Abstract
Abusive head trauma (AHT) is the leading cause of morbidity and mortality among abused children, yet the neuroanatomical underpinnings of AHT outcome is incompletely understood. The aim of this study was to characterize white matter (WM) abnormalities in infants with AHT using diffusion tensor imaging (DTI) and determine which microstructural abnormalities are associated with poor outcome. Retrospective DTI data from 17 infants (>3 months) with a diagnosis of AHT and a comparison cohort of 34 term infants of similar post-conceptual age (PCA) were compared using a voxel-based DTI analysis of cerebral WM. AHT cases were dichotomously classified into mild/moderate versus severe outcome. Clinical variables and conventional imaging findings were also analyzed in relation to outcome. Outcomes were classified in accordance with the Pediatric Cerebral Performance Category Score (PCPCS). Reduced axial diffusivity (AD) was shown in widespread WM regions in the AHT infants compared with controls as well as in the AHT severe outcome group compared with the AHT mild/moderate outcome group. Reduced mean diffusivity (MD) was also associated with severe outcome. Radial diffusivity (RD), conventional magnetic resonance findings, brain metric measurements, and clinical/laboratory variables (with the exception of Glascow Coma Scale) did not differ among AHT outcome groups. Findings support the unique role of DTI techniques, beyond conventional imaging, in the evaluation of microstructural WM injury of AHT. Reduced AD (likely reflecting axonal damage) and MD were associated with poor clinical outcome. DTI abnormalities may uniquely reflect AHT patterns of axonal injury that are not characterized by conventional imaging, which may have both therapeutic and prognostic implications.
Key words: : axial diffusivity, diffusion tensor imaging, pediatric brain injury
Introduction
Abusive head trauma (AHT) is a leading cause of death and disability among infants and children1–6 with an estimated mortality rate of 35.7%,7 and worse survivor neurological outcome compared with accidental traumatic brain injury (TBI).7–11 Among AHT survivors, the majority have permanent disability, including blindness, seizure disorder, cerebral palsy, and cognitive impairment.12 Abnormal neurological findings include (1) hemiplegia, quadriplegia, or diplegia (33–100%), (2) blindness or severe visual impairment (20–41%), and (3) persistent vegetative state or significant cognitive impairment (35–50%).11 Compared with children and adolescents with a diagnosis of accidental TBI who show significant increases in cognitive and motor scores during the first 6 months after injury, recovery curves among AHT survivors are flat.11,13–15 Long-term cognitive sequelae may not emerge for months or even years after AHT injury.16 Annually, the national economic burden of new fatal and nonfatal child maltreatment cases, including AHT, is approximately 124 billion dollars.17
Heterogeneous outcomes in AHT are likely reflective of heterogeneity in biomechanical injury. Among infants and toddlers, AHT injury is often the result of impact injury and/or violent shaking causing whipping of the head back and forth with severe rotational and angular acceleration/deceleration. These types of biomechanical forces have been associated with intracranial hemorrhage, cerebral edema, diffuse axonal injury, and/or retinal hemorrhages. Injuries to the craniocervical junction from hyperflexion and extension may be responsible for traumatic apnea and/or circulatory changes resulting in hypoxic ischemic injury16,18–21 or global ischemic encephalopathy.19,20,22 Interference with respiratory functions resulting in hypoxia may also account for additional brain injury.18
Given that AHT is further complicated by time-dependent (e.g., repeated injury) and age-specific factors, neuroimaging plays a crucial role in the evaluation of injury within the setting of AHT. Neuroimaging abnormalities associated with poor outcome include diffuse cerebral edema, cerebral hypoperfusion, brain infarction, and increased depth of parenchymal injury.9,23–30 Within the clinical setting, computed tomography (CT) scans, generally completed at initial presentation to an emergency department, are useful for revealing skull fractures, intracranial blood, and intraparenchymal hemorrhages. CT, however, is not sensitive for detecting microstructural injury. During the subacute phase of injury, magnetic resonance imaging (MRI) is superior to CT in assessing the extent of damage and potential clinical outcome.23
More recently, advanced imaging techniques such as susceptibility-weighted imaging (SWI), magnetic resonance spectroscopy (MRS), and diffusion-weighted imaging (DWI) have demonstrated additional utility in the evaluation of AHT. Clinically, DWI can be used to detect areas of acute injury defined by cytotoxic edema. With a greater number of diffusion encoding directions (i.e., ≥100), diffusion tensor imaging (DTI) metrics such as fractional anisotropy and axial diffusivity can be used to demonstrate changes in tissue microstructure such as axonopathy in the setting of trauma or hypoxic-ischemic injury.31
To date, no previous study that we are aware of has examined the utility of DTI within the setting of AHT evaluation. Given the sensitivity of DTI in detecting normative and atypical white matter (WM) patterns, as well as the dynamic WM changes occurring in infancy, we hypothesize that DTI will be a useful tool for the early evaluation and prognosis of AHT among infants and toddlers. The objective of our study was to characterize WM abnormalities in infants with AHT using DTI and determine which microstructural abnormalities are associated with poor outcome.
Methods
Patients
The Committee for Clinical Investigations approved this study, including review of medical records. General medical consent for treatment and/or an additional consent for sedation (if indicated) was obtained from parents and/or legal guardians.
AHT infants and toddlers
We conducted a retrospective review of term infants and toddlers greater than 3 months of age admitted to an academic children's hospital between 2005 and 2008, who underwent MRI with DTI during clinical assessment and had a subsequent diagnosis of AHT. AHT patients less than 3 months of age were excluded because different DTI protocols are used for this age group. For the purposes of this study we grouped all head injuries caused by abuse into the single category of AHT. In reaching a diagnosis of AHT,5,32,33 these infants/toddlers underwent a comprehensive assessment by the hospital's child protection team that included evaluation by a pediatrician board-certified in Pediatrics, Child Abuse Pediatrics, and Developmental-Behavioral Pediatrics and a social worker with extensive expertise in the field of child abuse.
Factors taken into account included histories provided by the caregivers, findings on physical examination, other unexplained injuries, consultation with subspecialists, review of available previous radiographic studies and/or medical records, and information provided from law enforcement and Child Protective Services. In all cases, underlying medical conditions and/or accidental trauma, which could mimic AHT, were ruled out. Defining abuse using the assessment of a child protection team is a commonly used standard employed in previous studies.
Classification of outcome within the AHT cohort
AHT outcomes were classified in accordance with the Pediatric Cerebral Performance Category Scale (PCPCS) based on medical chart review performed by the same pediatrician who conducted the acute comprehensive assessment and provided follow-up clinical care to the infants and toddlers in this cohort. The PCPCS was performed between 6 and 9 months after discharge. The PCPCS includes six categories used to summarize the level of neurological function in pediatric patients with TBI with the following outcomes: (1) normal (e.g., can perform all age-appropriate activities); (2) mild disability (e.g., conscious alert and able to interact in most age-appropriate activities but may have mild neurological deficit); (3) moderate disability (e.g., conscious, with sufficient cerebral function for age-appropriate independent activities of daily living, but has significant cognitive impairment); (4) severe disability (e.g., conscious but dependent on others for daily support because of impaired brain function including need for rehabilitation services and gastrostomy tube placement); (5) coma or vegetative state; and (6) brain death. Patients were dichotomized into two groups: (A) mild or moderate (categories 1–3 on the PCPCS) and (B) severe neurological dysfunction (categories 4–6 on the PCPCS).
Comparison cohort
For comparison purposes, we also included data from term infants of similar post-conceptual age (PCA) who underwent MRIs at the same institution during the same period. Clinical indication for MRIs included possible seizure activity, concern for meningitis, vascular malformations, and/or hydrocephalus, with findings indicating no structural abnormalities. Medical records were reviewed by a board certified neonatologist and neuropsychologist who determined that all comparison cohort participants did not have histories of chromosomal abnormalities, mitochondrial diseases, extracorporeal membrane oxygenation, neurologic symptoms, and/or other underlying medical conditions that would portend a poor neurologic outcome.
MR and CT imaging
A board certified neuroradiologist reviewed all neuroimaging. All imaging was obtained in a 1.5T General Electric System (GE-Medical Systems, Milwaukee, WI) with a neonatal or pediatric head coil and included the following sequences: Fast Spin Echo T2-weighted imaging (TE/TR=85/5000 msec, field of view=20 cm, matrix=320×160 or 256×128); Axial Fluid Attenuated Inversion Recovery FLAIR (TE/TR=120/9000 msec, field of view=20 cm, matrix=320×160 or 256×128); and diffusion tensor imaging (an echo-planar imaging sequence with the following parameters: TE/TR=80/10000 msec, field of view=22 cm, matrix=128×128, slice thickness=5 mm, spacing=0 applied along 25 non-collinear directions with a b-value of 700–1000 s/mm2. CT imaging protocol included a standard multi-detector 5 mm collimated slice acquisition using low-dose pediatric protocols.
Brain metric measurements
Brain metrics previously validated as measures of brain growth and/or atrophy in infants and toddlers were measured on T1- and T2-weighted images using Synapse software (Fuji) in accordance with previous publications.34 These 15 standard head and brain measurements (Supplementary Tables 1 and 2; see online supplementary material at ftp.liebertpub.com) were manually placed by a single person who was blinded to subject status and then confirmed by a neuroradiologist.
DTI metrics
This study focused on mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), and fractional anisotropy (FA). These metrics can be readily calculated from DTI data using available post-processing tools and reflect both the extent and directionality of water diffusion. Specifically, AD indicates the amount of diffusion along the principal axis, RD indicates the mean of the diffusivity along the two minor axes, and MD indicates the average diffusion along all three axes. FA indicates the strength of directional diffusion, ranging from 0 (isotropic) to 1 (anisotropic).
DTI post-processing—Tract-Based Spatial Statistics (TBSS)
DTI data analysis was performed using Oxford University's FMRIB FSL software (Version 4.1.4; www.fmrib.ox.ac.uk/fsl/fsl/downloading.html).35 Our protocol for TBSS36 was similar to other published methods previously optimized for infants and toddlers.37 Voxelwise statistics were performed using Threshold-Free Cluster Enhancement and corrected for age and multiple comparisons.
Analysis of DTI metrics in gray matter
Because the TBSS methods are only sensitive for analyzing microstructural changes in WM, we included additional regions of interest (ROI) measurements in select gray matter structures known to be vulnerable to injury in the setting of hypoxic-ischemia or trauma: basal ganglia (BG), thalamus (Thal) and medial occipital-parietal cortical gray matter. Consistent with previous publications in our laboratory,36 contours were placed on the anatomically defined ROIs using the combined information from the FA and MD maps.
Statistical analysis (brain metric measurements and clinical data)
Bilateral measurements were compared using the Student t test. Clinical variables and conventional imaging comparisons between outcome groups (mild/moderate vs. severe) were performed using the Mann-Whitney U test (non-parametric alternative to the Student t test for independent samples) and the Fisher exact test. Comparisons of DTI outcome data (mild/moderate vs. severe) were analyzed using the Mann-Whitney U test. The Statistical Package for the Social Sciences Version 19.5 (IBM SPSS, IBM Corporation, Armonk, NY, 2010) was used for all statistical analyses.
Results
Patients
A total of 17 term patients older than 3 months (mean PCA in weeks=76.53; standard deviation [SD]=22.34; range 54.57–124.43; median=70.86) fit the inclusion criteria for AHT and included the CT/MRI sequences pertinent to this study (mild/moderate outcome=9 patients; severe outcome=8 patients). A comparison cohort including 34 term infants of similar PCA (mean PCA in weeks=75.63; SD=17.10) was also identified. Comparisons between the AHT outcome groups (mild/moderate vs. severe) indicated no differences in the incidence of the following clinical variables on initial presentation: intubation, retinal hemorrhages, seizures, non-reactive/sluggish pupils, cardiopulmonary arrest, initial serum sodium value, initial blood glucose value, and initial hemoglobin level (Table 1). Initial Glasgow Coma Scale (GCS) score was significantly different (p=0.05) between the mild/moderate and the severe outcome groups (Table 1).
Table 1.
Clinical Variables in Abusive Head Trauma Cases: Comparison between Outcome Groups (N=16)
| Clinical variable | Mild/Mod. (n=9) | Severe (n=7) | p value |
|---|---|---|---|
| Intubated no. (%) | 3/9 (33.3) | 3/7 (42.86) | 1.00* |
| Retinal hemorrhage no. (%) | 7/9 (77.78) | 4/7 (57.14) | 0.60* |
| Seizures no. (%) | 5/9 (55.56) | 6/7 (85.71) | 0.31* |
| Non-reactive/sluggish pupils no. (%) | 3/9 (33.33) | 4/7 (57.14) | 0.62* |
| Cardiopulmonary arrest no. (%) | 0/9 (0.00) | 2/7 (28.57) | 0.18* |
| Initial GCS, mean (SD) | 11.67 (3.00) | 8.14 (3.08) | 0.05** |
| Initial serum Na+ (mEq/L), mean (SD) | 140.44 (5.13) | 142.29 (5.02) | 0.46** |
| Initial blood glucose (mg/dL), mean (SD) | 119.00 (28.81) | 98.43 (12.74) | 0.15** |
| Initial Hgb level (g/dL), mean (SD) | 9.47 (1.42) | 9.76 (1.42) | 0.92** |
Mod, moderate; GCS, Glasgow Coma Scale; SD, standard deviation; Na+, sodium; Hgb, hemoglobin;
By Fisher exact test.
By Mann-Whitney U test.
Note: Data for one participant in the Severe Group was unavailable because of inadequate documentation from an outside medical facility.
CT and conventional MR findings
We examined the incidence of extra-axial blood (e.g., subdural hemorrhages) and diffuse edema based on conventional CT scans and T1-, T2- and gradient echo MR images. A significant difference between AHT outcome groups was found among the incidence of diffuse edema on CT (p=0.04; Table 2). Our definition of “diffuse edema” was based on CT classic findings of brain edema that include diffuse hypoattenuation, effacement of multi-lobar sulci, effacement of basal cisterns, and possible “reversal sign.” No significant differences were found in the incidence of subdural hemorrhage on CT or among MRI variables (i.e., edema, subdural hemorrhage, enhancing subdural membrane, volume loss, and posterior fossa subdural hemorrhage) (Table 2).
Table 2.
Computed Tomography and Conventional Magnetic Resonance Imaging Findings for Outcome Groups (N=17)
| Imaging | Mild/Mod. (n=9) | Severe (n=7) | P*value |
|---|---|---|---|
| CTa | |||
| Diffuse edema no. (%) | 2/9 (22.0) | 6/7 (85.7) | 0.04 |
| Acute subdural hemorrhage no. (%) | 6/9 (66.7) | 6/7 (85.7) | 0.59 |
| MRIb | |||
| Edema no. (%) | 6/9 (66.7) | 8/8 (100) | 0.21 |
| Subdural hemorrhage no. (%)c | 9/9 (100.) | 6/8 (75) | 0.21 |
| Volume loss no. (%) | 4/9 (44.4) | 2/8 (25.0) | 0.62 |
| Posterior fossa hemorrhage no. (%) | 7/9 (77.8) | 6/8 (75) | 1.00 |
Mod, moderate; CT, computed tomography; MRI, magnetic resonance imaging.
By Fisher exact test.
Note: CT data for one participant in the severe group were unavailable.
MRI was performed 1–7 days post-hospital admission with one outlier at 16 months.
Subacute and chronic.
Brain metrics based on conventional T1- and T2-weighted MRI
Relative to the comparison cohort, there was a trend toward an increase in the interhemispheric distance (p=0.06) and the craniocaudal interopercular left (p=0.07) in the AHT patients suggesting mildly decreased brain volumes in the AHT patients (Supplementary Table 1; see online supplementary material at ftp.liebertpub.com). Among the AHT Mild/Moderate versus Severe Outcome Groups, brain metrics on conventional neuroimaging were not significantly different (Supplementary Table 2; see online supplementary material at ftp.liebertpub.com).
TBSS analysis: AHT cases versus comparison control cohort
Among AHT cases, significantly reduced AD (greater in the right hemisphere than left hemisphere) accounted for the majority of the atypical TBSS findings. Abnormal MD (i.e., AD plus RD) findings were characterized primarily by abnormally decreased AD rather than abnormally increased RD. Compared with the comparison control cohort, AHT cases had significantly reduced AD in regions associated with auditory and visual systems (i.e., centrum semiovale and optic radiations) expanding into the executive functioning systems (i.e., right external capsule and genu of the callosum) (Fig. 1). In contrast, there was relative sparing of central structures involving the motor system (i.e., posterior limb of the internal capsule) and central crossing structures (i.e., cortical spinal tract, brain stem, corona radiata as well as the body and splenium of the callosum). No corrected FA differences were found among AHT cases versus the comparison control cohort.
FIG. 1.
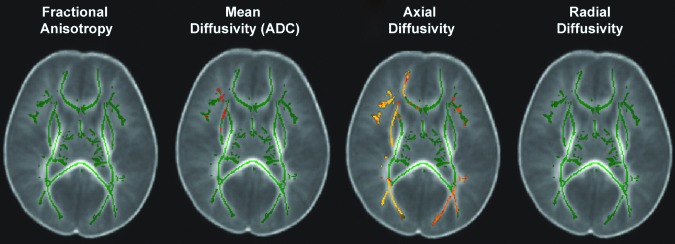
Tract-based spatial statistics (TBSS) is the spatial analysis of DTI data that compares one group to another. Thus this figure does not represent a single white matter skeleton of one brain but, rather, it depicts the overall mean of each group (in this case, abusive head trauma [AHT] cases and the comparison cohort) transposed on each other, with the red/yellow depicting significant group differences (p<0.05). Compared with the case controls, AHT cases had significantly reduced axial diffusivity in regions associated with auditory and visual systems expanding into the executive functioning systems. In contrast, there were no significant differences in radial diffusivity or fractional anisotropy, and there was relative sparing of central structures involving the motor system and central crossing structures. Color image is available online at www.liebertpub.com/neu
TBSS analysis: AHT cases and outcomes
Among AHT outcome groups, the Severe Outcome Group data indicated significantly abnormal bilateral AD and asymmetric RD (right greater than left) resulting in MD abnormalities. Compared to the Mild/Moderate Outcome Group, the Severe Outcome Group had significantly reduced AD in widespread regions of the executive system (i.e., genu of the callosum, external capsule and the anterior limb of the internal capsule) in addition to regions associated with auditory and visual systems (i.e., anterior limb of the internal capsule and fimbria, optic radiations and centrum semiovale) (Fig. 2). Relative sparing was seen in the central structures involving the motor system (i.e., posterior limb of the internal capsule) and central crossing structures (i.e., cortical spinal tract, brain stem, corona radiate, body and splenium of the callosum). There were no significant corrected FA abnormalities between outcome groups.
FIG. 2.
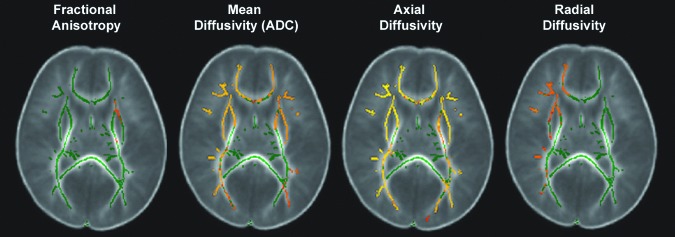
Tract-based spatial statistics (TBSS) is the spatial analysis of DTI data that compares one group to another. Thus this figure does not represent a single white matter skeleton of one brain but, rather, it depicts the overall mean of each group (in this case, abusive head trauma [AHT] cases with Mild/Moderate Outcomes and AHT cases with Severe Outcomes) transposed on each other, with the red/yellow depicting significant group differences (p<0.05). Compared with the Mild/Moderate Outcome Group, the Severe Outcome group had significantly reduced bilateral axial diffusivity (AD) in widespread regions of the executive system in addition to regions associated with auditory and visual systems. There were also asymmetric radial diffusivity (RD) abnormalities between outcome groups. Significant mean diffusivity (i.e., AD plus RD) differences between outcome groups were primarily driven by AD abnormalities. Relative sparing was seen in the central structures involving the motor system and central crossing structures. Color image is available online at www.liebertpub.com/neu
MD (DTI) data for gray matter structures
There were no significant reductions in MD involving the central gray matter structures between AHT cases versus controls, (p≥0.13; Table 3) nor between AHT outcome groups (p≥0.50; Table 3).
Table 3.
Mean Diffusivity Data Comparisons of Central Gray Matter Structures: Abusive Head Trauma Outcome Groups
| Mild/Mod. (n=9) | Severe (n=8) | p*value | Cases (n=17) | C-Cohort (n=34) | p**value | |
|---|---|---|---|---|---|---|
| Putamen left, mean (SD) | 0.80 (0.11) | 0.78 (0.13) | 0.74 | 0.81 (0.12) | 0.80 (0.12) | 0.77 |
| Putamen right, mean (SD) | 0.80 (0.11) | 0.78 (0.12) | 0.89 | 0.82 (0.12) | 0.80 (0.12) | 0.68 |
| Thalamus left, mean (SD) | 0.81 (0.11) | 0.84 (0.17) | 0.77 | 0.84 (0.13) | 0.81 (0.13) | 0.43 |
| Thalamus right, mean (SD) | 0.89 (0.29) | 0.85 (0.16) | 0.50 | 0.88 (0.21) | 0.81 (0.13) | 0.18 |
| OPGM left, mean (SD) | 0.82 (0.12) | 0.77 (0.15) | 0.56 | 0.83 (0.15) | 0.85 (0.14) | 0.49 |
| OPGM right, mean (SD) | 0.88 (0.18) | 0.85 (0.14) | 0.77 | 0.91 (0.18) | 0.93 (0.15) | 0.72 |
Mod, moderate; C-Cohort, comparison cohort; SD, standard deviation; OPGM, occipital-parietal gray matter.
By Mann-Whitney U test.
By Fisher exact test.
Discussion
Previous studies have demonstrated the utility of DTI in the evaluation and understanding of pediatric and adult head injuries. To date, however, no previous study that we are aware of has examined the utility of DTI in the evaluation of AHT in infants and toddlers. We retrospectively evaluated DTI data from 17 infants and toddlers with evidence of AHT who underwent medical evaluation at our institution and compared those results with a cohort of age-matched patients who underwent the same type of neuroimaging during the same period. We found that DTI provides additional microstructural characterization of the WM injury in AHT patients during a critical period of development and appears to have prognostic value.
Our study demonstrates that alterations in MD and associated AD, suggesting axonal injury,38–42 were associated with relatively poorer outcome. These microstructural abnormalities co-localize with WM tracts that mediate multiple neurocognitive domains including auditory, visual, and executive function. In comparison, microstructural alteration was not detected in central gray matter structures including the BG and Thal, which are known to be vulnerable to hypoxic-ischemic injury or trauma. Like DTI, initial GCS and edema on CT differentiated between AHT outcome groups; however, DTI imaging provided additional prognostic information (e.g., changes to tissue microstructure) compared with brain metric measurements, other clinical variables, and conventional imaging results. While GCS and cerebral edema are correlated with AHT outcome, DTI uniquely characterizes atypical changes to tissue microstructure during a critical period of WM development, which may have implications for sensory, cognitive, and behavioral outcomes.
TBSS revealed that not only reduced MD, but also reduced AD, likely consistent with axonal injury, was seen in the AHT infants compared with the comparison cohort and in infants with severe outcome compared with infants with mild/moderate outcome. Notably, this spatial pattern of reduced AD is relatively distinct among AHT infant brain injuries, unlike perinatal hypoxic-ischemic encephalopathy or neonatal stroke, in that it is neither confined to an arterial distribution nor heavily centered on central structures (e.g., callosum, corticospinal tract). However, the pattern does mirror the unique patterns of cytotoxic edema observed in AHT populations, however, and suggests that underlying axonal injury is an important mediator of outcome consistent with neuropathological findings among fatal AHT cases and animal studies.43 We did not find significant FA differences between the AHT cases and the comparison cohort nor between AHT outcome groups, which may be reflective of developing WM in the immature brain and/or the physiological mechanism of AHT injury among infants.
Previous studies in childhood and adolescent populations after accidental TBI have demonstrated reduced FA across widespread WM regions in the chronic epoch (>3 months) after the injury,44–48 which often correlated with neurocognitive functioning.38,40,49–51 For DTI data acquired in the acute epoch (weeks to 3 months after an accidental TBI), DTI results, including reduced FA in the corpus callosum, correlated with GCS scores, selected cognitive and functional outcome as well as global outcome.41,52 Alterations in AD in animal and human studies, however, have been inconsistent with studies finding increases,53,54 decreases,55 or no change.56 Directionality of AD diffusion changes may be related to timing of neuroimaging post-injury, mechanism(s) and/or region(s) of injury, and age of the patient. Future investigation, including those with animal models, will be necessary to better understand the precise meaning of AD alterations in the AHT population.
Very little data exist about correlating advanced neuroimaging techniques and outcome in AHT. Although studies are limited, SWI imaging, which is sensitive to microhemorrhages,21,57–60 and MRS, which is sensitive to metabolic alterations, have been shown to have prognostic values in this population. Our study's atypical DTI findings indicate microstructural abnormalities, which co-localize with WM tracts that mediate frontal executive, auditory, and visual functions, demonstrating the potential prognostic utility of DTI in the evaluation of AHT.
Limitations of our study include the retrospective design and small sample size. There was also a bias toward more severely injured AHT cases in that all of the imaging was performed primarily under clinical indications. In addition, the PCPCS measures broad clinical categories; more detailed neurocognitive outcome assessments are pending. We also recognize that patients may have had previous undocumented or “missed” episodes of injury resulting in acute findings being superimposed on previous TBI that could affect our DTI measurements. It is also important to emphasize that DTI was not used to discriminate etiology (e.g., abusive vs. accidental TBI), but only to identify the presence of TBI. Thus, use of DTI would not change the method of AHT diagnosis but alert physicians to the possibility of more widespread injury affecting both present and future development and function.
Conclusion
Our study supports the unique role of DTI techniques, beyond conventional imaging, in the evaluation of microstructural WM injury of AHT. Reduced AD (thought to be related to axonal injury) and MD (primarily from abnormal AD) were associated with poor outcomes. In contrast, brain metrics and clinical/laboratory values (with the exception of GCS and edema on CT) did not differ between AHT outcome groups. Our study demonstrates the utility of DTI to characterize WM microstructural alterations seen in the cerebrum of infants and toddlers with AHT, which may have both therapeutic and prognostic implications.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Rachel P. Berger for her constructive feedback on this manuscript and to Julie Castro for her assistance with data management. Support for this study is provided by: K23NS063371 (AP), Rudi Schulte Research Institute (SB), and USC-CHLA CTSI grant 1UL1RR031986 (AP, JLW).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bruce D.A., and Zimmerman R.A. (1989). Shaken impact syndrome. Pediatr. Ann. 18, 482–484, 486–489, 492–494 [DOI] [PubMed] [Google Scholar]
- 2.Caffey J. (1974). The whiplash shaken infant syndrome: manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics 54, 396–403 [PubMed] [Google Scholar]
- 3.Duhaime A.C., Alario A.J., Lewander W.J., Schut L., Sutton L.N., Seidl T.S., Nudelman S., Budenz D., Hertle R., and Tsiaras W., et al. (1992). Head injury in very young children: mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics 90, 179–185 [PubMed] [Google Scholar]
- 4.Fujiwara T., Barr R.G., Brant R.F., Rajabali F., and Pike I. (2012). Using International Classification of Diseases, 10th edition, codes to estimate abusive head trauma in children. Am. J. Prev. Med. 43, 215–220 [DOI] [PubMed] [Google Scholar]
- 5.Keenan H.T., Runyan D.K., Marshall S.W., Nocera M.A., Merten D.F., and Sinal S.H. (2003). A population-based study of inflicted traumatic brain injury in young children. JAMA 290, 621–626 [DOI] [PubMed] [Google Scholar]
- 6.Selassie A.W., Borg K., Busch C., Russell , and W.S. (2013). Abusive head trauma in young children: a population-based study. Pediatr. Emerg. Care. 29, 283–291 [DOI] [PubMed] [Google Scholar]
- 7.Shein S.L., Bell M.J., Kochanek P.M., Tyler-Kabara E.C., Wisniewski S.R., Feldman K., Makoroff K., Scribano P.V., and Berger R.P. (2012). Risk factors for mortality in children with abusive head trauma. J. Pediatr. 161, 716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keenan H.T., Runyan D.K., Marshall S.W., Nocera M.A., and Merten D.F. (2004). A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics 114, 633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hymel K.P., Makoroff K.L., Laskey A.L., Conaway M.R., and Blackman J.A. (2007). Mechanisms, clinical presentations, injuries, and outcomes from inflicted versus noninflicted head trauma during infancy: results of a prospective, multicentered, comparative study. Pediatrics 119, 922–929 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein B., Kelly M.M., Bruton D., and Cox C. (1993). Inflicted versus accidental head injury in critically injured children. Crit. Care Med. 21, 1328–1332 [DOI] [PubMed] [Google Scholar]
- 11.Ewing-Cobbs L., Prasad M., Kramer L., and Landry S. (1999). Inflicted traumatic brain injury: relationship of developmental outcome to severity of injury. Pediatr. Neurosurg. 31, 251–258 [DOI] [PubMed] [Google Scholar]
- 12.Newton A.W., and Vandeven A.M. (2005). Update on child maltreatment with a special focus on shaken baby syndrome. Curr. Opin. Pediatr. 17, 246–251 [DOI] [PubMed] [Google Scholar]
- 13.Chadwick O., Rutter M., Brown G., Shaffer D., Traub , and M.U. (1981). A prospective study of children with head injuries: II. Cognitive sequelae. Psychol. Med. 11, 49–61 [DOI] [PubMed] [Google Scholar]
- 14.Jaffe K.M., Fay G.C., Polissar N.L., Martin K.M., Shurtleff H., Rivara J.B., and Winn H.R. (1992). Severity of pediatric traumatic brain injury and early neurobehavioral outcome: a cohort study. Arch. Phys. Med. Rehabil. 73, 540–547 [PubMed] [Google Scholar]
- 15.Thompson N.M., Francis D.J., Steubing K.K., and Fletcher J.M. (1994). Motor, visual spatial, and somatosensory skills after closed head injury in children and adolescents: A study of change. Neuropsychology 8, 333–342 [Google Scholar]
- 16.Bonnier C., Nassogne M.C., and Evrard P. (1995). Outcome and prognosis of whiplash shaken infant syndrome; late consequences after a symptom-free interval. Dev. Med. Child Neurol. 37, 943–956 [DOI] [PubMed] [Google Scholar]
- 17.Fang X., Brown D.S., Florence C.S., and Mercy JA. (2012). The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse Negl. 36, 156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasier L.D. (2008). Abusive head trauma in infants and young children: a unique contributor to developmental disabilities. Pediatr. Clin. North Am. 55, 1269–1285 [DOI] [PubMed] [Google Scholar]
- 19.Geddes J.F., Hackshaw A.K., Vowles G.H., Nickols C.D., and Whitwell H.L. (2001). Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain 124, 1290–1298 [DOI] [PubMed] [Google Scholar]
- 20.Geddes J.F., Vowles G.H., Hackshaw A.K., Nickols C.D., Scott I.S., and Whitwell H.L. (2001). Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain 124, 1299–1306 [DOI] [PubMed] [Google Scholar]
- 21.Ghatan S., and Ellenbogen R.G. (2002). Pediatric spine and spinal cord injury after inflicted trauma. Neurosurg. Clin. N. Am. 13, 227–233 [DOI] [PubMed] [Google Scholar]
- 22.Liley W., Stephens A., Kaltner M., Larkins S., Franklin R.C., Tsey K., Stewart R., and Stewart S. (2012). Infant abusive head trauma—incidence, outcomes and awareness. Aust. Fam. Physician 41, 823–826 [PubMed] [Google Scholar]
- 23.Ashwal S., Wycliffe N.D., and Holshouser B.A. (2010). Advanced neuroimaging in children with nonaccidental trauma. Dev. Neurosci. 32, 343–360 [DOI] [PubMed] [Google Scholar]
- 24.Karandikar S., Coles L., Jayawant S., and Kemp A.M. (2004). The neurodevelopmental outcome in infants who have sustained a subdural haemorrhage from non-accidental head trauma. Child Abuse Review 13, 178–187 [Google Scholar]
- 25.Jaspan T., Griffiths P.D., McConachie N.S., and Punt J.A. (2003). Neuroimaging for non-accidental head injury in childhood: a proposed protocol. Clin. Radiol. 58, 44–53 [DOI] [PubMed] [Google Scholar]
- 26.Stoodley N. (2005) Neuroimaging in non-accidental head injury: if, when, why and how. Clin. Radiol. 60, 22–30 [DOI] [PubMed] [Google Scholar]
- 27.Stoodley N. (2006) Controversies in non-accidental head injury in infants. Br. J. Radiol. 79, 550-–55 [DOI] [PubMed] [Google Scholar]
- 28.Barlow K.M., and Minns R.A. (2000). Annual incidence of shaken impact syndrome in young children. Lancet 356, 1571–1572 [DOI] [PubMed] [Google Scholar]
- 29.Gilles E.E., and Nelson M.D., Jr. (1998). Cerebral complications of nonaccidental head injury in childhood. Pediatr. Neurol. 19, 119–128 [DOI] [PubMed] [Google Scholar]
- 30.Ichord R.N., Naim M., Pollock A.N., Nance M.L., Margulies S.S., and Christian C.W. (2007). Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion-weighted imaging. J. Neurotrauma 24, 106–118 [DOI] [PubMed] [Google Scholar]
- 31.Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., and Shenton M.E. (2012). White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 308, 1859–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger R.P., Adelson P.D., Richichi R., and Kochanek P.M. (2006). Serum biomarkers after traumatic and hypoxemic brain injuries: insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev. Neurosci. 28, 327–335 [DOI] [PubMed] [Google Scholar]
- 33.Duffy S.O., Squires J., Fromkin J.B., and Berger R.P. (2011). Use of skeletal surveys to evaluate for physical abuse: analysis of 703 consecutive skeletal surveys. Pediatrics 127, e47–e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen , The Tich S., Anderson P.J., Shimony J.S., Hunt R.W., Doyle L.W., and Inder T.E. (2009). A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am. J. Neuroradiol. 30, 125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., and Behrens T.E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 36.Paquette L.B., Wisnowski J.L., Ceschin R., Pruetz J.D., Detterich J.A., Del Castillo S., Nagasunder A.C., Kim R., Painter M.J., Gilles F.H., Nelson M.D, Williams R.G., Blüml S., and Panigrahy A. (2013). Abnormal cerebral microstructure in premature neonates with congenital heart disease. AJNR Am J Neuroradiol 34, 2026–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ball G., Counsell S.J., Anjari M., Merchant N., Arichi T., Doria V., Rutherford M.A., Edwards A.D., Rueckert D., and Boardman J.P. (2010). An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage 53, 94–102 [DOI] [PubMed] [Google Scholar]
- 38.Betz J., Zhuo J., Roy A., Shanmuganathan K., and Gullapalli R.P. (2012). Prognostic value of diffusion tensor imaging parameters in severe traumatic brain injury. J. Neurotrauma 29, 1292–1305 [DOI] [PubMed] [Google Scholar]
- 39.Farbota K.D., Bendlin B.B., Alexander A.L., Rowley H.A., Dempsey R.J., and Johnson S.C. (2012). Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front. Hum. Neurosci. 6, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander A.L., Lee J.E., Lazar M., and Field A.S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., and Cross A.H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 7, 1429–1436 [DOI] [PubMed] [Google Scholar]
- 42.Xie M., Tobin J.E., Budde M.D., Chen C.I., Trinkaus K., Cross A.H., McDaniel D.P., Song S.K., and Armstrong R.C. (2010). Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features. J. Neuropathol. Exp. Neurol. 69, 704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Case M.E. (2008). Inflicted traumatic brain injury in infants and young children. Brain Pathol. 18, 571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilde E.A., Chu Z., Bigler E.D., Hunter J.V., Fearing M.A., Hanten G., Newsome M.R., Scheibel R.S., Li X., and Levin H.S. (2006). Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma 23, 1412–1426 [DOI] [PubMed] [Google Scholar]
- 45.Yuan W., Holland S.K., Schmithorst V.J., Walz N.C., Cecil K.M., Jones B.V., Karunanayaka P., Michaud L., and Wade S.L. (2007). Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR Am. J. Neuroradiol. 28, 1919–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wozniak J.R., Krach L., Ward E., Mueller B.A., Muetzel R., Schnoebelen S., Kiragu A., and Lim K.O. (2007). Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 22, 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu Z., Wilde E.A., Hunter J.V., McCauley S.R., Bigler E.D., Troyanskaya M., Yallampalli R., Chia J.M., and Levin H.S. (2010). Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am. J. Neuroradiol. 31, 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu T.C., Wilde E.A., Bigler E.D., Li X., Merkley T.L., Yallampalli R., McCauley S.R., Schnelle K.P., Vasquez A.C., Chu Z., Hanten G., Hunter J.V., and Levin H.S. (2010). Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev. Neurosci. 32, 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levin H.S., Wilde E.A., Chu Z., Yallampalli R., Hanten G.R., Li X., Chia J., Vasquez A.C., and Hunter J.V. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 23, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ewing-Cobbs L., Prasad M.R., Swank P., Kramer L., Cox C.S, Jr., Fletcher J.M., Barnes M., Zhang X., and Hasan K.M. (2008). Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage 42, 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stipanicic A., Nolin P., Fortin G., and Gobeil M.F. (2008). Comparative study of the cognitive sequelae of school-aged victims of Shaken Baby Syndrome. Child Abuse Negl. 32, 415–428 [DOI] [PubMed] [Google Scholar]
- 52.Akpinar E., Koroglu M., and Ptak T. (2007). Diffusion tensor MR imaging in pediatric head trauma. J. Comput. Assist. Tomogr. 31, 657–661 [DOI] [PubMed] [Google Scholar]
- 53.Sidaros A., Engberg A.W., Sidaros K., Liptrot M.G., Herning M., Petersen P., Paulson O.B., Jernigan T.L., and Rostrup E. (2008). Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 131, 559–572 [DOI] [PubMed] [Google Scholar]
- 54.Tasker R.C., Westland A.G., White D.K., and Williams G.B. (2010). Corpus callosum and inferior forebrain white matter microstructure are related to functional outcome from raised intracranial pressure in child traumatic brain injury. Dev. Neurosci. 32, 374–384 [DOI] [PubMed] [Google Scholar]
- 55.Li J., Li X.Y., Feng D.F., and Gu L. (2011). Quantitative evaluation of microscopic injury with diffusion tensor imaging in a rat model of diffuse axonal injury. Eur. J. Neurosci. 33, 933–945 [DOI] [PubMed] [Google Scholar]
- 56.Mac Donald C.L., Dikranian K., Bayly P., Holtzman D., and Brody D. (2007). Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J. Neurosci. 27, 11869–11876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong K.A., Ashwal S., Holshouser B.A., Shutter L.A., Herigault G., Haacke E.M., and Kido D.K. (2003). Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 227, 332–339 [DOI] [PubMed] [Google Scholar]
- 58.Tong K.A., Ashwal S., Holshouser B.A., Nickerson J.P., Wall C.J., Shutter L.A., Osterdock R.J., Haacke E.M., and Kido D. (2004). Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann. Neurol. 56, 36–50 [DOI] [PubMed] [Google Scholar]
- 59.Babikian T., Freier M.C., Tong K.A., Nickerson J.P., Wall C.J., Holshouser B.A., Burley T., Riggs M.L., and Ashwal S. (2005). Susceptibility weighted imaging: neuropsychologic outcome and pediatric head injury. Pediatr. Neurol. 33, 184–194 [DOI] [PubMed] [Google Scholar]
- 60.Ashwal S., Babikian T., Gardner-Nichols J., Freier M.C., Tong K.A., and Holshouser B.A. (2006). Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch. Phys. Med. Rehabil. 87, Suppl 2, S50–S58 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


