Abstract
Background and Aims
Icacinaceae sensu stricto consist of a group of early branching lineages of lamiids whose relationships are not yet resolved and whose detailed floral morphology is poorly known. The most bizarre flowers occur in Emmotum: the gynoecium has three locules on one side and none on the other. It has been interpreted as consisting of three fertile and two sterile carpels or of one fertile carpel with two longitudinal septa and two sterile carpels. This study focused primarily on the outer and inner morphology of the gynoecium to resolve its disputed structure, and ovule structure was also studied. In addition, the perianth and androecium were investigated.
Methods
Flowers and floral buds of two Emmotum species, E. harleyi and E. nitens, were collected and fixed in the field, and then studied by scanning electron microscopy. Microtome section series were used to reconstruct their morphology.
Key Results
The gynoecium in Emmotum was confirmed as pentamerous, consisting of three fertile and two sterile carpels. Each of the three locules behaves as the single locule in other Icacinaceae, with the placenta of the two ovules being identical, which shows that three fertile carpels are present. In addition, it was found that the ovules are bitegmic, which is almost unique in lamiids, and that the stamens have monosporangiate thecae, which also occurs in the closely related family Oncothecaceae, but is not known from any other Icacinaceae sensu lato so far.
Conclusions
The flowers of Emmotum have unique characters at different evolutionary levels: the pseudotrimerous gynoecium at angiosperm level, the bitegmic ovules at lamiid level and the monosporangiate thecae at family or family group level. However, in general, the floral morphology of Emmotum fits well in Icacinaceae. More comparative research on flower structure is necessary in Icacinaceae and other early branching lineages of lamiids to better understand the initial evolution of this large lineage of asterids.
Keywords: Asterids, Emmotaceae, Emmotum harleyi, E. nitens, Garryales, Icacinaceae, early branching lamiids, anthers, floral morphology, gynoecium, ovules
INTRODUCTION
The former family Icacinaceae sensu lato (s.l.) turned out to be polyphyletic in molecular phylogenetic analyses (Karehed, 2001) and was provisionally subdivided into four distantly related families: Cardiopteridaceae and Stemonuraceae, both in Aquifoliales (campanulids), Pennantiaceae in Apiales (campanulids) and Icacinaceae sensu stricto (s.s.), which appear unresolved close to the base of lamiids (Karehed, 2001; Lens et al., 2008; Refulio-Rodriguez and Olmstead, 2014). Metteniusa, earlier also in Icacinaceae s.l., was placed in a fifth family, Metteniusaceae, also close to the base of lamiids (González et al., 2007), and is here considered to be in Icacinaceae s.s. as well.
Karehed (2001) recognized Icacinaceae s.s. along with Garryales s.s. (Aucuba and Garrya) within an expanded Garryales (s.l.) and divided the family into the ‘Icacina group’ (numerous genera), the ‘Emmotum group’ (Emmotum and Ottoschulzia) and the ‘Apodytes group’ (Apodytes and Raphiostylis). Lens et al. (2008), based on combined molecular and wood anatomical analyses, found very weak support for a clade consisting of the Apodytes and Emmotum groups plus Oncotheca and Cassinopsis, whereas Refulio-Rodriguez and Olmstead (2014), based on combined plastid and mitochondrial data, found Cassinopsis to be closer to the Icacina group. In neither of these studies, however, did Icacinaceae s.s. form a clade.
According to APG III (2009), Apodytes, Cassinopsis and Emmotum may not even belong to Icacinaceae s.s. and may be better placed in their own family Emmotaceae (a family proposed earlier just for Emmotum by van Tieghem, 1897), which might eventually be included in Garryales s.l., probably along with Metteniusaceae and Oncothecaceae. This ordinal circumscription, however, has not been confirmed in more recent phylogenetic analyses with molecular data. Whereas Lens et al. (2008) found the Icacina group closer to core lamiids, Refulio-Rodriguez and Olmstead (2014) found Garryales s.s. closer to the core lamiids, in both cases without good support. Figure 1 shows a summary of phylogenetic relationships among Icacinaceae s.l. and the classification adopted here.
Fig. 1.
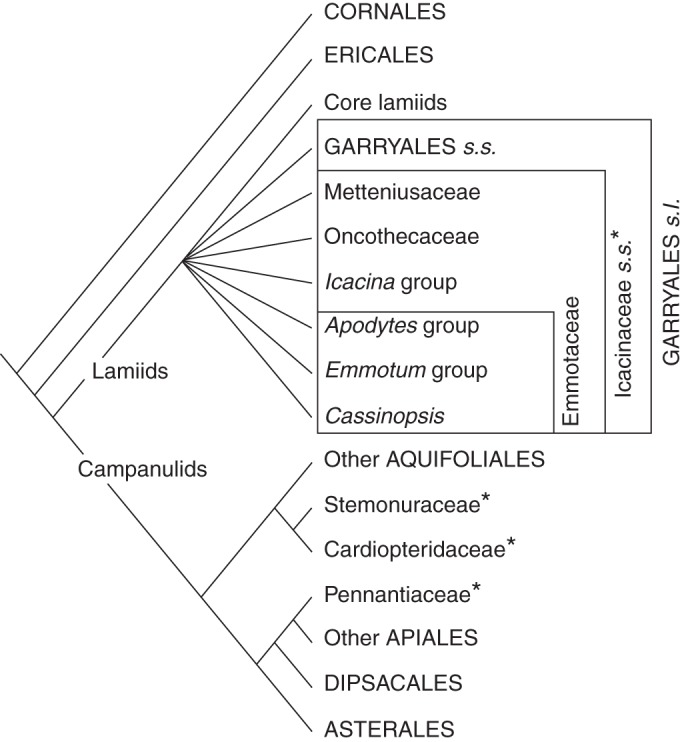
Summary phylogenetic tree of asterids showing the position of representatives of the former Icacinaceae s.l. (asterisks). Only supported clades are shown. The polytomy at the base of lamiids reflects lack of support and/or incongruent results in phylogenetic studies (Karehed, 2001; Lens et al., 2008; Refulio-Rodriguez and Olmstead, 2014), and the classification mapped on this area of the tree shows the taxonomic circumscriptions adopted in the text. Icacinaceae s.s. consist of all representatives of Icacinaceae s.l. that diverge close to the base of lamiids. The Apodytes, Emmotum and Icacina groups were recognized by Karehed (2001). The circumscription of the reinstated Emmotaceae was suggested by APG III (2009). Garryales s.l. represents the expanded order by including Garryales s.s. plus representatives of Icacinaceae s.s. as suggested by Karehed (2001) and APG III (2009).
The floral structure of Icacinaceae s.l. is in general extremely rudimentarily known. In contrast to other asterids, the flowers in this group seem to be less fancy and the high tannin content and/or woody tissues provide technical difficulties for the production of serial microtome sections. As a consequence, the floral structure has not been studied in detail in any of the former Icacinaceae s.l., except for Cardiopteris (Tobe, 2012; Kong et al., 2014) and Metteniusa (González and Rudall, 2010), but in both without analysis and reconstruction of the inner morphological surface of the gynoecium. Furthermore, drawings of the small flowers in revisionary works provide only minimal information (e.g. de Stefano et al., 2013). None of the taxa of Icacinaceae s.s. has been studied in detailed floral morphology. Ovary wall histology and fruit development have been investigated in Apodytes and Cassinopsis, but with a focus rather on ecological than on morphological aspects (Potgieter and van Wyk, 1994a, b).
In view of the systematic decomposition and profoundly new arrangement of the former family Icacinaceae (Fig. 1), it would be of interest to know more about the detailed floral structure of the newly established groups. The flowers of Emmotum are different from all other clades of Icacinaceae s.l. because of their trilocular ovary (Howard, 1942a; Sleumer, 1942), whereas the ovary is unilocular in Stemonuraceae, Cardiopteridaceae, Metteniusaceae and the other Icacinaceae s.s. (Sleumer, 1942; Potgieter and van Wyk, 1994a, b; de Stefano and Fernández-Concha, 2011). In addition, this trilocular ovary of Emmotum is strangely one-sided in a unique way (Howard, 1942a: plate I, fig. 9; plate IV, figs 6, 7). Its structure has been variously interpreted as pentamerous with two sterile and three fertile carpels (Engler, 1872; van Tieghem, 1897; Howard, 1942a), or as trimerous having two sterile carpels and a single fertile carpel with two septa (Stevens, 2001 onwards).
Fig. 9.
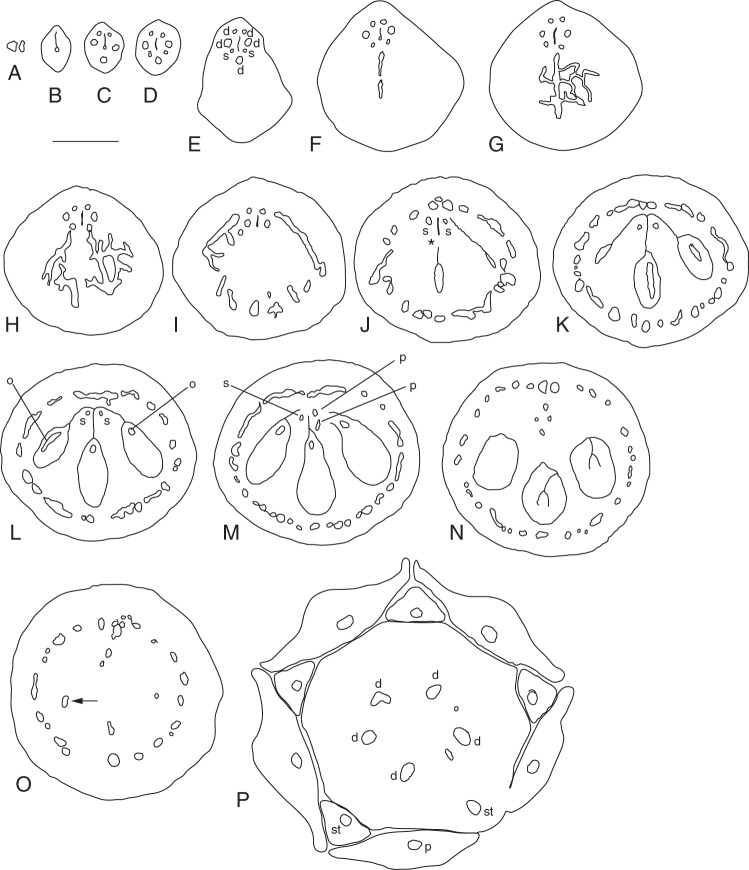
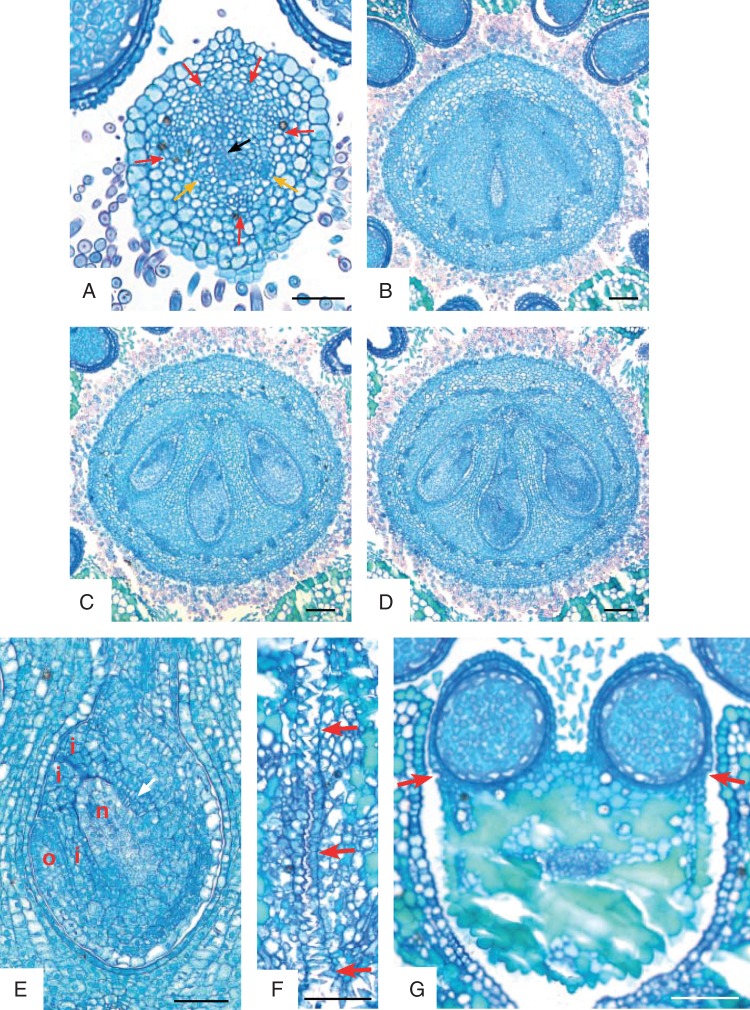
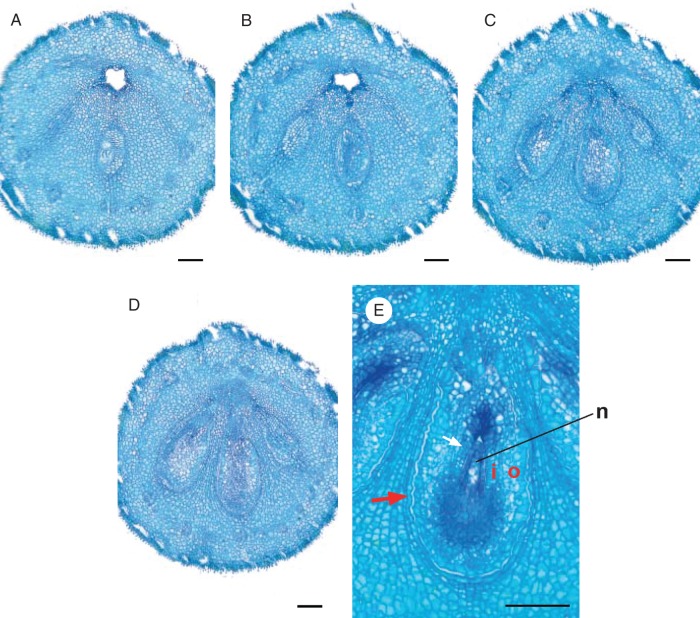
Emmotum nitens. Transverse microtome section series of gynoecium shortly before anthesis, line drawings of morphological outer and inner surfaces (thicker lines) and outlines of vascular bundles (thinner lines). (A) Stigma. (B) Style, above the zone with vascular bundles. (C) Style, zone with only the five dorsal vascular bundles visible. (D) Style, with the five dorsal and the two synlateral vascular bundles visible. (E) Style, transition to ovary. (F–H) Level of mainly dorsal bundle of median fertile carpel slanting and branching over bulged part of ovary. (I) Level of mainly dorsal bundles of lateral fertile carpels slanting and branching over bulged part of ovary. (J) Level of apical septum and arrangement of the dorsal carpel bundle network along the periphery of the ovary. (K) Slightly below level of apical septum. (L) Lowermost level of symplicate zone. (M) Uppermost level of synascidiate zone. (N) Mid-level of synascidiate zone. (O) Lowermost level of synascidiate zone, locule of the lateral fertile carpel on the left still present (arrow). (P) Level at the base of the gynophore with the five main (dorsal) carpel vascular bundles. In addition, petals and stamens and their vascular bundles are shown to visualize the alternation of the three inner organ whorls. Signatures: d, dorsal carpel vascular bundle; o, ovular vascular bundle; p, petal vascular bundle; s, synlateral carpel vascular bundle; st, stamen vascular bundle; asterisk, apical septum; dotted line, delimitation of nectariferous tissue. Scale bar = 0·3 mm.
Fig. 6.
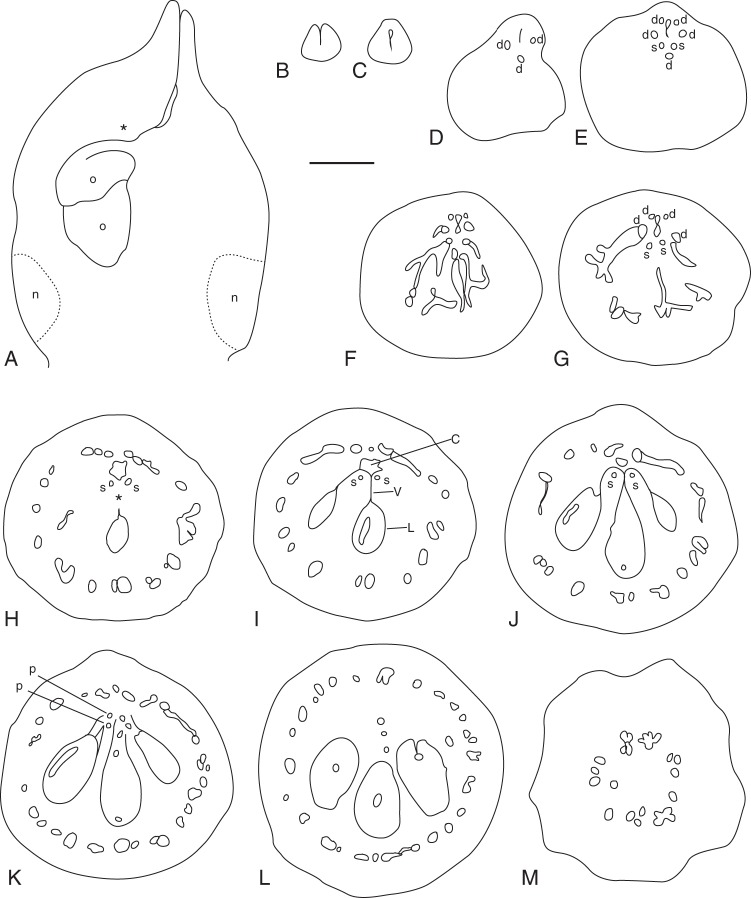
Emmotum harleyi. (A) Schematic reconstruction of median longitudinal section of gynoecium at anthesis, with entire (outer and inner) morphological surface. (B–M) Transverse microtome section series of anthetic gynoecium, line drawings of morphological outer and inner surfaces (thicker lines) and outlines of vascular bundles (thinner lines). (B) Stigma. (C) Style. (D) Upper level with only the three dorsal bundles of the fertile carpels visible. (E) Upper level with all five dorsal carpel bundles and the two synlateral bundles visible. (F) Level of mainly dorsal bundle of median fertile carpel slanting and branching over bulged part of ovary. (G) Level of mainly dorsal bundles of lateral fertile carpels slanting and branching over bulged part of ovary. (H) Level of apical septum and arrangement of the dorsal carpel bundle network along the periphery of the ovary. Open space between the five carpels and uppermost level of the locule of the median carpel. (I) Slightly below level of apical septum. (J) Lowermost level of symplicate zone. All three locules present. (K) Uppermost level of synascidiate zone. (L) Mid-level of synascidiate zone. (M) Basal level of gynophore. Signatures: c, central gaping space delimited by inner morphological surface of gynoecium; d, dorsal carpel vascular bundle; Lm locule; n, nectariferous tissue; O, ovule; p, placental vascular bundle; s, synlateral carpel vascular bundle; V, ventral slit; asterisk, apical septum; dotted line, delimitation of nectariferous tissue. Scale bar = 0·3 mm.
Fig. 7.
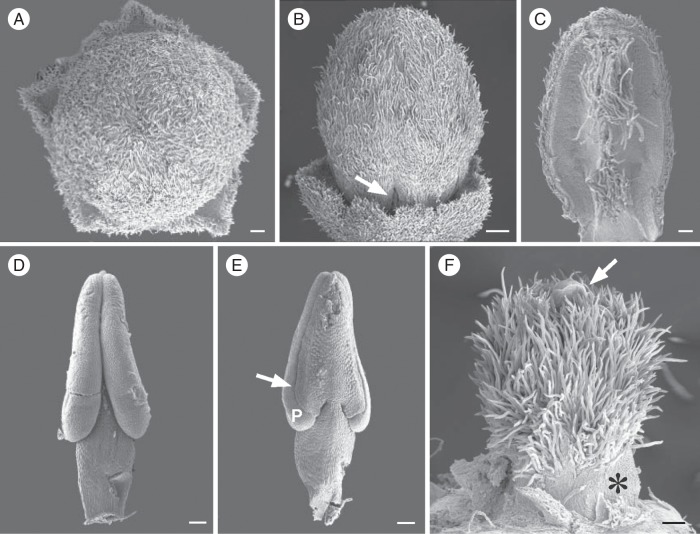
Emmotum nitens. Scanning electron micrographs. (A) Floral bud shortly before anthesis, from above. (B) Floral bud shortly before anthesis, from the side, sepals partly removed to show the free base of two petals (arrow). (C) Petal of floral bud shortly before anthesis, ventral side. Midrib with a lower and an upper group of hairs. (D, E) Stamens of floral bud shortly before anthesis. (D) Ventral side. (E) Dorsal side (P, ventral pollen sac; arrow, dehiscence line). (F) Gynoecium of floral bud shortly before anthesis, from the side (arrow, stigma; asterisk, nectary). Scale bars: (A, C–F) = 0·1 mm; (B) = 0·2 mm.
Gynoecium and ovule structure are of particular interest for investigation: the gynoecium because of the mentioned one-sidedness in Emmotum and the general trend of reduction of one or more carpels in Icacinaceae s.l., and the ovules because of reductions of nucellus and integument (Fagerlind, 1945). In addition, during this study we found an unusual feature of suprafamilial interest in the androecium so that we additionally focus on stamen structure. We also paid attention to the perianth, which is characterized in many Icacinaceae s.l. by its valvate petals.
This is the first study on the detailed outer and inner morphology of the highly unusual flowers of an enigmatic genus in early diverging lamiids. It is to be hoped that currently ongoing studies on the phylogeny of basal lamiids (Refulio-Rodriguez and Olmstead, 2014; Stull et al., 2014) and future structural studies will shed additional new light on the early evolution of this highly successful group of flowering plants.
MATERIAL AND METHODS
We collected and studied the two species of Emmotum that are known from the Chapada Diamantina (State of Bahia, Brazil) (Zappi et al., 2003; de Stefano et al., 2007):
Emmotum harleyi R. Duno (coll. A. Rapini and P.K. Endress 2005, 12 02 2013) (collection locality close to the type locality), floral buds and anthetic flowers.
Emmotum nitens (Benth.) Miers (coll. A. Rapini and P.K. Endress 2001, 06 02 2013), floral buds.
Other taxa of Icacinaceae s.s. were also studied cursorily for comparison:
Apodytes brachystylis F. Muell. (coll. P.K. Endress 9035, 07 11 1990, northern Queensland, Australia), floral buds.
Apodytes clusiifolia (Baill.) Villiers (coll. P.K. Endress 6306, 1981, New Caledonia), anthetic flowers.
Apodytes dimidiata E. Mey. ex Arn. (coll. P.K. Endress 2012-1, 04 07 2012, cultivated Botanic Garden of the University of Zurich), floral buds and anthetic flowers.
Cassinopsis madagascariensis Baill. (coll. P.K. Endress 7810, 10 1987, Madagascar), anthetic flowers.
The material was fixed and stored in 70 % ethanol. Flowers investigated with scanning electron microscopy (eight anthetic or slightly preanthetic flowers, of E. harleyi, four slightly preanthetic flowers of E. nitens) were critical-point dried, sputter-coated with platinum and studied at 10 kV with a Zeiss Supra-50 VP system. The material used for microtome section series (two anthetic flowers of E. harleyi, two slightly preanthetic flowers of E. nitens) was dehydrated and embedded in Kulzer's Technovit (2-hydroethyl methacrylate). It was infiltrated with Technovit solution in ethanol in several steps. Embedded material was sectioned using a Microm HM 355 rotary microtome with a conventional D knife. The 7-μm-thick sections (more than 1000 sections) were stained with ruthenium red and toluidine blue and mounted in Histomount.
RESULTS
Emmotum harleyi
Inflorescence
Each inflorescence in the axil of a foliage leaf consists of two branches of botryoids. Instead of single lateral flowers, there are often dyads or triads of flowers in the proximal region of each branch. Because there are not many flowers (fewer than ten per branch), such a branch could be seen as a poor thyrsoid or a poor panicle (for terminology, see Endress, 2010a). The two branches are of similar size and are situated one obliquely behind the other. We were not able to determine whether both branches are lateral to a very short aborting branch, whether one of the two branches is lateral to the other, or whether one of the two branches is an accessory branch. As the two branches branch right in the axil of the foliage leaf, the topology of this branching could only be determined in a developmental study.
Flowers
The flowers are pentamerous, probably including the gynoecium (for interpretation, see below), each organ category forming one whorl, and all whorls alternating. At anthesis, the flowers are widely open, the petals are spreading and slightly reflexed but the stamens are connivent and are more or less contiguous at their tips (Figs 2, 3 and 4A).
Fig. 2.

Emmotum harleyi. Flowers at anthesis.
Fig. 3.
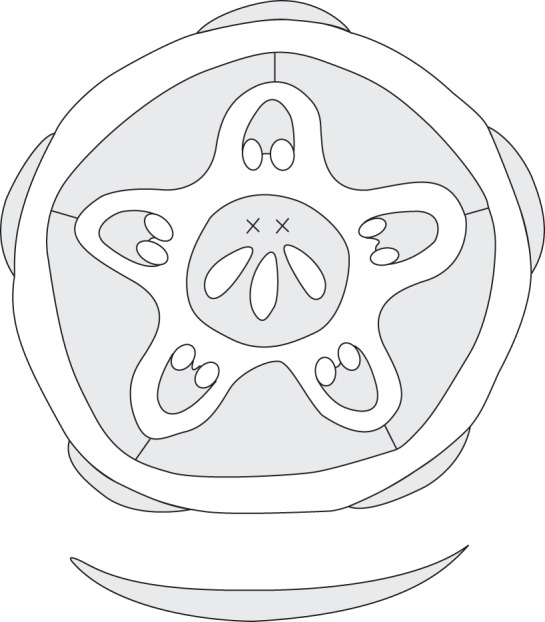
Emmotum harleyi. Floral diagram, including floral subtending bract (xx, two reduced carpels).
Fig. 4.
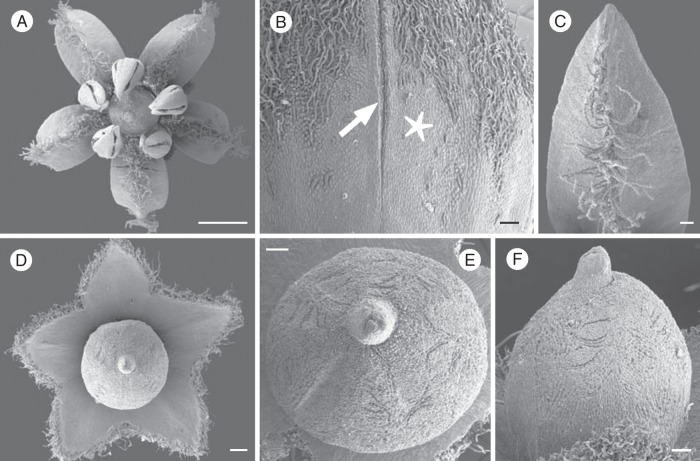
Emmotum harleyi. Scanning electron micrographs. (A) Flower at anthesis. (B) Floral bud, outer suface. Two petals with valvate aestivation and postgenital connection (arrow). Distribution of hairs reflects position of sepals in younger bud: in areas without hairs the sepals were appressed to the petals in bud (star). (C) Petal of anthetic flower, ventral side. Midrib with moniliform hairs. (D) Anthetic flower, showing synsepalous calyx and gynoecium (perianth and androecium removed). (E) Gynoecium of anthetic flower, from above, fertile side down. Surface with pressure marks of anthers and of moniliform hairs from petals. (F) Gynoecium of anthetic flower, from the side, fertile side at right. Surface with pressure marks of moniliform hairs from petals. Scale bars: (A) = 1 mm; (B, C, E, F) = 0·1 mm; (D) = 0·2 mm.
Calyx
The sepals are congenitally united in their lower half and are triangular in the upper half (Fig. 4D). They are only about half the length of the petals already in bud and are thus not protective for the inner floral organs except at the base. The outer surface is covered with curly unicellular, unlignified hairs. Sepals have more than one vascular trace. The main trace formed by the median bundle is accompanied by either two separate lateral traces or by synlateral traces.
Corolla
The petals are free without any congenitally united basal region. However, in bud, they are postgenitally connected by interdigitated epidermal cells at their margins, except for the very base, where they remain free from each other throughout development (Fig. 4B). The dorsal (outer) surface has a dense indumentum of curly unicellular hairs in the upper half, but is glabrous in the lower half where they were covered by the sepals in bud (Fig. 4B). The ventral (inner) surface has a median longitudinal rib with conspicuous long hairs, whereas the flanks are glabrous (Fig. 4C). The hairs have an irregularly moniliform shape. In the compact bud, the median rib of each petal protrudes between the anthers. The petals have a single vascular trace.
Androecium
The stamens are free from each other and also from the petals. At the floral base, they first fuse with the gynophore for a short distance. They have a short filament and an elongate, sagittate anther. In the anthetic flower, the filaments are more or less upright and the anthers are curved inward, their tips meeting in the centre of the flower over the gynoecium (Fig. 2). During critical-point drying anthers slightly curve backward so that they are no longer contiguous in the floral centre (Fig. 4A). Each of the two thecae has only one pollen sac. The dorsal pollen sac is not formed. That the remaining pollen sac represents the ventral one is shown by the position of the longitudinal dehiscence line, which is on the dorsal side of the pollen sac (figured for E. nitens, Figs 7D, E and 8G). The stamens have a single vascular trace.
Fig. 8.
Emmotum nitens. Transverse microtome sections of gynoecium shortly before anthesis (A–D) and sections of other floral parts (E–G). (A) Style. Poorly differentiated vascular bundles indicated (red arrows, dorsal carpel bundles; yellow arrows, synlateral carpel bundles); black arrow, slit-shaped inner morphological surface of gynoecium. (B) Level of apical septum (corresponding to Fig. 9J). (C) Lowermost level of symplicate zone (corresponding to Fig. 9L). (D) Uppermost level of synascidiate zone (corresponding to Fig. 9M). (E) Longitudinal section (more or less median) of bitegmic ovule (o, outer integument; i, inner integument; n, nucellus). (F) Transverse section of upper part of flower showing two petals that are postgenitally connected with interdigitated epidermal cells (arrows). (G) Transverse section of anther with monosporangiate thecae (arrows point to prospective dehiscence lines). Scale bars: (A, E, F) = 0·05 mm; (B–D, G) = 0·1 mm.
Gynoecium
The gynoecium consists of an obovoid main body and a short, stub-like style with a small stigma (Fig. 4D–F). The slightly narrower (tapering) basal part is at the level below the ovary locules and forms a gynophore (Fig. 6A). At anthesis, the entire gynoecium (including the gynophore) is approx. 1·6 mm long, and the gynophore is approx. 0·4. mm long. The gynoecium is glabrous; its surface is not smooth but has papillate epidermis cells and has imprints by the long hairs of the inner surface of the petals, which are tightly appressed to the gynoecium in bud (Fig. 4E, F). Likewise, the ovary surface may have five incomplete longitudinal edges caused by the anthers, which are appressed to the ovary surface in bud (Fig. 4E). The tissues of the gynoecium are conspicuous by their high tannin content at anthesis (Fig. 5). Idioblasts with oxalate druses are scattered especially in the ovary.
Fig. 5.
Emmotum harleyi. Transverse microtome section series of gynoecium at anthesis (A–D) and longitudinal section of ovule (E). (A) Level of apical septum (corresponding to Fig. 6H). (B) Level immediately below apical septum (corresponding to Fig. 6I). (C) Lowermost level of symplicate zone (corresponding to Fig. 6J). (D) Uppermost level of synascidiate zone (corresponding to Fig. 6 K). (E) Longitudinal section of bitegmic ovule. Signatures: o, outer integument; i, inner integument; n, nucellus; arrow, undulate contiguous surfaces of ovule and locular wall. Scale bars = 0·1 mm.
The ovary has three locules, each with two ovules, indicating the presence of three fertile carpels. For convenience, we call these ovule-bearing carpels fertile irrespective of whether all ovules develop into seeds. Most unusually, the three locules are all on the same side of the ovary (the abaxial side of the flower), which gives the ovary a conspicuously one-sided, monosymmetric shape (Figs 3, 4E, F, 5 and 6). The three locules are all at the same level and are of equal size. The outer contour of the side containing the ovules is slightly bulged, whereas the opposite side is less curved and less expanded (Fig. 4F). The latter appears to contain two reduced carpels, as explained below. Seen from above, there is a Y-shaped slit in the stigma (Fig. 4E). The forked part of the Y is on the side with the three locules. Thus, the fork probably marks the delimitations between the three fertile carpels whereas there are no delimitations between each lateral fertile carpel and its neighbouring sterile carpel and between the two sterile carpels. The long arm of the Y is tilted downwards so that in transverse sections it forms a gap between the two flanks of the gynoecium tip (and it gives the incorrect impression of a transverse section of a single carpel) (Fig. 6B).
Analysis of the internal morphology of the gynoecium shows that it is syncarpous along its whole length. The style is symplicate, and even the uppermost part that seems plicate at first sight (as explained above) (Fig. 6B, C). The locular part of the ovary is synascidiate in the lower approx. 85 % of its length (Figs 5D and 6K–M) and symplicate above (including the transition from ovary to style) (Figs 5A–C and 6D–J). There is a very short apical septum (as defined by Hartl, 1962) on the bulging side of the ovary (Figs 5A and 6H). At the level where the style is a tube, the inner morphological surface is a median slit, which is more or less gaping on the adaxial side (Fig. 6E). Lower down, in the roof of the ovary, this slit is also gaping on its abaxial side (Fig. 6F, G). Downwards, before the two gaps disappear at the level where the locules appear, the gaps open for a short distance into one. Its pentagonal shape in transverse section may reflect the inner surface of five carpels (Figs 5A, B and 6H, I). At the base of the symplicate zone, the inner morphological surface forms the three locules and the ventral slits of the three fertile carpels (Figs 5C and 6J), whereas the two sterile carpels are no longer apparent. In the synascidiate zone, the inner morphological surface no longer communicates between the three locules (Figs 5D and 6K, L). The placentae are at the uppermost level of the synascidiate zone (Fig. 6 K).
In the style, the slit formed by the inner morphological surface is surrounded by several cell layers of small-celled tissue with relatively thick, dark cell walls, but without distinct vascular bundles (Fig. 6B, C). At the transition level from the style to the ovary, outside of the dark-cell-walled area around the inner morphological surface, three vascular bundles appear in those radii where lower down the three locules are situated (Fig. 6D). According to their position, they represent the dorsal bundles of the three fertile carpels. At the other end of the slit, two weak bundles appear slightly lower down, which may be the bundles of two additional, reduced, carpels. Somewhat lower down, the three bundles of the three fertile carpels depart from close to the inner surface toward the dorsal side as three anastomosing vascular branches, which form a dense network of bundles around the ovary (Fig. 6F, G). In the symplicate zone of the upper ovary, each of the two septa between the three locules has a synlateral bundle close to its inner angle (Fig. 6I, J). Distally, these two bundles can be followed up to the transition toward the style (Fig. 6E–H). Downward, at the level of the placentae, these synlaterals branch and the branches serve the six ovules (Fig. 6 K). Below the placentae, few vascular bundles remain more or less in the centre of the ovary, before they connect to the closest part of the peripheral network of bundles lower down (Fig. 6L). At the base of the gynoecium, in the gynophore, the peripheral network is still present in a simple form (Fig. 6M) (but compare with Emmotum nitens). The arrangement of the vasculature does not have a monosymmetric pattern. Thus, there is no evidence of a difference between the abaxial side and the adaxial side of the gynoecium, which suggests a regular carpel arrangement around the circumference of the gynoecium, and thus the presence of five carpels.
Ovules
The two ovules in each locule are positioned one above and slightly beside the other (Fig. 6A). Both extend more or less in the median plane. The upper ovule is more or less horizontally directed, the lower one more vertically. The upper ovule has the micropyle turned slightly to the right or the left side. In the lower ovule, the micropyle is more or less in the median plane. The ovules are tightly appressed to each other and to the locular wall and their contiguous surfaces are undulate (Fig. 5E). They are bitegmic and crassinucellar, but with a thin nucellus (Fig. 5E). The micropyle is formed by the inner integument. At the level of the nucellus, the outer integument is three to five cell layers thick, the inner three or four cell layers. Above the nucellus, at the rim, both integuments are thicker. A well-differentiated endothelium is present. Each ovule is served by one vascular bundle, which ends in the chalaza. It is unknown whether there is always only a single seed per fruit and whether this seed develops predominantly in the median or a lateral locule (Sleumer, 1942).
Nectary
The periphery of the lower part of the ovary and the upper part of the gynophore is nectariferous (Fig. 6A). The tissue is full of starch grains. At anthesis, the nectariferous tissue loses coherence, which causes open spaces as artefacts in the microtome sections, especially in the upper part of the nectary.
Emmotum nitens
Inflorescence
Inflorescences in E. nitens appear in the axil of foliage leaves. They are thyrsoids or double thyrsoids. In addition, they have an obliquely transverse accessory branch (bud) at the base (i.e. without an individual subtending organ). Because they do not have many flowers (about 12), they can also be regarded as poor panicles (for terms, see Endress, 2010a).
Flowers
We studied advanced floral buds; anthetic flowers were not available. Flower morphology is as in E. harleyi. In bud, the epidermis of all contiguous organs seems to be somehow interdentated. Even the epidermis of the free thecae and the connective at the base of the sagittate anther are connected in this way.
Calyx
Sepals are short, congenitally united (Fig. 7A). The dorsal (outer) side of the calyx is densely strigose, with unicellular, unlignified hairs (Fig. 7B). Each sepal has a median and one or two smaller lateral bundles. The lateral bundles of adjacent sepals may or may not form synlateral bundles toward the base. The median, lateral and synlateral bundles form individual traces in the floral base. Thus, the number of traces per sepal is variable. The sepals are highly tanniniferous, especially on the dorsal (outer peripheral) side.
Corolla
Petals are valvate in bud, free from each other from the base, but postgenitally connected in bud with zipper-like interdigitated epidermis except for the floral base where they remain free (Figs 7B, 8F and 9P). The dorsal (outer) side is densely hairy with similar but longer hairs than on the sepals (Fig. 7A, B). Each petal has on its ventral (inner) surface a median longitudinal rib with long hairs (Fig. 7C). The hairs are concentrated in two groups (Fig. 7C). In the group of the upper half of the petal, they are moniliform but appear to be unicellular, as seen from microtome sections. In the group of the lower half of the petal, they are thinner and shorter and appear simpler. Each petal has a single vascular trace. The dorsal (outer peripheral) area of the petals is highly tanniniferous (Fig. 8F).
Androecium
Stamens are free from each other. At the floral base, they first fuse with the gynophore, and not with the perianth organs. Anthers are slender and sagittate, with monosporangiate thecae, each with a longitudinal dehiscence line (Figs 7D, E and 8G). In transverse section, they appear extremely introrse at first sight. However, the dehiscence line is toward the dorsal side of each theca, which indicates that the dorsal pollen sac has disappeared (Fig. 7E). Thus, the introrse appearance of the anther is mainly caused by the lack of the dorsal pollen sac on each side. Each stamen has a single collateral vascular bundle and a single vascular trace. Stamen filaments and connectives show high tannin contents in their tissues so that they easily tear in microtome sections (Fig. 8G).
Gynoecium
The overall shape of the gynoecium is as in E. harleyi. However, the outer surface is not glabrous but covered with long unicellular, lignified hairs, except for style, stigma, gynophore and nectary (Fig. 7F). The apical morphological mouth (opening) of the gynoecium has the shape of a more or less horizontal slit (Figs 7F and 9A). In contrast to E. harleyi, it is not forked. The gynophore is not attenuated at the base (Fig. 9O, P).
The analysis of the inner morphological surface shows about the same proportions as in E. harleyi. The style and transition to the ovary are symplicate (Fig. 9A–I), and the locular part of the ovary is synascidiate along ca. 75 % of its length in the lower part (Figs 8D and 9M-O) and symplicate above (Figs 8B, C and 9J–L). On the bulging side, there is also a very short apical septum (Figs 8B and 9J).
As in E. harleyi, the stigma and upper part of the style are devoid of vasculature (Fig. 9A, B). Toward the base of the style, five vascular bundles appear: the three on the abaxial side of the gynoecium are larger than the two on the adaxial side (Fig. 9C). They probably correspond to the primary bundles of the three fertile and the two sterile carpels, respectively. At the transition from the style to the ovary, in addition, a synlateral bundle appears between the median and each of the two lateral fertile carpels (Figs 8A and 9D). In the ovary, the vascular pattern (Fig. 9F–O) is as described for E. harleyi. Vascular bundles serving the ovules connect with the closest area of the peripheral bundle system basipetally (Fig. 9N, O). At the base of the gynoecium, in the gynophore, there are five major vascular strands alternating with the traces of the stamens. Thus, they correspond to the lower portions of the dorsal bundles of the five carpels.
Ovules
As in E. harleyi, the ovules are bitegmic and crassinucellar (or weakly crassinucellar) (Fig. 8E), and the number and position of the ovules in the ovary is also the same. The outer integument is three or four cell layers thick, the inner is three cell layers thick. The micropyle is formed by the inner integument (Fig. 8E). A distinct endothelium is present (Fig. 8E).
Nectary
As in E. harleyi, there is a nectary at the periphery of the gynophore. In floral bud, it is small-celled and cytoplasm-rich. Starch is not found at this stage.
DISCUSSION
Flower
Flowers with similarly expanded and somewhat recurved petals and connivent stamens as in Emmotum are also known from Ottoschulzia (Icacinaceae s.s.) (Santiago-Valentín and Viruet-Oquendo, 2013). Furthermore, the anthers may have a similar morphology in both genera (see below, under Androecium).
Calyx
As in Emmotum, in most Icacinaceae s.l., the calyx remains short, much shorter than the petals. The sepals are congenitally united in the lower portion and have a short free distal part. Thus, the sepals function as a protective device for the inner floral organs only in early stages of floral development.
Corolla
In Icacinaceae s.l., petals are commonly valvate and protective organs in flower buds, as in Emmotum (but they are quincuncial-imbricate in Cardiopteridaceae: Tobe, 2012; and Oncothecaceae: Dickison, 1986). Also, the presence of a longitudinal rib on the upper (ventral) side of the petals is common, and in a few genera, there are tufts of long hairs on this rib (Emmotum and Icacina) (Sleumer, 1942). That such hairs are moniliform, as observed here, was not noticed before in Emmotum (Howard, 1942a; Sleumer, 1942). The moniliform shape of these hairs appears not to be formed by multicellularity, in contrast to moniliform hairs in flowers of many other plants (Endress, 1994), but just by differential expansion of the wall of a single long cell. It should be studied in the field whether these hairs have a function in secondary pollen presentation, as they are located adjacent to the pollen sacs in bud. Petals with a longitudinal rib on the upper side are also known from some Stemonuraceae (Utteridge, 2011). The petals are mostly free from each other in Icacinaceae s.l. However, they are united in Calatola, Ottoschulzia and Platea of Icacinaceae s.s., and Acrocoelium, Dendrobangia, Gonocaryum and Leptaulus of Cardiopteridaceae (Sleumer, 1942), and in Metteniusaceae (Sleumer, 1942; González and Rudall, 2010) and Oncothecaceae (Dickison, 1986). Petals are lacking in Garryales, except for Aucuba, whose petals are shortly congenitally united (Reidt and Leins, 1994). Whether the basal petal union in these groups is congenital or only postgenital is, however, unknown in most of the other cases.
Androecium
As in Emmotum, the stamens are mostly free from the petals in Icacinaceae s.l. However, in some cases in which the petals are basally fused into a tube, the stamens are also fused with this tube, such as in Dendrobangia (Rusby, 1897), Gonocaryum (Sleumer, 1971) and Leptaulus (Rusby, 1897) (all three in Cardiopteridaceae), and in Metteniusaceae (Sleumer, 1942; González and Rudall, 2010).
That the thecae are monosporangiate in Emmotum has been stated earlier but seems to have been forgotten and is not mentioned in more recent literature (such as Takhtajan, 1997, 2009; Stevens, 2001 onward; de Stefano and Fernández-Concha, 2011). Miers (1851–1861, p. 53, plates 21 and 22) found that Emmotum (representing his tribe Emmoteae) differs from all other Icacinaceae s.l. ‘in its plurilocular ovarium, and the singular structure of its anthers, which are bilobed, and consist of two unilocular, evalvate and boat-shaped pollen cells’, and van Tieghem (1897, p. 120) clearly stated: ‘L’étamine n'a dans son anthère, de chaque côté de la côte médiane, qu'un seul sac pollinique, s'ouvrant par une fente longitudinale située dans l'angle externe’ (‘the stamen has on both sides of the median plane of the anther only one pollen sac, opening by a longitudinal slit on the outer side’). Howard (1942a, p. 482) mentioned that ‘[t]he thecae of the anthers are reduced to two’, thus confusing thecae with pollen sacs. The early description by Engler (1872, p. 43), ‘loculis … margine posteriore a connectivo omnino soluto extrorsum dehiscentibus’ (‘with the locules on the posterior margin completely detached from the connective and extrorsely dehiscing’), is not explicit but seems to point to this fact, and this vague phrasing is repeated in Engler (1896) and Sleumer (1942). However, Engler (1896, p. 240), in his general description of the family, mentioned that all Icacinaceae, except Polyporandra, have four-locular anthers. Thus, he appears to have overlooked the monosporangiate structure of the thecae in Emmotum.
The presence of monosporangiate thecae has not been described from any other Icacinaceae s.l. Howard (1942b, p. 32) mentioned for Ottoschulzia that ‘dehiscence of the anther is along the junction with the connective’, which may suggest similar monosporangiate thecae as in Emmotum. Although Howard (1942a, p. 482) also mentioned that the anthers of Poraqueiba and Oecopetalum have a similar dehiscence as Emmotum, for Poraqueiba, he explicitly mentioned dorsal and ventral pollen sacs (Howard, 1942b, p. 50) and for Oecopetalum, when distinguishing it from Poraqueiba, he did not mention a difference in the anthers (Howard, 1942b, p. 35). The only other recognized deviation from the ground pattern of angiosperm stamens among Icacinaceae s.l. is in Polyporandra, which has anthers with numerous microsporangia that open separately and thus lack thecal organization (Sleumer, 1942; Endress and Stumpf, 1990). In Metteniusa, the long, slender anthers have four pollen sacs but each pollen sac is transversally subdivided into several microsporangia (González and Rudall, 2010). Another speciality in Metteniusa is that the two pollen sacs of a theca are relatively far apart from each other and each pollen sac has a separate dehiscence line (González and Rudall, 2010).
Gynoecium
Pseudomonomerous gynoecia, i.e. gynoecia that seem to consist of one carpel but actually have more than one carpel, are known from a number of angiosperms. In many cases, these are bicarpellate gynoecia with one carpel sterile, as shown by Eckardt (1937), in his classical study, which concentrated mainly on families of the former Urticales (now in Rosales, APG III, 2009). But Eckardt (1937) also showed pseudomonomery in a number of gynoecia with more than two carpels. However, to our knowledge, one-sided pseudotrimerous gynoecia (with three fertile carpels on one side and additional, sterile, carpels on the other) as in Emmotum are not known from any other angiosperm. Also, pseudodimerous gynoecia are exceptional in angiosperms. Pelliciera (Tetrameristaceae, Ericales) has two locules (Kobuski, 1951; Stevens, 2001 onward; Kubitzki, 2004), but the flowers may be pentamerous as reported by Schönenberger et al. (2010) and confirmed in more detail to be pentamerous by von Balthazar and Schönenberger (2013). However, in contrast to Emmotum, the locules are, in addition, not one-sided in Pelliciera.
González and Rudall (2010, p. 203) stated that their ‘results [on Metteniusa] open up the possibility that, apart from Metteniusaceae and Oncothecaceae, other putatively basal lamiids that possess two pendant ovules (e.g. Garryales, Cardiopteridaceae, and Icacinaceae s.s.) could also show cryptic pseudomonomery’. In fact, it has long been assumed that representatives of these groups have pseudomonomerous gynoecia (Engler, 1872, p. 41; Baillon, 1874, p. 202; Sleumer, 1942, pp. 323, 337; Fagerlind, 1945, p. 350) and more conspicuously so if there are two stigmatic branches (Eucommia; Eckardt, 1957). These earlier authors noted that the seemingly unicarpellate gynoecia in these groups had additional, sterile carpels, although the term ‘pseudomonomerous’ was only introduced by Eckardt (1937). Garrya commonly has two styles and two ovules in the single locule or, more rarely, three styles accompanied by three ovules (Hallock, 1930; Eyde, 1964). This suggests that the gynoecium is not pseudomonomerous in Garrya but that each carpel has one ovule. However, there is no critical study focusing on this question in Garrya, and Aucuba has not been studied in this respect either. The gynoecium of Cardiopteris (Cardiopteridaceae, Aquifoliales) was interpreted as bicarpellate-pseudomonomerous (Tobe, 2012) or tricarpellate (Kong et al., 2014).
For Emmotum, van Tieghem (1897, p. 120) and Howard (1942a, p. 482) assumed a pentamerous gynoecium, of which three carpels are fertile and the other two are strongly reduced and without locules. However, according to Stevens (2001 onward), the gynoecium is trimerous and has two sterile but only one fertile carpel with two septa. The latter interpretation is likely to be incorrect because each of the three fertile portions has two obliquely collateral ovules, which would not be expected in a single septate carpel. Each of the three locules in Emmotum has the same structure concerning ovule position as the single fertile locule in other Icacinaceae s.l. However, it is unclear whether the gynoecium of Emmotum evolved from a pentamerous gynoecium with all carpels fertile (such as in Oncotheca) by reduction of fertility or from a trimerous gynoecium with one fertile carpel (such as in probably most Icacinaceae s.s.) by an increase in fertile carpel number.
The question remains whether there are one or two sterile carpels in the gynoecium of Emmotum and other Icacinaceae, and thus whether a trilocular gynoecium of Emmotum consists of four or five carpels. The outer ovary surface is slightly five-angular. However, this shape is superimposed by the five anthers, which imprint the slightly angular shape in bud (see Endress, 2008, for imprinted shape in general) and has probably nothing to do with gynoecium pentamery. The vasculature of the ovary is complex. However, in the upper part of the adaxial side of the gynoecium, there are two relatively distinct vascular bundles (in both Emmotum species here studied), which are best interpreted as the two main (dorsal) bundles of the two reduced carpels. These two bundles have three counterparts in the radii of the three fertile locules. At the base of the gynoecium, in the gynophore, there are five main vascular bundles, alternating with the stamen vascular bundles in Emmotum nitens (shortly before anthesis). They are in the same radii as the five primary (dorsal) carpel bundles in the style. Thus, they can only be interpreted as the lowermost portions of five carpel dorsal bundles. Thus, this is a strong argument that the gynoecium has two sterile carpels, in addition to the three fertile carpels. In E. harleyi there are several groups of bundles, which still form a network at this level (at anthesis). The gynoecium does not show separate tips of the carpels. However, in E. harleyi, two radial furrows on the abaxial side of the stigma may mark the borders between the three fertile carpels. The rim of the stigma is more or less horizontal in E. nitens, but somewhat oblique in E. harleyi. From drawings in the literature, the degree of obliqueness of the rim varies in Icacinaceae s.s., depending on stigma (and style) length, e.g. strongly oblique in Apodytes (Potgieter and van Wyk, 1994b, fig. 8) and Mappia (Sleumer, 1942, fig. 97E). Even within the genus Emmotum, there are also species with a longer style (Sleumer, 1940).
Emmotum is exceptional in Icacinaceae s.l. in having three locules formed by three fertile carpels. All other genera have a single locule formed by a single fertile carpel. The latter is even true for the genus Pyrenacantha, with numerous stigmatic lobes, which simulate a multicarpellate gynoecium (Endress, 2014).
In summary, the completely syncarpous gynoecium of Emmotum is most likely pseudotrimerous with three fertile and two sterile carpels, which is indicated by the following features: (1) the three fertile carpels radiate at such an angle that the presence of two additional, reduced carpels on the opposite side of the gynoecium leads to an approximately polysymmetric arrangement of the five carpels; (2) the inner morphological surface of the gynoecium on top of the ovary forms a five-angular opening, which fits with a pentamerous gynoecium; (3) there are five main vascular bundles in the style, which correspond to the primary vascular bundles of the five carpels; (4) there are five main vascular bundles in the gynoecium base (in E. nitens), above the level of the joining vasculature of the outer floral organs, alternating with the five stamens – the five main vascular bundles at this level are the lowermost parts of the primary vascular bundles of the five carpels.
Ovules
Ovules are poorly known in Icacinaceae s.l., especially in Icacinaceae s.s. Emmotum seems to be the only known Icacinaceae s.l. with two well-developed integuments (this study). According to Fagerlind (1945), Phytocrene (Icacinaceae s.s.) has a transient bitegmic phase during development, and in this phase bitegmy is only weakly expressed. Other Emmotaceae cursorily studied here, Apodytes clusiifolia and Cassinopsis madagascariensis, appear to be unitegmic. In the literature, unitegmic ovules are specifically mentioned from the following genera of Icacinaceae s.s.: Apodytes, Desmostachys, Leretia, Poraqueiba, Rhaphiostylis (Mauritzon, 1936a), Iodes and Pyrenacantha (Mauritzon, in Sleumer, 1942) and Metteniusa (Metteniusaceae; González and Rudall, 2010); of Stemonuraceae: Gomphandra (Fagerlind, 1945; Padmanabhan, 1961) and Stemonurus (Mauritzon, 1936a); of Cardiopteridaceae: Citronella (as Villaresia), Leptaulus (Mauritzon, 1936a) and Gonocaryum (Fagerlind 1945); and of Oncothecaceae: Oncotheca (Carpenter and Dickison, 1976; Dickison, 1986). Icacinaceae s.l. were generally characterized as unitegmic by Mauritzon (1936b). Cardiopteris (Cardiopteridaceae) has orthotropous, ategmic ovules (Kong et al., 2014).
The ovules of the studied species of Icacinaceae s.l. were described as crassinucellar (Padmanabhan, 1961), weakly crassinucellar (Mauritzon, 1936a), incompletely tenuinucellar or crassinucellar (Fagerlind, 1945), almost or completely tenuinucellar (Mauritzon, 1936b), tenuinucellar (Mauritzon in Sleumer, 1942), and probably tenuinucellar (González and Rudall, 2010). In Emmotum, they are crassinucellar, but with a relatively thin nucellus (this study). In Oncothecaceae, they are crassinucellar (Carpenter and Dickison, 1976; Dickison, 1986). Among Garryales in the circumscription of APG III (2009), all three genera have crassinucellar, unitegmic ovules and, as described by Endress (2010b), they are anticampylotropous, i.e. curved in the opposite direction of a campylotropous ovule (Garrya: Kapil and Rao, 1966; Eucommia: Tang, 1962; Eckardt, 1963; Sogo and Tobe, 2006; Aucuba: Palm and Rutgers, 1917; Satô, 1971, 1976; Alimova and Shinkina, 1987).
At a higher level, bitegmic ovules occur in some basal asterids (some Ericales), but are extremely rare in euasterids (see Endress, 2011; Friis et al., 2013). According to our extensive literature search, in lamiids they are known only from Vahlia (Vahliaceae) (Raghavan and Srinivasan, 1942) and perhaps weakly and transiently also in Phytocrene (Icacinaceae s.s.) (Fagerlind, 1945). Therefore, to discover pronounced bitegmic ovules also in Emmotum (Icacinaceae s.s. or Emmotaceae) in this study was a surprise. Vahlia is also otherwise unusual in lamiids and asterids because of the completely tenuinucellar ovules and the lack of a vascular bundle in the ovules (Raghavan and Srinivasan, 1942; Krach, 1976), both features more common in Lamiales and Gentianaceae (Endress, 2011), although the genus seems to be closer to Solanales (Refulio-Rodriguez and Olmstead, 2014). In campanulids, bitegmic ovules are only known from Quintinia (Paracryphiales) (Friis et al., 2013).
Floral structure of Icacinaceae s.l. and Oncothecaceae
We include the entire Icacinaceae s.l. to show what is similar and what is different in the segregated groups of this polyphyletic family. Emmotum (this study) and Metteniusa (González and Rudall, 2010) are similar in some respect. Floral organs are very crowded in bud so that there is a lot of imprinted shape in bud (see Endress, 2008) at the supracellular and the cellular level (wavy surfaces of contiguous organs, such as ovules and locule surface, imprint of edges of anthers and of petal hairs on the gynoecium surface and mutually indented epidermal cells of contiguous organs). Petals are valvate. The gynoecium appears to be basically pentamerous, but is different in detail (see below). Similarly indented epidermal cells in floral buds were also found in Ericales (basal asterids; von Balthazar and Schönenberger (2013).
Cardiopteris (Tobe, 2012) has quite different flowers. Floral organs are not crowded and not hairy. Petals are quincuncial-imbricate, not valvate. The gynoecium, although pseudomonomerous as commonly in Icacinaceae s.l., is different in detail (Tobe, 2012; Kong et al., 2014).
The potential relationship between Oncotheca and Emmotum (both in a possible clade also including the Apodytes group and Cassinopsis), as suggested by Lens et al. (2008), is supported by the shared presence of monosporangiate thecae in Emmotum and Oncotheca. In both cases, the dorsal pollen sacs are lacking (for Oncotheca, see Dickison, 1986; Endress and Stumpf, 1990). This lack of the dorsal pollen sacs may represent a synapomorphy or an apomorphic tendency for this clade.
The gynoecium in Icacinaceae s.l. is probably pseudomonomerous in general (pseudotrimerous in Emmotum). Although gynoecium structure is not known in detail for most genera, there are probably one or two (or more) sterile carpels involved in many cases (e.g. Sleumer, 1942; González and Rudall, 2010). The position of the fertile part of the gynoecium is abaxial in Emmotum in those lateral inflorescence branches without bracts (this study). Engler (1872) figured a floral diagram of Emmotum with the fertile part directed downward, which probably means directed to the abaxial side, but he did not indicate the position of the subtending bract of the flower. Also, it has not been studied whether the abaxial position of the fertile part is a common feature in Icacinaceae s.l. Neither Engler (1872, 1896) nor Sleumer (1942) provided any other floral diagrams of Icacinaceae s.l. However, Engler's floral diagram of Emmotum is incorrect in another aspect because it shows the carpels opposite the stamens, whereas in reality they alternate with the stamens, as expected (Figs 3 and 9P).
In Cardiopteris, the fertile carpel is adaxial according to Tobe (2012). González and Rudall (2010) constructed a diagram for the gynoecium of Metteniusa but did not determine its orientation in relation to the subtending bract of the flower. They interpreted the placentation in Metteniusa as parietal; thus, the placenta would be in the symplicate zone and the two ovules would belong to two carpels. If their interpretation is correct, Metteniusa would be very different from Emmotum, where the axile placentae are in the synascidiate zone and the three pairs of ovules are each in one carpel. They did not take into consideration the potential presence of solid reduced carpels as Eckardt (1937) showed for many pseudomonomerous gynoecia or the ontogenetic ‘shift’ of sterile carpels by differential growth of the gynoecium and resulting restriction of the sterile carpels to the upper part of the gynoecium as in some Anacardiaceae (Bachelier and Endress, 2007, 2009).
In a number of Icacinaceae s.l., the ovary is conspicuously one-sided, the sterile sector developing into a coloured, fleshy part in fruit. This is known, for example, from Apodytes (Potgieter and van Wyk, 1994b), which is presumably closely related to Emmotum (Lens et al., 2008; APG III, 2009). However, it also occurs in some genera that do not belong to Icacinaceae s.s.; one of the most conspicuous cases is Irvingbaileya (Stemonuraceae) (P.K. Endress, pers. observ.). Other genera with such one-sided fruits are listed in Potgieter and van Wyk (1994b). Interestingly, although the ovary in Emmotum is conspicuously one-sided at anthesis, it does not develop such a contrasting part in fruit and its fruits are described as drupes (Engler, 1896; Mazine-Capelo and Souza, 2006). The fruits of our collections of Emmotum are not mature.
Floral structure of basal lamiids in general
Flowers are mostly small, less than 1 cm long. Sepals are congenitally united at the base, short, not protecting the inner flower organs in advanced bud. Petals are mostly free, but lacking in Garryales (except for Aucuba). They are mostly valvate, postgenitally connected in bud but opening at anthesis (but not connected for a short distance at the very base). They are basally fused into a short tube in only few Icacinaceae s.l. (Sleumer, 1942) and in Aucuba (Reidt and Leins, 1994). In Metteniusa, they are fused together with the stamens into a short tube (Sleumer, 1942; González & Rudall, 2010). The same is true for Oncotheca, but here they are quincuncial-imbricate above the tube (Dickison, 1986).
Stamens are in a single whorl, free from each other, but fused with the corolla tube in Metteniusa and Oncotheca, as just mentioned. The anthers have monosporangiate thecae in Oncotheca (Dickison, 1986), Emmotum (this study) and, perhaps, in other unstudied Emmotaceae (see above). Gynoecia include one or two sterile carpels (not in Oncotheca), and mostly two or three carpels in total, rarely reaching five carpels (Emmotum, Metteniusa and Oncotheca). There are mostly two more or less collateral, anatropous, pendant ovules per fertile carpel, but only one in Aucuba and Garrya. Ovules are crassinucellar, but with relatively thin nucellus. They are unitegmic (except in Emmotum and perhaps weakly and transiently in Phytocrene).
Floral structure of basal asterids, basal lamiids and basal campanulids
Sepals are congenitally united, short, not protecting the inner floral organs in advanced bud in Cornaceae, Icacinaceae s.l. and Aquifoliaceae, but diverse in Ericales and other Cornales than Cornaceae. Petals are free or united. They are mostly valvate, postgenitally connected in bud in Cornaceae, Phyllonomaceae (Tobe, 2013) and Icacinaceae s.l., but not in Cardiopteridaceae or Oncothecaceae, where they are quincuncial-imbricate. Also in Ericales and Cornales other than Cornaceae, sepals and petals are often imbricate.
Stamens are in a single whorl in some Cornales, some Ericaceae and all Icacinaceae s.l. But two stamen whorls occur in some Ericales and some Cornales, and polyandry in some Hydrangeaceae and Loasaceae of Cornales and in some Ericales (especially Actinidiaceae, Ebenaceae, Lecythidaceae, Marcgraviaceae, Sapotaceae, Sarraceniaceae, Symplocaceae and Theaceae). Anthers with monosporangiate thecae occur in Grubbiaceae (Cornales), Oncothecaceae (Dickison, 1986; Endress & Stumpf, 1990), Emmotum (this study) and possibly in other Emmotaceae (or Icacinaceae s.s.).
Gynoecia with one or two of the carpels sterile generally occur in Icacinaceae s.l., and thus in both basal lamiids and basal campanulids, but are not common in either basal asterids or other lamiids and campanulids. Two collateral ovules (more rarely one) per locule are present generally in Icacinaceae s.l. but not in Cornales (in some only one ovule, in others more than two), Ericales (often one or more than two ovules), or other Aquifoliales (one ovule). Ovules are often crassinucellar (in contrast to more derived lamiids and campanulids) and have an endothelium (Endress, 2011).
CONCLUSIONS
The three outstanding floral features of Emmotum mentioned in the title of this paper are of particular interest at different evolutionary levels. (1) A pseudotrimerous gynoecium with three fertile carpels on one side and two reduced carpels on the other side is unique at the level of the angiosperms to our knowledge. This pattern was already described earlier for Emmotum, but in this paper it was critically analysed for the first time. (2) Conspicuously bitegmic ovules are exceptional at the level of the lamiids because they are so far only known from Emmotum, where they were found for the first time in this study, and from Vahlia (probably Solanales). All other lamiids have unitegmic ovules to our knowledge. Finally, (3) monosporangiate thecae were described 150 years ago in Emmotum but then forgotten in the later literature and refound again in the present study. They are of particular interest because they are not very common in angiosperms but also occur in Oncothecaceae. The function of this feature is unknown in these clades. Interestingly, similar monosporangiate thecae with disappearance of the dorsal pollen sacs are ubiquitous in Asclepiadoideae of Apocynaceae, a large clade of more advanced lamiids, where the reduced pollen sac took over an important function in pollination in forming guide rails for the application of pollinaria. However, the evolution of their monosporangiate thecae took an independent course from disporangiate thecae. At a lower systematic level, it would be of interest to know whether there are other Emmotaceae or Icacinaceae s.s. with monosporangiate thecae. We did not find any explicit mention in the literature. However, from how stamens are described in some genera, it is possible that there are many such cases. Ottoschulzia, Poraqueiba and Oecopetalum would be potential candidates for study.
It is puzzling that basal branches of the two large subclades of euasterids, lamiids and campanulids, have flowers with a corresponding suite of features, whereas this suite is largely lacking in the basal asterids on the one hand and in the more advanced lamiids and campanulids on the other. Is it a synapomorphy of euasterids? This will need broad comparative studies on all these groups for evolutionary reconstructions. However, elements of this suite of features are also common in some more advanced euasterids, such as only short sepals and valvate petals that are protective in floral buds.
ACKNOWLEDGEMENTS
For advice in sectioning flowers of Emmotum, we thank Merran Matthews. For preparing microtome sections of the genera other than Emmotum, we thank Ruth Jacob and Rosemarie Siegrist. For support with scanning electron microscopy, we thank Klaus Marquardt, Center for Microscopy and Image Analysis, University of Zurich. A.R. is supported by a PQ-1D research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
LITERATURE CITED
- Alimova GK, Shinkina NA. Aucubaceae. In: Yakovlev MS, editor. Comparative embryology of flowering plants: Davidiaceae-Asteraceae. Leningrad: Nauka; 1987. pp. 21–24. [Google Scholar]
- APG III (The Angiosperm Phylogeny Group) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Bachelier JB, Endress PK. Development of inflorescences, cupules and flowers in Amphipterygium, and comparison with Pistacia (Anacardiaceae) International Journal of Plant Sciences. 2007;168:1237–1253. [Google Scholar]
- Bachelier JB, Endress PK. Comparative floral morphology and anatomy of Anacardiaceae and Burseraceae (Sapindales), with a special focus on gynoecium structure and evolution. Botanical Journal of the Linnean Society. 2009;159:499–571. [Google Scholar]
- Baillon H. Deuxième étude sur les Mappiées (suite) Adansonia. 1874;11:187–203. [Google Scholar]
- Carpenter CS, Dickison WC. The morphology and relationships of Oncotheca balansae. Botanical Gazette. 1976;137:141–153. [Google Scholar]
- de Stefano RD, Fernández-Concha GC. Morphology-inferred phylogeny and revision of the genus Emmotum (Icacinaceae) Annals of the Missouri Botanical Garden. 2011;98:1–27. [Google Scholar]
- de Stefano RD, Angulo DF, Stauffer FW. Emmotum harleyi, a new species from Bahia, Brazil, and lectotypification of other Icacinaceae. Novon. 2007;17:306–309. [Google Scholar]
- de Stefano RD, Janovec JP, Can LL. Three decades to connect the sexes: Calatola microcarpa (Icacinaceae), a new species from the Southwestern Amazon. Phytotaxa. 2013;124:43–49. [Google Scholar]
- Dickison WC. Further observations on the floral anatomy and pollen morphology of Oncotheca (Oncothecaceae) Brittonia. 1986;38:249–259. [Google Scholar]
- Eckardt T. Untersuchungen über Morphologie, Entwicklungsgeschichte und systematische Bedeutung des pseudomonomeren Gynoeceums. Nova Acta Leopoldina, n.F. 1937;5:1–112. [Google Scholar]
- Eckardt T. Zur systematischen Stellung von Eucommia ulmoides. Berichte der Deutschen Botanischen Gesellschaft. 1957;69:487–498. [Google Scholar]
- Eckardt T. Some observations on the morphology and embryology of Eucommia ulmoides Oliv. Journal of the Indian Botanical Society. 1963;42A:27–34. :–. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Endress PK. The whole and the parts: relationships between floral architecture and floral organ shape, and their repercussions on the interpretation of fragmentary fossils. Annals of the Missouri Botanical Garden. 2008;95:101–120. [Google Scholar]
- Endress PK. Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms. Journal of Systematics and Evolution. 2010a;48:225–239. [Google Scholar]
- Endress PK. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden. 2010b;97:541–583. [Google Scholar]
- Endress PK. Angiosperm ovules: diversity, development, evolution. Annals of Botany. 2011;107:1465–1489. doi: 10.1093/aob/mcr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK. Multicarpellate gynoecia in angiosperms: occurrence, development, organization and architectural constraints. Botanical Journal of the Linnean Society. 2014;174:1–43. [Google Scholar]
- Endress PK, Stumpf S. Non-tetrasporangiate stamens in the angiosperms: structure, systematic distribution and evolutionary aspects. Botanische Jahrbücher für Systematik. 1990;112:193–240. [Google Scholar]
- Engler A. Icacineae. In: Martius CFP, Eichler AW, editors. Flora Brasiliensis. Leipzig: Fleischer; 1872. pp. 40–62. Vol. 12, pars 2. [Google Scholar]
- Engler A. Icacinaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. Leipzig: Engelmann; 1896. pp. 233–257. Part III, Section 5. [Google Scholar]
- Eyde RH. Inferior ovary and generic affinities of Garrya. American Journal of Botany. 1964;51:1083–1092. [Google Scholar]
- Fagerlind F. Bau des Gynöceums, der Samenanlage und des Embryosackes bei einigen Repräsentanten der Familie Icacinaceae. Svensk Botanisk Tidskrift. 1945;39:346–364. [Google Scholar]
- Friis EM, Pedersen KR, Endress PK. Floral structure of extant Quintinia (Paracryphiales, campanulids) compared with the Late Cretaceous Silvianthemum and Bertilanthus. International Journal of Plant Sciences. 2013;174:647–664. [Google Scholar]
- González FA, Rudall PJ. Flower and fruit characters in the early-divergent lamiid family Metteniusaceae, with particular reference to the evolution of pseudomonomery. American Journal of Botany. 2010;97:191–206. doi: 10.3732/ajb.0900194. [DOI] [PubMed] [Google Scholar]
- González FA, Betancur J, Maurin O, Freudenstein JV, Chase MW. Metteniusaceae, an early-diverging family in the lamiid clade. Taxon. 2007;56:795–800. [Google Scholar]
- Hallock FA. The relationships of Garrya. The development of the flowers and seeds of Garrya and its bearing on the phylogenetic position of the genus. Annals of Botany. 1930;44:771–812. [Google Scholar]
- Hartl D. Die morphologische Natur und die Verbreitung des Apikalseptums. Beiträge zur Biologie der Pflanzen. 1962;37:241–330. [Google Scholar]
- Howard RA. Studies of the Icacinaceae. III. A revision of Emmotum. Journal of the Arnold Arboretum. 1942a;23:479–494. [Google Scholar]
- Howard RA. Studies of the Icacinaceae. IV. Considerations of the New World genera. Contributions from the Gray Herbarium of Harvard University. 1942b;142:3–60. [Google Scholar]
- Kapil RN, Rao PRM. Studies of the Garryaceae. II. Embryology and systematic position of Garrya Douglas ex Lindley. Phytomorphology. 1966;16:564–578. [Google Scholar]
- Karehed J. Multiple origins of the tropical forest tree family Icacinaceae. American Journal of Botany. 2001;88:2259–2274. [PubMed] [Google Scholar]
- Kobuski CE. Studies in the Theaceae XXII. The genus Pelliciera. Journal of the Arnold Arboretum. 1951;32:256–262. [Google Scholar]
- Kong D-R, Schori M, Lu S-G, Li L, Peng H. Floral development of Cardiopteris, with emphasis on gynoecial structure and ovular morphology. Journal of Systematics and Evolution. 2014. in press. doi:10.1111/jse.12081 doi:10.1111/jse.12081.
- Krach JE. Samenanatomie der Rosifloren I. Die Samen der Saxifragaceae. Botanische Jahrbücher für Systematik. 1976;97:1–60. [Google Scholar]
- Kubitzki K. Pellicieraceae. In: Kubitzki K, editor. The families and genera of vascular plants. Berlin: Springer; 2004. pp. 297–299. Vol. 9. [Google Scholar]
- Lens F, Karehed J, Baas P, et al. The wood anatomy of the polyphyletic Icacinaceae s.l. and their relationships within asterids. Taxon. 2008;57:525–552. [Google Scholar]
- Mauritzon J. Embryologische Angaben über Stackhousiaceae, Hippocrateaceae, und Icacinaceae. Svensk Botanisk Tidskrift. 1936a;30:541–550. [Google Scholar]
- Mauritzon J. Zur Embryologie und systematischen Abgrenzung der Reihen Terebinthales und Celastrales. Botaniska Notiser. 1936b;1936:161–212. [Google Scholar]
- Mazine-Capelo FF, Souza VC. Icacinaceae. In: Cavalcanti TB, editor. Flora do Distrito Federal, Brasil. Brasília: Embrapa Recursos Genéticos e Biotecnologia; 2006. pp. 85–89. Vol. 5. [Google Scholar]
- Miers J. Contributions to botany. London: Williams and Norgate; 1851–1861. Vol. 1. [Google Scholar]
- Padmanabhan D. A contribution to the embryology of Gomphandra polymorpha. Proceedings of the National Institute of Science of India B. 1961;27:389–398. [Google Scholar]
- Palm B, Rutgers AAL. The embryology of Aucuba japonica. Recueil des Travaux Botaniques Néerlandais. 1917;14:119–126. [Google Scholar]
- Potgieter MJ, van Wyk AE. Fruit structure of the genus Cassinopsis Sond. (Icacinaceae) in Africa. South African Journal of Botany. 1994a;60:117–122. [Google Scholar]
- Potgieter MJ, van Wyk AE. Fruit structure of the southern African species of Apodytes E. Meyer ex Arn. (Icacinaceae) Botanical Journal of the Linnean Society. 1994b;115:221–233. [Google Scholar]
- Raghavan TS, Srinivasan VK. A contribution to the life-history of Vahlia viscosa Roxb. and Vahlia oldenlandioides Roxb. Proceedings of the Indian Academy of Sciences B. 1942;15:83–105. [Google Scholar]
- Refulio-Rodriguez NF, Olmstead RG. Phylogeny of Lamiidae. American Journal of Botany. 2014;101:287–299. doi: 10.3732/ajb.1300394. [DOI] [PubMed] [Google Scholar]
- Reidt G, Leins P. Das Initialstadium der sympetalen Krone bei Sambucus racemosa L. und Viburnum farreri Stearn. Botanische Jahrbücher für Systematik. 1994;116:1–9. [Google Scholar]
- Rusby HH. The affinities of Dendrobangia Rusby. Bulletin of the Torrey Botanical Club. 1897;24:79–81. [Google Scholar]
- Santiago-Valentín E, Viruet-Oquendo E. Notes on the flower, fruit, and the reproductive phenology of the elusive Ottoschulzia rhodoxylon. Harvard Papers in Botany. 2013;18:61–65. [Google Scholar]
- Satô Y. Embryological study in Aucuba japonica Thunb., with special reference to the unusual development of the embryo sac. Science Reports, Tôhoku University, Series IV (Biology) 1971;35:201–206. [Google Scholar]
- Satô Y. Embryological studies of some cornaceous plants. Science Reports, Tôhoku University, Series IV (Biology) 1976;37:117–130. [Google Scholar]
- Schönenberger J, von Balthazar M, Sytsma KJ. Diversity and evolution of floral structure among early diverging lineages in the Ericales. Philosophical Transactions of the Royal Society. 2010;B 365:437–448. doi: 10.1098/rstb.2009.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleumer H. Beiträge zur Kenntnis der Icacinaceen und Peripterygiaceen. Notizblatt des Botanischen Gartens und Museums Berlin-Dahlem. 1940;15:228–257. [Google Scholar]
- Sleumer H. Icacinaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1942. pp. 322–396. Vol. 20b. [Google Scholar]
- Sleumer H. Icacinaceae. In: van Steenis CGGJ, editor. Floral Malesiana, Ser. Leiden: Noordhoff; 1971. pp. 1–87. I, Vol. 7. [Google Scholar]
- Sogo A, Tobe H. Mode of pollen tube growth in pistils of Eucommia ulmoides (Eucommiaceae, Garryales) International Journal of Plant Sciences. 2006;167:933–941. [Google Scholar]
- Stevens PF. 2001. onward. Angiosperm phylogeny website, version 12 (July 2012). http://www.mobot.org/MOBOT/research/APweb. [accessed 15 November 2013]
- Stull GW, Refulio-Rodriguez NF, Olmstead RG, de Stefano RD, Soltis DE, Soltis PS. Resolving enigmatic basal lamiid relationships with a plastome-scale data set. Botanical Society of America, Botany. 2014. 2014 abstract, www.2014.botanyconference.org/engine/search/index.php?func=detail&aid=387 .
- Takhtajan A. Diversity and classification of flowering plants. New York: Columbia University Press; 1997. [Google Scholar]
- Takhtajan A. Flowering plants. Berlin: Springer; 2009. [Google Scholar]
- Tang SH. Sporogenesis and gametophyte development in Eucommia ulmoides Oliv. Acta Botanica Sinica. 1962;10:29–34. [Google Scholar]
- Tobe H. Floral structure of Cardiopteris (Cardiopteridaceae) with special emphasis on systematic and evolutionary implications. Journal of Plant Research. 2012;125:361–369. doi: 10.1007/s10265-011-0450-x. [DOI] [PubMed] [Google Scholar]
- Tobe H. Floral morphology and structure of Phyllonoma (Phyllonomaceae): systematic and evolutionary implications. Journal of Plant Research. 2013;126:709–718. doi: 10.1007/s10265-013-0556-4. [DOI] [PubMed] [Google Scholar]
- Utteridge TMA. A revision of the genus Medusanthera (Stemonuraceae, Icacinaceae s.l.) Kew Bulletin. 2011;66:1–31. [Google Scholar]
- van Tieghem P. Sur les Phanérogames sans graines, formant la division des Inséminées. Bulletin de la Société Botanique de France. 1897;44:99–139. [Google Scholar]
- von Balthazar M, Schönenberger J. Comparative floral structure and systematics in the balsaminoid clade including Balsaminaceae, Marcgraviaceae and Tetrameristaceae (Ericales) Botanical Journal of the Linnean Society. 2013;173:325–386. [Google Scholar]
- Zappi DC, Lucas E, Stannard BL, et al. Lista da plantas vasculares de Catolés, Chapada Diamantina, Bahia, Brasil. Boletim de Botânica da Universidade de São Paulo. 2003;21:345–398. [Google Scholar]