Abstract
Open innovation is the new buzz, with initiatives popping up left and right. Here, we give a personal perspective on a very successful, knowledge-driven innovation initiated in an academia- industry alliance, which culminated in technology platforms that enable the generation of therapeutic antibodies with novel properties. To start, we provide a general background on open innovation in the drug development field.
Keywords: Open innovation, immunotherapy, Fab-arm exchange, bispecific antibodies, Duobody technology, drug development, Technology platform
“Applied scientist seeks academic partner for an innovative encounter and possibly more. Future drug development not excluded.”
Soon Coming to a Pharma Near You…
Drug development requires multi-billion dollar investments in research and development (R&D). Bringing a new drug to the market is estimated to have a $1.8 billion price tag, or multiples thereof when taking drug failures into account.1-3 With costs soaring, the number of US Food and Drug Administration (FDA)-approved drugs per dollar spent steadily declined between the 1950s and 2010s.4,5 Several factors may have contributed to this apparent decrease in productivity, such as an increased regulatory focus on patient safety and cost-benefit, limited potential to develop improved products over existing treatments in increasingly crowded markets, and pressures to reduce internal research efforts as a result of downsizing, mergers and acquisitions.5,6 Large organizations furthermore often suffer from a lack of innovative power due to excessive bureaucracy and hierarchy, as well as slow decision-making combined with a low risk-appetite.
The pharmaceutical industry traditionally was locked into “not-invented-here” thinking, and therefore strongly relied on internal innovation. Yet, it has recently begun to seriously consider external innovation with biotechnology companies and academia. By accepting the notion that revolutionary discoveries were often “invented-there” anyway, the industry cracked the door to open innovation. This concept teaches that both internal and external ideas and resources may be, and should be, exploited to generate new drugs. Open innovation can be applied to all stages of drug discovery and development,7 in which the traditional boundaries between companies and academia are proactively being erased. Interestingly, common approaches to internalize new product opportunities are being integrated with novel innovation strategies leveraging knowledge and competences from academia and biotech in various ways (see BOX 1 and Figure 1).
BOX 1. Novel approaches to Open Innovation
Pre-competitive research
Pre-competitive research between academic and industry parties provides an opportunity to combine unique knowledge and capabilities and catalyze translational research into products or technologies. This may range from bilateral research collaborations to large private-public consortia. Currently, many consortia are actively focusing on key health care challenges, neglected diseases or providing access to large compound collections such as the Lead Factory initiative (http://www.europeanleadfactory.eu/) and the open lab at GSK Tres Cantos (http://www.gsk.com/partnerships/open-innovation/tres-cantos.html).9
Ideation and crowdsourcing
Combining the minds of researchers worldwide to unveil hidden knowledge has a large potential for solving technological challenges and the generation of new product ideas. A variety of approaches is used by the industry, such as various ideation approaches that have resulted in many useful solutions and proposals, catalyzing new drug developments (e.g., https://www.innocentive.com/, http://www.grants4targets.com/, http://openinnovation.astrazeneca.com/).43
Open use of assets or technologies
Another way to fuel innovations is to facilitate the use of technologies or assets by external scientists to allow the translation of scientific findings to potential product applications. This approach has been applied in drug discovery by larger Pharma such as Eli Lilly and Company in which external scientists can submit compounds for functional/pathway screening (https://openinnovation.lilly.com/dd). Alternatively, expertise, infrastructure or technology is made available to external parties for discovery or exploratory research such as in the Stevenage Catalyst ((http://www.stevenagecatalyst.com/). In the antibody arena, KyMab and Regeneron provide technology access for their transgenic animal technologies for antibody generation to academic researchers (www.kymabaccess.org, www.regeneron.com/techlicence) and Genmab for its bispecific antibody platform (www.duobody.com)
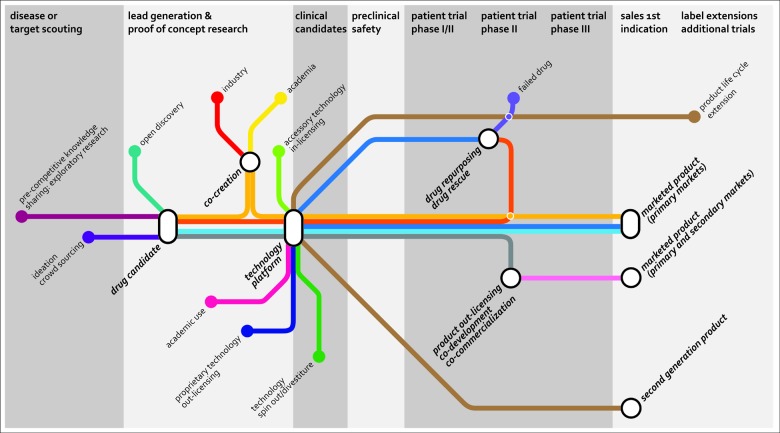
Figure 1. Current and emerging routes of open innovation in the pharmaceutical industry. Besides the traditional open innovation approaches (in/out-licensing, co-development, co-commercialization, acquisition, spin-out/divestiture), the industry is selectively opening up boundaries and is exploring open innovation approaches to bring in new ideas and product opportunities to fill or enrich its pipe-line. Source: Joost Bakker (Scicomvisuals).
It is now clear that new drug development dictates close interaction between large Pharma, biotech and academic research centers. Combining ideas, technologies, capabilities, assets and complementary knowledge is required to enable the successful translation of scientific concepts into products or technologies.8,9 In the past 3 y, new product approvals by FDA have again been on the increase.4,10 In antibody therapeutics, a novel generation of products is on its way, many of which encompass new therapeutic concepts.9,11 This may provide the first sign of an increased output from the observed shift in R&D approaches and the positive effects of the intensified collaborations between large Pharma, biotech and academia. Exemplary are a number of new therapeutic antibody approaches that recently came to fruition and that created exciting new treatment options for patients. First, there is the success of the antibody-induced activation of cellular immune responses against tumors, which was designated by Science as the breakthrough of the year 2013.12 This advance was the off-spring of a close collaboration between academic researchers at the University of California, Berkeley, and the biotech company Medarex13 that led to the approval of the anti-CTLA-4 antibody ipilimumab.14 Second, there have been strong advances with antibody-drug conjugates. A prominent example is the approval of brentuximab vedotin,15 which found its roots in the lifelong search for novel toxins in obscure sea creatures such as the sea hare by Prof. George Pettit,16 and was translated into practice by the biotech company Seattle Genetics.17 Finally, there is much attention for the promise of bispecific antibodies through the spectacular clinical data with bispecific T-cell engaging molecules obtained by Micromet.18 All of these approaches are now being enthusiastically embraced by Pharma. Genmab recently became a player in the field of bispecific antibodies via the development of the DuoBody® platform. The development of the platform, in our view, represents an attractive scientific and commercial success story of an academic – industry collaboration with many lessons learned. We provided the personal perspective below.
Passion For Innovation: The IgG4 Antibody Challenge
Continuous innovation is a life-line for biotechnology companies, not only as a necessity, but also to keep the entrepreneurial spirits of workers engaged and at the cutting edge. Without knowing it at the time, we essentially started our first open innovation project within Genmab in 2003. In the course of discovering antibody therapeutics for the treatment of autoimmune and inflammatory diseases, we were considering the characteristics of the optimal antibody isotype to achieve a maximal therapeutic window. Dogma at the time dictated that one should incorporate the human IgG4 subclass as the Fc-backbone of choice to prevent unwanted activation of antibody effector functions by therapeutic antibodies in immune related indications. However, a nagging issue was an unresolved hypothesis regarding instability of IgG4 antibodies in vivo: the Fab-arm exchange hypothesis (see BOX 2). A more detailed understanding of IgG4 biology therefore appeared essential prior to accepting the suitability of IgG4 antibodies for product development. To generate additional knowledge and test the hypothesis, we reinitiated IgG4 research in a Genmab-sponsored project with Prof. Rob Aalberse from the Department of Immunopathology at Sanquin Research in Amsterdam. In a parallel effort, in collaboration with the group of Prof. Marc De Baets from the Department of Neuroscience at the University of Maastricht, we started a project to study antibody therapy of the autoimmune disease Myastenia gravis. This work was based on the hypothesis that monovalent, effector-function-deficient antibody (fragments) against acetyl choline receptor (AChR), the relevant autoantigen in Myasthenia gravis, might be able to counteract the activity of pathogenic autoantibodies in vivo.19 A few years later, these two independent lines of research came together. In the collaboration with Sanquin Research, we refuted a long-standing belief in antibody biology by showing that IgG4 antibodies are indeed dynamic molecules that acquire bispecific properties by continuous exchange of half-molecules in vivo. Thereby we confirmed our IgG4 Fab-arm exchange hypothesis.20 Fab-arm exchange in the rhesus monkey Myasthenia gravis model was shown to be at the basis of the anti-inflammatory properties of IgG4.
BOX 2. Basis of the IgG4 Fab-arm exchange hypothesis
A number of key observations led to the development of the IgG4 Fab-arm exchange hypothesis.
Observation 1: The inability of serum-derived IgG4 to crosslink antigens and generate large immune complexes, indicated a functionally monovalent reactivity of IgG4.44,45 This reactivity conflicted with the behavior of recombinant IgG4 produced in cell culture.46 Recombinant IgG4 was perfectly capable of crosslinking identical antigens. This key difference suggested that the curious reactivity pattern might be due to a post-translational event in vivo rather than a protein structural characteristic.
Observation 2: The (partial) separation into IgG4 half-molecules when evaluated under non-reducing denaturing conditions (such as SDS-PAGE;47 due to the presence of mixed disulfide bonds).46,48 This distinct behavior of IgG4, as compared with other IgG, was shown to be caused by a single amino acid difference in the core hinge.49 Notably, no relevance for the presence of IgG4 half-molecules was indicated since under non-denaturing physiological conditions IgG4 behaved as a canonical 150 kD IgG molecule.
Toward a hypothesis: Combining these two observations led to the hypothesis that IgG4 inter-heavy chain disulfide bonds might become broken in vivo and the fraction of IgG4 half-molecules (observed on SDS-PAGE) might indeed somehow reflect an in vivo equilibrium. It was hypothesized that IgG4 molecules are produced as bivalent monospecific molecules, like other IgGs, but after secretion, half molecules (heavy-light chain pairs) between IgG4 molecules are exchanged. If this was correct, then this process would render IgG4 molecules bispecific. Assuming this to be a stochastic process with all polyclonal IgG4 molecules participating, then IgG4 would indeed become functionally monovalent in vivo as each individual IgG4 molecule would essentially bind two distinct antigens that are unlikely to be encountered simultaneously.46,50
Markedly, the initial hypothesis solely focused on the hinge region and essentially no contributions for the CH3 domains in the exchange process were envisioned.50 Elucidating the process, a critical role for both hinge and CH3 domain in the process became apparent20 and later the CH3 affinity constant in particular was identified as a critical requirement for the ability of IgG to engage in Fab-arm exchange.22
Concluding note: Understanding this mechanism, it is sometimes questioned whether the name IgG4 Fab-arm exchange is strictly correct as it is IgG4 half-molecules, and not just the Fab-arms, that exchange. This is indeed the case. We, however, favor the expression Fab-arm exchange as it aptly describes the major functional consequence of the process, that is the generation of novel antibody molecules who’s distinguishing feature is the presence of two different Fab (fragment antigen binding) domains (see also ref. 51).
The new insight in IgG4 biology led to follow-up questions and initiated new lines of research: in collaboration with Joep Killestein and Chris Polman from the Department of Neurology at the VU University Medical Center in Amsterdam, we demonstrated that a non-mutated IgG4 therapeutic antibody, natalizumab, engages in Fab-arm exchange with endogenous IgG4 in humans,21 and we elucidated the key molecular requirements of Fab-arm exchange in collaboration with Theo Rispens from the Department of Pathology at Sanquin Research, Ignace Lasters from Algonomics in Ghent, and Albert Heck from the Bijvoet Center for Biomolecular Research at Utrecht University.22,23 Our collaboration with Albert Heck, in addition, led to an unexpected spin-off into the realm of glycosylation. Here, we identified a novel CH3 mutation that strongly enhances Fc glycan complexity, providing new leads for antibody therapeutics.24 This work furthermore supported the development of a novel native high-resolution mass spectrometry method (orbitrap mass spectrometer), which enables facile mapping of whole antibody product microheterogeneity, including glycan profiles as well as antibody-drug conjugate adducts.25
Translating Science Into Technology Platform Applications
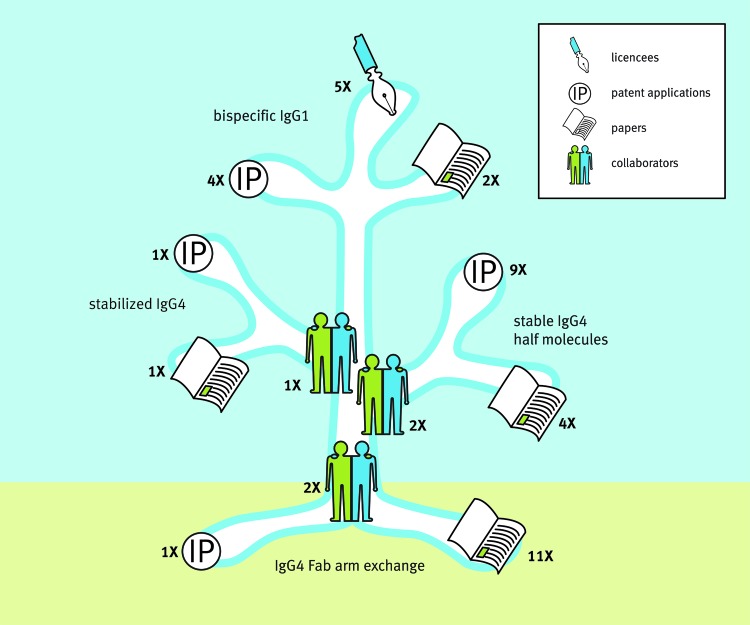
Although initiated by an apparently simple practical question (i.e., which antibody Fc backbone to use for therapy of immune diseases), we would like to stress that the drive forward was not necessarily motivated by traditional question-and-answer, but instead by scientific curiosity and a passion to solve basic antibody biology questions. Nevertheless, the culture in our company that fosters out-of-the-box thinking within a strong goal-oriented environment led us to eagerly look for potential applications of our new-found knowledge. Three major branches that led to intellectual property and potential applications in the form of novel antibody platforms were initiated to: 1) identify mutations that prevent IgG4 antibodies from engaging in Fab-arm exchange in order to generate IgG4 molecules that are stable in vivo;21-23,26 2) generate stable half antibodies in order to prepare monovalent antibody molecules with extended half-lifes relative to scFv and Fabs (UniBody® format).23,27-35 Notably, the first hint that UniBody molecules could be generated came from a cloning artifact which serendipitously introduced a novel splice site which led to deletion of the genetic hinge region and a researcher determined to understand an apparent mistake; and 3) develop a method to stabilize IgG molecules following Fab-arm exchange in order to allow the generation of therapeutic bispecific antibodies (DuoBody®) (Fig. 2).36-41
Figure 2. Science growing into applications. Insight in IgG4 biology and the unraveling of the IgG4 Fab-arm exchange process formed the roots for 3 major branches of research that led to the generation of scientific papers and intellectual property through various industry-academia collaborations. These culminated in 3 novel antibody technology platforms for the generation of stabilized IgG4, stable IgG4 half molecules (UniBody®) and bispecific IgG1 (DuoBody®). Numbers indicate: the number of collaborating parties involved; the number of patent applications filed26,27,29-35,38-41,52; the number of scientific papers published20-25,36,37,53-62; and the number of platform licensees that occurred between 2003 (the beginning of the IgG4 project within Genmab) and April 2014. Source: Joost Bakker (Scicomvisuals).
The path that led to the development of our DuoBody® platform turned out to be the most complex enterprise by far. The idea was fantastic, literally, as we initially had no clue as to how to translate the fascinating natural Fab-arm exchange process into a technologically and commercially-viable bispecific antibody platform. Our approach of leaving no stone unturned and having an open mind for the unexpected again provided a solution. In the rhesus monkey Myasthenia gravis model, we concluded that IgG4 anti-AChR provided protection because of in vivo Fab-arm exchange.20 This exchange reaction then necessarily involved the human IgG4 anti-AChR mAb and polyclonal rhesus monkey IgG4. The exact sequence of rhesus IgG4, however, was unknown at the time, and it was therefore not possible to perform a control experiment to prove interspecies IgG4 Fab-arm exchange with cloned antibodies. This gap remained an irritating loose end, so we set out to clone rhesus monkey IgG4. The “simple” control experiment, which took well over a year to perform, led to a number of surprises. First, rhesus monkeys from Indian and Chinese origin harbor a polymorphism42 in which IgG4 from the former has an allotype (containing an IgG1-type core hinge) that does not allow exchange, in stark contrast to Chinese-origin rhesus monkey IgG4, which does engage in exchange.22 Second, the CH3 domain of Chinese rhesus monkeys contained a distinct critical amino acid change for Fab-arm exchange compared with human IgG4. Third, the long-sought interspecies control experiment contained a key finding in that it unexpectedly resulted in an extremely high (> 95%) bispecific IgG4 yield.22 We understood then that matched mutations therefore apparently could drive IgG4 Fab-arm exchange to completion, which was a definite Eureka moment! This work was extended with a library approach to identify the optimal matched mutations at the CH3-CH3 interface allowing directional Fab-arm exchange to occur. We envisioned that the ensuing DuoBody® platform should be built on an IgG1 format, as IgG1 antibodies combine all the desired properties, including in vivo stability, Fc-mediated effector function, long half-life and well-understood manufacturability and developability. To enable this, we sought for appropriate in vitro reducing condition which, combined with the matched mutations identified above, allowed our controlled Fab-arm exchange process to be developed in the versatile DuoBody® platform (Fig. 3).36,37,40
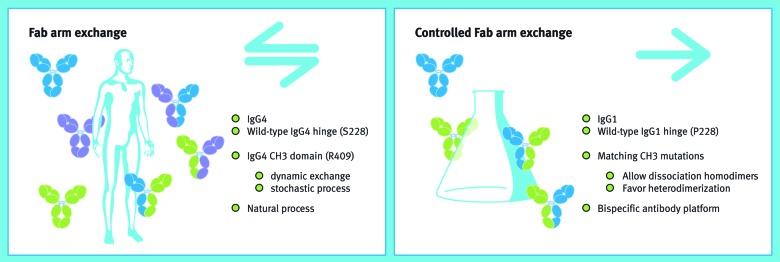
Figure 3. From nature to a bispecific technology platform. The left panel shows the major characteristics of the naturally occurring, bidirectional process of Fab-arm exchange as was discovered during the research described in this Perspective. This knowledge was applied to develop a platform of controlled, unidirectional Fab-arm exchange, of which the major characteristics are shown in the right panel. Source: Joost Bakker (Scicomvisuals).
One final step in building the platform was to demonstrate feasibility of the manufacturing process using standard unit operations for IgG1 and developability of DuoBody® products. We were struck by the robustness of the controlled Fab-arm exchange process as we could scale up from laboratory to manufacturing scale essentially without major adaptations (except for the use of diafiltration for buffer exchange at scale). We found that the DuoBody platform did not add any manufacturability liability on top of those already present in the original homodimeric parental antibody molecules. Indeed the DuoBody® platform generated bispecific IgG1 molecules with identical quality attributes to regular IgG1 products.36 We strongly believe that the robustness of the process is related to the fact that it was built on a process that comes naturally to antibodies, and which, surprisingly, only required two single matched CH3 interface mutations per antibody molecule.
Now, as of early 2014, the DuoBody® platform has evolved into a successful bispecific antibody platform for the discovery and development of new antibody drugs in internal and external projects. We have successfully executed a number of collaboration and licensing agreement with Pharma, including with Janssen Biotech, Novartis, Kirin-Kyowa Hakko and Eli Lilly and Co.
Getting the Full Picture: The Academic View
To evaluate the full experience from our journey into open innovation, it was critical to also portray the academic perspective. We were therefore delighted that Prof. Rob Aalberse, Prof. Marc De Baets and Prof. Albert Heck were willing to provide their views.
For these academic researchers, the main motivator for engaging in the collaboration was to generate knowledge, and they felt that they got the most out of the interaction when there was a sole focus on solving important scientific questions. However, once knowledge was gained and the potential for therapeutic or commercial applicability became apparent, this came with restrictions on sharing results with outside parties until intellectual property (IP) was secured. Although it was understood as being part of the deal, some experienced the accompanying change in ‘openness’ as a loss for the collaboration. Nevertheless, all indicated that the delay in being able to present or publish results due to IP issues was very acceptable as the requested periods were reasonable.
All three collaborations resulted in scientific articles in high-impact journals, abstracts, posters and presentation at numerous conferences. Publications were seen as important deliverables by all groups, and it was perceived as remarkable that also for Genmab as a company, publications were an important deliverable. Here, the underlying common aim to generate knowledge and share this with the scientific community was achieved at its best. Publications helped the academic groups to establish new research interactions within academia, as well as with other companies. The collaborations also resulted in co-inventorships on patents. Whereas in the past, patents were seen as ‘not done’ in academia, patents have become an important deliverable also in universities. In recent years, a specific need to describe ‘valorization’ of proposed research in grant applications has arisen; collaborating with a company helped to put this in perspective. Notably, interactions with Genmab allowed the researchers to become more aware of the enormous efforts required for developing scientific knowledge into products, indeed suggesting that the feasibility of valorization is often overestimated. The knowledge generated not only led to publications, but also paved the way for follow-up research and the development of new or improved techniques. Finally, working with a company provided an incentive to introduce or restructure processes for managing projects or decision-making within the academic laboratories.
All three collaborators indicated that working with a (biotech) company is an excellent experience for academic researchers. However, it’s not for everyone as one should be open to the commercial and result-oriented way of working. The opportunity for PhD students and post-docs to obtain a first-hand view of research in a commercial setting was seen as a valuable extra to their training. Working at the interface between academia and industry provided a steep but valuable learning curve that benefitted both the students, as well as the academic staff.
Innovation by Collaboration: Lessons Learned
In summary, we learned that for collaborations aiming at innovation to be successful, the following aspects are key:
-
A shared passion for the research topic
Focus on generating knowledge and not on potential valorization
Shared intention to publish and present at scientific conferences
-
Two-way exchange of knowledge with open and frequent communication
Avoidance of delays in sharing data
Acknowledgment that intellectual property aspects may be perceived as affecting “openness’ and reducing academic freedom
Respect each other’s expertise and responsibilities
Acknowledge and reward each other roles and understand responsibilities
Invest in trust and a respectful and long-term relationship
Open Innovation: Next Steps
The development of the DuoBody® platform was initiated with academia and we therefore felt it to be important that, next to its use in drug development, it should also be used to answer important research questions. Basic research employing the platform may lead to new insights, novel uses, or in vitro and in vivo proof-of-concepts in unforeseen applications. To take open innovation to the next level, we announced a challenge to the scientific community at the end of 2013, using crowdsourcing via the commercial site Innocentive.com, as well as by posting on Genmab’s DuoBody.com website and on social media (our social networks on LinkedIn). The incentive given for the most promising ideas and proposals was either a cash reward or grant funding. Interestingly, the response on our first challenge was tremendous, with more than three dozen ideas and proposals received. A scientific advisory board of internal and independent external experts identified a number of top proposals that were recommended for an award. Overall, the quality and innovative power of the top segment of proposals and ideas was remarkable. Genmab has the intention to continue this effort and future funding opportunities will be forthcoming. Finally, we wish to invite researchers in academia and industry to experience the full power of the DuoBody® platform for bispecific antibody research, as well as development. To further explore DuoBody®, we refer to the opportunities provided on www.duobody.com.
Concluding Remarks
The success story above would not have been possible without the enthusiastic contributions of outstanding researchers from a number of universities and research institutes, as well as from a number of contract research organizations and biotech companies. For the collaborations to be successful, we learned that it is very important to have common goals and be sensitive to each other’s needs and responsibilities. Working at the interface between academia and industry, each with its different goals and purpose, does have its own challenges. Yet, if all involved respect and understand each other’s intentions, there is no reason to hold back: just throw the door wide open to innovation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all our partners in research, both at Genmab and in academia, who shared our passion for antibody biology research and innovation. We are especially indebted to Prof. Rob Aalberse, who not only led us into this field, but was also willing to share his insights along the way. Next to Prof. Aalberse, we thank Prof. Marc De Baets and Prof. Albert Heck for their willingness to provide their academic perspective on collaborating with a biotech. From our Genmab colleagues, we need to give special mention to Ewald van de Bremer, Tom Vink, Michael Gramer and Patrick van Berkel who made seminal contributions to forward the IgG4 research and the DuoBody® platform and to Joyce Meesters, Muriel van Kampen, Luus Wiegman, Patrick Priem, Muriel van Kampen, Sandra Verploegen, Joost Neijssen, Kristin Strumane, Rob de Jong and Amitava Kundu for their enthusiasm and outstanding input. We thank Joost Bakker for graphics design. Finally, we are very grateful to Jan van de Winkel for his generous support and trusting us in following our scientific instincts even at times when practical applications were not immediately apparent.
References
- 1.Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–14. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 2.Herper M. The truly staggering cost of inventing new drugs. Forbes, 2012: http://www.forbes.com/sites/matthewherper/2012/02/10/the-truly-staggering-cost-of-inventing-new-drugs/
- 3.Strohl WR, Strohl LM. Therapeutic Antibody Engineering: Current and Future Advances Driving the Strongest Growth Area in the Pharmaceutical Industry. Woodhead Publishing, 2012. [Google Scholar]
- 4.Mullard A. 2012 FDA drug approvals. Nat Rev Drug Discov. 2013;12:87–90. doi: 10.1038/nrd3946. [DOI] [PubMed] [Google Scholar]
- 5.Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 6.Kessel M. The problems with today’s pharmaceutical business--an outsider’s view. Nat Biotechnol. 2011;29:27–33. doi: 10.1038/nbt.1748. [DOI] [PubMed] [Google Scholar]
- 7.Chesbourg H. Open Innovation: The New Imperative for creating and profiting from technology. Boston Harvard Business School Press, 2003. [Google Scholar]
- 8.Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov. 2013;12:489–92. doi: 10.1038/nrd4066. [DOI] [PubMed] [Google Scholar]
- 9.Mullard A. European lead factory opens for business. Nat Rev Drug Discov. 2013;12:173–5. doi: 10.1038/nrd3956. [DOI] [PubMed] [Google Scholar]
- 10.Mullard A. 2013 FDA drug approvals. Nat Rev Drug Discov. 2014;13:85–9. doi: 10.1038/nrd4239. [DOI] [PubMed] [Google Scholar]
- 11.Reichert JM. Antibodies to watch in 2014. MAbs. 2014;6:5–14. doi: 10.4161/mabs.27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–3. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 16.Pettit GR, Kamano Y, Fujii Y, Herald CL, Inoue M, Brown P, Gust D, Kitahara K, Schmidt JM, Doubek DL, et al. Marine animal biosynthetic constituents for cancer chemotherapy. J Nat Prod. 1981;44:482–5. doi: 10.1021/np50016a016. [DOI] [PubMed] [Google Scholar]
- 17.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–84. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 18.Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol. 2013;17:385–92. doi: 10.1016/j.cbpa.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Graus YF, de Baets MH, Parren PW, Berrih-Aknin S, Wokke J, van Breda Vriesman PJ, Burton DR. Human anti-nicotinic acetylcholine receptor recombinant Fab fragments isolated from thymus-derived phage display libraries from myasthenia gravis patients reflect predominant specificities in serum and block the action of pathogenic serum antibodies. J Immunol. 1997;158:1919–29. [PubMed] [Google Scholar]
- 20.van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–7. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 21.Labrijn AF, Buijsse AO, van den Bremer ET, Verwilligen AY, Bleeker WK, Thorpe SJ, Killestein J, Polman CH, Aalberse RC, Schuurman J, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009;27:767–71. doi: 10.1038/nbt.1553. [DOI] [PubMed] [Google Scholar]
- 22.Labrijn AF, Rispens T, Meesters J, Rose RJ, den Bleker TH, Loverix S, van den Bremer ET, Neijssen J, Vink T, Lasters I, et al. Species-specific determinants in the IgG CH3 domain enable Fab-arm exchange by affecting the noncovalent CH3-CH3 interaction strength. J Immunol. 2011;187:3238–46. doi: 10.4049/jimmunol.1003336. [DOI] [PubMed] [Google Scholar]
- 23.Rose RJ, Labrijn AF, van den Bremer ET, Loverix S, Lasters I, van Berkel PH, van de Winkel JG, Schuurman J, Parren PW, Heck AJ. Quantitative analysis of the interaction strength and dynamics of human IgG4 half molecules by native mass spectrometry. Structure. 2011;19:1274–82. doi: 10.1016/j.str.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Rose RJ, van Berkel PH, van den Bremer ET, Labrijn AF, Vink T, Schuurman J, Heck AJ, Parren PW. Mutation of Y407 in the CH3 domain dramatically alters glycosylation and structure of human IgG. MAbs. 2013;5:219–28. doi: 10.4161/mabs.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosati S, van den Bremer ET, Schuurman J, Parren PW, Kamerling JP, Heck AJ. In-depth qualitative and quantitative analysis of composite glycosylation profiles and other micro-heterogeneity on intact monoclonal antibodies by high-resolution native mass spectrometry using a modified Orbitrap. MAbs. 2013;5:917–24. doi: 10.4161/mabs.26282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van de Winkel JG, Vink T, Schuurman J, Parren PW, Aalberse RC, Van der Neut Kolfschoten M. WO2008145142. Stable IgG4 antibodies. 2007.
- 27.Parren PW, Neijssen J, Labrijn AF, Schuurman J, Vink T, Van de Winkel JG, Loverix S, Lasters I. WO2010084197. Methods for producing mixtures of antibodies. 2009.
- 28.Parren PW, Schuurman J, Vink T, Bleeker WK, van de Winkel JG, Van Berkel PH, Beurskens FJ. WO2007059782. Recombinant monovalent antibodies and methods for production thereof. 2005.
- 29.Losen M, Martinez-Martinez P, De Baets MH, Graus YF, Schuurman J, Parren PW. WO2007068255. Use of effector-function-deficient antibodies for treatment of auto-immune diseases. 2005.
- 30.Beurskens FJ, Bleeker WK, Labrijn AF, Parren PW, Schuurman J, Van Berkel PH, Van de Winkel JG, Vink T. WO2008145137. Recombinant non glycosylated monovalent half-antibodies obtained by molecular engineering. 2007.
- 31.Schuurman J, Vink T, Van de Winkel JG, Labrijn AF, Parren PW, Beurskens FJ, Bleeker WK, Van Berkel PH. WO2008145141. Method for extending the half-life of exogenous or endogenous soluble molecules. 2007.
- 32.Schuurman J, Vink T, van de Winkel JG, Labrijn AF, Parren PW, Bleeker WK, Beurskens FJ, Van Berkel PH. WO2008145138. Recombinant fucose modified monovalent half-antibodies obtained by molecular engineering. 2007.
- 33.Schuurman J, Vink T, Van de Winkel JG, Labrijn AF, Parren PW, Bleeker WK, Beurskens FJ, Van Berkel PH. WO2008145139. Fusion or linked proteins with extended half life. 2007.
- 34.Schuurman J, Vink T, Van de Winkel JG, Labrijn AF, Parren PW, Bleeker WK, Beurskens FJ, Van Berkel PH. WO2008145140. Transgenic animals producing monovalent human antibodies and antibodies obtainable from these animals. 2007.
- 35.Labrijn AF, Loverix S, Parren PW, Van de Winkel JG, Schuurman J, Lasters I. WO2010063785. Antibody variants having modifications in the constant region. 2008.
- 36.Gramer MJ, van den Bremer ET, van Kampen MD, Kundu A, Kopfmann P, Etter E, Stinehelfer D, Long J, Lannom T, Noordergraaf EH, et al. Production of stable bispecific IgG1 by controlled Fab-arm exchange: scalability from bench to large-scale manufacturing by application of standard approaches. MAbs. 2013;5:962–73. doi: 10.4161/mabs.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrijn AF, Meesters JI, de Goeij BE, van den Bremer ET, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013;110:5145–50. doi: 10.1073/pnas.1220145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Goeij B, Labrijn AF, Meesters J, Neijssen J, Parren PW, Schuurman J, Strumane K, Van Berkel PH. WO2012143523. Bispecific antibodies against her2. 2011.
- 39.Gramer M, Kundu A, Van den Bremer ET, Van Kampen M, Priem P, Labrijn AF, Meesters J, Neijssen J, Schuurman J, Parren PW, et al. WO2013060867. Production of heterodimeric proteins. 2011.
- 40.Labrijn AF, Meesters J, Van den Bremer ET, Neijssen J, Van Berkel PH, De Goeij B, Vink T, Van de Winkel JG, Schuurman J, Parren PW. WO2011131746. Heterodimeric antibody Fc-containing proteins and methods for production thereof. 2010.
- 41.Neijssen J, Meesters J, De Goeij B, Labrijn AF, Parren PW, Schuurman J. WO2012143524. Bispecific antibodies against her2 and CD3. 2011.
- 42.Scinicariello F, Engleman CN, Jayashankar L, McClure HM, Attanasio R. Rhesus macaque antibody molecules: sequences and heterogeneity of alpha and gamma constant regions. Immunology. 2004;111:66–74. doi: 10.1111/j.1365-2567.2004.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lessl M, Schoepe S, Sommer A, Schneider M, Asadullah K. Grants4Targets - an innovative approach to translate ideas from basic research into novel drugs. Drug Discov Today. 2011;16:288–92. doi: 10.1016/j.drudis.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Margni RA, Binaghi RA. Nonprecipitating asymmetric antibodies. Annu Rev Immunol. 1988;6:535–54. doi: 10.1146/annurev.iy.06.040188.002535. [DOI] [PubMed] [Google Scholar]
- 45.van der Zee JS, van Swieten P, Aalberse RC. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566–71. [PubMed] [Google Scholar]
- 46.Schuurman J, Van Ree R, Perdok GJ, Van Doorn HR, Tan KY, Aalberse RC. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology. 1999;97:693–8. doi: 10.1046/j.1365-2567.1999.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King DJ, Adair JR, Angal S, Low DC, Proudfoot KA, Lloyd JC, Bodmer MW, Yarranton GT. Expression, purification and characterization of a mouse-human chimeric antibody and chimeric Fab’ fragment. Biochem J. 1992;281:317–23. doi: 10.1042/bj2810317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuurman J, Perdok GJ, Gorter AD, Aalberse RC. The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Mol Immunol. 2001;38:1–8. doi: 10.1016/S0161-5890(01)00050-5. [DOI] [PubMed] [Google Scholar]
- 49.Angal S, King DJ, Bodmer MW, Turner A, Lawson AD, Roberts G, Pedley B, Adair JR. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol. 1993;30:105–8. doi: 10.1016/0161-5890(93)90432-B. [DOI] [PubMed] [Google Scholar]
- 50.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuurman J, Labrijn AF, Parren PW. Fab-arm exchange: what’s in a name? MAbs. 2012;4:636. doi: 10.4161/mabs.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aalberse RC, Van der Neut Kolfschoten M, Labrijn AF, Parren PW, Schuurman J, Vink T, Van de Winkel JG. WO2008119353. Bispecific antibodies and methods for production thereof. 2007.
- 53.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–77. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 54.Rispens T, Ooijevaar-de Heer P, Bende O, Aalberse RC. Mechanism of immunoglobulin G4 Fab-arm exchange. J Am Chem Soc. 2011;133:10302–11. doi: 10.1021/ja203638y. [DOI] [PubMed] [Google Scholar]
- 55.Davies AM, Rispens T, den Bleker TH, McDonnell JM, Gould HJ, Aalberse RC, Sutton BJ. Crystal structure of the human IgG4 C(H)3 dimer reveals the role of Arg409 in the mechanism of Fab-arm exchange. Mol Immunol. 2013;54:1–7. doi: 10.1016/j.molimm.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 56.Losen M, Martínez-Martínez P, Phernambucq M, Schuurman J, Parren PW, De Baets MH. Treatment of myasthenia gravis by preventing acetylcholine receptor modulation. Ann N Y Acad Sci. 2008;1132:174–9. doi: 10.1196/annals.1405.034. [DOI] [PubMed] [Google Scholar]
- 57.Rispens T, den Bleker TH, Aalberse RC. Hybrid IgG4/IgG4 Fc antibodies form upon ‘Fab-arm’ exchange as demonstrated by SDS-PAGE or size-exclusion chromatography. Mol Immunol. 2010;47:1592–4. doi: 10.1016/j.molimm.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Rispens T, Davies AM, Ooijevaar-de Heer P, Absalah S, Bende O, Sutton BJ, Vidarsson G, Aalberse RC. Dynamics of inter-heavy chain interactions in human immunoglobulin G (IgG) subclasses studied by kinetic Fab arm exchange. J Biol Chem. 2014;289:6098–109. doi: 10.1074/jbc.M113.541813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosati S, Yang Y, Barendregt A, Heck AJ. Detailed mass analysis of structural heterogeneity in monoclonal antibodies using native mass spectrometry. Nat Protoc. 2014;9:967–76. doi: 10.1038/nprot.2014.057. [DOI] [PubMed] [Google Scholar]
- 60.Labrijn AF, Aalberse RC, Schuurman J. When binding is enough: nonactivating antibody formats. Curr Opin Immunol. 2008;20:479–85. doi: 10.1016/j.coi.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Rispens T, Meesters J, den Bleker TH, Ooijevaar-De Heer P, Schuurman J, Parren PW, Labrijn A, Aalberse RC. Fc-Fc interactions of human IgG4 require dissociation of heavy chains and are formed predominantly by the intra-chain hinge isomer. Mol Immunol. 2013;53:35–42. doi: 10.1016/j.molimm.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Rispens T, Ooievaar-De Heer P, Vermeulen E, Schuurman J, van der Neut Kolfschoten M, Aalberse RC. Human IgG4 binds to IgG4 and conformationally altered IgG1 via Fc-Fc interactions. J Immunol. 2009;182:4275–81. doi: 10.4049/jimmunol.0804338. [DOI] [PubMed] [Google Scholar]