Summary
Tetrathiomolybdate (TM) is an oral copper chelator under development as an anti-angiogenic agent. We evaluated TM in combination with irinotecan, 5-fluorouracil, and leucovorin (IFL). Serum vascular endothelial growth factor (VEGF), basic fibroblast growth factor, interleukin 6 (IL-6), and IL-8 were measured to evaluate the anti-angiogenic effect. Twenty-four patients with metastatic colorectal cancer were treated. The combination with IFL was well tolerated and dose intensity of IFL was maintained during combination therapy with TM. By intention to treat analysis, the overall response rate (RR) was 25% (95% CI 9.8–46.7) and the median time to progression (TTP) was 5.6 months (95% CI 2.7–7.7). VEGF levels were correlated with TTP, as were changes in VEGF, IL-8, and IL-6. TM can be safely added to IFL without compromising dose intensity or diminishing the expected RR. Changes in serum VEGF, IL-8, and IL-6 after treatment may directly reflect changes in CRC tissue angiogenesis.
Keywords: Colorectal cancer, Anti-angiogenesis, Tetrathiomolybdate, Copper chelation, Vascular endothelial growth factor
Introduction
Tetrathiomolybdate (TM) is a non-cytotoxic, oral copper chelator, originally developed for use in Wilson’s Disease [1, 2]. TM complexes with serum copper, allowing clearance by the liver and excretion in bile, with a small amount eliminated in the urine. TM administered with food chelates dietary copper, preventing absorption from the gastrointestinal tract. Because copper is a necessary cofactor for many pro-angiogenic proteins, a strategy of copper depletion with TM to impair angiogenesis is under investigation [3-7]. TM has been shown in vitro and in vivo to decrease the activity of nuclear factor-κB (NF-κB), leading to lower levels of the pro-angiogenic cytokines, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin (IL-) 6, and IL-8 [6, 8]. Additionally, TM appears to directly inhibit the activity of NF-κB in vitro [6]. These cytokines are known to be present at high levels in patients with colorectal cancer (CRC) and higher levels of VEGF have been associated with poor prognosis [9-13]. Similarly, elevated tumor tissue vessel density, a direct measure of angiogenic activity, has been associated with a poor prognosis [14-16].
A phase I study of TM in patients with advanced cancer demonstrated the ability to produce copper deficiency in a majority of patients following 6–8 weeks of treatment [17]. Serum ceruloplasmin (Cp) level was used as a surrogate measure of total body copper, in lieu of serum free copper levels, because the copper-TM complex is detectable in serum measurements of copper, but is not bioavailable. The only significant toxicity associated with TM in this phase I study was anemia, defined as hematocrit <80% of baseline, occurring in approximately one-third of patients. Rapidly reversible neutropenia without infection was also observed. Anemia was deemed to be directly related to the degree of copper deficiency, rather than to the dose of TM, and the anemia resolved without need for transfusion within 5–7 days of TM discontinuation in all patients. Another treatment related toxicity in this phase I trial was sulfurous belching.
Primary treatment of patients with metastatic colorectal cancer currently consists of combination chemotherapy with an anti-angiogenic agent [18, 19]. At the time of development of this study, the irinotecan/5-fluorouracil/ leucovorin (IFL) combination was the standard first-line therapy for metastatic CRC [20]. While treatment using a relatively non-toxic anti-angiogenic agent, such as TM, alone is an attractive concept, initial clinical experience suggested that, as a class, anti-angiogenic agents are much more effective in combination with chemotherapy. In addition, TM requires several months of continuous use to deplete copper stores before therapeutic activity might be expected. Therefore, we combined TM with the standard IFL regimen in a pilot study for patients with metastatic CRC, the results of which are reported here. Objectives for this study included determination of the tolerability and toxicity of TM in combination with IFL, the success rate of TM leading to target levels of copper depletion in patients treated concurrently with chemotherapy, and evaluation of the time to disease progression (TTP) for these patients. Secondary objectives included measurement of the serum levels of the pro-angiogenic cytokines, VEGF, bFGF, IL-6, and IL-8, and the effect of TM and chemotherapy treatment on these cytokines.
Patients and methods
Eligibility criteria
Patients were required to have histologically proven adenocarcinoma primary to the colon or rectum with clinical and/or pathologic evidence of distant metastasis. Patients were permitted to have received adjuvant chemotherapy and up to one prior treatment for metastatic disease, but not previous treatment with IFL. Patients had to be at least 18 years of age at the time of enrollment, have a performance status (PS) of 0–2 and a life expectancy of at least 3 months. All patients were required to have adequate organ function, as defined by leukocyte count ≥3,000/μl, absolute neutrophil count ≥1,500/μl, hemoglobin ≥8 mg/dl, platelet count ≥150,000, serum creatinine ≤2.0 mg/dl, and serum bilirubin ≤1.1 mg/dl within 2 weeks of enrollment. Patients with active infection, concurrent serious medical or psychiatric conditions, a history of other active malignancy within 12 months of enrollment, and pregnant or lactating women were excluded from the study. Patients were not allowed to receive any other anti-neoplastic therapy while participating on this study. This study was approved by the University of Michigan General Clinical Research Center and Institutional Review Board. All patients provided written informed consent prior to treatment.
Study design
Prior to treatment, patients had a complete history and physical examination (PE), including assessment of PS, and baseline laboratory evaluation including carcinoembryonic antigen (CEA), Cp level, complete blood count with differential and platelet count (CBC), and serum chemistry panel with electrolytes, creatinine, and aspartate aminotransferase. Tumor measurements with appropriate radiographic studies were obtained. Serum levels of VEGF, bFGF, IL-6, and IL-8 were drawn at baseline.
Patients received standard doses of IFL (irinotecan 125 mg/m2, 5-FU 500 mg/m2, and leucovorin 20 mg/m2) on days 1, 8, 15, and 22 of a 6 week cycle [20]. Irinotecan and 5-FU doses were reduced 20% for grade 2 neutropenia, thrombocytopenia, diarrhea, or any other grade 2 non-hematologic toxicity. Treatment was held for grade 3/4 toxicity until recovery to grade 1, and resumed at a 20% dose reduction for both chemotherapeutic agents.
TM was initiated on day 1 of cycle 1 at a dose of 40 mg three times daily with meals and 60 mg before bed without food. Patients were instructed not to take vitamins or nutritional supplements containing copper. The TM dose was decreased to 20 mg twice daily with meals and 40 mg before bed without food when the target Cp level of 5–15 mg/dl was reached. Further dose adjustments were made as needed to maintain the Cp level at 5–15 mg/dl. With any hematocrit <80% of baseline and a Cp level at 5–15 mg/dl, TM was held for a period of 5 days, at which time the hematocrit was repeated. If the anemia was not improved, TM was deemed unlikely to have caused the anemia and it was restarted at the prior dose. If the anemia corrected, TM was felt likely to be the cause and it was resumed at a dose to achieve a new target Cp level of 10–15 mg/dl. For complicated grade 3/4 neutropenia or uncomplicated grade 3/4 neutropenia lasting at least 7 days, TM was held until recovery to grade 2 neutropenia. TM was also held as described above for grade 3/4 non-hematologic toxicity and resumed without dose reduction upon recovery from that toxicity.
During cycle 1, patients were monitored weekly with interval history, physical examination, and toxicity assessment and then biweekly for the duration of IFL treatment. Following discontinuation of IFL and during maintenance therapy with TM, history, PE, and PS were performed monthly. Cp levels were obtained weekly for the first 8 weeks of treatment and then biweekly, unless a dose adjustment of TM was required; then, the Cp levels were checked weekly until the target Cp level was again reached. A CBC was obtained weekly during IFL, then biweekly. Serum chemistries were monitored monthly. Patient appropriate radiographic studies and CEA levels for disease assessment were obtained every 10–12 weeks. Serum levels of VEGF, bFGF, IL-6, and IL-8 were repeated at the time of first copper deficiency (Cp 5–15 mg/dl) and then 3 months later or with progressive disease, whichever occurred first.
Patients received IFL for up to 6 months with stable disease and, for patients with partial response, for two cycles beyond the best response. TM was continued until disease progression. Patients were removed from treatment for documented progressive disease per RECIST Criteria, unacceptable toxicity, defined as an inability to receive at least 50% of the intended dose of IFL during the first 3 months of treatment, or patient choice. Patients were also removed from the study if they did not reach the target Cp level within 3 months of beginning treatment with TM.
Assays for VEGF, bFGF, IL-6, and IL-8
Blood from patients was collected in serum separator tubes and was allowed to clot for 30 min before centrifugation at 1,000×g for 10 min. Serum was immediately frozen (−70°C) in aliquots of 0.75 ml in microcentrifuge tubes. Human VEGF and human bFGF ELISAs were performed as directed by the manufacturer (R & D Systems, Minneapolis, MN). Briefly, serum (100 μl) was pipetted in triplicate into wells pre-coated for a monoclonal antibody specific for each factor and incubated for 2 h. After three washes to remove unbound substances, an enzyme-linked monoclonal antibody specific for each factor was added to the wells and incubated for 2 h. After a wash to remove unbound antibody-enzyme reagent, a substrate solution was added into the wells and allowed to incubate for 30 min. Optical intensity of each well was measured using a microplate reader. ELISAs for IL-6 and IL-8 were performed by the University of Maryland Cytokine Core Laboratory (Baltimore, MD).
Statistical methods
The endpoints of interest in the study were as follows: the tolerability and toxicity of TM in combination with IFL, the success rate of TM as measured by the ability of patients to reach their target Cp level and the time they spent within the target Cp window, and TTP. Secondary endpoints included survival time (OS), and serum levels of pro-angiogenic cytokines. All time-to-event endpoints were calculated from the date the patient began treatment on this clinical trial. All patients experienced disease progression and death due to disease during follow-up. The product-limit method of Kaplan and Meier was used to estimate the time-to-event probabilities. Patients not reaching the target Cp level were censored on the last date of TM administration for the estimate of the time to reach the target Cp level. The protocol called for measurements of serum angiogenic factors (VEGF, bFGF, IL-8, and IL-6) at baseline (before study treatment), when the patient reached target Cp level, and after 3 months of copper depletion or at the time of disease progression. As the effect of TM is thought to stabilize metastatic disease progression, the association between the pro-angiogenic cytokines and the time to disease progression was explored using the Spearman rank correlation coefficient. Changes in the pro-angiogenic cytokines were calculated between the baseline measurement, the measurement upon first reaching the target Cp level, and after 3 months of copper depletion.
Results
Twenty-four eligible patients were registered to the study between August 2001 and October 2003. Patient characteristics are shown in Table 1. The median age was approximately 57 years and 75% of the patients enrolled were male. The majority of patients had liver metastases and, in seven patients, the liver was the only site of disease. Eight patients had previously received standard adjuvant chemotherapy regimens with 5-FU and leucovorin. Six patients had previously received chemotherapy for metastatic disease: Five patients were treated with oxaliplatin and capecitabine on a clinical trial and one patient had received irinotecan with regional FUDR.
Table 1.
Patient characteristics
| Age, median (range) | 56.5 (34–73) |
| Months since metastatic diagnosis, median (range) | 2 (1–23) |
| Gender, N (%) | |
| Male | 18 (75.0) |
| Female | 6 (25.0) |
| Race, N (%) | |
| African American | 1 (4.2) |
| Caucasian | 23 (95.8) |
| Performance status, N (%) | |
| 0 | 5 (20.8) |
| 1 | 19 (79.2) |
| Primary site, N (%) | |
| Colon | 16 (66.6) |
| Rectum | 8 (33.3) |
| Sites of metastasis, N (%) | |
| Liver | 18 (75.0) |
| Lung | 8 (33.3) |
| Peritoneal | 11 (45.8) |
| Other | 10 (41.6) |
| Number of sites of metastasis, N (%) | |
| 1 | 11 (45.8) |
| 2–3 | 12 (50.0) |
| >3 | 1 (4.2) |
| Previous chemotherapy, N (%) | |
| Yes | 10 (41.7) |
| Adjuvant | 8 (33.3) |
| Metastatic | 6 (25.0) |
| None | 14 (58.3) |
| Surgery, N (%) | |
| Curative intent | 9 (37.5) |
| Palliative | 12 (50.0) |
| None | 3 (12.5) |
| Radiotherapy, N (%) | |
| Yes | 4 (16.7) |
| No | 20 (83.3) |
The median baseline Cp level was 29.7 mg/dl. Table 2 shows the effect of TM on serum Cp. Twenty-two of 24 patients (91.7%) achieved target levels of Cp within 3 months of initiation of study treatment, with a majority of patients reaching target Cp levels within 1 month. One patient was removed from the study at day 46 due to progressive disease and had not yet achieved copper deficiency. A second patient never achieved copper deficiency during 3 months on study and was withdrawn for that reason. The median percent time spent within the target Cp range for all patients during study treatment was 66.7% (range 0–92.3%). Patients were maintained in the target Cp range with divided doses of TM 40–180 mg/day. Most patients received TM 80–140 mg daily for the duration of their treatment. Overall, 14 of 24 patients reaching the target Cp required 47 dose adjustments to keep the serum Cp level within target range. The remaining patients did not require dose adjustments. The median average Cp level during TM treatment was 13.7 mg/dl. (range 8.7–19.6 mg/dl). There were ten incidences of TM levels falling below the target range among three patients. One patient experienced grade 3/4 neutropenia which was temporally associated with two of these incidences, but there were no other significant toxicities noted.
Table 2.
Effect of TM on serum Cp
| Received TM, N (%) | |
| Yes | 24 (100) |
| Achieved target Cpa | 22 (91.7) |
| Time to target Cpa, days | |
| Median | 28 |
| 95% CI | 21–35 |
| Time in target Cpa, days | |
| Median | 77 |
| Range | 0–182 |
| % time in target Cpa | |
| Median | 66.7% |
| Range | 0–92.3% |
5–15 mg/dl
CI confidence interval
All patients were evaluable for toxicity. There were no treatment related deaths. One patient withdrew from study in the third month of treatment secondary to persistent anorexia, nausea, and emesis, despite maximal medical management. Ten of the 24 patients required dose reductions after a first cycle of treatment due to grade 3/4 toxicity (9 neutropenia, 1 diarrhea). Four patients required an additional dose reduction for grade 3/4 toxicity after a second cycle of treatment (three neutropenia, one diarrhea). The median dose intensity received during the first 12 weeks of study treatment for 5-FU was 81.2% (range 46–100%) and for irinotecan was 81.3% (range 45–100%). One patient developed a deep venous thrombosis, which occurred during the first cycle of therapy. Anemia was generally mild (hematocrit 25.9–29.5) and occurred in six patients during the course of treatment. The anemia was felt to be related to TM in three patients, since they improved with adjustments to the TM dosing. The anemia in the other three patients was most likely related to the IFL and/or CRC. One of the six patients had a decrease in hematocrit to 18.9 during the second cycle of therapy, which was associated with a grade 4 neutropenia. Both toxicities resolved without complication after holding treatment for 3 weeks and dose reductions of both the TM and IFL. In general, the combination of IFL and TM was well tolerated. A summary of the toxicities experienced during treatment is provided in Table 3.
Table 3.
Graded toxicities per patient (all cycles)
| Grade 1/2 (95% CI) | Grade 3/4 (95% CI) | |
|---|---|---|
| Hematologic | ||
| Neutropenia | 12.5% (2.7–32.4) | 45.8%a (25.6–67.2) |
| Thrombocytopenia | 0 | 0 |
| Anemia | 25.0% (9.8–46.7) | 0 |
| Non-hematologic | ||
| Diarrhea | 16.7% (4.7–37.4) | 8.3% (1.0–27.0) |
| Nausea/vomiting | 4.2% (0.01–21.1) | 0 |
| Other | 20.8% (7.1–42.2) | 4.2% (0.01–21.1) |
CI confidence interval
Toxicities occurred during cycle 1–2 only
The best response achieved and the TTP for all patients is shown in Table 4. Using intention to treat analysis, the overall response rate (RR) was 25% (95% CI 9.8–46.7). All partial responses were observed in those patients without previous treatment for metastatic disease (RR 33%, 95% CI 13.3–59.0). Ten patients had at least a 50% reduction in baseline CEA level during study treatment. Six patients with stable disease and partial response discontinued chemotherapy following at least 6 months of IFL and continued TM alone, as specified in the study protocol. Each of these patients subsequently progressed on TM with a median TTP measured from the time of discontinuation of IFL of 2.4 months (range 1.8–4.7 months). All patients have died by the time of this analysis.
Table 4.
Response and survival (intention to treat)
| Best response, N (%) [95% CI] | |
| Complete | 0 |
| Partial | 6 (25) [9.8–46.7] |
| Stable disease | 9 (37.5) [18.8–59.4] |
| Median TTP, months (95% CI) | |
| All patients | 5.6 (2.7–7.7) |
| No prior metastatic treatment | 5.7 (3.7–8.3) |
| Median OS, months (95% CI) | 13.2 (9.8–18.8) |
CI confidence interval
The Spearman Rank Correlation Coefficients between serum cytokine levels and TTP are shown in Table 5. There was no significant correlation between baseline serum cytokine levels and TTP for any of the cytokines measured; however, the VEGF levels at the time of target Cp and at 3 months were moderately and significantly correlated with worsened TTP, indicating that higher levels of VEGF at these time points had a negative impact on TTP. When the change in each serum cytokine level between baseline and 3 months (for 12 patients still receiving treatment at 3 months) was considered, there was a strong, significant correlation between the degree of change and TTP for VEGF, IL-8, and IL-6. Greater decreases in these cytokine levels were associated with longer TTP. Figure 1 shows a representative scatter plot of the correlation between the relative changes in VEGF with TTP after 3 months of treatment.
Table 5.
Association between serum cytokine level and TTP
| Measurement | Spearman rank correlation coefficients with TTP
|
|||
|---|---|---|---|---|
| VEGF | bFGF | IL-6 | IL-8 | |
| Baseline | 0.064 | −0.054 | −0.326 | 0.300 |
| Time of target Cp | −0.470* | −0.230 | −0.419 | 0.026 |
| 3 months | −0.665* | 0.0270 | 0.490 | −0.427 |
| Relative change | ||||
| Baseline–time of target Cp | −0.407 | 0.007 | −0.172 | −0.220 |
| Baseline–3 months | −0.741* | 0.125 | −0.811* | −0.685* |
P<0.05
Fig. 1.
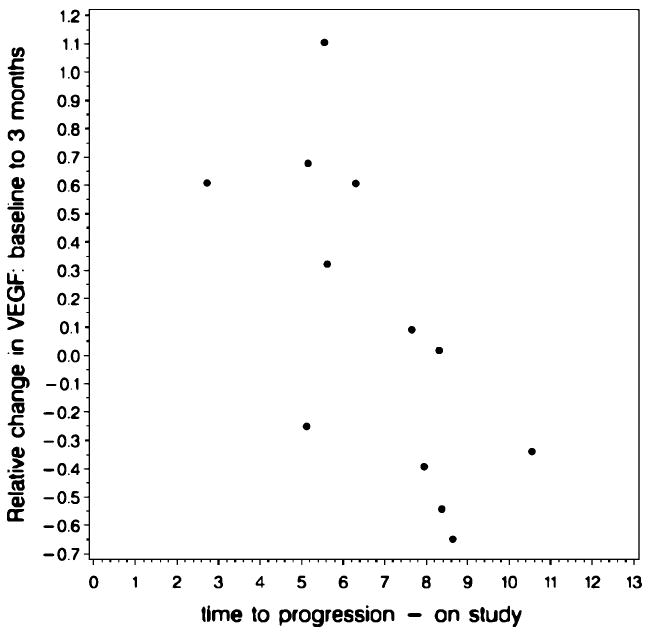
Representative scatter plot of the correlation between the relative changes in VEGF with TTP after 3 months of treatment
Discussion
TM as an anti-angiogenic therapy has previously been used as a single agent in phase I and phase II trials involving patients with advanced malignancy. This study demonstrated that TM can be safely added to combination chemotherapy for the treatment of advanced CRC. All but one patient in this pilot trial were able to reach copper deficiency as measured by serum Cp. The median time to target Cp level was 4 weeks (28 days) in this study, compared to 5 weeks in a phase II trial using TM alone in patients with renal cell carcinoma [7]. No patient had difficulty maintaining copper deficiency despite ongoing treatment, although 14 of 22 patients required at least one dose adjustment to the TM during that time.
TM was well tolerated and had no apparent impact on toxicity patterns observed with the IFL chemotherapy. Our patients experienced grade 3/4 diarrhea at a rate of 8.3% (95% CI 1.0–27.0%) and grade 3/4 neutropenia at a rate of 45.8% (95% CI 25.6–67.2%), compared with 22.7% and 53.8%, respectively, as reported by Saltz, et al. [20]. The median dose intensity of IFL during the first 12 weeks of treatment was >80% for both agents and similar to that previously reported. The most serious side effect attributable to TM has been anemia, which occurred in six patients in this trial at some time during the course of therapy. The anemia was felt to be directly related to TM in three of these cases and resolved after holding TM for approximately 1 week. For all six patients, the anemia was generally mild. One patient had deep venous thrombosis develop prior to copper deficiency, but there were no other serious adverse events attributable to VEGF inhibition, as has been seen with bevacizumab.
Preclinical studies have shown the importance of copper as a cofactor for NF-κB, which regulates the pro-angiogenic cytokines, VEGF, bFGF, IL-6, and IL-8 [6, 8]. Although serum cytokine levels are not currently accepted as surrogate markers for angiogenesis, we evaluated the impact of treatment with TM and chemotherapy on cytokine levels for exploratory purposes. Baseline levels of VEGF, bFGF, IL-6, and IL-8 had no correlation with TTP, however lower VEGF levels at the time of copper deficiency and at 3 months following copper deficiency were significantly correlated with longer TTP. Except for the lack of correlation between baseline VEGF levels and TTP, this result is similar to prior studies and is likely due to TM and chemotherapy having lowered tumor VEGF levels. Since the TM was given with chemotherapy when VEGF levels were obtained, it is impossible to determine the effect of TM alone. The correlation between levels of VEGF following 3 months of copper deficiency with TTP was stronger than the correlation with TTP at the time of copper deficiency (−0.665 and −0.470, respectively). Although given the small number of patients and exploratory nature of this analysis, these results may be artefactual, they may also indicate that maintaining lower VEGF levels throughout treatment is an important aspect of CRC treatment, a conclusion that is supported by other studies showing that TTP is related to VEGF levels at baseline and during treatment [21]. Additionally, there was a correlation between the changes in VEGF, IL-6, and IL- 8 levels after 3 months of therapy with TTP.
Since there was no correlation between baseline levels of any of the pro-angiogenic cytokines and TTP, serum cytokine levels are probably not reflective of the actual levels within the tumor microenvironment. However, TM is not expected to have an immediate effect on total body copper or angiogenesis and we attempted to control tumor progression with chemotherapy while TM treatment was leading to copper deficiency. We suggest that serum copper depletion occurs weeks before copper depletion in tissues and that changes in pro-angiogenic cytokine levels measured earlier in the course of TM treatment are less likely to correlate with levels within in the tumor tissue; however, VEGF, at least, is a marker for the effect of treatment on angiogenesis associated with the vascular system. We expect that a similar effect on angiogenesis would be seen within tumor tissue upon copper depletion within the tumor; therefore, we hypothesize that pro-angiogenic cytokine levels measured later in the course of treatment are more reflective of, if not directly related to, the degree of angiogenesis within the tumor tissue. This is also supported by our finding that there is a stronger correlation between lower VEGF levels after 3 months of copper deficiency than there is a the time of copper deficiency.
We believe that TM, as has been observed with other anti-angiogenic agents, would primarily act to prevent further tumor growth and metastasis, not reduce tumor size. The efficacy of this study treatment is difficult to gauge in a small pilot trial. It did not appear that TM and copper deficiency interfered with the response to chemotherapy. While the overall RR of 25% in this trial was lower than expected with IFL, in those patients previously untreated, a RR of 33% was observed with confidence intervals overlapping the observed response rate of IFL in larger trials. We did not expect the RR to be improved by TM. We hypothesized that the TTP might be improved with TM in this study cohort, compared to patients treated with IFL alone. The median TTP for all patients from the time of enrollment was 5.6 months (95% CI 2.7–7.7 months) for our patients, compared to a median progression free survival of 7.0 months using IFL only, as reported by Saltz, et al. [20]. The relatively low TTP in our study may be explained by the fact that some of our patients were previously treated. Furthermore, seven of the 24 patients discontinued the study treatment by the end of the first 3 months, which was the time at which tumor copper deficiency was expected. It is likely that these patients were progressing prior to copper deficiency and, therefore, the impact of TM could not truly be assessed. Finally, it’s possible that some patients in this study were treated with a suboptimal dose of TM, which would be expected to impact the results, especially TTP. This should be considered, since the most clinically efficacious dose of TM is unknown. The phase I trial of TM did not establish a maximum tolerated dose and changes in levels of the proangiogenic cytokines were not evaluated to see whether there was a correlation with dosages. In particular, patients who developed anemia were intentionally maintained at higher Cp levels than those patients who did not have anemia, which might have impacted the ability of TM to inhibit angiogenesis. Additionally, the relatively low rate of anemia among our patients might indicate that the target Cp level was too high and patients were not sufficiently copper deficient for anti-angiogenic effects to be present.
We attempted to determine whether continued copper depletion through TM treatment alone, in patients with stable or responsive disease, could prevent subsequent disease progression following 6 months of IFL and TM treatment. Only six patients had not progressed by 6 months and were continued on TM alone. Tumor progression was seen in each of these patients within the next 5 months. In future studies with TM and chemotherapy, we would suggest continuing both treatments in an attempt to control tumor progression.
In summary, the combination of TM and chemotherapy at the doses used in this study was well tolerated by patients with advanced CRC. Copper depletion was reliably achieved and maintained based on serial serum Cp levels. Infusional schedules of 5-FU have more recently been demonstrated to increase efficacy of chemotherapy in CRC. With its low toxicity profile, TM combined with FOLFOX or FOLFIRI, with or without additional targeted agents, is suggested for further study.
Acknowledgments
Dr. Sofia Merajver received partial funding for this research from grants CA77612, the Burroughs Wellcome Fund, the Breast Cancer Research Foundation, and Tempting Tables. The conduction of the clinical trial was supported by a National Cancer Institute General Cancer Research Center Grant to the University of Michigan Cancer Center.
Contributor Information
Elaina M. Gartner, Wayne State University, 4100 John R, 4HWCRC, Detroit, MI 48108, USA gartnere@karmanos.org
Kent A. Griffith, The University of Michigan, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA
Quintin Pan, The University of Michigan, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA.
George J. Brewer, The University of Michigan, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA
Gwen F. Henja, The University of Michigan, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA
Sofia D. Merajver, The University of Michigan, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA
Mark M. Zalupski, The University of Michigan, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA
References
- 1.Brewer GJ, Hedera P, Kluin KJ, Carlson MD, Askari F, Dick RB, et al. Treatment of Wilson’s disease with tetrathiomolybdate III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy. Arch Neurol. 2003;60:378–385. doi: 10.1001/archneur.60.3.379. [DOI] [PubMed] [Google Scholar]
- 2.Brewer GJ, Askari F, Lorincz MT, Carlson MD, Schilsky M, Kluin KJ, et al. Treatment of Wilson’s disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double blind study of treatment of the neurologic presentation of Wilson’s disease. Arch Neurol. 2006;63:521–527. doi: 10.1001/archneur.63.4.521. [DOI] [PubMed] [Google Scholar]
- 3.Cox C, Teknos TN, Barrios M, Brewer GJ, Dick RD, Merajver SD. The role of copper suppression as an antiangiogenic strategy in head and neck squamous cell carcinoma. Laryngoscope. 2002;111:696–701. doi: 10.1097/00005537-200104000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Goodman VL, Brewer GJ, Merajver SD. Copper deficiency as an anti-cancer strategy. Endocr Relat Cancer. 2004;11:255–263. doi: 10.1677/erc.0.0110255. [DOI] [PubMed] [Google Scholar]
- 5.Pan Q, Bao LW, Kleer CG, Brewer GJ, Merajver SD. Antiangiogenic tetrathiomolybdate enhances the efficacy of doxorubicin against breast carcinoma. Mol Cancer Ther. 2003;2:617–622. [PubMed] [Google Scholar]
- 6.Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 7.Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 8.Pan Q, Bao LW, Merajver SD. Tetrathiomolybdate inhibits angiogenesis and metastasis through suppression of the NFκB signaling cascade. Mol Cancer Res. 2003;1:701–706. [PubMed] [Google Scholar]
- 9.Ellis LM, Takahashi Y, Liu W, Shaheen RM. Vascular endothelial growth factor in human colon cancer: biology and therapeutic implications. Oncologist. 2000;5(suppl 1):11–15. doi: 10.1634/theoncologist.5-suppl_1-11. [DOI] [PubMed] [Google Scholar]
- 10.Broll R, Erdmann H, Duchrow M, Oevermann E, Schwandner O, Markert U, et al. Vascular endothelial growth factor (VEGF)—a valuable serum tumour marker in patients with colorectal cancer? Eur J Surg Oncol. 2001;27:37–42. doi: 10.1053/ejso.2000.1052. [DOI] [PubMed] [Google Scholar]
- 11.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 12.George ML, Dzik-Jurasz A, Padhani AR, Brown G, Tait DM, Eccles SA, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg. 2001;88:1628–1636. doi: 10.1046/j.0007-1323.2001.01947.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PAE, Robinson BA, et al. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200:183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- 14.Saclarides TJ, Speziale NJ, Drab E, Szeluga DJ, Rubin DB. Tumor angiogenesis and rectal carcinoma. Dis Colon Rectum. 1994;37:921–926. doi: 10.1007/BF02052599. [DOI] [PubMed] [Google Scholar]
- 15.Tanigawa N, Amaya H, Matsumura M, Lu C, Kitaoka A, Matsuyama K, et al. Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res. 1997;57:1043–1046. [PubMed] [Google Scholar]
- 16.Rajaganeshan R, Prasad R, Guillou PJ, Chalmers CR, Scott N, Sarkar R, et al. The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br J Cancer. 2007;96:1112–1117. doi: 10.1038/sj.bjc.6603677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer GJ, Dick RD, Grover DK, LeClaire B, Tseng M, Wicha M, et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: phase I study. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 18.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 20.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 21.Lissoni P, Rovelli F, Malugani F, Brivio F, Fumagalli L, Gardani GS. Changes in circulating VEGF levels in relation to clinical response during chemotherapy for metastatic cancer. Int J Biol Markers. 2003;18:152–155. doi: 10.1177/172460080301800209. [DOI] [PubMed] [Google Scholar]


