Abstract
Pluripotency manifests during mammalian development through formation of the epiblast, founder tissue of the embryo proper. Rodent pluripotent stem cells can be considered as two distinct states: naïve and primed. Naïve pluripotent stem cell lines are distinguished from primed cells by self-renewal in response to LIF signaling and MEK/GSK3 inhibition (LIF/2i conditions) and two active X chromosomes in female cells. In rodent cells, the naïve pluripotent state may be accessed through at least three routes: explantation of the inner cell mass, somatic cell reprogramming by ectopic Oct4, Sox2, Klf4, and C-myc, and direct reversion of primed post-implantation-associated epiblast stem cells (EpiSCs). In contrast to their rodent counterparts, human embryonic stem cells and induced pluripotent stem cells more closely resemble rodent primed EpiSCs. A critical question is whether naïve human pluripotent stem cells with bona fide features of both a pluripotent state and naïve-specific features can be obtained. In this review, we outline current understanding of the differences between these pluripotent states in mice, new perspectives on the origins of naïve pluripotency in rodents, and recent attempts to apply the rodent paradigm to capture naïve pluripotency in human cells. Unraveling how to stably induce naïve pluripotency in human cells will influence the full realization of human pluripotent stem cell biology and medicine.
Introduction
Pluripotency is defined as the capacity of a single cell to generate all cell lineages of the developing and adult organism. This is a property of a transient population of unrestricted cells known as the epiblast, which forms the embryo proper in vivo. Epiblast cells from pre-implantation rodent embryos can be perpetually expanded in culture as embryonic stem cell (ESC) lines [1,2]. In vitro, the ‘naïve’ ESCs self-renew indefinitely without genetic transformation, can be expanded clonally, and retain pluripotency. Furthermore, naïve ESCs are amenable to homologous recombination, which has allowed for extensive genetic dissection of the mouse genome.
The recent generation of primed epiblast stem cells (EpiSCs) evolved the view that pluripotent stem cell lines may exist as two distinct, stable pluripotent states: naïve and primed [3-5]. Both cell states exhibit features of bona fide pluripotent cell lines, including indefinite self-renewal, tri-germ layer potential and reliance on core transcription factors OCT4, SOX2, and NANOG. Human ESCs and iPSCs share defining features with primed mouse EpiSCs and not naïve mESCs, therefore embodying the primed state. Definitive evidence for a non-rodent naïve pluripotent state is lacking, which suggests that the naïve pluripotent state in vitro may be a rodent-specific phenomenon. However, there is growing interest in deriving naïve pluripotent stem cell lines from humans. Although recent advances in gene editing technology have improved the accessibility of primed hESCs/iPSCs for genetic intervention, naïve human pluripotent stem cells would further accelerate dissection of the human genome by permitting translation of gene targeting technologies previously limited to the mouse. In this review, we highlight advances in understanding rodent naïve and primed pluripotent stem cells and recent attempts to stabilize naïve human pluripotency via three routes: directly from pre-implantation embryos, through reprogramming of somatic cells, and through reversion of primed pluripotent cells.
Current ‘primed’ human ES and iPS cells
Naïve pluripotency is represented by the newly segregated pre-implantation epiblast and rodent naïve ESCs [6,7•]. The attainment of naïve pluripotency in vivo and ex vivo is demarcated by two active female X chromosomes and co-expression of pluripotent-associated transcription factors OCT4, SOX2, and NANOG. Following implantation, extraembryonic and autoinductive signaling prime the epiblast for differentiation and one female X chromosome is inactivated, but expression of OCT4, SOX2, and NANOG is retained. Primed pluripotency is exhibited in vitro by epiblast stem cell lines (EpiSCs) initially derived from post-implantation epiblasts and more recently, preimplantation blastocysts [4,5,8•]. Although human embryonic stem cells are derived from preimplantation blastocysts, they more closely resemble post-implantation EpiSCs and preimplantation blastocyst-derived EpiSCs [4,5,8•,9]. The derivation of EpiSCs from murine preimplantation blastocysts suggests human ICM outgrowth cells may progress to an EpiSC-like state during conventional hESC derivation protocols.
Naïve and primed pluripotent stem cells respond to different signaling pathways to sustain and exit the self-renewing state. Unlike mESCs, hESCs do not respond to LIF/STAT3 or 2i, cannot be efficiently propagated clonally, and respond to cooperative signaling between FGF and ACTIVIN/NODAL [4,10,11]. In both hESCs and mEpiSCs, NANOG expression depends on SMAD2/3 signaling. The state-specific divergence in self-renewal mechanism extends to differential response to differentiation-inducing cues. While FGF inhibition promotes mESC self-renewal, FGF/ERK inhibition promotes neuroectoderm commitment of hESCs, hiPSCs and EpiSCs [12•,13••]. Additionally, BMP4 cooperates with LIF to facilitate mESC self-renewal but induces extraembryonic phenotypes in mEpiSCs and hESCs/iPSCs [14,15]. The shared signaling responsiveness of mouse EpiSCs and human ESCs/iPSCs suggests that these cell lines represent mouse and human orthologs of a primed pluripotent cell state.
Because the chimera assay cannot be used in humans, recent studies have sought to clarify the relationship between naïve and primed rodent cell lines and hESCs/hiPSCs. A key property that distinguishes rodent naïve and primed pluripotent stem cells is the unique ability of naïve pluripotent stem cells to generate highgrade chimeras upon re-introduction into the pre-implantation blastocyst. Rodent EpiSCs cannot generate highgrade chimeras upon introduction into morula stage embryos or blastocysts, nor efficiently contribute to the germline. More recently, the rodent paradigm was tested in primates. Similarly to mEpiSCs, rhesus monkey ESCs also cannot efficiently home into inner cell mass of preimplantation blastocysts to generate high-grade chimeric monkeys [16••]. Additionally, when rhesus monkey ESCs are introduced into pre-blastocyst stage four-cell stage embryos, intermingling of rhesus monkey ESCs with ICM cells during blastocyst formation can be observed, but differentiation or death is observed in the pre-implantation environment [16••]. These data support the view that current primate ESCs correspond to a rodent primed pluripotent state (Figure 1).
Figure 1.
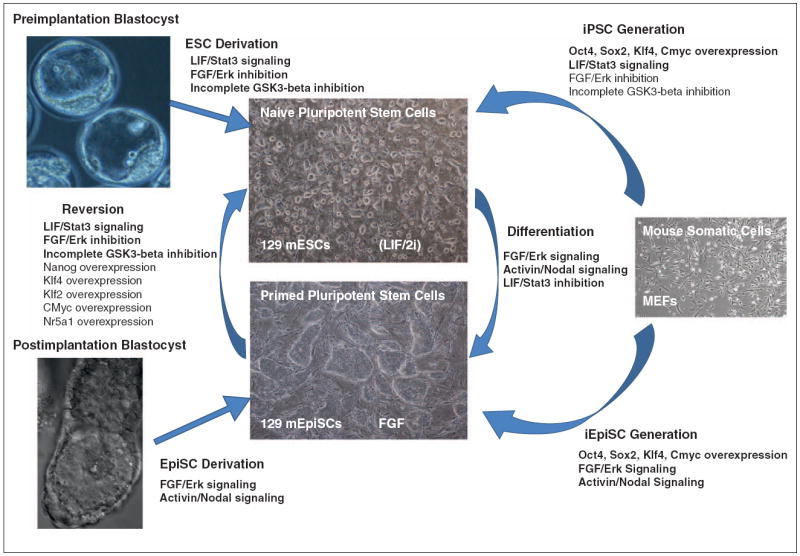
Accessing rodent naïve pluripotency through three different routes.
Pluripotent stem cells may be derived from in vivo sources such as the preimplantation blastocyst or the postimplantation epiblast, resulting in naïve mouse ESCs or primed EpiSCs respectively. Naïve iPSCs or primed iEpiSCs may be obtained by altering the cultural conditions during somatic cell reprogramming. Finally, naïve and primed pluripotent stem cells are directly interconvertible by differentiation of naïve pluripotent stem cells to primed pluripotent stem cells, or direct reversion of primed pluripotent stem cells to a naïve state. Therefore, naïve pluripotency may be captured in vitro in the form of (a) embryonic stem cells (ESCs) during ESC derivation, (b) induced pluripotent stem cells (iPSCs) during somatic cell reprogramming, or (c) Epi-iPSCs during reversion of EpiSCs to a naïve mESC-like state.
In certain respects regarding transcriptional regulation and marker expression, human ESCs appear to differ from mouse EpiSCs. Whereas disturbance of FGF2/ERK does not influence Nanog in mouse EpiSCs, inhibition of FGF/ERK signaling pathway in human ESCs rapidly downregulates NANOG [12•]. Additionally, in human ESCs, OCT4 binds to the FGF2 promoter establishing an autocrine loop, whereas in mouse EpiSCs, no evidence of regulation of Fgf2 by Oct4 was observed [12•]. Finally, human ESCs share several molecular features associated with mESCs, but not with EpiSCs. For instance, hESCs express the ICM-associated marker REX1, like naïve mESCs, but not EpiSCs; hESCs do not express FGF5, a key EpiSC-associated marker not expressed in mESCs. The tighter rewiring of FGF signaling to the core pluripotent transcription factors OCT4 and NANOG in humans may complicate simple application of the rodent paradigm to humans. An improved classification of the similarities and differences between hESCs and their rodent counterparts, EpiSCs, will sharpen our understanding of what it means to be naïve or primed.
Hence, a body of evidence suggests hESCs/iPSCs functionally resemble a primed EpiSC-like pluripotent state observed in rodents. Given the species-specific differences observed in analogous EpiSCs and hESCs/iPSCs, disentanglement of species-specific and state-specific differences is highly relevant for precisely defining naïve and primed pluripotent states in humans.
Capture of naïve pluripotency from rodent embryos
The relationship between cultured pluripotent stem cell lines and resident cells in the embryo is uncertain [17], but resolving the present gap in knowledge concerning the mechanisms that lead to stable pluripotency in vitro may facilitate the derivation of naïve human pluripotent cells.
Historically, naïve pluripotent stem cells are only readily derived from the 129 mouse strain, suggesting intrinsic genetic features in the 129 strain promote entry into or stabilization of naïve pluripotency [18]. It is curious that strain 129 is also predisposed toward testicular germ cell tumors (TGCTs). One study that investigated the genetic basis for 129 permissivity identified individual chromosomes that harbor susceptibility genes for TGCTs [19]. 129-Chr18(MOLF) males are resistant to spontaneous TGCTs, and four regions within chromosome 18 control this susceptibility. When ESC derivation efficiency in LIF/serum was investigated in 129-Chr 18(MOLF), derivation was significantly reduced. Thus, genetic elements contributing to the formation of TGCTs from primordial germ cells contribute to mESC derivation. Intriguingly, when EpiSCs are isolated from preimplantation embryos of 129-Chr18(MOLF) or NOD strains, EpiSCs were obtained at a similar frequencies to strain 129 (~25%) [8•]. Thus, while strain 129 genetic elements modulate entry or stabilization of the naïve pluripotent state, strain-specific genetic elements restricting access to the naïve state do not appear to impact access to the primed state. The generalizability of this principle can be witnessed in the observation that ESCs from other non-rodent mammalian species resemble EpiSCs and human ESCs.
A breakthrough in overcoming the barriers imposed by mouse genetic background was the development of 3i/2i, which involves inhibitors of FGFR, MEK, and GSK3 (3i) or MEK and GSK3 (2i) [20]. To identify Stat3-independent modes of pluripotency, Ying and colleagues applied 3i or 2i to Stat3-KO and recalcitrant mice strains, such as NOD, and successfully stabilized naïve pluripotency in recalcitrant mouse strains. Because chemokine stimulation of Stat3 was not required, Ying and colleagues claimed 2i-cultured mouse ESCs to reside in a novel state of ground state pluripotency, distinct from LIF/serum conditions. Thereafter, LIF/2i stabilized naive rat embryonic stem cells (rESCs) from the SD and DA rat strains [21,22]. These rESCs expressed naïve-associated markers such as Rex1, Klf4, and Tbx3. rESCs exhibited key functional features of naïve pluripotency such as chimerism potential, germ line colonization, and two active X chromosomes in females, a naïve molecular signature. Later, homologous recombination was applied and p53-KO rats were generated. These studies confirm capture of naïve pluripotent cells from a non-murine embryo and suggest the broader utility of naïve pluripotency for transgenesis [23••].
Are naïve pluripotency and ground state pluripotency fully interchangeable terms? Recent attention has focused on identifying intermediate states leading to stable naïve pluripotency. Using cell-fate mapping strategies and single-cell gene expression profiling to examine ICM outgrowths adaptation to LIF/serum, a germ cell-like precursor state was demonstrated as facultative for mESC generation [24]. ICM outgrowths showed primordial germ cell (PGC)-associated gene enrichment, functional resemblance to PGCs, and a propensity to transition to pluripotency. Interestingly, when 2i was applied to blastocysts carrying Blimp1-Cre and floxed-RFP, the cells were RFP-negative. Thus, classical LIF/serum and 2i may operate through distinct modes to promote entry into naïve pluripotency. Whereas LIF/serum involves transcriptional reprogramming of a few ICM cells with PGC-like potential, 2imay allow ‘direct capture’ of a broader population of epiblast cells with the potential to become naïve ESCs, circumventing the need to pass through a germ-cell state. In contrast to transitioning through a germcell state inLIF/serum conditions, 2i is believed to operate by altering the proportions of epiblast and hypoblast progenitors in favor of epiblast progenitors in both mouse and rat embryos, a bias that may also be operative during ICM outgrowths when ESCs are derived [25,26•]. The recent observation that mouse ESCs cultured in 2i or in LIF/serum conditions differ with respect to biallelic versus monoallelic expression of Nanog, with 2imESCs corresponding to the state of Nanog transcription in the mature epiblast, supports the view that 2i allows capture of cells that correspond very closely to pluripotent cells of the pre-implantation embryo [27••].
Toward naive human embryonic stem cells
Three studies have described attempts to isolate naïve ESCs from human preimplantation embryos. A recent study reported that female hESCs derived in 5% oxygen retain two active X chromosomes, whereas in atmospheric oxygen, XIST upregulation and an inactive X chromosome are found [28•]. These hESCs were derived in conventional hESC derivation medium containing FGF. These data complement a recent study [7] reporting that female human embryos contain a compartment with two active X chromosomes by demonstrating that ESC lines with two active X chromosomes, a feature of naïve ESCs, can be derived from human preimplantation embryos. However, these cell lines were maintained in conventional human ES conditions containing FGF, were not demonstrated to self-renew in 2i, nor were other features associated with naïve mESCs investigated.
Two additional studies examined whether human preimplantation embryos contain a compartment from which naïve human ES cells could be derived by applying 2i cultural conditions to thawed human embryos and fresh human embryos deemed unsuitable for transfer by preimplantation genetic diagnosis (PGD) analysis [26•,29]. In the first of these studies, the authors first investigated the timing of epiblast segregation in human embryos. The detection of exclusive staining of NANOG (epiblast-specific) and GATA6/GATA4/SOX17 (hypoblast-specific) in day 7 human embryos suggested this stage corresponds to the mouse embryo at E4.5 when all three embryonic lineages – trophectoderm, hypoblast, and epiblast – can be distinguished. The observation of human hypoblast at day 7 prompted various inhibitor treatments initiated at day 3 of development, presumably before ICM has segregated into epiblast and hypoblast. When treated with FGF inhibitors or 2i (in physiological oxygen concentrations), the GATA4-positive human hypoblast forms under conditions where it is blocked in mouse and rat embryos, indicating that human hypoblast specification does not rely on FGF. The second of these studies extended Roode et al.’s conclusions to fresh human embryos and the absence of physiological oxygen concentrations [29]. Whether the failure of 2i to suppress hypoblast or additional barriers explain the failure to derive naïveh ESCs remains an area of future investigation.
Nichols and colleagues found when human embryos are cultured in FGF/ERK inhibitors or in 2i, the NANOG-positive epiblast compartment is sustained, which would not be expected If the embryonic NANOG-positive cells corresponded to primed human ES cells. The survival of embryonic NANOG-positive cells provides hope for isolating cells independent of FGF/ERK signaling. Future investigation of whether these ERK-independent, NANOG-positive cells reside in a pre-XCI state would support existence of a transient naïve population in the early human epiblast [7•,26•]. Additionally, whether biallelic expression of NANOG also distinguishes the attainment of ground state pluripotency in the human epiblast, as has been observed in the mouse, remains to be clarified. Finally, a description of the response of the human ICM outgrowth to the combined effect of hypoxia and 2i could inform strategies to facilitate the derivation of naïve human ES cells from ICM outgrowths. These studies highlight the limitations of the rodent model and suggest how little is known about development of the human blastocyst and in particular, the human epiblast.
Direct reprogramming of somatic cells to naïve pluripotency
Direct reprogramming of human cells provides another platform to investigate derivation of naïve human pluripotent stem cell lines [30,31]. Ectopic expression of Oct4, Sox2, Klf4, and C-myc in LIF generates naïve mouse iPSCs [32], whereas ectopic expression of Oct4, Sox2, Klf4, and C-myc in EpiSC conditions yields iEpiSCs [33•]. These two studies suggest culture conditions dictate the terminal pluripotent state achieved following reprogramming factor induction in mice.
In the first report of human iPS cells, Yamanaka and colleagues stated that retroviral OCT4, SOX2, KLF4, and C-MYC fail to generate naïve hiPSCs in classical mouse ES cell conditions, but their attempts were not specified in detail [30]. Several groups have generated ‘mESC-like’ ‘hiPSCs’ by applying naïve culture conditions following reprogramming factor induction, but the cell lines lack the robust nature that distinguishes bona fide mouse ESCs/iPSCs (Figure 2; Table 1).
Figure 2.
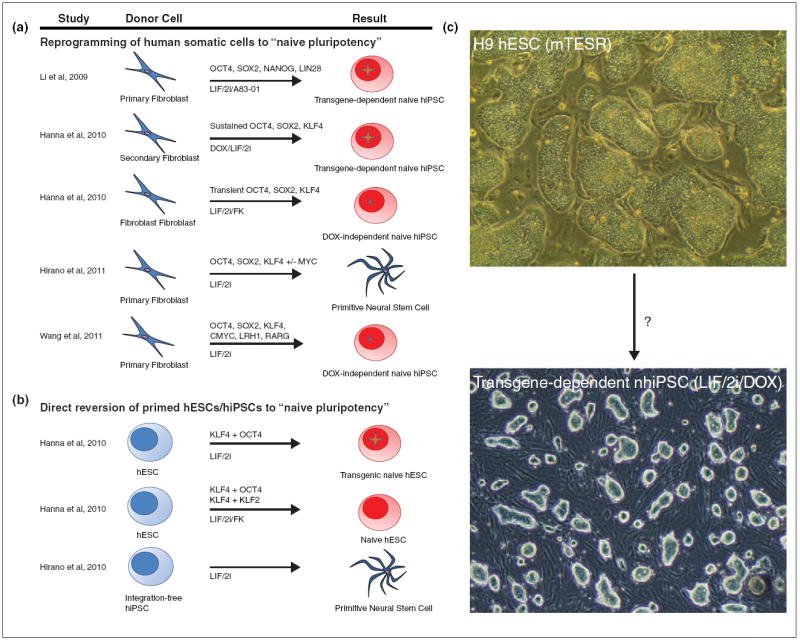
Attempts to generate naïve human pluripotent stem cells through direct reprogramming or reversion of hESCs/hiPSCs. Several groups have attempted to access the naïve human pluripotent state through (a) direct reprogramming of primary somatic cells or secondary fibroblasts, (b) reversion of conventional ‘primed’ human ESCs, iPSCs, or ‘secondary’ human iPSCs. (a) Direct reprogramming of human cells using constitutive lentiviruses, inducible lentiviruses, retroviruses, or Piggybac Transposon has given rise to transgene-dependent naïve human iPS cells, transgene-independent naïve human iPS cells and primitive neural stem cells. (b) Reversion of conventional human ESCs/iPSCs by stable or transfection of OCT4, KLF2, KLF4 and transfer into LIF/2i or LIF/2i/FK yields transgene-dependent or transgene-independent naïve human iPSCs; transfer and selection of integrationfree human iPS cells in LIF/2i yields primitive neural stem cells. (c) Can primed hESCs be converted directly into a stable naïve pluripotent state? Primed human ESC line H9 cultured in mTESR medium (top) and transgene-dependent naïve human iPS cell line cultured in LIF/2i/DOX conditions. The transgene-dependent nhiPSC line is dependent on continuous induction of a transgene-cassette encoding the four factors OCT4, SOX2, KLF4, and CMYC and was generated from the BJ fibroblast cell line. 2i = MEK inhibitor PD0325901 and GSK3-beta inhibitor CHIR99021. FK = Forskolin. rtTA = reverse tetracycline transactivator. Large star indicates self-renewal is sustained by high levels of transgenes. Small star indicates the presence of residual transgenes.
Table 1.
Attempts to generate naïve human iPS cells
| Transgene delivery method |
Transgene | Donor cell line |
Culture conditions |
Marker expression |
Functional pluripotency |
Functionally naïve? |
Stability | Transgene independence |
|
|---|---|---|---|---|---|---|---|---|---|
| Li et al., 2009 | pSIN-EF1A lentivirus | OCT4, SOX2, NANOG, LIN28 | IMR90 | LIF, PD03, CHIR, A-83-01 | SSEA3, SSEA4, TRA1-60, TRA1-81 | In vitro differentiation, Teratomas | Self-renewal in MEK/ALK5 inhibitors | >30 passages (mESC conditions) | Yes |
| Ware et al., 2009 | H9 human ESC | Sodium butyrate | SSEA3, SSEA4, TRA1-60, TRA1-81 | In vitro differentiation, Teratomas | XIST extinction | Enzymatic | Yes | ||
| Lengner et al., 2010 | Human blastocyst | FGF, 5% oxygen | SSEA3, SSEA4, TRA1-60, TRA1-81 | In vitro differentiation | XIST extinction | Sensitivity to cryopreservation and oxidative stress | Yes | ||
| Hanna et al., 2010 | FUW-tetO lentivirus | OCT4, SOX2, KLF4 | C1 secondary fibroblast | LIF, PD03, CHIR, DOX | SSEA3, SSEA4, TRA1-60, TRA1-81 | Teratomas | XIST extinction, signaling dependency | >50 passages (Trypsin) | No |
| Hanna et al., 2010 | pCAG | OCT4, KLF4 | WIBR3 hESC | LIF, PD03, CHIR | SSEA3, SSEA4, TRA1-60, TRA1-81 | Teratomas | XIST extinction, signaling dependency | No | |
| Hanna et al., 2010 | pCAG | OCT4, KLF4 | WIBR3 hESC | LIF, PD03, CHIR, Forskolin | SSEA3, SSEA4, TRA1-60, TRA1-81 | Teratomas | XIST extinction, signaling dependency | ~15 passages (Trypsin) | Yes |
| Hanna et al., 2010 | pCAG | KLF4, KLF2 | WIBR3 hESC | LIF, PD03, CHIR, Forskolin | SSEA3, SSEA4, TRA1-60, TRA1-81 | Teratomas | XIST extinction, signaling dependency | ~15 passages (trypsin) | Yes |
| Transgene delivery method | Transgene | Donor cell line | Culture conditions | Marker expression | Functional pluripotency | Functionally naïve? | Stability | Transgene independence | |
|
| |||||||||
| Buecker et al., 2010 | FUW-tetO | OCT4, SOX2, KLF4, CMYC, NANOG | Secondary fibroblast or primary human fibroblast | LIF, DOX | SSEA1 | Not pluripotent | LIF-responsiveness, amenability to homologous recombination | No | |
| Xu et al., 2010 | hESC | LIF, PD03, SB | SSEA3, SSEA4, TRA-1-60, TRA-1-81 | In vitro differentiation | Not determined | >30 passages | Yes | ||
| Transgene delivery metdod | Transgene | Donor cell line | Culture conditions | Marker expression | Functional pluripotency | Functionally naïve? | Stability | Transgene independence | |
|
| |||||||||
| Pompe et al., 2011 | pSIN-EF1A lentivirus | OCT4, SOX2, NANOG, LIN28 | LIF, PD03, CHIR, Forskolin | Mixed SSEA1+/TRA-1-60+ | Growth in 2i, Female H3K27me3 focus not observed | No | |||
| Hirano et al., 2011 | pMX retrovirus | OCT4, SOX2, KLF4 +/− CMYC | TIG1/TIG3 fetal lung fibroblasts, adult dermal fibroblasts | LIF, PD03, CHIR | Nestin-positive, but SSEA1-, TRA1-60- | Neural (but EB detection of T and GATA4) | No, Female H3K27me3 focus observed | ~50 passages (mechanical dissociation) | Transgenes detected |
| Hirano et al., 2011 | Nonintegrated hiPSC | LIF, PD03, CHIR | Nestin-positive, but SSEA1-, TRA1-60- | Neural (but EB detection of T and GATA4) | No, H3K27me3 focus observed | >50 passages (mechanical dissociation) | Transgenes detected | ||
| Wang et al., 2011 | Inducible PiggyBac | rtTA, OCT4, SOX2, KLF4, CMYC, LRH1, RARG | Neonatal fibroblast, adult fibroblast | LIF, PD03, CHIR | SSEA3, SSEA4, TRA-1-60, TRA-1-81 | In vitro differentiation, Teratomas | Upregulation of X-linked genes, XIST downregulation | >50 passages (Accutase) | Yes |
The first successful attempt to obtain hiPSCs that resemble mESCs used a combination of lentiviral OCT4, SOX2, NANOG and LIN28 and transfer into LIF/2i and a pan-ALK4/5/7-inhibitor A-83-01 [34]. The obtained cells were maintained for over 20 passages, exhibited reactivation of endogenous OCT4, SOX2 and NANOG, and generated teratomas. LIF-dependency and resistance to MEK inhibition and pan-ALK4/5/7- inhibition suggested a naïve signaling dependency but less efficient silencing of pSIN-EF1-alpha lentiviruses compared with the MMLV-based retroviruses confounds claims of transgene-independence [31]. Indeed, when OCT4 or NANOG is overexpressed in hESCs, the hESCs do not ‘default’ into neuroectoderm when challenged with FGF/ERK inhibition [13••]. Another study employed pSIN-EF1-alpha lentiviral delivery of OSNL, but used drug selection to select for high levels of OSNL transgenes, suggesting the unstable nature of these cell lines [35].
Jaenisch and colleagues reported the envisioned naïve hiPSCs derived from secondary human fibroblasts and hESCs [36,37]. The authors obtained transgene-dependent (OCT4/SOX2/KLF4) cells that required continuous transgene induction in combination with LIF/2i. The transgene-dependent cell lines expressed a hESC-like surface marker profile (SSEA3/SSEA4/TRA-1-60/TRA- 1-81-positive and SSEA1-negative), generated teratomas and exhibited extinction of XIST transcription, a feature associated with two female X chromosomes. To replace transgenes, the Protein Kinase A agonist Forskolin transiently replaced doxycycline and ‘transgene-independent’ cell lines were maintained in LIF/2i/Forskolin for about 15 passages.
The findings in Hanna et al., 2010 contrast with the findings of Tada and colleagues [38]. When retroviral OCT4, SOX2, KLF4 and C-MYC are delivered into primary human fibroblasts and cultured in LIF/2i, the obtained cell lines resemble primitive neural stem cells that retain low levels of OCT4 and NANOG, but high levels of SOX2 expression. Domed-shaped cell lines from integration-free human iPS cells but not human ES cells were obtained upon selection of domed-shaped colonies in LIF/2i. These cells could be mechanically passaged over 50 times. The neural observation resembles another group’s attempt to revert human ES cells to a naïve state [39]. The authors speculate that mouse and human cells differentially respond to LIF/2i and this could account for the failure to derive naïve human iPS cells.
Another study produced mESC-like human ‘iPSCs’ using DOX-inducible lentiviruses for OCT4, SOX2, KLF4, CMYC, and NANOG in LIF [40]. However, the hLR5 state relied on continuous expression of reprogramming factors and expressed high levels of SSEA-1, a mESC marker absent in hESCs. The cell lines generated possessed some mESC-like features such as high clonogenicity and amenability to homologous recombination. Critically, the endogenous OCT4 and NANOG regulatory regions were not reactivated (i.e. H3K4me3 mark), but were bivalent (i.e. H3K4me3 and H3K27me3 marks), suggesting these loci were ‘poised’ for activation. The bivalent status of OCT4 and NANOG prompted the authors to investigate conversion of hLR5 cells into primed human iPSCs. The resulting primed hLR5-iPSCs generated teratomas and expressed markers associated with bona fide primed human iPS cells.
A more recent report described the generation of naïve hiPSCs by inducible PiggyBac delivery of RARG and LRH1 in combination with OCT4, SOX2, KLF4, and CMYC [41]. Similar to Hanna et al., 2010, their cells could be propagated in the absence of doxycycline, grew in LIF/2i, and generated teratomas. However, PiggyBac transposons are not subjected to the same natural silencing process that diminishes retroviral (and less extensively lentiviral) expression. Like previous studies, their study failed to definitively demonstrate a transgene-independent state.
Quasi-pluripotent human cells that resemble mouse ES/iPSCs have been generated by combining canonical reprogramming factor overexpression with LIF and variations on the 2i cocktail. However, the resulting cells are unstable and probably nonequivalent across protocols. The cells described are transgene-dependent, lack stable endogenous expression of OCT4 and NANOG, or resemble primitive neural stem cells. Future attempts to generate naïve hiPSCs will require definitively ruling out the contribution of transgenic expression because stable transgene expression is not desirable for generating clinically relevant cell types.
Direct reversion of EpiSCs to naïve pluripotency
The murine naïve and primed states are readily inter-convertible. When naïve mESCs are cultured in FGF/Activin A, they adopt a primed state [42]. Conversely, gain-of-function studies with EpiSCs identified transcription that can reset primed pluripotency to naïve pluripotency (Table 2). Overexpression of Nanog, Klf4, Klf2, Stat3, Nr5a1, Nr5a2, C-Myc or continuous culture in LIF/serum, LIF/2i reverts 129 EpiSCs to naïve pluripotency [42-48]. Recalcitrant NOD EpiSCs can be reverted by Klf4 or Cmyc and/or culture in 2i or KP/CH culture [47]. The mechanism of the drug kenpaullone’s function remains unknown [49].
Table 2.
Proteins implicated in reversion of EpiSCs to a mESC-like state
| Protein | Tissue distribution | Gain-of-function or loss-of function phenotype in mice/ESCs | Reversion mouse background | Reversion culture condition | Reversion tissue origin | Refs |
|---|---|---|---|---|---|---|
| LIF/STAT3 | Gp130 knockout eliminates capacity for diapause; Stat3 activation significantly enhances EpiSC reversion to naïve pluripotency | 129 | LIF/serum | Postimplantation epiblast | Bao et al., 2009; Yang et al., 2010; All LIF/2i papers | |
| FGF/ERK | Nearly ubiquitous signaling pathway | Fgf4-null or Erk2-null ES cells blocked lineage commitment; | 129, NOD | N2B27-LIF/2i; KSR-LIF/2i | In vitro-derived EpiSC; Embryo-derived EpiSC | Kunath et al., 2007; Stavridis et al., 2007; All LIF/2i papers |
| WNT/GSK3-Beta | Nearly ubiquitous signaling pathway | Promotes self-renewal of mESCs/hESCs; GSK3 inhibition in combination with ERK inhibition allows efficient derivation of naïve mESCs | 129, NOD | N2B27-LIF/2i; KSR-LIF/2i | Sato et al., 2004; Ying et al., 2008; Ten Berge et al., 2011; All LIF/2i papers; | |
| Klf4 | High expression in gut; Highly expressed in naïve ESCs | Overexpression sustains LIF-independent self-renewal | 129, NOD | N2B27-LIF/2i | In vitro-derived EpiSC; iEpiSC; NOD ICM outgrowth; NOD EpiSC | Guo et al., 2009; Hanna et al., 2009; Han et al., 2011 |
| C-Myc | Highly expressed in proliferating cells | Overexpression sustains LIF-independent self-renewal | 129, NOD | N2B27-LIF/2i | NOD ICM outgrowth; NOD EpiSC | Cartwright et al., 2005; Hanna et al., 2009 |
| Nanog | Inner cell mass, germ cells; Highly expressed in naïve ESCs | In vivo knockout ICM fails to form; conditional knockout increases ES cell propensity for differentiation; Overexpression in mESCs sustains LIF-independent self-renewal; overexpression in EpiSCs reverts mESCs to pluripotency | 129 | N2B27-LIF/2i | In vitro-derived EpiSC | Silva et al, 2009 |
| Klf2 | High expression in lung; Highly expressed in naïve ESCs | Overexpression in mESCs sustains LIF-independent self-renewal; Knockdown reduces efficiency of EpiSC reversion to naïve pluripotency; replacement of exogenous Klf4 for somatic cell reprogramming | 129 | N2B27-LIF/2i | In vitro-derived EpiSC | Hall et al, 2009 |
| Nr5a1 | High expression in adrenal tissues | Overexpression reverts EpiSCs to naïve pluripotency; replacement of exogenous Oct4 for somatic cell reprogramming | 129 | N2B27-LIF/2i | In vitro-derived EpiSC | Guo et al, 2010 |
| Nr5a2 | High expression in liver cells; Expressed in naïve ESCs | Overexpression reverts EpiSCs to naïve pluripotency; replacement of exogenous Oct4 for somatic cell reprogramming | 129 | N2B27-LIF/2i | In vitro-derived EpiSC | Guo et al, 2010; Heng et al, 2010 |
| Mouse background | Donor cell type | TF transfection? | Transgene delivery method | Transgene | Culture conditions | Naïve pluripotency | |
|---|---|---|---|---|---|---|---|
| Guo et al., 2009 | 129 | EpiSC | Yes | PiggyBac | Klf4 | N2B27-LIF-PD03/CH | Yes |
| Hanna et al., 2009 | NOD | ICM outgrowth | Yes | FUW-Ubc or FUW-tetO | Klf4/Cmyc | Serum + LIF | Yes |
| Hanna et al., 2009 | NOD | EpiSC | Yes | FUW-Ubc or FUW-tetO | Klf4/Cmyc | Serum + LIF | Yes |
| Hanna et al., 2009 | NOD | ICM outgrowth | No | PD/CH or KP/CH | Yes | ||
| Hanna et al., 2009 | NOD | EpiSC | No | PD/CH or KP/CH | Yes | ||
| Silva et al., 2009 | 129 | EpiSC | Yes | PiggyBac | Nanog | PD/CH | Yes |
| Bao et al., 2009 | 129 | Postimplantationepiblast | No | LIF/serum | Yes | ||
| Hall et al., 2009 | 129 | EpiSC | Yes | PiggyBac | Klf2 | PD/CH | Yes |
| Greber et al., 2010 | 129 | EpiSC | No | KSR-PD/CH | Yes | ||
| Han et al., 2011 | 129 | iEpiSC | Yes | Lentivirus | Klf4 | Yes | |
| Yang et al., 2010 | 129 | EpiSC | Yes | PiggyBac | Stat3 | PD/CH | Yes |
| Guo et al., 2010 | 129 | EpiSC | Yes | PiggyBac | Nr5a1 | PD/CH | Yes |
Few attempts to revert primed hESCs to naïve pluripotency are described. Blau and colleagues used histone deacetylase (HDAC) inhibitors such as butyrate to pull hESCs toward an earlier stage [50]. Notably, sodium butyrate maintained hESCs in the absence of FGF. The butyrate-cultured human ES cells lost XIST expression but retained teratoma potential. However, findings were not extended beyond the H9 hESC line and butyrate hESCs did not possess other features of mESCs. Since extinction of XIST expression can be uncoupled from X chromosome status in female hESCs, additional assays to assess X chromosome reactivation status in female butyrate-cultured hESCs are needed [51]. Further, it will be interesting to determine whether sodium butyrate supports hESC derivation from preimplantation embryos. IDPPA5 is described as a key gene induced in butyrate hESCs because Dppa5 overexpression in EpiSCs endows EpiSCs with the potential to incorporate into preimplantation embryos, a feature associated with but not necessarily a definitive feature of naïve mESCs [52•].
Conclusions
The mouse has been the most important model organism for generating hypotheses about maintenance and differentiation of pluripotent stem cells, but the limited applicability of mouse ES cell principles across species, especially humans, challenges the relevance of rodent research to early human development. While naïve ESCs from multiple mouse strains and rats present new opportunities for genetic intervention and have clarified the nature of naïve pluripotency beyond mouse strain 129, the simple application of 2i to direct reprogramming or preimplantation human embryos fails to yield naïve human ES/iPS cells; indeed, definitive evidence for stable naïve human cells is lacking. Additional studies regarding the functional behavior of embryo-derived and reprogrammed-derived pluripotent cells will be needed to identify the best way to induce human pluripotency.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Evans M, Kaufman M. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols J, Smith A. Naïve and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;488:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 5.Brons LG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun P, Chuva de Sousa Lps SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science. 2003;303:644–649. doi: 10.1126/science.1092727. [DOI] [PubMed] [Google Scholar]
- 7•.Okamoto I, Patrat C, Thepot D, Peynot N, Fauque P, Daniel N, Diabangouaya P, Wolf JP, Renard JP, Duranthon V, Heard E. Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature. 2011;472:370–374. doi: 10.1038/nature09872. By directly investigating the status of the X chromosome in human embryos, the authors definitively show that cells with two active X chromosomes exist in human blastocysts. [DOI] [PubMed] [Google Scholar]
- 8•.Najm FJ, Chenoweth JG, Anderson PD, Nadeau JH, Redline RW, McKay RDG, Tesar PJ. Isolation of epiblast stem cells from preimplantation mouse embryos. Cell Stem Cell. 2011;8:318–325. doi: 10.1016/j.stem.2011.01.016. This paper shows that EpiSCs can be derived from preimplantation blastocysts, mimicking the derivation of human ESCs from preimplantation blastocysts. The paper also confirms the notion that signaling conditions, not developmental stage, dictates the phenotype of pluripotent cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiegiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 10.Daheron L, Opitz SL, Zaehres H, Lensch MW, Lensch WM, Andrews PW, Itskovitz-Eldor J, Daley GQ. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 11.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 12•.Greber B, Wu G, Bernemann C, Joo JY, Han DW, Ko K, Tapia N, Sabour D, Sterneckert J, Tesar P, Scholar HR. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010;5:215–226. doi: 10.1016/j.stem.2010.01.003. A key paper that describes how the FGF and ACTIVIN/NODAL signaling pathways have been rewired in human cells, and suggests key differences between mouse and human primed pluripotent stem cells. [DOI] [PubMed] [Google Scholar]
- 13••.Greber B, Coulon P, Zhang M, Moritz S, Frank S, Muller-Molina AJ, Arauzo-Bravo MJ, Han DW, Pape H-C, Scholer HR. FGF signaling inhibits neural induction in human embryonic stem cells. EMBO J. 2011;30:4874–4884. doi: 10.1038/emboj.2011.407. The first study to describe the response of human ES cells to FGF/ERK inhibition, an intervention that facilitates the direct conversion of primed murine pluripotent stem cells to naïve murine pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 15.Vallier L, Touboul T, Chng Z, Brimpari M, Hannan N, Millan E, Smithers LE, Trotter M, Rugg-Gunn P, Weber A, Pedersen RA. Early decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signaling pathways. PLoS ONE. 2009;4:e6082. doi: 10.1371/journal.pone.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Tachibana M, Sparman M, Ramsey C, Ma H, Lee H-S, Penedo MCT, Mitalipov S. Generation of chimeric rhesus monkeys. Cell. 2012;148:285–295. doi: 10.1016/j.cell.2011.12.007. The ability to participate in pre-implantation development is a defining feature of naïve pluripotent stem cells. The authors demonstrate that primate ESCs cannot contribute to chimeras either by re-introduction into the blastocyst or 4-cell stage embryos, suggesting that primate ESCs functionally resemble primed mEpiSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 18.Gardner RL, Brooks FA. Reflections on the biology of embryonic stem cells. Int J Dev Biol. 1997;41:235–243. [PubMed] [Google Scholar]
- 19.Anderson PD, Nelson VR, Tesar PJ, Nadeau JH. Genetic factors on mouse chromosome 18 affecting susceptibility to testicular germ cell tumors and permissiveness to embryonic stem cell derivation. Cancer Res. 2009;69:9112–9117. doi: 10.1158/0008-5472.CAN-09-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying QL, Wray J, Nichols J, Battle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying Q-L, Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh C-L, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Tong C, Li P, Wu NL, Yan Y, Ying QL. Production of p53 gene knockout rats by homologous recombination. Nature. 2010;467:211–213. doi: 10.1038/nature09368. A robust demonstration of the broader utility of naïve pluripotency for transgenesis in a species other than the mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu L-F, Surani MA, Jaenisch R, Zwaka TP. Blimp1 expression predicts embryonic stem cell development in vitro. Curr Biol. 2011;21:1759–1765. doi: 10.1016/j.cub.2011.09.010. A key paper that suggests that ES cell derivation involves transitioning through a germ cell-like state from the ICM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signaling promotes ground state pluripotency in the mouse. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Roode M, Blair K, Snell P, Elder K, Marchant S, Smith A, Nichols J. Human hypoblast formation is not dependent on FGF signaling. Dev Biol. 2012;361:358–363. doi: 10.1016/j.ydbio.2011.10.030. The first published description of the response of human embryos to FGFR inhibition, MEK inhibition, or 2i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Miyanari Y, Torres-Padilla E. Control of ground-state pluripotency by allelic regulation of Nanog. Nature. 2012 doi: 10.1038/nature10807. [epub 12 February 2012]. This paper identifies a novel assay for the attainment of ground state pluripotency in rodent cells, which is gain of biallelic expression of Nanog. The authors demonstrate that only pluripotent cells of the mature preimplantation epiblast and ES cells cultured in 2i express Nanog from both alleles. [DOI] [PubMed] [Google Scholar]
- 28•.Lengner CJ, Gimelbrant AA, Erwin JA, Cheng AW, Guenther MG, Welstead GG, Alagappan R, Frampton GM, Xu P, Muffat J, et al. Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell. 2010;141:872–883. doi: 10.1016/j.cell.2010.04.010. The first description of human ES cell lines with two active X chromosomes. [DOI] [PubMed] [Google Scholar]
- 29.Kuijk EW, van Tol LT, van de Velde H, Wubbolts R, Welling M, Geijsen N, Roelen BA. The roles of FGF and MAPK signaling in the segregation of the epiblast and hypoblast lineages in bovine and human embryos. Development. 2012;139:871–882. doi: 10.1242/dev.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cells derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 33•.Han DW, Greber B, Wu G, Tapia M, Arauzo-Bravo MJ, Ko K, Bernemann C, Stehling M, Scholer HR. Direct reprogramming of fibroblasts into epiblast stem cells. Nat Cell Biol. 2011;13:66–71. doi: 10.1038/ncb2136. The generation of iEpiSCs directly suggests that signaling conditions dictate the resulting pluripotent state of cells in the mouse. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Pomp O, Dreesen O, Leong DF, Meller-Pomp O, Tan TT, Zhou F, Colman A. Unexpected X chromosome skewing during culture and reprogramming of human somatic cells can be alleviated using telomerase. Cell Stem Cell. 2011;9:156–165. doi: 10.1016/j.stem.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to mouse ESCs. Proc Natl Acad Sci USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells. Cell Stem Cell. 2008;3:346–353. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirano K, Nagata S, Yamaguchi S, Nakagawa M, Okita K, Kotera H, Ainscough J, Tada T. Human and mouse induced pluripotent stem cells are differentially reprogrammed in response to kinase inhibitors. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0283. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, Talantova M, Lin T, Kim J, Wang X, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci USA. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buecker C, Chen HH, Polo JM, Daheron L, Bu L, Barakat TS, Okwleka P, Porter A, Gribnau J, Hochedlinger K, Geiisen N. A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell Stem Cell. 2010;6:535–546. doi: 10.1016/j.stem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Yang J, Liu H, Lu D, Chen X, Zenonos Z, Campos LS, Rad R, Guo G, Zhang S, et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc Natl Acad Sci USA. 2011;108:18283–18288. doi: 10.1073/pnas.1100893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall J, Guo G, Wray, Eyres I, Nichols J, Grotewold L, Morfopoulou S, Humphreys P, Mansfield W, Walker R, et al. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, van Oosten AL, Theunissen TW, Guo G, Silva JC, Smith A. Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell. 2010;7:318–328. doi: 10.1016/j.stem.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo G, Smith A. A genome-wide screen in EpiSCs identifies Nr5a nuclear receptors as potent inducers of ground state pluripotency. Development. 2010;137:3185–3192. doi: 10.1242/dev.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanna J, Markoulaki S, Mitalipova M, Cheng AW, Cassady JP, Staerk J, Carey BW, Lengner CJ, Foreman R, Love J, et al. Metastable pluripotent states in NOD-mouse derived ESCs. Cell Stem Cell. 2009;4:513–524. doi: 10.1016/j.stem.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. 2009;461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyssiotis CA, Foreman RK, Staerk J, Garcia M, Mathur D, Markoulaki S, Hanna J, Lairson LL, Charette BD, Bouchez LC, et al. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci USA. 2009;106:8912–8917. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ware CB, Wang L, Mecham BH, Shen L, Nelson AM, Bar M, Lambda DA, Dauphin DS, Buckhingham B, Askari B, et al. Histone deacetylase inhibition elicits an evolutionarily conserved self-renewal program in embryonic stem cells. Cell Stem Cell. 2009;4:359–369. doi: 10.1016/j.stem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva SS, Rowntree RK, Mekhoubad S, Lee JT. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:4820–4825. doi: 10.1073/pnas.0712136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Han DW, Tapia N, Joo JY, Greber B, Arauzo-Bravo MJ, Bernemann C, Ko K, Wu G, Stehling M, Do JT, Scholer HR. Epiblast stem cell subpopulations represent mouse embryos of distinct pregastrulation stages. Cell. 2010;143:617–627. doi: 10.1016/j.cell.2010.10.015. This paper demonstrates that primed pluripotency may be functionally demarcated into early and late primed pluripotent states, with early primed EpiSCs able to contribute to chimeras. This suggests that contribution to chimeras may not be restricted to naïve pluripotent stem cell lines and captures an unappreciated level of functional heterogeneity in rodent primed pluripotency. [DOI] [PubMed] [Google Scholar]


