Abstract
Background
This review updates parts of two earlier Cochrane reviews investigating effects of gabapentin in chronic neuropathic pain (pain due to nerve damage). Antiepileptic drugs are used to manage pain, predominantly for chronic neuropathic pain, especially when the pain is lancinating or burning.
Objectives
To evaluate the analgesic effectiveness and adverse effects of gabapentin for chronic neuropathic pain management.
Search methods
We identified randomised trials of gabapentin in acute, chronic or cancer pain from MEDLINE, EMBASE, and CENTRAL. We obtained clinical trial reports and synopses of published and unpublished studies from Internet sources. The date of the most recent search was January 2011.
Selection criteria
Randomised, double-blind studies reporting the analgesic and adverse effects of gabapentin in neuropathic pain with assessment of pain intensity and/or pain relief, using validated scales. Participants were adults aged 18 and over.
Data collection and analysis
Two review authors independently extracted data. We calculated numbers needed to treat to benefit (NNTs), concentrating on IMM-PACT (Initiative on Methods, Measurement and Pain Assessment in Clinical Trials) definitions of at least moderate and substantial benefit, and to harm (NNH) for adverse effects and withdrawal. Meta-analysis was undertaken using a fixed-effect model.
Main results
Twenty-nine studies (3571 participants), studied gabapentin at daily doses of 1200 mg or more in 12 chronic pain conditions; 78% of participants were in studies of postherpetic neuralgia, painful diabetic neuropathy or mixed neuropathic pain. Using the IMMPACT definition of at least moderate benefit, gabapentin was superior to placebo in 14 studies with 2831 participants, 43% improving with gabapentin and 26% with placebo; the NNT was 5.8 (4.8 to 7.2). Using the IMMPACT definition of substantial benefit, gabapentin was superior to placebo in 13 studies with 2627 participants, 31% improving with gabapentin and 17% with placebo; the NNT was 6.8 (5.6 to 8.7). These estimates of efficacy are more conservative than those reported in a previous review. Data from few studies and participants were available for other painful conditions.
Adverse events occurred significantly more often with gabapentin. Persons taking gabapentin can expect to have at least one adverse event (66%), withdraw because of an adverse event (12%), suffer dizziness (21%), somnolence (16%), peripheral oedema (8%), and gait disturbance (9%). Serious adverse events (4%) were no more common than with placebo.
There were insufficient data for comparisons with other active treatments.
Authors’ conclusions
Gabapentin provides pain relief of a high level in about a third of people who take if for painful neuropathic pain. Adverse events are frequent, but mostly tolerable. More conservative estimates of efficacy resulted from using better definitions of efficacy outcome at higher, clinically important, levels, combined with a considerable increase in the numbers of studies and participants available for analysis.
Medical Subject Headings (MeSH): Amines [adverse effects; *therapeutic use], Analgesics [adverse effects; *therapeutic use], Chronic Disease, Cyclohexanecarboxylic Acids [adverse effects; *therapeutic use], Fibromyalgia [*drug therapy], Neuralgia [*drug therapy], Randomized Controlled Trials as Topic, gamma-Aminobutyric Acid [adverse effects; *therapeutic use]
MeSH check words: Humans
BACKGROUND
This new review is an update of a previous Cochrane review titled ‘Gabapentin for acute and chronic pain’ (Wiffen 2005), which was an extension to a review previously published in The Cochrane Library on ‘Anticonvulsant drugs for acute and chronic pain’ (Wiffen 2011a). The effects of gabapentin in established acute postoperative pain have been published as a separate review in 2010 (Straube 2010).
The decision to split the review was undertaken after discussions with the Editor-in-Chief of The Cochrane Collaboration at a meeting in Oxford in early 2009. That meeting was in response to controversy in the USA over the effectiveness of gabapentin as an analgesic (Landefeld 2009) together with calls for the 2005 review to be updated with the inclusion of unpublished information made available through litigation (Vedula 2009). It was agreed to update the review by splitting the earlier one into two components: this review looking at the role of gabapentin in chronic neuropathic pain (including neuropathic pain of any cause, and fibromyalgia), and a second one to determine the effects of gabapentin in acute postoperative pain (Straube 2010). Other reviews may examine gabapentin in chronic musculoskeletal pain. Since the earlier review was published in 2005, unpublished data have been released by the licence holders of the first gabapentin product to be marketed, and these data have been included in this updated review.
Description of the condition
Chronic pain is a major health problem affecting one in five people in Europe (Breivik 2006). Chronic pain is usually defined by a period of about three to six months during which pain is felt every day or almost every day. Any pain that is not chronic is acute, though there are always special circumstances, using these definitions, where either or neither are entirely satisfactory. Data for the incidence of neuropathic pain (pain resulting from a disturbance of the central or peripheral nervous system) are difficult to obtain. Estimates in the UK indicate incidences per 100,000 person-years observation of 40 (95% confidence interval (CI) 39 to 41) for postherpetic neuralgia, 27 (26 to 27) for trigeminal neuralgia, 1 (1 to 2) for phantom limb pain, and 15 (15 to 16) for painful diabetic neuropathy, with rates decreasing in recent years for phantom limb pain and postherpetic neuralgia and increasing for painful diabetic neuropathy (Hall 2006). The prevalence of neuropathic pain in Austria was reported as being 3.3% (Gustorff 2008), 6.9% in France (Bouhassira 2008), and in the UK as high as 8% (Torrance 2006).
Antiepileptic drugs (also known as anticonvulsants) have been used in pain management since the 1960s, very soon after they were first used for their original indication. The clinical impression is that they are useful for neuropathic pain, especially when the pain is lancinating or burning (Jacox 1994). There is evidence for the effectiveness of a number of antiepileptics including carbamazepine, pregabalin, phenytoin and valproate; these have been considered in other reviews published by the Cochrane Pain, Palliative and Supportive Care review group (Moore 2009a; Wiffen 2005; Wiffen 2011a; Wiffen 2011b). The use of antiepileptic drugs in chronic pain has tended to be confined to neuropathic pain (like painful diabetic neuropathy), rather than nociceptive pain (like arthritis). Antepileptics are sometimes prescribed in combination with antidepressants, as in the treatment of postherpetic neuralgia (Monks 1994). In the UK carbamazepine and phenytoin are licensed for the treatment of pain associated with trigeminal neuralgia, and gabapentin and pregabalin more generally for the treatment of neuropathic pain, though licensed indications vary in different parts of the world.
Description of the intervention
Gabapentin (original trade name Neurontin) is licensed for the treatment of peripheral and central neuropathic pain in adults in the UK at doses up to 3.6 grams (3600 mg) daily. It is given orally, as tablets or capsules. Guidance suggests that gabapentin treatment can be started at a dose of 300 mg per day for treating neuropathic pain. Based on individual patient response and tolerability, the dosage may be increased by 300 mg per day until pain relief (or intolerable adverse effects) is experienced (EMC 2009). US marketing approval for gabapentin was granted in 2002 for postherpetic neuralgia; in Europe, the label was changed to include peripheral neuropathic pain in 2006. Gabapentin is also now available as generic products in some parts of the world.
How the intervention might work
Gabapentin is thought to act by binding to calcium channels and modulating calcium influx as well as influencing GABAergic neurotransmission (i.e. neurotransmission affected by gabapentin). This mode of action confers antiepileptic, analgesic and sedative effects. The most recent research indicates that gabapentin acts by blocking new synapse formation (Barres 2009).
Why it is important to do this review
Gabapentin is widely prescribed for neuropathic pain and it is common practice in some countries to aim for the maximum tolerated dose. There is growing controversy over whether this practice is justified by experimental evidence from double-blind randomised trials.
Neuropathic pain is a complex and often disabling condition in which many people suffer moderate or severe pain for many years. Conventional analgesics are usually not effective in alleviating the symptoms, though opioids may be effective in some individuals. Treatment is usually by unconventional analgesics such as antidepressants or antiepileptics. The reason is that neuropathic pain, unlike nociceptive pain (pain that arises from nerve endings detecting unpleasant or painful stimuli), such as arthritis, or gout, is caused by nerve damage, often accompanied by changes in the central nervous system.
There have been several changes in how efficacy of both conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes used today are better defined, particularly with new criteria of what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with ‘any improvement’. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be longer, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, we are now applying stricter criteria for inclusion of trials and assessment of outcomes, and we are more aware of problems that may affect our overall assessment.
To summarise, some of the recent insights into studies in neuropathic pain and chronic pain more generally that make a new review necessary, over and above including more trials are:
Pain relief results tend to have a U-shaped distribution rather than a bell-shaped distribution, with participants either achieving very good levels of pain relief, or little or none. This is the case for acute pain (Moore 2005a), fibromyalgia (Straube 2010), and arthritis (Moore 2009b); in all cases average results usually describe the actual experience of almost no-one in the trial. Continuous data expressed as averages should be regarded as potentially misleading, unless it can be proved to be suitable. Systematic reviews now frequently report results for responders (Lunn 2009; Moore 2010a; Straube 2008; Sultan 2008).
This means we have to depend on dichotomous results usually from pain changes or patient global assessments. The IMMPACT group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials shorter than 12 weeks, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2009b); the effect is particularly strong for less effective analgesics. What is not always clear is how withdrawals are reported. Withdrawals can be high in some chronic pain conditions (Moore 2005b; Moore 2010b).
The proportion with at least moderate benefit can be small, falling from 60% with an effective medicine in arthritis, to 30% in fibromyalgia (Moore 2009b; Straube 2008; Sultan 2008). A Cochrane Review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009a). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
Finally, individual patient analyses indicate that patients who get clinically useful pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a major way (Hoffman 2010; Moore 2010c). Good response to pain predicts good effects for other troublesome symptoms like sleep, fatigue and depression.
These are by no means the only issues of trial validity that have been raised recently. A summary of what constitutes evidence in trials and reviews in chronic pain has been published (Moore 2010d), and this review has attempted to address all of them, so that the review is consistent with current best practice.
This Cochrane Review concentrates on evidence in ways that make both statistical and clinical sense. Studies included and analysed meet a minima of reporting quality (blinding, randomisation), validity (duration, dose and timing, diagnosis, outcomes, etc.), and size (ideally a minimum of 500 participants in a comparison with the Number needed to treat to benefit (NNTs) of four or greater (Moore 1998)).
This review covers chronic neuropathic pain (including fibromyalgia), concentrating for efficacy on dichotomous responder outcomes. We consider conditions individually, as there is evidence of different effects in different neuropathic pain conditions for some interventions like pregabalin (Moore 2009a), though less so for others (Lunn 2009). The review also considers additional risks of bias. These include issues of withdrawal (Moore 2010b), size (Moore 1998; Nuesch 2010), and duration (Moore 2010a) in addition to standard risks of bias.
OBJECTIVES
To assess the analgesic efficacy of gabapentin for chronic neuropathic pain.
To assess the adverse effects associated with the clinical use of gabapentin for chronic neuropathic pain.
METHODS
Criteria for considering studies for this review
Types of studies
We included studies in this review if they were randomised controlled trials (RCTs) with double-blind (participant and observers) assessment of participant-reported outcomes, following two weeks of treatment or longer, though the emphasis of the review is on studies of 6 weeks or longer. Full journal publication was required, with the exception of extended abstracts of otherwise unpublished clinical trials (for example detailed information from PDFs of posters that typically include all important details of methodology used and results obtained). We did not include short abstracts (usually meeting reports with inadequate or no reporting of data). We excluded studies of experimental pain, case reports, and clinical observations.
Types of participants
We included adult participants aged 18 years and above. Participants could have one or more of a wide range of chronic neuropathic pain conditions including (but not limited to):
painful diabetic neuropathy (PDN);
postherpetic neuralgia (PHN);
trigeminal neuralgia;
phantom limb pain;
postoperative or traumatic neuropathic pain;
complex regional pain syndrome (CRPS);
cancer-related neuropathy;
HIV-neuropathy;
spinal cord injury;
fibromyalgia.
We also included studies of participants with more than one type of neuropathic pain. We analysed results according to the primary condition.
Types of interventions
Gabapentin in any dose, by any route, administered for the relief of neuropathic pain and compared to placebo, no interventionor any other active comparator. We did not include studies using gabapentin to treat pain resulting from the use of other drugs.
Types of outcome measures
Studies had to report pain assessment as either a primary or secondary outcome.
A variety of outcome measures were used in the studies. The majority of studies used standard subjective scales for pain intensity or pain relief, or both. Particular attention was paid to IMMPACT definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as at least 30% pain relief over baseline (moderate), at least 50% pain relief over baseline (substantial), much or very much improved on Patient Global Impression of Change (PGIC) (moderate), and very much improved on PGIC (substantial). These outcomes are different from those set out in the previous review, concentrating on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50%, and with pain not worse than mild (O’Brien 2010).
Primary outcomes
Patient reported pain intensity reduction of 30% or greater.
Patient reported pain intensity reduction of 50% or greater.
Patient reported global impression of clinical change (PGIC) much or very much improved.
Patient reported global impression of clinical change (PGIC) very much improved.
Secondary outcomes
Any pain-related outcome indicating some improvement.
Withdrawals due to lack of efficacy.
Participants experiencing any adverse event.
Participants experiencing any serious adverse event.
Withdrawals due to adverse events.
Specific adverse events, particularly somnolence and dizziness.
During the updating process we discussed and reached consensus concerning a common core data set for pain reviews, and to reflect that we also used a working set of seven outcomes that might form a core data set. This overlapped to some extent with outcomes already identified:
at least 50% pain reduction;
proportion below 30/100 mm on a visual analogue scale (no worse than mild pain);
patient global impression;
functioning;
adverse event (AE) withdrawal;
serious AE;
death.
We considered the possibility of using these outcomes, but aside from functioning they were already included in primary and secondary outcomes chosen (with death noted as a serious adverse event).
Search methods for identification of studies
Electronic searches
The following databases were searched:
the Cochrane Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, issue 12);
MEDLINE (via OVID) to January 2011; and
EMBASE (via OVID) to January 2011.
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy, and Appendix 3 for the CENTRAL search strategy. All relevant articles found were identified on PubMed and using the ‘related articles’ feature, a further search was carried out for newly published articles.
There was no language restriction. All relevant articles found were identified on PubMed and using the ‘related articles’ feature, a further search was carried out for newly published articles.
Searching other resources
We searched reference lists of retrieved articles and reviews for any additional studies. We searched the PhRMA clinical study results database (www.clinicalstudyresults.org) for trial results of gabapentin in painful conditions.
Data collection and analysis
Selection of studies
All potentially relevant studies identified by the search were read independently by two review authors to determine eligibility, and agreement reached by discussion. The studies were not anonymised in any way before assessment. All publications that could not clearly be excluded by screening the title and abstract were obtained in full and read.
Data extraction and management
Three review authors extracted data (RAM, PW, SD) using a standard data extraction form, and agreed data before entry into RevMan or any other analysis method. Data extracted included information about the pain condition and number of participants treated, drug and dosing regimen, study design, study duration and follow up, analgesic outcome measures and results, withdrawals and adverse events (participants experiencing any adverse event, or serious adverse event).
Assessment of risk of bias in included studies
We used the ‘Risk of bias’ tool to assess the likely impact on the strength of the evidence of various study characteristics relating to methodological quality (randomisation, allocation concealment and blinding), study validity (duration, outcome reporting, and handling of missing data), and size (Appendix 4).
We also scored each report independently for quality using a three-item scale (Jadad 1996). We then met to agree a ‘consensus’ score for each report. Quality scores were not used to weight the results in any way.
The three-item scale is as follows:
Is the study randomised? If ‘yes’, then score one point.
If described, is the randomisation appropriate? If ‘yes’ then add one point, if not deduct one point.
Is the study double-blind? If ‘yes’, then add one point.
Is the double-blind method appropriate? If ‘yes’ then add one point, if not deduct one point.
Are withdrawals and drop-outs described? (i.e. the number and reason for drop-outs for each of the treatment groups).
If ‘yes’, then add one point.
Low quality scores of two and below have been associated with greater estimates of efficacy than studies of higher quality (Khan 1996).
Measures of treatment effect
Relative risk (or ‘risk ratio’, RR) was used to establish statistical difference. NNT and pooled percentages were used as absolute measures of benefit or harm.
The following terms are used to describe adverse outcomes in terms of harm or prevention of harm:
When significantly fewer adverse outcomes occurred with gabapentin than with control (placebo or active) we use the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occurred with gabapentin compared with control (placebo or active) we use the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
The control treatment arm would be split between active treatment arms in a single study if the active treatment arms were not combined for analysis.
Dealing with missing data
We used intention-to-treat (ITT) analysis wherever possible. The ITT population consisted of participants who were randomised, took the assigned study medication and provided at least one post-baseline assessment. Missing participants were assigned zero improvement (baseline observation carried forward, BOCF) where this could be done. We were aware that imputation methods might be problematical and examined trial reports for information about them.
Assessment of heterogeneity
We dealt with clinical heterogeneity by combining studies that examined similar conditions. We assessed statistical heterogeneity visually (L’Abbe 1987) and with the use of the I2 statistic.
Assessment of reporting biases
The aim of this review was to use dichotomous data of known utility (Moore 2009b). The review did not depend on what authors of the original studies chose to report or not report, though clearly there were difficulties with studies failing to report any dichotomous results. Continuous data, which probably poorly reflect efficacy and utility, were extracted and used only when useful for illustrative purposes.
We undertook no statistical assessment of publication bias.
We looked for effects of possible enrichment, either complete or partial, in enrolment of participants into the studies. Enrichment typically means including participants known to respond to a therapy, and excluding those known not to respond, or to suffer unacceptable adverse effects, though for gabapentin no significant effects have been shown from partial enrichment (Straube 2008). Enriched enrolment randomised withdrawal studies, known to produce higher estimates of efficacy, would not be pooled (McQuay 2008).
Data synthesis
We used dichotomous data to calculate relative risk or benefit (risk ratio) with 95% CIs using a fixed-effect model, together with NNTs (Cook 1995). This was done for effectiveness, for adverse effects and for drug-related study withdrawal. We also undertook meta-analysis when sufficient clinically similar data were available. We calculated NNTs as the reciprocal of the absolute risk reduction (McQuay 1998). For unwanted effects, the NNT becomes the NNH (number needed to treat to harm), and is calculated in the same way. In the absence of dichotomous data, summary continuous data are reported where available and appropriate, but no analysis was carried out.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analysis for:
dose of gabapentin;
duration of studies; and
different painful conditions.
Sensitivity analysis
We planned no sensitivity analyses because the evidence base was known to be too small to allow reliable analysis.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
In this split update of the original review (Wiffen 2005) we made no attempt to contact authors or manufacturers of gabapentin. Clinical trial reports or synopses from previously unpublished studies became available as a result of legal proceedings in the USA. In the previous update, an author confirmed that one study was randomised but could provide no additional data (Perez 2000).
Included studies
The original chronic pain review included 14 studies with 1392 participants in 13 reports. Two of those studies were excluded in this review update: one postherpetic neuralgia (PHN) study because it was open (Dallocchio 2000), and one in Guillain Barré syndrome because it is now not considered to be a chronic neuropathic pain condition (Pandey 2002). Furthermore, the second of two studies in painful diabetic neuropathy (PDN) reported inSimpson 2001 was not considered because it was a test of additional venlafaxine, not of gabapentin.
An additional 18 studies with 2263 participants were included, bringing the total to 29 studies in 29 reports, involving 3571 participants. A number of chronic painful conditions were studied:
Postherpetic neuralgia; five studies, 1197 participants (Chandra 2006; Irving 2009; Rice 2001; Rowbotham 1998;Wallace 2010).
Painful diabetic neuropathy; eight studies, 1183 participants (Backonja 1998; CTR 945-1008; CTR 945-224;Gorson 1999; Morello 1999; Perez 2000; Sandercock 2009;Simpson 2001).
Mixed neuropathic pain; three studies, 418 participants (Gilron 2005; Gilron 2009; Serpell 2002).
Fibromyalgia; one study, 150 participants (Arnold 2007).
Complex regional pain syndrome type I; one study, 58 participants (van de Vusse 2004).
Spinal cord injury pain; three studies, 65 participants (Levendoglu 2004; Rintala 2007; Tai 2002).
Nerve injury pain; one study, 120 participants (Gordh 2008).
Phantom limb pain; two studies, 43 participants (Bone 2002; Smith 2005).
Cancer-related neuropathic pain; two studies, 236 participants (Caraceni 2004; Rao 2007).
HIV painful sensory neuropathy; one study, 26 participants (Hahn 2004).
Masticatory myalgia; one study, 50 participants (Kimos 2007).
Small fibre sensory neuropathy; one study, 54 participants (Ho 2009).
Three quarters of the participants (2398) were enrolled in studies of PHN, PDN, or mixed neuropathic pain. The other nine neuropathic pain conditions were studied in 802 participants, with the largest numbers in cancer-related neuropathic pain (236 participants), fibromyalgia (150) and nerve injury pain (120).
Sixteen of the studies had a parallel-group design and 13 had a cross-over design (Bone 2002; Gilron 2005; Gilron 2009; Gordh 2008; Gorson 1999; Ho 2009; Levendoglu 2004; Morello 1999;Rao 2007; Rintala 2007; Smith 2005; Tai 2002; van de Vusse 2004). We used whatever data were available from cross-over studies, including first period or multiple periods, though there are major issues with what constitutes the intention-to-treat (ITT) denominator where there are significant withdrawals.
Parallel-group trials were larger than cross-over trials. The 16 parallel-group studies involved 2967 participants (mean 185, median 154 participants, range 26 to 400), while the 13 cross-over studies involved 633 participants (mean 49, median 40 participants, range 7 to 120). Not all studies reported the results on an ITT basis, and this was particularly the case for cross-over studies with multiple comparisons.
Twenty-one studies either described enrolment processes that were not enriched, or had no exclusion criteria that would raise the possibility of enrichment (Straube 2008). Six studies were partially enriched (Caraceni 2004; Irving 2009; Rice 2001; Rowbotham 1998; Serpell 2002) or had previous treatment with gabapentin or pregabalin as an exclusion criterion, which may have led to enrichment (Arnold 2007; Wallace 2010). One study had complete enrichment (Ho 2009).
Twenty-five studies either made no mention of an imputation method for missing data (19) or declared use of last observation carried forward (LOCF) (6). Others performed analyses on completers only (van de Vusse 2004), one presented results without imputation (Rao 2007), and in one we could not decide how data had been treated (Ho 2009).
Details of all eligible studies are given in the ‘Characteristics of included studies’ table.
Excluded studies
Several other studies were considered but excluded for various reasons. These included open studies (Arai 2010; Dallochio 2000; Jean 2005; Keskinbora 2007; Ko 2010; Salvaggio 2008;Sator-Katzenschlager 2005; Yaksi 2007), studies in chronic conditions not considered for this review (McCleane 2001; Pandey 2002; Pandey 2005; Sator-Katzenschlager 2005; Yaksi 2007), acute treatment of herpes zoster (Berry 2005; Dworkin 2009), and trials in surgery to prevent chronic phantom pain (Nikolajsen 2006). We also excluded an n-of-1 study in chronic neuropathic pain (Yelland 2009) with complete enrichment, high withdrawals, and short (two-week) treatment periods because this design is rare and interpretation very difficult. Details of excluded studies are given in the ‘Characteristics of excluded studies’ table.
Risk of bias in included studies
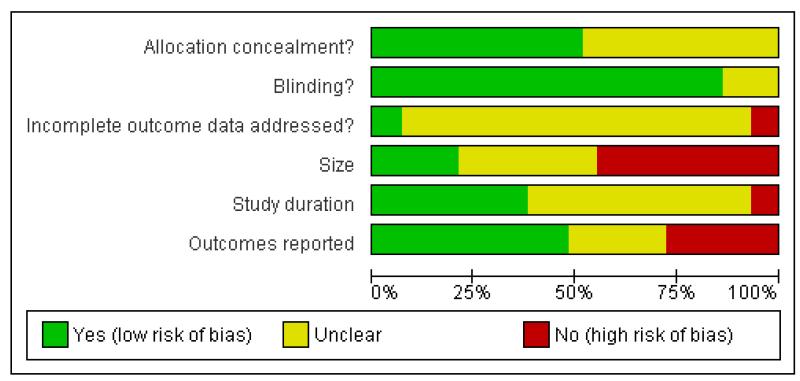
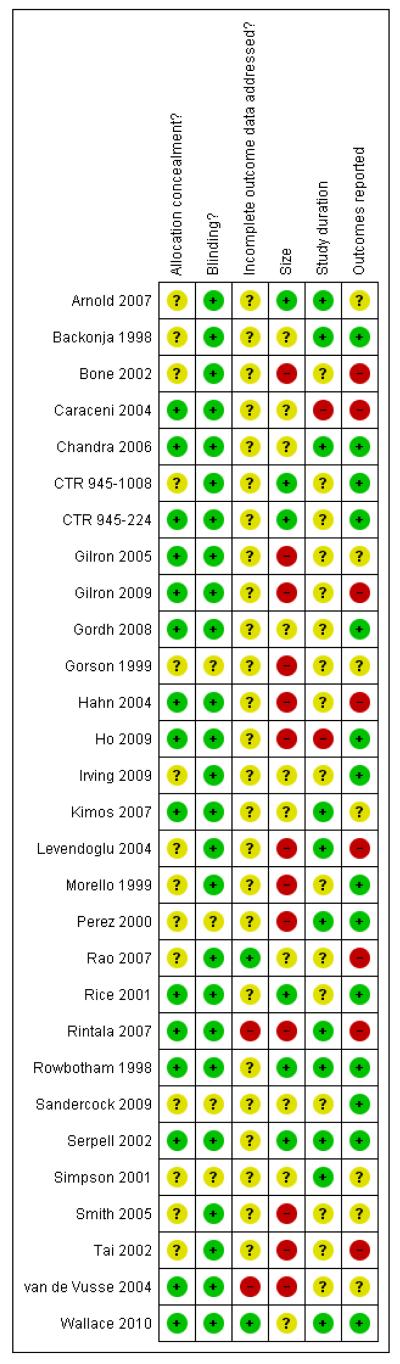
Reporting quality was largely good. On the five point Oxford Scale addressing randomisation, blinding, and withdrawals, two studies scored 2/5 points, two 3/5 points, eight 4/5 points, and 17 5/5 points. Studies with scores of 3/5 and above are considered unlikely to be subject to major systematic bias (Khan 1996). Points were lost mainly for inadequate descriptions of randomisation. The risk of bias assessments (Figure 1; Figure 2) emphasised this, with adequate sequence generation and allocation concealment being most often inadequately reported. Additional risk of bias also derived from studies being small, reporting unhelpful outcomes, rarely describing how efficacy data were handled on withdrawal, and being of short duration.
Figure 1.
Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Figure 2.
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Effects of interventions
Appendix 5 contains details of withdrawals, efficacy, and adverse events in the individual studies.
Efficacy outcomes
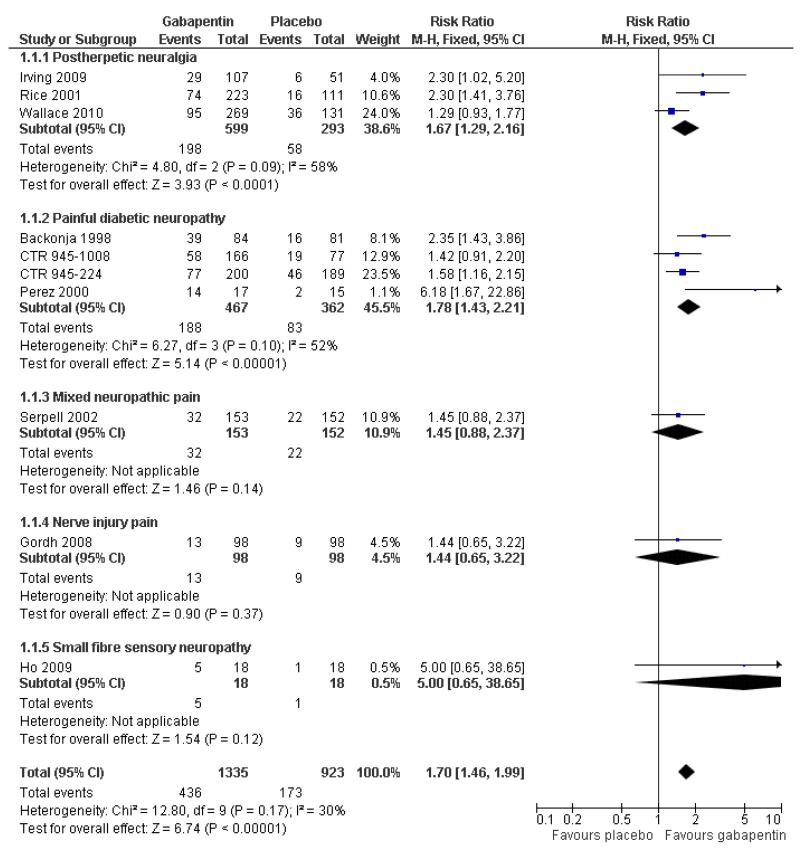
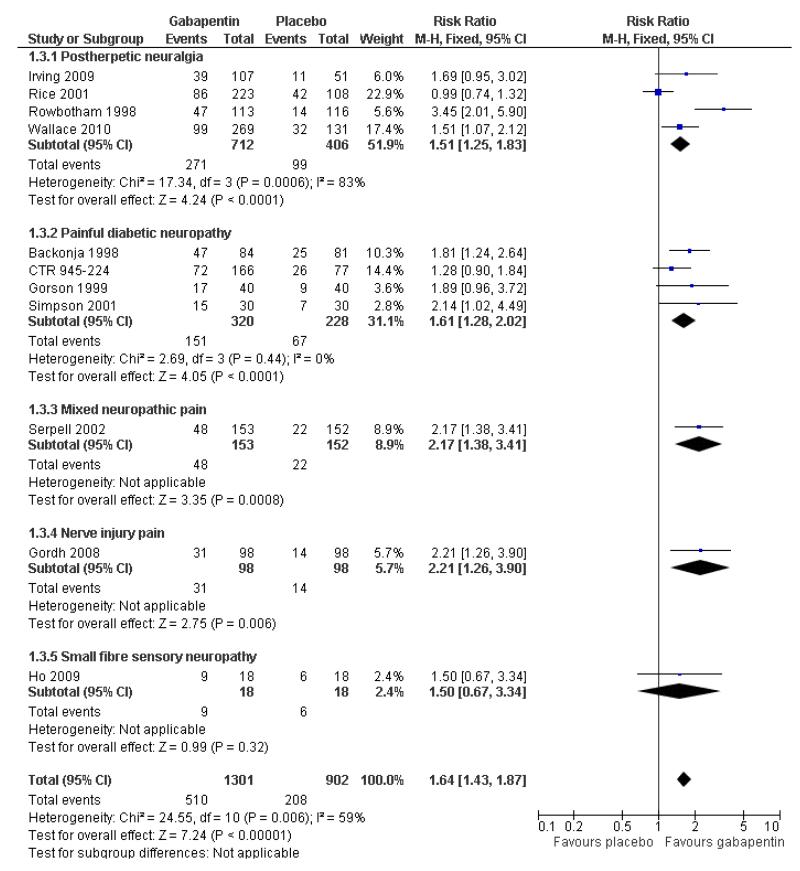
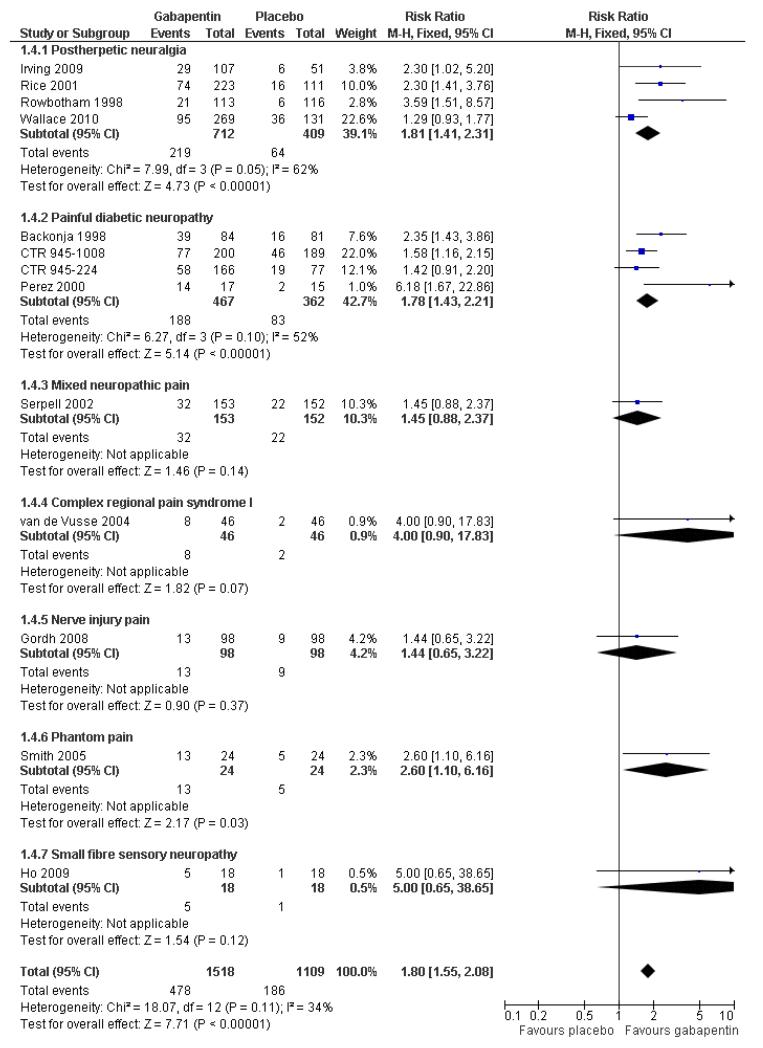
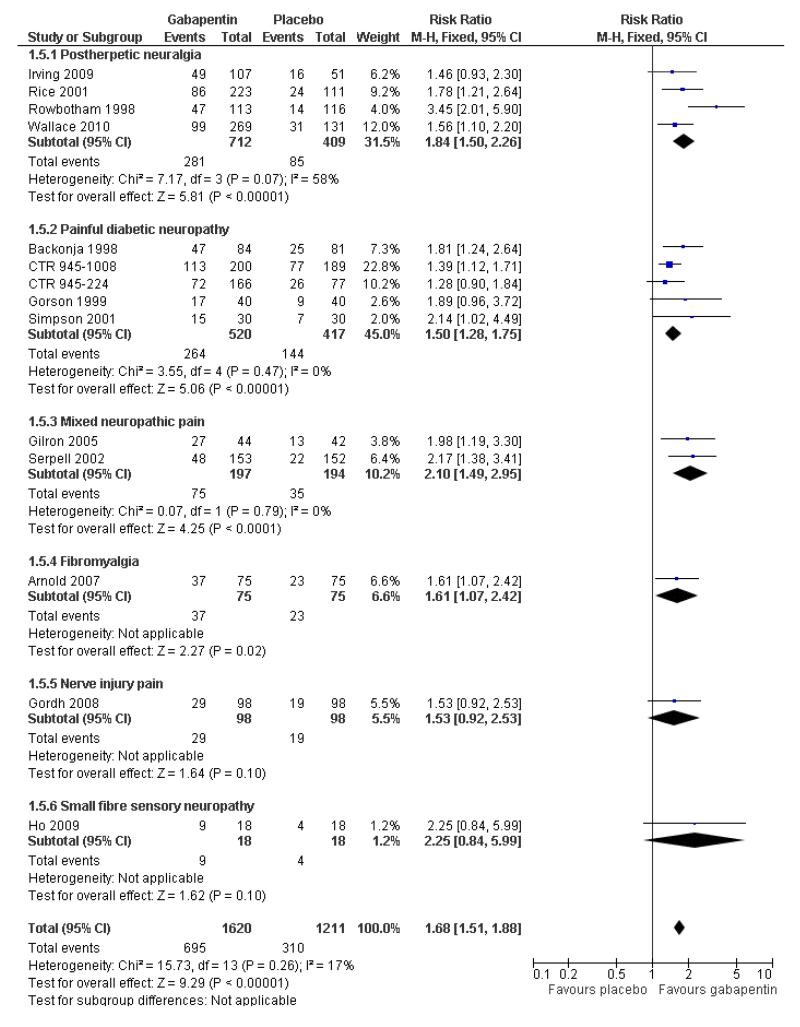
Analyses 1.1 to 1.5 show results for the following outcomes: at least 50% reduction in pain (Analysis 1.1; Figure 3); PGIC very much improved (Analysis 1.2; Figure 4); PGIC much or very much improved (Analysis 1.3; Figure 5); IMMPACT outcome of substantial improvement in pain (Analysis 1.4; Figure 6); IMMPACT outcome of at least moderate improvement in pain (Analysis 1.5; Figure 7).
Figure 3.
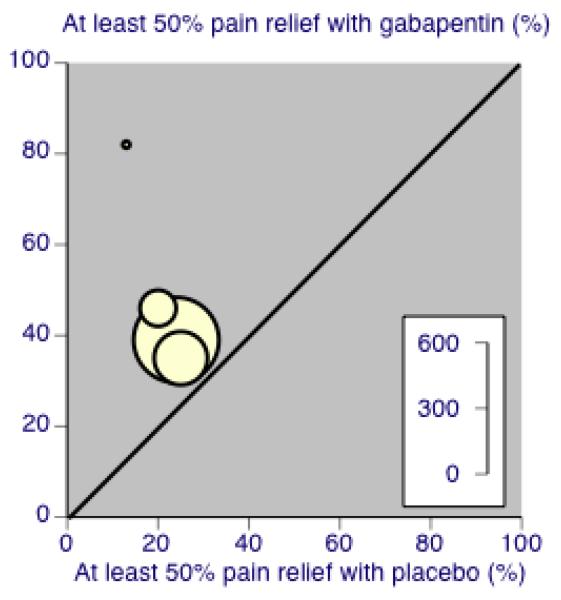
Forest plot of comparison: 1 All placebo-controlled studies, outcome: 1.1 At least 50% pain reduction over baseline.
Figure 4.
Forest plot of comparison: 1 All placebo-controlled studies, outcome: 1.2 Very much improved.
Figure 5.
Forest plot of comparison: 1 All placebo-controlled studies, outcome: 1.3 Much or very much improved.
Figure 6.
Forest plot of comparison: 1 All placebo-controlled studies, outcome: 1.4 IMMPACT outcome of substantial improvement.
Figure 7.
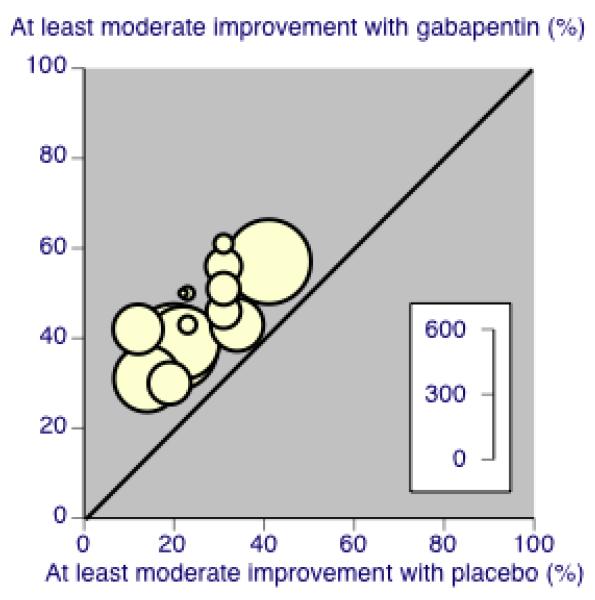
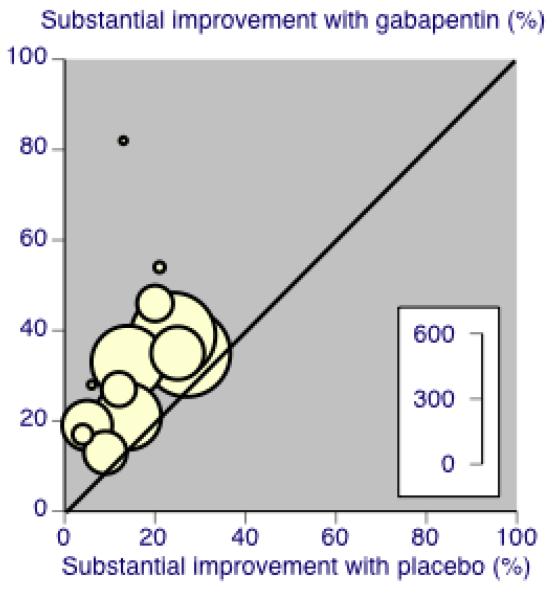
Percentage of participants achieving outcomes equivalent to IMMPACT at least moderate improvement, all doses, all conditions
Postherpetic neuralgia (PHN)
Of the five studies in PHN, four (Irving 2009; Rice Rowbotham 1998; Wallace 2010) had a placebo control only, one (Chandra 2006) an active control only. All four trolled studies had a parallel-group design, with study of four, seven, eight, and 10 weeks respectively; daily gabapentin doses varied between 1800 mg and 3600 mg.
A number of outcomes consistent with IMMPACT recommendations for substantial and moderate benefit were reported in two or more placebo-controlled studies, and the results showed gabapentin at doses of 1800 mg daily or more to be more effective than placebo (Summary of results A). For a PGIC (Patient Global Impression of Change) of much or very much improved; 39% of participants achieved this level of improvement with gabapentin and 18% with placebo. There were insufficient data for subgroup analyses based on dose or duration of studies.
Summary of results A: Efficacy outcomes with gabapentin in postherpetic neuralgia
| Number of | Percent with outcome | |||||
|---|---|---|---|---|---|---|
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio (95% CI) | NNT (95% CI) |
| Substantial benefit | ||||||
| At least 50% pain relief | 3 | 892 | 33 | 20 | 1.7 (1.3 to 2.2) | 7.5 (5.2 to 14) |
| PGIC very much improved | 2 | 563 | 15 | 6 | 2.7 (1.5 to 4.8) | 11 (7.0 to 22) |
| Moderate benefit | ||||||
| PGIC much or very much improved | 4 | 1121 | 38 | 20 | 1.9 (1.5 to 2.3) | 5.5 (4.3 to 7.7) |
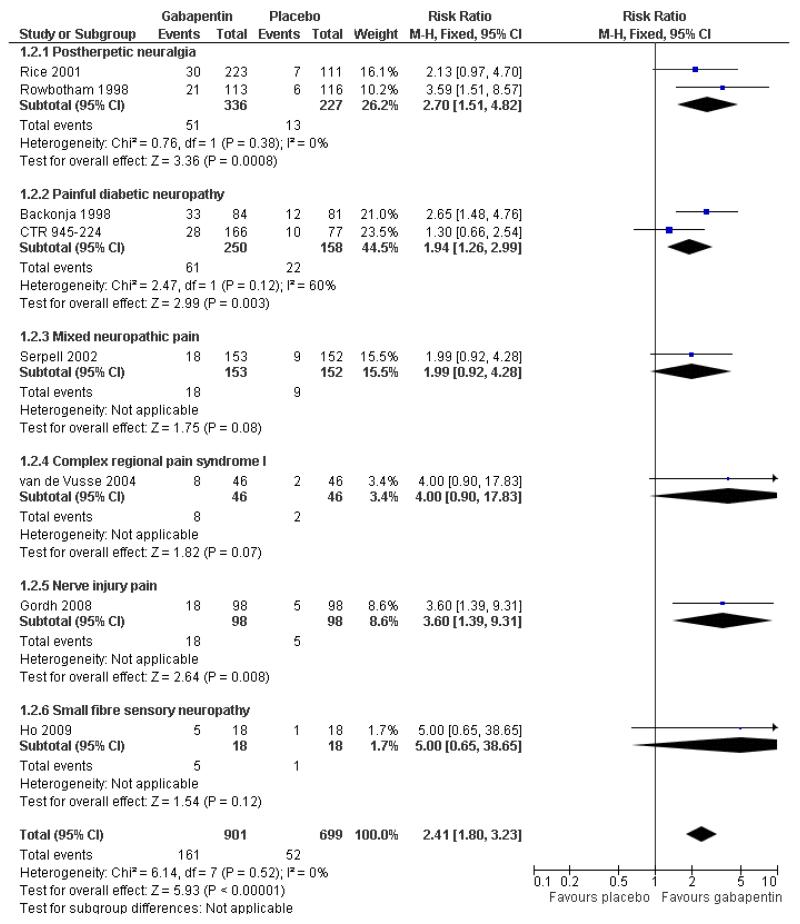
In the active controlled study involving 76 participants, gabapentin at doses of up to 2700 mg daily was compared to nortriptyline at doses of up to 150 mg daily over nine weeks. At least 50% improvement in pain over baseline using a VAS pain scale was achieved by 13/38 (34%) on gabapentin and 14/38 (37%) on nortriptyline, broadly in line with event rates in placebo-controlled studies (Chandra 2006).
Painful diabetic neuropathy (PDN)
Six of the eight studies in PDN were of parallel-group design (Backonja 1998; CTR 945-1008; CTR 945-224; Perez 2000;Sandercock 2009; Simpson 2001); two had a cross-over design (Gorson 1999; Morello 1999). Seven had a placebo comparator only, while one (Morello 1999) had an active control only. Six placebo-controlled parallel-group studies had a study duration between four and 14 weeks; all but one (Sandercock 2009) of seven weeks or longer. Daily gabapentin doses varied between 600 mg and 3600 mg; doses below 1200 mg were used in two studies, 900 mg daily as the only gabapentin dose in one (Gorson 1999), and 600 mg daily in one arm of another (CTR 945-224).
A number of outcomes consistent with IMMPACT recommendations for substantial and moderate benefit were reported in two or more placebo-controlled studies, and the results showed gabapentin at doses of 1200 mg daily or more to be more effective than placebo (Summary of results B). For PGIC much or very much improved; 43% of participants achieved this level of improvement with gabapentin and 31% with placebo, with very similar results when results from Simpson 2001 were omitted because of concerns one reviewer expressed about this study; no other efficacy outcome included data from this study. For the largest data set of at least 50% pain relief over baseline, there was consistency between studies (Figure 8). There were insufficient data for subgroup analyses based on dose or duration of studies.
Figure 8.
Painful diabetic neuropathy: Percentage of participants achieving at least 50% pain relief over baseline with gabapentin 1200-3600 mg daily, or placebo
Summary of results B: Efficacy outcomes with gabapentin in painful diabetic neuropathy (1200 mg daily or greater)
| Number of | Percent with outcome | |||||
|---|---|---|---|---|---|---|
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio (95% CI) | NNT (95% CI) |
| Substantial benefit | ||||||
| At least 50% pain relief | 4 | 829 | 40 | 23 | 1.8 (1.4 to 2.2) | 5.8 (4.3 to 9.0) |
| PGIC very much improved | 2 | 408 | 24 | 14 | 1.9 (1.3 to 3.0) | 9.6 (5.5 to 35) |
| Moderate benefit | ||||||
| PGIC much or very much improved | 3 | 466 | 43 | 31 | 1.5 (1.1 to 1.9) | 8.1 (4.7 to 28) |
| PGIC much or very much improved (excluding Simpson 2001) | 2 | 406 | 42 | 32 | 1.4 (1.1 to 1.8) | 9.9 (5.1 to 190) |
One other placebo-controlled study indicated that 41% of participants taking gabapentin 3000 mg daily achieved at least 50% reduction in average daily pain over four weeks compared with 12% with placebo (Sandercock 2009), but without giving the numbers of participants in each study treatment arm. Gabapentin 600 mg daily produced lesser effects than 1200 mg and 2400 mg daily in a study that compared them (CTR 945-224). In one placebo-controlled cross-over study involving 40 randomised participants, moderate or excellent pain relief was achieved by 17/40 (43%) with gabapentin 900 mg daily over six weeks, compared with 9/ 40 (23%) with placebo (Gorson 1999).
In one active controlled study involving 25 participants, gabapentin at 1800 mg daily was compared to amitriptyline 75 mg daily over six weeks. Complete or a lot of pain relief was achieved by 6/21 (29%) with gabapentin and 5/21 (24%) with amitripty-line (Morello 1999).
Mixed neuropathic pain
Three studies examined the effects of gabapentin in mixed neuropathic painful conditions (Gilron 2005; Gilron 2009; Serpell 2002); two included participants with PHN and PDN (Gilron 2005; Gilron 2009) and in the other the most common conditions were complex regional pain syndrome and PHN (Serpell 2002). One had a parallel-group comparison with placebo over eight weeks (Serpell 2002). The others had cross-over designs that included placebo and morphine alone and in combination with gabapentin over five weeks (Gilron 2005), and nortriptyline alone or in combination with gabapentin over six weeks (Gilron 2009). The parallel-group comparison with placebo (Serpell 2002) used gabapentin titrated to a maximum of 2400 mg daily in 305 participants. Only for the PGIC outcome of much or very much improved was there a significant benefit of gabapentin (Summary of results C).
Summary of results C: Efficacy outcomes with gabapentin in mixed neuropathic pain (Serpell 2002)
| Number of | Percent with outcome | |||||
|---|---|---|---|---|---|---|
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio (95% CI) | NNT (95% CI) |
| At least 50% pain relief | 1 | 305 | 21 | 14 | 1.5 (0.9 to 2.4) | not calculated |
| PGIC very much improved | 1 | 305 | 12 | 6 | 2.0 (0.9 to 4.3) | not calculated |
| PGIC much or very much improved | 1 | 305 | 31 | 14 | 2.2 (1.4 to 3.4) | 5.9 (3.8 to 13) |
One placebo-controlled cross-over study (Gilron 2005) over five weeks provided results for moderate pain relief for participants who completed a given treatment period. Gabapentin alone (target dose 3200 mg daily), morphine alone (target dose 120 mg daily), and the combination (target dose gabapentin 2400 mg plus 60 mg morphine daily) were significantly better than placebo (Summary of results D). These results were calculated from the numbers and percentages with a moderate response. The total is larger than the 57 randomised, because some will have participated in more than one treatment arm.
Summary of results D: Efficacy outcomes with gabapentin in mixed neuropathic pain (Gilron 2005)
| Number of | Percent with outcome | |||||
|---|---|---|---|---|---|---|
| At least moderate pain relief | Studies | Participants | Gabapentin | Placebo | Risk ratio (95% CI) | NNT (95% CI) |
| Gabapentin alone | 1 | 96 | 61 | 25 | 2.5 (1.5 to 4.2) | 2.8 (1.8 to 5.6) |
| Morphine alone | 1 | 96 | 80 | 25 | 3.2 (1.9 to 5.2) | 1.8 (1.4 to 2.7) |
| Gabapentin plus morphine | 1 | 93 | 78 | 25 | 3.1 (1.9 to 5.1) | 1.9 (1.4 to 2.8) |
The other cross-over study compared gabapentin alone (target dose 3600 mg daily), nortriptyline (target dose 100 mg daily) and the combination (target dose 3600 mg gabapentin plus 100 mg nortriptyline daily) over six weeks (Gilron 2009). Pain intensity was significantly lower with the combination, by less than one point out of 10 on a numerical rating pain scale.
Fibromyalgia
The efficacy of gabapentin in fibromyalgia at maximum doses of 2400 mg daily was compared with placebo in 150 participants in a single placebo (diphenhydramine) controlled parallel-group study lasting 12 weeks (Arnold 2007). The outcome of 30% reduction in pain over baseline was reported, with 38/75 participants (49%) achieving the outcome with gabapentin compared with 23/75 (31%) with placebo. The relative benefit was 1.6 (1.1 to 2.4) and the NNT was 5.4 (2.9 to 31).
Complex regional pain syndrome
The efficacy of gabapentin in complex regional pain syndrome at maximum doses of 1800 mg daily was compared with placebo in 58 participants in a single placebo-controlled cross-over study lasting three weeks in each period (van de Vusse 2004). Over both periods, and using per protocol reporting, “much” pain improvement (undefined) was achieved by 8/46 (17%) with gabapentin compared with 2/46 (4%) with placebo. There was no significant difference, with a relative benefit of 4.0 (0.9 to 18).
Spinal cord injury
The efficacy of gabapentin in spinal cord injury pain at maximum doses of 1800 mg or 3600 mg daily was compared with placebo in three cross-over trials (Levendoglu 2004; Rintala 2007; Tai 2002) over periods of four and eight weeks. None of the studies reported dichotomous outcomes equivalent to moderate or substantial pain relief.
One eight-week study randomised 20 participants to a maximum of 3600 mg gabapentin daily or placebo over eight weeks (Levendoglu 2004) and reported a 62% average fall in pain with gabapentin compared with a 13% fall with placebo.
A second eight-week study randomised 38 participants to a maximum of 3600 mg gabapentin daily, amitriptyline 150 mg daily, or placebo over eight weeks (Rintala 2007). It claimed statistical superiority for amitriptyline for the 22 participants completing all three phases, and no benefit of gabapentin over placebo.
The final study comparing gabapentin with placebo over four weeks in seven participants had no interpretable results (Tai 2002).
Nerve injury pain
A single cross-over study evaluated the efficacy of gabapentin at a maximum of 2400 mg daily compared with placebo over five-week treatment periods (Gordh 2008). Among the 98 participants of the 120 randomised and who completed both treatment periods, at least 50% pain relief was achieved by 13 (13%) on gabapentin and 9 (9%) on placebo, which did not reach statistical significance, risk ratio 1.4 (0.7 to 3.2). At least 30% pain relief was achieved by 29 (29%) on gabapentin and 19 (19%) on placebo, which did not reach statistical significance, risk ratio 1.5 (0.9 to 2.5).
Phantom limb pain
Two cross-over studies evaluated the efficacy of gabapentin compared with placebo in phantom limb pain (Bone 2002; Smith 2005). One (Bone 2002) randomised 19 participants to a maximum of 2400 mg gabapentin daily, or the maximum tolerated dose, with six-week treatment periods. Using an ITT approach, weekly VAS pain scores were lower at week six only with gabapentin, but not at any other time, nor with categorical pain measures. The other (Smith 2005) randomised 24 participants to gabapentin titrated to a maximum daily dose of 3600 mg. A “meaningful decrease in pain” (the top of a five-point scale) was achieved by 13 participants (54%) with gabapentin and 5 (21%) with placebo, a statistically significant difference, with risk ratio 2.6 (1.1 to 6.2).
Cancer-related neuropathic pain
Two studies examined gabapentin in the short term in cancer-related neuropathic pain (Caraceni 2004; Rao 2007). A parallel-group study (Caraceni 2004) randomised 121 participants to titration to a maximum of gabapentin 1800 mg daily or placebo, with 10 days of treatment. The average pain intensity was somewhat lower with gabapentin than with placebo, but the number of participants described as having pain under control was very similar with both treatments after six days, with 50% to 60% with pain under control over six to 10 days. A cross-over study (Rao 2007) compared gabapentin titrated to 2700 mg daily with placebo in chemotherapy-induced neuropathic pain over three weeks. There was no significant difference between gabapentin and placebo, but the study did recruit participants both with pain and sensory loss or paraesthesia, and baseline pain scores were only about 4/10 on a numerical rating scale. The study probably lacked sensitivity to detect any difference.
HIV-associated sensory neuropathies
A single parallel-group study compared gabapentin titrated to 2400 mg daily with placebo over four weeks in 24 participants with painful HIV-associated neuropathies (Hahn 2004). On average, pain and sleep improved substantially with gabapentin and placebo, though time courses differed.
Chronic masticatory myalgia
A single parallel-group study compared gabapentin titrated to 4200 mg daily with placebo over 12 weeks in 50 participants with painful chronic masticatory myalgia, where pain is associated with central sensitisation (Kimos 2007). Gabapentin was significantly better than placebo for VAS pain, pain reduction, and VAS function, and an NNT of 3.4 for gabapentin compared with placebo was reported, though no details were recorded about outcome.
Small fibre sensory neuropathies
A single cross-over study with complete enrichment, compared gabapentin at doses up to 4800 mg daily with tramadol 50 mg (probably four times a day), and placebo in 18 participants with small fibre sensory neuropathies using two-week treatment periods (Ho 2009). The number achieving at least 50% pain relief was 4/ 18 (22%) with gabapentin, 4/18 (22%) with tramadol, and 1/18 (6%) with placebo. Similar results were obtained for those feeling very much better.
Overall efficacy across all conditions
Assessing efficacy across all conditions was complicated by different reporting of outcomes, and the limited number of studies reporting the same outcome. This was possible for certain outcomes, including IMMPACT definitions of substantial and at least moderate improvement (Summary of results E). The following analyses include the single completely enriched study (Ho 2009), though this contributed 2% or fewer participants to the analyses, and its omission made no discernable difference to the results.
Nine studies with 1858 participants reported the outcome of at least 50% pain intensity reduction over baseline by the end of the study (Analysis 1.1; Figure 4). The outcome was achieved by 32% on gabapentin 1200 mg daily or greater, and 17% on placebo. The relative benefit was 1.8 (1.5 to 2.2) and the NNT 6.8 (5.4 to 9.2). Eight studies with 1600 participants reported the outcome equivalent to be very much improved (or top point on global rating scale) by the end of the study (Analysis 1.2; Figure 5). The outcome was achieved by 18% on gabapentin 1200 mg daily or greater, and 7% on placebo. The relative benefit was 2.4 (1.8 to 3.2) and the NNT 9.6 (7.4 to 14).
Ten studies with 1701 participants reported the outcome equivalent to be much or very much improved (or top two points on global rating scale) by the end of the study (Analysis 1.3; Figure 6). The outcome was achieved by 41% on gabapentin 1200 mg daily or greater, and 23% on placebo. The relative benefit was 1.7 (1.5 to 2.0) and the NNT 5.7 (4.6 to 7.6).
IMMPACT definitions (Summary of results E)
Two further analyses were conducted across all studies and all doses of gabapentin to assess efficacy according to IMMPACT definitions of substantial improvement (using at least 50% pain intensity reduction for preference over very much improved), and for moderate improvement (using at least 30% pain intensity reduction for preference over much or very much improved).
Twelve studies with 2227 participants reported the outcome equivalent to IMMPACT as “substantial” improvement by the end of the study (Analysis 1.4; Figure 6). The outcome was achieved by 31% on gabapentin 900 mg daily or greater, and 15% on placebo. The relative benefit was 1.9 (1.6 to 2.3) and the NNT 6.5 (5.3 to 8.4). Results were consistent across trials (Figure 9).
Figure 9.
Percentage of participants achieving outcomes equivalent to IMMPACT substantial improvement, all doses, all conditions
Thirteen studies with 2431 participants reported the outcome equivalent to IMMPACT of “at least moderate” improvement by the end of the study (Analysis 1.5; Figure 10). The outcome was achieved by 44% on gabapentin 900 mg daily or greater, and 26% on placebo. The relative benefit was 1.7 (1.5 to 1.9) and the NNT 5.5 (4.5 to 6.8). Results were consistent across trials (Figure 7).
Figure 10.
Forest plot of comparison: 1 All placebo-controlled studies, outcome: 1.5 IMMPACT outcome of at least moderate improvement.
Subgroup analyses for both IMMPACT definitions limited to parallel-group studies lasting six weeks or more produced virtually identical results as those for the ‘all studies’ analysis that included cross-over studies, and those shorter than six weeks.
Summary of results E: Efficacy outcomes across all conditions
| Number of | Percent with outcome | |||||
|---|---|---|---|---|---|---|
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio (95% CI) | NNT (95% CI) |
| Gabapentin doses 1200 mg daily or more | ||||||
| At least 50% pain relief | 10 | 2258 | 33 | 19 | 1.7 (1.5 to 2.0) | 7.2 (5.7 to 9.7) |
| PGIC very much improved | 8 | 1600 | 18 | 7 | 2.4 (1.8 to 3.2) | 9.6 (7.4 to 14) |
| PGIC much or very much improved | 11 | 2101 | 40 | 23 | 1.7 (1.5 to 1.9) | 6.1 (4.9 to 8.0) |
| Gabapentin doses 900 mg daily or more | ||||||
| IMMPACT definition - any substantial pain benefit | 13 | 2627 | 31 | 17 | 1.8 (1.6 to 2.1) | 6.8 (5.6 to 8.7) |
| IMMPACT definition - any substantial pain benefit parallel-group studies ≥ 6 weeks | 8 | 2097 | 33 | 19 | 1.7 (1.5 to 2.0) | 6.8 (5.4 to 9.0) |
| IMMPACT definition - any at least moderate pain benefit | 14 | 2831 | 43 | 26 | 1.7 (1.5 to 1.9) | 5.8 (4.8 to 7.2) |
| IMMPACT definition - any at least moderate pain benefit parallel-group studies ≥ 6 weeks | 9 | 2275 | 43 | 26 | 1.7 (1.4 to 2.0) | 5.9 (4.8 to 7.6) |
Withdrawals (see Summary of results F)
Adverse event withdrawals
Seventeen studies with 3022 participants reported on adverse event withdrawals, which occurred in 12% of participants on gabapentin at daily doses of 1200 mg or more, and in 8% on placebo (Analysis 2.1). The risk ratio was 1.4 (1.1 to 1.7), and the NNH 32 (19 to 100).
All-cause withdrawals
Seventeen studies with 3063 participants reported on withdrawals for any cause, which occurred in 20% of participants on gabapentin at daily doses of 1200 mg or more, and in 19% on placebo (Analysis 2.2). The risk ratio was 1.1 (0.9 to 1.2).
Adverse events (see Summary of results F)
Participants experiencing at least one adverse event
Eleven studies with 2356 participants reported on participants experiencing at least one adverse event, which occurred in 66% of participants on gabapentin at daily doses of 1200 mg or more, and in 51% on placebo (Analysis 3.1). The risk ratio was 1.3 (1.2 to 1.4), and the NNH was 6.6 (5.3 to 9.0).
Serious adverse events
Fourteen studies reported on 2702 participants experiencing a serious adverse event, which occurred in 4.0% of participants on gabapentin at daily doses of 1200 mg or more, and in 3.2% on placebo (Analysis 3.2). The risk ratio was 1.3 (0.9 to 2.0).
Particular adverse events
Somnolence, drowsiness, or sedation was reported as an adverse event in 16 studies with 2800 participants, and it occurred in 16% of participants on gabapentin at doses of 1200 mg daily or more, and in 5% on placebo (Analysis 3.3). The risk ratio was 3.2 (2.5 to 4.2), and the NNH was 9.2 (7.7 to 12).
Dizziness was reported as an adverse event in 16 studies with 3150 participants, and it occurred in 21% of participants on gabapentin at doses of 1200 mg daily or more, and in 7% on placebo (Analysis 3.4). The risk ratio was 3.2 (2.5 to 4.2), and the NNH was 7.0 (6.1 to 8.4).
Peripheral oedema was reported as an adverse event in nine studies with 2042 participants, and it occurred in 8.2% of participants on gabapentin at doses of 1200 mg daily or more, and in 2.9% on placebo (Analysis 3.5). The risk ratio was 3.4 (2.1 to 5.3), and the NNH was 19 (14 to 29).
Ataxia or gait disturbance was reported as an adverse event in five studies with 544 participants. It occurred in 26/295 (8.8%) participants on gabapentin at doses of 1200 mg daily or more, and in 3/249 (1.1%) on placebo, though all but one study reported no events with placebo (Analysis 3.6). This produced a risk ratio of 4.5 (1.9 to 11), and the NNH was 13 (9 to 24).
Summary of results F: Withdrawals and adverse events with gabapentin (1200 mg daily or more) compared with placebo
| Number of | Percent with outcome | |||||
|---|---|---|---|---|---|---|
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio (95% CI) | NNH (95% CI) |
| Withdrawal due to adverse events | 17 | 3022 | 12 | 8 | 1.4 (1.1 to 1.7) | 32 (19 to 100) |
| Withdrawal - all-cause | 17 | 3063 | 20 | 19 | 1.1 (0.9 to 1.2) | Not calculated |
| At least one adverse event | 11 | 2356 | 66 | 51 | 1.3 (1.2 to 1.4) | 6.6 (5.3 to 9.0) |
| Serious adverse event | 14 | 2702 | 4.0 | 3.2 | 1.3 (0.9 to 2.0) | Not calculated |
| Somnolence/drowsiness | 16 | 2800 | 16 | 5 | 3.2 (2.5 to 4.2) | 9.2 (7.7 to 12) |
| Dizziness | 16 | 3150 | 21 | 7 | 3.2 (2.6 to 4.1) | 7.0 (6.1 to 8.4) |
| Peripheral oedema | 9 | 2042 | 8.2 | 2.9 | 3.4 (2.1 to 5.3) | 19 (14 to 29) |
| Ataxia/gait disturbance | 5 | 544 | 8.8 | 1.2 | 4.5 (1.9 to 11) | 13 (9 to 24) |
Death
Deaths were rare in these studies. Four deaths occurred in PHN studies; two with placebo - one in 116 participants (Rowbotham 1998) and one in 133 (Wallace 2010); two with gabapentin - one in 223 participants (Rice 2001) and one in 107 (Irving 2009). An unpublished study (CTR 945-1008) reported two deaths; one of 200 participants treated with gabapentin, and one of 189 treated with placebo. A further study reported two deaths in 152 participants taking placebo (Serpell 2002). Overall, three deaths occurred with gabapentin and five with placebo.
DISCUSSION
Summary of main results
Gabapentin is a reasonably effective treatment for a variety of neuropathic pain conditions. It has been demonstrated to be better than placebo across all studies for IMMPACT outcomes of substantial and to have at least moderately important benefit, producing almost identical results for all trials and those in parallel-group studies lasting six weeks or longer. Numbers needed to treat to benefit (NNTs) were 6.8 (5.6 to 8.7) and 5.8 (4.8 to 7.2) for substantial and at least moderately important benefits, respectively. Results were consistent across the major neuropathic pain conditions tested, though some uncommon conditions could only be tested in small numbers.
Though gabapentin was tested in 12 different neuropathic pain conditions, only for three was there sufficient information to be confident that it worked satisfactorily, namely PHN, PDN, and mixed neuropathic pain, itself principally, though not exclusively, PHN and PDN.
Benefit was balanced by more withdrawals due to adverse events, and participants taking gabapentin experienced more adverse events, including somnolence, dizziness, peripheral oedema, and gait disturbance than did those taking placebo. Serious adverse events were no more common with gabapentin than placebo, and death was an uncommon finding in these studies.
Overall completeness and applicability of evidence
Efficacy and adverse event outcomes were not consistently reported across the studies, and this limited the analyses to some extent. However, for the most important efficacy and adverse event outcomes, analyses across all conditions were mostly based on between 1000 and over 3000 participants. All the larger studies (typically those with more than 100 participants) reported some efficacy outcome that fitted one or both of the IMMPACT outcomes of at least moderate or substantial benefit. Clearly, analysis at the level of the individual patient would facilitate a more robust estimate. There is one important unknown, namely whether the declaration of response in the trials was for participants who had both an analgesic response and were able to take gabapentin. If response included an LOCF assessment of efficacy from those who discontinued, this could have affected the results. Currently we have no knowledge of the size of any effect, and the practice in these studies is likely to have been the same as that in studies of other drug treatments in neuropathic pain - namely LOCF.
We understand that research has been done on a gabapentin pro-drug and a gabapentin gastric retention formulation. The total sample size of neuropathic pain subjects in these studies exceeds 1200 participants and so could meaningfully affect the numbers reported in this review. These studies are not yet published, but the review should be updated as soon as adequate data become available. Two studies of extended release gabapentin (Irving 2009;Wallace 2010) included in this review produced results not dissimilar from other formulations.
One difficulty is how to deal with relatively short term, relatively small, multiple cross-over studies that intensively study participants on a daily basis (Gilron 2005; Gilron 2009), but that do not report outcomes of clinical relevance (participants with adequate pain relief), but rather average pain scores, whose relevance has been questioned because of underlying skewed distributions (Moore 2010d). These studies can provide useful and clinically relevant information, like the relatively rapid onset of effect of therapies in neuropathic pain, even with average data.
There were almost no data for direct comparisons with other active treatments. This becomes important now that efficacy for gabapentin in neuropathic pain has been established, so that it’s place in relation to alternative therapy can be determined.
Quality of the evidence
The studies included in this review covered a large number of different painful conditions. For some, like HIV neuropathy for instance, it is unclear whether antiepileptic drugs such as gabapentin are effective in the condition. The main quality issues involve reporting of outcomes of interest, particularly dichotomous outcomes equivalent to IMMPACT, as well as better reporting of adverse events. The earliest study was published in 1998, and the past decade or so has seen major changes in clinical trial reporting. The studies themselves appear to be well-conducted, and individual patient analysis could overcome some of the shortcomings of reporting.
Potential biases in the review process
The review was restricted to randomised double-blind studies, thus limiting the potential for bias. Other possible sources of bias that could have affected the review included:
Duration - NNT estimates of efficacy in chronic pain studies tend to increase (get worse) with increasing duration (Moore 2010a). However, limiting studies to those of six weeks or longer did not change the main efficacy outcomes, mainly because most participants were in longer duration studies.
Outcomes may effect estimates of efficacy, but the efficacy outcomes chosen were of participants achieving the equivalent of IMMPACT-defined moderate or substantial improvement, and it is likely that lesser benefits, such as “any benefit” or “any improvement”, are potentially related to lesser outcomes, though this remains to be clarified.
The dose of gabapentin used differed between studies, in terms of maximum allowable dose, whether the dose was fixed, titrated to effect, or titrated up to the maximum irrespective of beneficial or adverse effects. We chose to pool data irrespective of dose, within broad limits, because it was the only practical way to deal with dose in a pooled analysis, and because of a lack of good evidence of any clear dose response for gabapentin in neuropathic pain.
The question of whether cross-over trials exaggerate treatment effects in comparison with parallel-group designs, as has been seen in some circumstances (Khan 1996), is unclear but unlikely to be the source of major bias (Elbourne 2002). Withdrawals meant that any results were more likely to be per protocol for completers than for a true ITT analysis. Parallel-group studies were larger than cross-over studies, and dominated analyses in terms of number of participants. The 15 parallel-group studies involved 2567 participants (median 150 participants), while the 13 cross-over studies involved 633 participants (median 40 participants). The cross-over studies were therefore dominated by results from larger parallel-group studies and, additionally, few cross-over studies reported outcomes that could be used in the analyses.
The absence of publication bias (unpublished trials showing no benefit of gabapentin over placebo) can never be proven. However, we can calculate the number of participants in studies of zero benefit (risk ratio of one) that would be required for the absolute benefit to reduce beneficial effects to a negligible amount (Moore 2008). If an NNT of 10 were considered a level that would make gabapentin clinically irrelevant, then for moderate benefit across all types of neuropathic pain there would have to be 1989 participants in zero effect studies, and for substantial benefit 1200 participants. With median study size for parallel-group studies, this would require a minimum of eight unavailable studies, or four studies of the largest size. This number of unavailable studies seems unlikely.
Agreements and disagreements with other studies or reviews
Previous version of this review
This review differs from the original review (Wiffen 2005) from which it was split in to two parts (acute pain (Straube 2010) and this review on Chronic neuropathic pain and fibromyalgia) in two major respects:
It uses strict definitions of what constitutes at least moderate and substantial benefit as defined by the 2008 IMMPACT criteria (Dworkin 2008). The previous review used a hierarchy of outcomes (pain relief of 50% or greater, global impression of clinical change, pain on movement, pain on rest or any other pain-related measure) that would have allowed any pain benefit to have been counted. That was reasonable, and continued a process of demonstrating that antiepileptic drugs effectively relieved pain in neuropathic pain conditions that began a decade earlier (McQuay 1995). This present review uses developing considerations that people with chronic pain want high levels of pain relief, ideally with more than 50% pain relief, and pain not worse than mild (O’Brien 2010), a result not dissimilar to that in cancer pain (Farrar 2000). Use of more stringent outcomes is likely to lead to lower estimates of efficacy, as has been described in acute migraine (Oldman 2002).
It has available many more studies and participants - at 3571 participants nearly two and a half times as many as before, including two previously unpublished studies with over 700 participants in PDN. The new information available derives from more modern studies with better reporting, and especially better reporting of dichotomous efficacy outcomes, and includes previously unpublished information, as has been recommended (Vedula 2009).
A consequence of the stringent definition of outcome and the larger numbers available has resulted in a reduction in estimates of efficacy over all studies, and for PHN and PDN analysed separately, as shown by increased NNTs (Summary of results G). Decreased estimate of efficacy was most noticeable for PDN, for which previously unpublished results made a major contribution to the updated review.
Summary of findings G: Comparison of NNTs from previous and present reviews
| Previous review | Current review | ||
|---|---|---|---|
| Outcomes | Improvement | IMMPACT moderate benefit | IMMPACT substantial benefit |
| All studies | 4.3 (3.5 to 5.7) | 5.8 (4.8 to 7.2) | 6.8 (5.6 to 8.7) |
| PHN | 3.9 (3.0 to 5.7) | 5.5 (4.3 to 7.7) | 7.5 (5.2 to 14) |
| PDN | 2.9 (2.2 to 4.3) | 8.1 (4.7 to 28) | 5.8 (4.3 to 9.0) |
Other systematic reviews
One other review has provided NNTs for gabapentin in different neuropathic pain conditions based on 50% pain relief, quoting NNTs of 4.7 and 4.3 for neuropathic pain and peripheral pain, and 4.6 and PHN and 3.9 for PDN (Finnerup 2005). A systematic review of therapies for PHN considered gabapentin effective, with an NNT of 4.6 (Hempenstall 2005). These efficacy estimates are also more optimistic than NNTs for IMMPACT substantial benefit calculated for this review, and more optimistic than NNTs calculated for the same outcome of at least 50% pain relief for PHN of 5.7 and PDN of 5.8. The use of more stringent criteria for efficacy, and availability of more information from longer duration studies has led to more conservative efficacy results. Both pregabalin and duloxetine produce NNTs in the region of five to six for at least 50% pain relief over eight to 12 weeks compared with placebo in PHN and PDN (Lunn 2009; Moore 2009a; Sultan 2008).
A number of other systematic reviews have examined efficacy of gabapentin in neuropathic pain. Systematic reviews of gabapentin for neuropathic pain in spinal cord injury (Tzellos 2008) and fibromyalgia (Hauser 2009) found no more studies than those reported here. An examination of the effects of enriched enrolment found no more studies, and produced similar results for withdrawals and adverse events based on a more limited data set (Straube 2008). A review comparing gabapentin and duloxetine in PDN was limited to two gabapentin studies, was statistical in nature, and restricted to average changes in some efficacy parameters (Quilici 2009). The most directly relevant was a comparison between gabapentin and tricyclic antidepressants (Chou 2009), in which a meta-analysis of six placebo-controlled gabapentin studies in PHN, PDN, and mixed neuropathic pain was performed. Using a mixture of outcomes the relative benefit compared with placebo was 2.2, similar to those found for the ‘all studies’ analysis and for analyses for PHN, PDN, and mixed neuropathic pain in this review. A systematic review of pregabalin and gabapentin in fibromyalgia (Hauser 2009) reported only on the single study identified in this review, but reported overall good reductions in pain and other outcomes, with no major difference between gabapentin and pregabalin.
There is one further review in the public domain (Perry 2008) which was performed as part of a legal case in the United States ending in 2009. Perry 2008 did consider similar outcomes to this review; NRS/VAS pain score was given hierarchical priority between >50% reduction in pain score (higher priority) and PGIC (lower priority) mainly because it was the pre-defined primary end point in almost all studies, and for some studies it was difficult to determine how the secondary endpoints were manipulated during changes in statistical analysis plans post hoc. The Perry conclusions are very similar to those of the present review. The likely real differences would lie in the fact that Perry excluded Perez 2000 and Simpson 2001, and did not have access to Sandercock 2009,Irving 2009, and Wallace 2010 (not yet published).
Perry’s conclusion on effectiveness was a clinical judgement based on balancing NNH against NNT, using the Cochrane glossary definition of effectiveness, and presuming that inherent biases in the studies (enrichment, exclusion of many typical real world patients) implied that on balance the benefit of gabapentin use on average does not exceed the harm, which is a somewhat different issue than addressed by this Cochrane review.
AUTHORS’ CONCLUSIONS
Implications for practice
Gabapentin at doses of 1200 mg or more is effective for some people with painful neuropathic pain conditions. About 43% (almost one in two) can expect a moderately important benefit with gabapentin, and 31% (almost one in three) can expect a substantial benefit. Over half of those treated with gabapentin will not have worthwhile pain relief. Results might vary between different neuropathic pain conditions, and the amount of evidence for gabapentin in some conditions (all except PHN, PDN, mixed) is low, excluding any confidence that it works or does not work.
The levels of efficacy found for gabapentin are consistent with those found for other drug therapies in these conditions.
Implications for research
The main research directions that would help:
Analysis of all gabapentin studies at the level of the individual participant in order to have consistent outcomes, and analyses based on them. Individual patient analyses can provide important information, for example showing that good pain response delivers large functional and quality of life benefits beyond pain (Moore 2010c).
More research in to the efficacy of gabapentin in some painful neuropathic pain conditions where there is insufficient information. These conditions tend to be uncommon, and studies can be difficult, with few possible participants. Others, though, like fibromyalgia, are common.
The main issue, though, is not whether gabapentin is effective, as it clearly is highly effective in a minority of patients, but how best to use it in clinical practice. New study designs have been proposed to examine this (Moore 2009c).
PLAIN LANGUAGE SUMMARY.
Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Antiepileptic drugs like gabapentin are commonly used for treating neuropathic pain, usually defined as pain due to damage to nerves. This would include postherpetic neuralgia (persistent pain experienced in an area previously affected by shingles), painful complications of diabetes, nerve injury pain, phantom limb pain, fibromyalgia and trigeminal neuralgia. This type of pain can be severe and long-lasting, is associated with lack of sleep, fatigue, and depression, and a reduced quality of life. In people with these conditions, gabapentin is associated with a moderate benefit (equivalent to at least 30% pain relief) in almost one in two patients (43%), and a substantial benefit (equivalent to at least 50% pain relief) in almost one in three (31%). Over half of those taking gabapentin for neuropathic pain will not have good pain relief, in common with most chronic pain conditions. Adverse events are experienced by about two-thirds of people taking gabapentin, mainly dizziness, somnolence (sleepiness), oedema (swelling), and gait disturbance, but only about 1 in 10 (11%) have to stop the treatment because of these unpleasant side effects. Overall gabapentin provides pain relief of a high level in about a third of people who take it for painful neuropathic pain. Adverse events are frequent, but mostly tolerable. This review looked at evidence from 29 studies involving 3571 participants.
ACKNOWLEDGEMENTS
We wish to thank Dr Thomas Perry for directing us to clinical trial reports and synopses of published and unpublished studies of gabapentin, and Dr Mike Clarke and colleagues for their advice and support.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
NHS Cochrane Collaboration Programme Grant Scheme, UK.
European Union Biomed 2 Grant no. BMH4 CT95 0172, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Multicentre, randomised, double-blind, placebo-controlled, parallel-group, partial enrichment, LOCF Titration to limit of tolerability or maximum 2400 mg daily over 6 weeks, then 6 weeks stable dose (12 weeks in total) |
|
| Participants | Fibromyalgia (ACR criteria for diagnosis). N = 150 , median age 48 years, 90% women. PI at randomisation ≥4/10, initial pain score 5.8/10 Excluded: individuals with prior treatment with gabapentin or pregabalin |
|
| Interventions | Gabapentin 2400 mg daily (max), n = 75 Placebo, n = 75 Maximum dose 2400 mg daily, placebo was diphenhydramine Paracetamol and OTC NSAIDs allowed (no dose limit stated) |
|
| Outcomes | ≥ 30% reduction in pain Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 4 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “matching placebo” |
| Incomplete outcome data addressed? Efficacy |
Unclear | LOCF |
| Size Efficacy |
Yes | 229 |
| Study duration Efficacy |
Yes | 8 weeks |
| Outcomes reported | Unclear | ≥ 30% reduction in pain |
| Methods | Multicentre, randomised, double-blind, placebo-controlled, parallel-group, not enriched, LOCF Titration to maximum tolerated dose or 3600 mg daily over 4 weeks, then stable dose for 4 weeks (8 weeks in total) |
|
| Participants | Painful diabetic neuropathy. N = 165, mean age 53 years, 40% women. Pain duration > 3 months before treatment, PI ≥40/100 at randomisation, initial mean pain score 6.4/10 | |
| Interventions | Gabapentin 3600 mg daily (max), n = 84 Placebo, n = 81 Medication for diabetes control remained stable during study. Paracetamol (max 3 g daily) allowed |
|
| Outcomes | PGIC much or moderately improved ≥ 50% reduction in pain (CTR) PGIC much improved (CTR) PGIC moderately or much improved (CTR) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 Parke-Davies/Pfizer sponsored |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not reported |
| Blinding? All outcomes |
Yes | “supplied in identical capsules in blinded fashion”. “All participants were supplied with an equal number of capsules” |
| Incomplete outcome data addressed? Efficacy |
Unclear | LOCF |
| Size Efficacy |
Unclear | 165 |
| Study duration Efficacy |
Yes | 8 weeks |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, not enriched. No imputation method mentioned Titration to maximum tolerated dose or 2400 mg daily over 1 week, then stable dose for 5 weeks (6 weeks total); 1-week washout, then cross-over |
|
| Participants | Established phantom limb pain ≥ 6 months, N = 19, mean age 56 years, 21% women. PI before treatment > 3/10, initial pain score 6.4/10 14 completed both treatment periods |
|
| Interventions | Gabapentin 2400 mg daily (max) Placebo Paracetamol + codeine 500 mg/30mg (max 12 tablets daily) allowed as rescue medication. Stable, low doses of TCAs continued |
|
| Outcomes | No dichotomous efficacy data Adverse events |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not described - but probably OK - remote |
| Blinding? All outcomes |
Yes | “identical, coded medication bottles containing identical tablets of gabapentin or placebo” |
| Incomplete outcome data addressed? Efficacy |
Unclear | No imputation mentioned |
| Size Efficacy |
No | 19 randomised |
| Study duration Efficacy |
Unclear | 6 weeks each period |
| Outcomes reported | No | No dichotomous data |
| Methods | Randomised, double-blind, placebo-controlled, parallel-group, partial enrichment. No imputation method mentioned Titration to pain ≤ 3/10 or limit of tolerability, or maximum 1800 mg daily (10 days in total) |
|
| Participants | Neuropathic cancer pain despite regular systemic opioid therapy. N = 121, mean age 60 years, 56% women. Pain at randomisation ≥ 5/10, initial pain intensity 7.3/10 | |
| Interventions | Gabapentin 1800 mg daily (max), n = 80 Placebo, n = 41 Any previous analgesics continued unchanged. One additional dose of opioid allowed for rescue medication |
|
| Outcomes | No dichotomous efficacy data Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Remote pharmacy department provided numbered containers |
| Blinding? All outcomes |
Yes | “identical capsules” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
Unclear | 121 randomised |
| Study duration Efficacy |
No | 10 days |
| Outcomes reported | No | No dichotomous outcomes |
| Methods | Randomised, double-blind, active controlled, parallel-group, no enrichment Dose escalation every 2 weeks until adequate pain relief obtained or limit of tolerability, to maximum nortriptyline 150 mg daily or gabapentin 2700 mg daily by 4 weeks, then stable dose for 5 weeks (9 weeks in total) |
|
| Participants | Postherpetic neuralgia. N = 76, mean age 54 years, 50% women. Pain > 2 months after healing of skin rash. PI at randomisation ≥ 40/100, initial average daily pain score 5.7/10 | |
| Interventions | Gabapentin 2700 mg daily (max), n = 38 Nortriptyline 150 mg daily (max), n = 38 Of ‘responders’ ~80% gabapentin took 2700 mg daily, ~66% nortriptyline took 75 mg daily |
|
| Outcomes | ≥ 50% pain relief over baseline pain ≥ 50% pain relief over (VAS) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 Sponsored Pfizer/independent |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | “code supplied in sealed envelopes, opened at time of enrolment”, “drugs dispensed in sealed envelopes” |
| Blinding? All outcomes |
Yes | “drugs placed in identical capsules”, “matching placebo of nortriptyline” to blind different dosing schedules |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
Unclear | 76 randomised |
| Study duration Efficacy |
Yes | 9 weeks |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Multicentre, randomised, double-blind, placebo-controlled, parallel-group, no obvious enrichment, LOCF Titration from 300 mg/day to maximum tolerated dose or 3600 mg daily over 3 weeks, then stable dose for 12 weeks (15 weeks total) |
|
| Participants | Painful diabetic neuropathy. N =389, mean age 58 years, “more men than women”. Pain duration > 3 months, PI at randomisation ≥ 40/100 | |
| Interventions | Gabapentin 3600 mg daily (max), n = 200 Placebo, n = 189 |
|
| Outcomes | ≥ 30% reduction in pain ≥ 50% reduction in pain Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 4 Pfizer sponsored |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | Matching placebo |
| Incomplete outcome data addressed? Efficacy |
Unclear | LOCF |
| Size Efficacy |
Yes | 389 randomised |
| Study duration Efficacy |
Unclear | 14 weeks |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Multicentre, randomised, double-blind, placebo-controlled, parallel-group, no enrichment, probably LOCF Titration over 3 weeks to 600, 1200, or 2400 mg daily, then stable dose to 4 weeks (7 weeks total) |
|
| Participants | Painful diabetic neuropathy for 1 to 5 years. N = 325, mean age 60 years, 44% women. PI at randomisation ≥ 40/100, initial pain score 6.2/10 |
|
| Interventions | Gabapentin 600 mg, n = 82 Gabapentin 1200 mg, n = 82 Gabapentin 2400 mg, n = 84 Placebo, n = 77 |
|
| Outcomes | ≥ 50% reduction in pain score PGIC very much improved PGIC much or very much improved Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 Parke-Davis/Pfizer sponsored |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Randomisation code broken after last patient completed |
| Blinding? All outcomes |
Yes | Matching placebo |
| Incomplete outcome data addressed? Efficacy |
Unclear | Probably LOCF |
| Size Efficacy |
Yes | 325 randomised |
| Study duration Efficacy |
Unclear | 7 weeks |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Randomised, double-blind, placebo-controlled 4-period cross-over, no enrichment. No imputation method mentioned (but if half of scores missing, outcome considered missing) Titration to target doses or limit of tolerability over 3 weeks, then stable dose for 1 week, and tapered dose for 1 week (5 weeks in total); 3-day washout and cross-over to next treatment |
|
| Participants | PDN and PHN. N = 57, median age 62 years, 44% women. Pain ≥ moderate for 3 months, initial mean pain score 5.8/10 | |
| Interventions | Gabapentin 3200 mg daily (max) Morphine 120 mg daily (max) Gabapentin plus morphine 2400 mg/60 mg daily (max) Placebo (lorazepam) 1.6 mg Mean maximum tolerated doses: gabapentin alone 2207 ± 89 mg, morphine alone 45. 3 ± 3.9 mg, gabapentin + morphine 1705 ± 83 + 34.4 ± 2.6 mg |
|
| Outcomes | Pain relief for those completing a given treatment (5-point scale) Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | “concealed allocation schedule” prepared remotely |
| Blinding? All outcomes |
Yes | “identical appearing blue and grey capsules …. in accord with a double-dummy design” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | Although 57 randomised, data available 40-44 completing a given treatment |
| Study duration Efficacy |
Unclear | 5 weeks each period |
| Outcomes reported | Unclear | At least moderate pain relief |
| Methods | Randomised, double-blind, placebo-controlled 3-period cross-over, no enrichment. No imputation method mentioned Titration to target doses or limit of tolerability over 24 days, then stable dose for 1 week, and tapered dose for 1 week (6 weeks in total); 6-day washout and cross-over to next treatment |
|
| Participants | PDN and PHN. N = 56, median age 64 years, 40% women. Pain ≥ moderate for 6 months, initial mean pain score 5.4/10 | |
| Interventions | Gabapentin 3600 mg daily (max) Nortriptyline 100 mg daily (max) Gabapentin plus nortriptyline 3600 mg/100 mg daily (max) Mean (SE) maximum tolerated doses: gabapentin alone 2433 ± 106 mg, nortriptyline alone 62 ± 3.6 mg, gabapentin + nortriptyline 2180 ± 108 + 50 ± 3.5 mg |
|
| Outcomes | Pain relief (average) Withdrawals Adverse events |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | “concealed allocation” |
| Blinding? All outcomes |
Yes | “double dummy” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | Reporting on < 50 completing 2 periods |
| Study duration Efficacy |
Unclear | 5-week period on treatment |
| Outcomes reported | No | No dichotomous data |
| Methods | Multicentre, randomised, double-blind, placebo-controlled, cross-over, not enriched. No imputation method mentioned Titration over 2 weeks from 300 mg to maximum pain reliefat a tolerable dose or 2400 mg daily, then stable dose for 3 weeks (5 weeks total); 3-week washout, then cross-over |
|
| Participants | Peripheral nerve injury with pain ≥ 6 months. N = 120, mean age 49 years, 53% women. PI at randomisation > 30/100, initial pain intensity 53/100 Efficacy analysis based on 98 who completed both treatment periods |
|
| Interventions | Gabapentin 2400 mg daily (max) Placebo Mean daily dose of gabapentin 2243 ± 402 mg Paracetamol ± codeine and dextropropoxyphene permitted as rescue medication Analgesics and NSAIDs used by ~50% during study |
|
| Outcomes | ≥ 50% pain relief (weekly mean pain score) ≥ 30% pain relief Marked pain relief (5-point scale) Marked or moderate pain relief (5-point scale) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Central, remote allocation, “sealed code envelope” |
| Blinding? All outcomes |
Yes | “capsules that were identical in appearance” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
Unclear | 120 randomised |
| Study duration Efficacy |
Unclear | 5-week period |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, not enriched. No imputation method mentioned Titration over 3 days to 900 mg, then fixed dose for remainder of 6-week period; 3 week washout, then cross-over |
|
| Participants | Painful diabetic neuropathy 1 to 5 years, pain ≥ moderate for over 3 months. N = 40, mean age 62 years, 23% women. Pain intensity at randomisation ≥ 40/100, initial pain intensity not reported | |
| Interventions | Gabapentin 900 mg, n = 19 (first phase) Placebo, n = 21 (first phase) Medication for diabetes control remained stable during study. Stable doses of NSAID or narcotics allowed |
|
| Outcomes | Pain relief at end of treatment (4-point global score) moderate or excellent Adverse events |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 0, Total = 3 Sponsored by Warner Lambert/Parke-Davis Note: no separate data for first period, small group sizes, non standard global scale |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not reported |
| Blinding? All outcomes |
Unclear | Not reported |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | 40 randomised |
| Study duration Efficacy |
Unclear | 6-week period |
| Outcomes reported | Unclear | Moderate or excellent pain relief |
| Methods | Randomised, double-blind, placebo-controlled, parallel-group, not enriched. No imputation method mentioned Titration over 2 weeks to adequate pain relief or 2400 mg daily, then stable dose for 2 weeks (4 weeks in total) |
|
| Participants | Painful HIV sensory neuropathy by standard definitions. N = 26, mean age 45 years, 23% women. Pain at any level including mild pain at randomisation, initial mean pain score 4.9/10 (lower limit of range 1.5) | |
| Interventions | Gabapentin 2400 mg daily (max), n = 15 (10 participants took max dose) Placebo, n = 11 |
|
| Outcomes | No dichotomous efficacy data Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Remote allocation |
| Blinding? All outcomes |
Yes | “identically appearing capsules” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | 26 randomised |
| Study duration Efficacy |
Unclear | 4 weeks |
| Outcomes reported | No | No dichotomous data |
| Methods | Randomised, double-blind, active placebo-controlled, cross-over. Analyses included all data available assuming that missing data were missing at random Titration over 1 week of gabapentin at pre-study dose (up to 4800 mg daily), tramadol 50 mg “q.i.d.” (probably once daily in USA - officially 4 times daily), or diphenhydramine 50 mg “qhs” (qh = every hour, but more likely 4 × daily) as active placebo, then stable dose for 1 week (2 weeks in total); 1-week washout, then cross-over to next treatment |
|
| Participants | Painful small fibre sensory neuropathy with gabapentin-sensitive pain that worsened with placebo, in a complete enrichment design. N = 18, mean age 59 years, 44% women. Pain at randomisation > 3, initial mean pain score 4.9/10 |
|
| Interventions | Gabapentin 4800 mg daily (max) Tramadol 200 mg daily (max) Placebo Stable pain medication other than gabapentin was continued Paracetamol (325 mg tablets, dose not specified) allowed for rescue medication. If inadequate patient could take additional 400 mg gabapentin, up to 1200 mg daily |
|
| Outcomes | ≥ 50 improvement in pain ≥ 30 improvement in pain PGIC very much better PGIC much or very much better Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Remote allocation |
| Blinding? All outcomes |
Yes | “matching capsules” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | 54 randomised to 3 groups. Gabapentin comparison with placebo 36 patients maximum |
| Study duration Efficacy |
No | 2 weeks |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Multicentre, randomised, double-blind, placebo-controlled, parallel-group, partial enrichment, LOCF, extended release formulation Gradual titration to 1800 mg over 2 weeks, then stable for 2 weeks (4 weeks in total) |
|
| Participants | Postherpetic neuralgia. N = 158, mean age 70 years, 53% women. Pain > 3 months after healing of skin rash, PI at randomisation ≥ 4/10, initial average daily pain score 6.5/10 | |
| Interventions | Gabapentin ER 1800 mg daily, n = 55 Gabapentin ER 1800 mg daily in split doses, n = 52 Placebo, n = 51 Rescue with paracetamol up to 4000 mg daily, or paracetamol plus hydrocodone 500 mg/5 mg up to 8 tablets daily |
|
| Outcomes | ≥ 50% reduction in pain score ≥ 30% reduction in pain score PGIC much or very much improved Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 Sponsored by Depomed |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | Double-dummy method |
| Incomplete outcome data addressed? Efficacy |
Unclear | LOCF |
| Size Efficacy |
Unclear | 158 randomised |
| Study duration Efficacy |
Unclear | 4 weeks |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Randomised, double-blind, placebo-controlled, parallel-group, not enriched. No imputation method mentioned Titration to adequate pain relief, limit of tolerability or 4200 mg daily, then stable dose for remainder of 12-week study |
|
| Participants | Chronic masticatory myalgia (pain classification based on defined criteria) lasting ≥ 6 months, not resulting from trauma or active inflammatory cause. N = 50, mean age 34 years, 100% women. PI at randomisation ≥ 50/100, initial average daily pain score 6. 2/10 | |
| Interventions | Gabapentin 4200 mg daily (max), n = 25 Placebo, n = 25 Stable doses of antidepressants continued Paracetamol (max 4000 mg daily) allowed as rescue medication |
|
| Outcomes | ≥ 30% reduction in pain Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 Note: withdrawals > 10% |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | “concealed randomization and the according allocation were implemented by a research assistant” (not involved with patients or investigators) |
| Blinding? All outcomes |
Yes | “identical looking capsules … packaged in identical clear bottles” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
Unclear | 50 randomised |
| Study duration Efficacy |
Yes | 12 weeks |
| Outcomes reported | Unclear | Pain reduction of 30% or more |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, not enriched. No imputation method mentioned Titration to limit of tolerability or maximum of 3600 mg over 4 weeks, then stable dose for remainder of 8-week period; 2-week washout then cross-over |
|
| Participants | Complete traumatic SCI at lumbar or thoracic level. N = 20, mean age 36 years, 35% women. Pain duration before treatment ≥ 6 months, PI at randomisation > 4/10, initial average daily pain 9/10 | |
| Interventions | Gabapentin 3600 mg daily (max) Placebo Mean max tolerated dose of gabapentin 2850 ± 751 mg No concurrent analgesics allowed |
|
| Outcomes | Pain reduction (mean data only) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 4 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not reported |
| Blinding? All outcomes |
Yes | “identically appearing capsules” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | 20 randomised |
| Study duration Efficacy |
Yes | 8-week period |
| Outcomes reported | No | No dichotomous data |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, not enriched. No imputation method mentioned Titration over 2 days and adjusted thereafter until adequate pain relief obtained or limit of tolerability to maximum 1800 mg gabapentin or 75 mg amitriptyline daily, then stable dose for remainder of 6-week period; 1-week washout, then cross-over |
|
| Participants | Painful diabetic neuropathy. N = 25, mean age 60 years, 4% women. Pain duration > 3 months before treatment, no initial PI at inclusion, initial pain intensity mild/moderate 19 completed 6 weeks with both study drugs | |
| Interventions | Gabapentin 1800 mg daily (max) Amitriptyline 75 mg daily (max) Paracetamol allowed as rescue medication (max 1300 mg daily) |
|
| Outcomes | Pain relief at end of treatment (6-point global score), complete or a lot Pain relief at end of treatment (6-point global score), at least moderate Adverse events Withdrawal |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 4 Note: no separate data for first period, small group sizes, non standard global scale |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not reported (all except clinical research pharmacist remained blinded until study termination) |
| Blinding? All outcomes |
Yes | “all capsules were identical in taste, color, size, and shape” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | 25 randomised |
| Study duration Efficacy |
Unclear | 6-week period |
| Outcomes reported | Yes | Complete, a lot of pain relief |
| Methods | Randomised, double-blind, placebo-controlled, parallel-group, not obviously enriched. No imputation method mentioned Dose adjusted on clinic successive visits, “based on clinical symptoms”, to a maximum of 1200 mg daily (12 weeks total) |
|
| Participants | Painful diabetic neuropathy. N = 32, mean age 54 years, 53% female. Failed conventional treatment. PI ≥ 60/100 at randomisation | |
| Interventions | Gabapentin 1200 mg daily (max), n = 17 Placebo, n = 15 All participants continued with non-opioid analgesia |
|
| Outcomes | ≥ 50% pain reduction | |
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 0, Total = 2 Published as letter, some details confirmed by correspondence |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not reported |
| Blinding? All outcomes |
Unclear | Not reported |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | 32 randomised |
| Study duration Efficacy |
Yes | 12-week period |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, not enriched. Missing data handled in a number of ways, and results presented without imputation Titration over 3 weeks to limit of tolerability or 2700 mg daily, then stable dose for 3 weeks (6 weeks total); then 2-week weaning-off and washout, and cross-over |
|
| Participants | Chemotherapy-induced peripheral neuropathy lasting ≥ 1 month. N = 115, mean age 59 years, 73% women. PI at randomisation ≥ 4/10, initial average daily pain 4/10 | |
| Interventions | Gabapentin 2700 mg daily (max) Placebo Usual cancer therapy continued |
|
| Outcomes | No dichotomous data Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 4 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “identical placebo capsules” |
| Incomplete outcome data addressed? Efficacy |
Yes | Results presented without imputation |
| Size Efficacy |
Unclear | 115 randomised |
| Study duration Efficacy |
Unclear | 6-week period |
| Outcomes reported | No | No dichotomous data |
| Methods | Multicentre, randomised, double-blind, placebo-controlled, parallel-group, partial enrichment, LOCF 4 day forced titration, then further titration over 2 weeks to target dose, and stable dose for 4 weeks (7 weeks in total). Participants unable to tolerate dosing regimen were withdrawn |
|
| Participants | Postherpetic neuralgia. N = 334, median age 75 years, 59% women. Pain > 3 months after healing of rash, PI ≥ 40/100 at randomisation, initial average daily pain 6.5/10 | |
| Interventions | Gabapentin 1800 mg daily, n = 115 Gabapentin 2400 mg daily, n = 108 Placebo, n = 111 |
|
| Outcomes | ≥ 50% reduction in mean pain score PGIC much or very much improved PGIC much and very much improved (CTR) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 Pfizer sponsored |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | List held securely and released only after study completion |
| Blinding? All outcomes |
Yes | “identical-appearing capsules” |
| Incomplete outcome data addressed? Efficacy |
Unclear | LOCF |
| Size Efficacy |
Yes | 334 randomised |
| Study duration Efficacy |
Unclear | 7-week period |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Randomised, double-blind, placebo-controlled, 3-way cross-over, not enriched. No imputation method mentioned Titration over 4 weeks to pain control, limit of tolerability, or maximum amitriptyline 150 mg daily, gabapentin 3600 mg daily, then stable dose for remainder of 8-week period; 1-week washout then cross-over Analysis for completers only |
|
| Participants | SCI at any level and degree of completeness. N = 38, only 22 patients completed all three cross-overs. Mean age 43 years, 9% women. Pain duration before treatment > 6 months, PI at randomisation > 5/10, initial pain intensity 5.6/10 | |
| Interventions | Amitriptyline 150 mg daily (max) Gabapentin 3600 mg daily (max) Placebo (diphenhydramine) 75 mg daily Oxycodone + paracetamol 5/325 mg (max 8 tablets daily) allowed for rescue medication |
|
| Outcomes | No dichotomous data for efficacy or harm Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Prepared, packaged and labelled by remote, commercial compounding pharmacy |
| Blinding? All outcomes |
Yes | “identical capsules” |
| Incomplete outcome data addressed? Efficacy |
No | Completers only |
| Size Efficacy |
No | 38 randomised |
| Study duration Efficacy |
Yes | 8-week period |
| Outcomes reported | No | No dichotomous data |
| Methods | Multicentre, randomised, double-blind, placebo-controlled, parallel-group, no enrichment, LOCF 4-week titration to maximum tolerated dose, or 3600 mg then stable dose for 4 weeks (8 weeks in total) |
|
| Participants | Postherpetic neuralgia. N = 229, median age 73 years, 48% women. Pain > 3 months after healing of rash, PI at randomisation > 40/100, initial average daily pain 6.4/10 | |
| Interventions | Gabapentin 3600 mg daily (max), n = 113. (83% had > 2400 mg daily) Placebo, n = 116 |
|
| Outcomes | PGIC moderate or much improved PGIC CTR moderate and much improved No change in pain SF36 and QoL Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 3 Parke-Davies sponsored |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | “subject-specific bottles based on randomisation schedule” |
| Blinding? All outcomes |
Yes | “identically appearing capsules” |
| Incomplete outcome data addressed? Efficacy |
Unclear | LOCF |
| Size Efficacy |
Yes | 229 randomised |
| Study duration Efficacy |
Yes | 8-week period |
| Outcomes reported | Yes | PGIC much improved (top level) |
| Methods | Randomised, double-blind, placebo-controlled, parallel-group, no obvious enrichment. No imputation method mentioned Gabapentin titrated over 2 weeks to 3000 mg daily, then stable dose for 2 weeks (4 weeks total) |
|
| Participants | Painful diabetic neuropathy. N = 147, mean age 59 years, 45% women. PI at randomisation > 4/10, initial PI 6.8/10 | |
| Interventions | Gabapentin ER, 3000 mg daily (as single dose) Gabapentin ER, 3000 mg daily (as divided dose) Placebo Numbers in each group not given |
|
| Outcomes | > 50% decrease in average daily pain | |
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 0, Total = 2 Published as letter |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Unclear | Not described |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
Unclear | 147 randomised |
| Study duration Efficacy |
Unclear | 4-week period |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Multicentre, randomised, double-blind, placebo-controlled, parallel-group, partial enrichment. No imputation method mentioned. Patients withdrawing due to lack of efficacy were defined as non-responders (n = 6), but treatment of substantial AE withdrawals (n = 49) and all-cause withdrawals (n = 73) not reported Titration over 5 weeks from 900 mg daily until pain controlled, or to maximum of2400 mg daily, then fixed dose (8 weeks in total) |
|
| Participants | Mixed neuropathic pain, most common conditions were CRPS (28%), PHN (14%). N = 305, median age 57 years, 53% women. PI at randomisation > 4/10, initial mean pain score 7.2/10 Excluded: individuals who had previously failed to respond to gabapentin at > 900 mg daily, or had experienced intolerable side effects at any dose |
|
| Interventions | Gabapentin 2400 mg daily (max), n = 153 Placebo, n = 152 101 took 2400 mg, 189 took 1800 mg, 27 took 900 mg Stable antidepressant therapy and NSAID/opioid therapy for other conditions allowed Paracetamol 500 mg/codeine 30 mg or paracetamol 500 mg (max 8 tablets daily) allowed as rescue medication |
|
| Outcomes | > 50% reduction in pain PGIC much or very much improved PGIC much improved and very much improved (CTR) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 Parke-Davies sponsored |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Randomisation list centrally held - remote allocation |
| Blinding? All outcomes |
Yes | “identical capsules” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
Yes | 305 randomised |
| Study duration Efficacy |
Yes | 8-week period |
| Outcomes reported | Yes | At least 50% reduction in pain |
| Methods | Randomised, double-blind, placebo-controlled, parallel-group, not obviously enriched (part 1 of study only) Titration over 4 weeks to maximum tolerated dose, then stable dose for 4 weeks (8 weeks in total) |
|
| Participants | Painful diabetic neuropathy. N = 60, mean age 50 years, 40% female. Pain duration > 3 months before treatment, PI > 40/100 at randomisation, initial pain score 6.5/10 | |
| Interventions | Gabapentin 3600 mg daily (max), n = 30 Placebo, n = 30 |
|
| Outcomes | PGIC moderate or much improved Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 1, W = 1, Total = 3 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not reported |
| Blinding? All outcomes |
Unclear | Not reported |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
Unclear | 60 randomised |
| Study duration Efficacy |
Yes | 8-week period |
| Outcomes reported | Unclear | Moderate or much improved |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, no enrichment. No imputation method mentioned Titration in 300 mg increments every 2 to 3 days until pain intensity of0 or uncomfortable side effects, or maximum 3600 mg daily, then stable dose for remainder of 6-week treatment period, followed by titration off medication in week 7; 5-week washout, then cross-over |
|
| Participants | Phantom limb pain and residual limb pain. N = 24, mean age 52 years, 25% women. Time since amputation > 6 months, PI before randomisation > 3/10, initial pain intensity 4.4/10 |
|
| Interventions | Gabapentin 3600 mg daily (max), (19/24 took max dose) Placebo |
|
| Outcomes | Meaningful decrease in pain (5-point scale) | |
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 0, Total = 4 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not described |
| Blinding? All outcomes |
Yes | “capsules that were identical in appearance” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | 24 randomised |
| Study duration Efficacy |
Unclear | 6-week period |
| Outcomes reported | Unclear | Meaningful decrease in pain (probably top of 5-point scale) |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, no enrichment. No imputation method mentioned Titration to limit of tolerability or maximum 1800 mg over 3 weeks, then stable for remainder of 4-week period; 2-week washout then cross-over |
|
| Participants | Traumatic spinal cord injury. N = 14, 7 patients with data, age 27 to 48 years, 6/7 male. Pain duration before treatment > 4/10 |
|
| Interventions | Gabapentin 1800 mg daily (max) Placebo NSAID, TCA and narcotics allowed for rescue medication as needed |
|
| Outcomes | Withdrawals | |
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not reported |
| Blinding? All outcomes |
Yes | Capsules with “identical shape and colour” |
| Incomplete outcome data addressed? Efficacy |
Unclear | Imputation not mentioned |
| Size Efficacy |
No | 7 patients with data of 14 |
| Study duration Efficacy |
Unclear | 4-week period |
| Outcomes reported | No | No dichotomous data |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, no enrichment Gabapentin titrated to maximum of 1800 mg daily over 5 days, then stable dose for remainder of 3-week treatment period; 2-week washout then cross-over |
|
| Participants | Complex regional pain syndrome type 1 (IASP criteria for diagnosis). N = 58, mean age 44 years, 17% women. Pain duration before treatment > 3/10, initial pain intensity 6. 3/10 46 patients completed both periods, with 12 excluded from analysis because they withdrew at some stage. Analysis performed only on complete data sets |
|
| Interventions | Gabapentin 1800 mg daily Placebo Usual analgesics continued without dose changes |
|
| Outcomes | Much improved (per protocol) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 2, DB = 2, W = 1, Total = 5 | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | “closed envelopes containing assignments were prenumbered and kept at the pharmacy” |
| Blinding? All outcomes |
Yes | “identical placebo capsules” |
| Incomplete outcome data addressed? Efficacy |
No | Analysis performed on completers |
| Size Efficacy |
No | Only 46 in final analysis |
| Study duration Efficacy |
Unclear | 3-week period |
| Outcomes reported | Unclear | Much improved |
| Methods | Randomised, double-blind, placebo-controlled, cross-over, partial enrichment, with exclusion of participants known not to respond to gabapentin or pregabalin, or who experienced dose limiting adverse events with gabapentin Gabapentin extended release given in fixed doses of 1800 mg, either as a single morning dose, or divided between 600 mg morning plus 1200 mg evening. No titration |
|
| Participants | neuropathic pain at least 3 months after healing of acute herpes zoster skin rash. N=400, mean age66 years, 52% women. Initial pain ≥4/10 on 0-10 scale. Mean initial pain 6. 5/10 | |
| Interventions | Gabapentin 1800 mg daily Placebo |
|
| Outcomes | A range of pain measures were used, but main results reported on numeric 0-10 rating scale, as well as patient global impression of change Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 4 Sponsored by Depomed |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Use of blinded medication carton |
| Blinding? All outcomes |
Yes | Identical blister packs |
| Incomplete outcome data addressed? Efficacy |
Yes | BOCF used for main results, with LOCF also |
| Size Efficacy |
Unclear | Over 100 per treatment group |
| Study duration Efficacy |
Yes | 10-week duration |
| Outcomes reported | Yes | At least 50% pain reduction over baseline |
AE = adverse event; CRPS = complex regional pain syndrome; DB = double-blinding; LOCF = last observation carried forward; BOCF = baseline observation carried forward; NSAID = non-steroidal anti-inflammatory drug; OTC = over the counter; PDN = painful diabetic neuropathy; PGIC = Patient Global Impression of Change; PHN = postherpetic neuralgia; QoL = quality of life; R = randomisation; W = withdrawals; ACR = American College of Rheumatology; CTR = clinical trial report; IASP = International Association for the Study of Pain; PI = pain intensity; SCI = spinal cord injury; TCA = tricyclic antidepressants; OTC = over the counter
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Arai 2010 | No mention of blinding of therapies in gabapentin plus imipramine additions to opioids in cancer pain |
| Berry 2005 | Single dose of gabapentin for treatment of acute herpes zoster |
| Dallochio 2000 | Painful diabetic neuropathy, open comparison of gabapentin and amitriptyline |
| Dworkin 2009 | Study for acute herpes zoster pain |
| Jean 2005 | Postherpetic neuralgia, with open administration of gabapentin |
| Keskinbora 2007 | Neuropathic cancer pain, with open administration of gabapentin |
| Ko 2010 | Open comparison of gabapentin and tramadol/paracetamol in painful diabetic neuropathy |
| McCleane 2001 | Low back pain |
| Nikolajsen 2006 | Trial of gabapentin in surgery to test whether use in surgery prevents development of phantom pain. There was no beneficial effect |
| Pandey 2002 | Guillain-Barré syndrome |
| Pandey 2005 | Guillain-Barré syndrome |
| Salvaggio 2008 | Facial pain, open administration of gabapentin plus tramadol |
| Sator-Katzenschlager 2005 | Chronic pelvic pain, with open administration of gabapentin |
| Yaksi 2007 | Lumbar spinal stenosis, with open administration of gabapentin |
| Yelland 2009 | No-of-1 study with short treatment periods of 2 weeks in chronic neuropathic pain, and with high withdrawal rate. Study design highly unusual and difficult to interpret |
| Yildrim 2003 | Not double-blind. Radiculopathy, not classic neuropathic pain |
DATA AND ANALYSES
Comparison 1.
Efficacy - placebo-controlled studies
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 At least 50% pain reduction over baseline | 10 | 2258 | Risk Ratio (M-H, Fixed, 95% CI) | 1.70 [1.46, 1.99] |
| 1.1 Postherpetic neuralgia | 3 | 892 | Risk Ratio (M-H, Fixed, 95% CI) | 1.67 [1.29, 2.16] |
| 1.2 Painful diabetic neuropathy | 4 | 829 | Risk Ratio (M-H, Fixed, 95% CI) | 1.78 [1.43, 2.21] |
| 1.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M-H, Fixed, 95% CI) | 1.45 [0.88, 2.37] |
| 1.4 Nerve injury pain | 1 | 196 | Risk Ratio (M-H, Fixed, 95% CI) | 1.44 [0.65, 3.22] |
| 1.5 Small fibre sensory neuropathy | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [0.65, 38.65] |
| 2 Very much improved | 8 | 1600 | Risk Ratio (M-H, Fixed, 95% CI) | 2.41 [1.80, 3.23] |
| 2.1 Postherpetic neuralgia | 2 | 563 | Risk Ratio (M-H, Fixed, 95% CI) | 2.70 [1.51, 4.82] |
| 2.2 Painful diabetic neuropathy | 2 | 408 | Risk Ratio (M-H, Fixed, 95% CI) | 1.94 [1.26, 2.99] |
| 2.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M-H, Fixed, 95% CI) | 1.99 [0.92, 4.28] |
| 2.4 Complex regional pain syndrome I | 1 | 92 | Risk Ratio (M-H, Fixed, 95% CI) | 4.0 [0.90, 17.83] |
| 2.5 Nerve injury pain | 1 | 196 | Risk Ratio (M-H, Fixed, 95% CI) | 3.6 [1.39, 9.31] |
| 2.6 Small fibre sensory neuropathy | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [0.65, 38.65] |
| 3 Much or very much improved | 11 | 2203 | Risk Ratio (M-H, Fixed, 95% CI) | 1.64 [1.43, 1.87] |
| 3.1 Postherpetic neuralgia | 4 | 1118 | Risk Ratio (M-H, Fixed, 95% CI) | 1.51 [1.25, 1.83] |
| 3.2 Painful diabetic neuropathy | 4 | 548 | Risk Ratio (M-H, Fixed, 95% CI) | 1.61 [1.28, 2.02] |
| 3.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M-H, Fixed, 95% CI) | 2.17 [1.38, 3.41] |
| 3.4 Nerve injury pain | 1 | 196 | Risk Ratio (M-H, Fixed, 95% CI) | 2.21 [1.26, 3.90] |
| 3.5 Small fibre sensory neuropathy | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 1.5 [0.67, 3.34] |
| 4 IMMPACT outcome of substantial improvement | 13 | 2627 | Risk Ratio (M-H, Fixed, 95% CI) | 1.80 [1.55, 2.08] |
| 4.1 Postherpetic neuralgia | 4 | 1121 | Risk Ratio (M-H, Fixed, 95% CI) | 1.81 [1.41, 2.31] |
| 4.2 Painful diabetic neuropathy | 4 | 829 | Risk Ratio (M-H, Fixed, 95% CI) | 1.78 [1.43, 2.21] |
| 4.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M-H, Fixed, 95% CI) | 1.45 [0.88, 2.37] |
| 4.4 Complex regional pain syndrome I | 1 | 92 | Risk Ratio (M-H, Fixed, 95% CI) | 4.0 [0.90, 17.83] |
| 4.5 Nerve injury pain | 1 | 196 | Risk Ratio (M-H, Fixed, 95% CI) | 1.44 [0.65, 3.22] |
| 4.6 Phantom pain | 1 | 48 | Risk Ratio (M-H, Fixed, 95% CI) | 2.6 [1.10, 6.16] |
| 4.7 Small fibre sensory neuropathy | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [0.65, 38.65] |
| 5 IMMPACT outcome of at least moderate improvement | 14 | 2831 | Risk Ratio (M-H, Fixed, 95% CI) | 1.68 [1.51, 1.88] |
| 5.1 Postherpetic neuralgia | 4 | 1121 | Risk Ratio (M-H, Fixed, 95% CI) | 1.84 [1.50, 2.26] |
| 5.2 Painful diabetic neuropathy | 5 | 937 | Risk Ratio (M-H, Fixed, 95% CI) | 1.50 [1.28, 1.75] |
| 5.3 Mixed neuropathic pain | 2 | 391 | Risk Ratio (M-H, Fixed, 95% CI) | 2.10 [1.49, 2.95] |
| 5.4 Fibromyalgia | 1 | 150 | Risk Ratio (M-H, Fixed, 95% CI) | 1.61 [1.07, 2.42] |
| 5.5 Nerve injury pain | 1 | 196 | Risk Ratio (M-H, Fixed, 95% CI) | 1.53 [0.92, 2.53] |
| 5.6 Small fibre sensory neuropathy | 1 | 36 | Risk Ratio (M-H, Fixed, 95% CI) | 2.25 [0.84, 5.99] |
Comparison 2.
Withdrawals - placebo-controlled studies
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event withdrawal | 17 | 3022 | Risk Ratio (M-H, Fixed, 95% CI) | 1.36 [1.09, 1.71] |
| 2 All-cause withdrawal | 17 | 3063 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 [0.91, 1.21] |
Comparison 3.
Adverse events
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 At least one adverse event | 11 | 2356 | Risk Ratio (M-H, Fixed, 95% CI) | 1.28 [1.20, 1.37] |
| 2 Serious adverse events | 14 | 2702 | Risk Ratio (M-H, Fixed, 95% CI) | 1.31 [0.88, 1.95] |
| 3 Somnolence | 16 | 2800 | Risk Ratio (M-H, Fixed, 95% CI) | 3.21 [2.48, 4.16] |
| 4 Dizziness | 16 | 3150 | Risk Ratio (M-H, Fixed, 95% CI) | 3.26 [2.62, 4.06] |
| 5 Peripheral oedema | 9 | 2042 | Risk Ratio (M-H, Fixed, 95% CI) | 3.40 [2.18, 5.32] |
| 6 Ataxia or gait disturbance | 5 | 544 | Risk Ratio (M-H, Fixed, 95% CI) | 4.47 [1.85, 10.82] |
Analysis 1.1. Comparison 1 Efficacy - placebo-controlled studies, Outcome 1 At least 50% pain reduction over baseline
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 1 Efficacy - placebo-controlled studies
Outcome: 1 At least 50% pain reduction over baseline
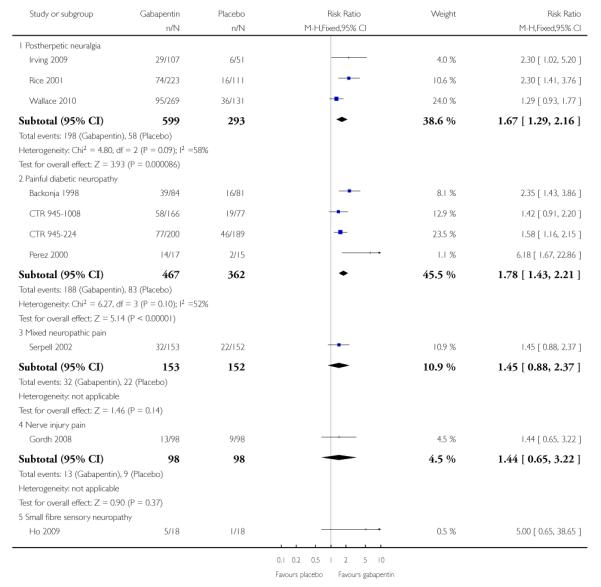 |
 |
Analysis 1.2. Comparison 1 Efficacy - placebo-controlled studies, Outcome 2 Very much improved
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 1 Efficacy - placebo-controlled studies
Outcome: 2 Very much improved
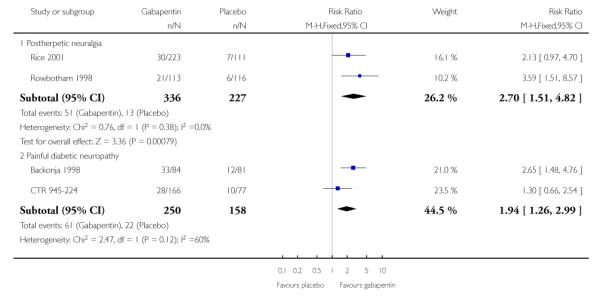 |
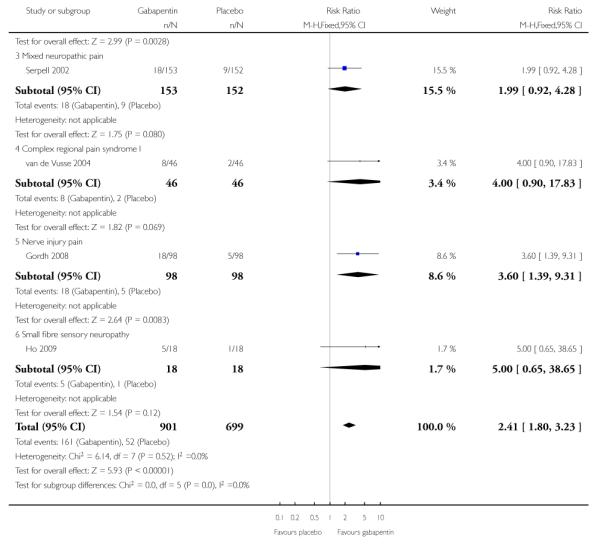 |
Analysis 1.3. Comparison 1 Efficacy - placebo-controlled studies, Outcome 3 Much or very much improved
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 1 Efficacy - placebo-controlled studies
Outcome: 3 Much or very much improved
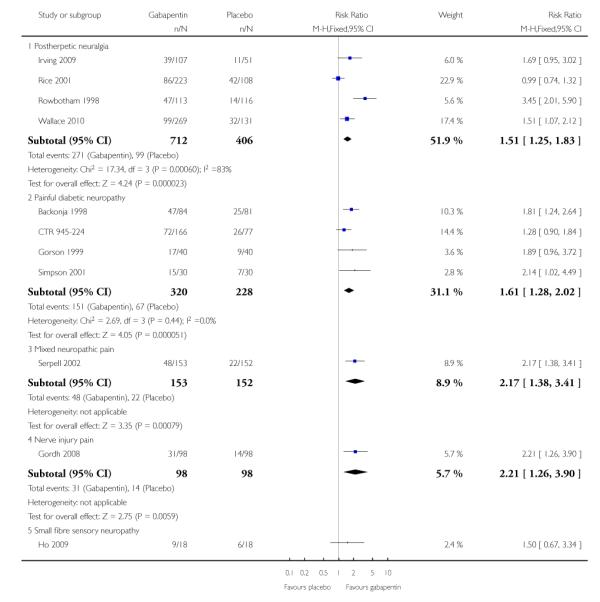 |
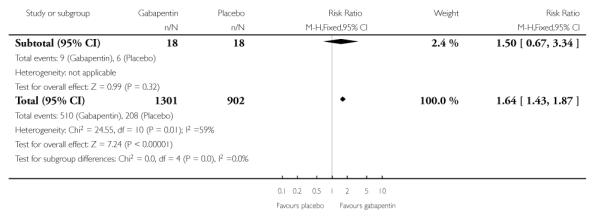 |
Analysis 1.4. Comparison 1 Efficacy - placebo-controlled studies, Outcome 4 IMMPACT outcome of substantial improvement
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 1 Efficacy - placebo-controlled studies
Outcome: 4 IMMPACT outcome of substantial improvement
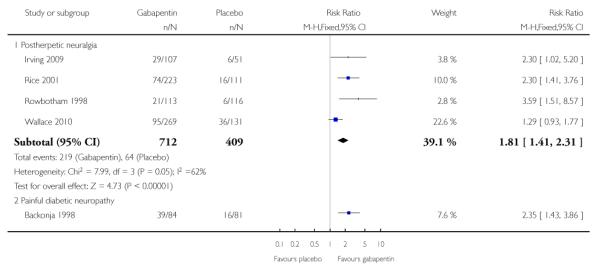 |
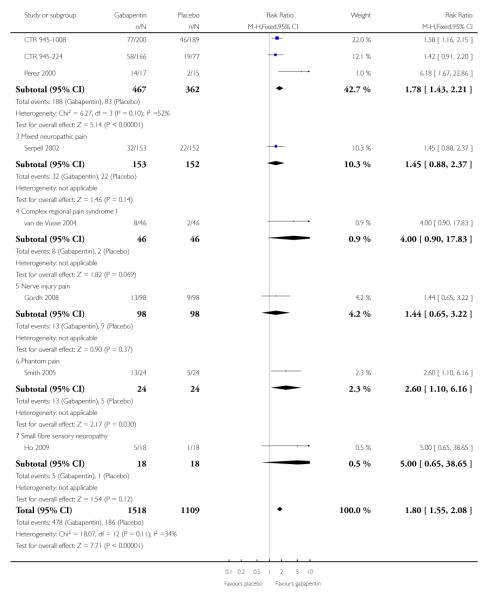 |
Analysis 1.5. Comparison 1 Efficacy - placebo-controlled studies, Outcome 5 IMMPACT outcome of at least moderate improvement
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 1 Efficacy - placebo-controlled studies
Outcome: 5 IMMPACT outcome of at least moderate improvement
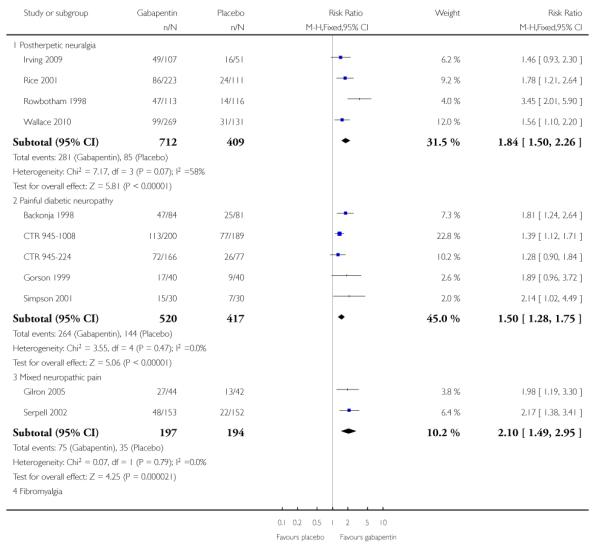 |
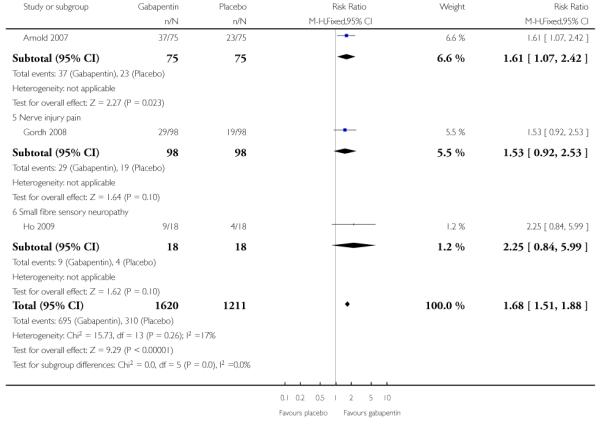 |
Analysis 2.1. Comparison 2 Withdrawals - placebo-controlled studies, Outcome 1 Adverse event withdrawal
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 2 Withdrawals - placebo-controlled studies
Outcome: 1 Adverse event withdrawal
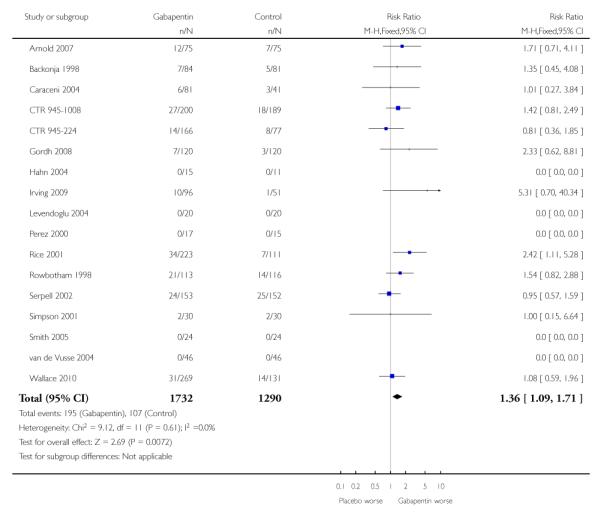 |
Analysis 2.2. Comparison 2 Withdrawals - placebo-controlled studies, Outcome 2 All-cause
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 2 Withdrawals - placebo-controlled studies
Outcome: 2 All-cause withdrawal
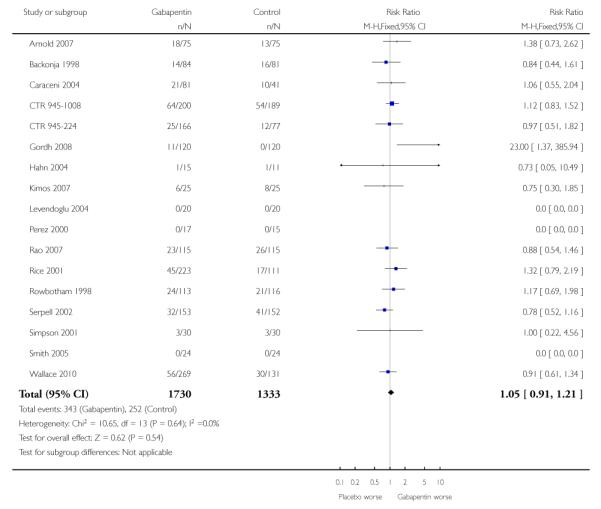 |
Analysis 3.1. Comparison 3 Adverse events, Outcome 1 At least one adverse event
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 3 Adverse events
Outcome: 1 At least one adverse event
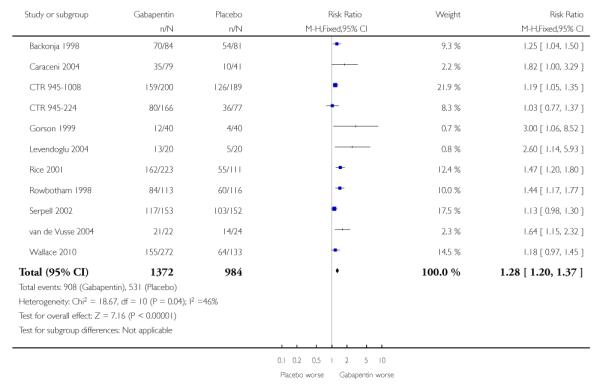 |
Analysis 3.2. Comparison 3 Adverse events, Outcome 2 Serious adverse events
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 3 Adverse events
Outcome: 2 Serious adverse events
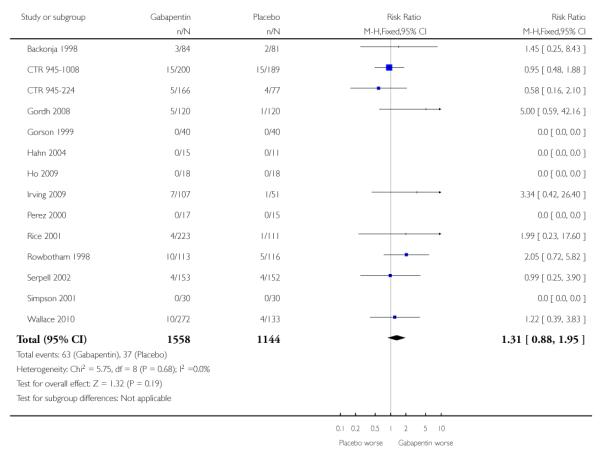 |
Analysis 3.3. Comparison 3 Adverse events, Outcome 3 Somnolence
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 3 Adverse events
Outcome: 3 Somnolence
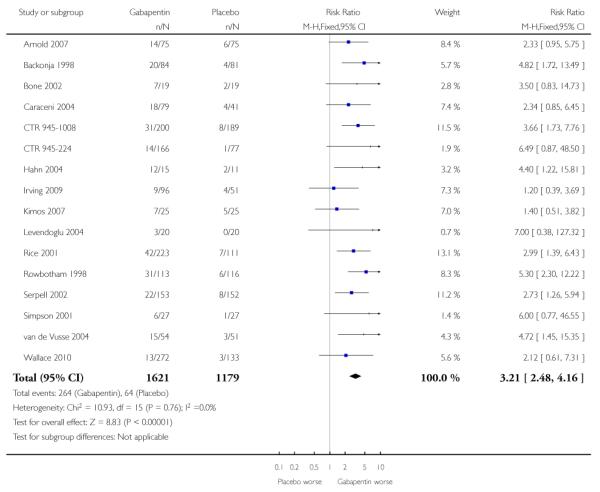 |
Analysis 3.4. Comparison 3 Adverse events, Outcome 4 Dizziness
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 3 Adverse events
Outcome: 4 Dizziness
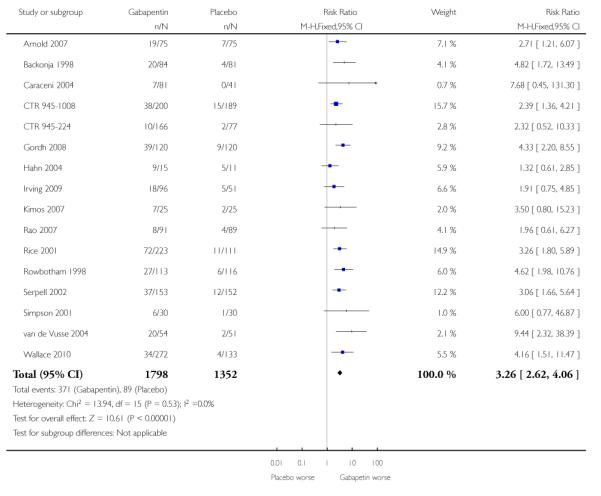 |
Analysis 3.5. Comparison 3 Adverse events, Outcome 5 Peripheral oedema
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 3 Adverse events
Outcome: 5 Peripheral oedema
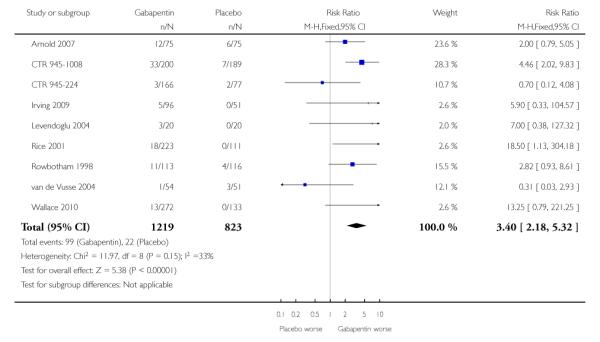 |
Analysis 3.6. Comparison 3 Adverse events, Outcome 6 Ataxia or gait disturbance
Review: Gabapentin for chronic neuropathic pain and fibromyalgia in adults
Comparison: 3 Adverse events
Outcome: 6 Ataxia or gait disturbance
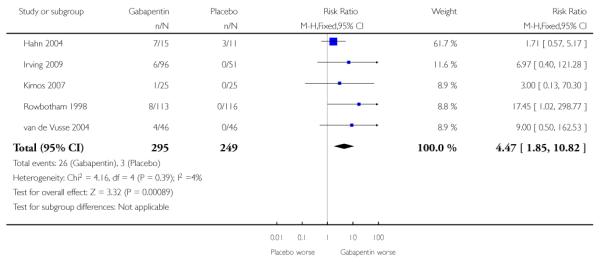 |
Appendix 1. MEDLINE (via Ovid) search strategy
(gabapentin* or neurontin* or neurotonin*).mp.
exp PAIN/
(pain* or discomfort* or analgesi*).mp.
2 OR 3
1 AND 4
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs
randomly.ab.
trial.ti.
groups.ab
OR/6-13
5 AND 13
Appendix 2. EMBASE (via OVID) search strategy
Gabapentin/ OR (gabapentin* or neurontin* or neurotonin*).mp.
exp PAIN/ OR exp chronic pain/ OR exp neuropathic pain/
(pain* or discomfort* or analgesi*).mp.
2 OR 3
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double-blind procedure.sh.
(clin* adj25 trial*)
((doubl* or trebl* or tripl*) adj25 (blind* or mask*))
placebo*
random*
OR/6-13
1 AND 4 AND 13
Appendix 3. CENTRAL search strategy
(gabapentin* or neurontin* or neurotonin*):ti,ab,kw
MESH descriptor PAIN explode all trees
(pain* or discomfort* or analgesi*):ti,ab,kw
2 OR 3
1 AND 4
Limit 5 to Clinical Trials (CENTRAL)
Appendix 4. Potential sources of bias in studies of chronic pain used in the ‘Risk of bias’ table
| Item | Red | Amber | Green |
|---|---|---|---|
| Randomisation | Not randomised | Claims randomisation, but no method given | Randomised by adequate method |
| Allocation concealment | Not reported | Reported but not described | Allocation undertaken independently and blind to investigator |
| Blinding | Not double-blind | Claims double-blind, but no method | Convincingly double-blind |
| Duration | 2 weeks or less | 3 to 6 weeks | 7 weeks or more |
| Outcome | Anything less than 30% pain intensity reduction Pain state ≥ 50/100 mm or equivalent or undefined |
Responder: pain intensity reduction of ≥ 30% from baseline State: final pain intensity < 50/100 mm, or equivalent |
Responder: pain intensity reduction of ≥ 50% from baseline State: final pain intensity < 30/100 mm, or equivalent State: no worse than mild pain |
| Incomplete outcome assessment | Average results only | Responder or state with last observation carried forward or imputation method for missing data or after withdrawal not stated | Responder or state response, using baseline observation carried forward (zero improvement after withdrawal) |
| Size | < 50 patients per treatment arm | 50 to 199 patients per treatment arm | ≥ 200 patients per treatment arm |
Appendix 5. Summary of outcomes in individual studies
| Study | Withdrawals | Efficacy | Adverse events (general) | Adverse events (specific) |
|---|---|---|---|---|
| Postherpetic neuralgia | ||||
|
Rowbotham 1998 Rowbotham et al. JAMA 1998 280: 1837-1842 Parke-Davis 945-211 CTR additional data Multicentre |
Gabapentin All-cause 24 AE 21 LoE 0 Placebo All-cause 21 AE 14 LoE 2 |
PGIC moderate or much improved Gaba: 47/113 Plac: 14/116 PGIC CTR much improved Gaba: 21/113 Plac: 6/116 PGIC CTR moderately improved Gaba: 26/113 Plac: 8/116 No change in pain 60% placebo, 23% gabapentin No change or worse in pain 68% placebo, 26% gabapentin Significant improvement over placebo in 5/9 SF-36QoL and 5/7 mood states |
At least one AE Gaba 84/113 Plac 60/116 Minor AE (treatment related) Gaba: 62/113 Plac: 32/116 SAE (treatment related) Gaba: 0/113 (10/113 CTR) Plac: 0/116 (5/116 CTR) Death: Gaba: 0/113 Plac: 1/116 |
Somnolence Gaba: 31/113 Plac: 6/116 Dizziness Gaba: 27/113 Plac: 6/116 Ataxia Gaba: 8/113 Plac: 0/116 Peripheral oedema Gaba: 11/113 Plac: 4/116 |
|
Rice 2001 Rice et al. Pain 2001 94: 215-224 Parke-Davis 945-295 CTR additional data Multicentre |
Gabapentin 1800 mg All-cause 22 AE 15 LoE 4 Gabapentin 2400 mg All-cause 23 AE 19 LoE 1 Placebo All-cause 17 AE 7 LoE 4 |
At least 50% reduction in mean pain score Gaba 1800: 37/115 Gaba 2400: 37/108 Plac: 16/111 PGIC very much or much improved Gaba 1800: 44/115 Gaba 2400: 42/108 Plac: 24/111 PGIC very much improved (CTR) Gaba 1800: 18/115 Gaba 2400: 12/108 Plac: 7/111 PGIC much improved (CTR) Gaba 1800: 26/115 Gaba 2400: 30/108 Plac: 17/111 Some significant differences in QoL measures and sleep |
At least one AE Gaba 1800: 81/115 Gaba 2400: 81/108 Plac: 55/111 SAE Gaba 1800: 3/115 Gaba 2400: 1/108 Plac: 1/111 Death: Gaba 1800: 0/115 Gaba 2400: 1/108 Plac: 0/111 |
Somnolence Gaba 1800: 20/115 Gaba 2400: 22/108 Plac: 7/111 Dizziness Gaba 1800: 36/115 Gaba 2400: 36/108 Plac: 11/111 Asthenia Gaba 1800: 7/115 Gaba 2400: 6/108 Plac: 4/111 Peripheral oedema Gaba 1800: 6/115 Gaba 2400: 12/108 Plac: 0/111 |
|
Irving 2009 Irving et al. Clin J Pain 2009 25: 185-192 Jensen et al. Clin J Pain 2009 25: 185-192 Multicentre Extended release |
All-cause withdrawal 15 total AE withdrawal Gabapentin 1800 single dose 4/44 Gabapentin 1800 split dose 6/52 Placebo 1/51 |
At least 50% reduction in pain score Gaba 1800 single dose 14/55 Gaba 1800 split dose 15/52 Placebo 6/51 At least 30% reduction in pain score Gaba 1800 single dose 24/55 Gaba 1800 split dose 25/52 Placebo 16/5 PGIC very much or much improved Gaba 1800 single dose 18/55 Gaba 1800 split dose 21/52 Placebo 11/5 Significantly better sleep with gabapentin compared with placebo |
Serious AE Gaba 1800 single dose 4/55 Gaba 1800 split dose 3/52 Placebo 1/51 Deaths Gaba 1800 single dose 0/55 Gaba 1800 split dose 1/52 Placebo 0/51 |
Somnolence Gaba 1800 single dose: 5/55 Gaba 1800 split dose: 4/52 Plac: 4/51 Dizziness Gaba 1800 single dose: 12/55 Gaba 1800 split dose: 6/52 Plac: 5/51 Gait disturbance Gaba 1800 single dose: 4/55 Gaba 1800 split dose: 2/52 Plac: 0/51 Peripheral oedema Gaba 1800 single dose: 4/55 Gaba 1800 split dose: 1/52 Plac: 0/51 |
|
Chandra 2006 Chandra et al. Int J Clin Pharm Ther 2006 44: 358-363 |
All-cause withdrawal Gabapentin 3/38 Nortriptyline 2/38 AE withdrawal Gabapentin 0/38 Nortriptyline 1/38 LoE withdrawal Gabapentin 0/38 Nortriptyline 1/38 |
At least 50% improvement over baseline pain (Likert) Gabapentin 7/38 Nortriptyline 9/38 At least 50% improvement over baseline pain (VAS) Gabapentin 13/38 Nortriptyline 14/38 |
No serious AE reported No deaths reported |
Sleepiness Gaba 4/38 Nort 6/38 Giddiness Gaba 1/38 Nort 0/38 |
|
Wallace 2010 Wallace et al. Clin Drug Invest 2010 30: 765-776 Extended release. Note that two different gabapentin regimens have been combined, both 1800 mg daily |
All-cause withdrawal Gabapentin 56/269 Placebo 30/131 AE withdrawal Gabapentin 31/269 Placebo 14/131 |
At least 50% improvement over baseline pain (Likert) Gabapentin 95/269 Placebo 36/131 Much or very much improved on PGIC Gabapentin 99/269 Placebo 32/131 |
At least one AE Gaba 155/272 Plac 64/133 Serious AE Gaba 10/272 Plac 4/133 Deaths Gaba 0/272 Plac 1/133 |
Dizziness Gaba 34/272 Plac 4/133 Somnolence Gaba 13/272 Plac 3/133 Peripheral oedema Gaba 13/272 Plac 0/133 |
| Painful diabetic neuropathy | ||||
|
Backonja 1998 Backonja et al. JAMA 1998 280: 1831-1836 Parke-Davis Pfizer 945-210 Multicentre |
All-cause withdrawal Gabapentin 14/84 Placebo 16/81 AE withdrawal Gabapentin 7/84 Placebo 5/81 LoE withdrawal Gabapentin 1/84 Placebo 5/81 |
PGIC much or moderately improved Gabapentin 47/84 Placebo 25/81 At least 50% reduction in pain (CTR) Gabapentin 39/84 Placebo 16/81 PGIC much improved (CTR) Gabapentin 33/84 Placebo 12/81 PGIC moderately or much improved (CTR) Gabapentin 47/84 Placebo 25/81 |
At least one AE Gaba 70/84 Plac 54/81 Serious AE Gaba 3/84 Plac 2/81 Deaths Gaba 0/84 Plac 0/81 |
Dizziness Gaba 20/84 Plac 4/81 Somnolence Gaba 19/84 Plac 5/81 |
|
Gorson 1999 Gorson et al. J Neurol, Neurosurg Psych 1999 66:251-252 |
Moderate or excellent pain relief (both phases) Gabapentin 17/40 Placebo 9/40 |
At least one AE Gaba 12/40 Plac 4/40 Serious AE Gaba 0/40 Plac 0/40 Deaths (inferred) Gaba 0/40 Plac 0/40 |
||
|
Morello 1999 Morello et al. Archives of Internal Medicine 1999 159: 1931-1937 |
All-cause withdrawal/early cross-over Gabapentin 3/25 Amitriptyline 4/25 AE withdrawal/early cross-over Gabapentin 2/25 Amitriptyline 3/25 LoE withdrawal/early cross-over Gabapentin 0/25 Amitriptyline 1/25 |
No significant difference at end of treatment Pain relief at end of treatment (6-point global score), complete, a lot Gabapentin 6/21 Amitriptyline 5/21 Pain relief at end of treatment (global score), at least moderate Gabapentin 11/21 Amitriptyline 14/21 |
At least one AE Gabapentin 18/23 Amitriptyline 17/24 No serious AEs or deaths noted |
Sedation Gaba 12/23 Amit 8/24 Dizziness Gaba 7/23 Amit 2/24 Ataxia Gaba 5/23 Amit 2/24 Peripheral oedema Gaba 3/23 Amit 2/24 |
|
CTR 945-224 Multicentre |
All-cause withdrawal Gabapentin 600 12/82 Gabapentin 1200 6/82 Gabapentin 2400 19/84 Placebo 12/77 AE withdrawal Gabapentin 600 8/82 Gabapentin 1200 3/82 Gabapentin 2400 11/84 Placebo 8/77 LoE withdrawal Gabapentin 600 0/82 Gabapentin 1200 0/82 Gabapentin 2400 4/84 Placebo 1/77 |
At least 50% reduction in pain score Gabapentin 600 13/82 Gabapentin 1200 33/82 Gabapentin 2400 25/84 Placebo 19/77 PGIC very much improved Gabapentin 600 9/82 Gabapentin 1200 14/82 Gabapentin 2400 14/84 Placebo 10/77 PGIC much or very much improved Gabapentin 600 22/82 Gabapentin 1200 36/82 Gabapentin 2400 36/84 Placebo 26/77 |
At least 1 AE Gabapentin 600 40/82 Gabapentin 1200 35/82 Gabapentin 2400 45/84 Placebo 36/77 Serious AE Gabapentin 600 5/82 Gabapentin 1200 2/82 Gabapentin 2400 3/84 Placebo 4/77 There were no deaths |
Somnolence Gabapentin 600 4/82 Gabapentin 1200 3/82 Gabapentin 2400 11/84 Placebo 1/77 Dizziness Gabapentin 600 7/82 Gabapentin 1200 4/82 Gabapentin 2400 6/84 Placebo 2/77 Peripheral oedema Gabapentin 600 4/82 Gabapentin 1200 1/82 Gabapentin 2400 2/84 Placebo 2/77 |
|
CTR 945-1008 Multicentre |
All-cause withdrawal Gabapentin 64/200 Placebo 54/189 AE withdrawal Gabapentin 27/200 Placebo 18/189 LoE withdrawal Gabapentin 1/200 Placebo 4/189 |
At least 30% reduction in pain Gabapentin 113/200 Placebo 77/189 At least 50% reduction in pain Gabapentin 77/200 Placebo 46/189 |
At least one AE Gaba 159/200 Plac 126/189 Serious AE Gaba 15/200 Plac 15/189 Deaths Gaba 1/200 Plac 1/189 |
Somnolence Gaba 31/200 Plac 8/189 Dizziness Gaba 38/200 Plac 15/189 Asthenia Gaba 22/200 Plac 8/189 Peripheral oedema Gaba 33/200 Plac 7/189 |
|
Simpson 2001 Simpson J Clin Neuromusc Dis 2001 3: 53-62. |
All-cause withdrawal Gabapentin 3/30 Placebo 3/30 Lack of efficacy Gabapentin 1/30 Placebo 1/30 Adverse event Gabapentin 2/30 Placebo 2/30 |
PGIC moderate or much improved Gaba: 15/30 Plac: 7/30 |
No deaths reported, and no serious adverse events reported | Somnolence Gaba 6/27 Plac 1/27 Dizziness Gaba 6/27 Plac 1/28 |
|
Perez 2000 Perez & Sanchez. American Journal of Medicine 2000 108: 689 |
No withdrawals apparent | At least 50% reduction in pain by 4 weeks Gabapentin 14/17 Placebo 2/15 |
No major side effects reported for gabapentin group | No data |
|
Sandercock 2009 Sandercock et al. Diabetes Care 2009 32: e20 |
Not mentioned | 41% with at least 50% decrease in average daily pain with gabapentin compared with 12% with placebo Similar results for sleep interference |
Not mentioned | No data |
| Mixed neuropathic pain | ||||
|
Serpell 2002 Serpell. Pain 2002 99: 557-566 Parke Davis/Pfizer 945-430-306 |
All-cause withdrawals Gabapentin 32/153 Placebo 41/152 AE withdrawals Gabapentin 24/153 Placebo 25/152 LoE withdrawals Gabapentin 1/153 Placebo 5/152 |
At least 50% reduction in pain Gabapentin 32/153 Placebo 22/152 PGIC very much or much improved Gabapentin 48/153 Placebo 22/152 PGIC very much improved CTR Gabapentin 18/153 Placebo 9/152 PGIC much improved CTR Gabapentin 30/153 Placebo 13/152 |
At least one AE Gabapentin 117/153 Placebo 103/152 Serious AE Gabapentin 4/153 Placebo 4/152 Deaths Gabapentin 0/153 Placebo 2/152 |
Somnolence Gabapentin 22/153 Placebo 8/152 Dizziness Gabapentin 37/153 Placebo 12/152 |
|
Gilron 2005 Gilron et al. NEJM 2005 352: 1324-1334. |
16 withdrawals during treatment | At least moderate pain relief (5-point scale) for those completing a given treatment: Placebo 13/42 Gabapentin 27/44 Morphine 35/44 gabapentin/morphine 32/41 |
Not interpretable | Not interpretable |
|
Gilron 2009 Gilron et al. Lancet 2009 374:1252-1261 |
All-cause withdrawals Gabapentin 8/54 Nortriptyline 2/52 Combination 1/52 AE withdrawals Gabapentin 7/54 Nortriptyline 1/52 Combination 1/52 |
Pain significantly lower with combination than either drug alone, by < 1/10 points | No serious AE recorded | Individual AE reporting showed higher incidence during titration than at maximum tolerated dose |
| Fibromyalgia | ||||
|
Arnold 2007 Arnold et al. Arthritis & Rheumatism 2007 56: 1336-1344 Multicentre |
All-cause withdrawals Gabapentin 18/75 Placebo 13/75 AE withdrawals Gabapentin 12/75 Placebo 7/75 LoE withdrawals Gabapentin 1/75 Placebo 2/75 |
At least 30% reduction in pain Gabapentin 38/75 Placebo 23/75 PG any improvement (7-point scale) Gabapentin 78% Placebo 36% |
“no significant differences in the percentage of serious treatment emergent adverse events” | Sedation Gaba 18/75 Somnolence Gaba 14/75 Placebo 6/75 Dizziness Gaba 19/75 Plac 7/75 Asthenia Gaba 6/75 Plac 5/751 Peripheral oedema Gaba 12/75 Plac 6/75 |
| Complex Regional Pain Syndrome type 1 | ||||
|
van de Vusse 2004 van de Vusse et al 20 BMC Neurology 2004 4:13 |
Both periods AE withdrawal Gabapentin 3/46 Placebo 0/46 LoE withdrawal Gabapentin 0/46 Placebo 0/46 |
Much improved (per protocol) both periods Gabapentin 8/46 Placebo 2/46 Much improved (per protocol) first period Gabapentin 3/22 Placebo 1/24 |
At least one AE First period Gaba 21/22 Placebo 14/24 |
Both periods Somnolence Gaba 15/54 Plac 3/51 Dizziness Gaba 20/54 Plac 2/51 Disturbed gait Gaba 4/54 Plac 0/51 Oedema Gaba 1/54 Plac 3/51 |
| Spinal cord injury | ||||
|
Tai 2002 Tai - J Spinal Cord Medicine 2002 25:100-5. |
Discontinuations All-cause 7/14 Urinary retention 1/14 |
Not interpretable | No data “No significant side effects noted at the maximum dosage” |
No data |
|
Levendoglu 2004 Levendoglu et al. Spine 2004 29: 743-751 |
All completed | Average fall in pain 62% with gabapentin, 13% with placebo Mean scores without SD. No dichotomous results |
All-cause AE Gaba 13/20 Plac 5/20 |
Sedation Gaba 3/20 Plac 0/20 Oedema Gaba 3/20 Plac 0/20 |
|
Rintala 2007 Rintala et al. Arch Phys Med Rehabil 2007 88: 1547-1560 |
16/38 withdrew | No dichotomous data. The paper claims statistical superiority of amitriptyline over gabapentin using paired t-tests for 22 patients completing all 3 phases. It also claims no benefit of gabapentin over placebo |
No dichotomous data | No dichotomous data |
| Nerve injury pain | ||||
|
Gordh 2008 Gordh et al. Pain 2008 138: 255-266 Multicentre |
All-cause withdrawal Gabapentin 11/120 Placebo 11/120 AE withdrawal Gabapentin 7/120 Placebo 3/120 LoE withdrawal Gabapentin 1/120 Placebo 2/120 |
Marked pain relief Gabapentin 18/98 Placebo 5/98 Marked or moderate pain relief Gabapentin 31/98 Placebo 14/98 No pain relief Gabapentin 54/98 Placebo 70/98 At least 50% pain relief Gabapentin 11 13/98 Placebo 7 9/98 At least 30% pain relief Gabapentin 20 29/98 Placebo 10 19/98 Benefits from gabapentin over placebo for sleep and some aspects of quality of life |
Serious AE Gaba 5/120 Plac 1/120 |
Dizziness Gaba 39/120 Plac 9/120 |
| Phantom | ||||
|
Smith 2005 Smith et al. Journal of Rehabilitation Research & Development 2005 42: 645-654 |
No apparent withdrawals | “Meaningful decrease in pain” (top of 5-point scale) Gabapentin 13/24 Placebo 5/24 |
No data | No data |
|
Bone 2002 Bone et al. Regional Anesthesia and Pain medicine 2002 27: 481-486 |
No data on where withdrawals occurred | No dichotomous data Significant benefit for gabapentin by week 6 for pain |
No data | Somnolence Gaba 7/19 Plac 2/19 Dizziness Gaba 2/19 Plac 1/19 |
| Cancer associated neuropathic pain | ||||
|
Caraceni 2004 Caraceni et al. Journal of Clinical Oncology 2004 22: 2909-2917 |
All-cause withdrawal Gabapentin 21/80 Placebo 10/41 AE withdrawal Gabapentin 6/80 Placebo 3/41 LoE withdrawal Gabapentin 0/80 Placebo 0/41 |
Somewhat better pain responses with gabapentin than placebo | No data Any AE Gaba 35/79 Placebo 10/41 |
Somnolence Gaba 18/79 Plac 4/41 Dizziness Gaba 7/89 Plac 0/41 |
|
Rao 2007 Rao et al. Cancer 2007 110: 2110-2118 |
All-cause withdrawal Gabapentin 23/115 Placebo 26/115 |
No significant difference between gabapentin and placebo, but pain scores were low and the study may have lacked sensitivity | No data | Dizziness Gaba 8/91 Plac 4/89 |
| HIV | ||||
|
Hahn 2004 Hahn et al. Journal of Neurology 2004 251: 1260-1266 |
All-cause withdrawal Gabapentin 1/15 Placebo 1/11 AE withdrawal Gabapentin 1/15 Placebo 0/11 |
Improvement in pain and sleep interference with gabapentin and placebo, with sustained difference in sleep but not pain | No serious AE or deaths reported | Somnolence Gaba 12/15 Plac 2/11 Dizziness Gaba 9/15 Plac 5/11 Disturbed gait Gaba 7/15 Plac 3/11 |
| Other | ||||
|
Kimos 2007 Kimos et al. Pain 2007 127: 151-160 Chronic masticatory myalgia |
All-cause withdrawal Gabapentin 6/25 Placebo 8/25 6 did not return after initial visit |
NNT calculated for clinically significant reported pain reduction (pain reduction of 30% or more) 3.4 | No data | Drowsiness Gaba 7/25 Plac 5/25 Dizziness Gaba 7/25 Plac 2/25 Ataxia Gaba 1/25 Plac 0/25 |
|
Ho 2009 Ho et al. Pain 2009 141: 19-24 Small fibre sensory neuropathy |
All-cause withdrawal 3/18 in first 4 weeks (withdrawn consent) | At least 50% improvement in pain Gabapentin 4/18 Tramadol 4/18 Placebo 1/18 At least 30% improvement in pain Gabapentin 9/18 Tramadol 8/18 Placebo 4/18 Very much better Gabapentin 5/18 Tramadol 3/18 Placebo 1/18 Much or very much better Gabapentin 9/18 Tramadol 6/18 Placebo 2/18 |
No serious AE or deaths reported | AE not ascribed consistently to drugs |
AE = adverse event; Amit = amitriptyline; Gaba = gabapentin; Nort = nortriptyline; PGIC = Patient Global Impression of Change; Plac = placebo; QoL = quality of life; SAE = serious adverse event; VAS = visual analogue scale; CTR = clinical trial report; LOE = lack of efficacy
HISTORY
Protocol first published: Issue 3, 2009
Review first published: Issue 3, 2011
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The protocol for the original gabapentin review (Wiffen 2005) was superceded and split, and an updated protocol produced for this review, to reflect, at least in part, the more recent developments in understanding of potential biases in chronic pain trials, and new outcomes of direct relevance to patients. The main difference between the original review and the updated protocol for this review, was more emphasis being given to a set of core outcomes, although all of those outcomes were included in the updated protocol.
Footnotes
DECLARATIONS OF INTEREST SD, RAM and HJM have received research support from charities, government and industry sources at various times. RAM and HJM have consulted for various pharmaceutical companies. RAM and HJM have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. PW is a full-time employee of the UK Cochrane Centre, funded by the UK National Institute of Health Research.
None of the authors have received any funds from any company with an interest in gabapentin for research on gabapentin, and none from any source for the production of this review. HJM at one time was paid by Pfizer to act on a Data Safety and Monitoring board (DSMB) for pregabalin trials (since abandoned).
References to studies included in this review
- Arnold 2007 {published data only} .Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE, Jr, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis and Rheumatism. 2007;56(4):1336–44. doi: 10.1002/art.22457. [DOI: 10.1002/art.22457] [DOI] [PubMed] [Google Scholar]
- Backonja 1998 {published data only} .*; Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280(21):1831–6. doi: 10.1001/jama.280.21.1831. [PUBMED: 9846777] [DOI] [PubMed] [Google Scholar]; Trial summary - Parke Davis/Pfizer 945-210. Unpublished report.
- Bone 2002 {published data only} .Bone M, Critchley P, Buggy DJ. Gabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over study. Regional Anesthesia and Pain Medicine. 2002;27(5):481–6. doi: 10.1053/rapm.2002.35169. [DOI: 10.1053/rapm.2002.35169] [DOI] [PubMed] [Google Scholar]
- Caraceni 2004 {published data only} .Caraceni A, Zecca E, Bonezzi C, Arcuri E, Yaya Tur R, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. Journal of Clinical Oncology. 2004;22(14):2909–17. doi: 10.1200/JCO.2004.08.141. [DOI: 10.1200/JCO.2004.08.141] [DOI] [PubMed] [Google Scholar]
- Chandra 2006 {published data only} .Chandra K, Shafiq N, Pandhi P, Gupta S, Malhotra S. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial - the GONIP Trial. International Journal of Clinical Pharmacology and Therapeutics. 2006;44(8):358–63. doi: 10.5414/cpp44358. [PUBMED: 16961166] [DOI] [PubMed] [Google Scholar]
- CTR 945-1008 {unpublished data only} .Anon Protocol A9451008. A 15 Week, randomized, double-blind, placebo-controlled, parallel-group, multi-center study of Neurontin (gabapentin) for efficacy and quality of life in patients with painful diabetic peripheral neuropathy. PhrmaWebSynopsis - Final. 2005 Jun 2; [Google Scholar]
- CTR 945-224 {unpublished data only} .Protocol 945-224. A double-blind placebo-controlled trial With 3 doses of gabapentin for treatment of painful diabetic peripheral neuropathy. 1998 1999 May 29 7;Sep 29 7; through. Unpublished. [Google Scholar]
- Gilron 2005 {published data only} .Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. New England Journal of Medicine. 2005;352(13):1324–34. doi: 10.1056/NEJMoa042580. [PUBMED: 15800228] [DOI] [PubMed] [Google Scholar]
- Gilron 2009 {published data only} .Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374(9697):1252–61. doi: 10.1016/S0140-6736(09)61081-3. [DOI: 10.1016/s0140-6736 (09)61081-3] [DOI] [PubMed] [Google Scholar]
- Gordh 2008 {published data only} .Gordh TE, Stubhaug A, Jensen TS, Arner S, Biber B, Boivie J, et al. Gabapentin in traumatic nerve injury pain: a randomized, double-blind, placebo-controlled, crossover, multi-center study. Pain. 2008;138(2):255–66. doi: 10.1016/j.pain.2007.12.011. [DOI: 10.1016/j.pain.2007.12.011] [DOI] [PubMed] [Google Scholar]
- Gorson 1999 {published data only} .Gorson KC, Schott C, Herman R, Ropper AH. Gabapentin in the treatment of painful diabetic neuropathy: a placebo controlled, double blind, crossover trial. Journal of Neurology, Neurosurgery and Psychiatry. 1999;66:251–2. doi: 10.1136/jnnp.66.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn 2004 {published data only} .Hahn K, Arendt G, Braun JS, von Giesen HJ, Husstedt IW, et al. German Neuro-AIDS Working Group. A placebo-controlled trial of gabapentin for painful HIV-associated sensory neuropathies. Journal of Neurology. 2004;251(10):1260–6. doi: 10.1007/s00415-004-0529-6. [DOI: 10.1007/s00415-004-0529-6] [DOI] [PubMed] [Google Scholar]
- Ho 2009 {published data only} .Ho TW, Backonja M, Ma J, Leibensperger H, Froman S, Polydefkis M. Efficient assessment of neuropathic pain drugs in patients with small fiber sensory neuropathies. Pain. 2009;14(1-2):19–24. doi: 10.1016/j.pain.2008.07.013. [DOI: 10.1016/j.pain.2008.07.013] [DOI] [PubMed] [Google Scholar]
- Irving 2009 {published data only} .*; Irving G, Jensen M, Cramer M, Wu J, Chiang YK, Tark M, et al. Efficacy and tolerability of gastric-retentive gabapentin for the treatment of postherpetic neuralgia: results of a double-blind, randomized, placebo-controlled clinical trial. Clinical Journal of Pain. 2009;25(3):185–92. doi: 10.1097/AJP.0b013e3181934276. [DOI] [PubMed] [Google Scholar]; Jensen MP, Chiang YK, Wu J. Assessment of pain quality in a clinical trial of gabapentin extended release for postherpetic neuralgia. Clinical Journal of Pain. 2009;25(4):286–92. doi: 10.1097/AJP.0b013e318192bf87. [DOI: 10.1097/AJP.0b013e318192bf87] [DOI] [PubMed] [Google Scholar]
- Kimos 2007 {published data only} .Kimos P, Biggs C, Mah J, Heo G, Rashiq S, Thie NM, et al. Analgesic action of gabapentin on chronic pain in the masticatory muscles: a randomized controlled trial. Pain. 2007;127(1-2):151–60. doi: 10.1016/j.pain.2006.08.028. [DOI: 10.1016/ j.pain.2006.08.028] [DOI] [PubMed] [Google Scholar]
- Levendoglu 2004 {published data only} .Levendoglu F, Ogun CO, Ozerbil O, Ogun TC, Ugurlu H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29(7):743–51. doi: 10.1097/01.brs.0000112068.16108.3a. [DOI: 10.1097/01.BRS.0000112068.16108.3A] [DOI] [PubMed] [Google Scholar]
- Morello 1999 {published data only} .Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Archives of Internal Medicine. 1999;159(16):1931–7. doi: 10.1001/archinte.159.16.1931. [PUBMED: 10493324] [DOI] [PubMed] [Google Scholar]
- Perez 2000 {published data only} .Perez HET, Sanchez GF. Gabapentin therapy for diabetic neuropathic pain. Journal of Medicine. 2000;108:689. doi: 10.1016/s0002-9343(00)00398-3. [DOI] [PubMed] [Google Scholar]
- Rao 2007 {published data only} .Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, et al. North Central Cancer Treatment Group. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110(9):2110–8. doi: 10.1002/cncr.23008. [DOI: 10.1002/cncr.23008] [DOI] [PubMed] [Google Scholar]
- Rice 2001 {published data only} .*; Rice AS, Maton S, Postherpetic Neuralgia Study Group Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94(2):215–24. doi: 10.1016/S0304-3959(01)00407-9. [DOI: 10.1016/S0304-3959(01)00407-9] [DOI] [PubMed] [Google Scholar]; Study detail and analysis, Parke-Davis 945-295. Unpublished Report No. RR-430-00124 2000.
- Rintala 2007 {published data only} .*; Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2007;88(12):1547–60. doi: 10.1016/j.apmr.2007.07.038. [DOI: 10.1016/j.apmr.2007.07.038] [DOI] [PubMed] [Google Scholar]
- Rowbotham 1998 {published data only} .Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280(21):1837–42. doi: 10.1001/jama.280.21.1837. [PUBMED: 9846778] [DOI] [PubMed] [Google Scholar]; Detailed summary, study No.4, Parke-Davis 945-211. Unpublished Report No. RR-995-00070 1998.
- Sandercock 2009 {published data only} .*; Sandercock D, Cramer M, Wu J, Chiang YK, Biton V, Heritier M. Gabapentin extended release for the treatment of painful diabetic peripheral neuropathy: efficacy and tolerability in a double-blind, randomized, controlled clinical trial. Diabetes Care. 2009;32(2):e20. doi: 10.2337/dc08-1450. [DOI: 10.2337/dc08-1450] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell 2002 {published data only} .Serpell MG, Neuropathic pain study group Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99(3):557–66. doi: 10.1016/S0304-3959(02)00255-5. [DOI: 10.1016/S0304-3959(02)00255-5] [DOI] [PubMed] [Google Scholar]; Parke Davis/Pfizer. 945-430-306. Unpublished 2000.
- Simpson 2001 {published data only} .Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. Journal of Clinical Neuromuscular Disease. 2001;3(2):53–62. doi: 10.1097/00131402-200112000-00002. [PUBMED: 19078655] [DOI] [PubMed] [Google Scholar]
- Smith 2005 {published data only} .Smith DG, Ehde DM, Hanley MA, Campbell KM, Jensen MP, Hoffman AJ, et al. Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. Journal of Rehabilitation Research and Development. 2005;42(5):645–54. doi: 10.1682/jrrd.2005.05.0082. [DOI: 10.1682/JRRD.2005.05.0082] [DOI] [PubMed] [Google Scholar]
- Tai 2002 {published data only} .Tai Q, Kirshblum S, Chen B, Millis S, Johnston M, DeLisa JA. Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. Journal of Spinal Cord Medicine. 2002;25(2):100–5. doi: 10.1080/10790268.2002.11753609. [PUBMED: 12137213] [DOI] [PubMed] [Google Scholar]
- van de Vusse 2004 {published data only} .van de Vusse AC, Stomp-van den Berg SG, Kessels AH, Weber WE. Randomised controlled trial of gabapentin in Complex Regional Pain Syndrome type 1 [ISRCTN84121379] BMC Neurology. 2004;4:13. doi: 10.1186/1471-2377-4-13. [DOI: 10.1186/1471-2377-4-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace 2010 {published data only} .Wallace MS, Irving G, Cowles VE. Gabapentin extended-release tablets for the treatment of patients with postherpetic neuralgia: a randomized, double-blind, placebo-controlled, multicentre study. Clinical Drug Investigation. 2010;30(11):765–76. doi: 10.2165/11539520-000000000-00000. [DOI: 10.2165/11539520-000000000-00000] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Arai 2010 {published data only} .Arai YC, Matsubara T, Shimo K, Suetomi K, Nishihara M, Ushida T, et al. Low-dose gabapentin as useful adjuvant to opioids for neuropathic cancer pain when combined with low-dose imipramine. Journal of Anesthesia. 2010;24(3):407–10. doi: 10.1007/s00540-010-0913-6. [DOI: 10.1007/s00540-010-0913-6] [DOI] [PubMed] [Google Scholar]
- Berry 2005 {published data only} .Berry JD, Petersen KL. A single dose of gabapentin reduces acute pain and allodynia in patients with herpes zoster. Neurology. 2005;65(3):444–7. doi: 10.1212/01.wnl.0000168259.94991.8a. [PUBMED: 16087911] [DOI] [PubMed] [Google Scholar]
- Dallochio 2000 {published data only} .Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs. amitriptyline in painful diabetic neuropathy: an open-label pilot study. Journal of Pain and Symptom Management. 2000;20(4):280–5. doi: 10.1016/s0885-3924(00)00181-0. [DOI: 10.1016/S0885-3924 (00)00181-0] [DOI] [PubMed] [Google Scholar]
- Dworkin 2009 {published data only} .Dworkin RH, Barbano RL, Tyring SK, Betts RF, McDermott MP, Pennella-Vaughan J, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain. 2009;142(3):209–17. doi: 10.1016/j.pain.2008.12.022. [DOI: 10.1016/j.pain.2008.12.022] [DOI] [PubMed] [Google Scholar]
- Jean 2005 {published data only} .Jean WH, Wu CC, Mok MS, Sun WZ. Starting dose of gabapentin for patients with post-herpetic neuralgia--a dose-response study. Acta Anaesthesiologica Taiwan. 2005;43(2):73–7. [PUBMED: 16060401] [PubMed] [Google Scholar]
- Keskinbora 2007 {published data only} .Keskinbora K, Pekel AF, Aydinli I. Gabapentin and an opioid combination versus opioid alone for the management of neuropathic cancer pain: a randomized open trial. Journal of Pain Symptom Management. 2007;34(2):183–9. doi: 10.1016/j.jpainsymman.2006.11.013. [: 10.1016/j.jpainsymman.2006.11.013] [DOI] [PubMed] [Google Scholar]
- Ko 2010 {published data only} .Ko SH, Kwon HS, Yu JM, Baik SH, Park IB, Lee JH, et al. Comparison of the efficacy and safety of tramadol/ acetaminophen combination therapy and gabapentin in the treatment of painful diabetic neuropathy. Diabetes Medicine. 2010;27(9):1033–40. doi: 10.1111/j.1464-5491.2010.03054.x. [DOI: 10.1111/ j.1464-5491.2010.03054.x] [DOI] [PubMed] [Google Scholar]
- McCleane 2001 {published data only} .McCleane GJ. Does gabapentin have an analgesic effect on background, movement and referred pain? A randomised, double-blind, placebo controlled study. The Pain Clinic. 2001;13:103. [Google Scholar]
- Nikolajsen 2006 {published data only} .Nikolajsen L, Finnerup NB, Kramp S, Vimtrup AS, Keller J, Jensen TS. A randomized study of the effects of gabapentin on postamputation pain. Anesthesiology. 2006;105(5):1008–15. doi: 10.1097/00000542-200611000-00023. [ISSN: 0003–3022] [DOI] [PubMed] [Google Scholar]
- Pandey 2002 {published data only} .Pandey CK, Bose N, Garg G, Singh N, Baronia A, Agarwal A, et al. Gabapentin for the treatment of pain in Guillain-Barre syndrome: a double-blinded, placebo-controlled, crossover study. Anesthesia and Analgesia. 2002;95(6):1719–23. doi: 10.1097/00000539-200212000-00046. [DOI] [PubMed] [Google Scholar]
- Pandey 2005 {published data only} .Pandey CK, Raza M, Tripathi M, Navkar DV, Kumar A, Singh UK. The comparative evaluation of gabapentin and carbamazepine for pain management in Guillain-Barre syndrome patients in the intensive care unit. Anesthesia and Analgesia. 2005;101(1):220–5. doi: 10.1213/01.ANE.0000152186.89020.36. [DOI: 10.1213/ 01.ANE.0000152186.89020.36] [DOI] [PubMed] [Google Scholar]
- Salvaggio 2008 {published data only} .Salvaggio I, Adducci E, Dell’Aquila L, Rinaldi S, Marini M, Zappia L, et al. Facial pain: a possible therapy with stellate ganglion block. Pain Medicine. 2008;9(7):958–62. doi: 10.1111/j.1526-4637.2008.00515.x. [DOI: 10.1111/j.1526-4637.2008.00515.x] [DOI] [PubMed] [Google Scholar]
- Sator-Katzenschlager 2005 {published data only} .Sator-Katzenschlager SM, Scharbert G, Kress HG, Frickey N, Ellend A, Gleiss A, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wiener Klinische Wochenschrift. 2005;117(21-22):761–8. doi: 10.1007/s00508-005-0464-2. [DOI: 10.1007/s00508-005-0464-2] [DOI] [PubMed] [Google Scholar]
- Yaksi 2007 {published data only} .Yaksi A, Ozgonenel L, Ozgonenel B. The efficiency of gabapentin therapy in patients with lumbar spinal stenosis. Spine. 2007;32(9):939–42. doi: 10.1097/01.brs.0000261029.29170.e6. [DOI: 10.1097/ 01.brs.0000261029.29170.e6] [DOI] [PubMed] [Google Scholar]
- Yelland 2009 {published data only} .Yelland MJ, Poulos CJ, Pillans PI, Bashford GM, Nikles CJ, Sturtevant JM, et al. N-of-1 randomized trials to assess the efficacy of gabapentin for chronic neuropathic pain. Pain Medicine. 2009;10(4):754–61. doi: 10.1111/j.1526-4637.2009.00615.x. [DOI: 10.1111/ j.1526-4637.2009.00615.x] [DOI] [PubMed] [Google Scholar]
- Yildrim 2003 {published data only} .Yildirim K, Siseciolglu M, Karatay S, Erdal A, Levent A, Ugur M. The effectiveness of gabapentin in patients with chronic radiculopathy. The Pain Clinic. 2003;15(3):213–8. [Google Scholar]
Additional references
- Barres 2009 .Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–92. doi: 10.1016/j.cell.2009.09.025. [DOI: 10.1016/j.cell.2009.09.025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhassira 2008 .Bouhassira D, Lant?ri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–7. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Breivik 2006 .Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European Journal of Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI: 10.1016/j.ejpain.2005.06.009] [DOI] [PubMed] [Google Scholar]
- Chou 2009 .Chou R, Carson S, Chan BK. Gabapentin versus tricyclic antidepressants for diabetic neuropathy and post-herpetic neuralgia: discrepancies between direct and indirect meta-analyses of randomized controlled trials. Journal of General Internal Medicine. 2009;24(2):178–88. doi: 10.1007/s11606-008-0877-5. [DOI: 10.1007/ s11606-008-0877-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook 1995 .Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452–4. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallocchio 2000 .Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs. amitriptyline in painful diabetic neuropathy: an open-label pilot study. Journal of Pain and Symptom Management. 2000;20(4):280–5. doi: 10.1016/s0885-3924(00)00181-0. [DOI: 10.1016/S0885-3924 (00)00181-0] [DOI] [PubMed] [Google Scholar]
- Dworkin 2008 .Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain. 2008;9(2):105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
- Elbourne 2002 .Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving crossover trials: methodological issues. International Journal of Epidemiology. 2002;31(1):140–9. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- EMC 2009 .Electronic Medicines Compendium [accessed 1 May 2009]; http://emc.medicines.org.uk/
- Farrar 2000 .Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88(3):287–94. doi: 10.1016/S0304-3959(00)00339-0. [DOI: 10.1016/S0304-3959(00)00339-0] [DOI] [PubMed] [Google Scholar]
- Finnerup 2005 .Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI: 10.1016/j.pain.2005.08.013] [DOI] [PubMed] [Google Scholar]
- Gustorff 2008 .Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiologica Scandinavica. 2008;52(1):132–6. doi: 10.1111/j.1399-6576.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- Hall 2006 .Hall GC, Carroll D, Parry D, McQuay HJ. Epidemiology and treatment of neuropathic pain: the UK primary care perspective. Pain. 2006;122(1-2):156–62. doi: 10.1016/j.pain.2006.01.030. [DOI: 10.1016/ j.pain.2006.01.030] [DOI] [PubMed] [Google Scholar]
- Hauser 2009 .Haüser W, Bernardy K, Uceyler N, Sommer C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin -a meta-analysis of randomized controlled trials. Pain. 2009;145(1-2):69–81. doi: 10.1016/j.pain.2009.05.014. [DOI: 10.1016/j.pain.2009.05.014] [DOI] [PubMed] [Google Scholar]
- Hempenstall 2005 .Hempenstall K, Nurmikko TJ, Johnson RW, A’Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Medicine. 2005;2(7):e164. doi: 10.1371/journal.pmed.0020164. [DOI: 10.1371/journal.pmed.0020164] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman 2010 .Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status 3 and function in patients with painful diabetic peripheral neuropathy? Pain. 2010;149(2):194–201. doi: 10.1016/j.pain.2009.09.017. [DOI: 10.1016/j.pain.2009.09.017] [DOI] [PubMed] [Google Scholar]
- Jacox 1994 .Jacox A, Carr DB, Payne R. Management of cancer pain. Clinical Practice Guideline No. 9. Agency for Health Care Policy and Research. US Department of Health and Human Services, Public Health Service; Rockville, Maryland: 1994. [Google Scholar]
- Jadad 1996 .Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Khan 1996 .Khan KS, Daya S, Jadad A. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine. 1996;156(6):661–6. [PubMed] [Google Scholar]
- L’Abbe 1987 .L’Abbé KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine. 1987;107:224–33. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- Landefeld 2009 .Landefeld CS, Steinman MA. The Neurontin legacy -marketing through misinformation and manipulation. New England Journal of Medicine. 2009;360(2):103–6. doi: 10.1056/NEJMp0808659. [PUBMED: 19129523] [DOI] [PubMed] [Google Scholar]
- Lunn 2009 .Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database of Systematic Reviews. 2009;(Issue 4) doi: 10.1002/14651858.CD007115.pub2. [DOI: 10.1002/ 14651858.CD007115.pub2] [DOI] [PubMed] [Google Scholar]
- McQuay 1995 .McQuay H, Carroll D, Jadad AR, Wiffen P, Moore A. Anticonvulsant drugs for management of pain: a systematic review. BMJ. 1995;311(7012):1047–52. doi: 10.1136/bmj.311.7012.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuay 1998 .McQuay H, Moore R. An evidence-based resource for pain relief. Oxford University Press; Oxford: 1998. An evidence-based resource for pain relief. [ISBN: 0–19–263048–2] [Google Scholar]
- McQuay 2008 .McQuay HJ, Derry S, Moore RA, Poulain P, Legout V. Enriched enrolment with randomised withdrawal (EERW): Time for a new look at clinical trial design in chronic pain. Pain. 2008;135(3):217–220. doi: 10.1016/j.pain.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Monks 1994 .Monks R. Psychotropic drugs. In: Wall PD, Melzack R, editors. Textbook of Pain. 3rd Edition. Churchill Livingstone; Edinburgh: 1994. pp. 963–89. [ISBN: 044304757X] [Google Scholar]
- Moore 1998 .Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78(3):209–16. doi: 10.1016/S0304-3959(98)00140-7. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
- Moore 2005a .Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta-analysis shows the impact of different ways of analysing and presenting results. Pain. 2005;116(3):322–31. doi: 10.1016/j.pain.2005.05.001. [DOI: 10.1016/j.pain.2005.05.001] [DOI] [PubMed] [Google Scholar]
- Moore 2005b .Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Research and Therapy. 2005;7(3):R644–65. doi: 10.1186/ar1704. [DOI: 10.1186/ar1704] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore 2008 .Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors. Systematic Reviews in Pain Research: Methodology Refined. IASP Press; Seattle: 2008. pp. 15–24. [ISBN: 978–0–931092–69–5] [Google Scholar]
- Moore 2009a .Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews. 2009;(Issue 3) doi: 10.1002/14651858.CD007076.pub2. [DOI: 10.1002/14651858.CD007076] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore 2009b .Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. [accessed 1 March 2009];Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases. 2009 doi: 10.1136/ard.2009.107805. http://ard.bmj.com/cgi/content/abstract/ard.2009.107805v1 [Epub ahead of print] [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed]
- Moore 2009c .Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P, et al. for the ACTINPAIN writing group of the IASP Special Interest Group (SIG) on Systematic Reviews in Pain Relief Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain. 2009 Sep 10; doi: 10.1016/j.pain.2009.08.007. Epub ahead of print. [DOI: 10.1016/j.pain.2009.08.007] [DOI] [PubMed] [Google Scholar]
- Moore 2010a .Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases. 2010;69(2):374–9. doi: 10.1136/ard.2009.107805. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore 2010b .Moore RA, Straube S, Derry S, McQuay HJ. Topical review: chronic low back pain analgesic studies - a methodological minefield. Pain. 2010;149(3):431–4. doi: 10.1016/j.pain.2010.02.032. [DOI: 10.1016/ j.pain.2010.02.032] [DOI] [PubMed] [Google Scholar]
- Moore 2010c .Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain. 2010;149(2):360–4. doi: 10.1016/j.pain.2010.02.039. [DOI: 10.1016/j.pain.2010.02.039] [DOI] [PubMed] [Google Scholar]
- Moore 2010d .Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, McQuay H, ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors “Evidence” in chronic pain--establishing best practice in the reporting of systematic reviews. Pain. 2010;150(3):386–9. doi: 10.1016/j.pain.2010.05.011. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
- Nuesch 2010 .Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, Egger M, Jüni P. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. British Medical Journal. 2010;341:c3515. doi: 10.1136/bmj.c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien 2010 .O’Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, et al. Patient-centered perspective on treatment: outcomes in chronic pain. Pain Medicine. 2010;11:6–15. doi: 10.1111/j.1526-4637.2009.00685.x. [DOI: 10.1111/ j.1526-4637.2009.00685.x] [DOI] [PubMed] [Google Scholar]
- Oldman 2002 .Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain. 2002;97(3):247–57. doi: 10.1016/S0304-3959(02)00024-6. [DOI: 10.1016/S0304-3959(02)00024-6] [DOI] [PubMed] [Google Scholar]
- Perry 2008 .Perry T. [accessed 2 March 2010];Neurontin - expert opinion on efficacy and effectiveness for pain. 2008 http://dida.library.ucsf.edu/tid/oxx18p10 Vol.
- Quilici 2009 .Quilici S, Chancellor J, Lothgren M, Simon D, Said G, Le TK, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurology. 2009;9:6. doi: 10.1186/1471-2377-9-6. [DOI: 10.1186/1471-2377-9-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube 2008 .Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology. 2008;66(2):266–75. doi: 10.1111/j.1365-2125.2008.03200.x. [DOI: 10.1111/ j.1365-2125.2008.03200.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube 2010 .Straube S, Derry S, Moore RA, Wiffen PJ, McQuay HJ. Single dose oral gabapentin for established acute postoperative pain in adults. Cochrane Database of Systematic Reviews. 2010;(Issue 5) doi: 10.1002/14651858.CD008183.pub2. [DOI: 10.1002/ 14651858.CD008183.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan 2008 .Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology. 2008;8:29. doi: 10.1186/1471-2377-8-29. [DOI: 10.1186/1471-2377-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance 2006 .Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Journal of Pain. 2006;7(4):281–9. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Tzellos 2008 .Tzellos TG, Papazisis G, Amaniti E, Kouvelas D. Efficacy of pregabalin and gabapentin for neuropathic pain in spinal-cord injury: an evidence-based evaluation of the literature. European Journal of Clinical Pharmacology. 2008;64(9):851–8. doi: 10.1007/s00228-008-0523-5. [DOI: 10.1007/s00228-008-0523-5] [DOI] [PubMed] [Google Scholar]
- Vedula 2009 .Vedula SS, Bero L, Scherer RW, Dickersin K. Outcome reporting in industry-sponsored trials of gabapentin for off-label use. New England Journal of Medicine. 2009;361(20):1963–71. doi: 10.1056/NEJMsa0906126. [DOI] [PubMed] [Google Scholar]
- Wiffen 2005 .Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database of Systematic Reviews. 2005;(Issue 3) doi: 10.1002/14651858.CD005452. [DOI: 10.1002/ 14651858.CD005452] [DOI] [PubMed] [Google Scholar]
- Wiffen 2011a .Wiffen P, Collins S, McQuay H, Carroll D, Jadad A, Moore A. Withdrawn - Anticonvulsant drugs for acute and chronic pain. Cochrane Database of Systematic Reviews. 2011;(Issue 1) [DOI: 10.1002/14651858.CD001133.pub3] [Google Scholar]
- Wiffen 2011b .Wiffen PJ, Derry S, Moore RA, McQuay HJ. Carbamazepine for acute and chronic pain. Cochrane Database of Systematic Reviews. 2011;(Issue 1) doi: 10.1002/14651858.CD005451.pub2. [DOI: 10.1002/14651858.CD005451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Indicates the major publication for the study