Abstract
Background
This is an updated version of the original Cochrane review first published in Issue 4, 2009. There is an ongoing debate about the indications for, and value of, adjuvant pelvic radiotherapy after radical surgery in women with early cervical cancer. Certain combinations of pathological risk factors are thought to represent sufficient risk for recurrence, that they justify the use of postoperative pelvic radiotherapy, though this has never been shown to improve overall survival, and use of more than one type of treatment (surgery and radiotherapy) increases the risks of side effects and complications.
Objectives
To evaluate the effectiveness and safety of adjuvant therapies (radiotherapy, chemotherapy followed by radiotherapy, chemoradiation) after radical hysterectomy for early‐stage cervical cancer (FIGO stages IB1, IB2 or IIA).
Search methods
For the original review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), Issue 4, 2008. The Cochrane Gynaecological Cancer Group Trials Register, MEDLINE (January 1950 to November 2008), EMBASE (1950 to November 2008). We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field. For this update, we extended the database searches to September 2011 and searched the MetaRegister for ongoing trials.
Selection criteria
Randomised controlled trials (RCTs) that compared adjuvant therapies (radiotherapy, chemotherapy followed by radiotherapy, or chemoradiation) with no radiotherapy or chemoradiation, in women with a confirmed histological diagnosis of early cervical cancer who had undergone radical hysterectomy and dissection of the pelvic lymph nodes.
Data collection and analysis
Two review authors independently abstracted data and assessed risk of bias. Information on grade 3 and 4 adverse events was collected from the trials. Results were pooled using random‐effects meta‐analyses.
Main results
Two RCTs, which compared adjuvant radiotherapy with no adjuvant radiotherapy, met the inclusion criteria; they randomised and assessed 397 women with stage IB cervical cancer. Meta‐analysis of these two RCTs indicated no significant difference in survival at 5 years between women who received radiation and those who received no further treatment (risk ratio (RR) = 0.8; 95% confidence interval (CI) 0.3 to 2.4). However, women who received radiation had a significantly lower risk of disease progression at 5 years (RR 0.6; 95% CI 0.4 to 0.9).
Although the risk of serious adverse events was consistently higher if women received radiotherapy rather than no further treatment, these increased risks were not statistically significant, probably because the rate of adverse events was low.
Authors' conclusions
We found evidence, of moderate quality, that radiation decreases the risk of disease progression compared with no further treatment, but little evidence that it might improve overall survival, in stage IB cervical cancer. The evidence on serious adverse events was equivocal.
Keywords: Female; Humans; Chemoradiotherapy, Adjuvant; Hysterectomy; Neoplasm Recurrence, Local; Neoplasm Staging; Radiotherapy, Adjuvant; Radiotherapy, Adjuvant/adverse effects; Radiotherapy, Adjuvant/methods; Radiotherapy, Adjuvant/mortality; Randomized Controlled Trials as Topic; Uterine Cervical Neoplasms; Uterine Cervical Neoplasms/mortality; Uterine Cervical Neoplasms/pathology; Uterine Cervical Neoplasms/radiotherapy; Uterine Cervical Neoplasms/surgery
Plain language summary
Radiotherapy, or a combination of radiotherapy and chemotherapy, after surgery for early‐stage cervical cancer
At present, doctors are not sure whether women with early cervical cancer who have had their womb and pelvic lymph nodes removed should be given radiotherapy. If the woman has a combination of certain risk factors that put her at high risk of having a recurrence of her cancer, doctors often think that it would be a good idea to give her radiotherapy. However, radiotherapy has never been shown to improve overall survival for these women and the combination of surgery and radiotherapy increases the risk of side effects and complications. We searched for all the available randomised controlled trials (RCTs) that assessed whether radiotherapy (with or without chemotherapy) could improve survival in these women.
We found only two trials that compared the use of radiotherapy with no radiotherapy in women with early cervical cancer who had had their womb and pelvic lymph nodes removed and who were at risk of having a recurrence of their cancer. These two trials enrolled 397 women. When we combined the findings from these two trials, we found that, on average, women who received radiotherapy were between 40% and 90% less likely to have a relapse of their cancer within 5 years than women who did not. However, because of the low number of deaths in the trials, we could not confirm whether radiotherapy helped to prolong life: our best estimate was that, 5 years after treatment, women who received radiotherapy were about 20% more likely to be alive than those who did not, but this estimate may not be very accurate and women's actual prospects could be anywhere between being three times more likely to be alive and being 60% more likely to be dead.
Although women who had radiotherapy tended to have more complications than women who did not, we could not be sure whether this was due to chance rather than the radiotherapy because few women reported complications.
The main limitations of the review were that we did not find any trials that evaluated a combination of radiotherapy and chemotherapy and that the two trials of radiotherapy gave very little information about side effects.
Summary of findings
Summary of findings for the main comparison. Summary of findings: comparison of radiotherapy with no further treatment.
| Adjuvant radiotherapy after surgery for cervical cancer | |||||
|
Patient or population: patients with early‐stage cervical cancer (FIGO stages IB1, IB2 or IIA) Settings: Inpatient or outpatient Intervention: Adjuvant radiotherapy after surgery | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Adjuvant radiotherapy after surgery | ||||
| Death within 5 years | Study population | RR 0.84 (0.3 to 2.36) | 397 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| 160 per 1000 | 134 per 1000 (48 per 1000 to 378 per 1000) | ||||
| Medium‐risk population | |||||
| 124 per 1000 | 104 per 1000 (37 per 1000 to 293 per 1000) | ||||
| Disease progression within 5 years | Study population | RR 0.58 (0.37 to 0.91) | 397 (2 studies) | ⊕⊕⊕⊝ moderate2,3 | |
| 210 per 1000 | 122 per 1000 (78 per 1000 to 191 per 1000) | ||||
| Medium‐risk population | |||||
| 164 per 1000 | 95 per 1000 (61 per 1000 to 149 per 1000) | ||||
| Haematological adverse events (grade 3 or 4) | Study population | RR 2.38 (0.63 to 9.05) | 388 (2 studies) | ⊕⊕⊕⊝ moderate4 | |
| 15 per 1000 | 36 per 1000 (9 per 1000 to 136 per 1000) | ||||
| Medium‐risk population | |||||
| 20 per 1000 | 48 per 1000 (13 per 1000 to 181 per 1000) | ||||
| Gastrointestinal adverse events (grade 3 or 4) | Study population | RR 7.32 (0.91 to 58.82) | 388 (2 studies) | ⊕⊕⊕⊝ moderate4 | |
| 0 per 1000 | 0 per 1000 (0 per 1000 to 0 per 1000) | ||||
| Medium‐risk population | |||||
| 0 per 1000 | 0 per 1000 (0 per 1000 to 0 per 1000) | ||||
| Genitourinary adverse events (grade 3 or 4) | Study population | RR 2.12 (0.54 to 8.37) | 388 (2 studies) | ⊕⊕⊕⊝ moderate4 | |
| 15 per 1000 | 32 per 1000 (8 per 1000 to 126 per 1000) | ||||
| Medium‐risk population | |||||
| 16 per 1000 | 34 per 1000 (9 per 1000 to 134 per 1000) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1 Inconsistent evidence about 5‐year survival, so the pooled estimate had wide CIs: thus uncertainty whether radiotherapy improves survival or increases the risk of death.
2 Inconsistent evidence about 5‐year progression‐free survival, so the pooled estimate had wide CIs: thus uncertainty whether radiotherapy improves time to disease progression or increases the risk of progression.
3 Imprecision in point estimate for Bilek 1982, indicated by large CI due to low number of women with disease progression, resulting in increased uncertainty in pooled estimate. However the overall precision of the pooled estimate is satisfactory as the larger study of GOG 92 is given substantially more weight.
4 Large CI in pooled estimate.
Background
This is an updated version of the review that was first published in the Cochrane Database of Systematic Reviews, Issue 4, 2009.
Description of the condition
Cervical cancer is the second most common cancer and the third most common cause of cancer death in women worldwide, and the leading cause of cancer death in women in developing countries (GLOBOCAN 2008). Worldwide it accounts for around 10% of all cancers diagnosed in women. A woman's risk of developing cervical cancer by age 65 years ranges from 0.69% in more‐developed countries to 1.38% in less‐developed countries (GLOBOCAN 2008). The risk of dying from cervical cancer is 0.2% and 0.8% in more‐ and less‐developed countries, respectively. In Europe, about 60% of women with cervical cancer are alive 5 years after diagnosis (EUROCARE 2003).
The International Federation of Gynecology and Obstetrics (FIGO) subdivides cervical cancers into four groups or stages, where stage I disease is confined to the cervix, and stage II tumours invade beyond the uterus, but not to the pelvic sidewall or lower third of the vagina. Stage III tumours extend to the pelvic sidewall and/or involve the lower third of the vagina and/or cause a swollen or a non‐functioning kidney (hydronephrosis), and stage IV tumours invade other pelvic organs or have distant metastases (Benedet 2000).
Description of the intervention
The treatment of cervical cancer is determined by the stage of the disease. Early cervical cancer (FIGO stage IA, IB and IIA) is a curable condition, and doctors aim to use as few types of treatment as possible to achieve cure, because using more than one increases treatment‐related side effects and complications. An adjuvant treatment is a supplementary treatment that is given to decrease the risk of the cancer recurring.
Microinvasive carcinoma of the cervix (FIGO stage IA1 and IA2) has a low risk of spread beyond the cervix, and is usually cured by non‐radical operations such as a cone biopsy, trachelectomy (excision of the cervix) or simple hysterectomy.
FIGO stage IB1, IB2 or IIA cervical cancer have no standard management, as both radical surgery and radiotherapy (RT) have been shown to be equally effective with 5‐year survival rates of 87% to 92% (Gray 2008; Peters 2000), though they differ in terms of side effects and complications. Stage IB1 disease is usually treated surgically, with radical hysterectomy and dissection of the pelvic lymph nodes (PLND), although the use of vaginal radical trachelectomy is increasing, to retain fertility (Kitchener 2010). There is conflicting evidence regarding the management of stage IB2 and stage IIA tumours: some clinicians treat these women with primary radical surgery, followed by adjuvant RT with or without chemotherapy, while others use chemoradiation as a primary therapy. The use of chemoradiation (as primary treatment or after surgery) is supported by evidence from a Cochrane review that showed that women with stage IB to IIA lesions who had chemoradiation had an absolute 5‐year survival benefit of 10% compared with women who just had RT (CCCMAC 2010). Neoadjuvant chemotherapy (NACT) followed by radical surgery may be used as an alternative therapy for bulkier tumours (Kesic 2006) although it remains unclear whether NACT offers benefits over surgery alone (Rydzewska 2010).
After radical surgery, certain pathological factors are thought to influence risk of recurrence and progression‐free survival (PFS), and are therefore indications for adjuvant therapy, which usually consists of RT with concurrent chemotherapy. These risk factors include: positive PLNDs, lower uterine segment involvement, involvement of lymphatics and blood vessels (lymphovascular space involvement (LVSI)), deep invasion of tumour into the substance or stroma of the cervix, involvement of the tissue next to the cervix (parametria), non‐squamous histological subtype, tumour grade, vaginal margin involvement and tumour size > 4 cm. When one or more of these factors is found, the 5‐year survival may drop to between 50% and 70% (Peters 2000).
It has long been recognised that using more than one treatment modality results in a very substantial increase in the number and severity of treatment complications and side effects, such as leg swelling due to lymphatic obstruction (lymphoedema), sexual dysfunction, urinary frequency, diarrhoea or constipation and bowel obstruction. GOG 92 2006 reported that while adjuvant RT reduced the risk of pelvic recurrence by only 39%, severe and life‐threatening toxicity was reported in 6% of irradiated patients compared to 2% in patients randomised to no further treatment (NFT) (Sedlis 1999). Peters 2000 reported 27 episodes of grade 4 toxicity in 21 of the 122 (17%) patients in the chemoradiation after radical surgery arm, most of which were haematological; while only 4 of 112 patients (4%) treated with radiation alone after radical surgery had grade 4 toxicity (Peters 2000). It is therefore important to weigh up the risks and benefits of the use of adjuvant RT and chemotherapy after radical surgery for each individual patient, in order to maximise their PFS while minimising their treatment‐related morbidity.
Why it is important to do this review
This is an update of an earlier review that aimed to establish the impact of adjuvant RT and chemoradiation after surgery compared with no supplementary treatment in early cervical cancer, on overall and disease‐free survival, as well as on treatment‐related morbidity and mortality, and quality of life (QoL). The role of adjuvant chemotherapy in early cervical cancer is the subject of a separate review (Rosa 2009).
Since the publication of Guttmann 1970 on the significance of postoperative irradiation in carcinoma of the cervix, doctors have been debating the indications for, and value of, adjuvant RT after radical surgery in early cervical cancer. After GOG 92 2006 showed that adjuvant RT reduced the number of recurrences, the debate changed to whether this benefit was enough to outweigh the attendant risks for early‐stage lesions. Two separate Cochrane reviews found chemoradiation to have survival benefits over RT for cervical cancer (CCCMAC 2010; Rosa 2009). However it remains unclear, in the context of primary radical surgery for early cervical cancer, whether the benefits of RT and chemoradiation outweigh the risks, compared with no adjuvant therapy.
Objectives
To evaluate the effectiveness and safety of adjuvant therapies (RT, chemotherapy followed by RT, chemoradiation) after radical hysterectomy for early‐stage cervical cancer (stages IB1, IB2 or IIA). In particular, we sought to evaluate whether these interventions improve survival and to assess any associated morbidity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with a confirmed histological diagnosis of early cervical cancer (FIGO stages IB1, IB2 or IIA) who had radical hysterectomy and PLND. The review included (but was not restricted to) women who may have had any of the following risk factors or any combination of them: positive PLNDs, parametrial or vaginal margin involvement, LVSI, lower uterine segment involvement, deep stromal invasion, non‐squamous histology, high‐grade tumours or tumours > 4 cm in size.
Types of interventions
Only studies that addressed RT or chemoradiation in the adjuvant setting were included. The following intervention and control groups were eligible:
Interventions:
Radiotherapy alone, or
Chemotherapy followed by RT, or
Chemoradiation (chemotherapy given concurrently with RT)
Controls:
No adjuvant chemotherapy or RT
Comparisons were restricted to those that compared an intervention with a control that is similar in all respects, except that RT or chemoradiation was not included in the treatment regimen. Chemotherapy was not limited to platinum‐based regimens only, as this would have excluded some earlier trials that may have utilised other chemotherapy regimens.
Types of outcome measures
Primary outcomes
Overall survival (OS) (time from entry into the trial until death from any cause)
Secondary outcomes
PFS (time from entry into the trial until progression of the disease or death)
Disease recurrence
QoL, measured by a validated scale
Adverse events, classified according to CTCAE 2006:
haematological or blood (leukopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage)
gastrointestinal or bowel (nausea, vomiting, anorexia, diarrhoea, proctitis, bowel obstruction)
genitourinary (sexual dysfunction, urinary frequency, haematuria, incontinence, renal failure)
skin (stomatitis, mucositis, desquamation, alopecia, allergy)
lymphoedema (swelling of the legs due to lymphatic obstruction)
infection
neurological or nervous system (peripheral and central)
pulmonary or lung (dyspnoea)
general (weakness, fatigue, lethargy, malaise)
Search methods for identification of studies
We sought papers in all languages and translations were carried out as necessary.
Electronic searches
We conducted searches to identify all published and unpublished RCTs addressing the use of adjuvant RT and chemoradiation for early‐stage cervix cancer. Trials were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library (Issue 4, 2008), MEDLINE (January 1950 to November 2008), EMBASE (1950 to November 2008), Cochrane Gynaecological Cancer Group (CGCRG) Specialised Register. For the updated review, these searches were extended as follows: MEDLINE to September week 4, 2011; EMBASE to week 40, 2011; CENTRAL Issue 4 2011 and the CGCRG Specialised Register. The updated search was performed by Jane Hayes of the CGCRG (see Acknowledgements).
The MEDLINE search strategy is presented in Appendix 1, EMBASE is presented in Appendix 2 and CENTRAL is presented in Appendix 3.
CENTRAL, The National Research Register (NRR) and Clinical Trials Register were searched in all fields using the following words: cervix cancer, cervical cancer, adjuvant RT, adjuvant chemoradiation, early stage.
Searching other resources
MetaRegister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, www.cancer.gov/clinicaltrials and Gynaecologic Oncologists of Canada (http://www.g‐o‐c.org) were searched for ongoing trials. The main investigators of any relevant ongoing trials were contacted for further information, as were any major cooperative trials groups active in this area.
The citation list of relevant publications, abstracts of scientific meetings and list of included studies were checked through handsearching and experts in the field were contacted to identify further reports of trials. Reports of conferences were handsearched in the following sources:
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologists)
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society)
British Journal of Cancer
British Cancer Research Meeting
Annual Meeting of European Society of Medical Oncology (ESMO)
Annual Meeting of the American Society of Clinical Oncology (ASCO)
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were removed and the remaining references were examined by two review authors (LR and SS) independently. Those studies that clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers was assessed independently by two review authors (LR and SS). Disagreements were resolved by discussion between the two review authors and if necessary by a third review author (DL). Reasons for exclusion were documented.
Data extraction and management
For included studies, data were extracted as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included data on:
Author, year of publication (if published) and journal citation (including language)
Country
Setting
Study design, methodology
-
Study population
Total number enrolled
Patient characteristics (inclusion and exclusion criteria, age, FIGO stage, histological cell type, comorbidity, previous treatment, number enrolled in each arm)
-
Intervention/control details
Type of chemotherapy, number of cycles and dose
Timing and dose of RT
Risk of bias in study ‐ see below
Duration of follow‐up
Deviations from protocol
and
Outcomes: data on all primary and secondary outcomes that are reported were extracted as below:
For time to event (OS or PFS) data, we extracted the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these were not reported, we estimated them from other reported statistics using the methods of Parmar 1998. We abstracted site of recurrence, where possible.
For dichotomous outcomes (e.g. adverse events, deaths and disease recurrences if it was not possible to use a hazard ratio (HR)), we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio (RR). We abstracted adverse events by grade of toxicity.
The time points at which outcomes were collected and reported were noted.
Both unadjusted and adjusted statistics were extracted, if reported. If adjusted statistics were reported, we noted the variables used in adjustment.
Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which participants were analysed in groups to which they were assigned.
Data were abstracted independently by two review authors (LR and SS) onto a data abstraction form specially designed for the review (see Appendix 4). Differences of opinion between review authors were resolved by discussion or by appeal to a third review author (DL or HD) if necessary.
Assessment of risk of bias in included studies
Risk of bias in included RCTs was assessed using the following criteria.
Sequence generation
We assessed the randomisation of participants to intervention groups as:
Low risk of bias: for example a computer‐generated random sequence or a table of random numbers
High risk of bias: for example date of birth, clinic identification number or surname
Unclear risk of bias: for example not reported
Allocation concealment
We assessed the concealment of allocation sequence from treatment providers and participants as:
Low risk of bias: for example where the allocation sequence could not be foretold
High risk of bias: for example allocation sequence could be foretold by patients, investigators or treatment provider
Unclear risk of bias: for example not reported
Blinding
We assessed the blinding of healthcare professionals who assessed disease progression as:
Low risk of bias, if outcome assessors were blind from the knowledge of which intervention a participant received
High risk of bias, If outcome assessors were not blind
Unclear risk of bias, if outcome assessor blinding was not described or was unclear
Incomplete reporting of outcome data
We recorded the proportion of participants whose outcomes were analysed.
We assessed loss to follow‐up for each outcome as:
Low risk of bias, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms
High risk of bias, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms
Unclear risk of bias if loss to follow‐up was not reported
Selective reporting of outcomes
We assessed whether studies are free of selective outcome reporting as follows:
Low risk of bias: for example if all outcomes that are specified above and also pre‐specified in the study were reported in the study
High risk of bias: for example if not all expected outcomes were reported
Unclear risk of bias
Other potential threats to validity
We assessed whether studies were apparently free of other problems that could have put them at a high risk of bias as:
Low risk of other bias
High risk of other bias
Unclear whether there was risk of other bias
Measures of treatment effect
We used the following measures of the effect of treatment:
For time to event data, we used HRs, if possible
For dichotomous outcomes, we used the RR
Dealing with missing data
We did not impute missing outcome data; if only imputed outcome data were reported, we planned to contact trial authors to request data on the outcomes only among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003) and by a formal statistical test of the significance of the heterogeneity (Deeks 2001).
Assessment of reporting biases
As only two trials met our inclusion criteria, we did not perform the planned assessment of reporting bias (see Differences between protocol and review).
Data synthesis
Results were pooled in meta‐analyses using random‐effects models with inverse variance weighting (DerSimonian 1986). Adjusted summary statistics were used if available; otherwise unadjusted results were used.
For time‐to‐event data (OS and PFS), HRs were pooled using the generic inverse variance facility of RevMan 5.1.
For dichotomous outcomes (deaths, disease recurrence, adverse events), RRs were pooled.
Subgroup analysis and investigation of heterogeneity
As only two trials met our inclusion criteria, we did not perform the planned subgroup analyses (see Differences between protocol and review).
Sensitivity analysis
As only two studies met our inclusion criteria, we did not perform the planned sensitivity analyses (see Differences between protocol and review).
Results
Description of studies
Results of the search
For the original review, the search strategy identified 824 unique references. The title and abstract screening of these references identified three studies as potentially eligible for this review. The full text screening of these three studies excluded one study for the reasons described in the table Characteristics of excluded studies. The remaining two RCTs met our inclusion criteria. The updated search identified 224 references after de‐duplication and we found no additional studies to classify for this update.
Searches of the grey literature did not identify any additional relevant studies.
Included studies
The two included trials, which are described in detail in Characteristics of included studies, randomised 397 women with stage IB cervical cancer (Bilek 1982 = 120 women; GOG 92 2006 = 277 women), all of whom were assessed at the end of the trials. Both trials compared adjuvant RT with no adjuvant RT.
GOG 92 2006 reported 67 deaths and 79 disease recurrences; Bilek 1982 reported six deaths and six disease recurrences; GOG 92 2006 reported 14 instances of severe adverse effects in 12 patients; Bilek 1982 reported 23 instances of adverse effects but it was unclear whether these were all in different women.
The proportion of women who died within 5 years was considerably lower in the trial of Bilek 1982 (6 of 120 women; 5%) than in GOG 92 2006 (48 of 277 women; 17%). This was largely because women in the Bilek 1982 trial had shorter average follow‐up, but probably also because the GOG 92 2006 trial included older women. It could also be due to different pathological risk factors among patients in the two trials; Bilek 1982 did not report these.
GOG 92 trial
The GOG 92 2006 trial was designed to establish whether postoperative pelvic RT would reduce recurrence rates and mortality in stage IB cervical cancer patients with negative lymph nodes, but any combinations of the following risk factors: large tumour diameter, deep stromal invasion and lymphovascular space invasion. Of the 277 eligible patients, 137 were randomly assigned to RT, and 140 to NFT. Patients in the RT group received external beam radiotherapy (EBRT) in doses between 46 Gy in 23 fractions to 50.4 Gy in 28 fractions, and no brachytherapy.
The median age of the included patients was 41 years (range: 20 to 80 years), and most tumours (79%) were squamous. The distribution of individual risk factors was not balanced between the two different treatment regimens, but the overall risk for recurrence was very similar for each regimen when all risk factors were considered as a group.
Women were followed up for a median of 120 months (range: 0 to 192 months).
Bilek 1982 trial
The trial of Bilek 1982 is a much older study, which aimed to report the treatment results and treatment‐related morbidity of 120 women with stage 1B cervical cancer. Sixty women were randomised to NFT after radical hysterectomy, while another 60 women received 52 Gy of whole pelvic EBRT, at a rate of 2 Gy per day.
The median age was 42 years (range: 23 to 59 years) in the NFT group, and 39 years (range: 23 to 60 years) in the RT group. All tumours in this study were squamous carcinomas. It was reported that there were no significant differences between the groups with regard to prognostic factors, but details of prognostic factors in the two groups were not presented.
Women were followed up for a mean of 44 months (range: 24 to 72 months).
Outcomes reported
Both studies reported OS. The GOG 92 2006 trial reported HRs for OS, disease recurrence (based on time to evidence of disease recurrence or date when patient was last seen) and PFS (survival until disease recurrence or death) and also the number of women who had disease recurrence or died after 5 years' and 12 years' follow‐up. The trial of Bilek 1982 did not report HRs; although it presented a survival plot, so we were unable to estimate an HR using the methods of Parmar 1998 since the plot was based on only six deaths. However, it was possible to deduce from the survival plot and supporting text the number of participants who died within 5 years; the number of women who had disease recurrence was also reported.
Adverse events (haematological, gastrointestinal and genitourinary side effects) were reported in both trials. Additionally the GOG 92 2006 trial reported neurological side effects, and the trial of Bilek 1982 reported lymphoedema, rectal or sigmoid strictures and hydronephrosis. The GOG 92 2006 trial reported only grade 3 and 4 adverse effects but the trial of Bilek 1982 had no such restriction.
Excluded studies
The trial of Lahousen 1999 was a multicentre RCT that randomised women who had undergone a radical hysterectomy for either chemotherapy, RT or observation. Radiotherapy consisted of total pelvic external irradiation with 50 Gy, where the treatment was given within 21 days of surgery. This study was excluded as 19 of the 76 women enrolled had stage IIB disease and we were unable to extract the outcomes separately for women without stage IIB disease.
Risk of bias in included studies
Both studies were at high risk of bias: they satisfied only one of the criteria that we used to assess risk of bias ‐ see Figure 1 and Figure 2.
1.
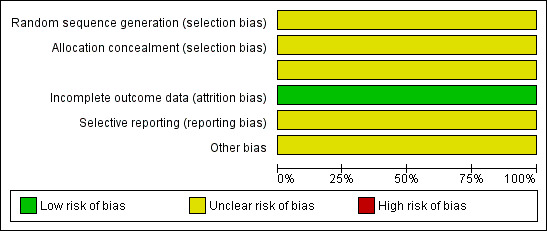
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
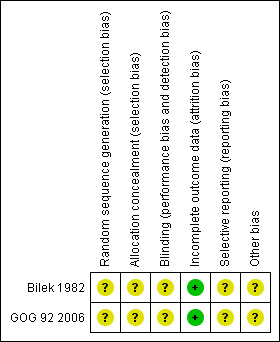
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Neither study reported the method of generation of the sequence of random numbers used to allocate women to treatment arms, or concealment of this allocation sequence from patients and healthcare professionals involved in the study, or blinding of the healthcare professionals who assessed disease progression. It was unclear whether the studies reported all the outcomes that they assessed or if any additional bias were present. However, in both studies, all women who were enrolled were assessed at endpoint.
Effects of interventions
See: Table 1
Survival
Overall survival
Analysis 1.1. Using an HR to compare the survival experience of women in the two treatment groups, the GOG 92 2006 trial found no statistically significant difference in OS between the radiation and control groups, after adjustment for capillary lymphatic space status, depth of invasion and tumour size (HR 0.7; 95% CI 0.5 to 1.1).
1.1. Analysis.
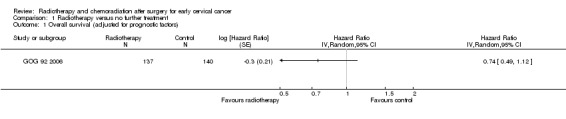
Comparison 1 Radiotherapy versus no further treatment, Outcome 1 Overall survival (adjusted for prognostic factors).
However, in a subgroup analysis of OS by prognostic category, GOG 92 2006 found that patients with a combination of negative capillary lymphatic space, deep stromal invasion and tumour size greater than 4 cm had a significantly lower risk of death if they received RT. Results were inconclusive for other subgroups.
Deaths within 5 years
Analysis 1.2. Meta‐analysis of both trials (Bilek 1982; GOG 92 2006) showed little difference in the risk of death within 5 years of treatment in women who received RT and those who received NFT (RR 0.8; 95% CI 0.3 to 2.4). There was moderate heterogeneity between trials (I2 = 43%).
1.2. Analysis.
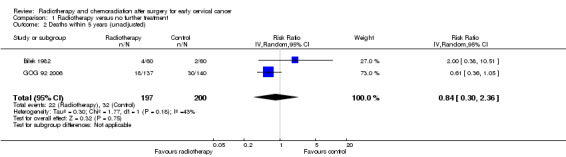
Comparison 1 Radiotherapy versus no further treatment, Outcome 2 Deaths within 5 years (unadjusted).
Progression‐free survival
Analysis 1.3 Using an HR to compare PFS of women in the two treatment groups the GOG 92 2006 trial found that women who received RT had a significantly lower risk of disease progression than women who received NFT (HR 0.6; 95% CI 0.4 to 0.9).
1.3. Analysis.
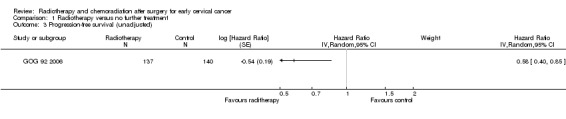
Comparison 1 Radiotherapy versus no further treatment, Outcome 3 Progression‐free survival (unadjusted).
Furthermore, only 9% (3 of 34 women) of the patients with adenocarcinoma or adenosquamous tumours in the RT arm had disease recurrence, compared with 44% (11 of 25) in the NFT arm, suggesting that RT may be beneficial for patients with non‐squamous histology.
Disease recurrence within 5 years
Analysis 1.4. Meta‐analysis of both trials (Bilek 1982; GOG 92 2006) showed that women who received RT had a significantly lower risk of disease progression within 5 years of treatment than women who received NFT (RR 0.6; 95% CI 0.4 to 0.9). There was no heterogeneity between trials (I2 = 0%).
1.4. Analysis.
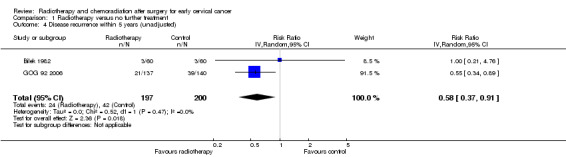
Comparison 1 Radiotherapy versus no further treatment, Outcome 4 Disease recurrence within 5 years (unadjusted).
Recurrence‐free survival
Analysis 1.5. Sensitivity analysis combining the unadjusted relative risk of recurrence in the trial of Bilek 1982 with HRs adjusted for prognostic factors for GOG 92 2006 yielded similar results (HR 0.6; 95% CI 0.4 to 1.0), with no heterogeneity between trials (I2 = 0%).
1.5. Analysis.
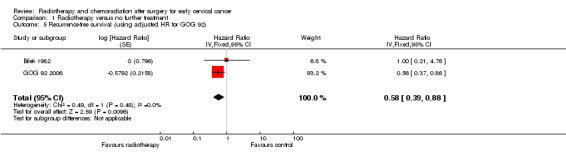
Comparison 1 Radiotherapy versus no further treatment, Outcome 5 Recurrence‐free survival (using adjusted HR for GOG 92).
Grade 3 and 4 adverse events
Haematological
Analysis 1.6. Meta‐analysis of both trials (Bilek 1982; GOG 92 2006) showed no statistically significant difference in the risk of haematological side effects (abnormalities of the blood) in women who received RT and those who received NFT (RR 2.4; 95% CI 0.6 to 9.0). There was no heterogeneity between trials (I² = 0%).
1.6. Analysis.
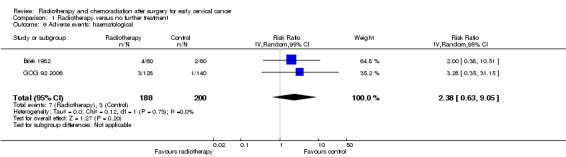
Comparison 1 Radiotherapy versus no further treatment, Outcome 6 Adverse events: haematological.
Gastrointestinal
Analysis 1.7. Meta‐analysis of both trials (Bilek 1982; GOG 92 2006) showed no statistically significant difference in the risk of gastrointestinal (bowel) side effects in women who received RT and those who received NFT (RR 7.3; 95% CI 0.9 to 58.8). There was no heterogeneity between trials (I² = 0%).
1.7. Analysis.
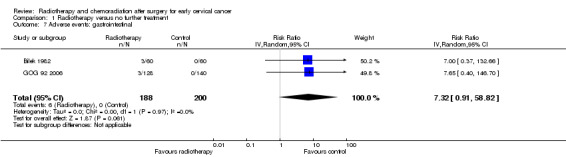
Comparison 1 Radiotherapy versus no further treatment, Outcome 7 Adverse events: gastrointestinal.
Rectal/sigmoid strictures
Analysis 1.8. The trial of Bilek 1982 showed no statistically significant difference in the risk of rectal or sigmoid strictures (scarring caused by RT, that can lead to bowel obstruction) in women who received RT and those who received NFT (RR 7.0; 95% CI 0.4 to 132.7).
1.8. Analysis.
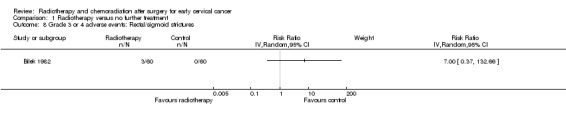
Comparison 1 Radiotherapy versus no further treatment, Outcome 8 Grade 3 or 4 adverse events: Rectal/sigmoid strictures.
Genitourinary
Analysis 1.9. Meta‐analysis of both trials (Bilek 1982; GOG 92 2006) showed no statistically significant difference in the risk of genitourinary side effects in women who received RT and those who received NFT (RR 2.1; 95% CI 0.5 to 8.4). There was no heterogeneity between trials (I² = 0%).
1.9. Analysis.
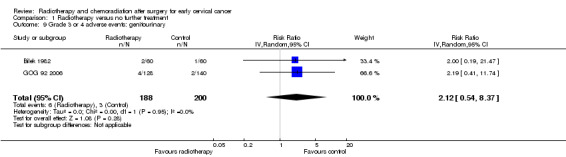
Comparison 1 Radiotherapy versus no further treatment, Outcome 9 Grade 3 or 4 adverse events: genitourinary.
Lymphoedema
Analysis 1.10Bilek 1982 showed no statistically significant difference in the risk of lymphoedema in women who received RT and those who received NFT (RR 2.4; 95% CI 0.9 to 6.4).
1.10. Analysis.
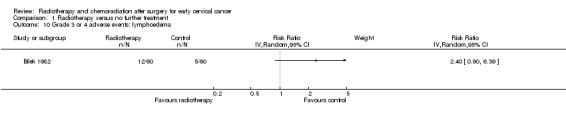
Comparison 1 Radiotherapy versus no further treatment, Outcome 10 Grade 3 or 4 adverse events: lymphoedema.
Hydronephrosis
Analysis 1.11. Bilek 1982 showed no statistically significant difference in the risk of hydronephrosis (swelling of the kidney due to obstruction of the ureters) in women who received RT and those who received NFT (RR 2.0; 95% CI 0.2 to 21.5).
1.11. Analysis.
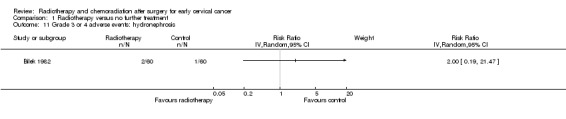
Comparison 1 Radiotherapy versus no further treatment, Outcome 11 Grade 3 or 4 adverse events: hydronephrosis.
Neurological
Analysis 1.12. GOG 92 2006 showed no statistically significant difference in the risk of neurological (nervous system) side effects in women who received RT and those who received NFT (RR 3.3; 95% CI 0.1 to 79.8).
1.12. Analysis.
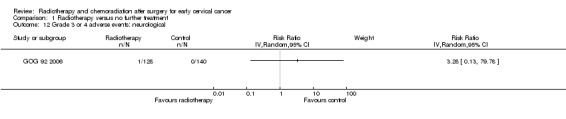
Comparison 1 Radiotherapy versus no further treatment, Outcome 12 Grade 3 or 4 adverse events: neurological.
Discussion
Summary of main results
We found only two trials, enrolling 397 women with stage IB cervical cancer, that met our inclusion criteria. These trials compared the use of RT with no RT in women with early cervical cancer who had radical hysterectomy and PLND and who were at high risk of disease recurrence.
These trials showed that adjuvant RT after radical surgery significantly decreased local recurrence rates, but provided only weak evidence that it might improve OS. When we combined the findings from these two trials, we found that, on average, the risk of relapse within 5 years among women who received RT was between 40% and 90% of the risk among women who did not receive RT (RR 0.6; 95% CI 0.4 to 0.9). However, because of the low number of deaths in the trials, we could not confirm whether this apparently beneficial effect translated into better survival: 5 years after treatment the risk of death among women who received RT was, on average, 80% of the risk among women who did not receive RT, but the 95% CI was wide, ranging from a much lower risk of death to over twice the risk (RR 0.8; 95% CI 0.3 to 2.4) ‐ see Table 1.
The trials had two major limitations. First, they gave very little information about adverse events. Although we found no statistically significant difference in risk of grade 3 and 4 adverse events in women who did and did not receive RT, this was largely because the trials reported very few side effects and so lacked the statistical power to detect any difference in risk that might be present. Overall the risk of adverse events was consistently higher among women who received RT. Second, the evidence from these trials does not assist us in determining which pathological risk factors, or combinations of risk factors, indicate that women should be treated with adjuvant RT.
Overall completeness and applicability of evidence
We did not find any randomised trials that assessed either chemoradiation or chemotherapy followed by RT compared with no adjuvant therapy in early cervical cancer. Hence the available evidence addresses RT alone. Although we specified QoL as an outcome of interest, neither trial reported this. QoL after treatment for cancer is an extremely important outcome, as treatment‐related morbidity very often degrades the quality of the time that patients live in the future.
Current practice definitely differs from centre to centre, and from population group to population group, and depends on such varied factors as local interpretation of evidence and complication rates, availability of resources and incidence of human immunodeficiency virus (HIV) infection. The two studies identified, with similar interventions, small numbers of patients, limited information about treatment‐related morbidity and QoL outcomes, and little information about patients' risk factors in one study (Bilek 1982), provide limited evidence that is relevant to the range of clinical practice.
Quality of the evidence
The amount of available evidence does not allow robust conclusions, especially as one of the included studies (Bilek 1982) had an extremely small number of patients and a dearth of information about those patients.
Both included studies had a high risk of bias, since they did not report the method of generation of the sequence of random numbers used to allocate women to treatment arms, or concealment of this allocation sequence from healthcare providers and patients, or blinding of outcome assessors. Inadequate concealment of allocation and lack of blinding are often associated with an exaggeration of the effects of treatment (Moher 1998; Schulz 1995). The evidence on OS is more robust than that for PFS, since blinding of outcome assessors is of less relevance for death than for disease progression.
Only one study reported an HR that is the best statistic to summarise the difference in risk in two treatment groups over the duration of a trial, when there is 'censoring', that is, the time to death (or disease progression) is unknown for some women as they were still alive (or disease free) at the end of the trial. The analyses of death (and disease recurrence) that are based on RRs are less reliable than those based on HRs because different women had different lengths of follow‐up and the RRs did not allow for this.
The two studies gave inconsistent evidence about 5‐year survival, so the pooled estimate of 5‐year survival had wide CIs: therefore we cannot be sure whether RT improves survival or increases the risk of death. Few women experienced disease progression, adverse events or death. Consequently the quality of the evidence is moderate and the findings of the review should be interpreted cautiously.
Furthermore, the available evidence does not assist us in deciding which women with high‐risk early cervical cancer are likely to benefit from adjuvant RT, apart from the subgroup in GOG 92 2006, which had the combination of negative capillary lymphatic space, deep stromal invasion and tumour size greater than 4 cm; these women had a significantly lower risk of death if they received RT. GOG 92 2006 also suggests that women with non‐squamous histology derive benefit from adjuvant RT. This is not strong evidence, as it is not confirmed by other studies, and several subgroup combinations of risk factors were examined, so it could be a chance finding.
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature and all studies were sifted and data extracted by two review authors independently. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence we have attempted to reduce bias in the review process.
The greatest threat to the validity of the review is likely to be the possibility of publication bias, that is, studies that did not find the treatment to have been effective may not have been published. We were unable to assess this possibility as we found only two included studies.
Agreements and disagreements with other studies or reviews
The excluded study of Lahousen 1999 concluded that adjuvant chemotherapy or RT does not improve survival or recurrence rates in high‐risk cervical cancer patients after radical hysterectomy. However, comparing this with the included studies is difficult as Lahousen 1999 randomised patients with high‐risk cervical cancers, including those with stage IIB cancers ‐ by definition a far more heterogenous and higher risk group of patients than those in the included studies.
In a review of chemotherapy for early cervical cancer, the addition of chemotherapy to RT was associated with improved OS and PFS compared with adjuvant RT alone (Rosa 2009). This evidence was considered to be of a moderate quality; however, evidence relating to adverse effects was limited and none of the included studies assessed QoL. To our knowledge adjuvant chemoradiation has not been compared with no adjuvant therapy for early‐stage cervical cancer in RCTs and there are no ongoing trials comparing RT with no adjuvant treatment. However there are currently two ongoing trials comparing adjuvant chemoradiation with adjuvant RT for early cervical cancer (see Rosa 2009 for further details). The outcome of these trials may help to establish whether there is a need for further trials of adjuvant RT or chemoradiation compared with no adjuvant treatment.
Authors' conclusions
Implications for practice.
1) The available evidence is not of high quality. It suggests that women with stage IB cervical cancer who have high risk factors after undergoing treatment with radical hysterectomy, should be carefully counselled about the risks and benefits of adjuvant RT, before a decision regarding adjuvant treatment is made. The counselling should emphasise not only the benefit of decreased local recurrence rates, but also the risks of increased treatment‐related side effects and the lack of evidence that RT improves survival.
2) The available evidence does not provide clear guidance in determining which women should be offered adjuvant RT after radical hysterectomy. Evidence from the GOG 92 2006 study suggests that women with a combination of negative capillary lymphatic space, deep stromal invasion and tumour size greater than 4 cm, and women with non‐squamous histology, might benefit from RT.
3) Based on limited evidence from an allied review (Rosa 2009), women with early cervical cancer and high‐risk factors for recurrence may also be offered adjuvant chemoradiation, pending further evidence from RCTs.
Implications for research.
Ideally, a large RCT with long‐term follow‐up is needed to assess the risks and benefits of adjuvant RT and adjuvant chemoradiation, compared to no adjuvant therapy, after radical hysterectomy for women with early‐stage cervical cancer. Trials should be large enough to have power to detect any benefit of RT in prognostic subgroups defined by capillary lymphatic space status, depth of invasion, tumour size and tumour type. Outcomes should include not only OS and PFS and adverse events, but also QoL. However, due to the decreasing incidence of cervical cancer in developed countries, which have the resources to run such trials, it seems unlikely that this research will be prioritised.
What's new
| Date | Event | Description |
|---|---|---|
| 26 February 2014 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 18 April 2012 | Amended | Minor correct made to the text. |
| 30 March 2012 | New citation required but conclusions have not changed | Review updated. No additional studies identified. Conclusions unchanged. |
| 25 September 2011 | New search has been performed | Search updated from November 2008 to September 2011 (283 records identified, 224 after de‐duplication). |
Acknowledgements
We thank Chris Williams for clinical and editorial advice, Jane Hayes for designing the search strategy, Gail Quinn and Clare Jess for their contribution to the editorial process, and the referees for their many helpful suggestions.
For the updated review, Tess Lawrie assisted with the updating of the text, Jane Hayes updated the search and Julia Dawson sifted the search results.
This work was carried out while LR and SS were working at the Pan‐Birmingham Gynaecological Cancer Centre.
Appendices
Appendix 1. MEDLINE search strategy
1 exp Uterine Cervical Neoplasms/ 2 (cervi* adj5 cancer*).mp. 3 (cervi* adj5 neoplas*).mp. 4 (cervi* adj5 carcinom*).mp. 5 (cervi* adj5 malignan*).mp. 6 (cervi* adj5 tumor*).mp. 7 (cervi* adj5 tumour*).mp. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp Radiotherapy, Adjuvant/ 10 radiotherapy.mp. 11 (chemoradiation or chemoradiotherapy).mp. 12 (chemo‐radiation or chemo‐radiotherapy).mp. 13 10 or 11 or 12 14 adjuvant.mp. 15 13 and 14 16 9 or 15 17 "randomized controlled trial".pt. 18 "controlled clinical trial".pt. 19 randomized.ab. 20 randomly.ab. 21 trial.ab. 22 groups.ab. 23 17 or 18 or 19 or 20 or 21 or 22 24 8 and 16 and 23 25 Animals/ 26 Humans/ 27 25 not (25 and 26) 28 24 not 27
key: mp = title, original title, abstract, name of substance word, subject heading word
ab = abstract
pt = publication type
Appendix 2. EMBASE search strategy
1 Uterine Cervix Tumor/ 2 (cervi* adj5 cancer*).mp. 3 (cervi* adj5 neoplas*).mp. 4 (cervi* adj5 carcinom*).mp. 5 (cervi* adj5 malignan*).mp. 6 (cervi* adj5 tumor*).mp. 7 (cervi* adj5 tumour*).mp. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 Adjuvant Therapy/ 10 radiotherapy.mp. 11 (chemoradiation or chemoradiotherapy).mp. 12 (chemo‐radiation or chemo‐radiotherapy).mp. 13 10 or 11 or 12 14 adjuvant.mp. 15 13 and 14 16 9 or 15 17 exp Controlled Clinical Trial/ 18 randomized.ab. 19 randomly.ab. 20 trial.ab. 21 groups.ab. 22 17 or 18 or 19 or 20 or 21 23 8 and 16 and 22 24 exp Animal/ 25 Human/ 26 24 not (24 and 25) 27 23 not 26
key: mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
ab = abstract
Appendix 3. Cochrane Central Register of Controlled Trials (CENTRAL) ,
#1 Mesh descriptor Uterine Cervical Neoplasms explode all trees #2 cervi* near/5 cancer* #3 cervi* near/5 neoplas* #4 cervi* near/5 carcinom* #5 cervi* near/5 malignan* #6 cervi* near/5 tumor* #7 cervi* near/5 tumour* #8 (#1 or #2 or #3 or #4 or #5 or #6 or #7) #9 Mesh descriptor Radiotherapy, Adjuvant explode all trees #10 radiotherapy #11 (chemoradiation or chemoradiotherapy) #12 (chemo‐radiation or chemo‐radiotherapy) #13 (#10 or #11 or #12) #14 adjuvant #15 (#13 and #14) #16 (#9 or #15) #17 (#8 and #16)
Appendix 4. Data abstraction form
Adjuvant radiotherapy and chemoradiation after surgery for cervical cancer
Paper ID:
THE DATA COLLECTION CHECKLIST
August 2008
DATA COLLECTION
Once potentially relevant studies have been identified for a review, the following data should be extracted independently by two reviewers.
Please record your name and the Study ID (first author and year of publication) in the space provided on this page and on any page(s) which may be separated from the main checklist, e.g. Results section.
For all items reviewers should mark an X against the appropriate response in each case. In addition it will be helpful if you cut and paste relevant supporting text and state its original location in the paper (page/column/paragraph). This facilitates later comparisons of extracted data. Any other comments can also be recorded in the right‐hand side boxes.
Data which is missing or 'UNCLEAR' in a published report should be marked clearly on the data collection form.
Items in the data extraction sheet which are clearly not applicable to the study in question should be marked accordingly (i.e. N/A).
Following data extraction, reviewers should compare their completed data extraction sheets and attempt to reach agreement for each item on the checklist before submitting their completed data records to LR. Decisions about clinical issues that cannot be resolved easily should be referred to SSNS. Decisions about methodological issues that cannot be resolved easily should be referred to AB.
SCOPE OF REVIEW: INCLUSION/EXCLUSION CRITERIA
| Inclusion criteria | Yes/No/Unclear | Relevant supporting text and location: (page/column/paragraph) |
| Did women have confirmed histological diagnosis of early‐stage cervical cancer (FIGO stage IB1, IB2 or IIA) and had radical hysterectomy and pelvic lymph nodes (PLNDs)? | ||
Was intervention one of the following?
Only studies that address radiotherapy or chemoradiation in the adjuvant setting will be included |
||
| Was there a concurrent control group restricting women to receive either no adjuvant chemotherapy or radiotherapy? | ||
| If any of the inclusion criteria are not satisfied, the study should be excluded from the review. COLLECT NO FURTHER DATA | ||
| INCLUSION CRITERIA | ||
| Type of study design, as described by authors: | Mark as appropriate | Relevant supporting text and location. (page/column/paragraph) |
| Randomised controlled trial (RCT) | ||
| Quasi‐randomised controlled trial (RCT) | ||
|
STUDY DETAILS |
Relevant supporting text and location (page/column/paragraph) |
|
Country: If multicentre please give details Please state UNCLEAR if information is not available |
|
|
Setting: |
|
|
Duration: Indicate N/A as appropriate |
|
| Median length of follow‐up: | |
| Mean length of follow‐up: | |
| Min length of follow‐up: | |
| Max length of follow‐up: | |
| Additional information: |
| Baseline characteristics of participants: | Relevant supporting text and location (page/column/paragraph) | |
| Age | Mean = Years SD = Median = Years Range: | |
| FIGO stage | Number (%) FIGO stage I: Number (%) FIGO stage II: | |
| Histological cell type | Number (%)
Number (%) Number (%) Number (%) Number (%) Number (%) |
|
| Tumour grade | [Just early stage so is this needed?] | |
| Comorbidity | |
|
| Previous treatment | ||
ASSESSMENT OF RISK OF BIAS:
|
Randomisation Was the allocation sequence adequately generated? Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. |
Tick one row | Relevant supporting text and location (page/column/paragraph) |
| Yes: for example, a computer‐generated random sequence or a table of random numbers | ||
| No: for example, non‐randomised or quasi‐randomised (participants allocated on basis of date of birth, clinic identification number or surname) | ||
| Unclear: Insufficient information about the sequence generation | ||
|
ALLOCATION CONCEALMENT Was the randomisation sequence for allocating participants to the different arms of the trial adequately concealed, to prevent both participants the clinicians providing treatment predicting in advance which arm of the trial a women would be assigned to? |
||
| Yes: for example, where the allocation sequence could not be foretold | ||
| No: for example, open random number lists or quasi randomisation such as alternate days, odd/even date of birth or hospital number | ||
| Unclear: for example, if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed | ||
|
BLINDING OF OUTCOME ASSESSORS: Were the clinicians who assessed disease progression at the end of follow‐up prevented from knowing which arm of the trial the women were assigned to? |
||
| Yes: outcome assessors were blinded | ||
| No: no blinding or incomplete blinding of outcome assessors | ||
| Unclear: insufficient information to permit judgement of 'yes' or 'no' |
| LOSS TO FOLLOW UP: | Enter numbers below | Relevant supporting text and location (page/column/paragraph) |
| How many participants were enrolled in each treatment arm? Intervention group: Comparison group: | ||
| How many participants were assessed at the end of follow‐up in each treatment arm? Intervention group: Comparison group: | ||
| What % of patients were lost to follow‐up? Intervention group: Comparison group: Overall: | ||
| Now code satisfactory level of loss‐to‐follow up as Yes/No/Unclear: | Tick one row below | |
| Yes: if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms | ||
| No: if more than 20% of patients were lost to follow or reasons for loss to follow‐up were different in different treatment arms | ||
| Unclear: if loss to follow was not reported |
CHARACTERISTICS OF THE INTERVENTIONS
| Describe the intervention(s) for each study group. Report this in the words of the paper and give specific details if they are provided, for example, details of radiotherapy, prior chemotherapy or concurrent combination of the two, etc. as appropriate | Location of text (page/column/paragraph) |
|
Intervention |
|
|
Comparison No adjuvant chemotherapy or radiotherapy Additional details: |
|
|
Did any women receive a different intervention from the one to which they were assigned? Yes/No/Unclear |
|
| If the answer to the question above is YES, record any reported changes of assigned treatment | |
|
Intervention |
|
|
Comparison |
|
|
If women received treatments different from those to which they were assigned, were outcomes reported in the groups to which they were assigned? Yes/No/Unclear |
OUTCOMES
|
Overall survival |
||
| If the following were reported, record the value | Value | Relevant supporting text and location (page/column/paragraph) |
| Unadjusted hazard ratio (HR) Was the comparison group the reference group for the estimate of the HR? | Yes/No/Unclear | |
| 95% confidence on unadjusted HR Lower 95% confidence interval Upper 95% confidence interval | ||
| Adjusted hazard ratio (HR) Was the comparison group the reference group for the estimate of the HR? List the factors for which the HR was adjusted: | Yes/No/Unclear | |
| 95% confidence on adjusted HR Lower 95% confidence interval Upper 95% confidence interval | ||
| If an HR was reported, record the number of women in each treatment arm on whom the estimated HR was based: Number of women in intervention arm: Number of women in comparison arm: |
||
| If an HR was reported, and if the study was based on a pre‐specified protocol for assigning women to intervention group or comparison group, was the HR based on an intention‐to‐treat (ITT) analysis? i.e. were women analysed in the groups to which they were assigned, regardless of what treatment they received? | Yes/No/Unclear | |
| SE(HR) | ||
| SE(ln(HR)) | ||
| Var(HR) | ||
| Var(ln(HR)) | ||
| Kaplan Meier plots | Yes/No | |
| Minimum follow up time | ||
| Maximum follow up time | ||
| Log rank P‐value | ||
| Was Cox regression reported? | Yes/No | |
| Cox P‐value |
| OUTCOMES | ||
|
Progression‐free survival |
||
| If the following were reported, record the value | Value | Relevant supporting text and location. (page/column/paragraph) |
| Unadjusted hazard ratio (HR) Was the comparison group the reference group for the estimate of the HR? | Yes/No/Unclear | |
| 95% confidence on unadjusted HR Lower 95% confidence interval Upper 95% confidence interval | ||
| Adjusted hazard ratio (HR) Was the comparison group the reference group for the estimate of the HR? List the factors for which the HR was adjusted: | Yes/No/Unclear | |
| 95% confidence on adjusted HR Lower 95% confidence interval Upper 95% confidence interval | ||
| If an HR was reported, record the number of women in each treatment arm on whom the estimated HR was based: Number of women in intervention arm: Number of women in comparison arm: |
||
| If an HR was reported, and if the study was based on a pre‐specified protocol for assigning women to intervention group or comparison group, was the HR based on an intention‐to‐treat (ITT) analysis? That is, were women analysed in the groups to which they were assigned, regardless of what treatment they received? | Yes/No/Unclear | |
| SE(HR) | ||
| SE(ln(HR)) | ||
| Var(HR) | ||
| Var(ln(HR)) | ||
| Kaplan Meier plots | Yes/No | |
| Minimum follow up time | ||
| Maximum follow up time | ||
| Log rank P‐value | ||
| Was Cox regression reported? | Yes/No | |
| Cox P‐value |
| Intervention group | Comparison group | Location of text (page/column/paragraph) | |||
| Total number of women enrolled in study | |||||
| For women enrolled in comparison of intervention/comparison | |||||
| Number of women enrolled | |||||
| Number of deaths | |||||
| Number of women whose vital status was known | |||||
| Time point at which deaths were recorded, for example, 1 year/5 years/end of study/not reported | |||||
| Median time to death | |||||
| Mean (SD) time to death | |||||
| Number (%) of women with disease progression | |||||
| Number of women whose disease was assessed | |||||
| Time point at which disease progression was recorded, for example, 1 year/5 years/end of study/not reported | |||||
| Median time to progression | |||||
| Mean (SD) time to progression | |||||
|
QoL outcome State 'not reported' if not given |
Response | Relevant supporting text and location (page/column/paragraph) | |||
| Validated scale Yes/No | |||||
| Name of scale | |||||
| Intervention group: Mean QoL at end of follow‐up SD of QoL at end of follow‐up Number of women assessed for QoL at end of follow‐up |
|||||
| Comparison group: Mean QoL at end of follow‐up SD of QoL at end of follow‐up Number of women assessed for QoL at end of follow‐up |
|||||
| Adverse events | |||
Grades of toxicity relating to chemotherapy, radiotherapy or a combination of both will be extracted and grouped as:
List below the specific types and numbers of adverse events reported. | |||
| Number | |||
| Intervention group | Comparison group | Location of text (page/column/paragraph) | |
|
|
|
||
|
Other: Give description and number of any other adverse events reported List below the specific types and numbers of adverse events reported | |||
|
|
|||
| Does the number of adverse events reported above refer to the number of women who experienced adverse events or to the number of episodes of adverse events? | Number of women/number of episodes | ||
Data and analyses
Comparison 1. Radiotherapy versus no further treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival (adjusted for prognostic factors) | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 2 Deaths within 5 years (unadjusted) | 2 | 397 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.30, 2.36] |
| 3 Progression‐free survival (unadjusted) | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Disease recurrence within 5 years (unadjusted) | 2 | 397 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.37, 0.91] |
| 5 Recurrence‐free survival (using adjusted HR for GOG 92) | 2 | Hazard Ratio (Fixed, 95% CI) | 0.58 [0.39, 0.88] | |
| 6 Adverse events: haematological | 2 | 388 | Risk Ratio (IV, Random, 95% CI) | 2.38 [0.63, 9.05] |
| 7 Adverse events: gastrointestinal | 2 | 388 | Risk Ratio (IV, Random, 95% CI) | 7.32 [0.91, 58.82] |
| 8 Grade 3 or 4 adverse events: Rectal/sigmoid strictures | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 9 Grade 3 or 4 adverse events: genitourinary | 2 | 388 | Risk Ratio (IV, Random, 95% CI) | 2.12 [0.54, 8.37] |
| 10 Grade 3 or 4 adverse events: lymphoedema | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 11 Grade 3 or 4 adverse events: hydronephrosis | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 12 Grade 3 or 4 adverse events: neurological | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bilek 1982.
| Methods | Multicentre RCT | |
| Participants | Country: German Democratic Republic 120 women with squamous cell carcinomas of the cervix uteri stage pT1bN0M0 previously treated by radical hysterectomy The mean age at study entry was 40.6 years (range: 23 to 60 years) All women presented with FIGO stage I. Tumour cell type was squamous in all 120 (100%) women Tumour grade: 1: 36 (30%), 2: 60 (50%), 3: 24 (20%) |
|
| Interventions | Women were randomised into 2 groups:
|
|
| Outcomes |
|
|
| Notes | Mean length of follow‐up was 44 months (range 24 to 72 months) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For all outcomes: % analysed: 120/120 (100%) "All patients (n = 120) entered into the study were evaluable for survival rate, date and anatomical location of recurrences, results of autopsy and morbidity of therapy" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
GOG 92 2006.
| Methods | Multicentre RCT | |
| Participants | Country: US 277 women were eligible for the study if they had primary stage IB squamous, adenosquamous carcinoma or adenocarcinoma of the cervix initially treated with a standard radical hysterectomy and who had negative lymph nodes but one of a specified combination of risk factors. The median age at study entry was 41 years (range 20 to 80 years) All patients had primary stage IB The tumour cell type was squamous in 218 (79%) women, adenosquamous in 32 (12%) women and adenocarcinoma in 27 (10%) women GOG Performance Grade: 0: 185 (67%) women, 1: 86 (31%) women, 2: 6 (2%) women |
|
| Interventions | Arm 1: RT RT was started within 4 to 6 weeks postoperatively. Patients received external beam irradiation and no brachytherapy. The pelvic irradiation was given with a 4‐field technique with a megavoltage beam, although cobalt‐60 was allowed if the SSD was greater than 80 cm. Radiation dose was from 46 Gy in 23 fractions to 50.4 Gy in 28 fractions, 5 fractions per week. Each patient was to be given daily fractions of 1.80 to 2.00 Gy over 4.5 to 6 weeks. Treatment breaks for clinical problems (vomiting or diarrhoea) were allowed to total no more than 1 week Arm 2: No adjuvant chemotherapy or RT Additional details: Follow‐up observation: patients were to be evaluated by physical examination, blood counts, blood chemistries and chest x‐rays, every 3 months during the first 2 years of follow‐up, and every 6 months during the subsequent years. Intravenous pyelogram, renal sonogram or CT scan with contrast was done at 6 months and then yearly. Results of these tests, as well as changes of therapy, adverse effects, progression or death, were reported |
|
| Outcomes |
|
|
| Notes | Of the 137 patients randomised to RT, 9 (6.6%) refused all RT and 6 (4.4%) refused to continue therapy after receiving less than 85% of the prescribed dose of 50.4 Gy (3.6, 3.6, 10.4, 14.4, 16.2, and 36.0 Gy). One patient discontinued RT due to an adverse reaction after receiving 21.6 Gy. In addition, 9 (6.6%) non‐compliant patients had acceptable radiation doses (85% of 50.4 Gy) but in excess of 20% protraction of overall treatment time. Two other patients exceeded 20% protraction of treatment time due to an adverse reaction to the radiation requiring interruption of therapy. Median length of follow‐up: 10.0 years (range: 0.003 to 16 years) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported, "After the eligibility criteria were verified, patients were randomly assigned to one of the two regimens: pelvic radiation or no further therapy" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For grade 3 or 4 adverse events: % analysed: 268/277 (97%) women RT: 128/137 (93%) women Control: 140/140 (100%) women Analysis of overall and PFS used survival methods that allowed for loss to follow‐up "There is a small but noteworthy imbalance in the follow‐up between the two treatment regimens. Of those who are alive, six patients are lost‐to‐follow‐up within the first year in the RT group while one is lost in the NFT group. Within 2 years on study, there are eight and three patients in the RT group and NFT group, respectively" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Abbreviations: CT: computed tomography; FIGO: International Federation of Gynecology and Obstetrics; HR: hazards ratio; NFT: no further treatment; OS: overall survival; PFS: progression‐free survival; RCT: randomised controlled trial; RT: radiotherapy; SSD: source‐surface difference.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lahousen 1999 | Study includes women with stage IIB disease ‐19/76 (25%) women |
Differences between protocol and review
This review includes only RCTs, however in the protocol we had planned to include non‐RCTs too.
If sufficient trials had been available, sub‐group analyses would have been performed, grouping the trials by high risk versus low risk patients. we would have considered factors such as age, stage, type of intervention, length of follow‐up and adjusted/unadjusted analysis in the interpretation of any heterogeneity. Similarly, if sufficient studies had been identified, we would have performed sensitivity analyses (i) excluding studies at high risk of bias and (ii) using unadjusted results.
Contributions of authors
LR, DL and SS drafted the clinical sections of the protocol; HD and AB drafted the methodological sections of the protocol. All authors agreed the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG‐506
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Bilek 1982 {published data only}
- Bilek K, Ebeling K, Leitsmann H, Seidel G. Radical pelvic surgery versus radical surgery plus radiotherapy for stage IB carcinoma of the cervix uteri: preliminary results of a prospective randomised clinical study. Archiv fur Geschwulstforschung 1982;52(3):223‐29. [PubMed] [Google Scholar]
GOG 92 2006 {published data only}
- Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow‐up of a gynaecologic oncology group study. International Journal of Radiation Oncology and Biological Physics 2006;65(1):169–76. [DOI] [PubMed] [Google Scholar]
- Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a gynecologic oncology group study. Gynecologic Oncology 1999;73:177‐83. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Lahousen 1999 {published data only}
- Lahousen M, Haas J, Pickel H, Hackl A, Kurz C, Ogris H, et al. Chemotherapy versus radiotherapy versus observation for high‐risk cervical carcinoma after radical hysterectomy: a randomized, prospective, multicenter trial. Gynecologic Oncology 1999;73:196–201. [DOI] [PubMed] [Google Scholar]
Additional references
Benedet 2000
- Benedet JL, Hacker NF, Ngan HYS. Staging classifications and clinical practice guidelines of gynaecologic cancers by FIGO Committee on Gynecologic Oncology and IGCS Guidelines Committee. International Journal of Gynaecology and Obstetrics 2000;70:207‐312. [PubMed] [Google Scholar]
CCCMAC 2010
- Vale C, Tierney JF, Stewart LA, Brady M, Dinshaw K, Jakobsen A, et al (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta‐analysis (Review). Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008285] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTCAE 2006
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30 (accessed 15 March 2012).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Egger M, Davey Smith G, Altman DG (editors). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). London: BMJ Publication Group, 2001:313‐35. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
EUROCARE 2003
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al and the EUROCARE Working Group. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14(Suppl 5):v61‐v118. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008. Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 10, version 2.0. IARC Press, Lyon, France, 2010. Available from http://globocan.iarc.fr.
Gray 2008
- Gray HJ. Primary management of early stage cervical cancer (IA1‐IB) and appropriate selection of adjuvant therapy. Journal of the National Comprehensive Cancer Network 2008;6(1):47‐52. [DOI] [PubMed] [Google Scholar]
Guttmann 1970
- Guttmann R. Significance of post‐operative irradiation in carcinoma of the cervix: a ten year survey. American Journal of Roentgenology 1970;108:102‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kesic 2006
- Kesic V. Management of cervical cancer. European Journal of Surgical Oncology 2006;32(8):832‐7. [DOI] [PubMed] [Google Scholar]
Kitchener 2010
- Kitchener HC, Hoskins W, Small, W, Thomas GM, Trimble EL and the Cervical Cancer Consensus Group. The development of priority cervical cancer trials: a Gynecologic Cancer InterGroup report. International Journal of Gynecological Cancer 2010;20:1092‐1100. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham D, Jones A, Cook DJ, Jadad AR, Moher M. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Peters 2000
- Peters WA, Liu PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high‐risk early‐stage cancer of the cervix. Journal of Clinical Oncology 2000;18(8):1606‐13. [DOI] [PubMed] [Google Scholar]
Rosa 2009
- Rosa DD, Medeiros LR, Bozzetti MC, Edelweiss MI, Pohlmann PR, Stein AT. Adjuvant chemotherapy for early stage cervix cancer. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD008285] [DOI] [PubMed] [Google Scholar]
Rydzewska 2010
- Rydzewska L, Tierney J, Vale CL, Symonds PR. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007406.pub2] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Sedlis 1999
- Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage Ib carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecologic Oncology 1999;73(2):177‐83. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rogers 2009
- Rogers L, Sui SSN, Luesley D, Bryant A, Dickinson HO. Adjuvant radiotherapy and chemoradiation after surgery for cervical cancer. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007583.pub2] [DOI] [PubMed] [Google Scholar]


