Abstract
Background and Aims
Considerable variation in seed size commonly exists within plants, and is believed to be favoured under natural selection. This study aims to examine the extent to which seed size distribution depends on the presence of competing neighbour plants.
Methods
Phaseolus vulgaris plants rooting with or without a conspecific neighbour were grown in soil with high or low nutrient availability. Seeds were harvested at the end of the growth cycle, the total nitrogen and phosphorus invested in seed production were measured and within-plant seed size distribution was quantified using a set of statistical descriptors.
Key Results
Exposure to neighbours' roots induced significant changes in seed size distribution. Plants produced proportionally more large seeds and fewer small ones, as reflected by significant increases in minimal seed size, mean seed size, skewness and Lorenz asymmetry coefficient. These effects were different from, and in several cases opposite to, the responses when the soil nutrient level was reduced, and were significant after correction for the amount of resources invested in seed production.
Conclusions
Below-ground neighbour presence affects within-plant seed size distribution in P. vulgaris. This effect appears to be non-resource-mediated, i.e. to be independent of neighbour-induced effects on resource availability. It implies that, based on current environmental cues, plants can make an anticipatory adjustment of their investment strategy in offspring as an adaptation to the local environment in the future.
Keywords: Anticipatory maternal effect, bet-hedging, game theory, neighbour detection, Phaseolus vulgaris, kidney bean, root competition, seed-setting, seed size variation, size inequality, skewness
INTRODUCTION
A considerable degree of variation in seed size within plants is commonly observed (Michaels et al., 1988; Silvertown, 1989; Ruiz de Clavijo, 2002; Völler et al., 2012). Such variation is often interpreted as an adaptive bet-hedging strategy (Harper et al., 1970; McGinley et al., 1987; McGinley and Charnov, 1988; Venable and Brown, 1988; Geritz, 1995). Many studies also reveal that plants modify the pattern of variation (i.e. distribution) to cope with their abiotic environmental conditions (e.g. temperature, Wulff, 1986; light, Galloway, 2001; nutrients, Galloway, 2001; water, Parciak, 2002). Here we demonstrate that seed size distribution may also be modified in response to the presence of a below-ground neighbour.
Within a species, seed size (following common practice, seed size refers to seed weight in this paper) often correlates positively with the competitiveness of the offspring (e.g. Houssard and Escarré, 1991; Eriksson, 1999; Lehtilä and Ehrlén, 2005; Dubois and Cheptou, 2012). Based on the trade-off, induced by resource limitation in plants, between competition (favours large seeds) and colonization (favours a large number of small seeds), Geritz (1995) extended an optimal offspring size model (Smith and Fretwell, 1974) by considering seedling competition and using evolutionary game theory. He assumed that (1) seedlings from larger seeds always outcompete seedlings from smaller seeds within a microsite, and (2) seeds are randomly dispersed over all microsites. He concluded that, due to the uncertainty of seed density (i.e. seedling competition intensity) in different microsites, plants producing seeds of various sizes would be favoured by natural selection. Meanwhile, they should adjust their seed size distributions in response to nutrient availability and the probability of seedling competition in microsites.
The most obvious impact of a below-ground neighbour is a reduction in soil resource availability (de Kroon et al., 2003). Thus, one might intuitively assume that introducing below-ground neighbours will have effects on the within-plant seed size distribution similar to those of limiting nutrients. The consequences often include a smaller mean seed size (e.g. in Desmodium paniculatum, Wulff, 1986; Campanula americana, Galloway, 2001; Sarcobatus vermiculatus, Breen and Richards, 2008) and a narrower seed size range caused by a decline in maximal seed size (Geritz, 1995).
However, the presence of a neighbour indicates not only a reduction in resource availability, but also, when seed dispersal is limited, future competition in the next generation (Geritz, 1995). That is, offspring of plants that grow close to neighbours are more likely to experience competition. A modelling study predicts that the adaptiveness of plant responses to environmental cues increases with the extent to which these cues accurately indicate future environmental conditions (Wong and Ackerly, 2005). Recent evidence shows that plant roots can detect neighbouring plants, independently of resource status (reviewed in Chen et al., 2012). This would leave open the possibility that plants can use neighbour cues to adjust their reproductive strategy to the probability of seedling density in next seasons. Indeed, the model of Geritz (1995) predicts that as total seed density within the seed shadow of a plant increases with neighbour density, its seeds will have a higher probability of dispersal to a microsite with several other seeds, and that the production of larger seeds owing to their advantage in competition should be favoured over the production of smaller ones. Empirical evidence shows that Bromus madritensis has fewer small seeds with more equal seed provisioning in response to higher competition intensity, but not to lower soil nutrient level (Violle et al., 2009). Similarly, in the marine animal Bugula neritina, mothers experiencing competition produce larger offspring (Allen et al., 2008).
Therefore, we hypothesize that (1) if plants only respond to resource reduction effects of below-ground neighbours, they should produce seeds with a smaller mean size and a smaller maximal size. On the other hand, (2) if they respond to non-resource effects of below-ground neighbours, which indicate a higher likelihood of seedling competition in the future, they should invest proportionally more in large seeds within the size distribution. This would imply that the perception of current environmental cues enables plants to adjust their offspring investment as an adaptation to the future environment.
To test these hypotheses, we performed an experiment using Phaseolus vulgaris plants. This species has been shown to detect its conspecific neighbours (Maina et al., 2002). Its limited seed dispersal distance, caused by the relatively large seed size, implies that seeds from such competing plants have a high chance of experiencing seedling competition with seeds from their neighbours. For this species, plants from large seeds outcompete plants from small seeds via faster seedling growth (Cipollini and Stiles, 1991).
The experiment was conducted with plants rooting either with or without a conspecific neighbour in soil with high or low nutrient level. In addition to the mean and coefficient of variation (CV), which cannot specify changes in investment in the production of small and large seeds, we applied a set of descriptors to comprehensively characterize the within-plant seed size distribution. Furthermore, we used the total amounts of nitrogen and phosphorus in seed production as covariates in the analyses to disentangle neighbour-induced non-resource effects from neighbour-induced resource reduction effects.
MATERIALS AND METHODS
Experimental design
The experiment was conducted at a plastic greenhouse facility of Utrecht University, Utrecht, the Netherlands. Commercially available seeds of Phaseolus vulgaris (red kidney bean, ‘Canadian wonder’) with similar size were selected and sown solitarily in small pots (0·25 L) with moist sand. Seven days later, seedlings with a height of ∼10 cm and having two healthy leaves were selected and transplanted into round plastic pots (19·5 cm height × 25 cm diameter, ∼7·5 L) filled with a mixture of potting soil and sand (1 : 2 in volume). These seedlings were randomly subjected to one of four treatments involving a below-ground neighbour (‘presence’, two plants in one pot; ‘absence’, two plants in two adjacent pots) and soil nutrient level (‘high’ or ‘low’ achieved with 1·0 or 0·3 g L–1 nutrient solution containing 19 % N + 6 % P2O5 + 20 % K2O + 3 % MgO + micronutrient fertilizer; Kristalon Blauwmerk, Yara Benelux, the Netherlands). To standardize above-ground interactions, the seedling pairs were planted so that the distance between the two (measured from the stem) was 12·5 cm in both treatments (i.e. within the pot or between the plants in their own pots). During the cultivation period, every pot received 240 mL nutrient solution (high or low) twice a week. Plants were grown in a plastic-roofed greenhouse where light availability was ∼50 % of natural daylight, and watered daily (except on the days when nutrient solutions were applied) from June to October. Each treatment combination consisted of 24 plants (i.e. 12 pairs), which were arranged in two blocks on benches to take microenvironmental variation in the plastic greenhouse into account.
Harvest and measurements
On 10 October 2012, at the end of the growth cycle when most of the leaves were yellow and dry, pods were harvested (∼114 d of growth). Most plants had many ripe pods and some aborted small pods, but on several plants we also found a few unripe ones. Hence, pods were divided into ripe, aborted and unripe groups, oven-dried at 70 °C for 4 d and weighed separately. We summed the mass of the three pod groups to determine total pod mass for each plant. We did not harvest the vegetative parts of the plants, as these were largely gone by the time of harvesting.
As seeds in aborted pods were undeveloped and seeds in unripe pods were still at intermediate developmental stages, they could not be treated in the same way as the mature seeds in ripe pods and included equally in the seed pool for the next generation. Consequently, only viable seeds (easily distinguishable from aborted ones by appearance and weight) from ripe pods (>95 % of the harvested pods) were weighed individually and used for subsequent analyses. After measuring the dry mass of individual seeds, all viable seeds of each mother plant were ground to a powder. Then total nitrogen content and total phosphorous content were determined with a continuous flow analyser (Skalar, Breda, the Netherlands) after Kjeldahl digestion.
To comprehensively characterize the within-plant seed size distribution for each plant, we not only calculated the mean and CV, but also applied additional descriptors, including maximal (i.e. heaviest) and minimal (i.e. lightest) sizes, which define the absolute range of the distribution; skewness, which quantifies the degree of number-related asymmetry of the distribution (Sokal and Rohlf, 1995); the Gini coefficient, which describes the degree of resource investment bias (i.e. size inequality or hierarchy) in the distribution (Weiner and Solbrig, 1984; see also the bias adjustment in Deltas, 2003); and the Lorenz asymmetry coefficient, which differentiates the major contributor (large seeds versus small seeds) to resource investment bias (Damgaard and Weiner, 2000).
Data analysis
In the experimental set-up, two adjacent plants were nested in each plant pair, and all plant pairs were nested in two blocks. We examined the overall effect of a below-ground neighbour on within-plant seed size distribution using linear mixed models with below-ground neighbour (presence versus absence), soil nutrient level (high versus low) and their interaction as fixed factors, with block and plant pair as random factors.
Since the amount of resource invested in seed production accurately represents the effects of plant nutrient availability (i.e. both soil nutrient level and neighbour-induced resource reduction), the neighbour-induced non-resource effect on seed size distribution can be examined by correcting for the level of resource investment in seed production in the analyses. This was done with linear mixed models, in which below-ground neighbour (presence versus absence) was introduced as a fixed factor, resource investment (i.e. total seed mass, and total amount of nitrogen or phosphorus in seed production) as covariate, with block and plant pair as random factors. In other words, the detection of a significant non-resource effect of a below-ground neighbour provides evidence that neighbour presence can affect the seed size distribution irrespective of its effect on resource availability for seed production, as represented by the level of resource invested in seeds.
Total pod mass, total seed mass, total nitrogen and total phosphorus content were log-transformed and seed number was square-root-transformed. For all the analyses, the interaction term was excluded from the full model if it did not show significant effects (Supplementary Data Tables S1–S4). All statistical analyses were performed using the lme4 (Bates et al., 2013) and lmerTest (Kuznetsova et al., 2013) packages in R version 3.0.2 (R Core Team, 2013).
RESULTS
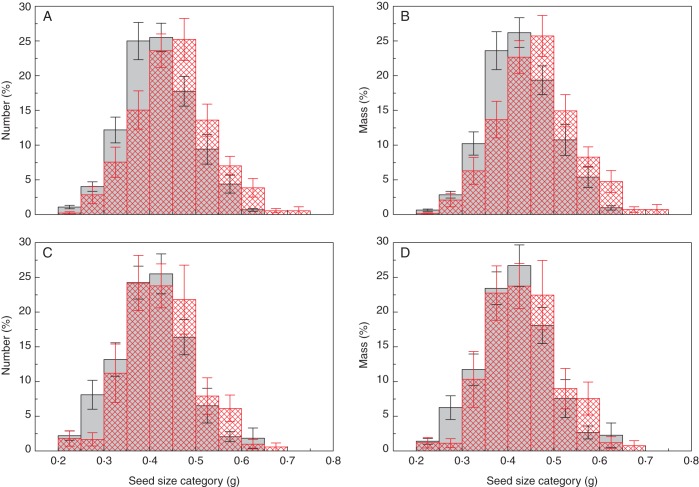
In total, 3001 mature seeds were collected and analysed. For each treatment combination, the within-plant seed size distribution (relative numbers and masses of seeds in different size categories) of P. vulgaris is presented in Fig. 1.
Fig. 1.
Relative numbers and masses of seeds in different size categories of Phaseolus vulgaris plants growing in the presence (open bars with red cross-hatching) and absence (grey bars) of a below-ground neighbour at (A, B) high and (C, D) low nutrient levels. Data were first standardized for each plant (as a percentage) and then averaged to represent the individual level. Data are means ± s.e.
Overall effect of a below-ground neighbour
Total pod mass, total seed mass, seed number and total amounts of nitrogen and phosphorus in the seeds produced by individual plants were significantly reduced by a below-ground neighbour and low soil nutrient level, but only for seed number was there a significant neighbour × nutrient interaction effect (Table 1, Fig. 2).
Table 1.
Overall effects of a below-ground neighbour (BN, i.e. joint effects of resource reduction and non-resource-related cues from neighbours) and soil nutrient level (NL) on total reproductive output and within-plant seed size distribution of Phaseolus vulgaris plants
| Total reproductive output | Fixed effects |
Descriptors of distribution | Fixed effects |
||||
|---|---|---|---|---|---|---|---|
| BN | NL | BN × NL | BN | NL | BN × NL | ||
| Total pod mass | <0·001 | <0·001 | – | Maximal size | 0·363 | <0·001 | – |
| Total seed mass | <0·001 | <0·001 | – | Minimal size | <0·001 | 0·292 | – |
| Seed number | <0·001 | <0·001 | <0·001 | Mean size | 0·013 | 0·077 | – |
| Total N content | <0·001 | <0·001 | – | CV | 0·180 | 0·914 | – |
| Total P content | <0·001 | <0·001 | – | Skewness | 0·025 | 0·189 | – |
| Adjusted Gini coefficient | 0·289 | 0·812 | – | ||||
| Lorenz asymmetry coefficient | 0·001 | 0·043 | – | ||||
P values (bold indicates P < 0·05) are based on type III sums of squares and Kenward–Roger approximation.
– denotes that the interaction term was excluded from the full model when its effect was not significant (Supplementary Data Table S1).
Fig. 2.
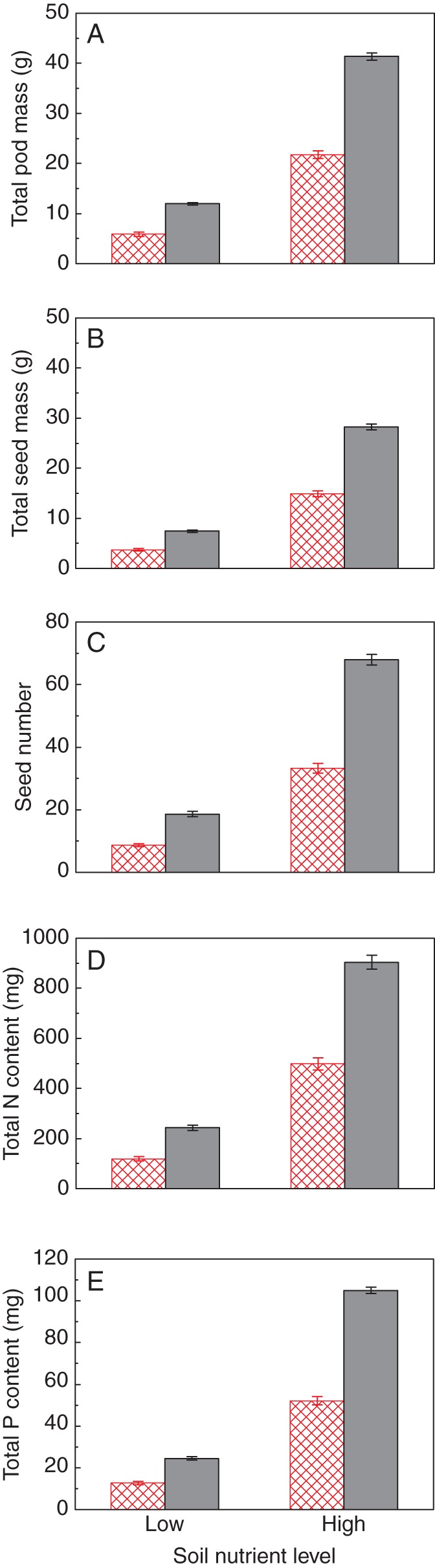
Total reproductive output, i.e. (A) total pod mass, (B) total seed mass, (C) seed number, and resource investment in seed production, i.e. total (D) nitrogen and (E) phosphorus content, of Phaseolus vulgaris plants growing in the presence (open bars with red cross-hatching) and absence (grey bars) of a below-ground neighbour at high and low nutrient levels. Data are means ± s.e.
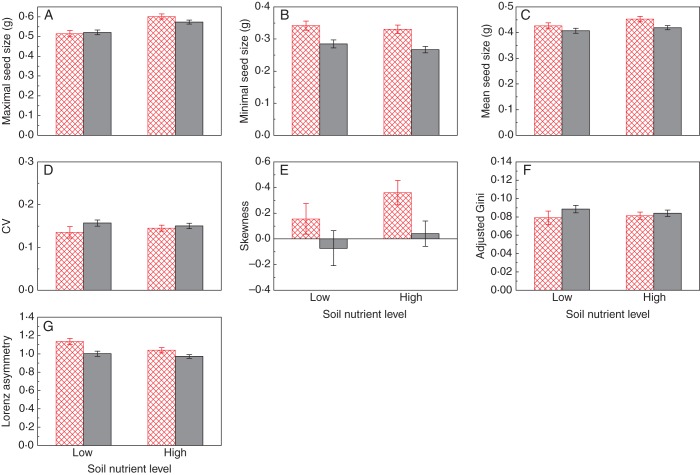
Lowering the nutrient level in the soil significantly reduced maximal seed size while leaving minimal seed size unchanged. In contrast, growing with a below-ground neighbour had no effect on maximal seed size, but significantly increased minimal seed size (Table 1, Fig. 3A, B). Mean seed size and skewness were significantly increased by the presence of a below-ground neighbour, but were not affected by lowering soil nutrient level (Table 1, Fig. 3C, E). Neither a below-ground neighbour nor soil nutrient level had an effect on the CV or adjusted Gini coefficient (Table 1), but they both significantly increased the Lorenz asymmetry coefficient (Table 1, Fig. 3G). The neighbour × nutrient interaction was not significant for any of the listed distribution descriptors (Table 1).
Fig. 3.
Descriptors of within-plant seed size distribution, i.e. (A) maximal seed size, (B) minimal seed size, (C) mean seed size, (D) coefficient of variation (CV), (E) skewness, (F) adjusted Gini coefficient and (G) Lorenz asymmetry coefficient, of Phaseolus vulgaris plants growing in the presence (open bars with red cross-hatching) and absence (grey bars) of a below-ground neighbour at high and low nutrient levels. Data are means ± s.e.
Non-resource effect of a below-ground neighbour
When the level of resource investment in seed production (i.e. total seed mass, total nitrogen content or total phosphorus content) was included in the analyses as a covariate to represent the effects of nutrient availability (i.e. both soil nutrient level and resource reduction caused by the neighbour), the non-resource effect of a below-ground neighbour was still significant (Table 2). As in the analyses of the overall effects of neighbours (Table 1), such non-resource effects entailed significant increases in minimal size, mean size, skewness and Lorenz asymmetry coefficient of the distribution (Table 2). On the other hand, the non-resource effects on maximal seed size depended on the level of resource investment in seeds (Table 2). Mean seed size was also significantly reduced by limiting the resource investment level (Table 2).
Table 2.
Non-resource effects of a below-ground neighbour (BN′) on the within-plant seed size distribution of Phaseolus vulgaris plants corrected for the amount of resource invested in seed production (in terms of total mass of seeds, total nitrogen content and total phosphorus content)
| Descriptors of distribution | The form of resource investment in seed production |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total seed mass (TSM) |
Total nitrogen content (TN) |
Total phosphorus content (TP) |
|||||||
| BN′ | TSM | BN′ × TSM | BN′ | TN | BN′ × TN | BN′ | TP | BN′ × TP | |
| Maximal size | 0·418 | <0·001 | 0·038 | 0·108 | <0·001 | 0·034 | 0·162 | <0·001 | 0·026 |
| Minimal size | 0·001 | 0·246 | – | <0·001 | 0·345 | – | 0·001 | 0·251 | – |
| Mean size | 0·002 | 0·045 | – | 0·001 | 0·021 | – | 0·002 | 0·042 | – |
| CV | 0·421 | 0·320 | – | 0·425 | 0·286 | – | 0·424 | 0·293 | – |
| Skewness | 0·008 | 0·127 | – | 0·008 | 0·120 | – | 0·009 | 0·142 | – |
| Adjusted Gini | 0·489 | 0·522 | – | 0·498 | 0·473 | – | 0·500 | 0·473 | – |
| Lorenz asymmetry | 0·045 | 0·020 | – | 0·038 | 0·024 | – | 0·033 | 0·037 | – |
P values (bold indicates P < 0·05) are based on type III sums of squares and Kenward–Roger approximation.
– denotes that the interaction term was excluded from the full model when its effect was not significant (Supplementary Data Tables S2–S4).
DISCUSSION
Our results show that the greatest plasticity in plant seed production in response to reduced resource availability occurred in terms of number reduction (Fig. 2C; see also Harper et al., 1970; Venable, 1992). However, a negative response of mean seed size to a lower resource investment level was also found (Table 2), which is consistent with many studies (e.g. Wulff, 1986; Galloway, 2001; Breen and Richards, 2008). This reduction was associated with a decline in maximal seed size, which is too costly to be maintained at high values (Geritz, 1995). Together, these results indicate that the quantity of seeds (i.e. seed number) tends to be favoured over their quality (i.e. size) when resources are limited. The maintenance of minimal seed size at low nutrient availability might imply that a basic level of resource storage should be achieved for seedling germination and survival at early developmental stages (Geritz, 1995).
As neighbour presence typically entails a reduction in resource availability and thus lower resource investment level in seeds (Fig. 2D, E), the effects of neighbour presence on seed size distribution could be mediated through reduced resource availability. However, in our study (1) the overall effects of a below-ground neighbour on various descriptors of the size distribution were different from, and in several cases opposite to, those of lowering nutrient availability in soil; (2) the non-resource effects of a below-ground neighbour were significant when plants were compared at the same level of resource investment in seed production; and (3) significant interaction effects were seldom found. These findings clearly indicate that the presence of a below-ground neighbour had effects that were independent of resource availability.
The responses of within-plant seed size distribution to non-resource effects of neighbours can be mainly interpreted as a maternal adaptation (Burgess and Marshall, 2014) to the future environment conditioned on current environmental cues (Wong and Ackerly, 2005). Due to the competitive advantage of large seeds (especially in dense populations; e.g. Houssard and Escarré, 1991; Eriksson, 1999), the presence of a neighbour, which indicates a higher likelihood of intensive seedling competition, would favour the production of large seeds. Indeed, in our study we found that plants with neighbours produced relatively fewer seeds in small size categories, as reflected by the increases in both skewness and minimal seed size (as shown in Fig. 1A, C). The larger values of the Lorenz asymmetry coefficient with an unchanged Gini coefficient suggest that plants with neighbours produced relatively fewer very small seeds and relatively more very large seeds. Together, this resulted in larger mean seed size in plants with neighbours, consistent with our second hypothesis.
The findings of non-resource effects also provide evidence of root-mediated neighbour detection in P. vulgaris plants. In the last decade, considerable research has shown that plants are capable of discriminating self from non-self (e.g. Falik et al., 2003) and even the relatedness (e.g. Dudley and File, 2007; Fang et al., 2013) of a conspecific neighbour via root interaction independently of nutrient availability. However, the mechanisms underlying this detection are still unclear (Chen et al., 2012). So far, most of these studies have mainly focused on root growth (e.g. Falik et al., 2003; Fang et al., 2013), although some have also paid attention to the responses in biomass allocation to reproduction (e.g. Gersani et al., 2001; Dudley and File, 2007). Our study extends current understanding by demonstrating that root-mediated neighbour detection can also influence plant reproductive strategy in terms of seed size distribution. Another example of the effect of neighbour presence on developmental processes in plant reproduction is provided by Viola tricolor, in which rooting with neighbours increases pollen tube growth rate much more than the availability of nutrients (Lankinen et al., 2013).
In contrast to our finding that competition with below-ground neighbours increased mean seed size but left the CV unchanged, some studies using other species have shown that mean seed size stabilizes (e.g. Weiner et al., 1997; Halpern, 2005; Violle et al., 2009) but the CV varies in response to neighbour density (e.g. Halpern, 2005; Violle et al., 2009). This may simply indicate that plant species differ in their adaptive traits related to seed size distribution (Harper et al., 1970). More importantly, however, our results suggest that the mean or CV of seed size is just one element of a complex in the distribution and may not be sufficient by itself to describe the changes. More descriptors that specify the range (maximal and minimal size), the degrees of asymmetry (skewness) and the size hierarchy structure (Gini and Lorenz asymmetry coefficients) of the distribution are needed to get more insight into the offspring investment strategy of plants.
To our knowledge, the current study is the first to demonstrate that the presence of a below-ground neighbour alters seed size distribution within plants. It provides an example of anticipatory maternal effects (Burgess and Marshall, 2014). That is, a neighbour in the current generation carries a reliable cue that increases the predictability of the next generation's density in the future, and plants can use it to adjust their bet-hedging (i.e. seed size distribution) accordingly (Wong and Ackerly, 2005). It also evokes the need for further studies of the mechanisms of below-ground neighbour detection, as well as the subsequent crosstalk between below- and above-ground plant parts in influencing reproductive strategy. Insight into such neighbour-induced responses may also be important for agriculture, as crops tend to grow in close proximity to conspecific neighbours, and seed size and uniformity therein are important quality measures in crops.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to G. A. Kowalchuk, C. Xu and two reviewers for their valuable comments. We thank F. H. J. Siesling, J. A. P. Kooiman and L. Xu for their technical help. This work was supported by a PhD fellowship (2010619022) from Chinese Scholarship Council to B.J.W.C.
LITERATURE CITED
- Allen RM, Bukley YM, Marshall DJ. Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. American Naturalist. 2008;171:225–237. doi: 10.1086/524952. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: linear mixed-effects models using Eigen and S4. 2013. R package version 1.0-5. http://CRAN.R-project.org/package=lme4 .
- Breen AN, Richards JH. Irrigation and fertilization effects on seed number, size, germination and seedling growth: implications for desert shrub establishment. Oecologia. 2008;157:13–19. doi: 10.1007/s00442-008-1049-3. [DOI] [PubMed] [Google Scholar]
- Burgess SC, Marshall DJ. Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos. 2014;123:769–776. [Google Scholar]
- Chen BJW, During HJ, Anten NPR. Detect thy neighbor: identity recognition at the root level in plants. Plant Science. 2012;195:157–167. doi: 10.1016/j.plantsci.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Cipollini ML, Stiles EW. Seed predation by the bean weevil Acanthoscelides obtectus on Phaseolus species: consequences for seed size, early growth and reproduction. Oikos. 1991;60:205–214. [Google Scholar]
- Damgaard C, Weiner J. Describing inequality in plant size or fecundity. Ecology. 2000;81:1139–1142. [Google Scholar]
- Deltas G. The small-sample bias of the Gini coefficient: results and implications for empirical research. Review of Economics and Statistics. 2003;85:226–234. [Google Scholar]
- Dubois J, Cheptou P-O. Competition/colonization syndrome mediated by early germination in non-dispersing achenes in the heteromorphic species Crepis sancta. Annals of Botany. 2012;110:1245–1251. doi: 10.1093/aob/mcs203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley SA, File AL. Kin recognition in an annual plant. Biology Letters. 2007;3:435–438. doi: 10.1098/rsbl.2007.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson O. Seed size variation and its effect on germination and seedling performance in the clonal herb Convallaria majalis. Acta Oecologica. 1999;20:61–66. [Google Scholar]
- Falik O, Reides P, Gersani M, Novoplansky A. Self/non-self discrimination in roots. Journal of Ecology. 2003;91:525–531. [Google Scholar]
- Fang S, Clark RT, Zheng Y, et al. Genotypic recognition and spatial responses by rice roots. Proceedings of the National Academy of Sciences of the USA; 2013. pp. 2670–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway LF. The effect of maternal and paternal environments on seed characters in the herbaceous plant Campanula americana (Campanulaceae) American Journal of Botany. 2001;88:832–840. [PubMed] [Google Scholar]
- Geritz SAH. Evolutionarily stable seed polymorphism and small-scale spatial variation in seedling density. American Naturalist. 1995;146:685–707. [Google Scholar]
- Gersani M, Brown JS, O'Brien EE, Maina GM, Abramsky Z. Tragedy of the commons as a result of root competition. Journal of Ecology. 2001;89:660–669. [Google Scholar]
- Halpern SL. Sources and consequences of seed size variation in Lupinus perennis (Fabaceae): adaptive and non-adaptive hypotheses. American Journal of Botany. 2005;92:205–213. doi: 10.3732/ajb.92.2.205. [DOI] [PubMed] [Google Scholar]
- Harper J, Lovell P, Moore K. The shapes and sizes of seeds. Annual Review of Ecology and Systematics. 1970;1:327–356. [Google Scholar]
- Houssard C, Escarré J. The effects of seed weight on growth and competitive ability of Rumex acetosella from two successional old-fields. Oecologia. 1991;86:236–242. doi: 10.1007/BF00317536. [DOI] [PubMed] [Google Scholar]
- de Kroon H, Mommer L, Nishiwaki A. Root competition: towards a mechanistic understanding. In: de Kroon H, Visser EJW, editors. Root ecology. Berlin: Springer; 2003. pp. 215–234. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package) 2013. R package version 2.0-3. http://CRAN.R-project.org/package=lmerTest . [Google Scholar]
- Lankinen Å, Larsson MC, Fransson A-M. Allocation to pollen competitive ability vs. seed production in Viola tricolor as an effect of plant size, soil nutrients and presence of a root competitor. Oikos. 2013;122:779–789. [Google Scholar]
- Lehtilä K, Ehrlén J. Seed size as an indicator of seed quality: a case study of Primula veris. Acta Oecologica. 2005;28:207–212. [Google Scholar]
- Maina GG, Brown JS, Gersani M. Intra-plant versus inter-plant root competition in beans: avoidance, resource matching or tragedy of the commons. Plant Ecology. 2002;160:235–247. [Google Scholar]
- McGinley MA, Charnov EL. Multiple resources and the optimal balance between size and number of offspring. Evolutionary Ecology. 1988;2:77–84. [Google Scholar]
- McGinley MA, Temme DH, Geber MA. Parental investment in offspring in variable environments: theoretical and empirical considerations. American Naturalist. 1987;130:370–398. [Google Scholar]
- Michaels HJ, Benner B, Hartgerink AP, et al. Seed size variation: magnitude, distribution, and ecological correlates. Evolutionary Ecology. 1988;2:157–166. [Google Scholar]
- Parciak W. Environmental variation in seed number, size, and dispersal of a fleshy-fruited plant. Ecology. 2002;83:780–793. [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. http://www.R-project.org/ [Google Scholar]
- Ruiz de Clavijo E. Role of within-individual variation in capitulum size and achene mass in the adaptation of the annual Centaurea eriophora to varying water supply in a Mediterranean environment. Annals of Botany. 2002;90:279–286. doi: 10.1093/aob/mcf188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertown J. The paradox of seed size and adaptation. Trends in Ecology & Evolution. 1989;4:24–26. doi: 10.1016/0169-5347(89)90013-X. [DOI] [PubMed] [Google Scholar]
- Smith CC, Fretwell SD. The optimal balance between size and number of offspring. American Naturalist. 1974;108:499–506. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. New York: Freeman; 1995. [Google Scholar]
- Venable DL. Size-number trade-offs and the variation of seed size with plant resource status. American Naturalist. 1992;140:287–304. [Google Scholar]
- Venable DL, Brown JS. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. American Naturalist. 1988;131:360–384. [Google Scholar]
- Violle C, Castro H, Richarte J, Navas M-L. Intraspecific seed trait variations and competition: passive or adaptive response? Functional Ecology. 2009;23:612–620. [Google Scholar]
- Völler E, Auge H, Prati D, Fischer M, Hemp A, Bossdorf O. Geographical and land-use effects on seed-mass variation in common grassland plants. Basic and Applied Ecology. 2012;13:395–404. [Google Scholar]
- Weiner J, Solbrig OT. The meaning and measurement of size hierarchies in plant populations. Oecologia. 1984;61:334–336. doi: 10.1007/BF00379630. [DOI] [PubMed] [Google Scholar]
- Weiner J, Martinez S, Müller-Schärer H, Stoll P, Schmid B. How important are environmental maternal effects in plants? A study with Centaurea maculosa. Journal of Ecology. 1997;85:133–142. [Google Scholar]
- Wong TG, Ackerly DD. Optimal reproductive allocation in annuals and an informational constraint on plasticity. New Phytologist. 2005;166:159–172. doi: 10.1111/j.1469-8137.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Wulff RD. Seed size variation in Desmodium paniculatum: I. Factors affecting seed size. Journal of Ecology. 1986;74:87–97. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.