Abstract
Objective
Assessment of the subjective and physiological effects of smoking cigarettes with different machine-smoked nicotine yields.
Methods
Eight volunteers rated the characteristics of cigarettes with varying levels of nicotine (Quest®). At 30 minute intervals, participants smoked one of three different Quest® brand cigarettes in a counterbalanced order (reported machine-smoked nicotine yield: 0.6 mg, 0.3 mg, or 0.05 mg). Smoking satisfaction and sensations were measured on a cigarette evaluation questionnaire. A mood questionnaire measured self-reported subjective changes in ‘happy’, ‘stimulated’, ‘anxious’, ‘desire to smoke’, and ‘desire not to smoke’. Heart rate and skin temperature were recorded continuously.
Results
As nicotine yield decreased, cigarettes produced smaller changes in subjective ratings on the evaluation questionnaire with the placebo nicotine cigarette always rated lower or less potent than the other two cigarettes evaluated. Heart rate was significantly increased by the reduced nicotine cigarettes, but was not affected by the nicotine-free cigarette.
Conclusion
These results indicate that machine-smoked yield is an important determinant of both the subjective and physiological effects of smoking. The use of reduced and nicotine free cigarettes in smoking cessation programs remains to be evaluated.
Keywords: nicotine, smoking, subjective sensations, physiological
Introduction
Nicotine is the major psychoactive constituent of tobacco and is generally accepted to be the primary reason for the addictive properties of cigarettes.1 As a drug of abuse, nicotine has many pharmacologic effects on the body and in the central nervous system that are important for understanding its addictive properties. Cigarette smoking, however, delivers not only nicotine but a set of intense sensations that have been shown to be an important part of the maintenance of smoking behavior.2–4 These sensations are a function of a complex interaction among the many components of smoke and tobacco, including tar, acetaldehyde, ammonia, flavorings such as menthol, and other factors such as pH.
Studies have been conducted to explore the contributions of nicotine yield vs. smoke sensations in smoking. Rose et al.5, for example, used a lidocaine rinse and spray mist to anesthetize the mouth and upper respiratory airway to reduce these sensations. Later work6 developed a bypass tubing and filtering system, and a regenerated smoke aerosol device that varied delivered nicotine and varied smoke sensations. Butschky et al.7 employed a 5 cigarette holder device and studied various mixes of regular cigarettes, denicotinized cigarettes, and non-tobacco (lettuce leaf) cigarettes. These studies and others8 showed that withdrawal effects and subjective cravings are significantly reduced by the robust sensory effects of inhaled smoke itself. Other subjective experiences such as cigarette liking and satisfaction were more related to nicotine yield than the type of smoke presented.
The ability to manipulate nicotine levels while keeping the sensory characteristics of smoking constant has been constrained to a certain extent by the lack of the same or very similar cigarettes that vary nicotine levels. Research attempting to delineate the relative contributions of nicotine versus the sensory characteristics of inhaled cigarette smoke often use different brands of cigarettes to vary nicotine levels.9 Different brand cigarettes have perceptibly different smell and taste qualities that are readily discernable to smokers, and smokers have distinct individual preferences for a particular brand or type of cigarette. These factors may complicate the parsing of nicotine vs. cigarette smoke characteristics in the analysis.
Recently, a brand of cigarettes (Quest®) was available from one manufacturer that varies nicotine levels while keeping levels of tar constant. The present experiment was conducted to assess the physiological and subjective effects of smoking these cigarettes in an attempt to further understand the contribution of nicotine vs. the sensations of smoke itself to the smoking experience. An understanding of the relative contributions of these two aspects of smoking may be useful in developing better quit smoking treatment programs.
Methods
Participants
Adult smokers were recruited via newspaper advertisements and internet postings for research subjects. Responders to the advertisements were given a description of the study and an initial screening over the telephone to see if they met the basic inclusion and exclusion criteria. Those passing the initial screen and expressing further interest were invited to the laboratory for a more in-depth assessment. Nine (9) adults were invited. They provided written informed consent and were screened according to the following criteria. Participants had to be healthy as determined by physical examination including ECG, blood chemistry analysis, urinalysis, and psychiatric screening according to DSM-IV criteria. Eight (5 women, 3 men) qualified. They ranged in age from 21–34 years with an average age of 24.9 ± 4.2 years, and smoked an average of 19.4 ± 8.4 cigarettes per day (range: 12–26). They reported an average Fagerstrom Tolerance Scale10 score of 4.9 ± 1.6. Their usual brands of cigarettes were well-known, commercially available cigarettes with an average nicotine yield of 0.92 ± 0.16 mg nicotine (range: 0.8–1.2) according to FTC 2000 test results. Average tar content of their usual brands was 12.25 ± 2.43 mg. Seven subjects smoked cigarettes formerly considered ‘light’ by FTC standards (7–15 mg tar) and one smoked a regular brand (> 15 mg tar). One participant smoked menthol cigarettes, while the remainder smoked non-menthol brands. Three participants reported current occasional marihuana use (once a month to once a week), two reported current cocaine use (1–2 times per month), and three reported no current occasional other drug use. No participant met DSM-IV criteria for abuse or dependence on any drug other than nicotine. All participants were moderate users of caffeine, reporting regular consumption of less than 300 mg of caffeine per day. The protocol and informed consent were approved by the McLean Hospital Institutional Review Board. All research procedures were conducted according to local, state, and federal regulations governing human research. Individuals were paid for their participation.
Materials
Subjective effects were measured using a 17-item cigarette evaluation questionnaire11 (Table 1). Additional effects were measured using a 5-item mood questionnaire. The mood questionnaire included the following items rated on a 10-point Likert-type scale from ‘not at all’ to ‘extremely’: How happy do you feel right now?, How stimulated do you feel right now?, How anxious do you feel right now?, How strong is your desire to smoke a cigarette right now?, How strong is your desire not to smoke a cigarette right now?
Table 1.
Cigarette evaluation questionnaire (after Rose et al, 1999).
| Satisfaction |
| 1. Was it satisfying? |
| 2. Did it taste good? |
| Similarity to usual brand |
| 3. How similar was it to your own brand? |
| Psychological reward |
| 4. Did it calm you down? |
| 5. Did it help you concentrate? |
| 6. Did it make you feel more awake? |
| 7. Did it reduce your hunger for food? |
| 8. Did it make you feel less irritable? |
| Aversion |
| 9. Did it make you nauseous? |
| 10. Did it make you dizzy? |
| Respiratory sensations |
| 11. Did you enjoy the sensations of the smoke in your throat and chest? |
| Craving reduction |
| 12. Did it immediately reduce your craving for cigarettes? |
| Perceived strength |
13. How strong was this cigarette on:
|
Notes: Smokers rated each cigarette smoked on a scale from 0—’Not at all’ to 7—’Extremely’ for each question. The 17-item questionnaire presented only the questions to the participants. The headings are presented here to show how this questionnaire assesses various aspects of cigarette smoking.
Continuous recordings of heart rate (beats per minute or bpm) and skin temperature (°C) were made with a Mini Logger Series 2000 device (Mini Mitter, Bend, OR). Heart rate was recorded via a Polar™ chest belt sensor attached to the left side of the participant’s chest below the heart using Cleartrace adult ECG electrodes (ConMed Corp, Utica, NY). Skin temperature was recorded using single-use temperature probes (Steri-Probe, Cincinnati Sub-Zero Products, Cincinnati, OH) attached to the stomach area just below the lowest right rib and connected to a port in the logger. Urine was tested for drugs of abuse using Triage® urine screen kits (Biosite Diagnostics, San Diego, CA). Breath alcohol content was measured using an AlcoSensor (Intoximeter, Saint Louis, MO) and expired carbon monoxide was measured using a Vitalograph BreathCO (Vitalograph, Lenexa, KS).
Commercially purchased Quest® cigarettes (Vector Tobacco, Inc.) with three levels of nicotine were used: ‘low nicotine’ #1 (yield of 0.6 mg nicotine, 10 mg tar), ‘extra-low nicotine’ #2 (yield of 0.3 mg nicotine, 10 mg tar), and denicotinized ‘nicotine-free’ #3 (yield of ≤0.05 nicotine, 10 mg tar).
Procedure
Participants were required to abstain from alcohol use from 10 pm the night before the experimental session and all other drug use except nicotine and caffeine for 72 hours before the experimental session. On the day of the study, breath alcohol levels (BAL) had to be below 0.002% for participation and urine samples negative for drugs of abuse. Participants were required to abstain from smoking cigarettes for at least 2 hours before coming to the laboratory. They reported the time of their last cigarette when they arrived and breath carbon monoxide levels were taken. Levels were not greater than 20 ppm for all participants upon arrival (average 9.9 ± 6.4 ppm, range 3–20). Participants maintained their usual caffeine intake on the days leading to the study and on the study day. All study day sessions began between 8 and 10 am.
Following the screening, participants completed a baseline 5-item questionnaire and were outfitted for continuous recording of heart rate and skin temperature with the Mini Logger device. They were escorted to an experimental room and allowed to sit quietly in a reclining chair. The two hour session began after a 15 minute period. They smoked 4 cigarettes, one every 30 minutes starting with their usual preferred cigarette (which they provided). At the appropriate time, participants were presented with a cigarette and allowed to light up. They had 5 minutes to smoke each cigarette. Individual Quest® cigarettes were placed in small plastic bags and presented to the subjects in a counterbalanced order. The cigarettes were clearly marked as Quest®, but participants were unaware of the strength.
The 13-question cigarette evaluation questionnaire comprised of 17 items was completed once for each cigarette smoked immediately after finishing the cigarette. The 5-item mood questionnaire was completed 15 minutes before presentation of the first cigarette and then at 5, 10, 15, and 24 minutes after finishing each cigarette.
Data analysis
Items on the cigarette evaluation questionnaire were analyzed with a 1-factor (dose) repeated measures analysis of variance with least significant difference post hoc analysis (SPSS 13 for Mac OS X). All other dependent variables were analyzed with a 2 factor (dose, time) repeated measures analysis of variance with least significant difference post hoc analysis (SPSS 13 for Mac OS X). An alpha of P ≤ 0.05 was set for significance. Due to equipment problems, complete heart rate data were not obtained on two of the participants and their data were not included in the heart rate analysis.
Results
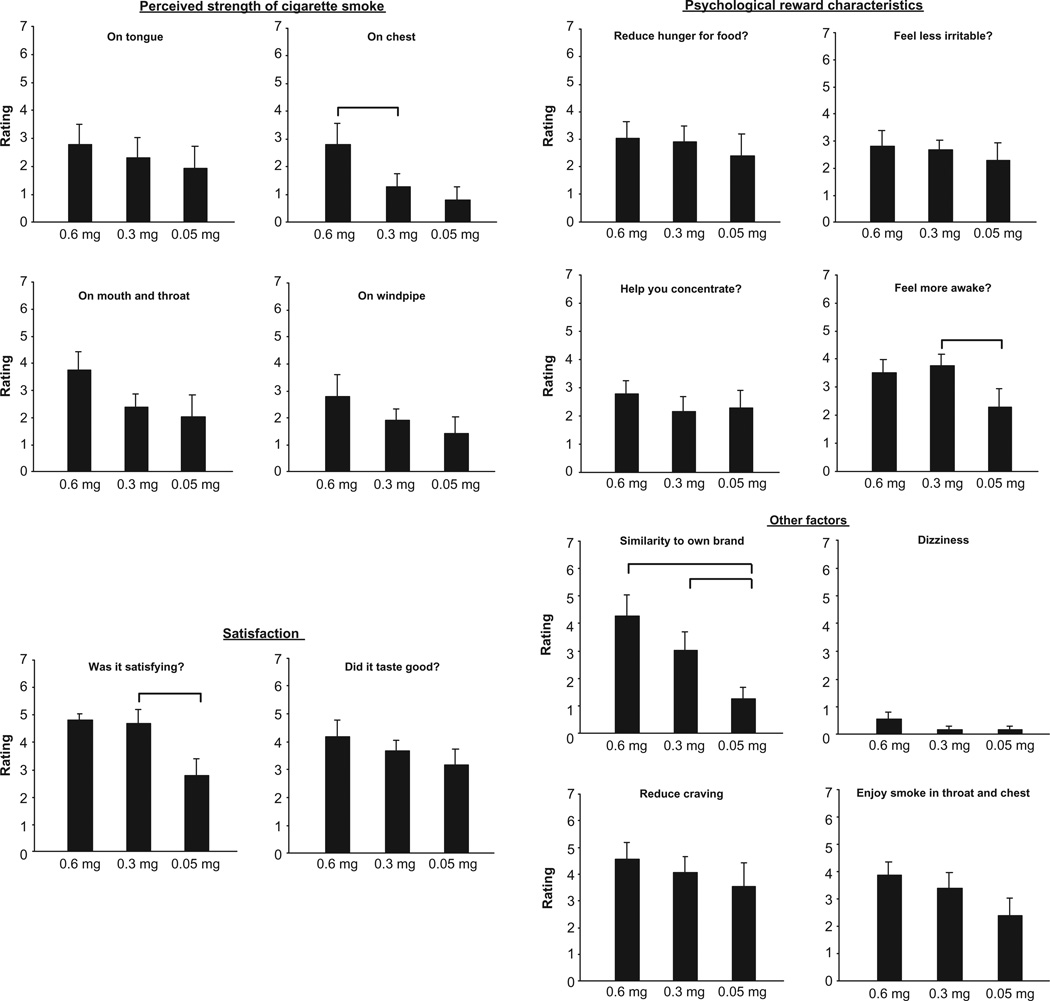
Significant reductions were observed on several items of the cigarette evaluation questionnaire as nicotine yield of the cigarettes decreased (Fig. 1) (statistical results are presented in Table 2). Ratings of ‘similarity to own brand’ showed the largest and most consistent decline. Additionally, there was a clear dose-response function. Post-hoc tests revealed that ratings of similarity to own brand for the 0.3 mg cigarette were significantly lower than those from the 0.6 mg cigarette, and the 0.05 mg nicotine cigarette ratings were significantly lower than those from the 0.3 mg cigarette. For ‘perceived strength on the chest’, the 0.3 mg nicotine yield cigarette was rated as significantly less strong than the 0.6 mg cigarette. The lowest yield cigarette was less ‘satisfying’ and participants reported that it made them feel less ‘awake’ than the 0.3 mg cigarette. Significant differences were observed between the 0.3 mg and 0.05 mg cigarettes on both of these measures. Other measures showed a pattern of decreasing values as machine-smoked nicotine yield was reduced, but these ratings were not significantly different overall.
Figure 1.
Subject ratings on 14 items of a 17-item questionnaire (means ± sem).
Notes: Ratings were on a scale of 0—’Not at all’ to 7—’Extremely’ and were conducted immediately following smoking a cigarette. Bars indicate where significant post hoc differences (P < 0.05) occurred between the different Quest cigarettes.
Table 2.
Cigarette evaluation questionnaire: ANOVA results.
| Was it satisfying? | F(2,14) =7.059 | P = 0.008 |
| Did it taste good? | F(2,14) = 2.0 | P = 0.172 |
| How similar was it to your own brand? | F(2,14) = 16.234 | P < 0.001 |
| Did it calm you down? | F(2,14) =0.69 | P = 0.518 |
| Did it help you concentrate? | F(214) = 1.195 | P = 0.332 |
| Did it make you feel more awake? | F(2,14) =7.483 | P = 0.006 |
| Did it reduce your hunger for food? | F(2,14) = 1.122 | P = 0.353 |
| Did it make you feel less irritable? | F(2,14) = 0.401 | P = 0.667 |
| Did it make you nauseous? | F(2,14) = 1.883 | P = 0.189 |
| Did it make you dizzy? | F(2,14) = 4.2 | P = 0.037 |
| Did you enjoy the sensations of the smoke in your throat and chest? | F(2,14) = 2.227 | P = 0.145 |
| Did it immediately reduce your craving for cigarettes? | F(2,14) = 0.6 | P = 0.562 |
| How strong was this cigarette on | ||
| a. Your tongue? | F(2,14) = 1.060 | P = 0.371 |
| b. Your nose? | F(2,14) = 1.258 | P = 0.314 |
| c. The back of your mouth and throat? | F(2,14) = 2.995 | P = 0.083 |
| d. Your windpipe? | F(2,14) = 1.648 | P = 0.228 |
| e. Your chest? | F(2,14) = 4.136 | P = 0.039 |
Note: Significant results are in bold font.
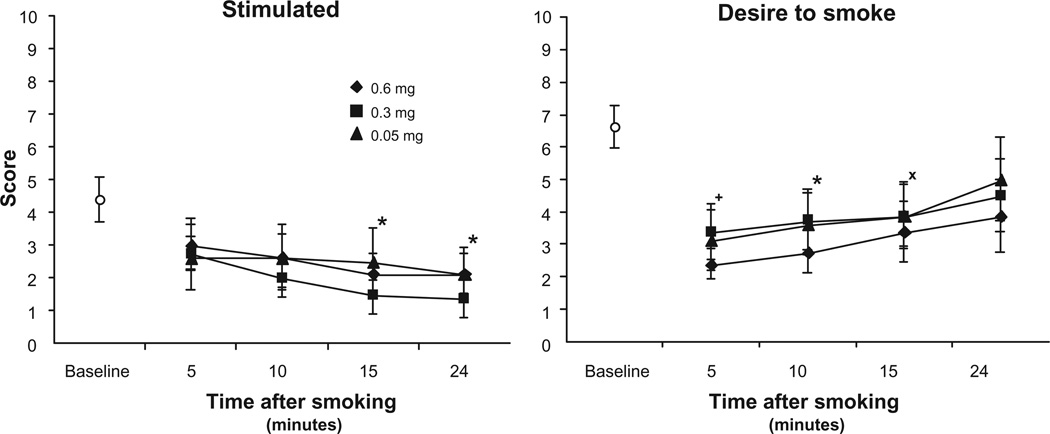
Subjective reports of ‘stimulation’ from the mood questionnaire decreased as a function of time after smoking [F(3,21) = 5.787, P = 0.005], but there were no differences among the cigarette strengths (Fig. 2). Ratings of ‘desire to smoke’ were not significantly affected by nicotine dose. These ratings were low immediately after finishing all cigarettes and showed time-related increases over the assessment period [F(3,21) = 4.892, P = 0.01] (Fig. 2). Conversely, ‘desire not to smoke’ showed time-related decreases following a cigarette [F(3,21) = 5.758, P = 0.005 (data not shown) but not an effect for dose. Ratings of ‘happy’ and ‘anxious’ remained relatively constant throughout the session and were not affected by nicotine dose (data not shown).
Figure 2.
Subject ratings on 2 items from the mood questionnaire (means ± sem).
Notes: Ratings were from 0—‘Not at all’ to 10—‘Extremely’. Data are means (±sem). Baseline measures were taken after at least a two hour abstinence period and just before the beginning of the smoking session. *Indicates significant post hoc differences from start values, P < 0.05; +P = 0.053; ×P = 0.051.
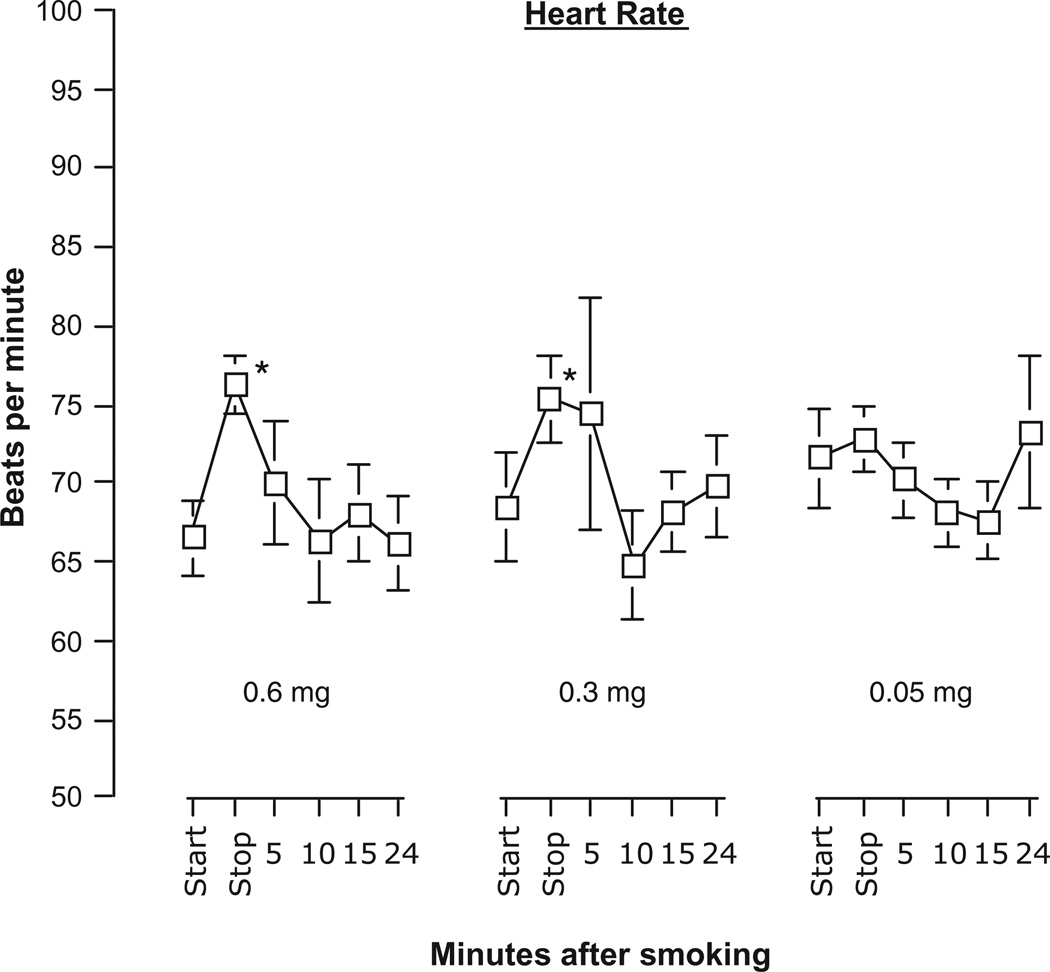
Heart rates significantly increased following the smoking of two of the Quest® cigarettes [Time: F(5,25) = 2.699, P = 0.044] (Fig. 3). For the 0.6 and 0.3 mg cigarettes, heart rates increased 9 beats per minute on average from the beginning of the smoking when compare to 5 minutes later at the time they finished the cigarette. Rates declined quickly and by 5 minutes following the end of smoking the cigarette, rates were not significantly different from the starting rates. Heart rate was not affected by smoking the 0.05 mg nicotine cigarette. Skin temperature remained steady throughout the experimental session and did not vary across cigarette conditions or time.
Figure 3.
Heart rate (mean beats per minute ± sem) at the start and stop (5-minute period) of smoking a cigarette and at 4 intervals following completion.
Note: *Indicates significant post hoc differences from start values, P < 0.05.
Discussion
The data collected in the present study demonstrate that the nicotine yield of cigarettes has a role in the subjective and physiological effects of smoking. On several measures of smoking satisfaction and other subjective effects associated with smoking, reducing nicotine yield in the cigarettes produced smaller effects in comparison to the effects of higher nicotine yield cigarettes, and heart rate increases were smaller. The results of this experiment are consistent with previous reports of smoking satisfaction and craving assessments as a function of nicotine levels12,13 and are in general agreement with prominent publications on the importance of nicotine as a contributing factor in cigarette smoking.1 The reduction in psychological and physiological sensations seen as a function of nicotine dose in this study point to the importance of nicotine in the perception of cigarette’s pleasurable qualities.
The sensations of smoke have been shown to be important determinants in subjective ratings of the smoking experience.5,14 Other research conducted with cigarettes with varying nicotine, tar, and menthol levels has shown that nicotine robustly influences physiology and most subjective ratings.15 The current study refines and extends this work by studying responses to lower-nicotine and de-nicotinized cigarettes that were all of one brand and all contained the same amount of tar. The use of these cigarettes (that have three different amounts of nicotine) potentially allows for clearer distinction between the sensory and physiological effects of smoking.
The results suggest that nicotine yield does not have a significant effect on smoking-induced reductions in craving; all three cigarettes reduced craving to the same degree. Previous studies have shown that smoking de-nicotinized cigarettes are effective in reducing cravings.7,14,16,17 These studies assessed cravings after an overnight abstinence period when, presumably, cravings would be very strong. The present study assessed cravings in a non-abstinence state as the lower nicotine cigarettes were smoked at 30 minute intervals. There may be differences in the relative contribution of nicotine vs. cigarette smoke sensations in reducing cravings that are dependent on the level of deprivation (abstinence period) which should be a topic for additional research.
Heart rates were similarly increased by the two low nicotine cigarettes (although the amount of the increase was less with the lower nicotine cigarette) and not at all with the de-nicotinized cigarette. These results are consistent with the fact that nicotine has potent physiological effects and with previous findings that lower nicotine yield cigarettes produce less physiological change.8,12
Several limitations should be mentioned concerning this study. First, the sample size was small. Despite the small sample, statistically significant results were obtained for several important measures of responses to smoking indicating that responses are a function of nicotine yield. Second, levels of delivered nicotine were not measured as we were attempting to obtain subjective and physiological data with a minimum of disruption and discomfort to the participants. Certainly, a more complete understanding of subjective responses to altering nicotine yield in the cigarette will be forthcoming with additional experiments that also assess nicotine levels in the body. Third, this study could not dissociate sensory from psychoactive nicotine effects. Important research by several research groups have developed methods for such an analysis, including comparisons of smoke-delivered nicotine via cigarettes vs. intravenous administration, and the blockade of airway sensations by peripheral nicotine antagonists (eg, trimethaphan).18,19
Nicotine is regarded by many as a necessary but not sufficient component for maintaining cigarette smoking behavior. In smoking cessation studies, nicotine replacement therapy, while improving quit rates and aiding in the process of quitting, is not effective for a large percentage of smokers.4 One reason why nicotine replacement therapies do not work for everyone may be that these therapies do not address the significant sensory cues associated with nicotine self administration via smoking.14,19,20 Several recent studies have use reduced nicotine cigarettes with and without other forms of nicotine replacement therapies in smoking cessation programs. Becker et al.21 used Quest cigarettes plus nicotine replacement therapy (patch). Their results indicate that a program of gradually reducing cigarette nicotine content plus a nicotine patch after the quit date was more effective than using the patch alone. Hatsukami et al.22 contrasted two types of Quest cigarettes with the nicotine lozenge in a six-week smoking cessation study and found that the 0.05 mg (‘nicotine-free’) cigarette produced abstinence rates similar to the lozenge. Interestingly, the 0.3 mg (‘reduced nicotine’) cigarette had significantly poorer abstinence rates than either of the other two experimental conditions.
Cigarettes that gradually reduce nicotine yield while keeping other characteristics as consistent as possible as done in the above cited references may prove to be an effective method of gradual nicotine withdrawal while maintaining the important sensations of smoking that research has shown to be important in reducing cravings. This may aid in a smoking cessation program of gradual reduction of nicotine without loss of smoking sensations that contribute to satisfaction. The effects of reducing nicotine yield cigarettes will need to be studied in additional populations before their effectiveness will be fully known.
Acknowledgments
This study was supported by National Institute on Drug Abuse grants DA 003994 (SEL) and DA KO5 00343 (SEL).
Footnotes
This is an open access article. Unrestricted non-commercial use is permitted provided the original work is properly cited.
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
The authors have no financial interests to disclose concerning the conduct or reporting of this study.
References
- 1.USDHHS. The Health Consequences of Smoking: Nicotine Addition. A Report of The Surgeon General. Washington, DC: US Government Printing Office; 1988. [Google Scholar]
- 2.Levin ED, Rose JE, Behm F, Caskey NH. The effects of smoking-related sensory cues on psychological stress. Pharmacology, Biochemistry, and behavior. 1991;39:265–268. doi: 10.1016/0091-3057(91)90177-4. [DOI] [PubMed] [Google Scholar]
- 3.Rose JE, Levin ED. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. British Journal of Addiction. 1991;86:605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 4.Roth MT, Andrus MR, Westman EC. Outcomes from an outpatient smoking-cessation clinic. Pharmacotherapy. 2005;25:279–288. doi: 10.1592/phco.25.2.279.56957. [DOI] [PubMed] [Google Scholar]
- 5.Rose JE, Tashkin DP, Ertle A, Zinser MC, Lafer R. Sensory blockade of smoking satisfaction. Pharmacology, Biochemistry, and behavior. 1985;23:289–293. doi: 10.1016/0091-3057(85)90572-6. [DOI] [PubMed] [Google Scholar]
- 6.Rose JE, Behm FM, Levin ED. Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacology, Biochemistry, and behavior. 1993;44:891–900. doi: 10.1016/0091-3057(93)90021-k. [DOI] [PubMed] [Google Scholar]
- 7.Butschky MF, Bailey D, Henningfield JE, Pickworth WB. Smoking without nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacology, Biochemistry, and Behavior. 1995;50:91–96. doi: 10.1016/0091-3057(94)00269-o. [DOI] [PubMed] [Google Scholar]
- 8.Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine and Tobacco Research. 1999;1:357–364. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- 9.Boren JJ, Stitzer ML, Henningfield JE. Preference among research cigarettes with varying nicotine yields. Pharmacology, Biochemistry, and behavior. 1990;36:191–193. doi: 10.1016/0091-3057(90)90148-b. [DOI] [PubMed] [Google Scholar]
- 10.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. British Journal of Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 11.Rose JE, Westman ED, Behm FM, Johnson MP, Goldberg JS. Blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan. Pharmacology, Biochemistry, and behavior. 1999;62(1):165–172. doi: 10.1016/s0091-3057(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 12.Gross J, Lee J, Stitzer ML. Nicotine-containing versus de-nicotinized cigarettes: Effects on craving and withdrawal. Pharmacology, Biochemistry and behavior. 1997;57:159–165. doi: 10.1016/s0091-3057(96)00309-7. [DOI] [PubMed] [Google Scholar]
- 13.Donny E, Hoursmuller E, Stitzer M. Smoking in the absence of nicotine: behavioral, subjective and physioloigcal effects over 11 days. Addiction. 2008;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- 14.Rose JE, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacology, Biochemistry, and behavior. 2003;76:243–250. doi: 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Pickworth WB, Moolchan ET, Murty R. Sensory and physiologic effects of menthol and nonmenthol cigarettes with differing nicotine delivery. Pharmacology Biochemistry behavior. 2002;71:55–61. doi: 10.1016/s0091-3057(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 16.Westman EC, Behm FM, Rose JE. Airway sensory replacement as a treatment for smoking cessation. Drug Development Research. 1996;38:257–262. [Google Scholar]
- 17.Rose JE, Behm FM, Westman EC, Johnson MP. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology, Biochemistry and behavior. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 18.Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharma-codynamic characteristics of intravenous and inhaled nicotine. Journal of Pharmacology and Experimental Therapeutics. 1985;234:1–12. [PubMed] [Google Scholar]
- 19.Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184(3–4):274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 20.Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology, Biochemistry, and behavior. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 21.Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine and Tobacco Research. 2008;10(7):1139–1148. doi: 10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- 22.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]