Abstract
Background and Aims
Floral secretions are common in Bulbophyllum Thouars, and the labella of a number of Asian species are said to produce secretions rich in lipids that act as food rewards for insect pollinators. Although some of these reports are based on simple histochemical tests, a much greater number are anecdotal and, hitherto, neither the ultrastructure of the labellum nor the secretory process has been investigated in detail. Furthermore, sophisticated histochemical approaches have generally not been applied. Here, both the labellar structure and the secretory process are investigated for four species of Asian Bulbophyllum sect. Racemosae Benth. & Hook. f., namely Bulbophyllum careyanum (Hook.) Spreng., B. morphologorum Kraenzl., B. orientale Seidenf. and B. wangkaense Seidenf., and compared with those of unequivocal lipid-secreting orchids.
Methods
Labellar, secretory tissue was investigated using light microscopy, scanning electron microscopy, transmission electron microscopy and histochemistry.
Key Results
The adaxial median longitudinal groove of the labellum contained secretory tissue comprising palisade-like epidermal cells, similar to those of certain lipid-secreting Oncidiinae Benth. However, these cells and their secretions gave positive results mainly for protein and mucilage, and their organelle complement was consistent with that of cells involved in protein and mucilage synthesis. Sub-cuticular accumulation of secretion resulted in cuticular distension and blistering. The sub-epidermal layer of isodiametric parenchyma contained starch and, like the epidermal cells, ultrastructure consistent with mucilage synthesis. Lipids were mainly confined to the cuticle, and hardly any intracellular lipid droplets were observed.
Conclusions
It is proposed that mucilage is produced by dictyosomes present in the palisade-like epidermal cells. Mucilage precursors may also be produced by these same organelles in sub-epidermal cells and are thought to pass along the symplast via plasmodesmata into the adjoining palisade-like secretory cells, which contain abundant arrays of rough endoplasmic reticulum. Here, they become chemically modified and form a protein-rich, mucilaginous secretion that, following vesicle-mediated transport across the cytoplasm, traverses the cell wall and accumulates in blisters formed from the distended cuticle. Rupture of these blisters releases the secretion onto the labellar surface. However, in certain species, there is some evidence that the secretion may traverse the cuticle via cuticular pores, and micro-channels may permit the passage of fragrance. Hydrolysis of sub-epidermal starch probably generates the carbohydrate and, together with mitochondria, much of the energy required for the secretory process. This anatomical organization resembles that found in certain lipid-secreting, Neotropical species of Bulbophyllum and Oncidiinae, but since the chemical composition of their secretions is different, and these taxa occur on a separate continent and have different insect pollinators, parallelism of floral anatomy is likely.
Keywords: Anatomy, Bulbophyllinae, Bulbophyllum, histochemistry, lipid, micromorphology, mucilage, orchid, Orchidaceae, protein, secretion, sect. Racemosae, ultrastructure
INTRODUCTION
Floral food rewards are relatively uncommon amongst orchids, and generally consist of nectar. Some species, however, reward insect pollinators with other food rewards, such as oil and resin-like secretions (van der Pijl and Dodson, 1969; van der Cingel, 2001; Davies and Stpiczyńska, 2008, and references therein). Floral oils are said to be produced by certain members of Cranichidinae Lindl. (Dressler, 1993), Satyrinae Schltr. (Garside, 1922), Coryciinae Benth. (Buchmann, 1987; Steiner, 1989, 1993; Pauw, 2006), Catasetinae (sensu Chase et al., 2003; Mickeliunas et al., 2006), Maxillariinae Benth. (Davies and Stpiczyńska, 2009) and especially Oncidiinae Benth. (van der Pijl and Dodson, 1969; Buchmann, 1987; Dressler, 1990; Singer and Cocucci, 1999; van der Cingel, 2001; Silvera, 2002; Singer et al., 2006; Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009; Pacek et al., 2012; Blanco et al., 2013), among others. To date, however, detailed ultrastructural and histochemical investigations of the secretion of lipid-rich or resinous compounds by orchid flowers have largely been confined to the oil-secreting epithelial and trichomal elaiophores of Neotropical Oncidiinae (Singer and Cocucci, 1999; Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2007, 2013; Davies and Stpiczyńska, 2008; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009; Pacek et al., 2012; Blanco et al., 2013; Gomiz et al., 2013), the epithelial elaiophore of Rudolfiella picta (Schltr.) Hoehne (Maxillariinae Benth.) and to resiniferous floral hairs and the poorly defined, resin-secreting regions that occur on the labella of certain species of Maxillariinae (Davies et al., 2003a, b; Davies and Turner, 2004; Davies and Stpiczyńska, 2008, 2012; Stpiczyńska and Davies, 2009).
One sub-tribe whose labellar anatomy has been largely neglected, but is said to secrete oil (Pohl, 1935; van der Cingel, 2001), is Bulbophyllinae Schltr.
The Pantropical orchid genus Bulbophyllum Thouars is one of the largest and is estimated to contain 1200–2000 species and some 5 % of all orchids. It occurs in Asia, Africa, Madagascar, the Neotropics and Australasia, although its distribution is not homogeneous throughout its entire range. Found mainly in the Palaeotropical region of south-east Asia, New Guinea is considered to be its centre of distribution, with hundreds of species of Bulbophyllum occurring in Asia, followed by Africa and then the Neotropics (Vermeulen, 1991; Dressler, 1993; Sieder et al., 2007, cited in Smidt et al., 2011). The genus includes some of the smallest plants in the orchid family, but has some of the most intricate flowers, often displaying very diverse and complex pollination strategies (van der Cingel, 2001).
Insect pollinators are attracted by a combination of floral scents (fruity or malodorous and often smelling of carrion), osmophores (often produced on ‘antennae’ or floral appendages), flower colour (often dull cream or yellow-green to purple-brown and frequently spotted), epidermal features (e.g. hairs, glands and secretions, such as oil and nectar) and mobile floral parts (e.g. labella and appendages), whereas some species have closed flowers with side slits (e.g. B. grandiflorum Blume).
Although some are said to be pollinated by foraging beetles, most species conform to the fly pollination syndrome, with several Asian members of sect. Racemosae Benth. & Hook. f., the subject of this paper, such as B. lilacinum Ridl., B. peninsulare Seidenf. together with B. penicillium C.S.P. Parish & Rchb.f. (sect. Hirtula Ridl.), being pollinated by fruit flies of the genus Drosophila (Diptera: Drosophilidae), such as D. ananassae. The flowers of these species often have a mild, fishy odour, and dipteran pollinators have been observed probing the liquid-containing, median, longitudinal labellar groove (Ong and Tan, 2012). Furthermore, it is now well established that those representatives of sections Beccariana Pfitzer and Stenochilus J.J. Sm. having a fruity or spicy floral scent, such as B. elevatopunctatum J.J. Sm., B. patens King ex Hook. f. and B. praetervisum J.J. Verm., are pollinated by males of the fruit fly genus Bactrocera [Diptera: Tephritidae (Ong, 2011; Ong et al., 2011, and references therein)]. Their scents primarily contain methyl eugenol (ME), raspberry ketone (RK) or zingerone that attract male fruit fly pollinators. Moreover, these phenylpropanoid compounds occur mainly in the labellum, and Tan and co-workers (Tan and Nishida, 2000, 2005; Tan et al., 2002) have demonstrated that the species of Bactrocera that are attracted depends on the composition of the secretion. For example, Bulbophyllum cheiri Lindl. produces ME and attracts Bactrocera dorsalis sensu lato, B. umbrosa and B. carambolae, whereas Bulbophyllum apertum Schltr. produces RK and attracts Bactrocera albistrigata, B. caudata, B. cucurbitae and B. tau. Conversely, Bulbophyllum patens releases floral zingerone that attracts both ME- and RK-sensitive species of Bactrocera. These compounds, which are found on the perianth lobes, including the labellum, are licked and used by the male pollinators to help boost their defence system and to make pheromones, thereby attracting female fruit flies (Tan and Nishida, 2000; Tan et al., 2002). The licking behaviour and related activity eventually triggers the lip mechanism and ensures pollination. In the non-resupinate flowers of B. praetervisum, the fruit flies, while probing and licking the flower parts, slip down the vertical, lateral sepals and cling to their margins, or to those of the labellum. If the latter, the insects are immediately catapulted against the column and dislodge the pollinia (Ong et al., 2011; Ong, 2013). Similar mechanisms occur in B. patens and B. stockeri J.J. Verm. (sect. Stenochilus), but in the latter species, unlike B. patens, the pollinator, once thrown against the column, becomes temporarily trapped between the stigma and the labellum, in the sticky stigmatic fluid (Ong, 2011, 2013). Other members of sect. Beccariana, including Bulbophyllum lasianthum Lindl., B. subumbellatum Ridl. and B. virescens J.J. Sm., and sect. Sestochilos (Breda) Benth. & Hook. f., e.g. B. lobbii Lindl., are mainly pollinated equally by both male and female dipterans known variously as blowflies, bluebottles or greenbottles (Diptera: Calliphoridae), in particular Chrysomya megacephala (B. lasianthum and B. virescens) and Hemipyrellia ligurriens (B. lobbii and B. virescens), despite the fact that flowers of B. lasianthum and B. virescens smell of carrion, whereas those of B. lobbii have a pleasant, rather fruity smell. The flesh fly Liopygia ruficornis (Diptera: Sarcophagidae) has also been observed pollinating both B. subumbellatum and B. virescens, together with the signal fly Scholastes sexvittatus (Diptera: Platystomatidae; Ong and Tan, 2011). Flesh flies are also known to pollinate B. mandibulare Rchb.f., [sect. Lepidorhiza Schltr. (Ong, 2012)]. Mimicry also occurs in some Asian species of Bulbophyllum. For example, in B. makoyanum (Rchb.f.) Ridl. [sect. Recurvae (Garay, Hamer & Siegerist) J.J. Verm.], the flowers are arranged in a circle on the inflorescence and collectively resemble a nectariferous, actinomorphic flower (Knerr, 1981), and Koehler and Davenport (1983) have demonstrated that the similar inflorescence of B. lepidum (Blume) J.J. Sm. [syn. B. flabellum veneris (J. Koenig) Aver.], when examined using UV light (visible to many insects), resembles the capitulum of Gazania uniflora (Asteraceae). Likewise, in B. cimicinum J.J. Verm. [sect. Epicrianthes (Blume) Hook. f.], the flower resembles a spider, and it has been suggested that it might attract spider-predatory or spider-parasitic flies or wasps (Christensen, 1994). Thus, the pollination syndrome here may involve pseudocarnivory or pseudoparasitism.
By contrast, some West African species of Bulbophyllum are pollinated by Hymenoptera, such as bees and wasps [including stingless bees and ctenuchid wasps (Johansson, 1974, cited in van der Cingel, 2001; Dressler, 1990, 1993)], whereas others such as B. magnibracteatum Summerh., B. imbricatum Lindl., B. maximum (Lindl.) Rchb.f., B. purpureorhachis (De Wild.) Schltr. and B. resupinatum Ridl. have flowers that, in terms of colour and form, would indicate sapromyophily. At the other extreme, B. schinzianum Kraenzl. var. phaeopogon (Schltr.) J.J. Verm. possesses a labellum that is thought to mimic the hairy body of an insect and may thus be pseudocopulatory (van der Cingel, 2001; Jongejan, 2004). However, this claim is speculative.
Relatively few observations have been made on the pollination of Neotropical species of Bulbophyllum. The flowers of B. correae Pabst (syn. B. setigerum Lindl.) are reported to be pollinated by Milichiidae flies that graze small, labellar hairs (Braga, 1977). Similarly, Sazima (1978) observed the pollination of B. warmingianum Cogn. (syn. B. involutum Borba, Semir & F. Barros) by female Milichiidae flies of the genus Pholeomyia. Although the weight and movement of the fly, as it licks the central labellar groove at the base of the very mobile labellum, are not in themselves sufficient to pitch the latter to the column, this is facilitated by air currents. Sazima (1978) claims this to be the first case of wind-assisted pollination in an entomophilous plant. A very similar mechanism involving both Pholeomyia and wind-assisted pollination is also known to occur in B. weddellii (Lindl.) Rchb.f. and B. ipanemense Hoehne (Borba and Semir, 1998).
As previously stated, the flowers of a significant number of Asian species have been reported to secrete an oily or resinous material. Amongst those documented are B. alticola Schltr. (sect. Codonosiphon Schltr.), B. auratum (Lindl.) Rchb.f., (syn. B. campanulatum Rolfe; sect. Recurvae) and B. lobbii and B. macranthum Lindl. [sections Sestochilos and Stenochilus, respectively - Pohl, 1935; Jongejan, 1994; van der Cingel, 2001)]. In B. alticola, the labellum produces glistening droplets of viscid fluid shortly after the flower opens. These attract insects that become caught in the sticky liquid. In attempting to escape, the insect grasps the column and effects pollination (Jongejan 1994). Bulbophyllum auratum produces copious fluid that coats and collects on the abaxial surface of the lateral perianth lobes. Insects trapped in this fluid grasp and draw down the labellum. As the insect frees itself from the fluid, the labellum, in regaining its original position, throws the insect against the column (Jongejan, 1994). Pohl (1935) observed that the flower of B. lobbii produces oil and sugar at the base of the lip and may have retained some features of bee-pollinated species (Christensen, 1994). Moreover, it traps flies in the tropics, much like B. virescens (Ong and Tan, 2011). In this last species, pollinating bluebottles landing on the flower initially face the column. On alighting on the hinged labellum, the latter tips towards the column, causing the insect to turn around so as to attempt to escape, whereupon the lip presses it tightly onto the column securing adhesion of the pollinia to its thorax. When the insect finally manages to escape, the pollinia are dislodged. Bulbophyllum lasianthum and B. subumbellatum have similar see-saw pollination mechanisms, except that here, the trapped fly, instead of turning, backs out of the flower (Ong and Tan, 2011). The pollination of B. macranthum closely resembles that of B. praetervisum (Ong et al., 2011; Ong, 2013) and was first described by Ridley (1890), followed by Millar (1978), Jongejan (1994) and, more recently, Ong et al. (2011). Again the flower is non-resupinate, resulting in a sliding-trap device, rather than the familiar see-saw device found in many plants of this genus. According to Pohl (1935), this species also produces oil and sugar at the base of the lip and adjacent floral parts. The pollinating fly licks the sepals, especially towards the dark-coloured, grooved apices, and grasps their margins. However, when it reaches the wider parts of the sepals, its purchase is compromised and it struggles wildly to grasp the labellum, which falls back, pitching the insect into the column between the stelidia. The latter separate slightly and close tightly onto its abdomen. The captive fly strikes the pollinia with its abdomen and these become attached to the first segment. The labellum, now released by the fly, returns to its original position and the pollinator escapes to deposit pollinia on the stigma of another flower. Thus, these presumed oily secretions are considered to play an integral part in pollination.
Remarkably, given the enormity and importance of the genus Bulbophyllum, very little is known about the labellar anatomy and the secretory activity of this, or indeed, any other fly-pollinated orchid genus. Furthermore, detailed micro-morphological studies of Bulbophyllum flowers are scarce (e.g. de Pádua Teixeira et al., 2004; Nunes et al., 2014). Histochemical, combined with ultrastructural investigations, would enable comparisons to be made of the structure of secretory tissues, the chemical nature of the secretion and the secretory process in this genus with those of other orchids for which oil secretion has been unequivocally demonstrated.
The present investigation of labellar structure and its secretory activity is confined to four representative members of the Asian sect. Racemosae (formerly assigned to sect. Careyana Pfitzer; Seidenfaden and Wood, 1992), including the type species Bulbophyllum careyanum (Hook.) Spreng. Section Racemosae contains approx. 38 provisionally accepted species (46 names) distributed throughout India, Nepal, Bhutan, Myanmar, China, Vietnam, Laos, Thailand, Peninsular Malaysia and the Philippines (J. J. Vermeulen, 2014, pers. comm.).
MATERIALS AND METHODS
Plants used in this study were obtained from the first author's collection. They include Bulbophyllum careyanum (Hook.) Spreng. (accession no. KLD 201304), B. morphologorum Kraenzl. (accession no. KLD 201308), B. orientale Seidenf. (accession nos KLD 201202, KLD 201203 and KLD 201309) and B. wangkaense Seidenf. (accession no. KLD 201313). Their identities were confirmed by J. J. Vermeulen (pers. comm., 2014). Spirit-preserved voucher material of each of the species was deposited at the herbarium of the Royal Botanic Gardens, Kew, UK under the accession nos Davies 2014–1 (B. careyanum), Davies 2014–2 (B. morphologorum), Davies 2014–3 (B. orientale KLD 201309) and Davies 2014–4 (B. wangkaense). Abbreviations for authors of plant names follow Brummitt and Powell (1992) throughout.
Localization of possible lipid-secreting floral tissues was performed on intact flowers by immersing them for 5–20 min in a saturated ethanolic solution of Sudan III. The presence of putative mucilage-secreting tissues was detected by immersing fixed labella (see below) in 0·05 % (w/v) aqueous ruthenium red solution. These tissues were subsequently examined using light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), as follows.
Representative pieces of secretory tissues (approx. 1 mm3) were excised and fixed in 2·5 % (v/v) glutaraldehyde/4 % (v/v) formaldehyde in 0·1 m phosphate buffer (pH 7·4) for 2 h at 4 °C, transferred and washed three times in 0·1 m sodium cacodylate buffer pH 6·8 and post-fixed in 1·5 % (w/v) osmium tetroxide solution for 1·5 h at 0 °C. The fixed material was then dehydrated using a graded ethanol series, and infiltrated and embedded in Spurr's resin (Spurr Low-Viscosity resin, Sigma). Following polymerization at 60 °C, sections were cut at 70 nm for TEM using a Leica EM UC7 ultramicrotome and a diamond knife, stained with uranyl acetate and lead citrate (Reynolds, 1963) and examined using a FEI Tecnai Spirit G2 transmission electron microscope, at an accelerating voltage of 90 kV.
Semi-thin sections (0·9–1·0 μm thick) were prepared for LM and were stained for general histology using aqueous 1 % (w/v) methylene blue/1 % (w/v) azure II (1:1) for 5–7 min (MB/AII).
The presence of lipids, starch and mucilage in hand-cut sections of floral tissue was detected by treating the latter with a saturated ethanolic solution of Sudan III, aqueous IKI (iodine–potassium iodide) solution and ruthenium red solution, respectively. Sections were also stained with Coomassie brilliant blue R250 (Fisher, 1968; Ruzin, 1999) and mercuric bromophenol blue [aqueous solution of 10 % (w/v) mercuric chloride, 0·1 % (w/v) bromophenol blue] for total proteins (Mazia et al., 1953; Pearse, 1985). A 10 % (w/v) aqueous solution of FeCl3 was used to test for catechol-type dihydroxyphenols (Gahan, 1984), and the periodic acid–Schiff (PAS) reaction was used to detect the presence of insoluble polysaccharides (Jensen, 1962).
Micrometry and photomicrography were accomplished using a Nikon Eclipse E200 (NIS-Elements AR software) for LM images and FEI Tecnai Spirit G2 (TEM Imaging & Analysis computer program) for TEM.
For SEM, whole labella were dehydrated and subjected to critical-point drying using liquid CO2. They were then sputter-coated with gold and examined using a Tescan Vega II LS scanning electron microscope, at an accelerating voltage of 30 kV.
RESULTS
Since the boundaries of many Bulbophyllum species are poorly delimited and may eventually be subject to realignment, the floral habit of each of the four species investigated here is shown for future reference (Figs 1–2).
Fig. 1.
Habit of three accessions of Bulbophyllum orientale. (A) Habit of accession KLD 201309 showing pendulous, lax and racemose inflorescence. (B) Detail of flowers of accession KLD 201309 showing a median longitudinal labellar groove, forwardly pointing lateral lobes to the labellum and spreading stelidia. (C) Single flower of accession KLD 201309 showing glistening secretory residues on the adaxial surface of the labellum (arrow) and on the sepals. (D, E) Floral inflorescences and flowers of accessions KLD 201203 and KLD 201202, respectively. Note the glistening sepals in each case. Scale bars = 5 mm in each case.
Fig. 2.
Three other species of Bulbophyllum sect. Racemosae showing habit of inflorescences and detail of flowers. (A) Habit of the compact, racemose inflorescence of B. careyanum (accession no. KLD 201304). (B) Detail of flowers showing the median groove of the labellum flanked by two pronounced ridges and distinct, yellow stelidia on the column. (C) Habit of the somewhat more pendulous, but rather truncated, racemose inflorescence of B. morphologorum (accession no. KLD 201308). (D) Detail of flowers, again showing the median longitudinal labellar groove flanked by two raised purple ridges, and the white stelidia. (E) Elongate, cylindrical and pendulous, racemose inflorescence of B. wangkaense (accession no. KLD 201313). (F) Again, note the presence of a median longitudinal groove on the labellum. In all three species, the labella and both adaxial and abaxial surfaces of the sepals are coated with a glistening, secretory residue. Scale bars = 5 mm in each case.
Bulbophyllum orientale
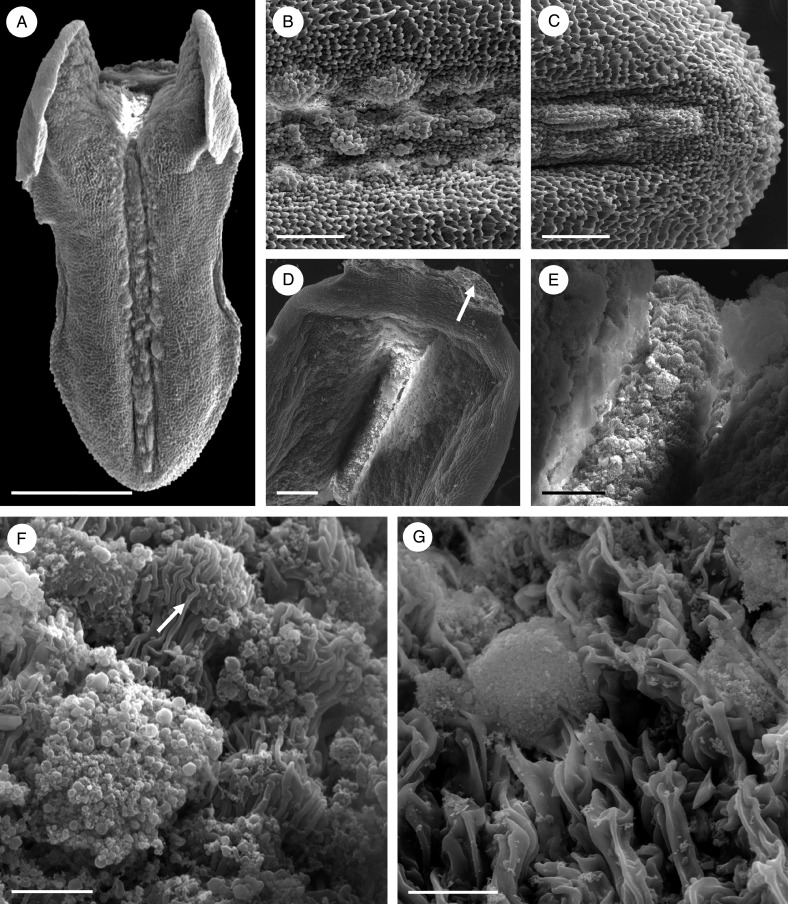
Inflorescences and flowers of all three accessions of this species were very similar (Fig. 1A–E). The inflorescence was pendulous, lax and racemose (Fig. 1A, B, D, E). The flowers, at first, smelled strongly of carrion, but then of over-ripe fruit. The rocking labellum had forwardly pointing lateral lobes and, adaxially, a median longitudinal groove, whereas the column had characteristically curved and spreading stelidia (Fig. 1B, C). Glistening residues of secretion were present on the linguiform labellum, as well as the margin and both abaxial and adaxial surfaces of sepals (Fig. 1B–E).
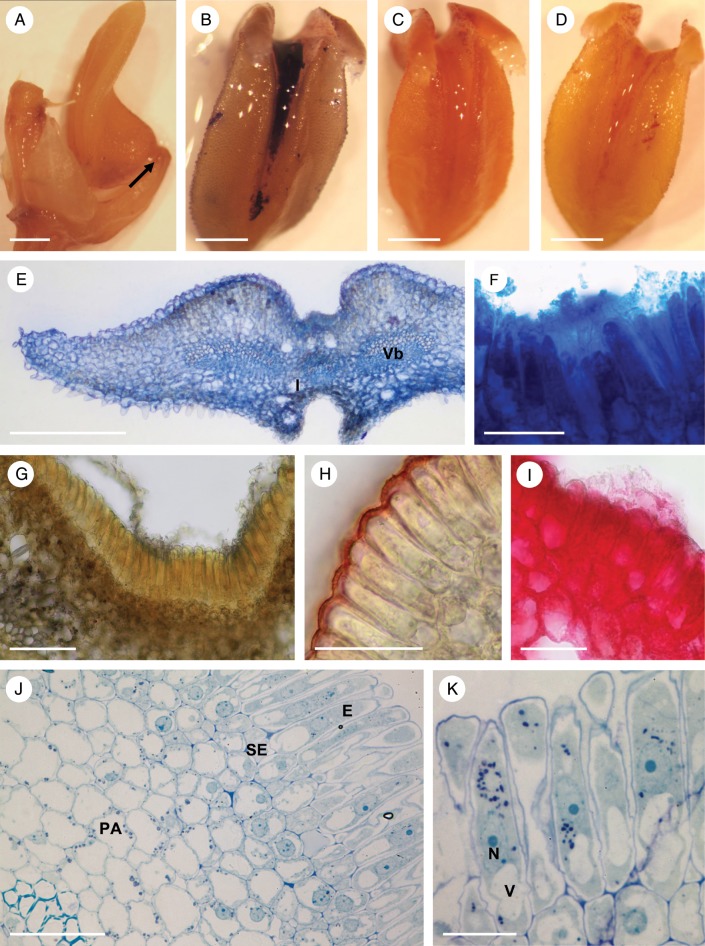
The rocking labellum was articulated to the column-foot (Fig. 3A). The labellar groove (Fig. 3A–E) contained palisade-like epidermal cells of mean dimensions 58·3 × 9·8 μm (Fig. 3F–K), beneath which were smaller sub-epidermal parenchyma cells. These isodiametric cells had a mean diameter of 11·2 μm, a relatively large nucleus and intensely staining cytoplasm (Fig. 3G–K). Most of the labellum consisted of ground parenchyma through which ran collateral vascular bundles. Large idioblasts containing raphides were also present (Fig. 3E, G).
Fig. 3.
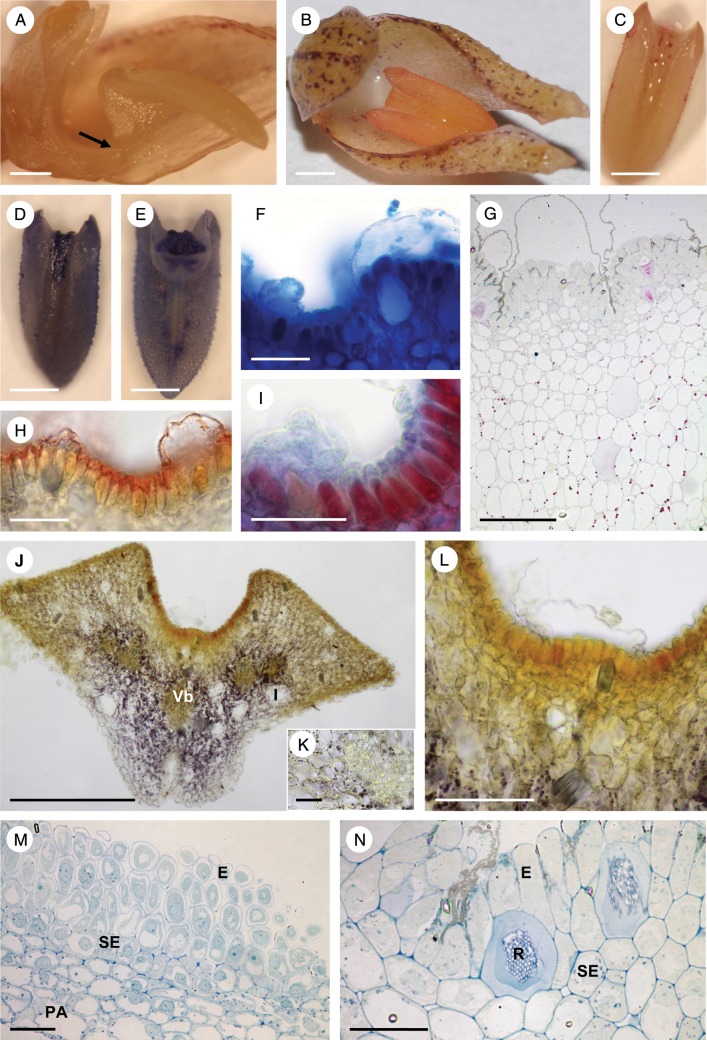
Bulbophyllum orientale – LM. (A) Lateral view of the labellum showing articulation (arrow) to the column-foot. (B) Adaxial view of the labellum stained with Coomassie brilliant blue. Note that the median longitudinal groove selectively stains for protein with this reagent. (C) Slight, but non-selective staining of the labellum with Sudan III. (D) Slight staining of the labellar groove for mucilage with ruthenium red. (E, F) Hand-cut transverse section of the labellum stained with Coomassie brilliant blue. Note staining of secreted material in F. (G) Section stained for starch using IKI. Note its absence from the palisade-like cells of the epidermis and its presence in the sub-epidermal and ground parenchyma. (H) Palisade-like epidermal cells with thick cuticle stained with Sudan III. (I) Blistered cuticle with sub-cuticular secreted material stained with ruthenium red. (J, K) Sections of palisade-like epidermis and sub-epidermal parenchyma stained with MB/AII. Note that the epidermal cells contain a central nucleus with nucleoli, plastids and basally located vacuoles. Scale bars = 1 mm, 1 mm, 1 mm, 1 mm, 500 μm, 50 μm, 100 μm, 50 μm, 50 μm, 50 μm and 20 μm, respectively. E, epidermis; I, idioblast; N, nucleus; PA, ground parenchyma; SE, sub-epidermal layer; V, vacuole; Vb, vascular bundle.
Similarly, palisade-like, epidermal cells also contained dense cytoplasm and a centrally located nucleus. Each usually contained several vacuoles, often concentrated towards the basal end of the cell (Fig. 3J, K). However, plastids tended to occur near the nucleus or in the apical part of the cell (Fig. 3K). The thin, outermost cellulosic cell walls had a thick, often blistered cuticle (Fig. 3G, H).
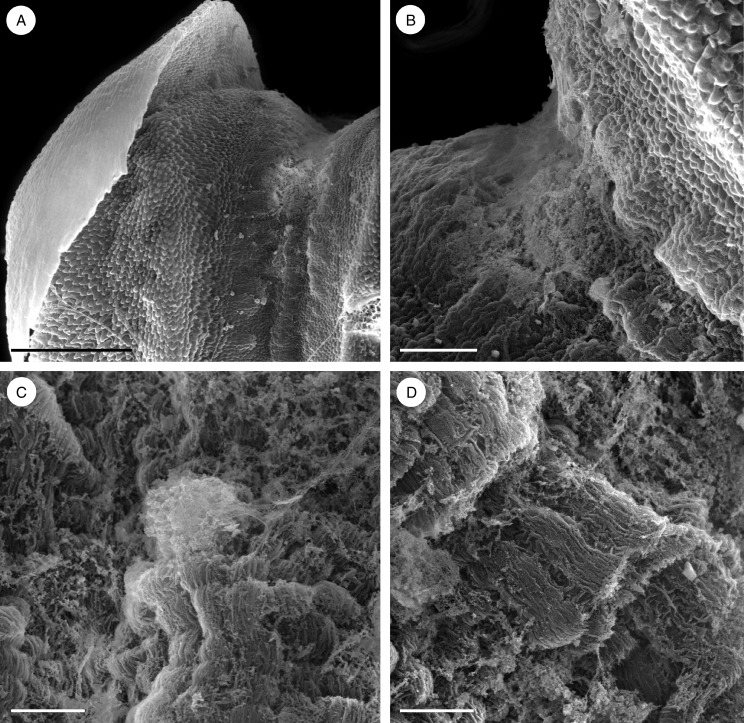
Observations by SEM revealed that the floor of the median longitudinal groove was verrucose and that the secretion accumulated mainly towards the basal part of the labellum (Fig. 4A, B), where it formed an amorphous film that coated the palisade-like, epidermal cells (Fig. 4C, D). The cuticle of these cells was finely striate (Fig. 4C, D). The secretion, which was largely restricted to the adaxial groove, selectively stained for proteins with Coomassie brilliant blue (Fig. 3B), but only weakly for mucilage with ruthenium red (Fig. 3D). Moreover, although entire flowers stained slightly with Sudan III, the degree of staining did not indicate the presence of elevated concentrations of lipid (Fig. 3C). However, the contents of the epidermal cells stained intensely for proteins with both mercuric bromophenol blue and Coomassie brilliant blue (Fig. 3E, F). Starch was largely absent from these cells, but occurred abundantly in the ground parenchyma (Fig. 3G). The cuticle had great affinity for Sudan III owing to its high lipid content (Fig. 3H), and this explains the slight staining of entire flowers following treatment with this reagent. By contrast, the secretion, including that which had accumulated beneath the blistered and detached cuticle, remained unstained with Sudan III, again demonstrating the absence of lipids in both epidermal cells and their secretion (Fig. 3H). The sub-cuticular secretion, however, stained slightly with ruthenium red (Fig. 3I), but treatment with FeCl3 did not reveal the presence of phenolic compounds.
Fig. 4.
Bulbophyllum orientale – SEM. (A, B) Basal part of the labellum with copious, amorphous secretion. (C) Palisade-like cells of the labellar groove coated with secretion. (D) Palisade-like cells with finely striate cuticle. Scale bars = 500 μm, 100 μm, 20 μm and 20 μm, respectively
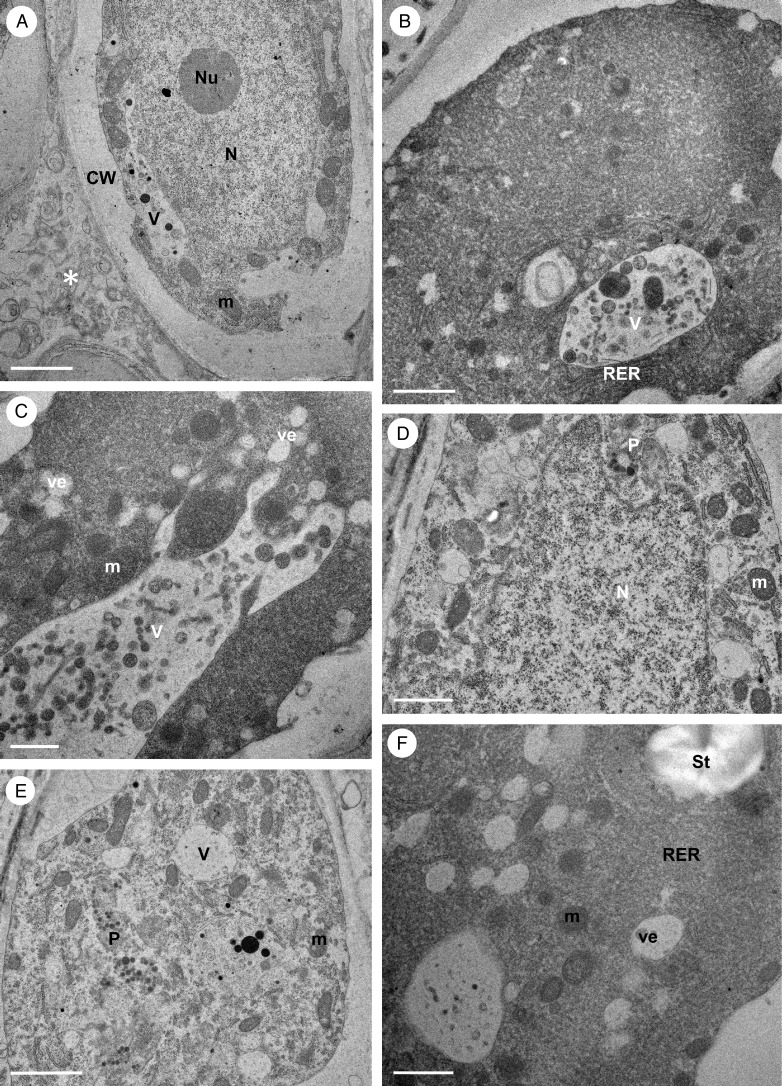
Observations by TEM showed the presence of secreted material in the intercellular spaces of the epidermis (Fig. 5A). Abundant rough endoplasmic reticulum (RER; with short profiles predominating) occurred in the cytoplasm of both epidermal and sub-epidermal cells, together with small vesicles, dictyosomes and numerous mitochondria (Fig. 5B–F). Plastids were only occasionally present in palisade-like epidermal cells. These were oval and contained several small plastoglobuli (Fig. 5D, E), whereas those found in the sub-epidermal parenchyma contained starch grains (Fig. 5F). Vacuoles contained osmiophilic, globular or irregularly shaped bodies of various sizes similar to those present in the intercellular spaces between adjacent palisade-like cells (Fig. 5A–C), but myelin-like figures were only occasionally observed.
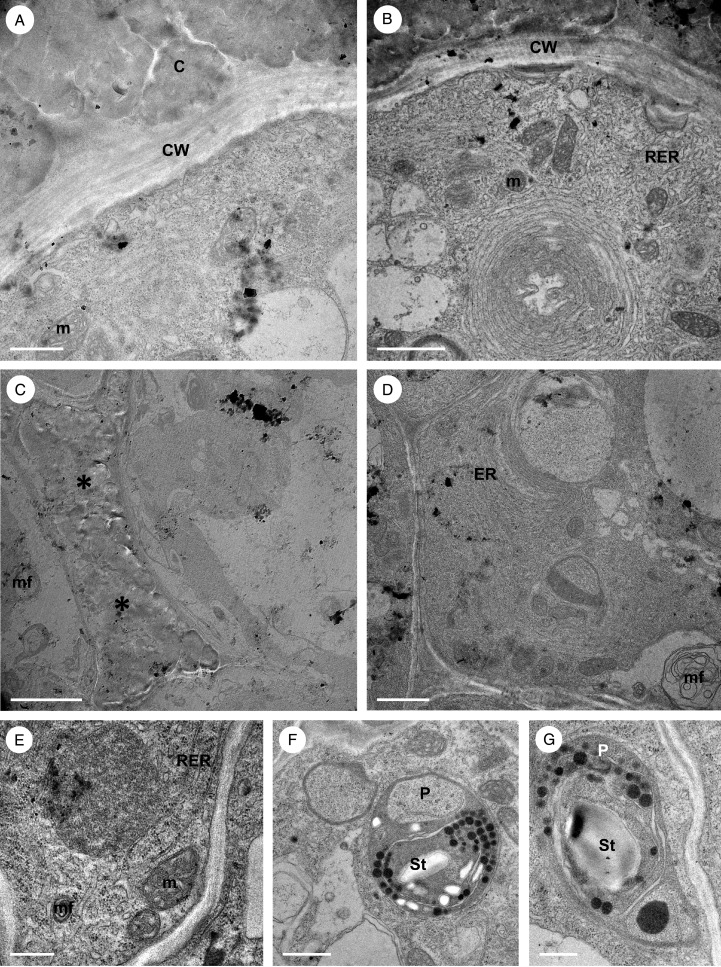
Fig. 5.
Bulbophyllum orientale – TEM. (A) Section through a palisade-like epidermal cell showing a large, central nucleus and nucleolus surrounded by mitochondria and a parietally located vacuole with contents resembling intercellular secreted material (asterisk). (B) Detail of cytoplasm with numerous RER profiles and a vacuole containing globular osmiophilic material. (C) Vacuole containing osmiophilic material. Vesicles, numerous mitochondria and RER profiles are present in the cytoplasm. (D) Nucleus surrounded by mitochondria, RER profiles and small, starchless plastids. (E) Detail of cytoplasm showing plastids with plastoglobuli, mitochondria, RER profiles and vacuoles. (F) Electron-dense cytoplasm of sub-epidermal cell with RER profiles, vesicles and a plastid containing starch. Scale bars = 2 μm, 2 μm, 1 μm, 2 μm, 2 μm and 1 μm, respectively. CW, cell wall; m, mitochondrion; N, nucleus; Nu, nucleolus; P, plastid; RER, rough endoplasmic reticulum; St, starch; V, vacuole; ve, vesicle.
Bulbophyllum careyanum
The racemose inflorescence was pendulous and compact (Fig. 2A, B), and the flowers produced a strong carrion-like smell. The rocking labellum was again linguiform and had a median longitudinal groove flanked by two pronounced, raised ridges, the column having two distinct, downwardly curved, but rigid, yellow stelidia (Fig. 2B). Glistening secretory residues were present on the adaxial surface of the labellum, especially within and adjacent to the median groove, but also adaxially and abaxially on the sepals (Fig. 2B). It was difficult to test entire flowers with Sudan III owing to their dark pigmentation, which often masked histochemical results.
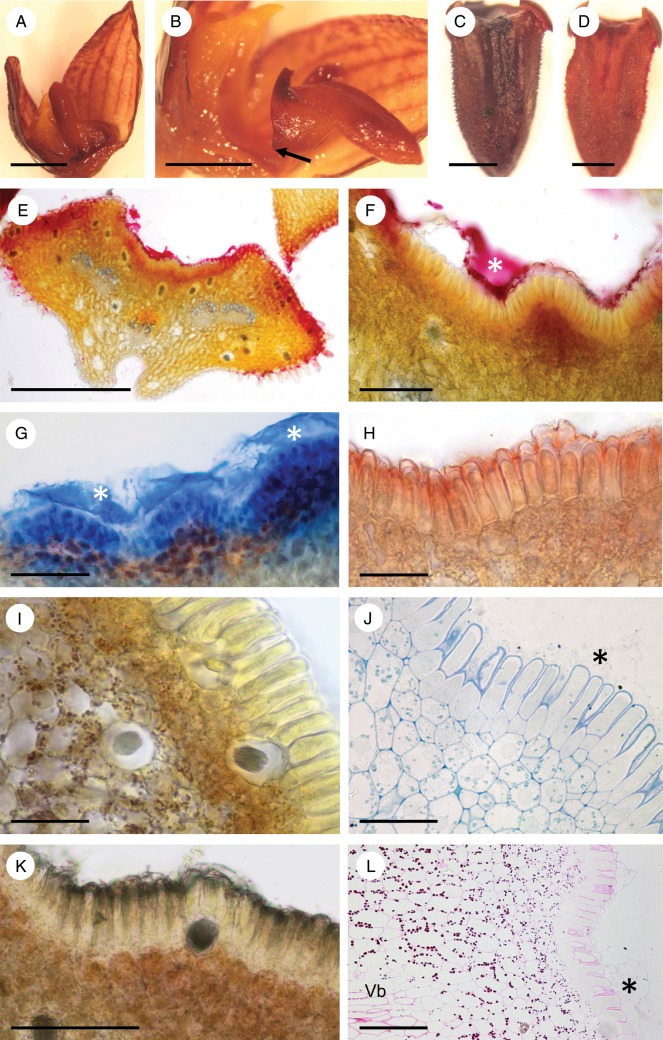
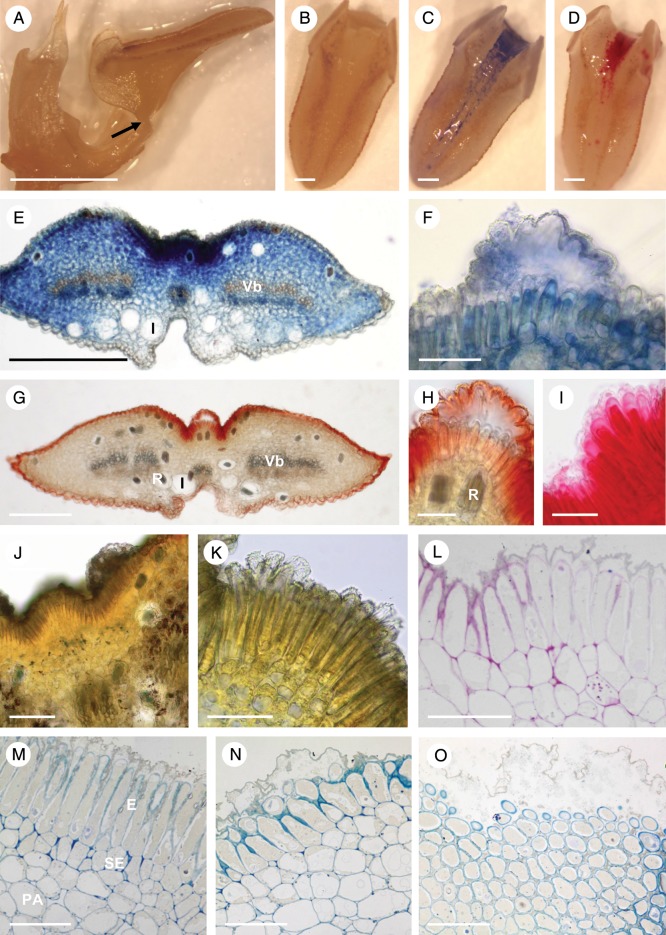
The rocking labellum was articulated to the base of the column-foot and, when in the upright position, was adpressed to the latter (Fig. 6A, B). The labellar groove (Fig. 6B–D) contained palisade-like epidermal cells of mean dimensions 51·2 × 8·3 μm (Fig. 6E–L). The isodiametric sub-epidermal parenchyma cells were smaller (mean diameter = 11·1 μm) and possessed intensely staining cytoplasm (Fig. 6E, F, I–L). Most of the labellum consisted of ground parenchyma through which ran collateral vascular bundles (Fig. 6E) composed mainly of sieve tubes and lignified, pitted xylem tracheids, the latter having relatively wide lumina measuring up to 26·6 μm in diameter. Idioblasts with raphides occurred scattered throughout the sub-epidermal and ground parenchyma (Fig. 6E, I, K), but larger idioblasts (up to 48 μm in diameter) were observed close to the abaxial epidermis.
Fig. 6.
Bulbophyllum careyanum – LM. (A, B) Lateral view of the labellum showing articulation (arrow) to the column-foot. In (A) the rocking labellum is in the upright position and adpressed to the column, whereas in (B) it is in its usual lowered position. (C) Adaxial view of the labellum with the median longitudinal groove selectively stained for protein with Coomassie brilliant blue. (D) Adaxial view of the labellum with the median longitudinal groove selectively stained for mucilage with ruthenium red. (E, F) Transverse section of the labellum stained with ruthenium red. Note the stained secreted material (asterisk) coating the palisade-like epidermal cells, and the cuticular blister. (G) Section of labellum selectively stained for protein with Coomassie brilliant blue. The secreted material (asterisks) that has accumulated beneath the detached cuticle has stained strongly for protein. (H) Transverse section of the labellum showing the epidermis with the cuticle stained red following treatment with Sudan III, and small sub-epidermal cells with numerous plastids. (I) Section stained with IKI revealing the presence of starch in the sub-epidermal tissue and ground parenchyma, but its general absence from epidermal cells. Note also the presence of idioblasts with raphides. (J) Section of palisade-like epidermal cells and sub-epidermal tissue stained with MB/AII. Note the granular appearance of the secretion coating the epidermal cells and the cuticular blister (asterisk). (K) Control (unstained) section. (L) Palisade-like epidermal cells and parenchymatous tissues following PAS staining to show the distribution of starch. Note also the cuticular blister (asterisk). Scale bars = 2 mm, 2 mm, 0·5 mm, 0·5 mm, 500 μm, 100 μm, 100 μm, 50 μm, 50 μm, 50 μm, 100 μm and 100 μm, respectively. Vb, vascular bundle.
Each palisade-like epidermal cell usually contained a centrally located nucleus and few plastids that showed a perinuclear distribution, together with vacuoles that generally occurred towards the basal end of the cell (Fig. 6J). Their thin, outer cellulosic cell walls had a relatively thick cuticle (Fig. 6H, I, K).
Both the labellar groove and its secretion stained for proteins with Coomassie brilliant blue (Fig. 6C, G) and for mucilage with ruthenium red (Fig. 6D–F), but hardly at all for lipids with Sudan III. Further histochemical testing with mercuric bromophenol blue and Coomassie brilliant blue revealed that the cytoplasm of epidermal cells contained protein (Fig. 6G), whereas IKI and PAS showed that starch was absent from these cells, but present in sub-epidermal and ground parenchyma (Fig. 6I, L). Treatment with FeCl3 did not reveal the presence of phenolic substances in cells of the labellum. Only the cuticle of epidermal cells stained for lipids with Sudan III, the cytoplasm and the secretion produced by this tissue remaining unstained, as did the contents of sub-secretory cells and ground parenchyma (Fig. 6H).
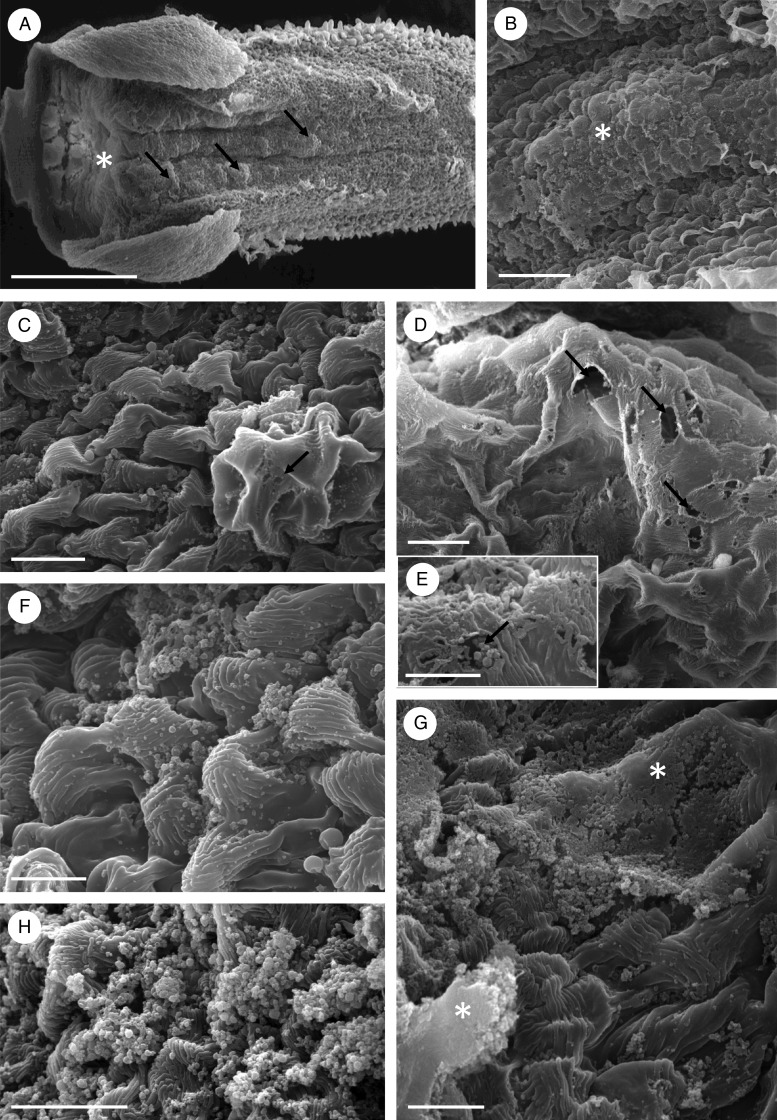
Scanning electron microscopy showed that the secretion was particularly abundant towards the basal part of the labellum (Fig. 7A, B) and that the floor of the entire length of the median longitudinal groove was verrucose (Fig. 7A, B). Cuticular blisters resulting from sub-cuticular accumulation of secretion (Fig. 7C, D) were often found on these verrucae. Discharge of secretion onto the adaxial epidermal surface probably results from stretching and tearing of the blister, although there is some evidence that it may also occur via cuticular pores (Fig. 7C–E). Once discharged, the secretion coats the striate (Fig. 7F) and disrupted cuticle. Pores and tears were seemingly confined to cuticular blisters (Fig. 7C, D) and secreted material was deposited on the surface of epidermal cells either as individual granules (Fig. 7C, E), as masses of granules (Fig. 7F, H) or as extensive, compacted sheets (Fig. 7G).
Fig. 7.
Bulbophyllum careyanum – SEM. (A) Labellum showing the median longitudinal groove with copious, amorphous secretion (asterisk) and verrucae (arrows). (B) Palisade-like epidermal cells coated with granular secretion (asterisk). (C) Palisade-like cells with finely striate cuticle. Note the presence of presumed pores (arrow) in the blistered cuticle. (D) Cuticular blister with numerous pores or tears (arrows). (E) Granules of secretion are seemingly released through the cuticular pore (arrow). (F) Granules of secreted material on the surface of the striate cuticle of palisade-like epidermal cells. (G) The copious secretion may appear as extensive, compacted sheets (asterisks) or (H) as masses of granules. Scale bars = 500 μm, 50 μm, 20 μm, 20 μm, 10 μm, 10 μm, 20 μm and 20 μm, respectively
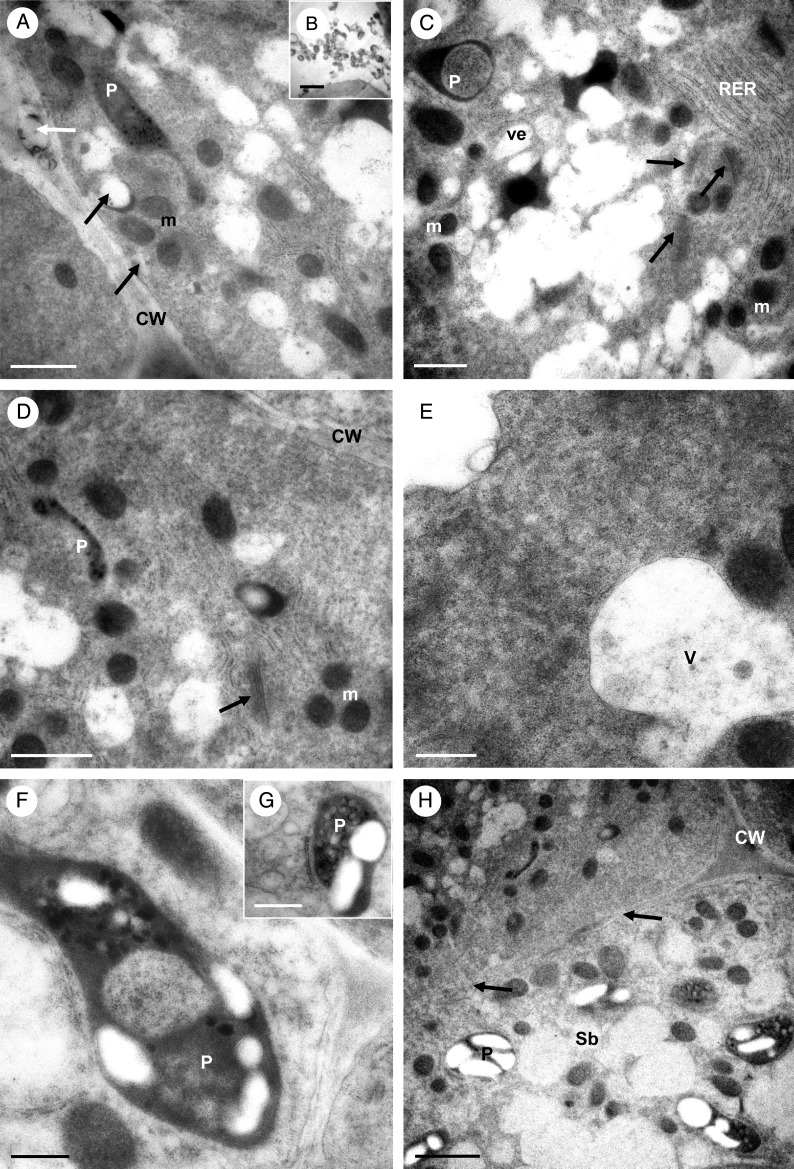
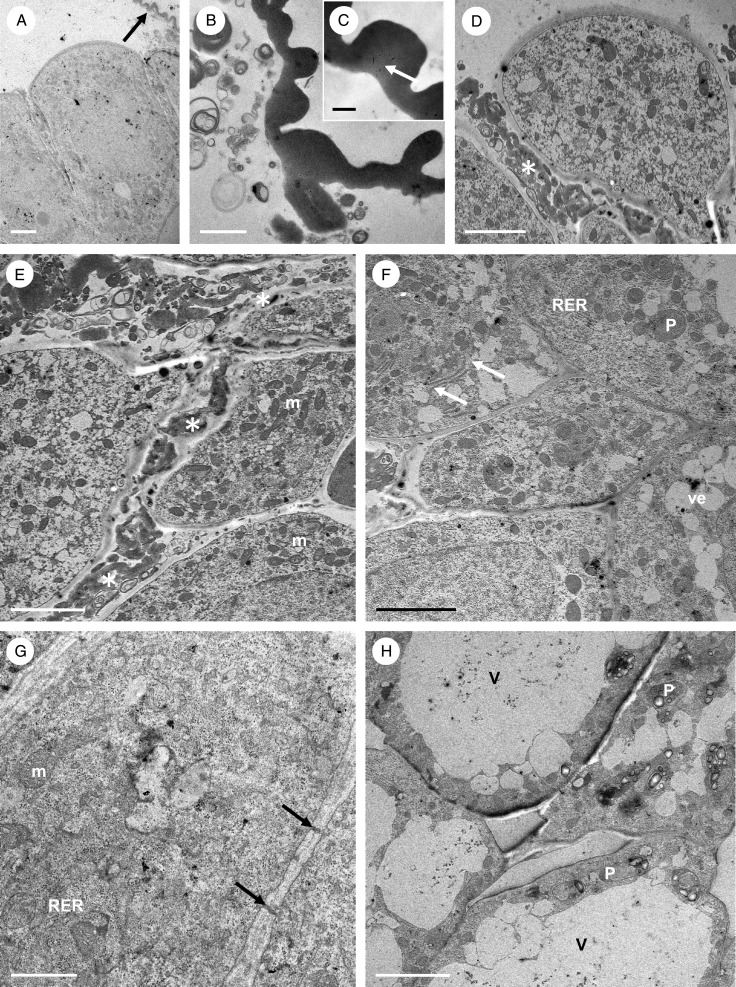
Transmission electron microscopy observations of the cytoplasm of palisade epidermal cells once more showed a predominance of RER (but both short and long profiles here) and ribosomes (both free and bound), together with abundant mitochondria, vesicles and dictyosomes (Fig. 8A, C–E). Occasional, irregularly shaped plastids were also observed, containing an electron-dense stroma, small plastoglobuli and, infrequently, minute starch grains not generally visible using LM (Fig. 8A, C, D, F, G). These plastids were often surrounded by long profiles of RER (Fig. 8D, F, G). Vacuoles often contained globular or irregular electron-dense bodies of various sizes, as well as flocculent material (Fig. 8E) and, occasionally, membranous profiles. Moreover, secretory vesicles of various sizes collected alongside the plasmalemma, and fused with the latter (Fig. 8A). It is proposed that these vesicles transport material similar to that found in the intercellular spaces, to the cell surface (Fig. 8B). Mitochondria and RER profiles were also abundant in sub-epidermal and ground parenchyma cells, but these cells possessed larger, starch-containing plastids (amyloplasts) than palisade cells (Fig. 8H). The cytoplasm of palisade-like epidermal cells and that of parenchymatous sub-epidermal cells was connected by means of numerous plasmodesmata contained within primary pit-fields (Fig. 8H).
Fig. 8.
Bulbophyllum careyanum – TEM. (A) Apical electron-dense cytoplasm of a palisade-like cell with numerous RER profiles and mitochondria. Secretory vesicles gather near to and fuse with the plasmalemma (black arrows). Secreted material present in the intercellular space is indicated by a white arrow. (B) Detail of secretory granules accumulating in the intercellular space. (C) Cytoplasm with numerous RER profiles, dictyosomes (arrows) and secretory vesicles. Note the irregularly shaped plastid with electron-dense stroma and enclave. (D) Palisade-like epidermal cell containing RER profiles, plastids, mitochondria and dictyosomes (arrow). (E) Cytoplasm of a similar cell with RER, free ribosomes and vacuoles containing globular material. (F) Plastid with mitochondria and RER. Such plastids contain starch grains and small plastoglobuli. (G) A similar plastid containing small starch grains and internal membranes. Note the secretory vesicle developing from the RER. (H) Palisade-like epidermal cell and adjoining sub-epidermal cell, the latter containing cytoplasm with less prominent RER profiles, but with well-developed amyloplasts containing starch and plastoglobuli. Plasmodesmata occurring in primary pit-fields of the cell wall (arrows) connect the adjoining cells. Scale bars = 1 μm, 1 μm, 1 μm, 1 μm, 500 nm, 500 nm, 1 μm and 2 μm, respectively. CW, cell wall; m, mitochondrion; P, plastid; RER, rough endoplasmic reticulum; Sb, sub-epidermal cell; V, vacuole; ve, vesicle.
Bulbophyllum morphologorum
In this species, the racemose inflorescence was pendulous, compact and rather more truncated (Fig. 2C) than those of the two above-described species. Flowers of this species had a mildly unpleasant, but nondescript scent. The stelidia were white and more broadly triangular than those of B. orientale, but seemingly less rigid than those of B. careyanum. A rocking labellum was present, the mid-lobe being linguiform with a median longitudinal groove flanked by two dark-red or purple, longitudinal ridges (Fig. 2D). Secreted material accumulated in this groove and, by day 2–3 of anthesis, completely filled the latter. This substance was viscid, glistening and lacked a sugary taste. Glistening droplets also occurred on both adaxial and abaxial surfaces of the sepals. On day 4, much less secretion was present in the labellar groove, suggesting that it might have been re-absorbed, but often a dry, silvery plug of residue could be seen at its distal end. Usually, by day 4 of anthesis, the labellar lamina had folded upwards and lengthwise along the median longitudinal axis and, finally, it withered. Entire flowers stained only very faintly with Sudan III, slightly more so along the proximal one-third of the length of the labellar ridges. However, the degree of staining was not sufficient to indicate the presence of lipid.
The adaxial, secretion-filled, median groove of the labellum (Fig. 9A–D) contained palisade-like, epidermal cells of mean dimensions 67·4 × 8·7 μm (Fig. 9E–N). These contained dense cytoplasm with a centrally located nucleus and several vacuoles which were mainly present in the basal part of the cells (Fig. 9M). Occasional plastids were seen close to the nucleus and in the apical part of the cells. The thin, cellulosic outer cell walls had a thick cuticle (Fig. 9F–O). This also extended along the radial walls of the palisade-like cells, often covering the apical half of these cells (Fig. 9L–N). The isodiametric cells of the sub-epidermal parenchyma were relatively small, with a mean diameter of 10·2 μm. They contained dense cytoplasm and a large nucleus (Fig. 9K–N). Collateral vascular bundles ran through the ground parenchyma of the labellum (Fig. 9E, G). Scattered idioblasts with raphides were found throughout the ground parenchyma, the larger (up to 94 μm in diameter) tending to occur near the abaxial surface of the labellum (Fig. 9E, G).
Fig. 9.
Bulbophyllum morphologorum – LM. (A) Labellum showing articulation (arrow) with the column-foot. (B) Adaxial view of the labellum, unstained. (C) Labellar groove stained for protein with Coomassie brilliant blue. (D) Labellar groove stained for mucilage with ruthenium red. (E) Transverse section of the labellum stained with Coomassie brilliant blue. Note the intense staining of palisade-like cells in the groove and the underlying parenchyma. Large idioblasts and collateral vascular bundles occur in the ground parenchyma. (F) Cuticular blister containing secretion that stains with Coomassie brilliant blue. (G) Transverse section of the labellum stained with Sudan III. (H) Detail showing intense staining of the cuticle with Sudan III. (I) Secretion beneath the detached cuticle stained for mucilage with ruthenium red. (J, K) Sections stained with IKI show that starch is absent from palisade-like epidermal cells. (L) The PAS reaction also failed to detect starch in palisade-like cells. (M, N) Palisade-like epidermis and sub-epidermal parenchyma stained with MB/AII. Note that the cuticle extends along half the length of the radial walls of palisade-like cells. (O) Paradermal section with secretion beneath the detached cuticle staining only slightly with MB/AII. Scale bars = 5 mm, 1 mm, 1 mm, 1 mm, 500 μm, 50 μm, 500 μm, 50 μm, 50 μm, 100 μm, 50 μm, 50 μm, 50 μm, 50 μm and 50 μm, respectively. E, epidermis; I, idioblast; PA, ground parenchyma; R, raphides; SE, sub-epidermal layer; Vb, vascular bundle.
The epidermal cells lining the median longitudinal groove of the labellum were coated with secretion that stained strongly for protein with Coomassie brilliant blue (Fig. 9C) and for mucilage with ruthenium red (Fig. 9D). The labellar groove and the associated secretion did not, however, stain for lipids with Sudan III. Nevertheless, the cytoplasm of epithelial cells stained intensely for protein with mercuric bromophenol blue and Coomassie brilliant blue (Fig. 9E, F). Starch was absent from these cells but was present in sub-epidermal and ground parenchyma cells, in particular those located close to vascular bundles (Fig. 9J–L). Sudan III stained only the cuticle of the epidermal cells, with the cytoplasm and sub-cuticular accumulations of secreted material remaining relatively unstained. Similarly, the cell contents of sub-epidermal and ground parenchyma did not stain for lipids (Fig. 9G, H). However, the sub-cuticular secretion stained for mucilage with ruthenium red (Fig. 9I), and to a lesser extent with MB/AII (Fig. 9M–O). Treatment with FeCl3 did not reveal the presence of phenolic compounds.
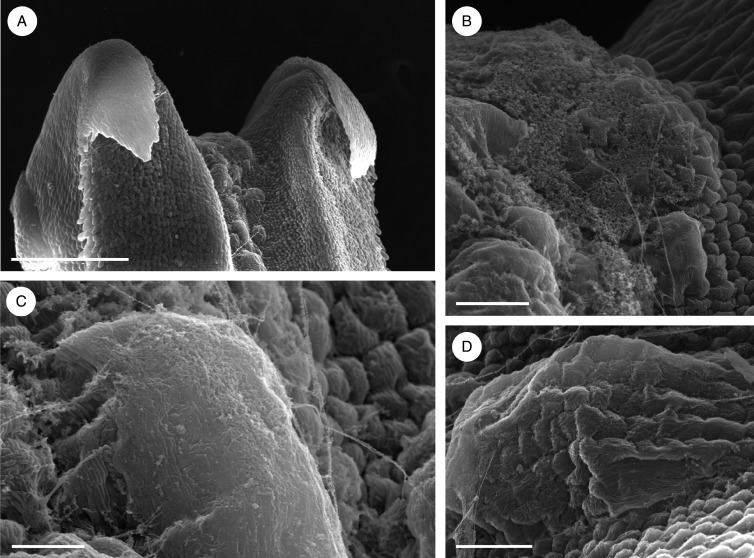
Observations by SEM showed the surface of the labellar groove to be verrucose (Fig. 10A–C), the secretion accumulating primarily as globules towards the basal part of the labellum (Fig. 10A, D, E). The cuticle was deeply striate and coated with secreted material. The spherical, striate bodies observed may represent osmophores (Fig. 10F). At higher magnifications, the adaxial labellar surface appeared squamous (Fig. 10G). Cuticular pores or cracks, however, were not observed (Fig. 10F, G).
Fig. 10.
Bulbophyllum morphologorum – SEM. (A–C) Adaxial view of the labellum showing the presence of verrucae along the entire length of the median longitudinal groove. (D) Base of the labellum showing articulation (arrow) with the column-foot. (E) Basal part of the labellum coated with copious, amorphous secretion. (F) Detail of secretion and possible osmophore (arrow) with striate cuticle. (G) Squamous adaxial surface formed of palisade-like cells with a deeply striate cuticle and a globule of secretion. Scale bars = 1 mm, 200 μm, 200 μm, 200 μm, 50 μm, 10 μm and 20 μm, respectively.
Observations by TEM of the cytoplasm of palisade-like epidermal cells revealed the presence of abundant RER, frequently as long or circular profiles, the latter often surrounded by small vacuoles. Shorter RER profiles, however, accumulated close to the plasmalemma where they formed dilated cisternae or secretory vesicles (Fig. 11A–E). The cytoplasm also contained numerous mitochondria, together with dictyosomes, but relatively few plastids. Intravacuolar, myelin-like figures were also present (Fig. 11C–E). The cell wall was lamellate, but lacked pores and cavities (Fig. 11A, B). In paradermal section (as in LM preparations – Fig. 9O), osmiophilic secreted material accumulated between the individual palisade-like cells (Fig. 11C). Plasmodesmata in primary pit-fields allowed continuity between the cytoplasm of palisade-like epidermal cells and that of sub-epidermal parenchyma cells (Fig. 11D). The cytoplasm of sub-epidermal parenchyma cells also contained numerous RER profiles, mitochondria and plastids with numerous, small plastoglobuli and starch grains (Fig. 11F, G).
Fig. 11.
Bulbophyllum morphologorum – TEM. (A) Section through lamellate cell wall of a palisade-like epidermal cell with cuticle. Dilated cisternae of endoplasmic reticulum occur in the parietal cytoplasm. (B) The cytoplasm also contains numerous RER profiles and mitochondria. (C). Secretion (asterisks) accumulates in the intercellular spaces of adjacent palisade-like epidermal cells. Myelin-like configurations protrude into the periplasmic space. (D) The endoplasmic reticulum forms perivacuolar, circular profiles (see also B), and numerous plasmodesmata in primary pit-fields traverse the cell walls. (E) Long and short profiles of RER, together with mitochondria, occur in the parietal cytoplasm. (F) Section of a sub-epidermal parenchyma cell showing a plastid containing small starch grains and plastoglobuli. (G) Similar section of a sub-epidermal parenchyma cell showing detail of a plastid with a starch grain, occasional internal membranes and plastoglobuli. Scale bars = 1 μm, 2 μm, 5 μm, 2 μm, 500 nm, 1 μm and 500 nm, respectively. C, cuticle; CW, cell wall; ER, endoplasmic reticulum; m, mitochondrion; mf, myelin-like figure; P, plastid; RER, rough endoplasmic reticulum; St, starch.
Bulbophyllum wangkaense
The racemose inflorescence was elongate, cylindrical and, like that of B. morphologorum, distinctly pendulous (Fig. 2E). The flowers all opened more or less simultaneously. On day 1 of anthesis, the flowers produced a mildly unpleasant, but sweet odour, not unlike that of over-ripe fruit, which disappeared at night. By day 2, the flowers were less malodorous and slightly less turgid. Movement of the linguiform labellum seemed to be more restricted than in the other three species, and the ridges flanking the median longitudinal groove distally (but not proximally) were less pronounced (Figs 2F and 12B). The relatively short stelidia were white. Droplets of glistening secretion were present on the adaxial surface of the labellum and both adaxial and abaxial surfaces of the sepals. However, these droplets were much smaller and, consequently, more difficult to observe, than those produced by the other three species investigated.
Fig. 12.
Bulbophyllum wangkaense – LM. (A) Lateral view of the labellum showing articulation (arrow) with the column-foot. (B) Adaxial view of the labellum showing absence of intense staining of the median longitudinal groove following treatment with Sudan III for lipids. (C) Slight staining of the labellar groove for mucilage with ruthenium red. (D, E) Intense staining of the adaxial surface of the labellar groove for proteins with Coomassie brilliant blue. In (E), note the point of articulation of the labellum with the column-foot. (F) Cuticular blister with secretion stained for protein with Coomassie brilliant blue. (G) Transverse section of the labellum following staining with PAS. Insoluble polysaccharides were not detected in secreted material, but starch was present in ground parenchyma cells. (H) Cuticle overlying palisade-like cells stained with Sudan III. (I) Secretion stained for protein with mercuric bromophenol blue. (J) Transverse section of the labellum base following treatment with IKI showing that starch occurs mainly in cells of the ground parenchyma. Note also the large idioblasts with raphides. (K) Transverse section of a vascular bundle and starch-rich ground parenchyma cells. (L) Secretion beneath the detached cuticle did not stain with IKI. Idioblasts with raphides also occur in the sub-epidermal parenchyma. (M) Paradermal section of palisade-like cells and sub-epidermal parenchyma cells stained with MB/AII. (N) Palisade-like cells of the epidermis with detached cuticle and sub-epidermal parenchyma cells with raphides stained with MB/AII. Scale bars = 1 mm, 1 mm, 1 mm, 1 mm, 1 mm, 50 μm, 100 μm, 50 μm, 50 μm, 500 μm, 50 μm, 100 μm, 100 μm and 50 μm, respectively. E, epidermis; I, idioblast; PA, ground parenchyma; R, raphides; SE, sub-epidermal layer; Vb, vascular bundle.
Again, a median longitudinal groove was present adaxially on the labellum (Fig. 12A–E), the secretion being particularly abundant at its basal end. This groove, as in the other three species, contained palisade-like epidermal cells of mean dimensions 35·6 × 11·1 μm (Fig. 12F–J, L, M). Also, as in the three other species, the isodiametric cells of the sub-epidermal parenchyma were relatively small (mean diameter = 12·1 μm) and contained intensely staining cytoplasm and a large nucleus (Fig. 12M). Collateral vacular bundles ran through the ground parenchyma (Fig. 12J, K), and large idioblasts with raphides were present in both the sub-epidermal and ground parenchyma (Fig. 12J, L, N).
The entire flower stained only faintly, but uniformly, with Sudan III, indicating the absence of elevated levels of lipids. The secreted material within the labellar groove did not stain strongly for lipids with Sudan III (Fig. 12B), but stained slightly more for mucilage with ruthenium red (Fig. 12C). However, both the labellar groove and secretion stained intensely for proteins with Coomassie brilliant blue (Fig. 12D, E). Further histochemical tests showed that the cytoplasm of epidermal cells stained for protein with Coomassie brilliant blue and with mercuric bromophenol blue (Fig.12F, I). Again, Sudan III stained only the cuticle (Fig. 12H). As the cuticle became detached from the epidermal cells, it formed prominent blisters (Fig. 12F–I, L), but the secreted material that had accumulated beneath it, together with the cytoplasm of palisade-like cells, remained unstained with this reagent (Fig. 12H). Although starch was absent from epidermal cells, it was present in the ground parenchyma (Fig. 12G, J, L). Treatment with FeCl3 did not reveal the presence of phenolic compounds.
Once more, SEM observations revealed the surface of the labellar groove to be verrucose (Fig. 13A). Cuticular blisters occurred most frequently at the basal part of the groove (Fig. 13B–D), and amorphous secretion was often visible on their surface. The cuticle overlying the cell walls of palisade-like epidermal cells was thick and distinctly striate (Figs 13C, D and 14A–C), homogeneous, electron-dense and osmiophilic (Fig. 14B, C). Observations using SEM and TEM did not reveal the presence of cuticular pores or cracks, but very fine micro-channels were visible in the cuticle at higher magnifications (Fig. 14C).
Fig. 13.
Bulbophyllum wangkaense; – SEM. (A) Proximal part of the labellum, the central groove containing verrucae. (B) Palisade-like epidermal cells that are coated with amorphous secretion are found in the median groove. Note the presence of cuticular blisters (C) Slightly striate, but mainly smooth cuticular blister with surface secretion. (D) An earlier stage of cuticular blister development. Scale bars = 500 μm, 50 μm, 20 μm and 50 μm, respectively.
Fig. 14.
Bulbophyllum wangkaense – TEM. (A) Cuticle (arrow) that has become detached from palisade-like cells. (B) Secreted material accumulates beneath the detached cuticle. (C) Micro-channels (arrow) may be present in the cuticle. (D) Secretion also collects between palisade-like cells (asterisk). (E) Palisade-like cells containing numerous mitochondria and vesicles. Note the presence of secretion (asterisks) between these cells. (F) Mitochondria and RER profiles with dilated ends (arrows) occur in the cytoplasm of palisade-like cells. (G) Cytoplasm with mitochondria and abundant RER profiles. Plasmodesmata occur in primary pit-fields of transverse walls (arrows). (H) Sub-epidermal parenchyma cells with plastids containing starch grains. Scale bars = 5 μm, 2 μm, 500 nm, 5 μm, 5 μm, 5 μm, 1 μm and 5 μm, respectively. m, mitochondrion; P, plastid; RER, rough endoplasmic reticulum; V, vacuole; ve, vesicle.
Mitochondria, short profiles of RER and numerous vesicles of various sizes (Fig. 14A, D–G) formed by the dilation of RER profiles (Fig. 14F) predominated. Dictyosomes were also present. Myelin-like figures were not observed. However, electron-dense, globular, osmiophilic material was seen in some vacuoles. Similar bodies, but of irregular shape, were also present in vacuoles, but these occurred in greater numbers extracellularly, both within cuticular blisters and in the intercellular spaces of palisade-like cells (Fig. 14B, D–E). The occasional, small plastids lacked starch and contained only a few plastoglobuli. Numerous plasmodesmata within primary pit-fields joined the cytoplasm of epidermal cells with that of adjoining cells (Fig. 14F, G). The cytoplasm of sub-epidermal cells also contained abundant mitochondria and RER. However, unlike epidermal cells, their plastids contained several starch grains (Fig. 14H).
DISCUSSION
The results were almost identical for all four species investigated in terms of labellar morphology and anatomy, and, indeed, de Pádua Teixeira et al. (2004) have commented on the highly conservative character of the Bulbophyllum labellum. In each case, the mid-lobe of the labellum was linguiform with an adaxially located, median longitudinal groove. The lateral lobes of the labellum were relatively small and forwardly pointing, and articulation to the column-foot allowed the labellum to rock. The adaxial groove contained palisade-like, epidermal secretory cells having a relatively thick, homogeneous and striate cuticle. At high magnifications, the cuticular striae of B. morphologorum were much more pronounced than those of the other species investigated and resembled those present on the sepals of Neotropical B. tripetalum Lindl. (fig. 2C in Nunes et al., 2014). Furthermore, under SEM, the adaxial labellar surface of B. morphologorum appeared squamous, similar to that of B. popayanense Kraenzl. (fig. 4H, I in Nunes et al., 2014). In all species investigated, a sub-epidermal, parenchymatous layer of smaller isodiametric cells was present, enclosing ground parenchyma containing collateral vascular bundles and idioblasts of varying dimensions, the latter containing raphides. The cuticle became blistered as secreted material accumulated between it and the wall of the epidermis. With the exception of B. careyanum, no cuticular cracks, tears or pores were evident. However, there was some evidence of micro-channels in the cuticle of B. wangkaense.
The palisade-like cells were nucleate and vacuolate with dense cytoplasm containing plastids, mitochondria, RER profiles and dictyosomes. These cells closely resembled both the oil-secreting cells of the labellar, epithelial elaiophores of Oncidiinae (Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2007, 2013; Stpiczyńska and Davies, 2008, Aliscioni et al., 2009; Davies and Stpiczyńska, 2009) and Rudolfiella picta (Maxillariinae; Davies and Stpiczyńska, 2009), as well as the secretory cells that occur in the adaxial labellar groove of Neotropical species of Bulbophyllum, such as members of sect. Didactyle (Lindl.) Cogn., including B. involutum Borba, Semir & F. Barros and B. meridense Rchb.f. (de Pádua Teixeira et al., 2004; fig. 4R and Q, respectively, in Nunes et al., 2014). This is of interest in that whereas oil-secreting Oncidiinae are pollinated by specialized oil-collecting bees, such as Tetrapedia, Paratetrapedia and Centris (Buchmann, 1987; Singer and Cocucci, 1999; van der Cingel, 2001; Singer et al., 2006), these Neotropical species of Bulbophyllum are pollinated by Milichiidae, Tachinidae and Sciaridae flies (Sazima, 1978; Borba and Semir, 1998, Silva et al., 1999; de Pádua Teixeira et al., 2004; Azevedo et al., 2006). In the species investigated here, both the contents of these cells and the material that they secrete stained strongly for protein, less so for mucilage, but showed no reaction whatsoever for polyphenolic compounds. Only the cuticle stained for lipids, and starch occurred mainly in the sub-epidermal and ground parenchyma. Thus, it would appear that in each case, the labellar secretion consisted of protein in a matrix of mucilage, but lacked lipids, the pink coloration which developed when entire flowers were immersed in Sudan III being due merely to the staining of the cuticle and/or droplets of volatile fragrances which were not visible in prepared sections, possibly because they were leached from the tissue by solvents during tissue processing. These histochemical results are consistent with myophily. The sub-epidermal cells had a similar organelle complement to the palisade-like epidermal cells. However, unlike the latter, they contained abundant amyloplasts with starch. These results differ from those obtained for Neotropical Bulbophyllum spp. In those species, the palisade-like epidermal cells mainly contained lipid, whereas the sub-epidermal cells contained starch or proteins (de Pádua Teixeira et al., 2004; Nunes et al., 2014). However, unlike the present study, neither of the last two studies involved ultrastructural investigations, and the TEM observations presented here for sect. Racemosae are consistent with our histochemical results. For example, the abundance of RER and the paucity of smooth endoplasmic reticulum (SER) profiles in secretory cells were consistent with protein, rather than lipid synthesis. Furthermore, lipid droplets were scant. Dictyosomes, organelles characteristic of carbohydrate metabolism, in particular mucilage production, were present in both palisade-like epidermal and sub-epidermal cells. It is thus proposed that mucilage precursors produced by the dictyosomes of sub-epidermal cells may pass along the symplast via plasmodesmata into the adjoining palisade-like cells, which contain extensive arrays of RER. Here, together with mucilage produced by the dictyosomes of epidermal cells, they undergo chemical modification to form a mucilaginous matrix that acts as a vehicle for the secretion and transport of protein produced by RER. Following vesicle-mediated transport across the cytoplasm, this material traverses the cell wall and accumulates in blisters formed by distension of the cuticle. These blisters may rupture, releasing the secretion onto the surface of the labellum. There is some evidence, however, that in certain species, the secretion may traverse the cuticle via cuticular pores. Hydrolysis of sub-epidermal starch probably generates the carbohydrate and, together with mitochondria, much of the energy required for this process. Once present on the labellar surface, high atmospheric relative humidity, coupled with the hygroscopic properties of mucilage, may cause the secretion to swell and become more obvious to pollinators. Micro-channels, similar to those present in the cuticle of the floral osmophores of Chloraea membranacea Lindl. (Sanguinetti et al., 2012), may permit the trans-cuticular passage of fragrances.
The presence of identical labellar anatomy in Asian and Neotropical species of Bulbophyllum, despite the differences in pollinators on those two continents, indicates parallelism of floral anatomy. Indeed, Gravendeel et al. (2004) demonstrated by means of phylogenetic analysis using plastid (matK) genome data that the genus probably arose on the Asian continent, with the African and Neotropical species forming two monophyletic groups sister to each other, and that this entire group is, in turn, sister to the Asian clade. Moreover, the African B. falcatum (Lindl.) Rchb.f. was recognized as sister to the Neotropical sections of Bulbophyllum, thus supporting the hypothesis that the New World was colonized from Africa (Gravendeel et al., 2004; Smidt et al., 2011).
Ong and Tan (2012), who investigated the pollination of three species of Bulbophyllum sect. Racemosae, including that of B. lilacinum and B. peninsulare, reported D. ananassae flies removing pollinia and feeding on the labellar secretion of all three taxa. It is therefore probable that the subjects of the present study are similarly pollinated and that in the absence of any other candidate, protein-rich mucilage produced by the secretory cells provides the only likely food reward. This contrasts strongly with the claim of earlier studies that Bulbophyllum spp. produce food rewards in the form of floral oils. Interestingly, Sazima (1978) and Borba and Semir (1998) have suggested that since only female flies were recorded visiting flowers of species of Bulbophyllum native to south-eastern Brazil, they were attracted to the flowers by their instinct for oviposition. This, however, did not occur in species of sect. Racemosae, nor was the sex of the flies recorded (Ong and Tan, 2012; P. T. Ong, 2014, pers. comm.). Nevertheless, it is easy to see how the labellar secretion could have provided these insects with a rich source of protein for egg production.
Thus, although many species of Asian Bulbophyllum have been reported to produce oil-rich food rewards, this does not appear to be the case for any of those species of sect. Racemosae studied to date.
ACKNOWLEDGEMENTS
The authors are grateful to the Stanley Smith (UK) Horticultural Trust (grant to K.L.D.) and the Faculty of Biology, University of Warsaw (BST grant to M.S.) for financially supporting this work. Thanks are also due to Marek Wróbel (Agroecological Laboratory, University of Life Sciences, Lublin, Poland for SEM support), Michał Rawski (Maria Curie-Skłodowska University, Lublin, Poland for TEM support), Agnieszka Krzyk (Botanic Garden, University of Warsaw, Poland for tissue preparation) and Alan Gregg (Swansea Botanical Complex, UK for assistance in compiling the paper) for their contributions.
LITERATURE CITED
- Aliscioni SS, Torretta JP, Bello ME, Galati BG. Elaiophores in Gomesa bifolia (Sims) M.W. Chase & N.H. Williams (Oncidiinae: Cymbideae: Orchidaceae): structure and oil secretion. Annals of Botany. 2009;104:1141–1149. doi: 10.1093/aob/mcp199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo CO, Borba EL, van den Berg C. Evidence of natural hybridization and introgression in Bulbophyllum involutum Borba, Semir & F. Barros and B. weddellii (Lindl.) Rchb.f. (Orchidaceae) in the Chapada Diamantina, Brazil, by using allozyme markers. Revista Brasileira de Botânica. 2006;29:415–421. [Google Scholar]
- Blanco MA, Davies KL, Stpiczyńska M, Carlsward BS, Ionta GM, Gerlach G. Floral elaiophores in Lockhartia Hook. (Orchidaceae: Oncidiinae): their distribution, diversity and anatomy. Annals of Botany. 2013;112:1775–1791. doi: 10.1093/aob/mct232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borba EL, Semir J. Wind-assisted fly pollination in three Bulbophyllum (Orchidaceae) species occurring in the Brazilian campos rupestres. Lindleyana. 1998;13:203–218. [Google Scholar]
- Braga P. Aspectos biologicos das Orchidaceae da Amazônica Central. Acta Amazonica, Manaus. 1977;7:1–89. [Google Scholar]
- Brummitt RK, Powell CE. Authors of plant names. Kew, UK: Royal Botanic Gardens, Kew; 1992. [Google Scholar]
- Buchmann SL. The ecology of oil flowers and their bees. Annual Review of Ecology and Systematics. 1987;18:343–396. [Google Scholar]
- Chase MW, Barret RL, Cameron KN, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KM, editor. Orchid conservation. Kota Kinabalu, Sabah, Malaysia: Natural History Publications; 2003. pp. 69–89. [Google Scholar]
- Christensen DE. Fly pollination in the Orchidaceae. In: Arditti J, editor. Orchid biology: reviews and perspectives. Vol. VI. New York: John Wiley & Sons; 1994. pp. 415–454. [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination – America, Africa, Asia and Australia. Rotterdam, The Netherlands: A.A. Balkema; 2001. [Google Scholar]
- Davies KL, Stpiczyńska M. The anatomical basis of floral, food-reward production in Orchidaceae. In: Texeira da Silva JA, editor. Floriculture, ornamental and plant biotechnology. Vol. V. Global Science Books; 2008. pp. 392–407. [Google Scholar]
- Davies KL, Stpiczyńska M. Comparative histology of floral elaiophores in the orchids Rudolfiella picta (Schltr.) Hoehne (Maxillariinae sensu lato) and Oncidium ornithorhynchum H.B.K. (Oncidiinae sensu lato) Annals of Botany. 2009;104:221–234. doi: 10.1093/aob/mcp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Stpiczyńska M. Comparative labellar anatomy of resin-secreting and putative resin-mimic species of Maxillaria s.l. (Orchidaceae: Maxillariinae) Botanical Journal of the Linnean Society. 2012;170:405–435. [Google Scholar]
- Davies KL, Turner MP. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae) Annals of Botany. 2004;93:75–86. doi: 10.1093/aob/mch007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP, Gregg A. Atypical pseudopollen-forming hairs in Maxillaria Ruiz & Pav. (Orchidaceae) Botanical Journal of the Linnean Society. 2003a;143:151–158. [Google Scholar]
- Davies KL, Turner MP, Gregg A. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae) Annals of Botany. 2003b;91:439–446. doi: 10.1093/aob/mcg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler RL. The orchids – natural history and classification. London: Harvard University Press; 1990. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Fisher DB. Protein staining of ribboned epon sections for light microscopy. Histochemie. 1968;16:92–96. doi: 10.1007/BF00306214. [DOI] [PubMed] [Google Scholar]
- Gahan PB. Plant histochemistry and cytochemistry: an introduction. London: Academic Press; 1984. [Google Scholar]
- Garside S. The pollination of Satyrium bicallosum Thunb. Annals of the Bolus Herbarium. 1922;3:147–154. [Google Scholar]
- Gomiz NE, Torretta JP, Aliscioni SS. Comparative anatomy of elaiophores in the genus Gomesa (Orchidaceae) Turkish Journal of Botany. 2013;37:859–871. [Google Scholar]
- Gravendeel B, Smithson A, Slik FJW, Schuiteman A. Epiphytism and pollinator specialization. Drivers for orchid diversity? Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359:1523–1535. doi: 10.1098/rstb.2004.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen WA. Botanical histochemistry: principles and practice. San Francisco: W.H. Freeman; 1962. [Google Scholar]
- Jongejan P. Specializations in ways of attracting insects for pollination in the genus Bulbophyllum. Proceedings of the 14th World Orchid Congress, Glasgow; Edinburgh: HMSO; 1994. pp. 383–388. [Google Scholar]
- Knerr JN. The genus Bulbophyllum: a living phantasy. American Orchid Society Bulletin. 1981;50:1051–1056. [Google Scholar]
- Koehler DJ, Davenport D. Ultraviolet mimicry by Bulbophyllum lepidum? American Orchid Society Bulletin. 1983;52:359–363. [Google Scholar]
- Mazia D, Brewer PA, Alfert M. The cytochemical staining and measurement of protein with mercuric bromophenol blue. Biological Bulletin. 1953;104:55–67. [Google Scholar]
- Mickeliunas L, Pansarin ER, Sazima M. Biologia floral, melitofilia, e influência de besouros Curculionidae no sucesso reprodutivo de Grobya amherstiae Lindl. (Orchidaceae: Cyrtopodiinae) Revista Brasileira de Botânica. 2006;29:251–258. [Google Scholar]
- Millar A. Orchids of Papua New Guinea, an introduction. Canberra: Australian National University Press; 1978. [Google Scholar]
- Nunes ELP, Smidt EC, Stützel T, Ike Coan A. What do floral anatomy and micromorphology tell us about Neotropical Bulbophyllum section Didactyle (Orchidaceae: Bulbophyllinae)? Botanical Journal of the Linnean Society. 2014;175:438–452. [Google Scholar]
- Ong PT. The pollination of Bulbophyllum patens. The Orchid Review. 2011;119:146–149. [Google Scholar]
- Ong PT. Notes on the pollination of Bulbophyllum mandibulare Rchb.f. Malayan Orchid Review. 2012;46:67–68. [Google Scholar]
- Ong PT. The pollination of two Bulbophyllum species. The Orchid Review. 2013;121:152–155. [Google Scholar]
- Ong PT, Tan KH. Fly pollination in four Malaysian species of Bulbophyllum (Section Sestochilus) – B. lasianthum, B. lobbii, B. subumbellatum and B. virescens. Malesian Orchid Journal. 2011;8:103–110. [Google Scholar]
- Ong PT, Tan KH. Three species of Bulbophyllum Section Racemosae pollinated by Drosophila flies. Malesian Orchid Journal. 2012;9:45–50. [Google Scholar]
- Ong PT, Hee AKW, Wee SL, Tan KH. The attraction of flowers of Bulbophyllum (Section Sestochilus) to Bactrocera fruit flies (Diptera: Tephritidae) Malesian Orchid Journal. 2011;8:93–102. [Google Scholar]
- Pacek A, Stpiczyńska M. The structure of elaiophores in Oncidium cheirophorum Rchb.f. and Ornithocephalus kruegeri Rchb.f. (Orchidaceae) Acta Agrobotanica. 2007;60:9–14. [Google Scholar]
- Pacek A, Stpiczyńska M, Davies KL, Szymczak G. Floral elaiophore structure in four representatives of the Ornithocephalus clade (Orchidaceae: Oncidiinae) Annals of Botany. 2012;110:809–820. doi: 10.1093/aob/mcs158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pádua Teixeira S, Borba EL, Semir J. Lip anatomy and its implications for the pollination mechanisms of Bulbophyllum species (Orchidaceae) Annals of Botany. 2004;93:499–505. doi: 10.1093/aob/mch072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauw A. Floral syndromes accurately predict pollination by a specialized oil-collecting bee (Rediviva peringueyi; Melittidae) in a guild of South African Orchids (Coryciinae) American Journal of Botany. 2006;93:917–926. doi: 10.3732/ajb.93.6.917. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. Histochemistry: theoretical and applied, 4th edition, Volume 2, Analytical technology. Edinburgh: Churchill Livingstone; 1985. [Google Scholar]
- van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press; 1969. [Google Scholar]
- Pohl F. Zwei Bulbophyllum-Arten mit besonders bemerkenswert gebauten Gleit- und Klemfallenblumen. Beiheft Botanisches Zentralblat. 1935;53:501–518. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley HN. On the method of fertilization in Bulbophyllum macranthum and allied orchids. Annals of Botany. 1890;4:327–336. [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. New York: Oxford University Press; 1999. [Google Scholar]
- Sanguinetti A, Buzatto CR, Pedron M, et al. Floral features, pollination biology and breeding system of Chloraea membranacea Lindl. (Orchidaceae: Chloraeinae) Annals of Botany. 2012;110:1607–1621. doi: 10.1093/aob/mcs221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazima M. Polinização por moscas em Bulbophyllum warmingianum Cogn. (Orchidaceae), na Serra de Cipó, Minas Gerais, Brasil. Revista Brasileira de Botânica. 1978;1:133–138. [Google Scholar]
- Seidenfaden G, Wood JJ. The orchids of Peninsular Malaysia and Singapore. Fredensborg, Denmark: Olsen & Olsen; 1992. [Google Scholar]
- Silva UF, Borba EL, Semir J, Marsaioli AJ. A simple solid injection device for the analyses of Bulbophyllum (Orchidaceae) volatiles. Phytochemistry. 1999;50:31–34. [Google Scholar]
- Silvera KI. 2002. Adaptive radiation of oil-rewarding compounds among Neotropical orchid species (Oncidiinae) MSc Thesis, University of Florida. [Google Scholar]
- Singer RB, Cocucci AA. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana. 1999;14:47–56. [Google Scholar]
- Singer RB, Marsaioli AJ, Flach A, Reis MG. The ecology and chemistry of pollination in Brazilian orchids: recent advances. In: Teixeira da Silva JA, editor. Floriculture, ornamental and plant biotechnology. Global Science Books; 2006. pp. 569–582. Vol. IV. [Google Scholar]
- Smidt EC, Borba EL, Gravendeel B, Fischer GA, van den Berg C. Molecular phylogeny of the Neotropical sections of Bulbophyllum (Orchidaceae) using nuclear and plastid spacers. Taxon. 2011;60:1050–1064. [Google Scholar]
- Steiner KE. The pollination of Disperis (Orchidaceae) by oil-collecting bees in southern Africa. Lindleyana. 1989;4:164–183. [Google Scholar]
- Steiner KE. Oil orchids and oil bees in southern Africa – Disperis and Rediviva. South African Orchid Journal. 1993;42:2–5. [Google Scholar]
- Stpiczyńska M, Davies KL. Elaiophore structure and oil secretion in flowers of Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb.f.) Garay & Pabst (Oncidiinae: Orchidaceae) Annals of Botany. 2008;101:375–384. doi: 10.1093/aob/mcm297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL. Floral resin-secreting trichomes in Maxillaria dichroma Rolfe (Orchidaceae: Maxillariinae) Acta Agrobotanica. 2009;62:43–51. [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Elaiophore diversity in three contrasting members of Oncidiinae Benth. (Orchidaceae) Botanical Journal of the Linnean Society. 2007;155:135–148. [Google Scholar]
- Stpiczyńska M, Davies KL, Pacek-Bieniek A, Kamińska M. Comparative anatomy of the floral elaiophore in representatives of the newly re-circumscribed Gomesa and Oncidium clades (Orchidaceae: Oncidiinae) Annals of Botany. 2013;112:839–854. doi: 10.1093/aob/mct149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KH, Nishida R. Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. Journal of Chemical Ecology. 2000;26:533–546. doi: 10.1023/a:1016277500007. [DOI] [PubMed] [Google Scholar]
- Tan KH, Nishida R. Synomone or kairomone? Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies during pollination. Journal of Chemical Ecology. 2005;31:509–519. doi: 10.1007/s10886-005-2023-8. [DOI] [PubMed] [Google Scholar]
- Tan KH, Nishida R, Toong YC. Floral synomone of a wild orchid, Bulbophyllum cheiri, lures Bactrocera fruit flies for pollination. Journal of Chemical Ecology. 2002;28:1161–1172. doi: 10.1023/a:1016277500007. [DOI] [PubMed] [Google Scholar]
- Vermeulen JJ. Orchids of Borneo, vol. 2. Bulbophyllum. Sabah, Malaysia: Toihaan; 1991. [Google Scholar]