Abstract
Background
Ovarian cancer is the sixth most common cancer among women and is usually diagnosed at an advanced stage. Bowel obstruction is a common feature of advanced or recurrent ovarian cancer. Patients with bowel obstruction are generally in poor physical condition with a limited life expectancy. Therefore, maintaining their QoL with effective symptom control is the main purpose of the management of bowel obstruction.
Objectives
To compare the effectiveness and safety of palliative surgery (surgery performed to control the cancer, reduce symptoms and improve quality of life for those whose cancer is not able to be entirely removed) and medical management for bowel obstruction in women with ovarian cancer.
Search methods
We searched the Cochrane Gynaecological Cancer Group Trials Register, The Cochrane Central Register of Controlled trials (CENTRAL), Issue 1 2009, MEDLINE and EMBASE up to February 2009. We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria
Studies that compared palliative surgery and medical interventions, in adult women diagnosed with ovarian cancer who had either full or partial obstruction of the bowel. Randomised controlled trials (RCTs) and non‐RCTs that used multivariable statistical adjustment for baseline case mix were eligible.
Data collection and analysis
Two review authors independently assessed whether potentially relevant studies met the inclusion criteria, abstracted data and assessed risk of bias. One non‐randomised study was identified so no meta‐analyses were performed.
Main results
The search strategy identified 183 unique references of which 22 were identified as being potentially eligible on the basis of title and abstract. Only one study met our inclusion criteria and was included in the review. It analysed retrospective data for 47 women who received either palliative surgery (n = 27) or medical management with Octreotide (n = 20) and reported overall survival and perioperative mortality and morbidity. Women with poor performance status were excluded from surgery. Although six (22%) women who received surgery had serious complications of the operation and three (11%) died of complications, multivariable analysis found that women who received surgery had significantly (p < 0.001) better survival than women who received Octreotide, after adjustment for important prognostic factors. However, the magnitude of this effect was not reported. Quality of life (QoL) was not reported and adverse events were incompletely documented.
Authors' conclusions
We found only low quality evidence comparing palliative surgery and medical management for bowel obstruction in ovarian cancer. Therefore we are unable to reach definite conclusions about the relative benefits and harms of the two forms of treatment, or to identify sub‐groups of women who are likely to benefit from one treatment or the other. However, there is weak evidence in support of surgical management to prolong survival.
Keywords: Adult; Female; Humans; Antiemetics; Antiemetics/therapeutic use; Antineoplastic Agents, Hormonal; Antineoplastic Agents, Hormonal/therapeutic use; Intestinal Obstruction; Intestinal Obstruction/drug therapy; Intestinal Obstruction/etiology; Intestinal Obstruction/surgery; Octreotide; Octreotide/therapeutic use; Ovarian Neoplasms; Ovarian Neoplasms/complications; Ovarian Neoplasms/pathology; Ovarian Neoplasms/surgery; Palliative Care; Palliative Care/methods
Plain language summary
Surgery compared to non‐surgical treatment to relieve symptoms of bowel obstruction in ovarian cancer
Ovarian cancer is the sixth most common cancer among women and is usually diagnosed at an advanced stage. Bowel obstruction is a common feature of advanced or recurrent ovarian cancer and causes vomiting, pain and diarrhoea. Patients with bowel obstruction are generally in poor physical condition with only a short time left to live. Therefore, maintaining their QoL with effective symptom control is the main purpose of the management of bowel obstruction.
We carried out a systematic review of published and unpublished studies that compared surgical and non‐surgical methods of managing bowel obstruction in women with ovarian cancer.
Women who are recommended for surgery are usually in better health than those who are not, so it can be difficult to disentangle the effects of surgery and the effects of their basic health. Therefore we only looked at studies that used statistical adjustment for the differences in underlying health between women who did and did not receive surgery.
We found only one relevant study. It included only 47 cases: 27 had an operation to relieve bowel obstruction and the 20 who did not have an operation were given a drug called Octreotide to control the amount of vomiting that often results from bowel obstruction.
Among the 27 women who had an operation, six women could not have their bowel obstruction corrected because the cancer had spread too far, six women had serious complications of surgery and three died of these complications. Nevertheless, the authors of the study reported that women who had the operation survived longer, on average, than those who did not, even after allowing for their underlying better health. It was unclear how much of the difference in survival could be ascribed to the differences in treatment and how much to the better health of women undergoing surgery.
Unfortunately the study did not assess their QoL or level of pain.
The study reported the numbers of women who could start eating again after their treatment (surgery or Octreotide) but it didn't analyse this allowing for the underlying difference in health of women in the two groups, so it is impossible to interpret these results.
We were therefore unable to reach definite conclusions about the relative benefits and harms of the two forms of treatment and we were unable to identify sub‐groups of women who are likely to benefit from one treatment or the other.
Background
Description of the condition
Ovarian cancer is the sixth most common cancer among women . A woman's cumulative risk of developing ovarian cancer by age 65 years is 0.5%: 0.4% in less developed countries and 0.7% in more developed countries. It is less common in women under the age of 35 years, and its incidence increases with age (GLOBOCAN 2002).
Ovarian cancer is characterised by its insidious onset and absence of early specific symptoms. About 70% of patients are diagnosed at International Federation of Gynecology and Obstetrics (FIGO) stages III and IV (Shepherd 1989), having widespread tumour dissemination within the abdominal cavity with or without tumour spread to the liver, lungs or distant organs (Jemal 2008).
In Europe, just over a third of women with ovarian cancer are alive five years after diagnosis (EUROCARE 2003), largely because most patients present with disease that has spread beyond the ovaries (Jemal 2008). Long term survival rates have improved little over the last thirty years: between 1971 to 1975, the relative five‐year survival rate for women diagnosed in England and Wales was 23% whereas in 1991 to 1993 it was 29% (Quinn 2001).
Although more than 70% of women with advanced cancer respond to initial chemotherapy, most patients suffer from recurrent disease within the peritoneal cavity and eventually become resistant to chemotherapy (Markman 2008). Once the disease recurs, it usually becomes incurable despite further chemotherapy and surgery.
Epithelial ovarian cancer (EOC) arises from the surface covering of the ovary or the lining of ovarian cysts and comprises 90% of all ovarian cancers in women in the USA, with the other 10% being non‐epithelial such as sex‐chord stromal and germ cell tumours (Quirk 2005). Like most other epithelial cancers, it initially spreads by direct extension to adjacent organs such as the uterus, fallopian tubes, adnexi (tubes and ovaries) and, more occasionally, the rectum. However, the mechanism of metastatic spread in epithelial ovarian cancer differs from other epithelial cancers. Following direct extension, ovarian cancer cells are seeded in the peritoneal cavity and fluid and disseminated to other pelvic and abdominal organs by the transcoelomic route (Sundar 2006), a route that provides direct access to the abdominal peritoneal cavity. Unlike other epithelial malignancies such as bowel cancers, distant spread via the blood is not common at the time of diagnosis in epithelial ovarian cancers. Therefore, dissemination of malignant cells into the abdominal cavity, largely contributes to the morbidity and mortality related to vital organs particularly gastrointestinal and genitourinary systems.
Primary peritoneal and fallopian tube cancers are considered to have similar mechanisms of metastatic spread and the management of these cancers is almost identical to the management of ovarian cancer. Histological features and tumour biology of primary peritoneal and fallopian tube cancers are also very similar to those of ovarian cancers (Clayton 2005; Rabban 2005).
Bowel obstruction is a common feature of advanced or recurrent ovarian cancer. Unlike primary colorectal cancers, in which the cause of obstruction is mostly due to intra‐luminal compression of the large bowel (Caceres 2008), ovarian cancers more commonly cause small bowel rather than large bowel obstruction by extrinsic compression of tumour mass and enlarged lymph nodes. Other causes of obstruction include the tumour infiltration of mesentery (a membrane connecting bowels to the major vessels of the abdomen), bowel muscle or nerves. Oedema of bowel wall, faecal impaction, and constipating drugs can contribute to the development and severity of bowel obstruction. Occasionally, the obstruction may be due to benign causes such as adhesions, postradiation bowel damage, inflammatory bowel disease, or hernia. These patients are usually suitable for surgical management.
Although the true incidence of bowel obstruction in ovarian cancer is not known, several retrospective studies have suggested that it occurs in 25 to 50% of all cases (Caprotti 2006; Dvoretsky 1988; Tunca 1981). Progressive external compression of the bowel and its mesentery by ovarian carcinoma results in obstruction and may eventually lead to death in these patients.
Description of the intervention
Patients with bowel obstruction have usually been heavily treated with multiple chemotherapeutic agents and surgery, and become resistant to chemotherapy. The aim of any further treatment is therefore to relieve the symptoms related to bowel obstruction and improve the quality of life (QoL). The life expectancy is limited with a median survival of approximately four months (Pothuri 2003).
Management options are surgery which may include the insertion of colorectal stents, gastrostomy, chemotherapy including hormonal treatment or treatment with intravenous fluids and pharmacologic agents to relieve the symptoms related to obstruction.
The purpose of palliative surgery is to relieve the symptoms of bowel obstruction by means of four procedures: stoma formation, bypassing the obstruction, resection of bowel (Pothuri 2003) and placement of colorectal stents (Caceres 2008). The surgery is associated with a high incidence of morbidity (5 to 90%) and mortality (5 to 40%) (Clarke‐Pearson 1987; Ooijen 1993; Redman 1988). Major surgical complications include entero‐cutaneous or entero‐vaginal fistulas, anastomosis leaks (leakage where two ends of bowel join together), short bowel syndrome (malabsorption disorder caused by the surgical removal of the small bowel) and sepsis (Clarke‐Pearson 1987; Caprotti 2006).
Once the obstruction is relieved, a small proportion of patients may become suitable for further treatment with chemotherapy.
Most patients are not well enough to receive chemotherapy or are resistant to most available chemotherapeutic agents. However, among patients well enough to tolerate treatment, one study (Bryan 2006) found chemotherapy was as effective as surgery in the management of bowel obstruction related to advanced ovarian cancer; platinum sensitivity was the best predictor of a successful outcome.
Percutaneous endoscopic gastrostomy (PEG) placement, usually under sedation, can be used to achieve intestinal decompression. PEG relieves gas pressure produced when intestinal obstruction is present by placing a tube in the intestinal tract, usually via the nasal passages and the stomach (nasogastric route) and provides nutrition when the obstruction is resolved. It is feasible to use PEG to relieve nausea and vomiting in palliative settings. It is considered for patients presenting with recurrent bowel obstruction and previously treated with surgery for small bowel obstruction. For selected patients, placement of PEG tubes can be followed by administration of chemotherapy (Pothuri 2005).
Somatostatin and its analogues, octreotide and vapreotide, have been used to alleviate symptoms from malignant bowel obstruction in ovarian cancer (Mangili 1996; Mercadante 2004). Somatostatin inhibits glucagon and insulin hormones, reduces acid secretion, slows mobility of intestines and decreases bile flow (Reichlin 1983). Octreotide acts in a similar way to somatostatin but has a longer half‐life. It inhibits growth hormone, glucagon, and insulin more potently. Octreotide also suppresses luteinising hormone response to gonadotropin‐releasing hormone and inhibits release of gastrin, secretin, vasoactive intestinal peptide, motilin, and pancreatic polypeptide (Fallon 1994; Reichlin 1983).
Steroids have also been used to relieve bowel obstruction. Their effect on bowel obstruction has been controversial: they may reduce the level of obstruction indirectly by reducing tumour oedema (Fainsinger 1994). However, a Cochrane systematic review of the use of corticosteroids in bowel obstruction related to gynaecological or gastrointestinal malignancies showed no evidence that corticosteroids were effective in treating bowel obstruction (Feuer 1999).
Why it is important to do this review
These patients are generally in poor physical condition with a limited life expectancy. Therefore, maintaining their QOL with effective symptom control is the main purpose of the management of bowel obstruction. Given the high morbidity and mortality of surgery in these patients, it is often difficult to decide whether to perform surgery. The practice of surgical management of these patients varies between different countries, cancer centres and hospitals (Feuer 2000). Therefore, a systematic review is needed to evaluate the evidence relating to surgery and its effects on short term symptom control and, in the longer term, prolongation of life, symptom free survival and ability to offer further chemotherapy. Such a review will provide guidance for clinicians and specify the areas for further research in this field.
Previous reviews have considered the role of surgery (Feuer 2000) and separately the value of corticosteroids (Feuer 1999) for the resolution of symptoms of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers. More importantly, the pathophysiology of the development of bowel obstruction in ovarian cancer and gastrointestinal cancers are very different and, for this reason, we believe that the role of palliative surgery in ovarian cancer related bowel obstruction should be evaluated separately.
Objectives
To compare the effectiveness and safety of palliative surgery and medical management for bowel obstruction in women with ovarian cancer.
Methods
Criteria for considering studies for this review
Types of studies
We searched for relevant randomised controlled trials (RCTs), but did not find any, so the following types of non‐RCTs with concurrent comparison groups were included:
Quasi‐RCTs, non‐randomised trials, prospective and retrospective cohort studies, and case series of 30 or more patients.
Case‐control studies and case series of fewer than 30 patients were excluded.
In order to minimise the effects of selection bias (systematic differences between baseline characteristics of the groups that are compared), we included only studies that used statistical adjustment for baseline case mix using multivariable analyses (e.g. adjusting for age, performance status, grade, etc) if any constraints were placed on treatment allocation (e.g. women with poor performance status would not be given surgery) or if treatment allocation was based on clinician preference. Studies that did not report the criteria for treatment allocation were excluded.
Types of participants
Adult women with a confirmed pathological diagnosis of ovarian cancer, fallopian tube or primary peritoneal cancer who have either full or partial obstruction of the bowel. Borderline ovarian tumours were included; there were no restrictions by FIGO stage at diagnosis, or by previous treatment.
Types of interventions
Intervention:
Any surgical procedure aimed at palliative care (including stoma formation, bypassing the obstruction, resection of bowel, placement of colorectal stents and gastrostomy).
Comparison:
Any medical intervention aimed at relieving bowel obstruction:
Chemotherapy;
Hormonal therapy;
Somatostatin and its analogues e.g. octreotide, vapreotide;
Steroids;
Any other medical treatment aimed at relieving bowel obstruction; or
Best supportive care.
Types of outcome measures
Primary outcomes
Overall survival: survival until death from all causes.
Secondary outcomes
Recurrence‐free survival (time to recurrence of bowel obstruction)
QoL, measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication.
-
Adverse events classified according to CTCAE 2006:
direct surgical morbidity (death within 30 days, injury to bladder, ureter, vascular, small bowel or colon), presence and complications of adhesions, febrile morbidity, haematoma, local infection)
surgically related systemic morbidity (chest infection, thrombo‐embolic events (deep vein thrombosis and pulmonary embolism), cardiac events (cardiac ischemias and cardiac failure), cerebrovascular accident
recovery: delayed discharge, unscheduled re‐admission
-
toxicity; grouped as:
haematological
gastrointestinal
genitourinary
skin
neurological
pulmonary.
other adverse events.
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews. The following electronic databases were searched:
The Cochrane Gynaecological Cancer Review Group's Trial Register
Cochrane Central Register of Controlled Trials (CENTRAL)
MEDLINE
EMBASE
The Medline, EMBASE and CENTRAL search strategies based on terms related to the review topic are presented in Appendix 1, Appendix 2 and Appendix 3 respectively.
Databases were searched from 1966 until February 2009.
All relevant articles found were identified on PubMed and using the 'related articles' feature, a further search was carried out for newly published articles.
Searching other resources
Unpublished and Grey literature
Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov and www.cancer.gov/clinicaltrials were searched for ongoing trials. The main investigators of any relevant ongoing trials were contacted for further information, as were any major co‐operative trials groups active in this area.
Handsearching
Reports of conferences were handsearched in the following sources:
British Journal of Cancer.
British Cancer Research Meeting.
Annual Meeting of the International Gynecologic Cancer Society.
Annual Meeting of the British Gynaecological Cancer Society (BGCS).
Annual Meeting of the American Society of Gynecologic Oncologist (SGO).
Annual Meeting of European Society of Medical Oncology (ESMO).
Annual Meeting of the American Society of Clinical Oncology (ASCO).
Reference lists and Correspondence
The citation lists of included studies were checked and experts in the field contacted to identify further reports of trials.
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were removed and the remaining references were examined by two review authors (AB, AK) independently.
Copies of the full text of relevant references were obtained. The eligibility of retrieved papers was assessed independently by two review authors (AB, AK). Disagreements were resolved by discussion between the review authors. Reasons for exclusion were documented.
Data extraction and management
For included studies, data were abstracted as follows:
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
-
Study population
Total number enrolled
Patient characteristics
Age
Co‐morbidities
-
Ovarian cancer details at diagnosis
FIGO stage
Histological cell type
Tumour grade
Extent of disease (at time of palliative surgery)
Total number of intervention groups
-
Intervention details
Details of palliative surgery
Type of surgeon (gynae‐oncologist, gynaecologist, general surgeon)
Experience of surgeon
-
Comparison details
Type of control
Dose (if appropriate)
Duration (if appropriate)
Risk of bias in study (see below)
Duration of follow‐up
-
Outcomes – Overall survival, recurrence‐free survival, QOL, patient satisfaction and adverse events.
For each outcome: Outcome definition (with diagnostic criteria if relevant);
Unit of measurement (if relevant);
For scales: upper and lower limits, and whether high or low score is good
Results: Number of participants allocated to each intervention group;
For each outcome of interest: Sample size; Missing participants
Only one study met the inclusion criteria and this did not report the magnitude of differences in outcomes between treatment groups, so we were unable to perform any quantitative synthesis. Should more studies be identified for updates of the review, the methods specified in Differences between protocol and review will be employed.
Assessment of risk of bias in included studies
The risk of bias in included studies was assessed using the Cochrane Collaboration's tool (Higgins 2008). This included assessment of:
sequence generation
allocation concealment
blinding (Assessment of blinding was restricted to blinding of outcome assessors, since it is generally not possible to blind participants and treatment providers to surgical interventions)
incomplete outcome data: We coded a satisfactory level of loss to follow‐up for each outcome as:
Yes, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms
No, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms
Unclear if loss to follow‐up was not reported
selective reporting of outcomes
other possible sources of bias
The risk of bias in non‐randomised studies was assessed in accordance with four additional criteria:
Cohort selection
-
Were relevant details of criteria for assignment of patients to treatments provided?
Yes
No
Unclear
-
Was the group of women who received the experimental intervention (palliative surgery) representative?
Yes, if representative of women with ovarian cancer undergoing treatment for bowel obstruction
No, if group of patients was selected
Unclear, if selection of group was not described
-
Was the group of women who received the comparison intervention representative?
Yes, if drawn from the same population as the intervention group
No, if drawn from a different source
Unclear, if selection of group not described
Comparability of treatment groups
-
Were there no differences between the two groups or were differences controlled for, in particular with reference to age, small/large bowel obstruction, FIGO stage, histology?
Yes, if at least two of these characteristics were reported and any reported differences were controlled for
No, if the two groups differed and differences were not controlled for.
Unclear, if fewer than two of these characteristics were reported even if there were no other differences between the groups, and other characteristics had been controlled for.
The risk of bias tool was applied independently by two review authors (AK, AB) and differences were resolved by discussion or by appeal to a third review author (RN or HD). Results were presented in a risk of bias graph.
Results
Description of studies
Results of the search
The search strategy identified 105 references in MEDLINE, 130 in Embase, two in CENTRAL and six in the specialised register. When the search results were merged into Endnote and duplicates were removed, 183 unique references remained. The title and abstract screening identified 22 references as potentially eligible. The full text screening excluded 21 of these for the reasons described in the table Characteristics of excluded studies. The one remaining reference (Mangili 2005) reported a study that met our inclusion criteria and is described in the table Characteristics of included studies.
Searches of the grey literature did not identify any additional relevant studies.
Included studies
Design
The one included study (Mangili 2005) reported retrospective analysis of data on forty‐seven patients from three centres in Italy, who had concomitant recurrent epithelial ovarian cancer and intestinal obstruction and received either palliative surgery (n = 27) or Octreotide (n = 20). Patients with poor performance status were excluded from surgery so this prognostic factor showed an imbalance at baseline between the two groups (p = 0.03).
Participants
Mean age at diagnosis of intestinal obstruction was 58.7 years (range 31 to 77 years). Mean interval from diagnosis of ovarian tumour to occlusion was 26 months (range 3 to 75 months). Two patients were stage IIC according to FIGO, 2 were stage IIIB, 38 were stage IIIC, and 5 were stage IV. All patients had undergone previous debulking surgery and successive chemotherapy. Five patients were also submitted to radiotherapy. Diagnosis of bowel occlusion was made on the basis of the symptoms reported by patients and clinical evaluation, and confirmed by supine and upright radiography, abdominal ecotomography, and hydrosoluble contrast medium radiography. All patients reported more than three episodes of vomiting daily.
With the exception of performance status, patients were well matched between the two arms in terms of important prognostic factors (age, pain and nausea symptoms, vomiting, diarrhoea, palpable masses, previous chemotherapy and radiotherapy, ascites, occlusion site and diagnosis and occlusion time). For the distribution of these factors at baseline by treatment arm see the table Characteristics of included studies.
Interventions
The surgical procedures included eight colostomies, nine intestinal bypasses, three intestinal resections, one bypass and colostomy and in six women surgical correction was not possible so they had surgical exploration only. No patients were submitted to any surgical procedure after these operations. For women receiving Octreotide, the starting dose ranged from 0.3 to 0.6 mg daily by subcutaneous bolus or continuous infusion, and the dosage was increased until the symptoms resolved. The median dosage was 0.6 mg daily and the maximum utilised dosage was 0.9 mg daily.
Outcomes reported
The study reported overall survival, whether the patients could tolerate any oral intake, and perioperative mortality and morbidity in the surgical group. No other side effects were reported. Quality of life was not reported. The mean interval from diagnosis of obstruction to death was 79 days (range: 9‐350 days).
A multivariable Cox proportional hazards model was used to compare overall survival in the two arms; the model adjusted for: age, performance status (0, 1, 2), pain (grade 1, 2, 3), nausea (grade 1, 2, 3), vomiting (Yes, No), diarrhoea (Yes, No), palpable masses (Yes, No), previous chemotherapy (1, 2, >2), previous radiotherapy (Yes, No), ascites (Yes, No), occlusion site (small, large or small and large bowel) and time from diagnosis of ovarian cancer to occlusion (months). However, hazard ratios (HR) comparing the relative risk of survival in the two treatment groups were not reported. We requested these from the corresponding author but did not receive a reply. Kaplan‐Meier plots were not presented: the survival plots presented were based on the numbers surviving at 100‐day intervals and therefore were not true Kaplan‐Meier plots. Although the statistical significance (p‐value) of the difference in mortality between treatment groups was reported, we were unable to estimate the (HR) using any of the recommended methods (Parmar 1998).
Excluded studies
Twenty‐one references, describing 20 studies, were excluded after obtaining the full text, for the following reasons:
Five studies (Bais 1995; Bryan 2006; Gadducci 1998; Lund 1989; Sartori 2009) had the potential for extreme selection bias as treatment allocation was based on clinician preference and no statistical adjustment was carried out.
Two studies (Li 2004; Onsrud 2001 ) did not provide relevant details of criteria for assignment of patients to treatments, and thus had the potential for extreme selection bias
One study (Arvieux 2005) had no comparison group.
Six studies (Glass 1973; Larson 1989; Medina‐Franco 2008; Miller 2000; Paganelli 1990; Ross 2006; Scheidbach 1999) had fewer than 30 patients or were case reports, and had other contributory factors that resulted in exclusion.
One study (Dinstl 1976) reported on patients with peritoneal cancer, but patients were not classified by ovarian cancer or by sex. In one RCT (Xinopoulos 2004), six patients were reported to have ovarian cancer, but a breakdown by treatment arm was not given.
One reference (Krouse 2002) was a narrative of three case reports and a later article by the same author (Krouse 2008) was a commentary on surgical approaches to malignant bowel obstruction.
Laval 2007 was a review of different treatments and did not yield any further studies.
For further details of all excluded studies see the table Characteristics of excluded studies.
Risk of bias in included studies
The one included study (Mangili 2005) was at high risk of bias: it satisfied only three of the ten criteria that we used to assess risk of bias (see Figure 1).
1.
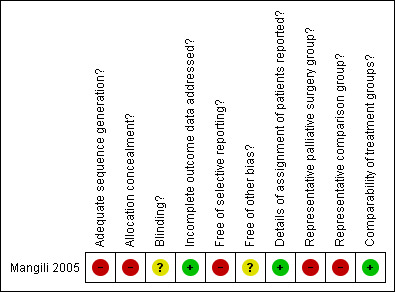
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
It reported a retrospective analysis so the method of sequence generation and concealment of allocation (which are relevant only to RCTs) were deemed to be unsatisfactory. It reported details of assignment of patients to groups: women with poor performance status were excluded from surgery. Consequently, the two treatment groups were not representative of women with recurrent ovarian cancer and bowel obstruction. The study did not report whether the outcome assessors were blinded. It was unclear whether all women with recurrent ovarian cancer and bowel obstruction who were treated at the three specified hospitals during a specified time period were included in the analysis; hence it was unclear whether any additional bias may have been present. A multivariable analysis was performed, adjusting for important prognostic factors including performance status for which there was an imbalance at baseline, so the two groups were deemed to be comparable. No loss to follow‐up was reported.
Effects of interventions
In the surgical group, six women (22%) died within 30 days of surgery and three of these deaths were due to surgical or anaesthetic complications. Six women (22%) experienced operative morbidity: two patients had wound infection, two had incision dehiscence, and two developed enterocutaneous fistula. In six women (22%), surgical correction of the bowel obstruction was not possible, because of diffuse carcinomatosis with concurrent infiltration of mesentery. Of the 21 patients who had definitive surgery, 16 (76%) patients were able to take a low‐residue diet. Of the remaining patients, three who survived tolerated only liquid intake, and the other two died without any oral intake. Of the six patients in whom surgical correction was not possible, the two patients who had percutaneous gastrostomy could take small amounts of fluid or ice. None of the other four patients was able to tolerate any oral intake.
In the Octreotide group, vomiting was controlled in all but one (5%) patient and one (5%) patient developed a fistula during treatment. Six (30%) patients were able to take a low‐residue diet. All patients received intravenous infusion as necessary. No other side effects were reported. Twelve patients (60%) were discharged from hospital and received medication at home. All the patients died with minimal distress.
Overall survival
In univariate analysis using the log‐rank test, the only significant predictors of survival were the type of treatment, performance status and the presence of palpable masses. Multivariable analysis using Cox regression) found that women who received surgery had a significantly (p < 0.001) better survival than women who received Octreotide, after adjustment for performance status, presence of palpable masses, age, presence of ascites, number of courses of previous chemotherapy, previous radiotherapy, site of occlusion, and diagnosis of tumour to occlusion time, but the HR was not reported.
Return to oral feeding and adverse events
Comparisons between the two groups in terms of return to oral feeding could not be made as this outcome was not analysed with statistical adjustment for the differences in prognostic factors between the two groups. Adverse events were inadequately reported and could not be compared between treatment arms.
Discussion
Summary of main results
We found only one study (Mangili 2005) that met our inclusion criteria. It reported retrospective data for 47 women who received either palliative surgery or medical management with Octreotide. Women with poor performance status were excluded from surgery. Although six (22%) women who received surgery had serious complications of the operation and three (11%) died of complications, multivariable analysis found that women who received surgery had significantly (p < 0.001) better survival than women who received Octreotide, after adjustment for important prognostic factors. However, the magnitude of this effect was not reported. Quality of life and adverse events were incompletely documented.
Overall completeness and applicability of evidence
Overall, the quality of the evidence was low (GRADE Working Group), because the review found only one relevant study, which was small and non‐randomised. This severely limits the conclusions that can be drawn. The one included study did not address many of the objectives of the review. Our primary outcome was incompletely documented as the study did not report the magnitude of the difference in overall survival between the two treatment groups e.g. using a HR or relative risk (RR). Although it reported the number of women who returned to oral feeding in each group, it did not analyse this outcome allowing for the differences in prognostic factors between the treatment groups. QoL was not reported using an instrument that assessed other aspects of QoL, in addition to return to oral feeding. Pain was not reported.
Women with bowel obstruction in ovarian cancer are generally in very poor health and have a short life expectancy. A good QoL after treatment should be of primary importance in what is a palliative treatment setting. Unfortunately this review was unable to assess this important outcome.
Quality of the evidence
The one included study included a small number of women (n = 47) and was at high risk of bias, largely because it was a non‐randomised retrospective study. Prognostic factors were reported so it was possible to assess imbalances at baseline. The only prognostic factor that differed significantly between the two groups at baseline was performance status: the treatment groups were well balanced with respect to other important factors. Analysis of survival — but not of other outcomes — was adjusted for differences in performance status between the groups. Nevertheless, it is difficult to be sure that the statistically significant difference in survival between the groups was due to the treatment they received rather than to the better performance status of women who received surgery, because the magnitude of the differences in survival were not reported for either unadjusted or adjusted analysis.
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature and all studies were sifted and data extracted by at least two reviewers independently. The review included non‐randomised studies and was not restricted to RCTs which provide the strongest level of evidence available. We made every attempt to minimise bias in the review process. We anticipated that selection bias was likely to be a real problem due to the non‐randomised assignment of patients to surgery. Patients suitable for palliative surgery tended to have better performance status and were in generally better health than those receiving the medical interventions, because treatment allocation depended on clinician preference. We attempted to minimise this bias by only including RCTs or quasi‐RCTs or non‐randomised studies of sufficient quality which included a multivariable analysis. Unfortunately we were able to include only one study of such quality that met the inclusion criteria.
A further threat to the validity of the review is likely to be the possibility of publication bias i.e. studies that did not find a statistically significant difference between treatments may not have been published. We were unable to assess this possibility as the analysis was restricted to a single study.
Agreements and disagreements with other studies or reviews
A qualitative systematic review (Feuer 2000) of surgery in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer found no evidence from a range of retrospective case series to favour surgery. Many of these case series were single arm studies. The review found that control of symptoms varied from 42% to over 80%, although it is often unclear how symptoms were measured and whether the symptom scores used were validated. It also found a large range in the rates of re‐obstruction, from 10 to 50%, although time to re‐obstruction was often not reported. There was a wide range of postoperative morbidity and mortality, although the definition of both of these surgical outcomes varied between papers. The pathophysiology of the development of bowel obstruction in ovarian cancer and gastrointestinal cancers are very different so our review evaluated the role of palliative surgery in ovarian cancer related bowel obstruction separately.
We excluded several studies from our review as they did not use multivariable analyses. The studies of Bais 1995, Gadducci 1998, Lund 1989 and Sartori 2009 all found that more women lived for at least two months if they received surgery than if they received medical management, but selection of treatment was based on clinician preference or performance status. Bryan 2006 found in a retrospective analysis of selected patients that surgery and chemotherapy had similar outcomes in terms of overall survival and re‐obstruction, but the surgical approach had higher morbidity.
However, the evidence from these excluded studies is dubious, as they did not allow for differences in baseline prognostic factors between women receiving surgery and those receiving medical management.
Authors' conclusions
Implications for practice.
We found only low quality evidence comparing palliative surgery and medical management for bowel obstruction in ovarian cancer. One small non‐randomised study found that women receiving surgery had longer survival than those receiving medical management, after adjustment for prognostic factors. The magnitude of the difference in survival was not reported and it was unclear how much of the difference in survival could be ascribed to the differences in treatment and how much to the better performance status of women undergoing surgery. Differences in QoL and adverse events subsequent to the two interventions were also unclear.
Therefore we are unable to reach definite conclusions about the relative benefits and harms of the two forms of treatment; we are unable to identify sub‐groups of women who are likely to benefit from one treatment or the other.
Implications for research.
Realistically, a sufficiently powered RCT comparing palliative surgery and medical management for women with bowel obstruction in ovarian cancer would be difficult. Alternatively, the relative benefits of surgery and medical management for bowel obstruction in ovarian cancer, as well as assessing the potential harms, adequately designed prospective non‐randomised studies would also be beneficial. Such studies should use multivariable analysis to adjust for differences in prognostic factors between the treatment groups and that the magnitude of differences in outcomes between the treatment groups is reported. It is debatable whether outcomes such as quality of life, pain and return to oral feeding should take preference over overall survival and time to further recurrence as the major objective of these studies.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 26 February 2014 | Amended | Contact details updated. |
Acknowledgements
We thank Chris Williams for clinical and editorial advice, Jane Hayes for designing the search strategy and Gail Quinn and Clare Jess for their contribution to the editorial process. We thank the referees for their many helpful suggestions.
Appendices
Appendix 1. MEDLINE search strategy
Medline Ovid 1950 to February week 2 2009
exp Ovarian Neoplasms/
exp Fallopian Tube Neoplasms/
exp Peritoneal Neoplasms/
((ovar* or fallopian or peritone*) adj5 (cancer* or neoplas* or carcinom* or malignan* or tumour* or tumour*)).mp.
exp Adnexal Diseases/
1 or 2 or 3 or 4 or 5
exp Intestinal Obstruction/
(intestin* adj5 obstruct*).mp.
(bowel* adj5 obstruct*).mp.
7 or 8 or 9
exp Palliative Care/
palliati*.mp.]
11 or 12
exp Surgical Procedures, Operative/
surg*.mp.
"surgery".fs.
exp Stents/
stent*.mp. [
14 or 15 or 16 or 17 or 18
6 and 10 and 13 and 19
key: mp=title, original title, abstract, name of substance word, subject heading word
fs=floating subheading
Appendix 2. EMBASE search strategy
Embase Ovid 1980 to 2009 week 08
exp Ovary Tumor/
exp Uterine Tube Tumor/
exp Peritoneum Tumor/
((ovar* or fallopian or peritone*) adj5 (cancer* or neoplas* or carcinom* or malignan* or tumour* or tumour*)).mp.
exp Adnexa Disease/
1 or 2 or 3 or 4 or 5
exp Intestine Obstruction/
(intestin* adj5 obstruct*).mp.
(bowel* adj5 obstruct*).mp.
7 or 8 or 9
exp Palliative Therapy/
palliati*.mp.
11 or 12
exp Surgery/
surg*.mp.
su.fs.
exp Stent/
stent*.mp.
14 or 15 or 16 or 17 or 18 or 19
6 and 10 and 13 and 19
key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
fs=floating subheading
Appendix 3. CENTRAL search strategy
CENTRAL Issue 1 2009
MeSH descriptor Ovarian Neoplasms explode all trees
MeSH descriptor Fallopian Tube Neoplasms explode all trees
MeSH descriptor Peritoneal Neoplasms explode all trees
(ovar* or fallopian or peritone*) near/5 (cancer* or neoplas* or carcinom* or malignan* or tumour* or tumour*)
MeSH descriptor Adnexal Diseases explode all trees
(#1 OR #2 OR #3 OR #4 OR #5)
MeSH descriptor Intestinal Obstruction explode all trees
intestin* near/5 obstruct*
bowel* near/5 obstruct*
(#7 OR #8 OR #9)
MeSH descriptor Palliative Care explode all trees
palliati*
(#11 OR #12)
MeSH descriptor Surgical Procedures, Operative explode all trees
surg*
Any MeSH descriptor with qualifier: SU
MeSH descriptor Stents explode all trees
stent*
(#14 OR #15 OR #16 OR #17 OR #18)
(#6 AND #10 AND #13 AND #19)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mangili 2005.
| Methods | Retrospective multi‐centre study. Data was collected from the following institutions: Gynecologic Department of S. Raffaele Hospital, University ‘‘Vita e Salute’’ of Milan and the Gynecologic Department of Varese University. |
|
| Participants | Patients with intestinal obstruction by ovarian cancer Mean age at diagnosis of intestinal obstruction was 58.7 years (range 31‐77 years) Two patients were stage IIC according to FIGO, 2 were stage IIIB, 38 were stage IIIC, and 5 were stage IV |
|
| Interventions |
Intervention Surgical procedure: this included eight colostomies, nine intestinal bypass, three intestinal resections, and one bypass and colostomy. No patients were submitted to any surgical procedure after these operations. Comparison Octreotide: The starting dose ranged from 0.3 to 0.6 mg daily by subcutaneous bolus or continuous infusion, and the dosage was increased until the symptoms resolved. Maximum utilized dosage was 0.9 mg daily. Median dosage was 0.6 mg daily. |
|
| Outcomes | Overall survival Perioperative mortality and morbidity |
|
| Notes | Six patients (18%) underwent only surgical exploration in the intervention group, because surgical correction was not possible. All patients were submitted to previous debulking surgery and successive chemotherapy. Five patients were also submitted to radiotherapy Patients with poor performance status were excluded from surgery. Performance status at baseline: 15 patients had status 0; 25 patients had status 1; 7 patients had status 2. Baseline characteristics, by treatment arm, of prognostic factors used in Cox model: Age (years): Surgery: Mean=54.8, Octreotide: Mean=59.6; no significant difference (nsd). Performance status (0, 1, 2): Surgery: 0: 11, 1: 15, 2: 1 , Octreotide: 0: 4, 1: 10, 2: 6; significance difference in performance status, p=0.03. Pain (grade 1, 2, 3): Surgery: 1: 10, 2:13, 3: 4, Octreotide: 1: 11, 2: 4, 3: 9; (nsd). Nausea (grade 1, 2, 3): Surgery: 1: 12, 2: 9, 3: 6, Octreotide: 1: 8, 2: 4, 3: 8; (nsd). Vomiting: Surgery: Yes: 27, no: 0, Octreotide: Yes: 20, No: 0; (nsd). Diarrhea: Surgery: Yes: 6, No: 21, Octreotide: Yes: 5, No: 15; (nsd). Palpable masses: Surgery: Yes: 20, No: 7, Octreotide: Yes: 15, No: 5; (nsd). Previous chemotherapy (1, 2, >2): Surgery: 1: 3, 2:10, >2: 14, Octreotide: 1: 1, 2: 16, >2: 5; (nsd). Previous RT: Surgery: Yes: 4, No: 23, Octreotide: Yes: 1, No: 19; (nsd). Ascites: Surgery: Yes: 19, No: 8, Octreotide: Yes: 16, No: 4; (nsd). Occlusion site: Surgery: Small: 7, Large: 7, Both: 13, Octreotide: Small: 8, Large: 5, Both: 7; (nsd). Diagnosis–occlusion time (months): Surgery: Mean=27, Octreotide: Mean=23; (nsd). We wrote to the authors in the hope of obtaining a hazard ratio or adjusted risk ratio for overall survival, but received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Not randomised |
| Allocation concealment? | High risk | Not randomised |
| Blinding? All outcomes | Unclear risk | Not reported |
| Incomplete outcome data addressed? All outcomes | Low risk | For all outcomes: % analysed: 47/47 (100%) |
| Free of selective reporting? | High risk | Progression of disease was discussed in the results section, but no survival plots or Cox regression was reported. |
| Free of other bias? | Unclear risk | Unclear whether all women treated at specified hospitals during a specified time period were included in study. |
| Details of assignment of patients reported? | Low risk | "Patients with poor performance status were excluded from surgery". |
| Representative palliative surgery group? | High risk | "Data of 47 patients with concomitant recurrent epithelial ovarian cancer and intestinal obstruction ... were reviewed". "All patients were submitted to previous debulking surgery and successive chemotherapy". However, patients receiving surgery were not representative of women with recurrent ovarian cancer and bowel obstruction because "Patients with poor performance status were excluded from surgery". |
| Representative comparison group? | High risk | "Patients with poor performance status were excluded from surgery". Hence the Octreotide group was not representative of women with recurrent ovarian cancer and bowel obstruction. |
| Comparability of treatment groups? | Low risk | No significant difference in age between groups, but no other factor (that was specified in the protocol) was reported. Multivariate analysis was used to adjust for other important prognostic factors. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arvieux 2005 | No comparison group |
| Bais 1995 | Extreme selection bias in treatment allocation and no statistical adjustment was carried out, "The primary criteria for surgical treatment were an estimated life expectancy of more than 8 weeks and at least a moderate general clinical condition ... Twelve of 31 patients were conservatively managed because of short life expectancy and/or extensive tumour". |
| Bryan 2006 | Extreme selection bias in treatment allocation and no statistical adjustment was carried out, "Treatments were approximately equally divided between chemotherapy, surgery, and supportive management, depending on clinician preference". |
| Dinstl 1976 | Peritoneal carcinoma details are reported, but not broken down by ovarian cancer or by sex. |
| Gadducci 1998 | Extreme selection bias in treatment allocation and no statistical adjustment was carried out, “The decision upon surgical or medical treatment of bowel obstruction was taken without any fixed protocol and the choice of therapy was individualised ... The selection of patients for surgery presumed that they will have a sufficiently long life expectancy”. |
| Glass 1973 | Only 15 women with ovarian cancer were included and there was no breakdown by treatments |
| Krouse 2002 | Narrative discussing three case reports |
| Krouse 2008 | Commentary on “Surgical Approaches to Malignant Bowel Obstruction,” by Helyer and Easson |
| Larson 1989 | Comparison of surgical procedures was possible, but only 19 operations for bowel obstruction were performed |
| Laval 2007 | Review paper of different treatments for patients with ovarian cancer and bowel obstruction. |
| Li 2004 | Relevant details of criteria for assignment of patients to treatments was not provided and only baseline characteristics of women receiving surgery were given. No statistical adjustment was used in any of the analyses so the study was open to selection bias. |
| Lund 1989 | "There was no significant difference in the distribution of primary prognostic factors as stage, size of residual tumour, or diffuse intestinal carcinomatosis at primary laparotomy. There was an equal distribution of clinical responders, patients with palpable abdominal tumour and ascites at intestinal obstruction, just as the median age was the same in the two groups”. However at the time of bowel obstruction, "The treatment ... was either conservative, ... or conservative treatment followed by surgical treatment if the patient was found to be operable". Extreme selection bias present in this study and no statistical adjustment was made. |
| Medina‐Franco 2008 | Only 11/130 women had ovarian cancer that induced bowel obstruction. |
| Miller 2000 | Only 9 patients had ovarian carcinoma and 1 had carcinoma of the fallopian tube. |
| Onsrud 2001 | Treatment was reported by cancer type, but no statistical adjustment was carried out so strong likelihood of selection bias. |
| Paganelli 1990 | Only 20 patients were included in the study |
| Ross 2006 | Case report |
| Sartori 2009 | Extreme selection bias in treatment allocation and no statistical adjustment was carried out, "The type of treatment of bowel obstruction, surgical or medical, was not decided based on a fixed protocol, but the choice of therapy was individualized". |
| Scheidbach 1999 | Of the 24 patients included in this study 12 were men and 12 were women. In all these patients, only three had received a Gynaecological operation for primary tumour. |
| Xinopoulos 2004 | This RCT which randomised 30 patients, included only 14 women, of whom only six had ovarian cancer. The paper did not report a breakdown of women with ovarian cancer by treatment. |
Differences between protocol and review
We added the following study constraint in the types of studies section, as it was apparent that selection bias would have considerably distorted results:
In order to minimise the effects of selection bias (systematic differences between baseline characteristics of the groups that are compared), we included only studies that used statistical adjustment for baseline case mix using multivariable analyses (e.g. age, performance status, grade, etc) if any constraints were placed on treatment allocation (e.g. women with poor performance status would not be given surgery) or it was based on clinician preference. Studies that did not report the assignment of treatment allocation were excluded.
We removed discussion of unadjusted results from the data synthesis, subgroup analysis and investigation of heterogeneity and sensitivity analysis sections as we do not plan to use unadjusted results in future updates due to the risk of selection bias.
Only one study met the inclusion criteria for the review and this did not report the magnitude of differences in outcomes between treatment groups, so we were unable to perform any quantitative synthesis. Should more studies be identified for updates of the review, the following methods will be employed:
Data on outcomes will be extracted as below:
For time to event (overall and recurrence‐free survival) data, we will extract the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these are not reported, we will attempt to estimate them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events and deaths), we will extract the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio (RR).
For continuous outcomes (e.g. quality of life measures), we will extract the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error.
Both unadjusted and adjusted statistics will be extracted, if reported.
Where possible, all data extracted will be those relevant to an intention‐to‐treat analysis, in which participants are analysed in groups to which they were assigned.
The time points at which outcomes were collected and reported will be noted.
Data will be abstracted independently by two review authors (AK, AB) onto a data abstraction form specially designed for the review. Differences between review authors will be resolved by discussion or by appeal to a third review author (RN) if necessary.
Measures of treatment effect
We will use the following measures of the effect of treatment:
For time to event data, we will use the HR, if possible. The HR summarises the chances of survival in women who received one type of treatment compared to the chances of survival in women who received another type of treatment. However, the logarithm of the HR, rather than the HR itself, is generally used in meta‐analyses.
For dichotomous outcomes, we will use the RR.
For continuous outcomes, we will use the mean difference between treatment arms if all trials measured the outcome on the same scale, otherwise standardised mean differences will be used.
Dealing with missing data
If data are missing or only imputed data are reported we will contact study authors to request data on the outcomes only among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between studies will be assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, if possible, by sub‐group analyses (see below). If there is evidence of substantial heterogeneity, the possible reasons for this will be investigated and reported.
Assessment of reporting biases
Funnel plots corresponding to meta‐analysis of the primary outcome will be examined to assess the potential for small study effects. When there is evidence of small‐study effects, publication bias will be considered as only one of a number of possible explanations. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random effects model, sensitivity analyses will be performed using fixed effects models.
Data synthesis
When sufficient, clinically similar studies are available, their adjusted results will be pooled in meta‐analyses.
For time‐to‐event data, HRs will be pooled using the generic inverse variance facility of RevMan 5.
For any dichotomous outcomes, the RR was calculated for each study and these were then pooled.
For continuous outcomes, the mean differences (or standardised mean differences) between the treatment arms at the end of follow‐up will be pooled.
If any studies have multiple treatment groups, the ‘shared’ comparison group will be divided into the number of treatment groups and comparisons between each treatment group and the split comparison group will be treated as independent comparisons.
Random effects models with inverse variance weighting will be used for all meta‐analyses (DerSimonian 1986).
If possible, indirect comparisons, using the methods of Bucher 1997 will be used to compare competing interventions that have not been compared directly with each other.
Subgroup analysis and investigation of heterogeneity
Sub‐group analyses will be performed, grouping the trials by:
Type of surgeon (general or specialist gynaecological)
Small or large bowel obstruction
Factors such as age, stage, type of intervention, length of follow‐up, will be considered in interpretation of any heterogeneity.
Sensitivity analysis
Sensitivity analyses will be performed (i) excluding non‐randomised studies if RCTs have been included (ii) excluding studies at high risk of bias.
Contributions of authors
AK and RN drafted the clinical sections of the protocol; AB and HD drafted the methodological sections of the protocol. AK and AB sifted the studies and abstracted data and assessed risk of bias. AB and HD wrote up the review. All authors agreed the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration Programme Grant Scheme CPG‐506
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Mangili 2005 {published data only}
- Mangili G, Aletti G, Frigerio L, Franchi M, Panacci N, Vigano` R, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. International Journal of Gynecological Cancer 2005;15:830–35. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arvieux 2005 {published data only}
- Arvieux C, Laval G, Mestrallet JP, Stefani L, Villard ML, Cardin N. Treatment of malignant intestinal obstruction.A prospective study over 80 cases. Annales de Chirurgie 2005;130(doi:10.1016/j.anchir.2005.05.011):470–476. [DOI] [PubMed] [Google Scholar]
Bais 1995 {published data only}
- Bais JM, Schilthuis MS, Slors JF, Lammes FB. Intestinal obstruction in patients with advanced ovarian cancer. International Journal of Gynecological Cancer 1995;5(5):346‐50. [DOI] [PubMed] [Google Scholar]
Bryan 2006 {published data only}
- Bryan DN, Radbod R, Berek JS. An analysis of surgical versus chemotherapeutic intervention for the management of intestinal obstruction in advanced ovarian cancer. International Journal of Gynecological Cancer 2006;16:125–134. [DOI] [PubMed] [Google Scholar]
Dinstl 1976 {published data only}
- Dinstl K, Hofbauer F, Schiessel R, Tuchmann A. [Mechanical ileus (author's transl)]. [German]. Zentralblatt fur Chirurgie 1976;101(23):1420‐6. [PubMed] [Google Scholar]
Gadducci 1998 {published data only}
- Gadducci A, Iacconi P, Fanucchi A, Cosio S, Miccoli P, Genazzani AR. Survival after intestinal obstruction in patients with fatal ovarian cancer: Analysis of prognostic variables. International Journal of Gynecological Cancer 1998;8(3):177‐82. [Google Scholar]
Glass 1973 {published data only}
- Glass RL, LeDuc RJ. Small intestinal obstruction from peritoneal carcinomatosis. American Journal of Surgery 1973;125:316–7. [DOI] [PubMed] [Google Scholar]
Krouse 2002 {published data only}
- Krouse RS, McCahill LE, Easson AM, Dunn GP. When the sun can set on an unoperated bowel obstruction: Management of malignant bowel obstruction. Journal of the American College of Surgeons 2002;195(1):117‐28. [DOI] [PubMed] [Google Scholar]
Krouse 2008 {published data only}
- Krouse RS. The Value of a Systematic Approach to Malignant Bowel Obstruction. The Journal of Supportive Oncology 2008;6(3):116‐17. [PubMed] [Google Scholar]
Larson 1989 {published data only}
- Larson J E, Podczaski E S, Manetta A, Whitney C W, Mortel R. Bowel obstruction in patients with ovarian carcinoma: analysis of prognostic factors. Gynecologic Oncology 1989;35(1):61‐5. [DOI] [PubMed] [Google Scholar]
Laval 2007 {published data only}
- Laval G, Beziaud N, Germain E, Rebischung C, Arvieux C. Management of obstructions due to carcinomatous peritonitis. Hepato‐Gastro 2007;14(6):465‐73. [Google Scholar]
Li 2004 {published data only}
- Li Z T, Wu X H, Fu SL. [Benefit of palliative surgery for bowel obstruction in recurrent ovarian carcinoma]. [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2004;39(4):260‐3. [PubMed] [Google Scholar]
Lund 1989 {published data only}
- Lund B, Hansen M, Lundvall F, Nielsen N C, Sorensen B L, Hansen HH. Intestinal obstruction in patients with advanced carcinoma of the ovaries treated with combination chemotherapy. Surgery, Gynecology & Obstetrics 1989;169(3):213‐8. [PubMed] [Google Scholar]
Medina‐Franco 2008 {published data only}
- Medina‐Franco H, Garcia‐Alvarez M N, Ortiz‐Lopez L J, Cuairan J Z. Predictors of adverse surgical outcome in the management of malignant bowel obstruction. Revista de Investigacion Clinica 2008;60(3):212‐6. [PubMed] [Google Scholar]
Miller 2000 {published data only}
- Miller G, Boman J, Shrier I, Gordon PH. Small‐bowel obstruction secondary to malignant disease: an 11‐year audit. Canadian Association of General Surgeons 2000;43(No. 5):353‐58. [PMC free article] [PubMed] [Google Scholar]
Onsrud 2001 {published data only}
- Onsrud M, Hagen B, Heimstad R. [Palliative treatment in gynecologic cancer]. [Norwegian]. Tidsskrift for Den Norske Laegeforening 2001;121(16):1896‐901. [PubMed] [Google Scholar]
Paganelli 1990 {published data only}
- Paganelli A M, Leone V, Malagutti V, Vescovo M, Sallusto A. Intestinal surgery in patients with ovarian carcinoma. European Journal of Gynaecological Oncology 1990;11(2):157‐60. [PubMed] [Google Scholar]
Ross 2006 {published data only}
- Ross AS, Semrad C, Waxman I, Dye C. Enteral stent placement by double balloon enteroscopy for palliation of malignant small bowel obstruction. Gastrointestinal Endoscopy 2006;64(5):835‐37 (doi:10.1016/j.gie.2006.03.001). [DOI] [PubMed] [Google Scholar]
Sartori 2009 {published data only}
- Sartori E, Chiudinelli F, Pasinetti B, Maggino T. Bowel Obstruction and Survival in Patients With Advanced Ovarian Cancer. Analysis of Prognostic Variables. International Journal of Gynecological Cancer 2009;19:54‐57. [DOI] [PubMed] [Google Scholar]
- Sartori E, Chiudinelli F, Pasinetti B, Zanagnolo V. Palliative care in advanced ovarian cancer patients with bowel obstruction. Gynecologic Oncology 2005;99(doi:10.1016/j.ygyno.2005.07.088):S215–S216. [DOI] [PubMed] [Google Scholar]
Scheidbach 1999 {published data only}
- Scheidbach H, Horbach TH, Groitl H, Hohenberger W. Percutaneous endoscopic gastrostomy/jejunostomy (PEG/PEJ) for decompression in the upper gastrointestinal tract: Initial experience with palliative treatment of gastrointestinal obstruction in terminally ill patients with advanced carcinomas. Surgical Endoscopy 1999;13:1103–5. [DOI] [PubMed] [Google Scholar]
Xinopoulos 2004 {published data only}
- Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, Tsamakidis K, Bitsakou G, Plataniotis G, et al. Stenting or stoma creation for patients with inoperable malignant colonic obstructions? Results of a study and cost‐effectiveness analysis. Surgical Endoscopy 2004;18(DOI: 10.1007/s00464‐003‐8109‐x):421–26. [DOI] [PubMed] [Google Scholar]
Additional references
Bryan 2006
- Bryan DN, Radbod R, Berek JS. An analysis of surgical versus chemotherapeutic intervention for the management of intestinal obstruction in advanced ovarian cancer. International Journal of Gynecological Cancer 2006;16:125‐34. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Caceres 2008
- Caceres A. Colorectal stents for palliation of large‐bowel obstructions in recurrent gynecologic cancer: an updated series. Gynecologic Oncology 2008;108(3):482‐5. [DOI] [PubMed] [Google Scholar]
Caprotti 2006
- Caprotti R. Palliative surgery for recurrent bowel obstruction due to advanced ovarian cancer. Minerva Ginecologica 2006;58(3):239‐44. [PubMed] [Google Scholar]
Clarke‐Pearson 1987
- Clarke‐Pearson DL. Surgical management of intestinal obstruction in ovarian cancer. I. Clinical features, postoperative complications, and survival. Gynecologic Oncology 1987;26(1):11‐8. [DOI] [PubMed] [Google Scholar]
Clayton 2005
- Clayton N L, Jaaback K S, Hirschowitz L. Primary fallopian tube carcinoma – the experience of a UK cancer centre and a review of the literature. Journal of Obstetrics and Gynaecology 2005;25(7):694‐702. [DOI] [PubMed] [Google Scholar]
CTCAE 2006
- CTCAE. Common Terminology Criteria for Adverse Events. (http://ctep.cancer.gov/forms/CTCAEv3.pdf) 9th August 2006; Vol. v3.0 (CTCAE).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG (eds) editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dvoretsky 1988
- Dvoretsky PM. Survival time, causes of death, and tumor/treatment‐related morbidity in 100 women with ovarian cancer. Human Pathology 1988;19(11):1273‐9. [DOI] [PubMed] [Google Scholar]
EUROCARE 2003
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J et al the EUROCARE Working Group. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14 (Supplement 5):v61‐v118. [DOI] [PubMed] [Google Scholar]
Fainsinger 1994
- Fainsinger RL, Spachynski K, Hanson J, Bruera E. Symptom control in terminally ill patients with malignant bowel obstruction. Journal of Pain and Symptom Management 1994;9:12‐8. [DOI] [PubMed] [Google Scholar]
Fallon 1994
- Fallon MT. The physiology of somatostatin and its synthetic analogue, octreotide. European Journal of Palliative Care 1994;1:20‐2. [Google Scholar]
Feuer 1999
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database of Systematic Reviews 1999, Issue 3. Art. No.: CD001219. DOI: 10.1002/14651858.CD001219.. [DOI: 10.1002/14651858.CD001219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feuer 2000
- Feuer DDJ, Broadley KE. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database of Systematic Reviews 2000, Issue 3. Art. No.: CD002764. DOI: 10.1002/14651858.CD002764.. [DOI: 10.1002/14651858.CD002764.] [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2002
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. Cancer incidence, mortality and prevalence worldwide. IARC CancerBase No. 5, version 2.0. IARCPress, Lyon 2004.
GRADE Working Group
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA: A Cancer Journal for Clinicians 2008;58:71‐96. [DOI] [PubMed] [Google Scholar]
Mangili 1996
- G. Mangili, Franchi M, Mariani A. Octreotide in the management of bowel obstruction in terminal ovarian cancer. Gynecologic Oncology 1996;61:345‐8. [DOI] [PubMed] [Google Scholar]
Markman 2008
- Markman M. Pharmaceutical management of ovarian cancer: current status. Drugs 2008;68(6):771‐89. [DOI] [PubMed] [Google Scholar]
Mercadante 2004
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. Journal of Pain and Symptom Management 2004;28:412‐6. [DOI] [PubMed] [Google Scholar]
Ooijen 1993
- Ooijen B, Burg ME, Planting AS, Siersema PD, Wiggers T. Surgical treatment of gastric drainage only for intestinal obstruction in patients with carcinoma of the ovary or peritoneal carcinomatosis of other origin. Surgery, Gynecology and Obstetrics 1993;176(5):469‐74. [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pothuri 2003
- Pothuri B. Palliative surgery for bowel obstruction in recurrent ovarian cancer:an updated series. Gynecologic Oncology 2003;89(2):306‐13. [DOI] [PubMed] [Google Scholar]
Pothuri 2005
- Pothuri B, Montemarano M, Gerardi M, Shike M, Ben‐Porat L, Sabbatini P, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecologic Oncology 2005;96(2):330‐4. [DOI] [PubMed] [Google Scholar]
Quinn 2001
- Quinn M, Babb P, Brock A, Kirby L, Jones J. Cancer Trends in England and Wales 1950–1999. Office for National Statistics 2001:http://www.statistics.gov.uk/downloads/theme_health/cancertrends_5099.pdf.
Quirk 2005
- Quirk JT, Natarajan N, Mettlin CJ. Age‐specific ovarian cancer incidence rate patterns in the United States. Gynecologic Oncology 2005;99(1):248‐50. [DOI] [PubMed] [Google Scholar]
Rabban 2005
- Rabban JT, Bell DA. Current issues in the pathology of ovarian cancer. Journal of Reproductive Medicine 2005 Jun;50(6):467‐74. [PubMed] [Google Scholar]
Redman 1988
- Redman CW. Survival following intestinal obstruction in ovarian cancer. Eurupean Journal of Surgical Oncology 1988;14(5):383‐6. [PubMed] [Google Scholar]
Reichlin 1983
- Reichlin S. Medical progress: somatostatin. New England Journal of Medicine 1983;309:1495‐501. [DOI] [PubMed] [Google Scholar]
Shepherd 1989
- Shepherd JH. Revised FIGO staging for gynaecological cancer. British Journal of Obstetrics and Gynaecology 1989;96(8):889‐92. [DOI] [PubMed] [Google Scholar]
Sundar 2006
- Sundar SS. Role of lymphangiogenesis in epithelial ovarian cancer. British Journal of Cancer 2006;94(11):1650‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunca 1981
- Tunca JC. The management of ovarian‐cancer‐caused bowel obstruction. Gynecologic Oncology 1981;12:186‐92. [DOI] [PubMed] [Google Scholar]


