Abstract
AIMS
To determine a brief, practical battery of tests that discriminate between children with a fetal alcohol spectrum disorder (FASD) and unexposed controls.
DESIGN
Children received dysmorphology exams, a targeted battery of cognitive and behavioral tests, and their mothers were interviewed about maternal risk factors. Children diagnosed with an FASD and children unexposed to alcohol prenatally were compared on cognitive/behavioral test results.
SETTING
A community in The Western Cape Province of South Africa.
PARTICIPANTS
Sixty-one, first grade children with FASD and 52 matched normal controls.
MEASURES
Statistical analyses of maternal drinking behavior and their child's test performance.
FINDINGS
Self-reported maternal drinking patterns before during and after pregnancy were used to confirm prenatal exposures to alcohol in the group of children diagnosed with FASD. With this sample of children diagnosed with FASD and completely unexposed controls, the adverse effects of maternal drinking on children's performance are reported. Results of the battery of standardized cognitive and behavioral tests indicate highly significant differences (p ≤ .001) between groups on: intelligence, perceptual motor, planning, and logical, spatial, short term, long term, and working memory abilities. Furthermore, a binary logistical regression model of only 3 specific cognitive and behavioral tests, including Digit Span A+B (Wald = 4.10), Absurd Situation (Wald = 3.57), and Word Association (Wald = 4.30) correctly classified 79.1% of the child participants as FASD or controls.
CONCLUSIONS
A brief, practical set of tests can discriminate children with and without FASD and provide useful information for interventions for affected children.
Keywords: fetal alcohol spectrum disorders (FASD), cognitive/behavioral testing, diagnosis, alcohol abuse
INTRODUCTION
Fetal Alcohol Spectrum Disorders (FASDs) have been the focus of collaborative studies in South Africa (ZA) for over 15 year and have contributed to an enhanced cross-cultural and population-based understanding of FASD. Because prenatal alcohol exposure impacts the developing fetus in a complex manner and the diagnosis of the spectrum of disorders is not straightforward, study of large groups of heavily alcohol-exposed children in ZA has advanced understanding of the range of affects on children and their ability to function effectively in the world. This article presents data from a study in the Western Cape Province (WCP) of ZA where a practical battery (commonly used and easily administered) of standardized tests was used to investigate the cognitive and behavioral patterns in a group of children diagnosed with FASD using the Revised IOM Diagnostic criteria (Hoyme et al, 2005)..
Since the diagnosis of fetal alcohol syndrome (FAS) was formalized and published in 1973 (Jones and Smith, 1973), further diagnostic delineation continues today. There is considerable agreement regarding important individual physical and behavioral components constituting the syndrome (Sokol and Clarren, 1989; Aase, 1994; Aase, et al., 1995; Stratton et al., 1996; Astley and Clarren, 2000; Chudley, 2005; Hoyme, et al. 2005).
According to the Institute of Medicine (IOM), children with FAS have a characteristic pattern of: 1) facial and body dysmorphology, 2) delayed physical growth and development, and 3) specific mental and behavioral deficits (Stratton et al.,1996). For a diagnosis of FAS, all three categories of problems must be present and the diagnosis should be made only after excluding other genetic and teratogenic anomalies (Hoyme, et al., 2005). All IOM-prescribed diagnoses of the FASD spectrum were utilized in this population-based study: FAS and partial fetal alcohol syndrome (PFAS), alcohol-related neurodevelopmental deficits (ARND), and alcohol-related birth defects (ARBD) (Warren et al., 2004).
Prenatal alcohol exposure can lead to brain damage resulting in cognitive and behavioral impairments. Specific deficit areas in the population of individuals exposed to alcohol prenatally (Conry, 1990; Kodituwakku, 2006), include: general intelligence (Streissguth et al., 2004; Mattson and Riley, 1998; Jacobson, et al., 2004; Bailey, et al., 2004), executive functioning (Kodituwakku, et al.1995, 2001; Kopera-Frye, et al., 1996; Mattson, et al., 1999(a); Schonfeld, et al.. 2006), information processing (Aragon, et al., 2008; Burden ,et al., 2005; Streissguth, 2007), attention (Steinhausen and Spohr,1998; Coles, et al., 1997; Coles, et al., 2002; Mattson, et al., 2006), language (both receptive and expressive) (Mattson, et al.. 2002; McGhee, et al., 2008; Janzen, 1995), learning and memory (Mattson and Riley, 1999(b); Roebuck, Spencer and Mattson, 2004; Kaemingk, et al., 2003), motor skills (Kalberg, et al., 2006; Korkman, 2003; Adnams, 2001), and behavior (Nash, et al., 2006; Whaley, et al., 2001; Thomas, et al., 1998; Bishop, et al., 2007).
Additionally, Mattson et al. (2010) indicated that tests of executive functioning and spatial processing distinguish children with prenatal alcohol-exposure and the physical features of FASD from children with no prenatal exposure. Aragon et al (2008) and Kodituwakku (2009) summarized the cognitive-behavioral phenotype associated with FASD as a generalized deficit in processing complex information, stemming from deficiencies in recruiting multiple regions of the brain to complete complex tasks.
South African FASD Epidemiology Studies
The first population-based study in this community produced the highest rates of FAS ever reported at that time, over 40.5 -46.4 per 1,000 (May, et al., 2000; Viljoen, et al., 2002). Two subsequent studies in this same community also found extremely high rates of FAS and PFAS 65.2 to 74.2 per 1,000 (Viljoen, et al., 2005; May, et al., 2005) and 68.0 to 89.2 per 1,000 (May, et al., 2007, 2008) as have other studies using similar methods in other ZA communities (Viljoen, et al., 2001; Urban, et al., 2008). These studies have raised many questions about the universal characteristics of FASD (Adnams, et al, 2001). For example, are deficits seen in children with FASD in ZA the same as deficits in children with FASD from other western countries? Adnams, et al, (2001) demonstrated that, while controlling for socioeconomic status, maternal depression, low parental education, violence and social disruption, children with FASD in a ZA community demonstrated a pattern of cognitive-motor deficits similar to those reported in the literature. This article summarizes testing results of a well-matched sub-sample of children with FASD and unexposed controls from a second, active-case ascertainment epidemiology study in a first grade cohort. The goal of the study was to define a battery of tests that discriminates between children with an FASD and unexposed controls. The rationale for the chosen battery was to determine a brief and practical set of cognitive and behavioral measures that identify the deficits common to all children with confirmed prenatal exposure to alcohol and a diagnosis of FASD and that are commonly used and available to school psychologists and developmental clinicians.
The hypothesis was that children with FASD perform poorly on tests that require greater mental effort and complex thinking. For example, cognitive planning tasks require conceptual set shifting and working memory, logical memory, and later recall. Although other studies have demonstrated that a set of neuropsychological measures can discriminate between exposed children and unexposed controls, the battery of tests used in many of those studies are extensive, quite time-consuming, expensive, and often are not readily available to those working in schools and communities (Mattson, 2010).
Many children affected with FASD are currently without appropriate educational and learning supports. Therefore, it is necessary to create bridges between the empirical evidence and real world practice to help the affected children. Evaluation of deficits for the affected children is the first step in determining a need for services. The field will benefit from empirically-determined evaluation guidelines for deficit areas of children with FASD. This study examines a battery of tests that are commonly available to school psychologists and developmental clinicians that target the known deficits in this population.
METHODS
Identifying Children with FASD in Schools
In the parent epidemiology study, 96% of all enrolled students in the targeted community's schools received guardian consent to participate. Screening and dysmorphology exams were completed for all consented children. After dysmorphology examinations, 92 children were developmentally tested and maternal prenatal risk factor questionnaires were completed. Those administering the developmental tests and the maternal interviewers were blinded to the reason for study entry and any results from the other study domains. Full methodology for the parent study is found in Viljoen, et al. (2005).
Child Control Group
Control children for the parent epidemiology study (n = 146) were randomly selected from consented first grade students enrolled in the same schools. Identical exams and testing were performed on subjects and potential controls (Viljoen, et al., 2005). After going through the examination and testing process and the selected child did not have an FASD, then he/she served as a control. Since some of the mothers of the normal controls were subsequently found to have consumed alcohol during the index pregnancy, and because prenatal alcohol exposure may impact performance on neuropsychological tests, a matching process was initiated to make sure that one completely unexposed control was matched to each child with an FASD by age (within 12 months) and sex. This necessitated recruitment of 25 additional, unexposed children who met age and sex criteria. These children were provided the same diagnostic exams and testing as the 36 children who originated from the random selection. This resulted in a sample of 61 seemingly unexposed controls; but, after a closer look at the maternal interviews, 9 of those 61 mothers drank some during pregnancy and after their children were eliminated, the result was the 52 unexposed control children reported on here.
Maternal Data for Cases and Controls
Available mothers of children with an FASD and controls were interviewed. Fifty-three of the 61 mothers of the FASD children included in this study were interviewed. For the remaining 8, prenatal alcohol consumption data were obtained via collateral sources (May, et al, 2005, May, et al., 1983; Streissguth, et al., 1985). Maternal data presented here focus primarily on confirmation of maternal drinking for case assessment/diagnosis. A detailed profile of maternal risk factors for FASD in this community are reported elsewhere (Viljoen, et al., 2002; May, et al., 2005; 2008). Structured maternal interviews contained items covering: reproduction; alcohol use before, during and after the index pregnancy; socio-economic status (SES); demographic variables; diet and nutrition; and physical status of the mother. Protocols utilized drinking questions in a timeline follow-back methodology (Sobell, et al., 1988; 2001) to elicit accurate reporting of alcohol consumed. All interviews were administered by experienced, Afrikaans-speaking interviewers.
Case Conferences for Final Diagnoses
Final diagnoses for all children (cases and controls) were made at a case conference based on dysmorphology exams, developmental testing, and maternal interviews documenting alcohol exposure and other risk factors (see Hoyme, et al., 2005; Viljoen, et al, 2005; May et al., 2000; 2007).
Test Battery
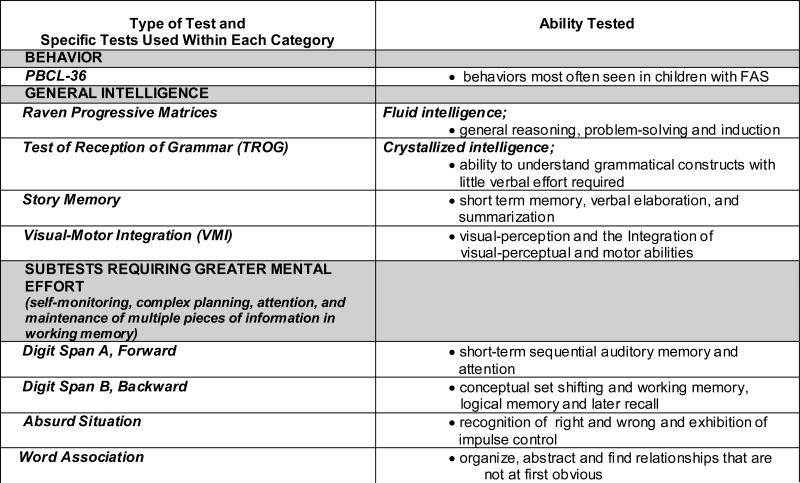
Intelligence tests, perceptual motor tests, planning tests, and logical, spatial, short term, long term, and working memory tasks were selected (see figure 1). The tests are normed and standardized except for the Personal Behavior Checklist, a frequently used checklist of children with FASD (Streissguth, 1998). The battery of standardized tests chosen and administered measure abilities shown to be affected in prenatally-exposed children, yet are generally available and practical to use by school and clinic staff.
Figure 1.
Testing Battery and Ability tested
Intellectual Abilities
The test battery selected included a measure of fluid intelligence, The Raven Progressive Matrices (Raven, 1947), for children 6 through 16 years measuring a person's ability to form perceptual relations and reason by analogy. This nonverbal test of intelligence was chosen for this South African population to offer a fluid intelligence measure that minimized the verbal comprehension load on the subject. The Raven has been used extensively for studies in diverse cultures because of the culturally-fair aspect of the measure (Sizemore and Amler, 1996; Sattler, 2001; Chiappedi, et al., 2012; Edwards, et al,, 2010). The Test for the Reception of Grammar (TROG) (Bishop, 1989), measures a child's ability to understand grammatical constructs using a multiple choice format which requires a minimal effort from the child. This is desirable for this student population for it provides an opportunity for the child to demonstrate his/her understanding of grammatical constructs separate from verbal abilities.
In addition, selected subtests from the Junior South African Individual Scales (JSAIS) (Robinson, 1989) were administered: Story Memory, Absurd Situation, Word Associations and Digit Span (forward and backward). The JSAIS was developed to provide a profile of an English or Afrikaans speaking child's cognitive abilities. The battery is standardized for school-aged Coloured, Afrikaans-speaking children from 6 years to 8 years, 11 months, making it very applicable for these children. The selected subtests were chosen to target empirically-based deficit areas in prenatally exposed children. It was hypothesized that these subtests tax the brain by requiring the involvement of multiple brain regions to successfully complete abstract reasoning, logical thinking, and simple memory/working memory tasks.
Motor and Visual-Perceptual Abilities
The Beery-Buktenica Developmental Test of Visual-Motor Integration (VMI) (Beery et al., 2003) was used to assess visual and motor integration abilities and evaluate visual perception. The VMI is a non-verbal test that is used among diverse environmental, educational, cultural, and linguistic groups.
Behavior
Behavior was assessed using the Personal Behavior Checklist (PBCL-36, Teacher Report Form) translated into Afrikaans (Streissguth et al, 1998). The PBCL-36 is a checklist developed to assess the behavior of children with FASD. The 36 questions resulted from the most commonly reported behaviors among families of children with FAS. The tool is not standardized, but it has adequate test-retest reliability.
Statistical Analyses
Data were analyzed using SPSS Version 19.0 (SPSS Inc., Chicago Ill, 2010). Chi square tests were performed on categorical level data, and one-way analysis of variance tests on interval level data, as noted in Tables 1, 2 and 3. Although we used standardized scores for the analysis, we also ran an analysis of covariance controlling for age to make certain our group significant differences were not accounted for by the age differential (see Table 3). Binomial logistic regression was used to determine which tests predict classification in either the FASD or Control group (see Table 4).
Table 1.
Child Physical and Dysmorphology Comparisons: FASD Cases versus Controls.
| Child Variables | FASD Children (n = 61)a | Control Children (n = 52) | Statistical Test | df | p |
|---|---|---|---|---|---|
| Sex (%) | |||||
| Males | 45.9 | 44.2 | |||
| Females | 54.1 | 55.8 | X2 = .03 | 1 | 1.000 |
| Age (months) | |||||
| Mean (SD) | 78.5 (7.6) | 88.3 (11.0) | F = 30.81 | 1/111 | .000 |
| Height (cm) | |||||
| Mean (SD) | 108.5 (4.6) | 119.8 (7.9) | F = 88.79 | 1/111 | .000 |
| Weight (kg) | |||||
| Mean (SD) | 15.7 (1.7) | 22.5 (5.1) | F = 93.61 | 1/111 | .000 |
| Occipital Circumference (OFC; in cm) | |||||
| Mean (SD) | 48.1 (1.3) | 51.3 (1.6) | F = 130.5 | 1/111 | .000 |
| Palpebral Fissure Length (cm) | |||||
| Mean (SD) | 2.25 (.12) | 2.54 (.18) | F = 97.16 | 1/108 | .000 |
| Smooth Philtrumb (%) | 78.7 | 34.7 | X2 = 21.76 | 1 | .000 |
| Narrow Vermilion Borderc (%) | 70.5 | 6.1 | X2 = 46.27 | 1 | .000 |
| Total Dysmorphology Score Mean (SD) | 17.1 (4.5) | 5.2 (4.3) | F = 196.03 | 1/108 | .000 |
Includes 8 ARND cases, 16 PFAS cases, and 37 FAS cases.
Score of 4 or 5 on Astley's Lip Philtrum Guide.
Score of 4 or 5 on Astley's Lip Philtrum Guide.
Table 2.
Maternal/Paternal Risk Factor Comparisons: FASD Cases versus Controls.
| Maternal/Paternal Variables | Mothers of FASD Children (n = 61) | Mothers of Control Children (n = 52) | Statistical Test | df | p |
|---|---|---|---|---|---|
| Drinking before index pregnancy (%) | 85.4 | 4.1 | X2 = 65.00 | 1 | .000 |
| Drinking Indicator - overall reported drinking during pregnancy (%) | 92.0 | 0.0 | X2 = 84.21 | 1 | .000 |
| Average No. drinks per day (during pregnancy) | 5.4 | 0.0 | F = 52.16 | 1/93 | .000 |
| Consumed 3 drinks or more per occasion during pregnancy (%) | 68.0 | 0.0 | X2 = 50.75 | 1 | .000 |
| Consumed 5 drinks or more per occasion during pregnancy (%) | 60.0 | 0.0 | X2 = 42.18 | 1 | .000 |
| Current drinker in last year (%) | 76.0 | 4.1 | X2 =53.16 | 1 | .000 |
| Drank during trimesters (%) | |||||
| 1st | 92.0 | 0.0 | X2 = 84.21 | 1 | .000 |
| 2nd | 92.0 | 0.0 | X2 = 83.23 | 1 | .000 |
| 3rd | 88.0 | 0.0 | X2 = 74.73 | 1 | .000 |
| Father's data | |||||
| Fathers of Index Children with drinking problems in past (%) | 35.7 | 3.4 | X2 = 10.23 | 1 | .001 |
TABLE 3.
Mean Standardizeda Scores on Developmental and Behavioral Indicators of Children with FAS versus Controls: Wave II.
| Child Variables | FASD Mean(SD) N = 61 | Controls Mean(SD) N = 52 | Test Score | d.f. | p | ANCOVA controlling for age Does test performance vary significantly by child's age? |
|---|---|---|---|---|---|---|
| Developmental Traits | ||||||
| Verbal Abilityb | 6.6 (9.5) | 20.3 (20.7) | F = 21.57 | 1/110 | .000 | No; F = .616, p = .434 |
| Non-Verbal Abilityc | 13.0 (12.8) | 24.2 (19.5) | F = 13.28 | 1/111 | .000 | No; F = .318, p = .574 |
| PBCL-36 | 14.2 (8.1) | 6.7 (6.4) | F = 21.96 | 1/94 | .000 | No; F = .304, p = .583 |
| VMI | 68.0 (12.4) | 79.3 (19.3) | F = 14.10 | 1/111 | .000 | No; F = .3.32, p = .071 |
| Digit Span A, Total Score | 5.8 (2.2) | 7.6 (2.3) | F = 18.29 | 1/111 | .000 | No; F = .995, p = .321 |
| Digit Span B, Total Score | 1.1 (1.4) | 2.1 (1.7) | F = 11.17 | 1/111 | .001 | No; F = 1.23, p = .270 |
| Digit Span AB, Scaled Score | 6.9 (2.9) | 10.3 (3.2) | F = 20.79 | 1/66 | .000 | No; F = ..293, p = .590 |
| Absurd Situation, Scaled Score | 55.6 (13.1) | 69.3 (15.7) | F = 25.20 | 1/111 | .000 | No; F = .926, p = .340 |
| Word Association, Scaled Score | 60.2 (14.9) | 69.2 (13.6) | F = 11.00 | 1/111 | .001 | No; F = .290, p = .592 |
| PBCL-36: Teacher-Rated | 12.3 (7.5) | 6.3 (5.9) | F = 21.10 | 1/110 | .000 | No; F = .1.01, p = .316 |
| Story Memory, Scaled Score | 65.9 (18.6) | 70.9 (21.8) | F = 1.01 | 1/66 | .318 | No; F = .062, p = .805 |
| Total Dysmorphology Score** | 17.1 (4.5) | 5.2 (4.3) | F = 196.03 | 1/111 | .000 | |
| Head Circumference (OFC) | 48.1 (1.3) | 51.3 (1.6) | F = 130.35 | 1/111 | .000 |
All scores standardized for age of child at time of testing.
Tests of the Reception of Grammar (TROG) percentile score used.
Raven Colored Progressive Matrices percentile score used.
Table 4.
Binary Logistic Regression of Cognitive and Behavioral testing to Predict Classification in FASD vs Control group.
| Variable | Wald | df | p |
|---|---|---|---|
| Digit Span A+B, scaled score | 3.90 | 1 | .048 |
| Absurd Situation, scaled score | 4.73 | 1 | .030 |
| Word Association, scaled score | 6.85 | 1 | .009 |
R2 = .36 (Cox & Snell), .48 (Nagelkerke). Model X2(3) = 30.10, p < .001
Overall ability of model to correctly classify participants as FASD or Control = 77.6%
RESULTS
Sample
Sixty one children with an FASD diagnosis and 52 non-exposed controls were used for this analysis. Of the 61 affected children, 37 or (60.7%) were FAS, 16 or (26.2%) were PFAS, and 8 or (13.1%) were ARND (See Table 1); 28 were male (45.9%) and 33 were female (54.1%), with an average age of 73 months. The average age of the 52 unexposed control group was 82 months and 23 were male (44.2%) and 29 were female (55.8%).
Table 1 compares physical and dysmorphology variables between FASD and control children. With the exception of sex, all variables discriminate between FASD and Control children at p ≤ .001.
Table 2 compares self-reported alcohol use by mothers of FASD children with mothers of control children. Each maternal/paternal risk factor significantly discriminates between mothers of FASD children and of control children as well as drinking before and after the index pregnancy, average number of drinks per day and drinking by each trimester, and fathers’ drinking problems.
Table 3 compares FASD and control children on developmental and behavioral tests. With the exception of the Story Memory Mental Age score, all neuropsychological test results are significant, including TROG, Raven non-verbal ability, and PBCL-36 personal behavior.
Binary logistic regression was performed to determine the cognitive and behavioral test variables that best discriminate between participants in the FASD and control groups. The Wald statistic, which is based on a z score, is used to test the significance of each predictor variable in the model (Table 4) showing the relative contribution of each. A model containing Digit Span A+B, absurd situation, and word associations correctly classified 77.6.1% of the child participants as either FASD or control.
DISCUSSION
Defining a battery of standardized tests that is useful for the FASD population is a vital goal; however, it is of particular importance in ZA and other under-served populations where resources are scarce and research has shown FASD to be both prevalent and severe. Most classrooms in ZA have multiple numbers of affected children enrolled, and children with an FASD can benefit from classroom and learning support (Adnams, 2008). The determination of needed supports begins with an accurate evaluation of each child's learning strengths and challenges.
Using maternal interview data, we were able to compare the testing results of definitively diagnosed children with those who were not exposed to alcohol at all. The battery of tests used was comprehensive, yet not lengthy or cumbersome. We included measures of verbal and nonverbal intelligence, visual-motor perception, behavior, and select subtests of the JSAIS felt to be discriminating for this population. With only one exception, the difference between the two groups was highly significant on all testing measures.
For the JSAIS subtests, The Absurd Situation, Word Association, and Digit Span subtests all discriminated well between the groups. The Absurd Situations subtest requires the subject to look at a picture and recognize what is going on in the picture. Each picture has incongruity that the subject is required to determine. In order to do this accurately, the subject must perceive detail correctly requiring alertness, attention and concentration, inference, knowledge of science, geography, and social understanding. Conceptually, the subject must have some understanding of what is right and wrong and must exhibit impulse control in order to correctly respond (Sattler, 2001).
To be successful with the Word Associations subtest, the subject must perceive the common elements of the paired words and bring those elements together into a concept. This measures concept formation and the ability to place objects and events together in meaningful groups (Lyon, 1995). In order to do this, the subject must organize, abstract, and find relationships that are not at first obvious. Memory is involved and success can be related to interests of the subjects and cultural opportunities.
Finally, Digit Span forward and Digit Span backward are considered separately as they tap into different abilities. Digit Span forward involves sequential processing and short-term memory. Digit Span backward involves planning, sequential processing and working memory (Borkowsi and Burke, 1996, Duncan et al., 2012). Working memory requires the subject to hold information in mind, manipulate the information into a different sequence or form before responding; requiring great mental effort and complex thinking.
The Story Memory subtest did not discriminate well between affected and unaffected children. The Absurd Situations, Digit Span, and Word Associations subtests, on the other hand, did discriminate well; they require more complex cognitive processes which is described by a number of researchers as a hallmark of children with an FASD (Aragon, et al., 2008, Kodituwakku, et al., 2006; Kodituwakku,2009; Duncan, et al, 2012).
The binary logistic regression analysis revealed that the JSAIS subtests of word associations, absurd situation, and digit span correctly classified 77.6% of the subjects with an FASD. These types of subtests are embedded not only in the JSAIS but within most well-normed, easily accessible, and widely-used intelligence tests such as the Wechsler Scales (Wechsler, 2003) and can therefore be used in a sensitive fashion for other populations. It is important to note the limitations of this study. Although we attempted to administer a comprehensive, yet feasible battery of tests, there were some aspects of performance that were not or were minimally evaluated. For example, the battery did not include a testing tool that looked at mathematical ability, a known area of difficulty for children affected by prenatal alcohol exposure. In addition, we did not include a comprehensive measure of behavior. Evaluation and assessment for appropriate educational programming should also include measures of academic performance and behavior. It would, therefore, be recommended that a comprehensive measure of academic performance and a behavioral measure be paired with this proposed battery of tests.
CONCLUSION
It is possible to utilize a practical and readily available set of evaluation tools to determine a learning profile for children diagnosed with an FASD. In most school districts in the USA and ZA, evaluations are conducted to better understand an individual child's strengths and areas of need. Well-known, frequently used, comprehensive tools and subtests measuring the domains of executive functioning, information processing, abstract reasoning, and complex thinking can be used effectively to evaluate children with a diagnosis within FASD to determine if the child is indeed struggling with tasks that require higher order, more complex cognitive processes. This assessment information could assist the schools in providing the appropriate learning supports for the affected child.
Acknowledgements
Appreciation goes to the National Institutes of Health (NIH), specifically the National Institute on Alcohol Abuse and Alcoholism (NIAAA) who funded this project via RO1 AA 09440, RO1/UO1 AA11685, UO1 AA14786, and RO1 AA15134.
Additional thanks go to Loretta Hendricks whose tireless work locating and transporting children for the study was instrumental to its success. Also, the authors would like to acknowledge the professional input from Ansie Kitching, Rubin Adams, and Piyadasa Kodituwakku.
REFERENCES
- Aase JM. Clinical recognition of FAS: difficulties of detection and diagnosis. Alcohol Health and Research World. 1994;18:5–9. [PMC free article] [PubMed] [Google Scholar]
- Aase JM, Jones KL, Clarren SK. Do we need the term “FAE”? Pediatrics. 1995;95:428–430. [PubMed] [Google Scholar]
- Adnams CM, Kodituwakku P, Hay A, Molteno C, Viljoen D, May PA. Patterns of Cognitive-Motor Development in Children with FAS from a Community in South Africa. Alcoholism Clinical and Experimental Research. 2001;25:557–562. [PubMed] [Google Scholar]
- Annett M. The growth of manual preference and speed. British Journal of Psychology. 1970;61:545–558. doi: 10.1111/j.2044-8295.1970.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Aragon AS, Kalberg WO, Buckley D, Barela-Scott LM, Tabachnick BG, May PA. Neuropsychological study of FASD in a sample of American Indian children: Processing simple versus complex information. Alcoholism Clinical and Experimental Research. 2008;32:2136–2148. doi: 10.1111/j.1530-0277.2008.00802.x. doi: 10.1111/j.1530-0277.2008.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong EM. Conceiving Risk, Bearing Responsibility: Fetal Alcohol Syndrome and the Diagnosis of Moral Disorder. The Johns Hopkins Press; Baltimore, Maryland: 2003. [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of Fetal Alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Bailey BN, Delaney-Black V, Covington CY, Ager J, Janisse J, Hannigan JH, Sokol RJ. Prenatal exposure to binge drinking and cognitive and behavioral outcomes at age 7 years. American Journal of Obstetrics and Gynecology. 2004;191:1037–1043. doi: 10.1016/j.ajog.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Beery EB, Buktenica NA, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration. 5th Edition. Psych Corp. Pearson Publishing; 1997. [Google Scholar]
- Bishop DVM. Test for Reception of Grammar. Age and Cognitive Performance Research Centre. University of Manchester; Manchester: 1989. [Google Scholar]
- Bishop S, Gahagan S, Lord C. Re-examining the core features of autism: a comparison of autism spectrum disorder and fetal alcohol spectrum disorder. Journal of Child Psychology and Psychiatry. 2007;48:1111–1121. doi: 10.1111/j.1469-7610.2007.01782.x. doi: 101111/j.1469-7610.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- Borkowski JG, Burke JE. Theories, models, and measurements of executive functioning: An information processing perspective. In: Lyon GR, Krasnegor NA, editors. Attention, memory and executive function. Paul H. Brookes; Baltimore: 1996. pp. 235–261. [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcoholism Clinical and Experimental Research. 2005;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Conry J, Cook JL, Lock C, Rosales T, LeBlanc N. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. Canadian Medical Assocoation Journal. 2005;172:S1–S21. doi: 10.1503/cmaj.1040302. doi: 10.1503/cmaj.1040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. New England Journal of Medicine. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Faek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcoholism Clinical and Experimental Research. 1997;21:150–161. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcoholism Clinical and Experimental Research. 2002;26:263–271. [PubMed] [Google Scholar]
- Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcoholism Clinical and Experimental Research. 1990;14:650–655. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Duncan J, Schramm M, Thompson R, Dumontheil I. Task rules, working memory, and fluid intelligence. Psychon Bull Rev. 2012;19:864–870. doi: 10.3758/s13423-012-0225-y. doi 10.3758/s13423-012-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, Flak E, Li Z, Wang S, Rauh V, Perera F. Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbons and Children's Intelligence at 5 Years of Age in a Prospective Cohort Study in Poland. Environmental Health Perspectives. 2010;118:1326–1331. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisses J, Ager JW, Greenwald MD, Delaney-Black V. A 14-Year Retrospective Maternal Report of Alcohol Consumption in Pregnancy Predicts Pregnancy and Teen Outcomes. Alcohol. 2010;44:583–594. doi: 10.1016/j.alcohol.2009.03.003. doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen LA, Nanson JL, Block GW. Neuropsychological evaluation of preschoolers with fetal alcohol syndrome. Neurotoxicology and Teratology. 1995;17:273–279. doi: 10.1016/0892-0362(94)00063-j. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcoholism Clinical and Experimental Research. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth AP. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the Fetal Alcohol Syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Mulvaney S, Tanner Halverson P. Learning following prenatal alcohol exposure: Performance on verbal and visual multi-trial tasks. Archives of Clinical Neuropsychology. 2003;18:33–47. [PubMed] [Google Scholar]
- Kalberg WO, Provost B, Tollison SJ, Tabachnick BG, Robinson LK, Hoyme HE, Trujillo PM, Buckley D, Aragon AS, May PA. Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcoholism Clinical and Experimental Research. 2006;30:2037–2045. doi: 10.1111/j.1530-0277.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- King AC. Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. American Journal of Public Health. 1994;84:294–296. doi: 10.2105/ajph.84.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcoholism Clinical and Experimental Research. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Kalberg WO, May PA. The effects of prenatal alcohol exposure on executive functioning. Alcohol Research and Health. 2001;25:92–198. [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku P, Coriale G, Fiorentino D, Aragon AS, Kalberg WO, Buckley D, Gossage JP, Ceccanti M, May PA. Neurobehavioral characteristics of children with fetal alcohol spectrum disorders in communities from Italy: Preliminary results. Alcoholism Clinical and Experimental Research. 2006;30:1551–1561. doi: 10.1111/j.1530-0277.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Neurocognitive profile in children with fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15:218–224. doi: 10.1002/ddrr.73. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera-Frye K, Dehaene S, Streissguth AP. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 1996;34:1187–1196. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kettunen S, Autti-Ramo I. Neurocognitive impairment in early adolescence following prenatal alcohol exposure of varying duration. Child Neuropsychology. 2003;0:117–128. doi: 10.1076/chin.9.2.117.14503. [DOI] [PubMed] [Google Scholar]
- Lyon MA. A comparison between WISC-III and WISC-R scores for learning disabilities reevaluations. Journal of Learning Disabilities. 1995;28:253–255. doi: 10.1177/002221949502800407. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism Clinical and Experimental Research. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis D, Jones KL, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism Clinical and Experimental Research. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. Journal of International Neuropsychology in Society. 1999;5:462–471. doi: 10.1017/s1355617799555082. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20:361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcoholism Clinical and Experimental Research. 2002;26:875–882. [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Auti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP, the CIFASD Toward a Neurobehavioral Profile of Fetal Alcohol spectrum Disorders. Alcoholism Clinical and Experimental Research. 2010;34:1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hymbaugh KJ, Aase JM, Samet JM. Epidemiology of Fetal Alcohol Syndrome among American Indians of the Southwest. Sociology and Biology. 1983;30:374–385. doi: 10.1080/19485565.1983.9988551. [DOI] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. American Journal of Public Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais A-S, Hendricks LS, Croxford JA, Viljoen DL. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. American Journal of Public Health. 2005;95:1190–1199. doi: 10.2105/AJPH.2003.037093. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug and Alcohol Dependence. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcoholism Clinical and Experimental Research. 2008;32:738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- McGhee CL, Bjorkquist OA, Riley EP, Mattson SN. Impaired language performance in young children with heavy prenatal alcohol exposure. Neurotoxicology and Teratology. 2008;31:71–75. doi: 10.1016/j.ntt.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash K, Rovet J, Greenbaum R, Fantus E, Nulman I, Koren G. Identifying the behavioral phenotype in fetal alcohol spectrum disorder: sensitivity, specificity and screening potential. Archives of Women's Mental Health. 2006;9:181–6. doi: 10.1007/s00737-006-0130-3. [DOI] [PubMed] [Google Scholar]
- Palmer C. Fetal alcohol effects – incidence and understanding in the Cape. South African Medical Journal. 1985;68:779–80. [PubMed] [Google Scholar]
- Raven JC. Raven coloured progressive matrices. Oxford Psychologists Press; Oxford: 1947. [Google Scholar]
- Robinson M. In: Junior South African Individual Scales (JSAIS) Robinson M. Pretoria., editor. Human Sciences Research Council; South Africa: 1989. [Google Scholar]
- Roebuck-Spencer TM, Mattson SN. Implicit strategy affects learning in children with heavy prenatal alcohol exposure. Alcoholism Clinical and Experimental Research. 2004;28:1424–1431. doi: 10.1097/01.alc.0000139826.25247.5b. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children, cognitive applications. Fourth Ed. Jerome M. Sattler, Publisher, Inc.; 2001. [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O'Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychology. 2006;12:439–452. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Sizemore 0, Amler R. Characteristics of ATSDR's adult and pediatric environmental neurobehavioral test batteries. Neurotoxicology. 1996;17:229–236. [PubMed] [Google Scholar]
- Sobell LC, Agrwal S, Annis H, Ayala-Velasquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, Zioikowski M. Cross cultural evaluation of two drinking assessment instruments: Alcohol timeline follow back and inventory of drinking situations. Substance Use and Misuse. 2001;36:313–331. doi: 10.1081/ja-100102628. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinker's reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sokol RF, Clarren SK. Guidelines for use of terminology describing the impact of prenatal alcohol on the offspring. Alcoholism Clinical and Experimental Research. 1989;13:597–598. doi: 10.1111/j.1530-0277.1989.tb00384.x. [DOI] [PubMed] [Google Scholar]
- SPSS 19.0 . Command Syntax Reference. SPSS Inc.; Chicago Ill.: 2010. [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC. Fetal alcohol syndrome diagnosis, epidemiology, prevention, and treatment. Institute of Medicine. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Steinhausen H-C, Spohr H-L. Long-term Outcome of Children with Fetal alcohol Syndrome: Psychopathology, Behavior, and Intelligence. Alcoholism Clinical and Experimental Research. 1998;22:334–338. doi: 10.1111/j.1530-0277.1998.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Clarren SK, Jones KL. Natural history of the Fetal Alcohol Syndrome, A ten-year follow-up of eleven patients. Lancet. 1985;2:85–92. doi: 10.1016/s0140-6736(85)90189-8. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Press S, Sampson PD. A fetal alcohol behavior scale. Alcoholism Clinical and Experimental Research. 1998;22:325–333. doi: 10.1111/j.1530-0277.1998.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O'Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Streissguth AP. Offspring effects of prenatal alcohol exposure from birth to 25 years: The Seattle prospective longitudinal study. Journal of Clinical Psychology in Medical Settings. 2007;14:81–101. [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcoholism Clinical and Experimental Research. 1998;22:528–533. [PubMed] [Google Scholar]
- Urban M, Chersich MF, Fourie LA, Chetty C, Olivier L, Viljoen D. Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: prevalence and risk factors. South African Medical Journal. 2008;98:877–882. [PubMed] [Google Scholar]
- Viljoen DL, Craig P, Hymbaugh K, Boyle C, Blount S. Fetal Alcohol Syndrome - South Africa, 2001. Morbidity and Mortality Weekly Report. 2003;52:660–662. [PubMed] [Google Scholar]
- Viljoen DL, Croxford J, Gossage JP, May PA. Characteristics of mothers of children with Fetal Alcohol Syndrome in the Western Cape Province of South Africa: a case control study. Journal of Studies on Alcohol. 2002;63:6–17. [PubMed] [Google Scholar]
- Viljoen DL, Gossage JP, Adnams CM, Jones KL, Robinson LK, Hoyme HE, Snell C, Khaole N, Asante KK, Findlay R, Quinton B, Brooke LE, May PA. Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. Journal of Studies on Alcohol. 2005;66:593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Bellugi U. Evidence from 2 genetic syndromes for dissociation between verbal and visual-spatial short-term-memory. Journal of Clinical and Experimental Neuropsychology. 1994;16:317–322. doi: 10.1080/01688639408402641. [DOI] [PubMed] [Google Scholar]
- Warren K, Floyd L, Calhoun F, Stone D, Bertrand J, Streissguth A, et al. Consensus statement on FASD. National Organization of Fetal Alcohol Syndrome; Washington, D.C.: 2004. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—4th Edition (WISC-IV®) Harcourt Assessment; San Antonio, TX: 2003. [Google Scholar]
- Whaley SE, O'Connor MJ, Gunderson B. Comparison of the adaptive functioning of children prenatally exposed to alcohol to a non-exposed clinical sample. Alcoholism Clinical and Experimental Research. 2001;25:1018–1024. [PubMed] [Google Scholar]