Abstract
Background
Fenoprofen is a non‐steroidal anti‐inflammatory drug (NSAID), available in several different countries, but not widely used.
Objectives
To assess the efficacy of single dose oral fenoprofen in acute postoperative pain, and associated adverse events.
Search methods
We searched Cochrane CENTRAL, MEDLINE, EMBASE and the Oxford Pain Relief Database for studies to December 2010.
Selection criteria
Single oral dose, randomised, double‐blind, placebo‐controlled trials of fenoprofen for relief of established moderate to severe postoperative pain in adults.
Data collection and analysis
Studies were assessed for methodological quality and data extracted by two review authors independently. Summed total pain relief (TOTPAR) or pain intensity difference (SPID) over 4 to 6 hours was used to calculate the number of participants achieving at least 50% pain relief. These derived results were used to calculate, with 95% confidence intervals, the relative benefit compared to placebo, and the number needed to treat (NNT) for one participant to experience at least 50% pain relief over 4 to 6 hours. Numbers of participants using rescue medication over specified time periods, and time to use of rescue medication, were sought as additional measures of efficacy. Information on adverse events and withdrawals was collected.
Main results
Five studies (696 participants) met the inclusion criteria; 24 participants were treated with fenoprofen 12.5 mg, 23 with fenoprofen 25 mg, 79 with fenoprofen 50 mg, 78 with fenoprofen 100 mg, 146 with fenoprofen 200 mg, 55 with fenoprofen 300 mg, 43 with zomepirac 100 mg, 30 with morphine 8 mg, 77 with codeine 60 mg, and 141 with placebo. Participants had pain following third molar extraction, laparoscopy, minor day surgery and episiotomy. The NNT for at least 50% pain relief over 4 to 6 hours with a single dose of fenoprofen 200 mg compared to placebo was 2.3 (1.9 to 3.0). There were insufficient data to analyse other doses or active comparators, time to use of rescue medication, or numbers of participants needing rescue medication. There was no difference in numbers of participants experiencing any adverse events between fenoprofen 200 mg and placebo. No serious adverse events or adverse event withdrawals were reported in these studies.
Authors' conclusions
Oral fenoprofen 200 mg is effective at treating moderate to severe acute postoperative pain, based on limited data for at least 50% pain relief over 4 to 6 hours. Efficacy of other doses, other efficacy outcomes, and safety and tolerability could not be assessed.
Plain language summary
Single dose oral fenoprofen for acute postoperative pain in adults
Fenoprofen is a non‐steroidal anti‐inflammatory drug (NSAID) that is used as a painkiller (analgesic). Five studies looking at a total of 696 participants were included. Because fewer than 200 participants were treated with any one dose of fenoprofen within each study, results must be treated with caution. A good level of pain relief was experienced by better than one in two (over half; 57%) of those with moderate or severe postoperative pain after a single dose of fenoprofen 200 mg, compared to about 1 in 7 (14%) with placebo. This level of pain relief is comparable to that experienced with ibuprofen 400 mg. The frequency of adverse events did not differ between fenoprofen 200 mg and placebo in these studies.
Background
Acute pain occurs as a result of tissue damage either accidentally due to an injury or as a result of surgery. Acute postoperative pain is a manifestation of inflammation due to tissue injury. The management of postoperative pain and inflammation is a critical component of patient care.
This review is for one of a series of reviews whose aim is to increase awareness of the range of analgesics that are potentially available, and to present evidence for relative analgesic efficacy through indirect comparisons with placebo, in very similar trials performed in a standard manner, with very similar outcomes, and over the same duration. Such relative analgesic efficacy does not in itself determine choice of drug for any situation or patient, but guides policy‐making at the local level.
Recent reviews include well established analgesics such as paracetamol (Toms 2008), naproxen (Derry C 2009b), diclofenac (Derry P 2009), and ibuprofen (Derry C 2009a), and newer cyclo‐oxygenase‐2 selective analgesics, such as lumiracoxib (Roy 2010), celecoxib (Derry 2008), etoricoxib (Clarke 2009), and parecoxib (Lloyd 2009).
Acute pain trials
Single dose trials in acute pain are commonly short in duration, rarely lasting longer than 12 hours. The numbers of participants is small, allowing no reliable conclusions to be drawn about safety. To show that the analgesic is working it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, commonly called rescue analgesia, if the pain has not diminished after about an hour. This is reasonable, because not all participants given an analgesic will have significant pain relief. Approximately 18% of participants given placebo will have significant pain relief (Moore 2006), and up to 50% may have inadequate analgesia with active medicines. The use of additional or rescue analgesia is hence important for all participants in the trials.
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years. Trials have to be randomised and double blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity scales immediately before the intervention, and then using pain intensity and pain relief scales over the following four to six hours for shorter acting drugs, and up to 12 or 24 hours for longer acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome. For patients given rescue medication it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over four to six hours (Moore 2005). Patients usually remain in the hospital or clinic for at least the first six hours following the intervention, with measurements supervised, although they may then be allowed home to make their own measurements in trials of longer duration.
Knowing the relative efficacy of different analgesic drugs at various doses can be helpful. An example is the relative efficacy in the third molar extraction pain model (Barden 2004).
Fenoprofen
Fenoprofen is a non‐steroidal anti‐inflammatory drug (NSAID), sporadically available in different countries, including Austria, Denmark, France, Ireland, Italy and the UK in Europe, as well as Brazil, Mexico, USA, Canada, and Hong Kong. There are no consistent licensed indications. In England in 2009 only 1200 prescriptions were issued in primary care. This compares with 2.4 million prescriptions for naproxen and 4.7 million prescriptions for ibuprofen in the same period (PACT 2010).
Clinicians prescribe NSAIDs on a routine basis for a range of mild‐to‐moderate pain. NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Moore 2003). They reversibly inhibit cyclooxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins (PGs) and thromboxane A2 (Fitzgerald 2001). PGs mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive processes. However, relatively little is known about the mechanism of action of this class of compounds aside from their ability to inhibit cyclooxygenase‐dependent prostanoid formation (Hawkey 1999).
Fenoprofen (trade names Nalfon, Nalfont, Nalgesic, Expron, Fenopront, Fenopron, Fepron) is used in the management of mild to moderate pain and for the relief of pain and inflammation associated with disorders such as osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. It is given as the calcium salt although doses are expressed in terms of the base; fenoprofen calcium (dihydrate) 1.2 g is approximately equivalent to 1 g of fenoprofen. A usual dose is the equivalent of 300 to 600 mg of fenoprofen three or four times daily, adjusted thereafter according to response. It has been recommended that the total daily dose should not exceed 3 g (UK) or 3.2 g (USA). Lower doses of 200 mg every 4 to 6 hrs are recommended for mild to moderate pain. Peak plasma concentrations occur 1 to 2 hours after a dose. The plasma half‐life is about 3 hours. Fenbufen 600 to 900 mg daily is at least as effective as ibuprofen 1200 to 1800 mg of fenoprofen 1800 to 2400 mg daily (Brogden 1978). Fenoprofen has been associated with agranulocytosis (Simon 1978), aplastic anaemia (Ashraf 1982) and thrombocytopenia (Katz 1980; Simpson 1978).
We could find no systematic review on the efficacy of fenoprofen in acute pain. This review looks at its efficacy in the setting of acute postoperative pain.
Objectives
To assess the efficacy and adverse effects of single dose oral fenoprofen for acute postoperative pain using methods that permit comparison with other analgesics evaluated in standardised trials using almost identical methods and outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were double blind trials of single dose oral fenoprofen compared with placebo for the treatment of moderate to severe postoperative pain in adults with at least ten participants randomly allocated to each treatment group. Multiple dose studies were included if appropriate data from the first dose was available. Cross‐over studies were included provided that data from the first arm were presented separately.
We excluded the following:
review articles, case reports, and clinical observations;
studies of experimental pain;
studies where pain relief is assessed only by clinicians, nurses or carers (i.e., not patient‐reported);
studies of less than four hours duration or studies that fail to present data over four to six hours post‐dose.
For postpartum pain, we included studies if the pain investigated was due to episiotomy or Caesarean section, irrespective of the presence of uterine cramps, but excluded studies investigating pain due to uterine cramps alone.
Types of participants
We included studies of adult participants (>15 yrs) with established postoperative pain of moderate to severe intensity following day surgery or in‐patient surgery. For studies using a visual analogue scale (VAS), pain of at least moderate intensity was equated to greater than 30 mm (Collins 1997).
Types of interventions
Fenoprofen or matched placebo administered as a single oral dose for postoperative pain.
Types of outcome measures
We collected data on the following outcomes if available:
participant characteristics;
patient reported pain at baseline (physician, nurse or carer reported pain will not be included in the analysis);
patient reported pain relief expressed at least hourly over four to six hours using validated pain scales (pain intensity and pain relief in the form of VAS or categorical scales, or both);
patient global assessment of efficacy (PGE), using a standard categorical scale;
time to use of rescue medication;
number of participants using rescue medication;
number of participants with one or more adverse events;
number of participants with serious adverse events;
number of withdrawals (all cause, adverse event).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases up to December 2010:
Cochrane CENTRAL,
MEDLINE via Ovid,
EMBASE via Ovid,
Oxford Pain Relief Database (Jadad 1996a).
Please see Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy and Appendix 3 for the Cochrane CENTRAL search strategy.
Searching other resources
Additional studies were sought from the reference lists of retrieved articles and reviews.
Language
No language restriction was applied to the searches.
Unpublished studies
We did not contact any manufacturing or distributing pharmaceutical company for unpublished trial data.
Data collection and analysis
Selection of studies
Two review authors independently assessed and agreed the search results for studies that might be included in the review.
Data extraction and management
Two review authors extracted data and recorded it on a standard data extraction form. Data suitable for pooling was entered into RevMan 5.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for quality using a five‐point scale (Jadad 1996b) that considers randomisation, blinding, and study withdrawals and dropouts.
The scale is used as follows:
Is the study randomised? If yes, give one point.
Is the randomisation procedure reported and is it appropriate? If yes, add one point; if no, deduct one point.
Is the study double‐blind? If yes, add one point.
Is the double‐blind method reported and is it appropriate? If yes, add one point; if no, deduct one point.
Are the reasons for patient withdrawals and dropouts described? If yes, add one point.
The scores for each study are reported in the 'Characteristics of excluded studies' table.
A Risk of bias table was completed using assessments of randomisation, allocation concealment and blinding.
Measures of treatment effect
Relative risk (or 'risk ratio', RR) were used to establish statistical difference. Numbers needed to treat (NNT) and pooled percentages were used as absolute measures of benefit or harm.
The following terms are used to describe adverse outcomes in terms of harm or prevention of harm:
When significantly fewer adverse outcomes occur with mefenamic acid than with control (placebo or active) we use the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occur with mefenamic acid compared with control (placebo or active) we use the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
We accepted randomisation to individual participant only.
Assessment of heterogeneity
Heterogeneity of studies was assessed visually (L'Abbe 1987).
Assessment of reporting biases
We assessed publication bias by examining the number of participants in studies with zero effect (relative risk of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level. In this case, we specified a clinically useful level as an NNT of ≥8 (Moore 2008).
Data synthesis
We calculated effect sizes and combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998).
For each study, the mean TOTPAR, SPID, VAS TOTPAR or VAS SPID (Appendix 4) values for active and placebo were converted to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). The proportion of participants in each treatment group who achieved at least 50%maxTOTPAR were calculated using verified equations (Moore 1996; Moore 1997a; Moore 1997b). These proportions were then converted into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. Information on the number of participants with at least 50%maxTOTPAR for active and placebo were used to calculate relative benefit, and number needed to treat to benefit (NNT).
Pain measures accepted for the calculation of TOTPAR or SPID were:
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
VAS for pain relief;
VAS for pain intensity.
If none of these measures were available, the number of participants reporting "very good or excellent" on a five‐point categorical global scale with the wording "poor, fair, good, very good, excellent" would be used for the number of participants achieving at least 50% pain relief (Collins 2001).
The number of participants reporting treatment‐emergent adverse effects was extracted for each treatment group.
Relative benefit or risk estimates were calculated with 95% confidence intervals (CI) using a fixed‐effect model (Morris 1995). NNT and number needed to treat to harm (NNH) and 95% CI were calculated using the pooled number of events by the method of Cook and Sackett (Cook 1995). A statistically significant difference from control was assumed when the 95% CI of the relative benefit or risk did not include the number one.
Subgroup analysis and investigation of heterogeneity
We analysed separately different doses of fenoprofen.
Sensitivity analysis
We planned sensitivity analyses to determine the effect of presenting condition (pain model), and low versus high (two versus three or more) quality studies. A minimum of two studies and 200 participants had to be available for any sensitivity analysis (Moore 1998). The z test (Tramer 1997) would be used to determine if there is a significant difference between NNTs for different groups in the sensitivity analyses when the 95% CIs do not overlap.
Results
Description of studies
Searches identified 15 potentially relevant studies published in 13 reports.
Included studies
Five studies (three publications) satisfied inclusion criteria and reported on a total of 696 participants. All studies used a single dose of study medication and were of parallel group design.
Cooper 1984 treated 129 participants with moderate or severe pain following surgical removal of impacted third molars, although 12 were lost to follow up or had major protocol violations so were not included in analyses. Thirty‐nine received 200 mg fenoprofen, 43 received 100 mg zomepirac, and 35 received placebo. Study duration was 4 hours.
Davie 1982 treated 90 participants with moderate or severe pain following minor outpatient surgery (mainly laparoscopy), with 30 receiving 200 mg fenoprofen, 30 receiving 8 mg morphine, and 30 receiving placebo. All participants received both a tablet (active or placebo) and an injection (active or placebo) to maintain blinding (double dummy technique). Study duration was 6 hours.
Laska 1981 reported on three studies that satisfied inclusion criteria. In study E1 (160 participants) 27 women with severe pain following episiotomy were treated with 50 mg fenoprofen, 27 with 100 mg fenoprofen, 26 with 200 mg fenoprofen, 27 with 300 mg fenoprofen, 26 with 60 mg codeine, and 27 with placebo. In study E2 (162 participants) 24 women with severe pain following episiotomy were treated with 12.5 mg fenoprofen, 23 with 25 mg fenoprofen, 23 with 50 mg fenoprofen, 23 with 100 mg fenoprofen, 23 with 200 mg fenoprofen, 23 with 60 mg codeine, and 23 with placebo. In study S (167 participants) 29 participants with moderate to severe pain following surgery (unspecified) were treated 50 mg fenoprofen, 28 with 100 mg fenoprofen, 28 with 200 mg fenoprofen, 28 with 300 mg fenoprofen, 28 with 60 mg codeine, and 26 with placebo. Study duration was 5 hours.
Further details of individual studies are in the 'Characteristics of included studies' table.
Excluded studies
Ten studies were excluded (Bettigole 1981; Burt 1982; Davie 1978; Derournay 1983; Gruber 1976; Gruber 1979; Kaiko 1984; Offen 1985; Sechzer 1977; Sunshine 1978). Details are in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Two studies (Cooper 1984; Davie 1982) scored 4/5, and three (Laska 1981, Studies E1, E2, S) scored 3/5 on the Oxford Quality Score, so all are considered to be of adequate methodological quality. Points were lost for failure to describe the method of randomisation in all studies, and for not reporting any information about withdrawals in Laska 1981.
The Risk of bias table assessing randomisation, allocation concealment and blinding, indicates that no studies were at high risk of bias (Figure 1).
1.
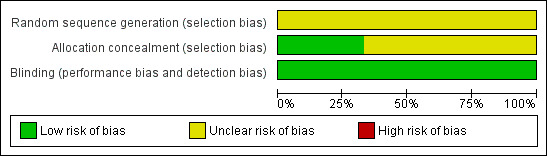
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
All studies compared fenoprofen with placebo, but only for the 200 mg dose were there sufficient data (≥ 2 studies, ≥ 200 participants) for statistical analysis. Although all studies compared fenoprofen with an active comparator, these were all different, so it was not possible to pool data for statistical analysis. Details of results in individual studies are in Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals).
Participants achieving at least 50% pain relief
Fenoprofen 200 mg versus placebo
Five studies compared fenoprofen 200 mg with placebo in 287 participants (Cooper 1984; Davie 1982; Laska 1981 (E1, E2, S)).
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with fenoprofen 200 mg was 57% (83/146, range 43% to 73%);
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with placebo was 13% (19/141, range 0% to 30%);
The relative benefit of treatment compared with placebo was 4.2 (2.7 to 6.4), giving an NNT for at least 50% pain relief over 4 to 6 hours for fenoprofen 200 mg compared with placebo of 2.3 (1.9 to 3.0) (Figure 2).
2.
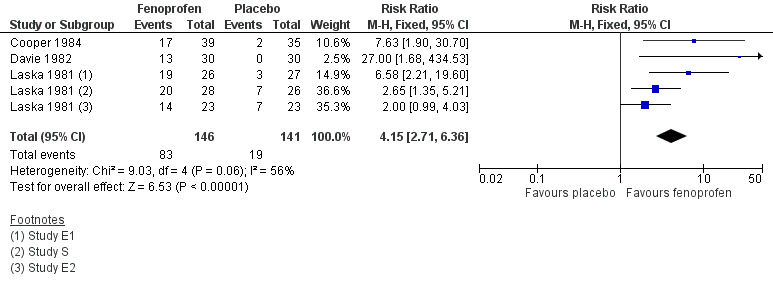
Forest plot comparing fenoprofen 200 mg versus placebo for the outcome of ≥50% total pain relief over 4 to 6 hours.
Assessment of potential publication bias
Studies with an additional 711 participants, demonstrating no difference between fenoprofen 200 mg and placebo, would be needed to give an NNT of 8, which we have specified as the limit of clinical usefulness.
Subgroup analysis
Different doses have been analysed separately, but only for 200 mg were there sufficient data to pool. No subgroup analysis for pain model was possible, again due to insufficient data.
Sensitivity analysis
There were insufficient data for the planned sensitivity analyses to determine the effect of presenting condition (pain model), and low versus high (two versus three or more) quality trials.
Use of rescue medication
Only one study (Cooper 1984) reported on mean time to use of rescue medication, and one (Davie 1982) on the number of participants using rescue medication. In both cases fenoprofen was better than placebo, but there were insufficient data for analysis.
Participants with one or more adverse events
Two studies (Laska 1981; E1 and S) did not report any adverse events, and we assumed that none occurred, since only two were reported in Laska 1981 (E2). In Cooper 1984 participants were asked to record on their report form "whether any adverse effects occurred during the observation period", but there were no details of the method of collection of adverse event data in the other studies. Specific events reported were nausea, vomiting, drowsiness and sweating, all of which may result from the surgery or anaesthetic rather than the analgesic medication given postoperatively.
Fenoprofen 200 mg versus placebo
The proportion of participants experiencing at least one adverse event over 4 to 6 hours with fenoprofen 200 mg was 6.2% (9/146, range 0% to 15%);
The proportion of participants experiencing at least one adverse event over 4 to 6 hours with placebo was 6.4% (9/141, range 0% to 17%);
The relative risk of treatment compared with placebo was 0.94 (0.42 to 2.1). There was no statistically significant difference and no NNH was calculated (Figure 3).
3.
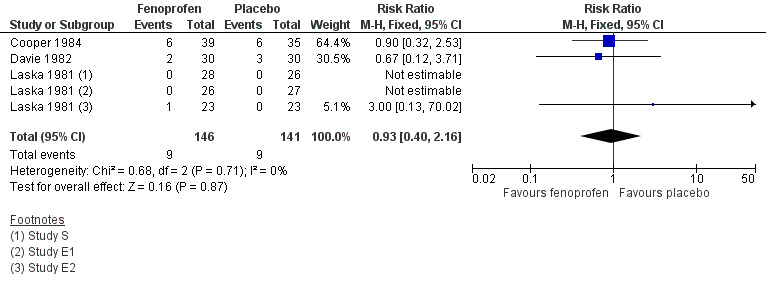
Forest plot comparing fenoprofen 200 mg versus placebo for the outcome of adverse effects over 4 to 6 hours.
No serious adverse events were reported in any study.
Withdrawals
Two studies reported on withdrawals and exclusions. Cooper 1984 reported that of those taking study medication, 12 participants excluded from analyses because they were lost to follow up (4), had un‐interpretable data (1), were not entered into the study (1), or had major protocol violations (6). Davie 1982 did not report any exclusions among participants who satisfied inclusion criteria and gave consent. Both studies reported that there were no adverse event withdrawals. Since these studies used single doses of study medication and were conducted over short time periods, it is unlikely that the exclusions reported here would significantly influence the results.
None of the three studies in Laska 1981 (E1, E2, S) mentioned any withdrawals or exclusions. It is unclear whether there were no withdrawals or exclusions or whether data are reported only for those who completed the study.
Discussion
Summary of main results
This review examined the efficacy of fenoprofen, a non‐steroidal anti‐inflammatory drug (NSAID), in providing postoperative pain relief. Five studies, published in three reports, satisfied inclusion criteria. Participants were experiencing moderate to severe pain following dental surgery (Cooper 1984), minor outpatient surgery (mainly laparoscopy) (Davie 1982) and unspecified surgery (Laska 1981; S), or severe pain following episiotomy (Laska 1981; E1 and E2). Although studies used doses of fenoprofen from 12.5 mg to 300 mg, there were sufficient data for analysis of only the 200 mg dose. Similarly, although zomepirac, codeine and morphine were used in direct comparisons, there were insufficient data for any one comparator for analysis. For fenoprofen 200 mg the relative benefit compared to placebo was 4.2 (2.7 to 6.4), and the NNT for at least 50% pain relief over six hours was 2.3 (1.9 to 3.0). There was no statistically significant difference in the number of participants experiencing one or more adverse events over the study period between fenoprofen 200 mg and placebo, and no serious adverse events or adverse event withdrawals were reported in any of the studies.
There were insufficient data to allow analysis of either time to use of rescue medication or number of participants using it.
Indirect comparisons of NNTs for at least 50% pain relief over 4 to 6 hours in reviews of other analgesics using identical methods indicate that fenoprofen 200 mg is less effective than etoricoxib 120 mg (NNT 1.9 (1.7 to 2.1) Clarke 2009), but may have similar efficacy to commonly used analgesics such as ibuprofen 400 mg (2.5 (2.4 to 2.6) Derry C 2009a), naproxen 500 mg (2.7 (2.3 to 3.2) Derry C 2009b) or diclofenac 50 mg (2.7 (2.4 to 3.0) Derry P 2009). It appears to have better efficacy than paracetamol 1000 mg (3.6 (3.4 to 4.0) Toms 2008). However, the results for fenoprofen are based on relatively few studies with small numbers of participants, so the magnitude of the effect must be interpreted with caution. We calculated that about 700 participants in similar trials would need to experience no benefit with fenoprofen to overturn the result of this review. While that is unlikely, it is not impossible, and much smaller numbers could easily increase the NNT for at least 50% pain relief.
Overall completeness and applicability of evidence
The most important limitation of this review is the small numbers of included studies and participants in each treatment arm. This restricted the analyses to a single dose of fenoprofen compared to placebo, and the numbers in those analyses were small resulting in wide confidence intervals for the point estimates for efficacy, and with consequent uncertainty over the true size of the treatment effect (Moore 1998). We were unable to make any direct comparison of fenoprofen with any active comparator. Furthermore, while all studies reported data for at least 50% pain relief and one or more adverse events, other outcomes were not reported at all, or inconsistently reported.
Participants in these studies had undergone various types of surgery, including third molar, laparoscopy, minor day surgery, and episiotomy, and fenoprofen demonstrated analgesic efficacy in each condition, although based on small numbers in single trials. We were unable to test for differences in response for different kinds of surgery.
No serious adverse events were reported by the included studies, but single dose studies are of limited use for determining the safety and tolerability of analgesics; they are underpowered to do so, and reporting of events is frequently inconsistent, making pooling of data impossible. Further complications arise when adverse event data continue to be collected after intake of rescue medication, which may be associated with its own adverse events.
Quality of the evidence
The methodological quality of the evidence was assessed using to the Oxford Quality Scoring System, based on whether the study was randomised, double‐blinded and if withdrawals were accounted for (Jadad 1996b). All scored ≥3/5 indicating that they are relatively free of bias (Jadad 1996b). Points were lost for failure to provide details of the method of randomisation in all studies, and also details of withdrawals in three studies (Laska 1981; E1, E2, S); these details were frequently not reported in older studies.
For participants who used rescue medication all studies carried forward the last pain measurement before remedication for subsequent evaluation points (last observation carried forward). Although baseline observation carried forward following use of rescue medication (or other missing data) gives a more conservative estimate of efficacy, it has been shown that in acute pain over four to six hours the imputation method makes little difference (Moore 2005).
Potential biases in the review process
Studies were identified from a comprehensive search of published papers, and all stages of the review process were carried out in duplicate, with data entry cross‐checked, according to established methods. We think it unlikely that we have introduced any systematic bias during the review process. While publication bias is always a potential problem when numbers of studies and participants are small, to reduce the result to no clinical significance would require more than three times the study participant numbers found in studies showing no effect at all.
Agreements and disagreements with other studies or reviews
We are not aware of any other reviews of fenoprofen in acute postoperative pain.
Authors' conclusions
Implications for practice.
Fenoprofen appears to be an effective analgesic for relief of postoperative pain following a variety of procedures, based on this very limited data set. No serious adverse events were reported in any of the studies, though numbers were too small to exclude rare but serious harm.
Implications for research.
Given the large number of available drugs of this and similar classes to treat postoperative pain, there is no urgent research agenda, and indeed the most recent studies identified were published in the mid 1980s. More studies could more accurately determine efficacy, but are unlikely to be performed because of well known alternatives. Such studies would need to improve reporting of outcomes other than pain relief and pain intensity difference, such as adverse events and time to remedication.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 17 April 2015 | Review declared as stable | No potentially relevant new studies identified after a restricted search (electronic search strategy run in selected databases). This review will no longer be updated. |
Appendices
Appendix 1. Search strategy for MEDLINE (via OVID)
Fenoprofen.sh
(fenoprofen or Nalfon or Nalfont or Nalgesic or Expron ro Fenopront or Fenopron or Fepron).ti,ab,kw.
OR/1‐2
Pain, postoperative.sh
((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* NEAR pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or ("post‐operative analgesi*")).ti,ab,kw.
((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).ti,ab,kw.
(("pain‐relief after surg*") or ("pain following surg*") or ("pain control after")).ti,ab,kw.
(("post surg*" or post‐surg*) AND (pain* or discomfort)).ti,ab,kw.
((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).ti,ab,kw.
((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).ti,ab,kw.
OR/4‐10
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
OR/12‐19
humans.sh.
20 AND 21
3 AND 11 AND 22
Appendix 2. Search strategy for EMBASE (via OVID)
Fenoprofen.sh.
(fenoprofen or Nalfon or Nalfont or Nalgesic or Expron or Fenopront or Fenopron or Fepron).ti,ab,kw.
OR/1‐2
Postoperative pain.sh.
((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* NEAR pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or ("post‐operative analgesi*")).ti,ab,kw.
((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).ti,ab,kw.
(("pain‐relief after surg*") or ("pain following surg*") or ("pain control after")).ti,ab,kw.
(("post surg*" or post‐surg*) AND (pain* or discomfort)).ti,ab,kw.
((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).ti,ab,kw.
((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).ti,ab,kw.
OR/4‐10
Clinical trials.sh
Controlled clinical trials.sh
Randomized controlled trial.sh
Double‐blind procedure.sh
(clin* adj25 trial*).ab.
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab.
placebo*.ab.
random*.ab.
OR/12‐19
3 AND 11 AND 20
Appendix 3. Search strategy for CENTRAL
MESH descriptor Fenoprofen
(fenoprofen or Nalfon or Nalfont or Nalgesic or Expron or Fenopront or Fenopron or Fepron):ti,ab,kw.
OR/1‐2
MESH descriptor Pain, Postoperative.
((postoperative near4 pain*) or (post‐operative near4 pain*) or post‐operative‐pain* or (post* near pain*) or (postoperative near4 analgesi*) or (post‐operative near4 analgesi*) or ("post‐operative analgesi*")):ti,ab,kw.
((post‐surgical near4 pain*) or ("post surgical" near4 pain*) or (post‐surgery near4 pain*)):ti,ab,kw.
(("pain‐relief after surg*") or ("pain following surg*") or ("pain control after")):ti,ab,kw.
(("post surg*" or post‐surg*) AND (pain* or discomfort)):ti,ab,kw.
((pain* near4 "after surg*") or (pain* near4 "after operat*") or (pain* near4 "follow* operat*") or (pain* near4 "follow* surg*")):ti,ab,kw.
((analgesi* near4 "after surg*") or (analgesi* near4 "after operat*") or (analgesi* near4 "follow* operat*") or (analgesi* near4 "follow* surg*")):ti,ab,kw.
OR/4‐10
3 AND 11
Limit 12 to Clinical Trials (CENTRAL)
Appendix 4. Glossary
Categorical rating scale:
The commonest is the five category scale (none, slight, moderate, good or lots, and complete). For analysis numbers are given to the verbal categories (for pain intensity, none=0, mild=1, moderate=2 and severe=3, and for relief none=0, slight=1, moderate=2, good or lots=3 and complete=4). Data from different subjects is then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. Good correlation was found, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
VAS:
Visual analogue scale: lines with left end labelled "no relief of pain" and right end labelled "complete relief of pain", seem to overcome this limitation. Patients mark the line at the point which corresponds to their pain. The scores are obtained by measuring the distance between the no relief end and the patient's mark, usually in millimetres. The main advantages of VAS are that they are simple and quick to score, avoid imprecise descriptive terms and provide many points from which to choose. More concentration and coordination are needed, which can be difficult post‐operatively or with neurological disorders.
TOTPAR:
Total pain relief (TOTPAR) is calculated as the sum of pain relief scores over a period of time. If a patient had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the composite trapezoidal rule. The trapezoidal rule is a simple method that approximately calculates the definite integral of the area under the pain relief curve by calculating the sum of the areas of several trapezoids that together closely approximate to the area under the curve.
SPID:
Summed pain intensity difference (SPID) is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the trapezoidal rule.
VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID.
See "Measuring pain" in Bandolier's Little Book of Pain, Oxford University Press, Oxford. 2003; pp 7‐13 (Moore 2003).
Appendix 5. Summary of efficacy outcomes in individual studies
| Analgesia | Rescue medication | |||||
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE: very good or excellent | Median time to use (h) | Number using |
| Cooper 1984 | (1) Fenoprofen 200 mg, n = 39 (2) Zomepirac 100 mg, n = 43 (3) Placebo, n = 35 |
TOTPAR 4: (1) 6.76 (2) 6.91 (3) 2.24 |
(1) 17/39 (2) 20/43 (3) 2/35 |
(1) 15/39 (2) 19/43 (3) 0/35 |
Mean: (1) 3.37 (2) 3.41 (3) 2.20 |
No data |
| Davie 1982 | (1) Fenoprofen 200 mg PO, n = 30 (2) Morphine 8 mg IV, n = 30 (3) Placebo, n = 30 |
VAS‐SPID 6: (1) 91.65 (2) 175.75 (3) 1.39 |
(1) 13/30 (2) 23/30 (3) 0/30 |
No data | No data | At 6 h: (1) 11/30 (2) 7/30 (3) 24/30 |
| Laska 1981 | (1) Fenoprofen 200 mg, n = 77 (E1 26, E2 23, Surg 28) (2) Fenoprofen 12.5 mg, n = 24 (E2 24) (3) Fenoprofen 25 mg, n = 23 (E2 23) (4) Fenoprofen 50 mg, n = 79 (E1 27, E2 23, S 29) (5) Fenoprofen 100 mg, n = 78 (E1 27, E2 23, S 28) (6) Fenoprofen 300 mg, n = 55 (E1 27, S 28) (7) Codeine 60 mg, n = 77 (E1 26, E2 23, S 28) (8) Placebo, n = 76 (E1 27, E2 23, S 26) |
TOTPAR 5: E1: (1) 13.0 (4) 11.6 (5) 12.1 (6) 12.3 (7) 7.8 (8) 3.5 E2: (1) 10.6 (2) 7.8 (3) 8.9 (4) 9.1 (5) 11.2 (7) 10.7 (8) 6.0 S: (1) 12.6 (4) 10.2 (5) 9.4 (6) 12.4 (7) 11.0 (8) 5.9 |
E1: (1) 19/26 (4) 18/27 (5) 19/27 (6) 19/27 (7) 10/26 (8) 3/27 E2: (1) 14/23 (4) 11/23 (5) 14/23 (7) 14/23 (8) 7/23 S: (1) 20/28 (4) 16/29 (5) 14/28 (6) 20/28 (7) 17/28 (8) 7/26 |
No data | No data | No data |
Appendix 6. Summary of adverse events, withdrawals and exclusions
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other/Exclusions |
| Cooper 1984 | (1) Fenoprofen 200 mg, n = 39 (2) Zomepirac 100 mg, n = 43 (3) Placebo, n = 35 |
(1) 6/39 (2) 7/43 (3) 6/35 Most mild. No causal relationship established between any drug and effect |
None | None | Exclusions: 19 because did not take medication (7), lost to follow‐up (4), remedicated before 1 h (3), medicated with slight pain (1) or next day (1), took confounding medication (1), uninterpretable results (1), or not entered on study (1) |
| Davie 1982 | (1) Fenoprofen 200 mg PO, n = 30 (2) Morphine 8 mg IV, n = 30 (3) Placebo, n = 30 |
(1) 2/30 (2) 4/30 (3) 3/30 Nausea/vomiting. Most mild, could have resulted from GA rather than study medications |
None | None | None |
| Laska 1981 | (1) Fenoprofen 200 mg, n = 77 (E1 26, E2 23, Surg 28) (2) Fenoprofen 12.5 mg, n = 24 (E2 24) (3) Fenoprofen 25 mg, n = 23 (E2 23) (4) Fenoprofen 50 mg, n = 79 (E1 27, E2 23, S 29) (5) Fenoprofen 100 mg, n = 78 (E1 27, E2 23, S 28) (6) Fenoprofen 300 mg, n = 55 (E1 27, S 28) (7) Codeine 60 mg, n = 77 (E1 26, E2 23, S 28) (8) Placebo, n = 76 (E1 27, E2 23, S 26) |
In study E2: (1) 1/23 severe sweating (3) 1/23 nausea/vomiting No adverse events reported for studies E1 and S |
None | None reported | None reported |
Data and analyses
Comparison 1. Fenoprofen 200 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with ≥ 50% pain relief over 4 to 6 hours | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.15 [2.71, 6.36] |
| 2 Participants with ≥1 adverse effect over 4 to 6 hours | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.40, 2.16] |
1.1. Analysis.
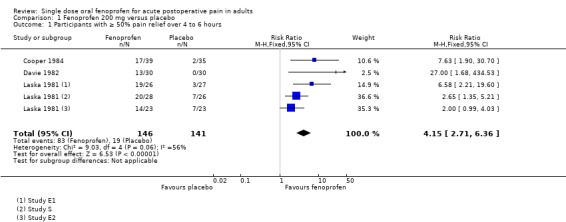
Comparison 1 Fenoprofen 200 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4 to 6 hours.
1.2. Analysis.
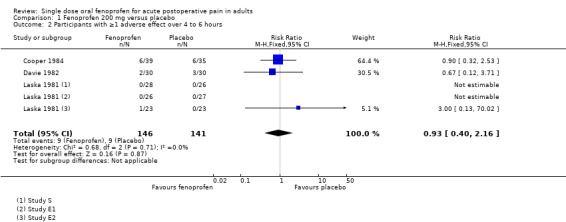
Comparison 1 Fenoprofen 200 mg versus placebo, Outcome 2 Participants with ≥1 adverse effect over 4 to 6 hours.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cooper 1984.
| Methods | RCT, DB, placebo and active controlled parallel‐group study. Medication administered when pain intensity was moderate to severe, with 4 hour single‐dose phase | |
| Participants | Surgical removal of impacted third molar Males (32) and females (85) Mean age 23 years N = 129 (117 analysed) |
|
| Interventions | Fenoprofen 200mg, n = 39 Zomepirac, n = 43 Placebo, n = 35 |
|
| Outcomes | patient reported pain relief patient reported pain intensity patient global assessment of efficacy use of rescue medication adverse events withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Low risk | opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | tablets and capsules indistinguishable by patients and not seen by surgeon and research assistant |
Davie 1982.
| Methods | RCT, DB, DD, placebo and active controlled parallel‐group study. Medication administered when pain intensity was moderate to severe, with 6 hour single‐dose phase | |
| Participants | Minor day case outpatient surgery Males (5) and females (85) Mean age 35 (range 20‐69) years N = 90 |
|
| Interventions | Fenoprofen 200 mg PO, n = 30 Morphine 8 mg IV, n = 30 Placebo, n = 30 |
|
| Outcomes | patient reported pain intensity use of rescue medication adverse events withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double dummy |
Laska 1981.
| Methods | RCT, DB, placebo and active controlled parallel‐group studies. Medication administered when pain intensity was moderate to severe, with 5 hour single‐dose phase | |
| Participants | Postpartum episiotomy pain (Studies E1 & E2) or postsurgical pain (Study S) Females only E1: Mainly age 18‐24 (range 18‐44) years. N = 160 E2: Mainly age 18‐24 (range 18‐44) years. N = 162 S: Mainly age over 44 (range 18+) years. N = 167 |
|
| Interventions | Fenoprofen 12.5 mg, n = 24 (E2) Fenoprofen 25 mg, n = 23 (E2) Fenoprofen 50 mg, n = 79 (E1 27, E2 23, S 29) Fenoprofen 100 mg, n = 78 (E1 27, E2 23, S 28) Fenoprofen 200 mg, n = 77 (E1 26, E2 23, S 28) Fenoprofen 300 mg, n = 55 (E1 27, S 28) Codeine 60 mg, n = 77 (E1 26, E2 23, S 28) Placebo, n = 76 (E1 27, E2 23, S 26) |
|
| Outcomes | patient reported pain relief patient reported pain intensity adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | identical appearance of medications |
DB ‐ double blind, DD ‐ Double Dummy, IV ‐ intravenous, N ‐ number of participants in study, n ‐ number of participants in treatment arm, PO ‐ oral, PR ‐ pain relief, R ‐ randomised, RCT ‐ Randomised Controlled Trial, W ‐ withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bettigole 1981 | Single dose data reported for episiotomy and uterine cramping combined. No single dose adverse event data |
| Burt 1982 | Single blind study. Includes participants with mild baseline pain |
| Davie 1978 | 2 hour data only. Pain relief scale not standard. Baseline pain not reported |
| Derournay 1983 | No placebo group |
| Gruber 1976 | Includes participants with mild baseline pain |
| Gruber 1979 | Baseline pain not reported. Pain intensity scale not standard |
| Kaiko 1984 | No placebo group |
| Offen 1985 | Baseline pain not reported. Includes participants with uterine cramping |
| Sechzer 1977 | Pain intensity and patient global evaluation scales not standard |
| Sunshine 1978 | Includes participants with somatic pain and fractures |
Contributions of authors
MXT and SD performed searching, data extraction, and analysis, including assessment of study quality. RAM helped with analysis and acted as arbitrator. All authors contributed to writing the final review. It was unlikely that an update of this review would be needed in the future.
Sources of support
Internal sources
Oxford Pain Research Funds, UK.
External sources
NHS Cochrane Collaboration Grant, UK.
NIHR Biomedical Research Centre Programme, UK.
Declarations of interest
SD and RAM have received research support from charities, government and industry sources at various times, but no such support was received for this work. RAM has consulted for various pharmaceutical companies and received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. MXT has no interests to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Cooper 1984 {published data only}
- Cooper SA, Gelb S, Goldman E, Cohn P, Dyer C. Comparative efficacy of fenoprofen calcium and zomepirac sodium in postsurgical dental pain. Oral Surgery Oral Medicine Oral Patholology 1984;57(5):485‐9. [DOI] [PubMed] [Google Scholar]
Davie 1982 {published data only}
- Davie IT, Slawson KB, Burt RA. A double‐blind comparison of parenteral morphine, placebo, and oral fenoprofen in management of postoperative pain. Anesthesia and Analgesia 1982;61(12):1002‐5. [PubMed] [Google Scholar]
Laska 1981 {published data only}
- Laska EM, Sunshine A. Fenoprofen and codeine analgesia. Clinical Pharmacology and Therapeutics 1981;29(5):606‐16. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bettigole 1981 {published data only}
- Bettigole JB. A double‐blind comparison of placebo, codeine, and fenoprofen in patients with postpartum pain. Current Therapeutic Research 1981;29(5):778‐84. [Google Scholar]
Burt 1982 {published data only}
- Burt RAP, Davie IT, Slawson KB. A pilot study comparing parenteral morphine, placebo, and oral fenoprofen for the relief of postoperative pain. Current Therapeutic Research 1982;32(2):295‐9. [Google Scholar]
Davie 1978 {published data only}
- Davie IT, Gordon NH. Comparative assessment of fenoprofen and paracetamol given in combination for pain after surgery. British Journal of Anaesthetics 1978;50:931‐5. [DOI] [PubMed] [Google Scholar]
Derournay 1983 {published data only}
- Detournay M. Fenoprofen as a treatment for post‐surgical pain [Le fénoprofène comme traitement des algies postchirurgicales]. Revue Médical de Liège 1983;38:855‐9. [PubMed] [Google Scholar]
Gruber 1976 {published data only}
- Gruber CM. Evaluating interactions between fenoprofen and propoxyphene: analgesia and adverse reports by postepisiotomy patients. Journal of Clinical Pharmacology 1976;August‐September:407‐17. [DOI] [PubMed] [Google Scholar]
Gruber 1979 {published data only}
- Gruber CM, Bauer RO. A multicentre study for analgesia involving fenoprofen, propoxyphene (alone or in combination) with placebo and aspirin controls in postpartum pain. Journal of Medicine 1979;10(1‐2):65‐98. [PubMed] [Google Scholar]
Kaiko 1984 {published data only}
- Kaiko RF, Wallenstein SL, Lapin J, Houde RW. Oral fenoprofen compared to intramuscular morphine and oral aspirin in cancer patients with postoperative pain. NIDA Research Monograph 1984;49:205‐11. [PubMed] [Google Scholar]
Offen 1985 {published data only}
- Offen WW, Gruber CM. Dose response to fenoprofen calcium using placebo and codeine controls. Journal of Medicine 1985;16(4):439‐52. [PubMed] [Google Scholar]
Sechzer 1977 {published data only}
- Sechzer PH. Evaluation of fenoprofen as a postoperative analgesic. Current Therapeutic Research 1977;21(2):137‐48. [PubMed] [Google Scholar]
Sunshine 1978 {published data only}
- Sunshine A, Slafta J, Gruber C. A comparative analgesic study of propoxyphene, fenoprofen, the combination of propoxyphene and fenoprofen, aspirin, and placebo. Journal of Clinical Pharmacology 1978;November‐December:556‐63. [DOI] [PubMed] [Google Scholar]
Additional references
Ashraf 1982
- Ashraf M, Pearson RM, Winfield DA. Aplastic anaemia associated with fenoprofen. BMJ (Clin Res Ed) 1982;284(6325):1301‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barden 2004
- Barden J, Edwards JE, McQuay HJ, Wiffen PJ. Relative efficacy of oral analgesics after third molar extraction. British Dental Journal 2004;197(7):407‐11. [DOI] [PubMed] [Google Scholar]
Brogden 1978
- Brogden RN, Heel RC, Speight TM, Avery GS. Fenbufen: a review of its pharmacological properties and therapeutic use in rheumatic diseases and acute pain. Drugs 1981;21(1):1‐22. [DOI] [PubMed] [Google Scholar]
Clarke 2009
- Clarke R, Derry S, Moore RA, McQuay HJ. Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004309.pub2] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins SL, Edwards J, Moore RA, Smith LA, McQuay HJ. Seeking a simple measure of analgesia for mega‐trials: is a single global assessment good enough?. Pain 2001;91(1‐2):189‐94. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. The Design of Analgesic Clinical Trials. Advances in Pain Research Therapy 1991;18:117‐24. [Google Scholar]
Derry 2008
- Derry S, Moore RA, McQuay HJ. Single dose oral celecoxib for acute postoperative pain. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004233] [DOI] [PubMed] [Google Scholar]
Derry C 2009a
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001548.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry C 2009b
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral naproxen and naproxen sodium for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry P 2009
- Derry P, Derry S, Moore RA, McQuay HJ. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004768.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 2001
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. New England Journal of Medicine 2001;345(6):433‐42. [DOI] [PubMed] [Google Scholar]
Hawkey 1999
- Hawkey CJ. Cox‐2 inhibitors. Lancet 199;353(9149):307‐14. [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore RA, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Katz 1980
- Katz ME, Wang P. Fenoprofen‐associated thrombocytopenia. Annals of Internal Medicine 1980;92(2 Pt 1):262. [DOI] [PubMed] [Google Scholar]
L'Abbe 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
Lloyd 2009
- Lloyd R, Derry S, Moore RA, McQuay HJ. Intravenous parecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004771.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2005
- McQuay HJ, Moore RA. Placebo. Postgraduate Medical Journal 2005;81:155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69(1‐2):127‐30. [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2003
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratio and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
PACT 2010
- The NHS Information Centre, Prescribing Support Unit. Prescription cost analysis, England 2009. 2010. [ISBN: 978‐1‐84636‐408‐2] [Google Scholar]
Roy 2010
- Roy YM, Derry S, Moore RA. Single dose oral lumiracoxib for postoperative pain. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD006865.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 1978
- Simon SD, Kosmin M. Fenoprofen and agranulocytosis. New England Journal of Medicine 1978;299(9):490(299):490. [DOI] [PubMed] [Google Scholar]
Simpson 1978
- Simpson RE 3rd, Goldstein DJ, Hjelte GS, Evans ER. Acute thrombocytopenia associated with fenoprofen. New England Journal of Medicine 1978;298(11):629‐30. [DOI] [PubMed] [Google Scholar]
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramer 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta‐analysis: a case study. BMJ 1997;315:635‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]


