Abstract
Calcitonin gene-related peptide (CGRP) is a well-validated target for migraine therapy and a known potent systemic vasodilator. LBR-101 is a monoclonal antibody against CGRP in clinical development for the preventive treatment of episodic and chronic migraine. Understanding the hemodynamic and cardiovascular consequences of chronic CGRP inhibition is therefore warranted. Given the conservation in CGRP sequence between monkeys and humans, addressing this question in monkeys is ideal as it allows dosing at super-therapeutic levels. To this end, two independent studies were conducted in monkeys: a single dedicated cardiovascular safety study and a repeat-dose, chronic study, both with electrocardiogram and hemodynamic assessments. LBR-101 was very well tolerated in both studies, with no clinically significant changes noted in any hemodynamic parameter, nor any relevant changes noted in any ECG parameter. In cynomolgus monkeys, cardiovascular and hemodynamic parameters do not appear to be affected by long-term inhibition of CGRP with LBR-101.
Keywords: CGRP, migraine, cardiovascular, hemodynamic, safety, antibody
Introduction
Calcitonin gene-related peptide (CGRP) is a neuropeptide that plays an important role in the pathophysiology of migraine.1,2 During migraine attacks, plasma CGRP levels are elevated in the external jugular vein and treatment with the acute migraine therapy sumatriptan will normalize CGRP to pre-attack levels.3 Intravenous administration of CGRP induces migraine headaches in migraine sufferers,4 while CGRP receptor antagonists (CGRP-RAs) have been shown to be effective acute migraine therapies, relieving not only migraine pain, but migraine-associated symptoms as well.5-7 The clinical development of CGRP-RAs, however, has been complicated by signs of liver toxicity associated with frequent use.8,9
The therapeutic utility of monoclonal antibodies (mAbs), which is a consequence of target-specificity, typically prolonged half-lives, as well as reduced potential for hepatotoxicity and drug-drug interactions,10 may make CGRP mAbs an option for the preventive treatment of migraine if efficacy and safety can be demonstrated.
CGRP is a potent endogenous vasodilator and its effects on blood pressure under both normal and abnormal circumstances have received considerable attention.11-14 Several consequences of CGRP inhibition were investigated during the extensive development of different CGRP-RAs. Of notice, in vitro and in vivo data suggest that administration of CGRP-RAs does not induce coronary vasoconstriction, in contrast to what was observed with 5-HT1B/D receptor agonists.15 Similarly, treatment with CGRP antagonists had no effect on dog coronary arteries under ischemic conditions, while treatment with 5-HT1B/D receptor agonists worsened the infarct area.16 Finally, CGRP antagonism did not seem to affect vasodilation induced by certain anti-hypertensive medications,17 or to influence treadmill-exercise-time in patients with angina.18
Most studies on the vascular consequences of inhibiting CGRP were conducted in short-duration exposure paradigms using medications that have short half-lives. Since mAbs typically have relatively longer half-lives, it is important to understand and characterize hemodynamic and cardiovascular parameters after long-term inhibition of CGRP. LBR-101 is a mAb directed against CGRP being developed for the preventive treatment of episodic and chronic migraine.19 Here, we describe the influence of single and multiple administrations of high doses of LBR-101 on hemodynamic and electrocardiographic parameters in cynomolgus monkeys.
Results
Baseline characteristics
In the single dose study at dose initiation, adult animals (males) ranged in weight from 9.6 to 13.7 kg. At the initiation of dosing in the multiple dose study, animal body weights ranged from 2.7 to 4.4 kg for males and 2.5 to 3.8 kg for females.
Effects on blood pressure and heart rate
Single dose: Telemetry Study
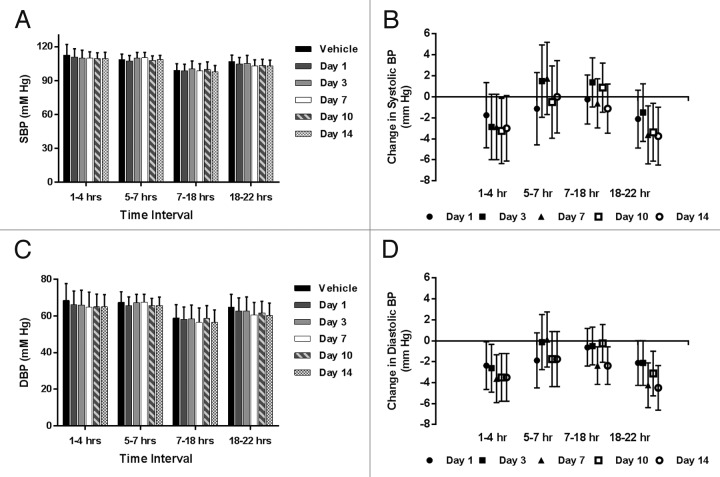
Figure 1A and B summarize the systolic blood pressure (SBP) as a function of treatment and time of day, to account for normal circadian influence on blood pressure and hemodynamic parameters. Group mean SBP (Fig. 1A) was remarkably similar before and after treatment with LBR-101 throughout the first day after dosing and on subsequent days (animals telemetered on Days 3, 7, 10 and 14 at identical time intervals as Day 1). At Hours 1–4 post-dosing when LBR-101 blood concentrations were at maximal levels (mean concentration of 3,500 μg/mL at 4 h), mean SBP was 111 mmHg compared with 113 mmHg at the same time interval following vehicle administration. Furthermore, SBP was 110 mmHg on Days 3 and 7, 109 mmHg on Day 10 and 110 mmHg on Day 14 after LBR-101 administration. Similar SBP data were recorded for other time intervals. Since this was a crossover designed study, the treated animals served as their own controls. When the data were analyzed as differences in blood pressure after LBR-101 administration compared with vehicle treatment, there are minor statistically significant reductions in SBP at the latter time interval on Days 7, 10 and 14 (Fig. 1B).
Figure 1. Hemodynamic data from single dose telemetry study of LBR-101 in monkeys. Data are shown with 95% confidence intervals. (A) group mean systolic blood pressure; (B) change in SBP vs vehicle; (C) group mean diastolic blood pressure; (D) change in DBP vs vehicle.
The effects of LBR-101 on the diastolic blood pressure (DBP) are summarized in Figure 1C and D. Following treatment with LBR-101, DBP was noted to be around 3 mmHg lower than the mean values obtained after vehicle administration (Fig. 1C). This result is consistent when the data are graphed as the mean change relative to vehicle (Fig. 1D). From Hours 5–22, the group mean for the vehicle and LBR-101 group were similar. The same trend was seen on other days, when a slight decrease in the DBP (ranging from 2.62–3.5 mmHg) occurred in the first interval measured, with a few changes of similar magnitude seen sporadically on Days 7–10 in the 7–22 h interval.
Similar to what was seen for the DBP, minor decreases in the heart rate were seen during the first assessment (Hours 1–4) relative to vehicle treatment (Fig. 2). Differences were undetectable during the intermediate assessments and were once more seen between Hours 18–22 on all days.
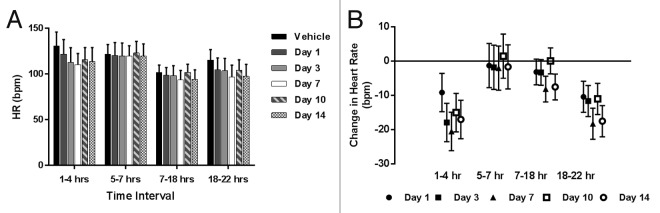
Figure 2. Heart rate data from single dose telemetry study of LBR-101 in monkeys. Data are shown with 95% confidence intervals. (A) group mean heart rate; (B) change in heart rate vs vehicle.
Multiple dose study
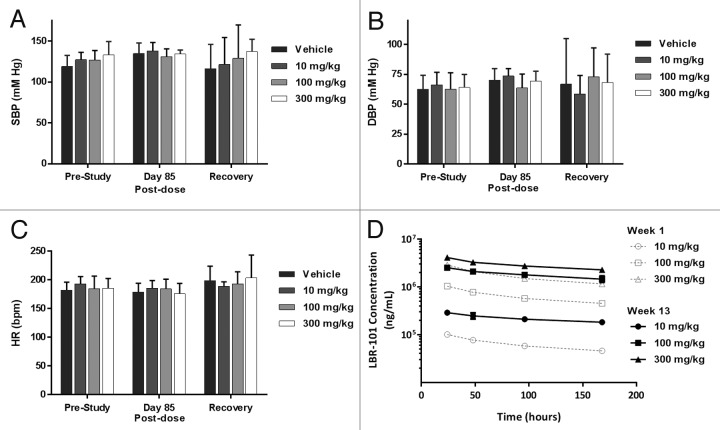
Blood pressure was recorded prior to the first dose, after 12 wk of dosing (13 doses) and approximately 1 wk after the end of dosing. No significant changes were noted in SBP or DBP in any of the treated groups of animals relative to vehicle-treated animals (Fig. 3A and B). Group mean heart rates were relatively consistent across the dose groups and time points measured, with no statistical differences measured (Fig. 3C). Plasma concentrations of LBR-101 were measured during the first week of dosing and at the time of blood pressure and ECG assessments (Fig. 3D), demonstrating accumulation with repeated, weekly dosing.
Figure 3. Hemodynamic data from a 14-wk, repeat dose study of LBR-101 in monkeys. Data are shown with 95% confidence intervals. (A) systolic blood pressure; (B) diastolic blood pressure; (C) heart rate; (D) time-concentration profile at Weeks 1 and 13.
ECG findings
Single dose: Telemetry study
QTc interval
There were no statistically significant changes in QTc interval at any time point, relative to vehicle treatment (Table 1).
Table 1. Group mean ECG parameters in single-dose cardiovascular safety study in monkeys.
| Time Interval | Treatment Day | RR (msec) | PR (msec) | QRS (msec) | QT (msec) | QTc (msec) |
|---|---|---|---|---|---|---|
| Hours 1 – 4 | Vehicle | 481.37 | 111.63 | 38.00 | 243.13 | 177.13 |
| Day 1 | 513.25* | 116.25* | 38.38 | 254.88* | 180.25 | |
| Day 3 | 553.00* | 118.38* | 38.13 | 264.75* | 171.63 | |
| Day 7 | 561.00* | 119.38* | 39.00* | 269.00* | 172.39 | |
| Day 10 | 538.00* | 116.88* | 38.88* | 261.25* | 174.63 | |
| Day 14 | 550.50* | 118.00* | 39.00* | 264.87* | 171.75 | |
| Hours 5 – 7 | Vehicle | 502.38 | 119.62 | 39.50 | 249.38 | 174.38 |
| Day 1 | 511.25 | 121.12 | 38.88 | 251.63 | 177.25 | |
| Day 3 | 514.25 | 118.62 | 38.75 | 254.13 | 174.13 | |
| Day 7 | 513.25 | 117.87 | 39.50 | 253.75 | 174.36 | |
| Day 10 | 498.38 | 117.25 | 39.00 | 251.38 | 180.38 | |
| Day 14 | 513.25 | 119.75 | 39.13 | 253.88 | 175.12 | |
| Hours 7 – 18 | Vehicle | 602.25 | 120.00 | 39.63 | 299.38 | 185.25 |
| Day 1 | 624.38 | 122.50* | 39.38 | 310.00 | 193.25 | |
| Day 3 | 631.50* | 119.88 | 39.25 | 306.38 | 180.13 | |
| Day 7 | 663.75* | 121.38 | 39.88 | 312.50* | 177.07 | |
| Day 10 | 602.38 | 120.38 | 39.63 | 299.88 | 190.00 | |
| Day 14 | 659.38* | 123.87* | 39.75 | 314.00* | 179.87 | |
| Hours 18 – 22 | Vehicle | 541.37 | 115.50 | 38.88 | 273.38 | 183.50 |
| Day 1 | 598.25* | 119.25* | 39.00 | 294.25* | 188.13 | |
| Day 3 | 604.62* | 117.00 | 39.00 | 295.38* | 178.75 | |
| Day 7 | 646.25* | 119.62* | 39.13 | 305.88* | 178.25 | |
| Day 10 | 597.37* | 119.50* | 39.25 | 296.00* | 188.25 | |
| Day 14 | 644.25* | 120.62* | 39.25 | 307.88* | 179.63 |
P < 0.05 compared with vehicle treatment
Other ECG parameters
lthough statistically significant changes in RR, PR, RS and QT were seen over the 14 d period when compared with vehicle (Table 1), they were all minor in absolute value.
Multiple dose study
QTc interval
There were no significant differences in QTc interval across all doses and time points (Table 2).
Table 2. Group mean ECG parameters in a 14-wk, repeat-dose safety study in monkeys.
| Time | Treatment | RR (msec) | PR (msec) | QRS (msec) | QT (msec) | QTc (msec) |
|---|---|---|---|---|---|---|
| Pre-Study | Vehicle | 334 | 81 | 38 | 197 | 341 |
| 10 mg/kg | 314 | 78 | 36 | 186 | 333 | |
| 100 mg/kg | 335 | 88 | 37 | 197 | 341 | |
| 300 mg/kg | 330 | 82 | 38 | 198 | 345 | |
| Pre-dose (Day 85) | Vehicle | 344 | 83 | 40 | 204 | 348 |
| 10 mg/kg | 340 | 83 | 36 | 198 | 341 | |
| 100 mg/kg | 337 | 91 | 38 | 199 | 343 | |
| 300 mg/kg | 328 | 80 | 37 | 197 | 344 | |
| Post-dose (Day 85) | Vehicle | 342 | 82 | 39 | 201 | 344 |
| 10 mg/kg | 327 | 82 | 37 | 196 | 343 | |
| 100 mg/kg | 331 | 92 | 38 | 200 | 344 | |
| 300 mg/kg | 349 | 86 | 37 | 204 | 345 | |
| Recovery Day 103 | Vehicle | 303 | 92 | 37 | 185 | 336 |
| 10 mg/kg | 318 | 83 | 33 | 188 | 334 | |
| 100 mg/kg | 313 | 99 | 40 | 189 | 338 | |
| 300 mg/kg | 299 | 90 | 34 | 183 | 335 |
Other ECG parameters
No significant or relevant ECG changes were seen for any of the ECG parameters assessed over the course of the study (Table 2).
Discussion
CGRP is a 37-amino acid neuropeptide that has received substantial attention as its role in migraine physiology has emerged,8,20 leading to a number of therapeutic candidates aimed at inhibiting or blocking its effects.21 Given its potent vasodilatory activity, the cardiovascular consequences of prolonged CGRP inhibition are important to understand when considering CGRP as a target for potential chronic administration.19
LBR-101 is a humanized mAb that potently and selectively binds to both isoforms (α and β) of CGRP, blocking its binding to the CGRP receptors. LBR-101 is specific for CGRP and does not bind to the closely related family members amylin, calcitonin or adrenomedullin peptides.22,23 In humans, the half-life of LBR-101 is ~45 d, while in monkeys the half-life is estimated to be between 10 and 26 d. Because of sequence conservation between monkey and human CGRP, the affinity for CGRP is also presumed to be maintained between the two species. As is the case with other mAbs,24 this extended pharmacokinetic half-life enables infrequent dosing to maintain therapeutic levels of drug in the body.
Because protein therapeutics are designed to be highly specific, adverse reactions are most commonly a result of exaggerated pharmacology (e.g., from prolonged target inhibition).25 Since CGRP is a vasodilator, its inhibition might be expected to translate into increased blood pressure and secondarily increased heart rate. Human Phase 1, single dose studies have been conducted using LBR-101 in doses up to 2,000 mg (~25 mg/kg/month), while Phase 2 studies are being conducted with a maximal LBR-101 dose of ~11 mg/kg/month. At doses up to 2,000 mg in human subjects, no clinically relevant changes in systolic or diastolic blood pressure, heart rate, or ECG parameters (RR, PR, QRS, or QTcF) were observed when comparing baseline vs. post-dose time-points, or between groups for any parameter or time-point. No statistically significant differences or clinically relevant abnormalities have been seen when comparing parameters obtained at Tmax vs. baseline, or Tmax vs. any other time-point.26
Herein we aimed to further explore the consequences of CGRP inhibition by giving much higher doses of LBR-101 to cynomolgus monkeys during a single dose safety pharmacology study and a repeat dose, 14-wk monkey study (up to 300 mg/kg/week). In both studies, we examined heart rate, diastolic and systolic blood pressure and the complement of ECG parameters.
Similar to the human experience, in these two monkey studies we failed to observe any meaningful effect of LBR-101 on ECG parameters. This result was not unexpected, since CGRP does not seem to influence cardiac electrical conduction or repolarization. We also failed to identify any relevant effect of CGRP inhibition on systolic blood pressure. A minor and clinically insignificant reduction in the diastolic blood pressure was seen in assessments conducted early in the test days in the single-dose study. This drop was accompanied by a coincident drop in the heart rate and minor decrease in activity (data not shown), both of which are possibly linked to the lack of in-room activites at these times. Nonetheless, these results are intriguing. Given the potent vasodilatory properties of CGRP and the minor nature of the findings, the biological plausibility for the changes come into question. In this regard, studies using αCGRP knockout mice may shed light on the topic, although they have reported conflicting results. An early study reported no differences between wild type and αCGRP knockout mice in terms of resting heart rate or blood pressure,27 while a more recent study reported increased mean arterial pressure and heart rate in αCGRP-null mice.28 A third study found that αCGRP knockout mice and wild animals displayed similar body weights, serum lipid markers, and insulin sensitivity, although the null mice had slightly higher core body temperatures. Interestingly, animals without CGRP were also less likely to become obese in response to fat feeding.29
The explanation for the discrepancy between αCGRP knockout blood pressure data may lie in the differential function of CGRP inside and outside the brain; it also highlights the challenges in applying data from genetic knockouts to predict therapeutic outcomes.
In terms of the pathophysiology of migraine, at the peripheral level (outside of the blood-brain barrier), CGRP release results in vasodilation and inflammation.30 At central synapses, CGRP is involved in modulating pain transmission.20 In pain modulation, CGRP seems to be crucially positioned at the intersection of peripheral events and central pain modulation.31 Similar dual effects may also help to explain the paradoxical minor effect observed on the blood pressure (minor reduction vs. increase) and heart rate (decrease), since there seem to exist differences, in terms of vasodilation, between inhibiting CGRP only at the nerve endings or throughout the entire central and peripheral nervous systems. It has been suggested that CGRP in the central nervous system is associated with regulation of various hemodynamic parameters,32,33 while peripheral inhibition of CGRP does not induce any hemodynamic changes, likely due to the number of overlapping compensatory mechanisms that are involved in the modulation of blood pressure.34 In other words, CGRP can interfere with the control of blood pressure via modulating central synapses but, at the peripheral level, its inhibition would be counterbalanced by other hemodynamically active substances (nitric oxide, brain natriuretic peptide and catecholamines). As mAbs do not penetrate the blood-brain barrier, this central pool of CGRP should be unaffected by CGRP mAb treatment. To date, there has been no conclusive evidence that the blood-brain barrier is disrupted in migraine attacks, thus the antibody should not be accessing the central nervous system.
Nonetheless, the small changes in diastolic blood pressure and heart rate seen in the monkeys need to be put in context. The changes were noted after very high doses of LBR-101 and were not seen in human studies with therapeutic doses of LBR-101. The magnitude of the observed changes were minor, substantially lower than what is seen with current migraine preventive medications, such as propranolol and verapamil, which are migraine medications with well-defined anti-hypertensive and bradycardic action often suggested to be used in migraineurs with hypertension.35
While providing provocative data, these two monkey studies are not without limitations. Baseline ECG parameters of RR, PR and QT differed between studies, potentially due to a combination of factors such as age and weight of the animals, use of anesthetic vs telemetered, conscious animals and differing testing laboratories. The data between studies should therefore be compared with caution; they were independently conducted studies and should be analyzed as such.
The chronic, multiple-dose study performed limited ECG assessments, unlike the continuous telemetry in the single-dose study. Nonetheless, once weekly dosing effectively maintained plasma levels of LBR-101 at a very high level (Fig. 3D), so the ECG assessments collected at Day 85 and during the recovery phase should be a reasonable representation of cardiac function during the study, when maximal amounts of drug were present. The small number of animals analyzed in the recovery group (2/gender/group) is also a limitation of the multiple-dose study. This is a standard design for chronic studies of this kind, but a larger group size would strengthen the observed findings. Due to the extended pharmacokinetic profile of LBR-101 in monkeys, the single dose study design required ECG assessments for multiple days following drug administration. However, only a single day of ECG monitoring was done following vehicle administration, so all of the data from the LBR-101 treated arm (over two weeks) must be compared with data from one day following vehicle treatment. Any single day abnormality that occurred following vehicle treatment would therefore be given much higher weight than appropriate.
In summary, inhibition of CGRP with very high doses of LBR-101 was associated with only minor and clinically insignificant changes in the diastolic blood pressure and heart rate. No changes in the systolic blood pressure and QTc were observed at any time in cynomolgus monkeys. These data add to the safety findings in humans and support further development of medications chronically inhibiting the CGRP pathway.
Methods
Two independent studies were conducted and are described below. The studies were conducted in accordance with current guidelines for animal welfare36 and were reviewed and approved by the Institutional Animal Care and Use Committee (Pfizer; Covance Laboratories).
Single-dose telemetry study
Eight adult male cynomolgus monkeys (Charles River Primates) were surgically instrumented with telemeters and allowed to recover for at least two weeks. Implants (DSI TL11M2-D70-PCT) and receivers (RMC-1) were manufactured by Data Sciences International.
Animals were acclimated to telemetry data acquisition cages at least overnight prior to dosing. During acclimation, pre-study recording of hemodynamic parameters was conducted to verify that the transducers and equipment were functioning correctly. During telemetered data acquisition, animals were housed individually in cages equipped with telemetry receivers. On non-collection days, animals were housed in cages without telemetry receivers. Animals were maintained on a 12-h light, 12-h dark day cycle, with ad libitum water and fed with certified primate diet.
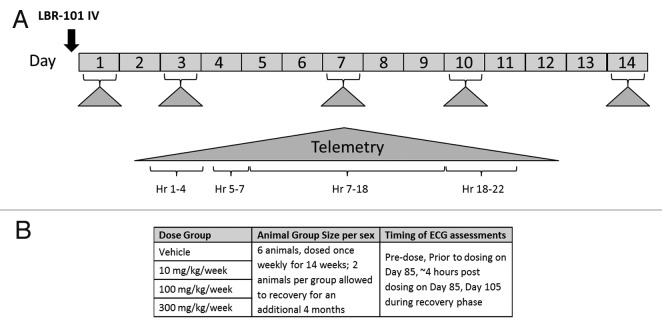
For the first phase of the study, animals (8 males) were administered vehicle only, and telemetry data were collected beginning ~1 h pre-dose through 22 h post-dose. Six days after vehicle administration, the same animals received a single IV administration of LBR-101 (100 mg/kg, an ~10-fold greater dose than the pharmacological EC50 in cynomolgus monkeys). Telemetered electrocardiographic and hemodynamic data were again continuously recorded from all animals. In addition, these animals were monitored for ~24 h on Days 3, 7, 10 and 14 after receiving their single dose of LBR-101 (Fig. 4A). Telemetered ECG and blood pressure signals were transmitted via the implanted radio-telemetry devices to receivers mounted in each cage. The acquired signals were passed through a data exchange matrix (DSI model DEM) and on to a PC-based data acquisition system (DSI software Ponemah P3 version 3.4); the data analysis software was Emka Technologies version 2.4.0.20 (Emka Technologies). The analog/digital sampling rate was 1,000 Hz for telemetered ECG data and 500 Hz for blood pressure data. Data were logged as 1 min means.
Figure 4. Study schematics. (A) single-dose study; (B): repeat-dose study.
Multiple-dose safety study
The repeat-dose safety study included 48 adult, gender-matched (6 per gender per group) LBR-101-naïve cynomolgus monkeys (Charles River Primates). Animals received vehicle or LBR-101 as an intravenous injection once weekly for 14 wk at doses of 10 mg/kg, 100 mg/kg, or 300 mg/kg. In each group, two animals of each gender were allowed to recover for an additional 4 mo following the end of dosing (Fig. 4B).
ECG and blood pressure measurements were recorded once during the pre-study phase, twice after steady-state was achieved (prior to dosing and 4 h post-dose on Day 85) and once ~1 wk after the end of dosing (Day 103 of the recovery phase). Animals were anesthetized with ketamine and ECGs were recorded using eight leads. Measurement of ECGs (including heart rate) was done with the captured data using the Life Science Suite Ponemah Physiology Platform software system via DSI, using leads I, II, aVF, CG4RL and CV4LL, as standard. A heart rate correction for the QT interval (QTc) was calculated using the Bazett formula.37
For both the single and repeat-dose studies, LBR-101 was formulated as a 51.4 mg/mL solution in 20 mM histidine, 84 mg/mL trehalose dihydrate, 0.2 mg/mL polysorbate 80, 0.05 mg/mL disodium EDTA dihydrate and 0.1 mg/mL l-methionine, pH ~5.5. Vehicle was formulated identically without the LBR-101. Additionally, in both studies, blood samples were taken periodically for analysis of LBR-101 plasma concentration using a validated ELISA method.
Data Analysis
Data were first aggregated in summary tables and figures using GraphPad Prism (version 6.0) and Excel 2010 (Microsoft). For the single exposure study, telemetry data were analyzed using ANOVA. Analysis was performed using SAS Release 8.2. In order to normalize the QT interval over a range of R-R intervals, Individual Animal Correction Factors (IACFs) were generated for each animal by relating each RR-interval with its associated QT-interval. The linear regression of this QT/RR-interval relationship was determined for the data set. The slope of this linear regression was used as the IACF for the associated animal across all treatments. This IACF was used to calculate the corrected QT-interval (QTc) using the following equation:
QT-I(c) = QT interval corrected for heart rate = QT-I - [(RR - 300)*(IACF)].
For the multiple exposure study, one-way ANOVA was also used to analyze data. If the ANOVA was significant (P ≤ 0.05), Dunnett’s post-test was used for in-between group comparisons. For each gender, the treated group was compared with the control (vehicle) group at the 5% two-tailed probability level.
Disclosure of Potential Conflicts of Interest
These studies were sponsored by Pfizer Inc. S.W., R.E. and M.E.B. are full time employees of Labrys Biologics, Inc.
Glossary
Abbreviations:
- ANOVA
analysis of variance
- CGRP
calcitonin gene-related peptide
- CGRP-RA
CGRP-receptor antagonist
- DBP
diastolic blood pressure
- ECG
electrocardiogram
- ELISA
enzyme-linked immunosorbent assay
- Hz
hertz
- IV
intravenous
- mAb
monoclonal antibody
- mm Hg
millimeters of mercury
- SBP
systolic blood pressure
- Tmax
time at maximal blood concentration
References
- 1.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 2.Edvinsson L, Goadsby PJ. Extracerebral manifestations in migraine. A peptidergic involvement? J Intern Med. 1990;228:299–304. doi: 10.1111/j.1365-2796.1990.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 4.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 5.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–23. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 6.Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–22. doi: 10.1177/0333102411398399. [DOI] [PubMed] [Google Scholar]
- 7.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM, BIBN 4096 BS Clinical Proof of Concept Study Group Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 8.Silberstein SD. Emerging target-based paradigms to prevent and treat migraine. Clin Pharmacol Ther. 2013;93:78–85. doi: 10.1038/clpt.2012.198. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann J, Goadsby PJ. New Agents for Acute Treatment of Migraine: CGRP Receptor Antagonists, iNOS Inhibitors. Curr Treat Options Neurol. 2012;14:50–9. doi: 10.1007/s11940-011-0155-4. [DOI] [PubMed] [Google Scholar]
- 10.Wu H, Dall’Acqua WF. Humanized antibodies and their applications. Methods. 2005;36:1–2. doi: 10.1016/j.ymeth.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Allen DM, Chen LE, Seaber AV, Urbaniak JR. Calcitonin gene-related peptide and reperfusion injury. J Orthop Res. 1997;15:243–8. doi: 10.1002/jor.1100150213. [DOI] [PubMed] [Google Scholar]
- 12.Källner G, Gonon A, Franco-Cereceda A. Calcitonin gene-related peptide in myocardial ischaemia and reperfusion in the pig. Cardiovasc Res. 1998;38:493–9. doi: 10.1016/S0008-6363(98)00016-9. [DOI] [PubMed] [Google Scholar]
- 13.Lynch JJ, Jr., Detwiler TJ, Kane SA, Regan CP. Effect of calcitonin gene-related peptide receptor antagonism on the systemic blood pressure responses to mechanistically diverse vasomodulators in conscious rats. J Cardiovasc Pharmacol. 2010;56:518–25. doi: 10.1097/FJC.0b013e3181f5d414. [DOI] [PubMed] [Google Scholar]
- 14.Supowit SC, Ethridge RT, Zhao H, Katki KA, Dipette DJ. Calcitonin gene-related peptide and substance P contribute to reduced blood pressure in sympathectomized rats. Am J Physiol Heart Circ Physiol. 2005;289:H1169–75. doi: 10.1152/ajpheart.00973.2004. [DOI] [PubMed] [Google Scholar]
- 15.Chan KY, Edvinsson L, Eftekhari S, Kimblad PO, Kane SA, Lynch J, Hargreaves RJ, de Vries R, Garrelds IM, van den Bogaerdt AJ, et al. Characterization of the calcitonin gene-related peptide receptor antagonist telcagepant (MK-0974) in human isolated coronary arteries. J Pharmacol Exp Ther. 2010;334:746–52. doi: 10.1124/jpet.110.165993. [DOI] [PubMed] [Google Scholar]
- 16.Lynch JJ, Shen YT, Pittman TJ, Anderson KD, Koblan KS, Gould RJ, Regan CP, Kane SA. Effects of the prototype serotonin 5-HT(1B/1D) receptor agonist sumatriptan and the calcitonin gene-related peptide (CGRP) receptor antagonist CGRP(8-37) on myocardial reactive hyperemic response in conscious dogs. Eur J Pharmacol. 2009;623:96–102. doi: 10.1016/j.ejphar.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Van der Schueren BJ, Blanchard R, Murphy MG, Palcza J, De Lepeleire I, Van Hecken A, Depré M, de Hoon JN. The potent calcitonin gene-related peptide receptor antagonist, telcagepant, does not affect nitroglycerin-induced vasodilation in healthy men. Br J Clin Pharmacol. 2011;71:708–17. doi: 10.1111/j.1365-2125.2010.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaitman BR, Ho AP, Behm MO, Rowe JF, Palcza JS, Laethem T, Heirman I, Panebianco DL, Kobalava Z, Martsevich SY, et al. A randomized, placebo-controlled study of the effects of telcagepant on exercise time in patients with stable angina. Clin Pharmacol Ther. 2012;91:459–66. doi: 10.1038/clpt.2011.246. [DOI] [PubMed] [Google Scholar]
- 19.Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache. 2013;53:1230–44. doi: 10.1111/head.12179. [DOI] [PubMed] [Google Scholar]
- 20.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–82. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 21.Vollbracht S, Rapoport AM. The pipeline in headache therapy. CNS Drugs. 2013;27:717–29. doi: 10.1007/s40263-013-0090-x. [DOI] [PubMed] [Google Scholar]
- 22.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fcgamma receptor I binding and monocyte triggering activities. Eur J Immunol. 1999;29:2613–24. doi: 10.1002/(SICI)1521-4141(199908)29:08<2613::AID-IMMU2613>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Zeller J, Poulsen KT, Sutton JE, Abdiche YN, Collier S, Chopra R, Garcia CA, Pons J, Rosenthal A, Shelton DL. CGRP function-blocking antibodies inhibit neurogenic vasodilatation without affecting heart rate or arterial blood pressure in the rat. Br J Pharmacol. 2008;155:1093–103. doi: 10.1038/bjp.2008.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingbeil C, Hsu DH. Pharmacology and safety assessment of humanized monoclonal antibodies for therapeutic use. Toxicol Pathol. 1999;27:1–3. doi: 10.1177/019262339902700101. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez I, Erdman A, Padhi D, Garnett CE, Zhao H, Targum SL, Balakrishnan S, Strnadova C, Viner N, Geiger MJ, et al. Electrocardiographic assessment for therapeutic proteins--scientific discussion. Am Heart J. 2010;160:627–34. doi: 10.1016/j.ahj.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Bigal ME, Escandon R, Bronson M, Walter S, Sudworth M, Huggins JP, Garzone P. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: Results of the Phase 1 program. Cephalalgia. 2013;34:483–92. doi: 10.1177/0333102413517775. [DOI] [PubMed] [Google Scholar]
- 27.Lu JT, Son YJ, Lee J, Jetton TL, Shiota M, Moscoso L, Niswender KD, Loewy AD, Magnuson MA, Sanes JR, et al. Mice lacking alpha-calcitonin gene-related peptide exhibit normal cardiovascular regulation and neuromuscular development. Mol Cell Neurosci. 1999;14:99–120. doi: 10.1006/mcne.1999.0767. [DOI] [PubMed] [Google Scholar]
- 28.Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, Imai Y, Kayaba Y, Nishimatsu H, Suematsu Y, et al. Elevated sympathetic nervous activity in mice deficient in alphaCGRP. Circ Res. 2001;89:983–90. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- 29.Walker CS, Li X, Whiting L, Glyn-Jones S, Zhang S, Hickey AJ, Sewell MA, Ruggiero K, Phillips AR, Kraegen EW, et al. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology. 2010;151:4257–69. doi: 10.1210/en.2010-0284. [DOI] [PubMed] [Google Scholar]
- 30.Eftekhari S, Edvinsson L. Possible sites of action of the new calcitonin gene-related peptide receptor antagonists. Ther Adv Neurol Disord. 2010;3:369–78. doi: 10.1177/1756285610388343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher LA, Kikkawa DO, Rivier JE, Amara SG, Evans RM, Rosenfeld MG, Vale WW, Brown MR. Stimulation of noradrenergic sympathetic outflow by calcitonin gene-related peptide. Nature. 1983;305:534–6. doi: 10.1038/305534a0. [DOI] [PubMed] [Google Scholar]
- 33.Le Mével JC, Lancien F, Mimassi N, Kermorgant M, Conlon JM. Central ventilatory and cardiovascular actions of calcitonin gene-related peptide in unanesthetized trout. J Exp Biol. 2012;215:1930–7. doi: 10.1242/jeb.070177. [DOI] [PubMed] [Google Scholar]
- 34.Casey DP, Joyner MJ. Compensatory vasodilatation during hypoxic exercise: mechanisms responsible for matching oxygen supply to demand. J Physiol. 2012;590:6321–6. doi: 10.1113/jphysiol.2012.242396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754–62. doi: 10.1212/WNL.55.6.754. [DOI] [PubMed] [Google Scholar]
- 36.Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996. [Google Scholar]
- 37.Roguin A. Henry Cuthbert Bazett (1885-1950)--the man behind the QT interval correction formula. Pacing Clin Electrophysiol. 2011;34:384–8. doi: 10.1111/j.1540-8159.2010.02973.x. [DOI] [PubMed] [Google Scholar]