Abstract
Weil's disease, the most severe form of leptospirosis, is characterized by jaundice, haemorrhage and renal failure. The mechanisms of jaundice caused by pathogenic Leptospira remain unclear. We therefore aimed to elucidate the mechanisms by integrating histopathological changes with serum biochemical abnormalities during the development of jaundice in a hamster model of Weil's disease. In this work, we obtained three-dimensional images of infected hamster livers using scanning electron microscope together with freeze-cracking and cross-cutting methods for sample preparation. The images displayed the corkscrew-shaped bacteria, which infiltrated the Disse's space, migrated between hepatocytes, detached the intercellular junctions and disrupted the bile canaliculi. Destruction of bile canaliculi coincided with the elevation of conjugated bilirubin, aspartate transaminase and alkaline phosphatase levels in serum, whereas serum alanine transaminase and γ-glutamyl transpeptidase levels increased slightly, but not significantly. We also found in ex vivo experiments that pathogenic, but not non-pathogenic leptospires, tend to adhere to the perijunctional region of hepatocyte couplets isolated from hamsters and initiate invasion of the intercellular junction within 1 h after co-incubation. Our results suggest that pathogenic leptospires invade the intercellular junctions of host hepatocytes, and this invasion contributes in the disruption of the junction. Subsequently, bile leaks from bile canaliculi and jaundice occurs immediately. Our findings revealed not only a novel pathogenicity of leptospires, but also a novel mechanism of jaundice induced by bacterial infection.
Keywords: bacterial invasion, cell junction, hepatocyte couplet, jaundice, Leptospira, scanning electron microscope
Leptospirosis is a zoonotic disease of global importance caused by pathogenic Leptospira. Weil's disease is the most severe form of human leptospirosis, which is characterized by jaundice, haemorrhage and renal failure (Levett 2001; Bharti et al. 2003). More than 50,000 cases of severe leptospirosis are reported each year with a case fatality rate higher than 10% (WHO 1999). Although jaundice has been one of the well-known symptoms of leptospirosis since its discovery, its mechanism still remains unknown.
Jaundice refers to the yellow discoloration of the sclerae, mucous membranes and skin and results from either enhanced bilirubin production or impaired bilirubin handling (uptake, conjugation or biliary excretion) by the liver (Roche & Kobos 2004). As jaundice is caused by dysfunctions at any steps of bilirubin metabolism, in some cases, it is difficult to elucidate its main mechanism. Several kinds of bacterial infection are known to cause jaundice through a variety of mechanisms (Trauner et al. 1998; Chand & Sanyal 2007).
Leptospira are spiral-shaped, extremely thin bacteria. They can bore through a gel-like medium in a cork-screw-like manner (Goldstein & Charon 1988). They are able to penetrate abraded skin and mucous membrane and migrate in host tissue (Bharti et al. 2003). Several functional and histopathological studies on leptospirosis-related jaundice have been made on human patients (Ramos-Morales et al. 1959; Arean 1962a; Kobayashi 2001; De Brito et al. 2006) and experimental animals (Arean 1962b; Miller & Wilson 1966; Higgin & Cousineau 1977; Van den Ingh & Hartman 1986; Nally et al. 2004). In human autopsies and animal models of acute Leptospira infection, hepatic cord disarray and dilation of intercellular space are commonly reported (Arean 1962a; Miller & Wilson 1966; De Brito et al. 2006). Degenerative lesions, inflammation and necrosis are minimal (Nally et al. 2004; De Brito et al. 2006). Serum biochemical studies on human patients have demonstrated that concentrations of aspartate transaminase (AST) and alanine transaminase (ALT) are moderately increased, accompanied by minor increase in alkaline phosphatase (ALP) concentration (Ramos-Morales et al. 1959; Kobayashi 2001). From these findings, most researchers have reported that impairment of bile excretion, intrahepatic cholestasis, is related to jaundice (Ramos-Morales et al. 1959; Higgin & Cousineau 1977; Kobayashi 2001; Bharti et al. 2003), but the mechanism is still unclear. Moreover, there are few reports that correlated histopathological findings of liver with serum biochemical studies and localization of the organisms in leptospirosis or that examine the time course of histopathological changes and its association with leptospiral localization.
In this study, we examined the association between serum biochemical, histopathological changes and leptospiral localization during the development of jaundice in a hamster model of Weil's disease. Hamsters inoculated with virulent Leptospira strains are used as animal models because their clinical manifestations and histopathological changes are similar to those of human leptospirosis (Haake 2006; Silva et al. 2008). For scanning electron microscope (SEM) observation, freeze-cracking and cross-cutting methods were used to obtain three-dimensional images of the liver. To investigate direct interactions between hepatocytes and the organism, hepatocyte couplets isolated from hamsters were co-cultured with either pathogenic or non-pathogenic leptospires.
Materials and methods
Bacteria and animals
Leptospira interrogans serovar Losbanos strain K37 was isolated from a rat trapped in Metro Manila, Philippines, in 2006 (Villanueva et al. 2010). The organism was passaged through hamsters to maintain virulence and then subcultured in Korthof's media less than three times prior to use in infection experiments. Leptospira biflexa serovar Patoc strain Patoc I was maintained in our laboratory through continuous passages in Korthof's medium. Prior to inoculation, these leptospires were grown at 30 °C to a stationary phase. The bacterial cell concentration was determined using a Thoma counting chamber under a dark-field microscope. Male golden Syrian hamsters were purchased from Japan SLC, Inc., Shizuoka, Japan. Hamsters were housed in an animal facility and fed standard hamster chow Labo H Standard (NOSAN Co., Yokohama, Japan) and water ad libitum.
Animal infection
Three- to five-week-old hamsters were injected subcutaneously with 104 of L. interrogans strain K37 in a final volume of 100 μl. The 50% lethal dose for this animal model was reported to be 100 (Villanueva et al. 2014). Uninfected control animals were injected with phosphate-buffered saline (PBS) alone. For survival assay, animals were monitored daily for signs of illness including weight loss and mobility loss and were euthanized when they appeared moribund. For the other experiments, hamsters were euthanized at the indicated days.
Enumeration of live leptospires in the liver
The number of leptospires present per cm3 of infected liver was determined by the limiting dilution method. Briefly, we euthanized the hamsters and obtained their livers at 3, 5 and 7 days postinfection. A lobe of the liver was collected and homogenized in the same volume of PBS at 4 °C. Fivefold serial dilutions of the homogenate were made. One hundred microlitres of these dilutions was mixed with the same volume of 2 × Korthof's media containing STAFF (sulfamethoxazole, trimethoprim, amphotericin B, fosfomycin and 5-fluorouracil) (Chakraborty et al. 2011) in 96-well plates. The plates were incubated at 30 °C for 21 days and examined for growth of leptospires using a dark-field microscope at 400× magnification.
Ethical Approval
Animal experiments were reviewed and approved by the Ethics Committee on Animal Experiments at the Faculty of Medical Sciences, Kyushu University. The experiments were carried out under the conditions indicated in the Regulations for Animal Experiments of Kyushu University and law 105 and notification 6 of the Government of Japan.
Serum biochemical analysis
Hamsters were anesthetized with sevoflurane (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan), and blood was obtained by cardiac puncture at the indicated time points. Serum was separated using Microtainer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and stored at 20 °C until analysis. Serum AST, ALT, ALP and γ-glutamyl transpeptidase (γ-GTP) levels were determined by Monolis, Inc., Tokyo, Japan. Serum total and direct bilirubin levels were measured using Nescoat Bilirubin Kit-K (Alfresa Pharma Corporation, Osaka, Japan).
Isolation and culture of hamster hepatocyte couplets
Hepatocyte couplets were isolated from 4-week-old male Syrian hamsters according to the two-step liver perfusion method as previously reported (Seglen 1976; Maslansky & Williams 1982) after modification. Briefly, the hamsters were anesthetized with sevoflurane. In Step 1 of the perfusion, the liver was washed out with a Ca++- and Mg++-free Hanks' balanced salt solution supplemented with 0.5 mM EGTA and 10 mM HEPES for 8 min at 6 ml/min. The portal vein was cannulated with a 23-gauge butterfly needle for injection. The subhepatic inferior vena cava was severed to allow the solution to escape. In Step 2 of the perfusion, a 0.05% type I collagenase (Sigma-Aldrich, St. Louis, MO, USA) solution in Leibovitz's L-15 (Gibco, Grand Island, NY, USA) supplemented with 4 mM CaCl2, MgSO4 and 10 mM HEPES was circulated through the liver for 8 min at 6 ml/min. At the beginning of this step, the subhepatic vena cava was clamped and the thoracic vena cava was severed. All perfused solutions were prewarmed at 37 °C. Gravity maintained the perfusion flow in both steps.
After the perfusion, the liver was excised and placed in cold L-15 supplemented with 10 mM HEPES (washing solution). Using two pairs of forceps, the lobes were torn apart and shaken in the solution to detach the cells. The cell suspension was filtered through one- and three-layer gauze and then centrifuged at 50 g for 2 min. The pellet was resuspended in 20 ml of the fresh washing solution. This centrifugation was repeated three times to remove non-parenchymal hepatocytes. After the final centrifugation, cells were suspended in L-15 supplemented with 1% newborn calf serum, 4 mM CaCl2, MgSO4 and 10 mM HEPES (culture medium). One hundred thousand cells were inoculated onto glass coverslips in 24-well plates and cultured at 37 °C. After an interval of 2 h, to allow the attachment of cells on the coverslips, the culture medium containing unattached cells was withdrawn and fresh culture medium was added. Then, the cells were cultured for 5 h to allow adjacent hepatocyte pairs to reorganize cell junctions.
Infection of hepatocyte couplets
For ex vivo infection, the hepatocytes on the coverslips were washed two times with washing solution. Leptospira interrogans strain K37 or L. biflexa strain Patoc I were centrifuged and suspended to 107 per ml in the washing solution. Five hundred microlitres of the bacterial suspension was added to the hepatocytes and incubated at 37 °C for 1 h. Cells were washed to remove unattached bacteria and fixed with a mixture of 4% paraformaldehyde (PFA) and 0.01% glutaraldehyde (GA) at 4 °C. Fixed cells were used for observation in both SEM and immunofluorescent microscope.
Scanning electron microscopy
For observation of liver tissue, infected hamsters were euthanized at 7 or 8 days postinfection. Food was withdrawn in the evening before the experiment, and water was available ad libitum. The liver was perfused from the left ventricle with 0.5% heparin in PBS and then a mixture of 4% PFA and 0.1% GA in PBS. After excision, they were cut into 2 × 2 × 10 mm pieces and kept in 2% GA at 4 °C. Prior to freeze-cracking or cross-cutting procedure, they were postfixed with 4% osmium tetroxide for 1 h. For the freeze-cracking method, after washing with PBS, the tissue pieces were immersed in 50% dimethyl sulfoxide and frozen on a metal plate chilled with liquid nitrogen. They were then split into about 2 × 2 × 2 mm pieces using a precooled razor blade and hammer. Cracked pieces were thawed in cold PBS. For the cross-cutting method, after washing in PBS, the tissue pieces were cut into approximately 2 × 2 × 2 mm pieces with a pair of blades crossed in PBS. The liver pieces that were processed as mentioned above or fixed hepatocytes on the coverslips were dehydrated in a graded series of acetone, freeze-dried with t-butyl alcohol and coated with osmium in an osmium-plasma coater HPC-1S (Vacuum Device Inc., Ibaraki, Japan). The samples were observed with JEOL JXA8600 M (JEOL, Tokyo, Japan).
Immunofluorescent microscopy of hamster liver tissue
For observation of liver tissue, the liver was obtained from infected hamsters at 9 days postinfection. After perfusion with a mixture of 4% PFA and 0.1% GA in PBS, the liver was cut (3 × 3 × 10 mm) and washed with PBS. The cut liver was blocked with 3% bovine serum albumin in PBS (blocking buffer), washed with PBS and incubated with a rabbit anti-strain K37 antiserum (1:200) at 4 °C overnight. After washing with PBS, the piece was incubated with a goat anti-rabbit Alexa Fluor 488 antibody (1:500; Molecular Probes Inc., Eugene, OR, USA) for 3 h at room temperature to stain the bacteria. The stained piece was washed with PBS and then freeze-cracked as described above. The cracked pieces were stained with 2 μg/ml 4′,6-diamidino-2-phenylindole (DAPI), mounted on microscopic slides and observed by Confocal Microscope A1 (Nikon Corporation, Tokyo, Japan).
Immunofluorescent microscopy of hepatocyte couplets isolated from hamsters
The fixed hepatocytes on coverslips were washed with PBS and permeabilized with 0.2% TritonX-100 in PBS for 20 min at room temperature. Then, the cells were blocked with the blocking buffer, washed with PBS and incubated with rabbit anti-strain K37 antiserum or rabbit anti-strain Patoc I antiserum (1:200) for 30 min at room temperature. After washing with PBS, the cells were incubated with a mixture of goat anti-rabbit Alexa Fluor 488 antibody (1:500) and 100 nM Acti-stain 555 phalloidin (Cytoskeleton, Inc., Denver, CO, USA) for 30 min at room temperature. After final washing, the coverslips were mounted on microscopic slides using Slowfade Gold Antifade Reagent (Molecular Probes Inc.) with 2 μg/ml DAPI and observed with Confocal Microscope A1.
For enumeration of leptospires adhering to the entire surface of each couplet, we observed the couplets on the coverslips in 24-well plate under immunofluorescent microscope IX71 (Olympus Corporation, Tokyo, Japan) changing the focal plane. We randomly selected 50 couplets that showed actin accumulation and no bleb formation. The entire surface of a couplet was divided into two distinct regions: total cell and perijunctional regions as previously reported (Crawford et al. 1991). The perijunctional region was defined as an ellipse with its long axis overlying the junction of the two hepatocytes with its transverse axis extending one-third of the transverse diameter of each cell. The total cell region included the perijunctional region. The surface area of each region was estimated from optical microscopic images. Based on the aforementioned conditions, the area of total cell region averaged 525 ± 97 μm2 and the perijunctional region constituted 19 ± 3% (means ± SD) of the total cell region. The density of adhering leptospires was defined as [number of adhering leptospires]/[area of region of interest].
Statistical analysis
The data were analysed using Welch's t-test. P values less than 5% were considered significant.
Results
Course of infection and progress of jaundice
We used hamsters infected subcutaneously with 104 of L. interrogans serovar Losbanos strain K37 (Villanueva et al. 2010) as an animal model of jaundice caused by pathogenic leptospires. The hamsters did not show any symptoms until 7 days postinoculation. After 8 days, loss of activity, ruffled fur, closing of eyes and weight loss were observed in the hamsters. By 10 days postinoculation, all hamsters were moribund (Figure 1a). Autopsy revealed jaundice, splenomegaly and haemorrhage in the lung, kidney and intestine of infected hamsters. Leptospires were recovered from the blood, kidney, liver and lung. From these data, we confirmed that strain K37 caused systemic infection and moribundity in hamsters.
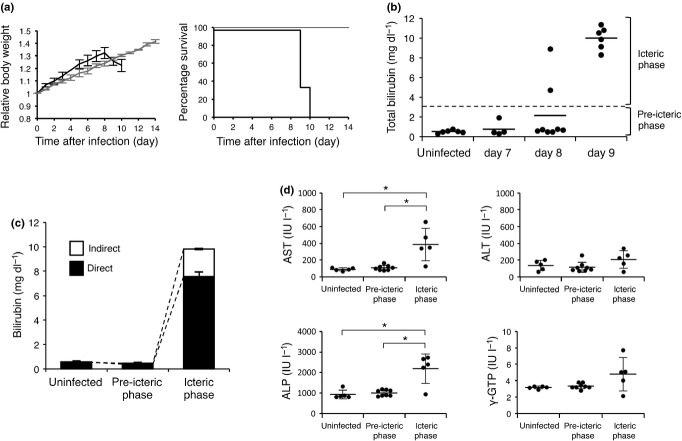
Figure 1.
Leptospira interrogans strain K37 infection causes sudden onset of jaundice and cholestasis-type abnormality of serum chemistries. (a) Relative body weight and survival of uninfected hamsters (n = 3, grey) or hamsters subcutaneously infected with L. interrogans strain K37 (n = 6, black). (b) Serum total bilirubin concentrations of uninfected (n = 6) or infected hamsters at seventh (n = 4), eighth (n = 8) or ninth (n = 6) day postinoculation. Horizontal dashed line indicates 3 mg/dl to distinguish pre-icteric and icteric phase. Horizontal bars indicate the mean. (c,d) Serum measurements of uninfected hamsters (n = 6) and infected hamsters, which were divided into two groups (pre-icteric phase; n = 10 and icteric phase; n = 8) according to serum bilirubin concentration. (c) Serum concentrations of direct and indirect bilirubin. (d) Serum concentrations of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (γ-GTP). Data are shown as the mean ± SD. Statistical significance is shown by *P < 0.05 as determined by Welch's t-test.
To analyse the progression of jaundice caused by strain K37 infection, we first measured the serum total bilirubin levels of the infected hamsters at 7, 8 and 9 days postinfection (Figure 1b). As a normal control, the serum total bilirubin levels of uninfected hamsters were less than 1 mg/dl. At 7 days, the serum total bilirubin levels were the same for the infected and uninfected hamsters. At 8 days, 25% (2 of 8) of infected hamsters were found to have elevated bilirubin levels (>3 mg/dl). At 9 days, the levels increased to around 10 mg/dl in all hamsters (6/6). These data indicated that the onset of jaundice caused by strain K37 was sudden and progressed within a day.
Serum biochemical analysis of Leptospira-induced jaundice
Hyperbilirubinaemia can result from either increased bilirubin production or impaired bilirubin handling (uptake, conjugation or biliary excretion) in the liver (Roche & Kobos 2004). To determine the type of Leptospira-induced jaundice and hepatic disorders, we measured serum direct bilirubin, AST, ALT, ALP and γ-GTP levels (Moseley 1996). Infected hamsters were divided into two groups according to serum total bilirubin concentrations (<3 mg/dl: pre-icteric phase and ≥3 mg/dl: icteric phase; Figure 1b), and they were compared with uninfected controls. In the icteric phase group, there was a predominant increase in direct bilirubin level rather than indirect bilirubin (Figure 1c). Serum AST, ALT, ALP and γ-GTP levels in the pre-icteric phase group were the same as the uninfected group (Figure 1d). In the icteric phase group, serum AST and ALP levels were significantly (P < 0.05) elevated in comparison with uninfected and pre-icteric phase groups. On the other hand, serum ALT and γ-GTP levels were only slightly, but not significantly, elevated (Figure 1d). These data indicate that elevation of serum direct bilirubin levels occurred with other serum abnormalities and that jaundice in the hamster model of leptospirosis closely matched cholestatic jaundice rather than increased bilirubin load from hemolysis or hepatocellular injury.
Histopathological analysis of the liver
By immunofluorescent staining, we confirmed the presence of many leptospires surrounding the hepatocytes of icteric hamsters (Figure 2a). We measured the number of leptospires in liver tissue at 3, 5 and 7 days postinfection. Colonization of the liver by leptospires was highly evident at 5 days. The number of leptospires increased to about 107 per cm3 of liver tissue at 7 days (Figure 2b). We thought that some structural changes in the liver due to bacterial load triggered acute jaundice. To examine the possibility, we studied the ultrastructure of infected and uninfected hamster livers by SEM (Figure 2d,e). Two different surfaces of the liver were observed using two methods of preparation: freeze-cracking and cross-cutting. The freeze-cracking method can crack the liver parenchyma and expose the flat fractured surfaces (Iida 1984) showing cross section of sinusoids, endothelial cells, Disse's space, hepatocytes and bile canaliculi. The cross-cutting method can separate hepatocytes along the intercellular junctions and expose the intercellular surfaces (Motta & Fumagalli 1975) showing the smooth surfaces of hepatocytes, and bile canaliculi running along the surfaces (Figure 2c). We assessed the morphological changes in intercellular contacts between adjoining hepatocytes using freeze-cracking method. We performed the cross-cutting method to assess the morphological changes in hepatocyte surface and bile canaliculi.
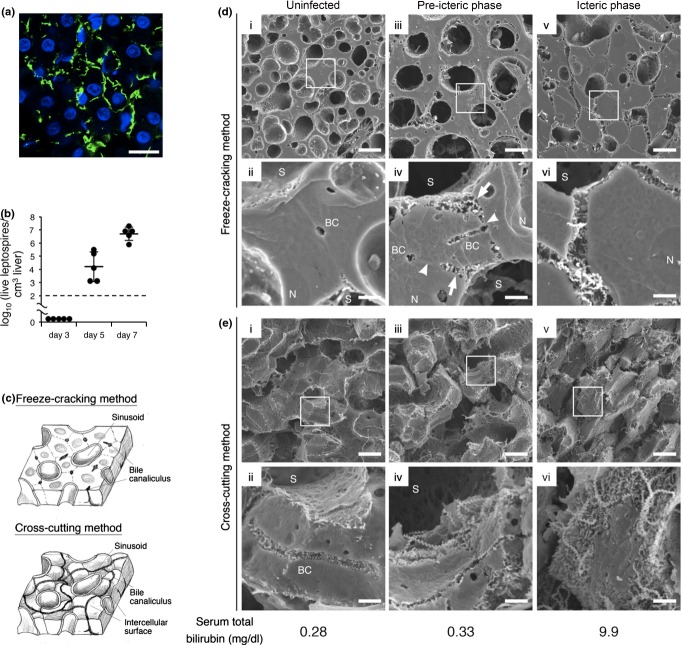
Figure 2.
Disruption of intercellular adhesion caused by leptospires in the liver triggers jaundice. (a) Representative confocal images of liver from hamsters infected with Leptospira interrogans strain K37 at eighth day postinoculation showing leptospires stained with rabbit polyclonal antisera and Alexa Fluor 488 conjugated anti-rabbit monoclonal antibody (green), and nuclei of liver cells stained with DAPI (blue) Scale bar, 10 μm. (b) Numbers of culturable leptospires in 1 cm3 of hamster livers at third, fifth and seventh day postinoculation determined by dilution method. Black dashed line represents the limit of detection. Data are the mean ± SD. (c) Schematic diagram of the liver structure exposed by two methods of sample preparation. Freeze-cracking method exposes flat fractured surface (top image). Cross-cutting method exposes intercellular surface (bottom image). (d,e) Representative scanning electron microscope images of the livers from uninfected hamsters (i and ii) or infected hamsters in pre-icteric (iii and iv) or icteric phase (v and vi) using freeze-cracking (d) and cross-cutting methods (e). The framed areas in the top images are enlarged in the bottom images. The scale bars represent 10 μm for the top images and 2 μm for the bottom images. The arrows show hepatocyte membrane detached from the adjacent cell. The arrowheads point to hepatocyte membranes that remained attached to the adjoining cell. Total bilirubin concentration of each hamster is indicated below the images. BC, bile canaliculi; N, nucleus of the hepatocyte; and S, sinusoid.
In the liver of an uninfected hamster, the flat fractured surface showed sinusoids and hepatic cords that were composed of hepatocytes tightly attached to each other. Bile canaliculi were observed at the centre of the attached cell membranes (Figure 2d, i and ii). Observation of the intercellular surface showed smooth surface of hepatocytes and bile canaliculi with microvilli along the surface (Figure 2e, i and ii).
In the liver of a pre-icteric phase hamster, we found a part of hepatocyte membrane around the sinusoid that was detached from the adjacent hepatocyte membrane (Figure 2d, iii and iv). The widened space between the endothelia and hepatocytes, which formed as a result of the detachment, was observed to be filled with many leptospires. The other part of intercellular contacts appeared to have remained attached (Figure 2d, iv). Cross-cutting results showed that the smooth surfaces were narrower than those of uninfected hamsters (Figure 2e, iii and iv), suggesting that the area of intercellular contacts shrunk. The bile canaliculi were neither tortuous nor dilated, and microvilli on the bile canaliculi were preserved (Figure 2e, iv).
In the liver of an icteric phase hamster, hepatocytes were almost completely detached from each other and hepatic cell cords were disarranged (Figure 2d, v and vi). Many leptospires were observed between the hepatocytes (Figure 2d, vi). Using the cross-cutting method, we could not distinguish the sinusoid, the intercellular surface or the bile canaliculi (Figure 2e, v and vi).
At higher magnification of the infected liver, leptospires were found not only in the widened space formed by cell detachment but also between adjoining hepatocytes (Figure 3). The leptospires seemed to have left grooves on the smooth surfaces of the hepatocytes, the shapes of which were similar to the organisms adhering to the membrane (Figure 3a). Occasionally, leptospires penetrated the hepatocyte membrane (Figure 3b). These data suggested that the development of jaundice is associated with the disruption of intercellular adhesion of hepatocytes caused by leptospiral invasion.
Figure 3.
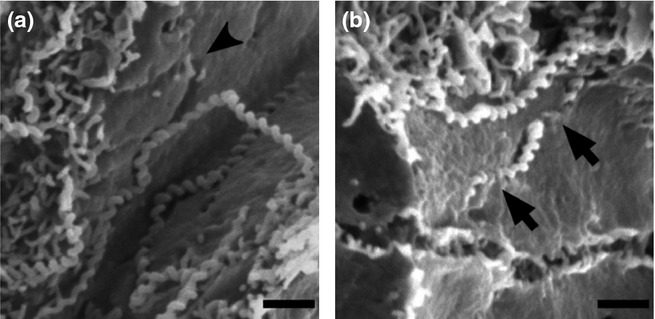
Leptospira invade liver parenchyma by paracellular route. (a,b) Representative scanning electron microscope images of liver from infected hamsters showing intercellular surface of hepatocytes exposed by cross-cutting method. The black arrowhead shows the trail formed by a leptospire on the intercellular surface (a). The black arrows point to penetration by a leptospire through cell membrane (b). Scale bars, 1 μm.
Interaction between leptospires and host hepatocytes ex vivo
Invasion of the intercellular spaces by leptospires is accompanied by their adhesion to the hepatocyte surface. To better understand the mechanisms of the migration of Leptospira from the bloodstream, we examined the interactions between the leptospires and hepatocyte couplets isolated from hamsters. Tissue culture is useful in the study of host–parasite interactions because it is independent of host immunological factors. Isolated hepatocyte couplets have been well characterized in rats (Gautam et al. 1987; Boyer et al. 1990; Thibault et al. 1992). The couplets retain intercellular junctions and polarities (Boyer et al. 1990).
We isolated hepatocyte from hamsters using the collagenase perfusion method (Seglen 1976). After 7-h incubation, actin filaments gathered between two adjacent hepatocytes (Figure 4a) as reported in the original rat hepatocyte couplets, suggesting that cell junctions and polarities were reorganized (Gautam et al. 1987; Thibault et al. 1992). We then co-cultured the hamster hepatocytes with pathogenic (L. interrogans) or non-pathogenic (L. biflexa) leptospires. After co-incubation for 1 h, pathogenic leptospires were associated with hepatocyte couplets mainly around intercellular junctions (Figure 4a,b,d). Scanning electron micrographs revealed some pathogenic leptospires entering the cell membrane at the cellular junction (Figure 4e). The cell association of non-pathogenic leptospires was significantly lower than that of pathogenic leptospires (Figure 4a,c).
Figure 4.
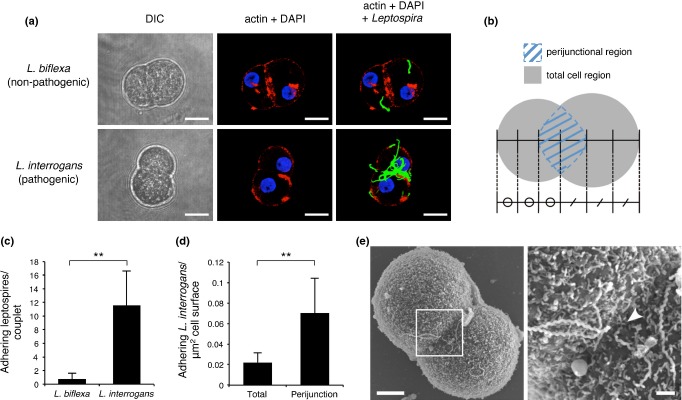
Attachment and invasion to host hepatocytes are characteristic of pathogenic leptospires. (a) Representative confocal images of isolated hamster hepatocyte couplets infected with pathogenic (Leptospira interrogans) or non-pathogenic (Leptospira biflexa) leptospires on coverslips for 1 h showing actin accumulation stained with Acti-stain 555 phalloidin (red), and leptospires stained with rabbit polyclonal antisera and Alexa Fluor 488 conjugated anti-rabbit monoclonal antibody (green). Nuclei of liver cells were stained with DAPI (blue). Non-adherent bacteria were washed out. Projections of three-dimensional reconstructions are shown Scale bar, 10 μm. (b) Schematic diagram of hepatocyte couplet indicating the total cell (grey) and perijunctional (blue striped) regions. The perijunctional region was defined as an ellipse with its long axis overlying the junction of the two hepatocytes, and its transverse axis extending over one-third of the transverse diameter of each cell. The total cell region included the perijunctional region. (c,d) Analysis of the number of pathogenic or non-pathogenic leptospires adhering to the total cell region of a couplet (c) and the number of adhering pathogenic leptospires per μm2 of total cell or perijunctional regions (d). n = 50 per group. Data are shown as the mean ± SD. Statistical significance is shown by **P < 0.001 as determined by Welch's t-test. (e) Representative scanning electron microscope images of isolated hamster hepatocyte couplet infected by L. interrogans strain K37. The framed area in the left image is enlarged in the right image. The scale bar represents 5 μm for the left image and 1 μm for the right image. The white arrowhead shows one end of a spirochete entering the intercellular junction (right).
Discussion
There are some previous studies that focused on the mechanism of jaundice caused by Leptospira infection in guinea pig (Arean 1962a, b; Higgin & Cousineau 1977) and hamster models (Miller & Wilson 1966; Van den Ingh & Hartman 1986). Histopathological changes in liver observed by optical microscopy or transmission electron microscopy are mainly shown as hepatic cord disarray (Arean 1962a, b; Miller & Wilson 1966; Nally et al. 2004; De Brito et al. 2006) and dilation of intercellular space (Miller & Wilson 1966; Van den Ingh & Hartman 1986). Leptospires are localized in the Disse's space and the dilated intercellular space (Miller & Wilson 1966; Van den Ingh & Hartman 1986; Matsunaga et al. 2006). Serum biochemical change is reported as moderate increase in transaminase (Arean 1962b). These previous studies, however, have been conducted on animals showing developed symptoms, and information about serum biochemical and histopathological changes in the early stage of the infection is limited. In our study, we analysed infected but pre-icteric hamsters as well as icteric hamsters. Our study demonstrated that the elevation of serum bilirubin level coincided with abnormality of other serum biochemistries and structural changes associated with Leptospira invasion of the liver. After co-incubation with hepatocyte couplets isolated from hamsters, pathogenic leptospires preferentially localized in the intercellular junction, which was in congruence with histological observations. These findings yielded important insights into the mechanism of jaundice caused by Leptospira infection.
On the 7, 8 and 9 days after infection, hamsters with elevated serum bilirubin levels showed only mild elevation of serum AST and ALP levels. Considering that the conjugated bilirubin was predominantly elevated, a series of abnormalities in liver function tests matched with a cholestatic pattern, rather than hepatocellular injury or hemolysis (Kamath 1996; Moseley 1996). Although the production of hemolysin by pathogenic leptospires was reported (Zhang et al. 2005), our results suggested that its contribution to jaundice was minimal. These abnormalities, except for ALP elevation, were consistent with previous reports on leptospirosis in human (Ramos-Morales et al. 1959; Kobayashi 2001) and guinea pigs (Ramos-Morales et al. 1959; Arean 1962b; Nally et al. 2004). ALP level was reported to be elevated in infected humans but low in guinea pigs.
Scanning electron microscope observation revealed that the advanced detachment of hepatocytes coincided with the elevation of serum bilirubin (Figure 2d,e). Intercellular junctions between hepatocytes are composed of gap, tight and adherence junctions and desmosomes and play important roles in hepatic function (Vinken et al. 2006). A tight junction seals the bile canaliculi and prevents bile from leakage to the bloodstream (Anderson 1996). Therefore, disruption of tight junction induces bile leakage and results in hyperbilirubinemia. In our model, partial detachment of the cells was observed in the pre-icteric phase (Figure 2d, iv), suggesting that structural change precedes bile leakage. This hepatocyte dissociation extended throughout the junction within the next 24 h, and the moment it was completed, jaundice occurred immediately (Figure 2d, vi). The partial detachments were shown to be initiated around the sinusoids with spirochetes (Figure 2d, iv). This indicates that partial detachment is a local change in membrane affected by leptospires and that adhesion of the hepatocytes is maintained in areas where leptospires are absent. Therefore, the mechanism of this change is different from necrosis or redistribution of cytoskeletons caused by toxins, as reported in several pathogenic bacteria (Balkovetz & Katz 2003). It was reported that Leptospira infection induced apoptosis of hepatocyte (Merien et al. 1998). We cannot exclude the possibility that there were some apoptotic cell in infected liver of our model. This apoptosis might explain the increase of unconjugated bilirubin. Merien et al. reported that around 5% of hepatocytes were apoptotic at 48 h after infection with 108 leptospires. However, we found cell junctions of almost all cells were disrupted. We therefore think that the main cause of jaundice in our animal model of experimental leptospirosis was not due to apoptosis of hepatocytes.
The three-dimensional details of the hepatocyte surface suggests that leptospiral invasion through intercellular junctions is directly involved in cell detachment. Bacterial-shaped grooves on the cell surface indicated that leptospires bore their way through the intercellular contacts and widened the space. Multiple boring through a region of membrane adhesion by numerous leptospires eventually resulted in the detachment of hepatocytes. Leptospires have a characteristic spiral shape and corkscrew motion, and these features are considered to relate with its pathogenicity for host tissue invasion (Barocchi et al. 2002). Penetration of cells such as human umbilical vein endothelial cells, Madin-Darby canine kidney cells, Vero cells and macrophage by leptospires were reported in in vitro studies (Barocchi et al. 2002; Merien et al. 1997, Thomas & Higbie 1990). Therefore, we carefully investigated whether leptospires localized in hepatocytes using SEM. We could find that some leptospires penetrated hepatocyte membrane by the cross-cutting method (Figure 3b), but none of the leptospires localized in hepatocytes by the freeze-cracking method (Figure 2e). We also found uptake of leptospires by Kupffer cells as previously reported (Nally et al. 2004). Our findings suggest that pathogenic leptospires invade host hepatocytes intercellularly rather than intracellularly. However, the substances attracting the spirochete towards the bile canaliculi are not known.
These histological changes are consistent with serum biochemical abnormalities. As long as the detachments were limited to the sinusoidal parts, and the tight junctions forming bile canaliculi were intact, bile is considered to flow through the canaliculi, and serum bilirubin levels were maintained at a normal level. Because bacterial invasion caused only mild damage to the hepatocytes, the serum transaminase level was also normal. When the detachments were extended to the bile canaliculi and tight junctions were disrupted, bile must have initiated leakage into the sinusoids, and serum-conjugated bilirubin levels were elevated because bile in the canaliculi contains mostly conjugated bilirubin. ALP localized in the bile canaliculi also appeared in the serum.
Previous histopathological studies of the liver on Weil's disease were mainly conducted using optical microscope (Arean 1962a, b; Nally et al. 2004; De Brito et al. 2006) or transmission electron microscope (Miller & Wilson 1966; Van den Ingh & Hartman 1986). These two methods, however, have disadvantages in the pathological analysis of leptospirosis. The resolution of optical microscopy is too low to observe the hepatocellular junction or fine spiral bacteria, such as Leptospira. Transmission electron microscope provides only cross-section images; therefore, it is hard to figure out overall lesion structure as well as distribution of leptospires. Scanning electron microscope can produce high-resolution images with a large depth of field. Together with two kinds of preparation methods, we successfully obtained three-dimensional images that display structural changes in the liver and the distribution of spirochetes in a relatively large field. These methods might be able to also reveal novel aspects of leptospiral pathogenicity in other organs.
Previous reports have demonstrated that L. interrogans adhere to host cells and the extracellular matrix and that pathogenic strains attached, to a greater degree, than non-pathogenic strains (Tsuchimoto et al. 1984; Ballard et al. 1986; Thomas & Higbie 1990; Martinez-Lopez et al. 2010; Pinne et al. 2012). We found that L. interrogans, but not L. biflexa, preferentially adhered to hepatocyte attachment sites and invaded intercellular junctions of hepatocytes ex vivo. These results indicate that not only specific attachment to host hepatocytes, but also invasion into hepatocyte junction are mechanisms characteristic of pathogenic leptospires. The molecular mechanism of the interaction between leptospires and the host cells, however, remains to be elucidated. Recently, it was reported that L. interrogans binds to chondroitin sulphate B proteoglycans (Breiner et al. 2009) and cadherin (Evangelista et al. 2014) on the surface of a mammalian cell. Because they are expressed on hepatocyte membranes (Toledo & Dietrich 1977; Vinken et al. 2006), these substances may play a role in Leptospira attachment to host hepatocytes.
Intrahepatic cholestasis is defined as impairment of bile formation induced by drugs or infections, and autoimmune, metabolic or genetic disorders (Trauner et al. 1998). Using several experimental models, mechanisms of intrahepatic cholestasis are explained as a functional impairment of hepatocellular uptake and canalicular excretion of bile constituents, which is associated with disfunction of membrane transport, decreased integrity of tight junction, loss of membrane fluidity and changes in the cytoskeleton. Increased paracellular permeability likely results in regurgitation of bile constituents into plasma (Chand & Sanyal 2007; Crocenzi et al. 2003; Mottino et al. 2007). In our model, the destruction of tight junction in the liver parenchyma, due to Leptospira invasion, causes leakage of bile. Therefore, the mechanism of Leptospira-induced jaundice is considered different from that of intrahepatic cholestasis reported previously. Supporting this consideration, our SEM observations revealed that bile canaliculi were neither tortuous nor dilated, which are features observed in intrahepatic cholestasis (Layden et al. 1975; Yoshino 1980). In conclusion, the migration of corkscrew-shaped bacteria with characteristic motility, from sinusoid to bile canaliculi, by paracellular routes, causes jaundice in Leptospira infected hamsters and is a novel mechanism of jaundice induced by Leptospira infection (Figure 5). Although this conclusion is based only on the hamster model, the similarities in histopathological (Arean 1962a; De Brito et al. 2006) and serum biochemical (Ramos-Morales et al. 1959; Kobayashi 2001) observations between our animal model and human patients support this mechanism of jaundice, and it can be applied to human Weil's disease. This is the first report that revealed three-dimensional structural change of the liver corresponding with abnormal serum chemistries, and leptospiral preference to perijunctional regions of hapatocytes.
Figure 5.
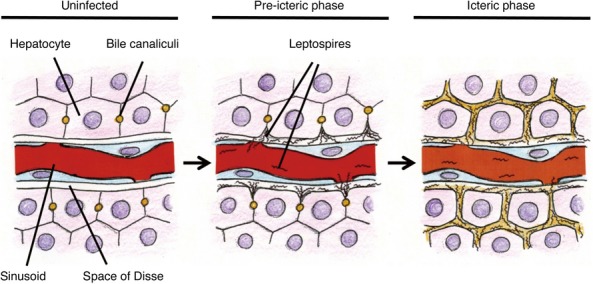
Schematic representation of jaundice caused by pathogenic Leptospira. In the normal state, a tight junction seals bile canaliculi and prevents leakage of bile to bloodstream (left). Once pathogenic leptospires reach the liver sinusoids, they have the property to attach to and invade intercellular junctions of hepatocytes and this invasion contributes to disrupt the junction (middle). When the tight junction is disrupted, bile leaks from bile canaliculi and this result in jaundice (right).
Acknowledgments
This study was supported by a grant of the Science and Technology Research Partnership for Sustainable Development program from Japan Science and Technology Agency and Japan International Cooperation Agency. We thank Takaya Segawa, Hirohumi Omura, Hideko Kameyama and Naomi Hidaka for their technical support.
References
- Anderson JM. Leaky junctions and cholestasis: a tight correlation. Gastroenterology. 1996;110:1662–1665. doi: 10.1053/gast.1996.v110.agast961662. [DOI] [PubMed] [Google Scholar]
- Arean VM. The pathologic anatomy and pathogenesis of fatal human leptospirosis (Weil's disease) Am. J. Pathol. 1962a;40:393–423. [PMC free article] [PubMed] [Google Scholar]
- Arean VM. Studies on the pathogenesis of leptospirosis. II. A clinicopathologic evaluation of hepatic and renal function in experimental leptospiral infections. Lab. Invest. 1962b;11:273–288. [PubMed] [Google Scholar]
- Balkovetz DF, Katz J. Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect. 2003;5:613–619. doi: 10.1016/s1286-4579(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Ballard SA, Williamson M, Adler B, Vinh T, Faine S. Interaction of virulent and avirulent leptospires with primary cultures of renal epithelial cells. J. Med. Microbiol. 1986;21:59–67. doi: 10.1099/00222615-21-1-59. [DOI] [PubMed] [Google Scholar]
- Barocchi MA, Ko AI, Reis MG, McDonald KL, Riley LW. Rapid translocation of polarized MDCK cell monolayers by Leptospira interrogans, an invasive but nonintracellular pathogen. Infect. Immun. 2002;70:6926–6932. doi: 10.1128/IAI.70.12.6926-6932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Phillips JM, Graf J. Preparation and specific applications of isolated hepatocyte couplets. Methods Enzymol. 1990;192:501–516. doi: 10.1016/0076-6879(90)92090-z. [DOI] [PubMed] [Google Scholar]
- Breiner DD, Fahey M, Salvador R, Novakova J, Coburn J. Leptospira interrogans binds to human cell surface receptors including proteoglycans. Infect. Immun. 2009;77:5528–5536. doi: 10.1128/IAI.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Miyahara S, Villanueva SYAM, Saito M, Gloriani NG, Yoshida S. A novel combination of selective agents for isolation of Leptospira species. Microbiol. Immunol. 2011;55:494–501. doi: 10.1111/j.1348-0421.2011.00347.x. [DOI] [PubMed] [Google Scholar]
- Chand N, Sanyal AJ. Sepsis-induced cholestasis. Hepatology. 2007;45:230–241. doi: 10.1002/hep.21480. [DOI] [PubMed] [Google Scholar]
- Crawford JM, Vinter DW, Gollan JL. Taurocholate induces pericanalicular localization of C6-NBD-ceramide in isolated hepatocyte couplets. Am. J. Physiol. 1991;260:G119–G132. doi: 10.1152/ajpgi.1991.260.1.G119. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Mottino AD, Sánchez Pozzi EJ, et al. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut. 2003;52:1170–1177. doi: 10.1136/gut.52.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito T, Menezes LF, Lima DMC, Lourenço S, Silva AMG, Alves VAF. Immunohistochemical and in situ hybridization studies of the liver and kidney in human leptospirosis. Virchows Arch. 2006;448:576–583. doi: 10.1007/s00428-006-0163-z. [DOI] [PubMed] [Google Scholar]
- Evangelista K, Franco R, Schwab A, Coburn J. Leptospira interrogans binds to cadherins. PLoS Negl. Trop. Dis. 2014;8:e2672. doi: 10.1371/journal.pntd.0002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A, Ng OC, Boyer JL. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987;7:216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Goldstein SF, Charon NW. Motility of the spirochete Leptospira. Cell Motil. Cytoskeleton. 1988;9:101–110. doi: 10.1002/cm.970090202. [DOI] [PubMed] [Google Scholar]
- Haake DA. Hamster model of leptospirosis. Curr. Protoc. Microbiol. 2006;12:12E.2.1–12E.2.13. doi: 10.1002/9780471729259.mc12e02s02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgin R, Cousineau G. The pathogenesis of leptospirosis II. Jaundice in experimental leptospirosis in guinea pigs. Can. J. Comp. Med. 1977;41:182–187. [PMC free article] [PubMed] [Google Scholar]
- Iida N. Freeze-fracture of biological specimens prior to conductive staining. Arch. Histol. Jpn. 1984;47:79–88. doi: 10.1679/aohc.47.79. [DOI] [PubMed] [Google Scholar]
- Kamath PS. Clinical approach to the patient with abnormal liver test results. Mayo Clin. Proc. 1996;71:1089–1094. doi: 10.4065/71.11.1089. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. Clinical observation and treatment of leptospirosis. J. Infect. Chemother. 2001;7:59–68. doi: 10.1007/s101560100011. [DOI] [PubMed] [Google Scholar]
- Layden TJ, Schwarz J, Boyer JL. Scanning electron microscopy of the rat liver. Studies of the effect of taurolithocholate and other models of cholestasis. Gastroenterology. 1975;69:724–738. [PubMed] [Google Scholar]
- Levett PN. Leptospirosis. Clin. Microbiol. Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez DG, Fahey M, Coburn J. Responses of human endothelial cells to pathogenic and non-pathogenic Leptospira species. PLoS Negl. Trop. Dis. 2010;4:e918. doi: 10.1371/journal.pntd.0000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslansky CJ, Williams GM. Primary cultures and the levels of cytochrome P450 in hepatocytes from mouse, rat, hamster, and rabbit liver. In Vitro. 1982;18:683–693. doi: 10.1007/BF02796423. [DOI] [PubMed] [Google Scholar]
- Matsunaga J, Werneid K, Zuerner RL, Frank A, Haake DA. LipL46 is a novel surface-exposed lipoprotein expressed during leptospiral dissemination in the mammalian host. Microbiology. 2006;152:3777–3786. doi: 10.1099/mic.0.29162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merien F, Baranton G, Perolat P. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect. Immun. 1997;65:729–738. doi: 10.1128/iai.65.2.729-738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merien F, Truccolo J, Rougier Y, Baranton G, Perolat P. In vivo apoptosis of hepatocytes in guinea pigs infected with Leptospira interrogans serovar Icterohaemorrhagiae. FEMS Microbiol. Lett. 1998;169:95–102. doi: 10.1111/j.1574-6968.1998.tb13304.x. [DOI] [PubMed] [Google Scholar]
- Miller NG, Wilson RB. Electron microscopy of the liver of the hamster during acute and chronic leptospirosis. Am. J. Vet. Res. 1966;27:1071–1081. [PubMed] [Google Scholar]
- Moseley RN. Evaluation of abnormal liver function tests. Med. Clin. North Am. 1996;80:887–906. doi: 10.1016/s0025-7125(05)70472-7. [DOI] [PubMed] [Google Scholar]
- Motta P, Fumagalli G. Structure of rat bile canaliculi as revealed by scanning electron microscopy. Anat. Rec. 1975;182:499–513. doi: 10.1002/ar.1091820408. [DOI] [PubMed] [Google Scholar]
- Mottino AD, Hoffman T, Crocenzi FA, Sánchez Pozzi EJ, Roma MG, Vore M. Disruption of function and localization of tight junctional structures and Mrp2 in sustained estradiol-17β-D-glucuronide-induced cholestasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G391–G402. doi: 10.1152/ajpgi.00496.2006. [DOI] [PubMed] [Google Scholar]
- Nally JE, Chantranuwat C, Wu XY, et al. Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am. J. Pathol. 2004;164:1115–1127. doi: 10.1016/S0002-9440(10)63198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinne M, Matsunaga J, Haake DA. Leptospiral outer membrane protein microarray, a novel approach to identification of host ligand-binding proteins. J. Bacteriol. 2012;194:6074–6087. doi: 10.1128/JB.01119-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Morales F, Díaz-Rivera RS, Cintrón-Rivera AA, Rullán JA, Benenson AS, Acosta-Matienzo J. The pathogenesis of leptospiral jaundice. Ann. Intern. Med. 1959;51:861–878. doi: 10.7326/0003-4819-51-5-861. [DOI] [PubMed] [Google Scholar]
- Roche SP, Kobos R. Jaundice in the adult patient. Am. Fam. Physician. 2004;69:299–304. [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Silva EF, Santos CS, Athanazio DA, et al. Characterization of virulence of Leptospira isolates in a hamster model. Vaccine. 2008;26:3892–3896. doi: 10.1016/j.vaccine.2008.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault N, Claude JR, Ballet F. Actin filament alternation as a potential marker for cholestasis: a study in isolated rat hepatocyte couplets. Toxicology. 1992;73:269–279. doi: 10.1016/0300-483x(92)90069-q. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Higbie LM. In vitro association of leptospires with host cells. Infect. Immun. 1990;58:581–585. doi: 10.1128/iai.58.3.581-585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo OM, Dietrich CP. Tissue specific distribution of sulfated mucopolysaccharides in mammals. Biochim. Biophys. Acta. 1977;498:114–122. doi: 10.1016/0304-4165(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N. Engl. J. Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- Tsuchimoto M, Niikura M, Ono E, Kida H, Yanagawa R. Leptospiral attachment to cultured cells. Zentralbl. Bakteriol. Mikrobiol. Hyg. A. 1984;258:268–274. doi: 10.1016/s0176-6724(84)80044-9. [DOI] [PubMed] [Google Scholar]
- Van den Ingh TS, Hartman EG. Pathology of acute Leptospira interrogans serotype Icterohaemorrhagiae infection in the Syrian hamster. Vet. Microbiol. 1986;12:367–376. doi: 10.1016/0378-1135(86)90086-6. [DOI] [PubMed] [Google Scholar]
- Villanueva SYAM, Ezoe H, Baterna RA, et al. Serologic and molecular studies of Leptospira and leptospirosis among rats in the Philippines. Am. J. Trop. Med. Hyg. 2010;82:889–898. doi: 10.4269/ajtmh.2010.09-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva SYAM, Saito M, Tsutsumi Y, et al. High virulence in hamsters of four dominantly prevailing Leptospira serovars isolated from rats in the Philippines. Microbiology. 2014;160:418–428. doi: 10.1099/mic.0.072439-0. [DOI] [PubMed] [Google Scholar]
- Vinken M, Papeleu P, Snykers S, et al. Involvement of cell junctions in hepatocyte culture functionality. Crit. Rev. Toxicol. 2006;36:299–318. doi: 10.1080/10408440600599273. [DOI] [PubMed] [Google Scholar]
- WHO. Leptospirosis worldwide, 1999. Wkly Epidemiol. Rec. 1999;74:237–242. [PubMed] [Google Scholar]
- Yoshino K. Scanning electron microscopy on the rat liver with alpha- naphthylisothiocyanate-induced cholestasis. Gastroenterol. Jpn. 1980;15:550–563. doi: 10.1007/BF02773758. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Geng Y, Bi B, et al. Identification and classification of all potential hemolysin encoding genes and their products from Leptospira interrogans serogroup Icterohaemorrhagiae serovar Lai. Acta Pharmacol. Sin. 2005;26:453–461. doi: 10.1111/j.1745-7254.2005.00075.x. [DOI] [PubMed] [Google Scholar]