Abstract
Background
Pre-eclampsia is a relatively common complication of pregnancy. HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome is a severe manifestation of pre-eclampsia with significant morbidity and mortality for pregnant women and their children. Corticosteroids are commonly used in the treatment of HELLP syndrome in the belief that they improve outcomes.
Objectives
To determine the effects of corticosteroids on women with HELLP syndrome and their children.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 June 2010).
Selection criteria
Randomized controlled trials comparing any corticosteroid with placebo, no treatment, or other drug; or comparing one corticosteroid with another corticosteroid or dosage in women with HELLP syndrome.
Data collection and analysis
Two review authors assessed trial quality and extracted data independently.
Main results
Eleven trials (550 women) compared corticosteroids with placebo or no treatment. There was no difference in the risk of maternal death (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.28 to 3.21), maternal death or severe maternal morbidity (RR 0.27, 95% CI 0.03 to 2.12), or perinatal/infant death (RR 0.64, 95% CI 0.21 to 1.97). The only clear effect of treatment on individual outcomes was improved platelet count (standardized mean difference (SMD) 0.67, 95% CI 0.24 to 1.10). The effect on platelet count was strongest for women who commenced treatment antenatally (SMD 0.80, 95% CI 0.25 to 1.35).
Two trials (76 women) compared dexamethasone with betamethasone. There was no clear evidence of a difference between groups in respect to perinatal/infant death (RR 0.95, 95% CI 0.15 to 6.17) or severe perinatal/infant morbidity or death (RR 0.64, 95% CI 0.27 to 1.48). Maternal death and severe maternal morbidity were not reported. In respect to platelet count, dexamethasone was superior to betamethasone (MD 6.02, 95% CI 1.71 to 10.33), both when treatment was commenced antenatally (MD 8.10, 95% CI 6.23 to 9.97) and postnatally (MD 3.70, 95% CI 0.96 to 6.44).
Authors’ conclusions
There was no clear evidence of any effect of corticosteroids on substantive clinical outcomes. Those receiving steroids showed significantly greater improvement in platelet counts which was greater for those receiving dexamethasone than those receiving betamethasone. There is to date insufficient evidence of benefits in terms of substantive clinical outcomes to support the routine use of steroids for the management of HELLP. The use of corticosteroids may be justified in clinical situations in which increased rate of recovery in platelet count is considered clinically worthwhile.
Medical Subject Headings (MeSH): Adrenal Cortex Hormones [*therapeutic use], Betamethasone [therapeutic use], Dexamethasone [therapeutic use], HELLP Syndrome [*drug therapy; mortality], Maternal Mortality, Perinatal Mortality, Platelet Count, Prednisolone [therapeutic use], Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Description of the condition
Pre-eclampsia (also referred to as gestational hypertension with proteinuria, or proteinuric hypertension) is a serious complication of pregnancy characterised by increased blood pressure and protein in the urine. It develops in 5% to 7% of pregnancies and is associated with poor maternal and perinatal outcomes. Although the cause has not been definitively determined, the final common pathway is vascular endothelial dysfunction with activation of the clotting cascade (Roberts 2000).
The syndrome of hemolysis, elevated liver enzymes and low platelets (HELLP) is a severe manifestation of pre-eclampsia and complicates approximately 0.5% to 0.9% of all pregnancies and 10% to 20% of cases with severe pre-eclampsia (Haram 2009). For HELLP syndrome to be diagnosed, there must be microangiopathic hemolysis, thrombocytopenia, and abnormalities of liver function. There is no consensus, however, on the specific thresholds of hematologic and biochemical values to use in establishing the diagnosis of HELLP syndrome. Sibai has used the following criteria: hemolysis as evidenced by an abnormal peripheral smear, lactate dehydrogenase (LDH) greater than 600 IU/L, or total bilirubin greater than 20.52 μmol/L; elevated liver enzymes as evidenced by an aspartate transaminase (AST) greater than 70 IU/L and platelets less than 100,000 cells/mm3. Those women who do not have all of these parameters are considered to have partial HELLP syndrome (Sibai 1993; Sibai 2004). Martin defines HELLP syndrome as hemolysis evidenced by an increased LDH level and progressive anemia; hepatic dysfunction as evidenced by an LDH level over 600 IU/L; elevated liver enzymes as evidenced by an AST greater than 40 IU/L, an alanine transaminase (ALT) greater than 40 IU/L, or both; and thrombocytopenia evidenced by a platelet nadir less than 150,000 cells/mm3. Further subclassification proposed by Martin and known as the Mississippi HELLP Classification System classifies women based on the lowest perinatal platelet count: class one HELLP syndrome - platelet nadir less than or equal to 50,000 cells/mm3, class two HELLP syndrome - platelet nadir less than or equal to 100,000 cells/mm3, class three HELLP syndrome - platelet nadir less than or equal to 150,000 cells/mm3 (Martin 1991; Martin 1999).
We will consider trials for inclusion in this review if they specify a definition of HELLP syndrome which includes generally accepted diagnostic criteria for hemolysis, elevated liver enzymes and thrombocytopenia.
The presence of HELLP syndrome is associated with significant maternal mortality and morbidity including acute renal and liver failure, disseminated intravascular coagulopathy, pulmonary edema, cerebrovascular accident, and sepsis (Sibai 1993). Additionally, perinatal morbidity and mortality are also markedly high and are related primarily to the complications of prematurity and growth restriction (Visser 1995). Approximately 70% of pregnancies complicated by HELLP syndrome require preterm delivery, with 15% occurring at extremely preterm gestational age (before 27 completed weeks’ gestation) (Abramovici 1999).
Description of the intervention
The intervention evaluated was the use of corticosteroids for the treatment of maternal HELLP syndrome. Various regimens have been reported using dexamethasone or betamethasone. The purpose of this review was to summarize the evidence from randomized controlled trials (RCTs) examining the maternal and perinatal effects of corticosteroid administration in women with HELLP syndrome.
How the intervention might work
Since adverse perinatal outcomes are increased at preterm gestations, interventions that would allow the potential for pregnancy prolongation without negatively impacting the maternal condition could result in increased fetal maturity and subsequently decreased perinatal morbidity and mortality. Corticosteroids have been well established in controlled trials to decrease perinatal morbidity and mortality in the context of preterm birth, specifically by decreasing the risk of respiratory complications (Roberts 2006). Although the goal of corticosteroid administration in this setting is to promote fetal pulmonary maturation, improvements in maternal platelet count have also been reported (Vigil-De Gracia 1997).
Why it is important to do this review
HELLP syndrome is a severe complication of pregnancy with considerable maternal and perinatal morbidity and mortality. There are suggestions from observational studies that steroid treatment in HELLP syndrome may improve disordered maternal hematological and biochemical features and perhaps perinatal mortality and morbidity (Clark 1986; Magann 1993; Yeast 1987).
The Cochrane review by Matchaba and Moodley was last updated in 2004 (search date 2003), and two of the seven trials identified were awaiting translation/more information (Matchaba 2004). The five studies reviewed showed improved biochemical profiles with steroid therapy, but were insufficient in numbers to address clinical outcomes adequately. Further research was called for as a matter of urgency.
For more information on eclampsia and HELLP syndrome, please refer to the ‘Interventions for treating pre-eclampsia and its consequences: generic protocol’ (Duley 2009).
OBJECTIVES
To determine, from the best available evidence, the effects of corticosteroids on maternal and perinatal mortality and morbidity in women with HELLP syndrome.
METHODS
Criteria for considering studies for this review
Types of studies
All published, unpublished, and ongoing RCTs. We excluded quasi-randomized trials (e.g. those randomized by date of birth or hospital number) from the analysis due to a high potential for bias. We examined studies published only as abstracts and included them only if they contained enough information to meet the inclusion criteria.
Types of participants
Women with hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome, as determined clinically or based on biochemical markers, both during pregnancy and after delivery, and their babies.
Types of interventions
Any corticosteroid versus placebo, no treatment, or other drug; or corticosteroid versus other corticosteroid or other dosage.
Types of outcome measures
Only outcomes with available data appear in the analysis tables. To avoid losing valuable data, we also included trials that used acceptable variations of the definitions of primary and secondary outcomes specified below, along with those that did not state their definitions.
We noted outcome data that were not pre-specified by the review authors, but which were reported by the trial authors, as non pre-specified and did not include them in the analysis or use them for the conclusions.
Primary outcomes
For the mother
Maternal death or severe maternal morbidity, defined as any one of the following: presence of liver hematoma, rupture or persistent liver failure; pulmonary edema; renal failure; abruptio placentae; eclampsia; or cerebrovascular accident.
For the child
Perinatal death: stillbirths (death in utero at or after 20 weeks’ gestation), perinatal deaths (stillbirths plus deaths in the first week of life), death before discharge from hospital, neonatal deaths (death in the first 28 days after birth).
Death or severe perinatal morbidity, defined as any one of the following: respiratory distress syndrome (RDS) with/without ventilatory support required; intracerebral hemorrhage; necrotizing enterocolitis; care in a special care nursery for seven days or more; or severe neonatal encephalopathy.
Secondary outcomes
For the mother
Presence of liver hematoma or rupture or liver failure.
Pulmonary oedema.
Renal failure.
Abruptio placenta.
Eclampsia.
Cerebrovascular accident.
Elective delivery: induction of labor or elective caesarean section.
Caesarean section and caesarean section performed under general anaesthesia.
Postpartum hemorrhage defined as blood loss of 500 mL or greater.
Change in platelet count.
Side effects or adverse events: any side effects or adverse events related to the intervention or intervention stopped due to side effects.
Use of hospital resources: admission to intensive care unit, length of stay, cost of care, use of mechanical ventilation, dialysis.
Woman’s experience and views of the interventions: childbirth experience, physical and psychological trauma, postnatal depression, breastfeeding, mother-infant interaction, and attachment.
For the child
Time from enrolment to birth.
RDS with/without ventilatory support required.
Intracerebral hemorrhage.
Necrotizing enterocolitis.
Care in a special care nursery for seven days or more.
Preterm birth defined as birth before 37 completed weeks’ gestation.
Very preterm birth defined as birth before 33 completed weeks’ gestation.
Extremely preterm birth defined as birth before 27 completed weeks’ gestation.
Infection.
Retinopathy of prematurity.
Apgar score at five minutes: low (seven or less) and very low (four or less).
Use of hospital resources: admission to special care nursery, length of stay, cost of care, endotracheal intubation, use of mechanical ventilation.
Long-term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay and cerebral palsy.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 June 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, consulted a third person. We entered data into Review Manager software (RevMan 2008) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non-random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re-included missing data in the analyses which we undertook. We assessed methods as:
adequate;
inadequate:
unclear.
We considered adequate a level of missing data up to 20%. We judged studies missing more than 20% of outcome data as inadequate.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
adequate (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
inadequate (where not all the study’s pre-specified outcomes had been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest were reported incompletely and so could be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses - see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardized mean difference to combine trials that measured the same outcome, but used different methods. (The standardized mean difference is the difference between two means divided by an estimate of the within-group standard deviation which allows the standardized values to be combined since they have no units.)
Unit of analysis issues
We included only individually RCTs in this review.
For neonatal outcomes, we adjusted the results for multiple pregnancies for clustering if sufficient information was available. If not, we performed sensitivity analysis to assess the impact of assuming independence or non-independence of the multiple birth babies. For neonatal outcomes, the denominator was the number of individual babies randomized rather than the number of pregnancies. In the case of trials comparing more than one type or dose of drug with placebo, each drug was compared individually with the placebo group. Where more than one comparison was included in the same analysis, the numbers in the placebo group were divided by the number of comparisons to avoid double-counting of cases.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention-to-treat basis, i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We used the I2, T2 and Chi2 statistics to examine heterogeneity among the trials in each analysis. If we identified substantial heterogeneity we explored it by pre-specified subgroup analysis. Heterogeneity greater than 50% as measured by the I2 statistic was considered substantial.
Assessment of reporting biases
Where we suspected reporting bias (see ‘Selective reporting bias’ above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2008). We used fixed-effect inverse variance meta-analysis for combining data where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials, we used random-effects meta-analysis.
If substantial heterogeneity was identified in a fixed-effect meta-analysis we noted this and repeated the analysis using a random-effects method. With random-effects analysis the point estimate represents the average treatment effect.
Subgroup analysis and investigation of heterogeneity
Where data were available we planned to carry out the following subgroup analyses.
Gestation at trial entry: greater than 37 completed weeks’ gestation, between 33 and 37 completed weeks’ gestation, between 27 and 33 completed weeks’ gestation, and less than 27 completed weeks’ gestation.
Type of intervention: type or class, dose, or duration of corticosteroid.
Corticosteroid versus no treatment, corticosteroid versus placebo.
We planned to use the following outcomes in subgroup analysis.
For the mother
Maternal death (during pregnancy or up to 42 days after end of pregnancy) or severe maternal morbidity, defined as any one of the following: presence of liver hematoma, rupture or liver failure; pulmonary edema; renal failure; abruptio placentae; eclampsia; or cerebrovascular accident.
For the child
Perinatal death: stillbirths (death in utero at or after 20 weeks’ gestation), perinatal deaths (stillbirths plus deaths in the first week of life), death before discharge from hospital, neonatal deaths (death in the first 28 days after birth).
Death or severe perinatal morbidity, defined as any one of the following: RDS with/without ventilatory support required; intracerebral hemorrhage; necrotizing enterocolitis; care in a special care nursery for seven days or more; or severe neonatal encephalopathy.
Time from enrolment to birth.
Severity of preterm birth (gestational age at time of birth). For fixed-effect meta-analyses we planned to conduct subgroup analyses classifying whole trials by interaction tests as described by (Deeks 2001). For random-effects meta-analyses we planned to assess differences between subgroups by inspection of the subgroups’ confidence intervals; non-overlapping confidence intervals indicating a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
We used sensitivity analysis when any significant sources of bias were identified in the methods of this review or of included studies.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search of the Pregnancy and Childbirth Group’s Trials Register found 24 reports representing 16 different studies (several studies resulted in more than one publication or report). After assessing eligibility we included 13 studies and excluded one (Barrilleaux 2005); we are seeking further information on one study before assessing eligibility (Morrison 1992); and awaiting translation of one study (Borekci 2008).
Included studies
All of the included studies recruited women with a diagnosis of hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. Criteria for recruitment in eight of the studies was a diagnosis of HELLP class one or two on the Mississippi HELLP classification system as defined by Martin 1999 (hemolysis (lactate dehydrogenase (LDH) greater than 600 IU/L), hepatic dysfunction (alanine transaminase (ALT) or aspartate transaminase (AST) greater than 70 IU/L), thrombocytopenia (platelets below 100,000 cells/mm3)), (Bouchnak 2005; Fonseca 2005; Isler 2001; Isler 2003; Magann 1994; Magann 1994a; Van Runnard 2006; Yalcin 1998). In the study by Katz 2008 85.7% of the women recruited were diagnosed with HELLP class one or two, although the sample also included women with less severe symptoms and where women had abnormal findings for at least one of the laboratory parameters used for diagnosis (described as partial cases). Ozer 2009 and Vigil-De 1997 recruited women with HELLP classes one, two, and three (i.e. with platelets below 150,000 cells/mm3) and it was not clear how many women were included with less severe disease. In two studies the diagnostic criteria were less clear (Kadanali 1997; Mould 2006).
In five of the included studies pregnant women were recruited, and treatment commenced, in the antenatal period (Isler 2001; Kadanali 1997; Magann 1994; Ozer 2009; Van Runnard 2006). In the study by Fonseca 2005 women were recruited and treatment commenced both antenatally and postnatally. In the remaining studies corticosteroid treatment was commenced just after delivery or in the postnatal period (although the diagnoses of HELLP syndrome may have been made before delivery) (Bouchnak 2005; Isler 2003; Katz 2008; Magann 1994a; Mould 2006; Vigil-De 1997; Yalcin 1998).
In the studies by Isler 2001 and Isler 2003, two different corticosteroids were compared (dexamethasone versus betamethasone). In all of the remaining studies corticosteroid therapy (with dexamethasone, betamethasone or prednisolone) was compared with placebo or no treatment. The drugs used in trials, routes of administration, dosing regimens, duration of treatment and criteria for discontinuation of treatment are set out in the Characteristics of included studies tables.
In most of the studies it was stated that women also received other standard treatment as required including magnesium sulfate and anti-hypertensive drugs, as well as standard doses of corticosteroids antenatally for fetal lung maturation in some studies.
Excluded studies
We excluded one study identified in our search as it did not meet our inclusion criteria for participants. It assessed the effect of steroids in severely pre-eclamptic women without HELLP syndrome (Barrilleaux 2005).
Risk of bias in included studies
The overall quality of these studies was very mixed, and this should be taken into account in the interpretation or results. We have set out details of the risk of bias assessment for each study in the Characteristics of included studies tables.
Allocation
Two of the included studies used random number tables to generate the allocation sequence (Isler 2001; Isler 2003) and four used computer-generated randomization sequences (Katz 2008; Magann 1994; Magann 1994a; Van Runnard 2006). Fonseca 2005 described using a block design (block size four) although it was not clear how the sequence was decided. In the remaining studies the method used to generate the randomization order was not described, or was not clear (Bouchnak 2005; Kadanali 1997; Mould 2006; Vigil-De 1997; Yalcin 1998).
In five of the included trials authors described using sealed opaque sequentially numbered envelopes to conceal the allocation sequence (Fonseca 2005; Isler 2001; Magann 1994; Magann 1994a; Ozer 2009) and Mould 2006 referred to the “sealed envelope system”. In two studies randomization was carried out by pharmacy, and in these placebo controlled studies drugs were provided in coded containers to conceal treatment group allocation (Katz 2008; Van Runnard 2006). In the Isler 2003 trial, group allocation was also described as being concealed by the use of sealed opaque envelopes; however, a number of women (it was not clear how many) were not assigned to groups on a random basis, but rather according to the type of corticosteroid treatment they had previously received; this is a potentially serious source of bias in this study.
The methods used to describe allocation concealment were not described in the trials by Bouchnak 2005, Kadanali 1997, Vigil-De 1997 and Yalcin 1998.
Blinding
Four of the included studies were placebo controlled trials, and women and clinical staff were likely to have been unaware of treatment group (Bouchnak 2005; Fonseca 2005; Katz 2008; Van Runnard 2006).
In the remaining studies, women in the two arms of the trials received either different types of corticosteroid treatment or women in control groups received no corticosteroid treatment, and treatment blinding was either not attempted or was not feasible (Isler 2001; Isler 2003; Kadanali 1997; Magann 1994; Magann 1994a; Mould 2006; Ozer 2009; Vigil-De 1997; Yalcin 1998). The lack of treatment blinding is a potentially serious source of bias in these trials as decisions about interventions (e.g. labor induction) may have been affected by knowledge of group allocation, and such clinical decisions may have had an impact on outcomes for mothers and babies.
Incomplete outcome data
Loss to follow up or missing data was not always well described in these studies, but in most trials all or most women appeared to be accounted for in the analysis. In the study by Magann 1994a, while all women were included in the analysis, for some outcomes (e.g. laboratory values) there were relatively high levels of missing data. In the study by Mould 2006 19% of the original sample were lost to follow up because of protocol violations or missing data, and there was no mention of an intention-to-treat analysis being carried out.
Selective reporting
We found it difficult to assess outcome reporting bias as study protocols were not available for most trials and study data were obtained directly from published reports; without access to original protocols it is difficult to determine whether results are reported for all study outcomes. Although most of the studies provided data on laboratory parameters it was surprising to us that morbidity (and even mortality) for mothers and babies were not reported in all of these trials.
We were not able to assess publication bias as there were too few studies contributing data to the analyses.
Other potential sources of bias
In two studies, data extraction and analysis was carried out from translation notes and our assessments of risk of bias are likely to be limited (Bouchnak 2005; Kadanali 1997).
In the studies by Isler 2003, Magann 1994, Magann 1994a and Vigil-De 1997 there appeared to be some baseline imbalance between groups at the commencement of treatment (either in disease severity or participant characteristics); it is difficult to assess the impact (if any) of such imbalance on results. For more details please see the full risk of bias assessments in the Characteristics of included studies tables.
Effects of interventions
We examined the effects of the interventions through two comparisons: the effect of corticosteroid versus placebo or no treatment, and the effect of one corticosteroid versus another corticosteroid. Within these comparisons we carried out subgroup analyses based on time of commencement of treatment - treatment commenced antenatally, treatment commenced postnatally, and treatment commencement mixed or uncertain. (The protocol of this review stated that we would perform subgroup analysis based on gestation at trial entry. However, methods of the identified trials varied considerably, and data were not available to allow for meaningful analysis based on gestation at trial entry. Therefore our analysis of subgroups was solely based on whether treatment was commenced antenatally, postnatally, or mixed or uncertain.) There were no studies that compared different doses of the same corticosteroid. All outcomes relating to the child are from trials that commenced treatment in the mother antenatally.
We assessed 48 outcomes based on the data of the included studies. We performed meta-analysis on 21 of the 48 outcomes as many of the prespecified outcomes were only described in a single study.
1. Corticosteroid versus placebo or control
A. Pre-specified outcomes
Maternal death
For the four trials (362 women) reporting maternal death, the risk ratio (RR) was 0.95 (95% confidence intervals (CI) 0.28 to 3.21). This lack of evidence for any overall effect was consistent across all subgroups.
Maternal death or severe maternal morbidity
Three trials (278 women) reported maternal death and severe maternal morbidity as defined above as the presence of liver hematoma, rupture or persistent liver failure; pulmonary oedema; persistent renal failure; abruptio placentae; eclampsia; or cerebrovascular accident. These trials included Fonseca 2005, Katz 2008, and Van Runnard 2006. Only Van Runnard 2006 provided sufficient information to determine if the recorded maternal deaths and maternal morbidity were mutually exclusive. The data provided by Fonseca 2005 and Katz 2008 did not allow us to determine whether events were mutually exclusive, or if multiple events could have occurred in the same woman. Both Fonseca 2005 and Katz 2008 found no evidence of any difference between treatment groups in respect to maternal deaths or individual maternal complications considered in the above definition of severe maternal morbidity. The one remaining trial (31 women), found no overall difference in the risk of maternal death or severe maternal morbidity (RR 0.27, 95% CI 0.03 to 2.12) (Van Runnard 2006).
Perinatal/infant death
Two studies (58 participants) reported perinatal or infant death (Magann 1994; Van Runnard 2006). There was no clear difference in perinatal/infant death between the two groups when treatment was commenced antenatally (RR 0.64, 95% CI 0.21 to 1.97).
Perinatal death or severe perinatal morbidity
Two trials (77 infants), Magann 1994 and Van Runnard 2006, reported perinatal death and perinatal morbidity as defined above as RDS with/without ventilatory support required; intracerebral hemorrhage; necrotizing enterocolitis; care in a special care nursery for seven days or more; or severe neonatal encephalopathy. Both studies administered corticosteroids to the mother antenatally. In each trial there was insufficient information to determine if the recorded outcomes and deaths were mutually exclusive. It was therefore not possible to create a composite outcome from these data. Neither study, however, found evidence of a difference between treatment groups in respect to perinatal death or any individual infant outcomes included in the above definition of severe perinatal morbidity.
Maternal liver hematoma, rupture, or failure
For the two trials (91 women) reporting maternal liver hematoma, rupture or failure the RR was 0.22 (95% CI 0.03 to 1.83). This demonstrated a lack of evidence for corticosteroids administered antenatally for HELLP syndrome. There were no studies that examined maternal liver hematoma, rupture or failure when treatment with corticosteroids was commenced postnatally, or when treatment commencement was mixed or uncertain.
Maternal pulmonary edema
There was no overall difference in maternal pulmonary edema in the three trials (297 women) that reported this outcome (RR 0.77, 95% CI 0.24 to 2.48). This lack of evidence was consistent across subgroups.
Maternal renal failure
Three trials (297 women) reported maternal renal failure. The RR for renal failure was 0.69 (95% CI 0.39 to 1.22) and this apparent lack of effect was similar across subgroups.
Eclampsia
Only one study (132 women) with corticosteroid commencement both antenatally and postnatally assessed eclampsia. There was no clear evidence of any difference between groups (RR 0.80, 95% CI 0.34 to 1.90).
Caesarean section or elective delivery including induction of labor
No clear effect was seen in two trials (46 women) that assessed the number of caesarean sections or elective deliveries. Both trials administered corticosteroids antenatally and found a RR of 1.01 (95% CI 0.79 to 1.29) (random effects analysis; heterogeneity: I2 = 56%, Tau2 = 0.02, Chi2 test for heterogeneity P = 0.13).
Length of stay in hospital or obstetrical delivery room for the mother
Five trials (354 women) assessed length of stay in hospital or in the obstetrical room. When meta-analysis was performed there was no effect shown overall (mean difference (MD) −1.15, 95% CI −2.77 to 0.46), (random effects analysis; heterogeneity; I2 = 63%, Tau2 = 1.87, Chi2 test for heterogeneity P = 0.03), and no effect in any subgroup individually. Sensitivity analysis showed only a single trial which demonstrated any effect (Yalcin 1998) but there was no indication to consider this trial alone in the subgroup of trials in which treatment was commenced postnatally. Two studies examined the effect of corticosteroids initiated postnatally (Katz 2008; Yalcin 1998) and the trial by Yalcin 1998 was a lower power trial and possibly affected by performance and selection bias as the randomization method was unclear as well as whether blinding was present. The Katz 2008 trial had a low risk of bias.
Need for dialysis
There was no evidence of effect in respect to need for dialysis in the one trial that assessed this outcome in women who received treatment antenatally (RR 3.00, 95% CI 0.13 to 70.83). There were no studies that examined need for dialysis when treatment with corticosteroids was commenced postnatally, or when treatment commencement was mixed or uncertain.
Time from enrolment to birth (hours)
Three trials (118 women) that commenced treatment with corticosteroids antenatally assessed the time from enrolment to birth. There was a high level of heterogeneity for this outcome suggesting different standards of care or differing treatment protocols and therefore we did not perform meta-analysis with data from these three trials. Each of the three trials (Magann 1994; Ozer 2009; Van Runnard 2006) assessed the effect of a different corticosteroid, (dexamethasone, betamethasone, and prednisolone respectively) commenced antenatally for HELLP syndrome. The Magann 1994 trial (25 women) was the only trial that assessed the effect of dexamethasone and while it did find a difference between groups (MD 26.00, 95% CI 17.17 to 34.83), this trial showed some baseline imbalance and was not blinded making it subject to performance bias. Additionally, this trial sample was too small (25 women) for any reliable conclusions about potential differential effects. No evidence of a treatment effect was seen in the remaining two trials. The Ozer 2009 trial (60 women) assessed the effect of betamethasone compared with placebo commenced antenatally and found no difference between treatment groups (MD 5.30, 95% CI −4.28 to 14.88) while the Van Runnard 2006 trial (33 women) assessed the effect of prednisolone compared with placebo commenced antenatally and found no evidence of effect (MD −26.40, 95% CI −135.61 to 82.81).
Abruptio placenta
There was no clear evidence of a difference between treatment groups in respect to frequency of abruptio placenta in women who had received corticosteroids antenatally in one trial (31 women). The RR was 1.07 (95% CI 0.07 to 15.57).
RDS with/without ventilatory support
There was no clear effect seen between treatment groups in regard to the frequency of RDS in two studies (58 infants) that assessed this outcome in infants of women who received treatment antenatally (RR 0.95, 95% CI 0.45 to 2.03).
Intracerebral hemorrhage
In two trials (58 infants) that measured the effect of corticosteroids on intracerebral hemorrhage the RR was 2.31 (95% CI 0.58 to 9.28). This lack of evidence was found in infants of women who commenced treatment antenatally.
Necrotizing enterocolitis
There was no clear evidence of any difference between groups in the one trial (33 infants) that reported necrotizing enterocolitis in infants of mothers who had received corticosteroids antenatally (RR 0.21, 95% CI 0.01 to 4.10).
Gestational age at delivery
One trial (33 infants) measured gestational age at delivery. There was no clear effect seen between the treatment groups in this trial (MD −0.30, 95% CI −1.30 to 0.70).
Retinopathy of prematurity/retrolental fibroplasia
In the one trial (25 infants) that measured retinopathy of prematurity/retrolental fibroplasia in infants of women who had received corticosteroids antenatally the RR was 0.36 (95% CI 0.02 to 8.05). This outcome does not indicate a clear difference between treatment groups.
Apgar score at five minutes less than seven
There was no differential effect seen in the two trials (58 infants) that measured Apgar scores at five minutes. The RR for an Apgar score less than seven was 0.89 (95% CI 0.27 to 2.95) in infants whose mothers had received corticosteroids antenatally.
Length of stay in hospital or special care nursery/neonatal intensive care unit
The MD in length of stay in hospital or special care nursery/neonatal intensive care unit in one trial (33 infants) was −3.80 (95% CI −19.60 to 12.00). This finding does not indicate a clear difference between treatment groups.
Long-term growth and development - head circumference less than two SD at 24 months
One trial (33 infants) measured the long-term effect of antenatal corticosteroids on growth and development of the infant at 24 months. No clear effect was seen in respect to head circumference less than two SD with a RR of 5.00 (95% CI 0.27 to 92.62).
Long-term growth and development - abnormal Griffiths or BSID Scales at 24 months
One trial (33 infants) measured the long-term effect of antenatal corticosteroids on growth and development of the infant at 24 months. No clear effect was seen in respect to abnormal Griffiths or BSID Scales with a RR of 0.75 (95% CI 0.22 to 2.52).
Days of mechanical ventilation required
No clear evidence of effect was seen between groups in respect to the number of days of mechanical ventilation required. In one trial (33 infants) the MD was 0.80 (95% CI −9.10 to 10.70).
Platelet count, change in platelet count, or rate of change of platelet count
Three trials (90 women) measured the effect of corticosteroids commenced both antenatally and postnatally on the absolute platelet count, change in platelet count, or rate of change of platelet count. As there were significant methodological differences between studies the effect on platelets was analysed using the standardized mean difference (SMD). There was clear evidence of an effect on platelets in women who received corticosteroids versus women who received placebo or no treatment. The overall effect demonstrated a SMD of 0.67 (95% CI 0.24 to 1.10). The strongest evidence is found in the group of women who commenced treatment antenatally; there was no clear evidence of an effect on platelet count on women who started treatment postnatally (one study, 17 women, SMD in platelet count at 72 hours postpartum 0.47, 95% CI −0.21 to 1.16). By contrast, in the group in which treatment was commenced antenatally, there was evidence of a treatment effect (SMD 0.80, 95% CI 0.25 to 1.35).
B. Non pre-specified outcomes
Several studies comparing the effect of corticosteroid against placebo or control also measured other hematologic outcomes that were not pre-specified in the protocol of this review but will be reported here. These include rates of change or serum levels of AST, ALT, and LDH as well as blood pressure and urinary output.
AST level or rate of change of AST
Two trials (56 women) measured the effect of corticosteroids commenced antenatally on AST level. Magann 1994 reported a rate of change of AST (units/hour) and Van Runnard 2006 reported AST levels (U/L) at nadirs following the start of trial medication. The combined data demonstrate a treatment effect with a SMD of −0.55 (95% CI −1.09 to −0.22).
ALT level or rate of change of ALT
Two trials (56 women) measured the effect of corticosteroids commenced antenatally on ALT level. Magann 1994 reported a rate of change of ALT (units/hour) and Van Runnard 2006 reported ALT levels (U/L) at nadirs following the start of trial medication. The combined data demonstrate a treatment effect with a SMD of −0.58 (95% CI −1.12 to −0.04).
LDH level or rate of change of LDH
Two trials (56 women) measured the effect of corticosteroids commenced antenatally on LDH level. Magann 1994 reported a rate of change of LDH (units/hour) and Van Runnard 2006 reported LDH levels (U/L) at nadirs following the start of trial medication. The combined data demonstrate a treatment effect with a SMD of −0.76 (95% CI −1.40 to −0.11).
Diastolic blood pressure or rate of change of mean arterial pressure
Two trials (56 women) measured the effect of corticosteroids commenced antenatally on blood pressure. Magann 1994 reported a rate of change of mean arterial pressure (units/hour) and Van Runnard 2006 reported mean diastolic blood pressure levels (mmHg). There was no clear difference demonstrated between groups with a SMD of −0.26 (95% CI −0.79 to 0.27).
Rate of change of urinary output
Only one trial (25 women) measured the effect of dexamethasone on the rate of change of urinary output in women with HELLP syndrome. Magann 1994 demonstrated a clear benefit in patients who received dexamethasone as compared to control with a MD of 3.49 (95% CI 1.83 to 5.15).
Planned subgroup analysis
For the review’s primary outcomes we had planned to carry out subgroup analysis by gestation at trial entry, by the dose and duration of the intervention, and by corticosteroid versus no treatment and corticosteroid versus placebo. In this version of the review insufficient data were available to allow us to carry out some of these additional analyses. If more data become available, we will carry out the planned analyses in future updates.
2. Dexamthasone versus betamethasone
A. Pre-specified outcomes
There was only one trial (Isler 2001) with 40 women (43 infants) that compared the effects of dexamethasone with betamethasone with treatment commenced antenatally. A second trial (Isler 2003), with 36 women, compared dexamethasone with betamethasone but commenced treatment postnatally. Participants included women with class one or class two HELLP syndrome at more than 22 weeks’ gestational age. Outcomes are therefore applicable to this group. The only pre-specified outcome that was examined by both studies was the adjusted time averaged change in platelet count. All other data are derived solely from the Isler 2001 trial which assessed treatment commenced antenatally.
i. Treatment commenced antenatally (Isler 2001)
There was no clear difference found between treatment groups receiving dexamethasone versus betamethasone in all pre-specified outcomes reported in this trial - details are listed below.
Maternal death
No maternal deaths were reported in either the dexamethasone or betamethasone group.
Severe maternal morbidity
No maternal outcomes were reported that met the pre-specified definition of severe maternal morbidity.
Perinatal/infant death
No difference was found in the rates of perinatal/infant death (43 infants) (RR 0.95, 95% CI 0.15 to 6.17).
Severe perinatal/infant morbidity or death
No difference was found in the rates of severe perinatal/infant morbidity or death (43 infants) (RR 0.64, 95% CI 0.27 to 1.48). The only measured outcome that met the definition of severe perinatal/infant morbidity was infant RDS. In the dexamethasone group there were four infants with RDS, in the betamethasone groups there were seven infants with RDS. Additionally, there were two deaths in the dexamethasone group, one intrauterine fetal death and one death from extreme prematurity. In the betamethasone group there were two deaths from extreme prematurity. From the text of the paper it was understood that these events were mutually exclusive. In the case that some or all of these deaths occurred in infants who had also been recorded as having RDS, sensitivity analysis was undertaken which showed no change in treatment effect.
Caesarean section
No difference was found in the caesarean section rates: 40 women (RR 0.79, 95% CI 0.47 to 1.33).
Length of stay in hospital or obstetrical delivery room for the mother
No difference found: 40 women (MD −7.50, 95% CI −24.29 to 9.29).
RDS with/without ventilatory support
No difference found: 43 infants (RR 0.55, 95% CI 0.19 to 1.60).
Gestational age at delivery
No difference found: 43 infants (MD −0.60, 95% CI −3.35 to 2.15).
Fetal sepsis or infection
No difference found: 43 infants (RR 4.78, 95% CI 0.24 to 94.12).
Apgar score at five minutes less than seven
No difference found: 43 infants (RR 0.95, 95% CI 0.22 to 4.21).
Length of stay in hospital or special care nursery/neonatal intensive care unit
No difference found: 43 infants (MD −5.40, 95% CI −18.86 to 8.06).
Use of mechanical ventilation
No difference found: 43 infants (RR 0.55, 95% CI 0.19 to 1.60).
ii. Treatment commenced antenatally (Isler 2001) and postnatally (Isler 2003)
Adjusted time averaged change in platelet count
There was clear evidence of a difference in effect between groups comparing dexamethasone with betamethasone in respect to the adjusted time averaged change in platelet count. Dexamethasone was found to be superior compared with betamethasone although there were high levels of heterogeneity of this outcome (76 women) (MD 6.02, 95% CI 1.71 to 10.33), (random-effects analysis; heterogeneity: I2 = 85%, Tau2 = 8.25, Chi2 test for heterogeneity P = 0.009). Subgroup analysis demonstrated this effect both when treatment was commenced antenatally, (MD 8.10, 95% CI 6.23 to 9.97) and when treatment was commenced postnatally (MD 3.70, 95% CI 0.96 to 6.44).
B. Non pre-specified outcomes
Isler 2001 (40 women) and Isler 2003 (36 women) also reported several other hematologic outcomes that were not pre-specified in the protocol of this review but will be reported here. These include adjusted time averaged change in AST level, LDH level, mean arterial pressure and urinary output. There were high levels of heterogeneity for several of these outcomes.
Adjusted time averaged change in AST level
There were differences in adjusted time averaged change in AST level between groups treated with dexamethasone and betamethasone although the overall difference between groups was not statistically significant (76 women) (MD −18.80, 95% CI −41.34 to 3.74), (random-effects analysis; heterogeneity: I2 = 97%, Tau2 = 255.79, Chi2 test for heterogeneity P < 0.00001).
Adjusted time averaged change in LDH level
Dexamethasone was found to be superior to betamethasone in respect to adjusted time averaged change in LDH level (76 women) (MD −73.40, 95% CI −113.13 to −33.67), (random-effects analysis; heterogeneity: I2 = 58%, Tau2 = 478.14, Chi2 test for heterogeneity P = 0.12).
Adjusted time averaged change in mean arterial pressure
There was clear evidence of a superior effect of dexamethasone as compared to betamethasone in relation to adjusted time averaged change in mean arterial pressure (76 women) (MD −7.64, 95% CI −8.27 to −7.01).
Adjusted time averaged change in urinary output
Greater treatment effect was evidenced in patients receiving dexamethasone as compared to patients receiving betamethasone in respect to adjusted time averaged change in urinary output (76 women) (MD 16.73, 95% CI 0.47 to 32.99), (random-effects analysis; heterogeneity: I2 = 92%, Tau2 = 126.89, Chi2 test for heterogeneity P = 0.0004).
DISCUSSION
Summary of main results
Corticosteroid versus placebo or no treatment
Pre-specified outcomes: there was no clear evidence of any treatment effect of corticosteroids on substantive clinical outcomes. Those receiving steroids showed significantly greater improvement in platelet counts.
Non pre-specified outcomes: there were also significantly greater improvements in aspartate transaminase, alanine transaminase (ALT), lactate dehydrogenase (LDH), and urinary output in women receiving corticosteroids.
Dexamethasone versus betamethasone
Pre-specified outcomes: there was no clear evidence of any difference between groups receiving dexamethasone versus betamethasone in respect to substantive clinical outcomes. Those receiving dexamethasone showed significantly greater improvements in platelet count than those receiving betamethasone.
Non pre-specified outcomes: those receiving dexamethasone versus betamethasone showed significantly greater improvements in LDH level, blood pressure, and urinary output. No difference was found between steroids in respect to ALT level.
Overall completeness and applicability of evidence
A limitation of the evidence was the limited number of outcomes reported in various trials, and different methods of measurement of continuous data.
Quality of the evidence
The trials were of varying quality; however, there was general consistency in the results.
Potential biases in the review process
The possibility of reporting bias could not be excluded, as not all trials reported all relevant outcomes.
Agreements and disagreements with other studies or reviews
The results of this review are consistent with previous findings reported by Matchaba and Moodley in a 2004 systematic review of the effect of corticosteroids in hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome (Matchaba 2004). They concluded that there was insufficient evidence to support the addition of corticosteroids to standard therapy in HELLP syndrome.
AUTHORS’ CONCLUSIONS
Implications for practice
There is to date insufficient evidence of benefits in terms of substantive clinical outcomes to support the use of steroids for the management of hemolysis, elevated liver enzymes and low platelets syndrome in routine clinical practice. The use of corticosteroids may be justified in clinical situations in which an increase, or increased rate of recovery, in platelet count is considered clinically worthwhile
Implications for research
The consistently greater improvement in platelet counts is reason for further research in this area. Future trials should be sufficiently powered to detect clinically meaningful differences in health outcomes. The very limited data reviewed here suggests that dexamethasone should be tested in preference to betamethasone in such trials.
PLAIN LANGUAGE SUMMARY.
Corticosteroids for HELLP syndrome in pregnancy
Pre-eclampsia is a serious complication of pregnancy characterized by high blood pressure with protein in the urine and sometimes progression to seizures (fits). HELLP syndrome is a more severe form of pre-eclampsia which can cause problems with liver function, blood clotting, and low platelets. HELLP may be diagnosed during pregnancy or after giving birth and is associated with ill health for the mother including liver hematoma, rupture, or failure; pulmonary edema; renal failure and death. Infant health may also be poor, primarily due to premature birth and growth restriction.This review examined the effect of treating women with HELLP syndrome using corticosteroids (which can reduce inflammation). The results of this review did not indicate that there was a clear effect on the health of pregnant women when treated with corticosteroids, or their babies. Corticosteroids did appear to improve some components of the women’s blood tests, but it is not clear that this had an effect on their overall health. The review identified 11 randomized controlled trials involving 550 women that compared corticosteroid (dexamethasone, betamethasone, or prednisolone) given during pregnancy, just after delivery or in the postnatal period, or both before and after birth, with placebo or no treatment. Two further trials showed that there was no clear difference between dexamethasone and betamethasone on the substantive clinical outcomes for women or their infants. Dexamethasone did improve maternal platelet count and some biochemical measures to a greater extent than betamethasone.
ACKNOWLEDGEMENTS
Translation of non-English studies was provided by volunteer translators through the Cochrane group. Alex Balistreri provided a translated summary of Kadanali 1997 and Alison Ledward provided a translated summary of Bouchnak 2005.
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
Department of Obstetrics and Gynaecology, University of Alberta, Canada. Support for summer studentship for primary author
The University of Liverpool, UK.
External sources
National Institute for Health Research (NIHR), UK.
TD is supported by the NIHR NHS Cochrane Collaboration Programme grant scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Double blinded, RCT. | |
| Participants | 20 women with pregnancy complicated by HELLP syndrome either antepartum or postpartum | |
| Interventions | Dexamethasone, 12 mg q12h × 2 doses, started immediately following delivery Control group: placebo. |
|
| Outcomes | Change in platelet count, change in hemolysis markers (LDH), change in hepatic cytolysis markers (AST), maternal morbidity | |
| Notes | This study was reported in French and a summarized English translation was provided | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Yes | (Placebo controlled trial) blinding adequate for participants and personnel, inadequate for outcome assessors |
| Incomplete outcome data addressed? All outcomes |
Yes | All women appear to be accounted for in the analysis. |
| Free of selective reporting? | Unclear | Not able to assess. |
| Overall risk of bias? | Unclear | Risk of bias difficult to assess from translation notes. |
| Methods | Prospective, double blind, placebo controlled randomized clinical trial | |
| Participants | 132 eligible women with gestational age > 20 weeks (60 pregnant, 72 postpartum, mean age 25.3 years (range 14-44), mean parity 2.4, gestation 20-41 weeks) Inclusion criteria: women diagnosed with class 1 or 2 HELLP syndrome pregnant or just after delivery (within 3 days of delivery) Exclusion criteria: oral temperature > 37.5 degrees celsius, diabetic ketoacidosis, for women postpartum - more than 24 hours elapsed since diagnosis (28% of the sample (women with GA 26-36 weeks) had betamethasone in the 2 weeks before delivery for fetal lung maturation.) |
|
| Interventions | Intervention: dexamethasone (pregnant women 10 mg doses IV every 12 hours until delivery and 3 doses after delivery; postpartum women 3 10 mg doses) Comparison group: placebo (sterile water IV) same regimen. Treatment was discontinued if temp rose above 37.5c. All women received 1-1.5 g/hr of magnesium sulfate IV and anti-hypertensive and/or hydration therapy as required |
|
| Outcomes | Duration of hospital stay (randomization to discharge). Platelet count > 100,000 cells/mm3, LDH, AST. Complications: acute renal failure, oliguria, pulmonary edema, eclampsia, infections, maternal death, need for platelet transfusion, need for plasma transfusion |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Stratified permuted blocks of 4. |
| Allocation concealment? | Yes | Consecutively numbered opaque envelopes, with active and placebo preparations packed in identical vials in sealed, coded boxes |
| Blinding? All outcomes |
Yes | Adequate for women, staff and outcome assessors; placebo controlled trial with codes not broken until after analysis |
| Incomplete outcome data addressed? All outcomes |
Yes | Adequate; 132 women were randomized, there were 6 protocol violations, (4 in intervention group and 2 in placebo) but authors report that they carried out an ITT analysis |
| Free of other bias? | Yes | No baseline imbalance apparent. |
| Overall risk of bias? | No | Low risk of bias. |
| Methods | Prospective, randomized clinical trial. | |
| Participants | 40 pregnant women (gestational age > 22 weeks) with a diagnosis of HELLP (HELLP defined as hemolysis (LDH > 600 IU/L) hepatic dysfunction (AST > 70 IU/L) thrombocytopenia (platelets < 100,000 cells/mm3). Class 1 and 2 HELLP syndrome Exclusions: gestational age < 22 weeks, women with diabetes, women who had had recent (within 7 days) corticosteroid therapy for fetal lung maturation or for maternal morbidity, women with class 3 HELLP not included |
|
| Interventions | Group 1: 12 mg combination of betamethasone acetate and betamethasone sodium phosphate IM every 24 hrs Group 2: 10 mg dexamethasone sodium phosphate IV every 12 hrs Treatment in both groups discontinued if symptoms resolved (no headache, vomiting, epigastric pain, platelet count > 100,000 cells/mm3 or 2 tests showing improvement) All women underwent IOL or where indicated CS. All received magnesium sulfate and IV fluids |
|
| Outcomes | Time-averaged changes in laboratory parameters (platelet count, LDH, AST) Clinical symptoms and indicators: headache, vision disturbance, epigastric pain, nausea, pulmonary edema, platelet transfusion, acute hypertensive therapy, oliguria, MAP Neonatal outcomes: gestational age at birth, birthweight, Apgar score at 5 minutes, need for ventilator, time to discharge, serious morbidity (ARDS, intraventricular hemorrhage, necrotizing enterocolitis, sepsis, hyperbilirubinemia) fetal or neonatal death |
|
| Notes | RESULTS: “Because patients had varying lengths of stay, dependent on individual clinical courses, the time-averaged change from baseline was computed for each of the above parameters. For each parameter, each patient’s post-baseline readings were compressed into a single measurement using trapezoid rule” | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table. |
| Allocation concealment? | Yes | Sequentially numbered, sealed opaque envelopes. |
| Blinding? All outcomes |
No | Different routes of drug administration. Not attempted. |
| Incomplete outcome data addressed? All outcomes |
Yes | All women accounted for in the analysis. |
| Free of other bias? | Yes | Baseline characteristics appear similar. |
| Overall risk of bias? | Unclear | Low risk of bias, possible performance bias with lack of blinding |
| Methods | Prospective mixed randomized/non-randomized clinical investigation | |
| Participants | 36 women with diagnosis of HELLP syndrome first manifesting in the postpartum period who had delivered at > 22 weeks (HELLP defined as hemolysis (LDH > 600 iu/l) hepatic dysfunction (AST > 40 IU/L). thrombocytopenia (platelets < 100,000 cells/mm3) with no underlying vascular disease Exclusions: women who developed HELLP antenatally, delivered before 22 weeks, required insulin therapy for diabetes or had evidence of infection at delivery |
|
| Interventions | Group 1: dexamethasone sodium phosphate 10 mg every 12 hours until criteria for discontinuation fulfilled Group 2: betamethasone 12 mg IM every 24 hrs until criteria for discontinuation fulfilled Criteria for discontinuation: symptoms resolved and lab tests returning to normal. No headache, vomiting, epigastric pain, stable BP ( < 160/110 mmHg without hypertensive drugs) platelet count > 100,000 cells/mm3 or 2 successive blood tests indicating upward trend (6 hr interval) AST and LDH downward trend, urine output > 50 ml/hr Both groups were intensively monitored and received IV magnesium sulfate for 24 hrs and anti-hypertensive medications as required (arterial pressure > 125 mm Hg) |
|
| Outcomes | Normalisation of MAP and urinary output; median duration of stay in special care (stable symptoms), urine output, lab parameters (time averaged changes in platelet count, LDH, AST) | |
| Notes | “Women who had received steroids antepartum for maturation of the fetal lungs before transfer to tertiary care at our facility were not randomized but included in the same study arm of the corticosteroid they had received antepartum.” “All patients who had not previously received corticosteroids for fetal lung maturation before delivery were prospectively randomized.” In the discussion, “the authors acknowledge that patients are randomized and non-randomized, however, the number of women with prior treatment was equivalent among groups and the disease severity at the onset of postpartum therapy was similar” | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random number table (but part of the sample were not randomly allocated) |
| Allocation concealment? | Unclear | Sequentailly numbered opaque sealed envelopes (part of the sample not randomized) |
| Blinding? All outcomes |
No | Different routes of drug administration. |
| Incomplete outcome data addressed? All outcomes |
Unclear | All women appear to be accounted for in the analysis but it was not clear how many of the women included in the analyses were not randomized |
| Free of other bias? | Unclear | Some baseline imbalance between groups at baseline (women in the betamethasone group had significantly lower mean LDH levels at baseline) |
| Overall risk of bias? | Unclear | Mixed randomized and non-randomized study. Not clear how many in the sample were not randomly allocated or whether non-random allocation was balanced across groups |
| Methods | Randomized blinded, placebo-controlled clinical trial (little information about study methods) | |
| Participants | 26 women with gestational age between 27-37 weeks diagnosed with HELLP syndrome (criteria for diagnosis not clear). Exclusions: women with chorioamnionitis. |
|
| Interventions | Intervention group: (13) 10 mg dexamethasone IV every 12 hrs (2 doses) then 5 mg every 12 hrs (2 doses), total dose of 30 mg over 36 hours Control group: (13) no corticosteroid. All women were given a 6 g dose of magnesium sulfate (approximately 1.5 g/hr). All women gave birth within 12 hours |
|
| Outcomes | MAP, urinary output, serum glutanic aspartate aminotransferase (AST), alamine aminotransferase (ALT), LDH, and platelet count | |
| Notes | Results were reported in graphs for platelet count, ALT levels, LDH levels and urine output. Not in a form in which we can enter results into RevMan ALT was listed as being measured along with AST but results for ALT were not reported in the results |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not stated (risk of bias assessment from translation notes). |
| Allocation concealment? | Unclear | “sealed envelopes were distributed randomly to patients” (translation notes) |
| Blinding? All outcomes |
Unclear | Not clear whether blinding or partial blinding was attempted |
| Incomplete outcome data addressed? All outcomes |
Yes | No attrition or withdrawals reported. |
| Free of other bias? | Yes | No baseline imbalance apparent. |
| Overall risk of bias? | Unclear | Risk of bias assessment from translation notes. Possible selection bias (randomization methods not clear). Possible performance bias (there is no mention of blinding or use of a placebo) |
| Methods | Prospective randomized double-blinded placebo-controlled clinical trial | |
| Participants | 114 women (analysis for 105) in the postpartum period with a diagnosis of HELLP (hemolysis (abnormal peripheral blood smear, total bilirubin > 1.2 mg/dL) LDH > 600 IU/L, AST > 70 IU/L, thrombocytopenia, platelets < 100,000 cells/mm3) Cases of complete syndrome (all parameters abnormal) and partial cases (1 or more parameters abnormal) were included. (Women with alterations in all lab parameters = 41.9%, according to Mississippi classification 85.7% were class I or II.) Women receiving antenatal steroid therapy for fetal lung maturation at < 34 weeks’ gestation were included, but earlier steroid use noted Exclusions: chronic user of corticosteroids, chronic liver disorders or with conditions likely to affect lab parameters (e.g. purpura), women receiving antenatal corticosteroids to treat HELLP |
|
| Interventions | Intervention: (61 women) 10 mg dexamethasone every 12 hrs for 4 days (after this at the discretion of the attending physician) Control group: (53 women) placebo following same regimen. If condition deteriorated women could receive dexamethasone at the discretion of the attending physician but it was reported that there was an ITT analysis. The study was described as double blinded so it was not clear whether randomization would be revealed if a woman’s condition deteriorated All women received magnesium sulfate for 24 hours following delivery or after last convulsion. Both groups had intensive monitoring of BP and urine output and lab tests at 24-hr intervals during and after treatment. All women received prophylactic heparin and, if required, anti-hypertensive drugs or hydration for oliguria |
|
| Outcomes | Frequency of complications: oliguria, pulmonary edema, hemorrhage, acute renal failure or death. Need for rescue therapy: blood transfusion. Length of hospitalizations. Laboratory indicators (platelet count, AST, LDH and diuresis per hour) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer random number generator. |
| Allocation concealment? | Yes | Sealed coded boxes containing study medication or placebo, prepared by pharmacy, identical in appearance |
| Blinding? All outcomes |
Yes | Described as randomized, double-blind placebo controlled trial. Reported that investigators women and caregivers were blind until after analysis |
| Incomplete outcome data addressed? All outcomes |
Unclear | Reported ITT analysis but 9 women were excluded because of protocol violations and not included in analysis so it was unclear if ITT was followed |
| Free of other bias? | Yes | No baseline imbalance apparent. |
| Overall risk of bias? | Unclear | Low risk of bias, placebo controlled trial. |
| Methods | Prospective RCT. | |
| Participants | 25 pregnant women, GA 24-37 weeks diagnosed with HELLP (Mississippi classification system HELLP class II or III) on admission to labor and delivery (platelets < 150,000 cells/mm3) Exclusion criteria: women with class 1 HELLP or evidence of fetal distress |
|
| Interventions | Intervention group: dexamethasone 10 mg IV every 12 hours until delivery (no women received therapy postpartum) Comparison group: standard care (no corticosteroids). All women were continuously monitored in labor and delivery, hourly mean arterial pressure and urinary output, lab tests every 6 hours. |
|
| Outcomes | Time to delivery. Change in MAP, urinary output, hematocrit, platelet count, LDH, AST, ALT. Neonatal death, Apgar score < 7, mean birthweight, morbidity (ARDS, intraventricular hemorrhage, retrolental fibroplasias) |
|
| Notes | Inclusion criteria - women with class III HELLP (platelets < 150,000 cells/mm3) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated sequence. |
| Allocation concealment? | Yes | Sealed opaque envelopes. |
| Blinding? All outcomes |
No | Different treatment regimens. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Not described. |
| Free of other bias? | Unclear | There was some baseline imbalance; there were more black women in the control group and the mean platelet count was higher in the control group suggesting that this group may have had less serious disease |
| Overall risk of bias? | Unclear | No blinding may represent a serious source of bias. |
| Methods | Prospective RCT. | |
| Participants | 40 women with diagnosis of HELLP in postpartum period. (HELLP defined using as presence of clinical signs and symptoms consistent with diagnosis of pre-eclampsia and lab evidence of hemolysis, hepatic dysfunction and thrombocytopenia. Mississippi triple class system: women with class 1 or 2 HELLP, i.e. platelets < 100,000 cells/mm3) Exclusions: women with clinical evidence of chorioamnionitis at delivery |
|
| Interventions | Intervention group: (20 women) immediately after delivery, 10 mg IV dexamethasone, then 10 mg 12 hrs later, 5 mg at 24 and 36 hrs. Total of 30 mg over 36 hrs. Control group: (20 women) routine care, no corticosteroids. Both groups were intensively monitored, MAP, urinary output every two hrs and lab tests every 6-12hrs. Both groups had magnesium sulfate IV 2 gm/hr |
|
| Outcomes | Symptom resolution and laboratory values returning to normal (adequate urine output, change in MAP, BP < 160/100, platelet trend upwards, LDH and liver enzymes downwards) Postpartum infectious morbidity. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated card. |
| Allocation concealment? | Yes | “computer generated card enclosed in an opaque envelope.” |
| Blinding? All outcomes |
Unclear | 1 group received no treatment, not clear whether partial blinding was attempted |
| Incomplete outcome data addressed? All outcomes |
No | While there was no mention of loss to follow up there were considerable missing data for some outcomes (e.g. results for 25/40 for urinary output) |
| Free of other bias? | Unclear | No baseline imbalance was reported although the graphs in the results section seem to suggest a different distribution of lab values in the 2 groups. This may not be meaningful as the numbers are small and the distribution may not be normal. Results are presented in graphs that are difficult to interpret and data are not in a form that allows us to analyse using Review Manager Software (RevMan 2008). |
| Overall risk of bias? | Unclear | Risk of performance bias as blinding not indicated. High levels of missing data |
| Methods | RCT. | |
| Participants | 37 postpartum women (outcome data available for 30). Women who had received betamethasone for fetal lung maturation (30%) waited 12 hours after delivery for entry to the study. |
|
| Interventions | Intervention (16 women): 10 mg dexamethasone every 12 hours until platelet levels recovered (> 100,000 cells/mm3) Comparison group (14): standard management (anti-hypertensive drugs and magnesium sulfate as indicated) |
|
| Outcomes | Time to recovery of platelet count. Recovery of ALT, LDH, time in high care unit and complications due to steroid use | |
| Notes | This study was reported in a very brief published report and there is little information on methods or results | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Yes | Described as the “sealed envelope system”. |
| Blinding? All outcomes |
Unclear | Not clear whether partial blinding was attempted. Different treatment regimens |
| Incomplete outcome data addressed? All outcomes |
No | 7/37 (19%) lost due to protocol violations or incomplete data |
| Free of selective reporting? | Unclear | Methods very brief so difficult to assess. |
| Free of other bias? | Yes | No baseline imbalance but methods reported too briefly to assess |
| Overall risk of bias? | Unclear | No blinding and relatively high attrition, randomization methods not clear. No placebo. This study may be at high risk of bias |
| Methods | Prospective, randomized placebo-controlled clinical trial (non-blinded) | |
| Participants | 60 pregnant women diagnosed with HELLP syndrome class 1, 2 and 3 (platelets < 150,000 cells/mm3, AST and ALT > 40 IU/L, LDH > 600 IU/L) Exclusions: women with pyrexia, postpartum HELLP syndrome, diabetes, epilepsy, hepatic or renal disease. |
|
| Interventions | Intervention group: 12 mg betamethasone IM every 12 hours until study criteria fulfilled (symptoms and signs in remission) Control: placebo (sterile water) IM - same regimen. Women with pregnancies 26-34 weeks’ gestation also had 2 doses of 12 mg betamethasone IM to encourage fetal lung maturation. All women received magnesium sulfate 4 g, then a maintenance infusion of 1-2 g/hr. Anti-hypertensives as required Monitoring of BP and urine output hourly. Lab tests 6 hourly IOL or CS if gestational age > 34 weeks or if condition worsened or there were signs of non-reassuring fetal status |
|
| Outcomes | Maternal complications and interventions (labor induction, vaginal delivery, CS, cardiopulmonary or hematological complications, need for transfusion, wound hematoma, DIC, neurological or renal complications, oliguria, need for dialysis, GI, infectious, metabolic complications, edema, ascites), length of hospitalisation. Change in laboratory values (ALT, AST, LDH, platelet count). MAP, systolic and diastolic BP, fetal distress, birthweight | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random number table. |
| Allocation concealment? | Yes | Consecutively numbered sealed opaque envelopes. |
| Blinding? All outcomes |
No | Women were told which treatment they were receiving. Blinding not attempted |
| Incomplete outcome data addressed? All outcomes |
Yes | No loss to follow up apparent. |
| Free of other bias? | Yes | No baseline imbalance apparent. |
| Overall risk of bias? | Unclear | This study had adequate randomization but no blinding. |
| Methods | Randomized double blind placebo controlled trial. | |
| Participants | 32 women randomized (31 women included in analyses). Inclusion criteria: pregnant women with diagnosis of HELLP syndrome with an onset before 30 weeks’ gestation in whom a prolongation of pregnancy was considered beneficial (HELLP defined as: hemolysis (LDH > 600 u/l and or haptoglobin less than or equal to 0.3 g/l), elevated liver enzymes (AST > 50 IU/L or ALT > 50 IU/L) low platelet count (< 100,000 cells/mm3)).Mean gestation at recruitment approximately 27.5 weeks. 1 twin pregnancy in each group. Exclusion criteria: women who required immediate delivery or if study medication contraindicated |
|
| Interventions | Interventions group (15): prednisolone IV, 50 mg over 12 hours in 100 ml of sodium chloride, for 2 days after delivery or for up to 14 days in antenatal period, then tapering off (4 day oral tapering protocol of 50, 20, 10 and 5 mg of medication). If women delivered during the tapering period a stress dose was given during and after delivery every 12 hours for 48 hours) Comparison group: (16) placebo (sodium chloride IV) same regimen Prior to randomization women received 2 IM doses of betamethasone 11.4 mg 24 hrs apart for fetal lung maturation. Magnesium sulfate and anti-hypertensive drugs according to protocol. Monitoring of BP, urine output, symptoms and laboratory tests every 6-24 hrs |
|
| Outcomes | Time to delivery, recurrent HELLP exacerbation, maternal, perinatal and neonatal infant outcomes including GA at delivery, Apgar scores, mortality, RDS grades III and IV, bronchopulmonary dysplasia, oxygen requirement at 28 days after birth, intraventricular hemorrhage, necrotizing enterocolitis, infant death in first year. Neurodevelopmental outcomes at 1 and 2 years | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated in blocks of 6. |
| Allocation concealment? | Yes | Randomization carried out by pharmacy. |
| Blinding? All outcomes |
Yes | “Nurses, physicians and researchers were blinded to the trial medication until the last patient was delivered and discharged.” |
| Incomplete outcome data addressed? All outcomes |
Yes | All but 1 woman accounted for in the analysis (1 woman had an emergency CS before the 1st dose of medication was administered) |
| Free of other bias? | Yes | No baseline imbalance apparent. |
| Overall risk of bias? | Unclear | Low risk of bias. |
| Methods | RCT. | |
| Participants | 34 postpartum women affected by HELLP (diagnosed before delivery with signs and symptoms and lab evidence of hemolysis (abnormal peripheral smear, increased bilirubin > 1.2 mg/dL) decreased hemoglobin and hematocrit, elevated liver enzymes (AST > 70 IU/L, ALT > 50 IU/L, LDH > 600 IU/L) low platelets - includes HELLP categories 1, 2, 3 (platelets < 150,000 cells/mm3) Exclusion criteria: clinical evidence of chorioamnionitis. |
|
| Interventions | Intervention group (17) : 10 mg IV dexamethasone, repeated at 12 and 24 hrs (total 30 mg) Control group: No treatment. All women were monitored for arterial BP, urinary output and lab tests every 12 hrs for 72 hrs No magnesium sulfate was given. Anti-hypertensives to treat severe hypertension as required. |
|
| Outcomes | Arterial BP, urinary output and laboratory tests (platelet count, LDH, AST and ALT). Postpartum infectious morbidity, metabolic disorder, wound infection, abnormal uterine bleeding or maternal death |
|
| Notes | No details on randomization methods. Not blinded. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No details of randomization methods. |
| Allocation concealment? | Unclear | Not described “random assignments”. |
| Blinding? All outcomes |
No | No blinding for women or clinical staff (different treatment regimens); it was not clear whether there was any attempt to blind outcome assessors |
| Incomplete outcome data addressed? All outcomes |
Yes | No loss to follow up apparent. |
| Free of other bias? | No | Baseline characteristics were described as similar but platelet counts were higher at baseline in the control group |
| Overall risk of bias? | Unclear | No details on randomization methods. Not blinded. |
| Methods | RCT. | |
| Participants | 30 postpartum women with a diagnosis of HELLP syndrome while pregnant from symptoms and laboratory findings. Hemolysis, elevated liver enzymes (AST > 48 IU/L, ALT > 24 IU/L) low platelets (< 100,000 cells/mm3) All women were delivered within 72 hrs of admission (IOL initiated with CS for fetal distress). Women randomized following delivery |
|
| Interventions | Intervention group: immediately after delivery 10 mg dexamethasone IV, then 10 mg at 12 hrs, and 5 mg at 24 and 36 hrs (total dose over 36 hrs = 30 mg) Control group: no steroids. Women in both groups had anti-convulsive therapy (IV magnesium sulfate - bolus 4 g then 1 g/hr over 48 hrs). Anti-hypertensive therapy as required (> 160/110 mmHg) Careful monitoring in both groups (arterial BP and urine output monitored 2 hrly) and lab tests 6-12 hrly All women remained in ICU until symptoms and lab tests showed signs of remission |
|
| Outcomes | Outcomes: Mean arterial BP, urine output, laboratory findings (AST, ALT, platelet count, hematocrit). Mean length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Women were “randomly assigned”. |
| Blinding? All outcomes |
Unclear | No blinding of women or clinical staff. It was not clear if outcome assessors were blind |
| Incomplete outcome data addressed? All outcomes |
Yes | All women seem to be accounted for in the analyses. 2 women died before delivery (before randomization) |
| Free of other bias? | Yes | Groups appear similar at baseline. Very little information on study methods |
| Overall risk of bias? | Unclear | Possible performance and selection bias, it was not clear how randomization was achieved or if blinding occurred |
ALT: alanine transaminase
ARDS: acute respiratory distress syndrome
AST: aspartate transaminase
BP: blood pressure
CS: caesarean section
DIC: disseminated intravascular coagulation
GA: gestational age
GI: gastrointestinal
HELLP: hemolysis, elevated liver enzymes and low platelets
hr: hour
hrly: hourly
ICU: intensive care unit
IOL: induction of labor
ITT: intention to treat
IM: intramuscular
IV: intravenous
LDH: lactate dehydrogenase
MAP: mean arterial pressure
RCT: randomized controlled trial
RDS: respiratory distress syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Barrilleaux 2005 | Does not meet inclusion criteria for participants. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | The abstract states that participants were “evaluated prospectively” in three groups “selected randomly” |
| Participants | 60 postpartum women with a diagnosis of HELLP. |
| Interventions | 3 intervention groups: Group 1: dexamethasone and magnesium sulfate infusion. Group 2: betamethasone and magnesium sulfate infusion. Group 3. magnesium sulfate infusion only. |
| Outcomes | Platelet count, urine output and other laboratory values. |
| Notes | We are awaiting a translation of this paper. |
| Methods | Proposed RCT. |
| Participants | Women with HELLP syndrome. |
| Interventions | Methylprednisolone versus placebo. |
| Outcomes | Maternal thrombocytopenia. |
| Notes | We have only a very brief note of this proposed trial. It was stated that the trial was scheduled to begin in 1992. We have no evidence that this trial was completed. We have searched for any published results and have attempted to contact the author for more information, but have not yet had any response (April 2010) |
HELLP: haemolysis elevated liver enzymes and low platelets
RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Dexamethasone efficacy in HELLP I syndrome, a multicentric, double-blind, placebo-controlled, randomized clinical trial |
| Methods | Multi-centre RCT. |
| Participants | Women with more than 20 weeks’ gestation or during the first three days postpartum with platelet count < or = 50,000/mm3, AST > or = 70 U/L and LDH > or = 600 U/L |
| Interventions | Experimental group: dexamethasone 10 mg. Dexamethasone sodium phosphate IV every 12 hours until delivery and 3 doses after delivery. Puerperal women three 10 mg doses after delivery Control group: placebo (sterile water); same regimen as experimental group |
| Outcomes | Duration of hospitalization. Recovery time of platelets to more than 100000/mm3. Recovery of AST, ALT and LDH. Transfusion of blood products |
| Starting date | October 2009. |
| Contact information | Dr Javier Fonseca, Universidad del Valle, Colombia jaenfo@gmail.com |
| Notes | Estimated completion date December 2011 |
ALT: alanine transaminase
AST: aspartate transaminase
HELLP: haemolysis elevated liver enzymes and low platelets
IV: intravenous
LDH: lactate dehydrogenase
RCT: randomized controlled trial
DATA AND ANALYSES
Comparison 1.
Any corticosteroid versus placebo or control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 5 | 362 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.28, 3.21] |
| 1.1 Treatment commenced antenatally | 2 | 91 | Risk Ratio (IV, Fixed, 95% CI) | 0.35 [0.02, 8.08] |
| 1.2 Treatment commenced postnatally | 2 | 139 | Risk Ratio (IV, Fixed, 95% CI) | 0.67 [0.13, 3.46] |
| 1.3 Treatment commencement mixed or uncertain | 1 | 132 | Risk Ratio (IV, Fixed, 95% CI) | 3.00 [0.32, 28.10] |
| 2 Maternal death or severe morbidity | 1 | 31 | Risk Ratio (IV, Fixed, 95% CI) | 0.27 [0.03, 2.12] |
| 2.1 Treatment commenced antenatally | 1 | 31 | Risk Ratio (IV, Fixed, 95% CI) | 0.27 [0.03, 2.12] |
| 2.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 2.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 3 Perinatal/infant death | 2 | 58 | Risk Ratio (IV, Fixed, 95% CI) | 0.64 [0.21, 1.97] |
| 3.1 Treatment commenced antenatally | 2 | 58 | Risk Ratio (IV, Fixed, 95% CI) | 0.64 [0.21, 1.97] |
| 3.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 3.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 4 Maternal liver hematoma, rupture or failure | 2 | 91 | Risk Ratio (IV, Fixed, 95% CI) | 0.22 [0.03, 1.83] |
| 4.1 Treatment commenced antenatally | 2 | 91 | Risk Ratio (IV, Fixed, 95% CI) | 0.22 [0.03, 1.83] |
| 4.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 4.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 5 Maternal pulmonary edema | 3 | 297 | Risk Ratio (IV, Fixed, 95% CI) | 0.77 [0.24, 2.48] |
| 5.1 Treatment commenced antenatally | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 5.2 Treatment commenced postnatally | 1 | 105 | Risk Ratio (IV, Fixed, 95% CI) | 0.35 [0.07, 1.72] |
| 5.3Treatment commencement mixed or uncertain | 1 | 132 | Risk Ratio (IV, Fixed, 95% CI) | 3.00 [0.32, 28.10] |
| 6 Maternal renal failure | 3 | 297 | Risk Ratio (IV, Fixed, 95% CI) | 0.69 [0.39, 1.22] |
| 6.1 Treatment commenced antenatally | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 0.67 [0.12, 3.71] |
| 6.2 Treatment commenced postnatally | 1 | 105 | Risk Ratio (IV, Fixed, 95% CI) | 0.66 [0.30, 1.42] |
| 6.3Treatment commencement mixed or uncertain | 1 | 132 | Risk Ratio (IV, Fixed, 95% CI) | 0.75 [0.28, 2.04] |
| 7 Eclampsia | 1 | 132 | Risk Ratio (IV, Fixed, 95% CI) | 0.8 [0.34, 1.90] |
| 7.1 Treatment commenced antenatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 7.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 7.3Treatment commencement mixed or uncertain | 1 | 132 | Risk Ratio (IV, Fixed, 95% CI) | 0.8 [0.34, 1.90] |
| 8 Caesarean section or elective delivery including induction of labor | 2 | 91 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.79, 1.29] |
| 8.1 Treatment commenced antenatally | 2 | 91 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.79, 1.29] |
| 8.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 8.3Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 9 Length of stay in hospital or obstetrical delivery room for the mother | 5 | 354 | Mean Difference (IV, Random, 95% CI) | −1.15 [−2.77, 0.46] |
| 9.1 Treatment commenced antenatally | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.04 [−0.87, 0.96] |
| 9.2 Treatment commenced postnatally | 2 | 135 | Mean Difference (IV, Random, 95% CI) | −2.27 [−6.38, 1.83] |
| 9.3Treatment commencement mixed or uncertain | 1 | 128 | Mean Difference (IV, Random, 95% CI) | −1.70 [−5.57, 2.17] |
| 10 Need for dialysis | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 3.00 [0.13, 70.83] |
| 10.1 Treatment commenced antenatally | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 3.00 [0.13, 70.83] |
| 10.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 10.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 11 Abruptio placenta | 1 | 31 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.07, 15.57] |
| 11.1 Treatment commenced antenatally | 1 | 31 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.07, 15.57] |
| 11.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 11.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 12 Respiratory distress syndrome with/without ventilatory support | 2 | 58 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.45, 2.03] |
| 12.1 Treatment commenced antenatally | 2 | 58 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.45, 2.03] |
| 12.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 13 Intracerebral hemorrhage | 2 | 58 | Risk Ratio (IV, Fixed, 95% CI) | 2.31 [0.58, 9.28] |
| 13.1 Treatment commenced antenatally | 2 | 58 | Risk Ratio (IV, Fixed, 95% CI) | 2.31 [0.58, 9.28] |
| 13.2 Treatement commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 13.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 14 Necrotizing enterocolitis | 1 | 33 | Risk Ratio (IV, Fixed, 95% CI) | 0.21 [0.01, 4.10] |
| 14.1 Treatment commenced antenatally | 1 | 33 | Risk Ratio (IV, Fixed, 95% CI) | 0.21 [0.01, 4.10] |
| 14.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 15 Gestational age at delivery | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [−1.30, 0.70] |
| 15.1 Treatment commenced antenatally | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [−1.30, 0.70] |
| 15.2 Treatment commenced postnatally | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Treatment commencement mixed or uncertain | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Retinopathy of prematurity/retrolental fibroplasia | 1 | 25 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.02, 8.05] |
| 16.1 Treatment commenced antenatally | 1 | 25 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.02, 8.05] |
| 16.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 16.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 17 Apgar score at 5 minutes < 7 | 2 | 58 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.27, 2.95] |
| 17.1 Treatment commenced antenatally | 2 | 58 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.27, 2.95] |
| 17.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 18 Length of stay in hospital or special care nursery/NICU (days) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | −3.80 [−19.60, 12.00] |
| 18.1 Treatment commenced antenatally | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | −3.80 [−19.60, 12.00] |
| 18.2 Treatment commenced postnatally | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18.3 Treatment commencement mixed or uncertain | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 19 Long-term growth and development - head circumference < 2 SD at 24 months | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 5.0 [0.27, 92.62] |
| 19.1 Treatment commenced antenatally | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 5.0 [0.27, 92.62] |
| 20 Long-term growth and development - Abnormal Griffiths or BSID scales at 24 months | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.75 [0.22, 2.52] |
| 20.1 Treatment commenced antenatally | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.75 [0.22, 2.52] |
| 20.2 Treatment commenced postnatally | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Treatment commencement mixed or uncertain | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 21 Days of mechanical ventilation required (days) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [−9.10, 10.70] |
| 21.1 Treatment commenced antenatally | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [−9.10, 10.70] |
| 21.2 Treatment commenced postnatally | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21.3 Treatment commencement mixed or uncertain | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 22 Platelet count or rate of change of platelet count | 3 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.24, 1.10] |
| 22.1 Treatment commenced antenatally | 2 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.25, 1.35] |
| 22.2 Treatment commenced postnatally | 1 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.47 [−0.21, 1.16] |
| 22.3 Treatment commencement mixed or uncertain | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23 AST level or rate of change of AST level (*non pre-specified outcome) | 2 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.55 [−1.09, −0.02] |
| 23.1 Treatment commenced antenatally | 2 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.55 [−1.09, −0.02] |
| 24 ALT level or rate of change of ALT level (*non pre-specified outcome) | 2 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.58 [−1.12, −0.04] |
| 24.1 Treatment commenced antenatally | 2 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.58 [−1.12, −0.04] |
| 25 LDH level or rate of change of LDH level (*non pre-specified outcome) | 2 | 46 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.76 [−1.40, −0.11] |
| 25.1 Treatment commenced antenatally | 2 | 46 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.76 [−1.40, −0.11] |
| 26 Diastolic blood pressure or rate of change of mean arterial blood pressure (*non pre-specified outcome) | 2 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.26 [−0.79, 0.27] |
| 26.1 Treatment commenced antenatally | 2 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | −0.26 [−0.79, 0.27] |
| 27 Rate of change of urinary output (*non pre-specified outcome) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 3.49 [1.83, 5.15] |
| 27.1 Treatment commenced antenatally | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 3.49 [1.83, 5.15] |
Comparison 2.
Dexamethasone versus betamethasone
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 1.1 Treatment commenced antenatally | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 2 Maternal death or severe morbidity | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 2.1 Treatment commenced antenatally | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 3 Perinatal/infant death | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.15, 6.17] |
| 3.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.15, 6.17] |
| 4 Severe perinatal/infant morbidity or death | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.64 [0.27, 1.48] |
| 4.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.64 [0.27, 1.48] |
| 5 Maternal pulmonary edema | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 5.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 6 Caesarean section | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | 0.79 [0.47, 1.33] |
| 6.1 Treatment commenced antenatally | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | 0.79 [0.47, 1.33] |
| 7 Length of stay in hospital or obstetrical delivery room for the mother | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | −7.50 [−24.29, 9.29] |
| 7.1 Treatment commenced antenatally | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | −7.50 [−24.29, 9.29] |
| 8 Respiratory distress syndrome with/without ventilatory support | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.19, 1.60] |
| 8.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.19, 1.60] |
| 9 Intracerebral hemorrhage | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 9.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 10 Necrotizing enterocolitis | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 10.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 11 Gestational age at delivery | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | −0.60 [−3.35, 2.15] |
| 11.1 Treatment commenced antenatally | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | −0.60 [−3.35, 2.15] |
| 12 Fetal sepsis or infection | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 4.78 [0.24, 94.12] |
| 12.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 4.78 [0.24, 94.12] |
| 13 Apgar score at 5 minutes < 7 | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.22, 4.21] |
| 13.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.95 [0.22, 4.21] |
| 14 Length of stay in hospital or special care nursery/NICU | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | −5.40 [−18.86, 8.06] |
| 14.1 Treatment commenced antenatally | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | −5.40 [−18.86, 8.06] |
| 15 Use of mechanical ventilation | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.19, 1.60] |
| 15.1 Treatment commenced antenatally | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.19, 1.60] |
| 16 Adjusted time averaged change in platelet count | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 6.02 [1.71, 10.33] |
| 16.1 Treatment commenced antenatally | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 8.1 [6.23, 9.97] |
| 16.2 Treatment commenced postnatally | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 3.70 [0.96, 6.44] |
| 17 Adjusted time averaged change in AST level (*non pre-specified outcome) | 2 | 76 | Mean Difference (IV, Random, 95% CI) | −18.80 [−41.34, 3.74] |
| 17.1 Treatment commenced antenatally | 1 | 40 | Mean Difference (IV, Random, 95% CI) | −30.30 [−36.06, −24.54] |
| 17.2 Treatment commenced postnatally | 1 | 36 | Mean Difference (IV, Random, 95% CI) | −7.30 [−13.11, −1.49] |
| 18 Adjusted time averaged change in LDH level (*non pre-specified outcome) | 2 | 76 | Mean Difference (IV, Random, 95% CI) | −73.40 [−113.13, − 33.67] |
| 18.1 Treatment commenced antenatally | 1 | 40 | Mean Difference (IV, Random, 95% CI) | −54.20 [−88.22, −20.18] |
| 18.2 Treatment commenced postnatally | 1 | 36 | Mean Difference (IV, Random, 95% CI) | −94.80 [−133.54, −56.06] |
| 19 Adjusted time averaged change in mean arterial pressure (mmHg) (*non pre-specified outcome) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | −7.64 [−8.27, −7.01] |
| 19.1 Treatment commenced antenatally | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | −7.50 [−8.37, −6.63] |
| 19.2 Treatment commenced postnatally | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | −7.80 [−8.71, −6.89] |
| 20 Adjusted time averaged change in urinary output (*non pre-specified outcome) | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 16.73 [0.47, 32.99] |
| 20.1 Treatment commenced antenatally | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 24.8 [19.58, 30.02] |
| 20.2 Treatment commenced postnatally | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 8.20 [0.69, 15.71] |
Analysis 1.1. Comparison 1 Any corticosteroid versus placebo or control, Outcome 1 Maternal death
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 1 Maternal death
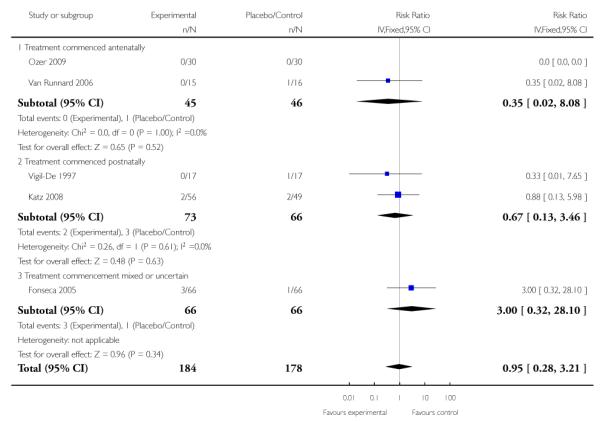 |
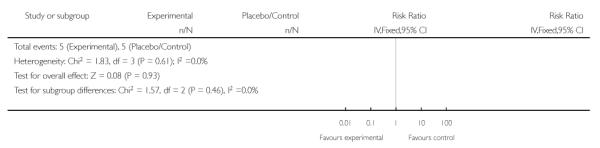 |
Analysis 1.2. Comparison 1 Any corticosteroid versus placebo or control, Outcome 2 Maternal death or severe morbidity
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 2 Maternal death or severe morbidity
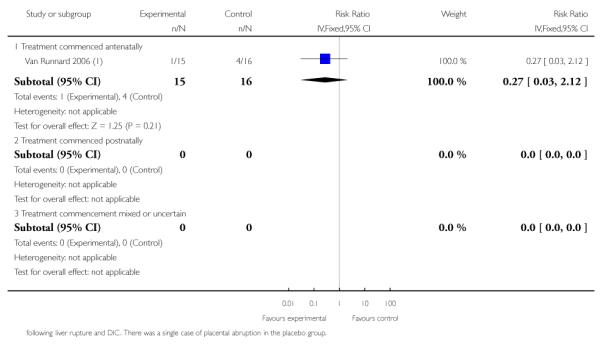 |
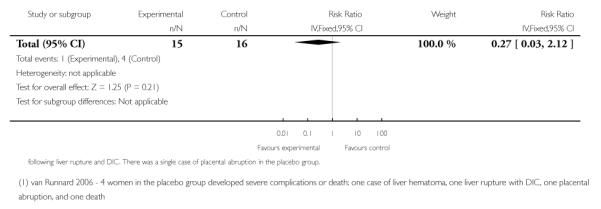 |
Analysis 1.3. Comparison 1 Any corticosteroid versus placebo or control, Outcome 3 Perinatal/infant death
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 3 Perinatal/infant death
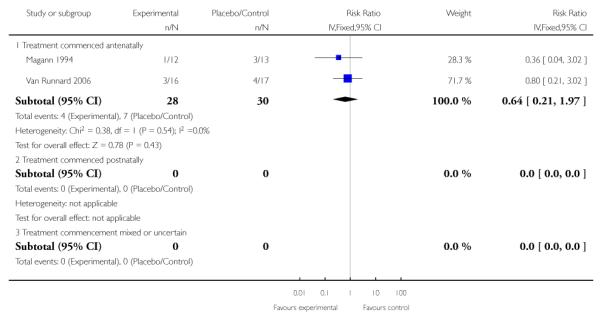 |
 |
Analysis 1.4. Comparison 1 Any corticosteroid versus placebo or control, Outcome 4 Maternal liver hematoma, rupture or failure
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 4 Maternal liver hematoma, rupture or failure
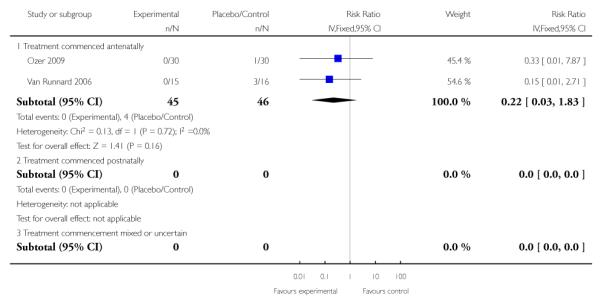 |
 |
Analysis 1.5. Comparison 1 Any corticosteroid versus placebo or control, Outcome 5 Maternal pulmonary edema
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 5 Maternal pulmonary edema
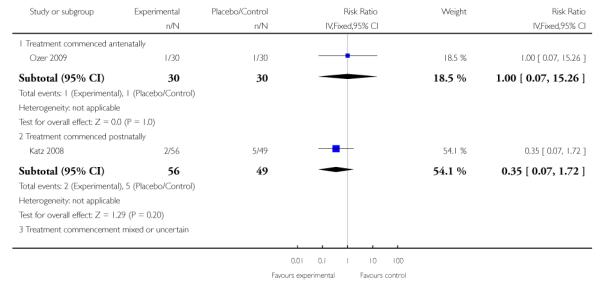 |
 |
Analysis 1.6. Comparison 1 Any corticosteroid versus placebo or control, Outcome 6 Maternal renal failure
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 6 Maternal renal failure
 |
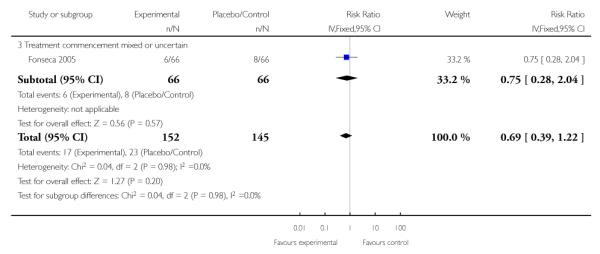 |
Analysis 1.7. Comparison 1 Any corticosteroid versus placebo or control, Outcome 7 Eclampsia
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 7 Eclampsia
 |
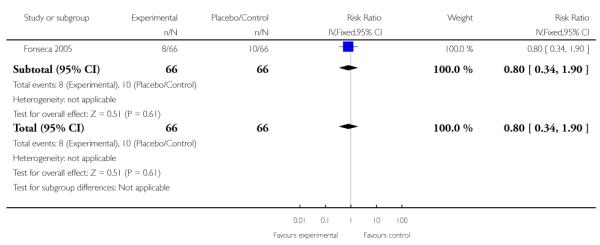 |
Analysis 1.8. Comparison 1 Any corticosteroid versus placebo or control, Outcome 8 Caesarean section or elective delivery including induction of labor
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 8 Caesarean section or elective delivery including induction of labor
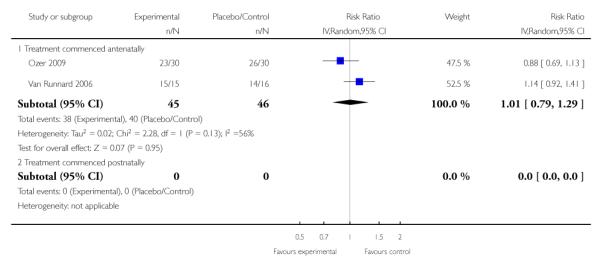 |
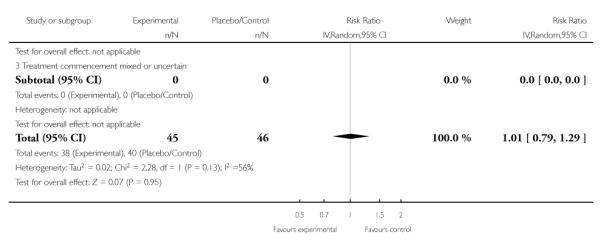 |
Analysis 1.9. Comparison 1 Any corticosteroid versus placebo or control, Outcome 9 Length of stay in hospital or obstetrical delivery room for the mother
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 9 Length of stay in hospital or obstetrical delivery room for the mother
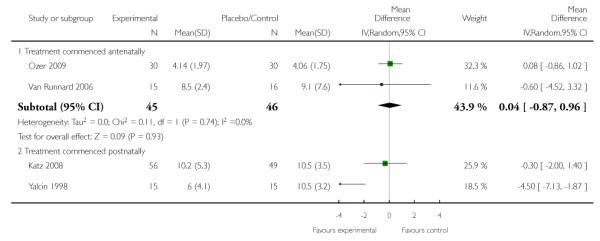 |
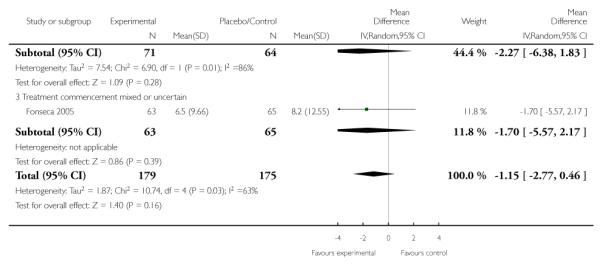 |
Analysis 1.10. Comparison 1 Any corticosteroid versus placebo or control, Outcome 10 Need for dialysis
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 10 Need for dialysis
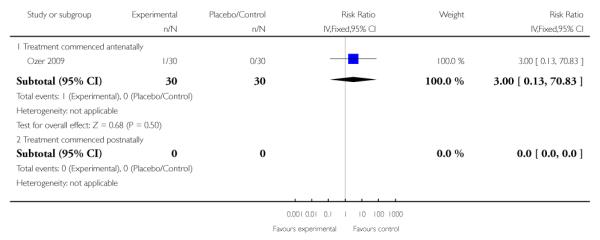 |
 |
Analysis 1.11. Comparison 1 Any corticosteroid versus placebo or control, Outcome 11 Abruptio placenta
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 11 Abruptio placenta
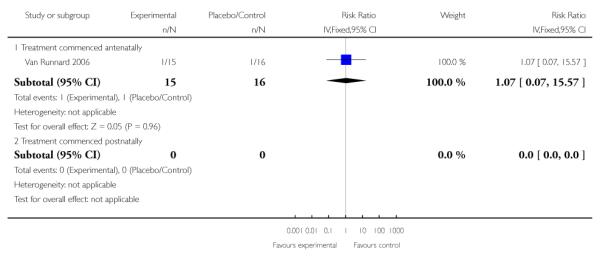 |
 |
Analysis 1.12. Comparison 1 Any corticosteroid versus placebo or control, Outcome 12 Respiratory distress syndrome with/without ventilatory support
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 12 Respiratory distress syndrome with/without ventilatory support
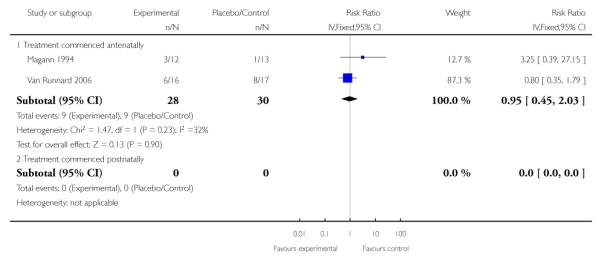 |
 |
Analysis 1.13. Comparison 1 Any corticosteroid versus placebo or control, Outcome 13 Intracerebral hemorrhage
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 13 Intracerebral hemorrhage
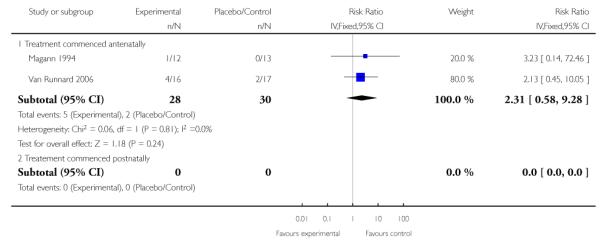 |
 |
Analysis 1.14. Comparison 1 Any corticosteroid versus placebo or control, Outcome 14 Necrotizing enterocolitis
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 14 Necrotizing enterocolitis
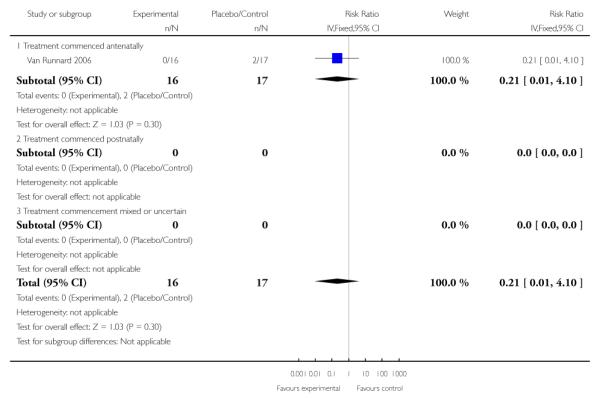 |
Analysis 1.15. Comparison 1 Any corticosteroid versus placebo or control, Outcome 15 Gestational age at delivery
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 15 Gestational age at delivery
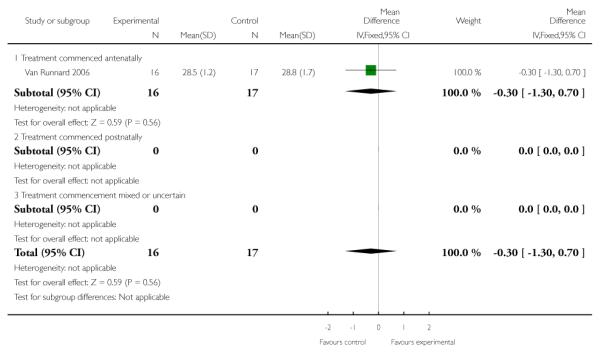 |
Analysis 1.16. Comparison 1 Any corticosteroid versus placebo or control, Outcome 16 Retinopathy of prematurity/retrolental fibroplasia
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 16 Retinopathy of prematurity/retrolental fibroplasia
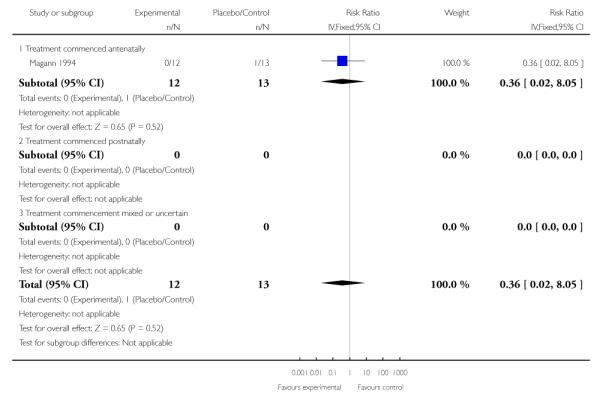 |
Analysis 1.17. Comparison 1 Any corticosteroid versus placebo or control, Outcome 17 Apgar score at 5 minutes < 7
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 17 Apgar score at 5 minutes < 7
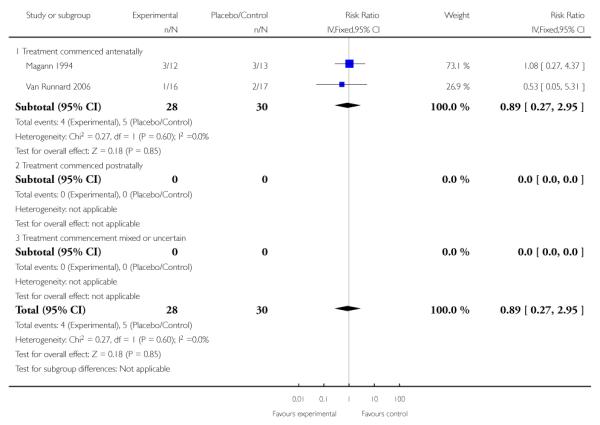 |
Analysis 1.18. Comparison 1 Any corticosteroid versus placebo or control, Outcome 18 Length of stay in hospital or special care nursery/NICU (days)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 18 Length of stay in hospital or special care nursery/NICU (days)
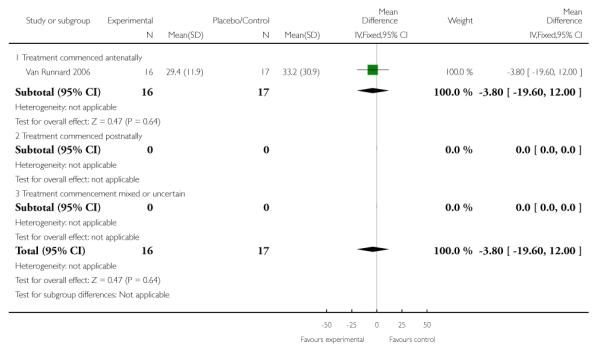 |
Analysis 1.19. Comparison 1 Any corticosteroid versus placebo or control, Outcome 19 Long-term growth and development - head circumference < 2 SD at 24 months
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 19 Long-term growth and development - head circumference < 2 SD at 24 months
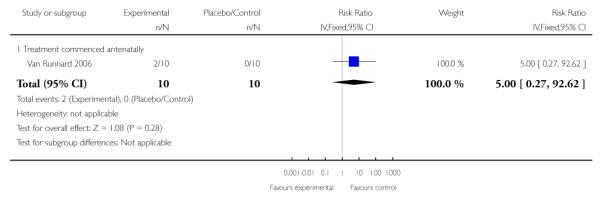 |
Analysis 1.20. Comparison 1 Any corticosteroid versus placebo or control, Outcome 20 Long-term growth and development - Abnormal Griffiths or BSID scales at 24 months
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 20 Long-term growth and development - Abnormal Griffiths or BSID scales at 24 months
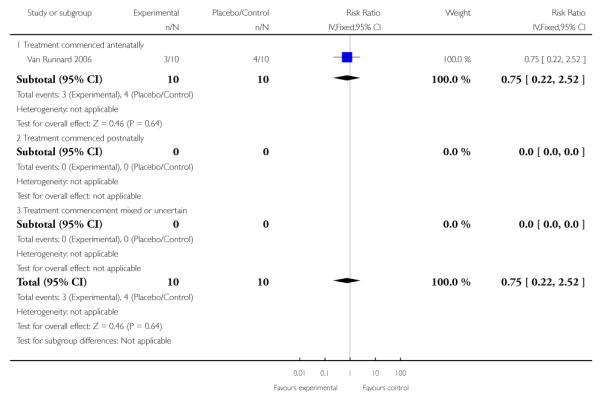 |
Analysis 1.21. Comparison 1 Any corticosteroid versus placebo or control, Outcome 21 Days of mechanical ventilation required (days)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 21 Days of mechanical ventilation required (days)
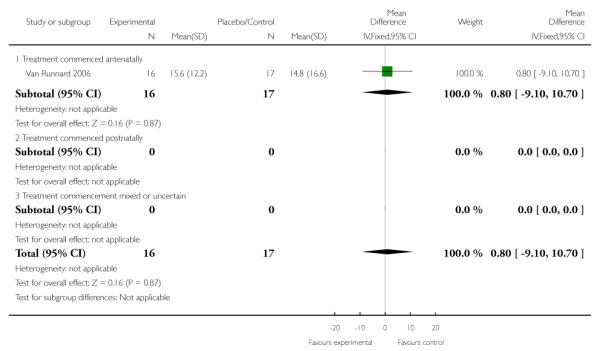 |
Analysis 1.22. Comparison 1 Any corticosteroid versus placebo or control, Outcome 22 Platelet count or rate of change of platelet count
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 22 Platelet count or rate of change of platelet count
 |
Analysis 1.23. Comparison 1 Any corticosteroid versus placebo or control, Outcome 23 AST level or rate of change of AST level (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 23 AST level or rate of change of AST level (*non pre-specified outcome)
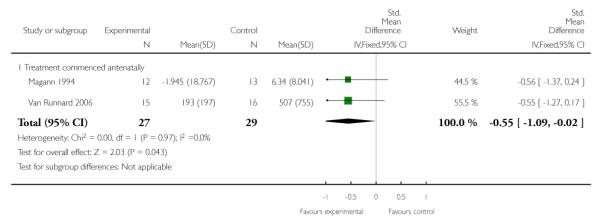 |
Analysis 1.24. Comparison 1 Any corticosteroid versus placebo or control, Outcome 24 ALT level or rate of change of ALT level (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 24 ALT level or rate of change of ALT level (*non pre-specified outcome)
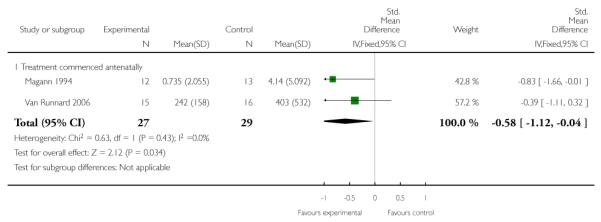 |
Analysis 1.25. Comparison 1 Any corticosteroid versus placebo or control, Outcome 25 LDH level or rate of change of LDH level (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 25 LDH level or rate of change of LDH level (*non pre-specified outcome)
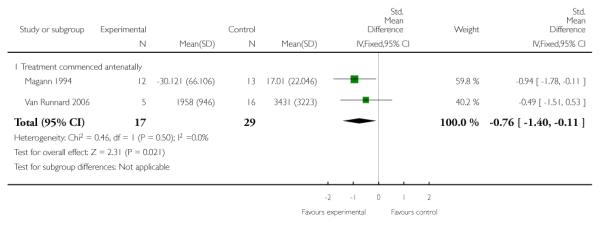 |
Analysis 1.26. Comparison 1 Any corticosteroid versus placebo or control, Outcome 26 Diastolic blood pressure or rate of change of mean arterial blood pressure (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 26 Diastolic blood pressure or rate of change of mean arterial blood pressure (*non pre-specified outcome)
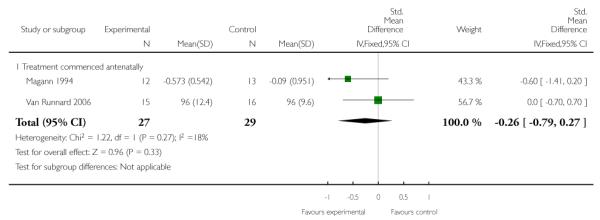 |
Analysis 1.27. Comparison 1 Any corticosteroid versus placebo or control, Outcome 27 Rate of change of urinary output (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 1 Any corticosteroid versus placebo or control
Outcome: 27 Rate of change of urinary output (*non pre-specified outcome)
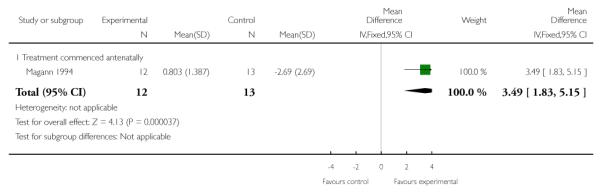 |
Analysis 2.1. Comparison 2 Dexamethasone versus betamethasone, Outcome 1 Maternal death
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 1 Maternal death
 |
Analysis 2.2. Comparison 2 Dexamethasone versus betamethasone, Outcome 2 Maternal death or severe morbidity
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 2 Maternal death or severe morbidity
 |
Analysis 2.3. Comparison 2 Dexamethasone versus betamethasone, Outcome 3 Perinatal/infant death
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 3 Perinatal/infant death
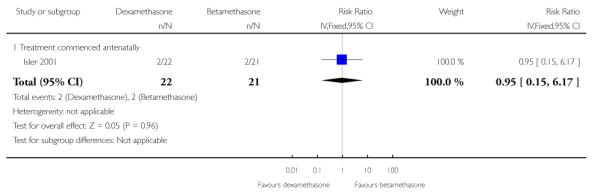 |
Analysis 2.4. Comparison 2 Dexamethasone versus betamethasone, Outcome 4 Severe perinatal/infant morbidity or death
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 4 Severe perinatal/infant morbidity or death
 |
Analysis 2.5. Comparison 2 Dexamethasone versus betamethasone, Outcome 5 Maternal pulmonary edema
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 5 Maternal pulmonary edema
 |
Analysis 2.6. Comparison 2 Dexamethasone versus betamethasone, Outcome 6 Caesarean section
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 6 Caesarean section
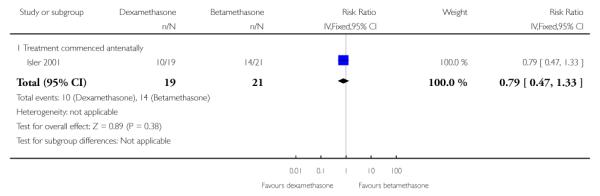 |
Analysis 2.7. Comparison 2 Dexamethasone versus betamethasone, Outcome 7 Length of stay in hospital or obstetrical delivery room for the mother
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 7 Length of stay in hospital or obstetrical delivery room for the mother
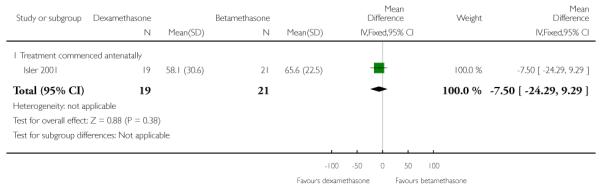 |
Analysis 2.8. Comparison 2 Dexamethasone versus betamethasone, Outcome 8 Respiratory distress syndrome with/without ventilatory support
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 8 Respiratory distress syndrome with/without ventilatory support
 |
Analysis 2.9. Comparison 2 Dexamethasone versus betamethasone, Outcome 9 Intracerebral hemorrhage
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 9 Intracerebral hemorrhage
 |
Analysis 2.10. Comparison 2 Dexamethasone versus betamethasone, Outcome 10 Necrotizing enterocolitis
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 10 Necrotizing enterocolitis
 |
Analysis 2.11. Comparison 2 Dexamethasone versus betamethasone, Outcome 11 Gestational age at delivery
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 11 Gestational age at delivery
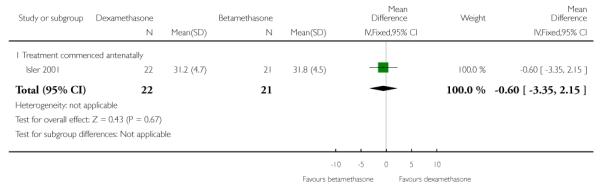 |
Analysis 2.12. Comparison 2 Dexamethasone versus betamethasone, Outcome 12 Fetal sepsis or infection
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 12 Fetal sepsis or infection
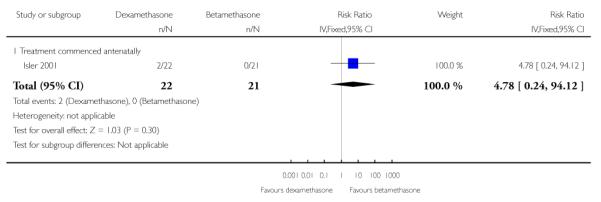 |
Analysis 2.13. Comparison 2 Dexamethasone versus betamethasone, Outcome 13 Apgar score at 5 minutes < 7
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 13 Apgar score at 5 minutes < 7
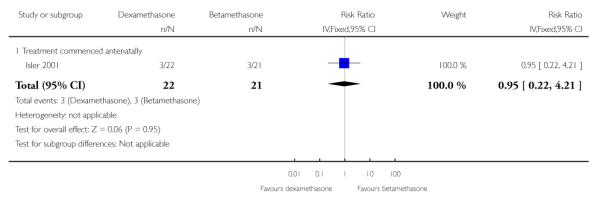 |
Analysis 2.14. Comparison 2 Dexamethasone versus betamethasone, Outcome 14 Length of stay in hospital or special care nursery/NICU
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 14 Length of stay in hospital or special care nursery/NICU
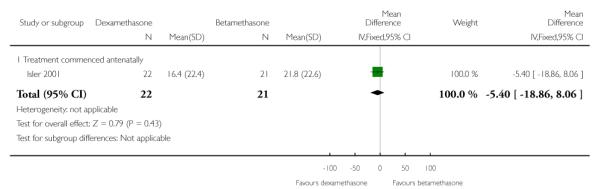 |
Analysis 2.15. Comparison 2 Dexamethasone versus betamethasone, Outcome 15 Use of mechanical ventilation
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 15 Use of mechanical ventilation
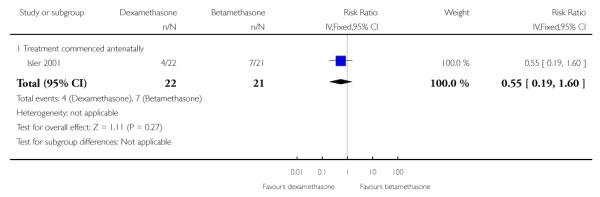 |
Analysis 2.16. Comparison 2 Dexamethasone versus betamethasone, Outcome 16 Adjusted time averaged change in platelet count
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 16 Adjusted time averaged change in platelet count
 |
Analysis 2.17. Comparison 2 Dexamethasone versus betamethasone, Outcome 17 Adjusted time averaged change in AST level (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 17 Adjusted time averaged change in AST level (*non pre-specified outcome)
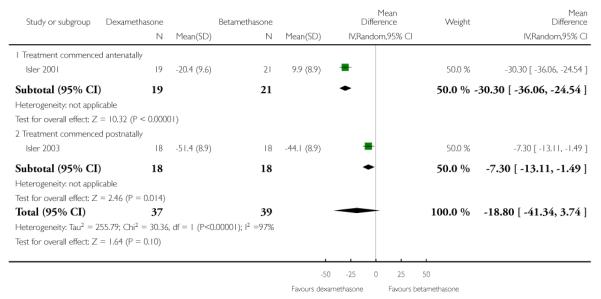 |
Analysis 2.18. Comparison 2 Dexamethasone versus betamethasone, Outcome 18 Adjusted time averaged change in LDH level (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 18 Adjusted time averaged change in LDH level (*non pre-specified outcome)
 |
Analysis 2.19. Comparison 2 Dexamethasone versus betamethasone, Outcome 19 Adjusted time averaged change in mean arterial pressure (mmHg) (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 19 Adjusted time averaged change in mean arterial pressure (mmHg) (*non pre-specified outcome)
 |
Analysis 2.20. Comparison 2 Dexamethasone versus betamethasone, Outcome 20 Adjusted time averaged change in urinary output (*non pre-specified outcome)
Review: Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy
Comparison: 2 Dexamethasone versus betamethasone
Outcome: 20 Adjusted time averaged change in urinary output (*non pre-specified outcome)
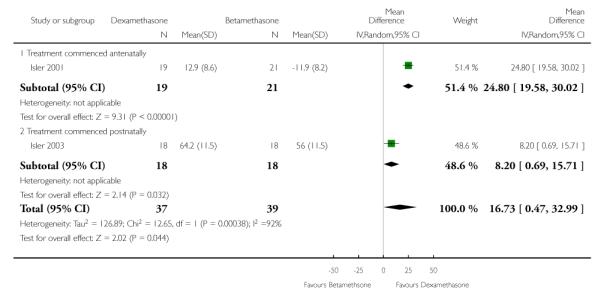 |
HISTORY
Protocol first published: Issue 4, 2009
Review first published: Issue 9, 2010
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
Inclusion of non pre-specified outcomes in the review, which are clearly identified. Authors planned to search PubMed and EMBASE; however, as the generic search strategy covers this, these searches were not carried out.
Footnotes
DECLARATIONS OF INTEREST None known.
References to studies included in this review
- Bouchnak 2005 {published data only} .Bouchnak M, Souissi K, Ouragini H, Abbes Z, Douiri H, Maghrebi H. Maternal benefit of postpartum corticosteroid therapy in patients with HELLP (hemolysis elevated liver enzymes low platelets count) syndrome [Interet de la corticotherapie en post partum dans le HELLP (hemolysis elevated liver enzymes low platelets count) syndrome] Tunisie Medicale. 2005;83(8):473–6. [PubMed] [Google Scholar]
- Fonseca 2005 {published data only} .Fonseca JE, Mendez F, Catano C, Arias F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. American Journal of Obstetrics & Gynecology. 2005;193(5):1591–8. doi: 10.1016/j.ajog.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Isler 2001 {published data only} .Isler CM, Barrilleaux PS, Magann EF, Bass JD, Martin JN. A prospective, randomized trial comparing the efficacy of dexamethasone and betamethasone for the treatment of antepartum hellp (hemolysis, elevated liver enzymes, and low platelet count) syndrome [Un estudio prospectivo, randomizado que compara la eficacia de la dexametasona y betametasona para el tratamiento anteparto del sindron de hellp (hemolisis, elevacion de enzimas hepaticas y plaquetopenia)] Revista Chilena de Obstetricia y Ginecologia. 2001;66(3):248–50. [Google Scholar]; *; Isler CM, Barrilleaux PS, Magann EF, Bass JD, Martin JN., Jr A prospective, randomized trial comparing the efficacy of dexamethasone and betamethasone for the treatment of antepartum hellp (hemolysis, elevated liver enzymes, and low platelet count) syndrome. American Journal of Obstetrics and Gynecology. 2001;184(7):1332–7. doi: 10.1067/mob.2001.115051. discussion 1337-9. [DOI] [PubMed] [Google Scholar]
- Isler 2003 {published data only} .*; Isler CM, Magann EF, Rinehart BK, Terrone DA, Bass JD, Martin JN., Jr Dexamethasone compared with betamethasone for glucocorticoid treatment of postpartum hellp syndrome. International Journal of Gynecology & Obstetrics. 2003;80(3):291–7. doi: 10.1016/s0020-7292(02)00394-6. [DOI] [PubMed] [Google Scholar]; Isler CM, Magann EF, Rinehart BK, Terrone DA, Bass JD, Martin JN., Jr Dexamethasone versus betamethasone for postpartum hellp syndrome: a randomized prospective clinical trial of comparative efficacy. American Journal of Obstetrics and Gynecology. 2000;182(1 Pt 2):S87. [Google Scholar]
- Kadanali 1997 {published data only} .Kadanali S, Kucukozkan T, Bukam B. Helpful effect of high-dose corticosteroid use on Hellp syndrome [Yuksek doz kortikosteroid kullaniminin HELLP sendromu seyrine olumlu etkileri] Jinekoloji Ve Obstetrik Dergisi. 1997;11:55–8. [Google Scholar]
- Katz 2008 {published data only} .Katz L, Amorim MM, Miranda GV, Pinto e Silva JL. Clinical and laboratorial profile and complications of patients with HELLP syndrome admitted in an obstetric intensive care unit. Revista Brasileira de Ginecologia e Obstetricia. 2008;30(2):80–6. doi: 10.1590/s0100-72032008000200006. [DOI] [PubMed] [Google Scholar]; *; Katz L, de Amorim MM, Figueiroa JN, Pinto e Silva JL. Postpartum dexamethasone for women with hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome: a double-blind, placebo-controlled, randomized clinical trial. American Journal of Obstetrics and Gynecology. 2008;198:282.e1–3.e8. doi: 10.1016/j.ajog.2007.10.797. [DOI] [PubMed] [Google Scholar]
- Magann 1994 {published data only} .*; Magann EF, Bass D, Chauhan SP, Sullivan DL, Martin RW, Martin JM. Antepartum corticosteroids: disease stabilization in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP) American Journal of Obstetrics and Gynecology. 1994;171:1148–53. doi: 10.1016/0002-9378(94)90054-x. [DOI] [PubMed] [Google Scholar]; Magann EF, Bass D, Chauhan SP, Sullivan DL, Martin RW, Martin JN. Antepartum corticosteroids: disease stabilization in patients with HELLP syndrome. American Journal of Obstetrics and Gynecology. 1994;170:410. doi: 10.1016/0002-9378(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Magann 1994a {published data only} .*; Magann EF, Perry KG, Meydrech EF, Harris RL, Chauhan SP, Martin JN. Postpartum corticosteroids: accelerated recovery from the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP) American Journal of Obstetrics and Gynecology. 1994;171:1154–8. doi: 10.1016/0002-9378(94)90055-8. [DOI] [PubMed] [Google Scholar]; Magann EF, Perry KG, Poist JE, Harris RL, Norman PF, Martin JN. Postpartum corticosteroids: accelerated recovery from HELLP syndrome. American Journal of Obstetrics and Gynecology. 1994;170:410. doi: 10.1016/0002-9378(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Mould 2006 {published data only} .Mould S, Paruk F, Moodley J. High-dose dexamethasone in the treatment of HELLP syndrome. International Journal of Gynecology & Obstetrics. 2006;93(2):140–1. doi: 10.1016/j.ijgo.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Ozer 2009 {published data only} .Ozer A, Kanat-Pektas M, Ozer S, Tapisiz OL, Zulfikaroglu EE, Danisman N. The effects of betamethasone treatment on clinical and laboratory features of pregnant women with HELLP syndrome. Archives of Gynecology and Obstetrics. 2009;280(1):65–70. doi: 10.1007/s00404-008-0865-3. [DOI] [PubMed] [Google Scholar]
- Van Runnard 2006 {published data only} .Van Runnard Heimel P, Huisjes A, Bots M, Bruinse H. A randomized placebo-controlled trial of prolonged prednisolone administration to patients with HELLP syndrome remote from term [abstract} Hypertension in Pregnancy. 2004;23(Suppl 1):13. doi: 10.1016/j.ejogrb.2005.11.041. [DOI] [PubMed] [Google Scholar]; *; Van Runnard Heimel PJ, Huisjes AJ, Franx A, Koopman C, Bots ML, Bruinse HW. A randomised placebo-controlled trial of prolonged prednisolone administration to patients with HELLP syndrome remote from term. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2006;128:187–93. doi: 10.1016/j.ejogrb.2005.11.041. [DOI] [PubMed] [Google Scholar]; Van Runnard Heimel PJ, Huisjes AJM, Franx A, Koopman C, Bots ML, Bruinse HW. A randomized placebo-controlled trial of prolonged prednisolone administration to patients with HELLP syndrome remote from term: maternal and neonatal complications [abstract] American Journal of Obstetrics and Gynecology. 2004;191(6 Suppl 1):S41. doi: 10.1016/j.ejogrb.2005.11.041. [DOI] [PubMed] [Google Scholar]; Van Runnard Heimel PJ, Schobben AF, Huisjes AJ, Franx A, Bruinse HW. The transplacental passage of prednisolone in pregnancies complicated by early-onset HELLP syndrome. Placenta. 2005;26(10):842–5. doi: 10.1016/j.placenta.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Vigil-De 1997 {published data only} .Vigil-De Gracia P, Garcia-Caceres E. Dexamethasone in the post-partum treatment of HELLP syndrome. International Journal of Gynecology & Obstetrics. 1997;59:217–21. doi: 10.1016/s0020-7292(97)00214-2. [DOI] [PubMed] [Google Scholar]
- Yalcin 1998 {published data only} .Yalcin OT, Sener T, Hassa H, Ozalp S, Okur A. Effects of postpartum corticosteroids in patients with hellp syndrome. International Journal of Gynecology & Obstetrics. 1998;61(2):141–8. doi: 10.1016/s0020-7292(98)00036-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Barrilleaux 2005 {published data only} .Barrilleaux PS, Martin JN, Jr, Klauser CK, Bufkin L, May WL. Postpartum intravenous dexamethasone for severely preeclamptic patients without hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome: a randomized trial. Obstetrics & Gynecology. 2005;105(4):843–8. doi: 10.1097/01.AOG.0000154887.57440.d1. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Borekci 2008 {published data only} .Borekci B, Bebek Z, Ingec M, Kadanali S. Effects of postpartum corticosteroids in patients with HELLP syndrome [HELLP sendromlu hastalarin tedavisinde postpartum kortikosteroid kullaniminin etkileri] Journal of the Turkish German Gynecology Association Artemis. 2008;9(2):79–83. [Google Scholar]
- Morrison 1992 {published data only} .Morrison JC. Effect on maternal thrombocytopenia in patients with HELLP syndrome of maternal steroid administration. Personal communication. 1992.
References to ongoing studies
- Fonseca 2010 {published data only} .Fonseca J. [accessed 12 July 2010];Dexamethasone efficacy in HELLP syndrome, a multicentric, double-blind, placebo-controlled, randomized clinical trial. http://clinicaltrials.gov/ct2/show/record/NCT01138839
Additional references
- Abramovici 1999 .Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation: does the HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? American Journal of Obstetrics and Gynecology. 1999;180(1 Pt 1):221–5. doi: 10.1016/s0002-9378(99)70178-x. [DOI] [PubMed] [Google Scholar]
- Clark 1986 .Clark SL, Phelan JR, Allen SH, Golde SR. Antepartum reversal of hematologic abnormalities associated with the HELLP syndrome. A report of three cases. Journal of Reproductive Medicine. 1986;31:70–2. [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. BMJ Books; London: 2001. [Google Scholar]
- Duley 2009 .Duley L, Henderson-Smart DJ, Walker GJA. Interventions for treating pre-eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews. 2009;(Issue 2) [DOI: 10.1002/14651858.CD007756] [Google Scholar]
- Haram 2009 .Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A Review. BMC Pregnancy and Childbirth. 2009;9:8. doi: 10.1186/1471-2393-9-8. [PUBMED: 19245695] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2009 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2. The Cochrane Collaboration; [updated September 2009]. 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Magann 1993 .Magann EF, Martin RW, Issacs JD, Blake PG, Morrison JC, Martin JN., Jr Corticosteroids for the enhancement of fetal lung maturity: impact on the gravida with preeclampsia and the HELLP syndrome. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1993;33:127–31. doi: 10.1111/j.1479-828x.1993.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Martin 1991 .Martin JN, Jr, Blake PG, Perry KG, McCaul JF, Hess LW, Martin RW. The natural history of HELLP syndrome: patterns of disease progression and regression. American Journal of Obstetrics and Gynecology. 1991;164:1500–9. doi: 10.1016/0002-9378(91)91429-z. [DOI] [PubMed] [Google Scholar]
- Martin 1999 .Martin JN, Jr, Rinehart BK, May WL, Magann EF, Terrone DA, Blake PG. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. American Journal of Obstetrics and Gynecology. 1999;180(6 Pt 1):1373–84. doi: 10.1016/s0002-9378(99)70022-0. [DOI] [PubMed] [Google Scholar]
- RevMan 2008 .The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan) 5.0 The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Roberts 2000 .Roberts JM. Preeclampsia: what we know and what we do not know. Seminars in Perinatology. 2000;24(1):24–8. doi: 10.1016/s0146-0005(00)80050-6. [DOI] [PubMed] [Google Scholar]
- Roberts 2006 .Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD004454.pub2. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
- Sibai 1993 .Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome) American Journal of Obstetrics and Gynecology. 1993;169(4):1000–6. doi: 10.1016/0002-9378(93)90043-i. [DOI] [PubMed] [Google Scholar]
- Sibai 2004 .Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstetrics & Gynecology. 2004;103(5 Pt 1):981–91. doi: 10.1097/01.AOG.0000126245.35811.2a. [DOI] [PubMed] [Google Scholar]
- Vigil-De Gracia 1997 .Vigil-De Gracia P, Garcia-Caceres E. Dexamethasone in the postpartum treatment of HELLP syndrome. International Journal of Gynecology & Obstetrics. 1997;59:217–21. doi: 10.1016/s0020-7292(97)00214-2. [DOI] [PubMed] [Google Scholar]
- Visser 1995 .Visser W, Wallenburg HC. Temporising management of severe pre-eclampsia with and without the HELLP syndrome. British Journal of Obstetrics and Gynaecology. 1995;102(2):111–7. doi: 10.1111/j.1471-0528.1995.tb09062.x. [DOI] [PubMed] [Google Scholar]
- Yeast 1987 .Yeast JD, Coronado S. Hepatic dysfunction, thrombocytopenia and late onset preeclampsia. A report of three cases. Journal of Reproductive Medicine. 1987;32:781–4. [PubMed] [Google Scholar]
References to other published versions of this review
- Matchaba 2004 .Matchaba P, Moodley J. Corticosteroids for HELLP syndrome in pregnancy. Cochrane Database of Systematic Reviews. 2004;(Issue 1) doi: 10.1002/14651858.CD002076.pub2. [Art. No.: CD002076. DOI: 10.1002/14651858.CD002076.pub2] [DOI] [PubMed] [Google Scholar]
- Matchaba 2009 .Matchaba PT, Moodley J. Corticosteroids for HELLP syndrome in pregnancy. Cochrane Database of Systematic Reviews. 2009;(Issue 3) doi: 10.1002/14651858.CD002076.pub3. [Art. No.: CD002076. DOI: 10.1002/14651858.CD002076.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Indicates the major publication for the study


