine. In those studies, when individuals started choosing cocaine (because the monetary alternative was low), they tended to take all available infusions. However, if monetary alternatives started high, participants were more likely to choose money and therefore make fewer overall choices for cocaine during the session. In our study, however, when monetary alternatives were presented in the ascending sequence, a majority of participants were readily able to cease taking methamphetamine once the dollar amount reached medium dollar values ($1–7). Alternatively, when monetary alternatives were presented in the descending sequence, the participants exhibited a remarkable ability to wait a significant amount of time (2 hours) until the monetary values were sufficiently low ($0.05) before choosing methamphetamine. These results are inconsistent with the hypothesis that methamphetamine-addicted individuals exhibit impulsive and uncontrolled drug-taking behavior. This outcome was especially unexpected given that these individuals had agreed to forced abstinence from methamphetamine for almost two weeks (as part of the protocol), and yet remained capable of turning down a majority of opportunities to infuse their drug of choice.
Importantly, the participants’ preferred route of administration of methamphetamine in their natural environment did not influence the number of choices for intravenous methamphetamine in the laboratory. Specifically, participants who generally smoked methamphetamine, as compared to participants who generally used methamphetamine intravenously, did not self-report that intravenous methamphetamine was more reinforcing. These data suggest that preferred route of administration did not have a significant effect on behavior in this laboratory model of methamphetamine self-administration.
We hypothesized that participants who used greater quantities of methamphetamine recently, or for a greater number of years, would be more likely to choose methamphetamine in the laboratory. Yet, the data indicate that frequency and duration of self-reported use, which may be measures of severity of dependence, had no effect on self-administration behavior in the laboratory setting. In addition, baseline desire recorded 15 min prior to the self-administration procedure was not associated with increased choices for methamphetamine during that session. This result is consistent with previous research demonstrating that drug craving is not necessarily predictive of increased choices for cocaine in the laboratory (Johanson and Uhlenhuth 1980; 1981)Fischman et al., 1990; Leyton et al., 2005; Haney et al., 2006). However, it is important to note that self-reported craving for methamphetamine (Hartz et al., 2001) and cocaine (Weiss et al., 2003) among individuals in treatment programs has been shown to predict subsequent use during the following week. The discrepancy between desire and action in the current study may be due to multiple factors. First, the presence of alternative reinforcers (i.e. money) may outweigh initial levels of craving and attenuate drug-taking behavior, though we are unaware of published data that specifically support this hypothesis. Second, the laboratory setting is distinct from the environment users are accustomed to and this also likely influenced participants’ behavior.
A secondary aim of this report was to evaluate the effects of rivastigmine on choices for methamphetamine. The data indicate that rivastigmine (1.5 and 3 mg), as compared to placebo, did not significantly alter methamphetamine self-administration behavior. In a separate report (O'Laco et al. 2006), we showed that rivastigmine attenuated some positive subjective effects produced by methamphetamine; a result in apparent contrast to the findings shown here. This may not be too disconcerting since several research groups have shown a disconnect between outcomes for subjective effects (typically measured by VAS reports) versus reinforcing effects (measured by self-administration) produced by stimulants (Haney et al. 1999; Hart et al. 2001; Sofuoglu et al. 2000; Ward et al. 1997b). In terms of treatment implications, the reduction in methamphetamine-induced subjective effects by rivastigmine should not (O’Laco et al. 2006) be discounted in light of the current data showing no effects of rivastigmine on self-administration behavior. This recommendation is made given that choices for very low doses of methamphetamine in the laboratory (3 mg) are unlikely to equate with drug-taking behavior during outpatient clinical trials, and for this reason future experiments should investigate choice behavior for higher doses of methamphetamine. It is important to mention that subjective responses produced by 30 mg methamphetamine may be quite informative to outcomes in outpatient clinical trials, since our group has shown that bupropion (a DAT/NET inhibitor) attenuated methamphetamine-induced subjective responses in the laboratory (Newton et al. 2006), and this finding was substantiated in an outpatient trial in which bupropion treatment was associated with reduced methamphetamine use (Elkashef et al. 2007). Evaluation of methamphetamine choice behavior using other AChE inhibitors (e.g., donepezil) or compounds that alter ACh systems (e.g., varenicline) would help to determine whether the current findings should be generalized.
In this report, a preliminary analysis revealed that 3 mg rivastigmine, as compared to 0 mg, was associated with reductions in methamphetamine-induced subjective responses during the self-administration session. These new data coincide with the finding that rivastigmine attenuated positive subjective effects produced by methamphetamine 30 mg (O’Laco et al. 2006).
Some limitations of the current study should be highlighted. First, the connection between the sample dose (30 mg) and actual self-administration doses (3 mg) may have accounted for the reason that some subjects chose no methamphetamine. Newer designs employed in our laboratory have since made the sample sessions and choice sessions identical in the presentation of 3 mg dosages. Second, only a single dose of methamphetamine was tested in the choice sessions. This decision was based entirely on the realization by our IRB and medical staff that no research group had ever shown the effects of multiple repeated doses of intravenous methamphetamine. As a result, we proceeded with utmost caution. The preliminary outcomes obtained, however, will give us the opportunity to explore higher doses of methamphetamine in future experiments.
In summary, the current report indicates that there were no effects of rivastigmine on total choices for methamphetamine, that there were low levels of methamphetamine self-administration but these were 8 times greater than saline, and that choice behavior was insensitive to alternative reinforcers. In addition, we showed that rivastigmine may reduce the positive subjective effects produced by methamphetamine during self-administration. Finally, this laboratory model of methamphetamine self-administration may provide a method for identifying putative treatments prior to evaluation using costly randomized clinical trials technologies.
Acknowledgements
The authors thank Timothy Fong, M.D., Roger Donovick, M.D. and Gilles Fleury, M.D. for medical supervision of participants during this study. This work was supported by the National Institutes of Health (DA 18185, DA 14593, DA 17754, RR-00865).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–1478. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- Bentue-Ferrer D, Tribut O, Polard E, Allain H. Clinically significant drug interactions with cholinesterase inhibitors: a guide for neurologists. CNS Drugs. 2003;17:947–963. doi: 10.2165/00023210-200317130-00002. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, McGregor IS. Intravenous methamphetamine self-administration in rats: Effects of intravenous or intraperitoneal MDMA co-administration. Pharmacol Biochem Behav. 2006 doi: 10.1016/j.pbb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Comer SD, Haney M, Foltin RW, Fischman MW. Amphetamine self-administration by humans: modulation by contingencies associated with task performance. Psychopharmacology (Berl) 1996;127:39–46. doi: 10.1007/BF02805973. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Effects of repeated oral methamphetamine administration in humans. Psychopharmacology (Berl) 2001;155:397–404. doi: 10.1007/s002130100727. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. The reinforcing properties of amphetamine in overweight subjects and subjects with depression. Clin Pharmacol Ther. 1987;42:127–136. doi: 10.1038/clpt.1987.122. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug Alcohol Depend. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine self-administration in humans during abstinence: effects of dose, alternative reinforcement, and priming. Psychopharmacology (Berl) 2004;172:316–323. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Self-administration of cocaine by humans: a laboratory perspective. Ciba Found Symp. 1992;166:165–173. doi: 10.1002/9780470514245.ch10. discussion 173-80. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther. 1990;253:760–770. [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology (Berl) 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology (Berl) 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology (Berl) 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 2003;100:6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacology. 1980;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: repeated assessment of d-amphetamine. Pharmacol Biochem Behav. 1981;14:159–163. doi: 10.1016/0091-3057(81)90237-9. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sughondhabirom A, Pittman B, Gueorguieva R, Kalayasiri R, Joshua D, Morgan P, Coric V, Malison RT. A paradigm to investigate the regulation of cocaine self-administration in human cocaine users: a randomized trial. Psychopharmacology (Berl) 2006;185:306–314. doi: 10.1007/s00213-006-0323-5. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Laurent CL, de Wit H. Interaction of expectancy and the pharmacological effects of d-amphetamine: subjective effects and self-administration. Psychopharmacology (Berl) 1996;125:371–378. doi: 10.1007/BF02246020. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. Neither non-contingent electric footshock nor administered corticosterone facilitate the acquisition of methamphetamine self-administration. Pharmacol Biochem Behav. 2005;80:333–339. doi: 10.1016/j.pbb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Fong T, Chiang N, Holmes TH, Bloch DA, Anderson A, Elkashef A. A comprehensive assessment of the safety of intravenous methamphetamine administration during treatment with selegiline. Pharmacol Biochem Behav. 2005a;82:704–711. doi: 10.1016/j.pbb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav. 2005b;82:90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Newton TF, Reid MS, De La Garza R, Palamar J, Mahoney JJ, Fong T, Elkashef A, Mojsiak J, Chiang N, Anderson A. A double-blind, placebo-controlled assessment of potential interactions between intravenous methamphetamine and aripiprazole; Presented at the 2005 Annual Meeting of the Society for Neuroscience; 2005. [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005c;182:426–435. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- O'Laco E, De La Garza R, II, Mahoney JJ, III, Shoptaw S, Newton TF. A double-blind, placebo-controlled assessment of potential interactions between intravenous methamphetamine and rivastigmine: cardiovascular and subjective effects; Abstract presented at the 68th Annual Scientific Meeting of the College on the Problems of Drug Dependence; Scottsdale, AZ. 2006. [Google Scholar]
- Pan Y, Xu X, Wang X. Rivastigmine blocks voltage-activated K+ currents in dissociated rat hippocampal neurons. Br J Pharmacol. 2003;140:907–912. doi: 10.1038/sj.bjp.0705503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl) 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Clin Ther. 1998;20:634–647. doi: 10.1016/s0149-2918(98)80127-6. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology (Berl) 2006;185:505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Brown S, Babb DA, Pentel PR, Hatsukami DK. Carvedilol affects the physiological and behavioral response to smoked cocaine in humans. Drug Alcohol Depend. 2000;60:69–76. doi: 10.1016/s0376-8716(99)00143-x. [DOI] [PubMed] [Google Scholar]
- Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ, Self D, Jatlow P, Malison RT. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology (Berl) 2005 doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- Walsh SL, Haberny KA, Bigelow GE. Modulation of intravenous cocaine effects by chronic oral cocaine in humans. Psychopharmacology (Berl) 2000;150:361–373. doi: 10.1007/s002130000439. [DOI] [PubMed] [Google Scholar]
- Ward AS, Haney M, Fischman MW, Foltin RW. Binge cocaine self-administration by humans: smoked cocaine. Behav Pharmacol. 1997a;8:736–744. doi: 10.1097/00008877-199712000-00009. [DOI] [PubMed] [Google Scholar]
- Ward AS, Haney M, Fischman MW, Foltin RW. Binge cocaine self-administration in humans: intravenous cocaine. Psychopharmacology (Berl) 1997b;132:375–381. doi: 10.1007/s002130050358. [DOI] [PubMed] [Google Scholar]
- Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clin Ther. 2003;25:1634–1653. doi: 10.1016/s0149-2918(03)80160-1. [DOI] [PubMed] [Google Scholar]
- Winger GD, Yasar S, Negus SS, Goldberg SR. Intravenous self-administration studies with l-deprenyl (selegiline) in monkeys. Clin Pharmacol Ther. 1994;56:774–780. doi: 10.1038/clpt.1994.208. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Cervo L, Johanson CE. Effects of repeated methamphetamine administration on methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1984;21:737–741. doi: 10.1016/s0091-3057(84)80012-x. [DOI] [PubMed] [Google Scholar]


