Abstract
Background. In this study, the radiosensitizing effect of resveratrol as a natural product was investigated on cell toxicity induced by 131I in thyroid cancer cell. Methods. Human thyroid cancer cell and human nonmalignant fibroblast cell (HFFF2) were treated with 131I and/or resveratrol at different concentrations for 48 h. The cell proliferation was measured by determination of the percent of the survival cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Results. Findings of this study show that resveratrol enhanced the cell death induced by 131I on thyroid cancer cell. Also, resveratrol exhibited a protective effect on normal cells against 131I toxicity. Conclusion. This result indicates a promising effect of resveratrol on improvement of cellular toxicity during iodine therapy.
1. Introduction
Radioiodine-131 (131I) as a radioactive iodine is widely used for treatment of patients with thyroid diseases, such as thyroid cancer and Graves' disease. It emits beta particles and has a physical half-life of 8.02 days [1]. DNA damage and chromosomal breaks are main reasons for cell damage and death. Reactive oxygen species (ROS) are generated by 131I [2, 3]; these toxic products can attack critical macromolecules, such as DNA, leading to cell damage and death [4, 5]. However, 131I concentrates at high level in thyroid tissue with a high target to nontarget ratio which is perfect for thyroid cancer therapy; it has side effects, such as sialadenitis, haematological depression, xerostomia, and radiation thyroiditis [6–11]. Several studies have shown that genetic damage is increased in patients after 131I therapy, with the frequency of micronuclei being elevated [12–14]. The occurrence of secondary malignancies and leukaemia might increase with higher radioactive iodine doses [7]. Thus, protection of normal cells may mitigate side effects induced by 131I. Resveratrol is a natural polyphenol compound that is found in fruits, such as grapes. Several pharmacological properties were reported for resveratrol, such as neuroprotective, chemosensitive, anti-inflammatory, anticancer, antitumourigenic, chemopreventive, and antioxidant actions [15–20]. Recently we showed that resveratrol protected genotoxicity induced by 131I on normal human lymphocytes; it significantly reduced the DNA damage induced by 131I in vitro [21]. Sebastià reported that resveratrol protected human lymphocytes against genotoxicity induced by gamma radiation [22]. Scavenging of free radicals is proposed as the main mechanisms for protective effects of resveratrol [23, 24]. However, resveratrol exhibited protective effects on cellular toxicity induced by beta particle on normal cells; its effect is unclear on thyroid cancer cell during iodine-131 therapy. To further explore the beneficial effects of resveratrol, the aim of this study was to investigate its therapeutic effects on cell death induced by 131I in thyroid human cancer and human nonmalignant fibroblast cells in vitro.
2. Materials
2.1. Chemicals
Resveratrol (RSV) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma (USA). 131I-Na in sterile solution was prepared by AEOI, Tehran, Iran, and was used freshly.
2.2. Cell Culture
Human thyroid cancer (Thr.C1-PI 33) and human nonmalignant skin fibroblast (HFFF2) cells were got from the Pasture Institute of Iran. These cells were cultured at 37°C and 5% CO2 in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Paisley, UK) supplemented with 10% fetal bovine serum (FBS) and 100 μg/mL penicillin-streptomycin (Gibco). Experiments on cells were performed in the exponential growth phase.
2.3. Cell Antiproliferation Assay
Untreated and treated thyroid cancer and HFFF2 cells were subjected to cell proliferation assay using MTT to quantify the metabolic activity to cleave tetrazolium salts [25, 26]. Cells (20,000) were seeded in 96-well plates. After 24 h incubation, cells were treated with various concentrations of RSV (0.5, 5, 10, and 50 μg/mL) and incubated for 48 h at 37°C and 5% CO2. RSV was dissolved in ethanol and diluted with medium. After 48 hours of culture, 20 μL MTT (5 mg/mL in phosphate buffer saline) was added to each well, and culturing was continued for 4 hours. Then, culture supernatant was discarded and replaced by DMSO, and the cell plates were shaken for 10 minutes. The absorbance of every culture well was read on an ELISA Reader (Bioteck, USA).
2.4. Irradiation Protocol
Cells were seeded in 96-well plates. After 24 h incubation, cells were treated with various concentrations of RSV (0.5, 5, 10, and 50 μg/mL) and incubated for 2 h at 37°C and 5% CO2. After incubation, the solution of 131I was added at dose 10 μCi in 100 μL to each well and incubated for 48 h. MTT assay was performed according to above protocol.
2.5. Statistical Analysis
Data were presented as mean ± standard deviation (SD) of three independent experiments. Data were compared with student t-test and the differences were considered significant if the P value < 0.05.
3. Results
3.1. Effect of Resveratrol on Cell Proliferation in Thyroid Cancer and HFFF2
Effects of RSV on cell proliferation in thyroid cancer and HFFF2 were determined by MTT assay. Thyroid cell proliferation was significantly inhibited by RSV at concentrations 10 and 50 μg/mL (P < 0.02). A statistical difference between concentrations of RSV at doses 5 and 50 μg/mL was observed. RSV exhibited a reduction of 12% in cellular growth in thyroid cells when cells were treated with 10 and 50 μg/mL of RSV. Figure 1(a) shows the percentage of cell proliferation in the thyroid cancer cells treated by RSV. In the comparison of cancer cell, human nonmalignant fibroblast cell (HFFF2) was used for any effect of RSV on cell proliferation. RSV did not cause significant cellular toxicity in HFFF2 cell (Figure 1(b)).
Figure 1.
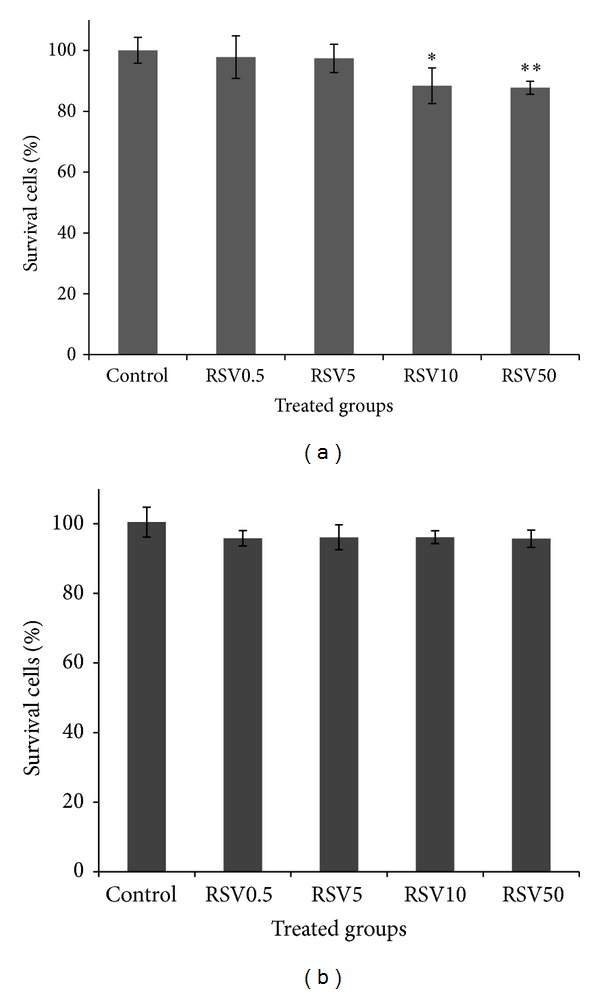
Effect of resveratrol (RSV) at different concentrations (0.5, 5, 10, and 50 μg/mL) on thyroid cancer cells (a) and nonmalignant fibroblast cell (HFFF2) (b). Cell proliferation was assayed with MTT test (n = 4). *P < 0.05, comparing RSV10 with control. **P < 0.05, comparing RSV50 with RSV5.
3.2. Effect of Resveratrol and 131I on Cell Proliferation in Thyroid Cancer and HFFF2
Figure 2 shows the combination effect of RSV and 131-iodine on percentage of cell proliferation in control, RSV-pretreated, and/or 131I in thyroid cancer and HFFF2 cells. 131I significantly reduced survival rate in thyroid cancer cell by 87%. Thyroid cancer cell proliferation was significantly reduced in RSV treated groups. RSV significantly reduced percentage of cell survival to 60% and 63% at concentrations 5 and 10 μg/mL, respectively. These results indicate that RSV has synergetic effects with 131I on inhibition of cell growth on thyroid cancer cell. A radiosensitive effect by RSV in thyroid cancer cells treated with 131I was observed. It is interesting that RSV was not shown any enhancement of toxicity on HFFF2 cell in combination with 131I. RSV exhibited an increase of cell growth in combination with 131I in HFFF2 cells at concentrations of 0.5, 10, and 50 μg/mL when these RSV treated groups were compared to 131I alone (P < 0.05).
Figure 2.
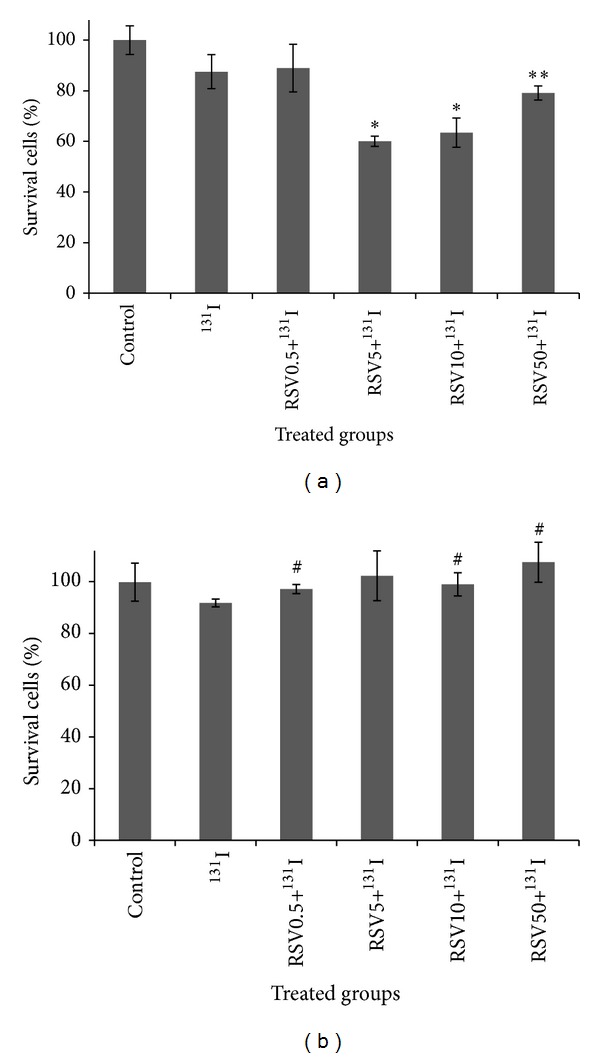
Effect of resveratrol (RSV) at different concentrations (0.5, 5, 10, and 50 μg/mL) in combination with 131I on thyroid cancer cells (a) and nonmalignant fibroblast cell (HFFF2) (b). Cell proliferation was assayed with MTT test (n = 4). *P < 0.05, comparing RSV5 and RSV10 with 131I. **Nonsignificant, comparing RSV50 and 131I. # P < 0.05, comparing RSV0.5, 10, and 50 with 131I.
4. Discussion
In this study, RSV exhibited a radiosensitizing effect on thyroid cancer cell; it reduced cell growth in combination with 131I. RSV increased cell growth in nonmalignant fibroblast cell (HFFF2) treated with 131I. Then RSV exhibited a radiosensitive effect on cancer cell and radioprotective effect on normal cell. This dual effect of RSV is dependent on type of cell. Radioactive iodine-131 (131I) is widely used for the treatment of thyroid-related diseases. 131I is taken up almost exclusively by thyroidal tissue, and high-dose radioiodine treatment is associated with limited side effects such as sialadenitis and xerostomia. Patients suffer from these side effects. Pharmacological treatment can be a promising strategy for protecting patients from side effects induced by 131I therapy [3]. Recently we showed RSV significantly protected human lymphocytes from genotoxicity induced by 131I. RSV reduced micronuclei frequency in lymphocytes in combination with 131I [21]. In this study we tried to evaluate the effects of RSV on thyroid cancer cells, because it is hypothesised that RSV may have a protective effect on thyroid cancer cells, which will be contraindicated in thyroid cancer therapy with 131I. Our results indicate that RSV has radiosensitizing effects on thyroid cancer cell and radioprotective effects on normal cells against cellular toxicity induced by 131I. These results are promising for using of this natural product in 131I therapy in patients. Resveratrol has been shown to have several biological properties such as antioxidant activity, induction apoptosis in cancer cells, and inflammation [27–29]. RSV induced apoptosis in several cancer cells such as human colorectal and bladder cancers. This effect was through activation of caspase and regulation of the Akt/Bcl-2 signaling pathway [30, 31]. RSV sensitized colon cancer cell lines to 5-fluorouracil treatment on the increase of apoptotic effect and exhibited stronger antitumor effect [32]. In our study, RSV significantly sensitized thyroid cancer cell to 131I at concentrations 5 and 10 μg/mL, while this cellular toxicity was not increased at higher concentration 50 μg/mL. RSV maximally enhanced cell death induced by 131I at concentration 5 μg/mL. RSV probably has tumor cell toxicity through activation or inhibition of cellular signal pathways, which was established in other studies. RSV induced apoptosis in thyroid carcinoma cells; it acts via a Ras-MAPK kinase pathway to increase p53 expression [28, 33–35]. RSV suppressed anaplastic thyroid carcinoma cell growth via S-phase cell-cycle arrest and apoptosis; it induced functional Notch1 protein expression and activated the pathway by transcriptional regulation [36]. Also, resveratrol increased iodide trapping in FRTL-5 cells, iodide influx, and rNIS protein level even in the absence of TSH. These mechanisms may contribute to enhancement of cell toxicity through increasing of 131I uptake by thyroid cancer cells [37]. Antioxidant activity against cellular oxidative stress is one of the main mechanisms related to protection of RSV. RSV directly scavenges reactive oxygen species produced by oxidative stress and it is probably related to the presence of hydroxyl groups on the chemical structure of RSV [38, 39]. Resveratrol strongly prevented C6 cells from H2O2-induced toxicity by modulating glial, oxidative, and inflammatory responses. Resveratrol increased heme oxygenase 1 (HO1) expression and extracellular GSH content [40]. Ionizing radiation produces free radicals that damages macromolecules such as DNA leading to cell death in normal tissues. RSV protected normal cells against genotoxicity induced by ionizing radiation. Antioxidant activity is a main mechanism for radioprotective of RSV in normal cells [21, 41]. Also resveratrol inhibits IL-1β expression induced by radiation via the activation of Sirt1; this mechanism participates in radioprotection [42].
Our findings indicate that resveratrol is a promising natural product in patients on radioiodine therapy; it sensitizes thyroid cancer cell to 131I. Also, RSV is an effective protective agent on normal cells against toxicity induced by radioiodine therapy. With these two beneficial actions, RSV may improve the treatment of patients with thyroid cancer during radioiodine therapy.
Acknowledgments
This study was supported by a grant from Mazandaran University of Medical Sciences. This research was the subject of a Pharm.D. thesis of Seyed Amir Hossein Hosseini as a student of Mazandaran University of Medical Sciences.
Conflict of Interests
The authors declared no potential conflict of interests with respect to the authorship and/or publication of this study.
References
- 1.Robbins RJ, Schlumberger MJ. The evolving role of 131I for the treatment of differentiated thyroid carcinoma. Journal of Nuclear Medicine. 2005;46(supplement 1):28S–37S. [PubMed] [Google Scholar]
- 2.Hosseinimehr SJ. Flavonoids and genomic instability induced by ionizing radiation. Drug Discovery Today. 2010;15(21-22):907–918. doi: 10.1016/j.drudis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Noaparast Z, Hosseinimehr SJ. Radioprotective agents for the prevention of side effects induced by radioiodine-131 therapy. Future Oncology. 2013;9(8):1145–1159. doi: 10.2217/fon.13.79. [DOI] [PubMed] [Google Scholar]
- 4.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21(3):397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 5.Hosseinimehr SJ. Potential utility of radioprotective agents in the practice of nuclear medicine. Cancer Biotherapy and Radiopharmaceuticals. 2009;24(6):723–731. doi: 10.1089/cbr.2009.0635. [DOI] [PubMed] [Google Scholar]
- 6.Bohuslavizki KH, Brenner W, Lassmann S, et al. Quantitative salivary gland scintigraphy—a recommended examination prior to and after radioiodine therapy. NuklearMedizin. 1997;36(3):103–109. [PubMed] [Google Scholar]
- 7.Chow SM. Side effects of high-dose radioactive iodine for ablation or treatment of differentiated thyroid carcinoma. Journal of the Hong Kong College of Radiologists. 2005;8(3):127–135. [Google Scholar]
- 8.van Nostrand D, Neutze J, Atkins F. Side effects of “rational dose” iodine-131 therapy for metastatic well-differentiated thyroid carcinoma. Journal of Nuclear Medicine. 1986;27(10):1519–1527. [PubMed] [Google Scholar]
- 9.Samuel AM, Unnikrishnan TP, Baghel NS, Rajashekharrao B. Effect of radioiodine therapy on pulmonary alveolar-capillary membrane integrity. Journal of Nuclear Medicine. 1995;36(5):783–787. [PubMed] [Google Scholar]
- 10.Walter MA, Turtschi CP, Schindler C, Minnig P, Müller-Brand J, Müller B. The dental safety profile of high-dose radioiodine therapy for thyroid cancer: long-term results of a longitudinal cohort study. Journal of Nuclear Medicine. 2007;48(10):1620–1625. doi: 10.2967/jnumed.107.042192. [DOI] [PubMed] [Google Scholar]
- 11.Nakada K, Ishibashi T, Takei T, et al. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? Journal of Nuclear Medicine. 2005;46(2):261–266. [PubMed] [Google Scholar]
- 12.Ballardin M, Gemignani F, Bodei L, et al. Formation of micronuclei and of clastogenic factor(s) in patients receiving therapeutic doses of iodine-131. Mutation Research. 2002;514(1-2):77–85. doi: 10.1016/s1383-5718(01)00323-0. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez S, Carbonell E, Galofré P, Creus A, Marcos R. Cytogenetic damage after 131-iodine treatment for hyperthyroidism and thyroid cancer: a study using the micronucleus test. European Journal of Nuclear Medicine. 1999;26(12):1589–1596. doi: 10.1007/s002590050499. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe N, Kanegane H, Kinuya S, et al. The radiotoxicity of 131I therapy of thyroid cancer: assessment by micronucleus assay of B lymphocytes. Journal of Nuclear Medicine. 2004;45(4):608–611. [PubMed] [Google Scholar]
- 15.Csiszar A. Anti-inflammatory effects of resveratrol: Possible role in prevention of age-related cardiovascular disease. Annals of the New York Academy of Sciences. 2011;1215(1):117–122. doi: 10.1111/j.1749-6632.2010.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szekeres T, Saiko P, Fritzer-Szekeres M, Djavan B, Jäger W. Chemopreventive effects of resveratrol and resveratrol derivatives. Annals of the New York Academy of Sciences. 2011;1215(1):89–95. doi: 10.1111/j.1749-6632.2010.05864.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernández AF, Fraga MF. The effects of the dietary polyphenol resveratrol on human healthy aging and lifespan. Epigenetics. 2011;6(7):870–874. doi: 10.4161/epi.6.7.16499. [DOI] [PubMed] [Google Scholar]
- 18.Zhu H-L. Resveratrol and its analogues: promising antitumor agents. Anti-Cancer Agents in Medicinal Chemistry. 2011;11(5):479–490. doi: 10.2174/187152011795677427. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Park JH, Kim YJ, Park KH. The neuroprotective effect of resveratrol on retinal ganglion cells after optic nerve transection. Molecular Vision. 2013;19:1667–1676. [PMC free article] [PubMed] [Google Scholar]
- 20.Shi X-P, Miao S, Wu Y, et al. Resveratrol sensitizes tamoxifen in antiestrogen-resistant breast cancer cells with epithelial-mesenchymal transition features. International Journal of Molecular Sciences. 2013;14(8):15655–15668. doi: 10.3390/ijms140815655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedayati M, Shafaghati N, Hosseinimehr SJ. Resveratrol mitigates genotoxicity induced by iodine-131 in primary human lymphocytes. Radiation and Environmental Biophysics. 2013;52(2):287–291. doi: 10.1007/s00411-013-0461-1. [DOI] [PubMed] [Google Scholar]
- 22.Sebastià N, Montoro A, Montoro A, et al. Assessment in vitro of radioprotective efficacy of curcumin and resveratrol. Radiation Measurements. 2011;46(9):962–966. [Google Scholar]
- 23.Chen C, Jiang X, Hu Y, Zhang Z. The protective role of resveratrol in the sodium arsenite -induced oxidative damage via modulation of intracellular GSH homeostasis. Biological Trace Element Research. 2013;155(1):119–131. doi: 10.1007/s12011-013-9757-x. [DOI] [PubMed] [Google Scholar]
- 24.Kavas GÖ, Ayral PA, Elhan AH. The effects of resveratrol on oxidant/antioxidant systems and their cofactors in rats. Advances in Clinical and Experimental Medicine. 2013;22(2):151–155. [PubMed] [Google Scholar]
- 25.Singh M, Bhui K, Singh R, Shukla Y. Tea polyphenols enhance cisplatin chemosensitivity in cervical cancer cells via induction of apoptosis. Life Sciences. 2013;93(1):7–16. doi: 10.1016/j.lfs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Ashrafi SA, Hosseinimehr SJ, Varmira K, Abedi SM. Radioimmunotherapy with 131I-bevacizumab as a specific molecule for cells with overexpression of the vascular endothelial growth factor. Cancer Biotherapy and Radiopharmaceuticals. 2012;27(7):420–425. doi: 10.1089/cbr.2012.1224. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Yang S, Troup S, et al. Resveratrol induces apoptosis in breast cancer cells by E2F1-mediated up-regulation of ASPP1. Oncology Reports. 2011;25(6):1713–1719. doi: 10.3892/or.2011.1248. [DOI] [PubMed] [Google Scholar]
- 28.Shih A, Davis FB, Lin H-Y, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. The Journal of Clinical Endocrinology & Metabolism. 2002;87(3):1223–1232. doi: 10.1210/jcem.87.3.8345. [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, Liu Q, Wang M, et al. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PloS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027081.e27081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B, Zhou Z, Zhou W, et al. Resveratrol inhibits proliferation in human colorectal carcinoma cells by inducing G1/Sphase cell cycle arrest and apoptosis through caspase/cyclinCDK pathways. Molecular Medicine Reports. 2014;10(4):1697–1702. doi: 10.3892/mmr.2014.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou C, Ding J, Wu Y. Resveratrol induces apoptosis of bladder cancer cells via miR21 regulation of the Akt/Bcl2 signaling pathway. Molecular Medicine Reports. 2014;9(4):1467–1473. doi: 10.3892/mmr.2014.1950. [DOI] [PubMed] [Google Scholar]
- 32.Hotnog D, Mihaila M, Lancu IV, et al. Resveratrol modulates apoptosis in 5-fluorouracyl treated colon cancer cell lines. Roumanian Archives of Microbiology and Immunology. 2013;72(4):255–264. [PubMed] [Google Scholar]
- 33.Duntas LH. Resveratrol and its impact on aging and thyroid function. Journal of Endocrinological Investigation. 2011;34(10):788–792. doi: 10.3275/7926. [DOI] [PubMed] [Google Scholar]
- 34.Kang HJ, Youn Y-K, Hong M-K, Kim LS. Antiproliferation and redifferentiation in thyroid cancer cell lines by polyphenol phytochemicals. Journal of Korean Medical Science. 2011;26(7):893–899. doi: 10.3346/jkms.2011.26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin H-Y, Tang H-Y, Shih A, et al. Thyroid hormone is a MAPK-dependent growth factor for thyroid cancer cells and is anti-apoptotic. Steroids. 2007;72(2):180–187. doi: 10.1016/j.steroids.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Yu XM, Jaskula-Sztul R, Ahmed K, Harrison AD, Kunnimalaiyaan M, Chen H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Molecular Cancer Therapeutics. 2013;12(7):1276–1287. doi: 10.1158/1535-7163.MCT-12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebai H, Hovsépian S, Ristorcelli E, Aouani E, Lombardo D, Fayet G. Resveratrol increases iodide trapping in the rat thyroid cell line FRTL-5. Thyroid. 2010;20(2):195–203. doi: 10.1089/thy.2009.0171. [DOI] [PubMed] [Google Scholar]
- 38.Frombaum M, le Clanche S, Bonnefont-Rousselot D, Borderie D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and NO bioavailability: potential benefits to cardiovascular diseases. Biochimie. 2012;94(2):269–276. doi: 10.1016/j.biochi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Iuga C, Alvarez-Idaboy JR, Russo N. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: a quantum chemical and computational kinetics study. Journal of Organic Chemistry. 2012;77(8):3868–3877. doi: 10.1021/jo3002134. [DOI] [PubMed] [Google Scholar]
- 40.Quincozes-Santos A, Bobermin LD, Latini A, et al. Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064372.e64372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sebastià N, Almonacid M, Villaescusa JI, et al. Radioprotective activity and cytogenetic effect of resveratrol in human lymphocytes: an in vitro evaluation. Food and Chemical Toxicology. 2013;51(1):391–395. doi: 10.1016/j.fct.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Fu Y, Wang Y, Du L, et al. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating Sirt1 and limiting NLRP-3 inflammasome activation. International Journal of Molecular Sciences. 2013;14(7):14105–14118. doi: 10.3390/ijms140714105. [DOI] [PMC free article] [PubMed] [Google Scholar]


