Abstract
Hydrophobic interaction chromatography-high performance liquid chromatography (HIC-HPLC) is a powerful analytical method used for the separation of molecular variants of therapeutic proteins. The method has been employed for monitoring various post-translational modifications, including proteolytic fragments and domain misfolding in etanercept (Enbrel®); tryptophan oxidation, aspartic acid isomerization, the formation of cyclic imide, and α amidated carboxy terminus in recombinant therapeutic monoclonal antibodies; and carboxy terminal heterogeneity and serine fucosylation in Fc and Fab fragments. HIC-HPLC is also a powerful analytical technique for the analysis of antibody-drug conjugates. Most current analytical columns, methods, and applications are described, and critical method parameters and suitability for operation in regulated environment are discussed, in this review.
Keywords: Enbrel®, HIC-HPLC, biosimilar, deamidation, etanercept, formulation, hydrophobic interaction chromatography, oxidation, succinimide, tryptophan
Introduction
Therapeutic proteins are complex recombinant biopolymers typically composed of an arrangement of the 20 genetically-encoded amino acids.1 The amino acid sequence defines the protein three-dimensional structure and functional interface with interacting proteins. In the course of fermentation, purification, upon storage and handling, and during forced degradation studies, protein products will undergo a number of degradations. These degradations may include a combination of amino acid side chain enzymatic or chemical post-translational modification (PTM), aggregation, and proteolytic cleavage.2 Monitoring these post-translational modifications during manufacturing and stability is required by health authorities. The availability of selective and robust analytical methods designed for quantifying these PTMs is therefore of prime importance. A wide array of analytical techniques are readily available to achieve extensive characterization of recombinant proteins in the non-GMP environment, but may require subsequent qualification, and full validation according to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines, as products are launched on the market.3
A technique critical to molecular variant characterization is hydrophobic interaction chromatography (HIC)-high performance liquid chromatography (HPLC). Retention and elution mechanisms of protein therapeutics from HIC matrices are well-understood and have been thoroughly described.4,5 Similar to RP-HPLC, retention of a protein of interest reflects its overall hydrophobicity; the more hydrophobic proteins needing less hydrophobic column matrices or less retentive mobile phase conditions. However, HIC-HPLC has four major differences compared with RP-HPLC: (1) retention to the HIC column is solely due to the interaction between amino acid residues located at the surface of the molecule and the column matrix; (2) differences in retention time between variants of the same molecule may therefore be the result of conformational changes, triggered or not, by a PTM; (3) HIC-HPLC is a non-destructive analytical method, where collected fractions can be assessed for their biological function; and (4) elution from the HIC-HPLC column is not driven by an increasing relative amount of organic solvent, but rather by gradual weakening of matrix/solute hydrophobic-hydrophobic interactions by a reverse gradient of ammonium sulfate or equivalent.
HIC-HPLC Columns Commercially Available
HIC-HPLC remains a specialty application and a limited number of columns and manufacturers are available. Columns, solid phase description and applications are indicated in Table 1.
Table 1. HIC-HPLC Columns and Analytical Applications for mAbs and related molecules.
| Column | Solid phase | Particle/pore size | Application | References |
|---|---|---|---|---|
| Dionex Propac HIC-10 | proprietary ethyl/amide based chemistry on non-endcaped silica | 5 μm, 300Å pore size | Trp oxidation, Asp isomerization, succinimide in mAbs, Carboxy terminus processing in Fc, serine O-fucosylation | 6–9 |
| TSKgel butyl-NPR | butyl on polymethacrylate base material | 2.5 μm, (non-porous) | proteolytic cleavage aggregates, misfolded domains. | 10,11 |
| TSKgel phenyl- 5PW | phenyl on polymethacrylate base material | 10 µm, 1000Å pore size | Asp isomerization in mAbs, Fab N-glycosylation, free thiol in Fab | 12–14 |
| TSKgel ether-5PW | poly ethyl ether on polymethacrylate base material | 10 µm, 1000Å pore size | (Fab)2 purification, Antibody drug conjugates | 15,16 |
| POROS HP2/20 | phenyl on Polystyrenedivinylbenzene particles | 20 µm, 500–10000Å pore size | N/A | 17 |
| PolyLC ethyl Aspartamide | ethyl/aspartamide on silica | 5 μm, 1000Å pore size | N/A | 18 |
| PolyLC methyl Aspartamide | methyl/aspartamide on silica | 5 μm, 1000Å pore size | N/A | 18 |
| PolyLC propyl Aspartamide | propyl/aspartamide on silica | 5 μm, 1000Å pore size | N/A | 18 |
N/A, no reported applications for antibodies and variants thereof.
Analytical Applications:Monitoring proteolytic fragment, aggregation, and misfolding of a Fc fusion protein
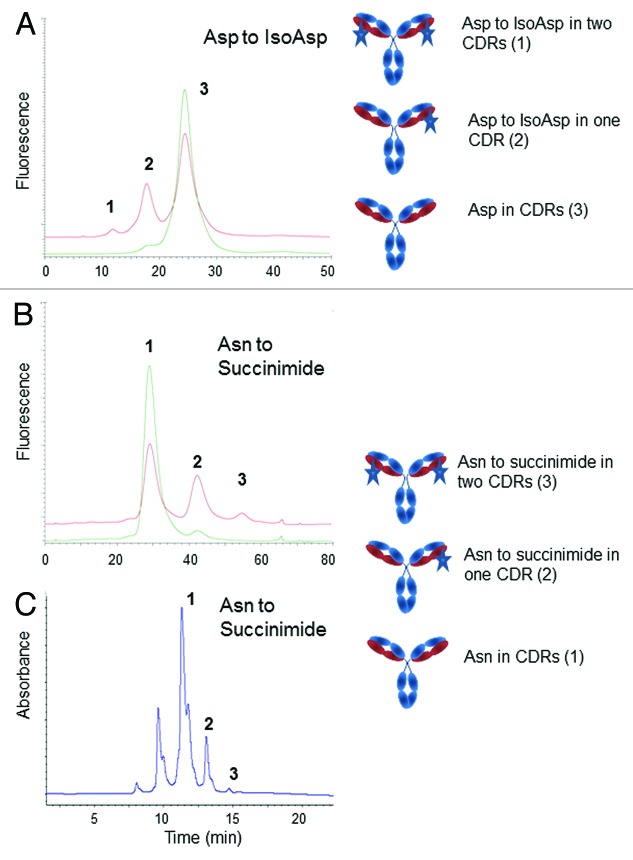
Etanercept (Enbrel®) is a marketed recombinant therapeutic protein. It is a fusion protein between the Fc domain of a human IgG1 antibody and two monomers of the human tumor necrosis factor (TNF) receptor-2 soluble domain.19 Each of the TNF receptor monomers are complex structures subject to misfolding, incorrect formation of the possible 22 disulfide bridges and proteolytic cleavage during fermentation. HIC-HPLC using the TSKgel butyl-NPR column is a method implemented for describing the molecule purity during fermentation, purification and batch release.10 Etanercept and its molecular variants are eluted in a 50 min reverse gradient of ammonium sulfate buffered at pH 7.0 with sodium phosphate. Isolation and characterization of variants showed that the early eluting peak contains truncated variants at Ser186 and Asp235 within the TNF domain (Fig. 1). Species eluting under peak 3 include dimers, etanercept associated with Chinese hamster ovary (CHO) host cell proteins and TNF receptor domain misfolded variants. Optimization of the fermentation and purification process results in a decrease of peaks 1 and 3 (Fig. 1). This method is particularly valuable to demonstrate similarity between the commercial Enbrel® and recombinant etanercept products seeking approval under the biosimilar regulatory pathway.20
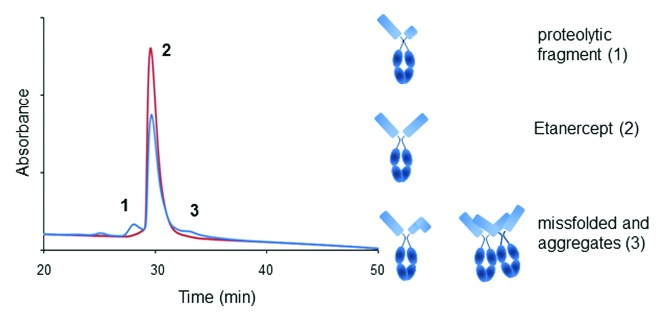
Figure 1. Elution profile of Etanercept on a butyl NPR HIC-HPLC column at initial and after one month at 40 °C (blue and red traces, respectively). Species eluting under each peak (numbered 1, 2, and 3) are shown in cartoon as per ref. 10.
Oxidation of Tryptophan in CDRs of a Monoclonal Antibody
Oxidation of exposed amino acid side chains such as tryptophan and methionine is a common occurrence in recombinant therapeutic proteins. Monitoring oxidation is of particular importance when residues undergoing this type of degradation are located in the complementarity-determining region (CDR) of a monoclonal antibody (mAb) because it may affect binding to the target antigen. Only few methods are available to monitor such PTMs. Methionine oxidation located in the Fab domain of mAbs has been shown for instance to be detectable by IEX-HPLC.21,22 While IEX-HPLC is easy to use, the method is rarely selective enough to allow reliable quantitation of oxidation. Conversely, peptide mapping is the preferred method for most PTM quantitation. However, low throughput and complex sample preparation may limit the use of this method during process development. In a recent publication, HIC-HPLC was shown to offer ease of use and reasonable selectivity to monitor the oxidation of tryptophan residues located in the CDR of a recombinant mAb.6 The elution profile presents two well-resolved pre-peaks eluting before the main. Forced oxidation studies, isolation and characterization by LC-MS peptide mapping of the species eluting under each peaks were performed. Results show prepeak 2 to contain mAb species oxidized at about 50% of the heavy chain tryptophan 47 residues; a figure consistent with the oxidation of one site per mAb.6 Oxidation of two of the strictly conserved Fc methionines is also observed in both pre-peaks (Fig. 2).6 The method uses two 100 mm Dionex Propac HIC-10 columns in series to improve recovery and resolution.6 Analytes are eluted via a reverse gradient of ammonium sulfate buffered at pH 5.5 and supplemented with 10% acetonitrile as organic modifier.6 The column is equilibrated in the presence of 500 mM arginine, ensuring analyte stability and elution, as well as column conditioning.23
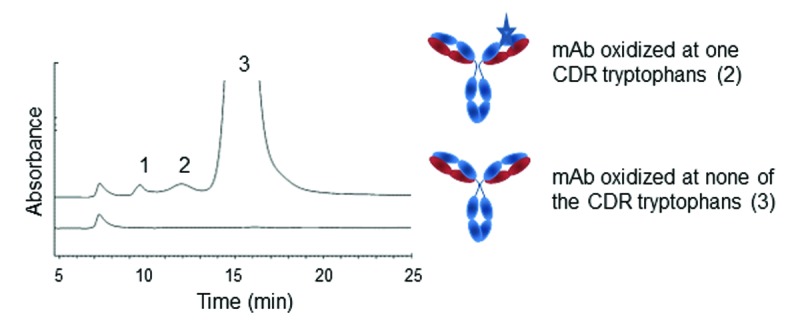
Figure 2. Elution profile on two Dionex Propac HIC-10 columns in series of a mAb oxidized at one CDR tryptophan residues (indicated as stars in the cartoon, pre-peak 2) and conserved Fc methionines (pre-peak 1 and 2). Chromatogram reproduced with permission from Elsevier (ref. 6).
Monitoring Carboxy Terminal Micro-Heterogeneity in mAbs
Amino and carboxy terminal processing account for a significant portion of the charge micro-heterogeneity found in therapeutic proteins.21 N-terminus micro-heterogeneity is due to the near complete cyclization of the first heavy chain glutamine residue to pyroglutamic acid.21 Carboxy terminal charge micro-heterogeneity comes from the enzymatic processing of the heavy chain carboxy terminal lysine residue during fermentation, followed in certain cases by α-amidation of the ante-penultimate amino acid.21 Because these posttranslational modifications may occur at different levels and in different combinations, multiple charge variants are likely to coelute during IEX-HPLC chromatography, complicating the characterization of each species eluting under each peak. Valliere-Douglas and colleagues developed a HIC-HPLC based method to be used as second dimension following IEX-HPLC fractionation and papain digest.7 The method uncovers the coelution in a basic peak of two carboxy terminal mAb variants, one with an un-processed carboxy terminal terminal lysine, the second with an α-amidated proline.7 These two species carry the same nominal charge but present a hydrophobic profile different enough to be separated by HIC-HPLC.7 The method uses two 100 mm Dionex Propac HIC-10 columns in series and a reverse gradient of ammonium sulfate buffered at pH 5.2.7 While a 2D-HPLC method is particularly appealing to further separate the variants that may co-elute during IEX-HPLC chromatography, the necessity to perform papain digest may be cumbersome.
Monitoring Aspartic Acid Isomerization and Succinimides in mAbs
Deamidation of asparagine and isomerization of aspartic acid residues are also a common occurrence in therapeutic proteins.21,24 The level at which these PTMs occur is a function of the flexibility of the peptide containing the asparagine or aspartic acid residues, as well as the nature of the neighboring amino acids.25 Similar to oxidation, deamidation and isomerization occurring within a CDR or in the direct vicinity of a CDR may cause a reduction or complete loss of potency, depending on the severity of the phenomenon.24 Asparagine deamidation results in a net protein charge difference due to the conversion of an amide group to a carboxylic moiety and is therefore usually detected by IEX-HPLC. However, analytical methods to monitor isomerization of aspartic acid into iso-aspartic acid (isoAsp) are harder to come by. Valliere-Douglas and colleagues developed a HIC-HPLC method to separate variants containing an isoAsp residues in each of the two light chains of an IgG2 mAb and eluting as two well-resolved pre-main peaks (Fig. 3A).7,8 The method uses two Dionex Propac HIC-10 columns in series and an ammonium sulfate gradient buffered at pH 4.85. Isomerization of aspartate residues into isoAsp in the CDRs of two mAbs were also successfully monitored using a TSKgel phenyl-5PW column after papain digest of the samples.12
Figure 3. Elution profile on two Dionex Propac HIC-10 columns in series of a mAb presenting an isoAsp residue (A) or succinimide (B) in one and both of a light chain CDR (indicated as stars in the cartoon). Panels A and B, show an overlay of mAbs controls and stressed at 40 °C. Elution profile on TSKgel butyl-NPR of a mAb presenting a succinimide (C) in the Fab region of a mAb. Species eluting under each peak (numbered 1, 2, and 3) are shown in cartoons. Chromatograms reproduced with permission from Elsevier and David Ouellette (Refs. 6 and 11, respectively).
The Dionex Propac HIC-10 based method was also used to isolate variants containing succinimide structures in the light chain of a different IgG2 mAb (Fig. 3B). Succinimide moieties are formed when the electron pair of the nitrogen on the peptide backbone attacks the carboxylic group of an asparagine or aspartic acid side chain.25 This cyclic intermediate is usually stable at mildly acidic pH, particularly when stabilized by a specific amino acid context. It is readily hydrolyzed into aspartic acid or isoAsp under neutral and basic conditions.25 The HIC method developed for succinimide monitoring also uses two HIC-10 columns in series, elution with ammonium acetate instead of sulfate, optimized mobile phase pH and gradient, and an organic content of 7% acetonitrile.7 Detection and quantitation of succinimide structures in an IgG1 mAb by HIC-HPLC was also reported by Ouellette and colleagues.11 The method relies on the TSKgel butyl-NPR column and analytes are eluted by a reverse gradient of ammonium sulfate buffered at pH 6.0. The molecular variants containing one or two succinimide moieties elute, like on the HIC-10 column, as well-resolved post-main peaks (Fig. 3C).
Detection of Unpaired Cysteines in mAbs
HIC-HPLC was also used to monitor levels of unpaired cysteine residues in the Fab of an IgG1 mAb.14 Harris and coauthors report the use of the TSKgel phenyl-5PW column and a gradient of ammonium sulfate buffered with TRIS-HCl at pH 7.5 to separate omalizumab Fab with unpaired Cys22 and Cys96 from the corresponding Fab, where the two cysteines are engaged in the expected disulfide bridge.14
Purification and Characterization of N- and O-Glycosylated mAbs
HIC-HPLC methods can also be used for the characterization of very low abundance variants. Valliere-Douglas and colleagues describe a HIC-HPLC based method to be used as second dimension after IEX-HPLC fractionation and papain digest.9 This method is designed for the separation of an O-fucosylated serine on the light chain and is analogous to the method used by the same group for the characterization of Fc carboxy terminal variants described above using two Dionex Propac HIC-10 columns in series.7,8 Fab variants are eluted with a reverse gradient of ammonium sulfate buffered at pH 5.2. The authors show that an acidic variant peak isolated by IEX-HPLC contains a species eluting before the unmodified Fab by HIC-HPLC. Isolation of this HIC-HPLC early eluting peak and subsequent in-depth molecular characterization by peptide mapping and Electron-transfer dissociation ion-trap MS established the structure of the variant and exact site of the O-fucosylation.
HIC-HPLC was also used to monitor N-glycosylation in the Fab domain of murine monoclonal IgG antibodies.13 The separation method relies on the TSKgel phenyl-5PW column and a 0 to 100% linear gradient of ammonium sulfate buffered with 20 mM sodium phosphate at pH 7.5. The Fab glycosylated murine mAb species elute as pre-peaks to the Main.
Prediction of mAbs Relative Hydrophobicity and Solubility Optima
Protein aggregation is one of the most common degradation pathways for therapeutic recombinant proteins stored as liquid formulations.26 The amount of oligomers in each batch of manufactured therapeutic protein needs to meet strict specifications due to the inherent immunogenicity of large molecular complexes.26 The aggregation mechanism of a given molecule may be difficult to fully understand because it results from the interplay of a number of factors, including electrostatic and hydrophobic interactions, the later mechanism being exacerbated upon local domain unfolding.27,28 While surface hydrophobicity may not be sufficient to explain mAbs aggregation alone, most of the aggregation-prone motifs identified in mAbs by in silico means, and later confirmed by mutagenesis studies, are rich in hydrophobic residues, especially β-branched aliphatic residues (Ile, Val, Leu) and aromatic residues (Phe, Trp, Tyr).29-31 Based on these efforts, protein structure-based engineering is now taking place to decrease surface hydrophobicity to optimize stability, solubility, and viscosity, and subsequently improve the manufacturability of potential drug candidates.29-33 While hydrophobicity assessment is performed using fluorescent probes,34 HIC-HPLC may also be used to rank relative surface hydrophobicity of entirely different mAbs by calculating their relative hydrophobicity index.35
HIC may also be used for the estimation of mAbs solubility optima in different formulations.36 The method takes advantage of the well-established relationship between protein precipitation/aggregation and retention on HIC supports. A HIC chromatography column is run under mobile phase conditions where the soluble mAb of interest is not retained.36 Under these conditions, the fully soluble mAb sample presents the highest possible flow-through peak height. Conversely, partial or complete mAb aggregation results in retention on the column and in the corresponding reduction of the flow-through peak height. Using this approach, the authors verify the expected protein salting-in/salting out phenomenon where increasing salt concentration in the formulation first improves protein solubility and then reduces it.36 The effect of different sodium salts, pH, and the presence of glycine as excipient on mAb solubility are also explored.36 The method may be particularly appealing for the screening of mAb candidates during early phases of development.
Monitoring the Degree of Payload Conjugation in Antibody-Drug Conjugates
Antibody-drug conjugates (ADC) are an emerging class of therapeutic agents in which a small molecule or peptide is attached to a mAb.37 Attaching small molecule payloads to mAbs is a natural alliance of the strength of these two classes of therapeutic solutions. The antibody provides target specificity of the small molecule payload, while the latter potentiates the efficacy of the mAb. ADCs therefore may substantially increase the therapeutic index of small molecules by improving their safety and efficacy profiles. Analytical strategies typically used for mAbs need to be adapted to address the specific challenges of the ADCs.16 HIC is an analytic solution reported to be particularly suitable to describing the antibody-to-drug ratio.38 Determination of the number of payload small molecules per mAb was performed on a TSKgel ether-5PW column using a reverse NaCl linear gradient elution buffered with sodium phosphate at pH 7.0 supplemented by 10% acetonitrile and 10% isopropanol.38 The method yielded baseline resolved peaks representing up to eight drug molecules per mAb vehicle (Fig. 4).
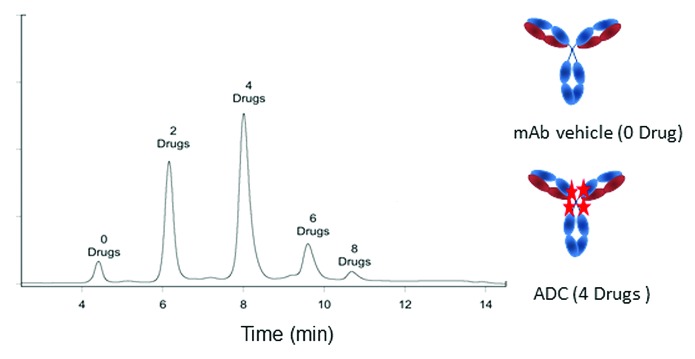
Figure 4. Antibody to drug ratio determined for an ADC on a TSKgel ether-5PW HIC-HPLC column. Stars represent drug attached to the mAb. Chromatogram reproduced with permission from Fredric S. Jacobson (ref. 16).
HIC-HPLC Method Development and Method Critical Parameters
Development of a HIC-HPLC method is relatively straightforward. However, HIC-HPLC cannot be considered a platform method in the same way that SEC-HPLC and CE-SDS might, but HIC-HPLC could perhaps be compared, in terms of development time and efforts, to IEX-HPLC.
The quality of the separation of hydrophobic variants during HIC-HPLC is dictated first and foremost by the nature of the solid phase used. Screening of the commercially available columns using a standard ammonium sulfate gradient is therefore generally advised (Table 1). Critical method parameters for the Dionex Propac HIC-10 HPLC-based method requiring optimization during development have been reported and include the concentration and nature of salt, pH of the mobile phase, percentage of organic modifier, gradient shape, and flow rate.7,8 Ammonium sulfate is the most common salt used for HIC-HPLC. It may be replaced by sodium sulfate without anticipating loss in performance;7 however, the purity of the ammonium sulfate is important. Ghost peaks and disturbances in the baseline may occur with use of reagents of lower purity. Dionex HIC-10 column uses non-endcaped silica, resulting in interactions between a charged analyte and the silica resin may occur. Changes in mobile phase pH or consistency of resin manufacturing may therefore modify the elution profile. The pH should be adjusted to maximize separation and recovery of the desired variants; the best binding is achieved at pHs approaching the pI of the analyte.7,8 Because separation performance may vary from column to column, multiple lots should be tested to ensure robustness of the analytical method. The amount of organic modifier was shown to have a profound effect on retention time. Increasing the relative amount of acetonitrile or n-propanol in the mobile phase results in a significant shift toward early retention times.7,8
The separation of mAb variants by HIC-HPLC reported in the literature tends to remain a characterization exercise rather than operation in regulated GMP environments. Full ICH validation and operation in GMP environments is possible, however, when certain critical quality attributes have to be measured, as is the case for etanercept. HIC-HPLC columns manufacturers are few but well established, and therefore column supply does not represent an impediment.
Conclusion
HIC-HPLC is a less widely used analytical technique compared with standard methods for separating variants based on size, charge or electrophoretic mobility. However, in a number of instances, HIC-HPLC methods display enough ease of use and selectivity toward targeted variants to be used as a valuable orthogonal method for characterization applications. In addition, upon validation, HIC-HPLC methods may be amenable to use in regulated environment as release and stability tests.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to express their gratitude to Merck Biologics colleagues, particularly Steve Farrand, for their encouragements and support. The authors are grateful to David Oullette, Fredric S Jacobson and Elsevier for permission to reproduce figures from their original publications.
References
- 1.Ambrogelly A, Palioura S, Söll D. Natural expansion of the genetic code. Nat Chem Biol. 2007;3:29–35. doi: 10.1038/nchembio847. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz SA, Engen JR, Mazzeo JR, Jones GB. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov. 2012;11:527–40. doi: 10.1038/nrd3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chirino AJ, Mire-Sluis A. Characterizing biological products and assessing comparability following manufacturing changes. Nat Biotechnol. 2004;22:1383–91. doi: 10.1038/nbt1030. [DOI] [PubMed] [Google Scholar]
- 4.Queiroz JA, Tomaz CT, Cabral JM. Hydrophobic interaction chromatography of proteins. J Biotechnol. 2001;87:143–59. doi: 10.1016/S0168-1656(01)00237-1. [DOI] [PubMed] [Google Scholar]
- 5.Geng X, Wang L. Liquid chromatography of recombinant proteins and protein drugs. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;866:133–53. doi: 10.1016/j.jchromb.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Boyd D, Kaschak T, Yan B. HIC resolution of an IgG1 with an oxidized Trp in a complementarity determining region. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:955–60. doi: 10.1016/j.jchromb.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Valliere-Douglass J, Wallace A, Balland A. Separation of populations of antibody variants by fine tuning of hydrophobic-interaction chromatography operating conditions. J Chromatogr A. 2008;1214:81–9. doi: 10.1016/j.chroma.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 8.Valliere-Douglass J, Jones L, Shpektor D, Kodama P, Wallace A, Balland A, Bailey R, Zhang Y. Separation and characterization of an IgG2 antibody containing a cyclic imide in CDR1 of light chain by hydrophobic interaction chromatography and mass spectrometry. Anal Chem. 2008;80:3168–74. doi: 10.1021/ac702245c. [DOI] [PubMed] [Google Scholar]
- 9.Valliere-Douglass JF, Brady LJ, Farnsworth C, Pace D, Balland A, Wallace A, Wang W, Treuheit MJ, Yan B. O-fucosylation of an antibody light chain: characterization of a modification occurring on an IgG1 molecule. Glycobiology. 2009;19:144–52. doi: 10.1093/glycob/cwn116. [DOI] [PubMed] [Google Scholar]
- 10.US patent Enbrel 7,294,481 B1.
- 11.Ouellette D, Chumsae C, Clabbers A, Radziejewski C, Correia I. Comparison of the in vitro and in vivo stability of a succinimide intermediate observed on a therapeutic IgG1 molecule. MAbs. 2013;5:432–44. doi: 10.4161/mabs.24458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakankar AA, Borchardt RT, Eigenbrot C, Shia S, Wang YJ, Shire SJ, Liu JL. Aspartate isomerization in the complementarity-determining regions of two closely related monoclonal antibodies. Biochemistry. 2007;46:1534–44. doi: 10.1021/bi061500t. [DOI] [PubMed] [Google Scholar]
- 13.Grebenau RC, Goldenberg DM, Chang CH, Koch GA, Gold DV, Kunz A, Hansen HJ. Microheterogeneity of a purified IgG1 due to asymmetric Fab glycosylation. Mol Immunol. 1992;29:751–8. doi: 10.1016/0161-5890(92)90185-Z. [DOI] [PubMed] [Google Scholar]
- 14.Harris RJ. Heterogeneity of recombinant antibodies: linking structure to function. Dev Biol (Basel) 2005;122:117–27. [PubMed] [Google Scholar]
- 15.Inouye K, Morimoto K. Single-step purification of F(ab’)2 mu fragments of mouse monoclonal antibodies (immunoglobulins M) by hydrophobic interaction high-performance liquid chromatography using TSKgel ether-5PW. J Biochem Biophys Methods. 1993;26:27–39. doi: 10.1016/0165-022X(93)90019-K. [DOI] [PubMed] [Google Scholar]
- 16.Wakankar A, Chen Y, Gokarn Y, Jacobson FS. Analytical methods for physicochemical characterization of antibody drug conjugates. MAbs. 2011;3:161–72. doi: 10.4161/mabs.3.2.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheich C, Leitner D, Sievert V, Leidert M, Schlegel B, Simon B, Letunic I, Büssow K, Diehl A. Fast identification of folded human protein domains expressed in E. coli suitable for structural analysis. BMC Struct Biol. 2004;4:4. doi: 10.1186/1472-6807-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alpert AJ. High performance hydrophobic interaction chromatography of proteins on a series of poly(alkyl aspartamide)-silicas. J Chromatogr A. 1986;359:85–97. doi: 10.1016/0021-9673(86)80064-4. [DOI] [Google Scholar]
- 19.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–61. [PubMed] [Google Scholar]
- 20.Declerck PJ. Biosimilar monoclonal antibodies: a science-based regulatory challenge. Expert Opin Biol Ther. 2013;13:153–6. doi: 10.1517/14712598.2012.758710. [DOI] [PubMed] [Google Scholar]
- 21.Du Y, Walsh A, Ehrick R, Xu W, May K, Liu H. Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies. MAbs. 2012;4:578–85. doi: 10.4161/mabs.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teshima G, Li MX, Danishmand R, Obi C, To R, Huang C, Kung J, Lahidji V, Freeberg J, Thorner L, et al. Separation of oxidized variants of a monoclonal antibody by anion-exchange. J Chromatogr A. 2011;1218:2091–7. doi: 10.1016/j.chroma.2010.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arakawa T, Tsumoto K, Nagase K, Ejima D. The effects of arginine on protein binding and elution in hydrophobic interaction and ion-exchange chromatography. Protein Expr Purif. 2007;54:110–6. doi: 10.1016/j.pep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Xu W, Dukleska S, Benchaar S, Mengisen S, Antochshuk V, Cheung J, Mann L, Babadjanova Z, Rowand J, et al. Developability studies before initiation of process development: improving manufacturability of monoclonal antibodies. MAbs. 2013;5:787–94. doi: 10.4161/mabs.25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson NE, Robinson AB. in Molecular Clocks: Deamidation of Asparaginyl and Glutaminyl Residues in Peptides and Proteins. Althouse Press, Cave Junction, OR (2004) [Google Scholar]
- 26.Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W. Structure-immunogenicity relationships of therapeutic proteins. Pharm Res. 2004;21:897–903. doi: 10.1023/B:PHAM.0000029275.41323.a6. [DOI] [PubMed] [Google Scholar]
- 27.Kumar V, Dixit N, Zhou LL, Fraunhofer W. Impact of short range hydrophobic interactions and long range electrostatic forces on the aggregation kinetics of a monoclonal antibody and a dual-variable domain immunoglobulin at low and high concentrations. Int J Pharm. 2011;421:82–93. doi: 10.1016/j.ijpharm.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Joubert MK, Luo Q, Nashed-Samuel Y, Wypych J, Narhi LO. Classification and characterization of therapeutic antibody aggregates. J Biol Chem. 2011;286:25118–33. doi: 10.1074/jbc.M110.160457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Design of therapeutic proteins with enhanced stability. Proc Natl Acad Sci U S A. 2009;106:11937–42. doi: 10.1073/pnas.0904191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chennamsetty N, Helk B, Voynov V, Kayser V, Trout BL. Aggregation-prone motifs in human immunoglobulin G. J Mol Biol. 2009;391:404–13. doi: 10.1016/j.jmb.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Perchiacca JM, Ladiwala AR, Bhattacharya M, Tessier PM. Aggregation-resistant domain antibodies engineered with charged mutations near the edges of the complementarity-determining regions. Protein Eng Des Sel. 2012;25:591–601. doi: 10.1093/protein/gzs042. [DOI] [PubMed] [Google Scholar]
- 32.Wu SJ, Luo J, O’Neil KT, Kang J, Lacy ER, Canziani G, Baker A, Huang M, Tang QM, Raju TS, et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel. 2010;23:643–51. doi: 10.1093/protein/gzq037. [DOI] [PubMed] [Google Scholar]
- 33.Lauer TM, Agrawal NJ, Chennamsetty N, Egodage K, Helk B, Trout BL. Developability index: a rapid in silico tool for the screening of antibody aggregation propensity. J Pharm Sci. 2012;101:102–15. doi: 10.1002/jps.22758. [DOI] [PubMed] [Google Scholar]
- 34.Kayser V, Chennamsetty N, Voynov V, Helk B, Trout BL. Conformational stability and aggregation of therapeutic monoclonal antibodies studied with ANS and Thioflavin T binding. MAbs. 2011;3:408–11. doi: 10.4161/mabs.3.4.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rustandi R, Beck A, eds. Glycosylation Engineering of Biopharmaceuticals: Methods and Protocols, Methods in Molecular Biology, (2013) vol. 988. [Google Scholar]
- 36.Gagnon P, Mayes T, Danielsson A. An adaptation of hydrophobic interaction chromatography for estimation of protein solubility optima. J Pharm Biomed Anal. 1997;16:587–92. doi: 10.1016/S0731-7085(97)00153-2. [DOI] [PubMed] [Google Scholar]
- 37.Ambrogelly A, Antochshuk V, Shi S, Liu Z, Shameem M. A path to building quality during monoclonal antibody candidate selection in Monoclonal antibodies - development, delivery and applications. Future Science. 2014 in the press. [Google Scholar]
- 38.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10:7063–70. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]