Abstract
Canine visceral leishmaniasis (CVL) is a severe and fatal systemic chronic inflammatory disease. We investigated the alterations in, and potential associations among, antioxidant enzymes, trace elements and histopathology in CVL. Blood and tissue levels of Cu-Zn superoxide dismutase, catalase and glutathione peroxidase were measured in mixed-breed dogs naturally infected with Leishmania infantum chagasi, symptomatic (n = 19) and asymptomatic (n = 11). Serum levels of copper, iron, zinc, selenium and nitric oxide, and plasma lipid peroxidation were measured. Histological and morphometric analyses were conducted of lesions in liver, spleen and lymph nodes. We found lower blood catalase and glutathione peroxidase activity to be correlated with lower iron and selenium respectively. However, higher activity of Cu-Zn superoxide dismutase was not correlated with the increase in copper and decreased in zinc observed in infected animals compared to controls. Organ tissue was characterized by lower enzyme activity in infected dogs than in controls, but this was not correlated with trace elements. Lipid peroxidation was higher in symptomatic than in asymptomatic and control dogs and was associated with lesions such as chronic inflammatory reaction, congestion, haemosiderin and fibrosis. Systemic iron deposition was observed primarily in the symptomatic dogs showing a higher tissue parasite load. Dogs with symptomatic CVL displayed enhanced LPO and Fe tissue deposition associated with decreased levels of antioxidant enzymes. These results showed new points in the pathology of CVL and might open new treatment perspectives associated with antioxidants and the role of iron in the pathogenesis of CVL.
Keywords: antioxidant enzymes, canine visceral leishmaniasis, iron, trace elements
Canine visceral leishmaniasis (CVL) is a chronic zoonotic disease in Brazil caused by Leishmania (Leishmania) infantum chagasi (syn L. infantum; Mauricio et al. 2000; Shaw 2006) transmitted by the phlebotomine sandfly Lutzomyia longipalpis. Dogs are both natural hosts and the main reservoir of the parasite (Deane & Deane 1955). A typical mononuclear inflammation has been described primarily in organs rich in cells of the mononuclear phagocytic system such as liver, spleen, bone marrow and lymph nodes (Deane & Deane 1955; Alvar et al. 2004).
Macrophages generate highly toxic molecules such as reactive oxygen species (ROS) including superoxide radicals (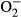 ), hydrogen peroxide (H2O2) and hydroxyl radicals (OH−) and reactive nitrogen species including nitric oxide (NO) to combat Leishmania (Mauel et al. 1991; Assrue et al. 1994; Biswas et al. 1997). This can disturb cell structure and function through lipid peroxidation (LPO) which leads to the formation of degradation products, including malonyl dialdehyde (Assrue et al. 1994; Biswas et al. 1997). Leishmania inhibit ROS production and can replicate within macrophages (Cunningham 2002; Paltrinieri et al. 2010). However, according to Paltrinieri et al. (2010), an increase in ROS depends on inflammation, rather than directly on the presence of Leishmania.
), hydrogen peroxide (H2O2) and hydroxyl radicals (OH−) and reactive nitrogen species including nitric oxide (NO) to combat Leishmania (Mauel et al. 1991; Assrue et al. 1994; Biswas et al. 1997). This can disturb cell structure and function through lipid peroxidation (LPO) which leads to the formation of degradation products, including malonyl dialdehyde (Assrue et al. 1994; Biswas et al. 1997). Leishmania inhibit ROS production and can replicate within macrophages (Cunningham 2002; Paltrinieri et al. 2010). However, according to Paltrinieri et al. (2010), an increase in ROS depends on inflammation, rather than directly on the presence of Leishmania.
To protect against ROS damage, vertebrate hosts possess a variety of antioxidant defences that include metal redistribution and antioxidant enzyme systems requiring trace elements such as copper (Cu), zinc (Zn), iron (Fe) and selenium (Se) for their activity. Antioxidant enzymes such as Cu-Zn superoxide dismutase (SOD) remove damaging ROS from the environment by catalysing the dismutation of superoxide radicals to H2O2 and O2. Catalase (CAT) requires iron (Fe), glutathione peroxidase (GSH-Px) requires selenium (Se), and both catalyse the reduction in H2O2 to H2O and O2 (Britti et al. 2008).
Although studies of human forms of leishmaniasis (Araujo et al. 2008; Mishra et al. 2010) and experimental animal models (Anstead et al. 2001) show an association between trace element serum levels and oxidative stress, there are only a few reports of trace elements associated with CVL (Nieto et al. 2003; Pasa et al. 2003; Adamama-Moraitou et al. 2005). These studies do not report an association of trace elements and oxidative stress with histological alterations, tissue antioxidant enzymes, tissue iron deposition or clinical symptoms in dogs with visceral leishmaniasis.
The aim of this study was to investigate the alterations in, and potential associations among, antioxidant enzymes, histopathology and trace elements in dogs naturally infected with L. infantum to determine whether imbalance in oxidative/antioxidant systems leads to greater oxidative stress and more severe tissue lesions in target organs of infected dogs. These are important factors in understanding the pathology and pathogenesis of CVL and may contribute to possible treatment strategies.
Materials and methods
Dogs
Thirty mixed-breed dogs of unknown age naturally infected with Leishmania (L.) infantum chagasi were obtained from the Control Zoonosis Center of the Municipality of Ribeirão das Neves, Belo Horizonte Metropolitan Area, Minas Gerais (MG) State, Brazil. The parasite was identified by indirect immunofluorescence antibody testing (IFAT; titre, 1:40 dilution) and enzyme-linked immunosorbent assay (ELISA; optical density, 100; 1:400 dilutions) and subsequently quarantined. Nine uninfected dogs with negative IFAT and ELISA were obtained from a non-endemic geographical area from the Zoonoses Center of Carandaí, MG, Brazil. Parasitological examinations using bone marrow impression smears, ear biopsies for histology and immunohistochemistry, and conventional PCR using spleen samples obtained following necropsy were also negative.
Dogs were quarantined for at least 40 days at the experimental kennel of the Instituto de Ciências Biológicas (ICB), Universidade Federal de Minas Gerais (UFMG) and provided with drinking water and a balanced commercial food (Nero Refeição, Total Alimentos, Brazil) ad libitum prior to inclusion in the study. Dogs were treated for intestinal helminths (Helfine cães®, 133 Agener União, Brazil) and ectoparasite infestations (Frontline Top Spot®, Merial, Brazil) and vaccinated against rabies (Defensor®, Pfizer Saúde Animal, Brazil) and other infectious diseases (Vanguard HTLP 5/CV-L®, Pfizer Saúde Animal, Brazil).
At the end of the quarantine period, infected dogs were physically examined and classified as asymptomatic (n = 11) with no clinical signs of disease or symptomatic (n = 19) with classical disease symptoms as follows: (i) body condition (weight loss or cachexia and clinical anaemia); (ii) enlarged cervical and popliteal lymph nodes; and (iii) dermatological symptoms (alopecia, dry exfoliative dermatitis or ulcers, onychogryphosis, keratoconjunctivitis; Alvar et al. 1994; Mancianti et al. 1998; da Silva 2007; de Amorin et al. 2010). All animals (infected and controls) showed negative serological results for Erlichia and Babesia infection.
Ethical Approval
All experimental procedure involving animals were performed in accordance with National Institute of Health Guide for Care and use of animal and with approval of our institutional ethics committee, which also reviewed and approved this work (CETEA, Universidade Federal de Minas Gerais, protocol no. 213/2007).
Necropsy and parasitological and serological diagnosis of Leishmania
To confirm the presence of Leishmania after quarantine, dogs were anaesthetized with 2.5% (0.5 ml/kg) intravenous thiopental for obtaining bone marrow aspirates and ear skin tissue (5 mm punch). Smears were air-dried and stained with 10% Giemsa. The entire slide from each bone marrow sample was examined for Leishmania amastigotes by light microscopy at 1000×. Control dogs were free of Leishmania. Ear skin samples obtained from all dogs used in the study were immediately fixed in 10% neutral buffered formalin for immunohistochemistry to detect Leishmania amastigotes (Tafuri et al. 2004).
For ELISA determination of anti-Leishmania IgG, soluble Leishmania antigen was derived from L. infantum strain MHOM/BR/1967/BH46 promastigote forms ruptured ultrasonically (optical density > 100 > 1:400 dilutions). IFAT, employing the same L. infantum antigen used in ELISA, was used to detect Leishmania antibodies, with titres >1:40 considered positive.
For PCR analysis, fragments of tissue were extracted with the aid of the ‘DNeasy® Blood & Tissue Kit (Qiagen Inc., Valencia, CA, USA) used according to the manufacturer's instructions. DNA amplification was performed using oligonucleotide primers (forward, 5′ TGT CGC TTG CAG ACC AGA TG 3′ and reverse, 5′ GCA TCG CAG GTG TGA GCA C 3′) amplifying a 90 bp fragment of a single-copy-number gene of DNA polymerase of L. infantum (GenBank accession number AF009147; Bretagne et al. 2001). The PCR mixture consisted of 15 pmol of DNA template, 15 pmol of each primer, 7 μl of Go Taq® Green Master Mix (Promega, San Luis Obispo, CA, USA) and 3.5 μl of nuclease-free water (Go Taq® Green Master Mix). Amplification was carried out using an initial denaturation step at 95 °C for 3 min; followed by 30 cycles of annealing at 51 °C for 60 s, extension at 72 °C for 30 s and denaturation at 94 °C for 60 s; and a final extension step at 72 °C for 2 min (da Silva et al. 2009). PCR products were analysed by gel electrophoresis on a 5% non-denaturing polyacrylamide gel in 89 mM Tris–borate buffer (pH 8.0) containing 2 mM EDTA. Fragments were visualized by silver nitrate staining.
Necropsy was conducted immediately after serological and parasitological examination. Dogs were anaesthetized with 0.5 ml/kg thiopental i.v. (2.5%) and killed with thiopental at 1.0 ml/kg. Samples of liver, spleen and cervical lymph nodes were collected for histology.
Laboratory procedures
SOD, CAT and GSH-Px activity
SOD, CAT and GSH-Px were measured in blood, liver, spleen and lymph node, and GSH-Px was measured in plasma. Samples were lysed for 1 min in an IKA T10 homogenizer and centrifuged for 15 min at 10,000 g. The supernatant was used for biochemical assays. Protein concentration in all samples was determined according to Lowry et al. (1951) using bovine serum albumin as standard.
Superoxide dismutase activity was measured according to Gioda et al. (2010). In brief, 40 μl of supernatant was added to 50 mM PBS (1 ml, pH 7.8, 37 °C) containing 1 mM diethylenetriaminepentaacetic acid. The reaction was initiated by adding 0.2 mM pyrogallol, and samples were incubated at 37 °C for 3 min. The absorbance was determined at 420 nm. Superoxide dismutase activity was calculated as units per mg protein, with one unit constituting the amount of enzyme resulting in 50% inhibition of pyrogallol autoxidation.
Catalase activity was measured according to Gioda et al. (2010). Briefly, 0.3 M H2O2 was added as substrate to 0.1 ml of sample supernatant and 2.0 ml of potassium phosphate buffer (50 mM, pH 7.0) to a final H2O2 concentration of 6 mM, and the reaction was run for 1 min at room temperature. Decomposition of H2O2 by CAT was measured by the change in absorbance at 240 nm (Δ,E). Catalase activity was expressed as mM of H2O2 decomposed per min per mg protein.
Glutathione peroxidase activity was measured from supernatant preserved in ice-cold Tris–HCl buffer (50 mM, pH 7.5, containing 5 mM EDTA and 1 mM dithiothreitol) and centrifuged at 10,000 g for 15 min at 4 °C. The experiment was performed using a Cayman Kit (No. 703002) according to the manufacturer's instructions and Gioda et al. (2010). Results were expressed in nmol NADPH/min/ml.
Serum levels of Cu, Fe, Zn and Se
Serum was diluted with 0.05% Triton X-100 in 0.01% HNO3 for trace element determination. Cu, Fe and Zn were measured by inductive plasma spectrometry emission coupled with optical emission detection (ICP OES), and Se was assessed by inductively coupled plasma mass spectrometry (ICP MS). All methods were validated according to recommendations of the international and national organizations of the Instituto de Metrologia (INMETRO, 2010), International Organization for Standardization (ISO, 1999) and National Association of Testing Authorities (NATA, 1997). The performance parameters adopted were selectivity, linear range, detection and quantification limits, precision and accuracy at the 95% CI. Recovery was close to 100%, and the accuracy was determined by reference material analysis using Seronorm L1 201405. The quality of results was confirmed by standard reference materials and statistical tools such as media and recovery control charts. All assays were performed in the trace metal laboratory of Fundação Centro Tecnológico de Minas Gerais (CETEC) in a clean area in ISO 5 and ISO 7 class laminar airflow cabinets using validated methods (Araujo et al. 2008; Souza et al. 2013).
TBAR as LPO measures
Lipid peroxidation was determined by measuring the concentration in plasma of thiobarbituric acid-reactive substance (TBARS; Gioda et al. 2010). Briefly, 200 μl of plasma, 1100 μl of 1.4% H3PO4 and 500 μl of 0.6% thiobarbituric acid were combined and incubated for 45 min at 90 °C. Subsequently, 200 μl of 8.7% sodium dodecyl sulphate was added, and the mixture was centrifuged at 3000 g for 10 min and read in a spectrophotometer at 532 nm.
Nitric oxide serum levels
Nitrite (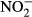 ) concentration, an indirect measure of NO, was measured in serum aliquots from all dogs using the Griess reaction (Green et al. 1982). Twenty-five μl of serum was mixed with 25 μl nitrate reductase and incubated overnight or 6 h at 37 °C. Subsequently, 50 μl of Griess reagent [1% sulphanylamide, 0.1% naphthylethylene-diamide-dihydrochloride and 2.5% phosphoric acid (Sigma, St. Louis, MO, USA)] was added. The absorbance was measured at 540 nm using a microplate reader. Results were expressed as μM
) concentration, an indirect measure of NO, was measured in serum aliquots from all dogs using the Griess reaction (Green et al. 1982). Twenty-five μl of serum was mixed with 25 μl nitrate reductase and incubated overnight or 6 h at 37 °C. Subsequently, 50 μl of Griess reagent [1% sulphanylamide, 0.1% naphthylethylene-diamide-dihydrochloride and 2.5% phosphoric acid (Sigma, St. Louis, MO, USA)] was added. The absorbance was measured at 540 nm using a microplate reader. Results were expressed as μM 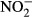 .
.
Histology and histomorphometric analysis
Samples of liver, spleen and cervical lymph nodes were fixed in 10% neutral buffered formalin, dehydrated, cleared, embedded in paraffin, cut into 4–5 μm sections and stained with haematoxylin and eosin (H&E), with Perls' Prussian blue to detect Fe, Gomori's ammoniacal silver for collagen detection (Melo et al. 2009) and immunohistochemistry for Leishmania amastigote detection (Tafuri et al. 2004).
Histological screening focused on the detection and grading of the following abnormalities: (i) liver: inflammation, intralobular granulomas, degenerative hepatocyte lesions (hydropic and steatotic), hypertrophy and hyperplasia of Kupffer cells, haemosiderin deposits and congestion; (ii) spleen: inflammation, hypertrophy and hyperplasia of the red and white pulp; granuloma, congestion and haemosiderin deposits; and (iii) cervical lymph node: thickening and chronic inflammatory reaction of the capsule and subcapsular sinus, hyperplasia and hypertrophy of cells (macrophages) in the medullar layer (cords and sinus), congestion and haemosiderin deposits. Changes were evaluated semi-quantitatively as presence or absence and graded according to intensity over the entire tissue slide: 1, slight (20–30%); 2, moderate (31–60%); and 3, severe (>60%).
Tissue sections were stained with Prussian blue to detect Fe and Gomori's ammoniacal silver for collagen. Forty-five randomly chosen images of histological sections were examined. Analysis was carried out using an Axiolab light microscope (Zeiss) at 440× and a computer-assisted image analysis system (Kontron Elektronic Image Analyzer, Carl Zeiss, Germany – KS300 software). Using a digital pad, the area stained for Fe or collagen was measured from real images and segmented to generate binary images. The results are expressed in μm2 according to Melo et al. (2009). For detection and assessment of Leishmania amastigotes, parasites in the entire slide sample were quantified under light microscopy at 1000× according to Tafuri et al. (2001).
Statistical analysis
The analyses were performed using GraphPad Prisma 5.0, GraphPad Software, Inc., La Jolla, CA, USA. All analyses were carried out randomly, and dogs were categorized as symptomatic infected, asymptomatic infected or uninfected (controls). The results obtained for trace elements, enzymes, NO, TBARS, tissue iron deposition and fibrosis were compared among groups using one-way anova and Tukey′s post hoc test. The analysis was followed by residual analyses to check for the error distribution and suitability of the normal model. Histological screening results were compared among groups using the Kruskal–Wallis test, as the residuals did not fit the assumptions of homoscedasticity and normality. Spearman's correlation coefficients were calculated to assess correlation between variables of group. Differences were considered significant when P ≤ 0.05.
Results
Antioxidant enzyme activity and levels of trace elements
We found significantly lower CAT activity in blood and GSH-Px in plasma (P < 0.001 and P < 0.01 respectively) of infected dogs, both symptomatic and asymptomatic, than in controls (Figure 1a,c). GSH-Px activity in blood was also lower in symptomatic (P < 0.0001) and asymptomatic dogs (P < 0.001) than in controls. Symptomatic dogs showed significantly lower (P < 0.01) GSH-Px activity compared to asymptomatic (Figure 1d). Infected dogs showed significantly higher SOD activity than controls (P < 0.01; Figure 1b).
Figure 1.
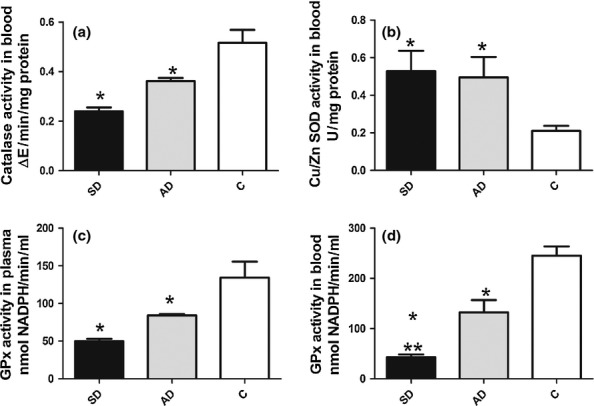
Antioxidant enzyme activity in blood or plasma of uninfected dogs and L. infantum infected dogs. CAT (a), SOD in blood (b) GSH-Px in plasma (c) and GSHPx in blood activity in symptomatic (SD) and asymptomatic (AD) dogs compared to uninfected control dogs (c). Significant differences (P < 0.05) from control are represented by*.
Liver, spleen and lymph node CAT, GSH-Px and SOD were significantly lower (P < 0.05) in infected dogs than in controls, with no difference between asymptomatic and symptomatic (Table 1).
Table 1.
Antioxidant enzyme activity and iron deposition in organs of uninfected dogs and those with canine visceral leishmaniasis. Infected dogs: symptomatic (SD) and asymptomatic (AD) compared to uninfected control dogs (C)
| Liver | Spleen | |||||
|---|---|---|---|---|---|---|
| Parameters | SD | AD | C | SD | AD | C |
| CAT | 0.222 ± 0.01* | 0.280 ± 0.02* | 0.3799 ± 0.05 | 0.239 ± 0.01* | 0.312 ± 0.02* | 0.475 ± 0.03 |
| GSH-Px | 66.7 ± 8.0* | 88.3 ± 8.3* | 174.4 ± 6.2 | 60.84 ± 6.5* | 89.1 ± 3.3* | 127.4 ± 17.9 |
| SOD | 0.210 ± 0.02* | 0.272 ± 0.04* | 0.518 ± 0.05 | 0.178 ± 0.03* | 0.266 ± 0.02* | 0.364 ± 0.04 |
| Fe | 546.7 ± 60.8* ** | 303.8 ± 63* | 101.7 ± 25.3 | 2356 ± 529.7* ** | 791.1 ± 215.1* | 483.5 ± 57.9 |
| Lymph node | |||
|---|---|---|---|
| Parameters | SD | AD | C |
| CAT | 0.219 ± 0.01* | 0.334 ± 0.02* | 0.453 ± 0.03 |
| GSH-Px | 58.3 ± 5.7* | 83.8 ± 1.3* | 128.1 ± 18.6 |
| SOD | 0.132 ± 0.01* | 0.306 ± 0.03* | 0.373 ± 0.03 |
| Fe | 1237 ± 216.7* ** | 358.5 ± 148.6* | 226 ± 63.13 |
Values are shown as mean ± standard deviation. Significant differences (P ≤ 0.05) relative to control are represented by * and relative to AD by ** P < 0.01 for all. Parameters: CAT, catalase; GSH-Px, glutathione peroxidase; SOD, Cu-Zn superoxide dismutase; Fe, iron.
Fe and Zn were significantly lower in blood of symptomatic dogs than in either asymptomatic or controls (P < 0.01; Figure 2a,c). In contrast, serum Cu concentrations were higher in symptomatic dogs than in asymptomatic and control dogs (P < 0.01 and P < 0.001 respectively; Figure 2b). The Se concentration was significantly lower in infected dogs than in controls (P < 0.001 and P < 0.01 respectively) and lower in symptomatic than in asymptomatic dogs (P < 0.01; Figure 2d).
Figure 2.
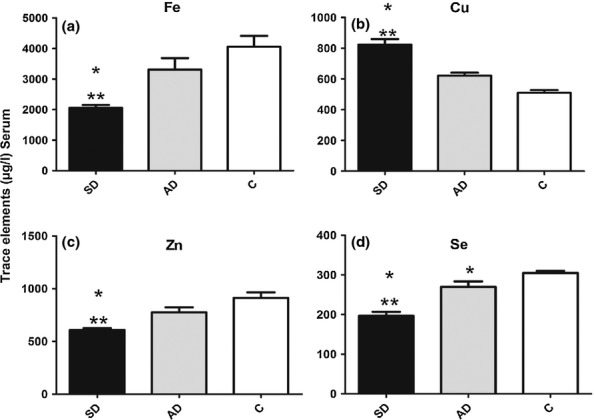
Serum levels of trace elements in uninfected dogs and in dogs infected with L. infantum. Serum Fe (a), Cu (b), Zn (c), and Se (d) concentrations in symptomatic (SD) and asymptomatic (AD) dogs, compared to uninfected control dogs (c). Significant differences (P < 0.05) relative to control are represented by * and relative to AD by**.
We observed significant positive correlation of GSH-Px with Se in blood (r = 0.758 and P = 0.001) and plasma (r = 0.793 and P = 0.01) and significant positive correlation of CAT with Fe (r = 0.658 and P = 0.001) of infected dogs but no correlation between SOD and Cu, Zn or serum trace elements with liver, spleen and lymph node antioxidant enzyme activity.
Lipid peroxidation
Lipid peroxidation was higher in plasma of all infected (symptomatic and asymptomatic) dogs than in controls (P < 0.0001 and P < 0.01) and higher in symptomatic than in asymptomatic dogs (P < 0.001; Figure 3b).
Figure 3.
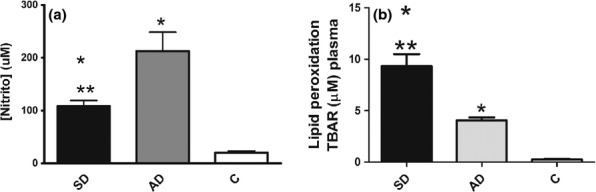
Nitrite and lipid peroxidation (LPO) in uninfected dogs and dogs infected with L. infantum. NO in serum (a), LPO in plasma (b) of symptomatic (SD) and asymptomatic (AD) dogs compared to uninfected control dogs (c). Significant differences (P < 0.05) from control are represented by * from AD by **.
Histopathological and parasitological aspects
Liver
Liver of infected dogs showed severe chronic inflammation involving the capsule, portal area and hepatic lobules with intralobular granulomas comprising macrophages (epithelioid cells), lymphocytes and plasma cells (Table 2). Congestion and haemosiderin pigment deposits were present (Figure 4a). Swelling and vacuolization of hepatocytes, identified as hydropic degeneration and steatosis, were found (Figure 4b). Semi-quantitative analysis showed higher score lesions (P < 0.05) in infected dogs than the control group (Table 2). The chronic inflammatory reaction (P < 0.04) was more intense in symptomatic dogs than asymptomatic (Figure 4b). In contrast, we observed higher numbers of hepatic intralobular granulomas in asymptomatic than symptomatic dogs (P = 0.01). Widespread haemosiderin pigment deposits mainly in hepatic sinusoids (haemosiderosis) were significantly greater in symptomatic than in asymptomatic and control dogs (P = 0.04; P < 0.02 respectively). Fibrosis was more widely distributed in symptomatic than in asymptomatic and controls dogs (P < 0.05 and P < 0.1 respectively; Table 2). Tissue parasite load was higher in symptomatic dogs (P = 0.006; Table 2).
Table 2.
Histopathological and parasitological evaluation of liver in canine visceral leishmaniasis. Analyses of presence quantification (P) and grading (G) of tissues abnormalities following the distribution: slight (S), moderate (M) and severe (SV)
| Symptomatic n = 19 | Asymptomatic n = 11 | Control n = 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grading | aP ≤ 0.05 | Grading | aP ≤ 0.05 | Grading | |||||||
| Histopathology | S | M | SV | G/P | S | M | SV | G/P | S | M | SV |
| Inflammation | 8 | 5 | 3 | */* ** | 5 | 2 | 2 | */* | 0 | 0 | 0 |
| Hypertrophy/hyperplasia of Kupffer | 5 | 10 | 3 | */* | 3 | 6 | 2 | */* | 0 | 0 | 0 |
| Intralobular granulomas | 5 | 3 | 1 | */* ** | 3 | 3 | 2 | */* | 0 | 0 | 0 |
| Degeneration | 5 | 8 | 6 | */* | 2 | 6 | 3 | */* | 2 | 0 | 0 |
| Congestion | 4 | 10 | 2 | */* | 5 | 3 | 3 | */* | 2 | 0 | 0 |
| Haemosiderin | 5 | 3 | 0 | * **/* ** | 2 | 0 | 0 | */* | 0 | 0 | 0 |
| Fibrosis (μm2)b | 2751 ± 866*, ** | 2039 ± 597 | 1567 ± 303 | ||||||||
| Parasitological diagnosisb | 26.4 ± 5** | 10.6 ± 2 | |||||||||
Significant differences (P ≤ 0.05) relative to control are represented by * and relative to asymptomatic by **.
Values are shown as mean ± standard deviation.
Figure 4.
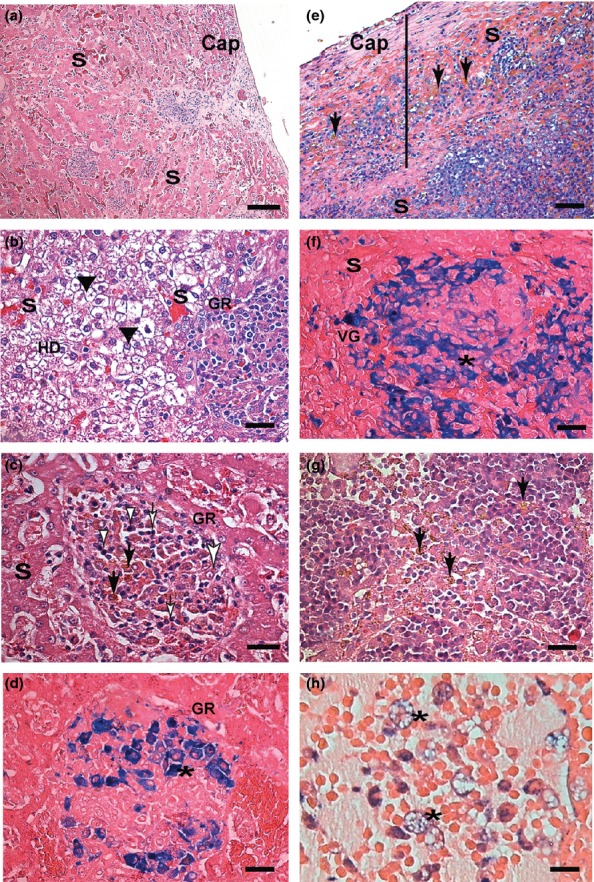
Liver, spleen and lymph node sections of symptomatic dogs naturally infected with L. infantum. Liver (a, b, c, d): (a) Note congestion of sinusoid vessels (S). Inflammation and capsule thickness (Cap). (b) Intense hydropic degeneration (HD), (black arrow heads) granuloma (GR) and congestion. (c) In the centre of the micrograph is an intralobular granuloma (GR) comprising epithelioid cells with an elongated or oval nucleus (large white arrow), plasma cells (white arrow heads) and lymphocytes (white small arrows). Haemosiderin deposition as circular brown structures can be noted (black arrows). (d) Perls' Prussian blue staining confirmed haemosiderin deposition inside macrophages of granulomas (*) where it has been visualized as homogeneous circular blue structures. (e, f) Spleen sections: (e) Note capsule (Cap) thickening (black bar) and congestion of the red pulp sinusoid vessels (S). Haemosiderin deposition as brown (black arrows). (f) Perls' Prussian blue staining confirming Virchow's granuloma (VG) haemosiderin deposition inside cells of the red pulp (macrophages*) visualized as homogeneous circular blue structures. (g, h) Lymph node sections: (g) Haemosiderin in macrophages (black arrows) of medullar and sinus cords. (h) Perls' Prussian blue staining confirming haemosiderin deposition inside foam macrophages (*). H&E (a, b, c, e and g); Perls' Prussia blue. (d, f, h); a, e (Bar = 32 μm), and b, c, d, f, g, h (Bar = 16 μm).
Spleen
Semi-quantitative analysis showed that all tissue changes had more frequent presence and higher grading in infected dogs than in the control group (P < 0.05; Figure 4e and Table 3). Inflammation was more intense in symptomatic dogs than in asymptomatic (P = 0.01; Table 3). Virchow's granulomas were more frequently present and of higher grade in symptomatic than in asymptomatic dogs (P = 0.001 and P = 0.01). Hyperplasia and hypertrophy of macrophages of red pulp, with and without Leishmania amastigotes, were also found, with no differences observed between symptomatic and asymptomatic dogs. As in liver, the parasite load in spleen was higher in symptomatic animals (P = 0.0269; Table 3). Higher presence and grade of haemosiderin deposits and congestion was observed in red pulp of symptomatic than of asymptomatic dogs (P = 0.01 and P < 0.01; Figure 4f and Table 3). Splenic fibrosis was more widely distributed in symptomatic dogs than in asymptomatic and controls (P < 0.01 and P < 0.001; Table 3).
Table 3.
Histopathological and parasitological characteristics of the spleen in cases of canine visceral leishmaniasis. Analyses of presence quantification (P) and grading (G) of tissue abnormalities following the distribution: slight (S), moderate (M) and severe (SV)
| Symptomatic n = 19 | Asymptomatic n = 11 | Control n = 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grading | aP ≤ 0.05 | Grading | aP ≤ 0.05 | Grading | |||||||
| Histopathology | S | M | SV | G/P | S | M | SV | G/P | S | M | SV |
| Inflammation | 6 | 5 | 3 | */* ** | 2 | 1 | 1 | */* | 0 | 0 | 0 |
| Granuloma de Virchow | 12 | 4 | 2 | ** */* ** | 3 | 1 | 0 | */* | 0 | 0 | 0 |
| Hypertrophy/hyperplasia white/red pulp | 5 | 5 | 0 | */* | 2 | 6 | 1 | */* | 0 | 0 | 0 |
| Haemosiderin | 2 | 5 | 3 | ** */* ** | 2 | 1 | 0 | */* | 0 | 0 | 0 |
| Congestion | 7 | 6 | 6 | ** */* | 4 | 5 | 0 | */* | 0 | 0 | 0 |
| Fibrosis (μm2)b | 3064 ± 881* ** | 2022 ± 385 | 1516 ± 345 | ||||||||
| Parasitological diagnosis b | 32 ± 6** | 8.4 ± 2 | |||||||||
Significant differences (P ≤ 0.05) relative to control are represented by * and relative to asymptomatic by**.
Values are shown as mean ± standard deviation.
Lymph nodes
Cervical lymph nodes showed chronic lymphadenitis in all infected dogs. However, chronic inflammatory reaction (P < 0.01) was higher in symptomatic dogs (Table 4) than in asymptomatic and control dogs. The primary alterations in all cases were hyperplasia and hypertrophy of macrophages of the medullary cords and sinuses. Congestion was intense (P < 0.05) in all infected dogs (Table 4). Haemosiderin pigment deposition (Figure 4g) as confirmed by Prussian blue staining (Figure 4h) was more intense (P < 0.01; P = 0.01) in symptomatic than in asymptomatic dogs. The tissue parasite load was greater in symptomatic dogs (P = 0.03). Fibrosis was more widely distributed in symptomatic dogs than in controls (P < 0.001; Table 2).
Table 4.
Histopathological and parasitological evaluation of the cervical lymph nodes in cases of canine visceral leishmaniasis. Analyses of presence quantification (P) and grading (G) of tissue abnormalities following the distribution: slight (S), moderate (M) and severe (SV)
| Symptomatic n = 19 | Asymptomatic n = 11 | Control n = 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grading | aP ≤ 0.05 | Grading | aP ≤ 0.05 | Grading | |||||||
| Histopathology | S | M | SV | G/P | S | M | SV | G/P | S | M | SV |
| Inflammation | 7 | 9 | 3 | * **/* ** | 4 | 3 | 0 | */* | 0 | 0 | 0 |
| Macrophages hyperplasic | 2 | 12 | 2 | */* | 2 | 8 | 1 | */* | 0 | 0 | 0 |
| Hypertrophy/hyperplasic of nodules (lymphatic follicles) | 2 | 4 | 4 | */* | 4 | 2 | 2 | */* | 0 | 0 | 0 |
| Hypertrophy/hyperplasia medullar sinus/cords | 3 | 11 | 5 | */* | 3 | 8 | 0 | */* | 0 | 0 | 0 |
| Haemosiderin | 8 | 3 | 4 | *.**/* ** | 1 | 1 | 1 | */* | 0 | 0 | 0 |
| Congestion | 3 | 5 | 2 | */* | 2 | 4 | 2 | */* | 0 | 0 | 0 |
| Fibrosis (μm2)b | 1943 ± 392* | 1596 ± 261 | 1280 ± 3217 | ||||||||
| Parasitological diagnosisb | 28 ± 3** | 6.7 ± 2 | |||||||||
Significant differences (P ≤ 0.05) relative to control are represented by * and relative to asymptomatic by**.
Values are shown as mean ± standard deviation.
A significant negative correlation was found between tissue antioxidant enzyme activity and the inflammatory response in all three studied organs. However, pathologies such as congestion and/or haemosiderosis were only correlated to tissue antioxidant enzymes in spleen. Fibrosis, although present in all studied organs, was only correlated tissue antioxidant enzymes in liver and spleen. Lymph nodes showed correlation of inflammation with CAT only (Table 5).
Table 5.
Correlation between presence of histopathology and tissue antioxidant enzyme or iron in canine visceral leishmaniasis
| Liver | Spleen | Lymph node | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue histopathology | CAT | SOD | GSH-Px | Fe | CAT | SOD | GSH-Px | Fe | CAT | Fe |
| Inflammation | −0.735 | −0.768 | −0.781 | 0.659 | −0.723 | −0.584 | −0.787 | 0.780 | −0.729 | − |
| Degeneration | − | − | − | 0.758 | − | − | − | − | − | − |
| Haemosiderin | − | − | − | 0.597 | −0.659 | −0.653 | −0.783 | 0.677 | − | 0.729 |
| Congestion | − | – | − | − | −0.696 | −0.624 | −0.774 | 0.723 | − | − |
| Fibrosis | −0.677 | −0.598 | −0.699 | 0.598 | −0.648 | −0.685 | −0.598 | 0.678 | 0.726 | |
Table represented only Spearman's significant correlation coefficients (P < 0.01). Without significant correlation (−). Antioxidant enzyme: CAT, catalase; SOD, Cu-Zn superoxide dismutase, GSH-Px, glutathione peroxidase; Fe, iron; NO, nitric oxide; LPO, lipid peroxidation.
Nitric oxide production
Nitric oxide was higher in all infected dogs than in controls (P < 0.001) and higher in asymptomatic than in symptomatic dogs (P = 0.001; Figure 3a).
Iron in tissue
Iron deposition was observed in liver, spleen and lymph node, accompanied by decreased serum levels without significant cell necrosis. Prussian blue staining showed Fe inside Kupffer cells and hepatocytes (Figure 4d). More prominent deposits of Fe in the liver of symptomatic dogs were observed than in the asymptomatic group and controls (Figure 4d and Table 1). In spleen, Fe deposition was found in red pulp sinusoids, but not in the white pulp (Figure 4f). Symptomatic dogs showed higher Fe deposition than asymptomatic and controls (Table 1). In cervical lymph nodes (Figure 4h), Fe deposition was higher in symptomatic dogs than in asymptomatic and controls (Table 1).
In infected animals, a significant positive correlation was seen between LPO and iron deposition in liver (P = 0.003, r = 0.659), spleen (P < 0.002, r = 0.728) and cervical lymph node (P < 0.01, r = 0.765). A significant positive correlation was observed in infected animals between Fe and tissue damage in (i) liver: capsule inflammation, portal inflammation, haemosiderin, hepatocyte degeneration; (ii) spleen: capsule inflammation, congestion, haemosiderin; and (iii) cervical lymph node: haemosiderin (Table 5).
Discussion
We observed low antioxidant enzyme activity and altered oxidant/antioxidant balance and trace elements associated with tissue iron deposition and damage in CVL.
The body's ability to counteract oxidative stress depends on the status and activity of the antioxidant system including CAT, SOD and GSH-Px (de Luna et al. 2000; Heidarpour et al. 2012; Samanta et al. 2012). The importance of Zn, Cu, Se and Fe on the immune response to CVL, and their redistribution during the course of infection is well documented (Nieto et al. 2003; Pasa et al. 2003; Heidarpour et al. 2012).
In our study, we found higher SOD activity in blood of infected dogs than in controls, along with lower CAT and GSH-Px activity. These results were also reported by Britti et al. (2008) in CVL and Dimri et al. (2012) in dogs infected with Dirofilaria immitis. In addition, Dimri et al. (2012) suggest that SOD activity increase might be attributed to upregulation of its synthesis to counteract free radicals. As a consequence, there is an increase in H2O2 production accompanied by a decrease GSH-Px and CAT, which can result in tissue damage. We found decreased activity of the antioxidant enzymes SOD, CAT and GSH-Px, which have not been previously studied in CVL.
As these antioxidant enzymes require trace elements for activity, we evaluated those elements in serum. Lower serum levels of Zn, Fe and Se and higher serum Cu were found in all infected dogs, especially those symptomatic, compared to controls. Changes in trace mineral metabolism are an integral aspect of acute-phase response and have been shown in CVL (Nieto et al. 2003; Pasa et al. 2003), possibly related to host susceptibility (Faryadi & Mohebali 2003; Weyenbergh et al. 2004). We did not observe a correlation of Cu or Zn serum levels with SOD activity in any group. However, GSH-Px activity was correlated with lower Se plasma/blood levels, and CAT activity with lower Fe blood levels, in all infected dogs. We did not observe a correlation of these elements with low enzyme activity in liver, spleen or lymph node tissue.
Classical histological alterations in CVL have been generally described as an intense chronic inflammatory reaction, fibrosis and degeneration of organs such as liver, spleen, lymph node, kidney and lung (Alvar et al. 2004; Alexandre-Pires et al. 2006; Melo et al. 2009). Our results were in accordance with previous reports and symptomatic dogs exhibiting a more pronounced chronic inflammatory reaction than controls. This indicates that severity of the disease is a consequence of histological alterations directly associated with increasing utilization or redistribution of trace elements and deficiency in antioxidant enzyme activity.
Tissue changes observed may result from ROS but also from NO production by inflammatory cells (Mauel et al. 1991; Assrue et al. 1994; Kocyigit et al. 2005). Research has shown that symptomatic dogs show a heavier parasite load and lower serum levels of NO than do asymptomatic (Zafra et al. 2008). In the present study, low NO levels with high parasite load in symptomatic dogs seemed to be related to Fe tissue deposition and severe lesions. We found systemic iron deposition in all infected dogs, with widely distributed haemosiderin deposits in symptomatic dogs. Prussian blue staining showed intracytoplasmatic granules especially inside macrophage inflammatory exudate or granulomas in liver, spleen and lymph node. Iron level was positively correlated with grade of inflammation, haemosiderin, and fibrous connective tissue in all organs of symptomatic dogs and negatively correlated with tissue antioxidant enzymes. Iron is an important component of biological processes and has been associated with ROS production, erythrocatheresis, localized tissue lesions and stimulating fibrinogenesis, probably due to oxygen free radical formation (Arthur 1996; Biswas et al. 1997; Britti et al. 2008). Although Fe2+ and Fe3+ may provoke LPO with subsequent cell membrane damage (Huynh and Andrew 2008), studies of lesions associated with chronic tissue Fe increase in CVL were not considered until this work.
Although bone marrow, potentially involved in iron metabolism, was not evaluated, our results may provoke discussion of increase in tissue iron deposition and cell damage in association with low antioxidant enzyme activity in CVL that could explain differences in clinical signs and factors associated with severity.
Organ tissue Fe deposits may result from multiple distinct but overlapping conditions: (i) Systemic oxidative stress may decrease RBC lifespan (senescent erythrocytes; de Luna et al. 2000; Samanta et al. 2012) and increase LPO levels in plasma of infected dogs, as demonstrated by higher LPO levels together with a positive correlation of LPO with Fe tissue deposits. Senescent erythrocytes are taken up through erythrophagocytosis and destroyed within macrophages, and Fe is released from haemoglobin and rapidly shifted to reutilization by incorporation into iron proteins or iron storage within ferritin (Weiss et al. 1999). (ii) CVL is a chronic infection characterized by a systemic inflammatory reaction in organs rich in cells of the mononuclear phagocyte system (Alvar et al. 2004). Tissue damage or inflammation in liver and spleen of infected dogs showed a positive correlation with LPO in plasma and Fe in tissue and negative correlation with antioxidant enzymes. (iii) According to Alexandre-Pires et al. (2006) and Melo et al. (2009), major abnormalities in the splenic microvascular system and liver architecture result in changes to circulatory dynamics, bringing about a reduction in blood flow directly related to congestion and haemosiderosis. (iv) Moreover, intracellular Fe overload may block the transcription of inducible NO synthase as described by Weiss et al. (1999). Thus, the lower NO formation in symptomatic dogs might be due to higher tissue Fe deposits.
In addition, we have shown evidence that dogs with symptomatic VL display enhanced LPO and Fe tissue deposition associated with decreased levels of antioxidant enzymes. These alterations in oxidant balance may further aggravate infection-associated pathology in canine VL as observed by Faryadi and Mohebali (2003) and Weyenbergh et al. (2004) in human VL. Together these results suggest a functional derangement in infected animals that may be responsible for the clinical features and open new approaches for treatment perspectives associated with antioxidants and the role of iron in the pathogenesis of CVL.
Acknowledgments
The authors thank Dr Marco Antonio Alves Carneiro for statistical advice; Miss Vânia Aparecida N. Silva and Olinda D. Rodrigues for histological technical support and Mr Weder Gomes de Oliveira for animal care. This study was supported by research grants from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (APQ00068-08; APQ-01355-09), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (473601/2009-5) and Pro-reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq-UFMG). We also thank Lucidus Consultancy (edit@lucidusconsultancy.com) for correction of English.
References
- Adamama-Moraitou KK, Saridomichelakis MN, Polizopoulou Z, Kritsepi M, Tsompanakou A, Koutinas AF. Short-term exogenous glucocorticosteroidal effect on iron and copper status in canine leishmaniosis (Leishmania infantum. Can. J. Vet. Res. 2005;69:287–292. [PMC free article] [PubMed] [Google Scholar]
- Alexandre-Pires G, Pais D, Correia M, Pina JA. Leishmaniosis: a report about the microvascular and cellular architecture of the infected spleen in Canis familiaris. Microsc. Res. Tech. 2006;69:227–235. doi: 10.1002/jemt.20267. [DOI] [PubMed] [Google Scholar]
- Alvar J, Molina R, San Andres M, et al. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann. Trop. Med. Parasitol. 1994;88:371–378. doi: 10.1080/00034983.1994.11812879. [DOI] [PubMed] [Google Scholar]
- Alvar J, Cañavate C, Molina R, Moreno J, Nieto J. Canine leishmaniasis. Adv. Parasitol. 2004;57:1–88. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- de Amorin IFG, Freitas E, Alves CF, et al. Humoral immunological profile and parasitological statuses of Leishmune® vaccinated and visceral leishmaniasis infected dogs from an endemic area. Vet. Parasitol. 2010;173:55–63. doi: 10.1016/j.vetpar.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Anstead GM, Chandrasekar B, Zhao W, Yang J, Perez L, Melby P. Malnutrition alters the innate immune response and increases early visceralization following Leishmania donovani infection. Infect. Immun. 2001;69:4709–4718. doi: 10.1128/IAI.69.8.4709-4718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo AP, Mayrink W, Machado-Coelho GL. The influence of copper, selenium and zinc on the response to Montenegro skin test in subjects vaccinated against American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2008;102:64–69. doi: 10.1016/j.trstmh.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Arthur MJ. Iron overload and liver fibrosis. J. Gastroenterol. Hepatol. 1996;11:1124–1129. doi: 10.1111/j.1440-1746.1996.tb01840.x. [DOI] [PubMed] [Google Scholar]
- Assrue J, Cunha FQ, Epperlein M. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur. J. Immunol. 1994;24:672–676. doi: 10.1002/eji.1830240328. [DOI] [PubMed] [Google Scholar]
- Biswas T, Ghosh DK, Mukherjee N, Ghosal J. Lipid peroxidation of erythrocytes in visceral leishmaniasis. J. Parasitol. 1997;83:151–152. [PubMed] [Google Scholar]
- Bretagne S, Durand R, Olivi M, et al. Real-time PCR as a new tool for quantifying Leishmania infantum in liver in infected mice. Clin. Diagn. Lab. Immunol. 2001;4:828–831. doi: 10.1128/CDLI.8.4.828-831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britti D, Sconza S, Morittu VM, Santori D, Boari A. Superoxide dismutase and Glutathione peroxidase in the blood of dogs with Leishmaniasis. Vet. Res. Commun. 2008;32:S251–S254. doi: 10.1007/s11259-008-9121-3. [DOI] [PubMed] [Google Scholar]
- Cunningham AC. Parasitic adaptive mechanisms in infection by Leishmania. Exp. Mol. Pathol. 2002;72:132–141. doi: 10.1006/exmp.2002.2418. [DOI] [PubMed] [Google Scholar]
- Deane LM, Deane MP. Sobre a biologia do Phlebotomus longipalpis, transmissor da leishmaniose visceral em zona endêmica do Estado do Ceará. I. Distribuição, predominância e variação estacional. Rev. Bras. Biol. 1955;15:83–95. [Google Scholar]
- Dimri U, Singh SK, Sharma MC, Behera SK, Kumar D, Tiwari P. Oxidant/antioxidant balance, minerals status and apoptosis in peripheral blood of dogs naturally infected with Dirofilaria immitis. Res. Vet. Sci. 2012;93:296–299. doi: 10.1016/j.rvsc.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Faryadi M, Mohebali M. Alterations of serum zinc, copper and iron concentration in patients with acute and chronic cutaneous leishmaniasis. Iran. J. Publ. Health. 2003;32:53–58. [Google Scholar]
- Gioda CR, de Oliveira-Barreto T, Prímola-Gomes TN, et al. Cardiac oxidative stress is involved in heart failure induced by thiamine deprivation in rat. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H2039–H2045. doi: 10.1152/ajpheart.00820.2009. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS. Analysis of nitrate, nitrite, and nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Heidarpour M, Soltani S, Mohri M, Khoshnegah J. Canine visceral leishmaniasis: relationships between oxidative stress, liver and kidney variables, trace elements, and clinical status. Parasitol. Res. 2012;111:1491–1496. doi: 10.1007/s00436-012-2985-8. [DOI] [PubMed] [Google Scholar]
- Huynh C, Andrews NW. Iron acquisition within host cells and the pathogenicity of Leishmania. Cell Microbiol. 2008;10:293–300. doi: 10.1111/j.1462-5822.2007.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Metrologia Normalizalização e Qualidade Industrial – INMETRO. 2010. Orientações sobre validação de métodos de ensaios químicos-DOQ-CGCRE-008. Rio de Janeiro. 2. ed. 33 p.
- International Organization for Standardization – ISO. 1999. Part 1: Water quality – Calibration and evaluation of analytical methods and estimation of performance characteristics. 8466-1 Switzerland, 11 pp.
- Kocyigit A, Keles H, Selek S, Guzel S, Celik H, Erel O. Increased DNA damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat. Res. 2005;585:71–78. doi: 10.1016/j.mrgentox.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough HJ, Farra L, Randall RJ. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- de Luna R, Ferrante M, Severino L, et al. Decreased lipid fluidity of the erythrocyte membrane in dogs with leishmaniasis associated anaemia. J. Comp. Pathol. 2000;122:213–216. doi: 10.1053/jcpa.1999.0357. [DOI] [PubMed] [Google Scholar]
- Mancianti F, Gramiccia M, Gradoni L, Pieri S. Studies on canine leishmaniasis control. I. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans. R. Soc. Trop. Med. Hyg. 1998;82:566–567. doi: 10.1016/0035-9203(88)90510-x. [DOI] [PubMed] [Google Scholar]
- Mauel J, Ransijn A, Buchmuller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on n L-arginine-dependent process that produces nitrogen derivatives. J. Leukoc. Biol. 1991;49:73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- Mauricio IL, Stothard JR, Miles MA. The strange case of Leishmania chagasi. Parasitol. Today. 2000;16:188–189. doi: 10.1016/s0169-4758(00)01637-9. [DOI] [PubMed] [Google Scholar]
- Melo FA, Moura EP, Ribeiro RR, et al. Hepatic extracellular matrix alterations in dogs naturally infected with Leishmania (Leishmania) chagasi. Int. J. Exp. Pathol. 2009;90:538–548. doi: 10.1111/j.1365-2613.2009.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Carpenter S, Singh S. Low serum zinc levels in an endemic area of visceral leishmaniasis in Bihar, India. Indian J. Med. Res. 2010;131:793–798. [PubMed] [Google Scholar]
- National Association of Testing Authorities – NATA. 1997. (Australia), Format and content of test methods and procedures for validation and verification of chemical test methods, technical note. 8.
- Nieto J, Alvar J, Mullen AB, et al. Pharmacokinetics, toxicities, and efficacies of sodium stibogluconate formulations after intravenous administration in animals. Antimicrob. Agents Chemother. 2003;47:2781–2787. doi: 10.1128/AAC.47.9.2781-2787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrinieri S, Ravicini S, Rossi G, Roura X. Serum concentrations of the derivatives of reactive oxygen metabolites (d-ROMs) in dogs with leishmaniosis. Vet J. 2010;186:393–395. doi: 10.1016/j.tvjl.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Pasa S, Kargin F, Bildik A, Seyrek K, Ozbel Y, Ozensoy S. Serum and hair levels of zinc and other elements in dogs with visceral leishmaniasis. Biol. Trace Elem. Res. 2003;94:141–147. doi: 10.1385/BTER:94:2:141. [DOI] [PubMed] [Google Scholar]
- Samanta S, Ghoshal A, Bhattacharya K, Saha B, Walden P, Mandal C. Sialoglycosylation of RBC in visceral leishmaniasis leads to enhanced oxidative stress, calpain-induced fragmentation of spectrin and hemolysis. PLoS ONE. 2012;7:1–10. doi: 10.1371/journal.pone.0042361. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shaw JJ. Further thoughts on the use of the name Leishmania (Leishmania) infantum chagasi for the aetiological agent of American visceral leishmaniasis. Mem. Inst. Oswaldo Cruz. 2006;101:577–579. doi: 10.1590/s0074-02762006000500017. [DOI] [PubMed] [Google Scholar]
- da Silva SM. Avaliação clínica e laboratorial de cães naturalmente infectados por Leishmania (Leishmania) chagasi (CUNHA & CHAGAS, 1937) submetidos a um protocolo terapêutico em clínica veterinária de Belo Horizonte. Belo Horizonte: Universidade Federal de Minas Gerais; 2007. [Google Scholar]
- da Silva SM, Ribeiro VM, Ribeiro RR, Tafuri WL, Melo MN, Michalick MS. First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet. Parasitol. 2009;166:159–162. doi: 10.1016/j.vetpar.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Souza CC, Fabrino JHF, Beinner MA, et al. Development and validation of methods for the determination of copper and iron in serum of dogs with canine visceral leishmaniasis using multivariate optimization and GFAAS. Anal. Methods. 2013;5:3129–3135. [Google Scholar]
- Tafuri WL, Santos RL, Arantesa RM, et al. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J. Immunol. Methods. 2004;292:17–23. doi: 10.1016/j.jim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Tafuri WL, De Oliveira MR, Melo MN. Canine visceral leishmaniasis: a 726 remarkable histopathological picture of one case reported from Brazil. Vet. Parasitol. 2001;96:203–212. doi: 10.1016/s0304-4017(00)00436-2. [DOI] [PubMed] [Google Scholar]
- Weiss G, Umlauft F, Urbanek M, et al. Associations between cellular immune effector function, iron metabolism and disease activity in patients with chronic hepatitis C virus infection. J. Infect. Dis. 1999;180:1452–1458. doi: 10.1086/315052. [DOI] [PubMed] [Google Scholar]
- Weyenbergh JV, Santana G, D'Oliveira A, Jr, et al. Zinc/copper imbalance reflects immune dysfunction in human leishmaniasis: an ex vivo and in vitro study. BMC Infect. Dis. 2004;4:1–17. doi: 10.1186/1471-2334-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra R, Jaber JR, Pérez-Écija RA, Barragán A, Martínez-Moreno A, Pérez J. High iNOS expression in macrophages in canine leishmaniasis is associated with low intracellular parasite burden. Vet. Immunol. Immunopathol. 2008;123:353–359. doi: 10.1016/j.vetimm.2008.02.022. [DOI] [PubMed] [Google Scholar]


