Abstract
Purpose
Apoptosis plays an important role in neoplastic processes. Bcl-B is an antiapoptotic Bcl-2 family member, which is known to change its phenotype upon binding to Nur77/TR3. The expression pattern of this protein in human malignancies has not been reported.
Experimental Design
We investigated Bcl-B expression in normal human tissues and several types of human epithelial and nonepithelial malignancy by immunohistochemistry, correlating results with tumor stage, histologic grade, and patient survival.
Results
Bcl-B protein was strongly expressed in all normal plasma cells but found in only18%of multiple myelomas (n = 133). Bcl-B immunostaining was also present in normal germinal center centroblasts and centrocytes and in approximately half of diffuse large B-cell lymphoma (n =48) specimens, whereas follicular lymphomas (n = 57) did not contain Bcl-B. In breast (n = 119), prostate (n = 66), gastric (n = 180), and colorectal (n = 106) adenocarcinomas, as well as in non – small cell lung cancers (n = 82), tumor-specific overexpression of Bcl-B was observed. Bcl-B expression was associated with variables of poor prognosis, such as high tumor grade in breast cancer (P = 0.009), microsatellite stability (P = 0.0002), and left-sided anatomic location (P = 0.02) of colorectal cancers, as well as with greater incidence of death from prostate cancer (P = 0.005) and shorter survival of patients with small cell lung cancer (P = 0.009). Conversely, although overexpressed in many gastric cancers, Bcl-B tended to correlate with better outcome (P = 0.01) and more differentiated tumor histology (P < 0.0001).
Conclusions
Tumor-specific alterations in Bcl-B expressionmay define subsets of nonepithelial and epithelial neoplasms with distinct clinical behaviors.
Defective apoptosis represents one of the six recognized cardinal features of cancer (1). Bcl-2 family proteins are evolutionarily conserved regulators of cell life and death. In humans, six antiapoptotic members of the Bcl-2 family have been identified, including Bcl-2, Bcl-XL, Mcl-1, Bcl-W, Bfl-1, and Bcl-B (2). Overexpression of Bcl-2 and some other antiapoptotic members of the Bcl-2 family has been documented in human cancers (reviewed in ref. 3). Given that investigational therapies targeting specific Bcl-2 family proteins or their encoding mRNAs are now in clinical trials, it is important to define which Bcl-2 family proteins are overexpressed in various types of cancer, so that appropriate targeted therapies can be matched to specific malignancies.
Bcl-B (also known as Bcl2-L-10) was the last antiapoptotic member of the human Bcl-2 family to be identified (4, 5), and relatively little is known about its in vivo functions. Bcl-B contains conserved BH1, BH2, BH3-like, and BH4 domains, as well as a COOH terminal transmembrane domain, typical of antiapoptotic Bcl-2 family proteins that target intracellular membranes of mitochondria (4, 5). Dimerization of proapoptotic and antiapoptotic Bcl-2 family proteins plays an important role in controlling their activity (6, 7). The Bcl-B protein was shown to differentially bind proapoptotic Bcl-2 family members (4). Thus, Bcl-B may have a unique pattern of selectivity for binding to various proapoptotic members of the Bcl-2 family, suggesting a specific role for this protein in controlling cell life and death.
Although initially recognized for its antiapoptotic activity, the mouse orthologue of Bcl-B reportedly displays either antiapoptotic or proapoptotic activity, depending on cellular context (8, 9). In this regard, we recently reported that Bcl-B binds orphan nuclear receptor Nur77/TR3, which converts the phenotype of Bcl-B from antiapoptotic to proapoptotic (10). Thus, Bcl-B is similar to Bcl-2 in its ability to display opposing effects on apoptosis based on protein interactions and other factors (11).
Heretofore, analysis of Bcl-B protein expression in human tissues or cancers has not been described. A variety of publicly available DNA microarray datasets have suggested Bcl-B mRNA is widely expressed in human tissues and many cancers but have attracted little attention and permitted few conclusions. The closest orthologue of Bcl-B in mice is Boo/Diva, with 49% amino acid identity. However, Boo/Diva expression in mice seems to be limited to ovary and testis (8, 9), suggesting that the murine gene is regulated differently than its closest human counterpart and highlights an important species-specific difference. By generating monospecific antibodies that recognize Bcl-B, we have investigated Bcl-B protein expression in normal human tissues and in several types of human malignancy by immunohistochemistry, correlating the expression results with clinically relevant variables.
Materials and Methods
Patient specimens
Informed consent was obtained in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Boards of each institution that participated.
Bone marrow biopsies from 165 patients, 114 with symptomatic multiple myeloma (MM), 19 with indolent MM, 13 with monoclonal gammopathy of undetermined significance (MGUS), and 19 with reactive plasmacytosis, were obtained from Veterans Affairs Medical Center (VA Hospital) of Los Angeles. Patients were categorized according to WHO criteria, assessing the plasma count in bone marrow (group 1, 0-10%; group 2, 11-30%; group 3, >30%; ref. 12).
Tissue microarrays (TMA) comprising paraffin-embedded lymph node specimens from 48 patients diagnosed with diffuse large B-cell lymphomas (DLBCL) and from 57 patients with follicular lymphoma were provided by the Robert H. Lurie Comprehensive Cancer Center, Northwestern University.
Tumor specimens were obtained from 79 small cell lung cancer (SCLC) patients with limited disease who were treated by surgery followed by chemotherapy using various multidrug regimens at the Thoracic Surgery Department of Medical University of Gdañsk between 1984 and 2001. In addition, thoracic radiation was given to 4% of the patients and prophylactic cranial irradiation to 8% of the patients. Patients ranged from clinical stages I to IIIA and were of good performance status (Karnofsky score, 80-100). Clinical data represent a median follow up of 1.3 y.
TMAs containing specimens from 82 non-SCLC patients were obtained from Princess Margaret Hospital and Ontario Cancer Institute in Toronto. The specimens represented 22 adenocarcinomas, 32 squamous cell carcinomas, and 16 large cell carcinomas (12 unspecified tumors).
Clinicopathologic characteristics related to paraffin-embedded tissue specimens containing cervical, colorectal, gastric, breast, prostate, and ovarian cancers were described elsewhere (13-16). In addition, a TMA was produced for 26 cases of Crohn’s disease that had been obtained from the Department of Pathology at Yonsei University Medical Center.
Tissue preparation
The tissues were prepared for paraffin embedding, as described elsewhere (17). TMAs were produced for all investigated tumors and tissues, as described previously (14).
Antibodies
Glutatione S-transferase (GST)–Bcl-B fusion protein was produced in bacteria and purified by affinity chromatography as described (18). A polyclonal antibody specific for Bcl-B (BR-49) was raised in rabbits using the affinity-purified recombinant GST–Bcl-B protein as the immunogen. An additional anti–Bcl-B serum (AR-77) was generated in rabbits using a synthetic peptide (NH2-REPGTPEPAPSTPEAAVLR- amide) corresponding to residues 32 to 50 of human Bcl-B. Commercial mouse monoclonal antibodies included anti-CD138 (Serotec), anti-CD68 (DakoCytomation), anti-CD10 (Novocastra), anti–Bcl-6 (Novocastra), anti-MUM1 (DakoCytomation), anti–Bcl-2 (DakoCytomation), anti-Hsp60 (Nventa), anti–β-actin (Sigma-Aldrich), and anti-GST (BD PharMingen).
Immunohistochemistry
Dewaxed tissue sections were immunostained as reported previously (19). To determine specificity, the immunostaining procedure was done in parallel using preimmune Bcl-B serum and immune serum (1:1,000) preabsorbed with 10 μg of GST–Bcl-B, GST–Bcl-XL recombinant protein, or synthetic peptide immunogens. The immunostaining scoring system was described previously (20).
For double-labeling procedure, tissue sections were stained as above using Bcl-B rabbit polyclonal antiserum (3,3′-diaminobenzidine chromagen, DAKOCytomation; brown) followed by mouse monoclonal CD138 antibody (Serotec; SG chromagen, Vector Lab., Inc.; black). Nuclear red (DAKOCytomation) was used for counterstaining of the double-labeled slides. Automated image analysis system (Aperio Technology, Inc.) was used to visualize Bcl-B and CD138 staining separately, applying a color deconvolution algorithm (21). Quantification of immunostaining was done using color translation and an automated thresholding algorithm (Aperio Technology, Inc).
Immunohistochemistry results for CD10, Bcl-6, and MUM1 were used to subclassify DLBCL cases into GCB and non-GCB categories (22): cases immunopositive for CD10 alone or for both CD10 and Bcl-6 were assigned to the GCB group, whereas cases that were CD10-/Bcl-6- or that were CD10-/Bcl-6+/MUM1+ were considered non-GCB.
Expression plasmids
Bcl-B encoding cDNA in pcDNA3–Bcl-B plasmid was digested with BamHI and XhoI (Promega), purified (Qiagen) from a 1% agarose gel, and then ligated with modified pTRE2hyg plasmid (Clontech) previously digested with the same restriction enzymes. Proper plasmid construction was confirmed by restriction enzyme digestion and DNA sequencing.
Stable transfection
Stable transfection was conducted using the HeLa Tet-On cell line (Clontech), which was derived from the HeLa cells. This cell line had been stably transfected with the pTet-On plasmid, which encoded the tetracycline repressor and allowed the inserted sequence to be inducibly expressed by tetracycline or doxycycline. The HeLa Tet-On cells were seeded at 50% confluency and cultured overnight. Transfection was conducted for 3 h using LipofectAMINE Plus. Transfected cells were cultured in complete media for 24 h and then split into fresh media. The split cells were seeded to 10% confluence and cultured in media containing G418 (100 μg/mL) to maintain the integration of the pTet-On construct and hygromycin B (300 μg/mL) to select stable transfectants of pTRE2hyg/Bcl-B. Positive foci resistant to both antibiotics were isolated and expanded. The transfected cells were cultured in the presence or absence of doxycycline (1 μg/mL) for 16 h for immunoblot studies.
Immunoblotting
Specimens derived from normal and malignant human tissues with high ratios of cancer cells relative to stroma (>70%) were provided by M. D. Anderson Cancer Center-Orlando for immunoblotting analysis. The protein lysate preparations, immunoblotting procedures, and antigen detection were described previously (23). Blots were probed with rabbit anti–Bcl-2 antisera (1:2,000 to 1:3,000 v/v), mouse anti-Hsp60, or β-actin antibodies. Expression and purification of recombinant Bcl-2 family proteins are described elsewhere (10).
Microsatellite instability
Specimens were analyzed for microsatellite instability, as described previously (14).
Statistical analysis
Data were analyzed using the STATISTICA software package (StatSoft) as described elsewhere (14). Median Bcl-B immunopercentage and immunoscore (IS) were applied as cutoffs for Kaplan-Meier survival analyses.
Results
Characterization of Bcl-B antibodies and immunoblot analysis of normal and malignant human tissues
The specificity of the BR-49 antibody was documented previously, showing reactivity with Bcl-B but not Bcl-2, Bcl-XL, Mcl-1, Bcl-W or Bfl-1 (10). To characterize the specificity of the AR-77 antibody, immunoblot analysis was done using recombinant Bcl-2 family proteins generated in bacteria (Fig. 1A and B). The AR-77 antibody was determined to be specific for Bcl-B, detecting GST fusion protein containing Bcl-B and lacking cross-reactivity with other Bcl-2 family members (Fig. 1A and B). Note that the two bands seen with GST–Bcl-B likely correspond to intact fusion protein (~ 45-50 kDa) and proteolyzed protein separating Bcl-B (~ 23 kDa) from GST.
Fig. 1.
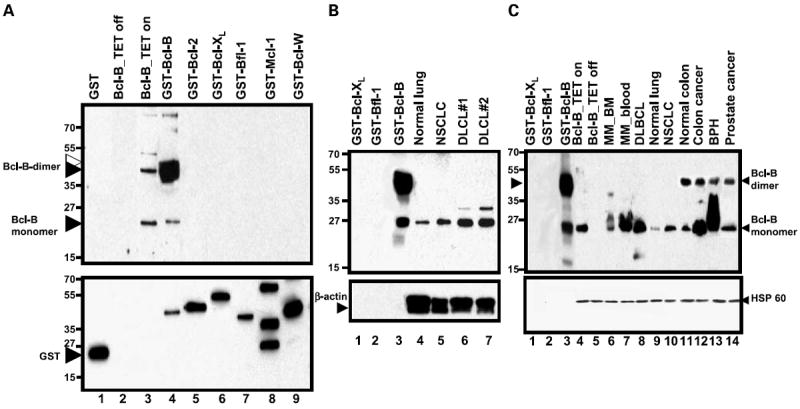
Characterization of Bcl-B antibodies and immunodetection of Bcl-B protein in human tissue lysates. A, GST fusion proteins containing Bcl-XL, Bfl-1, Bcl-2, Mcl-1, Bcl-W, and Bcl-B (0.1 μg/lane) were analyzed by immunoblotting using AR-77 antiserum (top). The blot was reprobed with anti-GST (bottom). Cell lysates from HeLa cells with tetracycline-inducible Bcl-B are also included (tet on/off). B and C, selected GST fusion proteins (0.05 μg/lane) and human tissue lysates normalized for total protein content (50 μg/lane) were subjected to SDS-PAGE/immunoblot analysis, using AR-77 (1:2,000, v/v; B) or BR-49 (1:3,000, v/v; C) antibodies to Bcl-B (top). Blots were reprobed with anti-HSP60 and anti −β-actin antibodies (bottom). Antibody detection was accomplished using an enhanced chemiluminescence method. Black arrows, Bcl-B monomers or SDS-resistant Bcl-B dimers; white/black double arrow, GST – Bcl-B fusion protein.
Probing tissue lysates with the AR-77 or BR-49 antibodies showed reactivity with a protein at ~ 23 kDa, corresponding to the predicted molecular mass of Bcl-B, as well as ~ 45 to 50 kDa band that seems to be an SDS-resistant dimer, based on studies that have detected this species even when using epitopetagged Bcl-B protein that was detected using antibodies directed against the tag (not shown). Also, a dimeric form of Bcl-B was shown by SDS-PAGE analyses of purified recombinant Bcl-B produced without GST tag (not shown). In some tumor lysates, bands were detected that may correspond to posttranslationally modified forms of Bcl-B, which migrate at a few kilo-Daltons larger apparent molecular mass than monomeric Bcl-B in SDS-PAGE (see, for example, diffuse large cell lymphoma, MM, and benign prostatic hyperplasia samples). The specificity of the anti–Bcl-B antibodies was further confirmed by analysis of lysates from HeLa cells containing a tetracycline-inducible Bcl-B construct, revealing the presence of the expected ~ 23 kDa Bcl-B band only when the tetracycline analogue doxycyclin was added to cultures (Fig. 1).
Levels of endogenous Bcl-B varied among tissues and tumor specimens analyzed by immunoblotting. Bcl-B was elevated in non-SCLC compared with normal lung in two of two paired specimens and also higher in a colorectal cancer compared with normal colonic tissue from the same patient (Fig. 1C). In contrast, Bcl-B protein levels were higher in benign prostatic hyperplasia specimen compared with a prostate cancer. Bcl-B protein was also present in three of four diffuse large-cell lymphoma and two of three MM specimens tested. Reprobing blot with anti-Hsp60 or anti–β-actin antibodies confirmed equivalent loading of tissue lysates.
Immunohistochemical analysis of Bcl-B protein expression in normal human tissues
To lay a foundation for assessing Bcl-B expression in cancers, we first ascertained the in vivo patterns of Bcl-B protein expression in normal human tissues by immunohistochemical analysis. The most intense Bcl-B immunoreactivity was found in plasma cells. Strongly stained plasma cells were found in bone marrow, lymphoid tissues, at sites of inflammation, and infiltrating some tumors (Fig. 2). In contrast, erythroid cells, myeloid cells, and megakaryocytes in bone marrow, as well as macrophages, dendritic cells, and most lymphocytes in nodes and extranodular sites of inflammation were Bcl-B negative. Immunoblotting analysis of plasma cells isolated from bone marrow also confirmed the presence of Bcl-B protein (Supplementary data). Moreover, comparisons of immune (Fig. 2A) and preimmune serum (Fig. 2B), as well as preabsorption experiments using GST–Bcl-B (Fig. 2C) or GST– Bcl-XL fusion proteins (Fig. 2D), confirmed the specificity of plasma cell immunostaining (also see Supplementary data). The plasma cell phenotype of the Bcl-B–positive cells was also confirmed by two-color immunohistochemical analysis, showing coexpression of Bcl-B with CD138 (syndecan-1) expressing cells (Fig. 2I-L). In contrast, the Bcl-B–positive cells were negative for the macrophage marker CD68 (not shown) in twocolor immunostainings.
Fig. 2.
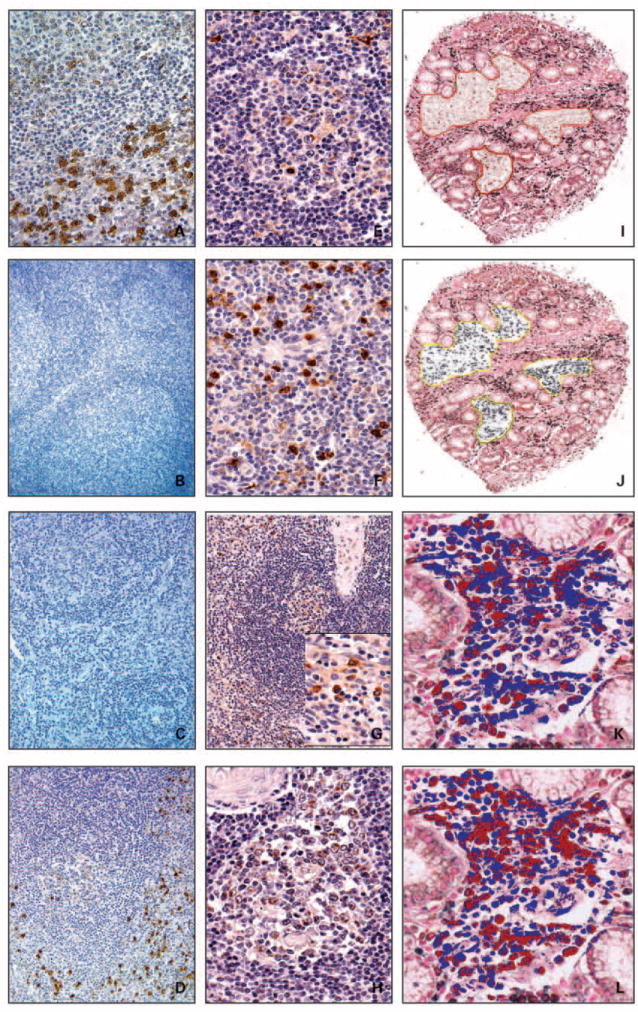
Immunohistochemical detection of Bcl-B expression in B cells and plasma cells. A-D, serial sections of normal human lymph node specimen were stained with anti – Bcl-B antiserum (A; raised against recombinant Bcl-B protein; 400×), preimmune serum (B; 100×), anti – Bcl-B antiserum preabsorbed with GST – Bcl-B (C; 200×), and anti – Bcl-B antiserum preabsorbed with GST – Bcl-xL (D; 200×). Specimens were counterstained with hematoxylin. Human lymph node (E and F) and spleen (G and H) sections containing secondary follicles (E and H) were stained with the Bcl-B antibody to visualize immunopositive B cells in germinal centers (E and H) and plasma cells in medullary cords (F) and red pulp (G). Photomicrographs were taken at original magnifications ranging from 100× to 400×. I-L, TMA containing gut specimens from patients with Crohn’s disease were double stained with the Bcl-B (3,3′-diaminobenzidine; brown) and CD138 (SG; black) antibodies and counterstained with nuclear red. The brown (I) and black (J) colors were separated in the annotated regions using a color deconvolution algorithm (Aperio). Quantification of immunohistochemical staining for Bcl-B (K) and CD138 (L) was done using color translation and an automated thresholding algorithm (Aperio). Dark red pixels visualize positive immunostaining, whereas blue pixels depict immunonegative areas. Note colocalization of Bcl-B and CD138 cells. Original magnifications are 100× (I and J) and 400× (K and L).
In addition to plasma cells, Bcl-B also immunolocalized to centroblasts and centrocytes in germinal centers, but not to other types of cells in lymphoid and hematopoietic tissues (Fig. 2E and H), including bone marrow, spleen, nodes, and thymus. The intensity of Bcl-B immunostaining in these lymphocytes, however, was substantially less than plasma cells (Fig. 2F-G). Among other normal human tissues, Bcl-B expression was detected in hepatocytes, renal tubule epithelium, bronchial and nasopharyngeal epithelium, and type II pneumocytes, as well as cytotrophoblasts in the placenta and some neuronal cells, again with immunointensity much less than observed in plasma cells. In the prostate gland, Bcl-B immunoreactivity was strong in the luminal secretory cells but was absent in basal cells (Supplementary data), thus constituting a pattern of expression opposite of Bcl-2 (24). In all cells examined, the Bcl-B staining pattern was predominantly cytosolic, with a punctate or granular organellar distribution.
Bcl-B protein expression in hematopoietic malignancies
Due to the predominant expression of Bcl-B protein in plasma cells, we investigated expression of Bcl-B in plasma cell dyscrasias. Bone marrow biopsies were immunohistochemically evaluated from 165 patients, 114 with symptomatic MM, 19 with indolent MM, 13 with MGUS, and 19 with reactive plasmacytosis. Unlike normal plasma cells which seemed to be uniformly Bcl-B–positive, Bcl-B protein was immunolocalized only to a proportion of plasma cells in the 165 specimens of plasma cell dyscrasia, with an average prevalence of 29 ± 2.1% immunopositive cells. Only 16% (26 of 165) of all tumors showed at least 50% immunopositivity for Bcl-B, with 21 of these high Bcl-B expressors belonging to symptomatic MM (21 of 114, 18%), 3 to the indolent MM group (3 of 19, 16%), and 1 each to the MGUS (1 of 13, 8%) and the reactive plasmacytosis (1 of 19, 5%) groups. Using 5% cutoff, 30% (49 of 165) of the plasma cell dyscrasia cases were negative for Bcl-B. Figure 3A-D illustrates examples of Bcl-B immunostaining in malignant plasma cells. No significant differences in Bcl-B IS values were observed when specimens from MM, MGUS, and reactive plasmacytosis cases were compared. Similarly, Bcl-B immunopositivity and IS were comparable in grades 1 to 3, categorized according to WHO criteria regarding plasma cell content. Bcl-B expression was not significantly associated with patient age, gender, overall survival, or response to therapy in MM.
Fig. 3.
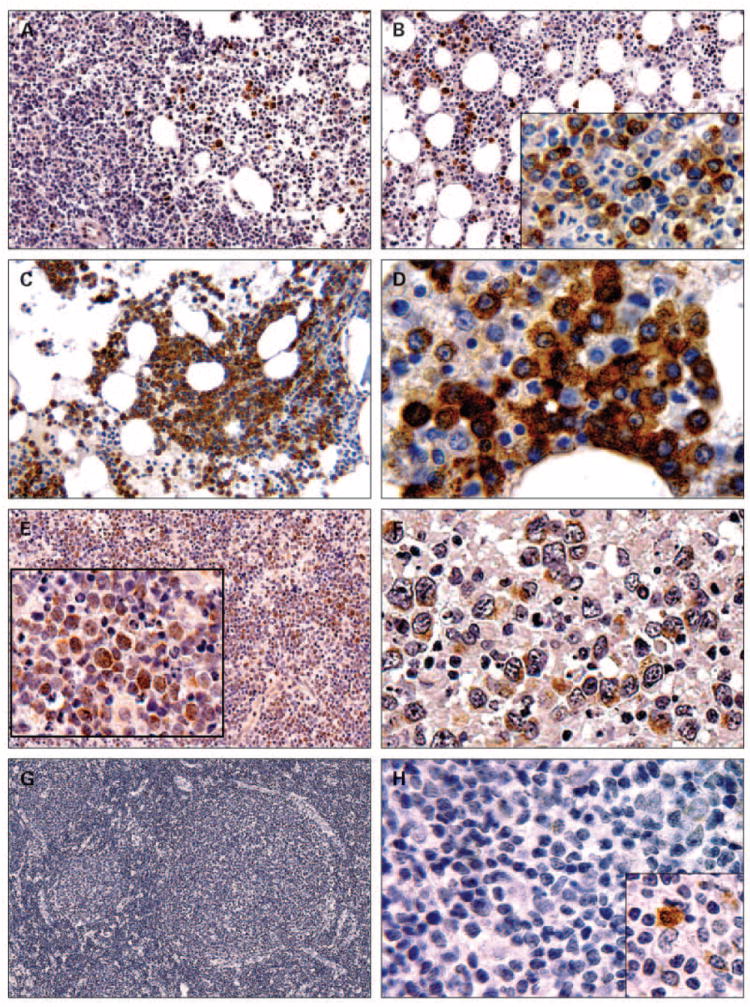
Immunohistochemical detection of Bcl-B expression in human hematolymphoid malignancies. Bone marrow biopsies from multiple myeloma patients (A-D) and lymph node specimens from DLBCL (E and F) and follicular lymphoma (G and H) cases were immunostained using Bcl-B antiserum. Original magnifications are 150× (G), 200× (A-C and E), 1000× (D, F, and H), and 800× to 1,200× (insets).
Lymph node specimens from 48 DLBCL patients were investigated by immunohistochemistry for Bcl-B expression (Fig. 3E-F). Cases were considered positive if ≥30% of tumor cells were Bcl-B immunoreactive. Of 48 DLBCL cases, 25 (52%) were Bcl-B immunopositive, using the 30% cutoff that corresponded to the median percentage of Bcl-B–positive cells in this cohort. CD10, Bcl-6, and MUM1 immunostainings were applied to classify the DLBCL cases into GCB and non-GCB groups (22), using the same cutoff of ≥30% (22). In the investigated patient cohort, 19 of 48 (40%) were considered GCB and 29 (60%) were classified as non-GCB cases. Both groups contained almost identical proportions of Bcl-B – negative and Bcl-B–positive cases (47% and 53%, respectively, in the GCB group; 48% and 52% in the non-GCB category). Thus, analysis of the expression of Bcl-B protein in the GCB and non-GCB lymphomas did not reveal significant differences. The Bcl-B immunostaining data also did not correlate with Bcl-2 staining in these DLBCL specimens.
None of the specimens derived from 57 patients with follicular lymphoma contained detectable Bcl-B immunostaining in malignant B cells (Fig. 3G-H). Positive Bcl-B immunoreactivity in plasma cells observed in these specimens provided an internal staining control.
Bcl-B protein expression in solid tumors
In addition to hematopoietic malignancies, Bcl-B protein expression was investigated in breast, cervical, ovarian, prostate, lung, gastric, and colorectal cancers. The findings provide evidence of alterations in Bcl-B protein expression in several types of solid tumors.
Breast cancer
TMAs containing specimens derived from 119 stages I to III breast cancer patients were immunostained for Bcl-B. The tissue samples comprised 28 cases of DCIS and 104 ductal, 12 lobular, and 3 mucinous invasive carcinomas. In addition, 12 normal mammary epithelium specimens, excised from surgical margins, were included on the arrays, as well as four independent samples of normal mammary gland tissue. Whereas expression of Bcl-B was below the level of detection in normal mammary epithelium, Bcl-B immunostaining was prevalent in 64% of in situ carcinomas and in 89% of invasive cancers (cutoff 10% immunopositive cells), suggesting increasing expression with breast cancer progression. Comparison of Bcl-B immunostaining results for transformed versus normal mammary epithelium was highly significant (P < 0.0001), using either immunopositivity or IS data (Figs. 4A-C and 5A).
Fig. 4.
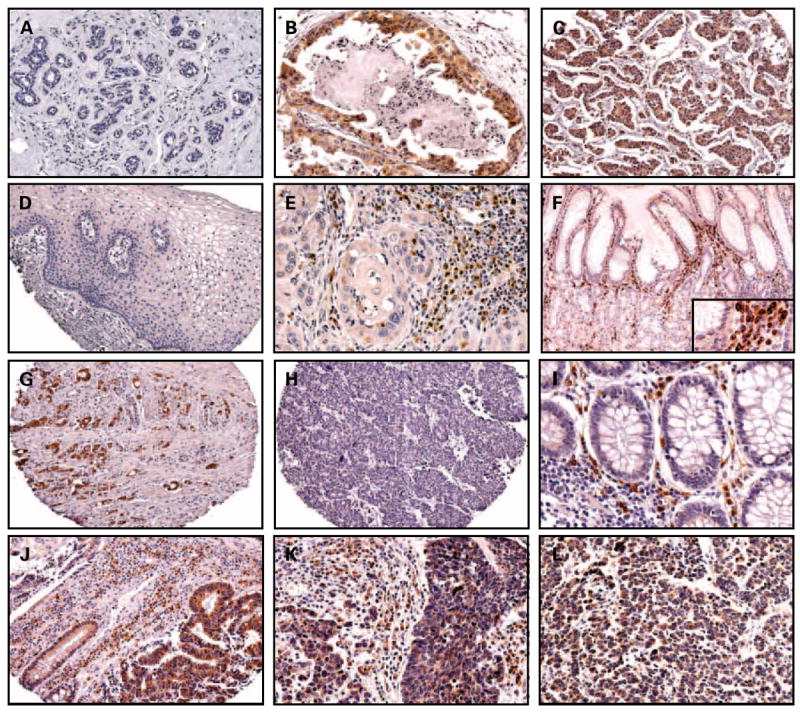
Distribution of Bcl-B immunostainings in human nonlymphoid malignancies. Representative Bcl-B immunostaining results are presented for microarrays of breast specimens in normal mammary epithelium (A; 60×), DCIS (B; 200×), and ductal adenocarcinoma (C; 60×); uterine cervix specimens in normal cervix (D; 60×) and squamous cervical carcinoma (E; 200×); gastric specimens in normal gastric epithelium (F; 100×; inset, 300×) and gastric cancers (G and H; 60×); colon specimens in normal colonic epithelium (I; 200×) and colon cancer (J; 60×); and SCLC in primary tumor (K; 200×) and LN metastasis (L; 200×). Note Bcl-B immunopositive plasma cells in normal (F and I) and malignant (E, J-L) tissues.
Fig. 5.
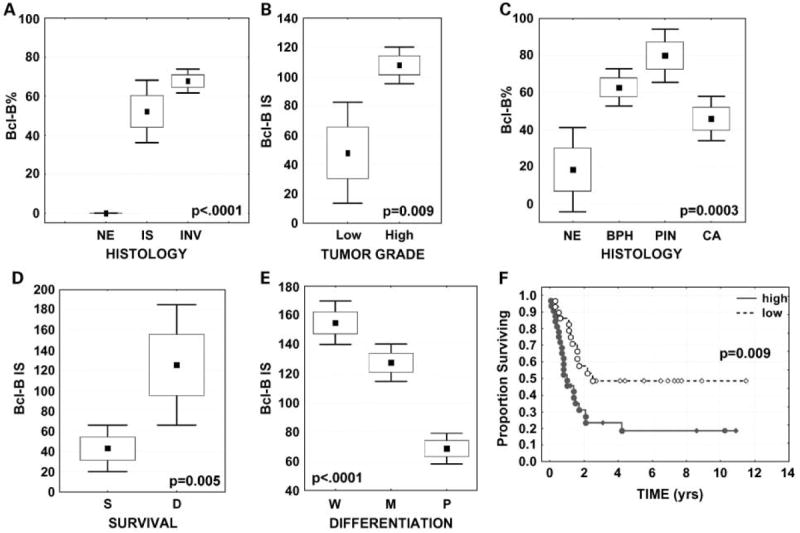
Graphic presentation of Bcl-B immunohistochemistry results in human nonlymphoid malignancies. Box and whisker plots display the distribution of immunopercentage data for Bcl-B in normalmammary epithelium (NE) versus in situ (IS) and invasive (INV) breast carcinoma (A), and in normal prostatic epithelium (NE) versus benign prostatic hyperplasia (BPH), prostatic intraepithelial neoplasia (PIN), and prostate cancer (CA; C). Box and whisker plots depict Bcl-BIS data for low-grade versus high-grade breast cancers (B), for prostate tumors from patients who survived (S) or died (D) from cancer (D), and for well (W), moderately (M), and poorly (P) differentiated gastric cancers (E). The mean immunopercentage/IS is plotted as amarker; whiskers reflect ± 1.96 SE from the mean. F, Bcl-Bimmunopercentage data for SCLC were dichotomized into high (red) versus low (blue) expression groups based on the median values. The percentage of patients remaining alive (ordinate) was compounded over time (abscissa; in y) by the Kaplan-Meier method. The log-rank test was used for correlating the immunostaining data with the patient survival.
Significantly higher Bcl-B immunostaining was observed in invasive ductal compared with invasive lobular carcinoma (70% versus 47% mean immunopercentage, P = 0.03; 108 versus 59 mean IS, P = 0.02). Higher Bcl-B immunostaining was associated with more advanced stage of disease (P = 0.01 for immunopercentage; P = 0.004 for IS), more involved lymph nodes (P = 0.04 for IS), and higher histologic grade of tumors, with high-grade tumors containing significantly higher levels of Bcl-B protein as determined by immunostaining (70 ± 3.2% versus 42 ± 14.1% immunopositive, P = 0.02; 108 ± 6.4 versus 48 ± 17.6 IS, P = 0.009; Fig. 5B). Bcl-B elevation in invasive tumors was correlated with higher ER-β expression (r = 0.42, P < 0.0001 for immunopercentage; r = 0.37, P < 0.0001 for IS) and with lower PR expression (r = -0.22, P = 0.02 for IS), but not with ER-α expression. Breast tumors that showed lymphatic vessel invasion contained higher levels of Bcl-B protein (P = 0.005 for immunopositivity; P = 0.0006 for IS).
No correlations were found between Bcl-B expression and patient age, tumor size, or patient survival. In this particular cohort of breast cancer patients, only PR was an independent prognostic factor for overall and disease-free survival in univariate and multivariate analysis (P = 0.008; P = 0.01, respectively; Cox regression) among all variables assessed (patient age, clinical stage, tumor grade, lymphatic vessel invasion, ER-α, ER-β, PR, Bcl-B% or IS).
Cervical cancer
TMAs containing cervical specimens derived from Asian women diagnosed with cervical intraepithelial neoplasia 1 (low-grade squamous intraepithelial lesions; mild dysplasia; n = 47), cervical intraepithelial neoplasia 2 (high-grade squamous intraepithelial lesion; moderate dysplasia; n = 46), cervical intraepithelial neoplasia 3 (high-grade squamous intraepithelial lesion; severe dysplasia-carcinoma in situ; n = 137), and invasive squamous cell carcinoma (n = 109) were stained for Bcl-B. Normal cervical epithelium adjacent to the transformed cells was available for each histologic entity (n = 328) for all patients in the precancerous groups and 30 of 109 in women diagnosed with invasive cancer. Barely detectable Bcl-B immunostaining was observed in normal epithelium of the exocervix (Fig. 4D) and all stages of the malignant progression (cervical intraepithelial neoplasia 1-3), indicating that Bcl-B expression is not a characteristic of cervical cancer in this Asian cohort. Strongly stained plasma cells were found infiltrating many cervical tumors serving as a positive control (Fig. 4E).
Ovarian cancer
Bcl-B expression was investigated using TMAs containing tissue specimens from 91 ovarian carcinoma patients and six normal ovarian surface epithelium or fallopian tube specimens. The patient cohort comprised 62 individuals with serous carcinomas and 29 cases of nonserous tumors, including mucinous (n = 13), endometrioid (n = 11), clear cell (n = 2), granulosa (n = 1), dysgerminoma (n = 1), and carcinosarcoma (n = 1) types.
Low-intensity Bcl-B immunostaining was detected in ovarian surface epithelium and normal tubal epithelium, with similar levels of Bcl-B expression found in ovarian cancers. No significant differences in Bcl-B protein levels were noted between the two broad histologic categories of ovarian cancer—serous and nonserous. Among these patients, Bcl-B immunostaining did not correlate with patient age, histologic grade of tumor, CA125 serum marker, overall or disease-free survival, or response to therapy. International Federation of Gynecology and Obstetrics stages II to IV showed elevated levels of Bcl-B compared with stage I tumors (87 ± 7.6 versus 49 ± 18.4, for IS) but the difference was statistically insignificant. Statistical comparisons done for a more homogenous cohort of ovarian cancers, namely the 64 serous carcinoma cases, failed to reveal significant associations between Bcl-B expression and the clinical variables. Thus, Bcl-B overexpression is not a common trait of ovarian cancers.
Prostate cancer
To characterize the expression of Bcl-B in prostate cancers, we used TMAs containing patient specimens representing the full range of prostate malignant transformation, including specimens of benign prostatic hyperplasia (n = 38), prostatic intraepithelial neoplasia (n = 11), and prostate adenocarcinoma (n = 41) derived from 66 patients. Gleason score data were available for all tumors, whereas clinical stage information (T2-T3; according to International Union against Cancer criteria) was known for 48% of patients. In addition, nonneoplastic prostate epithelium from 14% of cases was available for comparison of protein expression in nontransformed versus neoplastic epithelium.
Low expression of Bcl-B was found in the normal prostatic epithelium (mean immunopercentage, 18 ± 11.6). Bcl-B expression was markedly increased in benign prostatic hyperplasia (63 ± 5%) and prostatic intraepithelial neoplasia (80 ± 7%) lesions, but less so in the invasive cancers (46 ± 6%; P = 0.0003 for immunopercentage, P = 0.01 for IS; Fig. 5C). Immunohistochemical analysis of specimens revealed higher Bcl-B levels in high-grade tumors (Gleason grade 4) compared with tumors with Gleason grade 3; however, the difference did not reach statistical significance. Higher Bcl-B ISs correlated with poor clinical outcome, with Bcl-B significantly up-regulated in tumors from patients who died from prostate cancer compared with those who survived without relapse during the follow-up period (median follow-up, 2.7 years; 65 ± 14% versus 33 ± 9% for immunopositivity, P = 0.05; 126 ± 30 versus 43 ± 18 for IS, P = 0.005; Fig. 5D). Comparison of preoperational PSA levels suggested that intratumoral Bcl-B is higher in patients with higher PSA levels, but the results did not reach statistical significance.
Gastric cancer
Archival gastric specimens from 169 Asian patients who underwent surgical resection for localized gastric cancer were analyzed immunohistochemically for Bcl-B protein expression. Bcl-B was expressed in normal gastric surface epithelium, but glands deep within the gastric mucosa were negative for Bcl-B or contained only trace amounts of this protein (Fig. 4F).
Expression of Bcl-B was prevalent in gastric cancers, with 89% of tumors showing ≥10% immunopositive cells. Bcl-B protein was significantly associated with histologic architecture and cellular differentiation of gastric adenocarcinomas, with higher levels of Bcl-B expression in well-differentiated tumors compared with poorly differentiated tumors (90 ± 2.4% versus 52 ± 3%, P < 0.0001 and 155 ± 8 versus 69 ± 5 IS, P < 0.0001; Fig. 5E) and higher Bcl-B levels in intestinal-type (Fig. 4G) compared with diffuse-type cancers (Fig. 4H; P < 0.0001). Similarly, Bcl-B levels in tumors containing signet ring cells were lower than those in non–signet ring cell tumors (43 ± 10.0% versus 75 ± 3%, P < 0.0001 and 59 ± 17 versus 118 ± 6 IS, P < 0.0001). Interestingly, tumors with prominent lymphoid infiltration showed higher Bcl-B protein content compared with those that were not infiltrated by lymphocytes (P = 0.01 for immunopositivity, P = 0.009 for IS).
Although tumors from patients who died from cancer contained slightly lower levels of Bcl-B protein compared with tumors from those who survived (P = 0.01 for IS), no significant association with overall survival or disease-free survival was observed for this patient cohort using Kaplan-Meier survival analysis and log-rank test analysis. Also, Bcl-B expression did not correlate with the clinical stage or mucin content in tumors.
Colorectal cancer
TMAs were constructed using primary tumor specimens derived from a cohort of 106 Asian patients with stage II colorectal cancer, who were treated by surgical resection with curative intent. Of the 106 selected cases, 63 patients survived without recurrence, 7 patients had recurrent disease, and 36 patients died from colorectal cancer. Thus, while not an unbiased sequential case series, the survival profile of this cohort closely resembles that of a random population of stage II colorectal cancer patients, with 72.5% of individuals alive at 5 years. Adjacent normal colonic mucosa was present in 65% of the 106 tumor specimens on the array, permitting side-by-side comparisons of immunostaining results for morphologically normal versus malignant epithelium. In addition, four specimens of normal colon derived from individuals who were not diagnosed with colon cancer were stained separately.
Significantly higher Bcl-B protein expression was found in colorectal cancers (Fig. 4J) compared with normal colonic epithelium (Fig. 4I), as assessed by percentage of immunopositive cells (64 ± 5% versus 92 ± 1%) and IS (82 ± 7 versus 181 ± 6, P < 0.0001 and P = 0.004, respectively). To explore if differences in Bcl-B protein expression may correlate with previously identified prognostic features (25), we compared Bcl-B immunostaining with microsatellite instability status, anatomic location of tumors, patient gender, and age. In the investigated cohort, higher Bcl-B levels were found in microsatellite stable compared with microsatellite instability tumors (P = 0.0002 for immunopositivity, P = 0.004 for IS) and in the left-sided compared with right-sided adenocarcinomas (P = 0.02 for immunopositivity; P = 0.04 for IS). Age and gender were not associated with Bcl-B expression. In univariate analysis, no correlations were observed between overall or disease-free survival and Bcl-B immunostaining data.
Because sections from this same TMA had previously been analyzed by immunohistochemistry for expression of some other Bcl-2-family proteins (13), we compared Bcl-B immunostaining data with Bcl-2, Bcl-XL, Bax, and Bid. Bcl-B immunostaining in colorectal cancers correlated with Bcl-XL (r = 0.43, P < 0.0001 for IS) and Bax (r = 0.24, P = 0.03 for IS), but not with Bcl-2 or Bid.
SCLC
Immunostaining for Bcl-B was done on SCLC primary tumors obtained by hemithoracotomy from 79 patients with limited stage disease. The cohort comprised 76% men and 24% women with median age of 57 years, among whom 53% were diagnosed with nonspecified SCLC type, 27% with intermediate, 11% mixed intermediate, 5% fusiform/spindle, 3% oat cell, and 1% with polygonal carcinoma. In addition to primary tumors, matching metastatic mediastinal lymph nodes derived from 24 patients in this cohort were available for investigation.
No significant differences in Bcl-B levels were observed between primary (Fig. 4K) and metastatic tumors (Fig. 4L), as determined by paired t test. Higher Bcl-B content was found in primary tumors from men (P = 0.004 for immunopositivity, P = 0.02 for IS) and from older patients (P = 0.003 for immunopositivity, P = 0.01 for IS). No association was noted between performance status, clinical stage (tumor-node metastasis and Unio Internationale Contra Cancrum stage), tumor size or site (left/right lung), and Bcl-B protein levels in tumors.
To investigate possible association of Bcl-B expression with clinical outcome, Bcl-B immunopercentage and IS data were dichotomized at the median values into “low” versus “high” expression groups. High Bcl-B prevalence correlated with shorter overall survival (log-rank test P = 0.009; Fig. 5F) and increased relative risk of death due to SCLC (univariate Cox proportional hazards analysis, hazard ratio 2.4 and 95% confidence interval 1.3-4.7; P = 0.008). However, in multivariate Cox analysis, which included patient age, gender, clinical stage, and tumor size and site, Bcl-B did not show independent prognostic significance. Bcl-B immunostaining data also did not significantly correlate with time to recurrence after surgery and chemotherapy, although a trend toward higher Bcl-B and shorter time was noted (median, 4.9 versus 3.5 years).
Non-SCLC
TMAs containing specimens from 82 non-SCLC patients were immunohistochemically analyzed for Bcl-B protein expression, including 22 adenocarcinomas, 32 squamous cell carcinomas, 16 large cell carcinomas, and 12 unspecified tumors. Large cell tumors contained relatively low Bcl-B expression (81 ± 15 IS), adenocarcinomas showed intermediate expression (126 ± 20 IS), and squamous cell carcinomas revealed higher Bcl-B content (154 ± 14 IS; P = 0.005). No follow-up data were available for these non-SCLC patients.
Discussion
We surveyed Bcl-B protein expression by immunohistochemical methods in normal human tissues and several types of malignancy. Our findings provide evidence of cancer-associated alterations in Bcl-B expression, suggesting that Bcl-B may contribute to pathogenesis or progression of human cancers and indicating that Bcl-B should be considered when tailoring selection of targeted therapies directed against Bcl-2 family members.
Although most hematopoietic cells exhibited negligible Bcl-B immunoreactivity, normal plasma cells uniformly contained intense Bcl-B immunostaining, suggesting that expression of this member of the Bcl-2 family is induced during terminal stages of B-cell differentiation. Modest intensity Bcl-B immunoreactivity was found in a subpopulation of germinal center B cells, raising the possibility that Bcl-B is initially induced in these antigenic response regions of nodes, from which plasmablasts arise. The presence of Bcl-B protein, not only in the short-lived plasma cells in splenic red pulp and lymph node medullary cords but also in the long-lived plasma cells in bone marrow, suggests a possible role for Bcl-B in maintaining survival of these cells in vivo. Given that autoantibody-producing plasma cells are a recognized source of persistent allergy and autoimmunity (26), Bcl-B might be an attractive target for novel therapies for diseases where autoantibodies secreted by long-lived plasma cells contribute to pathology (reviewed in refs. 27, 28). Therapies designed to target B lineage cells, such as radiation, immunosuppression (prednisone, cyclophosphamide), and anti-CD20 antibodies do not eradicate nondividing long-lived plasma cells in their survival niches (29). Heretofore, the elimination of these cells has been achieved only by complete immunoablation using antithymocyte globulin, but specific targeting of long-lived plasma cells remains unattainable (reviewed in ref. 28).
MM, which arises because of clonal expansion of plasma cells, remains largely incurable, with only 10% of patients surviving 10 years after diagnosis. In multiple studies, increased levels of antiapoptotic Bcl-2, Bcl-XL, and Mcl-1 proteins were linked to survival of MM cells and resistance to chemotherapy (reviewed in ref. 30). Experimentally reducing expression or function of antiapoptotic Bcl-2 famly proteins was shown to sensitize MM cells to various chemotherapeutic agents (30-32). Expression of Bcl-B in myeloma may indicate a role for this protein in the pathogenesis of this malignancy or could simply be a concomitant of a plasma cell phenotype. In fact, the observation that essentially all normal plasma cells express Bcl-B, whereas only 18% of myelomas show prominent Bcl-B expression, suggests that Bcl-B expression is not pathologically elevated in myelomas and implies that Bcl-B–negative myelomas may represent a more immature stage of plasma cell differentiation compared with terminally differentiated, nonproliferating, normal plasma cells. Alternatively, because Bcl-B is converted from a protector to a killer through interactions with Nur77/TR3 (10), absence of Bcl-B may reflect a proapoptotic role for Bcl-B in MM.
Among epithelial neoplasms examined in this study, cancerspecific overexpression of Bcl-B protein was commonly observed in breast, prostate, gastric, colorectal, and lung adenocarcinomas, where tumor immunostaining was clearly stronger in intensity than corresponding normal epithelial cells in those tissues. Prominent expression of Bcl-B was also found for SCLC. However, it is unclear whether the high Bcl-B immunostaining in SCLC is indicative of tumor-associated overexpression versus a reflection of a normal profile for the neuroendocrine cells from which these cancers arise. Nevertheless, these findings document for the first time that high level expression of Bcl-B is a common feature of several types of human malignancy.
The functional significance of Bcl-B expression in epithelial malignancies remains to be determined. From a correlative stand-point, Bcl-B expression was associated with variables of poor prognosis in breast and colorectal cancer, greater incidence of death from prostate cancer, and shorter survival as well as increased relative risk of death from SCLC, suggesting Bcl-B may contribute to aggressive behaviors of these tumors. Conversely, Bcl-B protein expression was not pronounced in cervical or ovarian cancers, suggesting that Bcl-B is not commonly involved in these malignancies. Also, whereas Bcl-B was overexpressed in many gastric cancers, it tended to correlate with better outcome and more differentiated (lower grade) histology.
In considering the consequence of elevated Bcl-B expression in human tumors, it should also be considered that both proapoptotic and antiapoptotic Bcl-2 family proteins are known to change their respective phenotypes in certain scenarios (reviewed in ref. 33). For example, Bcl-2 and Bcl-XL can be converted by caspases from cytoprotective to lethal proteins (34, 35). Also, binding of Nur77/TR3 to Bcl-2, Bfl-1, or Bcl-B converts these normally antiapoptotic proteins to proapoptotic killers (10, 11). Additionally, Bcl-XL expression was associated with increased apoptosis under circumstances where K-Ras is targeted to mitochondria (36). Thus, Bcl-B may play opposing roles in apoptosis regulation depending on cellular context.
Acknowledgments
We thank M. Hanaii and T. Siegfried for manuscript preparation.
Grant support: NIH grants CA-113318, GM-60554, CA-81534 (J. Reed), P30CA06055 (J.Winter), and CA114810 (S.Krajewski).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Mechanisms of apoptosis (Warner/Lambert Award) Am J Pathol. 2000;157:1415–30. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed JC. Mechanisms of Bcl-2 family protein function and dysfunction in health and disease. Behring Inst Mitt. 1996;97:72–100. [PubMed] [Google Scholar]
- 4.Ke N, Godzik A, Reed JC. Bcl-B: a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J Biol Chem. 2001;276:12481–4. doi: 10.1074/jbc.C000871200. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Holzgreve W, De Geyter C. Bcl2-L-10, a novel anti-apoptotic member of the Bcl-2 family, blocks apoptosis in the mitochondria death pathway but not in the death receptor pathway. Hum Mol Genet. 2001;10:2329–39. doi: 10.1093/hmg/10.21.2329. [DOI] [PubMed] [Google Scholar]
- 6.Chao DT, Korsmeyer SJ. Bcl-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 7.Wang H-G, Reed JC. Mechanisms of Bcl-2 protein function. Histol Histopathol. 1998;13:521–30. doi: 10.14670/HH-13.521. [DOI] [PubMed] [Google Scholar]
- 8.Inohara N, Gourley TS, Carrio R, et al. Diva, a Bcl-2 homologue that binds directly to Apaf-1 and induces BH3-independent cell death. J Biol Chem. 1998;273:32479–86. doi: 10.1074/jbc.273.49.32479. [DOI] [PubMed] [Google Scholar]
- 9.Song Q, Kuang Y, Dixit VM, Vincenz C. Boo, a novel negative regulator of cell death, interactst with Apaf-1. EMBO. 1999;18:167–78. doi: 10.1093/emboj/18.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luciano F, Krajewska M, Ortiz-Rubio P, et al. Nur77 converts phenotype of Bcl-B, an antiapoptotic protein expressed in plasma cells and myeloma. Blood. 2007;109:3849–55. doi: 10.1182/blood-2006-11-056879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin B, Kolluri SK, Lin F, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor TR3/NGFI-B/Nur77. Cell. 2004;116:527–40. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 12.Went P, Mayer S, Oberholzer M, Dirnhofer S. Plasma cell quantification in bone marrow by computer-assisted image analysis. Histol Histopathol. 2006;21:951–6. doi: 10.14670/HH-21.951. [DOI] [PubMed] [Google Scholar]
- 13.Krajewska M, Kim H, Kim C, et al. Analysis of apoptosis protein expression in early-stage colorectal cancer suggests opportunities for new prognostic biomarkers. Clin Cancer Res. 2005;11:5451–61. doi: 10.1158/1078-0432.CCR-05-0094. [DOI] [PubMed] [Google Scholar]
- 14.Krajewska M, Kim H, Shin E, et al. Tumor-associated alterations in caspase-14 expression in epithelial malignancies. Clin Cancer Res. 2005;11:5462–71. doi: 10.1158/1078-0432.CCR-04-2527. [DOI] [PubMed] [Google Scholar]
- 15.Krajewska M, Olson AH, Mercola D, Reed JC, Krajewski S. Claudin-1immunohistochemistry for distinguishing malignant from benign epithelial lesions of prostate. Prostate. 2007;67:907–10. doi: 10.1002/pros.20578. [DOI] [PubMed] [Google Scholar]
- 16.Meinhold-Heerlein I, Stenner-Liewen F, Liewen H, et al. Expression and potential role of Fas-associated phosphatase-1 (FAP-1) in ovarian cancer. Am J Pathol. 2001;158:1335–44. doi: 10.1016/S0002-9440(10)64084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krajewska M, Krajewski S, Banares S, et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9:4914–25. [PubMed] [Google Scholar]
- 18.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–21. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 19.Krajewski S, Krajewska M, Ellerby LM, et al. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:5752–7. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krajewska M, Zapata JM, Meinhold-Heerlein I, et al. Expression of Bcl-2 family member Bid in normal and malignant tissues. Neoplasia. 2002;4:129–40. doi: 10.1038/sj.neo.7900222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–9. [PubMed] [Google Scholar]
- 22.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 23.Krajewski S, Zapata JM, Reed JC. Detection of multiple antigens on Western blots. Anal Biochem. 1996;236:221–8. doi: 10.1006/abio.1996.0160. [DOI] [PubMed] [Google Scholar]
- 24.Krajewski S, Bodrug S, Krajewska M, et al. Immunohistochemical analysis of Mcl-1 protein in human tissues: differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1in control of programmed cell death in vivo. Am J Pathol. 1995;146:1309–19. [PMC free article] [PubMed] [Google Scholar]
- 25.Elsaleh H. The microsatellite instability phenotype in human colorectal carcinoma: relationship to sex, age, and tumor site. Gastroenterology. 2001;121:230–1. doi: 10.1053/gast.2001.26044. [DOI] [PubMed] [Google Scholar]
- 26.Manz RA, Moser K, Burmester GR, Radbruch A, Hiepe F. Immunological memory stabilizing autoreactivity. Curr Top Microbiol Immunol. 2006;305:241–57. doi: 10.1007/3-540-29714-6_12. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–42. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 28.Radbruch A, Muehlinghaus G, Luger EO, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med. 2005;202:1471–6. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oancea M, Mani A, Hussein MA, Almasan A. Apoptosis of multiple myeloma. Int J Hematol. 2004;80:224–31. doi: 10.1532/IJH97.04107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauhan D, Velankar M, Brahmandam M, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26:2374–80. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 32.Le Gouill S, Podar K, Harousseau JL, Anderson KC. Mcl-1 regulation and its role in multiple myeloma. Cell Cycle. 2004;3:1259–62. doi: 10.4161/cc.3.10.1196. [DOI] [PubMed] [Google Scholar]
- 33.Cheng WC, Berman SB, Ivanovska I, et al. Mitochondrial factors with dual roles in death and survival. Oncogene. 2006;25:4697–705. doi: 10.1038/sj.onc.1209596. [DOI] [PubMed] [Google Scholar]
- 34.Cheng E, Clem R, Ravi R, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–8. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 35.Clem RJ, Cheng EH, Karp CL, et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci U S A. 1998;95:554–9. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bivona TG, Quatela SE, Bodemann BO, et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell. 2006;21:481–93. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]


